Abstract
Introduction
as late-life depression is associated with poor somatic health, we aimed to investigate the role of depression severity and symptom phenotypes in the progression of somatic multimorbidity.
Methods
we analysed data from 3,042 dementia-free individuals (60+) participating in the population-based Swedish National Study on Aging and Care in Kungsholmen. Using the baseline clinical assessment of 21 depressive symptoms from the Comprehensive Psychopathological Rating Scale, we: (i) diagnosed major, minor (in accordance with DSM-IV-TR) and subsyndromal depression; (ii) extracted symptom phenotypes by applying exploratory network graph analysis. Somatic multimorbidity was measured as the number of co-occurring chronic diseases over a 15-year follow-up. Linear mixed models were used to explore somatic multimorbidity trajectories in relation to baseline depression diagnoses and symptom phenotypes, while accounting for sociodemographic and behavioural factors.
Results
in multi-adjusted models, relative to individuals without depression, those with major (β per year: 0.33, 95% confidence interval [CI]: 0.06–0.61) and subsyndromal depression (β per year: 0.21, 95%CI: 0.12–0.30) experienced an accelerated rate of somatic multimorbidity accumulation, whereas those with minor depression did not. We identified affective, anxiety, cognitive, and psychomotor symptom phenotypes from the network analysis. When modelled separately, an increase in symptom score for each phenotype was associated with faster multimorbidity accumulation, although only the cognitive phenotype retained its association in a mutually adjusted model (β per year: 0.07, 95%CI: 0.03–0.10).
Conclusions
late-life major and subsyndromal depression are associated with accelerated somatic multimorbidity. Depressive symptoms characterised by a cognitive phenotype are linked to somatic health change in old age.
Keywords: depression, symptom network, comorbidity, psychosomatic, epidemiology, older people, longitudinal
Key Points
Major and subsyndromal depression are associated with accelerated disease accumulation in late life.
Cognitive symptoms of depression in old age are markers of worse multimorbidity trajectory.
Acknowledging and targeting the clinical heterogeneity in late-life depression may translate in healthier ageing.
Introduction
Depression in old age has negative consequences for quality of life and wellbeing, but it is also detrimental for somatic health [1–3]. Individuals with depression exhibit higher risk of developing several somatic diseases, and those already presenting diseases experience worsening of their clinical status and premature mortality [4, 5]. Although unhealthy behaviours, common in depressed individuals, may partly explain such findings, depression is also believed to reflect accelerated biological ageing due to immune-inflammatory, metabolic, and cardiovascular dysregulations [6–8].
The co-occurrence of multiple chronic diseases, i.e. multimorbidity, embodies one of the defining features of the ageing process [9]. The speed at which diseases accumulate over time has been proposed as a metric of ageing, expressing the underlying homeostatic dysregulation and loss of resilience of biologically old individuals [10–12]. Although emerging evidence suggests that individuals with depression in young adulthood and midlife may accumulate diseases more rapidly, this relationship is yet to be fully examined in old age [13–15]. Uncovering to what extent late-life depression is associated with the speed of disease accumulation would advance our understanding of the interplay between mental and somatic health, likely contributing to improved management and care of older adults with complex health issues.
Any assessment of the impact of depression on chronic disease accumulation must consider its specific manifestation in old age. Older adults often present depressive symptoms without fulfilling the operational criteria for major depression, as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM) [16, 17]. This is consistent with a characterisation of depression as a continuum on which less symptomatic states, such as minor and subsyndromal depression are also present. Importantly, these milder states are frequent in older people [16] and have significant implications in terms of healthcare use, morbidity, and mortality [18–20].
In addition to the higher occurrence of subclinical depression, the clinical presentation of depression in older people is believed to differ from their younger counterparts. Somatic and cognitive symptoms are more prevalent in old age, potentially reflecting the co-presence of somatic diseases [21]. Analysing late-life outcomes associated with depression must therefore account for its expression in old age, considering not only subclinical symptomatology but also specific symptom burden. To this end, a network perspective that envisions depression as a syndrome arising from multiple interacting symptoms may be helpful [22, 23]. Within the network approach, a technique to detect coherent phenotypes from interconnected symptoms has been suggested as a promising tool to unravel the clinical complexity of psychiatric and geriatric syndromes [24–27].
Within an urban population-based setting, we aimed (i) to assess whether depression, including its subclinical states, was associated with the speed of somatic disease accumulation, and (ii) to examine whether symptom phenotypes differentially contributed to somatic disease trajectories.
Methods
Study population
This study is based on longitudinal data from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), an ongoing population-based cohort comprising community-dwellers and nursing home residents aged 60+ from central Stockholm [28]. Of the 3,363 individuals participating in the baseline examination (participation rate 73%), we excluded 310 individuals with prevalent dementia, 1 with intellectual disability and 10 who refused to attend the medical examination. The eligible study population amounted to 3,042 individuals (Supplementary Figure S1), whereas the analysed sample ranged from 3,018 to 2,851 due to missing data (actual sample sizes are provided alongside model estimates). These individuals were subsequently reassessed every 3 (participants aged ≥78 years) or 6 years (participants aged <78 years) using a standardised protocol that included interviews, clinical assessment, and laboratory testing [28]. In this study, we analysed data from baseline (2001–2004) until Wave 5 (2016–2019), for a maximum follow-up time of 15 years (Supplementary Figure S1).
Written informed consent was gathered from participants or their next of kin if cognitively impaired. SNAC-K received approval from the Regional Ethical Review Board in Stockholm.
Depressive symptomatology
Depressive symptoms
Depressive symptoms were assessed using the Comprehensive Psychopathological Rating Scale (CPRS), a semi-structured tool to explore psychiatric symptoms and signs. In SNAC-K, a subset of the original scale is administered, and each symptom is examined by trained physicians on a 0–6 scale (i.e. absent to severe) based on their frequency, duration, and intensity [29]. Twenty-one depressive symptoms were used to derive symptom phenotypes through network analysis (Supplementary Text and Statistical analyses for details). For descriptive purposes, the Montgomery–Åsberg Depression Rating scale (MADRS) was also employed [30].
Depression diagnoses
Major and minor depression were diagnosed according to DSM-IV-TR using an algorithm developed in previous population-based studies [31, 32]. Briefly, selected items from CPRS were employed to appraise the presence of the DSM-IV-TR diagnostic criteria [32]. Diagnoses were derived as follows: major depression defined by the presence of at least one core symptom (low mood and/or loss of interest), and at least five symptoms; minor depression defined by the presence of at least one core symptom, and two to four symptoms in total. We further derived the depressive state of subsyndromal symptomatic depression (SSD) defined by Judd et al. as the presence of at least two DSM symptoms in the absence of any other depression diagnoses (i.e. major or minor depression) [33].
Somatic multimorbidity
The measure of somatic multimorbidity was based on extensive clinical information gathered from SNAC-K medical examination, nurse interview, laboratory tests, and medication review. Linkage to the Swedish National Patient Register enabled to retrieve additional medical diagnoses through ICD-10 codes in both inpatient and outpatient health services. A previously described operationalisation in SNAC-K was used to define chronic diseases into 60 broader categories with clinical relevance for older people [34]. From this count, we excluded psychiatric categories and dementia, leaving 54 somatic disease categories to be explored (Supplementary Table S1) [35]. The count of somatic diseases was available at baseline and at each follow-up wave, allowing the examination of longitudinal trajectories of disease accumulation over time.
Covariates
Sociodemographic factors included age, sex, marital status, education, and occupation-based socioeconomic status (manual vs. non-manual). Lifestyle factors and anthropometrics comprised alcohol consumption (no/occasional, light to moderate, and heavy consumption), smoking (ever vs. never), and body mass index (BMI). In sensitivity analyses, we furthermore explored: physical activity (light/moderate-to-vigorous intensity physical activity vs. sedentary), global cognitive function (Mini Mental State Examination [MMSE]), disability (composite score combining instrumental and basic activities of daily living [ADL]) and antidepressant use.
Statistical analyses
Depressive symptoms phenotypes were estimated with Exploratory Graph Analysis (EGA), a clustering procedure applied to network analysis (details in Supplementary Text) [36]. Briefly, network analysis is a methodology to visualise correlations between nodes (i.e. symptoms), which are depicted as connecting lines. EGA was applied to the network of 21 depressive symptoms to identify groups of highly interrelated depressive symptoms (i.e. symptom phenotypes). Akin to factor analysis, EGA enables to estimate symptom loadings, which are then used to derive group scores for each participant (Supplementary Text) [25, 36].
Linear mixed effect models were employed to explore the change in the number of somatic diseases over 15 years in relation to depression diagnoses and depressive phenotypes at baseline. We applied random effects for both individuals and follow-up time, with unstructured covariance between random parameters to account for inter-individual variability over time. We estimated the interaction between the exposure and time as fixed effect, which indicated the annual average increase in somatic disease count in relation to depressive diagnoses and phenotypes. For example, a statistically significant positive interaction between a given category of depression diagnosis and time would indicate an additional number of somatic diseases per year experienced on average by that group, relative to the average annual change in somatic diseases among individuals with no depression. Model estimates from both basic-adjusted (age, sex, and education) and fully adjusted models (age, sex, education, smoking, alcohol consumption, socioeconomic status, and BMI) are presented. When examining depressive phenotypes as exposure, we estimated models where each phenotype was entered separately, along with a mutually adjusted one.
Sensitivity analyses
To test whether cognitive decline or incipient dementia drove the association between depressive status and somatic disease accumulation, we ran additional models excluding participants with MMSE<24 score at baseline and participants developing dementia within 3 years from baseline. We repeated the analysis additionally adjusting for physical activity and the burden of disability at baseline to help account for potential reverse causation between overall health status and depression. Further, to verify potential misclassification of depression diagnosis due to antidepressant use at baseline, we repeated the analyses after excluding antidepressant users. Finally, to reduce parameter estimate bias by accounting for informative study dropout, we applied joint models to simultaneously analyse longitudinal change in somatic disease count and mortality in relation to depressive status [37].
A P < 0.05 was considered statistically significant. All analyses were carried out with STATA 17 and R (version 4.0.0).
Results
Compared with the non-depressed group, individuals with any depression diagnosis were more likely to be older, female, less educated, not married, ever smoker, with lower socioeconomic status, and higher somatic, cognitive, and disability burden (Table 1). Across depression diagnoses groups, a gradient with respect to the MADRS and antidepressant use was observed, whereas no difference in baseline age, BMI, or somatic disease burden was detected. Furthermore, for all 21 depressive symptoms, the proportion of individuals reporting at least occasional symptoms (CPRS ≥2) was lowest in those with no depression and highest in those with major depression, although the gradient minor>subsyndromal was not present for several somatic and cognitive symptoms including reduced appetite, reduced sleep, lack of initiative, and concentration difficulties (Supplementary Table S2).
Table 1.
Descriptive characteristics of analytical sample according to depression status at baseline
| No depression (noDep) |
Subsyndromal depression (SSD) |
Minor depression (MinDep) | Major depression (MajDep) | P-value | |
|---|---|---|---|---|---|
| N = 2,741 (90%) | N = 122 (4%) | N = 140 (5%) | N = 23 (1%) | ||
| Age, mean (SD) | 72.6 (10.3) | 78.7 (10.4) | 76.5 (11.1) | 76.6 (10.1) | <0.01b |
| Sex (female), n (%) | 1,707 (62) | 91 (74) | 88 (63) | 18 (78) | <0.01a |
| Education (university), n (%) | 992 (36) | 21 (17) | 37 (26) | 2 (8) | <0.01a |
| Marital status (not married), n (%) | 1,432 (52) | 95 (78) | 101 (72) | 19 (82) | <0.01a |
| Socioeconomic status (non-manual), n (%) | 2,216 (82) | 89 (75) | 109 (78) | 11 (50) | <0.01a |
| Smoking (ever), n (%) | 381 (14) | 25 (21) | 31 (25) | 8 (36) | <0.05a |
| Alcohol (no or occasional use), n (%) | 869 (32) | 65 (53) | 64 (46) | 16 (73) | <0.01a |
| (Light to moderate use), n (%) | 1,405 (51) | 34 (28) | 52 (37) | 4 (18) | |
| (Heavy use), n (%) | 447 (16) | 22 (18) | 23 (16) | 2 (9) | |
| BMI, mean (SD) | 25.8 (4.1) | 24.6 (4.2) | 24.0 (4.0) | 23.7 (4.4) | <0.01b |
| Somatic disease count, mean (SD) | 3.6 (2.5) | 5.1 (2.7) | 4.4 (2.5) | 5.6 (3.5) | <0.01b |
| Disability (ADL + IADL impairments), mean (SD) | 0.4 (1.3) | 1.47 (2.4) | 1.6 (2.7) | 2.1 (3.1) | <0.01b |
| MMSE, mean (SD) | 28.7 (1.6) | 27.8 (2.9) | 27.9 (2.2) | 26.3 (3.9) | <0.01b |
| MADRS, mean (SD) | 1.8 (2.4) | 8.5 (3.8) | 12.4 (4.5) | 24.3 (5.0) | <0.01b |
| Antidepressant use, n (%) | 182 (6) | 23 (19) | 33 (24) | 9 (40) | <0.01a |
SD: standard deviation; ADL: activities of daily living; IADL: instrumental activities of daily living; MADRS: Montgomery–Åsberg Depression Rating scale; MMSE: Mini Mental State Examination.
Missing data: depression status (n = 16), education (n = 10), marital status (n = 9), Socioeconomic status (n = 46), smoking (n = 26), alcohol use (n = 24), Antidepressant use (n = 1), BMI (n = 122), MMSE (n = 5), ADL (n = 7), MADRS (n = 101).
aChi-square test for categorical variables.
bANOVA for continuous variables with Tukey–Kramer test for multiple comparisons. Statistically significant differences:
Age (SSD > noDep; MinDep>noDep);
BMI (SSD > noDep; MinDep>noDep);
Somatic disease count (SSD > noDep; MinDep>noDep; MajDep>noDep);
MMSE (all comparisons except SSD vs. MinDep);
Disability (SSD > noDep; MinDep>noDep; MajDep>noDep); MADRS (all comparisons significant).
Network-based depressive phenotypes
The network of 21 depressive symptoms is represented in Figure 1. The clustering procedure identified four groups of highly interrelated symptoms, which were labelled as affective, cognitive, anxiety, and psychomotor phenotypes given their underlying symptom composition (see Supplementary Table S3 for network loadings). Descriptive characteristics of the identified phenotypes are presented in Supplementary Table S4, whereas additional information regarding their robustness is provided in Supplementary Figure S2 and Supplementary text.
Figure 1.
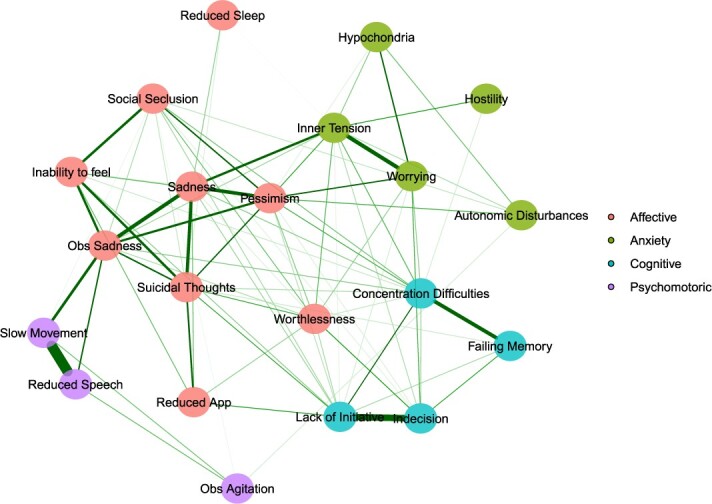
Four phenotypes derived from the partial correlation network of 21 depressive symptoms estimated from the population with full information on all symptoms (N = 2,860). Exploratory graph analysis employed the walktrap algorithm to detect groups of highly intercorrelated nodes, which were depicted in different colours.
Association of depressive diagnoses and phenotypes with multimorbidity trajectories
The presence of any depression diagnosis was associated with accelerated multimorbidity (β*time: 0.12, 95% confidence interval [CI]: 0.06–0.18) compared with non-depressed individuals (Supplementary Table S5). The trajectories of somatic disease burden according to depressive status are depicted in Figure 2. Compared with the non-depressed group, individuals with major (β*time: 0.33, 95%CI: 0.06–0.61) and subsyndromal depression (β*time: 0.21, 95%CI: 0.12–0.30) presented a faster annual change in somatic disease count, whereas those with minor depression did not (β*time: 0.03, 95%CI: −0.05–0.11). Full adjustment for covariates only minimally attenuated the estimates’ magnitude and precision, without any significant change (Supplementary Table S5).
Figure 2.
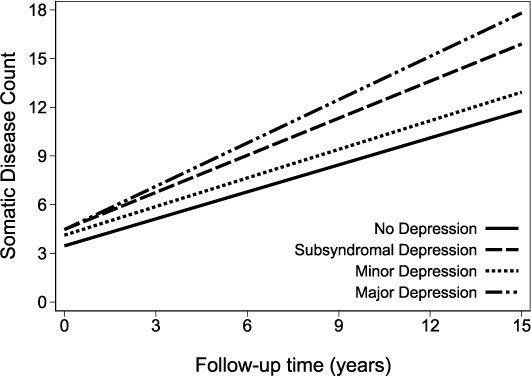
Predicted number of somatic diseases over time according to depression status estimated through linear mixed model adjusted for time, age, sex, education, marital status, socioeconomic status, smoke, alcohol and body mass index.
The association of depressive phenotypes with somatic disease count over time is presented in Table 2. All phenotypes exhibited a positive association with the speed of somatic disease accumulation in separate models, with cognitive and affective showing the largest point estimates. When entered into a mutually adjusted model, only the cognitive phenotype remained associated with the speed of somatic disease accumulation over time (β*time, per unit increase: 0.07, 95%CI: 0.03–0.10).
Table 2.
Association of depressive symptom phenotypes (continuous score per phenotype) and the speed of somatic disease accumulation over the follow-up
| Separate models | Mutually adjusted model | |
|---|---|---|
| Total population, N = 2,697 | ||
| Affective phenotype, per unit increase | 0.04 (0.02 – 0.06) | −0.01 (−0.05 – 0.03) |
| Anxiety phenotype, per unit increase | 0.03 (0.01 – 0.05) | −0.01 (−0.04 – 0.01) |
| Cognitive phenotype, per unit increase | 0.05 (0.03 – 0.07) | 0.07 (0.03 – 0.10) |
| Psychomotor phenotype, per unit increase | 0.02 (0.004 – 0.04) | 0.005 (−0.01 – 0.03) |
Coefficients with 95% confidence intervals from linear mixed models representing an interaction between symptom phenotype and follow-up time.
P < 0.05 is indicated using bold formatting.
Missing data: education (n = 8), marital status (n = 9), Socioeconomic status (n = 42), smoking (n = 25), alcohol use (n = 23), BMI (n = 107).
Models adjusted for: time, age, sex, education, marital status, SES, smoke, alcohol, BMI.
Symptom phenotypes’ interquartile range (IQR): affective (−0.562 to 0.1); anxiety (−0.712 to 0.283); cognitive (−0.705 to 0.3665); psychomotoric (−0.327 to −0.1635).
Sensitivity analyses
The estimates were not affected after (a) adjusting for baseline disability, (b) adjusting for physical activity at baseline, (c) excluding participants with MMSE<24 at baseline or incident dementia at 3 years’ follow-up, and (d) excluding antidepressant users (Supplementary Tables S6 and S7). Finally, joint modelling to account for informative dropout due to mortality did not modify the pattern of results, pointing to an underestimation of the association (Supplementary Table S5).
Discussion
In this population-based cohort study, individuals with major depression experienced a worst multimorbidity trajectory, followed by those with subsyndromal depression. Upon examining which depressive symptom phenotype presented with faster multimorbidity accumulation, we found that cognitive burden was independently associated with worse disease development. This study extends previous research on late-life depression by highlighting the aspects of severity and symptom diversity that underlie the link between depression and somatic health change.
Depression and multimorbidity accumulation
Individuals with any level of depression experienced an accelerated accumulation of somatic diseases over time, even after adjustment for several sociodemographic, behavioural, and clinical factors. This finding is in line with previous literature, although most studies have investigated this association in young and middle-aged adults or employed dissimilar methodologies [13]. Two studies from ageing cohorts have shown that the presence of depressive syndromes, defined using rating scales, was associated with increased risk of incident multimorbidity [38, 39]. In contrast, we employed clinical diagnoses of depression along the severity spectrum, which enabled to compare the differential strength of the association with somatic disease accumulation. Furthermore, we modelled the trajectory of disease accumulation rather than the incidence of multimorbidity. Given the overwhelming prevalence of multimorbidity in old-age (89% with 2+ diseases in SNAC-K) [34], the rate of change in disease accumulation has been proposed as a metric of ageing that can meaningfully describe the health trajectory, even in older adults with already high disease burden [10, 40].
As expected, we found that major depression was associated with the fastest speed of somatic disease accumulation. The long-term implications of major depression have been documented, both in terms of disease development and mortality [2, 41]. Mood disorders and biological burden are closely intertwined, with inflammation, cardiovascular burden, and disrupted stress response constituting converging mechanisms between depression and most age-related diseases [42, 43]. Unfavourable lifestyle behaviours may also explain part of the link between major depression and somatic disease accumulation, although by accounting for factors such as smoking, alcohol consumption, and physical activity our findings were nevertheless confirmed. Furthermore, care-related factors including low medication compliance, inadequate use of somatic healthcare services, and antidepressant side-effects may contribute to poor health in individuals with severe depression [3]. Despite its seemingly lower occurrence of major depression in old age compared with earlier life phases [44], our findings suggest its detrimental consequences in terms of multimorbidity accumulation. Therefore, timely identification, treatment, and monitoring of major depression may be critical to limit the health deterioration in this vulnerable population.
We found that relative to no depression, subsyndromal, but not minor depression, was associated with accelerated multimorbidity trajectories. This result was unexpected and the lack of association of multimorbidity change with minor depression remains to be explained. Indeed, evidence suggests that subclinical depression, despite its various operationalisations, is linked to poor health outcomes in terms of morbidity and healthcare utilisation in old age [16]. However, no specific study to our knowledge has compared different definitions of subclinical depression in relation to multimorbidity development. Therefore, these findings require confirmation.
Subsyndromal depression may include a combination of unspecific depressive symptoms, which may closely relate to the burden of age-related diseases. Although some isolated symptoms, such as fatigue and reduced appetite, may overlap with the clinical presentation of several somatic diseases, others may reasonably arise as a reaction to the somatic burden, such as impaired sleep, feelings of worthlessness and death thoughts. Further, although the prevalence of subsyndromal depression seems to increase with age, a proportion of older individuals with SSD has been shown to transition to minor and major depression over time [45]. This suggests that depressive states during ageing are dynamic and are likely intertwined with the burden of somatic health. Our finding, although preliminary, supports the link between subclinical depressive symptomatology and multimorbidity development, which may prove clinically relevant for healthcare providers working with older people.
Symptom phenotypes and multimorbidity accumulation
Investigating depressive symptom phenotypes is important in older adults, as they tend to exhibit heterogeneous clinical presentations, with more cognitive and somatic symptoms that often overlap with somatic health [21, 35, 46]. Few studies have investigated symptom phenotypes in relation to the development of somatic diseases. A study of individuals aged 18–65 found that mood and somatic/vegetative symptoms were associated with higher incidence of somatic diseases, while cognitive symptoms were not [47]. A study from an older population (mean age 63 years) reported that somatic but not affective symptoms were associated with incident chronic illness burden [38]. However, differences in study populations and characterisation of symptom phenotypes make the comparison of findings challenging.
By using network analysis to derive symptom phenotypes, we were able to tackle the symptom–symptom correlations across phenotypes, which reflects the perspective of depression as a syndrome arising from symptoms’ interactions [22]. All four symptom phenotypes were associated with the speed of multimorbidity accumulation with varying degrees of strength, albeit only the cognitive one retained its association in mutually adjusted models. In old age, cognitive difficulties are highly prevalent, and often co-occur with depressive and somatic burden, as multiple biological underpinnings are shared across these phenomena, including inflammation, cardiovascular burden, and neurodegeneration [42, 48, 49]. Although cognitive difficulties may arise due to a depressive syndrome, they may also express underlying neurodegenerative processes leading to dementia, which may partially explain the observed health deterioration over a long period of time [50]. In addition, reduced engagement in healthy lifestyle behaviours and poorer medical compliance may also contribute to disease development in individuals with cognitive difficulties. Regardless of its causes, cognitive difficulties may represent a marker of increased physical and mental vulnerability that can translate into accelerated multimorbidity development [51], thus warranting attention from healthcare providers.
Strengths and limitations
Strengths of this study include: the large size and population-based design with high participation rate; the thorough assessment of somatic multimorbidity combining detailed clinical information from multiple resources; repeated follow-ups over a timeframe of 15 years; the extensive psychiatric examination which allowed ascertainment of both depression diagnoses and symptomatology. Several limitations require acknowledgement. First, the study population at baseline included participants with somatic disease burden, which could affect depression and the rate of multimorbidity development. To partly mitigate this issue, additional adjustment for disability levels and physical activity was performed, with no change in the pattern of results. Second, we did not account for the development of depression over the follow-up, and future studies may explore the concurrent association of multimorbidity and depression over time, given the evolving nature of depression. Third, dementia, and its preclinical phase, may drive the association between depression and multimorbidity. We explored the association in cognitively intact participants, and after excluding those with incident dementia after 3 years, without any change in the results. Fourth, selective censoring over the long follow-up may have impacted the results. We ran joint analysis of longitudinal change and mortality, which did not lead to a meaningful change of estimates. Last, generalisability of these findings is restricted to older people living in urban setting and with relatively high socioeconomic status, which could contribute to a relatively low prevalence of all depression diagnoses in this population.
Conclusions
In this population-based study, older adults with major and subsyndromal depression experienced an accelerated accumulation of somatic diseases compared with the non-depressed. Furthermore, a higher burden of cognitive symptoms was associated with a faster multimorbidity trajectory. These findings suggest that not only depression severity but also symptom composition is relevant for somatic health in old age. Acknowledging and addressing late-life depression in its heterogenous clinical presentation may represent a critical step towards better mental and somatic health of older adults.
Supplementary Material
Acknowledgement
We are grateful to SNAC-K participants and caregivers for their participation in the study, and to all the people involved in its management.
Contributor Information
Federico Triolo, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Linnea Sjöberg, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Amaia Calderón-Larrañaga, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Stockholm Gerontology Research Center, Stockholm, Sweden.
Martino Belvederi Murri, Institute of Psychiatry, Department of Neuroscience and Rehabilitation, University of Ferrara, Ferrara, Italy.
Davide Liborio Vetrano, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Stockholm Gerontology Research Center, Stockholm, Sweden.
Laura Fratiglioni, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Stockholm Gerontology Research Center, Stockholm, Sweden.
Serhiy Dekhtyar, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Data Availability
SNAC-K data (http://www.snac-k.se/) can be accessed by the scientific community upon approval provided by the SNAC-K management and maintenance committee. Applications can be submitted to Maria Wahlberg (Maria.Wahlberg@ki.se) at the Aging Research Center, Karolinska Institutet.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
SNAC-K receives financial support from the Swedish Research Council (https://www.vr.se/; grant no.: 2011–6,243; 2017–06088), the Swedish Research Council for Health, Working Life and Welfare (https://forte.se/; grant no.: 2016–07175), and is supported by the Swedish Ministry of Health and Social Affairs and the participating County Councils and Municipalities. We further acknowledge support from the Swedish Research Council (grant no.: 2016–00981 [ACL]; 2017–00639 [LS]; 2021–06398 [ACL]) and the Swedish Research Council for Health, Working Life and Welfare (grant no.: 2017–01764 [ACL]; 2019–01076 [FT, SD]; 2020–01544 [ACL], 2021–00256 [ACL]). This project received financial support from Stiftelsen 1759 (project ‘Depression och kroniska sjukdomar hos äldre’) and Lindhes Advokatbyrå AB (grant no.: LA2022–0080). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alexopoulos GS. Depression in the elderly. Lancet 2005; 365: 1961–70. [DOI] [PubMed] [Google Scholar]
- 2. Penninx B, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gold SM, Köhler-Forsberg O, Moss-Morris R et al. Comorbid depression in medical diseases. Nat Rev Dis Primers 2020; 6: 69. 10.1038/s41572-020-0200-2. [DOI] [PubMed] [Google Scholar]
- 4. Momen NC, Plana-Ripoll O, Agerbo E, Benros ME, Borglum AD, Christensen MK. Association between mental disorders and subsequent medical conditions. N Engl J Med 2020; 382: 1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leone M, Kuja-Halkola R, Leval A et al. Association of Youth Depression with subsequent somatic diseases and premature death. JAMA Psychiat 2021; 78: 302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han LKM, Verhoeven JE, Tyrka AR et al. Accelerating research on biological aging and mental health: current challenges and future directions. Psychoneuroendocrinology 2019; 106: 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci 2011; 13: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2014; 19: 895–901. [DOI] [PubMed] [Google Scholar]
- 9. Vetrano DL, Calderon-Larranaga A, Marengoni A et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci 2018; 73: 1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and Frontiers for integrated Gerontological and clinical research. J Am Med Dir Assoc 2015; 16: 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dekhtyar S, Vetrano DL, Marengoni A et al. Association between speed of multimorbidity accumulation in old age and life experiences: a cohort study. Am J Epidemiol 2019; 188: 1627–36. [DOI] [PubMed] [Google Scholar]
- 12. Calderon-Larranaga A, Santoni G, Wang HX et al. Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med 2018; 283: 489–99. [DOI] [PubMed] [Google Scholar]
- 13. Triolo F, Harber-Aschan L, Belvederi Murri M et al. The complex interplay between depression and multimorbidity in late life: risks and pathways. Mech Ageing Dev 2020; 192: 111383. 10.1016/j.mad.2020.111383. [DOI] [PubMed] [Google Scholar]
- 14. Arias-de la Torre J, Ronaldson A, Prina M et al. Depressive symptoms during early adulthood and the development of physical multimorbidity in the UK: an observational cohort study. Lancet Healthy Longevity 2021; 2: e801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bobo WV, Grossardt BR, Virani S, St Sauver JL, Boyd CM, Rocca WA. Association of Depression and Anxiety with the accumulation of chronic conditions. JAMA Netw Open 2022; 5: e229817. 10.1001/jamanetworkopen.2022.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord 2011; 129: 126–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biella MM, Borges MK, Strauss J, Mauer S, Martinelli JE, Aprahamian I. Subthreshold depression needs a prime time in old age psychiatry? A narrative review of current evidence. Neuropsychiatr Dis Treat 2019; 15: 2763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson M, Bernfort L, Marcusson J, Wressle E, Milberg A. Direct costs of very old persons with subsyndromal depression: a 5-year prospective study. Am J Geriatr Psychiatry 2018; 26: 741–51. [DOI] [PubMed] [Google Scholar]
- 19. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry 2013; 202: 22–7. [DOI] [PubMed] [Google Scholar]
- 20. Ludvigsson M, Marcusson J, Wressle E, Milberg A. Morbidity and mortality in very old individuals with subsyndromal depression: an 8-year prospective study. Int Psychogeriatr 2019; 31: 1569–79. [DOI] [PubMed] [Google Scholar]
- 21. Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: meta-analysis. Br J Psychiatry 2012; 200: 275–81. [DOI] [PubMed] [Google Scholar]
- 22. Cramer AOJ, van Borkulo CD, Giltay EJ et al. Major depression as a complex dynamic system. PLoS One 2016; 11: e0167490. 10.1371/journal.pone.0167490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belvederi Murri M, Amore M, Respino M, Alexopoulos GS. The symptom network structure of depressive symptoms in late-life: results from a European population study. Mol Psychiatry 2020; 25: 1447–56. [DOI] [PubMed] [Google Scholar]
- 24. Christensen AP, Golino H, Silvia PJ. A psychometric network perspective on the validity and validation of personality trait questionnaires. Eur J Pers 2020; 34: 1095–108. [Google Scholar]
- 25. Christensen AP, Golino H. On the equivalency of factor and network loadings. Behav Res Methods 2021; 53: 1563–80. [DOI] [PubMed] [Google Scholar]
- 26. Belvederi Murri M, Grassi L, Caruso R et al. Depressive symptom complexes of community-dwelling older adults: a latent network model. Mol Psychiatry 2022; 27: 1075–82. [DOI] [PubMed] [Google Scholar]
- 27. Olde Rikkert MGM, Melis RJF, Cohen AA, Geeske PG. Why illness is more important than disease in old age. Age and Ageing 2022; 51: afab267. 10.1093/ageing/afab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lagergren M, Fratiglioni L, Hallberg IR et al. A longitudinal study integrating population, care and social services data. The Swedish national study on aging and care (SNAC). Aging Clin Exp Res 2004; 16: 158–68. [DOI] [PubMed] [Google Scholar]
- 29. Åsberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand 1978; 57: 5–27. [DOI] [PubMed] [Google Scholar]
- 30. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–9. [DOI] [PubMed] [Google Scholar]
- 31. Skoog I, Nilsson L, Landahl S, Steen B. Mental disorders and the use of psychotropic drugs in an 85-year-old urban population. Int Psychogeriatr 1993; 5: 33–48. [DOI] [PubMed] [Google Scholar]
- 32. Sjöberg L, Karlsson B, Atti A-R, Skoog I, Fratiglioni L, Wang H-X. Prevalence of depression: comparisons of different depression definitions in population-based samples of older adults. J Affect Disord 2017; 221: 123–31. [DOI] [PubMed] [Google Scholar]
- 33. Judd LL, Rapaport MH, Paulus MP, Brown JL. Subsyndromal symptomatic depression: a new mood disorder? J Clin Psychiatry 1994; 55: 18–28. [PubMed] [Google Scholar]
- 34. Calderon-Larranaga A, Vetrano DL, Onder G et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci 2017; 72: 1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Triolo F, Belvederi Murri M, Calderón-Larrañaga A et al. Bridging late-life depression and chronic somatic diseases: a network analysis. Transl. Psychiatry 2021; 11: 557. 10.1038/s41398-021-01686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golino H, Christensen AP. EGAnet: exploratory graph analysis – a framework for estimating the number of dimensions in multivariate data using network psychometrics, 2021; R package version 0.9.8. ed.
- 37. Crowther MJ, Abrams KR, Lambert PC. Flexible parametric joint modelling of longitudinal and survival data. Stat Med 2012; 31: 4456–71. [DOI] [PubMed] [Google Scholar]
- 38. Poole L, Steptoe A. Depressive symptoms predict incident chronic disease burden 10years later: findings from the English longitudinal study of ageing (ELSA). J Psychosom Res 2018; 113: 30–6. [DOI] [PubMed] [Google Scholar]
- 39. Ye B, Xie R, Mishra SR et al. Bidirectional association between physical multimorbidity and subclinical depression in Chinese older adults: findings from a prospective cohort study. J Affect Disord 2022; 296: 169–74. [DOI] [PubMed] [Google Scholar]
- 40. Calderon-Larranaga A, Fratiglioni L. Multimorbidity research at the crossroads: developing the scientific evidence for clinical practice and health policy. J Intern Med 2019; 285: 251–4. [DOI] [PubMed] [Google Scholar]
- 41. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 2014; 171: 453–62. [DOI] [PubMed] [Google Scholar]
- 42. Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry 2019; 9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018; 15: 505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haigh EAP, Bogucki OE, Sigmon ST, Blazer DG. Depression among older adults: a 20-year update on five common myths and misconceptions. Am J Geriatr Psychiatry 2018; 26: 107–22. [DOI] [PubMed] [Google Scholar]
- 45. Sigström R, Waern M, Gudmundsson P, Skoog I, Östling S. Depressive spectrum states in a population-based cohort of 70-year olds followed over 9 years. Int J Geriatr Psychiatry 2018; 33: 1028–37. [DOI] [PubMed] [Google Scholar]
- 46. Hegeman JM, de Waal MW, Comijs HC, Kok RM, van der Mast RC. Depression in later life: a more somatic presentation? J Affect Disord 2015; 170: 196–202. [DOI] [PubMed] [Google Scholar]
- 47. Gaspersz R, Lamers F, Beekman ATF, van Hemert AM, Schoevers RA, Penninx B. The impact of depressive disorder symptoms and subtypes on 6-year incidence of somatic diseases. Psychother Psychosom 2018; 87: 308–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol 2011; 7: 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 2012; 139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh-Manoux A, Dugravot A, Fournier A et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiat 2017; 74: 712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fabbri E, An Y, Zoli M et al. Association between accelerated multimorbidity and age-related cognitive decline in older Baltimore longitudinal study of aging participants without dementia. J Am Geriatr Soc 2016; 64: 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SNAC-K data (http://www.snac-k.se/) can be accessed by the scientific community upon approval provided by the SNAC-K management and maintenance committee. Applications can be submitted to Maria Wahlberg (Maria.Wahlberg@ki.se) at the Aging Research Center, Karolinska Institutet.


