Abstract
We intended to summarize the most recent research pertaining to the use of phosphodiesterase-5 (PDE5) inhibitors in pulmonary hypertension in light of recent developments in the knowledge of the pathophysiological mechanisms and treatments for pulmonary hypertension, with major contributions in the area in the last decade. The aim of this meta-analysis is to determine the efficacy of PDE5 inhibitors for pulmonary hypertension in adults. We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to carry out this meta-analysis. Online database searching to identify eligible trials was performed in MEDLINE, EMBASE, and the Cochrane Library by two authors independently. Outcomes assessed in the current meta-analysis included change in the cardiac index from baseline in liters per minute per square meter (L/min/m2), mean peripheral arterial pressure (PAP) in mm Hg, mortality, hospitalization, and six-minute walking distance (6MWD) in meters (m). Overall, 17 articles met the inclusion criteria and were included in the current meta-analysis. PDE5 inhibitors significantly improve cardiac index (mean difference: 0.18, 95% CI: 0.04, 0.32, p-value: 0.01), mean PAP (mean difference: −5.61, 95% CI: −7.60, −3.62, p-value: 0.01), and 6MWD (mean difference: 26.26, 95% CI: 16.95, 35.57, p-value: 0.001) as compared to the patients in the control group. No significant difference was found in terms of risk of mortality (risk ratio (RR): 0.51, 95% CI: 0.17, 1.54) and risk of hospitalization (RR: 0.59, 95% CI: 0.23, 1.55) between the two groups. The current meta-analysis concluded that PDE5 inhibitors improve 6MWD, mean PAP, and cardiac index in patients with pulmonary hypertension. However, no significant difference was reported in terms of mortality and hospitalization between the two groups.
Keywords: cardiac index, meta-analysis, peripheral arterial pressure, phosphodiesterase 5 inhibitors, pulmonary hypertension
Introduction and background
Pulmonary hypertension (PH) comprises a complex group of diseases that are characterized by enhanced right ventricular afterload. It ultimately leads to right heart failure. The enhanced afterload may be because of passive transmission of high left-sided pressures, obstruction of the pulmonary arterial bed, or a combination of both [1,2]. PH is a deadly disease with unknown associations or causes with connective tissue disorders. Individuals with PH, who do not get targeted treatment, have a poor quality of life with high rates of mortality [3]. The prevalence of PH is estimated to be 10 to 52 cases per million [4].
Pulmonary arterial hypertension (PAH) is a group of diseases where PH occurs in the setting of enhanced vascular resistance. The promise of recovery from what had formerly been a very poor prognosis has been made possible by the more recent availability of drugs and therapies that target the pulmonary arterial bed. Even though PAH is uncommon, PH caused by lung and heart disease is significantly more frequent. The emergence of medications effective in treating PAH has generated significant interest in their use in treating other types of pulmonary hypertension. Mechanisms of the development of PAH are undergoing investigations with certain pathways being implicated. Potential mechanisms included enhanced local concentrations of endothelin-1, causing fibroblast proliferation and vasoconstriction [5], increased levels of serotonin, leading to mitogenesis of vascular cells and proliferation of vascular smooth muscles [6], and decreased concentrations of vasodilators such as nitric oxide and prostacyclin [7].
In the last decade, new therapies have been studied and approved for use in PAH patients, such as prostacyclins and endothelin (ET) inhibitors. With these treatments, patients have shown improvements in exercise tolerance and symptoms. However, none of these treatment options provides a cure for PAH and has shown maximum long-term outcomes [8]. Another treatment option is phosphodiesterase-5 (PDE-5) inhibitors that preserve cyclic guanosine monophosphate in the nitric oxide-cyclic guanosine monophosphate-protein kinase G signaling pathway leading to vasodilation [8]. The objectives of treatment are to attain a state associated with exercise tolerance and good quality of life with a reduced rate of mortality and to maintain right ventricular function by utilizing supplemental oxygen and treating the underlying cause [9]. PDE5 inhibitors may improve function in group 2 patients with left-side heart disease. Past studies have shown that nitric oxide is responsible for vascular tone regulation and is an inhibitor of nitric oxide synthase, causing less vasoconstriction in patients with heart failure compared to those individuals with normal pulmonary vascular resistance [10].
We intended to summarize the most recent research pertaining to the use of PDE5 inhibitors in pulmonary hypertension in light of recent developments in the knowledge of the pathophysiological mechanisms and treatments for pulmonary hypertension, with major contributions in the area in the last decade. The aim of this meta-analysis is to determine the efficacy of PDE5 inhibitors for pulmonary hypertension in adults.
Review
Methodology
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to carry out this meta-analysis.
Search Strategy
Online database searching to identify eligible trials was performed in MEDLINE, EMBASE, and the Cochrane Library by two authors independently. Keywords used in the search included a combination of “phosphodiesterase type 5 inhibitors,” “pulmonary arterial hypertension,” “pulmonary hypertension,” “mortality,” “hemodynamics,” and “outcomes.” The search was limited to human and adult studies, irrespective of geography and publication year. Two authors reviewed all the titles and abstracts of the articles identified in the initial search and excluded studies that were duplicates or did not fulfill the eligibility criteria. Full-text articles of all remaining studies were retrieved and assessed for pre-defined inclusion and exclusion criteria. Any disagreement in the process of article selection between two authors was resolved via discussions. Reference lists of all selected articles were manually searched for additional studies.
Study Selection
We included randomized control trials (RCTs) in which PDE5 inhibitors were compared to a placebo or any other treatment in adults with pulmonary hypertension from any cause, irrespective of the World Health Organization (WHO) functional class and group of pulmonary hypertension. We excluded studies conducted in the pediatric population. We also excluded observational studies, non-randomized trials, cross-over studies, reviews, and case reports. Lastly, we did not include studies that were published in a language other than English.
Data Extraction and Quality Assessment
The data from each study were extracted by a pre-defined data collection sheet developed using Microsoft Excel (Microsoft Corporation, New York, USA). Data extracted included author name, publication year, study setting, study, groups, sample size, follow-up duration, type of PDE5 inhibitors, and participants’ characteristics. One author extracted the data and the second author cross-checked it and enter it into Review Manager Software (Version 5.4.0, The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
All included studies were assessed for quality and risk of bias by two authors independently using the Cochrane Collaboration tool (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) for risk of bias in randomized trials. The final decision on the overall risk of bias was made through discussion, and all differences were resolved through discussions with the co-researcher.
Outcomes
Outcomes assessed in the current meta-analysis included change in the cardiac index from baseline in liters per minute per square meter (L/min/m2), mean peripheral arterial pressure (PAP) in mm Hg, mortality, hospitalization, and six-minute walking distance (6MWD) in meters (m).
Statistical Analysis
Statistical analysis was done using the Review Manager Version 5.4.0 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). For dichotomous outcomes, pooled estimates were calculated as a risk ratio (RR) along with a 95% confidence interval using a random-effects model. For continuous outcomes, we used the mean difference and 95% confidence interval using the random effect model. The level of significance was kept at 0.05. The heterogeneity among the study results was assessed using the I-square statistics and Cochran-Q statistics. A p-value of less than 0.1 was considered significant for heterogeneity.
Results
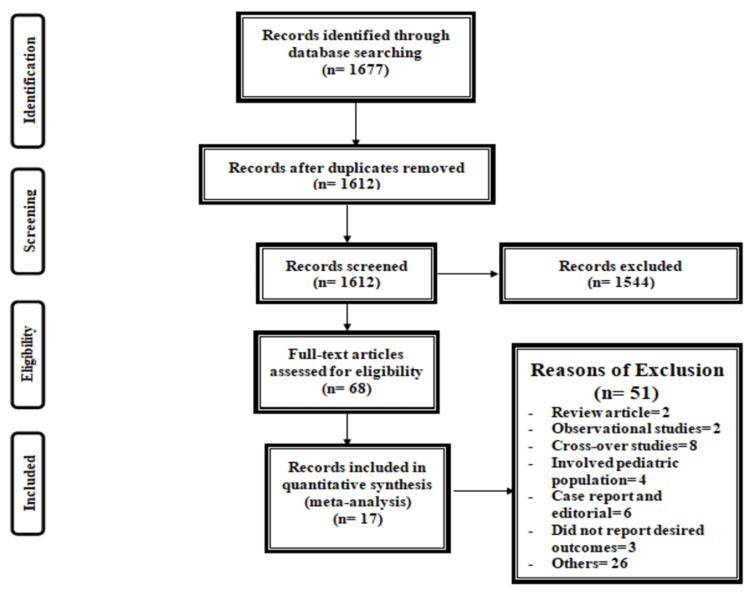
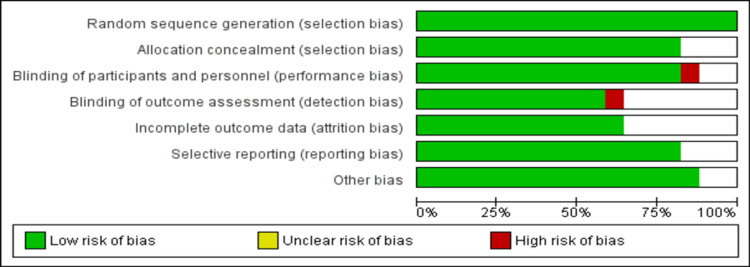
Figure 1 shows the PRISMA flowchart of the selection of RCTs for the meta-analysis. The initial search yielded 1677 articles. After removing duplicates, titles, and abstract screening, 1612 were done. In total, 1544 articles were excluded in the process of title, and abstract screening. Full-text of 68 articles was retrieved to assess for eligibility criteria. Overall, 17 articles met the inclusion criteria and were included in the current meta-analysis [8,11-25], which enrolled 1685 patients with pulmonary hypertension. Table 1 shows the characteristics of the included studies. Among all the included studies, five were multicenter and 12 were conducted in single centers only. Thirteen studies used sildenafil, three used tadalafil, and one used vardenafil. Figure 2 shows the risk of bias in the assessment of included studies.
Table 1. Characteristics of included studies.
PDE5: phosphodiesterase-5 (PDE5) inhibitors; COPD: chronic obstructive pulmonary disease, HFpEF: heart failure with preserved ejection fraction.
| Author Name | Year | Setting | Population | Groups | Sample size | Type of PDE5 | Follow-up |
| Albini et al. [11] | 2017 | Single center | Patients with pulmonary arterial hypertension | PDE5 | 6 | Tadalafil | Six months |
| Placebo | 5 | ||||||
| Belyavsky et al. [12] | 2020 | Single center | Patients with heart failure with preserved ejection fraction and combined pre- and post-capillary pulmonary hypertension | PDE5 | 30 | Sildenafil | Six months |
| Placebo | 20 | ||||||
| Bermejo et al. [13] | 2018 | Multicenter | Patients with valvular heart disease and persistent pulmonary hypertension | PDE5 | 104 | Sildenafil | Six months |
| Placebo | 96 | ||||||
| Galie et al. [14] | 2005 | Multicenter | Patients with pulmonary arterial hypertension | PDE5 | 70 | Sildenafil | Three months |
| Placebo | 65 | ||||||
| Galie et al. [15] | 2009 | Single center | Patients with pulmonary arterial hypertension | PDE5 | 323 | Tadalafil | Four months |
| Placebo | 82 | ||||||
| Guazzi et al. [16] | 2010 | Single center | Patients with HFpEF and pulmonary hypertension | PDE5 | 22 | Sildenafil | Twelve months |
| Placebo | 22 | ||||||
| Hoendirmis et al. [17] | 2015 | Single center | Patients with HFpEF and pulmonary hypertension | PDE5 | 21 | Sildenafil | Three months |
| Placebo | 22 | ||||||
| Jing et al. [18] | 2011 | Single center | Patients with pulmonary arterial hypertension | PDE5 | 42 | Vardenafil | Three months |
| Placebo | 16 | ||||||
| Lewis et al. [19] | 2007 | Single center | Patients with systolic heart failure and secondary pulmonary hypertension | PDE5 | 17 | Sildenafil | Three months |
| Placebo | 17 | ||||||
| Palazzini et al. [20] | 2010 | Single center | Patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension | PDE5 | 56 | Sildenafil | Four months |
| Placebo | 50 | ||||||
| Rao et al. [21] | 2011 | Single center | Patients with pulmonary arterial hypertension and COPD | PDE5 | 15 | Sildenafil | Three months |
| Placebo | 18 | ||||||
| Simonneau et al. [22] | 2008 | Multicenter | Patients with pulmonary arterial hypertension | PDE5 | 133 | Sildenafil | Four months |
| Placebo | 134 | ||||||
| Suntharalingam et al. [23] | 2008 | Single center | Patients with chronic thromboembolic pulmonary hypertension | PDE5 | 9 | Sildenafil | Three months |
| Placebo | 10 | ||||||
| Vitulo et al. [24] | 2016 | Multicenter | Patients with pulmonary arterial hypertension and COPD | PDE5 | 10 | Sildenafil | Four months |
| Placebo | 18 | ||||||
| Vizza et al. [25] | 2017 | Multicenter | Patients with pulmonary arterial hypertension | PDE5 | 50 | Sildenafil | Three months |
| Placebo | 53 | ||||||
| Wilkins et al. [26] | 2005 | Single center | Patients with pulmonary arterial hypertension | PDE5 | 13 | Sildenafil | Four months |
| Placebo | 12 | ||||||
| Zhuang et al. [8] | 2014 | Single center | Patients with pulmonary arterial hypertension | PDE5 | 60 | Tadalafil | Four months |
| Placebo | 64 |
Figure 1. PRISMA flowchart of selection of studies.
PRISMA: preferred reporting items for systematic reviews and meta-analyses.
Figure 2. Risk of bias assessment.
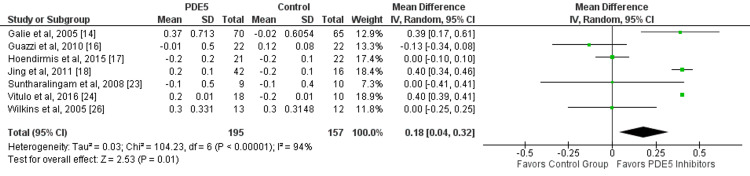
Overall, seven studies assessed the effect of PDE5 inhibitors on the cardiac index in patients with pulmonary hypertension. In patients with pulmonary hypertension, PDE5 inhibitors significantly improve cardiac index (mean difference: 0.18, 95% CI: 0.04, 0.32, p-value: 0.01), as shown in Figure 3. Significant heterogeneity was found among the study results (I-square: 94%, p-value: 0.001). Three out of seven studies found significant improvement in patients receiving PDE5 inhibitors.
Figure 3. Effect of PDE5 inhibitors on the cardiac index.
PDE5: phosphodiesterase-5.
Source: References [14,16-18,23,24,26].
Green squares representing individual study estimates, black diamond representing pooled estimates.
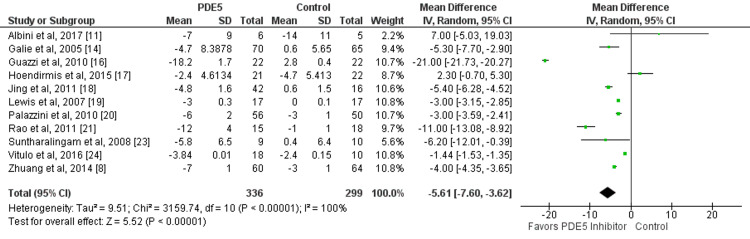
Eleven studies compared the change in mean PAP between patients who received PDE5 inhibitors and patients in the control group. The mean reduction of PAP from baseline was significantly greater in patients receiving PDE5 inhibitors compared to their counterparts (mean difference: −5.61, 95% CI: −7.60, −3.62, p-value: 0.01) as shown in Figure 4. Significant heterogeneity was found among the study results (I-square: 100%, p-value: 0.001).
Figure 4. Effect of PDE5 inhibitors on mean peripheral arterial pressure.
PDE5: phosphodiesterase-5.
Source: References [8,11,14,16-21,23,24].
Green square representing individual study estimates, black diamond representing pooled estimates.
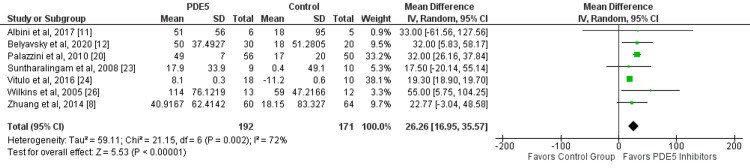
In this meta-analysis, seven out of 17 studies evaluated improvements in 6MWD between two study groups. There was a significantly greater improvement of 6MWD in patients receiving PDE5 inhibitors compared to the control group (mean difference: 26.26, 95% CI: 16.95, 35.57, p-value: 0.001) as shown in Figure 5. Significant heterogeneity was reported among the study results (I-square: 72%, p-value: 0.001).
Figure 5. Effect of PDE5 inhibitors on six-minute walking distance.
PDE5: phosphodiesterase-5.
Source: References [8,11-12,20,23,24,26].
Green square representing individual study estimates, black diamond representing pooled estimates.
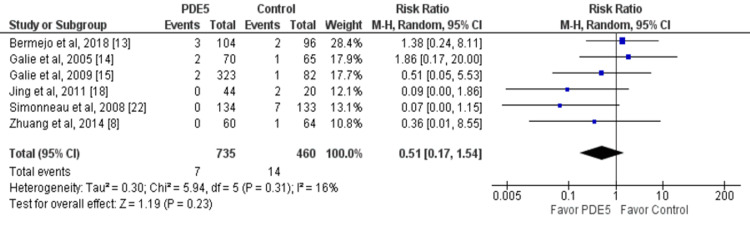
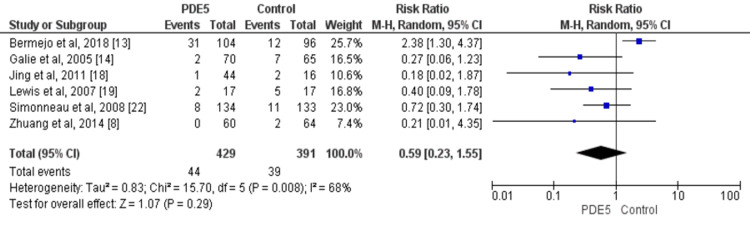
Mortality and hospitalization were reported in six studies. No significant difference was reported between the two groups in relation to the risk of mortality (RR: 0.51, 95% CI: 0.17, 1.54) and the risk of hospitalization (RR: 0.59, 95% CI: 0.23, 1.55) as shown in Figure 6 and Figure 7, respectively.
Figure 6. Effect of PDE5 inhibitors on the risk of mortality.
PDE5: phosphodiesterase-5.
Source: References [8,13-15,18,22].
Blue square representing individual study estimates, black diamond representing pooled estimates.
Figure 7. Effect of PDE5 inhibitors on the risk of hospitalization.
PDE5: phosphodiesterase-5.
Source: References [8,13,14,18-19,22].
Blue square representing individual study estimates, black diamond representing pooled estimates.
Subgroup Analysis
We conducted a subgroup analysis based on the type of PDE5 inhibitor given to patients. Table 2 shows the results of the subgroup analysis. In all three groups, the reduction of mean PAP was significantly lower in patients receiving PDE5 inhibitors compared to the placebo group. No significant difference was reported between the two groups in terms of risk of hospitalization and mortality between two arms in all three subgroups.
Table 2. Results of subgroup analysis.
PDE5: phosphodiesterase-5 inhibitors; PAP: peripheral arterial pressure; 6MWD: six-minute walking distance.
*Significant at p-value<0.05.
^Presented as risk ratio (95% CI).
| Type of PDE5 | Outcomes | MD (95% CI) | I-square |
| Sildenafil | PAP | −6.21 (−8.62, −3.20)* | 100% |
| Mortality^ | 0.68 (0.09-4.81) | 54% | |
| Hospitalization^ | 0.76 (0.27-2.16) | 75% | |
| 6MWD | 26.76 (16.25, 37.27)* | 81% | |
| Vardenafil | PAP | −5.40 (−6.28, −4.52)* | - |
| Mortality^ | 0.09 (0.00-1.86) | - | |
| Hospitalization^ | 0.18 (0.02-1.87) | - | |
| Tadalafil | PAP | −3.99 (−4.34, −3.64)* | 49% |
| Mortality^ | 0.45 (0.07-3.01) | 0% | |
| Hospitalization^ | 0.21 (0.01-4.35) | - | |
| 6MWD | 23.48 (−1.42, 48.38) | 0% |
Discussion
The current meta-analysis showed clear clinical and statistical benefits for the utilization of PDE5 inhibitors in patients with PH compared to placebo in relation to 6MWD, mean PAP, and cardiac index. However, no significant difference was reported in terms of mortality and hospitalization between the two groups.
One of the main outcomes assessed in this study was mean peripheral arterial pressure. The study found that the reduction in mean PAP was significantly greater in patients receiving PDE5 inhibitors. Most of the impact of PDE5 inhibitors is because of vascular smooth muscle vasodilation and relaxation. When this occurs in the systemic circulation, it could possibly reduce blood pressure. That reduction in pressure could cause a reflex cardiac stimulation, enhanced heart rate, and cardiac output. When vasodilation happens in the pulmonary circulation, it results in a reduction in pulmonary arterial blood pressure [27].
The first placebo-controlled research assessing the impact of intravenous sildenafil in pulmonary hypertension patients showed a significant reduction in pulmonary vascular resistance and pulmonary pressure [28]. Following certain clinical trials and case reports confirming the anti-pulmonary hypertensive impacts of sildenafil [28], this increasing body of evidence concluded in the pivotal sildenafil usage in pulmonary hypertension (SUPER-1) study in 2005: a large, multicenter, RCT showed improvements in both hemodynamic and functional outcomes to week 12 from baseline following three times daily administration of sildenafil [14]. In long-term extension research, improvements were maintained after three years of treatment [29]. Oral sildenafil was approved for the treatment of pulmonary atrial hypertension in both the European Union and the United States in 2005, followed by tadalafil in 2009. In spite of an increase in the frequency of side effects like flushing, headache, and myalgia, a recent Cochrane systematic review and meta-analysis of 36 studies involving 2999 patients found that patients taking PDE5 inhibitors were more likely to noticeably enhance their WHO functional class and six-minute walking distance (6MWD), a gauge of exercise capacity, and were less likely to die [30]. It is encouraging to note that these advantages are observed across the PH group 1 subclass, irrespective of the underlying etiology of the pulmonary arterial hypertension, be it idiopathic, and connected to connective tissue disease, congenital heart disease, or HIV infection [30].
Three drugs were used, including vardenafil, tadalafil, and sildenafil. Even though, in none of the studies, head-to-head comparisons of these drugs were made, but beneficial impacts of PDE5 inhibitors were visible across all three drugs. For instance, effects on PAP, mortality, and hospitalization were consistent across all three drugs. However, the magnitude of effect seemed greater in sildenafil as compared to vardenafil and tadalafil, possibly due to the large number of studies that included sildenafil drugs. In terms of 6MWD, no significant impact of tadalafil was reported in this meta-analysis. However, considering the number of studies and the number of participants included, this finding needs to be interpreted with cautious. Even though all PDE5 inhibitors employ a similar mechanism of action and show similar therapeutic safety and efficacy [31], they are differentiated on the basis of their pharmacodynamic and pharmacokinetic properties [32].
The current meta-analysis has certain limitations. Firstly, all RCTs include a small sample of patients. All the included RCTs for the meta-analysis shared the common weakness of heterogeneity in patient demographics, small samples, study duration, and dosage of medications. More prospective and multicenter RCTs need to be conducted to warrant the findings.
Conclusions
The current meta-analysis concluded that PDE5 inhibitors improve 6MWD, mean PAP, and cardiac index in patients with pulmonary hypertension. However, no significant difference was reported in terms of mortality and hospitalization between the two groups. Considering the limitations of the current meta-analysis related to studies with small sample sizes, more RCTs need to be conducted that should be sufficiently powered, with a long-term follow-up period, and need to include hemodynamic data along with other significant outcomes.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Primary pulmonary hypertension. Rubin LJ. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Primary pulmonary hypertension. Runo JR, Loyd JE. Lancet. 2003;3:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 3.Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. D'Alonzo GE, Barst RJ, Ayres SM, et al. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 4.Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Ling Y, Johnson MK, Kiely DG, et al. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 5.Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. Giaid A, Yanagisawa M, Langleben D, et al. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 6.Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. Eddahibi S, Humbert M, Fadel E, et al. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellular microparticles in the pathogenesis of pulmonary hypertension. Amabile N, Guignabert C, Montani D, Yeghiazarians Y, Boulanger CM, Humbert M. Eur Respir J. 2013;42:272–279. doi: 10.1183/09031936.00087212. [DOI] [PubMed] [Google Scholar]
- 8.Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Zhuang Y, Jiang B, Gao H, Zhao W. Hypertens Res. 2014;37:507–512. doi: 10.1038/hr.2014.28. [DOI] [PubMed] [Google Scholar]
- 9.ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. McLaughlin VV, Archer SL, Badesch DB, et al. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Role of nitric oxide in the local regulation of pulmonary vascular resistance in humans. Cooper CJ, Landzberg MJ, Anderson TJ, Charbonneau F, Creager MA, Ganz P, Selwyn AP. Circulation. 1996;93:266–271. doi: 10.1161/01.cir.93.2.266. [DOI] [PubMed] [Google Scholar]
- 11.Hemodynamic and exercise effects of different types of initial oral combination therapy in pulmonary arterial hypertension. Albini A, Dardi F, Rinaldi A, et al. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A3112 Am Thorac Soc. 2017;195:0. [Google Scholar]
- 12.Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre- and postcapillary pulmonary hypertension: a randomized open-label pilot study. Belyavskiy E, Ovchinnikov A, Potekhina A, Ageev F, Edelmann F. BMC Cardiovasc Disord. 2020;20:408. doi: 10.1186/s12872-020-01671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Bermejo J, Yotti R, García-Orta R, et al. Eur Heart J. 2018;39:1255–1264. doi: 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sildenafil citrate therapy for pulmonary arterial hypertension. Galiè N, Ghofrani HA, Torbicki A, et al. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 15.Tadalafil therapy for pulmonary arterial hypertension. Galiè N, Brundage BH, Ghofrani HA, et al. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 16.Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 17.Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Hoendermis ES, Liu LC, Hummel YM, et al. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 18.Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Jing ZC, Yu ZX, Shen JY, et al. Am J Respir Crit Care Med. 2011;183:1723–1729. doi: 10.1164/rccm.201101-0093OC. [DOI] [PubMed] [Google Scholar]
- 19.Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Lewis GD, Shah R, Shahzad K, et al. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 20.A randomized open label study comparing bosentan to sildenafil first-line treatment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Palazzini M, Mazzanti G, Gotti E, et al. Am Thorac Soc. 2010;181:0. [Google Scholar]
- 21.Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. https://pubmed.ncbi.nlm.nih.gov/21545068/ Indian J Chest Dis Allied Sci. 2011;53:81–85. [PubMed] [Google Scholar]
- 22.Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Simonneau G, Rubin LJ, Galiè N, et al. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 23.Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Suntharalingam J, Treacy CM, Doughty NJ, et al. Chest. 2008;134:229–236. doi: 10.1378/chest.07-2681. [DOI] [PubMed] [Google Scholar]
- 24.Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. Vitulo P, Stanziola A, Confalonieri M, et al. J Heart Lung Transplant. 2017;36:166–174. doi: 10.1016/j.healun.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial. Vizza CD, Jansa P, Teal S, Dombi T, Zhou D. BMC Cardiovasc Disord. 2017;17:239. doi: 10.1186/s12872-017-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Wilkins MR, Paul GA, Strange JW, et al. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 27.Cardiovascular risk, drugs and erectile function-a systematic analysis. Baumhäkel M, Schlimmer N, Kratz M, Hackett G, Jackson G, Böhm M. Int J Clin Pract. 2011;65:289–298. doi: 10.1111/j.1742-1241.2010.02563.x. [DOI] [PubMed] [Google Scholar]
- 28.Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Ghofrani HA, Osterloh IH, Grimminger F. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Rubin LJ, Badesch DB, Fleming TR, et al. Chest. 2011;140:1274–1283. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- 30.Phosphodiesterase 5 inhibitors for pulmonary hypertension. Barnes H, Brown Z, Burns A, Williams T. Cochrane Database Syst Rev. 2019;1:0. doi: 10.1002/14651858.CD012621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The ENDOTRIAL study: a spontaneous, open-label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. Jannini EA, Isidori AM, Gravina GL, et al. J Sex Med. 2009;6:2547–2560. doi: 10.1111/j.1743-6109.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 32.A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Hatzimouratidis K, Hatzichristou DG. Drugs. 2005;65:1621–1650. doi: 10.2165/00003495-200565120-00003. [DOI] [PubMed] [Google Scholar]