ABSTRACT
The Advisory Committee on Immunization Practices (ACIP) recommends recombinant zoster vaccine (RZV) to prevent against herpes zoster (HZ) and related complications in immunocompetent adults ≥50 y and immunocompromised adults ≥19 y. In 2019, a statistical safety signal for Guillain-Barré syndrome (GBS) following RZV was identified using data from the Vaccine Safety Datalink (VSD). Subsequently, the U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and collaborators undertook additional analyses using Centers for Medicare & Medicaid Services (CMS) Medicare data to further investigate the potential risk of GBS following RZV. Concurrently, epidemiologic data suggested a potentially elevated risk of GBS following HZ in U.S. adults. Using data from these sources and a published simulation model, this study evaluated the health benefits and risks associated with vaccinating immunocompetent adults ≥50 y with RZV compared to no vaccination. In the base case analysis, RZV vaccination averted 43,000–63,000 cases of HZ, including GBS complications, per million vaccinated per 10-y age cohort compared to 3–6 additional cases of GBS projected following RZV per million vaccinated in the same population. This analysis highlights the projected health benefits of RZV vaccination compared to the relatively low potential risk of GBS following RZV.
KEYWORDS: Herpes zoster vaccination, recombinant zoster vaccine, Shingrix, adult vaccination, risk-benefit, vaccine safety
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends the use of recombinant zoster vaccine (RZV) to prevent against herpes zoster (HZ) and related complications in immunocompetent adults ≥50 y. In October 2021, the ACIP updated these recommendations to include immunocompromised adults ≥19 y.1,2 Since RZV licensure and the ACIP recommendations that followed, the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) have engaged in safety monitoring through established post-marketing surveillance systems including the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD).3,4 In January 2019, rapid cycle analysis of VSD data identified a statistical safety signal for Guillain-Barré syndrome (GBS) following vaccination with RZV; however, results of the chart-confirmed analyses indicated that the evidence was insufficient to confirm the initial signal.5 FDA, CDC, and collaborators conducted additional safety assessment studies using Centers for Medicare & Medicaid Services (CMS) Medicare data to further investigate the potential risk of GBS following RZV administration.6 Concurrently, epidemiologic data suggested a potentially elevated risk of GBS following an episode of HZ in the U.S. adult population.7 Considering these data and as part of the continual process of evidence evaluation,8 the objective of this study was to evaluate the health benefits and risks associated with vaccinating immunocompetent adults aged 50 y and older with RZV compared to no vaccination. Using a HZ vaccination simulation model previously developed in 2017 to inform ACIP recommendations.9 this study compared the projected estimates of averted HZ cases and related complications, including GBS, and potential adverse events, specifically GBS, associated with RZV vaccination. The previously published state-transition model9 was modified to incorporate two new health states, one for GBS following vaccination with RZV and one for GBS as a complication of HZ. This analysis compared projected outcomes for a hypothetical cohort of individuals aged 50 y and older vaccinated with RZV compared to no vaccination, stratified by 10-y age cohorts (50–59, 60–69, 70–79, 80–89, and 90–99 years). Each age-stratified simulation included a cohort size of 1 million U.S. immunocompetent adults. In each annual cycle, a hypothetical individual in the model could experience an episode of HZ and related complications (postherpetic neuralgia (PHN), ocular complications, HZ-associated GBS, HZ-related death) or no zoster illness (Figures 1(a,b)). In the vaccination submodel, vaccinated individuals also had a probability of experiencing GBS following RZV administration (Figure 1(b)). Outcome measures were projected cases of uncomplicated HZ, HZ-related complications (postherpetic neuralgia, ocular complications, GBS, and death), and GBS following vaccination with RZV.
Figure 1.
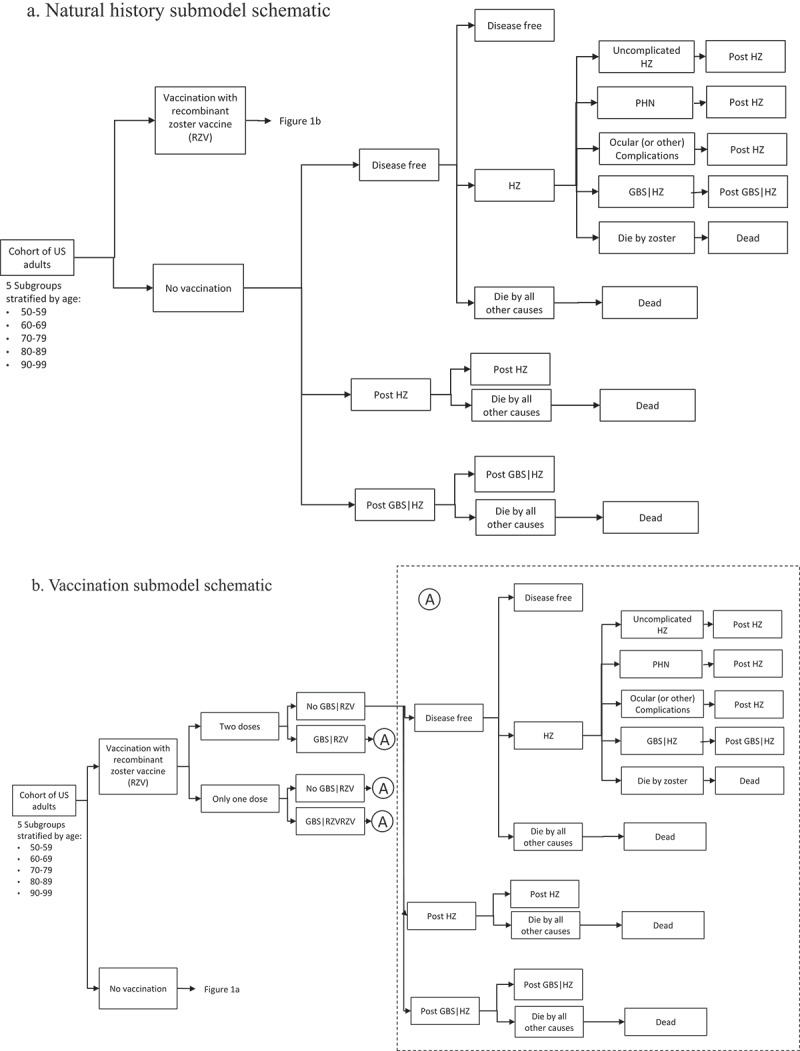
(a) Natural history submodel schematic; (b) Vaccination submodel schematic.
Model inputs that were added or updated since the previous analysis included the risk of GBS following RZV vaccination, the risk of GBS associated with an episode of HZ, the assumed completion rate for the two-dose RZV series, and the effectiveness of a single dose of RZV (Appendix Table A1). The risk of GBS associated with RZV vaccination was derived from two unpublished studies based on post-marketing vaccine safety surveillance and investigations that were presented to ACIP in October 2020 and February 2021.5,10 At the time of analysis, the Vaccine Adverse Reporting System (VAERS) had reported no unexpected severe adverse events or unanticipated patterns of safety reporting associated with RZV administration.4 However, both the Vaccine Safety Datalink (VSD) and an investigation conducted by the Food and Drug Administration (FDA) identified a statistical safety signal for GBS.5,6,10 Due to differences in study methods, these data sources were used to define two plausible scenarios to inform the risk of GBS following RZV vaccination (Appendix A1a). The risk of GBS associated with an episode of HZ was derived from a self-controlled case series analysis of claims data using two large national data sources in the U.S.7 This claims-based study reported rate ratios for 18–64 y and 65+ y age groups and was presented to the ACIP in October 2020 (Appendix A2b). Additionally, two studies were used to update inputs on the overall proportion of vaccine-eligible individuals who were assumed to complete a two-dose RZV series (Leung et al. manuscript under review)8 and the effectiveness of a single dose of RZV11 (Appendix A2).
In the base case analysis, outcomes were projected for each age cohort over a 20-y analytic time horizon and outcomes were discounted at 1.5% per year.12 For the vaccination submodel, vaccination was initiated at the start of the time horizon and individuals were followed for the 20-y analytic time horizon, which corresponds to the assumed duration of vaccine protection, or until death. Uncertainty analyses explored the robustness of results to variation in the parameter inputs over plausible ranges. Probabilistic sensitivity analysis using 10,000 random draws from defined probability distributions for each parameter (Appendix Table A2) generated 95% confidence intervals for projected health outcomes. The model was programmed using TreeAge Pro 2021, version R1.0.
For the base case analysis results, vaccination was projected to avert 43,000 to 63,000 cases of HZ and related complications, depending on age, per million vaccinated per 10-y age cohort (Table 1). These projected vaccination benefits included the prevention of 50 to 170 HZ-related deaths, depending on age, per million vaccinated per 10-y age cohort (Table 1). Projected cases of GBS potentially associated with vaccination were 3.3 cases per million vaccinated using input data derived from the VSD study5 or 6.3 cases per million vaccinated using FDA-derived inputs10 (Table 1). Estimates for projected cases of GBS following vaccination did not vary by age as it was not possible to stratify the risk by age due to sparse data. Incremental cases of GBS, defined as the number of total GBS cases under vaccination minus the number of total GBS cases under no vaccination, ranged from 2.5 to 2.9 cases per million vaccinated using VSD-derived inputs5 and 5.5 to 5.9 per million vaccinated using FDA-derived inputs.10 Across all 10-y age cohorts (Table 1). For all 10-y age cohorts and using the two data scenarios for the risk of GBS following RZV vaccination, there were small numbers of incremental cases of GBS compared to the substantial numbers of HZ-related complications and deaths averted due to vaccination (Table 1). The projected number of incremental GBS cases was sensitive to parameter uncertainty with wide confidence intervals (Appendix Table A6; Appendix Figure A1). Results for averted HZ outcomes, however, were robust to uncertainty analyses (Appendix Tables A3 and A4). For example, when conducting one-way sensitivity analysis using the upper-bound range for the probability of GBS after RZV vaccination derived from the VSD data source, more than 61,000 HZ episodes were averted per million vaccinated compared to fewer than 13 projected cases of GBS related to RZV vaccination per million vaccinated.
Table 1.
Projected cases and cases averted of GBS or HZ per million vaccinated, base case assumptions, 20-y time horizon, discounted.
| (a) Projected cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group (years) | Strategy | Projected GBS|RZV cases (per million vaccinated*) |
Projected HZ cases (per million) † |
|||||||
| GBS|RZV (VSD) |
GBS|RZV (FDA) |
Uncomplicated | PHN | Ocular Complications | GBS | Deaths | Total HZ (cases+deaths) | |||
| 50–59 | Not vaccinated | - | - | 87,137 | 12,562 | 9,871 | 0.9 | 110 | 109,681 | |
| Vaccinated | 3.3 | 6.3 | 49,578 | 7,038 | 5,606 | 0.5 | 62 | 62,284 | ||
| 60–69 | Not vaccinated | - | - | 98,034 | 19,211 | 11,609 | 1.4 | 129 | 128,984 | |
| Vaccinated | 3.3 | 6.3 | 51,387 | 9,861 | 6,064 | 0.7 | 67 | 67,380 | ||
| 70–79 | Not vaccinated | - | - | 88,131 | 22,350 | 10,959 | 1.6 | 329 | 121,771 | |
| Vaccinated | 3.3 | 6.3 | 42,756 | 10,412 | 5,274 | 0.8 | 158 | 58,600 | ||
| 80–89 | Not vaccinated | - | - | 61,355 | 19,148 | 7,986 | 1.1 | 240 | 88,730 | |
| Vaccinated | 3.3 | 6.3 | 21,581 | 6,728 | 2,808 | 0.4 | 84 | 31,202 | ||
| 90–99 | Not vaccinated | - | - | 35,907 | 13,650 | 4,916 | 0.7 | 147 | 54,621 | |
| |
Vaccinated |
3.3 |
6.3 |
7,735 |
2,897 |
1,055 |
0.2 |
32 |
11,719 |
|
| (b) Projected HZ cases averted compared to incremental GBS cases | ||||||||||
| Incremental GBS cases (per million vaccinated)** |
Averted HZ cases (per million vaccinated*) |
|||||||||
| Age group (years) |
Strategy |
GBS (VSD) |
GBS (FDA) |
Uncomplicated |
PHN |
Ocular Complications |
Deaths |
Total HZ |
||
| 50–59 | Vaccinated | 2.9 | 5.9 | 37,559 | 5,524 | 4,266 | 47 | 47,397 | ||
| 60–69 | Vaccinated | 2.6 | 5.6 | 46,648 | 9,350 | 5,544 | 62 | 61,604 | ||
| 70–79 | Vaccinated | 2.5 | 5.5 | 45,375 | 11,938 | 5,685 | 171 | 63,170 | ||
| 80–89 | Vaccinated | 2.6 | 5.6 | 39,774 | 12,421 | 5,178 | 155 | 57,528 | ||
| 90–99 | Vaccinated | 2.8 | 5.7 | 28,171 | 10,753 | 3,861 | 116 | 42,902 | ||
*Cases per million RZV vaccinated (1-dose or 2-dose series).
†Cases per cohort. Each cohort includes 1 million individuals, some proportion of whom experience HZ and some who do not. For example, for unvaccinated individuals 60–69 y, this includes 128,984 total cases HZ and 871,016 individuals without HZ, over the 20-y time horizon.
**Cases of GBS|RZV + GBS|HZ per million RZV vaccinated (1-dose or 2-dose series).
HZ= Herpes zoster; PHN= Postherpetic neuralgia; GBS=Guillain-Barré syndrome; RZV= Recombinant zoster vaccine; VSD=Vaccine Safety Datalink; FDA=U.S. Food and Drug Administration.
This analysis highlights the projected health benefits associated with RZV vaccination compared to the relatively low potential risk of GBS following RZV. Risk-benefit analysis is commonly conducted to inform vaccine policy recommendations and to support the continuous critical appraisal of evidence regarding vaccine benefits and harms as it emerges.13 This type of data-driven analysis may be particularly useful when safety signals are detected in surveillance systems or in special studies because a well-designed risk-benefit model can capture a more holistic picture of the impacts of a vaccination program. As such, a risk-benefit analysis can provide a more contextualized and appropriate evaluation of potential risks, like GBS cases associated with RZV.
Risk-benefit modeling of rare events like GBS is challenging due to the limited data available to define underlying and attributable risks with and without vaccination. In the case of this analysis, key parameters relied on limited evidence to define the probability of GBS following either RZV vaccination or an HZ episode. Where assumptions were needed, however, this limitation was addressed by conservatively defining the base case in a way that biased away from vaccination. Additionally, parameter uncertainty was rigorously evaluated using deterministic one-way and probabilistic sensitivity analyses.
Risk-benefit modeling of rare events like GBS is challenging due to the limited data available to define underlying and attributable risks with and without vaccination. In the case of this analysis, key parameters relied on limited evidence to define the probability of GBS following either RZV vaccination or an HZ episode. Where assumptions were needed, however, this limitation was addressed by conservatively defining the base case in a way that biased away from vaccination. Additionally, parameter uncertainty was rigorously evaluated using deterministic one-way and probabilistic sensitivity analyses.
Risk-benefit modeling of rare events like GBS is challenging due to the limited data available to define underlying and attributable risks with and without vaccination. In the case of this analysis, key parameters relied on limited evidence to define the probability of GBS following either RZV vaccination or an HZ episode. Where assumptions were needed, however, this limitation was addressed by conservatively defining the base case in a way that biased away from vaccination. Additionally, parameter uncertainty was rigorously evaluated using deterministic one-way and probabilistic sensitivity analyses.
In summary, this analysis explored the balance of potential risks and benefits associated with RZV vaccination, demonstrating that vaccination yields substantial health benefits compared to the small risk of a rare adverse event. Future work should continue to investigate estimates of GBS risk following RZV or an episode of HZ. Any empirical work in this area can then be paired with additional modeling work to continually evaluate the tradeoffs between the risks and benefits of vaccination. In February 2021, following review of this analysis and other RZV safety assessments, the ACIP concluded that the benefit–risk balance remained favorable14 and continued to recommend RZV for immunocompetent adults 50 y and older.
Supplementary Material
Acknowledgments
Richard Forshee (Food and Drug Administration [FDA]), Jennifer Nelson (Kaiser Permanente), Angela Rose (UM CHEAR), Acham Gebremariam (UM CHEAR), members of the Advisory Committee on Immunization Practices (ACIP) Herpes Zoster Work Group, and the CDC Immunization Safety Office.
Funding Statement
This study was funded by Centers for Disease Control and Prevention (Contract # 75D30121P10141).
Disclosure statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2060668.
References
- 1.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R.. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Morb Mortal Wkly Rep. 2018;67(3):103–5. doi: 10.15585/mmwr.mm6703a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL.. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesse EM, Shimabukuro TT, Su JR, Hibbs BF, Dooling KL, Goud R, et al. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix) — United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(4):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su JR. Zoster Vaccine Session: Update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix) [Internet]. 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-10/ZosterVaccine-02-Su-508.pdf
- 5.Nelson JC. Zoster Vaccine Session: Vaccine Safety Datalink (VSD) update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix) [Internet]. 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-10/ZosterVaccine-03-Nelson-508.pdf
- 6.Goud R, Lufkin B, Duffy J, Whitaker B, Wong H-L, Liao J, et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in medicare beneficiaries. JAMA Intern Med [Internet]. 2021;181(12):1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson TC, Leung JW, Harpaz R, Dooling KL. Risk of Guillain-Barré syndrome following herpes zoster, United States, 2010–2018. Hum Vaccin Immunother [Internet]. 2021;1–7. doi: 10.1080/21645515.2021.1985890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson T. Zoster Vaccine Session : Summary and planned risk-benefit analysis regarding use of RZV in immunocompetent adults Post-licensure Safety Monitoring of RZV [Internet]. 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-10/ZosterVaccine-05-Anderson-508.pdf
- 9.Prosser LA, Harpaz R, Rose AM, Gebremariam A, Guo A, Ortega-Sanchez IR, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170(6):380–88. [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration (FDA) . Assessment of the risk of Guillain-Barre Syndrome (GBS) following recombinant zoster vaccine (RZV) in Medicare data. [Internet]. 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/02-Zoster-Vaccines-Forshee.pdf
- 11.Izurieta HS, Wu X, Forshee R, Lu Y, Sung H-M, Agger PE, Chillarige Y, Link-Gelles R, Lufkin B, Wernecke M, et al. Recombinant Zoster Vaccine (Shingrix) real-world effectiveness in the first two years post-licensure. Clin Infect Dis Internetciab125. 2021. ;73(6):941–48. doi: 10.1080/00498254.2020.1737890. [DOI] [PubMed] [Google Scholar]
- 12.Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics Internet. 2018;36(7):745–58. doi: 10.1007/s40273-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Carr W, Reingold A, Hunter P, Lee G, Temte J, Campos-Outcalt D, Rubin L, O’-Leary S, Savoy M, et al. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67(45):1271–72. doi: 10.15585/mmwr.mm6745a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson T. Zoster Vaccines Session: summary of the HZ work group’s interpretation of recombinant zoster vaccine safety data [Internet]. 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/04-Zoster-Vaccines-Anderson.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


