Abstract
We investigate the link between topology of the hydrogen bond network (HBN) and large-scale density fluctuations in water from ambient conditions to the glassy state. We observe a transition from a temperature-dependent topology at high temperatures, to a steady-like topology below the Widom temperature TW ∼ 220 K signaling the fragile-to-strong crossover and the maximum in structural fluctuations. As a consequence of the steady topology, the network suppresses large-scale density fluctuations much more efficiently than at higher temperatures. Below TW, the contribution of coordination defects of the kind A2D1 (two acceptors and one donor) to the kinetics of the HBN becomes progressively more pronounced, suggesting that A2D1 configurations may represent the main source of dynamical heterogeneities. Below the vitrification temperature, the freezing of rotational and translational degrees of freedom allow for an enhanced suppression of large-scale density fluctuations and the sample reaches the edges of nearly hyperuniformity. The formed network still hosts coordination defects, hence implying that nearly hyperuniformity goes beyond the classical continuous random network paradigm of tetrahedral networks and can emerge in scenarios much more complex than previously assumed. Our results unveil a hitherto undisclosed link between network topology and properties of water essential for better understanding water’s rich and complex nature. Beyond implications for water, our findings pave the way to a better understanding of the physics of supercooled liquids and disordered hyperuniform networks at large.
Keywords: network topology, supercooled water, glassy water, large-scale density fluctuations
Significance Statement.
We expose a hitherto undisclosed link between topology of the hydrogen bond network (HBN) and properties of water, and we identify a coordination defect as the source of dynamical heterogeneities. The HBN in supercooled liquid water preserves a temperature-independent topology that efficiently suppresses large-scale density fluctuations. The steady topology of the network implies the existence of collective bonds transformations with long-range correlations. Hyperuniformity, a state of matter that suppresses large-scale density fluctuations, can be accommodated in networks hosting as much as 20% coordination defects, hence going beyond the paradigm of continuous random networks. Our results enrich our understanding of the complex properties of water and supercooled liquids, and pave the way to a deeper understanding of nearly hyperuniform networks.
Introduction
The simple molecular structure of water hides a remarkably wide list of anomalous behaviors that stretch over the most complex phase diagram of any pure substance (1), and whose origin lies in a critical point located at low temperatures and low pressures (2–6), in a region of the phase diagram known as “no-man’s land.” At these thermodynamic conditions, below the line of homogeneous ice nucleation, supercooled water undergoes very rapid crystallization (7, 8), on time scales too short for experiments to accurately probe the liquid phase, from which the epithet “no-man’s land.” The absence of an experimental proof of the existence of a liquid–liquid critical point (LLCP) led to contrasting interpretations of numerical results sparking heated debates that lasted for several years (2,9–13); the major criticism moved against the existence of a LLCP was the possibility that observations of the transition between two liquid forms were due to undetected crystalline structures (11, 12). Later simulations discarded such possibility (14) and showed that arguments against the LLCP scenario were based on imprecise sampling of the rotational degrees of freedom in the liquid phase (15). More recently, further proofs of the validity of the LLCP scenario have been reported from computational investigations of realistic models of water (4), and experimental efforts have pushed the boundaries of the no-man’s land providing important evidences supporting the existence of the critical point (5, 6). From the LLCP, a bundle of lines emanates in the phase diagram towards the more familiar regions of ambient conditions; such lines, commonly known as Widom lines, are the loci of maxima of thermodynamic response functions which tend to diverge upon approaching the LLCP. The maxima in thermodynamic response functions are strictly linked to the spatial arrangement and clustering of water molecules into ordered and disordered local environments and their dynamical properties (16, 17).
At ambient pressure, upon cooling, liquid water undergoes a fragile-to-strong transition occurring at TW ∼ 220 K (18). This dynamical transition is connected to the percentage of hydrogen-bonds that gradually build up upon cooling (19,20) and signals the breakdown of the Stokes–Einstein relationship crossing over from a non-Arrhenius (fragile) to an Arrhenius (strong) behavior (21). The breakdown of the Stokes–Einstein relationship seems to be related to the development of dynamical and spatial heterogeneities that cause a decoupling of rotational and translational degrees of freedom, with the development of clusters of molecules rotating faster than the average (22–24).
At deeply supercooled conditions, water exhibits polyamorphism, i.e. it can exist in more than one glassy state. Indeed, the most common forms of glassy solid water are the low-density and high-density amorphous ices (25–34), which are connected by a first-order phase transition (25, 26, 35–38) and which are structurally linked to the liquid phases at equilibrium conditions (39). Moreover, the complex behavior of glassy solid water is reflected in the fact that amorphous ices seem to encompass a larger set of subfamilies characterized by different structural properties (40–44). Recently, it has been shown that amorphous ices are able to suppress, up to some extent, large-scale density fluctuations (37, 38) and are, therefore, nearly hyperuniform. Central to the concept of hyperuniformity (45) is the structure factor S(k). In the thermodynamic limit, 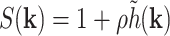 , where
, where 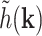 is the Fourier transform of the total correlation function (pair correlation function minus one) and
is the Fourier transform of the total correlation function (pair correlation function minus one) and 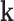 is the wavevector. The vanishing of normalized long-range density fluctuations in hyperuniform systems implies that S(k) → 0 for k → 0, where
is the wavevector. The vanishing of normalized long-range density fluctuations in hyperuniform systems implies that S(k) → 0 for k → 0, where 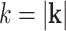 is the wavenumber and S(k) is the structure factor
is the wavenumber and S(k) is the structure factor 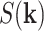 averaged over all directions at wavevector
averaged over all directions at wavevector 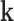 . A useful practical measure of the degree of hyperuniformity in a system is provided by the hyperuniformity index
. A useful practical measure of the degree of hyperuniformity in a system is provided by the hyperuniformity index 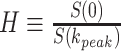 , where kpeak is the wavenumber k at which S(k) has its maximal peak value. It is worth to remark that, in the thermodynamic limit, S(k) is related to the isothermal compressibility kT via the relation S(0) = kTρkBT, being ρ the density and kB the Boltzmann constant. Crystals, for which S(0) = 0, and hence H = 0, are trivially hyperuniform: being uncompressible, they are devoid of large-scale density fluctuations. On the other hand, disordered systems in which H ∼ 10−3 or smaller are deemed to be nearly hyperuniform (46) and are characterized by the appearance of some degree of long-range correlations. Hyperuniformity in disordered systems is linked to an increased packing efficiency with respect to random distributions, and many natural systems, from the structure of the early universe (47) to prime numbers (48) to biological systems (49,50), find such configurations to be efficient arrangements (51). The reference state that is used in the definition of H, i.e. the the normalization of S(0) with respect to S(kpeak), derives from how the scattering intensity evolves as density increases from a low-density phase to a perfectly hyperuniform state. At very low densities, the scattering pattern is very uniform because the particles are spatially uncorrelated, but as the density increases, there is increasingly less scattering around the origin and increasingly a dominant higher-intensity concentric ring emerges around the origin located in the vicinity of wavenumber kpeak. Thus, the value S(kpeak) is an important reference state to be compared to S(0).
, where kpeak is the wavenumber k at which S(k) has its maximal peak value. It is worth to remark that, in the thermodynamic limit, S(k) is related to the isothermal compressibility kT via the relation S(0) = kTρkBT, being ρ the density and kB the Boltzmann constant. Crystals, for which S(0) = 0, and hence H = 0, are trivially hyperuniform: being uncompressible, they are devoid of large-scale density fluctuations. On the other hand, disordered systems in which H ∼ 10−3 or smaller are deemed to be nearly hyperuniform (46) and are characterized by the appearance of some degree of long-range correlations. Hyperuniformity in disordered systems is linked to an increased packing efficiency with respect to random distributions, and many natural systems, from the structure of the early universe (47) to prime numbers (48) to biological systems (49,50), find such configurations to be efficient arrangements (51). The reference state that is used in the definition of H, i.e. the the normalization of S(0) with respect to S(kpeak), derives from how the scattering intensity evolves as density increases from a low-density phase to a perfectly hyperuniform state. At very low densities, the scattering pattern is very uniform because the particles are spatially uncorrelated, but as the density increases, there is increasingly less scattering around the origin and increasingly a dominant higher-intensity concentric ring emerges around the origin located in the vicinity of wavenumber kpeak. Thus, the value S(kpeak) is an important reference state to be compared to S(0).
The network topology in disordered materials is strongly related to  and is an important structural descriptor for understanding the nature of disorder that is usually hidden in pairwise correlations. Correlations between dynamics, anomalous behaviors, and hydrogen bond network (HBN) have been reported in the case of water (16,52), but a direct link between the topology of the network (the geometric configurations of the network) and the degree of suppression of large-scale density fluctuations is still a missing piece of information, crucial for a deeper understanding of the properties of network forming materials at large.
and is an important structural descriptor for understanding the nature of disorder that is usually hidden in pairwise correlations. Correlations between dynamics, anomalous behaviors, and hydrogen bond network (HBN) have been reported in the case of water (16,52), but a direct link between the topology of the network (the geometric configurations of the network) and the degree of suppression of large-scale density fluctuations is still a missing piece of information, crucial for a deeper understanding of the properties of network forming materials at large.
In this article, we quantify the large-scale density fluctuations of liquid water and of glassy water modeled via classical molecular dynamics simulations and we link it to the topology of the HBN measured via the ring statistics, a theoretical tool that has been instrumental in understanding the properties of water at different thermodynamic conditions (2, 16, 36, 52–61). We show that, in correspondence with the fragile-to-strong transition, the topology of the HBN becomes steady and the corresponding fluctuations strongly damped, implying that the kinetics of the HBN at supercooled conditions is governed by collective rearrangements with long-range correlations. Moreover, we show that the decoupling between translational and rotational degrees of freedom occurring in correspondence with the fragile-to-strong transition is linked to the activity of coordination defects of the kind A2D1, in which a water molecule accepts two HBs and donates one.
Our results shed light on the complex nature of water and on the delicate balance between structural and dynamical properties developing at supercooled conditions, with possible applications to other network forming materials at large.
Methodology
We have performed out-of-equilibrium classical MD simulations of a sample of N = 50,000 water molecules interacting with the TIP4P/2005 potential (62) in the NPT ensemble. We have employed Nosé–Hoover thermostat (63, 64) with 0.2 ps relaxation time to maintain constant temperature, and Parrinello–Rahman barostat (65) with 2 ps relaxation time to maintain constant pressure. We have performed simulations with the GROMACS 18.0.1 package (66). We have truncated short-range interactions at 9.5 Å, and we have computed long-range electrostatic terms using particle mesh Ewald with a grid spacing of 1.2 Å. We have equilibrated liquid water at T = 300 K and simulated the cooling and vitrification of the sample implementing a quenching rate qc = 1 K/ns until the final temperature of T = 100 K is reached. All results are the average of five independent simulations. Following Giovambattista et al. (35), we simulate the vitrification of our samples cooling liquid water from ambient temperature to T = 100 K at a quenching rate qc = 1 K/ns. While different quenching rates produce glassy water on different regions of the potential energy landscapes (35,38, 67), the overall conclusions of this work are transferable to such other scenarios.
In order to probe the topology of the HBN we have adopted the ring statistics. Starting from a water molecule, we construct rings recursively traversing the HBN until the starting point is reached again or the path exceeds the maximal ring size considered (12 water molecules in our case). We do not distinguish between the donor/acceptor character of the starting water molecules (53).
Results
In Fig. 1 we report the profiles of the hyperuniformity index H and of the coefficient C measuring the percentage of water molecules that change at least one hydrogen bonded neighbors in the time window of 1 ps. At temperatures close to ambient, the value of H reported in Fig. 1(a), only slightly decreases upon decreasing the temperature, as expected for a system in the fluid phase pervaded by large-scale density fluctuations. A clear crossover occurs roughly in correspondence with the Widom temperature (TW ∼ 220 K, or 1000/T = 4.5/K), which signals the maximum kT and in structural fluctuations, (39, 68–70) and the onset of the fragile-to-strong crossover (18, 19). Below TW, we observe a remarkable increase in slope of the values of H indicating that the supercooled liquid—although still hosting large-scale density fluctuations—is endowed with an enhanced capability to dampen large-scale density fluctuations. In the following discussion, we shall see that this remarkable enhanced efficiency is linked to the topology of the HBN and its fluctuations.
Fig. 1.

Panel (a): H as a function of the 1/T upon cooling the liquid sample from T = 300 K to T = 100 K. The cyan straight lines emphasize the presence of the crossover occurring at TW and are obtained from fitting the data points below and above TW. Panel (b): profile of C as a function of 1/T and computed as follows. At a given time step, we compute the list of water molecules that are hydrogen bonded to a target water and we compute the number of entries in the list of neighbors that differ from the previous time step. Therefore, C represents the percentage of water molecules changing one or more hydrogen bonded neighbor in the time window between t and t + dt, with dt = 1 ps. The green line represents the fit with a four-points logistic function.
For this model of water, at the simulated quenching rate which is two order magnitude faster than the estimated minimum experimental rate (71), vitrification occurs at Tv ∼ 200 K (35,37, 38, 72), ∼60 K above the experimental data (71, 73). The value of H continuously decreases while decreasing the temperature from TW down to T ∼ 180 K, corresponding to 1,000/T ∼ 5/K. Upon further cooling we observe an initial small increase in the value of H, which eventually stabilizes at H ∼ 8 × 10−3. We posit that the increase in the value of H in the temperature window 1,000/T ∈ [∼5.5 − 6.0] K−1 is indicative of a rearrangement occurring at the level of the HBN, which induces large-scale density fluctuations. Considering the error on the measure, we can qualitatively state that glassy water is nearly hyperuniform, in agreement with previous investigations (37,38).
In Fig. 1(b), we report the percentage of water molecules that change one or more hydrogen bonded neighbor on a time window of 1 ps. The value of 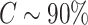 at ambient temperature indicates that
at ambient temperature indicates that 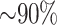 of water molecules change one or more hydrogen bonded neighbor every ps. As translational and rotational degrees of freedom become progressively less accessible upon cooling, C decreases accordingly. Such decrease follows the typical S-shaped profile of logistic functions describing populations dynamics, like surface growth, bacteria population, deposition of materials, clusters growth, and so on (74). The logistic function fitting our computational data is reported in Fig. 1(b) as a green continuous curve. Remarkably, the flex of the logistic function, defining where the rate of change switches sign, is located in correspondence with TW, hence proving that the fragile-to-strong transition is intimately connected to the dynamics of the HBs.
of water molecules change one or more hydrogen bonded neighbor every ps. As translational and rotational degrees of freedom become progressively less accessible upon cooling, C decreases accordingly. Such decrease follows the typical S-shaped profile of logistic functions describing populations dynamics, like surface growth, bacteria population, deposition of materials, clusters growth, and so on (74). The logistic function fitting our computational data is reported in Fig. 1(b) as a green continuous curve. Remarkably, the flex of the logistic function, defining where the rate of change switches sign, is located in correspondence with TW, hence proving that the fragile-to-strong transition is intimately connected to the dynamics of the HBs.
Dynamical arrest occurs at Tv = (1,000/T) ∼ 5. Although rotational and translational degrees of freedom are frozen below Tv, thermal fluctuations allow water molecules to change their connectivity through localized vibration, implying that the HBN is a dynamic system that keeps rearranging. Nearly hyperuniformity emerges and the value of C decreases very slowly, reaching 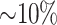 at 1,000/T = 10/K. As we will see in the forthcoming discussion, the requirement for nearly hyperuniformity to be accommodated in dynamic networks is that the overall network topology is preserved.
at 1,000/T = 10/K. As we will see in the forthcoming discussion, the requirement for nearly hyperuniformity to be accommodated in dynamic networks is that the overall network topology is preserved.
In Fig. 2 we report P(n), the normalized probability of having a ring of a given length n. Panel (a) reports the distribution P(n) computed in the temperature window T ∈ [300 − 220] K, corresponding to the temperature window in which the liquid sample is pervaded by large-scale density fluctuations and H decreases very slowly decreasing the temperature (Fig. 1a). At T = 300 K, the distribution P(n) is very broad, with an almost equal contribution of heptagonal (n = 7) and hexagonal (n = 6) rings, being n = 6 the configuration of the ground state which, at ambient pressure, are cubic and hexagonal ices. Both n = 6 and n = 7 account for 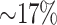 of the overall topology. The network is also rich in pentagonal rings (n = 5), which account for
of the overall topology. The network is also rich in pentagonal rings (n = 5), which account for 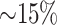 of P(n), while n = 8 and n = 9 for
of P(n), while n = 8 and n = 9 for 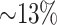 and
and 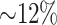 , respectively. The network accommodates also a non-negligible percentage of longer rings, namely n = 10 and n = 11, and a smaller percentage of n = 12 and squared rings (n = 4). Upon cooling down to T = 220 K (cyan symbols, corresponding to the Widom temperature) we observe a gradual—but consistent—depletion of longer (n ≥ 9) rings from the network and a corresponding increase in shorter rings, especially in n = 6. This reshaping of the HBN toward topologies richer in n ∈ [5 − 7] and poorer in longer ones is a consequence of the reduction of translational and rotational degrees of freedom, which cause a contraction of the available configurational space and, overall, of the configurational entropy.
, respectively. The network accommodates also a non-negligible percentage of longer rings, namely n = 10 and n = 11, and a smaller percentage of n = 12 and squared rings (n = 4). Upon cooling down to T = 220 K (cyan symbols, corresponding to the Widom temperature) we observe a gradual—but consistent—depletion of longer (n ≥ 9) rings from the network and a corresponding increase in shorter rings, especially in n = 6. This reshaping of the HBN toward topologies richer in n ∈ [5 − 7] and poorer in longer ones is a consequence of the reduction of translational and rotational degrees of freedom, which cause a contraction of the available configurational space and, overall, of the configurational entropy.
Fig. 2.
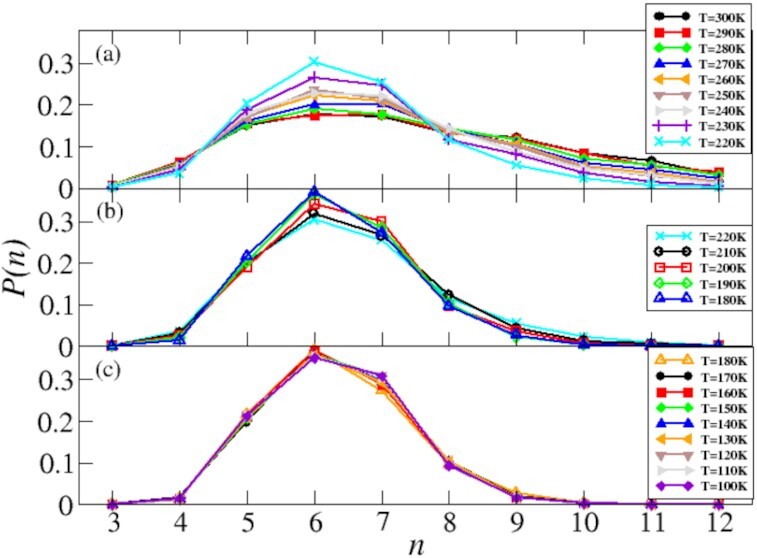
Probability P(n) of having a ring of length n as a function of the temperature. Panel (a) reports P(n) in the temperature window T ∈ [300 − 220] K corresponding to the slow decrease in the index H. Panel (b) shows P(n) in the temperature window T ∈ [220 − 180] K corresponding to the enhanced reduction in the index H. Panel (c) reports P(n) in the temperature window T ∈ [180 − 100] K in correspondence of the emergence of nearly hyperuniformity.
Panel (b) reports one of the main results of this work, namely the distribution P(n) in the temperature window T ∈ [220 − 180] K, i.e. in the temperature range in which the supercooled liquid shows a enhancement in suppressing large-scale density fluctuations as marked by the rapid decrease in H (Fig. 1a). Remarkably, upon cooling below TW, the overall topology of the HBN stabilizes towards a steady topology—in terms of preserved percentage of n-member rings—and does not show any significant modification, but only a mild enhancement in the contribution of n = 6 occurring mostly at T = 180 K. In this temperature range, the networks are dominated by n ∈ [5 − 8], with only a minor contribution of n = 4 and n = 9, and are mostly deprived of longer (n > 9) rings. We posit that the absence of longer rings is another key factor in the enhanced suppression of large-scale density fluctuations as longer rings account for higher densities. (16,53, 60).
Considering that the sample is liquid and 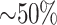 of water molecules changing one or more HBs every ps at TW and
of water molecules changing one or more HBs every ps at TW and 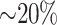 at T = 180 K (Fig. 1b), the steady topology of the HBN is a remarkably surprising property that boosts the sample’s efficiency in suppressing large-scale density fluctuations. Such steady topology implies that the underlying kinetics governing networks transformations is strongly correlated and of long-range in nature. A pictorial representation of this effect is reported in Fig. 3, showing two snapshots with different connectivity but the same topology and, therefore, the same distribution P(n).
at T = 180 K (Fig. 1b), the steady topology of the HBN is a remarkably surprising property that boosts the sample’s efficiency in suppressing large-scale density fluctuations. Such steady topology implies that the underlying kinetics governing networks transformations is strongly correlated and of long-range in nature. A pictorial representation of this effect is reported in Fig. 3, showing two snapshots with different connectivity but the same topology and, therefore, the same distribution P(n).
Fig. 3.

Pictorial representations of two snapshots at two different time steps. Water molecules are depicted as red spheres, and bonds with sticks. The connectivity moving from panel (a) to panel (b), corresponding to two consecutive time steps, has changed, but the overall topology is preserved. This snapshot reports the specific case in which the total number of rings is conserved.
The striking evidence of a steady-like topology of the HBN in supercooled liquid water allows us to conclude that the dynamics of the HBN undergoes a transition from a uncorrelated kinetics at T > TW where Stokes–Einstein relationship holds, to a correlated one, at T < TW where Stokes–Einstein relations breaks down. Rationalizing the correlated nature of bond breaking / making at T < TW is a beguiling nontrivial task on which we will try to shed light in the forthcoming discussion.
Panel (c) reports the distribution P(n) in the temperature range T ∈ [180 − 100] K, i.e. below the temperature of vitrification and in correspondence with the emergence of nearly hyperuniformity. In this temperature window, the sample is a glass and the topology of the HBN does not show any appreciable change; P(n)’s are peaked on n = 6 and the networks accommodate also n = 5, n = 7, and n = 8 rings. The absence of n = 4 is also particularly interesting. Squared rings are stiff configurations difficult to anneal at low temperature; they increase the local strain of the network and increase the fluctuations of bond angles decreasing the overall “quality” of the network (75). At these conditions, 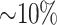 of water molecules change one or more hydrogen bonded neighbor (Fig. 1b). Therefore, the kinetics of the HBN follows the same correlated kinetics described above and pictorially represented in Fig. 3.
of water molecules change one or more hydrogen bonded neighbor (Fig. 1b). Therefore, the kinetics of the HBN follows the same correlated kinetics described above and pictorially represented in Fig. 3.
The dynamic nature of the HBN that gives rise to density fluctuations can be quantified accessing the network’s fluctuations. In Fig. 4 we report σ, the value of the standard deviations of each ring in the P(n)’s reported in Fig. 2.
Fig. 4.

Fluctuations σ of the HBN reports σ in the temperature window T ∈ [300 − 220] K corresponding to the slow decrease in the index H. Panel (b) shows σ in the temperature window T ∈ [220 − 180] K corresponding to the enhanced reduction in the index H. Panel (c) reports σ in the temperature window T ∈ [180 − 100] K in correspondence of the emergence of nearly hyperuniformity.
Panel (a) shows σ in the temperature window T ∈ [300 − 220] K, when the liquid is pervaded by large-scale density fluctuations. It is possible to appreciate the noisy, intense degree of fluctuations of the HBN. In particular, the degree of fluctuations at the level of long (n > 9) rings is as high as at the level of short (n ∈ [4, 5]) rings, indicating that the HBN as a whole undergoes pervasive rearrangements that cause density fluctuations.
Panel (b) reports σ in the temperature window T ∈ [220 − 180] K, when the value of H in the supercooled liquid decreases. The reduction of fluctuations in the HBN is here very noticeable, a temperature-dependent trend very clear. Fluctuations dampen strongly upon cooling and tend to become negligible on the few longer (n > 9) rings. This observation further demonstrates that the HBN suppresses large-scale density fluctuations in the supercooled liquid phase. Overall, the clear temperature-dependent reduction of σ explains the increased rate at which the index H decreases in this temperature window.
Panel (c) shows σ in the temperature window T ∈ [180 − 100] K, i.e. in correspondence of the glassy phase of water in which nearly hyperuniformity emerges. The fluctuations of the HBN are considerably damped, hence accounting for the reduced large-scale density fluctuations.
To delve deeper into the correlated nature of the HBN emerging at supercooled conditions, we inspect the “quality” of the HBN in terms of coordination defects. We focus on the simplest coordination defects, in which a water molecule has three HBs. Given a water molecule, two configuration give rise to this geometry, namely A2D1 (two acceptors “A” and one donor “D”), and A1D2 (one acceptor “A” and two donors “D”). Besides the perfectly four-folded coordinated case A2D2 characterizing a CRN, A2D1 and A1D2 defects represent the mostly occurring configurations in liquid water at ambient conditions (52,76). However, since the A2D1 defects occur with a frequency almost double than A1D2 defects in bulk water at ambient conditions (52,76), we focus on A2D1 configurations only.
Upon cooling, the network of bonds gradually build up (19) and the percentage of coordination defects decreases. On the other hand, spatial and dynamical heterogeneities develop affecting transport properties (19, 22–24) and water molecules keep changing neighbors (Fig. 1b). We here posit that A2D1 defects represent the source of the decoupling between rotational and translational degrees of freedom leading to the breakdown of the Stokes–Einstein relation occurring at TW, and govern the kinetics of the HBN below TW. In Fig. 5(a), we report the percentage of A2D1 coordination defects upon cooling. As expected, the percentage of defects decreases with decreasing the temperature, but such reduction is not linear; rather, as for the profile of C (Fig. 1b), also the profile of A2D1 defects follows a logistic profile (green line) with a flex roughly located in correspondence of TW. Interestingly, the percentage of A2D1 defects drops in the temperature window 1,000/T ∈ [∼5.0 − 5.2] K−1 followed by a sudden increase. This increase is unexpected, considering that it occurs in the proximity of the temperature of dynamical arrest and, upon lowering the temperature, coordination defects should disappear. On the other hand, this increase explains and accounts for the sudden increase of the hyperuniformity index H reported in Fig. 1(a). Interestingly, although A2D1 defects decrease upon cooling, they acquire an increasingly pivotal role in the dynamics of the HBN. In Fig. 5(b), we report 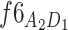 , the percentage of A2D1 defects involved in the dynamics of hexagonal rings at every ps. We compute
, the percentage of A2D1 defects involved in the dynamics of hexagonal rings at every ps. We compute 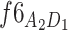 as follows: at a given time step t we list all hexagonal rings containing an A2D1 defect. After 1 ps, we count how many of these rings have preserved the hexagonal topology but exchanged the A2D1 defect with another water molecule.
as follows: at a given time step t we list all hexagonal rings containing an A2D1 defect. After 1 ps, we count how many of these rings have preserved the hexagonal topology but exchanged the A2D1 defect with another water molecule. 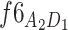 is the percentage of A2D1 defects involved in such mechanism. Remarkably, we can observe that
is the percentage of A2D1 defects involved in such mechanism. Remarkably, we can observe that 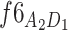 increases from
increases from 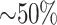 at 1,000/T ∼ 3.5/K to
at 1,000/T ∼ 3.5/K to 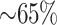 at T ∼ 1,000/T ∼ 4.5/K, to stabilize at
at T ∼ 1,000/T ∼ 4.5/K, to stabilize at 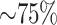 at lower temperatures. Therefore, considering that the overall dynamics of the HBN slows down (Fig. 1b) and that the percentage of A2D1 defects hosted in the network decreases upon cooling (Fig. 5a), the profile of
at lower temperatures. Therefore, considering that the overall dynamics of the HBN slows down (Fig. 1b) and that the percentage of A2D1 defects hosted in the network decreases upon cooling (Fig. 5a), the profile of 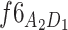 allows us to conclude that A2D1 defects play a pivotal role in the kinetics of the HBN and, possibly, in the development of the dynamical heterogeneities below TW. Although the inspection of
allows us to conclude that A2D1 defects play a pivotal role in the kinetics of the HBN and, possibly, in the development of the dynamical heterogeneities below TW. Although the inspection of 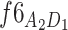 is not an exhaustive investigation of the many possible mechanisms underlying the overall kinetics of the HBN, it nonetheless highlights the central role of A2D1 defects in determining the complex behavior of water. Moreover, considering the central molecule in A2D1 defects has one dangling hydrogen, as pictorially reported in the snapshot in Fig. 5, we posit that rotations are relatively easier compared to translations; rotations could occur breaking only one of the HBs, compared to the breaking of three HB required to escape the cage of the neighbors. Therefore, we posit that A2D1 defects might be related to the supposed decoupling of translational and rotational degrees of freedoms occurring in correspondence with and below TW.
is not an exhaustive investigation of the many possible mechanisms underlying the overall kinetics of the HBN, it nonetheless highlights the central role of A2D1 defects in determining the complex behavior of water. Moreover, considering the central molecule in A2D1 defects has one dangling hydrogen, as pictorially reported in the snapshot in Fig. 5, we posit that rotations are relatively easier compared to translations; rotations could occur breaking only one of the HBs, compared to the breaking of three HB required to escape the cage of the neighbors. Therefore, we posit that A2D1 defects might be related to the supposed decoupling of translational and rotational degrees of freedoms occurring in correspondence with and below TW.
Fig. 5.
Panel (a): percentage-wise profile as a function of 1/T for A2D1 defects (black circles). The green line represents the fit with a four point logistic fit. The flex occurs roughly in correspondence with TW. Panel (b): percentage-wise profile as a function of 1/T for 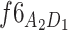 , the percentage of A2D1defects involved in changes in local topology preserving hexagonal configurations. The snapshot represents the A2D1 geometry, with the central water molecules accepting two bonds emphasized by the blue arrows and donating one bond emphasized by the green arrow. Panel (c) reports the gOO(r)’s computed between oxygen atoms of A2D1 defects only in the temperature window T ∈ [300 − 220] K, panel (d) in the temperature window T ∈ [220 − 180] K, and and panel (e) in the temperature window T ∈ [180 − 100] K.
, the percentage of A2D1defects involved in changes in local topology preserving hexagonal configurations. The snapshot represents the A2D1 geometry, with the central water molecules accepting two bonds emphasized by the blue arrows and donating one bond emphasized by the green arrow. Panel (c) reports the gOO(r)’s computed between oxygen atoms of A2D1 defects only in the temperature window T ∈ [300 − 220] K, panel (d) in the temperature window T ∈ [220 − 180] K, and and panel (e) in the temperature window T ∈ [180 − 100] K.
In order to delve deeper into the role of A2D1 defects, in Fig. 5(c)–(e) we report the two-body pair correlation function gOO(r) at different temperatures and computed between oxygen of A2D1 defects only. In panel (c), we report the gOO(r) in the temperature window T ∈ [300 − 220] K, i.e. when the HBN is strongly fluctuating. We can observe that the gOO(r)’s lack long-range spatial correlation, as one would expect for a liquid (as well as for a glass). On the other hand, the gOO(r)’s clearly deviates from that of a random distribution, that one would expect to observe in a uncorrelated system. At high temperatures, the gOO(r)’s are characterized by a low intensity first peak at ∼2.7 Å and a very mild second peak at ∼4.2 Å. Upon approaching TW = 220 K, we observe an enhancement of the second hydration peak and signatures of a third shell appearing at ∼6.7 Å. In panel (d), we report the gOO(r)’s in the temperature window T ∈ [220 − 180] K, i.e. when the HBN of the supercooled liquid allows an enhanced suppression of large-scale density fluctuations, and the overall topology acquires a steady-like configuration. We can observe the development of long-range (beyond the second shell of neighbors) correlations with hints of a fourth coordination shell emerging at ∼8.5 Å. Correspondingly, the interstitial space between first and second shells of neighbors become less populated. In panel (e) we report the gOO(r)’s in the temperature window T ∈ [180 − 100] K, i.e. in correspondence with the dynamical arrest and the emergence of nearly hyperuniformity. We can observe a further development of long-range spatial correlation and an enhanced structurization of the third and fourth hydration shells. Overall, our observations explain, end expand upon, the observations of clustered dynamical heterogeneities observed in supercooled water (22–24). Similar observations can be drawn inspecting the spatial correlation between A1D2 defects.
Conclusions
We have performed large-scale out-of-equilibrium molecular dynamics simulations to model the cooling and vitrification of liquid water at ambient pressure. By tracking large-scale density fluctuations via the hyperuniformity index H and the HBN via the ring statistics, we have unveiled a hitherto hidden connection between the ability of suppressing large-scale density fluctuations and the topology of the HBN. At high temperatures the liquid sample is pervaded by large-scale density fluctuations, the HBN strongly fluctuates and the corresponding topology changes configurations. In correspondence with the Widom temperature (TW ∼ 220 K) the sample undergoes a crossover and below TW the liquid phase is endowed by a enhanced suppression of large-scale density fluctuations. Correspondingly, the fluctuations of the HBN are strongly reduced and the topology almost steady. The preserved topology, i.e. the roughly temperature-independent rings composition of the HBN and the concomitant development of long-range spatial correlations between coordination defects, signal the existence of cooperative bonds breaking / making mechanisms with long-range correlations that govern the kinetics of the HBN in the supercooled liquid. A detailed analysis of the dynamical and spatial behavior of A2D1 coordination defects indicates that these defects play a pivotal role in the complex kinetics of the HBN, and such role increases upon decreasing the temperature. We also posit that A2D1 defects may be the source of the decoupling between translational and rotational degrees of freedom occurring in correspondence with the fragile-to-strong transition. From an experimental perspective, deconvolving absorption spectra to identify the contribution of A2D1 configurations could help in verifying our hypothesis.
Nearly hyperuniformity emerges below the temperature of vitrification Tv, as the fluctuations of the HBN are strongly suppressed and the topology of the HBN steady. Nonetheless, the network is dynamic and hosts a considerable amount of coordination defects. Inspecting the profile of structure factors obtained experimentally could help in validating our findings.
Rationalizing the concerted mechanisms underlying the HBN transformations in the supercooled liquid and in the glassy state is an alluring perspective (and a nontrivial task) that would deepen our understanding of the physics of supercooled liquids, and of water in particular. The concerted bond-switch mechanism initially proposed by Wooten et al. (77) to produce amorphous silicon and germanium (and related to the occurrence of Stone–Wales defects in 2D hyperuniform networks (78)) may be present, but they are only one of the several possible and more complex mechanisms involving longer-range correlations. Likewise, the discovery of nearly hyperuniformity in 3D networks hosting a considerable amount of coordination defects opens new avenues for the design of experimental set ups to make and test such kind of networks, as well as for better understanding the relationship between stiffness in 3D network forming materials and hyperuniformity.
ACKNOWLEDGEMENTS
The author acknowledges the use of supercomputing facilities provided by the STFC-Hartree Centre. The author gratefully thanks Professor Francesco Mallamace for the support, encouragement, and many insightful discussions.
Notes
Competing Interest: The author declares no competing interest.
Funding
The author declares no funding.
Authors' Contributions
F.M. conceived the and conducted the research, analyzed the results, and wrote the manuscript.
Data Availability
The data underlying this article are available upon request to the author.
References
- 1. Salzmann CG. 2019. Advances in the experimental exploration of water’s phase diagram. J Chem Phys. 150(6):060901. [DOI] [PubMed] [Google Scholar]
- 2. Palmer JC, et al. 2014. Metastable liquid– liquid transition in a molecular model of water. Nature. 510:385–388. [DOI] [PubMed] [Google Scholar]
- 3. Sellberg JA, et al. 2014. Ultrafast x-ray probing of water structure below the homogeneous ice nucleation temperature. Nature. 510:381–384. [DOI] [PubMed] [Google Scholar]
- 4. Debenedetti PG, Sciortino F, Zerze GH. 2020. Second critical point in two realistic models of water. Science. 369:289–292. [DOI] [PubMed] [Google Scholar]
- 5. Kringle L, Thornley WA, Kay BD, Kimmel GA. 2020. Reversible structural transformations in supercooled liquid water from 135 to 245 K. Science. 369:1490–1492. [DOI] [PubMed] [Google Scholar]
- 6. Kim KH, et al. 2020. Experimental observation of the liquid-liquid transition in bulk supercooled water under pressure. Science. 370:978. [DOI] [PubMed] [Google Scholar]
- 7. Martelli F, Palmer JC. 2022. Signatures of sluggish dynamics and local structural ordering during ice nucleationz. J Chem Phys. 156:114502. [DOI] [PubMed] [Google Scholar]
- 8. Yagasaki T, Matsumoto M, Tanaka H. 2014. Spontaneous liquid-liquid phase separation of water. Phys Rev E. 89:020301(R). [DOI] [PubMed] [Google Scholar]
- 9. Palmer JC, Car R, Debenedetti PG. 2013. The liquid–liquid transition in supercooled ST2 water: a comparison between umbrella sampling and well-tempered metadynamics. Faraday Discuss. 167:77–94. [DOI] [PubMed] [Google Scholar]
- 10. Palmer JC, et al. 2016. Metastability and no criticality reply. Nature. 531:E2–E3. [DOI] [PubMed] [Google Scholar]
- 11. Chandler D. 2016. Metastability and no criticality. Nature. 531:E1–E2. [DOI] [PubMed] [Google Scholar]
- 12. Limmer DT, Chandler D. 2011. The putative liquid-liquid transition is a liquid-solid transition in atomistic models of water. J Chem Phys. 135:134503. [DOI] [PubMed] [Google Scholar]
- 13. Limmer DT, Chandler D. 2014. Theory of amorphous ices. Proc Natl Acad Sci USA. 111:9413–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer JC, Singh RS, Chen R, Martelli F, Debenedetti PG. 2016. Density and bond-orientational relaxations in supercooled water. Mol Phys. 114:2580–2585. [Google Scholar]
- 15. Palmer JC, et al. 2018. Comment on “The putative liquid-liquid transition is a liquid-solid transition in atomistic models of water” [I and II: J. Chem. Phys. 135, 134503 (2011); J. Chem. Phys. 138, 214504 (2013)]. J Chem Phys. 148:137101. [DOI] [PubMed] [Google Scholar]
- 16. Martelli F. 2019. Unravelling the contribution of local structures to the anomalies of water: the synergistic action of several factors. J Chem Phys. 150:094506. [DOI] [PubMed] [Google Scholar]
- 17. Russo J, Tanaka H. 2014. Understanding water’s anomalies with locally favoured structures. Nat Commun. 5:3556. [DOI] [PubMed] [Google Scholar]
- 18. Ito K, Moynihan CT, Angell CT. 1999. Thermodynamic determination of fragility in liquids and a fragile-to- strong liquid transition in water. Nature. 398:492–494. [Google Scholar]
- 19. Mallamace F, et al. 2007. Evidence of the existence of low-density liquid phase in supercooled, confined water. Proc Natl Acad Sci USA. 104:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito S, Bagchi B, Ohmine I. 2018. Crucial role of fragmented and isolated defects in persistent relaxation of deeply supercooled water. J Chem Phys. 149:124504. [DOI] [PubMed] [Google Scholar]
- 21. Chen SH, et al. 2006. The violation of the Stokes–Einstein relation in supercooled water. Proc Natl Acad Sci USA. 103:12974–12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giovambattista N, Mazza MG, Buldyrev SV, Starr FW, Stanley HE. 2004. Dynamic heterogeneities in supercooled water. J Phys Chem B. 188:6655–6662. [Google Scholar]
- 23. Mazza MG, Giovambattista N, Starr FW, Stanley HE. 2996. Relation between rotational and translational dynamic heterogeneities in water. Phys Rev Lett. 96:057803. [DOI] [PubMed] [Google Scholar]
- 24. Mazza MG, Giovambattista N, Stanley HE, Starr FW. 2007. Connection of translational and rotational dynamical heterogeneities with the breakdown of the Stokes-Einstein and Stokes-Einstein-Debye relations in water. Phys Rev E. 76:031203. [DOI] [PubMed] [Google Scholar]
- 25. Mishima O, Calvert L, Whalley E. 1985. An apparent first-order transition between two amorphous phases of ice induced by pressure. Nature. 324:76. [Google Scholar]
- 26. Mishima O, Calvert L, Whalley E. 1984. Melting ice I at 77 K and 10 kbar: a new method of making amorphous solids. Nature. 310:393–395. [Google Scholar]
- 27. Hemley RJ, Chen LC, Mao HK. 1989. New transformations between crystalline and amorphous ice. Nature. 338:638–640. [Google Scholar]
- 28. Mishima O. 1996. Relationship between melting and amorphization of ice. Nature. 384:546–549. [Google Scholar]
- 29. Debenedetti PG. 2003. Supercooled and glassy water. J Phys Condens Matter. 15:R1669–R1726. [Google Scholar]
- 30. Yuanzheng Y, Austen AC. 2004. Clarifying the glass-transition behaviour of water by comparison with hyperquenched inorganic glasses. Nature. 427:717. [DOI] [PubMed] [Google Scholar]
- 31. Loerting T, Giovambattista N. 2006. Amorphous ices: experiments and numerical simulations. J Phys Condens Matter. 18:R919. [Google Scholar]
- 32. Mishima O, Stanley HE. 1998. Decompression-induced melting of ice IV and the liquid-liquid transition in water. Nature. 392:164–168. [Google Scholar]
- 33. Loerting T, et al. 2015. The glass transition in high-density amorphous ice. J Non-Cryst Solids. 407:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amann-Winkel K, et al. 2016. Colloquium: water’s controversial glass transitions. Rev Mod Phys. 88(1):011002. [Google Scholar]
- 35. Giovambattista N, Sciortino F, Starr FW, Poole PH. 2016. Potential energy landscape of the apparent first-order phase transition between low-density and high-density amorphous ice. J Chem Phys. 145:224501. [DOI] [PubMed] [Google Scholar]
- 36. Martelli F, Giovambattista N, Torquato S, Car R. 2018. Searching for crystal-ice domains in amorphous ices. Phys Rev Mater. 2:075601. [Google Scholar]
- 37. Martelli F, Torquato S, Giovambattista N, Car R. 2017. Large-scale structure and hyperuniformity of amorphous ices. Phys Rev Lett. 119:136002. [DOI] [PubMed] [Google Scholar]
- 38. Gartner TE III, Torquato S, Car R, Debenedetti PG. 2021. Manifestations of metastable criticality in the long-range structure of model water glasses. Nat Comm. 12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martelli F, Leoni F, Sciortino F, Russo J. 2020. Connection between liquid and non-crystalline solid phases in water. J Chem Phys. 153:104503. [DOI] [PubMed] [Google Scholar]
- 40. Winkel K, Bowron DT, Loerting T, Mayer E, Finney JL. 2009. Relaxation effects in low density amorphous ice: two distinct structural states observed by neutron diffraction. J Chem Phys. 130:204502. [DOI] [PubMed] [Google Scholar]
- 41. Shephard JJ, Klotz S, Vickers M, Salzmann CG. 2016. A new structural relaxation pathway of low-density amorphous ice. J Chem Phys. 144:204502. [DOI] [PubMed] [Google Scholar]
- 42. Loerting T, et al. 2011. How many amorphous ices are there?. Phys Chem Chem Phys. 13:8783–8794. [DOI] [PubMed] [Google Scholar]
- 43. Loerting T, Salzmann C, Kohl I, Mayer E, Hallbrucker A. 2001. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys Chem Chem Phys. 3:5355–5357. [Google Scholar]
- 44. Finney JL, et al. 2002. Structure of a new dense amorphous ice. Phys Rev Lett. 89:205503. [DOI] [PubMed] [Google Scholar]
- 45. Torquato S, Stillinger FH. 2003. Local density fluctuations, hyperuniformity, and order metrics. Phys Rev E. 64:041113. [DOI] [PubMed] [Google Scholar]
- 46. Atkinson S, Zhang G, Hopkins AB, Torquato S. 2016. Critical slowing down and hyperuniformity on approach to jamming. Phys Rev E. 94:012902. [DOI] [PubMed] [Google Scholar]
- 47. Pietronero L. 1987. The fractal structure of the universe: correlations of galaxies and clusters and the average mass density. Phys A: Stat Mech Appl. 114:257–284. [Google Scholar]
- 48. Zhang G, Martelli F, Torquato S. 2018. The structure factor of primes. J Phys A Math Theor. 51:115001. [Google Scholar]
- 49. Jiao Y, et al. 2014. Avian photoreceptor patterns represent a disordered hyperuniform solution to a multiscale packing problem. Phys Rev E. 89:022721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang M, Hu W, Yang S, Liu QX, Zhang HP. 2021. Circular swimming motility and disordered hyperuniform state in an algae system. Proc Natl Acad Sci USA. 118:e2100493118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torquato S. 2018. Hyperuniform states of matter. Phys Rep. 745:1–95. [Google Scholar]
- 52. Martelli F. 2021. Topology and complexity of the hydrogen bond network in classical models of water. J Mol Liq. 329:115530. [Google Scholar]
- 53. Formanek M, Martelli F. 2020. Probing the network topology in network–forming materials: the case of water. AIP Adv. 10:055205. [Google Scholar]
- 54. Camisasca G, Schlesinger D, Zhovtobriukh I, Pitsevich G, Pettersson LGM. 2019. A proposal for the structure of high- and low-density fluctuations in liquid water. J Chem Phys. 151:034508. [DOI] [PubMed] [Google Scholar]
- 55. Santra B, DiStasio RA Jr, Martelli F, Car R. 2015. Local structure analysis in abinitio liquid water. Mol Phys. 113:2829–2841. [Google Scholar]
- 56. Castagna J, Martelli F, Jordan KE, Crain J. 2021. Simulation of large molecular systems with electronically-derived forces. Comp Phys Comm. 264:107959. [Google Scholar]
- 57. Martelli F, Crain J, Franzese G. 2020. Network topology in water nanoconfined between phospholipid membranes. ACS Nano. 14:8616–8623. [DOI] [PubMed] [Google Scholar]
- 58. Chiricotto M, Martelli F, Giunta G, Carbone P. 2021. The role of long-range electrostatic interactions and local topology of the hydrogen bond network in the wettability of wetted and partially wetted single and multilayer graphene. J Phys Chem C. 125:6367–6377. [Google Scholar]
- 59. Foffi R, Sciortino F. 2021. Structure of high-pressure supercooled and glassy water. Phys Rev Lett. 12:175502. [DOI] [PubMed] [Google Scholar]
- 60. Foffi R, Russo J, Sciortino F. 2021. Structural and topological changes across the liquid–liquid transition in water. J Chem Phys. 154:184506. [DOI] [PubMed] [Google Scholar]
- 61. Wei Z, Chiricotto M, Elliott JD, Martelli F, Carbone P. 2022. Wettability of graphite under 2D confinement. arXiv:220507693v1 [physicsflu-dyn]. 10.48550/arXiv.2205.07693. [DOI] [Google Scholar]
- 62. Abascal JLF, Vega C. 2005. A general purpose model for the condensed phases of water: TIP4P/2005. J Chem Phys. 123:234505. [DOI] [PubMed] [Google Scholar]
- 63. Nosé S. 1984. A molecular dynamics method for simulations in the canonical ensemble. Mol Phys. 52:255–268. [Google Scholar]
- 64. Hoover WG. 1985. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A. 31:1695. [DOI] [PubMed] [Google Scholar]
- 65. Parrinello M, Rahman A. 1981. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 52:7182. [Google Scholar]
- 66. Abraham MJ, et al. 2015. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 1:19–25. [Google Scholar]
- 67. Handle PH, Sciortino F. 2018. Potential energy landscape of TIP4P/2005 water. J Chem Phys. 148:134505. [DOI] [PubMed] [Google Scholar]
- 68. Russo J, Tanaka H. 2014. Understanding water’s anomalies with locally favoured structures. Nat Commun. 5:3556. [DOI] [PubMed] [Google Scholar]
- 69. Shi R, Russo J, Tanaka H. 2018. Origin of the emergent fragile-to-strong transition in supercooled water. Proc Natl Acad Sci USA. 115:9444–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi R, Russo J, Tanaka H. 2018. Common microscopic structural origin for water’s thermodynamic and dynamic anomalies. J Chem Phys. 149:224502. [DOI] [PubMed] [Google Scholar]
- 71. Kohl I, Bachmann L, Hallbrucker A, Mayer E, Loerting T. 2005. Liquid-like relaxation in hyperquenched water at ≤140 K. Phys Chem Chem Phys. 7:210–3220. [DOI] [PubMed] [Google Scholar]
- 72. Martelli F, Ko HY, Oğuz EC, Car R. 2016. Local-order metric for condensed phase environments. Phys Rev B. 97:064105. [Google Scholar]
- 73. Bhat SN, Sharma A, Bhat SV. 2005. Vitrification and glass transition of water: Insights from spin probe ESR. Phys Rev Lett. 95:235702. [DOI] [PubMed] [Google Scholar]
- 74. Barabási AL, Stanley HE. Fractal concepts in surface growth. 1st edn. Cambridge: Cambridge University Press. [Google Scholar]
- 75. Barkema GT, Mousseau N. 2000. High-quality continuous random networks. Phys Rev B. 62:4985–4990. [Google Scholar]
- 76. DiStasio RA Jr, Santra B, Li Z, Wu X, Car R. 2014. The individual and collective effects of exact exchange and dispersion interactions on the ab initio structure of liquid water. J Chem Phys. 141:084502. [DOI] [PubMed] [Google Scholar]
- 77. Wooten F, Winer K, Weaire D. 1985. Computer generation of structural models of amorphous Si and Ge. Phys Rev Lett. 54:1392. [DOI] [PubMed] [Google Scholar]
- 78. Chen D, et al. 2021. Stone–wales defects preserve hyperuniformity in amorphous two-dimensional networks. Proc Natl Acad Sci USA. 119:e2016862118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available upon request to the author.



