Abstract
Studies have shown that 40 individuals out of 100,000 are diagnosed with rheumatoid arthritis (RA) yearly, with a total of 1.3 million in the United States. Furthermore, the impact of RA in some cases can extend to cardiovascular diseases (CVD), as the studies showed that 84% of RA patients are at risk of developing hypertension. This study aims to design and develop different dosage forms (capsule-in-capsule and three-dimensional (3D) printed tablet) of nifedipine/indomethacin fixed-dose combination (FDC). The hot-melt extrusion (HME) was utilized alone and with fused deposition modeling (FDM) techniques The developed dosage forms were intended to provide delayed-extended and immediate release profiles for indomethacin and nifedipine, respectively. FDC dosage forms were successfully developed and characterized. Nifedipine formulations showed significant improvement in release profiles, having 94% of the drug release at 30 minutes compared with pure nifedipine, which had a percent release of 2%. Furthermore, the release of indomethacin was successfully delayed at a pH of 1.2 and extended at a pH of 6.8. Differential scanning calorimetry results showed endothermic crystalline peaks at 165 °C and 176 °C for indomethacin and nifedipine, respectively. Moreover, the thermal analysis of all formulations showed the absence of the endothermic peaks indicating complete solubilization of indomethacin and nifedipine in the polymeric carriers. All formulations had post-processing drug content in the range of 95% to 98%. Moreover, results from the stability study showed that all formulations were able to remain chemically and physically stable with no signs of recrystallization or degradation. The designed FDC dosage forms could improve the quality of life by enhancing patient compliance and preventing the need for polypharmacy.
Keywords: Hot-melt extrusion, 3D printing, Fused deposition modeling (FDM), Fixed dose combination, Indomethacin, Nifedipine
Graphical Abstract
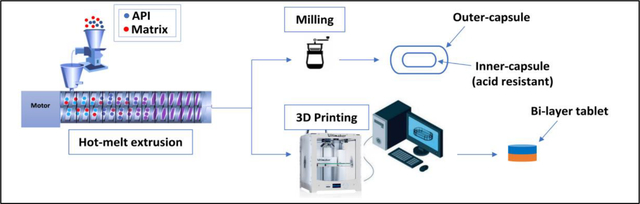
1. Introduction
Geriatric patients have the highest rate of drug and supplement consumption in the United States. This group of patients is susceptible to alterations in pharmacokinetics and pharmacodynamics due to an increase in comorbidity, age-related changes in physiology, and organ dysfunction.[1] In addition to challenges in geriatrics, such as adherence related to frailty and cognitive disorders, the administration of multiple medicines (polypharmacy) has been associated with increases in the health care cost because of adverse drug reactions. Moreover, polypharmacy has negative impacts on patients’ compliance and adherence to medication regimens.[2] Every year 40 individuals out of 100,000 are diagnosed with rheumatoid arthritis (RA) with a total of 1.3 million in the United States. RA is a chronic inflammatory disease that affects and damages the joints leading to a physical disability. Additionally, the impact of RA in some cases can extend to cardiovascular diseases, since the studies showed that 84% of rheumatoid arthritis patients are at risk of developing hypertension.[3] This is caused by the increase in arterial stiffness and the reduction of blood vessel elasticity caused by RA medications. Furthermore, RA patients suffer from pain and morning stiffness that lasts for at least one hour, which can be a burden on the patient’s health.[3]
There are currently four classes of drugs used for the treatment of RA: nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and biologic response modifiers. NSAIDs and DMARDs are the common standard medications for RA treatment.[4] However, NSAID increases water and sodium retention, and this side effect can lead to a decrease in the patient’s repones to some antihypertensive drugs, including diuretics and angiotensin-converting enzyme (ACE) inhibitors.[4] A clinical study was conducted to assess the drug-drug interactions of indomethacin in patients treated with the ACE inhibitor enalapril and calcium channel blockers amlodipine. Patients who received indomethacin and enalapril treatments have shown significant increases in supine and systolic blood pressure (BP). On the other hand, there were no significant increases in blood pressure in the patients who received indomethacin and amlodipine treatments, compared to placebo.[5] Crossover studies investigated the interaction between nifedipine and indomethacin.[6,7] It has been found that there was no drug-drug interaction between the combination. Moreover, nifedipine’s hypotensive effect was not altered by the inhibition of prostaglandin synthesis induced by indomethacin. This makes nifedipine to be the appropriate drug candidate in patients requiring concomitant therapy of calcium channel blockers with NSAIDs.[6,7] This can be explained by the fact that indomethacin is a non-selective cyclooxygenase inhibitor, and it can cause an increase in sodium retention by interfering with prostaglandin synthesis which can affect the efficiency of the ACE inhibitors.[8,9] Furthermore, calcium channel blockers such as amlodipine, and nifedipine are acting on the inhibition of voltage-dependent entry of calcium ions. Therefore, indomethacin would be preferable with calcium channel blockers for patients who have both arthritis and hypertension.[6]
Fixed-dose combination (FDC) is a well-known approach for combining at least two different active pharmaceutical ingredients in a single unit to provide treatment for different pathologies. From a poly-medicated patient’s point of view, FDC improves patient adherence and decreases dosing frequency. Besides, it`s cost-effective. From an economical point of view, manufacturing drug combinations as FDC has benefits in reducing the cost of manufacturing, distribution, and packaging.[10] Moreover, the FDC approach was successfully applied in the treatment of several conditions including hypertension, immunodeficiency viral infections, diabetes, and tuberculosis. Depo-Testadiol® was the first FDC drug approved in 1954, it contains a combination of estradiol and testosterone. According to the Physicians’ Desk Reference and the United States Pharmacopeia compendium, there were 150 FDC products available in the market in 2005.[11] Recently, there are more than 600 clinical studies to evaluate FDC products for different diseases, and this can emphasize the importance and the need for FDC products by pharmaceutical industries to improve patient health outcomes.[12] However, there are a few drawbacks to this approach. These include the need for considering the dose titrations of the drugs based on the patient’s need, and if an adverse drug reaction occurs from the FDC, it may be difficult to identify which active pharmaceutical ingredient (API) is responsible for the reaction.[13,14]
Several pharmaceutical techniques such as hot-melt extrusion (HME), spray drying, and three-dimensional (3D) printing are commonly used for combining drugs in different systems to achieve different release profiles.[15] These systems are monolithic, multi-layer, and multiarticulate systems.[15–17] The HME process involves three major steps, melting, mixing, and shaping. First, the mixture (usually a carrier and an active pharmaceutical ingredient) is fed through a feeder. Then, the mixture softens due to the high extrusion temperature and shear force, and it moves to the mixing, where the drug is dispersed within the polymer. [18] The shaping zone is important in reducing the pulsation flow and giving and maintaining extrudate uniformity. Extruders come in various sizes that include 11mm, 16 mm, 24 mm, and 36 mm, among others, in which the number refers to the diameter of the twin screw used in the instrument. In terms of applications, the HME technique has been used to develop different dosage forms for different drug delivery systems. These include sustained released tablets, pellets, granules, transdermal dosage forms, and implants.[19]
Additive manufacturing (AM) is an alternative approach utilized to develop personalized drugs or complex dosage forms.[20] The interest in AM for pharmaceutical applications has grown since it has benefits for both the patient and the economy.[20] Different AM approaches have been utilized for developing pharmaceutical products. Examples of these techniques include inkjet, laser-based, and nozzle-based printing. Nozzle-based printing is the most used since it is a straightforward process. Advantages include lower equipment costs compared to other printing techniques, the ease of producing dosage forms, and the availability of numerous choices for compatible excipients. The nozzle-based printer is subdivided into pressure-assisted microsyringe (PAM) and fused deposition modeling (FDM).[21–23] 3D printing technology has shown the potential to revolutionize the pharmaceutical manufacturing process by providing a path for personalized medicine and complex dosage forms such as fixed-dose combinations, implants, and microneedles for transdermal routes.[24] Three steps are involved in the 3D printing process: designing the product (using specific software), slicing the design into several layers, and finally printing the drug product using the desired processing parameters such as printing speed, temperature, and infill percentage. [25] Interestingly, the oral disintegrating tablet SPRITAM® (levetiracetam) was the first 3D-printed drug approved by U.S. Food and Drug Administration (FDA) using the ZipDose® technology. Recently, using HME and FDM has shown the potential to improve the bioavailability of the drugs and simplify the downstream process for economic purposes.[26] By these two techniques, the downstream process is reduced to only three steps; a) preparation of filaments utilizing HME, b) designing the dosage form using software and translating the design to a printable format, and c) printing the desired dosage form. [25]
The aim of our study is to design and develop a fixed-dose combination (FDC) of nifedipine/indomethacin. Two different approaches were used to prepare FDCs, a capsule-in-capsule dosage form and a 3D-printed tablet utilizing HME alone and with fused deposition modeling (3D printing). The FDC dosage forms were designed to achieve an extended-release profile of indomethacin for up to 10 hours and an immediate-release profile of nifedipine. The prepared FDCs can be administered once a day at bedtime, thereby providing pain relief for RA patients that often suffer from morning stiffness.
2. Materials and Methods
2.1. Materials
Nifedipine (NIF) and indomethacin (IND) were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and Sigma-Aldrich (Milwaukee, WI, USA), respectively. Polyethylene oxide N80 grade was gifted from Coloron Inc (West Point, PA, USA). Hydroxypropyl cellulose (HPC LF) and Polyplasedone were donated from Ashland (Ashland, Wilmington, DE), and Kollidon grades (12 PF, SR, VA 64, and CL) were gifted from BASF (Ludwigshafen, Germany). All other solvents and reagents used in this study were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA).
2.2. Methods
2.2.1. Hot-melt extrusion
2.2.1.1. Capsule-in-capsule dosage form
Polymeric carriers were different for each drug. Kollidon® SR and Kollidon® VA 64 was selected for indomethacin, and Kollidon® 12 PF was selected for nifedipine. The hot-melt extrusion processes were conducted using 11 mm twin-screw co-rotating extruders (Thermo Fisher Scientific, Waltham, MA, USA). A standard screw configuration consisting of three mixing zones and four conveying zones was utilized. Furthermore, an extrusion temperature of 180 °C with a screw speed of 100 rpm (for NIF formulations) and 75 rpm (for IND formulations) was applied.
2.2.1.2. 3D printed tablets dosage form
Two physical mixtures for NIF and IND formulations were prepared. The first consisted of 10% of NIF, 10% (w/w) polyplasdone, and 80% of polyethylene oxide N80, while the second consisted of 30% of IND, 45.5% HPMC E4, 19.5 % HPC LF, and 5% Kollidon® CL. NIF and IND filaments were extruded using 11 mm twin-screw co-rotating extruders (Thermo Fisher Scientific, Waltham, MA, USA) with a standard screw configuration consisting of three mixing zones and four conveying zones selected for all formulations. Extrusion temperatures were 140 °C for NIF and 180 °C for IND, and the screw speed was kept at 100 rpm for both formulations.
2.2.2. 3D Printing process
The 3D designs of the tablets were created using Tinkercad software, then exported to std files. All tablets had a diameter of 12 mm and IND had a thickness dimension of 2 mm, while NIF’s diameter was 2.1 mm. The tablets were fabricated using the Ultimaker 3 dual extrusion with a 0.4 mm nozzle (Ultimaker, Geldermalsen, Netherlands). Different printing settings were optimized to achieve the best fabrication for NIF and IND tablets, as seen in Table 5.
Table 5.
Values of the coefficient of determination (R2) of zero-order, first-order, Higuchi, and Korsmeyer-Peppas.
| Formulation | Zero-order | First order | Higuchi | Korsmeyer-Peppas | Hixson-Crowell |
|---|---|---|---|---|---|
|
| |||||
| NIF-2 | 0.8897 | 0.6089 | 0.8255 | 0.8786 | 0.9317 |
| NIF-3D-2 | 0.9038 | 0.7374 | 0.8339 | 0.8904 | 0.9374 |
| IND-2 | 0.9081 | 0.9289 | 0.8519 | 0.9774 | 0.9546 |
| IND-3D-2 | 0.9633 | 0.8902 | 0.8610 | 0.9767 | 0.9230 |
2.2.3. Differential scanning calorimetry (DSC)
The physical states of the components before and after the extrusion processes were evaluated using differential scanning calorimetry (DSC) (TA instruments DSC 25 Discovery series) with a heating rate of 10 °C /min and a temperature ranging from 20 °C to 200 °C. Furthermore, the two compartments in the 3D-printed tablet were separated and analyzed. All formulations were blended, the weights of the samples were between 3 to 6 mg, and the samples were placed in aluminum pans. The Trios software was used to analyze the thermograms of the samples.
2.2.4. Scanning electron microscopy (SEM)
The printing efficiency of the NIF and IND tablets was evaluated using the JSM-7200FLV Field-Emission Scanning Electron Microscope (JOEL, Peabody, MA, USA) with an accelerating voltage of 5 kV. For acquiring cross-sectional images of the 3D-printed tablets, the samples were mounted on a carbon pad, placed on an aluminum stub, and sputter-coated with platinum under an argon atmosphere using a fully automated Denton Desk V TSC Sputter Coater (Denton Vacuum, Moorestown, NJ, USA) before imaging.
2.2.5. Filament characterization
The TA-XT2i analyzer (Texture Technologies, Hamilton, MA, USA) with a TA-95N 3-point bend probe set (Texture Technologies) were used to characterize the mechanical properties of the filaments. Seven samples from each formulation were placed on the 3-point test holder with a 25 mm gap, and the blade’s speed was set at 10 mm/s.[27] The Exponent software 6.15.0 (Stable Micro Systems, Godalming, UK) was used to analyze the data.[27]
2.2.6. Drug content
Three samples from each formulation were dissolved in 20 mL of acetonitrile, followed by vortex stirring (Scientific industries vortex genie-120V) for one minute. Standard calibration curves were generated with r2 being between 0.99 and 1. The HPLC consisting of a Waters 2695 separation Module and a Waters 2489 UV/Visible detector (Waters Technologies Corporation, Milford, USA) was used. Two different HPLC methods were applied to separately analyze nifedipine and indomethacin. The mobile phases for both APIs consisted of acetonitrile (ACN) and water (H2O), as seen in Table 1. The retention times were 6.7 min and 5.3 min for nifedipine and indomethacin, respectively.[28,29]
Table 1.
HPLC parameters used in the in-vitro drug release studies.
| APIs | Mobile phase ACN:H2O % (v/v) | Column | Flow rate (mL/min) | Wavelength (nm) | Injection volume (μL) |
|---|---|---|---|---|---|
| IND | 70:30 | C 18 | 1.2 | 225 | 10 |
| NIF | 60:40 | C 18 | 1 | 235 | 10 |
2.2.7. In-vitro drug release studies
Drug release studies of the APIs (NIF and IND) and the formulations were performed using the USP apparatus II. The dissolution medium was 800 mL of 0.1 N HCl (pH 1.2) with 0.5% of Sodium lauryl sulfate (SLS).[29] After two hours, 100 mL of 0.2 M sodium phosphate dibasic (pH 9) was added to the medium to provide a final pH of 6.8 for the remaining 10 hours. The pH of the medium after adding 0.2 M sodium phosphate buffer was checked. The dissolution medium was kept at 37 °C and a speed of 50 rpm. A sample volume of 2 mL was retrieved at time points 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, and 12 hours. A 2 mL of fresh medium was added each time to maintain a total volume of 900 mL. The samples were centrifuged for 10 min at 13,000 rpm, then the supernatants were analyzed using the HPLC. All dissolution experiments were conducted in triplicates. Statistical analysis was performed using t test (SPSS Inc, Chicago, IL, USA). The confidence interval set for the statistical analysis was 95%, and the p-value for the significant difference should be less than 0.05.
2.2.8. Stability studies
All formulations were sealed in vials with screw caps and stored in the stability chamber under conditions of 25 °C / 60% RH. DSC and drug content tests were performed at 0, 1, 2, and 3 months. The purpose of the studies was to evaluate the physical and chemical integrity of the formulations under specific storage conditions.
3. Results and discussion
3.1. Hot-melt extrusion
3.1.1. Capsule-in-capsule dosage form
Different physical mixtures of NIF and Kollidon® 12PF with varying ratios were prepared (Table 2). Formulations NIF-1 and NIF-2 with drug loading of 30 and 25 % (w/w), respectively, could not be extruded using an extrusion temperature of 180 °C and a 100 rpm screw speed. The mixtures were entrapped inside the HME instrument due to high drug loading and exceeding the polymer’s capacity. Formulation NIF-3 with the ratio of 20 % NIF and 80 % Kollidon® 12PF was successfully extruded indicating that 20% is the acceptable drug loading for the formulation to be extruded using the applied parameters, as seen in table 2. Moreover, two physical mixtures of IND formulations IND-1 (IND and Kollidon® SR) and IND-2 (IND and Kollidon® SR, Kollidon® VA 64) were prepared with a drug loading of 30 % and 25 %, respectively. Formulation IND-1 had the highest torque (65%) during the extrusion process. On the other hand, formulation IND-2 was successfully extruded with a lower torque around 20% (Table 2). Furthermore, the final weight of the formulations combined was 350 mg containing 75 mg of IND and 10 mg of NIF (Figure 1). The drugs’ doses were chosen based on the marketed products of IND and NIF.
Table 2.
The composition of capsule-in-capsule formulations.
| Formulation | API %(w/w) | Kollidon® 12PF % (w/w) | Kollidon® SR % (w/w) | Kollidon® VA 64 % (w/w) | HME processing parameters | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Screw speed (rpm) | |||||
| NIF-1 | 30 | 70 | - | - | 180 | 100 |
| NIF-2 | 25 | 75 | - | - | 100 | |
| NIF-3 | 20 | 80 | - | - | 100 | |
| IND-1 | 30 | - | 70 | - | 75 | |
| IND-2 | 25 | - | 45 | 30 | 75 | |
Figure 1.
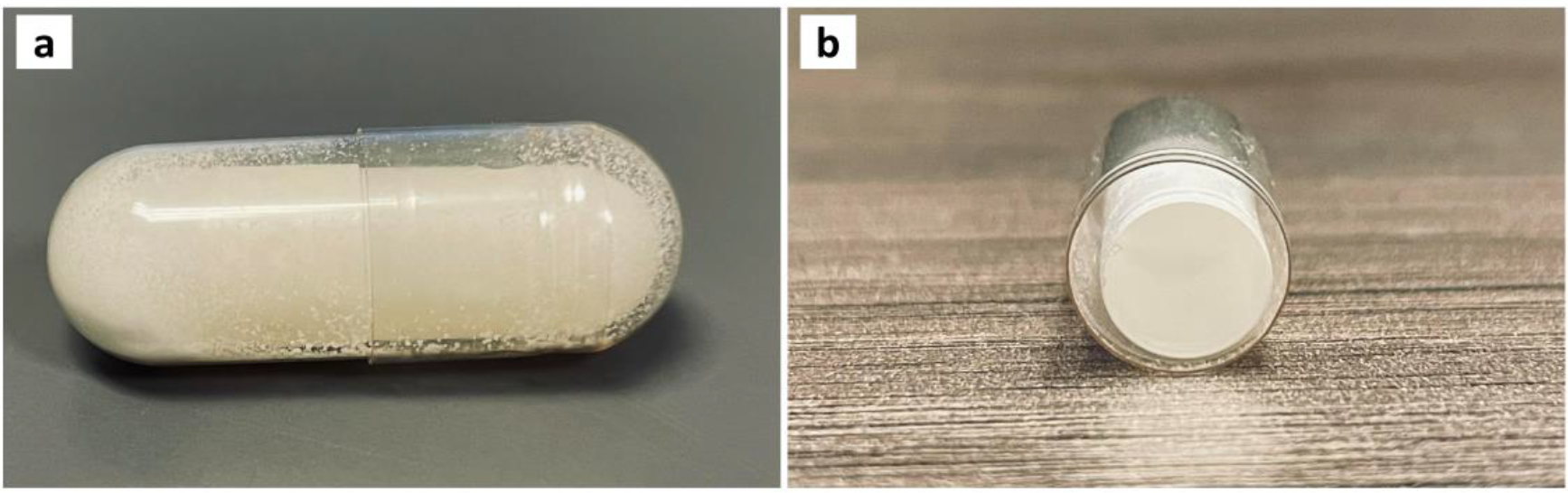
Images of the overall (a) and cross-section (b) for the capsule-in-capsule dosage form.
3.1.2. 3D-Printed tablet dosage form
Different physical mixtures were prepared for NIF and IND to obtain formulations that can be both extrudable and printable, as seen in Table 3. Two physical mixtures of IND with different formulation compositions were prepared. Formulations IND-3D-1 and IND-3D-2 were successfully extruded at 130 °C and 180 °C, respectively, and the screw speed was kept at 100 rpm. High extrusion temperatures were applied for formulation IND-3D-2 since HPMC E4 has a high glass transition temperature (165 °C). Moreover, both formulations have an extrusion torque lower than 20 % due to the low shear force that was been generated during the extrusion process. For NIF formulations, two physical mixtures with different compositions were prepared (NIF-3D-1, and NIF-3D-2). An extrusion temperature of 130 °C was used for NIF-3D-1, while a temperature of 145 °C was used for NIF-3D-2. A speed screw of 100 rpm was used for the extrusion of both formulations. Furthermore, two different extrusion dies were used (2.4 mm, and 1.94 mm) for IND and NIF, respectively.
Table 3.
The composition of 3D-printed tablet formulations.
| Formulation | API % (w/w) | Composition % (w/w) | HME processing parameters | |
|---|---|---|---|---|
| Temperature (°C) | Screw speed (rpm) | |||
| IND-3D-1 | 25 | TPGS (10) Kollidon® SR (65) |
130 | 100 |
| IND-3D-2 | 30 | HPMC E4 (45.5) HPC LF (19.5) Kollidon® CL (5) |
180 | 100 |
| NIF-3D-1 | 20 | PEG (20) Kollidon® 12PF (30) Kollidon® VA 64 (30) |
130 | 100 |
| NIF-3D-2 | 10 | Polyplasedone™ (10) PEO N80 (80) |
145 | 100 |
3.2. Filament characterization
The study was conducted to evaluate the mechanical properties of the filaments. Brittleness and softness are important factors that can affect the filament’s printability. The ability of the filament to withstand deformation after applying force was tested, and two parameters were used for that (breaking force, and breaking distance).[30] Formulation IND-3D-2 showed a high breaking force and a low breaking distance, compared to formulation NIF-3D-2 (Figure 2).[27] This finding indicates that IND-3D-2 had low ductility. Formulation NIF-3D-2 showed high resistance to the applied force and deformed plastically before it fractured due to the high ductility. Both filaments had good mechanical properties for 3D printing.[31]
Figure 2.
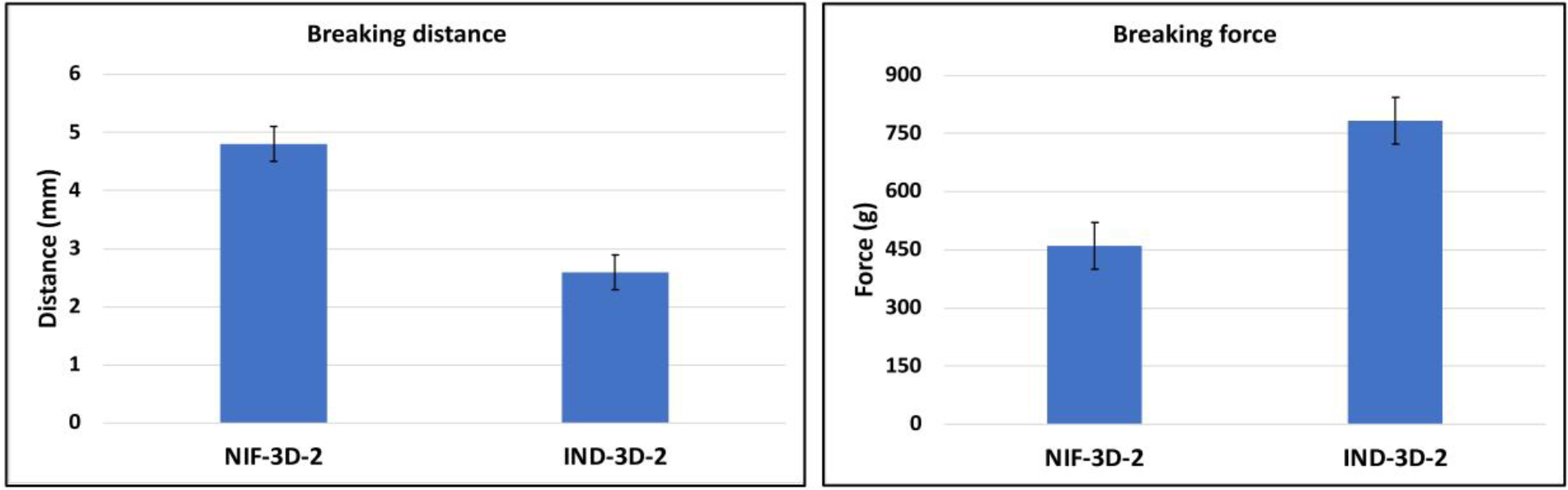
Mechanical properties of 3D-filaments; a) breaking distance, b) breaking force.
3.3. 3D printing process
Extrusion filaments with adequate mechanical properties (stiffness and brittleness) can be fabricated using the 3D printer. These two parameters can help determine filament printability. Different printing settings were used for formulations IND-3D-1 and NIF-3D-1; however, neither of them could be successfully printed, and further work was not carried out on these formulations. On the other hand, formulations IND-3D-2 and NIF-3D-2 were successfully printed using the settings seen in Table 4, since they have good mechanical and rheological properties. Filament swelling was measured by comparing the filament diameter to the HME die diameter. NIF filaments had higher swelling (0.8 mm) compared to IND filaments (0.4 mm). A high printing temperature (250 °C) was applied for the IND-3D-2 formulation since HPMC E4 has a high glass transition temperature (165 °C).[30] In previous work, the extrusion of HPMC E4 produced non-printable filaments since it has a high molecular weight (80,000 Da) and melt viscosity. [32] Moreover, incorporating HPC improved the flexibility and stickiness of IND formulation filaments. [30,32] NIF and IND tablets were designed and printed as a stack of two tablets, the first compartment had the IND-3D-2 formulation and the second one had the NIF-3D-2 formulation. The total thickness of both compartments was 4.1 mm with an average weight of 350 mg containing 75 mg of IND and 10 mg NIF, as seen in Figure 3.
Table 4.
3D printing settings of NIF and IND formulations.
| Printer settings | NIF-tablet | IND-tablet |
|---|---|---|
| Printing temperature (°C) | 200 | 250 |
| Layer height (mm) | 0.2 | 0.2 |
| Wall thickness (mm) | 0.8 | 0.8 |
| Infill density (%) | 40 | 80 |
| Print speed (mm/s) | 50 | 50 |
| Infill speed (mm/s) | 20 | 50 |
| Travel speed (mm/s) | 50 | 50 |
| Build plate temperature(°C) | — | 60 |
Figure 3.
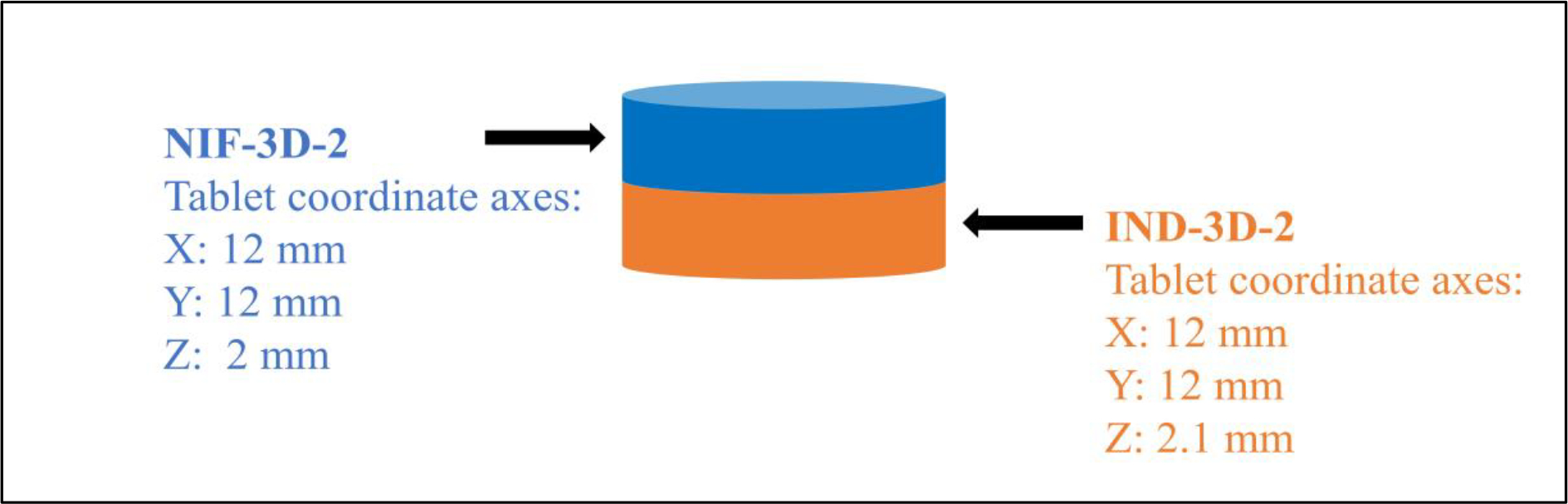
The dimensions of the 3D-printed tablet.
3.4. Differential scanning calorimetry (DSC)
The thermal analysis was conducted to evaluate the physical state before and after the extrusion process. The DSC results showed thermal peaks at 165 °C and 176 °C corresponding to the melting points of IND and NIF, respectively which indicate the presence of the APIs in the crystal states. Moreover, thermal analysis of formulations NIF-2, IND-2, NIF-3D-2, and IND-3D-2 revealed the absence of the APIs melting peaks, which indicates that the APIs were in amorphous states. The thermogram of NIF-3D-2 showed a melting point peak at 70 °C for PEO N80 (Figure 4). This is because PEO is classified as a semicrystalline polymer with two-phase spherulitic crystals scattered in an amorphous state.[33] The glass transition temperatures of the polymer blends of formulations IND-3D-2 and IND-2 were difficult to determine due to the similarities in the chemical structures of the polymer blends used.[34]
Figure 4.
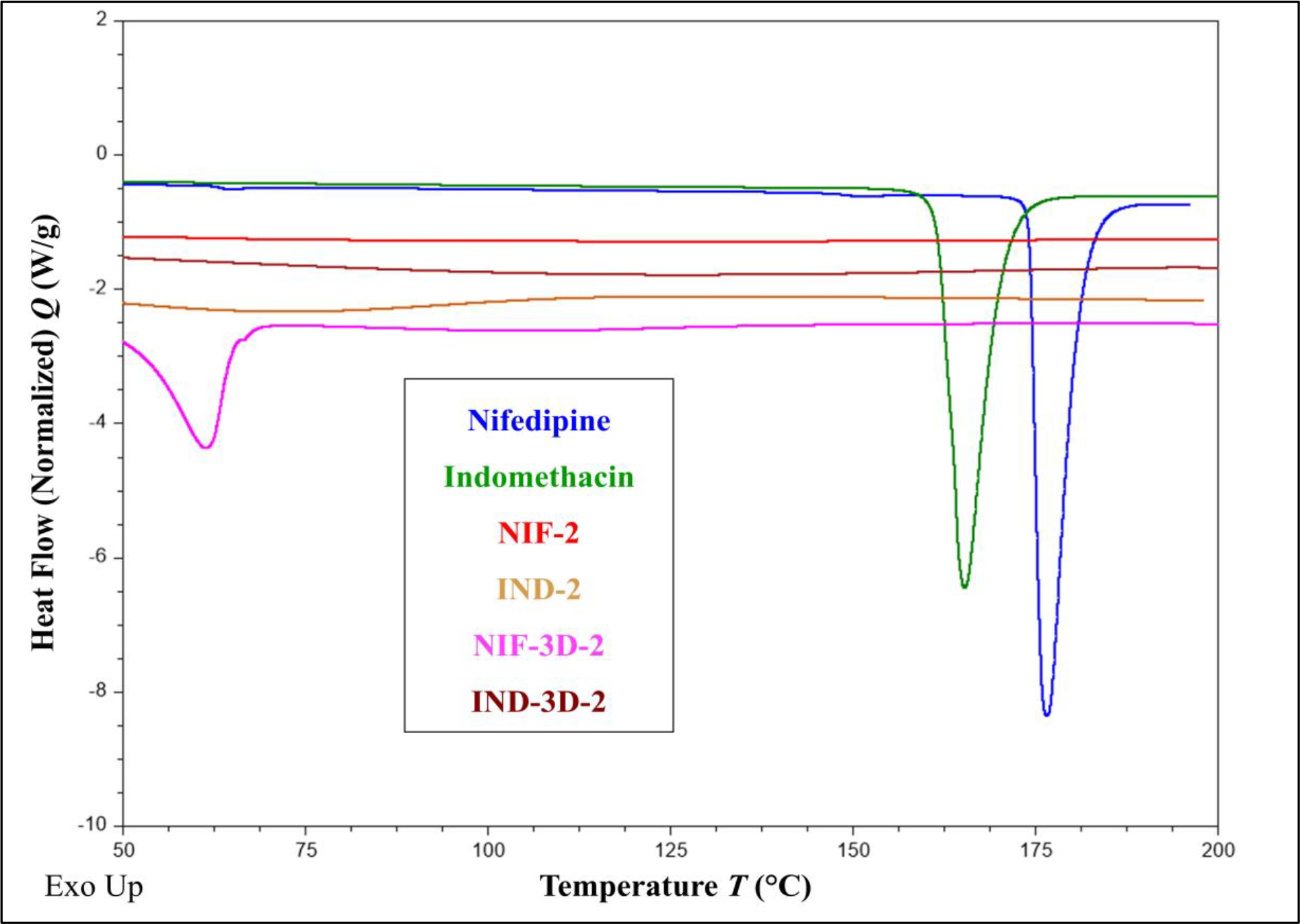
Thermograms of IND, NIF, and the formulations (NIF-2, IND-2, NIF-3D-2, and IND-3D-2).
3.5. Scanning electron microscopy (SEM)
In this study, the 3D-printed tables were split to have a deeper understanding of the tablet layer structure. The SEM images showed that NIF-3D-2 and IND-3D-2 tablets have tight layer structures (Figure 5). This can be attributed to several factors including filament homogeneity and diameter, feeding rate, and melt viscosity during the HME process. Moreover, SEM images of the IND-3D-2 tablet showed that the layers are rough which be due to high drug loading (30 %) or the high printing temperature.[35]
Figure 5.
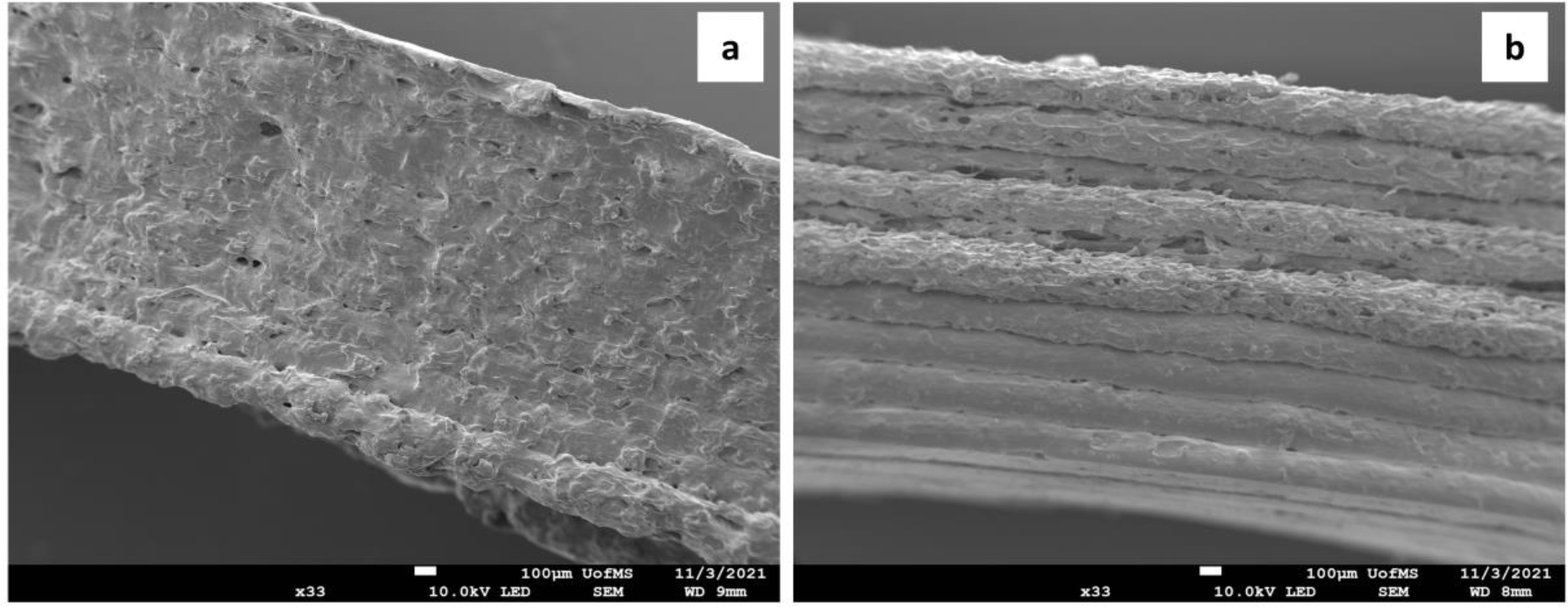
SEM images for formulations NIF-3D-2 (a), and IND-3D-2 (b).
3.6. Drug content
Formulations NIF-2 and IND-2 had an average drug of contents of 95% and 97%, respectively. Furthermore, drug content studies for formulations NIF-3D-2 and IND-3D-2 were conducted before and after the printing process, to evaluate the effect of the printing temperature on the APIs degradation. Results showed that formulations NIF-3D-2 and IND-3D-2 had average drug contents of 98% and 95%, respectively, indicating that the printing temperature did not affect the formulation content.
3.7. In-vitro drug release studies
3.7.1. Capsule-in-capsule dosage from
IND was encapsulated in a delayed release-acid-resistant capsule (inner capsule) to minimize its release in the stomach, thereby lowering the potential for adverse effects such as stomach irritation. NIF was encapsulated in the outer capsule. Before the release study, the acid-resistant capsules were evaluated in terms of the ability of the capsule to resist the acidic medium before reaching deformation. Three capsule-in capsules were tested in a dissolution medium of 800 mL with a pH of 1.2 and paddle speed of 50 rpm, to mimic the stomach conditions. The average time for resisting the acidic medium was calculated. Results showed that the average time was around 90 minutes.
The release study was conducted in a dissolution medium of 800 mL with a pH of 1.2 for the first two hours, then the pH was adjusted to 6.8 pH with a final volume of 900 mL. For the first two hours, only 2% of pure NIF was released, which can be explained by the low solubility of NIF and its presence in the crystal state, as seen in solubility and DSC results. Kollidon® 12PF (polyvinylpyrrolidone) was chosen to be the matrix for NIF due to its low molecular weight (2500 g/mol), low glass transition temperature (90 °C), extrudability, and high water solubility to provide an immediate release of the drug.[36] Moreover, formulation NIF-2 showed a significant improvement in the release of NIF; around 100% in 30 min (Figure 6). This improvement can be caused by the presence of the NIF in an amorphous state within the polymer, which requires lower energy to break the crystal lattice compared to the crystal state (pure NIF).[37] Furthermore, the distributive and dispersive mixing during the extrusion can cause a reduction in the APIs particle size, which leads to an improvement of the wettability due to the increase in the surface area and reducing the agglomeration. [38] Additionally, the solubilizations and formation of hydrogen bonds between NIF and the water-soluble carrier (Kollidon® PF12) can improve the drug release in the dissolution medium.[39] Indomethacin release was successfully delayed in the acidic medium with a release of only 5 % in the first two hours, caused by its encapsulation in the acid-resistant capsule. Furthermore, extending IND’s release in a basic medium (pH 6.8) is challenging because of its weakly acidic nature and its significantly enhanced solubility in the basic medium (pH 6.8). Kollidon® SR is a combination of polyvinyl acetate and polyvinyl pyrrolidone (80% / 20%), which is suitable for prolonged drug release. The purpose of polyvinyl pyrrolidone is to form small pores so that the API can slowly diffuse into the dissolution medium.[40] As seen in Figure 6, an extended drug release of up to 10 hours in the basic medium was successfully achieved for formulation IND-2 (API 25%, Kollidon® VA 30%, and Kollidon® SR 45%).
Figure 6.
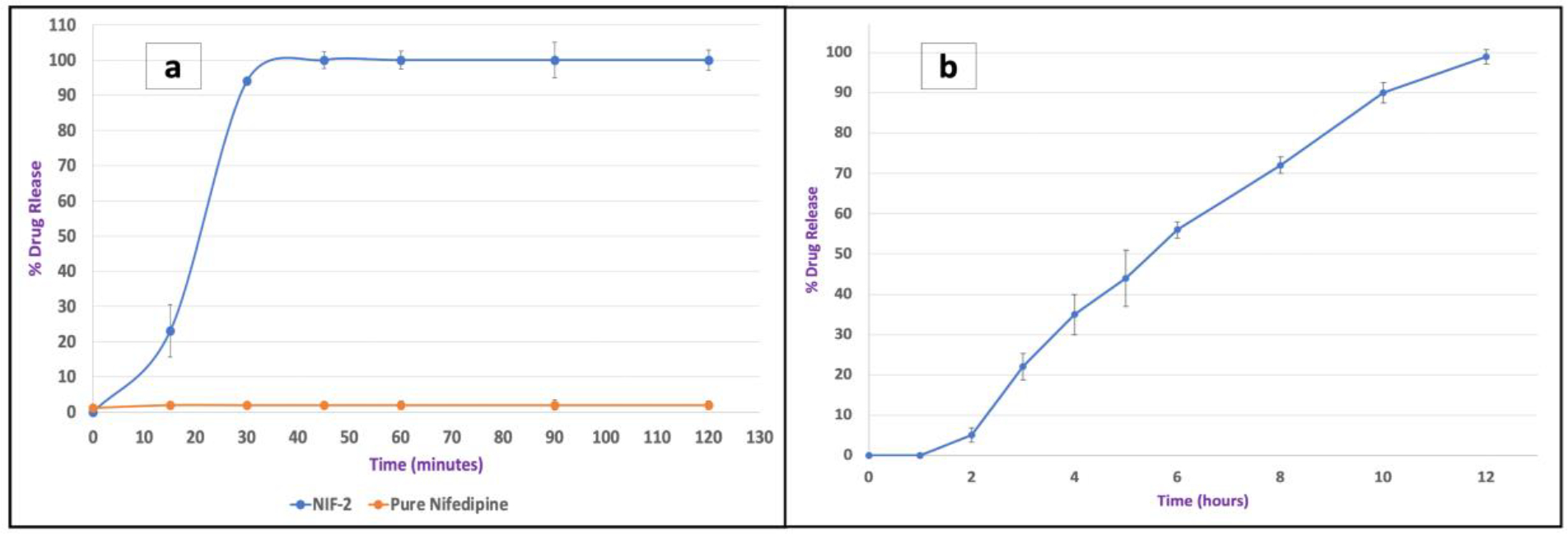
The release profiles of pure NIF, formulation NIF-2 (a), and formulation IND-2 (b).
3.7.2. 3D tablet dosage form
NIF-3D-2 tablets showed an immediate drug release (95 % after 30 minutes) compared to pure NIF (2 % after two hours). The faster drug release of 3D-printed tablets contributed to the dispersion of the poorly water-soluble API in the hydrophilic polymer forming a homogenous solid dispersion, and the formation of hydrogen bonds between the formulation components. Furthermore, the drug release rate can be significantly affected by the infill density. A tablet with 100% infill density can show a delay in the API release due to the lack of pores and prevention of the dissolution medium from passing through the tablet.[41] Based on that, the infill density for tablet NIF-3D-2 was set at 40% to achieve an immediate release profile (Figure 7). Furthermore, IND-3D-2 tablets showed delayed-extended release profiles (for 12 hours), which can be caused by the combination of HPMC and HPC. Moreover, HPMC is considered a more hydrophilic matrix compared to HPC LF, due to the differences in their molecular weights (80,000 Da, and 95,000 Da), respectively. Moreover, a polymer blend of HPMC and HPC may influence the matrix hydration and erosion rate to provide retardation in the drug release profile compared to a single polymer system. [42–44] The tight 3D structure and the infill density (100%) also have roles in extending the API release.[30] IND-3D-2 tablets with components ratios of 30% of the API, 45.5% HPMC E4, 19.5% HPC LF, and 5 % of Kollidon® CL were found to be printable and successfully provided a delayed release in the first two hours with (only 8 %), and an extended-release profile for 12 hours (Figure 7).
Figure 7.
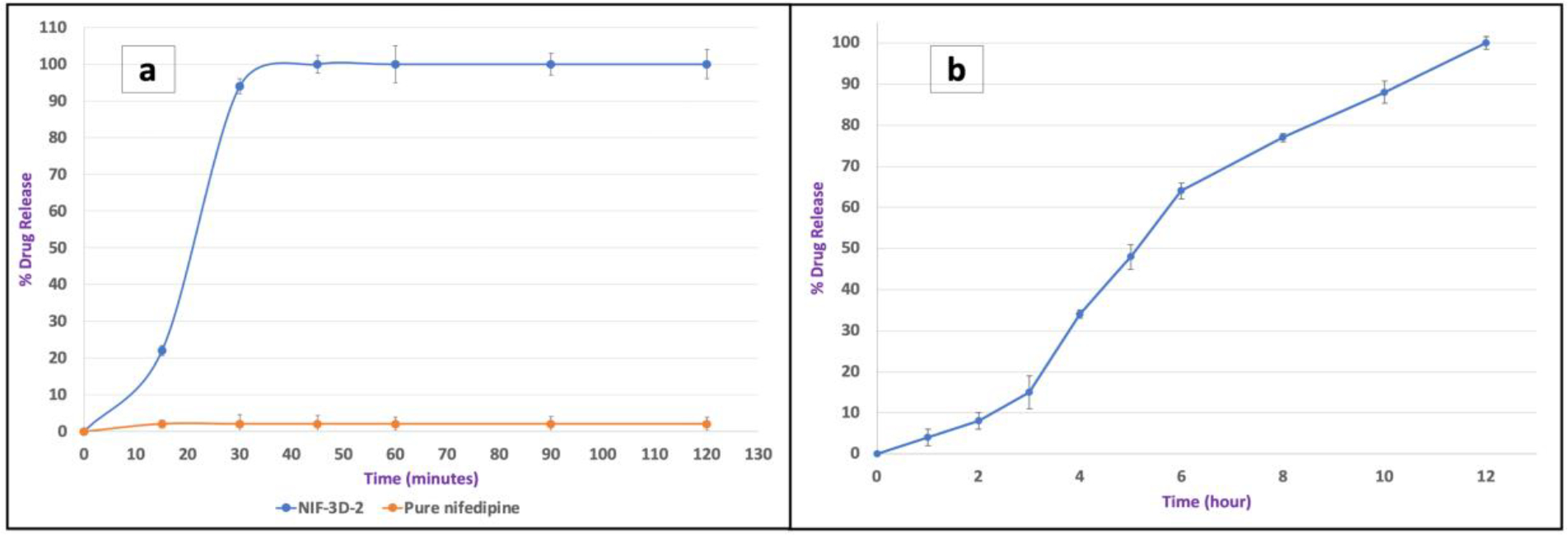
The release profiles of pure NIF, formulation NIF-3D-2 (a), and formulation IND-3D-2 (b).
The statistical analysis of IND formulations (IND-2 and IND-3D-2) and NIF formulations (NIF-3 and NIF-3D-2) showed p values above 0.05. The p values indicate that there is no significant difference between the release profiles. The release kinetics of all formulations were evaluated using the DDSolver program and the data were fitted into four release models zero-order, first-order, Higuchi, and Korsmeyer-Peppas. Moreover, the release kinetics were determined by the coefficient of determination (R2) for each formulation, the highest coefficient of determination (R2) is the proper model for describing the release kinetics.[45] The results showed that formulations NIF-2 and NIF-3D-2 best fit in the Hixson-Crowell model which suggests that the release of the API contributed to the decrease in the surface area as a function of time (Table 6).[46] Formulations IND-2 and IND-3D-2 had the highest coefficient of determination (R2) with the Korsmeyer-Peppas model which describes some release mechanisms including diffusion of the API from the matrix to the dissolution medium or by the matrix erosion, as seen in table 6. Furthermore, the release exponent (n) of formulations IND-2 and IND-3D-2 were calculated to characterize the release mechanisms. Both formulations IND-2 and IND-3D-2 had release exponent (n) higher than 1.0 which is considered as a super case II transport in which erosion is the predominant release mechanism.[47,48]
Table 6.
Values of the coefficient of determination (R2) of zero-order, first-order, Higuchi, and Korsmeyer-Peppas
| Formulation | Zero-order | First order | Higuchi | Korsmeyer-Peppas | Hixson-Crowell |
|---|---|---|---|---|---|
| NIF-2 | 0.8897 | 0.6089 | 0.8255 | 0.8786 | 0.9317 |
| NIF-3D-2 | 0.9038 | 0.7374 | 0.8339 | 0.8904 | 0.9374 |
| IND-2 | 0.9081 | 0.9289 | 0.8519 | 0.9774 | 0.9546 |
| IND-3D-2 | 0.9633 | 0.8902 | 0.8610 | 0.9767 | 0.9230 |
3.8. Stability studies
The purpose of the stability study was to evaluate the physicochemical properties of the formulations during storage time. Results from drug content studies (Figure 8) and thermal analysis (Figure 9) were similar for the initial formulations and the third month. This indicates that all formulations remained physically and chemically stable during this period, with no signs of degradation or recrystallization. Furthermore, the improvement in their stability is attributed to the formation of hydrogen bonds between the components and the ability of the carrier to keep the APIs in amorphous states, thereby inhibiting recrystallization during storage time.
Figure 8.
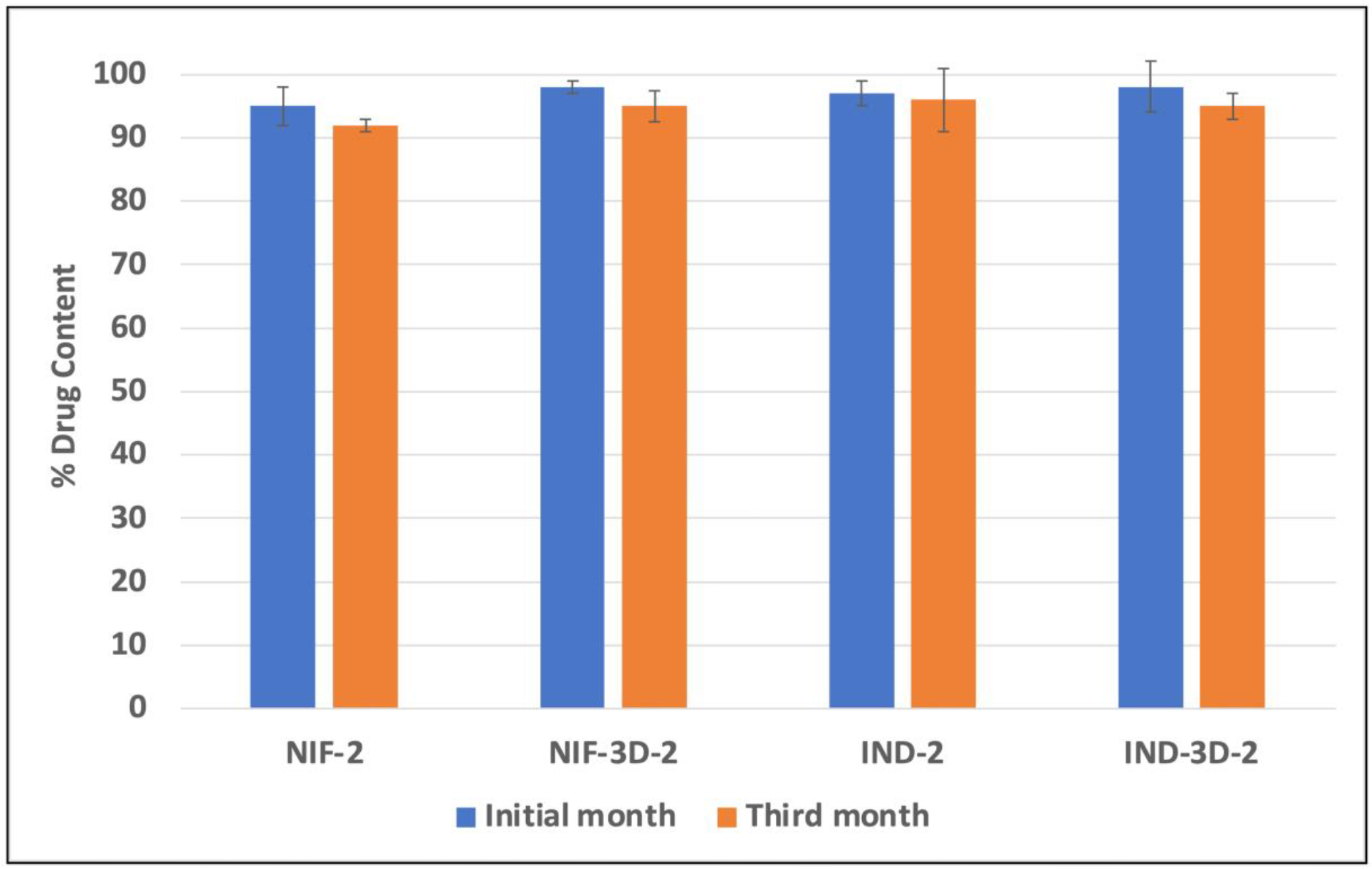
Percent drug content of NIF and IND during the stability study.
Figure 9.
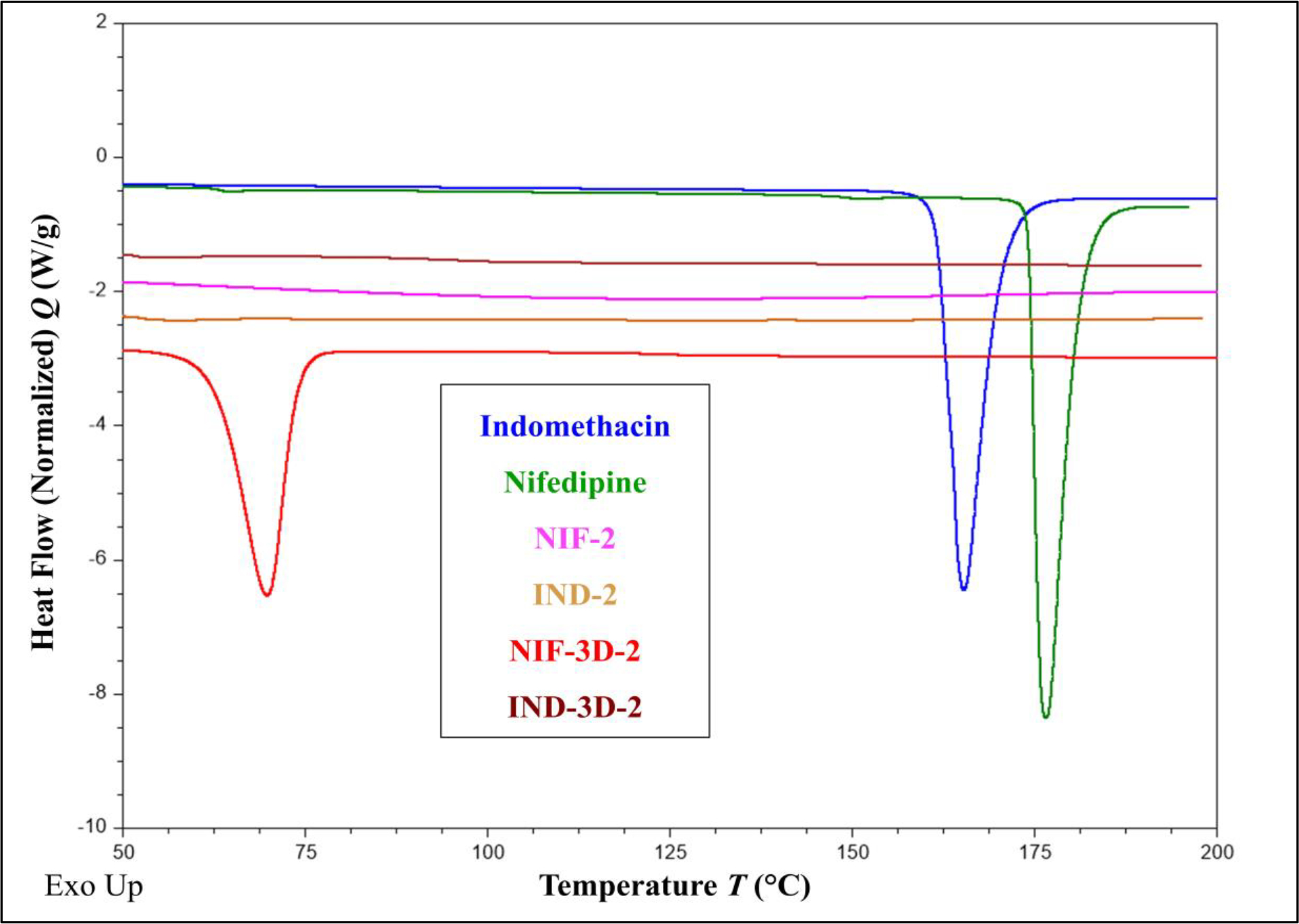
Thermogram analysis to assess the stability of the formulations in the third month.
Conclusion
The fixed-dose combinations (capsule-in-capsule and 3D-printed tablet) were successfully developed and characterized using hot-melt extrusion alone and with fused deposition modeling (3D Printer). NIF release profiles in both formulations (NIF-2 and NIF-3D-2) were significantly improved compared to the pure drug. Moreover, formulations IND-2 and IND-3D-2 showed delayed-extended release profiles for 10 hours. All formulations remained chemically and physically stable with no signs of recrystallization or degradation. The designed FDCs could improve the quality of life by reducing morning stiffness, enhancing patient compliance, and preventing the need for polypharmacy.
Acknowledgment
This project was partially supported by Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE). Scanning Electron Microscopy images presented in this work were generated at the Microscopy and Imaging Center, The University of Mississippi. This facility is supported in part by grant 1726880, National Science Foundation.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or conflicts of interest or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest
The authors believe that there are no conflicts of interest to declare for the research article entitled “Development and Characterization of Different Dosage Forms of Nifedipine/Indomethacin Fixed-Dose Combinations”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. Oxford University Press; 2012;67:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MS, Roberts MS. Challenges and innovations of drug delivery in older age. Adv Drug Deliv Rev. Elsevier; 2018;135:3–38. [DOI] [PubMed] [Google Scholar]
- 3.Perrie Y, Badhan RKS, Kirby DJ, Lowry D, Mohammed AR, Ouyang D. The impact of aging on the barriers to drug delivery. Journal of controlled release. Elsevier; 2012;161:389–98. [DOI] [PubMed] [Google Scholar]
- 4.Jagpal A, Navarro-Millán I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment, and treatment. BMC Rheumatol. BioMed Central; 2018;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am J Hypertens. Oxford University Press; 2000;13:1161–7. [DOI] [PubMed] [Google Scholar]
- 6.Polónia J, Boaventura I, Gama G, Camões I, Bernardo F, Andrade P, et al. Influence of non-steroidal anti-inflammatory drugs on renal function and 24h ambulatory blood pressure-reducing effects of enalapril and nifedipine gastrointestinal therapeutic system in hypertensive patients. J Hypertens. 1995;13:925–31. [DOI] [PubMed] [Google Scholar]
- 7.Salvetti A, Magagna A, Abdel-Haq B, Lenzi M, Giovannetti R. Nifedipine interactions in hypertensive patients. Cardiovasc Drugs Ther. Springer; 1990;4:963–8. [DOI] [PubMed] [Google Scholar]
- 8.Watkins J, Abbott EC, Hensby CN, Webster J, Dollery CT. Attenuation of the hypotensive effect of propranolol and thiazide diuretics by indomethacin. Br Med J. British Medical Journal Publishing Group; 1980;281:702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiwah AC, Mactier RA. Antagonistic effect of non-steroidal anti-inflammatory drugs on frusemide-induced diuresis in cardiac failure. Br Med J (Clin Res Ed). BMJ Publishing Group; 1981;283:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-García R, Prada M, Bolás-Fernández F, Ballesteros MP, Serrano DR. Oral fixed-dose combination pharmaceutical products: Industrial manufacturing versus personalized 3D printing. Pharm Res. Springer; 2020;37:1–22. [DOI] [PubMed] [Google Scholar]
- 11.Mitra A, Wu Y. Challenges and opportunities in achieving bioequivalence for fixed-dose combination products. AAPS J. Springer; 2012;14:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.11. NIH. FixedDoseCombination.ClinicalTrials.gov.https://clinicaltrials.gov/ct2/result/fixed-dosecombination (accessed 23rd March 2020).
- 13.Khouri AS, Realini T, Fechtner RD. Fixed-combination drugs. OPHTHALMOLOGY MONOGRAPHS. AMERICAN ACADEMY OF OPHTHALMOLOGY; 2008;13:139. [Google Scholar]
- 14.Aronson JK. Medication errors result from the confusion of drug names. Expert Opin Drug Saf. Taylor & Francis; 2004. p. 167–72. [DOI] [PubMed] [Google Scholar]
- 15.Desai D, Wang J, Wen H, Li X, Timmins P. Formulation design, challenges, and development considerations for fixed-dose combination (FDC) of oral solid dosage forms. Pharm Dev Technol. Taylor & Francis; 2013;18:1265–76. [DOI] [PubMed] [Google Scholar]
- 16.Mandal U, Pal TK. Formulation and in vitro studies of a fixed-dose combination of a bilayer matrix tablet containing metformin HCl as sustained release and glipizide as an immediate release. Drug Dev Ind Pharm. Taylor & Francis; 2008;34:305–13. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell WB, Kaushal AM. Multiparticulate Technologies for Fixed-Dose Combinations. Multiparticulate Drug Delivery. Springer; 2017. p. 155–68. [Google Scholar]
- 18.Patil H, Tiwari R v, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. Springer; 2016;17:20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. Taylor & Francis; 2007;33:1043–57. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Vo AQ, Feng X, Bandari S, Repka MA. Pharmaceutical additive manufacturing: a novel tool for complex and personalized drug delivery systems. AAPS PharmSciTech. Springer; 2018;19:3388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norman J, Madurawe RD, Moore CM v, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. Elsevier; 2017;108:39–50. [DOI] [PubMed] [Google Scholar]
- 22.Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled-release pharmaceutical bilayer tablets. Int J Pharm. Elsevier; 2014;461:105–11. [DOI] [PubMed] [Google Scholar]
- 23.Daly R, Harrington TS, Martin GD, Hutchings IM. Inkjet printing for pharmaceutics–a review of research and manufacturing. Int J Pharm. Elsevier; 2015;494:554–67. [DOI] [PubMed] [Google Scholar]
- 24.Prasad LK, Smyth H. 3D Printing technologies for drug delivery: a review. Drug Dev Ind Pharm. Taylor & Francis; 2016;42:1019–31. [DOI] [PubMed] [Google Scholar]
- 25.Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int J Pharm. Elsevier; 2016;499:376–94. [DOI] [PubMed] [Google Scholar]
- 26.Ashour EA, Majumdar S, Alsheteli A, Alshehri S, Alsulays B, Feng X, et al. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. Journal of Pharmacy and Pharmacology. Oxford University Press; 2016;68:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omari S, Ashour EA, Elkanayati R, Alyahya M, Almutairi M, Repka MA. Formulation development of loratadine immediate-release tablets using hot-melt extrusion and 3D printing technology. J Drug Deliv Sci Technol. Elsevier; 2022;74:103505. [Google Scholar]
- 28.O’Connor TF, Lawrence XY, Lee SL. Emerging technology: A key enabler for modernizing pharmaceutical manufacturing and advancing product quality. Int J Pharm. Elsevier; 2016;509:492–8. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Guirales SA, Jurado N, Kara A, Lalatsa A, Serrano DR. Understanding direct powder extrusion for fabrication of 3D printed personalized medicines: A case study for nifedipine minitablets. Pharmaceutics. MDPI; 2021;13:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Feng X, Patil H, Tiwari R v, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. Elsevier; 2017;519:186–97. [DOI] [PubMed] [Google Scholar]
- 31.Rösler J, Harders H, Bäker M. Mechanical behavior of engineering materials: metals, ceramics, polymers, and composites. Springer Science & Business Media; 2007. [Google Scholar]
- 32.Xu P, Li J, Meda A, Osei-Yeboah F, Peterson ML, Repka M, et al. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int J Pharm. Elsevier; 2020;588:119760. [DOI] [PubMed] [Google Scholar]
- 33.Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials. Elsevier; 2002;23:4241–8. [DOI] [PubMed] [Google Scholar]
- 34.Nyamweya N, Hoag SW. Assessment of polymer-polymer interactions in blends of HPMC and film-formingpolymers by modulated temperature differential scanning calorimetry. Pharm Res. Springer; 2000;17:625–31. [DOI] [PubMed] [Google Scholar]
- 35.Verstraete G, Camaro A, Grymonpré W, Vanhoorne V, van Snick B, Boone MN, et al. 3D printing of high drug-loaded dosage forms using thermoplastic polyurethanes. Int J Pharm. Elsevier; 2018;536:318–25. [DOI] [PubMed] [Google Scholar]
- 36.Alsulays BB, Park J-B, Alshehri SM, Morott JT, Alshahrani SM, Tiwari R v, et al. Influence of molecular weight of carriers and processing parameters on the extrudability, drug release, and stability of fenofibrate formulations processed by hot-melt extrusion. J Drug Deliv Sci Technol. Elsevier; 2015;29:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dierickx L, van Snick B, Monteyne T, de Beer T, Remon JP, Vervaet C. Co-extruded solid solutions as immediate release fixed-dose combinations. European Journal of Pharmaceutics and Biopharmaceutics. Elsevier; 2014;88:502–9. [DOI] [PubMed] [Google Scholar]
- 38.K. Kolter MKAG. Hot Melt Extrusion with BASF Pharma Polymers. 2nd Revised. 2012. [Google Scholar]
- 39.Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. Elsevier; 2006;321:1–11. [DOI] [PubMed] [Google Scholar]
- 40.Reza MS, Quadir MA, Haider SS. Development of theophylline sustained release dosage form based on kcollidedSR. Pak J Pharm Sci. 2002;15:63–70. [PubMed] [Google Scholar]
- 41.Kadry H, Al-Hilal TA, Keshavarz A, Alam F, Xu C, Joy A, et al. Multi-purpose filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int J Pharm. Elsevier; 2018;544:285–96. [DOI] [PubMed] [Google Scholar]
- 42.Tiwari SB, Rajabi-Siahboomi AR. Applications of complementary polymers in HPMC hydrophilic extended release matrices. Drug Deliv Technol. 2009;9:20–7. [Google Scholar]
- 43.Nyamweya NN. Applications of polymer blends in drug delivery. Futur J Pharm Sci. Springer; 2021;7:1–15. [Google Scholar]
- 44.Gusler G, Berner B, Chau M, Padua A. Optimal polymer mixtures for gastric retentive tablets. Google Patents; 2004. [Google Scholar]
- 45.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. Elsevier; 1983;15:25–35. [Google Scholar]
- 46.Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. ACS Publications; 1931;23:923–31. [Google Scholar]
- 47.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. Journal of controlled release. Elsevier; 1987;5:23–36. [PubMed] [Google Scholar]
- 48.Singh J, Gupta S, Kaur H. Prediction of in vitro Drug Release Mechanisms from Extended Release Matrix Tablets using SSR/R^ sup 2^ Technique. Trends Appl Sci Res. Academic Journals Inc.; 2011;6:400. [Google Scholar]


