Abstract
In-vitro fertilization is an effective treatment for various causes of infertility. However, management of women with poor ovarian response or premature ovarian insufficiency remains challenging because these women have underdeveloped small ovarian follicles that do not respond to hormone treatment. In-vitro activation of small follicles has been developed but its efficiency has much room for improvement. In the current study, we provide several lines of evidence showing that curcumin, an FDA-approved traditional medicine, can specifically promote the development of mouse ovarian follicles from the primary to secondary stage, which greatly potentiates these small follicles for subsequent in-vivo development into antral follicles that can be ovulated. Mechanistically, we show that curcumin promotes the proliferation and differentiation of granulosa cells and the growth of oocytes by activating the phosphatidylinositol 3 kinase (PI3K) signaling pathway. Most importantly, we show that in-vitro treatment of human ovarian tissues with curcumin can promote the in-vivo survival and development of small human ovarian follicles, showing that curcumin can be used as a potential drug to increase the success rate of in-vitro activation of small human follicles. We thus identify curcumin as a novel potential drug for promoting the development of small human ovarian follicles for infertility treatment.
Keywords: in-vitro activation of ovarian follicles, novel drug, female infertility treatment
Significance Statement.
In-vitro activation (IVA) of small follicles has been developed for activating small ovarian follicles in vitro as a way of treating infertility in women with poor ovarian response or premature ovarian insufficiency. However, all currently used IVA methods share the common issue of low efficiency. Thus, new ways to improve the success rate of IVA are needed. In the current study, we provide several lines of mechanistic and practical evidence showing that the in-vitro use of the FDA-approved drug curcumin can promote the growth of small human ovarian follicles and thus increase the success rate of IVA in treating infertility.
Introduction
In mammals, the ovaries are essential for the production of fertilizable eggs as well as various hormones and other factors (1, 2). The pool of primordial follicles in a mammalian ovary is formed early in life and is considered to be the only source of ovarian follicles over the course of the animal’s life. To produce mature eggs, only a small proportion of primordial follicles are recruited into the growing follicle pool at any given time (3, 4), and the survival of primordial follicles serves as the determining factor of the female reproductive lifespan [for reviews, see (2, 5, 6)].
The mechanisms of primordial follicle activation have been investigated in numerous studies over the past decade. In oocytes, the phosphatidylinositol 3 kinase (PI3K)/phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling pathway governs primordial follicle activation (7, 8). The mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway in pregranulosa cells plays an important role in enhancing the expression of Kit ligand in granulosa cells, which, in turn, activates the PI3K signaling pathway in oocytes by binding to the Kit receptor on the oocyte surface, and the subsequent signaling cascade activates the primordial follicle into a growing primary follicle (9). Moreover, in recent years, various other molecular networks and signaling pathways have been reported to be involved in the activation of primordial follicles, such as the Hippo signaling pathway (10–12), the MAPK3/1 signaling pathways, etc [for a recent review, see (13)].
With a better understanding of the molecular mechanisms of primordial follicle activation, in-vitro activation (IVA) methods have been developed for activation of small ovarian follicles in vitro as a way of treating infertile patients with premature ovarian insufficiency (POI) or poor ovarian response (POR) (10, 14). For IVA treatment, ovarian cortices from POI patients are fragmented to disrupt the Hippo signaling pathway, followed by treatment with the PTEN inhibitor bpv and the PI3K activator 740Y-P, which is a cell-permeable phospho-peptide that can bind to the SH2 domain of the p85 regulatory subunit of PI3K to trigger the enzymatic activity of PI3K. These ovarian tissues are then autografted beneath the serosa of the Fallopian tubes for in-vivo development into antral follicles that can respond to hormone treatment during in-vitro fertilization (IVF) (10). Using this IVA method, live births have been reported in several IVF centers around the world (10, 15, 16). In recent years, a simpler drug-free IVA method has been introduced. Drug-free IVA only includes the fragmentation of the obtained ovarian cortex without tissue culture and drug treatment, and thus only involves one surgery, and several live births have been reported using this method (17–19). However, all currently used IVA methods share the common issue of low efficiency. Thus, new ways to improve the success rate of IVA are needed.
Curcumin is an active biological compound of Curcuma longa and has been used as a traditional medicine for many centuries (20, 21). Curcumin has been reported to have antioxidant (22), anti-inflammatory (23), and anti-carcinogenic properties (24, 25), and the pharmacological safety of curcumin has been reported (26) and human consumption is “generally recognized as safe” by the US Food and Drug Administration (27) and the China National Medical Products Administration (28). In studies with rodent models of premature ovarian failure induced by different artificial means, curcumin has been reported to be a potential protective compound that reduces oxidative stress and inflammatory responses in the ovaries such that the damage to the ovaries by ionizing radiation, alkylating agents, or reactive oxygen species is to some extent prevented (29–31). Other studies on how the ovaries are affected by curcumin have produced contrasting results. For example, curcumin was reported in one study to reverse rat ovarian injury caused by ischemia-reperfusion (32), but this protective effect was not seen in another similar study (33). Also, curcumin was reported to activate the PI3K/PTEN signaling pathway in ovaries (31), while another study reported that curcumin reverses the inhibited phosphatase activity of PTEN and increases the protein level of PTEN, thus suggesting that curcumin suppresses the activation of the PI3K signaling pathway (34). In terms of follicular development, conflicting results also exist between different animal models and even between in-vivo and in-vitro results in the same study (34, 35). In addition, in a human ovarian granulosa cell line and in cultured primary human granulosa cells, low concentrations and high concentrations of curcumin seemed to have opposite effects on cell viability (36), making the effects of curcumin on ovarian follicular development an intriguing topic for further study.
Another important issue is the bioavailability of curcumin (37, 38), which limits its therapeutic efficiency (39). Our results in the current study also showed that intraperitoneal (i.p.) injection of curcumin in mice at a dose of 100 mg/kg body weight resulted in solid yellow clumps of curcumin in the abdominal cavity, showing that curcumin cannot be efficiently absorbed by the body, although this dosage of 100 mg/kg body weight or higher has been used in other in-vivo studies (31–34).
In the current study, we aimed to demonstrate whether curcumin can affect the follicular development of the ovary, and if it does, the best way of administration of curcumin in order to promote follicular development. We found that curcumin can only be applied in vitro to exert its effects on promoting ovarian follicular development in mice, and that curcumin specifically promotes the transition from primary follicles to secondary follicles in in-vitro-cultured mouse ovaries. However, this effect was not seen when curcumin was administrated through i.p. injection or intragastric (i.g.) administration in mice, which again suggests very limited bioavailability. It is worth noting that curcumin not only promotes the transition from primary to secondary follicles, but also potentiates the future development of these follicles into antral follicles, which occurs after curcumin treatment has ceased. Most importantly, in-vitro treatment of human ovarian tissues with curcumin can lead to increased survival and activation of human ovarian follicles, showing that curcumin may be used to improve current IVA techniques in order to obtain more activated small follicles for fertility treatment.
Materials and methods
Animals
C57BL/6 J mice were purchased from the Medical Laboratory Animal Centre of Guangdong (Guangzhou, China). Neonatal female pups were housed together with their nursing female mouse under controlled environmental conditions with free access to water and food. All animal protocols were approved by the Committee on Ethics at the Hong Kong University Shenzhen Hospital (protocol code: hkuszh2020031 and date of approval: 2020-04-12).
Mouse ovary culture
Ovaries and oviducts were collected from postnatal day (PD) 7 mice, then dissected by a pair of 29-gauge needles in phosphate-buffered saline (PBS). Ovaries were isolated and cultured on Millicell inserts (MCEP24H48, Millipore, Burlington, MA, USA) in 24-well culture plates containing 500 µl of Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F-12; 1:1, v/v; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 1% insulin–transferrin–selenium (ITS, 41400–045, Invitrogen, Waltham, MA, USA) and 3 mg/ml bovine serum albumin (BSA, A1933, Sigma, St. Louis, MO, USA) at 37°C in an atmosphere of 5% CO2. The medium was supplemented with penicillin and streptomycin and was changed every 2 d. The concentration of curcumin (08511, Sigma, USA) was 50 µM for in-vitro culture and was 100 µM for in-vitro culture before transplantation.
Human fetal ovarian tissue culture
All protocols were approved by the Committee on Ethics at the Hong Kong University Shenzhen Hospital (protocol code: hkuszh2020031 and date of approval: 2020-04-12), and informed consent was obtained from all subjects who participated in the study after counseling. Human ovarian samples were obtained from the fetuses of eight women who underwent second trimester medical abortion for nonmedical reasons in the Department of Obstetrics and Gynaecology of Hong Kong University Shenzhen Hospital. Ovarian fragments from each fetus were carefully cut into small cubes (∼1 mm3). Some of the pieces were fixed for histological examination, and the remaining cubes were cultured on Millicell inserts (MCEP24H48, Millipore, Burlington, MA, USA) in 24-well culture plates containing 500 µl of DMEM/F-12 supplemented with 1% ITS and 3 mg/ml BSA at 37°C in an atmosphere of 5% CO2. The medium was supplemented with penicillin and streptomycin and changed every 2 d. The concentration of curcumin (08511, Sigma, USA) was 100 µM for in-vitro culture before transplantation.
Ovarian tissue transplantation
Host animals were anesthetized with avertin (250 mg/kg) (T48402, Sigma, USA), and their kidneys were externalized through a horizontal incision. The two ovaries of a mouse (control group and curcumin group) or human fetal ovarian tissues from the same women were randomly inserted under the kidney capsule of the same host mouse that had been ovariectomized to increase endogenous gonadotropin levels. Three days after transplantation, the hosts were treated with 2 IU FSH (Puregon, Merck, Hoddesdon, UK) every 36 h by i.p. injection. For the mouse study, the C57BL/6 J mice were sacrificed to detect follicular development at 14 to 28 d after transplantation. For the human fetal ovarian samples, severe combined immunodeficiency (SCID) mice were sacrificed at 3 to 6 months after transplantation.
Morphological analysis and quantification of ovarian follicles
Quantification of ovarian follicles was performed as previously described (8). Briefly, ovaries were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned to a thickness of 8 µm. The sections were then stained with hematoxylin for morphological observation. Ovarian follicles at different developmental stages—including primordial, primary, secondary, and antral follicles—were counted in all sections of an ovary or tissue section. In each section, follicles that contained oocytes with clearly visible nuclei were scored, as previously reported (40). As judged from careful morphological analysis, the incidence of counting the same follicle twice or missing a follicle was low.
Immunofluorescence
Paraffin-embedded mouse ovarian tissues were sectioned to a thickness of 5 µm as described above (13). Subsequently, these sections were dewaxed, hydrated, and subjected to antigen retrieval using 0.01% sodium citrate buffer (pH 6.0). After cooling, the sections were incubated with primary antibodies overnight at 4°C and thoroughly rinsed with PBS. The sections were then incubated with Alexa Fluor 488-conjugated secondary antibodies (1:200, Thermo Fisher Scientific) at 37°C for 1 h and then washed with PBS and stained with DAPI. The antibodies used were anti-BrdU (#G3G4; Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA, USA) at 1:200 dilution and anti-MVH (ab27591; Abcam, Cambridge, UK) at 1:200 dilution.
Granulosa cell isolation and culture
Ovaries at PD13 were harvested, washed in PBS to rinse off the impurities, and then incubated in 0.02% collagenase (17018029, Invitrogen, Waltham, MA, USA) with DMEM/F-12 medium at 37°C. After the tissues had mostly been digested by collagenase, usually within 15 to 20 min, the mixture was incubated at 37ºC with frequent pipetting for another 5 min until clusters of granulosa cells or other cells were completely dispersed. The digestion reaction was filtered through a 40 µm cell strainer, and the filtered cells were collected by centrifugation and resuspended in DMEM/F-12 medium with 10% fetal bovine serum (FBS, 10099–141, Invitrogen, Waltham, MA, USA). The cells obtained contained mostly granulosa cells and were then cultured overnight at 37°C in 6-well culture plates.
On the second day, the cells were washed three times and cultured in FBS-free DMEM/F-12 medium for 24 h to starve the granulosa cells. The cells were then treated with 10 µM LY294002 (a PI3K inhibitor) or 10 µM rapamycin (an mTORC1 inhibitor) for 1 h and with 100 µM curcumin for 20 min.
Western blotting
Total protein was extracted from the ovaries or cells with a lysis buffer (41) (50 mM HEPES-KOH (pH 7.5), 100 mM KCl, 2 mM EDTA, 10% glycerol, 0.1% NP-40, 10 mM NaF, 0.25 mM Na3VO4, and 50 mM β-glycerolphosphate) supplemented with cOmplete Protease Inhibitor (Roche #04,693,116,001). The proteins were then separated via sodium dodecyl sulphate–polyacrylamide gel electrophoresis with a 5% stacking gel and a 10% separating gel and were subsequently electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore, MA, USA). The membranes were first incubated for 1 h in 5% skim milk in Tris–HCl containing 0.1% Tween 20 and subsequently incubated with primary antibodies against the following proteins overnight at 4°C: Akt (#4691; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), phospho-Akt (#4060; Ser473; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), Foxo3a (#12829; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), phospho-Foxo3a (ab26649; T32; 1:1,000 dilution; Abcam, Cambridge, UK), p70 S6K1 (#9202; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), phospho-p70 S6K1 (#9234; Thr389; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), rpS6 (#2217; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), phospho-rpS6 (#2211; Ser-235/Ser-236; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), 4EBP1 (#9644; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), phospho-4EBP1 (#9451; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), cleaved caspase-3 (#9664; 1:1,000 dilution; Cell Signaling Technologies, Danvers, MA, USA), and β-Actin (ab227387; 1:2,500 dilution; Abcam, Cambridge, UK). The membranes were then washed with Tris–HCl containing 0.1% Tween 20 and incubated with the corresponding secondary antibody (1:2,500 dilution; Invitrogen, Waltham, MA, USA) at room temperature for 1 h. The levels of β-Actin expression were detected as the internal control.
Treatment of mice in vivo
Mice at PD7 received curcumin (100 mg/kg) or sesame oil by i.p. injection for 7 consecutive days. On the last day of curcumin administration (day 14), all mice were sacrificed and their ovaries were collected for morphological analysis and quantification of ovarian follicles.
For the 5-bromo-2-deoxyuridine (BrdU) assay, hosts at 7 or 13 d after transplantation were treated with a single i.p. injection of BrdU (B5002, Sigma, USA) at 120 mg/kg before collecting the grafts. Two hours later, the amount of BrdU labeling was determined by immunofluorescence using the BrdU antibody.
Statistical analysis
All mouse experiments were repeated at least three times, and the values are presented as the means ± SD. t-tests were performed to determine significant differences between the treatment and control groups. When multiple sets of data were examined, significant results were determined using the ANOVA test with the SAS software (SAS Institute, Inc., Cary, NC). A P-value < 0.05 was considered statistically significant.
Results
Curcumin treatment promotes the growth of small follicles in mouse ovaries in vitro but not in vivo
We first studied the possible effects of curcumin on follicular development when administered in vivo. We injected curcumin i.p. (100 mg/kg) into PD7 female mice, which is when most of the primordial follicles have been formed (2, 8), for 7 consecutive days. After 7 d of treatment, we collected the ovaries and made serial paraffin sections of 8 µm thickness of the entire ovaries, and we systematically counted the numbers of follicles of different types in all serial sections, as reported earlier (8). Our results showed that there was no significant difference in ovarian size (Supplementary Fig. S1A), ovarian weight (Supplementary Fig. S1B), or numbers of primordial, primary, or secondary follicles between the curcumin-treated group and the control group (Supplementary Fig. S1C–G). However, it is worth noting that we observed apparently unabsorbed clumps of curcumin in the abdominal cavities of the curcumin-treated recipient mice (Supplementary Fig. S1H), showing that in-vivo injection of curcumin at 100 mg/kg might not be an effective way of administrating curcumin into the body. We concluded that, as previously reported (42, 43), there is indeed an issue of bioavailability when administering curcumin in vivo.
Then, we performed i.g. administration of curcumin (150 mg/kg) to PD13 mice, one time per day for 5 consecutive days and collected the ovaries after 7 and 14 d of administration. The results showed that there was no significant difference in ovarian sizes (Supplementary Fig. S2A and S2B), ovarian weights (P = 0.308, P = 0.472) (Supplementary Fig. S2C and S2D), or the development of various stages of follicles (Supplementary Fig. S2E–H) between the curcumin treated group and the control group after 7 and 14 d of treatment. Similar results were seen with ovarian samples that were collected after 7 and 14 d of curcumin treatment. The results showed that i.g. administration of curcumin had no effect on the development of ovarian follicles in mice.
We next examined the possible effect of in-vitro treatment with curcumin on follicular development. We obtained mouse ovaries at PD7 and cultured them for a period of 6 d as we described earlier (9) with or without curcumin (50 µM). After 6 d of culture, the ovaries were collected and fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin for morphological analysis.
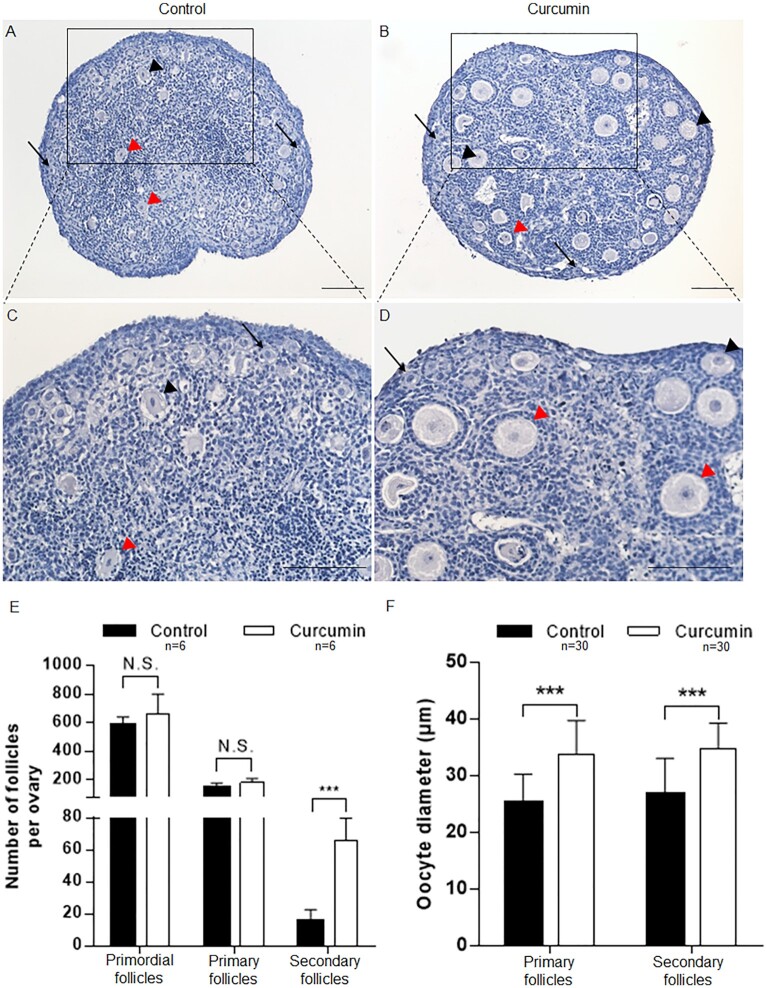
Morphological analysis with hematoxylin staining showed that in the control group without curcumin the mouse ovaries contained mostly primordial follicles (Fig. 1A and C, arrows) and a few activated primary and secondary follicles (Fig. 1A and C, black arrowheads, red arrowheads). In comparison, although many primordial follicles were still observed in the cultured mouse ovaries with curcumin (Fig. 1B and D, arrows), many more activated primary and secondary follicles were seen, with cuboidal granulosa cells (Fig. 1B and D, black arrowheads, red arrowheads). We further quantified the follicles by making serial 8 µm paraffin sections of the cultured ovaries and counting the different types of follicles in all sections of all analyzed ovaries (n = 6 for the control group; n = 6 for the curcumin group), as we described previously (8). Our results showed that there was no significant difference in the numbers of primordial follicles or primary follicles per ovary between the curcumin-treated group and the control group (Fig. 1E). However, the average number of secondary follicles in curcumin-treated ovaries (66 ± 13) was significantly increased (P = 1.22e–05) compared to the control ovaries (16 ± 6) (Fig. 1E). This showed that curcumin can specifically promote the development from primary to secondary follicles in cultured mouse ovaries, but not the survival or activation of primordial follicles.
Fig. 1.
The effects of curcumin on stimulating the development of ovaries and follicles in vitro. Ovaries at PD7 were cultured for 6 d with or without 50 µM curcumin. (A–D) Comparison of the morphology of curcumin-treated (B and D) and control ovaries (A and C) (arrows, primordial follicles; black arrowheads, primary follicles; red arrowheads, secondary follicles). Hematoxylin staining indicates nuclei. Scale bars: 50 µm. (E) Ovarian follicles at different developmental stages were counted. (F) The diameters of the oocytes in the primary and secondary follicles were measured. Data are shown as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
Because curcumin promoted the growth from primary follicles into secondary follicles in mouse ovaries, we wondered whether curcumin might also stimulate the growth of oocytes. Indeed, we found that curcumin treatment significantly increased the oocyte diameter of primary and secondary follicles (P = 1.40e–007, P = 5.87e–007). The average diameter of the oocytes in primary follicles of the untreated group was 25.1 ± 5.1 µm, whereas in the curcumin treated group the diameters of oocytes in primary follicles were significantly enlarged to 33.9 ± 5.9 µm (Fig. 1F). Similarly, in secondary follicles of curcumin treated mouse ovaries, the diameter of oocytes (34.9 ± 4.4 µm) was significantly higher than that in the control group (26.8 ± 6.3 µm) (Fig. 1F). These results indicated that curcumin has an effect on promoting the growth of oocytes, and thus it is clear that curcumin promotes the development from primary to secondary follicles.
Treatment of small mouse ovarian follicles with curcumin in vitro potentiates their subsequent development into antral follicles in vivo
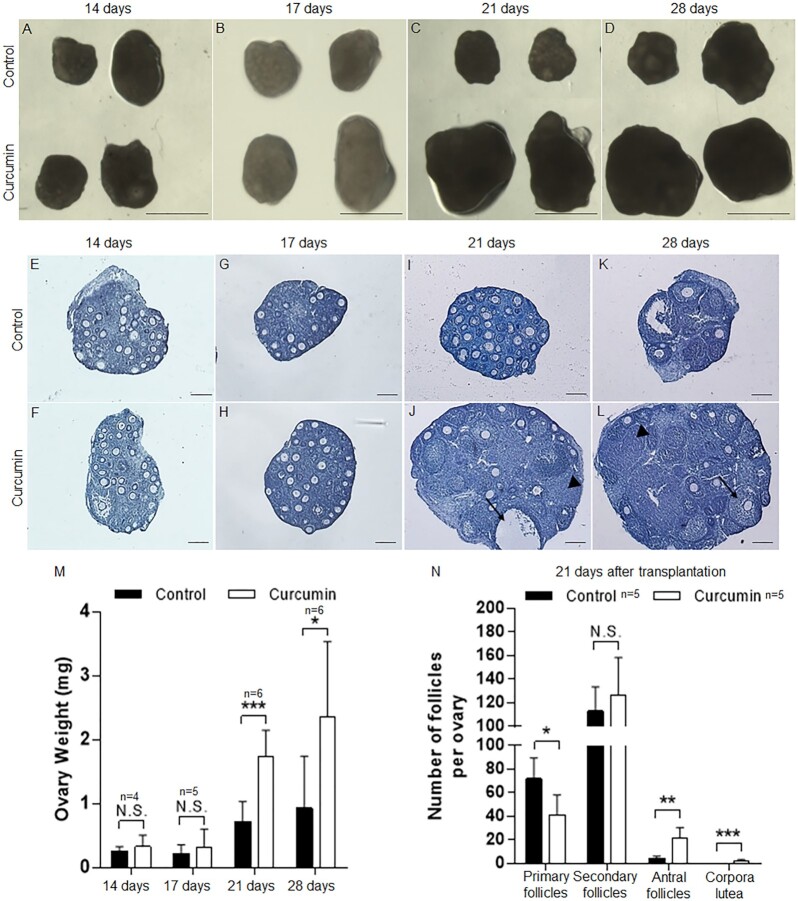
In order to study the physiological significance of in-vitro curcumin treatment in mouse ovaries, we transplanted the curcumin-treated mouse ovaries under the kidney capsules of ovariectomized adult female recipient mice, as previously described (44). We separated paired ovaries and treated them with or without curcumin for 6 d in culture, followed by transplanting the ovaries under the kidney capsules of the recipient mice. These ovaries were then collected after 14, 17, 21, and 28 d of engraftment (n = 4 mice for 14 d of engraftment; n = 5 mice for 17 d of engraftment; n = 6 mice for 21 d of engraftment; and n = 6 mice for 28 d of engraftment, with one or two recipient mice per experiment and two or three ovaries transplanted under each kidney capsule). As shown in Fig. 2A and B, after 14 or 17 d of transplantation no obvious differences were seen in the size of the transplanted ovaries between the control group and the curcumin-treated group. However, the ovarian volumes were significantly increased in the curcumin-treated ovaries after 21 and 28 d of transplantation compared to the control ovaries (Fig. 2C and D). As quantified in Fig. 2M, after 14 or 17 d of engraftment the ovarian weights were similar for the curcumin-treated group and the control group. Nevertheless, after 21 and 28 d of engraftment, greater ovarian weights were observed in the curcumin-treated ovarian group compared to the control group (P = 6.85e–04, P = 3.31e–02) (Fig. 2M).
Fig. 2.
Increased ovarian and follicular development in vivo after curcumin treatment in vitro. Paired PD7 ovaries were treated with or without curcumin (100 µM) for 6 d followed by transplantation under the kidney capsules of ovariectomized recipient mice. Transplanted ovarian grafts were collected 14 to 28 d later. (A–D) Isolated paired ovaries for curcumin-treated versus controls after transplantation for 14, 17, 21, and 28 d. Scale bars: 1 mm. (E–L) The morphology of curcumin-treated (F, H, J, and L) and control (E, G, I, and K) ovaries after 14 to 28 d of engraftment (arrowheads, corpora lutea; arrows, antral follicles). Hematoxylin staining indicates nuclei. Scale bars: 50 µm. (M) The weight of the ovaries was measured 14 to 28 d after transplantation. (N) Distribution of follicles in ovaries treated with or without curcumin after transplantation for 21 d. Data are shown as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
We examined the follicular development of the embedded ovaries and found that after 14 and 17 d of engraftment follicular development in the curcumin-treated ovaries and control ovaries was similar (Fig. 2E–H), and the majority of follicles in both groups were small follicles and no antral follicles were seen. However, after 21 and 28 d of engraftment, the curcumin treatment had apparently accelerated follicular development, resulting in more antral follicles and notably more corpora lutea (Fig. 2I–L). At 21 d of engraftment, follicle counting of serial ovarian sections showed a 5.1-fold increase in antral follicles in the curcumin-treated group, and corpora lutea were also present in the curcumin-treated group (3 ± 1) compared with the control group (0 ± 0) (Fig. 2N). Moreover, a decreased number of primary follicles was seen in the curcumin-treated group (42 ± 17) compared with the control group (72 ± 20) (Fig. 2N), implying that more primary follicles may have proceeded to develop into antral follicles and corpora lutea.
These results together suggest that in-vitro treatment of mouse ovaries with curcumin at the small follicle stage can potentiate the follicles for several weeks for further development into advanced stages, suggesting the potential clinical use of in-vitro curcumin treatment in promoting the development of small follicles in human ovaries.
Curcumin promotes the proliferation of mouse ovarian granulosa cells and suppresses their apoptosis
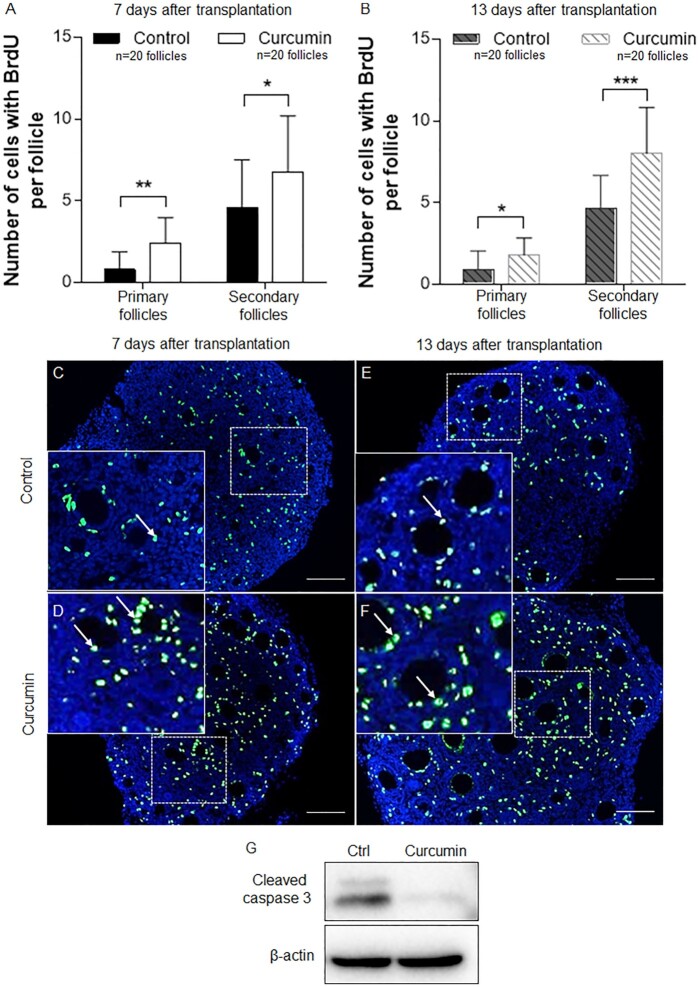
The differentiation and proliferation of granulosa cells are essential for follicular development (1), so we examined the effect of in-vitro curcumin treatment on in-vivo granulosa cell proliferation using a BrdU incorporation assay. We separated paired ovaries from PD7 mice and cultured them with or without curcumin (100 µM) for 6 d and then transplanted the cultured ovaries under the kidney capsules of ovariectomized adult female mice as described above. After 7 and 13 d of engraftment, the recipient mice were given an i.p. injection of BrdU (120 mg/kg) 2 h prior to being sacrificed. The ovaries were then collected and fixed for BrdU staining.
As shown in Fig. 3A–F, after 7 and 13 d of transplantation greater numbers of BrdU-positive granulosa cells were seen in the curcumin-treated ovaries (Fig. 3D and F) compared to the control group (Fig. 3C and E). Quantitative analyses showed significantly greater numbers of BrdU-positive cells in the curcumin-treated groups at both 7 and 13 d after the embedding (Fig. 3A and B). At 7 d after the embedding, the control group had 0.8 ± 1.0 and 4.6 ± 2.9 BrdU-positive cells per follicle in primary and secondary follicles, respectively, while the curcumin-treated group had 2.4 ± 1.6 and 6.8 ± 3.4 BrdU-positive cells per primary and secondary follicle, respectively (Fig. 3A). Likewise, at 13 d after transplantation, primary and secondary follicles contained 1.8 ± 1.0 and 8.0 ± 2.8 BrdU-positive cells per follicle, respectively, in the curcumin-treated ovaries, and these were significantly higher than the 0.9 ± 1.1 and 4.7 ± 2.0 BrdU-positive cells per follicle in primary and secondary follicles of the control ovaries (Fig. 3B). These results indicate that the in-vitro treatment with curcumin promotes the subsequent in-vivo proliferation of granulosa cells both in primary and secondary follicles for at least 2 weeks.
Fig. 3.
The effect of curcumin on granulosa cell proliferation and ovarian cell apoptosis. (A) The numbers of BrdU-positive granulosa cells in primary and secondary follicles were counted after transplantation for 7 d. (B) The numbers of BrdU-positive granulosa cells in primary and secondary follicles were counted after transplantation for 13 d. (C–F) Immunofluorescence staining for BrdU-positive granulosa cells (arrows) in curcumin-treated (100 µM) and control ovaries is shown. BrdU-positive staining for curcumin (D) and control (C) ovaries after 7 d of transplantation. BrdU-positive staining for curcumin (F) and control (E) ovaries after 13 d of transplantation. Scale bars: 50 µm. (G) Western blot analysis of the expression of cleaved caspase-3. The expression of β-Actin was used as the internal control. Data are shown as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 versus control.
We next evaluated the level of apoptosis in ovarian cells after culture with or without curcumin. We cultured ovaries from PD7 mice, and after 6 d, the ovaries were collected for Western blot. Our results showed that curcumin treatment significantly reduced the level of cleaved caspase-3, an apoptosis marker (Fig. 3G), suggesting that curcumin may suppress apoptosis in ovarian cells.
In summary, the above results indicate that curcumin promotes the proliferation of granulosa cells and reduces the apoptosis of ovarian cells.
Curcumin promotes mouse follicular development by stimulating PI3K signaling and suppressing mTORC1 signaling in granulosa cells
In order to determine how curcumin might promote the transition from primary to secondary follicles, we examined two signaling pathways that are crucial to the growth of follicles, namely the PI3K signaling pathway and the mTORC1 signaling pathway (6). We obtained granulosa cells from ovaries of PD13 mice by digesting with collagenase as described previously (45, 46). Different from our previous studies where we activated or suppressed mTOR signaling in pregranulosa cells of primordial follicles (9), here most of the granulosa cells came from primary and secondary follicles. The granulosa cells were first cultured in FBS-free medium for 24 h to starve the cells. The cells were then treated with curcumin (100 µM) for 10, 20, or 60 min before the cells were chilled on ice and lysed. As shown in Fig. 4A, Western blot analyses showed that the levels of phosphorylated Akt (indicating an activated PI3K signaling pathway) were significantly elevated at 10 min after curcumin treatment, and they reached maximum levels at 20 min (Fig. 4A). The level of phosphorylated Foxo3 was also elevated at 20 min after curcumin treatment (Fig. 4A). At 60 min after curcumin treatment, the levels of both phosphorylated Akt and phosphorylated Foxo3 decreased (Fig. 4A) showing a typical temporary phosphorylation pattern similar to stimulation with growth factors (45). These results showed that curcumin activated the PI3K signaling pathway in the granulosa cells of primary and secondary follicles in mice.
Fig. 4.
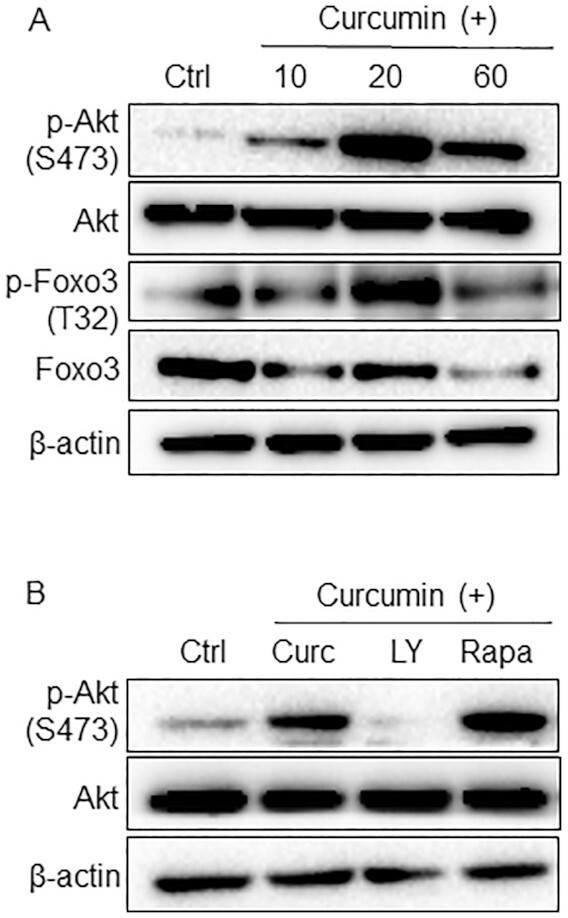
Activation of the PI3K signaling pathway in granulosa cells after treatment with curcumin. (A) Granulosa cells were collected from PD13 mouse ovaries and treated with or without curcumin (100 µM) for 10, 20, and 60 min. Western blot of granulosa cell proteins with specific antibodies against p-Akt (S473), Akt, p-Foxo3 (T32), Foxo3, and β-Actin. β-Actin was used as the internal control. (B) Granulosa cells were cultured with LY294002 (10 µM) or rapamycin (10 µM) for 1 h and treated with curcumin for 20 min. The levels of p-Akt (S473) and Akt were analyzed by Western blotting. The experiments were repeated at least three times.
We also measured the activation of the mTORC1signaling pathway. As previously reported, mTORC1 activates p70 S6 kinase 1 (S6K1) and ribosomal protein S6 (rpS6) and inactivates eukaryotic translation initiation factor 4E (4E-BPs) (47). Curcumin significantly decreases the phosphorylation levels of S6K1, rpS6, and 4E-BP1 (Supplementary Fig. S3), showing that curcumin suppresses the activation of mTORC1 signaling in the granulosa cells of primary and secondary follicles. The role of the mTORC signaling pathway in regulating the differentiation of helper T cell has been widely reported (48, 49), indicating a promoting role of the mTORC signaling pathway in cell differentiation. In our study, the proliferation of granulosa cells in primary and secondary follicles is the most important. The possible reason for the inhibition of the mTORC1 signaling pathway after curcumin treatment could be that it can inhibit the differentiation of granulosa cells, thus enabling granulosa cells to proliferate better.
We then pretreated the cultured granulosa cells with the PI3K inhibitor LY294002 and the mTORC1 inhibitor rapamycin before curcumin was added. Western blot analyses showed that the elevated level of phosphorylated Akt was completely suppressed by LY294002 (Fig. 4B), showing that the activation of Akt by curcumin was solely achieved through the PI3K pathway. However, rapamycin showed no effect on curcumin-stimulated Akt phosphorylation (Fig. 4B). These results further indicate that curcumin promotes follicular development through the PI3K signaling pathway rather than the mTORC1 signaling pathway.
Curcumin enhances the survival and development of small ovarian follicles in humans
In the present study, we demonstrated that initial in-vitro treatment of cultured mouse ovaries with curcumin enhanced subsequent in-vivo development of ovarian follicles, and this effect was mediated through the PI3K signaling pathway in granulosa cells. We wondered, then, whether curcumin might also affect follicular development in human ovaries. Due to difficulties in obtaining adult human ovarian tissues containing small follicles for study, we used the ovaries of fetuses at 16 to 23 weeks’ gestation from the second trimester medical abortion for non-medical reasons. These stages of fetal ovaries contain numerous female germ cells that are about to form primordial follicles at around 18 weeks of gestation (50, 51). Thus, fetal ovaries can provide materials for studying how curcumin might affect the development of human follicles.
After obtaining the fetal ovarian tissues, we cut them into ∼1 mm3 cubes and cultured them in DMEM/F-12 medium supplemented with 1% ITS and 3 mg/ml BSA with or without curcumin for 6 d. After culturing, the tissues were transplanted under the kidney capsules of ovariectomized female SCID mice for 3 to 6 months, with i.p. injections of FSH (2 IU per animal) every 36 h to facilitate the growth of small human follicles (10, 52). The embedded human ovarian tissues were collected at 3 to 6 months after transplantation.
We observed the formation of ovarian follicles in four fetal ovarian samples from 16, 23, 17, and 20 weeks’ gestation after 3 to 6 months of engraftment under the kidney capsules of ovariectomized female SCID mice. However, another four samples (16, 23, 19, and 22 gestational weeks) showed no follicular structures and were abandoned. The reason for the samples with no follicles could be that these samples were in worse condition when collected from surgery.
In the four fetal ovarian samples that showed the formation of follicular structures, three samples (16, 23, and 17 gestational weeks) exhibited more activated follicles after curcumin treatment, while the remaining sample (20 gestational weeks) showed no significant difference in follicular numbers in the curcumin-treated group compared to the control group. The results are described in detail below and in the Supplementary Information.
The first case originated from a fetal ovary at 16 gestational weeks. After treating with curcumin for 6 d in culture followed by 6 months of engraftment under the kidney capsules of SCID mice, the sizes of the ovarian tissues in the curcumin-treated group were comparable to those of the control group (Fig. 5A) with similar tissue weights (Fig. 5B). As shown by histological analysis, more activated follicles were seen in the curcumin-treated ovarian tissues compared to the control tissues (Fig. 5C and D, arrowheads). Similar results were also obtained by immunofluorescence staining of the germ cell marker mouse vasa homologue (MVH), and more oocytes were seen compared to the control tissues (Fig. 5E and F, arrows). The relative average numbers of follicles counted in serial sections of each tissue showed more follicles in the curcumin-treated group, including 2 times the number of primordial follicles, 5.5 times the number of primary follicles, and 4.5 times the number of secondary follicles in the curcumin-treated group compared to the control group (Fig. 5G). In addition, the average number of total follicles per tissue in the curcumin-treated group was 3.7 times the number in the control group (Fig. 5G). Curcumin seemed to promote both the development and survival of primordial, primary, and secondary follicles in this sample. In addition, the other two samples (23 and 17 gestational weeks) also showed that curcumin can potentially promote the survival and subsequent development of small follicles (Suppelementary Figs. S4 and S5), with a detailed description in the Supplementary Information.
Fig. 5.
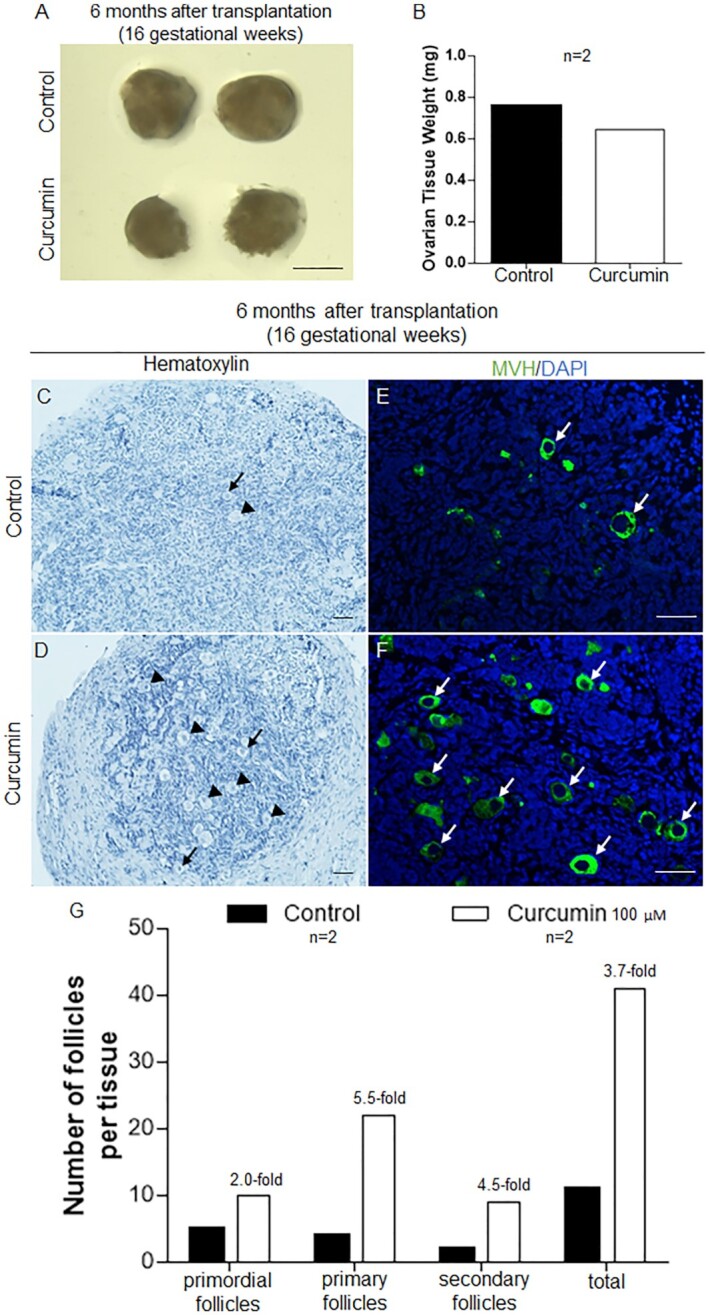
Curcumin treatment enhanced the survival and development of human follicles in the 16 gestational weeks fetal ovarian tissues. Ovarian fragments (16 gestational weeks) were carefully cut into small cubes, treated with or without curcumin (100 µM) for 6 d, and transplanted under the kidney capsule of ovariectomized SCID mice. (A) Isolated ovarian tissues for curcumin-treated versus controls after transplantation for 6 months. Scale bars: 1 mm. (B) The weight of the ovarian tissues was measured 6 months after transplantation. (C and D) The morphology of curcumin-treated (D) and control (C) ovarian tissues after 6 months of engraftment (arrowheads, growing follicles; arrows, primordial follicles). Hematoxylin staining indicates nuclei. Scale bars: 50 µm. (E and F) Immunofluorescent labeling of germ cells showing oocytes in the control (E) and curcumin-treated (F) tissues (arrows, oocytes; green: MVH, blue: DAPI). Scale bars: 50 µm. (G) Distribution of follicles in ovarian tissues treated with or without curcumin after transplantation for 6 months.
In the four fetal ovarian samples that showed formation of follicular structures, three samples (16, 23, and 17 gestational weeks) (Fig. 5, Supplementary Figs. S4 and S5) exhibited more activated follicles after curcumin treatment. However, in the remaining sample (20 gestational weeks), follicle development and survival of the curcumin-treated group were not found to be enhanced (Supplementary Data, Fig. S6). We believe that this is due to individual differences. This also reminds us that further experiments with adult ovarian samples are needed in future studies.
The above results suggest that like in the case of cultured and grafted mouse ovaries, curcumin can potentially promote the survival and subsequent development of small follicles, including primordial follicles, primary follicles, and secondary follicles, in human ovaries.
Discussion
Assisted reproduction is an effective treatment for patients with different causes of infertility. However, management of women with POR or POI is still challenging because these women have mostly underdeveloped small ovarian follicles that do not respond to hormone treatment. Based on studies that the PI3K/PTEN signaling network controls the activation of primordial follicles (7, 8), IVA has been developed to assist in the assisted reproduction of women with POR or POI by activating their small follicles in vitro. Researchers also disrupted the Hippo signaling pathway by fragmenting ovaries followed by a PTEN inhibitor (bpv) and PI3K activator (740Y-P) for treating infertility in women with POI (10). However, the activation of underdeveloped small ovarian follicles is still at its initial stage, and more mechanistic studies as well as more clinical treatments using small follicles for fertility purposes are very much needed.
In this study, we first showed that curcumin can promote the transition of primary follicles into secondary follicles in mice. Importantly, we showed that the in-vitro treatment of cultured mouse ovarian tissues with curcumin can potentiate the future development of ovarian follicles into antral follicles in vivo even when prior curcumin treatment is stopped for several weeks, implying that curcumin may be an effective in-vitro treatment for promoting the development of small follicles in women with POR or even POI. Indeed, our results showed that, like in the case of mouse ovaries, curcumin can enhance the survival and development of small human ovarian follicles. These findings suggest that curcumin might be useful as a drug for the in-vitro treatment of cultured human ovarian tissue and might enhance the development of follicles after retransplantation back into patients. The pharmacological safety of curcumin for human consumption was approved by the FDA and NMPA, making curcumin a safe drug for the treatment of female infertility in POR and POI patients.
In recent years, the use of curcumin has attracted some attentions due to its antioxidative, anti-inflammatory, and anti-carcinogenic properties (22–25). Previous studies have provided incomplete and even contradictory information on the role of curcumin in follicular development in different animal models (34, 35), and its mechanisms of action remain poorly understood (31, 34). Our study provides the first systematic evaluation of how curcumin might affect ovarian follicular development and suggests that curcumin affects follicular growth through activation of the PI3K signaling pathway in the granulosa cells. Importantly, we propose that curcumin might be used as a novel drug to promote the growth of small human ovarian follicles and thus to increase the success rate of current IVA of small human follicles.
In addition, our results showed that i.p. injection of curcumin (100 mg/kg) was not an effective way of administrating curcumin because of apparent precipitation of the dissolved curcumin as yellow solid clumps in the abdominal cavities of the recipient mice. Using such i.p. administration, Lv et al. reported that more primordial follicles were seen in the curcumin-treated group. The discrepancy between our results and those of Lv et al. may be due to differences in follicle counting. Lv et al. counted follicles in one of every five serial paraffin sections, but primordial follicles are not evenly distributed throughout an ovary but are mostly located at the ovarian cortex (2).
In summary, the current study shows that curcumin can effectively promote the development from primary to secondary follicles in cultured mouse ovaries and can potentiate their future development into antral follicles and corpora lutea. More importantly, curcumin can promote the survival and development of small follicles in human ovarian tissues, suggesting that curcumin has the potential to be used as an in-vitro drug to facilitate the clinical treatment of infertility caused by POR and POI.
Supplementary Material
Notes
Competing Interest: The authors declare no competing interests.
Contributor Information
Yu Zhao, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Yihui Zhang, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Dongteng Liu, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Haiwei Feng, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China.
Xiaohui Wang, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China.
Jiajun Su, Department of Anatomical Pathology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China.
Yuanqing Yao, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China.
Ernest H Y Ng, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
William S B Yeung, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Raymond H W Li, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Kenny A Rodriguez-Wallberg, Department of Oncology–Pathology, The Karolinska Institute, Stockholm 14186, Sweden.
Kui Liu, Shenzhen Key Laboratory of Fertility Regulation, Center of Assisted Reproduction and Embryology, The University of Hong Kong—Shenzhen Hospital, Haiyuan First Road 1, Shenzhen, Guangdong 518053, China; Department of Obstetrics and Gynecology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR 999077, China.
Funding
This work was supported by the Shenzhen–Hong Kong Innovation Circle Type D (SGDX2019081623080755 to K.L.), the Shenzhen Science and Technology Program (KQTD20190929172749226 to K.L.), the Sanming Project of Medicine in Shenzhen, China (SZSM201612083), the Shenzhen Science and Technology Program (Grant No. RCBS20200714114941343), the Guangdong Basic and Applied Basic Research Foundation, China (No. 2021A1515110762), the National Natural Science Foundation of China and the Swedish Research Council Collaboration Research Programme (NSFC-VR 8211101255 to K.L. and K.R.W), the National Natural Science Foundation of China (82071706 to K.L.).
Authors’ contributions
K.L. was responsible for the study design and conception; Y.Z., Y.Z., D.L., and H.F. were responsible for data collection and the accuracy of the data; Y. Z. was responsible for drafting the manuscript; X.W. and J.S. were responsible for human sample collection; and Y.Y., E.H.Y.N., W.S.B.Y., R.H.W.L., K.A., R.-W., and K.L. were responsible for critically revising the manuscript for important intellectual content. All authors approved the final version to be published.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. McGee EA, Hsueh AJ. 2000. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 21(2):200–214. [DOI] [PubMed] [Google Scholar]
- 2. Adhikari D, Liu K. 2009. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 30(5):438–464. [DOI] [PubMed] [Google Scholar]
- 3. Skinner MK. 2005. Regulation of primordial follicle assembly and development. Hum Reprod Update. 11(5):461–471. [DOI] [PubMed] [Google Scholar]
- 4. Broekmans FJ, et al. 2007. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 18(2):58–65. [DOI] [PubMed] [Google Scholar]
- 5. Zhang H, Liu K. 2015. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 21(6):779–786. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y, et al. 2021. Current understandings of core pathways for the activation of mammalian primordial follicles. Cells. 10(6):1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castrillon DH, et al. 2003. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 301(5630):215–218. [DOI] [PubMed] [Google Scholar]
- 8. Reddy P, et al. 2008. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 319(5863):611–613. [DOI] [PubMed] [Google Scholar]
- 9. Zhang H, et al. 2014. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 24(21):2501–2508. [DOI] [PubMed] [Google Scholar]
- 10. Kawamura K, et al. 2013. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 110(43):17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah JS, et al. 2018. Biomechanics and mechanical signaling in the ovary: a systematic review. J Assist Reprod Genet. 35(7):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SY, Kurita T. 2018. New insights into the role of phosphoinositide 3-kinase activity in the physiology of immature oocytes: lessons from recent mouse model studies. Eur Med J Reprod Health. 3(2):119–125. [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, et al. 2018. MAPK3/1 participates in the activation of primordial follicles through mTORC1-KITL signaling. J Cell Physiol. 233(1):26–237. [DOI] [PubMed] [Google Scholar]
- 14. Kawamura K, Kawamura N, Hsueh AJ. 2016. Activation of dormant follicles: a new treatment for premature ovarian failure?. Curr Opin Obstet Gynecol. 28(3):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki N, et al. 2015. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 30:608–615. [DOI] [PubMed] [Google Scholar]
- 16. Zhai J, et al. 2016. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 101:4405–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabregues F, et al. 2018. Pregnancy after drug-free in vitro activation of follicles and fresh tissue autotransplantation in primary ovarian insufficiency patient: a case report and literature review. J Ovarian Res. 11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawamura K, Ishizuka B, Hsueh AJW. 2020. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod Biomed Online. 40(2):245–253. [DOI] [PubMed] [Google Scholar]
- 19. Ferreri J, et al. 2020. Drug-free in-vitro activation of follicles and fresh tissue autotransplantation as a therapeutic option in patients with primary ovarian insufficiency. Reprod Biomed Online. 40(2):254–260. [DOI] [PubMed] [Google Scholar]
- 20. Sharma R, Gescher A, Steward W. 2005. Curcumin: the story so far. Eur J Cancer. 41(13):1955–1968. [DOI] [PubMed] [Google Scholar]
- 21. Park J, Conteas CN. 2010. Anti-carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol. 2(4):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trujillo J, et al. 2013. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 1:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aggarwal BB, Harikumar KB. 2009. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 41(1):40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swamy MV, et al. 2008. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer. 60:81–89. [DOI] [PubMed] [Google Scholar]
- 25. Wilken R, et al. 2011. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ammon HP, Wahl MA. 1991. Pharmacology of Curcuma longa. Planta Med. 57(1):1–7. [DOI] [PubMed] [Google Scholar]
- 27. FDA . 2018. GRAS Notice (GRN) No. 822. [Accessed 2022 Jul 16]. https://www.fda.gov/media/132575/download. [Google Scholar]
- 28. NMPA . 2020. National medicine permission number Z20040032. [Accessed 2022 Jul 16]. https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9MTQwMjY2Jml0ZW1JZD1mZjgwODA4MTdjODMxMmM0MDE3YzliYmZjOGRlMDM2MA==. [Google Scholar]
- 29. Aktas C, Kanter M, Kocak Z. 2012. Antiapoptotic and proliferative activity of curcumin on ovarian follicles in mice exposed to whole body ionizing radiation. Toxicol Ind Health. 28(9):852–863. [DOI] [PubMed] [Google Scholar]
- 30. Melekoglu R, et al. 2018. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yan Z, et al. 2018. Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol. 60(3):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sak ME, et al. 2013. The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int J Surg. 11(9):967–970. [DOI] [PubMed] [Google Scholar]
- 33. Eser A, et al. 2015. Effects of curcumin on ovarian ischemia-reperfusion injury in a rat model. Biomed Rep. 3(6):807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lv Y, et al. 2021. Single-oocyte gene expression suggests that curcumin can protect the ovarian reserve by regulating the PTEN-AKT-FOXO3a pathway. Int J Mol Sci. 22(12):6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sirotkin AV, et al. 2018. Effect of turmeric on the viability, ovarian folliculogenesis, fecundity, ovarian hormones and response to luteinizing hormone of rabbits. Animal. 12(6):1242–1249. [DOI] [PubMed] [Google Scholar]
- 36. Moreira-Pinto B, et al. 2020. Dissimilar effects of curcumin on human granulosa cells: beyond its anti-oxidative role. Reprod Toxicol. 95:51–58. [DOI] [PubMed] [Google Scholar]
- 37. Cheng AL, et al. 2001. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 21(4B):2895–2900. [PubMed] [Google Scholar]
- 38. Sharma RA, et al. 2001. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin Cancer Res. 7(7):1894–1900. [PubMed] [Google Scholar]
- 39. Sasaki H, et al. 2011. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 34(5):660–665. [DOI] [PubMed] [Google Scholar]
- 40. Johnson J, et al. 2004. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 428(6979):145–150. [DOI] [PubMed] [Google Scholar]
- 41. Li M, et al. 2019. The histone modification reader ZCWPW1 is required for meiosis prophase I in male but not in female mice. Sci Adv. 5(8):eaax1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pan MH, Huang TM, Lin JK. 1999. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 27(4):486–494. [PubMed] [Google Scholar]
- 43. Begum AN, et al. 2008. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 326(1):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang H, et al. 2015. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 21(10):1116–1118. [DOI] [PubMed] [Google Scholar]
- 45. Reddy P, et al. 2005. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 281(2):160–170. [DOI] [PubMed] [Google Scholar]
- 46. Wang J, et al. 2019. Procr-expressing progenitor cells are responsible for murine ovulatory rupture repair of ovarian surface epithelium. Nat Commun. 10(1):4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fingar DC, Blenis J. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 23(18):3151–3171. [DOI] [PubMed] [Google Scholar]
- 48. Delgoffe GM, et al. 2011. The mammalian Target of Rapamycin (mTOR) regulates T helper cell differentiation through the selective activation of mTORC1 and mTORC2 signaling. Nat Immunol. 12(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng H, et al. 2016. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity. 45(3):540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bayne RA, Martins da Silva SJ, Anderson RA. 2004. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod. 10(6):373–381. [DOI] [PubMed] [Google Scholar]
- 51. Maheshwari A, Fowler PA. 2008. Primordial follicular assembly in humans—revisited. Zygote. 16(4):285–296. [DOI] [PubMed] [Google Scholar]
- 52. Li J, et al. 2010. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 107(22):10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.