Abstract
BACKGROUND
PVDOMICS (Pulmonary Vascular Disease Phenomics) is a precision medicine initiative to characterize pulmonary vascular disease (PVD) using deep phenotyping. PVDOMICS tests the hypothesis that integration of clinical metrics with omic measures will enhance understanding of PVD and facilitate an updated PVD classification.
OBJECTIVES
The purpose of this study was to describe clinical characteristics and transplant-free survival in the PVDOMICS cohort.
METHODS
Subjects with World Symposium Pulmonary Hypertension (WSPH) group 1–5 PH, disease comparators with similar underlying diseases and mild or no PH and healthy control subjects enrolled in a cross-sectional study. PH groups, comparators were compared using standard statistical tests including log-rank tests for comparing time to transplant or death.
RESULTS
A total of 1,193 subjects were included. Multiple WSPH groups were identified in 38.9% of PH subjects. Nocturnal desaturation was more frequently observed in groups 1, 3, and 4 PH vs comparators. A total of 50.2% of group 1 PH subjects had ground glass opacities on chest computed tomography. Diffusing capacity for carbon monoxide was significantly lower in groups 1–3 PH than their respective comparators. Right atrial volume index was higher in WSPH groups 1–4 than comparators. A total of 110 participants had a mean pulmonary artery pressure of 21–24 mm Hg. Transplant-free survival was poorest in group 3 PH.
CONCLUSIONS
PVDOMICS enrolled subjects across the spectrum of PVD, including mild and mixed etiology PH. Novel findings include low diffusing capacity for carbon monoxide and enlarged right atrial volume index as shared features of groups 1–3 and 1–4 PH, respectively; unexpected, frequent presence of ground glass opacities on computed tomography; and sleep alterations in group 1 PH, and poorest survival in group 3 PH. PVDOMICS will facilitate a new understanding of PVD and refine the current PVD classification. (Pulmonary Vascular Disease Phenomics Program PVDOMICS [PVDOMICS]; NCT02980887)
Keywords: phenotyping, pulmonary hypertension
Diagnosis and clinical classification of pulmonary hypertension (PH) requires chest and cardiac imaging, pulmonary function testing, and cardio-pulmonary hemodynamics according to the World Symposium on Pulmonary Hypertension (WSPH).1 In this framework, results of clinical testing assign patients to 1 of 5 groups based on presumed common disease characteristics, similar pathological features, and predicted response to treatment. Although this approach has defined treatment and prognosis, the entire spectrum of pulmonary vascular disease (PVD) and PH may not be fully captured by the traditional WSPH classification.2–4 The potential to improve understanding and clinical care of PVD coupled with advances in translational science led the National Institutes of Health/National Heart, Lung and Blood Institute to support PVDOMICS (Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics), a precision medicine initiative to intensively characterize PH and PVD utilizing deep clinical phenotyping and comprehensive “omics” analysis. This approach may refine the traditional WSPH groups 1–5 classification by potentially identifying new, more precise phenotypes within PVD that will enhance scientific understanding and lead to novel targeted therapeutics.5
PVDOMICS6 tests the general hypothesis that integration of clinical metrics with detailed omic measures in the blood will enhance understanding of PVD, identify cohorts for novel therapies, and potentially facilitate an updated classification scheme for PVD. PVDOMICS enrolled subjects with WSPH groups 1–5 according to the 2013 classification guidelines1 and allowed for mixed group etiologies, a novel approach reflecting real-world clinical practice. In addition, PVDOMICS enrolled healthy control subjects and also comparators with similar underlying diseases but with milder or no pulmonary vascular phenotype, including cardiopulmonary disease with a mean pulmonary artery pressure (mPAP) of 21–24 mm Hg, pulmonary vascular resistance (PVR) between 2.2 and 3.0 WU, and group 1 pulmonary arterial hypertension (PAH) with exercise-induced PH. The inclusion of disease comparators allows distinction of PVD clinical and omic features from those of the underlying disease, which has not been possible in other large cohort studies of pulmonary vascular disease and may ultimately uncover precursors to PVD. All participants in PVDOMICS underwent a deep phenotyping protocol as previously described.6 This comprehensive phenotyping protocol, performed across the full spectrum of PVD, disease comparator groups, and healthy control subjects, uniquely positions the PVDOMICS cohort to better understand PH as a spectrum and appreciate heterogeneity within a WSPH group and similarities across WSPH groups.
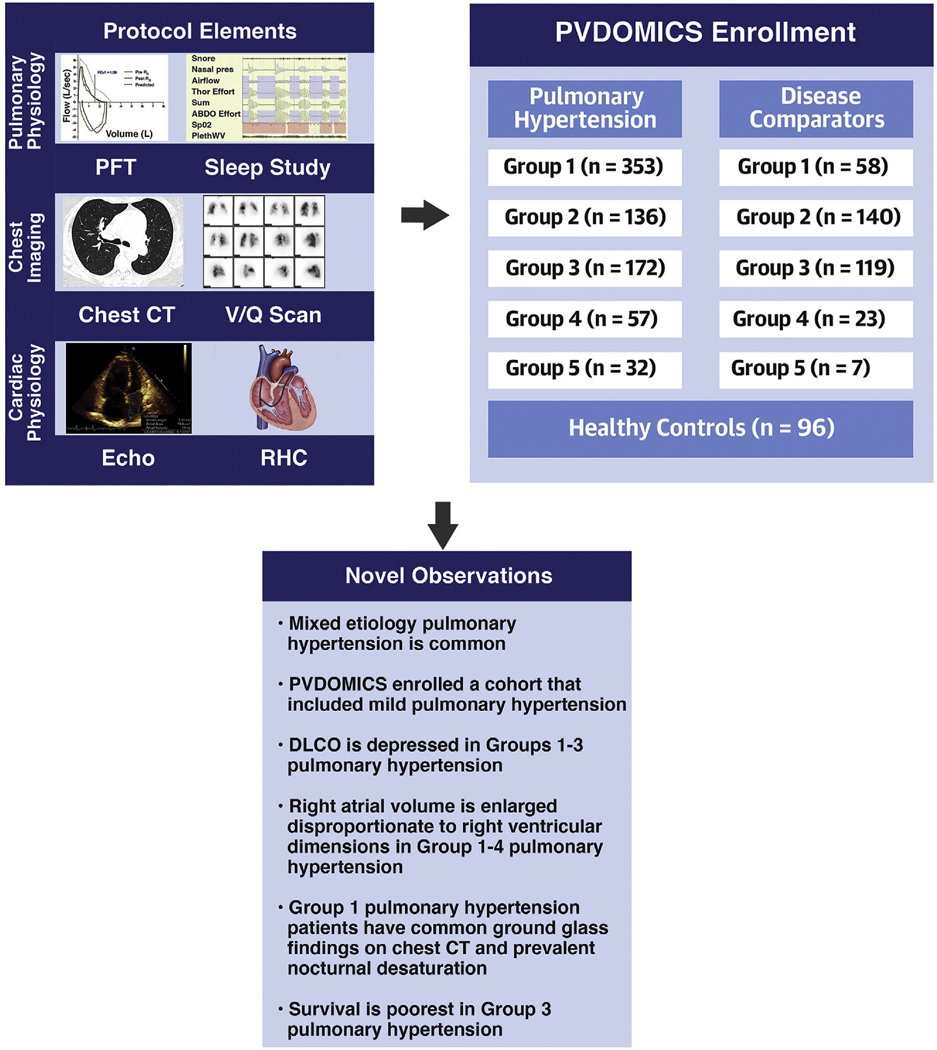
Here we describe the following: 1) the PVDOMICS cohort, summarizing the basic characteristics and concomitant diseases, conventional cardiopulmonary physiological and imaging assessments in healthy control subjects, disease comparators, and traditional WSPH categories according to an individual’s primary group assignment at enrollment; 2) early clinical observations from this cohort related to pulmonary and cardiac phenotypes; and 3) death and transplant outcomes in each group (Central Illustration).
CENTRAL ILLUSTRATION PVDOMICS Protocol Elements, Enrollment, and Key Findings.
PVDOMICS is a precision medicine initiative to characterize pulmonary vascular disease using deep phenotyping. PVDOMICS tests the hypothesis that integration of clinical metrics with omic measures will enhance understanding of pulmonary vascular disease and facilitate an updated pulmonary vascular disease classification. Subjects across the spectrum of pulmonary vascular disease, comparators, and healthy control subjects were enrolled including mild and mixed etiology pulmonary vascular disease. Novel findings include enlarged right atrial volume in groups 1–4 pulmonary hypertension; unexpected, frequent presence of ground glass opacities on computed tomography and sleep alterations in group 1 pulmonary hypertension; and poorest survival in group 3 PH. PVDOMICS will facilitate new understanding of pulmonary vascular disease and refine the current pulmonary vascular disease classification.
Hemnes AR, et al. J Am Coll Cardiol. 2022;80(7):697–718.
METHODS
This clinical research network included 7 centers across the United States (Brigham and Women’s Hospital, Harvard Medical School; Columbia University Irving Medical Center; Weill Cornell Medical Center; Johns Hopkins Hospital; Mayo Clinic [Rochester]; University of Arizona [Tucson]; and Vanderbilt University Medical Center) and a Data Coordinating Center at the Cleveland Clinic. Participants were enrolled into a prospective, longitudinal cohort study (NCT02980887). Phenotyping protocol was approved by local institutional review boards (see Supplemental Appendix for full data), and subjects were enrolled after informed consent from November 30, 2016, to October 18, 2019. Detailed inclusion/exclusion criteria are in the Supplemental Appendix. Both incident and prevalent cases of PVD were included. We have included clinical data available as of May 19, 2021, and survival data as of November 22, 2021. Although >2,000 clinical variables were collected on each participant, this paper presents a limited but representative data set that includes commonly used metrics required to classify WSPH or comparator type and to define disease severity.
PHENOTYPING PROTOCOL.
Details of the protocol have been previously published.6–8 Briefly, subjects with known or suspected PVD and disease comparators as well as age-, sex-, race-, and Hispanic ethnicity-matched healthy control subjects ≥18 years of age were recruited from the enrolling centers. All subjects underwent clinical phenotyping including collection of demographics, quality of life surveys, medication use, and comorbidities. Also collected were 6-minute walk testing, pulmonary function testing, home overnight sleep study, chest computed tomography (CT), ventilation/perfusion lung scan, electrocardiogram, echocardiogram, cardiac magnetic resonance imaging, routine clinical laboratory measurements, and cardiopulmonary exercise testing. Enrollees with known or suspected PVD underwent protocolized right heart catheterization (RHC) with confrontational testing including oxygen, nitric oxide, and either fluid bolus or invasive exercise challenge.7 Blood for “omic” analyses was collected in the fasting state. Cores at the Data Coordinating Center performed central reading for echocardiography, cardiac magnetic resonance imaging, cardiopulmonary exercise testing, electrocardiogram, chest CT, overnight sleep study, pulmonary function testing, and right heart catheterization. Vital status and presence or absence of heart, lung, or heart-lung transplantation was assessed annually after enrollment.
PULMONARY VASCULAR DISEASE DEFINITION.
Participants were classified according to 2013 WSPH guidelines,1 which were in place at the inception of the study. As such, milder PH patients with mPAP (21–24 mm Hg) or PVR <3 WU could be included with the comparator groups. Previously established group 1 PVD on therapy with PVR <3 WU were included in the PH category with review by an Adjudication Committee comprised of at least 3 principal investigators including at least 1 cardiologist and 1 pulmonologist from the clinical centers and members of the Data Coordinating Center (E.M.H., J.A.L., A.B.W., S.C.M., R.P.F., F.P.R., S.G.S., M.K.R., E.B.R., A.R.H.). Hemodynamic definitions used in the cohort have been previously published6,7 and are presented in Supplemental Table 1. Subjects were classified as single or mixed etiology PH at the discretion of the site principal investigator. Subjects with mixed PH were allowed up to 2 secondary contributing conditions to PVD from the WSPH classification. Mixed group designations were ranked in order of predominant features and were reviewed by the Adjudication Committee.
COMPARATOR DEFINITION.
Enrollees with mild elevation in mean pulmonary arterial pressure (21–24 mm Hg), normal mean pulmonary arterial pressure or mPAP >24 mm Hg, with PVR <3 WU were assigned to the appropriate WSPH 1–5 comparator groups, representing groups at risk for PVD with similar underlying cardiopulmonary disease. Healthy control subjects underwent all noninvasive testing and blood sampling but did not undergo RHC or ventilation/perfusion scan. Hemodynamic definitions are in Supplemental Table 2. Comparators were allowed 1 secondary contributor to PVD similar to mixed PH groups. Mixed group comparator designation was also reviewed by the Adjudication Committee as in the previous text.
STATISTICAL ANALYSIS.
PVD status and PH group could not be determined for 2 participants, and they are excluded from this analysis. Descriptive results are presented as median with 25th and 75th percentiles (P25, P75) for continuous variables. Categorical variables are presented with the number of observations and percentage of total. Each comparator group was compared with its respective PH group using the Pearson chi-square or Fisher exact test for categorical variables, and 2-sample Student’s t-test or Wilcoxon rank sum test for continuous and ordinal variables. To compare across comparator groups 1–4 or PH groups 1–5, the Kruskal-Wallis test was used for continuous and ordinal variables and Pearson chi-square test for categorical variables. Group 5 comparators were not included for these or within-group comparisons because of having only 7 subjects; however, their data are presented for descriptive purposes only. Significance of comparisons across PH or comparator groups is given by the P values or, for the case of comparing comparators to PH within group, significance is indicated if the P value is ≤0.05. P values and 95% CIs presented in this report have not been adjusted for multiplicity, and therefore, inferences drawn from these statistics may not be reproducible. Comparing the relative magnitude of PH-comparator differences from one marker to another was performed using a nonparametric bootstrap approach for semiparametric repeated measures and general MANOVA designs.9 Time to first lung and/or heart transplant or death are summarized by Kaplan-Meier curves and also the median and 25th and 75th percentiles of time to transplant or death. PH group-related survival was compared using log-rank tests with HRs (and 95% confidence limits) using the PH group 1 as the reference group, or PH group overall when comparing to comparators and healthy control subjects.
RESULTS
SUBJECT CHARACTERISTICS.
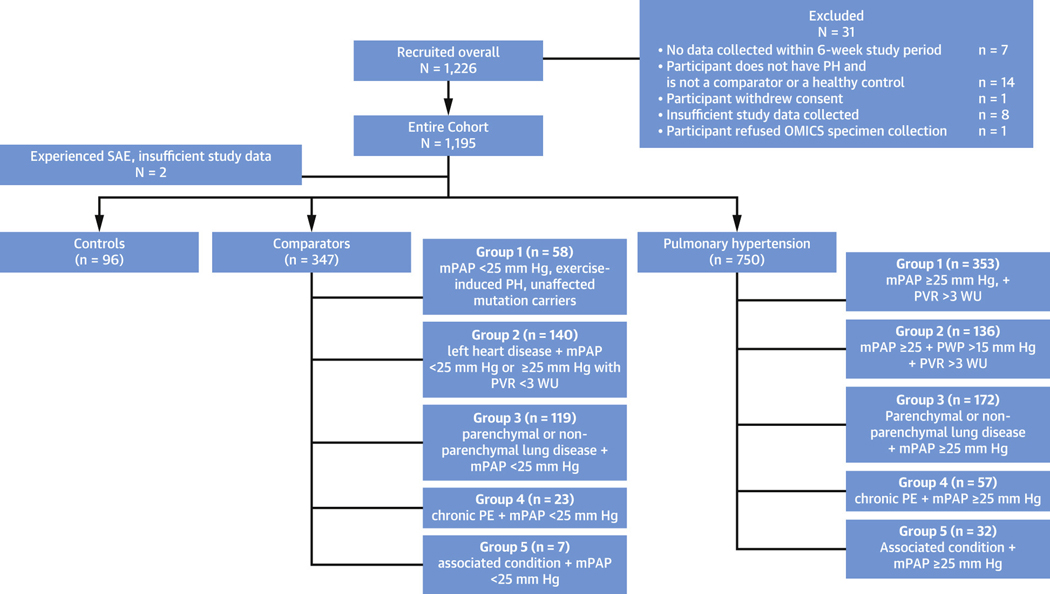
PVDOMICS enrolled 1,226 subjects with 1,193 included in the final analysis, including 96 healthy control subjects, 347 comparators, and 750 patients with PH (Figure 1). By WSPH groups, there were 353 group 1,136 group 2,172 group 3, 57 group 4, and 32 group 5 enrollees (Figure 1). The comparators included 58 group 1, 140 group 2, 119 group 3, 23 group 4, and 7 group 5 subjects. For this paper, all PH and comparator participants were listed according to their primary WSPH or comparator group.
Figure 1. Enrollment and Subject Final Diagnosis.
STROBE diagram depicting enrollment and patient classification according to primary World Symposium on Pulmonary Hypertension diagnosis or disease comparator. mPAP = mean pulmonary artery pressure; PE = pulmonary embolism; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; PWP = pulmonary wedge pressure; SAE = serious adverse event.
DISEASE ETIOLOGY ACCORDING TO WSPH GROUPS.
Disease duration, functional class, and medication use are presented in Table 1 for comparators and PH groups. The cohort of PH patients included 200 (26.7%) incident cases. Use of PAH-directed medications was common in the PH group (56.5%, 424 of 750 subjects) while only present in 20 of 347 (5.8%) of comparators. Focusing on primary PH etiology (Table 2), in group 1 PH the most common etiology was idiopathic PAH (n = 158) followed by connective tissue disease-associated PAH (n = 93). In group 2 PH, 100 of 136 (73.5%) subjects were categorized as having heart failure with preserved ejection fraction. In group 3 enrollees, 58 had obstructive sleep apnea, 66 chronic obstructive pulmonary disease, and 90 had interstitial lung disease (idiopathic pulmonary fibrosis or other). Mixed etiology PH was identified by investigators in 38.9% of the PH enrollees (292 of 750) (Table 3). In both groups 1 and 2 PH, among those with mixed disease, the most common secondary diagnosis was group 3, present in 62 of 93 (66.7%) of mixed group 1 and 66 of 78 (84.6%) of mixed group 2.
Table 1.
History of Disease, Functional Class, PH Medications
| Group 1 WSPH (n = 353) Comparators (n = 58) | Group 2 WSPH (n = 136) Comparators (n = 140) | Group 3 WSPH (n = 172) Comparators (n = 119) | Group 4 WSPH (n = 57) Comparators (n = 23) | Group 5 WSPH (n = 32) Comparators (n = 7) | Overall PH or Comparator P Value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age at diagnosis, y | PH | 47.4 [34.0, 59.2] {1} | 68.6 [58.7, 74.8] {0} | 64.2 [55.4, 72.1] {0} | 55.6 [44.3, 67.1] {0} | 56.2 [43.0, 64.9] {1} | <0.001 |
| Prevalent PH, n | PH | 305 (86.4) {0} | 84 (61.8) {0} | 91 (52.9) {0} | 46 (80.7) {0} | 24 (75.0) {0} | <0.001 |
| Prevalent participants: duration of PH at enrollment, y | PH | 4.6 [1.5, 9.7] {0} | 1.3 [0.37, 3.9] {0} | 2.0 [0.50, 4.2] {0} | 1.6 [0.59, 4.4] {0} | 3.2 [1.4, 7.5] {0} | <0.001 |
| Functional class, n | PH | {2} | {7} | {17}a | {1}a | {0} | <0.001 |
| Class I | 43 (12.3) | 1 (0.78) | 3 (1.9)a | 2 (3.6)a | 3 (9.4) | ||
| Class II | 145 (41.3) | 35 (27.1) | 33 (21.3)a | 22 (39.3)a | 13 (40.6) | ||
| Class III | 145 (41.3) | 86 (66.7) | 108 (69.7)a | 31 (55.4)a | 16 (50.0) | ||
| Class IV | 18 (5.1) | 7 (5.4) | 11 (7.1)a | 1 (1.8)a | 0 (0.0) | ||
| Functional class, n | Comparator | {4} | {9} | {12} | {1} | {1} | <0.001 |
| Class I | 3 (5.6) | 6 (4.6) | 1 (0.93) | 3 (13.6) | 0 (0.0) | ||
| Class II | 37 (68.5) | 44 (33.6) | 41 (38.3) | 13 (59.1) | 1 (16.7) | ||
| Class III | 13 (24.1) | 72 (55.0) | 62 (57.9) | 6 (27.3) | 4 (66.7) | ||
| Class IV | 1 (1.9) | 9 (6.9) | 3 (2.8) | 0 (0.0) | 1 (16.7) | ||
| PH medication use, n | PH | 296 (84.3) {2}a | 29 (21.5) {1}a | 52 (30.4) {1}a | 27 (47.4) {0}a | 20 (62.5) {0} | <0.001 |
| Comparator | 7 (12.1) {0} | 8 (5.8) {1} | 2 (1.7) {0} | 3 (13.0) {0} | 0 (0.0) {0} | 0.019 | |
| Endothelin receptor antagonists, n | PH | 197 (56.1) {2}a | 6 (4.4) {1} | 16 (9.4) {1}a | 3 (5.3) {0} | 11 (34.4) {0} | <0.001 |
| Comparator | 2 (3.4) {0} | 4 (2.9) {1} | 0 (0.0) {0} | 1 (4.3) {0} | 0 (0.0) {0} | 0.11 | |
| PDE5i, n | PH | 237 (67.5) {2}a | 24 (17.8) {1}a | 43 (25.1) {1}a | 8 (14.0) {0} | 14 (43.8) {0} | <0.001 |
| comparator | 5 (8.6) {0} | 7 (5.0) {1} | 1 (0.84) {0} | 2 (8.7) {0} | 0 (0.0) {0} | 0.067 | |
| Soluble guanylate cyclase stimulator, n | PH | 17 (4.8) {2} | 1 (0.74) {1} | 3 (1.8) {1} | 18 (31.6) {0}a | 1 (3.1) {0} | <0.001 |
| Comparator | 0 (0.0) {0} | 0 (0.0) {1} | 0 (0.0) {0} | 1 (4.3) {0} | 0 (0.0) {0} | 0.068 | |
| Prostanoids, n | PH | 151 (43.0) {2}a | 6 (4.4) {1} | 12(7.0) {1}a | 6 (10.5) {0} | 5 (15.6) {0} | <0.001 |
| Comparator | 0 (0.0) {0} | 1 (0.72) {1} | 1 (0.84) {0} | 0 (0.0) {0} | 0 (0.0) {0} | 0.99 | |
| On CCB medications for PH | PH | 16 (4.6) {2} | 1 (0.74) {1} | 3(1.8) {1} | 0 (0.0) {0} | 0 (0.0) {0} | 0.046 |
| Comparator | 0 (0.0) {0} | 0 (0.0) {1} | 0 (0.0) {0} | 0 (0.0) {0} | 0 (0.0) {0} | - | |
Values are median [P25, P75] {n of missing}, or n (%) {n of missing}. P values calculated as follows: Kruskal-Wallis test for continuous and ordinal variables, Pearson’s chi-square or Fisher exact test for categorical variables.
Significant difference at level 0.05 between comparators and PH participants within the group. No corrections for multiple testing were applied. 7 group 5 comparators are shown for descriptive purposes only, they were not included in the statistical comparisons.
CCB = calcium-channel blocker; PDE5i = phosphodiesterase type 5 inhibitor; PH = pulmonary hypertension; WSPH = World Symposium on Pulmonary Hypertension.1
Table 2.
Etiology of Disease: Risk Factors Reported
| PH Overall (n = 750) |
Comparator Overall (n = 347) |
|||
|---|---|---|---|---|
| Group 1 Risk Factors | Primary G1 (n = 353) | G1 Risk Factors (n = 83) | Primary G1 Comparator (n = 58) | G1 Risk Factors (n = 72) |
|
| ||||
| Idiopathic PAH | 158 | 19 | 0 | 2 |
| Connective tissue disease | 93 | 54 | 41 | 31 |
| Systemic sclerosis | 42 | 23 | 21 | 16 |
| Mixed connective tissue disease | 16 | 7 | 5 | 3 |
| Rheumatoid arthritis | 7 | 10 | 9 | 9 |
| Systemic lupus erythematosus | 16 | 12 | 5 | 7 |
| Other connective tissue disease (Sjögren’s, antisynthetase syndrome, undifferentiated) | 15 | 5 | 8 | 8 |
| Congenital heart disease | 36 | 2 | 6 | 9 |
| Shunt repaired | 15 | 1 | 3 | 6 |
| Familial PAH | 27 | 3 | 1 | 0 |
| HHT | 4 | 2 | 0 | 0 |
| HIV | 10 | 4 | 0 | 2 |
| Drug- and toxin-induced PAH | 16 | 1 | 1 | 1 |
| Portal hypertension | 18 | 2 | 0 | 1 |
| Mild PH (mPAP 21-<25 mm Hg) | 0 | 0 | 10 | 27 |
| ePAH (exercise mPAP ≤30 mm Hg, flow <10 L/min, and mPAP-Q slope >3) (mm Hg·min/L) | 0 | 0 | 9 | 9 |
| Connective tissue disease with mild (mPAP 21-<25 mm Hg) or no PH | 0 | 0 | 43 | 27 |
| Othera | 10 | 0 | 0 | 0 |
|
| ||||
| Group 2 Risk Factors | Primary G2 (n = 136) | G2 Risk Factors (n = 105) | Primary G2 Comparator (n = 140) | G2 Risk Factors (n = 46) |
|
| ||||
| Heart failure with preserved ejection fraction | 100 | 83 | 76 | 28 |
| Heart failure with reduced ejection fraction | 26 | 13 | 19 | 2 |
| Valvular heart disease | 35 | 13 | 28 | 5 |
| Stenotic | 19 | 4 | 13 | 2 |
| Regurgitant | 29 | 12 | 19 | 3 |
| Cardiomyopathy | 14 | 2 | 28 | 2 |
| Hypertrophic | 7 | 1 | 6 | 0 |
| Restrictive | 6 | 0 | 2 | 0 |
| Mild PVD risk associated with left heart disease (left heart disease with mPAP <25 mm Hg) | 0 | 0 | 56 | 14 |
| Moderate PVD risk associated with left heart disease | 0 | 0 | 77 | 15 |
| Isolated postcapillary pulmonary hypertension mPAP ≥25 mm Hg, PVR <3, DPG <7 (mm Hg) | 0 | 0 | 56 | 8 |
| Provocable mPCW >18 with V waves <5 mm Hg after challenge, significant change from resting hemodynamics | 0 | 0 | 23 | 9 |
| Provocable mPCW >15 with V waves >5 mm Hg after challenge, significant change from resting hemodynamics | 0 | 0 | 45 | 6 |
| Othera | 3 | 2 | 3 | 0 |
|
| ||||
| Group 3 Risk Factors | Primary G3 (n = 172) | 3 Risk Factors (n = 218) | Primary G3 Comparator (n = 119) | G3 Risk Factors (n = 116) |
|
| ||||
| Obstructive sleep apnea/obesity hypoventilation | 58 | 127 | 43 | 82 |
| COPD | 66 | 56 | 35 | 16 |
| Mild to no PVD risk (mPAP <21 mm Hg) and COPD | 0 | 0 | 7 | 3 |
| Moderate PVD risk (mPAP 21-<25 mm Hg) and COPD | 0 | 0 | 12 | 2 |
| Idiopathic pulmonary fibrosis | 15 | 7 | 13 | 1 |
| Other ILD (including CPFE, other ILD, SSc-ILD, hypersensitivity pneumonitis) | 75 | 49 | 35 | 14 |
| Mild to no PVD risk (mPAP <21 mm Hg) and other ILD, including CPFE and SSc-ILD | 0 | 0 | 12 | 3 |
| Moderate PVD risk (mPAP 21-<25 mm Hg) and other ILD, including CPFE and SSc-ILD | 0 | 0 | 6 | 5 |
| Nonparenchymal restrictive lung disease/thoracic cage abnormality | 8 | 9 | 8 | 7 |
| Cystic fibrosis | 3 | 0 | 3 | 0 |
| Othera | 2 | 2 | 1 | 3 |
|
| ||||
| Group 4 Risk Factors | Primary G4 (n = 57) | G4 Risk Factors (n = 14) | Primary G4 Comparator (n = 23) | G4 Risk Factors (n = 10) |
|
| ||||
| Chronic thromboembolic pulmonary hypertension | 57 | 14 | 0 | 0 |
| Pulmonary thromboendarterectomy | 18 | 0 | 5 | 3 |
| Balloon pulmonary angioplasty | 4 | 0 | 0 | 1 |
| Chronic PE with mild to no PVD (mPAP <25 mm Hg) | 0 | 0 | 21 | 6 |
| Othera | 0 | 0 | 2 | 1 |
|
| ||||
| Group 5 Risk Factors | Primary G5 (n = 32) | G5 Risk Factors (n = 9) | Primary G5 Comparator (n = 7) | G5 Risk Factors (n = 14) |
|
| ||||
| Sarcoidosis | 19 | 3 | 3 | 8 |
| Myeloproliferative disease | 3 | 3 | 1 | 5 |
| Hemoglobinopathy | 8 | 2 | 2 | 2 |
| Sickle cell | 7 | 1 | 2 | 2 |
| Thalassemia | 1 | 2 | 0 | 0 |
| Othera | 2 | 0 | 1 | 0 |
Values are n within group.
Other = schistosomiasis, pulmonary capillary hemangiomatosis, pulmonary veno-occlusive disease, or other conditions not otherwise listed.
CPFE = combined pulmonary fibrosis and emphysema; DPG = diastolic pressuregradient; G = group; ILD = interstitial lung disease; mPAP = mean pulmonary artery pressure; mPCW = mean pulmonary capillary wedge pressure; PAH = pulmonary arterial hypertension; PE = pulmonary embolism; PH = pulmonary hypertension; PVD = pulmonary vascular disease; PVR = pulmonary vascular resistance; SSc-ILD = scleroderma-associated interstitial lung disease.
Table 3.
Mixed PH and Comparator Groups
| Secondary WSPH group (n) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Primary WSPH Group (n) | Group 1 | Group 2 | Group | 3 Group 4 | Group 5 | Total Mixed |
No Secondary Group | Total |
|
| ||||||||
| PH participants | ||||||||
| Group 1 | 0 | 28 | 62 | 2 | 1 | 93 (26) | 260 | 353 |
| Group 2 | 9 | 0 | 66 | 2 | 1 | 78 (57) | 58 | 136 |
| Group 3 | 34 | 41 | 0 | 2 | 1 | 78 (45) | 94 | 172 |
| Group 4 | 2 | 9 | 12 | 0 | 1 | 24 (42) | 33 | 57 |
| Group 5 | 2 | 3 | 11 | 3 | 0 | 19 (59) | 13 | 32 |
| Total | 47 | 81 | 151 | 9 | 4 | 292 (39) | 458 | 750 |
| Comparators | ||||||||
| Group 1 | 0 | 3 | 21 | 0 | 2 | 26 (45) | 32 | 58 |
| Group 2 | 9 | 0 | 71 | 2 | 4 | 86 (61) | 54 | 140 |
| Group 3 | 20 | 21 | 0 | 1 | 0 | 42 (35) | 77 | 119 |
| Group 4 | 2 | 1 | 5 | 0 | 0 | 8 (35) | 15 | 23 |
| Group 5 | 0 | 2 | 0 | 2 | 0 | 4 (57) | 3 | 7 |
| Total | 31 | 27 | 97 | 5 | 6 | 166 (48) | 181 | 347 |
Values are n or n (% of group total) within group.
PH = pulmonary hyperten5ion.
DEMOGRAPHICS AND ANTHROPOMETRICS.
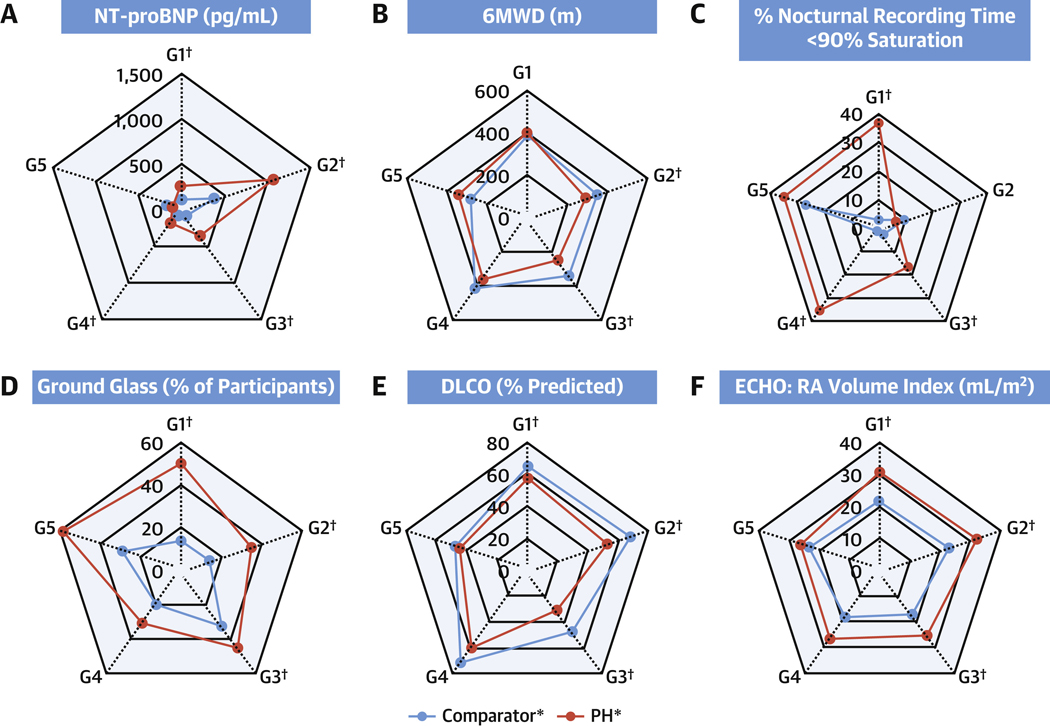
Demographic, anthropometric, and laboratory data are presented in Table 4. There were differences in age across both PH and comparators. Additionally, group 1 PH enrollees were younger than their comparators, and group 2 and 3 PH enrollees were older than their comparators (P < 0.05 both). Female sex was more common than male in all forms of PH with the exception of group 5, although this sex difference was most pronounced in groups 1 (73.4% women) and 4 (61.4% women). Although there were no differences between PH groups and their comparators in body mass index (BMI), there were differences amongst PH subtypes, with groups 2 and 4 PH having the highest BMI (P < 0.001). There were no differences between comparator groups in systolic or diastolic systemic blood pressure or pulse; however, there were differences in each of these metrics across WSPH groups (P < 0.001 systolic blood pressure and pulse; P = 0.005 diastolic blood pressure). Group 1 PH enrollees had lower systolic blood pressure than group 1 comparators (P < 0.05). Laboratory analysis demonstrated that PH groups 1–4 had higher NT-proBNP than their disease comparators (P < 0.05 for all) (Figure 2A) and positive antinuclear antibody tests were common in both PH and comparators with significantly higher percent of positive antinuclear antibody in group 1 comparators vs PH.
Table 4.
Demographics, Anthropometrics, Clinical Laboratory Values
| Factor | Healthy Control Subjects (n = 96) | Group 1 WSPH (n = 353) Comparators (n = 58) | Group 2 WSPH (n = 136) Comparators (n = 140) | Group 3 WSPH (n = 172) Comparators (n = 119) | Group 4 WSPH (n = 57) Comparators (n = 23) | Group 5 WSPH (n = 32) Comparators (n = 7) | Overall PH or Comparator P Value | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Demographics | ||||||||
| Age, y | PH | 48.4 [36.0, 58.7] {0} | 54.1 [41.4, 63.3] {0}a | 69.8 [61.2, 76.5] {0}a | 65.1 [57.1, 72.6] {0}a | 56.7 [48.5, 68.7] {0} | 58.6 [47.1, 70.3] {0} | <0.001 |
| Comparator | - | 63.5 [54.7, 72.4] {0} | 63.0 [53.8, 71.9] {0} | 62.9 [56.2, 68.8] {0} | 52.7 [39.3, 61.9] {0} | 61.6 [43.5, 66.8] {0} | 0.004 | |
| Female | PH | 67 (69.8) {0} | 259 (73.4) {0} | 78 (57.4) {0} | 88 (51.2) {0} | 35 (61.4) {0} | 11 (34.4) {0} | <0.001 |
| Comparator | - | 46 (79.3) {0} | 76 (54.3) {0} | 64 (53.8) {0} | 12 (52.2) {0} | 6 (85.7) {0} | 0.000 | |
| Hispanic ethnicity | PH | {0} | {0} | {0} | {0} | {0} | {0} | 0.058 |
| No | 81 (84.4) | 302 (85.6) | 128 (94.1) | 149 (86.6) | 50 (87.7) | 26 (81.3) | ||
| Yes | 14 (14.6) | 43 (12.2) | 5 (3.7) | 16 (9.3) | 6 (10.5) | 5 (15.6) | ||
| Unknown or not reported | 1 (1.0) | 8 (2.3) | 3 (2.2) | 7 (4.1) | 1 (1.8) | 1 (3.1) | ||
| Hispanic ethnicity | Comparator | - | {0} | {0} | {0} | {0} | {0} | 0.34 |
| No | - | 49 (84.5) | 128 (91.4) | 107 (89.9) | 19 (82.6) | 6 (85.7) | ||
| Yes | - | 6 (10.3) | 6 (4.3) | 6 (5.0) | 2 (8.7) | 1 (14.3) | ||
| Unknown or not reported | - | 3 (5.2) | 6 (4.3) | 6 (5.0) | 2 (8.7) | 0 (0.0) | ||
| Race | PH | {0} | {0} | {0} | {0} | {0} | {0} | <0.001 |
| Black or African American | 9 (9.4) | 37 (10.5) | 15 (11.0) | 22 (12.8) | 13 (22.8) | 19 (59.4) | ||
| White | 85 (88.5) | 268 (75.9) | 115 (84.6) | 138 (80.2) | 41 (71.9) | 11 (34.4) | ||
| Other | 2 (2.1) | 37 (10.5) | 5 (3.7) | 7 (4.1) | 1 (1.8) | 2 (6.3) | ||
| Unknown or not reported | 0 (0.0) | 11 (3.1) | 1 (0.74) | S (2.9) | 2 (3.5) | 0 (0.0) | ||
| Race | Comparator | - | {0} | {0} | {0} | {0} | {0} | 0.008 |
| Black or African American | - | 6 (10.3) | 8 (5.7) | 6 (5.0) | 7 (30.4) | 3 (42.9) | ||
| White | - | 49 (84.5) | 128 (91.4) | 106 (89.1) | 13 (56.5) | 4 (57.1) | ||
| Other | - | 2 (3.4) | 4 (2.9) | 4 (3.4) | 1 (4.3) | 0 (0.0) | ||
| Unknown or not reported | - | 1 (1.7) | 0 (0.0) | 3 (2.5) | 2 (8.7) | 0 (0.0) | ||
| Anthropometrics | ||||||||
| BMI, kg/m2 | PH | 27.4 [22.1, 31.5] {2} | 27.4 [23.7, 32.9] {0} | 32.7 [26.3, 37.3] {0} | 28.2 [23.6, 34.2] {0} | 31.1 [26.4, 36.1] {0} | 26.4 [23.2, 33.4] {0} | <0.001 |
| Comparator | - | 27.0 [23.8, 32.0] {0} | 31.2 [27.2, 36.6] {0} | 28.7 [24.4, 32.8] {0} | 32.5 [28.1, 36.5] {0} | 29.1 [25.2, 35.1] {0} | <0.001 | |
| Systolic blood pressure, mm Hg | PH | 122.0 [112.0, 134.0] {1} | 112.0 [103.0, 124.0] {2}a | 127.0 [112.0, 146.0] {1} | 122.5 [109.0, 136.0] {0} | 127.0 [113.0, 138.0] {0} | 115.5 [106.5, 133.5] {0} | <0.001 |
| Comparator | - | 122.0 [113.0, 132.0] {0} | 123.0 [110.0, 137.0] {1} | 121.0 [109.0, 136.0] {1} | 125.0 [119.0, 137.0] {0} | 127.0 [108.0, 154.0] {0} | 0.51 | |
| Diastolic blood pressure, mmHg | PH | 74.0 [69.0, 84.0] {1} | 69.0 [63.0, 76.0] {2} | 70.0 [64.0, 79.0] {1} | 72.0 [64.0, 81.5] {0} | 74.0 [65.0, 81.0] {0} | 71.0 [65.5, 79.0] {0} | 0.005 |
| Comparator | - | 70.0 [63.0, 78.0] {0} | 72.0 [65.0, 79.0] {1} | 71.0 [65.0, 83.0] {1} | 75.0 [71.0, 85.0] {0} | 76.0 [61.0, 78.0] {0} | 0.11 | |
| Heart rate, beats/min | PH | 69.0 [61.0, 79.0] {1} | 76.0 [66.0, 86.0] {3}a | 70.0 [61.0, 78.0] {1} | 79.5 [70.0, 91.0] {0}a | 75.0 [68.0, 83.0] {0} | 79.0 [69.5, 90.5] {0} | <0.001 |
| Comparator | - | 70.5 [64.0, 82.0] {0} | 69.0 [63.0, 80.0] {1} | 75.0 [66.0, 83.0] {1} | 69.0 [61.0, 76.0] {0} | 69.0 [66.0, 76.0] {0} | 0.052 | |
| Clinical laboratory values | ||||||||
| NT-proBNP, pg/mL | PH | 48.6 [25.9, 74.6] {7} | 256.5 [96.8, 994.9] {15}a | 1073.0 [370.6, 2,284.0] {5}a | 359.5 [118.1, 1,441.0] {6}a | 184.1 [83.1, 871.8] {1}a | 99.7 [34.7, 713.4] {1} | <0.001 |
| Comparator | - | 112.8 [64.1, 222.0] {1} | 382.9 [111.2, 920.7] {2} | 85.8 [44.7, 193.6] {3} | 72.1 [44.4, 126.4] {2} | 163.6 [73.5, 925.0] {0} | <0.001 | |
| Positive rheumatoid factor, n | PH | 3 (3.3) {6} | 32 (9.4) {13} | 15 (11.5) {5} | 18 (10.8) {6} | 4 (7.1) {1} | 2 (6.5) {1} | 0.83 |
| Comparator | - | 7 (12.1) {0} | 8 (5.7) {0} | 11 (9.5) {3} | 2 (9.5) {2} | 0 (0.0) {0} | 0.47 | |
| Positive antinuclear antibody, ANA, n | PH | 15 (17.9) {12} | 113 (39.0) {63}a | 58 (55.2) {31} | 57 (42.2) {37} | 13 (28.9) {12} | 3 (11.5) {6} | <0.001 |
| Comparator | - | 29 (63.0) {12} | 50 (42.7) {23} | 39 (40.6) {23} | 4 (20.0) {3} | 2 (33.3) {1} | 0.007 | |
Values are median [P25, P75] {n of missing}, or n (column %) {n of missing}. P values calculated as follows: Kruskal-Wallis test for continuous and ordinal variables, Pearson’s chi-square or Fisher exact test for categorical variables.
Significant difference at level 0.05 between comparators and PH participants within the group. No corrections for multiple testin0 were applied. 7 group 5 comparators are shown for descriptive purposes only, they were not included in the statistical comparisons. For race and ethnicity, the “other/unknown” category is shown for descriptive purposes only, they were not included in the statistical comparisons. Positive ANA is defined as titer ≥1:160 or positive ANA value obtained by immunoassay. Positive RA factor is defined as >20 lU/ml
Figure 2. Selected Clinical Variables Across the Spectrum of Pulmonary Vascular Disease.
Spider plots of selected clinical variables across the spectrum of pulmonary vascular disease including disease comparators. Presented are characteristics of cardiac physiology: (A) N-terminal-pro-brain natriuretic peptide (NT-proBNP), (B) 6-minute walk distance (6MWD), (C) sleep study (% recording time <90% saturation), (D) ground glass on chest computed tomography (CT), (E) diffusing capacity for carbon monoxide (DLCO), and (F) echocardiographic (ECHO) right atrial (RA) volume index. *Significant difference across PH or comparator groups. Median values shown. Comparator group 5 not included in statistical comparisons. †Significant difference at level 0.05 between comparators and PH participants within group. G = group.
PULMONARY PHYSIOLOGY.
Pulmonary physiology metrics are presented in Table 5. There were significant differences in 6-minute walk distance between WSPH groups, with groups 2 and 3 having the numerically lowest distances and both being lower than their respective comparators (P < 0.05 across PH and groups 2 and 3 vs comparators) (Figure 2B). A total of 703 of 1,193 enrollees underwent sleep study and were largely similar to the enrolled subjects (see Supplemental Table 3 for comparison of enrollees with and without sleep study). Nocturnal desaturation was prevalent in PH and more frequently observed as a percentage of recording time <90% in groups 1, 3, and 4 PH vs comparators (P < 0.05 for all) (Figure 2C) with a higher % of enrollees experiencing 60 or more desaturation minutes in WSPH groups 1, 3, and 4 vs comparators (P < 0.05). Differences were noted in apnea/hypopnea index across PH groups (P < 0.001), with the numerically highest values in group 2. Parenchymal lung disease findings on chest CT were common in group 1 PH with emphysema in 9.8%, interstitial lung disease in 17.7%, and 50.2% ground glass in PH vs only 13.7% in comparators (P < 0.05). Although there were no differences in disease duration or oral therapy use in group 1 PH subjects with and without ground glass opacities, prostanoid use was more common in those with ground glass opacities (32.5% vs 52.8%; P < 0.001) (Supplemental Table 4). WSPH groups 2 and 3 also had more ground glass than their comparators (Figure 2D). As expected, WSPH group 3 had more emphysema and interstitial lung disease than other WSPH groups. Pulmonary function testing differences were present (except for forced expiratory volume/forced vital capacity % predicted10) across the PH groups with the lowest diffusing capacity for carbon monoxide (DLCO) in group 3 PH (P < 0.001) (Figure 2E). Groups 1–3 PH all had significantly lower DLCO vs their respective comparators (P < 0.05). DLCO was reduced to similar levels in groups 1, 2, and 4 PH.
Table 5.
Pulmonary Physiology
| Healthy Control Subjects (n = 96) | Group 1 WSPH (n = 353) Comparators (n = 58) | Group 2 WSPH (n = 136) Comparators (n = 140) | Group 3 WSPH (n = 172) Comparators (n = 119) | Group 4 WSPH (n = 57) Comparators (n = 23) | Group 5 WSPH (n = 32) Comparators (n = 7) | Overall PH or Comparator p Value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 6-min walk test | ||||||||
| 6 min walk distance, m | PH | 531.3 [469.0, 594.7] {2} | 406.7 [320.3, 479.2] {43} | 298.7 [210.0, 368.0] {29}a | 253.6 [182.9, 356.0] {46}a | 364.2 [280.4, 442.3] {7} | 347.0 [299.1, 420.0] {4} | <0.001 |
| Comparator | - | 394.0 [306.0, 440.0] {5} | 352.3 [280.6, 451.4] {23} | 339.0 [256.0, 405.0] {20} | 423.5 [318.4, 526.0] {3} | 283.7 [245.0, 327.0] {0} | 0.007 | |
| SpO2 at the end of the walk, % | PH | 98.0 [97.0, 99.0] {2} | 93.0 [88.0, 96.0] {45}a | 96.0 [92.0, 98.0] {29}a | 89.5 [85.0, 95.0] {46}a | 91.0 [87.0, 95.0] {7}a | 91.0 [87.5, 96.0] {4} | <0.001 |
| Comparator | - | 98.0 [96.0, 99.0] {5} | 97.0 [95.0, 98.0] {23} | 93.0 [89.0, 97.0] {20} | 98.0 [96.0, 99.0] {3} | 97.0 [92.0, 99.0] {0} | <0.001 | |
| Sleep study | ||||||||
| Apnea hypopnea index, events/h of sleep | PH | 3.3 [1.3, 8.8] {18} | 4.4 [1.07,13.5] {168} | 11.5 [4.2, 26.9] {52} | 4.4 [0.91,11.9] {76} | 7.2 [2.5, 23.2] {22} | 7.5 [3.5,14.7] {12} | <0.001 |
| Comparator | - | 8.1 [2.5,16.6] {27} | 8.4 [2.9, 20.3] {57} | 4.8 [0.87,15.3] {53} | 7.8 [2.5, 21.7] {8} | 7.9 [0.71, 22.8] {3} | 0.082 | |
| Apnea hypopnea index ≥5 | PH | 31 (39.7) {18} | 92 (49.7) {168} | 60 (71.4) {52} | 47 (49.0) {76} | 22 (62.9) {22} | 12 (60.0) {12} | 0.008 |
| Comparator | 17 (54.8) {27} | 57 (68.7) {57} | 33 (50.0) {53} | 10 (66.7) {8} | 2 (50.0) {3} | 0.11 | ||
| O2 or PAP device used during PVDOMICS sleep study | PH | 5 (6.5) {19} | 77 (49.7) {198}a | 35 (52.2) {69} | 59 (72.0) {90}a | 19 (59.4) {25}a | 9 (56.3) {16} | 0.021 |
| Comparator | 3 (14.3) {37} | 27 (37.0) {67} | 24 (46.2) {67} | 1 (9.1) {12} | 1 (33.3) {4} | 0.017 | ||
| O2 or PAP device used during PVDOMICS sleep study | PH | 19 | {198}a | {69} | 0 {9 | fN | to | <0.001 |
| O2 only used | 1 (1.3) {0} | 46 (29.7)a | 10 (14.9) | 45 (54.9)a | 7 (21.9)a | 4 (25.0) | ||
| PAP device only used | 4 (5.2) {0} | 21 (13.5)a | 21 (31.3) | 3 (3.7)a | 8 (25.0)a | 2 (12.5) | ||
| O2 and PAP used | 0 (0.0) {0} | 10 (6.5)a | 4 (6.0) | 11 (13.4)a | 4 (12.5)a | 3 (18.8) | ||
| O2 and PAP not used | 72 (93.5) {0} | 78 (50.3)a | 32 (47.8) | 23 (28.0)a | 13 (40.6)a | 7 (43.8) | ||
| O2 or PAP device used during PVDOMICS sleep study | Comparator | - | {37} | {67} | {67} | {12} | {4} | 0.001 |
| O2 only used | - | 1 (4.8) | 5 (6.8) | 17 (32.7) | 0 (0.0) | 1 (33.3) | ||
| PAP device only used | - | 2 (9.5) | 18 (24.7) | 4 (7.7) | 1 (9.1) | 0 (0.0) | ||
| O2 and PAP used | - | 0 (0.0) | 4 (5.5) | 3 (5.8) | 0 (0.0) | 0 (0.0) | ||
| O2 and PAP not used | - | 18 (85.7) | 46 (63.0) | 28 (53.8) | 10 (90.9) | 2 (66.7) | ||
| Experienced desaturation, O2 <90% | PH | 55 (70.5) {18} | 165 (91.7) {173} | 71 (89.9) {57} | 82 (86.3) {77} | 31 (88.6) {22} | 17 (89.5) {13} | 0.74 |
| Comparator | - | 25 (83.3) {28} | 74 (91.4) {59} | 48 (76.2) {56} | 13 (92.9) {9} | 3 (75.0) {3} | 0.066 | |
| Recording time, min | PH | 399.5 [350.0, 442.0] {18} | 386.5 [334.0, 444.0] {171}a | 377.0 [292.0, 439.0] {54}a | 392.0 [320.0, 444.5] {76}a | 400.0 [334.0, 468.0] {22}a | 362.0 [324.0, 431.7] {11} | 0.66 |
| Comparator | - | 414.4 [276.0, 465.0] {27} | 387.0 [341.0, 453.0] {57} | 411.0 [361.9, 462.4] {54} | 381.0 [316.0, 463.0] {8} | 425.5 [364.5, 532.5] {3} | 0.86 | |
| % of recording time <90% O2 saturation | PH | 0.12 [0.00, 2.8] {18} | 37.0 [2.2, 87.3] {173}a | 6.4 [0.61, 41.8] {57} | 17.1 [0.42, 64.5] {77}a | 35.7 [5.3, 82.9] {22}a | 35.3 [5.1, 58.0] {13} | 0.009 |
| Comparator | - | 2.8 [0.19,13.1] {28} | 6.3 [0.80, 24.6] {59} | 2.3 [0.01, 31.2] {56} | 1.2 [0.10, 6.1] {9} | 26.8 [0.01, 56.9] {3} | 0.29 | |
| Severity index for participants experiencing desaturation, O2 <90% | PH | {18} | {173}a | {57} | {77}a | {22}a | {13} | 0.086 |
| No desaturation | 23 (29.5) | 15 (8.3)a | 8 (10.1) | 13 (13.7)a | 4 (11.4)a | 2 (10.5) | ||
| Desaturation for 20 min or less of recording time | 42 (53.8) | 47 (26.1)a | 30 (38.0) | 23 (24.2)a | 4 (11.4)a | 3 (15.8) | ||
| Desaturation for 20+ to 60 min of recording time | 9 (11.5) | 17 (9.4)a | 10 (12.7) | 13 (13.7)a | 7 (20.0)a | 1 (5.3) | ||
| Desaturation for 60+ min of recording time | 4 (5.1) | 101 (56.1)a | 31 (39.2) | 46 (48.4)a | 20 (57.1)a | 13 (68.4) | ||
| Severity index for participants experiencing desaturation, O2 <90% | Comparator | {28} | {59} | {56} | {9} | {3} | 0.23 | |
| No desaturation | 5 (16.7) | 7 (8.6) | 15 (23.8) | 1 (7.1) | 1 (25.0) | |||
| Desaturation for 20 min or less of recording time | 13 (43.3) | 33 (40.7) | 23 (36.5) | 9 (64.3) | 1 (25.0) | |||
| Desaturation for 20+ to 60 min of recording time | 7 (23.3) | 14 (17.3) | 5 (7.9) | 2 (14.3) | 0 (0.0) | |||
| Desaturation for 60+ mins of recording time | 5 (16.7) | 27 (33.3) | 20 (31.7) | 2 (14.3) | 2 (50.0) | |||
| Lung imaging | ||||||||
| Computed tomography | ||||||||
| Emphysema | PH | 1 (1.2) {10} | 31 (9.8) {36} | 15 (12.2) {13} | 74 (47.1) {15}a | 3 (5.8) {5} | 5 (16.1) {1} | <0.001 |
| Comparator | - | 4 (7.8) {7} | 8 (6.8) {22} | 27 (25.0) {11} | 0 (0.0) {3} | 0 (0.0) {0} | <0.001 | |
| Interstitial Lung disease | PH | 0 (0.0) {10} | 56 (17.7) {36} | 14 (11.4) {13} | 69 (43.9) {15} | 2(3.8) {5} | 17(54.8) {1} | <0.001 |
| Comparator | - | 4 (7.8) {7} | 7 (5.9) {22} | 42 (38.9) {11} | 2(10.0) {3} | 1 (14.3) {0} | <0.001 | |
| Ground glass | PH | 1 (1.2) {10} | 159 (50.2) {36}a | 43 (35.0) {13}a | 71 (45.2) {15}a | 16 (30.8) {5} | 18 (58.1) {1} | 0.005 |
| Comparator | - | 7 (13.7) {7} | 17 (14.4) {22} | 35 (32.4) {11} | 4 (20.0) {3} | 2 (28.6) {0} | 0.004 | |
| Ventilation/perfusion scan | ||||||||
| Probability of pulmonary embolism | PH | 93 | 46 | 30 | 44 | 6 | 6 | <0.001 |
| None/other | 3 (100.0) | 297 (96.7) | 101 (95.3) | 119 (93.0) | 16 (31.4) | 23 (88.5) | ||
| High | 0 (0.0) | 10 (3.3) | 5 (4.7) | 9 (7.0) | 35 (68.6) | 3(11.5) | ||
| Probability of pulmonary embolism | Comparator | - | {21} | {29} | {29} | {1} | {2} | <0.001 |
| None/other | - | 37 (100.0) | 109 (98.2) | 87 (96.7) | 10 (45.5) | 3 (60.0) | ||
| High | - | 0 (0.0) | 2 (1.8) | 3 (3.3) | 12 (54.5) | 2 (40.0) | ||
| Pulmonary function test | ||||||||
| Forced expiratory volume/ forced vital capacity, % predicted | PH | 97.6 [92.4, 101.6] {4} | 92.8 [86.3, 98.6] {30}a | 92.8 [84.4,101.0] {13} | 91.5 [71.3, 102.8] {19} | 91.2 [84.1, 97.2] {8}a | 90.1 [83.8, 96.6] {2} | 0.49 |
| Comparator | - | 97.6 [94.2,104.2] {9} | 95.1 [88.5,100.4] {17} | 96.3 [83.9,105.4] {15} | 99.5 [95.6,104.0] {5} | 95.8 [93.7, 98.7] {0} | 0.059 | |
| DLCO, % predicted | PH | 88.7 [78.7,101.7] {4} | 58.6 [42.4, 72.5] {26}a | 53.2 [40.6, 66.1] {20}a | 31.4 [21.9, 42.6] {37}a | 60.0 [47.9, 74.9] {7} | 45.4 [30.3, 57.6] {3} | <0.001 |
| Comparator | - | 66.0 [57.0, 83.8] {8} | 68.7 [55.0, 80.6] {19} | 47.9 [31.6, 73.2] {22} | 72.0 [57.6, 79.1] {4} | 48.2 [41.7, 89.1] {1} | <0.001 | |
| TLC, % predicted | PH | 102.1 [93.9, 111.6] {6} | 89.8 [79.4, 100.0] {36} | 81.9 [69.4, 92.6] {25}a | 76.5 [59.4, 96.6] {49} | 90.8 [75.1,100.7] {9} | 74.3 [62.8, 82.7] {5} | <0.001 |
| Comparator | - | 92.9 [80.1,101.9] {12} | 87.9 [72.8, 97.9] {36} | 84.9 [59.1,103.7] {22} | 88.5 [67.5, 97.2] {5} | 73.7 [67.1, 99.7] {2} | 0.29 | |
Values are median [P25, P75] {n of missing}, or n (column %) {n of missing}. P values calculated as follows: Kruskal-Wallis test for continuous and ordinal variables, Pearson chi-square or Fisher exact test for categorical variables.
Significant difference at level 0.05 between comparators and PH participants within the group. 7 group 5 comparators are shown for descriptive purposes only, they were not included in the statistical comparisons.
CARDIAC PHYSIOLOGY.
Details of cardiac physiology are shown in Table 6. Sinus rhythm was identified with lowest frequency in group 2 PH and comparators (P < 0.001) and was less prevalent in group 2 PH vs comparators (P < 0.05). On echocardiogram differences were seen in left ventricular ejection fraction by visual estimate, left ventricular end-systolic and -diastolic diameters, and left atrial volume across comparators and PH groups (P < 0.001 all). Group 2 had the highest numerical left atrial volume levels, as well as the highest proportion of subjects with moderate or severe reduction in visually estimated left ventricular ejection fraction. Right atrial volume index (RAVI) differed between WSPH groups (P = 0.010) (Figure 2F) and between comparators (P < 0.001) with groups 1 and 2 PH having the largest RAVI across the groups. In each of the groups 1–4, RAVI was greater in WSPH groups vs their respective comparators (P < 0.05). Right ventricular end-diastolic dimension (RVEDD) did not differ between WSPH groups, whereas it did between comparators (P = 0.020). Overall, right ventricular (RV) size (normal, mild, moderate, severe enlargement on an integrated visual RV assessment) differed between WSPH groups (P < 0.001) and between PH and comparators for groups 1–4. Similarly, RV function by visual estimate (normal, mild, moderate, severe) differed between WSPH groups 1–4 (P = 0.003) and their respective comparators. Approximately one-half of group 1 PH patients had normal or only mildly decreased RV function and normal or only mildly dilated RVs, and these findings were also common in other forms of PH. Interestingly, the percentage difference in RAVI between PH and their respective comparators in groups 1–4 was greater than the difference in basal RVEDD between the same groups (40%−47% RAVI difference vs 7%−21% RVEDD difference). Tricuspid annular plane systolic excursion differed between WSPH groups 1–3 and their comparators and was lowest in group 2 PH.
Table 6.
Cardiac Physiology and Hemodynamics (RHC)
| Healthy Control Subjects (n = 96) | Group 1 WSPH (n = 353) Comparators (n = 58) | Group 2 WSPH (n = 136) Comparators (n = 140) | Group 3 WSPH (n = 172) Comparators (n = 119) | Group 4 WSPH (n = 57) Comparators (n = 23) | Group 5 WSPH (n = 32) Comparators (n = 7) | Overall PH or Comparator P Value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Electrocardiography | ||||||||
| Sinus rhythm | PH | 89 (98.9) {6} | 327 (94.2) {6} | 73 (57.0) {8}a | 146 (89.6) {9} | 50 (92.6) {3} | 29 (96.7) {2} | <0.001 |
| Comparator | - | 54 (98.2) {3} | 96 (69.6) {2} | 103 (88.0) {2} | 21 (95.5) {1} | 7 (100.0) {0} | <0.001 | |
| Cardiac imaging | ||||||||
| Echo | ||||||||
| Left atrial volume 4 chamber, mL | PH | 37.1 [30.7, 45.0] {4} | 38.5 [29.1, 51.9] {27} | 69.1 [48.7, 84.5] {12} | 40.2 [30.8, 54.2] {17} | 41.3 [30.9, 49.8] {4} | 41.5 [29.3, 66.0] {5} | <0.001 |
| Comparator | - | 39.0 [28.7, 49.4] {10} | 59.7 [43.5, 85.4] {18} | 36.2 [27.7, 53.9] {24} | 47.7 [38.0, 56.3] {3} | 54.4 [44.3, 59.2] {0} | <0.001 | |
| LV end-diastolic diameter, cm | PH | 4.5 [4.2, 4.8] {4} | 4.4 [3.9, 4.7] {19} | 4.9 [4.3, 5.3] {14} | 4.6 [4.1, 4.8] {17} | 4.5 [4.1, 5.0] {5} | 4.6 [4.1, 5.5] {3} | <0.001 |
| Comparator | 4.4 [4.0, 4.8] {10} | 4.9 [4.4, 5.4] {23} | 4.6 [4.1, 4.9] {26} | 4.8 [4.4, 5.2] {3} | 4.7 [4.5, 5.2] {0} | <0.001 | ||
| LV end-systolic diameter, cm | PH | 3.0 [2.7, 3.2] {4} | 2.7 [2.4, 3.1] {24} | 3.3 [2.9, 4.0] {17} | 3.0 [2.7, 3.3] {19} | 3.0 [2.5, 3.3] {5} | 3.1 [2.7, 3.6] {3} | <0.001 |
| Comparator | - | 2.8 [2.3, 3.1] {12} | 3.2 [2.9, 3.8] {24} | 3.0 [2.6, 3.4] {27} | 3.2 [2.7, 3.5] {3} | 3.0 [2.7, 3.5] {0} | <0.001 | |
| LV visual ejection fraction, % | PH | {4} | {16} | {10} | {11} | {4} | {4} | <0.001 |
| Normal | 92 (100.0) | 321 (95.3) | 96 (76.2) | 149 (92.5) | 49 (92.5) | 25 (89.3) | ||
| Mild | 0 (0.0) | 10 (3.0) | 11 (8.7) | 8 (5.0) | 3 (5.7) | 2 (7.1) | ||
| Moderate | 0 (0.0) | 5 (1.5) | 10 (7.9) | 3 (1.9) | 0 (0.0) | 0 (0.0) | ||
| Severe | 0 (0.0) | 1 (0.30) | 9 (7.1) | 1 (0.62) | 1 (1.9) | 1 (3.6) | ||
| LV visual ejection fraction, % | Comparator | - | {9} | {17} | {18} | {3} | {0} | <0.001 |
| Normal | - | 49 (100.0) | 96 (78.0) | 91 (90.1) | 20 (100.0) | 6 (85.7) | ||
| Mild | - | 0 (0.0) | 14 (11.4) | 7 (6.9) | 0 (0.0) | 0 (0.0) | ||
| Moderate | - | 0 (0.0) | 10 (8.1) | 3 (3.0) | 0 (0.0) | 1 (14.3) | ||
| Severe | - | 0 (0.0) | 3 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| RA volume index, mL/m2 | PH | 18.4 [15.4, 23.2] {5} | 31.0 [22.4, 44.0] {24}a | 32.3 [22.8, 40.2] {13}a | 25.3 [18.9, 43.0] {20}a | 26.7 [20.9, 47.3] {3}a | 26.2 [15.8, 38.1] {4} | 0.010 |
| Comparator | - | 22.1 [15.2, 27.4] {13} | 22.9 [16.7, 38.1] {20} | 17.2 [12.7, 22.6] {28} | 18.5 [16.0, 23.5] {4} | 23.5 [16.5, 26.6] {0} | <0.001 | |
| RV end-diastolic basal dimension, cm | PH | 3.5 [3.3, 3.9] {6} | 4.4 [3.9, 5.0] {34}a | 4.3 [3.8, 4.9] {25}a | 4.3 [3.8, 4.8] {32}a | 4.5 [3.8, 5.0] {8} | 4.3 [3.7, 4.9] {7} | 0.36 |
| Comparator | - | 3.6 [3.4, 4.1] {12} | 4.0 [3.5, 4.6] {27} | 3.7 [3.5, 4.1] {33} | 4.0 [3.7, 4.4] {4} | 3.5 [3.4, 4.2] {0} | 0.020 | |
| RV size, visual | PH | {4} | {15}a | {10}a | {11}a | {4}a | {3} | <0.001 |
| Normal | 83 (90.2) | 72 (21.3)a | 39 (31.0)a | 47 (29.2)a | 14 (26.4)a | 11 (37.9) | ||
| Mildly dilated | 9 (9.8) | 102 (30.2)a | 56 (44.4)a | 53 (32.9)a | 16 (30.2)a | 10 (34.5) | ||
| Moderately dilated | 0 (0.0) | 93 (27.5)a | 27 (21.4)a | 40 (24.8)a | 16 (30.2)a | 5 (17.2) | ||
| Severely dilated | 0 (0.0) | 71 (21.0)a | 4 (3.2)a | 21 (13.0)a | 7 (13.2)a | 3 (10.3) | ||
| RV size, visual | Comparator | - | {9} | {19} | {20} | {3} | {0} | 0.29 |
| Normal | - | 37 (75.5) | 77 (63.6) | 70 (70.7) | 15 (75.0) | 5 (71.4) | ||
| Mildly dilated | - | 9 (18.4) | 27 (22.3) | 22 (22.2) | 4 (20.0) | 1 (14.3) | ||
| Moderately dilated | - | 2 (4.1) | 16 (13.2) | 6 (6.1) | 1 (5.0) | 1 (14.3) | ||
| Severely dilated | - | 1 (2.0) | 1 (0.83) | 1 (1.0) | 0 (0.0) | 0 (0.0) | ||
| RV function, visual | PH | {4} | {15}a | {10}a | {12}a | {4}a | {3} | 0.005 |
| Normal | 90 (97.8) | 92 (27.2)a | 45 (35.7)a | 54 (33.8)a | 18 (34.0)a | 12 (41.4) | ||
| Mild decrease | 2 (2.2) | 90 (26.6)a | 42 (33.3)a | 43 (26.9)a | 16 (30.2)a | 9 (31.0) | ||
| Moderate decrease | 0 (0.0) | 90 (26.6)a | 32 (25.4)a | 42 (26.3)a | 13 (24.5)a | 6 (20.7) | ||
| Severe decrease | 0 (0.0) | 66 (19.5)a | 7 (5.6)a | 21 (13.1)a | 6 (11.3)a | 2 (6.9) | ||
| Right ventricle function, visual | Comparator | - | {9} | {19} | {20} | {3} | {0} | <0.001 |
| Normal | - | 46 (93.9) | 77 (63.6) | 82 (82.8) | 17 (85.0) | 6 (85.7) | ||
| Mild decrease | - | 2 (4.1) | 35 (28.9) | 15 (15.2) | 2 (10.0) | 0 (0.0) | ||
| Moderate decrease | - | 0 (0.0) | 6 (5.0) | 2 (2.0) | 1 (5.0) | 1 (14.3) | ||
| Severe decrease | - | 1 (2.0) | 3 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Tricuspid annular plane systolic excursion, cm | PH | 2.2 [2.0, 2.6] {8} | 1.9 [1.6, 2.2] {55}a | 1.7 [1.3, 2.0] {37}a | 1.9 [1.6, 2.2] {46}a | 1.9 [1.4, 2.1] {12} | 1.9 [1.7, 2.3] {7} | 0.002 |
| Comparator | - | 2.2 [1.9, 2.5] {18} | 1.9 [1.6, 2.4] {44} | 2.0 [1.7, 2.4] {48} | 2.1 [1.7, 2.5] {6} | 2.2 [2.1, 2.5] {2} | 0.13 | |
| Tricuspid valve regurgitation degree | PH | {6} | {17} | {11}a | {12}a | {4} | {3} | <0.001 |
| None/mild | 90 (100.0) | 241 (71.7) | 63 (50.4)a | 115 (71.9)a | 39 (73.6) | 22 (75.9) | ||
| Moderate/severe | 0 (0.0) | 95 (28.3) | 62 (49.6)a | 45 (28.1)a | 14 (26.4) | 7 (24.1) | ||
| Tricuspid valve regurgitation degree | Comparator | - | {9} | {18} | {18} | {3} | {0} | 0.032 |
| None/mild | - | 39 (79.6) | 91 (74.6) | 90 (89.1) | 18 (90.0) | 6 (85.7) | ||
| Moderate/severe | - | 10 (20.4) | 31 (25.4) | 11 (10.9) | 2 (10.0) | 1 (14.3) | ||
| Hemodynamics | ||||||||
| Resting RHC | ||||||||
| Right mean pressure, mm Hg | PH | N/A | 6.0 [4.0,10.0] {17}a | 12.0 [8.5,15.0] {4}a | 7.0 [4.0,10.0] {3}a | 6.0 [4.0,10.0] {0}a | 6.0 [3.5, 9.0] {0} | <0.001 |
| Comparator | - | 4.0 [2.0, 6.0] {1} | 8.0 [5.0,11.0] {3} | 5.0 [3.0, 7.0] {2} | 3.0 [3.0, 5.0] {1} | 3.0 [3.0, 4.0] {0} | <0.001 | |
| mPAP, mm Hg | PH | N/A | 43.0 [33.0, 54.0] {17}a | 39.0 [33.0, 46.0] {3}a | 35.0 [29.0, 43.5] {4}a | 34.0 [27.0, 43.0] {0}a | 32.0 [25.0, 39.5] {0} | <0.001 |
| Comparator | - | 17.0 [15.0,19.0] {1} | 23.0 [18.0, 28.0] {3} | 19.0 [15.0, 23.0] {1} | 17.5 [14.0, 20.0] {1} | 11.0 [11.0, 23.0] {0} | <0.001 | |
| Pulmonary arterial wedge, PCW pressure, mm Hg | PH | N/A | 10.0 [7.0,13.0] {20}a | 20.0 [16.0, 25.0] {4}a | 11.0 [8.0,15.0] {4}a | 12.0 [7.0,15.0] {0} | 12.0 [8.0,16.0] {1} | <0.001 |
| Comparator | - | 8.0 [6.0,10.0] {1} | 15.0 [10.0,19.0] {3} | 10.0 [6.0,12.0] {1} | 8.0 [6.0,10.0] {1} | 8.0 [4.0,11.0] {0} | <0.001 | |
| Cardiac output, L/min | PH | N/A | 5.0 [4.1, 6.2] {20} | 4.5 [3.6, 5.4] {7}a | 5.1 [4.2, 6.5] {6} | 5.0 [4.0, 6.0] {0} | 6.2 [5.2, 9.1] {3} | <0.001 |
| Comparator | - | 5.1 [4.5, 6.3] {1} | 5.3 [4.4, 6.4] {4} | 5.2 [4.2, 6.5] {3} | 5.4 [4.7, 5.9] {1} | 5.7 [4.4, 7.7] {0} | 0.86 | |
| Cardiac index, L/min/m2 | PH | N/A | 2.7 [2.2, 3.2] {12} | 2.2 [1.9, 2.6] {7}a | 2.8 [2.2, 3.2] {4} | 2.4 [2.0, 2.8] {0} | 3.0 [2.5, 4.4] {3} | <0.001 |
| Comparator | - | 2.7 [2.4, 3.3] {1} | 2.6 [2.2, 3.1] {4} | 2.6 [2.3, 3.1] {3} | 2.5 [2.2, 2.6] {1} | 3.2 [2.0, 3.4] {0} | 0.13 | |
| Pulmonary vascular resistance, WU | PH | N/A | 6.2 [4.0, 9.4] {25}a | 3.7 [2.6, 5.5] {8}a | 4.7 [3.0, 6.4] {8}a | 4.4 [3.0, 7.4] {0}a | 2.6 [2.1, 4.4] {4} | <0.001 |
| Comparator | - | 1.7 [1.1, 2.2] {1} | 1.5 [1.04, 2.0] {4} | 1.8 [1.2, 2.5] {3} | 1.7 [0.98, 2.0] {1} | 1.4 [0.92, 2.1] {0} | 0.072 | |
Values are median [P25, P75] {n of missing}, or N (column %) {n of missing}. P values calculated as follows: Kruskal-Wallistest for continuous and ordinal variables, Pearson’s chi-square or Fisher exact test for categorical variables.
Significant difference at level 0.05 between comparators and PH participants within the group. No corrections for multiple testing were applied. 7 group 5 comparators are shown for descriptive purposes only, they were not included in the statistical comparisons. Cardiac output and cardiac index are calculated based on thermodilution in 90% of the cases or more.
HEMODYNAMIC ASSESSMENT.
Right heart catheterization data are presented in Table 6. In WSPH groups 1–4 the mPAP and PVR were higher than in their respective comparators (P < 0.05 for all). There were significant differences in reported hemodynamic values across PH groups (P < 0.001 for all); notably, cardiac index was lowest and right atrial pressure highest in group 2 PH, whereas PVR and mPAP were highest in group 1 PH. There were 110 study participants with an mPAP of 21–24 mm Hg and 154 with a PVR between 2.2 and 3.0 WU.
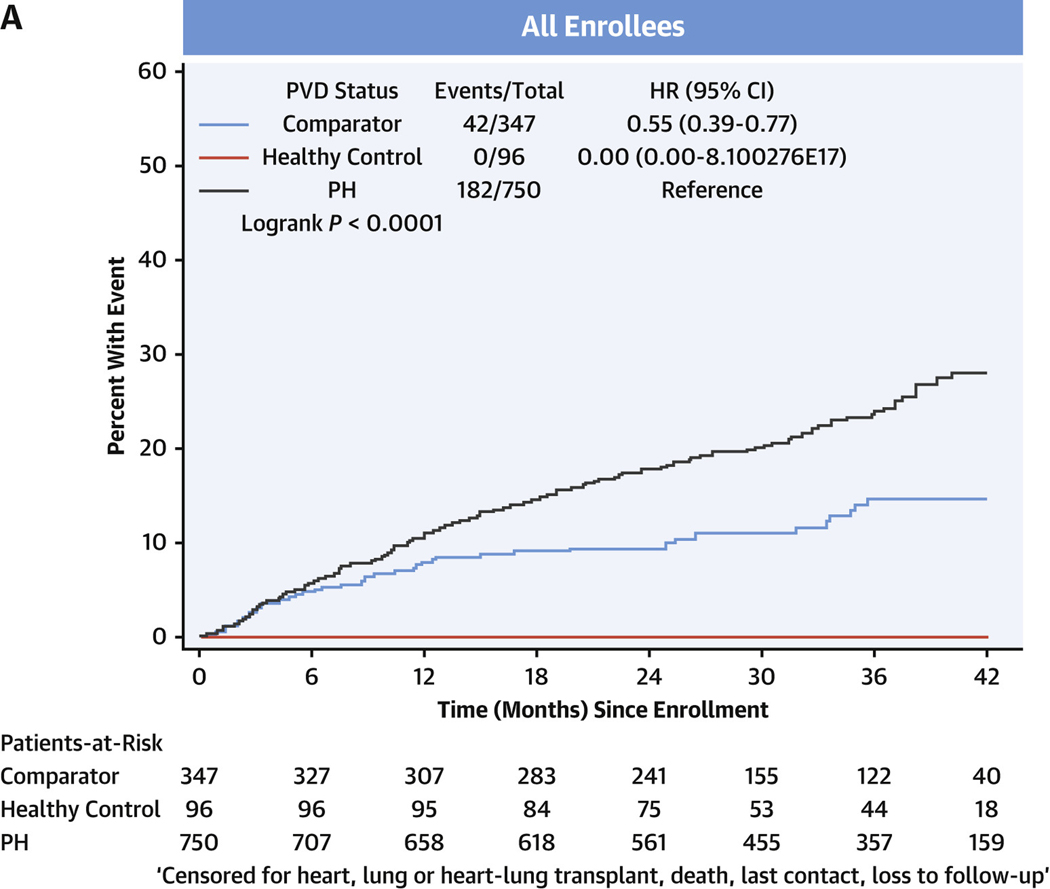
SURVIVAL.
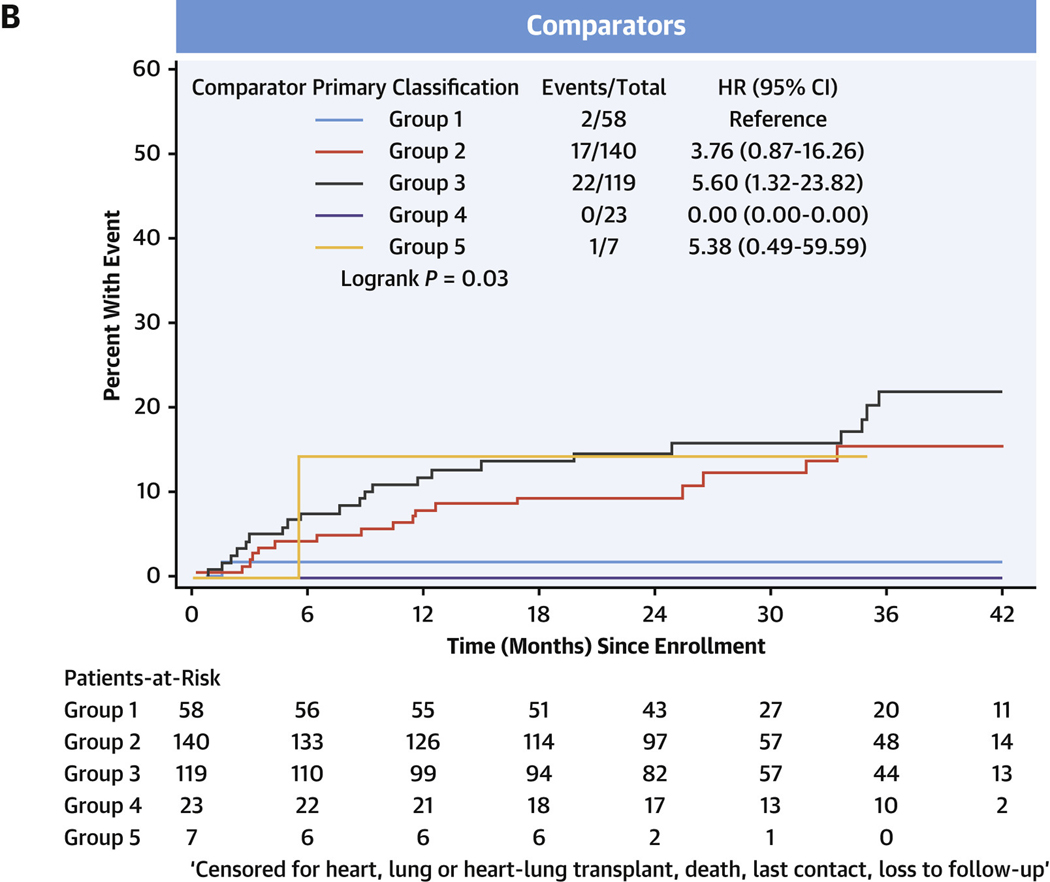
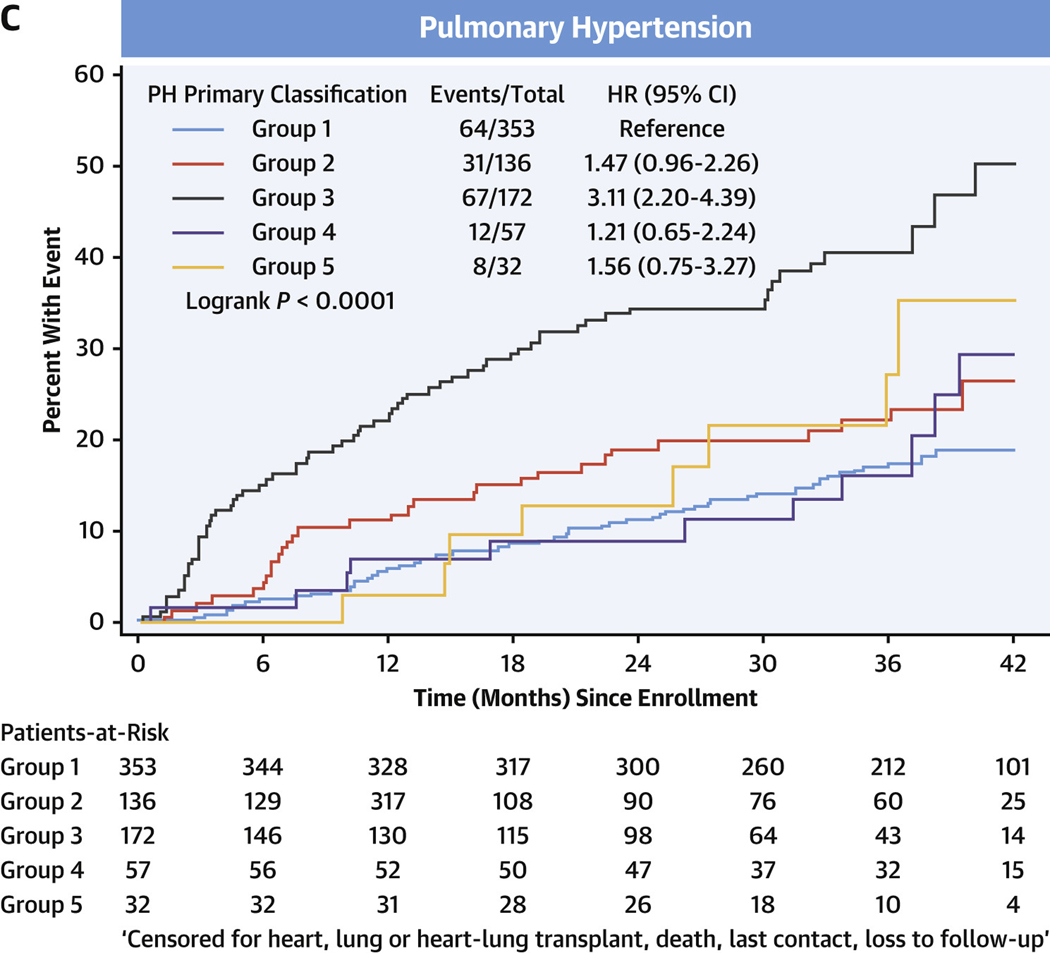
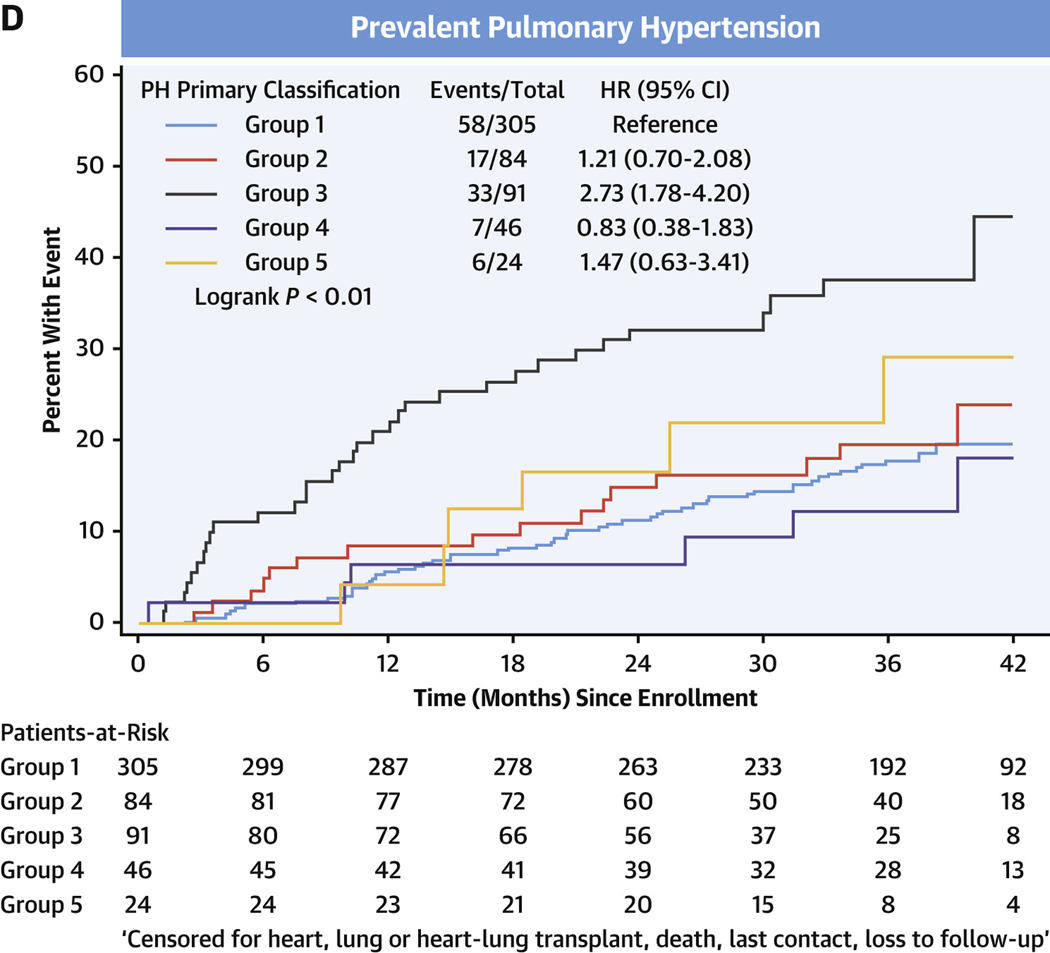
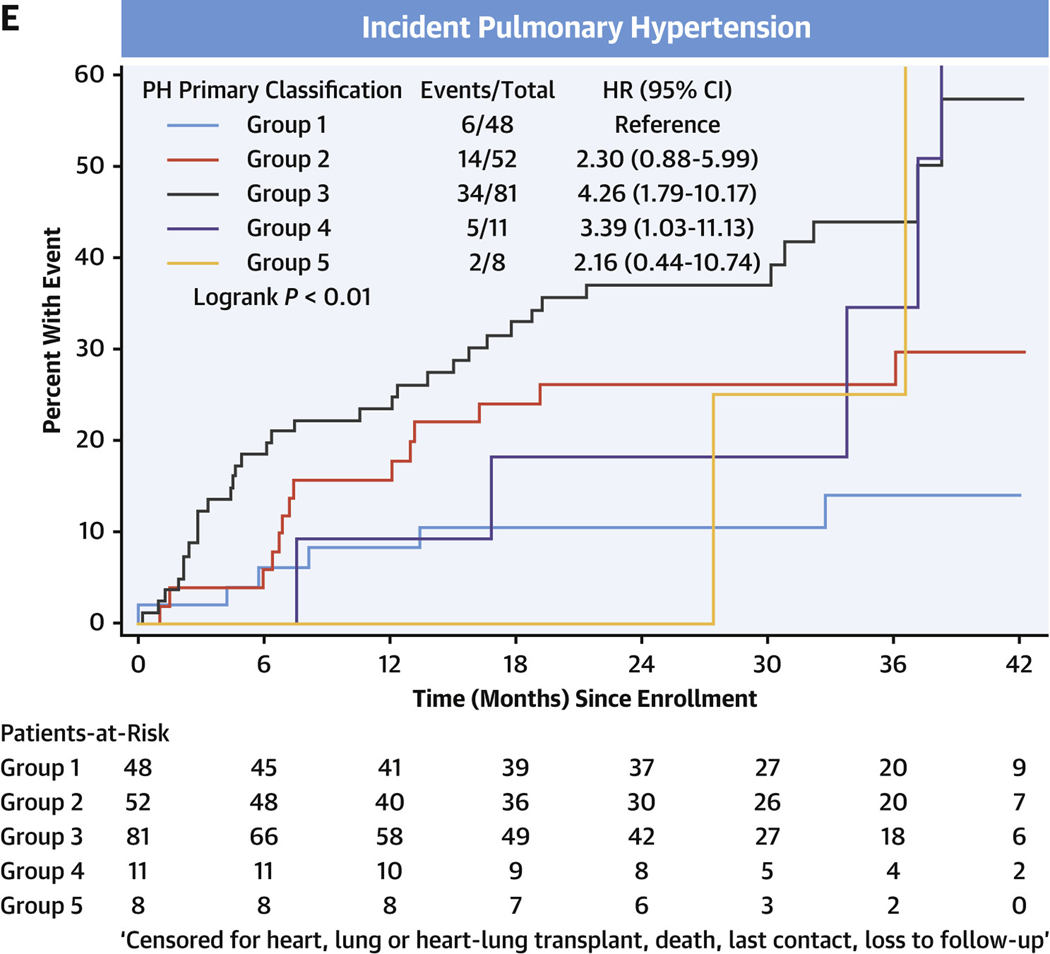
In the entire cohort, transplant-free survival was poorest in PH, intermediate in comparators, and there were no events in the healthy control subjects (Figure 3A, Supplemental Tables 5A to 5D). Among comparators, group 3 subjects averaged a >5-fold increased risk of death or heart/lung transplant over the follow-up period compared with group 1 subjects (HR: 5.60; 95% CI: 1.32–23.82) (Figure 3B). In the PH group, using group 1 PH as the reference group, the HR for death or relevant transplant was largest in group 3 PH at 3.11 (95% CI: 2.20–4.39) (Figure 3C) and moderately elevated, but not significantly, in group 2 PH at 1.47 (95% CI: 0.96–2.26). Compared with group 1 PH, transplant-free survival was significantly lower for both prevalent PH group 3 (HR: 2.73; 95% CI: 1.78–4.20) and incident group 3 PH (HR: 4.26; 95% CI: 1.79–10.17) (Figures 3D and 3E, respectively).
FIGURE 3. Transplant-free Survival in PVDOMICS Cohort.
Kaplan-Meier estimates of transplant-free survival given with HRs and 95% CIs. (A) Transplant-free survival in all enrollees. (B) Transplant-free survival in comparator group. (C) Transplant-free survival in pulmonary hypertension group. (D) Transplant-free survival in prevalent pulmonary hypertension. (E) Transplant-free survival in incident pulmonary hypertension. PH = pulmonary hypertension; PVD = pulmonary vascular disease.
DISCUSSION
Seeking to understand the breadth of PVD and refine the current classification system, PVDOMICS enrolled the first prospective U.S.-based protocol driven cohort of subjects representing the spectrum of PVD, inclusive of all WSPH groups, disease comparators, and healthy control subjects. PH subjects and comparators underwent a deep clinical phenotyping protocol including imaging of the lungs and heart and standardized right heart catheterization that will ultimately lead to identification of new phenotypes of PVD using both the basic clinical phenotyping, presented here, and omic analyses in the future. Early observations of this cohort include not only a description of the spectrum of pulmonary vascular disease and precursors in the United States, but also offer novel observations of similarities and differences across PH groups and comparators. A surprising observation is the nearly 40% prevalence of mixed etiology PH. Other new features are that low DLCO is a feature of groups 1–4 PH, lessening its discriminatory value; the high prevalence of nocturnal desaturation and abnormalities on chest CT in group 1 PH; elevated RAVI in groups 1–4 PH to a greater degree than RV dilation, suggesting this may be a key clue to the presence of PH; and poorer transplant-free survival in group 3 PH.
Although there have been several modern registry studies of PAH previously published, PVDOMICS is unique for several reasons. First, PVDOMICS included all forms of PH by design, which is distinct from most registries that exclusively focused on group 1 PAH.11–15 Moreover, rigorous adjudication to the diagnostic standards of the protocol was made by a committee of experienced physicians. This gives confidence that the assignment of patients to their respective WSPH category met general consensus. Second, although there are other registries that enrolled patients with non–group 1 PH, these either focused on groups 1 and 4 PH16–18 or were studies of treatment with PAH-specific therapy.19 Third, PVDOMICS sought to understand the spectrum of PVD, including precursors to and predictors of PVD, and as such, enrolled disease comparators with similar clinical presentation and predisposing conditions, eg, similar heart or lung pathology with either milder PVD or without manifest pulmonary hypertension. These crucial comparator groups have not previously been extensively studied and serve as more appropriate comparisons in assessing markers of PVD in non–group 1 cohorts. Additionally, although the enrollment criteria specified a definition of PH consistent with contemporary criteria from the fifth WSPH,1 comparators included enrollees with mPAP 21–24 mm Hg (110 enrollees met this definition), 20 PVR <3.0 WU, as well as those with mPAP <21 mm Hg. Planned longitudinal follow-up in PVDOMICS will allow early understanding of this little-studied and highly clinically relevant group.20–23 Fourth, PVDOMICS only enrolled subjects residing in the United States, in contrast to other registries that were based in Europe11,9,24 or the United Kingdom,25 where there may be differences in management. Further, REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management),12 the major prior U.S.-based registry, focused on group 1 PH and thus cannot comment on the full spectrum of PVD. Finally, in contrast to prior registries, PVDOMICS was a prospective cohort study with an extensive predefined phenotyping protocol for parameters measured with central interpretation for enrollees. This extensive protocol enhances our ability to illustrate phenotypic heterogeneity potentially classified within a single WSPH group in registry studies (ie, mixed WSPH groups 1 and 2).26 Thus, PVDOMICS combines the strength of a multicenter study with extensive and uniformly interpreted, high-quality data. These strengths of PVDOMICS will allow the data to answer impactful, relevant questions about clinical and omic phenotypes of PVD in a manner not possible in prior registries.
The enrollment data in PVDOMICS present a timely snapshot of PH in the United States and its complexity. First, despite diverse disease etiologies, PH subjects shared many similarities. Lung imaging demonstrated a high prevalence of parenchymal findings in all WSPH groups and a reduction in DLCO in groups 1–3 PH compared with comparators, suggesting that low DLCO may be an indicator of PH broadly, but cannot define which type to clinicians at the bedside. WSPH groups 1–3 also shared significant RA enlargement with relatively less proportional increase in RVEDD, suggesting that RAVI could be a more universal characteristic of PH rather than RV dimension, which was normal or mildly increased in more than one-half of enrollees with groups 1–3 PH, although this requires further study. Finally, even with expert adjudication by PH specialists, a substantial proportion of both PH patients and comparators had >1 diagnosis that could be causative of PH. This so-called “mixed etiology” PH is little understood in terms of prognosis or treatment, particularly in the case of group 1 PH with a high degree of prevalent enrollees, and illustrates the limitations of our current classification schema that allows for only a single diagnosis.20
There are also germane observations about the WSPH groups that were enrolled and how they differed. The largest PH group in PVDOMICS is group 1, and the distribution of etiologies is similar to published registry studies.11–13 Group 1 PH patients, despite having higher PVR than comparators, had no significant difference in resting cardiac index or 6-minute walk distance, perhaps reflecting enrollment of primarily successfully treated, prevalent disease. Group 1 PH patients had a number of alterations in pulmonary physiology including the following: 1) a high prevalence of nocturnal desaturation compared with comparators that was not explained by obstructive sleep apnea; and 2) a high prevalence of parenchymal findings on CT, including 17.6% with interstitial lung disease and one-half of patients with ground glass opacities. On further analysis, this finding could not be explained by disease duration, and treatment with oral therapies was not different between those with ground glass opacities and those without. Interestingly, prostacyclin use was more common in group 1 with ground glass opacities. Further study is warranted to understand if this is a marker of disease severity necessitating prostacyclin, an effect of prostacyclin therapy, or a marker of end-stage disease. Traditionally, group 1 PH requires exclusion of parenchymal lung disease27,28; thus, further study will be required to understand the cause and implications of these ground glass opacities. In group 2 PH, more than one-half of enrollees had a diagnosis of heart failure with preserved ejection fraction. This enrollment feature will facilitate new understanding of the poorly understood PVD of heart failure with preserved ejection fraction. Additionally, group 2 PH had severely impaired hemodynamics with numerically lowest cardiac index and highest right atrial pressure, with the lowest prevalence of sinus rhythm. In group 3 PH and comparators, there was a similar prevalence of obstructive lung disease and interstitial lung disease, which will facilitate future comparisons between these different subphenotypes. The observation that approximately one-half of all group 3 PH enrollees and comparators also were diagnosed with obstructive sleep apnea demonstrates the complicated and less well understood interactions among sleep-disordered breathing, intermittent hypoxia, pulmonary parenchymal disease, and pulmonary vascular pathologies. Finally, 6-minute walk distance was lowest in groups 2 and 3 PH, where it may be a valuable clinical assessment tool.
Follow up-status suggested significant differences in transplant-free survival. First, the low-risk state of many group 1 enrollees with prevalent disease and use of PAH-directed therapy was confirmed with improved survival compared with other PH groups. Transplant recipients were most common in group 3 PH, perhaps reflecting a bias in enrollment of patients undergoing pre-lung transplant evaluations in this group, and compared with group 1 PH, transplant-free survival was statistically lower in group 3 PH. In this group 39 of 172 (22.7%) either died or underwent transplant in the first year of follow-up.
STUDY LIMITATIONS.
First, enrollees in group 1 PH had predominantly prevalent disease with a median of 4.6 years of PH duration at enrollment, whereas other groups tended to have a shorter duration of PH diagnosis before enrollment, with medians ranging from 1.3–3.2 years. Consistent with this observation, 84% of group 1 PH subjects were also on PH-directed medical therapy. Although these enrollees will inform on the natural history of treated, prevalent PAH, observations on incident PAH cases may be more limited. Alternatively, this predominantly prevalent population may be relevant to clinical experience that heavily favors prevalent PVD. Although 59% of enrollees had sleep study, these data are not available for all participants. Minor differences in race, ethnicity, and BMI were found in enrollees who did not have a sleep study from those who did. Follow-up data in the PVDOMICS cohort is presently limited to vital status and solid organ transplantation; thus, data on medication changes, hospitalization, and other markers of disease severity is not present. A more detailed follow-up study visit is planned for a subset that will include echocardiography, quality of life metrics, 6-minute walk, and omic analysis. Finally, although we did successfully enroll across the spectrum of PVD, there are fewer enrollees in WSPH groups 4 and 5, and comparators for both groups and the enrollment reflects patients referred for specialist evaluation of PVD and also the funding-specific enrollment targets.29 Nonetheless, the detailed imaging, physiological, and omics data from the whole cohort will facilitate finding novel discoveries about the etiology, classification, and outcomes in all WSPH groups.
CONCLUSIONS
PVDOMICS achieved its goal of enrolling subjects across the spectrum of PVD including a subset with mild disease defined by mPAP and PVR and nearly 40% with mixed etiology PH, 2 understudied groups. Early findings among PH patients identified low DLCO as a common feature in groups 1–3 PH, the unexpected presence of ground glass opacities in one-half of group 1 enrollees, and a common finding of enlarged RA in groups 1–4 PH even without RV changes, suggesting that this may be a sensitive indicator of PH. This rich data set is facilitating new understanding of the full spectrum of PVD and will allow refinement of the current PVD classification.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Classification of PH requires cardiac and pulmonary imaging, functional, and hemodynamic assessments. The current classification system has limitations, and more than 1 potential etiology is identified in many patients.
TRANSLATIONAL OUTLOOK:
Longer-term cohort studies are needed to refine the system used for classification of PH and clarify implications for management and prognosis.
ACKNOWLEDGMENTS
The authors thank the members of the OSMB: Sharon Rounds, MD, Raymond Benza, MD, Todd Bull, MD, Jean Cadigan, PhD, James Fang, MD, Mardi Gomberg-Maitland, MD, and Grier Page, PhD.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The study received grants U01 HL125218 (Principal Investigator: Dr Rosenzweig), U01 HL125205 (Principal Investigator: Dr Frantz), U01 HL125212 (Principal Investigator: Dr Hemnes), U01 HL125208 (Principal Investigator: Dr Rischard), U01 HL125175 (Principal Investigator: Dr Hassoun), U01 HL125215 (Principal Investigator: Dr Leopold), and U01 HL125177 (Principal Investigator: Dr Beck) and was supported by the Pulmonary Hypertension Association. Dr Hemnes has served as a consultant for Bayer, United Therapeutics, Janssen, GossamerBio, and Tenax Therapeutics; holds stock in Tenax Therapeutics; and has received grants from the National Institutes of Health, CMREF, and Imara. Dr Leopold is supported by the National Institutes of Health grant U01 125215 and the American Heart Association 19AIML34980000; receives salary support from the Massachusetts Medical Society; has received research funding (to her institution) from Astellas; has served as a consultant for Abbott Vascular and United Therapeutics; and has served as a site principal investigator for a study sponsored by Aria CV. Dr Abidov is supported by research grants from Astellas Pharma, Boehringer Ingelheim, and Kiniksa outside of the submitted work. Dr Rosenzweig has received consulting fees from Acceleron for a scientific advisory board meeting; and her institution receives grant support from Bayer, United Therapeutics, Janssen, and SonVie. Dr Borlaug has received research grants from National Institutes of Health/NHLBI, AstraZeneca, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and has received consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr Dubrock has received consulting fees from Janssen Pharmaceuticals; and has served on advisory boards for Janssen Pharmaceuticals and United Therapeutics. Dr Finet is a consultant to Wolters Kluwer Health, Clinical Drug Information Ad Honorem. Dr Frantz has consulting, steering committee, and advisory board relationships with Altavant Sciences, Bayer, Gossamer Bio, Janssen, Shouti, France Foundation, IQVIA, Tenax, UpToDate, and United Therapeutics. Dr Garcia is CEO and founder of Aqualing Therapeutics. Dr Hassoun has served as scientific advisor for Merck Sharp & Dohme, an activity unrelated to the current work. Dr Highland has grants/contracts with Acceleron Pharmaceuticals, Actelion Pharmaceuticals (Janssen), Bayer Healthcare, Boehringer Ingelheim, Eiger Pharmaceuticals, Eli Lilly, Gossamer Bio, United Therapeutics, and Viela Bio (Horizon); has served as a consultant and/or member of a steering or advisory committee with Acceleron Pharmaceuticals, Actelion Pharmaceuticals (Janssen), Boehringer Ingelheim, Forsee, Genentech, Gossamer Bio, and United Therapeutics; and has served on the Speakers Bureau for Actelion Pharmaceuticals (Janssen), Bayer Healthcare, Boehringer Ingelheim, and United Therapeutics. Dr Hill has received research grants for Acceleron, Aerovate. Altavant, Gossamer. Liquidia, Merck, and United Therapeutics; and has served on advisory boards for Acceleron, Aerovate, Altavant, Gossamer, and Liquidia. Dr Maron has relationships with Deerfield Corporation, Actelion Sciences, and Tenax Therapeutics; and has U.S. Patent #9,605,047, Patent pending PCT/US2019/059890, Patent application 2021/133937. Dr Mathai has served as a consultant for Acceleron, Actelion, Bayer, and United Therapeutics. Dr Mehra is supported by National Institutes of Health grants U01HL125177 and UH3HL140144 and the American Heart Association AHA 18SFRN34170013; has received royalties from Up to Date; has received compensation from the American Board of Internal Medicine; and has received an honorarium from the American Academy of Sleep Medicine. M.M. Park has served on the Speakers Bureau of Lantheus Medical Imaging (Definity contrast). Dr Rischard has consulting relationships with Acceleron and United Therapeutics; is on a Steering Committee for Acceleron; and receives research support from Ismed, United Therapeutics, Bayer, Acceleron, Janssen, and AADI. Dr Thomas has served as a consultant for GE, Abbott, egnite, EchoIQ, and Caption Health; as well as spouse employment for Caption Health. Dr Horn has served on the Data and Safety Monitoring Board of AADi Biosciences and SoniVie; has served on the Clinical Events Committee for V-wave; and has served as a consultant for Biotronik. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PVD
pulmonary vascular disease
- RAVI
right atrial volume index
- RV
right ventricle
- RVEDD
right ventricular end-diastolic dimension
- WSPH
World Symposium on Pulmonary Hypertension
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. [DOI] [PubMed] [Google Scholar]
- 2.Newman JH, Rich S, Abman SH, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach. A joint NHLBI-Cardiovascular Medical Research and Education FundWorkshop report. Am J Respir Crit Care Med. 2017;195:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erzurum S, Rounds SI, Stevens T, et al. Strategic plan for lung vascular research: an NHLBI-ORDR Workshop Report. Am J Respir Crit Care Med. 2010;182:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robbins IM, Moore TM, Blaisdell CJ, Abman SH. Improving outcomes for pulmonary vascular disease. Am J Respir Crit Care Med. 2012;185:1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leopold JA, Maron BA, Loscalzo J. The application of big data to cardiovascular disease: paths to precision medicine. J Clin Invest. 2020;130:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hemnes AR, Beck GJ, Newman JH, et al. PVDOMICS: a multi-center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res. 2017;121:1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WHW, Wilcox JD, Jacob MS, et al. Comprehensive diagnostic evaluation of cardiovascular physiology in patients with pulmonary vascular disease: insights from the PVDOMICS program. Circ Heart Fail. 2020;13: e006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellis CL, Park MM, Abidov A, et al. Comprehensive echocardiographic evaluation of the right heart in patients with pulmonary vascular diseases: the PVDOMICS experience. Eur Heart J Cardiovasc Imaging. 2022;23(7):958–969. 10.1093/ehjci/jeab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrich S, Konietschke F PM, MANOVA RM. Resampling-based analysis of multivariant data and reported measures designs. R package version 0.3.4. Accessed June 22, 2022. https://CRANR-projectorg/package=MANOVARM 2019 [Google Scholar]
- 10.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137: 376–387. [DOI] [PubMed] [Google Scholar]
- 13.Lee WT, Ling Y, Sheares KK, Pepke-Zaba J, Peacock AJ, Johnson MK. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J. 2012;40:604–611. [DOI] [PubMed] [Google Scholar]
- 14.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132:373–379. [DOI] [PubMed] [Google Scholar]
- 15.Zhang R, Dai LZ, Xie WP, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest. 2011;140:301–309. [DOI] [PubMed] [Google Scholar]
- 16.Escribano-Subias P, Blanco I, Lopez-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40:596–603. [DOI] [PubMed] [Google Scholar]
- 17.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J. 2012;39:945–955. [DOI] [PubMed] [Google Scholar]
- 18.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. [DOI] [PubMed] [Google Scholar]
- 19.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–880. [DOI] [PubMed] [Google Scholar]
- 20.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron BA, Brittan EL, Hess E, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med. 2020;8:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assad TR, Maron BA, Robbins IM, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol. 2017;2:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs G, Avian A, Tscherner M, et al. Characterization of patients with borderline pulmonary arterial pressure. Chest. 2014;146:1486–1493. [DOI] [PubMed] [Google Scholar]
- 24.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36:957–967. [DOI] [PubMed] [Google Scholar]
- 25.Corris PA. The UK National Pulmonary Hypertension Service, Registry and Research Collaboration. Glob Cardiol Sci Pract. 2015;2015:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badagliacca R, Papa S, Rischard F. Multidimensional assessment and cluster analysis for idiopathic pulmonary arterial hypertension phenotyping. J Heart Lung Transplant. 2021;40:166–167. [DOI] [PubMed] [Google Scholar]
- 27.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904. 10.1183/13993003.01904-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed). 2016;69:177. [DOI] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services. NIH/NHLBI. FOA RFA-HL-14–027: Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics: Clinical Centers (CC) (U01). Accessed June 22, 2022. https://grants.nih.gov/grants/guide/rfa-files/rfa-hl-14-027.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.