Abstract
Background and objective:
Drug use type and frequency may affect Anti-Retroviral Therapy (ART) uptake for HIV-infected people who inject drugs (PWID). This paper assesses the association between self-reported baseline drug use and ART among HIV-infected PWID in Indonesia, Ukraine and Vietnam.
Methods:
Data on self-reported baseline drug use and ART among HIV-infected PWID at the 26- and 52-week follow-ups were extracted from the HIV Prevention Trials Network (HPTN) 074, a randomized, controlled vanguard study to facilitate HIV treatment for PWID in Indonesia, Ukraine, and Vietnam. Multivariable logistic regression models were fit by study site and the whole HPTN 074 sample, using a 0.5 type I error rate.
Results:
The response rate were 83.3% and 77.0% at 26th and 52th weeks. At 26-week, baseline use of over one non-opiate/non-stimulant drug was associated with lower odds of ART use among Indonesian participants (OR = 0.21, 95%CI: 0.05–0.82); and baseline injecting drugs for over 20 days in the previous month was associated with lower odds of ART use among all HPTN 074 sample (OR = 0.59, 95% CI: 0.36–0.97).
Conclusion:
The association of a specific drug use pattern with later ART uptake implies the importance of medication-assisted treatment to enhance ART uptake and adherence among participants.
Keywords: People living with HIV, people who inject drugs, drugs, substance use, antiretroviral therapy, HPTN074
Introduction
People who inject drugs (PWID) are among the most vulnerable groups for HIV infection due to the sharing of needles, syringes, and other injecting equipment. It is estimated that PWID are 22 times more likely to acquire HIV than those who do not inject drugs (UNAIDS, 2018). In 2008, 16 million PWID were identified in 148 countries, with the largest populations of PWID residing in East and Southeast Asia (about 4 million) and Eastern Europe (about 3 million). Among them, about 3 million people were living with HIV (Bradley M. Mathers et al., 2008), accounting for 17.8% of the HIV prevalence in the world (Degenhardt et al., 2017). In Indonesia, Ukraine and Vietnam the prevalence of HIV in PWID in each country is approximately 30% (UNAIDS, 2020 Health, 2018).
HIV prevention and treatment efforts for HIV infected PWID have effectively combined approaches, including harm reduction programs and antiretroviral treatment (ART) (Adedinsewo et al., 2014). However, suboptimal access to HIV care and treatment among PWID persist due to structural, social, and individual barriers that hinder access and adherence to HIV treatment (Davis et al., 2018; Go et al., 2019; Latkin et al., 2017; Wolfe et al., 2010). In addition, the effects of drug addiction may reduce the ability to adhere to treatment, such as timely medication or medical checkup (Kuchinad et al., 2016). The empirical literature has shown that PWID tend to use several drugs simultaneously. However, most studies among PWID focus on the effect of a single substance on ART initiation. For example, one effect of cocaine, specifically in the withdrawal process, is intense craving, which causes concentration deficits and difficulty in adhering to ART medication among PWID (Gonzalez et al., 2013; Stein et al., 2000). Low ART adherence among methamphetamine users may be attributable to the combined effect of methamphetamine and HIV on the brain, leading to impaired neurocognitive functioning, which, in turn, negatively influences ability to comply with antiretroviral medication (Moore et al., 2012). The use of short- and long-acting opioids alters physiological and behavioral functions (Stimmel & Kreek, 2000). The combined impact of poly-drug use on ART initiation among PWID is unknown.
Results of the HPTN 074 trial in 2017 (Miller et al., 2018) showed that most PWID used two or more drugs at baseline and the drug use patterns were remarkably different across study sites (Lancaster et al., 2018). Herein we examine the association between self-reported drug use patterns at baseline and ART use at 26 and 52-week visits for each study site separately and the HPTN study combined.
Methods
Parent study and population
This secondary analysis was nested within HPTN074, a vanguard study for a network-based randomized HIV prevention trial comparing an integrated intervention of supported ART to the standard of care among HIV-infected PWID, in three locations Jakarta (Indonesia), Kyiv (Ukraine) and Thai Nguyen (Vietnam) from February 5, 2015 to June 30, 2017.
Inclusion criteria of participants were 1) age 18–45 years at the screening visit; 2) able to provide informed consent; 3) active injection drug user, defined as self-reported injecting drugs and the anatomical location of the most recent injection site identified by study staff; 4) sharing needles/syringes or drug solutions at least once in the last month; 5) HIV-infected based on a study-defined testing algorithm; 6) HIV viral load ≥1,000 copies/mL at Screening; 7) Willing and able to identify, recruit, and have enrolled at least one HIV uninfected network injection partner who was eligible to be study participation according to the inclusion criteria 8) Have no plans to move outside the study area for at least one year after study enrollment; 9) Willing to participate in intervention activities, including the provision of regular phone contact (Miller et al., 2018).
The trial enrolled 502 HIV infected PWID who were randomized into the intervention group (126 participants) or the standard of care group (376 participants). Study visits included screening, enrollment (baseline), and follow-up at 4, 13, 26, 39, 52, 78, 91, and 104 weeks. We analyzed data from the baseline, 26, and 52-week visits among participants who were not on ART and not virally suppressed (≥1000 copies/mL) at baseline. The detailed procedures of data collection are described in (Miller et al., 2018).
Measures
The dependent outcome variable was self-reported ART use among participants at 26 and 52-week visits. Independent variables were self-reported drug use patterns at baseline, including the type and number of drugs used in the last 3 months and the number of days injected drugs in the previous month at baseline. The National Institute on Drug Abuse (NIDA) categorization of drug use was applied to create three variables of drug use: 1) Number of injected/non-injected opiates (including heroin, opium, Buprenorphine, illegally manufactured methadone, homemade opioids and desomorphine, codeine, Subutex); 2) Number of injected/non-injected stimulants (including amphetamine, methamphetamine, cocaine, short and long action stimulants, Spices, spice, vint (pervitin), vint (pezisibin); and 3) Number of injected/non-injected other drugs (non-opiate and non-stimulant drugs, including marijuana, ketamine and benzodiazepines, central nervous system depressants and other drugs as indicated in each study site) (Shah & McCann, 2011). Each of these three variables contained two subgroups (0–1 drug versus >1 drugs). The number of days injected any types of drug were categorized into two groups (injected < 20 days in the last month and ≥ 20 days in the last month).
Other covariates included demographic characteristics at baseline, namely age (<35 and ≥35 years), sex (male and female), employment status (employed full-time and not full-time), education status (secondary school or less and technical training, college or higher), and marital status (married/live with sex partner and others as separated/divorced/ widowed/ single). In addition, the study arm (Intervention and Standard of care) and CD4 count at screening (<200 cell/mm3 and ≥200 cell/mm3) were included in the analysis to control their effect on the association between baseline drug use pattern and later ART uptake.
Statistical analysis
Data were analyzed using Statistical Analysis Software (SAS) 9.4 (Vourli et al., 2018). Descriptive analysis was conducted to describe the demographic characteristics of study participants, their patterns of drug use at baseline and ART use at 26 and 52-week follow-up visits. Chi-square analyses and multiple logistic regression models were conducted to assess the association between baseline drug use patterns and ART use at 26 and 52-weeks among the whole sample, controlling for baseline characteristics (described above). A type I error rate of 0.05 was used for the analysis. Missing data were excluded from the analysis (i.e., a complete case approach was used). Because of the substantial differences in drug use patterns across the three study sites, multiple logistic regression models were also performed separately within each site at both follow-ups.
Results
Distribution of study participants at 26 and 52-week follow-up visits by baseline characteristics
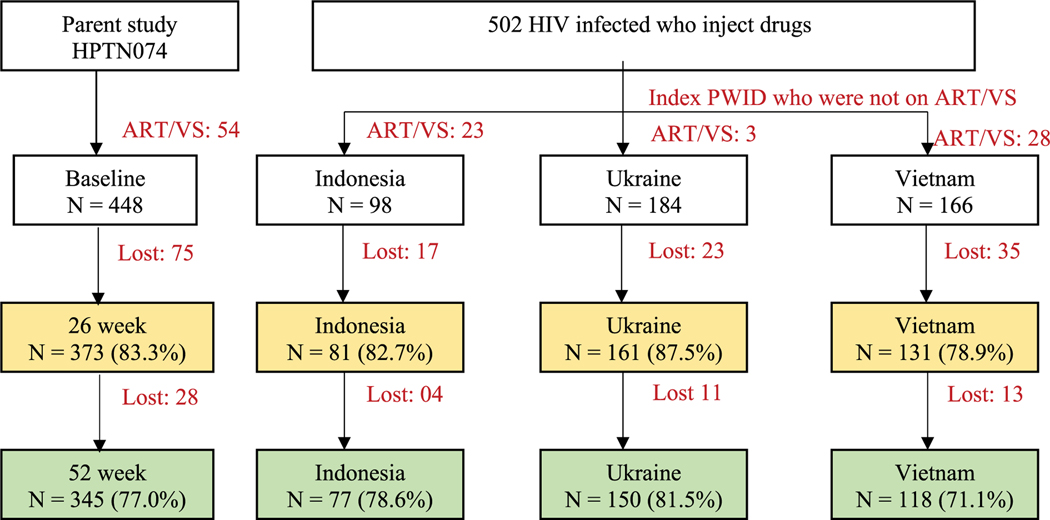
Among 448 participants who were not on ART and not virally suppressed at baseline, 373 and 345 participants were followed at 26 and 52 weeks (83.3% and 77.0%, respectively). In comparison to the whole sample, the retention rates were slightly higher in Ukraine (87.5% and 81.5%), following by Indonesia (82.7% and 78.6%) and lower in Vietnam (78.9% and 71.1%) at both visits (Figure 1).
Figure 1.
Number of participants at baseline, 26-week and 52-week visits. Note:-ART: Antiretroviral therapy-VS: Viral suppression
Overall, at baseline, most participants were male (>82%) with CD4 count ≥200 cells/mm3 (>73%). Approximately half of the study participants were over 35 years old, had a highest level of education completed of secondary education or lower, and were married/lived with partners. Less than 30% of participants were employed full-time and received intervention from the parent study. However, the characteristics of participants were considerably different across study sites (Table 1).
Table 1.
Distribution of study participant baseline characteristics at 26- and 52-week follow-up visits.
| Total |
Indonesia |
Ukraine |
Vietnam |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 w | 52 w | 26 w | 52 w | 26 w | 52 w | 26 w | 52 w | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Overall | 373 | 100.0 | 345 | 100.0 | 81 | 100.0 | 77 | 100.0 | 161 | 100.0 | 150 | 100.0 | 131 | 100.0 | 118 | 100.0 |
| Personal characteristics | ||||||||||||||||
| ≥ 35 years old | 179 | 48.0 | 169 | 49.0 | 31 | 38.3 | 30 | 39.0 | 77 | 47.8 | 71 | 47.3 | 71 | 54.2 | 68 | 57.6 |
| Male | 309 | 82.8 | 284 | 82.3 | 73 | 90.1 | 69 | 89.6 | 105 | 65.2 | 97 | 64.7 | 131 | 100.0 | 118 | 100.0 |
| Employed full-time | 109 | 29.2 | 98 | 28.4 | 23 | 28.4 | 22 | 28.6 | 22 | 13.7 | 19 | 12.7 | 64 | 48.9 | 57 | 48.3 |
| Secondary school, or less | 228 | 61.1 | 203 | 58.8 | 21 | 25.9 | 18 | 23.4 | 82 | 50.9 | 73 | 48.7 | 125 | 95.4 | 112 | 94.9 |
| Married/live with sex partner | 187 | 50.1 | 172 | 49.9 | 28 | 34.6 | 30 | 39.0 | 101 | 62.7 | 95 | 63.3 | 58 | 44.3 | 47 | 39.8 |
| Intervention group | 94 | 25.2 | 92 | 26.7 | 19 | 23.5 | 18 | 23.4 | 42 | 26.1 | 39 | 26.0 | 33 | 25.2 | 35 | 29.7 |
| CD4 count ≥ 200 cells/mm3 | 274 | 73.5 | 253 | 73.3 | 50 | 61.7 | 50 | 64.9 | 118 | 73.3 | 109 | 72.7 | 106 | 80.9 | 94 | 79.7 |
| Drug use pattern by type and number of drugs | ||||||||||||||||
| Combination of drug within 1 group | ||||||||||||||||
| Any use of opiate | 372 | 99.7 | 343 | 99.4 | 81 | 100.0 | 77 | 100.0 | 160 | 99.4 | 148 | 98.7 | 131 | 100.0 | 118 | 100.0 |
| Any use of stimulant | 167 | 44.8 | 161 | 46.7 | 59 | 72.8 | 57 | 74.0 | 78 | 48.4 | 78 | 52.0 | 30 | 22.9 | 26 | 22.0 |
| Any use of other drug | 218 | 58.4 | 201 | 58.3 | 63 | 77.8 | 62 | 80.5 | 130 | 80.7 | 120 | 80.0 | 25 | 19.1 | 19 | 16.1 |
| At least 2 opiates | 150 | 40.2 | 143 | 41.4 | 18 | 22.2 | 20 | 26.0 | 115 | 71.4 | 106 | 70.7 | 17 | 13.0 | 17 | 14.4 |
| At least 2 stimulants | 45 | 12.1 | 46 | 13.3 | 1 | 1.2 | 1 | 1.3 | 39 | 24.2 | 40 | 26.7 | 5 | 3.8 | 5 | 4.2 |
| At least 2 other drugs | 67 | 18.0 | 65 | 18.8 | 22 | 27.2 | 23 | 29.9 | 45 | 28.0 | 42 | 28.0 | 0 | 0.0 | 0 | 0.0 |
| Combination of different groups of drugs | ||||||||||||||||
| At least 2 groups of drugs | 167 | 44.8 | 161 | 46.7 | 59 | 72.8 | 57 | 74.0 | 78 | 48.4 | 78 | 52.0 | 25 | 19.1 | 19 | 16.1 |
| Drug use pattern by days injecting the previous month | ||||||||||||||||
| >20 days | 230 | 61.7 | 221 | 64.1 | 44 | 54.3 | 44 | 57.1 | 100 | 62.1 | 96 | 64.0 | 86 | 65.6 | 81 | 68.6 |
Regarding the drug use patterns, among the overall sample of participants at the 26-week follow-up, the percentages of baseline use of any opiates or any other drugs were 99.7% and 58.4%, respectively. Nearly half (44.8%) of the participants had any use of stimulants 3 months before the baseline survey, with the highest use of stimulants present in Indonesia (72.8%). Concurrent use of at least two opiates during the 3 months before baseline was reported for 40.2% of participants, followed by users of at least two other drugs (18.0%) and at least two stimulants (12.1%). 44.8% of participants used substances from at least two different groups of drugs. About 60% of participants injected drugs more than 20 days in 1 month before the baseline survey (Table 1).
Sub-group analysis by site showed a unique pattern of drug use in each study site which was different from that of the whole sample and the other sites. Specifically, less than 20% of Vietnamese participants concurrently used several drugs within or between the drug categories. All participants in Vietnam reported using at least one opiate at baseline, and 19.1% of them used at least one additional type of drug (either stimulant and/or other drugs). In Indonesia, over 70% of participants concurrently used drugs from two or more different drug categories (opiate with stimulants or other drugs), while less than 30% of them used more than one drug within one category. In Ukraine, a combined use of at least two opiates was the most common drug use pattern (over 70%). Between- group drug use (opiate with stimulants or other drugs) was approximately 50%. The proportion of participants who injected drugs more than 20 days in the last month was highest in Vietnam (65.6%), followed by Ukraine (62.1%) and Indonesia (54.3%) (Table 1).
Effects of drug use on follow-up ART use among PWIDs
The association of drug use patterns upon ART use, controlling for covariates, is presented in Tables 2, 3, 4 and 5. Tables 2 and 4 present results for the overall sample at 26 and 52 weeks, respectively. Tables 3 and 5 present results separately by study site at 26 and 52 weeks, respectively. At the 26 and 52-week visits, the percent of participants on ART by the date of that visit was 59.5% and 68.6% in Vietnam, 32.3% and 47.3% in Ukraine, and 39.5% to 37.7% in Indonesia, respectively (Tables 3 and 5).
Table 2.
Association between baseline drug use and ART use at 26-week follow-up visit of all sites (n = 373)
| Covariates | Whole sample |
Unadjusted Model |
Multivariable Model |
||||
|---|---|---|---|---|---|---|---|
| On ART(%) | OR | 95% Cl | p-value | aOR | 95% Cl | p-value | |
| Overall | 162/373 (43.4%) | ||||||
| Number of injected/non-injected opiates in last 3 months | |||||||
| 0–1 | 110/223 (49.3%) | REF | 0.36, 0.84 | 0.01 | REF | 0.42, 1.42 | NS |
| >1 | 52/150 (34.7%) | 0.55 | 0.77 | ||||
| Number of injected/non-injected stimulants in last 3 months | |||||||
| 0–1 | 147/328 (44.8%) | REF | 0.32, 1.19 | NS | REF | 0.58, 3.11 | NS |
| >1 | 15/45 (33.3%) | 0.62 | 1.34 | ||||
| Number of injected/non-injected other drugs in last 3 months | |||||||
| 0–1 | 146/306 (47.7%) | REF | 0.19, 0.63 | <0.01 | REF | 0.22, 1.00 | 0.05 |
| >1 | 16/67 (23.9%) | 0.34 | 0.47 | ||||
| Days injected drugs last month | |||||||
| 0–20 days last month | 68/143 (47.6%) | REF | 0.50, 1.16 | NS | REF | 0.36, 0.97 | 0.04 |
| >20 days last month | 94/230 (40.9%) | 0.76 | 0.59 | ||||
| CD4 count at screening | |||||||
| <200 cells/mm3 | 50/99 (50.5%) | REF | 0.43, 1.08 | NS | REF | 0.26, 0.80 | 0.01 |
| > = 200 cells/mm3 | 112/274(40.9%) | 0.68 | 0.45 | ||||
| Arm | |||||||
| SOC | 90/279 (32.3%) | REF | 4.01,11.79 | <0.01 | REF | 5.47,18.60 | <0.01 |
| Intervention | 72/94 (76.6%) | 6.87 | 10.08 | ||||
| Age Group | |||||||
| 18–34 | 79/194 (40.7%) | REF | 0.84, 1.90 | NS | REF | 0.70, 1.89 | NS |
| 35+ | 83/179 (46.4%) | 1.26 | 1.15 | ||||
| Sex | |||||||
| Male | 143/309 (46.3%) | REF | 0.27, 0.88 | 0.02 | REF | 0.41, 1.87 | NS |
| Female | 19/64 (29.7%) | 0.49 | 0.88 | ||||
| Employment | |||||||
| Employed full-time | 57/109 (52.3%) | REF | 0.38, 0.94 | 0.03 | REF | 0.44, 1.35 | NS |
| Not fulltime employed | 105/264 (39.8%) | 0.60 | 0.77 | ||||
| Education Status | |||||||
| Secondary school, or less | 104/228 (45.6%) | REF | 0.52, 1.21 | NS | REF | 0.78, 2.79 | NS |
| Technical training, college | 58/145 (40.0%) | 0.79 | 1.48 | ||||
| Marriage Status | |||||||
| Married/live with sex partner | 85/187 (45.5%) | REF | 0.56, 1.28 | NS | REF | 0.38, 1.05 | NS |
| Others | 77/186 (41.4%) | 0.85 | 0.63 | ||||
| Study Site | |||||||
| Indonesia | 32/81 (39.5%) | REF | REF | ||||
| Ukraine | 52/161 (32.3%) | 0.73 | 0.42, 1.27 | NS | 0.76 | 0.35, 1.65 | NS |
| Vietnam | 78/131 (59.5%) | 2.25 | 1.28, 3.97 | 0.01 | 3.03 | 1.30, 7.07 | 0.01 |
NS: Not significant, p > 0.05
Table 3.
Association between baseline drug use and ART use at 26-week follow-up visit separately by study site.
| Covariates | Indonesia |
Ukraine |
Vietnam |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On ART (%) | aOR | 95% CI | p-value | On ART (%) | aOR | 95% CI | p-value | On ART (%) | aOR | 95% CI | p-value | |
| Overall | 32/81 (39.5%) | 52/161 (32.3%) | 78/131 (59.5%) | |||||||||
| Number of injected/non-injected opiates in last 3 months | ||||||||||||
| 0–1 | 25/63 (39.7%) | REF | 0.21, 3.36 | 0.80 | 17/46 (37.0%) | REF | 0.33, 2.31 | NS | 68/114 (59.6%) | REF | 0.23, 2.54 | NS |
| >1 | 7/18 (38.9%) | 0.84 | 35/115 (30.4%) | 0.87 | 10/17 (58.8%) | 0.77 | ||||||
| Number of injected/non-injected stimulants in last 3 months | ||||||||||||
| 0–1 | 31/80 (38.8%) | 42/122 (34.4%) | REF | 0.24, 2.09 | NS | 74/126 (58.7%) | REF | 0.65,69.04 | NS | |||
| >1 | 1/1 (100.0%) | 10/39 (25.6%) | 0.71 | 4/5 (80.0%) | 6.68 | |||||||
| Number of injected/non-injected other drugs in last 3 months | ||||||||||||
| 0–1 | 27/59 (45.8%) | REF | 0.05, 0.82 | 0.03 | 41/116 (35.3%) | REF | 0.37, 2.60 | NS | 78/131 (59.5%) | |||
| >1 | 5/22 (22.7%) | 0.21 | 11/45 (24.4%) | 0.98 | 0/0 (-%) | |||||||
| Days injected drugs last month | ||||||||||||
| 0–20 days last month | 19/37 (51.4%) | REF | 0.12, 1.06 | 0.06 | 23/61 (37.7%) | REF | 0.24, 1.46 | NS | 26/45 (57.8%) | REF | 0.34, 1.80 | NS |
| >20 days last month | 13/44 (29.5%) | 0.35 | 29/100 (29.0%) | 0.59 | 52/86 (60.5%) | 0.78 | ||||||
| CD4 count at screening | ||||||||||||
| <200 cells/mm3 | 14/31 (45.2%) | REF | 0.26, 2.67 | 0.75 | 19/43 (44.2%) | REF | 0.12, 0.76 | 0.01 | 17/25 (68.0%) | REF | 0.17, 1.36 | NS |
| > = 200 cells/mm3 | 18/50 (36.0%) | 0.83 | 33/118 (28.0%) | 0.3 | 61/106 (57.5%) | 0.48 | ||||||
| Arm | ||||||||||||
| SOC | 21/62 (33.9%) | REF | 1.14, 19.61 | 0.03 | 21/119 (17.6%) | REF | 6.26,41.14 | <0.01 | 48/98 (49.0%) | REF | 3.69,50.64 | <0.01 |
| Intervention | 11/19 (57.9%) | 4.72 | 31/42 (73.8%) | 16.05 | 30/33 (90.9%) | 13.67 | ||||||
| Age Group | ||||||||||||
| 18–34 | 16/50 (32.0%) | REF | 0.45, 4.23 | 0.57 | 32/84 (38.1%) | REF | 0.23, 1.36 | NS | 31/60 (51.7%) | REF | 0.77, 3.93 | NS |
| 35+ | 16/31 (51.6%) | 1.38 | 20/77 (26.0%) | 0.56 | 47/71 (66.2%) | 1.74 | ||||||
| Sex | ||||||||||||
| Male | 30/73 (41.1%) | REF | 0.07, 4.20 | 0.55 | 35/105 (33.3%) | REF | 0.33, 2.00 | NS | 78/131 (59.5%) | |||
| Female | 2/8 (25.0%) | 0.53 | 17/56 (30.4%) | 0.81 | 0/0 (-%) | |||||||
| Employment | ||||||||||||
| Employed full-time | 10/23 (43.5%) | REF | 0.19, 2.70 | 0.63 | 8/22 (36.4%) | REF | 0.23, 2.46 | NS | 39/64 (60.9%) | REF | 0.32, 1.60 | NS |
| Not fulltime employed | 22/58 (37.9%) | 0.72 | 44/139 (31.7%) | 0.75 | 39/67 (58.2%) | 0.72 | ||||||
| Education Status | ||||||||||||
| Secondary school, or less | 6/21 (28.6%) | REF | 0.52, 9.30 | 0.29 | 26/82 (31.7%) | REF | 0.31, 1.79 | NS | 72/125 (57.6%) | |||
| Technical training, college | 26/60 (43.3%) | 2.19 | 26/79 (32.9%) | 0.75 | 6/6 (100.0%) | |||||||
| Marriage Status | ||||||||||||
| Married/live with sex partner | 16/28 (57.1%) | REF | 0.11, 1.12 | 0.08 | 31/101 (30.7%) | REF | 0.46, 2.52 | NS | 38/58 (65.5%) | REF | 0.24, 1.28 | NS |
| Others | 16/53 (30.2%) | 0.35 | 21/60 (35.0%) | 1.07 | 40/73 (54.8%) | 0.55 | ||||||
NS: Not significant, p > 0.05
Table 4.
Association between baseline drug use and ART use at 52-week follow-up of all sites (n = 345).
| Covariates | Whole sample |
Unadjusted Model |
Multivariable Model |
||||
|---|---|---|---|---|---|---|---|
| On ART(%) | OR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Overall | 181/345 (52.5%) | ||||||
| Number of injected/non-injected opiates in last 3 months | |||||||
| 0–1 | 115/202 (56.9%) | REF | 0.42, 1.00 | 0.05 | REF | 0.39, 1.26 | NS |
| >1 | 66/143 (46.2%) | 0.65 | 0.7 | ||||
| Number of injected/non-injected stimulants in last 3 months | |||||||
| 0–1 | 156/299 (52.2%) | REF | 0.59, 2.03 | NS | REF | 0.86, 3.96 | NS |
| >1 | 25/46 (54.3%) | 1.09 | 1.85 | ||||
| Number of injected/non-injected other drugs in last 3 months | |||||||
| 0–1 | 157/280 (56.1%) | REF | 0.26, 0.80 | 0.01 | REF | 0.35, 1.32 | NS |
| >1 | 24/65 (36.9%) | 0.46 | 0.68 | ||||
| Days injected drugs last month | |||||||
| 0–20 days last month | 72/124 (58.1%) | REF | 0.45, 1.10 | NS | REF | 0.37, 1.03 | NS |
| >20 days last month | 109/221 (49.3%) | 0.7 | 0.62 | ||||
| CD4 count at screening | |||||||
| <200 cells/mm3 | 53/92 (57.6%) | REF | 0.47, 1.22 | NS | REF | 0.34, 1.03 | NS |
| > = 200 cells/mm3 | 128/253 (50.6%) | 0.75 | 0.59 | ||||
| Arm | |||||||
| SOC | 111/253 (43.9%) | REF | 2.37, 6.98 | <0.01 | REF | 2.57, 8.28 | <0.01 |
| Intervention | 70/92 (76.1%) | 4.07 | 4.61 | ||||
| Age Group | |||||||
| 18–34 | 90/176 (51.1%) | REF | 0.73, 1.70 | NS | REF | 0.53, 1.42 | NS |
| 35+ | 91/169 (53.8%) | 1.11 | 0.87 | ||||
| Sex | |||||||
| Male | 156/284 (54.9%) | REF | 0.33, 1.00 | 0.05 | REF | 0.39, 1.58 | NS |
| Female | 25/61 (41.0%) | 0.57 | 0.79 | ||||
| Employment | |||||||
| Employed full-time | 55/98 (56.1%) | REF | 0.51, 1.30 | NS | REF | 0.69, 2.16 | NS |
| Not fulltime employed | 126/247 (51.0%) | 0.81 | 1.22 | ||||
| Education Status | |||||||
| Secondary school, or less | 109/203 (53.7%) | REF | 0.58, 1.36 | NS | REF | 1.33, 4.56 | <0.01 |
| Technical training, college | 72/142 (50.7%) | 0.89 | 2.46 | ||||
| Marriage Status | |||||||
| Married/live with sex partner | 91/172 (52.9%) | REF | 0.63, 1.47 | NS | REF | 0.46, 1.24 | NS |
| Others | 90/173 (52.0%) | 0.97 | 0.75 | ||||
| Study Site | |||||||
| Indonesia | 29/77 (37.7%) | REF | REF | ||||
| Ukraine | 71/150 (47.3%) | 1.49 | 0.85, 2.61 | NS | 1.97 | 0.95, 4.11 | NS |
| Vietnam | 81/118 (68.6%) | 3.62 | 1.98, 6.62 | <0.01 | 7.54 | 3.17,17.94 | <0.01 |
NS: Not significant, p > 0.05
Table 5.
Association between baseline drug use and ART use at 52-week follow-up visit separately by study site
| Covariates | Indonesia |
Ukraine |
Vietnam |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On ART (%) | aOR | 95% CI | p-value | On ART (%) | aOR | 95% CI | p-value | On ART (%) | aOR | 95% CI | p-value | |
| Overall | 29/77 (37.7%) | 71/150 (47.3%) | 81/118 (68.6%) | |||||||||
| Number of injected/non-injected opiates in last 3 months | ||||||||||||
| 0–1 | 23/57 (40.4%) | REF | 0.20, 2.47 | NS | 22/44 (50.0%) | REF | 0.36, 2.11 | NS | 70/101 (69.3%) | REF | 0.13, 1.78 | NS |
| >1 | 6/20 (30.0%) | 0.71 | 49/106 (46.2%) | 0.87 | 11/17 (64.7%) | 0.48 | ||||||
| Number of injected/non-injected stimulants in last 3 months | ||||||||||||
| 0–1 | 28/76 (36.8%) | 51/110 (46.4%) | REF | 0.64, 3.64 | NS | 77/113 (68.1%) | REF | 0.42,44.53 | NS | |||
| >1 | 1/1 (100.0%) | 20/40 (50.0%) | 1.52 | 4/5 (80.0%) | 4.31 | |||||||
| Number of injected/non-injected other drugs in last 3 months | ||||||||||||
| 0–1 | 23/54 (42.6%) | REF | 0.12, 1.35 | NS | 53/108 (49.1%) | REF | 0.43, 2.41 | NS | 81/118 (68.6%) | |||
| >1 | 6/23 (26.1%) | 0.41 | 18/42 (42.9%) | 1.02 | 0/0 (-%) | |||||||
| Days injected drugs last month | ||||||||||||
| 0–20 days last month | 16/33 (48.5%) | REF | 0.17, 1.41 | NS | 28/54 (51.9%) | REF | 0.32, 1.66 | NS | 28/37 (75.7%) | REF | 0.18, 1.33 | NS |
| >20 days last month | 13/44 (29.5%) | 0.48 | 43/96 (44.8%) | 0.73 | 53/81 (65.4%) | 0.49 | ||||||
| CD4 count at screening | ||||||||||||
| <200 cells/mm3 | 11/27 (40.7%) | REF | 0.38, 3.95 | NS | 23/41 (56.1%) | REF | 0.20, 1.08 | NS | 19/24 (79.2%) | REF | 0.12, 1.35 | NS |
| > = 200 cells/mm3 | 18/50 (36.0%) | 1.23 | 48/109 (44.0%) | 0.47 | 62/94 (66.0%) | 0.41 | ||||||
| Arm | ||||||||||||
| SOC | 21/59 (35.6%) | REF | 0.38, 4.54 | NS | 41/111 (36.9%) | REF | 2.50,15.50 | <0.01 | 49/83 (59.0%) | REF | 2.56,34.91 | <0.01 |
| Intervention | 8/18 (44.4%) | 1.32 | 30/39 (76.9%) | 6.23 | 32/35 (91.4%) | 9.46 | ||||||
| Age Group | ||||||||||||
| 18–34 | 15/47 (31.9%) | REF | 0.58,5.13 | NS | 42/79 (53.2%) | REF | 0.26, 1.26 | NS | 33/50 (66.0%) | REF | 0.45, 2.75 | NS |
| 35+ | 14/30 (46.7%) | 1.73 | 29/71 (40.8%) | 0.57 | 48/68 (70.6%) | 1.12 | ||||||
| Sex | ||||||||||||
| Male | 28/69 (40.6%) | REF | 0.02, 2.51 | NS | 47/97 (48.5%) | REF | 0.41, 2.03 | NS | 81/118 (68.6%) | |||
| Female | 1/8 (12.5%) | 0.23 | 24/53 (45.3%) | 0.91 | 0/0 (-%) | |||||||
| Employment | ||||||||||||
| Employed full-time | 8/22 (36.4%) | REF | 0.30, 3.86 | NS | 9/19 (47.4%) | REF | 0.54,5.14 | NS | 38/57 (66.7%) | REF | 0.51, 2.99 | NS |
| Not fulltime employed | 21/55 (38.2%) | 1.08 | 62/131 (47.3%) | 1.67 | 43/61 (70.5%) | 1.24 | ||||||
| Education Status | ||||||||||||
| Secondary school, or less | 6/18 (33.3%) | REF | 0.33, 4.49 | NS | 28/73 (38.4%) | REF | 1.14,5.38 | 0.02 | 75/112 (67.0%) | |||
| Technical training, college | 23/59 (39.0%) | 1.22 | 43/77 (55.8%) | 2.48 | 6/6 (100.0%) | |||||||
| Marriage Status | ||||||||||||
| Married/live with sex partner | 13/30 (43.3%) | REF | 0.20, 1.87 | NS | 42/95 (44.2%) | REF | 0.62, 2.88 | NS | 36/47 (76.6%) | REF | 0.16, 1.11 | NS |
| Others | 16/47 (34.0%) | 0.62 | 29/55 (52.7%) | 1.34 | 45/71 (63.4%) | 0.42 | ||||||
NS: Not significant, p > 0.05
At the 26-week follow-up visit, among the whole sample, participants who used more than one other drugs (eg., marijuana, ketamine, benzodiazepines) had lower odds of ART use than those who used only one or less other drug (aOR = 0.47, 95% CI 0.22–1.00, p = .05). Participants who injected drugs more than 20 days in the last month were less likely to use ART than those who reported ≤20 days of drug injection (aOR = 0.59, 95% CI 0.36–0.97, p = .04) (Table 2). Among participants in Indonesia, HIV-infected PWID who used more than one other drugs (had considerably lower odds of being on ART compared to those who used 0 or 1 other drugs (aOR = 0.21, 95% CI 0.05–0.82, p = .03), and those who injected drugs more than 20 days in the last month had lower odds of initiating ART than those who injected ≤20 days (aOR = 0.35, 95%CI 0.12–1.06; p = .06) (Table 3).
In the whole sample and within the Ukraine and Vietnam sites, using more than one opiate or stimulant was not strongly associated with ART use at 26 weeks (Tables 2 and 3).
At the 52-week follow up visit, the unadjusted analysis of the whole sample showed that participants using more than one opiate or other drug had lower odds of ART use (aOR = 0.65, 95% CI 0.42–1.00, p = .05; aOR = 0.46, 95% CI 0.26–0.80, p = .01). However, the associations did not remain after adjusting for potential confounders in the multivariable logistic regression (p = .24 and p = .25, respectively) (Table 4). The specified drug use patterns were also not associated with ART use within each study site after 52 weeks of follow-up (Table 5).
Discussion
The result showed that drug use patterns at baseline, specifically using other (non-opiate/non-stimulant drugs eg., marijuana, ketamine, benzodiazepines) in the last 3 months and injecting any drug for more than 20 days in the last month at baseline, were associated with lower odds of self-reported ART use at the 26 week visit, but the association was less evident at 52 weeks, for the whole study sample as well as by study site.
At the 26-week follow up, using more than one other drugs (non-opiate/non-stimulant drugs) was associated with lower odds of self-reported ART use in Indonesian participants and in the whole sample. In this study, nearly all participants used at least one opiate (99.7%), thus the use of any stimulant or any other drug essentially constituted poly-drug use with opiates in this sample. These results suggest that using more than one other drug may reduce the odds of future ART initiation among HIV infected PWID. In addition, it possibly reflects the combined impact of using both other drugs and opiates on lowering the likelihood of study participants to initiate ART.
Some other drugs such as sedatives (Waller, 2018), are commonly used with opiates to increase feelings of arousal, reduce heroin withdrawal symptoms and relieve psychological stress, anxiety, sleep deprivation, and chronic pain among drug users (Fatseas et al., 2009; Kerr et al., 2012). This may explain the common use of other drugs among PWID in this study. The poly-drug use may further inflict adverse effects on the mental, physical, and social aspects of the individual’s life. Studies in the US reported that individuals who used heroin and sedatives faced challenges in stable housing, employment, overdose, injury, education, and general health, compared with those who only used heroin (Fatseas et al., 2009; Moses & Greenwald, 2019). Results from the HPTN 074 baseline analysis showed that Indonesian participants used opioids with drugs in other categories, namely marijuana and benzodiazepines, more frequently than the other countries in which 41.5% participants in Indonesia used opioids with Marijuana and 52.7% with benzodiazepine compared to that of 64% and 0.4% in Ukraine and 0.9% and 0% in Vietnam (Lancaster et al., 2018). Our results in Indonesia further confirmed this phenomenon, which revealed that a higher number of other drugs (non-opiate/non-stimulant drugs) used by participants was inversely associated with their ART use at 26 weeks. This association was not evident in the other two sites. In Vietnam, it might be because the use of other drugs was not very common (19.1% used at least one other drug, no one used more than one other drug). The use of other drugs in Ukraine (80.7% used at least one other drug, 28% used more than one other drugs) was similar to that among Indonesian participants (77.8% used at least one drug and 27.2% used more than one drugs), but the considerably different drug use patterns (opioid, stimulants and other drugs) among Ukrainian and Indonesian participants (Table 1) might result in different effect on ART initiation between these two sites.
Specific patterns of drug use in a given site considerably differed depending on the availability of local drugs such as illegally manufactured methadone (84.2%) and home-made opioids (75.7%) in Ukraine, heroin (81.8%) in Indonesia, and heroin (99.5%) in Vietnam (Lancaster et al., 2018). Among the Ukrainian PWID, the use of opiates and other drugs was highly common (99.4% and 80.7% respectively), but the prevalence of stimulants use was much lower than that among Indonesian PWID (48.4% and 72.8%, respectively). The difference in drug use patterns between Indonesia and Ukraine sites might result in the differences in effects on ART use between these two sites.
Our finding that the higher frequency of injection drug use at baseline (more than 20 days per month) was associated with lower odds of ART use in the whole analysis was consistent with previous studies, which reported that increased use of any drug might result in a lower rate of ART initiation and higher rates of ART interruptions among PWID (Azar et al., 2015; Hicks et al., 2007). This finding emphasizes the importance of integrating substance use treatment with HIV care and treatment for PWID, because reducing drug use frequency may facilitate the initiation and adherence of ART among this key population (Gregory M. Lucas, 2011; Rosen et al., 2012).
However, the association between baseline drug use patterns and ART use at 26 weeks was not evident at the 52-week visit. The underlying reason for this result might be the change in drug use patterns over time. With improved ART use and reduction in substance use resulting from the HPTN 074 intervention effects, the mental and physical health of PWID, as well as their social relations, might be improved. Then, the improvement of PWID’s well-beings would facilitate and sustain the effect on follow-up ART, which eventually modifies the effects of drug use before treatment on later ART performance after a period of time (Carl Latkin et al., 2015; Davis et al., 2018; Go et al., 2019; Kalichman et al., 1999; Latkin et al., 2017; Wolfe et al., 2010).
There are several limitations to interpret this paper’s findings. First, information on drug use and ART status were collected via self-report using a face-to-face interview. Thus, data might be susceptible to recall and social desirability bias. Second, the lost-to-follow-up rate might result in overestimation of ART use in follow-up surveys, particularly in Vietnam. This paper used data from a randomized controlled trial HPTN 074, with an intervention that might affect the association between drug use and ART uptake among PWID. The sample size of each study site was too small to detect associations between a regional-specific drug use patterns and follow-up ART use.
Conclusion
The use of more than one non-opiate/non-stimulant drug, combined with opiates, within three months before baseline was associated with lower odds of ART use at 26-week follow-up. The injection of any drugs for more than 20 days in the last month at baseline reduced the odds of ART initiation at the 26-week visit. The results emphasized the importance of integrated substance use treatment and HIV intervention programs for HIV infected PWID. It also suggested that the initial addiction assessment should focus on the drug use patterns, which combines new drugs and traditional ones such as opiates and stimulants so that appropriate strategies for referral to ART and substance use treatment should be made.
Acknowledgments
The authors would like to express our gratitude to the HPTN 074 protocol team and the research teams from Indonesia, Ukraine and Vietnam and all the outreach community workers from each of the three sites. Also, we are grateful for the support in Vietnam from the Vietnam Administration of HIV/AIDS Control, Thai Nguyen Department of Health and the Pho Yen Health District Center; and from Ukraine the NGO “Club Eney” and the Gromashevsky Institute of Epidemiology and Infectious Diseases. Lastly, we thank the participants for their invaluable contributions to this research.
Funding
This study take data from the parent trial which were funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH); award numbers UM1AI068619 [HPTN Leadership and Operations Center], UM1AI068617 [HPTN Statistical and Data Management Center], UM1AI068613 [HPTN Laboratory Center] and the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure statement
The authors declare that they have no competing interests. Additional data and materials are available upon requests sent to the corresponding author.
Ethics and consent
The study protocol, available at clinicaltrials.gov (NCT02935296), was approved by at least one local IRB in each site, namely University of Indonesia, Ukrainian Institute of Public Health Policy, Vietnam Administration of HIV/AIDS Control, University of North Carolina at Chapel Hill and Faculty of Medicine. All study participants provided written informed consent in their local languages, or English if preferred, upon joining the study.
Paper context
Drug use pattern detrimentally affects the well-beings of PWID, resulting in delay in ART use. Our study’s results demonstrate that using more non-opiate/non-stimulant drug and more days injecting drugs were associated with lower odds of ART use at 26-week follow-up. This implies the importance of medication-assisted treatment in association with HIV treatment to enhance ART uptake and adherence among PWID.
References
- Adedinsewo DA, Wei SC, Robertson M, Rose C, Johnson CH, Dombrowski J, & Skarbinski J. (2014). Timing of antiretroviral therapy initiation in a nationally representative sample of HIV-infected adults receiving medical care in the United States. AIDS Patient Care and STDs, 28(12), 613–621. 10.1089/apc.2014.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar P, Wood E, Nguyen P, Luma M, Montaner J, Kerr T, & Milloy MJ (2015). Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: A longitudinal analysis. BMC Infectious Diseases, 15(1), 193. 10.1186/s12879-015-0913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, McCrimmon T, Dasgupta A, Gilbert L, Terlikbayeva A, Hunt T, Primbetova S, Wu E, Darisheva M, & El-Bassel N. (2018). Individual, social, and structural factors affecting antiretroviral therapy adherence among HIV-positive people who inject drugs in Kazakhstan. The International Journal on Drug Policy, 62, 43–50. 10.1016/j.drugpo.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, & Larney S. (2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. The Lancet Global Health, 5(12), e1192–e1207. 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M, Lavie E, Denis C, & Auriacombe M. (2009). Self-perceived motivation for benzodiazepine use and behavior related to benzodiazepine use among opiate-dependent patients. Journal of Substance Abuse Treatment, 37(4), 407–411. 10.1016/j.jsat.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Go VF, Hershow RB, Kiriazova T, Sarasvita R, Bui Q, Latkin CA, Rose S, Hamilton E, Lancaster KE, Metzger D, Hoffman IF, & Miller WC (2019). Client and provider perspectives on antiretroviral treatment uptake and adherence among people who inject drugs in Indonesia, Ukraine and Vietnam: HPTN 074. AIDS and Behavior, 23 (4), 1084–1093. 10.1007/s10461-018-2307-y [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Mimiaga MJ, Israel J, Andres Bedoya C, & Safren SA (2013). Substance use predictors of poor medication adherence: The role of substance use coping among HIV-infected patients in opioid dependence treatment. AIDS and Behavior, 17(1), 168–173. 10.1007/s10461-012-0319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, A. (2018). Assessment of HIV services packages for key populations in Indonesia. Washington, DC: APMG Health. [Google Scholar]
- Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, Hellinger J, Gaist P, Gebo KA, & Network HIVR (2007). The impact of illicit drug use and substance abuse treatment on adherence to HAART. AIDS Care, 19(9), 1134–1140. 10.1080/09540120701351888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Catz S, & Ramachandran B. (1999). Barriers to HIV/ AIDS treatment and treatment adherence among African-American adults with disadvantaged education. Journal of the National Medical Association, 91(8), 439–446. [PMC free article] [PubMed] [Google Scholar]
- Kerr T, Marshall BDL, Milloy MJ, Zhang R, Guillemi S, Montaner JSG, & Wood E. (2012). Patterns of heroin and cocaine injection and plasma HIV-1 RNA suppression among a long-term cohort of injection drug users. Drug and Alcohol Dependence, 124(1–2), 108–112. 10.1016/j.drugalcdep.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad KE, Hutton HE, Monroe AK, Anderson G, Moore RD, & Chander G. (2016). A qualitative study of barriers to and facilitators of optimal engagement in care among PLWH and substance use/misuse. BMC Research Notes, 9(1), 229. 10.1186/s13104-016-2032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KE, Hoffman IF, Hanscom B, Ha TV, Dumchev K, Susami H, Rose S, Go VF, Reifeis SA, Mollan KR, Hudgens MG, Piwowar-Manning EM, Richardson P, Dvoriak S, Djoerban Z, Kiriazova T, Zeziulin O, Djauzi S, Ahn CV, Latkin C, . . . Team, H. S. (2018). Regional differences between people who inject drugs in an HIV prevention trial integrating treatment and prevention (HPTN 074): A baseline analysis. Journal of the International AIDS Society, 21(10), e25195. 10.1002/jia2.25195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C, Zelaya C, Lancaster K, & Tobin K. (2017). Intervention manual - A research guide for counselors and system navigators conducting the intervention for HIV prevention trials Network 074. https://www.hptn.org/sites/default/files/inline-files/HPTN074SSPAppA_Intervention%20Manual_28Sep2017_V2.3.pdf
- Lucas GM (2011). Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sciences, 88(21–22), 948–952. 10.1016/j.lfs.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, & Mattick RP (2008). Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. The Lancet, 372(9651), 1733–1745. 10.1016/S0140-6736(08)61311-2 [DOI] [PubMed] [Google Scholar]
- Miller WC, Hoffman IF, Hanscom BS, Ha TV, Dumchev K, Djoerban Z, Rose SM, Latkin CA, Metzger DS, Lancaster KE, Go VF, Dvoriak S, Mollan KR, Reifeis SA, Piwowar-Manning EM, Richardson P, Hudgens MG, Hamilton EL, Sugarman J, Eshleman SH, . . . Burns DN (2018). A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment (HPTN 074): A randomised, controlled phase 3 feasibility and efficacy study. The Lancet, 392(10149), 747–759. 10.1016/s0140-6736(18)31487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, & Grant I. (2012). Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care, 24(12), 1504–1513. 10.1080/09540121.2012.672718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses TEH, & Greenwald MK (2019). History of regular nonmedical sedative and/or alcohol use differentiates substance-use patterns and consequences among chronic heroin users. Addictive Behaviors, 97, 14–19. 10.1016/j.addbeh.2019.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Black AC, Arnsten JH, Simoni JM, Wagner GJ, Goggin K, Remien RH, Golin CE, Wang Y, Bangsberg D, & Liu HH (2012). ART adherence changes among patients in community substance use treatment: A preliminary analysis from MACH14. AIDS Research and Therapy, 9(1), 30. 10.1186/1742-6405-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RZ, & McCann K. (2011). 54 - Substance abuse and child abuse. In Jenny C. (Ed.), Child abuse and neglect (pp. 529–538). W.B. Saunders. 10.1016/B978-1-4160-6393-3.00053-1 [DOI] [Google Scholar]
- Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, & Clarke J. (2000). Adherence to antiretroviral therapy among HIV-infected methadone patients: Effect of ongoing illicit drug use. The American Journal of Drug and Alcohol Abuse, 26(2), 195–205. 10.1081/ADA-100100600 [DOI] [PubMed] [Google Scholar]
- Stimmel B, & Kreek MJ (2000). Neurobiology of addictive behaviors and its relationship to methadone maintenance. The Mount Sinai Journal of Medicine, New York, 67(5–6), 375–380. [PubMed] [Google Scholar]
- UNAIDS. (2018). Miles to go: Closing gaps breaking barriers righting injustices. UNAIDS. [Google Scholar]
- UNAIDS. (2019). HIV and AIDS estimates. Country Factsheets Vietnam. https://www.unaids.org/en/regionscountries/countries/vietnam
- Vourli G, Nikolopoulos G, Paparizos V, Skoutelis A, Metallidis S, Gargalianos P, Papadopoulos A, Chini M, Sipsas NV, Psychogiou M, Chrysos G, Sambatakou H, Gogos C, Katsarou O, Paraskeva D, Dedes N, Touloumi G, & Greek HIVPG (2018). HIV cascade of care in Greece: Useful insights from additional stages. PLoS One, 13(11), e0207355. 10.1371/journal.pone.0207355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller DG (2018). Medical pharmacology and therapeutics E-Book. Substance Abuse and Dependence, 685. [Google Scholar]
- Wolfe D, Carrieri MP, & Shepard D. (2010). Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. The Lancet, 376(9738), 355–366. 10.1016/S0140-6736(10)60832-X [DOI] [PubMed] [Google Scholar]