ABSTRACT
National Immunization Technical Advisory Groups (NITAGs) and Health Technology Assessment (HTA) agencies evaluate the value of vaccines and provide decision-making authorities with recommendations. The availability of information on disease-burden evidence considered or required for the assessment of vaccines included in national immunization programs (NIPs) is limited. The aim of this review is to summarize the epidemiologic and health economic (HE) evidence considered by NITAGs/HTA agencies when evaluating pediatric pneumococcal conjugate vaccine (PCV) NIPs. A systematic literature review of national recommendation reports for PCV NIPs in children in 31 European countries, published since 2001, was performed using NITAG/HTA agency websites, Google, MEDLINE, and EMBASE. The presence of epidemiological data was mapped, HE data was extracted, and findings were summarized. A total of 46 records for 19 countries were identified. Fifteen countries’ records included a recommendation concerning implementation of PCV NIP, switching from one PCV to another or a change in vaccination schedule within an existing NIP. All of these included epidemiological invasive pneumococcal disease data, and to varying degree epidemiological data on acute otitis media and pneumonia. HE data was referenced in 13 countries’ records, with 8 countries providing in-depth details on cost-effectiveness analyses. Pediatric PCV NIP recommendations were published by 61% of European countries, with varying degree of details and decision rationale. Some countries only publish the HE aspect of their rationale. The identified material can provide insight and support local policymakers and clinicians how data influenced the decision-making process in their countries.
KEYWORDS: PCV, pneumococcal vaccine, national immunisation program, recommendation, NITAG
Background
Pneumococcal disease (PD) is a cause of morbidity and mortality worldwide, especially affecting children under 5 years, elderly aged 65 years or older, and immunocompromised individuals.1–4 PD is caused by a group of bacteria called Streptococcus pneumoniae (S. pneumoniae), and can be divided into noninvasive and invasive diseases.1 The invasive pneumococcal disease (IPD) burden is mainly determined by bacteremic pneumonia, bacteremia and meningitis.1,5 The clinical spectrum of noninvasive PD ranges from sinusitis and acute otitis media (AOM) to pneumococcal pneumonia (PP). AOM is one of the most common childhood infections, of which 30–60% are caused by S. pneumoniae.6
The burden of disease associated with S. pneumoniae is largely preventable through routine vaccination. Pneumococcal conjugate vaccines (PCV) provide protection from IPD as well as noninvasive pneumococcal infection. Thus far, three different pneumococcal conjugate vaccines (PCV) have existed on the global market. PCV7 was approved in 2001 in Europe and between 2006 and 2009 many European countries introduced PCV7 into their childhood national immunization programs (NIP). In 2009, higher-valent PCVs (PCV10 and PCV13), replacing PCV7, became available.7 PCV is included in NIPs in 150 countries across the world, and in 41 countries in Europe.8 Following the introduction of PCV7 as routine vaccination, a number of studies have observed a reduction in IPD cases related to vaccine serotypes alongside the emergence of non-vaccine serotypes.7,9–12
National Immunization Technical Advisory Groups (NITAGs) and Health Technology Assessment (HTA) agencies evaluate the value of vaccines and provide the decision-making authorities with recommendations. The effectiveness of PCV vaccination has been evaluated in a variety of countries and populations.13–16 Furthermore, a majority of European countries have established national communicable disease surveillance systems for IPD allowing for utilization of local evidence when forming recommendations. However, there is limited and inconsistent information available to the public on which types of disease-burden evidence the agencies require or consider important when forming their recommendations. Specifically, it has been unclear to what extent the use of epidemiological and health economic data are involved in these decisions.
Much of the information regarding current criteria for assessments of vaccination programs is not comprehensive or is out of date. Furthermore, information regarding these processes for PCVs is very limited. Additional research is needed that focuses on the disease burden evidence used for assessments of vaccine programs.
In this study, we conduct a systematic literature review of published recommendations and government reports by NITAG and HTA agencies in 31 European countries. The aim is to collect and summarize the evidence on burden of disease, epidemiology, and health economic assessments that national agencies consider when evaluating PCV vaccination programs.
Methods
The systematic literature review was carried out in line with Center for Reviews and Dissemination’s (CRD) guidance for undertaking reviews in health care.17 A study protocol was developed before conducting the review describing the methods, which followed the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18
Data sources and search strategy
HTA agencies and NITAG websites were searched for evidence and data to use in this study. The agency website list (Table S1 in Supplementary Material) was developed and compiled based on information from several sources including the Global NITAG Network website,19 and the publicly available lists of HTA and advisory agencies from the International Network of Agencies for Health Technology Assessment (INAHTA)20 and European Network for Health Technology Assessment (EUNetHTA).21
As a complement to the agency website search, MEDLINE® and EMBASE® were searched based on protocol-defined search strings to identify additional relevant HTA and advisory reports. Lastly, one Google search per country was performed to identify any further gray literature. The first 20 hits were screened by title and pre-view information. See the Supplementary Material for more details on the search strategy.
Eligibility criteria
The eligibility criteria for inclusion were pre-defined in the protocol and designed to include national recommendation reports by NITAGs and HTA agencies for PCV vaccination in children in any of the EU-27 countries in addition to Iceland, Norway, Switzerland, and the UK. All types of technology appraisal guidance reports for PCV vaccines (PCV7, PCV10, PCV13), including extensions of recommendations, updates of and new reports, published by NITAGs/HTA agencies between 1 January 2001 to 31 October 2020, and available in full text, were considered for inclusion. Reports concerned with regional recommendations and pediatric risk groups were excluded.
All identified recommendation reports that fulfilled the eligibility criteria were included. Material such as published articles or links to other websites that were referred to in the agencies reports and/or websites were included in the review if deemed relevant based on the eligibility criteria.
Selection methods
Study selection followed a two-step process. In step one, identified reports (or title and abstracts for scientific published material) were screened and categorized as ‘include’ and ‘exclude’, by two independent reviewers based on eligibility criteria. Discrepancies between reviewers were resolved by consensus. Unresolved disputes were referred to a third reviewer and a consensus reached. In step two, two reviewers independently reviewed the full text reports in the ‘included’ categories against the eligibility criteria. Reasons for exclusions of studies were recorded for all full text reviewed records. Any disagreements were referred to a third reviewer.
Data collection and synthesis
All relevant data from the included full text reports was extracted into a pre-specified data extraction grid in MS Excel® (Table 1). The two reviewers extracted data independently and in duplicate. Discrepancies between reviewers were resolved by consensus, with unresolved disputes referred and resolved through a third reviewer. Quality of the extraction process was ensured by the independent review of the records and consensus by third reviewer to ensure that interpretation was aligned across the records. Data was organized in summary tables providing an overview on collected evidence. No quantitative analysis of the data was performed.
Table 1.
Data collection table.
| Feature | Variable |
|---|---|
| General information | • HTA/NITAG agency • Year • Report Title • Country • Population • Recommendation: • Taking a stance (positive/negative) either in relation to the implementation of a PCV into the countries NIP, the change of one PCV to another in an existing NIP, or the change in vaccination schedule for an already implemented PCV NIP • No recommendation; neutral assessment |
| Epidemiological data for IPD, AOM and pneumonia | • Epidemiological context (type of PCV, year of introduction etc.) Inclusion of evidence on: • Incidence • Prevalence • Mortality • Clinical presentation (for IPD, pneumonia) • Serotype distribution • % attributable to S. pneumoniae |
| Health economic data | • Description of health economic models • Description of major assumptions in model • Model inputs for costs, utilities, epidemiologic, resource use, etc. • Outcomes presented • Conclusion |
| Other | • HTA/NITAG critiques of evidence |
Results
Included studies
In total, 101 records were identified through HTA/NITAG website searches, 91 records through EMBASE, 57 through MEDLINE, and 75 through Google searches (Figure 1). After removal of duplicates, 256 records were screened for inclusion. In the next step 112 records were reviewed in full, with 46 records included in the final data extraction. The reasons for the exclusion of 66 full-text records were that the record did not concern the population of interest, that the record was not a recommendation report and/or not issued by a NITAG or HTA agency. See Table S2 in Supplementary Material for a full list of excluded studies.
Figure 1.
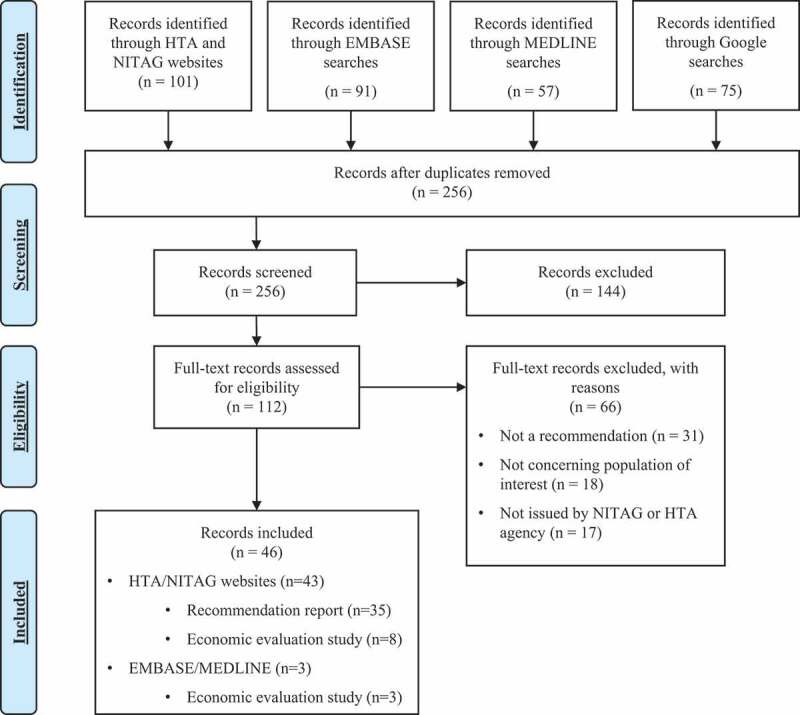
PRISMA flow chart.
Summary statistics of included records
Relevant records were identified for 19 of 31 countries included in the search. No records were included for Bulgaria, Cyprus, Czech Republic, Greece, Hungary, Iceland, Latvia, Lithuania, Malta, Romania, Slovakia, and Slovenia. The highest number of records (five) were found for France, the Netherlands, and the UK (Figure 2). Of the 46 included records, 32 (70%) were issued by NITAGs (including records issued by other public entities such as public health authorities or institutions), and four records (9%) were issued by HTA bodies. Ten (22%) records were peer-reviewed published PCV cost-effectiveness (CE) analyses that were either published by members of a NITAG or HTA agency or that stated that they were used as part of the national recommendation.
Figure 2.
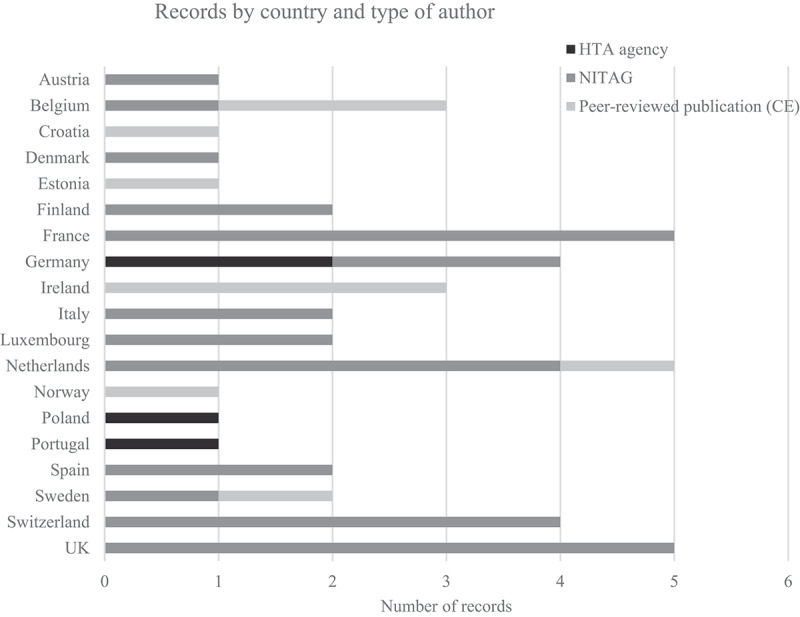
Number of records included by country and authoring institution.
Within these records, 30 (65%) included a positive recommendation for at least one vaccine, 11 (22%) took no identifiable position and five (8%) issued a negative recommendation. Ten of the 11 records without a position were CE analyses. Of the five with negative recommendations, one record from Portugal (from 2008) concluded that there was not enough evidence in the Portuguese setting to include PCV7.22 Two records from France (from 2012 and 2018) recommended to not include PCV10 in addition to PCV13, which had already been approved, as both records concluded there was no added clinical benefit.23,24 One record from 2002 in the Netherlands decided not to include PCV7 due to high budget impact and exceeding the stated threshold for cost-effectiveness.25 One other Dutch record (2005) recommended against a dosing change for PCV7 (once it had been included).26
Table 2 presents an overview of the evidence included in each of the identified records. For all countries at least one included record had some form of epidemiological data for either IPD, AOM, or pneumonia, with IPD being the most common (100% of countries). For 12 countries (63%) we identified at least one record that included epidemiological data for all three diseases. Thirteen countries (68%) included health economic evidence in at least one of the included records, all of them also including epidemiological evidence for at least one of AOM or pneumonia, in addition to IPD. Thirteen countries (68%) assessed more than one of the vaccines, either in the same or in separate included records.
Table 2.
Overview of evidence included in each record, by country.
| Country | Year | PCV | Recommendation | Epidemiological evidence |
HE-evidence | Ref | ||
|---|---|---|---|---|---|---|---|---|
| IPD | AOM | Pneumonia | ||||||
| Austria | 2020 | PCV13 | Positive | x | 27 | |||
| Belgium | 2018 | PCV10, PCV13 | Positive | x | x | x | 28 | |
| Belgium | 2011 | PCV10, PCV13 | N/A | x | x | x | x | 29 |
| Belgium | 2006 | PCV7 | N/A | x | x | x | x | 30 |
| Croatia | 2015 | PCV10, PCV13 | N/A | x | x | x | x | 31 |
| Denmark | 2007 | PCV7 | Positive | x | x | x | 32 | |
| Estonia | 2015 | PCV10, PCV13 | N/A | x | x | x | x | 33 |
| Finland | 2018 | PCV10, PCV13 | N/A | x | x | x | 34 | |
| Finland | 2008 | PCV7 | Positive | x | x | x | x | 35 |
| France | 2018 | PCV10 | Negative | x | x | x | 24 | |
| France | 2012 | PCV10, PCV13 | Negative (PCV10) Positive (PCV13) | x | 23 | |||
| France | 2009 | PCV13 | Positive | x | 36 | |||
| France | 2008 | PCV7 | Positive | x | x | 37 | ||
| France | 2006 | PCV7 | Positive | x | x | 38 | ||
| Germany | 2015 | PCV10, PCV13 | Positive | x | 39 | |||
| Germany | 2008 | PCV7 | N/A | x | x | x | x | 40 |
| Germany | 2006 | PCV7 | Positive | x | 41 | |||
| Germany | 2005 | PCV7 | Positive | x | x | x | 42 | |
| Ireland | 2012 | PCV10, PCV13 | N/A | x | 43 | |||
| Ireland | 2008 | PCV7 | N/A | x | x | x | x | 44 |
| Ireland | 2007 | PCV7 | N/A | x | 45 | |||
| Italy | 2019 | PCV13 (PCV10 for one region) | Positive | x | 46 | |||
| Italy | 2017 | PCV13 | Positive | x | 47 | |||
| Luxembourg | 2016 | PCV10, PCV13 | Positive | x | x | x | 48 | |
| Luxembourg | 2011 | PCV13 | Positive | x | x | x | 49 | |
| Netherlands | 2013 | PCV10, PCV13 | Positive | x | x | 50 | ||
| Netherlands | 2010 | PCV10, PCV13 | Positive | x | x | x | 51 | |
| Netherlands | 2005 | PCV7 | Negative | x | x | x | x | 26 |
| Netherlands | 2003 | PCV7 | N/A | x | x | x | x | 52 |
| Netherlands | 2002 | PCV7 | Negative | x | x | 25 | ||
| Norway | 2006 | PCV7 | N/A | x | x | x | x | 53 |
| Poland | 2014 | PCV13 | Positive | x | x | x | 54 | |
| Portugal | 2008 | PCV7 | Negative | x | x | 22 | ||
| Spain | 2009 | PCV10, PCV13 | Positive | x | x | x | 55 | |
| Spain | 2006 | PCV7 | Positive | x | 56 | |||
| Sweden | 2008 | PCV7 | N/A | x | x | x | x | 57 |
| Sweden | 2008 | PCV7 | Positive | x | x | 58 | ||
| Switzerland | 2020 | PCV13 | Positive | 59 | ||||
| Switzerland | 2019 | PCV13 | Positive | x | x | 60 | ||
| Switzerland | 2010 | PCV13 | Positive | x | 61 | |||
| Switzerland | 2007 | PCV7 | Positive | x | x | x | 62 | |
| UK | 2019 | PCV13 | Positive | 63 | ||||
| UK | 2018 | PCV13 | Positive | 64 | ||||
| UK | 2017 | PCV13 | Positive | x | 65 | |||
| UK | 2014 | PCV13 | Positive | 66 | ||||
| UK | 2005 | PCV7 | Positive | 67 | ||||
Epidemiological evidence
All countries had an included record that provided some form of epidemiological data for IPD (Table 3). The most common types of epidemiological evidence found for IPD were IPD incidence or prevalence (all countries), serotype distribution (all countries except Denmark and Croatia) and clinical presentation (all countries except Poland). AOM evidence was included in the records of 13 countries (68%), all of which included incidence evidence; seven of those countries included other types of evidence in addition to incidence. Fourteen countries (74%) had records that included epidemiological evidence for pneumonia, with incidence and clinical presentation being the most common types of evidence encountered.
Table 3.
Epidemiological evidence, by type, disease, and country.
| Country/Dimension | Austria | Belgium | Croatia | Denmark | Estonia | Finland | France | Germany | Ireland | Italy | Luxembourg | Netherlands | Norway | Poland | Portugal | Spain | Sweden | Switzerland | UK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPD | |||||||||||||||||||
| Incidence | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Prevalence | x | x | x | x | x | x | x | x | |||||||||||
| Mortality | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Serotype distribution | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| % attributable to S. pneumoniae | x | x | x | x | x | ||||||||||||||
| Clinical presentation | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| AOM | |||||||||||||||||||
| Incidence | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Prevalence | x | x | x | ||||||||||||||||
| Mortality | x | x | |||||||||||||||||
| Serotype distribution | x | x | |||||||||||||||||
| % attributable to S. pneumoniae | x | x | x | x | x | ||||||||||||||
| Pneumonia | |||||||||||||||||||
| Incidence | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||
| Prevalence | x | x | x | x | x | ||||||||||||||
| Mortality | x | x | x | x | x | x | x | ||||||||||||
| Serotype distribution | x | ||||||||||||||||||
| % attributable to S. pneumoniae | x | x | x | x | |||||||||||||||
| Clinical presentation | x | x | x | x | x | x | x | x | x | x |
The sources of epidemiological evidence used in records differed by country and recommendation. All records that included positive recommendations sourced at least some of the evidence from national or in-country surveillance data; some of those records additionally included global, European, US, or UK data. Records with negative recommendations varied in the sources and content of epidemiological data used for assessment. A French record from 2018 with a negative recommendation (PCV1024) included epidemiological evidence for IPD, AOM, and pneumonia, gathered from both national and international sources. In contrast, a Portuguese record from 2008 with a negative recommendation (PCV722) included only IPD evidence sourced from surveillance and regional data, with the lack of reliable national IPD evidence being the reason for a negative recommendation.
Evidence for AOM was the least common epidemiological evidence found in the included records. Twelve of the 30 records with positive recommendations reported AOM evidence, with ten of those records sourcing their AOM evidence from surveillance or national data. In contrast, records in France (2008, PCV737) and the Netherlands (2010, PCV10 & PCV1351) only sourced AOM evidence from external data (e.g., US or Finnish data). Of the 20 records that studied PCV7, 13 included AOM evidence, while only eight of 25 records studying PCV13 and seven of the 14 records assessing PCV10 included epidemiological evidence for AOM, respectively.
Health economic evidence
Twenty-two records mentioned health economic aspects. Eleven (50%) of these all referred to externally conducted health economic analyses and reported to a varying degree on model details and results. The remaining 11 were either peer-reviewed published CE papers (10) or an HTA-report (1, Germany). A summary of the available data in the latter 11 records can be found in Table 4.
Table 4.
Summary of CE-records.
| Country | Year | Type of analysis (perspective) | Intervention | Comparator | Type of model | CE results |
|---|---|---|---|---|---|---|
| Belgium 29 |
2011 | Cost-utility analysis (payer) | PCV10 and PCV13 | PCV7 | Markov model | Both PCV13 and PCV10 likely to be cost-effective in the base case setting. Varying results in sensitivity analyses. |
| Belgium 30 |
2006 | Cost-effectiveness analysis (payer) | PCV7, either 3 + 1 or 2 + 1 dosing schedule | 1) No vaccination 2) Incrementally between dosing schedules |
Markov model | The 2 + 1 schedule carries an incremental cost per QALY that is likely to be acceptable in the Belgian health care system. The 3 + 1 schedule does not in relation to the 2 + 1 schedule. |
| Croatia 31 |
2015 | Cost-effectiveness analysis (societal) | PCV10 and PCV13 | No vaccination | Markov model | In the base case, vaccination likely carries a cost per QALY that is higher than what is commonly acceptable in Croatia. Scenario analyses suggest that a lower price of the evaluated vaccines would shift the cost per QALY toward acceptable levels. |
| Estonia 33 |
2015 | Cost-effectiveness analysis (societal) | PCV10 and PCV13 | No vaccination | Markov model | It is not possible to favor one of the interventions over the other. The incremental cost per QALY varies through scenarios. From highly likely to be at an acceptable level to less likely. |
| Germany 40 |
2008 | Cost-effectiveness analysis (payer and societal) | PCV7 | No vaccination | Markov model | General vaccination up to two years of life is cost saving. The assumptions regarding herd protection greatly impact these results. |
| Ireland 43 |
2012 | Cost-effectiveness analysis (payer) | PCV13, PCV10 and PCV7 | No vaccination | Markov model | PCV13 is referred to as the “most cost-effective option for childhood pneumococcal vaccination”. |
| Ireland 44 |
2008 | Cost-effectiveness analysis (payer) | PCV7 | No vaccination | Markov model | Implementation is cost-effective when including herd protection effects, otherwise not cost-effective. |
| Ireland 45 |
2007 | Cost-effectiveness analysis (payer) | PCV7 | No vaccination | Markov model | In the base case the cost per life year gained is deemed too high to be acceptable in the Irish health care system. With the inclusion of herd protection, the cost per life year gained is on a level which would potentially be acceptable. |
| Netherlands 52 |
2003 | Cost-effectiveness analysis (societal) | PCV7 | No vaccination | Decision tree | Implementation of PCV7 is not cost-effective given that the ICER is higher than what the authors state as the suggested willingness to pay (WTP) threshold in the Netherlands. |
| Norway 53 |
2006 | Cost-effectiveness analysis (societal) | PCV7 | No vaccination | Markov model | A three-dose regimen may be cost saving if it has the same effect as a four-dose regimen. A four-dose regimen is only cost-effective if both herd protection and indirect costs are considered. |
| Sweden 57 |
2008 | Cost-effectiveness analysis (societal) | PCV7 | No vaccination | Markov model | A three-dose regimen was found to be cost saving. A four-dose regimen without herd protection being considered carried an incremental cost per QALY gained that is likely acceptable in Sweden. When herd protection is considered, the incremental cost per QALY gained is low enough to be objectively seen as cost-effective. |
All the identified records conducted cost-effectiveness analyses where the incremental costs and effects of two (or more) alternatives are estimated to calculate an incremental cost-effectiveness ratio (ICER). The ICER is a measure of the cost of producing one more unit of health (measured as quality-adjusted life years (QALY)). The interpretation of this incremental cost per unit of health is in relation to a threshold value which varies by country, the willingness to pay (WTP) threshold. As such, there is no mean of comparing the cost-effectiveness between countries. It should be interpreted only as cost-effective (or cost-ineffective) in the setting of the specified country.
In 10 (91%) of the analyses a Markov model was used, one (9%) record reported using a decision tree model. Nine (82%) records reported using a scenario with no vaccination as the comparator, one (9%) used PCV7 as comparator and one (9%) had both no vaccination as well as the two investigated PCV7 dosing schedules as comparators. Seven (64%) records included a figure or description of the model structure and the accompanied health states. Eight (73%) analyses had a lifetime horizon, one (9%) had a 20-year horizon, one had a 10-year horizon, and one had a 5-year horizon. The decision tree model used the 10-year time horizon.
C7ost-effectiveness varied with scenarios in the different analyses, with two of the identified reasons for tipping one way or the other were the assumptions regarding the dosing schedule (2 + 1 or 3 + 1) and herd protection. Inclusion of herd protection, through, e.g., risk reduction assumptions in the model, favored cost-effectiveness and by extension implementation as this would increase the efficacy of the vaccination without adding extra costs. It is inherent to decision modeling that there is a degree of uncertainty in the estimates generated attributable to, e.g., the used data and the assumptions made by the investigator.
Discussion
To our knowledge, this is the first study assessing the disease burden evidence included in national childhood PCV vaccination recommendations developed by national NITAGs and/or HTA agencies, in the EU-27 countries including Iceland, Switzerland, and the UK.
The recommendation forming processes and the ways of assessing and including available evidence in the decision-making process has previously been shown to differ among European countries. In 2010, a survey was performed among gatekeepers of the Vaccine European New Integrated Collaboration Effort (VENICE), an EU/EEA Member States network of experts in vaccine-preventable diseases, on what evidence countries have available to perform an HTA assessment specifically within the field of pneumococcal vaccines.68 Eleven (41%) of 27 responding countries reported that at that time had performed an HTA for the pneumococcal vaccine. The survey found a wide variation between countries on the extent and content of evaluations by the respective HTA agencies, making comparisons between countries difficult.
In another survey performed in 201369 among the VENICE network, 85% (23) of the 27 responding countries reported having an established NITAG. Among these, 45% (10) had formal frameworks in place for the systematic development of vaccine recommendations. In a follow-up survey in 2014,70 93% (26) of the 28 responding countries reported having a NITAG or expert group in place, and among these 77% (20) reported applying a systematic approach to the assessment of evidence when forming recommendations for inclusion of vaccines in the NIP, indicating a rapid development toward establishment of NITAGs and evidence-based vaccination recommendations.
In both surveys, all responding countries listed country-specific epidemiology and burden of disease as key elements considered in the decision-making process, along with vaccine effectiveness/efficacy and safety. Results from health economic evaluations were considered as key elements by around 50% of countries in the decision-making process.69,70
Despite common key criteria, the framework and working processes for assessment varied between countries, and this together with other country-specific key factors (e.g., availability of local disease incidence, healthcare system in place, consideration of health economic evaluation), has over the years contributed to differences in vaccination schedules and policies across Europe.69,70 In 2018, Sheikh et al.71 found in a study with 16 European countries, that despite the presence of standardized assessment frameworks, vaccination programs varied across countries, with only pediatric recommendations being comparable. For pneumococcal vaccination specifically, inclusion recommendations are comparable between most European countries, with the difference lying instead in the choice of PCV vaccine.
As part of the 2014 VENICE survey,70 13 countries (50%) reported that their NITAG usually published a background paper with the decision rationale, with varying degree of details, either as peer-reviewed published papers or non-peer reviewed online reports. In line with this, the present study identified some type of PCV report for 61% (19) of 31 included countries. Fifteen (48%) countries’ records included a recommendation for or against either the implementation of PCV into the countries NIP, the switch of one PCV to another within an existing NIP, or a change in vaccination schedule within an existing NIP. The majority of the records expressed a positive recommendation. Only five of identified records, from three countries, expressed a negative recommendation. When only PCV7 was available, i.e., between 2001 and 2008, some countries stated in their records that they took a negative position due to lack of epidemiological IPD evidence and evidence of cost-effectiveness, however these data gaps seem to have been filled over time as no such positions were identified regarding PCV13, i.e., from 2009 and forward. The results also show that even though PCV10 was not evaluated as frequently as PCV13 in the identified records, the clinical efficacy was considered similar enough that one could not be directly preferred over the other. However, as PCV13 covers more serotypes than PCV10, it was considered in the records to have a slight advantage.
In line with findings in the published literature69–71 and the fact that IPD surveillance systems with compulsory reporting and national coverage have been implemented in a majority of European countries over the years,72 almost all records included in the present study contained country-specific national epidemiological evidence for IPD. Serotype data specifically constitutes one of the most important decision factors when implementing or switching PCV in the NIP, demonstrated by, e.g., Belgium experiencing an increase in emerging serotypes after the switch from PCV13 to PCV10.73 Surveillance systems for AOM and pneumonia are not as readily implemented as for IPD, and determination of etiology is not considered standard practice in many European countries. Therefore, epidemiological evidence on AOM and pneumonia varied with country and year of recommendation, even though a lack of this evidence was not highlighted in any of the included records as a detrimental factor for forming a recommendation. The lack of epidemiological evidence in some records could also be explained by the nature of those records, e.g., for the UK only meeting minutes from NITAG meetings with high level summaries of discussion and decisions were available. A lack of consistent data reporting between countries, as well as a lack of homogeneity in the type of records that were identified made any deeper comparisons related to the use of disease evidence between countries difficult.
All health economic evaluations, and specifically those of (pediatric) vaccines, are sensitive to model assumptions as well as decisions on whether to include discounting and societal costs and benefits, such as income/productivity loss from parents due to caring for a sick child. In the context of vaccines, benefits of the vaccine are often more discounted than its disadvantages since the disease prevented by the vaccine might occur years ahead in the future while possible adverse effects usually occur shortly after vaccination. Despite the dynamic nature of the impact of vaccination on disease occurrence, the present study found that the identified health economic analyses utilized static cohort models, with only half of these including assumptions around herd protection. Ideally, a dynamic modeling approach that can account for the transmission between individuals and in turn accounts for indirect effects such as herd protection on disease transmission should be used as to not underestimate the full value of vaccination.74 Since simpler and thereby potentially more transparent models often are favored by NITAGs and HTA agencies, the cost-effectiveness evidence should ideally be combined with other evidence such as national disease burden, acceptability of the vaccine, etc., when forming the decision.75
A review of European recommendation documents for PCV in line with the present study has to the best of our knowledge not been done previously. Schuurman et al.76 searched for and reviewed recommendation documents for a gender-neutral (GN) human papillomavirus (HPV) vaccination program in 32 countries worldwide where GN HPV was recommended. In line with our findings all identified records included epidemiological data, and slightly more than half of records reported health economic results. St-Martin et al.77 reviewed and compared the medical and epidemiological evidence used during the decision-making processes for introduction of rotavirus (RV) vaccine into NIPs in Sweden, Norway, Finland and Denmark. They found that all countries included disease burden as a key criterion, but interpreted it differently, and that health economic evaluations differed due to the use of different type of models, inclusion of indirect effects, and specific national inputs. The study showcased that four countries with comparable public health-care systems and RV burden, interpreting the same or similar scientific evidence still arrived at different decisions regarding vaccine inclusion in NIP.
One of the limitations of this study is that it is restricted to those countries that have publicly available PCV recommendations by a NITAG or HTA agency. The European countries that do not have a NITAG or HTA agency and base their PCV decisions on tender criteria only are thus not represented here. Secondly, the study is restricted to the availability of records at the date of search. This is especially relevant in this setting, where the main data source are websites that are subject to continuous updates. Furthermore, websites and records were, if needed, translated to English using Google Translate which further adds risk of misinterpretation of the recommendations albeit such a risk could be considered small. Lastly, the vast majority of included records were HTA agency/NITAG reports and do as such not warrant a formal quality assessment. The peer-reviewed published CE studies included in the review were all either identified through the website search or specifically state that they were used as part of the national recommendation forming process, and thus no quality assessment of these were performed. Since there is little consistency in the way countries review and recommend PCV, the data reported in the included records were notably heterogeneous. This limited the ability to compare between reports and countries, and further highlights the standardization of future assessments. The present review showed that 60% of 31 European countries publish reports on childhood PCV vaccination recommendations, with varying degree of details and decision rationale. Some of the countries only published the health economic aspect of their rationale. All countries included in-country epidemiological evidence on IPD, but further research on the differences in the use of specifically AOM and pneumonia evidence is needed. CE analyses were based exclusively on static cohort models, with results being sensitive to the inclusion of herd protection, societal costs, and vaccine price. The material identified through this review can provide knowledge and support local policymakers and clinicians to understand which data has influenced the decision-making process for inclusion and choice of PCV into NIPs in their countries.
Supplementary Material
Funding Statement
This work was funded by MSD.
Disclosure statement
ET, SS and GB are employees of MSD subsidiaries of Merck & Co., Inc., Kenilworth, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. HFW is an employee of Quantify Research and has received research project funding from MSD for the current work.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2060017.
References
- 1.Blasi F, Mantero M, Santus P, Tarsia P.. Understanding the burden of pneumococcal disease in adults. Clini Microbiol Infect. 2012;18:7–11. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright K. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr. 2002;161:188–95. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) . Pneumococcal Disease - Risk Factors; 2017. https://www.cdc.gov/pneumococcal/clinicians/risk-factors.html.
- 4.European Centre for Disease Prevention and Control (ECDC) . Disease factsheet about pneumococcal disease; 2020. https://www.ecdc.europa.eu/en/pneumococcal-disease/facts.
- 5.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27(29):3802–10. doi: 10.1016/j.vaccine.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Htar MTT, Christopoulou D, Schmitt H-J. Pneumococcal serotype evolution in Western Europe. BMC infect dis. 2015;15(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The World Health Organization (WHO) . Vaccine in National Immunization Programme Update October 2020; 2020. https://cdn.who.int/media/docs/default-source/immunization/hpv/vaccineintrostatus.pdf?sfvrsn=a705e49c_5.
- 9.Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O’brien KL, Moore MR, Serotype Replacement Study Group . Serotype-Specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10(9):e1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanquet G, Krizova P, Valentiner-Branth P, Ladhani SN, Nuorti JP, Lepoutre A, Mereckiene J, Knol M, Winje BA, Ciruela P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74(5):473–82. doi: 10.1136/thoraxjnl-2018-211767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–e209. doi: 10.1016/j.ijid.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–68. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 13.Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22(1):10–16. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29(49):9127–31. [DOI] [PubMed] [Google Scholar]
- 15.Urueña A, Pippo T, Betelu MS, Virgilio F, Giglio N, Gentile A, Jimenez SG, Jáuregui B, Clark AD, Diosque M, et al. Cost-Effectiveness analysis of the 10- and 13-valent pneumococcal conjugate vaccines in Argentina. Vaccine. 2011;29(31):4963–72. doi: 10.1016/j.vaccine.2011.04.111. [DOI] [PubMed] [Google Scholar]
- 16.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist A-C, Gershman KA, Vazquez M, Bennett NM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 17.Centre for Reviews and Dissemination CRD . Systematic Reviews - CRD’s guidance for undertaking reviews in health care; 2009.
- 18.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Preferred Reporting Items for Systematic Reviews and Meta-Analyses. http://www.prisma-statement.org/.
- 19.National Immunization Technical Advisory Groups . 2020; https://www.nitag-resource.org/.
- 20.International Network of Agencies for Health Technology Assessment (INAHTA) . Members List. http://www.inahta.org/members/members_list/.
- 21.European Network for Health Technology Assessment EUnetHTA Network; 2020. Jun 02. https://eunethta.eu/about-eunethta/eunethtanetwork/.
- 22.Comissão Técnica de Vacinação (CTV) . Parecer técnico sobre a introdução da vacina pneumocócica conjugada heptavalente no PNV em 2009; 2008.
- 23.Haut Conseil de la santé publique . Avis relatif à une éventuelle évolution des recommandations de vaccination des nourrissons contre les infections invasives à pneumocoque par le vaccin pneumococcique conjugué 13-valent; 2012.
- 24.Haute Autorité de Santé (HAS) . Place du vaccin SYNFLORIX™ dans la stratégie vaccinale contre les infections à pneumocoques chez l’enfant âgé de moins de 5 ans; 2018.
- 25.Gezondheidsraad (Health Council of the Netherlands) . Algemene vaccinatie tegen meningokokken C en pneumokokken; 2002.
- 26.Gezondheidsraad (Health Council of the Netherlands) . Dossier vaccinatie van zuigelingen tegen pneumokokkeninfecties; 2005.
- 27.Bundesministerium Für Soziales G. Pflege und Kosumentschutz. Impfplan Österreich 2020; 2020.
- 28.Council SH . Vaccinatie tegen pneumokokken; 2018.
- 29.Gezondheidszorrg) KFKVD . Cost-Effectiveness of 10- and 13-valent pneumococcal conjugate vaccines in childhood; 2011.
- 30.KCE (Federaal Kenniscentrum voor de Gezondheidszorrg) . Effects and costs of pneumococcal conjugate vaccination of Belgian children; 2006.
- 31.Vucina VV, et al. Cost-Effectiveness of pneumococcal conjugate vaccination in Croatia. Vaccine. 2015;33(Suppl 1):A209–18. [DOI] [PubMed] [Google Scholar]
- 32.Sundhetsstyrelsen . ANBEFALING OM INDFØRELSE AF PNEUMOKOKVACCINATION I DET DANSKE BØRNEVACCINATIONSPROGRAM; 2007.
- 33.Tartu University Department of Public Health . Pneumokokkinfektsioonivastase vaktsineerimise kulutõhusus; 2015.
- 34.THL - Terveyden ja hyvinvoinnin laitos . Pneumokokkirokotus strategiat; 2018.
- 35.KTL (National Public Health Institute) . Kansanterveyslaitoksen asettaman lasten pneumokokkirokotustyöryhmän selvitys [Report of the Pediatric Pneumococcal Vaccination Task Force set up by the National Institute of Public Health]; 2008.
- 36.Haut Conseil de la santé publique . Avis relatif à la vaccination par le vaccin pneumococcique conjugué 13-valent; 2009.
- 37.Haut Conseil de la santé publique . Avis relatif à la réévaluation des recommandations vaccinales du vaccin anti-pneumococcique conjugué heptavalent dans les suites de l’extension d’AMM1 à la prévention des otites moyennes aiguës et des pneumonies à pneumocoque; 2008.
- 38.Conseil supérieur d’hygiène publique de France . Avis du Conseil supérieur d’hygiène publique de France (section maladies transmissibles) relatif à la vaccination par le vaccin anti-pneumococcique conjugué chez les enfants de moins de deux ans et les enfants de deux à cinq ans (séance du 19 mai 2006); 2006.
- 39.STIKO (Ständigen Impfkommission) at Robert Koch-Institut . Wissenschaftliche Begründung zur Änderung der Pneumokokken-Impfempfehlung für Säuglinge; 2015.
- 40.Claes C, Reinert RR, Vauth C, Greiner W. Health technology assessment heptavalenter pneumokokkenkonjugat-impfstoff (PCV7); 2008.
- 41.STIKO (Ständigen Impfkommission) at Robert Koch-Institut . Begründung der STIKO-Empfehlungen zur Impfung gegen Pneumokokken und Meningokokken vom Juli 2006: Pneumokokken-Impfung mit 7-valentem Konjugatimpfstoff für Kinder unter 2 Jahren; 2006.
- 42.Deutsche Agentur für Health Technology Assessment des Deutschen Instituts für Medizinische Dokumentation und Information (DIMDI) . Medizinische und ökonomische Effektivität der Pneumokokkenimpfung für Säuglinge und Kleinkinder; 2005.
- 43.National Centre for Pharmacoeconomics . Economic evaluation of a universal childhood pneumococcal conjugate vaccination strategy in Ireland; 2012. [DOI] [PubMed]
- 44.Tilson L, Usher C, Butler K, Fitzsimons J, O’Hare F, Cotter S, O’Flanagan D, Johnson H, Barry M. Economic evaluation of a universal childhood pneumococcal conjugate vaccination strategy in Ireland. Value Health. 2008;11(5):898–903. [DOI] [PubMed] [Google Scholar]
- 45.National Centre for Pharmacoeconomics . Economic Evaluation of a Universal Childhood Pneumococcal Conjugate Vaccination Strategy in Ireland; 2007. [DOI] [PubMed]
- 46.Società Italiana d’Igiene Medicina Preventiva e Sanità Pubblica . Calendario Vaccinale per la Vita 4° edizione 2019; 2019.
- 47.Ministero della Salute . Piano Nazionale Prevenzione Vaccinale; 2017.
- 48.CSMI . Avis du CSMI pour la vaccination universelle contre le pneumocoque : vaccin 10 ou 13-valent ? 2016.
- 49.CSMI . Vaccination universelle des nourrissons et des enfants contre les infections invasives à Streptococcus pneumoniae; 2011.
- 50.Gezondheidsraad (Health Council of the Netherlands) . Dossier vaccinatie van zuigelingen tegen pneumokokkeninfecties; 2013.
- 51.Gezondheidsraad (Health Council of the Netherlands) . Dossier vaccinatie van zuigelingen tegen pneumokokkeninfecties; 2010.
- 52.Bos JM, et al. Epidemiologic impact and cost-effectiveness of universal infant vaccination with a 7-valent conjugated pneumococcal vaccine in the Netherlands. Clin Ther. 2003;25(10):2614–30. doi: 10.1016/S0149-2918(03)80322-3. [DOI] [PubMed] [Google Scholar]
- 53.Wisloff T, Abrahamsen TG, Bergsaker MAR, Løvoll Ø, Møller P, Pedersen MK, Kristiansen IS. Cost effectiveness of adding 7-valent pneumococcal conjugate (PCV-7) vaccine to the Norwegian childhood vaccination program. Vaccine. 2006;24(29–30):5690–99. doi: 10.1016/j.vaccine.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 54.Agencja Oceny Technologii Medycznych . Prezesa Agencji Oceny Technologii Medycznych w sprawie objęcia refundacją produktu leczniczego Prevenar 13, szczepionka przeciw pneumokokom polisacharydowa, skoniugowana (13-walentna, adsorbowana); zawiesina do wstrzykiwań; 0,5 ml; 1 ampułko-strzykawka (0,5 ml) + 1 igła; kod EAN: 5909990737420; 2014.
- 55.Grupo de Trabajo de Neumococo . Nuevas vacunas antineumocócicas conjugadas; 2009.
- 56.Grupo de trabajo de la Ponencia de Registro y Programa de Vacunas . Enfermedad invasora por streptococcus pneumoniae; 2006.
- 57.Socialstyrelsen . A cost-effectiveness analysis of introducing pneumococcal vaccine in the Swedish vaccination programme; 2008.
- 58.Socialstyrelsen . Bör Sverige införa ett sju-valent konjugerat pneumokockvaccin (PCV7) i det allmänna barnvaccinations-programmet? 2008.
- 59.Bundesamt für Gesundheit und Eidgenössische Kommission für Impffragen . Schweizerischer Impfplan 2020; 2020.
- 60.Bundesamt für Gesundheit (BAG) . Pneumokokkenimpfung von Kindern unter 5 Jahren neu als Basisimpfung empfohlen; 2019.
- 61.Bundesamt für Gesundheit (BAG) . Empfehlungen zur Pneumokokkenimpfung bei Kindern unter 5 Jahren: Wechsel vom 7- zum 13-valenten konjugierten Impfstoff; 2010.
- 62.Bundesamt für Gesundheit (BAG) . Empfehlungen zur Pneumokokkenimpfung bei Kindern unter 5 Jahren; 2007.
- 63.The Joint Committee on Vaccination and Immunisation (JCVI) . Minute of the meeting held on 05 June 2019; 2019.
- 64.The Joint Committee on Vaccination and Immunisation (JCVI) . Minute of the meeting on 07 February 2018; 2018.
- 65.The Joint Committee on Vaccination and Immunisation (JCVI) . Minute of the meeting on 04 October 2017; 2017.
- 66.The Joint Committee on Vaccination and Immunisation (JCVI) . Minute of the meeting on 04 June 2014; 2014.
- 67.The Joint Committee on Vaccination and Immunisation (JCVI) . Proposed changes to the routine childhood immunisation schedule; 2005.
- 68.Vaccine European New Integrated Collaboration Effort (VENICE) II . HTA for pneumococcal vaccines: Survey on the availability of Health Technology Assessment in the field of pneumococcal vaccines and availability of information to perform one. VENICE II, in collaboration with the ECDC; 2011.
- 69.Nohynek H, Wichmann O, D’Ancona F. National advisory groups and their role in immunization policy-making processes in European countries. Clin Microbiol Infect. 2013;19(12):1096–105. doi: 10.1111/1469-0691.12315. [DOI] [PubMed] [Google Scholar]
- 70.Takla A, Wichmann O, Carrillo-Santisteve P, Cotter S, Lévy-Bruhl D, Paradowska-Stankiewicz I, Valentiner-Branth P, D’-Ancona F. Characteristics and practices of National Immunisation Technical Advisory Groups in Europe and potential for collaboration, April 2014. Euro Surveill. 2015;20(9). doi: 10.2807/1560-7917.ES2015.20.9.21049. [DOI] [PubMed] [Google Scholar]
- 71.Sheikh S, Biundo E, Courcier S, Damm O, Launay O, Maes E, Marcos C, Matthews S, Meijer C, Poscia A, Postma M. A report on the status of vaccination in Europe. Vaccine. 2018;36(33):4979–92. [DOI] [PubMed] [Google Scholar]
- 72.European Centre for Disease Prevention and Control , Annual epidemiological report for 2017. Surveillance systems overview for 2017 [Internet; Excel workbook]. 2018, ECDC. [Google Scholar]
- 73.Desmet S, Lagrou K, Wyndham-Thomas C, Braeye T, Verhaegen J, Maes P, Fieuws S, Peetermans WE, Blumental S. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2021;21(1):127–36. doi: 10.1016/S1473-3099(20)30173-0. [DOI] [PubMed] [Google Scholar]
- 74.Ray GT. Pneumococcal conjugate vaccine: review of cost-effectiveness studies in Australia, North America and Europe. Expert Rev Pharmacoecon Outcomes Res. 2008;8:373–93. [DOI] [PubMed] [Google Scholar]
- 75.Ultsch B, Damm O, Beutels P, Bilcke J, Brüggenjürgen B, Gerber-Grote A, Greiner W, Hanquet G, Hutubessy R, Jit M, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European vaccine economics community. Pharmacoeconomics. 2016;34(3):227–44. doi: 10.1007/s40273-015-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schuurman S, et al. Abstract: human papillomavirus gender-neutral vaccination recommendations: do health technology assessment agencies or national immunization technical advisory groups include head and neck cancer burden in their appraisal? 33rd International Papillomavirus Conference; 2020 Jul 20–24; Virtual. [Google Scholar]
- 77.St-Martin G, Lindstrand A, Sandbu S, Fischer TK. Selection and Interpretation of Scientific Evidence in Preparation for Policy Decisions: A Case Study Regarding Introduction of Rotavirus Vaccine Into National Immunization Programs in Sweden, Norway, Finland, and Denmark. Front Public Health. 2018;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


