Significance
Vibrio cholerae remains a significant problem of global health. Current oral cholera vaccines (OCVs) confer over 65% protection to adults and children older than 5 y but show poor efficacy in children younger than 5 y in endemic regions. Safe and effective cholera vaccines for all age groups are needed. Here, we reported a polyvalent protein immunogen that induced broad and functional antibodies, reduced over 99% vibrio colonization of small intestines, and protected 88% of any diarrhea and 100% of severe diarrhea from O1, O139, and non-O1/non-O139 V. cholerae infection in an adult rabbit colonization model and an infant rabbit passive protection model. This cross-protective polyvalent protein and the rabbit models (adult and infant) potentially overcome hurdles in cholera vaccine development.
Keywords: cholera, MEFA, polyvalent, rabbit, vaccine
Abstract
Using epitope- and structure-based multiepitope fusion antigen vaccinology platform, we constructed a polyvalent protein immunogen that presents antigenic domains (epitopes) of Vibrio cholerae toxin-coregulated pilus A, cholera toxin (CT), sialidase, hemolysin A, flagellins (B, C, and D), and peptides mimicking lipopolysaccharide O-antigen on a flagellin B backbone. Mice and rabbits immunized intramuscularly with this polyvalent protein immunogen developed antibodies to all of the virulence factors targeted by the immunogen except lipopolysaccharide. Mouse and rabbit antibodies exhibited functional activities against CT enterotoxicity, CT binding to GM1 ganglioside, bacterial motility, and in vitro adherence of V. cholerae O1, O139, and non-O1/non-O139 serogroup strains. When challenged orogastrically with V. cholerae O1 El Tor N16961 or a non-O1/non-O139 strain, rabbits IM immunized with the immunogen showed a 2-log (99%) reduction in V. cholerae colonization of small intestines. Moreover, infant rabbits born to the mother immunized with the protein immunogen acquired antibodies passively and were protected from bacterial intestinal colonization (>2-log reduction), severe diarrhea (100%), and mild diarrhea (88%) after infection with V. cholerae O1 El Tor (N16961), O1 classical (O395), O139 (Bengal), or a non-O1/non-O139 strain. This study demonstrated that this polyvalent cholera protein is broadly immunogenic and cross-protective, and an adult rabbit colonization model and an infant rabbit passive protection model fill a gap in preclinical efficacy assessment in cholera vaccine development.
Vibrio cholerae infection continues to be a threat to public health, especially in South and Southeast Asia and sub-Saharan Africa, causing 1.3 to 4 million clinical cholera cases and 21,000 to 143,000 deaths annually (1), with about half of the cases and deaths being in children younger than 5 y (2). In Bangladesh, V. cholerae is one of the top three leading etiologies of diarrheal disease requiring hospitalization across all age groups (3).
V. cholerae consists of over 200 serogroups, but serogroup O1 (classical biotype caused the first six pandemics and El Tor biotype caused the seventh pandemic) and serogroup O139 (which caused a large outbreak in the early 1990s but waned afterward) are associated with epidemic cholera. Serogroup O1 is the main target in cholera vaccine development historically; however, serogroup O139 remains a concern, and other serogroups (non-O1/non-O139) are also able to cause sporadic gastrointestinal infections (4, 5). An ideal cholera vaccine would protect against all major V. cholerae serogroups that cause gastrointestinal infections.
Current cholera vaccines target O1 or O1 and O139 O-specific polysaccharides (OSP) of lipopolysaccharide (LPS) and thus are serogroup specific. Three killed whole-cell oral cholera vaccines (OCVs; Dukoral, Euvichol, and Shanchol) prequalified by the World Health Organization (WHO) confer >65% efficacy in adults and children older than 5 y (6–8), but these OCVs provide significantly less or minimal protection to children younger than 5 y in endemic countries (8–11). Unlike Dukoral, which includes a killed serogroup O1 strain (and recombinant cholera toxin (CT) B subunit), Euvichol and Shanchol are “bivalent” as they carry killed bacteria of O1 and O139 serogroup strains to induce antibodies to the LPS of both serogroups, although their protection against serogroup O139 has not been determined. In addition to the killed oral vaccines, a live oral vaccine, Vaxchora, which carries an attenuated O1 serogroup strain, is licensed for travelers in the United States and assumed to protect against only the O1 serogroup (12).
While immunity to OSP has been the primary target for cellular cholera vaccines, there is evidence that immunity to some protein antigens of V. cholerae may also be important (13–15). Unlike OSP, which is serogroup specific, many virulence factor proteins are shared by V. cholerae serogroups and thus can serve as conserved antigens to provide cross-protection. CT and toxin-coregulated pilus A (TcpA) proteins produced by V. cholerae O1 and O139 serogroup strains are the virulence determinants in cholera (16–18). CT, an AB5 toxin after binding to monosialotetrahexosylganglioside (GM1) gangliosides of small intestinal epithelial cells and entering host cells via endocytosis, elevates epithelial cell cyclic adenosine monophosphate (cAMP) levels to cause fluid hypersecretion into the gut lumen and profuse watery diarrhea. TcpA mediates V. cholerae adherence to host cells and enhances bacterial colonization of small intestines by aggregating bacteria into microcolonies. Other factors attributing to virulence include sialidase, flagella, and possible hemolysin. Sialidase plays a role in CT binding to host receptors and CT uptake by host cells (19–21); flagella composed of five flagellins (A, B, C, D, and E) mediate bacterial motility, alter morphology, and enhance biofilm development and virulence (22–25); hemolysin causes vacuolation in host cells (26).
Antibody responses to the abovementioned virulence factors of V. cholerae were mounted in cholera patients or vaccinees (27, 28). Earlier studies indicated that individuals immunized with Dukoral developed robust anti-CT and anti-LPS antibody responses (9, 29, 30). A recent study profiling immune responses in Bangladesh adult patients showed that V. cholerae infection raised systemic and mucosal immunoglobulin M (IgM), immunoglobulin G (IgG), and immunoglobulin A (IgA) antibodies to CT, TcpA, LPS O-antigen, sialidase (neuraminidase; NanH), hemolysin A (HlyA), and flagellins (FlaB, FlaC, and FlaD; although FlaA is essential for flagellar biosynthesis, antibodies to FlaA were not mounted after natural exposure to V. cholerae) (31). While vibriocidal antibodies, which largely represent antibodies to LPS, are associated with moderate-to-long-term protection (12, 32) and anti-CT antibodies appear to confer short-term protection (9), correlates to protection from antibodies to the other virulence factors are yet to be established.
In this study, we constructed a polyvalent cholera protein immunogen by applying an epitope- and a structure-based vaccinology platform called multiepitope fusion antigen (MEFA) (33), then characterized the broad immunogenicity of the polyvalent MEFA protein, and examined protection from the MEFA protein–induced antibodies against V. cholerae intestinal colonization and clinical diarrhea preclinically. Mice immunized with this polyvalent protein were examined for antibody responses to the virulence factors targeted by the immunogen, and the derived antibodies were then evaluated for in vitro functions, including prevention of adherence from different V. cholerae serogroup strains, neutralization of CT enterotoxicity, blocking of CT binding to host receptor GM1, inhibition of vibrio motility, vibriocidal activity, and the activity against hemolysis. Furthermore, we applied two animal models to assess this MEFA immunogen for broad protection. First, we intramuscularly (IM) immunized adult rabbits and then challenged the rabbits orogastrically with a V. cholerae O1 or non-O1/non-O139 strain to assess protection against bacterial colonization of small intestines. Second, we IM immunized pregnant rabbits and challenged their infant rabbits with a V. cholerae O1, O139, or a non-O1/non-O139 serogroup strain to evaluate protection against vibrio intestinal colonization and clinical cholera.
Results
Cholera MEFA Protein Carried Epitopes of CT, TcpA, Sialidase, HlyA, Flagellins (B, C, and D), and Peptides Mimicking O1 OSP.
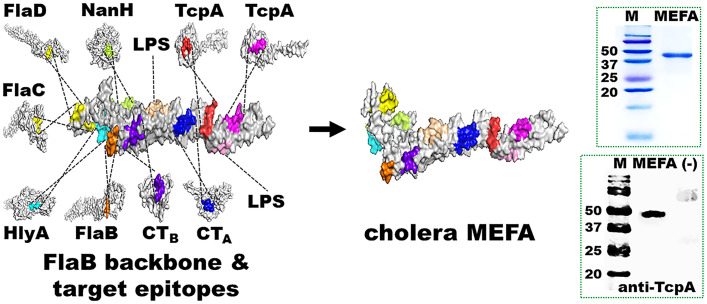
A polyvalent MEFA protein immunogen named cholera MEFA was produced (Fig. 1). Immunodominant B cell epitopes ADSRPPDEIKQS (CTA subunit), SQHIDSQKKA (CTB subunit), PATADATAASK (TcpA of O1 classical 395), GKVSADEAKNP (TcpA of O1 El Tor N16961, O139 Bengal, and non-O1/non-O139), MQDNTNNGSGV (sialidase), SNGSNSSSERR (FlaB), LQSQSANGSNSKSE (shared by FlaC and FlaD), and TGGVEVSGDGPK (HlyA) were identified in silico. These epitopes were predicted antigenic and exposed on the surface of the representing virulence factor proteins. Additionally, two peptides, LPSAGRGVCYEA and QHLNSILLVTK mimicking O1 OSP (mimotopes) (34), were included. Flagellin B (FlaB), which is structurally stable and strongly antigenic and possesses multiple surface-exposed and well-separated continuous epitopes, was selected as the backbone to present foreign epitopes. After substitutions of backbone epitopes with the epitopes from the target virulence factors, a cholera MEFA protein was in silico constructed and confirmed for structural stability, epitope surface exposure, and antigenicity. Subsequently, a chimeric cholera MEFA gene was synthesized after codon optimization, cloned in plasmid pUC57, subcloned into expression vector pET28a, and verified by DNA sequencing. Cholera MEFA protein (39.2 kDa) expressed in Escherichia coli BL21 (DE3) was extracted and refolded, at a yield of 40 mg per liter culture broth and purity of greater than 90% based on visual assessment of sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) Coomassie blue staining, and recognized by anti-CT or anti-TcpA polyclonal antibodies in western blot (Fig. 1).
Fig. 1.
Construction and characterization of a polyvalent cholera MEFA protein immunogen. B cell immunodominant epitopes of V. cholerae CT subunit A (CTA) and subunit B (CTB), TcpA, sialidase (NanH), flagellin C (FlaC), flagellin D (FlaD), hemolysin A (HlyA), and O1 OSP mimotopes were integrated into backbone protein flagellin B (FlaB). Recombinant cholera MEFA protein was detected in SDS-PAGE with Coomassie blue staining and western blot with rabbit anti-TcpA antiserum. M, molecule marker (kDa); MEFA, cholera MEFA protein; (−), E. coli BL21 host cell proteins.
Cholera MEFA Protein Induced Broad Antigen-Specific Antibody Responses in Mice.
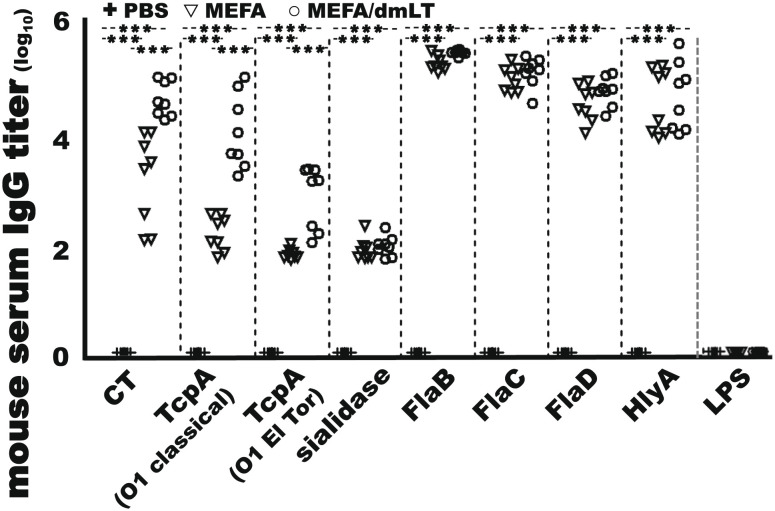
Mice IM immunized with 25 µg cholera MEFA protein, with or without adjuvant double-mutant heat labile toxin (dmLT) [LTR192G/L211A, a double-mutant heat-labile toxin of enterotoxigenic E. coli (ETEC)], developed IgG responses to the target antigens (Fig. 2). Anti-CT, anti-TcpA (O1 classical), anti-TcpA (O1 El Tor and O139), anti-sialidase, anti-FlaB, anti-FlaC, anti-FlaD, and anti-HlyA IgG titers were 3.3 ± 0.84, 2.3 ± 0.32, 1.9 ± 0.09, 2.0 ± 0.20, 5.4 ± 0.13, 5.1 ± 0.23, 4.7 ± 0.34, and 4.7 ± 0.60 (log10), respectively, from serum samples of the group immunized with the MEFA protein (without dmLT adjuvant). When adjuvant dmLT was included in mouse immunization, the IgG titers were 4.8 ± 0.33, 4.2 ± 0.68, 3.0 ± 0.58, 2.1 ± 0.18, 5.6 ± 0.05, 5.3 ± 0.28, 4.9 ± 0.26, and 4.8 ± 0.63 (log10), respectively. Anti-CT and anti-TcpA IgG responses were significantly greater in the immunized group with dmLT adjuvant (P < 0.001) (Fig. 2). IgG response to LPS was not detected from either immunized group. No antigen-specific IgG responses were detected from serum samples of the control mice [IM injected with phosphate buffered saline (PBS)].
Fig. 2.
Serum IgG titers (in log10) in the mice IM immunization with cholera MEFA (▽; n = 8), cholera MEFA and adjuvant dmLT (○; n = 8), or PBS as the control (+; n = 8). Serum samples from each mouse were twofold diluted (1:200 to 1:25,600), in triplicates, and examined in ELISAs for IgG responses to CT, TcpA (O1 classical or O1 El Tor), sialidase, flagellin B (FlaB), flagellin C (FlaC), flagellin D (FlaD), hemolysin A (HlyA), and O1 LPS. *** indicates a P value <0.001 based on two-way ANOVA with the Bonferroni post hoc test.
When a reduced antigen dose (10 µg cholera MEFA protein) or the WHO-prequalified Shanchol was used, at a 3-wk interval, antigen-specific IgG titers in the serum samples from the mice IM immunized with 10 µg cholera MEFA were the same as the titers in the mice IM immunized with 25 µg cholera MEFA (at a 2- or 3-wk interval) but significantly greater than the titers in the mice orogastrically immunized with Shanchol (P < 0.001) except for the titers to anti-TcpA (O1 El Tor or O139; P > 0.05) (SI Appendix, Fig. S1).
Cholera MEFA-Induced Mouse Antibodies Were Functional against the Target Virulence Factors.
Sera from the mice immunized with cholera MEFA protein showed functional activities against V. cholerae adherence, CT enterotoxicity, CT binding to GM1, and V. cholerae motility.
Antibodies inhibited adherence of V. cholerae O1, O139, and non-O1/non-O139 serogroup strains.
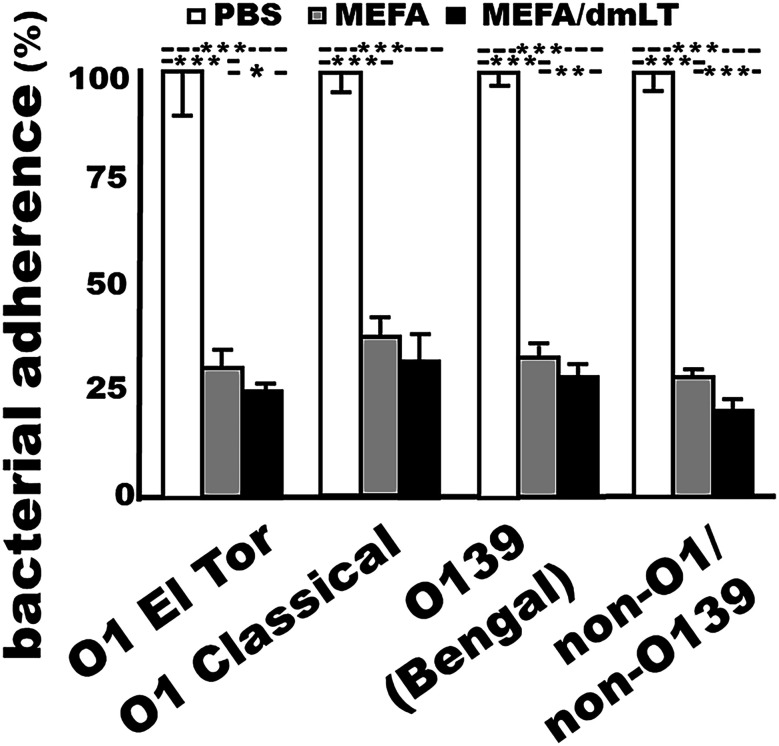
Bacterial adherence to host receptors initiates V. cholerae intestinal colonization and infection. Antibodies inhibiting bacterial adherence serve as the first line of defense against cholera. Mouse serum samples (heat-inactivated) from each immunized group (with or without adjuvant dmLT) significantly inhibited adherence of V. cholerae to Caco-2 cells compared with the serum samples from the control mice that were injected with PBS (P < 0.01) (Fig. 3). Incubated with the sera from the mice immunized with cholera MEFA, V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 34 D-23 bacteria (in colony forming units; CFUs) adherent to Caco-2 cells were reduced by 70%, 62%, 67%, and 72%, respectively, compared with the bacteria incubated with the serum from the control mice. Treated with the sera from the mice immunized with the MEFA protein adjuvanted with dmLT, adherence of the four serogroup strains (CFUs) was decreased by 74%, 68%, 72%, and 80%.
Fig. 3.
V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 El Tor 34 D23 bacteria (CFUs in %; with the CFUs from the group treated with the control mouse sera referred to as 100%) adhered to Caco-2 cells after incubation with the sera of the mice IM injected with PBS (control; n = 8), cholera MEFA protein alone (n = 8), or cholera MEFA protein and adjuvant dmLT (n = 8); five replicates for each treatment. *, **, and *** indicate P values < 0.05, 0.01, and <0.001, respectively, based on two-way ANOVA with the Bonferroni post hoc test. Boxes and bars are means and SDs.
Antibodies neutralized CT enterotoxicity.
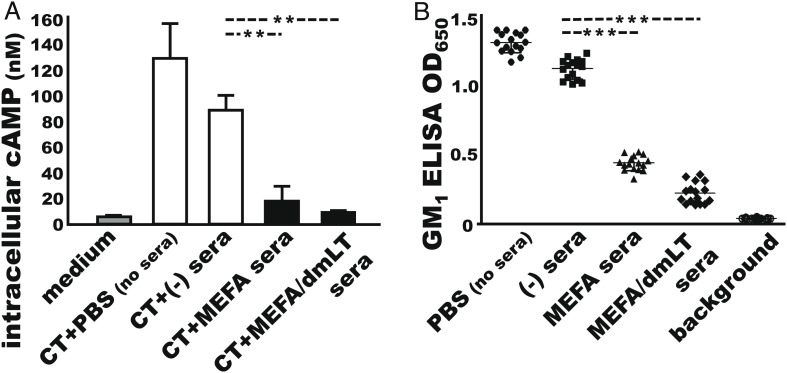
CT enterotoxicity elevates intracellular cAMP levels in intestinal epithelial cells, leading to fluid and water hypersecretion and watery diarrhea. Neutralizing antibodies can prevent CT enterotoxicity from cAMP elevation and water hypersecretion. Mouse serum samples from the group immunized with cholera MEFA (with or without adjuvant dmLT) prevented CT from elevating intracellular cAMP levels in T-84 cells (Fig. 4A). The intracellular cAMP concentrations in the T-84 cells exposed to CT premixed with the sera of mice immunized with cholera MEFA or cholera MEFA and adjuvant dmLT were 17.5 ± 16.8 nM and 8.3 ± 2.2 nM (picomole per ml), respectively, which were significantly lower than the cAMP levels in the cells exposed to CT premixed with the sera of the control mice (88.5 ± 16.8 nM; P < 0.01).
Fig. 4.
Antibody neutralization of CT enterotoxicity and blocking CT from binding to GM1. (A) Direct cAMP EIA Kit (Enzo Life) measured intracellular cyclic AMP levels (nM, pico mole per ml) in T-84 cells exposed to CT and the serum from the mice IM immunized with cholera MEFA (MEFA), cholera MEFA and adjuvant dmLT (MEFA/dmLT), or PBS the control (−); n = 2 and two replicates for each treatment. Cells treated with culture medium indicate cAMP baseline level, and cells exposed to CT (+ PBS, no sera) show stimulation of intracellular cAMP in T-84 cells. (B) Competitive GM1 ELISA to measure mouse serum antibodies for blocking CT from binding to GM1, after incubation with CT, and the serum of the mice IM immunized with cholera MEFA protein (▲), cholera MEFA protein with adjuvant dmLT (♦), or PBS (■); wells incubated with CT (+ PBS, no sera;●) as a positive control. ** and *** indicate the P values < 0.01 and <0.001 based on one-way ANOVA with Tukey’s test. Boxes and bars are means and SDs.
Antibodies blocked CT from binding to GM 1 ganglioside.
CT binds to GM receptors at host intestinal epithelial cells, via the B subunit pentamer, enters host cells, and releases the toxic CTA subunit to cause fluid secretion. Functional antibodies block CT from binding to GM1 receptors and prevent CT from entering host cells. Competitive GM1 enzyme-linked immunosorbentassay (ELISA) showed that CT, after incubation with the sera from the mice immunized with MEFA protein (with or without dmLT adjuvant), exhibited a significant reduction in binding to GM1 ganglioside (Fig. 4B). The OD650 readings were 0.44 ± 0.05 and 0.22 ± 0.08, respectively, in the wells incubated with CT and the serum samples of the group immunized with cholera MEFA protein alone or along with dmLT adjuvant, significantly lower than the optical density (OD) in the wells incubated with CT and the control mouse serum samples (1.12 ± 0.07; P < 0.001) or CT and PBS (1.30 ±0.07; P < 0.001).
Antibodies impaired V. cholerae motility.
Bacterial motility assists V. cholerae to penetrate intestinal mucosal barriers and enhances biofilm development and virulence. Functional antibodies can inhibit or impair bacterial motility to alleviate infections. Microscopic examination observed that motility of V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 34 D-23 was reduced when the sera from the mice immunized with cholera MEFA protein (with or without dmLT adjuvant) were added to the bacterial suspension. Bacterial motility was unaltered or less altered when the sera of the control mice were added (SI Appendix, Fig. S2, and Video S1).
Vibriocidal antibody titers and antibody activity against hemolysis were not detected in mouse serum samples. The OD values in the wells with V. cholerae O1 El Tor N16961 incubated with the sera of the immunized mice or the control mice were 0.32 ± 0.07 and 0.33 ± 0.03, respectively, showing no reduction compared with the OD values in the growth control wells (0.24 ± 0.04). Colonies of V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 34 D-23 exhibited hemolytic characteristics on sheep blood agar plates coated with sera from the immunized or control mice after overnight growth at 30°C, showing no morphological alteration compared with the colonies on the plates without mouse serum treatment.
Cholera MEFA Protein Induced Broadly Functional Antibodies in Adult Rabbits.
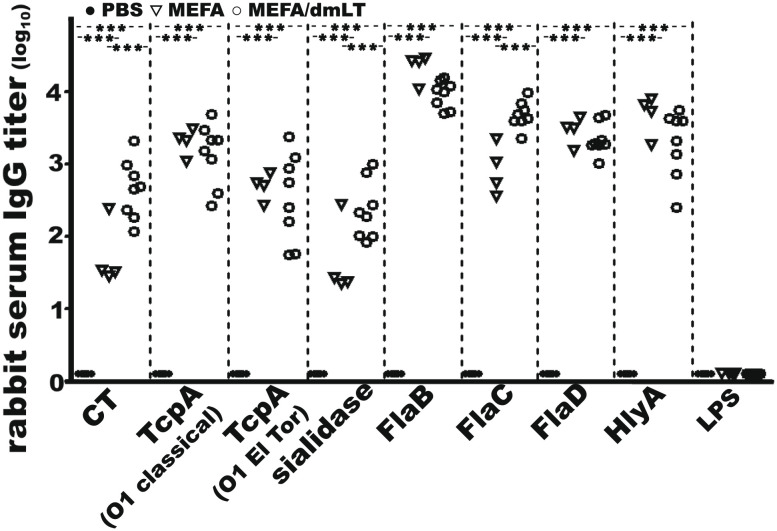
Rabbits IM immunized with cholera MEFA protein developed broad antibody responses to the V. cholerae virulence factors targeted by the immunogen (Fig. 5). Anti-CT, anti-TcpA (O1 classical), anti-TcpA (O1 El Tor and O139), anti-sialidase, anti-FlaB, anti-FlaC, anti-FlaD, and anti-HlyA IgG titers (log10) were 1.7 ± 0.44, 3.3 ± 0.19, 2.7 ± 0.19, 1.6 ± 0.53, 4.3 ± 0.20, 2.9 ± 0.35, 3.5 ± 0.20, and 3.7 ± 0.28, respectively, in the serum samples of the group immunized with cholera MEFA protein. The IgG titers in the sera of the group immunized with cholera MEFA and dmLT adjuvant were 2.6 ± 0.41, 3.1 ± 0.43, 2.5 ± 0.61, 2.4 ± 0.40, 4.0 ± 0.19, 3.7 ± 0.19, 3.3 ± 0.22, and 3.3 ± 0.47 (log10), respectively. Anti-LPS response was not detected in the immunized rabbits. No antigen-specific IgG responses were detected from the serum samples of the rabbits injected with PBS (control).
Fig. 5.
The IgG titers (in log10) from the serum samples of the NZW adult rabbit IM immunized with cholera MEFA (▽; n = 4), cholera MEFA and adjuvant dmLT (○; n = 8), or PBS (●; n = 8). Rabbit serum dilutions (1:200 to 1:25,600) were titrated for IgG to CT, TcpA (O1 classical or O1 El Tor), sialidase, flagellin B (FlaB), flagellin C (FlaC), flagellin D (FlaD), hemolysin A (HlyA), and O1 LPS. Five replicates for each treatment. *** indicates a P value <0.001 based on two-way ANOVA with the Bonferroni post hoc test.
Mild IgA antibody responses were detected from the cecum content of the rabbits immunized with cholera MEFA protein. Anti-CT, anti-TcpA (O1 classical), anti-TcpA (O1 El Tor and O139), anti-FlaB, anti-FlaC, anti-FlaD, and anti-HlyA IgA titers (in log2) were 0.54 ± 0.24, 0.30 ± 0.87, 0.46 ± 0.37, 0.62 ± 0.49, 0.60 ± 0.31, 0.58 ± 0.34, and 0.57 ± 0.50, respectively, from the cecum content suspensions of the rabbits IM immunized with cholera MEFA and were 1.1 ± 0.30, 0.43 ± 0.26, 0.95 ± 0.79, 1.0 ± 0.43, 0.95 ± 0.57, 1.4 ± 0.57, and 1.0 ± 0.30 (log2), respectively, in the cecum content suspensions of the rabbits IM immunized with cholera MEFA adjuvanted with dmLT. IgA to sialidase or O1 OSP was not detected from either immunized group. No antigen-specific IgA was detected in the cecum contents of the control rabbits. Additionally, the cecum content supernatants of the two adult rabbits orogastrically administered with the live attenuated Vaxchora had mild IgA responses detected (SI Appendix, Fig. S3).
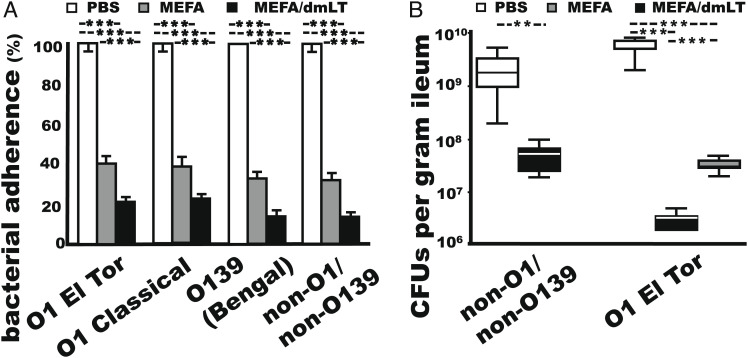
Rabbit serum samples from the group immunized with cholera MEFA protein (with or without dmLT adjuvant) neutralized CT enterotoxicity, blocked CT from binding to GM1, impaired bacterial motility, and significantly inhibited adherence of V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, and non-O1/non-O139 34 D-23 to Caco-2 cells (P < 0.001; Fig. 6A). Sera from the rabbits immunized with MEFA protein and dmLT adjuvant showed significantly better adherence inhibition activities than the sera of the rabbits immunized with MEFA protein without dmLT adjuvant (P < 0.001).
Fig. 6.
Rabbit antibody protection against V. cholerae adherence and intestinal colonization. (A) In vitro adherence of O1 El Tor N16961, O1 classical O395, O139 Bengal, and non-O1/non-O139 El Tor 34 D23 (CFUs in %; with the number of bacteria treated with the control serum referred to as 100%) to Caco-2 cells, after incubation with the sera of the adult rabbits IM immunized with PBS (control; n = 4), cholera MEFA protein alone (n = 4), or cholera MEFA protein and dmLT adjuvant (n = 4), in five replicates. (B) V. cholerae colonization in the small intestine (CFUs in %) of the rabbits (n = 4) IM immunized with cholera MEFA and dmLT (black) or PBS control (white) after orogastric inoculation with non-O1/non-O139 strain El Tor 34 D-23 or in the rabbits (n = 4) IM immunized with cholera MEFA (gray), cholera MEFA adjuvanted with dmLT (black), or PBS control (white) after orogastric inoculation with O1 El Tor N16961, with five replicates for each rabbit. ** and *** indicate P values of <0.01 and 0.001 based on two-way ANOVA with the Bonferroni post hoc test. Boxes and bars are means and SDs.
Adult Rabbits IM Immunized with Cholera MEFA Protein Showed a 2-log Reduction in Intestinal Colonization by the Challenge V. cholerae O1 or non-O1/non-O139 Strain.
Adult rabbits IM immunized with cholera MEFA protein, with or without dmLT adjuvant, showed a 2-log reduction in intestinal colonization by V. cholerae O1 El Tor N16961 or non-O1/non-O139 34 D-23 strain (Fig. 6B) (O1 classical and O139 Bengal strains were not examined). Rabbits remained normal and showed no diarrhea or fluid accumulation in the intestine or cecum after orogastric inoculation with V. cholerae O1 El Tor N16961. Vibrio bacteria recovered from the distal ileal segment (CFUs per gram) of the rabbits immunized with cholera MEFA alone or cholera MEFA and dmLT adjuvant were (3.4 ± 0.88) × 107 and (3.0 ± 0.89) × 106, respectively. This was more than 2 logs of reduction (>99%) compared with the rabbits injected with PBS (5.5 ± 1.54) × 109 CFUs (P < 0.001). PCR screening with TcpA-specific primers showed that the colonies were 100% positive for V. cholerae. Similarly, adult rabbits IM immunized with a reduced dose of cholera MEFA protein (100 µg dose, two doses) or orogastrically inoculated with Vaxchora (5 × 109 CFUs, two doses) showed over two logs of reduction in vibrio colonization of the small intestine when they were challenged with V. cholerae El Tor N16961 (SI Appendix, Fig. S4).
After challenge with non-O1/non-O139 strain El Tor 34-D 23, the rabbits IM immunized with cholera MEFA and dmLT adjuvant remained healthy and had (4.0 ± 1.6) × 107 CFUs V. cholerae isolated per gram of the distal ileal segment. In contrast, two of the four control rabbits shed loose feces and had yellowish fluid accumulated in the small intestine and cecum; the rabbits in this group had significantly more vibrio bacteria recovered from the distal segment of the small intestine (2.2 ± 1.5) × 109 (CFUs per gram) compared with the immunized rabbits (P <0.01).
Infant Rabbits Born to the Mothers IM Immunized with Cholera MEFA Protein Were Protected from Diarrhea after Challenge with O1, O139, or non-O1/non-O139 V. cholera.
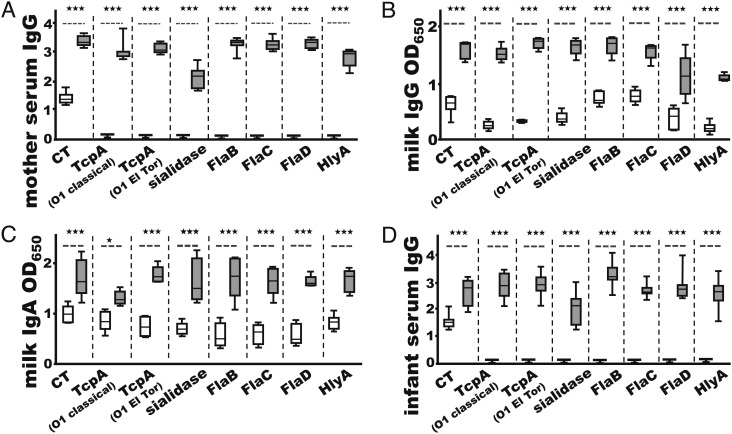
Thirteen rabbits of timed pregnancy were included to reproduce infant rabbits for assessing the preclinical efficacy of cholera MEFA-derived antibodies against clinical diarrhea. With two immunized rabbits showing false pregnancy, five immunized (with cholera MEFA protein and dmLT adjuvant) and six control (PBS and dmLT adjuvant) mothers delivered 34 and 40 infant rabbits, respectively. After IM administration with two doses of cholera MEFA protein and adjuvant dmLT, robust IgG (titers in log10) in serum samples and moderate IgG and IgA (OD650 values were shown due to high background) in milk samples, to all of the targeted virulence factors except LPS, were detected from the immunized mother rabbits (Fig. 7 A–C). Antigen-specific IgG antibodies (except anti-LPS) were detected from the sera of the infant rabbits born to the immunized mothers (Fig. 7D). In contrast, no antigen-specific IgG or IgA antibodies, except moderate anti-CT serum IgG, were detected from the control mother rabbits (which were IM injected with PBS and dmLT adjuvant) or the infant rabbits born to the control mothers.
Fig. 7.
IgG and IgA responses from the pregnant rabbits immunized with cholera MEFA and dmLT adjuvant (gray box) or PBS and dmLT (white box) or from the infant rabbits born to immunized (gray box) or control (white box) rabbits. (A) Antigen-specific IgG titers (log10) from the sera of the immunized (n = 7) or control (n = 6) mother rabbits. (B) The OD650 values of IgG ELISAs from the milk samples of the immunized (n = 5) or the control (n = 6) mother rabbits. (C) The OD650 values of IgA ELISAs from the milk samples of the immunized (n = 5) or the control (n = 6) mother rabbits. (D) Antigen-specific IgG titers (log10) from the serum samples of infant rabbits born to the immunized (n = 34) or control (n = 40) mothers. * and *** indicate P values <0.05 and <0.001, respectively, based on two-way ANOVA with the Bonferroni post hoc test. Boxes and bars are means and SDs.
The infant rabbits born to the immunized mothers or the control mothers were divided into four groups and infected with V. cholerae O1 El Tor N16961, O1 classical O395 strain, O139 Bengal strain, or non-O1/non-O139 El Tor 34-D 23 strain. After orogastric inoculation, the infant rabbits born to the immunized mothers were protected from diarrhea. Only 3 (of 15 challenged with V. cholerae El tor N16961) and 1 (of 6 inoculated with O139 Bengal strain) infant rabbits born to immunized mothers developed mild diarrhea. In contrast, all of the infant rabbits (n = 40) born to the control mothers (IM immunized with PBS and dmLT adjuvant) developed diarrhea (6 with mild diarrhea and 34 with severe diarrhea) after the challenge with the four serogroup strains (Table 1). The efficacy against any diarrhea was 88.4%, with 80%, 100%, 83.3%, and 100% for V. cholerae O1 El Tor N16961, O1 classical O395 strain, O139 Bengal strain, and non-O1/non-O139 El Tor 34-D 23 individually. The efficacy against severe diarrhea from these four V. cholerae strains was 100%.
Table 1.
Infant rabbit passive protection model to show cholera MEFA-induced antibodies for cross-protection against V. cholerae O1, O139, and non-O1/non-O139 challenge
| Infant rabbits | No. of rabbits with clinical outcome | % efficacy against | |||||
|---|---|---|---|---|---|---|---|
| Clinical outcome | Healthy | Mild diarrhea | Severe diarrhea | Any diarrhea | Severe diarrhea | Infant rabbit daily wt. gain (%) | V. cholerae colonized at small intestine (CFUs) |
| Challenged with O1 El Tor N16961 | |||||||
| Immunized (n = 15) | 12 | 3 | 0 | 80 | 100 | 1.54 ± 3.64*** | 2.4 ± 1.6 (×106)*** |
| Control (n = 16) | 0 | 2 | 14 | −18.0 ± 3.92 | 1.7 ± 0.7 (×109) | ||
| Challenged with O1 classical O395 | |||||||
| Immunized (n = 7) | 7 | 0 | 0 | 100 | 100 | 6.84 ± 6.51*** | 1.0 ± 1.3 (×106)*** |
| Control (n = 9) | 0 | 0 | 9 | −17.92 ± 6.05 | 4.0 ± 1.7 (×108) | ||
| Challenged with O139 (Bengal) | |||||||
| Immunized (n = 6) | 5 | 1 | 0 | 83.3 | 100 | 1.89 ± 1.49*** | 1.9 ± 0.6 (×106)** |
| Control (n = 8) | 0 | 3 | 5 | −20.57 ± 8.43 | 7.8 ± 6.9 (×108) | ||
| Challenged with non-O1/non-O139 El Tor 34-D 23 | |||||||
| Immunized (n = 6) | 6 | 0 | 0 | 100 | 100 | 2.16 ± 1.00*** | 1.5 ± 0.9 (×106)* |
| Control (n = 7) | 0 | 1 | 6 | −13.91 ± 6.64 | 2.5 ± 4.6 (×109) | ||
NZW rabbits of timed pregnancy were IM immunized with cholera MEFA protein (adjuvanted with dmLT) (n = 5) or PBS (with dmLT adjuvant as the control) (n = 6), and the born infant rabbits were orogastrically inoculated with V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 strain El Tor 34-D 23. Mild diarrhea was defined as yellow-stained hindquarters or pasty feces, and severe diarrhea is defined as liquid stool and loss of 20% of body weight within 24 h. Efficacy = [(% with diarrhea in the control group − % with diarrhea in the immunized group)/% with diarrhea in the control group] × 100. *, **, and *** indicate P values less than 0.05, 0.01, and 0.001, respectively.
Additionally, the infant rabbits born to the immunized mothers showed a positive weight gain 24-h postchallenge (3.1 ± 2.5; %), whereas the infant rabbits born to the control mothers lost body weight (−17.6 ± 2.8; P < 0.001). Furthermore, the infant rabbits born to the immunized mothers had a 2-log reduction in vibrio bacteria colonization of small intestines compared with the infant rabbits born to the control mothers; the reduction was 99.9%, 99.7%, 99.8%, and 99.9%, respectively, for the challenge strain V. cholerae O1 El Tor N16961, O1 classical O395 strain, O139 Bengal strain, and non-O1/non-O139 El Tor 34-D 23 (Table 1).
Discussion
Cholera progresses from V. cholerae adherence to host receptors on the epithelial cells and colonization of the small intestine, followed by the delivery of CT into epithelial cells. CT secreted by bacteria binds to host GM1 receptors (by CTB pentamer) and enters host cells to elevate intracellular cyclic AMP levels (by the CTA subunit), causing a massive efflux of fluid into the intestinal lumen and clinical cholera. A vaccine can prevent cholera if it induces antibodies to prevent this cascade of events by preventing V. cholerae adherence to host small intestinal enterocytes and colonization of small intestines, blocking CT from binding to host GM1 receptors, and neutralizing CT enterotoxicity. Data from this study showed that a polyvalent multiepitope fusion protein induces broadly functional antibodies to multiple V. cholerae virulence factors and protects rabbits from V. cholerae intestinal colonization and clinical diarrhea. Similar to cholera patients after natural exposure to V. cholerae (35), mice and rabbits IM immunized with this cholera MEFA protein developed robust antibody responses to CT, TcpA, HlyA, FlaB, FlaC, FlaD, and sialidase. The MEFA-derived antibodies neutralized CT enterotoxicity, blocked CT from binding to GM1 receptor, impaired V. cholerae motility, and inhibited adherence of O1, O139, and non-O1/non-O139 serogroup strains. Moreover, intramuscular immunization of cholera MEFA protein leads to a 99% reduction in V. cholerae intestinal colonization in adult rabbits and passively protects infant rabbits from 88.4% any diarrhea and 100% severe diarrhea when challenged with an O1, an O139, or a non-O1/non-O139 V. cholerae strain. These results may suggest a potential application of this cholera MEFA protein immunogen for the development of a cross-protective protein-based cholera vaccine.
Cholera MEFA-induced antibodies are functional, demonstrated by antibody in vitro activities against CT enterotoxicity and GM1 binding, vibrio adherence, and bacterial motility, and the precise role of the antibodies to each target epitope can be further defined. Data from current studies showed that mouse or rabbit antibodies, derived from IM immunization of cholera MEFA alone (without dmLT adjuvant), neutralized CT enterotoxicity and blocked CT from binding to GM1 receptor, indicating that the representative CTA epitope or at least the CTB epitope is functional. CT enterotoxicity is mediated by the toxin A subunit, and CT binding to GM1 receptor is controlled by the B subunit pentamer; antibodies from a neutralizing CTA epitope can prevent CT from stimulating cAMP levels in epithelial cells, whereas antibodies from a protective CTB epitope block CT from binding to GM1. Blocking of binding to GM1 can prevent CT from entering host cells and thus CT toxic activity. The TcpA epitopes carried by this MEFA immunogen appear functional since MEFA-induced antibodies inhibited V. cholerae adherence. Similarly, the flagellin epitopes can be functional because MEFA-induced antibodies impaired vibrio motility, although it is unknown whether each flagellin epitope (FlaB, FlaC, or FlaD) plays a role or whether two or all of the three epitopes (potentially the remaining peptides of FlaB backbone) worked synergistically.
The OSP and hemolysin epitopes carried by this MEFA, by contrast, are not functional, and the functional activity of the sialidase epitope was not examined. Despite the two peptides mimicking V. cholerae O1 LPS O-antigen were reported to induce protective anti-LPS antibodies after being conjugated to bovine serum albumin (34), the current study showed that these mimotopes failed in inducing anti-LPS antibodies or vibriocidal antibodies when presented on this cholera MEFA protein. While a vibriocidal antibody titer is considered a marker of protection (12, 36), it is not a true correlate of protection (37, 38). Results from the current study suggest that protein antigens can contribute to immune protection. This cholera MEFA immunogen, nevertheless, can be improved to carry OSP mimotopes that induce vibriocidal antibodies or to be combined with protective OSP antigens or OSP conjugates. Additionally, although the sialidase or hemolysin epitope induced antigen-specific antibody response, antibody function against hemolytic activity was not detected, and assays to test anti-sialidase antibody functional activity were not developed in the laboratory. Ideally, using functional hemolysin and OSP epitopes, as well as better functional epitopes from the other virulence factors which can be identified from future empirical epitope mapping studies, can further improve the immunogenicity and protection of this polyvalent protein antigen.
An infant rabbit passive protection model and an adult rabbit colonization model were applied synergistically to characterize cholera MEFA antigen protection against V. cholerae small intestinal colonization and clinical diarrhea. Early studies indicated that rabbit small intestines can be colonized by O1 and non-O1 V. cholerae and that inoculation with V. cholerae protects against subsequent homologous or heterologous infections (39, 40). Moreover, rabbits and humans likely share similar or identical mechanisms of intestinal colonization by V. cholerae (41). Therefore, surgical or nonsurgical adult and infant rabbit models were applied to study V. cholerae intestinal colonization or clinical cholera in the past. In this study, we modified the adult rabbit colonization model by anesthetizing rabbits during orogastric inoculation and 2–3 h afterward to slow down peristalsis (instead of using opium to temporarily introduce hypoperistalsis) to improve V. cholerae intestinal colonization. This modification enabled V. cholerae to effectively colonize small intestines, shown by over 108 to 109 CFU (per gram of the ground distal ileum segment) V. cholerae colonized in rabbits 24-h postinoculation. Our results showed that immunization with cholera MEFA protein (with or without adjuvant dmLT or the live attenuated Vaxchora) reduced 99% intestinal colonization of V. cholerae O1 El Tor N16961 or non-O1/non-O139 strain 34 D-23. We need to point out that because V. cholerae colonizes the ileum more effectively, only rabbit distal ileal segments were collected to assess MEFA-mediated protection against V. cholerae colonization. Additional challenge studies using other serogroup strains including O1 classical O395 and O139 Bengal, at an increased sampling size, will allow us to better assess MEFA-mediated antibodies for cross-protection against V. cholerae intestinal colonization.
The infant rabbit passive protection model developed in this study may benefit cholera vaccine development. Currently, there are no suitable animal models to assess vaccine preclinical efficacy against symptomatic cholera. The adult rabbit model, although it can be used to assess protection against V. cholerae intestinal colonization, is unable to evaluate protection against clinical diarrhea or cholera because adult rabbits rarely develop or sustain cholera unless an extremely high inoculum dose (1011 bacteria) is used (40). In contrast, infant rabbits developed diarrhea after V. cholerae inoculation, and they have been used to evaluate a live vaccine candidate (42); however, such protection against clinical diarrhea in infant rabbits was from the probiotic effect or physical exclusion from the vaccine live bacteria but not vaccine-induced immunity. By immunizing mother rabbits and challenging the suckling newborn, this infant rabbit passive protection model allows us to measure protection from vaccine-induced antibodies against clinical disease and thus evaluate the candidacy of this polyvalent immunogen for a cross-protective cholera vaccine. While the current infant rabbit passive protection model represents an applicable animal model to assess the preclinical efficacy of cholera vaccine candidates, it measures protection from passively acquired antibodies rather than active immunity. Future studies with a WHO-prequalified OCV product as the reference can further validate this infant rabbit passive protection model and its application in evaluating the efficacy of this cholera MEFA vaccine candidate. Eventually, controlled human infection model studies will determine the protection of this protein-based vaccine candidate against clinical cholera.
Although the mechanism and correlates to protection against cholera are not fully understood, IgA antibodies particularly mucosal secretory IgA (sIgA), even at a low level, are believed to play an important role in persistent protection against cholera (28). While we were unable to collect enough fecal or intestinal wash samples to titrate sIgA antibodies in the current study, we detected mild IgA antibody responses from the cecum content or milk samples of the rabbits immunized with the cholera MEFA protein. Rabbit sIgA to flagellins (B, C, and D), TcpA, and CT may play roles in inhibiting V. cholerae motility, virulence, and adherence to host receptors and thus preventing V. cholerae from intestinal colonization, and the passive IgA (and IgG) antibodies likely contributed to cross-protection of infant rabbits from V. cholerae intestinal colonization and clinical diarrhea.
The double-mutant heat-labile toxin of enterotoxigenic E. coli (dmLT, LTR192G/L211A) used as the adjuvant also served as an antigen. Because of the genetic and antigenic homology between LT and CT, anti-LT antibodies from adjuvant dmLT can supplement MEFA-induced anti-CT antibodies to neutralize CT enterotoxicity and prevent CT from binding to GM1. Indeed, mice or rabbits immunized with cholera MEFA and dmLT adjuvant developed greater anti-CT IgG titers (Figs. 2 and 5). CT was reported to enhance V. cholerae intestinal colonization in adult rabbits (41); therefore, anti-LT antibodies derived from dmLT adjuvant likely assisted MEFA-induced anti-CT antibodies in inhibiting V. cholerae bacterial colonization in rabbit small intestines. Rabbit sera of the group immunized with cholera MEFA and dmLT adjuvant exhibited significantly better activities against adherence from O1, O139, and non-O1/non-O139 strains (Fig. 6A) compared with the sera of the rabbits immunized with cholera MEFA alone (a lack of significance in antibody adherence inhibition from mouse sera in Fig. 3 is likely caused by use of a lower dmLT adjuvant dose, 0.1 µg dmLT used in mice versus 1 µg in rabbits). More, when challenged with strain O1 El Tor N16961, the rabbit immunized with cholera MEFA and dmLT adjuvant showed one more log reduction in bacterial colonization of the small intestine than the rabbits immunized without dmLT adjuvant (Fig. 6B). Passive antibodies from dmLT adjuvant (at a dose of 1 µg) alone, however, were insufficient to protect infant rabbits from clinical diarrhea, shown by the infant rabbits born to the mothers immunized with PBS and dmLT developed severe diarrhea after the challenge with V. cholerae. Whether the passive anti-dmLT antibodies alleviated disease severity was unknown since a group immunized with PBS only was not included in the infant rabbit passive protection study.
Data from this study support the application of the epitope- and structure-based MEFA vaccinology platform for the construction of broadly protective polyvalent immunogens. This MEFA vaccinology platform combines epitope vaccinology and structural vaccinology concept and has been used to construct polyvalent immunogens for the development of a broadly protective vaccine against enterotoxigenic E. coli diarrhea (43–45). Results from the current study showed that IM administration of polyvalent cholera MEFA protein elicited broadly functional antibodies in mice and rabbits. This cholera MEFA protein, ideally improved with functional LPS mimotopes and hemolysin epitope(s), can be explored as an add-on to a parenteral vaccine or be combined with other polyvalent antigens, for example, ETEC MEFA antigens 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV(MecVax) (46), for the potential development of a combination vaccine against two or more groups of enteric pathogens.
Protection from protein antigens in the absence of an immunity to OSP found in this study and an early study (47) argues with our current understanding of immune protection from cholera. Previously, inoculation with a live attenuated vaccine Vaxchora induced protection against a wild-type O1 V. cholerae but not O139 (48), and patients convalescent from cholera due to serogroup O1 or O139 V. cholerae developed vibriocidal antibodies only to the homologous serogroup (49), indicating that protection is serogroup specific and mainly because of OSP. Although current OCVs including Vaxchora also carry most of the virulence proteins targeted by this cholera MEFA protein and the fact that natural exposure to V. cholerae does not provide cross-protection, the high dose of specific antigens (represented by epitopes) and perhaps the absence of immune mask effect from excessive somatic antigens may amplify the protection of this cholera MEFA protein, leading to protection against different V. cholerae serogroups.
Methods and Materials
Ethics Statement.
Animal immunization and challenge studies complied with the Animal Welfare Act (1996 National Research Council Guidelines), the United State Department of Agriculture Animal Welfare Act Regulations, and the public health service Policy on Humane Care and Use of Laboratory Animals. Protocols were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign; animal studies were supervised by institutional attending veterinarians and staff.
Bacteria and Plasmids.
V. cholerae and recombinant E. coli strains used in this study are listed in SI Appendix, Table S1. V. cholerae O1, O139, and non-O1/non-O139 strains provided by the Biodefense and Emerging Infections Resources Repository (BEI Resources, Manassas, VA) were used for PCR amplification of virulence genes tcpA, flaB, flaC, flaD, nanH, and hlyA (PCR primers in SI Appendix, Table S2), in vitro antibody function assays, adult rabbit colonization, and infant rabbit challenge studies. Vector pET28α (Novagen, Madison, WI) and E. coli BL21-CodonPlus (DE3) RP strain (Agilent Technologies, Santa Clara, CA) were used to express cholera MEFA and V. cholerae virulence factor proteins.
Cholera MEFA Gene Construction and Expression.
Backbone protein and immunodominant B cell epitopes from CT, TcpA, FlaB, FlaC, FlaD, sialidase, and HlyA were identified in silico by using Immune Epitope Database epitope prediction program (https://www.iedb.org) (50), protein homology/analogY Recognition Engine (51), and Python Moledular Modelling (PyMol) (https://www.pymol.org) (52). Linear epitopes with the highest antigenicity score and surface exposure were selected. Additionally, two peptides mimicking O1 OSP antigens (mimotopes) (34) were included. Epitope homology was examined among the four V. cholerae serogroup strains that were included in this study as well as strains randomly selected from the NCBI GenBank database, and the conserved epitopes were chosen for cholera MEFA construction. A second TcpA epitope was added to overcome the heterogeneity among serogroups.
To generate polyvalent cholera MEFA protein, we selected virulence factors that have been demonstrated to induce protective antibodies or to mount dominant host immune responses after V. cholerae infection. Using FlaB as the backbone, we replaced the surface-exposed and immunodominant backbone epitopes (except the most immunodominant FlaB epitope) with linear epitopes of CT, TcpA, FlaC (or FlaD, an epitope shared by FlaC and FlaD), sialidase (neuraminidase), hemolysin A, and two OSP mimotopes. The resultant cholera MEFA was examined in silico for protein structure and stability with PyMol and Expert Protein Analysis System (https://www.expasy.org) and modified by alternating epitope positions on the backbone (53).
The cholera MEFA gene was synthesized and cloned into vector pUC57 after codon optimization by GenScript, then subcloned into vector pET28α, and expressed in E. coli BL21-CodonPlus (DE3) RP strain. To extract recombinant cholera MEFA protein, a single colony grown overnight at an Lysogeny Broth/Kan agar plate was cultured in 5 mL Lysogeny Broth containing kanamycin (30 μg/ml) in a 37°C incubator shaker (220 rpm). Then, 3 mL overnight growth was transferred to 200 mL 2 × Yeast Extract Tryptone (YT; Fisher Scientific) with kanamycin (30 μg/mL) and grew for approximately 3 h or until the OD595 reached 0.5 to 0.7. Bacteria were then induced with isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma; 1 mM) for 4 h and were harvested by centrifugation (13,000 × g) at 4°C for 15 min. Bacterial pellets frozen overnight in a −80°C freezer were thawed and incubated with 10 mL bacterial protein extraction reagent (B-PER; Thermo Fisher Scientific). Bacterial suspension, after vigorous vortex, was incubated at 4°C on a shaker (120 rpm) for 30 min and sonicated on ice. Bacterial lysates were centrifuged (16,200 × g at 4°C for 15 min), and inclusion body proteins were extracted using B-PER by following the manufacturer’s protocol.
Inclusion body proteins were solubilized and refolded by following the manufacturer’s protocol (Novagen). Briefly, inclusion body protein suspension was incubated with 50-volume freshly made 1× IB solubilization buffer (50 mM CAPS, pH 11.0) supplemented with 0.3% N-lauroylsarcosine and 1 mM dithiothreitol (DTT) at room temperature for 1 h. Solubilized protein in the supernatant was collected after centrifugation (16,200 × g for 10 min), transferred to molecular porous membrane tubing (Spectrum Laboratories, Inc., Rancho Dominguez), and dialyzed in dialysis buffer (1M Tris-HCl at pH 8.5) supplemented with DTT (0.1 mM in final concentration) at 4°C for 4 h. After two exchanges of dialysis buffer without DTT, approximately 8 h apart, refolded protein in the supernatant was collected and stored in a −80°C freezer.
Cholera MEFA protein was characterized in 15% sodium dodecyl SDS-PAGE with Coomassie blue staining and western blot using rabbit anti-CT polyclonal antibodies (Sigma) and anti-TcpA antisera.
Mouse Intramuscular Immunization with Cholera MEFA Protein.
Eight-wk-old female BALB/c mice (Charles River Laboratories International, Inc.), in three groups (eight mice per group), were IM immunized with 25 µg cholera MEFA protein (in 25 µL PBS), 25 µg cholera MEFA protein (in 25 µL PBS) and 0.1 µg adjuvant dmLT (in 1 µL; double-mutant heat-labile toxin of ETEC, LTR192G/L211A supplied by PATH), or 25 µL PBS as the control. Two boosters of the same dose as the primary were followed at an interval of 2 wk. All mice were euthanized 2 wk after the second booster. Serum samples were collected from each mouse and stored at −20°C until use.
In a second study, four groups of mice, eight per group, were IM immunized with PBS as the control, 10 µg MEFA protein or 25 µg MEFA protein, or orogastrically immunized with 100 µL Shanchol (Shantha), with three doses at a 3-wk interval.
Mouse Serum Antigen-Specific Antibody Titration.
Mouse serum IgG responses to each virulence factor targeted by cholera MEFA were titrated in ELISAs, and 96-well microtiter 2HB plates (Thermo Fisher Scientific) coated with CT (Sigma), recombinant protein TcpA (of O1 El Tor), TcpA (of O1 classical), FlaB, FlaC, FlaD, sialidase (Nan H) or HlyA, or O1 LPS, 100 ng per well (in 100 µL coating buffer; 15 mM Na2CO3 and 35 mM NaHCO3 at pH 9.6) were incubated 1 h at 37°C, washed with PBS-0.05% Tween-20 (PBST), blocked with 10% skim milk (in PBST), and then incubated with two-fold serum dilutions (1:200 to 1:25,600) 1 h at 37°C in triplicates. Wells were washed and then incubated with horseradish peroxidase (HRP)–conjugated goat anti-mouse IgG (1:5,000; Sigma) for 1 h at 37°C. After three washes with PBST, wells were incubated with the 3,3′,5,5′-tetramethylbenzidine (TMB) microwell peroxidase substrate system (2-C) (KPL). Antibody titers were calculated by multiplying the highest serum dilution which gave an OD650 that is three times the control OD or >0.3 (after subtraction with background OD value, typically 0.05–0.1) with the adjusted OD and expressed in a log10 scale (44, 54–56).
Mouse Serum Antibody In Vitro Assays.
Mouse serum samples were examined for antibody functional activities against CT enterotoxicity, CT binding to GM1, bacterial adherence, bacterial motility, vibriocidal activity, and hemolytic activities.
Neutralization against CT enterotoxicity.
Cyclic AMP EIA Kit (Enzo Life Sciences) and T-84 cells American Type Culture Collection (ATCC, #CCL-248) were used to examine mouse serum antibody neutralization against CT enterotoxicity. As described (57, 58), T84 cells (1–2 × 105 per well) cultured in a 24-well BD Falcon cell culture plate (Fisher Scientific), in Dulbecco's Modified EagleMedium/Nutrient Mixture/F12 medium containing 0.3 mM 3-Isobutylxanthine-1-Methyl (per well) to 95 to 100% confluence, were incubated with 10 ng CT (10 µL), which was premixed with 30 µL heat-inactivated mouse serum sample, in a CO2 incubator at 37°C for 3 h. After gentle washes with PBS, cells were lysed with 300 µL 0.1M HCl with 0.5% Triton X-100 on a shaker (130 rpm) at room temperature for 30 min and collected by centrifugation (1,000 x g for 10 min). Intracellular cAMP levels were measured following the manufacturer’s protocol (Enzo Life).
Blocking CT from binding to GM1.
Competitive GM1 ELISA was used to measure the cholera MEFA-induced (anti-CTB) antibodies against CT (B pentamer) binding to GM1. As described previously (59, 60), Maxisorb microtiter plates (Nunc) coated with GM1 ganglioside (Sigma; 80 ng per well) overnight at 4°C were washed with PBST and incubated with 5% skim milk at 37°C for 1 h. After 3× washes with PBST, wells were incubated with CT (20 ng) and mouse serum dilutions (1:200–1:12,800). Washed with PBST, wells were incubated with rabbit anti-CT polyclonal antiserum (Sigma; 1:3,000), followed by HRP-conjugated goat-anti-rabbit secondary antibodies (Sigma; 1:5,000 dilution). OD650 was measured with TMB peroxidase substrate system (2-C) (KPL).
Vibrio adherence inhibition.
Mouse serum antibody functional activity against V. cholerae adherence was examined with Caco-2 cells (ATCC #HT-37). As described (43, 54–56), with minor modification, V. cholerae strain O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 El Tor 34-D 23 (1.25 × 104 bacteria), after incubation with 15 µL mouse serum sample (heat-inactivated) at room temperature for 30 min on a shaker incubator (50 rpm), was added to 95 to 100% confluent Caco-2 cells and incubated in a 37°C CO2 incubator for 1 h. Cells were washed (to remove nonadherent bacteria), dislodged, and harvested. Collected cells (with adherent bacteria) were suspended, serially diluted, and plated on LB agar plates. V. cholerae bacteria (CFUs) were counted after overnight growth at 30°C.
Vibrio motility inhibition.
Ten microliter V. cholerae bacteria (of OD 0.1) incubated with heat-inactivated mouse serum dilution (40 µL) at room temperature for 30 min were transferred to a glass slide. Bacterial motility was visually examined using a microscope (Zeiss Axiovert 200M with the Apotome Structured Illumination Optical Sectioning System) at 100X objective and recorded with Axiocam 506 with a high-resolution black and white camera.
Vibriocidal and hemolytic activity.
Serum samples from different treatment groups were examined for vibriocidal activity. Briefly, 25 µL (in 1:10 initial dilution) heat-inactivated serum samples were twofold serially diluted in a 96-well flat-bottom microtiter plate, mixed with an indicator [a bacteria–complement–saline mixture composed of 150 µL V. cholerae (1 × 106 CFU, OD600 = 0.3), 300 µL guinea pig complement serum, and 2,550 µL PBS, for a full plate], 25 µL per well, and incubated at 37°C for 1 h on a shaker (50 rpm). After being added with brain heart infusion broth (150 µL per well), the mixture was cultured for 2 to 4 h at 37°C until OD595 in the growth control well (no serum) reached 0.2 to 0.28. The highest serum dilution with greater than 50% OD reduction was considered the vibriocidal antibody titer.
To examine antibody function against the hemolytic activity, V. cholerae inoculum was streaked on a TSA w/5% sheep blood agar plate (Thermo Scientific), which was spread with 100 µL heat-inactivated mouse (or rabbit) sera. Cultured at 30°C for 24 to 36 h, colonies were visually examined for hemolytic characteristics (61).
Adult Rabbit Immunization and Challenge, a Rabbit Colonization Model to Assess Antibody Protection against Vibrio Colonization of the Small Intestine.
Adult rabbits were IM immunized with cholera MEFA protein and then orogastrically challenged with V. cholerae serogroup strains. Rabbit serum and cecum content samples were collected and examined for antigen-specific antibody responses, and rabbit intestinal distal segments were collected to quantify vibrio colonization to assess the protection of MEFA-induced antibodies against V. cholerae intestinal colonization.
Rabbit immunization.
Twenty New Zealand White (NZW) rabbits weighed between 1.5 and 2 kg (Charles River Laboratories) were divided into five groups, four rabbits per group, and included in two studies. In the first study, one group was IM immunized with 200 µg cholera MEFA protein (in 200 µL) and 1 µg dmLT adjuvant (1 µL), and the other was IM injected with 200 µL PBS as the control. In the second study to examine whether dmLT adjuvant enhances MEFA in vivo protection against V. cholerae intestinal colonization, three groups were included for IM immunization, cholera MEFA alone, cholera MEFA and adjuvant dmLT, and PBS (as the control). Two booster injections were followed with the same dose as the primary at an interval of 2 wk. Serum samples were collected from each rabbit before the primary and every 2 wk afterward and stored at −80°C until use.
In a separate pilot study, two rabbits per group were IM administered with 100 µg cholera MEFA protein or 100 µL PBS (as the control), or orogastrically inoculated with Vaxchora (5 × 109 CFUs), at a regimen of two doses (one primary and one booster) and a 2-wk interval.
Rabbit orogastric challenge with V. cholera.
Two weeks after the final dose, each rabbit was orogastrically inoculated with 5 × 1010 CFU V. cholerae O1 El Tor strain (N16961) or non-O1/non-O139 strain (34-D 23) (in 1 mL PBS). Rabbits were IV administered with famotidine (Pepcid, 0.75 mg per kg body weight) via ear vein 3 h before inoculation, sedated with dexmedetomidine (0.10 mg per kg body weight; IM), and anesthetized with inhalation of 3 to 5% isoflurane. With a 16’ intermittent red rubber catheter, rabbits were administered with 3 mL 5% sodium bicarbonate, then bacterial inoculum (5 × 1010 CFUs in 1 ml PBS), and followed with 3 mL sodium bicarbonate. Rabbits were monitored for abnormal signs including loose feces 24-h postinoculation.
Rabbits were euthanized 24-h postinoculation. Rabbits were sedated with dexmedetomidine, followed by inhalation of isoflurane, then exsanguinated with a cardiac puncture, and finally intracardiac injection of KCl (2 mg/mL). At necropsy, fecal formation and fluid accumulation in intestines and cecum were examined, and the distal ileal segment (10 cm) and cecum content were collected.
Rabbit antibody titration and antibody in vitro protection.
Rabbit serum and cecum content suspension samples were examined in ELISAs for IgG and IgA responses to the virulence factors targeted by the MEFA immunogen, as described in the above mouse antibody titration except for the use of HRP-conjugated goat anti-rabbit IgG and IgA secondary antibodies. Rabbit cecum content was suspended in fecal suspension buffer (10 mM Tris, 100 mM NaCl, 0.05% Tween-20, and 5 mM sodium azide at pH 7.4) supplemented with 0.5 mM phenylmethylsulfonyl fluoride and one-gram cecum content in 5 mL buffer (1:6 dilution). After centrifugation, the supernatant was collected and used for antibody titration.
Rabbit serum samples were examined for antibody functional activities against CT enterotoxicity, CT binding to GM1, vibrio adherence, vibrio motility, and vibriocidal and hemolytic activities, as described in the above mouse serum antibody in vitro assays. Cecum content suspensions were also used for bacterial adherence inhibition assays.
Rabbit small intestine quantitative colonization assay.
The distal ileal segment collected from each rabbit at necropsy was cut open and ground in PBS (1 g tissue in 9 mL sterile PBS) in a glass grinder after a gentle but thorough rinse with PBS to remove feces as we previously described in pig quantitative colonization assay (59). The homogenized solution was serially diluted and plated on LB agar plates. Bacteria (CFUs) grown overnight at 30°C were counted and recorded. Twenty colonies randomly selected from a plate were screened in PCRs with TcpA- or CT-specific primers to verify V. cholerae.
Immunization of Pregnant Rabbits with Cholera MEFA Protein and Challenge of the Born Infant Rabbits with V. cholerae, an Infant Rabbit Passive Protection Model to Assess Preclinical Efficacy against Clinical Diarrhea.
We developed an infant rabbit passive protection model to evaluate cholera MEFA-induced antibodies for cross-protection against clinical diarrhea by immunizing pregnant rabbits with the MEFA protein and challenging born infant rabbits with different V. cholerae serotype strains.
Pregnant rabbit immunization.
NZW rabbits of timed pregnancy (Charlies River Laboratories) were randomly divided into two groups. Four days after mating, one group was IM immunized with 250 µg cholera MEFA protein (in 250 µL) and 1 µg dmLT adjuvant (1 µL) and the other group with 250 µL PBS and 1 µg dmLT as the control. One booster was followed 2 wk later at the same dose as the primary. Sera collected before the primary and 2 wk after the booster and milk collected at necropsy were examined in ELISAs for IgG and IgA responses to the target virulence factors.
Infant rabbit orogastric challenge with different V. cholerae serogroup strains.
After suckling for 3 d, the infant rabbits born to the immunized or the control mother rabbits were anesthetized with IV administration of 50 to 100 µL famotidine 2-3 h before the challenge inoculation. Anesthetized infant rabbits received 250 µL 5% sodium carbonate before orogastric inoculation with V. cholerae O1 El Tor N16961, O1 classical O395, O139 Bengal, or non-O1/non-O139 El Tor 34-D 23 (2.5x109 CFUs in 500 µL PBS) and then 250 µL 5% sodium carbonate after the vibrio challenge.
The challenged infant rabbits were monitored every 6 h during the first 12 h and then hourly afterward during 24-h postinoculation. Clinical outcomes were recorded as cholera or severe diarrhea (profuse diarrhea and dehydration and loss of 20% body weight), watery diarrhea (watery feces), moderate diarrhea (loose and unformed feces), mild diarrhea (semiformed feces), or normal (with formed fecal pellets). Infant rabbits were euthanized 24-h postinoculation or earlier if they developed cholera or severe dehydration. All rabbits were measured for body weight before the challenge and at 6-, 18-, and 24-h postinoculation or the time of euthanasia.
At necropsy, fecal formation and fluid accumulation in the small intestine, colon, and cecum were examined and recorded; additionally, blood and an ileal distal segment were collected from each infant rabbit. Serum samples were titrated for IgG or IgA responses to the virulence factors targeted by the cholera MEFA protein. Ileal distal segments were homogenized, diluted, and plated on LB agar plates. After overnight growth at 30°C, CFUs were counted. Twenty colonies were screened in PCR with primers specific to TcpA.
Assessment of antibody passive protection against clinical cholera or diarrhea.
Antigen-specific antibody responses from the immunized mothers and the born infant rabbits were titrated and examined for correlates to clinical protection in infant rabbits, including reduction in V. cholerae intestinal colonization, prevention of weight loss, and most importantly protection against clinical diarrhea.
Statistical Analyses.
Antibody titration, antibody neutralization activity, and rabbit colonization data were presented as means and SDs. Statistical differences among treatment groups were analyzed using two-way ANOVA with the Bonferroni post hoc test or one-way ANOVA with Tukey’s test, based on the number of the variance, by using GraphPad Prism 7 software (San Diego, CA). A calculated P value of less than 0.05 indicated a significant difference.
Supplementary Material
Appendix 01 (PDF)
Microscopic video clips to show antibody inhibition activity against motility of Vibrio cholerae O1 El Tor N16961. Bacteria (10 μl, OD = 0.1), after incubation with PBS (no sera), or the sera of the mice IM immunized with PBS (control sera) or the cholera MEFA protein and adjuvant dmLT (MEFA/dmLT sera), were observed under a Zeiss Axiovert 200M microscope with the Apotome Structured Illumination Optical Sectioning System. Video was recorded with Axiocam 506 using a high-resolution black and white camera.
Acknowledgments
The following reagents were obtained through BEIResources, NIAID, NIH: Vibrio cholerae, Strain N16961, NR-147; Vibrio cholerae, Strain 395, NR-9906; Vibrio cholerae, Strain MO45, NR-144; Vibrio cholerae, Strain El Tor 34-D 23,NR-150; and genomic DNA of Vibriocholerae, Strain 395. We thank Dr. Nicole L. Herndon (University of Illinois,Veterinary Medicine College) for her assistance in rabbit studies. This work issupported by the University of Illinois at Urbana-Champaign and NIHR01AI121067-01A1.
Author contributions
D.A.S. and W.Z. designed research; I.U., S.L., G.P., and H.S. performed research; I.U. analyzed data; and D.A.S. and W.Z. wrote the paper.
Competing interests
The authors have patent filings to disclose: PCT_US2020_023521, the cholera MEFA immunogen and its application as a subunit vaccine against cholera.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Ali M., Nelson A. R., Lopez A. L., Sack D. A., Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 9, e0003832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization W. H., Cholera vaccines: WHO position paper - August 2017. Wkly. Epidemiol. Rec. 92, 477–498 (2017). [PubMed] [Google Scholar]
- 3.Taniuchi M., et al. , Etiology of diarrhea requiring hospitalization in Bangladesh by quantitative PCR, 2014–2018. Clin. Infect. Dis. 73, e2493–e2499 (2020), 10.1093/cid/ciaa840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris J. G. Jr., et al. , Non-O group 1 Vibrio cholerae gastroenteritis in the United States: Clinical, epidemiologic, and laboratory characteristics of sporadic cases. Ann. Intern. Med. 94, 656–658 (1981). [DOI] [PubMed] [Google Scholar]
- 5.Dutta D., et al. , Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerg. Infect. Dis. 19, 464–467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S. K., et al. , 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: A cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 13, 1050–1056 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Franke M. F., et al. , Long-term effectiveness of one and two doses of a killed, bivalent, whole-cell oral cholera vaccine in Haiti: An extended case-control study. Lancet Glob. Health 6, e1028–e1035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi Q., et al. , Protection against cholera from killed whole-cell oral cholera vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 17, 1080–1088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens J. D., et al. , Field trial of oral cholera vaccines in Bangladesh: Results from three-year follow-up. Lancet 335, 270–273 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Luquero F. J., Azman A. A., Protection of young children with cholera vaccine. Lancet Infect. Dis. 18, 947–948 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Qadri F., et al. , Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: Results from 2 years of follow-up of a randomised trial. Lancet Infect Dis. 18, 666–674 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Levine M. M., et al. , PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Expert Rev. Vaccines 16, 197–213 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Price G. A., Holmes R. K., Immunizing adult female mice with a TcpA-A2-CTB chimera provides a high level of protection for their pups in the infant mouse model of cholera. PLoS Negl. Trop. Dis. 8, e3356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souod N., Kargar M., Hoseini M. H., Jafarinia M., Fusion-expressed CtxB-TcpA-C-CPE improves both systemic and mucosal humoral and T-cell responses against cholera in mice. Microb. Pathog. 157, 104978 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Molaee N., Mosayebi G., Amozande-Nobaveh A., Soleyman M. R., Abtahi H., Evolution of the immune response against recombinant proteins (TcpA, TcpB, and FlaA) as a candidate subunit cholera vaccine. J. Immunol. Res. 2017, 2412747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren J., Lonnroth I., Ouchterlony O., Identification and characterization of cholera exotoxin in culture filtrates of V. cholerae. Acta Pathol. Microbiol. Scand B Microbiol. Immu nol. 79, 448 (1971). [PubMed] [Google Scholar]
- 17.Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J., Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U.S.A. 84, 2833–2837 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrington D. A., et al. , Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168, 1487–1492 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaisar M. H., et al. , Vibrio cholerae sialidase-specific immune responses are associated with protection against cholera. mSphere 6, e01232-20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galen J. E., et al. , Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect. Immun. 60, 406–415 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almagro-Moreno S., Boyd E. F., Sialic acid catabolism confers a competitive advantage to pathogenic vibrio cholerae in the mouse intestine. Infect. Immun. 77, 3807–3816 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freter R., Allweiss B., O’Brien P. C., Halstead S. A., Macsai M. S., Role of chemotaxis in the association of motile bacteria with intestinal mucosa: In vitro studies. Infect. Immun. 34, 241–249 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson K., Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: Analysis of motility mutants in three animal models. Infect. Immun. 59, 2727–2736 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mekalanos J. J., Sadoff J. C., Cholera vaccines: Fighting an ancient scourge. Science 265, 1387–1389 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Watnick P. I., Lauriano C. M., Klose K. E., Croal L., Kolter R., The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39, 223–235 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueroa-Arredondo P., et al. , Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 69, 1613–1624 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauffman R. C., et al. , Single-cell analysis of the plasmablast response to vibrio cholerae demonstrates expansion of cross-reactive memory B cells. mBio 7, e02021-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris J. B., Cholera: Immunity and prospects in vaccine development. J. Infect. Dis. 218, S141–S146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jertborn M., Svennerholm A. M., Holmgren J., Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine 14, 1459–1465 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Shamsuzzaman S., et al. , Robust gut associated vaccine-specific antibody-secretingcell responses are detected at the mucosal surface of Bangladeshi subjectsafter immunization with an oral killed bivalent V. cholerae O1/O139 whole cellcholera vaccine: Comparison with other mucosal and systemic responses. Vaccine 27, 1386–1392 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Charles R. C., et al. , The plasma and mucosal antibody response to the complete vibrio cholerae O1 protein immunome and O-specific polysaccharide in adults with Inaba or Ogawa cholera in Bangladesh. Am. J. Trop. Med. Hygiene 95, 4 (2017). [Google Scholar]
- 32.Mosley W. H., Benenson A. S., Barui R., A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titer to the pattern of endemic cholera. Bull. World Health Organ. 38, 327–334 (1968). [PMC free article] [PubMed] [Google Scholar]
- 33.Li S., Lee K. H., Zhang W., Multiepitope fusion antigen: MEFA, an epitope- and structure-based vaccinology platform for multivalent vaccine development. Methods Mol. Biol. 2414, 151–169 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghazi F. M. P., Gargari S. L. M., Synthetic peptides mimicking lipopolysaccharide as a potential vaccine candidates against Vibrio cholerae serogroup O1. Iran. J. Microbiol. 9, 244–250 (2017). [PMC free article] [PubMed] [Google Scholar]
- 35.Aaboud M., et al. , Measurements of the production cross section of a [Formula: see text] boson in association with jets in pp collisions at [Formula: see text] TeV with the ATLAS detector. Eur. Phys. J. C Part Fields 77, 361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akter A., et al. , Induction of systemic, mucosal and memory antibody responses targeting Vibrio cholerae O1 O-specific polysaccharide (OSP) in adults following oral vaccination with an oral killed whole cell cholera vaccine in Bangladesh. PLoS Negl. Trop. Dis. 13, e0007634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha D., et al. , Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189, 2318–2322 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Haney D. J., et al. , Lipopolysaccharide-specific memory B cell responses to an attenuated live cholera vaccine are associated with protection against Vibrio cholerae infection. Vaccine 36, 2768–2773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spira W. M., Fedorkacray P. J., Pettebone P., Colonization of the rabbit small-intestine by clinical and environmental isolates of non-O1 Vibrio-cholerae and Vibrio-mimicus. Infect. Immun. 41, 1175–1183 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cray W. C., Tokunaga E., Pierce N. F., Successful colonization and immunization of adult-rabbits by oral inoculation with Vibrio-cholerae-O1. Infect. Immun. 41, 735–741 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C. Jr., Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect. Immun. 50, 813–816 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubbard T. P., et al. , A live vaccine rapidly protects against cholera in an infant rabbit model. Sci. Transl. Med. 10, eaap8423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan X., Knudsen D. E., Wollenberg K. M., Sack D. A., Zhang W., Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin. Vaccine Immunol. 21, 243–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nandre R., et al. , Enterotoxigenic Escherichia coli adhesin-toxoid multiepitope fusion antigen CFA/I/II/IV-3xSTaN12S-mnLTR192G/L211A-derived antibodies inhibit adherence of seven adhesins, neutralize enterotoxicity of LT and STa toxins, and protect piglets against diarrhea. Infect. Immun. 86, e00550-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan Q., et al. , A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 51, 93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo H., et al. , Preclinical characterization of immunogenicity and efficacy against diarrhea from MecVax, a multivalent enterotoxigenic E. coli vaccine candidate. Infect. Immun. 89, e0010621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price G. A., Holmes R. K., Immunizing adult female mice with a Tcpa-A2-CTB chimera provides a high level of protection for their pups in the infant mouse model of Cholera. PLoS Negl. Trop. Dis. 8, e3356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albert M. J., Alam K., Rahman A. S., Huda S., Sack R. B., Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169, 709–710 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Qadri F., et al. , Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65, 3571–3576 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vita R., et al. , The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J. E., The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janson G., Zhang C., Prado M. G., Paiardini A., PyMod 2.0: Improvements in protein sequence-structure analysis and homology modeling within PyMOL. Bioinformatics 33, 444–446 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Duan Q., et al. , MEFA (multiepitope fusion antigen)-novel technology for structural vaccinology, proof from computational and empirical immunogenicity characterization of an enterotoxigenic Escherichia coli (ETEC) adhesin MEFA. J. Vaccines Vaccin. 8, 367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan Q., et al. , Co-administered tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (multiepitope fusion antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1-CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front. Microbiol. 9, 1198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu T., Moxley R. A., Zhang W., Mapping the neutralizing epitopes of enterotoxigenic Escherichia coli (ETEC) K88 (F4) fimbrial adhesin and major subunit FaeG. Appl. Environ. Microbiol. 85, e00329-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo H., et al. , Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: Dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6). Hum. Vaccin. Immunother. 16, 419–425 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W. P., Francis D. H., Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin b (STb) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STb antibodies. Clin. Vaccine Immunol. 17, 1223–1231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruan X., et al. , Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect. Immun. 82, 1823–1832 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W. P., et al. , Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78, 316–325 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J. C., Duan Q. D., Zhang W. P., Significance of enterotoxigenic Escherichia coli (ETEC) heat-labile toxin (LT) enzymatic subunit epitopes in LT enterotoxicity and immunogenicity. Appl. Environ. Microbiol. 84, e00849-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buxton R., Blood Agar Plates and Hemolysis Protocols American Society for Microbiology, Washing. DC 1–9, (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Microscopic video clips to show antibody inhibition activity against motility of Vibrio cholerae O1 El Tor N16961. Bacteria (10 μl, OD = 0.1), after incubation with PBS (no sera), or the sera of the mice IM immunized with PBS (control sera) or the cholera MEFA protein and adjuvant dmLT (MEFA/dmLT sera), were observed under a Zeiss Axiovert 200M microscope with the Apotome Structured Illumination Optical Sectioning System. Video was recorded with Axiocam 506 using a high-resolution black and white camera.
Data Availability Statement
All study data are included in the article and/or SI Appendix.