Abstract
Many patients with symptoms and signs of heart failure have a left ventricular ejection fraction ≥50%, termed heart failure with preserved ejection fraction (HFpEF). HFpEF is a heterogeneous syndrome mainly affecting older people who have many other cardiac and non-cardiac conditions that often cast doubt on the origin of symptoms, such as breathlessness, or signs, such as peripheral oedema, rendering them neither sensitive nor specific to the diagnosis of HFpEF. Currently, management of HFpEF is mainly directed at controlling symptoms and treating comorbid conditions such as hypertension, atrial fibrillation, anaemia, and coronary artery disease.
HFpEF is also characterized by a persistent increase in inflammatory biomarkers. Inflammation may be a key driver of the development and progression of HFpEF and many of its associated comorbidities. Detailed characterization of specific inflammatory pathways may provide insights into the pathophysiology of HFpEF and guide its future management. There is growing interest in novel therapies specifically designed to target deregulated inflammation in many therapeutic areas, including cardiovascular disease. However, large-scale clinical trials investigating the effectiveness of anti-inflammatory treatments in HFpEF are still lacking. In this manuscript, we review the role of inflammation in HFpEF and the possible implications for future trials.
Keywords: Epidemiology, Hypertension, Global, International, Cardiovascular
Graphical Abstract
Graphical Abstract.
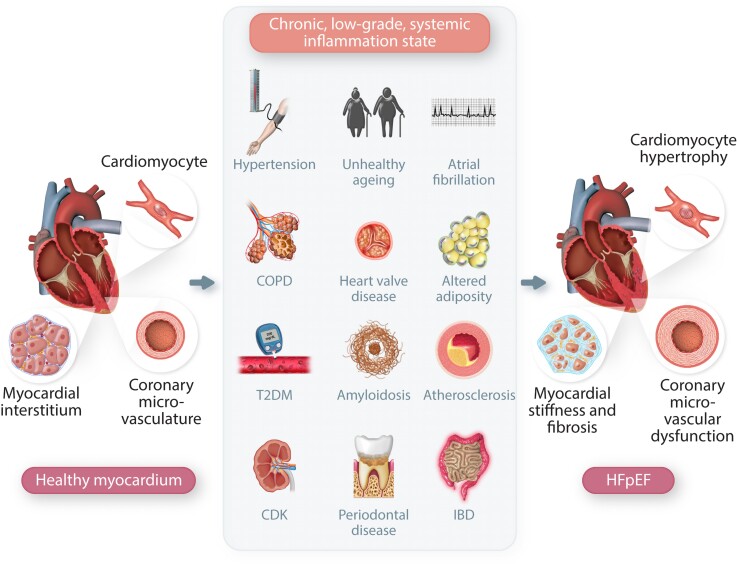
The comorbidity-inflammation paradigm in heart failure with preserved ejection fraction (HFpEF). Unhealthy ageing and highly prevalent comorbidities induce a chronic, low-grade, systemic inflammatory state, which drives the progression from healthy myocardium towards HFpEF. CKD, chronic kidney disease; CMD, coronary microvascular dysfunction; COPD, chronic obstructive pulmonary disease; HFpEF, heart failure with preserved ejection fraction; IBD, inflammatory bowel disease; T2DM, Type 2 diabetes mellitus.
This manuscript was handled by Guest Editor Carolyn S.P. Lam.
This article is part of the Spotlight Issue on Heart Failure.
1. Introduction
Many patients diagnosed with heart failure (HF) have a left ventricular ejection fraction (LVEF) ≥50%, i.e. HF with preserved LVEF (HFpEF).1 Nevertheless, making a diagnosis of HFpEF is challenging. Most patients are elderly, with a high proportion of women and several cardiovascular and non-cardiovascular comorbidities.1 Outpatients with HFpEF have a better overall prognosis and a much lower rate of cardiovascular events than those with HF with reduced LVEF (HFrEF), but a higher proportion of non-cardiovascular deaths.2 However, among patients with decompensated HF, the outcome is similar for HFpEF and HFrEF, although the prognosis for HFpEF might often be driven by comorbid disease rather than HF itself.3 Also, HFpEF and HFrEF are not entirely distinct entities. Indeed, impaired myocardial contraction is expected in both, e.g. circumferential for HFrEF and long axis for HFpEF.4,5 However, measurement of LVEF is prone to substantial error—hence the introduction of the HFmrEF phenotype, which acts as a buffer zone to reduce classification error between HFrEF and HFpEF in research and clinical practice. HFrEF may recover, especially for younger patients with little myocardial scar (e.g. dilated cardiomyopathy) who receive guideline-recommended therapy; this should not be considered HFpEF but rather HF with recovered LVEF.6,7 For patients with HFpEF, LVEF may also drop over time due to measurement error [increased with the onset of atrial fibrillation (AF)]8 or additional myocardial insults (e.g. myocardial infarction).5
A series of randomized control trials (RCTs) of neurohormonal antagonists failed to show clear cardiovascular benefits in patients with HFpEF.1 Recently, a RCT on a factor Xa antagonist demonstrated a substantial reduction in mortality for patients with HFpEF and coronary artery disease (CAD),9 while two others found that sodium-glucose cotransporter-2 inhibitors (SGLT2i) reduce hospitalizations for worsening HF.10,11 These trials showing that the natural history of HFpEF can be favourably modified encourage exploring other therapeutic avenues, including inflammation, which might have a key role in the pathophysiology underlying HFpEF.12 A thorough characterization of inflammatory pathways involved in HFpEF might help identify therapeutic targets and interventions. Herein, we provide an overview of the role of inflammation in the pathogenesis of HFpEF, summarize the available evidence for anti-inflammatory treatments, and discuss the potential implications for the design of future HFpEF trials. We deliberately avoid a detailed discussion of antifibrotic therapies because fibrosis is a non-specific downstream consequence of myocardial damage.12,13
2. The inflammatory-metabolic phenotype and HFpEF
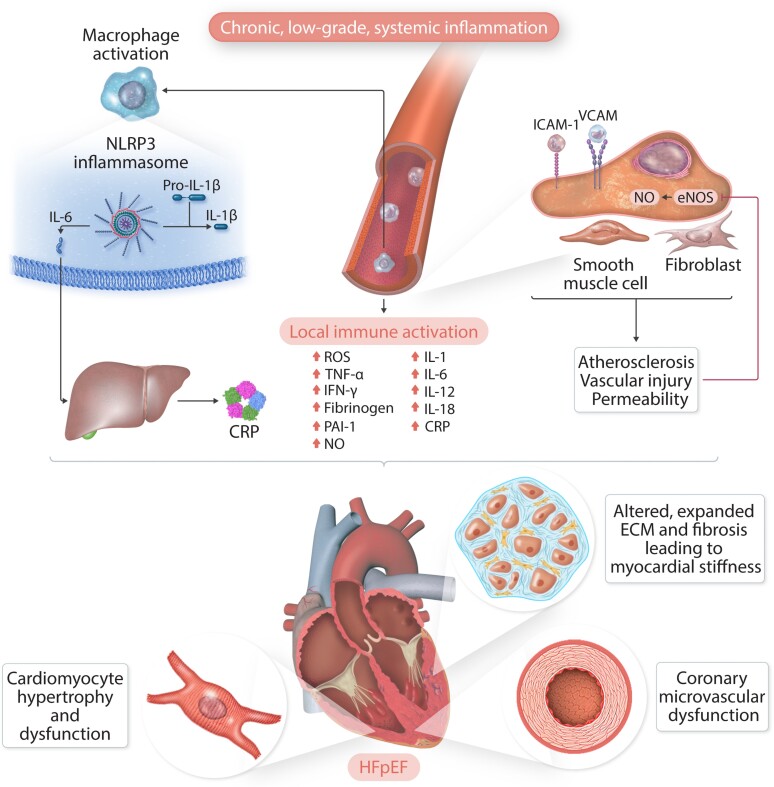
Inflammatory biomarkers, including tumour necrosis factor (TNF)-α and its receptors (TNFR1 and TNFR2), interleukin (IL)-6 and IL-8, high-sensitivity C-reactive protein (hsCRP), pentraxin-3 and the chemokine (C–C motif) ligand 2 (CCL2), also referred to as monocyte chemoattractant protein-1 (MCP-1), are often elevated in patients with HFpEF.14,15 Chronic, low-grade, systemic inflammation might have detrimental effects on myocardial structure and function (Graphical Abstract). Experimental models suggest that increased production of pro-inflammatory cytokines enhances oxidative stress, drives the differentiation of fibroblasts into collagen-secreting myofibroblasts, and induces extracellular matrix degradation, leading to increased myocardial stiffness and coronary microvascular dysfunction (CMD).16,17 Local inflammation also reduces nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) availability, resulting in the hypo-phosphorylation of the giant sarcomeric protein titin, which further increases myocardial stiffness and worsens diastolic function.16 Oxidative stress might also be implicated in the development of metabolic heart disease, indicating bidirectional links between inflammation and cardiac dysfunction.18
Whether the persistent inflammation that characterizes HFpEF represents a causal factor or an epiphenomenon due to one or more pro-inflammatory comorbid conditions is not well understood.16 Indeed, inflammation is frequently associated with unhealthy ageing and several cardio-metabolic comorbidities (Graphical Abstract), e.g. obesity and altered adiposity, chronic kidney disease (CKD), hypertension, CAD, Type 2 diabetes mellitus (T2DM), AF, elevated serum uric acid (SUA) concentrations, and chronic obstructive pulmonary disease (COPD).19–22 Chronic low-grade inflammation might also contribute to the development of sarcopaenia and frailty, which are very common among outpatients with HFpEF, with a prevalence varying from 30 to 52%.23 All these features, particularly when combined, might lead to a poorer quality of life and drive cardiovascular and non-cardiovascular outcomes.24,25 Recently, proteomic bioprofiles have been investigated to identify potential mechanistic pathways associated with HF development. Systemic inflammation might mediate the association between several comorbidities and cardiac dysfunction, promoting the progression of disease.25–27
AF and HFpEF frequently co-exist. Chronically elevated left atrial pressures might produce adverse structural atrial remodelling and dysfunction that increase the risk of developing AF. On the other hand, AF, especially with a rapid ventricular response, leads to a decreased LV filling time and the loss of atrial contribution to LV filling; therefore, it might precipitate the onset or worsening of HFpEF.28 Biomarkers reflecting systemic congestion, inflammation, and fibrosis predict clinically overt AF as well as HFpEF and are associated with abnormal diastolic filling and reduced exercise capacity.29 Endothelial inflammation can cause CMD and myocardial fibrosis, which can induce both atrial and ventricular myopathy.30 In addition, systemic inflammatory and metabolic disorders have been linked to an expansion and pro-inflammatory transformation of epicardial adipose tissue (EAT)31–35 EAT accumulation may also impair atrial distension and contraction by its proximity to the myocardium, leading to mechanical dysfunction, electroanatomical fragmentation, and ultimately AF.36,37
Patients with HFpEF may exhibit different inflammation patterns according to their comorbidities. Inflammation in hypertension is often driven by renin–angiotensin–aldosterone system activation.38,39 On the other hand, the inflammatory milieu of obese patients is primarily due to adipokines, i.e. cytokines secreted by both adipocytes and macrophages resident in adipose tissue.40 Patients with HFpEF who are obese have higher serum concentrations of many pro-inflammatory proteins (galectin-9, CD4, and TNF-related apoptosis-inducing ligand receptor 2).41 This heterogeneity in inflammatory phenotypes could lead to the application of precision medicine in HF treatment, with different therapeutic approaches according to the pattern of comorbidity.42
Other non-metabolic diseases cause a chronic, low-grade, systemic inflammatory response, and produce a clinical picture that fits the HFpEF definition. Amyloidosis is a systemic disorder characterized by the progressive deposition of fibrillary proteins that cause immune cell infiltration into tissues and pro-inflammatory cytokine production in various organs, eventually resulting in their failure.43 Amyloid deposition might be common in patients with HFpEF,44 but, until recently, the diagnosis of amyloidosis has seldom been considered and rarely investigated. Heart valve diseases, even if not severe, can worsen cardiac structure and function and contribute to HF symptoms in the presence of a normal LVEF: it is noteworthy that degenerative valve disease is the most frequent valvular disorder in Western countries and is characterized by calcification, which might, in turn, be related to inflammation.45 Chronic inflammatory disorders, such as inflammatory bowel disease and rheumatoid arthritis, have been linked to an increased risk of cardiovascular disease (CVD) and HF, especially during flares in disease activity.46,47 Periodontal disease is extremely common and associated with increased cardiovascular risk.48 The more severe the periodontitis, the higher the risk of developing HF; conversely, good oral hygiene reduces it.49,50
3. How to identify and quantify inflammation in clinical practice
3.1. Inflammatory biomarkers
The inflammatory profile of patients with HFpEF can be investigated by measuring plasma concentrations of inflammatory biomarkers. However, plasma concentrations reflect a steady-state between production and disposal and are not necessarily evidence of changes in inflammation. In the presence of renal or hepatic dysfunction, elevated plasma concentrations may reflect reduced clearance rather than increased production.51
The most explored inflammatory pathway involves the nucleotide oligomerization domain-like receptor family, pyrin domain-containing (NLRP3) inflammasome (Figure 1), with the subsequent cleavage and activation of IL-1β, IL-6, IL-12, IL-18 and, finally, the production of CRP in the liver.52 This pathway is driven by endogenous stimuli and defined as ‘sterile’ inflammation instead of exogenous-induced inflammation due to infection. In routine clinical practice, hsCRP is widely available, relatively stable in peripheral blood and the most commonly measured inflammatory biomarker. High concentrations of hsCRP are associated with more comorbidity and greater disease severity in HFpEF and HFrEF,53,54 and predict a worse outcome, although for HFpEF this is mainly for non-cardiovascular events.53
Figure 1.
The inflammatory pathways. Chronic, low-grade systemic inflammation activates nucleotide oligomerization domain-like receptor family, pyrin domain-containing (NLRP3) inflammasome, leading to the cleavage and activation of pro-inflammatory cytokines. Inflammation also promotes endothelial dysfunction, atherosclerosis, and vascular injury. All these alterations may contribute to the development of heart failure with preserved ejection fraction (HFpEF). CMD, coronary microvascular dysfunction; eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin-1β; IL-6, interleukin-6; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; TNF-a, tumour necrosis factor-a; VCAM, vascular cell adhesion molecule; VSMC, vascular smooth muscle cell.
In several population-based longitudinal registries including patients with cardiovascular risk factors, SUA levels are associated with an increased risk of all-cause and cardiovascular mortality at serum concentrations substantially lower than those used to define hyperuricaemia in clinical practice.55,56 One potential explanation for this association is the induction of systemic inflammation due to oxygen-free radical production during the conversion of purines to uric acid by xanthine oxidase.22,57 The ESC-EORP-HF-LT registry showed that higher SUA concentrations are associated with an adverse prognosis for both HFrEF and HFpEF.58 However, uric acid itself is a powerful antioxidant,59 which may account for why randomized trials of xanthine oxidase inhibitors have, so far, failed to show convincing cardiovascular benefits.60 A large randomized trial of allopurinol for patients with ischaemic heart disease (IHD) should report soon.61
Galectin-3 is a lectin expressed by activated cardiac macrophages and induces the secretion of IL-6. Elevated serum concentrations of galectin-3 are associated with adverse LV remodelling, cardiomyocyte hypertrophy, and myocardial fibrosis.62 In the Aldo-DHF trial, baseline serum concentrations of galectin-3 were inversely correlated with functional capacity and directly associated with NYHA class in HFpEF patients.62 Data from multiple RCTs and registries suggest that galectin-3 is an independent predictor of adverse outcomes in chronic and acutely decompensated HF, regardless of LVEF.62,63 Thus, galectin-3 has been proposed as a marker of fibrotic activity,64 potentially mediated by inflammation. However, it is excreted by the kidney, and not all studies have corrected for renal function.
High serum concentrations of the soluble suppression of tumourigenicity 2 (sST2, the circulating form of the cellular ST2 receptor, expressed by cardiac and vascular cells together with its ligand IL-33 following cardiovascular injury) are also associated with increased myocardial fibrosis and inflammation as well as with poorer outcomes in patients HFpEF.65–67 Many other markers of inflammation, metabolic dysfunction, and extracellular matrix remodelling (e.g. tissue inhibitors of metalloproteinases, procollagen Type I C-terminal propeptide, and procollagen Type III N-terminal propeptide (PIIINP), adrenomedullin, cystatin C, and resistin) have prognostic significance in HFpEF68–70 but have yet to find a role in clinical practice.
Ferritin binds to iron in cells. In the absence of other diseases, iron deficiency is associated with low release of ferritin, and low serum concentrations signify iron deficiency. However, in the presence of inflammation, serum ferritin leaks from cells rendering it unreliable as a marker of iron deficiency, which might mask a diagnosis of iron deficiency in HFpEF.71 Iron deficiency is common in HF, irrespective of LVEF and associated with adverse outcomes.72 Inflammation can also inhibit iron absorption and iron mobilization and/or utilization even when iron stores are not depleted. Pro-inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6) upregulate the protein hepcidin, leading to reduced intestinal iron absorption and decreased iron mobilization from bone marrow stores.73 Intravenous iron administration in patients with systolic HF improves symptoms and clinical outcomes, but evidence of benefit is still lacking for those with HFpEF.74
Omics techniques such as single-cell RNA sequencing are an emerging tool for studying the transcriptional heterogeneity in both healthy and diseased hearts75 and the diversity of immune cells implicated in the development of CVD.76 Multi-omics approaches may soon uncover novel inflammatory cardiac pathways that offer new therapeutic opportunities.
3.2. Imaging techniques
Echocardiography is the most widely available imaging technique and provides valuable information regarding structural and functional alterations in HFpEF. The E/e’ ratio is a widely used surrogate marker of LV filling pressures, but its clinical utility remains controversial, and it has not been used to select patients for any of the successful landmark trials of HFpEF.77 Left atrial volume and function might be the best marker to integrate left ventricular systolic and diastolic dysfunction, including the mitral apparatus and heart rhythm78,79 but will be agnostic to the underlying pathophysiology. Echocardiography can also evaluate EAT accumulation,80 which is associated with greater inflammatory activity, impaired haemodynamics, worse symptoms, and a poorer prognosis in patients with HF.81
Echocardiography and, more generally, ultrasound represent a valuable tool to estimate congestion,82 which is central to the diagnosis and prognosis of HF and is also associated with increased inflammatory activity.83 In experimental conditions, the development of venous congestion activates the innate immune system and the secretion of pro-inflammatory cytokines in healthy individuals.84 Therefore, treatment targeted at congestion might improve inflammation. Intensification of therapy with loop diuretics had a mixed effect on biomarkers of immune activation and inflammation in one small study of HFrEF, but it normalized endotoxin, which is often increased in congestive HF due to altered gut permeability and subsequent translocation of lipopolysaccharide into the circulation.85,86
Cardiac magnetic resonance (CMR) represents the gold standard for evaluating chamber geometry and structure, as well as quantifying EAT volume with great precision.87,88 CMR can also be used to estimate the extracellular volume and myocardial oedema.89 In HFpEF, the former correlates with myocardial stiffness and the extent of interstitial collagen deposition evaluated at the histopathological level,89 while the latter could be related to increased microvascular permeability or impaired lymphatic function.90
Localized myocardial inflammation can be assessed by positron emission tomography using [18F]fluorodeoxyglucose, which accumulates in activated inflammatory cells (monocyte, macrophage, lymphocyte) due to increased glucose uptake. Mechanistic studies suggest that [18F]fluorodeoxyglucose imaging after myocardial infarction (MI) and pressure-overload HF may provide additional prognostic information.91
4. Inflammation as a therapeutic target in clinical trials of HFpEF
Many large RCTs have investigated the effect of anti-inflammatory agents on cardiovascular events in patients with atherosclerosis, hypertension, and other cardiovascular disorders (Table 1).92 Most of these trials excluded patients with moderate or severe HF, did not always include HF-related endpoints and often did not provide data on LVEF,93,95–100,102,104,105 limiting extrapolation of their results to patients with HFpEF. Nevertheless, IHD is frequently undiagnosed and sub-optimally treated in patients with HFpEF.1 Not many patients with HFpEF undergo coronary angiography due to their advanced age, the high number of comorbidities, particularly renal dysfunction, and the lack of evidence that revascularization is beneficial in the absence of acute ischaemia. Of those who are investigated, many (>50%) have obstructive epicardial CAD, and most (85%) have evidence of CMD.111 The presence of CAD is not surprising, given the high proportion of patients with HFpEF who have hypertension, T2DM, obesity, and CKD. These comorbidities also account for the high prevalence of CMD, which may play a key pathophysiological role in the development of HFpEF, independent of atherosclerotic burden.112,113 Inflammation promotes all stages of atherosclerosis, from plaque formation to rupture, leading to macrovascular and microvascular ischaemic events.76,114 Also, chronic inflammation, even in the absence of epicardial stenoses and traditional coronary risk factors, is associated with CMD.115
Table 1.
Clinical trials investigating anti-inflammatory agents in ischaemic heart disease
| Trial (year) | Setting | Intervention | No. of Patients | FU (months) | Primary endpoint | Results | Study limitations |
|---|---|---|---|---|---|---|---|
| Antioxidants | |||||||
| ARISE (2008)93 | Recent ACS; HF: 15% |
Succinobucol vs. placebo | 6144 | 24 | CVM, RCA, MI, CVA, UA, or Revasc. | No difference. | No data on LVEF. |
| Immunosuppressive agents | |||||||
| METIS (2009)94 | IHD; HF: 100% LVEF: ∼35% |
Methotrexate vs. placebo | 50 | 3 | Difference in 6MWT. | No difference. | Small trial Median hs-CRP at baseline only 2.8 mg/L. |
| CIRT (2019)95 | IHD with T2DM or metabolic syndrome; HF: 13%. |
Methotrexate vs. placebo | 4786 | 28 | Non-fatal MI, RCA, CVM, or urgent Revasc. | No difference. Safety: ↑ cancer (mostly skin basal-cell) with methotrexate. |
No data on LVEF. |
| Colchicine | |||||||
| LoDoCoa (2013)96 | Chronic CAD | Colchicine vs. standard care | 532 | 36 | ACS, RCA, or ischaemic CVA. | 5.3% on colchicine vs. 16.0% with standard care (HR: 0.33, 0.18–0.59; P < 0.001). | History of HF not reported. |
| COLCOTa (2019)97 | Recent MI; HF: 1.9% |
Colchicine vs. placebo | 4745 | 23 | CVM, RCA, MI, CVA, or urgent Revasc. | 5.5% on colchicine vs. 7.1% on placebo (HR: 0.77, 0.61–0.96; P = 0.02). Safety: more pneumonia with colchicine v. placebo 0.9% vs. 0.4%, P = 0.03). |
Relatively short follow-up. No data on LVEF. |
| LoDoCo2a (2020)98 | Chronic CAD (severe HF excluded) | Colchicine vs. placebo | 5522 | 29 | CVM, MI, ischaemic CVA, or urgent Revasc. | 6.8% on colchicine vs. 9.6% on placebo (HR: 0.69, 0.57–0.83; P < 0.001). | History of HF not reported. No baseline CRP. |
| COPS (2020)99 | ACS and evidence of CAD managed with PCI or medical therapy. | Colchicine vs. placebo | 795 | 12 | All-cause mortality, ACS, urgent Revasc, or ischaemic CVA. | No difference. Safety concern: ↑ACM (8 vs. 1, P = 0.017) and non-CVM with colchicine (5 vs. 0, P = 0.024). |
History of HF not reported. |
| Phospholipase A2 inhibitors | |||||||
| SOLID-TIMI 52 (2014)100 | Recent ACS (NYHA III–IV HF excluded). | Darapladib vs. placebo | 13 026 | 30 | CAD-related death, MI, or urgent Revasc. | No difference. | History of HF not reported. No data on LVEF. |
| STABILITY (2014)101 | Chronic CAD (NYHA III–IV HF excluded). | Darapladib vs. placebo | 15 828 | 444 | CVM, MI, or CVA. | No difference. | History of HF not reported. No LVEF data |
| VISTA-16 (2014)102 | ACS; HF: 17.8%. |
Varespladib vs. placebo | 5145 | 6 | CVM, MI, CVA, or UA. | No difference. | Termination of the trial for futility and possible harm. No LVEF data. |
| Cholesterylester transfer protein inhibitors | |||||||
| Dal-GenE (2022)103 | Recent ACS and the AA genotype at variant rs1967309 in the ADCY9 gene. | Dalcetrapib or placebo | 6149 | 39.9 | CVM, RCA, non-fatal MI, and non-fatal stroke. | No difference. | COVID-19 pandemic during study conduct. |
| Anti-IL-1 | |||||||
| MRC-ILA Heart studya (2015)104 | Recent NSTE-ACS. | Anakinra vs. placebo | 182 | 12 | AUC for CRP over the first 7 days. | AUC for CRP ↓ with anakinra vs. placebo (P = 0.003). With IL-1ra, at 14 days ↓ hs-CRP (P < 0.0001) and ↓ IL-6 (P = 0.02). | History of HF not reported. Small sample size. |
| CANTOSa (2017)105,106 | Prior MI and hs-CRP ≥2 mg/L; HF: 22%. |
Canakinumab (three different doses) vs. placebo | 10 061 | 444 | MI, CVA, or CVM. | 50 mg dose, no difference vs. placebo; 150 mg dose (0.85: 0.74–0.98, P = 0.021); 300 mg dose (0.86: 0.75–0.99, P = 0.03). Safety: Canakinumab associated with ↑ of fatal infection but ↓ (lung) cancer. |
No data on LVEF. |
| VCU-ART 3a (2020)107 | STEMI within 12 h of symptom onset (mean LVEF: 51%). | Anakinra vs. placebo | 99 | 12 | AUC for hs-CRP after 14 days. | AUC for CRP ↓ with Anakinra vs. placebo (P < 0.001). | History of HF not reported. Small sample size. Missing data. |
| Anticoagulation | |||||||
| COMMANDER-HF | Chronic HFrEF (LVEF ≤40%), CAD and sinus rhtythm, recently hospitalized for HF. | Rivaroxaban 2.5 mg bid vs. placebo | 5022 | 21 | ACM, MI, or CVA. | No difference. | Only HFrEF. |
| COMPASS pre-planned subanalysisa (2019)9 | CAD or peripheral artery disease; HF: 22%. |
Rivaroxaban 2.5 mg bid + ASA 100 mg and rivaroxaban 5 mg bid alone, vs. ASA 100 mg alone | 27 395 | 23 | CVM, CVA, or MI. | Rivaroxaban + ASA reduced endpoint in patients without (0.79: 0.68–0.93) and with HF (0.68: 0.53–0.86, P for interaction 0.28) with larger absolute risk reduction in those with HF (2.4 vs. 1.0%) vs. ASA alone. No significant differences with rivaroxaban alone. | Only 84% of HF patients had LVEF recorded at baseline (only 12% had LVEF <40%). |
| Influenza vaccination | |||||||
| IAMIa (2021)108 | Recent ACS; Acute HF: 3.8%. |
Influenza vaccine vs. placebo | 2532 | 12 | ACM, MI, or stent thrombosis. | 5.3% after influenza vaccine vs. 7.2% with placebo (0.72: 0.52–0.99, P = 0.04). ACM: 2.9 vs. 4.9% (0.59: 0.39–0.89, P = 0.01). CVD: 2.7 vs. 4.5% (0.59: 0.39–0.90, P = 0.014). MI: 2.0 vs. 2.4% (0.86: 0.50–1.46, P = 0.57). Stent thrombosis: 0.5 vs. 0.2% (1.94: 0.48–7.76, P = 0.34). | History of HF not reported. LVEF ≥50% at discharge in 60.5% of partecipants. |
| IVVE109 (2022) | Chronic HF in low and/or middle income countries. | Influenza vaccine vs. placebo | 2569 | 36 | CVM, non-fatal MI, non-fatal stroke. | No difference for primary. All hospitalizations and pneumonia were reduced. Reductions in the primary endpoint were noted during peak influenza season. | |
| Epigenetic regulators | |||||||
| BETonMACE pre-planned subanalysisa (2021)110 | T2DM up to 3 months after ACS. | Apabetalone vs. placebo | 2425 | 26 | Hosp. for HF. | Apatabetalone ↓ first HF hosp.: 2.4 vs. 4.0% (0.59: 0.38–0.94, P = 0.03), total HF hosps.: 35 vs. 70 (0.47: 0.27–0.83, P = 0.01), and the composite of CVM or HF hosp.: 5.7 vs. 7.8% (0.72: 0.53–0.98, P = 0.04). | No data on LVEF. |
Results are presented as (hazard ratio: 95% confidence interval, P-value). ACM, all-cause mortality; ACS, acute coronary syndrome; ASA, acetylsalicylic acid; AUC, area under the curve; CAD, coronary artery disease; CI, confidence interval; CK-MB, creatin kinase-muscle brain; CMR, cardiac magnetic resonance; CVA, cerebrovascular accident; CVM, cardiovascular mortality; EF, ejection fraction; HDL-C, high-density lipoprotein-cholesterol; HF, heart failure; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; Lp-PLA2, lipoprotein-associated phospholipase A2; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MACE, major adverse cardiovascular events; MI, myocardial infarction; NSTE, non-ST elevation; PCI, percutaneous coronary intervention; PROBE, prospective randomized observer-blinded endpoint; RCA, resuscitated cardiac arrest; Revasc, coronary revascularization; sPLA2, soluble phospholipase A2; STEMI, ST-elevation myocardial infarction; SVG, saphenous vein graft; T2DM, Type 2 diabetes mellitus; TnI, troponin I; UA, unstable angina.
The trial met the primary endpoints.
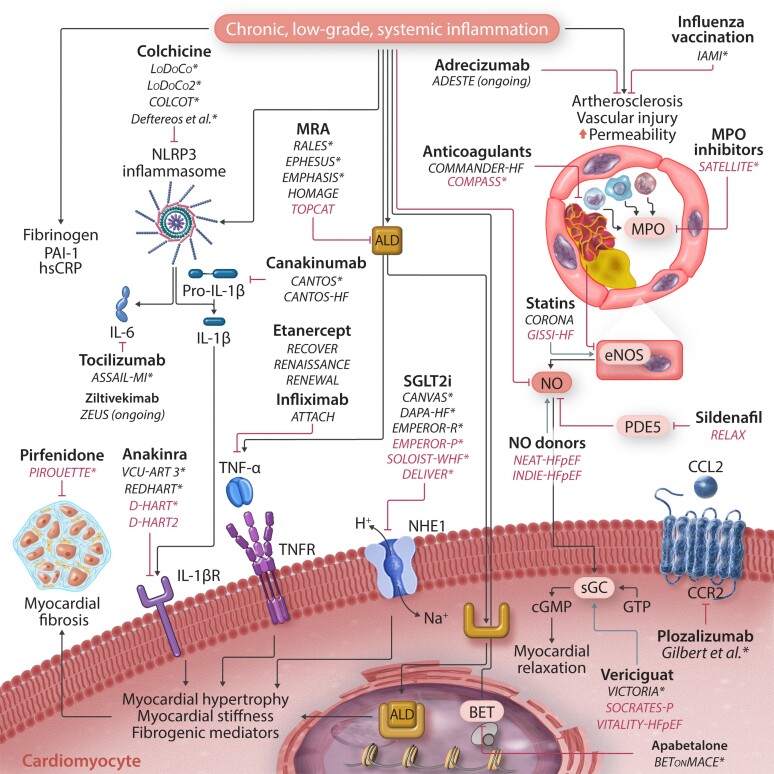
Inflammation has been the therapeutic target for many RCTs that enrolled only patients with HF (Table 2 and Figure 2), but, until now, most of them were focused on the HFrEF phenotype.116–118
Table 2.
Clinical trials investigating anti-inflammatory agents in heart failure
| Trial (year) | Setting | Treatment groups | No. of patients | Follow-up | Primary endpoint | Results | Study limitations |
|---|---|---|---|---|---|---|---|
| Anti-TNF-α | |||||||
| ATTACH (2003)116 | Chronic HF (NYHA III–IV, LVEF ≤35%) | Infliximab vs. placebo | 150 | 6 | Change in clinical status at 14 weeks. | No difference. | Only HFrEF |
| RENAISSANCE, RECOVER, RENEWAL (2004)117 | Chronic HF (NYHA II–IV, LVEF ≤30%) | Etanercept vs. placebo | 2048 | 6 | RENAISSANCE and RECOVER: clinical status at 24 weeks. RENEWAL: CVM or HF hosp. from RENAISSANCE and RECOVER. |
All neutrals. Safety: more infections with etanercept. |
Only HFrEF |
| Statins | |||||||
| CORONA (2007)118 | Chronic HFrEF and IHD >60 years, | Rosuvastatin vs. placebo | 5011 | 33 | CVM, MI, or CVA. | No difference. | Only HFrEF |
| GISSI-HF (2008)119 | Chronic HF | Rosuvastatin vs. placebo | 4574 | 9 | ACM | No difference | Only 10% of patients had LVEF >40%. Sub-optimal compliance to treatment. |
| Nitric oxide signalling promoters | |||||||
| RELAX (2013)24 | Chronic HF (LVEF ≥50%, elevated NT-proBNP or filling pressures). | Sildenafil vs. placebo | 216 | 6 | Change in peak VO2 after 24 weeks. | No difference. | |
| NEAT-HFpEF (2015)120 | Chronic HFpEF | Isosorbide mononitrate vs. placebo | 110 | 3 | Daily activity level assessed by accelerometry | No difference. | Small trial. Possible induction of nitrate tolerance. |
| SOCRATES-PRESERVED (2017)121 | HF with recent exacerbation LVEF >45% | Vericiguat vs. placebo | 477 | 3 | Change from in NT-proBNP and LAV. | No difference. | Short treatment duration. |
| INDIE-HFpEF (2018)122 | Chronic HF LVEF ≥50% Peak VO2 <75% predicted |
Inhaled inorganic nitrite vs. placebo | 105 | 3 | Peak VO2 | No difference. | |
| VICTORIAa (2020)123 | Chronic HF (NYHA II–IV, LVEF < 45%) | Vericiguat vs. placebo | 5050 | 11 | CVM or HF hosp. | 35.5% on vericiguat vs. 38.5% on placebo (0.90: 0.82–0.98, P = 0.02). HF hosp.: 27.4 vs. 29.6% (0.90: 0.81–1.00). CVM: 16.4 vs. 17.5% (0.93: 0.81–1.06). |
Only HFrEF |
| VITALITY-HFpEF (2020)124 | Chronic HF LVEF ≥45% |
Vericiguat vs. placebo | 789 | 6 | KCCQ | No difference. | |
| Colchicine | |||||||
| Deftereos et al. (2014)125 | Chronic HF Mean LVEF: 28% |
Colchicine vs. placebo | 267 | 6 | NYHA | No difference. No difference. |
Single-center study. `Only HFrEF. |
| Anti-IL-1 | |||||||
| D-HARTa (2014)126 | Chronic HFpEF and CRP >2 mg/L. | Anakinra vs. placebo | 12 | 1 | Peak VO2 | ↑ Peak VO2 (+1.2 mL/kg/min, P = 0.009) and ↓ in CRP (−74%, P = 0.006). | Single centre?? Small sample size and short FU. |
| D-HART2 (2018)127 | Chronic HFpEF and CRP >2 mg/L | Anakinra vs. placebo | 31 | 3 | Peak VO2; VE/VCO2 slope | No difference. | Small sample size and short FU. |
| REDHART (2017)128 | Recent HF hosp. LVEF <50% CRP >2 mg/L. | Anakinra vs. placebo | 60 | 3 | Peak VO2 | No effect after 2 weeks; patients treated for 12 weeks had ↑ in peak VO2 (P = 0.009). | Small sample size and short FU. |
| CANTOS-VO2a (2018)129 | Chronic HF (LVEF <50%) with prior MI and hs-CRP ≥2 mg/L. | Canakinumab vs. placebo | 15 | 12 | Peak VO2 and LVEF | Within group analysis: canakinumab ↑ in peak VO2 (P = 0.023) and LVEF (P = 0.012). No changes on placebo. | Single-centre substudy. Small sample size. Within group analyses. |
| CANTOS-HF pre-planned subanalysisa (2019)106 | Chronic HF with prior MI and hs-CRP ≥2 mg/L. | Canakinumab vs. placebo | 385 | 444 | Time to first HF hosp. | 50 mg (1.04: 0.79–1.36); 150 mg (0.86: 0.65–1.13); 300 mg (0.76: 0.57–1.01, P for trend =0.025). | No data on LVEF. |
| SGLT2 inhibitors | |||||||
| DAPA-HFa (2019)130 | HFrEF | Dapagliflozin vs. placebo | 4744 | 18.2 | Worsening HF or CVM | 16.3% on dapagliflozin vs. 21.2% on placebo (0.74: 0.65–0.85, P < 0.001). Worsening HF: 10.0% vs. 13.7% (0.70: 0.59–0.83). CVM: 9.6% vs. 11.5% (0.82: 0.69–0.98). Effects similar in presence or absence of T2DM. |
Only HFrEF |
| EMPEROR-Reduceda (2020)131 | HFrEF | Empagliflozin vs. placebo | 3730 | 16 | CVM or HF hosp. | 19.4% on empagliflozin vs. 24.7% on placebo (0.75: 0.65–0.86, P < 0.001). CVM: 10 vs. 10.8% (0.92: 0.75–1.12). HF hosp.: 13.2 vs. 18.3% (0.70: 0.58–0.85, P < 0.001). Effects similar in presence or absence of T2DM. Safety: Uncomplicated genital tract infection more frequent with empagliflozin. |
Only HFrEF |
| EMPEROR-Preserveda (2021)10 | HF (NYHA II–IV, LVEF >40%) | Empagliflozin vs. placebo | 5988 | 26.2 | CVM or HF hosp. | 13.8% on empagliflozin vs. 17.1% on placebo (0.79: 0.69–0.90, P < 0.001). CVM: 7.3 vs. 8.2% (0.91: 0.76–1.06). HF hosp.: 8.6 vs. 11.8% (0.71: 0.60–0.83). Effects similar in presence or absence of T2DM. Safety: Uncomplicated genital and urinary tract infections and hypotension more frequent with empagliflozin. |
|
| SOLOIST-WHFa (2021)132 | T2DM recently hospitalized for HF | Sotagliflozin vs. placebo | 1222 | 9 | CVM or urgent visits for HF | 51.0 per 100 patient-years on sotagliflozin vs. 76.3 on placebo (0.67: 0.52–0.85, P < 0.001). CVM: 10.6 vs. 12.5 (0.84: 0.58–1.22). | Few patients with HFpEF (∼80% of the population had LVEF <50%). |
| DELIVERa (2022)11 | HF (NYHA II–IV, LVEF >40%) | Dapagliflozin vs. placebo | 6263 | CVM, HF hosp, or urgent HF visits | To be published. | ||
Results are presented as (hazard ratio: 95% confidence interval, P-value). 6MWT, six minutes walking test; ACS, acute coronary syndrome; CAD, coronary artery disease; CI, confidence interval; CITP, collagen Type 1 C-terminal telopeptide; CKD, chronic kidney disease; CVM, cardiovascular mortality; EAT, epicardial adipose tissue; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IQR, interquartile range; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAV, left atrial volume; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP; aminoterminal-pro-brain natriuretic peptide; NYHA, New York Heart Association; PICP, procollagen Type 1 C-terminal propeptide; PIIINP, procollagen Type III N-terminal propeptide; PUFA, polyunsaturated fatty acids; QoL, quality of life; T2DM, Type 2 diabetes mellitus; VE/VCO2, minute ventilation-carbon dioxide production; VO2, volume of oxygen consumption.
The trial met the primary endpoints.
Figure 2.
Clinical trials targeting inflammatory pathways in patients with heart failure. Many different biochemical pathways are involved in inflammation-driven heart injury and can be targeted at different levels. Drugs are in bold, while clinical trials are in italics (trials that included patients with HFpEF are marked blue). ‘*’ denotes trials/studies that met their primary endpoint. BET, bromodomain and extra-terminal motif; CCL2, C–C chemokine ligand 2; CCR2, C–C chemokine receptor Type 2; cGMP, cyclic guanosine monophosphate; ECM, extracellular matrix; eNOS, endothelial nitric oxide synthase; GTP, guanosine triphosphate; HFpEF, heart failure with preserved ejection fraction; IL-1β-R, interleukin-1β receptor; MPO, myeloperoxidase; NLRP3, nucleotide oligomerization domain-like receptor family, pyrin domain-containing; NO, nitric oxide; PDE5, phosphodiesterase-5; sGC, soluble guanylate cyclase; TNFR, tumour necrosis factor receptor.
4.1. Statins
This drug class has several anti-inflammatory properties, including induction of endothelial NO synthase, inhibition of adhesion molecules expression, and reduction of immune cells chemotaxis.133 Administration of rosuvastatin is associated with a reduction in hsCRP.134 Nevertheless, in patients with HF, the effect of statins on disease progression and death remains uncertain.118 In the GISSI-HF trial, rosuvastatin showed no impact on time to death or admission to hospital for cardiovascular reasons in HF patients, irrespective of LVEF; however, only 10% of those enrolled had LVEF >40%.119 Interestingly, in the same trial, administration of n-3 polyunsaturated fatty acids reduced cardiovascular events by a small amount compared with placebo.135 In the CORONA trial, patients in the lowest tertile of aminoterminal-pro-brain natriuretic peptide (NT-proBNP) (roughly <1000 ng/L) had less severe disease and a better prognosis but appeared to benefit from a statin.136 Further analyses of the Heart Protection Study confirmed this finding, showing that as NT-proBNP and absolute cardiovascular risk increase, the relative risk reduction with statins shrinks, reaching a point where statin therapy is futile.137 A recent collaborative meta-analysis of unpublished data from major primary and secondary prevention RCTs showed that statins modestly reduced the risks of non-fatal HF hospitalization but not HF death.138 However, the authors did not have data on LVEF; relatively few were likely to have had HFpEF.
4.2. Nitric oxide signalling pathway
NO is an intercellular messenger synthesized and released into the endothelial cells by NO synthases while converting arginine into citrulline. Impaired NO signalling is one of the cardinal features of endothelial dysfunction and atherosclerosis,139 as NO inhibits platelet aggregation and promotes vascular smooth muscle cell relaxation.140 NO regulates myocardial stiffness and diastolic function in healthy myocardium through the cGMP-protein kinase G pathway.16,141,142 Moreover, NO can also balance the functional activity, growth, and death of many immune and inflammatory cell types, including macrophages, T lymphocytes, antigen-presenting cells, mast cells, neutrophils, and natural killer cells.143 NO signalling could represent a therapeutic target for myocardial stiffness and inflammation, which are typical stigmata of HFpEF. In an inflammatory milieu, NO bioavailability is reduced by reactive oxygen species142 and the inactivation of endothelial NO synthase.144 However, in the RELAX trial, 216 patients with chronic HFpEF were randomized to a phosphodiesterase-5 inhibitor known to prolong NO half-life (sildenafil) or placebo. Sildenafil did not improve exercise performance.24 Likewise, in the NEAT-HFpEF120 and INDIE-HFpEF122 trials, nitrates/nitrites failed to improve daily activity level and exercise capacity in patients with HFpEF. Cimlanod, a nitroxyl donor, has been studied in patients with HFrEF in the STAND-UP AHF trial: compared with placebo, this drug led to decreased NT-proBNP plasma concentrations during infusion.145 In patients with chronic HFrEF, cimlanod showed haemodynamic effects similar to those of nitroglycerin, i.e. venodilatation and preload reduction without additional inotropic or lusitropic effects.146 Ongoing trials of cimlanod will further define its potential role in the treatment of HF.
Vericiguat, an oral soluble guanylate cyclase stimulator, enhances the cGMP pathway by acting synergistically with NO. For patients with HFrEF, the VICTORIA trial suggested a reduction in morbidity and mortality, except in those with a very high NT-proBNP (>5314 ng/L) who appeared to be harmed.123 However, for patients with HFpEF (SOCRATES-PRESERVED, n = 477 and VITALITY-HFpEF, n = 789), vericiguat failed to reduce plasma NT-proBNP concentrations, left atrial volume, or physical activity.121,124
4.3. Colchicine
Colchicine interferes with cytosolic microtubule assembly inhibiting immune cells chemotaxis and cytokines secretion,147 decreasing neutrophil L-selectin expression, thereby inhibiting diapedesis148 and inhibiting activation of the NLRP3 inflammasome, which is indirectly responsible for the cleavage of pro-IL-1β to active IL-1β.149 Colchicine has been extensively studied with encouraging findings in patients with IHD96–99 but with no apparent benefit for patients with chronic HFrEF.125 Two ongoing trials could provide valuable insights into the use of colchicine for HFpEF: the COLCOT-T2D will recruit 10 000 patients with T2DM but without known CAD and evaluate whether colchicine reduces cardiovascular risk and progression to HF; the COLpEF (NCT04857931) will enrol 426 patients with HFpEF to assess the effects of colchicine on hsCRP concentrations (primary outcome), symptoms, and other secondary outcomes.
4.4. Anti-IL-1
More than 20 years ago, Ridker et al.150,151 described the significant association of serum concentrations of inflammatory biomarkers (e.g. IL-6 and CRP) with an increased risk of cardiovascular events. Recently, IL-6 has been associated with new-onset HFpEF in community-dwelling individuals.152 IL-1β is a key determinant of IL-6 production and has adverse effects on myocardial function in animal models, impairing systolic function, interfering with mitochondrial energy production and uncoupling β-adrenergic receptors, and L-type calcium channels.153
Canakinumab is a human monoclonal antibody targeting IL-1β, approved for treating many auto-immune diseases.154 In the CANTOS trial, 10 061 patients with a history of MI and hsCRP ≥2 mg/L were randomized to receive canakinumab in three different doses or placebo. After a median follow-up of 4 years, patients treated with the 150 mg and 300 mg dose had a lower incidence of the primary endpoint, a composite of non-fatal MI, non-fatal stroke, or cardiovascular death.105 In a prespecified analysis, the use of canakinumab led to a lower incidence of HF hospitalization and HF-related mortality; patients who achieved a hsCRP of <2 mg/L with treatment appeared to derive more benefit.106 However, there were no data on LVEF, precluding an analysis by LVEF phenotype.
Anakinra is a recombinant antagonist of the IL-1 receptor, developed as a disease-modifying intervention for rheumatoid arthritis.155 The VCU-ART-3 trial enrolled 99 patients admitted for ST-elevation MI with a mean LVEF of 51%; anakinra reduced hsCRP compared with placebo.107 The D-HART enrolled 12 patients with HFpEF (median BNP 32 pg/mL) and hsCRP >2 mg/L were assigned to receive anakinra vs. matching placebo. After 14 days, patients assigned to anakinra had a lower hsCRP and greater peak VO2.126 However, the subsequent D-HART2, which included 32 obese patients with HFpEF and hsCRP >2 mg/L (median NT-proBNP 98 ng/L and 166 ng/L if assigned to placebo and anakinra, respectively), did not confirm an effect on VO2, but anakinra reduced hsCRP and NT-proBNP concentrations after 4 weeks.127
4.5. Sodium-glucose cotransporter-2 inhibitors
SGLT2i, or gliflozins, inhibit the kidney reabsorption of glucose but also appear to have anti-inflammatory effects.156 They were primarily developed to treat patients with T2DM, but recent landmark RCTs of HFrEF demonstrated that SGLT2i also reduce HF hospitalizations and cardiovascular death, whether or not the patients have T2DM.130,131,157 In the EMPEROR-Preserved trial, nearly 6000 patients with chronic HF and a LVEF >40% were randomized to empagliflozin or placebo. Empagliflozin reduced the incidence of the composite primary endpoint (hospitalization for HF or cardiovascular death), mostly due to a reduction in hospitalization. Haematocrit increased, NT-proBNP fell, and the decline in the estimated glomerular filtration rate was slowed. The benefit was observed for both HFmrEF (LVEF 40–49%) and HFpEF (LVEF ≥50%) regardless of a diagnosis of T2DM.10 The ongoing DELIVER trial will assess the effects of another SGLT2i, dapagliflozin, in HFpEF, probably establishing this class of drugs as a cornerstone for treating HFpEF.158,159
SGLT2i cause a diuresis, resulting in a reduction in plasma volume and interstitial fluid, an increase in haematocrit and a reduction in body weight. Longer-term, SGLT2i might cause a further rise in haematocrit due to stimulation of erythropoietin and improved iron absorption and further loss in weight due to glycosuria and glucose wasting. However, diverse other potential mechanisms have been proposed, including an anti-inflammatory effect, reduction in oxidative stress and fibrosis, reduced deposition of advanced-glycation end-products, inhibition of the sodium/hydrogen exchanger-1 expressed on cardiomyocyte sarcolemma, and an increase in ketone body production as an energy substrate.160–167 Applying artificial intelligence to a cohort of patients with HFpEF, Bayes-Genis et al.168 recently proposed that SGLT2i act at a molecular level, reducing systemic inflammation by lowering the plasma concentration of NO synthase Type 2 and NLRP3 inflammasome. Improvement in cardiac function with administration of empagliflozin to nondiabetic patients with HFrEF has been linked to a reduction in serum markers of inflammation and EAT volume.169,170
4.6. Anticoagulants
Activation of inflammatory pathways induces microvascular dysfunction and increases the risk of thrombotic events contributing to the progression of HF.171 The generation of thrombin can amplify the effects of other stimuli on inflammatory pathways, which might be ameliorated by reducing its production. The COMPASS trial randomized >27 000 patients with CAD in sinus rhythm to a combination of rivaroxaban 2.5 mg bd and aspirin compared with aspirin and rivaroxaban alone. The combination reduced cardiovascular events and mortality.172 The overall result was driven by a large benefit in a subgroup of 4250 patients with HFpEF and mild symptoms;9 patients with severe symptoms had been excluded. In contrast, the COMMANDER-HF, conducted in patients with HFrEF, CAD and in sinus rhythm who had a recent decompensation, showed no improvement in the primary endpoint (all-cause mortality, MI, or stroke) with low-dose rivaroxaban vs. placebo.173 Overall, these data suggest that the efficacy of this intervention, as for many others, depends on the patient profile. In patients with mild, stable HFpEF (and probably HFrEF), low-dose rivaroxaban may be rather effective, but in patients with more severe HF the dose is either too low or too late because other factors are driving progression.174
4.7. Influenza and COVID vaccination
Observational studies suggest that influenza vaccination may reduce mortality in patients with HF, and guidelines recommend considering this intervention in patients with HF.1,175,176 In a recent double-blind RCT including 2571 patients with a recent MI or severe CAD, influenza vaccination reduced the risk of all-cause mortality, MI or stent thrombosis after 12 months; benefits appeared very early, suggesting a therapeutic effect on the post-MI inflammatory phase.108 Unfortunately, the study was terminated prematurely due to the COVID-19 pandemic, but an observational study on >7000 patients with HF showed that COVID-19 vaccination was associated with a substantial reduction in all-cause hospitalization rates and mortality, irrespective of LVEF.177 RCTs are ongoing in patients with recent MI or HF.178
5. Emerging anti-inflammatory targets with potential benefit in HFpEF
HFpEF is associated with the activation of many inflammatory pathways; whether any of these are therapeutic targets is uncertain (Table 2).
5.1. Anti-IL-6
Recent data suggest cross-talk between IL-1 and IL-6 signalling pathways in HF,106,179 leading to increased hepatic CRP production.52 Elevated plasma IL-6 concentrations are a hallmark of persistent low-level ‘sterile’ inflammation related to unhealthy ageing, which is characterized by an augmented risk of metabolic and CVD (‘inflamm-ageing’).180,181 Indeed, IL-6 can increase vascular smooth muscle cell stiffness and mitochondrial dysfunction, which explains the link between inflammation and impaired vascular function.52,182 Interestingly, Tet-2-mutated macrophages secrete higher amounts of IL-6, and these mutations are frequently seen in clonal haematopoiesis of indeterminate potential (CHIP), which is associated with an increased risk of cardiovascular events. In the BIOSTAT-HF study, more than half of the enrolled population had elevated serum concentrations of IL-6, associated with higher NT-proBNP and TNF-α, more iron deficiency, and poorer cardiovascular outcomes.183 Moreover, IL-6 administration in animal models was associated with myocardial hypertrophy and fibrosis, promoting diastolic dysfunction.184 Thus, IL-6 blockade might reduce the cardiovascular burden in HFpEF, but robust data are lacking.182 A subanalysis of the CANTOS trial showed the beneficial effects of canakinumab were more pronounced in those who achieved on-treatment IL-6 concentrations below the study median value of 1.65 ng/L.179 In patients with rheumatoid arthritis without CVD, inhibition of IL-6 receptor with tocilizumab was associated with improved LV systolic function and reduced LV mass.185 In the ASSAIL-MI trial, tocilizumab increased myocardial salvage as measured by CMR in patients with acute ST-segment elevation MI.186 Ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, reduced biomarkers of inflammation and thrombosis among patients with high cardiovascular risk, elevated hsCRP and CKD187; a RCT is ongoing to evaluate its clinical value in patients with CV and renal disease (NCT05021835).
5.2. C–C chemokine receptor Type 2 modulation
In mouse models, two subsets of cardiac macrophages can be identified according to the surface expression of C–C chemokine receptor 2 (CCR2). Tissue-resident CCR2– macrophages are the most represented subset in a normal heart, showing many cardioprotective properties, like promoting tissue regeneration and coronary angiogenesis. Conversely, only a small amount of inflammatory monocyte-derived CCR2+ macrophages is present in healthy mice. The latter initiate inflammation because they can induce neutrophil and monocyte migration into damaged tissues. Mice that developed chronic HF following coronary artery ligation had an increase in CCR2+/CCR2– ratio188; findings subsequently confirmed in human hearts. CCR2 + macrophage abundance was associated with LV remodelling and more advanced systolic dysfunction in myocardial specimens obtained from HFrEF patients who underwent LV assist device implantation.189 An intense proliferation of CCR2+ macrophages has also been described in murine models of pressure overload obtained by aortic constriction.190 CCR2 modulation could represent a potential target to reduce inflammation and block the development of HFpEF: in murine models of angiotensin-II-induced HFpEF, inhibition of CCR2+ macrophages improved diastolic function,191 while in humans, the anti-CCR2 humanized monoclonal antibody MLN1202 (plozalizumab) reduced hsCRP in 112 individuals with CV risk factors.192 Ongoing exploratory trials targeting CCR2 in different diseases (e.g. T2DM and COPD) could hopefully pave the way to study this therapy in the whole HF spectrum.12
5.3. Immunomodulation
The systemic inflammatory state can be reduced by targeting cytokine pathways and a generalized modulation of the immune system activity. Cardiosphere-derived cells are a population of cardiac progenitor cells with prominent anti-inflammatory properties. In a rat model of hypertensive HFpEF, intracoronary treatment with cardiosphere-derived cells reduced serum concentrations of inflammatory cytokines, improved diastolic function, and decreased myocardial fibrosis, despite persistent hypertension.193 The ongoing REGRESS-HFpEF trial (NCT02941705) will assess whether intracoronary administration of cardiosphere-derived cells can reduce pro-inflammatory and pro-fibrotic signalling, as well as improve functional status and haemodynamics in patients with HFpEF.
5.4. Exercise training
Impaired exercise capacity is a hallmark of HFpEF,194 which is likely to be multifactorial, including cardiac dysfunction, skeletal muscle deconditioning, obesity, co-existent lung or joint disease, and psychological factors.195 In patients with HFpEF, endurance exercise training was associated with increased functional capacity (as assessed by peak VO2) and quality of life but no improvement in endothelial function or arterial stiffness.196,197 Physical training reduces systemic vascular resistance198 and increases skeletal muscle perfusion, peripheral oxygen utilization, and mitochondrial function.199,200 These effects further modulate inflammatory and oxidative processes201 that may benefit patients with HFpEF.202,203
5.5. Epigenetics regulators
The term ‘epigenetics’ encompasses the changes that affect gene activity and expression without involving alterations in the genome (DNA sequence).204 The administration of epigenetics regulators in animal models of hypertensive cardiomyopathy reduced TNF-α concentrations and interstitial myocardial fibrosis.205 The BETonMACE trial enrolled 2425 patients with T2DM up to 3 months after an acute coronary syndrome: participants were randomized to placebo and apabetalone, an inhibitor of bromodomain and extra-terminal motif proteins, which are epigenetic modulators of inflammation, thrombogenesis, and lipoprotein metabolism implicated in atherothrombosis.206 Although the trial missed its primary endpoint (MI, stroke, or cardiovascular death), in a prespecified secondary analysis, treatment with apabetalone was associated with a lower incidence of HF hospitalization than placebo110 {hospitalization for HF or cardiovascular death [5.7 vs. 7.8%, hazard ratio 0.72 (95% confidence interval 0.53–0.98), P = 0.04]}. Unfortunately, there were no data about LVEF; thus, further RCTs are needed to evaluate whether epigenetic modulators represent another promising therapeutic approach to preventing and treating HFpEF.
Micro-RNA (miRNA) are small, non-coding RNAs involved in the RNA-induced silencing complex, which binds messenger RNA either inducing its degradation or inhibiting its translation at the ribosomal level.207 A single miRNA can act as an epigenetic modulator, regulating the expression of hundreds of different mRNAs without modifying the gene sequences.208 Several cardiovascular conditions seem to be associated with specific miRNAs: for example, serum concentrations of miR-210 and miR-1 correlate with symptom severity in HF.209,210 Furthermore, inhibition of miRNA-21 prevented the development of HFpEF in an experimental model.211
5.6. Myeloperoxidase inhibitors
Extracellular deposition of granulocyte-derived myeloperoxidase (MPO) can cause oxidative stress, leading to microvascular dysfunction, inflammation, tissue damage, and fibrosis. A novel MPO inhibitor (AZD4831) reduced inflammation and improved microvascular function in preclinical models.212 Target engagement and safety of AZD4831 have been tested in a Phase 2a study of HFpEF (NCT03756285), supporting further development.
5.7. Adrecizumab
Adrenomedullin is a peptide hormone synthesized by endothelial and vascular smooth muscle cells. Its production is stimulated by volume overload to maintain endothelial barrier function, while the disruption of the adrenomedullin system results in vascular leakage and systemic and pulmonary oedema. Adrenomedullin is markedly elevated in patients with sepsis and in patients with acute HF, probably as a compensatory mechanism against fluid overload and tissue congestion.213 Adrecizumab is a monoclonal, non-neutralizing antibody that stabilizes adrenomedullin, ‘trapping’ it in the circulation without blocking adrenomedullin receptor signalling. In addition, adrecizumab translocates adrenomedullin from the tissue into the circulation. In animal models of systemic inflammation and septic shock, adrecizumab-induced increases in plasma adrenomedullin improved haemodynamics, renal function, and reduced markers of inflammation.213 A Phase II proof of concept study in patients hospitalized for acute HF is ongoing (NCT04252937).
5.8. Epicardial adipose tissue
In patients with HFpEF, EAT accumulation is often marked, and it promotes haemodynamic derangements,31–33 altered adipogenesis by secretion of pro-inflammatory and pro-atherogenic adipokines,34 and an adverse prognosis.81 Noteworthy, increased biventricular hypertrophy and EAT exacerbate pericardial restraint in HFpEF, resulting in higher LV filling pressure to achieve a given transmural pressure, particularly during exercise.32 Enhanced pericardial restraint in patients with HFpEF partially explains the lower concentration of natriuretic peptides observed in this cohort, showing pathophysiology similar to that observed in constrictive pericarditis.
Thus, in selected HFpEF patients, non-pharmaceutical interventions (e.g. exercise, diet, or bariatric surgery)214 or pharmacological therapies (e.g. SGLT2i and GLP-1 agonists)35 targeting excessive EAT accumulation might decrease inflammation and potentially provide meaningful clinical benefits.
6. Conclusions
Subclinical inflammation is common in patients with HFpEF, regardless of underlying aetiology and associated comorbidities. A deeper understanding and detailed characterization of inflammatory mechanisms responsible for disease onset and progression may lead to new therapeutic opportunities to improve the well-being and outcomes of those with or at risk of developing HFpEF.
Contributor Information
Nicola Riccardo Pugliese, Department of Clinical and Experimental Medicine, University of Pisa, Pisa 56126, Italy.
Pierpaolo Pellicori, Robertson Institute of Biostatistics and Clinical Trials Unit, University of Glasgow, Glasgow G12 8QQ, UK.
Francesco Filidei, Department of Clinical and Experimental Medicine, University of Pisa, Pisa 56126, Italy.
Nicolò De Biase, Department of Clinical and Experimental Medicine, University of Pisa, Pisa 56126, Italy.
Pasquale Maffia, Centre for Immunobiology, Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8TA, UK; Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; Department of Pharmacy, School of Medicine and Surgery, University of Naples Federico II, Naples 80138, Italy.
Tomasz J Guzik, Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; Department of Internal and Agricultural Medicine, Jagiellonian University, Collegium Medicum, Krakow 31-008, Poland.
Stefano Masi, Department of Clinical and Experimental Medicine, University of Pisa, Pisa 56126, Italy.
Stefano Taddei, Department of Clinical and Experimental Medicine, University of Pisa, Pisa 56126, Italy.
John G F Cleland, Robertson Institute of Biostatistics and Clinical Trials Unit, University of Glasgow, Glasgow G12 8QQ, UK.
Funding
P.M. is supported by the British Heart Foundation (BHF) (grants PG/19/84/34771, FS/19/56/34893A, PG/21/10541, PG/21/10634); TJG is funded by European Research Council (ERC-InflammaTension: ERC-2016-COG-726318) and ERA-Net CVD: Brain-Gut-Immune funded by NCBiR, Poland. Other authors have no relationships relevant to the contents of this paper to disclose.
Data availability
There are no new data associated with this article.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Anker MS, Hülsmann M, Cleland JG. What do patients with heart failure die from? A single assassin or a conspiracy? Eur J Heart Fail 2020;22:26–28. [DOI] [PubMed] [Google Scholar]
- 3. Cleland JGF, McDonagh T, Rigby AS, Yassin A, Whittaker T, Dargie HJ. The national heart failure audit for England and Wales 2008–2009. Heart 2011;97:876–886. [DOI] [PubMed] [Google Scholar]
- 4. Pellicori P, Kallvikbacka-Bennett A, Khaleva O, Carubelli V, Costanzo P, Castiello T, Wong K, Zhang J, Cleland JGF, Clark AL. Global longitudinal strain in patients with suspected heart failure and a normal ejection fraction: does it improve diagnosis and risk stratification? Int J Cardiovasc Imaging 2014;30:69–79. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JGF, Pellicori P, Clark AL, Petrie MC. Time to take the failure out of heart failure: the importance of optimism. JACC: Heart Fail 2017;5:538–540. [DOI] [PubMed] [Google Scholar]
- 6. Halliday BP, Cleland JGF. Maintaining success for patients with dilated cardiomyopathy and remission of heart failure. JACC Basic Transl Sci 2022;7:500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarke CL, Grunwald GK, Allen LA, Barón AE, Peterson PN, Brand DW, Magid DJ, Masoudi FA. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes 2013;6:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;36:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Branch KR, Probstfield JL, Eikelboom JW, Bosch J, Maggioni AP, Cheng RK, Bhatt DL, Avezum A, Fox KAA, Connolly SJ, Shestakovska O, Yusuf S. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease the COMPASS trial. Circulation 2019;140:529–537. Epub ahead of print 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone S V, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Belohlavek J, Chiang CE, Willem Borleffs CJ, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O’Meara E, Kerr Saraiva JF, Tereschenko SN, Thierer J. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Hear Fail 2022;10:184–197. [DOI] [PubMed] [Google Scholar]
- 12. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020;17:269–285. [DOI] [PubMed] [Google Scholar]
- 13. Cleland JGF, Pellicori P, González A. A novel treatment for heart failure targets myocardial fibrosis. Nat Med 2021;27:1343–1344. [DOI] [PubMed] [Google Scholar]
- 14. Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol 2011;57:861–869. [DOI] [PubMed] [Google Scholar]
- 15. Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 2011;13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 16. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 17. Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu L, Balzarolo M, Robinson EL, Lorenz V, Della Verde G, Joray L, Mochizuki M, Kaufmann BA, Valstar G, de Jager SCA, den Ruijter HM, Heymans S, Pfister O, Kuster GM. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc Res 2022;118:2973–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 2020;17:559–573. [DOI] [PubMed] [Google Scholar]
- 20. Siedlinski M, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel RB, Welsh P, Maffia P, Erdmann J, Tomaszewski M, Caulfield MJ, Sattar N, Holmes M V, Guzik TJ. White blood cells and blood pressure: a Mendelian randomization study. Circulation 2020;141:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 22. Spiga R, Marini MA, Mancuso E, Di Fatta C, Fuoco A, Perticone F, Andreozzi F, Mannino GC, Sesti G. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler Thromb Vasc Biol 2017;37:1241–1249. [DOI] [PubMed] [Google Scholar]
- 23. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. JACC Hear Fail 2019;7:291–302. [DOI] [PubMed] [Google Scholar]
- 24. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanders-Van Wijk S, Tromp J, Beussink-Nelson L, Hage C, Svedlund S, Saraste A, Swat SA, Sanchez C, Njoroge J, Tan RS, Fermer ML, Gan LM, Lund LH, Lam CSP, Shah SJ. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS-HFpEF study. Circulation 2020:2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira JP, Verdonschot J, Wang P, Pizard A, Collier T, Ahmed FZ, Brunner-La-Rocca HP, Clark AL, Cosmi F, Cuthbert J, Díez J, Edelmann F, Girerd N, González A, Grojean S, Hazebroek M, Khan J, Latini R, Mamas MA, Mariottoni B, Mujaj B, Pellicori P, Petutschnigg J, Pieske B, Rossignol P, Rouet P, Staessen JA, Cleland JGF, Heymans S, Zannad F.. Proteomic and mechanistic analysis of spironolactone in patients at risk for HF. JACC Hear Fail 2021;9:268–277. [DOI] [PubMed] [Google Scholar]
- 27. Santema BT, Arita VA, Sama IE, Kloosterman M, van den Berg MP, Nienhuis HLA, Van Gelder IC, van der Meer P, Zannad F, Metra M, Ter Maaten JM, Cleland JG, Ng LL, Anker SD, Lang CC, Samani NJ, Dickstein K, Filippatos G, van Veldhuisen DJ, Lam CSP, Rienstra M, Voors AA. Pathophysiological pathways in patients with heart failure and atrial fibrillation. Cardiovasc Res 2022;118:2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Packer M, Lam CSP, Lund LH, Redfield MM. Interdependence of atrial fibrillation and heart failure with a preserved ejection fraction reflects a common underlying atrial and ventricular myopathy. Circulation 2020;141:4–6. [DOI] [PubMed] [Google Scholar]
- 29. Pellicori P, Urbinati A, Kaur K, Zhang J, Shah P, Kazmi S, Capucci A, Cleland JGF, Clark AL. Prevalence and incidence of atrial fibrillation in ambulatory patients with heart failure. Am J Cardiol 2019;124:1554–1560. [DOI] [PubMed] [Google Scholar]
- 30. Godo S, Takahashi J, Yasuda S, Shimokawa H. Role of inflammation in coronary epicardial and microvascular dysfunction. Eur Cardiol Rev 2021;16:1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obokata M, Reddy YNV, Pislaru S V, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Hear Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Hear Fail 2020;8:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–2372. [DOI] [PubMed] [Google Scholar]
- 35. Packer M. Drugs that ameliorate epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J Card Fail 2019;25:986–1003. [DOI] [PubMed] [Google Scholar]
- 36. Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res 2014;102:205–213. [DOI] [PubMed] [Google Scholar]
- 37. Arakelyan M, Golukhova EZ, Gromova OI, Bulaeva NI. Epicardial adipose tissue and nonvalvular atrial fibrillation. Eur Heart J 2021:42. Epub ahead of print 12 October 2021. [Google Scholar]
- 38. Pugliese NR, Masi S, Taddei S. The renin–angiotensin–aldosterone system: a crossroad from arterial hypertension to heart failure. Heart Fail Rev 2019:1–12. [DOI] [PubMed] [Google Scholar]
- 39. Murray EC, Nosalski R, MacRitchie N, Tomaszewski M, Maffia P, Harrison DG, Guzik TJ. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res 2021;117:2589–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res 2020;126:1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kresoja KP, Rommel KP, Wachter R, Henger S, Besler C, Klöting N, Schnelle M, Hoffmann A, Büttner P, Ceglarek U, Thiele H, Scholz M, Edelmann F, Blüher M, Lurz P. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:1633–1644. [DOI] [PubMed] [Google Scholar]
- 42. Maffia P, Guzik TJ. When, where, and how to target vascular inflammation in the post-CANTOS era? Eur Heart J 2019;40:2492–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siegismund CS, Escher F, Lassner D, Kühl U, Gross U, Fruhwald F, Wenzel P, Münzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail 2018;20:751–757. [DOI] [PubMed] [Google Scholar]
- 44. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Hear Fail 2014;2:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, Bönner F, Zimmer S, Nickenig G, Jansen F. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol 2020;40:885–900. [DOI] [PubMed] [Google Scholar]
- 46. Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Lamberts M, Khalid U, Nielsen OH, Torp-Pedersen C, Gislason GH, Hansen PR. Inflammatory bowel disease is associated with an increased risk of hospitalization for heart failure a Danish nationwide cohort study. Circ Hear Fail 2014;7:717–722. [DOI] [PubMed] [Google Scholar]
- 47. Ahlers MJ, Lowery BD, Farber-Eger E, Wang TJ, Bradham W, Ormseth MJ, Chung CP, Stein CM, Gupta DK. Heart failure risk associated with rheumatoid arthritis-related chronic inflammation. J Am Heart Assoc 2020;9: e014661. Epub ahead of print 18 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, Guzik TJ, Hingorani AD, Nart J, D’Aiuto F. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res 2020;116:28–39. [DOI] [PubMed] [Google Scholar]
- 49. Sharma S, Sridhar S, McIntosh A, Messow CM, Aguilera EM, Del Pinto R, Pietropaoli D, Gorska R, Siedlinski M, Maffia P, Tomaszewski M, Guzik TJ, D’Aiuto F, Czesnikiewicz-Guzik M. Periodontal therapy and treatment of hypertension-alternative to the pharmacological approach. A systematic review and meta-analysis. Pharmacol Res 2021;166:105511. [DOI] [PubMed] [Google Scholar]
- 50. Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G, Mikolajczyk TP, Schramm-Luc A, Furtak A, Matusik P, Koziol J, Drozdz M, Munoz-Aguilera E, Tomaszewski M, Evangelou E, Caulfield M, Grodzicki T, D'Aiuto F, Guzik TJ.. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J 2019;40:3459–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Defilippi CR, Herzog CA. Interpreting cardiac biomarkers in the setting of chronic kidney disease. Clin Chem 2017;63:59–65. [DOI] [PubMed] [Google Scholar]
- 52. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:1324–1340. [DOI] [PubMed] [Google Scholar]
- 53. Pellicori P, Zhang J, Cuthbert J, Urbinati A, Shah P, Kazmi S, Clark AL, Cleland JGF. High-sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes, and mode of death. Cardiovasc Res 2020;116:91–100. [DOI] [PubMed] [Google Scholar]
- 54. Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca H-P. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 2015;17:1006–1014. [DOI] [PubMed] [Google Scholar]
- 55. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell'Oro R, Bruno B, Lippa L, D'Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G, Palatini P.. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension 2020;75:302–308. [DOI] [PubMed] [Google Scholar]
- 56. Pugliese NR, Mengozzi A, Virdis A, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, Dell'Oro R, Bruno B, Lippa L, D'Elia L, Verdecchia P, Mallamaci F, Cirillo M, Rattazzi M, Cirillo P, Gesualdo L, Mazza A, Giannattasio C, Maloberti A, Volpe M, Tocci G, Georgiopoulos G, Iaccarino G, Nazzaro P, Parati G.. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin Res Cardiol 2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carluccio E, Coiro S, Ambrosio G. Unraveling the relationship between serum uric acid levels and cardiovascular risk. Int J Cardiol 2018;253:174–175. [DOI] [PubMed] [Google Scholar]
- 58. Ambrosio G, Leiro MGC, Lund LH, Coiro S, Cardona A, Filippatos G, Ferrari R, Piepoli MF, Coats AJS, Anker SD, Laroche C, Almenar-Bonet L, Poder P, Valero DB, Frisinghelli A, Maggioni AP. Serum uric acid and outcomes in patients with chronic heart failure through the whole spectrum of ejection fraction phenotypes: analysis of the ESC-EORP heart failure long-term (HF LT) registry. Eur J Intern Med 2021;89:65–75. [DOI] [PubMed] [Google Scholar]
- 59. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki S, Yoshihisa A, Yokokawa T, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Tsuda A, Tsuda T, Ishibashi T, Konno I, Yamaguchi O, Machii H, Nozaki N, Niizeki T, Miyamoto T, Takeishi Y. Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial. J Int Med Res 2021;49:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. MacKenzie IS, Ford I, Walker A, Hawkey C, Begg A, Avery A, Taggar J, Wei L, Struthers AD, MacDonald TM. Multicentre, prospective, randomised, open-label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL-HEART study. BMJ Open 2016;6:e013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher-Krainer E, Duvinage A, Unkelbach I, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Stough WG, Pieske BM. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the aldo-DHF trial. Eur J Heart Fail 2015;17:214–223. [DOI] [PubMed] [Google Scholar]
- 63. van der Velde AR, Gullestad L, Ueland T, Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ, de Boer RA. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 2013;6:219–226. [DOI] [PubMed] [Google Scholar]
- 64. Calvier L, Martinez-Martinez E, Miana M, Cachofeiro V, Rousseau E, Sádaba JR, Zannad F, Rossignol P, López-Andrés N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Hear Fail 2015;3:59–67. [DOI] [PubMed] [Google Scholar]
- 65. Cunningham JW, Claggett BL, O’Meara E, Prescott MF, Pfeffer MA, Shah SJ, Redfield MM, Zannad F, Chiang L-M, Rizkala AR, Shi VC, Lefkowitz MP, Rouleau J, McMurray JJ V, Solomon SD, Zile MR. Effect of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFpEF. J Am Coll Cardiol 2020;76:503–514. [DOI] [PubMed] [Google Scholar]
- 66. Rios FJ, Zou ZG, Harvey AP, Harvey KY, Nosalski R, Anyfanti P, Camargo LL, Lacchini S, Ryazanov AG, Ryazanova L, McGrath S, Guzik TJ, Goodyear CS, Montezano AC, Touyz RM. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc Res 2020;116:721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res 2021;117:1450–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, Petutschnigg J, Ahmed FZ, Cosmi F, Brunner La Rocca H-P, Mamas MA, Clark AL, Edelmann F, Pieske B, Khan J, McDonald K, Rouet P, Staessen JA, Mujaj B, González A, Diez J, Hazebroek M, Heymans S, Latini R, Grojean S, Pizard A, Girerd N, Rossignol P, Collier TJ, Zannad F.. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. Eur Heart J 2021;42:684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pellicori P, Ferreira JP, Mariottoni B, Brunner-La Rocca HP, Ahmed FZ, Verdonschot J, Collier T, Cuthbert JJ, Petutschnigg J, Mujaj B, Girerd N, González A, Clark AL, Cosmi F, Staessen JA, Heymans S, Latini R, Rossignol P, Zannad F, Cleland JGF.. Effects of spironolactone on serum markers of fibrosis in people at high risk of developing heart failure: rationale, design and baseline characteristics of a proof-of-concept, randomised, precision-medicine, prevention trial. The heart OMics in AGing (HO). Eur J Heart Fail 2020;22:1711–1723. [DOI] [PubMed] [Google Scholar]
- 70. Zhao B, Bouchareb R, Lebeche D. Resistin deletion protects against heart failure injury by targeting DNA damage response. Cardiovasc Res 2021. Epub ahead of print 29 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Masini G, Graham FJ, Pellicori P, Cleland JGF, Cuthbert JJ, Kazmi S, Inciardi RM, Clark AL. Criteria for iron deficiency in patients with heart failure. J Am Coll Cardiol 2022;79:341–351. [DOI] [PubMed] [Google Scholar]
- 72. Cleland JGF, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, Wong K, Rigby A, Goode K, Clark AL. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol 2016;1:539–547. [DOI] [PubMed] [Google Scholar]
- 73. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 74. Graham FJ, Pellicori P, Ford I, Petrie MC, Kalra PR, Cleland JGF. Intravenous iron for heart failure with evidence of iron deficiency: a meta-analysis of randomised trials. Clin Res Cardiol 2021;110:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gladka MM. Single-Cell RNA sequencing of the adult mammalian heart—state-of-the-art and future perspectives. Curr Heart Fail Rep 2021;18:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vallejo J, Cochain C, Zernecke A, Ley K. Heterogeneity of immune cells in human atherosclerosis revealed by scRNA-seq. Cardiovasc Res 2021;117:2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 2017;69:1451–1464. [DOI] [PubMed] [Google Scholar]
- 78. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, Carter R, Borlaug BA. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891–900. [DOI] [PubMed] [Google Scholar]
- 79. Pugliese NR, De Biase N, Conte L, Gargani L, Mazzola M, Fabiani I, Natali A, Dini FL, Frumento P, Rosada J, Taddei S, Borlaug BA, Masi S. Cardiac reserve and exercise capacity: insights from combined cardiopulmonary and exercise echocardiography stress testing. J Am Soc Echocardiogr 2021;34:38–50. [DOI] [PubMed] [Google Scholar]
- 80. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 2009;22:1311–1319. [DOI] [PubMed] [Google Scholar]
- 81. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, Flammer A, Borlaug BA, Ruschitzka F, Masi S. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 2021;23:1858–1871. [DOI] [PubMed] [Google Scholar]
- 82. Pellicori P, Platz E, Dauw J, ter Maaten JM, Martens P, Pivetta E, Cleland JGF, McMurray JJV, Mullens W, Solomon SD, Zannad F, Gargani L, Girerd N. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail 2021;23:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Van Linthout S, Tschöpe C. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 2017;14:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, Lejemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J 2014;35:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Andrew J, Coats S, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999;353:1838–1842. [DOI] [PubMed] [Google Scholar]
- 86. Kim M, Huda MN, Bennett BJ. Sequence meets function—microbiota and cardiovascular disease. Cardiovasc Res 2022;118:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tromp J, Bryant JA, Jin X, van Woerden G, Asali S, Yiying H, Liew OW, Ching JCP, Jaufeerally F, Loh SY, Sim D, Lee S, Soon D, Tay WT, Packer M, van Veldhuisen DJ, Chin C, Richards AM, Lam CSP.. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail 2021;23:835–838. [DOI] [PubMed] [Google Scholar]
- 88. Van Woerden G, Van Veldhuisen DJ, Manintveld OC, Van Empel VPM, Willems TP, De Boer RA, Rienstra M, Westenbrink BD, Gorter TM. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ Heart Fail 2022;15:E009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nagueh SF, Chang SM, Nabi F, Shah DJ, Estep JD. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017;10. Epub ahead of print 1 August 2017. [DOI] [PubMed] [Google Scholar]
- 90. Cuijpers I, Simmonds SJ, van Bilsen M, Czarnowska E, González Miqueo A, Heymans S, Kuhn AR, Mulder P, Ratajska A, Jones EAV, Brakenhielm E. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res Cardiol 2020;115:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thackeray JT, Bengel FM. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: a determinant of subsequent remodeling or recovery. JACC Cardiovas Imaging 2018;11:1340–1355. [DOI] [PubMed] [Google Scholar]
- 92. Liberale L, Montecucco F, Schwarz L, Lüscher TF, Camici GG. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res 2021;117:411–422. [DOI] [PubMed] [Google Scholar]
- 93. Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, Cooper J, Scott R, Lewis EF, L’Allier PL, Pfeffer MA. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:1761–1768. [DOI] [PubMed] [Google Scholar]
- 94. Moreira DM, Vieira JL, Mascia Gottschall CA. The effects of METhotrexate therapy on the physical capacity of patients with ISchemic heart failure: a randomized double-blind, placebo-controlled trial (METIS trial). J Card Fail 2009;15:828–834. [DOI] [PubMed] [Google Scholar]
- 95. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, May ML, Bertrand O, Johnston J, Paynter NP, Glynn RJ. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 97. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 98. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X-F, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 99. Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, van Gaal W, Howes L, Collins N, Yong A, Bhindi R, Whitbourn R, Lee A, Hengel C, Asrress K, Freeman M, Amerena J, Wilson A, Layland J. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation 2020;142:1890–1900. [DOI] [PubMed] [Google Scholar]
- 100. O’Donoghue ML, Braunwald E, White HD, Steen DP, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im KA, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, Steen DP, Lamp JM, McCourt A, Barakat D, Mezzetti J, Morrison C, Stevens M, Ward C, Ardissino D. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. J Am Med Assoc 2014;312:1006–1015. [DOI] [PubMed] [Google Scholar]
- 101. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014; 370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 102. Nicholls SJ, Kastelein JJP, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE, Hennekens C, Brown W V, DeMets D, Pfeffer M, Roleau J, Abraham J, Gebel J, Huff C, Katzan I, Shishehbor M, Rassi A, Uchino K, Vest A, Zishiri E, Heckman MJ, Balog C. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. J Am Med Assoc 2014;311:252–262. [DOI] [PubMed] [Google Scholar]
- 103. Tardif JC, Pfeffer MA, Kouz S, Koenig W, Maggioni AP, McMurray JJ V, Mooser V, Waters DD, Grégoire JC, L’Allier PL, Jukema WJ, White HD, Heinonen T, Black DM, Laghrissi-Thode F, Levesque S, Guertin MC, Dubé MP. Investigators for the dal-G. Pharmacogenetics-guided dalcetrapib therapy after an acute coronary syndrome: the dal-GenE trial. Eur Heart J 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morton AC, Rothman AMK, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA heart study. Eur Heart J 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 106. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 107. Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, Canada JM, Carbone S, Roberts CS, Abouzaki N, Melchior R, Christopher S, Turlington J, Mueller G, Garnett J, Thomas C, Markley R, Wohlford GF, Puckett L, de Chazal HM, Chiabrando JG, Bressi E, Del Buono MG, Schatz A, Vo C, Dixon DL, Biondi-Zoccai GG, Kontos MC, Van Tassell BW. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc 2020;9:e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fröbert O, Götberg M, Erlinge D, Akhtar Z, Christiansen EH, MacIntyre CR, Oldroyd KG, Motovska Z, Erglis A, Moer R, Hlinomaz O, Jakobsen L, Engstrøm T, Jensen LO, Fallesen CO, Jensen SE, Angerås O, Calais F, Kåregren A, Lauermann J, Mokhtari A, Nilsson J, Persson J, Stalby P, Islam AKMM, Rahman A, Malik F, Choudhury S, Collier T, Pocock SJ. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021;144:1476–1484. [DOI] [PubMed] [Google Scholar]
- 109. Loeb M, Dokainish H, Dans A, Palileo-Villanueva LM, Roy A, Karaye K, Zhu J, Liang Y, Goma F, Damasceno A, AlHabib KF, Yonga G, Mondo C, Almahmeed W, Al Mulla A, Yusuf S. Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): rationale and design. Am Heart J Mosby 2019;212:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nicholls SJ, Schwartz GG, Buhr KA, Ginsberg HN, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Sweeney M, Ray KK. Apabetalone and hospitalization for heart failure in patients following an acute coronary syndrome: a prespecified analysis of the BETonMACE study. Cardiovasc Diabetol 2021;20. Epub ahead of print 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, Lang NN, Jhund PS, Campbell RT, McMurray JJV, Petrie MC. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021;6:1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Taqueti VR. Coronary microvascular dysfunction in heart failure with preserved ejection fraction – common, unrecognized, and prevalent in patients with or without epicardial cad. JAMA Cardiol 2021;6:1118–1120. [DOI] [PubMed] [Google Scholar]
- 113. Mordi IR, Pearson ER, Palmer CNA, Doney ASF, Lang CC. Differential association of genetic risk of coronary artery disease with development of heart failure with reduced versus preserved ejection fraction: a GoDARTS Mendelian randomization study and meta-analysis. Circulation 2019;139:986–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Welsh P, Grassia G, Botha S, Sattar N, Maffia P. Targeting inflammation to reduce cardiovascular disease risk: a realistic clinical prospect? Br J Pharmacol 2017;174:3898–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30:1837–1843. [DOI] [PubMed] [Google Scholar]
- 116. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH). Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 117. Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, Van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T.. Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized etanercept worldwide evaluation (RENEWAL). Circulation 2004;109:1594–1602. [DOI] [PubMed] [Google Scholar]
- 118. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JGF, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson Å, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJV, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 119. GISSI-HF investigators . Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 120. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WHW, McNulty SE, Velazquez EJ, Shah MR, Braunwald E. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, Lewinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. J Am Med Assoc 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O’Connor CM. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 124. Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O’Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. J Am Med Assoc 2020;324:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, Karageorgiou S, Papadimitriou C, Vastaki M, Kaoukis A, Angelidis C, Pagoni S, Pyrgakis V, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW. Anti-inflammatory treatment with colchicine instable chronic heart failure. A prospective, randomized study. JACC Hear Fail 2014;2:131–137. [DOI] [PubMed] [Google Scholar]
- 126. Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki NA, Dixon D, Biondi-Zoccai G, Arena R, Abbate A. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Erdle CO, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Del BM, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E, Arena R, Abbate A. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure anakinra response trial). Circ Hear Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi-Erdle C, Kadariya D, Christopher S, Viscusi M, Del Buono M, Kontos MC, Arena R, Van Tassell B, Abbate A. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol 2018;122:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McMurray J, Solomon S, Inzucchi S, Køber L, Kosiborod M, Martinez F, Ponikowski P, Sabatine M, Anand I, Bělohlávek J, Böhm M, Chiang C, Chopra V, de Boer R, Desai A, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Investigators D-HTC . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 131. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 132. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 133. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005;4:977–987. [DOI] [PubMed] [Google Scholar]
- 134. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AMJ, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 135. GISSI-HF investigators . Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 136. Cleland JGF, McMurray JJV, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, Hjalmarson Å, Korewicki J, Lindberg M, Ranjith N, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin. A report from CORONA (controlled rosuvastatin multinational trial in heart). J Am Coll Cardiol 2009;54:1850–1859. [DOI] [PubMed] [Google Scholar]
- 137. Cleland JGF, Squire I, Ng L. Interpretation of amino-terminal pro-brain natriuretic peptide levels in the HPS and the CORONA study. J Am Coll Cardiol 2008;52:1104–1105. [DOI] [PubMed] [Google Scholar]
- 138. Preiss D, Campbell RT, Murray HM, Ford I, Packard CJ, Sattar N, Rahimi K, Colhoun HM, Waters DD, LaRosa JC, Amarenco P, Pedersen TR, Tikkanen MJ, Koren MJ, Poulter NR, Sever PS, Ridker PM, MacFadyen JG, Solomon SD, Davis BR, Simpson LM, Nakamura H, Mizuno K, Marfisi RM, Marchioli R, Tognoni G, Athyros VG, Ray KK, Gotto AM, Clearfield MB.. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J 2015;36:1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 140. Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 2018;15:292–316. [DOI] [PubMed] [Google Scholar]
- 141. Hamdani N, Bishu KG, Von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 2013;97:464–471. [DOI] [PubMed] [Google Scholar]
- 142. Van Heerebeek L, Franssen CPM, Hamdani N, Verheugt FWA, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 2012;9:293–302. [DOI] [PubMed] [Google Scholar]
- 143. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007;15:252–259. [DOI] [PubMed] [Google Scholar]
- 144. Yamamoto E, Hirata Y, Tokitsu T, Kusaka H, Sakamoto K, Yamamuro M, Kaikita K, Watanabe H, Hokimoto S, Sugiyama S, Maruyama T, Ogawa H. The pivotal role of eNOS uncoupling in vascular endothelial dysfunction in patients with heart failure with preserved ejection fraction. Int J Cardiol 2015;190:335–337. [DOI] [PubMed] [Google Scholar]
- 145. Felker GM, McMurray JJV, Cleland JG, O'Connor CM, Teerlink JR, Voors AA, Belohlavek J, B M, Borentain M, Bueno H, Cole RT, DeSouza MM, Ezekowitz JA, Filippatos G, Lang NN, Kessler PD, Martinez FA, Mebazaa A, Metra M, Mosterd A, Pang PS, Ponikowski P, Sato N, Seiffert D, Ye J.. Effects of a novel nitroxyl donor in acute heart failure: the STAND-UP AHF study. JACC Hear Fail 2021;9:146–157. [DOI] [PubMed] [Google Scholar]
- 146. Lang NN, Ahmad FA, Cleland JG, O'Connor CM, Teerlink JR, Voors AA, Taubel J, Hodes AR, Anwar M, Karra R, Sakata Y, Ishihara S, Senior R, Khemka A, Prasad NG, DeSouza MM, Seiffert D, Ye JY, Kessler PD, Borentain M, Solomon SD, Felker GM, McMurray JJV.. Haemodynamic effects of the nitroxyl donor cimlanod (BMS-986231) in chronic heart failure: a randomized trial. Eur J Heart Fail 2021;23:1147–1155. [DOI] [PubMed] [Google Scholar]
- 147. Andreu JM, Timasheff SN. Tubulin bound to colchicine forms polymers different from microtubules. Proc Natl Acad Sci U S A 1982;79:6753–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest 1995;96:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJF, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin TT, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA, Tocci MJ.. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 1992;356:768–774. [DOI] [PubMed] [Google Scholar]
- 150. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 151. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98:731–733. [DOI] [PubMed] [Google Scholar]
- 152. Chia YC, Kieneker LM, van Hassel G, Binnenmars SH, Nolte IM, van Zanden JJ, van der Meer P, Navis G, Voors AA, Bakker SJL, De Borst MH, Eisenga MF. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc 2021;10:18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Van Tassell BW, Raleigh JMV, Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep 2015;12:33–41. [DOI] [PubMed] [Google Scholar]
- 154. De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, Koné-Paut I, Lachmann HJ, Ozen S, Simon A, Zeft A, Calvo Penades I, Moutschen M, Quartier P, Kasapcopur O, Shcherbina A, Hofer M, Hashkes PJ, Van der Hilst J, Hara R, Bujan-Rivas S, Constantin T, Gul A, Livneh A, Brogan P, Cattalini M, Obici L, Lheritier K, Speziale A, Junge G.. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018;378:1908–1919. [DOI] [PubMed] [Google Scholar]
- 155. Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 2009;36:1118–1125. [DOI] [PubMed] [Google Scholar]
- 156. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, Mayer G. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019;62:1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, De Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, Wilderäng U, Öhrn F, Claggett B, Langkilde AM, Petersson M, McMurray JJV. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail 2021;23:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Savarese G, Butler J, Lund LH, Bhatt DL, Anker SD. Cardiovascular effects of non-insulin glucose-lowering agents: a comprehensive review of trial evidence and potential cardioprotective mechanisms. Cardiovasc Res 2021. Epub ahead of print 15 August 2021. [DOI] [PubMed] [Google Scholar]
- 160. Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metab Clin Exp 2018;85:32–37. [DOI] [PubMed] [Google Scholar]
- 161. Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Iborra-Egea O, Santiago-Vacas E, Yurista SR, Lupón J, Packer M, Heymans S, Zannad F, Butler J, Pascual-Figal D, Lax A, Núñez J, de Boer RA, Bayés-Genís A. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic Transl Sci 2019;4:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cowie MR, Fisher M. SGLT2 Inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020;17:761–772. [DOI] [PubMed] [Google Scholar]
- 164. Packer M. Critical examination of mechanisms underlying the reduction in heart failure events with SGLT2 inhibitors: identification of a molecular link between their actions to stimulate erythrocytosis and to alleviate cellular stress. Cardiovasc Res 2021;117:74–84. [DOI] [PubMed] [Google Scholar]
- 165. Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ. Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [na + ]i in the heart. Cardiovasc Res 2021;117:2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ. SGLT2 Inhibitors and the cardiac Na+/H+ exchanger-1: the plot thickens. Cardiovasc Res 2021;117:2702–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 Inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bayes-Genis A, Iborra-Egea O, Spitaleri G, Domingo M, Revuelta-López E, Codina P, Cediel G, Santiago-Vacas E, Cserkóová A, Pascual-Figal D, Núñez J, Lupón J. Decoding empagliflozin’s molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci Rep 2021;11. Epub ahead of print 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, Rodriguez-Cordero A, Zafar MU, Fergus I, Atallah-Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ.. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021;77:243–255. [DOI] [PubMed] [Google Scholar]
- 170. Antonio R-IJ GS-GC, Anderly R-C PV-DA, Donna M, Samantha S, Farah A-L, Chiara G, Frank M, Anuradha L, Javier S, Valentin F, José BJ. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF. JACC Hear Fail 2021;9:578–589. [DOI] [PubMed] [Google Scholar]
- 171. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 2021;18:666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, St S, Keltai M, Ryden L, Pogosova N.. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 173. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–1342. [DOI] [PubMed] [Google Scholar]
- 174. Cleland JGF, Pellicori P. Myocardial dysfunction and coronary artery disease as therapeutic targets in heart failure COMPASS directions. Circulation 2019;140:538–541. [DOI] [PubMed] [Google Scholar]
- 175. Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett B, Hegde SM, Solomon SD, Torp-Pedersen C, Biering-Sørensen T. Influenza vaccine in heart failure: cumulative number of vaccinations, frequency, timing, and survival: a Danish nationwide cohort study. Circulation 2019;139:575–586. [DOI] [PubMed] [Google Scholar]
- 176. Behrouzi B, Bhatt DL, Cannon CP, Vardeny O, Lee DS, Solomon SD, Udell JA. Association of influenza vaccination with cardiovascular risk: a meta-analysis. JAMA Netw Open 2022;5:E228873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Johnson KW, Patel S, Thapi S, Jaladanki BS SK, Rao A, Nirenberg S, Lala A. Association of reduced hospitalizations and mortality among COVID-19 vaccinated patients with heart failure. J Card Fail 2022. Epub ahead of print June 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pellicori P, Khan MJI, Graham FJ, Cleland JGF. New perspectives and future directions in the treatment of heart failure. Heart Fail Rev 2020;25:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur Heart J 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 180. Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kosmopoulos M, Chiriacò M, Stamatelopoulos K, Tsioufis C, Masci PG, Kontogiannis C, Mengozzi A, Pugliese NR, Taddei S, Virdis A, Masi S, Georgiopoulos G. The relationship between telomere length and putative markers of vascular ageing: a systematic review and meta-analysis. Mech Ageing Dev 2021;201:111604. [DOI] [PubMed] [Google Scholar]
- 182. Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol 2020;18:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P.. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail 2019;21:965–973. [DOI] [PubMed] [Google Scholar]
- 184. Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertens (Dallas, Tex 1979) 2010;56:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kobayashi H, Kobayashi Y, Giles JT, Yoneyama K, Nakajima Y, Takei M. Tocilizumab treatment increases left ventricular ejection fraction and decreases left ventricular mass index in patients with rheumatoid arthritis without cardiac symptoms: assessed using 3.0 tesla cardiac magnetic resonance imaging. J Rheumatol 2014;41:1916–1921. [DOI] [PubMed] [Google Scholar]
- 186. Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, Berg ES, Bjørkelund E, Bendz C, Hopp E, Kleveland O, Stensæth KH, Opdahl A, Kløw NE, Seljeflot I, Andersen GØ, Wiseth R, Aukrust P, Gullestad L.. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2021;77:1845–1855. [DOI] [PubMed] [Google Scholar]
- 187. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, Libby P, Davidson M. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021;397:2060–2069. [DOI] [PubMed] [Google Scholar]
- 188. Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 2019;124:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial JA, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 2010;49:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, Wyant T, Davidson M. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol 2011;107:906–911. [DOI] [PubMed] [Google Scholar]
- 193. Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marbán E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci 2016;1:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Pugliese NR, De Biase N, Gargani L, Mazzola M, Conte L, Fabiani I, Natali A, Dini FL, Frumento P, Rosada J, Taddei S, Borlaug BA, Masi S. Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol 2021;28:1650–1661. [DOI] [PubMed] [Google Scholar]
- 195. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2209–2225. [DOI] [PubMed] [Google Scholar]
- 196. Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 2013;62:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Mann N, Rosenzweig A. Can exercise teach US how to treat heart disease? Circulation 2012;126:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. J Am Med Assoc 2000;283:3095–3101. [DOI] [PubMed] [Google Scholar]
- 199. Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 1997;29:1067–1073. [DOI] [PubMed] [Google Scholar]
- 200. Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 2011;58:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 2017;47:600–611. [DOI] [PubMed] [Google Scholar]
- 202. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;2019:CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Pugliese NR, Mazzola M, Madonna R, Gargani L, De Biase N, Dini FL, Taddei S, De Caterina R, Masi S. Exercise-induced pulmonary hypertension in HFpEF and HFrEF: different pathophysiologic mechanism behind similar functional impairment. Vascul Pharmacol 2022;144:106978. [DOI] [PubMed] [Google Scholar]
- 204. Kim SY, Morales CR, Gillette TG, Hill JA. Epigenetic regulation in heart failure. Curr Opin Cardiol 2016;31:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Felisbino MB, McKinsey TA. Epigenetics in cardiac fibrosis: emphasis on inflammation and fibroblast activation. JACC: Basic Transl Sci 2018;3:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ray KK, Nicholls SJ, Ginsberg HD, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Cummings JL, Sweeney M, Schwartz GG. Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: rationale, design, and baseline characteristics of the BETonMACE trial. Am Heart J 2019;217:72–83. [DOI] [PubMed] [Google Scholar]
- 207. Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005;122:553–563. [DOI] [PubMed] [Google Scholar]
- 208. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 209. Endo K, Naito Y, Ji X, Nakanishi M, Noguchi T, Goto Y, Nonogi H, Ma X, Weng H, Hirokawa G, Asada T, Kakinoki S, Yamaoka T, Fukushima Y, Iwai N. MicroRNA 210 as a biomarker for congestive heart failure. Biol Pharm Bull 2013;36:48–54. [DOI] [PubMed] [Google Scholar]
- 210. Sygitowicz G, Tomaniak M, Blaszczyk O, Koltowski L, Filipiak KJ, Sitkiewicz D. Circulating microribonucleic acids miR-1, miR-21 and miR-208a in patients with symptomatic heart failure: preliminary results. Arch Cardiovasc Dis 2015;108:634–642. [DOI] [PubMed] [Google Scholar]
- 211. Dong S, Ma W, Hao B, Hu F, Yan L, Yan X, Wang Y, Chen Z, Wang Z. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating bcl-2. Int J Clin Exp Pathol 2014;7:565–574. [PMC free article] [PubMed] [Google Scholar]
- 212. Gan LM, Lagerström-Fermér M, Ericsson H, Nelander K, Lindstedt EL, Michaëlsson E, Kjaer M, Heijer M, Whatling C, Fuhr R. Safety, tolerability, pharmacokinetics and effect on serum uric acid of the myeloperoxidase inhibitor AZD4831 in a randomized, placebo-controlled, phase I study in healthy volunteers. Br J Clin Pharmacol 2019;85:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Voors AA, Kremer D, Geven C, ter Maaten JM, Struck J, Bergmann A, Pickkers P, Metra M, Mebazaa A, Düngen HD, Butler J. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail 2019;21:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Launbo N, Zobel EH, von Scholten BJ, Færch K, Jørgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: a systematic review and meta-analysis. Obes Rev 2021;22. Epub ahead of print 1 January 2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data associated with this article.