Abstract
Expanding human populations favors a few species while extinguishing and endangering many others (Maxwell et al., 2016; Pimm et al., 2014). Understanding how animals perceive and learn about dangers and rewards can aid conservationists seeking to limit abundant or restore rare species (Schakner and Blumstein, 2016; Greggor et al., 2014; Angeloni et al., 2008; Fernández-Juricic and Schulte, 2016). Cognition research is informing conservation science by suggesting how naïve prey learn novel predators (Griffin et al., 2000; Moseby et al., 2015; Schakner et al., 2016; Blumstein, 2016), the mechanisms underlying variation in tolerance of human disturbance (Bostwick et al., 2014), and when natural aversions and fear learning can be leveraged to humanely control predators (Nielsen et al., 2015; Colman et al., 2014; Norbury et al., 2014; Lance et al., 2010; Cross et al., 2013). Insights into the relationships between cognition and adaptability suggest that behavioral inflexibility often presages species rarity (Amiel et al., 2011; Reif et al., 2011; Sol et al., 2008; Zhang et al., 2014; but see Kellert, 1984). Human compassion and restraint are ultimately required to conserve species. Cognitive science can therefore further inform conservation by revealing the complex inner worlds of the animals we threaten and, in partnership with environmental psychologists, explore how such newfound knowledge affects our empathy for other species and ultimately the public’s actions on behalf of species in need of conservation (Collado et al., 2013; Zhang et al., 2014).
Introduction
Conservation of rare species in the Anthropocene seeks to maintain and restore populations threatened by over-exploitation, habitat loss and degradation, invasive species, pollution, climate change, and myriad other side products of a rapidly growing and hyper-consumptive human population [1]. Efforts to forestall the extinction of species, which today occurs at a rate that is 1000 times greater than in the past [2], often seek to understand how animals perceive their environment and consider how to maintain natural behaviors in captivity or modify them in the wild to augment rare, or limit abundant, animals [3,4]. The application of animal behavior to pressing environmental problems gave rise to the sub-discipline of Conservation behavior barely two decades ago [5,6]. Central to this field of study is the realization that cognitive mechanisms, such as perception, attention, neophobia, categorization, and various forms of learning underlie many conservation-relevant behaviors [4,7••]. Here we review the growing integration of animal cognition and conservation biology within the field of conservation behavior. We move beyond discussions of why behavior and conservation need to be integrated [8•] to review recent examples where integration advances conservation goals and suggest that greater understanding of other animal’s cognitive abilities may ultimately build the human compassion and restraint needed to slow the current extinction crisis (Figure 1).
Figure 1.
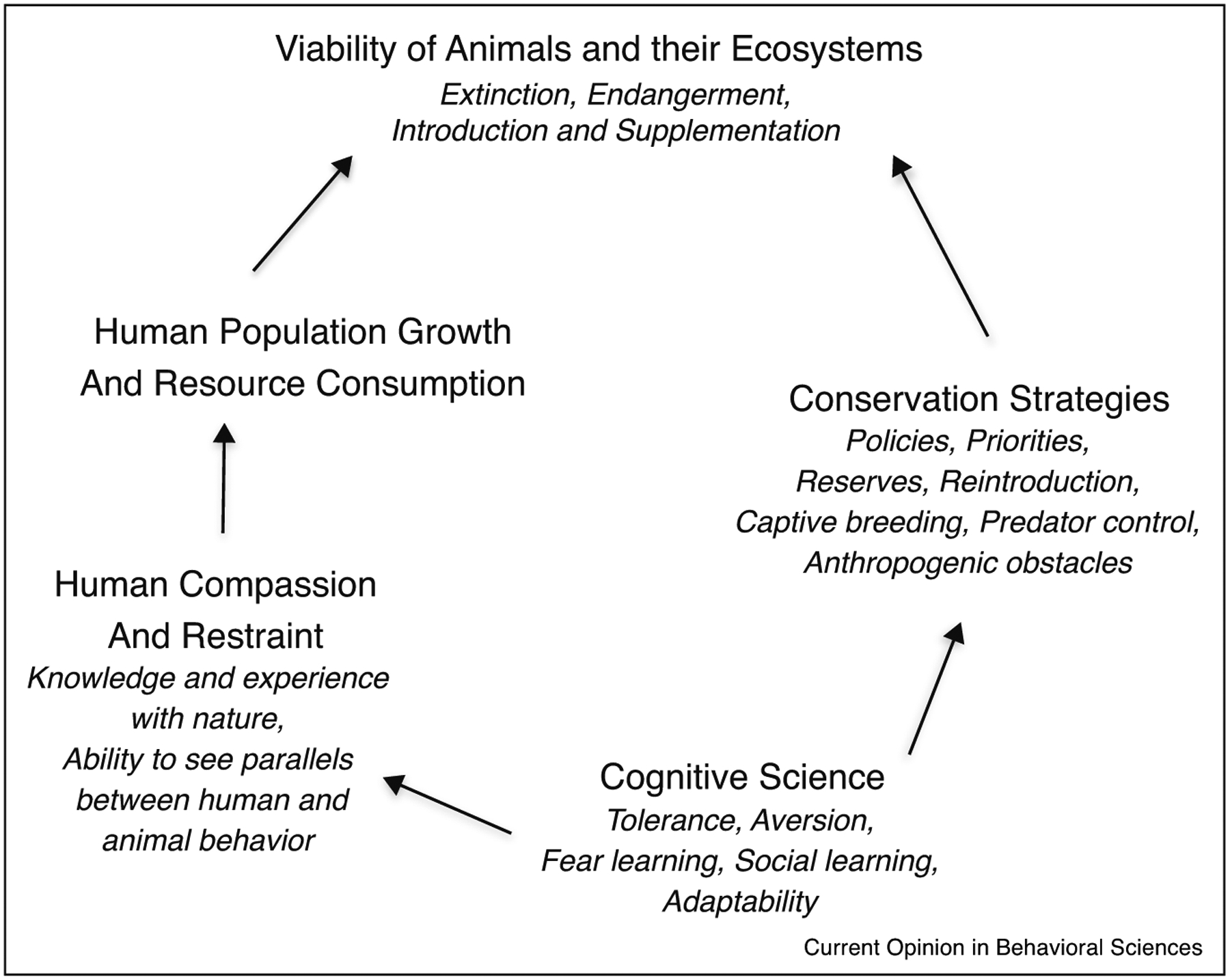
How cognition research can inform conservation action and human behavior to restore rare species and limit further degradation of Earth’s ecosystems.
Perceiving novel threats
Understanding the cognitive world of an animal can help craft effective conservation strategies. Invasive, novel predators, for example, have threatened and extinguished a wide variety of prey species because naïve prey do not recognize the cues emitted by predators that do not resemble those with whom they have co-evolved [9]. Understanding that many animals learn to recognize predators by individual or social experience [10,11] has enabled conservation biologists to teach wild [12] and captive-reared [13,14] animals to coexist with new threats. Learning is typically accomplished through classical conditioning of natural fear responses by pairing an unconditioned stimulus such as a simulated mobbing event [12], alarm vocalization [13], or a familiar predator [14] with the sight of the novel predator. Conditioning is most effective in expanding existing natural anti-predator responses to a novel predator rather than creating responses de novo [15]. Eradication of invasive predators is unlikely in many situations and exposing enough prey to paired observations of unconditioned and conditioned stimuli may be insufficient to save large and naïve wild populations. Therefore, allowing small numbers of novel predators to kill some rare species within protected wild populations, such as those on some islands or reserves, may be needed if naïve populations are to evolve appropriate predator recognition and anti-predator responses across vast landscapes [16••].
While conditioning species in the lab is routine, two major questions remain in applying this technique in real-world, conservation settings. First, because nature provides a wide range of contextual stimuli that may disrupt the ability of prey to associate the conditioned stimulus with the unconditioned stimulus, research on the perceptibility, detectability, and biological relevance of conditioned stimuli in natural landscapes is needed [17]. Second, because typically effective stimuli such as recordings of alarm or distress vocalizations may not exist for extremely rare species, especially those now existing only in captivity (e.g., Hawaiian Crow, Corvus hawaiensis), documentation of distress displays in rare species while they still exist in the wild should be prioritized and their similarity to the displays of more common species better understood.
Learning to live with humans
Animals differ in their tolerance of human actions. Conservationists facing this issue must understand the interplay between habituation and sensitization [18]. Both forms of learning are responses to a single stimulus that increases (sensitization) or reduces (sensitization) an animal’s tolerance to the stimulus through time. Hazing with stimuli that startle animals, such as loud and unexpected noise, bright lights, or non-lethal shocks and bullets can effectively sensitize animals, which is used to promote coexistence with human activities such as farming and ranching [19]. Habituation occurs most readily with repeated, frequent, and strong stimulation; these principles can guide its application in conservation settings [18]. For example, habituating animals to the presence of humans by frequent, nonthreatening encounters could reduce disturbance of rare species by ecotourists interested in viewing or photographing them. Discovering why some animals appear unable to habituate to human actions may suggest ways to adjust human encounters to increase coexistence or improve the ability of conservationists to predict which species require seclusion.
Leveraging a species’ innate response to a fearful stimulus can reduce human-wildlife conflicts (e.g., using a shark model to keep sea turtles from commercial fishing rigs, [20]); however, other solutions engage the animal’s associative learning capacity [21]. For example, when native predators exploit rare prey, managers may use the principles of conditioned taste aversion to protect the prey. Predators that consume mimics of the prey that are laced with emetic chemicals, such as carbachol or Levamisole hydrochloride, quickly reduce consumption of actual prey in laboratory and field settings. In this way, egg predation by corvids [22,23,24••] and foxes [25] has been reduced to the benefit of threatened songbirds and seabirds. Where lethal control of wildlife occurs, conditioned taste aversion may also be used to protect non-target species, such as foxes and badgers [26,27]. In some situations conditioned taste aversions were unsuccessful at protecting nesting birds because predators recognized tainted eggs [28] or because they removed and cached eggs far from the nesting area [29]. Efficacy of conditioning taste aversions in the wild is highest when emetics are tasteless and odorless, sickness is induced relatively quickly following ingestion, the proportion of treated mimics is approximately equal to untreated prey, and when stopping consumption of food (e.g., eggs or grains) rather than attack or killing of mobile prey (e.g., livestock) is the desired outcome [19,23].
As society comes to appreciate the cognitive complexity of predators, such as wolves and bears that threaten human enterprise, or corvids that eat the eggs of rare species, the ethics and efficacy of lethal control is increasingly questioned. Rare prey or human commerce and safety may not benefit when predators are removed from an ecosystem because the perturbed territorial or social dynamic of the predator results in increased reproduction or immigration, which leads to heightened predation [30,31••,32]. In addition, the loss of apex predators can undermine ecosystem function in unanticipated ways [33]. As such, managers seek creative, non-lethal ways to aversively condition smart, often long-lived and social creatures. Exclusion of predators using selectively porous fences [32,34•] or electrified flags (fladry; [31••,35]) is simple, economical and effective for small areas. Increasing human contact with livestock or employing guard animals is effective and humane in larger settings [31••,36]. Taste aversion, electric barriers, and harassment have been less effective at deterring marine mammals, such as seals that prey upon rare salmon, than terrestrial animals [37]. Greater understanding of how factors such as reinforcement schedule, a species’ sensory ability, prior experience, and environmental distractors influence the efficacy of non-lethal deterrents is needed.
Cognitive research is revealing a complex, and as yet little understood, response that a wide range of animals have to dead conspecifics [38]. To the conservation biologist this complexity offers both special challenges and opportunities. For example, the activities of invasive rodents, which threaten biodiversity on many islands [39], may be reduced in sensitive areas by exploiting their avoidance of conspecific carcasses [40] and their sensitivity to puterscine and cadaverine odors [41]. Similarly, some birds learn to avoid aspects of their environment associated with a conspecific’s death. American crows (Corvus brachyrhynchos), for example, quickly associate a person or place with the sight of a dead crow [42]. This association is coincident with activation of the crow’s hippocampus, suggesting the formation of spatial or social memories [43]. Lasting memories of associated dangers cause crows to avoid places where dead crows have been observed and to mob people seen near dead crows, which suggests that managers can manipulate the occurrence of corvids that prey upon rare species by deploying effigies or scarecrows that represent individuals previously associated with deadly scenes [42,44]. For animals whose populations are limited to captivity, maintaining an environment that promotes appropriate natural behavior is crucial to long-term success [45]. However, traditional husbandry practices dictate that corpses must be promptly removed which may limit the ability of captive animals to learn about predators or dangerous areas, and may affect social behavior in animals such as elephants, bison, and non-human primates that respond strongly to dead conspecifics [38,46]. Providing opportunities for captive animals to interact with dead members of their social group may enhance their wellbeing in captivity and increase their fitness when reintroduced into the wild.
The adaptive significance of cognition
Many of the above examples illustrate the importance of behavioral modification as a tool for conservation. However, understanding the degree to which animals naturally adjust their behavior when environments change can also inform conservation. Many of the hallmarks of a cognitive life, such as the ability to innovate and rapidly adjust behaviors to prevailing environmental conditions, provide the flexibility necessary for animals to survive in our human-dominated world. Indeed, wild apes quickly learn to avoid many anthropogenic dangers [47•], experienced turtles use their knowledge of the landscape to escape when their home ponds are rapidly drained [48], and large brained individual eiders are more likely to avoid predation than their small brained neighbors [49•]. Though there is some controversy over the advantages of a large brain in challenging environments [50], several studies report that large-brained vertebrates are better at invading and surviving in novel environments than small-brained ones [51–53]. Understanding that a species’ cognitive toolkit, including its capacity to learn (both individually and socially), innovate, forecast, and gauge the knowledge possessed by others, determines in part its ability to adapt to variation may be useful to the conservation biologist charged with predicting species’ responses to climate change, planned land use actions, and other environmental challenges.
Linking scientific understanding to conservation action
As scientists document the cognitive capacity of other species and communicate this information widely, the general public may become more conservation minded. Environmental psychologists have long noted that animals we perceive as most similar to ourselves, including with respect to cognition and consciousness, invoke affective responses of love and sympathy in humans [54,55]. Other aspects of the animal (e.g., cuteness, predatory nature, utility, and vulnerability) and the human (e.g., gender, education, religiosity, knowledge, culture, and childhood experience) modify our emotional responses, making them far from universal. Thus, humans who perceive wolves and coyotes as intelligent favor their conservation, while those that perceive them as indifferent killers do not [56]. Human environmental attitudes are malleable, as evidenced by the link between attitude and experience or knowledge. The more humans know [55,57] and the more they experience [58•,59•] concerning nature, the stronger are their positive feelings towards nature. Human attitudes may not reliably translate into conservation actions [60], especially by organizations [61], but there is evidence that children with favorable attitudes towards animals are more likely to engage in environmentally friendly behaviors, such as recycling, saving energy, modeling to their peers, and volunteering for conservation organizations [58•,59•]. Adults are also more willing to pay for conservation of species they perceive as favorable [57]. These studies suggest that cognitive scientists that make their knowledge available to the general public, especially children, can enhance conservation by affecting human attitudes. Contributing to nature-based learning situations, such as summer camps, field trips, or citizen science programs may be especially impactful. Synthesizing technical findings for the general public through writing and social media outlets, or engaging those who do so most proficiently, such as journalists, museum curators, and film producers, may also increase the curiosity and conservation leanings of people. However, to broaden one’s reach, cognitive scientists may wish to seek collaborations with non-traditional partners, such as poets, artists, musicians, architects, and game developers.
Conclusion and prospectus
Scientists studying the cognition and behavior of animals have been slow to engage with today’s pressing need to conserve the very species we find so fascinating. The usual reasons for this reticence, including perceived lack of scientific rigor in applied studies, are easily dismissed [62], and the above examples illustrate many ways in which high caliber research can inform conservation action. We have stressed the utility of research into the learning abilities of animals because these capacities directly affect behavioral flexibility, which is important to a population’s viability in a changing world [63]. As human action enhances the viability of some species and severely limits the viability of others, understanding of a species’ cognitive abilities can inform strategies to limit invasive species, restore rare species, modify dangerous environments, inform harvest quotas, design effective conservation reserves and policies, and prioritize conservation actions [64]. Perhaps the most important aspect of research that expands our understanding of the cognitive worlds of animals, however, is that it promotes compassion and humane approaches to the control of overly abundant, and restoration of rare, species. As our species learns more about whom we are endangering, we may find the will to limit our population growth and resource consumption thereby slowing the current mass extinction event.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Maxwell SL, Fuller RA, Brooks TM, Watson JEM: The ravages of guns, nets and bulldozers. Nature 2016, 536:143–145. [DOI] [PubMed] [Google Scholar]
- 2.Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO: The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 334:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Schakner Z, Blumstein DT: Learning and conservation behavior: an introduction and overview. In Conservation Behavior: Applying Behavioral Ecology to Wildlife Conservation and Management. Edited by Berger-Tal O, Saltz D. Cambridge University Press; 2016:66-92. [Google Scholar]
- 4.Greggor AL, Clayton NS, Phalan B, Thornton A: Comparative cognition for conservationists. Trends Ecol. Evol 2014, 29: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angeloni L, Schlaepfer MA, Lawler JJ, Crooks KR: A reassessment of the interface between conservation and behavior. Anim. Behav 2008, 75:731–737. [Google Scholar]
- 6.Fernández-Juricic E, Schulte BA: Conservation behavior: continued application, development and expansion. Anim. Behav 2016, 120:195–196. [Google Scholar]
- 7.••.Greggor AL, Berger-Tal O, Blumstein DT, Angeloni L, Bessa-Gomes C, Blackwell BF, Cassady St Clair C, Crooks K, de Silva S, Fernández-Juricic E, Goldenberg SZ, Mesnick SL, Owen M, Price CJ, Saltz D, Schell CJ, Suarez AV, Swaisgood RR, Winchell CS, Sutherland WJ: Research priorities from animal behavior for maximizing conservation progress. Trends Ecol. Evol 2016, 31:953–964. [DOI] [PubMed] [Google Scholar]; This article synthesizes priority areas where animal behavior research can inform conservation needs. The areas include fifty key questions, several of which concern animal cognition, derived from extensive dialog among a group of animal behavior researchers and conservation practitioners.
- 8.•.Roth T II, Krochmal AR: Cognition-centered conservation as a means of advanced integrative animal behavior. Curr. Opin. Behav. Sci 2015, 6:1–6. [Google Scholar]; In this review, the authors illustrate the potential gains to the field of animal behavior by adopting a more integrated research framework that better values the contributions of conservation driven studies.
- 9.Carthey AJR, Banks PB: Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev 2014, 89:932–949. [DOI] [PubMed] [Google Scholar]
- 10.Griffin AS: Social learning about predators: a review and prospectus. Learn. Behav 2004, 32:131–140. [DOI] [PubMed] [Google Scholar]
- 11.Cornell HN, Marzluff JM, Pecararo S: Social learning spreads knowledge about dangerous humans among American crows. Proc. R. Soc. Lond. B 2012, 279:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean IG, Hölzer C, Studholme BJS: Teaching predator-recognition to a naïve bird: implications for management. Biol. Conserv 1999, 87:123–130. [Google Scholar]
- 13.Shier DM, Owings DH: Effects of predator training on behavior and post-release survival of captive prairie dogs (Cynomys ludovicianus). Biol. Conserv 2006, 132:126–135. [Google Scholar]
- 14.McLean IG, Schmitt NT, Jarman PJ, Duncan C, Wynne CDL: Learning for life: training marsupials to recognize introduced predators. Behaviour 2000, 137:1361–1376. [Google Scholar]
- 15.Griffin AS, Blumstein DT, Evans C: Training captive-bred or translocated animals to avoid predators. Conserv. Biol 2000, 14:1317–1326. [Google Scholar]
- 16.••.Moseby K, Blumstein DT, Letnic M: Harnessing natural selection to tackle the problem of prey naïveté. Evol. Appl 2015, 9:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this perspective piece, the authors argue for a paradigm shift in reintroduction biology away from conserving prey species by excluding predators, towards improving the antipredator response of naïve individuals via in situ predator exposure. By allowing predators, not people, to drive the selection process, the remaining individuals should be equipped with traits that offer the greatest long-term fitness.
- 17.Schakner ZA, Petelle MB, Berger-Tal O, Owen MA, Blumstein DT: Developing effective tools for conservation behaviorists: Reply to Greggor et al.. Trends Ecol. Evol 2016, 29:651–652. [DOI] [PubMed] [Google Scholar]
- 18.Blumstein DT: Habituation and sensitization: new thoughts about old ideas. Anim. Behav 2016, 120:235–262. [Google Scholar]
- 19.Shivik JA: Non-lethal alternative for predation management. Sheep Goat Res. J 2004, 19:64–71. [Google Scholar]
- 20.Bostwick A, Higgins BM, Landry AM Jr, McCracken ML: Novel use of a shark model to elicit innate behavioral responses in sea turtles: application to bycatch reduction in commercial fisheries. Chelonian Conserv. Biol 2014, 13:237–246. [Google Scholar]
- 21.Shivik JA, Treves A, Callahan P: Nonlethal techniques for managing predation: primary and secondary repellents. Conserv. Biol 2003, 17:1531–1537. [Google Scholar]
- 22.Nicolaus LK, Cassel JF, Carlson RB, Gustavson CR: Taste-aversion conditioning of crows to control predation on eggs. Science 1983, 220:212–214. [DOI] [PubMed] [Google Scholar]
- 23.Dimmick CR, Nicolaus LK: Efficiency of conditioned aversion in reducing depredation by crows. J. Appl. Ecol 1990, 27:200–209. [Google Scholar]
- 24.••.Gabriel PO, Golightly RT: Aversive conditioning of Steller’s jays to improve marbled murrelet nest survival. J. Wildl. Manag 2014, 78:894–903. [Google Scholar]; This study demonstrated the potential for wild Steller’s jays to be taste conditioned to avoid mimic eggs of marbled murrelets, an endangered seabird whose reproductive success is harmed by Steller’s jay depredation. The authors cite the inability of Steller’s jays to detect the noxious agent, and the relative insignificance of eggs in the overall diet of Steller’s jays as important factors for successful taste conditioning.
- 25.Maguire GS, Stojanovic D, Weston MA: Conditioned taste aversion reduces fox depredation on model eggs on beaches. Wildl. Res 2009, 36:702–708. [Google Scholar]
- 26.Nielsen S, Travaini A, Vassallo AI, Procopio D, Zapata SC: Conditional taste aversion in the grey fox (Pseudalopex griseus), in Southern Argentine Patagonia. Appl. Anim. Behav. Sci 2015, 163:167–174. [Google Scholar]
- 27.Baker SE, Johnson PJ, Slater D, Watkins RW, MacDonald DW: Learned food aversion with and without odour cue for protecting untreated baits from wild mammal foraging. Appl. Anim. Behav. Sci 2007, 102:410–428. [Google Scholar]
- 28.Catry T, Granadeiro JP: Failure of Methiocarb to produce conditioned taste aversion in carrion crows consuming littler tern eggs. Waterbirds 2006, 29:211–214. [Google Scholar]
- 29.Skarphédinsson KH: Ravens in Iceland: Population Ecology, Egg Predation in Eider Colonies, and Experiments with Conditioned Taste-aversion. MSc. Thesis Madison: University of Wisconsin; 1993. [Google Scholar]
- 30.Minnie L, Gaylard A, Kerley GIH: Compensatory life-history responses of a mesopredator may undermine carnivore management efforts. J. Appl. Ecol 2016, 53:379–387. [Google Scholar]
- 31.••.Treves A, Krofel M, McManus J: Predator control should not be a shot in the dark. Front. Ecol. Environ 2016, 14:380–388. [Google Scholar]; The authors conducted a comprehensive study comparing both the quality and findings of lethal and non-lethal predator controls studies. They found that none of the lethal-control studies met the highest standards of experimental design, and that non-lethal methods were more effective at reducing livestock depredation.
- 32.Doherty TS, Ritchie EG: Stop jumping the gun: a call for evidence-based invasive predator management. Conserv. Lett 2016, 0:1–8. [Google Scholar]
- 33.Colman NJ, Gordon CE, Crowther MS, Letnic M: Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc. R. Soc. B 2014, 281:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.•.Norbury G, Hutcheon A, Reardon J, Daigneault A: Pest fencing or pest trapping: a bio-economic analysis of cost-effectiveness. Austr. Ecol 2014, 39:795–807. [Google Scholar]; In this study, the authors evaluated the cost-effectiveness of three means of predator control: exclusion fences, leaky fences and trapping, using 50 years of data from two predator control programs in new Zealand. They found that exclusion fences were cost-effective for <1 ha areas, leaky fences for 1–219 ha, and trapping for >219 ha.
- 35.Lance NJ, Breck SW, Sime C, Callahan P, Shivik JA: Biological, technical, and social aspects of applying electrified fladry for livestock protection from wolves (Canis lupus). Wildl. Res 2010, 37:708–714. [Google Scholar]
- 36.Gehring TM, Hawley JE, Davidson SJ, Rossler ST, Cellar AC: Are viable non-lethal management tools available for reducing wolf-human conflict? Preliminary results from field experiments. In In Proceedings of the 22nd Vertebrate Pest Conference. Edited by Timm RM, O’Brien JM. Proceedings of the 22nd Vertebrate Pest Conference University of California Press; 2006:2–6. [Google Scholar]
- 37.Schakner ZA, Blumstein DT: Behavioral biology of marine mammal deterrents: a review and prospectus. Biol. Conserv 2013, 167:380–389. [Google Scholar]
- 38.Anderson JR: Comparative thanatology. Curr. Biol 2016, 26: R553–R556. [DOI] [PubMed] [Google Scholar]
- 39.Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR: Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. U. S. A 2016, 113:11261–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prounis GS, Shields WM: Necrophobic behavior in small mammals. Behav. Processes 2012, 94:41–44. [DOI] [PubMed] [Google Scholar]
- 41.Pinel JPJ, Gorzalka BB, Ladak F: Cadaverine and putrescine initiate the burial of dead conspecifics by rats. Physiol. Behav 1981, 27:819–824. [DOI] [PubMed] [Google Scholar]
- 42.Swift KN, Marzluff JM: Wild American crows gather around their dead to learn about danger. Anim. Behav 2015, 209:187–197. [Google Scholar]
- 43.Cross DJ, Marzluff JM, Palmquist I, Minoshima S, Shimizu T, Miyaoka R: Distinct neural circuits underlie assessment of a diversity of natural dangers by American crows. Proc. R. Soc. B 2013, 280:20131046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson SA, Colwell MA: Experimental evidence that scare tactics and effigies reduce corvid occurrence. Northwestern Nat. 2014, 95:103–112. [Google Scholar]
- 45.Kiik K, Maran T, Kneidinger N, Tammaru T: Social behavior of endangered European mink (Mustela lutreola) litters in captivity. Appl. Anim. Behav. Sci 2016, 182:61–71. [Google Scholar]
- 46.Anderson JR: A primatological perspective on death. Am. J. Primatol 2011, 73:410–414. [DOI] [PubMed] [Google Scholar]
- 47.•.Hockings KJ, McLennan MR, Carvalho S, Ancrenaz M, Bobe R, Bryne RW, Dunbar RIM, Matsuzawa T, McGrew WCW, Williamson EA, Wilson ML, Wood B, Wrangham RW, Catherine MH: Apes in the anthropocene: flexibility and survival. Trends Ecol. Evol 2015, 30:215–222. [DOI] [PubMed] [Google Scholar]; In this review, the authors argue that although cognitively flexible animals such as apes may be better equipped to adapt in human dominate landscapes, there exists a tipping point which may be species dependent. Conservation and management must therefore recognize that thresholds of anthropogenic tolerance are continuous, not binary.
- 48.Krochmal A, Roth TC II, Rush S, Wachter K: Turtles outsmart rapid environmental change: the role of cognition in navigation. Commun. Integr. Biol 2015, 8:e1052922 10.1080/19420889.2015.1052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Ost M, Jaatinen K: Smart and safe? Antipredator behavior and breeding success are related to head size in a wild bird. Behav. Ecol 2015, 26:1371–1378. [Google Scholar]; This study is one of the first to evaluate the cognitive buffer hypothesis at the individual level in a wild population. The authors found that free-ranging female eiders with greater head volume were better able to evaluate risk, and that this was beneficial during seasons of heightened predation, but was costly in seasons with low predation.
- 50.Dale S, Lifjeld JT, Rowe M: Commonness and ecology, but not bigger brains, predict urban living in birds. BMC Ecol. 2015, 15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amiel JJ, Tingley R, Shine R: Smart moves: effects of relative brain size on establishment success of invasive amphibians and reptiles. PLoS One 2011, 6:e18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reif J, Bohning-Gase K, Flade M, Schwarz J, Schwager M: Population trends of birds across the iron curtain: brain matters. Biol. Conserv 2011, 144:2524–2533. [Google Scholar]
- 53.Sol D, Bacher S, Reader SM, Lefebvre L: Brain size predicts the success of mammal species introduced into novel environments. Am. Nat 2008, 172:S63–S71. [DOI] [PubMed] [Google Scholar]
- 54.Kellert SR: American attitudes toward and knowledge of animals: an update. In Advances in Animal Welfare Science. Edited by Fox MW, Mickley LD. Human Society of the United States; 1984:177–213. [Google Scholar]
- 55.Serpell JA: Factors influencing human attitudes to animals and their welfare. Anim. Welfare 2004, 13:S145–S151. [Google Scholar]
- 56.Kellert SR: Perceptions of predators, particularly the wolf and coyote. Biol. Conserv 1985, 31:167–189. [Google Scholar]
- 57.Martín-López B, Montes C, Benayas J: The non-economic motives behind willingness to pay for biodiversity conservation. Biol. Conserv 2007, 139:67–82. [Google Scholar]
- 58.•.Collado S, Staats H, Corraliza JA: Experiencing nature in children’s summer camps: affective, cognitive and behavioural consequences. J. Environ. Psychol 2013, 33:37–44. [Google Scholar]; Direct exposure to nature during childhood increases children’s emotional affinity toward nature and their willingness to carry out environmentally friendly behaviors.
- 59.•.Zhang W, Goodale E, Chen J: How contact with nature affects children’s biophilia, biophobia and conservation attitude in China. Biol. Conserv 2014, 177:109–116. [Google Scholar]; In China, contact with nature is reduced in urban settings and this correlated with a reduction in children’s willingness to conserve biodiversity.
- 60.Heberlein TA: Navigating environmental attitudes. Conserv. Biol 2012, 26:583–585. [DOI] [PubMed] [Google Scholar]
- 61.Knegtering E, Hendrickx L, van der Windt HJ, Schoot Uiterkamp AJM: Effects of species characteristics on nongovernmental organizations’ attitudes toward species conservation policy. Environ. Behav 2002, 34:378–400. [Google Scholar]
- 62.Caro T, Sherman PW: Eighteen reasons animal behaviourists avoid involvement in conservation. Anim. Behav 2013, 85:305–312. [Google Scholar]
- 63.Macdonald DW: Animal behavior and its role in carnivore conservation: examples of seven deadly threats. Anim. Behav 2016, 120:197–209. [Google Scholar]
- 64.Brooker RM, Feeney WE, White JR, Manassa RP, Johansen JL, Dixson DL: Using insights from animal behaviour and behavioural ecology to inform marine conservation initiatives. Anim. Behav 2016, 120:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]


