Abstract
Aim(s) We tested the following hypotheses: would better oral hygiene self-care (OHS) influence cardiovascular (CVD) mortality? Will using mouthwash in addition to OHS affect CVD mortality? How does mouthwash usage impact the oral microbes?
Design and methods Among 354 dentate subjects from the Kuopio Oral Health and Heart study, the association of OHS with CVD mortality was assessed using Cox regression analyses, adjusting for age, sex, smoking, dyslipidemia, diabetes, hypertension and education. Additionally, whether using mouthwash would affect this relationship was evaluated.
Results In the multivariable-adjusted models, OHS was associated with a 51% reduction in the risk of CVD mortality (hazard ratio [HR] 0.49 [0.28-0.85]; p = 0.01). Even those who had coronary artery disease at baseline showed a marginally significant benefit (0.50 [0.24-1.06]; p = 0.07). However, mouthwash usage did not change OHS effects (HR = 0.49 [0.27-0.87]; p = 0.01), indicating no additional benefits nor detriments. All tested microbes trended to decrease with mouthwash usage in the short term, but none were statistically significant.
Conclusion Good OHS significantly lowered the risk of CVD mortality relative to poor OHS. Mouthwash usage did not show any long-term harm or benefit on CVD mortality beyond the benefits rendered by brushing and flossing.
Key points
Good oral hygiene self-care (OHS) that encompasses both brushing and flossing was associated with significantly lower risk of cardiovascular mortality compared with poor OHS during a median follow-up of 18.8 years.
The patients who had coronary artery disease at baseline also experienced a marginally significant decrease in the risk of cardiovascular mortality with good OHS (p = 0.07).
The additional use of mouthwash with OHS did not influence the risk of cardiovascular mortality.
Introduction
The clinical health benefits of brushing and flossing have been controversial.1 Poor oral hygiene was reported to be associated with significant shifts in the composition and function of the oral microbiome.2 Moreover, several recent randomised trials have demonstrated that oral hygiene performance using an interdental brush decreased periodontal pathogens identified by the real time polymerase chain reaction (rt-PCR).3 It is, however, not established whether good oral hygiene will result in systemic health benefits. Thus, we examined if oral hygiene self-care (OHS) at baseline is associated with reduced risk of cardiovascular (CVD) mortality.
Some commensal bacteria possess nitrate reductase that can generate vasodilator nitric oxide (NO) from fruits and vegetables.4 This may provide a basis for the beneficial effects of fruit and vegetable intake in diabetes prevention as we reported previously.5,6 Several studies claimed that the use of mouthwash can decrease NO producing bacteria and increased blood pressure,7,8 while other studies did not find any change in blood pressure.9,10 Blood pressure increase induced by the mouthwash use was small (2.3 mm Hg) but statistically significant.8,11 This minute level of systolic blood pressure (SBP) change can be due to diurnal variation,12 sodium intake,13 psychological stress,14 coffee consumption,15 or oestrogen levels.16 The biggest flaw among the studies linking mouthwash use and elevated SBP is that none of the studies considered the influence of endogenous NO production which contributes more than 80% total NO production in humans.17
Based on the reports that mouthwash usage increased blood pressure18 and the incidence of diabetes,19 we postulated that mouthwash usage may increase the risk of cardiovascular mortality. Thus, we investigated whether mouthwash usage in addition to good OHS would influence its association to CVD mortality in a longitudinal study with 18.8 years of follow-up. Additionally, we tested whether mouthwash usage would alter the oral bacterial population.
Although we have assessed the OHS only once at baseline, the brushing and flossing habits have been proven to be stable for three years,20 or as long as for 40 years,21 with remarkable reproducibility.22,23 The aims of the current study are:
Primary aim - to determine if brushing and flossing affect the risk of CVD mortality in multivariable adjusted models
Secondary aims - a) to determine if mouthwash usage has independent impact on CVD mortality; b) to determine if mouthwash usage affects some periodontal pathogens and cariogenic bacteria proportions.
Materials and methods
Human subjects' protection
The details of the methods section have been published previously.24 This study was approved by the Ethics (institutional review) Board of Kuopio University Hospital and the University of Kuopio in 1995. Written informed consent was obtained from all participants. The longitudinal portion of the study was approved by the Boston University Institutional Review Board in 2005. This project adhered to the guidelines set forth by the Declaration of Helsinki and the Belmont Accord to assure the protection of human research subjects.
Study population
The description of the study population and the endpoint have been published previously24 but are briefly reiterated here. The Kuopio Oral Health and Heart (KOHH) study was started in 1995-1996 to explore the association between oral health and coronary artery disease (CAD). For the longitudinal part of the study, the mortality data (median follow-up of 18.8 years) were added to the baseline data to create a prospective follow-up study assessing oral infection impacts on CVD mortality. At baseline, 256 consecutive patients attending the Kuopio University Hospital coronary angiography unit and with a confirmed diagnosis of CAD were recruited to participate in the KOHH study. Also, 250 age- and sex-matched controls were recruited from the general surgery or otorhinolaryngology departments at the same hospital. The controls were determined by 'not having heart disease' based on their medical history and the pre-admission tests. The controls resided in the same geographic area where the cases arose. The same exclusion and inclusion criteria were applied to the control subjects. For further details regarding this cohort, please refer to the previous publications.24,25
Endpoint determination
The CVD mortality data were obtained from the Finnish Death Registry in every year from 2009-2015. The current study used the mortality report of 2015. Using the World Health Organisation's International Classification of Diseases-10 codes, I00 through I99 were considered CVD mortality due to atherosclerotic heart disease and stroke. The reliability of these data was very high, with 99% after comparing the 2009 and 2011 records in a random sample of 100 records.
Predictor assessment
At the initiation of this study (1995-1996), a single examiner (MS) conducted dental examinations. For the current study, the edentulous subjects who cannot floss were excluded. The exposure, that is, OHS, was assessed by questionnaire. Toothbrushing was assessed in four categories: 1) brush once or less frequently a week; 2) brush several times a week; 3) brush once a day; and 4) brush more than once daily. We created a dichotomy of brushing by combining the lower two and upper two groups. Similarly, a dichotomy of flossing was created from the four categories by collapsing the first two and the last two categories: 1) never; 2) once a week; 3) several times per week; and 4) daily.
Although large amounts of plaque accumulation on teeth surfaces are a risk factor for periodontitis,26 host factors, such as genetics, and lifestyle factors, such as smoking, obesity, diabetes and OHS, also contribute to periodontitis development.27 To assess how mouthwash changes oral microbe proportions, we collected plaque samples from the worst-affected periodontal sites and analysed by rapid multiplex rt-PCR tests using species-specific 16S rRNA gene primers.28 The periodontal pathogens assessed were Porphyromonas gingivalis, Prevotella intermedia, Actinobacillus actinomycetemcomitans and Tannerella forsythia.28 Similarly, gram-positive microbes were tested from the same plaque samples. The samples were cultured and Streptococcus mutans and Lactobacilli spp. were identified using the analytical profile index kits (Biomerieux, Espoo, Finland)29 at the Kuopio University Hospital laboratory.
Mouthwash usage was assessed by questionnaire. If the patient used mouthwash daily or several times a week, it was considered exposed, and never used or used less frequently than several times weekly were considered as controls. We do not know which patients used what brand, but the brand names of the mouthwashes include chlorhexidine, Listerine (essential oils), products containing 0.05% cetylpyridinium chloride and Meridol (amine fluoride).
Confounding factors
Age in years and smoking in three categories (never, past and current smokers) were assessed. Total cholesterol, triglyceride and high-density lipoprotein cholesterol (HDL) were measured by the automated enzymatic technique. We assessed dyslipidemia by total/HDL cholesterol ratio which was proven the best predictor of future atherosclerosis.30 Diabetes was ascertained by medical record review. Subjects were considered to have diabetes if documented diagnoses were in the medical records or if they were being treated for diabetes. To avoid confounding by affluence and high socioeconomic status, we adjusted educational levels, income and private insurance status.
Inflammatory markers
C-reactive protein (CRP) was measured by immunoturbidimetry utilising the Hitachi 717 analyser (Boehringer Mannheim, Mannheim, Germany). All blood samples were collected after fasting if required and analysed immediately in the hospital laboratory.
Salivary lysozyme (SLZ) is a proteolytic enzyme in the oral innate immune system expressed by neutrophil leucocytes and macrophages in response to exposure to bacteria.31 SLZ is capable of cleaving peptidoglycan of the bacterial cell wall, resulting in bactericidal action and activates the nucleotide-binding oligomerisation domain-like receptors. Thus, SLZ is a marker for oral innate immune activation, which can rupture both gram-positive and gram-negative bacterial cell walls. SLZ was quantified utilising Micrococcus lysodeikticus (Sigma Chemical Co., St. Louis, MO, USA), human milk lysozyme (Sigma Chemical Co.) and bovine serum albumin (Sigma Chemical Co.) as standards according to the methods described previously.32
Dental plaque scores were created, assigning: 0 = if no visible plaque was present; 1 = if plaque covered gingival 1/3 of the tooth surface; 2 = if plaque covered gingival 2/3; and 3 = if plaque covered the whole surface evaluated. Then, mean dental plaque indices were calculated by summing all plaque indices and dividing by the sum of the surfaces evaluated. Mean gingival bleeding indices were created similarly by summing all surfaces with gingival bleeding and dividing by the sum of the surfaces evaluated.
Statistical analyses
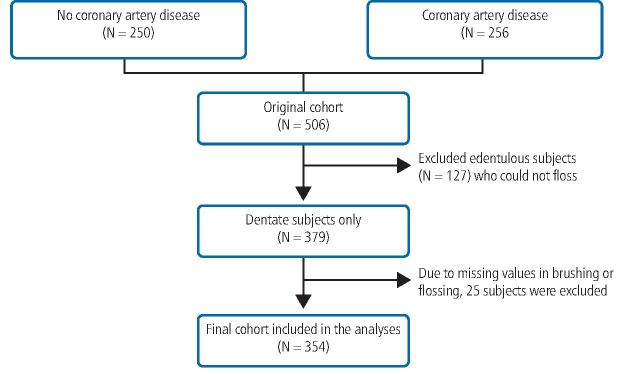
Of the 506 subjects in the original cohort, 127 edentulous subjects who could not perform flossing (the predictor) were excluded, yielding a sample size of 379. Due to missing values in brushing and flossing data, an additional 25 subjects were excluded and a final sample of 354 was included in the analyses. Using Statistical Analysis System version 9.4, we combined the binary variable of brushing daily 1/0 and flossing daily 1/0, and categorised the cohort into four mutually exclusive groups: group 0 consisted of those who never brushed or flossed; group 1 consisted of those who flossed but not brushed; group 2 consisted of those who brushed but not flossed; and group 3 consisted of those who brushed daily and flossed. There were only four people in group 1 and we merged them into group 0. Thus, OHS group 0, 1 and 2 were created: 0 being the poorest oral hygiene group (poor OHS and reference); 1 being those who brushed daily but not flossed (good OHS); and 2 being those who brushed and flossed (better OHS). Because the sample size in Cox regression is event rate (that is, CVD mortality), the sample size in this study is very small between 40-50. Thus, to save the degree of freedom (statistical power), we used linear trend models whenever possible. Combining people who never brushed and brushed seldom led to the reference group (n = 41). Next, we generated two levels of OHS, namely, who only brushed daily (n = 261) (OHS level 1) and the 57 people who brushed and flossed became OHS level 2. The baseline characteristics were stratified by OHS categories and compared by non-parametric three-group comparison by Kruskal-Wallis test or chi-squared test.
In the multivariable proportional hazard analyses, OHS was tested in relation to CVD mortality adjusting for age, sex, smoking (never, current and past smoking in linear trend), diabetes, hypertension and education. Although this study was initiated as a matched case-control study, we used unmatched analyses, which were proven to be more precise and appropriate.33
We then evaluated whether good OHS can decrease CVD mortality among those who had CAD at baseline. Because systemic inflammation from multiple sources, such as autoimmune diseases, psychological stresses and metabolic inflammation, may influence total inflammatory output, we adjusted the serum CRP levels along with established confounding factors. Next, we tested whether mouthwash usage would enhance or attenuate OHS impact on CVD mortality. Additionally, we evaluated whether the mouthwash usage would influence microbes positively or negatively.
Results
The cohort evolvement is illustrated in Figure 1. In this cohort of 354 dentate subjects, only 57 subjects had good OHS. There were 96 all-cause mortalities accrued in 18.8 years of follow-up and 56 of these were CVD-related, while 40 were non-CVD-related deaths. Of the CVD mortalities, 73% occurred in those who had CAD at baseline and thus safely presumed that CAD is on the causal pathway to CVD mortality. Brushing was highly prevalent, showing 88.2% of the cohort brushed daily while flossing had opposite distribution, showing only 17% flossed daily and 83% did not.
Fig. 1.
Cohort evolvement flow chart
The baseline characteristics are presented in Table 1. Age, BMI, education and income were not statistically different between the groups. The proportions of women and the number of teeth were higher in the better OHS groups. (Table 1). CRP, a metabolic inflammation marker, was marginally significant between the OHS groups, while oral innate immunity marker SLZ was significantly and inversely correlated with OHS.
Table 1.
Baseline characteristics stratified by oral self-care practice
| Characteristic | Seldom or no brushing/flossing (n = 41) | Daily brushing only (n = 261) | Daily brushing and flossing (n = 57) | p-value |
|---|---|---|---|---|
| Age (mean ± SD) | 56.3 (± 8.6) | 58.0 (± 9.6) | 58.9 (± 8.0) | 0.35 |
| Female sex (N %) | 1 (2.4%) | 81 (31.0%) | 31 (54.3%) | 0.0001* |
| Body Mass Index (mean ± SD) | 25.8 (± 3.1) | 25.1(± 3.7) | 24.8 (± 3.1) | 0.20 |
| Number of teeth, mean (± SD) | 15.3 (± 7.7) | 17.1 (± 8.9) | 20.4 (± 7.5) | 0.0001** |
| Mean plaque score (± SD) | 4.7 (± 2.6) | 3.8 ± 2.07) | 3.1(± 1.7) | 0.01* |
| Mean gingival bleeding index, mean (± SD) | 3.33 (± 2.00) | 2.21 (± 1.25) | 1.69 (± 1.05) | 0.0001* |
| Frequency of mouthwash usage (N %) | 5 (12.2%) | 51 (20.2%) | 16 (28.6%) | 0.14 |
| Education (years), mean (± SD) | 11 (± 2.2) | 12 (± 3.2) | 12 (± 3.1) | 0.06 |
| Annual income, mean (± SD) | 31.0 (± 7.1) | 34.6 (± 12.5) | 32.9 (± 10.9) | 0.12 |
| Periodontitis (N %) | 7 (19.4%) | 56 (21.6%) | 13 (22.0%) | 0.97 |
| Ever smoking (N %) | 21(58.3%) | 81(31.3%) | 14(23.7%) | 0.002 |
| Baseline CAD (N %) | 26 (63.4%) | 116 (44.4%) | 14 (24.6%) | 0.0004* |
| CVD mortality (N %) | 13 (36.1%) | 39 (15.1%) | 4 (6.8%) | 0.0006* |
| Diabetes (N %) | 5 (12.8%) | 19 (7.5%) | 2 (3.6%) | 0.25 |
| Hypertension | 15 (38.5%) | 82(32.8%) | 14(25.5%) | 0.38 |
| C-reactive protein, mg/L median, (interquartile range) | 8.5 (3.0-10.5) | 5 (3-10) | 5 (3-6) | 0.05* |
| Salivary lysozyme, mg/L (median, interquartile range) | 36.9 (13.1-75.2) | 21.0 (7.3-38.3) | 11.4 (6.0-38.5) | 0.009* |
| Dyslipidemia (median, interquartile range) | 5.3 (4.2-6.4) | 4.8 (3.9-5.8) | 4.2 (3.6-5.2) | 0.002* |
|
Key: * = denotes significance. Continuous variables were tested by Kruskal-Wallis test. Categorical variables were tested by chi-squared test. Unit of income is 1,000 euros. This analysis does not include 40% of diabetes cases which occurred in edentulous group. Dyslipidemia is measured by total/HDL cholesterol ratio. Due to missing values, some variables have n <379. | ||||
The CVD survival curves stratified by the OHS are presented in Figure 2. Better OHS portended a longer survival (green line) compared with shorter survival associated with poor OHS (red line). The CVD mortality risk was the lowest in the best OHS group (both brushing and flossing) (hazard ratio = 0.25 [confidence interval: 0.07-0.89]; p = 0.03) and in the brushing only group (HR = 0.72 [CI: 0.37-1.41]; p = 0.34). This suggests that flossing presented significantly greater benefits in CVD mortality reduction than brushing alone. These are presented in Table 2; model 1 and 2. We next tested if this beneficial impact of oral hygiene performance is persistent among those who already had CAD at baseline. In a stratified analysis, the CAD group had a sufficient number of CVD mortality and we observed beneficial effects of OHS remained (HR = 0.50 [0.24-1.06]; p = 0.07) (Table 2; model 3).
Fig. 2.
Survival curves from CVD mortality stratified by oral self-care
Table 2.
Multivariable adjusted hazard ratio for CVD mortality according to the oral hygiene self-care
| Model | Predictor(s) | Hazard ratio (95% confidence interval) |
P-value |
|---|---|---|---|
| Model 1 | Oral hygiene self-care (linear trend) | 0.49 (0.28-0.85) | 0.01* |
| Model 2 | Brushing only | 0.72 (0.37-1.41) | 0.34 |
| Brushing and flossing | 0.25 (0.07-0.89) | 0.03* | |
| Model 3: restricted to CAD group | Oral hygiene self-care (linear trend) | 0.50 (0.24-1.06) | 0.07 |
| Model 4: median CRP levels adjusted | Oral hygiene self-care (linear trend) | 0.48 (0.26-0.86) | 0.01* |
| Model 5: mouthwash adjusted | Oral hygiene self-care (linear trend) | 0.49 (0.27-0.87) | 0.01* |
|
Key: * = significant p-values. Model 3 - restricted to CAD patients and age, sex, smoking, hypertension, and dyslipidemia. Sample size is decreased by ~50%. Model 4 - adjusted for median CRP in addition to age, sex, smoking, diabetes, dyslipidemia, and education. Model 5 - adjusted for mouthwash usage in addition to age, sex, smoking, diabetes, dyslipidemia, and education. Reference group consists of subjects who brushed 'never' or 'seldom' (n = 41). Oral hygiene self-care in linear trend: 'no brushing or flossing' → 'brushing only' → 'brushing and flossing' in ordinal trend. Full models are adjusted for age, sex, smoking, diabetes, dyslipidemia, hypertension and education as confounders. | |||
When we adjusted serum CRP levels in the multivariable model, it showed more salient oral hygiene benefit with a tight confidence interval (HR = 0.44 [0.25-0.78]; p = 0.004) (Table 2; model 4). When we adjusted mouthwash usage in the model, the HR remained the same (Table 2; model 5). The effect of independent mouthwash usage on CVD mortality was not meaningful (HR 0.95 [0.45-2.01]; p = 0.89). Hypertensive patients appear to use mouthwash more frequently, especially those who are taking diuretics (34%) versus those not taking diuretics (11%). This may be due to xerostomic effects of diuretics.34 The relationship of mouthwash usage and tested oral microbes are presented in Table 3.
Table 3.
Microbiota detection rates stratified by mouthwash usage
| Microbiota detection rate N (%) | Non-users of mouthwash | Users of mouthwash | P-value |
|---|---|---|---|
| P. gingivalis | 109 (51.4%) | 26 (47.3%) | 0.58 |
| P. intermedia | 202 (96.19%) | 50 (90.9%) | 0.11 |
| A. actinomycetemcomitans | 34 (16.04%) | 6 (10.91%) | 0.34 |
| T. forsythia | 182 (86.3%) | 45 (81.8%) | 0.41 |
| Lactobacilli spp. | 85 (29.9%) | 26 (34.7%) | 0.43 |
| S. mutans | 95 (33.6%) | 26 (34.7%) | 0.85 |
Discussion
Better oral hygiene performance demonstrated significant survival benefits against CVD mortality. Moreover, there was a trend of reduced CVD with OHS in patients who already had heart disease, but it was marginally significant (p = 0.07). This is consistent with a previous report which indicated that interdental brush or floss use were associated with lower secondary CVD incidences among coronary heart disease patients.35
The study that reported mouthwash use increasing the risk of diabetes appear to have some methodological flaws.19 As the average duration for developing overt diabetes from obesity is approximately ten years,36,37 it is highly likely that the obese subjects in that cohort had undiagnosed diabetic pathologies already. Noticeably, twice or more daily use of mouthwash19 further suggests that these individuals already experience polydipsia, a cardinal sign of diabetes.38 Thus, reverse causation is quite possible, which is common in diseases with long latency, such as diabetes, cardiovascular diseases and cancer.39
A short-term antimicrobial action by mouthwash was reported and it suppressed SARS-CoV-2 virus.40 However, ascribing the microbial changes and subsequent NO production to mouthwash use appears to be highly speculative. Moreover, the endogenous NO production which generates 80% of total NO is intricately controlled through the renin-angiotensin-aldosterone system,41 kallikrein-kinin pathway,42 or neuronal mechanisms.43
In another study, the summary score that combined all the nitrate-reducing microbes was associated with lower blood pressure.44 If the humans ate more fruits and vegetables, the nitrate-reducing microbes would proliferate and produce more NO, which will lower the blood pressure. Thus, the nitrate-reducing microbes are the markers for the higher fruits and vegetable intake.
Good oral self-care may decrease innate immune activation involving both gram-positive and gram-negative microorganisms.45,46 This theorem was corroborated by a small randomised controlled trial, among post-stroke nursing home patients, where the experimental group received intense oral care twice daily including toothbrushing, tongue brushing, flossing, mouth rinse and lip care while control patients received usual oral care.47 The results demonstrated that Staphylococcus aureus colonisation almost doubled (from 4.8% to 9.5%) in the control group, while colonisation of the same microorganism in the intervention group decreased (from 20.8% to 16.7%). A recent large cohort (n = 247,696) study showed that oral hygiene was associated with reduced major CVD incidences.48
Although it is possible that residual confounding from smoking or a generally healthy lifestyle might have influenced our results, confounding by smoking or socioeconomic factors are unlikely because the prevalence of current smoking at the time of data collection was only 11%,49 and socioeconomic factors did not influence the outcomes in affluent Scandinavian countries where healthcare is largely state-provided.50 Additionally, when we adjusted income or private health insurance as markers for healthy lifestyle, the results did not change.
Limitations
Some limitations of our study need to be discussed. Our current study is an extension of an original case-control study and potential risk amplification is possible.51 We have, however, proven that the semi-non-parametric 'time to event' proportional hazard analyses used in the current study are impervious from this concern.24 The second limitation is that we made an assumption that increased frequency of oral hygiene practice equates to improved cleansing efficacy. This may require validation.
Conclusions
We conclude that brushing and flossing, that is, better OHS, is associated with reduced risk of CVD mortality. However, the additional use of mouthwash did not provide any further advantages or disadvantages to OHS alone. Our results have high public health importance because brushing and flossing are relatively inexpensive and have low risk of adverse effects. Moreover, even those who already have heart disease can lower the risk of CVD mortality by maintaining good oral hygiene. Further large-scale studies are warranted.
Author contributions
Sok-Ja Janket: designed the study, analysed data, prepared the manuscript and edited and approved the submission to BDJ. Caitlyn Lee: created figures, edited the manuscript, approved the submission to BDJ and performed the actual submission. Markku Surakka: collected the data, edited the manuscript and approved the submission. Tejasvini G. Jangam; created figures, edited the manuscript and approved the submission. Thomas E. Van Dyke: participated in the discussion and edited and approved the submission. Alison E. Baird: contributed to oversight of the data analyses, interpretation of the results and the extensive editing of the manuscript. Jukka H. Meurman designed the study, edited manuscript and approved the submission.
Alison E. Baird and Jukka H. Meurman share senior authorship.
Funding information
This study was supported by the grant # 0635351N from the American Heart Association awarded to Sok-Ja Janket.
Jukka H. Meurman was supported in part by a grant from the Finnish Medical Society.
Thomas E. Van Dyke was supported NIH/NIDCR grants DE025020 and DE25383.
Ethics declaration
At the time of this study, Caitlyn Lee was a high school student who completed a summer externship at Sok-Ja Janket's laboratory, at Boston University School of Dental Medicine
A portion of this study was submitted as a thesis in partial fulfilment of the requirements for the degree of Master of Science for Tejasvini G. Jangam at the Boston University School of Medicine.
The abstract was presented at the 96th International Association for Dental Research conference in July 2018.
Sok-Ja Janket received a post hoc travel support from SunStar Company to present her results at the International Association for Dental Research conference. This support was made after the data were analysed and manuscript was partially written. Thus, this support could not have affected the results.
The study was approved by the Ethics (institutional review) Board of Kuopio University Hospital and the University of Kuopio in 1995. Written informed consent was obtained from all participants.
No other conflicts of interest are declared.
References
- 1.Donn J. Medical benefits of floss unproven. The Associated Press (New York) 2016 August 2.
- 2.Börnigen D, Ren B, Pickard R et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep 2017; 7: 17686. [DOI] [PMC free article] [PubMed]
- 3.Bourgeois D, Bravo M, Llodra J-C et al. Calibrated interdental brushing for the prevention of periodontal pathogens infection in young adults - a randomized controlled clinical trial. Sci Rep 2019; 9: 15127. [DOI] [PMC free article] [PubMed]
- 4.Bondonno C P, Liu A H, Croft K D et al. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free Radic Biol Med 2014; 77: 353-362. [DOI] [PubMed]
- 5.Janket S-J, Conte H A, Diamandis E P. Do Prevotella copri and Blastocystis promote euglycaemia? Lancet Microbe 2021; DOI: 10.1016/S2666-5247(21)00215-9. [DOI] [PubMed]
- 6.Liu S, Serdula M, Janket S-J et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004; 27: 2993-2996. [DOI] [PubMed]
- 7.Pignatelli P, Fabietti G, Ricci A, Piattelli A, Curia M C. How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. Int J Mol Sci 2020; 21: 7538. [DOI] [PMC free article] [PubMed]
- 8.Tribble G D, Angelov N, Weltman R et al. Frequency of Tongue Cleaning Impacts the Human Tongue Microbiome Composition and Enterosalivary Circulation of Nitrate. Front Cell Infect Microbiol 2019; 9: 39. [DOI] [PMC free article] [PubMed]
- 9.Ashworth A, Cutler C, Farnham G et al. Dietary intake of inorganic nitrate in vegetarians and omnivores and its impact on blood pressure, resting metabolic rate and the oral microbiome. Free Radic Biol Med 2019; 138: 63-72. [DOI] [PubMed]
- 10.Sundqvist M L, Lundberg J O, Weitzberg E. Effects of antiseptic mouthwash on resting metabolic rate: A randomized, double-blind, crossover study. Nitric Oxide 2016; 61: 38-44. [DOI] [PubMed]
- 11.Bondonno C P, Liu A H, Croft K D et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens 2015; 28: 572-575. [DOI] [PubMed]
- 12.Kawano Y. Diurnal blood pressure variation and related behavioural factors. Hypertens Res 2011; 34: 281-285. [DOI] [PubMed]
- 13.Neal B, Wu Y, Feng X et al. Effect of Salt Substitution on Cardiovascular Events and Death. N Engl J Med 2021; 385: 1067-1077. [DOI] [PubMed]
- 14.Spruill T M. Chronic psychosocial stress and hypertension. Curr Hypertens Rep 2010; 12: 10-16. [DOI] [PMC free article] [PubMed]
- 15.Mesas A E, Leon-Muñoz L M, Rodriguez-Artalejo F, Lopez-Garcia E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr 2011; 94: 1113-1126. [DOI] [PubMed]
- 16.Zhao Z, Wang H, Jessup J A, Lindsey S H, Chappell M C, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol 2014; DOI: 10.1152/ajpheart.00859.2013. [DOI] [PMC free article] [PubMed]
- 17.Kleinbongard P, Dejam A, Lauer T et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 2003; 35: 790-796. [DOI] [PubMed]
- 18.Kapil V, Haydar S M A, Pearl V, Lundberg J O, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 2013; 55: 93-100. [DOI] [PMC free article] [PubMed]
- 19.Joshipura K J, Muñoz-Torres F J, Morou-Bermudez E, Patel R P. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide 2017; 71: 14-20. [DOI] [PMC free article] [PubMed]
- 20.Astrøm A N, Jakobsen R. Stability of dental health behaviour: a 3-year prospective cohort study of 15-, 16-and 18-year-old Norwegian adolescents. Community Dent Oral Epidemiol 1998; 26: 129-138. [DOI] [PubMed]
- 21.Winterfeld T, Schlueter N, Harnacke D et al. Toothbrushing and flossing behaviour in young adults - a video observation. Clin Oral Investig 2015; 19: 851-858. [DOI] [PubMed]
- 22.Ganss C, Schlueter N, Preiss S, Klimek J. Tooth brushing habits in uninstructed adults - frequency, technique, duration and force. Clin Oral Investig 2009; 13: 203-208. [DOI] [PubMed]
- 23.Norderyd O, Kochi G, Papias A et al. Oral health of individuals aged 3-80 years in Jonkoping, Sweden, during 40 years (1973-2013). I. Review of findings on oral care habits and knowledge of oral health. Swed Dent J 2015; 39: 57-68. [PubMed]
- 24.Janket S-J, Baird A E, Jones J A et al. Number of teeth, C-reactive protein, fibrinogen and cardiovascular mortality: a 15-year follow-up study in a Finnish cohort. J Clin Periodontol 2014; 41: 131-140. [DOI] [PMC free article] [PubMed]
- 25.Meurman J H, Janket S-J, Surakka M et al. Lower risk for cardiovascular mortality for patients with root filled teeth in a Finnish population. Int Endod J 2017; 50: 1158-1168. [DOI] [PubMed]
- 26.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994; 5: 78-111. [DOI] [PubMed]
- 27.Tran S D, Rudney J D. Multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J Clin Microbiol 1996; 34: 2674-2678. [DOI] [PMC free article] [PubMed]
- 28.Wahlfors J, Meurman J H, Väisänen P et al. Simultaneous detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis by a rapid PCR method. J Dent Res 1995; 74: 1796-1801. [DOI] [PubMed]
- 29.Smith P B, Tomfohrde K M, Rhoden D L, Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol 1972; 24: 449-452. [DOI] [PMC free article] [PubMed]
- 30.Millán J, Pintó X, Muñoz A et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009; 5: 757-765. [PMC free article] [PubMed]
- 31.Gajda E, Bugla-Płoskońska G. Lysozyme - occurrence in nature, biological properties and possible applications. Postepy Hig Med Dosw (Online) 2014; 68: 1501-1515. [DOI] [PubMed]
- 32.Rudney J D, Smith Q T. Relationships between levels of lysozyme, lactoferrin, salivary peroxidase, and secretory immunoglobulin A in stimulated parotid saliva. Infect Immun 1985; 49: 469-475. [DOI] [PMC free article] [PubMed]
- 33.Pearce N. Analysis of matched case-control studies. BMJ 2016; DOI: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed]
- 34.Janket S-J, Jones J, Rich S et al. The effects of xerogenic medications on oral mucosa among the Veterans Dental Study participants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 223-230. [DOI] [PubMed]
- 35.Reichert S, Schlitt A, Beschow V et al. Use of floss/interdental brushes is associated with lower risk for new cardiovascular events among patients with coronary heart disease. J Periodontal Res 2015; 50: 180-188. [DOI] [PubMed]
- 36.Abdullah A, Stoelwinder J, Shortreed S et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr 2011; 14: 119-126. [DOI] [PubMed]
- 37.Meigs J B, Muller D C, Nathan D M, Blake D R, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003; 52: 1475-1484. [DOI] [PubMed]
- 38.Fournier A. Diagnosing diabetes. A practitioner's plea: keep it simple. J Gen Intern Med 2000; 15: 603-604. [DOI] [PMC free article] [PubMed]
- 39.Janket S-J, Ackerson L K, Meurman J H. Potential reverse causation? Int J Cancer 2017; 140: 2168. [DOI] [PubMed]
- 40.Muñoz-Basagoiti J, Perez-Zsolt D, León R et al. Mouthwashes with CPC Reduce the Infectivity of SARS-CoV-2 Variants In Vitro. J Dent Res 2021; 100: 1265-1272. [DOI] [PubMed]
- 41.Manrique C, Lastra G, Gardner M, Sowers J R. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am 2009; 93: 569-582. [DOI] [PMC free article] [PubMed]
- 42.Sohaei D, Hollenberg M, Janket S-J, Diamandis E P, Poda G, Prassas I. The therapeutic relevance of the Kallikrein-Kinin axis in SARS-cov-2-induced vascular pathology. Crit Rev Clin Lab Sci 2023; 60: 25-40. [DOI] [PubMed]
- 43.Bredt D S. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res 1999; 31: 577-596. [DOI] [PubMed]
- 44.Goh C E, Trinh P, Colombo P C et al. Association Between Nitrate-Reducing Oral Bacteria and Cardiometabolic Outcomes: Results From ORIGINS. J Am Heart Assoc 2019; DOI: 10.1161/JAHA.119.013324. [DOI] [PMC free article] [PubMed]
- 45.Davis K M, Nakamura S, Weiser J N. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest 2011; 121: 3666-3676. [DOI] [PMC free article] [PubMed]
- 46.Janket S-J, Meurman J H, Nuutinen P et al. Salivary lysozyme and prevalent coronary heart disease: possible effects of oral health on endothelial dysfunction. Arterioscler Thromb Vasc Biol 2006; 26: 433-434. [DOI] [PMC free article] [PubMed]
- 47.Chipps E, Gatens C, Genter L et al. Pilot study of an oral care protocol on poststroke survivors. Rehabil Nurs 2014; 39: 294-304. [DOI] [PubMed]
- 48.Park S-Y, Kim S-H, Kang S-H et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J 2019; 40: 1138-1145. [DOI] [PubMed]
- 49.Janket S-J, Qvarnström M, Meurman J H, Baird A E, Nuutinen P, Jones J A. Asymptotic dental score and prevalent coronary heart disease. Circulation 2004; 109: 1095-1100. [DOI] [PubMed]
- 50.Cabrera C, Hakeberg M, Ahlqwist M et al. Can the relation between tooth loss and chronic disease be explained by socio-economic status? A 24-year follow-up from the population study of women in Gothenburg, Sweden. Eur J Epidemiol 2005; 20: 229-236. [DOI] [PubMed]
- 51.Sommerfelt H, Steinsland H, van der Merwe L et al. Case/control studies with follow-up: Constructing the source population to estimate effects of risk factors on development, disease, and survival. Clin Infect Dis 2012; DOI: 10.1093/cid/cis802. [DOI] [PMC free article] [PubMed]