Abstract
Examinination of microbial diversity in environments of increasing salt concentrations indicates that certain types of dissimilatory metabolism do not occur at the highest salinities. Examples are methanogenesis for H2 + CO2 or from acetate, dissimilatory sulfate reduction with oxidation of acetate, and autotrophic nitrification. Occurrence of the different metabolic types is correlated with the free-energy change associated with the dissimilatory reactions. Life at high salt concentrations is energetically expensive. Most bacteria and also the methanogenic archaea produce high intracellular concentrations of organic osmotic solutes at a high energetic cost. All halophilic microorganisms expend large amounts of energy to maintain steep gradients of NA+ and K+ concentrations across their cytoplasmic membrane. The energetic cost of salt adaptation probably dictates what types of metabolism can support life at the highest salt concentrations. Use of KCl as an intracellular solute, while requiring far-reaching adaptations of the intracellular machinery, is energetically more favorable than production of organic-compatible solutes. This may explain why the anaerobic halophilic fermentative bacteria (order Haloanaerobiales) use this strategy and also why halophilic homoacetogenic bacteria that produce acetate from H2 + CO2 exist whereas methanogens that use the same substrates in a reaction with a similar free-energy yield do not.
OSMOTIC ADAPTATION IN MICROORGANISMS— TWO STRATEGIES
Microbial life can be found over the whole range of salt concentrations from freshwater and marine biotopes to hypersaline environments with NaCl concentrations up to saturation. Halophilic and halotolerant microorganisms are found in all three domains of life: Archaea, Bacteria, and Eucarya. Colonization of hypersaline environments such as salt lakes and salted food products by these microorganisms is often highly successful, and salt-loving and/or salt-tolerant microorganisms may reach high population densities in such ecosystems.
Since biological membranes are permeable to water, cells cannot maintain the water activity of their cytoplasm higher than that of the surrounding brine, because this would lead to a rapid loss of water to the environment (12). Therefore, any microorganism living at high salt concentrations may be expected to keep its cytoplasm at least isoosmotic with the extracellular environment. Buildup of a turgor pressure requires a hyperosmotic cytoplasm. With the possible exception of the halophilic archaea of the order Halobacteriales (135), all halophilic microorganisms maintain a turgor pressure.
Two fundamentally different strategies exist within the microbial world that enable microorganisms to cope with the osmotic stress inherent to the presence of high salt concentrations. (i) Cells may maintain high intracellular salt concentrations, osmotically at least equivalent to the external concentrations (the “salt-in” strategy). All intracellular systems should then be adapted to the presence of high salt concentrations. (ii) Cells may maintain low salt concentrations within their cytoplasm (the “compatible-solute” strategy). The osmotic pressure of the medium is then balanced by organic compatible solutes. No special adaptation of the intracellular systems is required.
The salt-in strategy is used by two phylogenetically unrelated groups: the aerobic extremely halophilic archaea of the order Halobacteriales (6, 58, 61) and the anaerobic halophilic bacteria of the order Haloanaerobiales (83, 87, 88, 92, 108). With a single exception (discussed below), no organic osmotic solutes have been found in representatives of these groups, and most studies report that the intracellular ionic concentrations are similar to those of the surrounding medium. The ionic composition of the cytoplasm generally differs greatly from that of the medium, which in most cases contains NaCl as the main salt. The intracellular environment is characterized by the presence of molar concentrations of KCl (58, 61, 83, 92).
In cells using this strategy for osmotic adaptation, all enzymes and structural cell components have to be adapted to the presence of high salt concentrations to ensure the proper functioning of the intracellular enzymatic machinery. Salt-tolerant enzymes are the rule, both in the aerobic archaea (61) and in the anaerobic bacteria of the order Haloanaerobiales (90, 108). Such halophilic proteins show unique molecular adaptations. These include the presence of a large excess of acidic amino acids and small amounts of hydrophobic amino acids. The low content of hydrophobic amino acids is offset by a high content of the “borderline hydrophobic amino acids” serine and threonine (61). Aspects of the evolutionary processes that may have led to the present highly salt-adapted state of these proteins were recently reviewed (19). Most proteins of the halophilic archaea depend on the presence of relatively high salt concentrations for the maintenance of their proper conformation and activity. These microorganisms thus depend on the continuous presence of high salt concentrations in their environment.
In most other halophilic and halotolerant microorganisms, the osmotic balance is provided by small organic molecules that are either synthesized by the cells or taken up from the medium when available. The compatible-solute strategy does not involve the need for specially adapted proteins. Compatible solutes are defined as solutes which, at high concentrations, allow enzymes to function efficiently (12). “Conventional” enzymes appear to function well in the presence of molar concentrations of these solutes. A great diversity exists in the types of solutes encountered. Compatible solutes detected in halophilic and halotolerant microorganisms include polyols such as glycerol and arabitol, sugars and sugar derivatives (sucrose, trehalose, glucosylglycerol), amino acids and derivatives, and quaternary amines such as glycine betaine. The list of known organic osmotic solutes is steadily growing (24, 25). Compatible solutes are typically low-molecular-weight compounds, soluble at high concentrations in water, and either uncharged or zwitterionic at the physiological pH. Beyond these common denominators, they have little in common structurally. Certain compatible solutes seem to be more efficient than others in protecting enzymes from the harmful effects of exposure to high salt concentrations and other stressful treatments (24, 25). The nature of the interactions of compatible solutes with proteins is still poorly understood, and therefore it is not yet possible to predict from the molecular structure how efficient any compound may be in providing osmotic stabilization and maintaining enzyme structure and activity. Intracellular concentrations of organic osmotic solutes are regulated according to the salinity of the medium, and the strategy of using organic compatible solutes thus allows a high degree of adaptability of the cells to changes in the salt concentration of their environment.
Many bacteria that accumulate organic solutes for osmotic stabilization were reported to contain also quite high intracellular Na+ and K+ concentrations, in some cases even in the molar range (see Table 5 in reference 133). A discussion on whether these bacteria use a combination of both strategies or whether the apparently high ionic concentrations are due to artifacts related to the technical problems involved in the measurement of intracellular ionic concentrations is beyond the scope of this review. However, the fact that the cellular proteins of Halomonas elongata are somewhat richer in acidic amino acids than those of nonhalophilic microorganisms (28) may signify that some degree of salt adaptation of the intracellular machinery is required in organisms that primarily use compatible solutes for osmotic adaptation.
Whatever strategy is used—maintaining a high salt cytoplasm with the buildup and maintenance of steep Na+ and K+ gradients across the cell membrane or the biosynthesis of large amounts of organic osmotic compounds—it is obvious that life at high salt concentrations is costly from a bioenergetic point of view. The energy requirement for adaptation of microbial life to high salt concentrations was recognized as early as 1956. Relating to the physiology of the bacterium Halomonas halodenitrifians (then named Micrococcus halodenitrificans), Baxter and Gibbons (5) described “adaptation at the cellular level, in a mechanism that is probably energy-dependent that maintains the intracellular salt concentration at a level considerably below that of the environment.” However, in spite of the general recognition that halophily is an energetically costly process, there do not appear to have been any systematic calculations of the amount of energy needed for bacteria and other microorganisms to be able to cope with the presence of molar concentrations of salts in their environment. Moreover, the energy requirements for the two opposed strategies have never been compared.
The writing of this review was inspired by the publication of two mutually exclusive statements in the recent literature: one opinion being that production of organic compatible solutes is energetically cheaper than maintaining high KCl concentrations in the cytoplasm, and the other proposing the opposite view. In a study of the alkaliphilic halophilic archaea Natronococcus occultus, Natronobacterium gregoryi, Natrialba magadii, and Natronomonas pharaonis, it was observed that cells grown in a nutrient-poor medium replaced part of their intracellular KCl with a novel organic osmotic solute, 2-sulfotrehalose (20). Cultures grown in medium rich in organic nutrients relied on KCl alone to provide osmotic equilibrium. The authors stated that, “Accumulation and maintenance of an inorganic ion gradient are energetically costly. In rich medium, there is presumably a larger amount of substrate for growth (or more easily metabolized substrates), so that an ion gradient can be easily established and maintained. In the defined medium, the cost of the ion gradient may now be comparable to that of synthesizing sulfotrehalose.”
A different opinion was presented during discussions on the mode of life of anaerobic halophilic bacteria of the order Haloanaerobiales, organisms that do not accumulate organic solutes but maintain high intracellular ionic concentrations. It was proposed that synthesis of organic osmotic solutes is not feasible for a fermentative bacterium that obtains only a very limited amount of energy from its substrates and that therefore the energetically cheaper solution of balancing the salinity of the medium with high internal salt concentrations is the only available option. Thus, Haloanaerobium acetoethylicum was stated to adapt to growth at high salt concentrations “not by producing compatible solutes (e.g., betaine or glycerol), which would further tax their already constrained energy conservation mechanisms” (108). A similar statement was made by Oren (88): “It may be speculated that the synthesis of organic osmotic solutes, which is the strategy of osmotic adaptation used by the other eubacteria, is too energy consuming for fermentative bacteria that obtain only limited amounts of energy from the substrates degraded” (also see references 70 and 73).
In this review I compare the energetic costs of the two different modes of adaptation to life at high salt concentrations. The conclusions obtained not only solve the controversy exposed above but also lead to some ideas why certain groups of microorganisms prefer certain organic osmotic solutes. Moreover, understanding the energetic aspects of halophilism enables some speculations on why certain physiological types of microorganisms are able to grow at salt concentrations of up to NaCl saturation while others (such as aceticlastic methanogenic bacteria and nitrifying bacteria) have not been shown to occur above a relatively low-salinity threshold.
ORGANIC OSMOTIC SOLUTES—ENERGETIC ASPECTS
Energetic Cost of Organic Osmotic-Solute Biosynthesis
A large number of different osmotic solutes have been detected in different halophilic and halotolerant microorganisms (25). In the following paragraphs, a representative selection is discussed, chosen because of their chemical structure, their distribution in the microbial world, and/or their ability to be accumulated at the highest salt concentration range of the host microbe. The compounds discussed (Fig. 1) are (i) glycerol, which enables growth of the unicellular green alga Dunaliella at salt concentrations up to NaCl saturation (8, 9) and is also found in a number of fungi (1, 12, 132); (ii) sucrose and trehalose, found in many nonhalophilic and slightly halophilic bacteria when grown at elevated salt concentrations and also found in the least halophilic among the cyanobacteria (71, 105); (iii) glucosylglycerol, the main osmotic stabilizer of cyanobacteria adapted to intermediate salt concentrations (71) but also identified as an osmotic solute in the heterotrophic bacterium Pseudomonas mendocina (98); (iv) glycine betaine, produced by many halophilic anoxygenic photosynthetic bacteria (25, 27, 127) and by the most salt tolerant among the cyanobacteria (71, 106) (many halophilic and halotolerant heterotrophic bacteria take up glycine betaine from the medium when the compound is available to concentrations sufficient to provide osmotic balance [45, 133]; only a few heterotrophs can synthesize glycine betaine de novo, Actinopolyspora halophila being a notable example [24, 25]; glycine betaine is also produced, together with additional compounds, by certain halophilic methanogenic archaea [59, 60, 109, 110, 112]); and (v) ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid), the main osmotic solute produced by heterotrophic halophilic bacteria and also found as a minor osmoticum in photosynthetic bacteria of the genus Halorhodospira (24, 25).
FIG. 1.
Some organic compatible solutes found in halophilic and halotolerant microorganisms.
Since the biochemical pathways leading to the biosynthesis of the above-mentioned osmotic solutes are known, the energetic cost in terms of the number of ATP molecules required for the production of one molecule of solute can easily be estimated. Glycerol is formed via reduction of dihydroxyacetone phosphate (9), sucrose and trehalose are produced by coupling of UDP-glucose with fructose-6-phosphate and glucose-1-phosphate, respectively, and the biosynthesis of glucosylglycerol is based on the reaction of ADP-glucose with glycerolphosphate (36). Two alternative modes of glycine betaine biosynthesis have been shown to operate in nature. Halorhodospira methylates glycine, using S-adenosylmethionine as a methyl donor (27), and methanogens may use a similar pathway (72, 109, 113). However, cyanobacteria produce glycine betaine by oxidation of choline (27). The formation of ectoine proceeds from aspartate semialdehyde and acetyl coenzyme A (95), and the enzymology and molecular genetics involved in the pathway are now well established (14, 69).
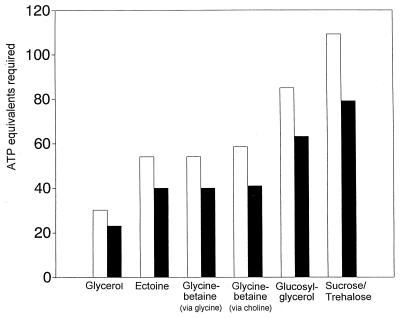
Figure 2 presents the results of these calculations. For each solute, two separate calculations were made, one relating to autotrophic growth and one relating to aerobic heterotrophic growth. For autotrophic microorganisms, the cost of synthesis of the compatible solutes from CO2 was estimated, based on the reactions of the Calvin cycle and the specific reactions leading to the formation of the product. For heterotrophs, glucose was chosen as the model substrate, to be degraded via the Embden-Meyerhof pathway and the tricarboxylic acid cycle. Calculations were then based on the decreased production of ATP when organic osmotic solutes were made as compared with the maximal ATP yield during complete oxidation of the equivalent amount of glucose to CO2. Consumption and formation of one NADH or NADPH molecule was taken to be equivalent to the requirement for and generation of three ATP molecules, respectively, since transfer of two electrons from NAD(P)H to oxygen coupled with electron transport phosphorylation may yield up to three ATP molecules. (The value 3 is a consensus value. Values between 2 and 4 H+/ATP have been reported for different energy-transducing membrane systems.) Between 30 and 109 molecules of ATP appear to be required for the autotrophic biosynthesis of one molecule of the different compatible solutes, and for the heterotrophs between 23 and 79 potential ATP molecules that could otherwise have been generated during respiration are “wasted” per molecule of compatible solute synthesized.
FIG. 2.
Energy requirement (in ATP equivalents) for the synthesis of selected organic compatible solutes from CO2 (open bars) and the cost of the production of these solutes for an aerobic heterotrophic microorganism growing on glucose (solid bars). For autotrophic microorganisms, the cost of synthesis of compatible solutes from CO2 was calculated based on the reactions of the Calvin cycle and the specific reactions leading to the formation of the product. Calculations of the energetic cost for heterotrophs were based on the assumption that each glucose molecule spent in the synthesis of osmotic solutes would have yielded 38 ATP equivalents upon complete oxidation to CO2 via the Embden-Meyerhof pathway and the tricarboxylic acid cycle. In all cases, the consumption and formation of one NADH or NADPH molecule was taken to be equivalent to the requirement for and generation of three ATP molecules, respectively. Calculations of the energetic cost of the biosynthesis of glycine betaine were based in part on Fig. 5 in reference 104, and separate calculations were made for synthesis of glycine betaine by the oxidation of choline and by stepwise methylation of glycine. For nitrogen-containing solutes, ammonia was assumed to be assimilated via the glutamine synthetase/glutamate synthase system, adding the need for one ATP molecule and one NADH molecule (three potential ATP equivalents) per atom of nitrogen (104).
To assess the implications of the above-calculated values for the energy household of the cell, the amount of ATP needed for compatible-solute production should be compared with the requirement for biosynthesis of the structural cell components. A theoretical study of the amount of ATP required for the synthesis of cell material in (nonhalophilic) bacteria was made by Stouthamer (124, 125). His calculations yielded a maximum value of 6.5 g (dry weight) of autotrophic microbial cells formed from CO2 per mol of ATP. When estimating that nonhalophilic microorganisms consist of 80% water (by weight) and that the density of the dry cell material is around 2 g/cm3, the production of 1 cm3 of biovolume of nonhalophilic bacteria, containing 0.2 g of dry material of structural cell components and 0.9 ml of water, would cost a minimum of 30 mmol of ATP.
Compared to this value, the energetic cost of producing organic osmotic solutes is huge. An organism living in 4 M salt (by no means the highest salt concentration allowing the growth of many species that exclude salt from their cytoplasm) may contain close to 8 M organic solute in its cytoplasm to maintain osmotic equilibrium, with the exact value depending on the activity coefficients of the ions and the organic solutes involved. Thus, Dunaliella salina grown in 4 M NaCl contained 7.8 M glycerol intracellularly (12). When using glycerol, glycine betaine, ectoine, glucosylglycerol, and sucrose or trehalose as the osmotic solute, the theoretically added dry weight per 0.2 g of structural cell material not directly involved in osmotic regulation would be 0.66, 0.85, 1.03, 1.83, and 2.46 g, respectively, of osmotic solute (7.2 mmol/0.9 ml) (assuming for the moment that all these compounds are soluble at the 8 M concentration). The synthesis of these quantities of solutes from CO2 would cost 216, 389 to 421, 389, 612, and 785 mmol of ATP, respectively, as calculated from the data presented in Fig. 2. It is thus clear that the strategy of accumulating osmotic solutes is energetically very costly, the more so when larger organic molecules such as disaccharides (12 carbon atoms) are to provide osmotic balance. The use of smaller molecules such as glycerol (three carbons) or glycine betaine or ectoine (five and six carbons, respectively) is less energy consuming. The amount of energy needed for the production of the osmotic solutes may thus greatly exceed the energy requirement for the biosynthesis of proteins, nucleic acid, cell walls, etc.
A similar calculation can be made for the heterotrophs, which have a higher YATPMAX and need less ATP for the synthesis of the organic solutes. A nonhalophilic microorganism growing on glucose at maximal efficiency (YATPMAX = 28.8 g [dry weight] per mol of ATP) (124, 125) needs for the formation of 0.2 g of dry cell material about 0.2 g of glucose for assimilation (assuming a 1:1 ratio by weight of glucose assimilated and cell material formed) and, in addition, 0.033 g of glucose for the formation of ATP (at 38 molecules of ATP/molecule of glucose oxidized), which makes a total of 0.233 g of glucose. If such an organism also produces glycerol at an intracellular concentration of 8 M (7.2 mmol/0.2 g of structural dry weight [see above]), an additional 0.648 g of glucose has to be assimilated and 0.136 g of glucose has to be spent to supply the energy (as ATP and NADH equivalents) needed for glycerol biosynthesis. The total amount of glucose required for the formation of 0.2 g of structural cell material is thus increased to 1.017 g, i.e., 4.4 times as much as for a nonhalophilic microorganism. A similar calculation for the accumulation of 8 M ectoine in the cytoplasm shows that 1.364 g of glucose would be needed for the production of the compatible solute, thus increasing the total requirement to 1.597 g, or 6.9 times as much as in the absence of salt.
In view of the above calculation, it should be expected that the cell yield of halophilic and halotolerant heterotrophic microorganisms (Y, expressed as the amount of structural cell material synthesized, the biovolume, or the number of new cells produced per gram of organic substrate used) will decrease sharply as the salt concentration of the growth medium increases. When expressed on the basis of cell dry weight formed, including the intracellular osmotic solutes, the difference should be much smaller or altogether absent. It is curious that this hypothesis never seems to have been tested in laboratory models; a few simple measurements on cultures of microorganisms with a broad salinity range and simple growth requirements (such as Halomonas elongata, which in defined medium produces ectoine as its main compatible solute) could easily supply valuable information on the energetic cost of this type of adaptation to life at high salt concentrations.
It is obvious that of all known compatible solutes, glycerol is the simplest and cheapest to produce. Its solubility in molar terms exceeds that of all other solutes, since it is miscible with water at all ratios. Moreover, even at extremely high concentrations (4 M and more) it still supports excellent activity of intracellular enzymes in an organism such as Dunaliella (7). Therefore, the question should be asked why glycerol is not more widespread as a compatible solute and why it seems to be restricted to the domain Eucarya in particular to certain green algae (8, 136) and yeasts (1, 12, 132). The reason may be sought in the special adaptations of the cell membrane required. Most biological membranes are highly permeable to glycerol, and therefore a specially adapted membrane structure is needed to keep the glycerol produced within the cell. Indeed, the Dunaliella cell membrane was found to be several orders of magnitude less permeable to glycerol than were all other membranes known (13, 31). However, the permeability to water was not unusually low (18). The reason for the unusual behavior of the Dunaliella membrane has never been definitively elucidated, but it has been suggested that the high sterol content may be a key factor (118). Since (with very few exceptions) sterols are lacking in the membranes of prokaryotes, glycerol accumulation may not be possible in the domains Bacteria and Archaea, and these organisms must therefore use other solutes, whose production is more costly.
It is interesting that solutes that are cheaper to produce are generally found in organisms that grow at the highest salt concentrations, i.e., organisms that have to synthesize compatible solutes in the highest concentrations. This is especially pronounced in the cyanobacteria. A clear trend has been documented in which the least salt-tolerant species produce sucrose and/or trehalose, the somewhat more halotolerant ones balance the external salinity with glucosylglycerol, and the truly halophilic types use glycine betaine (71, 89, 105, 106). The higher cost dictated by the higher concentrations needed is thus offset at least in part by the choice of cheaper alternatives. This is probably not the sole reason for the preferential use of such solutes as glycine betaine and ectoine in the prokaryotic world. It was also documented that the more energetically “expensive” solutes sucrose and trehalose are not highly effective in supporting the activity of salt-sensitive enzymes at high concentrations (24). The reason for this is unknown. In fact, our understanding of the function of organic osmotic solutes and of the factors that determine whether a compound with a given chemical structure will make a good compatible solute is still very limited (24, 25).
Maintenance of suitable cell buoyancy and limits to the solubility of the compounds may be an additional reason why “lighter” solutes are made by microorganisms thriving at the highest salt concentrations. The solubility of sucrose in water is limited to about 3 M at room temperature. A 2 M solution of sucrose has a density of 1.24 g/ml, which is already higher than that of the most saline brines harboring life (about 1.235 g/ml in saturated NaCl solutions and in the Dead Sea). Therefore, the hypothetical cells that maintain intracellular sucrose concentrations above 2 M may be expected to sink rapidly to the bottom of salt lakes, thus limiting their access to important resources such as oxygen or light. In view of all this, it is understandable that disaccharides alone have very limited potential as osmoadaptors (also see references 24 and 25). For comparison, 7.8 M glycerol in water has a density of 1.16 g/ml.
Compatible Solutes as Organic Carbon Reserves
It may be argued that the real energy cost involved in the accumulation of organic osmotic solutes may be lower than what appears from calculations such as given above, since the osmotic solutes may also represent a carbon and energy reservoir, ready to be used when required. Such a statement is true only to a limited extent. In an environment of constant salinity, the cells have to maintain a constant and high osmolarity of the cytoplasm, and therefore they will not have the possibility to recruit part of their intracellular osmotic solutes for other purposes such as energy generation. Only when the cells encounter a decrease in salinity (such as may occur in salt lakes when rainwater mixes with the highly saline brines) may part of the intracellular osmotic solutes become available for other purposes. Such events are expected to be relatively rare in most habitats in which halophilic microorganisms thrive. Even then the possibility of the direct utilization of organic compatible solutes for energy generation, thereby recovering part of the energy invested in their biosynthesis, is not always exploited in full. Certain solutes, such as glycine betaine and ectoine, are excreted from the cells upon salt downshock and are therefore lost to the cell, at least temporarily (22, 127, 128). However, (energy-dependent) transport systems are present in the cell membrane, enabling uptake of the solutes from the medium at a later stage (see below). Part of the loss may thus be recovered at a relatively low cost.
For glycerol in Dunaliella, return of an excess of compatible solutes to the energy metabolism of the cell is possible. When subjected to dilution stress, excess glycerol is converted into starch (after oxidation to dihydroxyacetone followed by phosphorylation to dihydroxyacetone phosphate). Being osmotically inactive, the starch may serve as a storage polymer to be used in the production of structural cell material or, alternatively, to be converted again to glycerol when additional glycerol is needed for cell growth or upon an increase in medium salinity (9).
Similarly, in the photosynthetic alkaliphilic halophile Halorhodospira halochloris, which uses glycine betaine as its main osmotic solute, the minor component trehalose can be degraded intracellularly under conditions of dilution stress (41). It has been argued that in many microorganisms trehalose might serve primarily as an energy reserve rather than as an osmotic stabilizer (131).
In most cases, microorganisms regulate their osmotic solute content separately from their biosynthetic and energy-generating processes. Thus, Rhizobium (a bacterium with a relatively low salt tolerance) can synthesize glycine betaine from choline or take up glycine betaine from the medium. While glycine betaine can serve as both a nutrient and an osmoprotector, its degradation is repressed at high salt concentrations, when it serves as an osmoticum only (23, 67). Halorhodospira cells use glycine betaine only as osmotic solute. The compound cannot serve as a nitrogen source, even under the most stringent nitrogen depletion. The physiological basis of this one-way-only biosynthetic pathway is not understood, but it emphasizes the importance of betaine not only as an inert solute but also as a cytoplasmic protectant. However, nitrogen starvation induces the breakdown of ectoine, mobilizing two nitrogen atoms per molecule; trehalose then replaces ectoine as the osmotic solute (26). The advantage of being able to choose between different osmotic solutes is obvious. Many halophilic and halotolerant microorganisms maintain “cocktails” of osmotic solutes, and the regulation of the synthesis of each of the solutes is optimized according to the needs of the cells (25, 133).
How Permeable Are Membranes to Osmotic Solutes?
Membranes are not perfectly impermeable to low-molecular-weight osmotic solutes, and some of the material produced at a high energetic cost may leak out of the cells. Some microorganisms are surprisingly effective in retaining the solutes produced. Dunaliella, which has a membrane virtually impermeable to glycerol, loses less than 5% of the glycerol produced to the outer medium at physiological temperatures (136). Only at supraoptimal temperatures (>40°C) do substantial amounts of glycerol appear in the medium, probably due to an increase in membrane permeability. A similar phenomenon has been documented in Asteromonas, another glycerol-accumulating green alga (136). Turnover of glycerol in Dunaliella is also very slow: a turnover time of about 6 h was measured in Dunaliella tertiolecta grown in 0.7 M NaCl (35). This also suggests that the quantities lost by leakage through the membrane are negligible.
Bacteria that produce compatible solutes often possess transport proteins in the membrane that enable them to take these solutes up from the medium, which is energetically more favorable than de novo synthesis. Such transport systems may be intended primarily to salvage osmotic solutes that would otherwise be lost to the environment from leaky membranes. Thus, glycine betaine-producing cyanobacteria have an efficient glycine betaine transporter in their membrane (76). Similar glycine betaine transport systems have been demonstrated in Halorhodospira halochloris (96) and in salt-tolerant and salt-requiring methanogenic archaea (43, 101). A transport system for glucosylglycerol was characterized in the cyanobacterium Synechococcus strain PCC 6803. A mutant lacking a functional transporter leaked significant amounts of glucosylglycerol to the medium when grown at high salt concentrations. This indicates that the membrane is not completely impermeable to the compound and that the glucosylglycerol transport system may serve to recover the loss. Transcription of the gene encoding the transporter was increased in cells adapted to high salt (37, 75).
The presence of transport systems for osmotic compounds reduces the energy to be spent on salt adaptation when suitable compounds are available in the medium, thus relieving the organisms of much of the cost of de novo solute synthesis. Glycine betaine, produced as an osmotic solute by halophilic cyanobacteria and leaking out of the membranes of healthy cells or being released from lysing cells, may be available to the heterotrophic community that accompanies the cyanobacteria in microbial mats (85). Glycine betaine transporters are widespread in heterotrophic bacteria, especially in microbes that are incapable of de novo synthesis of the compound. When glycine betaine is available, bacteria that have the transport system will take it up and accumulate it as an osmotic solute, relieving them of the need to produce other compounds such as ectoine. That is also the reason why halophilic bacteria grown in rich media contain glycine betaine as their main osmoticum (45); glycine betaine is found abundantly in yeast extract used in such media.
ION GRADIENTS—ENERGETIC ASPECTS
Energetic Cost of Na+ and K+ Gradient Generation
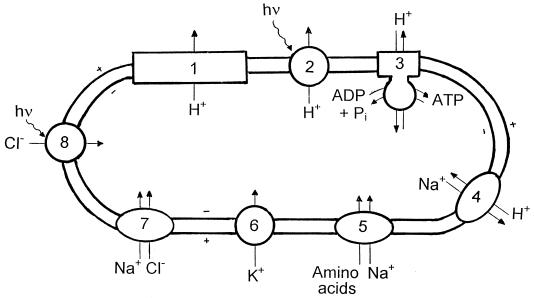
Two groups of heterotrophic bacteria balance the osmotic pressure of the medium with high intracellular KCl concentrations: the halophilic archaea of the order Halobacteriales (Fig. 3) and the anaerobic halophilic bacteria of the order Haloanaerobiales.
FIG. 3.
Ion movements in the aerobic halophilic archaea (family Halobacteriaceae). 1, proton extrusion via respiratory electron transport; 2, light-driven proton extrusion mediated by bacteriorhodopsin; 3, ATP formation by ATP synthase, driven by the proton gradient (alternatively, this system can serve to generate a proton gradient at the expense of ATP during fermentative growth on l-arginine); 4, electrogenic sodium/proton antiporter; 5, sodium gradient-driven inward amino acid transport; 6, potassium uniport, driven by the membrane potential; 7, light-independent chloride transport system, probably coupled with inward transport of sodium; 8, halorhodopsin, a light-driven inward chloride pump. For details, see the text.
The primary energy source for the extrusion of Na+ and accumulation of K+ by most microorganisms is the proton electrochemical gradient (Δμ̃H+) across the cytoplasmic membrane. This proton electrochemical gradient is derived either from respiratory electron transport (in the Halobacteriales during aerobic growth) or at the expense of ATP formed during substrate-level phosphorylation by activity of the membrane ATPase (F-type H+-ATPase in bacteria, A-type H+-ATPase in halophilic archaea) (42). The exceptional case of the direct generation of a proton motive force by light, mediated by bacteriorhodopsin in Halobacterium salinarum and a few additional members of the Halobacteriaceae, is not discussed here; even among the halophilic archaea, the presence of bacteriorhodopsin seems to be the exception rather than the rule, and, moreover, they all grow well heterotrophically in the dark. An overview of the modes of energy generation in the Halobacteriaceae was recently given by Bickel-Sandkötter et al. (10).
The Na+ gradient (Δμ̃Na+) is established at the expense of the proton gradient via Na+/H+ antiporters. Activity of such antiporters has been documented both in halophilic archaea (62, 65) and in halophilic bacteria (38) and eukaryotes (49, 50). In-depth physiological and molecular genetic studies of the Na+/H+ antiporter systems of Escherichia coli have contributed extensively to our understanding of the physiology of these transporters. Both electroneutral and electrogenic Na+/H+ antiporters have been described. The NhaA transporter of E. coli is an example of the latter type, extruding one Na+ ion for every two protons entering the cell (94, 117). A second electrogenic antiporter (NhaB) identified in E. coli has a stoichiometry of 3H+/2Na+ (93). The main Na+/H+ antiporter of Halobacterium salinarum is also electrogenic (61), and a stoichiometry of 2H+/Na+ has been suggested (57, 65). In addition to their function in osmoregulation, Na+/H+ antiporters play essential roles in the homeostasis of intracellular pH (93, 94).
For the sake of completeness, it should be mentioned that an Na+ gradient may also be established by primary Na+ pumps without using the proton electrochemical gradient as an intermediary. Primary respiration-driven Na+ pumps were shown to occur in a number of slightly halophilic (e.g., Vibrio alginolyticus) (126) and moderately halophilic (e.g., Salinivibrio costicola) (126, 129) bacteria. It may be expected that the energy requirement for the establishment of a Na+ gradient will not be greatly different whether a primary or secondary Na+ transport system is used. All calculations below are therefore based on the formation of a primary proton electrochemical gradient.
In Halobacterium, K+ probably enters the cells passively via a uniport system and is accumulated in accordance with the size of the membrane potential (29, 134). The cell membrane of Halobacterium salinarum is highly permeable to potassium ions (63). The transmembrane potential difference (Δψ) can provide the driving force for the accumulation of a monovalent cation according to the Nernst equation. Such a passive uniport mechanism can be invoked whenever the equilibrium potential for the cation is equal to or less negative than the Δψ of the membrane. The nature of K+ influx as a simple diffusion process was demonstrated in bacteriorhodopsin-containing membrane vesicles of H. salinarum, which, upon illumination, accumulated K+ proportionally to the external K+ concentration (66).
Accumulation of chloride is expected to be an energy-demanding process in cells that maintain an inside-negative membrane potential. The finding that intracellular chloride concentrations in Halobacterium cells are similar to those in the outside medium signifies that the distribution of chloride is far from equilibrium. Halobacterium was shown to transport Cl− against the Δψ, supposedly based on cotransport with Na+ (21). The additional possibility of light-dependent Cl− transport mediated by the retinal protein halorhodopsin exists for a number of halophilic archaea. Halorhodopsin seems to be more widely distributed than bacteriorhodopsin within the Halobacteriales. As stated above, the halophilic archaea all grow well in darkness, and therefore the light-driven processes is not taken into account in the energetic comparisons below.
The number of ATP equivalents needed to extrude Na+ ions from a cell and to replace them with K+, with additional energy requirement for the entry of an equimolar amount of Cl−, can be estimated on the basis of our understanding of the transport systems involved, as outlined above. For these calculations, the membrane is assumed to be impermeable to Na+. Entrance of Na+ coupled to energy-requiring processes not related to the buildup and maintenance of the ion gradients, such as occurs during cotransport with amino acids (38, 133), is not taken into account. In these cases, the Na+ gradient is used as an energy reservoir to drive endergonic processes that any cell has to perform, whatever its degree of halophilism and its mode of osmoregulation may be. It was further assumed that the membrane is permeable to K+, which enters the cell passively by a uniport system (134) (an assumption that is further evaluated in the following section), and that Cl− enters the cell against the membrane potential via cotransport with Na+ (21) at a stoichiometry of 1 Na+/Cl−. The stoichiometry of the membrane ATPase/ATP synthase was taken to be 3H+/ATP. At a stoichiometry of 1Na+/2H+ of the antiporter (65), three molecules of KCl can then be accumulated for every two ATP hydrolyzed. Assuming the stoichiometry of the antiporter to be 3H+/2Na+, similar to that of the NhaB antiporter of E. coli (93, 94), one molecule of ATP should suffice for the accumulation of two K+ and two Cl− ions.
Whatever inaccuracies there may be in the calculation presented above, it is clear that the energy cost of the salt-in strategy (0.5 to 0.67 ATP equivalent per KCl accumulated, depending on the stoichiometry of the antiporter) is only a small fraction of that of the “salt-out, organic solutes in” mode of halophilic life discussed above.
Does K+ Transport in Halobacterium Require ATP?
The above model based on K+ influx in halophilic archaea as a simple uniport-mediated diffusion process may require some modification. Studies with Haloferax volcanii showed that the size of the K+ gradient over the membrane is not in equilibrium with the Δψ, suggesting that K+ transport cannot be accounted for simply by a passive process. When the Δψ of the cation is more negative than the Δψ of the membrane, either of two processes should be active: K+ transport energized by symport or antiport with other ions, or primary transport driven by ATP hydrolysis. ATP is required to enable H. volcanii to accumulate up to 3.6 M KCl, representing a 500- to 1,000-fold gradient of K+ (74). Cells can be partially depleted of their intracellular K+ down to about 1.5 M. The accumulation of the remainder is energy dependent. The presence of an energy-dependent active K+ transport system with a Km of 1 to 2 mM was documented. The system showed a requirement for ATP and was found to be comparable to the low-affinity K+ transporter (the Trk system) of E. coli. It was proposed that in H. volcanii part of the intracellular K+ content (up to about 1.5 M) could be in an osmotically inactive bound form and that the remainder is accumulated by using a Δψ-driven K+ transport system, whose activity is regulated by ATP (74). This view differs from that proposed by Lanyi et al. (66), who claimed that K+ uniport is sufficient to explain the K+ gradient observed, based on experiments with membrane vesicles of Halobacterium salinarum in the absence of ATP.
In the Trk K+ transport system of E. coli, K+ is transported in symport with H+, mediated by ATP (2, 3). ATP is not used as the energy source for the transporter but, rather, as a regulator of its activity. ATP consumption may be explained either by a cyclic phosphorylation and dephosphorylation of the system or by its binding to an allosteric site of the transport complex. However, the possibility that K+ uptake is carried out by an electrogenic pump fueled by ATP cannot yet be ruled out (74). If at least part of the K+ enters the cell by using such an active transport process, allowance for the (as yet unknown) quantity of ATP consumed for the activation of the transport system should be made in the calculation presented above. However, no evidence has been reported for the involvement of an ATP-driven K+ transport system in the Halobacteriales resembling the well-characterized Kdp K+ transport system of E. coli, shown to be present in many other bacteria as well (119).
Even when K+ transport is not a simple Δψ-driven facilitated diffusion but involves an active process, such as was suggested for Haloferax volcanii, the overall cost of the establishment of the ion gradients is still much lower than that required to fill the cytoplasm with organic osmotic solutes.
How Much Energy Is Required To Maintain the Ion Gradients in Halobacterium?
The main process causing reentry of Na+ into the cells seems to be an Na+-driven cotransport process (94). The Na+ gradient is used in this case as an energy reservoir to drive endergonic processes that the cell has to perform anyhow, unrelated to osmotic adaptation. Therefore, the cost involved in pumping this Na+ out again is not taken into account here.
Little is known about the extent of the occurrence of passive Na+ “leaks” in the membranes (94). Studies with E. coli showed that downhill Na+ movement from deenergized cells (under anaerobic conditions or in the presence of an uncoupler) is very slow. This movement is probably mediated by Na+/H+ antiporter activity, but contributions of Na+ leaks cannot be ruled out (15). It was suggested that a genetic approach involving deletion of Na+-coupled transport systems be used to investigate the extent of leakiness of the membranes associated with the presence of these carriers (94). To my knowledge, such experiments have yet to be performed. However, when liposomes prepared from the membranes of a variety of bacteria and archaea were loaded with 22Na+ and suspended in medium with an equal concentration of unlabeled Na+, efflux of the label was very slow, suggesting that passive permeability of the membranes for Na+ is low at physiological temperatures (130).
The maintenance of the K+ gradient in halophilic archaea does not seem to require much energy. Much of the K+ may be strongly bound to proteins and other intracellular components. It was claimed that in Haloarcula marismortui most of the intracellular K+ was tightly and specifically bound to the cell matrix (32, 33). In Haloferax volcanii, only about half of the intracellular K+ could be exchanged (74). However, no bound K+ was detected in lysed Halobacterium salinarum cells, suggesting that in this organism most of the internal K+ is osmotically active and is held inside due to the properties of the cell membrane (64).
For the moderately halophilic bacteria, which generally maintain low intracellular ionic concentrations, the question has been asked whether the exclusion of salt and the maintenance of steep ionic gradients is achieved through the constant pumping of ions by active, energy-dependent mechanisms or by tightening the permeability barrier of the membrane. This important question does not seem to have been pursued in depth. Continuous-culture experiments with Halomonas elongata strongly indicate that the maintenance energy of such an organism is relatively independent of the salinity of the medium, providing evidence in favor of an ion-tight membrane (25; also see reference 133).
Ion Pumping in Microorganisms That Accumulate Organic Solutes
Microorganisms that balance the osmotic pressure of the medium with high concentrations of organic osmotic solutes also have to expend energy in ion pumps to keep intracellular ionic concentrations low and to counteract the diffusion of inorganic salts through their membranes.
Na+/H+ antiporters have been characterized from moderately halophilic bacteria that mainly use organic solutes, such as Salinivibrio costicola (38, 39). These antiporters play a key role in keeping the intracellular Na+ concentrations low. The activity of the Na+/H+ antiporter activity in the cytoplasmic membrane of Dunaliella increased when the cells were grown at increasing NaCl concentrations (49, 50).
The need for Na+ pumping thus may increase the energy cost of the “compatible-solutes” strategy beyond that of the synthesis of the compounds only.
ALKALIPHILIC HALOPHILIC ARCHAEA AND FERMENTATIVE HALOPHILIC BACTERIA RECONSIDERED
As stated above, one of the goals of writing of this essay was to reevaluate statements made in the literature about the energy cost of different modes of osmoregulation in the halophilic alkaliphilic archaea and of the salt-in strategy of the halophilic anaerobic bacteria. Based on the calculations made in the previous sections, it is now possible to reassess the value of these statements.
2-Sulfotrehalose in Halophilic Alkaliphilic Archaea— Is Its Synthesis Energetically Less Costly than Establishment of an Ion Gradient?
When grown in rich media, Natronococcus occultus and other halophilic alkaliphilic archaea accumulate KCl in molar concentrations, similar to the neutrophilic halophilic archaea. However, when these organisms were grown in nutrient-poor media, an organic osmotic solute, 2-sulfotrehalose, was produced. Because of the dependency of the intracellular enzymes on high salt concentrations, sulfotrehalose can replace KCl only in part (20).
It was argued that the formation of 2-sulfotrehalose in nutrient-poor media may be dictated by bioenergetic restrictions: the establishment and maintenance of an ion gradient was assumed to be more costly than the production of an organic solute, and therefore the organisms supposedly replaced part of their intracellular KCl with the disaccharide when subjected to energy-limiting conditions (20). In view of the considerations presented above, this assumption is probably incorrect.
Biosynthesis of 2-sulfotrehalose is energetically costly. The nutrient-poor medium used for growth of Natronococcus was based on amino acids as organic nutrients. Since Natronococcus was found to grow on acetate and to possess functional glyoxylic acid and tricarboxylic acid cycles (51), I based the calculation of the ATP requirement on the use of acetate as the building block for the production of 2-sulfotrehalose, assuming that the sulfate group is derived from adenosine-5′-phosphosulfate. The bottom line of the calculation is that the synthesis of 1 molecule of 2-sulfotrehalose from 12 acetate ions should cause the cell a loss of 83 ATP molecules relative to the amount of energy that could have been gained by complete oxidation of the acetate to CO2. Similar calculations can be made for other growth substrates. In addition, it must be taken into account that sulfotrehalose is an anion and that the cell also has to provide suitable counterions (K+ ?), which may require additional energy. In any case, the cost of sulfotrehalose biosynthesis appears to exceed by far the cost of building up a high intracellular KCl concentration (assuming that the amount of energy required for the establishment and maintenance of the Na+ and K+ gradients in these alkaliphiles is similar to that in the neutrophilic halophilic archaea [discussed above]). The reason why these halophilic alkaliphilic archaea synthesize 2-sulfotrehalose mainly during nutrient starvation remains thus far from clear.
Energetic Considerations on Osmoregulation in Halophilic Fermentative Bacteria
It was mentioned above that the anaerobic halophilic bacteria of the order Haloanaerobiales are exceptional within the domain Bacteria with respect to their mode of osmoregulation, using the salt-in strategy and lacking organic osmotic solutes (83, 92, 108). Accordingly, they have adapted their intracellular enzymatic machinery to the presence of high salt concentrations (90, 108). The unusual behavior of these bacteria was previously explained by the low level of energy generated by these organisms in the course of their fermentations. It was argued that biosynthesis of large amounts of osmotic solutes would be energetically too costly for these bacteria and that therefore the salt-in option was the only possibility for microorganisms possessing such a type of metabolism (88, 108). A critical reassessment confirms this intuitive conclusion.
The fermentative anaerobes do not possess respiration-driven primary proton pumps, and ATP produced by fermentation is the primary energy source driving all bioenergetic processes. Membrane ATPases are expected to convert part of the ATP to a secondary proton electrochemical gradient, which in turn may fuel the activity of specific ion transport systems such as Na+/H+ antiporters. An Na+/H+ antiporter of the homoacetogenic representative Acetohalobium arabaticum was partially characterized (102).
Most representatives of the Haloanaerobiales ferment simple sugars such as glucose to products such as ethanol, acetate, H2, and CO2. The ATP yield per molecule of glucose fermented may vary according to the amount of acetate formed and can theoretically be anywhere between 2 and 4. From the data on Haloanaerobium acetoethylicum presented by Rengpipat et al. (107), a value of 2.8 to 2.9 can be estimated; for Halobacteroides halobius, a value of about 2.6 can be calculated (91).
Assuming a YATP value of 10.5 g (dry weight)/mol of ATP (4, 124, 125) and an average of 2.7 ATP molecules formed per glucose, 6.3 g of glucose has to be fermented to supply the energy for the production of 1 g (dry weight) of cells for a nonhalophilic microorganism. I assume again that 1 g (dry weight) of structural cell components is equivalent to 4.5 ml. This value is in good agreement with experimental data for halophilic bacteria: values of 1.8 and 2.7 ml of intracellular volume per g (dry weight) (including salts and compatible solutes) were measured for Salinivibrio costicola (38) and calculated from the data presented for Ectothiorhodospira mobilis (44), respectively. Then 18 mmol of compatible solutes (to yield an intracellular concentration of 4 M) would be needed per g of structural cell material to satisfy the osmotic need of organisms such as Haloanaerobium or Halobacteroides species, which grow optimally at NaCl concentrations of 2 M. In the hypothetical case in which such organisms used sucrose or trehalose as their osmotic solutes, this would increase the amount of glucose required for the production of the same biovolume of cells by 10 g (a calculation based on the need for 2 glucose molecules to supply the building blocks for the biosynthesis of 1 molecule of disaccharide and an additional 1.1 glucose molecules to be fermented to supply the 3 ATP equivalents required for its biosynthesis). If the same 10 g of glucose were all fermented, the 150 mmol of ATP formed would suffice for the accumulation of between 225 and 300 mmol KCl, depending on the stoichiometry of the Na+/H+ antiporter system. Therefore, the salt-in strategy again appears to be the cheaper option. If ectoine were produced instead of disaccharides, 1.74 molecules of glucose would suffice to generate the precursors aspartate semialdehyde and acetyl coenzyme A and the energy requirement for the production the solute. With the “salt-in” option, the same amount of glucose would yield 4.7 molecules of ATP during fermentation, sufficient for accumulation of 7.1 to 9.4 molecules of KCl. The bottom line of these calculations is that in the anaerobes also, the salt-in strategy appears to require much less of the expensive substrate than does the production of organic osmotic solutes. The salt-in option should then be the preferred one for a fermentative bacterium that requires large amounts of substrate which supply only little energy and which may not always be available in excess. This also explains how Halobacterium salinarum, an archaeon that uses the salt-in strategy, is able to grow fermentatively on l-arginine (10, 40).
There is some evidence that the halophilic anaerobic bacteria may invest as little energy as possible in the maintenance of ion gradients. Not only are the apparent intracellular Na+ concentrations in exponentially growing cells relatively high (83, 92), but also, when entering the stationary growth phase, Na+ replaces K+ to a large extent as the dominant intracellular cation (92). Measurements of intracellular ionic concentrations in Haloanaerobium acetoethylicum even suggested that this organism may contain Na+ as the main cation even in exponentially growing cells (73, 108). If this is true, H. acetoethylicum would be the only known organism that does not attempt to keep Na+ out of its cytoplasm, thereby reducing to a minimum the energetic cost of withstanding the high salinity of its environment. The regulation of intracellular ion concentrations in this interesting microorganism surely deserves renewed examination.
WHY CERTAIN PHYSIOLOGICAL GROUPS OF MICROORGANISMS ARE ABSENT IN HYPERSALINE ENVIRONMENTS—ENERGETIC CONSIDERATIONS
As documented above, life at high salt concentrations is energetically expensive since it involves the buildup and maintenance of steep ion concentration gradients across the cell membrane, whether or not this is accompanied by the biosynthesis or accumulation of organic osmotic compounds. The calculations presented show that the salt-in option is energetically more favorable than the maintenance of a low salt cytoplasm with organic osmotic solutes to provide osmotic balance. It should also be remembered that the intracellular concentration of uncharged or zwitterionic osmotic solutes exceeds that of the salt outside, since NaCl and other salts are ionized, thus increasing osmolarity. Moreover, the accumulation of osmotic solutes while keeping a low-salt cytoplasm requires strong ion pumps to counteract leakage of ions into the cytoplasm in addition to the cost of compatible-solute synthesis or transport, if available in the medium.
The energetically relatively cheap solution of balancing salt-out with salt-in is not widely used in nature. The presence of high intracellular salt concentrations in the Halobacteriales and the Haloanaerobiales requires a complete adaptation of all intracellular components to function at high salt concentrations. One can only wonder why evolution did not use this strategy more widely in a greater variety of phylogenetic groups and metabolic types of bacteria.
When organic solutes are used, glycerol seems to be the ideal compound: it is the least costly to make, and its solubility in water has no limits. Brown (12) could therefore state that glycerol “may be regarded as God’s gift to solute-stressed eukaryotes.” In spite of these advantages, glycerol is used by only a few halophilic microorganisms, all belonging to the domain Eucarya. The reason why glycerol is not used in the prokaryotes must probably be sought in the special adaptations of the cell membrane required to provide a permeability barrier against a compound that normally freely penetrates biological membranes, as discussed above.
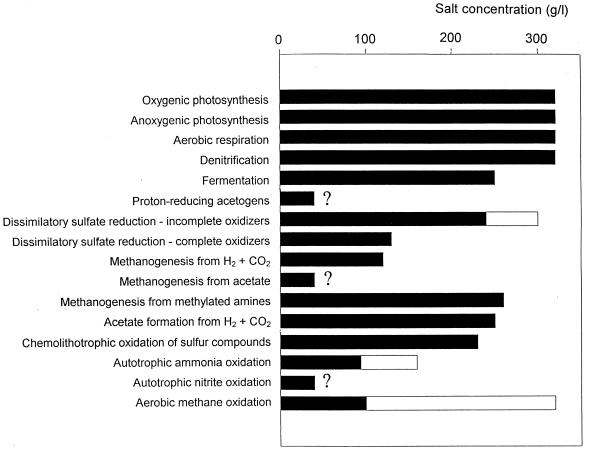
A survey of the halophilic microorganisms shows that not all known metabolic types function in the presence of high salt concentrations. While processes such as oxygenic and anoxygenic photosynthesis, aerobic respiration, and denitrification can occur at or close to NaCl saturation, other physiological groups have never been shown to thrive at high salinities. Figure 4 summarizes the approximate upper limits of salt concentrations for different microbial types, based on both laboratory experiments with isolated cultures and on measurements of the processes in natural samples. Examples of bacterial groups yet to be found at salinities above 10 to 15% are methanogens growing autotrophically with hydrogen as the electron donor, aceticlastic methanogens, dissimilatory sulfate reducers that perform complete oxidation of their substrates, and autotrophic ammonia and nitrite oxidizers. No obvious reasons for the apparent lack of these types have been put forward. However, it is possible that the bioenergetic consequences of the need to cope with the high salt concentrations may preclude the existence of the groups of microorganisms that obtain only a little energy from their dissimilatory processes.
FIG. 4.
Approximate upper salt concentration limits for the occurrence of selected microbial processes. Values presented are based in part on laboratory studies of pure cultures (solid bars) and in part on activity measurements of microbial communities in hypersaline environments in nature (open bars). Data are based on references 11, 17, 82, 84, 121, 141, and many other sources. For additional information, see the text.
The autotrophic nitrifiers may present a prime example. Reports of the occurrence of autotrophic oxidation of ammonia to nitrite or nitrite to nitrate at elevated salt concentrations are lacking. Autotrophic oxidation of NH4+ to NO2− does not seem to occur above 150 g of salt per liter, and the salt limit for the oxidation of NO2− to NO3− may even be lower (114), nor have (to my knowledge) bacterial isolates performing these processes ever been shown to grow at above 10% salt. A “halophilic” isolate named Nitrosococcus halophilus has its growth optimum at 4% NaCl and grows up to 9.4% NaCl only (54, 55). An attempt to demonstrate nitrification in the Great Salt Lake, Utah, at a total salt concentration above 30% by using a microcosm simulation yielded negative results (100). Lack of an energy source is probably not the main reason: halophilic proteolytic heterotrophs, aerobic as well as anaerobic, do exist, and ammonia is often present at high concentrations in hypersaline lakes. The Dead Sea contains between 0.43 and 0.55 mM NH4+ (78). Ammonia concentrations of up to 64 μM were measured in the Great Salt Lake north arm (99). In the south arm (259 g of total dissolved salts per liter), ammonia concentrations were between 7 and 45 μM, the nitrate concentration was as low as 1 to 3.5 μM, and nitrite was never detected. Concentrations varied during the seasons but were uniform with depth (123). Substantial ammonia concentrations were also reported to occur in saltern ponds of low to intermediate salt concentration (47, 48, 68). Ammonia, and not nitrate, is the dominant inorganic nitrogen species in most or all hypersaline water bodies. Oxygen is generally available as the electron acceptor, albeit at relatively low concentrations due to the reduced solubility of gases in concentrated brines.
Nitrifying bacteria obtain very small amounts of energy from their dissimilatory metabolism. Not only are their electron donors (ammonia and nitrite) relatively oxidized, so that only little energy can be gained from electron transport to oxygen, but also most of the energy generated has to be used to drive uphill electron transport to produce NADPH, the reducing power required for autotrophic CO2 fixation in the Calvin cycle (34). It was estimated that Nitrosomonas has to oxidize about 30 g of ammonia for the production of 1 g of cell material (116). Since these organisms are autotrophs, the theoretical maximum YATP value (YATPMAX) value for the nitrifiers should also be low, about 6.5 g of cell dry weight/mol of ATP (in contrast to the YATP consensus value of 10.5 determined for fermentative growth and the theoretically calculated YATPMAX of 28.8 g [dry weight] per mol of ATP for aerobic growth on glucose plus inorganic salts) (4, 124, 125). In view of all this, the energetic burden of halophilism may be too great for ammonia- or nitrite-oxidizing autotrophs. Autotrophs that obtain their energy from the oxidation of reduced sulfur compounds are much better off because their substrates are more reduced that those of the nitrifying bacteria. Thus far, only one halophilic autotrophic sulfur oxidizer, Thiobacillus halophilus, has been isolated; this organism oxidizes thiosulfate, tetrathionate, and elemental sulfur, has its optimum at 5 to 6% NaCl, but grows at up to 24% NaCl (138). No data have been published on its intracellular salt or organic osmotic-solute content.
Thermodynamic constraints cannot explain the apparent lack of aerobic methane oxidation in hypersaline environments. Methane oxidation is a highly exergonic process (CH4 + 2 O2→HCO3− + H+ + H2O; ΔG°′ = −813.1 kJ). However, even in environments with relatively low salinities, such as the cyanobacterial mats in the Solar Lake, Sinai (about 8.5% salt), and saltern evaporation ponds in Eilat, Israel (13.2% salt), no methane oxidation could be measured in spite of the availability of both methane and oxygen (17). Recent reports on the occurrence of methane oxidation in sediments of hypersaline reservoirs in Ukraine and Tuva (up to 33% salts) and the isolation of halophilic methanotrophs from these environments (52, 53) indicate that the existence of halophilic methanotrophs is at least thermodynamically feasible and that the earlier reported lack of methane oxidation in other hypersaline environments (17, 120) should have other reasons. A reexamination of the occurrence of methanotrophic bacteria in different hypersaline environments, using both more sensitive techniques for the detection of methane oxidation and molecular approaches to detect and characterize the bacteria involved, is therefore recommended.
Dissimilatory sulfate reduction occurs up to quite high salt concentrations. Black sediments are often found on the bottom of salt lakes and saltern ponds approaching NaCl saturation. Direct evidence of the occurrence of sulfate reduction comes from measurements of the formation of H235S from 35SO42− added to the sediments (84). A comparison of the stable-isotope composition of the sulfate and the sulfide that was present in the past in the lower water layers of the Dead Sea pointed to dissimilatory sulfate reduction as the source of the sulfide (77). A number of halophilic sulfate reducers, such as Desulfovibrio halophilus (optimum NaCl concentration, 6 to 7%; maximum concentration, 18%) (16) and Desulfovibrio oxyclinae (optimum NaCl concentration, 5 to 10%; maximum concentration, 22.5%), have been isolated (56). The most halophilic strain known thus far is Desulfohalobium retbaense, isolated from Lake Retba in Senegal, which has its growth optimum at 10% NaCl and grows up to 24% NaCl (79; also see reference 80).
Previously it was suggested that Desulfovibrio halophilus cannot synthesize compatible solutes and that it grows in defined mineral medium while accumulating salts into the cytoplasm (79). However, it was later demonstrated that organic solutes (trehalose and glycine betaine) provide the osmotic balance (137), as expected for a member of the proteobacteria.
No information is available on the nature of the electron donors used by the communities of sulfate-reducing bacteria in the sediments of salt lakes. However, it is interesting that, with a single exception, all halophilic and halotolerant strains of sulfate reducers isolated are incomplete oxidizers, growing on substrates such as lactate. Once more there may be a bioenergetic reason: oxidation of lactate to acetate and CO2 yields relatively much energy (2 lactate + SO42−→2 acetate + 2HCO3− + HS− + H+; ΔG°′ = −160.1 kJ), so that organisms performing such a process may also have energy available for the biosynthesis of compatible solutes. The oxidation of acetate with sulfate as the electron acceptor yields only little energy (acetate + SO42−→2HCO3− + HS−; ΔG°′ = −47.7 kJ). An acetate-oxidizing sulfate reducer, Desulfobacter halotolerans, was recently isolated from the sediments of the Great Salt Lake, Utah (11). Its salt optimum is 1 to 2%, and the maximum NaCl concentration tolerated is 13%.
The situation for the methanogens is less clear. While the reduction of CO2 with hydrogen and the aceticlastic split are the main methanogenic reactions in freshwater environments, neither of these have yet been shown to occur at high salt concentrations. Solar Lake (Sinai) sediments (7 to 7.4% salt) did not show methanogenesis from acetate or from H2 + CO2 (30). The highest salt concentration at which methanogenesis from H2 + CO2 was demonstrated in nature was 8.8% (Mono Lake, Calif.) (82). The most halotolerant isolate that grows on H2 + CO2 is the recently described Methanocalculus halotolerans, obtained from an oil well. This organism grows at up to 12% NaCl, with optimum growth at 5% NaCl (81). The upper salinity boundary for the use of acetate as a methanogenic substrate is probably even lower, but few data are available. Methanogenesis does occur, however, at much higher salt concentrations in species such as Methanohalophilus mahii, Methanohalophilus halophilus, Methanohalophilus portocalensis, and Methanohalophilus zhilinae—all moderate halophiles that grow optimally at 4 to 12% salt. Methanohalobium evestigatum and Methanohalophilus strain Z7302 grow well up to 24 to 25% NaCl (59, 140). Their energy sources are methylated amines, methanol, and dimethyl sulfide (82, 140, 141; also see reference 80).
Methanogens, even nonhalophilic ones, may contain substantial intracellular K+ concentrations: values as high as 1.23 M were reported for the nonhalophilic Methanobrevibacter arboriphilus, 1.1 M was reported for Methanobacterium thermoautotrophicum, and 0.86 M was reported for Methanobacterium bryantii (46). This K+ serves as the counterion to the trianionic cyclic 2,3-diphosphoglycerate, a compound that may play a role in the thermostability of cytoplasmic enzymes rather than in osmoregulation. The halophilic species use organic compatible solutes mainly to provide osmotic balance, and as such they are fundamentally different from the aerobic halophilic archaea (the members of the Halobacteriales), which accumulate KCl. A variety of organic osmotic compounds have been detected in halophilic methanogens: β-glutamine, β-glutamate (110), Nɛ-acetyl-β-lysine, and glycine betaine (59, 60, 110, 111, 112, 122).
Comparison of the free-energy changes associated with the different methanogenic reactions (Table 1) in an attempt to assess their potential occurrence at high salt concentrations is not straightforward. Reaction stoichiometries are complex, and it is difficult to compare methanogenesis from CO2, involving reduction reactions only, with the disproportionation reactions involved in methanogenesis from methanol or from methylated amines. It is clear that methanogenesis from acetate yields very little energy only (−31.1 kJ/mol of acetate). Accordingly, aceticlastic methanogens are notoriously slow growers. Thus, the apparent lack of halophilic representatives in this case may also be due to energetic constraints. When calculated per molecule of energy source, the standard free-energy change during growth on hydrogen is −34 kJ per mol of hydrogen, not very different from that on acetate. Calculated per mole of substrate transformed, the energy yield on the methanogenic substrates that do enable growth at high salt concentrations is much larger (between −79 and −191 kJ/mol). However, as stressed above, the apparent correlation may here be based on unjustified speculations, first because it is difficult to compare the methanogenic reactions and second because little is known about the efficiency at which the free-energy change as calculated from thermodynamic data is conserved in the form of ATP and/or a proton (or sodium) electrochemical gradient during growth on methylated amines.
TABLE 1.
Standard free-energy change of different reactions performed by methanogenic bacteria (for comparison, selected reactions of dissimilatory sulfate reducers and homoacetogenic bacteria are given as well)
| Reaction | Free-energy change (kJ)
|
|
|---|---|---|
| Total | Per mol of substrate | |
| Methanogenic bacteria | ||
| H2 + CO2 | ||
| 4H2 + H+ + HCO3−→CH4 + 3H2O | 135.9 | −34 |
| Acetate | ||
| Acetate + H2O→CH4 + HCO3− | −31.1 | −31.1 |
| Methanol | ||
| 4 Methanol→3CH4 + HCO3− + H2O + H+ | −314.7 | −78.7 |
| Monomethylamine | ||
| 4 Methylamine + 3H+ + 3H2O→3CH4 + HCO3− + 4NH4+ | −368.3 | −92.1 |
| Dimethylamine | ||
| 2 Dimethylamine + H+ + 3H2O→3CH4 + HCO3− + 2NH4+ | −286.5 | −143.3 |
| Trimethylamine | ||
| 4 Trimethylamine + 9H2O + H+→9CH4 + 3HCO3− + 4NH4+ | −764.5 | −191.1 |
| Sulfate-reducing bacteria | ||
| H2 + SO42− | ||
| 4H2 + SO42− + H+→HS− + 4 H2O | −152.3 | |
| Lactate + SO42− | ||
| 2 Lactate + SO42−→2 acetate + HCO3− + HS− + H+ | −160.1 | |
| Acetate + SO42− | ||
| Acetate + SO42−→2HCO3− + HS− | −47.7 | |
| Homoacetogenic bacteria | ||
| H2 + CO2 | ||
| 4H2 + H+ + 2HCO3−→acetate + 4H2O | −104.6 | |
While methanogens growing on H2 + CO2 seem to be absent in hypersaline environments, halophilic homoacetogenic bacteria that use the same substrates for the production of acetate have been isolated (139, 142). Indications have even been obtained that homoacetogens may occur in the sediments of the Dead Sea (86). Acetohalobium arabaticum is able to grow between 10 to 25% NaCl with an optimum at 15 to 18% (141), and the alkaliphilic Natroniella acetigena grows in the range of 10 to 26% NaCl with an optimum at 12% (142).
At first sight the thermodynamic parameters of the reactions do not explain why halophilic homoacetogenic bacteria occur while CO2-reducing methanogens do not. The acetogenic reaction yields even less energy than the methanogenic process (−26.1 and −34 kJ per mol of hydrogen oxidized, respectively). However, it should be kept in mind that the halophilic homoacetogens belong to the order Haloanaerobiales (103, 142). All representatives of this group examined thus far accumulate inorganic ions to establish osmotic balance (83, 92, 108), an option that is energetically much cheaper than the synthesis of organic osmotic solutes. No analyses of the intracellular salt and solute content of the halophilic homoacetogens have yet been performed to my knowledge.
EPILOGUE
The previous sections demonstrate that bioenergetic considerations, generally neglected in surveys of halophilic life, are very important for the understanding of the possibilities and limitations of microbial life at high salt concentrations.
The energetically cheaper salt-in option requires extensive adaptations of the intracellular machinery (61), and these can be achieved only in a long and complex evolutionary process (19). This option is used by a few specialized groups of prokaryotes only. All other organisms use organic osmotic solutes, which are expensive to produce. In a review on osmolarity and turgor pressure in higher plants, Raven (104) made a similar statement: “For osmolarity generation, the computations show that compatible solutes cost at least an order of magnitude more than inorganic solutes on either an energy (protons absorbed per osmol generated) or an H2O (H2O lost per osmol generated) basis.” When organic solutes are accumulated, there is a clear preference at the highest salinities for the smaller solutes, which are energetically cheaper to produce. Other factors such as the nutrient status may be important as well. Thus, Halorhodospira halochloris shifts from ectoine to trehalose production under nitrogen limitation (26). The use of complex cocktails of organic compatible solutes by many microorganisms allows separate regulation of their concentrations in response to the environmental conditions.
Energy constraints may provide a plausible explanation why certain metabolic types known to inhabit freshwater and marine environments have not yet been found in hypersaline environments. Since the production of osmotic solutes at molar concentrations requires large amounts of energy, microorganisms that obtain only a little energy in the course of their dissimilatory metabolism may be unable to cope with high salt concentrations. Growth would be exceedingly slow, and a proportionally high percentage of the energy generated would have to be used for maintenance purposes. In this context, it may also be noteworthy that dinitrogen fixation, an energetically extremely expensive process, was never shown to occur at high salt concentrations, except in cyanobacteria (89) and photosynthetic purple bacteria such as Halorhodospira halochloris (26), which in the light are probably not energy limited.
In most cases a good correlation exists between the upper salt concentration at which the different microbial processes have been shown to occur in nature and the ability of the corresponding microbial isolates to grow at high salt concentrations in culture (Fig. 4). For some processes, however, the upper salinity limit in nature exceeds that of the most halophilic or halotolerant species available in culture. Most microorganisms that produce organic compatible solutes are also able to take up such solutes from the environment when available, thereby reducing the energy cost of life at high salt concentrations. Such an option is expected to be advantageous in complex microbial communities in which different metabolic types of microorganisms coexist, some of which may produce and release potential osmotic solutes in excess. The extent to which halophilic microorganisms exist in such microbial consortia that are strictly dependent on a steady supply of osmotic solutes from their neighbors is yet unknown. Inclusion of potential osmotic solutes in the enrichment media in the search for the “missing” metabolic types may therefore be recommended.
In the above discussions, bioenergetic considerations were used as a possible explanation for the apparent lack of certain metabolic types of microorganisms able to function at high salt concentrations. This does not imply that such types do not exist. It has been proven again and again that microorganisms that obtain only very small amounts of energy in the course of their dissimilatory metabolism do have their place in nature. Striking examples are the oxidation of acetate with elemental sulfur as the electron acceptor by Desulfuromonas acetoxidans (acetate + 4S0 + 4H2O→2HCO3− + 4HS− + 5H+; ΔG°′ = −6.7 kJ) (97) and the even more extreme case of the proton-reducing acetogens that grow even though the ΔG°′ of their dissimilatory reactions is positive (115). Therefore, the option should be kept open that the “missing” types of halophilic microorganisms may still be discovered in the future. The above discussions are therefore also intended to stimulate microbiologists in their search for novel types of halophilic microorganisms.
ACKNOWLEDGMENTS
I thank Carol D. Litchfield (George Mason University, Fairfax, Va.), Etana Padan and Shimon Schuldiner (The Hebrew University of Jerusalem), and Karlheinz Altendorf and Evert Bakker (University of Osnabrück) for critically reading the manuscript and for invaluable comments.
This study was supported in part by grant 95-00027 from the United States–Israel Binational Science Foundation (BSF, Jerusalem, Israel).
REFERENCES
- 1.Adler L, Blomberg A, Nilsson A. Glycerol metabolism and osmoregulation in the salt-tolerant yeast Debaryomyces hansenii. J Bacteriol. 1985;162:300–306. doi: 10.1128/jb.162.1.300-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker E P. Cell K+ and K+ transport systems in prokaryotes. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 205–224. [Google Scholar]
- 3.Bakker E P. Low affinity K+ uptake systems. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 253–276. [Google Scholar]
- 4.Bauchop T, Elsden S R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- 5.Baxter R M, Gibbons N E. Effects of sodium and potassium chloride on certain enzymes of Micrococcus halodenitrificans and Pseudomonas salinaria. Can J Microbiol. 1956;2:599–606. doi: 10.1139/m56-072. [DOI] [PubMed] [Google Scholar]
- 6.Bayley R M, Morton R A. Recent developments in the molecular biology of extremely halophilic bacteria. Crit Rev Microbiol. 1978;6:151–205. doi: 10.3109/10408417809090622. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Amotz A. Adaptation of the unicellular alga Dunaliella parva to a saline environment. J Phycol. 1975;11:50–54. [Google Scholar]
- 8.Ben-Amotz A, Avron M. The role of glycerol in the osmotic regulation of the halophilic alga Dunaliella parva. Plant Physiol. 1973;51:875–878. doi: 10.1104/pp.51.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Amotz A, Avron M. Glycerol and β-carotene metabolism in the halotolerant alga Dunaliella: a model system for biosolar energy conversion. Trends Biochem Sci. 1981;6:297–299. [Google Scholar]
- 10.Bickel-Sandkötter S, Gärtner W, Dane M. Conversion of energy in halobacteria: ATP synthesis and phototaxis. Arch Microbiol. 1996;166:1–11. doi: 10.1007/s002030050349. [DOI] [PubMed] [Google Scholar]
- 11.Brandt K K, Ingvorsen K. Desulfobacter halotolerans sp. nov., a halotolerant acetate-oxidizing sulfate-reducing bacterium isolated from sediments of Great Salt Lake, Utah. Syst Appl Microbiol. 1997;20:366–373. [Google Scholar]
- 12.Brown A D. Microbial water stress physiology. Principles and perspectives. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1990. [Google Scholar]
- 13.Brown F F, Sussman I, Avron M, Degani H. NMR studies of glycerol permeability in lipid vesicles, erythrocytes and the alga Dunaliella. Biochim Biophys Acta. 1982;690:165–173. doi: 10.1016/0005-2736(82)90319-4. [DOI] [PubMed] [Google Scholar]
- 14.Canovas D, Vargas C, Iglesias-Guerra F, Csonka L N, Rhodes D, Ventosa A, Nieto J J. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Mol Biol. 1997;272:25794–25801. doi: 10.1074/jbc.272.41.25794. [DOI] [PubMed] [Google Scholar]
- 15.Castle A M, Macnab R M, Schulman R G. Coupling between the sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem. 1986;261:7797–7806. [PubMed] [Google Scholar]
- 16.Caumette P, Cohen Y, Matheron R. Isolation and characterization of Desulfovibrio halophilus sp. nov., a halophilic sulfate-reducing bacterium isolated from Solar Lake (Sinai) Syst Appl Microbiol. 1990;14:33–38. [Google Scholar]
- 17.Conrad R, Frenzel P, Cohen Y. Methane emission from hypersaline microbial mats: lack of aerobic methane oxidation activity. FEMS Microbiol Ecol. 1995;16:297–305. [Google Scholar]
- 18.Degani H, Avron M. The differential water permeability in the halotolerant alga Dunaliella as measured by nuclear magnetic resonance. Biochim Biophys Acta. 1982;690:174–177. doi: 10.1016/0005-2736(82)90320-0. [DOI] [PubMed] [Google Scholar]
- 19.Dennis P P, Shimmin L C. Evolutionary divergence and salinity-mediated selection in halophilic Archaea. Microbiol Mol Biol Rev. 1997;61:90–104. doi: 10.1128/mmbr.61.1.90-104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desmarais D, Jablonski P E, Fedarko N S, Roberts M F. 2-Sulfotrehalose, a novel osmolyte in haloalkaliphilic Archaea. J Bacteriol. 1997;179:3146–3153. doi: 10.1128/jb.179.10.3146-3153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duschl A, Wagner G. Primary and secondary chloride transport in Halobacterium halobium. J Bacteriol. 1986;168:548–552. doi: 10.1128/jb.168.2.548-552.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischel U, Oren A. Fate of compatible solutes during dilution stress in Ectothiorhodospira marismortui. FEMS Microbiol Lett. 1993;113:113–118. [Google Scholar]
- 23.Fougère F, Le Rudulier D. Glycine betaine biosynthesis and catabolism in bacteroids of Rhizobium meliloti: effect of salt stress. J Gen Microbiol. 1990;136:2503–2510. doi: 10.1099/00221287-136-1-157. [DOI] [PubMed] [Google Scholar]
- 24.Galinski E A. Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experientia. 1993;49:487–496. [Google Scholar]
- 25.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 26.Galinski E A, Herzog R M. The role of trehalose as a substitute for nitrogen-containing compatible solutes (Ectothiorhodospira halochloris) Arch Microbiol. 1990;153:607–613. [Google Scholar]
- 27.Galinski E A, Trüper H G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- 28.Gandbhir M, Rashed I, Marlière P, Mutzel R. Convergent evolution of amino acid usage in archaebacterial and eubacterial lineages adapted to high salt. Res Microbiol. 1995;146:113–120. doi: 10.1016/0923-2508(96)80889-8. [DOI] [PubMed] [Google Scholar]
- 29.Garty H, Caplan S R. Light-dependent rubidium transport in intact Halobacterium halobium cells. Biochim Biophys Acta. 1977;459:532–545. doi: 10.1016/0005-2728(77)90052-4. [DOI] [PubMed] [Google Scholar]
- 30.Giani D, Giani L, Cohen Y, Krumbein W E. Methanogenesis in the hypersaline Solar Lake (Sinai) FEMS Microbiol Lett. 1984;25:219–224. [Google Scholar]
- 31.Gimmler H, Hartung W. Low permeability of the plasma membrane of Dunaliella parva for solutes. J Plant Physiol. 1988;133:165–172. [Google Scholar]
- 32.Ginzburg M. Ion metabolism in whole cells of Halobacterium halobium and H. marismortui. In: Caplan S R, Ginzburg M, editors. Energetics and structure of halophilic microorganisms. Amsterdam, The Netherlands: Elsevier; 1978. pp. 561–577. [Google Scholar]
- 33.Ginzburg M, Sachs L, Ginzburg B Z. Ion metabolism in Halobacterium halobium. J Membr Biol. 1971;5:78–101. doi: 10.1007/BF01870826. [DOI] [PubMed] [Google Scholar]
- 34.Gottschalk G. Bacterial metabolism. 2nd. ed. New York, N.Y: Springer-Verlag; 1986. [Google Scholar]
- 35.Goyal A, Lilley R, Brown A D. The size and turnover of the glycerol pool in Dunaliella. Plant Cell Environ. 1986;9:703–706. [Google Scholar]
- 36.Hagemann M, Erdmann N. Activation and pathway of glucosylglycerol synthesis in the cyanobacterium Synechococcus sp. PCC 6803. Microbiology. 1994;140:1427–1431. [Google Scholar]
- 37.Hagemann M, Richter S, Mikkat S. The gytA gene encodes a subunit for the transport system for the osmoprotective compound glucosylglycerol in Synechocystis sp. strain PCC 6803. J Bacteriol. 1997;179:714–720. doi: 10.1128/jb.179.3.714-720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamaide F, Kushner D J, Sprott G D. Proton motive force and Na+/H+ antiport in a moderate halophile. J Bacteriol. 1983;156:537–544. doi: 10.1128/jb.156.2.537-544.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamaide F, Kushner D J, Sprott G D. Proton circulation in Vibrio costicola. J Bacteriol. 1985;161:681–686. doi: 10.1128/jb.161.2.681-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartmann R, Sickinger H-D, Oesterhelt D. Anaerobic growth of halobacteria. Proc Natl Acad Sci USA. 1980;77:3821–3825. doi: 10.1073/pnas.77.7.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzog R M, Galinski E A, Trüper H G. Degradation of the compatible solute trehalose in Ectothiorhodospira halochloris: isolation and characterization of trehalase. Arch Microbiol. 1990;153:600–606. [Google Scholar]
- 42.Hochstein L I, Bogomolni R. What do extreme halophiles tell us about the evolution of the proton-translocating ATPases? In: Oren A, editor. Microbiology and biogeochemistry of hypersaline environments. Boca Raton, Fla: CRC Press, Inc.; 1999. pp. 273–280. [Google Scholar]
- 43.Hong T Y, Lai M C. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Glycine betaine transport in the halophilic methanogen, abstr. K-144; p. 366. [Google Scholar]
- 44.Imhoff J F, Riedel T. Requirements for, and cytoplasmic concentrations of, sulphate and chloride, and cytoplasmic volume spaces in the halophilic bacterium Ectothiorhodospira mobilis. J Gen Microbiol. 1989;135:237–244. [Google Scholar]
- 45.Imhoff J F, Rodriguez-Valera F. Betaine is the main compatible solute of halophilic eubacteria. J Bacteriol. 1984;160:478–479. doi: 10.1128/jb.160.1.478-479.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarrell K F, Sprott G D, Matheson A T. Intracellular potassium concentration and relative acidity of the ribosomal proteins of methanogenic bacteria. Can J Microbiol. 1984;30:663–668. [Google Scholar]
- 47.Javor B J. Plankton standing crop and nutrients in a saltern ecosystem. Limnol Oceanogr. 1983;28:153–159. [Google Scholar]
- 48.Javor B J. Nutrients and ecology of the Western Salt and Exportadora de Sal saltern brines. In: Schreiber B C, Harner H L, editors. Proceedings of the Sixth International Symposium on Salt. Vol. 1. Toronto, Canada: The Salt Institute; 1983. pp. 195–205. [Google Scholar]
- 49.Katz A, Pick U, Avron M. Characterization and reconstitution of the Na+/H+ antiporter from the plasma membrane of the halophilic alga Dunaliella. Biochim Biophys Acta. 1989;983:1–14. doi: 10.1016/0005-2736(89)90373-8. [DOI] [PubMed] [Google Scholar]
- 50.Katz A, Pick U, Avron M. Modulation of Na+/H+ antiporter activity by extreme pH and salt in the halotolerant alga Dunaliella salina. Plant Physiol. 1992;100:1224–1229. doi: 10.1104/pp.100.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kevbrina M V, Plakunov V K. Acetate metabolism in Natronococcus occultus. Mikrobiologiya. 1992;61:770–775. . (In Russian; English translation, 61:534–538, 1993.) [Google Scholar]
- 52.Khmelenina V N, Starostina N G, Tsvetkova M G, Sokolov A P, Suzina N E, Trotsenko Y A. Methanotrophic bacteria in saline reservoirs of Ukraina and Tuva. Mikrobiologiya. 1996;65:696–703. . (In Russian.) [Google Scholar]
- 53.Khmelenina V N, Kalyuzhneya M G, Starostina N G, Suzina N E, Trotsenko Y A. Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr Microbiol. 1997;35:257–261. [Google Scholar]
- 54.Koops H-P, Möller U. The lithotrophic ammonia-oxidizing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 2625–2638. [Google Scholar]
- 55.Koops H-P, Böttcher B, Möller U, Pommerening-Röser A, Stehr G. Description of a new species of Nitrosococcus. Arch Microbiol. 1990;154:244–248. [Google Scholar]
- 56.Krekeler D, Sigalevich P, Teske A, Cypionka H, Cohen Y. A sulfate-reducing bacterium from the oxic layer of a microbial mat from Solar Lake (Sinai), Desulfovibrio oxyclinae sp. nov. Arch Microbiol. 1997;167:369–375. [Google Scholar]
- 57.Krulwich T A. Na+/H+ antiporters. Biochim Biophys Acta. 1983;726:245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- 58.Kushner D J. The Halobacteriaceae. In: Woese C R, Wolfe R S, editors. The bacteria. A treatise on structure and function. VIII. Archaebacteria. Orlando, Fla: Academic Press, Inc.; 1985. pp. 171–214. [Google Scholar]
- 59.Lai M-C, Gunsalus R P. Glycine betaine and potassium ion are the major compatible solutes in the extremely halophilic methanogen Methanohalophilus strain Z7302. J Bacteriol. 1992;174:7474–7477. doi: 10.1128/jb.174.22.7474-7477.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai M-C, Sowers K R, Robertson D E, Roberts M F, Gunsalus R P. Distribution of compatible solutes in the halophilic methanogenic archaebacteria. J Bacteriol. 1991;173:5352–5358. doi: 10.1128/jb.173.17.5352-5358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanyi J K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974;38:272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lanyi J K, McDonald R E. Existence of electrogenic hydrogen/sodium antiport in Halobacterium cell envelope vesicles. Biochemistry. 1976;15:4608–4614. doi: 10.1021/bi00666a010. [DOI] [PubMed] [Google Scholar]
- 63.Lanyi J K, Hilliker K. Passive potassium ion permeability of Halobacterium halobium envelope membranes. Biochim Biophys Acta. 1976;448:181–184. doi: 10.1016/0005-2736(76)90086-9. [DOI] [PubMed] [Google Scholar]
- 64.Lanyi J K, Silverman M P. The state of binding of intracellular K+ in Halobacterium cutirubrum. Can J Microbiol. 1972;18:993–995. doi: 10.1139/m72-154. [DOI] [PubMed] [Google Scholar]
- 65.Lanyi J K, Silverman M P. Gating effects in Halobacterium halobium membrane transport. J Biol Chem. 1979;254:4750–4755. [PubMed] [Google Scholar]
- 66.Lanyi J K, Helgerson S L, Silverman M P. Relationship between proton motive force and potassium ion transport in Halobacterium halobium envelope vesicles. Arch Biochem Biophys. 1979;193:329–339. doi: 10.1016/0003-9861(79)90037-7. [DOI] [PubMed] [Google Scholar]
- 67.Le Rudulier D, Pocard J-A. Osmoregulation in Rhizobium meliloti: control of glycine betaine biosynthesis and catabolism. In: Rodriguez-Valera F, editor. General and applied aspects of halophilic microorganisms. New York, N.Y: Plenum Press; 1991. pp. 89–96. [Google Scholar]
- 68.Litchfield C D, Irby A, Vreeland R H. The microbial ecology of solar salt plants. In: Oren A, editor. Microbiology and biogeochemistry of hypersaline environments. Boca Raton, Fla: CRC Press, Inc.; 1999. pp. 39–52. [Google Scholar]
- 69.Louis P, Galinski E A. Characterization of the genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology. 1997;143:1141–1149. doi: 10.1099/00221287-143-4-1141. [DOI] [PubMed] [Google Scholar]
- 70.Lowe S E, Jain M K, Zeikus J G. Biology, ecology, and biotechnological applications of anaerobic bacteria adapted to environmental stresses in temperature, pH, salinity, or substrates. Microbiol Rev. 1993;57:451–509. doi: 10.1128/mr.57.2.451-509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackay M A, Norton R S, Borowitzka L J. Organic osmoregulatory solutes in cyanobacteria. J Gen Microbiol. 1984;130:2177–2191. [Google Scholar]
- 72.Menaia J A G F, Duarte J C, Boone D R. Osmotic adaptation of moderately halophilic methanogenic Archaeobacteria, and detection of cytosolic N,N-dimethylglycine. Experientia. 1993;49:1047–1054. [Google Scholar]
- 73.Mermelstein L D, Zeikus J G. Anaerobic nonmethanogenic extremophiles. In: Horikoshi K, Grant W D, editors. Extremophiles. Microbial life in extreme environments. New York, N.Y: Wiley-Liss; 1998. pp. 255–284. [Google Scholar]
- 74.Meury J, Kohiyama M. ATP is required for K+ active transport in the archaebacterium Haloferax volcanii. Arch Microbiol. 1989;151:530–536. [Google Scholar]
- 75.Mikkat S, Effmert U, Hagemann M. Uptake and use of the osmoprotective compounds trehalose, glucosylglycerol, and sucrose by the cyanobacterium Synechocystis sp. PCC 6803. Arch Microbiol. 1997;167:112–118. [PubMed] [Google Scholar]
- 76.Moore D J, Reed R H, Stewart W D P. A glycine betaine transport system in Aphanothece halophytica and other glycine betaine synthesising cyanobacteria. Arch Microbiol. 1987;147:399–405. [Google Scholar]
- 77.Nissenbaum A, Kaplan I R. Sulfur and carbon isotopic evidence for biogeochemical processes in the Dead Sea. In: Nriagu J O, editor. Environmental biogeochemistry. Vol. 1. Ann Arbor, Mich: Ann Arbor Science Publishers; 1976. pp. 309–325. [Google Scholar]
- 78.Nissenbaum A, Stiller M, Nishry A. Nutrients in pore waters from Dead Sea sediments. Hydrobiologia. 1990;197:82–87. [Google Scholar]
- 79.Ollivier B, Hatchikian C E, Prensier G, Guezennec J, Garcia J-L. Desulfohalobium retbaense gen. nov. sp. nov., a halophilic sulfate-reducing bacterium from sediments of a hypersaline lake in Senegal. Int J Syst Bacteriol. 1991;41:74–81. [Google Scholar]
- 80.Ollivier B, Caumette P, Garcia J-L, Mah R A. Anaerobic bacteria from hypersaline environments. Microbiol Rev. 1994;58:27–38. doi: 10.1128/mr.58.1.27-38.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ollivier B, Fardeau M-L, Cayol J-L, Magot M, Patel B K C, Prensier G, Garcia J-L. Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. Int J Syst Bacteriol. 1998;48:821–828. doi: 10.1099/00207713-48-3-821. [DOI] [PubMed] [Google Scholar]
- 82.Oremland R S, King G M. Methanogenesis in hypersaline environments. In: Cohen Y, Rosenberg E, editors. Microbial mats. Physiological ecology of benthic microbial communities. Washington, D.C: American Society for Microbiology; 1989. pp. 180–190. [Google Scholar]
- 83.Oren A. Intracellular salt concentrations of the anaerobic halophilic eubacteria Haloanaerobium praevalens and Halobacteroides halobius. Can J Microbiol. 1986;32:4–9. [Google Scholar]
- 84.Oren A. Anaerobic degradation of organic compounds at high salt concentrations. Antonie Leeuwenhoek. 1988;54:267–277. doi: 10.1007/BF00443585. [DOI] [PubMed] [Google Scholar]
- 85.Oren A. Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments. Antonie Leeuwenhoek. 1990;58:291–298. doi: 10.1007/BF00399342. [DOI] [PubMed] [Google Scholar]
- 86.Oren A. Anaerobic degradation of organic compounds in hypersaline environments: possibilities and limitations. In: Wise D L, editor. Bioprocessing and biotreatment of coal. New York, N.Y: Marcel Dekker, Inc.; 1990. pp. 155–175. [Google Scholar]
- 87.Oren A. The genera Haloanaerobium, Halobacteroides and Sporohalobacter. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 1893–1900. [Google Scholar]
- 88.Oren A. Anaerobic heterotrophic bacteria growing at extremely high salt concentrations. In: Guerrero R, Pedrós-Alió C, editors. Trends in microbial ecology. Spanish Society for Microbiology; 1993. pp. 539–542. [Google Scholar]
- 89.Oren, A. Salts and brines. In B. A. Whitton and M. Potts (ed.), Ecology of cyanobacteria: their diversity in time and space, in press. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 90.Oren A, Gurevich P. The fatty acid synthetase of Haloanaerobium praevalens is not inhibited by salt. FEMS Microbiol Lett. 1993;108:287–290. [Google Scholar]
- 91.Oren A, Weisburg W G, Kessel M, Woese C R. Halobacteroides halobius gen. nov., sp. nov., a moderately halophilic anaerobic bacterium from the bottom sediments of the Dead Sea. Syst Appl Microbiol. 1984;5:58–70. [Google Scholar]
- 92.Oren A, Heldal M, Norland S. X-ray microanalysis of intracellular ions in the anaerobic halophilic eubacterium Haloanaerobium praevalens. Can J Microbiol. 1997;43:588–592. [Google Scholar]
- 93.Padan E. The molecular mechanism of regulation of the NhaA Na+/H+ antiporter of Escherichia coli, a paradigm for an adaptation to Na+ and H+ In: Oren A, editor. Microbiology and biogeochemistry of hypersaline environments. Boca Raton, Fla: CRC Press, Inc.; 1999. pp. 163–175. [Google Scholar]
- 94.Padan E, Schuldiner S. Na+ transport systems in prokaryotes. In: Bakker E P, editor. Alkali transport systems in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 3–24. [Google Scholar]
- 95.Peters P, Galinski E A, Trüper H G. The biosynthesis of ectoine. FEMS Microbiol Lett. 1990;71:157–162. [Google Scholar]
- 96.Peters P, Tel-Or E, Trüper H G. Transport of glycine betaine in the extremely haloalkaliphilic sulphur bacterium Ectothiorhodospira halochloris. J Gen Microbiol. 1992;138:1993–1998. [Google Scholar]
- 97.Pfennig N, Biebl H. Desulfotomaculum acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976;110:3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- 98.Pocard J-A, Smith L T, Smith G M, Le Rudulier D. A prominent role for glucosylglycerol in the adaptation of Pseudomonas mendocina SKB70 to osmotic stress. J Bacteriol. 1994;176:6877–6884. doi: 10.1128/jb.176.22.6877-6884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Post F J. The microbial ecology of the Great Salt Lake. Microb Ecol. 1977;3:143–165. doi: 10.1007/BF02010403. [DOI] [PubMed] [Google Scholar]
- 100.Post F J, Stube J C. A microcosm study of nitrogen utilization in the Great Salt Lake, Utah. Hydrobiologia. 1988;158:89–100. [Google Scholar]
- 101.Proctor L M, Lai R, Gunsalus R P. The methanogenic archaeon Methanosarcina thermophila TM-1 possesses a high-affinity glycine betaine transporter involved in osmotic adaptation. Appl Environ Microbiol. 1997;63:2252–2257. doi: 10.1128/aem.63.6.2252-2257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pusheva M A, Detkova E N. Bioenergetic aspects of acetogenesis on various substrates by the extremely halophilic acetogenic bacterium Acetohalobium arabaticum. Microbiologiya. 1996;65:589–593. . (In Russian; English translation, 65:516–520.) [Google Scholar]
- 103.Rainey F A, Zhilina T N, Boulygina E S, Stackebrandt E, Tourova T P, Zavarzin G A. The taxonomic status of the fermentative halophilic anaerobic bacteria: description of Haloanaerobiales ord. nov., Halobacteroidaceae fam. nov., Orenia gen. nov. and further taxonomic rearrangements at the genus and species level. Anaerobe. 1995;1:185–199. doi: 10.1006/anae.1995.1018. [DOI] [PubMed] [Google Scholar]
- 104.Raven J A. Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985;101:25–77. doi: 10.1111/j.1469-8137.1985.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 105.Reed R H, Richardson D L, Warr S R C, Stewart W D P. Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol. 1984;130:1–4. [Google Scholar]
- 106.Reed R H, Chudek J A, Foster R, Stewart W D P. Osmotic adjustment in cyanobacteria from hypersaline environments. Arch Microbiol. 1984;138:333–337. [Google Scholar]
- 107.Rengpipat S, Langworthy T A, Zeikus J G. Halobacteroides acetoethylicus sp. nov., a new obligately anaerobic halophile isolated from deep subsurface hypersaline environments. Syst Appl Microbiol. 1988;11:28–35. [Google Scholar]
- 108.Rengpipat S, Lowe S E, Zeikus J G. Effect of extreme salt concentrations on the physiology and biochemistry of Halobacteroides acetoethylicus. J Bacteriol. 1988;170:3065–3071. doi: 10.1128/jb.170.7.3065-3071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts M F, Lai M-C, Gunsalus R P. Biosynthetic pathways of the osmolytes Nɛ-acetyl-β-lysine, β-glutamine, and betaine in Methanohalophilus strain FDF1 suggested by nuclear magnetic resonance analysis. J Bacteriol. 1992;174:6688–6693. doi: 10.1128/jb.174.20.6688-6693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robertson D E, Noll D, Roberts M F, Menaia J A G F, Boone D R. Detection of the osmoregulator betaine in methanogens. Appl Environ Microbiol. 1990;56:563–565. doi: 10.1128/aem.56.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robertson D E, Roberts M F, Belay N, Stetter K O, Boone D R. Occurrence of β-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl Environ Microbiol. 1990;56:1504–1508. doi: 10.1128/aem.56.5.1504-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robertson D E, Lai M-C, Gunsalus R P, Roberts M F. Composition, variation, and dynamics of major osmotic solutes in Methanohalophilus strain FDF1. Appl Environ Microbiol. 1992;58:2438–2443. doi: 10.1128/aem.58.8.2438-2443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robinson P M, Roberts M F. Effects of osmolyte precursors on the distribution of compatible solutes in Methanohalophilus portocalensis. Appl Environ Microbiol. 1997;63:4032–4038. doi: 10.1128/aem.63.10.4032-4038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rubentschik L. Zur Nitrifikation bei hohen Salzkonzentrationen. Zentbl Bakteriol II Abt. 1929;77:1–18. [Google Scholar]
- 115.Schink B. Energetics of syntrophic cooperation and methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schlegel H G. General microbiology. 6th ed. Cambridge, United Kingdom: Cambridge University Press; 1986. [Google Scholar]
- 117.Schuldiner S, Padan E. Na+/H+ antiporters in Escherichia coli. In: Bakker E P, editor. Alkali cation transport in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 25–51. [Google Scholar]
- 118.Sheffer M, Fried A, Gottlieb H, Tietz A, Avron M. Lipid composition of the plasma-membrane of the halotolerant alga Dunaliella salina. Biochim Biophys Acta. 1986;857:165–172. [Google Scholar]
- 119.Siebers A, Altendorf K. K+-translocating Kdp-ATPases and other bacterial P-type ATPases. In: Bakker E P, editor. Alkali cation transport in prokaryotes. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 225–252. [Google Scholar]
- 120.Slobodkin A I, Zavarzin G A. Methane production in halophilic cyanobacterial mats in lagoons of Sivash Lake. Mikrobiologiya. 1992;61:294–298. . (In Russian; English translation, 61:198–201.) [Google Scholar]
- 121.Sokolov A P, Trotsenko Y A. Methane consumption in (hyper)saline habitats of Crimea (Ukraine) FEMS Microbiol Ecol. 1995;18:299–304. [Google Scholar]
- 122.Sowers K R, Robertson D E, Noll D, Gunsalus R P, Roberts M F. Nɛ-acetyl-β-lysine: an osmolyte synthesized by methanogenic archaebacteria. Proc Natl Acad Sci USA. 1990;87:9083–9087. doi: 10.1073/pnas.87.23.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stephens D W, Gillespie D M. Phytoplankton productivity in the Great Salt Lake, Utah, and a laboratory study of algal response to enrichment. Limnol Oceanogr. 1976;21:74–87. [Google Scholar]
- 124.Stouthamer A H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Leeuwenhoek. 1973;39:545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- 125.Stouthamer A H. Energetic aspects of the growth of micro-organisms. In: Haddock B A, Hamilton W A, editors. Microbial energetics. Cambridge, United Kingdom: Cambridge University Press; 1977. pp. 285–315. [Google Scholar]
- 126.Tokuda H, Unemoto T. Growth of a marine Vibrio alginolyticus and moderately halophilic V. costicola becomes uncoupler resistant when the respiration-dependent Na+ pump functions. J Bacteriol. 1983;156:636–643. doi: 10.1128/jb.156.2.636-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trüper H G, Galinski E A. Biosynthesis and fate of compatible solutes in extremely halophilic phototrophic eubacteria. FEMS Microbiol Rev. 1990;75:247–254. [Google Scholar]
- 128.Tschichholz I, Trüper H G. Fate of compatible solutes during dilution stress in Ectothiorhodospira halochloris. FEMS Microbiol Ecol. 1990;73:181–186. [Google Scholar]
- 129.Udagawa T, Unemoto T, Tokuda H. Generation of Na+ electrochemical potential by the Na+-motive NADH oxidase and Na+/H+ antiport system of a moderately halophilic Vibrio costicola. J Biol Chem. 1986;261:2616–2622. [PubMed] [Google Scholar]
- 130.van de Vosseberg J L C M, Ubbink-Kok T, Elferink M H L, Driessen A J M, Konings W N. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol. 1995;18:925–932. doi: 10.1111/j.1365-2958.1995.18050925.x. [DOI] [PubMed] [Google Scholar]
- 131.van Laere A J. Trehalose: reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 132.van Laere A J, Hulsmans E. Water potential, glycerol synthesis, and water content of germinating Phycomyces spores. Arch Microbiol. 1987;147:257–262. [Google Scholar]
- 133.Ventosa A, Nieto J J, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagner G, Hartmann R, Oesterhelt D. Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem. 1978;89:169–179. doi: 10.1111/j.1432-1033.1978.tb20909.x. [DOI] [PubMed] [Google Scholar]
- 135.Walsby A E. The pressure relationships of gas vacuoles. Proc R Soc London Ser B. 1971;178:301–326. [Google Scholar]
- 136.Wegmann K, Ben-Amotz A, Avron M. Effect of temperature on glycerol retention in the halotolerant algae Dunaliella and Asteromonas. Plant Physiol. 1980;66:1196–1197. doi: 10.1104/pp.66.6.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welsh D T, Lindsay Y E, Caumette P, Herbert R A, Hannan J. Identification of trehalose and glycine betaine as compatible solutes in the moderately halophilic sulfate reducing bacterium Desulfovibrio halophilus. FEMS Microbiol Lett. 1996;140:203–207. [Google Scholar]
- 138.Wood A P, Kelly D P. Isolation and characterisation of Thiobacillus halophilus sp. nov., a sulphur-oxidising autotrophic eubacterium from a Western Australian hypersaline lake. Arch Microbiol. 1991;156:277–280. [Google Scholar]
- 139.Zavarzin G A, Zhilina T N, Pusheva M A. Halophilic acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman & Hall; 1994. pp. 432–444. [Google Scholar]
- 140.Zhilina T N, Zavarzin G A. Methanohalobium evestigatum gen. nov., sp. nov., extremely halophilic methane-producing archaebacteria. Dokl Akad Nauk SSSR. 1987;293:464–468. . (In Russian.) [Google Scholar]
- 141.Zhilina T N, Zavarzin G A. Extremely halophilic, methylotrophic, anaerobic bacteria. FEMS Microbiol Rev. 1990;87:315–322. [Google Scholar]
- 142.Zhilina T N, Zavarzin G A, Detkova E N, Rainey F A. Natroniella acetigena gen. nov. sp. nov., an extremely haloalkaliphilic, homoacetic bacterium: a new member of Haloanaerobiales. Curr Microbiol. 1996;32:320–326. doi: 10.1007/s002849900057. [DOI] [PubMed] [Google Scholar]