Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has a serious impact on HIV-infected individuals due to limited access to treatment services. This report aimed to describe four cases of oral lesions in HIV-infected antiretroviral-naive patients found during the COVID-19 pandemic.
Case
Four patients, males, with an age ranged from 29 to 53 years, came to Oral Medicine Department with chief complaints of lesions on their mouth. They had postponed their visit to healthcare services due to limited access during pandemic. Three patients had just been diagnosed with HIV and had not yet received anti-retrovirus, while 1 patient had not yet been detected with HIV. From the clinical examination and laboratory findings, we diagnosed the lesions with mucous patches, chronic atrophic candidiasis, angular cheilitis, necrotizing ulcerative gingivitis, linear gingival erythema, cytomegalovirus-associated ulcers, and oral hairy leukoplakia.
Case Management
We gave chlorhexidine gluconate 0.2% mouthwash for mucous patches, nystatin oral suspension for chronic atrophic candidiasis, miconazole cream 2% for angular cheilitis, debridement with hydrogen peroxide 1.5% and rinsed with normal saline for necrotizing ulcerative gingivitis, and diphenhydramine hydrochloride and 0.2% chlorhexidine gluconate for CMV ulcers. All patients showed good clinical improvement after the treatments.
Conclusion
Oral lesions are still commonly found in HIV-infected patients during COVID-19 pandemic. Dentists remain to have a crucial role in the early diagnosis and treatment of HIV-associated oral lesions during COVID-19 pandemic that will have an impact on HIV treatments, also in implementing the Bali Declaration on oral health in HIV/AIDS 2019 to support UNAIDS goal to end AIDS by 2030.
Keywords: ARV-naive patients, COVID-19 pandemic, HIV, oral lesions
Introduction
HIV (human immunodeficiency virus) is a virus that can cause AIDS (acquired immunodeficiency virus) and is still a serious health problem. HIV is a member of the lentivirus family and a subgroup of retroviruses.1 Oral manifestations are the earliest and the most important indicator of HIV infection.2,3 Oral lesions can not only indicate HIV infection but are also one of the earliest clinical features and can predict the progression of HIV to AIDS. Therefore, oral lesions can be used to determine anti-HIV therapy and are used in staging and classification systems.2 The factors related to oral manifestations that occur in HIV patients are a CD4+ lymphocyte count of fewer than 200 cells/mm3 and a high viral load.2 A diagnosis of oral lesions may point to a positive HIV/AIDS status. The progression of HIV infection is associated with certain oral lesions, including candidiasis, hairy leukoplakia, and Kaposi’s sarcoma. The severity of lesions depends on a low CD4+ cell count.4
The oral manifestations of HIV infection can be categorized into infections, neoplasms, immune mediated, other diseases (parotid disease, nutritional, xerostomia), and oral manifestations as adverse effects of anti-retroviral therapy. Oral or pharyngeal candidiasis are the commonest fungal infections observed as the initial manifestation of symptomatic HIV infection. Oral hairy leukoplakia has been shown to be associated with Epstein–Barr virus (EBV) infection. Oral candidiasis and oral hairy leukoplakia are associated very frequently and are considered AIDS defining disease and have also been included in the clinical classification of HIV by Centers for Disease Control and Prevention (CDC). Other infectious diseases commonly occurring in HIV patients are herpes simplex and varicella zoster virus infection, Cytomegalovirus-related ulcerations, oral warts associated with human papilloma virus, linear erythematous gingivitis, necrotizing ulcerative gingivitis, necrotizing ulcerative periodontitis, and syphilis. Kaposi’s sarcoma and non-Hodgkin’s lymphoma are the most common neoplasms occur in HIV patients. The disorders of the immune system also lead to various oral manifestations, such as aphthous ulcers, xerostomia and necrotizing stomatitis.5,6 Oral manifestations such as adverse effect of anti-retroviral therapy can also be found. They are oral hyperpigmentation, erythema multiforme, xerostomia, paresthesia, lip edema, cheilitis, and taste disturbances.6
Oral lesions occurred in up to 50% of the HIV-infected patients and up to 80% of the AIDS patients. Since oral lesions are considered the first clinical features of HIV infection and markers of highly predictive immunosuppression, they can be useful for early testing, diagnosis, and treatment of HIV/AIDS patients. In 1993, EC-Clearinghouse arranged the classification and diagnostic criteria for oral lesions in HIV infection. It consists of three major groups, namely lesions strongly associated with HIV infection, lesions less commonly associated with HIV infection, and lesions seen in HIV infection. One of the lesions from each group will be described in this article.5
COVID-19 is a respiratory infectious disease that first appeared in China in early December 2019. In March 2020, WHO declared it a pandemic.7 The pandemic in Indonesia started in early March 2020 and reached 56,385 confirmed cases, with 2876 deaths by the end of June 2021.8 The COVID-19 pandemic situation makes people afraid because the disease is very contagious. People avoid going to health facilities if they are not in a really life-threatening situation.7
One year of COVID-19 Pandemic, COVID-19 cases continued to fluctuate. The existing healthcare infrastructure in Indonesia is inadequate to deal with the increasing demands for healthcare services.9 The government even asked to postpone routine health checks to avoid people from being exposed to COVID-19. A recent survey showed that almost one-third of respondents who needed consultation about their health was postponed by health facilities to avoid COVID-19. About a quarter of respondents stated they have difficulty getting health services because many health facilities were closed.9 As well as health facilities, at the beginning of the pandemic they limited the number of patient visits. People living with HIV (PLWH) in many recent studies have serious impact due to a limitation of health services in the early COVID-19 pandemic specifically limited in HIV testing and ART initiations. Delayed in examining the patient’s condition allowed the patient’s condition to be unknown so that HIV treatment is also delayed. Many people remain at high risk of HIV infection due to lack of or limited access to prevention and treatment services.10
Antiretroviral therapy (ART) allowed a significant decrease in the appearance of opportunistic infections, together with a decrease in mortality and an increase in the survival and quality of life in HIV-infected patients. The decrease in some oral manifestations, such as oral candidiasis, Kaposi’s sarcoma, and oral hairy leukoplakia, are considered efficacy markers of ART.3
Since oral manifestations are the earliest clinical appearance of HIV infection and markers of immune suppression, dentists have a crucial role to play in early detection screening for oral lesions associated with HIV infection and AIDS, and to contribute in ending the HIV epidemic as stated in the Bali Declaration on oral health in HIV/AIDS 2019.11,12 Early recognition can reduce patients’ morbidity and improve their welfare.13 The purpose of this article is to describe four cases of oral lesions in HIV-infected antiretroviral (ARV)-naive patients during the COVID-19 pandemic.
Case Presentation
Case 1
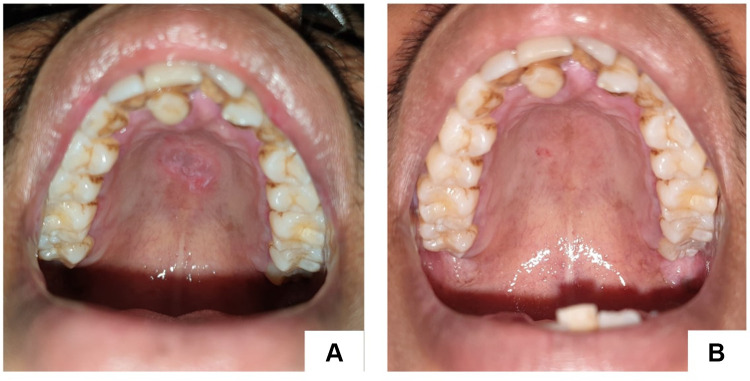
A 35-year-old man diagnosed with HIV stage II was referred to the Oral Medicine Specialist, Dr. Central General Hospital Hasan Sadikin, Bandung, with secondary syphilis, suspected neurosyphilis, Ocular syphilis, and atypical optic neuritis et causa panuveitis ocular dextra sinistra, hepatitis B, and pruritic papular eruption. He had a history of drinking alcohol from the age of 15 years until he was 29 years, but did not consume alcohol again until now. He started to have free sex in the last 3 years, both with men and women. There were multiple lesions, excoriations, confluent, irregular shape, well defined, partially raised with hypopigmented macules and erythematous papules on the chest, abdomen, back, and both upper and lower limbs. Intraoral examination showed irregular painless multiple ulcers on the palate with white margins, and yellow reddish base (Figure 1). Immunoserological examination of anti-HIV two weeks ago was reactive, hepatitis b surface antigen (HBsAg) chromatography was reactive, Treponema pallidum hemagglutination assay (TPHA) titer was reactive with titer 1:10,240, and quantitative venereal disease research laboratory test (VDRL) was reactive with titer 1:256. From the medical history and the clinical feature, we diagnosed with a mucous patch. He was given chlorhexidine gluconate 0.2% mouthwash. The lesions healed on the second visit, 2 weeks later (Figure 1).
Figure 1.
Painless single ulcer, with a red base, white edges on the palate (A). Lesions healed in second visit, 14 days later (B).
Case 2
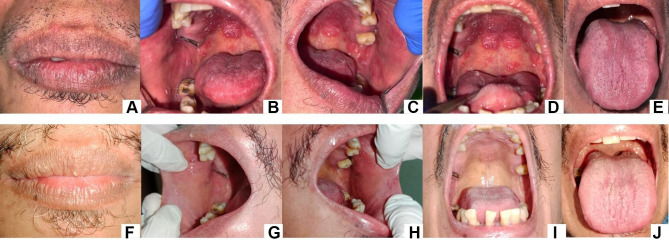
A 53-year-old man with no history of systemic disease came to the Oral Medicine Specialist, Dr. Hasan Sadikin Hospital, Bandung, with complaints of discomfort and pain in the oral cavity, especially when eating for 1 month. The patient has visited a general practitioner and was given many medicines but has not recovered. He had no fever history in the last month. Extra oral examination showed reddish fissure at both corners of the mouth (Figure 2A). Intra-oral examination revealed white plaques and erythematous lesions on the right and left buccal mucosa, palate and the tongue (Figure 2B–E). The diagnosis of chronic atrophic candidiasis and angular cheilitis were made. The patient was asked to take an anti-HIV test and the result was reactive. He was referred to Internal Medicine Department for further HIV management. Three weeks later, on the second visit, all lesions were completely healed (Figure 2F–J).
Figure 2.
White plaque with erythematous area on the palate, buccal mucosa right and left, and tongue (A–E). Fissured on the corners of the lip. Second visit (three weeks), the lesions disappeared (F–J).
Case 3
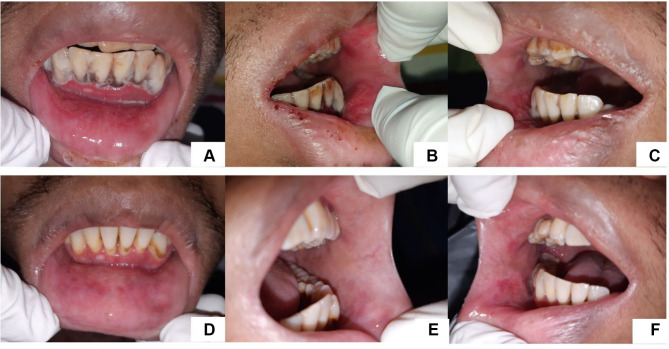
A 29-year-old man was referred by the Department of Internal Medicine regarding lesions in his oral cavity. He complained about pain in the lower anterior jaw gum and lower lip for a week and had a fever history in the last 2 weeks. He was diagnosed by the Internist with HIV stage IV with wasting syndrome, hypothyroidism, latent syphilis, chronic hepatitis B, moderate dehydration et causa inadequate intake, stage 1 acute kidney injury (AKI), inflammatory anemia, and malnutrition. On extra oral examination, we found exfoliative lips, and the lips looked pale. From the intra-oral examination, we found multiple irregular painful erosion on the lower labial mucosa. The anterior mandibular gingiva was edematous and tended to bleed and there was an excruciating grayish-white plaque in the region of teeth 33 to 43 (Figure 3). On the upper anterior gingiva, reddish bands appeared on the attached gingiva of teeth 11 and 21 (Figure 3). Laboratory examinations showed reactive TPHA with titer 1:5120 and reactive VDRL with titer 1:64, reactive hepatitis B e antigen (HBeAg), reactive HBsAg, non-reactive anti-hepatitis c virus (HCV). Examination of Mycobacterium tuberculosis using the Assay method did not find Mycobacterium tuberculosis bacteria. The lesions on the gingiva were diagnosed with necrotizing ulcerative gingivitis (NUG). The lesion on the maxillary anterior gingiva was diagnosed as linear gingival erythema.
Figure 3.
Greyish white plaques on mandibular anterior gingiva, erosion on lower labial mucosa (A). Erosion on left buccal mucosa (B). Erosion on left buccal mucosa (C). The lesion has improved (7 days) (D–F).
Necrotizing ulcerative gingivitis in this patient was treated with 1.5% hydrogen peroxide irrigation and rinsed with saline solution. Patients were also asked to rinse their mouth with 0.2% chlorhexidine gluconate 3 times a day and regularly clean their teeth and tongue at least 2 times a day. We did not prescribe any antibiotic because Internal Medicine had given azithromycin for systemic infection. After 7 days of therapy, the lesions improved (Figure 3).
Case 4
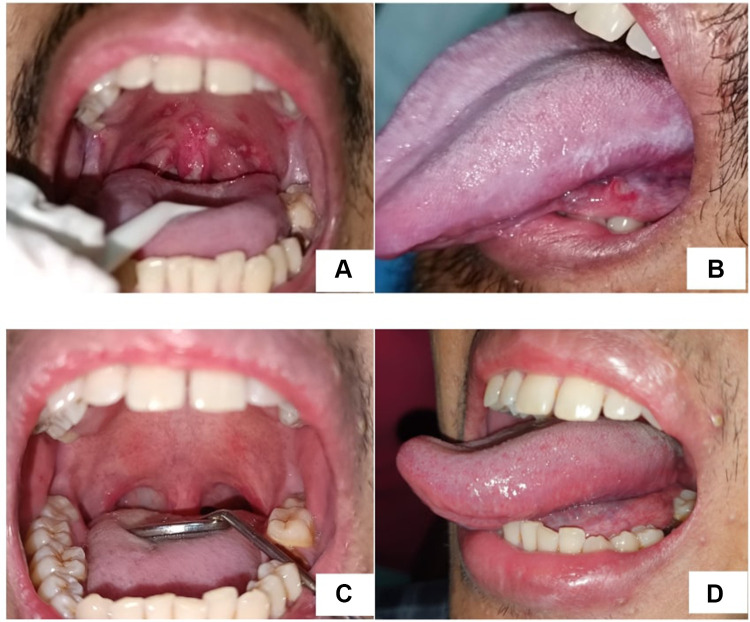
A 29-year-old man referred from the Department of Internal Medicine regarding lesions in his oral cavity and complained of pain when swallowing. He was diagnosed with HIV stage IV with wasting syndrome, sol intracranial et causa toxoplasmosis cerebri, anemia et causa gastrointestinal bleeding, hyponatremia et causa Syndrome of Inappropriate Antidiuretic Hormone secretion (SIADH), and malnutrition. The intraoral examination found a painful single ulcer on the ventrolateral of the tongue, a white-yellowish base surrounded by a halo erythematous. There were also painful multiple ulcers, white yellowish base, shallow, irregular, confluent, surrounded by halo erythematous on the soft palate extending to the oropharynx and uvula (Figure 4A). We also found painless white corrugated, adherent plaques. It could not be scrapped (Figure 4B).
Figure 4.
Multiple ulcers on soft palate, oropharynx, uvula (A), single ulcer on ventrolateral of the tongue and white corrugated, adherent plaques on left lateral of the tongue (B) two months after first visit, the lesions improved (C and D).
We suspected CMV ulcers, so we suggested the patient to take some laboratory tests to establish the diagnosis of ulcers. Laboratory examination showed reactive antibody cytomegalovirus (CMV) IgG, nine times higher than the threshold. Polymerase chain reaction (PCR) of CM to detect viral DNA was reactive and showed 7.11×102 copies/mL. Therefore, the ulcers in the oral cavity were diagnosed as CMV ulcers. The painless adherent white corrugated plaques that cannot be scrapped on the lateral of the tongue were diagnosed as oral hairy leukoplakia.
Folic acid, clindamycin, co-trimoxazole, and fluconazole were prescribed by the internist, and ARV was given three weeks later. We gave diphenhydramine hydrochloride and gargling with 0.2% chlorhexidine gluconate. Two months after therapy, the ulcers disappeared (Figure 4C), and the pain while swallowing has gone. Oral hairy leukoplakia on the lateral of the tongue was also healed (Figure 4D).
Discussion
The European Clearinghouse updated in 2009 classified oral lesions associated with HIV infections into 3 groups. In this case report, we found some lesions belong to group I that are strongly associated with HIV infections including oral hairy leukoplakia, oral candidiasis, angular cheilitis, necrotizing ulcerative gingivitis, and linear gingival erythema. We also found lesions seen in HIV infections classified in group III including secondary syphilis-related oral mucous patches and CMV ulcers. Oral lesions can be seen early in HIV infection, so the typical lesions that often occur in immunocompromised states can lead to suspicion of HIV. These oral lesions can occur more often in people who have not been diagnosed with HIV so they have not received antiretroviral therapy, as seen in all cases. Another factor for the occurrence of oral lesions is a low of CD4+ lymphocyte levels,14 as found in case 3. HIV screening carried out early can be very useful in providing overall patient health, infection control, and lifestyle improvements, and in diseases that are often transmitted through sexual activity such as HIV. The best management is to consider the immunological status through preventive measures and regular oral dental examinations to maintain health and achieve a better quality of life.15
Secondary syphilis, which is referred to as “the great imitator”, can manifest clinically in many forms involving different organs including the oral mucosa, and resembles several diseases clinically and histologically making it difficult and challenging for clinicians to diagnose.16 In the third case where the patient showed an oral lesion in the form of an ulcer on the palate area without pain. People with genital syphilis ulcers are 3 times more likely to get HIV infection than people who do not have syphilis and HIV-seropositive people with genital syphilis ulcers are 2–3 times more likely to transmit HIV than people without syphilis ulcers.17 In HIV patients, it can also be suspected that there are other sexually transmitted infections such as syphilis and gonorrhea. VDRL and TPHA examination is the right combination for syphilis screening. Elevated VDRL titters may indicate a new infection, reinfection, or latent syphilis. In the intra-oral examination, the patient was found to have an ulcer that resembles a snail track and this lesion is a hallmark of the oral manifestations of secondary syphilis. From the VDRL examination, the results were reactive with a titter level of 1:256 and the patient was diagnosed with secondary syphilis. This is in line with the finding of oral lesions in the form of snail track ulcers. The features of secondary syphilis are often painless lesions, grayish-white mucous patches, or irregular linear erosion called “snail-track” ulcers. The second stage of syphilis, also called secondary syphilis, often indicates the involvement of the mucous membranes of the oral cavity. In secondary syphilis, oral lesions may appear in 30% of cases.17 The characteristic appearance of the mucous patch is a slightly raised oval or round plaque with an eroded area in the center covered by a thin grayish-white pseudo membrane.17
Oral candidiasis is the most common opportunistic infection in HIV patients and occurs in 80–90% of the patients.18 The clinical feature of oral pseudo-membrane candidiasis is a creamy white pseudo-membrane plaque that can be scrapped. The pseudo membrane consists of a collection of hyphae and yeast cells, inflammatory cells, bacteria, epithelial cells, food debris, and necrotic tissues.19 Due to the weakened immune system, HIV patients tend to be more at risk of developing opportunistic infections such as oral candidiasis. Candida is commensal, but in immunocompromised conditions, it can become pathogenic. This happens because the host’s immune system is reduced in this case in the oral cavity so that it can cause oral candidiasis. As we know, saliva is a medium where interactions occur between the oral microbiome, innate immune cells, and secreted immune modulators and are carriers of salivary proteins so that they become a defense against C. albicans. Decreased salivary secretion can cause dysbiosis, therefore, favoring the overgrowth of C. albicans. In healthy individuals, salivary mucin and secretory IgA bind to the cell wall of C. albicans, and these bonds are removed by ingestion.20 This situation also occurred in the first case where the patient also had xerostomia. This causes sIgA to decrease. Under normal conditions, sIgA inhibits the adhesion of C. albicans to polystyrene by binding to the cell wall mannoprotein and reducing the adherence of C. albicans to epithelial cells. Like sIgA, mucin can also bind and aggregate with C. albicans and is lost by ingestion. Mucin also harms the growth of C. albicans by suppressing the expression of its virulence gene. In addition, saliva itself has the activity of killing candida (candidacidal) because it has various antifungal proteins including histatin 5, LL-37, calprotectin, and lactoferrin which in different ways can kill C. albicans.20 In the case of HIV where CD4+ levels are reduced, this can lead to an overgrowth of C. albicans. Although CD4+ is the host’s dominant defense mechanism against candidiasis, CD8+ cells become important when CD4+ cells drop below the protective limit that occurs in HIV patients progressing to AIDS. Looking at epithelial cell responses, intact oral epithelial cells exhibited fungistatic activity through acid-labile proteins. The hypothesis that occurs in this situation is that in conditions where CD4+ T lymphocytes are decreased, HIV patients are protected against candida infection by CD8+ T cells that migrate to the area of infection under normal conditions of E-cadherin expression, whereas in patients with oropharyngeal candidiasis there is decreased E-cadherin which inhibits CD8+ T cell migration. Oral epithelial cells together with annexin A1 keep Candida in a commensal form but can be overwhelmed so that it can no longer hold it to become a pathogen and eventually cause oral candidiasis.21
Angular cheilitis is characterized by erythema, moist maceration, ulceration, and crusting at the commissures of the mouth. It is caused by multifactorial and could range from local to systemic etiologies. The local factors that caused angular cheilitis are classified into four categories: anatomical, mechanical, allergic, chemical, and infectious. These local factors can either act alone or combine in developing the lesion. Systemic factors include nutritional deficiencies, systemic diseases, and side effects of drugs. Saliva pooling and stasis at the commissures of the mouth produce a chronic, conducive, and moist environment for microbial growth. This condition can cause infection and clinically manifest as angular cheilitis. Candida albicans, Staphylococcus aureus, and/or β-hemolytic streptococci are the most common culprits among microbial agents in causing angular cheilitis.22
Nystatin belongs to the polyene group. This polyene group exhibits broad-spectrum antifungal activity in treating superficial and deep fungal infections.23 Mechanism of action from polyene is by inhibiting the production of ergosterol which is very important for the integrity of the yeast cell membrane. Polyenes can also affect the attachment of fungi and epithelial cells.24 Polyene is not absorbed by the gastrointestinal tract. Nystatin is derived from Streptomyces noursei. It binds to the ergosterol of fungal and establishes a pore, so the membrane is more permeable, leading to leakage of K+, Na+, and H+ so that it is fungicidal.25,26 Nystatin can also cause secondary cellular damage via autoxidation. This topical preparation of nystatin is also effective because it can be absorbed into the oral epithelium so that it can kill yeast hyphae in the tissue. Nystatin also exhibits a good post-antifungal effect, whereby regrowth of the fungus can be inhibited after brief exposure to antifungal agents.27 The selection of nystatin oral suspension for the management of oral candidiasis was in accordance with the recommendations of the Infectious Diseases Society of America (IDSA) of nystatin oral suspension (100,000 U/mL, 4–6 mL four times a day) for 7–14 days for the treatment of candidiasis in clinical practice guidelines updated in 2016.25 Miconazole is an imidazole that is useful against candidiasis caused by C. albicans. It is also effective against both candida and gram-positive cocci; therefore, it is an appropriate first-line agent for angular cheilitis. Miconazole shows antifungal properties by ergosterol synthesis inhibition.23
Other lesions that can be found in the oral cavity of HIV patients and are lesions that are closely related to HIV infection are periodontal disease, namely necrotizing ulcerative gingivitis, necrotizing ulcerative periodontitis, and linear gingival erythema.5 As happened in the third case-patient where necrotizing ulcerative gingivitis (NUG) and linear gingival erythema (LGE) were found. Periodontal diseases are common in people who live with HIV. It is well recognized that the development of periodontal disease depends on the interaction between the resident oral microbiota in the dentogingival plaque and the host response. The bacteria colonize and invade the periodontal tissue, and the host uses a variety of defense mechanisms to maintain a dynamic equilibrium with the resident oral microbial flora.28 NUG is characterized by marginal gingival necrosis, gingival bleeding, and pain. Additional common features, but not pathognomonic, NUG include pseudo-membrane formation, cervical lymphadenopathy, and fetid breath.29
Necrotizing ulcerative gingivitis caused by specific microflora and low immune response can play an important role in initiating this disease. Necrotizing ulcerative gingivitis, caused by multifactorial, includes infection of the gingiva by periodontopathic bacteria, mainly spirochetes and fusiform bacilli, a compromised immune response in the host and other predisposing factors such as physical and emotional stress, malnutrition, and other general debilitating states.29
Linear gingival erythema (LGE) has been found as an early stage of HIV-associated periodontal disease which presents as a fiery red, distinct linear erythema along the free gingival margin, attached gingiva, and alveolar mucosa.30 LGE and is limited to the soft tissues of the periodontium.31 Although many people believe LGE to result from an abnormal host immune response to subgingival bacteria, more recent data suggest that it is an unusual variant of candidiasis.30
As the condition in this patient where laboratory examination of CD4+ level was only 35, causing the body’s defense against infection greatly decreases. The patient was also diagnosed with latent syphilis, the VDRL examination showed a reactive result with a titter of 1:64 and TPHA with a titter of 1:5120.
Treatment of necrotizing ulcerative gingivitis in the acute phase has two main objectives: to stop the disease process and tissue destruction and to control the patient’s general feeling of discomfort and pain that interfere with nutrition and oral hygiene practices. These targets can be achieved by a careful superficial ultrasonic debridement and chemical agent of the necrotic lesions with oxygen-releasing agents.32 Hydrogen peroxide, an oxidizing agent, has been widely used in the management of necrotizing ulcerative gingivitis. It releases oxygen as an active intermediate, loosening debris in inaccessible areas. It also uses as an antiseptic by attacking membrane lipids, DNA, and other essential cell components.23 Since NUG is caused by periodontopathic bacteria, mainly in the form of spirochetes and Fusobacteria, Prevotella, and Peptostreptococcus species, antibiotic is commonly used.33 Azithromycin, a second-generation macrolide antibiotic, a broad-spectrum antibacterial that inhibits bacterial protein synthesis, quorum-sensing and reduces the formation of biofilm. It is accumulated effectively in cells, particularly phagocytes, and delivered in high concentrations to the sites of infection, as reflected in rapid plasma clearance and extensive tissue distribution.34 It is effective against anaerobes and gram-negative bacilli.35 It is also used in treating periodontal disease.35
In the fourth case, there were multiple ulcers spread from the palate to the oropharynx. Ulcers that are difficult to heal and present in immunocompromised patients may suggest lesions due to cytomegalovirus. Human cytomegalovirus (CMV) is a virus that belongs to the Herpesviridae subfamily Betaherpesvirinae or commonly known as human herpesvirus type 5 (HHV-5).1 Transmission can occur through contact with viruses such as semen, cervical secretions of urine, saliva, milk, and blood, or organ or tissue transplantation. Primary CMV infection usually occurs in childhood. Like other types of herpes viruses, this CMV virus can be latent and can reactivate under possible conditions such as stress, immunosuppression, autoimmune diseases, and during chemotherapy.36 CMV infection is an opportunistic infection that often occurs in HIV patients.37 When CD4+ T lymphocytes are reduced in number as occurs in immunocompromised patients, CMV can be reactivated and cause disease.38 By exploiting the memory compartment of the immune system, HIV can escape detection by the immune system. CMV which is a chronic viral infection, often together with HIV occupies a large portion of T cell memory. CMV and HIV together have a strategy of manipulating the immune system and hijacking cytokine and chemokine signals so that they can escape and cause chronic infection. CMV is a virus that can cause infectious mononucleosis syndrome, infections of the central nervous system and retinitis. In the oral cavity, the manifestation can be in the form of ulcers.36 To establish the diagnosis of CMV ulcers, clinical examination and laboratory tests are carried out to determine antibodies to CMV or by detection of viral DNA by polymerase chain reaction (PCR).
Oral hairy leukoplakia (OHL) is a lesion strongly associated with HIV infection caused by the Epstein–Barr virus (EBV).5 Oral candidiasis and Oral hairy leukoplakia within the oral cavity not only suggest an HIV infection but also predict the first signs of developing into AIDS in HIV patients. Clinically, OHL is a painless white corrugated plaque with a shaggy surface, and cannot be removed. EBV can be detected by several techniques, such as polymerase chain reaction (PCR), immunohistochemistry, electron microscopy, and in situ hybridization (ISH), but the gold-standard exam is ISH.39 Lateral border of the tongue is the commonest site of OHL.39,40 It is unclear why OHL is so localized on the lateral of the tongue, but many studies showed lateral tongue epithelium has low numbers of Langerhans cells.40 The pathogenesis of oral hairy leukoplakia is complex. It includes an interaction of persistent Epstein–Barr virus replication and virulence, systemic immunosuppression, and suppression of the local host immunity.41 OHL does not require specific treatment and frequently resolves under Highly Active Anti-Retroviral Therapy (HAART), if associated with HIV infection.41
After the treatment was done, the patients were satisfied. We suggested them to visit the HIV Clinic regularly and consume anti-retrovirals so they will have a better quality of life. They have approved and written the informed consent of case details and all images for the publication of this report. This case series had complied with the Declaration of Helsinki. The institution has also approved to publication of this case report.
All cases in this report showed that oral lesions are still commonly found in HIV/AIDS patients during COVID-19 pandemic. The 8th World Workshop on Oral Health and Disease in HIV/AIDS several months before the pandemic held in Indonesia has issued the Bali declaration on oral health in HIV/AIDS. It stated that the role of dental professionals is recognized as an integral part of the healthcare team committed to achieving the aims of UNAIDS in ending the global HIV/AIDS epidemic by 2030. Therefore, dentists remain to have a crucial role in the early diagnosis and treatment of HIV-associated oral lesions and their awareness to support HIV prevention and control in the COVID-19 era. They should have the appropriate knowledge and attitudes to manage HIV/AIDS patients, if detection and treatment of oral lesions is delayed it will have an impact on HIV treatment.12
Conclusion
Strongly HIV-associated oral lesions (such as oral candidiasis and oral hairy leukoplakia) and other oral lesions (such as necrotizing ulcerative gingivitis, CMV ulcers, mucous patches, angular cheilitis, linear gingival erythema) can be an early warning and can be a reference for suspicion of HIV. In the COVID-19 era, oral lesions are still commonly found in HIV/AIDS patients. Dentists remain to have a crucial role in the early diagnosis and treatment of HIV-associated oral lesions and their awareness to support HIV prevention and control in the COVID-19 era, and implementing the Bali declaration on oral health in HIV/AIDS 2019 to support UNAIDS goal in ending global AIDS by 2030.
Acknowledgments
The authors would like to thank the patients who have agreed to take part in this case report. The authors would also like to thank the staff of Teratai Clinic Hasan Sadikin General Hospital for their support.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Riedel S, Morse S, Mietzner T, Miller S. Jawetz Melnick & Adelbergs Medical Microbiology 28 E Medical Microbiology. McGraw Hill Professional; 2019. [Google Scholar]
- 2.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Prim. 2015;1:1–22. doi: 10.1038/nrdp.2015.35 [DOI] [PubMed] [Google Scholar]
- 3.Lomelí-Martínez SM, González-Hernández LA, Ruiz-Anaya AD, et al. Oral manifestations associated with HIV/AIDS patients. Medicina. 2022;58:1214. doi: 10.3390/medicina58091214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berberi A, Aoun G. Oral lesions associated with human immunodeficiency virus in 75 adult patients: a clinical study. J Korean Assoc Oral Maxillofac Surg. 2017;43:388. doi: 10.5125/jkaoms.2017.43.6.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aškinytė D, Matulionytė R, Rimkevičius A. Oral manifestations of HIV disease: a review. Stomatologija. 2015;17:21–28. [PubMed] [Google Scholar]
- 6.Bajpai S. Oral manifestations of HIV disease. Contemp Clin Dent. 2010;1:1–5. doi: 10.4103/0976-237X.62510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sürme Y, Özmen N, Ertürk Arik B. Fear of COVID-19 and related factors in emergency department patients. Int J Ment Health Addict. 2021. doi: 10.1007/s11469-021-00575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jumadi J, Fikriyah VN, Hadibasyir HZ, et al. Spatiotemporal accessibility of COVID-19 healthcare facilities in Jakarta, Indonesia. Sustainability. 2022;14(21):14478. doi: 10.3390/su142114478 [DOI] [Google Scholar]
- 9.Mahendradhata Y, Andayani NL, Hasri ET, et al. The capacity of the Indonesian healthcare system to respond to COVID-19. Front Public Health. 2021;9:1–9. doi: 10.3389/fpubh.2021.649819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sufiawati I, Rafi MA, Putri FM. Evaluating knowledge, attitude, and behavior of dentists on HIV/AIDS in West Java, Indonesia, in the COVID-19 era. Int J Dent. 2021;2021. doi: 10.1155/2021/1901887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santella AJ, Conway DI, Watt RG. The potential role of dentists in HIV screening. Br Dent J. 2016;220:229–233. doi: 10.1038/sj.bdj.2016.172 [DOI] [PubMed] [Google Scholar]
- 12.Tappuni AR, Sufiawati I. The Bali declaration on oral health in HIV/AIDS. Oral Dis. 2020;26(S1):172. doi: 10.1111/odi.13404 [DOI] [PubMed] [Google Scholar]
- 13.Noujeim Z, Mantash A, El-Outa A, Doumit M. Oral manifestations of HIV infection and aids: an update on clinical diagnosis and management. Int J Cur Adv Res. 2017;6:6256–6263. doi: 10.24327/ijcar.2017.6263.0905 [DOI] [Google Scholar]
- 14.Boasso A, Shearer GM, Chougnet C. Immune dysregulation in human immunodeficiency virus infection: know it, fix it, prevent it? J Intern Med. 2009;265(1):78–96. doi: 10.1111/j.1365-2796.2008.02043.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HIkmah N. Kejadian Hiv/Aids dengan manifestasi oral pada anak-anak. Stomatognatic. 2010;7(1):20, 68–73. [Google Scholar]
- 16.de Paulo LFB, Servato JPS, Oliveira MTF, Durighetto AF, Zanetta-Barbosa D. Oral manifestations of secondary syphilis. Int J Infect Dis. 2015;35:40–42. doi: 10.1016/j.ijid.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Feller L, Chandran R, Marnewick JC, et al. Syphilis in the context of HIV infection. SADJ. 2011;66(6):288–291. [PubMed] [Google Scholar]
- 18.Chadwick DR, Sutherland RK, Raffe S, Pool ERM, Beadsworth MBJ. British HIV association guidelines on the management of opportunistic infection in people living with HIV: the clinical management of candidiasis 2019. HIV Med. 2020;21(S5):1–19. doi: 10.1111/hiv.13004 [DOI] [PubMed] [Google Scholar]
- 19.Lestari PE. Infeksi jamur candida pada penderita Hiv/Aids. J Kedokt Gigi Unej. 2013;10:35–38. [Google Scholar]
- 20.Salvatori O, Puri S, Tati S, Edgerton M. Innate immunity and saliva in candida albicans -mediated oral diseases. J Dent Res. 2016;95:365–371. doi: 10.1177/0022034515625222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidel PL. Candida-host interactions in HIV disease: implications for oropharyngeal candidiasis. Adv Dent Res. 2011;23:45–49. doi: 10.1177/0022034511399284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandarathodiyil AK, Anil S, Vijayan SP. Angular cheilitis-an updated overview of the etiology, diagnosis, and management. Int J Dent Oral Sci. 2021;8:1437–1442. [Google Scholar]
- 23.Dowd FJ, Johnson BS. Pharmacology and Therapeutics for Dentistry. Elsevier; 2017. [Google Scholar]
- 24.Glick M, Greenberg MS, Lockhart PB, Challacombe SJ. Introduction to oral medicine and oral diagnosis: patient evaluation. In: Burket’s Oral Medicine. Wiley Online Library; 2021. [Google Scholar]
- 25.Quindos G, Gil-Alonso S, Marcos-Arias C, et al. Therapeutic tools for oral candidiasis: current and new antifungal drugs Guillermo. Med Oral Patol Oral Cir Bucal. 2019;24:e172–e180. doi: 10.4317/medoral.22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevor JA, Katzung GB. Pharmacology Examination & Board Review. McGraw Hill; 2015. [Google Scholar]
- 27.Lyu X, Zhao C, Yan ZM, Hua H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1161–1171. doi: 10.2147/DDDT.S100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alpagot T, Konopka K, Bhattacharyya M, Gebremedhin S, Düzgüneş N. The association between gingival crevicular fluid TGF-β1 levels and periodontal status in HIV-1 + patients. J Periodontol. 2015;79:123–130. doi: 10.1902/jop.2008.070312 [DOI] [PubMed] [Google Scholar]
- 29.Phiri R, Feller L, Blignaut E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4+ T cell count. J Int Acad Periodontol. 2014;12:98–103. [PubMed] [Google Scholar]
- 30.Peters SM, Heinz MJ, Koslovsky DA, Yoon AJ, Philipone EM. Necrotizing ulcerative stomatitis as initial presentation of undiagnosed HIV infection: a case report and review of literature. J Oral Maxillofac Surg Med Pathol. 2017;29:570–574. doi: 10.1016/j.ajoms.2017.07.005 [DOI] [Google Scholar]
- 31.Menon S. Periodontal diseases in HIV. Int J Dent Sci. 2000;14:1–6. [Google Scholar]
- 32.Malek R, Amina G, Nadia K. Necrotizing ulcerative gingivitis abstract. Contemp Clin Dent. 2017;8:496–500. doi: 10.4103/ccd.ccd_1181_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H, Imamura A. Unexpected acute necrotizing ulcerative gingivitis in a well-controlled hiv-infected case. Intern Med. 2017;56:2223–2227. doi: 10.2169/internalmedicine.8409-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi: 10.1016/j.pharmthera.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 35.Kapoor A, Malhotra R, Grover V, Grover D. Systemic antibiotic therapy in periodontics. Dent Res J. 2012;9:505–515. doi: 10.4103/1735-3327.104866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribas R, Lima AAS. Oral ulcers induced by cytomegalovirus infection: report on two cases. J Dent Indones. 2017;24(2):50–54. doi: 10.14693/jdi.v24i2.1002 [DOI] [Google Scholar]
- 37.Whitley RJ, Jacobson MA, Friedberg DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. Arch Intern Med. 1998;158:957–969. doi: 10.1001/archinte.158.9.957 [DOI] [PubMed] [Google Scholar]
- 38.Lalonde RG, Bovivin G, Deschênes J, et al. Canadian consensus guidelines for the management of cytomegalovirus disease in HIV/AIDS. Can J Infect Dis. 2004;15:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins LL, Rosseto JHF, Andrade NS, et al. Diagnosis of oral hairy leukoplakia: the importance of EBV in situ hybridization. Int J Dent. 2017;2017:1–6. doi: 10.1155/2017/3457479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenspan JS, Greenspan D, Webster-Cyriaque J. Hairy leukoplakia; lessons learned: 30-plus years. Oral Dis. 2016;22:120–127. doi: 10.1111/odi.12393 [DOI] [PubMed] [Google Scholar]
- 41.Alexander Kreuter MD. Oral hairy leukoplakia: a clinical indicator of immunosuppression Alexander. CMAJ. 2011;183(4):932. doi: 10.1503/cmaj.100841 [DOI] [PMC free article] [PubMed] [Google Scholar]