Abstract
Background
Cellulite is an aesthetic condition affecting the appearance of skin in certain body regions and is associated with body dissatisfaction, psychosocial stress, and decreased quality of life. Previous studies established the safety and feasibility of a novel, minimally invasive device to identify and release septa responsible for cellulite depressions: targeted verifiable subcision (TVS).
Objectives
The objective of this single-arm, open-label, multicenter study was to evaluate the safety and efficacy of TVS for reducing the appearance of moderate to severe cellulite in adult women.
Methods
Adult women aged 21 to 55 years and a BMI < 30 kg/m2 with moderate or severe cellulite on the buttocks and/or thighs were eligible to enroll at 9 sites. Endpoint data included results from 4 of the postprocedural follow-up visits at 24 hours, 7 days, 30 days, and 90 days. The primary endpoints were a mean ≥1 point reduction in the Cellulite Severity Scale at 90 days and no related serious adverse events at 30 days.
Results
Seventy-four female participants with a mean BMI of 24.8 ± 2.7 and age of 41.4 ± 7.4 years received this single procedure. The mean improvement in Cellulite Severity Scale (N = 68) was 1.5 ± 0.9 (P < 0.0001). There were no device-related serious adverse events at 30 days.
Conclusions
TVS for selectively identifying and verifiably releasing septa responsible for cellulite depressions is an effective and safe means to improve the appearance of moderate to severe cellulite in adult women.
Level of Evidence: 2

The dimpled appearance of skin, known as cellulite, occurs in approximately 80% to 90% of postpubertal females.1,2 Cellulite rarely occurs in men, which may be due to specific anatomical differences in the number, thickness, and orientation of septal connections in the superficial fascia.3 Although cellulite can be located in any region of the body with subcutaneous adipose tissue, specific areas tend to have greater susceptibility, including the buttocks and thighs.4,5 Several factors contribute to the development of cellulite in women, such as age, thickened fibrous septa, enlarged fat lobules, stress, hormonal imbalance, decreased dermal collagen, and a sedentary lifestyle.6,7
Successfully addressing cellulite has proven challenging. Anatomical studies of the structure of cellulite have increased the understanding of its pathophysiology, leading to the development of additional treatment options.3,8,9 Increasing attention has been focused on the septa because they are thought to cause the skin surface irregularities that characterize cellulite.10,11 Radiological studies have demonstrated that thickened fibrous septa connect the dermis to the underlying superficial fascia with superficial fat located in the space between.7,11,12 The septa between the superficial fascia and the dermal undersurface are not simplistic, 2-dimensional structures similar to a single pillar or string; rather, they are 3-dimensional structures with densely grouped, thickened fibrous lattices and structural configurations similar to the hexagonal cells in a honeycomb.13-15 With changes in the fat lobules, the fibrous septa restrain portions of the dermis to the superficial fascia, resulting in depressions and dimpled appearance of the skin.
To further explore positive results from earlier safety and feasibility studies, including an open-label multicenter study, a single-arm, multicenter, open-label pivotal study, CONtrolled Focal Fibrous Band Release Method (CONFFIRM) [NCT04743635], was initiated to assess a novel, minimally invasive device (now known as Avéli; Revelle Aesthetics, Inc., Mountain View, CA) for targeted verifiable subcision (TVS).16,17 The objective of this study was to evaluate the efficacy and safety of the device in a single procedure for the reduction of cellulite in the buttock and thigh areas of adult females. Primary and secondary endpoint data from study initiation through postprocedure follow-up at 90 days (3 months) for all participants was analyzed and is reported herein.
METHODS
Study Participants and Enrollment
Female participants (n = 74) between 26 and 54 years with moderate to severe cellulite, a BMI < 30.0, and absent, mild, or moderate skin laxity were enrolled in the study. No male participants were enrolled in the study. Key exclusion criteria included participants who had undergone a cellulite procedure on the buttocks or thighs within the last 12 months, previous liposuction on the buttocks and/or thighs, >10% increase or decrease in body weight within the last 6 months, history of weight loss >60 kg, severe skin laxity, known clotting defects or bleeding disorders, and nicotine use within 6 months. Participants did not pay to participate in this research study and received reasonable compensation for their time to complete follow-up activities.
There were a total of 9 study sites in the United States and Australia, including 6 plastic surgeons and 3 dermatologists. The study was enrolled between January and February 2021.
Ethics and Compliance
The protocol utilized in this study and related materials were approved by an IRB/ethics committee (WCG IRB in the US [Puyallup, WA] and Bellberry in Australia [Eastwood, NSW]). The CONFFIRM study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki. Each participant provided written informed consent prior to the procedure and were advised they could voluntarily withdraw from the study at any time. The trial was carried out in accordance with the International Conference on Harmonisation Good Clinical Practice, ISO 14155, the US Code of Federal Regulations (CFR) applicable to clinical studies (45 CFR Part 46, 21 CFR Parts 11, 50, 54, 56), and all applicable local laws, regulations, and guidelines.
Efficacy Assessment and Study Endpoints
The validated Cellulite Severity Scale (CSS) was developed by Hexsel et al and is based on 5 key morphologic aspects of cellulite.7 For the purposes of this study based on FDA precedent, only the first 2 components were utilized: number of depressions (Part A) and depth of depressions (Part B). The primary efficacy endpoint for the study was considered reached with a mean ≥1 point reduction in the total CSS score (Part A + Part B—1) at day 90 (Table 1). Efficacy was determined by digital participant images evaluated by 3 independent, blinded physicians. The independent physician evaluators were provided with baseline and Day 90 images, which were graded utilizing the CSS scoring system. The primary safety endpoint was evaluated based on device-related serious adverse events (SAEs) at 30 days.
Table 1.
CSS Scoring
| Part A: No. of evident depressions | |
|---|---|
| 0 | None |
| 1 | Mild (≤4 depressions) |
| 2 | Moderate (5 to 9 depressions) |
| 3 | Severe (≥10 depressions) |
| Part B: Average depth of depressions | |
| 0 | None |
| 1 | Mild (1-2 mm) |
| 2 | Moderate (3-4 mm) |
| 3 | Severe (≥5 mm) |
| Total CSS score | = (Part A + Part B) – 1 |
CSS, Cellulite Severity Scale.
A secondary efficacy endpoint was considered reached if >60% of participants improved according to the Global Aesthetic Improvement Scale (GAIS) (Table 2). Although the GAIS was not originally designed specifically to assess cellulite, it was adapted for utilization in clinical trials evaluating changes in cellulite severity.18-20 A participant is considered improved if the GAIS assessment is improved (1), much improved (2), or very much improved (3). The blinded, independent physician evaluators rated the overall improvement employing the baseline and 90-day follow-up digital images. In addition, participant-reported outcomes, such as procedural pain severity (utilizing a Visual Analog Scale [VAS] score of 0-10) at 15 minutes after anesthesia administration, 5 minutes after device insertion, and immediately after the procedure, were also assessed.
Table 2.
Global Aesthetic Improvement Scale
| Rating | Assessment | Description |
|---|---|---|
| −3 | Very much worse | Treated area appearance obviously worse than before procedure |
| −2 | Much worse | Treated area appearance markedly (or significantly) worse than before procedure |
| −1 | Worsened | Treated area appearance slightly worse than before procedure |
| 0 | Unaltered (no change) | Treated area appearance essentially the same |
| +1 | Improved | Noticeable improvement in appearance of treated areas but subtle in magnitude |
| +2 | Much improved | Marked or significant improvement in appearance in treated areas |
| +3 | Very much improved | Optimal cosmetic result in treated areas for this participant; no additional treatment indicated |
Study Photography and Image Evaluation
The primary and secondary study efficacy endpoints were reliant on quality and consistent imaging. The images were taken under strict, standardized environments with only qualified photographers trained to the same methods. A chief photographer was identified to develop and conduct training for all study site photographers. A training manual outlined the standardized set-up, including equipment, lighting, participant angles, and camera positioning.
At study photography visits, each participant was photographed per the study photography manual to generate a minimum of 3 images from a minimum of 3 different angles. The efficacy outcomes were specific to the treated regions only, and these regions were disclosed to the evaluators.
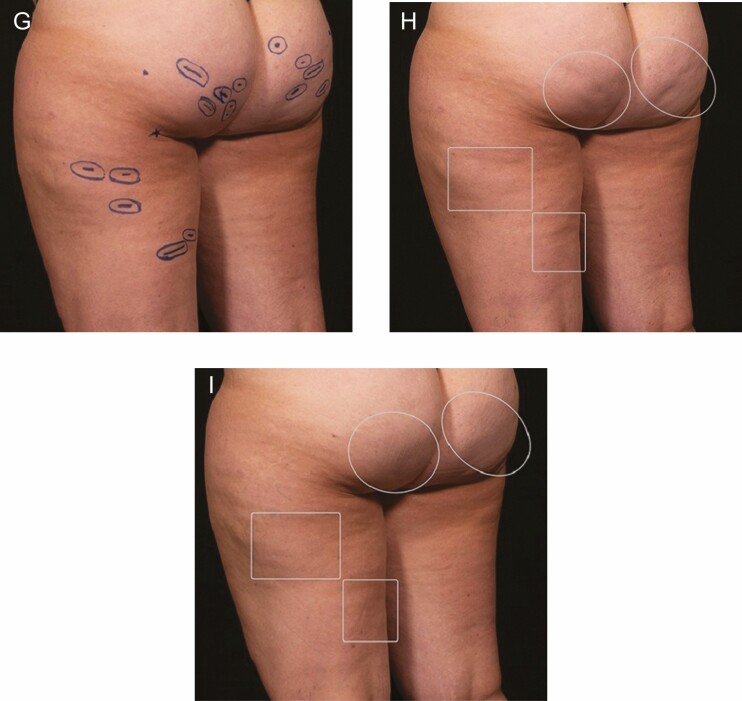
For the CSS assessment, independent evaluators were blinded, and the before and after images were assigned a unique identifier and randomized by ABio Clinical Research Partners, LLC (Midlothian, VA), an independent, third party so that the evaluators did not know which images were before or after the procedure. Once the CSS image evaluations were completed, the correct baseline and follow-up images for each participant were provided for the GAIS unblinded assessment. The scheme for outlining the images for blinded CSS evaluations is shown in Figure 1.
Figure 1.
Sample participant photos of this 34-year-old female patient with BMI = 29: (A, D, G) With patient in a relaxed standing position, the planned treatment sites on the thighs and buttocks were marked. (B, E, H) In these baseline photos, outlines were applied around a group of treated depressions for the blinded reviewer evaluations; and (C, F, I) shown 3 months after treatment.
Procedure
Assessment and marking of depressions were performed by the investigator with a marker while the participant stood in a relaxed position (Figure 1). The procedure was conducted using a minimally invasive device for TVS. The novel procedure was performed by a single physician investigator with local anesthesia. Most procedures utilized a limited dilute lidocaine technique with a lidocaine concentration of 0.3% and average volumes of 350 mL, administered with an auto-refilling syringe and a 20G spinal needle as described by Green and Layt.21 Anesthesia was delivered thoroughly from the skin entry site, generally in the gluteal crease, to and below the marked cellulite depressions. Anesthetic was delivered at least 35 mm past the marked targets to prevent discomfort when positioning the device at the marked cellulite depressions. The limited dilute lidocaine anesthesia technique facilitates a time-efficient procedure and minimizes leakage of excess fluid during recovery.
The device consisted of a handle that housed the slider, home button, and active button that were utilized to deploy a hook (Figure 2). The device was advanced through a small skin entry site (approximately 3.5 mm) to the procedure location. The distal end of the device contained an integrated light source that provided illumination and allowed the user to track and advance to the procedure location and aided in estimating depth (Figure 3). The investigators kept the device close to the dermis in the superficial plane with the light visible.
Figure 2.
Schematic of minimally invasive device for targeted verifiable subcision.
Figure 3.
(A) Smaller, brighter spot, indicating device is in the superficial plane. (B) Dimmer, larger spot, indicating the device is in a deeper plane.
The focal release of septa in a step-wise procedure is illustrated in Figure 4. The hook is integral to the performance of the focal release. With the light positioned under the marked procedure location, the hook was deployed. Septa responsible for the cellulite depression were identified by engaging the septa with the hook and visualizing a recreated depression on the skin surface. If pulling the septa recreated the appearance of a depression in the marked target area, the device was employed to release the septa. Release of the contributing septa was verified by passing through the area again with the hook deployed. The steps were repeated until the depression could no longer be recreated on the skin surface. If passing through the area again did not recreate a depression in the target area, the septa were confirmed as released. The length of the device permits treatment of multiple cellulite depressions on the buttock and thigh from a single skin insertion site. The procedure was repeated until all marked depression areas were treated. Following the procedure, the entry site(s) was bandaged.
Figure 4.
(A) The light enabled correct positioning of the distal end of the device. (B) After advancing to the target septa, (C) pulling with the deployed blunt hook confirmed its correct location. (D) The septa were then released by deploying and pulling with the sharpened link. Stevens WG, Kaminer MS, Fabi SG, Fan L. Study of a New Controlled Focal Septa Release Cellulite Reduction Method. Reproduced from Stevens et al. by permission of Oxford University Press on behalf of The Aesthetic Society.17
Postprocedure Follow-Up
Participants were evaluated by phone 24 hours following the procedure. Assessments also occurred 7, 30, 90 days, 180 days, and 365 days postprocedure. During each follow-up visit, safety assessments were conducted to assess for any adverse events (AEs). Patient-reported pain, discomfort, and soreness were also evaluated through Day 90, and participants were asked to report, when they were able to return to procedural activity levels via an online and blinded questionnaire to minimize potential bias. All AEs were assessed for severity employing the Common Terminology Criteria for Adverse Events.22
Statistical Analysis
The primary effectiveness endpoint was the change in CSS score at 3 months from baseline. The CSS (Table 1) was determined by 3 independent, blinded physician evaluators of participant images obtained before and 3 months after treatment. Success was achieved when the mean change across all treated participants was at least 1 point. For the primary endpoint, the reduction in CSS was evaluated on the modified intent-to-treat (mITT) group (excludes 6 roll-in participants, 2 per each investigator who had not performed a TVS procedure in the earlier safety and feasibility studies). The 2-sided 95% confidence interval for the mean reduction in CSS was calculated along with the corresponding P value (Student t test).
Descriptive statistics were employed to summarize the secondary endpoints, including mean, standard deviation, median, range for continuous data, and frequency and percentage for category values. The secondary endpoints were split into 2 categories, either inferential or simply descriptive. The inferential secondary endpoints were hypothesis tested employing the hierarchical approach of gatekeeping to ensure the overall alpha for the trial was maintained. Descriptive secondary endpoints did not have any hypothesis testing performed.
RESULTS
The demographics and baseline characteristics of enrolled participants are included in Table 3. The enrolled participants had a mean BMI of 24.8 ± 2.7 and age of 41.4 ± 7.4 years. The majority of participants (67/68, 98.5%) had either mild or moderate skin laxity, as characterized by the treating physician. The mITT group consisted of 68 participants who underwent the procedure. The 6 roll-in participants were not included in the mITT data. However, all participants who received the procedure, including roll-ins, underwent evaluations at the Day 90 visit.
Table 3.
Demographics and Baseline Characteristics
| Parameter | Summary, n = 68a |
|---|---|
| Mean age (SD), range, y | 41.4 (7.4), 26 to 54 |
| Mean (SD) BMI, range, kg/m2 | 24.8 (2.7), 19 to 29.8 |
| Fitzpatrick skin type,b no. (%) | |
| I | 1 (1.5%) |
| II | 19 (27.9%) |
| III | 24 (35.3%) |
| IV | 15 (22.1%) |
| V | 6 (8.8%) |
| VI | 3 (4.4%) |
| Skin laxityb | |
| None | 1 (1.5%) |
| Mild | 37 (54.4%) |
| Moderate | 30 (44.1%) |
SD, standard deviation; y, years.
aThe roll-in participants are not included in the modified intent-to-treat participant data.
bAssessed by treating physician.
Skin entry sites were planned during the marking phase and placed strategically to reach all targeted depressions. The primary entry sites were well concealed within the gluteal crease, with a mean of 2.9 access sites in each participant.
Most participants were treated in both the buttocks and the thigh (88.2%, 60/68). The other 8 (11.8%) participants were treated in the buttocks only. The mean number of depressions treated was 20 (range, 6-45) (Table 4), which is double the most severe CSS-A category (≥10 depressions). In this study, 94.1% of the participants fit the most severe CSS-A category at baseline.
Table 4.
Range of Depressions Treated
| Range of depressions | No. (%) | Cellulite Severity Score, depressions |
|---|---|---|
| <5 | 0.0 | |
| 5-9 | 4 (5.8%) | Moderate 4 (5.8%) |
| 10-15 | 18 (26.5%) | Severe 64 (94.1%) |
| 16-20 | 18 (26.5%) | |
| 21-25 | 13 (19.1%) | |
| 26-30 | 6 (8.8%) | |
| 31-35 | 7 (10.3%) | |
| 35-45 | 2 (2.9%) |
The primary efficacy endpoint was surpassed with a mean improvement in CSS of 1.50 ± 0.9 (P < 0.0001). The secondary GAIS endpoint was met with an overall improvement rate of 95.6% (P < 0.0001). Most participants (80.9%) were very much improved (26.5%) or much improved (54.4%). Improvement from baseline to 3 months was demonstrated in sample participant images in Figures 1, 5-7.
Figure 5.
Sample participant photos of this 39-year-old female patient with BMI = 19.9: (A, C, E) before treatment and (B, D, F) 3 months after treatment.
Figure 7.
Sample participant photos of this 51-year-old patient with BMI = 20.7: (A, C, E) before treatment and (B, D, F) 3 months after treatment.
Figure 6.
Sample participant photos of this 37-year-old female patient with BMI = 23.7: (A, C, E) before treatment and (B, D, F) 3 months after treatment.
The participant-reported outcomes demonstrated that all participants found the procedure tolerable. The mean VAS pain scores (0, no pain to 10, unbearable pain) during the procedure were 1.1. The mean pain was 3.8 within 24 hours of the procedure and improved to 1.7 by Day 7. Oral analgesics were taken postprocedure by 53% of participants for an average of 4.8 days. The recovery period after the study procedure overall was short; most participants (75%) returned to normal activities within 1 day.
The primary safety endpoint was also met with an absence of device-related SAEs at 30 days. All AEs were reported and monitored by investigators beginning at the procedure through all follow-up visits. Importantly, the definition of AEs in CONFFIRM is defined as any undesirable medical occurrence providing a comprehensive, real-world safety profile.
A total of 210 related AEs were reported in the 68 mITT participants through the Day 90 follow-up visit. All the reported related AEs were mild (Grade 1, 90.5%) or moderate (Grade 2, 9.5%) on the 5-grade Common Terminology Criteria for Adverse Events scale. The reported types and incidence of related AEs are typical and expected for this type of procedure. The related AEs experienced by >3% of participants are presented in Table 5. The most common AEs were ecchymosis, occurring in 59 participants (86.8%, mean duration 34.4 days), followed by tenderness (35, 51.5%, mean duration 19.9 days), pain (26, 38.2%, mean duration 17.4 days), and induration (24, 35.3%, mean duration 63.6 days).
Table 5.
Adverse Events Experienced by >3% of Participants
| Adverse event | No. (%) |
|---|---|
| Ecchymosis | 59 (86.8) |
| Tenderness | 35 (51.5) |
| Pain | 26 (38.2) |
| Induration | 24 (35.3) |
| Numbness | 11 (16.2) |
| Incision-site bleeding | 11 (16.2) |
| Edema | 8 (11.8) |
| Fluid discharge | 6 (8.8) |
| Hemosiderin stain | 5 (7.4) |
| Swelling | 4 (5.8) |
| Burning sensation | 3 (4.4) |
| Tingling | 3 (4.4) |
The majority of related AEs did not require any action (140/210, 66.7%). Most AEs requiring intervention (33 uses in 31 participants) involved over-the-counter pain medication (mean duration 4.6 days) or at-home recovery management, including compression garments (participant preference) massage, or icing. For example, the ecchymosis AEs were mild, with all but 1 evaluated as grade 1, and were treated with simple measures such as ice and compression. Induration was not bothersome to participants, not visible, and only felt on palpating the area. Some participants (20.8%, 5/24) were advised to massage the area. Only 2 AEs (1%, 2/210) required minor intervention.
DISCUSSION
This study demonstrated that utilization of a minimally invasive device in a single TVS procedure under local anesthesia was highly effective in reducing cellulite on the buttocks and thighs of adult women having an average of 20 depressions (range, 6-45). With strong safety results, all the reported related AEs were mild (90.5%) or moderate (9.5%) and expected for this type of procedure. All participants found the procedure tolerable. The mean VAS pain scores during the procedure were very low at 1.1. The recovery period after the study procedure overall was short; most participants (75%) returned to normal activities within 1 day. Taken together, these results indicate that the procedure is safe and highly effective for treating the appearance of cellulite.
A pilot study of the device by Stevens et al also demonstrated the safety, efficacy, and feasibility of the procedure in 20 female participants out to 180 days.17 One of the key discoveries in the pilot study was that septa arrangement and structure vary across cellulite depressions and participants.17 Previously, septa were conceptualized as a single fibrous strand that would result in a single cellulite depression. However, the 3-dimensional septa structure consists of a complex framework of webbing, walls, and branching structures that extend beyond the center of the depression.13,14 Because these septa complexes vary in location and orientation relative to the center of a cellulite depression, the device for TVS is designed to test and discover the extent of the septa complex responsible for a particular cellulite depression beyond the center of the depression. This publication expands on 2 pilot studies by demonstrating a statistically significant improvement in reducing the appearance of cellulite in a powered multi-center pivotal study.16,17
Current treatments for cellulite include injectable biologic treatments, dermal fillers, topical agents, massage, laser and light, acoustic and vacuum assisted subcision, and radiofrequency devices.23,24 Although some of these treatments may require minimal downtime or no local anesthesia, most require multiple treatments to achieve some level of efficacy.11 Additionally, nonsubcision treatments do not directly impact the fibrous septa, which are believed to be the primary cause of cellulite.25 Subcision techniques, such as by needle or by acoustic or vacuum-assisted and injectable biologic treatments, are directed at the fibrous septa but have a nonverifiable approach and do not recreate skin depressions while the patient is prone. The device for TVS is unique in that it provides tactile and visual feedback that allows for identification of the culprit septa.17 Movement of the handle after engaging the septa creates resistance as the septa is put under tension. The tensioned septa complex recreates a depression that can then be visualized on the skin. If the recreated depression is inside the marked target, the user exposes the blade to release the septa. When the septa complex is released, the user will feel the resistance subside and the recreated depression will no longer be visible on the skin. The device allows for disengagement of septa that recreate depressions outside of the marked target, enabling focal treatment of the culprit septa and leaving the general supporting structure of the region intact.
The light source on the device uniquely allows the user to track the position of the device and determine the depth between the dermis and superficial fascia. Maintaining a superficial depth allows the user to be confident that they have identified all septa, even with their complex 3-dimensional structure, which contributes to the tethering of the dermis creating the cellulite depression.
Because there is more than 1 fibrous strand per cellulite depression, it is very important to treat each depression in a methodical manner employing the identify, release, and verify process (see Figure 4).17 Ensuring all culprit septa have been released is a critical element of a positive outcome. Full release can be verified by advancing to the same location, deploying the hook, and pulling to identify any remaining septa. If any depression can be recreated in the marked target during the verification pass, the septa should be released. If the device passes freely through the area, the user can be confident all contributing septa have been released. A procedure video for 1 participant demonstrates this technique (Video).
Limitations of this study include the open-label study design and the lack of a comparator group. The study population included participants with normal and overweight BMI ranges with an age range of 26 to 54 years; therefore, the results may not apply to older patients or those with obesity. Future studies that include additional patient-reported outcomes will be considered. Nine sites in the United States and Australia enrolled participants, which may not be generalizable to all patients and clinicians. The TVS procedure may be operator dependent. Future publications will include the extended follow-up results with evaluations of the duration of response.
CONCLUSIONS
The primary and secondary results of this CONFFIRM study through 90 days demonstrate that the minimally invasive device for TVS is safe and effective in reducing the appearance of moderate to severe cellulite on the buttocks and thighs of adult women under local anesthesia in a single procedure with a 1.5-point mean reduction in CSS, no device-related SAEs, and 95.6% improvement on the GAIS.
Acknowledgments
The authors acknowledge the editorial assistance of Shireen Dunwoody of Dunwoody Consulting. This study was sponsored by Revelle Aesthetics, Inc. (Mountain View, CA).
Contributor Information
W Grant Stevens, University of Southern California, Keck School of Medicine, Marina del Rey, CA, USA.
Mark R Magnusson, Griffith University, School of Medicine, Southport, Queensland, Australia and is an international editor for Aesthetic Surgery Journal.
Disclosures
The authors are consultants to and/or shareholders of the study sponsor, Revelle Aesthetics, Inc. (Mountain View, CA). The authors were principal investigators in the study and participated in the research, data collection, and writing of the manuscript.
Funding
The study is sponsored by Revelle Aesthetics, Inc. (Mountain View, CA).
REFERENCES
- 1. Christman M, Belkin D, Oula ML, Geronemous R, Brauer J. An anatomical approach to evaluating and treating cellulite. J Drugs Dermatol. 2017;16:58–61. [PubMed] [Google Scholar]
- 2. Emanuele E. Cellulite: advances in treatment: facts and controversies. Clin Dermatol. 2013;31:725–730. doi: 10.1016/j.clindermatol.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 3. Rudolph C, Hladik C, Hamade H, Frank K, et al. Structural gender dimorphism and the biomechanics of the gluteal subcutaneous tissue—implications for the pathophysiology of cellulite. Plast Reconstr Surg. 2019;143:1077–1086. doi: 10.1097/PRS.0000000000005407 [DOI] [PubMed] [Google Scholar]
- 4. Schweritz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230–234. doi: 10.1111/j.1524-4725.1978.tb00417.x [DOI] [PubMed] [Google Scholar]
- 5. Friedman DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cometic Invest Dermatol. 2017;10:17–23. doi: 10.2147/CCID.S95830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: part I. Pathophysiology. J Am Acad Dermatol. 2010;62:361–370; quiz 371. doi: 10.1016/j.jaad.2009.10.042 [DOI] [PubMed] [Google Scholar]
- 7. Hexsel DM, Dal’Forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23:523–528. doi: 10.1111/j.1468-3083.2009.03101.x [DOI] [PubMed] [Google Scholar]
- 8. Mirrashed F, Sharp JC, Krause V, Morgan J, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161–168. doi: 10.1111/j.1600-0846.2004.00072.x [DOI] [PubMed] [Google Scholar]
- 9. Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118–124. doi: 10.1034/j.1600-0846.2002.00331.x [DOI] [PubMed] [Google Scholar]
- 10. Young VL, DiBernardo BE. Comparison of cellulite severity scales and imaging methods. Aesthetic Surg J. 2021;41(6):521–537. doi: 10.1093/asj/sjaa226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bass LS, Kaminer MS. Insights into the pathophysiology of cellulite: a review. Dermatol Surg. 2020;46:S77–S85. doi: 10.1097/DSS.0000000000002388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amore R, Amuso D, Leonardi V, et al. Treatment of dimpling from cellulite. Plast Reconstr Surg Glob Open. 2018;6:e1771. doi: 10.1097/GOX.0000000000001771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song AY, Askari M, Azemi E, Alber S, et al. Biomechanical properties of the superficial fascial system. Aesthetic Surg J. 2006;26:395–403. doi: 10.1016/j.asj.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 14. Lozano JNJ, Vacas-Jacques P, Anderson R, Franco W. Effect of fibrous septa in radiofrequency heating of cutaneous and subcutaneous tissues: computational study. Lasers Surg Med. 2013;45:326–338. doi: 10.1002/lsm.22146 [DOI] [PubMed] [Google Scholar]
- 15. Rudolph C, Hladik C, Hamade H, et al. Structural gender dimorphism and the biomechanics of the gluteal subcutaneous tissue: implications for pathophysciology of cellulite. Plast Reconstr Surg. 2019;143:1077–1086. doi: 10.1097/PRS.0000000000005407 [DOI] [PubMed] [Google Scholar]
- 16. Layt C. A safety and feasibility study of a novel controlled focal fibrous septa release method for improving the appearance of moderate and severe cellulite. Plast Reconstr Surg Global Open. 2022;10(4):e4237. doi: 10.1097/GOX.0000000000004237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevens WG, Kaminer MS, Fabi SG, Fan L. Study of a new controlled focal septa release cellulite reduction method. Aesthet Surg J. 2022;42(8):937–945. doi: 10.1093/asj/sjac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narins RS, Brandt F, Leyden J, Lorenc ZP, et al. A randomized, double blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. doi: 10.1046/j.1524-4725.2003.29150.x [DOI] [PubMed] [Google Scholar]
- 19. Kaminer MS, Coleman WP, Weiss RA, et al. Multicenter pivotal study of vacuum-assisted precise tissue release for the treatment of cellulite. Dermatol Surg. 2015;41(3):336–347. doi: 10.1097/DSS.0000000000000280 [DOI] [PubMed] [Google Scholar]
- 20. Petti C, Stoneburner J, McLaughlin L. Laser cellulite treatment and laser-assisted lipoplasty of the thighs and buttocks: combined modalities for single stage contouring of the lower body. Lasers Surg Med. 2016;48:14–22. doi: 10.1002/lsm.22437 [DOI] [PubMed] [Google Scholar]
- 21. Green JB, Layt C. Limited dilute lidocaine anesthesia: a useful technique with many practical applications. J Cosmet Dermatol. 2022;21(4):1445–1447. doi: 10.1111/jocd.14825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Department of Health and Human Services. NCI, Common Terminology Criteria for Adverse Events (CTCAE), in Version 5.0. Published Nov 27, 2017. Accessed July 13, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 23. Sadick N. Treatment for cellulite. Int J Womens Dermatol. 2019;5:68–72. doi: 10.1016/j.ijwd.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Resonic Rapid Acoustic Pulse Device. K212502 510(k) Summary. Soliton, Inc. Accessed July 13, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf21/K210964.pdf
- 25. Kaminer MS. New approaches in managing cellulite: EXPERT INSIGHTS. Cutis. 2021;107(5 Suppl 1):2–7. doi: 10.12788/cutis.0208 [DOI] [PubMed] [Google Scholar]