About 50% of human genes contain multiple active alternative promoters (1). Since transcription and splicing are tightly coupled (2), transcripts from different promoters often lead to changes in exon inclusion and produce different splicing variants. Multiple promoters and alternative splicing greatly amplify the complexity of the protein variants and regulation within our limited human genome to meet the enormous need during human development or in response to environmental challenges. Unfortunately, cancer cells have evolved to highjack the usage of alternative promoters for their own gains (3). Notable examples include an alternative transcription initiation (ATI) isoform of anaplastic lymphoma kinase ALKATI in melanoma (4), an estrogen receptor isoform ERα36 in breast cancer (5, 6), and multiple isoforms of p53 family proteins in a wide variety of cancers (7), to name a few. Identification of new oncogenic protein isoforms in each type of cancer will certainly enhance our understanding of cancer development and more importantly provide new opportunities for therapy.
Triple-negative breast cancer (TNBC) does not express hormone receptors, such as estrogen receptor and progesterone receptor, and also does not have human epidermal growth factor receptor 2 (HER2) gene amplification. Therefore, patients with TNBC do not respond to endocrine therapy or anti-HER2 targeted therapy. Moreover, TNBC is usually more aggressive and tends to recur or metastasize. Thus, innovative approaches are urgently needed for TNBC treatment. In PNAS, Yang et al. (8) report a previously unidentified variant of macrophage receptor with a collagenous structure (MARCO) in some TNBCs and show that the presence of this variant is associated with poor patient outcomes but also with a better response to inhibitors of BET (bromodomain and extra-terminal motif).
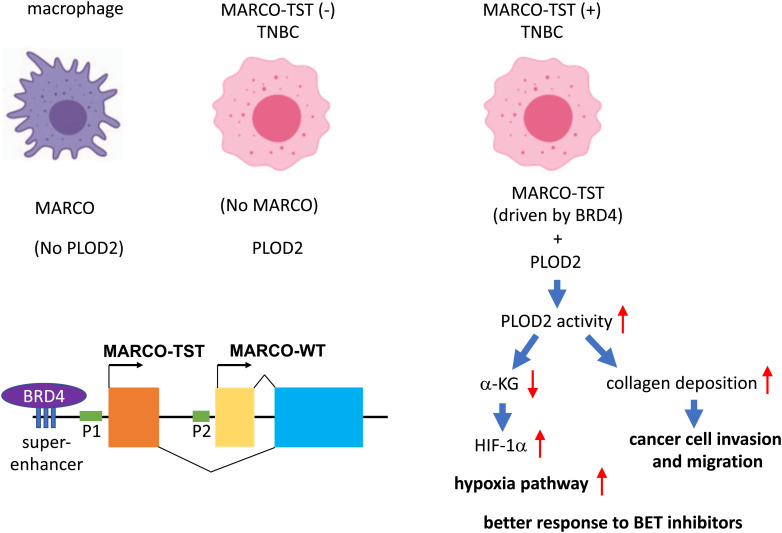
The authors carried out a comprehensive analysis of RNA splicing from 360 TNBC patients in Fudan University Shanghai Cancer Center TNBC (FUSCCTNBC) cohort and found that tumor-specific transcripts (TSTs) are associated with poor patient outcomes. Among 227 TSTs in TNBC, they focused on a new splicing variant of MARCO (named MARCO-TST), which showed the highest frequency (16.4%) in the FUSCCTNBC cohort. MARCO is a class A scavenger receptor present on the cell surface of some immune cells, such as macrophages, monocytes, and dendritic cells, and mediates opsonin-independent phagocytosis (9). There are eight classes (A-H) of scavenger receptors, all with the ability to bind polyanions for clearance (9). MARCO contains a cytoplasmic domain, a transmembrane domain (TM), a spacer domain, and a long extracellular fibrillary collagenous domain ended with a scavenger receptor cysteine-rich (SRCR) domain. MARCO binds exogenous pathogens (such as bacteria) or modified endogenous ligands (such as acetylated or oxidized low-density lipoprotein and apoptotic cells) and promotes phagocytosis and clearance of these ligands. MARCO-TST is derived from an alternative promoter and is expressed in some TNBCs but not in macrophages (Fig. 1). MARCO-TST and MARCO share the same TM, spacer, collagenous domain, and SRCR domain but have different cytoplasmic domains. The authors demonstrate that MACRO-TST can promote tumor progression of TNBC. Through transcriptome profiling and immunoprecipitation combined with stable isotope labeling with amino acids in cell culture (SILAC)-based quantitative mass spectrometry, they elucidate the mechanism of action.
Fig. 1.
In normal tissues, MARCO and procollagen-lysine, 2-oxoglutarate 5-dioxygenases 2 (PLOD2) are expressed in different cell types. MARCO is solely expressed in macrophages, monocytes, and dendritic cells, whereas PLOD2 is widely expressed in different cell types but not immune cells. In some TNBCs, the BRD4-driven super-enhancer activates an alternative promoter (P1) of MARCO, resulting in the transcription of a splicing variant MARCO-TST. MARCO-TST interacts with PLOD2 in TNBC and promotes PLOD2 dimerization and activation. Enhancement of PLOD2 activity can drive cancer progression by promoting collagen cross-linking. It also reduces the levels of intracellular α-KG and inhibits the degradation of HIF-1α, thereby activating the hypoxic response. Consequently, these tumors respond better to BETi.
MARCO-TST interacts with procollagen-lysine, 2-oxoglutarate 5-dioxygenases (PLOD), particularly PLOD2, to enhance hypoxia-inducible factor-1α (HIF-1α) stability. PLOD2 catalyzes the hydroxylation of lysyl residues in collagen to form stable collagen cross-links. The enzyme activity of PLOD2 depends on its dimerization (10). The interaction between MACRO-TST and PLOD2 enhances PLOD2 dimerization and therefore promotes collagen deposition. Upregulation of PLODs expression or activity promotes cancer progression and metastasis (11). Thus, MACRO-TST may promote tumor progression through enhancing the procollagen activity of PLOD2. Enhancement of PLOD2 activity also leads to depletion of α-ketoglutarate (α-KG) since α-KG is used as a cofactor in the hydroxylation of lysyl residues in collagen. On the other hand, the proline hydroxylation of HIF-1α by prolyl hydroxylation domain proteins also requires α-KG. Proline hydroxylation of HIF-1α induces VHL-dependent ubiquitination, leading to HIF-1α degradation (12). Thus, MARCO-TST overexpression also inhibits proline hydroxylation of HIF-1α and therefore stabilizes HIF-1α, which is known to be able to drive tumor progression (Fig. 1).
Since MARCO-TST and MARCO-wild-type (WT) share most of the functional domains (except for the cytoplasmic domain), MARCO-TST does not gain new functions. In fact, when ectopically expressed in tumor cells, both MARCO-TST and MARCO-WT share the same ability in PLOD2 binding and HIF-1α stabilization (8). In normal tissues, the expression of MARCO and PLOD2 is mutually exclusive (Fig. 1). MARCO is expressed in immune cells where PLOD2 is not present. On the other hand, PLOD2 is regulated by HIF-1α (13), E2 promoter binding factors (E2Fs) (14), etc., and is overexpressed in many types of cancer, such as breast cancer, bladder cancer, renal cell carcinoma, non-small cell lung cancer, etc. (11). Unlike MARCO-WT, the expression of MARCO-TST is aberrantly activated in some TNBCs by a separate promoter, which resides upstream of the promoter of MARCO-WT. Thus, PLOD2 is coexpressed with MARCO-TST in some TNBCs but not macrophages (Fig. 1). The interaction between MARCO-TST and PLOD2 and its functional consequences therefore only exist in MARCO-TST-expressing TNBC.
The authors went on to demonstrate that the expression of MARCO-TST is activated by a BRD4-driven super-enhancer and thus can be inhibited by BET inhibitors (BETi). The sensitivity to a BETi OTX015 is correlated with the expression of MARCO-TST among several TNBC patient-derived organoids, supporting a connection between MARCO-TST expression and BETi sensitivity. Super-enhancers are large clusters of enhancers that drive very high levels of transcription and often control genes important for cell-type specification (15). There is growing evidence supporting that super-enhancers also play a vital role in the tumorigenesis. BRD4, one of the BET protein family members, binds acetylated histones at transcription start sites and super-enhancers and mediates transcriptional coactivation and elongation via RNA polymerase II and a mediator. BETi disrupts the communication between super-enhancers and their target promoters along with a subsequent cell-specific repression of oncogenes, which is considered to be the main mechanism of sensitivity to BETi (16).
This study identifies an oncogenic splicing variant of MARCO in some TNBCs and elucidates the mechanism by which the aberrant expression of MARCO-TST activates the hypoxia pathway. It has significant clinical implications. Several BETi have been tested in clinical trials. But one of the challenges is to identify good biomarkers to predict response to BETi since not all of the overexpressed BET proteins are oncogenic drivers (17). This study suggests that MARCO-TST may serve as a biomarker to predict the BETi response in TNBC. Besides BETi, this study also suggests that the HIF-1 inhibitors can be used for the treatment of MARCO-TST-positive TNBC. Although HIF-2α was not studied in this paper, one would expect a similar stabilization of HIF-2α by MARCO-TST in these cancer cells given the essential role of proline hydroxylation in the degradation of both HIF-1α and HIF-2α. Nevertheless, the role of HIF-1α vs. HIF-2α in TNBC needs to be formally tested since both can have different or even contrasting activities (18). Should HIF-2α also play a role in these TNBC cells, a HIF-2α inhibitor belzutifan, which was approved by FDA recently (19), can be immediately tested in clinical trials for MARCO-TST-positive TNBC. Furthermore, the unique cytoplasmic domain in MARCO-TST may serve as a tumor neoantigen for the future development of tumor immunotherapy. The study by Yang et al. (8) undoubtedly has opened up opportunities to develop new therapies against TNBC.
“In PNAS, Yang et al. (8) report a previously unidentified variant of macrophage receptor with collagenous structure (MARCO) in some TNBC, and show that the presence of this variant is associated with a better response to inhibitors of BET (Bromodomain and Extra-Terminal motif).”
The study also raises several intriguing questions: First, since BRD4-directed super-enhancers are involved in a wide range of cancers (20), one would expect to see the aberrant expression of MARCO-TST in other types of cancer besides TNBC. Indeed, MARCO-TST can be detected in several other types of cancer, such as HER2+ breast cancer, esophageal cancer, glioblastoma multiforme, etc. However, the frequency is much lower (below 5%). Therefore, additional factors besides BRD4-directed super-enhancers may be required for the accumulation of MARCO-TST. Second, MARCO-TST contains a long collagen-like domain. Can PLOD2 catalyze the hydroxylation of lysyl residues in the collagen-like peptides of MARCO-TST and regulate MARCO-TST? If so, the interaction between MARCO-TST and PLOD2 may lead to a reciprocal regulation. Third, the enhancer, alternative promoter, and additional exons of MARCO-TST are inherently present in our genome. Is MARCO-TST really tumor specific, i.e., can it be expressed in some normal tissues at certain stages of development? If so, does MARCO-TST play any role in normal tissues or during development? Many splice variants play different but important roles. Future study will be required to address this question. After all, it is still possible that MARCO-TST is not totally an “evil me.”
Acknowledgments
W.-C.L. and F.-T.L. are supported by funding from NIH R01CA203824, Department of Defense Grants W81XWH-19-1-0369, W81XWH-22-1-0226, and W81XWH-22-1-0534, and Rivkin Center for Ovarian Cancer Pilot Award.
Author contributions
W.-C.L. and F.-T.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
See companion article, “Superenhancer drives a tumor-specific splicing variant of MARCO to promote triple negative breast cancer progression,” 10.1073/pnas.2207201119.
Contributor Information
Weei-Chin Lin, Email: weeichil@bcm.edu.
Fang-Tsyr Lin, Email: flin@bcm.edu.
References
- 1.Singer G. A., et al. , Genome-wide analysis of alternative promoters of human genes using a custom promoter tiling array. BMC Genomics 9, 349 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer P., et al. , Coupling of transcription with alternative splicing: RNA pol II promoters modulate SF2/ASF and 9G8 effects on an exonic splicing enhancer. Mol. Cell 4, 251–258 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Demircioglu D., et al. , A pan-cancer transcriptome analysis reveals pervasive regulation through alternative promoters. Cell 178, 1465–1477.e1417 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Wiesner T., et al. , Alternative transcription initiation leads to expression of a novel ALK isoform in cancer. Nature 526, 453–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garan L. A. W., Xiao Y., Lin W. C., 14-3-3tau drives estrogen receptor loss via ERalpha36 induction and GATA3 inhibition in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 119, e2209211119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z. Y., Yin L., Estrogen receptor alpha-36 (ER-alpha36): A new player in human breast cancer. Mol. Cell. Endocrinol. 3, 193–206 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Joruiz S. M., Bourdon J. C., p53 Isoforms: Key regulators of the cell fate decision. Cold Spring Harb. Perspect. Med. 6, a026039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y. S., et al. , Superenhancer drives a tumor-specific splicing variant of MARCO to promote triple-negative breast cancer progression. Proc. Natl. Acad. Sci. U.S.A. 119, e2207201119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowdish D. M., Gordon S., Conserved domains of the class A scavenger receptors: Evolution and function. Immunol. Rev. 227, 19–31 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Guo H. F., et al. , Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe(2+)-binding. Nat. Commun. 9, 512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi Y., Xu R., Roles of PLODs in collagen synthesis and cancer progression. Front. Cell Dev. Biol. 6, 66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivan M., et al. , HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292, 464–468 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Gilkes D. M., Bajpai S., Chaturvedi P., Wirtz D., Semenza G. L., Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Hollern D. P., Honeysett J., Cardiff R. D., Andrechek E. R., The E2F transcription factors regulate tumor development and metastasis in a mouse model of metastatic breast cancer. Mol. Cell Biol. 34, 3229–3243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pott S., Lieb J. D., What are super-enhancers? Nat. Genet. 47, 8–12 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Donati B., Lorenzini E., Ciarrocchi A., BRD4 and Cancer: Going beyond transcriptional regulation. Mol. Cancer 17, 164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shorstova T., Foulkes W. D., Witcher M., Achieving clinical success with BET inhibitors as anti-cancer agents. Br J. Cancer 124, 1478–1490 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raval R. R., et al. , Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell Biol. 25, 5675–5686 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallah J., et al. , FDA approval summary: Belzutifan for von Hippel-Lindau disease associated tumors. Clin. Cancer Res. doi: 10.1158/1078-0432.CCR-22-1054. Online ahead of print. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sengupta S., George R. E., Super-enhancer-driven transcriptional dependencies in Cancer. Trends Cancer 3, 269–281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]