Abstract
Superficial tumors are still challenging to overcome due to the high risk and toxicity of surgery and conventional chemotherapy. Microneedles (MNs) are widely used in the treatment of superficial skin tumors (SST) due to the high penetration rate of the stratum corneum (SC), excellent biocompatibility, simple preparation process, high patient compliance, and minimal invasion. Most importantly, MNs can provide not only efficient and rarely painful delivery carriers, but also combine multi-model strategies with photothermal therapy (PTT), immunotherapy, and gene therapy for synergistic efficacy. To promote an in-depth understanding of their superiorities, this paper systematically summarized the latest application progress of MNs in the treatment of SST by delivering various types of photosensitizers, immune signal molecules, genes, and chemotherapy drugs. Just as important, the advantages, limitations, and drug release mechanisms of MNs based on different materials are introduced in the paper. In addition, the application of MN technology to clinical practice is the ultimate goal of all the work. The obstacles and possible difficulties in expanding the production of MNs and achieving clinical transformation are briefly discussed in this paper. To be anticipated, our work will provide new insights into the precise and rarely painful treatment of SST in the future.
Graphical Abstract
Keywords: Microneedles (MNs), Superficial tumors, Photothermal therapy (PTT), Immunotherapy, Synergistic therapy
Introduction
Superficial skin tumors (SST), such as breast cancer, basal or squamous cell carcinoma (BCC), head and neck cancer, human oral epidermoid carcinoma, and melanoma, impose a great burden on patients both physically and psychologically due to the specificity of tumors color, shape, and location [1, 2]. Surgical excision is a traditional treatment method. However, in most cases, surgical treatment is often affected by changes in the patient’s internal indicators, so it cannot be the first choice for treatment after tumor diagnosis [3–5]. At the same time, surgical treatment is required to accurately diagnose the tumor sites before surgery and to ensure that the tumor tissue can be completely removed after surgery, which undoubtedly increases the difficulty of the treatment process. In the same way, not all patients with SST are suitable for surgical resection. Therefore, there is an urgent need to develop effective, safe, and easy-to-use methods to increase the cure rate of SST.
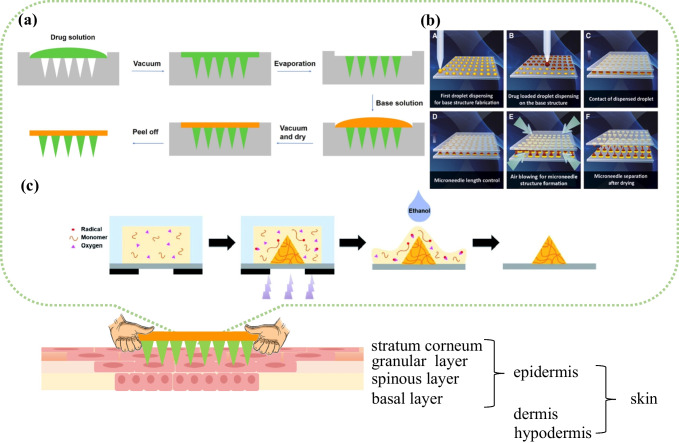
At present, the research on the treatment of SST is tending to adopt percutaneous drug delivery and locally targeted therapy. The prototype and preparation techniques of MNs were first proposed around 1950 [6]. As an emerging drug delivery carrier, MNs are small in shape, convenient to use, low in cost, safe and non-toxic. Compared with oral administration, MNs can avoid the metabolic effect of the digestive system and improve drug bioavailability. For another thing, compared with injection administration, MNs can effectively reduce patients’ pain sensation, improve patients’ compliance, and avoid the occurrence of needle sickness. At present, MNs have been applied to medical beauty [7], tumor treatment [8], diabetes treatment [9–11], disease screening and diagnosis [12], antibacterial [13], and other fields, which have achieved good feedback. With the improvement of industrial technology, MNs have also been successfully applied in the vaccination of influenza vaccines in clinical practice [14, 15]. The mechanism of action between MNs and skin based on different preparation methods is shown in Fig. 1 [16–20].
Fig. 1.
Schematic of the mechanism of action between MNs and skin. The preparation process of drug-loaded solid MNs with a two-step casting method [24], Copyright 2021 American Chemical Society; b droplet-born air-blowing method [25], Copyright 2021 Elsevier; and c free radical polymerization method [26], Copyright 2022 Royal Society of Chemistry
In the treatment of SST, the combined application of chemotherapy, immunotherapy, gene therapy, photothermal therapy (PTT), photodynamic therapy (PDT), and other multiple treatment methods provides an important prerequisite for the cure of tumors [21, 22]. Taking chemotherapy as an example, paclitaxel (PTX), 5-fluorouracil (5-FU), doxorubicin (DOX), and cisplatin (DDP) are commonly used in clinical cancer treatment. However, due to the difference in drug solubility and the limitation of responsive release, it is often difficult to successfully deliver all drugs to tumor targets. The skin is a barrier between the body and the external environment. In this case, MNs can interact with fibrin tissue at the superficial surface of the skin and successfully deliver the loaded drugs to the SST targets to achieve intelligent drug release [23]. Therefore, the combination of MNs and multiple tumor diagnosis and treatment strategies has special significance for the treatment of SST.
Based on the above points, we reviewed the MN-mediated different diagnostic and therapeutic approaches for SST, looking forward to laying a solid knowledge foundation for increasing the cure rate of clinical SST.
Superficial skin tumors
Breast cancer, BCC or squamous cell carcinoma, head and neck carcinoma, human oral epidermoid carcinoma, and melanoma are currently high incidences of SST. Taking melanoma as an example, surgical resection, chemotherapy, gene intervention, and locally targeted therapy are all effective treatments [27]. In the nineteenth century, surgical resection and lymphadenectomy were almost the only treatment for primary melanoma. However, once melanoma had metastasized or spread throughout the body, surgery would not be able to meet its therapeutic effect. In the middle of the twentieth century, chemotherapy drugs became the next breakthrough in the treatment of melanoma. However, the toxicity of chemotherapy drugs made the survival rate of melanoma patients extremely low. With the accumulation of treatment and survival data of melanoma patients and the improvement of treatment methods, BRAF mutation and MAPK mutation are considered to be the causes of melanoma. BRAF mutation occurs in about half of melanoma patients. It is easily targeted and inactive in healthy cells. Therefore, BRAF intervention has become a breakthrough in melanoma treatment [28].
Different from the treatment of melanoma, non-melanoma, mainly represented by cell carcinoma, adopt targeted therapy, which is mainly because BCC and squamous cell carcinoma rarely show metastasis. Most of the clinical manifestations were local tumors, which were relieved after radical surgery or radiotherapy [29]. At present, immunosuppression and radiotherapy can be used to treat rare cell carcinoma. Targeted delivery of coded gene molecules, enzyme inhibitors, chemotherapy drugs, monoclonal antibodies, and other strategies have shown better and better therapeutic prospects in the treatment of non-melanoma skin cancers [29–31].
The different types of MNs in the treatment of tumors
Solid MNs for tumors treatment
The MNs are shaped like an upside-down pushpin with sharp tips, which is the key point of penetration during interaction with the skin. The idea of MNs was first put forward in the 1970s. It was not established research till with the rise of microfabrication technology in the 1990s [32]. In recent years, various materials including polymers, ceramics, stainless steel, glass, metals, and non-metals have been used for the preparation of solid MNs [33]. After solid MNs form drug delivery channels on the skin surface, the drug is slowly released under the action of ISF and pH gradient and then absorbed by the body [32]. Currently, although solid MNs are widely used in the treatment of tumors, the release efficiency of loaded drugs is often affected by the MN carrier matrix. Last but not least, when metal solid MNs are inserted into the skin, there is a chance of causing irritation, swelling, erythema, discoloration, or further side effects, which will be fatal for those patients who are sensitive to certain matrix materials. Repeated insertion of MNs at the same location also may cause the above problems, even at the tumors targets [32, 34–36].
Hollow MNs for tumors treatment
To solve the problems of low drug loading, slow drug release, and material degradability of solid MNs, experts designed and developed hollow MNs for the delivery of drugs and small molecules in the 1990s. The design of the hollow MNs is to retain the head of the MNs, hollow out the center matrix of the sharp MN tails, and then replace them with the delivered small molecule substances and drugs [37, 38]. At present, hollow MN-mediated vaccine intradermal delivery and drug intradermal delivery have been proved to be promising methods to improve the effectiveness of drug preparations [39–42]. Compared with intravenous administration, the drug delivered by hollow MNs could achieve 10 h of sustainable release while maintaining the same dosage [42]. At the same time, hollow MNs have been proved to be able to completely deliver up to 2 mL of liquid and deposit 75% of the administered drug in the skin [39, 41]. However, the preparation of hollow MNs requires a series of complex means, such as the use of vacuum pumping. It is also important for researchers to effectively solve the problem of mutual penetration between matrix and drugs in the preparation process. With the end of the central drug release process, the mechanical strength of the MNs is greatly weakened based on the original strength, which will undoubtedly lead to the toxic side effects of MNs residues in the tumor sites [37, 38].
Dissolving MNs for the treatment of tumors
At the beginning of the twenty-first century, dissolving MNs were developed as new green and environmentally friendly drug carriers. The dissolving MNs are made of some biocompatible, water-soluble matrix materials, regardless of whether they will fracture in the skin and biocompatibility issues [25]. Currently, materials such as maltose, polyvinylpyrrolidone (PVP), trehalose, dextran, hyaluronic acid (HA), and carboxymethyl cellulose (CMC) can be used to prepare the dissolving MNs [43–46]. Natural polymers and natural polysaccharides can be dissolved or degraded when they come into contact with body fluids due to their special structure and existing state in nature. The main advantage of polysaccharides is that there are a large number of hydrophilic groups in their structure, such as hydroxyl, carboxyl, sulfonic group, or other branched chain groups, which provide conditions for them to become excellent soluble MN matrix [47]. Many studies on the safety of dissolving MN patches have shown that animal organs and cells will not be affected by the matrix materials after repeated application of MNs, which indicates that dissolving MNs are extremely safe and widely used with relatively lower limitations [25, 48, 49].
Hydrogel MNs for tumors treatment
Hydrogel healing has long been regarded as a good means of repairing damaged tissues. At the beginning of the twenty-first century, hydrogel MNs were first developed as an ideal drug delivery vehicle. In the preparation of hydrogel MNs, polysaccharides, polypeptides, and polymers are high-quality raw materials. These materials are loose, porous, and hydrophilic to a certain extent. When MNs are partially inserted into the skin surface, they will absorb water and swell. Due to the above characteristics, based on the osmotic pressure difference or temperature difference between ISF and the MN matrix, after fully absorbing the ISF, the pores between the polymer materials are opened larger, leading to drug release [50–53]. It is also not negligible that the porous characteristics of hydrogel MNs make it possible to adjust the release rate of drugs through different degrees of cross-linking, which has more advantages than soluble MNs in drug delivery [54–56]. However, most of the existing hydrogel MNs are used for the extraction and detection of ISF. Hydrogel MNs play a more prominent role in terms of tumor diagnosis and prognosis instead of tumor treatment, which may become a major obstacle to hydrogel MNs in tumor intervention [51, 57, 58].
Coated MNs for the treatment of tumors
To make the drug-loaded MNs penetrate the skin more quickly and improve the stability of the MNs, experts have developed a type of coated MNs by directly coating the drug around the tips of the MNs at the beginning of the twenty-first century. The coated drug can be a molecular prototype, polymer film-modified, or nano-modified drug so that the drug coated on the surface of the MNs can be released quickly and accurately when it contacts the ISF [59–61].
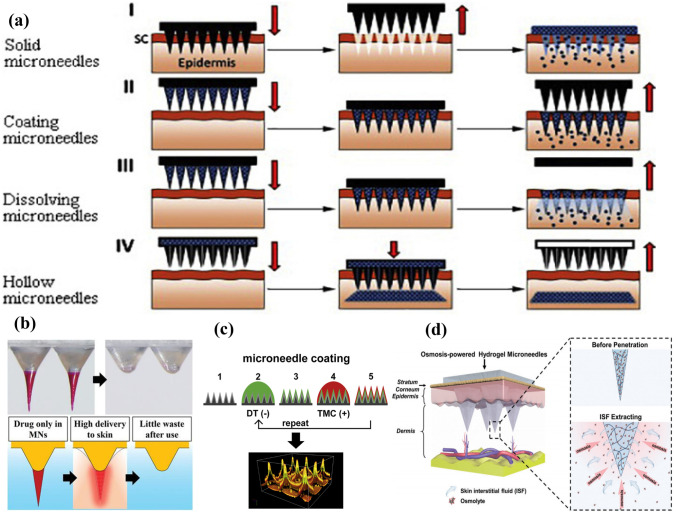
The boundary of different types of MNs gradually becomes indistinct in the application. The combination of coated MNs, solid MNs, hollow MNs, and hydrogel MNs can further enhance the mechanical properties of the MNs while maintaining good drug release properties [62, 63]. At the same time, MNs for treatment by changing the structure have also been developed, such as the use of double-layer MNs. In general, to reduce the dissolution of the backing layer as much as possible, the double-layer MNs prepared by the two-step pouring method can make it possible that the needle tips of the lower layer has a stronger drug release capacity [63, 64]. A schematic diagram of different types of MNs is shown in Fig. 2. The years, advantages, disadvantages, and preparation methods of different types of MNs have been summarized in Table 1.
Fig. 2.
Schematic diagram of different types of MNs. a Flow chart of drug delivery by solid MNs, coated MNs, dissolving MNs, and hollow MNs [65].
Copyright 2018 Elsevier. Representative images of b dissolving MNs [66], Copyright 2021 Wiley; c coated MNs [67], Copyright 2017 Elsevier; and d hydrogel MNs [52], Copyright 2020 Wiley
Table 1.
The summary of the years, advantages, disadvantages, and preparation methods of different types of MNs
| Types of MNs | Years | Advantages | Disadvantages | Preparation methods | Refs |
|---|---|---|---|---|---|
| Solid MNs |
1970s put forward 1990s rise |
Excellent mechanical performance Strong puncture ability |
Low security High toxicity Complex preparation process Serious matrix residue |
Two-step etching method Free radical polymerization method Laser technology |
[26, 36, 68–70] |
| Hollow MNs | 1990s |
High drug loading High drug delivery efficiency |
Poor mechanical performance Complex preparation process |
Solvent-casting method Microelectromechanical technology Laser technology |
[38, 39, 41, 70, 71] |
| Dissolving MNs | At the beginning of the twenty-first century |
Good biodegradability High security Low toxicity Wide range of use |
Low drug loading |
Solvent-casting method Three-step centrifugation method Droplet-born air-blowing method Two-layer centrifugation method |
[72–79] |
| Hydrogel MNs | At the beginning of the twenty-first century |
ISF extraction test Excellent swelling performance Adjustable mechanical strength Adjustable drug release rate |
Narrow scope of application Complex matrix composition Insoluble matrix materials |
Freeze–thaw cycle method Solvent-casting method |
[51, 79–81] |
| Coated MNs | At the beginning of the twenty-first century |
High drug delivery efficiency Excellent mechanical performance |
Low drug loading Complex preparation process Low security |
Wet etch process Dip coating method |
[22, 59, 82–84] |
MN-mediated treatment and diagnosis of superficial tumors
PDT and PTT
In recent years, to deal with the occurrence of various skin cancers, synergy strategy [85, 86], drug function modification strategy [87], spatiotemporal controllable drug release strategy [50, 73, 88], local targeted drug delivery strategy, [5, 89], and other methods have been developed. Among them, the local targeted therapy strategy has become a potential treatment mode due to its remarkable drug concentration and few side effects. In the treatment of skin tumors, the targeted delivery of photosensitizers into tumor cells will be an effective therapeutic strategy [90]. Under the irradiation of a light source with a specific wavelength, cytotoxic substances are produced, which act on the target tissue to kill pathological cells, induce microvascular injury, and stimulate the immune response, which is PDT [91–93]. It is worth noting that after photosensitizers take effect in the body, the target position is often accompanied by an increase in local temperature, which can destroy the tumor cell membrane and denature proteins and then induce tumor cell apoptosis. In general, this above process is called PTT, which is inseparable from the PDT [94]. In addition, heating can also increase blood flow at the tumor sites, and the rapid flow of blood drives the drug deep into the tumor to play a therapeutic role.
Conjugated phthalocyanine
Phthalocyanine is a near-infrared dye containing a phthalocyanine structure, which belongs to the second generation of photosensitizers. It is widely used due to its excellent photodynamic efficiency while reducing the photosensitivity of the skin. Tham et al. [95] combined phthalocyanine with a self-developed mesoporous nanocarrier. To maximize the therapeutic efficacy, MNs are combined to facilitate the penetration of the substance into the deeper tissues of the skin. The results showed that the penetration was 27.2% and 63.1% respectively for the pretreatment without MNs and the treatment with MNs.
Indocyanine green (ICG) is a special phthalocyanine dye that can generate reactive oxygen species after absorbing near-infrared energy, which can produce high cytotoxicity and destroy the cellular structure of tumors. On the other hand, the absorbed energy is further converted into heat energy, which increases the local temperature of the tumor to achieve the purpose of killing tumor cells [96]. To improve the stability of ICG, Pei et al. [97] developed a safe and effective delivery system to ensure the synergistic effect of simultaneous delivery of chemotherapy drugs and photothermal agents to the tumor sites. The dissolving MN patches based on PVP showed excellent skin penetration and the tips of the MN patch were dissolved by ISF to release DOX and ICG at the tumor sites, which reached a local high temperature of 50 °C within 2 min. At the same time, it was observed that the MNs were completely dissolved without residue.
Metal compound
The operation of irradiation often causes side effects such as itching, blisters, and dermatitis on the skin. The means of PTT for the treatment of tumors avoid the symptoms of extracellular leakage and dermatitis. Metals have good optical properties and strong cellular uptake. In terms of practical ability, metal complexes have more excellent structural plasticity. In these studies, the doping of metal compounds can enhance the photothermal conversion effect of polymer photosensitizers [98–102].
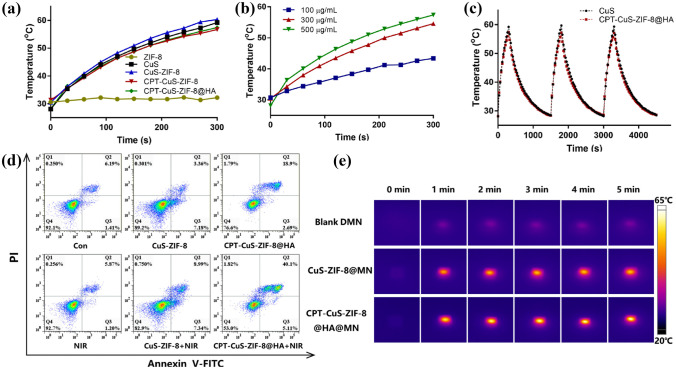
CuS is an ideal photosensitizer. Zhao et al. [94] used MNs as the delivery carrier of the photosensitizer CuS and the anticancer drug camptothecin (CPT), achieving the goal of intelligent time–space drug release. As shown in Fig. 3, the MNs coated with CuS showed excellent photothermal stability after repeated irradiation. Under the irradiation of the laser, the structure of MNs begins to be destroyed, thus releasing anticancer drugs. Apoptosis experiment showed that near-infrared (NIR) radiation was the main cause of cell apoptosis, which indicated that the delivery of MNs would not produce substances harmful to the body (Fig. 3d). The composition of dissolving MN matrix PVP and PVA also provided an argument for the safety of MNs. According to the in vivo photothermal effect evaluation experiment of B16 tumor-bearing mice, the existence of MNs and photosensitizers made the body temperature reached 50 °C within 1 min after irradiation, which indicated that the drug delivery channels produced by MNs were effective (Fig. 3e).
Fig. 3.
Schematic diagram of temperature change of MN PTT loaded with metal photosensitizers. a Temperature change curves of CPT-CuS-ZIF-8@HA, CPT-CuS-ZIF-8, CuS-ZIF-8, CuS, and ZIF-8 under laser irradiation (1.0 W/cm2) at different time points. b Temperature change curves of CPT-CuS-ZIF-8@HA at different concentrations under NIR irradiation. c Photothermal conversion stability curves of CuS and CPT-CuS-ZIF-8@HA for three cycles. d Schematic diagram of apoptosis analysis. e The thermographic images of blank DMN, CuS-ZIF-8@MN, and CPT-CuS-ZIF-8@HA@MN under NIR irradiation at different time points [94].
Copyright 2021 Elsevier
Moreira et al. [103] co-encapsulated DOX and AuSS nanorods in PVP MNs system for PTT of tumor-bearing mice. The research showed that the existence of AuSS nanorods can make the system produce higher temperatures within 5 min, which can enhance the sensitivity of cancer cells to chemotherapy drugs, and accelerate protein denaturation and cell membrane destruction. Among numerous photothermal materials, AuNCs have good chemical stability, a simple preparation process, and an excellent photothermal effect. On the one hand, the AuNCs can be taken up by cells within 10 min, and the cell vitality decreased by 50% after being irradiated by near-infrared. On the other hand, the existence of AuNCs can also significantly improve the mechanical strength of the MNs, which is mainly due to the enhanced aggregation of polymer materials by the photosensitizer [104].
Precursor photosensitizers
The direct delivery of photosensitizers required for PTT treatment of skin tumors by MNs is a very effective strategy. However, it is also affected by the stability and drug loading of the photosensitizers. Delivery of precursor photosensitizers would be a good solution. For example, 5-aminolevulinic acid is gradually converted to its metabolite protoporphyrin IX (PPIX) after delivery into the human body. This is a highly efficient photosensitizer, which can combine with oxygen under the irradiation of excitation light, resulting in a certain photochemical reaction, which eventually leads to cell death [105]. While promoting the activation of the pro-drug at the subcutaneous tumor site, it does not hinder the restoration of the anticancer properties of its parent drug [106, 107].
Gene therapy
At present, experts believe that gene therapy has great potential in the treatment of tumors or other inflammatory diseases, especially after the outbreak of COVID-19, which can be seen in the general acceptance of nucleic acid [108]. Gene transfection and gene silencing are the main means of gene intervention. Silencing the expression of mutant genes may become the key to curing SST.
However, the stability of nucleic acids cannot be ignored. The storage date, storage method, usage, dosage, etc., will become a thought-provoking problem in the large-scale production of nucleic acid-loaded pharmaceutical dosage forms. Now, the problems listed above will be solved by the advent of MNs. Nucleic acid stability is maintained by the dryness of the MN encapsulation process. MNs can coat various low molecular and macromolecular substances on the surface during the preparation process, which will facilitate self-administration in terms of clinical. In addition, in the case of loading unstable biomolecules or vaccines, the improvement of the stability of dry materials can reduce the requirements for cold chain storage, which will facilitate the use of genetic means and MNs for tumor treatment [83, 108].
Gene transfection
Gene transfection is defined as the process of delivering nucleic acid-containing biological functions into cells of living organisms and further maintaining their biological functions. P53DNA, siRNA, and other synthetic DNAs with tumor suppressor effects are commonly used nucleic acids that can intervene in the cell cycle of tumor cells and inhibit the growth, metastasis, and spread of cancer cells. Physical injection, electroporation, and ballistic delivery are common gene delivery methods. However, the gene transfection efficiency is limited due to factors such as DNA molecular weight, charge, and enzymatic degradation. At the same time, the above methods are usually accompanied by limitations such as pain, high price, and professional operation. The emergence of MNs successfully solves the safety problems of other viral vectors and effectively reduces the side effects caused by the carrier materials. Studies have shown that MNs for gene transfection can simultaneously improve DNA stability within solid matrices and increase DNA delivery. Compared with the PVP matrix, the PVA matrix can enhance the in vitro transfection ability of genes better [109, 110].
In the process of gene therapy, how to enhance the uptake of genetic material is a major problem for experts. Ionic polymers with opposite charges are a perfect solution [81]. Li et al. [23] and his team took advantage of the acidic skin environment to coat the pH-responsive polyelectrolyte multilayer membrane (PEM) on the surface of polycaprolactone (PCL) MNs for the delivery of p53 plasmids, thereby achieving rapid gene delivery freed. In vivo experiments showed that the tumor-inhibiting effect of the MN experimental group was about twice that of the injection group. Grace Cole’s research showed that the use of the freeze-drying method increased the loading of DNA nanoparticles in soluble MNs, and maintained long-term stability and ideal tumor inhibition effect while ensuring mechanical properties [111].
Gene silencing
In addition to delivering tumor suppressor genes into cells to inhibit tumor growth, RNA interference is also considered one of the effective ways to inhibit the growth cycle of tumor cells. To examine the efficiency of gene silencing exhibited by siRNA when it is delivered into the body employing MN carriers, the delivered siRNA is modified with cholesterol to better penetrate the stratum corneum of the skin and into the cytoplasm. The study of Deng et al. showed that GAPDH gene expression was reduced by 66% after MN injection [68]. Tang and his team members used MNs for siRNA delivery to achieve targeted enrichment of anticancer genes at xenograft sites. Combining with MNs allowed siRNA to have higher stability and better permeability. The team successfully achieved the silencing of the HPV-16-E6 oncogene, and at the same time, it did not show major adverse reactions and biological toxicity [112].
Because the main cause of melanoma is BRAF gene mutation, silencing the expression of this gene may become the key to curing the disease. At the same time, the gene silencing efficiency can be further evaluated by calculating the protein expression of the target gene and the relevant control group. Peptide nano-complexes can promote the transfection of target genes and accelerate the silencing of target genes, which makes MNs express more obvious advantages in the treatment of skin melanoma [84]. Due to the relatively simple preparation process of siRNA, it is expected to achieve large-scale industrial production in the future.
Chemotherapy
Chemotherapy combined with different medical technologies to treat tumors has become an important means of clinical cancer treatment. Though chemotherapy methods have great toxicity and adverse reactions, for now, the status of chemotherapy methods will not be replaced by other methods in the next few years or even decades [113].
Chemotherapy combined with nanotechnology
Nanocarriers including liposomes are widely used due to their excellent drug delivery properties and low adverse drug reactions in recent years [114]. A growing number of studies have found that single-agent chemotherapy shows more limitations for intractable tumor diseases. For this problem, Ahmed et al. [115] used MNs for the delivery of the dual anticancer drug DOX and celecoxib, which showed that the drug delivery method of the MN carrier could make the anticancer drug penetrate the skin more effectively and have stronger cytotoxicity than the common type of drug delivery method.
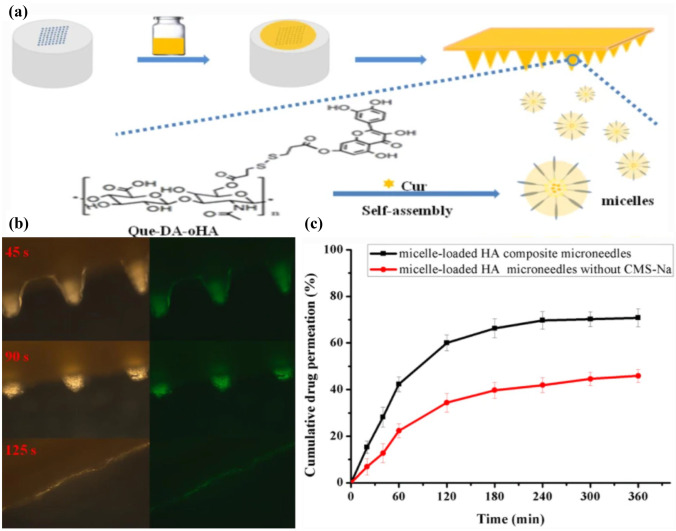
The incorporation of nanotechnology and novel drug formulations has led to further advances in MNs in the treatment of oncological diseases. This conclusion is supported by Abdelghany and members of his team. They gave a systematic review of the results comparing a single chemotherapeutic drug with curcumin (Cur) MNs with nanosuspension technology. In vitro studies showed that the use of MNs greatly enhances the intradermal penetration of Cur. The delivered chemotherapeutics penetrated the skin up to 5 times the depth of MN insertion. Meanwhile, as shown in Fig. 4a, Cur-loaded soluble HA MNs combined with micelles were also successfully prepared. Experiments showed that the MNs released more than 70% of the drug within 6 h, and could be completely dissolved in about 2 min after insertion (Fig. 4b, c). Cur-loaded micelles enabled targeted aggregation at tumor sites, resulting in drug accumulation [75, 87].
Fig. 4.
Preparation process and in vitro release studies. a Principle of preparation of Cur-loaded soluble HA MNs. b Dissolution after 45 s, 90 s, and 125 s of insertion into pigskin. c In vitro drug release profiles of Cur-loaded micellar MNs [87].
Copyright 2020 Springer
Mojeiko et al. [116] also tried to use a combination of MNs and microemulsions to explore innovative ways to treat breast cancer, and to use the MN carriers to assist the penetration of the drug-loaded microemulsions. The higher frequency of use of the MN roller and the shorter MN length innovatively reduced moisture loss and damage to the skin barrier. Lan et al. [117] reported the MN-mediated targeted delivery of the first-line anticancer drug DDP. Under the delivery effect of the soluble MNs, the inhibition degree of DDP on head and neck cancer tumor cells reached 58.6%. In addition, no hepatotoxicity, pulmonary toxicity, and nephrotoxicity were detected except in tumor tissue.
Chemotherapy combined with osmotic solubilized technology
The researchers evaluated the potential of hydrogel MNs to deliver anticancer drugs to prove the feasibility of MNs combined with osmotic enhancement technology for the treatment of SST. Drug accumulation in skin, plasma, and visceral tissues was observed within 1 week of anticancer drugs delivered using MNs [79]. Huang et al. [118] encapsulated DOX and trametinib into self-prepared dextran methacrylate hydrogel MNs, which could penetrate 600 μm into the skin for drug release. Compared with the injection group and the single-administration group, the dual-delivery MN group showed a more outstanding melanoma inhibitory effect. The presence of MNs and some chemical penetration enhancers can enhance the delivery of targeted drugs. Under the action of MNs and oleic acid, a chemical permeation enhancer, anticancer drugs, and honokiol could increase drug delivery by 3 times and 27 times respectively [119]. One of the main goals of curing BCC is to enhance the permeability of specific drugs for the treatment of BCC, such as imiquimod. Sabri et al. [120] developed imiquimod-loaded polyvinylpyrrolidone-vinyl acetate (PVPVA) MNs. The lesion site of the disease is mainly located at about 400 μm below the skin surface, which makes it possible to realize the delivery of BCC-targeted drugs with MNs. Sabri et al. used MN device to evaluate the permeability of imiquimod cream, which showed that only a small amount of effective penetration occurred when a simple cream was applied to pig skin. Most of the drug was recovered during subsequent skin cleansing. After pretreatment with MNs, the amount of imiquimod delivered through the stratum corneum barrier was about 3 times that of the ointment, and the skin permeability was significantly improved [69, 121, 122]. In addition, Naguib et al. [123] demonstrated a 4.5-fold increase in 5-Fu passing through the skin when the skin was pretreated with MNs.
Immunotherapy
From a physiological point of view, making good use of the immune ability of patients when they are sick is a breakthrough point of many experts’ research. At present, the combination of MNs and immunotherapy has successfully delivered some therapeutic agents required for tumor treatment into human cancer cells, such as monoclonal antibodies, vaccines, and immune checkpoint inhibitors [79, 124, 125].
The delivery of immune signaling molecules
It is well known that the growth rate of tumors has a great relationship with the environment and location. Generally speaking, the environment in which the tumor is located will have a great impact on the growth of breast cancer tumor cells, and even the environment in which the tumor is located can be reshaped. As a specific immune signaling molecule, PD-L1, when combined with PD-1 on lymphocytes, allows tumor cells to escape immune in vivo [126, 127]. Thus, experts have developed a new idea to deliver a large amount of anti-PD-1 (aPD-1) antibodies in vitro into the tumor tissue microenvironment to competitively bind to PD-1, thereby reducing the chance of tumor cell immune escape. Lan et al. [76] successfully developed MN arrays loaded with pH-responsive tumor-targeting lipid NPs. It can precisely deliver aPD-1 and DDP to cancer foci. To improve the efficiency of aPD-1 delivery, a physiologically responsive MN delivery device was simultaneously developed for the treatment of cutaneous melanoma. Wang et al. [78] reported an innovative self-degradable MN patch that continuously transported aPD-1 in a physiologically controlled way. In this system, MNs encapsulated aPD-1 and glucose oxidase (GOx), which converts blood sugar to gluconic acid. The production of the acidic environment promoted the self-dissociation of NPs, leading to the release of aPD-1. It was found that a single administration of the MN patch induced a strong immune response in a B16F10 mouse melanoma model compared to MNs without GOx or intratumoral injection of free aPD-1 with the same dose, which demonstrated that the MN patch-assisted system improved cancer immunogenicity significantly.
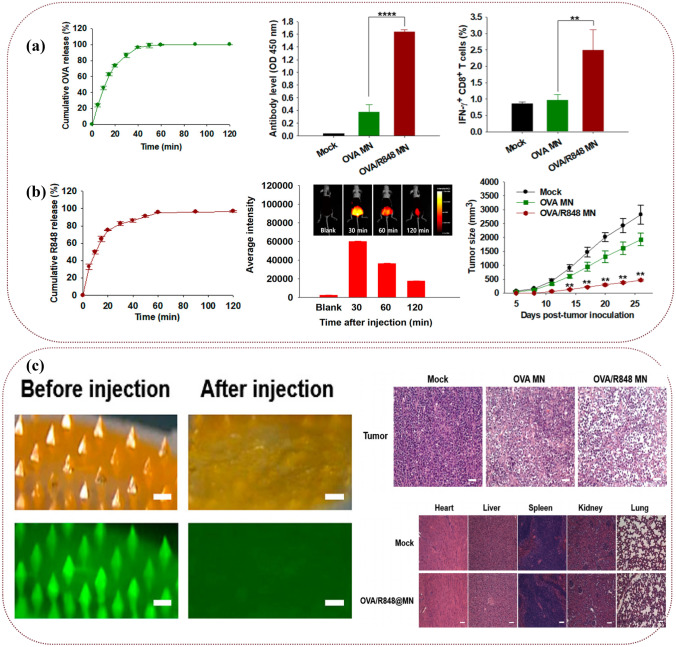
MN-assisted delivery of DNA vaccines [89, 128, 129], tumor cell lysates [130, 131], immune adjuvants required for immunotherapy, or immune checkpoint inhibitors [71, 132] has also been developed for the treatment of superficial tumors. Subunit vaccines are an ideal choice for immunotherapy due to their good biosafety. However, there is still a common problem of low immunogenicity. Auxiliary delivery of immune adjuvants may be a logical solution to the problem. Many researchers used soluble MNs as the delivery carriers of ovalbumin (OVA). Duong et al. [133] proposed a dissolvable MN mixture for triggering vaccine and adjuvant release in skin tissue, which elicited stronger antigen-specific antibody responses than subcutaneous administration. Dissolving MN cocktail enhances antibody recall memory after challenge compared to traditional vaccination. Compared with intramuscular injection, immune signaling molecules delivered through the hollow MN array elicited significantly higher antibody responses and higher numbers of interferon-secreting lymphocytes [39, 74, 134, 135]. As shown in Fig. 5, the experimental group of receptor agonists (R848) delivered by MNs showed better immune effects than the control group and the subcutaneous injection group, specifically in the increase of the number of antibodies, immune factors, and immune cells and the decrease of tumors size. Most importantly, the insertion and dissolution of MNs were highly safe and low toxic, which could be observed from the safety evaluation of the experiment [135].
Fig. 5.
Effectiveness and safety evaluation after treatment with OVA/R848 MNs. a Effectiveness evaluation: in vivo immune response after treatment with OVA/R848 MNs. The schematic diagrams are respectively the cumulative release of OVA, the expression of IgG specifically generated by OVA after MNs treatment, and the percentage of CD8+ T cells specifically generated by OVA in the spleen. n = 5 (**P < 0.01, ****P < 0.0001). b Effectiveness evaluation: cumulative release of R848, local distribution of photothermal effect in vivo, and the change of tumor size after implantation of MNs. c Safety evaluation: dissolution of MNs, H&E staining of tumors, and important organs of mice treated with OVA and R848. Scale bar = 100 μm [135].
Copyright 2018 American Chemical Society
Immune monitoring and diagnosis
In addition to using immunotherapy to treat tumors, pro-drug strategy and nano-drug delivery strategy have also been proved to be ideal treatment methods in the early diagnosis and treatment of tumors [136–139]. However, it is undoubtedly more beneficial for patients to be able to accurately diagnose corresponding diseases through specific indicators at the early stage of tumor onset. Traditional tumor detection and diagnosis mainly rely on the identification of tumor biomarkers, which usually come from patients’ blood, urine, and other biological samples [140]. Taking melanoma as an example, the biomarkers of melanoma include P16, BRAF V600E, S100B, NRAS, and ALK. The detection of these markers can effectively diagnose melanoma [70]. However, the routine means of extracting biological samples not only brings serious physical trauma but also requires corresponding histological treatment and nursing of patients [141, 142]. Compared with traditional biomarker detection methods, the MN monitoring and diagnosis devices have the advantages of simplicity, rapidity, and strong patient compliance [143], which is meaningful for patients.
Currently, Yang et al. [144] introduced a hydrogel MN patch for rapid and easy capture of Epstein-Barr virus cell-free DNA from ISF in situ around 15 min, with a maximum capture efficiency of 93.6%. Dervisevic et al. [143] fabricated an electrochemical transdermal immunesensor using high-density silicon MN arrays, which achieved the simultaneous extraction and detection of breast cancer biomarker ErbB2 successfully. The Si-MN-based immunosensor with an Au coating indicated excellent skin model penetration and good analytical performance in the extraction and electrochemical quantification of ErbB2 biomarkers. Chen et al. [145] achieved a blood-free sample process by inserting MNs into the animal detection area. The MN-based assay could detect the occurrence of breast cancer within 7 days. For comparison, the classical blood test needed 14 days to validate the occurrence of breast cancer.
In addition to monitoring and diagnosis, prevention is also an indispensable step in the process of tumor discovery and treatment. How to deliver the vaccine to the tumor site becomes the key to solving the problem. The degradable MNs can deliver the composition into the tumor tissue site, to carry out transdermal vaccine inoculation, achieve significant inhibition of the growth and proliferation of mouse melanoma, and further prevent the occurrence of tumors [146].
Other methods used to treat tumors
The growth, metastasis, and spread of tumors require more energy and glucose than normal tissues. Depleting the glucose and energy near the tumor tissue will become one of the very potential tumor treatment methods. Therefore, a method called “starvation therapy” come into being. Glucose oxidase can specifically oxidize glucose and reduce the energy and oxygen near the tumor tissues. Therefore, it has been widely used in the “starvation therapy” of various tumor diseases. Glucose oxidase has poor stability and is easily enzymatically hydrolyzed. When it was encapsulated in dissolving MNs, drug release could be achieved through the dissolution of MN carriers in vivo, which could effectively enhance the stability of the enzyme. Therefore, the leakage and safety risks associated with local intratumoral injections are avoided [72]. The application of MNs ensured the continuous delivery of glucose oxidase to achieve accumulation at the tumor site [147].
However, despite the innovativeness of this treatment method, due to the complexity and intractability of tumor diseases, it is difficult to cure tumors only by a single starvation therapy. Moreover, in the long-term application of this method for tumor treatment, adverse reactions such as hypoglycemia will inevitably occur. Therefore, today’s research trend is increasingly favoring starvation therapy as a topical adjuvant therapy to assist other treatments such as PTT and immunotherapy.
Synergistic approach to tumors therapy
A single skin tumor treatment approach exhibits various limitations under the test of clinical translation. Combining two or more treatments can have a positive effect, improving treatment efficiency while greatly reducing side effects.
Chemotherapy is the most basic and common cancer treatment. At the same time, PTT is relatively in-depth research in recent years, and it is also the most potential tumor treatment method. When the chemotherapeutic drugs are delivered to the skin surface by various types of MNs, the infrared photothermal agents required for PTT are delivered simultaneously. After being irradiated by infrared lasers, these materials will produce a certain photothermal effect, which synergizes with the chemotherapeutic effects of the abovementioned chemotherapeutic drugs to destroy tumors [73, 99, 104]. Sun et al. [77] co-encapsulated PTX and excellent photosensitizer IR780 in self-developed self-assembled nanomicelle soluble MNs for photothermal-chemotherapy synergistic treatment of melanoma. After the MNs are dissolved in the skin, the self-assembled nanomicelle can deliver the first-line anticancer drug PTX to the deep part of the tumor to the greatest extent. Compared with the injection method, the delivery of MNs enabled the drug to accumulate at the tumor site without reservation and effectively achieve a cure rate of more than 80% while ensuring certain biocompatibility. Chen et al. [148] used MNs for the co-delivery of lanthanum hexaboride and DOX. When the local tumor was exposed to near-infrared light, lanthanum hexaboride could quickly induce a thermal ablation area, the local temperature could reach 50 °C, and DOX could be released in a large range while heating, thereby further inducing tumor cells to ablate die.
The purpose of the combination of gene therapy and PTT is to deliver some target genes that can produce anti-cancer effects together with photothermal agents into human tumor cells. Xu et al. [149] chose MNs to co-deliver p53 DNA and ICG derivative IR820 into skin cells for the treatment of human oral epidermoid carcinoma. The study found that the local temperature of the part irradiated by near-infrared light increased by about 15 °C.
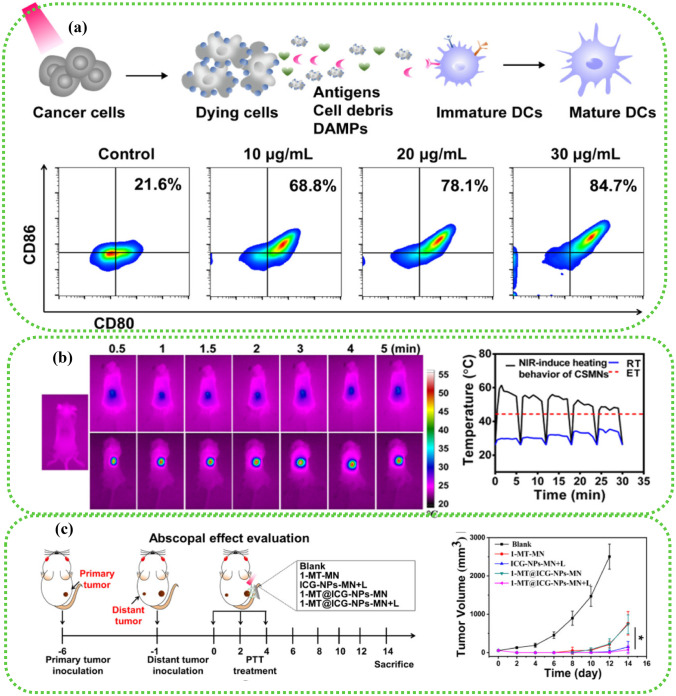
The combination of PTT and immunotherapy can inhibit the growth and spread of tumors and has no significant effect on the safety of experimental animals. The MN system loaded with immunologic agents and photosensitizers is expected to show great therapeutic potential in clinical translation [85, 86, 150]. He et al. [24] delivered semiconducting polymer nanoparticles co-modified with poly and immune adjuvant PIC HA to the tissue site of mouse melanoma tumors through the soluble MNs. Semiconductor polymer NPs have good photothermal conversion efficiency. The existence of dissolving MNs enables the NPs to have higher efficacy of PTT while reducing the toxic and side effects of delivery. As shown in Fig. 6, the experimental results showed that the immune signal molecules and photosensitizers delivered by MNs could significantly increase the local temperature of tumors and the number of immune cells. The synergistic therapy based on the MN delivery platform has a significant tumor-suppressive effect, providing a potential tumor treatment modality in the treatment of SST.
Fig. 6.
The immunity study of tumors in vivo. a ICD induced by ICG with irradiation in vitro. Percentage of CD86CD80 DCs after MN treatments (means ± SD, n = 3). b Infrared thermographic maps and temperature elevation profile of MN-treated mice under laser irradiation (808 nm, 0.35 W/cm2). c Schematic illustration of a bilateral B16 tumor model and the administration schedule. Tumor volume after being treated with MNs [86].
Copyright 2020 American Chemical Society
In conclusion, we summarized the study of MNs combined with multiple therapeutic strategies against SST in this paper, which was expected to provide insights into the development of more potential therapeutic platforms for targeting superficial tumors. Different types of MNs, characteristics of MNs, matrixes, the loaded drugs, the diseases treated with MNs, and the treatment methods in cancer therapy are summarized in Table 2.
Table 2.
Different types of MNs, characteristics of MNs, matrixes, the loaded drugs, the diseases treated with MNs, and the treatment methods in cancer therapy
| MN types | Characteristics | Matrixes | The loaded drugs | Diseases | Treatment methods | Refs |
|---|---|---|---|---|---|---|
| Solid MNs |
High mechanical strength Low biosafety Low biocompatibility |
Stainless steel | Dabrafenib, trametinib | Melanoma | PTT | [95] |
| Silicon | Cholesterol-modified housekeeping gene (Gapdh) siRNA | Genetic disease | Gene therapy | [68] | ||
| Oscillating MN device dermapen | Imiquimod | BCC | Chemotherapy | [69] | ||
| HA, stainless steel | Itraconazole | BCC | Chemotherapy | [122] | ||
| Dissolving MNs |
Excellent biocompatibility Excellent biosafety Release the loaded drug rapidly Wide range of applications Simple preparation process |
HA |
5-Fu; ICG |
Human epidermoid cancer, Melanoma |
PTT Chemotherapy |
[96] |
| PVP K30: PVA 103 = 6: 1, PVP K90 | CuS NPs, CPT | Melanoma |
PTT; Chemotherapy |
[94] | ||
| HA | 5-ALA | Skin lesions | PTT | [105] | ||
| PVP | Cationic delivery peptide (RALA), DNA | Immunogenic disease | Gene therapy | [109] | ||
| PVA | RALA/pDNA NPs | Cervical Cancer | Gene therapy | [111] | ||
| PVA | CU-NS | Skin cancer | Chemotherapy | [75] | ||
| HA | Cur | Melanoma | Chemotherapy | [87] | ||
| Sodium carboxymethylcellulose (SCMC) | LCC-NPs | Head and neck cancer | Chemotherapy | [117] | ||
| Maltose | Honokiol, Oleic acid | Breast cancer | Chemotherapy | [119] | ||
| PVP | Imiquimod | BCC | Chemotherapy | [120] | ||
| PVP | aPD-1, DDP | Oral squamous cell carcinoma |
Chemotherapy; Immunotherapy |
[76] | ||
| PVA | RALA/pDNA NPs | CRPC | Immunotherapy | [128] | ||
| CS: NMP = 2 g/80 ml | Polymeric nanocomplex of paclitaxel-encapsulated sulfobutylether-β-cyclodextrin (SBE)/mannosylated N,N,N-trimethylchitosan (mTMC)/DNA | Melanoma | Immunotherapy | [89] | ||
| P127: PEG = 7: 3 | Toll-like receptor 7/8 agonist (R848), ovalbumin protein | EG-7 lymphoma | Immunotherapy | [135] | ||
| HA | Ovalbumin protein | EG-7 lymphoma | Immunotherapy | [74] | ||
| HA: PVP K30 = 15%: 40% | GOx | Melanoma | Starvation therapy | [72] | ||
| PVP K30: PVA 103 = 1: 4, PVP K90 | GOx, CAT, ICG | Melanoma | Starvation therapy | [147] | ||
| PVP K90 | PTX, IR780 | Melanoma |
PTT Chemotherapy |
[73] | ||
| HA | AuNC, DOX | Melanoma |
PTT Chemotherapy |
[104] | ||
| HA | Chloroquine, CQ, IR780 | Melanoma |
PTT Immunotherapy |
[150] | ||
| PVA: PVP = 2: 1 | Lanthanum hexaboride, DOX | Breast Cancer |
PTT Chemotherapy |
[148] | ||
| HA | p53 DNA, IR820 | Human oral epidermoid carcinoma |
PTT Gene therapy |
[149] | ||
| PVP K90 | Poly(cyclopentadithiophene-alt-benzothiadiazole), immune adjuvant polyinosinic–polycytidylic acid | Melanoma |
PTT Immunotherapy |
[24] | ||
| Coated MNs |
High transdermal efficiency Prepare the coating solution separately Complex preparation process and mold The coating tends to become uneven |
PCL | p53 DNA, PEI | Human oral epidermoid carcinoma | Gene therapy | [82] |
| PCL | Dimethylmaleic anhydride-modified polylysine (PLL-DMA), p53 DNA | Human oral epidermoid carcinoma | Gene therapy | [23] | ||
| Stainless steel | BRAF siRNA | Melanoma | Gene therapy | [84] | ||
| Polyethylene glycol (PEG) | Gold nanorod, DOC | Human oral epidermoid carcinoma |
PTT Chemotherapy |
[99] | ||
| Hydrogel MNs |
Controlled drug release Excellent biocompatibility Excellent biosafety High risk of drug leakage |
GelMA | pDNA, poly(β-aminoester) (PBAE) NPs | Tissue regeneration and cancer therapy | Gene therapy | [81] |
| PVA: PEG = 20%: 7.5% | Bevacizumab |
Lymphomas Secondary metastatic tumors |
Chemotherapy | [79] | ||
| DexMA | DOX, Tra | Melanoma | Chemotherapy | [118] | ||
| Biodegradable MNs |
Excellent biodegradability Low toxicity |
HA | aPD1, GOx | Melanoma | Immunotherapy | [78] |
| HA | Cytotoxic T-cell epitope peptide | Melanoma | Immunotherapy | [146] | ||
| Core–shell structure MNs |
Complex preparation process High drug loading relatively |
Oligomeric sodium hyaluronate, PVP: HA = 31.25%: 33%; 40%; 50% | ICG, aPD-L1 | Melanoma |
PTT Immunotherapy |
[85] |
| PVP: PVA = 25%: 15% | 1-Methyl-tryptophan, ICG, indoleamine 2,3-dioxygenase (IDO) | Melanoma |
PTT Immunotherapy |
[86] | ||
| Injectable MNs | Complex use process | Stainless steel | Cholesterol-modified housekeeping gene (Gapdh) siRNA | Cervical Cancer | Gene therapy | [112] |
| Derma roller® MNs | High market transformation efficiency | HA | DOX, CEL | Melanoma | Chemotherapy | [115] |
Current status of clinical research mediated by minimally invasive MNs
In recent years, several clinical trials on the efficacy and safety of MNs have been published. MNs have been used to treat skin diseases [151, 152], vaccines, or insulin delivery [153, 154], which all showed similar or more effective results than controls. In other specific studies, MN-mediated drug delivery was more effective than drug application alone or placebo MNs. In the treatment of some non-tumor diseases, MNs are relatively safe, causing only mild adverse reactions. MNs have advantages over traditional therapies, such as requiring no specialized training to implement, reducing costs through the use of fewer vaccines, and avoiding needle phobia [155]. However, we are infrequent in the treatment of any tumors or superficial tumors. We suspect that it is related to the severity and complexity of the tumor diseases. For now, many indicators have not been adequately compared between groups, and further research is needed to be concluded.
Conclusion and outlook
In short, tumors that develop on the body’s surface can try to use MNs as an ideal drug delivery system. MNs can form unobstructed drug release channels while interacting with the skin so that the loaded drugs or other adjuvant therapy components can penetrate deep into tumor cells. At present, experts’ researches on MNs provide a clear idea for the clinical treatment of skin cancer. We reviewed the current research progress of many minimally invasive MN-mediated tumor therapies, including PTT, gene therapy, immunotherapy, and starvation therapy. Their developments hold promise for the treatment of superficial tumors and have shown high potential for clinical conversion. The synergistic use of multiple methods also holds great promise for the treatment of patients. However, despite the latest exciting outcomes from the minimally invasive MNs for superficial tumor diseases therapy, several challenges and limitations remained that need to be developed before further clinical translations. First of all, the drug loading of MNs still has a small killing effect on tumor cells, especially for patients with advanced cancer. In addition, even if the MNs achieve the ideal drug loading, due to the limitations of the use of the MNs, it will be difficult to use the MNs to treat some internal tumors after the occurrence of the tumors. Although there have been reports of biodegradable MNs applied between teeth and gingival tissue to treat periodontitis, and reports of MN patches applied to cardiac stromal cells [80, 156], there are also many difficulties in realizing the real clinical translation of these theoretically ideal methods.
To solve these problems and meet the special needs of tumor therapy, more methods and strategies should be explored to improve the drug load of MNs, including the combination of new nanomaterials, chemical modification of drug molecules, and so on. In addition, more MN-mediated treatments for superficial tumors should be widely developed and their corresponding therapeutic mechanisms should be elucidated to realize multi-modal treatments against these diseases. In addition, to maintain the inherent properties of the MNs and pay attention to the biosafety of the material itself, more materials should be developed. Last but not least, there is also an urgent need to develop some minimally invasive in vivo transplantation treatments and apply this system to the treatments of more diseases, which will be the direction of our lives to strive for.
Acknowledgements
We tender our apologies to those authors whose deserving research was not cited in this manuscript. And we would like to express our deep gratitude to all the authors of this manuscript.
Abbreviations
- MNs
Microneedles
- SST
Superficial skin tumors
- SC
Stratum corneum
- PTT
Photodynamic therapy
- BCC
Basal cell carcinoma
- LHRHa
Luteinizing hormone-releasing hormone analogs
- NPs
Nanoparticles
- DLP
Digital light processing
- DOX
Doxorubicin
- DOC
Docetaxel
- PDT
Photodynamic therapy
- MAPK
MAP kinase
- ICG
Indocyanine green
- PTX
Paclitaxel
- MSN
Mesoporous silica nanoparticles
- 5-Fu
5-Fluorouracil
- CaO2@Mn-PDA NFs
Calcium peroxide-polydopamine MN nanoformulation
- PPIX
Protoporphyrin IX
- PEM
Polyelectrolyte multilayer membrane
- PCL
Polycaprolactone
- NIR
Near-infrared
- PVPVA
Polyvinylpyrrolidone-vinyl acetate
- aPD-1
Anti-PD-1
- OVA
Ovalbumin
- NK cells
Natural killer cells
- HA
Polyglycolic acid
- CPT
Camptothecin
- 5-ALA
5-Aminolevulinic acid
- CU-NS
Curcumin nanosuspension
- Cur
Curcumin
- LCC-NPs
Lipid-coated cisplatin nanoparticles
- CS
Chitosan
- NMP
N-Methylpyrrolidone
- GOx
Glucose oxidase
- PVP
Polyvinylpyrrolidone
- CAT
Catalase
- AuNC
Gold nanocage
- CRPC
Castrate resistant prostate cancer
- p53 DNA
p53 expression plasmid
- PEI
Polyethylenimine
- pDNA
Plasmid DNA
- GelMA
Gelatin methacryloyl
- DexMA
Dextran methacrylate
- Tra
Trametinib
- CEL
Celecoxib
Author contribution
Meng Wang: investigation, conceptualization, writing—original draft, writing—review and editing. Xiaodan Li: writing—review and editing. Wenzhen Du: writing—review and editing. Minge Sun: writing—review and editing. Guixia Ling: conceptualization, supervision. Peng Zhang: supervision.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papke DJ, Jr, Hornick JL. Recent advances in the diagnosis, classification and molecular pathogenesis of cutaneous mesenchymal neoplasms. Histopathology. 2022;80(1):216–232. doi: 10.1111/his.14450. [DOI] [PubMed] [Google Scholar]
- 2.Campana LG, Miklavčič D, Bertino G, Marconato R, Valpione S, Imarisio I, Dieci MV, Granziera E, Cemazar M, Alaibac M, Sersa G. Electrochemotherapy of superficial tumors - Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin Oncol. 2019;46(2):173–191. doi: 10.1053/j.seminoncol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Cho WK, Chang JS, Park SG, Kim N, Choi DH, Kim H, Kim YB, Park W, Suh CO. Internal mammary node irradiation in node-positive breast cancer treated with mastectomy and taxane-based chemotherapy. Breast. 2021;59:37–43. doi: 10.1016/j.breast.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei S, Zheng R, Zhang S, Chen R, Wang S, Sun K, Zeng H, Wei W, He J. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18(3):900–909. doi: 10.20892/j.issn.2095-3941.2020.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan J, Liu B, Long Y, Wang Z, Tong C, Wang W, You P, Liu X. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554–569. doi: 10.1016/j.actbio.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Reaume SE. The use of hydrofluoric acid in making glass microneedles. Science. 1952;116(3023):641. doi: 10.1126/science.116.3023.641. [DOI] [PubMed] [Google Scholar]
- 7.Biesman BS, Cohen JL, DiBernardo BE, Emer JJ, Geronemus RG, Gold MH, Lehman AS, Pilcher BK, Monheit GD, Schlesinger TE, Teller CF. Treatment of atrophic facial acne scars with microneedling followed by polymethylmethacrylate-collagen gel dermal filler. Dermatol Surg. 2019;45(12):1570–1579. doi: 10.1097/DSS.0000000000001872. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Park J, Chow SYA, Kasuya MCZ, Ikeuchi Y, Kim B. Localised light delivery on melanoma cells using optical microneedles. Biomed Opt Express. 2022;13(2):1045–1060. doi: 10.1364/BOE.450456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Ji W, Zhang B, Ma L, Fu W, Qian W, Zhang X, Li J, Sheng E, Tao Y, Zhu D. A theranostic microneedle array patch for integrated glycemia sensing and self-regulated release of insulin. Biomater Sci. 2022;10(5):1209–1216. doi: 10.1039/D1BM01834E. [DOI] [PubMed] [Google Scholar]
- 10.Zong Q, Guo R, Dong N, Ling G, Zhang P. Design and development of insulin microneedles for diabetes treatment. Drug Deliv Transl Res. 2022;12(5):973–980. doi: 10.1007/s13346-021-00981-y. [DOI] [PubMed] [Google Scholar]
- 11.Zong Q, Zhou R, Zhao Z, Wang Y, Liu C, Zhang P. Glucose-responsive insulin microneedle patch based on phenylboronic acid for 1 diabetes treatment. Eur Polymer J. 2022;173:111217. doi: 10.1016/j.eurpolymj.2022.111217. [DOI] [Google Scholar]
- 12.Qiao Y, Du J, Ge R, Lu H, Wu C, Li J, Yang S, Zada S, Dong H, Zhang X. A sample and detection microneedle patch for psoriasis microRNA biomarker analysis in interstitial fluid. Anal Chem. 2022;94(14):5538–5545. doi: 10.1021/acs.analchem.1c04401. [DOI] [PubMed] [Google Scholar]
- 13.Chao X, Zhang C, Li X, Lv H, Ling G, Zhang P. Synthesis and characterization of ionic liquid microneedle patches with different carbon chain lengths for antibacterial application. Biomaterials science. 2022;10(4):1008–1017. doi: 10.1039/D1BM01661J. [DOI] [PubMed] [Google Scholar]
- 14.Wise J. Microneedle patch for flu vaccination proves successful in human clinical trial. BMJ. 2017;357:j3120. doi: 10.1136/bmj.j3120. [DOI] [Google Scholar]
- 15.Azukizawa H, Hirobe S, Hanafusa T, Matsuo K, Quan Y-S, Kamiyama F, Katayama I, Okada N, Nakagawa S. A Clinical study of influenza vaccine using a dissolving microneedle array. J Dermatol Sci. 2013;69(2):e33. doi: 10.1016/j.jdermsci.2012.11.399. [DOI] [Google Scholar]
- 16.Roger M, Fullard N, Costello L, Bradbury S, Markiewicz E, O'Reilly S, Darling N, Ritchie P, Määttä A, Karakesisoglou I, Nelson G, von Zglinicki T, Dicolandrea T, Isfort R, Bascom C, Przyborski S. Bioengineering the microanatomy of human skin. J Anat. 2019;234(4):438–455. doi: 10.1111/joa.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choe C, Lademann J, Darvin ME. Lipid organization and stratum corneum thickness determined in vivo in human skin analyzing lipid–keratin peak (2820–3030 cm−1) using confocal. Raman Microscopy. 2016;47(11):1327–1331.
- 18.Liu Y, Lunter DJ. Optimal configuration of confocal Raman spectroscopy for precisely determining stratum corneum thickness: evaluation of the effects of polyoxyethylene stearyl ethers on skin. Int J Pharm. 2021;597:120308. doi: 10.1016/j.ijpharm.2021.120308. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Lunter DJ. Confocal Raman microspectroscopy as an alternative method to investigate the extraction of lipids from stratum corneum by emulsifiers and formulations. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2018;127:61–71. [DOI] [PubMed]
- 20.Franzen L, Windbergs M, Hansen S. Assessment of near-infrared densitometry for in situ determination of the total stratum corneum thickness on pig skin: influence of storage time. Skin Pharmacol Physiol. 2012;25(5):249–256. doi: 10.1159/000339905. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Li X, Zhang P, Du J, Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J Control Release. 2018;286:201–209. doi: 10.1016/j.jconrel.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Jain AK, Lee CH, Gill HS. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release. 2016;239:72–81. doi: 10.1016/j.jconrel.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Xu Q, Zhang P, Zhao X, Wang Y. Cutaneous microenvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J Control Release. 2019;314:72–80. doi: 10.1016/j.jconrel.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 24.He T, Luo Y, Zhang Q, Men Z, Su T, Fan L, Chen H, Shen T. Hyalase-mediated cascade degradation of a matrix barrier and immune cell penetration by a photothermal microneedle for efficient anticancer therapy. ACS Appl Mater Interfaces. 2021;13(23):26790–26799. doi: 10.1021/acsami.1c06725. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Guo R, Wang S, Yang X, Ling G, Zhang P. Fabrication, evaluation and applications of dissolving microneedles. Int J Pharm. 2021;604:120749. doi: 10.1016/j.ijpharm.2021.120749. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Lee H, Choi H, Yoo K-Y, Yoon H. Investigation on photopolymerization of PEGDA to fabricate high-aspect-ratio microneedles. RSC Adv. 2022;12(16):9550–9555. doi: 10.1039/D2RA00189F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smetana K, Lacina L, Kodet O. Targeted therapies for melanoma. Cancers. 2020. [DOI] [PMC free article] [PubMed]
- 28.Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366–1379. doi: 10.1080/15384047.2019.1640032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalhout SZ, Emerick KS, Kaufman HL, Miller DM. Immunotherapy for non-melanoma skin cancer. Curr Oncol Rep. 2021;23(11):125. doi: 10.1007/s11912-021-01120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garbutcheon-Singh KB, Veness MJ. The role of radiotherapy in the management of non-melanoma skin cancer. Australas J Dermatol. 2019;60(4):265–272. doi: 10.1111/ajd.13025. [DOI] [PubMed] [Google Scholar]
- 31.Benkhaled S, Van Gestel D, Gomes da Silveira Cauduro C, Palumbo S, Del Marmol V, Desmet A. The state of the art of radiotherapy for non-melanoma skin cancer: a review of the literature. Front Med (Lausanne). 2022;9:913269. 10.3389/fmed.2022.913269. [DOI] [PMC free article] [PubMed]
- 32.Tariq N, Ashraf MW, Tayyaba S. A review on solid microneedles for biomedical applications. J Pharm Innov. 2021.
- 33.Singh V, Kesharwani P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. Journal of controlled release : official journal of the Controlled Release Society. 2021;338:394–409. doi: 10.1016/j.jconrel.2021.08.054. [DOI] [PubMed] [Google Scholar]
- 34.Liu T-T, Chen K, Wang Q. Skin drug permeability and safety through a vibrating solid micro-needle system. Drug Deliv Transl Res. 2018;8(5):1025–1033. doi: 10.1007/s13346-018-0544-2. [DOI] [PubMed] [Google Scholar]
- 35.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Pradeep Narayanan S, Raghavan S. Solid silicon microneedles for drug delivery applications. Int J Adv Manuf Technol. 2017;93(1):407–422.
- 37.Cárcamo-Martínez Á, Mallon B, Domínguez-Robles J, Vora LK, Anjani QK, Donnelly RF. Hollow microneedles: a perspective in biomedical applications. Int J Pharm. 2021;599:120455. doi: 10.1016/j.ijpharm.2021.120455. [DOI] [PubMed] [Google Scholar]
- 38.Ita K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: two decades of research. J Drug Deliv Sci Techno. 2018;44:314–322. doi: 10.1016/j.jddst.2018.01.004. [DOI] [Google Scholar]
- 39.Niu L, Chu LY, Burton SA, Hansen KJ, Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Mönkäre J, Pontier M, van Kampen EEM, Du G, Leone M, Romeijn S, Nejadnik MR, O'Mahony C, Slütter B, Jiskoot W, Bouwstra JA. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur J Pharm Biopharm. 2018;129:111–121. doi: 10.1016/j.ejpb.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Abd-El-Azim H, Tekko IA, Ali A, Ramadan A, Nafee N, Khalafallah N, Rahman T, McDaid W, Aly RG, Vora LK, Bell SJ, Furlong F, McCarthy HO, Donnelly RF. Hollow microneedle assisted intradermal delivery of hypericin lipid nanocapsules with light enabled photodynamic therapy against skin cancer. J Control Release. 2022;348:849–869. doi: 10.1016/j.jconrel.2022.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Jung YS, Koo D-H, Yang J-Y, Lee H-Y, Park J-H, Park JH. Peri-tumor administration of 5-fluorouracil sol-gel using a hollow microneedle for treatment of gastric cancer. Drug Deliv. 2018;25(1):872–879. doi: 10.1080/10717544.2018.1455760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore LE, Vucen S, Moore AC. Trends in drug- and vaccine-based dissolvable microneedle materials and methods of fabrication. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2022;173:54–72. [DOI] [PubMed]
- 44.Vora LK, Moffatt K, Tekko IA, Paredes AJ, Volpe-Zanutto F, Mishra D, Peng K, Thakur RR, Donnelly RF. Microneedle array systems for long-acting drug delivery. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2021;159:44–76. [DOI] [PubMed]
- 45.Chen MY, Chen YY, Tsai HT, Tzai TS, Chen MC, Tsai YS. Transdermal delivery of luteinizing hormone-releasing hormone with chitosan microneedles: a promising tool for androgen deprivation therapy. Anticancer Res. 2017;37(12):6791–6797. doi: 10.21873/anticanres.12139. [DOI] [PubMed] [Google Scholar]
- 46.Sabri AH, Kim Y, Marlow M, Scurr DJ, Segal J, Banga AK, Kagan L, Lee JB. Intradermal and transdermal drug delivery using microneedles - Fabrication, performance evaluation and application to lymphatic delivery. Adv Drug Deliv Rev. 2020;153:195–215. doi: 10.1016/j.addr.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Ali M, Namjoshi S, Benson HAE, Mohammed Y, Kumeria T. Dissolvable polymer microneedles for drug delivery and diagnostics. J Control Release. 2022;347:561–589. doi: 10.1016/j.jconrel.2022.04.043. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XP, Wang BB, Hu LF, Fei WM, Cui Y, Guo XD. Safety evaluation of 3-month effects of microneedle patches prepared from hyaluronic acid in mice. Biochem Eng J. 2021;176:108157. doi: 10.1016/j.bej.2021.108157. [DOI] [Google Scholar]
- 49.Bhatnagar S, Bankar NG, Kulkarni MV, Venuganti VVK. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int J Pharm. 2019;556:263–275. doi: 10.1016/j.ijpharm.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Li JY, Feng YH, He YT, Hu LF, Liang L, Zhao ZQ, Chen BZ, Guo XD. Thermosensitive hydrogel microneedles for controlled transdermal drug delivery. Acta Biomater. 2022;153:308–319. doi: 10.1016/j.actbio.2022.08.061. [DOI] [PubMed] [Google Scholar]
- 51.Sivaraman A, Banga AK. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv Transl Res. 2017;7(1):16–26. doi: 10.1007/s13346-016-0328-5. [DOI] [PubMed] [Google Scholar]
- 52.Zheng M, Wang Z, Chang H, Wang L, Chew SWT, Lio DCS, Cui M, Liu L, Tee BCK, Xu C. Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within minutes. Adv Healthcare Mater. 2020;9(10):e1901683. doi: 10.1002/adhm.201901683. [DOI] [PubMed] [Google Scholar]
- 53.Cao Y, Tao Y, Zhou Y, Gui S. Development of sinomenine hydrochloride-loaded polyvinylalcohol/maltose microneedle for transdermal delivery. J Drug Delivery Sci Techno. 2016;35:1–7. doi: 10.1016/j.jddst.2016.06.007. [DOI] [Google Scholar]
- 54.Meng F, Hasan A, Babadaei MM, Kani PH, Talaei AJ, Sharifi M, Cai T, Falahati M, Cai Y. Polymeric-based microneedle arrays as potential platforms in the development of drugs delivery systems. J Adv Res. 2020;26:137–147. [DOI] [PMC free article] [PubMed]
- 55.Zhou X, Luo Z, Baidya A, Kim HJ, Wang C, Jiang X, Qu M, Zhu J, Ren L, Vajhadin F, Tebon P, Zhang N, Xue Y, Feng Y, Xue C, Chen Y, Lee K, Lee J, Zhang S, Xu C, Ashammakhi N, Ahadian S, Dokmeci MR, Gu Z, Sun W, Khademhosseini A. Biodegradable β-cyclodextrin conjugated gelatin methacryloyl microneedle for delivery of water-insoluble drug. Adv Healthcare Mater. 2020;9(11):e2000527. [DOI] [PMC free article] [PubMed]
- 56.Luo Z, Sun W, Fang J, Lee K, Li S, Gu Z, Dokmeci MR, Khademhosseini A. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv Healthcare Mater. 2019;8(3):e1801054. [DOI] [PMC free article] [PubMed]
- 57.Turner JG, White LR, Estrela P, Leese HS. Hydrogel-forming microneedles: current advancements and future trends. Macromol Biosci. 2021;21(2):2000307. doi: 10.1002/mabi.202000307. [DOI] [PubMed] [Google Scholar]
- 58.He R, Niu Y, Li Z, Li A, Yang H, Xu F, Li F. A hydrogel microneedle patch for point-of-care testing based on skin interstitial fluid. Adv Healthcare Mater. 2020;9(4):1901201. doi: 10.1002/adhm.201901201. [DOI] [PubMed] [Google Scholar]
- 59.Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res. 2014;31(9):2393–2403. doi: 10.1007/s11095-014-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Boese SE, Luo Z, Nitin N, Gill HS. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: development and in vitro evaluation. Biomed Microdevice. 2015;17(2):44. doi: 10.1007/s10544-015-9944-y. [DOI] [PubMed] [Google Scholar]
- 61.Zeng Q, Gammon JM, Tostanoski LH, Chiu YC, Jewell CM. In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater Sci Eng. 2017;3(2):195–205. doi: 10.1021/acsbiomaterials.6b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Miyazaki T, Itoh M, Matsumoto H, Moro-Oka Y, Tanaka M, Miyahara Y, Suganami T, Matsumoto A. A porous reservoir-backed boronate gel microneedle for efficient skin penetration and sustained glucose-responsive insulin delivery. Gels (Basel, Switzerland). 2022;8(2):74. [DOI] [PMC free article] [PubMed]
- 63.Jeon EY, Lee J, Kim BJ, Joo KI, Kim KH, Lim G, Cha HJ. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials. 2019;222:119439. doi: 10.1016/j.biomaterials.2019.119439. [DOI] [PubMed] [Google Scholar]
- 64.Ono A, Azukizawa H, Ito S, Nakamura Y, Asada H, Quan Y-S, Kamiyama F, Katayama I, Hirobe S, Okada N. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int J Pharm. 2017;532(1):374–383. doi: 10.1016/j.ijpharm.2017.08.110. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Wang L, Yu H, Li C, Feng J, Haq F, Khan A, Khan RU. Preparation, properties and challenges of the microneedles-based insulin delivery system. J Control Release. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 66.Li S, Xia D, Prausnitz MR. Efficient drug delivery into skin using a biphasic dissolvable microneedle patch with water-insoluble backing. Adv Functional Mater. 2021;31(44). [DOI] [PMC free article] [PubMed]
- 67.Schipper P, van der Maaden K, Groeneveld V, Ruigrok M, Romeijn S, Uleman S, Oomens C, Kersten G, Jiskoot W, Bouwstra J. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J Control Release. 2017;262:28–36. doi: 10.1016/j.jconrel.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Deng Y, Chen J, Zhao Y, Yan X, Zhang L, Choy K, Hu J, Sant HJ, Gale BK, Tang T. Transdermal delivery of siRNA through microneedle array. Sci Rep. 2016;6:21422. doi: 10.1038/srep21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabri A, Ogilvie J, McKenna J, Segal J, Scurr D, Marlow M. Intradermal delivery of an immunomodulator for basal cell carcinoma; expanding the mechanistic insight into solid microneedle-enhanced delivery of hydrophobic molecules. Mol Pharm. 2020;17(8):2925–2937. doi: 10.1021/acs.molpharmaceut.0c00347. [DOI] [PubMed] [Google Scholar]
- 70.Liu C, Zhao Z, Lv H, Yu J, Zhang P. Microneedles-mediated drug delivery system for the diagnosis and treatment of melanoma. Colloids Surf, B. 2022;219:112818. doi: 10.1016/j.colsurfb.2022.112818. [DOI] [PubMed] [Google Scholar]
- 71.Chen G, Chen Z, Wen D, Wang Z, Li H, Zeng Y, Dotti G, Wirz RE, Gu Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc Natl Acad Sci USA. 2020;117(7):3687–3692. doi: 10.1073/pnas.1917891117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng Y, Zhou H, Ding J, Zhou W. Cell membrane inspired nano-shell enabling long-acting Glucose Oxidase for Melanoma starvation therapy via microneedles-based percutaneous delivery. Theranostics. 2021;11(17):8270–8282. doi: 10.7150/thno.60758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin W, Quan G, Sun Y, Chen M, Yang P, Feng D, Wen T, Hu X, Pan X, Wu C. Dissolving microneedles with spatiotemporally controlled pulsatile release nanosystem for synergistic chemo-photothermal therapy of melanoma. Theranostics. 2020;10(18):8179–8196. doi: 10.7150/thno.44194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S-J, Lee H-S, Hwang Y-H, Kim J-J, Kang K-Y, Kim SJ, Kim HK, Kim JD, Jeong DH, Paik M-J, Yee S-T. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLoS ONE. 2019;14(8):e0220382. doi: 10.1371/journal.pone.0220382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdelghany S, Tekko IA, Vora L, Larraneta E, Permana AD, Donnelly RF. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics. 2019;11(7):308. doi: 10.3390/pharmaceutics11070308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lan X, Zhu W, Huang X, Yu Y, Xiao H, Jin L, Pu JJ, Xie X, She J, Lui VWY, Chen H-J, Su Y-X. Microneedles loaded with anti-PD-1-cisplatin nanoparticles for synergistic cancer immuno-chemotherapy. Nanoscale. 2020;12(36):18885–18898. doi: 10.1039/D0NR04213G. [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Chen M, Yang D, Qin W, Quan G, Wu C, Pan X. Self-assembly nanomicelle-microneedle patches with enhanced tumor penetration for superior chemo-photothermal therapy. Nano Res. 2022;15(3):2335–2346. doi: 10.1007/s12274-021-3817-x. [DOI] [Google Scholar]
- 78.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16(4):2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 79.Courtenay AJ, McCrudden MTC, McAvoy KJ, McCarthy HO, Donnelly RF. Microneedle-mediated transdermal delivery of bevacizumab. Mol Pharm. 2018;15(8):3545–3556. doi: 10.1021/acs.molpharmaceut.8b00544. [DOI] [PubMed] [Google Scholar]
- 80.Shi H, Xue T, Yang Y, Jiang C, Huang S, Yang Q, Lei D, You Z, Jin T, Wu F, Zhao Q. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci Adv. 2020;6(25):eaaz3621. [DOI] [PMC free article] [PubMed]
- 81.Qu M, Kim H-J, Zhou X, Wang C, Jiang X, Zhu J, Xue Y, Tebon P, Sarabi SA, Ahadian S, Dokmeci MR, Zhu S, Gu Z, Sun W, Khademhosseini A. Biodegradable microneedle patch for transdermal gene delivery. Nanoscale. 2020;12(32):16724–16729. doi: 10.1039/D0NR02759F. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Xu Q, Wang J, Zhang P, Wang Y, Ji J. A gene-coated microneedle patch based on industrialized ultrasonic spraying technology with a polycation vector to improve antitumor efficacy. J Mater Chem B. 2021;9(27):5528–5536. doi: 10.1039/D1TB00512J. [DOI] [PubMed] [Google Scholar]
- 83.Pearton M, Saller V, Coulman SA, Gateley C, Anstey AV, Zarnitsyn V, Birchall JC. Microneedle delivery of plasmid DNA to living human skin: Formulation coating, skin insertion and gene expression. J Control Release. 2012;160(3):561–569. doi: 10.1016/j.jconrel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruan W, Zhai Y, Yu K, Wu C, Xu Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553(1–2):298–309. doi: 10.1016/j.ijpharm.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 85.Yang P, Chen M, Qin W, Shi C, Bai X, Quan G, Pan X, Wu C. Effective photothermal therapy mediated by indocyanine green nanoparticle tip-loaded microneedles to enhance checkpoint inhibitor immunotherapy for melanoma treatment. ACS applied nano materials. 2021;4(6):5921–5931. doi: 10.1021/acsanm.1c00832. [DOI] [Google Scholar]
- 86.Chen M, Quan G, Wen T, Yang P, Qin W, Mai H, Sun Y, Lu C, Pan X, Wu C. Cold to hot: binary cooperative microneedle array-amplified photoimmunotherapy for eliciting antitumor immunity and the abscopal effect. ACS Appl Mater Interfaces. 2020;12(29):32259–32269. doi: 10.1021/acsami.0c05090. [DOI] [PubMed] [Google Scholar]
- 87.Cheng Z, Lin H, Wang Z, Yang X, Zhang M, Liu X, Wang B, Wu Z, Chen D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv Transl Res. 2020;10(5):1520–1530. doi: 10.1007/s13346-020-00735-2. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Li S, Wang X, Chen Q, He Z, Luo C, Sun J. Smart transformable nanomedicines for cancer therapy. Biomaterials. 2021;271:120737. [DOI] [PubMed]
- 89.Xu J, Xu B, Tao J, Yang Y, Hu Y, Huang Y. Microneedle-assisted, DC-targeted codelivery of pTRP-2 and adjuvant of paclitaxel for transcutaneous immunotherapy. Small. 2017;13(28):1700666. doi: 10.1002/smll.201700666. [DOI] [PubMed] [Google Scholar]
- 90.Yang F, Ji Q, Liao R, Li S, Wang Y, Zhang X, Zhang S, Zhang H, Kan Q, Sun J, He Z, Sun B, Luo C. Precisely engineering a dual-drug cooperative nanoassembly for proteasome inhibition-potentiated photodynamic therapy. Chin Chem Lett. 2022;33(4):1927–1932. doi: 10.1016/j.cclet.2021.11.056. [DOI] [Google Scholar]
- 91.Zhang P, Han T, Xia H, Dong L, Chen L, Lei L. Advances in photodynamic therapy based on nanotechnology and its application in skin cancer. Front Oncol. 2022;12:836397. [DOI] [PMC free article] [PubMed]
- 92.Li S, Yang F, Sun X, Wang Y, Zhang X, Zhang S, Zhang H, Kan Q, Sun J, He Z, Luo C. Precisely engineering a carrier-free hybrid nanoassembly for multimodal DNA damage-augmented photodynamic therapy. Chem Eng J. 2021;426:130838. doi: 10.1016/j.cej.2021.130838. [DOI] [Google Scholar]
- 93.Zhang S, Wang Z, Kong Z, Wang Y, Zhang X, Sun B, Zhang H, Kan Q, He Z, Luo C, Sun J. Photosensitizer-driven nanoassemblies of homodimeric prodrug for self-enhancing activation and synergistic chemo-photodynamic therapy. Theranostics. 2021;11(12):6019–6032. doi: 10.7150/thno.59065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y, Zhou Y, Yang D, Gao X, Wen T, Fu J, Wen X, Quan G, Pan X, Wu C. Intelligent and spatiotemporal drug release based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 2021;135:164–178. doi: 10.1016/j.actbio.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Tham HP, Xu K, Lim WQ, Chen H, Zheng M, Thng TGS, Venkatraman SS, Xu C, Zhao Y. Microneedle-assisted topical delivery of photodynamically active mesoporous formulation for combination therapy of deep-seated melanoma. ACS Nano. 2018;12(12):11936–11948. doi: 10.1021/acsnano.8b03007. [DOI] [PubMed] [Google Scholar]
- 96.Hao Y, Chen Y, He X, Yang F, Han R, Yang C, Li W, Qian Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact Mater. 2020;5(3):542–552. doi: 10.1016/j.bioactmat.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pei P, Yang F, Liu J, Hu H, Du X, Hanagata N, Zhao S, Zhu Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater Sci. 2018;6(6):1414–1423. doi: 10.1039/C8BM00005K. [DOI] [PubMed] [Google Scholar]
- 98.Song G, Sun Y, Liu T, Zhang X, Zeng Z, Wang R, Li P, Li C, Jiang G. Transdermal delivery of Cu-doped polydopamine using microneedles for photothermal and chemodynamic synergistic therapy against skin melanoma. Chem Eng J. 2021;426:130790. doi: 10.1016/j.cej.2021.130790. [DOI] [Google Scholar]
- 99.Hao Y, Dong M, Zhang T, Peng J, Jia Y, Cao Y, Qian Z. Novel approach of using near-infrared responsive PEGylated gold nanorod coated poly(L-lactide) microneedles to enhance the antitumor efficiency of docetaxel-loaded MPEG-PDLLA micelles for treating an A431 tumor. ACS Appl Mater Interfaces. 2017;9(18):15317–15327. doi: 10.1021/acsami.7b03604. [DOI] [PubMed] [Google Scholar]
- 100.Hamdan IMN, Tekko IA, Bell SEJ. Gold nanorods-loaded hydrogel-forming needles for local hyperthermia applications: Proof of concept. Eur J Pharm Biopharm. 2022;179:105–117. doi: 10.1016/j.ejpb.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 101.Hao Y, Chen Y, Lei M, Zhang T, Cao Y, Peng J, Chen L, Qian Z. Near-infrared responsive PEGylated gold nanorod and doxorubicin loaded dissolvable hyaluronic acid microneedles for human epidermoid cancer therapy. Adv Ther. 2018;1(2):1800008. doi: 10.1002/adtp.201800008. [DOI] [Google Scholar]
- 102.Chen J, Cao Y, Lin S, Niu H, Zhang H, Guan L, Shu C, Wu A, Bian Y, Zhu Y. A responsive microneedle system for efficient anti-melanoma by combining self-enhanced chemodynamic therapy with photothermal therapy. Chem Eng J. 2022;431:133466. doi: 10.1016/j.cej.2021.133466. [DOI] [Google Scholar]
- 103.Moreira AF, Rodrigues CF, Jacinto TA, Miguel SP, Costa EC, Correia IJ. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int J Pharm. 2020;576:118907. doi: 10.1016/j.ijpharm.2019.118907. [DOI] [PubMed] [Google Scholar]
- 104.Dong L, Li Y, Li Z, Xu N, Liu P, Du H, Zhang Y, Huang Y, Zhu J, Ren G, Xie J, Wang K, Zhou Y, Shen C, Zhu J, Tao J. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl Mater Interfaces. 2018;10(11):9247–9256. doi: 10.1021/acsami.7b18293. [DOI] [PubMed] [Google Scholar]
- 105.Champeau M, Jary D, Vignion-Dewalle A-S, Mordon S, de Lassalle EM, Vignoud S, Mortier L. Introduction of a model of skin lesions on rats and testing of dissolving microneedles containing 5-aminolevulinic acid. Int J Pharm. 2021;594:120115. doi: 10.1016/j.ijpharm.2020.120115. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z, Li H, Bian Y, Wang Z, Chen G, Zhang X, Miao Y, Wen D, Wang J, Wan G, Zeng Y, Abdou P, Fang J, Li S, Sun CJ, Gu Z. Bioorthogonal catalytic patch. Nat Nanotechnol. 2021;16(8):933–941. doi: 10.1038/s41565-021-00910-7. [DOI] [PubMed] [Google Scholar]
- 107.Quirk B, Olasz E, Kumar S, Basel D, Whelan H. Photodynamic therapy for benign cutaneous neurofibromas using aminolevulinic acid topical application and 633 nm red light illumination. Photobiomodulation, photomedicine, and laser surgery. 2021;39(6):411–417. doi: 10.1089/photob.2020.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noh I, Lee K, Rhee YS. Microneedle systems for delivering nucleic acid drugs. J Pharm Invest. 2022;1–20. [DOI] [PMC free article] [PubMed]
- 109.Cole G, McCaffrey J, Ali AA, McBride JW, McCrudden CM, Vincente-Perez EM, Donnelly RF, McCarthy HO. Dissolving microneedles for DNA vaccination: improving functionality via polymer characterization and RALA complexation. Hum Vaccin Immunother. 2017;13(1):50–62. doi: 10.1080/21645515.2016.1248008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee Y, Dugansani SR, Jeon SH, Hwang SH, Kim J-H, Park SH, Jeong J-H. Drug-Delivery System Based on Salmon DNA Nano- and Micro-Scale Structures. Sci Rep. 2017;7(1):9724. doi: 10.1038/s41598-017-09904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cole G, Ali AA, McCrudden CM, McBride JW, McCaffrey J, Robson T, Kett VL, Dunne NJ, Donnelly RF, McCarthy HO. DNA vaccination for cervical cancer: strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2018;127:288–297. [DOI] [PubMed]
- 112.Tang T, Deng Y, Chen J, Zhao Y, Yue R, Choy KW, Wang CC, Du Q, Xu Y, Han L, Chung TKH. Local administration of siRNA through microneedle: optimization, bio-distribution, tumor suppression and toxicity. Sci Rep. 2016;6:30430. doi: 10.1038/srep30430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kilgallen AB, Stibler U, Printezi MI, Putker M, Punt CJA, Sluijter JPG, May AM, van Laake LW. Comparing conventional chemotherapy to chronomodulated chemotherapy for cancer treatment: protocol for a systematic review. JMIR research protocols. 2020;9(10):e18023. doi: 10.2196/18023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11(13):6370–6392. doi: 10.7150/thno.57828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahmed KS, Shan X, Mao J, Qiu L, Chen J. Derma roller (R) microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater Sci Eng C Mater Biol Appl. 2019;99:1448–1458. doi: 10.1016/j.msec.2019.02.095. [DOI] [PubMed] [Google Scholar]
- 116.Mojeiko G, de Brito M, Salata GC, Lopes LB. Combination of microneedles and microemulsions to increase celecoxib topical delivery for potential application in chemoprevention of breast cancer. Int J Pharm. 2019;560:365–376. doi: 10.1016/j.ijpharm.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 117.Lan X, She J, Lin D-A, Xu Y, Li X, Yang W-F, Lui VWY, Jin L, Xie X, Su Y-X. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl Mater Interfaces. 2018;10(39):33060–33069. doi: 10.1021/acsami.8b12926. [DOI] [PubMed] [Google Scholar]
- 118.Huang S, Liu H, Huang S, Fu T, Xue W, Guo R. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohyd Polym. 2020;246:116650. doi: 10.1016/j.carbpol.2020.116650. [DOI] [PubMed] [Google Scholar]
- 119.Gao X, Patel MG, Bakshi P, Sharma D, Banga AK. Enhancement in the transdermal and localized delivery of honokiol through breast tissue. AAPS PharmSciTech. 2018;19(8):3501–3511. doi: 10.1208/s12249-018-1158-1. [DOI] [PubMed] [Google Scholar]
- 120.Sabri AH, Cater Z, Gurnani P, Ogilvie J, Segal J, Scurr DJ, Marlow M. Intradermal delivery of imiquimod using polymeric microneedles for basal cell carcinoma. Int J Pharm. 2020;589:119808. doi: 10.1016/j.ijpharm.2020.119808. [DOI] [PubMed] [Google Scholar]
- 121.Al-Mayahy MH, Sabri AH, Rutland CS, Holmes A, McKenna J, Marlow M, Scurr DJ. Insight into imiquimod skin permeation and increased delivery using microneedle pre-treatment. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2019;139:33–43. [DOI] [PubMed]
- 122.Zhang J, Wang Y, Jin JY, Degan S, Hall RP, Boehm RD, Jaipan P, Narayan RJ. Use of drawing lithography-fabricated polyglycolic acid microneedles for transdermal delivery of itraconazole to a human basal cell carcinoma model regenerated on mice. JOM (Warrendale, Pa. : 1989). 2016;68(4):1128–1133. [DOI] [PMC free article] [PubMed]
- 123.Naguib YW, Kumar A, Cui Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm Sin B. 2014;4(1):94–99. doi: 10.1016/j.apsb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368(1):109–115. doi: 10.1016/j.ijpharm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 125.Yu Y, Wang H, Guo B, Wang B, Wan Z, Zhang Y, Sun L, Yang F. Microneedle-based two-step transdermal delivery of Langerhans cell-targeting immunoliposomes induces a Th1-biased immune response. Eur J Pharm Biopharm. 2022;177:68–80. doi: 10.1016/j.ejpb.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng H, Siddharth S, Parida S, Wu X, Sharma D. Tumor Microenvironment: Key Players in Triple Negative Breast Cancer Immunomodulation. Cancers. 2021;13(13):3357. doi: 10.3390/cancers13133357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hei Y, Chen Y, Li Q, Mei Z, Pan J, Zhang S, Xiong C, Su X, Wei S. Multifunctional immunoliposomes enhance the immunotherapeutic effects of PD-L1 antibodies against melanoma by reprogramming immunosuppressive tumor microenvironment. Small. 2022;18(9):e2105118. doi: 10.1002/smll.202105118. [DOI] [PubMed] [Google Scholar]
- 128.Cole G, Ali AA, McErlean E, Mulholland EJ, Short A, McCrudden CM, McCaffrey J, Robson T, Kett VL, Coulter JA, Dunne NJ, Donnelly RF, McCarthy HO. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019;96:480–490. doi: 10.1016/j.actbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 129.Duong HT, Yin Y, Thambi T, Nguyen TL, Phan VG, Lee MS, Lee JE, Kim J, Jeong JH, Lee DS. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. [DOI] [PubMed]
- 130.Chablani L, Tawde SA, Akalkotkar A, D'Souza MJ. Evaluation of a particulate breast cancer vaccine delivered via skin. AAPS J. 2019;21(2):12. doi: 10.1208/s12248-018-0285-7. [DOI] [PubMed] [Google Scholar]
- 131.Ye Y, Wang C, Zhang X, Hu Q, Zhang Y, Liu Q, Wen D, Milligan J, Bellotti A, Huang L, Dotti G. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692. [DOI] [PubMed]
- 132.Gilardi M, Saddawi-Konefka R, Wu VH, Lopez-Ramirez MA, Wang Z, Soto F, Ramms DJ, Proietto M, Mikulski Z, Miki H, Sharabi A, Kupor D, Rueda R, Hollern DP, Wang J, Gutkind JS. Microneedle-mediated intratumoral delivery of anti-CTLA-4 promotes cDC1-dependent eradication of oral squamous cell carcinoma with limited irAEs. Mol Cancer Ther. 2022;21(4):616–624. doi: 10.1158/1535-7163.MCT-21-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duong HTT, Yin Y, Thambi T, Kim BS, Jeong JH, Lee DS. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J Mater Chem B. 2020;8(6):1171–1181. doi: 10.1039/C9TB02175B. [DOI] [PubMed] [Google Scholar]
- 134.Zhao J-H, Zhang Q-B, Liu B, Piao X-H, Yan Y-L, Hu X-G, Zhou K, Zhang Y-T, Feng N-P. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomed. 2017;12:4763–4772. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim NW, Kim SY, Lee JE, Yin Y, Lee JH, Lim SY, Kim ES, Duong HT, Kim HK, Kim S, Kim JE. Enhanced cancer vaccination by in situ nanomicelle-generating dissolving microneedles. Acs Nano. 2018;12(10):9702–9713. [DOI] [PubMed]
- 136.Li Z, Xu Q, Lin X, Yu K, Lin L, Liu Y, Yu Z, Liu T, Luo D. Integrating of lipophilic platinum(IV) prodrug into liposomes for cancer therapy on patient-derived xenograft model. Chin Chem Lett. 2022;33(4):1875–1879. doi: 10.1016/j.cclet.2021.10.077. [DOI] [Google Scholar]
- 137.Yang Y, Liu X, Ma W, Xu Q, Chen G, Wang Y, Xiao H, Li N, Liang X-J, Yu M, Yu Z. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265:120456. doi: 10.1016/j.biomaterials.2020.120456. [DOI] [PubMed] [Google Scholar]
- 138.He M, Yu L, Yang Y, Zou B, Ma W, Yu M, Lu J, Xiong G, Yu Z, Li A. Delivery of triptolide with reduction-sensitive polymer nanoparticles for liver cancer therapy on patient-derived xenografts models. Chin Chem Lett. 2020;31(12):3178–3182. doi: 10.1016/j.cclet.2020.05.034. [DOI] [Google Scholar]
- 139.Yu L, Wang Z, Mo Z, Zou B, Yang Y, Sun R, Ma W, Yu M, Zhang S, Yu Z. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm Sin B. 2021;11(7):2004–2015. doi: 10.1016/j.apsb.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shallan AI, Guijt RM, Breadmore MC. Electrokinetics for sample preparation of biological molecules in biological samples using microfluidic systems. Bioanalysis. 2014;6(14):1961–1974. doi: 10.4155/bio.14.140. [DOI] [PubMed] [Google Scholar]
- 141.Compton LA, Murphy GF, Lian CG. Diagnostic immunohistochemistry in cutaneous neoplasia: an update. Dermatopathology. 2015;2(1):15–42. doi: 10.1159/000377698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fuertes L, Santonja C, Kutzner H, Requena L. Inmunohistoquímica en dermatopatología: revisión de los anticuerpos utilizados con mayor frecuencia (parte i) Actas Dermosifiliogr. 2013;104(2):99–127. doi: 10.1016/j.ad.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 143.Dervisevic M, Alba M, Adams TE, Prieto-Simon B, Voelcker NH. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens Bioelectron. 2021;192:113496. doi: 10.1016/j.bios.2021.113496. [DOI] [PubMed] [Google Scholar]
- 144.Yang B, Fang X, Kong J. In situ sampling and monitoring cell-free DNA of the Epstein-Barr virus from dermal interstitial fluid using wearable microneedle patches. ACS Appl Mater Interfaces. 2019;11(42):38448–38458. doi: 10.1021/acsami.9b12244. [DOI] [PubMed] [Google Scholar]
- 145.Chen L, Zhang C, Xiao J, You J, Zhang W, Liu Y, Xu L, Liu A, Xin H, Wang X. Local extraction and detection of early stage breast cancers through a microneedle and nano-Ag/MBL film based painless and blood-free strategy. Mater Sci Eng C Mater Biol Appl. 2020;109. [DOI] [PubMed]
- 146.Kim H, Seong K-Y, Lee JH, Park W, Yang SY, Hahn SK. Biodegradable microneedle patch delivering antigenic peptide-hyaluronate conjugate for cancer immunotherapy. ACS Biomater Sci Eng. 2019;5(10):5150–5158. doi: 10.1021/acsbiomaterials.9b00961. [DOI] [PubMed] [Google Scholar]
- 147.Zhou Y, Niu B, Zhao Y, Fu J, Wen T, Liao K, Quan G, Pan X, Wu C. Multifunctional nanoreactors-integrated microneedles for cascade reaction-enhanced cancer therapy. J Control Release. 2021;339:335–349. doi: 10.1016/j.jconrel.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 148.Chen MC, Lin ZW, Ling MH. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10(1):93–101. doi: 10.1021/acsnano.5b05043. [DOI] [PubMed] [Google Scholar]
- 149.Xu Q, Li X, Zhang P, Wang Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J Mater Chem B. 2020;8(19):4331–4339. doi: 10.1039/D0TB00105H. [DOI] [PubMed] [Google Scholar]
- 150.Chen M, Yang D, Sun Y, Liu T, Wang W, Fu J, Wang Q, Bai X, Quan G, Pan X, Wu C. In situ self-assembly nanomicelle microneedles for enhanced photoimmunotherapy via autophagy regulation strategy. ACS Nano. 2021;15(2):3387–3401. doi: 10.1021/acsnano.0c10396. [DOI] [PubMed] [Google Scholar]
- 151.Juhasz MLW, Cohen JL. Microneedling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997–1003. doi: 10.2147/CCID.S267192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tan C, Yeo Chen Long D, Cao T, Tan Wei Ding V, Srivastava R, Yow AP, Tan WP, Wong Wing Kee D, Xu C, Tey HL. Drug-free microneedles in the treatment of keloids: a single-blinded intraindividual controlled clinical trial. British J Dermatol. 2018;179(6):1418–1419. [DOI] [PubMed]
- 153.Lee G, Ma Y, Lee Y-H, Jung H. Clinical evaluation of a low-pain long microneedle for subcutaneous insulin injection. BioChip J. 2018;12(4):309–316. doi: 10.1007/s13206-018-2411-0. [DOI] [Google Scholar]
- 154.Wise J. Microneedle patch for flu vaccination proves successful in human clinical trial. 2017;357:j3120.
- 155.Jeong SY, Park JH, Lee YS, Kim YS, Park JY, Kim SY. The current status of clinical research involving microneedles: a systematic review. Pharmaceutics. 2020;12(11). [DOI] [PMC free article] [PubMed]
- 156.Zhang X, Hasani-Sadrabadi MM, Zarubova J, Dashtimighadam E, Haghniaz R, Khademhosseini A, Butte MJ, Moshaverinia A, Aghaloo T, Li S. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter. 2022;5(2):666–682. doi: 10.1016/j.matt.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.