Background
In the last years, 18F-fluorodeoxyglucose PET/computed tomography (18F-FDG PET/CT) has demonstrated its utility for the evaluation of gastric cancer; however, considering some histotypes such as gastric signet ring cell carcinoma (GSRCC) the results are limited. The aim of this review is to analyze the diagnostic performance of 18F-FDG PET and PET/CT for the assessment of GSRCC.
Methods
A wide literature search of the PubMed/MEDLINE, Scopus, Embase and Cochrane library databases was made to find relevant published articles about the diagnostic performance of 18F-FDG PET or PET/CT for the evaluation of GSRCC.
Results
The comprehensive computer literature search revealed 179 articles. On reviewing the titles and abstracts, 162 articles were excluded because the reported data were not within the field of interest. Nine studies were included in the review and references were also screened for additional articles. Finally, 26 articles were selected and retrieved in full-text version.
Conclusion
Despite some limitations affect our review, GSRCC seems to have low 18F-FDG uptake, and therefore 18F-FDG PET or PET/CT reveals impaired sensitivity for its evaluation. However, a correlation between 18F-FDG uptake and some clinico-pathologic features (such as stage, depth of invasion, size and presence of nodal metastasis) has been demonstrated. Besides, a possible prognostic role of PET/CT features is starting to emerge.
Keywords: 18F-fluorodeoxyglucose, gastric cancer, gastric signet ring cell cancer, PET, SRCC
Introduction
Gastric cancer is one of the most common and aggressive cancer worldwide. Despite the decrease in incidence in the last decades, the survival rate remains low because the clinical presentation is often absent or nonspecific [1,2].
Gastric cancer can be histologically divided according to a different classification. World Health Organization (WHO) is one of the most used such as Lauren classification that divides gastric cancer mainly into intestinal and diffuse cancer, comprising gastric signet ring cell cancer (GSRCC) and other types [3].
Complete surgical removal of the primitive tumor with nodal dissection is a pivotal part of the treatment of disease. However, recurrence is often present, ranging from 22 to 48% of the cases [4].
Accurate and early staging of the disease is fundamental for treatment planning and nowadays a complete workup includes computed tomography (CT), gastroscopy and laparoscopy [1]. In the last years, 18F-fluorodeoxyglucose PET/computed tomography (18F-FDG PET/CT) has increased its role for staging and restaging of disease, because of its ability to provide both anatomic and functional information. Its use has been widely demonstrated especially in treatment planning [5].
Stomach evaluation with 18F-FDG PET/CT may be challenging due to the risk of having heterogeneous gastric uptake distribution owing to different inflammatory and physiologic states. Besides, diabetic patients, especially in therapy with oral hypoglycemic drugs, may present an increased tracer uptake in the bowel, which affects and limits the evaluation of the scan in the gastrointestinal tract [6,7].
Despite the proven usefulness of 18F-FDG PET/CT for the evaluation of patients affected by gastric cancer, its role remains controversial given its low general sensitivity [8]. In particular, gastric cancer seems to be non 18F-FDG-avid in a high percentage of cases, up to 53% as reported in the literature, and impaired 18F-FDG uptake is particularly evident when GSRCC is present [1]. The aim of this systematic review is to analyze the performance of 18F-FDG PET or PET/CT for the assessment of GSRCC.
Materials and methods
Search strategy
A wide literature search of the PubMed/MEDLINE, Scopus, Embase and Cochrane library databases was made to find significant published articles concerning the usefulness of PET and PET/CT for the evaluation of GSRCC. Search algorithms were the following: (1) “signet ring cell” AND “PET”, (2) “SRCC” and “PET”, (3) “signet” AND “ring” AND “cell” AND “PET”, (4) “signet ring cell” AND “PET”, (5) “SRCC” and “PET” and (6) “signet” AND “ring” AND “cell” AND “positron” AND “emission” AND “tomography”.
No beginning date limit was applied to the search and it was updated until 31 October 2020. Only articles in the English language were considered; preclinical studies, conference proceedings, reviews and editorials were excluded. To expand our search, the references of the retrieved articles were also screened for additional papers.
Study selection
Two researchers (F.D. and D.A.) independently reviewed the titles and the abstracts of the retrieved articles. The same two researchers then independently reviewed the full-text version of the remaining articles to determine their eligibility for inclusion. The studies with less than eight patients affected by GSRCC were conventionally excluded from the review due to the low sample analyzed. The quality assessment, including the risk of bias and applicability concerns, was carried out using Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 evaluation [9].
Data abstraction
For each included study, data concerning the basic study were collected (author names, year of publication, country of origin and type of study) and PET device used, number of patients evaluated and number of patients affected by GSRCC. The main findings of the articles included in this review are reported in the Results.
Results
Literature search
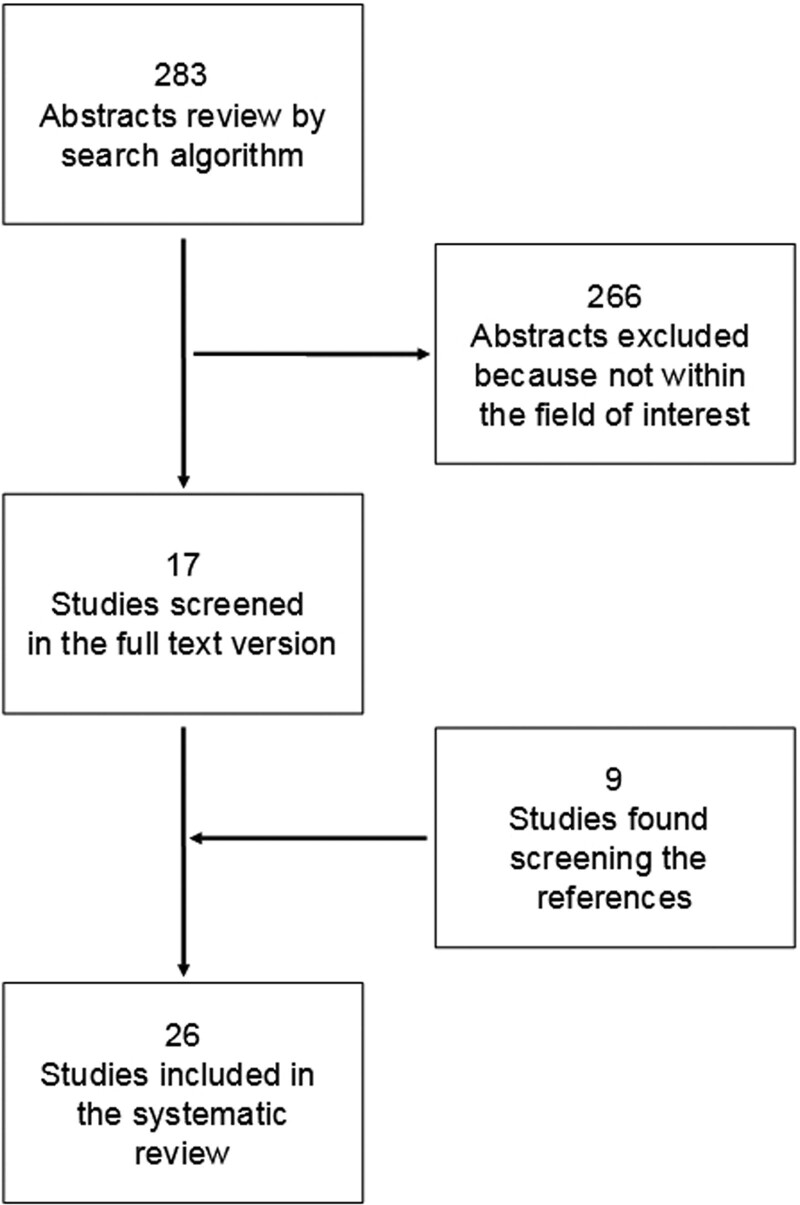
A total of 283 articles were extrapolated with the computer literature search; by reviewing the titles and abstracts, 266 of them were excluded because the reported data were not within the field of interest of this review. Seventeen articles were selected and retrieved in full-text version [10–26]; nine additional studies were also found screening the references of these articles [27–35]. A total of 26 articles were then included in the systematic review [10–35] (Fig. 1).
Fig. 1.
Flow chart of the search of eligible studies on the diagnostic performance of 18F-FDG PET or PET/CT for the assessment of GSRCC.
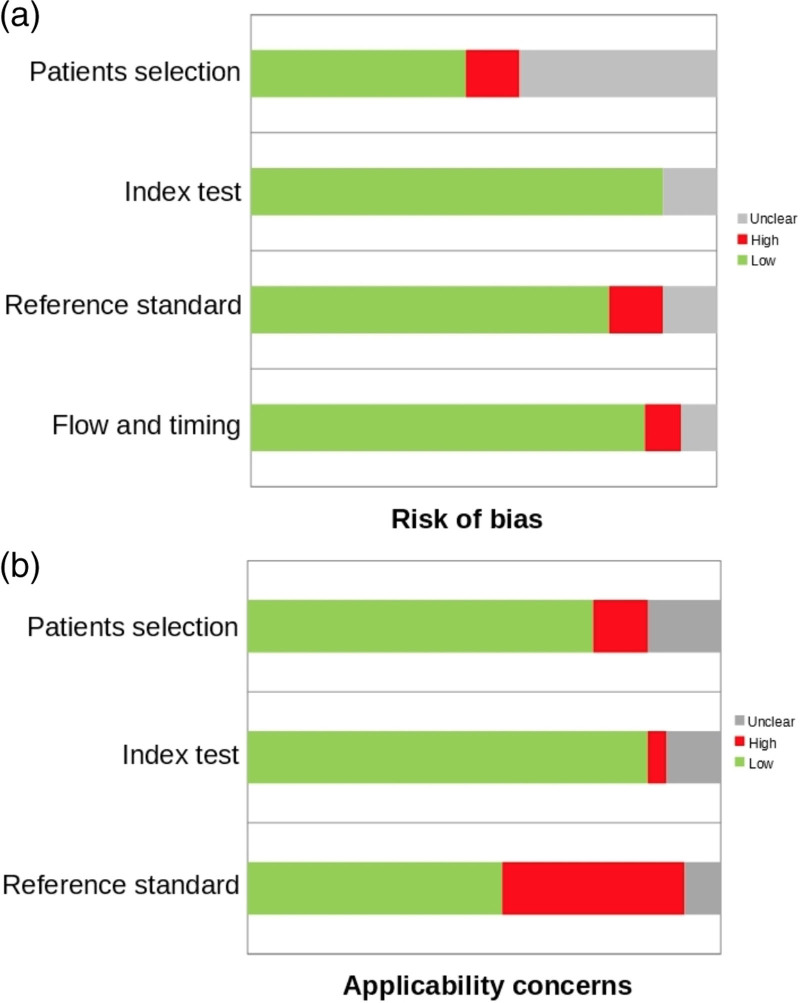
In general, the quality assessment using QUADAS-2 evaluation underlined a low risk of bias (Fig. 2a) and low problem regarding applicability concerns (Fig. 2b).
Fig. 2.
QUADAS-2 quality assessment for risk of bias (2a) and applicability concerns (2b). QUADAS, Quality Assessment of Diagnostic Accuracy Studies.
Among the 26 studies included in the systematic review, 24 were of retrospective nature [10–17,19–31,33–35] while two were prospective one [18,32]. In 9 studies the device used was PET only [10,11,13–15,17,27,28,30], in 1 study both PET and PET/CT were used [18], while in the remaining 16 studies hybrid PET/CT tomograph was used [12,16,19–26,29,31–35]. 18F-FDG was used in all the considered studies, except for one work in which both 18F-FDG and 18F-fluorothymidine (18F-FLT) were used [18]. The main characteristics of the studies and their results are briefly presented in Tables 1 and 2.
Table 1.
Characteristics of the studies considered for the review
| First author | References | Year | Country | Study Design | Isotope | N. Pts | Gender male:Female | GSRCC pts |
|---|---|---|---|---|---|---|---|---|
| De Potter et al. | 10 | 2002 | Belgium | Retrospective | 18F-FDG | 20 | 16:4 | 8 (40.0%) |
| Yoshioka et al. | 11 | 2003 | Japan | Retrospective | 18F-FDG | 42 | 29:13 | 8 (19.0%) |
| Stahl et al. | 28 | 2003 | Germany | Retrospective | 18F-FDG | 40 | 27:13 | 11 (27.5%) |
| Mochiki et al. | 27 | 2004 | Japan | Retrospective | 18F-FDG | 85 | 55:30 | 9 (10.6%) |
| Chen et al. | 15 | 2005 | South Korea | Retrospective | 18F-FDG | 73 | 61:12 | 9 (12.3%) |
| Kim et al. | 14 | 2006 | Korea | Retrospective | 18F-FDG | 68 | 49:19 | 12 (18%)a |
| Yamada et al. | 13 | 2006 | Japan | Retrospective | 18F-FDG | 35 | 24:11 | 9 (25.7%) |
| Herrmann et al. | 18 | 2007 | Germany | Prospective | 18F-FDG, 18F-FLT | 45 | 31:14 | 27 (60%) |
| Park et al. | 34 | 2009 | Korea | Retrospective | 18F-FDG | 105 | 75:30 | 23 (21.9%) |
| Hur et al. | 33 | 2010 | Korea | Retrospective | 18F-FDG | 133 | 92:41 | 25 (18.8%)a |
| Alakus et al. | 17 | 2010 | Germany | Retrospective | 18F-FDG | 35 | 28:7 | 17 (48.6%) |
| Choi et al. | 19 | 2010 | Korea | Retrospective | 18F-FDG | 40 | 28:12 | 40 (100%) |
| Kim et al. | 35 | 2011 | Korea | Retrospective | 18F-FDG | 136 | 88:51 | 19 (14.0%) |
| Ha et al. | 12 | 2011 | Korea | Retrospective | 18F-FDG | 78 | 53:25 | 17 (21.8%) |
| Pak et al. | 16 | 2011 | Korea | Retrospective | 18F-FDG | 41 | 19:22 | 41 (100.0%) |
| Youn et al. | 31 | 2012 | Korea | Retrospective | 18F-FDG | 396 | 278:118 | 92 (23.2%) |
| Smyth et al. | 32 | 2012 | USA | Prospective | 18F-FDG | 113 | 68:45 | 52 (46.0%)c |
| Lee et al. | 20 | 2012 | Korea | Retrospective | 18F-FDG | 271 | 171:100 | 31 (11.4%)a |
| Takebayashi et al. | 30 | 2013 | Japan | Retrospective | 18F-FDG | 50 | 29:21 | 25 (50.0%)b |
| Park et al. | 21 | 2014 | Korea | Retrospective | 18F-FDG | 74 | 56:18 | 16 (22%) |
| Charalampakis et al. | 23 | 2015 | USA | Retrospective | 18F-FDG | 60 | 38:22 | 31 (51.6%) |
| Chen et al. | 25 | 2016 | China | Retrospective | 18F-FDG | 64 | 38:26 | 19 (29.7%) |
| Park et al. | 29 | 2018 | Korea | Retrospective | 18F-FDG | 124 | 80:44 | 28 (22.6%) |
| Chon et al. | 22 | 2019 | Korea | Retrospective | 18F-FDG | 727 | 494:233 | 110 (15.1%) |
| Harada et al. | 24 | 2020 | USA | Retrospective | 18F-FDG | 59 | 43:16 | 26 (44.1%) |
| Arslan et al. | 26 | 2020 | Turkey | Retrospective | 18F-FDG | 341 | 256:85 | 92 (26.9%) |
; 18F-FDG, 18F-fluorodeoxyglucose; 18F-FLT, 18F- fluorothymidine; GSRCC, gastric signet ring cell carcinoma; Pts, patients.
GSRCC and mucinous cancer.
Nonintestinal cancers.
Diffuse histology cancer.
Table 2.
Results and main findings of the studies considered for the review
| First author | Device | Activity mean (MBq) | Uptake time (min) | PET analysis | Setting | GSRCC PET positive | Mean SUV | Main findings |
|---|---|---|---|---|---|---|---|---|
| De Potter et al. | PET | 6.5 MBq/kg | 60 | Qualitative | Restaging | 5 (62.5%) | NS | Detection rate for GSRCC is lower than non-GSRCC |
| Yoshioka et al. | PET | 222 ± 72 | 30–45 | Qualitative and semiquantitative | Staging and restaging | NS | 7.7 ± 2.6 | Lower SUV for GSRCC and nodal metastasisa |
| Stahl et al. | PET | 300 | 40 | Qualitative and semiquantitative | Staging, pretreatment | NS | 4.8 ± 2.8b | Lower detection rate and SUV for nonintestinal cancers. |
| Mochiki et al. | PET | 275–370 | 40 | Qualitative and semiquantitative | Staging, preoperative | 7 (77.8%) | NS | Detection rate for GSRCC is similar to non-GSRCC |
| Chen et al. | PET | 370–555 | 60 | Qualitative, 3-point scale and semiquantitative | Staging, preoperative | NS | 4.2 | Lower SUVmax for GSRCC and mucinous cancer |
| Kim et al. | PET | 555 | 60 | Qualitative, 5-point scale and semiquantitative | Staging, preoperative | 8 (88.9%) | 4.7 ± 1.7 | Lower sensitivity and SUVmax for nodal GSRCC metastasis |
| Yamada et al. | PET | 200–300 | 50–60 | Qualitative and 4-point scale | Staging, preoperative | 6 (66.6%) | NS | Detection rate for GSRCC is lower than non-GSRCC. Low GLUT-1 expression for GSRCCc |
| Herrmann et al. | PET and PET/CT | 300–370 MBq FGD, 300 MBq FLT | 60 for FDG, after injection for FLT | Qualitative and semiquantitative | Staging, preoperative | 27 (100%) for 18F -FLT 16 (59.3%) for 18F -FDG | 5.4 for 18F-FLT, 6.4 for 18F-FDG | Higher sensitivity for 18F-FLT than 18F-FDG, but lower 18F-FLT and FDG uptake for GSRCC than non-GSRCC |
| Park et al. | PET/CT | 370 | 45 | Qualitative and semiquantitative | Restaging | NS | NS | Higher rate of false negative for GSRCC |
| Hur et al. | PET/CT | 440 | 60 | Qualitative and semiquantitative | Staging, preoperative | 22 (88.0%)d | NS | No differences in detection rate for primary and nodal metastasis between GSRCC and non-GSRCC |
| Alakus et al. | PET | 370 | 60 | Qualitative and semiquantitative | Staging, preoperative | NS | 3.0 | Lower SUVmax for GSRCC. Correlation between SUVmax and GLUT-1 expression |
| Choi et al. | PET/CT | 370 | 60 | Qualitative and semiquantitative | Staging, preoperative | 17 (42.5%) | NS | Correlation between SUVmax and some clinicopathological features |
| Kim et al. | PET/CT | 296–444 | 55–60 | Qualitative and semiquantitative | Restaging | NS | NS | No different accuracy in detecting recurrence between GSRCC and non-GSRCC |
| Ha et al. | PET/CT | 5–6 MBq/kg | 60 | Qualitative and semiquantitative | Staging, preoperative | 6 (35.3 %) | NS | Detection rate for primary and nodal localization in GSRCC is lower than non-GSRCC |
| Pak KH | PET/CT | 30–444 | 60 | Qualitative and semiquantitative | Staging, preoperative | NS | 3.8 | Correlation between some clinicopathological features and SUVmax. Worse survival for higher SUVmax |
| Youn et al. | PET/CT | NS | 60 | Qualitative | Staging, preoperative | 29 (31.5%) | NS | Lower detection rate for SRCC and diffuse carcinomas |
| Smyth et al. | PET/CT | NS | 65 ± 10 | Qualitative and 5-point scale | Staging, preoperative | 23 (44.2%)e | NS | Lower detection rate for diffuse carcinomas and low utility for metastatic lesions |
| Lee et al. | PET/CT | 5.18 MBq/kg | 60 | Qualitative and semiquantitative | Staging, prevision of recurrence | 12 (38.7%) | 7.4 ± 9.3 | Lower sensitivity for GSRCC than non-GSRCC. Marginal correlation between 18F-FDG uptake and prognosis in GSRCC. |
| Takebayashi et al. | PET | NS | NS | Qualitative and semiquantitative | Staging, preoperative | NS | 9.2 ± 1.0b | Nonintenstinal gastric cancer show higher SUVmax and GLUT-1 expression |
| Park et al. | PET/CT | 7.4 MBq/kg | 60 | Qualitative and semiquantitative | Staging, preoperative | NS | NS | Similar sensitivity for PET/CT and CECT in GSRCC for primary tumor and nodal metastasis assessment, in contrast with non-GSRCC |
| Charalampakis et al. | PET/CT | NS | NS | Qualitative and semiquantitative | Staging, previous to therapy | NS | NS | Lower SUV for GSRCC and tendency for shorter OS. |
| Chen et al. | PET/CT | 3.7 MBq/kg | 50 ± 6 | Qualitative and semiquantitative | Staging, preoperative | NS | 3.2 ± 1.2 | GSRCC has lower SUVmax and lower HER2 expression than non-GSRCC. |
| Park et al. | PET/CT | 5.5 MBq/kg | 60 | Qualitative and semiquantitative | Restaging | NS | NS | GSRCC has low HER2 expression and lower semiquantitative parameters. |
| Chon et al. | PET/CT | 5.5 MBq/kg | 60 | Qualitative and semiquantitative | Staging, preoperative | 75 (68.2%) | 4.5 ± 1.9 | Lower sensitivity and SUVmax for GSRCC and diffuse cancer. Weak correlation between SUVmax and size. High SUVmax is a poor prognostic factor. |
| Harada et al. | PET/CT | 333–629 | 60–90 | Qualitative and semiquantitative | Staging, prechemoradiation | NS | 5.8 | Lower SUVmax and TLG for GSRCC. Absence of GSRCC is associated with response to therapy. |
| Arslan et al. | PET/CT | 3.7–5.2 MBq/kg | 60 | Qualitative and semiquantitative | Staging, preoperative | 90 (97.8%) | 9.7 ± 7.6 | GSRCC has lower SUVmax. Higher SUVmax for primary GSRCC in presence of nodal and distant metastasis |
18F-FDG, 18F-fluorodeoxyglucose; 18F-FLT, 18F- fluorothymidine; CECT, contrast-enhanced computed tomography; GLUT-1, glucose transporter 1; GSRCC, gastric signet ring cell carcinoma; HER2, human epidermal growth factor receptor 2; kg, kilograms; MBq, megabecquerel; NS, not specified; OS, overall survival; Pts, patients; SUV, standardized uptake value; TLG, total lesion glycolysis.;
GSRCC and poorly differentiated carcinoma.
Nonintestinal histology.
GSRCC, nonsolid carcinoma and poorly differentiated carcinoma.
SRCC and mucinous cancer.
Diffuse histology.
18F-FDG avidity and diagnostic accuracy of PET and PET/CT
Several studies have reported that 18F-FDG PET and PET/CT imaging has impaired accuracy for the evaluation of GSRCC, compared to other gastric cancer histotypes.
In particular, the sensitivity and specificity of 18F-FDG imaging are higher for non-GSRCC compared to GSRCC [10,12,18,22,31]. Furthermore, a higher rate of false-negative scans is described in patients affected by GSRCC [10,14,26,34]. In general, the criteria used to define PET scan positivity or negativity were comparable among the studies considered in the review and consisted of visual and semiquantitative evaluation for most of the studies. PET scan was considered positive when an increased FDG uptake higher than background activity in the surrounding tissue and blood pool was present; positron imaging was also compared to other imaging modalities or hystopathologic evidence when available.
Regarding diagnostic accuracy of 18F-FDG PET/CT for the evaluation of GSRCC, the data included in the review are very heterogeneous. The detection rate of PET/CT ranges from 14 to 98% of the cases, depending on hystopatologic classification used in each study. Furthermore, the sensitivity varies from 15 to 63% and the specificity from 60 to 100% considering only GSRCC, but only small amounts of the studies clearly report these informations.
Moreover, the lower 18F-FDG uptake of GSRCC has been underlined by many studies and standardized uptake value (SUV) is usually lower compared to other gastric cancer subtypes [10,11,18,22,25,28]. Significant differences in terms of the detection rate and SUV values between intestinal and nonintestinal gastric cancer have also been reported such as a strong correlation between the presence of mucus and impaired 18F-FDG uptake, with the presence of higher SUV in nonmucus-containing tumors [15,20,28]. These findings are also true when comparing GRSCC and poorly-differentiated gastric cancer to well-differentiated forms [11,32]. Moreover, diffuse gastric cancer presented low positivity of primary lesions compared to other histotypes [32]. Interestingly, some clinicopathologic parameters such as stage, depth of invasion, size, presence of nodal metastasis and lymphovascular invasion are demonstrated to be correlated with the degree of 18F-FDG uptake [16]. Despite the similarity of SUV between different gastric cancer histotypes, diffuse-type tumors tend to have larger primary tumor than other types [14]. Higher SUVmax, SUVmean, total lesion glycolysis (TLG) and metabolic tumor volume (MTV) have also been reported for well-differentiated and moderately-differentiated cancer compared to poorly-differentiated and GSRCC cancer [29].
Otherwise, some studies report no differences in terms of 18F-FDG uptake and sensitivity between GSRCC and non-GSRCC cancer and surprisingly in some cases GSRCC demonstrates higher SUV [27,30,33,35].
As primary lesions, lymph node localization of GSRCC tends to be less 18F-FDG avid than other gastric cancer nodal metastasis and therefore PET imaging has impaired sensitivity [11,14,21,26].
Correlation of 18F-FDG uptake and glucose transporter-1 or human epidermal growth factor receptor 2 expression
Correlation between 18F-FDG uptake and glucose transporter-1 (GLUT-1) expression in gastric cancer has been reported. In particular, GSRCC has significantly lower expression of this transporter than other gastric cancer histotypes, resulting in impaired 18F-FDG uptake and detection [4,17,19].
Expression of human epidermal growth factor receptor 2 (HER2) in GSRCC has also been studied in the past. In particular, a lower HER2 expression rate in GSRCC compared to well-differentiated and moderately-differentiated cancer has been demonstrated. Moreover, this difference is not present when considering SRCC and poorly-differentiated forms of gastric cancer. [25,29]. Furthermore, PET/CT parameters such as SUVmax, SUVmean, TLG and MTV are significantly higher in HER2 positive patients [29]. In contrast to what was previously reported, increased GLUT-1 expression for nonintestinal cancers has been also reported in literature [30].
Prognostic value of 18F-FDG PET/CT
18F-FDG PET/CT is recently starting to emerge as a promising imaging method of prognostic utility in GSRCC. When considering all gastric cancer together, the presence of GSRCC or lower SUVmax are correlated with shorter overall survival (OS) [23,29]. However, when considering GSRCC or diffuse histology, patients with higher SUVmax tend to have more relapse, shorter relapse free survival, lower cancer-specific survival rate and shorter OS [16,22]. Moreover, the absence of GSRCC, higher SUVmax and higher TLG were associated with pathologic complete response [24]. However, these insights are not clearly supported by other findings when evaluating the role of PET/CT in predicting the recurrence of GSRCC [20].
Discussion
Several studies have demonstrated that GSRCC generally presents lower 18F-FDG uptake compared to other gastric cancer histotypes; therefore, PET and PET/CT imaging has impaired accuracy for the evaluation of GSRCC. [10,12,14,18–20,22,25,26,31,32,34]. Moreover, the risk of false-negative reports is high when considering GSRCC and PD cancers [10,14,26,34]. The sensitivity and specificity of 18F-FDG PET or PET/CT is generally higher for non-GSRCC (75 and 75%) compared to GSRCC (63 and 60%) [10].
As previously mentioned, GSRCC is classified as nonintestinal, diffuse and mucus-containing cancer [3]. Different uptake between intestinal and nonintestinal gastric cancer have been described by Stahl et al., [28] despite the fact that nonintestinal tumor were higher in grade than intestinal types. Moreover, a strong correlation between the presence of mucus and impaired 18F-FDG uptake was also reported. Similar findings were also confirmed by other authors [14,15,18,22,23,31]. Kim et al., [14] reported that despite the similarity of SUV between different gastric cancer histotypes, diffuse-type tumors had significantly larger primary tumor compared to other types. Partially in contrast, in their article, Chon et al., [22] reported that the degree of correlation between SUVmax and size of primary lesion was relatively weak for patients with GSRCC or diffuse histology.
18F-FDG uptake, expressed as SUVmax, may be related with some clinical and histologic features, such as depth of invasion, size, presence of nodal metastasis and lymphovascular invasion, proposed by Pak et al., [16]. Moreover, they reported that patients with higher SUVmax had larger tumors, more total gastrectomies and higher stages compared to the group of lower SUVmax. In this context, Harada et al., [24] showed that high SUVmax and TLG were associated with the absence of GSRCC.
Nodal localizations of GSRCC, especially if regional, tends to be less 18F-FDG avid than other gastric tumor nodal metastasis resulting in impaired sensitivity for PET and PET/CT imaging [11,12,14,21,26]. For example, Kim et al., [14], underlined reduced sensitivity and SUV for GSRCC nodal metastasis and furthermore univariate analysis reported GSRCC and SUV to be significant variables for nodal metastasis detection. However, multivariate analysis confirmed only SUV as an independent predictive variable. In a recent article, Arslan et al., [26] demonstrated that SUVmax of positive regional lymph nodes were significantly lower for GSRCC. Interestingly, this difference was not confirmed when considering distant lymph nodes as also suggested by Smyth et al., [32] reporting that in patients with diffuse cancer, metastatic disease was more likely to be detected by laparoscopy than PET/CT.
It is known that the GLUT-1 overexpression plays a major role for 18F-FDG uptake by cancer cells [36]. In this context, studies about the expression of this transporter in GSRCC have been performed reporting in general reduced GLUT1 presence [13,17,19]. Yamada et al., [13] reported that tracer uptake and detection rate on 18F-FDG PET/CT differed depending on GLUT-1 expression between the GSRCC and other subtypes of gastric cancer. When applying multiple regression analysis, GLUT-1 expression was the most influential factor for predicting the degree of 18F-FDG uptake. Choi et al., [19] reported that, in GSRCC, group of membranous pattern of GLUT-1 staining had significantly higher SUVmax than group of cytoplasmic pattern. Significant differences were also reported in tracer uptake when comparing depth of invasion, extent of nodal metastasis and stage, where higher stage demonstrated increased SUVmax.
Also, anti-HER2 antibodies are starting to emerge as a tool for gastric cancer treatment [37] and therefore, given the ability of HER2 cancer status to predict response to treatment, correlation between 18F-FDG uptake and receptor expression has been researched through the years. In general, GSRCC demonstrated lower HER2 expression compared to other gastric cancer [25,29]. In this context, Park et al., [29], reported that SUVmax, SUVmean, TLG and MTV were significantly higher in HER2 positive patients. Interestingly, the authors also reported that in negative HER2 patients, no correlation between prognosis and PET/CT parameters was highlighted.
In the last years, general implications on the prognostic value of 18F-FDG PET/CT in GSRCC are starting to arise. Pak et al., [16] reported that for GSRCC patients with higher SUVmax had more relapse, shorter relapse-free survival, lower cancer-specific survival rate and lower OS. Univariate analysis demonstrated tumor nodes and metastases stage, type of gastrectomy and SUVmax as significant variables for the prediction of survival. More recently, Lee et al., [20] evaluated the possible role of 18F-FDG PET/CT in predicting recurrence after curative surgical resection, reporting only marginal significance was present for GRSCC when considering a negative scan as predictive of longer recurrence-free survival. The difference was significant when considering other gastric cancer subtypes. Similarly, Charalampakis et al., [23] underlined that patients with lower SUV and presence of GSRCC had the tendency to shorter OS. Moreover, Harada et al., [24] reported that in absence of GSRCC, SUVmax and TLG were associated with pathologic complete response. Lastly, Chon et al., [22] reported that in presence of diffuse or GSRCC, higher SUVmax was correlated to shorter disease-free survival and OS.
A singular study was proposed by Herrmann et al., [18] when comparing 18F-FDG and 18F-FLT PET or PET/CT for the staging of gastric cancer. 18F-FLT demonstrated lower uptake in GSRCC compared to other gastric cancer subtypes but higher sensitivity compared to 18F-FDG. The authors than suggested 18F-FLT as a promising tool for the evaluation of the particular aggressive histologic type of gastric cancer, such as GSRCC.
As previously mentioned, some works are partially in contrast to the findings reported. For example, Mochiki et al., [27] reported that histology of primary gastric cancer did not significantly influence the detection of disease on PET imaging. Similar results were obtained by Hur et al., [33]. Higher uptake of 18F-FDG by GSRCC compared to other gastric cancer was otherwise reported by Takebayashi et al., [30]. Surprisingly, the authors reported increased GLUT-1 expression for nonintestinal cancers. Similar results were also underlined by Kim et al., [35] reporting that adenocarcinoma had lower accuracy than GSRCC. In general, the low percentage of GSRCC presented in these studies is suggested by the authors as a possible explanation for these results.
Conclusion
In conclusion, 18F-FDG PET or PET/CT demonstrated impaired diagnostic accuracy for the evaluation of primary GSRCC and nodal localization compared to other gastric cancer histotypes. Interestingly, the uptake and the main semiquantitative parameters (such as SUV) are generally lower for GSRCC. Reduced GLUT-1 and HER2 expression is also present in GSRCC. Lastly, a possible prognostic role of 8F-FDG PET/CT features is starting to emerge.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaneko Y, Murray WK, Link E, Hicks RJ, Duong C. Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer. J Nucl Med. 2015; 56:523–529. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014; 20:5679–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunisaki C, Makino H, Akiyama H, Otsuka Y, Ono HA, Kosaka T, et al. Clinical significance of the metastatic lymph-node ratio in early gastric cancer. J Gastrointest Surg. 2008; 12:542–549. [DOI] [PubMed] [Google Scholar]
- 5.Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients’ treatment decision making? Eur J Nucl Med Mol Imaging. 2011; 38:64–73. [DOI] [PubMed] [Google Scholar]
- 6.Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008; 35:95–99. [DOI] [PubMed] [Google Scholar]
- 7.Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case-control analysis. Clin Nucl Med. 2011; 36:452–456. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Zhao J, Gao P, Song Y, Sun J, Chen X, et al. Prognostic value of pretreatment standardized uptake value of F-18-fluorodeoxyglucose PET in patients with gastric cancer: a meta-analysis. BMC Cancer. 2017; 17:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536. [DOI] [PubMed] [Google Scholar]
- 10.De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002; 29:525–529. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003; 44:690–699. [PubMed] [Google Scholar]
- 12.Ha TK, Choi YY, Song SY, Kwon SJ. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc. 2011; 81:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006; 20:597–604. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005; 103:2383–2390. [DOI] [PubMed] [Google Scholar]
- 16.Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol. 2011; 104:566–570. [DOI] [PubMed] [Google Scholar]
- 17.Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010; 31:532–538. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007; 48:1945–1950. [DOI] [PubMed] [Google Scholar]
- 19.Choi BH, Song HS, An YS, Han SU, Kim JH, Yoon JK. Relation between fluorodeoxyglucose uptake and glucose transporter-1 expression in gastric signet ring cell carcinoma. Nucl Med Mol Imaging. 2011; 45:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012; 39:1425–1434. [DOI] [PubMed] [Google Scholar]
- 21.Park K, Jang G, Baek S, Song H. Usefulness of combined PET/CT to assess regional lymph node involvement in gastric cancer. Tumori. 2014; 100:201–206. [DOI] [PubMed] [Google Scholar]
- 22.Chon HJ, Kim C, Cho A, Kim YM, Jang SJ, Kim BO, et al. The clinical implications of FDG-PET/CT differ according to histology in advanced gastric cancer. Gastric Cancer. 2019; 22:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charalampakis N, Xiao L, Elimova E, Wadhwa R, Shiozaki H, Shimodaira Y, et al. Initial standardized uptake value of positron emission tomography influences the prognosis of patients with localized gastric adenocarcinoma treated preoperatively. Oncology. 2015; 89:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada K, Patnana M, Wang X, Iwatsuki M, Murphy MAB, Zhao M, et al. Low metabolic activity in primary gastric adenocarcinoma is associated with resistance to chemoradiation and the presence of signet ring cells. Surg Today. 2020; 50:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Zhou X, Liu J, Huang G. Relationship between 18F-FDG PET/CT Findings and HER2 expression in gastric cancer. J Nucl Med. 2016; 57:1040–1044. [DOI] [PubMed] [Google Scholar]
- 26.Arslan E, Aksoy T, Gündoğan C, Şen Ç, Yilmaz Tatar S, Dursun N, et al. Metabolic characteristics and diagnostic contribution of 18F-FDG PET/CT in gastric carcinomas. Mol Imaging Radionucl Ther. 2020; 29:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004; 28:247–253. [DOI] [PubMed] [Google Scholar]
- 28.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003; 30:288–295. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Lee N, Beom SH, Kim HS, Lee CK, Rha SY, et al. The prognostic value of volume-based parameters using 18F-FDG PET/CT in gastric cancer according to HER2 status. Gastric Cancer. 2018; 21:213–224. [DOI] [PubMed] [Google Scholar]
- 30.Takebayashi R, Izuishi K, Yamamoto Y, Kameyama R, Mori H, Masaki T, et al. [18F]Fluorodeoxyglucose accumulation as a biological marker of hypoxic status but not glucose transport ability in gastric cancer. J Exp Clin Cancer Res. 2013; 32:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youn SH, Seo KW, Lee SH, Shin YM, Yoon KY. 18F-2-Deoxy-2-Fluoro-D-glucose positron emission tomography: computed tomography for preoperative staging in gastric cancer patients. J Gastric Cancer. 2012; 12:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG, et al. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012; 118:5481–5488. [DOI] [PubMed] [Google Scholar]
- 33.Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol. 2010; 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009; 34:441–447. [DOI] [PubMed] [Google Scholar]
- 35.Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011; 26:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001; 42:1551–1555. [PubMed] [Google Scholar]
- 37.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697. [DOI] [PubMed] [Google Scholar]