Significance
Phototrophic organisms provide most of the metabolic energy powering life on this planet. There exist two fundamentally different systems for harvesting light energy: (bacterio)chlorophyll-based photosynthetic complexes and proton-pumping rhodopsins. Different phototrophic groups use one system or the other. Here, using transcriptomics, infrared variable fluorescence, and flash photolysis measurements, we show that the bacterium Sphingomonas glacialis AAP5 isolated from an alpine lake is able to use xanthorhodopsin and bacteriochlorophyll-based photosystems simultaneously. Our results suggest that the possession of two light-harvesting systems may also be beneficial in other environments where organisms are exposed to extreme changes of light and low temperature.
Keywords: anoxygenic photosynthesis, xanthorhodopsin, dual phototrophy, light energy, bacteriochlorophyll a
Abstract
Photoheterotrophic bacteria harvest light energy using either proton-pumping rhodopsins or bacteriochlorophyll (BChl)-based photosystems. The bacterium Sphingomonas glacialis AAP5 isolated from the alpine lake Gossenköllesee contains genes for both systems. Here, we show that BChl is expressed between 4°C and 22°C in the dark, whereas xanthorhodopsin is expressed only at temperatures below 16°C and in the presence of light. Thus, cells grown at low temperatures under a natural light–dark cycle contain both BChl-based photosystems and xanthorhodopsins with a nostoxanthin antenna. Flash photolysis measurements proved that both systems are photochemically active. The captured light energy is used for ATP synthesis and stimulates growth. Thus, S. glacialis AAP5 represents a chlorophototrophic and a retinalophototrophic organism. Our analyses suggest that simple xanthorhodopsin may be preferred by the cells under higher light and low temperatures, whereas larger BChl-based photosystems may perform better at lower light intensities. This indicates that the use of two systems for light harvesting may represent an evolutionary adaptation to the specific environmental conditions found in alpine lakes and other analogous ecosystems, allowing bacteria to alternate their light-harvesting machinery in response to large seasonal changes of irradiance and temperature.
Life on Earth essentially depends on the supply of solar energy harvested by phototrophic organisms. They divide into two fundamentally distinct groups: chlorophototrophs and retinalophototrophs. Chlorophototrophs harvest light using chlorophyll (Chl) or bacteriochlorophyll (BChl) molecules integrated into photosynthetic (PS) complexes (1). The captured excitation energy is then transferred to the reaction center (RC) where it serves to initiate the charge separation reaction. Cyanobacteria, the most important phototrophic group, employs two kinds of Chl-based RCs working in series, and these confer the ability to evolve oxygen and fix inorganic carbon. Oxygenic cyanobacteria were later recruited as plastids by eukaryotic organisms giving rise to the enormous diversity of plants and algae (2). Anoxygenic phototrophic organisms with BChl are present in several phyla of the domain Bacteria. Some of these organisms, such as purple or green sulfur bacteria, harvest light energy and conduct complete anoxygenic photosynthesis. However, there also exist a number of photoheterotrophic species (aerobic anoxygenic phototrophs) that harvest light energy only as a supplement to their predominantly heterotrophic metabolism (3, 4). These organisms are common in many marine (5–7) and freshwater environments (8–10).
A fundamentally different mechanism for capturing light energy is used by retinalophototrophs. They contain rhodopsins, simple transmembrane proteins with a single chromophore, retinal (11). Xanthorhodopsins (XRs) have an additional carotenoid antenna to extend the light spectrum that can be harvested (12). When illuminated, rhodopsins translocate protons across the membrane. The established gradient is then used for ATP synthesis (13). Proton-pumping rhodopsins were first discovered in halophilic Archaea (14) but later were also found in Proteobacteria (15). Subsequent research established that photoheterotrophic rhodopsin-containing bacteria are widely distributed in the oceans (6, 16–18) and in freshwater lakes (19).
Zeng et al. recently isolated two heterotrophic Tardiphaga sp. strains from a glacier in Greenland encoding a complete set of genes for anoxygenic photosynthesis organized in the photosynthesis gene cluster (PGC) as well as the XR gene (20). Additionally, they identified eight metagenome-assembled genomes (MAGs) from Greenland glaciers that also contained PGC and XR genes. The authors speculated that these organisms may use the two systems to capture solar energy, which they termed as dual phototrophy, under specific spectral conditions in the glacier. However, none of the systems were expressed in the isolates grown in the laboratory, and consequently, their functional role remained uncertain.
Another photoheterotrophic bacterium, Sphingomonas glacialis AAP5, has been isolated from the high mountain lake Gossenköllesee (GKS) located in the Tyrolean Alps (21, 22). This isolate also contained the PGC and an XR operon in its genome, but no RuBisCO. The strain produced a small amount of BChl a, but no XR expression was detected under laboratory conditions. Three more Sphingomonas strains with the PGC and the XR genes were cultured from the high mountain lake Namco in Tibet (21). Sphingomonas strains originating from low-elevation lakes contained the PGC but lacked the XR gene (21). This led to the hypothesis that the presence of two different systems for light harvesting might be beneficial in alpine lakes or other analogous ecosystems such as glacial environments. Therefore, we decided to test whether S. glacialis AAP5 expresses its phototrophic apparatus under conditions typical for these habitats, namely low temperature and low nutrients. In addition, we characterized how XR and PS complexes contribute to its metabolism.
Results
Phototrophic Sphingomonas in the GKS.
Since S. glacialis AAP5 was originally isolated from the GKS (Fig. 1 A, Left), we first attempted to identify the presence of phototrophic species in metagenomes from this lake that were obtained in October 2017 and February and April 2018 (23). During this period, daily mean temperatures for the water column ranged between 0.1 ± 0.0°C and 7.5 ± 0.3°C, and the period of ice cover was clearly visible from the inversion of the temperature gradient along the water column (Fig. 1 A, Right).
Fig. 1.
MAG of a dual phototrophic Sphingomonas recovered from GKS metagenomes. (A) GKS (Left). Temperature profiles of the GKS between July 2017 and July 2018 (Right). (B) Phylogenetic position of XR and PufM from one MAG in relation to the S. glacialis AAP5 proteins. For full trees, see Dataset S1. (C) Mapping of metagenome reads to the chromosome sequence of S. glacialis AAP5. MAG, metagenome-assembled genome; CPM, counts per million reads. (D) Relative abundance of Sphingomonas XR and pufM genes in three GKS metagenomes from autumn 2018 to spring 2019.
From the assembled data, we extracted several sequences related to XR and pufM genes from S. glacialis AAP5 (SI Appendix, Fig. S1). The most similar genes were found in MAG141 (Fig. 1 B and Dataset S1). MAG141 also contained a second pufM gene, which indicates that it represents a hybrid between at least two closely related species. However, full coverage of the genome of S. glacialis AAP5, isolated in 2012, by reads obtained from sampling in 2017–2018 (with the highest peaks at the rRNA gene clusters) further indicated that potential dual phototrophic Sphingomonas strains are stable members of the GKS bacterial community (Fig. 1 C). The relative abundance of the XR and pufM genes was higher in February and April when the GKS was covered with ice compared with that in October before the lake started to freeze (Fig. 1 D). This suggests that dual phototrophic Sphingomonas strains may be particularly adapted to conditions when the ice cover and snow cover reduce light penetration.
Temperature Dependence of XR and PGC Expression.
To investigate the temperature dependence of XR and BChl-based photosystem biosynthesis, cultures were grown under four different regimes: at 7°C or 22°C with constant illumination and at 7°C or 22°C with a 12-h light–12-h dark regime. Analyses of the pigment composition revealed that BChl a was produced in cultures grown under a light–dark regime independently of the growth temperatures. By contrast, cultures grown under continuous light produced no BChl a, consistent with previous results (21). A different pattern was observed for retinal, as a marker of XR, as this was observed only in cultures grown at 7°C independently of the light regime. Cultures grown under a light–dark regime at 7°C contained both retinal and BChl a, documenting the presence of both systems (Fig. 2 A). Based on the HPLC data, we calculated that the cultures (n = 3) contained between 1,330 and 7,610 XRs and 400–888 photosystems per cell (assuming 36 BChl a molecules per photosystem).
Fig. 2.
Induction of XR- and BChl a–based phototrophy. (A) Reversed-phase chromatography of pigments from cells grown under different conditions. Cells were grown at low (7°C) or high (22°C) temperature either under continuous illumination or under 12-h dark–12-h light regime. Traces are shifted vertically. Numbers above peaks indicate: 1, retinal; 2, nostoxanthin; 3, unknown carotenoid(s); 4–6, BChl a (isomers); and 7, neurosporene. (B) Expression of the XR and pufM genes in cells grown under different temperatures. Upper panel: values for XR gene, Lower panel: values for pufM gene. The ΔCt mean values and standard deviations calculated from three parallel biological replicates for different growth phases (defined in the graphical legend) are shown. Cells were grown under 12-h dark–12-h light regime and harvested 4 h after changing the light regime to light (for XR) or to dark (for pufM).
To further assess the influence of temperature on the expression of both systems, cultures were grown under a light–dark regime at temperatures ranging from 4°C to 25°C. Cells were harvested 4 h after changing the light regime (to light for XR and to dark for pufM) in the mid-exponential, early stationary, and late stationary phases. Using reverse transcription quantitative PCR (RT-qPCR), we monitored changes in XR (E2E30_RS05030) and pufM (E2E30_RS16295) expression (Fig. 2 B). The XR gene was clearly expressed at low temperatures from 4°C to 10°C, with a maximum at 4°C in the late stationary phase. At 13°C and 16°C, expression was observed only during the late stationary phase. At higher temperatures, the expression stopped. A much broader temperature optimum, 7°C to 22°C, was observed in the case of the pufM gene, which was clearly up-regulated during all three growth phases. In contrast, at 25°C, the pufM transcription was clearly inhibited. Notably, in contrast to pufM, XR expression was independent of the carbon content in the medium (SI Appendix, Fig. S2).
Transcriptome Dynamics in a Light–Dark Regime.
To further understand how light exposure influences gene expression, we monitored the transcriptome dynamics of S. glacialis AAP5 grown in batch culture at 7°C under a light–dark regime (Fig. 3 A). Samples were taken four times per day in the mid-exponential (day 4), early stationary (day 5), and late stationary (day 6) phases (SI Appendix, Fig. S3 A and B and Dataset S2). The number of differentially expressed genes between 6-h light and 6-h dark and their range of expression change declined as the culture progressed into the stationary phase (Fig. 3 B and Dataset S2). The XR operon (E2E30_RS05020-05030) was the most up-regulated, and the PGC (E2E30_RS16220-16405) was the most down-regulated chromosomal locus on all days. In the late stationary phase, it remained the only locus responsive to light–dark transition. Thus, light regulation of both systems was maintained, even when transcriptional dynamics of all other genes was reduced to a minimum.
Fig. 3.
Transcriptome dynamics at low temperature under changing light regimes. (A) Growth at 7°C under 12-h light–12-h dark regime (gray bar: light and black bar: dark). The time points for sampling are indicated by black arrows. The mean values and standard deviations from three biological replicates are shown. (B) Transcriptome dynamics of chromosomal genes during different growth phases when 6 h of light is compared with 6 h of dark. Significantly differentially expressed genes are shown as blue dots. Genes of PGC and the XR locus are highlighted in pink and orange, respectively. (C) Expression of XR locus and PGC from samples taken after 1 h and 6 h in the light compared with the previous dark period. Individual and groups of genes are defined in the graphical legend. (D) Normalized RNA-seq read mapping to the XR locus with single-nucleotide resolution. (E) Leader (rv1) and read-through (rv2) expression profile from RNA-seq (Upper panel) compared with RT-qPCR data (Lower panel). Day 4: exponential, day 5: early stationary, and day 6: late stationary phase.
Except for the XR and PGC, there was little overlap between the differentially expressed genes in the exponential and early stationary phases, indicating that many of these changes were due to the progressing culture age (SI Appendix, Fig. S3 C). Translation-related genes were up-regulated in the light in both phases. Stress response genes were exclusively up-regulated in the exponential phase. Different TonB receptors were differentially expressed in both growth phases indicating a shift in nutrient availability that is also reflected by a shift in the expression of degradation pathways (Dataset S2).
PGC expression was suppressed by light, but the response of individual operons was not uniform. Three operons (E2E30_RS16260-16275, 16360-16375, and 16380-16405) coding for BChl synthesis genes showed an immediate down-regulation already after 1 h of illumination, whereas the central operon (E2E30_RS16280-16355) containing genes for structural proteins responded slower (Fig. 3 C). The same trend was observed on day 6, but with a smaller amplitude for the central operons, while the peripheral operons did not change in expression. The acsF gene (E2E30_RS16320) and regulatory genes ppsR (E2E30_RS16385) (24) and ppaA (E2E30_RS16380) were not differentially expressed in response to light on any given day.
In the early stationary phase, the XR operon was strongly up-regulated after 1 h in light compared with the previous dark period (Fig. 3 C). In the late stationary phase, activation was slower, and the maximum expression was reached after 6 h of illumination. The upstream neighboring operon of two genes coding for an NAD(P)-binding (E2E30_RS05015) protein and a fasciclin (E2E30_RS05010) protein with a potential role in photooxidative stress (25) showed a similar expression pattern. The constitutively expressed gene (E2E30_RS05035) downstream of the XR operon is a likely candidate for regulating expression of this locus. It encodes a protein with blue light–sensing LOV- and DNA-binding domains (26). It was highly similar (84% coverage, 66% identity, and e-value 2e-92) to the EL222 protein of Erythrobacter litoralis that is known to undergo conformational changes necessary to bind to DNA in the presence of light (27).
Analysis of the XR locus with single-nucleotide resolution revealed an 86-bp transcript, starting 185 bp upstream (1,092,603 bp) from and on the same strand as the XR gene, that was strongly expressed at 7°C (Fig. 3 D). In the presence of light, a full read through of the promoter and the gene was observed, with coverage of around 3.5% of the upstream “leader” sequence. In the dark, expression was terminated abruptly 99 bp upstream of the XR start codon. The observation was also confirmed using RT-qPCR (Fig. 3 E). Whereas expression of both was dependent on temperature, the ratio of leader and read-through expression was determined by light. The 5′-UTR sequence was conserved in Sphingomonas strains with the same XR locus architecture as S. glacialis AAP5, suggesting that its function is connected to the LOV transcription factor (SI Appendix, Fig. S4).
The inverse regulation of PGC and XR had a contrasting effect on the carotenoid biosynthesis pathway. All genes (E2E30_RS05025, 15535, 15540, and 15550) encoding the pathway from geranylgeranyl pyrophosphate to retinal were light activated, whereas the branch from lycopene to spirilloxanthin (the latter carotenoid is present in the RC) pathway encoded by genes E2E30_RS16245-16255 was expressed in the dark (SI Appendix, Fig. S5).
PS Apparatus and Activity.
The phototrophic activity of the cells expressing both systems was tested by a suite of biophysical measurements. The absorption spectra of the isolated membranes from cells grown under a light–dark regime at 7°C revealed a clear band at 871 nm documenting the presence of BChl a–containing complexes. Unfortunately, the large absorption of nostoxanthin overlies the smaller XR signal (SI Appendix, Fig. S6 A). To better visualize the XR complexes, the membranes were solubilized and separated using sucrose density step-gradient centrifugation. The procedure yielded two distinct bands, orange and purple, documenting the presence of XR and BChl a–containing complexes (SI Appendix, Fig. S6 B).
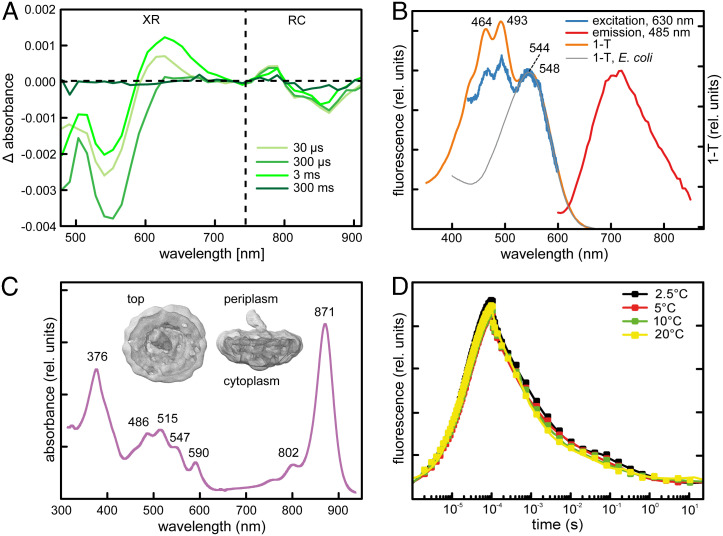
The photochemical activity of both systems in the isolated membranes was investigated using flash photolysis in the visible and the near-infrared regions (Fig. 4 A). The absorbance change in the visible region resembled the previously detected signal of XR (21); the latter is the result of the charge separation in the PS RC (28). The positive electrochromic shift around 800 nm originated from the accessory BChl a, whereas the reduction at 865 nm reflected the bleaching of the primary donor BChl a. These data prove that the RC and XR were photochemically active at the same time.
Fig. 4.
PS apparatus. (A) Light-induced absorption changes elicited by a 2-ms broadband pulse in suspension of membranes covering the range where the activity of both XR and the bacterial RC can be observed. Delays are given in the legend. (B) Fluorescence excitation (blue line) and emission (red line) spectra of XR from AAP5. Orange line represents 1–T absorption spectrum. Gray line represents 1–T absorption spectrum of the heterologously expressed XR gene. Due to the presence of tetrapyrrols in the sample, the excitation spectrum was measured at 630 nm. The emission spectrum was excited by 485 nm. (C) Absorption spectrum of BChl-based RC. (Inset) Contour density of the PS complex determined by the cryo-EM. (D) BChl a fluorescence induction–relaxation kinetics recorded at various temperatures.
In order to further characterize the XR from S. glacialis AAP5, XR was purified from cells grown at 7°C under continuous light. The absorption spectrum revealed two major absorption bands at 464 and 493 nm (Fig. 4 B). These bands were absent in the heterologously expressed XR in E. coli, which had only one broad absorption band in the green part of the spectrum. The XR pigment analysis revealed that, in addition to retinal, it also contains the blue-absorbing carotenoid nostoxanthin. Excitation close to the maximum of the carotenoid band produced a broad emission spectrum peaking at about 710 nm that can be ascribed to retinal (29, 30). To quantify the transfer efficiency from nostoxanthin to retinal, an excitation spectrum was also acquired (Fig. 4 B) and compared with the absorption spectrum of nostoxanthin-free heterologously expressed XR. We estimated the yield of energy transfer to be ~50%. This proves that nostoxanthin serves as an auxiliary antenna for XR and expands absorption into the blue part of the spectrum, which is less covered by BChl a (Fig. 4 C). In addition, the photochemical function of purified XR was confirmed by flash photolysis. The absorbance change kinetics had the main components of 9 μs, 480 μs, 6.3 ms, and 154 ms (SI Appendix, Fig. S7 A) similar to the heterologously expressed XR (21). The difference spectra were also similar, but in contrast to the heterologously expressed XR (without a nostoxanthin antenna), the flash-induced difference spectra of the native XR had a distinct absorption shoulder at ~490 nm (SI Appendix, Fig. S7 B).
The BChl-containing complexes were purified from cells grown at 22°C in the dark. The absorption spectrum had a single absorption band in the near-infrared region suggesting that the obtained complex is a standard RC–LH1 complex (Fig. 4 C). This was confirmed by a low-resolution contour map determined using cryoelectron microscopy (cryo-EM) (Fig. 4 C, Inset). The obtained complexes were approximately circular with a diameter of 150 Å (including detergent micelle) and a total height of ~114 Å. They consisted of LH1 ring surrounding a type 2 RC with a cytochrome c subunit on the periplasmic side and a protruding hydrophilic H-subunit domain on the cytoplasmic side.
In Vivo Phototrophic Activity.
The in vivo activity of the PS complexes was investigated using variable BChl a fluorescence measurements. First, the photochemical activity of cells grown at 7°C in the light–dark regime was tested using single-turnover flash induction–relaxation measurements in the dark. The RC primary photochemistry and electron transfer activity remained unchanged over the wide range of temperatures from 2.5°C to 20°C (Fig. 4 D and SI Appendix, Fig. S8). This documents that the RC is well adapted for the temperatures it encounters in its natural environment. To further analyze the PS electron transport, its activity was probed using a multiple flash protocol. The RCs exhibited a stable catalytic turnover rate (RC reopening) of 1100 ± 80 s−1 (n = 4) within the temperature range of 12°C to 25°C, regardless of light or dark adaptation (SI Appendix, Fig. S8). Below 10°C, however, the RC reopening rate declined in cells exposed to light due to a slowed down cyclic electron flow between the RC and the cytochrome bc1 complexes. The PS complexes were capable of physiologically relevant activity at constant high light at 2.5°C; however, the RC reopening rate was reduced by more than 50% to 370 ± 150 s−1 (Fig. 4 D).
The contribution of XR phototrophy to the cellular metabolism of S. glacialis AAP5 was investigated in the XR-containing cells grown under continuous light at 7°C. When these cells were exposed to white light, the respiration rate decreased in an intensity-dependent manner. In contrast, cells grown under light at 22°C and missing both XR and BChl a maintained constant respiration rates irrespective of light intensity (Fig. 5 A). At 950 µmol photons m−2 s−1, the respiration rate in XR-containing cells was reduced by 78.3% ± 4.3% (n = 4) in comparison with that in the control cells. This result indicated that a large part of the oxidative phosphorylation was replaced by light-derived energy captured by XR. To assess ATP production directly, we measured the cellular ATP content in XR-containing cells kept in the dark and in cells exposed to light. After 6 min of illumination, the luciferase signal increased by almost 50%, which corresponds to an increase of 9.41 × 106 ATP molecules cell−1 (Fig. 5 B). Finally, the stimulatory effect of light was confirmed in a regrowth experiment. The XR-containing cultures grown with light reached approximately 40% higher density when compared with cultures grown in the dark (Fig. 5 C).
Fig. 5.
Impact of XR-based phototrophy. (A) Respiration activity of S. glacialis AAP5 exposed to increasing light intensity at 7°C. Orange symbols represent the activities of XR-containing cells; black symbols represent heterotrophic cells (mean values of four biological replicates). (B) ATP content in the XR-containing cells kept in the dark or exposed to white light of 500 μmol photons m−2 s−1. Three technical replicates were carried out. (C) Cultivation of S. glacialis AAP5 at 7°C under continuous light and dark. Light intensity was 500 μmol photons m−2 s−1. The mean values and standard deviations from three biological replicates are shown.
Discussion
Here, we have demonstrated that the bacterium S. glacialis AAP5 is capable of dual phototrophy. It possesses all the necessary genes for BChl- and XR-based light energy conversion and, under appropriate physiological conditions, uses them both and even at the same time to generate a proton gradient across the membrane for the production of ATP. Thereby, the need to generate energy through aerobic respiration is reduced and saves available organic carbon for growth, which is scarce in oligotrophic aquatic ecosystems such as alpine lakes (31). This effect has been documented for both BChl-containing (32–34) and rhodopsin-containing photoheterotrophs (35–37).
Yet, why does S. glacialis AAP5 possess two different phototrophic systems that basically fulfill the same function? Zeng et al. proposed that differences in the absorption spectra of rhodopsins and BChl a–based photosystems might explain the simultaneous genomic presence of both (20). The penetration of blue and green light differs between snow and ice and might favor one system over the other (20). We think that this hypothesis is not correct. While BChl a molecules absorb in the UV and IR parts of the spectrum, the photosystems contain the additional carotenoid spirilloxanthin, which absorbs blue–green light and transfers the energy to BChl a (21). XR only utilizes the visible part of the spectrum as it contains a green-absorbing rhodopsin and blue-absorbing nostoxanthin antenna (Fig. 4 B). Thus, the absorption spectra of XR and BChl a–based photosystems largely overlap in the visible spectrum (Fig. 4 B and C), and small changes of the spectral irradiance should not have any dramatic effect.
BChl-based systems are large pigment–protein complexes, which require a complex machinery for its synthesis, assembly, and regulation (1). On the other hand, due to the large number of pigments, they can effectively operate even under low-light conditions. In contrast, XR has only two chromophores: retinal and nostoxanthin antenna. The utilization of simple xanthophyll nostoxanthin is unusual as the original XR from Salinibacter ruber contains a more complex glycosylated ketocarotenoid salinixanthin (30). While XR is much more straightforward to assemble, metabolic energy balance calculations suggest that rhodopsins are efficient only at higher irradiance (13).
Another remarkable difference between XR and BChl-based photosynthesis is their contrasting regulation (Fig. 6 A). In a previous study, we documented that PGC expression was inhibited by the presence of light, glucose, or galactose (21). By contrast, XR is not inhibited by the presence of sugars, and the XR promoter is activated only at temperatures below 16°C, with the gene being fully transcribed only in the presence of light. The studied lake is an oligotrophic lake with temperatures usually lower than the determined threshold for XR activity for the whole year (9). Thus, it is likely that S. glacialis AAP5 cells contain and utilize both XR and BChl-based photosystems in its natural habitat.
Fig. 6.
Expression regulation of dual phototrophy in S. glacialis AAP5. (A) Proposed scheme of anoxygenic photosynthesis (AP) and XR regulation by nutrients, light, and temperature. (B) Hypothetical model of an alpine lake with the main environmental factors (temperature, irradiance, and snow cover) affecting the expression of dual phototrophy during the seasons. The thin black lines indicate ice cover. Cyt, cytochrome; QH2, ubiquinol.
Physical conditions in alpine lakes periodically change from moderate temperatures and high light intensities in summer to cold temperatures and coverage by ice and snow in winter that alters the light spectrum and greatly reduces its penetration (38). The possibility to use two phototrophic systems with different photochemical properties and contrasting regulation may represent an adaptation strategy allowing for perpetual growth in this challenging, dynamically changing habitat. The counterbalanced regulation of BChl- and XR-based phototrophy may in particular help to tune the ratio of both systems to light intensity, day length, and temperature (Fig. 6 B). During short winter days, light reaching the lake water column is strongly attenuated by snow and ice, which is favorable for the PGC expression and synthesis of the BChl a–containing photosystems. In late spring to early summer, ice starts to melt, and the water is still cold especially at the surface, where it is in contact with ice (39, 40). Photosystems probably cannot use the full potential of increasing light intensities as the cyclic electron transport is slower at these temperatures. Thus, XR expression becomes more advantageous. During long summer days, both systems work equally well. However, due to its simple biosynthesis, XR is the more cost-effective system (13). Reaching autumn, warmer water temperatures and shorter days with lower light influx might favor PGC expression again. This is consistent with the peak of BChl-containing bacteria in the GKS found in the middle of September (9).
In addition to the natural seasonal cycle, light intensity may rapidly fluctuate during the day due to cloud cover changes (41), which may impact the transcriptional regulation of both photosystems differently. The constitutive high activity of the XR promoter at low temperatures and the light-dependent full transcription of the gene seem to be very costly regulatory strategies but could be beneficial due to a rapid reactivation in response to light. In contrast, reactivation of PGC expression in the dark takes hours (42–44), making its repression insensitive to fluctuations in light intensity.
Are dual phototrophic organism present also in other environments? A survey of 215,874 bacterial genomes identified both PGC and rhodopsin genes in 55, mostly alphaproteobacterial, genomes. Almost half of them were of alpine or glacial origin (20), but they were also found in other environments. Recently, a BChl a–producing mesophilic Rhodobacter strain M37P was isolated from Yellowstone springs. It also contains an XR gene, but its expression has not yet been documented (45).
BChl a–based phototrophy is inherited mostly vertically, (46) and distant horizontal transfers are very rare (47). In contrast, rhodopsin genes have been found in Bacteria, Archaea, Eukaryota, and even Viruses (SI Appendix, Fig. S9) and are relatively easy to transfer horizontally (48). Thus, a BChl a–containing bacterium may eventually receive rhodopsin genes horizontally. This process may have occurred repeatedly during the evolution. However, whether these species retain and express the obtained rhodopsin gene will depend on the new genes providing a competitive advantage in a particular environment. Thus, dual phototrophy may also be beneficial in other environments with highly dynamic physicochemical conditions with extremes favoring one system over the other.
Materials and Methods
Metagenomic Analysis.
Illumina sequencing data (150-bp paired-end reads) were previously generated from the GKS (latitude 47.2298° N and longitude 11.0140° E) samples taken on October 25th, 2017, February 28th, 2018, and April 26th, 2018, in 5-m depth (23). Water temperature was 4°C for all samples. In February and April, the lake was covered by snow and ice. Metagenome assembly binning and functional annotation were performed using the SqueezeMeta pipeline with default settings (49). Binning results from the MaxBin2 (50) and MetaBAT 2 (51) were combined using the DAS Tool (52). Bin statistics were computed using the CheckM (53). Mapping of reads to the S. glacialis AAP5 genome (GenBank accession GCF_004354345.1) was performed using Bowtie 2 in the local mode and allowing for one mismatch in the seed alignment.
Phylogenetic Analyses.
Nucleotide sequences of the conserved 300-bp upstream intergenic region, the XR gene, and the PS RC subunit M (pufM) gene were retrieved from genomes downloaded from NCBI GenBank (October 2021) or from metagenomes (23) and aligned using ClustalW (54). Ambiguously aligned regions and gaps were manually excluded from further analysis. The phylogenetic trees were computed using both neighbor-joining (NJ) (55) and maximum likelihood (ML) (56) algorithms included in MEGA 6.06 software (57). The Tamura–Nei model (58) was used for inferring the NJ trees. The ML trees were constructed using the GTR nucleotide substitution model (59). A uniform rate of nucleotide substitution was used.
Cultivation Conditions.
S. glacialis AAP5 (=DSM 111157=CCUG 74776) was grown aerobically in Erlenmeyer flasks placed in a temperature-controlled incubator on an orbital shaker. Illumination was provided by a bank of Dulux L 55W/865 luminescent tubes (Osram GmbH, Munich, Germany, spectral temperature of 6,500 K). At the beginning of each experiment, the inoculum (approximately OD600 = 0.8) was grown in full organic medium at 22°C in darkness and then diluted to OD600 = 0.003 with organic medium containing 10× diluted organic components (21). The cultures were illuminated (photon flux density ~100 µmol photons m−2 s−1) in either continuous or 12-h light–12-h dark regime. For XR and pufM transcription experiments, sampling was done 4 h after turning the light on and 4 h after turning it off. For the transcriptome experiment, sampling was done 1 h and 6 h after the light was switched on and 1 h and 6 h after the light was switched off. E. coli heterologously expressing XR was grown as described earlier (21). For spectroscopic analyses, respiration assay, ATP assay, and the cultivation experiment, S. glacialis AAP5 was incubated under continuous light (~500 µmol photons m−2 s−1) to prevent BChl a formation. As a control for the respiration assay, cells were grown at 22°C. Control cultures for the cultivation experiment were incubated in the dark.
Pigment Analysis.
The cells were collected by centrifugation (10,000 × g for 5 min); the pellet was gently resuspended in 40 µl water and then extracted with 1 ml acetone:methanol (7:2 vol:vol). The pigment extracts were analyzed using a high-performance liquid chromatography system Nexera LC-40 HPLC system (Shimadzu Inc., Tokyo, Japan) equipped with a diode-array UV–VIS detector as described earlier (21).
RNA Isolation and Purification.
Cells were harvested by centrifugation. Pellets were resuspended in 1 ml PGTX extraction solution (60) and immediately frozen in liquid nitrogen. RNA was extracted and processed as described earlier (21).
Reverse Transcription Quantitative PCR (RT-qPCR).
XR and pufM transcripts were reverse-transcribed and relatively quantified in triplicates as described earlier (21). Primers and PCR conditions for the XR leader sequence can be found in SI Appendix, Table S1. The comparative Ct method (61) was used to quantify changes in gene expression.
RNA Sequencing and Transcriptome Analysis.
Cultivations for RNA sequencing were performed in triplicates. Due to the low yield of RNA, replicates of the day 4 samples had to be pooled. Libraries were generated according to Shishkin et al. (62). including rRNA removal with the Ribo-Zero Kit (Illumina Inc., USA). The library was sequenced on a NovaSeq 6000 (Illumina Inc., USA) in paired-end mode with 100 cycles in total. Raw reads were processed, and differential gene expression was assessed as described before (21). Briefly, quality-filtered reads were mapped to the S. glacialis AAP5 genome (GenBank accession GCF_004354345.1) using Bowtie 2. FeatureCounts was used to assess the number of reads per gene. Normalization and identification of significantly differentially regulated genes (FDR < 0.05) were performed with edgeR.
XR Purification.
The cell pellet was resuspended in 20 mM Tris-Cl, pH 8.0, and homogenized thoroughly with a few grains of DNase and a few milligrams of MgCl2. The cells were broken by passaging three times through an Emulsiflex-C5 cell disrupter (Avestin, Inc., Canada), after which for all the following steps, the sample was kept as dark as possible. The ruptured cell suspension was ultracentrifuged (120 min, 180,000 × g, and 4°C), and the resulting membrane pellet was gently resuspended in 20 mM Tris-Cl at pH 8.0 before the optical density (OD) was adjusted to ~5 cm−1 at the XR absorption maximum (~490 nm). The resuspended membranes were solubilized with 2.0% n-dodecyl-β-D-maltoside (DDM), 0.2% Triton X-100 for 60 min stirring at RT, and then centrifuged (19,600 × g, 30 min, 4°C) to remove any non-solubilized material. The resulting translucent sample was gently loaded onto a stepwise sucrose gradient (0.4 M (5 ml), 0.6 M (6.5 ml), 0.8 M (6.5 ml), and 1.2 M (5.0 ml) sucrose in TD buffer (0.02% DDM, 20 mM Tris-Cl, and pH 8.0)) and ultracentrifuged overnight (175,000 × g at 4°C). The orange XR-containing band was gently removed from the gradient, loaded on an anion-exchange gravity column at 10°C with TOYOPEARL Phenyl-650S resin (Tosoh), and eluted with increasing concentrations of NaCl in TD buffer. The resulting fractions were assayed spectrophotometrically for purity, then pooled, concentrated, and run on a Sephacryl S-300 (GE Healthcare) size-exclusion column. The best fractions (protein absorption 275 nm/XR absorption 548 nm ≤ 2.0) were pooled and concentrated to the required OD. For comparison, XR was also heterologously expressed in E. coli and purified (21). Absorption spectra were recorded using a Shimadzu UV-2600 spectrometer equipped with an integrating sphere.
Fluorescence Spectroscopy.
The excitation spectrum of XR was measured using Fluorolog 3.2.2. (Horiba Ltd., Kyoto, Japan) in 3 × 3-mm quartz cuvettes. Spectra bandwidth for the measurement was set to 5 nm for excitation and 8 nm for emission. Fluorescence was detected at 630 nm. To obtain the emission spectrum, fluorescence was excited at 485 nm. Due to a minor contamination of the sample by tetrapyrroles, the steady-state spectrum of XR emission could not be easily obtained due to the low fluorescence yield of retinal. Hence, a time-resolved picosecond measurement of fluorescence was performed using a FluoTime 300 (PicoQuant, Berlin, Germany), and the emission spectrum of XR (emission bandwidth 5 nm) was acquired as the fastest decay component. In this way, the contributions of other pigments to the fluorescence signal were straightforwardly eliminated as a result of the several orders of magnitude difference in lifetimes.
Infrared Variable Fluorescence Measurements.
BChl a fluorescence induction and relaxation were measured using an FL-3000 double-modulation fluorometer (Photon Systems Instruments Ltd., CZ) as described by Koblížek et al. (63). Temperature-dependent measurements using a TR 2000 thermoregulator (Photon Systems Instruments Ltd., CZ) were performed according to Kaftan et al. (64). The cell pellet was resuspended in 2.4 ml fresh medium to a final concentration of 100 nM BChl a. The dark-adapted cells were equilibrated for 5 min at the selected temperature and then subjected to a single saturating pulse (100 μs, 330 ± 7 mmol photons m−2 s−1, λMAX = 460 nm, and FWHM = 25 nm, limited by a short-pass interference filter λ < 650 nm). The fluorescence relaxation was probed by logarithmically spaced flashlets commencing at 10 μs after the saturating pulse. A train of saturating pulses simulating a continuous light excitation of 1.6 mmol photons m−2 s−1 followed by fluorescence relaxation measurements probed the PS activity of light-adapted cells. The BChl a fluorescence signal was registered using a silicon photodiode detector (λ > 850 nm) with 100-ns time resolution. The induction part was analyzed assuming the restricted energy transfer between photosystem units. The relaxation was fitted using three exponential kinetics as described earlier (63)
Time-Resolved Absorption Spectroscopy.
Flash photolysis experiments with microsecond resolution were performed in the 400- to 700-nm spectral range using a locally built spectrometer (65). The measurement was performed, and the data were analyzed as described previously (21, 66). Mostly, the measurements were performed using the intermediate spectral resolution of the instrument, 6.4 nm/pixel; however, some experiments on membranes were done using a lower spectral resolution (12.8 nm/pixel) in order to be able to simultaneously cover the whole range of 480–900 nm in a single measurement to capture processes in XR and the RC.
Cryoelectron Microscopy.
To obtain the contour density map, the purified RC–LH1 complexes were deposited on glow-discharged holey carbon grids and rapidly frozen in liquid ethane with plunge freezer LEICA EM GP2 (Leica Microsystems, Germany). For sample visualization, a 200-kV JEOL JEM 2100F transmission electron microscope (TEM) was used (JEOL, Tokyo, Japan, at 25,000× magnification). TEM images were recorded using a Gatan K2 Summit direct detection camera, with a resolution corresponding to 1.4 Å per pixel. A dataset of 320,000 particles was collected, and image analysis was carried out using cryoSPARC.
Respiration.
Respiration was measured using an OX1LP Dissolved Oxygen Clark-type oxygen electrode (Qubit Systems Inc., Kingston, Canada) in a thermostat-controlled measuring chamber at 7°C. Illumination was provided by an A113 LED light source (Qubit Systems Inc., ON, Canada) delivering “white” light with two major emission peaks at 455 nm (FWHM = 35 nm) and 570 nm (FWHM = 130 nm). Each set of experiments was carried out with a freshly cleaned polarographic electrode fitted with a new High-Sens semipermeable membrane (YSI Inc., Yellow Springs, OH, USA). The two-point calibration was done using sterile growth medium bubbled first with nitrogen (no oxygen) and then saturated with air. The respiration rate was calculated as a slope of the linear fit of time-dependent measurements of the oxygen concentration inside the measuring chamber.
ATP Assay.
ATP content in cells was determined using the BacTiter-Glo™ Microbial Cell Viability Assay (Promega, Madison, USA). Cells were grown in a diluted organic medium at low temperature (7°C) under continuous illumination to induce XR biosynthesis but at the same time to inhibit BChl a synthesis. Cells reaching their early stationary phase were incubated for 24 h in the dark, subsequently harvested, and resuspended in buffer. The resulting suspension (~1.62 × 108 cells ml−1) was pipetted in a 96-well opaque plate and exposed to 500 µmol photons m−2 s−1 of white light provided by an RSL 231 LED lamp (Retlux Ltd., Říčany, Czech Republic) for 2 to 10 min. Then, it was mixed with an equal volume of assay reagent and incubated at RT for 5 min, and luminescence was recorded using a FLUOstar Omega plate reader (BMG LABTECH GmbH, Offenburg, Germany). Cells incubated in the dark were used as a reference.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Acknowledgments
This study was mainly financed by the Czech Science Foundation, EXPRO project 19-28778X PhotoGemm+ (K.K., J.T., A.T.G., and M.K.). JT was also partially financed by the mobility grant CZ.02.2.69/0.0/0.0/18_053/0017705 of the Czech Ministry of Education. D.B. acknowledges the Czech Science Foundation grant 20-01159S and Institutional Research Concept (RVO: 60077344). D.K. and Z.G. were supported by the European Regional Development Fund project no. CZ.02.1.01/0.0/0.0/15_003/0000441. Z.G. was also supported by the LM2018129 Czech-BioImaging project. We thank Jason Dean and Adéla Vaňková for technical assistance, Kateřina Delawská for her help with the ATP assay, Sahana Shivaramu for her expertise in the measurements using the flow cytometry, and Eric Weninger for providing in situ temperature data. Funding: The authors received research support from the Czech Science Foundation, EXPRO project 19-28778X PhotoGemm+, mobility grant CZ.02.2.69/0.0/0.0/18_053/0017705 of the Czech Ministry of Education, the Czech Science Foundation grant 20-01159S and Institutional Research Concept (RVO: 60077344), the European Regional Development Fund project no. CZ.02.1.01/0.0/0.0/15_003/0000441, and the LM2018129 Czech-BioImaging project.
Author contributions
K.K., J.T., and M.K. designed research; K.K., J.T., D.K., A.T.G., D.B., Z.G., A.D., and M.K. performed research; K.K., J.T., D.K., D.B., Z.G., and C.B. analyzed data; C.B., R.G., and R.S. provided data; and K.K., J.T., D.K., D.B., R.S., and M.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
RNA sequencing data are publicly available at the National Center for Biotechnology Information gene expression omnibus database under accession number GSE196609 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE196609).
Supporting Information
References
- 1.Gardiner A. T., Nguyen-Phan T. C., Cogdell R. J., A comparative look at structural variation among RC–LH1 ‘Core’ complexes present in anoxygenic phototrophic bacteria. Photosynth. Res. 145, 83–96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankenship R. E., Early evolution of photosynthesis. Plant Physiol. 154, 434–438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yurkov V. V., Csotonyi J. T., “New light on aerobic anoxygenic phototrophs” in The Purple Phototrophic Bacteria, Hunter C. N., Daldal F., , M. C. Thurnauer, Beatty J. T., Eds. (Advances in Photosynthesis and Respiration, Springer, 2009), vol. 28, pp. 31–55. [Google Scholar]
- 4.Koblížek M., Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 39, 854–870 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kolber Z. S., et al. , Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292, 2492–2495 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Consarnau L., et al. , Microbial rhodopsins are major contributors to the solar energy captured in the sea. Sci. Adv. 5, eaaw8855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazulla C. R., et al. , Global diversity and distribution of aerobic anoxygenic phototrophs in the tropical and subtropical oceans. Environ. Microbiol. 24, 2222–2238 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Mašín M., Nedoma J., Pechar L., Koblížek M., Distribution of aerobic anoxygenic phototrophs in temperate freshwater systems. Environ. Microbiol. 10, 1988–1996 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Čuperová Z., Holzer E., Salka I., Sommaruga R., Koblížek M., Temporal changes and altitudinal distribution of aerobic anoxygenic phototrophs in mountain lakes. Appl. Environ. Microbiol. 79, 6439–6446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrera I., et al. , Diversity and distribution of freshwater aerobic anoxygenic phototrophic bacteria across a wide latitudinal gradient. Front. Microbiol. 8, 175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinhassi J., DeLong E. F., Béjà O., González J. M., Pedrós-Alió C., Marine bacterial and archaeal ion-pumping rhodopsins: Genetic diversity, physiology, and ecology. Microbiol. Mol. Biol. Rev. 80, 929–954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balashov S. P., et al. , Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science 309, 2061–2064 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchman D. L., Hanson T. E., Bioenergetics of photoheterotrophic bacteria in the oceans. Environ. Microbiol. Rep. 5, 188–199 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Oesterhelt D., Stoeckenius W., Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature New Biol. 233, 149–152 (1971). [DOI] [PubMed] [Google Scholar]
- 15.Béjà O., et al. , Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science 289, 1902–1906 (2000), 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 16.Rusch D. B., et al. , The Sorcerer II Global Ocean Sampling expedition: Northwest Atlantic through eastern tropical Pacific. PLoS Biol. 5, e77 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell B. J., Waidner L. A., Cottrell M. T., Kirchman D. L., Abundant proteorhodopsin genes in the North Atlantic Ocean. Environ. Microbiol. 10, 99–109 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Frias-Lopez J., et al. , Microbial community gene expression in ocean surface waters. Proc. Natl. Acad. Sci. U.S.A. 105, 3805–3810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atamna-Ismaeel N., et al. , Widespread distribution of proteorhodopsins in freshwater and brackish ecosystems. ISME J. 2, 656–662 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y., et al. , Potential rhodopsin- and bacteriochlorophyll-based dual phototrophy in a high Arctic glacier. mBio 11, e02641-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopejtka K., et al. , Simultaneous presence of bacteriochlorophyll and xanthorhodopsin genes in a freshwater bacterium. mSystems 5, 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopejtka K., et al. , Characterization of the aerobic anoxygenic phototrophic bacterium Sphingomonas sp. AAP5. Microorganisms 9, 768 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellas C. M., Sommaruga R., Polinton-like viruses are abundant in aquatic ecosystems. Microbiome 9, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovács Á. T., Rákhely G., Kovács K. L., The PpsR regulator family. Res. Microbiol. 156, 619–625 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Dubey A. P., Pandey P., Mishra S., Gupta P., Tripathi A. K., Role of a fasciclin domain protein in photooxidative stress and flocculation in Azospirillum brasilense Sp7. Res. Microbiol. 172, 103875 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Glantz S., et al. , Functional and topological diversity of LOV domain photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 113, 201509428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash A. I., et al. , Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl. Acad. Sci. U.S.A. 108, 9449–9454 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bína D., Litvin R., Vácha F., Kinetics of in vivo bacteriochlorophyll fluorescence yield and the state of photosynthetic apparatus of purple bacteria. Photosynth. Res. 99, 115–125 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Lewis A., Spoonhower J. P., Perreault G. J., Observation of light emission from a rhodopsin. Nature 260, 675–678 (1976). [DOI] [PubMed] [Google Scholar]
- 30.Balashov S. P., Imasheva E. S., Wang J. M., Lanyi J. K., Excitation energy-transfer and the relative orientation of retinal and carotenoid in xanthorhodopsin. Biophys. J. 5, 2402–2414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez M. T., Rofner C., Sommaruga R., Dissolved organic monomer partitioning among bacterial groups in two oligotrophic lakes. Environ. Microbiol. Rep. 7, 265–272 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harashima K., Shiba T., Totsuka T., Simidu U., Taga N., Occurrence of bacteriochlorophyll a in a strain of an aerobic heterotrophic bacterium. Agric. Biol. Chem. 42, 1627–1628 (1978). [Google Scholar]
- 33.Hauruseu D., Koblížek M., Influence of light on carbon utilization in aerobic anoxygenic phototrophs. Appl. Environ. Microbiol. 78, 7414–7419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piwosz K., Villena-Alemany C., Mujakić I., Photoheterotrophy by aerobic anoxygenic bacteria modulates carbon fluxes in a freshwater lake. ISME J. 16, 1046–1054 (2021), 10.1038/s41396-021-01142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez-Consarnau L., et al. , Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature 445, 210–213 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Lami R., Cottrell M. T., Campbell B. J., Kirchman D. L., Light-dependent growth and proteorhodopsin expression by Flavobacteria and SAR11 in experiments with Delaware coastal waters. Environ. Microbiol. 11, 3201–3209 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Feng S., Powell S. M., Wilson R., Bowman J. P., Light-stimulated growth of proteorhodopsin-bearing sea-ice psychrophile Psychroflexus torquis is salinity dependent. ISME J. 7, 2206–2213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sommaruga R., The role of solar UV radiation in the ecology of alpine lakes. J. Photochem. Photobiol. B 1–2, 35–42 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Felip M., Wille A., Sattler B., Psenner R., Microbial communities in the winter cover and the water column of an alpine lake: System connectivity and uncoupling. Aquat. Microb. Ecol. 29, 123–134 (2002). [Google Scholar]
- 40.Bertilsson S., et al. , The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr. 58, 1998–2012 (2013). [Google Scholar]
- 41.Blumthaler M., Ambach W., Cede A., Staehelin J., Attenuation of erythemal effective irradiance by cloudiness at low and high altitude in the Alpine region. Photochem. Photobiol. 63, 193–196 (1996). [Google Scholar]
- 42.Yurkov V. V., van Gemerden H., Impact of light/dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch. Microbiol. 159, 84–89 (1993). [Google Scholar]
- 43.Tomasch J., Gohl R., Bunk B., Diez M. S., Wagner-Döbler I., Transcriptional response of the photoheterotrophic marine bacterium Dinoroseobacter shibae to changing light regimes. ISME J. 5, 1957–1968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottesen E. A., et al. , Ocean microbes. Multispecies diel transcriptional oscillations in open ocean heterotrophic bacterial assemblages. Science 345, 207–212 (2014), 10.1126/science.1252476. [DOI] [PubMed] [Google Scholar]
- 45.Kyndt J. A., Robertson S., Shoffstall I. B., Ramaley R. F., Meyer T. E., Genome sequence and characterization of a xanthorhodopsin-containing, aerobic anoxygenic phototrophic Rhodobacter species, isolated from mesophilic conditions at Yellowstone National Park. Microorganisms 10, 1169 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imhoff J. F., Rahn T., Künzel S., Neulinger S. C., Phylogeny of anoxygenic photosynthesis based on sequences of photosynthetic reaction center proteins and a key enzyme in bacteriochlorophyll biosynthesis, the chlorophyllide reductase. Microorganisms 7, 576 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y., Feng F., Medová H., Dean J., Koblížek M., Functional type 2 photosynthetic reaction centers found in the rare bacterial phylum Gemmatimonadetes. Proc. Natl. Acad. Sci. U.S.A. 111, 7795–7800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma A. K., Spudich J. L., Doolittle W. F., Microbial rhodopsins: Functional versatility and genetic mobility. Trends Microbiol. 14, 463–469 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Tamames J., Puente-Sánchez F., SqueezeMeta, A highly portable, fully automatic metagenomic analysis pipeline. Front. Microbiol. 9, 3349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y.-W., Simmons B. A., Singer S. W., MaxBin 2.0: An automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Kang D. D., et al. , MetaBAT 2: An adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ. 7, e7359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sieber C. M. K., et al. , Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 3, 836–843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks D. H., Imelfort M., Skennerton C. T., Hugenholtz P., Tyson G. W., CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson J. D., Higgins D. G., Gibson T. J., CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitou N., Nei M., The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987). [DOI] [PubMed] [Google Scholar]
- 56.Felsenstein J., Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 17, 368–376 (1981). [DOI] [PubMed] [Google Scholar]
- 57.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S., MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura K., Nei M., Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Tavare S. “Some probabilistic and statistical problems in the analysis of DNA sequences” in Some Mathematical Questions in Biology/DNA Sequence Analysis, Miura R. M., Ed. (AMS, 1986). [Google Scholar]
- 60.Pinto F., Thapper A., Sontheim W., Lindblad P., Analysis of current and alternative phenol based RNA extraction methodologies for cyanobacteria. BMC Mol. Biol. 10, 79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livak K. J., Schmittgen T. D., Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Shishkin A. A., et al. , Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods 12, 323–325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koblížek M., et al. , Utilization of light energy in phototrophic Gemmatimonadetes. J. Photochem. Photobiol. B 213, 112085 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Kaftan D., Bína D., Koblížek M., Temperature dependence of photosynthetic reaction centre activity in Rhodospirillum rubrum. Photosynth. Res. 142, 181–193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bína D., Litvín R., Vácha F., Šiffel P., New multichannel kinetic spectrophotometer-fluorimeter with pulsed measuring beam for photosynthesis research. Photosynth. Res. 88, 351–356 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Saccon F., et al. , A flexible LHCII structure allows for fine-tuning of excitation energy dissipation. SSRN Electron. J. (2020), 10.2139/ssrn.3600541. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Data Availability Statement
RNA sequencing data are publicly available at the National Center for Biotechnology Information gene expression omnibus database under accession number GSE196609 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE196609).