Abstract
Transient receptor potential (TRP) channels are a widely expressed family of 28 evolutionarily conserved cationic ion channels that operate as primary detectors of chemical and physical stimuli and secondary effectors of metabotropic and ionotropic receptors. In vertebrates, the channels are grouped into six related families: TRPC, TRPV, TRPM, TRPA, TRPML, and TRPP. As sensory transducers, TRP channels are ubiquitously expressed across the body and the CNS, mediating critical functions in mechanosensation, nociception, chemosensing, thermosensing, and phototransduction. This article surveys current knowledge about the expression and function of the TRP family in vertebrate retinas, which, while dedicated to transduction and transmission of visual information, are highly susceptible to non-visual stimuli. Every retinal cell expresses multiple TRP subunits, with recent evidence establishing their critical roles in paradigmatic aspects of vertebrate vision that include TRPM1-dependent transduction of ON bipolar signaling, TRPC6/7-mediated ganglion cell phototransduction, TRP/TRPL phototransduction in Drosophila and TRPV4-dependent osmoregulation, mechanotransduction, and regulation of inner and outer blood-retina barriers. TRP channels tune light-dependent and independent functions of retinal circuits by modulating the intracellular concentration of the 2nd messenger calcium, with emerging evidence implicating specific subunits in the pathogenesis of debilitating diseases such as glaucoma, ocular trauma, diabetic retinopathy, and ischemia. Elucidation of TRP channel involvement in retinal biology will yield rewards in terms of fundamental understanding of vertebrate vision and therapeutic targeting to treat diseases caused by channel dysfunction or over-activation.
1. Introduction
The primary function of the vertebrate retina is to intercept photons, support the percolation of electro-chemical signals across specialized circuits composed of neurons and glia, and distribute retinal output to visual centers in the midbrain via transmission of action potentials in the optic nerve. The types of retinal cells, their organization, and communication with endothelial and pigment epithelial cells (RPE) that constitute the outer and inner blood-retina barriers are conserved from cyclostomes to mammals. At first approximation, the retina appears to be utterly devoted to processing light-evoked signaling downstream from photoreceptors (rods, cones and ipRGCs), Still, it is becoming increasingly evident that light-independent signals (mechanical stimuli, temperature, ionic composition, and the inflammatory milieu) profoundly influence retinal development, function, and pathology (Grüsser et al., 1989; Križaj et al., 2014; Križaj, 2019). The biomechanical milieu alone consists of an assortment of signals - mechanical loading from intraocular pressure (IOP), blinking, sneezing, eye rubbing, activity-dependent changes in ion gradients and shear forces within blood vessels – that may interact with light-evoked signals at every stage of retinal processing, but current literature leaves many gaps concerning our understanding of molecular mechanisms through which mechanical, ionic and inflammatory stimuli contribute to vision loss in diseases such as glaucoma, diabetic retinopathy, ischemia, and retinal detachment. This review shows that many types of retinal dysfunction involve pathological over- or underactivation of evolutionarily conserved sensory transducers within the light-independent signaling circuits. Functioning predominantly as conduits for the second messenger calcium, these ion channels – belonging to the transient receptor potential (TRP) channel family - mediate the cells’ responsiveness to touch, temperature, osmolarity, taste, pheromones, acidity, pain, inflammation, oxidation, metabolic energy, and polyunsaturated fatty acids across the body. Transcriptomic analyses show that every retinal cell type expresses multiple TRP subunits, some of which may interact (Gilliam and Wensel, 2011; Choi et al., 2015; Jo et al., 2022).
The vertebrate TRP superfamily has been initially identified by homology with the TRP channel gene encoding a light-activated channel in Drosophila photoreceptors (Cosens and Manning, 1969; Wu et al., 2010; Montell et al., 2002). Gene sequencing revealed the family to be ancient, with many individual members traced to the Cnidaria-Bilateria junction more than half a billion years ago (Peng et al., 2015) (Fig. 1). All TRP channels have in common six transmembrane domains with a pore-forming loop in a hydrophobic stretch between transmembrane segment 5 and 6 (S5 and S6) and motifs that are conserved across some isoforms (e.g., ankyrin (ANK) repeats, proline-rich domains (PRD); coiled-coil domains; and the TRP-box) (Clapham, 2003). In humans, the superfamily encompasses six families with 27 members: Canonical (TRPC1-7), Vanilloid (TRPV1-6), Melastatin (TRPM1-8), Ankyrin (TRPA1), polycystin (TRPP1-3) and Mucolipin (TRPML1-3). The canonical family was identified first, with the founding member TRPC1 as the homolog of TRP. The remaining subfamilies are named following the original designation of the first family member. Thus, the vanilloid family tracks TRPV1, identified initially through expression cloning for a channel activated by vanilloid compounds such as capsaicin, and the melastatin family tracks TRPM1, a putative tumor suppression protein (Montell et al., 2002). While the majority of TRP proteins function as non-selective cation channels with isoform-specific calcium vs. sodium permeability (PCa/PNa ~10), TRPV5, TRPV6, and TRPM3α2 function as Ca2+–selective channels and TRPM4, TRPM5 and TRPM3α1 function as monovalent cation (mainly Na+) channels. The C–terminal domains of TRPC and TRPV channels have binding sites for Ca2+ and Ca2+–regulated proteins and are therefore susceptible to modulation by changes in Ca2+ induced by other cell receptors and channels. The cytoplasmic domains of TRP channels recruit large complexes of proteins, lipids, and small molecules into macromolecular complexes. Nearly all TRP families have potential mechanosensory members, with most isoforms responding to mechanical stimuli in a context– and cell-type-dependent manner.
Fig. 1. Phylogenetic tree of the mammalian TRP family.

(A) TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin), TRPP (polycystin) (Gees et al., 2014). (B) Subunit-specific organization of amino acid domains. TRPC, TRPV and TRPA possess N-terminal ankyrin repeat domains. TRPC, TRPM and TRPV contain the “TRP box” believed to modulate gating. TRPP and TRPML have endoplasmic reticulum (ER) retention domains. CIRB, calmodulin/inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) receptor binding domain; NUDIX, nucleoside diphosphate-linked moiety X; PDZ, acronym for postsynaptic density protein 95 (PSD95), Drosophila disc large tumour suppressor (DLGA) and zonula occludens protein 1 (ZO1). from Moran et al. (2011), and Gees et al. (2014).
A primary functional role for TRP channels is as a conduit for Ca2+, a key 2nd messenger that regulates many cellular signaling mechanisms. Due to their voltage independence, TRP channels subserve Ca2+ influx and control the membrane potential in resting excitable and non-excitable cells. Background Ca2+ entry mediated by TRP channels modulates voltage-operated signaling, neurite outgrowth, hormone secretion, and contraction in neurons and myocytes, whereas ‘non-excitable cells’ utilize TRP channels as a major source of calcium-based excitation that regulates gliotransmitter release, vascular tone, cell volume, Mg2+ reabsorption, contractility, fibrosis, and many other functions. TRP isoforms may function in receptor-operated, store-operated, and/or stretch-operated modes and adopt multiple activation mechanisms via association with G protein-coupled receptors (GPCRs), growth factor receptors (e.g., ET-1), protease-activated receptors, the STIM1 complex and the cytoskeleton (Moran et al., 2011; Nilius and Szallasi, 2014). Some TRP channels (TRPV1, TRPM8, TRPP, TRPML) operate in the plasma membrane and as Ca2+ release channels from intracellular organelles (lysosomes, ER, Golgi apparatus, and mitochondria) (Gees et al., 2014). While detailed analyses of TRP signaling in the retina are relatively sparse, investigations in heart, lung, brain, vasculature, immune system, bone, and gut used global KOs, floxed mice, and selective shRNA/pharmacological modulators to document a wide range of sensory functions that include mechanosensation, thermosensation, phototransduction, chemosensation and nociception (Clapham, 2003; Nilius and Szallasi, 2014; White et al., 2016; Križaj, 2020). In recognition of the fundamental importance of TRP signaling, the Nobel Prize Committee awarded the 2021 Medicine Prize to University of California San Francisco’s David Julius for the pioneering work on heat-sensitive “capsaicin receptors” (TRPV1) TRPV2 and TRPM8 channels.
Mammalian retinas express most if not all TRP isoforms. Northern blots in the paper by Gilliam and Wensel (2011), the first to conduct a systematic analysis of retinal TRP expression, showed the highest signals for TRPM1, TRPM3, TRPV4, TRPP2 > TRPC2, TRPC6, TRPM2, TRPM4, TRPM7, TRPML1, TRPV1, TRPV2, and relatively weak expression of TRPM6, TRPV5, TRPV6, and TRPA1. Retinal neurons, glia, epithelial and endothelial cells express cell type-specific subunits (e.g., TRPM1 in bipolar neurons), whereas other subunits (notably, TRPC channels) show broad expression across all layers (Gilliam and Wensel, 2011; Molnar et al., 2012). Mutation studies revealed that TRP signaling plays fundamental and irreplaceable functions in vertebrate vision: TRPM1 mutations cause congenital stationary night blindness type 2 in mice, horses, and humans (Koike et al., 2010a); TRPML mutations, photoreceptor degeneration (Sun et al., 2000); TRPV4 mutations, retinal degeneration (Thibodeau et al., 2017) while TRPM3 mutations were associated with early-onset cataracts and primary open angle glaucoma (Bennett et al., 2014). In general, however, our understanding of retinal TRP signaling lags far behind the body of work on other organs. Despite the ubiquitous expression, we do not know the identity of physiological inputs, modulatory mechanisms, and roles in pathology for the large majority of TRP subunits, and the pool of existing data includes studies with conflicting results. The absence of information about TRP channel signaling in vertebrate retinas contrasts sharply with the in-depth characterization of dTRP channel expression and function in Drosophila phototransduction. It is clear that TRP channels equip retinal cells – neurons and non-excitable cells alike - with the capacity to integrate light-activated signals with many types of mechanical stimulus (strain, shear, compression, swelling), endogenous and extracellular chemical agents, toxic compounds and/or temperature. Because many if not most cell types in the vertebrate retina express multiple TRP isoforms, polysensory integration of light-induced and light-independent signals is likely to take place at every stage of retinal processing.
2. TRP signaling in Müller glia
Müller cells constitute ~90% of the retinal glial population with crucial functions in trafficking, release/recycling of neurotransmitters, synaptogenesis, water transport, immune signaling, retinal (lactate, glucose, and glutamine) metabolism, vascular function, and K+ siphoning (Bringmann et al., 2006; MacDonald et al., 2015; Vecino et al., 2016; Musada et al., 2020). Their ovoid cell body emanates two processes towards the OLM and the ILM (Fig. 2A and B), with lateral offshoots that tile the OPL and IPL, ensheath retinal neurons, contact microglia and blood vessels, and fasciculate optic nerve head axons. The apical process connects to photoreceptor inner segments with adherens and tight junctions to form the outer limiting membrane (OLM), whereas endfeet contacts with endothelial cells and astrocytes contribute to the inner limiting membrane (ILM) and the inner blood-retina barrier (Wang et al., 2017; Reichenbach and Bringmann, 2010; Shen et al., 2012). Müller cells can therefore be visualized as nonoverlapping columnar units that control signaling, metabolism, and viability of all neurons while constituting a functional link between retinal, vitreal, and subretinal spaces. In addition to being ideally positioned to regulate mechanical, metabolic, and chemomodulatory signaling under homeostatic and pathological conditions, this radial glia are amongst the first retinal cells to respond to environmental, genetic, and signaling stressors. The reactive response of Müller glia is characterized by hypertrophy and release of gliotransmitters and cytokines (Reichenbach and Bringmann, 2010; Vecino et al., 2016), with cells in some species exhibiting a remarkable potential for proliferation, pluripotency, and neurogenic programming (Lahne et al., 2020).
Fig. 2. TRP and Müller cell function.

(A) Dissociated mouse Müller cell labeled with an anti-glutamine synthetase antibody. (B) Schematic representation of a generic Müller cell, with apical microvilli within the outer limiting membrane (OLM), lateral processes in the OPL and IPL, INL cell body and the endfoot in the RGCL, abutting the ILM. The inset expands the endfoot region, with strong expression of TRPV4 and TRPC1 in the proximity to ER cisternae containing inositol triphosphate receptors (IP3R) and ryanodine receptors (RyR). Coupling between TRPV4, TRPC1, Orai and store release (RyR, IPR3) channels underlies TRP-dependent Calcium-Induced Calcium Release and Store-operated Calcium Entry mechanisms that contribute to glial excitation. Coupling between TRPV4, inwardly rectifying potassium (Kir2.1) channels and bidirectional water transport across aquaporin (AQP4) channels mediates cell swelling and volume regulation. TRPC3, TRPC6 and TRPV1 channels are probably localized to Müller glial soma and processes, with expression in the endfoot remaining unclear. (C) Calcium imaging. Simultaneous recording from a mouse Müller cell and ganglion cell loaded with the calcium indicator dye Fura-2 AM. The TRPV4 agonist GSK1016790A (GSK101), but not glutamate (Glu), evokes a slow and sustained [Ca2+]i elevation in the glia whereas the RGC responds GSK101 with a rapid inactivating [Ca2+]i signal, and is also excited by glutamate. (D) Possible mechanism of TRPV4 activation in glia. Ca2+ influx via TRPV4 stimulates membrane phospholipase A2 (PLA2), which may also be sensitive to lipid distension. PLA2-dependent cleavage of membrane lipids generates arachidonic acid, metabolized by cytochrome P450 and epoxygenases into EETs (epoxyeicosatrienoic acids), the final common activators of the TRPV4 pore. (E) Müller endfoot is a SER-rich compartment (MC), in contact with astrocytes (AsC) and the capillary endothelium (Endo). The yellow marker denotes the basement membrane; green denotes an astrocyte process that abuts the endfoot. (F) Calcium imaging in a dissociated mouse Müller cell. The cell responds to OAG (1-oleoyl-2-acetyl-sn-glycerol), a membrane-permeable analog of 1,2-diacylglycerol (DAG), a messenger activator of TRPC3/6/7 channels, with a large [Ca2+]i increase. The bars in panels C and F denote the timing of drug application. From Ryskamp et al., 2014a); Molnar et al. (2016); P. Barabas, D.K. unpublished data (Created in Biorender.com).
2.1. TRP channels and calcium signaling in Müller glia
Müller cells in many species are not electrically excitable due to minimal expression of voltage-gated channels and Ca2+-permeable glutamate receptors but nonetheless exhibit excitability via spontaneous and activity-dependent activation of voltage-independent Ca2+-permeable channels and Ca2+ release from internal compartments (Keirstead and Miller, 1995; Huang et al., 2011; Grosche et al., 2016). Cytosolic Ca2+ signals often occur as waves that regulate the biology of adjacent neurons, endothelial cells, pericytes, microglia, and astrocytes via the release of gliotransmitters (glutamate, D-serine, and ATP/adenosine) and cytokines (TNFα, MCP-1, IL-1β) (Newman and Zahs, 1998; Metea and Newman, 2006a; Kurth-Nelson et al., 2009; Yarishkin et al., 2018a; Tworig et al., 2021). Ca2+ waves can be initiated by TRP channels, Orai channels, and purinergic P2X receptors and amplified by the substantial driving force from the hyperpolarized (~−90 to − 120 mV) membrane potential. Presently the most studied TRP subunit in Müller glia is TRPV4, with expression documented for mouse (Ryskamp et al., 2014a; Jo et al., 2015), rat (Sappington et al., 2015), pig (Taylor et al., 2017), non-human primate (Gao et al., 2019) and human (Ryskamp et al., 2011) preparations. TRPC1, TRPV4, and TRPC6 subunits have been linked to CICR and SOCE, and TRPC1 and TRPV4 were implicated in the propagation of Ca2+ waves, Ca2+-dependent release of gliotransmitters, cytokines, eicosanoids, as well as regulation of the blood-retina barrier (Phuong et al., 2016; Thébault, 2021). mRNA/antibody analyses additionally documented Müller cell expression of TRPA1, TRPV2, TRPV6, TRPM3 and TRPP1 subunits (Ryskamp et al., 2014b; Jo et al., 2015, 2022; Križaj, 2019; Souza Monteiro de Araújo et al., 2020) which remain to be investigated functionally. Immortalized cell lines such as QMMuC1 may differ from native cells with respect to TRP signaling during tonic and evoked activity and TRP channel coupling to downstream signaling pathways.
2.2. TRPV4 and TRPC1-dependent mechanosensing in Müller glia
Müller glia continuously experience tensile stretch, tugging, and changes in cell volume (Fortune, 2019). The cells also provide mechanical support to the retina (MacDonald et al., 2015) and are exquisitely sensitive to every type of mechanical stress. Experimental stretch is a powerful regulator of Müller cell gene expression (Wang et al., 2013) and retinal detachment, intraocular pressure, and indentation were shown to evoke inward currents and [Ca2+]i increases that were linked to gliotransmitter/cytokine release and reactive gliosis (Puro 1991; Woldemussie et al., 2004; Lindqvist et al., 2010; Agte et al., 2017; Matsumoto et al., 2018). Extracellular matrix (ECM) stretch evokes dose-dependent [Ca2+]i elevations that are partially suppressed by pharmacological inhibition of TRPV4 and TRPC1 channels and by ablation of Trpv4 and Trpc1 genes (Jo et al., 2022). TRPV4 agonists mimic, and antagonists inhibit, mechanically evoked calcium signals (Ryskamp et al., 2014a; Jo et al., 2022). TRPV4−/− Müller cells show reduced sensitivity to stretch, swelling, and detachment and reduced sensitivity to chronic ocular hypertension (Ryskamp et al., 2014a). Similar to mouse Müller cells, their guinea pig counterparts respond to mechanical poking with [Ca2+]i responses that are insensitive to the Piezo1 channel blocker GsMTx4 (Agte et al., 2017); they have not yet been tested for TRPV4. ~35% reduction in the amplitude of calcium responses induced by moderate (~6%) ECM strains was reported in TRPC1−/− Müller cells (Jo et al., 2022). Because stretch-induced signals in TRPV4−/− Müller glia are unaffected by TRPC1 antagonists, and responses in TRPC1−/− cells are suppressed by TRPV4 blockers (Jo et al., 2022), TRPV4 homotetramers may co-exist with TRPC1-TRPV4 heteromers (Greenberg et al., 2019; Cullimore et al., 2022). The precise sequence of mechanical-biophysical events that link membrane stress to Müller glial TRP activity remains to be elucidated, with possibilities including gating by lipid bilayer distension/curvature, integrin tethering, ECM deflection, and/or activation via stretch-sensitive PLA2 and STIM1 proteins (Ryskamp et al., 2014a; White et al., 2016; Servin Vences et al., 2017; Potla et al., 2020). IOP-induced gliosis is decreased in TRPV4−/− retinas but augmented in TRPC1/3−/− retinas (Ryskamp et al., 2014a; Molnar et al., 2016), suggesting that TRPV4 and TRPC1-containing channels stimulate different downstream signaling pathways.
Given that both overactivation and underactivation of TRPV4 signaling in Müller cells induce reactive gliosis, we propose that the channel participates in homeostatic maintenance and inflammatory activation. Another exciting aspect of TRPV4 signaling is polymodal additivity that can be unmasked by facilitation of the cells’ pressure response by body temperature (Matsumoto et al., 2018) and by combining its activation by swelling with exposure to chemical agonists (Toft-Bertelsen et al., 2017). Polymodality of gating probably reflects signal integration at different molecular domains (Vriens et al., 2004; Garcia-Elias et al., 2008).
Mechanosignaling in Müller cells bears similarities to brain astrocytes, which respond to indentation and pressure with TRPV4 and TRPC1-dependent Ca2+ influx, ATP, and glutamate release. TRP-dependent gliotransmitter release might help synchronize local neuronal and microglial activity (Malarkey et al., 2008; Diaz et al., 2019; Shibasaki, 2020; Turovsky et al., 2020). The retina is an ideal preparation to investigate such processes: in contrast to 20–30% of the brain astrocyte population that expresses TRPV4 (Shibasaki et al., 2014; Pivonkova et al., 2018), all retinal Müller cells manifest robust TRPV4-ir and functional responsiveness (Ryskamp et al., 2014a, 2015; Jo et al., 2015). It should also be noted that TRP channels usually collaborate with other mechanosensitive channels (such as Piezo and tandem-pore potassium families) and that cellular mechano-homeostasis requires synergistic activation of transducers with opposing effects on intracellular signaling (Yarishkin et al., 2019, 2021).
2.3. Osmoregulation in Müller glia reflects dynamic communication between TRP and AQP channels
Retinal osmolyte and water content are continuously monitored and regulated by the inner and outer retina-blood barriers constituted by Müller glia and RPE, respectively. Changes in osmolarity deform the cell membrane via swelling or shrinking, which in turn may affect many membrane-delimited processes and contribute to mechanosensation. Over the past several decades, many studies highlighted the multifaceted contributions of Müller cells to redistribution of ions and water between the retina, vitreous, and subretinal spaces (reviewed in Reichenbach and Bringmann, 2010). Given their central role in osmoregulation, it stands to reason that Müller glia sense changes in cell volume, with TRPV4 - a nonselective cation channel that was identified based on its sensitivity to swelling (Strotmann et al., 2000) and subsequently implicated in systemic and cellular osmotransduction (White et al., 2016) - at its nexus. The cells indeed strongly react with TRPV4 antibodies. By far the strongest TRPV4-ir was detected in the endfoot compartment (Jo et al., 2015; Ryskamp et al., 2014a; Li et al., 2021a,b) where proximity to AQP4, ER, and mitochondrial compartments subserves interactions with CICR, SOCE, K+ and water transport (Fig. 2B, E). Antibody labeling is punctate, presumably due to TRPV4 aggregation into macromolecular complexes that may include aquaporins, PACSIN 3, actin, microtubules, phosphatidylinositol biphosphate (PIP2), IP3 receptors, TRPM4 and anoctamin channels (e.g., Garcia-Elias et al., 2008; Jo et al., 2015; White et al., 2016; Redmon et al., 2017).
Wild-type Müller cells respond to synthetic TRPV4 agonists (GSK1016790A and 4α-PDD) and the endogenous agonist arachidonic acid with a nonselective cationic conductance that reverses at 0 mV (Ryskamp et al., 2014a, 2015; Toft-Bertelsen et al., 2019). In contrast to moderate outward rectification, typical of “generic” TRPV4-mediated currents (Watanabe et al., 2003), TRPV4 currents evoked by agonists and swelling in Müller glia exhibit a linear I-V relationship (Lapajne et al., 2022). The onset of the TRPV4-mediated current and the attendant increase in [Ca2+]i are slow (~minutes; Fig. 2C) due to obligatory activation of a polyunsaturated fatty acid messenger cascade downstream from phospholipase A2 (PLA2) (Fig. 2D). PLA2 is a Ca2+- and stretch-dependent enzyme that cleaves endocannabinoids to produce arachidonic acid, which in turn is metabolized by cytochrome P450 (CYP450) and epoxygenases into epoxyeicosatrienoic acids (5′6′-EET, 8′9′-EET, and 11′12′ EET) that function as final common activators of TRPV4 (Berna-Erro et al., 2017; Watanabe et al., 2003). The PLA2-EET cascade amplifies the original stimulus (Ca2+ influx across the channel pore), thereby equipping the glia with an extraordinary sensitivity to mechanical, thermal, lipid, and osmotic inputs (Matsumoto et al., 2018; Toft-Bertelsen et al., 2019; Redmon et al., 2021).
As in astrocytes and ex vivo brain tissue (Križaj et al., 1996; Risher et al., 2009), the volume of Müller cells is roughly proportional to the tonicity of the extracellular milieu (Jo et al., 2015). The cells swell in response to a reduction in extracellular tonicity or under the condition of energy depletion, which produces cytotoxic edema of Müller cell processes due to net ion and water entry into the retina. Swelling is associated with an increase in cytosolic [Ca2+]i that is sensitive to TRPV4 antagonists and Trpv4 gene deletion. TRPV4 inhibition obliterates calcium signals induced by weak (~5–15 mOsm) stimuli and suppresses 40–70% of the calcium response evoked by large (120–190 mOsm) hypotonic gradients. Regulatory volume decrease (RVD) observed when cells are exposed to large hypotonic gradients, is inhibited by BAPTA, and missing in TRPV4−/− cells (Jo et al., 2015; Toft-Bertelsen et al., 2019). Although Müller cell volume regulation shows strong Ca2+-dependence, osmosensitivity of the RVD response may be too low to play significant roles under physiological circumstances. In cell-based assays, hypotonicity activated TRPV4 channels via tyrosine phosphorylation at the N-terminus, PLA2 activation, and generation of lipid metabolites (Vriens et al., 2004; White et al., 2016). Another critical factor is the rate of lipid distension that works together with PLA2 activation to control the extent of channel activation (Ryskamp et al., 2014a; Toft-Bertelsen et al., 2017). The rate of cell swelling is governed by the density, location, and hydraulic permeability of AQP4 channels (Jo et al., 2015; Toft-Bertelsen and Macaulay, 2021), with deletion of Aqp genes slowing TRPV4 activation by reducing the rate of water influx and membrane expansion (Liu et al., 2006; Jo et al., 2015; Toft-Bertelsen et al., 2017). The latter involves PLA2-dependence of TRPV4 activation and activation of the eicosanoid cascade. Despite the instantaneous osmotic equilibration mediated by AQPs, the onset of Ca2+ signaling in hypotonically challenged cells is likely to reflect the time course of PLA2-EET signaling. We do not yet know whether AQP4 forms a macromolecular complex with TRPV4 (as proposed for cortical astrocytes; Benfenati et al., 2011) and cPLA2, whether AQP4 surface localization involves Ca2+/CaM-dependent step downstream from TRPV4 (Kitchen et al., 2020) and/or whether AQP4 controls the TRPV4 pore indirectly through expansion of the plasma membrane (Mola et al., 2021; Toft-Bertelsen et al., 2017).
Current evidence implicates Ca2+ influx through TRP channels in AQP-dependent swelling and RVD, with likely additional involvement of channel trafficking and activation of Ca2+ -dependent K+ and Cl− channels. TRPV4 agonists such as GSK1016790A and arachidonic acid facilitate swelling, whereas calcium chelation and Trpv4 gene ablation reduce its rate and extent (Pannicke et al., 2006; Ryskamp et al., 2014a; Jo et al., 2015). AQP4 trafficking may be regulated by calcium-dependent and independent steps (Sidhaye et al., 2006). The positive feedback loop involving AQP-dependent swelling, TRPV4 activation, and Ca2+ influx may be exacerbated by the swelling-induced release of the agonist arachidonic acid (Pannicke et al., 2006; Reichenbach et al., 2007) to contribute to edema formation and breakdown of inner and outer blood-retina barriers in diabetic retinas (Arredondo Zamarippa et al., 2017; Orduna Rios et al., 2019). Within this context, AQP4-dependent swelling functions as a driver of retinal edema (e.g., Manley et al., 2000; Thrane et al., 2011). Conversely, chelation of intracellular calcium, PLA2 blockade, or deletion of the TRPV4 gene reduced the excessive influx of calcium into Müller cell processes, suppressed swelling (Ryskamp et al., 2014a; Jo et al., 2015; Pannicke et al., 2006) and ameliorated the reactive response to diabetes (Acharya et al., 2017). TRPV4 inhibition has been similarly effective in counteracting edema in the retina, brain, lung, and other tissues (Balakrishna et al., 2014; Hoshi et al., 2018; Orduna Rios et al., 2019; Chmelova et al., 2019; Haywood et al., 2022; Michinaga et al., 2021), suggesting that targeting TRPV4 channels might constitute an alternative anti-edema strategy to AQP4 downregulation/inhibition (Manley et al., 2000; Da and Verkman, 2004; Kitchen et al., 2020). In an additional benefit, this strategy might preserve homeostatic ion/water fluxes through the retinal glymphatic pathway.
In contrast to wild-type cells in which TRPV4 functions to sense and mediate swelling-evoked [Ca2+]i signals (Ryskamp et al., 2014a; Iuso and Križaj, 2016), hypotonicity-induced Ca2+ increases in immortalized Müller cells do not involve TRPV4 activity (Netti et al., 2017).
2.4. TRPC1, store-operated calcium entry (SOCE), and calcium-induced calcium release (CICR)
Glial excitability is organized around communication between the plasma membrane and release channels in the endoplasmic reticulum (ER). Müller glia express ryanodine and inositol triphosphate receptors that are coupled to metabotropic receptor pathways and TRP channels (Keirstead and Miller, 1995; Da Silva et al., 2008; Lipp et al., 2009; Huang et al., 2010) (Fig. 3). The central CICR/SOCE hub is at the endfoot due to its packing with smooth ER, and extensive network of flat subplasmalemmal cisternae (Fig. 3D). [Ca2+]i signals evoked by the TRPV4 agonist GSK1016790A are reduced ~40% in the presence of CICR blockers such as thapsigargin (Ryskamp et al., 2014a). TRP subunits are thus functionally coupled to ER Ca2+ release, phospholipase C (PLC) signaling and store-operated Ca2+ entry (SOCE) (Figs. 2D and 3D).
Fig. 3. Store operated Ca2+ entry (SOCE), Ca2+ induced Ca2+ release (CICR) and excitability in mouse Müller cells.

(A) Mouse Müller glia, whole cell patch clamp recording. Depletion of the calcium endoplasmic reticulum (ER) store evokes a slowly developing inward current mediated by store-operated channels. Cyclopiazonic acid (CPA) inhibits calcium sequestration into the ER, resulting in its gradual emptying of stored Ca2+ in Ca2+-free saline. The sensor proteins within the ER membrane (‘STIM1’ in panel D) communicate the depletion status to the plasma membrane to activate ‘store-operated channels (TRPC1 and Orai in panel D). The slow inward current reflects Na+ influx through TRPC1. Restoration of 2 mM Ca2+ to the superfusing saline results in a transient activation of TRPC1 and Ca2+-permeable Orai channels and large inward current that subsides when the ER compartment is reloaded with Ca2+. (B) Calcium imaging, dissociated mouse Müller cell. The depletion-activated current marked with red arrow in panel A mediates [Ca2+]i elevations mediated by TRPC1 and Orai channels and propagation of a Ca2+ wave from the endfoot towards the cell body that is presumably mediated by calcium store release channels (RyR and InsP3R in panel D). Scale bar, 5 μm. (C) The amplitude of the depletion-evoked inward current is reduced by targeted deletion of Trpc1/3 genes. (D) Schematic model of store-operated signaling in Müller endfeet. Ca2+ -Induced Ca2+ release (CICR) is mediated by RyR and IP3R channels. In response to their activation by Ca2+ (RyRs) and IP3 (IP3Rs), Ca2+ is released from the ER lumen into the cytosol. Lowering of the lumenal [Ca2+]ER triggers the depletion sensor, STIM1, which in turn activates TRPC1 and Orai channels within the plasma membrane and induces plasma membrane influx of cations, shown as inward current and calcium elevation in panels A–C. Store-operated and CICR mechanisms may interact with additional TRP subtypes such as TRPV4. TRPC3/6 channels are independently activated by diacylglycerol (DAG) downstream from phospholipase C (PLC) and G protein-coupled receptors (GPCRs). PLA2 (phospholipase A2), AA (arachidonic acid), EETs (epoxyeicosatrienoic acids), P450 (cytochrome P450) are obligatory proteins in the glial TRPV4 activation pathway.
Store release-dependent excitability requires ER refilling with Ca2+ through store-operated Ca2+ entry (SOCE) – a cellular pathway through which plasma membrane cation channels are activated by the STIM1 (Stromal interaction molecule 1) ER depletion sensor. Müller cell SOCE is associated with a slowly developing inward cationic current that reverses at 0 mV (Fig. 3A and C), is accompanied by a rise in [Ca2+]i, and often takes the form of Ca2+ waves that propagate from the endfoot towards the soma (Molnar et al., 2016; Phuong et al., 2016) (Fig. 3B). The depletion-induced current is ~50% suppressed by pharmacological inhibition of TRPC1 or deletion of the Trpc1 gene (Fig. 3C), with the other component mediated by Orai channels (Molnar et al., 2016). In situ hybridization and transcript analyses corroborated strong Trpc1 expression in the INL and purified Müller cells (Da Silva et al., 2008; Gilliam and Wensel, 2011; Molnar et al., 2012; Jo et al., 2022).
TRPC1 homotetramers cannot form a functional pore. Like heteromerization between Drosophila TRP and TRPL channels (Hardie, 2014; Katz and Minke, 2018), vertebrate TRPC1 subunits form obligatory heteromeric associations with canonical associations, vanilloid, or polycystin subunits. I-V properties of the TRPC1-containing channel pore in Müller cells (Fig. 3C) resemble the currents mediated by heteromeric TRPC1:V4 channels (e.g., Ma et al., 2011; Du et al., 2014). Indeed, TRPC1−/− and TRPV4−/− cells show reduced responsiveness to mechanical stimuli. Because TRPC1 subunits are also sensitive to stretch (Jo et al., 2022), they may serve as excitability conduits between mechanical stimuli, intracellularly stored calcium, and calcium signaling that combines mechanical stimulation, metabotropic receptors, CICR, PLC-dependent activation of TRPC3/6 channels, and SOCE (Fig. 3D).
Kelly and coworkers suggested that store-operated signaling in Müller glia involves TRPC6 channels (Da Silva et al., 2008), which belong to the diacylglycerol (DAG)-sensitive group of canonical TRP channels (together with TRPC3 and TRPC7 isoforms). Purified mouse Müller cell preparations express Trpc1 and Trpc6 transcripts (Da Silva et al., 2008; Jo et al., 2022) and their [Ca2+]i response to DAG analogs (Fig. 2F) confirms functional expression of the TRPC3/6/7 subfamily. Interestingly, DAG is converted by lipases into arachidonic acid and metabolized into epoxygenase-derived eicosanoids (EETs), hydroxyeicosatetraenoic acids (HETEs), and leukotrienes that activate multiple vanilloid TRP isoforms. Hence, lipid signaling likely modulates multiple glial TRP subunits in series and in parallel.
2.5. Inflammation and disease
One of the earliest retinal responses to injury and stress is reactive gliosis that precedes neuronal phenotypes in glaucoma and diabetes and exacerbates neuronal injury from elevated IOP, neovascularization, BRB breakdown, and cytokine/chemokine release (Woldemussie et al., 2004; Inman and Horner, 2007; Devoldere et al., 2019). Müller glia respond to retinal detachment, intraocular pressure, and mechanical trauma with upregulation of glial fibrillary acidic protein (GFAP), vimentin, and complement proteins (Reichenbach and Bringmann, 2010). While retinal development and function appear unaffected in global TRPC1, TRPC3, TRPC6, and TRPA1 knockout mice (Molnar et al., 2012, 2016; Yarishkin et al., 2018a; Araújo et al., 2020), a range of structural and functional phenotypes emerges when the tissue is pushed out of homeostatic range. For example, reactive gliosis evoked by intravitreal injection of TRPV4 agonists simulates the effects of mechanical/thermal stress (Ryskamp et al., 2014a), possibly due to activation of the JAK2/STAT3/NFkB pathway (Li et al., 2021a,b). Conditional TRPV4 ablation protects neurons from detachment-induced reactivity and inflammation by reducing the sensitivity of Müller cells to swelling, detachment, and temperature (Matsumoto et al., 2018). The cells were suggested to regulate inflammation angiogenesis via MCP-1, IL-1β, IL-6, VEGF-A, and TNFα cytokines (Almasieh et al., 2012; Vecino et al., 2016; Devoldere et al., 2019), with TRPV4 antagonists and gene deletion inhibiting swelling-induced cytokine release (Matsumoto et al., 2018). As noted above, reactive gliosis in normotensive retinas can be triggered by TRPV4 overactivation or by ablating TRPV4 channels (Ryskamp et al., 2014a). Thus, tonic TRP signaling is required to maintain a healthy homeostatic state, and inflammatory activation can follow under- and overactivation of homeostatic sensing.
A Trpc1 SNP (rs7638459) was identified as a risk factor for type 2 diabetes (Chen et al., 2013), yet diabetic (STZ-treated) TRPC1/4/5/6 quadruple KO retinas showed protection against neuronal/pericyte loss (Sachdeva et al., 2018). In contrast to the proinflammatory effects of TRPV4 stimulation, TRPC1/3−/− retinas exposed to chronic ocular hypertension upregulate gliotic markers (Molnar et al., 2016), suggesting that TRPC1/3 activity helps maintain an anti-inflammatory state in the presence of chronic mechanical stress.
2.6. TRPV4 and lipid modulation
TRP channels tend to be very sensitive to membrane cholesterol, responding to depletion and supplementation with isoform-specific up/downregulation (Levitan and Barrantes, 2012; Lakk et al., 2021). Müller glia are the leading retinal producer and supplier of cholesterol which constitutes ~98% of total sterols in the retina, is required for glia-dependent synapse formation, and has been linked to acquired and inherited retinal degenerations such as Smith-Lemli-Opitz Syndrome, Niemann-Pick type C disease, diabetic retinopathy, macular degeneration (Fliesler and Bretillon, 2010) as well as glaucoma (Marcus et al., 2012). Retinas from animals with altered cholesterol levels and diabetes (Trivino et al., 2006) exhibit deficits - reactivity, hypertrophy, pathological swelling of Müller cells, and altered permeability of the microvascular endothelial barrier – that resemble the effects of excessive TRPV4 activation (Jo et al., 2015; Phuong et al., 2016; Ryskamp et al., 2014a; 2016). It would be interesting to explore whether ameliorating the reactive Müller glial response to hypercholesterolemia by PLA2 blockers (Acharya et al., 2017) includes suppressive or modulatory effects on TRPV4 activation (Ryskamp et al., 2014a) and whether channel activation is impacted by statins. Consistent with the multi-domain model of TRPV4 activation (Vriens et al., 2004), depleting free membrane cholesterol with scavenger cyclodextrins reduced the amplitude of agonist-, swelling, and temperature-evoked TRPV4 signals - effects that were reversed by cholesterol supplementation (Lakk et al., 2017). In contrast to endothelial cells (Saliez et al., 2008) but like trabecular meshwork cells (Lakk et al., 2017, 2021), TRPV4 in Müller glia does not partition into cholesterol-enriched lipid rafts and caveolar domains and thus the mechanism of its cholesterol sensitivity remains to be ascertained. It is possible if not likely that the channel is modulated by annular lipids that form hydrophobic pockets around the pore, by phosphatidylinositol-4,5-biphosphate (PIP2), and through cholesterol binding to Cholesterol Recognition/Interaction Amino acid Consensus (CRAC/CARC) domains that span the loop between TM4 and TM5 of TRPV4 (Kumari et al., 2015).
2.7. Müller cells, endothelial cells, and the blood-retina barrier (BRB)
The inner blood-retina barrier consists of a tight non-fenestrated endothelial monolayer that maintains ionic and water homeostasis across the retina-vitreous space and is regulated by interactions between the endothelium, Müller cells, pericytes, ganglion cells, and astrocytes. The interdependence of signaling between these cell types is critical for BRB integrity, with TRPV4 channels expressed on multiple sides of the neurovascular junction. A detailed review of TRP-dependent modulation of endothelial function and blood flow is beyond the scope of this review, but the reader is directed to recent summaries (Thébault, 2021; Lapajne et al., 2022). Release of gliotransmitters, arachidonic acid, nitric oxide and eicosanoids from Müller endfeet may result in vasoconstriction or vasodilation (Newman, 2015; Someya et al., 2019), with the epoxygenase pathway upstream from TRPV4 gating functioning as a potent blood vessel dilator (Metea and Newman, 2006b; Hu et al., 2004) that may affect local hyperemia. The sensitivity of endothelial cells to TRPV4 agonists appears to be ~25-fold higher relative to Müller glial endfeet (Phuong et al., 2017; Jo et al., 2015), suggesting that blood vessels are highly attuned to changes in local osmotic and mechanical gradients. Overactivation of microvascular endothelial channels was associated with loss of barrier permeability (Phuong et al., 2016). Involvement of TRPV4 channels in diabetic pathology remains to be studied further, as high glucose-treated endothelial cells and cells studied in streptozotocin-induced rats show reduced TRPV4 expression (Monaghan et al., 2015) yet TRPV4 antagonists and channel ablation were reported to be protective in diabetic animals (Arredondo Zamarippa et al., 2019; Orduña Ríos et al., 2019). Cappelli et al. (2021) showed that ablation of TRPV4 channels increases pathological angiogenesis and reduces pericyte coverage without impacting normal postnatal development. Another layer of complexity may involve cell type- or context-dependent interactions between TRPV4 channels and resident TRPV1, TRPV2, TRPC3 and TRPC6 isoforms (Sachdeva et al., 2018; O’Leary et al., 2019; Guarino et al., 2020). Finally, the glymphatic paravascular compartment between the endfeet and the endothelium may constitute a TRPV4- and AQP4-dependent venue for rapid fluid exchange. An analogy with brain astrocytes (Butenko et al., 2012; Diaz et al., 2019; Turovsky et al., 2020) implicates TRPV4-dependent signaling in Müller glial endfeet in the modulation of intravascular pressure, neurovascular coupling, functional hyperemia and barrier permeability and retinal pathophysiology. Consistent with this, isolated and intact microvascular retinal endothelial cells respond to TRPV4-mediated Ca2+ influx with dissolution of adherens/occludens junctions, loss of barrier integrity and blood leakage that is restored by genetic and pharmacological suppression of TRPV4 activity (Phuong et al., 2016) in which TRPV4−/− retinas are protected against diabetes-induced edema and BRB breakdown (Orduna Rios et al., 2019). Another essential aspect of gliovascular signaling involves endothelial TRPV1, TRPV2 and TRPV4 channels that modulate angiogenesis, myogenic constriction, cell migration and neurovascular coupling (O’Leary et al., 2019; Monaghan et al., 2015; Barabas et al., 2020).
TRPC6 may be upregulated in Müller cells from hyperglycemic STZ-treated retinas (Sachdeva et al., 2018). RNAi-mediated Trpc6 knockdown suppressed VEGF secretion and abolished glucose- and calcium-dependent decreases in glutamate uptake in an immortalized cell line (rMC-1; Ma et al., 2020), suggesting potential functions at the neurovascular junction.
2.8. Other TRP subunits in Müller glia
TRPV1.
TRPV1 antibodies labeled Müller cells in mouse, rabbit (Martínez-García et al., 2013), and vervet monkey (Bouskila et al., 2020) but not rat (Leonelli et al., 2009) retinas. Transgenic TRPV1Cre:Ai9 and TRPV1Cre:Ai3 retinas show fluorescent TRPV1 reporter tags in a subpopulation of Müller cells (Lakk et al., 2018). Non-uniform expression of TRPV1 lineage markers in transgenic retinas could reflect differential expression of the reporter transgene or actual differences in regional Trpv1 expression. It is worth noting that Müller cells represent a major retinal site for anandamide (AEA) uptake and degradation by fatty acid amide hydrolases (Glaser et al., 2005). Activity-dependent AEA release also modulates Müller glial TRPV1 signaling (Ryskamp et al., 2014b; M. Lakk and DK, unpublished observations). Comparison to other tissues (Alessandri-Haber et al., 2009; Balakrishna et al., 2014; Nilius and Szallasi, 2014) suggests potential roles in inflammatory and neuropathic sensitization.
TRPA1 regulates resting [Ca2+]i levels in cortical astrocytes (Shigetomi et al., 2011) and was localized to Müller cells with immunohistochemistry (Araújo et al., 2020), but functional validation is lacking.
TRPM3 may be expressed in mouse Müller glia, with potential roles in gliotransmitter release (Webster et al., 2020).
TRPP1 was proposed to regulate osmotic swelling in Müller cells (Vogler et al., 2016).
TRPC4 antibodies labeled vimentin-positive radial processes in the chick retina (Crousillac et al., 2003), and Trpc5 transcripts and immunosignals were detected in INL/GCL layers in the mouse retina (Oda et al., 2020; Witkovsky et al., 2008). There is no functional information about TRPC3-5,7 channels in Müller glia.
2.9. Summary
TRP channels in Müller cells, microglia and astrocytes regulate a vast range of context-specific signaling responses within which Ca2+ influx controls the transcriptome, metabolism, gliotransmitter and cytokine/chemokine release. The most studied isoforms, TRPC1 and TRPV4, have been linked to glial sensing of the biomechanical milieum regulation of blood-retina barrier and reactive gliosis. The function of the majority of TRP subunits expressed in retinal glia remains unknown.
2.10. TRP channels in the retinal pigment epithelium
Regulation of TRP channel activity is advantageous because it generates multimodal signal pathways by the same TRP channel. As the retinal pigment epithelium (RPE) serves many functions in maintaining photoreceptor function (Bok, 1985; Strauss, 2005), we hypothesize that the multimodal TRP channels are ideal for coordinating these different functions.
2.11. Ca2+ conducting ion channels in the RPE: TRP channels show a large variety
The RPE supports the photoreceptor function in several ways (Bok, 1985; Strauss, 2005; Wimmers et al., 2007). Among these functions are epithelial transport of Cl− and water from the retina to the blood side, phagocytosis of shed photoreceptor outer segments during their renewal process and the secretion of angiogenic, neurotrophic, or immunogenic factors. Evidence from several groups showed the Ca2+-dependence of these functions (Strauss, 2005; Wimmers et al., 2007). For that reason, RPE expresses a variety of Ca2+-conducting ion channels, among them voltage-dependent Ca2+ channels and TRP channels. There are independent reports of the TRP channel expression pattern in the RPE, but these papers do not seem to support each other. Marked differences appear primarily at the mRNA and Western blot/immunohistochemistry levels. Zhao et al. used an RPE model based on human fetal cells (Zhao et al., 2015) to report the expression of TRPC4, TRPM1, TRPM3, TRPM7 and TRPV4 at the mRNA and Western blot levels. TRPM3 was found at the primary cilium, TRPV4 in the apical microvilli. TRPM3 and TRPC4 are localized close to the tight junctions. The group tried to show the functional role by measurements of transepithelial transport parameters such as transepithelial resistance and voltage. Although they showed that the broad range TRP blocker La3+ inhibited transepithelial transport by 70%, the group could not identify a specific contribution from a specific TRP channel isoform. Bollimuntha et al. added the expression of TRPC1 in ARPE19 cells (Bollimuntha et al., 2005). The expression of TRPM3 was supported by Gilliam and Wensel (2011) but not found in another study (Brown et al., 2015). The expression of TRPC4 and TRPV4 was supported by data from Crousillac and colleagues (Crousillac et al., 2003). Based on the use of antibodies, Gilliam et al. verified the expression of TRPM3 but could not confirm the expression of TRPV2 (Gilliam and Wensel, 2011). Kennedy et al. reported the expression of TRPV5 and 6 and tried to verify this in recordings of intracellular free Ca2+ (Kennedy et al., 2010). Using large (up to 5 mM) increases in extracellular Ca2+, the group evoked responses of intracellular free Ca2+ sensitive to the nonselective TRP channel blocker ruthenium red. The group of Mitchell demonstrated functional expression of TRPML1 channels in mouse primary cell culture RPE cells, in ARPE-19 cells and in human embryonic stem cell based RPE cells (Beckel et al., 2018; Gómez et al., 2018). We studied TRP expression profiles in freshly isolated human primary culture RPE cells as well in RPE cells from mouse and pig in primary culture. Our published and unpublished data support the observations at the mRNA level (Barro-Soria et al., 2012; Cordeiro et al., 2010; Reichhart et al., 2015b; Wimmers and Strauss, 2007) (Fig. 4A). We found the expression of Trpc1, 4, 7; Trpm1, 2, 3, 7; Trpv1-4 (but not V5, V6). The verification at the functional level exists so far for only a few TRP channels in the RPE.
Fig. 4. TRPV2 activation modalities and differential regulation of the RPE.

(A) Spectrum of TRP channels expressed in freshly isolated human RPE cells analyzed by means of RT-PCR. (B) Heat-evoked Ca2+ transient in a human RPE cell is blocked by ruthenium red; indication for TRPV2 channel activation (Cordeiro et al., 2010). (C) Modalities of TRPV2 activation in the RPE by different G-protein coupled receptors by direct activation of the TRPV2 channel and/or by increase in surface expression of TPRV2 channel proteins mediated by PI3 kinase; activated TRPV2 channels promotes secretion of VEGF-A, MCP1 or IL-6 from the RPE. (D) AngII activated TRPV2 and the downregulation of renin expression in the RPE: AngII binds to AT1 receptors in the basolateral membrane of RPE and leads to an increase in [Ca2+]i through activation of ATRAP under participation of an InsP3-sensitive intracellular Ca2+ store. The mechanism of ATRAP -dependent TRPV2 activation is unclear. [Ca2+]i increase downregulates renin expression in the RPE via an unknown mechanism (Created in Biorender.com).
2.12. Functional evidence of TRP channels in the RPE
The direct evidence for the activity of TRP channels in the RPE stems from patch-clamp experiments and measurements of intracellular free Ca2+ with Ca2+-imaging techniques using Ca2+-sensitive fluorescence dyes such as fura-2. These methods provided functional evidence for the activity of TRPC and TRPV2 channels.
TRPC1/4:
When investigating the Ca2+ permeability of the plasma membrane for Ca2+ in the human RPE (human primary and ARPE19 cell cultures) under resting conditions, we were surprised to find that TRPC channels are tonically active (Wimmers and Strauss, 2007). The mean value of the basic level of the intracellular free Ca2+ concentration in the RPE was measured at 100 nM. The Ca2+-sensitive fluorescence dye used in these measurements is distributed throuough the complete inner space of the cell. It might be possible that there are subcellular spaces in the RPE with much higher intracellular Ca2+ concentrations; although this has not been investigated so far. Under these conditions, in the absence of active second-messenger pathways, inhibition of L-type channels had no effect on the Ca2+ level whereas La3+, Gd3+ or Ni2+ reduced the baseline intracellular Ca2+ concentration down to 20 nM. A concentration/effect curve showed a half-maximal effect of La3+ at a concentration of 1 μM. In addition to TRP channels, ARPE19 cells functionally express voltage-dependent L-type channels of the Cav1.3 subtype as another type of Ca2+ conducting channel (Reichhart et al., 2015a). L-type channels might be inhibited by La3+, Gd3+ or Ni2+ as well but a specific inhibition of these channels by dihydropyridines had no effect on the intracellular free Ca2+ baseline levels. Sensitivity to La3+, Gd3+ and Ni2+ indicates TRP channel activity at baseline conditions. To obtain more insight into the TRP subtype, we tested the effects of blockers SKF96365 and 2-APB at concentrations of 50 μM and 75 μM, respectively. Similar to La3+, SKF9365 and 2-APB reduced basal [Ca2+]i to 20–50 nM. As TRPM channels are Ni2+- insensitive (Penner and Fleig, 2007; Enyeart et al., 2002) and TRPV channels are activated by 2-APB (Hu et al., 2004; Clapham et al., 2005), the effects of blockers on baseline [Ca2+]i can be attributed to TRPC channels. The subtype(s) of TRP channels that provide the Ca2+-conductance under basal conditions remain to be ascertained.
TRPV2:
Our PCR results indicated the expression of heat-sensitive TPRV channels in human RPE. To show the contribution of these channels to the Ca2+ signaling, we developed an experiment in which we could provide a rapid and short-time temperature increase of the extracellular milieu at different values while we measured intracellular free Ca2+ (Cordeiro et al., 2010; Reichhart et al., 2015b). We were able to evoke an increase in intracellular free Ca2+ with temperature pulses to 56 °C and in membrane conductance with temperature pulses to 45 °C (higher temperatures destabilize the whole-cell configuration), a temperature range specific for TRPV1 and TRPV2 channels. At temperatures of 56 °C, a larger contribution by TRPV2 in Ca2+ rises is expected. La3+ and ruthenium red blocked temperature-evoked Ca2+ transients whereas high concentrations of 2-APB evoked large [Ca2+]i elevations.
Downregulation of TRPV2 expression by anti-TRPV2 siRNA reduced the temperature-evoked Ca2+ transients by the same magnitude as mRNA reduction. In whole-cell recordings of the patch-clamp technique, we showed that an increase in extracellular temperature induced a mildly rectifying current with a ruthenium red-sensitive inward component. Activation at high temperatures, block by La3+/ruthenium red, Ca2+ rises in response to 2-APB and current/voltage relation of whole-cell currents substantiated the functional expression of TRPV2 channels in the RPE (Cordeiro et al., 2010; Hu et al., 2004). The TRPV1 agonist capsaicin and the TRPV3 agonist camphor (Clapham et al., 2005) had no effects on intracellular Ca2+. Thus, TRPV2 appears to be the main thermoTRP channel in RPE cells.
2.13. RPE functions that are regulated by Ca2+ signaling: potential roles of TRP channels
Among the different functions of the RPE, the ones with evidenced Ca2+-signaling as major regulator are epithelial transport of Cl− and water, the circadian regulation of phagocytosis of shed photoreceptor out segments and secretion of growth factors (Wimmers et al., 2007). For epithelial transport and for regulation of secretion exist functional data that indicated a contribution of TRP channels. For phagocytosis the data for contribution of TRP channels are rather weak.
2.13.1. Transepithelial transport regulation by an unknown TRP channel
Epithelial transport of water is driven by a transepithelial gradient of Cl− across the RPE and serves to eliminate water from the outer retina. The transport starts with a net uptake of Cl− by the Na+/2Cl−/K+-co-transport across the apical membrane into the RPE cell to establish a high intracellular Cl− concentration. Cl− leaves the cell across the basolateral membrane through a variety of Cl channels towards the blood side driven by a transmembrane gradient for Cl− and among these basolateral Cl channels are Ca2+-activated Cl channels. Transepithelial Cl− transport can be induced by increasing intracellular free Ca2+ with an apical application of ATP (Peterson et al., 1997). Indeed, Zhao et al. showed that transepithelial transport parameters in Ussing chamber experiments of a fetal RPE-based RPE model might depend on the activation of TRP channels by inhibitory effects of La3+, a broad-range TRP channel blocker (Zhao et al., 2015), suggesting involvement of a TRP subtype. However, it is unclear whether the RPE cell model used in the study by Zhao represents the mature RPE.
2.13.2. TRPV2 channels in the RPE: a multitude of activation mechanisms and functions
The contribution of TRPV2 channels to the regulation of RPE function gives a good example of how TRP channels have different activation pathways and that each activation pathway results in different RPE cell reactions (Fig. 4). In general, two different mechanisms activate TRPV2 channels in heterologeous expression and in the RPE (Kanzaki et al., 1999; Reichhart et al., 2015b). One of the direct activation mechanism acts by increasing the current through the ion channel pore; the other acts through an increase in the surface expression of the channel proteins. When elucidating the activation mechanisms for TRPV2 in more detail, we used a heat pulse to 45 °C to increase whole-cell currents as well as [Ca2+]i (Fig. 4 B) in RPE cells and used the increased membrane conductance as a specific measure of functionally available TRPV2 channels in the plasma membrane. However, given that such temperature shifts do not commonly occur in the retina, we investigated TRPV2 activating pathways (Fig. 4 C). When stimulating the cells with IGF-1, we found that heat and IGF-1 activated TRPV2 in the same order of magnitude. In contrast, cannabidiol did not change the membrane conductance while it increases the response to heat-evoked stimulation. The total activatable TRPV2 membrane conductance is larger with cannabidiol than without. Thus, the effects of cannabidiol seem to be limited to increasing TRPV2 channel density in the plasma membrane. Using an immunocytochemistry-based assay, we analyzed the surface expression of TRPV2. Cannabidiol increased the surface expression by 4-fold whereas IGF-1 by ~2-fold. Thus, IGF-1 increases the membrane conductance by both TRPV2 channel activity and surface expression whereas cannabidiol increases surface expression only. However, given that such temperature shift do not commonly occur in the retina, we investigated TRPV2 activating pathways.
Together with heat, we identified three agonists that activated TRPV2 channels as part of Ca2+-signaling cascade. In whole-cell recordings of the patch-clamp technique and measurements of intracellular free Ca2+, we demonstrated that insulin-like growth factor-1 (IGF-1) strongly activates TRPV2 in human RPE cells. In mouse RPE cells, we showed that TRPV2 contributes to angiotensin-2 evoked Ca2+ signals under the contribution of angiotensin- receptor associated protein (ATRAP) (Barro-Soria et al., 2012) (Fig. 4D). TRPV2 channels may also contribute to immune-related Ca2+ signaling. Using human serum as a source for activated complement proteins, we found that RPE cells react with a highly reproducible Ca2+ signal that integrates the activities of the different complement proteins including anaphylatoxins and terminal complex (Genewsky et al., 2015). This signal was blocked by ruthenium red and by isradipine and nifedipine further implicating TRPV2 channels and voltage-dependent L-type channels. Furthermore, an investigation of anaphylatoxins-activated Ca2+ transients in RPE cells revealed that TRPV2 channels mainly contribute to the signal pathways of C3a and C5a receptor activation because anaphylatoxins-evoked Ca2+ rises were insensitive to nifedipine, an L-type channel blocker (Busch et al., 2017). IGF-1 and the anaphylatoxins-dependent activation of TRPV2 channels are both mediated by the activation of PI3-kinase.
The multimodal activation of TRPV2 enables the participation into a variety of functional changes in the RPE. Stimulation of RPE cells with angiotensin-2 results in a reduction of renin expression and secretion by RPE cells via activation of AT1 receptor and an increase in intracellular free Ca2+ (Barro-Soria et al., 2012). Using a siRNA approach to reduce the TRPV2 channel expression in primary cultures of porcine RPE cells, we showed reduced angiotensin-2-evoked Ca2+ transients (Barro-Soria et al., 2012). Thus, the angiotensin-2-dependent Ca2+signals require TRPV2 activation. In animal studies, we showed that the renin production by the RPE controls the local renin-angiotensin-system in the retina (Milenkovic et al., 2010). Thus, the TRPV2 channel in the RPE is essential to regulate intraretinal renin-angiotensin system.
Complement-evoked Ca2+ signals in the RPE also lead to changes in secretion (Genewsky et al., 2015; Busch et al., 2017). The complement component C5a activates intracellular Ca2+ signals that are dependent on PI3-kinase activation sharing the same siganling pathway than IGF-1 (Busch et al., 2017). This leads to increased C3 expression in RPE cells as well and secretion of MCP1, VEGF-A and IL-8 (Busch et al., 2017). VEGF-A secretion is also under TRPV2-dependent control by IGF-1 (Cordeiro et al., 2010). Either increase in temperature or IGF-1 lead to an increased release of VEGF-A by the RPE. The observation that heat increases the VEGF- A secretion might have an implication for our understanding of the effects of laser treatment of the retina. Areas that are not directly affected by the laser but receive a heat impact might change the secretion by RPE cells through activation of TRPV2 channels.
2.13.3. Unknown functions of TRP channels for RPE physiology
The basal intracellular Ca2+ concentration is at a “physiological” concentration level of 100 nM, presumably due to tonic activity of TRPC1/4 channels (Wimmers and Strauss, 2007). Constant Ca2+ influx must be counterbalanced by ATP-driven Ca2+ clearance and/or sequestration. Indeed, we found that inhibition of PMCAs (plasma membrane Ca2+-ATPases) strongly elevates [Ca2+]i. We speculate that tonic Ca2+ influx quickly delivers Ca2+ to the extracellular space, most likely to the retinal side, as TRPC4 channels were located on the apical membrane of RPE cells. Ca2+ release across the apical membrane might thus serve for Ca2+-dependent signaling mechanisms in the photoreceptor outer segments.
Cultured RPE cells from mouse or human functionally express TRPML1 channels (Beckel et al., 2018; Gómez et al., 2018). These channels seem to participate in intracellular Ca2+ signaling involing lysosomes integrated in to toll-receptor signaling. The group discussed the potential functional relevance for RPE cells but further evidence is lacking.
2.14. Summary
Own data and data from other groups suggest a large variety of TRP channels expressed in the RPE. Most of the data are at the mRNA level, evidence of protein expression or functional validation are still lacking. So far, the best investigated channel is the TRPV2 in terms of regulation, functional role and functional analysis. Functional evidence exists for TRPC channels. For the majority of TRP in the RPE exists a large gap of knowledge.
3. TRP expression in microglia
As the resident immune cells of the brain and retinal parenchyma, microglia continually monitor tissue homeostasis and respond to a wide variety of stressors by transitioning into a reactive state to orchestrate the inflammatory response (Prinz et al., 2019). Under healthy circumstances, microglial phagocytosis of dying cells, debris and pathogens and secretion of cytokines/chemokines help limit the spread of tissue damage, modulate neurogenesis and prune excess synapses, whereas overactivation promotes retinal injury (Li and Barres, 2018; Silverman and Wong, 2018). Mild local insults attract microglial processes and affect synaptic remodeling without unleashing a full-blown reactivity, whereas strong activation occurs within a neurodegeneration-promoting context associated with cytokine release (Silverman and Wong, 2018). Calcium is essential for microglial chemotaxis, with changes in [Ca2+]i correlating with process motility in intact retinas (Redmon et al., 2021). We propose that TRP channels work together with P2X and Orai channels as the main modes of plasmalemmal Ca2+ influx in retinal microglia.
3.1. The transcriptional profile for TRP genes in retinal microglia diverges from CNS expression
A comparison of vanilloid subfamily expression in retinal vs. cortical microglia shows a remarkable divergence. Retinal cells show the following expression sequence: Trpv2>Trpv1>Trpv3>Trpv4 whereas their cortical cognates exhibit Trpv4>Trpv6>Trpv2>>Trpv3>Trpv1 (Redmon et al., 2021). Overall Trpv abundance in the retina is markedly lower vis a vis cortex (Fig. 5A), an observation consistent with the reported variability between microglial sensomes across the CNS (Bennett et al., 2018; De Biase and Bonci, 2019). Thus, retinal microglia likely sense and respond differently to biomechanical and chemical cues than their cortical and PNS counterparts.
Fig. 5. TRP channel expression in retinal microglia.

(A) Expression of simultaneously measured vanilloid TRP transcripts in retinal (red bars) and cortical (gray bars) microglia shows overall transcript expression to be significantly lower in the retinal cohort. (B) TRPV4eGFP retinas show lineage TRPV4 signals in RGCs and ramified IPL/OPL microglia. (C) TRPV1, calcium imaging. Dissociated retinal microglia respond to the agonist capsaicin with an increase in [Ca2+]i (Redmon et al., 2021).
3.2. TRPV4 mediates the microglial response to osmotic and mechanical inputs
Low TRPV4 expression in retinal microglia relative to RGC and Müller glial expression (Figs. 5 and 9B) caused it to be missed in early immunohistochemical analyses (Ryskamp et al., 2011). The expression was subsequently unmasked with lineage-specific markers and 2-photon microscopy (Redmon et al., 2021) which demonstrated it for both reactive and quiescent microglia (Fig. 5B). TRPV4-mediated calcium influx correlated with retraction of secondary and tertiary processes but without significant changes in surveillance. Interestingly, TRPV4 agonists suppressed lamellipodial ruffling in primary microglial cultures (Redmon et al., 2021). Mechanisms that mediate microglial surveillance may be distinct from those that serve motility.
Fig. 9. TRPV1 signaling in RGCs.

(A) Confocal image from the retinal ganglion cell layer (RGCL) and inner plexiform layer (IPL) of transgenic adult Trpv1Cre:Ai9 retina expressing the fluorescent marker tdTomato and immunolabelled with an antaibody raised against the Cannabinoid Receptor type 1 (CB1R). tdTomato signal shows sparse TRPV1 expression in RGC somata and processes (arrows), together with a presumed amacrine cell (arrowhead). Scale = 20 μm (Jo et al., 2017). (B) Hypothetical model of TRPV1 signaling in RGCs. The TRPV1 pore is activated by endocannabinoids (anandamide, AEA), protons and arachidonic acid metabolites such as 20-HETE and 12-HpETE. Another endocannabinoid, 2-acylglycerol, binds the CB1 receptor to initiate a Gi cascade that inhibits protein kinase A and suppresses cAMP-dependent facilitation of the TRPV1 channel. Endocannabinoid modulation of [Ca2+]RGC therefore reflects the relative activation of the AEA vs 2-AG legs of RGC signal transduction.
The high input resistance, low resting conductance and low [Ca2+]i in unstimulated retinal microglia (Redmon et al., 2021) predict that the cells’ membrane potential and [Ca2+]i will be impacted by even a limited amount of cation Ca2+ influx. Small (10–20 pA) TRPV4 currents were indeed associated with substantial [Ca2+]i increases and significant (~10 mV) hyperpolarizations presumably mediated by a Ca2+-activated potassium conductance. Perfusion of intact retinas with the TRPV4 antagonist HC06047 at room temperature did not affect microglial [Ca2+]i, resting membrane potential or branching but microglial motility might be TRPV4-dependent at body temperature (Nishimoto et al., 2021). Similar to Müller cells, microglial TRPV4 activation requires eicosanoid signaling (Redmon et al., 2021).
3.3. TRPV1 may be involved in inflammatory activation
Cortical microglia express TRPV1 channels, which may contribute to proinflammatory cytokine release, oxidative stress, and neurotoxicity (Marrone et al., 2017). TRPV1 has been similarly linked to pressure-induced release of the proinflammatory cytokine IL-6 from retinal microglia (Sappington and Calkins, 2008). Functional analyses of calcium signaling in these cells show substantial [Ca2+]i responses to the TRPV1 agonist capsaicin (Fig. 5C). The channel may be functionally active in plasma membrane as well as intracellular compartments (Miyake et al., 2015).
3.4. Summary
Low Trpv expression in retinal microglia may reflect the specifics of the retinal biomechanical milieu in which cells must contend with IOP fluctuations and ionic changes associated with the tonic activity of ON and OFF pathways. TRPV channels equip microglia with responsiveness to mechanical and chemical stimuli, with potential roles in retinal inflammatory signaling.
4. The Drosophila Trp channels: signal transduction cascade in the photoreceptor
4.1. An introduction
The “A-type” Drosophila mutation, isolated by Cosens and Manning shows a transient light response in the fly’s electroretinogram (Cosens and Manning, 1969). Thus, the signaling cascade that transduces light into an electrophysiological signal is active but is not maintained active in the mutant fly. Baruch Minke and colleagues demonstrated that the phenotype is based on a transient photoreceptor potential (Trp) based on a defect in signal cascade and gave the mutation the name Trp (Barash et al., 1988; Minke, 1977, 1982, 2002; Minke et al., 1975; Selinger and Minke, 1988). The defective gene was isolated and, at the end of 1989, identified as an integral membrane protein with unknown function (Montell and Rubin, 1989; Wong et al., 1989). Its identification as ion channel that conducts Na+ and Ca2+ was achieved 3 years later by Hardie and Minke (1992). This gene turned out to be a founder of a complete gene family of homologs in the vertebrate system (Wes et al., 1995; Zhu et al., 1995). The product of the TRP gene codes for an ion channel that conducts Ca2+ and Na+, generating the light-induced current (LIC) in the Drosophila photoreceptor. Upon opening of this channel, the membrane potential of the photoreceptor becomes depolarized. The TRP channel would permit a constant depolarization during the period of illumination. This property fails due to the mutation (Hardie and Minke, 1992).
The research on Drosophila TRP activation/deactivation showed that the unique property of all TRP channels, the multimodal regulation, is fully exploited to generate and fine-tune the LIC (Hardie and Juusola, 2015; Hardie and Raghu, 2001; Katz and Minke, 2018; Montell et al., 2002). The research relies on detecting and functional analysis of more genes that change photoreceptor activity when mutated (Pak and Leung, 2003). Furthermore, proteins were analyzed in heterologous expression systems. Of great advantage was the unique structural arrangement of the light transduction cascade-forming proteins (Voolstra and Huber, 2020). This arrangement permitted the analysis of the complete light transduction cascade in one membrane patch from the photoreceptors’ light-sensitive compartment, the rhabdomere (Minke and Parnas, 2006). This seems to be important as the analysis of Drosophila TRP channels in expression might result in confounding data (Lev et al., 2012). Still, basic questions need clarification. However, the specialized activation and gating regulation modalities of Drosophila TRP channels substantially contribute to the fly photoreceptor’s unique properties (Voolstra and Huber, 2020; Hardie and Raghu, 2001; Katz and Minke, 2018).
4.2. The light-evoked signaling transduction cascade in Drosophila; an overview
The structure of the Drosophila photoreceptor resembles that of vertebrates (Hardie and Raghu, 2001). Attached to a cell body is a compartment with a greatly enlarged surface area that contains densely packed proteins of the light transduction cascade. Its evolutionary origin is distinct from photosensitive compartments in vertebrates. The vertebrate photoreceptor outer segment evolved from a cilium, whereas the Drosophila rhabdomere evolved from microvilli. Vertebrate photoreceptors hyperpolarize upon light perception whereas Drosophila photoreceptors depolarize. The Drosophila’s photoreceptor light response results from a lipid-based signal transduction cascade ignited by light-dependent activation of Drosophila rhodopsin that, in turn, activates phospholipase Cβ4 (PLCβ4) through a Gαq G-protein subunit. PLC generates inositol-1,4,5-trisphosphate (InsP3) and diacyl glycerol (DAG) from phosphatidylinositol-4,5-bisphosphate (PIP2), a membrane lipid (Cook et al., 2000; Devary et al., 1987; Hardie et al., 2015; Hardie and Juusola, 2015; Katz and Minke, 2018; Katz et al., 2017; Minke, 2012; Minke and Selinger, 1992; Montell et al., 2002; Raghu et al., 2012; Tian et al., 2012; Voolstra and Huber, 2020). These metabolites amplify the single-photon response and serve critical roles in photoreceptor depolarization. DAG activates protein kinase C (eye-specific PKC) and represents a substrate to refill PIP2 levels in the plasma membrane (Balakrishnan et al., 2015; Voolstra et al., 2017). PKC itself signals inactivation and adaptation. The reduced concentration of PIP2 in the plasma membrane, which changes its lipid packing, generates mechanosensitive force by a force-from-lipid mechanism (Cox et al., 2019) that may activate TRP in the rhabdomere membrane (Hardie and Franze, 2012). TRP activity itself is additionally facilitated by rise in intracellular Ca2+ but gets inactivated at higher Ca2+ concentrations (Hardie, 1991; Chu et al., 2013a; Hardie and Juusola, 2015; Hardie and Raghu, 2001; Katz and Minke, 2012). InsP3 might have several roles in light transduction cascades of insect photoreceptors. InsP3 activates a release of Ca2+ from intracellular Ca2+ stores upon binding to the InsP3 receptor, a ligand-gated intracellular Ca2+ channel. In bees or the ventral photoreceptors of Limulus, intracellular Ca2+-rises resulting from InsP3 receptor activation represent a significant proportion of the overall light-induced Ca2+ signal that amplifies TRP activity (Brown and Blinks, 1974; Walz and Baumann, 1995; Walz et al., 1995). In Drosophila, InsP3 receptor-dependent Ca2+ release seems not to be a part of the light transduction cascade (Acharya et al., 1997). It might contribute to adjustment of the photoreceptor’s light sensitivity, a hypothesis that is not fully proven as publications about the problem are still controversial (Voolstra and Huber, 2020). However, the understanding of the role of essential signaling molecules and their metabolites is originated/verified by analysis of different Drosophila mutations: ninaE (“neither inactivation nor afterpotential E”) for rhodopsin, norpA (“no receptor potential A”) for PLCβ4, inaC (“inactivation no afterpotential C”) for PKC, rdgA (“retinal degeneration A”) for DAG kinase (Pak and Leung, 2003).
The mutation inaD (“inactivation no afterpotential D”) is not among the genes that directly contribute to the light transduction cascade (Montell and Montell, 1998; Montell, 2012; Pak et al., 2012; Tsunoda and Zuker, 1999; Wang and Montell, 2007). However, it plays a vital role for the unique properties of the Drosophila’s light transduction cascade. The photoreceptor response to light shows fast kinetics, with high specificity, sensitivity, and reproducibility (Chu et al., 2013a; Henderson et al., 2000). The single photon-response of the Drosophila photoreceptor is five times larger than in mammalian rods. Its kinetics - a fully processed reaction within 0.1s - is five times faster than that of mammalian rods (Hardie and Raghu, 2001). These qualities are associated with function of inaD, a scaffolding protein (Sanxaridis et al., 2007; Wang et al., 2005a) that assembles via five PDZ domains (PDZ = post-synaptic density protein). PSD-95, disc large tumor suppressor 1 (dlg1) and zona-occludens1 (ZO-1) may assemble into a “transducisome” or “signalplex” (Wang and Montell, 2007) that includes the G-protein Gαq subunit, PLCβ4, PKC and TRP. In this way, inaD ensures the high concentration of the light transduction proteins, their stoichiometry and diffusion-less interaction with, therefore, high-speed light transduction. Recent studies showed that this complex is not a fixed entity and might underlie regulatory changes that possibly modulate the light transduction cascade. Several studies led to the hypothesis that the activation of the light transduction cascade also leads to a conformational change of inaD. However, functional role of this mechanism remains unclear. Patch-clamp analysis of the light-evoked photoreceptor response has proven this signaling complex’s functional relevance (Delgado et al., 2019; Minke and Parnas, 2006). Here, excised patches from the rhabdomere membrane can fully generate a light response. This type of investigation has led essential advances in the understanding of the regulation of TRP channels during the light response.
4.3. TRP and TRPL in phototransduction
4.3.1. Ion permeability of TRP channels in Drosophila photoreceptors
The LIC is generated by two TRP channels, TRP and TRPL (Niemeyer et al., 1996; Reuss et al., 1997). To date, the literature describes three TRP channel proteins in the Drosophila photoreceptors (Hardie, 2014; Hardie and Juusola, 2015; Katz and Minke, 2018; Minke and Parnas, 2006; Raghu and Hardie, 2009), with the TRP family related to the vertebrate TRPC group (Raghu and Hardie, 2009). The TRPC channels in vertebrates are the ones with the highest homology to the Drosophila TRP’s (Clapham et al., 2005). TRP activation generates light-dependent photoreceptor depolarization at a more significant proportion than TRPL. The third Trp gene, Trpγ, has no known function. The investigation of the functional properties of these channels is not easy as they change their properties depending on the expression system (Lev et al., 2012; Minke and Parnas, 2006). The TRP channel subunits generally form tetramers to build a functional channel. Indeed, Montell’s group concluded from co-immunoprecipitation experiments with heterologously expressed TRP- and TRPL-proteins and from in situ analysis of Drosophila photoreceptors that TRP and TRPL form heterotetramers. This conclusion was challenged by in vivo/in situ data. Co-immunoprecipitation experiments and the binding affinities to the scaffold protein inaD concluded that only TRP binds to inaD and that therefore TRP and TRPL subunits form only homotetramers and not heterotetramers as known for other members of the mammalian TRPC family (Bahner et al., 2002; Chevesich et al., 1997; Paulsen et al., 2000; Tsunoda et al., 1997). This is supported by the observation that only TRPL shows a light-dependent regulation of surface expression that would not function when TRP and TRPL form heterotetramers (Bahner et al., 2002; Niemeyer et al., 1996). However, Bahner et al. and Xu et al. (Bahner et al., 2002; Xu et al., 1997) considered the existence of both homotetramers and heterotetramers of TRP and TRPL. Given the observation that TRP is expressed in large excess over TRPL, the TRP/TRPL heterotetramers represent a smaller proportion among the functional channels and their possible existence was challenged by Katz et al. (2013). The role of TRPγ is unknown, but it might possibly form heterotetramers with TRPL (Xu et al., 2000; but see Katz et al., 2017). TRP and TRPL strongly differ in their permeability properties.TRP shows a 50-fold higher permeability for Ca2+ than for Cs+ whereas for TRPL, this permeability ratio is 4:1 (Chu et al., 2013b; Liu et al., 2007; Reuss et al., 1997). The trp mutation leads primarily to a loss of Ca2+ permeability (Hardie and Minke, 1992). These data stem only from in vivo measurements because TRP needs inaD to form tetramers to build a full functional channel pore and cannot be investigated as an isolated channel in heterologous expression systems. Furthermore, in vivo TRP current recordings are impossible in the absence of Na+ because this leads to a fast Ca2+ overload and disruption of the cell so that the permeability ratio of Ca2+ versus monovalent cations is estimated in a bi-ionic approach. Thus, the permeability ratio is given as Ca2+/Cs+. The TRP/TRPL channel activation might occur by mechanical forces in the plasma membrane and by phosphorylation, as there are 28 possible phosphorylation sites in the TRP protein (Voolstra et al., 2013). Phosphorylation of TRP is light-dependent and seems important for shaping LIC under flicker light conditions (Voolstra et al., 2010, 2017). As described below, TRP/TRPPL activation by light may be associated with mechanical forces inside the plasma membrane, and protons.
4.3.2. Effects of Na+ and Ca2+ entering the photoreceptor cell
Photoreceptor stimulation by light generates LIC representing the combined activity of TRP, TRPL (Leung et al., 2000; Niemeyer et al., 1996), and the Na+/Ca2+ exchanger (Wang et al., 2005b). The proportion of Ca2+ in the LIC was estimated between 26%, and thus Na+ influx mediates the majority of transmembrane flux across the rhabdomere membrane (Chu et al., 2013b). On one hand, permeability ratio for Ca2+/Na+ of the TRP and of TRPL accounts for proportion between Ca2+ and Na+flux in the LIC. The Ca2+ permeability of TRP is about 50 times higher than that for monovalent cations. (Reuss et al., 1997). However, the permeability of TRPL for Ca2+ is only about 4 times higher that for Na+ (Liu et al., 2007). In this way, TRPL contributes significantly to LIC. The ratio of Na+/Ca2+ in the extracellular fluid of the rhabdomere is 120/1.5 mM (Hofstee and Stavenga, 1996) and increases the proportion of Na+ in LIC. Thus, Ca2+ stimulates LIC and the Na+ flux in LIC generates the receptor potential that represents the physiological equivalent of light. The receptor stimulates the release of histamine from the photoreceptor synapse (Gengs et al., 2002) to spread the signal into the visual information computing neuronal network.
The increase in [Ca2+]i that results from the proportion of Ca2+ in the LIC serves many functions determined by the different Ca2+-dependent proteins. We refer the reader to excellent reviews by Hardie and Raghu, Voolstra and Huber or Minke or Montell for more detail (Gutorov et al., 2019; Hardie and Juusola, 2015; Katz and Minke, 2018; Montell et al., 2002; Voolstra and Huber, 2020). Ca2+ modulates the rhodopsin cycle and the chain of interacting proteins in the light transduction cascade. Positive and negative Ca2+ -mediated feedback mechanisms regulate acceleration of the light response, light sensitivity, light adaptation, and termination of the photoreceptor response. Ca2+ also triggers a light-dependent “pupil mechanism” by activating myosin V-dependent migration of pigment granules from the cytosol towards the rhabdomere basement (Satoh et al., 2008). At concentrations lower than 1 μM, Ca2+ accelerates the light transduction cascade by activating PLC, TRP and PKC whereas higher concentrations of Ca2+ inhibit the phototransduction cascade. The rhodopsin cycle includes activation and deactivation of rhodopsin as well as the exchange of retinal isomers. Thus, regulation of TRP activity and its ability to increase intracellular free Ca2+ is of central importance for photoreceptor function.
4.3.3. Regulation of TRP/TRPL activity and its role in photoreceptor function
The plasma membrane of the rhabdomere contains two types of TRP channels, TRP and TRPL. Both have different ion permeability properties and serve different roles in photoreceptor signal transduction. Loss-of-function mutation of TRP has a greater impact onto the photoreceptor potential than TRPL (Gutorov et al., 2019; Hardie and Juusola, 2015; Katz and Minke, 2018; Minke, 2002; Raghu and Hardie, 2009). Different experimental approaches have led to different conclusions and interpretations of the data and the precise sequence of molecular steps remains to be ascertained.
The activity of TRP and TRPL channels in the dark:
Isolated photoreceptors that are prepared and separated from the pigmented cells show spontaneous activity in the dark that requires TRP and TRPL channels (Hardie and Minke, 1994). Hardie and Minke followed the hypothesis that this effect might be due to an inefficient inhibition of TRP and TRPL under metabolic stress. They showed that anoxia or uncoupling of mitochondrial ATP production leads to TRP/TRPL activation in recording from photoreceptor preparations (Agam et al., 2000; Minke and Agam 2003), a conclusion that was supported by Hardie et al. (2003). PKC seems to be one of the critical enzymes for this effect (Agam et al., 2004). A reduction in intracellular ATP leads to reduced PKC activity and subsequent spontaneous active TRP/TRPL in the dark. Further evidence for the role of PKC comes from experiments in which intracellular Ca2+ buffering by BAPTA completely abolished the effects of ATP depletion (Agam et al., 2004). Another pathway for metabolism-induced spontaneous TRP/TRPL activity links PLC activity and TRP/TRPL activation (Hardie et al., 2003). Here, Hardie and colleagues suggest a model in which ATP depletion reduces the turnover of DAG to regenerate PIP2 by DAG kinase. Increased levels of DAG lead to a higher number of active TRP/TRPL. Studies of spontaneous photoreceptor activity in the dark are thus important for the understanding of TRP/TRPL activation in response to light.
The light-dependent activation of TRP:
The light-dependent activation mechanisms for TRP/TRPL determine LIC’s properties, amplitude and kinetics. Several parallel acting pathways serve different functions to activate TRP/TRPL. The initial and basal activation occurs via the lipid metabolism. Initiation of lipid metabolism occurs via light-dependent activation of PLC that cleaves DAG and InsP3 from PIP2 (Hardie, 2003, 2004). DAG itself possibly mediates the excitation of the photoreceptor by activation of TRP/TRPL channels. Evidence of DAG’s role in activating TRP came from a mutation that leads to photoreceptor degeneration in Drosophila. The rdgA gene mutation leads to blindness in Drosophila. Interestingly, a rdgA/trp or a rdgA/norpA (norpA = PLC) double mutant rescue photoreceptors from degeneration (Raghu et al., 2000). Ca2+ that enters the photoreceptor cell can explain the effect (Bloomquist et al., 1988; Hardie et al., 2003). The rdgA codes for DAG kinase that is inactive due to the mutation. This leads to DAG accumulation and subsequent overstimulated TRP channels. In turn, the overstimulated TRP channels lead to toxic overload of photoreceptor cells with Ca2+ with subsequent cell death. Non-functional TRP channels of the Trp mutant rescue the degenerative phenotype in the rdgA/trp double mutant because mutant TRP has lost its Ca2+ permeability (Hardie and Minke, 1992). In same way, the absence of PLC (norpA) prevents DAG accumulation so that DAG kinase activity has no effects on TRP activation (Hardie et al., 2003). How DAG leads to activation of TRP/TRPL is not clear. Application of DAG to freshly isolated rhabdomers evoked a very small response that is in speed and amplitude not comparable to LIC (Delgado et al., 2014; Raghu and Hardie, 2009). Thus, DAG might not directly activate TRP by binding to the protein as is known from mammalian TRP channels. The second activation mechanism might result from its cleavage by DAG lipase. The products of this reaction, poly-unsaturated fatty acids (PUFA), might represent a parallel stimulator for TRP channels (Chyb et al., 1999; Parnas et al., 2009b). However, blocking DAG lipase is not blocking TRP channel activation (Delgado et al., 2014, 2019). Hardie et al. investigated TRP/TRPL activation in situ in photoreceptors from flies carrying a null mutant of PLC (norpA) (Hardie et al., 2003). In this study, TRP channels could be activated by the application of polyunsaturated fatty acids. However, the reaction is much weaker than the light-dependent stimulation of TRP. Thus, PUFA does not necessarily activate TRP channels in a direct manner. Work that is more recent further challenged the role of PUFA. Although Leung et al. showed that the NinaE gene functions as a DAG lipase localizing to the inner segment of the rhabdomere and that reduced levels in the mutant fly leads to abnormal light responses (Leung et al., 2008), the TRP/TRPL can still be activated and the PUFA levels do not change after light stimulation (Delgado et al., 2014). A more recent publication showed that here might be the activating mechanism of polyunsaturated fatty acids. Randall et al. showed that changing the fatty acid composition in the Drosophila photoreceptors with a yeast diet that did not affect PLC activity leads to reduced light sensitivity and slower light-induced contractions (Randall et al., 2015). It might be possible that PUFAs have multiple targets in the photoreceptor cell that overcome the absence of PLC. The experiment with the double mutant rdgA/norpA suggests that PLC activation is essential for TRP activation (Hardie et al., 2003). If DAG can so far not be verified, the PLC-dependent PIP2 depletion represents a major mechanism (Hardie et al., 2004). First, the PLC reaction produces protons that can lead to a significant acidification due to the narrow spaces in the rhabdomere microvilli (Huang et al., 2010). Acidification is a TRP activator. Second, the PIP2 depletion generates mechanical forces in the plasma membrane because after PIP2 degradation the plasma membrane requires less space and proteins move closer to each other. Indeed, atomic-force microscopy reveals light-induced shrinkage of rhabdomere microvilli (Hardie and Franze, 2012). That activates Trp channels but also other ion channels that are involved in the light transduction cascade, such as inward rectifier K+ channels (Hardie and Franze, 2012). Also passively induced mechanical impacts onto the plasma membrane by hypotonic challenge activated TRPL (Parnas et al., 2009a). A recent review by Gutorov et al., (2019) concluded a role of the cholesterol content in the plasma membrane for termination of TRPL channel activity. The mechanism was detected by the effects of the drug methyl--β-cyclodextrin. TRPL channels are fast deactivated by methyl-β-cyclodextrin (Peters et al., 2017). Methyl-β-cyclodextrin leads to extrusion of cholesterol from the plasma membrane and therefore, to disruption of lipid rafts. This further supports the idea of TRP/TRPL activation by changes in the lipid environment of the plasma membrane. Protons and PLC-dependent PIP2 depletion are currently viewed the most important activators of TRP/TRPL (Gutorov et al., 2019; Huang et al., 2010) (Fig. 6).
Fig. 6. Activation of TRP/TRPL channels in the rhabdomere of the Drosophila compound eye.

(A) Ommatidium of an insect eye represents a single eye of the compound eye; in dark brown the photosensitive cell, the rhabdomere. (B) Light-evoked signal transduction cascade in the microvilli membrane where all compounds of the cascade form a unit through binding with inaD. Light-dependent stimulation of rhodopsin leads to activation of PLC4β through Gαq type G protein. PLC cleaves InsP3 and DAG from the PIP2. This reaction leads to acidification and structural changes in the membrane in which phospholipids come closer and generate a mechanical force; the acidification and the mechanical force lead to activation of TRP/TRPL to generate the light-activated current (LIC) (Created in Biorender.com).
The lipid-based activation mechanisms open in the single-photon response only a few TRP channels that might not be strong enough for a good signal-to-noise ratio to define physiologically relevant photoreceptor response to one photon (Hardie and Juusola, 2015; Hardie and Raghu, 2001; Katz and Minke, 2018). An amplification step is required. (Hardie et al., 2002). The key for this amplification is the Ca2+ that enters the rhabdomere through the TRP channels. At concentrations up to 1 μM Ca2+ is an activator of TRP. In the initial phase of the light-response Ca2+ entering the rhabdomere opens more TRP channels that, in turn lead to more robust Ca2+ rise and depolarization of the photoreceptor. The exact mechanism how Ca2+ activates TRP/TRPL is not clear. As Ca2+ increases activate a variety of kinases it was hypothesized that Ca2+/CaMK increases TRP/TRPL activity by phosphorylation. A recent paper concluded that CamK does not directly activate TRP/TRPL although these channel proteins harbor two CamK binding sites (Chen et al., 2021). A direct Ca2+ effect leading to TRP/TRPL activation is the most likely one. At this stage, Ca2+-dependent dark noise reduction establishing an activation threshold for TRP/TRPL has to be overcome (Chu et al., 2013a; Katz and Minke, 2012). Calculations resulted in estimating 15 TRP channels being activated in the rising phase (first 10–20 ms; quantum bump) of single-photon response (Henderson et al., 2000). Furthermore, PLC shows a comparable Ca2+ dependence to TRP. With increasing intracellular Ca2+ concentrations, the light-evoked PLC activity increases up to 1 μM and decreases at concentrations higher than 1 μM (Running Deer et al., 1995). Thus, Ca2+ not only increases TRP open probability but also fosters PIP2 depletion of the plasma membrane (Voolstra and Huber, 2020). Using computer-based modeling of TRP/TRPL activation, the role of Ca2+ and DAG kinase in the shape of LIC could be simulated (Pumir et al., 2008; Song et al., 2012).
Deactivation of TRP channels and termination of LIC:
The generation of a physiologically relevant signal in response to one photon requires the precise activation of TRP/TRPL channels and the precise termination of the process and the deactivation process depends on the free intracellular Ca2+. Due to the narrow space in the microvillus, even a relatively weak Ca2+ inward current will generate huge Ca2+ concentrations that go up to 200 μM (Oberwinkler and Stavenga, 2000). This high level terminates LIC and puts the photoreceptor into a refractive phase that will last for appr. 100 ms (Oberwinkler and Stavenga, 2000); until Ca2+ levels are decreased by Ca2+ extruding mechanisms such as the Na+/Ca2+ exchanger (Voolstra and Huber, 2020). At high Ca2+ concentration, Ca2+-dependent proteins maintain LIC in a refractive period. First, Ca2+-dependent kinases, CamKII and PKC (due to ninaD being in close proximity of TRP and additionally activated by DAG) become activated at higher Ca2+ concentrations leading to TRP/TRPL inactivation upon channel phosphorylation (Hardie et al., 1993). Through the proximity of PKC and TRP, TRP becomes phosphorylated leading to Ca2+-dependent inactivation (Hardie et al., 1993). Another important kinase is PLC (Balakrishnan et al., 2015; Gu et al., 2005; Hardie et al., 2004; Katz and Minke, 2012, 2018), with comparable Ca2+ sensitivity to TRP channel modulation (Running Deer et al., 1995). At concentrations below 1 μM PLC is activated by Ca2+, whereas at higher concentrations (50 μM), it becomes inhibited. This would increase the PIP2 abundance in the plasma membrane with subsequent TRP/TRPL deactivation. The increase in PIP2 abundance is further supported by DAG kinase (rdgA). The first step to recycle DAG for PIP2 regeneration is the phosphorylation of DAG by DAG kinase so that DAG-P can re-enter the PIP2 synthesis (Balakrishnan et al., 2015; Hardie et al., 2015). Again, the increase of PIP2 in the plasma membrane will reduce TRP/TRPL activity. In the case of inactive DAG kinase (rdgA mutation), refilling of the PIP2 levels is impaired and TRP activity cannot be reduced, leading to photoreceptor degeneration (Sengupta et al., 2013). The Ca2+-dependence of PLC might explain the effect of the TRP mutation (Hardie et al., 2001). The absence of a Ca2+ influx through TRP and the low Ca2+ permeability of TRPL lead to a weak PLC activation and after a low PIP2 depletion of the plasma membrane so that the receptor potential cannot be maintained.
4.4. Regulation of TRP/TRPL channel activation by translocation from the plasma membrane
The above-described short-term regulation of the TRP/TRPL channel activity has limitations in adaptation of the photoreceptor activity to various constant light scenarios. Long-term regulation of TRP/TRPL activity for tuning photoreceptor sensitivity to work under constant light or darkness conditions might occur via modulation of the integration into the inaD complex and by translocation mechanisms that regulate the number of channels in the plasma membrane. Regulation by inaD modulation applies for the TRP channel. As a base, the integration into inaD is a prerequisite for TRP activity (Minke and Parnas, 2006), anchors the TRP channel in the plasma membrane as well as in the signaling complex with all advantages for fast and efficient light transduction (Tsunoda et al., 2001). However, it appears that the inaD complex is a rather dynamic complex that seems to be regulated by light too. Liu et al. (2011) demonstrated that the redox status of inaD changes upon illumination and affects the binding properties of the PDZ domains. The light-induced oxidation of the PDZ domain that binds to TRP results in the release of TRP from the inaD complex. However, although the integration of TRP into inaD is required for its activation, a significant physiological effect for this mechanism is unknown. TRPL is the channel that is regulated by its surface expression (Bahner et al., 2002). In the first few minutes under constant light TRPL is moved away from the “signalplex” to the basement of the rhabdomere and from there TRPL is transported over the next hours in vesicles to the basolateral membrane (Cronin et al., 2006; Oberegelsbacher et al., 2011). TRPL is not subjected to digestion/degradation because the total of TRPL stays constant (Bahner et al., 2002). Rearing in the dark for 2 h redistributes TRPL into the plasma membrane of the rhabdomere (Bahner et al., 2002). The higher number of TRPL channels in the rhabdomere plasma membrane might correlate with a higher sensitivity of the photoreceptors and would therefore serve the dark adaptation. The structural basis is the presence of TRPL (Bahner et al., 2002). Thr TRPL presence is regulated by both the N- and C-terminus of the TRPL channel protein (Richter et al., 2011). The translocation process appears to involve multiple mechanisms that are ignited by the phototransduction cascade (Meyer et al., 2006). The mediator is Ca2+ that enters the cells through TRP channels. The second mechanism is a vesicle bound transportation guided by rab proteins resembling that of rhodopsin distribution (Oberegelsbacher et al., 2011). That also includes the participation of the CULD protein (CUB/LDLa- domain) known for regulation of rhodopsin trafficking in Drosophila. Recently, new work added another important protein for the TRPL translocation. Identification and characterization of protein encoded by the ttd14 gene revealed new information about the TRPL trafficking mechanism (Cerny et al., 2015). ttd14 phenotype includes an impairment of TRPL translocation process. In the light, there is a partial inhibition of TRPL removal from the plasma membrane and failure in redistribution in the dark. The mechanism is not clear but might rely onto binding properties of TTD14 including binding sites for G-proteins and phospholipids. Furthermore, the ttd14 mutant shows retinal degeneration and increased mortality of the larvae so that the TRPL-related phenotype might have explanations that are more complex.
4.5. Summary
The first isolation of a TRP channel gene occurred by investigation of a Drosophila mutation. Since then, the analysis of Drosophila’s TRP channels generated blueprints for the functional understanding of vertebrate TRP channels in general, especially in retinal neurons and glia cells. Still new impacts into the TRP channel research come from Drosophila TRP’s. Thus, we gave this chapter a special emphasis.
5. TRP expression in the photoreceptors
Rod and cone photoreceptors are the primary light-sensitive neurons in vertebrate retinas. The tonic release of the neurotransmitter glutamate at bipolar and horizontal cell contacts is controlled by Ca2+ influx through non-inactivating voltage-dependent Cav1.4 (L-type) Ca2+ channels that act in combination with CICR (release of Ca2+ from ryanodine ER stores), mitochondrial sequestration and SOCE (store-operated calcium entry) (Witkovsky et al., 1997; Križaj et al., 1999; Križaj, 2012; Szikra and Križaj, 2009b; Suryanarayanan and Slaughter, 2006; Thoreson, 2021). Rapid, Cav1.4-dependent release predominates at ribbon sites, while non-ribbon sites manifest the slower, ryanodine receptor-dependent component (Chen et al., 2014).
A fascinating aspect of photoreceptor Ca2+ homeostasis is the dramatic difference in influx, sequestration, and clearance mechanisms in the primary cilium (OS) and the inner segment (IS)/synaptic compartments. OS [Ca2+]i reflects balanced activation of CNG channels and NKCX transporters, in contrast to IS/terminal [Ca2+]i which is maintained by Cav1.4 channels, mitochondrial and ER stores, SOCE and PMCAs (Križaj and Copenhagen, 2002; Križaj, 2005); intersegmental Ca2+ diffusion is prevented by the sequestration barrier within ellipsoid mitochondria (Szikra and Križaj, 2007, 2009). Together, influx and clearance pathways maintain [Ca2+]i within an operating range of 50–700 nM (Sampath et al., 1999; Križaj et al., 2003; Szikra and Križaj, 2006). An interesting challenge, given high-affinity Ca2+clearance mediated by plasma membrane calcium ATPases PMCA1/2 (Križaj and Copenhagen, 1998; Duncan et al., 2006), has been to identify the source of Ca2+ influx under light-saturated conditions when both CNG and voltage-operated Ca2+ channels are closed yet the hyperpolarized cells maintain a large gradient for Ca2+ influx. TRP channels represent potential candidates for this voltage-independent Ca2+ influx (Fig. 7).
Fig. 7. Schematic presentation of a vertebrate photoreceptor.
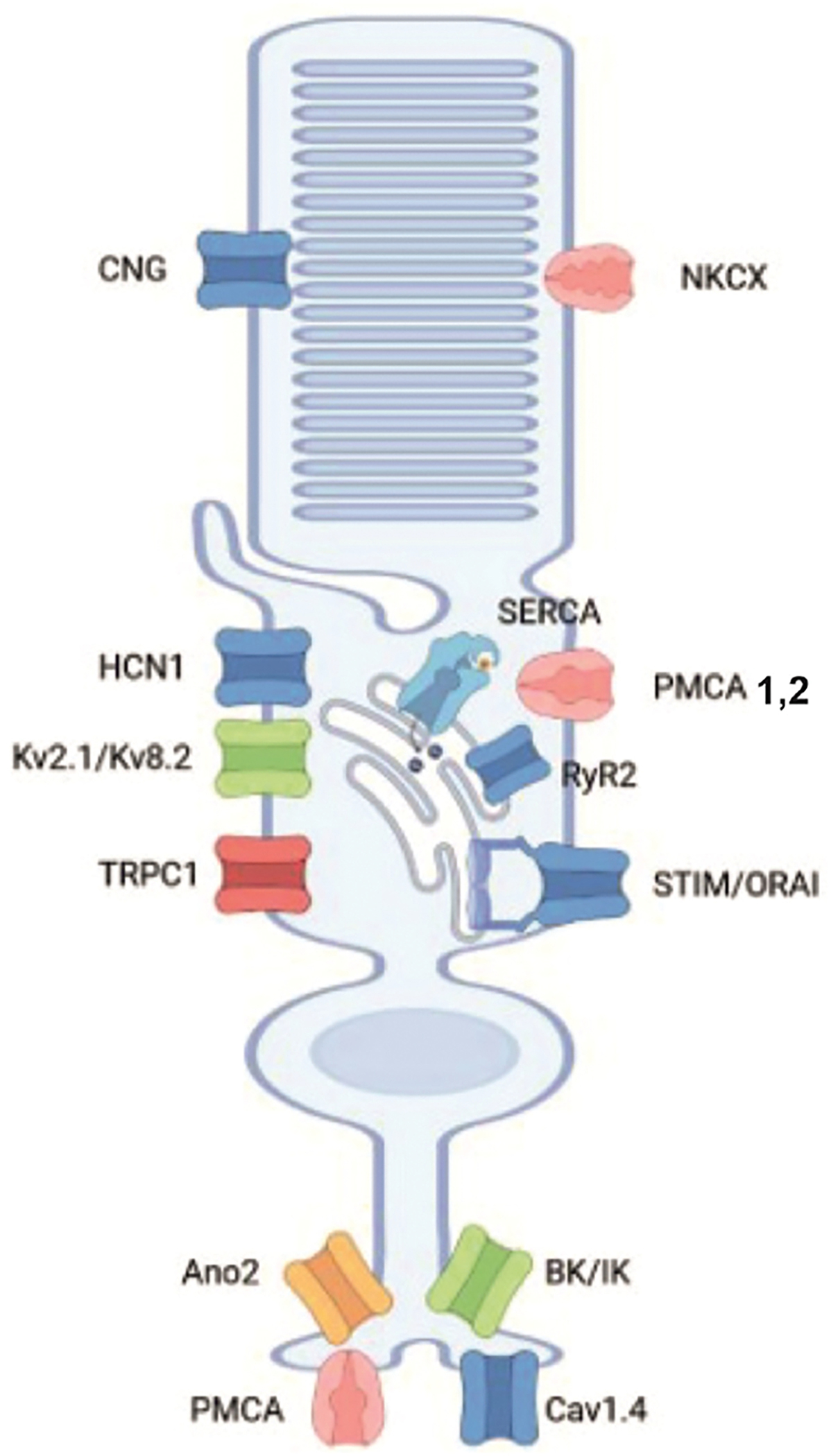
Shown are those ion channels and transporters involved in signal processing and Ca2+ transport in photoreceptors. TRPC1 channels are shown as homomers in the photoreceptor cell body though it seems likely that they exist as heteromers of unknown composition. CNG: cyclic nucleotide-gated cation channel; NKCX: Na+,K+-Ca2+ exchanger; HCN1: hyperpolarization-activated cyclic nucleotide-gated channel 1; SERCA: sarco-/endoplasmic reticulum Ca2+ ATPase; PMCA: plasma membrane Ca2+ ATPase: RyR2: ryanodine receptor 2; STIM: stromal interaction molecule; Ano2: anoctamine 2; BK/IK: Ca2+-activated K+ channels (Created in Biorender.com).
5.1. TRPC channels in the vertebrate photoreceptors
TRPC1 is the vertebrate homolog of the Drosophila TRP gene and the first cloned mammalian TRP gene. Its expression at mRNA and protein levels has been reported in photoreceptors for all species studied so far, including chicken (Crousillac et al., 2003), Ambystoma (Szikra et al., 2008), Xenopus (Bocchero et al., 2020) and mouse (Gilliam and Wensel 2011; Molnar et al., 2012). Taking into account the usual caveats regarding TRP antibody labeling (Gilliam and Wensel, 2011; Ryskamp et al., 2015), TRPC1-ir was detected in frog and salamander rod inner segments, terminals and proximal outer segments, and cone terminals additionally labeled with a TRPC6 antibody (Szikra et al., 2008; Bocchero et al., 2020). In light-saturated cells, voltage-operated Ca2+ channels are closed, and ER stores are largely emptied of Ca2+ (Križaj et al., 2003) while powerful high-affinity PMCA and SERCA pumps continue with the clearance of free Ca2+ from the cytosol (Križaj and Copenhagen, 1998; Duncan et al., 2006). To maintain Ca2+-dependent folding, modification and sorting of newly synthesized proteins and protect the cells from ER stress, the cellular machinery requires maintained access to calcium ions. TRPC may subserve this role in combination with Orai channels. Pharmacological experiments in amphibian photoreceptors implicated TRPC1 in store-operated Ca2+ entry (SOCE) and maintenance of baseline calcium levels in inner segments of light-saturated cells (Szikra et al., 2008, 2009). Hence, TRPC1 activation could plausibly counterbalance cytosolic [Ca2+]i decreases caused by prolonged saturation of the phototransduction cascade. We and others also found that ryanodine stores and TRPCs in amphibian rods and cones contribute to neurotransmission under certain conditions (Suryanarayanan and Slaughter, 2006; Szikra et al., 2008, 2009; Chen et al., 2014). While TRPC channels may modulate calcium signaling in all photoreceptor compartments in amphibians, this does not seem to be the case in mammalian rods and cones. Trpc1 and Trpc3 genes are strongly expressed in the mouse outer nuclear layer, yet their ablation did not affect scotopic and photopic ERG b-waves. We interepret this result as indicating that TRPC1 and TRPC3 channels do not contribute to phototransduction and kinetics of the light response in mouse rods (Molnar et al., 2012). It remains to be determined whether TRPCs directly interact with Orai channels and the STIM1 depletion sensors and whether activation of TRPC1 relies on the presence of functional Orai channels.
5.2. TRPML channels in the vertebrate photoreceptors
With in situ hybridization experiments TRPML1 (also known as MCOLN1) a member of the mucolipin subfamily was detected in photoreceptors as another member of the TRP channels (Gilliam and Wensel 2011). Mutations in TRPML1 cause mucolipidosis type IV (Sun et al., 2000), a neurodegenerative disorder with severe clinical manifestations (Goldin et al., 2008; Venugopal et al., 2007). The TRPML1−/− knock out mouse displays prominent thinning of the photoreceptor layer, suggesting a role for TRPML, a lysosomal protein, in postnatal development of photoreceptors (Grischuk et al., 2016). In a rat model of retinal degeneration caused by retinal detachment the subretinal injection of the TRPML1 agonist ML-SA1 attenuated photoreceptor degeneration, possibly due to reduction in the production of reactive oxygen species (Yan et al., 2021). As intracellular channels located to late endosomes and lysosomes (Venkatachalam et al., 2006), TRPML1 may participate in redox signaling. It should be mentioned that other studies provide evidence that the retinal degeneration caused by TRPML1 mutations may be attributed to a possible localization of the channel to lysosomes of the retinal pigment epithelium (Coblentz et al., 2013; Gómez et al., 2018).
5.3. TRPV channels in the vertebrate photoreceptors
A fascinating recent study has implicated TRPC1 (and Piezo) channels in the regulation of the frog rod photoresponse and in mechanical and light-induced OS deformations (Bocchero et al., 2020). Calcium imaging and whole-cell recording suggest that TRP channels (predominantly TRPC1, TRPV4 and possibly TRPV2) contribute to Ca2+ influx into amphibian (salamander, frog) photoreceptor OSs (Bocchero et al., 2020; Pang et al., 2021; Lapajne et al., 2022). Their function may include regulation of retinomotor movements and potentially phototransduction. Salamander (Ambystoma tigrinum) rod OSs indeed show temperature-, hypotonicity- and pressure-evoked inward currents (Pang et al., 2021) and respond to TRPV4 agonists with [Ca2+]i elevations (Lapajne et al., 2022). In addition, immunohistochemistry showed expression of TRPV1 and TRPV2 members of the vanilloid subfamily in synaptic terminals and outer segments of photoreceptors in goldfish and zebrafish retinas (Zimov and Yazulla 2004).
5.4. Summary
TRP channel expression in invertebrate, teleost, amphibian and mammalian photoreceptors shows substantial differences in the type of expressed subtype, and channel localization. A good example is TRPC1 – its fruit fly homolog mediates phototransduction; TRPC1 was suggested to mediate mechanotransduction and store-operated signals in amphibians and the channel is strongly expressed in mouse rods (Molnar et al., 2012) but without known function.
6. TRP channel expression in RGCs
Retinal ganglion cells (RGCs) are projection neurons that transfer visual information from the retina to at least 50 midbrain areas (Martersteck et al., 2017). The >30 types of RGCs have distinct patterns of dendritic stratification, excitatory/inhibitory input, rod/cone input, and axonal projections. Their output integrates glutamatergic (AMPA- and NMDA receptor-mediated) inputs from presynaptic bipolar cells, inhibition by amacrine (glycinergic and GABAergic) cells and their modulation by dopaminergic, purinergic, and peptidergic retinal circuits. RGCs appear uniquely sensitive to mechanical stressors, with modest (3–5 mm Hg) increases in IOP sufficient to dramatically impact RGC firing and survival (Grüsser et al., 1989; Della Santina et al., 2013; El-Danaf and Huberman, 2015). Chronic IOP elevations, prolonged hypoxia, and inflammatory stress increase the risk of neurodegeneration and blindness by injuring RGCs (Almasieh et al., 2012; Križaj, 2019). Literature shows disagreements concerning the functional subpopulations affected by mechanical stress, yet it has become clear that even brief periods of ocular hypertension are sufficient to result in dose-dependent pruning of dendrites and apoptosis (Della Santina et al., 2013; Ou et al., 2016; Feng et al., 2013; El-Danaf and Huberman, 2015; Risner et al., 2018). RGC excitability can be time-dependently enhanced (Risner et al., 2018) or suppressed (Della Santina et al., 2013) by mechanical stress. The unique sensitivity to IOP, chemical and temperature inputs suggests that the cells express transducer molecules that are absent or minimally expressed in other retinal neurons. TRP subunits that are known to be important in neuronal sensing of touch, pain, temperature, osmolarity, pheromones, and taste, such as TRPV4, are indeed strongly expressed in RGCs (Gilliam and Wensel, 2011; Jo et al., 2017) (Figs. 9 and 10) and have been linked to light-independent sensory transduction (Ryskamp et al., 2011; Križaj, 2016; Jo et al., 2017; Lakk et al., 2017). TRP channel-mediated integration of synaptic and intrinsic light responses with thermal, chemical, and mechanical inputs (e.g., body temperature, endocannabinoids, IOP and ion gradients) indicates that retinal output reflects an integrated response to multiple types of sensory stimuli (Križaj, 2016; Lapajne et al., 2022).
Fig. 10. Expression and function of vanilloid TRP channels in retinal ganglion cells.
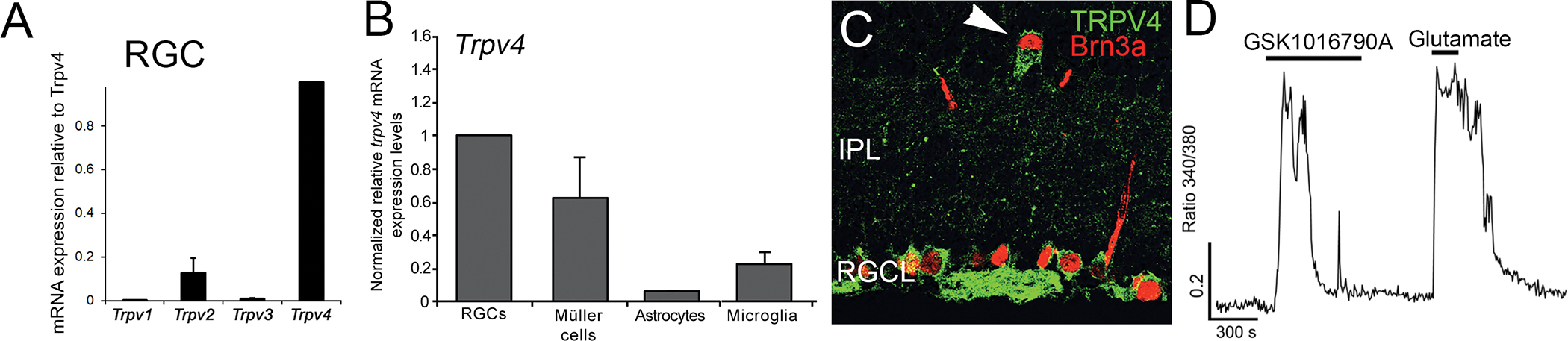
(A) Relative expression of main vanilloid Trp genes in mouse RGCs. (B) Comparison of Trpv4 transcript abundance between RGCs, Müller glia, astrocytes and microglia shows prominent signal in RGCs. (C) Immunolabeling of mouse retinas for TRPV4 (green) and Brn3a (red) shows the channel is expressed in most RGCs, including the subpopulation of displaced cells in the proximal IPL. (D) TRPV4-mediated [Ca2+]i signals in RGCs are large – comparable to responses obtained with saturating concentrations of glutamate.
6.1. Rhabdomeric phototransduction by TRPC6/7 channels
One of the most exciting developments in retinal biology over the past two decades has been elucidating molecular pathways that mediate light-evoked signaling in intrinsically photosensitive RGCs (ipRGCs). A landmark study from King Wai Yau’s group (Xue et al., 2011) conclusively identified DAG-sensitive TRPC6 and TRPC7 channels as obligatory mediators of the light-evoked current downstream from melanopsin, a light-sensitive rhabdomeric (R-)opsin. A first clue was obtained in a study in which melanopsin, co-expressed with TRPC3, impelled light sensitivity to HEK293 cells (Melyan et al., 2005). The observation suggested that DAG-sensitive TRP channels mediating the light response in fruit fly photoreceptors might contribute to mammalian vision as transducers of the ipRGC light response. Consistent with this, Trp transcript analyses in purified cell preparations (Hartwick et al., 2007) and immunohistochemical studies localized multiple TRPC signals to the mouse IPL and ipRGCs (Warren et al., 2006; Sekaran et al., 2007; Witkovsky et al., 2008) but identification of the transducer was confounded by the absence of phenotypes in TRPC3−/− and TRPC7−/− mice, questionable antibody selectivity and reduced but not absent, light responses in TRPC6−/− cells (Gilliam and Wensel, 2011; Perez-Leighton et al., 2011). The issue was resolved by Xue et al. (2011), who reported that the light response is obliterated in double KO (TRPC6/7−/−) ipRGCs. It is now clear that light-evoked excitation in mouse ipRGCs reflects cation influx through heteromultimeric channels composed of (interchangeable) TRPC6 and TRPC7 subunits.
A detailed overview of ipRGC phototransduction models is beyond the scope of this review. Still, it is worth noting that rodent ipRGCs include at least 6 classes that comprise 3% of the total RGC population and have been categorized based on process stratification, projection, melanopsin expression, cell body size and receptor fields into M1-M6 subpopulations (Contreras et al., 2021) whereas the human transcriptome so far seems to be limited to a single, M1-like, population (Mure, 2021). TRPC6/7 transduction characterizes light-evoked signaling in mouse M1 cells, Brnb3-negative cells that stratify in the OFF sublamina and project to non-image-forming regions of the visual system associated with circadian photoentrainment, sleep induction and the pupillary reflex (Xue et al., 2011; Sonoda et al., 2018). M1 cells are the only RGC type with high levels of Trpc6/7 expression (Tran et al., 2019). Neonatal mice lacking TRPC6/7 show reduced photosensitivity in M1 but not other subtypes of RGCs and fail to show photoaversion (Caval-Holme et al., 2022). The M1 population thus appears to constitute a separate information channel for transferring information from the retina to the midbrain. Similar to the Drosophila phototransduction pathway described in Section 6, the ipRGC signaling cascade involves R-opsin, transactivation of a Gq/11 -like protein, PLCβ4 -mediated hydrolysis of membrane phosphatidylinositol-4,5-bisphosphate (PIP2), and generation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) lipid messengers (Fig. 8). M1 TRPC6/7 channels are probably gated via PIP2 hydrolysis (Itsuki et al., 2014) downstream from Gαq, Gα11, or Gα14 (Xue et al., 2011; Hughes et al., 2015; Jiang et al., 2018). Consistent with this, the ipRGCs light response is potentiated by membrane-permeant analogs of DAG that activate TRPC3/6/7 channels (Warren et al., 2006, but see Graham et al., 2008).
Fig. 8. TRP signal transduction in intrinsically photosensitive retinal ganglion cells (ipRGC).

(A) M1 ipRGC: Fast reacting M1 ipRGC’s signal cascade starts with melanopsin (Opn4) that after perception of a photon activates Gαq/11 G protein that in turn leads to activation of PLCβ4 (PLC). PLCβ4 cleaves PIP2 into InsP3 and DAG, a reaction step that leads to activation of TRPC6/7 channels and/or inhibition of a background conductance K+ channel (depending on the light intensity). As a result, the cell depolarizes and subsequenctly voltage-operated Ca2+ channels (VOCC) become active leading to increased action potential rates. The link betweem PLCβ4 activation and ion channel modulation is not clear. (B) M2 ipRGC: an ipRGC that reacts with slower amplitude and kinetics, using similar signaling cascade as the M1 ipRGC; participation of the K+ channel (TASK1 or HCN) is not shown. M2 melanopsin activates an unknown Gα? to activate HCN channels and depolarize the cell. (Created in Biorender.dom).
M2 ipRGCs stratify in the ON sublamina, project to SCN and LGN, and utilize HCN (hyperpolarization-activated cyclic nucleotide-gated) channels in parallel with the TRPC6/7 rhabdomeric pathway for the light response (Jiang et al., 2018). Cyclic nucleotide-dependent signaling in these cells appears independent from PLCβ1-4−/− and is insensitive to Ruthenium Red, a nonselective TRP blocker. How PLCβ1-4-dependent TRPC mechanisms interact with PLCβ1-4-independent pathways in ipRGCs is not well understood.
M4 ipRGCs are monostratified contrast-sensitive cells that form ON αRGC mosaics, project to the LGN and contribute to visual acuity tracking. Similar to M1s, M4 phototransduction relies on a Gq-coupled PLC, which increases the cells’ excitability by closing a TASK-like two-pore potassium (K2P) leak conductance (Sonoda et al., 2018) or a HCN conductance (Jiang et al., 2018). The I-V relationship of the light-evoked current becomes more linear in TRPC 3/6/7 KO retinas, which display impaired responsiveness in the presence of bright light (Sonoda et al., 2018). TASK vs. TRPC6/7 signaling might regulate M4 responses under scotopic vs. photopic conditions. To the best of our knowledge, transduction mechanisms and projection pathways in M3, M5, and M6 cells that mediate chromatic opponency (UV-green) and other ipRGC modalities remain unknown, and different transduction pathways may exist across species. There is an ongoing debate concerning the redundancy of PLC, G proteins, K+ channels, TRPs and intracellular Ca2+ store components, and it is unclear whether PIP2 is required for the closing of TRPC6/7 channels as shown in other preparations.
Immunolabeling for TRPC3, TRPC6, and TRPC7 subunits suggests that DAG-sensitive canonical TRPs are expressed across the IPL/RGCL in non-photosensitive classes of RGC and presumably in amacrine cells (Wang et al., 2010; Witkovsky et al., 2008). The channels could play a role in a wide range of molecular contexts that engage Gαq/11 -coupled receptors but their functions remain to be ascertained.
6.2. TRPV1 signaling, RGC excitability and glaucoma
TRPV1 was among the first TRP channels studied in the retina and remains one of the most widely studied if little understood isoforms in the retinal TRP armamentarium. TRPV1 expression has been reported in teleost (Zimov and Yazulla, 2004; Nisembaum et al., 2022), mouse (Weitlauf et al., 2014; Jo et al., 2017; Lakk et al., 2017; Risner et al., 2020), rat (Nucci et al., 2007; Leonelli et al., 2009; Sappington et al., 2009), vervet monkey (Bouskila et al., 2020, 2021) and human (Sappington et al., 2015) RGCs. Analyses of the effects of TRPV1 agonists and inhibitors and TRPV1−/− phenotypes in ex vivo and intact retinas (Ryskamp et al., 2014b) are consistent with an assortment of TRPV1 functions in mammalian RGCs.
TRPV1-expressing neurons in the central mouse RGCL are mostly displaced amacrine cells, with the density of TRPV1-expressing RGCs increasing towards the periphery (Jo et al., 2017). Vervet monkeys show a similar expression pattern to mouse, with the strongest signal observed in the peripheral retina (Bouskila et al., 2020). Ca2+ imaging studies in mouse showed capsaicin responsiveness in ~20% of dissociated mouse RGCs (Jo et al., 2017). Similarly, TRPV1-lineage cells in transgenic Trpv1Cre:Ai9 and Trpv1Cre:AAV−Flex-tdTomato reporter mice exhibited TRPV1 signals in 20–40% of total RGCs (Jo et al., 2017), with ~36% of mouse RGCs that immunolabel for the intermediary filament SMI-32 (i.e., often used as a marker for the alpha ganglion cell subtype that can be recognized by large cell bodies, monostratified dendritic arbors, and fast conducting axons) also expressing TRPV1 (Lakk et al., 2018). Roughly 70% of RGCs immunolabeled for SMI-32 in human retinas express Trpv1 mRNA (Sappington et al., 2015), indicating the channel is confined to a subpopulation of αRGCs. It remains to be seen whether TRPV1 localization and expression in human RGCs differs from their mouse counterparts, as recently shown for human and rodent corneal epithelial cells (Lapajne et al., 2020). The overall levels of mouse RGC Trpv1 mRNA compared to cognate Trpv2, Trpv4, and Trpv6 expression are minuscule (Gilliam and Wensel, 2011; Lakk et al., 2018). Nonetheless, capsaicin evokes [Ca2+]i signals in a significant cohort of RGCs, indicating that biologically meaningful expression is mediated by a low density of channels. Knocking out the Trpv4 gene does not produce compensatory changes in Trpv1 expression and does not affect the amplitude or kinetics of TRPV4-evoked signals (Lakk et al., 2018). Thus, TRPV1 does not form an obligatory complex with the more abundant TRPV4 subunit.
Analyses of TRPV1 signaling in mouse αRGCs by the Calkins laboratory (Risner et al., 2020) brought insights into how the channel shapes neuronal excitability, dendritic remodeling and axonal function in a known glaucoma-susceptible RGC subpopulation. Deletion of the Trpv1 gene reduced the somatic area, dendritic length and complexity of sustained αON and αOFF cells. TRPV1 knockdown did not affect spontaneous activity in ON cells but reduced it in OFF αRGCs (Risner et al., 2020), suggesting involvement of a signaling pathway that is tonically active in the dark. Firing of TRPV1−/− sustained αON-S RGC increased in the presence of light, suggesting that TRPV1 activity suppresses RGC excitation. TRPV1−/− sustained αOFF-S RGCs similarly showed decreased firing (Risner et al., 2020), but it remains unclear whether these responses include non-autonomous contributions from adjacent TRPV1-expressing amacrine and bipolar cells, integration of bipolar inputs in ON vs. OFF sublaminae, or endocannabinoid inputs. ON TRPV1−/− cells from eyes with elevated IOP show increased depolarization and light-evoked spiking due to upregulation of axonal NaV1.6 channels (McGrady et al., 2020). This effect disappeared by 4 weeks of ocular hypertension (Weitlauf et al., 2014). Another interesting TRPV1−/− phenotype involves reduced dendritic pruning and deterioration of IPL synapses in hypertensive (Risner et al., 2021) relative to wild-type retinas (Della Santina et al., 2013; El-Danaf and Huberman, 2015; Feng et al., 2013; Ou et al., 2016). The neuroprotective effect of TRPV1 during mechanical stress was suggested to involve the Akt/mTOR pathway (Sakamoto et al., 2017). In contrast to the somatic anti-excitotoxic effect of TRPV1 deletion, TRPV1−/− optic nerves in mouse glaucoma models showed accelerated degeneration (Ward et al., 2014) together with an increase in the amplitude of the optic nerve compound action potential (CAP) and augmented expression of voltage-gated Na+ channels (McGrady et al., 2020). It remains to be seen whether the vulnerability of TRPV1−/− axons reflects axon-intrinsic gain-of-function effects or loss-of-function due to altered signaling in ONH astrocytes, microglia and endothelial cells. It will also be interesting to ascertain the biophysical properties of the TRPV1-mediated current in RGCs, its biological agonists, binding-modulatory partners, targeting into lipid raft domains, and activity-dependent trafficking and potential parallels to cortical counterparts, which were extensively documented to modulate synaptic transmission via presynaptic and postsynaptic modulation (Bialecki et al., 2020).
In contrast to the protective role of TRPV1 in living mice, 1-day exposure of cultured rat RGCs to 70 mm Hg hydrostatic pressure induced apoptosis that was suggested to be driven by TRPV1-mediated Ca2+ influx (Sappington et al., 2009, 2015). Use of hydrostatic pressure as a mechanical modulator of biological responses in RGCs and astrocytes has been questioned (Lei et al., 2011; Osborne et al., 2015) and thus the precise mechanism of TRPV1-dependence of RGC apoptosis remains to be ascertained. High-IOP DBA-2J eyes show increased TRPV1-immunoreactivity in RGC dendrites (Sappington et al., 2009), with mechanical stress in RGC cultures associated with ~12-fold increase in Trpv1 mRNA expression (Sappington et al., 2015). Ischemia-reperfusion (Nucci et al., 2007) and optic nerve transection (Leonelli et al., 2009), in contrast, reduced TRPV1 expression, indicating that the gene is under stringent modulatory control by the external milieu. Cells that overexpress TRPV1 channels generally do not respond to pressure steps with mechanosensitive currents (Nikolaev et al., 2019) and we were unable to discern direct roles for TRPV1 activation in RGCs stimulated with stretch, shrinking, or pressure (Ryskamp et al., 2011; A Jo, O. Yarishkin, and D. K., unpublished data). TRPV1 channels in wild-type mouse RGCs cannot be activated by cell shrinking (Ryskamp et al., 2011; Jo et al., 2017), suggesting that the cells express the full-length protein variant. However, iPSC -derived human RGCs may express the truncated N-terminal variant, as indicated by TRPV1 sensitivity to hyperosmotic stress (Hsu et al., 2019; Zaelzer et al., 2015).
Similar to TRPV4, TRPV1 in RGCs almost certainly behaves as a molecular integrator of chemical, mechanical, heat and nociceptive stimuli that may include sensitivity to noxious (>43 °C) temperature, acid pH, and a wide assortment of lipids - endocannabinoids (“endovanilloids” anandamide (AEA; N-arachidonoylethanolamine) and N-arachidonoyl dopamine, N-oleoyl dopamine)), 2-arachidonoylglycerol (2-AG), arachidonic acid metabolites (12(S)-hydroxyglutaric acid, 12-hydroxyhexanedienic acid and 20-hydroxyeicosatetraenoic acid (20-HETE)), lipoxygenase products, monoacylglycerols and retinoids (Nilius and Szallasi, 2014). Their function may include detection of local inflammation (Marinelli, 2017), regulators of intracellular ion concentration (Miraucourt et al., 2016), synaptic plasticity (Egaña-Huguet et al., 2021) and sensing the local modulatory milieu. RGCs co-express (ionotropic) TRPV1 and (metabotropic) CB1 receptors, which are simultaneously stimulated by activity-dependent release of endocannabinoids anandamide (AEA) and 2-AG (Ryskamp et al., 2014b; Bouchard et al., 2016). AEA elevates [Ca2+]i directly via TRPV1 activation, whereas 2-AG lowers the amplitude of AEA-induced [Ca2+]i elevations through CB1R-dependent lowering of [cAMP]i (Jo et al., 2017). This suggests a sequential mechanism whereby activity-dependent endocannabinoid release within the bipolar-amacrine-RGC circuit results in transient TRPV1-mediated excitation followed by sustained 2-AG-mediated activation of CB1R- and Gi-dependent pathways (Fig. 9).
Endocannabinoids may simultaneously suppress presynaptic release and stimulate postsynaptic TRPV1 channels (Middleton and Protti, 2011; Middleton et al., 2019; Jo et al., 2017). An investigation of spontaneous RGC firing by Protti’s group additionally found that endocannabinoids augment the (amacrine-mediated inhibitory) surround of ON-S RGCs (Middleton et al., 2019), suggesting that endocannabinoids regulate the postsynaptic signal gain through parallel yet independent modulation of receptive field center and surround mechanisms. The calcium-dependent modulation might accentuate contrast sensitivity while preserving the response bandwidth of the bipolar-RGC synapse (Middleton et al., 2019). In addition, analogy with sensory and central neurons (Alessandri-Haber et al., 2009) predicts roles for TRPV1 in adaptive potentiation/suppression of excitatory/inhibitor balance (Egaña-Huguet et al., 2021), inflammatory sensitization that reflects intra-retinal release of prostaglandins, ATP, TNFα, bradykinin and protons. Another intriguing candidate for TRPV1 activation are retinoids that are continually recycled by Müller glia. The vitamin A metabolite 9-cis retinoic acid has been implicated, for example, in TRPV1-dependent induction of neuronal sensitivity and neurogenic inflammation (Yin et al., 2013).
6.3. RGC TRPV4 signaling, volume regulation and pathology
Mouse RGCs show the following vanilloid expression pattern: Trpv4>Trpv2>Trpv1 = Trpv3 (Lakk et al., 2018) (Fig. 10A). TRPV4 expression has been reported in teleost (salmon; Nisembaum et al., 2022), tiger salamander (D. Ryskamp, D. K., unpublished observations), rat (Sappington et al., 2015; Li et al., 2021a,b), pig (Taylor et al., 2017), non-human primate (Gao et al., 2019) and human (Ryskamp et al., 2011) RGCs. In general, expression seems to be strongest in cells with large somata (Ryskamp et al., 2011; Lakk et al., 2018; Gao et al., 2019). TRPV4-ir signals in cell bodies and proximal dendrites are more pronounced compared to distal dendrites (Ryskamp et al., 2011; Lakk et al., 2018) (Fig. 10C), whereas axons are labeled by some but not all TRPV4 antibodies. Consistent with the absence of TRPV4-ir in the distal retina (Ryskamp et al., 2011), global ablation of TRPV4 channels has no effect on retinal ERG a- and b-waves, and shows a minimal effect on oscillatory potentials (Yarishkin et al., 2018). This points at RGCs as the main generators of retinal neuronal TRPV4 activity.
Exposure to TRPV4 agonists such as GSK1016790A (Fig. 10D) and phorbol esters evokes a nonselective inward cationic current, promotes excitability and elevates [Ca2+]i in rodent and primate RGCs (Ryskamp et al., 2011, 2014; Lakk et al., 2018; Gao et al., 2019). In contrast to sustained TRPV4 activation in non-excitable cells (Jo et al., 2015; Phuong et al., 2016; Lapajne et al., 2020; Redmon et al., 2021), TRPV4 channels in RGCs activate with rapid onset and show significant inactivation/desensitization. The attendant increase in [Ca2+]i is similarly transient (Ryskamp et al., 2014a; Lakk et al., 2018). The differences in activation between retinal neuronal and glial TRPV4 channels may reflect cell-type-specific engagement of the PLA2-EET cascade and differential roles of Ca2+-dependent inactivation.
Swelling-induced TRPV4 activation in RGCs occurs at Δ > 30 mOsm, suggesting that the RGC TRPV4 channels are not sensitive to osmotic gradients under physiological conditions. Because the RGC response desensitizes in continuous presence of hypotonic stress (Ryskamp et al., 2011; Lakk et al., 2018; Toft-Bertelsen et al., 2019), TRPV4 channels are unlikely to significantly contribute to cells’ volume regulation. Sensitivity to stretch may be augmented during excessive swelling in glaucoma, ischemia and diabetes (Reichenbach and Bringmann, 2010; Pinar-Sueiro et al., 2011).
A hereditary channelopathy caused by missense variants of the TRPV4 gene produces a dominant autosomal phenotype associated with loss of hearing and blindness (Thibodeau et al., 2017). TRPV4 mutations have also been associated with debilitating sensory and motor neuropathies that include hereditary motor and sensory neuropathy type 2C (also known as Cgarcot Marie Tooth type 2C), scapuloperoneal spinal muscular atrophy and congenital distal spinal muscular atrophy (Echaniz-Laguna et al., 2014; Nilius and Szallasi, 2014). It remains to be seen whether high TRPV4 expression in αRGCs (Ryskamp et al., 2011; Lakk et al., 2018) predisposes them to injury from mechanical stress. It is worth noting that TRPV4 agonists induce RGC apoptosis (Ryskamp et al., 2011) whereas TRPV4 inhibition and gene deletion improve neuronal survival in pig (Taylor et al., 2017) and mouse (Matsumoto et al., 2018) models of retinal detachment.
6.4. Open questions regarding TRP isoforms in RGCs
TRPV2.
Analysis of thermoTRP transcripts in mouse RGCs showed relatively abundant Trpv2 expression (Fig. 9A). Antibody labeling in rodent retinas was inconclusive (Gilliam and Wensel, 2011; Leonelli et al., 2009).
TRPC1.
In situ hybridization shows moderate Trpc1 expression in all nuclear layers of the mouse retina, including RGCL (Molnar et al., 2012).
TRPC5.
TRPC5 may suppress the length of RGC axons in developing retinas, whereas its antagonist clemizole enhances axonal outgrowth (Oda et al., 2020). How the channel is activated under physiological conditions is unknown.
TRPM3.
Antibody staining (Brown et al., 2015), reporter expression (Hughes et al., 2012), and functional assays (Brown et al., 2015) documented TRPM3 expression in a subset of RGCs. Altered pupillary light reflex in TRPM3-null mice under photopic and scotopic conditions (Hughes et al., 2012) predicted expression in ipRGCs yet a TRPM3 promoter-driven reporter did not co-localize with melanopsin (Hughes et al., 2012). Synthetic TRPM3 agonists evoke calcium responses and retinal waves in developing retinas but TRPM3 KO mice have normal ERGs (Brown et al., 2015).
TRPA1 is a redox-sensitive channel activated by the mustard oil component allyl isothiocyanate (AITC) and by intense cooling. It has been localized to RGCs from chick, mouse and human retinas (Araújo et al., 2020). We find that dissociated adult RGCs respond to AITC with large [Ca2+]i elevations that are comparable to the effects of saturating concentrations of glutamate (Fig. 10). Deletion and pharmacological inhibition of TRPA1 attenuated the increase in the apoptosis biomarker, active caspase-3, reduced retinal cell death and preserved retinal tissue thickness in retinal models of oxidative stress (Araújo et al., 2020) whereas TRPV1−/− and TRPV4−/− retinas showed no difference from controls.
TRPM8.
Literature search shows no studies on TRPM8 channel expression in the retina. Our pilot experiments (A. JO and D.K.) demonstrate that TRPM8-selective agonists such as icillin evoke large calcium signals in a subset of RGCs (Fig. 11).
Fig. 11. RGCs functionally express thermoTRP channels.

Calcium imaging. Background-subtracted 340/380 ratio of calcium signals in Fura-2 indicator-loaded mouse RGCs. The TRPA1 channel agonist allyl isothiocyanate (AITC) and TRPM8 agonist icillin induce [Ca2+]i elevations comparable in size to the signal induced by glutamate. Data acquired by A. Jo. Inset: Schematic of temperature optima of thermoTRP channels known to be expressed in mammalian RGCs (Created in Biorender.com).
6.5. Summary: multisensory transduction and thermosensing in RGCs
TRP channels are emerging as important non-synaptic regulators of RGC signaling, survival and output. They regulate cell swelling (TRPV4; Ryskamp et al., 2011, 2014b, 2015; Toft-Bertelsen et al., 2019), response to mechanical stress (TRPC1, TRPV1; TRPV4; Sappington et al., 2009; Molnar et al., 2016), membrane lipids (TRPV1/4; Ryskamp et al., 2014b, 2016), cholesterol (Lakk et al., 2017), endocannabinoids (TRPV1; TRPV4; Jo et al., 2017); Ca2+ store repletion (TRPC1, TRPC6; Da Silva et al., 2008; Szikra et al., 2009; Molnar et al., 2016) and transduction of the ipRGC light response (TRPC6/7; Xue et al., 2011). It is unclear to which extent TRP subunits heteromerize to form functional channels. For example, ~10% of RGCs coexpress TRPV1 and TRPV4 subunits (Lakk et al., 2018), and Sappington et al. (2015) showed that TRPV4 antibodies co-immunoprecipitate TRPV1 in a rat RGC preparation (Sappington et al., 2015). Several TRPs, including TRPV1-4, A1, C6, and M3 may sensitize RGCs to pathological stressors whereas others (TRPC1, TRPV1) might be protective in other contexts (Molnar et al., 2016; Risner et al., 2020). Although the retina is isothermic and not expected to experience thermal shifts, it is remarkable that RGCs express most if not all “thermoTRP” channels that cover the spectrum of temperature activations from <10 °C (TRPA1) to > 40 °C (TRPV1, TRPV2) (Fig. 10). Transcriptionwise, the dominant channel is TRPV4, with the activation optimum around the body temperature (Jo et al., 2017), with the overall pattern of transcription similar to dorsal root and trigeminal ganglion neurons in which thermoTRP channels engage in multiple modes of sensory transduction (Clapham, 2003).
7. TRPM channels in retinal bipolar cells
Two TRPM subunits with reliable detection in mammalian retinas are TRPM1 and TRPM3 (Brown et al., 2015; Gilliam and Wensel, 2011; Ribelayga et al., 2010). TRPM3 locates to the inner plexiform layer, close to OFF system synapses. The ERG b-wave of Trpm3−/− mice is normal, supporting a role for ganglion cell function (Brownet al., 2015). In contrast, analysis of TRPM1 in the retina has led to a quantum jump in our understanding of visual information processing (Almutairi et al., 2021; Koike et al., 2010a; Oancea and Wicks, 2011). The TRPM1 gene produces two isoforms, TRPM1-L (long form = full-length protein predicted from the gene) and TRPM-S (short form = N-terminal truncated form) (Duncan et al., 1998; Fang and Setaluri, 2000; Koike et al., 2010b). TRPM1-S localizes to the inner plexiform layers and during embryonic development to the RPE. All known TRPM1 splicing variants can produce ionic currents including TRPM1-S (Oancea et al., 2009) and presumably contribute to the membrane conductance in these structures. The ion channel encoded by TRPM1-L is expressed by ON bipolar cells and responsible for the signal inversion from light-induced hyperpolarization of photoreceptors into depolarization of ON bipolar cells.
7.1. TRPM1 channels and an old problem in bipolar cell functions
Vision starts with light-dependent activation of photoreceptors. Parallel processing by the neuronal network that is activated by photoreceptors identifies essential elements of visual information such as color, shape or movements (Boycott and Wassle, 1999; Wassle, 2004; Wassle and Boycott, 1991). The parallel processing starts with the bipolar cells, which consists of two basic functional types, the ON and the OFF cells. ON bipolar cells mediate the light signal by depolarizing in response to glutamate released from photoreceptors. TRPM1 was identified as the ON bipolar transduction channel following a pioneering study of the spotting coat pattern in Appaloosa horses (Bellone et al., 2008; Koike et al., 2010a; Schneider et al., 2015). Expression of the TRPM1 channel in melanocytes determines the coat pattern in these animals (Devi et al., 2013; Guo et al., 2012; Hwang et al., 2016; Jia et al., 2020).
Bellone et al. (2008) noted that the horses suffer from congenital stationary night-blindness, sparking investigations by several research groups in retinas across species. They localized the TRPM1 channel to ON bipolar cells. (Gilliam and Wensel, 2011; Klooster et al., 2011; Koike et al., 2010a, 2010b; Morgans et al., 2009, 2010). Heterologous expression of TRPM1 reveals that this is a non-selective cation channel that conducts Ca2+, shows a double rectification in current-voltage plots and leads to cell depolarization (Koike et al., 2010b; Lambert et al., 2011; Oancea et al., 2009). TRPM1 deficient mice showed a no-b-wave phenotype in the scotopic ERG (Koike et al., 2010b; Morgans et al., 2009; Shen et al., 2009) together with an intact a-wave response (rod activation) and loss of transduction currents in ON- but not OFF bipolar cells (Koike et al., 2010b; Morgans et al., 2009). Genetic analyses in humans revealed that mutations in the TRPM1 gene represents a primary cause for the complete form of CSNB1 (Almutairi et al., 2021; Audo et al., 2009; Li et al., 2009; Malaichamy et al., 2014; Nakamura et al., 2010; van Genderen et al., 2009; Zeitz et al., 2015). Thus, the TRPM1 channel in ON bipolar cells mediates the long-sought mechanism that translates light-dependent rod hyperpolarization into ON excitation.
7.2. Mechanisms of TRPM1 activation by glutamate receptor deactivation
Subcellular localization analysis of TRPM1 in bipolar cells provided the first clues into the underlying signaling cascade that drives the light-dependent ON bipolar depolarization. TRPM1 co-localizes with the Gαo, G-protein regulators the metabotropic glutamate receptor mGluR6 in bipolar dendritic tips (Cao et al., 2009; Dhingra et al., 2008; Klooster et al., 2013; Koike et al., 2010a, 2010b; Križaj et al., 2010; Morgans et al., 2010). Localization requires rod photoreceptor activity (Križaj et al., 2010). TRPM1 expression and localization in mice take place following the eye opening. Three papers from Scott Nawy implicated a non-selective CNG cation channel in mGluR6-dependent activation of Gαo (Nawy, 1999; Nawy and Jahr, 1990, 1991). mGluR6, RGS7 (regulator of G protein signaling 7) or RGS11 deficient mice also show loss or reductions in the scotopic b-wave (Cao et al., 2012; Chen et al., 2010; Koyasu et al., 2008; Mojumder et al., 2009; Shim et al., 2012). A hypothetic final pathway could be developed via heterologous expression experiments using CHO or HEK293 cells as an expression system. Heterologous expression of TRPM1 leads to small currents so the deeper biophysical/electrophysiological characterization of TRPM1 was possible but difficult (Koike et al., 2010b; Lambert et al., 2011; Oancea et al., 2009; Shen et al., 2012). The group of Oberwinkler (Lambert et al., 2011) found an approach to record currents from the TRPM1 gene product heterologous expression strong enough for biophysical analysis. The group found that exon 11 in the TRPM1 gene is not present in the TRPM3 gene, which shows normally robust current in heterologous expression. Lambert et al. (2011) removed exon 11 from the TRPM1 gene and could measure currents from this gene product that permitted an electrophysiological and pharmacologic characterization. The group of Furukawa (Koike et al., 2010b) mimicked the situation of signaling proteins in CHO cells via heterologous expression of TRPM1L, mGluR6. Koike et al. al (Koike et al., 2010b). showed suppression of TRPM1 currents by glutamate via a Gαo-dependent mechanism. Finally, the group showed in inside-out patch recordings a decrease in the TRPM1 single-channelopen probability when purified Gαo protein was applied from the intracellular side of the membrane patch (Koike et al., 2010a, 2010b). Thus, a possible signal cascade in ON bipolar might look as follows: the light-dependent depolarization of ON bipolar cells occurs by deactivation of mGluR6 receptor and subsequent reduction in the number active Gαo proteins that are suppressors of the TRPM1 channel.
The above constructed model for the signaling cascade bases onto data from heterologous expression systems, from expression in cell types that are not neurons. The question is whether this model applies also to the in vivo situation of bipolar cells. Both mGluR6 and the TRPM1 knockout show loss of the ERG b-wave (Koike et al., 2010b; Koyasu et al., 2008; Morgans et al., 2009). Electrophysiological recordings from ON bipolar cells revealed that the loss of b-wave is caused by the inability of these cells to react with hyperpolarization (Koike et al., 2010b; Morgans et al., 2009). The role of G protein-dependent signaling is also substantiated by the RGS7 and RGS11 knockout models (Cao et al., 2009, 2012; Chen et al., 2010; Shim et al., 2012). The reduction of ERG b-waves but also reduced ON bipolar activity in single cell recordings generated a consistent picture of the role of G protein signaling in light-dependent depolarization of ON bipolar cells. RGS7 and/or RGS11 knockout phenotypes are more complex than those of TRPM1 and mGluR6 deficient mice. The b-waves are not fully absent but are reduced or delayed, possibly involving compensatory upregulation. At the far end is the inhibition of TRPM1 by G protein subunits. ON bipolar cells of Gαo knockout mice show absent glutamate induced currents (Dhingra et al., 2000; Okawa et al., 2010). Okawa et al. (2010) used knockout mouse models of splice variants of the Gαo proteins: Gαo1 and Gαo2. The group recorded single flash responses from ON bipolar cells and found that deletion of a single splice variant did not disrupt the ON bipolar response but seem to regulate the ON bipolar cell’s sensitivity to light stimuli. The study by Dhingra et al. (2002) investigated the two Gαo splice variants in specific knockout models at the level of single flash Ganzfeld ERG and concluded that only Gαo1 is the critical mediator for the ON bipolar light response. However, it is to mention that Dhingra et al. used only three light intensities, one scotopic, one mesopic and one photopic response. So, the authors did not analyze the luminance response curve that might have provided information about the sensitivity of the b-wave response. Furthermore, the a-wave response in the mesopic response was smaller, indicating effect of the Gαo1 knockout at the photoreceptor level. Shen et al. showed that intracellular dialysis of ON bipolar cells with Gβ1γ2 dimers reduced the TRPM1 currents (Shen et al., 2012). Using transgenic approaches that enable a valid ON bipolar cell identification by GFP showed that ON bipolar cells display a specific expression of Gβ and Gγ protein expression: Gβ3, Gβ4 and Gγ13 (Dhingra et al., 2008; Huang et al., 2003). Indeed, a Gγ13 knockout mouse displays a reduced b-wave (Ramakrishnan et al., 2015). This group also concluded by co-localization experiments that the combination of Gβ3γ13 is the primary Gβγ dimer in the light signaling cascade in ON bipolar cells.
7.3. TRPM1 forms a signaling complex in ON bipolar cells
With more genes identified that lead to congenital stationary night blindness new properties of TRPM1 channels in ON bipolar cells appeared. These properties would permit a better understanding of the mechanisms that lead to congenital stationary night blindness and would integrate the function of the products of genes that are mutated in disease. Surprisingly, these data lead to conlcusoin that the TRPM1 signaling pathway is arranged in a signaling complex that resembles to the one known in Drosophila photoreceptors. The protein that has permitted a new insight is nyctalopin. Mutations in the nyctalopin coding gene cause congenital stationary night blindness (Bech-Hansen et al., 2000; Zeitz et al., 2003). Nyctalopin is expressed in ON bipolar cells (Morgans et al., 2006; O’Connor et al., 2005). Co-immunoprecipitation experiments showed that TRPM1, mGluR6 and nyctalopin are parts of a signaling complex in ON bipolar cells (Cao et al., 2011; Pearring et al., 2011). Via the binding nyctalopin anchors this complex to the plasma membrane either by a transmembrane region (rodents) or by a GPI anchor (O’Connor et al., 2005; Zeitz et al., 2003). mGluR6 itself forms complexes with a transmembrane G protein-coupled receptor GPR179 (Orlandi et al., 2013, 2018); a receptor with so far unknown ligand that is required for the ON bipolar cell light response and its gene can cause stationary night blindness (Klooster et al., 2013; Peachey et al., 2012). More important for the TRPM1 activity is the ability of GPR179 also to form complexes with RGS7 and RGS11 that modulate the light transduction cascade in ON bipolar cells. As GPR179 might regulate the mGluR6 sensitivity (Klooster et al., 2013) it might modulate the G protein dependent inhibition of TRPM1.
7.4. Summary and open questions
Negative regulation of TRPM1 generates the light response of ON bipolar cells. The unsolved issues include the need to characterize TRPM1 channel properties: pharmacology, potential multimeric channel proteins and the confounding issue of the effects of TRPV1 modulators. The antagonist capsazepine blocked both capsaicin- and glutamate- elicited cation conductance of ON bipolar cells yet TRPV1 knockout mice show normal ERG (Shen et al., 2009). Heterologously expressed TRPM1 channels were unaffected by capsaicin but inhibited by capsazepine (Lambert et al., 2011; Schneider et al., 2015). The difference between native TRPM1 and heterologously expressed channels was ascribed to the unique protein environment in ON bipolar cells (Lambert et al., 2011; Schneider et al., 2015). In contrast to other TRPM channels, TRPM1 lacks an amino acid sequence that permits formation of homotetramers (Agosto et al., 2014; Tsuruda et al., 2006).
The interaction between the G protein and TRPM1 is under debate. Gαo knockdown abolished the b-wave and ON bipolar light response (Dhingra et al., 2000, 2002; Okawa et al., 2010) and intracellular application of Gαo in ON bipolar cells demonstrated the inhibition of bipolar cell cation current (Nawy, 1999). However, expression profiles and heterologous expression suggest that the Gβγ dimer may have similar function to Gαo (Ramakrishnan et al., 2015; Shen et al., 2012). Xu et al. (2016) showed that Gαo and the Gβ3γ13 dimer bind to TRPM1 to synergistically modulate TRPM1 activity. Identification of the G protein binding domain might resolve the issue.
Another novel mechanism involves protein kinase Cα (PKCα) modulation. ON bipolar cells are the retina’s only cell type expressing PKCα (Ruether et al., 2010; Xiong et al., 2015). PKCα knockout mice show comparable b-wave amplitudes to wild-type mice, however, the b-wave is characterized by delayed rise-time and decay (Ruether et al., 2010; Xiong et al., 2015). PKCα is thus likely to enhance the ON bipolar light response, possibly by relieving the Mg2+ block of TRPM1 (Rampino and Nawy, 2011). Another unexplored mechanism involves protein kinase Cα (PKCα) modulation. ON bipolar cells are the only cell type in the retina that expresses PKCα (Ruether et al., 2010; Xiong et al., 2015). PKCα knockout mice show comparable b-wave amplitudes to wildtype mice, however, the b-wave is characterized by delayed rise-time and decay (Ruether et al., 2010; Xiong et al., 2015). PKCα is thus likely to enhance the ON bipolar cell light response, possibly by relieving the Mg2+ block of TRPM1 (Rampino and Nawy, 2011).
8. TRP expression in horizontal and amacrine cells
Horizontal and amacrine cells are responsible for the initial signal processing within the retina. Through feed-back (cone - horizontal cell – cone - bipolar cell) and feed-forward inhibition (cone -horizontal cell – bipolar cell) at the first synapse, horizontal cells influence the activity of photoreceptors and bipolar cells. The feedback inhibition is mediated by either acidification of the synaptic cleft, or the ephaptic influence of connexins. Bipolar cells may be inhibited by GABA release from horizontal cells through GABAC receptors. The role of TRP channels is almost entirely unknown. There have been no functional studies, with antibody labeling suggesting potential TRPV1 expression in monkey horizontal cells (Bouskila et al., 2020).
Amacrine cells have been categorized, depending on the species, into 23 to 63 subtypes (Shekhar and Sanes 2021). Accordingly, they were proposed to fulfill a range of different functions. A systematic screen of TRP channels in the mouse retina attributed a positive signal for TRPC5 to amacrine cells (Gilliam and Wensel 2011). Various TRPC subunits were identified in primary cultures and retina slices from chicken (Sosa et al., 2002; Crousillac et al., 2003; Maddox et al., 2018). These have been implicated in NO dependent GABA release by amacrine cells (Maddox et al., 2018). Analysis of transgenic TRPV1-tdT retinas shows ubiquitous TRPV1 expression in regular and displaced mouse amacrine cells (Jo et al., 2017). Overall, the data concerning the involvement of TRP channels in both horizontal and amacrine cells appear to be sparse yet a substantial role for TRP channels in amacrine signaling seems likely and we suggest that further investigation of TRP channel signaling in horizontal and amacrine cells would be of considerable benefit for the understanding of retinal signal processing.
9. Summary: the multimodal potential of TRP channels in retinal research
Multimodal activation of TRP channels represents a gateway to understanding how the retina integrates light-evoked and light-independent information. TRP channel research has generated breakthroughs in understanding key retinal and biological functions such as circadian photoentrainment, non-image-forming vision, pupillary light reflex, ON bipolar transmission, mechanotransduction, and regulation of retinal barriers (Fig. 12). Multimodality of TRP channel activation undergirds signaling pathways that drive pathological remodeling in glaucoma, diabetes, macular degeneration, mucolipidosis together with reactive gliosis, remodeling of retinal circuits and altered outer/inner barrier permeability. Understanding these mechanisms will aid the development of conceptual and translational strategies to restore vision by targeting specific TRP isoforms. This will take investment and time. TRP channels in the fruit fly rhabdomere were the first TRP with a defined function, yet it took more than 15 years to gain insight into their activation mechanism, which continues to be investigated to this day. Many questions remain regarding the signal transduction mechanism in different types of ipRGC, rod bipolar cells, cone ON bipolar cells, and the role of TRPs in biomechanical milieu sensing by Müller glia, microglia, retinal and optic nerve head astrocytes, RPE and endothelial cells. We note that the biological functions of the large majority of known TRP channels in the retina and the eye remain to be explored and defined. The biology of retinal TRP channels is thus likely to remain a productive area of research for years to come.
Fig. 12. Summarizing overview of the TRP channel expression in the different cell types of the retina.

TRP family members written in capitals refer to evidence for protein expression and/or functional evidence; those in italics refer to gene expression data only; question marks indicate involvement of TRP channel subtypes with open questions. (created in Biorender.com).
Acknowledgements
Supported by: DK: National Institutes of Health (R01EY027920, R01EY031817, P30EY014800), ALSAM-Skaggs Foundation, Rankin-Stauss Foundation, USAMRAA and unrestricted support from Research to Prevent Blindness to the Moran Eye Institute at the University of Utah. OS: Deutsche Forschungsgemeinschaft DFG STR/480 and SFB 699, Einstein Foundation Berlin; ProRetina.
Abbreviations
- AAV
adeno-associated virus
- 2-AG 2
arachidonoylglycerol
- 2- APB
2-aminoethoxydiphenyl borate
- AEA
endocannabinoids anandamide
- AKT
a serine/threonine protein kinase = protein kinase B
- AMPA
aminomethylphosphonic acid
- ANK
ankyrin
- AQP
aquaporin
- ARPE19
RPE cell line
- AT1
angiotensin-2 receptor type 1
- ATRAP
angiotensin-receptor associated protein ATP adenosine triphosphate
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N’-tetraacetic acid = Ca2+ chelator BRB blood retina barrier
- C3a, C5a
complement reaction products - anaphylatoxins
- CaM
calmodulin
- CaMKII
calmodulin kinase II
- CAP
optic nerve compound action potential
- CB1R
cannabinoid receptor 1
- CHO
Chinese hamster ovary cells
- cGMP
cyclic guanosine monophosphate
- CICR
Ca2+-induced Ca2+ release
- CNG
cyclic nucleotide gated
- CNS
central nervous system
- CRAC
Ca2+ release-activated channel
- CRAC/CARC
cholesterol recognition/interaction amino acid consensus
- CULD
C1r/C1s, Uegf, Bmp1 domain/Low Density Lipoprotein Receptor Class A domain
- CYP450
cytochrome P450
- DAG
diacyl glycerol
- ECM
extracellular matrix
- EET
epoxygenase-derived eicosanoids
- ERG
electroretinogram
- Fura-2
Ca2+-sensitive fluorescence dye
- GABA
γ-Aminobutyric acid
- GFAP
green fluorescent protein
- GPCR
G protein coupled receptor
- GPR179
G protein coupled receptor 179
- HCN
channel hyperpolarization-activated cyclic nucleotide-gated cation channel
- HEK293
human embryonic kidney cells 293
- HETE
hydroxyeicosatetraenoic acids
- HMGB1
high-mobility-group-protein B1
- IGF-1
insulin-like growth factor-1
- IL-1, -6, -8
interleukin-1, -6, -8
- ILM
inner limiting membrane
- inaC
inactivation no afterpotential C
- inaD
inactivation no afterpotential D
- InsP3
inositol-1,4,5-trisphosphate
- IOP
intraocular pressure
- IPL
inner plexiform layer
- ipRGC
intrinsically photosensitive retinal ganglion cell JAK2 Janus kinase 2
- Kv2.1; 8.2
voltage-gated K+ channel 2.1 or 8.2
- LCN
lateral cerebellar nucleus
- LGN
lateral geniculate nucleus
- LIC
light activated current
- L-type
low voltage-activated, long lasting Ca2+ channel subtype
- MβCD
methyl-β-cyclodextrin
- MCP1
monocyte chemoattractive protein-1
- mGluR1, 6
metabotropic glutamate receptor-1, -6
- ninaE
neither inactivation nor afterpotential
- E mTOR
mammalian Target of Rapamycin
- NKCX
Na+,K+-Ca2+ exchanger
- NMDA
N-methyl-D-aspartate
- NMDG+
N-methyl-D-glucamine
- NO
nitrogen mono oxide
- norpA
no receptor potential A
- OLM
outer limiting membrane
- OPL
outer plexiform layer
- QMMuC1
Müller cell line
- PACSIN
3 protein kinase C and casein kinase II substrate
- PAR2
protease-activated receptor 2
- PDZ
post-synaptic density protein 95, disc large tumor suppressor 1, zona occludens1
- PI3-kinase
phosphoinositide-3-kinase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PKC
proteinkinase C
- PLA2
phospholipase-A2
- PLC
phospholipase C
- PLCβ4
phospholipase Cβ4
- PMCA
plasma membrane Ca2+ ATPase
- PNS
peripheral nervous system
- PUFA
poly-unsaturated fatty acids
- rdgA
retinal degeneration A
- rdgC
retinal degeneration C
- RGC
retinal ganglion cell
- RGS7, 11
regulator of G-protein signaling 7, 11
- RPE
retinal pigment epithelium
- RT-PCR
reverse transcription polymerase chain reaction
- RVD
regulatory volume decrease
- SCN
suprachiasmatic nucleus
- SERCA
sarcoplasmic Ca2+ ATPase
- SOCE
store-operated Ca2+ entry
- SNP
single-nucleotide polymorphism
- STAT
signal transducers and activators of transcription
- STZ
streptozocin
- STIM1
stromal interaction molecule 1
- TASK
tandem pore domain K+ channels
- TNFα
tumor necrosis factor alpha
- TRP
transient receptor potential
- TRPC
canonical transient receptor potential channel
- TRPL
L-subtype of Drosophila’s TRP channel
- TRPM
melastatin subtype of transient receptor potential channel
- TRPN
transient receptor potential channel/nompC subtype
- TRPP
polycystin subtype of transient receptor potential channel
- TRPV
vanilloid-subtype of transient receptor potential channel
- ttd14
GTP- and phospholipid-binding protein
- VEGF-A
vascular endothelial growth factor-A
Data availability
Data will be made available on request.
References
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS, 1997. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron 18, 881–887. [DOI] [PubMed] [Google Scholar]
- Acharya NK, Qi X, Goldwaser EL, Godsey GA, Wu H, Kosciuk MC, Freeman TA, Macphee CH, Wilensky RL, Venkataraman V, Nagele RG, 2017. Retinal pathology is associated with increased blood-retina barrier permeability in a diabetic and hypercholesterolaemic pig model: beneficial effects of the LpPLA2 inhibitor Darapladi. Diab Vasc Dis Res 14 (3), 200–213. [DOI] [PubMed] [Google Scholar]
- Agam K, Frechter S, Minke B, 2004. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium 35, 87–105. [DOI] [PubMed] [Google Scholar]
- Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B, 2000. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J. Neurosci. 20, 5748–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto MA, Zhang Z, He F, Anastassov IA, Wright SJ, McGehee J, Wensel TG, 2014. Oligomeric state of purified transient receptor potential melastatin-1 (TRPM1), a protein essential for dim light vision. J. Biol. Chem. 289, 27019–27033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agte S, Pannicke T, Ulbricht E, Reichenbach A, Bringmann A, 2017. Two different mechanosensitive calcium responses in Müller glial cells of the Guinea pig retina: differential dependence on purinergic receptor signaling. Glia 65, 62–74. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Chen X, Levine JD, 2009. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 29 (19), 6217–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A, 2012. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2, 152–181. [DOI] [PubMed] [Google Scholar]
- Almutairi F, Almeshari N, Ahmad K, Magliyah MS, Schatz P, 2021. Congenital stationary night blindness: an update and review of the disease spectrum in Saudi Arabia. Acta Ophthalmol. 99 (6), 581–591. [DOI] [PubMed] [Google Scholar]
- Araújo D, De Logu F, Adembri C, Rizzo S, Janal MN, Landini L, Magi A, Mattei G, Cini N, Pandolfo P, Geppetti P, Nassini R, Calaza KDC, 2020. TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death Dis. 15 (8), 633, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo Zamarripa D, Noguez Imm R, Bautista Cortes AM, Vazquez Ruiz O, Bernardini M, Fiorio Pla A, Gkika D, Prevarskaya N, Lopez-Casillas F, Liedtke W, Clapp C, Thebault S, 2017. Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: diabetic milieu makes a difference. Sci. Rep. 7, 13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Said S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Leveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS, Zeitz C, 2009. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 85, 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A, 2002. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron 34, 83–93. [DOI] [PubMed] [Google Scholar]
- Balakrishna S, Song W, Achant AW, Doran SF, Liub B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE, 2014. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 307, L158–L172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan SS, Basu U, Raghu P, 2015. Phosphoinositide signalling in Drosophila. Biochim. Biophys. Acta 1851, 770–784. [DOI] [PubMed] [Google Scholar]
- Barabas P, Augustine J, Fernández JA, McGeown JG, McGahon MK, Curtis TM, 2020. Ion channels and myogenic activity in retinal arterioles. Curr. Top. Membr. 85, 187–226. [DOI] [PubMed] [Google Scholar]
- Barash S, Suss E, Stavenga DG, Rubinstein CT, Selinger Z, Minke B, 1988. Light reduces the excitation efficiency in the nss mutant of the sheep blowfly Lucilia. J. Gen. Physiol. 92, 307–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro-Soria R, Stindl J, Muller C, Foeckler R, Todorov V, Castrop H, Strauss O, 2012. Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin- receptor-associated-protein and TRPV2 channel. PLoS One 7, e49624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG, 2000. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat. Genet. 26, 319–323. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Gómez NM, Lu W, Campagno KE, Nabet B, Albalawi F, Lim JC, Boesze-Battaglia K, Mitchell CH, 2018. Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels. Sci. Rep. 8 (1), 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B, 2008. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus). Genetics 179, 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Siegfried CJ, Shiels A, 2014. Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One 4 (8), e104000, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED, Barres BA, 2018. A combination of ontogeny and CNS environment establishes microglial identity. Neuron 27 (6), 1170–1183, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry- Moghaddam M, 2011. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Izquierdo-Serra M, Sepúlveda RV, Rubio-Moscardo F, Doñate-Macián P, Serra SA, Carrillo-Garcia J, Perálvarez-Marín A, González-Nilo F, Fernández-Fernández JM, Valverde MA, 2017. Structural determinants of 5’,6’-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci. Rep. 7 (1), 10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki J, Werner A, Weilinger NL, Tucker CM, Vecchiarelli HA, Egaña J, Mendizabal-Zubiaga J, Grandes P, Hill MN, Thompson RJ, 2020. Suppression of presynaptic glutamate release by postsynaptic metabotropic NMDA receptor signalling to pannexin-1. J. Neurosci. 40 (4), 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL, 1988. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54 (5), 723–733. [DOI] [PubMed] [Google Scholar]
- Bocchero U, Falleroni F, Mortal S, Li Y, Cojoc D, Lamb T, Torre V, 2020. Mechanosensitivity is an essential component of phototransduction in vertebrate rods. PLoS Biol. 18, e3000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, 1985. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 26, 1659–1694. [PubMed] [Google Scholar]
- Bollimuntha S, Cornatzer E, Singh BB, 2005. Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells. Vis. Neurosci. 22, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard JF, Casanova C, Cécyre B, Redmond WJ, 2016. Expression and function of the endocannabinoid system in the retina and the visual brain. Neural Plast, 9247057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskila J, Bleau M, Micaelo-Fernandes C, Bouchard JF, Ptito M, 2021. The vertical and horizontal pathways in the monkey retina are modulated by typical and atypical cannabinoid receptors. Cells 13 (11), 3160, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskila J, Micaelo-Fernandes C, Palmour RM, Bouchard J-F, Ptito M, 2020. Transient receptor potential vanilloid type 1 is expressed in the horizontal pathway of the vervet monkey retina. Sci. Rep. 10, 12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B, Wassle H, 1999. Parallel processing in the mammalian retina: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 40, 1313–1327. [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A, 2006. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25, 397–424. [DOI] [PubMed] [Google Scholar]
- Brown JE, Blinks JR, 1974. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J. Gen. Physiol. 64, 643–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Xiong WH, Peters JH, Tekmen-Clark M, Strycharska-Orczyk I, Reed BT, Morgans CW, Duvoisin RM, 2015. TRPM3 expression in mouse retina. PLoS One 10, e0117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C, Annamalai B, Abdusalamova K, Reichhart N, Huber C, Lin Y, Jo EAH, Zipfel PF, Skerka C, Wildner G, Diedrichs-Möhring M, Rohrer B, Strauß O, 2017. Anaphylatoxins activate Ca2+, Akt/PI3-kinase, and FOXO1/FoxP3 in the retinal pigment epithelium. Front. Immunol. 15 (8), 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M, 2012. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7, e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA, 2009. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J. Neurosci. 29, 9301–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA, 2012. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc. Natl. Acad. Sci. U. S. A. 109, 7905–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Posokhova E, Martemyanov KA, 2011. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J. Neurosci. 31, 11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli HC, Guarino BD, Kanugula AK, Adapala RK, Perera V, Smith MA, Paruchuri S, Thodeti CK, 2021. Transient receptor potential vanilloid 4 channel deletion regulates pathological but not developmental retinal angiogenesis. J. Cell. Physiol. 236 (5), 3770–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caval-Holme FS, Aranda ML, Chen AQ, Tiriac A, Zhang Y, Smith B, Birnbaumer L, Schmidt TM, Feller MB, 2022. The retinal basis of light aversion in neonatal mice. J. Neurosci. 42, 4101–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny AC, Altendorfer A, Schopf K, Baltner K, Maag N, Sehn E, Wolfrum U, Huber A, 2015. The GTP- and phospholipid-binding protein TTD14 regulates trafficking of the TRPL ion channel in Drosophila photoreceptor cells. PLoS Genet. 11, e1005578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Križaj D, Thoreson WB, 2014. Intracellular calcium stores drive slow non-ribbon vesicle release from rod photoreceptors. Front. Cell. Neurosci. 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Jin X, Li Q, Wang W, Wang Y, Zhang J, 2013. Association of TRPC1 gene polymorphisms with type 2 diabetes and diabetic nephropathy in Han Chinese population. Endocr. Res. 38 (2), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Shen Z, Asteriti S, Chen Z, Ye F, Sun Z, Wan J, Montell C, Hardie RC, Liu W, Zhang M, 2021. Calmodulin binds to Drosophila TRP with an unexpected mode. Structure 29, 330–344 e334. [DOI] [PubMed] [Google Scholar]
- Chen FS, Shim H, Morhardt D, Dallman R, Krahn E, McWhinney L, Rao A, Gold SJ, Chen CK, 2010. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest. Ophthalmol. Vis. Sci. 51, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevesich J, Kreuz AJ, Montell C, 1997. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 18, 95–105. [DOI] [PubMed] [Google Scholar]
- Chmelova M, Sucha P, Bochin M, Vorisek I, Pivonkova H, Hermanova Z, Anderova M, Vargova L, 2019. The role of aquaporin-4 and transient receptor potential vaniloid isoform 4 channels in the development of cytotoxic edema and associated extracellular diffusion parameter changes. Eur. J. Neurosci. 50 (1), 1685–1699. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Sun D, Jakobs TC, 2015. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 21, 749–766. [PMC free article] [PubMed] [Google Scholar]
- Chu B, Liu CH, Sengupta S, Gupta A, Raghu P, Hardie RC, 2013a. Common mechanisms regulating dark noise and quantum bump amplification in Drosophila photoreceptors. J. Neurophysiol. 109, 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Postma M, Hardie RC, 2013b. Fractional Ca2+ currents through TRP and TRPL channels in Drosophila photoreceptors. Biophys. J. 104, 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC, 1999. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 397, 255–259. [DOI] [PubMed] [Google Scholar]
- Clapham DE, 2003. TRP channels as cellular sensors. Nature 426, 517–524. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G, 2005. International Union of Pharmacology, XLIX: nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev 57, 427–450. [DOI] [PubMed] [Google Scholar]
- Coblentz J, St Croix C, Kiselyov K, 2013. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem. J. 457, 361–368. [DOI] [PubMed] [Google Scholar]
- Contreras E, Nobleman AP, Robinson PR, Schmidt TM, 2021. Melanopsin phototransduction: beyond canonical cascades. J. Exp. Biol. 1 (23), 224 jeb226522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook B, Bar-Yaacov M, Cohen, Ben-Ami ,H, Goldstein RE, Paroush Z, Selinger Z, Minke B, 2000. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat. Cell Biol. 2 (5), 296–301. [DOI] [PubMed] [Google Scholar]
- Cordeiro S, Seyler S, Stindl J, Milenkovic VM, Strauss O, 2010. Heat-sensitive TRPV channels in retinal pigment epithelial cells: regulation of VEGF-A secretion. Invest. Ophthalmol. Vis. Sci. 51, 6001–6008. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A, 1969. Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287. [DOI] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B, 2019. Biophysical principles of ion-channel-mediated mechanosensory transduction. Cell Rep. 29 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Lieu MH, Tsunoda S, 2006. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J. Cell Sci. 119, 2935–2944. [DOI] [PubMed] [Google Scholar]
- Crousillac S, LeRouge M, Rankin M, Gleason E, 2003. Immunolocalization of TRPC channel subunits 1 and 4 in the chicken retina. Vis. Neurosci. 20, 453–463. [DOI] [PubMed] [Google Scholar]
- Cullimore B, Baumann J, Rudzitis CN, Jo AO, Kirdajova D, Križaj D, 2022. Mechanotransduction mechanisms in CNS glia. Neural Reg Res (in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da T, Verkman A, 2004. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Invest. Ophthalmol. Vis. Sci. 45, 4477–4483. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Herron CE, Stevens K, Jollimore CA, Barnes S, Kelly ME, 2008. Metabotropic receptor-activated calcium increases and store-operated calcium influx in mouse Muller cells. Invest. Ophthalmol. Vis. Sci. 49, 3065–3073. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Bonci A, 2019. Region-specific phenotypes of microglia: the role of local regulatory cues. Neuroscientist 25 (4), 314–333. [DOI] [PubMed] [Google Scholar]
- Delgado R, Delgado MG, Bastin-Heline L, Glavic A, O’Day PM, Bacigalupo J, 2019. Light-induced opening of the TRP channel in isolated membrane patches excised from photosensitive microvilli from Drosophila photoreceptors. Neuroscience 396, 66–72. [DOI] [PubMed] [Google Scholar]
- Delgado R, Munoz Y, Pena-Cortes H, Giavalisco P, Bacigalupo J, 2014. Diacylglycerol activates the light-dependent channel TRP in the photosensitive microvilli of Drosophila melanogaster photoreceptors. J. Neurosci. 34, 6679–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO, 2013. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J. Neurosci. 33, 17444–17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z, 1987. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. U. S. A. 84 (19), 6939–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S, Markandeya Y, Maddodi N, Dhingra A, Vardi N, Balijepalli RC, Setaluri V, 2013. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment Cell Melanoma Res 26, 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoldere J, Peynshaert K, De Smedt SC, Remaut K, 2019. Müller cells as a target for retinal therapy. Drug Discov. Today 24, 1483–1498. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N, 2002. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o). J. Neurosci. 22, 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN Jr., Birnbaumer L, Sterling P, Vardi N, 2000. The light response of ON bipolar neurons requires G[alpha]o. J. Neurosci. 20, 9053–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, Vardi N, 2008. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J. Comp. Neurol. 510, 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JR, Kim KJ, Brands MW, Filosa JA, 2019. Augmented astrocyte microdomain Ca2+ dynamics and parenchymal arteriole tone in angiotensin II-infused hypertensive mice. Glia 67 (3), 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X, 2014. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. Faseb. J. 28 (11), 4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW, 1998. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 58, 1515–1520. [PubMed] [Google Scholar]
- Duncan JL, Yang H, Doan T, Silverstein RS, Murphy GJ, Nune G, Liu X, Copenhagen D, Tempel BL, Rieke F, Krizaj D, 2006. Scotopic visual signaling in the mouse retina is modulated by high-affinity plasma membrane calcium extrusion. J. Neurosci. 26, 7201–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Danaf RN, Huberman AD, 2015. Characteristic patterns of dendritic remodeling in early- stage glaucoma: evidence from genetically identified retinal ganglion cell types. J. Neurosci. 35, 2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echaniz-Laguna A, Dubourg O, Carlier P, Carlier RY, Sabouraud P, Péréon Y, Chapon F, Thauvin-Robinet C, Laforêt P, Eymard B, Latour P, Stojkovic T, 2014. Phenotypic spectrum and incidence of TRPV4 mutations in patients with inherited axonal neuropathy. Neurology 82, 1919–1926. [DOI] [PubMed] [Google Scholar]
- Egaña-Huguet J, Saumell-Esnaola M, Achicallende S, Soria-Gomez E, Bonilla-Del Río I, García Del Caño G, Barrondo S, Sallés J, Gerrikagoitia I, Puente N, Elezgarai I, Grandes P, 2021. Lack of the transient receptor potential vanilloid 1 shifts cannabinoid-dependent excitatory synaptic plasticity in the dentate gyrus of the mouse brain Hippocampus. Front. Neuroanat. 7 (15), 701573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart JJ, Xu L, Enyeart JA, 2002. Dual actions of lanthanides on ACTH inhibited leak K(+) channels. Am. J. Physiol. Endocrinol. Metab. 282, E1255–E1266. [DOI] [PubMed] [Google Scholar]
- Fang D, Setaluri V, 2000. Expression and Up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem. Biophys. Res. Commun. 279, 53–61. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhao Y, Yoshida M, Chen H, Yang JF, Kim TS, Cang J, Troy JB, Liu X, 2013. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Invest. Ophthalmol. Vis. Sci. 7 (2), 1106–1117, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Bretillon L, 2010. The ins and outs of cholesterol in the vertebrate retina. J. Lipid Res. 51 (12), 3399–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune B, 2019. Pulling and Tugging on the Retina: mechanical impact of glaucoma beyond the optic nerve head. Invest. Ophthalmol. Vis. Sci. 60, 26–35. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA, 2008. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J. Biol. Chem. 283 (46), 31284–31288. [DOI] [PubMed] [Google Scholar]
- Gao F, Yang Z, Jacoby RA, Wu SM, Pang JJ, 2019. The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell Death Dis. 7 (5), 364, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gees M, Alpizar YA, Luyten T, Parys JB, Nilius B, Bultynck G, Voets T, Talavera K, 2014. Differential effects of bitter compounds on the taste transduction channels TRPM5 and IP3 receptor type 3. Chem. Senses 39 (4), 295–311. [DOI] [PubMed] [Google Scholar]
- Genewsky A, Jost I, Busch C, Huber C, Stindl J, Skerka C, Zipfel PF, Rohrer B, Strauß O, 2015. Activation of endogenously expressed ion channels by active complement in the retinal pigment epithelium. Pflügers Archiv 467 (10), 2179–2191. [DOI] [PubMed] [Google Scholar]
- Gengs C, Leung HT, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL, 2002. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA). J. Biol. Chem. 277 (44), 42113–42120. [DOI] [PubMed] [Google Scholar]
- Gilliam JC, Wensel TG, 2011. TRP channel gene expression in the mouse retina. Vis. Res. 51, 2440–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Deutsch DG, Studholme KM, Zimov S, Yazulla S, 2005. Endocannabinoids in the intact retina: 3 H-anandamide uptake, fatty acid amide hydrolase immunoreactivity and hydrolysis of anandamide. Vis. Neurosci. 22 (6), 693–705. [DOI] [PubMed] [Google Scholar]
- Goldin E, Caruso RC, Benko W, Kaneski CR, Stahl S, Schiffmann R, 2008. Isolated ocular disease is associated with decreased mucolipin-1 channel conductance. Invest. Ophthalmol. Vis. Sci. 49, 3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez NM, Lu W, Lim JC, Kiselyov K, Campagno KE, Grishchuk Y, Slaugenhaupt SA, Pfeffer BA, Fliesler SJ, Mitchell CH, 2018. Robust lysosomal calcium signaling through channel TRPML1 is impaired by lysosomal lipid accumulation. Faseb. J. 32, 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM, 2008. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 99 (5), 2522–2532. [DOI] [PubMed] [Google Scholar]
- Greenberg HZE, Carlton-Carew SRE, Zargaran AK, Jahan KS, Birnbaumer L, Albert AP, 2019. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced relaxations and nitric oxide production in mesenteric arteries: comparative study using wild-type and TRPC1−/− mice. Channels 13 (1), 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk Y, Stember KG, Matsunaga A, Olivares AM, Cruz NM, King VE, Humphrey DM, Wang SL, Muzikansky A, Betensky RA, Thoreson WB, Haider N, Slaugenhaupt SA, 2016. Retinal dystrophy and optic nerve pathology in the mouse model of mucolipidosis IV. Am. J. Pathol. 186, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche A, Hauser A, Lepper MF, Mayo R, von Toerne C, Merl-Pham J, Hauck SM, 2016. The proteome of native adult müller glial cells from murine retina. Mol. Cell. Proteomics 15 (2), 462–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser OJ, Grüsser-Cornehls U, Kusel R, Przybyszewski AW, 1989. Responses of retinal ganglion cells to eyeball deformation: a neurophysiological basis for “pressure phosphenes. Vis. Res. 29, 181–194. [DOI] [PubMed] [Google Scholar]
- Gu Y, Oberwinkler J, Postma M, Hardie RC, 2005. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 15, 1228–1234. [DOI] [PubMed] [Google Scholar]
- Guarino BD, Paruchuri S, Thodeti CK, 2020. The role of TRPV4 channels in ocular function and pathologies. Exp. Eye Res. 201, 108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Carlson JA, Slominski A, 2012. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 21, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutorov R, Peters M, Katz B, Brandwine T, Barbera NA, Levitan I, Minke B, 2019. Modulation of transient receptor potential C channel activity by cholesterol. Front. Pharmacol. 10, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, 1991. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc. Roy. Soc. Lond. B 245203–245210. [Google Scholar]
- Hardie RC, 2003. TRP channels in Drosophila photoreceptors: the lipid connection. Cell Calcium 33, 385–393. [DOI] [PubMed] [Google Scholar]
- Hardie RC, 2004. Regulation of Drosophila TRP channels by lipid messengers, 160–167 Novartis Found. Symp. 258.; discussion 167–171, 263–166. [PubMed] [Google Scholar]
- Hardie RC, 2014. Photosensitive TRPs. Handb. Exp. Pharmacol. 223, 795–826. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Franze K, 2012. Photomechanical responses in Drosophila photoreceptors. Science 338, 260–263. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Gu Y, Martin F, Sweeney ST, Raghu P, 2004. In vivo light-induced and basal phospholipase C activity in Drosophila photoreceptors measured with genetically targeted phosphatidylinositol 4,5-bisphosphate-sensitive ion channels (Kir2.1). J. Biol. Chem. 279, 47773–47782. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Juusola M, 2015. Phototransduction in Drosophila. Curr. Opin. Neurobiol. 34, 37–45. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Liu CH, Randall AS, Sengupta S, 2015. In vivo tracking of phosphoinositides in Drosophila photoreceptors. J. Cell Sci. 128, 4328–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Chyb S, Raghu P, 2003. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J. Biol. Chem. 278, 18851–18858. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P, 2002. Molecular basis of amplification in Drosophila phototransduction: roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron 36, 689–701. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B, 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B, 1994. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J. Gen. Physiol. 103, 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Peretz A, Suss-Toby E, Rom-Glas A, Bishop SA, Selinger Z, Minke B, 1993. Protein kinase C is required for light adaptation in Drosophila photoreceptors. Nature 363, 634–637. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P, 2001. Visual transduction in Drosophila. Nature 413, 186–193. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST, 2001. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30, 149–159. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE, 2007. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J. Neurosci. 5 (49), 13468–13480, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood N, Ta HQ, Zhang A, Charles EJ, Rotar E, Noona S 4th, Salmon M, Daneva Z, Sonkusare SK, Laubach VE, 2022. Endothelial transient receptor potential vanilloid 4 channels mediate lung ischemia-reperfusion injury. Ann. Thorac. Surg. 113 (4), 1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SR, Reuss H, Hardie RC, 2000. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J. Physiol. 524 Pt 1, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstee CA, Stavenga DG, 1996. Calcium homeostasis in photoreceptor cells of Drosophila mutants inaC and trp studied with the pupil mechanism. VisNeurosci 13 (2), 257–263. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Okabe K, Shibasaki K, Funatsu T, Matsuki N, Ikegaya Y, Koyama R, 2018. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J. Neurosci. 38, 5700–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Chien KH, Yarmishyn AA, Buddhakosai W, Wu WJ, Lin TC, Chiou SH, Chen JT, Peng CH, Hwang DK, Chen SJ, Chang YL, 2019. Modulation of osmotic stress-induced TRPV1 expression rescues human iPSC-derived retinal ganglion cells through PKA. Stem Cell Res. Ther. 23 (1), 284, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, et al. , 2004. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741–35748. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC, 2010. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr. Biol. 20, 189–197. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T, 2003. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J. Comp. Neurol. 455, 1–10. [DOI] [PubMed] [Google Scholar]
- Huang W, Xing W, Ryskamp DA, Punzo C, Križaj D, 2011. Localization and phenotype- specific expression of ryanodine calcium release channels in C57BL6 and DBA/2J mouse strains. Exp. Eye Res. 93, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Jagannath A, Hickey D, Gatti S, Wood M, Peirson SN, Foster RG, Hankins MW, 2015. Using siRNA to define functional interactions between melanopsin and multiple G Protein partners. Cell. Mol. Life Sci. 72 (1), 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Pothecary CA, Jagannath A, Foster RG, Hankins MW, Peirson SN, 2012. Profound defects in pupillary responses to light in TRPM-channel null mice: a role for TRPM channels in non-image-forming photoreception. Eur. J. Neurosci. 35 (1), 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E, Lee TH, Lee WJ, Shim WS, Yeo EJ, Kim S, Kim SY, 2016. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell Melanoma Res 29, 81–91. [DOI] [PubMed] [Google Scholar]
- Inman DM, Horner PJ, 2007. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia 55, 942–953. [DOI] [PubMed] [Google Scholar]
- Itsuki K, Imai Y, Hase H, Okamura Y, Inoue R, Mori MX, 2014. PLC-mediated PI (4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. J. Gen. Physiol. 143 (2), 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuso A, Križaj D, 2016. TRPV4-AQP4 interactions ‘turbocharge’ astroglial sensitivity to small osmotic gradients. Channels 10 (3), 172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Hu S, Jiao D, Li X, Qi S, Fan R, 2020. Synaptotagmin-4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca2+ influx via TRPM1 channels. Cell Biochem. Funct. 38, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Yue WWS, Chen L, Sheng Y, Yau KW, 2018. Cyclic-nucleotide- and HCN-channel-mediated phototransduction in intrinsically photosensitive retinal ganglion cells, 175 Cell 18 (3), 652–664. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N, Križaj D, 2015. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal müller glia. J. Neurosci. 35, 13525–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Noel JM, Lakk M, Yarishkin O, Ryskamp DA, Shibasaki K, McCall MA, Križaj D, 2017. Mouse retinal ganglion cell signalling is dynamically modulated through parallel anterograde activation of cannabinoid and vanilloid pathways. J. Physiol. 15 (20), 6499–6516, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Rudzitis CN, Križaj D, 2022. TRPV4 and TRPC1 channels mediate the response to tensile strain in mouse Müller cells. Cell Calcium 5 (104), 102588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I, 1999. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1, 165–170. [DOI] [PubMed] [Google Scholar]
- Katz B, Minke B, 2012. Phospholipase C-mediated suppression of dark noise enables single- photon detection in Drosophila photoreceptors. J. Neurosci. 32, 2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Oberacker T, Richter D, Tzadok H, Peters M, Minke B, Huber A, 2013. Drosophila TRP and TRPL are assembled as homomultimeric channels in vivo. J. Cell Sci. 15 (Pt 14), 3121–3133, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Minke B, 2018. The Drosophila light-activated TRP and TRPL channels - targets of the phosphoinositide signaling cascade. Prog. Retin. Eye Res. 66, 200–219. [DOI] [PubMed] [Google Scholar]
- Katz B, Payne R, Minke B, 2017. TRP channels in vision. In: Emir TLR (Ed.), Neurobiology of TRP Channels, Boca Raton (FL, pp. 27–63. [Google Scholar]
- Keirstead SA, Miller RF, 1995. Calcium waves in dissociated retinal glial (Müller) cells are evoked by release of calcium from intracellular stores. Glia 14 (1), 14–22. [DOI] [PubMed] [Google Scholar]
- Kennedy BG, Torabi AJ, Kurzawa R, Echtenkamp SF, Mangini NJ, 2010. Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in retinal pigment epithelium. Mol. Vis. 16, 665–675. [PMC free article] [PubMed] [Google Scholar]
- Kitchen P, Salman MM, Halsey AM, Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan A, Kreida S, Al-Jubair T, Winkel Missel J, Gourdon P, Törnroth-Horsefield J, Conner MT, Ahmed Z, Conner AC, Bill RM, 2020. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 181 (4), 784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Blokker J, Ten Brink JB, Unmehopa U, Fluiter K, Bergen AA, Kamermans M, 2011. Ultrastructural localization and expression of TRPM1 in the human retina. Invest. Ophthalmol. Vis. Sci. 52 (11), 8356–8362. [DOI] [PubMed] [Google Scholar]
- Klooster J, van Genderen MM, Yu M, Florijn RJ, Riemslag FC, Bergen AA, Gregg RG, Peachey NS, Kamermans M, 2013. Ultrastructural localization of GPR179 and the impact of mutant forms on retinal function in CSNB1 patients and a mouse model. Invest. Ophthalmol. Vis. Sci. 54, 6973–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Numata T, Ueda H, Mori Y, Furukawa T, 2010a. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium 48, 95–101. [DOI] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T, 2010b. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. U. S. A. 107, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, Nishizawa Y, Terasaki H, 2008. Photopic electroretinograms of mGluR6-deficient mice. Curr. Eye Res. 33, 91–99. [DOI] [PubMed] [Google Scholar]
- Križaj D, Copenhagen DR, 2002. Calcium regulation in photoreceptors. Front. Biosci. 7, d2023–d2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, 2005. Compartmentalization of calcium entry pathways in mouse rods. Eur. J. Neurosci. 22, 3292–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, 2012. Calcium stores in vertebrate photoreceptors. Adv. Exp. Med. Biol. 740, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, 2016. Polymodal sensory integration in retinal ganglion cells. Adv. Exp. Med. Biol. 854, 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, 2019. What is glaucoma? In: Kolb H, Fernandez E, Nelson R (Eds.), The Organization of the Retina and Visual System, Salt Lake City (U.T.). University of Utah Health Sciences Center. [PubMed] [Google Scholar]
- Križaj D, 2020. No cell is an island: trabecular meshwork ion channels as sensors of the ambient milieu. In: Samples John R., Knepper Paul A. (Eds.), Glaucoma Research and Clinical Advances, vol. 3. Kugler Publications Amsterdam. [Google Scholar]
- Križaj D, Copenhagen DR, 1998. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron 21 (1), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR, 1999. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J. Neurosci. 19 (17), 7249–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, Lai FA, Copenhagen DR, 2003. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J. Physiol. 547, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, Huang W, Furukawa T, Punzo C, Xing W, 2010. Plasticity of TRPM1 expression and localization in the wild type and degenerating mouse retina. Vis. Res. 50, 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, Rice ME, Wardle RA, Nicholson C, 1996. Water compartmentalization and extracellular tortuosity after osmotic changes in cerebellum of Trachemys scripta. J. Physiol. 1 (3), 887–896, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI, 2014. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr. Eye Res. 39, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Kumar A, Sardar P, Yadav M, Majhi RK, Kumar A, Goswami C, 2015. Influence of membrane cholesterol in the molecular evolution and functional regulation of TRPV4. Biochem. Biophys. Res. Commun. 456 (1), 312–319. [DOI] [PubMed] [Google Scholar]
- Kurth-Nelson ZL, Mishra A, Newman EA, 2009. Spontaneous glial calcium waves in the retina develop over early adulthood. J. Neurosci. 29, 11339–11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF, 2020. Reprogramming müller glia to regenerate retinal neurons. Ann. Rev. Vis. Sci. 6, 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Hoffmann GF, Gorusupudi A, Enyong E, Lin A, Bernstein PS, Toft-Bertelsen T, MacAulay N, Elliott MH, Križaj D, 2021. Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. J. Lipid Res. 62, 100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Križaj D, 2021. TRPV4-Rho signaling drives cytoskeletal and focal adhesion remodeling in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 320, C1013–C1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Vazquez-Chona F, Yarishkin O, Križaj D, 2018. Dyslipidemia modulates Müller glial sensing and transduction of ambient information. Neural Regen Res 13, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Yarishkin O, Baumann JM, Iuso A, Križaj D, 2017. Cholesterol regulates polymodal sensory transduction in Müller glia. Glia 65, 2038–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Drews A, Rizun O, Wagner TF, Lis A, Mannebach S, Plant S, Portz M, Meissner M, Philipp SE, Oberwinkler J, 2011. Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J. Biol. Chem. 286, 12221–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapajne L, Lakk M, Yarishkin O, Gubeljak L, Hawlina M, Križaj D, 2020. Polymodal sensory transduction in mouse corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 61, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapajne L, Rudzitis CN, Cullimore B, Ryskamp D, Lakk M, Redmon SN, Yarishkin O, Križaj D, 2022. TRPV4: cell type-specific activation, regulation and function in the vertebrate eye. Curr. Top. Membr. the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Rajabi S, Pedrigi RM, Overby DR, Read AT, Ethier CR, 2011. In vitro models for glaucoma research: effects of hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 52, 6329–6339. [DOI] [PubMed] [Google Scholar]
- Leonelli M, Martins DO, Kihara AH, Britto LR, 2009. Ontogenetic expression of the vanilloid receptors TRPV1 and TRPV2 in the rat retina. Int. J. Dev. Neurosci. 27 (7), 709–718. [DOI] [PubMed] [Google Scholar]
- Leung HT, Geng C, Pak WL, 2000. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J. Neurosci. 20, 6797–6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL, 2008. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 58, 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Minke B, 2012. The activity of the TRP-like channel depends on its expression system. Channels 6, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I, Barrantes FJ, 2012. Cholesterol Regulation of Ion Channels and Receptors. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- Li Q, Barres BA, 2018. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18 (4), 225–242. [DOI] [PubMed] [Google Scholar]
- Li Q, Cheng Y, Zhang S, Sun X, Wu J, 2021a. TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. J Neuroinflammation N17 18 (1), 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sergouniotis PI, Michaelides M, Mackay DS, Wright GA, Devery S, Moore AT, Holder GE, Robson AG, Webster AR, 2009. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am. J. Hum. Genet. 85, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Xu Y, Liu Z, Shi M, Zhang Y, Deng Y, Zhong X, Chen L, He J, Zeng J, Luo M, Cao W, Wan W, 2021b. TRPV4 inhibitor HC067047 produces antidepressant-like effect in LPS-induced depression mouse model. Neuropharmacology 201 (15), 108834. [DOI] [PubMed] [Google Scholar]
- Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A, 2010. Retinal glial (Muller) cells: sensing and responding to tissue stretch. Invest. Ophthalmol. Vis. Sci. 51, 1683–1690. [DOI] [PubMed] [Google Scholar]
- Lipp S, Wurm A, Pannicke T, Wiedemann P, Reichenbach A, Chen J, Bringmann A, 2009. Calcium responses mediated by type 2 IP3-receptors are required for osmotic volume regulation of retinal glial cells in mice. Neurosci. Lett. 457, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar I, 2006. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 281, 15485–15495. [DOI] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, Hardie RC, 2007. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J. Neurosci. 27, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wen W, Wei Z, Yu J, Ye F, Liu CH, Hardie RC, Zhang M, 2011. The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell 145 (7), 1088–1101. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhao S, Zhang J, Sun T, Fan Y, Zheng Z, 2020. High glucose-induced TRPC6 channel activation decreases glutamate uptake in rat retinal müller cells. Front. Pharmacol. 10, 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cheng K-T, Wong C-O, O’Neil RG, Birnbaumer L, Ambudkar IS, Yao X, 2011. Heteromeric TRPV4-C1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium 50, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RB, Randlett O, Oswald J, Yoshimatsu T, Franze K, Harris WA, 2015. Muller glia provide essential tensile strength to the developing retina. J. Cell Biol. 210, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox JW, Khorsandi N, Gleason E, 2018. TRPC5 is required for the NO-dependent increase in dendritic Ca2+ and GABA release from chick retinal amacrine cells. J. Neurophysiol. 119, 262–273. [DOI] [PubMed] [Google Scholar]
- Malaichamy S, Sen P, Sachidanandam R, Arokiasamy T, Lancelot ME, Audo I, Zeitz C, Soumittra N, 2014. Molecular profiling of complete congenital stationary night blindness: a pilot study on an Indian cohort. Mol. Vis. 20, 341–351. [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V, 2008. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56, 821–835. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS, 2000. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 6, 159–163. [DOI] [PubMed] [Google Scholar]
- Marcus MW, Muskens RP, Ramdas WD, Wolfs RC, De Jong PT, Vingerling JR, Hofman A, Stricker BH, Jansonius NM, 2012. Cholesterol-lowering drugs and incident open-angle glaucoma: a population-based cohort study. PLoS One 7, e29724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, 2017. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 10 (8), 15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone MC, Morabito A, Giustizieri M, Chiurchiu V, Leuti A, Mattioli M, Marinelli S, Riganti L, Lombardi M, Murana E, Totaro A, Piomelli D, Ragozzino D, Oddi S, Maccarrone M, Verderio C, Marinelli S, 2017. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 8, 15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martersteck EM, Hirokawa KE, Evarts M, Bernard A, Duan X, Li Y, Ng L, Oh SW, Ouellette B, Royall JJ, Stoecklin M, Wang Q, Zeng H, Sanes JR, Harris JA, 2017. Diverse central projection patterns of retinal ganglion cells. Cell Rep. 18 (8), 2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García MC, Martínez T, Pañeda C, Gallego P, Jimenez AI, Merayo J, 2013. Differential expression and localization of transient receptor potential vanilloid 1 in rabbit and human eyes. Histol. Histopathol. 28 (11), 1507–1516. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Sugio S, Seghers F, Krizaj D, Akiyama H, Ishizaki Y, Gailly P, Shibasaki K, 2018. Retinal detachment-induced muller glial cell swelling activates TRPV4 ion channels and triggers photoreceptor death at body temperature. J. Neurosci. 38, 8745–8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady NR, Risner ML, Vest V, Calkins DJ, 2020. TRPV1 tunes optic nerve axon excitability in glaucoma. Front. Physiol. 11, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW, 2005. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 433, 741–745. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA, 2006a. A Calcium signaling in specialized glial cells. Glia 54, 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA, 2006b. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 26, 2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NE, Joel-Almagor T, Frechter S, Minke B, Huber A, 2006. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J. Cell Sci. 119, 2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michinaga S, Onishi K, Shimizu K, Mizuguchi H, Hishinuma S, 2021. Pharmacological inhibition of transient receptor potential vanilloid 4 reduces vasogenic edema after traumatic brain injury in mice. Biol. Pharm. Bull. 44, 1759–1766. [DOI] [PubMed] [Google Scholar]
- Middleton TP, Huang JY, Protti DA, 2019. Cannabinoids modulate light signaling in ON- sustained retinal ganglion cells of the mouse. Front. Neural Circ. 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton TP, Protti DA, 2011. Cannabinoids modulate spontaneous synaptic activity in retinal ganglion cells. Vis. Neurosci. 28, 393–402. [DOI] [PubMed] [Google Scholar]
- Milenkovic VM, Brockmann M, Meyer C, Desch M, Schweda F, Kurtz A, Todorov V, Strauss O, 2010. Regulation of the renin expression in the retinal pigment epithelium by systemic stimuli. Am. J. Physiol. Ren. Physiol 299 (2), F396–F403. [DOI] [PubMed] [Google Scholar]
- Minke B, 1977. Drosophila mutant with a transducer defect. Biophys. Struct. Mech. 3, 59–64. [DOI] [PubMed] [Google Scholar]
- Minke B, 1982. Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J. Gen. Physiol. 79, 361–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, 2002. The TRP calcium channel and retinal degeneration. Adv. Exp. Med. Biol. 514, 601–622. [DOI] [PubMed] [Google Scholar]
- Minke B, 2012. The history of the prolonged depolarizing afterpotential (PDA) and its role in genetic dissection of Drosophila phototransduction. J. Neurogenet. 26 (2), 106–117. [DOI] [PubMed] [Google Scholar]
- Minke B, Agam K, 2003. TRP gating is linked to the metabolic state and maintenance of the Drosophila photoreceptor cells. Cell Calcium 33, 395–408. [DOI] [PubMed] [Google Scholar]
- Minke B, Parnas M, 2006. Insights on TRP channels from in vivo studies in Drosophila. Annu. Rev. Physiol. 68, 649–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Selinger Z, 1992. The inositol-lipid pathway is necessary for light excitation in fly photoreceptors. Soc. Gen. Physiol. 47, 201–217. [PubMed] [Google Scholar]
- Minke B, Wu C, Pak WL, 1975. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258, 84–87. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Tsui J, Gobert D, Desjardins JF, Schohl A, Sild M, Spratt P, Castonguay A, De Koninck Y, Marsh-Armstrong N, Wiseman PW, Ruthazer ES, 2016. Endocannabinoid signaling enhances visual responses through modulation of intracellular chloride levels in retinal ganglion cells. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Shirakawa H, Nakagawa T, Kaneko S, 2015. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 63, 1870–1882. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Qian Y, Wensel TG, 2009. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J. Neurosci. 29, 7753–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mola MG, Saracino E, Formaggio F, Amerotti AG, Barile B, Posati T, Cibelli A, Frigeri A, Palazzo C, Zamboni R, Caprini M, Nicchia GP, Benfenati V, 2021. Cell volume regulation mechanisms in differentiated astrocytes. Cell. Physiol. Biochem. 55, 196–212. [DOI] [PubMed] [Google Scholar]
- Molnar T, Yarishkin O, Iuso A, Barabas P, Jones B, Marc RE, Phuong TT, Krizaj D, 2016. Store-operated calcium entry in muller glia is controlled by synergistic activation of TRPC and Orai channels. J. Neurosci. 36, 3184–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar T, Barabas P, Birnbaumer L, Punzo C, Kefalov V, Križaj D, 2012. Store-operated channels regulate intracellular calcium in mammalian rods. J. Physiol. 590, 3465–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, McGeown JG, Curtis TM, 2015. Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS One 10, e0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, 1998. TRP trapped in fly signaling web Drosophila visual transduction, 35, 356–363 Curr. Opin. Neurobiol. 8, 389–397. Montell, C. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM, 1989. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323. [DOI] [PubMed] [Google Scholar]
- Montell C, 2012. Drosophila visual transduction. Trends Neurosci. 35, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX, 2002. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 9, 229–231. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Bíró T, Szallasi A, 2011. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10 (8), 601–620. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brown RL, Duvoisin RM, 2010. TRPM1: the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays 32, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L, 2006. Localization of nyctalopin in the mammalian retina. Eur. J. Neurosci. 23, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL, 2009. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. U. S. A. 106, 19174–19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure LS, 2021. Intrinsically photosensitive retinal ganglion cells of the human retina. Front. Neurol. 12, 636330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musada GR, Dvoriantchikova G, Myer C, Ivanov D, Bhattacharya SK, Hackam AS, 2020. The effect of extrinsic Wnt/beta-catenin signaling in Muller glia on retinal ganglion cell neurite growth. Dev Neurobiol 80, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sanuki R, Yasuma TR, Onishi A, Nishiguchi KM, Koike C, Kadowaki M, Kondo M, Miyake Y, Furukawa T, 2010. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol. Vis. 16, 425–437. [PMC free article] [PubMed] [Google Scholar]
- Nawy S, 1999. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J. Neurosci. 19, 2938–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE, 1990. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature 346, 269–271. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE, 1991. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron 7, 677–683. [DOI] [PubMed] [Google Scholar]
- Netti V, Fernandez J, Kalstein M, Pizzoni A, Di Giusto G, Rivarola V, Ford P, Capurro C, 2017. TRPV4 contributes to resting membrane potential in retinal muller cells: implications in cell volume regulation. J. Cell. Biochem. 118, 2302–2313. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR, 1998. Modulation of neuronal activity by glial cells in the retina. J. Neurosci. 18 (11), 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2015. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS, 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 85, 651–659. [DOI] [PubMed] [Google Scholar]
- Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vasquez V, Laver DR, Martinac B, 2019. Mammalian TRP ion channels are insensitive to membrane stretch. J. Cell Sci. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Szallasi A, 2014. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 66, 676–814. [DOI] [PubMed] [Google Scholar]
- Nisembaum LG, Loentgen G, L’Honore T, Martin P, Paulin CH, Fuentes M, Escoubeyrou K, Delgado MJ, Besseau L, Falcon J, 2022. Transient receptor potential- vanilloid (TRPV1-TRPV4) channels in the atlantic salmon, Salmo salar. A focus on the pineal gland and melatonin production. Front. Physiol 12, 784416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto R, Derouiche S, Eto K’, Deveci A, Kashio M, Kimori Y, Matsuoka Y, Morimatsu H, Nabekura J, Tominaga M, 2021. Thermosensitive TRPV4 channels mediate temperature-dependent microglia movement. Proc. Natl. Acad. Sci. U. S. A. 118, e2012894118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci C, Gasperi V, Tartaglione R, Cerulli A, Terrinoni A, Bari M, De Simone C, Agro AF, Morrone LA, Corasaniti MT, Bagetta G, Maccarrone M, 2007. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Invest. Ophthalmol. Vis. Sci. 48, 2997–3004. [DOI] [PubMed] [Google Scholar]
- O’Connor E, Eisenhaber B, Dalley J, Wang T, Missen C, Bulleid N, Bishop PN, Trump D, 2005. Species specific membrane anchoring of nyctalopin, a small leucine-rich repeat protein. Hum. Mol. Genet. 14, 1877–1887. [DOI] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE, 2009. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2, ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E, Wicks NL, 2011. TRPM1: new trends for an old TRP. Adv. Exp. Med. Biol. 704, 135–145. [DOI] [PubMed] [Google Scholar]
- Oberegelsbacher C, Schneidler C, Voolstra O, Cerny A, Huber A, 2011. The Drosophila TRPL ion channel shares a Rab-dependent translocation pathway with rhodopsin. Eur. J. Cell Biol. 90, 620–630. [DOI] [PubMed] [Google Scholar]
- Oberwinkler J, Stavenga DG, 2000. Calcium transients in the rhabdomeres of dark- and light- adapted fly photoreceptor cells. J. Neurosci. 20, 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M, Yamamoto H, Matsumoto H, Ishizaki Y, Shibasaki K, 2020. TRPC5 regulates axonal outgrowth in developing retinal ganglion cells. Lab. Invest. 100, 297–310. [DOI] [PubMed] [Google Scholar]
- Okawa H, Pahlberg J, Rieke F, Birnbaumer L, Sampath AP, 2010. Coordinated control of sensitivity by two splice variants of Galpha(o) in retinal ON bipolar cells. J. Gen. Physiol. 136, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary C, McGahon MK, Ashraf S, McNaughten J, Friedel T, Cincolà P, Barabas P, Fernandez JA, Stitt AW, McGeown JG, Curtis TM, 2019. Involvement of TRPV1 and TRPV4 channels in retinal angiogenesis. Invest. Ophthalmol. Vis. Sci. 60 (10), 3297–3309. [DOI] [PubMed] [Google Scholar]
- Orduna Rios M, Noguez Imm R, Hernandez Godinez NM, Bautista Cortes AM, Lopez Escalante DD, Liedtke W, Martinez Torres A, Concha L, Thebault S, 2019. TRPV4 inhibition prevents increased water diffusion and blood-retina barrier breakdown in the retina of streptozotocin-induced diabetic mice. PLoS One 14, e0212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Cao Y, Martemyanov KA, 2013. Orphan receptor GPR179 forms macromolecular complexes with components of metabotropic signaling cascade in retina ON- bipolar neurons. Invest. Ophthalmol. Vis. Sci. 54, 7153–7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi C, Omori Y, Wang Y, Cao Y, Ueno A, Roux MJ, Condomitti G, de Wit J, Kanagawa M, Furukawa T, Martemyanov KA, 2018. Transsynaptic binding of orphan receptor GPR179 to dystroglycan-pikachurin complex is essential for the synaptic organization of photoreceptors. Cell Rep. 25, 130–145 e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A, Aldarwesh A, Rhodes JD, Broadway DC, Everitt C, Sanderson J, 2015. Hydrostatic pressure does not cause detectable changes in survival of human retinal ganglion cells. PLoS One 10 (1), e0115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Jo RE, Ullian EM, Wong RO, Della Santina L, 2016. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J. Neurosci. 36, 9240–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak WL, Leung HT, 2003. Genetic approaches to visual transduction in Drosophila melanogaster. Recept. Channel 9, 149–167. [PubMed] [Google Scholar]
- Pak WL, Shino S, Leung HT, 2012. PDA (prolonged depolarizing afterpotential)-defective mutants: the story of nina’s and ina’s–pinta and santa maria, too. J. Neurogenet. 26, 216–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM, 2021. Generators of pressure-evoked currents in vertebrate outer retinal neurons. Cells 10, 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes HP, Bringmann A, 2006. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 55, 633–639. [DOI] [PubMed] [Google Scholar]
- Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, Metzner H, Yaka R, Minke B, 2009a. Membrane lipid modulations remove divalent open channel block from TRP- like and NMDA channels. J. Neurosci. 29, 2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas M, Peters M, Minke B, 2009b. Linoleic acid inhibits TRP channels with intrinsic voltage sensitivity: implications on the mechanism of linoleic acid action. Channels 3, 164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen R, Bahner M, Huber A, 2000. The PDZ assembled “transducisome” of microvillar photoreceptors: the TRP/TRPL problem. Pflügers Archiv 439, R181–183. [PubMed] [Google Scholar]
- Peachey NS, Ray TA, Florijn R, Rowe LB, Sjoerdsma T, Contreras-Alcantara S, Baba K, Tosini G, Pozdeyev N, Iuvone PM, Bojang P Jr., Pearring JN, Simonsz HJ, van Genderen M, Birch DG, Traboulsi EI, Dorfman A, Lopez I, Ren H, Goldberg AF, Nishina PM, Lachapelle P, McCall MA, Koenekoop RK, Bergen AA, Kamermans M, Gregg RG, 2012. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 90, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring JN, Bojang P Jr., Shen Y, Koike C, Furukawa T, Nawy S, Gregg RG, 2011. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J. Neurosci. 31, 10060–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Shi X, Kadowaki T, 2015. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 84, 145–157. [DOI] [PubMed] [Google Scholar]
- Penner R, Fleig A, 2007. The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7. Handb. Exp. Pharmacol. 2, 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, Kofuji P, 2011. Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol- sensitive TRPC subunits. Eur. J. Neurosci. 33, 856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Katz B, Lev S, Zaguri R, Gutorov R, Minke B, 2017. Depletion of membrane cholesterol suppresses Drosophila transient receptor potential-like (TRPL) channel activity. Curr. Top. Membr. 80, 233–254. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Meggyesy C, Yu K, Miller SS, 1997. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J. Neurosci. 17, 2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TT, Yarishkin O, Krizaj D, 2016. Subcellular propagation of calcium waves in Muller glia does not require autocrine/paracrine purinergic signaling. Channels 10, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TTT, Redmon SN, Yarishkin O, Winter JM, Li DY, Krizaj D, 2017. Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. J. Physiol. 595, 6869–6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar-Sueiro S, Urcola H, Rivas MA, Vecino E, 2011. Prevention of retinal ganglion cell swelling by systemic brimonidine in a rat experimental glaucoma model. Clin. Exp. Ophthalmol. 39, 799–807. [DOI] [PubMed] [Google Scholar]
- Pivonkova H, Hermanova Z, Kirdajova D, Awadova T, Malinsky J, Valihrach L, Zucha D, Kubista M, Galisova A, Jirak D, Anderova M, 2018. The contribution of TRPV4 channels to astrocyte volume regulation and brain edema formation. Neuroscience 394, 127–143. [DOI] [PubMed] [Google Scholar]
- Potla R, Hirano-Kobayashi M, Wu H, Chen H, Mammoto A, Matthews BD, Ingber DE, 2020. Molecular mapping of transmembrane mechanotransduction through the beta1 integrin-CD98hc-TRPV4 axis. J. Cell Sci. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Jung S, Priller J, 2019. Microglia biology: one century of evolving concepts. Cell 179 (2), 292–311. [DOI] [PubMed] [Google Scholar]
- Pumir A, Graves J, Ranganathan R, Shraiman BI, 2008. Systems analysis of the single photon response in invertebrate photoreceptors. Proc. Natl. Acad. Sci. U. S. A. 105, 10354–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, 1991. Stretch-activated channels in human retinal Muller cells. Glia 4, 456–460. [DOI] [PubMed] [Google Scholar]
- Raghu P, Hardie RC, 2009. Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium 45, 566–573. [DOI] [PubMed] [Google Scholar]
- Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC, 2000. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26, 169–179. [DOI] [PubMed] [Google Scholar]
- Raghu P, Yadav S, Mallampati NB, 2012. Lipid signaling in Drosophila photoreceptors. Biochim. Biophys. Acta 1821, 1154–1165. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan H, Dhingra A, Tummala SR, Fina ME, Li JJ, Lyubarsky A, Vardi N, 2015. Differential function of Ggamma13 in rod bipolar and ON cone bipolar cells. J. Physiol. 593, 1531–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampino MA, Nawy SA, 2011. Relief of Mg2+-dependent inhibition of TRPM1 by PKCalpha at the rod bipolar cell synapse. J. Neurosci. 31, 13596–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AS, Liu CH, Chu B, Zhang Q, Dongre SA, Juusola M, Franze K, Wakelam MJ, Hardie RC, 2015. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J. Neurosci. 35, 2731–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmon SN, Shibasaki K, Križaj D, 2017. Transient receptor potential cation channel subfamily V member 4. In: Choi Sangdun (Ed.), Encyclopedia of Signaling Molecules, second ed. Springer Verlag. [Google Scholar]
- Redmon SN, Yarishkin O, Lakk M, Jo A, Mustafic E, Tvrdik P, Krizaj D, 2021. TRPV4 channels mediate the mechanoresponse in retinal microglia. Glia 69, 1563–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A, 2010. Müller Cells in the Healthy and Diseased Retina. Springer Science & Business Media, Springer Verlag, New York. [Google Scholar]
- Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A, 2007. Muller cells as players in retinal degeneration and edema. Graefes Arch. Clin. Exp. Ophthalmol. 245, 627–636. [DOI] [PubMed] [Google Scholar]
- Reichhart N, Markowski M, Ishiyama S, Wagner A, Crespo-Garcia S, Schorb T, Ramalho JS, Milenkovic VM, Föckler R, Seabra MC, Strauß O, 2015a. Rab27a GTPase modulates L-type Ca2+ channel function via interaction with the II-III linker of CaV1.3 subunit. Cell. Signal. 27 (11), 2231–2240. [DOI] [PubMed] [Google Scholar]
- Reichhart N, Keckeis S, Fried F, Fels G, Strauss O, 2015b. Regulation of surface expression of TRPV2 channels in the retinal pigment epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 253, 865–874. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC, 1997. In vivo analysis of the drosophila light- sensitive channels, TRP and TRPL. Neuron 19, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, 2010. Vertebrate vision: TRP channels in the spotlight. Curr. Biol. 20, R278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Katz B, Oberacker T, Tzarfaty V, Belusic G, Minke B, Huber A, 2011. Translocation of the Drosophila transient receptor potential-like (TRPL) channel requires both the N- and C-terminal regions together with sustained Ca2+ entry. J Biol Chem 286, 34234–34243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA, 2009. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two- photon microscopy. Glia 57, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ML, McGrady NR, Boal AM, Pasini S, Calkins DJ, 2020. TRPV1 supports axogenic enhanced excitability in response to neurodegenerative stress. Front. Cell. Neurosci. 14, 603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ML, Pasini S, Cooper ML, Lambert WS, Calkins DJ, 2018. Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc. Natl. Acad. Sci. U. S. A. 115 (10), E2393–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruether K, Feigenspan A, Pirngruber J, Leitges M, Baehr W, Strauss O, 2010. PKC {alpha} is essential for the proper activation and termination of rod bipolar cell response. Invest. Ophthalmol. Vis. Sci. 51, 6051–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running Deer JL, Hurley JB, Yarfitz SL, 1995. G protein control of Drosophila photoreceptor phospholipase C. J. Biol. Chem. 270, 12623–12628. [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Frye AM, Phuong TT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY, Krizaj D, 2016. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci. Rep. 6, 30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Iuso A, Krizaj D, 2015. TRPV4 links inflammatory signaling and neuroglial swelling. Channels 9, 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, Krizaj D, 2014a. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J. Neurosci. 34, 15689–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Redmon S, Jo AO, Krizaj D, 2014b. TRPV1 and endocannabinoids: emerging molecular signals that modulate mammalian vision. Cells 3, 914–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Renteria RC, Liedtke W, Krizaj D, 2011. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 31, 7089–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva R, Schlotterer A, Schumacher D, Matka C, Mathar I, Dietrich N, Medert R, Kriebs U, Lin J, Nawroth P, Birnbaumer L, Fleming T, Hammes HP, Freichel M, 2018. TRPC proteins contribute to development of diabetic retinopathy and regulate glyoxalase 1 activity and methylglyoxal accumulation. Mol. Metabol 9, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Kuroki T, Sagawa T, Ito H, Mori A, Nakahara T, Ishii K, 2017. Opioid receptor activation is involved in neuroprotection induced by TRPV1 channel activation against excitotoxicity in the rat retina. Eur. J. Pharmacol. 812, 57–63. [DOI] [PubMed] [Google Scholar]
- Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C, 2008. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117 (8), 1065–1074. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Bandarchi J, Fain GL, 1999. Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J. Gen. Physiol. 113, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanxaridis PD, Cronin MA, Rawat SS, Waro G, Acharya U, Tsunoda S, 2007. Light- induced recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila photoreceptors. Mol. Cell. Neurosci. 36, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Calkins DJ, 2008. Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 49, 3004–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Sidorova T, Long DJ, Calkins DJ, 2009. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 50, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, Calkins DJ, 2015. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels 9, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Li BX, Xia H, Ready DF, 2008. Calcium-activated Myosin V closes the Drosophila pupil. Curr. Biol. 18, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider FM, Mohr F, Behrendt M, Oberwinkler J, 2015. Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells. Eur. J. Cell Biol. 94, 420–427. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, Hankins MW, 2007. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J. Neurosci. 27, 3981–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger Z, Minke B, 1988. Inositol lipid cascade of vision studied in mutant flies. Cold Spring Harbor Symp. Quant. Biol 53 (Pt 1), 333–341. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Barber TR, Xia H, Ready DF, Hardie RC, 2013. Depletion of PtdIns (4,5)P(2) underlies retinal degeneration in Drosophila trp mutants. J. Cell Sci. 126, 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin-Vences MR, Moroni M, Lewin GR, Poole K, 2017. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 6, e21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Sanes JR, 2021. Generating and using transcriptomically based retinal cell atlases. Annu. Rev. Vis. Sci. 7, 43–72. [DOI] [PubMed] [Google Scholar]
- Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK, Lee S, Coorey NJ, Killingsworth M, Sherman LS, Gillies MC, 2012. Conditional Mullercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J. Neurosci. 32, 15715–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S, 2009. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J. Neurosci. 29, 6088–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, 2020. TRPV4 activation by thermal and mechanical stimuli in disease progression. Lab. Invest. 100, 218–223. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, Ishizaki Y, 2014. A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J. Biol. Chem. 289, 14470–14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS, 2011. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15 (1), 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Wang CT, Chen YL, Chau VQ, Fu KG, Yang J, McQuiston AR, Fisher RA, Chen CK, 2012. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J. Biol. Chem. 287, 14873–14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye VK, Guler AD, Schweitzer KS, D’Alessio F, Caterina MJ, King LS, 2006. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. U. S. A. 103, 4747–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman SM, Wong WT, 2018. Microglia in the retina: roles in development, maturity, and disease. Ann. Rev. Vis. Sci 4, 45–77. [DOI] [PubMed] [Google Scholar]
- Someya E, Akagawa M, Mori A, Morita A, Yui N, Asano D, Sakamoto K, Nakahara T, 2019. Role of Neuron(−)Glia signaling in regulation of retinal vascular tone in rats. Int. J. Mol. Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Postma M, Billings SA, Coca D, Hardie RC, Juusola M, 2012. Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr. Biol. 22, 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda T, Lee SK, Birnbaumer L, Schmidt TM, 2018. Melanopsin phototransduction is repurposed by ipRGC subtypes to shape the function of distinct visual circuits. Neuron 99, 754–767 e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa R, Hoffpauir B, Rankin ML, Bruch RC, Gleason EL, 2002. Metabotropic glutamate receptor 5 and calcium signaling in retinal amacrine cells. J. Neurochem. 81, 973–983. [DOI] [PubMed] [Google Scholar]
- Souza Monteiro de Araujo D, De Logu F, Adembri C, Rizzo S, Janal MN, Landini L, Magi A, Mattei G, Cini N, Pandolfo P, Geppetti P, Nassini R, Calaza KDC, 2020. TRPA1 mediates damage of the retina induced by ischemia and reperfusion in mice. Cell Death Dis. 11, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, 2005. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD, 2000. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2, 695–702. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS Jr., Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA, 2000. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, 2471–2478. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Slaughter MM, 2006. Synaptic transmission mediated by internal calcium stores in rod photoreceptors. J. Neurosci. 26, 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Cusato K, Thoreson WB, Barabas P, Bartoletti TM, Križaj D, 2008. Depletion of calcium stores regulates calcium influx and signal transmission in rod photoreceptors. J. Physiol. 586, 4859–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Križaj D, 2006. The dynamic range and domain-specific signals of intracellular calcium in photoreceptors. Neuroscience 141, 143–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Križaj D, 2007. Intracellular organelles and calcium homeostasis in rods and cones. Vis. Neurosci. 24 (5), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Križaj D, 2009. Calcium signals in inner segments of photoreceptors. In: Tombran-Tink J, Barnstable C (Eds.), Visual Transduction and Non-visual Light Perception. Humana Press, Totowa, NJ, pp. 197–223. [Google Scholar]
- Szikra T, Barabas P, Bartoletti TM, Huang W, Akopian A, Thoreson WB, Križaj D, 2009. Calcium homeostasis and cone signaling are regulated by interactions between calcium stores and plasma membrane ion channels. PLoS One 4 (8), e6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Arner K, Ghosh F, 2017. Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Exp. Eye Res. 154, 10–21. [DOI] [PubMed] [Google Scholar]
- Tian Y, Hu W, Tong H, Han J, 2012. Phototransduction in Drosophila. Sci. China Life Sci. 55, 27–34. [DOI] [PubMed] [Google Scholar]
- Thébault S, 2021. Minireview: insights into the role of TRP channels in the retinal circulation and function. Neurosci. Lett. 20, 136285, 765. [DOI] [PubMed] [Google Scholar]
- Thibodeau ML, Peters CH, Townsend KN, Shen Y, Hendson G, Adam S, Selby K, Macleod PM, Gershome C, Ruben P, Jones SJM, Consortium FC, Friedman JM, Gibson WT, Horvath GA, 2017. Compound heterozygous TRPV4 mutations in two siblings with a complex phenotype including severe intellectual disability and neuropathy. Am. J. Med. Genet. 173, 3087–3092. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, 2021. Transmission at rod and cone ribbon synapses in the retina. Pflügers Archiv 473, 1469–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo Thrane V, Enger R, Haj-Yasein NN, Skare O, Holen T, Klungland A, Ottersen OP, Nedergaard M, Nagelhus EA, 2011. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. U. S. A. 108, 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, Krizaj D, MacAulay N, 2017. When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. J. Physiol. 595, 3287–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, MacAulay N, 2021. TRPing on cell swelling - TRPV4 senses it. Front. Immunol. 12, 730982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, Yarishkin O, Redmon S, Phuong TTT, Krizaj D, MacAulay N, 2019. Volume sensing in the transient receptor potential vanilloid 4 ion channel is cell type- specific and mediated by an N-terminal volume-sensing domain. J. Biol. Chem. 294, 18421–18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, Levin JZ, Lin D, Wang C, Lieber CM, Regev A, He Z, Sanes JR, 2019. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron 104, 1039–1055 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivino A, Ramirez AI, Salazar JJ, de Hoz R, Rojas B, Padilla E, Tejerina T, Ramirez JM, 2006. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Exp. Eye Res. 83, 357–366. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS, 1997. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 388, 243–249. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sun Y, Suzuki E, Zuker C, 2001. Independent anchoring and assembly mechanisms of INAD signaling complexes in Drosophila photoreceptors. J. Neurosci. 21, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Zuker CS, 1999. The organization of INAD-signaling complexes by a multivalent PDZ domain protein in Drosophila photoreceptor cells ensures sensitivity and speed of signaling. Cell Calcium 26, 165–171. [DOI] [PubMed] [Google Scholar]
- Tsuruda PR, Julius D, Minor DL Jr., 2006. Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovsky EA, Braga A, Yu Y, Esteras N, Korsak A, Theparambil SM, Hadjihambi A, Hosford PS, Teschemacher AG, Marina N, Lythgoe MF, Haydon PG, Gourine AV, 2020. Mechanosensory signaling in astrocytes. J. Neurosci. 40, 9364–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworig JM, Coate CJ, Feller MB, 2021. Excitatory neurotransmission activates compartmentalized calcium transients in Muller glia without affecting lateral process motility. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M, 2009. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am. J. Hum. Genet. 85, 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Hofmann T, Montell C, 2006. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 281, 17517–17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal B, Browning MF, Curcio-Morelli C, Varro A, Michaud N, Nanthakumar N, Walkley SU, Pickel J, Slaugenhaupt SA, 2007. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am. J. Hum. Genet. 81, 1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler S, Pannicke T, Hollborn M, Kolibabka M, Wiedemann P, Reichenbach A, Hammes HP, Bringmann A, 2016. Impaired purinergic regulation of the glial (müller) cell volume in the retina of transgenic rats expressing defective polycystin-2. Neurochem. Res. 41 (7), 1784–1796. [DOI] [PubMed] [Google Scholar]
- Voolstra O, Bartels JP, Oberegelsbacher C, Pfannstiel J, Huber A, 2013. Phosphorylation of the Drosophila transient receptor potential ion channel is regulated by the phototransduction cascade and involves several protein kinases and phosphatases. PLoS One 8, e73787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Beck K, Oberegelsbacher C, Pfannstiel J, Huber A, 2010. Light-dependent phosphorylation of the drosophila transient receptor potential ion channel. J. Biol. Chem. 285, 14275–14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Huber A, 2020. Ca2+ signaling in Drosophila photoreceptor cells. Adv. Exp. Med. Biol. 1131, 857–879. [DOI] [PubMed] [Google Scholar]
- Voolstra O, Rhodes-Mordov E, Katz B, Bartels JP, Oberegelsbacher C, Schotthofer SK, Yasin B, Tzadok H, Huber A, Minke B, 2017. The Phosphorylation state of the Drosophila TRP channel modulates the frequency response to oscillating light in vivo. J. Neurosci. 37, 4213–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B, 2004. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U. S. A. 101, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz B, Baumann O, 1995. Structure and cellular physiology of Ca2+ stores in invertebrate photoreceptors. Cell Calcium 18, 342–351. [DOI] [PubMed] [Google Scholar]
- Walz B, Baumann O, Zimmermann B, Ciriacy-Wantrup EV, 1995. Caffeine- and ryanodine-sensitive Ca2+-induced Ca2+ release from the endoplasmic reticulum in honeybee photoreceptors. J. Gen. Physiol. 105, 537–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O’Sullivan ML, Mukherjee D, Punal VM, Farsiu S, Kay JN, 2017. Anatomy and spatial organization of Muller glia in mouse retina. J. Comp. Neurol. 525, 1759–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jiao Y, Montell C, 2005a. Dissecting independent channel and scaffolding roles of the Drosophila transient receptor potential channel. J. Cell Biol. 171, 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C, 2007. Phototransduction and retinal degeneration in Drosophila. Pflügers Archiv 454, 821–847. [DOI] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C, 2005b. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron 45, 367–378. [DOI] [PubMed] [Google Scholar]
- Wang X, Fan J, Zhang M, Sun Z, Xu G, 2013. Gene expression changes under cyclic mechanical stretching in rat retinal glial (Muller) cells. PLoS One 8, e63467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Teng L, Li A, Ge J, Laties AM, Zhang X, 2010. TRPC6 channel protects retinal ganglion cells in a rat model of retinal ischemia/reperfusion-induced cell death. Invest. Ophthalmol. Vis. Sci. 51, 5751–5758. [DOI] [PubMed] [Google Scholar]
- Ward NJ, Ho KW, Lambert WS, Weitlauf C, Calkins DJ, 2014. Absence of transient receptor potential vanilloid-1 accelerates stress-induced axonopathy in the optic projection. J. Neurosci. 34, 3161–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW, 2006. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur. J. Neurosci. 23, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, 2004. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 5, 747–757. [DOI] [PubMed] [Google Scholar]
- Wassle H, Boycott BB, 1991. Functional architecture of the mammalian retina. Physiol. Rev. 71, 447–480. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B, 2003. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438. [DOI] [PubMed] [Google Scholar]
- Webster CM, Tworig J, Caval-Holme F, Morgans CW, Feller MB, 2020. The impact of steroid activation of TRPM3 on spontaneous activity in the developing retina. eNeuro 7 (2). ENEURO.0175–19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Ward NJ, Lambert WS, Sidorova TN, Ho KW, Sappington RM, Calkins DJ, 2014. Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J. Neurosci. 34, 15369–15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C, 1995. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U. S. A. 92, 9652–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I, 2016. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 96, 911–973. [DOI] [PubMed] [Google Scholar]
- Wimmers S, Karl MO, Strauss O, 2007. Ion channels in the RPE. Prog. Retin. Eye Res. 26, 263–301. [DOI] [PubMed] [Google Scholar]
- Wimmers S, Strauss O, 2007. Basal calcium entry in retinal pigment epithelial cells is mediated by TRPC channels. Invest. Ophthalmol. Vis. Sci. 48, 5767–5772. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Gabriel R, Krizaj D, 2008. Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas. J. Comp. Neurol. 510, 158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Schmitz Y, Akopian A, Krizaj D, Tranchina D, 1997. Gain of rod to horizontal cell synaptic transfer: relation to glutamate release and a dihydropyridine-sensitive calcium current. J. Neurosci. 17 (19), 7297–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemussie E, Wijono M, Ruiz G, 2004. Muller cell response to laser-induced increase in intraocular pressure in rats. Glia 47, 109–119. [DOI] [PubMed] [Google Scholar]
- Wong F, Schaefer EL, Roop BC, LaMendola JN, Johnson-Seaton D, Shao D, 1989. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron 3, 81–94. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE, 2010. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WH, Pang JJ, Pennesi ME, Duvoisin RM, Wu SM, Morgans CW, 2015. The effect of PKCalpha on the light response of rod bipolar cells in the mouse retina. Invest. Ophthalmol. Vis. Sci. 56, 4961–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Li HS, Guggino WB, Montell C, 1997. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 27 (7), 1155–1164, 89. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Chie, n F, Butler A, Salkoff L, Montell C, 2000. TRPgamma, a drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron 26 (3), 647–657. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW, 2011. Melanopsin signalling in mammalian iris and retina. Nature 479, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Wang Y, Ding J, Lu L, Ke G-J, Dong K, 2021. TRPML1 inhibited photoreceptor apoptosis and protected the retina by activation of autophagy in experimental retinal detachment. Ophthalmic Res. 64, 587–594. [DOI] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Lakk M, Krizaj D, 2018. TRPV4 does not regulate the distal retinal light response. Adv. Exp. Med. Biol. 1074, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Baumann JM, Krizaj D, 2019. Mechano-electrical transduction in trabecular meshwork involves parallel activation of TRPV4 and TREK-1 channels. Channels 13, 168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Baumann JM, De Ieso ML, Vazquez-Chona F, Rudzitis CN, Sundberg C, Lakk M, Stamer WD, Krizaj D, 2021. Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. J. Physiol. 599, 571–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M, Crandall A, Heurteaux C, Hartnett ME, Krizaj D, 2018a. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J. Gen. Physiol. 150, 1660–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Luo J, Qian A, Du J, Yang Q, Zhou S, Yu W, Du G, Clark RB, Walters ET, Carlton SM, Hu H, 2013. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J. Clin. Invest. 123 (9), 3941–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, Bourque CW, 2015. DeltaN-TRPV1: a molecular Co-detector of body temperature and osmotic stress. Cell Rep. 13, 23–30. [DOI] [PubMed] [Google Scholar]
- Zeitz C, Robson AG, Audo I, 2015. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog. Retin. Eye Res. 45, 58–110. [DOI] [PubMed] [Google Scholar]
- Zeitz C, Scherthan H, Freier S, Feil S, Suckow V, Schweiger S, Berger W, 2003. NYX (nyctalopin on chromosome X), the gene mutated in congenital stationary night blindness, encodes a cell surface protein. Invest. Ophthalmol. Vis. Sci. 44, 4184–4191. [DOI] [PubMed] [Google Scholar]
- Zhao PY, Gan G, Peng S, Wang SB, Chen B, Adelman RA, Rizzolo LJ, 2015. TRP channels localize to subdomains of the apical plasma membrane in human fetal retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 56, 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L, 1995. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 373, 193–198. [DOI] [PubMed] [Google Scholar]
- Zimov S, Yazulla S, 2004. Localization of vanilloid receptor 1 (TRPV1/VR1)-like immunoreactivity in goldfish and zebrafish retinas: restriction to photoreceptor synaptic ribbons. J. Neurocytol 33, 441–452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


