Abstract
Pulmonary arterial hypertension (PAH) is a complex multifactorial disease with poor prognosis characterized by functional and structural alterations of the pulmonary circulation causing marked increase in pulmonary vascular resistance (PVR), ultimately leading to right heart failure and death. Mutations in the gene encoding Bone Morphogenetic Protein Receptor type 2 (BMPR2), a receptor for the transforming growth factor-beta (TGF-β) superfamily, account for over 70% of families with PAH, and approximately 20% of sporadic cases. In recent years, however, less common or rare mutations in other genes have been identified. This review will consider how these newly discovered PAH genes could help to provide a better understanding of the molecular and cellular bases of the maintenance of the pulmonary vascular integrity, as well as their role in the PAH pathogenesis underlying occlusion of arterioles in the lung. We will also discuss how insights into the genetic contributions of these new PAH-related genes may open up new therapeutic targets for this, currently incurable, cardiopulmonary disorder.
Keywords: pulmonary hypertension, pulmonary vascular remodeling, functional genetic, gene mutation, familial
1. Introduction
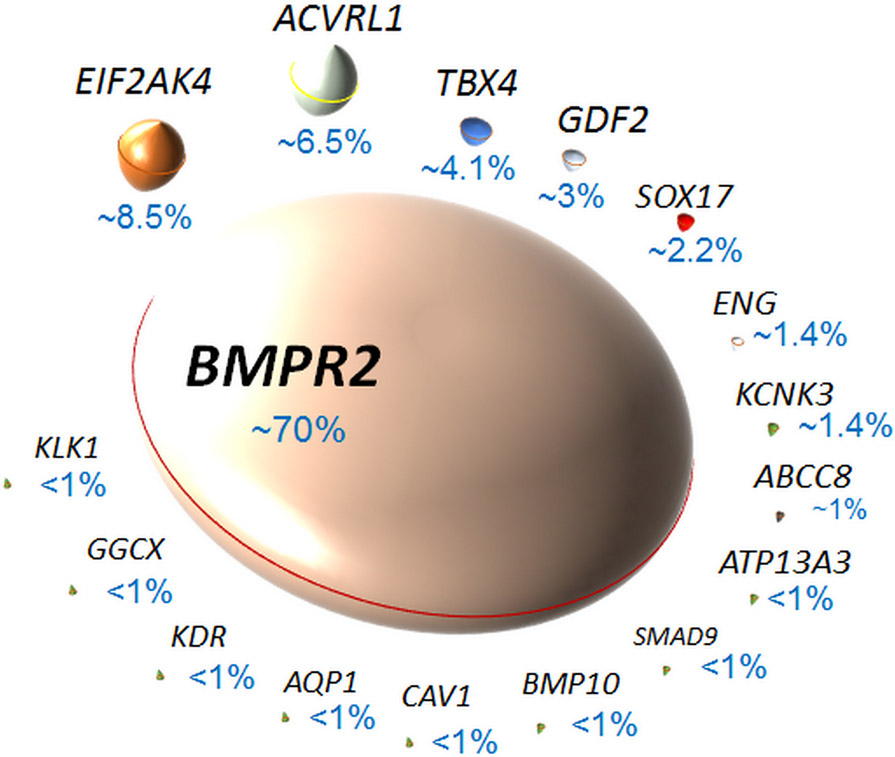
The term pulmonary arterial hypertension (PAH) encompasses a heterogeneous group of devastating and incurable cardiopulmonary vascular disorders that are characterized by vasoconstriction and remodeling in the distal pulmonary arteries 1, 2. PAH may occur in a number of different clinical contexts (Table 1): sporadically, without an identifiable underlying cause, called idiopathic PAH, or in association with exposure to certain weight reducing drugs and certain toxins, or other diseases (aPAH) like congenital heart disease, connective tissue disease, human immunodeficiency virus (HIV), schistosomiasis, portal hypertension or as a pulmonary veno-occlusive disease (PVOD). When PAH occurs in a familial context (called heritable PAH), heterozygous germline mutations in the Bone Morphogenetic Protein Receptor type 2 (BMPR2) gene are detected in at least 70 to 80% of cases 3, 4. Recently, mutations located in other genes of different members of the transforming growth factor (TGF)-β superfamily have been reported (Table 2 and Figure 1, 2, and 3), including genes encoding general control nonderepressible (GCN)-2 (EIF2AK4), activin receptor-like kinase (ALK)-1 (ACVRL1), transcription factor T-box 4 (TBX4), BMP9 (GDF2), SRY-related HMG-box transcription factor (SOX17), endoglin (ENG), a potassium two pore domain channel called TWIK-related acid-sensitive potassium channel (TASK)-1 (KCNK3), sulfonylurea receptor (SUR)-1 (ABCC8), ATPase 13A3 (ATP13A3), SMAD family member 8 (SMAD9), BMP10 (BMP10), aquaporin-1 (AQP1), SMAD1 (SMAD1), SMAD4 (SMAD4), and caveolin-1 (CAV1). Currently, it remains unclear how mutations in these newly discovered genes in certain familial PAH cases affect the maintenance of the pulmonary vascular integrity and lead to occlusion of arterioles in the lung. We review here the current understanding of the connections between these newly discovered PAH-predisposing gene mutations, disease pathology and clinical manifestation. We also discuss how dissecting the connections between these newly identified PAH-related genes could increase our current understanding of the PAH pathogenesis, and offer new therapeutic targets.
Table 1:
Classification of Pulmonary Hypertension (From 2)
| 1. Pulmonary Arterial Hypertension (PAH) |
|
| 2. PH due to left heart disease | |
| 3. PH due to lung diseases and/or hypoxia | |
| 4. PH due to pulmonary artery obstructions | |
| 5. PH with unclear and/or multifactorial mechanisms | |
Table 2:
Gene name, functions of 16 genes related to pulmonary arterial hypertension (PAH)
| Gene Symbol | Location | Product name | Function |
|---|---|---|---|
| ABCC8 | 11p15.1 (38 exons) | sulfonylurea receptor 1 (SUR1) | This gene encodes SUR1 that is a member of the ATP-binding cassette (ABC) family of transporter proteins which function as transporters, ion channels and channel regulators. |
| ACVRL1 | 12q13.13 (11 exons) | Activin receptor-like kinase-1 (ALK1) | This gene encodes a transmembrane type I receptor that is abundantly expressed by endothelial cells and that leads to activation of SMAD1/5/8. |
| AQP1 | 7p14.3 (4 exons) | Aquaporin-1 | This gene encodes a protein that is a membrane channel that allows rapid water movement driven by a transmembrane osmotic gradient. |
| ATP13A3 | 3q29 (36 exons) | ATPase 13A3 | This gene encodes a P-type ATPase that transport a variety of cations across membranes. |
| BMP10 | 2p13.3 (2 exons) | Bone morphogenetic protein 10 (BMP-10) | This gene encodes BMP-10 that is a secreted protein that is predominantly expressed by the right atria. Among BMPs, BMP9 and BMP10 are two high affinity ligands for ALK1 and BMPRII present in a heterotetrameric complex on pulmonary endothelial cells. |
| BMPR2 | 2q33.1-q33.2 (13 exons) | Bone morphogenetic protein receptor, type II (BMPRII) | This gene encodes a transmembrane type II receptor with an intrinsic serine/threonine kinase domain that is expressed on pulmonary vascular endothelial and smooth muscle cells and different inflammatory cell types. On ligand binding, BMPRII phosphorylates and activates type I receptors which then activates SMAD1/5/8. |
| CAV1 | 7q31.2 (4 exons) | Caveolin-1 | This gene encodes a small, oligomeric scaffolding protein, typically required to generate membrane curvature in structures, such as caveolae. In addition, caveolin-1 has been reported to bind to multiple other proteins, to control cholesterol homeostasis, and to modulate different cell functions, such as endocytosis, cholesterol accumulation, receptor internalization, and cell signaling, proliferation, and cell apoptosis. |
| EIF2AK4 | 15q15.1 (39 exons) | General control nonderepressible 2 (GCN2) | This gene encodes the protein kinase GCN2 that predominantly responds to amino acid deprivation by binding uncharged transfer RNAs. When activated, GCN2 phosphorylates the alpha subunit of eukaryotic translation initiation factor-2 (EIF2), resulting in the downregulaton of protein synthesis. |
| ENG | 9q34.11 (15 exons) | Endoglin | This gene encodes the transmembrane type III receptor endoglin that is predominantly expressed on proliferating endothelial cells in vitro and on angiogenic blood vessels in vivo. |
| GDF2 | 10q11.22 (2 exons) | Bone morphogenetic protein 9 (BMP-9) | This gene encodes BMP-9 that is a secreted protein that is predominantly expressed by the liver. Among BMPs, BMP9 and BMP10 are two high affinity ligands for ALK1 and BMPRII present in a heterotetrameric complex on pulmonary endothelial cells. |
| GGCX | 2p11.2 (15 exons) | Gamma-glutamyl carboxylase (GGCX) | This gene encodes an integral membrane protein of the rough endoplasmic reticulum that carboxylates glutamate residues of vitamin K-dependent proteins to gamma carboxyl glutamate. |
| KCNK3 | 2p23.3 (3 exons) | TWIK-related acid-sensitive potassium channel (TASK-1) | This gene encodes TASK-1, which is an acid-sensitive potassium (K+) channel containing two pore-forming P domains and contributing to resting membrane potentials. |
| KDR | 4q12 (30 exons) | Vascular endothelial growth factor receptor 2 (VEGFR2) | This gene encodes the vascular endothelial growth factor receptor 2 (VEGFR2) that is considered to be one of the main receptors involved in endothelial cell proliferation, migration and survival |
| KLK1 | 19q13.33 (6 exons) | Kallikrein 1 | This gene encodes a member of the kallikrein subfamily of serine proteases that are involved in diverse physiological functions. |
| SMAD1 | 4q31.21 (12 exons) | SMAD family member 1 (SMAD1) | This gene encodes SMAD1. Once phosphorylated, SMAD1 binds with Smad4 and migrates to the nucleus. |
| SMAD4 | 18q21.2 (12 exons) | SMAD family member 4 (SMAD4) | This gene encodes SMAD4 that plays a pivotal role in mediating the downstream effects of the BMP/TGF-β superfamily signaling. |
| SMAD9 | 13q13.3 (10 exons) | SMAD family member 8 (SMAD8) | This gene encodes SMAD8. Once phosphorylated, SMAD8 binds with Smad4 and migrates to the nucleus. |
| SOX17 | 8q11.23 (2 exons) | SRY-related HMG-box transcription factor (SOX17) | This gene encodes a member of the Sry-related high mobility group domain family F (SoxF) transcription factors that is a key developmental regulator of endothelial and hematopoietic lineage. |
| TBX4 | 17q23.2 (11 exons) | T-box transcription factor 4 (TBX4) | This gene encodes the T-domain transcription factors TBX4 that is expressed in the lung mesenchyme in which it plays a central role in the transcriptional regulation of genes required for mesoderm differentiation. |
Figure 1: Genetic mutations associated with familial pulmonary arterial hypertension (PAH):
(adapted from 4).
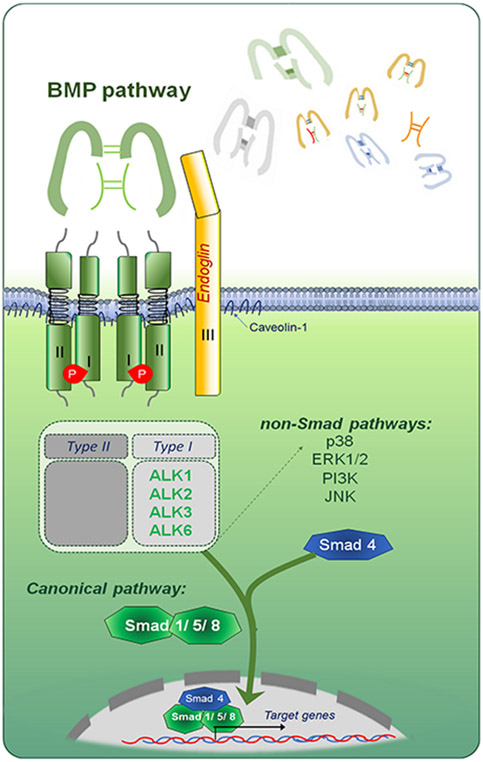
Figure 2: The BMP signalling pathway.
BMP ligands can form homo- or heterodimers which signal through a tetrameric set of receptors, comprising two type I and two type II receptors. Binding of BMP dimers to the receptors triggers a phosphorylation cascade that result in phosphorylation of Smad 1/5/8 and players of various non-SMAD pathways to initiate SMAD and non-SMAD signaling cascades. The non-SMAD pathways include signaling by mitogen-activated proteins kinases (MAPKs p38, ERK, and JNK), PI3K/AKT, and small Rho-like GTPases. Phosphorylated Smads are able to form complexes with Smad4 and translocate to the nucleus. (Illustration credit: Ben Smith)
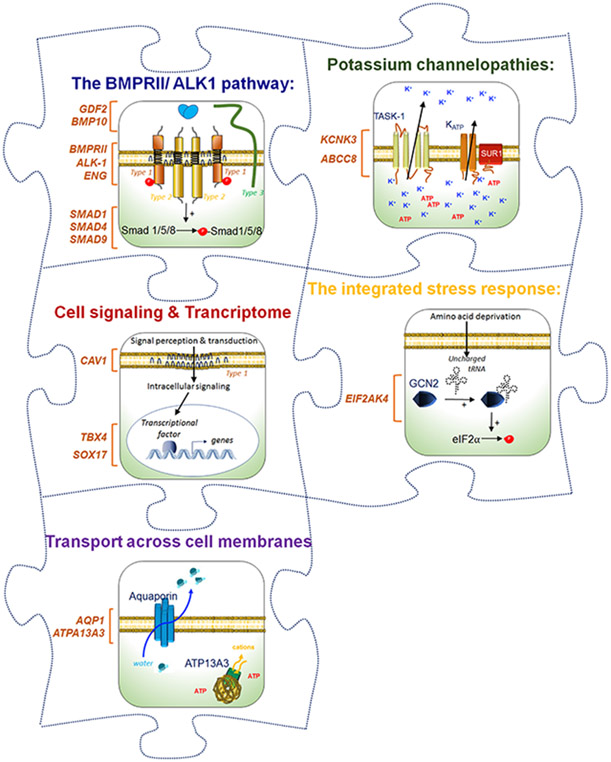
Figure 3: PAH physiopathology: a puzzle with many missing pieces.
(Illustration credit: Ben Smith)
2. Genes implicated in PAH
Heritable PAH (HPAH) includes not only cases of familial PAH defined by the presence of two or more members of the family with PAH with or without identified germline variant, but also idiopathic PAH cases when a pathogenic variant in one of the known PAH-related genes has been identified. Usually, HPAH is inherited by heterozygous mutations in an autosomal dominant manner with an incomplete penetrance (approximately 10-30%) 5, 6, which means that not all mutation carriers develop PAH during their life. Notably, the penetrance in males is estimated to be about three times lower than in females 5. Therefore, HPAH may require a second hit or a further stress for it to manifest clinically such as chronic exposure to hypoxia, oxidative stress, inflammatory signals, or to certain drugs, toxins or hormones, or even general changes in expression of genes that occur with aging of the pulmonary circulation. Additional genetic or epigenetic factors, diet, lifestyle, or a combination of all these factors may also contribute to the onset and progression of the pulmonary vascular remodeling in HPAH patients. Some pedigrees may appear to show genetic anticipation, where the disease presents at a younger age in successive generations, but this likely represents an artefact of incomplete time of observation 5. The notable exception to the dominant inheritance pattern is pulmonary veno-occlusive disease (PVOD) or pulmonary capillary hemangiomatosis (PCH), which are inherited as an autosomal recessive trait. Other, more complex genetic patterns are now starting to emerge, and will be discussed in more detail below.
2.1. Bone morphogenetic protein pathway genes in Heritable PAH
2.1.1. BMPR2
BMPR2 was the first gene identified in families with PAH 7, 8, and accounts for around 70-80% of heritable cases. It encodes the bone morphogenetic protein (BMP) receptor type-II (Figure 2). The majority of pathogenic variants in BMPR2 are nonsense, frameshift or splice-site mutations that are predicted to lead to degradation of the mutant transcript by nonsense-mediated mRNA decay. Deletions or duplications involving one or more exons, which would significantly disrupt protein structure, are also common. This indicates that haploinsufficiency - a 50% reduction in functional protein - is the primary molecular mechanism 9. The remaining mutations are amino acid substitutions, often occurring in functionally-important domains, such as the kinase domain. Some missense mutations may act in a dominant negative manner, downregulating the function of the remaining wildtype allele, although evidence for this remains contradictory and may be mutation-specific 10, 11.
2.1.2. ACVRL1 and Endoglin
ACVRL1 encodes activin A receptor-like kinase I, known as ALK1, which is the predominant type-I receptor in endothelial cells (Figure 2). ALK1 complexes with BMPRII to activate signaling in response to BMP9 and BMP10 12. Endoglin (gene symbol ENG) is an accessory protein, also known as CD105, that is highly expressed in endothelial cells and interacts with ALK1 to promote BMP signaling. Heterozygous loss-of-function mutations in ACVRL1 or ENG primarily cause hemorrhagic telangiectasia (HHT), a multi-system vascular disorder characterized by epistaxis, multiple telangiectases on the skin and mucosal surfaces, and arteriovenous malformations (AVMs) in major organs such as the lung, liver or brain 13, 14. PAH has been reported in a small proportion of individuals with HHT, predominantly in association with ACVRL1 mutations 15, 16. ENG mutations are less commonly associated with PAH, but have been reported in several large studies 15-18. The penetrance of HHT is considerably higher than for PAH, and thus almost all of these PAH cases also show signs of HHT. However, pulmonary hypertension may in some cases pre-date the onset of HHT, and is therefore not solely attributable to high cardiac output associated with liver AVMs 19.
2.1.3. Smads
Smad proteins operate downstream of the receptor complex, transmitting the signal to the nucleus, where they bind Smad-responsive elements to regulate gene expression (Figure 2). In the BMP pathway, Smad-1, −5 and −8 (encoded by SMAD1, SMAD5 and SMAD9 genes, respectively) are the receptor-Smads, while Smad-4 (SMAD4) is common to both the BMP and TGF-β pathways. Of these genes, SMAD9 mutations have been reported most frequently in PAH patients, although the overall prevalence is low. Interestingly, loss of function mutations in SMAD4 primarily lead to a syndrome of juvenile polyposis of the colon and HHT, with few reports of PAH 20. This may suggest that non-canonical signaling pathways downstream of BMPRII activation may predominate over canonical BMP signaling in the pathogenesis of PAH.
2.1.4. BMP9 and BMP10
BMP9 (encoded by GDF2) and BMP10 are the two major ligands of the ALK1-BMPRII receptor complex 12. Mutations in GDF2 account for up to 3-5% of HPAH cases 17, 21, although incomplete penetrance and variable expressivity have been recently reported 6, as discussed in more detail below. While some of these variants are clear loss of function that lead to premature truncation of the protein, many are missense substitutions that change a single amino acid. In vitro overexpression studies of these mutations have demonstrated that they interfere with normal protein processing. BMP9 and BMP10 are both synthesized as a pro-protein, which then dimerizes and undergoes furin cleavage to generate the mature protein that is secreted from the cell 22. Modeling of GDF2 mutations found in HPAH patients showed a significant reduction in mature BMP9, with most of the protein remaining uncleaved 17, 21, 23. This has been confirmed in vivo, with significantly lower levels of BMP9 in the plasma of PAH patients carrying pathogenic GDF2 variants compared to controls 21, 23. Recently it has been shown that BMP9 and BMP10 can form heterodimers that are biologically active in plasma 24. Interestingly, those patients with a GDF2 pathogenic variant and reduced plasma BMP9 levels also showed reduced levels of prodomain-bound BMP10 (pBMP10). Indeed, plasma BMP9 and pBMP10 levels were highly correlated, both in controls and PAH patients, with significantly lower levels of both cytokines in males than females 23. Overall, however, there were no differences in PAH versus controls, except for a slight reduction in pBMP10 in female patients, although some patients without GDF2 mutations also exhibited very low levels of BMP9 23. In contrast, plasma BMP9 levels were markedly reduced in patients with portopulmonary hypertension, even in the absence of genetic mutation, and predicted transplant-free survival 25. BMP10 mutations have also been reported in PAH patients, but less frequently than GDF2 23, 26 (Figure 1).
2.2. Other PAH risk genes
Caveolin-1 (CAV1) and KCNK3, encoding a potassium channel known as TASK1, were the first HPAH genes identified by whole exome sequencing in families that were negative for mutations in BMPR2 27, 28. More recently, whole exome or genome sequencing has identified several additional novel genes, including aquaporin-1 (AQP1), ATPase 13A3 (ATP13A3), SRY-box transcription factor 17 (SOX17), ATP binding cassette subfamily C member 8 (ABCC8), gamma-glutamyl carboxylase (GGCX), kallikrein 1 (KLK1), and vascular endothelial growth factor receptor 2 (KDR) 17, 18, 29, 30. SOX17 is especially interesting, with rare loss of function variants identified in HPAH patients, but also more common variants in an enhancer region were associated with a modest increase in PAH risk in a large meta-analysis of genome wide association studies 31 (Figure 1).
Recently, TBX4 mutations have also been described in HPAH. TBX4 encodes the T-Box Transcription Factor 4, one of a family of transcription factors that share a common DNA-binding domain known as the T-box. T-box genes are important in developmental processes, including bone development and lung branching morphogenesis. Loss-of-function mutations in TBX4 were originally found to cause small patella syndrome (SPS), a bone developmental abnormality characterized by small or absent knee-caps, and in some cases abnormalities of the pelvic bones and/or feet. The first potential link with PAH came with the identification of a microdeletion at 17q22q23.2 in a child with multiple congenital anomalies and PAH 32. The deletion encompassed TBX2 and TBX4, as well as multiple other genes. A subsequent study of patients with PAH and developmental delays or dysmorphic features narrowed down the common deletion region to suggest TBX2 and TBX4 as candidate genes 33. Sequencing of these two genes in non-syndromic PAH cases then identified intragenic loss-of-function mutations in TBX4 but not TBX2. Skeletal surveys revealed unrecognized SPS in all of the patients, and in two parents who carried the TBX4 mutation but were unaffected with PAH 33. Since then, multiple studies have validated TBX4 as a significant cause of PAH, with or without SPS, and it is now recognized as the major locus in pediatric cases 17, 18, 34.
2.3. EIF2AK4 mutations in PVOD/PCH
Familial PVOD and PCH, classified as group 1.6 at the 6th World Symposium of PH 2 (Table 1), shows autosomal recessive inheritance, and therefore differs from HPAH. EIF2AK4, encoding eukaryotic translation initiation factor 2 alpha kinase 4 also known as general control nonderepressible 2 (GCN2), was identified as the causative gene for heritable PVOD/PCH, with biallelic mutations reported in familial cases, and up to 25% of sporadic cases 35, 36. Interestingly, biallelic EIF2AK4 mutations were also identified in approximately 1% of idiopathic PAH patients in a large whole genome sequencing study 37. These patients could not be distinguished by chest CT, but showed significantly reduced diffusing capacity for carbon monoxide (DLCO) and earlier age at diagnosis. As discussed below, correct classification is important for clinical management, and therefore genetic testing for EIF2AK4 is valuable in making a differential diagnosis between PVOD/PCH and idiopathic PAH.
2.4. Mutations in Associated forms of PAH
Early reports suggested that BMPR2 mutations were found not only in familial or idiopathic PAH, but also in PAH associated with congenital heart defects or anorexigen exposure 38, 39. However, the only large systematic study of associated PAH has been from the US-based PAH Biobank, which included 1239 associated PAH cases analyzed by whole exome sequencing 18. Mutations were identified in 8 of 110 cases (7.2%) of drugs and toxins-associated PAH, in the genes BMPR2, ACVRL1, TBX4 and SOX17. For connective tissue disease-associated PAH, the frequency was 33 of 722 cases (4.6%), involving all genes except SOX17. ABCC8 was particularly frequent, representing 9 (27%) of these variants. And in CHD-associated PAH, mutations were identified in 22 of 268 cases (8.2%) with BMPR2, ACVRL1, SMAD9, TBX4, SOX17, and ABCC8 accounting for 2 or more variants each. A subset of these cases was also included in a prior publication that concluded SOX17 may account for up to 3% of CHD-PAH cases 40.
2.5. Genetic complexities: possible semi-dominant genes and digenic inheritance
Recent large-scale next generation sequencing efforts have started to uncover more complex modes of inheritance. One childhood-onset PAH case was reported with a homozygous GDF2 nonsense mutation 6, and a second case was homozygous for the Arg110Trp substitution 41, previously determined to be functionally pathogenic 23. Two other homozygous cases with protein truncating variants have been reported with pulmonary arteriovenous malformations (AVMs) but not PAH 42, 43, while a sibling of one was homozygous for the same mutation, yet unaffected. The four affected individuals showed spider-like telangiectases on the skin. All heterozygous parents were unaffected. This underlines that the incomplete penetrance of PAH extends beyond BMPR2 to other PAH genes, and also illustrates considerable variable expressivity of GDF2 mutations. This is further illustrated by several cases with heterozygous GDF2 variants and an HHT-like vascular anomaly syndrome 44, and a family with typical HHT, including childhood-onset pulmonary AVMs in the proband 45.
The occurrence of more severe disease in homozygous cases compared with heterozygotes suggests that the inheritance pattern may be semi-dominant, rather than a true dominant where homozygous cases are not more severe. However, since PAH varies considerably in the age of onset and clinical course, it can be difficult to distinguish these two scenarios. With respect to GDF2, only two of five homozygous individuals developed PAH, albeit in childhood, suggesting that the presentation is not necessarily more severe than the many documented heterozygous cases. However, it remains unclear how mutations that appear functionally equivalent can give rise to different phenotypes, either PAH and/or a spectrum of vascular anomalies that overlap with HHT.
ATP13A3 provides more convincing evidence of a dose-dependent effect, with biallelic mutations reported in three families 46. All homozygous or compound heterozygous individuals presented with PAH in early childhood and had a severe clinical course with high mortality. Their heterozygous parents and siblings were unaffected. A homozygous mutation in KCNK3 has also been reported in a consanguineous PAH family 47. The proband, who was homozygous for the variant, presented at two months of age and required lung transplantation at five years-old, whereas the heterozygous mother presented at age 19, a year after the child's birth. The heterozygous father was unaffected. Although only a single family, this suggests that KCNK3 may also show semi-dominance.
In the reverse scenario, whereas heterozygous carriers of recessive EIF2AK4 mutations are expected to be healthy, Hadinnapola et al found a significantly higher frequency of heterozygous rare, likely deleterious, EIF2AK4 variants in patients with idiopathic PAH compared with controls, suggesting that the heterozygous state may contribute to PAH risk 37. Two of these cases also had mutations in BMPR2. Similarly, co-occurrence of a BMPR2 mutation and heterozygous EIF2AK4 variant was documented in two members of a family affected with PAH 48. Two other individuals carried only the BMPR2 mutation and were unaffected. This raises the possibility that heterozygous EIF2AK4 mutations might act as modifiers of disease penetrance, although in this family, the two unaffected BMPR2 carriers were both male, where the penetrance is known to be lower. At least five other cases have been reported with likely deleterious variants in BMPR2 plus one other gene (TBX4 in one case, ACVRL1 in two, and SMAD9 in two) 17, 18. Further studies are required to understand these examples of potential digenic inheritance, and whether the second mutation increases the overall likelihood of developing PAH.
3. What does this mean for the patient or clinician?
Currently, PAH-specific therapies should only be used in patients diagnosed with PAH and not in unaffected mutation carriers. The search for the presence of a mutation in one of the known predisposing genes provides information for both the patient and clinician.
First, the presence and the type of mutation can give information about the clinical phenotype and outcomes and thus is recommended. It is indeed known that PAH patients carrying a BMPR2 3, ACVRL1 49, and EIF2AK4 50 are younger at diagnosis than non-carriers. PAH patients with BMPR2 mutations are also known to have a more severe hemodynamic at diagnosis and a worse prognosis 3. They are also less likely to show an acute vasodilator response and their diffusing capacity of the lung for carbon monoxide (DLCO) values are relatively preserved 3, 51. In addition, even with a similar afterload, right-ventricular (RV) function is more severely affected in BMPR2 mutation carriers than in noncarriers 52. In explanted lungs from BMPR2 patients with PAH, a more pronounced bronchial vascular changes (including bronchial arterial hypertrophy/ dilatation and bronchial microvessel density) have been reported, along with formation of large fibrous vascular structures that appeared to connect the systemic vasculature to pulmonary veins 53. The resultant increase in communication between systemic and pulmonary circulations could explain why higher amounts of hemoptysis are seen in PAH patients with BMPR2 mutations. Despite less severe initial hemodynamics and similar management, ACVRL1 patients with PAH had also worse prognosis compared with other patients with PAH, suggesting more rapid disease progression 49. Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis (PVOD/PCH) very often share an overlapping disease phenotype with PAH, but a genetic test identifying a bi-allelic EIF2AK4 mutation could serve as a tool for the early diagnosis of PVOD 50. Heritable PVOD/PCH due to bi-allelic EIF2AK4 mutations display similar disease severity compared with mutation non-carriers, but the response to therapy approved for PAH drugs in PVOD/PCH is rare 50. The identification of a bi-allelic EIF2AK4 mutation can therefore allow an appropriate and early referral for lung transplantation.
Second, the presence of a mutation can also help to detect PAH in an early phase and to identify predictors of outcomes in asymptomatic mutation carriers. A systematic screening for PAH in adults carrying a BMPR2 mutation demonstrates that asymptomatic BMPR2 mutation carriers have a significant risk of developing incident PAH ranging from 0.99% per year in males to 3.5% per year in females 54. Due to the severity of this devastating disease and the potential impact of abortion on parents, screening program might also lead to the consideration of pre-natal diagnosis or pre-implantation diagnosis 55, 56.
The development of genetic counselling and the analysis of the phenotypic profile of patients according to their genotype have already helped to develop preventive medicine and a specific monitoring for BMPR2 mutation carriers. This explains why the 2015 European Society of Cardiology (ESC)/ European Respiratory Society (ERS) guidelines currently recommend that “individuals who test positive for PAH-causing mutations and first-degree relatives of heritable PAH cases may be considered to have an annual screening echocardiogram (IIb-C)”. The modality for genetic testing varies between countries, but often the first step is to perform testing of a panel of the known HPAH genes. This should ideally be performed in an accredited clinical testing laboratory to ensure the highest quality assurance and correct interpretation of the results. If there is a strong suspicion of a genetic cause, a negative test may be followed up by whole exome or whole genome sequencing, either in a clinical lab or on a research basis. Practices vary as to whether the patient may be informed of the results from a research test. Due to the complexities of incomplete penetrance and variable expressivity discussed above, as well as the implications of a positive test result, it is important that patients and their families are fully informed prior to genetic testing. Such education should be provided by a genetics professional, or a PAH physician with in-depth genetic knowledge. It is important to note that a negative test result in a patient with PAH does not exclude the possibility of heritable PAH, since there are still families in whom the causative gene has not been identified. When a pathogenic variant is identified, asymptomatic family members may choose to be tested for the familial mutation, and here, a negative test result returns their PAH risk to population level (i.e., very low). Genetic counseling from a specialist clinical genetics provider is essential for asymptomatic family members who are considering a genetic test.
Pediatric PAH differs from the adult PAH in several important aspects, including sex bias, clinical presentation, etiology, response to therapy, but the genetic basis in children is also very different from adult. Indeed, children have a greater predominance of rare pathogenic inherited variants including in the TBX4 and SOX17 genes 57. Thus, there is a strong case for offering genetic counseling and testing in children with PAH.
4. Rare genetic mutations shed light on the pathogenesis of PAH: one or more paths to pathogenesis?
4.1. Experimental evidence supporting a role of BMPR2 mutations on PAH precipitation and progression
To date there is compelling in vitro and in vivo evidence for a contribution of the loss and/or alterations of the BMP pathway in the characteristic pathogenic pulmonary vascular remodeling in PAH. Defects in the BMP pathway have been indeed associated with modulation of cell proliferation, survival, and differentiation, but also with glycolytic metabolism, persistent inflammation and genome instability. Despite enormous progress, however, our understanding of the detailed mechanisms by which reduction or alteration of the BMP pathway contribute to the pulmonary vascular remodeling in PAH is still far from complete. BMP activation is indeed highly context- and dose-dependent, and the cellular response depends on which ligands are present and what receptors are expressed by the signal-receiving cell. For example, a recent study has shown that BMP9 acts as an angiogenic switch that can either promote or prevent endothelial proliferation, depending on the level of BMPRII at the endothelial cell surface 58. The activities of BMPs are dynamically and precisely regulated at different molecular levels through for example the action of highly regulated diffusible and cell surface-bound agonists and antagonists or via caveolin-1, a cell membrane protein involved in the formation of caveolin-rich microdomains in the plasma membrane essential not only for endocytosis, transcytosis, mechanotransduction, but also for the transmission of the BMP signalling and other signaling pathways. Recently, it was also shown that pulmonary artery smooth muscle cells (PA-SMCs) exposed to tumour necrosis factor (TNF)-α exhibit a reduction of BMPRII receptor presence on their cell surface, leading to BMP6-mediated PA-SMC proliferation via preferential activation of an ALK2/ACTRIIA signalling axis 59. These experimental evidences highlight how slight changes to the cellular microenvironment can result in dramatic changes to the response of pulmonary vascular cells. Therefore, a better understanding of these precise molecular mechanisms behind the subversion of the BMP signaling in PAH is essential and might help to understand the low penetrance of the BMPR2 mutations.
Transgenic animals, especially mice, have been extensively used to study the susceptibility of various knockout (KO), knockin (KI) animals to remodel pulmonary vessels in response to different known PH inducers such as chronic hypoxia, monocrotaline or SU5416 combined with chronic hypoxia (Table 3). Even if they do not reproduce all aspects of human PAH pathology, they have enriched our understanding of biology behind the BMP signaling and their role in the homeostasis and remodeling of the pulmonary circulation. For example, the demonstration that mice deficient in ALK1, BMPRII, endoglin, Smad1, Smad8 or in KLF develop spontaneous PH or were more prone to develop experimental PH relative to their wild type littermates underlines their importance in the PAH physiopathology. However, some of these results are still unclear, and much remains to be learned. Many important questions regarding the contributions of these different PAH-related genes in the pathophysiology indeed remain unanswered, not only in relation to how they regulate pulmonary vascular cell behaviors involved in vascular function, but also why they predominantly affect the pulmonary vasculature. It is also important to know to what extent the regulation of these pathways controlled by these PAH-related genes is the same as in humans.
Table 3:
Susceptibility of various transgenic animals to remodel pulmonary vessels
| Gene Symbol | Animal | Spontaneous PH | Susceptibility to chronic- hypoxia-induced PH |
References |
|---|---|---|---|---|
| ABCC8 | --- | --- | --- | --- |
| ACVRL1 | • Alk1+/− mice | ↑ RVSP ↑ Fulton index ↑ Vessel wall area |
--- | 101 |
|
AQP1
|
• Aqp1 −/− mice | = RVSP = Fulton index = % Wall area = % Muscularized PAs |
↓ RVSP ↓ Fulton index ↓ % Wall area ↓ % Muscularized PAs |
64 |
| ATP13A3 | --- | --- | --- | --- |
|
BMP10
|
• Bmp10
−/− mice (tamoxifen inducible) |
= RVSP = Fulton index = % Wall thickness = % Muscularized PAs |
↓ RVSP = Fulton index = % Wall thickness = % Muscularized PAs |
60 |
|
BMPR2
|
• Bmpr2+/− mice | ↑ mPAP ↑ % Wall thickness |
= mPAP ↓ % Wall thickness |
102 |
| • Bmpr2+/R899X mice | ↑ RVSP ↑ Fulton index ↑ % Muscularized PAs |
--- | 103 | |
| • Bmpr2ΔEx2/+ mice | = RVSP = Fulton index = % Muscularized PAs |
↑ RVSP ↑ Fulton index = % Muscularized PAs |
104 | |
| • Bmpr2+/− mice | = RVSP = Fulton index = % Muscularized PAs |
--- | 105 | |
| • Bmpr2+/Δ527bp rat • Bmpr2+/Δ16bp rat |
= RVSP = Fulton index = % Muscularized PAs |
= RVSP = Fulton index = % Muscularized PAs |
106 | |
| • Bmpr2+/Δ71 rat • Bmpr2+/Δ140 rat |
↑ mPAP ↑ Fulton index ↑ % Muscularized PAs |
↑ mPAP ↑ Fulton index ↑ % Muscularized PAs |
70 | |
| • L1cre+; Bmpr2f/f and L1cre+; Bmpr2 f/+ mice |
in a small proportion of mice: ↑ RVSP ↑ Fulton index ↑ Muscularized vessels ↑ % Wall thickness |
--- | 107 | |
| • SM22-rtTA x TetO7-BMPR2R899X mice | ↑ RVSP ↑ Fulton index ↑ Muscularized vessels |
--- | 108 | |
|
CAV1
|
• Cav-1 −/− mice | ↑ RVSP ↑ Fulton index |
↓ RVSP ↑ Fulton index |
109 |
| • EC-Cav-1 −/− mice | = RVSP = Fulton index = Vessel area = Vessel thickness |
↑ RVSP ↑ Fulton index ↑ Vessel area ↑ Vessel thickness |
110 | |
|
EIF2AK4
|
• Eif2ak4 −/− rat | = RVSP = Fulton index = % Muscularized PAs |
--- | 111 |
| ENG | • Eng+/− mice | ↑ RVSP ↑ Fulton index ↑ Vessel wall area |
= RVSP = Fulton index = Vessel wall area |
112 |
| • Eng+/− mice | = RVSP = Fulton index = % Wall thickness = % Muscularized PAs |
↓ RVSP ↓ Fulton index ↓ % Wall thickness ↓ % Muscularized PAs |
113 | |
| GDF2 | • Bmp9 +/− mice | = RVSP = Fulton index = % Wall thickness = % Muscularized PAs |
↓ RVSP ↓ Fulton index ↓ % Wall thickness ↓ % Muscularized PAs |
60, 114 |
| GGCX | --- | --- | --- | --- |
|
KCNK3
|
• Kcnk3 mutated rat | ↑ RVSP = Fulton index ↑ % Muscularized PAs |
↑ RVSP |
115 |
| KDR | • KdrΔend mice | ↑ RVSP ↑ Fulton index ↑ % Wall thickness |
↑ RVSP ↑ Fulton index ↑ % Wall thickness ↑ % smoth muscle + area |
78 |
| KLK1 | --- | --- | --- | --- |
| SMAD1 | • L1Cre(+); Smad1 mice • Tagln-Cre(+);Smad1 mice |
in a small proportion of mice: ↑ RVSP in a small proportion of mice ↑ Fulton index ↑ Muscularized vessels ↑ % Wall thickness |
--- | 116 |
| SMAD4 | --- | --- | --- | --- |
| SMAD9 | • Smad8ex4,5 mice | Presence of remodeled vessels in lungs | --- | 117 |
| SOX17 | --- | --- | --- | --- |
| TBX4 | --- | --- | --- | --- |
Because the recruitment or modulation of the BMP signalling is critical for proper function of the cardiovascular system with partial redundancy among members and complex interrelationship with other pathways, many unexpected and unexplained phenotypes can be observed in transgenic animals. For example, a recent study has highlighted redundant roles for BMP9 and BMP10 in cardiovascular homeostasis under normoxic conditions, but has also underlined specific roles under chronic hypoxic conditions 60. These authors have shown that adult Bmp9 and Bmp10 KO mice do not develop spontaneous PH under unstressed conditions, but that the combined deficiency in Bmp9 and Bmp10 lead to vascular defects resulting in a decrease in peripheral vascular resistance and blood pressure and the progressive development of high-output heart failure (HOHF) and pulmonary hemosiderosis 60. Therefore, the use of genetically engineered animals and preclinical PAH animal models, combined with functional analyses of human pulmonary vascular cell responses to exogenous BMPs in different environment not only greatly improved our understanding of the regulatory mechanisms and signaling of the different BMPs but also provided a powerful strategy to decipher their critical roles in the maintenance of the pulmonary vascular integrity and remodeling.
4.2. Other PAH-related genes offering avenues or new therapies
Although the discovery of these novel PAH related genes plays a crucial part in furthering our understanding of the basis of disease, the challenge remains whether these pathways can be targeted by therapeutic interventions. Such interventions might target a related part of the pathway or specifically correct the signaling defect brought about by a mutation. Of the recently reported PAH genes, receptors, transporters and ion channels in particular represent druggable targets, whereas transcription factors are more challenging. This section will focus on those pathways and genes that suggest potential druggable targets.
ATP13A3.
Recently, whole genome sequencing identified rare variants in ATP13A3 in PAH patients, accounting for 1.1% of a large I/HPAH cohort 17. Missense mutations in ATP13A3 are enriched within the catalytic domains, suggesting a potential disruption of normal ATP13A3 function. The finding of protein truncating variants in PAH patients suggests that loss-of-function of ATP13A3 could contribute to the pathogenesis of PAH. ATP13A3 is a member of the P-type ATPase family of transporters 61. Although the function of ATP13A3 was largely unknown, it has recently been shown to be a polyamine transporter 62. Polyamines, such as putrescine, spermidine, and spermine, are physiologically important polycations that have previously been shown to play a role in the pathobiology of PAH 63. The mechanisms by which mutations in ATP13A3 perturb polyamine metabolism remain to be determined but offer the possibility that modulation of polyamine metabolism might offer new therapeutic avenues in PAH.
AQP1.
Aquaporin 1 (AQP1) is a widely expressed water channel and is predominantly recognised for maintaining water homeostasis in the kidney. In the UK/EU PAH Cohort 9 patients were found to harbour heterozygous mutations in AQP1. Of note, 5 of these unrelated individuals had the recurrent mutation p.Arg195Trp (R195W). Structural analysis of the mutations based on the high-resolution bovine AQP1 structure predicts that the majority of identified human AQP1 rare variants are situated within the critical water channel. In particular, the recurrent variant R195W locates to the hydrophilic face of the pore. The arginine at position 195 helps define the constriction region of the AQP1 pore structure and is conserved across the water-specific aquaporins. Recently, knockout of AQP1 was shown to alleviate hypoxia-induced PAH in mice 64. Reduced angiogenesis was also observed in Aqp1 knockout mice 65. Conversely, aquaporin 1 overexpression induced proliferation and migration of pulmonary artery smooth muscle cells 66. Collectively, these findings suggest an essential role of AQP1 in vascular homeostasis and suggest that inhibitors of AQP1 are worthy of further investigation in PAH 67.
KCNK3.
Impaired potassium channel function had become a well-recognized feature of PAH pulmonary artery smooth muscle cells even prior to the identification of mutations in the KCNK3 gene, which encodes 2-pore domain potassium channel, TASK-1. The role of potassium channels in PAH has been reviewed recently 68, 69. Activators of TASK-1 have been shown to restore function and warrant further study in PAH 28. Moreover, dysfunction of BMPRII has been associated with reduced KCNK3 expression and function in rats with targeted disruption of BMPRII 70.
4.3. Possible developmental origins for PAH: TBX4, SOX17 and KDR
TBX4 is known to play a crucial role in embryonic lung development. In mouse, Tbx4 and the closely related gene Tbx5 are strongly expressed in the trachea and lung mesenchyme, and are important in lung branching morphogenesis 71. Homozygous knockout Tbx4 embryos die around day E10.5 due to failure of allantois development and placental insufficiency 72. Conditional alleles are therefore needed to study the effects of loss of Tbx4 expression. Loss of Tbx4/Tbx5 expression leads to reduced expression of Fgf10, which in turn reduces expression of Bmp4, a target of Fgf10 71. As noted earlier, TBX4 mutations are more frequent in childhood-onset PAH than adults, suggesting that developmental lung defects may directly contribute to PAH susceptibility. Galambos et al. 73 published a retrospective series of 19 neonates and children with pulmonary hypertension and either an intragenic TBX4 mutation or a large heterozygous deletion that encompassed the entire TBX4 locus and a variable number of neighboring genes. Ten of these children presented with persistent pulmonary hypertension of the newborn (PPHN), a condition that is distinct from PAH and reflects a failure to transition from the fetal to postnatal circulatory pattern. PPHN initially improved or resolved in eight cases, but the other two died before the age of one year. Subsequently, all 17 remaining children developed PH, with a variable clinical course that ranged from death or lung transplantation to resolution of their disease. Histological samples obtained via biopsy or at transplant/death showed significant defects in lung development, elements of interstitial lung disease and vascular remodeling that in some cases extended to the bronchial circulation. In one case, where a biopsy was performed at age 2 and transplant at 18, longitudinal histology showed marked progression of alveolar growth defects, as well as vascular remodeling, over time. These studies suggest that although the neonatal period may be unremarkable, abnormal lung development resulting from loss of TBX4 function can predispose to subsequent development of PH.
At the other end of the age spectrum, KDR mutations were associated with an older age of onset than other HPAH cases, many with low DLCO and evidence of interstitial lung disease 30, 74. KDR encodes the receptor for vascular endothelial growth factor (VEGF-A). It is essential for embryonic blood vessel development, as evidenced by the embryonic lethal phenotype in Kdr loss-of-function mice 75. In the neonatal period, inhibition of VEGF signaling in rats leads to reduced alveolarization and vascular density 76, suggesting that PH due to KDR mutation may have its roots in a subtle developmental abnormality, and with diminished ability to maintain a healthy vasculature during adult life 74. Extreme KDR blockade with SU5416, a VEGF-receptor antagonist, is used to generate one of the well-studied rodent models of PH 77, and endothelial-specific deletion also leads to pulmonary vascular remodeling and PH (Table 3) 78. Further studies of animals with more subtle defects in VEGF signaling are needed to understand the origins of PAH resulting from heterozygous loss of KDR function, and the stressors that may precipitate development of the disease.
SOX17 is one of a family of SRY-box transcription factors containing a DNA-binding domain with homology to that of the sex-determining region Y (SRY) gene. SOX genes are involved in various developmental processes, and SOX17 plays specific roles in cardiac development and pulmonary vascular morphogenesis (among others). Thus, SOX17 variants - both common and rare - may give further insight into the early origins of PAH. Indeed, rare SOX17 variants are a significant contributor to CHD-associated pediatric PAH 40. We refer the reader to a recent review for a comprehensive summary of the current state of knowledge regarding SOX17 and PAH 79. Lastly, preterm birth may itself be a risk factor for pulmonary vascular disease in adult life 80, 81.
5. Current therapeutic developments related to the genetic pathways
5.1. Targeting the BMPRII pathway
The fact that BMPR2 mutation is the most common genetic finding in patients with PAH, coupled with the finding that loss of expression or function of BMPRII is a feature common to many forms of PAH in animals and patients, has led to attempts to restore function in this pathway as a potential therapeutic approach. There are various approaches that have been proposed to achieve restoration of BMPRII signalling based on insights into the transcription, translation, cell surface trafficking, and degradation of BMPRII. These approaches have been reviewed extensively recently 82, 83. One promising approach is developing a version of human BMP9 to agonize the deficient BMPRII/ALK1 pathway in PAH, but this is yet to be tested in the clinic. Here, we will focus on approaches that are already being actively explored in the clinic.
Sotatercept.
Early studies of BMP and TGF-β signalling in PAH showed that loss of BMP signalling was a common finding in patients with PAH as well as animal models of disease. In addition, these changes were often accompanied by an increase in signalling mediated by TGF-β 84. Classically, TGF-β signals via Smads 2 and 3, whereas BMPs signal via Smads 1,5 and 8, although both pathways utilise Smad4. Activins are members of the TGF-β superfamily and also signal predominantly via Smad2/3. Sotatercept is a recombinant fusion protein consisting of the extracellular domain of the human activin receptor type IIA (ACTRIIA) linked to the Fc portion of human IgG1 85. A number of TGF-β family ligands bind with high affinity to ACTRIIA including among others activin A, activin B, GDF8 and GDF11. Thus, sotatercept acts as a soluble ligand trap for these factors and blocks their engagement with cell surface receptor complexes, thereby inhibiting their signalling. The application of sotatercept analogues in preclinical rodent models of PAH reverses aspects of disease pathology and is associated with a reduction in Smad2/3 activation in the pulmonary vasculature 86. The precise mechanism of action of sotatercept in PAH remains unclear, although the observed efficacy suggests that activins play a more significant role in PAH than previously appreciated. Previous preclinical studies have targeted Smad2/3 activation by TGF-β in PAH using, for example, small molecule inhibitors of ALK5 84, 87 or Fc-TGFβRII 88, but it appears that activin-induced Smad2/3 activation is also playing an important role in PAH pathobiology. The available data from animals with experimental PAH (e.g., Sugen-hypoxia model) treated with a murine sotatercept analogue does not suggest any rescue of the suppressed BMP signalling pathway. No matter what the precise mechanism of action is, sotatercept has shown very promising results in the Phase 2 (PULSAR) trial 89. The trial met its primary endpoint of a reduction in pulmonary vascular resistance, despite many patients already being on double or triple therapy with existing vasodilator agents. On the basis of these promising data, Phase 3 trials of sotatercept are now underway in PAH: STELLAR [NCT04576988], HYPERION, [NCT04811092] and ZENITH [NCT04896008].
Tacrolimus.
Since the discovery of loss of function mutations in BMPR2 in 2000, efforts to identify approaches to restore BMPRII signalling have gained momentum. Most of these approaches remain in the preclinical stages of development, and have been extensively reviewed recently, but at least two approaches have been tested in the clinic. The first potential BMPRII modulator to be tested in the clinic was low dose tacrolimus (FK506). This small molecule was identified from an in vitro screen for BMP signalling activity from a panel of FDA approved drugs 90, 91. The mechanism of action involves the displacement of FKBP12 from between the type I and type II BMP receptors, leading to phosphorylation of the type I receptor and subsequent downstream Smad phosphorylation. This effect is thought to be independent of calcineurin inhibition and the immunosuppressive effects of tacrolimus, and may occur at a lower dose than that required for immunosuppression. In rodent models of experimental PAH, low dose tacrolimus was shown to reverse aspects of the pathology and physiology of PAH. Building on these findings, a small open label Phase 2 (n=23 subjects, 16 weeks) study was conducted with tacrolimus in patients with PAH 91. Although primarily a safety and tolerability study, the trial was negative for efficacy endpoints overall, but this was a small-scale single centre study which included patients with diverse forms of PAH. The investigators developed biomarkers of tacrolimus target engagement to determine whether the drug was actually increasing BMP signalling in individual patients. These assays comprised transcriptional readouts of known BMP targets in peripheral mononuclear cells. There was some suggestion that patients in whom target engagement could be demonstrated had a better response to the drug. This approach is undergoing further clinical development for PAH (https://vivus.com).
Genetically-targeted therapies.
Small molecule approaches to correct specific types of mutation, such as ribosomal readthrough of nonsense mutations or molecular chaperones that correct abnormal protein trafficking, are already in clinical use for some genetic diseases, including Duchenne muscular dystrophy and cystic fibrosis (CF). They have also shown promise for HPAH, although they remain in the early pre-clinical phases 92-94. One of the challenges is that, unlike CF, no mutation in BMPR2 or other HPAH gene occurs at a high frequency, and thus these approaches need to be tested on tens or even hundreds of different mutations. Bmpr2 gene replacement therapy has been successfully tested in animal models, but presents challenges for human translation 95. Likewise, the potential promise of gene editing approaches such as Crispr-Cas9 is offset by the challenges of delivering these therapies to the lung. In contrast, gene editing of autologous hematopoietic stem cells is showing great promise in early clinical trials for sickle cell disease and beta-thalassemia 96. Multiple groups have shown that bone marrow-derived cells play a role in the pathogenesis of PAH 97-100, raising the intriguing possibility that perhaps gene editing of hematopoietic cells might be an alternative route for genetically-targeted PAH therapy.
6. Conclusion
Recent whole-exome and whole-genome sequencing efforts in large patient cohorts have transformed our understanding of the genetic basis of Group 1 pulmonary hypertension. Despite these advances, many questions still remain. Some of these new genes have a clear functional relevance to what we already know about vascular remodeling and PAH, whereas others are obscure. One of the biggest puzzles is why many PAH genes have such low penetrance and variable expressivity, and what are the additional factors that contribute to disease initiation and progression? How do variants in HPAH genes interact with co-morbidities in APAH? For example, as we identify pathogenic variants in known HPAH genes in cases of CTD-PAH, is it coincidence that the patient has connective tissue disease (i.e., they would have developed PAH anyway, and been classified as idiopathic PAH), or did the co-occurrence of CTD hasten the onset of PAH in someone who is genetically predisposed? In CHD-PAH, to what extent does the HPAH gene play a role in cardiac development? At the individual case level, it is impossible to know; careful genetic epidemiological studies will be required in even larger cohorts to unravel these complexities.
The identification of BMPR2 mutations, and our subsequent understanding of the pivotal role of BMP signaling, is translating into novel therapies for PAH that are relevant beyond just those patients with a heritable cause. To some, progress may seem slow, but this reflects the detailed level of study that is needed to understand the pathways and the effects of individual mutations, followed by drug development and rigorous clinical trials. The timeline from the identification of CFTR to the new modulator therapies now transforming the lives of patients with cystic fibrosis is similar. The concerted effort of the field to further improve our understanding behind these newly discovered genes in PAH will continue to enhance our understanding of this disease and drive the development of disease-modifying therapies.
Sources of Funding:
MAA acknowledges support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R35HL140019. CG acknowledges support from the French National Agency for Research grant n°ANR-16-CE17-0014 and the Fondation du Souffle.
Nonstandard Abbreviations and Acronyms:
- ACTRIIA
activin receptor type IIA
- ALK
activin receptor-like kinase
- AQP1
aquaporin-1
- BMP
bone morphogenetic protein
- BMPRII
bone morphogenetic protein receptor type-II
- GCN2
general control nonderepressible-2
- HHT
hemorrhagic telangiectasia
- HPAH
heritable pulmonary arterial hypertension
- KI
knockin
- KO
knockout
- PAH
pulmonary arterial hypertension
- pBMP10
prodomain-bound bone morphogenetic protein 10
- PCH
pulmonary capillary hemangiomatosis
- PVOD
pulmonary veno-occlusive disease
- SUR1
sulfonylurea receptor-1
- TASK-1
TWIK-related acid-sensitive potassium channel-1
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures:
Over the last three years, C.G. reports grants from Acceleron, ShouTi, and Janssen and grants and personal fees from Merck outside the submitted work. MAA has no disclosures.
References:
- 1.Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur Respir J. 2019;53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans JD, Girerd B, Montani D, Wang XJ, Galie N, Austin ED, Elliott G, Asano K, Grunig E, Yan Y, Jing ZC, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Graf S, Kaptoge S, Di Angelantonio E, Humbert M, Morrell NW. Bmpr2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir Med. 2016;4:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM, West JD, Phillips JA 3rd, Hamid R, Loyd JE. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:892–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Fan R, Ji R, Zou W, Penny DJ, Varghese NP, Fan Y. Novel homozygous bmp9 nonsense mutation causes pulmonary arterial hypertension: A case report. BMC Pulm Med. 2016;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene pph1) is caused by mutations in the bone morphogenetic protein receptor-ii gene. American journal of human genetics. 2000;67:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International PPHC, Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84 [DOI] [PubMed] [Google Scholar]
- 9.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, Chung WK, Benjamin N, Elliott CG, Eyries M, Fischer C, Graf S, Hinderhofer K, Humbert M, Keiles SB, Loyd JE, Morrell NW, Newman JH, Soubrier F, Trembath RC, Viales RR, Grunig E. Pulmonary arterial hypertension: A current perspective on established and emerging molecular genetic defects. Human mutation. 2015;36:1113–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin ED, Phillips JA, Cogan JD, Hamid R, Yu C, Stanton KC, Phillips CA, Wheeler LA, Robbins IM, Newman JH, Loyd JE. Truncating and missense bmpr2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir Res. 2009;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girerd B, Montani D, Eyries M, Yaici A, Sztrymf B, Coulet F, Sitbon O, Simonneau G, Soubrier F, Humbert M. Absence of influence of gender and bmpr2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 2010;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of bmp9 and bmp10 as functional activators of the orphan activin receptor-like kinase 1 (alk1) in endothelial cells. Blood. 2007;109:1953–1961 [DOI] [PubMed] [Google Scholar]
- 13.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a tgf-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351 [DOI] [PubMed] [Google Scholar]
- 14.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195 [DOI] [PubMed] [Google Scholar]
- 15.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, Gruenig E, Kermeen F, Halme M, Raisanen-Sokolowski A, Laitinen T, Morrell NW, Trembath RC. Molecular and functional analysis identifies alk-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, Aldred MA, Trembath RC. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441 [DOI] [PubMed] [Google Scholar]
- 17.Graf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Aldred M, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani HA, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, MacKenzie Ross RV, Moledina S, Montani D, Newnham M, Olschewski A, Olschewski H, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Stein DF, Suntharalingam J, Swietlik EM, Toshner MR, van Heel DA, Vonk Noordegraaf A, Waisfisz Q, Wharton J, Wort SJ, Ouwehand WH, Soranzo N, Lawrie A, Upton PD, Wilkins MR, Trembath RC, Morrell NW. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N, Pauciulo MW, Welch CL, Lutz KA, Coleman AW, Gonzaga-Jauregui C, Wang J, Grimes JM, Martin LJ, He H, Investigators PAHBEC, Shen Y, Chung WK, Nichols WC. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med. 2019;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mache CJ, Gamillscheg A, Popper HH, Haworth SG. Early-life pulmonary arterial hypertension with subsequent development of diffuse pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia type 1. Thorax. 2008;63:85–86 [DOI] [PubMed] [Google Scholar]
- 20.Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in madh4 (smad4). Lancet. 2004;363:852–859 [DOI] [PubMed] [Google Scholar]
- 21.Wang XJ, Lian TY, Jiang X, Liu SF, Li SQ, Jiang R, Wu WH, Ye J, Cheng CY, Du Y, Xu XQ, Wu Y, Peng FH, Sun K, Mao YM, Yu H, Liang C, Shyy JY, Zhang SY, Zhang X, Jing ZC. Germline bmp9 mutation causes idiopathic pulmonary arterial hypertension. Eur Respir J. 2019;53 [DOI] [PubMed] [Google Scholar]
- 22.Bidart M, Ricard N, Levet S, Samson M, Mallet C, David L, Subileau M, Tillet E, Feige JJ, Bailly S. Bmp9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell Mol Life Sci. 2012;69:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson J, Swietlik EM, Salmon RM, Hadinnapola C, Nikolic I, Wharton J, Guo J, Liley J, Haimel M, Bleda M, Southgate L, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani HA, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, Lawrie A, MacKenzie Ross RV, Moledina S, Montani D, Olschewski A, Olschewski H, Ouwehand WH, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Suntharalingam J, Toshner MR, Trembath RC, Vonk Noordegraaf A, Wort SJ, Wilkins MR, Yu PB, Li W, Graf S, Upton PD, Morrell NW. Characterization of gdf2 mutations and levels of bmp9 and bmp10 in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tillet E, Ouarne M, Desroches-Castan A, Mallet C, Subileau M, Didier R, Lioutsko A, Belthier G, Feige JJ, Bailly S. A heterodimer formed by bone morphogenetic protein 9 (bmp9) and bmp10 provides most bmp biological activity in plasma. J Biol Chem. 2018;293:10963–10974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolic I, Yung LM, Yang P, Malhotra R, Paskin-Flerlage SD, Dinter T, Bocobo GA, Tumelty KE, Faugno AJ, Troncone L, McNeil ME, Huang X, Coser KR, Lai CSC, Upton PD, Goumans MJ, Zamanian RT, Elliott CG, Lee A, Zheng W, Berasi SP, Huard C, Morrell NW, Chung RT, Channick RW, Roberts KE, Yu PB. Bone morphogenetic protein 9 is a mechanistic biomarker of portopulmonary hypertension. Am J Respir Crit Care Med. 2019;199:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyries M, Montani D, Nadaud S, Girerd B, Levy M, Bourdin A, Tresorier R, Chaouat A, Cottin V, Sanfiorenzo C, Prevot G, Reynaud-Gaubert M, Dromer C, Houeijeh A, Nguyen K, Coulet F, Bonnet D, Humbert M, Soubrier F. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur Respir J. 2019;53 [DOI] [PubMed] [Google Scholar]
- 27.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA 3rd, Palomero T, Sumazin P, Kim HR, Talati MH, West J, Loyd JE, Chung WK. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohnen MS, Ma L, Zhu N, Qi H, McClenaghan C, Gonzaga-Jauregui C, Dewey FE, Overton JD, Reid JG, Shuldiner AR, Baras A, Sampson KJ, Bleda M, Hadinnapola C, Haimel M, Bogaard HJ, Church C, Coghlan G, Corris PA, Eyries M, Gibbs JSR, Girerd B, Houweling AC, Humbert M, Guignabert C, Kiely DG, Lawrie A, MacKenzie Ross RV, Martin JM, Montani D, Peacock AJ, Pepke-Zaba J, Soubrier F, Suntharalingam J, Toshner M, Treacy CM, Trembath RC, Vonk Noordegraaf A, Wharton J, Wilkins MR, Wort SJ, Yates K, Graf S, Morrell NW, Krishnan U, Rosenzweig EB, Shen Y, Nichols CG, Kass RS, Chung WK. Loss-of-function abcc8 mutations in pulmonary arterial hypertension. Circ Genom Precis Med. 2018;11:e002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swietlik EM, Greene D, Zhu N, Megy K, Cogliano M, Rajaram S, Pandya D, Tilly T, Lutz KA, Welch CCL, Pauciulo MW, Southgate L, Martin JM, Treacy CM, Penkett CJ, Stephens JC, Bogaard HJ, Church C, Coghlan G, Coleman AW, Condliffe R, Eichstaedt CA, Eyries M, Gall H, Ghio S, Girerd B, Grunig E, Holden S, Howard L, Humbert M, Kiely DG, Kovacs G, Lordan J, Machado RD, Mackenzie Ross RV, McCabe C, Moledina S, Montani D, Olschewski H, Pepke-Zaba J, Price L, Rhodes CJ, Seeger W, Soubrier F, Suntharalingam J, Toshner MR, Vonk Noordegraaf A, Wharton J, Wild JM, Wort SJ, Lawrie A, Wilkins MR, Trembath RC, Shen Y, Chung WK, Swift AJ, Nichols WC, Morrell NW, Graf S. Bayesian inference associates rare kdr variants with specific phenotypes in pulmonary arterial hypertension. Circ Genom Precis Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, Pauciulo MW, Hadinnapola C, Aman J, Girerd B, Arora A, Knight J, Hanscombe KB, Karnes JH, Kaakinen M, Gall H, Ulrich A, Harbaum L, Cebola I, Ferrer J, Lutz K, Swietlik EM, Ahmad F, Amouyel P, Archer SL, Argula R, Austin ED, Badesch D, Bakshi S, Barnett C, Benza R, Bhatt N, Bogaard HJ, Burger CD, Chakinala M, Church C, Coghlan JG, Condliffe R, Corris PA, Danesino C, Debette S, Elliott CG, Elwing J, Eyries M, Fortin T, Franke A, Frantz RP, Frost A, Garcia JGN, Ghio S, Ghofrani HA, Gibbs JSR, Harley J, He H, Hill NS, Hirsch R, Houweling AC, Howard LS, Ivy D, Kiely DG, Klinger J, Kovacs G, Lahm T, Laudes M, Machado RD, MacKenzie Ross RV, Marsolo K, Martin LJ, Moledina S, Montani D, Nathan SD, Newnham M, Olschewski A, Olschewski H, Oudiz RJ, Ouwehand WH, Peacock AJ, Pepke-Zaba J, Rehman Z, Robbins I, Roden DM, Rosenzweig EB, Saydain G, Scelsi L, Schilz R, Seeger W, Shaffer CM, Simms RW, Simon M, Sitbon O, Suntharalingam J, Tang H, Tchourbanov AY, Thenappan T, Torres F, Toshner MR, Treacy CM, Vonk Noordegraaf A, Waisfisz Q, Walsworth AK, Walter RE, Wharton J, White RJ, Wilt J, Wort SJ, Yung D, Lawrie A, Humbert M, Soubrier F, Tregouet DA, Prokopenko I, Kittles R, Graf S, Nichols WC, Trembath RC, Desai AA, Morrell NW, Wilkins MR, Consortium UNBRD, Consortium UPCS, Consortium UPB. Genetic determinants of risk in pulmonary arterial hypertension: International genome-wide association studies and meta-analysis. Lancet Respir Med. 2019;7:227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmakayalu M, Major H, Sheffield V, Solomon DH, Smith RJ, Patil SR, Shchelochkov OA. Microdeletion of 17q22q23.2 encompassing tbx2 and tbx4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am J Med Genet A. 2011;155A:418–423 [DOI] [PubMed] [Google Scholar]
- 33.Kerstjens-Frederikse WS, Bongers EM, Roofthooft MT, Leter EM, Douwes JM, Van Dijk A, Vonk-Noordegraaf A, Dijk-Bos KK, Hoefsloot LH, Hoendermis ES, Gille JJ, Sikkema-Raddatz B, Hofstra RM, Berger RM. Tbx4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet. 2013;50:500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu N, Gonzaga-Jauregui C, Welch CL, Ma L, Qi H, King AK, Krishnan U, Rosenzweig EB, Ivy DD, Austin ED, Hamid R, Nichols WC, Pauciulo MW, Lutz KA, Sawle A, Reid JG, Overton JD, Baras A, Dewey F, Shen Y, Chung WK. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med. 2018;11:e001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Best DH, Sumner KL, Austin ED, Chung WK, Brown LM, Borczuk AC, Rosenzweig EB, Bayrak-Toydemir P, Mao R, Cahill BC, Tazelaar HD, Leslie KO, Hemnes AR, Robbins IM, Elliott CG. Eif2ak4 mutations in pulmonary capillary hemangiomatosis. Chest. 2014;145:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, Chelghoum N, Coulet F, Bonnet D, Dorfmuller P, Fadel E, Sitbon O, Simonneau G, Tregouet DA, Humbert M, Soubrier F. Eif2ak4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69 [DOI] [PubMed] [Google Scholar]
- 37.Hadinnapola C, Bleda M, Haimel M, Screaton N, Swift A, Dorfmuller P, Preston SD, Southwood M, Hernandez-Sanchez J, Martin J, Treacy C, Yates K, Bogaard H, Church C, Coghlan G, Condliffe R, Corris PA, Gibbs S, Girerd B, Holden S, Humbert M, Kiely DG, Lawrie A, Machado R, MacKenzie Ross R, Moledina S, Montani D, Newnham M, Peacock A, Pepke-Zaba J, Rayner-Matthews P, Shamardina O, Soubrier F, Southgate L, Suntharalingam J, Toshner M, Trembath R, Vonk Noordegraaf A, Wilkins MR, Wort SJ, Wharton J, Consortium NB-RD, Idiopathic UKNCSo, Heritable PAH, Graf S, Morrell NW. Phenotypic characterization of eif2ak4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation. 2017;136:2022–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts KE, McElroy JJ, Wong WP, Yen E, Widlitz A, Barst RJ, Knowles JA, Morse JH. Bmpr2 mutations in pulmonary arterial hypertension with congenital heart disease. Eur Respir J. 2004;24:371–374 [DOI] [PubMed] [Google Scholar]
- 39.Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA, Morse JH. Bmpr2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523 [DOI] [PubMed] [Google Scholar]
- 40.Zhu N, Welch CL, Wang J, Allen PM, Gonzaga-Jauregui C, Ma L, King AK, Krishnan U, Rosenzweig EB, Ivy DD, Austin ED, Hamid R, Pauciulo MW, Lutz KA, Nichols WC, Reid JG, Overton JD, Baras A, Dewey FE, Shen Y, Chung WK. Rare variants in sox17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med. 2018;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallego N, Cruz-Utrilla A, Guillen I, Bonora AM, Ochoa N, Arias P, Lapunzina P, Escribano-Subias P, Nevado J, Tenorio-Castano J. Expanding the evidence of a semi-dominant inheritance in gdf2 associated with pulmonary arterial hypertension. Cells. 2021;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Yang J, Tang X, Li H, Shen Y, Gu W, Zhao S. Homozygous gdf2-related hereditary hemorrhagic telangiectasia in a chinese family. Pediatrics. 2020;146 [DOI] [PubMed] [Google Scholar]
- 43.Hodgson J, Ruiz-Llorente L, McDonald J, Quarrell O, Ugonna K, Bentham J, Mason R, Martin J, Moore D, Bergstrom K, Bayrak-Toydemir P, Wooderchak-Donahue W, Morrell NW, Condliffe R, Bernabeu C, Upton PD. Homozygous gdf2 nonsense mutations result in a loss of circulating bmp9 and bmp10 and are associated with either pah or an "hht-like" syndrome in children. Mol Genet Genomic Med. 2021;9:e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wooderchak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fulop GT, Langa C, Morrell NW, Botella LM, Bernabeu C, Stevenson DA, Runo JR, Bayrak-Toydemir P. Bmp9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. American journal of human genetics. 2013;93:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balachandar S, Graves TJ, Shimonty A, Kerr K, Kilner J, Xiao S, Slade R, Sroya M, Alikian M, Curetean E, Thomas E, McConnell VPM, McKee S, Boardman-Pretty F, Devereau A, Fowler TA, Caulfield MJ, Alton EW, Ferguson T, Redhead J, McKnight AJ, Thomas GA, Genomics England Research C, Aldred MA, Shovlin CL. Identification and validation of a novel pathogenic variant in gdf2 (bmp9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Am J Med Genet A. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado RD, Welch CL, Haimel M, Bleda M, Colglazier E, Coulson JD, Debeljak M, Ekstein J, Fineman JR, Golden WC, Griffin EL, Hadinnapola C, Harris MA, Hirsch Y, Hoover-Fong JE, Nogee L, Romer LH, Vesel S, Diseases NB-R, Graf S, Morrell NW, Southgate L, Chung WK. Biallelic variants of atp13a3 cause dose-dependent childhood-onset pulmonary arterial hypertension characterised by extreme morbidity and mortality. J Med Genet. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navas Tejedor P, Tenorio Castano J, Palomino Doza J, Arias Lajara P, Gordo Trujillo G, Lopez Meseguer M, Roman Broto A, Lapunzina Abadia P, Escribano Subia P. An homozygous mutation in kcnk3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet. 2017;91:453–457 [DOI] [PubMed] [Google Scholar]
- 48.Eichstaedt CA, Song J, Benjamin N, Harutyunova S, Fischer C, Grunig E, Hinderhofer K. Eif2ak4 mutation as "second hit" in hereditary pulmonary arterial hypertension. Respir Res. 2016;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girerd B, Montani D, Coulet F, Sztrymf B, Yaici A, Jais X, Tregouet D, Reis A, Drouin-Garraud V, Fraisse A, Sitbon O, O'Callaghan DS, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in patients carrying an acvrl1 (alk1) mutation. Am J Respir Crit Care Med. 2010;181:851–861 [DOI] [PubMed] [Google Scholar]
- 50.Montani D, Girerd B, Jais X, Levy M, Amar D, Savale L, Dorfmuller P, Seferian A, Lau EM, Eyries M, Le Pavec J, Parent F, Bonnet D, Soubrier F, Fadel E, Sitbon O, Simonneau G, Humbert M. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: A population-based study. Lancet Respir Med. 2017;5:125–134 [DOI] [PubMed] [Google Scholar]
- 51.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, Montani D, Souza R, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in carriers of bmpr2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383 [DOI] [PubMed] [Google Scholar]
- 52.van der Bruggen CE, Happe CM, Dorfmuller P, Trip P, Spruijt OA, Rol N, Hoevenaars FP, Houweling AC, Girerd B, Marcus JT, Mercier O, Humbert M, Handoko ML, van der Velden J, Vonk Noordegraaf A, Bogaard HJ, Goumans MJ, de Man FS. Bone morphogenetic protein receptor type 2 mutation in pulmonary arterial hypertension: A view on the right ventricle. Circulation. 2016;133:1747–1760 [DOI] [PubMed] [Google Scholar]
- 53.Ghigna MR, Guignabert C, Montani D, Girerd B, Jais X, Savale L, Herve P, Thomas de Montpreville V, Mercier O, Sitbon O, Soubrier F, Fadel E, Simonneau G, Humbert M, Dorfmuller P. Bmpr2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J. 2016;48:1668–1681 [DOI] [PubMed] [Google Scholar]
- 54.Montani D, Girerd B, Jais X, Laveneziana P, Lau EMT, Bouchachi A, Hascoet S, Gunther S, Godinas L, Parent F, Guignabert C, Beurnier A, Chemla D, Herve P, Eyries M, Soubrier F, Simonneau G, Sitbon O, Savale L, Humbert M. Screening for pulmonary arterial hypertension in adults carrying a bmpr2 mutation. Eur Respir J. 2021;58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girerd B, Montani D, Jais X, Eyries M, Yaici A, Sztrymf B, Savale L, Parent F, Coulet F, Godinas L, Lau EM, Tamura Y, Sitbon O, Soubrier F, Simonneau G, Humbert M. Genetic counselling in a national referral centre for pulmonary hypertension. Eur Respir J. 2016;47:541–552 [DOI] [PubMed] [Google Scholar]
- 56.Frydman N, Steffann J, Girerd B, Frydman R, Munnich A, Simonneau G, Humbert M. Pre-implantation genetic diagnosis in pulmonary arterial hypertension due to bmpr2 mutation. Eur Respir J. 2012;39:1534–1535 [DOI] [PubMed] [Google Scholar]
- 57.Welch CL, Chung WK. Genetics and genomics of pediatric pulmonary arterial hypertension. Genes (Basel). 2020;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theilmann AL, Hawke LG, Hilton LR, Whitford MKM, Cole DV, Mackeil JL, Dunham-Snary KJ, Mewburn J, James PD, Maurice DH, Archer SL, Ormiston ML. Endothelial bmpr2 loss drives a proliferative response to bmp (bone morphogenetic protein) 9 via prolonged canonical signaling. Arterioscler Thromb Vasc Biol. 2020;40:2605–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, Inman GJ, Bradley JR, Rana AA, Upton PD, Morrell NW. Tnfalpha drives pulmonary arterial hypertension by suppressing the bmp type-ii receptor and altering notch signalling. Nat Commun. 2017;8:14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouvard C, Tu L, Rossi M, Desroches-Castan A, Berrebeh N, Helfer E, Roelants C, Liu H, Ouarne M, Chaumontel N, Mallet C, Battail C, Bikfalvi A, Humbert M, Savale L, Daubon T, Perret P, Tillet E, Guignabert C, Bailly S. Different cardiovascular and pulmonary phenotypes for single- and double-knock-out mice deficient in bmp9 and bmp10. Cardiovasc Res. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmgren MG, Nissen P. P-type atpases. Annu Rev Biophys. 2011;40:243–266 [DOI] [PubMed] [Google Scholar]
- 62.Hamouda NN, Van den Haute C, Vanhoutte R, Sannerud R, Azfar M, Mayer R, Cortes Calabuig A, Swinnen JV, Agostinis P, Baekelandt V, Annaert W, Impens F, Verhelst SHL, Eggermont J, Martin S, Vangheluwe P. Atp13a3 is a major component of the enigmatic mammalian polyamine transport system. J Biol Chem. 2021;296:100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gillespie MN, Olson JW. Polyamine regulatory pathways as pharmacologic targets in pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:375–389 [DOI] [PubMed] [Google Scholar]
- 64.Liu M, Liu Q, Pei Y, Gong M, Cui X, Pan J, Zhang Y, Liu Y, Liu Y, Yuan X, Zhou H, Chen Y, Sun J, Wang L, Zhang X, Wang R, Li S, Cheng J, Ding Y, Ma T, Yuan Y. Aqp-1 gene knockout attenuates hypoxic pulmonary hypertension of mice. Arterioscler Thromb Vasc Biol. 2019;39:48–62 [DOI] [PubMed] [Google Scholar]
- 65.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792 [DOI] [PubMed] [Google Scholar]
- 66.Yun X, Jiang H, Lai N, Wang J, Shimoda LA. Aquaporin 1-mediated changes in pulmonary arterial smooth muscle cell migration and proliferation involve beta-catenin. American journal of physiology. Lung cellular and molecular physiology. 2017;313:L889–L898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patil RV, Xu S, van Hoek AN, Rusinko A, Feng Z, May J, Hellberg M, Sharif NA, Wax MB, Irigoyen M, Carr G, Brittain T, Brown P, Colbert D, Kumari S, Varadaraj K, Mitra AK. Rapid identification of novel inhibitors of the human aquaporin-1 water channel. Chem Biol Drug Des. 2016;87:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McClenaghan C, Woo KV, Nichols CG. Pulmonary hypertension and atp-sensitive potassium channels. Hypertension. 2019;74:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Ribeuz H, Capuano V, Girerd B, Humbert M, Montani D, Antigny F. Implication of potassium channels in the pathophysiology of pulmonary arterial hypertension. Biomolecules. 2020;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hautefort A, Mendes-Ferreira P, Sabourin J, Manaud G, Bertero T, Rucker-Martin C, Riou M, Adao R, Manoury B, Lambert M, Boet A, Lecerf F, Domergue V, Bras-Silva C, Gomez AM, Montani D, Girerd B, Humbert M, Antigny F, Perros F. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932–948 [DOI] [PubMed] [Google Scholar]
- 71.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of tbx4 and tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naiche LA, Papaioannou VE. Loss of tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693 [DOI] [PubMed] [Google Scholar]
- 73.Galambos C, Mullen MP, Shieh JT, Schwerk N, Kielt MJ, Ullmann N, Boldrini R, Stucin-Gantar I, Haass C, Bansal M, Agrawal PB, Johnson J, Peca D, Surace C, Cutrera R, Pauciulo MW, Nichols WC, Griese M, Ivy D, Abman SH, Austin ED, Danhaive O. Phenotype characterisation of tbx4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur Respir J. 2019;54 [DOI] [PubMed] [Google Scholar]
- 74.Eyries M, Montani D, Girerd B, Favrolt N, Riou M, Faivre L, Manaud G, Perros F, Graf S, Morrell NW, Humbert M, Soubrier F. Familial pulmonary arterial hypertension by kdr heterozygous loss of function. Eur Respir J. 2020;55 [DOI] [PubMed] [Google Scholar]
- 75.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376:62–66 [DOI] [PubMed] [Google Scholar]
- 76.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. American journal of physiology. Lung cellular and molecular physiology. 2000;279:L600–607 [DOI] [PubMed] [Google Scholar]
- 77.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the vegf receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438 [DOI] [PubMed] [Google Scholar]
- 78.Winter MP, Sharma S, Altmann J, Seidl V, Panzenbock A, Alimohammadi A, Zelniker T, Redwan B, Nagel F, Santer D, Stieglbauer A, Podesser B, Sibilia M, Helbich T, Prager G, Ilhan-Mutlu A, Preusser M, Lang IM. Interruption of vascular endothelial growth factor receptor 2 signaling induces a proliferative pulmonary vasculopathy and pulmonary hypertension. Basic Res Cardiol. 2020;115:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y, Wharton J, Walters R, Vasilaki E, Aman J, Zhao L, Wilkins MR, Rhodes CJ. The pathophysiological role of novel pulmonary arterial hypertension gene sox17. Eur Respir J. 2021;58 [DOI] [PubMed] [Google Scholar]
- 80.Goss KN, Beshish AG, Barton GP, Haraldsdottir K, Levin TS, Tetri LH, Battiola TJ, Mulchrone AM, Pegelow DF, Palta M, Lamers LJ, Watson AM, Chesler NC, Eldridge MW. Early pulmonary vascular disease in young adults born preterm. Am J Respir Crit Care Med. 2018;198:1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naumburg E, Axelsson I, Huber D, Soderstrom L. Some neonatal risk factors for adult pulmonary arterial hypertension remain unknown. Acta Paediatr. 2015;104:1104–1108 [DOI] [PubMed] [Google Scholar]
- 82.Guignabert C, Humbert M. Targeting transforming growth factor-beta receptors in pulmonary hypertension. Eur Respir J. 2021;57 [DOI] [PubMed] [Google Scholar]
- 83.Morrell NW, Bloch DB, ten Dijke P, Goumans MJ, Hata A, Smith J, Yu PB, Bloch KD. Targeting bmp signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13:106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: Potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–576 [DOI] [PubMed] [Google Scholar]
- 85.Raje N, Vallet S. Sotatercept, a soluble activin receptor type 2a igg-fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther. 2010;12:586–597 [PubMed] [Google Scholar]
- 86.Yung LM, Yang P, Joshi S, Augur ZM, Kim SSJ, Bocobo GA, Dinter T, Troncone L, Chen PS, McNeil ME, Southwood M, Poli de Frias S, Knopf J, Rosas IO, Sako D, Pearsall RS, Quisel JD, Li G, Kumar R, Yu PB. Actriia-fc rebalances activin/gdf versus bmp signaling in pulmonary hypertension. Sci Transl Med. 2020;12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas M, Docx C, Holmes AM, Beach S, Duggan N, England K, Leblanc C, Lebret C, Schindler F, Raza F, Walker C, Crosby A, Davies RJ, Morrell NW, Budd DC. Activin-like kinase 5 (alk5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am J Pathol. 2009;174:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yung LM, Nikolic I, Paskin-Flerlage SD, Pearsall RS, Kumar R, Yu PB. A selective transforming growth factor-beta ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, Souza R, Waxman A, Escribano Subias P, Feldman J, Meyer G, Montani D, Olsson KM, Manimaran S, Barnes J, Linde PG, de Oliveira Pena J, Badesch DB, Investigators PT. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384:1204–1215 [DOI] [PubMed] [Google Scholar]
- 90.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. Fk506 activates bmpr2, rescues endothelial dysfunction, and reverses pulmonary hypertension. The Journal of clinical investigation. 2013;123:3600–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, Haddad F, Long-Boyle J, Hedlin H, Zamanian RT. Randomised placebo-controlled safety and tolerability trial of fk506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50 [DOI] [PubMed] [Google Scholar]
- 92.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense bmpr2 and smad9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;49:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC, Morrell NW. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: Potential for rescue. Hum Mol Genet. 2008;17:3180–3190 [DOI] [PubMed] [Google Scholar]
- 94.Dunmore BJ, Yang X, Crosby A, Moore S, Long L, Huang C, Southwood M, Austin ED, Rana A, Upton PD, Morrell NW. 4pba restores signaling of a cysteine-substituted mutant bmpr2 receptor found in patients with pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2020;63:160–171 [DOI] [PubMed] [Google Scholar]
- 95.Feng F, Harper RL, Reynolds PN. Bmpr2 gene delivery reduces mutation-related pah and counteracts tgf-beta-mediated pulmonary cell signalling. Respirology. 2016;21:526–532 [DOI] [PubMed] [Google Scholar]
- 96.Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, Foell J, de la Fuente J, Grupp S, Handgretinger R, Ho TW, Kattamis A, Kernytsky A, Lekstrom-Himes J, Li AM, Locatelli F, Mapara MY, de Montalembert M, Rondelli D, Sharma A, Sheth S, Soni S, Steinberg MH, Wall D, Yen A, Corbacioglu S. Crispr-cas9 gene editing for sickle cell disease and beta-thalassemia. N Engl J Med. 2021;384:252–260 [DOI] [PubMed] [Google Scholar]
- 97.Yan L, Chen X, Talati M, Nunley BW, Gladson S, Blackwell T, Cogan J, Austin E, Wheeler F, Loyd J, West J, Hamid R. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;193:898–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, Cheng Y, Goldberg L, Ventetuolo CE, Liang O, Klinger JR, Quesenberry PJ. Bone marrow endothelial progenitor cells are the cellular mediators of pulmonary hypertension in the murine monocrotaline injury model. Stem Cells Transl Med. 2017;6:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asosingh K, Wanner N, Weiss K, Queisser K, Gebreab L, Kassa B, Stuehr E, Graham B, Erzurum S. Bone marrow transplantation prevents right ventricle disease in the caveolin-1-deficient mouse model of pulmonary hypertension. Blood Adv. 2017;1:526–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crosby A, Toshner MR, Southwood MR, Soon E, Dunmore BJ, Groves E, Moore S, Wright P, Ottersbach K, Bennett C, Guerrero J, Ghevaert C, Morrell NW. Hematopoietic stem cell transplantation alters susceptibility to pulmonary hypertension in bmpr2-deficient mice. Pulm Circ. 2018;8:2045894018801642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jerkic M, Kabir MG, Davies A, Yu LX, McIntyre BA, Husain NW, Enomoto M, Sotov V, Husain M, Henkelman M, Belik J, Letarte M. Pulmonary hypertension in adult alk1 heterozygous mice due to oxidative stress. Cardiovasc Res. 2011;92:375–384 [DOI] [PubMed] [Google Scholar]
- 102.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. Bmpr-ii heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. American journal of physiology. Lung cellular and molecular physiology. 2004;287:L1241–1247 [DOI] [PubMed] [Google Scholar]
- 103.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial bmpr-ii with bmp9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. American journal of physiology. Lung cellular and molecular physiology. 2008;294:L98–109 [DOI] [PubMed] [Google Scholar]
- 105.Song Y, Jones JE, Beppu H, Keaney JF Jr., Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous bmpr2-mutant mice. Circulation. 2005;112:553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tian W, Jiang X, Sung YK, Shuffle E, Wu TH, Kao PN, Tu AB, Dorfmuller P, Cao A, Wang L, Peng G, Kim Y, Zhang P, Chappell J, Pasupneti S, Dahms P, Maguire P, Chaib H, Zamanian R, Peters-Golden M, Snyder MP, Voelkel NF, Humbert M, Rabinovitch M, Nicolls MR. Phenotypically silent bone morphogenetic protein receptor 2 mutations predispose rats to inflammation-induced pulmonary arterial hypertension by enhancing the risk for neointimal transformation. Circulation. 2019;140:1409–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the bmpr2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing bmpr2r899x transgene in smooth muscle develop pulmonary vascular lesions. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cruz JA, Bauer EM, Rodriguez AI, Gangopadhyay A, Zeineh NS, Wang Y, Shiva S, Champion HC, Bauer PM. Chronic hypoxia induces right heart failure in caveolin-1−/− mice. Am J Physiol Heart Circ Physiol. 2012;302:H2518–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oliveira SDS, Chen J, Castellon M, Mao M, Raj JU, Comhair S, Erzurum S, Silva CLM, Machado RF, Bonini MG, Minshall RD. Injury-induced shedding of extracellular vesicles depletes endothelial cells of cav-1 (caveolin-1) and enables tgf-beta (transforming growth factor-beta)-dependent pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2019;39:1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manaud G, Nossent EJ, Lambert M, Ghigna MR, Boet A, Vinhas MC, Ranchoux B, Dumas SJ, Courboulin A, Girerd B, Soubrier F, Bignard J, Claude O, Lecerf F, Hautefort A, Florio M, Sun B, Nadaud S, Verleden SE, Remy S, Anegon I, Bogaard HJ, Mercier O, Fadel E, Simonneau G, Vonk Noordegraaf A, Grunberg K, Humbert M, Montani D, Dorfmuller P, Antigny F, Perros F. Comparison of human and experimental pulmonary veno-occlusive disease. Am J Respir Cell Mol Biol. 2020;63:118–131 [DOI] [PubMed] [Google Scholar]
- 112.Toporsian M, Jerkic M, Zhou YQ, Kabir MG, Yu LX, McIntyre BA, Davis A, Wang YJ, Stewart DJ, Belik J, Husain M, Henkelman M, Letarte M. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arterioscler Thromb Vasc Biol. 2010;30:509–517 [DOI] [PubMed] [Google Scholar]
- 113.Gore B, Izikki M, Mercier O, Dewachter L, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F, Eddahibi S. Key role of the endothelial tgf-beta/alk1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One. 2014;9:e100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tu L, Desroches-Castan A, Mallet C, Guyon L, Cumont A, Phan C, Robert F, Thuillet R, Bordenave J, Sekine A, Huertas A, Ritvos O, Savale L, Feige JJ, Humbert M, Bailly S, Guignabert C. Selective bmp-9 inhibition partially protects against experimental pulmonary hypertension. Circulation research. 2019;124:846–855 [DOI] [PubMed] [Google Scholar]
- 115.Lambert M, Capuano V, Boet A, Tesson L, Bertero T, Nakhleh MK, Remy S, Anegon I, Pechoux C, Hautefort A, Rucker-Martin C, Manoury B, Domergue V, Mercier O, Girerd B, Montani D, Perros F, Humbert M, Antigny F. Characterization of kcnk3-mutated rat, a novel model of pulmonary hypertension. Circulation research. 2019;125:678–695 [DOI] [PubMed] [Google Scholar]
- 116.Han C, Hong KH, Kim YH, Kim MJ, Song C, Kim MJ, Kim SJ, Raizada MK, Oh SP. Smad1 deficiency in either endothelial or smooth muscle cells can predispose mice to pulmonary hypertension. Hypertension. 2013;61:1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang Z, Wang D, Ihida-Stansbury K, Jones PL, Martin JF. Defective pulmonary vascular remodeling in smad8 mutant mice. Hum Mol Genet. 2009;18:2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]