Abstract
Hypertension is a major contributor to the global burden of disease. Unfortunately, hypertension is controlled in less than one fifth of patients worldwide due to either failure to treat or lack of compliance to medication. An ideal therapy would be administered one time only and yield life-long blood pressure control. We investigated our hypothesis that Crispr-Cas9 mediated disruption of a key gene in the Renin-Angiotensin-System (RAS), Angiotensinogen (AGT), specifically in the liver, would result in sustained, and possibly life long, reduction in blood pressure. We demonstrated in vitro, that the Crispr-Cas9 system led to a significant reduction in AGT expression in hepatocytes. Delivery of the Crispr-Cas9 system into the liver via the hepatocyte-targeting AAV8 reduced both AGT expression (40% decrease) and circulating AGT levels (30% decrease). In the Spontaneously Hypertensive Rat (SHR) model of hypertension, Crispr-Cas9 mediated loss of AGT expression reduced blood pressure in adult animals with established hypertension and prevented the spontaneous development of hypertension in young SHR. Moreover, reductions in blood pressure were prolonged and sustained up to one year of follow up. In addition, the partial disruption of the hepatic AGT gene was sufficient to control hypertension but did not affect the homeostatic response to cardiovascular stress such as sodium depletion and furosemide. In summary, we have demonstrated that targeting the Crispr-Cas9 system to hepatic AGT results in sustained reduction of blood pressure and is a potential therapy to achieve sustained and possibly life-long control of human hypertension.
Keywords: Hypertension, Gene-editing, Angiotensinogen, RAAS, Liver, AAV
Introduction
Hypertension affects more than one billion people worldwide. Complications of hypertension includes stroke, renal failure, cardiac hypertrophy, myocardial infarction and cardiac failure. Despite the development of effective antihypertensive therapies, the number of people with uncontrolled hypertension continues to rise 1 due to either failure to treat or lack of compliance to medication. The latter is often due to the inconvenience of having to take multiple drugs, several times daily or with frequent side effects. Consequently, there has been much interest in developing new therapies that can provide sustained control of hypertension with fewer side effects. With this in mind, studies using antisense technology targeting AGT were employed with some degree of success 2 however, antisense molecules are rapidly degraded by endonucleases and this approach may require frequent antisense administration. Indeed, initial studies using natural antisense or deoxyphosphorothioated antisense targeting AGT resulted in transient decreases in blood pressure3–5. In a recent study with state-of-the-art modifications to stabilize the antisense molecules6, blood pressure reduction for 2–3 weeks was possible. While this is an improvement over past antisense approaches or current pharmaceutical approaches, it would still require several treatments each month. In addition, siRNA mediated silencing of genes involved in hypertension has also been investigated. In the short term, studies with direct injection of siRNAs were found to be moderately successful 7. Viral based methods led to prolong siRNA expression and more sustained blood pressure reduction. To date, injection of AAVs carrying siRNAs that target Angiotensinogen 8, 9; β1 Adrenergic receptor 10; Angiotensinogen-II Type-1 receptor 11; Endothelin-1 12 and several others13–18, have all been demonstrated to significantly reduce blood pressure; however, the need for AAV persistence to maintain siRNA expression is problematic as the AAV can be lost during cell proliferation 19. Moreover, despite longer term blood pressure reduction, siRNAs would still be considered a treatment, not a cure.
In contrast to pharmacological, antisense or siRNA-based approaches, gene ablation offers the possibility of a single and sustained treatment for hypertension. For a therapeutic modality, gene ablation has to be efficient, reliable and precise. The necessary efficiency, reliability and precision is found with the Crispr-Cas9 gene editing system. To date, the Crispr-Cas9 system has been used in a limited way in the hypertension field to investigate the effect of single nucleotide polymorphisms on hypertension 20, 21; as well as several genes such as CPI-17 and Gper with variable and modest effects on blood pressure 22–25.
In this study, we wanted to determine the effects of targeting the Crispr-Cas9 system to the Renin–Angiotensin system (RAS). To that end, we first demonstrated effective Crispr-Cas9 targeting of Angiotensinogen (AGT) in vitro. In vivo, effective Crispr-Cas9 targeting of Angiotensinogen (40% ablation) in liver hepatocytes was associated with significant reductions of blood pressure in the SHR. The effects on blood pressure were sustained and prolonged. Importantly, the partial disruption of the hepatic AGT gene was sufficient to control hypertension but did not block the homeostatic response to cardiovascular stress such as sodium depletion and furosemide. In summary, we are the first to demonstrate that targeting the Crispr-Cas9 to RAS is an effective, and safe method for sustained reduction in blood pressure.
Methods
Transparency and Openness Promotion (TOP):
This manuscript was prepared in accordance with the AHA Transparency and Openness Promotion (TOP) Guidelines.
Animal models:
Wistar Kyoto (WKY) and Spontaneously Hypertensive (SHR) rats were purchased from Charles River (Wilmington, MA, USA). All studies were approved by the Duke University Division of Laboratory Animals (DLAR) and the Duke Institutional Animal Care and Use Committee (IACUC). Protocol number is A056-19-03.
Crispr-Cas9:
sgRNA sequences were designed using online software provided by Benchling (See next section for sequences). Plasmids: pX601-AAV-CMV: NLS-SaCas9-NLS-3xHA-bGHpA; U6: BsaI-sgRNA and pX601-GFP were obtained from Addgene. The paired gRNAs were synthesized and annealed according to standard protocols. Once annealed, the gRNAs were cloned into the vector by using BsaI restriction site.
Primer sequences used in this study:
gRNA1-forward 5’CACCGTGCTGTAGTAGAGGAGATGAA3’; gRNA1-reverse 5’AAACTTCATCTCCTCTACTACAGCAC3’; gRNA2-forward 5’CACCGGTCTGGCTGCTGCTTCCACC3’; gRNA2-reverse: 5’AAACGGTGGAAGCAGCAGCCAGACC 3’; gRNA3-forward 5’CACCGTAGTAGAGGAGATGAAAGGG3’; gRNA3-reverse: 5’AAACCCCTTTCATCTCCTCTACTAC3’; T7-forward: 5’AAGCAAGTCCACAGATCCGTGA3’; T7-reverse: 5’CTCTGTCCCTCTCACGCATGAA3’; ITR primer-forward: 5’GGAACCCCTAGTGATGGAGTT3’; ITR primer-reverse: 5’CGGCCTCAGTGAGCGA3’.
BRL 3A rat liver cell line culture:
BRL 3A cell line was purchased from ATCC (ATCC® CRL-1442). The cells were cultured in DMEM (ThermoFisher) supplemented with 10% fetal bovine serum (ThermoFisher). Cells were used between passages 3 and 5.
Transfection in vitro:
BRL3A cells were transfected with pX601-GFP plasmids containing the gRNAs via Lipofectamine® 2000 Reagent (ThermoFisher). The transfection was carried out according to the manufacturer’s instructions.
Genomic DNA isolation:
Genomic DNA was purified by DNeasy Blood & Tissue Kits following the manufacturer’s instructions (Genessee). DNA was amplified by Q5® Hot Start High-Fidelity 2X Master Mix (NEB) according to the manufacturer’s instructions.
Indel assay:
The ~1 kb amplicons of target sites were purified and digested by T7 Endonuclease I (NEB) following the manufacturer’s recommendations. Digestions were then electrophoresed on a 2%w/v agarose gel and imaged on a gel imager (Bio-Rad).
Immunoblotting:
Rat liver was isolated, cut into small pieces, and minced via a turrax homogenizer. Following homogenization, the minced tissue was lysed by adding 1/10th volume of 10x lysis buffer (625mM Tris pH7.4; 10% v/v SDS; 10x Protease Inhibitor Cocktail-I (Sigma)). Samples were lysed by repeated passaging through a 25-guage needle. The supernatant containing the cellular protein was then cleared of insoluble debris via centrifugation (14,000g, 4°C, 10 min). The Bradford assay method was used to determine the protein concentration. Cellular protein (20μg per sample) was first electrophoresed on 4–12% SDS-PAGE gel (ThermoFisher) and then transferred to a PVDF membrane. The PVDF membrane was then incubated with a anti-AGT antibody (1:1000, Abcam) in antibody incubation buffer (5% w/v fat-free milk powder, 20mM Tris-HCl pH7.4, 133mM NaCl). An anti-GAPDH monoclonal antibody (1:2000; Cell signaling technology) was used as an internal loading control. Proteins were visualized via ECL-Prime (GE Healthcare).
Delivery of the gene-editing machinery in vivo:
To generate AAV8 viral particles, 293T cells in a 15cm dish (1 million cells, culture media: DMEM supplemented with 10%v/v fetal bovine serum) were transfected with pAAV2/8 (Addgene #112864), pAdDeltaF6 (Addgene #112867), and pX601 (Addgene #61591). Seventy-two hours later, media was removed and AAV particles were purified by Iodixanol gradient ultracentrifugation method (Millipore Sigma) according to the manufacturer’s instructions. The viral particles were further concentrated by Amicon® Ultra-15 centrifugal filter units (Millipore Sigma) according to the manufacturer’s instructions. Viral titers were determined via a PCR based method utilizing the primers described above. The AAV8 viral particles (2.0×1012 genome copies/rat diluted in 400μl PBS) were injected into a lateral tail vein.
AGT, ANGI, ANGII ELISA assays and renin activity assays:
Whole blood was collected from rat tail vein into tubes with EDTA at pre-set time-point. Blood was centrifugated for 15 minutes at 2,000 x g to collect plasma. AGT ELISA kit was from NOVUS biologicals. Rat renin activity fluorometric assay kit was from BioVision. All assays were performed according to the manufacturer’s instructions.
ANGI and ANGII ELISA kits were purchased from Enzo. The ANG I assay is specific for Ang I, exhibiting very low cross-reactivity with related angiotensin peptides. The Ang II assay does show cross reactivity with degradation products of Ang II (i.e. Ang III and IV which are present at levels significantly lower than that of Ang II, thus the cross reactivity is unimportant) 26.
Blood pressure measurements:
The CODA™ Monitor rat tail-cuff system (Kent Scientific Corporation) was employed to measure the rat blood pressure in accordance with manufacturer’s protocol. Firstly, rat was trained to get used to the measurement one week before AAV injection. They were restrained by clear holders with a dark nosecone and the values were obtained while non-sedated. Three readings were obtained and averaged.
Blinding:
Animal studies were conducted in a blinded fashion. AAV containing either the control or AGT gene-editing machinery was de-identified and given random designations. Information on the relationship between random designation and group identity was held in confidence by an individual not involved in the study. De-identified viruses were injected into the rats by the first author (HS), who also carried out the tail-cuff measurements. Following data collection and archival, statistical analysis was performed blinded. Only after statistical analysis had been performed was the key provided to match the randomly designated group to the identity of the virus.
Low salt diet and furosemide regimen:
animals were provided with NaCl (0.02% w/v) and furosemide (2.28 mmol/L) in their drinking water for 10 days27. The standard diet contained 0.4% NaCl.
Statistical Analysis
All experiments were performed at least 3 times. Each individual experiment was an average of a technical triplicate. Data were analyzed GraphPad Prism Version 5.0. Student’s t-test and ANOVA were used to examine differences between groups. The results were expressed as mean ± standard deviation (SD). Values were considered significantly different if p < 0.05.
Results
In this study we wanted to determine if targeting Angiotensinogen (AGT) gene in the liver with Crispr-Cas9 would reduce blood pressure. Three guide-RNAs (gRNAs) were designed to the first coding exon, exon 2, of the Angiotensinogen gene (Figure 1A). The gRNAs were then sub-cloned into a plasmid vector containing the expression cassette for Cas9. Once sub-cloned, the gRNA-Cas9 constructs were transiently transfected into cultured hepatocytes. Cas9 expression was robust (Figure 1B). All three gRNAs led to efficient cleavage of the AGT gene; however, gRNA-1 displayed the greatest potency (Figure 1C). All future experiments used gRNA-1.
Figure 1. Development of Crispr-Cas9 system for targeting the liver Angiotensinogen-II (AGT) gene.
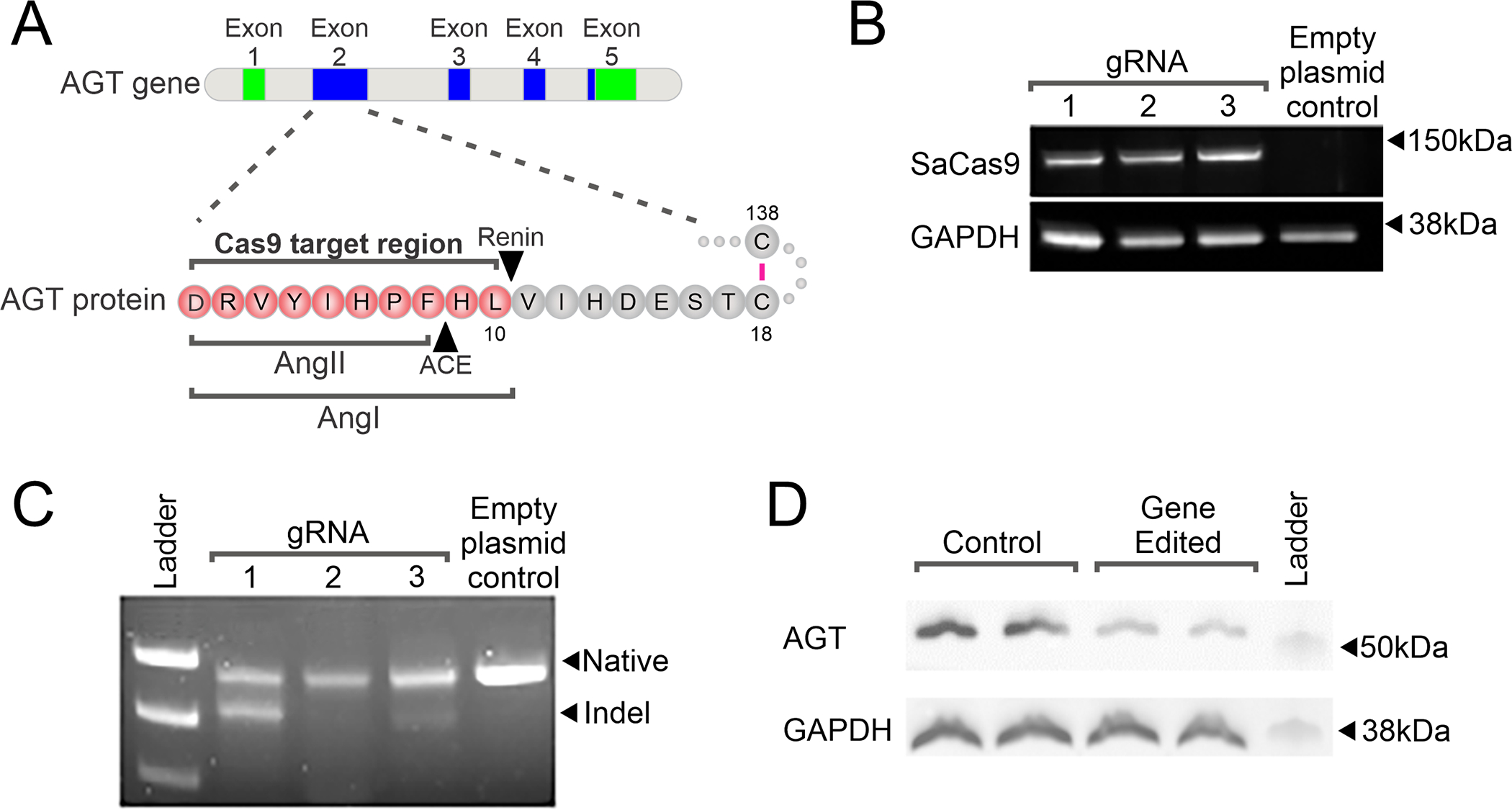
(A) A schematic of the AGT gene and the region targeted by the gRNAs used in this study. Coding exons are shown in green. Exons that give rise to untranslated regions are shown in blue.
(B) Expression of Cas9-gRNA constructs in cultured hepatocytes. gRNAs targeting Cas9 to the AGT gene were cloned into an AAV8 expression plasmid. An empty vector was used as a control. Following infection of hepatocytes with the AAV8-Cas9-AGTgRNA constructs; protein extracts (20μg) were analyzed for expression of the Cas9 protein. GADPH was used as a loading control. Representative images are shown. N=3.
(C) Targeting efficiency of the test gRNA. One week after AAV8-Cas9-AGTgRNA infection, hepatocyte genomic DNA was isolated and analyzed for INDELs (insertions/deletions) via T7 endonuclease. Genomic DNA was amplified using specific primers flanking the cut site in the AGT gene. A representative image is shown. N=3.
(D) AAV8-Cas9-AGTgRNA (2×1012 viral particles) was injected into the tail vein of 12-week old SHR. Control animals received an equivalent dose of AAV8-Cas9. One week after infection, liver tissue was analyzed for the expression of AGT protein (20mg) by immunoblotting. GAPDH was used as a loading control. N=3. Representative image shown.
Various AAV serotypes have been demonstrated to show cell selectivity. We employed one serotype, AAV8, which has been shown to preferentially target hepatocytes 28, 29. To carry out in vivo experiments, the Cas9-gRNA cassette was sub-cloned into an AAV plasmid and AAV8-Cas9-AGTgRNA particles subsequently generated. To measure the effects of hepatic AGT gene targeting on blood pressure, the spontaneously hypertensive rat (SHR) model was used. SHR develop hypertension during adolescence 30. Experiments with a GFP tracer indicated that AAV8 localized expression to the liver hepatocytes and not other tissues (data not shown). Moreover, the viral dose was optimized to give rise to ~50% depletion of circulating AGT levels (Figure S1) as complete disruption of the AGT gene would likely render the SHR devoid of RAS response to stress. Injection of these AAV8-Cas9-AGTgRNA particles into the tail vein was effective in reducing AGT protein levels in the liver (Figure 1D). Following Cas9 cleavage of the genomic DNA the nuclear machinery repairs the damage by removing a random number of nucleotides. When the repair removes three nucleotides (one codon), or multiples thereof, the reading frame will stay in-frame and a protein produced. To validate the immunoblotting results, circulating AGT levels were measured. We found that 2 weeks following injection of the AAV8-Cas9-AGTgRNA particles; liver AGT levels were significantly reduced in both young SHR (Figure 2A) and adult SHR (Figure 2B). Concomitant with a loss in AGT expression, plasma levels of both AngI and AngII were reduced in the gene edited SHR (Figure 2C and 2D). There no effect on plasma renin activity (Figure 2E).
Figure 2. AGT Gene-editing reduces AGT levels.
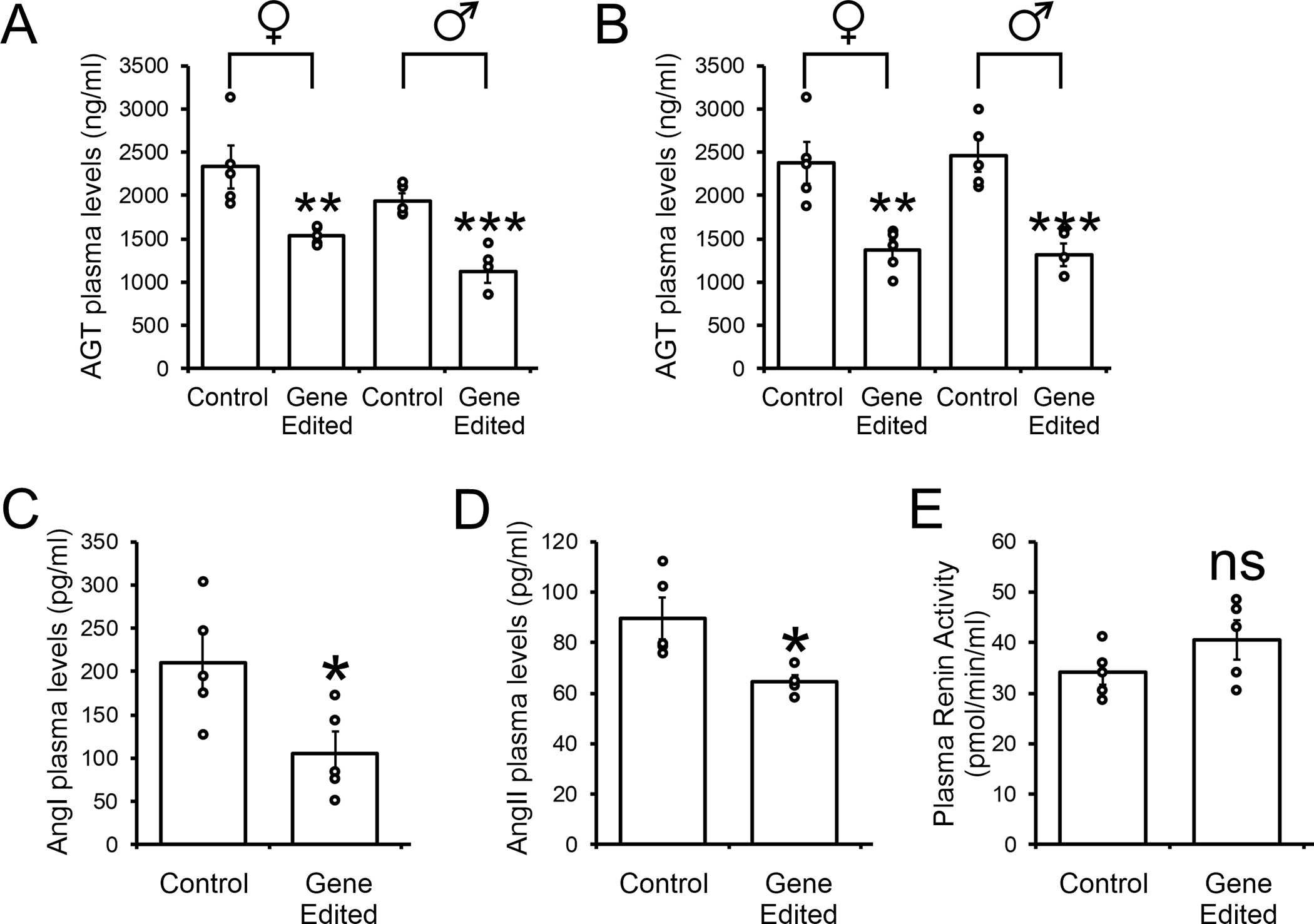
(A and B) AAV8-Cas9-AGTgRNA (2×1012 viral particles) was injected into the tail vein of (A) 5-week old adolescent SHR and (B) 12-week old adult SHR. Control animals received an equivalent dose of AAV8-Cas9. One week after injection, blood was removed by tail-vein and plasma analyzed for AGT levels by ELISA. N=5 per group. Mean ± standard deviation is shown by the bar graph. Individual data points are plotted. Student T-test: **P<0.01, ***P<0.001.
(C, D and E) AAV8-Cas9-AGTgRNA (2×1012 viral particles) was injected into the tail vein of 12-week old adult SHR. Control animals received an equivalent dose of AAV8-Cas9. One week after injection, blood was removed by tail-vein and plasma analyzed for (C) Angiotensinogen-I levels, (D) Angiotensinogen-II levels and (E) Renin activity by ELISA. N=5 per group. Mean ± standard deviation is shown by the bar graph. Individual data points are plotted. Student T-test: *P<0.05, **P<0.01, ***P<0.001
Following the demonstration that Crispr-Cas9 reduced AGT expression we measured the effect on blood pressure. In control SHR, hypertension begins to be apparent around week 5 and progressively increased over time. Notably, injection of AAV8-Cas9-AGTgRNA particles into 5-week old SHR significantly prevented the progression of hypertension in these animals (Figure 3A). This effect was observed in both female and male SHR. Moreover, the effect was stronger on systolic versus diastolic blood pressure (Figure 3A). Importantly, the effects on hypertension were sustained. Significant reductions in blood pressure were still observed 1-year after injection of the AGT gene editing machinery (Figure 3B).
Figure 3. AGT gene editing prevents the acquisition of high blood pressure in the spontaneously hypertensive rat.
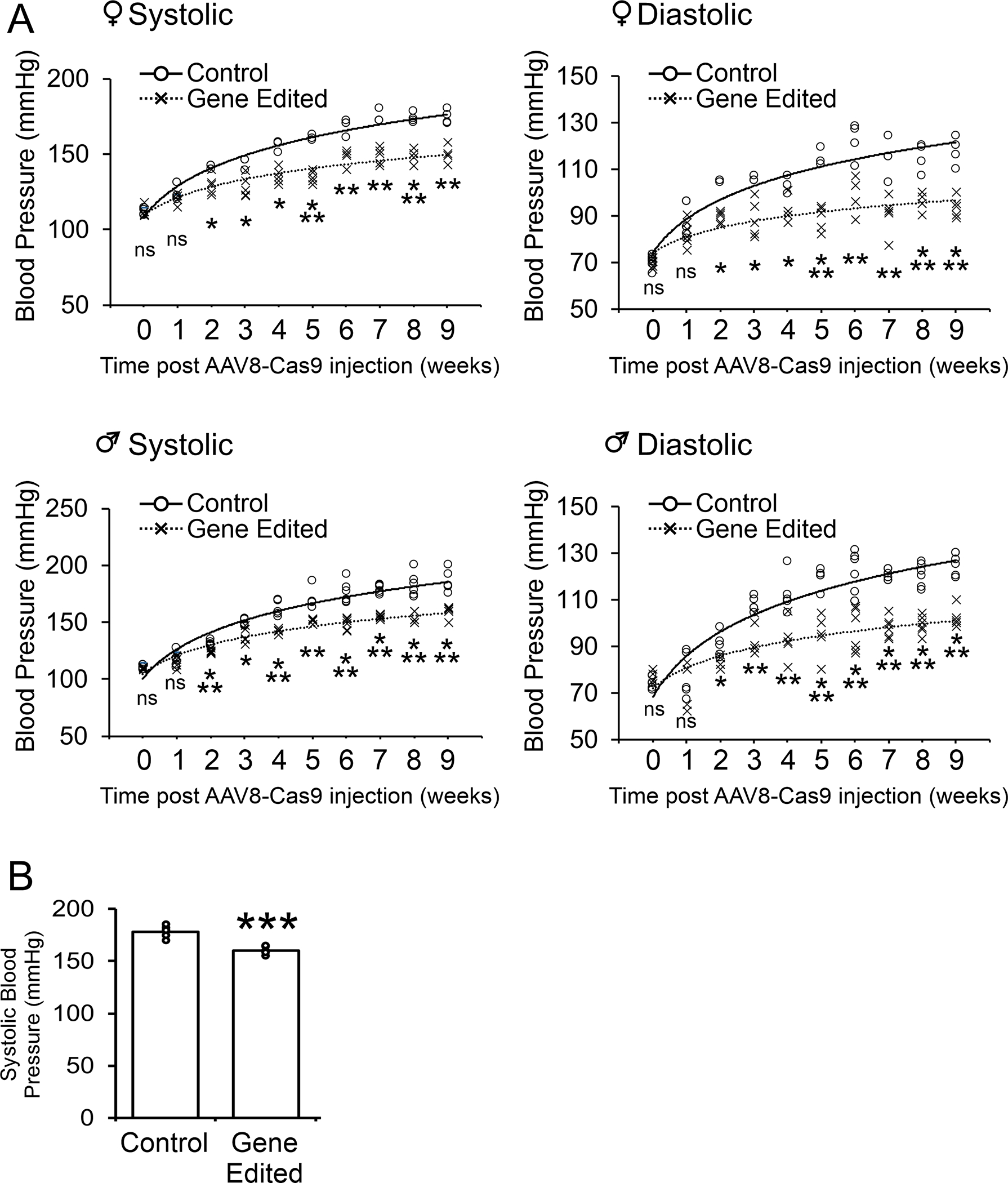
(A) AAV8-Cas9 (control) or AAV8-Cas9-AGTgRNA (gene-editing) viral particles (2×1012 viral particles) were injected into the tail vein of adolescent (5-week old) female (top panels) and male (bottom panels) SHR. Blood pressure measurements were made by tail-cuff for up to 9 weeks post-injection. N=4–5 per group. ANOVA with Post-Hoc Bonferroni correction: Ns not significant, *P<0.05, **P<0.01, ***P<0.001. Individual points are plotted. The mean of the control and gene-edited groups is shown by the solid and dashed line respectively. The curve was fitted as a polynomial of the 1st order.
(B) Analysis of blood pressure 1 year after AAV8-Cas9 (control) or AAV8-Cas9-AGTgRNA (gene-editing) viral particle injection. N=4. Mean ± standard deviation is shown. Student T-test: ***P<0.001.
We then wanted to determine if AGT gene targeting would be effective in reducing blood pressure in adult SHR with established hypertension. In 12 week old SHR, hypertension was apparent and sustained (Figure 4). Injection of AAV8-Cas9-AGTgRNA particles led to a rapid and progressive decrease in both systolic and diastolic blood pressure (Figure 3). Blood pressure in AGT gene-edited SHR was comparable to Wistar-Kyoto (WKY) control rats. Importantly, the reductions in blood pressure were sustained up to one year (Figure 4). In the WKY, gene editing of the AGT gene was similarly associated with prolonged reductions in both diastolic and systolic blood pressure (Figure 5).
Figure 4. AGT gene editing reduces blood pressure in hypertensive SHR.
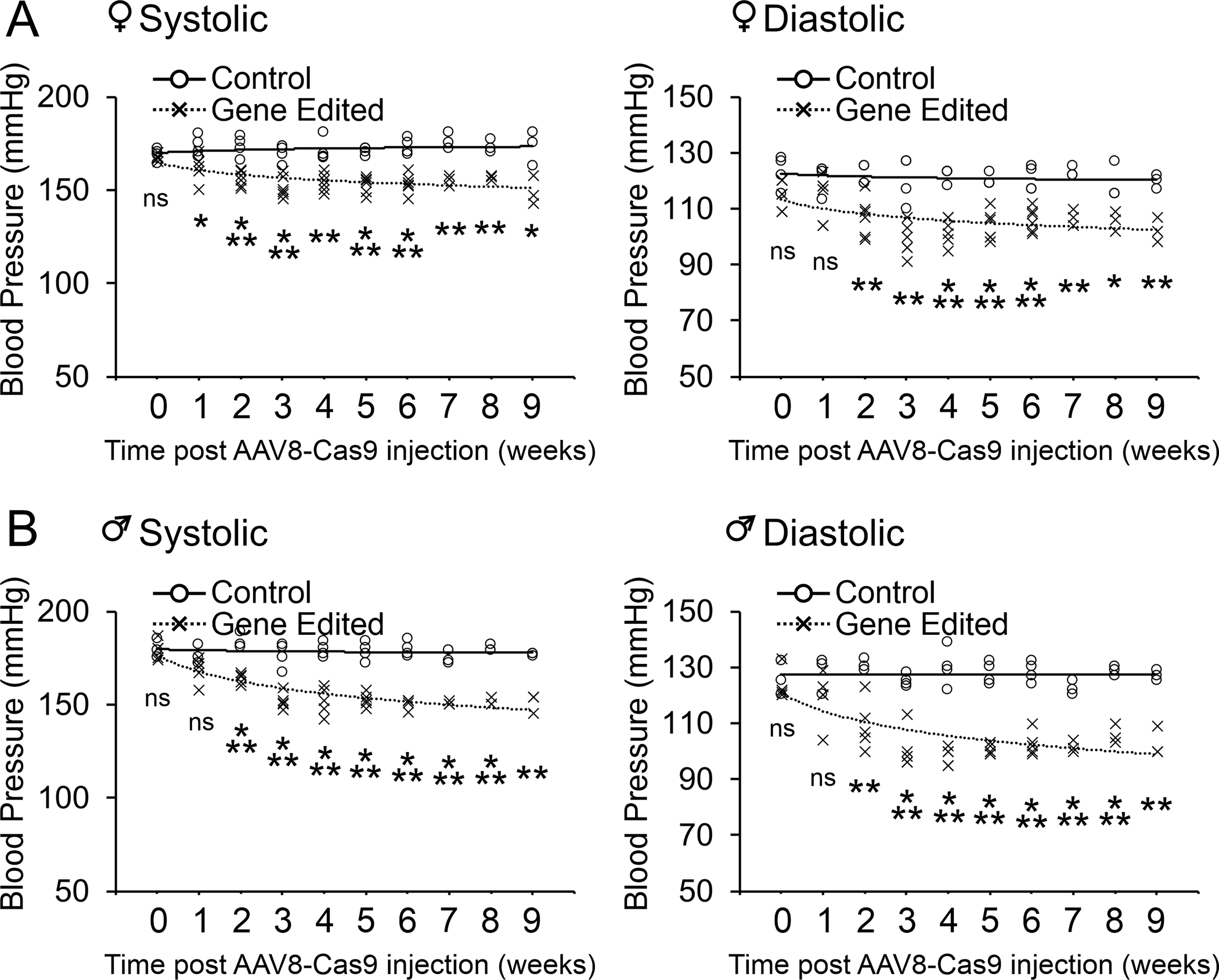
AAV8-Cas9 (control) or AAV8-Cas9-AGTgRNA (gene-editing) viral particles (2×1012 viral particles) were injected into the tail vein of adult (12-week old) female (top panels) and male (bottom panels) SHR with established hypertension. Blood pressure measurements were made by tail-cuff for up to 9 weeks post-injection. N=4–5 per group. ANOVA with Post-Hoc Bonferroni correction: Ns not significant, *P<0.05, **P<0.01, ***P<0.001. Individual data points are plotted. The mean of the control and gene-edited groups is shown by the solid and dashed line respectively. The curve was fitted as a polynomial of the 1st order.
Figure 5. AGT gene-editing reduces blood pressure in WKY rats.
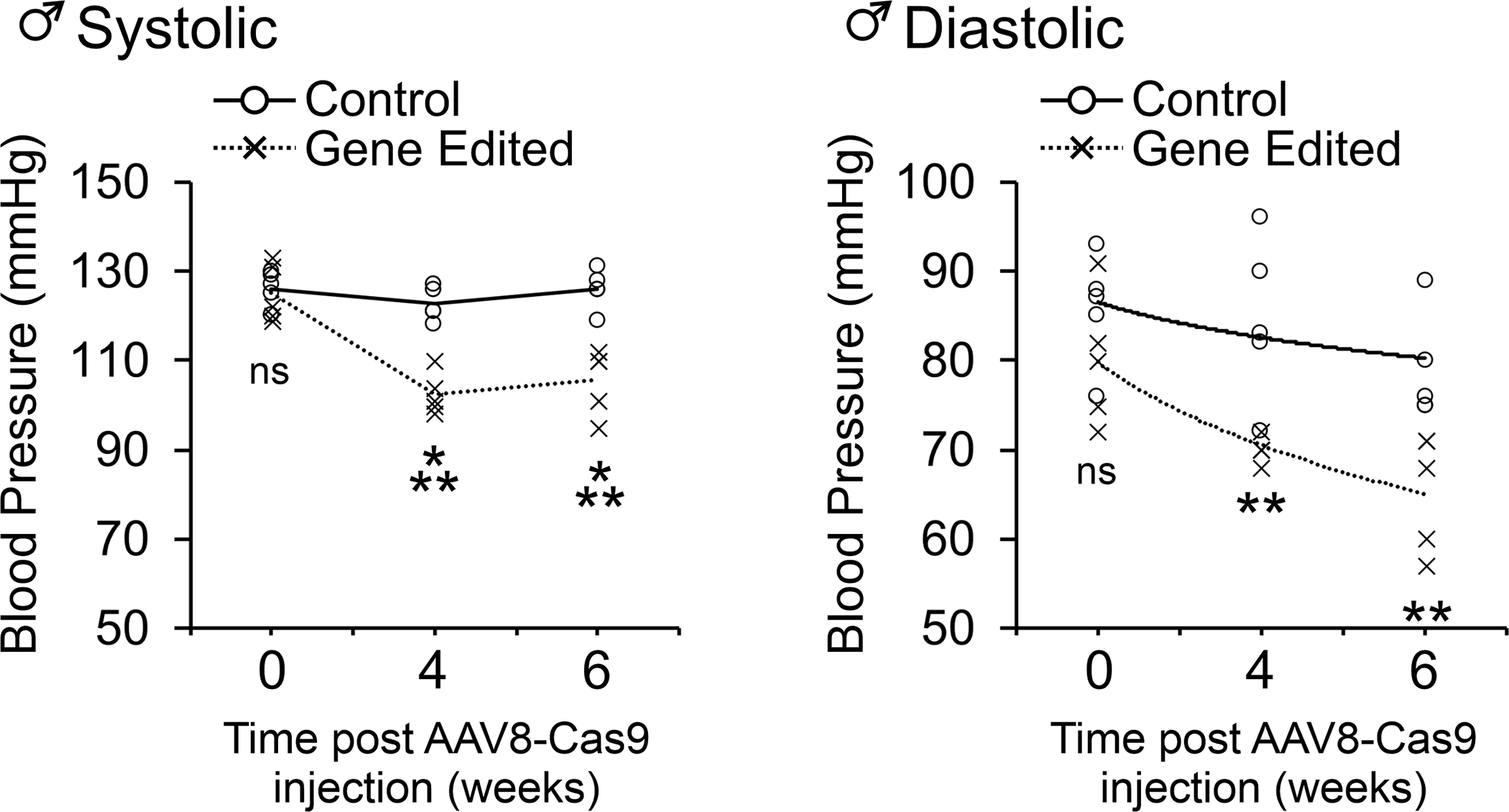
AAV8-Cas9-AGTgRNA was injected into the tail vein of adult (12-week old) of male WKY rats. Blood pressure measurements were made by tail-cuff 4- and 6-weeks post-injection. N=5 per group. ANOVA with Post-Hoc Bonferroni correction: **P<0.01, ***P<0.001. Individual data points are plotted. The mean of the control and gene-edited groups is shown by the solid and dashed line respectively. The curve was fitted as a polynomial of the 1st order.
Finally, we wanted to examine the safety of AGT gene editing by studying the rats’ ability to adapt to stress stimuli. Adult SHR were injected with either control AAV8-Cas9 or AAV8-Cas9-AGTgRNA. Twenty-eight days after viral injection, by which point AGT gene editing had occurred, the SHR were placed on a low salt diet plus furosemide regimen for 10 days 27. At baseline, prior to starting the low salt diet plus furosemide regimen, systolic and diastolic blood pressures were significantly different between control and AGT gene-edited SHR (Figure 6A, P<0.0001). In response to low salt diet plus furosemide regimen, blood pressures decreased in both SHR receiving the control AAV8-Cas9 and AGT gene-editing machinery (Figure 6A). The blood pressure in control SHR was 149±4/103±4 (mean ± SEM) and for gene edited SHR was 132±6/91±4 mm Hg (P<0.05). Importantly, gene edited SHR did not develop hypotension. When the animals returned to their normal diet, blood pressure returned to baseline values for both groups (Figure 6A). Plasma was analyzed for AGT, Angiotensin-I (Ang-I) and Angiotensin-II (Ang-II) levels; as well as renin activity. Low salt diet plus furosemide regimen resulted in significant increases in plasma renin activity in the control SHR associated with increases in plasma Ang-I and Ang-II levels but had no effect on plasma AGT levels (Figure 6B–E). The gene edited SHR exhibited similar Ang I and Ang II responses, albeit more attenuated (p=0.025 and 0.01 respectively). Importantly the plasma renin activity responses were not different between the 2 groups (P=0.424) (Figure 6B–D).
Figure 6. AGT gene editing does not affect dietary induced changes in blood pressure in hypertensive SHR.
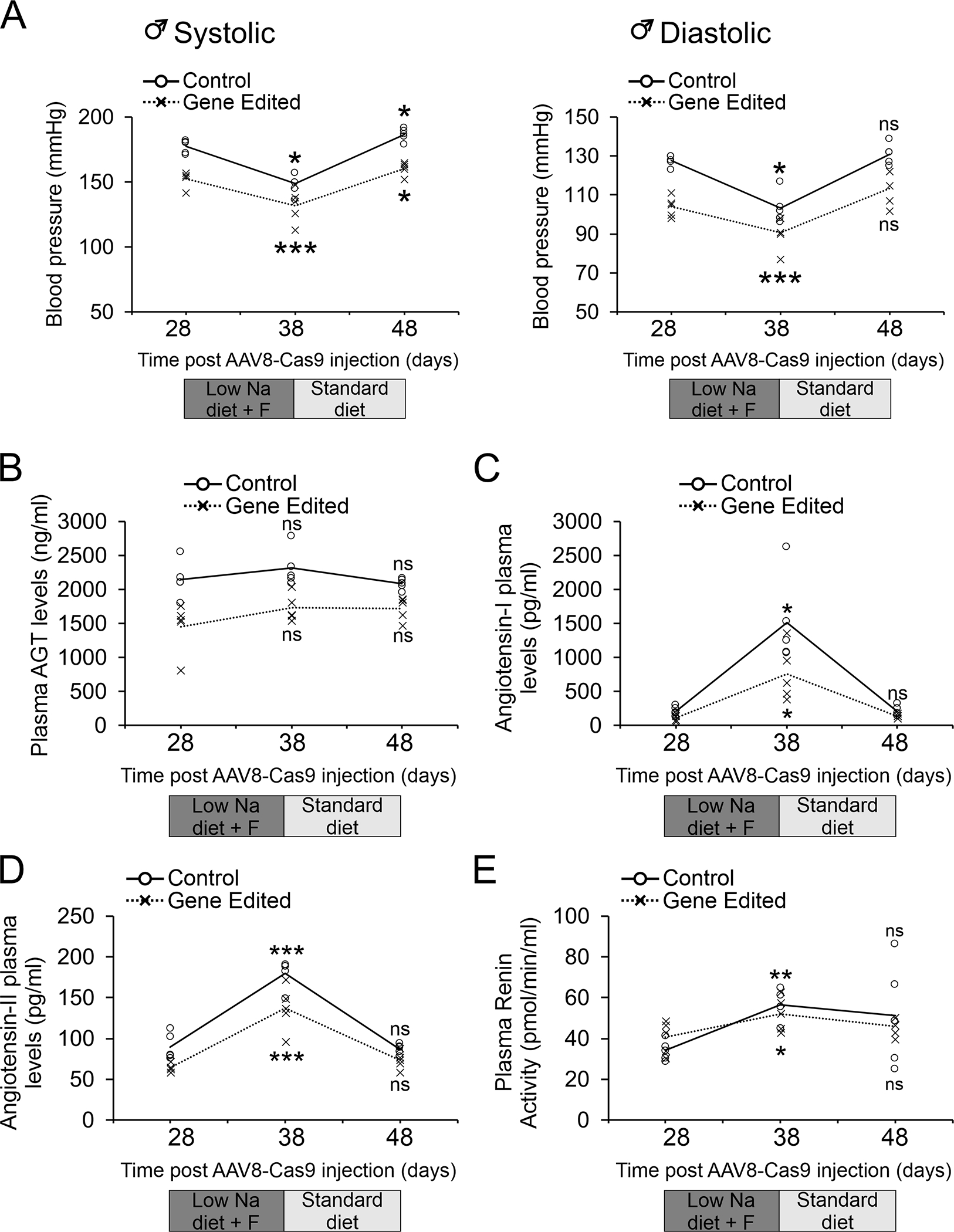
AAV8-Cas9 (control) or AAV8-Cas9-AGTgRNA (gene-editing) viral particles (2×1012 viral particles) were injected into the tail vein of adult (12-week old) male SHR. After 28 days on a standard diet, the animals were placed on a low salt diet plus furosemide (Low Na + F) regimen. The animals were maintained on the low salt diet furosemide (Low Na + F) regimen for 10 days and then returned to a standard diet for a further 10 days.
(A) Blood pressure measurements were made by tail-cuff at the indicated time-points.
(B-E) In addition to blood pressure measurements, blood was taken from the animals by tail-vein and serum analyzed by ELISA for plasma (B) AGT levels; (C) Angiotensinogen-I levels; (D) Angiotensinogen-II levels; as well as (E) Renin activities. N=5 per group.
N=5 per group.
Comparisons were made to standard chow immediately prior to the low salt diet plus furosemide regimen for each group (control and AGT gene-edited SHR). ANOVA with Post-Hoc Bonferroni correction was employed: ns, not significant, *P<0.05, **P<0.01, ***P<0.001. Individual data points are shown. The mean of the control and gene-edited groups is shown by the solid and dashed line respectively. The curve was fitted as a line of best-fit.
Discussion
This is the first report of the Crispr-Cas9 system being employed to target RAS for the effective control of hypertension. In this context, our findings that gene editing of AGT results in sustained reuction in SHR are of important significance.
Our gene-editing approach is distinctive from other approaches such as siRNA since Crispr-Cas9 system permanently disrupts the targeted gene without the need for continual expression of the Cas9 enzyme and repeated AAV administration or associated chronic effect on the immune system. Thus, Crispr-Cas9 mediated control of blood pressure is an important breakthrough for hypertension treatment and control.
An important demonstration of our study is the safety of AGT deletion using Crispr system. We observed that the partial deletion of hepatic AGT gene, sufficient to control hypertension, did not block the renin angiotensin response to cardiovascular stress such as sodium depletion and furosemide. Our data confirms the seminal work of Oliver Smithies which demonstrated that reducing AGT gene copies had a direct relationship on blood pressure reduction 31. Our data demonstrated that in response to sodium depletion and furosemide, the fall in blood pressure was protected by increased plasma renin activity with the associated generation of plasma angiotensin I and II in both control and Crispr treated SHRs. Indeed, our data showed that 40 % ablation of hepatic AGT provided sufficient AGT as substrate for increased plasma renin to generate plasma angiotensin. Beyond the effects of gene-editing hepatic AGT, one major safety concern with Crispr-Cas9 mediated gene-editing is the potential for off-target events. Off-target events, defined as events at unintended genomic sequences, are relatively rare 32–34. However, while rare, the possibility of random alterations to the genome has spurred further development of the gene-editing toolkit. In this regard, modification of the DNA nuclease has been an important area of focus with substitutions of Cas9 with alternative nucleases such as Cpf-1 34 and Fok-1 35 further reducing the rate of random mutations. Nevertheless, if in the future, gene-editing of AGT is contemplated for human hypertension application, more work will be needed to demonstrate precision and no off-target effects. Irrespective of the precision of gene-editing, the choice of vector to deliver the gene-editing machinery is also important for safety. In our study, we chose to AAV8 to deliver the gene-editing machinery specifically to the liver. AAV8 is known to specifically target the liver 36, 37. As such, we do not expect any extrahepatic effect based on many published studies that AAV8 delivery of transgenes have no other tissue effects 38–40. However, this will have to be carefully evaluated if our approach is contemplated for human hypertension application.
The incomplete ablation of angiotensinogen can be explained by the mechanism of Cas9 mediated DNA cleavage. Following cleavage, the cell repairs the damage by removing one, two, or three nucleotides from the gene. Removal of one or two nucleotides will disrupt the coding sequence and prevent the formation of a functional protein. In contrast, the removal of three nucleotides does not disrupt the coding sequence and, unless the removed amino acid was critical, would give rise to a functional protein. This inherent inability of the Crispr-Ca9 system to fully ablate gene expression is important for clinical applications of the technique as it allows for optimization of the dose. Once a plateau has been reached, increasing the dose will have no further effect on protein expression. Indeed, we injected various doses of the Crispr-Cas9 virus into rats and measured plasma angiotensinogen levels. While each dose continued to decrease plasma angiotensinogen levels, no significant further decrease in blood pressure was observed. It is unclear if this is due to activation of other regulatory pathways or demonstration that angiotensinogen is present at overwhelming excess.
A limited number of studies have shown that targeting of several genes via the Crispr-Cas9 system resulted in variable and modest effect of blood pressure. Yang et al utilized the Crispr-Cas9 system to ablate the CPI-17 gene as well as to prevent the activation of the CPI-17 protein by preventing the phosphorylation of a key residue 24. In both models, mean blood pressure was reduced by between 3 and 6mmHg 24. The reduction in blood pressure was found to be associated with reduced blood vessel contractility 24. Another study demonstrated that Crispr-Cas9 mediated ablation of Gper (G protein coupled estrogen receptor) reduced blood pressure in the SHR model by affecting the gut microbiome 25. Indeed, the authors were able to reverse the reductions in blood pressure by transplanting the gut microbiota from a wild-type SHR into the gut of the Gper knockout animals 25. This may explain why previous Gper gene ablation models have shown both increased and decreased blood pressure measurements 41, 42.
Perspectives
In conclusion, we demonstrate that targeting RAS with the Crispr-Cas9 system is an effective method for achieving a sustainable reduction in blood pressure that appears to be safe; yielding possible life-long control of hypertension. Over the years, there have been significant efforts in finding a method of lifelong control and possibly cure for hypertension, such as the development of a vaccine, but without success. Crispr- AAV8 is a technology that can be delivered systemically as somatic therapy at any age. Our results raise an intriguing and provocative question: is gene editing of liver AGT a breakthrough and provide a potential cure for hypertension?
Supplementary Material
Novelty and Significance.
What Is New?
Gene-editing of a key gene involved in hypertension
Prolonged control of blood pressure
What Is Relevant?
AGT gene-editing prevented the development of hypertension
AGT gene-editing reduced blood pressure in SHR with established hypertension
Summary
AGT gene-editing is a potential cure for hypertension
Acknowledgements
We thank the Mandel Center for their continued support.
Sources of Funding
Funding was provided from the NIH (R01HL131814 and R01HL139718) as well as the Edna and Fred L. Mandel, Jr. Foundation.
Footnotes
Disclosures
None.
References
- 1.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 american college of cardiology/american heart association (acc/aha) guideline. J Am Heart Assoc. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CH, Wang Y, Ma M, Mullick AE, Crooke RM, Graham MJ, Daugherty A, Lu HS. Antisense oligonucleotides targeting angiotensinogen: Insights from animal studies. Biosci Rep. 2019;39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomita N, Morishita R, Higaki J, Aoki M, Nakamura Y, Mikami H, Fukamizu A, Murakami K, Kaneda Y, Ogihara T. Transient decrease in high blood pressure by in vivo transfer of antisense oligodeoxynucleotides against rat angiotensinogen. Hypertension. 1995;26:131–136 [DOI] [PubMed] [Google Scholar]
- 4.Makino N, Sugano M, Ohtsuka S, Sawada S. Intravenous injection with antisense oligodeoxynucleotides against angiotensinogen decreases blood pressure in spontaneously hypertensive rats. Hypertension. 1998;31:1166–1170 [DOI] [PubMed] [Google Scholar]
- 5.Makino N, Sugano M, Ohtsuka S, Sawada S, Hata T. Chronic antisense therapy for angiotensinogen on cardiac hypertrophy in spontaneously hypertensive rats. Cardiovasc Res. 1999;44:543–548 [DOI] [PubMed] [Google Scholar]
- 6.Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP, Crooke RM. Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension. 2017;70:566–576 [DOI] [PubMed] [Google Scholar]
- 7.Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, Hoorn EJ, Foster D, Danser AHJ. Strong and sustained antihypertensive effect of small interfering rna targeting liver angiotensinogen. Hypertension. 2019;73:1249–1257 [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Mohuczy D, Zhang YC, Kimura B, Galli SM, Phillips MI. Intravenous angiotensinogen antisense in aav-based vector decreases hypertension. Am J Physiol. 1999;277:H2392–2399 [DOI] [PubMed] [Google Scholar]
- 9.Haase N, Foster DJ, Cunningham MW, Bercher J, Nguyen T, Shulga-Morskaya S, Milstein S, Shaikh S, Rollins J, Golic M, Herse F, Kraker K, Bendix I, Serdar M, Napieczynska H, Heuser A, Gellhaus A, Thiele K, Wallukat G, Muller DN, LaMarca B, Dechend R. Rna interference therapeutics targeting angiotensinogen ameliorate preeclamptic phenotype in rodent models. J Clin Invest. 2020;130:2928–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Liu XL, Wen J, Huang LH, Lu Y, Miao RJ, Liu X, Li Y, Xing XW, Yuan H. Downregulation of the beta1 adrenergic receptor in the myocardium results in insensitivity to metoprolol and reduces blood pressure in spontaneously hypertensive rats. Mol Med Rep. 2017;15:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A, Huang BS, Wang HW, Ahmad M, Leenen FH. Knockdown of mineralocorticoid or angiotensin ii type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin ii hypertension in rats. J Physiol. 2014;592:3523–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen PG, Sun Z. Aav delivery of endothelin-1 shrna attenuates cold-induced hypertension. Hum Gene Ther. 2017;28:190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp BA, Howell NL, Padia SH. Intrarenal ghrelin receptor inhibition ameliorates angiotensin ii-dependent hypertension in rats. Am J Physiol Renal Physiol. 2018;315:F1058–F1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosswhite P, Chen K, Sun Z. Aav delivery of tumor necrosis factor-alpha short hairpin rna attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension. 2014;64:1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z, Bello-Roufai M, Wang X. Rnai inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H1880–1887 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Skelley L, Cade R, Sun Z. Aav delivery of mineralocorticoid receptor shrna prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther. 2006;13:1097–1103 [DOI] [PubMed] [Google Scholar]
- 17.Hao M, Li M, Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep. 2017;15:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Li X, Zheng S, Chen Y, Chen C, Wang J, Tong H, Zhou L, Yang J, Zeng C. Downregulation of renal g protein-coupled receptor kinase type 4 expression via ultrasound-targeted microbubble destruction lowers blood pressure in spontaneously hypertensive rats. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colella P, Ronzitti G, Mingozzi F. Emerging issues in aav-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X, Waghulde H, Mell B, Morgan EE, Pruett-Miller SM, Joe B. Positional cloning of quantitative trait nucleotides for blood pressure and cardiac qt-interval by targeted crispr/cas9 editing of a novel long non-coding rna. PLoS Genet. 2017;13:e1006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai X, Mangum KD, Dee RA, Stouffer GA, Lee CR, Oni-Orisan A, Patterson C, Schisler JC, Viera AJ, Taylor JM, Mack CP. Blood pressure-associated polymorphism controls arhgap42 expression via serum response factor DNA binding. J Clin Invest. 2017;127:670–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasagawa S, Nishimura Y, Sawada H, Zhang E, Okabe S, Murakami S, Ashikawa Y, Yuge M, Kawaguchi K, Kawase R, Mitani Y, Maruyama K, Tanaka T. Comparative transcriptome analysis identifies ccdc80 as a novel gene associated with pulmonary arterial hypertension. Front Pharmacol. 2016;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahal Z, Fujikawa K, Matsuo H, Zahid HM, Koike M, Misumi M, Kaneko T, Mashimo T, Ohara H, Nabika T. Effects of the prdx2 depletion on blood pressure and life span in spontaneously hypertensive rats. Hypertens Res. 2019;42:610–617 [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Fujii W, Kaji N, Kakuta S, Kada K, Kuwahara M, Tsubone H, Ozaki H, Hori M. The essential role of phospho-t38 cpi-17 in the maintenance of physiological blood pressure using genetically modified mice. FASEB J. 2018;32:2095–2109 [DOI] [PubMed] [Google Scholar]
- 25.Waghulde H, Cheng X, Galla S, Mell B, Cai J, Pruett-Miller SM, Vazquez G, Patterson A, Vijay Kumar M, Joe B. Attenuation of microbiotal dysbiosis and hypertension in a crispr/cas9 gene ablation rat model of gper1. Hypertension. 2018;72:1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chappell MC. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:H137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Gomez JA, Herrera M, Perez-Marco R, Repenning P, Zhang Z, Payne A, Pratt RE, Koller B, Beierwaltes WH, Coffman T, Mirotsou M, Dzau VJ. Salt restriction leads to activation of adult renal mesenchymal stromal cell-like cells via prostaglandin e2 and e-prostanoid receptor 4. Hypertension. 2015;65:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krooss SA, Dai Z, Schmidt F, Rovai A, Fakhiri J, Dhingra A, Yuan Q, Yang T, Balakrishnan A, Steinbruck L, Srivaratharajan S, Manns MP, Schambach A, Grimm D, Bohne J, Sharma AD, Buning H, Ott M. Ex vivo/in vivo gene editing in hepatocytes using “all-in-one” crispr-adeno-associated virus vectors with a self-linearizing repair template. iScience. 2020;23:100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Xu Y, Jadhav K, Zhu Y, Yin L, Zhang Y. Hepatic forkhead box protein a3 regulates apoa-i (apolipoprotein a-i) expression, cholesterol efflux, and atherogenesis. Arterioscler Thromb Vasc Biol. 2019;39:1574–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doris PA. Genetics of hypertension: An assessment of progress in the spontaneously hypertensive rat. Physiol Genomics. 2017;49:601–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A. 2002;99:4602–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J, Xue D, Chuai G, Gao Y, Zhang G, Liu Q. Benchmarking and integrating genome-wide crispr off-target detection and prediction. Nucleic Acids Res. 2020;48:11370–11379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Wang M, Zheng T, Hou Y, Zhang P, Tang T, Wei J, Du Q. Specificity profiling of crispr system reveals greatly enhanced off-target gene editing. Sci Rep. 2020;10:2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Wu Y, Yee JK. Detection of crispr/cas9-generated off-target effect by integration-defective lentiviral vector. Methods Mol Biol. 2021;2162:243–260 [DOI] [PubMed] [Google Scholar]
- 35.Saifaldeen M, Al-Ansari DE, Ramotar D, Aouida M. Crispr foki dead cas9 system: Principles and applications in genome engineering. Cells. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sands MS. Aav-mediated liver-directed gene therapy. Methods Mol Biol. 2011;807:141–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markusic DM, Herzog RW. Liver-directed adeno-associated viral gene therapy for hemophilia. J Genet Syndr Gene Ther. 2012;1:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen GN, Everett JK, Kafle S, Roche AM, Raymond HE, Leiby J, Wood C, Assenmacher CA, Merricks EP, Long CT, Kazazian HH, Nichols TC, Bushman FD, Sabatino DE. A long-term study of aav gene therapy in dogs with hemophilia a identifies clonal expansions of transduced liver cells. Nat Biotechnol. 2021;39:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samelson-Jones BJ, Arruda VR. Translational potential of immune tolerance induction by aav liver-directed factor viii gene therapy for hemophilia a. Front Immunol. 2020;11:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, Lindsey SH. Gper activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2016;310:H953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and g-1 in aged female and adult male rats is associated with gpr30 downregulation. Am J Physiol Endocrinol Metab. 2013;305:E113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


