Abstract
Objective:
In response to persistent public health concerns regarding prescription opioids, many states and healthcare systems have implemented legislation and policies intended to regulate or guide opioid prescribing. The overall impact of these policies is still uncertain. The aim of this systematic review was to examine the existing evidence of provider-level and patient-level outcomes preimplementation and postimplementation of policies and legislation constructed to impact provider prescribing practices around opioid analgesics.
Design:
A systematic search of MEDLINE, EMBASE, the Web of Science, and the Cochrane Database of Systematic Reviews was conducted to identify studies evaluating the impact of opioid prescribing policies on provider-level and patient-level outcomes. The systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results:
Eleven studies were included in the review. A meta-analysis was not possible due to between-study heterogeneity. Six of the studies assessed state-level policies, and five were at the level of the healthcare system or hospital. Studies showed temporal associations between policy implementation and reductions in opioid prescribing, as well as opioid-related overdoses. Results were mixed regarding the impact of policies on misuse. The majority of the studies were judged to be of low quality based on the GRADE criteria.
Conclusions:
There is low to moderate quality evidence suggesting that the presence of opioid prescribing policy will reduce the amount and strength of opioid prescribed. The presence of these policies may impact the number of overdoses, but there is no clear evidence to suggest that it reduces opioid misuse.
Keywords: Opioids, prescription drug misuse, inappropriate prescribing, overdose, policy, legislation
INTRODUCTION
Misuse and abuse of prescription opioids present an ongoing public health crisis. More than 1 million emergency department visits per year are directly related to prescription drug misuse and abuse.1 Even more alarming, the number of unintentional overdose deaths from prescription pain relievers has increased substantially in the United States, more than quadrupling since 1999.2 Coinciding with the rise in opioid-related morbidity and mortality is an increase in opioid prescribing, primarily for chronic noncancer pain. Hydrocodone is now the most commonly prescribed medication in the United States; it is prescribed more frequently than any blood pressure, cholesterol, or diabetes medication.3 The number of prescriptions for opioids has increased from approximately 76 million, in 1991, to nearly 207 million in 2013, with the United States their biggest consumer globally, accounting for almost 100 percent of the world total for hydrocodone and 81 percent for oxycodone.4 Literature suggests a parallel relationship between the availability of prescription opioids and opioid-related deaths,5 as well as a possible association between opioid-related mortality and daily opioid dose.6–10 The increased availability of prescription opioids, daily dose thresholds, opioid type, polysubstance use, and many other demographic and geographical factors contribute to the observed increase in opioid-related mortality.11,12
Prescription opioids can be useful for the management of both acute and chronic pain, but it is essential to balance the need for effective pain management with the prevention of opioid-related harms such as abuse and overdose. The prescribing practices surrounding opioids have therefore been placed squarely in the center of the debate between the benefits and harms of prescription opioids. For the purposes of this review, we focus on the extent to which state-level prescribing policies may have impacted clinician’s prescribing practices and the resultant rates of adverse opioid-related outcomes.
Responsible opioid prescribing is not a new concept however; in 1997, the Federation of State Medical Boards (FSMB) introduced the “Model Policy for the Use of Controlled Substances for the Treatment of Pain,” which was also adopted by several state medical boards. Subsequent to this, there have been several revisions to this policy and in response to growing pressure to respond escalating opioid prescribing, several states and healthcare systems implemented more specific legislation or policies on opioid prescribing intended to guide clinician practices. The Washington (WA) State Agency Medical Director’s Group (AMDG) implemented the first state-level opioid dosing guideline in the United States, followed by legislation in 2010 aimed at creating opioid prescription practices which were able to manage and reduce chronic noncancer pain, without significantly increasing the patient’s opioid-related morbidity and mortality.8
Policies such as the Washington State guidelines are distinct from prescription drug monitoring programs (PDMPs), which allow providers to track patient and prescriber information related to controlled substances. The implementation of PDMPs has been demonstrated to decrease the prescribing of schedule II controlled substances.13 Currently, 49 states have different versions of PDMPs that are operational, but opioid prescribing policies are not as ubiquitous and are currently available in only 11 states.14 In addition, the policies themselves are heterogeneous in nature, addressing components of prescribing such as dosing or duration, the use of high dose opioids, initiation and maintenance of therapy, pain contracts, and patient monitoring.15 There are several possible reasons why more states have not adopted prescribing policies, including policies are often implemented at the level of the healthcare system, concern of balancing physician autonomy and oversight, and lack of evidence supporting their effectiveness.
However, evidence-based clinical practice guidelines could assist clinicians in making informed prescribing decisions. The content of prescribing guidelines can vary but often include statements on initial dosing, duration, dose adjustments, and use of high dose opioids (often defined as ≥120 mg/d of MED).16 There is a growing body of literature examining the effectiveness of opioid prescribing policies and it is now necessary to evaluate and summarize the best available evidence on the topic.13
The goal of this systematic review was to examine the existing evidence of provider-level and patient-level outcomes preimplementation and postimplementation of policies and legislation constructed to impact provider prescribing practices in the United States and Canada. Provider-level outcomes focused on prescribing metrics such as average daily morphine equivalents prescribed, whereas patient-level outcomes focused on negative health outcomes such as overdose deaths.
METHODS
Eligibility criteria
It was anticipated that few randomized controlled trials would exist; therefore, the search included other study designs (cohort, time series, and cross sectional) to evaluate policies, legislation, or guidelines (referred to hereafter as “policies”) on the prescribing of opioids at the state or system level. To be eligible, a policy had to be specifically crafted to dictate or influence provider prescribing practices. System-level policies could occur at the level of a practice group, hospital, or healthcare system (eg, Veterans Affairs [VA]). The search was limited to the English language and only manuscripts where the full text could be obtained were eligible. Studies were excluded if they were conducted outside the United States or Canada, evaluated policies before 2007 (were not evaluated because they are considered outdated after 5–6 years),8 or included only patients with cancer-related pain. Studies of strictly educational interventions, measurements of adherence only, PDMPs, and pharmacy benefit strategies were also excluded, as these were not directly evaluating the impact of prescribing policies on the outcomes of interest.
Data sources and search strategy
We performed a comprehensive search of select databases to identify all relevant articles published on opioid prescribing policies between January 2007 and February 2015. A systematic search of MEDLINE, EMBASE, the Web of Science, and the Cochrane Database of Systematic Reviews was undertaken using a predefined strategy based on the combination of relevant terms. The following MEDLINE search was employed: (opioid OR opioids OR opiate OR narcotic OR narcotics) AND (policy OR policies OR guideline OR guidelines OR law OR laws OR legislation OR rule OR rules) AND (prescribing OR prescription OR prescribe*).
In addition, conference proceedings from the past 5 years of the American Pain Society Annual Meeting and the International Association for the Study of Pain Annual Meeting were reviewed. Last, hand checking of references was performed of any review articles on the topic of opioid prescribing policies, as well as any eligible studies identified in the search.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)17 flow diagram was used to illustrate the process for screening, eligibility assessment, and inclusion in the review.
Outcome measures
We evaluated both the provider and patient outcomes. Provider-level outcomes focused on opioid prescribing: 1) opioid prescribing as a proportion of patient visits, 2) opioid prescriptions as a proportion of all prescriptions, 3) total number of opioid prescriptions, 4) average quantity of opioids prescribed, 5) average amount of opioids prescribed (in milligrams of MED), and 6) total number of opioid prescriptions exceeding 120 mg/d of morphine equivalents (a daily upper limited specified in many policies). We did not assess secondary measures such as adherence to guidelines or provider knowledge. Although these outcomes are frequently reported, their measurement is not standardized or directly clinically relevant.
Patient-level outcomes focused on negative health outcomes related to opioids: opioid overdose deaths, nonfatal overdoses, healthcare visits related to opioid abuse, cases of diversion, substance abuse treatment admissions, and self-reported misuse or abuse.
We recorded provider-level and patient-level outcomes for both study arms (policy and standard care) or for both study periods (prepolicy and postpolicy).
Data extraction
Data extraction was performed by two independent reviewers using a standardized extraction tool. Discrepancies were resolved by an open discussion between the two reviewers and disagreements were adjudicated by a predetermined third party. Data extracted from the full-text manuscripts included study duration in weeks; participant characteristics (age, gender, and race/ethnicity); number of participants in the sample; subpopulation studied, if any (eg, chronic pain or back pain) and the main study results (eg, opioid prescriptions as a proportion of all prescriptions; total number of opioid prescriptions; average quantity of opioids prescribed; average amount of opioids prescribed; total number of opioid prescriptions exceeding 120 mg/d of morphine equivalents; number of overdose deaths; number of opioid overdoses; number of healthcare visits at which an opioid was received; and number of new opioid users;). All data were recorded for the prepolicy and postpolicy periods, or for the policy and standard care arms in a randomized controlled trial. Detailed information about the intervention (policy) was recorded.
Data analysis and synthesis
The primary analysis consisted of evaluating the change in 1) provider-level outcomes (prescribing) and 2) patient-level outcomes (eg, overdose and overdose deaths) in the prepolicy and postpolicy periods.
A qualitative assessment of the included studies was performed and results summarized in table format. The qualitative assessment focused on 1) risk of bias, 2) quality of the evidence, and 3) possible sources of between-study heterogeneity.
The risk of bias was assessed using standardized instruments adapted from the Cochrane Collaboration. Different instruments were used for different study designs. For randomized controlled trials, the methodological quality of study components was assessed, including randomization, blinding, allocation concealment, analyses, and censoring. For cohort or time-series studies, this assessment included sampling methods, exposure and outcome assessment, control for confounding, loss to follow-up, and selection bias.
The quality of evidence was assessed using the GRADE guidelines.18 Randomized controlled trials were initially assigned a rating of high quality, whereas observational study designs were initially assigned a rating of low quality. Ratings were downgraded or upgraded based on several factors. Ratings were modified down due to risk of bias, inconsistency, indirectness, imprecision, or publication bias. Ratings were modified upward if there was a large magnitude of effect, dose response, and when residual confounding would play a minimal role. The final rating categorized studies as high, moderate, low, or very low quality. GRADE scores were assigned by two independent reviewers; discrepancies were reso-lved by an open discussion between the two reviewers, and disagreements were adjudicated by a predetermined third party.
Qualitatively, potential sources of heterogeneity were assessed via open discussion between the two reviewers. Due to the heterogeneity of the studies, a quantitative meta-analysis could not be conducted.
RESULTS
Overview of the studies
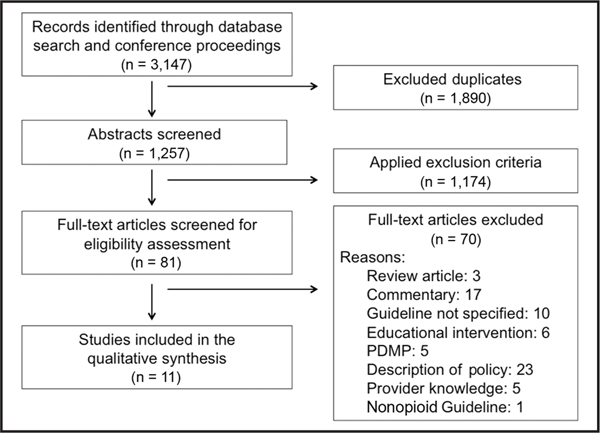
After screening of titles, abstracts, and full-text articles, 11 articles were ultimately included in the analysis. Figure 1 details the flow diagram of the initial search, eligibility assessment, and inclusion of studies.
Figure 1.
PRISMA flow diagram.
Six of the studies were assessments of state-level policies, and five were at the system level (healthcare system or hospital). In aggregate, the studies examined from 2000 to 2014. The periods of analysis ranged from 17 months to 15 years. The studies ranged in size from a randomized controlled trial of 135 participants to a large study utilizing claims data with 161,283 participants. The following geographic areas were involved in the studies: Washington (3 studies), Florida (2), Ontario (2), California, Maine, Minnesota, and Utah.
The state-level interventions evaluated policies in Florida (2 studies), Ontario (2), and Washington (2). The Florida policy was designed to regulate the operation of pain clinics specifically. It stipulated (i) limitations on pain clinic ownership; (ii) mandating registration and inspection of pain clinics; (iii) placing limits on prescribing; and (iv) restricting on-site dispensing of controlled substances.
The Washington State policy is a set of guidelines set forth by the state’s AMDG and makes specific recommendations regarding the prescribing of opioids for chronic noncancer pain. The policies evaluated in Ontario involved two interventions which occurred in concert with one another: (1) prescribing guidelines issued by the Ontario College of Physicians and Surgeons (CPSO) and (2) “Narcotics Safety and Awareness Act” (NSAA).
Three of the studies evaluating the system-level policies evaluated changes in prescribing before and after the implementation of guidelines designed to place limits on opioid prescribing.19–21 One prospective study evaluated the impact of limiting postoperative opioid prescriptions to 6 weeks following orthopedic surgery in conjunction with patient counseling22; and one RCT compared dose escalation and stable dose prescribing strategies in chronic noncancer pain.23 This latter study was included as the authors intended to test the impact of a liberal versus a conservative prescribing policy.
The majority of the studies were judged to be of low quality based on the GRADE criteria. Two studies were assessed to be of very low quality,22,24 and three studies were assessed as moderate quality.20,21,23 There were no studies that were determined to be high-quality evidence.
Effectiveness of opioid prescription policies
Tables 1 and 2 summarize state-level and system-level interventions, respectively. Each of the 11 studies reported reductions in either the quantity or amount of prescribed opioids following policy implementation, or improvements in patient-level outcomes such as diversion, misuse, or overdose deaths. The majority of the studies reported descriptive analysis only without formal quantitative comparisons of the policy vs control.
Table 1.
Summary and GRADE score for studies of state-level opioid prescribing guidelines
| Study | Target | Study design | Intervention | Main results | Bias | GRADE |
|---|---|---|---|---|---|---|
| Johnson et al. (2014)25 | State of Florida | Time series | Legislation to improve operation of pain clinics, mandatory reporting to prescription monitoring program. | Prescription opioid overdose deaths declined 27.0 percent, from 13.6 to 9.9 per 100,000 persons. Heroin overdose death rates did increase, but overdose death rate for all drugs declined 17.7 percent. | Unmeasured confounding. Other factors could have influenced opioid prescribing such as media coverage on the rise in opioid misuse. | Low |
| Gomes et al. (2014)7 | Ontario residents | Time series | Narcotics Safety and Awareness Act (NSAA) | Prevalence of potentially inappropriate opioid prescriptions decreased 40.3 percent, from 1.6 percent to 1.0 percent. | Study only applies to publicly funded prescription drugs. Inappropriate prescribing of controlled substances may have declined before NSAA implemented. | Low |
| Surratt et al. (2014)26 | State of Florida | Time series | Legislation to improve operation of pain clinics. | Decrease in the number of cases of oxycodone, morphine, and methadone diversion investigated by the state. | Prescription monitoring program implemented at the same time, so cannot attribute specifically to legislation. Not all areas in Florida covered. | Low |
| Garg et al. (2013)27 | Injured workers with a dispensed opioid prescription, Washington State | Time series | The Washington State Agency Medical Director’s Group (AMDG) Guidelines | Fewer users became chronic in the post-Guideline period (4.7 percent, 95% CI = 4.5–5.0 percent) than in the pre-Guideline period (6.3 percent, 95% CI = 6.1–6.6 percent; p < 0.001). | Unmeasured confounding. Other factors could have influenced opioid prescribing such as media coverage on the rise in opioid misuse. | Low |
| Fischer et al. (2013)28 | Respondents to the Ontario Centre for Addiction and Mental Health annual survey | Cross sectional | Prescribing guidelines issued by the Ontario College of Physicians and Surgeons (CPSO); NSAA | Nonmedical prescription opioid use decreased from 7.7 percent to 4.0 percent from 2010 to 2011. No significant change in opioid use (26.6 percent to 23.9 percent). | Limitations of survey methodology—response, recall bias, etc. Broad definition of nonmedical use. | Low |
| Franklin et al. (2012)24 | Worker’s compensation claimants, Washington State | Time series | The Washington State Agency Medical Director’s Group (AMDG) Guidelines | Decline in MED of long-acting opioids (−27 percent); 35 percent decrease in the proportion of workers on doses >120 mg/d MED; 50 percent decrease from 2009 to 2010 in the number of deaths (32 --> 16). | Policy implemented in 2007, but period described is from 2009 to 2010. | Very low |
CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation rating of evidence; MED, morphine equivalent dose; mg, milligrams.
Table 2.
Summary and GRADE score for studies of system-level opioid prescribing guidelines
| Study | Target | Study design | Intervention | Results | Bias | GRADE |
|---|---|---|---|---|---|---|
| Saunders et al. (2015)20 | Physicians in Group Health (GH), health plan in Washington State | Prospective cohort | Chronic opioid therapy guidelines instituted with training on opioid prescribing. Control group of in-network physicians not exposed to intervention. | Intervention physicians decreased the daily MED by 35 percent (74.1–48.3 mg) over the study period. In contrast, the control group of network physicians fell 14 percent (88.2–75.7 mg). | Did not adjust for physician characteristics or patient characteristics. | Moderate |
| Westanmo et al. (2015)21 | VA Health System (one hospital and 11 clinics), Minnesota | Time series | Opioid Safety Initiative: guidelines to decrease high dose opioid prescriptions. Involved provider education and detailing. | Total number of patients on >200 mg/d MED decreased from 342 (0.67 percent) to 65 (0.12 percent); mean dose among those who received an opioid decreased from 43 to 23 mg MED daily (−47 percent). | Did not adjust for physician characteristics or patient characteristics. Patients could have left the VA health system. | Moderate |
| Holman et al. (2014)22 | Orthopedic patients in Utah | Prospective cohort | Perioperative counseling and opioids prescribed for a maximum of 6 weeks post-op. | Patients in the intervention group were more likely to stop using opioids by week 6 compared with the control group (RR = 0.74 (95% CI 0.58–0.93)). However, there was no difference in opioid use past 12 weeks (RR = 0.98 (0.71–1.35)). | Treatment assigned based on one of two surgeons; the surgeon is a major source of confounding. Patients in the control group tended to be slightly older, with a lower proportion of women. Preoperative opioid use not measured. | Very low |
| Fox et al. (2013)9 | Emergency physicians treating patients with dental pain | Retrospective chart review | Emergency department opioid prescribing guidelines | Opioid prescribing for patients with dental pain decreased by 17 percent (95% CI: 7–25 percent) after the policy. | Abstractors not blinded to study aims. No adjustment for physician or patient characteristics. Only 16 physicians in the practice. | Low |
| Naliboff et al. (2011)23 | Pain clinic at a VA hospital in California | RCT | Compared liberal (dose escalation) and conservative opioid (stable dosing) prescribing guidelines for treatment of chronic pain. | No difference in prevalence of misuse the stable dose (22/67 (33 percent)) and the escalating dose group (16/62 (26 percent)) of the escalating dose group. The opioid dose in the escalating group increased 80 percent compared to 16 percent in the stable group. No difference in functional outcomes. | Loss to follow-up. | Moderate |
GRADE, Grading of Recommendations Assessment, Development and Evaluation rating of evidence; VA, veterans affairs; MED, morphine equivalent dose; mg, milligrams; CI, confidence interval; RR, relative risk.
Five studies examined provider-level outcomes such as the number or proportion of opioids prescribed before and after the policy implementation. Three studies demonstrated declines in the dose amount of daily MEDs; average doses of prescribed opioids dropped anywhere from 27 to 47 percent following implementation of the policy.20,21,24 Two of these studies also concluded that fewer proportions of patients were prescribed high dose opioids (≥120 mg/d MED and ≥ 200 mg/d MED).21,24
Six studies examined patient-level outcomes such as misuse and overdose deaths. Two studies concluded that overdose deaths decreased following policy implementation with declines of 27 and 50 percent, respectively.24,25 Results evaluating misuse were mixed. The RCT by Naliboff et al.23 demonstrated that two opioid prescribing policies for chronic noncancer pain (escalating and stable dose plans) had similar rates of misuse. A serial cross-sectional study in Ontario conducted before and after implementation of the Narcotic Safety Awareness Act found that self-reported nonmedical use decreased by 3.7 percent and another study in Florida demonstrated a decrease in the number of cases of diversion.26,28
Bias
Recent policy measures combined with extensive media coverage and extensive social discourse focusing on the harms of prescription opioid abuse are all possible factors in explaining the observed decreases in prescription opioid prescribing.28 Indicators of prescription opioid abuse need to be evaluated over long time periods and in a variety of both patient and provider subgroups. Physician training is a factor that could contribute significantly to prescribing patterns, as physicians’ medical training, residency, and continuing education can contribute to his or her attitudes about prescribing opioids. A lack of pain-management training as well as physicians being inconvenienced by scheduling multiple patient visits or writing multiple prescriptions during long-term pain treatment cannot be discounted as possibly influencing both physicians’ ability to implement/understand opioid prescribing policies as well as their prescribing trends.29
The relationship between opioid prescribing policies and patient-level and provider-level outcomes may be confounded by other variables. Patient and provider characteristics, as well as media or professional society attention to opioid prescribing, are examples of possible confounding factors that need to be controlled for if to estimate direct and indirect effects of opioid prescribing policies without bias.30 At present, it is difficult to imply causation between policy implementation and outcomes using primarily descriptive statistics of prepolicy and postpolicy metrics; future studies should use more robust statistical methodology to deal with potential confounding.
Heterogeneity
There were several sources of heterogeneity between studies, including study populations, duration of studies, the policy intervention itself, the provider-level and patient-level outcomes, and the statistical analyses. Some studies evaluated the outcomes in VA patients21,23; one study focused on worker’s compensation recipients,24 while others looked at dental pain patients in the emergency department,19 orthopedic patients,22 or physicians themselves.20 As the majority of studies did not include standard errors or confidence intervals along with the prepolicy and postpolicy changes, it was not possible to synthesize effect estimates.
DISCUSSION
Without up-to-date information and training, providers may be unwittingly contributing to community risk by failing to adequately monitor patients on prescription opioids, prescribing at too high a dose and duration, and prescribing more than patients will use/need. To date, several states and many healthcare systems have implemented either legislation or guidelines to attempt to standardize the way that opioid prescribing is conducted. As the introduction of opioid prescribing policies is a relatively recent phenomenon, the body of evidence on this topic is still being generated. To our knowledge, this review is one of the first to synthesize the currently available evidence examining the effectiveness of opioid prescribing policies.
In general, opioid prescribing policies appear to be temporally associated with decreases in opioid prescribing, as well as patient-centered outcomes such as overdose deaths. This would suggest that implementation of opioid prescribing policies and legislation can have a positive impact. However, enthusiasm for the success of opioid prescribing policies is tempered by the inherent bias present in studying this agenda, as well as the large degree of heterogeneity present between studies.
This question at hand lends itself to study through observational data. This makes it difficult to tease out the effects of the policy itself versus other unmeasured factors such as media coverage of the opioid epidemic and overdose, educational initiatives through professional organizations, and scientific literature on opioid misuse. In addition, many policies were implemented concurrently or around the same time as PDMPs. Therefore, it is extremely difficult to establish a causal relationship between opioid prescribing policies and the provider-level and patient-level outcomes of interest.
The study by Saunders et al. in 2015 is unique in that it establishes a control group that was not exposed to the intervention but would have been exposed to other factors such as media coverage. During the study period, average prescribing by the control group of physicians fell 14 percent (88.2–75.7 mg), suggesting other factors besides the policy might decrease prescribing. However, any additional decline in the intervention group could be attributed to the intervention itself; this group decreased their average prescribed amount by 35 percent (74.1–48.3 mg) over the study period. A limitation of this study is that the intervention included both the policy itself, along with an in educational session. Therefore, it is not clear if we are seeing the effects of the policy or the educational intervention.
We only identified one RCT examining the impact of different policies on prescribing.23 This study was very specific in that it examined a conservative (stable dose) versus liberal (escalating dose) approach to long-term opioid prescribing. This study was included as it was an analysis of a specific component of many opioid prescribing policies addressing titration of opioids. Interestingly, during the short-term study period, there was no difference in opioid misuse between these two strategies. More studies of this nature need to be conducted to determine which aspects of the policy work. Many of the existing guidelines are based on consensus of expert panels and not derived from primary evidence. Additional work is needed to develop evidence-based guidelines regarding dose, duration, and titration of prescription opioids for both acute and chronic pain.
LIMITATIONS
A quantitative meta-analysis was not possible due to between-study heterogeneity. This systematic review is inherently limited by any methodological weakness present in the original studies. This task was further complicated by the fact that there is lack of standardized and universally accepted metrics of substance misuse, abuse, and overdose.31 Additionally, all but one of the studies was observational in nature and the majority of trials were assessed to be of low quality.
Even with a comprehensive search strategy, there still exists the possibility that relevant articles were missed. Publication bias is a potential factor as well; negative studies on the topic may not have been published. This area is also an emerging area of interest given the recent development of many policies and new literature on this topic is likely in progress.
We did not include studies of educational initiatives informing prescribers about the policies, as this was considered to be a separate research question and we were specifically interested in the impact of the policy itself. The impact of educational interventions has to do with the means to best disseminate policy information and is in and of itself a viable area of study. In addition, we did not examine the impact of opioid prescribing policies on pain or functional outcomes due to a paucity of literature addressing this outcome. This is an important consideration which needs to be addressed; the negative consequences of opioid prescribing must be balanced with its benefits.
Last, this was not an evaluation and analysis of the content of the policies themselves. However, given the heterogeneous nature of the policies, there is a need for this type of work as well.
CONCLUSIONS
There is low to moderate quality evidence suggesting that the presence of opioid prescribing policy will reduce the amount and strength of opioid prescribed. The presence of these policies may impact the number of overdoses, but there is no clear evidence to suggest that it reduces opioid misuse. While the findings from this review generally support the implementation of opioid prescribing policies overall, the heterogeneity between the reviewed studies makes it too difficult to make specific policy recommendations at this time. Additional work is needed to determine which policy components are most effective and if the changes seen in the postpolicy periods can be sustained.
Contributor Information
Francesca L. Beaudoin, Department of Emergency Medicine, Brown University, Providence, Rhode Island..
Geetanjoli N. Banerjee, Department of Epidemiology, Brown University, Providence, Rhode Island..
Michael J. Mello, Departments of Emergency Medicine and Health Services, Policy and Practice, Brown University, Providence, Rhode Island..
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13–4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. Available at http://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf Accessed February 12, 2016. [Google Scholar]
- 2.Volkow ND: Prescription Opioid and Heroin Abuse. Washington, DC: National Institute on Drug Abuse, 2014. Available at https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2015/prescription-opioid-heroin-abuse. Accessed February 12, 2016. [Google Scholar]
- 3.Manchikanti L, Helm S II, Fellows B, et al. : Opioid epidemic in the United States. Pain Physician. 2012; 15(3 suppl): ES9–E38. [PubMed] [Google Scholar]
- 4.Raymond Y, Francisco T, Andrés F: International Narcotics Control Board Report 2008. United Nations Pubns. 2009: 20. [Google Scholar]
- 5.Dart RC, Surratt HL, Cicero TJ, et al. : Trends in opioid analgesic abuse and mortality in the United States. NEngl JMed. 2015; 372(3): 241–248. [DOI] [PubMed] [Google Scholar]
- 6.Nuckols TK, Anderson L, Popescu I, et al. : Opioid prescribing: A systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014; 160(1): 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes T, Mamdani MM, Dhalla IA, et al. : Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011; 171(7): 686–691. [DOI] [PubMed] [Google Scholar]
- 8.Franklin GM, Fulton-Kehoe D, Turner JA, et al. : Changes in opioid prescribing for chronic pain in Washington State. J Am BoardFam Med. 2013; 26(4): 394–400. [DOI] [PubMed] [Google Scholar]
- 9.Mack KA, Zhang K, Paulozzi L, et al. : Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015; 26(1): 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnert A, Valenstein M, Bair M, et al. : Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011; 305(13): 1315–1321. [DOI] [PubMed] [Google Scholar]
- 11.King NB, Fraser V, Boikos C, et al. : Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: A systematic review. Am J Public Health. 2014; 104(8): e32–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler S: The proliferation of dosage thresholds in opioid prescribing policies and their potential to increase pain and opioid-related mortality. Pain Med. 2015; 16: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 13.Sigler K, Guernsey B, NB I, et al. : Effect of a triplicate prescription law on prescribing of Schedule II drugs. Am J Health Syst Pharm. 1984; 41(1): 108–111. [PubMed] [Google Scholar]
- 14.Reifler LM, Droz D, Bailey JE, et al. : Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012; 13(3): 434–442. [DOI] [PubMed] [Google Scholar]
- 15.Trescot A, Boswell M, Atluri S, et al. : Opioid guidelines in the management of chronic non-cancer pain. Pain Physician. 2006; 9: 1–40. [PubMed] [Google Scholar]
- 16.Chou R, Fanciullo GJ, Fine PG, et al. : Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. JPain. 2009; 10(2): 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. : Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. BMJ. 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64(4): 401–406. [DOI] [PubMed] [Google Scholar]
- 19.Fox TR, Li J, Stevens S, et al. : A performance improvement prescribing guideline reduces opioid prescriptions for emergency department dental pain patients. Ann Emerg Med. 2013; 62(3): 237–240. [DOI] [PubMed] [Google Scholar]
- 20.Saunders K, Shortreed S, Thielke S, et al. : Evaluation of health plan interventions to influence chronic opioid therapy prescribing. Clin JPain. 2015; 31(9): 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westanmo A, Marshall P, Jones E, et al. : Opioid dose reduction in a VA health care system—Implementation of a primary care population-level initiative. Pain Med. 2015; 16: 1–8. [DOI] [PubMed] [Google Scholar]
- 22.Holman J, Stoddard G, Horwitz D, et al. : The effect of pre-operative counseling on duration of postoperative opiate use in orthopaedic trauma surgery: A surgeon-based comparative cohort study. J Orthop Trauma. 2014; 28: 502–506. [DOI] [PubMed] [Google Scholar]
- 23.Naliboff BD, Wu SM, Schieffer B, et al. : A randomized trial of 2 prescription strategies for opioid treatment of chronic nonma lignant pain. J Pain. 2011; 12(2): 288–296. [DOI] [PubMed] [Google Scholar]
- 24.Franklin GM, Mai J, Turner JA, et al. : Bending the prescription opioid dosing and mortality curves: Impact of the Washington State opioid dosing guideline. Am JInd Med. 2012; 55: 325–331. [DOI] [PubMed] [Google Scholar]
- 25.Johnson H, Paulozzi LJ, Porucznik C, et al. : Decline in drug overdose deaths after state policy changes—Florida, 2010–2012. MMWRMorb Mortal Wkly Rep. 2014; 63(26): 569–574. [PMC free article] [PubMed] [Google Scholar]
- 26.Surratt HL, O’Grady C, Kurtz SP, et al. : Reductions in prescription opioid diversion following recent legislative interventions in Florida. PharmacoepidemiolDrug Saf 2014; 23(3): 314–320. [DOI] [PubMed] [Google Scholar]
- 27.Garg RK, Fulton-Kehoe D, Turner JA, et al. : Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013; 14(12):1620–1628. [DOI] [PubMed] [Google Scholar]
- 28.Fischer B, Ialomiteanu A, Kurdyak P, et al. : Reductions in non-medical prescription opioid use among adults in Ontario, Canada: Are recent policy interventions working? Subst Abuse Treat Prev Policy. 2013; 8(7): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victor TW, Alvarez NA, Gould E: Opioid prescribing practices in chronic pain management: Guidelines do not sufficiently influence clinical practice. J Pain. 2009; 10(10): 1051–1057. [DOI] [PubMed] [Google Scholar]
- 30.Vanderweele TJ, Vansteelandt S: Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010; 172(12): 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanch B, Buckley NA, Mellish L, et al. : Harmonizing post-market surveillance of prescription drug misuse: A systematic review of observational studies using routinely collected dated (2000–2013). Drug Saf. 2015; 38(6): 553–564. [DOI] [PubMed] [Google Scholar]