Abstract
Objective:
Black Americans (BA) in the United States are disproportionately exposed to childhood adversity compared to White Americans (WA). Such disparities may contribute to race-related differences in brain structures involved in regulating the emotional response to stress, such as the amygdala, hippocampus, and prefrontal cortex (PFC). The present study investigated neuroanatomical consequences of racial disparities in adversity.
Methods:
The present analysis utilized self-reports of adversity and structural magnetic resonance imaging data of 7,350 White American (WA) and 1,786 Black American (BA) children (ages 9–10) from the Adolescent Brain Cognitive Development (ABCD) Study public data release 2.0.
Results:
BA children experienced more traumatic events, family conflict, and material hardship on average compared to WA children. The parents/caregivers of BA children also had lower educational attainment, lower income, and more unemployment compared to parents/caregivers of WA children. Further, BA children showed lower amygdala, hippocampus, and PFC gray matter volume compared to WA children. The volume of the PFC and amygdala, but not the hippocampus, also varied with metrics of childhood adversity with income being the most common predictor of brain volume differences. Importantly, accounting for differences in childhood adversity attenuated the magnitude of some race-related differences in gray matter volume.
Conclusions:
The results suggest that disparities in childhood adversity contribute to race-related differences in gray matter volume within key brain regions for threat-related processes. Structural alterations of these same regions are linked to cognitive-affective dysfunction observed in disorders such as PTSD. More granular assessments of structural inequities across racial/ethnic identities are needed for a thorough understanding of their impact on the brain. Together, the present findings may provide insight into potential systemic contributors to disparate rates of psychiatric disease among Black and White individuals in the US.
Keywords: Race disparities, MRI, Adversity, brain structure
Children across the United States (U.S.) grow up in vastly different environments that shape their responses to stress and ability to function later in life. Uncontrollable factors such as the neighborhood children are born into can contribute to significant early life adversity such as enduring socioeconomic disadvantage or increased risk of violence exposure. In the U.S., Black American (BA) children are disproportionately burdened with these adverse life experiences compared to White American (WA) children (1). Current U.S. census data shows BA households, on average, have a lower median income, lower educational attainment, and higher rates of unemployment and poverty compared to WA households (2). Moreover, prior research suggests that BA children are more likely to be exposed to trauma and domestic violence and are more likely to have a parent who died, an incarcerated parent, or divorced or separated parents compared to WA children (3–5). Additionally, research has shown that BA children live in disproportionately disadvantaged neighborhoods and are more likely to be exposed to neighborhood violence than WA children (6, 7). These racial disparities are not random. Rather, they are deep-rooted structural inequalities that result from a history of disenfranchisement of racially minoritzed groups (e.g., slavery, segregation) that reinforce themselves through societal norms and practices (i.e., systemic racism) (8).
Early life adversity can have lasting negative consequences on mental health in adulthood. Several studies have found positive associations between childhood adversity (e.g., witnessing violence and low socioeconomic status) and later life prevalence of poor psychosocial and behavioral outcomes, including posttraumatic stress disorder (PTSD), anxiety, and depression development, problematic drug and alcohol use, low life satisfaction, suicidal attempts and ideation, and violence perpetration (9–15). Thus, the prior literature demonstrates a strong relationship between adverse life experiences and outcomes such that more adversity experienced in childhood is tied to a greater risk of deleterious mental health outcomes later in life. Further, recent literature has emphasized that different types of adversity are associated with distinct outcomes. Specifically, “threat” type adversity (e.g., physical or sexual abuse, witnessing violence) is more often associated with dysregulated emotional responses whereas “deprivation” type adversity (e.g., poverty, neglect) is more typically associated with language and cognitive deficits (16–18).
Prior work has shown that early exposure to adversity (i.e., either threat or deprivation) is associated with structural alterations of brain regions such as the prefrontal cortex (PFC), amygdala, and hippocampus which support healthy emotional functioning in response to threat and stress (19–21). Therefore, racial disparities in childhood adversity may contribute to race-related differences in the structure of the PFC, hippocampus, and amygdala. The Adolescent Brain and Cognitive Development (ABCD) study, a large MRI study of childhood development, may be well-suited to investigate the impacts of racial disparities of adversity on the brain. Prior ABCD studies have found that socioeconomic status (22) and trauma exposure (23) are associated with differences in thickness and volume of threat-related brain regions, and that greater neighborhood disadvantage is associated with greater amygdala reactivity in response to faces (24). Further, socioeconomic status partially mediates the association between race and some aspects of gray matter morphology (25, 26). Relatedly, prior work outside of the ABCD study found lower neural reactivity to threat within the PFC, hippocampus and amygdala in BA participants compared to WA participants and these differences were partially attributable to racial disparities in negative life experiences (27). The previous literature thus suggests that adversity is associated with differential structure and functional responses within threat-related neural circuitry, though no work that we are aware of has investigated the relationship between racial disparities in adversity and the structure of this circuitry as a whole during childhood. While emergent research has investigated the impacts of racial discrimination on the brain, it is also important to understand how contextual factors (e.g., systemic racism) may also impact threat neurocircuitry (28–31). Understanding the potential effects of such disparities on these brain structures is critical for a fuller understanding of the impacts of stress on the developing brain and creating generalizable neurobiological models of disease.
Therefore, the present study investigated the relationship between racial disparities in adversity exposure and race-related differences in brain structure within the ABCD study. We hypothesized that BA children would have experienced more adversity than WA children in the sample. We further hypothesized that greater exposure to adverse life experiences would be related to lowered gray matter volume in the amygdala, hippocampus, and several subregions of the PFC. Finally, we anticipated that BA and WA children would show differences in gray matter volume of these regions, and these differences would be partially explained by racial differences in exposure to adversity.
Methods and Materials
Participants
The present study utilized data from the Adolescent Brain Cognitive Development (ABCD) annual curated NIH public release 2.0 (released March 2019, accessed July 2019 from the NIMH Data Archive; NDA) (32). Participants (N = 11,878) aged 9–10 were recruited from 21 research sites across the United States. The present analyses included 9,382 participants (WA = 7,516; BA = 1,866; Male = 4,921/Female = 4,461) (descriptive statistics are available in Table 1). Children were primarily contacted and recruited through U.S. public and private schools within the 21 catchment areas. Less than 10% of the sample was recruited through other methods including mailing lists, affiliates and referrals, summer programs, and twin registries. Further methods for sampling and recruiting are described in prior publications (33).
Table 1.
Participant demographics
| Total N | White American | Black American | Statistics | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | % or M (SD) | % or M (SD) | χ2 or t(df) | p-value | |||
| Age* | 9382 | 119.03 | (7.50) | 118.82 | (7.26) | t(9380) = 1.09 | 0.28 |
| Sex | 9382 | χ2 = 5.86 | 0.02 | ||||
| Male | 53.1% | 50.1% | |||||
| Female | 46.9% | 49.9% | |||||
| Parent education | 9373 | t(2802) =33.15§ | < 0.001 | ||||
| Grade school | 3.8% | 11.9% | |||||
| High school diploma or equiv. | 6.9% | 24.1% | |||||
| Some college | 14.0% | 23.4% | |||||
| Associate degree | 12.1% | 16.9% | |||||
| Bachelor’s degree | 33.1% | 12.7% | |||||
| Master’s degree | 22.9% | 9.6% | |||||
| Doctoral or professional deg. | 7.1% | 1.3% | |||||
| Parent employment | 9121 | χ2 =344.90 | < 0.001 | ||||
| Not currently employed | 5.6% | 19.0% | |||||
| Currently employed | 94.4% | 81.0% | |||||
| Family income | 8654 | t(1985) =40.30§ | < 0.001 | ||||
| Less than $5,000 | 1.2% | 14.2% | |||||
| $5,000 through $11,999 | 1.8% | 11.2% | |||||
| $12,000 through $15,999 | 1.4% | 5.9% | |||||
| $16,000 through $24,999 | 3.2% | 9.8% | |||||
| $25,000 through $34,999 | 4.3% | 12.2% | |||||
| $35,000 through $49,999 | 6.5% | 13.3% | |||||
| $50,000 through $74,999 | 14.0% | 13.9% | |||||
| $75,000 through $99,999 | 16.5% | 7.7% | |||||
| $100,000 through $199,999 | 36.9% | 9.7% | |||||
| $200,000 and greater | 14.2% | 2.1% | |||||
| Neighborhood disadvantage | 8840 | 90.30 | (23.91) | 105.94 | (22.25) | t(2706) =−25.66§ | < 0.001 |
| Family conflict | 9363 | 1.96 | (1.94) | 2.43 | (2.01) | t(2786) =−9.17§ | < 0.001 |
| Financial hardship | 9296 | 0.30 | (0.89) | 1.01 | (1.49) | t(2166) =−19.63§ | < 0.001 |
| Trauma history | 9043 | 0.48 | (1.10) | 0.67 | (1.02) | t(2965) =−7.26§ | < 0.001 |
Note.
Age presented in months. WA and BA participants statistically differed in all demographic variables except age.
Symbol indicates that the test was corrected for unequal variances due to violation of Levene’s test for homogeneity of variance.
Measures
Demographic history
Family demographic data was acquired using a standardized survey completed by participants’ parents (NDA: pdem02) that assessed both parent and child race/ethnicity, parental education and employment, and family income, among other variables. Parents identified their children as a member of one or more racial identities from 16 categories (e.g., White, Black/African American, Alaska Native, Samoan, Vietnamese). The present analyses focused on environmental and brain structure relationships specifically in White American (WA) and Black American (BA) children. Children who were identified by their parents as both Black and White were excluded from our analysis.
Parents and caregivers self-reported their current employment status, their highest educational attainment, and their total family income at the time of the interview. Parent educational attainment was self-reported for 22 levels from “Never attended/Kindergarten only” through “Doctoral degree” and recoded into 7 ordinal groups (see Table 1) for the present analyses. Employment status was recategorized from 11 possible categories into two groups of “currently employed” or “not currently employed”. The “currently employed” group consisted of parents/caregivers who endorsed “working now”, “stay at home parent”, “student”, “maternity leave”, or “sick leave” as their employment status. The “not currently employed” group consisted of those who endorsed “temporarily laid off”, “looking for work”, “disabled”, or “unemployed not looking for work” as their employment status. Retired individuals and those who did not provide employment information were excluded from analyses. Family income was self-reported for 10 levels from less than $5,000 to $200,000 and greater. The family income variable was not modified for analysis.
Neighborhood disadvantage
Neighborhood disadvantage was measured using the Area Deprivation Index (ADI) (34) included as part of the ABCD study assessments of residential history (NDA: abcd_rhds01). Briefly, the ADI is a factor-based index that uses 17 socioeconomic indicators from the U.S. Census Survey (e.g., poverty, housing, employment) to characterize a given neighborhood. Parents/caregivers of participants were asked to provide up to 3 primary addresses and the first address was used to derive regional U.S. Census information to determine the ADI. Data for each census region were queried from the 2011–2015 American Community Survey 5-year summary database (U.S. Census Bureau, 2016). A weighted ADI sum score that represented a participant’s level of neighborhood disadvantage was used in statistical analyses (described further in Kind et al., 2014)(35). Greater weighted ADI sum score represented higher neighborhood disadvantage. In exploratory analyses, given emerging research of both racial disparities in exposure and impacts on the brain (36, 37), we further assessed potential impacts of neighborhood inequities on the brain by including measures of particulate matter (PM2.5) and ground pollution indexed via nitrogen dioxide (NO2) from the residential history of participants in exploratory analyses (methods and analyses described in the supplement).
Family conflict
Family conflict was assessed with the Family Conflict Subscale (FCS) of the Youth Family Environment Scale (NDA: abcd_fes01). The FCS consists of 9 items completed by the children that assessed physical and emotional conflicts within the household (e.g., the extent to which family members become openly angry, criticize, or hit each other). Participants rated each item as either “true” (1) or “false” (0) and three items with negative phrasing (e.g., “family members rarely become openly angry”) were reverse coded for analyses. The sum score from the FCS items served as an index of family conflict and was included in statistical analyses.
Material hardship
Family material hardship was assessed using a material hardship questionnaire collected as part of the parent demographic survey (NDA: pdem02). The questionnaire consists of 7 items related to economic insecurity (e.g., “couldn’t afford to pay rent”, “had utilities shut off due to non-payment”, “couldn’t afford to go to the doctor”). The sum score of the material hardship items was used in statistical analyses.
Trauma history
Participants’ trauma history was assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-V (KSADS-5). Trauma history was obtained from parent reports based on the 17-item traumatic events module of the KSADS-5 (NDA: abcd_ptsd01). The items included events such as motor vehicle accident, natural disaster, and sexual/non-sexual assault. Endorsed items were summed for each child to create a trauma history score.
Structural brain imaging
Structural MRI data was collected across 21 sites on Siemens Prisma, General Electric 750, and Phillips 3T scanners, using prospective motion correction when available. Detailed imaging protocols, parameters, and processing of the structural imaging data have been previously published (38, 39). Briefly, structural MRI (T1w and T2w) data were preprocessed by the ABCD team using FreeSurfer v5.3.0 (https://surfer.nmr.mgh.harvard.edu). Images were corrected for gradient nonlinearity distortions and head motion and resampled into alignment with an averaged reference brain. The cortical surface was then reconstructed, and subcortical regions of the brain were segmented. For the present study, gray matter volume (GMV) of cortical regions of interests (ROIs) based on the Desikan-Killiany atlas (40) and GMV of subcortical ROIs and estimated intracranial volume based on FreeSurfer segmentations (41) were used in analyses. Participants who failed T1 or T2 quality control checks (NDA: mriqcrp102) or who failed FreeSurfer quality control (NDA: freesqc01) were excluded from analyses (N = 832). An independent samples t-test demonstrated racial groups differed in intracranial volume [t(8235) = 19.44, p < 0.001]. Thus, GMV of our a priori ROIs (i.e., PFC, hippocampus, amygdala, and insula) was normalized as a proportion of estimated intracranial volume [(region volume/intracranial volume) x 100] and averaged across left and right hemispheres. Subdivisions of the PFC based on the Desikan-Killiany atlas (i.e., frontal pole, superior frontal gyrus, rostral anterior cingulate, pars opercularis, medial orbitofrontal cortex, lateral orbitofrontal cortex, caudal middle frontal gyrus, caudal anterior cingulate, rostral middle frontal gyrus, pars orbitalis, and pars triangularis) were used as separate ROIs given these regions may have differing functions and thus show differing relationships. Given growing understanding of the role of the insula in threat processing (42, 43), we included the insula as another ROI for analysis. In total, GMV of fourteen ROIs were included in statistical analyses (NDA: abcd_smrip101; abcd_smrip201).
Statistical analyses
Statistical analyses were completed using IBM SPSS Statistics version 24.0 (Armonk, NY). The number of participants available for statistical tests varied due to incomplete data on some measures. T-tests were corrected for unequal variances where appropriate and Bonferroni corrections were applied for each family of tests to correct for multiple comparisons. We assessed group differences in adversity measures using chi-square tests for categorical variables (i.e., employment status) and independent samples t-tests for continuous and ordinal variables (i.e., income, educational attainment, neighborhood disadvantage, family conflict, material hardship, trauma history). A Bonferroni correction was applied to control for multiple comparisons within this family of tests (7 tests, p = 0.05/7 = 0.007). We also completed exploratory analyses with participant PTSD symptoms reported by the caregivers which are detailed in the supplemental material.
Next, we completed 14 linear mixed-effects models to assess race-related differences in GMV of the a priori ROIs. The models accounted for nesting of families (NDA: acpsw03) and covaried for age, gender, and scanner type (NDA: abcd_mri01) with restricted maximum likelihood estimation. A Bonferroni correction was applied to control for multiple comparisons within this family of tests (14 tests, p = 0.05/14 = 0.0035). We completed additional mixed-effects models to assess the relationship between regional GMV and the measures of childhood adversity (one brain region per model, 14 models total). The models included the seven indices of adversity (i.e., educational attainment, employment status, income, neighborhood disadvantage, family conflict, material hardship, trauma history) as independent variables and GMV for each brain region as the dependent variable. We again covaried for family relatedness, age, gender, and scanner type. We also completed separate independent samples t-tests between the racial groups using the Destrieux atlas to validate the robustness of the effect across brain parcellations and covariate approaches (Table S1).
Further, we investigated whether accounting for childhood adversity modulated race-related differences in regional GMV similar to prior work (39). We completed parallel mediation analyses within the JASP statistical package to calculate the standardized estimates of the total, direct, and indirect effects of racial group on regional GMV as well as the percentage of variance mediated by the adversity metrics. Parallel mediation models used full information maximum likelihood for estimation. Participant racial group was included as the predictor variable and metrics of adversity (i.e., educational attainment, employment status, income, neighborhood disadvantage, family conflict, material hardship, trauma history) were included as mediators. The dependent variables for the mediation models were the residual GMV values estimated from linear mixed effects models that accounted for age, scanner, gender, and family relatedness (equivalent to the above models without including racial group). An exploratory parallel mediation was also completed to determine if accounting for other neighborhood variables such as pollutant/toxin exposure further explained race-related variability in GMV.
Results
Race-related differences in adversity
Chi-square and independent samples t-tests revealed BA and WA children in the present study differed in parent employment status, parent educational attainment, and family income (Table 1). Specifically, WA children’s parents were 3 times more likely to be currently employed. Further WA children’s parents had higher educational attainment, and greater family income compared to BA children’s parents. Specifically, 75.2% of WA parents had a college degree compared to 40.6% of BA parents and 88.1% of WA parents made $35,000 a year or more compared to 46.7% of BA parents. Further, WA children experienced less family conflict, less material hardship, less neighborhood disadvantage, and fewer traumatic events compared to BA children (Table 1). Racial differences in trauma exposure remained significant when removing non-traumatized individuals (t(3194) = −2.18, p = 0.03).
Race-related differences in GMV
Linear mixed-effects models revealed BA and WA children in the present sample differed in GMV within 11 of the 14 a priori ROIs, after covarying for family relatedness, gender, age, and scanner type (Figure 1; Table 2). An alternative visualization of the results is available in the supplement (Figure S1). Specifically, WA children showed greater GMV compared to BA children within the amygdala, hippocampus, frontal pole, superior frontal gyrus, rostral anterior cingulate, pars opercularis, pars orbitalis, lateral orbitofrontal cortex, caudal middle frontal gyrus, and the caudal anterior cingulate and smaller GMV compared to BA in the pars triangularis (all p values < 0.001). No difference was observed in GMV of the insula, rostral middle frontal gyrus, or medial orbitofrontal cortex between the groups. Similar results were observed in the Destrieux parcellation (Table S1).
Figure 1. Race-related differences in regional gray matter volume.
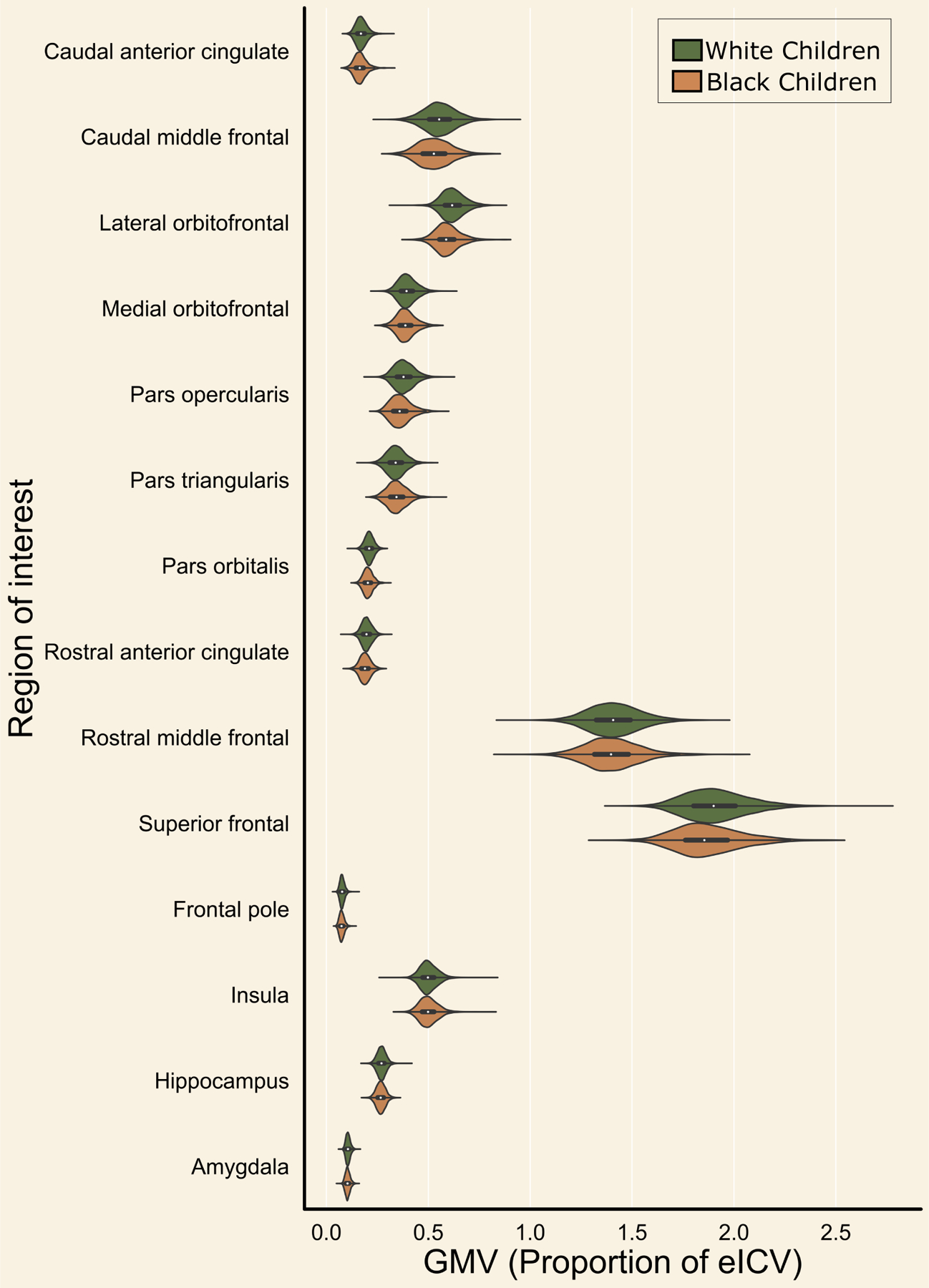
Gray matter volume (X-Axis) was calculated for several brain regions of interest (Y-Axis) in White American (Green) and Black American (Orange) children. Plots show the distribution of values for each group. Dashed lines inside distributions represent the quartiles for each group per brain region.
Table 2.
Race-related differences in gray matter volume (in mm3) of a priori regions of interest
| White American | Black American | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Region | MM | SE | MM | SE | t-statistic | p-value |
| Caudal anterior cingulate┼ | 0.173 | 0.000 | 0.168 | 0.001 | 6.00 | < 0.001 |
| Caudal middle frontal┼ | 0.558 | 0.001 | 0.536 | 0.002 | 10.53 | < 0.001 |
| Lateral orbitofrontal┼ | 0.631 | 0.001 | 0.610 | 0.001 | 16.13 | < 0.001 |
| Medial orbitofrontal | 0.406 | 0.001 | 0.405 | 0.001 | 0.95 | 0.340 |
| Pars opercularis┼ | 0.385 | 0.001 | 0.370 | 0.001 | 11.32 | < 0.001 |
| Pars triangularis┼ | 0.346 | 0.001 | 0.353 | 0.001 | −4.77 | < 0.001 |
| Pars orbitalis┼ | 0.210 | 0.000 | 0.207 | 0.001 | 6.85 | < 0.001 |
| Rostral anterior cingulate┼ | 0.199 | 0.000 | 0.191 | 0.001 | 10.54 | < 0.001 |
| Rostral middle frontal | 1.421 | 0.002 | 1.423 | 0.003 | −0.53 | 0.593 |
| Superior frontal┼ | 1.939 | 0.002 | 1.912 | 0.004 | 7.09 | < 0.001 |
| Frontal pole┼ | 0.080 | 0.000 | 0.078 | 0.000 | 6.77 | < 0.001 |
| Insula | 0.502 | 0.001 | 0.504 | 0.001 | −1.53 | 0.127 |
| Hippocampus┼ | 0.272 | 0.000 | 0.270 | 0.001 | 4.26 | < 0.001 |
| Amygdala┼ | 0.109 | 0.000 | 0.108 | 0.000 | 4.93 | < 0.001 |
Note. MM = estimated marginal mean, SE = standard error.
Symbol indicates the t-test result was significant after Bonferroni Correction (0.05/14 = 0.0035). N = 8,237. T-statistic obtained from linear mixed effect models that also accounted for effects of scanner type, age, gender, and family relatedness.
Relationships between adversity and GMV
Linear mixed-effects models assessed the effects of the indices of adversity (i.e., income, education, employment, neighborhood disadvantage, material hardship, trauma history, family conflict) on GMV for each ROI, while covarying for family relatedness, age, gender, and scanner type. Childhood adversity was associated with GMV within the caudal anterior cingulate, caudal middle frontal gyrus, lateral orbitofrontal cortex, medial orbitofrontal cortex, pars opercularis, pars orbitalis, rostral anterior cingulate, rostral middle frontal gyrus, superior frontal cortex, frontal pole, insula, and amygdala (Table 3). Specifically, we observed unique effects of all adversity indices except trauma history and family conflict which were not uniquely related to GMV in any of the models. Income was the most frequent predictor, having effects on GMV in 8 of 14 regions.
Table 3.
Summary of mixed-effects analyses predicting gray matter volume
| Material Hardship | Employment | Family income | Education | Family conflict | Neighborhood Disadvantage | Trauma history | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Region | b | t-statistic | b | t-statistic | b | t-statistic | b | t-statistic | b | t-statistic | b | t-statistic | b | t-statistic |
| Caudal anterior cingulate | −0.001 | −2.34* | 0.002 | 1.22 | 0.000 | 2.21* | 0.000 | 0.72 | −0.000 | −0.60 | 0.000 | 0.37 | −0.000 | −1.03 |
| Caudal middle frontal | −0.002 | −2.48* | −0.001 | −0.14 | 0.002 | 4.54*** | 0.000 | 0.11 | −0.001 | −1.61 | −0.000 | −1.05 | −0.000 | −0.14 |
| Lateral orbitofrontal | −0.000 | −0.36 | 0.001 | 0.32 | 0.002 | 6.26*** | −0.000 | −0.31 | −0.000 | −1.34 | −0.000 | −0.32 | 0.001 | 2.00* |
| Medial orbitofrontal | −0.000 | −0.87 | −0.001 | −0.52 | 0.001 | 2.20* | −0.001 | −1.49 | 0.000 | 1.07 | 0.000 | 0.77 | 0.000 | 0.90 |
| Pars opercularis | 0.000 | 0.09 | −0.000 | −0.05 | 0.002 | 4.67*** | −0.001 | −2.92** | 0.000 | 0.55 | 0.000 | 0.10 | 0.000 | 0.56 |
| Pars triangularis | −0.000 | −0.46 | −0.002 | −0.86 | −0.000 | −0.94 | −0.001 | −1.81 | 0.000 | 1.01 | 0.000 | 2.01* | −0.000 | −0.18 |
| Pars orbitalis | −0.000 | −0.49 | −0.001 | −1.25 | 0.001 | 3.67*** | 0.000 | 1.06 | 0.000 | 1.22 | −0.000 | −0.43 | 0.000 | 1.41 |
| Rostral anterior cingulate | −0.000 | −0.83 | −0.002 | −1.55 | 0.001 | 4.61*** | 0.000 | 0.63 | 0.000 | 0.33 | 0.000 | 0.30 | 0.000 | 0.71 |
| Rostral middle frontal | −0.003 | −1.92 | −0.009 | −1.44 | 0.001 | 1.51 | 0.003 | 2.88** | −0.001 | −1.26 | 0.000 | 0.94 | 0.002 | 1.79 |
| Superior frontal | −0.002 | −1.04 | −0.000 | −0.03 | 0.005 | 5.30*** | 0.000 | 0.16 | −0.001 | −0.84 | 0.000 | 0.37 | 0.001 | 1.09 |
| Frontal pole | −0.000 | −1.59 | −0.001 | −1.18 | 0.000 | 3.06** | 0.000 | 0.81 | −0.000 | −0.19 | 0.000 | 1.08 | −0.000 | −0.57 |
| Insula | −0.000 | −0.34 | −0.003 | −1.55 | 0.000 | 1.30 | −0.001 | −2.45* | 0.000 | 0.11 | 0.000 | 3.10** | −0.000 | −0.31 |
| Hippocampus | −0.000 | −0.14 | 0.001 | 0.48 | 0.000 | 1.23 | −0.000 | −0.12 | 0.000 | 0.36 | 0.000 | 1.51 | −0.000 | −1.37 |
| Amygdala | 0.000 | 0.41 | 0.001 | 2.35* | 0.000 | 0.73 | 0.000 | 0.66 | −0.000 | −0.44 | −0.000 | −0.65 | −0.000 | −1.51 |
Note.
= p < 0.05
= p < 0.01
= p < 0.001. Linear mixed effects models also accounted for effects of scanner type, age, gender, and family relatedness.
We next sought to determine if accounting for childhood adversity affected the magnitude of race-related differences in brain structure. Standardized estimates from the parallel mediation models are provided in Table 4. Standardized estimates for total and direct effects for each brain region are shown for each brain region and plotted in Figure 2. Direct effects of racial group for several brain regions were smaller than total effects, with significant partial mediation observed for the caudal anterior cingulate, caudal middle frontal gyrus, lateral orbitofrontal gyrus, pars triangularis, pars orbitalis, superior frontal gyrus, and frontal pole (Figure 3). Exploratory parallel mediation models that accounted for additional neighborhood variables of pollutant/toxin exposure showed similar effects, but in these models there was no mediation for the pars triangularis or frontal pole, however full mediation was observed for the superior frontal gyrus (described in the supplement). These findings demonstrate that racial disparities in adversity partially mediate some of the race-related differences in regional GMV.
Table 4.
Summary of parallel mediation analyses of race-related effects on GMV accounting for adversity.
| Brain Region | Total Effect (c) | p-value | Total Indirect Effect (ab) | p-value | Direct Effect (c’) | p-value | Percentage Mediated |
|---|---|---|---|---|---|---|---|
| Caudal anterior cingulate+ | −0.17 | < .001 | −0.04 | 0.006 | −0.13 | < .001 | 26.04% |
| Caudal middle frontal+ | −0.29 | < .001 | −0.09 | < .001 | −0.20 | < .001 | 30.58% |
| Lateral orbitofrontal+ | −0.45 | < .001 | −0.03 | 0.034 | −0.41 | < .001 | 7.40% |
| Medial orbitofrontal | −0.03 | 0.333 | −0.02 | 0.287 | −0.01 | 0.748 | - |
| Pars opercularis | −0.31 | < .001 | 0.01 | 0.613 | −0.32 | < .001 | 2.57% |
| Pars triangularis+ | 0.13 | < .001 | 0.06 | < .001 | 0.08 | 0.02 | 42.42% |
| Pars orbitalis+ | −0.19 | < .001 | −0.04 | 0.008 | −0.15 | < .001 | 21.88% |
| Rostral anterior cingulate | −0.29 | < .001 | −0.03 | 0.098 | −0.27 | < .001 | 8.93% |
| Rostral middle frontal | 0.02 | 0.597 | −0.09 | < .001 | 0.10 | 0.001 | - |
| Superior frontal+ | −0.20 | < .001 | −0.10 | < .001 | −0.10 | 0.003 | 50.76% |
| Frontal pole+ | −0.19 | < .001 | −0.04 | 0.006 | −0.15 | < .001 | 23.28% |
| Insula | 0.05 | 0.116 | 0.02 | 0.155 | 0.02 | 0.501 | - |
| Hippocampus | −0.12 | < .001 | −0.01 | 0.765 | −0.11 | < .001 | 4.27% |
| Amygdala | −0.14 | < .001 | −0.01 | 0.582 | −0.13 | < .001 | 6.67% |
Note: Gray matter volume (GMV) estimated from residuals of linear mixed effects models that included age, gender, scanner, and family relatedness (i.e., the isolated race-related effect). Percentage mediated is calculated by ab/c * 100.
Symbol indicates model met criteria for partial or full mediation. Percentage mediated omitted for regions in which no significant total effect was observed.
Figure 2. Effects of racial disparities in adversity on race-related differences in brain structure.
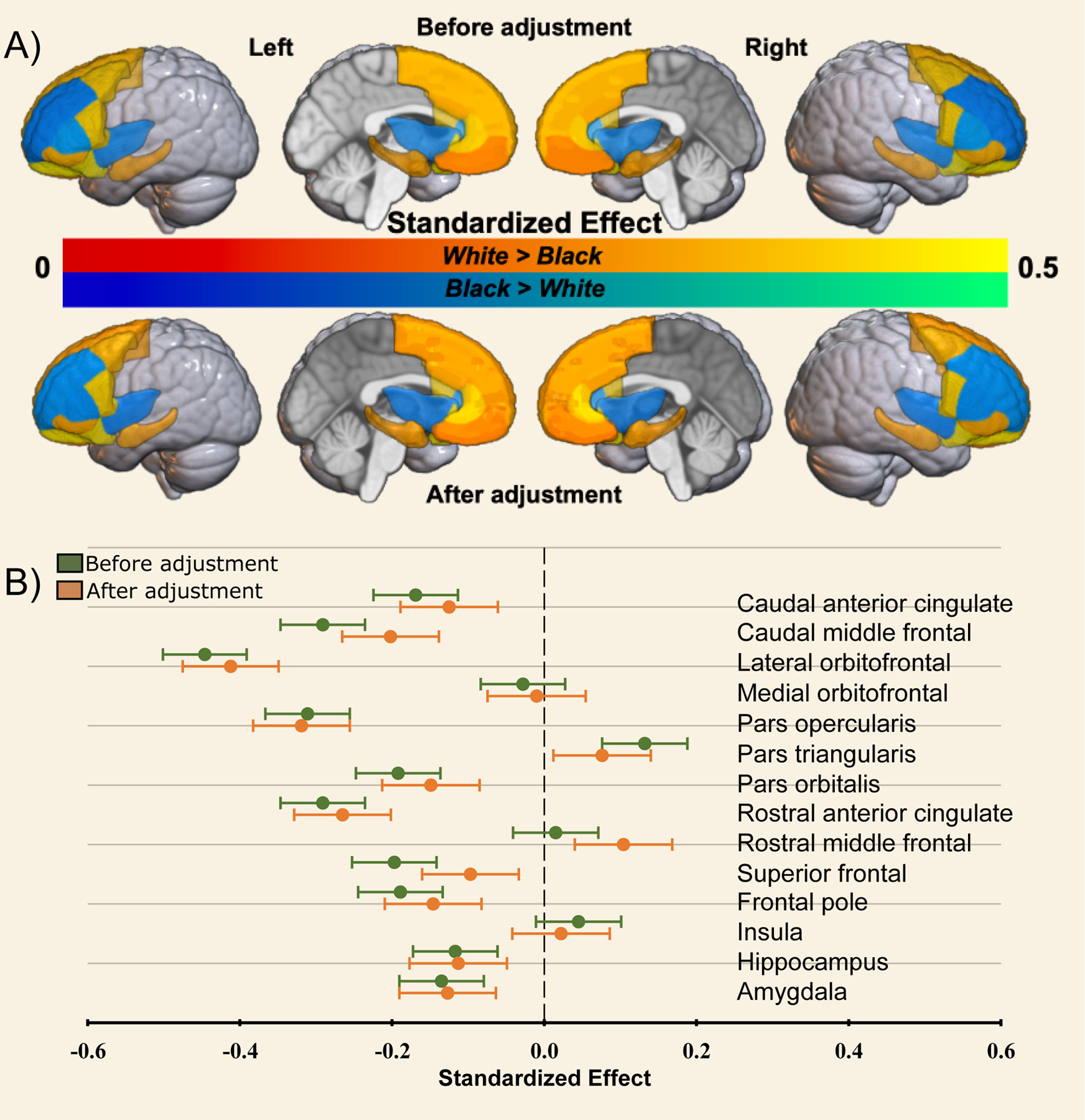
Standardized estimates were calculated from the parallel mediation analyses for differences in gray matter volume (GMV) between Black American and White American children before (Total Effect) and after (Direct Effect) accounting for disparities in sociodemographic factors. Panel A provides a graphical representation of estimates where White > Black (warm colors) and Black > White (cool colors) before (top) and after (bottom) accounting for racial disparities. Panel B shows a plot of the Standardized estimates (dots) per region for the total (green) and direct (orange) effects on GMV data. Bars represent the 95% confidence interval for the effect.
Figure 3. Graphical representation of parallel mediation results.
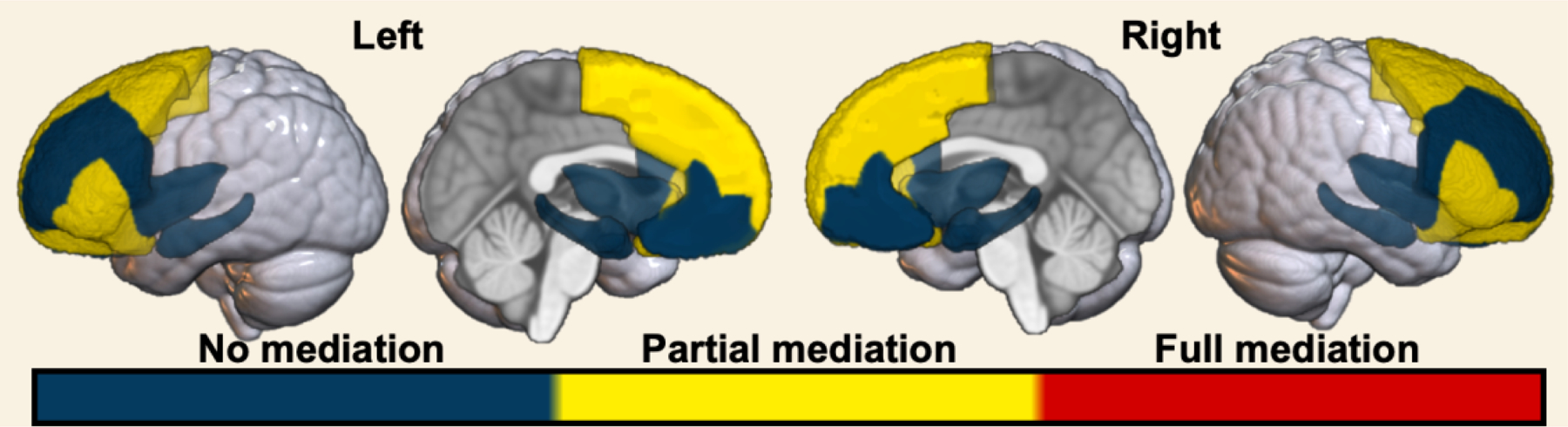
Parallel mediation modeling revealed no, partial, or full mediation of race-related differences on regional GMV by the adversity metrics. Blue – no significant total and/or indirect effect. Yellow – significant total, indirect, and direct effect. Red – significant total and indirect, but not direct, effect.
Associations between adversity and reported PTSD symptoms
Given the prior findings, we completed supplementary analyses on race-related differences in PTSD symptoms and the relationship to adversity which are described in the supplementary materials. Black children had significantly greater PTSD symptoms and these symptoms were further predicted by adversity (Table S2). Accounting for adversity partially mediated race-related differences in PTSD symptoms, but also attenuated correlations between regional GMV and PTSD symptom severity (Table S3).
Discussion
The present study investigated the neuroanatomical consequences of racial disparities in adversity during childhood. We found that, compared to WA children, BA children endorsed more traumatic events, material hardship, and family conflict, lived in more disadvantaged neighborhoods, and their caregivers had lower income, educational attainment, and were more likely to be unemployed. Greater exposure to these adversities was linked to lowered GMV within the amygdala and several subregions of the PFC. Accordingly, BA children showed lower GMV within the amygdala, hippocampus and several subregions of the PFC compared to WA children. Importantly, accounting for racial disparities in exposure to adversity partially mediated race-related differences in a number of regions including the caudal anterior cingulate, lateral orbitofrontal gyrus, and superior frontal gyrus. However, although our findings held when considering other adversity disparities such as pollution exposure, there remain other structural inequities that may contribute to race-related differences in the brain necessary to investigate in future research. Taken together, our findings highlight the impact that disparities in early life adversity have on race-related differences in the structure of neural circuitry important for PTSD and other trauma- and stress-related disorders.
One way to conceptualize the present findings is that a significant portion of the GMV differences reflect racial disparities in toxic stress. Toxic stress refers to prolonged exposure to adverse experiences that leads to excessive activation of stress response systems and an accumulation of stress hormones which in turn disrupt the immune and metabolic regulatory systems and ultimately the developing architecture of the brain (44–46). Importantly, the effects of toxic stress may be dependent on the relative timing of stress exposure. The PFC, amygdala, and hippocampus experience rapid development beginning in early childhood and continuing until early adulthood (47), and this development is punctuated by sensitive periods where stress may have larger impacts (48, 49). In fact, prior work suggests that exposure to adversity during these sensitive periods may have direct effects on the PFC, amygdala, and hippocampus as well as subsequent threat responses and regulation (50–54). Moreover, our results showed that income was the most common predictor of GMV disparities, aligning with prior literature showing the effects of low SES and specifically low income have profound effects on neurobiological trajectories (22, 24, 25, 55–57). Taken together, early life adversity may act as a toxic stressor that disproportionately impacts BA children, due to their significant increased exposure to adversity, and contributes to differential neural development of key threat processing regions.
Importantly, the impacts of toxic stress may be immediate or temporally delayed depending on the specific brain region. For example, one study examining the effect of childhood sexual abuse on regional brain development found an association between abuse and lower hippocampal volume at ages 3–5 but with lower frontal cortex volume at ages 14–16 (58). In the present study, no effects of adversity were found in the hippocampus, however effects were found in the amygdala and the prefrontal cortex, potentially reflecting the impact of differential sensitive periods of brain development for these regions. A potential delayed effect may partially explain the relatively small magnitude of racial differences in gray matter volume of threat-related regions. Specifically, it may be that the disparities in adversity do not lead to major immediate differences, but these will be potentiated into adulthood either in brain structure or brain function (27). Future analyses of the longitudinal ABCD dataset may shine light as to what potential long-term impacts these disparities may have on the brain and behavior. In sum, our findings may reflect the neuroanatomical consequences of racially disparate environments of toxic stress.
We note here that many of the observed race-related and adversity effects had relatively small effect sizes despite many findings being highly statistically significant. The ABCD study has high statistical power for small effects afforded by its large sample size and these effects are likely more accurate to the general population than traditionally large effects in small sample sizes. A recent review of effect sizes in ABCD analyses has demonstrated that the median in-sample effect size across multiple instruments (161 variables representing all questionnaires and tasks) was 0.03 (59). The authors found a slightly larger median effect size (0.05) when mimicking “real world” analyses of ABCD data. Thus, the observed effects of race-related disparities on brain structure are in line with, and larger than, other observations from analyses of ABCD data.
The present findings should be considered in light of several limitations. Our analyses were limited to parent-identified BA and WA participants and did not include participants with other racial identities. Although the ABCD study is one of the largest studies of children’s brains, there was a limited amount of data on non-WA and non-BA children (note only 15.7% of the present sample were BA and only 17.6% were not BA or WA). Unequal sample sizes can impact statistical group comparisons. Further, many neuroimaging studies have demographically unrepresentative samples that can impact the generalizability of research findings. Thus, we echo the recommendations of prior work to increase representation of non-White racial/ethnic groups to address broader questions on the impact of racial and ethnic disparities across groups (60). Another limitation to the present study is the lack of longitudinal MRI data. The current analyses were focused on the impact of racial disparities on the earliest available assessment of brain structure. However, future analyses of the longitudinal MRI data in combination with potential changes in adversity may be useful to test nuanced questions about the role of adversity on race-related differences in brain development. An additional limitation is the potential role of other adversity types on race-related differences in brain structure. We focused on structural adversities but could not capture certain aspects (e.g., nutritional differences or direct toxin exposure) nor did the present analyses focus on other factors such as racial discrimination (61). Nutritional and racial discrimination data were collected 1-year after the baseline visit, precluding any meaningful interpretations with the baseline MRI data. Further, although we assessed pollutant exposure at the neighborhood level, more direct measures of toxin exposure such as those available from baby teeth collected in the ABCD study may provide more granular information in future analyses. Recent studies demonstrate racism and racial discrimination directly affect brain structure and function and are associated with poor mental health outcomes (28–30, 62–64) and thus future research should further explore these relationships in children. Finally, although we assessed adversity, it is unclear when these adversities occurred or for how long. Information on the timing and duration of the children’s adversity exposure could allow us to draw stronger conclusions about its effect on brain development.
In summary, we have shown that differential exposure to childhood adversity contributes to racial differences between BA and WA children in GMV of brain regions key to emotion regulation. The disparities in gray matter volume observed in this study may be a consequence of long-term dysregulation of threat-related neural circuitry. The current findings thus have important implications for our understanding of the impact of socioeconomic and environmental inequalities on mental health in the United States and our understanding of racial differences in psychiatric disorder development, particularly PTSD for which the literature on lifetime prevalence is mixed (65–70). Although more research is needed on the neurobiological consequences of racial disparities in childhood adversity, the present findings offer new insight into biological impacts of disproportionate stress exposure.
Supplementary Material
Acknowledgements:
We are grateful to Drs. Negar Fani and Sierra Carter for their valuable insight during the analyses, to the ABCD study team for their important research efforts, and to the reviewers for their helpful comments during drafting of the manuscript.
Funding and Disclosures:
This research was supported by the National Institute of Mental Health K00 MH119603 (NGH), K01MH118467 (LAML) and U01MH110925 (KJR). Dr. Lebois reports unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation (ISSTD) and spousal license payment for Vanderbilt IP from Acadia Pharmaceuticals unrelated to the present manuscript. Dr. Ressler serves on advisory boards or has performed scientific consultation for Takeda, Janssen, Bioxcel and Verily, and he has received sponsored research support from Takeda, Alkermes, Alto Neuroscience, Genomind, and Brainsway all of which are unrelated to the present work. He receives funding from NIH and the Brain and Behavior Research Fund. The other authors report no biomedical financial interests or conflicts of interest. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1522634 and 10.15154/1503209.
References
- 1.Sacks V, Murphey D: The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity [Internet]. Child Trends 2018; [cited 2021 Jan 11] Available from: https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity/
- 2.Fontenot K, Semega J, Kollar M: Income and Poverty in the United States: 2017. US Census Bur Curr Popul Rep 2018; [cited 2020 Jan 22] [Google Scholar]
- 3.Hatch SL, Bruce AE, Dohrenwend P: Distribution of Traumatic and Other Stressful Life Events by Race/Ethnicity, Gender, SES and Age: A Review of the Research. Am J Community Psychol 2007; 40:313–332 [DOI] [PubMed] [Google Scholar]
- 4.Maguire-Jack K, Lanier P, Lombardi B: Investigating Racial Differences in Clusters of Adverse Childhood Experiences. Am J Orthopsychiatry 2020; 90:106–114 [DOI] [PubMed] [Google Scholar]
- 5.Slopen N, Shonkoff JP, Albert MA, et al. : Racial Disparities in Child Adversity in the U.S. - Interactions With Family Immigration History and Income. Am J Prev Med 2016; 50:47–56 [DOI] [PubMed] [Google Scholar]
- 6.Williams DR, Collins C: Racial Residential Segregation: A Fundamental Cause of Racial Disparities in Health. Public Health Rep 2001; 116:404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman GM, Messner SF: Individual, family background, and contextual explanations of racial and ethnic disparities in youths’ exposure to violence. Am J Public Health 2013; 103:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banaji MR, Fiske ST, Massey DS: Systemic racism: individuals and interactions, institutions and society. Cogn Res Princ Implic 2021; 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aisenberg E, Herrenkohl T: Community Violence in Context Risk and Resilience in Children and Families. J Interpers Violence 2008; 23:296–315 [DOI] [PubMed] [Google Scholar]
- 10.Elmore AL, Crouch E: The Association of Adverse Childhood Experiences With Anxiety and Depression for Children and Youth, 8 to 17 Years of Age. Acad Pediatr 2020; 20:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackman DA, Farah MJ, Meaney MJ: Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci 2010; 11:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes K, Bellis MA, Hardcastle KA, et al. : The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017; 2:e356–e366 [DOI] [PubMed] [Google Scholar]
- 13.Mclaughlin KA, Breslau J, Green JG, et al. : Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Soc Sci Med 2011; 73:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petruccelli K, Davis J, Berman T: Adverse childhood experiences and associated health outcomes: A systematic review and meta-analysis. Child Abuse Negl 2019; 97:104127. [DOI] [PubMed] [Google Scholar]
- 15.Reiss F: Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc Sci Med 2013; 90:24–31 [DOI] [PubMed] [Google Scholar]
- 16.Machlin L, Miller AB, Snyder J, et al. : Differential Associations of Deprivation and Threat With Cognitive Control and Fear Conditioning in Early Childhood. Front Behav Neurosci 2019; 13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin KA, Sheridan MA, Lambert HK: Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 2014; 47:578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheridan MA, McLaughlin KA: Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci 2014; 18:580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calem M, Bromis K, McGuire P, et al. : Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clin 2017; 14:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Brito SA, Viding E, Sebastian CL, et al. : Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 2013; 54:105–112 [DOI] [PubMed] [Google Scholar]
- 21.Hanson JL, Nacewicz BM, Sutterer MJ, et al. : Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry 2015; 77:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor RL, Cooper SR, Jackson JJ, et al. : Assessment of Neighborhood Poverty, Cognitive Function, and Prefrontal and Hippocampal Volumes in Children. JAMA Netw Open 2020; 3:e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong HJ, Durham EL, Moore TM, et al. : The association between latent trauma and brain structure in children. Transl Psychiatry 2021; 11:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gard AM, Maxwell AM, Shaw DS, et al. : Beyond family-level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev Sci 2021; 24:e12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assari S: Socioeconomic Status Inequalities Partially Mediate Racial and Ethnic Differences in Children’s Amygdala Volume. Stud Soc Sci Res 2020; 1:p62. [DOI] [PubMed] [Google Scholar]
- 26.Assari S: Race, Ethnicity, Family Socioeconomic Status, and Children’s Hippocampus Volume. Res Health Sci 2020; 5:p25. [DOI] [PubMed] [Google Scholar]
- 27.Harnett NG, Wheelock MD, Wood KH, et al. : Negative life experiences contribute to racial differences in the neural response to threat. NeuroImage 2019; 202:116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark US, Miller ER, Hegde RR: Experiences of Discrimination Are Associated With Greater Resting Amygdala Activity and Functional Connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fani N, Harnett NG, Bradley B, et al. : Racial Discrimination and White Matter Microstructure in Trauma-Exposed Black Women. Biol Psychiatry 2022; 91:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fani N, Carter SE, Harnett NG, et al. : Association of Racial Discrimination With Neural Response to Threat in Black Women in the US Exposed to Trauma. JAMA Psychiatry 2021; 78:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neblett EW Jr: Racism and health: Challenges and future directions in behavioral and psychological research. Cultur Divers Ethnic Minor Psychol 2019; 25:12–20 [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Koob GF, Croyle RT, et al. : The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci 2018; 32:4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garavan H, Bartsch H, Conway K, et al. : Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci 2018; 32:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh GK: Area Deprivation and Widening Inequalities in US Mortality, 1969–1998. Am J Public Health 2003; 93:1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kind AJH, Jencks S, Brock J, et al. : Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Intern Med 2014; 161:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tessum CW, Apte JS, Goodkind AL, et al. : Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci U S A 2019; 116:6001–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan CC, Marshall A, Smolker H, et al. : Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): Protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci 2021; 52:101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey BJ, Cannonier T, Conley MI, et al. : The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018; 32:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagler DJ, Hatton SN, Cornejo MD, et al. : Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 2019; 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desikan RS, Ségonne F, Fischl B, et al. : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31:968–980 [DOI] [PubMed] [Google Scholar]
- 41.Fischl B, Salat DH, Busa E, et al. : Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355 [DOI] [PubMed] [Google Scholar]
- 42.Wood KH, Ver Hoef LW, Knight DC: Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage 2012; 60:787–799 [DOI] [PubMed] [Google Scholar]
- 43.Patrick F, Kempton MJ, Marwood L, et al. : Brain activation during human defensive behaviour: A systematic review and preliminary meta-analysis. Neurosci Biobehav Rev 2019; 98:71–84 [DOI] [PubMed] [Google Scholar]
- 44.Gunnar M, Quevedo K: The Neurobiology of Stress and Development. Annu Rev Psychol 2007; 58:145–173 [DOI] [PubMed] [Google Scholar]
- 45.Shonkoff JP, Garner AS: The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Am Acad Pediatr 2012; 129:e232–e246 [DOI] [PubMed] [Google Scholar]
- 46.Middlebrooks J, Audage N: The Effects of Childhood Stress on Health Across the Lifespan. Cent Dis Control Prev Natl Cent Inj Prev Control 2008; [cited 2020 Jul 30] [Google Scholar]
- 47.Gogtay N, Giedd JN, Lusk L, et al. : Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 2004; 101:8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bick J, Nelson CA: Early adverse experiences and the developing brain. Neuropsychopharmacology 2016; 41:177–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tottenham N, Galván A: Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 2016; 70:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lupien SJ, McEwen BS, Gunnar MR, et al. : Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10:434–445 [DOI] [PubMed] [Google Scholar]
- 51.McEwen BS, Nasca C, Gray JD, et al. : Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacol Rev 2016; 41:3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pechtel P, Lyons-Ruth K, Anderson CM, et al. : Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage 2014; 97:236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teicher MH, Samson JA, Anderson CM, et al. : The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016; 17:652–666 [DOI] [PubMed] [Google Scholar]
- 54.Tottenham N, Sheridan MA: A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci 2010; 3:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanson JL, Albert WD, Skinner AT, et al. : Resting state coupling between the amygdala and ventromedial prefrontal cortex is related to household income in childhood and indexes future psychological vulnerability to stress. Dev Psychopathol 2019; 31:1053–1066 [DOI] [PubMed] [Google Scholar]
- 56.Noble KG, Houston SM, Brito NH, et al. : Family income, parental education and brain structure in children and adolescents. Nat Neurosci 2015; 18:773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomasi D, Volkow ND: Associations of family income with cognition and brain structure in USA children: prevention implications. Mol Psychiatry 2021; 26:6619–6629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersen SL, Tomada A, Vincow ES, et al. : Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008; 20:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owens MM, Potter A, Hyatt CS, et al. : Recalibrating expectations about effect size: A multi-method survey of effect sizes in the ABCD study. PLoS ONE 2021; 16:e0257535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hyde LW, Gard AM, Tomlinson RC, et al. : An ecological approach to understanding the developing brain: Examples linking poverty, parenting, neighborhoods, and the brain. Am Psychol 2020; 75:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagata JM, Ganson KT, Sajjad OM, et al. : Prevalence of Perceived Racism and Discrimination Among US Children Aged 10 and 11 Years: The Adolescent Brain Cognitive Development (ABCD) Study. JAMA Pediatr 2021; 175:861–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter RT: Racism and Psychological and Emotional Injury: Recognizing and Assessing Race-Based Traumatic Stress. Couns Psychol 2007; 35:13–105 [Google Scholar]
- 63.Cheng H-L, Mallinckrodt B: Racial/ethnic discrimination, posttraumatic stress symptoms, and alcohol problems in a longitudinal study of Hispanic/Latino college students. J Couns Psychol 2015; 62:38. [DOI] [PubMed] [Google Scholar]
- 64.Paradies Y, Ben J, Denson N, et al. : Racism as a Determinant of Health: A Systematic Review and Meta-Analysis. PLOS ONE 2015; 10:e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breslau J, Aguilar-Gaxiola S, Kendler KS, et al. : Specifying race-ethnic differences in risk for psychiatric disorder in a USA national sample. Psychol Med 2006; 36:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elkins J, Briggs HE, Miller KM, et al. : Racial/Ethnic Differences in the Impact of Adverse Childhood Experiences on Posttraumatic Stress Disorder in a Nationally Representative Sample of Adolescents. Child Adolesc Soc Work J 2018; 36:449–457 [Google Scholar]
- 67.Alegría M, Fortuna LR, Lin JY, et al. : Prevalence, risk, and correlates of posttraumatic stress disorder across ethnic and racial minority groups in the United States. Med Care 2013; 51:1114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall-Clark B, Sawyer B, Golik A, et al. : Racial/Ethnic Differences in Symptoms of Posttraumatic Stress Disorder. Curr Psychiatry Rev 2016; 12:124–138 [Google Scholar]
- 69.Mclaughlin KA, Alvarez K, Fillbrunn M, et al. : Racial/ethnic variation in trauma-related psychopathology in the United States: a population-based study. Psychol Med 2019; 49:2215–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roberts AL, Gilman SE, Breslau J, et al. : Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med 2010; 41:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


