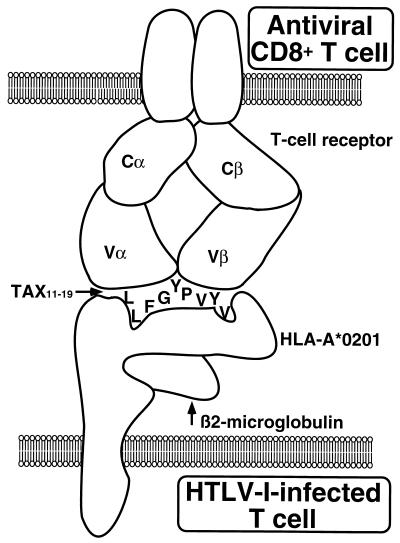
Abstract
The interactions between human T-cell lymphotropic virus type I (HTLV-I) and the cellular immune system can be divided into viral interference with functions of the infected host T cell and the subsequent interactions between the infected T cell and the cellular immune system. HTLV-I-mediated activation of the infected host T cell is induced primarily by the viral protein Tax, which influences transcriptional activation, signal transduction pathways, cell cycle control, and apoptosis. These properties of Tax may well explain the ability of HTLV-I to immortalize T cells. It is not clear, though, how HTLV-I induces T-cell transformation (interleukin-2 [IL-2] independence). Recent evidence suggests that Tax may promote the G1- to S-phase transition, although this may involve additional proteins. A role for other viral proteins that may constitutively activate the IL-2 receptor pathway has also been suggested. By virtue of their activated state, HTLV-I-infected T cells can nonspecifically activate resting, uninfected T cells via virus-mediated upregulation of adhesion molecules. This may favor viral dissemination. Moreover, the induction of a remarkably high frequency of antiviral CD8+ T cells does not appear to eliminate the infection. Indeed, individuals with a high frequency of virus-specific CD8+ T cells have a high viral load, indicating a state of chronic immune system stimulation. Thus, while an activated immune system is needed to eradicate the infection, the spread of the HTLV-I is also accelerated under these conditions. A detailed knowledge of the molecular interactions between virus-specific CD8+ T cells and immunodominant viral epitopes holds promise for the development of specific antiviral therapy.
The cellular immune response constitutes the specific host defense toward an established viral infection. Unlike the humoral immune response, which may neutralize and prevent the infection, the cellular immune response attempts to eliminate virus-infected cells. Typically, this is executed by cytotoxic CD8+ T lymphocytes (CTLs) that recognize viral peptides on the surface of the infected cells in the context of major histocompatibility complex (MHC) class I antigens. An unusual virus-host relationship occurs, however, when the virus persistently infects cells regulating the immune response, as exemplified by certain human herpesviruses and retroviruses.
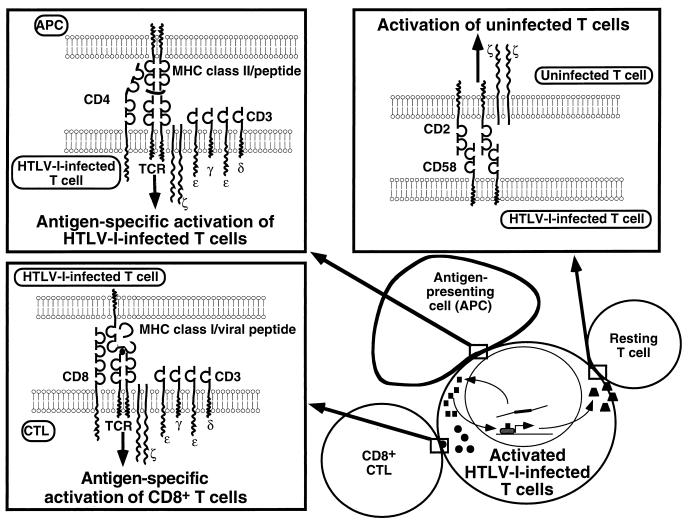
Human T-cell lymphotropic virus type I (HTLV-I) is a retrovirus that resides in and functionally alters immune cells of central importance for immunoregulation (Fig. 1). First, HTLV-I infects activated T cells and incorporates into their genome, where it persists; second, HTLV-I regulatory proteins alter activation and cell death pathways in the host T cell; third, HTLV-I-infected T cells may activate resting T cells, facilitating propagation of the infection; and finally, HTLV-I infection induces a strong antiviral immune response, which nonetheless appears incapable of eradicating the infection.
FIG. 1.
Activation of T cells by HTLV-I. Infection of CD4+ T cells influences immune system T-cell activation by at least four separate pathways. (i) The HTLV-I-infected T cells are activated by viral interference with signaling pathways and transcriptional regulation (bottom right). (ii) The HTLV-I-infected T cell interacts with and activates resting T cells (top right, activation of uninfected T cells) in a viral antigen-independent manner. The CD58-CD2 interaction (shown) is critical, but other molecular interactions and cytokines (not shown) are likely to contribute. (iii) Virus-specific CD8+ T cells (and, to a lesser degree, CD4+ T cells [not shown]) are activated by recognition of viral peptide epitopes (bottom left, antigen-specific activation of CD8+ T cells). (iv) APC may present MHC class II-restricted peptide antigens that activate the HTLV-I-infected T cell (top left, antigen-specific activation of HTLV-I-infected T cells). This activation process is altered by virtue of viral interference with the signaling cascade or the transcriptional regulation of the HTLV-I-infected T cell, or both.
In a small percentage of infected individuals, HTLV-I causes disease (121), most often either adult T-cell leukemia/lymphoma (ATL) or a chronic inflammatory disease of the central nervous system (HTLV-I-associated myelopathy/tropical spastic paraparesis, HAM/TSP). Less frequently, the joints (HTLV-I arthropathy), the eyes (HTLV-I uveitis), the skin (infective dermatitis in children), the muscles (polymyositis), or the lungs (pulmonary infiltrative pneumonitis) are affected (90). While the pathogeneses of these diseases are unknown, they all appear to involve activated, HTLV-I-infected CD4+ T cells.
In this review the interaction between HTLV-I and the cellular immune system is analyzed, with special emphasis on the multiple ways in which HTLV-I maintains an active immune system that favors viral dissemination.
INFECTION OF T CELLS BY HTLV-I
HTLV-I particles form by budding through the host cell membrane, thereby incorporating cell membrane molecules into the viral envelope. Free HTLV-I particles have extremely low infectivity (314), and transmission of HTLV-I usually requires virus-producing T cells, which allow cell-to-cell contact. The presence of 3′-azido-3′-deoxythymidine at the time of infection appears to have a protective effect on uninfected peripheral blood mononuclear cells (192). Although the receptor for HTLV-I is unknown, a putative receptor or cofactor for HTLV-I entry is thought to be encoded by a gene on chromosome 17 (273). Indirect evidence for this comes from studies with mouse-human somatic cell hybrids infected by a vesicular stomatitis virus (VSV)/HTLV-I pseudotype virus. This chimeric virus is made up of the HTLV-I envelope and the VSV core particle and therefore displays tropism identical to HTLV-I but cytopathic effects like those of VSV. Whereas mouse cells are much more resistant to HTLV-I infection than are human T cells, mouse-human somatic hybrid cells containing a region of the long arm of human chromosome 17 displayed increased susceptibility to infection by the VSV/HTLV-I pseudotype virus (273). The region on chromosome 17 has been mapped to 17q21-q23 (282), although the gene encoding the cofactor or receptor for HTLV-I entry is still unknown.
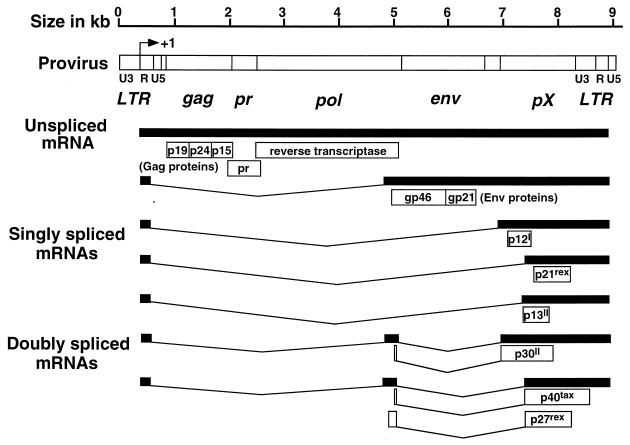
The core particle of HTLV-I carries two copies of genomic RNA as well as viral enzymes (reverse transcriptase, protease, RNase H, and integrase), which are essential for establishing the viral infection. Upon viral entry into the T cell, RNA is reverse transcribed into DNA and integrates in the host cell genome as a provirus. Although insertion of HTLV-I into the host cell DNA may have a slight preference for G+C-rich regions (325), HTLV-I does not incorporate at specific sites in the genome (260). The integrated HTLV-I provirus consists of 9,032 bp (261) and is organized in 5′ and 3′ long terminal repeats (LTR), a gag region encoding the structural proteins, a pol region encoding the reverse transcriptase, an env region encoding the envelope proteins, and a region at the 3′ end of the provirus known as pX, encoding regulatory proteins (reviewed in reference 74), which are responsible for the altered host cell functions (Fig. 2).
FIG. 2.
HTLV-I genomic organization and encoded proteins. The approximate sizes of HTLV-I proviral genes are shown for mRNAs encoding the structural Gag, protease (pr), reverse transcriptase (RT), envelope (Env), and regulatory proteins (open boxes). The protease is encoded by the 3′ end of gag mRNA in a different reading frame from the Gag proteins extending into the 5′ end of pol. The RNase H and integrase (not shown) are encoded by pol. The pX gene has four ORFs. ORF-I may encode p12I; ORF-II may encode p13II and p30II; ORF-III encodes p21rex and p27rex; and ORF-IV encodes p40tax. The LTR R region begins at the initiation of transcription site.
In vivo, the vast majority of HTLV-I provirus is found in CD4+ CD45RO+ T cells (240, 246) although CD8+ T cells can also be infected (105, 246, 309). Infection of dendritic cells has been demonstrated (191), but its importance in propagating the viral infection has been difficult to evaluate because of the complicated technical procedures involved in obtaining uncultured dendritic cells. Likewise, it has been reported that glial cells can be productively infected in vivo (173). Although this is a potentially important observation, its significance is not clear (215). HTLV-I transcription is higher in primary CD4+ T cells than in CD8+ T cells, which may explain why HTLV-I-induced leukemia and lymphoma are of the CD4+ phenotype (222). It is not known, however, what restricts the viral tropism to predominantly CD4+ T cells, since a broad range of cell types can be infected in vitro. These cell types include B cells (61), monocytes/macrophages (58, 116, 162), NK cells (187), glial cells (116, 303), endothelial cells (115, 126), promyelocytic HL-60 cells (114), and a human osteosarcoma cell line (46). Moreover, coinfection with HTLV-I and human immunodeficiency virus (HIV) broadens the spectrum of HIV cellular tropism to include CD8+ T cells, B cells, epithelial cells, and skeletal muscle cells (190).
A number of reports have described antibodies that interfere with HTLV-I syncytium formation and infection. An antibody known as 34-23 recognizes proteins of 31, 45, 55, and 70 kDa and shows increased binding to mouse-human hybrid cells containing human chromosome 17 (86). Inhibition of HTLV-I syncytium formation and infection was also achieved by an antibody to an 80-kDa glycoprotein (2). However, it is important to bear in mind that antibodies to adhesion molecules may inhibit HTLV-I infection because of interference with cell-cell contact. Recently, an antibody to vascular cell adhesion molecule 1 (VCAM-1) has been shown to prevent HTLV-I syncytium formation, although antibodies to its ligand, very late antigen 4 (VLA-4), did not (111). Moreover, cell-to-cell fusion is not sufficient to ensure viral entry (250). By examining the infectivity of HTLV-I with point mutations in the envelope glycoprotein, Rosenberg et al. (250) defined fusion-competent mutants with severe defects in infectivity. This suggests that the viral envelope glycoprotein may be involved in postfusion events required for full infectivity of HTLV-I.
Incorporation of HTLV-I into the CD4+-T-cell genome may result in either a silent or a productive infection. A silent infection is defined by the presence of HTLV-I sequences in the host cell genome in the absence of detectable HTLV-I-encoded mRNA. Thus, if the virus does not insert into critical genes, a nonproductively infected T cell is functionally indistinguishable from an uninfected T cell. Alternatively, CD4+ T cells may be productively infected by HTLV-I, resulting in viral mRNA transcription and the production of viral particles. Nevertheless, most infected T-cell clones contain a single integrated provirus, indicating that they do not reinfect themselves (247).
Single-cell cloning under limiting-dilution conditions of T cells from HAM/TSP patients indicated a frequency of HTLV-I-infected T cells between 15 and 18%, as determined by PCR amplification of pol or LTR viral sequences from genomic DNA (124, 247, 309). Unless the single-cell cloning is performed with allogeneic, uninfected feeder cells, the frequency is overestimated because of in vitro infection of the T cells (247, 309). The frequency estimate by single-cell cloning is in accordance with independent estimates by limiting-dilution PCR analysis, as well as by Southern blot analysis of genomic DNA from peripheral blood T cells (246). Since most infected T-cell clones contain a single integrated provirus (247), these analyses indicate that HAM/TSP patients have between 3 and 30% (typically 10%) HTLV-I-infected leukocytes. The majority of HTLV-I-infected T cells are silently infected (124, 246, 247, 309), and very few cells (1 in 5,000) express high levels of HTLV-I in vivo (91). It is not clear whether silently infected T cells may later reactivate viral transcription in vivo.
ACTIVATION OF HTLV-I-INFECTED T CELLS
Activation of the host T cell by HTLV-I occurs through several independent mechanisms, the most intensively studied of which is mediated through activation of cellular transcription factors by the viral trans-activator Tax. Activation of transcription factors may be viewed as the “end” signal of a transduction cascade from the membrane to the nucleus during activation, although a pathway may activate multiple transcription factors and, conversely, a transcription factor may be activated by multiple pathways. Molecular aspects of transcriptional activation by Tax have been reviewed recently (27) and are only summarized here in the context of a signaling pathway activated by HTLV-I.
Besides activation of transcription factors, HTLV-I alters signaling pathways. Typically, T-cell activation requires two signals: an antigen-specific signal mediated via the T-cell receptor (TCR) and a non-antigen-specific costimulatory signal. These signals initiate transcriptional activation of a number of genes and drive the T cell into the mid- to late G1 phase of the cell cycle, the completion of which requires cytokine signaling. HTLV-I regulatory proteins interfere with the control of each of these steps during T-cell activation.
T-Cell Receptor-Mediated Activation
Although infection by HTLV-I may lead to organ-specific inflammatory diseases, the mechanisms that target tissue destruction to the central nervous system, the joints, the eyes, the muscles, etc., are unknown (118). It is conceivable, though, that autoreactive T cells are randomly infected and cause organ-specific disease by virtue of their chronic activation and altered requirements for antigen-specific triggering (118). This hypothesis is difficult to test because of the inherent problems of generating antigen-specific T-cell clones from HTLV-I-infected individuals. That is, since mononuclear cells from these patients undergo spontaneous proliferation following 3 to 9 days in culture (133, 139), it is virtually impossible to determine antigen-specific responses, because the “background” of spontaneous proliferation often amounts to more than that of an antigen-specific response.
Recent advances in generating MHC-peptide complexes and peptide-loaded soluble MHC class I-immunoglobulin complexes make it feasible to directly isolate antigen-specific T cells (11, 101). This approach may clarify the possible role of antigen-specific T cells in HTLV-I-induced diseases. So far, however, the only way to analyze the impact of HTLV-I infection on antigen-specific T-cell responses relies on in vitro infection of established antigen-specific T-cell clones. Mitsuya et al. (206) examined the functional properties of tetanus toxoid-specific T cells infected by HTLV-I. The HTLV-I-infected T-cell clones proliferated in response to soluble tetanus toxoid, but, unlike uninfected T-cell clones, they could do so in the absence of accessory cells. This may be explained by upregulation of MHC class II on HTLV-I-infected T cells (276) followed by T-cell presentation of antigen. Thus, Scholz et al. (256) found that an HTLV-I-infected T-cell clone specific for a myelin basic protein peptide responded to an approximately 100-fold-lower concentration of soluble peptide antigen than did the parental uninfected T-cell clone. The mechanism of the enhanced response involved upregulation of MHC class II and lymphocyte function-associated antigen 3 (LFA-3; CD58) on the infected T cells, which allowed them to present the peptide antigen to other T cells. Nevertheless, compared to uninfected T cells, the response of HTLV-I-infected T cells to antigenic peptide presented by Epstein-Barr virus (EBV)-transformed B cells was slightly impaired. This demonstrated that the responsiveness of the HTLV-I-infected T cells was not enhanced; rather, the HTLV-I-infected T cells were better antigen-presenting cells (APCs).
Popovic et al. (239) examined the consequences of infecting a keyhole limpet hemocyanin (KLH)-specific CD4+ T-helper cell (SR2) with an HTLV-I-infected isolate (TK). SR2 cells proliferated and provided “help” to B lymphocytes in the presence of KLH presentation in the context of the appropriate MHC class II. However, following HTLV-I infection, TK-infected SR2 cells displayed spontaneous proliferation in the absence of antigenic peptide. Importantly, the TK-infected SR2 cells gained the ability to provide promiscuous antigen-independent help to B cells, resulting in polyclonal immunoglobulin production. The mechanism of the promiscuous B-cell help was not examined, but interleukin-4 (IL-4), IL-5, and gamma interferon (IFN-γ) are known to enhance immunoglobulin secretion, and these cytokines were spontaneously secreted by a myelin basic protein-specific HTLV-I-infected T-cell clone (255). Nevertheless, Yarchoan et al. (318) found that supernatant from an infected T-cell clone, 8.8H, which provided promiscuous antigen-independent B-cell help, did not provide help for immunoglobulin production. Although this may not entirely rule out cytokines, it suggests that cognate T-cell–B-cell interaction is required for the promiscuous B-cell help provided by HTLV-I-infected T cells.
In contrast, loss of function was demonstrated in two alloreactive cytotoxic CD4+-T-cell clones. Following infection, the number of HTLV-I p19-expressing T cells increased concomitantly with a loss of cytotoxicity (239). Although it was not shown that the HTLV-I-infected T cells were of the same origin as the parental cytotoxic T-cell clone, the observation suggested that HTLV-I infection interfered with the cytotoxic effector mechanism. Subsequent studies confirmed the loss of cytotoxicity in antigen-specific HTLV-I-infected T cells (131, 277, 318, 322) and additionally provided evidence for identical β-chain rearrangement of the TCR in the infected T-cell clones with impaired cytotoxicity and their parental uninfected T-cell clones, indicating that they were of the same origin (131, 277, 322).
During the early phase after HTLV-I infection, the expression of CD2, CD3, CD4, CD26, and CD28 remains normal whereas the expression of the IL-2 receptor α (IL-2Rα) chain and human leukocyte antigen (HLA)-DR is upregulated (276, 322). Following this stage, the HTLV-I-infected T cells may become IL-2 independent (i.e., transformed). This is usually accompanied by downregulation of CD3 expression and loss of antigen responsiveness (131, 322). Nevertheless, the loss of cytotoxic activity may be an effect on the lytic machinery, since HTLV-I-infected T cells had lost serine esterase activity (322) and since the loss of cytotoxic function occurred with normal levels of CD3 expressed on the cell surface (131).
In summary, complex alterations may influence the antigen response of HTLV-I-infected T cells and lead to both gain of function and loss of function: CD4+ T-helper cells may gain APC-like functions and the ability to provide indiscriminate B-cell help, whereas cytotoxic CD4+ T cells may lose their cytotoxic effector function (Table 1).
TABLE 1.
Alterations of antigen-specific responses of HTLV-I-infected T cells
| T-cell clone | Antigen specificitya | MHC restriction | Function | HTLV-I-infected subclone | Functional alterations induced by HTLV-I infection | Reference |
|---|---|---|---|---|---|---|
| SR2 | KLH | HLA-DR4 | Helper-inducer | SR2/TK | Antigen-independent B-cell help | 239 |
| YT | TT | ? | Helper-inducer | YTH3 | Response to soluble antigen | 205 |
| YT | TT | ? | Helper-inducer | YTH5 | Response to soluble antigen | 205 |
| 19 | (Allo) | HLA-DR1 | Helper-inducer | 19TK | Antigen-independent B-cell help, gain of NK-like activity | 277 |
| 207 | (Allo) | HLA-DR1 | Helper-inducer, cytotoxic | 207TK | Antigen-independent B-cell help, loss of cytotoxicity | 277 |
| 8.8 | (Allo) | HLA-DPw2 | Helper-inducer, cytotoxic | 8.8H | Antigen-independent B-cell help, loss of cytotoxicity | 318 |
| Ob1A12.8 | MBP(84–102) | HLA-DRB1*1501 | ? | G4 | Response to soluble antigen, partial reduced antigen response, loss of IL-10 secretion, gain of IFN-γ secretion | 256 |
| DM322A | (Allo) | HLA-DR2 | Cytotoxic | DM322A | Loss of cytotoxicity | 239 |
| AE15.3 | (Allo) | HLA-DR7 | Cytotoxic | AE15.3 | Loss of cytotoxicity | 239 |
| KN6 | HSV-1 | HLA-DR | Cytotoxic | KN6-HT | Loss of cytotoxicity | 131 |
| MY1 | HSV-1, HSV-2 | HLA-DR | Cytotoxic | MY1-HT | Loss of cytotoxicity, partial reduced antigen response | 131 |
| 827 | TT | HLA-DR3 | Cytotoxic | 827-p19-I | Response to soluble antigen, loss of cytotoxicity | 322 |
| 8.7 | (Allo) | HLA-DPw2 | Cytotoxic | 8.7H | Loss of cytotoxicity | 318 |
TT, tetanus toxoid; MBP(84–102), myelin basic protein, peptide 84–102; HSV-1, herpes simplex virus type 1. (Allo), alloantigen that has not been defined.
Recently, Mahana et al. (194) demonstrated that the phosphorylation state of the protein Vav can be influenced by proteins from the pX region of HTLV-I. Using molecular clones, they were able to associate the ability of an infected T-cell clone to induce asymptomatic infection with a downregulation of Vav phosphorylation. In contrast, a T-cell clone which induced lethal leukemia differed in two nucleotides in the pX region and displayed constitutive tyrosine phosphorylation of Vav. Since tyrosine-phosphorylated Vav is involved in the signal transduction from the TCR, this suggests the possibility—contrary to the general assumption—that minor differences in the HTLV-I sequence may be important in the pathogenesis.
PKA signaling pathway.
The second-messenger cyclic AMP (cAMP) influences T-cell signaling via a cAMP-dependent protein kinase (PKA). PKA is composed of two catalytic (C) subunits and two regulatory (R) subunits, which exist in two isoforms, giving rise to type I and type II PKA. Each regulatory subunit can bind cAMP at two distinct binding sites, which dissociates the PKA complex into R2(cAMP)4 and two catalytically active C subunits. Since type I PKA is dissociated more easily than type II PKA and since the localization of the isotypes may differ (for example, type I PKA colocalizes with the TCR, in contrast to type II PKA), differential activation of the two types of PKA may shape the response of a given cell to a variety of stimuli. Thus, it has been suggested that type I PKA is involved in the response to proliferative signals whereas type II PKA is involved in cell differentiation and the response to antiproliferative signals (44).
Activation of the catalytic subunit of PKA leads to phosphorylation of cAMP response element (CRE) binding proteins (CREBs) on Ser-133. In addition to cellular CREs, a CRE-like domain is found in each of three 21-bp imperfect repeats in the HTLV-I LTR promoter, which are known as the Tax responsive elements. Tax-mediated transactivation of the viral LTR occurs through interaction with the CREB/activating transcription factor (ATF) family of proteins (1, 278, 319). In vitro, Tax also interacts with and facilitates dimerization of other basic-region leucine zipper (bZIP)-containing proteins, thereby enhancing their DNA binding activity (16, 235, 299), although this may not be important in vivo.
Tax may activate both cellular CREs and HTLV-I LTR CREs, although the mechanisms of transactivation of these CRE sites differ (319). Murine thymoma cell lines deficient in either the catalytic subunit (lacking PKA activity) or in the adenylate cyclase (lacking endogenous cAMP, but with normal PKA) were used to evaluate the significance of the PKA signaling pathway on Tax transactivation (146, 241). Whereas the response of the viral LTR to cAMP depended on PKA, the Tax-mediated transactivation of the LTR did not require PKA activity. Nevertheless, Tax-induced transactivation decreased in the absence of PKA activity and was restored by the catalytic subunit of bovine PKA. Moreover, a single-amino-acid substitution in CREB at Ser-133, an essential phosphorylation site for transcriptional activation, attenuated both Tax- and PKA-mediated activation of the HTLV-I promoter (26). In contrast, Kwok et al. (164) found that mutation of Ser-133 in CREB did not impair Tax-mediated transactivation of the LTR but significantly impaired Tax-mediated transactivation of cellular CREs.
The difference between the HTLV-I CREs and cellular CREs may be explained by a differential requirement for CREB phosphorylation in recruiting CREB binding protein. Thus, association of Tax with cellular CRE occurs through CREB binding protein, which is recruited only in the presence of phosphorylated CREB. In contrast, Tax-mediated activation of viral CRE may occur in the absence of CREB phosphorylation (164).
CRE, together with serum response elements, is also involved in Tax-mediated activation of immediate-early genes, including c-fos, fra-1, c-jun, junD, erg-1, and erg-2 (9, 77, 78). Tax mediates activation through interaction with the serum response factor p67SRF (79, 279). Consistently, constitutively high-level expression of c-Fos (10, 77), Erg-1, and Erg-2 (9, 77) has been found in HTLV-I-transformed T cells and Tax-expressing cell lines. This provides a mechanism by which Tax may in part replace growth signals in HTLV-I-infected T cells.
PKC signaling pathway.
Activation of T cells through the TCR but not through the IL-2R (295) results in protein kinase C (PKC) activation (reviewed in reference 281). The family of PKC isoenzymes includes at least 12 members, some of which are not Ca2+ dependent. PKC isoenzymes are usually divided into three groups based on their primary structure and their activation requirements: (i) Ca2+-dependent or conventional PKCs (PKCs) include PKC-α, PKC-β1, PKC-β2, and PKC-γ; (ii) Ca2+-independent or novel PKCs (PKC) include PKC-δ, PKC-ɛ, PKC-η, PKC-θ, and PKC-μ; and (iii) atypical PKCs (PKCs), which do not respond to phorbol esters, include PKC-ζ, PKC-λ, and PKC-ι (32, 281).
In HTLV-I-infected T cells, Tax physically associates with at least three separate PKC isoforms: PKC-α, PKC-δ, and PKC-η (183). The association results in phosphorylation of Tax and an increase in autophosphorylation of PKC in vitro, indicating that Tax activates PKC activity. The significance of the phosphorylation of Tax is unclear (73, 227). However, Tax-mediated activation of PKC may explain the activation of nuclear factor κB (NF-κB)/Rel in HTLV-I-infected T cells. The NF-κB/Rel family of transcription factors includes p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), c-Rel, and RelB (185), which occur as dimers sequestered in the cytoplasm through association with NF-κB inhibitor proteins IκBα, IκBβ, IκBɛ, IκBγ, and Bcl-3. In addition, p100 (NF-κB2) and p105 (NF-κB1), precursors of p52 and p50, respectively, possess IκB domains (266). Following T-cell activation, NF-κB separates from IκB and translocates to the nucleus. The NF-κB/IκB dissociation is thought to occur following phosphorylation of IκB, but dephosphorylation of IκB may also be involved in NF-κB/Rel activation (184). Calphostin C, a PKC inhibitor, prevented both phorbol ester- and Tax-induced NF-κB DNA binding activity (183). Moreover, transfection of Jurkat T cells with a Tax mutant (M22) that fails to activate NF-κB-dependent transcription failed to induce membrane translocation of PKC (183). Tax did not appear to increase PKC phosphorylation of IκBα, suggesting the possibility that Tax activates the PKC pathway and that downstream events lead to phosphorylation of IκB and subsequent NF-κB activation. A role for Tax in activating signal transduction pathways upstream of IκBα was also suggested by Kanno et al. (149), who found that IκBα mutants which were defective in extracellular signal-induced degradation also blocked Tax-mediated NF-κB activation. Recently, several IκB kinases have been identified (62, 172, 200, 245, 258, 308, 323), and Tax may also associate with and activate these kinases (45, 88).
It has also been demonstrated that Tax may activate the NF-κB/Rel system by direct interaction with its members. Thus, Tax was found to activate NF-κB/Rel by associating with ankyrin motifs in IκBγ (113) and by interacting directly with different NF-κB/Rel members, including p50 (279), p65 (166), p100 (20, 171), and c-Rel (171). Tax has also been reported to transactivate the c-rel promoter, leading to increased c-Rel expression (179).
Collectively, these data suggest that Tax may use several mechanisms to activate NF-κB/Rel proteins: (i) by activation of PKC, (ii) by interaction with NF-κB/Rel and IκB proteins, and (iii) by activation of IκB kinases.
Activation of NF-κB has been implicated in HTLV-I-induced tumorigenesis, since the growth of both the HTLV-I-transformed T-cell line MT-2 and of fibroblastic tumors in Tax transgenic mice were inhibited by antisense oligodeoxynucleotides to mRNA of either p50 or p65 (155).
The requirements for Tax-mediated transactivation of the CREB/ATF or NF-κB/Rel pathways can be separated. Smith and Greene (271) generated Tax mutants by site-directed mutagenesis affecting two consecutive codons. Tax mutants that selectively induced either CREB/ATF but not NF-κB/Rel activity or NF-κB/Rel but not CREB/ATF activity could be defined. Similarly, Semmes and Jeang (263) generated 47 single-amino-acid Tax mutants and analyzed their transactivation ability, confirming the observation by Smith and Greene that Leu320 was important for CREB/ATF activity but not for NF-κB/Rel activity. Both studies indicated that the N-terminal 50 amino acids and a C-terminal region between amino acids 275 and 325 are important for the transactivating function of Tax. Using Tax mutants deficient in inducing either CREB/ATF or NF-κB/Rel activity, Smith and Greene found that transformation of rat fibroblasts was achieved by transfection of Tax or by transfection of a Tax mutant deficient in activation of NF-κB/Rel but not by transfection of a Tax mutant deficient in activation of CREB/ATF (272); suggesting that CREB/ATF, but not NF-κB/Rel, was critical for Tax-mediated transformation of rat fibroblasts. Since this appears to be in conflict with the data from Tax transgenic mice (155), it is likely that cell-specific factors determine the relative importance of CREB/ATF and NF-κB/Rel in transformation. Thus, the role of CREB/ATF and NF-κB/Rel proteins in the induction of ATL is unclear.
Ca2+ signaling pathway.
Activation of NF-κB/Rel or CREB/ATF is not sufficient for Tax-mediated activation of the CD28 enhancer of the IL-2 gene. LiFeng et al. (181) found that nuclear factor of activated T cells (NF-AT) complexes induced by Tax bound to the CD28 response element in the IL-2 promoter, implicating NF-AT in Tax-mediated transactivation. In contrast to the cooperation between NF-AT and the transcription factors c-Fos and c-Jun (AP1) (242), the Tax-induced NF-AT complex does not contain c-Fos or c-Jun (181). Moreover, constitutive dephosphorylation and activation of NF-ATp, a member of the NF-AT family, was found in Tax-expressing and HTLV-I-infected T-cell lines (180). The constitutive dephosphorylation of NF-ATp was reversed in the presence of cyclosporin A (CsA), an inhibitor of the calcium/calmodulin-dependent phosphatase calcineurin. This suggests that Tax activates the Ca2+ signaling pathway proximal to or at the level of calcineurin. Interestingly, activation of the Ca2+ signaling pathway downregulates IL-10 production. In particular, the combination of Ca2+ ionophores and phorbol esters results in poor IL-10 induction but significant IFN-γ production (321). Indeed, HTLV-I infection of an IL-10-producing T-cell clone resulted in a loss of its ability to secrete IL-10 but in acquisition of the ability to constitutively secrete IFN-γ (256). In contrast, transfection of Jurkat T cells with a Tax expression plasmid induced IL-10 mRNA expression and IL-10 secretion (213), and this was partially inhibited by antisense oligonucleotides to the p65 subunit of NF-κB. The reason for this discrepancy in IL-10 secretion between Tax-transfected Jurkat T cells and HTLV-I-infected T-cell clones is unclear, but a similar discrepancy in IL-2 secretion can be found between these cells (124, 199), suggesting that the level of expression of Tax or of other viral or cellular proteins may explain the difference.
MAP kinase pathways.
At least three pathways have been delineated via the small GTPases Ras, Rac, CDC42, and Rho. Ras activates extracellular signal-regulated kinases 1 and 2 (ERK-1 and ERK-2) via Raf and mitogen-activated protein (MAP) kinase/ERK kinase 1 and 2 (MEK-1 and MEK-2); Rac and CDC42 activate c-Jun N-terminal kinase (JNK) via p21-activated kinase (PAK), MEK kinase (MEKK), and JNK kinase; and Rho activates p38 via a less well characterized pathway. However, cross talk between the pathways exists; Ras may activate JNK, and CDC42 and Rac may activate p38 (reviewed in reference 182).
The transition from IL-2-dependent to IL-2-independent growth in HTLV-I-infected T cells is associated with constitutive activation of JNK (142, 311). A downstream target of JNK is the transcription factor ATF2, which, together with CREB, is important for activation of the HTLV-I promoter (75). Thus, activation of the JNK pathway by Tax helps to increase the transcription of HTLV-I. The mechanism of JNK activation was examined by Jin et al. (142), who identified a novel protein, named G-protein pathway suppressor 2 (GPS2), which interacted physically with Tax and inhibited its activation of JNK. GPS2 also inhibited tumor necrosis factor alpha (TNF-α) activation of JNK. In contrast, GPS2 did not prevent TNF-α-induced activation of p38, nor did it prevent MEKK- or JNK kinase-mediated JNK activity (142).
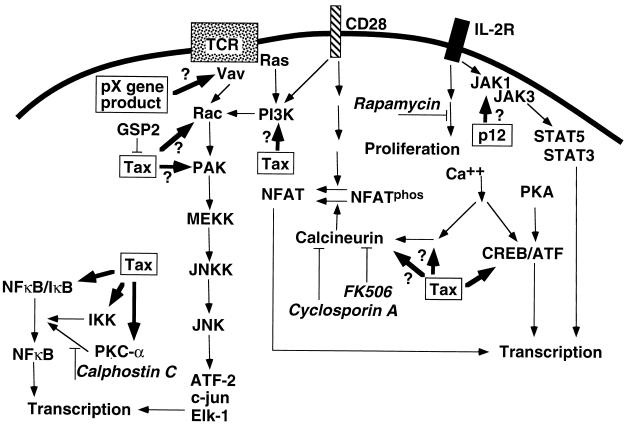
This indicates that GPS2 acts between the TNF-α receptor and MEKK and hence suggests that Tax-induced activation of the MAP kinase pathway occurs proximal to MEKK, perhaps via Ras, phosphatidylinositol-3-kinase, Rac, or PAK (Fig. 3).
FIG. 3.
Role of different signal transduction pathways in HTLV-I infection. HTLV-I-encoded proteins (predominantly, if not exclusively, Tax) may interfere with multiple intracellular signal transduction pathways. HTLV-I-encoded proteins are boxed. The bold arrow indicates the target of the viral protein, and a suggested target is indicated with a question mark. Inhibitory agents are indicated in italic. The candidate viral gene product, p12I, is shown as a possible viral mechanism of IL-2R pathway activation. A protein encoded by pX but separate from Tax may interfere with Vav. See the text for details and abbreviations.
Costimulatory Signaling Pathways
Several signaling pathways and transcription factors involved in TCR-CD3 signal transduction are activated in HTLV-I-infected T cells. However, while separate surface receptors may activate a distinct set of kinases, signaling pathways often converge on a common pathway. Hence, the presence of activated proteins in a common pathway is compatible with activation of several upstream pathways. This becomes an issue when analyzing the evidence for activation of costimulatory pathways in HTLV-I-infected T cells, since the membrane-proximal signaling molecules in these pathways have not been well defined.
CD28 costimulation.
A number of molecules expressed on T cells may enhance or costimulate T-cell activation; however, special emphasis has been placed on the CD28 molecule, since mice deficient in the CD28 gene have significantly impaired T-cell activation (98). This indicates that other costimulatory pathways cannot completely compensate for the loss of CD28 signaling (98). The salient functions of the CD28 costimulatory pathway are to enhance IL-2 transcription, stabilize IL-2 mRNA, and promote T-cell survival by upregulating the antiapoptotic protein Bcl-xL (274).
The CD28 signaling pathway is resistant to inhibition by CsA but sensitive to rapamycin (22, 145), a phenotype also observed for the IL-2R pathway (66). Nontransformed and nonimmortalized HTLV-I-infected T-cell clones were resistant to CsA and sensitive to rapamycin (124), consistent with virus-mediated activation of either the CD28 or IL-2R signaling pathway. The CD28 ligands, CD80 and CD86, are upregulated on HTLV-I-infected T cells (169, 255, 296), suggesting the possibility that the CD28 signaling pathway is constitutively active. The CD28 costimulatory requirements of HTLV-I-infected T cells were analyzed by comparing an HTLV-I-infected, antigen-specific T-cell clone with the uninfected parental T-cell clone (255). As APCs, Chinese hamster ovary (CHO) cells transfected with the restricting MHC class II element alone or in combination with CD80 or CD86 were used. These experiments demonstrated that the HTLV-I-infected T-cell clone was independent of CD80 or CD86 costimulation for proliferation and for IL-5 and IFN-γ secretion, in contrast to the uninfected T-cell clone (255). Moreover, the presence of antibodies to CD80 and CD86 prevented proliferation induced by CD80- or CD86-transfected CHO cells in uninfected but not HTLV-I-infected T cells (255). Similarly, Tax-transduced or Tax-transfected T cells cooperate with CD3-mediated activation, suggesting that Tax modulates the same costimulatory pathway as does CD28 signaling.
Taken together, these observations suggest that HTLV-I-induced T-cell activation substitutes for CD28 costimulation. However, CD80- or CD86-induced costimulation is a potent inducer of IL-2 mRNA, but HTLV-I-infected T-cell clones (124) and Tax-transduced primary T cells (8) did not express IL-2 mRNA by Northern blotting analysis. Thus, the FK506- and CsA-resistant and rapamycin-sensitive pathway is more likely to involve the IL-2R pathway (late CD28 pathway) than the early CD28 pathway.
CD2 costimulation.
The CD58-CD2 interaction is important for activation of resting and uninfected T cells by HTLV-I-infected T cells (152, 153, 309), as discussed later in this review. However, the CD2 pathway is not critical for HTLV-I-induced activation of infected T-cell clones, since FK506 and CsA inhibit the CD2 signaling pathway (22) but not the HTLV-I-induced activation of the host T cell (124).
OX40 costimulation.
A contribution from other costimulatory pathways to HTLV-I-induced T-cell activation cannot be excluded. The interaction between OX40, a TNF/nerve growth factor receptor family member, and its ligand, gp34 (OX40L), is costimulatory for T cells in the presence of mitogens (18, 94). OX40L was initially detected on HTLV-I-infected T cells as a 34-kDa glycoprotein transactivated by Tax (207, 286, 291). OX40 is induced on activated T cells and constitutively expressed on HTLV-I-transformed T cells (130); nevertheless, the significance of the OX40-OX40L interaction for HTLV-I-induced T-cell activation remains to be determined. Since OX40 mediates adhesion to OX40L expressed on vascular endothelial cells (129, 130), it is possible that this interaction is important for HTLV-I-mediated inflammatory diseases.
IL-2R Signaling Pathway
In normal T cells, the cytokine IL-2 induces the G1-to-S phase transition (36). Since this is essential for T-cell cycling, there has been interest in the possibility that HTLV-I-infected T cells use an IL-2 autocrine mechanism to traverse the G1 restriction point. The high-affinity IL-2R complex is composed of three subunits: the α, βc, and γc chains; the subscript c indicates that these chains are shared (common) among several cytokine receptors: βc is used by IL-2R and IL-15R; γc is used by IL-2R, IL-4R, IL-7R, IL-9R, and IL-15R (reviewed in reference 287). The signaling module of the IL-2R comprises βcγc, which itself is an intermediate-affinity IL-2R. The IL-2Rα chain does not participate in signal transduction, but its association with βcγc increases the receptor affinity for IL-2 by approximately 100-fold (287).
The possibility that HTLV-I particles or surface proteins can activate the IL-2R pathway was initially suggested based on an association between HTLV-I virions and the IL-2Rα chain (170); furthermore, it was shown that the HTLV-I envelope glycoprotein contains a region homologous to a segment of IL-2 that binds βc (160). Whether these features of the HTLV-I virion are important for activation of the IL-2R signaling pathway remains to be demonstrated.
The IL-2R chains are absent or expressed at very low levels in resting T cells, but their expression is inducible upon T-cell activation (53). IL-2Rα chains are expressed in large numbers on HTLV-I-transformed T cells from patients with ATL (107). The mechanism involves Tax transactivation of the promoter for the IL-2Rα chain (50, 132, 197, 268) and is mediated by activation of NF-κB (15, 176, 252). In addition, transient-transfection studies linking the promoter of IL-2 to a chloramphenicol acetyltransferase (CAT) reporter gene demonstrated that Tax may also transactivate the IL-2 promoter (132, 197, 199, 268). Although the Tax-mediated transactivation of the IL-2 promoter is not very strong, it may synergize with a TCR- or phorbol ester-mediated signal or with the HTLV-I regulatory protein Rex (197, 199).
Nonetheless, analysis of IL-2 secretion and IL-2 mRNA in HTLV-I-infected T-cell lines or clones has not implicated IL-2 autocrine growth in HTLV-I-induced T-cell activation. Arya et al. (14) did not detect IL-2 mRNA expression in HTLV-I-transformed T cells (HuT-102) by Northern blot hybridization of cloned IL-2 DNA to poly(A) isolated RNA. Likewise, Northern blot analysis of HTLV-I-infected T-cell clones at a time when they displayed spontaneous clonal proliferation did not detect IL-2 mRNA (124). Moreover, the presence of a blocking antibody to the IL-2Rα chain (anti-Tac) did not prevent the HTLV-I-induced proliferation (124). The transcription factor NF-AT is important for the initiation of IL-2 gene transcription, and CsA and FK506 inhibit IL-2 production by preventing the dephosphorylation and nuclear translocation of NF-AT. CsA or FK506 did not inhibit the spontaneous clonal proliferation of HTLV-I-infected T-cell clones, although they did inhibit TCR-CD3-mediated superimposed proliferation of these clones (124). CD28-induced signals may, however, activate NF-AT and lead to IL-2 secretion in a CsA-resistant manner (92), and CsA may not inhibit Tax-induced transactivation of the IL-2 gene (268).
Taken together, however, the data on HTLV-I-infected T-cell clones suggest that autocrine IL-2 secretion is not involved in HTLV-I-induced spontaneous clonal proliferation. In addition, Akagi and Shimotohno (8) found IL-2-independent proliferation of Tax-transduced T cells after CD3 cross-linking.
To investigate IL-2 mRNA expression in single cells, Goebels et al. (95) examined three HTLV-I-transformed T-cell lines by in situ hybridization with an IL-2 cRNA probe. Whereas 2% of HuT-102, 0.8% of MT-2, and 0.5% of MT-4 HTLV-I-transformed T-cell lines expressed IL-2 mRNA, 28 to 35% of uninfected but phorbol myristate acetate- and phytohemagglutinin-stimulated Jurkat T cells expressed IL-2 mRNA. Moreover, using a system with inducible expression of an endoplasmic reticulum-targeted single-chain antibody to knock out surface expression of IL-2Rα, Richardson et al. (248) found that IL-2Rα expression is dispensable for in vitro growth of HTLV-I-transformed T-cell lines. Thus, proliferation of HTLV-I-transformed T cells is not mediated by autocrine IL-2 secretion.
A more complex question is the role of the IL-2R pathway during the transformation process. The lack of detectable IL-2 mRNA in HTLV-I-infected T-cell clones, which are neither completely immortalized nor transformed, suggests that the transformation is not the direct result of aberrant autocrine IL-2 secretion. Nevertheless, this does not exclude an important role of the IL-2–IL-2R pathway in the early phase following HTLV-I infection. Kimata and Ratner (153) examined the presence of IL-2 mRNA and IL-2 activity following HTLV-I infection of human primary lymphocytes. While IL-2 was transiently expressed during the early phase of the infection (days 7 to 49, when viral integration is polyclonal), it was undetectable at later stages (days 100 to 150, when viral integration is oligoclonal). In contrast, expression of the viral tax-rex mRNA was low in the polyclonal phase and high in the oligoclonal phase, indicating that Tax expression did not induce autocrine IL-2 secretion. Indeed, the source of IL-2 during the polyclonal phase of the infection is uncertain, since HTLV-I-infected T cells can induce IL-2 production from uninfected T cells via T-cell–T-cell interaction (152, 310). In summary, evidence supporting a critical role for an autocrine IL-2 growth loop in HTLV-I-induced T-cell transformation is lacking.
Importantly, the development of IL-2 independence (i.e., transformation) may be associated with a constitutive IL-2-independent activation of the IL-2R signaling pathway. The ability of IL-2 to induce a signal in T cells is due to dimerization of the βc and γc chains and subsequent phosphorylation of signal transduction proteins. IL-2R signaling involves tyrosine phosphorylation and activation of the Janus family of kinase 1 and 3 (JAK1 and JAK3), which are associated with the βc and γc chains, respectively. Upon activation, JAKs phosphorylate tyrosine residues in the cytoplasmic tail of the IL-2R, which serve as docking sites for latent cytoplasmic transcription factors termed signal transducers and activators of transcription (STATs). STATs are then tyrosine phosphorylated and activated by JAKs, resulting in dimerization and nuclear translocation of STATs (52). IL-2R signaling activates STAT5 in resting T cells and activates STAT1, STAT3, and STAT5 in preactivated T cells. In contrast to nontransformed HTLV-I-infected T cells and Tax-transfected T cells, HTLV-I-transformed T-cell lines displayed constitutive tyrosine phosphorylation of JAK3 (202, 312), JAK1, STAT3, and STAT5 (202). In addition, STAT3 and STAT5 displayed constitutive DNA binding activity, and both γc and JAK3 associated with the IL-2R βc, indicating an activated IL-2R signaling pathway in the absence of IL-2 (202).
HTLV-I-infected but nonimmortalized and nontransformed T-cell clones expressed slightly elevated levels of JAK3 and STAT3 tyrosine phosphorylation but showed diminished induction of further tyrosine phosphorylation following IL-2 stimulation (255). Importantly, uncultured leukemic cells from patients with ATL expressed constitutive tyrosine phosphorylation, constitutive DNA binding activity, or both, of one or more of JAK3, STAT1, STAT3, STAT5, and STAT6, and there was a correlation between proliferation of ATL cells and activation of JAK3, STAT1, STAT3, and STAT5 (284). Since JAK1/JAK3 and STAT3/STAT5 activation is not observed in Tax-transfected T cells or newly HTLV-I-infected cord blood T cells (202), the constitutive activation of JAK and STAT may be associated with the process of transformation. In support of this notion, the transition to IL-2-independence of HTLV-I-infected cord blood T cells occurred concomitantly with an increase in constitutive STAT activity (202). Despite this association, the mechanism of JAK and STAT activation has not been linked to a viral protein yet.
A candidate viral protein that may induce IL-2R activation is p12I, which may be encoded by the first open reading frame (ORF) of the pX region of HTLV-I (161) (Fig. 2). When overexpressed, p12I physically associates with both the βc and γc chains (216) and may dimerize them, thereby initiating constitutive JAK and STAT activation and IL-2-independent proliferation (i.e., transformation). Nonetheless, alternatively spliced mRNAs of ORF-I (encoding p12I) can be found in both IL-2-independent and IL-2-dependent HTLV-I-infected T-cell lines with significant variability between cell lines (38). Although the variability in the level of p12I mRNA may indicate that splice site regulation is an important viral regulatory pathway, it also suggests that transformation cannot be explained simply by a shift in splice site utilization to ORF-I. However, it is clear that p12I is not necessary for immortalization of HTLV-I-infected T cells, since deletion of ORF-I and ORF-II in an infectious molecular clone does not affect its ability to immortalize T cells (59), and, furthermore, Tax is both necessary and sufficient for in vitro immortalization of primary human CD4+ peripheral and cord blood lymphocytes (8, 96, 97). However, these Tax-immortalized T cells remain IL-2 dependent (8, 96), suggesting a possible role for additional proteins in the transformation process.
In summary, autocrine IL-2 production may play a role early after infection, causing clonal expansion, but its production diminishes and little if any IL-2 is produced at later stages in the nontransformed, HTLV-I-infected T cell. Concomitantly with transformation, however, activation of the JAK-STAT pathway of the IL-2R is activated by an unknown mechanism.
Besides JAK1 and JAK3, the protein tyrosine kinases Syk, Lck, and Fyn associate with the IL-2R and contribute to its signal transduction (106, 156, 204). Lck and Fyn are dispensable for IL-2R-mediated signaling in HTLV-I-infected T cells (203). The transition from an IL-2-dependent state to an IL-2-independent state (i.e., transformation) in HTLV-I-infected T-cell lines correlated with downregulation of lck mRNA (159) (Table 2), and although IL-2-dependent HTLV-I-infected T-cell lines expressed lck mRNA, they scarcely expressed Lck protein (228). Consistently, Tax-transfected Jurkat T cells expressed diminished levels of Lck protein and repressed lck mRNA levels (174). Genes that are known to be repressed by Tax contain binding sites (E-boxes) for basic helix-loop-helix proteins in their promoter regions (292, 293). Whereas uninfected T cells may use two separate promoters for lck transcription, HTLV-I-infected and IL-2-dependent T cells use the upstream promoter exclusively (221). Transfection of a CAT construct under control of the distal lck promoter demonstrated that Tax downregulated this promoter, but not if a putative E-box was deleted (174). The Tax-mediated downregulation of lck mRNA was proportional to the level of pX mRNA (174). Conversely, Lck suppresses the HTLV-I promoter (229), suggesting that downregulation of Lck may further enhance viral transcription. In contrast to Lck and Fyn, altered expression of IL-2R-associated Syk in HTLV-I-infected T cells has not been reported. Syk may be a mediator of IL-2-induced activation of c-Myc (204, 208).
TABLE 2.
Alterations in signal transduction-related proteins associated with transformation of HTLV-I-infected T cells
HTLV-I-Induced Cell Cycling
Incorporation of [3H]thymidine in the absence of exogenous IL-2 in HTLV-I-infected but not uninfected T-cell clones indicates that the virus is capable of inducing the G1/S-phase transition. In its hypophosphorylated form, the retinoblastoma protein (pRb) is a negative regulator of the G1/S-phase transition, in part through its sequestering of members of the E2F family of transcription factors (267). Following T-cell activation, pRb is inactivated by phosphorylation and releases E2F, which promotes S-phase entry. During the early G1 phase, cyclins D2 and D3 and cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) are synthesized by an IL-2-independent pathway (189, 209), whereas IL-2 stimulation late in G1 induces de novo synthesis of CDK2 (209), the kinase partner of cyclin E. Initially, D-type cyclin complexes are responsible for pRb phosphorylation, whereas cyclin E-CDK2 becomes the major pRb kinase close to the G1/S-phase transition (267). The activity of cyclin-CDK complexes is regulated by a group of CDK inhibitors, of which two families have been described. One family, including p21WAF1/CIP1, p27KIP1, and p57KIP2, inhibits all CDK-cyclin complexes, whereas the other family, including p16INK4a, p15INK4b, p18INK4c, and p19INK4d, specifically inhibits the kinase activity of cyclin D-CDK4 and cyclin D-CDK6 (244, 267).
HTLV-I-mediated interference with cell cycle-regulating proteins was initially demonstrated in T-cell clones from patients with HAM/TSP; in contrast to uninfected T-cell clones, pRb was constitutively hyperphosphorylated in HTLV-I-infected T-cell clones (119). The hyperphosphorylation of pRb correlates with Tax expression in a tetracycline repressor-based Tax expression system (254). Importantly, although transforming growth factor β (TGF-β) completely abolished hyperphosphorylation of pRb in CD3-TCR-stimulated, uninfected T-cell clones, it did not prevent pRb phosphorylation in HTLV-I-infected T-cell clones (119). These observations suggest that HTLV-I activates T cells via a TGF-β-insensitive pathway. TGF-β interferes with pRb phosphorylation by its ability to (i) induce an inhibitor, p15INK4b, of CDK4 and CDK6 (104); (ii) inhibit CDK4 synthesis (70); (iii) inhibit CDK2 synthesis (89); (iv) inhibit cyclin A synthesis (89); (v) inhibit cyclin E synthesis (89); and (vi) prevent the assembly of active cyclin E-CDK2 complexes (158) by releasing sequestered p27KIP1 (238, 269). Tax does not significantly alter the expression of CDK2, CDK4, CDK6, p27KIP1, or cyclin A (7).
Suzuki et al. (280) and Low et al. (188) found that Tax associates with p16INK4a. Whereas p16INK4a inhibits CDK4 kinase activity, the Tax-p16INK4a complex has lost this function. This provides direct evidence for Tax-mediated interference with cell cycle progression (Fig. 4). p16INK4a contains four ankyrin motifs, and it is possible that Tax binds to p16INK4a via these motifs, since Tax binding to IκB can be mediated by ankyrin motifs (113). It remains to be determined whether Tax also inhibits p15INK4b, a mediator of TGF-β inhibition, which is 97% homologous to p16INK4a in the last three of its four ankyrin motifs (264). Inhibition of p16INK4a may explain the Tax-induced activity of CDK4 and CDK6 and thus the ability of Tax to induce G1- to S-phase progression in lymphocytes (254), although Tax can also activate E2F-mediated transcription independently of p16INK4a (175). Tax may also enhance cyclin D-CDK4 activity by decreasing the expression of p18INK4c (7). Interestingly, HTLV-I-infected T-cell lines expressed high levels of cyclin D2 mRNA, in contrast to uninfected T-cell lines, which predominantly expressed cyclin D3 mRNA (7). The significance of this is unknown. Tax does not appear to switch the cyclin D isotype from D2 to D3 (7, 254).
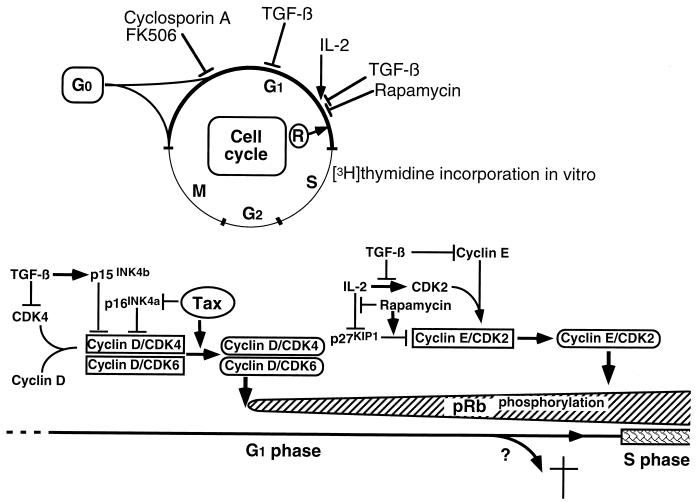
FIG. 4.
Cell cycle in HTLV-I-infected T cells. Immortalization and transformation of HTLV-I-infected T cells correlate with specific events in the cell cycle. T cells immortalized by HTLV-I infection require exogenous IL-2 to approach the restriction point (R). Transformation occurs when the infected cells no longer need exogenous IL-2 for cell cycling. Inhibitors of cell cycling and their sites of action are shown at the top. HTLV-I-infected T-cell clones are resistant to CsA, FK506, and TGF-β but sensitive to rapamycin (119, 124). A simplistic representation of selected proteins and drugs with relevance to HTLV-I-infected T-cell activation is shown at the bottom. Arrows indicate a stimulatory signal. See the text for further details.
The CDK inhibitor p27KIP1 is a critical regulator of the G1 restriction point, since (i) IL-2R signaling eliminates p27KIP1 (72, 165, 226) through a rapamycin-sensitive pathway (226); (ii) rapamycin-sensitive cells become rapamycin resistant if p27KIP1 synthesis is inhibited by antisense oligonucleotides (150); (iii) antisense inhibition of p27KIP1 synthesis prevents the cells from becoming quiescent (47, 249); and (iv) p27KIP1 links TGF-β to cell cycle arrest in mink epithelial cells (238). Despite the central role of p27KIP1 in cell cycle regulation, it is not known whether the function of p27KIP1 is altered in HTLV-I-infected T cells. Low et al. (188) did not detect an association of Tax with p27KIP1 under conditions where Tax associated with p16INK4a. Nevertheless, HTLV-I-mediated spontaneous proliferation is inhibited by rapamycin (124) but not by TGF-β (119). This indicates that p27KIP1 regulation is normal in HTLV-I-infected T-cell clones and hence not involved in their lack of inhibition by TGF-β.
In contrast, the level of the CDK inhibitor p21WAF1/CIP1 is elevated in HTLV-I-transformed T cells by a mechanism involving Tax-mediated transactivation of the promoter for p21WAF1/CIP1 (7, 39), but Tax does not physically associate with p21WAF1/CIP1 (188). The expression of p21WAF1/CIP1 is normally regulated by p53 and is responsible for p53-induced G1 arrest following DNA damage (31, 57), but Tax-induced p21WAF1/CIP1 expression is p53 independent, since it occurs in p53-null cells (39). Despite the presence of the wild-type p53 gene in most HTLV-I-transformed T cells (39), Tax inactivates p53 by inhibiting its transcription (293) and by interfering with its transactivation domain (237). The lack of fully functional p53 in HTLV-I-infected T cells may contribute to HTLV-I-induced tumorigenesis.
Thus, Tax may induce G1- to S-phase progression in lymphocytes by directly interacting with the cell cycle machinery and by influencing the transcription of cell cycle proteins and transcription factors. Most recently, Tax has also been shown to bind to a mitotic checkpoint protein, MAD1 (141). This suggest that Tax may also interfere with the G2-M phase of the cell cycle, and the specific interaction with MAD1 may explain the ability of Tax to induce multinucleated cells (141).
PROGRAMMED CELL DEATH IN HTLV-I-INFECTED T CELLS
One mechanism used to control cell growth is programmed cell death (apoptosis). T cells may undergo apoptosis by at least two separate mechanisms: (i) withdrawal of growth factors and (ii) activation-induced cell death (AICD). Withdrawal of growth factors, for example IL-2, is antigen independent and can be inhibited by the antiapoptotic proteins Bcl-xL and Bcl-2. In contrast, AICD is antigen dependent, is mediated by CD95 (Fas) or TNF-α, and is only partially inhibited by Bcl-xL or Bcl-2 (298). The CD95-CD95L interaction plays a crucial role in peripheral AICD, as demonstrated by experiments with gld mice (deficient in CD95L) and lpr mice (deficient in CD95), both of which develop a lymphoproliferative disease (219). Since HTLV-I can induce a T-cell leukemia/lymphoma and HTLV-I-infected T-cell clones proliferate spontaneously in the absence of exogenous growth factors (124), an HTLV-I-mediated interference with normal T-cell apoptosis might explain the tumorigenic ability of the virus. Indeed, proteins encoded by EBV (108), adenovirus (243), and Sindbis virus (177) have been shown to inhibit apoptosis.
Nevertheless, the effect of HTLV-I infection on T-cell survival is controversial. Copeland et al. (48) examined the sensitivity of HTLV-I-infected T-cell lines to anti-CD95 antibody-mediated apoptosis. Despite expression of high levels of CD95, the HTLV-I-infected cell lines showed reduced susceptibility to anti-CD95-induced apoptosis (at antibody concentrations between 1 and 100 ng/ml). The resistance could be transferred to susceptible Jurkat T cells by transfection of a Tax-expressing vector or by treatment with soluble Tax, suggesting that Tax conferred resistance to CD95-CD95L-mediated apoptosis. Brauweiler et al. (28) also found that HTLV-I-infected T-cell lines (SLB, MT-2, MT-4, and HuT-102) were more resistant to apoptosis-inducing stimuli, such as anti-CD95 antibodies (250 ng/ml), taxol, or UV irradiation. Importantly, Tax repressed bax gene expression, and this was mediated by a 27-bp sequence in the bax promoter containing a putative basic helix-loop-helix binding site. Bax is known to promote apoptosis by inhibiting Bcl-xL and Bcl-2, suggesting that Tax-mediated repression of bax may provide a molecular mechanism for the antiapoptotic effect of Tax. In addition, HTLV-I-infected T cells secrete thioredoxin, a small protein regulating the reduction-oxidation status in the cell. Thioredoxin has been reported to protect against oxidative stress-induced apoptosis (reviewed in reference 220).
Several reports have demonstrated that HTLV-I-infected T cells can be induced to undergo apoptosis. Fresh mononuclear cells from ATL patients are activated (CD25+) and sensitive to CD95-mediated apoptosis (55), and IL-2-dependent HTLV-I-infected T-cell lines are susceptible to anti-CD95-induced (54) and activation (CD2)-induced apoptosis (103). These apparently conflicting results may be due in part to differences in anti-CD95 antibodies and the concentrations used. Thus, Debatin et al. (54) used 10- to 100-fold-higher concentrations of anti-CD95 antibodies than did Copeland et al. (48) and Brauweiler et al. (28). Moreover, Debatin et al. (55) examined the feasibility of inducing apoptosis in freshly obtained peripheral blood lymphocytes from ATL patients but did not evaluate whether HTLV-I-infected T cells were more or less susceptible than uninfected T cells. In addition to anti-CD95, adriamycin appears to induce apoptosis in HTLV-I-infected T cells by a p53-independent pathway (85).
While these reports demonstrated the feasibility of inducing apoptosis in HTLV-I-infected T cells by exogenous stimuli, other observations have suggested that Tax itself may induce apoptosis. Chlichlia et al. (42, 43) expressed a fusion protein of Tax either N-terminal or C-terminal to the hormone binding domain of the estrogen receptor. Addition of estrogen or hydroxytamoxifen induced Tax transactivation and upregulation of CD28, CD69, and CD5 but not CD25, which required additional stimulation through the TCR-CD3 complex (43). This is surprising, since Tax has been shown to upregulate CD25 (8, 15, 50, 132, 252, 268) and increased expression of CD25 is even detected on HTLV-I-infected T-cell clones with a modest expression of Tax (124, 247). A potential concern, therefore, is whether the hormone-mediated induction of Tax had additional side effects. Importantly, Chlichlia et al. (42, 43) found that induction of Tax promoted apoptosis in T cells through a pathway that critically required the protease function of the IL-1β-converting enzyme (42). A similar conclusion was reached by Chen et al. (41) using a Cd2+-inducible Tax-system (JPX-9). Although Tax induced CD95 ligand expression (41, 42) and the CD95-CD95 ligand interaction is known to activate IL-1β-converting enzyme–proteases, blocking experiments failed to implicate this pathway in Tax-mediated induction of apoptosis (42). In contrast to these observations, a tetracycline repressor-based Tax expression system failed to detect apoptosis in lymphocytes expressing Tax (254).
Tax can induce oncogenic transformation in Rat-1 cells, a cell line derived from rat fibroblasts (285). Nevertheless, in contrast to wild-type Rat-1 cells, Tax-transformed Rat-1 cells underwent apoptosis within 7 days of incubation in serum-free medium, and this was inhibited by overexpression of Bcl-2 (313). This suggests that the expression of Tax makes Rat-1 cells growth factor dependent and susceptible to withdrawal apoptosis. Although the level of Tax expression may be critical for its biologic activities, comparative analysis of the apoptosis-inducing properties of Tax, c-Myc, and c-Fos suggested that Tax possesses relatively low apoptosis-inducing activity (80).
In conclusion, the outcome of HTLV-I infection on T-cell survival is controversial. The ability of Tax to prevent apoptosis of infected T cells is appealing, since Tax may also transform cells and since data from transgenic mice demonstrate that splenic T cells are more resistant to apoptosis induced by anti-CD95 antibodies (154). It is interesting, though, that the adenovirus E1A protein may transform cells and induce apoptosis, which is inhibited by the adenovirus E1B 19-kDa protein (243). If a similar mechanism operates in HTLV-I-infected T cells, it appears to require interaction with a cellular protein in order to explain the conflicting results obtained with Tax-transfected cells (28, 42, 43, 48). Moreover, as mentioned above, it is possible that the concentration of Tax determines the T-cell phenotype.
IMMORTALIZATION AND TRANSFORMATION OF T CELLS BY HTLV-I
Peripheral or cord blood T cells can be immortalized and eventually transformed following coculture with HTLV-I-producing T cells. Here, immortalization means the ability of the T cells to grow continuously. This may require the presence of exogenous growth factors (usually IL-2) as in the case of CTLL-2 cells. If, however, exogenous growth factors are not required, the T cells are transformed, as in the case of Jurkat cells. The distinction between immortalization and transformation is important when analyzing the impact of viral infection on T-cell activation (Table 3).
TABLE 3.
Comparison of HTLV-I-infected T-cell clones, HTLV-I-transformed T-cell lines, and peripheral blood mononuclear cells from HTLV-I-infected individualsa
| Characteristic | Uninfected T cells, T-cell clones, or PBMCsb | HTLV-I-infected T-cell clones | HTLV-I-transformed T-cell lines | PBMCs from HTLV-I-infected individuals |
|---|---|---|---|---|
| IL-2-independent growth | − | ±c | + | − |
| Spontaneous proliferationd | − | + | + | + |
| Inhibition of spontaneous proliferation by: | ||||
| Rapamycin | NAe | + | − | + |
| FK506 | NA | − | − | + |
| CsA | NA | − | − | + |
| TGF-β | NA | − | − | + |
| IFN-β | NA | − | ±f | + |
| Anti-IL-2Rα MAbg | NA | − | − | + |
| Inhibition of CD3/TCR-mediated proliferation by: | ||||
| Rapamycin | + | + | NA | + |
| FK506 | + | + | NA | + |
| CsA | + | + | NA | + |
| TGF-β | + | + | NA | + |
| IFN-β | + | + | NA | + |
| Anti-IL-2Rα MAb | + | + | − | + |
PBMC, peripheral blood mononuclear cells.
HTLV-I-infected T-cell clones require IL-2 for growth but may proliferate for a limited time in the absence of IL-2.
Spontaneous proliferation of T-cell clones is measured by IL-2 independent [3H]thymidine incorporation 7 to 14 days after stimulation. Spontaneous proliferation of peripheral blood mononuclear cells from HTLV-I-infected individuals is measured by [3H]thymidine incorporation after 5 to 7 days in culture in the absence of exogenous growth factors.
NA, not applicable.
The degree of inhibition of HTLV-I-infected T-cell lines by IFN-β depends on the T-cell line but is less than that of uninfected T-cell lines.
MAb, monoclonal antibody.
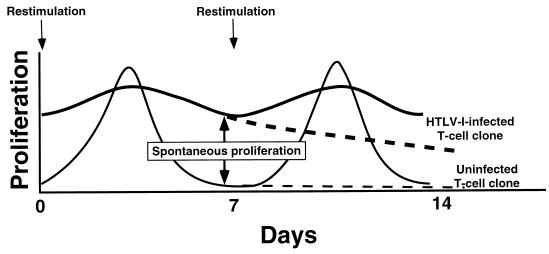
The initial stages of HTLV-I-induced T-cell activation can be studied by analyzing in vivo HTLV-I-infected T-cell clones derived by limiting-dilution single-cell cloning of peripheral blood T cells from patients with HAM/TSP (124). T-cell clones are maintained in culture by periodic restimulation with irradiated feeder cells and antigen or mitogen. Whereas uninfected T-cell clones do not incorporate significant amounts of [3H]thymidine 1 week after restimulation, productively infected T-cell clones strikingly incorporate [3H]thymidine in the absence of exogenous growth factors, a phenomenon termed spontaneous clonal proliferation (Fig. 5). This reflects an HTLV-I-induced prolonged state of T-cell activation (124, 206, 239, 309, 322). Nevertheless, HTLV-I-infected T-cell clones are not immortalized, since they do not grow continuously without restimulation with irradiated feeder cells and phytohemagglutinin. Despite their ability to enter S phase 7 to 12 days after restimulation in the absence of exogenous IL-2 (124), the HTLV-I-infected T-cell clones need exogenous IL-2 for growth beyond 12 days and thus are not transformed. It is interesting that HTLV-I-infected T-cell clones are not immortalized, since they are capable of immortalizing peripheral blood lymphocytes in vitro, although with variable efficiency (247).
FIG. 5.
Spontaneous clonal proliferation of HTLV-I-infected T-cell clones. A schematic representation of proliferation (measured by [3H]thymidine incorporation) following restimulation (arrows) of uninfected and HTLV-I-infected T-cell clones is shown. Dashed lines indicate proliferation of the T-cell clones if they are not restimulated. Spontaneous clonal proliferation is defined as the ability of HTLV-I-infected T cells to incorporate [3H]thymidine in the absence of exogenous growth factors 7 days or more after stimulation (124, 309).
The in vitro immortalization process occurs in defined stages. Initially, T-cell growth may decrease and reach a crisis stage which, at about 4 weeks following infection (96), will result in either cell death or increased cell growth. HTLV-I-infected T cells surviving the crisis display upregulated expression of CD25 and MHC class II but remain IL-2 dependent. The initial proliferative phase is characterized by polyclonal proviral integration and transient expression of IL-2 mRNA and IL-2 activity, which is undetectable at later time points (153). At approximately 100 days after infection, proviral integration is oligoclonal, with upregulation of CD25 surface expression but not of IL-2 mRNA. In contrast to IL-2 mRNA, viral tax-rex mRNA is scarcely expressed in the initial phase but is expressed abundantly at later time points (153).
The immortalization is caused by Tax. By inserting the pX region of HTLV-I into transformation-defective but replication competent herpesvirus saimiri, Grassmann et al. (97) demonstrated that the pX region was sufficient for immortalizing human thymocytes and cord blood lymphocytes. Although it cannot be excluded that proteins encoded by herpesvirus saimiri influence the function of HTLV-I proteins, subsequent analyses deleting or inserting nucleotides to generate constructs deficient in the expression of Tax or Rex, or both, have shown that Tax is both necessary and sufficient for immortalizing CD4+ cord blood lymphocytes in this system (96).
An interpretation of the requirements for immortalization and transformation from a cell cycle perspective is shown in Fig. 4. In normal T cells, TCR-mediated activation brings T cells into the G1 phase of the cell cycle, which is associated with activation of tyrosine and serine/threonine protein kinases, Ca2+ flux, and subsequent activation of transcription factors. In addition cyclin D2, D3, CDK4, and CDK6 are synthesized prior to IL-2R signaling (189, 209), which, however, is required to bring T cells beyond the G1 restriction point (36). Phosphorylation of pRb has been proposed to correspond to the restriction point (324), and IL-2R signaling allows pRb phosphorylation by eliminating a critical regulator of the restriction point, the CDK inhibitor p27KIP1 (47, 72, 150, 165, 226, 249).
Tax-immortalized but nontransformed T cells are dependent upon IL-2; hence, they cannot pass the G1 restriction point in the absence of exogenous growth factors. This indicates that overexpression of Tax alone does not induce S-phase progression but that additional events are required. Tax-immortalized T cells are able to enter G1 and, more importantly, do not exit the cell cycle by apoptosis in the presence of appropriate growth factors. The ability of Tax to activate pathways and transcription factors known to be activated in G1 during normal T-cell activation may thus be responsible for the G1 progression (immortalized phenotype).
The transformation of T cells may require the concerted action of several viral and cellular proteins. Tax may transcriptionally repress and inactivate the tumor suppressor p53 (39, 237, 293) and may repress the DNA repair enzyme β-polymerase (140), thus enhancing the accumulation of gene mutations. The transformation process, however, is expected to compensate for an IL-2R signal and hence to phosphorylate pRb and promote S-phase entry in the absence of exogenous growth factors. Constitutive activation of IL-2R-associated STAT3 and STAT5 has been demonstrated in HTLV-I-transformed T cells (202), but STAT5 does not regulate E2F (29) and thus does not induce pRb phosphorylation and S-phase entry. Recent evidence, however, suggests that Tax expression may promote pRb phosphorylation and the G1/S-phase transition (254). However, it is unclear whether this is the function of Tax alone, since control cells immortalized by Tax remained IL-2 dependent. IL-2 induces CREB/ATF1 activity late in G1 in a cAMP-independent but rapamycin-dependent manner (71). This activity may be compensated for by Tax expression, since Tax interacts with CREB/ATF1 and thereby increases its transcription activity. This interaction is essential for the ability of Tax to transform rat fibroblasts (272) and to clonally expand CD4+ T cells (6). Tax-mediated activation of cellular CREs may be important in T-cell transformation. The mechanism of CREB phosphorylation in HTLV-I-transformed T cells then becomes important, since Tax transactivation of cellular CREs is dependent on phosphorylated CREB (164).
T cells approaching the G1 restriction point may either commit to the cell cycle (if p27KIP1 is downregulated, pRb is hyperphosphorylated, and cyclin E-CDK2 is activated) or undergo apoptosis (if these conditions are not met) (186). Thus, it may be hypothesized that HTLV-I-immortalized T cells may undergo apoptosis in the absence of exogenous IL-2 because they do not approach the G1 restriction point in an appropriate way; i.e., they have not downregulated p27KIP1, which would allow activation of the pRb kinases (cyclin D-CDK4, cyclin D-CDK6, and cyclin E-CDK2). Hence, transformation is an ability to escape apoptosis in late G1, in the absence of an exogenous growth factor. Transduction in early G1 of either p16INK4a or the human papillomavirus E7 protein prevents pRb phosphorylation, further G1 progression, and subsequent activation-induced cell death (186). Interpreted in this way, the ability of Tax to inhibit p16INK4a may activate the cyclin D-CDK complexes and promote cell cycle progression to the restriction point. This may be a critical element in immortalization and perhaps in transformation. In contrast, if the T cell is not ready to enter S phase, forced cell cycle progression may provoke apoptosis. It is conceivable that the apparently contradictory results obtained by analyzing apoptosis in Tax-expressing cells, as discussed above, can be explained by different outcomes of Tax-p16INK4a interaction.
In addition to the G1-S-phase deregulation, the ability of Tax to disturb the mitotic checkpoint protein MAD1 may contribute to the transformation process. Indeed, p53 induces MAD1, and thus Tax may target both G1-S and M checkpoints via p53 (141).
ACTIVATION OF THE CELLULAR IMMUNE SYSTEM BY HTLV-I-INFECTED T CELLS
An important consequence of HTLV-I-mediated activation of the host T cell is its ability to further amplify the immune system activation to uninfected, resting T cells in an antigen-nonspecific manner and to antiviral T cells in an antigen-specific manner. Consequently, HTLV-I-infected individuals express several markers of immune system activation (reviewed in reference 49). This amplification of immune system activation may be important for both the spread of the virus and progression to disease.
Activation of Non-Virus-Specific T Cells
Wainberg et al. (300) were the first to detect the T-cell-activating properties of purified HTLV-I. HTLV-I particles were purified from the culture supernatant of an HTLV-I-producing cell line, C10/MJ2, by initial low-speed centrifugation followed by 143,000 × g for 2 h. Subsequently, virus particles were banded by overnight centrifugation through 22 to 53% sucrose gradients and detected by the presence of reverse transcriptase activity. This virus preparation had mitogenic activity when added to mononuclear cells, as assessed by [3H]thymidine incorporation and direct cell counting, but the mechanism was not further explored (300).
Extending this observation, Gazzolo and Duc Dodon (65, 87) demonstrated that an HTLV-I preparation made by ultracentrifugation (32,000 × g) of culture medium from any of three different HTLV-I-infected and virus-producing cell lines, C91/PL, HuT-102, and MT-2, induced T-cell activation mediated by autocrine IL-2 production. In contrast, the pelleted fraction from a Tax-producing but non-virus-producing cell line, C8166/45, failed to induce T-cell activation. Preincubation with 10 to 20 μg of anti-Env antibody (0.5α) per ml inhibited the mitogenic activity of a purified virion preparation made from cell-free C91/PL supernatant run through a Sepharose CL-4B chromatography column (37). Pooled fractions, with A260/A280 absorbance greater than 2, were defined as purified HTLV-I. This purification method preserves envelope glycosylation (198), which may be important, since pretreatment of C91/PL cells with an N-linked glycosylation inhibitor, tunicamycin, reduces the mitogenic activity of the preparation (37). This does not, however, specifically implicate Env, since tunicamycin also prevents appropriate glycosylation of other cell surface proteins. Despite the partial inhibition by 0.5α of the mitogenic activity of the purified virion fractions, C8166/45 T cells infected by a vaccinia virus construct expressing recombinant HTLV-I envelope proteins were unable to induce resting T cells to proliferate (37). Thus, Env proteins expressed on the virions were found to be mitogenic, in contrast to Env proteins expressed on the cell surface.
The pathway activated by the viral preparation was inhibited by antibodies to CD2 (64). The CD2 pathway was initially described as an alternative T-cell activation pathway (201). The ligand for CD2 is CD58 (LFA-3) (262, 301). Its interaction with CD2 is of very low affinity and has an extremely high dissociation rate (297), which may prevent CD2-CD58 interaction among resting T cells. Nevertheless, upregulated CD58 expression may facilitate clustering. In addition, an isoform of CD58 is glycosylphosphatidylinositol anchored (67), which allows extra lateral mobility in the membrane. When present on the APC, CD58 can efficiently enhance TCR signaling via CD2 interaction (23–25, 125, 210, 211). The CD2-mediated activation of T cells is dependent on the intracytoplasmic tail of CD2 (24) and on CD3ζ, which mediates CD2-induced T-cell activation (125, 211). Thus, the ability of anti-CD2 antibodies to prevent T-cell activation induced by an HTLV-I preparation (64) implicates CD2 signal transduction in HTLV-I-induced activation of resting T cells.
The hypothesis that HTLV-I particles are intrinsically mitogenic has been contested by others (152, 309). Using sucrose density gradient purification, Kimata et al. did not detect mitogenic activity in purified fractions of MT-2 cell supernatant, although these fractions were positive for both p19 Gag by enzyme-linked immunosorbant assay (ELISA) and gp46 Env by Western blot analysis. In addition, none of nine different antibodies to Env, including the 0.5α antibody, inhibited the activation of resting T cells mediated by HTLV-I-infected T cells (152). Similarly, Wucherpfennig et al. (309) were unable to inhibit the THTLV-I–T-cell activation by antibodies to Env. Hence, HTLV-I Env proteins expressed on the surface of T cells are not required for activation of resting T cells.
It is not settled how the CD2 pathway is activated by the HTLV-I preparation (33). It has been demonstrated that cellular proteins are associated with HIV particles (13); thus, CD58 associated with HTLV-I particles following budding through the cell membrane might permit cross-linking of CD2 by free virus. Alternatively, CD58 associated with membranes contaminating the viral preparation may lead to activation of the resting T cells. Indeed, cell membrane fragments from activated T cells and activated T cells fixed by paraformaldehyde can induce [3H]thymidine incorporation in resting T cells. This T-cell–T-cell activation is blocked by antibodies to CD58 and intercellular cell adhesion molecule 1 (ICAM-1) (CD54) (30). Importantly, Kimata and Ratner (153) found that paraformaldehyde fixation still allowed HTLV-I-producing T cells to activate resting peripheral blood T cells. Moreover, Wucherpfennig et al. (309) found that not only anti-CD2 antibodies but also anti-CD58 antibodies inhibited THTLV-I–T-cell activation and the mitogenic activity of HTLV-I virions. This strongly suggests that HTLV-I-induced activation of resting T cells is mediated by CD58-CD2 interaction.
Further characterization of the THTLV-I–T-cell activation demonstrated its partial dependence on the interaction between CD11a/CD18 (LFA-1) and CD54. Although anti-CD54 antibodies had only a marginal blocking effect alone, they enhanced the inhibition achieved by anti-CD58 antibodies. Similarly, a cooperative inhibitory effect was observed between antibodies to CD11a and CD58 (309). Kimata et al. (152) also found that antibodies to CD58 and CD2 blocked the THTLV-I–T-cell activation by using paraformaldehyde-fixed MT-2 cells as stimulator cells and peripheral blood lymphocytes as responder cells. However, they were unable to inhibit the THTLV-I–T cell activation by anti-CD11a or anti-CD54 alone. Combinations of these antibodies and anti-CD58 or anti-CD2 were not examined. However, antibodies to a number of other T-cell surface molecules, including CD80, CD28, CD4, CD8, MHC class I, and MHC class II (anti-HLA-DR, L243, and anti-HLA-DQ, S3/4), and serum with high-titer antibodies to Env and Gag had no significant blocking effect compared to control ascites (309).
Imai et al. (128) examined the expression of 19 different cell adhesion molecules in 14 HTLV-I-infected and 6 uninfected T-cell lines. Except for one HTLV-I-infected T-cell line (MT-1), the mRNA and surface protein expression of CD58 was consistently enhanced in HTLV-I-infected T cells. In addition, the surface expression of CD54 was found to be increased in HTLV-I-transformed T cells (81) and in HTLV-I-infected T-cell clones (256), probably because of Tax-mediated transactivation of the CD54 promoter (232). These observations provide a possible explanation for the ability of HTLV-I-infected T cells to adhere to and activate resting T cells via the CD2 signaling pathway.
Consistent with an IL-2 autocrine mechanism of activation (87), anti-Tac antibodies to the IL-2Rα (309) or anti-IL-2 antibodies (152) inhibited THTLV-I–T-cell activation. Moreover, Kimata et al. (152) were able to detect IL-2 mRNA induction in responding Jurkat T cells, and this was blocked by antibodies to CD58. These findings suggest that CD58 expressed on HTLV-I-infected T cells interacts with CD2 on the responding T cells, thereby inducing IL-2 production and IL-2R expression and subsequent IL-2 autocrine T-cell proliferation. Mononuclear cells from patients with HAM/TSP express activation markers in vivo and incorporate [3H]thymidine in the absence of exogenous growth factors when cultured in vitro (133, 139), a phenomenon termed spontaneous proliferation. The term “spontaneous” does not imply that the mononuclear cells are transformed, since they do not incorporate [3H]thymidine immediately after being placed in culture, nor does it imply that these cells will grow in the absence of exogenous growth factors for a prolonged period. Instead “spontaneous” refers to the incorporation of [3H]thymidine on days 3 to 9 in the absence of exogenous growth factors, which is not seen in mononuclear cells from normal individuals. Wucherpfennig et al. (309) demonstrated that antibodies to the adhesion molecules CD11a, CD54, and CD58 and to the IL-2Rα chain (CD25) inhibited the spontaneous proliferation, corroborating the significance of the CD58-CD2 interaction. Since the THTLV-I–T-cell interaction is antigen nonspecific, it may potentially occur between any preactivated and resting T cells where T-cell–T-cell interaction is established. This may explain why the number of activated T cells expressing MHC class II and IL-2Rα far exceeds the number of HTLV-I-infected T cells in many patients with HAM/TSP (139).
Interestingly, anti-MHC class I (W6/32) and anti-β2-microglobulin antibodies were shown to inhibit the spontaneous proliferation and HTLV-I expression in peripheral blood mononuclear cells (196). The mechanism of this inhibition is unknown but may involve negative signaling mediated by MHC class I cross-linking. Spontaneous proliferation was not affected by CD8 depletion (196), indicating that it does not reflect a CD8+-T-cell response to viral epitopes expressed by HTLV-I-infected CD4+ T cells.
Guyot et al. (102) examined the consequence of CD2-mediated activation of HTLV-I-infected T cells for viral transcription. Antibodies to CD2 but not to CD3 induced a 1.5- to 5.7-fold increase in the level of p24 capsid protein in cell culture supernatant concomitant with a 2- to 4-fold increase in the levels of all species of viral mRNA. Moreover, Jurkat T cells cotransfected with a Tax-expressing plasmid and a CAT reporter gene construct under the control of the HTLV-I promoter demonstrated an 11-fold increase in CAT activity following CD2 stimulation. Thus, by inference, the THTLV-I–T-cell interaction, leading to activation of resting T cells and autocrine IL-2 production, may result in upregulation of CD58 on resting T cells and a reverse interaction involving CD2 activation of the HTLV-I-infected T cells. This would then lead to enhanced viral production and possibly to infection of more T cells. Whereas antibody-mediated activation of the CD2 pathway may result in apoptosis in HTLV-I-infected T cells (103, 305), it is unclear whether this occurs after CD2-CD58 interaction.
The CD58-CD2 interaction has been established as an important costimulatory pathway in THTLV-I–T-cell interaction. In contrast, CD80/CD86-CD28 interaction was found to be important in T-cell–T-cell activation by some investigators (169, 283) but not by others (122), who demonstrated that the combined addition of antibodies to CD80 and CD86 was able to inhibit the costimulatory activity of CD80- and CD86-transfected CHO cells but not the THTLV-I–T-cell interaction. Moreover, in contrast to EBV-transformed B cells, CD86 expressed on T cells was hypoglycosylated and had significantly reduced binding affinity for CTLA4 and no detectable binding to CD28 (122), indicating the presence of cell-type-specific posttranslational modifications of CD86. Although CTLA4 may mediate a negative signal (289, 304), CD86-CTLA4 interaction did not prevent the T-cell–T-cell activation, probably because of the reduced binding affinity and the CD2-CD58 stimulatory signals. However, this finding raises the interesting possibility that CD86 on tumorigenic T cells mediates a negative signal via CTLA4 to effector cells, preventing the elimination of the tumor cells (100).
In addition to membrane-associated molecules, HTLV-I-infected T cells may induce a number of cytokines, including granulocyte-macrophage colony-stimulating factor, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-15, TGF-β, TNF-α, lymphotoxin, and IFN-γ (reviewed in reference 33), although a single HTLV-I-infected cell may not produce all of them. By using various cell types and conditions, different cytokine profiles may be produced. However, IL-6 appears to be frequently upregulated, even in nontransformed T cells, by a mechanism involving Tax-mediated transactivation of the IL-6 promoter (167, 168, 214, 224, 247, 251, 316). The significance of HTLV-I-induced cytokines is unclear, although they are likely to influence immunoregulation.
In summary, the CD2-CD58 interaction is critical for the activation of uninfected T cells by HTLV-I-infected T cells. This interaction may potentially also be initiated by virus particles which have incorporated CD58 into the envelope during the budding process through the T-cell membrane. In addition, other adhesion molecules (e.g., CD54-CD11a/CD18) and cytokines (e.g., IL-2 and IL-6) are likely to influence the activation of the uninfected T cells.
Activation of Virus-Specific CD8+ T Cells
Infection by a virus usually elicits an antiviral CD8+ CTL response, which recognizes viral peptide fragments on the surface of the infected cell. Intracellular viral proteins are degraded by proteases in the cytosol, generating peptides of variable length. Peptides of 8 to 10 residues are preferentially transported into the endoplasmic reticulum (ER) by an ATP-dependent transporter enzyme, TAP. In the ER, the peptides bind in the groove between the two α-helices of MHC class I, stabilizing the interaction between MHC class I heavy chain and β2-microglobulin. The complex between MHC class I and the viral peptide is transported to the Golgi apparatus, where further posttranslational modifications occur, and the MHC class I-viral peptide complex is subsequently expressed on the cell surface. Here it may indicate to the immune system that the cell is infected and thereby may induce the education of antigen-specific precursor CTLs.
Importantly, the binding of viral peptide to the MHC class I molecule is restricted in several ways. First, the digestion of the protein in the cytosol must generate 8 to 10 residue peptides that are preferentially transported to the ER and form stable MHC class I-peptide complexes. Second, the MHC class I molecules contain “pockets” with preferences for certain amino acid anchor residues in the peptide (69). Algorithms to predict potential MHC binding peptides from a protein exist, although this does not ensure that the peptide is naturally processed and presented.
Many viruses, especially herpesviruses, have evolved proteins that interfere with the presentation of viral proteins on the cell surface, thereby preventing a strong antiviral CTL response. For example, EBV-infected B cells may restrict viral gene expression to the nuclear antigen, EBNA1, which is poorly processed and therefore not presented on the cell surface (40, 151, 178, 218). Herpes simplex virus type 1 encodes a 9-kDa protein, known as infected-cell polypeptide 47, which inhibits peptide translocation to the ER and thus inhibits MHC class I assembly (5, 76, 112, 290, 320). Infection by cytomegalovirus downregulates MHC class I expression on the cell surface (17, 19, 56, 302, 317) by expression of various viral proteins: the pp65 matrix protein inhibits the presentation of the otherwise abundantly expressed immediate-early gene product p72 (93); the US6 protein inhibits peptide transport into the ER lumen (4, 109, 110); the US11 and U2 proteins dislocate MHC class I molecules from the ER back into the cytosol (144, 306, 307); and the U3 protein results in retention of the MHC class I complex in the ER (3). The adenovirus E3 protein binds to MHC class I and results in its retention in the ER (12, 34, 60, 135, 275, 313), and the HIV protein Nef is implicated in downregulation of MHC class I on HIV-infected T cells (259). Similar mechanisms have not been detected in HTLV-I-infected T cells; rather, infection of glial cells by HTLV-I induced the expression of MHC class I by Tax-mediated transactivation of the MHC class I gene (253).
MHC class I-restricted virus-specific CD8+ T cells can be generated from HTLV-I-infected individuals and may kill cultured tumor cells infected by HTLV-I (148, 206). Indeed, analysis of the CTL response to HTLV-I may be a very sensitive measure of previous viral exposure. In tests of coded peripheral blood lymphocyte samples from HTLV-I-seronegative and PCR-negative individuals previously exposed to HTLV-I, Nishimura et al. (225) found that 7 of 19 had detectable CD8+-T-cell response to Env and Tax proteins, as opposed to 0 of 16 matched controls from individuals without risk factors.
The CD8+-T-cell response to HTLV-I is oligoclonal in patients with HAM/TSP, although the TCR-Vβ usage differs between individual patients (82, 99, 117). Jacobson et al. analyzed the HTLV-I-specific CD8+-T-cell immune response by using EBV-transformed B cells infected with vaccinia virus expressing HTLV-I recombinants as target cells (138). The response was directed primarily against Tax, with a minor response against Env and Gag. The immunodominant epitope of Tax presented by HLA-A2 was mapped to amino acids 11 to 19 (147, 157, 294). Surprisingly, the response to Tax could be demonstrated by using freshly isolated CD8+ T cells, indicating a high precursor frequency of virus-specific CD8+ T cells (138). In patients with HAM/TSP, the precursor frequencies of antiviral cytotoxic CD8+ T cells were higher than 1/400 in peripheral blood lymphocytes and were between 1/125 and 1/488 in cerebrospinal fluid cells (136). Indeed, the real number of virus-specific CD8+ T cells is likely to be much greater (35, 217). The vaccinia virus approach has the advantage that it may detect T-cell responses to all the processed and presented peptides as long as sufficient expression of the recombinant protein allows for adequate loading of the MHC class I molecules. A comparison of the CD8+-T-cell response to target cells, either infected with the vaccinia virus-Tax construct or pulsed with synthetic peptides, demonstrated that the major response was directed against Tax 11–19 in HLA-A2 expressing patients and against Tax 186–195 in HLA-B14 expressing patients (68). Importantly, the frequencies of Tax-reactive CD8+ T cells in HTLV-I-infected asymptomatic carriers were at least 10-fold lower, 1/2,900 to 1/3,620, and were as low as 1/20,000 or undetectable in some individuals (68). Taken together, these data demonstrate a high frequency of Tax-specific CD8+ T cells in some healthy HTLV-I-infected carriers but a significantly higher frequency in patients with HAM/TSP.
Studies by Daenke et al., however (51), did not detect a clear difference in the frequency between peripheral blood Tax-specific CD8+ T cells in HAM/TSP patients and healthy HTLV-I-infected carriers. These investigators used target cells pulsed with 15-residue peptides (overlapping by 5 amino acids) spanning Tax and Rex. Since the size of these peptides would be expected to preclude binding in the groove of MHC class I, they may be degraded in the culture medium or may be taken up by the target cell for further processing and presentation. The frequencies of Tax-specific CD8+ T cells in peripheral blood from asymptomatic HTLV-I-infected carriers were on the order of 1/1,400 to 1/3,200 (mean, 1/2,438) (51), comparable to those previously reported (68). However, the frequencies of Tax-specific CD8+ T cells from HAM/TSP patients were more variable (between 1/223 and 1/4,176; mean, 1/906), overlapping with the CD8+-T-cell frequencies of some asymptomatic HTLV-I carriers (51). Thus, while the frequency of virus-specific CD8+ T cells in some HAM/TSP patients is higher than in asymptomatic HTLV-I-infected carriers, it remains to be determined whether this is important for the development of disease.
Using a novel technology, Greten et al. (101) directly examined the frequency of Tax 11–19-specific CD8+ T cells in peripheral blood and cerebrospinal fluid of HAM/TSP patients by Tax 11–19-loaded soluble HLA-A2–immunoglobulin complexes. This confirmed the presence of a high frequency (14%) of these cells in both peripheral blood and cerebrospinal fluid. In one patient, the frequency of Tax 11–19-reactive CD8+ T cells in the cerebrospinal fluid approached 1/4 (101). A high frequency of Tax 11–19-reactive CD8+ T cells in HLA-A2+ HAM/TSP patients has been confirmed by others by direct visualization with HLA-A2/Tax 11–19 tetramer complexes (21).
Additional epitopes of HTLV-I proteins and their MHC restriction have been characterized, as shown in Table 4. Using the TAP-defective cell line 174CEM.T2, Pique et al. (236) characterized potential HLA-A2 binding peptides from HTLV-I. They found that the strongest binding peptides originate from the Tax, Env, and Pol proteins, although they did not address whether the HLA-A2 binding peptides were naturally processed and presented in an infected cell. Similarly, Schönbach et al. (257) examined 64 HTLV-I peptides, which matched the predicted binding motif for HLA-B*3501. The majority of peptides with high-affinity binding for HLA-B*3501 originated from the Env protein (Table 4). When the peptides were tested in HLA-B*3501 transgenic mice for their ability to induce a CTL response, all except one were classified as medium- to high-affinity HLA-B*3501 binders, suggesting a rough correlation between HLA affinity and immunogenicity of the peptides, as previously reported for HLA-A*0201 (265). However, more important but unresolved questions are whether these peptides are naturally processed and presented, inducing an immune response in humans expressing HLA-B*3501.
TABLE 4.
CD8+-T-cell recognition of HTLV-I peptide epitopes
| Epitope sequence | Protein | Amino acid positions | MHC binding | CTL response | Reference(s) |
|---|---|---|---|---|---|
| LLFGYPVYV | Tax | 11–19 | HLA-A2 | + | 68, 123, 147, 157, 294 |
| ALFGYPVYV | Tax | 11–19 (11L→A) | HLA-A2 | + | 123 |
| CLFGYPVYV | Tax | 11–19 (11C→A) | HLA-A2 | + | 84 |
| LLFVYPVYV | Tax | 11–19 (14G→V) | HLA-A2 | − | 84 |
| LLFGAPVYV | Tax | 11–19 (15Y→A) | HLA-A2 | ± | 84, 123 |
| LLFGYAVYV | Tax | 11–19 (16P→A) | HLA-A2 | − | 123 |
| LLFGYQVYV | Tax | 11–19 (16P→Q) | HLA-A2 | − | 84 |
| LLFGYPVAV | Tax | 11–19 (18Y→A) | HLA-A2 | − | 84, 123 |
| PFGYPVYV | Tax | 12–19 (12L→P) | HLA-A2 | − | 223 |
| LFRYPVYV | Tax | 12–19 (14G→R) | NTa | − | 223 |
| LFGCPVYV | Tax | 12–19 (15Y→C) | NT | − | 223 |
| GDCVQGDWCPISGGLb | Tax | 21–35 | HLA-A2 | + | 223 |
| GDYVQGDWCPISGGL | Tax | 21–35 (23C→Y) | NT | − | 223 |
| GDCVQGDWCSISGGL | Tax | 21–35 (30P→S) | NT | − | 223 |
| GDCVQGDWCPVSGGL | Tax | 21–35 (31I→V) | NT | − | 223 |
| ISGGLCSSARLHRHALbc | Tax | 31–46 | HLA-A2 | + | 223 |
| VSGGLCSSARLHRHAL | Tax | 31–46 (31I→V) | NT | − | 223 |
| GRVIGSALQFLIPRL | Tax | 61–75 | HLA-B15 | + | 234 |
| IPPSFLQAMRKYSPFbc | Tax | 101–115 | HLA-A2 | + | 223 |
| IPLSFLQAMRKYSPF | Tax | 101–115 (103P→L) | NT | − | 223 |
| KYSPFRNGYMEPTLGbc | Tax | 111–125 | HLA-A2 | + | 223 |
| KYSPFRSGYMEPTLG | Tax | 111–125 (117N→S) | NT | − | 223 |
| KYSPFRNGCMEPTLG | Tax | 111–125 (119Y→C) | NT | − | 223 |
| LSFPDPGLRPQNLYTd | Tax | 131–145 | HLA-B35 | + | 51 |
| QNLYTLWGGSVVCMYLY | Tax | 141–157 | HLA-B35 | + | 51 |
| VVCMYLYQLSPPITWe | Tax | 151–165 | HLA-A2 | + | 233 |
| PPITWPLLPHVIFCHb | Tax | 161–176 | HLA-A2 | + | 51 |
| VIFCHPGQLGAFLTN | Tax | 172–186 | NT | + | 51 |
| GAFLTNVPY | Tax | 181–189 | HLA-B35 | +f | 257 |
| VPYKRIELL | Tax | 187–195 | HLA-B14 | + | 68, 157 |
| DCLPTTLFQPVRAPV | Tax | 211–225 | HLA-B35 | + | 51 |
| LTTPGLIWTFTDGTP | Tax | 241–255 | NT | + | 51 |
| SFHNLHLLFEEYTNI | Tax | 301–315 | HLA-B35 | + | 51 |
| EYTNIPISLLFNEKE | Tax | 311–325 | HLA-B35 | + | 51 |
| DALSAQLY | Rex | 80–87 | HLA-B35 | +f | 257 |
| YPGRVNEIL | Gag | 76–84 | HLA-B35 | +f | 257 |
| VPSSSSTPLg | Env | 247–255 | HLA-B35 | +f | 257 |
| VPSPSSTPLLh | Env | 247–255 | HLA-B35 | +f | 257 |
| SPPSTPLLYi | Env | 249–257 | HLA-B35 | +f | 257 |
| YPSLALAPAg | Env | 257–265 | HLA-B35 | +f | 257 |
| YPSLALAPHg | Env | 257–265 (265A→H) | HLA-B35 | +f | 257 |
| QAFPQCTIL | Pol | 177–185 | HLA-B35 | +f | 257 |
| SAQWIPWRLLKRAAC | Pol | 863–877 | HLA-A30 | + | 233 |
NT, not tested.
Not found to be an HLA-A2 (HLA-A*0201?) binding peptide by Pique et al. (236).
Not found to be an HLA-A*0201 binding peptide by Koenig et al. (157).
Tax 130–138 is an HLA-A2 binding peptide (236).
Tax 155–163 is likely to be the presented epitope (236).
CTL response tested in HLA-B*3501 transgenic mice.
HTLV-I ATK and MT-2.
HTLV-I HS35.
HTLV-I 1010/3.
Structural Analysis of CD8+-T-Cell Recognition of a Viral Peptide
Immunohistochemistry on autopsy material from the spinal cords of HAM/TSP patients indicates that CD8+ T cells are the predominant cell type found in the inflammatory lesions (212), although CD4+ T cells are found in very early lesions (134). There are two opposing explanations for the observed high frequency of virus-specific CD8+ T cells in HAM/TSP patients (118): (i) CD8+ T cells may kill HTLV-I-infected glial cells directly or may cause demyelination indirectly by initiating an antigen-specific inflammatory response in the central nervous system, causing nonspecific bystander killing of oligodendrocytes; or, conversely, (ii) virus-specific CD8+ T cells may play only a marginal pathogenic role and their high frequency merely represents an ineffective attempt to control the infection. In the first case, it would be desirable to eliminate or anergize the CD8+ T cells, whereas in the latter case, it would be desirable to strengthen the effector functions of the CD8+ T cells. Despite this dilemma in determining the precise function of the CD8+-T-cell response in HAM/TSP, a detailed knowledge about the viral recognition by the CD8+ T cells is necessary to attempt to rationally interfere with the antigen-specific response.
In HLA-A2-expressing individuals, the majority of the CD8+-T-cell response is directed toward the Tax 11–19/HLA-A2 complex (68, 136, 138). Crystallization of the Tax 11–19 peptide (LLFGYPVYV) in the groove of HLA-A2 led to the prediction of four potential TCR contact residues: P1 (Leu11), P5 (Tyr15), P6 (Pro16), and P8 (Tyr18) (193). The structure of the ternary complex TCR/Tax 11–19/HLA-A2 confirmed the importance of P5 (Tyr15), which protrudes into the TCR between the third variable loop of TCR Vα and Vβ (Fig. 6) and forms contacts with TCR Vα1, Vα2, Vα3, and Vβ3 (83). The TCR CDRβ3 loop extends across the peptide binding site and contacts the HLA-A2 α1 domain, P5 to P8 of the Tax 11–19 peptide, and the HLA-A2 α2 domain. The contact between P7 (Val17) and the TCR was unexpected based on the initial crystal structure of the Tax 11–19/HLA-A2 binary complex (193). However, binding of TCR to Tax 11–19/HLA-A2 alters the conformation of this MHC class I-peptide complex, pressing P6 2.7 Å down and moving P7 4.6 Å up, which is sufficient to contact the TCR (83). In contrast, P1 was not critical for TCR contact, as predicted by functional data (123, 157). The TCR binds in a diagonal manner to the HLA-A2/Tax 11–19 complex and this may be a general feature, since a separate Tax 11–19-reactive TCR, which differed in 16 of 17 residues that contacted the MHC-peptide complex, binds in a similar diagonal mode (63).
FIG. 6.
Schematic representation of CD8+-T-cell recognition of an immunodominant HTLV-I peptide. A diagram of presentation of Tax 11–19 (LLFGYPVYV) by HLA-A*0201 to a virus-specific CD8+ T cell is shown. Tax 11–19 is anchored in HLA-A*0201 by Leu12 and Val19, whereas the middle of the peptide bulges toward the TCR (193). Virtually the entire peptide is buried in the interface between HLA-A*0201 and the TCR, which contacts the binding site in a diagonal orientation (83). The TCR third variable loops of both Vα and Vβ contact the protruding Tyr15 in the middle of the viral peptide. The CDR1 loops of the TCR are also positioned over the peptide, with CDR1α extending from the N-terminal peptide to Tyr15 and CDR1β extending from the C-terminal peptide to Tyr15, whereas the TCR CDR2α loop is placed over the α2 domain of the HLA-A*0201 α-helix and the CDR2β loop is placed over the α1 domain of the α-helix (83). The central position of Tyr15 explains why altering this amino acid influences the TCR interaction with the viral peptide/HLA-A*0201 complex (84, 123).
Detailed knowledge of the TCR-peptide-MHC class I interaction allows modulation of the immune response. Single-amino-acid substitutions in the peptide antigen may generate altered peptide ligands (APLs) with altered functional properties. Altering the viral peptide antigen may, however, generate APLs with poor or no ability to bind either MHC class I or the TCR. Garboczi et al. (84) evaluated the ability of the ternary complex to assemble with APLs of Tax 11–19 by using a native gel band shift assay (84). Whereas Tax 11–19, Tax 11–19 (11L→C), and Tax 11–19 (15Y→A) allowed assembly of the ternary complex, Tax 11–19 (14G→V), Tax 11–19 (16P→Q), and Tax 11–19 (18Y→A) did not, although they did bind to HLA-A2.
Analyses of Tax-specific CD8+-T-cell clones with a TCR identical to that crystallized in the ternary complex with Tax 11–19/HLA-A2 demonstrated that a Tax 11–19 (15Y→A) substitution altered the response of the virus-specific CD8+ T cells, since this peptide did not induce clonal expansion or IL-2 production although it did induce a cytolytic response (123). Similarly, Tax 11–19 (15Y→A) induced a split in chemokine secretion, with virtually normal MIP-1α secretion but impaired MIP-1β secretion (84) (Table 5). Moreover, when Tax 11–19 (15Y→A) was presented by B cells or T cells, it induced unresponsiveness (anergy) in the CD8+ T cells (123). Anergic CD8+ T cells maintained the ability to lyse target cells (120, 231), to proliferate in response to IL-2, and to respond to TCR ligation by protein tyrosine phosphorylation and upregulation of CD40L. The induction of anergy can be prevented by bystander mononuclear cells (120). It is possible that novel costimulatory molecules (143), cytokines secreted by professional APCs, or both, are required during the primary stimulation in order to prevent CD8+-T-cell anergy induction. Thus, anergy induction by B-cell presentation of antigen indicates that the types of APC that can induce a productive immune response are limited and further suggests that the tissue localization of an immune response to HTLV-I may perhaps be restricted by the availability of monocytes or dendritic cells. Since Tax presentation by CD4+ T cells also induced CD8+-T cell anergy (120), an important question is whether HTLV-I-infected CD4+ T cells can serve as APCs for educating CD8+ T cells in vivo. Dendritic cells can be infected by HTLV-I (191) and would be expected to be much more efficient APCs. Thus, the APC responsible for educating the large number of antiviral CD8+ T cells in HAM/TSP remains to be determined.
TABLE 5.
Altered functional response of virus-specific CD8+-T-cell clones by a single-amino-acid mutation in an immunodominant Tax peptidea
| Function | Response with:
|
|
|---|---|---|
| Tax 11–19 | Tax 11–19 (15Y→A) | |
| [3H]thymidine incorporation | + | − |
| Cytotoxicity | + | + |
| p56lck activity | + | + |
| IL-2 secretion | + | − |
| IFN-γ secretion | + | (+) |
| MIP-1α secretion | + | + |
| MIP-1β secretion | + | (+) |
Activation of Virus-Specific CD4+ T Cells
Although antiviral cellular immune responses usually reside in the CD8+ subset of T cells, virus-specific CD4+-T-cell responses have also been described. However, a predominantly CD4+ virus-specific T-cell response may indicate a defect in the antigen presentation pathway, since cytosolic viral proteins are presented by MHC class I (230). By using synthetic peptides from Env gp46, CD4+ CTL lines from two HAM/TSP patients from different geographical areas demonstrated responses to the same immunogenic region spanning amino acids 196 to 209 (137). This sequence was also shown to elicit a proliferative response in immunized mice (163). The CD4+-T-cell response in naive (uninfected) individuals to HTLV-I Env was directed toward Env gp46 peptides spanning amino acids 76 to 90, 136 to 160, 171 to 185, and 196 to 210 and toward Env gp21 peptides spanning amino acids 366 to 400 and 436 to 485. The gp21 epitopes were restricted by HLA-DRB1*0101 and HLA-DRB1*1502, alleles that are frequently found in HAM/TSP patients from Japan (315). The CD4+-T-cell response to Env peptides in HTLV-I seronegative individuals is characterized by heterogeneous TCR Vβ usage (195), whereas the TCR Vβ usage in Env-specific CD4+ T cells from HAM/TSP patients has not been analyzed.
CONCLUSIONS
HTLV-I infects CD4+ T cells and incorporates into the genome as a provirus. Whereas more than 10% of CD4+ T cells may be infected in vivo, only a small fraction of productively infected T cells cause activation of the immune system. While an activated immune system is needed to eradicate the infection, the spread of the virus is also accelerated under these conditions. The interactions between HTLV-I and the cellular immune system can be divided into viral interference with functions of the infected host T cell and the subsequent interactions between the infected T cell and the cellular immune system.
HTLV-I-mediated activation of the infected host T cell is induced primarily by the viral protein Tax, which influences transcriptional activation, signal transduction pathways, cell cycle control, and apoptosis. These properties of Tax recapitulate T-cell activation events during the G1 phase of the cell cycle and may well explain the ability of Tax to immortalize T cells. However, it is not yet clear how HTLV-I induces T-cell transformation. An important function of IL-2 may be to activate CREB/ATF, which are also activated by Tax. Furthermore, recent evidence suggests that Tax may promote the G1/S-phase transition, although this may involve additional proteins. A role for other viral proteins that may constitutively activate the IL-2R pathway in HTLV-I-immortalized T cells has also been suggested. In addition, Tax may abrogate a mitotic checkpoint.
By virtue of their activated state, HTLV-I-infected T cells can nonspecifically activate resting, uninfected T cells via virus-mediated upregulation of adhesion molecules. This may also favor viral dissemination, since transmission of HTLV-I usually requires T-cell–T-cell interaction. Moreover, the induction of a remarkably high frequency of antiviral CD8+ T cells does not appear to eliminate or control the HTLV-I infection. Indeed, individuals with a high frequency of virus-specific CD8+ T cells have a high viral load, indicating a state of chronic immune system stimulation. It is not understood why such a high frequency of antiviral CD8+ T cells (1 in 4 CD8+ T cells may be specific for a single viral epitope) can coexist with such a high frequency of virus-infected CD4+ T cells. Perhaps the circulation of infected CD4+ T cells allows them to escape CD8+-T-cell-mediated killing.
Thus, the complex interaction of HTLV-I with T cells allows the virus to persist in the host by expanding the population of infected T cells and by enhancing the spread of the virus to uninfected resting T cells. The host responds to the infection by a vigorous education of virus-specific CD8+ T cells but fails to eliminate the virus. Our detailed knowledge of the molecular interactions between virus-specific CD8+ T cells and immunodominant viral epitopes holds promise for the development of specific antiviral therapy.
ACKNOWLEDGMENTS
I thank G. J. Buckle and D. A. Hafler for discussions.
I thank the Danish Multiple Sclerosis Society, the Danish Medical Research Council, and the National Multiple Sclerosis Society for supporting part of my work referenced in this review.
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agadjanyan M G, Ugen K E, Wang B, Williams W V, Weiner D B. Identification of an 80-kilodalton membrane glycoprotein important for human T-cell leukemia virus type I and type II syncytium formation and infection. J Virol. 1994;68:485–493. doi: 10.1128/jvi.68.1.485-493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10994. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J H J, Ploegh H L, Peterson P A, Yang Y, Früh K. The ER luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 5.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Früh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus protein ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 6.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 7.Akagi T, Ono H, Shimotohno K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene. 1996;12:1645–1652. [PubMed] [Google Scholar]
- 8.Akagi T, Shimotohno K. Proliferative response to tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J Virol. 1993;67:1211–1217. doi: 10.1128/jvi.67.3.1211-1217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandre C, Charnay P, Verrier B. Transactivation of Krox-20 and Krox-24 promoters by HTLV-I Tax protein through common regulatory elements. Oncogene. 1991;6:1851–1857. [PubMed] [Google Scholar]
- 10.Alexandre C, Verrier B. Four regulatory elements in the human c-fos promoter mediate transactivation by HTLV-I Tax protein. Oncogene. 1991;6:543–551. [PubMed] [Google Scholar]
- 11.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 12.Anderson M, Pääbo P, Nilsson T, Peterson P A. Impaired intracellular transport of class I MHC antigen as a possible means for adenoviruses to evade immune surveillance. Cell. 1985;43:215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 13.Arthur L O, Bess J W, Sowder R C, Benveniste H, Mann R E, Chermann J C, Enderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 14.Arya S K, Wong-Staal F, Gallo R C. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science. 1984;223:1086–1087. doi: 10.1126/science.6320374. [DOI] [PubMed] [Google Scholar]
- 15.Ballard D W, Böhnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I Tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 16.Baranger A M, Palmer C R, Hamm M K, Glebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 17.Barnes P D, Grundy J E. Down-regulation of the class HLA heterodimer and β2-microglobulin on the surface of cells infected with cytomegalovirus. J Gen Virol. 1992;73:2395–2403. doi: 10.1099/0022-1317-73-9-2395. [DOI] [PubMed] [Google Scholar]
- 18.Baum P R, Gayle III R B, Ramsdell F, Srinivasan S, Sorensen R A, Watson M L, Seldin M F, Baker E, Sutherland G R, Clifford K N, Alderson M R, Goodwin R G, Fanslow W C. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beersma M F C, Bijlmakers M J E, Ploegh H L. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J Immunol. 1993;151:4455–4464. [PubMed] [Google Scholar]
- 20.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-kappa B2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieganowska K, Höllsberg P, Buckle G J, Lim D-G, Greten T J, Schneck J, Altman J, Jacobson S, Ledis S L, Hanchard B, Chin J, Morgan O, Roth P A, Hafler D A. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus type I-associated myelopathy. J Immunol. 1999;162:1765–1771. [PubMed] [Google Scholar]
- 22.Bierer B, Schreiber S, Burakoff S. The effect of the immunosuppressant FK506 on alternate pathways of T cell activation. Eur J Immunol. 1991;21:439–445. doi: 10.1002/eji.1830210228. [DOI] [PubMed] [Google Scholar]
- 23.Bierer B E, Barbosa J, Herrmann S, Burakoff S J. Interaction of CD2 with its ligand, LFA-3, in human T cell proliferation. J Immunol. 1988;140:3358–3363. [PubMed] [Google Scholar]
- 24.Bierer B E, Peterson A, Gorga J C, Herrmann S H, Burakoff S J. Synergistic T cell activation via the physiological ligands for CD2 and the T cell receptor. J Exp Med. 1988;168:1145–1156. doi: 10.1084/jem.168.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bockenstedt L K, Goldsmith M A, Dustin M, Olive D, Springer T A, Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988;141:1904–1911. [PubMed] [Google Scholar]
- 26.Bodor J, Walker W, Flemington E, Spetz A L, Habener J F. Modulation of Tax and PKA-mediated expression of HTLV-I promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 1995;377:413–418. doi: 10.1016/0014-5793(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 27.Brady J N. Biology of HTLV-I: host cell interactions. In: Höllsberg P, Hafler D A, editors. Human T cell lymphotropic virus type I. Chichester, England: John Wiley & Sons, Ltd.; 1996. pp. 79–112. [Google Scholar]
- 28.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-I Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 29.Brennan P, Babbage J W, Burgering B M, Groner B, Reif K, Cantrell D A. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 30.Brod S, Purvee M, Benjamin D, Hafler D. T-T cell interactions are mediated by adhesion molecules. Eur J Immunol. 1990;20:2259–2268. doi: 10.1002/eji.1830201015. [DOI] [PubMed] [Google Scholar]
- 31.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 32.Buchner K. Protein kinase C in the transduction of signals toward and within the cell nucleus. Eur J Biochem. 1995;288:211–221. [PubMed] [Google Scholar]
- 33.Buckle G J, Hafler D A, Höllsberg P. HTLV-I-induced T cell activation. J Acquired Immune Defic Syndr Hum Retroviruses. 1996;13(Suppl 1):S107–S113. doi: 10.1097/00042560-199600001-00018. [DOI] [PubMed] [Google Scholar]
- 34.Burgert H G, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41:987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 35.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantrell D A, Smith K A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 37.Cassé H, Girerd Y, Gazzolo L, Duc Dudon M. Critical involvement of human T cell leukaemia virus type I virions in mediating the viral mitogenic effect. J Gen Virol. 1994;75:1909–1916. doi: 10.1099/0022-1317-75-8-1909. [DOI] [PubMed] [Google Scholar]
- 38.Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman M E. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- 39.Cereseto A, Diella F, Mulloy J C, Cara A, Michieli P, Grassmann R, Franchini G, Klotman M E. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- 40.Chen F, Zou J Z, DiRenzo L, Winberg G, Hu L F, Klein E, Klein G, Ernberg I. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt’s lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Zachar V, Zdravkovic M, Guo M, Ebbesen P, Liu X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J Gen Virol. 1997;78:3277–3285. doi: 10.1099/0022-1317-78-12-3277. [DOI] [PubMed] [Google Scholar]
- 42.Chlichlia K, Busslinger M, Peter M E, Walczak H, Krammer P H, Schirrmacher V, Khazaie K. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene. 1997;14:2265–2272. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- 43.Chlichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-I tax function: T cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 44.Cho-Chung Y S, Pepe S, Clair T, Budillon A, Nesterova M. cAMP-dependent protein kinase: role in normal and malignant growth. Crit Rev Oncol Hematol. 1995;21:33–61. doi: 10.1016/1040-8428(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 45.Chu Z L, DiDonato J A, Hawiger J, Ballard D W. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 46.Clapham P, Nagy K, Cheingsong-Popov R, Exley M, Weiss R A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983;222:1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- 47.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 48.Copeland K F T, Haaksma A G M, Goudsmit J, Krammer P H, Heeney J L. Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–1268. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 49.Copeland K F T, Heeney J L. T helper cell activation and human retroviral pathogenesis. Microbiol Rev. 1996;60:722–742. doi: 10.1128/mr.60.4.722-742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross S L, Feinberg M B, Wolf J B, Holbrook N J, Wong-Staal F, Leonard W J. Regulation of the human interleukin-2 receptor α chain promoter: activation of α nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 51.Daenke S, Kermode A G, Hall S E, Taylor G, Weber J, Nightingale S, Bangham C R M. High activated and memory cytotoxic T-cell responses to HTLV-1 in healthy carriers and patients with tropical spastic paraparesis. Virology. 1996;217:139–146. doi: 10.1006/viro.1996.0101. [DOI] [PubMed] [Google Scholar]
- 52.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 53.David D, Bani L, Moreau J-L, Demaison C, Sun K, Salvucci O, Nakarai T, de Montalembert M, Chouaïb S, Joussemet M, Ritz J, Théze J. Further analysis of interleukin-2 receptor subunit expression on the different human peripheral blood mononuclear cell subsets. Blood. 1998;91:165–172. [PubMed] [Google Scholar]
- 54.Debatin K-M, Goldmann C K, Bamford R, Waldmann T A, Krammer P H. Monoclonal-antibody-mediated apoptosis in adult T-cell leukemia. Lancet. 1990;335:497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- 55.Debatin K-M, Goldmann C K, Waldmann T A, Krammer P H. APO-1-induced apoptosis of leukemia cells from patients with adult T-cell leukemia. Blood. 1993;81:2972–2977. [PubMed] [Google Scholar]
- 56.Del Val M, Hengel H, Häcker H, Hartlaub U, Ruppert T, Lucin P, Koszinowski U H. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J Exp Med. 1992;172:729–738. doi: 10.1084/jem.176.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 58.de Revel T, Mabondzo A, Gras G, Delord B, Roques P, Boussin F, Neveux Y, Bahuau M, Fleury H J, Dormont D. In vitro infection of human macrophages with human T-cell leukemia virus type 1. Blood. 1993;81:1598–1606. [PubMed] [Google Scholar]
- 59.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 60.Deryckere F, Burgert H G. Early region 3 of adenovirus type 19 (subgroup D) encodes an HLA-binding protein distinct from that of subgroups B and C. J Virol. 1996;70:2832–2841. doi: 10.1128/jvi.70.5.2832-2841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhawan S, Streicher H Z, Wahl L M, Miller N, Louie A T, Goldfarb I S, Jackson W L, Casali P, Notkins A L. Model for studying virus attachment. II. Binding of biotinylated human T cell leukemia virus type I to human blood mononuclear cells potential targets form human T cell leukemia virus type I infection. J Immunol. 1991;147:102–108. [PubMed] [Google Scholar]
- 62.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 63.Ding Y H, Smith K J, Garboczi D N, Utz U, Biddison W E, Wiley D C. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 64.Duc Dodon M, Bernard A, Gazzolo L. Peripheral T-lymphocyte activation by human T-cell leukemia virus type I interferes with the CD2 but not the CD3/TCR pathway. J Virol. 1989;63:5413–5419. doi: 10.1128/jvi.63.12.5413-5419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duc Dodon M, Gazzolo L. Loss of interleukin-2 requirement for the generation of T colonies defines an early event of human T-lymphotropic virus type I infection. Blood. 1987;69:12–17. [PubMed] [Google Scholar]
- 66.Dumont F J, Staruch M J, Koprak S L, Melino M R, Sigal N H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–258. [PubMed] [Google Scholar]
- 67.Dustin M L, Selvaraj P, Mattaliano R J, Springer T A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. Nature. 1987;329:846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- 68.Elovaara I, Koenig S, Brewah Y, Woods R M, Lehky T, Jacobson S. High human T cell lymphotropic virus type I (HTLV-I)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-I-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelhard V. Structure of peptides associated with class I and class II MHC molecules. Annu Rev Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- 70.Ewan M, Sluss H, Whitehouse L, Livingston D. TGF-β inhibition of cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 71.Feuerstein N, Huang D, Hinrichs S H, Orten D J, Aiyar N, Prystowsky M B. Regulation of cAMP-responsive enhancer binding proteins during cell cycle progression in T lymphocytes stimulated by IL-2. J Immunol. 1995;154:68–79. [PubMed] [Google Scholar]
- 72.Firpo E J, Koff A, Solomon M J, Roberts J M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fontes J D, Strawhecker J M, Bills N D, Lewis R E, Hinrichs S H. Phorbol esters modulate the phosphorylation of human T-cell leukemia virus type I Tax. J Virol. 1993;67:4436–4441. doi: 10.1128/jvi.67.7.4436-4441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franchini G. Molecular mechanisms of human T cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 75.Franklin A A, Kubik M F, Uittenbogaard M N, Brauweiler A, Utaisincharoen P, Matthews M-A H, Dynan W S, Hoeffler J P, Nyborg J K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–21231. [PubMed] [Google Scholar]
- 76.Früh K, Ahn K, Djaballah H, Sampe P, van Endert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 77.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-I Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 78.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter transactivation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 80.Fujita M, Shiku H. Differences in sensitivity to induction of apoptosis among rat fibroblast cells transformed by HTLV-I tax gene or cellular nuclear oncogenes. Oncogene. 1995;11:15–20. [PubMed] [Google Scholar]
- 81.Fukedome K, Furuse M, Fukuhara N, Orita S, Imai T, Takagi S, Nagaira M, Hinuma Y, Yoshie O. Strong induction of ICAM-1 in human T cells transformed by human T-cell leukemia virus type 1 and depression of ICAM-1 or LFA-1 in adult T-cell leukemia-derived cell lines. Int J Cancer. 1992;52:418–427. doi: 10.1002/ijc.2910520316. [DOI] [PubMed] [Google Scholar]
- 82.Furukawa K, Mori M, Ohta N, Ikeda H, Shida H, Furukawa K, Shiku H. Clonal expansion of CD8+ cytotoxic T lymphocytes against human T cell lymphotropic virus type I (HTLV-I) genome products in HTLV-I-associated myelopathy/tropical spastic paraparesis patients. J Clin Investig. 1994;94:1830–1839. doi: 10.1172/JCI117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garboczi D N, Ghosh R, Utz U, Fan Q R, Biddison W E, Wiley D C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 84.Garboczi D N, Utz U, Ghosh P, Seth A, Kim J, VanTienhoven E A E, Biddison W E, Wiley D C. Assembly, specific binding, and crystallization of a human TCR-ab with an antigenic Tax peptide from human T lymphotropic virus type 1 and the class I MHC molecule HLA-A2. J Immunol. 1996;157:5403–5410. [PubMed] [Google Scholar]
- 85.Gartenhaus R B, Wang P, Hoffmann P. Induction of the WAF1/CIP1 protein and apoptosis in human T-cell leukemia virus type I-transformed lymphocytes after treatment with adriamycin by using a p53-independent pathway. Proc Natl Acad Sci USA. 1996;93:265–268. doi: 10.1073/pnas.93.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gavalchin J, Fan N, Lane M J, Papsidero L, Poiesz B J. Identification of a putative cellular receptor for HTLV-I by a monoclonal antibody, Mab 34-23. Virology. 1993;194:1–9. doi: 10.1006/viro.1993.1228. [DOI] [PubMed] [Google Scholar]
- 87.Gazzolo L, Dudon M D. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- 88.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Conelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geng Y, Weinberg R. Transforming growth factor β effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci USA. 1993;90:10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gessain A. Epidemiology of HTLV-I and associated diseases. In: Höllsberg P, Hafler D A, editors. Human T cell lymphotropic virus type I. Chichester, England: John Wiley & Sons, Ltd.; 1996. pp. 33–64. [Google Scholar]
- 91.Gessain A, Louie A, Gout O, Gallo R C, Franchini G. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-I associated myelopathy. J Virol. 1991;65:1628–1633. doi: 10.1128/jvi.65.3.1628-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosh P, Sica A, Cippitelli M, Subleski J, Lahesmaa R, Young H A, Rice N R. Activation of nuclear factor of activated T cells in a cyclosporin A-resistant pathway. J Biol Chem. 1996;271:7700–7704. doi: 10.1074/jbc.271.13.7700. [DOI] [PubMed] [Google Scholar]
- 93.Gilbert M J, Riddell S R, Plachter B, Greenberg P D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 94.Godfrey W R, Fagnoni F F, Harara M A, Buck D, Engleman E G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goebels N, Waase I, Pfizenmaier K, Krönke M. IL-2 production in human T lymphotropic virus I-infected leukemic T lymphocytes analyzed by in situ hybridization. J Immunol. 1988;141:1231–1235. [PubMed] [Google Scholar]
- 96.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grassmann R, Dengler C, Müller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M-C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Green J M, Noel P J, Sperling A I, Walunas T L, Gray G S, Bluestone J A, Thompson C B. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 99.Greenberg S J, Jacobson S, Waldmann T A, McFarlin D E. Molecular analysis of HTLV-I proviral integration and T cell receptor arrangement indicates that T cells in tropical spastic paraparesis are polyclonal. J Infect Dis. 1989;159:741–744. doi: 10.1093/infdis/159.4.741. [DOI] [PubMed] [Google Scholar]
- 100.Greenfield E A, Howard E, Paradis T, Nguyen K, Benazzo F, McLean P, Höllsberg P, Davis G, Hafler D A, Sharpe A H, Freeman G J, Kuchroo V K. B7.2 expressed by T cells does not induce CD28-mediated costimulatory activity but retains CTLA4 binding. J Immunol. 1997;158:2025–2034. [PubMed] [Google Scholar]
- 101.Greten T F, Slansky J E, Kubota R, Soldan S S, Jaffee E M, Leist T P, Pardoll D M, Jacobson S, Schneck J P. Direct visualization of antigen-specific T cells: HTLV-I Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guyot D J, Newbound G C, Lairmore M D. Signaling via the CD2 receptor enhances HTLV-1 replication in T lymphocytes. Virology. 1997;234:123–129. doi: 10.1006/viro.1997.8636. [DOI] [PubMed] [Google Scholar]
- 103.Guyot D J, Trask J, Andrews J M, Newbound G C, Lairmore M D. Stimulation of the CD2 receptor pathway induces apoptosis in human T lymphotropic virus type I-infected cell lines. J Acquired Immune Defic Syndr Hum Retroviruses. 1996;11:317–325. doi: 10.1097/00042560-199604010-00001. [DOI] [PubMed] [Google Scholar]
- 104.Hannon G, Beach D. p15ink4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 105.Harper M E, Kaplan M H, Marselle L M, Pahwa S G, Chayt K J, Sarngadharan M G, Wong-Staal F, Gallo R C. Concomitant infection with HTLV-I and HTLV-III in a patient with T8 lymphoproliferative disease. N Engl J Med. 1986;315:1073–1078. doi: 10.1056/NEJM198610233151707. [DOI] [PubMed] [Google Scholar]
- 106.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin S D, Perlmutter R M, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 107.Hattori T, Uchiyama T, Toibana T, Takatsuki K, Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981;58:645–647. [PubMed] [Google Scholar]
- 108.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 109.Hengel H, Flohr T, Hämmerling G J, Koszinowski U H, Momburg F. Human cytomegalovirus inhibits peptide translocation into the endoplasmic reticulum for MHC class I assembly. J Gen Virol. 1996;77:2287–2296. doi: 10.1099/0022-1317-77-9-2287. [DOI] [PubMed] [Google Scholar]
- 110.Hengel H, Koopmann O J, Flohr T, Muranyi W, Goulmy E, Hämmerling G J, Koszinowski U H, Momburg F. A viral ER resident glycoprotein inactivates the MHC encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 111.Hildreth J E, Subramanium A, Hampton R A. Human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation mediated by vascular cell adhesion molecule-1: evidence for involvement of cell adhesion molecules in HTLV-I biology. J Virol. 1997;71:1173–1180. doi: 10.1128/jvi.71.2.1173-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hill A, Jugovic P, York I, Russ G, Bennik J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 113.Hirai H, Suzuki T, Fujisawa J I, Inoue J I, Yoshida M. Tax protein of human T-cell leukemia virus type I binds to the ankyrin motifs of inhibitory factor kB and induces nuclear translocation of transcription factor NF-kB proteins for transcriptional activation. Proc Natl Acad Sci USA. 1994;91:3584–3588. doi: 10.1073/pnas.91.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiramatsu K, Masuda M, Yoshikura H. Mode of transmission of human T-cell leukemia virus type I (HTLV-I) in a human promyelocytic HL60 cell. Int J Cancer. 1986;37:601–606. doi: 10.1002/ijc.2910370420. [DOI] [PubMed] [Google Scholar]
- 115.Ho D D, Rota T R, Hirsch M S. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc Natl Acad Sci USA. 1984;81:7588–7590. doi: 10.1073/pnas.81.23.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoffman P M, Dhib-Jalbut S, Mikovits J A, Robbins D S, Wolf A L, Bergey G K, Lohrey N C, Weislow O S, Ruscetti F W. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc Natl Acad Sci USA. 1992;89:11784–11788. doi: 10.1073/pnas.89.24.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Höger T A, Jacobson S, Kawanishi T, Kato T, Nishioka K, Yamamoto K. Accumulation of human T lymphotropic virus (HTLV)-I-specific T cell clones in HTLV-I-associated myelopathy/tropical spastic paraparesis patients. J Immunol. 1997;159:2042–2048. [PubMed] [Google Scholar]
- 118.Höllsberg P. Pathogenesis of chronic progressive myelopathy associated with human T-cell lymphotropic virus type I. Acta Neurol Scand Suppl. 1997;169:86–93. doi: 10.1111/j.1600-0404.1997.tb08156.x. [DOI] [PubMed] [Google Scholar]
- 119.Höllsberg P, Ausubel L J, Hafler D A. Human T cell lymphotropic virus type I-induced T cell activation. Resistance to TGF-β1-induced suppression. J Immunol. 1994;153:566–573. [PubMed] [Google Scholar]
- 120.Höllsberg P, Batra V, Dressel A, Hafler D A. Induction of anergy in CD8 T cells by B cell presentation of antigen. J Immunol. 1996;157:5269–5276. [PubMed] [Google Scholar]
- 121.Höllsberg P, Hafler D A. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 122.Höllsberg P, Scholz C, Anderson D E, Greenfield E A, Kuchroo V K, Freeman G J, Hafler D A. Expression of a hypoglycosylated form of B7-2 on human T cells with altered binding to CD28 and CTLA-4. J Immunol. 1997;159:4799–4805. [PubMed] [Google Scholar]
- 123.Höllsberg P, Weber W E J, Dangond F, Batra V, Sette A, Hafler D A. Differential activation of proliferation and cytotoxicity in human T-cell lymphotropic virus type I Tax-specific CD8 T cells by an altered peptide ligand. Proc Natl Acad Sci USA. 1995;92:4036–4040. doi: 10.1073/pnas.92.9.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Höllsberg P, Wucherpfennig K W, Ausubel L J, Calvo V, Bierer B E, Hafler D A. Characterization of HTLV-I in vivo infected T cell clones. IL-2 independent growth of nontransformed T cells. J Immunol. 1992;148:3256–3263. [PubMed] [Google Scholar]
- 125.Howard F D, Moingeon P, Moebius U, McConkey D J, Yandava B, Gennert T E, Reinherz E L. The CD3ζ cytoplasmic domain mediates CD2-induced T cell activation. J Exp Med. 1992;176:139–145. doi: 10.1084/jem.176.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoxie J A, Matthews D M, Cines D B. Infection of human endothelial cells by human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1984;81:7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ijichi S, Izumo S, Nagai M, Shinmyozu K, Hall W W, Osame M. Anti-viral and immunomodulatory effects of interferon-α on cultured lymphocytes from patients with human T lymphotropic virus type I-associated myelopathy (HAM/TSP) J Neuroimmunol. 1995;61:213–221. doi: 10.1016/0165-5728(95)00101-7. [DOI] [PubMed] [Google Scholar]
- 128.Imai T, Tanaka Y, Fukudome K, Takagi S, Araki K, Yshie O. Enhanced expression of LFA-3 on human T-cell lines and leukemic cells carrying human T-cell-leukemia virus type I. Int J Cancer. 1993;55:811–816. doi: 10.1002/ijc.2910550520. [DOI] [PubMed] [Google Scholar]
- 129.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Imura A, Hori T, Imada K, Kawamata S, Tanaka Y, Imamura S, Uchiyama T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–2958. [PubMed] [Google Scholar]
- 131.Inatsuki A, Yasukawa M, Kobayashi Y. Functional alterations of herpes simplex virus-specific CD4+ multifunctional T cell clones following infection with human T lymphotropic virus type I. J Immunol. 1989;143:1327–1333. [PubMed] [Google Scholar]
- 132.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type I. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Itoyama Y, Kira J, Fujii N, Goto I, Yamamoto N. Increases in helper inducer T cells an activated T cells in HTLV-I-associated myelopathy. Ann Neurol. 1989;26:257–262. doi: 10.1002/ana.410260212. [DOI] [PubMed] [Google Scholar]
- 134.Iwasaki Y, Ohara Y, Kobayashi I, Akizuki S. Infiltration of helper/inducer T lymphocytes heralds central nervous system damage in human T-cell leukemia virus infection. Am J Pathol. 1992;140:1003–1008. [PMC free article] [PubMed] [Google Scholar]
- 135.Jackson M R, Nilsson T, Peterson P A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacobson S, McFarlin D E, Robinson S, Voskuhl R, Martin R, Brewah A, Newell A J, Koenig S. HTLV-I-specific cytotoxic T lymphocytes in the cerebrospinal fluid of patients with HTLV-I-associated neurological disease. Ann Neurol. 1992;32:651–657. doi: 10.1002/ana.410320508. [DOI] [PubMed] [Google Scholar]
- 137.Jacobson S, Reuben J S, Streilein R D, Palker T J. Induction of CD4+ human T lymphotropic virus type-1-specific cytotoxic T lymphocytes from patients with HAM/TSP. J Immunol. 1991;146:1155–1162. [PubMed] [Google Scholar]
- 138.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 139.Jacobson S, Zaninovic V, Mora C, Rodgers-Johnson P, Sheremata W A, Gibbs C J, Jr, Gajdusek D C, McFarlin D E. Immunological findings in neurologic diseases associated with antibodies to HTLV-I activated lymphocytes in tropical spastic paraparesis. Ann Neurol. 1988;23(Suppl):S196–S200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- 140.Jeang K-T, Widen S G, Semmes O J, Wilson S H. HTLV-I trans-activator protein, Tax, is a trans-repressor of the human β-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 141.Jin D Y, Spencer F, Jeang K T. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 142.Jin D Y, Teramoto H, Giam C Z, Chun R F, Gutkind J S, Jeang K T. A human suppressor of c-Jun N-terminal kinase 1: activation by tumor necrosis factor alpha. J Biol Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 143.Johnson J G, Jenkins M K. Monocytes provide a novel costimulatory signal to T cells that is not mediated by the CD28/B7 interaction. J Immunol. 1994;152:429–437. [PubMed] [Google Scholar]
- 144.Jones T, Sun L. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J Virol. 1997;71:2970–2979. doi: 10.1128/jvi.71.4.2970-2979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.June C H, Ledbetter J A, Gillispie M M, Lindsten T, Thompson C B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kadison P, Poteat H T, Klein K M, Faller D V. Role of protein kinase A in tax transactivation of the human T-cell leukemia virus type I long terminal repeat. J Virol. 1991;64:2141–2148. doi: 10.1128/jvi.64.5.2141-2148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kannagi M, Shida H, Igarashi H, Kuruma K, Murai H, Aono Y, Maruyama I, Osame M, Hattori T, Inoko H, Harada S. Target epitope in the Tax protein of human T-cell leukemia virus type I recognized by class I major histocompatibility complex-restricted cytotoxic T cells. J Virol. 1992;66:2928–2933. doi: 10.1128/jvi.66.5.2928-2933.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kannagi M, Sugamura K, Sato H, Okochi K, Uchino H, Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983;130:2942–2946. [PubMed] [Google Scholar]
- 149.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-kappa B via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 150.Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. The upregulation of p27kip by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood. 1998;91:561–569. [PubMed] [Google Scholar]
- 151.Khanna R, Burrows S R, Kurilla M G, Jacob C A, Misko I S, Sculley T B, Kieff E, Moss D J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992;176:169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kimata J, Palker T, Ratner L. The mitogenic activity of human T-cell leukemia virus type I is T-cell associated and requires the CD2/LFA-3 activation pathway. J Virol. 1993;67:3134–3141. doi: 10.1128/jvi.67.6.3134-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kimata J, Ratner L. Temporal regulation of viral and cellular gene expression during human T-lymphotropic virus type I-mediated lymphocyte immortalization. J Virol. 1991;65:4398–4407. doi: 10.1128/jvi.65.8.4398-4407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kishi S, Saijyo S, Arai M, Karasawa S, Ueda S, Kannagi M, Iwakura Y, Fujii M, Yonehara S. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphotropic virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J Exp Med. 1997;186:57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kitajima I, Sjinohara T, Bilakovics J, Brown D A, Xu X, Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–1795. doi: 10.1126/science.1299224. . (Erratum, 259:1523, 1993.) [DOI] [PubMed] [Google Scholar]
- 156.Kobayashi N, Kono T, Hatakeyama M, Minami Y, Miyazaki T, Perlmutter R M, Taniguchi T. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc Natl Acad Sci USA. 1993;90:4201–4205. doi: 10.1073/pnas.90.9.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koenig S, Woods R, Brewah Y, Newell A, Jones G, Boone E, Adelsberger J, Baseler M, Robinson S, Jacobson S. Characterization of MHC class I restricted cytotoxic T cell responses to Tax in HTLV-I-infected patients with neurologic disease. J Immunol. 1993;156:3874–3883. [PubMed] [Google Scholar]
- 158.Koff A, Ohtsuki M, Polyak K, Roberts J, Massague J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- 159.Koga Y, Oh-Hori N, Sato H, Yamamoto N, Kimura G, Nomoto K. Absence of transcription of lck (lymphocyte specific protein tyrosine kinase) message in IL-2-independent, HTLV-I-transformed T cell lines. J Immunol. 1989;142:4493–4499. [PubMed] [Google Scholar]
- 160.Kohtz D S, Altman A, Kohtz J D, Puszkin S. Immunological and structural homology between human T-cell leukemia virus type I envelope glycoprotein and a region of human interleukin-2 implicated in binding the beta receptor. J Virol. 1988;62:659–662. doi: 10.1128/jvi.62.2.659-662.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koralnik I J, Gessain A, Klotman M E, Monico A L, Berneman Z N, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc Natl Acad Sci USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koralnik I J, Lemp J F, Jr, Gallo R C, Franchini G. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type I (HTLV-I) AIDS Res Hum Retroviruses. 1992;8:1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- 163.Kurata A, Palker T J, Steilein R D, Scearce R M, Haynes B F, Berzofsky J A. Immunodominant sites of human T cell lymphotropic virus type I envelope protein for murine helper T cells. J Immunol. 1989;143:2024–2030. [PubMed] [Google Scholar]
- 164.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 165.Kwon T K, Buchholz M A, Ponsalle P, Chrest F J, Nordin A A. The regulation of p27Kip1 expression following the polyclonal activation of murine G0 T cells. J Immunol. 1997;158:5642–5648. [PubMed] [Google Scholar]
- 166.Lacoste J, Lanoix J, Pepin N, Hiscott J. Interactions between HTLV-I Tax and NF-kappa B/Rel proteins in T cells. Leukemia. 1994;8(Suppl. 1):S71–S76. [PubMed] [Google Scholar]
- 167.Lal R B, Rudolph D, Buckner C, Pardi D, Hooper W C. Infection with human T-lymphotropic viruses leads to constitutive expression of leukemia inhibitory factor and interleukin-6. Blood. 1993;81:1827–1832. [PubMed] [Google Scholar]
- 168.Lal R B, Rudolph D L. Constitutive production of interleukin-6 and tumor necrosis factor alpha from spontaneously proliferating T cells in patients with human T-cell lymphotropic virus type-I/II. Blood. 1991;78:571–574. [PubMed] [Google Scholar]
- 169.Lal R B, Rudolph D L, Dezzutti C S, Linsley P S, Prince H E. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J Immunol. 1996;157:1288–1296. [PubMed] [Google Scholar]
- 170.Lando Z, Sarin P, Megson M, Greene W C, Waldmann T A, Gallo R C, Broder S. Association of human T-cell leukemia/lymphoma virus with the Tac antigen marker for the human T-cell growth factor receptor. Nature. 1983;305:733–736. doi: 10.1038/305733a0. [DOI] [PubMed] [Google Scholar]
- 171.Lanoix J, Lacoste J, Pepin N, Rice N, Hiscott J. Overproduction of NFKB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signaling pathway. Oncogene. 1994;9:841–852. [PubMed] [Google Scholar]
- 172.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkB kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 173.Lehky T J, Fox C H, Koenig S, Levin M C, Flerlage N, Izumo S, Sato E, Raine C S, Osame M, Jacobson S. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann Neurol. 1995;37:167–175. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- 174.Lemasson I, Robert-Hebmann V, Hamaia S, Duc Dodon M, Gazzolo L, Devaux C. Transrepression of lck gene expression by human T-cell leukemia virus type I-encoded p40tax. J Virol. 1997;71:1975–1983. doi: 10.1128/jvi.71.3.1975-1983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lemasson I, Thebault S, Sardet C, Devaux C, Mesnard J M. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16 (INK4A)-negative T-cell line. J Biol Chem. 1998;273:23598–23604. doi: 10.1074/jbc.273.36.23598. [DOI] [PubMed] [Google Scholar]
- 176.Leung K, Nabel G. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 177.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–740. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 178.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 179.Li C-C H, Ruscetti F W, Rice N R, Chen E, Yang N-S, Mikovits J, Longo D L. Differential expression of Rel family members in human T-cell leukemia virus type I-infected cells: transcriptional activation of c-rel by Tax protein. J Virol. 1993;67:4205–4213. doi: 10.1128/jvi.67.7.4205-4213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.LiFeng G, Maggirwar S B, Harhaj E W, Sun S-C. Constitutive dephosphorylation and activation of a member of the nuclear factor of activated T cells, NF-AT1, in Tax expressing and type I human T-cell leukemia virus-infected human T cells. J Biol Chem. 1997;272:1425–1428. doi: 10.1074/jbc.272.3.1425. [DOI] [PubMed] [Google Scholar]
- 181.LiFeng G, Maggirwar S B, Sun S-C. Activation of the IL-2 gene promoter by HTLV-I Tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 1996;15:3744–3750. [PMC free article] [PubMed] [Google Scholar]
- 182.Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases: the p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 183.Lindholm P F, Tamami M, Makowski J, Brady J N. Human T-cell lymphotropic virus type 1 Tax1 activation of NF-κB: involvement of the protein kinase C pathway. J Virol. 1996;70:2525–2532. doi: 10.1128/jvi.70.4.2525-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Link E, Deer L D, Shreck R, Zabel U, Verma I, Baeuerle P A. Purified IkB-β is inactivated upon dephosphorylation. J Biol Chem. 1992;267:239–246. [PubMed] [Google Scholar]
- 185.Liou H-C, Baltimore D. Regulation of the NF-kB/rel transcription factor and IkB inhibitor system. Curr Opin Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 186.Lissy N A, Van Dyk L F, Becker-Hapak M, Vocero-Akbani A, Mendler J H, Dowdy S F. TCR antigen-induced cell death occurs from a late G1 phase cell cycle check point. Immunity. 1998;8:57–65. doi: 10.1016/s1074-7613(00)80458-6. [DOI] [PubMed] [Google Scholar]
- 187.Lo K M, Vivier E, Rochet N, Dehni G, Levine H, Haseltine W A, Anderson P. Infection of human natural killer (NK) cells with replication-defective human T cell leukemia virus type I provirus. Increased proliferative capacity and prolonged survival of functionally competent NK cells. J Immunol. 1992;149:4101–4108. [PubMed] [Google Scholar]
- 188.Low K G, Dorner L F, Fernando D B, Grossman J, Jeang K-T, Comb M J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;71:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lucas J J, Szepesi A, Modiano J F, Domemico J, Gelfand E W. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk 6)), a major cyclin D-associated ckd4 homologue in normal human T lymphocytes. J Immunol. 1995;154:6275–6284. [PubMed] [Google Scholar]
- 190.Lusso P, Lori F, Gallo R C. CD4-independent infection by human immunodeficiency virus type 1 after phenotypic mixing with human T-cell leukemia viruses. J Virol. 1990;64:6341–6344. doi: 10.1128/jvi.64.12.6341-6344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Macatonia S E, Cruikshank J K, Rudge P, Knight S C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-I and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 192.Macchi B, Faraoni I, Zhang J, Grelli S, Favalli C, Mastino A, Bonmassar E. AZT inhibits the transmission of human T cell leukemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J Gen Virol. 1997;78:1007–1016. doi: 10.1099/0022-1317-78-5-1007. [DOI] [PubMed] [Google Scholar]
- 193.Madden D, Garboczi D, Wiley D. The antigenic identity of peptide-MHC complexes: a comparison of the conformation of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- 194.Mahana W, Zhao T M, Teller R, Robinson M A, Kindt T J. Genes in the pX region of human T cell leukemia virus I influence Vav phosphorylation in T cells. Proc Natl Acad Sci USA. 1998;95:1782–1787. doi: 10.1073/pnas.95.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Manca F, Li Pira G, Fenoglio D, Valle M T, Lunkl A, Ferraris A, Lancia F, Saverino D, Mortara L, Balderas R, Arp J, Dekaban G, Dalgleish A G, Lozzi L, Theofilopoulos A N. Recognition of human T-cell leukemia virus (HTLV-1) envelope by human CD4+ T-cell lines from HTLV-1 seronegative individuals: specificity and clonal heterogeneity. Blood. 1995;85:1547–1554. [PubMed] [Google Scholar]
- 196.Mann D L, Martin P, Hamlin-Green G, Nalewaik R, Blattner W. Virus production and spontaneous cell proliferation in HTLV-I-infected lymphocytes. Clin Immunol Immunopathol. 1994;72:312–320. doi: 10.1006/clin.1994.1147. [DOI] [PubMed] [Google Scholar]
- 197.Maruyama M, Shibuya H, Harada H, Hatakeyama M, Seiki M, Fujita T, Inoue J, Yoshida M, Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-I-encoded p40x and T3/Ti complex triggering. Cell. 1987;48:343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- 198.McGrath M, Witte O, Pincus T, Weissman I L. Retrovirus purification: a method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978;25:923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McGuire K, Curtiss V, Larson E, Haseltine W. Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J Virol. 1993;67:1590–1599. doi: 10.1128/jvi.67.3.1590-1599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 201.Meuer S C, Hussey R E, Fabbi M, Fox D, Acuto O, Fitzgerald K A, Hodgdon J C, Protentis J P, Schlossman S F, Reinherz E L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984;36:897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 202.Migone T-S, Lin J-X, Cereseto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 203.Mills G B, Arima N, May C, Hill M, Schmandt R, Li J, Miyamoto N G, Greene W C. Neither the LCK nor the FYN kinases are obligatory for IL-2-mediated signal transduction in HTLV-I-infected human T cells. Int Immunol. 1992;4:1233–1243. doi: 10.1093/intimm/4.11.1233. [DOI] [PubMed] [Google Scholar]
- 204.Minami Y, Nakagawa Y, Kawahara A, Miyazaki T, Sada K, Yamamura H, Taniguchi T. Protein tyrosine kinase Syk is associated with and activated by the IL-2 receptor: possible link with the c-myc induction pathway. Immunity. 1995;2:89–100. doi: 10.1016/1074-7613(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 205.Mitsuya H, Guo H-G, Cossman J, Megson M, Reitz M S, Jr, Broder S. Functional properties of antigen-specific T cells infected by human T-cell leukemia-lymphoma virus (HTLV-I) Science. 1984;225:1484–1486. doi: 10.1126/science.6206569. [DOI] [PubMed] [Google Scholar]
- 206.Mitsuya H, Matis L A, Megson M, Bunn P A, Murray C, Mann D L, Gallo R C, Broder S. Generation of an HLA-restricted cytotoxic T cell line reactive against cultured tumor cells from a patient infected with human T cell leukemia/lymphoma virus. J Exp Med. 1983;158:994–999. doi: 10.1084/jem.158.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miura S, Ohtani K, Numata N, Niki M, Ohbo K, Ina Y, Gojobori T, Tanaka Y, Tozawa H, Makamura M, Sugamura K. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol Cell Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miyazaki T, Liu Z J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Perlmutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 209.Modiano J F, Domenico J, Szepesi A, Lucas J J, Gelfand E W. Differential requirements for interleukin-2 distinguish the expression and activity of the cyclin-dependent kinases Cdk4 and Cdk2 in human T cells. J Biol Chem. 1994;269:32972–32978. [PubMed] [Google Scholar]
- 210.Moingeon P, Chang H-C, Wallner B P, Stebbins C, Frey A Z, Reinherz E L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989;339:312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- 211.Moingeon P, Lucich J L, McConkey D J, Letourneur F, Malissen B, Kochan J, Chang H-C, Rodewald H-R, Reinherz E. CD3ζ dependence of the CD2 pathway of activation in T lymphocytes and natural killer cells. Proc Natl Acad Sci USA. 1992;89:1492–1496. doi: 10.1073/pnas.89.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moore G R W, Traugott U, Scheinberg L C, Raine C S. Tropical spastic paraparesis: a model of virus-induced cytotoxic T-cell mediated demyelination. Ann Neurol. 1989;26:523–530. doi: 10.1002/ana.410260405. [DOI] [PubMed] [Google Scholar]
- 213.Mori N, Gill P S, Mougdil T, Murakami S, Eto S, Prager D. Interleukin-10 gene expression in adult T-cell leukemia. Blood. 1996;88:1035–1045. [PubMed] [Google Scholar]
- 214.Mori N, Shirakawa F, Shimizu H, Murakami S, Oda S, Yamamoto K, Eto S. Transcriptional regulation of the human interleukin-6 gene promoter in human T-cell leukemia virus type I-infected T-cell lines: evidence for the involvement of NF-kB. Blood. 1994;84:2904–2911. [PubMed] [Google Scholar]
- 215.Moritoyo T, Reinhart T A, Moritoyo H, Sato E, Izumo S, Osame M, Haase A T. Human T-lymphotropic virus type I-associated myelopathy and tax gene expression in CD4 T lymphocytes. Ann Neurol. 1996;40:84–90. doi: 10.1002/ana.410400114. [DOI] [PubMed] [Google Scholar]
- 216.Mulloy J C, Crowley R W, Fullen J, Leonard W J, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 218.Murray R J, Kurilla M G, Griffin H M, Brooks J M, Mackett M, Arrand J R, Rowe M, Burrows S R, Moss D J, Kieff E, Rickinson A B. Human cytotoxic T cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci USA. 1990;87:2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 220.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 221.Nakamura K, Koga Y, Yoshida H, Kimura G, Nomoto K. Differential expression of two lck transcripts directed from the distinct promoters in HTLV-I+ and HTLV-I− T-cells. Int J Cancer. 1991;48:789–793. doi: 10.1002/ijc.2910480526. [DOI] [PubMed] [Google Scholar]
- 222.Newbound G C, Andrews J M, O’Rourke J P, Brady J N, Lairmore M D. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J Virol. 1996;70:2101–2106. doi: 10.1128/jvi.70.4.2101-2106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Niewiesk S, Daenke S, Parker C E, Taylor G, Weber J, Nightingale S, Bangham C R M. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J Virol. 1995;69:2649–2653. doi: 10.1128/jvi.69.4.2649-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nishimoto N, Yoshizaki K, Eiraku N, Machigashira K, Tagoh H, Ogata A, Kuritani T, Osame M, Kishimoto T. Elevated levels of interleukin-6 in serum and cerebrospinal fluid of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurol Sci. 1990;97:183–193. doi: 10.1016/0022-510x(90)90217-b. [DOI] [PubMed] [Google Scholar]
- 225.Nishimura M, Kermode A G, Clerici M, Shearer G M, Berzofsky J A, Uchiyama T, Wiktor S Z, Pate E, Maloney B, Manns A, Blattner W, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I)-specific T cell responses from seronegative and polymerase chain reaction-negative persons exposed to HTLV-I. J Infect Dis. 1994;170:334–338. doi: 10.1093/infdis/170.2.334. [DOI] [PubMed] [Google Scholar]
- 226.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M-H, Massague J, Crabtree G R, Roberts J M. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 227.Nyunoya H, Akagi T, Ogura T, Maeda S, Shimotohno K. Evidence for phosphorylation of trans-activator p40x of human T-cell leukemia virus type I produced in insect cells with a baculovirus expression vector. Virology. 1988;167:538–544. [PubMed] [Google Scholar]
- 228.Oh-Hori N, Koga Y, Yoshida H, Morita M, Kimura G, Nomoto K. Human T-cell leukemia virus type-I-infected T-cell lines scarcely produce p56lck, whether or not they express lck mRNA. Int J Cancer. 1990;46:315–319. doi: 10.1002/ijc.2910460229. [DOI] [PubMed] [Google Scholar]
- 229.Ohta M, Morita T, Shimotohno K. lck suppresses gene expression from various promoters including human T-cell leukemia virus type I promoter. Jpn J Cancer Res. 1990;81:440–444. doi: 10.1111/j.1349-7006.1990.tb02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Oldstone M B A. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 231.Otten G, Germain R. Split anergy in a CD8 T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 232.Owen S M, Rudolph D L, Dezutti C S, Shibata N, Naik S, Caughman S W, Lal R B. Transcriptional activation of the intercellular adhesion molecule 1 (CD54) gene by human T lymphotropic virus types I and II is mediated through a palindromic response element. AIDS Res Hum Retroviruses. 1997;13:1429–1437. doi: 10.1089/aid.1997.13.1429. [DOI] [PubMed] [Google Scholar]
- 233.Parker C E, Daenke S, Nightingale S, Bangham C R M. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992;188:628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- 234.Parker C E, Nightingale S, Taylor G P, Weber J, Bangham C R M. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–2868. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 236.Pique C, Connan F, Levilain J-P, Choppin J, Dokhélar M-C. Among all human T-cell leukemia virus type 1 proteins, Tax, polymerase and envelope proteins are predicted as preferential targets for the HLA-A2-restricted cytotoxic T-cell response. J Virol. 1996;70:4919–4926. doi: 10.1128/jvi.70.8.4919-4926.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pise-Masison C A, Choi K-S, Radinovich M, Dittmer J, Kim S-J, Brady J N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Polyak K, Kato J-Y, Solomon M J, Sherr C J, Massagué J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 239.Popovic M, Flomenberg N, Volkman D J, Mann D, Fauci A S, Dupont B, Gallo R C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984;226:459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- 240.Popovic M, Lange-Wantzin G, Sarin P S, Mann D, Gallo R C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci USA. 1983;80:5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Poteat H T, Chen F Y, Kadison P, Sodroski J G, Haseltine W A. Protein kinase A-dependent binding of a nuclear factor to the 21-base-pair repeat of the human T-cell leukemia virus type I long terminal repeat. J Virol. 1990;64:1264–1270. doi: 10.1128/jvi.64.3.1264-1270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 243.Rao L, Debbas M, Sabbatini P, Hockenberry D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reed S I. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 245.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 246.Richardson J H, Edwards A J, Cruickshank J K, Rudge P, Dalgleish A G. In vivo cellular tropism of human T-cell leukemia virus type I. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Richardson J H, Höllsberg P, Windhagen A, Child L A, Hafler D A, Lever A M L. Variable immortalizing potential and frequent virus latency in blood-derived T-cell clones infected with human T-cell leukemia virus type I. Blood. 1997;89:3303–3314. [PubMed] [Google Scholar]
- 248.Richardson J H, Waldmann T A, Sodroski J G, Marasco W A. Inducible knockout of the interleukin-2 receptor alpha chain: expression of the high-affinity IL-2 receptor is not required for the in vitro growth of HTLV-I-transformed cell lines. Virology. 1997;237:209–216. doi: 10.1006/viro.1997.8779. [DOI] [PubMed] [Google Scholar]
- 249.Rivard N, L’Allemain G, Bartek J, Pouysségur J. Abrogation of p27Kip1 by cDNA antisense suppresses quiescence (G0 state) in fibroblasts. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- 250.Rosenberg A R, Delamarre L, Pique C, Pham D, Dokhelar M C. The extodomain of the human T-cell leukemia virus type 1 TM glycoprotein is involved in postfusion events. J Virol. 1997;71:7180–7186. doi: 10.1128/jvi.71.10.7180-7186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rott O, Tontsch U, Fleischer B, Cash E. Interleukin-6 in “normal” and HTLV-I tax-expressing brain-specific endothelial cells. Eur J Immunol. 1993;23:1987–1991. doi: 10.1002/eji.1830230839. [DOI] [PubMed] [Google Scholar]
- 252.Ruben S, Tan H P T-H, Kawakami K, Roeder R, Haseltine W, Rosen C A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988;241:89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- 253.Sawada M, Suzumura A, Yoshida M, Marunouchi T. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J Virol. 1990;64:4002–4006. doi: 10.1128/jvi.64.8.4002-4006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schmitt I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Scholz C, Freeman G J, Greenfield E A, Hafler D A, Höllsberg P. Activation of human T cell lymphotropic virus type I-infected T cells is independent of B7 costimulation. J Immunol. 1996;157:2932–2938. [PubMed] [Google Scholar]
- 256.Scholz C, Hafler D A, Höllsberg P. Downregulation of IL-10 secretion and enhanced antigen-presenting abilities following HTLV-I infection of T cells. J Neurosci Res. 1996;45:786–794. doi: 10.1002/(SICI)1097-4547(19960915)45:6<786::AID-JNR15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 257.Schönbach C, Nokihara K, Bangham C R M, Kariyone A, Karaki S, Shida H, Takatsu K, Egawa K, Wiesmüller K-H, Takiguchi M. Identification of HTLV-I-specific CTL directed against synthetic and naturally processed peptides in HLA-B*3501 transgenic mice. Virology. 1996;226:102–112. doi: 10.1006/viro.1996.0632. [DOI] [PubMed] [Google Scholar]
- 258.Schouten G J, Vertegaal A C, Whiteside S T, Israel A, Toebes M, Dorsman J C, van der Eb A J, Zantema A. IκBα is a target for the mitogen-activated 90 kD ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schwartz O, Marechal V, Legall S, Lomonier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 260.Seiki M, Eddy R, Shows T B, Yoshida M. Nonspecific integration of the HTLV provirus genome into adult T-cell leukaemia cells. Nature. 1984;309:640–642. doi: 10.1038/309640a0. [DOI] [PubMed] [Google Scholar]
- 261.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Selvaraj P, Plunkett M L, Dustin M, Sanders M E, Shaw S, Springer T A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature. 1987;326:400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- 263.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 265.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J M, Oseroff C, Yuan L, Ruppers J, Sidney J, del Guercio M-F, Southwood S, Kubo R T, Chesnut R W, Grey H M, Chisari F V. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 266.Sha W C. Regulation of immune responses by NF-κB/Rel transcription factors. J Exp Med. 1998;187:143–146. doi: 10.1084/jem.187.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sherr C J. Cancer and cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 268.Siekevitz M, Feinberg M B, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Slingerland J M, Hengst L, Pan C-H, Alexander D, Stanper M R, Reed S I. A novel inhibitor of cyclin-cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smith, D., G. J. Buckle, D. A. Hafler, D. A. Frank, and P. Höllsberg. HTLV-I-infected T cells evade the antiproliferative action of IFN-β. Virology, in press. [DOI] [PubMed]
- 271.Smith M R, Greene W C. Identification of HTLV-I tax transactivator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 272.Smith M R, Greene W C. Type I human T cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sommerfelt M A, Williams B P, Clapham P R, Solomon E, Goodfellow P N, Weiss R A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988;242:1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- 274.Sperling A I, Bluestone J A. The complexities of T-cell co-stimulation: CD28 and beyond. Immunol Rev. 1996;153:155–182. doi: 10.1111/j.1600-065x.1996.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 275.Sprengel J, Schmitz B, Heuss-Nietzel D, Zock C, Doerfler W. Nucleotide sequence of human adenovirus 12 DNA: comparative functional analysis. J Virol. 1994;68:379–389. doi: 10.1128/jvi.68.1.379-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Suciu-Foca N, Rubinstein P, Popovic M, Gallo R C, King D W. Reactivity of HTLV-transformed human T-cell lines to MHC class II antigens. Nature. 1984;312:275–277. doi: 10.1038/312275a0. [DOI] [PubMed] [Google Scholar]
- 277.Suciu-Foca N, Rubinstein P, Rohowsky-Kochan C, Cai J, Popovic M, Gallo R C, King D W. Functional modifications of alloreactive T cell clones infected with HTLV-I. J Immunol. 1986;137:1115–1119. [PubMed] [Google Scholar]
- 278.Suzuki T, Fujisawa J-I, Toita M, Yoshida M. The trans-activator Tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Suzuki T, Hirai H, Fujisawa J-I, Fujita T, Yoshida M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 280.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-I Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 281.Szamel M, Resch K. T-cell antigen receptor-induced signal-transduction pathways. Activation and function of protein kinases C in T lymphocytes. Eur J Biochem. 1995;228:1–15. doi: 10.1111/j.1432-1033.1995.tb20221.x. [DOI] [PubMed] [Google Scholar]
- 282.Tajima Y, Tashiro K, Camerini D. Assignment of the possible HTLV-I receptor gene to chromosome 17q21-q23. Somatic Cell Mol Genet. 1997;23:225–227. doi: 10.1007/BF02721374. [DOI] [PubMed] [Google Scholar]
- 283.Takamoto T, Makino M, Azuma M, Kanzaki T, Baba M, Sonoda S. HTLV-I-infected T cells activate autologous CD4+ T cells susceptible to HTLV-I infection in a costimulatory molecule-dependent fashion. Eur J Immunol. 1997;27:1427–1432. doi: 10.1002/eji.1830270620. [DOI] [PubMed] [Google Scholar]
- 284.Takemoto S, Mulloy J C, Cereseto A, Migone T S, Patel B K R, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S, White J D, Leonard W J, Waldmann T, Franchini G. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tanaka Y, Takeshi I, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I. Int J Cancer. 1985;36:549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 287.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 288.Tendler C L, Greenberg S J, Blattner W A, Manns A, Murphy E, Fleisher T, Hanchard B, Morgan O, Burton J D, Nelson D L, Waldmann T A. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogeneic implications and a rationale for immunotherapy. Proc Natl Acad Sci USA. 1990;87:5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tivol E, Borriello F, Schweitzer A, Lynch W, Bluestone J, Sharpe A. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 290.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 291.Tozawa H, Andou S, Takayama Y, Tanaka B, Lee B, Nakamura H, Hayami M, Hinuma Y. Species-dependent antigenicity of the 34-kDa glycoprotein found on the membrane of various primate lymphocytes transformed by human T-cell leukemia virus type-I (HTLV-I) and simian T-cell leukemia virus (STLV-I) Int J Cancer. 1988;41:231–238. doi: 10.1002/ijc.2910410213. [DOI] [PubMed] [Google Scholar]
- 292.Uittenbogaard M, Armstrong A P, Chiaramello A, Nyborg J K. HTLV-I Tax protein represses gene expression through the bHLH family of transcription factors. J Biol Chem. 1994;269:22466–22469. [PubMed] [Google Scholar]
- 293.Uittenbogaard M, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type I tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 294.Utz U, Koenig S, Coligan J E, Biddison W E. Presentation of three different viral peptides, HTLV-1 Tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J Immunol. 1992;149:214–221. [PubMed] [Google Scholar]
- 295.Valage V E, Wong J G P, Datlof B M, Sinskey A J, Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988;55:101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]
- 296.Valle A, Garrone P, Yssel H, Bonnefoy J Y, Freedman A S, Freeman G, Nadler L M, Banchereau J. mAb 104, a new monoclonal antibody recognizes the B7 antigen that is expressed on activated B cells and HTLV-I-transformed T cells. Immunology. 1990;69:531–535. [PMC free article] [PubMed] [Google Scholar]
- 297.van der Merwe P A, Barcley A N, Mason D W, Davies E A, Morgan B P, Tone M, Krishnam A K C, Ianelli C, Davis S J. Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry. 1994;33:10149–10160. doi: 10.1021/bi00199a043. [DOI] [PubMed] [Google Scholar]
- 298.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wagner S, Green M R. HTLV-I tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 300.Wainberg M A, Spira B, Boushira M, Margolese R G. Inhibition by human T-lymphotropic virus (HTLV-I) of T-lymphocyte mitogenesis: failure of exogenous T-cell growth factor to restore responsiveness to lectin. Immunology. 1985;54:1–7. [PMC free article] [PubMed] [Google Scholar]
- 301.Wallner B P, Frey A Z, Tizard R, Mattaliano R J, Hession C, Sanders M E, Duston M L, Springer T A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987;166:923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Warren A P, Ducroq D H, Lehner P J, Borysiewicz L K. Human cytomegalovirus-infected cells have unstable assembly of major histocompatibility complex class I complexes and are resistant to lysis by cytotoxic T lymphocytes. J Virol. 1994;68:2822–2829. doi: 10.1128/jvi.68.5.2822-2829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Watabe K, Saida T, Kim S U. Human and simian glial cells infected by human T-lymphotropic virus type I in culture. J Neuropathol Exp Neurol. 1989;48:610–619. doi: 10.1097/00005072-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 304.Waterhouse P, Penninger J, Timms E, Wakeham A, Shahinian A, Lee K, Thompson C, Griesser H, Mak T. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 305.Wesselborg S, Prüfer U, Wild M, Schraven B, Meuer S C, Kabelitz D. Triggering via the alternate CD2 pathway induces apoptosis in activated human T lymphocytes. Eur J Immunol. 1993;23:2707–2710. doi: 10.1002/eji.1830231050. [DOI] [PubMed] [Google Scholar]
- 306.Wiertz E J H J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 307.Wiertz E J H J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 308.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 309.Wucherpfennig K W, Höllsberg P, Richardson J H, Benjamin D, Hafler D A. T-cell activation by autologous human T-cell leukemia virus type I-infected T-cell clones. Proc Natl Acad Sci USA. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wucherpfennig K W, Ota K, Endo N, Seidman J G, Rosenzweig A, Weiner H L, Hafler D A. Shared human T cell receptor Vβ usage to immunodominant regions of myelin basic protein. Science. 1990;248:1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- 311.Xu X, Heidenreich O, Kitajima I, McGuire K, Li Q, Su B, Nerenberg M. Constitutively activated JNK is associated with HTLV-I mediated tumorigenesis. Oncogene. 1996;13:135–142. [PubMed] [Google Scholar]
- 312.Xu X, Kang S-H, Heidenreich O, Okerholm M, O’Shea J J, Nerenberg M I. Constitutive activation of different Jak tyrosine kinases in human T cell leukemia virus type 1 (HTLV-1) Tax protein or virus-transformed cells. J Clin Investig. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 315.Yamano Y, Kitze B, Yashiki S, Usuku K, Fujiyoshi T, Kaminagayoshi T, Unoki K, Izumo S, Osame M, Sonoda S. Preferential recognition of synthetic peptides from HTLV-I gp21 envelope protein by HLA-DRB1 alleles associated with HAM/TSP (HTLV-I-associated myelopathy/tropical spastic paraparesis) J Neuroimmunol. 1997;76:50–60. doi: 10.1016/s0165-5728(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 316.Yamashita I, Katamine S, Moriuchi R, Miyamoto T, Eguchi K, Nagataki S. Transactivation of the human interleukin-6 gene by human T-lymphotropic virus type 1 tax protein. Blood. 1994;84:1573–1578. [PubMed] [Google Scholar]
- 317.Yamashita Y, Shimokata K, Mizuno S, Yamaguchi H, Nishiyama Y. Down-regulation of the surface expression of class I MHC antigens by human cytomegalovirus. Virology. 1993;193:727–736. doi: 10.1006/viro.1993.1181. [DOI] [PubMed] [Google Scholar]
- 318.Yarchoan R, Guo H-G, Reitz M, Molyish A, Mitsuya H, Broder S. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus type I. J Clin Investig. 1986;77:1466–1473. doi: 10.1172/JCI112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yin M-J, Paulssen E J, Seeler J-S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 321.Yssel H, de Waal Malefyt R. IL-10 and human T cells. In: de Vries J E, de Waal Malefyt R, editors. Interleukin 10. R. G. Austin, Tex: Landes Co.; 1995. pp. 19–27. [Google Scholar]
- 322.Yssel H, de Waal Malefyt R, Duc Dodon M, Blanchard D, Gassolo L, de Vries J E, Spits H. Human T cell leukemia/lymphoma virus type I infection of a CD4 proliferative/cytotoxic T cell clone progresses in at least two distinct phases based on changes in function and phenotype of the infected cells. J Immunol. 1989;142:2279–2289. [PubMed] [Google Scholar]
- 323.Zandi E, Rotwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 324.Zetterberg A, Larsson O, Wiman K G. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 325.Zoubak S, Richardson J H, Rynditch A, Höllsberg P, Hafler D A, Boeri E, Lever A M L, Bernardi G. Regional specificity of HTLV-I proviral integration in the human genome. Gene. 1994;143:155–163. doi: 10.1016/0378-1119(94)90091-4. [DOI] [PubMed] [Google Scholar]