Abstract
Introduction
Low back pain (LBP) is a musculoskeletal disorder that affects more than 80% of people in the United States at least once in their lifetime. LBP is one of the most common complaints prompting individuals to seek medical care. The purpose of this study was to determine the effects of spinal stabilization exercises (SSEs) on movement performance, pain intensity, and disability level in adults with chronic low back pain (CLBP).
Methods
Forty participants, 20 in each group, with CLBP were recruited and randomly allocated into one of two interventions: SSEs and general exercises (GEs). All participants received their assigned intervention under supervision one to two times per week for the first four weeks and then were asked to continue their program at home for another four weeks. Outcome measures were collected at baseline, two weeks, four weeks, and eight weeks, including the Functional Movement ScreenTM (FMSTM), Numeric Pain Rating Scale (NPRS), and Modified Oswestry Low Back Pain Disability Questionnaire (OSW) scores.
Results
There was a significant interaction for the FMSTM scores (p = 0.016), but not for the NPRS and OSW scores. Post hoc analysis showed significant between-group differences between baseline and four weeks (p = 0.005) and between baseline and eight weeks (p = 0.026) favor SSEs over GEs. Further, the results demonstrated that all participants, regardless of group, had significant improvements in movement performance, pain intensity, and disability level over time.
Conclusion
The results of the study favor SSEs over GEs in improving movement performance for individuals with CLBP, specifically after four weeks of the supervised SSE program.
Keywords: functional movement screen, pain intensity, disability level, lumbar spine, movement impairments, movement system
INTRODUCTION
Low back pain (LBP) is a musculoskeletal disorder that affects more than 80% of people in the United States at least once in their lifetime.1,2 LBP is considered to be one of the most common complaints prompting individuals to seek medical care. It is a very costly condition as the total direct and indirect medical spending for LBP is estimated between $100 and $200 billion a year.2 In addition, LBP is a leading cause of disability, contributing to work absenteeism and loss of productivity worldwide.3,4 Clinically, aberrant movement patterns such as a painful arc, lateral shifting, or Gower’s sign are associated with lumbar instability or movement coordination impairment.5,6 Furthermore, patients with CLBP often develop compensatory movement patterns to complete functional tasks, such as stepping over an obstacle and squatting.7 Therefore, observation and analysis of movement quality may be key elements in LBP management, particularly for patients with subacute and chronic LBP.8
The quality of movement has been measured in different ways, including the use of self-reported measures, impairment measures, and movement performance measures. Self-reported questionnaires are commonly administered because they are based on the patients’ own evaluation of their pain and function.9 However, these self-reported questionnaires do not always distinguish whether or why a specific task is performed properly.10 The self-reported questionnaires lack the description of movements and how the patient will perform the specific task and only address whether the patient is able to do it or not. Therefore, to address the inadequacy of self-reported questionnaires, functional performance measures that are capable of assessing the patient’s ability to perform specific functional tasks, as well as the ease and efficiency of performing these tasks, may be more appropriate to determine the quality of movement.
The Functional Movement ScreenTM (FMSTM) is a quantitative assessment tool that was developed to assess movement performance by identifying limitations and restrictions of movement patterns and to determine whether abnormal movements are present.11,12 Individuals or athletes with lower FMSTM scores also have been found to be associated with a higher risk of musculoskeletal injury.13–16 Because of its ability to evaluate and treat patients with injuries, the FMSTM has been advocated as a tool to be incorporated in rehabilitation.11 The FMSTM has been used as an outcome measure to examine the effects of an exercise program on healthy people and was found to be capable of capturing the improvement of functional movement patterns after an exercise program.17,18 Moreover, a recent study found that patients with CLBP demonstrated lower FMSTM scores as compared to healthy controls.7 Therefore, the FMSTM appears to be a useful functional assessment measure to identify movement deficits in patients with CLBP.7
A variety of treatments have been used by physical therapists for treating CLBP, including manual therapy, exercise programs (e.g., trunk coordination, strengthening, and endurance exercises), lower quarter nerve mobilization, traction, and patient education.19,20 Given the high prevalence of CLBP and high recurrence of LBP and the associated costs, clinicians have been advised to place a priority on interventions which can prevent recurrences and transitions of acute and subacute to CLBP.20 Among conservative treatments, therapeutic exercises are the most widely used for the management of LBP.21 A plethora of evidence has shown that therapeutic exercises are moderately effective for CLBP.22 A meta-analysis of exercise therapy for the treatment of LBP reported that therapeutic exercise was effective in decreasing pain in patients with CLBP.23 However, debates continue regarding what specific type of exercise may be most effective. More recently, spinal stabilization exercises (SSEs) have been advocated as the optimal choice in the rehabilitation of LBP because SSEs have a positive effect on supporting and stabilizing the lumbar spine, reducing pain, and enhancing proprioception as a result of LBP.24,25 In addition, SSEs were found to be more effective than GEs in decreasing pain and improving physical function in patients with LBP and were more effective than a placebo intervention in lumbar segmental instability in patients with LBP.26,27
However, the FMSTM has not yet been used to examine the effectiveness of physical therapy interventions in the LBP population. Although SSEs have been shown to be effective in treating patients with LBP, it is not known if SSEs would improve movement performance. To date, no study has been conducted for assessing the effects of SSEs on the quality of movement performance. Therefore, a randomized clinical trial was warranted to examine whether or not SSEs would have a favorable outcome on movement performance assessed by the FMSTM. The purpose of this study was to determine the effects of SSEs on movement performance, pain intensity, and disability level in adults with CLBP.
METHODS
Study Design and Participants
This study was a double-blinded randomized clinical trial, comparing two exercise programs: SSE vs. GE. Approval from the investigators’ institutional review board was obtained prior to participant enrollment and data collection. To determine adequate sample size for this study, an a priori power analysis was performed using G*Power 3.1.9.28 Using a small-to-medium effect size of 0.20 and an alpha level of 0.05, a sample size of 40 participants was needed to ensure an adequate power level of 0.80 for a mixed-model 2 x 4 analysis of variance (ANOVA) test. Participants of any ethnicity, sex, or race who did not receive physical therapy at the time and within the prior three months, were recruited for this study from the local communities through flyers, word-of-mouth marketing, emails, and direct mail advertisements. Participants were adults of 18 to 65 years of age with LBP for a duration of more than 12 weeks.29 In addition, the eligible participants must have the ability to understand and speak English and had a minimum pain score of 2/10 in the past week using the NPRS. Participants who met inclusion criteria and agreed to participate in the study were asked to sign a written informed consent form.
Participants were excluded if they had (1) serious spinal conditions, such as fracture, infection, or tumor, (2) signs of nerve root compression, (3) a history of the lower extremity or lumbar spine surgery, (4) a history of hip, knee, or ankle pain in the previous two years, (5) current pregnancy by self-report, (6) systemic joint disease (e.g., rheumatologic or neurological disorders), (7) vestibular or other balance disorders, (8) ongoing treatment for the inner ear, sinus, or upper respiratory infection, (9) a history of falls or fear of falling, or (10) a need for any form of walking aids (e.g., cane or walker). A neurological examination was performed to further screen for each participant’s eligibility. Once the participants were deemed to be eligible for this study, their demographic characteristics (i.e., age, sex, height, weight, leg dominance) and pain history (e.g., pain duration, pain intensity) were collected. In addition, participants completed two questionnaires, the Fear-Avoidance Beliefs Questionnaire (FABQ) and Patient-Reported Outcomes Measurement Information System®-29 (PROM-29), which were used to describe the participants of this study.
Investigators
Two investigators were responsible for data collection for this study. The principal investigator, investigator #1, was the treating therapist who was responsible for group allocation and intervention administration and was blinded to the results of the FMSTM, NPRS, and OSW. Investigator #2 was responsible for collecting outcome measures and was blinded to each participant’s group assignment.
Functional Movement Screen TM
The FMSTM Test Kit (Functional Movement Systems Inc., Chatham, VA) was used to assess the movement performance of seven different movement patterns for this study. The FMSTM Test Kit consists of a two-inch by six-inch board, one four-foot-long dowel, two shorter dowels, and an elastic cord.30 The FMSTM includes seven test components: the deep squat, hurdle step, in-line lunge, shoulder mobility, active straight-leg-raise, trunk stability push-up, and rotary stability. Additionally, there are three clearance screens, including the impingement-clearing test, press-up clearing test, and posterior-rocking clearing test. These three clearance screen tests are used to determine the presence of pain associated with internal rotation and flexion of the shoulder, spinal flexion, and spinal extension. However, because this study focused on the LBP population, the impingement-clearing test was excluded (Appendix A).
In the original FMSTM scoring system, each of the seven test components is scored on a scale of 0 to 3: 3 when the test component is performed correctly without compensations, 2 when completion of the test component required compensatory movement, 1 when the participant is unable to perform the test component as required, and 0 when there is an occurrence of pain during the test component. However, as all of the participants had LBP in this study, the FMSTM scores were modified so that a zero score was given only when the participant reported an increase in the LBP, not simply for the presence of LBP. The validity and reliability of this modified FMSTM scoring system have been established previously with excellent inter-rater reliability in those with LBP (ICC = 0.99).31 Lastly, a composite score ranging from 0 to 21 is calculated to indicate the overall quality of movement performance, with a higher score indicating higher quality of movement performance. A score of 14 or lower on the original FMSTM scoring system indicates that the participant could have a higher risk for future injury.11,13
Outcome Measures
Each participant was asked to complete the two clinical outcome measures, the NPRS and the OSW, prior to the FMSTM test. During the FMSTM, each participant performed all seven test components in the same order as described by Cook et al. (2010). No warm-up was required before the start of the measurement. Each participant performed three trials for each of the seven FMSTM test components, and the best score from the three trials was recorded. However, the participants performed the two clearance screens only once. Therefore, when a participant had no pain with a clearance screen, the screen was considered negative. If there was an increase in LBP, not simply the presence of LBP with a clearance screen, the screen was considered positive, and the associated test was scored zero. Two FMSTM test components are associated with a clearance screen: the push-up test with the press-up clearance screen and the rotator stability test with the posterior rocking clearance screen. Five of the seven FMSTM test components were performed bilaterally: hurdle step, in-line lunge, shoulder mobility, active straight leg raise, and rotary stability test. Each participant performed these five tests first on the right side and then on the left side. For movements that were scored on both limbs simultaneously, the lower score was used to compute the composite score. The total score of the seven test components was added together to obtain a composite score of the FMSTM.
For each participant, the FMSTM, NPRS, and OSW measurements scores were collected at baseline and then two weeks, four weeks, and eight weeks after the initiation of treatment. Additionally, NPRS measurements were collected at the beginning of each session, before and after each test, and any aggravation of LBP was recorded throughout the entire testing procedure.
Interventions
Once the participants completed the FMSTM, they were assigned randomly into either the SSE group or the GE group. Participants in the SSE group were instructed in the SSEs, which were modeled on the SSE program designed by Hicks et al. (Appendix B).32 The SSE program of this study targeted the spinal stabilizer muscles, including the transversus abdominus, erector spine, lumbar multifidus, quadratus lumborum, and oblique abdominal muscles. The SSEs consisted of four categories. The exercises in the first category were abdominal bracing exercises, which were designed primarily to target the transversus abdominus muscle. The participant performed each abdominal bracing exercise up to 30 repetitions with a target hold time of eight seconds. The SSEs in the second category were quadruped exercises, which were designed to target both the erector spinae and multifidus. The participant performed each quadruped exercise up to 30 repetitions with a target hold time of eight seconds. The SSEs in the third category were prone-plank exercises, which were designed to primarily target the quadratus lumborum muscle. The participant performed each prone plank exercise up to 30 repetitions with a target hold time of eight seconds. Lastly, the SSEs in the fourth category were side-plank exercises, which were designed to train the oblique abdominal muscle. The participant performed each side-plank exercise up to 30 repetitions with a target hold time of eight seconds.
The GE program consisted of ROM and flexibility exercises of low back and lower extremities (Appendix C). There were four exercise categories, including knee-to-chest, lower trunk rotation, prone press-ups, and hamstring stretch. Each participant in this group was asked to perform each exercise up to 20 times with a target hold time of 10 seconds. The participants were instructed to perform all of the four exercises within a pain-free range.
At the initial treatment session, all participants were instructed to perform four exercises, one from each category. The exercise intensity progressed to the next level when the participant could perform the exercise with proper form and for the required repetitions and hold time. Once they progressed to the next level of the exercise, they discontinued the previous level of the exercise.
On the first visit, all participants were instructed in the exercises at a level that they were able to perform without pain. All participants were given an exercise log based on the assigned group to report their exercise compliance (Appendix D). In addition, all participants were given an exercise handout, which illustrated the exercises and listed the required exercise repetitions and holds time. All participants were asked to return one to two times per week for four weeks for exercise progression and to ensure that they were performing the exercises properly. The intervention frequency and duration were chosen to reflect common physical therapy practice. However, each participant was asked to perform their assigned exercise program at least five times per week, and exercises during the on-site visits were counted toward the required exercise frequency. After the four-week intervention, all participants were asked to continue their exercise program at home five times a week for another four weeks until their final follow-up visit at week 8. In addition, the participants were instructed on how to progress their exercises at home.
Data Analysis
All statistical analyses were performed using SPSS Statistics, Version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics, including frequency, means, and standard deviations, were calculated for the demographic data of the participants, including age, gender, height, weight, body mass index (BMI), the participants’ characteristics (e.g., duration of pain, distribution of pain, FABQ scores, and PROMIS scores), and the results of outcome measures (i.e., FMSTM, NPRS, and OSW scores). Independent t-and chi-square tests were used to determine if there was a difference in participants’ characteristics at baseline. Three separate 2 (group) x 4 (time) repeated measure (RM) ANOVAs were used to analyze the three outcome measures collected over four different time points. Post hoc analysis was performed if there was a significant interaction. The α level was set at 0.05 for all statistical analyses.
RESULTS
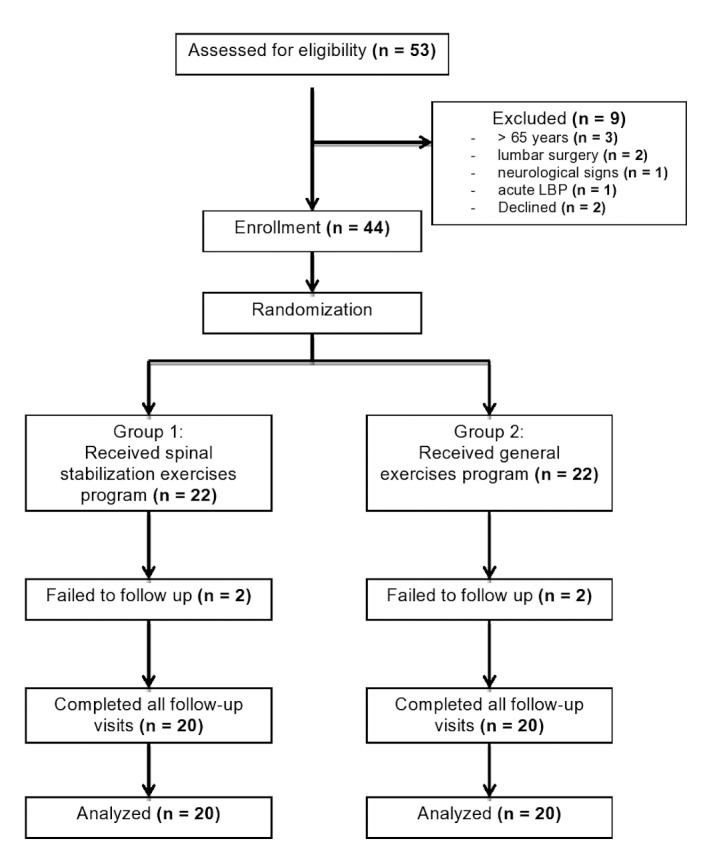
A total of 40 participants met the inclusion criteria and completed the eight-week exercise program (Figure 1).
Figure 1. Consort diagram of participants’ screening, enrollment, and randomization.
The characteristics of the participants and baseline outcome measurements are summarized in (Table 1).
Table 1. Participants’ Characteristics and Outcome Measurements (count or mean ± SD) at Baseline.
| All participants (n = 40) | SSE Group (n = 20) |
GE Group (n = 20) |
p-value (SSE vs. GE) |
|
|---|---|---|---|---|
| Age (years) | 39.9 ± 12.5 | 38.8 ± 11.8 | 41.0 ± 13.3 | 0.583 |
| Gender (male/female) | 23/17 | 13/7 | 10/10 | 0.337 |
| Weight (kg) | 79.8 ± 15.7 | 78.6 ± 15.6 | 81.1 ± 15.8 | 0.625 |
| Height (cm) | 169.7 ± 10.1 | 167.2 ± 9.8 | 172.3 ± 10.0 | 0.112 |
| BMI | 28.0 ± 6.6 | 28.6 ± 7.7 | 27.5 ± 5.5 | 0.612 |
| Average pain | 4.7 ± 1.8 | 4.5 ± 1.4 | 5.0 ± 2.2 | 0.397 |
| LBP onset symptoms (Insidious/Traumatic) | 36/4 | 18/2 | 18/2 | 1.000 |
| Duration of LBP (months) | 95.2 ± 87.5 | 78.6 ± 87.7 | 111.9 ± 86.3 | 0.234 |
| Side of LBP(central/right/left) | 14/13/13 | 6/7/7 | 8/6/6 | 0.803 |
| Distribution of pain | ||||
| LBP only | 30 (75%) | 15 (75%) | 15 (75%) | 1.000 |
| LBP + leg pain above the knee | 1 (2.5%) | 0 | 1 (5%) | |
| LBP + leg pain below the knee | 9 (22.5%) | 5 (25%) | 4 (20%) | 0.705 |
| PA duration (minute/week) | 99.7 ± 145.1 | 86.0 ± 162.9 | 113.5 ± 127.8 | 0.556 |
| FABQ | ||||
| Work | 8.4 ± 7.4 | 9.5 ± 6.7 | 7.2 ± 8.1 | 0.344 |
| Physical activity | 10.1 ± 6.5 | 10.1 ± 7.4 | 10.1 ± 5.6 | 1.000 |
| PROMIS-29 | ||||
| Physical function | 43.4 ± 2.4 | 43.4 ± 2.4 | 43.4 ± 2.4 | 0.512 |
| Anxiety | 51.2 ± 3.1 | 53.7 ± 2.8 | 51.2 ± 3.1 | 0.795 |
| Depression | 49.0 ± 3.2 | 49.0 ± 3.2 | 49.0 ± 3.2 | 0.815 |
| Fatigue | 55.1 ± 2.4 | 57.0 ± 2.3 | 53.1 ± 2.4 | 0.229 |
| Sleep disturbance | 52.4 ± 3.4 | 52.4 ± 3.4 | 52.4 ± 3.4 | 0.695 |
| Social roles | 51.9 ± 2.2 | 51.9 ± 2.2 | 53.7 ± 2.3 | 0.487 |
| Pain interference | 57.1 ± 1.9 | 55.6 ± 1.9 | 57.1 ± 1.9 | 0.558 |
| Average pain intensity | 4.3 ± 1.8 | 4.1 ± 1.2 | 4.4 ± 2.3 | 0.549 |
| Impact score | 20.1 ± 6.6 | 19.9 ± 6.9 | 20.3 ± 6.5 | 0.852 |
| NPRS | 3.5 ± 1.6 | 3.4 ± 1.3 | 3.5 ± 1.9 | 0.846 |
| OSW | 18.1 ± 9.1 | 18.2 ± 9.1 | 18.1 ± 9.4 | 0.973 |
| Modified FMSTM score | 10.7 ± 3.4 | 10.9 ± 3.2 | 10.6 ± 3.8 | 0.788 |
Note: SSE = spinal stabilization exercises, GE = general exercises, BMI = body mass index, LBP = low back pain, PA = physical activity, FABQ = Fear Avoidance Beliefs Questionnaire, PROMIS-29 = Patient-Reported Outcomes Measurement Information System, NPRS = Numeric Pain Rating Scale, OSW= Modified Oswestry Low Back Pain Disability Questionnaire, FMSTM = Functional Movement ScreenTM.
In general, the participants had mild LBP with an average NPRS score of 3.5 ± 1.6. There was no significant difference (p < 0.05) in any of the baseline participants’ characteristics, and outcome measurements between participants in the SSE group and those in the GE group (Table 1). Therefore, the two groups were considered similar at the beginning of the study.
Outcome Measurements
The means and standard deviations of all three outcome measurements at all four-time points are shown in (Table 2).
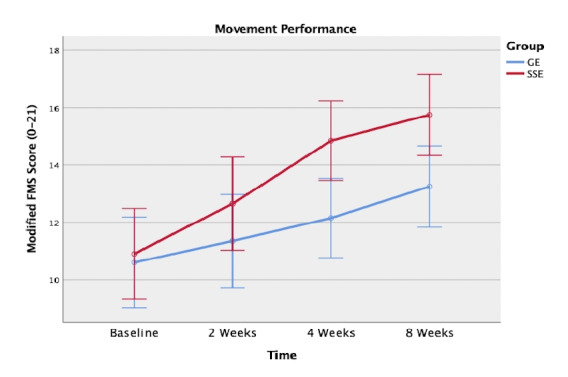
The ANOVA results showed a significant interaction of group by time, F (3, 114) = 3.599, p = 0.016, indicating that there was a significant difference in the modified FMSTM scores between groups over eight weeks. Next, separate 2 x 2 RM ANOVAs followed to examine the between-group differences between each two-time point. Consequently, significant between-group differences were found between baseline and four weeks (p = 0.005) and between baseline and eight weeks (p = 0.026) favor SSEs over GEs. In addition, there was a significant main effect of time. Post-hoc pair-wise comparisons showed that all participants made significant improvements in the modified FMSTM scores between the two adjacent time points: from baseline to two weeks (p = 0.011), from two weeks to four weeks (p = 0.001), and from four weeks to eight weeks (p = 0.008) (Figure 2).
Figure 2. Movement performance using the modified Functional Movement Screen scoring system between the spinal stabilization exercise (SSE) group and the general exercise (GE) group at baseline, 2 weeks, 4 weeks, and 8 weeks.
The RM ANOVA for the NPRS and OSW scores showed no significant interaction of group by time: F (3, 114) = 1.185, p = 0.319 and F (3, 114) = 0.538, p = 0.605, respectively.
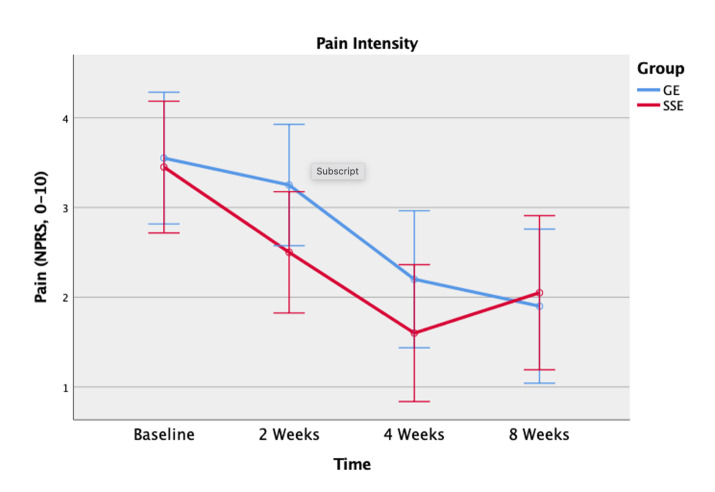
However, there was a significant main effect of time (p < 0.001) for both the NPRS and OSW scores. Post-hoc pair-wise comparisons showed significantly lower NPRS scores from baseline to two weeks (p = 0.007), from two weeks to four weeks (p < 0.001), but no significant difference from four weeks to eight weeks (p = 0.818) (Figure 3).
Figure 3. Pain intensity using the Numeric Pain Rating Scale (NPRS) between the spinal stabilization exercise (SSE) group and the general exercise (GE) group at baseline, 2 weeks, 4 weeks, and 8 weeks.
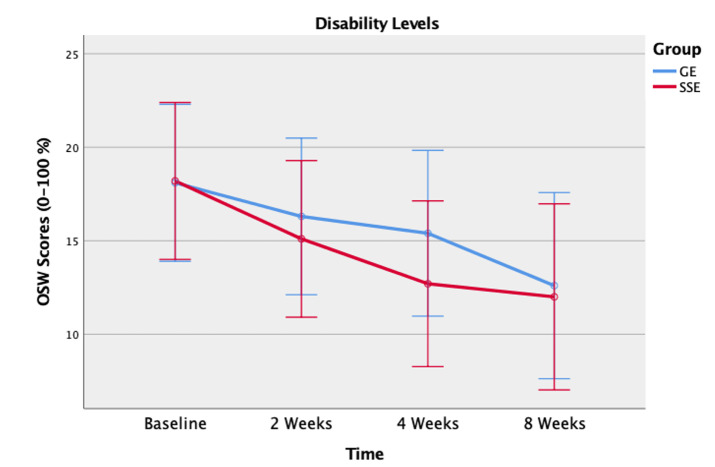
Similarly, post-hoc pairwise showed significantly lower OSW scores from baseline to two weeks (p = 0.017), from two weeks to four weeks (p = 0.047), but no significant difference from four weeks to eight weeks (p = 0.117) (Figure 4).
Figure 4. Disability levels using the Modified Oswestry Low Back Pain Disability Questionnaire (OSW) between the spinal stabilization exercise (SSE) group and the general exercise (GE) group at baseline, 2 weeks, 4 weeks, and 8 weeks.
Home Exercise Program Compliance
There were no significant differences between the two groups in the supervised phase and the unsupervised phase. However, both groups had significantly better exercise compliance in the supervised phase (first four weeks) than they did in the unsupervised phase (last four weeks) (p = 0.044 for the SSE group, p = 0.025 for the GE group) (Table 3).
Table 3. Home Exercise Compliance Rates (%, Mean ± SD).
| All (n = 40) |
SSE Group (n = 20) |
GE Group (n = 20) |
p-value (SSE vs. GE) |
|
|---|---|---|---|---|
| Supervised phase (0 – 4 weeks) |
85.9 ± 14.8 | 83.7 ± 13.9 | 88.0 ± 15.6 | 0.369 |
| Unsupervised phase (5 – 8 weeks) |
76.1 ± 22.7 | 74.7 ± 20.4 | 77.5 ± 25.4 | 0.707 |
| Entire study 0 – 8 weeks |
81.0 ± 16.7 | 79.2 ± 14.8 | 82.7 ± 18.8 | 0.516 |
|
p-value (supervised vs. unsupervised) |
0.002* | 0.044* | 0.0024* |
Note: SSE = spinal stabilization exercise, GE = general exercise.
DISCUSSION
Functional Performance
The modified FMSTM scores showed that participants who received SSEs had made significantly greater improvement on movement performance than those who received a GE program. The results suggest that the exercises prescribed to the individuals with CLBP should be specific to and target spinal stabilizers in order to improve quality of movement as compared to GEs, such as ROM and flexibility exercises. There is no published evidence regarding the effectiveness of the SSE program on movement performance in patients with LBP. However, this finding is in agreement with a previously published study by Bagherian et al., who demonstrated that the SSE program enhanced functional movement patterns in healthy, pain-free collegiate athletes, particularly for those who had low baseline FMSTM scores (i.e., ≤ 14).17 In contrast to the participants in the Bagherian et al. study, the participants in this study were those with CLBP. Although the participants in this study had low disability levels, the SSEs designed in this study were at a low level of difficulty and intensity as compared to those in the Bagherian et al. study, which included high-level exercises, such as back extension and sit-ups. Considering the improvement made by the participants in the SSE group, the dosage and progression of the SSEs seemed to be appropriate for this patient population.
The SSE group demonstrated a significantly greater improvement than the GE group in movement performance at the conclusion of the supervised phase (i.e., 4 weeks), and the differential effects were maintained at the end of the study period (i.e., 8 weeks). This finding is consistent with other studies which also found that a four-week SSE program was effective for enhancing stability and functional capabilities, as well as for reducing pain intensity in patients with CLBP.33,34 It was hypothesized that four weeks were necessary to alter neuromuscular control of the spinal column, and therefore improve inter-segmental spinal stability.34 Further examination of each of the seven test components at baseline, week 4, and week 8 revealed that the SSE group appeared to have greater improvement on the rotary stability test and the trunk stability push-up test than the GE group. Not surprisingly, these two test components were designed specifically to assess an individual’s spinal stability, which is consistent with the goal of the SSE program.12
The results of this study support that SSEs were effective in enhancing the spinal stabilizers, thus improving movement performance. Deficits in the spinal stabilizers are considered to be the primary cause of spinal instability leading to LBP.35 Specifically, the TrA and LM muscles are considered to play an essential role in lumbopelvic stabilization. Impairments inactivation and coordination of the TrA and LM muscles have been identified in patients with CLBP and are believed to contribute to their poor movement coordination.36–38 Therefore, strength and proper activation of these muscles are necessary for the stability of the lumbar spine in order to restore proper functional movements for this patient population, as indicated by the results of this study.37–41 Furthermore, the literature supports the use of SSEs for individuals with LBP for improving neuromuscular control and endurance, retraining and strengthening deep spinal muscles, reducing pain, and enhancing proprioception related to the dysfunction.24,25,42
Pain Intensity and Disability Level
At eight weeks, the SSE group had a reduction in NPRS score of 1.4 points from baseline, and the GE group had a reduction in NPRS score of 1.6 points from baseline. Neither group demonstrated a clinically meaningful change in pain intensity that exceeded the minimal detectable change (MDC) or minimal clinically important change (MCID) score of 2 for the NPRS in individuals with LBP.43 Although there was no difference in pain between groups, there were differences in the modified FMSTM scores between groups. For individuals with CLBP who have low levels of pain, the NPRS may not be a useful outcome measure to examine treatment effects. Instead, a high functional level test, such as the FMSTM, may be necessary to detect different treatment effects.
Similar to the result of the NPRS scores, the results of the study indicated no significant differences in disability reduction between the SSE program and the GE program over eight weeks. This result implies that both exercise interventions had an equivalent effect on functional improvement and disability reduction. Both groups were considered to have a minimal disability level at baseline (OSW score:18.2 for the SEE group and 18.1 for the GE group). Although the participants in this study reported minimal disability levels at baseline, all participants demonstrated significant improvement in disability levels, in the first four weeks. Although neither group demonstrated a clinically meaningful change in their disability level that exceeded the MDC of 10.5 for the OSW in individuals with LBP, only the SSE group’s disability improvement (OSW score: 6.2 points) exceeded the MCID of 6 points.6,44 The minimal pain intensity and disability levels at baseline could have contributed lack of clinically meaningful changes. However, participants with high pain and disability levels may not be able to complete the FMSTM.
Implications for Clinical Practice
The FMSTM with the modified scoring system may be a useful outcome measure for assessing the quality of movement, specifically in adults with LBP. Identification and quantification of abnormal movement patterns may allow therapists to address movement impairments in their plan of care.
Limitations of the Study
There were limitations in this study. The participants in this study had low NPRS and OSW scores. Therefore, the results of this study only can be generalized to those individuals with CLBP with low pain intensity and mild disability levels. However, participants with a moderate or moderate-to-high level of pain may not be able to complete or perform the FMSTM tests or SSE program. Furthermore, this study was conducted on participants between 18 and 65 years old. Therefore, this study might not be generalized to patients over 65 years old. The other limitation is that the participants were not restricted from other physical activities although participants were advised not to engage in any activity that might increase their LBP. However, the randomization procedure may have minimized this uncontrolled factor. Lastly, medication use was not controlled in this study in order to reflect the current clinical practice collected at baseline. However, at each follow-up visit, all participants were asked if they had taken any medication because of their LBP. Future studies should examine the effects of eight-week supervised treatments (e.g., SSEs) in order to achieve better outcomes and maximize the benefits of the treatment. In addition, it is recommended that future studies should examine the effectiveness of SSEs on the movement performance of individuals who have moderate and higher pain intensity of LBP and disability levels. Furthermore, longer-term follow-ups are recommended for future studies to examine the effects of physical therapy interventions on movement performance in patients with CLBP.
Conclusion
The results of this study suggest that SSEs are more effective in enhancing movement performance than GEs over a period of eight weeks in individuals with CLBP. In addition, all participants in both groups demonstrated a significant reduction in pain intensity and disability level while attending supervised PT sessions in the first four weeks of the study. However, these significant improvements seemed to be diminished during the unsupervised PT sessions for the last four weeks when the participants stopped meeting regularly with the investigators. Moreover, this study demonstrated that supervised SSE sessions seemed to maximize the benefits of this treatment including improving the quality of movement and reducing the aberrant movement that is associated with CLBP.
DISCLOSURE STATEMENT
The authors declare that there is no conflict of interest. In addition, the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and the statement that the results of the present study do not constitute an endorsement by ACSM.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
The authors would like to thank the Texas Physical Therapy Foundation for funding this project.
References
- Non-specific low back pain. Balagué Federico, Mannion Anne F, Pellisé Ferran, Cedraschi Christine. Feb;2012 The Lancet. 379(9814):482–491. doi: 10.1016/s0140-6736(11)60610-7. doi: 10.1016/s0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- The rising prevalence of chronic low back pain. Freburger Janet K., Holmes George M., Agans Robert P., Jackman Anne M., Darter Jane D., Wallace Andrea S., Castel Liana D., Kalsbeek William D., Carey Timothy S. Feb 9;2009 Archives of Internal Medicine. 169(3):251–258. doi: 10.1001/archinternmed.2008.543. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The global burden of cancer 2013. Fitzmaurice Christina, Dicker Daniel, Pain Amanda, Hamavid Hannah, Moradi-Lakeh Maziar, MacIntyre Michael F., Allen Christine, Hansen Gillian, Woodbrook Rachel, Wolfe Charles, Hamadeh Randah R., Moore Ami, Werdecker Andrea, Gessner Bradford D., Te Ao Braden, McMahon Brian, Karimkhani Chante, Yu Chuanhua, Cooke Graham S., Schwebel David C., Carpenter David O., Pereira David M., Nash Denis, Kazi Dhruv S., De Leo Diego, Plass Dietrich, Ukwaja Kingsley N., Thurston George D., Yun Jin Kim, Simard Edgar P., Mills Edward, Park Eun-Kee, Catalá-López Ferrán, deVeber Gabrielle, Gotay Carolyn, Khan Gulfaraz, Hosgood H. Dean III, Santos Itamar S., Leasher Janet L., Singh Jasvinder, Leigh James, Jonas Jost B., Sanabria Juan, Beardsley Justin, Jacobsen Kathryn H., Takahashi Ken, Franklin Richard C., Ronfani Luca, Montico Marcella, Naldi Luigi, Tonelli Marcello, Geleijnse Johanna, Petzold Max, Shrime Mark G, Younis Mustafa, Yonemoto Naohiro, Breitborde Nicholas, Yip Paul, Pourmalek Farshad, Lotufo Paulo A., Esteghamati Alireza, Hankey Graeme J., Ali Raghib, Lunevicius Raimundas, Malekzadeh Reza, Dellavalle Robert, Weintraub Robert, Lucas Robyn, Hay Roderick, Rojas-Rueda David, Westerman Ronny, Sepanlou Sadaf G., Nolte Sandra, Patten Scott, Weichenthal Scott, Abera Semaw Ferede, Fereshtehnejad Seyed-Mohammad, Shiue Ivy, Driscoll Tim, Vasankari Tommi, Alsharif Ubai, Rahimi-Movaghar Vafa, Vlassov Vasiliy V., Marcenes W. S., Mekonnen Wubegzier, Melaku Yohannes Adama, Yano Yuichiro, Artaman Al, Campos Ismael, MacLachlan Jennifer, Mueller Ulrich, Kim Daniel, Trillini Matias, Eshrati Babak, Williams Hywel C., Shibuya Kenji, Dandona Rakhi, Murthy Kinnari, Cowie Benjamin, Amare Azmeraw T., Antonio Carl Abelardo, Castañeda-Orjuela Carlos, van Gool Coen H., Violante Francesco, Oh In-Hwan, Deribe Kedede, Soreide Kjetil, Knibbs Luke, Kereselidze Maia, Green Mark, Cardenas Rosario, Roy Nobhojit, Tillmann Taavi, Li Yongmei, Krueger Hans, Monasta Lorenzo, Dey Subhojit, Sheikhbahaei Sara, Hafezi-Nejad Nima, Kumar G. Anil, Sreeramareddy Chandrashekhar T., Dandona Lalit, Wang Haidong, Vollset Stein Emil, Mokdad Ali, Salomon Joshua A., Lozano Rafael, Vos Theo, Forouzanfar Mohammad, Lopez Alan, Murray Christopher, Naghavi Mohsen. Jul 1;2015 JAMA Oncology. 1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low back pain in healthy postmenopausal women and the effect of physical activity: A secondary analysis in a randomized trial. Marini Mirca, Bendinelli Benedetta, Assedi Melania, Occhini Daniela, Castaldo Maria, Fabiano Jacopo, Petranelli Marco, Migliolo Mario, Monaci Marco, Masala Giovanna. May 10;2017 PloS One. 12(5):e0177370. doi: 10.1371/journal.pone.0177370. doi: 10.1371/journal.pone.0177370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical observation of standing trunk movements: What do the aberrant movement patterns tell us? Biely Scott A., Silfies Sheri P., Smith Susan S., Hicks Gregory E. Apr;2014 Journal of Orthopaedic & Sports Physical Therapy. 44(4):262–272. doi: 10.2519/jospt.2014.4988. doi: 10.2519/jospt.2014.4988. [DOI] [PubMed] [Google Scholar]
- A comparison of five low back disability questionnaires: Reliability and responsiveness. Davidson Megan, Keating Jennifer L. Jan 1;2002 Physical Therapy. 82(1):8–24. doi: 10.1093/ptj/82.1.8. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]
- Differences in performance on the functional movement screen between chronic low back pain patients and healthy control subjects. Ko Min-Joo, Noh Kyung-Hee, Kang Min-Hyeok, Oh Jae-Seop. 2016Journal of Physical Therapy Science. 28(7):2094–2096. doi: 10.1589/jpts.28.2094. doi: 10.1589/jpts.28.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Description of movement quality in patients with low back pain: A qualitative study as a first step to a practical definition. van Dijk Margriet J.H., Smorenburg Nienke T.A., Visser Bart, Nijhuis–van der Sanden Maria W.G., Heerkens Yvonne F. Feb 14;2017 Physiotherapy Theory and Practice. 33(3):227–237. doi: 10.1080/09593985.2017.1282998. doi: 10.1080/09593985.2017.1282998. [DOI] [PubMed] [Google Scholar]
- The assessment of function: How is it measured? A clinical perspective. Reiman Michael P, Manske Robert C. May;2011 Journal of Manual & Manipulative Therapy. 19(2):91–99. doi: 10.1179/106698111x12973307659546. doi: 10.1179/106698111x12973307659546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measuring and managing pain and performance. Simmonds Maureen J. Aug;2006 Manual Therapy. 11(3):175–179. doi: 10.1016/j.math.2006.03.002. doi: 10.1016/j.math.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Functional movement screening: The use of fundamental movements as an assessment of function‐part 1. Gray Cook L B, Hoogenboom B J, Voight M. 2014Int J Sports Phys Ther. 9(3):396. [PMC free article] [PubMed] [Google Scholar]
- Functional movement screening: The use of fundamental movements as an assessment of function‐part 2. Gray Cook L B, Hoogenboom B J, Voight M. 2014Int J Sports Phys Ther. 9(4):549. [PMC free article] [PubMed] [Google Scholar]
- Modifiable risk factors predict injuries in firefighters during training academies. Butler R.J., Contreras M., Burton L.C., Plisky P.J., Goode A., Kiesel K. 2013Work. 46(1):11–17. doi: 10.3233/WOR-121545. [DOI] [PubMed] [Google Scholar]
- Use of a functional movement screening tool to determine injury risk in female collegiate athletes. Chorba R.S., Chorba D.J., Bouillon L.E., Overmyer C.A., Landis J.A. 2010N Am J Sports Phys Ther. 5(2):47. [PMC free article] [PubMed] [Google Scholar]
- Association between ROWING injuries and the functional movement SCREEN™ in female collegiate division I ROWERS. Clay H., Mansell J., Tierney R. 2016Int J Sports Phys Ther. 11(3):345. [PMC free article] [PubMed] [Google Scholar]
- Functional movement test scores improve following a standardized off-season intervention program in professional football players. Kiesel K., Plisky P., Butler R. Mar 10;2011 Scandinavian Journal of Medicine & Science in Sports. 21(2):287–292. doi: 10.1111/j.1600-0838.2009.01038.x. doi: 10.1111/j.1600-0838.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- The effect of core stability training on functional movement patterns in college athletes. Bagherian Sajad, Ghasempoor Khodayar, Rahnama Nader, Wikstrom Erik A. Jul;2019 Journal of Sport Rehabilitation. 28(5):444–449. doi: 10.1123/jsr.2017-0107. doi: 10.1123/jsr.2017-0107. [DOI] [PubMed] [Google Scholar]
- Active duty firefighters can improve functional movement screen (FMS) scores following an 8-week individualized client workout program. Stanek Justin M., Dodd Daniel J., Kelly Adam R., Wolfe Alex M., Swenson Ryan A. Mar 14;2017 Work. 56(2):213–220. doi: 10.3233/wor-172493. doi: 10.3233/wor-172493. [DOI] [PubMed] [Google Scholar]
- Non-pharmacological interventions for chronic pain in multiple sclerosis. Amatya Bhasker, Young Jamie, Khan Fary. Dec 19;2018 Cochrane Database of Systematic Reviews. 2018(12) doi: 10.1002/14651858.cd012622.pub2. doi: 10.1002/14651858.cd012622.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low back pain: Clinical practice guidelines linked to the international classification of functioning, disability, and health from the Orthopaedic section of the american physical therapy association. Delitto Anthony, George Steven Z., Van Dillen Linda R., Whitman Julie M., Sowa Gwendolyn, Shekelle Paul, Denninger Thomas R., Godges Joseph J. Apr;2012 Journal of Orthopaedic & Sports Physical Therapy. 42(4):A1–A57. doi: 10.2519/jospt.2012.42.4.a1. doi: 10.2519/jospt.2012.42.4.a1. [DOI] [PubMed] [Google Scholar]
- Exercises for nonspecific low back pain treatment. Lizier Daniele Tatiane, Perez Marcelo Vaz, Sakata Rioko Kimiko. Dec;2012 Revista Brasileira de Anestesiologia. 62(6):842–846. doi: 10.1590/s0034-70942012000600008. doi: 10.1590/s0034-70942012000600008. [DOI] [PubMed] [Google Scholar]
- Medications for acute and chronic low back pain: A review of the evidence for an american pain society/american college of physicians clinical practice guideline. Chou Roger, Huffman Laurie Hoyt. Oct 2;2007 Annals of Internal Medicine. 147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- Exercise therapy for treatment of non-specific low back pain. Hayden Jill, van Tulder Maurits W, Malmivaara Antti, Koes Bart W. Jul 20;2005 Cochrane Database of Systematic Reviews. 2005(3) doi: 10.1002/14651858.cd000335.pub2. doi: 10.1002/14651858.cd000335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core stability: The centerpiece of any training program. Bliss Lisa S., Teeple Peter. Jun;2005 Current Sports Medicine Reports. 4(3):179–183. doi: 10.1097/01.csmr.0000306203.26444.4e. doi: 10.1097/01.csmr.0000306203.26444.4e. [DOI] [PubMed] [Google Scholar]
- Association of physical performance and fear-avoidance beliefs in adults with chronic low back pain. Panhale V.P., Gurav R.S., Nahar S.K. 2016Ann Med Res. 6(6):375–379. doi: 10.4103/amhsr.amhsr_331_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A meta-analysis of core stability exercise versus general exercise for chronic low back pain. Wang Xue-Qiang, Zheng Jie-Jiao, Yu Zhuo-Wei, Bi Xia, Lou Shu-Jie, Liu Jing, Cai Bin, Hua Ying-Hui, Wu Mark, Wei Mao-Ling, Shen Hai-Min, Chen Yi, Pan Yu-Jian, Xu Guo-Hui, Chen Pei-Jie. Dec 17;2012 PloS One. 7(12):e52082. doi: 10.1371/journal.pone.0052082. doi: 10.1371/journal.pone.0052082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efficacy of segmental stabilization exercise for lumbar segmental instability in patients with mechanical low back pain: A randomized placebo controlled crossover study. Kumar Senthil P. 2011North American Journal of Medical Sciences. 3(10):456–461. doi: 10.4297/najms.2011.3456. doi: 10.4297/najms.2011.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Faul Franz, Erdfelder Edgar, Lang Albert-Georg, Buchner Axel. May;2007 Behavior Research Methods. 39(2):175–191. doi: 10.3758/bf03193146. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Multidisciplinary biopsychosocial rehabilitation for subacute low back pain. Marin Teresa J, Van Eerd Dwayne, Irvin Emma, Couban Rachel, Koes Bart W, Malmivaara Antti, van Tulder Maurits W, Kamper Steven J. Jun 28;2017 Cochrane Database of Systematic Reviews. 2017(6) doi: 10.1002/14651858.cd002193.pub2. doi: 10.1002/14651858.cd002193.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. Movement: Functional movement systems: Screening, assessment. Corrective Strategies. On Target Publications; Aptos, CA: [Google Scholar]
- Reliability and validity of the functional movement screen™ with a modified scoring system for young adults with low back pain. Alkhathami Khalid, Alshehre Yousef, Wang-Price Sharon, Brizzolara Kelli. Jun 1;2021 International Journal of Sports Physical Therapy. 16(3):620–627. doi: 10.26603/001c.23427. doi: 10.26603/001c.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Hicks Gregory E., Fritz Julie M., Delitto Anthony, McGill Stuart M. Sep;2005 Archives of Physical Medicine and Rehabilitation. 86(9):1753–1762. doi: 10.1016/j.apmr.2005.03.033. doi: 10.1016/j.apmr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: A randomized clinical trial. Inani Sumit B., Selkar Sohan P. Jan 28;2013 Journal of Back and Musculoskeletal Rehabilitation. 26(1):37–43. doi: 10.3233/bmr-2012-0348. doi: 10.3233/bmr-2012-0348. [DOI] [PubMed] [Google Scholar]
- Effect of spinal stabilization exercise on dynamic postural control and visual dependency in subjects with chronic non-specific low back pain. Salavati Mahyar, Akhbari Behnam, Takamjani Ismail Ebrahimi, Bagheri Hossein, Ezzati Kamran, Kahlaee Amir Hossein. Apr;2016 Journal of Bodywork and Movement Therapies. 20(2):441–448. doi: 10.1016/j.jbmt.2015.10.003. doi: 10.1016/j.jbmt.2015.10.003. [DOI] [PubMed] [Google Scholar]
- The effects of gluteus muscle strengthening exercise and lumbar stabilization exercise on lumbar muscle strength and balance in chronic low back pain patients. Jeong Ui-Cheol, Sim Jae-Heon, Kim Cheol-Yong, Hwang-Bo Gak, Nam Chan-Woo. 2015Journal of Physical Therapy Science. 27(12):3813–3816. doi: 10.1589/jpts.27.3813. doi: 10.1589/jpts.27.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunk muscles strength as a risk factor for nonspecific low back pain: A pilot study. Cho Kang Hee, Beom Jae Won, Lee Tae Sung, Lim Jun Ho, Lee Tae Heon, Yuk Ji Hyun. 2014Annals of Rehabilitation Medicine. 38(2):234–240. doi: 10.5535/arm.2014.38.2.234. doi: 10.5535/arm.2014.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inefficient muscular stabilization of the lumbar spine associated with low back pain: A motor control evaluation of transversus abdominis. Hodges Paul W., Richardson Carolyn A. Nov;1996 Spine. 21(22):2640–2650. doi: 10.1097/00007632-199611150-00014. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Clinical spinal instability and low back pain. Panjabi Manohar M. Aug;2003 Journal of Electromyography and Kinesiology. 13(4):371–379. doi: 10.1016/s1050-6411(03)00044-0. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Core strength training for patients with chronic low back pain. Chang Wen-Dien, Lin Hung-Yu, Lai Ping-Tung. 2015Journal of Physical Therapy Science. 27(3):619–622. doi: 10.1589/jpts.27.619. doi: 10.1589/jpts.27.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The effects of abdominal muscle coactivation on lumbar spine stability. Gardner-Morse Mack G., Stokes Ian A. Jan;1998 Spine. 23(1):86–91. doi: 10.1097/00007632-199801010-00019. doi: 10.1097/00007632-199801010-00019. [DOI] [PubMed] [Google Scholar]
- Electromyographic and kinetic analysis of two abdominal muscle performance tests. Haladay Douglas E., Denegar Craig R., Miller Sayers J., Challis John. Oct 14;2015 Physiotherapy Theory and Practice. 31(8):587–593. doi: 10.3109/09593985.2015.1062945. doi: 10.3109/09593985.2015.1062945. [DOI] [PubMed] [Google Scholar]
- Quantification of lumbar stability by using 2 different abdominal activation strategies. Grenier Sylvain G., McGill Stuart M. Jan;2007 Archives of Physical Medicine and Rehabilitation. 88(1):54–62. doi: 10.1016/j.apmr.2006.10.014. doi: 10.1016/j.apmr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Responsiveness of the numeric pain rating scale in patients with low back pain. Childs John D., Piva Sara R., Fritz Julie M. Jun;2005 Spine. 30(11):1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Fritz Julie M, Irrgang James J. Feb 1;2001 Physical Therapy. 81(2):776–788. doi: 10.1093/ptj/81.2.776. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.