Abstract
The insect-pathogenic fungal pathogen Entomophaga maimaiga is endemic to northeastern Asia and was first found in North America in 1989. Due to repeated epizootics and spread within populations of the major forest defoliator in northeastern North America, the gypsy moth (Lymantria dispar), this pathogen has gained much notoriety. Although this pathogen was purposely introduced to North America for biological control of L. dispar in 1910 to 1911, it is questionable whether it became established at the time of release and then remained at innocuous levels until relatively recently. Alternatively, the fungal strain present in North America today could be a more recent accidental introduction. DNA analysis demonstrates that this pathogen differs significantly from North American members of the same species complex (the Lepidoptera-specific Entomophaga aulicae species complex), and, to date, isolates of this introduced pathogen display little heterogeneity in North America. Nonsusceptible lepidopteran larvae have been identified, and either E. maimaiga is unable to penetrate the cuticle or the fungus cannot survive within the hemocoel. In the latter case, although E. maimaiga grows as protoplasts lacking cell walls in the host hemolymph, glycoproteins on plasma membranes of the protoplasts could lead to host recognition. Epizootiological studies demonstrate a clear association between fungal activity and environmental moisture but little association with host density under hypothesized conditions of high fungal density. Prediction of the occurrence of epizootics is not yet possible. E. maimaiga is easily established in new areas by releasing azygospores, but the ability to use this pathogen further for biological control will depend, in large part, on the development of mass production systems.
Entomophthoralean Fungi as Arthropod Pathogens
The fungal order Entomophthorales in the class Zygomycetes is composed principally of obligate pathogens that infect arthropods. The >200 species infect a wide variety of hosts, and nearly every fungal species or strain is quite host specific. Species within the Entomophthorales are well known for their ability to cause dramatic epizootics in populations of aphids, leafhoppers and planthoppers, flies, grasshoppers, cicadas, and coleopteran and lepidopteran larvae. Sexual spores are generally soil borne and vegetative spores are airborne, as might be expected since many of the hosts occupy epigeal (i.e., above yet near the ground) habitats. Because many insect hosts are pestiferous, species within the Entomophthorales have frequently been studied to evaluate their potential for pest control. Mass production for application as mycoinsecticides is presently not feasible for most species, largely due to difficulties with in vitro growth and stability. Nonetheless, manipulation of entomophthoralean species for classical biological control, augmentation to induce epizootics, and conservation to promote infection are alternative uses that are being explored. However, to optimize the manipulation of these pathogens for pest control, extensive knowledge of their biology and epizootiology is required.
In recent years, the establishment of an entomophthoralean pathogen infectious to larvae of a major nonindigenous North American forest defoliator has provided researchers with an opportunity to concentrate studies on one member of this fungal group. Entomophaga maimaiga was originally described from northern Asia and was first discovered infecting Lymantria dispar, the gypsy moth, in North America in 1989. Previously, relatively little was known of this pathogen from northern Asia, and the majority of publications described epizootics in L. dispar populations in Japan (3, 82, 136, 154). Due to the importance of L. dispar outbreaks in North America and the recurrence of epizootics caused by E. maimaiga, studies of the biology, pathology, and epizootiology of this fungus have been conducted. There are many gaps in our knowledge of this fungus, however, because E. maimaiga has been studied intensively for only a relatively short time. Therefore, when possible, information on other members of the lepidopteran-specific E. aulicae species complex, the orthopteran-specific sister group Entomophaga grylli, or other species of Entomophthorales is discussed to provide possible scenarios for E. maimaiga activity.
GENERAL DISTRIBUTION AND BIOLOGY OF E. MAIMAIGA
Taxonomy and Distribution
E. maimaiga belongs to the E. aulicae species complex. This complex occurs in temperate areas of the northern hemisphere, and its strains infect only lepidopteran larvae. Members of this complex lack morphological features that can be used to differentiate species or strains; therefore, biochemical assays have been used to identify groupings within the complex. There are two named species within the complex, E. aulicae and E. maimaiga (143) (Table 1). E. aulicae is now considered to be composed of at least three groups.
TABLE 1.
Groupings of isolates of the E. aulicae species complex, their host affinities, collection dates, and storage locationsa
| Fungal group | Lepidopteran family | Host species | Collection location | Collection yr | Isolate no.b |
|---|---|---|---|---|---|
| Group I | Geometridae | Lambdina fiscellaria | Newfoundland, Canada | 1970 | FPMI 458 |
| Lambdina fiscellaria | Newfoundland, Canada | 1985 | FPMI 893 | ||
| Rheumaptera hastata | Ontario, Canada | 1982 | FPMI 717 | ||
| Tortricidae | Choristoneura fumiferana | Newfoundland, Canada | 1973 | FPMI 521 | |
| Choristoneura fumiferana | Newfoundland, Canada | 1978 | FPMI 646 | ||
| Choristoneura fumiferana | Ontario, Canada | 1979 | FPMI 877 | ||
| Choristoneura fumiferana | Ontario, Canada | 1984 | ARSEF 1995 | ||
| Choristoneura fumiferana | Ontario, Canada | 1986 | FPMI 934 | ||
| Choristoneura fumiferana | Maine, USA | 1975 | ARSEF 51 | ||
| Choristoneura occidentalis | British Columbia, Canada | 1976 | FPMI 599 | ||
| Lasiocampidae | Dendrolimus spectabilis | Ibaraki, Japan | 1984 | ARSEF 1655 | |
| Noctuidae | Aedia leucomelas | Tokyo, Japan | 1976 | ARSEF 198 | |
| Mamestra brassicae | Ibaraki, Japan | 1984 | ARSEF 1751 | ||
| Notodontidae | Heterocampa guttivitta | Vermont, USA | 1989 | ARSEF 1843 | |
| Saturniidae | Dryocampa rubicunda | Ontario, Canada | 1977 | FPMI 626 | |
| Group II | Noctuidae | Heliothis sp. | Georgia, USA | 1978 | ARSEF 251 |
| Arctiidae | Hyphantria cunea | Ontario, Canada | 1974 | FPMI 558 | |
| Lymantriidae | Orgyia vetusta | California, USA | 1993 | ARSEF 4004–4008 | |
| Group III | ? | Unidentified lepidopteran larva | Switzerland | 1979 | FPMI 770 |
| E. maimaiga | Lymantriidae | Lymantria dispar | Ishikawa, Japan | 1984 | ARSEF 1390 |
| Lymantria dispar | Connecticut, USA | 1989 | ARSEF 1775 |
E. maimaiga was initially separated from the E. aulicae species complex based on differences in isozymes, its ability to infect L. dispar, and its endemism in northeastern Asia (130). Of 11 enzyme systems that were evaluated, E. maimaiga differed from E. aulicae in 5: glucosephosphate isomerase (C), glucosephosphate isomerase (R-4), glyceraldehyde-3-phosphate dehydrogenase (R), peptidase with glycyl-leucine (R), and glutathione reductase-1 (R). Subsequent studies comparing restriction fragment length polymorphisms demonstrated that E. maimaiga was clearly differentiated from other E. aulicae isolates in the composition of rDNA at 4 of 10 restriction enzyme loci: DraI, EcoRI, EcoRV, and HindIII (144). Interestingly, E. maimaiga and E. aulicae occur sympatrically in Japan yet only E. maimaiga has been isolated from L. dispar (Table 1). In fact, none of the isolates of E. aulicae that have been tested to date are able to infect L. dispar (61, 71, 130).
E. maimaiga was originally described as a fungal strain from L. dispar japonensis, the Japanese gypsy moth, collected in central Honshu, Japan, in 1984 (130). L. dispar is native across temperate Asia and Europe, as well as northern Africa (40). There are reports of an otherwise unidentified entomophthoralean pathogen infecting L. dispar in South Korea (108, 109) and Jilin and Heilongjiang Provinces, northern China (121). Entomophthoralean resting spores have also been found in larvae of L. dispar from the Sakhalin Islands, north of Japan, and the Primorsky region of far-eastern Russia (44). Entomophthoralean epizootics also occur in populations of the closely related Lymantria obfuscata in Kashmir (115).
Curiously, there are very few records of entomophthoralean pathogens infecting L. dispar in Europe. An entomophthoralean epizootic has been recorded in an L. dispar population in a marsh in northeastern Poland (42, 126), and entomophthoralean spores have been isolated from L. dispar cadavers in Yugoslavia (78).
In one collection of L. dispar in Romania, entomophthoralean resting spores were found in one cadaver of L. dispar. rDNA isolated from these resting spores differed from that of both E. aulicae and E. maimaiga in restriction fragment length polymorphisms, restriction sites, and nucleotide sequences (143). However, the lack of morphological characteristics associated with most entomophthoralean resting spores makes these structures notoriously unreliable for identification purposes. Whether this Romanian fungus was even a member of the E. aulicae species complex is questionable because of its uncharacteristically high divergence from all other members of the E. aulicae species complex (143). For example, sequence analysis of two rDNA regions (the 3′ end of the 18S rRNA gene and a region of the 25S rRNA gene) showed that different species of Entomophaga contained 5 or 6 nucleotide differences in each of these regions whereas the fungus from Romania differed from Entomophaga species by 36 or 38 nucleotides.
Due to the lack of their availability in culture, we have not been able to compare the DNA from European or other Asian L. dispar-infecting entomophthoraleans with the isolates of E. maimaiga from central Japan. Fungal epizootics in larval L. dispar populations are more conspicuous when host populations are abundant. In areas where L. dispar is endemic, larval populations do not reach outbreak densities as frequently as in North America, where this pest has been introduced and many natural enemies are absent. Therefore, we do not know whether the entomophthoralean species infecting L. dispar in Asia (other than central Japan), rarely occurring in European L. dispar, or causing epizootics in L. obfuscata in Kashmir are the same as E. maimaiga present in Japan and North America.
History In North America
L. dispar is native to Europe and across temperate Asia and was accidentally introduced from France to Medford, Mass., a suburb of Boston, in 1868 or 1869 (90). By the early 1900s, North American scientists had learned that a fungal pathogen of L. dispar was present in Japan. During 1909, an American scientist travelled to Japan and returned with two L. dispar larvae infected by this entomophthoralean fungus. This fungus was released in the Boston area in 1910 to 1911 by exposing L. dispar larvae to germinating resting spores, placing bags containing infected larvae in trees hosting L. dispar populations, and opening the bags to allow infected larvae to disperse among the resident population and, hopefully, transmit the disease. By the end of 1911, no transmission had been documented and a viral epizootic had decimated the local L. dispar populations, and so this program was discontinued (131). In the summary report, it was stated that “Should it [E. maimaiga] obtain a foothold in the field … it might be expected to prove continuously effective from season to season, owing to its habit of forming resting spores in great abundance, which the experiments have shown are able to survive the New England winter, and a very slight increase in virulence, such as often appears in parasitic fungi in successive seasons, might bring about quite different results from those above reported.”
Throughout the monograph describing these early attempts to release this fungus, Speare and Colley (131) referred to the fungus only as the gypsy fungus. In the same monograph, these authors described releases of an entomophthoralean fungus that infected browntail moth, Euproctis chrysorrhoea, and referred to this second fungus as Entomophthora aulicae. The authors did not discuss why they used different names for these two fungi but we infer that they considered that the fungi were different. We know that the gypsy fungus infected L. dispar during these early studies. However, a list of 18 hosts for E. aulicae given within the monograph does not include L. dispar (131), although this species was abundant in the region at that time. We know of no historical specimens that could be used to confirm that the fungus released in 1910 to 1911 was E. maimaiga. However, because to date all strains of E. maimaiga can infect L. dispar while all strains of E. aulicae that have been tested cannot (61, 71, 130), it seems certain that the gypsy fungus of 1910 to 1911 was actually E. maimaiga.
After the 1910 to 1911 releases, as L. dispar populations spread, surveys for pathogens of L. dispar were conducted in northeastern North America. However, entomophthoralean spores were never detected within L. dispar larvae (25, 92, 111).
Following exceptionally damaging L. dispar outbreaks in the United States in 1981, another trip to Japan was made with the aim of isolating the entomophthoralean pathogen of L. dispar for potential introduction into North America. In 1984, this fungus was successfully isolated on egg yolk/Sabouraud maltose agar (EYSMA) (129), and it was from these isolates that the species E. maimaiga was described (130). In experimental-scale plots in Allegany State Park, N.Y., in 1985 and Shenandoah National Park, Va., in 1986, laboratory colony and field-collected L. dispar larvae were infected by microinjection with E. maimaiga protoplasts and released into the field. Transmission of E. maimaiga to the native L. dispar populations was nonexistent (1985) or extremely low (1986), and these releases were considered to have failed based on absence of E. maimaiga infections at release sites in 1987 and 1989 to 1991 (59).
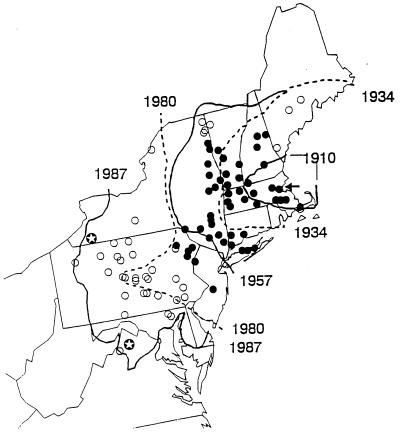
During early June 1989, E. maimaiga was first found causing high levels of infection in L. dispar populations in southwestern Connecticut (2, 60). Allozymes and restriction fragment length polymorphisms were used to confirm that three northeastern isolates were the same as the 1984 isolates of E. maimaiga from Japan and differed substantially from isolates of E. aulicae from North America (60). Further collections across the L. dispar distribution during 1989 demonstrated that E. maimaiga was present in seven northeastern states (2, 60) (Fig. 1). The large area where the fungus was found was isolated from the 1985 to 1986 release sites, suggesting that these deliberate introductions were unrelated to the 1989 epizootics. By 1990 E. maimaiga was found in 10 northeastern states (33) and Ontario (147), and by 1992 it had spread across most of the contiguous distribution of L. dispar in the northeastern United States (59) (Fig. 2). Beginning in 1990, small quantities of this fungus were introduced to localized areas where it was not already present (46). While some of the fungal spread from 1989 to 1992 was due to these human manipulations, the cause of much of this large-scale spread cannot be definitively determined (see below). The distribution of E. maimaiga was again evaluated in 1996, and it had continued to spread, although it was not found in those areas most recently colonized by the constantly spreading L. dispar populations (122).
FIG. 1.
Distribution of E. maimaiga in the United States in 1989. Open circles, locations where E. maimaiga was not found; solid circles, locations where E. maimaiga was found during June and July 1989; circles containing stars, locations where the 1984 isolate of E. maimaiga from Japan was released in 1985 and 1986. Lines indicate limits of defoliation caused by L. dispar as it spread from its original introduction site near Boston (arrow). Reprinted from reference 60 with permission of the publisher.
FIG. 2.
Spread of E. maimaiga in the United States from 1989 to 1992. Reprinted from reference 47 with permission of the publisher.
The source of this virulent and fast-spreading strain of E. maimaiga, first found in North America in 1989, remains a lingering and perplexing question. Unfortunately, no samples remain from the 1910 to 1911 introductions that could be used to help track the origin of the strains present today in North America. Whether the E. maimaiga strain first detected in North America in 1989 originated from the 1910 to 1911 introductions remains debatable because there was no evidence of this fungus between 1910 and 1989 (59). Some have hypothesized a slow spread by this fungus from the Boston area between 1910 and 1989. However, because the spread of this fungus was so rapid between 1989 and 1992, it seems unlikely that the E. maimaiga strain introduced in 1910 to 1911 would have spread so slowly between 1911 and 1989 (59). Alternatively, long-lived azygospores from the 1910 to 1911 introductions might have survived, possibly causing an occasional infection but undergoing a gradual or sudden change to a fast-spreading, virulent strain. The ability of the strain in North America to spread rapidly suggests that it is possible that E. maimaiga was successfully introduced only accidentally in the more recent past. The microscopic E. maimaiga azygospores occur in soil and tree bark and could have been transported to North America from northern Asia in many ways (soil on the soles of shoes, dunnage from shipping, etc.). Another hypothesis is that viable spores of E. maimaiga from the 1985 (New York) or 1986 (Virginia) release programs were either accidentally transported by humans or carried on the wind to an area(s) within the 1989 fungal distribution, with subsequent spread by E. maimaiga between 1985 or 1986 and 1989.
In the face of these hypotheses regarding the origin of E. maimaiga presently in North America, a model was used to investigate the times and places where we would assume that both E. maimaiga and L. dispar would have been very active and thus where and when E. maimaiga would have been detected if it was present (148). Aside from 1989, during 1971 the weather conditions and host populations in Connecticut would have allowed the detection of extensive fungal activity, yet the fungus was not detected then. Therefore, Weseloh (148) concluded that E. maimaiga must have become well established and started spreading some time after 1971. Unfortunately, at present this study constitutes the only objective evidence to help explain the origin(s) of the current strain(s) of E. maimaiga present in North America.
General Life Cycle
E. maimaiga has two spore forms: conidia, produced externally on cadavers (Fig. 3A), and azygospores (resting spores), produced within cadavers (Fig. 3B). The fungus, host-related factors, and environmental conditions all determine the type of spore formed after host death. Hosts are infected either by germ conidia produced from azygospores or by conidia discharged from cadavers. If hosts are infected by infective germ conidia produced from azygospores, the resulting cadaver will bear only conidia (48). For infections initiated by conidia discharged from cadavers, host instar, temperature, humidity, host molting status, fungal isolate and dose have been associated with spore form (68, 123, 125). The most important determinant of spore form was host age. Early-instar cadavers produce conidia almost exclusively, while cadavers of later instars usually bear azygospores but can also discharge conidia, especially at lower temperatures.
FIG. 3.
(A) Pear-shaped conidia actively discharged from the surface of cadavers (average conidial dimensions, 20.6 by 26.6 μm). (B) Double-walled azygospores containing lipid droplets, produced within later-instar cadavers (average azygospore diameter, 32.1 μm).
Conidia of E. maimaiga are hyaline, thin-walled and pear-shaped (averaging 20.6 by 26.6 μm), and each contains 21 to 34 nuclei (Fig. 3A). After being discharged, these spores are ready to germinate under favorable conditions but are generally short-lived (see below for more detail). The multinucleate azygospores, averaging 32.1 μm in diameter (Fig. 3B), are constitutively dormant when produced in late spring or early summer, and their 2-μm-thick double wall creates an environmentally resistant barrier. In early spring, azygospores germinate to produce germ conidia that are similar in size and appearance to conidia. Unfortunately, the germ conidia of the members of the Entomophthorales have been only poorly studied, although we now know that the germ conidia of E. maimaiga differ fundamentally in their biology from cadaver-produced conidia.
The general mode of infection by fungal entomopathogens is entry through the host cuticle. Infective fungal spores must first adhere to the cuticle and then differentiate and begin penetration by using a combination of mechanical pressure and enzymatic degradation (132). Adhesion by E. maimaiga conidia is most probably initiated by their external mucus coating. While appressoria are formed for penetration in some cases (Fig. 4), these specialized structures do not always seem to be necessary (44). The enzymes used for cuticular penetration by hyphomycete entomopathogens have been extensively studied (132), but the methods used by the Entomophthorales for cuticular penetration have not been elucidated. The median time from inoculation of conidia onto L. dispar larvae until successful infection is 8.7 h at 25°C (62).
FIG. 4.
Conidia of E. maimaiga that have germinated and produced germ tubes, at the end of which appressoria (arrows) have formed near the base of a secondary seta on the L. dispar cuticle. Bar, 10 μm. Reprinted from reference 47 with permission of the publisher.
After cuticular penetration, E. maimaiga grows in the host hemocoel as naturally occurring protoplasts, lacking cell walls (Fig. 5), until shortly before host death, when the cell walls begin to be regenerated, forming hyphal bodies. The closely related E. grylli has also been reported to grow as protoplasts throughout infections and to acquire cell walls only after host death (36). In contrast, immediately after cuticular penetration by E. aulicae, only protoplasts occur in the hemolymph, but hyphal bodies with cell walls are subsequently also present throughout most of an infection (105). Due to the close taxonomic relationship among these fungal pathogens and the reported disparity in development, the types of cells of E. aulicae throughout an infection should be investigated further.
FIG. 5.
Multinucleate protoplasts of E. maimaiga within infected L. dispar, late in an infection. Bar, 20 μm.
As infections progress after initial penetration, the density of E. maimaiga cells within the insect increases daily (53). During early infections of grasshoppers with E. grylli, fungal cells were found in the hemolymph and associated with the fat body, and a limited number of protoplasts occurred in the neural ganglia (36). Late in infection, E. grylli protoplasts were more abundant in both the fat body and nervous tissue, but the gastrointestinal tract and skeletal muscle were not invaded significantly until after host death. In seeming agreement with the very low concentration of fungal cells in vital organs until after host death, insects infected by entomophthoralean fungi frequently display few overt behavioral changes until they are moribund. While no obvious changes in L. dispar larval activity until shortly before death have been noted, detailed studies revealed that larvae eat less during the last 2 days of infection before they die (45).
At the time of death, fat body cells of grasshoppers infected with E. grylli contained no glycogen deposits, suggesting that one factor contributing to host death is nutrient depletion (36). In a study of two lepidopteran hosts infected with E. aulicae, a short-lived cell-lytic factor whose concentration peaked at or shortly after death was hypothesized to be the cause of death and subsequent host cell lysis (98); the onset of cell-lytic activity was detected as larvae appeared to become paralyzed ca. 1 h before death. Preliminary studies with E. maimaiga suggested the presence and activity of a similar cell-lytic factor (98).
The period of activity of E. maimaiga in the field is closely allied to the ca. 2-month period each spring when L. dispar larvae are present. E. maimaiga is known to infect only larvae, although if late-stage larvae are infected, they can die as pupae that contain relatively few azygospores or conidiophores can grow out through the pupal intersegmental membranes to discharge conidia (44). Bioassays with E. maimaiga have been conducted principally by showering conidia, discharged either from cadavers or from cultures grown on EYSMA, into a weak detergent solution and then briefly dipping larvae into conidial suspensions of known concentrations (62, 125). The bioassay results have shown that fourth-instar larvae are the most susceptible, although at optimal doses the mortality rate is high for all instars tested (62, 125). Studies have also shown 20°C to be an optimal temperature for E. maimaiga (62). For the one isolate tested across a range of dosages (ARSEF 1400), the 50% lethal concentration was 3.63 × 103 conidia/ml (confidence interval, 2.24 × 103 to 5.89 × 103) for fourth-instar larvae at 20°C (125).
The time to death for L. dispar larvae infected with E. maimaiga varies with the fungal dose (125), isolate (65), temperature, and instar at infection (58, 125), but this fungus generally kills its hosts within several days of exposure to conidia. For example, E. maimaiga ARSEF 3052 kills fourth-instar larvae eating Quercus rubra leaves at 20°C in 4.8 days (65). In this experiment larvae infected with a normally lethal dose but maintained at 5°C did not become infected by 25 days.
Constant exposure of fungal cells within infected L. dispar larvae to temperatures of ≥30°C caused fungal mortality, thus curing the insect of infection. The fungus can withstand shorter intervals of exposure to ≥30°C and still cause host death and subsequently reproduce; e.g., when infected larvae were held at 30°C for a 48-h period 3 days after E. maimaiga protoplasts had been injected, conidia were discharged from cadavers of 30% of injected larvae (54). While other hosts of the Entomophthorales are known to cure themselves of fungal infections by basking to elevate their temperatures, e.g., flies infected with Entomophthora muscae (145) and grasshoppers infected with Entomophaga grylli (27), L. dispar is a thermal conformer (28) and basking by infected larvae has never been reported. Therefore, we hypothesize that it is unlikely that L. dispar larvae would normally cure themselves of infections by basking. On the contrary, late-instar L. dispar larvae tend to avoid heat and sunlight by resting on the ground during the day and feeding in tree canopies at night (23).
At the time of host death, conidia are not discharged immediately from cadavers and azygospores are not mature. Conidiophores have emerged through the cuticle of the dead host (Fig. 6) and are ready to discharge conidia beginning at 14 h after host death. Formation of azygospores is a slower process, with only 60% of fungal cells within cadavers developing into mature azygospores by 5 days after host death (57).
FIG. 6.
Scanning electron micrograph of conidiophores of E. maimaiga bearing conidia. Reprinted from reference 47 with permission of the publisher. (Image by T. M. Butt.)
POPULATION GENETICS
Molecular Biology of E. aulicae
The genome of E. maimaiga has not yet been evaluated, but the genomes of two isolates from the same species complex have been studied extensively. These two isolates, both belonging to E. aulicae species group I (Table 1), have genomes 2 orders of magnitude larger than those usually expected of fungi (103, 104). The genome size of 4.3 pg yields an average DNA content for each of the 15 chromosomes of 0.3 pg, which is exceptionally high for fungal chromosomes (104). Whether this large genome is related to a high proportion of repetitive DNA is not yet known. Also unusual for many fungal genomes, an estimated 10% of the cytosines are 5-methylcytosine (Silver and Hattman, unpublished data cited in reference 143) and extensive heterochromatin is found in interphase nuclei and chromosomes undergoing condensation during mitosis (101, 102, 114).
Sexual Reproduction
Studies of E. maimaiga demonstrate that the vegetative growth of this fungus is haploid (60). E. maimaiga produces azygospores as multinucleate hyphal bodies that individually round up at one end (57). These spores are azygospores and not zygospores, because there is no conjugation of hyphae at the time of spore formation. Hyphal anastomosis within infected insects to allow the exchange of nuclei has also never been reported. For three other species of the Entomophthorales, as hyphal bodies develop into dormant azygospores, the number of nuclei is reduced from as many as 25 to 2, so that dormant azygospores contain only 2 nuclei (96). The most straightforward speculation would be that the reduction in nuclear number results from the destruction of the extraneous nuclei.
During the brief period when the spore has been activated and pregermination processes have begun, the cytoplasm becomes granular, large lipid droplets are broken down, and only one larger nucleus has been seen. This nucleus is presumably a fusion of the two nuclei present during dormancy. Subsequently, germinating azygospores contain multiple nuclei that are the same size as those of immature azygospores or binucleate dormant azygospores. Although synaptonemal complexes have not been observed, it is presumed that the change from the binucleate to the uninucleate state during activation is due to karyogamy and that the immediately subsequent divisions are meiotic (96). Therefore, although not formed by the conjugation of two independent hyphae, these azygospores could still be considered sexual spores due to conjugation of nuclei.
Genetic Variability of Host and Pathogen
Studies have not been conducted to evaluate the genetic variability within E. maimaiga populations. Genetic variability largely occurs due to mutation and/or recombination during sexual reproduction. However, the extent to which sexual reproduction in E. maimaiga might yield genomic changes is questionable. Although evidence suggests that meiosis occurs in entomophthoralean species having azygospores (see above) (96), meiosis most probably occurs between nuclei from within the same parent cell in the absence of anastomosis. We do not know whether nuclei within multinucleate E. maimaiga cells differ. If they do, variability in this fungus could be maintained principally through differential allocation of nuclei in the multinucleate fungal cells, recombination during meiosis, and mutation.
We assume that only a limited diversity of genotypes of E. maimaiga was introduced from Asia to North America, regardless of the timing of fungal introduction. As a consequence, the gene pools for this pathogen in North America would be limited. In contrast, we would assume that there would be much greater diversity in virulence among E. maimaiga strains in Japan. For E. aulicae native to North America, cDNA probes demonstrated that strains isolated from eastern spruce budworm from different geographic areas could be differentiated but were very similar (143). In contrast, strains of E. aulicae isolated from eastern hemlock looper from the same area showed only 58 to 61% similarity (143). This subspecific variability could be representative of clonal lineages of potentially selfing strains. One study of E. aulicae during a 1984 epizootic of eastern spruce budworm in Ontario identified the presence of 15 distinct phenotypes, with one predominant phenotype by the end of the epizootic (Tyrrell and Harvey, unpublished data cited in reference 143). The E. maimaiga-L. dispar system could provide an excellent comparison of variability in pathogenicity before and after a biological invasion as well as before and after an epizootic. The genotypic diversity in E. maimaiga in Japan should be compared with the diversity in North America, on the molecular level and by using comparisons of virulence, for an excellent contrast between coevolved and naive host-pathogen systems. Preliminary data suggests that genotypic diversity of E. maimaiga in North America is presently very limited (143). Walsh (143) hypothesized that this may result from the limited number of host species infected in North America or the short length of time that E. maimaiga has been present in North America.
Based on the history of repeated epizootics in North America since 1989, the strain of E. maimaiga now present in North America is considered highly virulent against North American L. dispar. Interestingly, L. dispar was initially introduced into North America in 1868 or 1869 as just a few insects (90) and was reintroduced from Europe in the 1910s (39). Although in the 1990s an Asian race of L. dispar was introduced into the Pacific Northwest (14) and a mixture of Asian and European races was introduced into North Carolina (112), control programs are thought to have eradicated both of these populations. Studies to date have shown that North American populations of L. dispar display little genetic variability (75) and are thought to have originated principally from the early European introductions. The strain of L. dispar initially introduced into North America originated from France, where entomophthoralean pathogens have never been reported to infect L. dispar. Therefore, the host race of E. maimaiga present in North America that is clearly highly susceptible evolved in the absence of this pathogen.
The greatest amount of diversity within L. dispar is found in Japan, where numerous subspecies have been named (79). There is the possibility that diversity in susceptibility to E. maimaiga occurs among different Japanese L. dispar genotypes, although this has not been tested. Little quantitative data on the frequency of epizootics caused by E. maimaiga in Japanese L. dispar populations is available. Whether the susceptibility of European L. dispar to E. maimaiga differs from that of Japanese L. dispar has also not been tested to ascertain whether susceptibility differs between L. dispar genotypes. E. maimaiga is biotrophic (does not grow saprophytically in the environment), suggesting that host resistance to this pathogen would constitute a strong force for selection of more virulent strains.
HOST SPECIFICITY
Range of Susceptible Hosts
A high level of host specificity is desirable for use of natural enemies in biological control, in order to limit adverse impacts on nontarget species. Because of the potential for expanding the application of E. maimaiga for pest control, extensive studies have been conducted on the host specificity of this fungus. Species of Coleoptera, Orthoptera, and Hymenoptera that were dipped into suspensions of 105 conidia/ml (an optimal dose for infecting L. dispar) did not become infected (130, 140), supporting the specificity of E. maimaiga for species of Lepidoptera.
The same infection protocol was used to bioassay lepidopteran larvae of 78 species in 10 superfamilies (52). The majority of these test insects were reared from insects collected in areas of West Virginia where E. maimaiga had not yet been found. Fungal reproduction after host death was accepted as proof of successful infection. Fungal spores were produced from cadavers of 35.6% of the species tested and in a total of 7 of 10 lepidopteran superfamilies. Although these results suggest broad host specificity, levels of infection at this optimal dose differed substantially among the superfamilies. Infection levels were <50% for all superfamilies except Bombycoidea, Sphingoidea, and Noctuoidea (52). In the Bombycoidea, only one species in the Lasiocampidae (Malacosoma disstria) was infected at >50%, and in the Sphingoidea, of the two species tested, only Manduca sexta from a laboratory colony was heavily infected, while the other sphingid species tested, reared from a wild female, was not infected. The only group that consistently demonstrated high levels of susceptibility was the tussock moth family (Lymantriidae; four species tested), to which L. dispar belongs. Similar results had previously been found during a much smaller study of 16 species of Lepidoptera within eight families by using a variety of infection techniques (130). Among larvae dipped into conidial suspensions, the Douglas fir tussock moth (Orgyia pseudotsugata) was the only species other than L. dispar consistently infected at high levels but was also the only nontarget lymantriid tested. Lower levels of infection were seen in two noctuids and one arctiid. Among species showered with conidia, only three species of lymantriids became infected (O. pseudotsugata, Dasychira dorsipennata, and an unidentified species) (130).
Our standard method used to test specificity involves briefly dipping lepidopteran larvae into a suspension of E. maimaiga conidia, allowing them to dry, and then maintaining them at high relative humidity for 2 days. This technique was used to standardize the exposure rate, which is difficult to do with the Entomophthorales because conidia are actively discharged and not long-lived. When the 78 lepidopteran species were tested by this technique, controls of five species did not survive immersion in the conidial suspension (52). Curiously, for 7 of the 78 species in six different families, treatment mortality was significantly greater than control mortality, yet no spores were produced in cadavers. The majority of these test insects died within a few days of exposure instead of the standard 4 to 7 days at 20°C (62), the fungus did not reproduce, and the cadavers were full of bacteria. We hypothesize that excessive cuticular penetration by conidia may have allowed such abundant bacterial invasion of the hemocoel that the immune system could not respond adequately. We do not know whether E. maimaiga would have been able to successfully reproduce within these host species if bacteria had not invaded. Larval mortality without subsequent fungal sporulation has occasionally been noted during immersion bioassays with L. dispar, but spores are usually produced from the majority of cadavers (44). Premature host death and lack of fungal reproduction have previously been attributed to attenuated fungal strains, bacterial contamination of conidial suspensions, or excessive dosage; therefore, these results for L. dispar have been considered artifacts of this type of bioassay. Whether this pattern of mortality without fungal reproduction in the seven nontarget species in this study is a laboratory artifact remains to be determined.
Interactions with Nonsusceptible Hosts
Nontarget studies also included challenges of 34 of the 78 test species by injection with E. maimaiga protoplasts (52). For eight of the species challenged, E. maimaiga did not develop successfully within injected larvae and conidial inoculation also had not produced infections. For six species that had not become infected after conidial inoculation, E. maimaiga developed successfully when injected. Therefore, we have identified two types of nonpermissive Lepidoptera: ones within which E. maimaiga cannot survive, and ones that E. maimaiga cannot infect but within which it can survive if injected.
Cuticular penetration.
Due to the intense setation of L. dispar, we investigated the association between setation and conidial infection. Interestingly, species not infected by conidial inoculation generally were those with sparse setation (only primary setae or short secondary setae) (52). E. maimaiga consistently caused the highest levels of infection in lymantriid larvae (the tussock moths), which are densely covered with setae. Of course, increased setation would also influence the surface area for conidial attachment, essentially increasing the conidial dose for hairy larvae. It is also possible that abundant setation increases the relative humidity at the level of the cuticle and thereby favors germination of conidia. Whether cuticular topography influences the ability of E. maimaiga conidia to recognize host cuticle is currently under investigation. Studies with Metarhizium anisopliae have shown that appressoria are formed at the bases of setal sockets or after extensive growth over the microfolds of the cuticle of <1-day-old fifth-instar Manduca sexta larvae (133). For E. aulicae, dense setation alone is not associated with successful infection, because a strain of E. aulicae isolated from an exceptionally setateous host, western tussock moth (Orgyia vetusta), could not successfully infect L. dispar (71).
For some entomophthoralean species, lipids associated with the insect cuticle can stimulate conidia. Germination of conidia of Erynia variabilis was associated with fatty acids from cuticles of the fly hosts (80). This response, however, was found to be nonspecific in a study exposing Conidiobolus obscurus and Nomuraea rileyi to cuticular extracts from an aphid and a larval lepidopteran (15). Whether E. maimaiga uses chemicals occurring on insect cuticle to recognize its correct host has not yet been determined.
Fungal survival postpenetration.
The insect immune response is composed of both humoral and cellular defenses. To understand factors determining E. maimaiga survival within potential hosts, studies have investigated both defense systems. When E. maimaiga protoplasts were injected into L. dispar larvae, only a few protoplasts were melanized and encapsulated by lysed coagulocytes and subsequently enveloped by several layers of flattened plasmatocytes (21). This encapsulation response was heightened in larvae injected with a less virulent E. maimaiga isolate (22). However, protoplasts must have survived, because all the inoculated larvae died and fungal spores were produced from the cadavers. In fact, a hemocytic response to the presence of entomophthoralean cells appears to be a poor indicator of the success of an infection; when the nonpermitted grasshopper pathogen E. grylli was injected into L. dispar larvae, the melanization and encapsulation responses were seen only occasionally. Neither E. aulicae nor E. grylli kills L. dispar, yet the fates of fungal cells injected into larvae usually could not be established (21). The authors (21) suggest that the majority of cells of these nonpermitted fungi were lysed in nonhosts.
At least initially after infection, E. maimaiga is found within the host hemocoel as protoplasts lacking cell walls. Studies with E. aulicae (9) and E. maimaiga (22) have shown that walled cells are detected by susceptible hosts while protoplasts frequently escape detection. It is generally accepted that these fungi are found as protoplasts in the hemocoel to avoid detection through absence of the sugar-rich cell walls containing chitin or β(1-3)-glucan (9, 22). However, among entomophthoralean fungi present as wall-less protoplasts within infected insects, there is still intense host specificity; e.g., E. aulicae cannot infect L. dispar, and E. maimaiga cannot infect the geometrid Lambdina fiscellaria (13).
In E. maimaiga and its sister species E. aulicae, glycoproteins have been detected in the plasma membranes of protoplasts (12). The pattern, type, and magnitude of mannose glycosylation differed between these species, with greater levels of mannose glycosylation in E. maimaiga plasma membranes. However, both fungi showed a single protein that was glycosylated with both sialic acid and galactose β(1-4) linked to N-acetyl-d-glucosamine. Beauvais et al. (9), working with E. aulicae, speculated that protoplasts were destroyed in nonhosts by nonspecific adhesion or by an unspecified enzyme inducing a nonspecific defense reaction. As an alternative, we hypothesize that sugars on protoplast surfaces could be used for recognition of nonself in nonpermissive hosts, based on both the differential glycosylation in protoplast plasma membranes and increasing evidence of use of sugars for nonhost recognition in insects, crustacea, plant pathogens, and mycoparasites (12).
Other factors that could influence interactions between fungal cells and potential hosts have also been investigated. No evidence of production of fungal toxins specific to host insects or fungus-specific induction of plasma proteins by nonhosts was found (13). Fungal growth was not restricted in cell-free hemolymph from nonhosts, suggesting that the serum is not inhibitory to fungal cells. The activity of both phenoloxidase-activating trypsin and phenoloxidase increased for up to 96 h after fungal challenge in L. dispar larvae challenged with E. aulicae (nonpermissive) but not E. maimaiga (13). Moreover, three isoforms of phenoloxidase (pI 5.0 to 5.5) and at least six isoforms of trypsin (four basic trypsins [pI 8 to 10] and two acidic trypsins [pI 4 to 6]), with preferences for small amino acid residues, were activated in L. dispar after challenge with E. aulicae. We suggest that differences in the sugar moieties of glycoproteins on protoplast plasma membranes potentially activate zymogenic trypsins that, in turn, activate the prophenoloxidase cascade to create a nonpermissive humoral response (13). While we hypothesize that the glycosylation of plasma membranes of E. maimaiga protoplasts conceals these fungal cells within permissive hosts, e.g., L. dispar, the same glycoproteins potentially lead to fungal recognition in nonpermissive hosts. We assume that as with E. aulicae in nonpermissive L. dispar, humoral responses are also activated against E. maimaiga when it is injected into nonpermissive hosts, e.g., hemlock looper (L. fiscellaria).
Ecological Host Specificity
During epizootics caused by E. maimaiga in L. dispar populations, cadavers of late-instar larvae cover lower tree trunks (135) but cadavers of other lepidopteran species are seen only very rarely (44). In fact, in its area of endemism, E. maimaiga is found only from L. dispar. Based on the results of laboratory bioassays (presented above), we assumed that E. maimaiga would be found infecting other lepidopteran species to some extent during epizootics. During epizootics caused by E. maimaiga in L. dispar populations in seven plots in Virginia, 1,511 larvae from 52 species in seven lepidopteran families were collected from the foliage and reared (51). Only 2 individuals (1 of 96 Catocala ilia [Noctuidae] and 1 of 318 M. disstria [Lasiocampidae]) died from infection by E. maimaiga. Of the 20 species collected in the field and also assayed in the laboratory, only 6 had been susceptible in the laboratory but only 1 of these (M. disstria) was found infected in the field (51). Especially based on these field results, we concluded that E. maimaiga is highly host specific and therefore has good potential for development as a biological control agent.
Although thousands of cadavers of L. dispar from across the area of distribution of this insect were diagnosed for cause of death from 1989 to 1995, non-L. dispar cadavers were noted and collected only very rarely during epizootics. Cadavers of nontarget larvae were evaluated by using DNA probes to confirm whether death had been caused by E. aulicae or E. maimaiga (51). The only cadavers other than L. dispar that had been killed by E. maimaiga were four individuals of three different species of lymantriids (51). These data do not provide estimates of the percentage of infection of these species in the field; however, the small number of cadavers found in the field during a time when abundant E. maimaiga-killed L. dispar were collected gives some indication that while E. maimaiga can infect other lymantriids in the field, this is probably uncommon.
Field evaluations were continued both to help explain the discrepancy between laboratory bioassay data and infection of nontarget species in the field and to help predict the susceptibility of species not included in laboratory and field studies. We hypothesized that the spatial and temporal activity of larval lepidopterans would result in differential exposure to fungal inoculum. To what extent L. dispar larvae are infected by airborne conidia when feeding on the foliage is not known, yet other foliage-feeding larvae collected in the forest were not infected (51), including those known to be susceptible in the laboratory (52). Indeed, studies of the behavior of L. dispar document their wandering on the soil briefly as early instars and wandering and resting on the soil during daylight hours for later instars, as well as larval aggregation (23, 24). Presumably, these larval behaviors promote exposure to E. maimaiga due to the occurrence of spores in the soil and the potential discharge of conidia from sporulating cadavers within larval aggregations. While it is difficult to generalize about the behavior of other sympatric species of forest Lepidoptera, the use of dark hiding places (such as leaf litter or bark flaps) during daylight hours, as practiced by L. dispar, is a relatively uncommon behavior (20). Although definitive studies have not been conducted, evidence to date suggests that the field specificity of E. maimaiga for L. dispar may result, in part, from the behavior of the larvae of this species increasing their exposure to fungal inoculum in the soil.
EPIZOOTIOLOGY
Spatial Distribution
For numerous entomophthoralean fungi, before death the infected hosts climb to elevated locations, where they often produce rhizoids to hold the resulting cadaver in place (118). It is thought that this behavior is caused by the infecting fungus and allows the pathogen to distribute actively ejected conidia over a larger area. Neither a pronounced climbing behavior nor rhizoid formation, however, has been noted in E. maimaiga-infected hosts; in fact, if any taxis occurs, infected L. dispar larvae seem to be found in lower tree canopies. Third- through fifth- to sixth-instar L. dispar larvae infected with E. maimaiga were more abundant in understory vegetation and on lower tree branches than high in the tree canopy (73).
In the understory foliage, cadavers of early-instar L. dispar larvae bearing conidia are predominantly found attached to undersides of branches, twigs, and petioles (72, 149) (Fig. 7). Prolegs are attached to the substrate, and, frequently, the anterior portion of the body is flexed at 90° to the surface of attachment. While these locations are elevated, this is also the location where early-instar larvae are normally found (24). Perhaps because early-instar larvae of these forest lepidopterans already are normally found in somewhat elevated locations when conidia are produced, climbing is unnecessary. Cadavers of later-instar larvae principally containing azygospores are usually found attached to tree bark by larval prolegs with their heads oriented downward on lower tree trunks (72, 135) (Fig. 8). Healthy later-instar larvae frequently spend daylight hours in dark locations on tree trunks with their heads directed upward (23). Therefore, the general locations of both early- and late-instar larval cadavers are not atypical of healthy insects (69), suggesting that no strong predeath taxis occurs, although the specific orientation of larval bodies at death clearly is not typical of healthy larvae.
FIG. 7.
Early-instar L. dispar larva killed by E. maimaiga, with conidia being discharged. The cadaver is attached to a twig in the understory vegetation. Photograph by D. Specker. Reprinted from reference 72 with permission of the publisher.
FIG. 8.
Later-instar L. dispar larva killed by E. maimaiga and containing azygospores. There is no external indication of fungal mortality, and the cadaver is positioned on tree bark with its head facing downward. Photograph by D. Specker.
Once azygospore-filled cadavers desiccate, they fall from trees. Azygospores are leached from ruptured cadavers and can be found in high titers at the bases of trees (74). Density gradient centrifugation has been used to evaluate the distribution of azygospores at increasing distances from tree trunks and increasing depths in soil. The highest titers of azygospores (average = 4,751 resting spores/g of dry soil) are found in the organic soil layer within 10 cm of tree bases (Fig. 9) (50). Lower densities of azygospores also occur on tree bark after an epizootic (50, 66).
FIG. 9.
Densities of E. maimaiga azygospores at different soil depths and distances from tree trunks. Data from reference 50.
Azygospore Survival and Germination
Azygospores mature asynchronously within their hosts over several days after host death, changing from readily permeable multinucleate cells to thick-walled cells impermeable to stains and containing only two nuclei, large lipid droplets, and condensed cytoplasm (57). These azygospores are dormant immediately after their production during June or early July. The requirements for cessation of dormancy of eight other entomophthoralean fungi have been identified (47). In most instances, several months at 4°C is required, although a lengthened photophase is necessary to break the dormancy of an aphid pathogen from northern Canada (142). For E. maimaiga azygospores retained in the field through one winter and brought to the laboratory, germination was possible only on 12 March (149) of the year after spore formation. During a second study, cadavers containing E. maimaiga azygospores were collected in the field during mid-July, placed in small bags for easy retrieval, and replaced in the field on the soil surface. Bags were brought to the laboratory, and resting spores were placed at 20°C monthly beginning in November. Germination by these spores was first seen only during tests begun after 30 March (57). After that time, germination rates were higher under a 14-h light/10-h dark photoperiod than at 13 h/11 h or 12 h/12 h. The exact requirements for cessation of dormancy and activation of E. maimaiga azygospore germination have not been identified, but under field conditions, these spores are able to germinate ca. 9 months after production and ca. 1 to 2 weeks before L. dispar eggs begin hatching.
What makes azygospores environmentally resistant? The 30-μm-diameter azygospores have one to several prominent central lipid droplets at maturation. It has been suggested that lipids protect against damage from freezing (134). Preliminary studies have shown that azygospores are also rich in sugars, with the most abundant sugar being trehalose at 203.4 μg/g (wet weight) (86). Trehalose is presumed to be important in desiccation and freeze tolerance (87), not only by appending itself to membranes but also by being able to promote the freezing of intracellular water in an amorphous (vitrified) rather than crystalline (icy) form, which is thought to protect the cells from freezing damage. Thermally stimulated depolarization currents were used to detect the presence of a vitrified state (glassy state) within E. maimaiga azygospores that had been stored at 4°C (86).
The 2-μm-thick wall of E. maimaiga azygospores that aids in spore protection has not been chemically characterized. This wall does not dissolve after treatment with proteinase K (11), and Conidiobolus obscurus azygospore walls do not dissolve in either acid or alkali (85). E. maimaiga azygospores are not recognized by antibodies raised against the plasma membranes of protoplasts (53). In agreement, the only entomophthoralean azygospore walls that have been characterized, those of the aphid pathogen C. obscurus, were found to contain four layers including a surface layer of β-glucan (85). The surface layer was underlaid by a layer of sclerotized protein and chitin, with additional chitin and protein layers to the interior. The chitin-protein complex was associated with a very small amount of hexoses. Upon initial stages of germination, the thick walls appear to be thinned or digested from within the cell, presumably by endogenously produced enzymes.
E. maimaiga passes most of the year as azygospores in the soil. At present, we know little about survival of these resistant spores throughout this period, in part because of difficulties in determining the viability of these dormant spores. Unfortunately, the vital stains tested to date do not penetrate the thick azygospore wall, and assays to detect metabolic activity have been unsuccessful due to the negligible metabolic activity in the spores while dormant. Germination studies designed to evaluate azygospore survival are compromised by a lack of understanding of the cues for breaking dormancy; i.e., if azygospores do not germinate, is it because they have not received proper cues or because they are dead? E. maimaiga azygospores clearly survive some degree of drying; azygospores on tree trunks are known to survive over the winter (66, 124), although the possibility of a reduction in survival of these environmentally exposed spores has not been evaluated.
Many azygospores do not germinate during the first year after production; in fact, infections initiated by E. maimaiga azygospores has been documented for up to 6 years after their production (151). While tests of the persistence of E. maimaiga azygospores in the soil for more than 6 years have yet to be made, evidence from other entomophthoralean fungi suggests the potential for such extended viability. For example, azygospores of the entomophthoralean infecting 17-year cicadas, Massospora cicadina, are thought to survive in the soil for at least 17 years between cicada broods (91). No studies, however, have yet shown what percentage of azygospores in the field remain ungerminated yet viable from year to year or even if biotic or abiotic factors are associated with decreasing survival. For germination studies conducted in the laboratory, maximal germination of E. maimaiga azygospores under optimal conditions was only ca. 72% (57).
Field investigations of E. maimaiga reveal the first infections initiated by azygospores ca. 1 to 2 weeks before L. dispar eggs begin to hatch (52, 57). Also in conjunction with the L. dispar life cycle, E. maimaiga azygospores cease germinating in mid to late June, when L. dispar is present as late instars (57). Although azygospore germination occurs over ca. 1.5 months in the field, germination in the laboratory occurs from 2 to 16 days after azygospores are brought to 15 or 20°C. While azygospore germination is slow, this fact alone does not account for the long period of azygospore germination in the field. The asynchrony in azygospore germination in the field might be due to both microenvironment and microclimate. Throughout the period that E. maimaiga azygospores are active, infection of larvae caged on soil containing azygospores varies positively with soil moisture (57).
Germinating azygospores each produce one infective germ conidium that is actively ejected from the end of a germ tube. Whether germ conidia become airborne is unresolved. Germ conidia are morphologically indistinguishable from conidia discharged from cadavers, and so testing whether they are airborne must be conducted before any conidia are released from larval cadavers. The majority of azygospores are soil borne, and germ conidia would therefore be discharged only slightly above the soil. Without knowing the weather conditions (especially the moisture levels and wind speeds) under which germ conidia are discharged, it is difficult to estimate the extent to which germ conidia from soil-borne azygospores become airborne. However, many actively discharged fungal spores are released under high-moisture conditions at night when wind speeds are low and the chances of becoming airborne are decreased (6). Of course, germ conidia produced by azygospores trapped on tree bark could become wind borne after discharge more readily than those from soil-borne azygospores.
Azygospores have been found in L. dispar egg masses in Japan (4) and North America (44). We assume that these azygospores washed into egg masses when a cadaver bearing azygospores was positioned above an egg mass, broke open, and released its contents. Whether these spores lead to infection of neonates after egg hatch has not been adequately investigated (49), although trials attempting to study this have been conducted (155). L. dispar egg masses are covered with hairs from the mother and are very hydrophobic. Azygospores of E. maimaiga have been germinated only in free water (57), and studies have shown that azygospores of the entomophthoralean Conidiobolus obscurus require free water for germination (110). It is therefore questionable whether azygospores within L. dispar egg masses would receive adequate moisture for germination.
Airborne Conidia
Assays demonstrated that oak-fed fourth-instar cadavers discharge an average of 2.1 × 105 conidia at 20°C (62). After the infected insects die, E. maimaiga matures within the cadavers, and at 20°C and 100% relative humidity, half of the cadavers are ready to discharge conidia 17 h after death (54). Moisture is critical for conidial discharge, and as the humidity is lowered from 100 to 70%, progressively fewer cadavers form and discharge conidia. Conidial discharge was correlated with relative humidity for the previous 2 h or leaf wetness for the previous 3 h (70). If ambient moisture declines after conidial discharge has begun, conidial discharge can stop but can then resume when the relative humidity increases again (70). This may explain, in part, why the majority of conidia are discharged between 8 p.m. and 8 a.m. under field conditions.
Conidia are actively discharged when the osmotic pressure within both the conidium and conidiophore increases, causing rupture of the wall enclosing the conidium around the conidial base. As is typical of members of the Entomophthorales, discharged conidia can be numerous enough to form a visible aureole around an undisturbed cadaver. For E. maimaiga, cadavers producing conidia usually occur in the foliage (see above), where conidia would presumably readily become airborne once discharged. Aerial sampling was conducted over 28 days beginning at ca. mid-third-instar during the 1992 field season. Collections from aerial spore samplers demonstrated two major periods of conidial abundance in the air, each consisting of higher densities of airborne conidia for >12 h/day (64). Conidial flux was autocorrelated, positively associated with leaf wetness and wind and negatively associated with temperature. Conidial flux was also associated with leaf wetness at lags of 5 to 14 and 16 h. After each of the intervals of higher densities of airborne conidia, E. maimaiga infection levels increased in the resident L. dispar population. Bioassays with L. dispar larvae caged at 0.5 m demonstrated a higher association of infection with rainfall and temperature than with density of airborne conidia, suggesting that microclimatic conditions are critical for successful infection of larva in the understory vegetation (64). Interestingly, a separate study sampling airborne conidia after 10 June 1997 and 1998, when second- and third-instar larvae (1997) or third- and fourth-instar larvae (1998) were present, also demonstrated two periods of abundant airborne conidia (99). However, the number of days included in each period of conidial abundance varied, with more conidia collected over more days during the wetter year (1997). Whether peaks in the abundance of airborne conidia also occur earlier in the season has not yet been investigated.
After conidia are discharged from a cadaver, they generally fall slowly within the small volume of air they occupy, although this unit of air may be moving in any direction (119). Whether conidial dispersal contributes to initiation of disease at another location depends on numerous processes including transport, dilution, survival, and deposition (5). These processes are poorly understood for spores of entomopathogenic fungi as a whole. The pyriform shape of E. maimaiga conidia is thought to have only a minor influence on transport of these small structures, even though shape is critical to processes influencing deposition (119).
The survival of airborne conidia is critical for successful disease transmission and therefore for the development of epizootics, as suggested from results of 1992 bioassays. Studies with other members of the Entomophthorales suggest that low moisture and high temperature limit conidial survival (117). From preliminary studies, we know that E. maimaiga conidia are sensitive to low moisture levels, yet the conidia of some species of entomophthoralean fungi can survive for several weeks at 40 to 50% relative humidity (16, 138). Studies have demonstrated that some E. maimaiga conidia can survive for at least 2 weeks at 15°C on moist unsterilized soil (44). How long conidia remain viable on other substrates and under warmer, drier conditions is not known. Based on laboratory and field studies, sunlight has been suggested as being the dominant factor affecting the survival of E. grylli conidia (26). While it seems likely that the hyaline, thin-walled conidia of E. maimaiga might be sensitive to sunlight, it is questionable whether sunlight plays a major role in conidial degradation, because E. maimaiga infects insects living within forests, in contrast to E. grylli, which infects rangeland grasshoppers. Under field conditions, studies were conducted to estimate how many conidia actually survived and infected hosts. By using a strain of E. maimaiga isolated in Japan in 1984, different ratios of healthy and infected L. dispar larvae were released on caged oak saplings in a central New York woodlot. Results from 1987 and 1988 yielded the estimate that one in 4 million potential penetration events (based only on production of primary conidia by cadavers) had caused each successful infection (62).
As is typical of the Entomophthorales, E. maimaiga conidia are ready to germinate soon after production, although adequate moisture is essential for germination. Under 100% relative humidity at 20°C, the median time to germination in the dark was 3.4 h whereas the median time to germination in the light was 9.0 h (54). It is not surprising that germination is faster in the dark, since conidia are discharged mainly during scotophase (70).
Upon deposition, if primary conidia do not receive the as yet unidentified cues necessary to initiate preinfection processes, primary conidia can produce secondary conidia. Secondary conidia are similar in shape to, although slightly smaller than, primary conidia and are actively ejected from primary conidia. If secondary conidia do not contact a host, they can, in turn, eject tertiary conidia, which are smaller yet. Although quaternary conidia may be produced, it is not certain whether these are infective. Therefore, for each cadaver bearing conidia, the chances of successfully locating a host could be trebled by the repetitive (successive) production of further infective conidia.
Spread of E. maimaiga.
The rate of E. maimaiga spread was quantified over small-scale distances during introduction and establishment of this fungus for biological control of L. dispar in Virginia, West Virginia, Pennsylvania, and Maryland (56). Transects from release sites during the dry spring of 1991 demonstrated that E. maimaiga spread up to 350 m, whereas in 1992, E. maimaiga spread >1,000 m from both the 1991 and 1992 release plots. A study conducted in Michigan in 1993 also documented spread from release site epicenters, with infections found at least 425 m from release sites 1 year after release and at decreasing rates with increased distance from the epicenter (127). It has been hypothesized that less fungal spread was documented in Michigan than in the mid-Atlantic states due to below-average June precipitation (8).
Aerial sampling demonstrates that E. maimaiga conidia are wind borne within the forest (64), but other mechanisms besides wind-borne dispersal of conidia can also possibly account for short-range spread, including dispersal of infected insects, such as ballooning by neonates or wandering by late-instar larvae. Vertebrates are known to consume and subsequently to defecate resting spores of other species of Entomophthorales (43), but whether this facilitates the dispersal of E. maimaiga has not been determined. Humans may also be unwitting vectors of E. maimaiga; after sampling in areas where epizootics had occurred the previous season, E. maimaiga azygospores were abundant in the meager amount of mud on soles of shoes (59).
How far can E. maimaiga travel in one season? Extrapolating from data collected between 1989 and 1990 at scattered sampling locations, we estimate that E. maimaiga spread >100 km in one season (33). This is in contrast to the 1991 spread of only 350 m from release points (56). Moreover, a distributional survey in 1992 demonstrated apparent simultaneous appearance of E. maimaiga across areas far from locales where establishment of E. maimaiga had been documented. Due to the simultaneous appearance of disease for the first time in 1992 throughout most of Virginia and surrounding areas, we hypothesized that airborne conidia might have spread long distances. Potential long-range dispersal of conidia is infinitely more difficult to investigate and to prove than short-range dispersal. Much remains to be studied to prove whether viable E. maimaiga conidia can survive wind dispersal over the long distances of documented spread by this pathogen between 1989 and 1992. This leads to another question: could E. maimaiga conidia have travelled by wind from Japan to North America? Laboratory experiments have revealed that E. maimaiga conidia could not have survived the several days of freezing temperatures that they would have experienced during intercontinental movement on the wind (59).
A mathematical model was developed to investigate the discrepancy between the rate of spread by E. maimaiga between 1989 and 1990 and the short distance spread during 1991. The results of experiments with this model led to the opinion that the discrepancy in spread rate at different scales is very possibly due to missing information about differential mechanisms for long- versus short-distance conidial dispersal (32). The large differences in wind speed within and above the forest canopy may be one factor helping to explain the faster long-range than short-range spread by E. maimaiga. Long-distance dispersal of fungal spores has not been categorically proven, even for fungal plant pathogens that have been the subjects of extensive research efforts over many years. At this time, we can only conclude that long-distance spread of E. maimaiga conidia on the wind appears very likely but has not been proven.
Occurrence of Epizootics
An epizootic is defined as “an unusually large number of cases of disease” (37). We are only now beginning to understand the level of E. maimaiga infection in a North American L. dispar population that could be considered “usual.” Operationally, ca. 30 to 100% infection across the season is often considered an epizootic. However, this range of disease prevalence can have very different impacts on the host population. Since 1989, E. maimaiga infections have been found each year throughout the distribution of this fungus but in different abundances and always accompanied by variable levels of mortality due to other natural enemies. Therefore, except at very high levels of infection, quantification of the impact of E. maimaiga cannot always help predict the densities of L. dispar at the end of the season.
For infection to occur, pathogen, host, and environmental conditions must act in concert. Therefore, to understand the development of an epizootic, all three must be included in analyses. Of this triad, studying L. dispar in mature forests where larvae feed on foliage high above the ground presents challenges for quantifying population densities and infection prevalence. Ecological studies of natural enemies that are insects have frequently demonstrated that natural enemy-caused mortality is dependent on host density (113). Therefore, the remarkably similar high levels of E. maimaiga infection across a range of host densities (from >15,000 egg masses/ha [49] to <50 egg masses/ha [56]) is initially puzzling. Work with other insect pathogens, including other entomophthoralean species, has suggested that density independence can result if the pathogen is ubiquitous and environmental conditions are favorable (38, 137). The activity of the same pathogen can be dependent on host density at lower pathogen densities, under aggregated distributions of the pathogen, or under harsher environmental conditions (137). Numerous studies have found no association between E. maimaiga infection prevalence and L. dispar density (10, 19, 56, 146, 150) or only a weak association (149). Many of these studies were conducted directly after the occurrence of fungal epizootics, and it can be hypothesized that infection was abundant because E. maimaiga azygospores were already broadly distributed in the environment at high titers. In other instances, lack of an association between disease and host density during epizootics was correlated with favorable weather conditions, e.g., frequent and abundant leaf wetness, that would have allowed unchecked amplification of E. maimaiga conidia in the environment.
Dwyer and Buonaccorsi (31) have shown that variability in the susceptibility of L. dispar to L. dispar nuclear polyhedrosis virus (LdMNPV) greatly reduces the density dependence of the mortality caused by this viral pathogen in its host. To determine whether such variation in susceptibility (or virulence) accounts for the observed lack of density dependence of E. maimaiga in L. dispar, Malakar (93) measured this variation in laboratory and field bioassays. She found that the heterogeneity in the susceptibility of L. dispar to E. maimaiga was considerably lower than the heterogeneity in susceptibility to LdMNPV. Consequently, such variation cannot account for the evidently weaker density dependence of E. maimaiga in L. dispar than that of LdMNPV. The authors suggest that other factors such as airborne dispersal of conidia, larval behavior that differs by host density (with less larval movement off trees in lower-density populations [83, 88]), or the short period during which E. maimaiga and L. dispar have coexisted in North America, might help explain the weak dependence of E. maimaiga on L. dispar density (93). As can be seen, the density of the pathogen must also be considered in understanding the dynamics of epizootics.
The threshold densities of azygospores in the environment that result in development of epizootics are unknown but most certainly would vary depending on the environmental conditions. Although bioassays can be conducted by exposing larvae to soil, with our present knowledge of azygospore dormancy, this can be done only during, or just before, the field season, when azygospores are germinating. As an alternative to bioassays for monitoring E. maimaiga azygospores in the environment, quantification in soil by wet sieving and subsequent density gradient centrifugation has been used. These procedures are very labor-intensive and time-consuming (74), making quantification of large numbers of soil samples a major undertaking. Density gradient centrifugation also cannot differentiate E. maimaiga resting spores from resting spores of other members of the Entomophthorales that are similar in appearance. To use this method, we assume that all azygospores found are E. maimaiga, usually based on knowledge that areas from which soil samples were taken had hosted E. maimaiga epizootics.
To evaluate the abundance of E. maimaiga in the environment, quantifying the density of E. maimaiga conidia by sampling the airborne microflora would also be necessary. As a caveat, quantifying airborne conidia might not take into account microrange movement of the fungus, e.g., healthy larvae could become infected when resting next to a sporulating cadaver. As with sampling azygospores, techniques for sampling airborne conidia assume that all pear-shaped conidia of the appropriate size are E. maimaiga, although other entomophthoralean species have similarly shaped conidia. At present, the principal method used to quantify the prevalence of E. maimaiga infections in a host population is to collect and rear L. dispar larvae to determine the percentage of larvae infected. This can also provide information on whether the insect was infected by germ conidia or conidia, depending on the types of spores produced from cadavers (48).
Each year since 1989, when E. maimaiga was first found in North America, epizootics have occurred somewhere in North American L. dispar populations (44), providing researchers with repeated opportunities to affirm that the development of epizootics is often correlated with environmental conditions. Numerous studies have demonstrated correlations between environmental moisture and E. maimaiga infection (Table 2). During 1989, record levels of rain fell during spring in much of the area where epizootics also occurred. Observers were quick to assume that abundant rainfall is necessary for development of epizootics. However, during subsequent years with closer to average rainfall, epizootics also occurred, and in 1991, when rainfall was low, abundant infection was still obvious in several localized areas.
TABLE 2.
Relationships between activity of E. maimaiga and moisture
| Property | Association with moisture | Reference(s) |
|---|---|---|
| Individual processes | ||
| Type of spore formed | More azygospores with higher humidity | 123 |
| Conidial production and discharge | Increased with higher relative humidity or leaf wetness, also with lagged effects | 54, 70 |
| Density of airborne conidia | Greater with higher humidity or following rainfall, also with lagged effects | 64, 99 |
| Conidial germination | Requires free water | 54 |
| Infection by azygospores | Increased with greater soil moisture | 57 |
| Levels of infection in manipulated experiments | Greater with artificial rainfall for both field plots and larvae caged in mesh bags | 93 |
| Slightly higher with artificial rainfall, and significantly higher in irrigated areas | 149 | |
| Greater with soil exposure 1–2 days after rainfall but increased the same days as rain for larvae caged in the foliage | 150 | |
| Increased with application of water to azygospores in soil at tree bases | 44, 56, 66 | |
| Levels of infection associated with field conditions | Positively associated with May but not June rainfall for 2 years | 56 |
| Positively associated with June but not May rainfall (although May rain was constantly abundant) | 149 | |
| Positively associated with rainfall and relative humidity of ≥90% 2 weeks before samples were taken | 127 |
During 1991, the percentage of E. maimaiga infection was positively associated with rainfall during May and negatively associated with temperature during both May and June across plots in four states (56). In contrast, the percentage of infection was positively associated with rainfall in June but not May in 1990 in Connecticut (149); however, rainfall was abundant throughout May, so that this data might not have been able to demonstrate an effect for May. Clearly, calendar months do not adequately describe the period when L. dispar larvae are present in the field, and the instars that are present during each spring month vary from year to year (49). By using more appropriate analyses than infection by month, infection in Michigan was positively correlated with precipitation and relative humidity 2 weeks before sampling (127).
During an experimental study in Connecticut, infection of larvae caged in the foliage (assumed to be infected by airborne conidia) occurred on the same day as rainfall while infection of larvae caged on the soil occurred 1 to 2 days after rainfall (the latter larvae were assumed to be infected predominantly by soil-borne azygospores) (150). A model of this system has demonstrated that throughout the field season, the pattern as well as the amount of rainfall is critical to the development of epizootics (152). There is tremendous interest in developing the ability to predict the activity of E. maimaiga and thereby minimize the use of insecticides for control of L. dispar. While one cannot predict what weather will occur during an upcoming season, we hope that in the future, levels of infection can be associated with weather data to develop a generalized picture of ambient conditions amenable to E. maimaiga activity. However, to complete this picture, reliable estimates of host and pathogen densities are also required.
As an epizootic develops, little infection is generally observed among early-instar larvae, although infections become increasingly abundant during later-instar larvae (49, 66, 146, 149, 150). Azygospores germinate throughout most of this time, but germination ceases approximately halfway through the last larval instar (57). We can only estimate when and where most L. dispar larvae become infected in the field. Spore production from cadavers collected in the field can assist in answering this question, because any cadavers containing azygospores resulted from larvae infected by conidia discharged from cadavers. In general, cadavers of earlier-instar larvae collected from the field do not contain azygospores while cadavers of later-instar larvae primarily contain azygospores (146, 149). These data suggest that the exponential increase in infection among later-instar larvae is driven mainly by infections from conidia discharged from cadavers. A simulation model of the E. maimaiga-L. dispar system demonstrated that although azygospores are important for initiating infections, cycles of secondary infection by conidia lead to the exponential increase in infection characteristic of epizootics (62). Between 14 and 26°C, this model demonstrated four to nine cycles of infection (from infection to death) within one field season (84). Experiments with this simulation model yielded results consistent with the existence of a host density threshold below which the rapid increase in secondary infection, characteristic of epizootics in dense host populations, does not occur.
Both this simulation model (62) and a second model investigating the E. maimaiga-L. dispar system (152) more closely mimicked the development of epizootics in the field when instar-specific behavior was added. Neonates are exposed to germinating azygospores in the forest soil during early-spring dispersal. Infection initiated by azygospores does not occur again until fourth- and higher-instar larvae, which begin to wander on the forest floor and hide in the litter during the day (23). Neither model incorporated the information that infections initiated by germ conidia from azygospores yielded only conidia after host death, because this fact was not known when these models were developed. However, addition of this information to these models would clearly increase the number of conidia available to cause cycles of infection during the season.
To what extent do L. dispar populations decrease after E. maimaiga epizootics? Since numerous natural enemies as well as abiotic conditions affect L. dispar populations, it is difficult to determine the impact of E. maimaiga alone. In addition, studies of releases of E. maimaiga have been plagued by infection in control plots (10, 56, 127), so that comparisons between plots with and without fungus are difficult. In one instance, L. dispar egg masses could not be found after epizootics (56), although it is more common that resulting L. dispar densities are substantially decreased from pre-epizootic densities and some egg masses can still be found (10, 49, 56, 127, 146, 149, 150). In one well-cited example, L. dispar populations increased after an epizootic, but the population densities still remained below 50 egg masses/ha (60), a very low population density for this insect. In two examples, although sparse, L. dispar egg masses could still be found after epizootics occurring in 1992 (49) and 1995 to 1996 (146), and host populations have remained low since these epizootics. Following these epizootics, E. maimaiga infections were detected each year among the few larvae that could be found (49).
Interactions between E. maimaiga and the Biotic Community
L. dispar is not endemic to North America and has been the focus of one of the longest ongoing programs of classical biological control in North America. The primary emphasis for many years was the introduction and establishment of insect predators and parasitoids rather than pathogens (29). As a result of these importation programs, 1 exotic predator and 12 exotic parasitoids have become established in North America (35). LdMNPV was first reported in North America in 1907, although it had not been purposely introduced (41). It is possible that this virus was introduced accidentally with parasitoids being introduced from Europe (41). In addition to the exotic natural enemies that have been introduced, several natural enemies native to North America have also become associated with L. dispar. The majority of these native North American enemies are generalist predators, e.g., deer mice consume L. dispar pupae, stinkbugs and long-billed cuckoos consume larvae, and other bird species feed on eggs.
Investigations of the influence of the newly arrived E. maimaiga on the established community of natural enemies already associated with L. dispar in North America have only begun. There is concern that E. maimaiga might outcompete and displace other natural enemies but would not be as effective at controlling L. dispar (139, 155). Under this supposition, if E. maimaiga were for some reason inactive (potentially during a severe drought), these other natural enemies would no longer be present to respond as L. dispar populations increase. To allay these fears, further field studies, as well as laboratory studies, of the coexistence of major natural enemies of L. dispar with E. maimaiga should be undertaken.
Pathogens.
E. maimaiga (reported as E. aulicae) is known to cause epizootics in L. dispar populations in conjunction with the fungal pathogen Paecilomyces canadensis (possibly P. farinosus [55]) in Japan (3). Mixed infections with these two fungi were found in ca. 20% of dead larvae. In North America, several species of entomopathogenic fungi have been found in association with L. dispar (55, 63, 92) although none has been found coinfecting with E. maimaiga. All entomopathogenic fungi infecting L. dispar in North America, besides E. maimaiga, are hyphomycetes, and none is ever very common. Of these, P. farinosus is by far the most consistently present and causes infection rates averaging from 4.6 to 12.2% (55).
Before E. maimaiga was discovered in North America, LdMNPV was by far the most commonly occurring pathogen. This pathogen acts relatively slowly, and populations of L. dispar generally achieved unacceptably high densities before viral transmission was adequate to cause a “population crash.” In outbreak populations of L. dispar, insecticide treatments have generally preceded this collapse. However, naturally occurring LdMNPV epizootics have often run their course and eventually controlled outbreak populations of L. dispar in nonrecreational forested areas. E. maimaiga and LdMNPV can coinfect individual L. dispar larvae; however, percentages of field-collected cadavers in which both pathogens reproduce have been recorded as only <8% in three instances (10, 67, 146), although 44% coinfection was reported in a fourth instance (10).
Coinfection has been studied in the laboratory with both simultaneous and lagged infection by fungus and virus (93, 94). Most of the L. dispar larvae simultaneously infected with both pathogens and maintained at 20°C died within 5 to 7 days, and cadavers bore only E. maimaiga spores and no viral occlusion bodies. Larvae that were not killed by E. maimaiga died from LdMNPV infections in the expected time of 14 days. Some of the cadavers of larvae dying at ca. 14 days contained both fungal spores and LdMNPV occlusion bodies, but because the experimental containers allowed contact between sporulating cadavers and living insects, these fungal infections most probably resulted from secondary infection after the first group of cadavers produced conidia. Laboratory coinfections were also conducted with E. maimaiga exposure after larvae had been infected with LdMNPV for 10 days. In these bioassays, coinfection shortened the time to death, with coinfected larvae dying 1 to 2 days sooner than positive controls infected only with LdMNPV. However, coinfected larvae produced fewer viral occlusion bodies than did positive larval controls. In summary, bioassays have demonstrated that for simultaneous infection or for cases when E. maimaiga infection precedes LdMNPV infection, E. maimaiga will dominate, killing the coinfected insect and subsequently reproducing, whereas for larvae infected by LdMNPV and subsequently E. maimaiga, death occurs sooner than it would in larvae with LdMNPV infection alone, but viral reproduction may decline, resulting in less deposition of viral inoculum in the environment. This is due to more rapid pathogenesis from E. maimaiga than LdMNPV.
These excellent studies, however, do not answer the epizootiological question asked by researchers and land managers concerning L. dispar: Will E. maimaiga prevent LdMNPV from being a principal cause of population collapses, and/or will E. maimaiga provide equivalent epizootics? At present, this is an open question, and many years could pass before long-term empirical evidence to suggest trends is available. To date, field studies have shown that E. maimaiga and LdMNPV coexisted in L. dispar populations in central New York, with LdMNPV being the principal pathogen during 1991 epizootics and E. maimaiga being the principal pathogen during 1992 (49). Is the extent to which the L. dispar population declined in 1991 or crashed in 1992 equivalent to the population decline that would occur due to LdMNPV acting alone? Examination of more field examples of the epizootiology of LdMNPV before the advent of E. maimaiga in North America may improve our ability to compare the dynamics of epizootics caused by these two pathogens.
Parasitoids.
Interactions between E. maimaiga and hymenopteran or dipteran parasitoids within L. dispar larvae have not been investigated. Empirical studies have documented relatively low levels of parasitism during E. maimaiga epizootics, mostly by Cotesia melanoscelus, a hymenopteran parasitoid attacking earlier-instar larvae, and tachinid flies, which parasitize later-instar larvae (49, 149). In these examples, it is unknown whether parasitoids were scarce due to direct antagonism with the fungus, lack of healthy hosts, or site-specific features. In contrast, a recent study documented high rates of L. dispar mortality due to C. melanoscelus and a tachinid that attacks later-instar larvae (Compsilura concinnata), concurrent with high levels of infection by E. maimaiga (76). C. concinnata is well known as being polyphagous (23) and multivoltine, utilizing many other species of forest Lepidoptera. Throughout the spring and summer, therefore, this parasitoid would be present even if L. dispar populations were kept low by E. maimaiga.
Studies have demonstrated that within hosts some fungal entomopathogens can adversely affect parasitoids, although in some instances cohabitation of fungus and parasitoid in an insect host has no observed effect on either (17). Regardless, L. dispar parasitoids in North America are rarely considered effective at controlling regional increases in host populations (34).
Host plants eaten by L. dispar.
One indirect impact of the biotic community on E. maimaiga could occur as a tritrophic effect. L. dispar is highly polyphagous, and we investigated whether feeding by larvae on different species of foliage influenced E. maimaiga (65). Negative effects of host plant on entomopathogenic fungi have previously been linked to the presence of alkaloids in plants (47). Although L. dispar larvae feed principally on host plants with a diversity of secondary plant compounds, they tend to avoid alkaloid-containing plants (7). L. dispar larvae given foliage from five different species of trees (red oak, red maple, quaking aspen, Japanese larch, and white pine) and then infected with E. maimaiga showed equivalent percentages of mortality across the tree species (65). However, the percentage of cadavers producing spores was significantly lower for maple-fed than larch-fed larvae, and the time to death was shortest for maple-fed larvae. L. dispar larvae fed maple also developed more slowly than did larvae fed foliage from other tree species. These results suggested no overt inhibition of E. maimaiga by the different host plants consumed by L. dispar larvae. We hypothesize that the minimal negative effects observed could result principally from differential development of this obligate fungal pathogen in a host growing suboptimally on a nonpreferred host plant.
USE FOR BIOLOGICAL CONTROL
L. dispar is a pest of forests, including recreational areas and sensitive ecological preserves, as well as being a pest of urban and suburban trees. There is tremendous interest in the use of tactics to control L. dispar that do not disturb other members of forested and urban ecosystems. At present, the bacterial pathogen Bacillus thuringiensis is aerially sprayed as a microbial insecticide over large areas for control and eradication efforts (116). The use of B. thuringiensis-based insecticides is controversial because it may also kill other members of the order Lepidoptera (97, 141). As an alternative, LdMNPV is highly host specific and has been registered as the microbial insecticide Gypchek. However, the production costs of Gypchek prohibit commercial production and use, and Gypchek is now produced mainly for experimental work. With the relatively recent discovery of E. maimaiga in North America, interest in the potential of this fungus for L. dispar control is high.
Availability of Inoculum
Entomophthoralean species are generally not as simple to grow in vitro as many hyphomycetes. E. maimaiga requires rich media for in vitro growth but can readily be grown as protoplasts (Fig. 10; protoplasts grown in vitro and those occurring in vivo differ in morphology) and hyphal bodies in liquid tissue culture media or as mycelium on EYSMA. It is important to note that repeated subculture of this fungus in tissue culture media may result in a loss of virulence (58), and any potential mass production method would need to focus on preventing attenuation.
FIG. 10.
Phase-contrast image of E. maimaiga protoplasts grown in Grace’s insect tissue culture medium plus 5% fetal bovine serum. Bar, 10 μm. Reprinted from reference 58 with permission of the publisher.
For entomopathogenic hyphomycetes, conidia are generally the stage that is mass produced. E. maimaiga conidia are fairly short-lived and not very environmentally resistant; this stage is generally not considered for mass production and release for any of the Entomophthorales. For some of the Entomophthorales, growth of hyphal bodies under aerated conditions with subsequent drying and milling yields preparations that, upon field release, will rehydrate under moist conditions and produce conidia (95, 128). To date, this “marcescent” process has not been pursued for production of E. maimaiga, although E. maimaiga was able to grow as mycelia in 25-liter bubblers with yeast extract, maltose, peptone, and sunflower oil (130). The method used for mass production of E. aulicae hyphal bodies in a medium consisting of 13 amino acids, tryptic soy broth, and calcium caseinate has been developed and optimized for 14-liter fermentors (106, 107). E. maimaiga also grew well by this method in this medium (100). Unfortunately, survival and conidial production of E. aulicae or E. maimaiga hyphal bodies grown under these conditions has not been evaluated (100).
The growth stage of E. maimaiga principally used to date for release has been the azygospore. Several programs have previously concentrated on releases of entomophthoralean azygospores for pest control, with varying degrees of success. Releases of field-produced azygospores have been successful against a homopteran orchard pest in Nova Scotia (30) but unsuccessful against forest tent caterpillar (M. disstria) in Alabama swamps (1). Unsuccessful programs to control a complex of cereal aphid species relied on releases of in vitro-produced azygospores (153). The lack of success during the cereal aphid and M. disstria field studies may have resulted in part because each program was conducted for only 1 year. Low germination and asynchrony of azygospore germination were considered the causes for failure of fungal releases to control cereal aphids (153). When initially deciding on the optimal E. maimaiga stage for release, asynchronous azygospore germination was not considered detrimental, since L. dispar is univoltine, and a relatively high threshold of damage by this pest is often tolerated.
For E. maimaiga release programs to date, azygospores had to be collected in the field, in part because germinable azygospores could not be produced in the laboratory. However, azygospores have now been produced in tissue culture media in the laboratory (81). In vitro azygospore production is slow (azygospore production begins only at 7 days), varies by fungal isolate, and is enhanced by increased aeration. Much remains to be learned in order to optimize in vitro azygospore production, but studies are being conducted in parallel to ensure that in vitro-produced spores are germinable. In fact, azygospores can be produced in the laboratory by microinjection of L. dispar larvae, but the exact storage conditions necessary to satisfy dormancy requirements and optimize spore survival have not yet been identified.
During initial studies investigating methods for releasing E. maimaiga, azygospores were collected in soil from the bases of tree trunks that had been covered with fungus-killed cadavers the previous year (66). Azygospores released in contaminated soil at the bases of trees and watered each week caused the highest levels of L. dispar infection. Moreover, further studies demonstrated that even weekly application of 1 liter of water to azygospore-laden soil around the bases of trees improved infection levels (56).
A study comparing releases of soil bearing azygospores with releases of infected larvae that would produce E. maimaiga conidia after host death demonstrated increased infection with azygospore releases (66). One study based only on releases of earlier-instar L. dispar larvae injected with E. maimaiga protoplasts (producing conidia after host death) concluded that while this method resulted in the establishment of E. maimaiga, microinjection of protoplasts was not operationally feasible for larger-scale releases (10). Interestingly, it was this method of releasing larvae injected with E. maimaiga protoplasts that was used to release the 1984 Japanese isolate in 1985 and 1986 (55). We hypothesize that lack of establishment from these releases was not due to the release of method but instead was probably due to confounding factors, e.g., drought (1986), occurrence of a viral epizootic (1985), and possible attenuation of the fungal isolate being used (1985 and 1986).
Injection of larvae with protoplasts for release is more difficult and labor-intensive than collection of soil bearing azygospores. However, knowing where to collect soil containing high titers of azygospores can be problematic, and movement of soil is strictly regulated by state and federal agencies and may not be an option for some states or areas. To ascertain whether azygospores are present in soil, time-consuming assays are necessary that do not unequivocally determine the densities of E. maimaiga azygospores that will germinate the following spring. Perhaps the most benign alternative to transplanting E. maimaiga-contaminated soil is the movement of cadavers bearing azygospores collected from tree trunks. While this is presently the preferred method for field collection of E. maimaiga azygospores, it is not without its own difficulties. The majority of cadavers containing azygospores remain attached to tree trunks for only a few weeks and then fall to the ground, after which time the azygospores are quickly leached into the soil (72). Therefore, azygospore-containing cadavers must be field-collected during the brief period directly after massive larval mortality. Locations where epizootics have occurred must be rapidly identified and visited for cadaver or soil collection. After collection, either cadavers or soil bearing azygospores must be maintained to maximize fungal survival until release sites are identified. In fact, storage of cadavers is usually necessary, because they must be collected in the field long before sites have been identified for release based on fall counts of L. dispar egg masses. Unfortunately, we have little information at present about conditions that optimize azygospore survival after collection, and such studies are complicated due to their long-term nature and the difficulty of determining whether azygospores are alive (see above).
Use of Naturally Occurring Epizootics for Control
Releases for establishment.
When E. maimaiga was first found in North America in 1989, surveys revealed that it was not yet established in areas more recently colonized by L. dispar (2, 60). At that time, no one knew how long it would take for E. maimaiga to spread on its own. Consequently, there was great interest in developing methods to introduce and establish this fungus in new locations. Small-scale studies in seven 0.01-ha plots in central New York demonstrated that it was fairly easy to establish E. maimaiga in new locations by using soil-borne azygospores (66) (see above). Subsequently, field-collected azygospores in soil were introduced at 41 locations in four mid-Atlantic states in 1991 and 1992. The release sites were separated by at least 3 km. At each location, 6 × 105 azygospores were released around the bases of oaks in 0.01-ha plots. The majority of releases were made in spring 1991, but ensuing rainfall was abnormally low. Nevertheless, L. dispar larvae infected with E. maimaiga were recovered at 28 of the 34 1991 release sites (56), and levels of infection were >40% at 6 sites. Surprisingly, extremely low levels of infection were found at 4 of the 15 control sites. This was interpreted as probably being caused by movement of E. maimaiga azygospores when samplers wearing contaminated shoes visited control sites. During 1992, when spring rainfall was approximately normal, E. maimaiga infections were recovered at 40 of the total 41 release sites and at a majority of the control sites. For 1992 release sites, infection levels averaged 72.4%, with infection being >70% in 24 of the 28 1991 release sites monitored for a second year.
Unexpectedly, in 1992, E. maimaiga spread across large areas of northern Virginia where L. dispar was established but E. maimaiga had not previously been found (59). Seven of the sites where E. maimaiga was introduced in 1991 and 1992 were sampled again in 1994 to investigate fungal persistence. In keeping with expectations regarding long-term survival of E. maimaiga azygospores, E. maimaiga-infected L. dispar larvae were collected at all sites, and infection levels were higher in 1994 than in 1992 in five of the seven sites (47).
In Michigan, E. maimaiga was released at two sites in 1991 and one site in 1992 (127). At each site, the eight 0.04-ha release plots and four control plots were separated by only 53 m. During 1991, at four of the release plots per site, 2.5 × 106 field-collected azygospores were released, and at the remaining four release plots per site, 60 larvae that had been injected with E. maimaiga protoplasts were released. During 1992, all fungal releases consisted of soil containing azygospores. Due to the ability of this fungus to spread and the relatively small distances between plots within sites, it is questionable to what extent each release plot can be considered to have been isolated, and therefore we will consider the results by site. During the first year of fungal release in Michigan, very low levels of infection were found at two of the three sites. The second year after release, E. maimaiga was found at low to intermediate levels of infection (9 to 40%) in two of the sites (the host population in the third area collapsed as a result of an LdMNPV epizootic). By the third year, infection was >90% at one site and 30 to 43% at the second site. Interestingly, infection levels in control plots frequently were fairly similar to those in release plots, suggesting that for future studies of this type, the release plots must be located farther from the control plots. During 1993, defoliation at one site was quantified and was negatively correlated with infection by E. maimaiga (127). A subsequent Michigan study also found that E. maimaiga prevalence was high in forest stands that experienced little defoliation while LdMNPV prevalence was positively associated with defoliation (19).
One study of the establishment and persistence of E. maimaiga in a marsh in the Niagara area of Ontario was based on releases of approximately 129 protoplast-injected L. dispar larvae at each of 8 0.01-ha plots hosting 100 to 5,400 egg masses/ha (10). Release plots were separated by ≥300 m, and the seven control plots were ≥400 m away from the release plots. Unfortunately, the collections of L. dispar cadavers from this area before fungal release were only macroscopically examined, and so we cannot be certain that fungal establishment was due solely to releases. However, E. maimaiga had not been found in this area previously. E. maimaiga was released in late June 1992, probably when late instars were present. Among larvae collected to evaluate disease transmission, 86% died of LdMNPV infections. Although LdMNPV infections were abundant in 1992, E. maimaiga-infected larvae were collected from seven of eight release plots but also from five of seven control plots. E. maimaiga infection averaged 13.7% for release plots, which was significantly higher than the 5.1% infection found in control plots. The year following fungal releases, an average of 46.8% E. maimaiga infection was found, with no difference between release and control plots. This study supports the previous accounts that relatively small amounts of inoculum are necessary for fungal establishment of E. maimaiga across a range of host densities. Furthermore, this fungus appears quite resilient, becoming established during both LdMNPV epizootics (10, 127) and dry springs (56).
Releases of E. maimaiga for establishment along the leading edge of L. dispar spread are not confined to those described above (18, 44, 46). In the United States, either cadavers or soil containing E. maimaiga azygospores were known to have been introduced at 146 locations from 1990 to 1994 (46). Unfortunately, these programs were usually operational, the sources of E. maimaiga were varied, methods were not standardized, and the results for fungal establishment and its impact were usually not quantified. Nevertheless, introductions along the leading edge of L. dispar spread continue, in part because we cannot predict how quickly E. maimaiga will colonize new areas on its own and reach densities that will often initiate epizootics (the actual occurrence of which will also depend on environmental conditions). Between 1991 and 1995, E. maimaiga was released at 45 locations in Michigan, yet by 1996 this fungus had not spread throughout the state, being found in only 78% of counties sampled (99).
L. dispar populations in North America are constantly spreading, and as this pest spreads, there is usually a lag before its natural enemies also colonize these new areas. When L. dispar populations first disperse into new areas, tree death and damage to trees, as well as the unsightliness of large hairy caterpillars and the abundant frass, often cause intense public concern. While some may be interested in releasing E. maimaiga with the goal of establishment and subsequent natural control, others frequently want immediate control of outbreak pest populations.
At present, studies have not been conducted to evaluate the optimal concentrations to release for specific types or sizes of areas. In addition, the conditions optimizing fungal establishment and enhancing infection need to be investigated. As L. dispar populations continue to spread, the demand for sources of E. maimaiga for establishment in newly colonized areas will continue, and the need for information about how, when, and where to best release the fungus will persist.
Releases to augment fungal populations.
Based on Japanese dynamics of the E. maimaiga-L. dispar system, we hypothesize that once L. dispar has become established in an area, the populations will continue to reach outbreak densities at irregular intervals. In addition, as E. maimaiga becomes established in a new area, outbreak L. dispar populations do not always collapse immediately. We asked whether E. maimaiga could be used to augment preexisting fungal inocula and cause earlier initiation of epizootics, thereby preventing defoliation largely caused by later-instar larvae. Azygospores in soil were released in small woodlots, characteristic of homeowner properties (73). Because the amount of E. maimaiga available for release was restricted due to limited availability, only 106 resting spores were released per site. In the first year that this study was conducted (1995), survival of fifth-instar L. dispar larvae was lower in treatment than control plots and severe defoliation occurred only in control plots. As an example of the unpredictability of L. dispar dynamics, in the second year of this study (1996) L. dispar populations collapsed throughout the area and no defoliation occurred in any plot. These results suggested that fungal augmentation might constitute a promising control strategy as the availability of E. maimaiga azygospores for biological control applications improves. Further studies should potentially concentrate on releasing higher doses of E. maimaiga in larger numbers of plots to evaluate treatments.
Interactions with B. thuringiensis.
B. thuringiensis, a bacterium occurring worldwide that produces an insecticidal toxin, has been used extensively for biological control of L. dispar. Interactions between B. thuringiensis and E. maimaiga were studied on a population level to determine whether the efficacy of E. maimaiga would be impacted by B. thuringiensis and vice versa (99). Working at sites with low host densities, when B. thuringiensis was applied against early-instar larvae, fewer early-instar larvae were infected by E. maimaiga, leading to an overall reduction in fungal infection in B. thuringiensis-treated plots. The authors suggested that lower mortality due to E. maimaiga would be due to the presence of fewer early-instar larvae after B. thuringiensis-induced mortality. Lower fungal infections could also be due to reduced larval contact with soil-borne E. maimaiga azygospores, because when they are present at lower densities, fewer L. dispar larvae move off of trees (99). Final L. dispar egg mass density and defoliation did not differ between plots receiving standard B. thuringiensis applications and control plots that experienced naturally occurring E. maimaiga epizootics (99). However, mortality caused by E. maimaiga occurred much later in the season than did B. thuringiensis-caused mortality, and this would potentially be unacceptable for control under higher host population densities.
OUTSTANDING QUESTIONS
Studies of E. maimaiga have been conducted for a relatively brief period, and the research effort has been intensive due to the importance of the lepidopteran pest that this fungus attacks. However, the impact of E. maimaiga on L. dispar populations across space and over time is not resolved. At present, across most of the area in North America where L. dispar is established, E. maimaiga has caused epizootics and L. dispar populations are at innocuous densities. In fact, since 1989, most declines in L. dispar populations have been attributed to the activity of E. maimaiga (122), although this has been well documented for few locations and times. Before E. maimaiga was known to occur in North America, it was common for northeastern L. dispar populations to decline precipitously and remain at low densities for variable periods between outbreaks. In fact, this host is well known to have a “boom or bust” dynamic; insects having such patterns are generally termed “outbreak” insects. It has been suggested that virulent pathogens can create instability in populations of forest defoliators, resulting in outbreaks (139). Whether the presence of E. maimaiga in North America will alter this overall pattern of outbreaks by L. dispar is unknown. It is also possible that with E. maimaiga in North America, L. dispar outbreaks will become less frequent or peak outbreak densities will be lower. We do know that L. dispar populations occasionally reach outbreak densities in Japan, where both this host and pathogen are native, suggesting that the presence of E. maimaiga will not completely change the ability of L. dispar to reach outbreak densities. Due to the historical unpredictability in the dynamics of North American L. dispar populations, it has been suggested that at least several decades of study will be required before we can begin to understand the impact of E. maimaiga on L. dispar populations (89).
We are still far from being able to predict the prevalence of E. maimaiga infections. Host-pathogen models could assist with investigating the principal factors limiting the occurrence of epizootics and identifying the potential thresholds necessary for development of epizootics. In particular, land managers want to be able to predict the occurrence of fungal epizootics in order to prevent unnecessary insecticide applications. It is clear that the activity of E. maimaiga is dependent to some extent on the weather. If the weather can be approximated several months before the period of L. dispar activity, can the activity of E. maimaiga be predicted with a high degree of accuracy? How frequently do weather conditions facilitating the occurrence of epizootics occur, and does this vary significantly between different regions colonized by L. dispar?
Another factor useful for the prediction of E. maimaiga activity would be the density of azygospores present at the beginning of a season. We know that E. maimaiga azygospores can survive for extended periods. However, will azygospore persistence vary by site? Studies have demonstrated that the highest titers of azygospores are found in the organic layer of soil at tree bases (Fig. 9) (50). Will the persistence of E. maimaiga azygospores be lower at sites without thick organic soil layers, and will general soil type alter retention or activity of azygospores? The conidia of Nomuraea rileyi, a hyphomycete pathogen of noctuids, are not well retained in sandy soils (77). One factor critical to the development of epizootics is survival of conidia. Although few studies have investigated conidial survival, it appears that at least some conidia survive long enough to fall to the forest floor and to cause infections there.
With the heavy selection pressure by this virulent fungus against L. dispar in North America, will this pest evolve some type of resistance to E. maimaiga? Resistance mechanisms might include altered physiological relations with the pathogen or altered behavior to reduce exposure to resting spore-laden soil. We know that late-instar larvae of L. dispar in northern China, where E. maimaiga is thought to occur, can be found resting at ground level (120), but whether this behavior is as common as for North American L. dispar is unknown. It is also not known whether variability in susceptibility to E. maimaiga occurs within Asian populations of L. dispar.
Although host specificity is critical to the use of a natural enemy for biological control, the mechanisms that control host specificity are poorly understood for entomopathogenic fungi. Building on basic information on the host range of E. maimaiga, this system could provide an excellent model to identify the factors responsible for host recognition by conidia and cuticular penetration by conidia as well as the factors responsible for the recognition of fungal cells as nonself within infected insects.
Finally, widespread use of this pathogen for biological control will require an economically feasible method for mass production. If a product is developed, will persistence of the long-lived azygospores of this fungus after establishment hamper its development for biological control because areas might not need to be treated to the same extent on a regular basis? Whether this fungus is eventually developed as a product or not, it is clear that E. maimaiga can be an extremely effective biological control agent on its own. Whether we will be able to harness this obligate pathogen more efficiently for pest control practices remains to be investigated and demonstrated.
ACKNOWLEDGMENTS
I thank L. Bauer, J. Elkinton, M. Filotas, R. Humber, J. Vandenberg, and R. Weseloh for their helpful comments on the manuscript and J. Liebherr for assistance with figures. D. Aylor, A. Hunter, and R. Webb provided unpublished data and useful insights.
The work in my laboratory was supported in part by USDA, NRICGP 9604343 and USDA Forest Service Cooperative Agreements 42-96-0009 and 23-95-23.
REFERENCES
- 1.Abrahamson L P, Harper J D. Microbial insecticides control forest tent caterpillar in southwestern Alabama. Forest Service research note SO-157. U.S. Washington, D.C.: Department of Agriculture; 1973. [Google Scholar]
- 2.Andreadis T G, Weseloh R M. Discovery of Entomophaga maimaiga in North American gypsy moth, Lymantria dispar. Proc Natl Acad Sci USA. 1990;87:2461–2465. doi: 10.1073/pnas.87.7.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki J. Mixed infection of the gypsy moth, Lymantria dispar japonica Motschulsky (Lepidoptera: Lymantriidae), in a larch forest by Entomophthora aulicae (Reich.) Sorok. and Paecilomyces canadensis (Vuill.) Brown et Smith. Appl Entomol Zool. 1974;9:185–190. [Google Scholar]
- 4.Aoki J, Yanase K, Yanbe T, Koyama R. Hibernation of resting spores of Entomophthora aulicae in egg masses of the gypsy moth Porthetria dispar. J Invertebr Pathol. 1976;27:395–396. [Google Scholar]
- 5.Aylor D E. A framework for examining inter-regional aerial transport of fungal spores. Agric For Meteorol. 1986;38:263–288. [Google Scholar]
- 6.Aylor D E. The role of intermittent wind in the dispersal of fungal pathogens. Annu Rev Phytopathol. 1990;28:73–92. [Google Scholar]
- 7.Barbosa P, Krischik V A. Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth, Lymantria dispar. Am Nat. 1987;130:53–69. [Google Scholar]
- 8.Bauer, L. S. Personal communication.
- 9.Beauvais A, Latgé J-P, Vey A, Prevost M-C. The role of surface components of the entomopathogenic fungus Entomophaga aulicae in the cellular immune response of Galleria mellonella. J Gen Microbiol. 1989;135:489–498. [Google Scholar]
- 10.Belme D M. Gypsy moths (Lymantria dispar) in the Niagara Region: population density variation, introduction of an entomopathogenic fungus (Entomophaga maimaiga) and occurrence of nuclear polyhedrosis virus. M.S. thesis. St. Catherine’s, Ontario, Canada: Brock University; 1995. [Google Scholar]
- 11.Bidochka, M. J. Personal communication.
- 12.Bidochka M J, Hajek A E. Protoplast plasma membrane glycoproteins in two species of entomophthoralean fungi. Mycol Res. 1996;100:1094–1098. [Google Scholar]
- 13.Bidochka M J, Hajek A E. A nonpermissive entomophthoralean fungal infection increases activation of insect prophenoloxidase. J Invertebr Pathol. 1998;72:231–238. doi: 10.1006/jipa.1998.4782. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanowicz S M, Wallner W E, Bell J, ODell T M, Harrison R G. Asian gypsy moths (Lepidoptera: Lymantriidae) in North America: evidence from molecular data. Ann Entomol Soc Am. 1993;86:710–715. [Google Scholar]
- 15.Boucias D G, Latgé J-P. Nonspecific induction of germination of Conidiobolus obscurus and Nomuraea rileyi with host and non-host cuticle extracts. J Invertebr Pathol. 1988;51:168–171. [Google Scholar]
- 16.Brobyn P J, Wilding N, Clark S J. Laboratory observations on the effect of humidity on the persistence of infectivity of conidia of the aphid pathogen Erynia neoaphidis. Ann Appl Biol. 1987;110:579–584. [Google Scholar]
- 17.Brooks W M. Host-parasitoid-pathogen interactions. In: Beckage N E, Thompson S N, Federici B A, editors. Parasites and pathogens of insects. 2. Pathogens. San Diego, Calif: Academic Press, Inc.; 1993. pp. 231–272. [Google Scholar]
- 18.Buss L J. Evaluation of three egg mass survey methods and two biological control agents for gypsy moth management. M.S. dissertation. East Lansing: Michigan State University; 1997. [Google Scholar]
- 19.Buss L J, McCullough D G, Ramm C W. A comparison of three egg mass survey methods and frequency of two pathogens in stands with varying gypsy moth populations in Michigan. Environ Entomol. 1999;28:485–495. [Google Scholar]
- 20.Butler L, Kondo V. Impact of dimilin on non-target Lepidoptera: results of an operational gypsy moth suppression program at Coopers Rock State Forest, West Virginia. West Virginia Agricultural and Forestry Experiment Station Bulletin 710. Morgantown: West Virginia University; 1993. [Google Scholar]
- 21.Butt T M, Humber R A. Response of gypsy moth hemocytes to natural fungal protoplasts of three Entomophaga species (Zygomycetes: Entomophthorales) J Invertebr Pathol. 1989;53:121–123. [Google Scholar]
- 22.Butt T M, Humber R A, Hajek A E. Gypsy moth immune response to hyphal bodies and natural protoplasts of entomophthoralean fungi. J Invertebr Pathol. 1996;68:278–285. doi: 10.1006/jipa.1996.0097. [DOI] [PubMed] [Google Scholar]
- 23.Campbell R W. The gypsy moth and its natural enemies. Forest Service, Agricultural Information Bulletin 381. U.S. Washington, D.C.: Department of Agriculture; 1975. [Google Scholar]
- 24.Campbell R W, Hubbard D L, Sloan R J. Patterns of gypsy moth occurrence within a sparse and numerically stable population. Environ Entomol. 1975;4:535–542. [Google Scholar]
- 25.Campbell R W, Podgwaite J D. The disease complex of the gypsy moth. I. Major components. J Invertebr Pathol. 1971;18:101–107. doi: 10.1016/0022-2011(91)90015-i. [DOI] [PubMed] [Google Scholar]
- 26.Carruthers R I, Feng Z, Ramos M E, Soper R S. The effect of solar radiation on the survival of Entomophaga grylli (Entomophthorales: Entomophthoraceae) conidia. J Invertebr Pathol. 1988;52:154–162. [Google Scholar]
- 27.Carruthers R I, Larkin T S, Firstencel H. Influence of thermal ecology on the mycosis of a rangeland grasshopper. Ecology. 1992;73:190–204. [Google Scholar]
- 28.Casey T M, Knapp R. Caterpillar thermal adaptations and behavioral differences reflect metabolic thermal sensitivities. Comp Biochem Physiol Ser A. 1987;86:679–682. [Google Scholar]
- 29.Clausen C P. Lymantriidae. In: Clausen C P, editor. Introduced parasites and predators of arthropod pests and weeds: a world review. USDA Agricultural Handbook 480. U.S. Washington, D.C.: Department of Agriculture; 1978. pp. 195–204. [Google Scholar]
- 30.Dustan A G. The artificial culture and dissemination of Entomophthora sphaerosperma Fres., a fungus parasite for the control of the European apple sucker (Psyllia mali Schmdb.) J Econ Entomol. 1927;20:68–75. [Google Scholar]
- 31.Dwyer G, Buonaccorsi J P. Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am Nat. 1997;150:685–707. doi: 10.1086/286089. [DOI] [PubMed] [Google Scholar]
- 32.Dwyer G, Elkinton J S, Hajek A E. Spatial scale and the spread of a fungal pathogen of gypsy moth. Am Nat. 1998;152:485–494. doi: 10.1086/286185. [DOI] [PubMed] [Google Scholar]
- 33.Elkinton J S, Hajek A E, Boettner G H, Simons E E. Distribution and apparent spread of Entomophaga maimaiga (Zygomycetes: Entomophthorales) in gypsy moth (Lepidoptera: Lymantriidae) populations in North America. Environ Entomol. 1991;20:1601–1605. [Google Scholar]
- 34.Elkinton J S, Liebhold A M. Population dynamics of gypsy moth in North America. Annu Rev Entomol. 1990;35:571–596. [Google Scholar]
- 35.Fuester, R. W. Unpublished results.
- 36.Funk C J, Ramoska W A, Bechtel D B. Histopathology of Entomophaga grylli pathotype 2 infections in Melanoplus differentialis. J Invertebr Pathol. 1993;61:196–202. [Google Scholar]
- 37.Fuxa J R, Tanada Y. Epidemiological concepts applied to insect epizootiology. In: Fuxa J R, Tanada Y, editors. Epizootiology of insect diseases. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 3–21. [Google Scholar]
- 38.Galaini-Wraight S, Wraight S P, Carruthers R I, Magalhaes B P, Roberts D W. Description of a Zoophthora radicans (Zygomycetes: Entomophthoraceae) epizootic in a population of Empoasca kraemeri (Homoptera: Cicadellidae) on beans in central Brazil. J Invertebr Pathol. 1991;58:311–326. [Google Scholar]
- 39.Gerardi M H, Grimm J K. The history, biology, damage, and control of the gypsy moth, Porthetria dispar (L.). Cranbury, N.J: Association of University Presses; 1979. [Google Scholar]
- 40.Giese R L, Schneider M L. Cartographic comparisons of Eurasian gypsy moth distribution (Lymantria dispar L.; Lepidoptera: Lymantriidae) Entomol News. 1979;90:1–16. [Google Scholar]
- 41.Glaser R W. Wilt of gypsy-moth caterpillars. J Agric Res. 1915;4:101–128. [Google Scholar]
- 42.Glowacka-Pilot B. Role of pathogens during a population build-up of Lymantria dispar in the Biebrza marshes in 1976–78. Pr Inst Badaw Lesn. 1982;608/612:29–42. . (In Polish.) [Google Scholar]
- 43.Gugnani H C, Okafor J I. Mycotic flora of the intestine and other internal organs of certain reptiles and amphibians with special reference to characterization of Basidiobolus isolates. Mykosen. 1980;23:260–268. doi: 10.1111/j.1439-0507.1980.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 44.Hajek, A. E. Unpublished results.
- 45.Hajek A E. Food consumption of Lymantria dispar (Lepidoptera: Lymantriidae) larvae infected with Entomophaga maimaiga (Zygomycetes: Entomophthorales) Environ Entomol. 1989;18:723–727. [Google Scholar]
- 46.Hajek A E. Proceedings of the Annual Gypsy Moth Review. 1995. The changing face of gypsy moth biological control: Entomophaga maimaiga; pp. 78–81. [Google Scholar]
- 47.Hajek A E. Ecology of terrestrial fungal entomopathogens. Adv Microb Ecol. 1997;15:193–249. [Google Scholar]
- 48.Hajek A E. Entomophaga maimaiga reproductive output is determined by the spore type initiating infection. Mycol Res. 1997;101:971–974. [Google Scholar]
- 49.Hajek A E. Fungal and viral epizootics in gypsy moth (Lepidoptera: Lymantriidae) populations in central New York. Biol Control. 1997;10:58–68. [Google Scholar]
- 50.Hajek A E, Bauer L, McManus M L, Wheeler M M. Distribution of resting spores of the Lymantria dispar pathogen Entomophaga maimaiga in soil and on bark. BioControl. 1998;43:189–200. [Google Scholar]
- 51.Hajek A E, Butler L, Walsh S R A, Silver J C, Hain F P, Hastings F L, ODell T M, Smitley D R. Host range of the gypsy moth (Lepidoptera: Lymantriidae) pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) in the field versus laboratory. Environ Entomol. 1996;25:709–721. [Google Scholar]
- 52.Hajek A E, Butler L, Wheeler M M. Laboratory bioassays testing the host range of the gypsy moth fungal pathogen Entomophaga maimaiga. Biol Control. 1995;5:530–544. [Google Scholar]
- 53.Hajek A E, Butt T M, Strelow L I, Gray S M. Detection of Entomophaga maimaiga (Zygomycetes: Entomophthorales) using enzyme linked immunosorbent assay (ELISA) J Invertebr Pathol. 1991;58:1–9. [Google Scholar]
- 54.Hajek A E, Carruthers R I, Soper R S. Temperature and moisture relations of sporulation and germination of Entomophaga maimaiga (Zygomycetes: Entomophthoraceae), a fungal pathogen of Lymantria dispar. Environ Entomol. 1990;19:85–90. [Google Scholar]
- 55.Hajek A E, Elkinton J S, Humber R A. Entomopathogenic hyphomycetes associated with gypsy moth larvae. Mycologia. 1997;89:825–829. [Google Scholar]
- 56.Hajek A E, Elkinton J S, Witcosky J J. Introduction and spread of the fungal pathogen Entomophaga maimaiga along the leading edge of gypsy moth spread. Environ Entomol. 1996;25:1235–1247. [Google Scholar]
- 57.Hajek A E, Humber R A. Formation and germination of Entomophaga maimaiga azygospores. Can J Bot. 1997;75:1739–1745. [Google Scholar]
- 58.Hajek A E, Humber R A, Griggs M H. Decline in virulence of Entomophaga maimaiga (Zygomycetes: Entomophthorales) with repeated in vitro subculture. J Invertebr Pathol. 1990;56:91–97. [Google Scholar]
- 59.Hajek A E, Humber R A, Elkinton J S. The mysterious origin of Entomophaga maimaiga in North America. Am Entomol. 1995;41:31–42. [Google Scholar]
- 60.Hajek A E, Humber R A, Elkinton J S, May B, Walsh S R A, Silver J C. Allozyme and RFLP analyses confirm Entomophaga maimaiga responsible for 1989 epizootics in North American gypsy moth populations. Proc Natl Acad Sci USA. 1990;87:6979–6982. doi: 10.1073/pnas.87.18.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajek A E, Humber R A, Walsh S R A, Silver J C. Sympatric occurrence of two Entomophaga aulicae (Zygomycetes: Entomophthorales) complex species attacking forest Lepidoptera. J Invertebr Pathol. 1991;58:373–380. [Google Scholar]
- 62.Hajek A E, Larkin T S, Carruthers R I, Soper R S. Modeling the dynamics of Entomophaga maimaiga (Zygomycetes: Entomophthorales) epizootics in gypsy moth (Lepidoptera: Lymantriidae) populations. Environ Entomol. 1993;22:1172–1187. [Google Scholar]
- 63.Hajek A E, Nelson P E, Humber R A, Perry J L. Two Fusarium species pathogenic to gypsy moth, Lymantria dispar. Mycologia. 1993;85:937–940. [Google Scholar]
- 64.Hajek A E, Olsen C H, Elkinton J S. Dynamics of airborne conidia of the gypsy moth (Lepidoptera: Lymantriidae) fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) Biol Control. 1999;16:111–117. [Google Scholar]
- 65.Hajek A E, Renwick J A A, Roberts D W. Effects of larval host plant on the gypsy moth fungal pathogen, Entomophaga maimaiga. Environ Entomol. 1995;24:1307–1314. [Google Scholar]
- 66.Hajek A E, Roberts D W. Pathogen reservoirs as a biological control resource: Introduction of Entomophaga maimaiga to North American gypsy moth, Lymantria dispar, populations. Biol Control. 1991;1:29–34. [Google Scholar]
- 67.Hajek A E, Roberts D W. Field diagnosis of gypsy moth (Lepidoptera: Lymantriidae) larval mortality caused by Entomophaga maimaiga and the gypsy moth nuclear polyhedrosis virus. Environ Entomol. 1992;21:706–713. [Google Scholar]
- 68.Hajek A E, Shimazu M. Types of spores produced by Entomophaga maimaiga infecting the gypsy moth Lymantria dispar. Can J Bot. 1996;74:708–715. [Google Scholar]
- 69.Hajek A E, Soper R S. Within-tree location of gypsy moth, Lymantria dispar, larvae killed by Entomophaga maimaiga (Zygomycetes: Entomophthorales) J Invertebr Pathol. 1991;58:468–469. [Google Scholar]
- 70.Hajek A E, Soper R S. Temporal dynamics of Entomophaga maimaiga after death of gypsy moth (Lepidoptera: Lymantriidae) larval hosts. Environ Entomol. 1992;21:129–135. [Google Scholar]
- 71.Hajek A E, Walsh S R A, Silver J C, Strong D R. A disjunct Californian strain of Entomophaga aulicae infecting Orygia vetusta. J Invertebr Pathol. 1996;68:260–268. doi: 10.1006/jipa.1996.0094. [DOI] [PubMed] [Google Scholar]
- 72.Hajek A E, Tatman K M, Wanner P H, Wheeler M M. Location and persistence of gypsy moth, Lymantria dispar, cadavers containing Entomophaga maimaiga azygospores. Mycologia. 1998;90:754–760. [Google Scholar]
- 73.Hajek A E, Webb R E. Inoculative augmentation of the fungal entomopathogen Entomophaga maimaiga as a homeowner tactic to control gypsy moth (Lepidoptera: Lymantriidae) Biol Control. 1999;14:11–18. [Google Scholar]
- 74.Hajek A E, Wheeler M M. Application of techniques for quantification of soil-borne entomophthoralean resting spores. J Invertebr Pathol. 1994;64:71–73. [Google Scholar]
- 75.Harrison R G, Wintermeyer S F, ODell T M. Patterns of genetic variation within and among gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae), populations. Ann Entomol Soc Am. 1983;76:652–656. [Google Scholar]
- 76.Hunter, A. F., and J. S. Elkinton. Complex effects of synchrony with the host plant on population growth of a spring-feeding lepidopteran. Ecology, in press.
- 77.Ignoffo C M, Garcia C, Hostetter D L, Pinnell R E. Vertical movement of conidia of Nomuraea rileyi through sand and loam soils. J Econ Entomol. 1977;70:163–164. [Google Scholar]
- 78.Injac M, Vasiljevic L. Lutte contre le bombyx disparate (Lymantria dispar L.) par les virus de polyedrose nucleaire (Baculovirus) a l’aide de l’avion. Zast Bilja. 1978;29:43–56. [Google Scholar]
- 79.Inoue H, Sugi S, Kuroko H, Moriuti S, Kawabe A. Moths of Japan. 2. Plates and synoptic catalogue. Tokyo, Japan: Kodansha; 1982. [Google Scholar]
- 80.Kerwin J L. Fatty acid regulation of the germination of Erynia variabilis conidia on adults and puparia of the lesser housefly, Fannia canicularis. Can J Microbiol. 1984;30:158–161. [Google Scholar]
- 81.Kogan, P. H., and A. E. Hajek. Formation of azygospores of the insect pathogenic fungus Entomophaga maimaiga in cell culture. Submitted for publication. [DOI] [PubMed]
- 82.Koyama R. Two epizootic diseases of the gypsy moth. Shirin-Boeki. 1954;27:296–298. . (In Japanese.) [Google Scholar]
- 83.Lance D R, Barbosa P. Dispersal of larval Lepidoptera with special references to forest defoliators. Biologist. 1979;61:90–110. [Google Scholar]
- 84.Larkin, T. S. Unpublished results.
- 85.Latgé J P, Fournet B, Cole G, Dubourdieu D, Tong N. Composition chimique et ultrastructure des parois des corps hyphaux et des azygospores de Conidiobolus obscurus. Can J Microbiol. 1984;30:1507–1521. [Google Scholar]
- 86.Leopold, A. C. Unpublished results.
- 87.Leopold A C. Coping with desiccation. In: Alscher R G, Cumming J R, editors. Stress responses in plants: adaptation and acclimation mechanisms. New York, N.Y: Wiley-Liss; 1990. pp. 37–56. [Google Scholar]
- 88.Liebhold A M, Elkinton J S, Wallner W E. Effect of burlap bands on between-tree movement of late-instar gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae) Environ Entomol. 1986;15:373–379. [Google Scholar]
- 89.Liebhold A, Sharov A. Current decline in gypsy moth outbreaks and its significance to the Slow the Spread project. Gypsy Moth News. 1998;44:8–9. [Google Scholar]
- 90.Liebhold A, Mastro V, Schaefer P W. Learning from the legacy of Léopold Trouvelot. Bull Entomol Soc Am. 1989;2:20–22. [Google Scholar]
- 91.Lloyd M, Dybas H S. The periodical cicada problem. I. Population ecology. Evolution. 1966;20:133–149. doi: 10.1111/j.1558-5646.1966.tb03350.x. [DOI] [PubMed] [Google Scholar]
- 92.Majchrowicz I, Yendol W G. Fungi isolated from the gypsy moth. J Econ Entomol. 1973;66:823–824. [Google Scholar]
- 93.Malakar R D. Interactions between two gypsy moth (Lymantria dispar L.) pathogens—nuclear polyhedrosis virus and Entomophaga maimaiga (Entomophthorales: Zygomycetes). Ph.D. dissertation. Amherst: University of Massachusetts; 1997. [Google Scholar]
- 94.Malakar R D, Elkinton J S, Hajek A E, Burand J P. Within-host interactions of Lymantria dispar (Lepidoptera: Lymantriidae) nucleopolyhedrosis virus and Entomophaga maimaiga (Zygomycetes: Entomophthorales) J Invertebr Pathol. 1999;73:91–100. doi: 10.1006/jipa.1998.4806. [DOI] [PubMed] [Google Scholar]
- 95.McCabe, D. E., and R. S. Soper. July 1985. Preparation of an entomopathogenic fungal insect control agent. U.S. patent 4,530,834.
- 96.McCabe D E, Humber R A, Soper R S. Observation and interpretation of nuclear reductions during maturation and germination of entomophthoralean resting spores. Mycologia. 1984;76:1104–1107. [Google Scholar]
- 97.Miller J C. Field assessment of the effects of a microbial pest control agent on nontarget Lepidoptera. Am Entomol. 1990;36:135–139. [Google Scholar]
- 98.Milne R, Wright T, Welton M, Budau C, Gringorten L, Tyrrell D. Identification and partial purification of a cell-lytic factor from Entomophaga aulicae. J Invertebr Pathol. 1994;64:253–259. [Google Scholar]
- 99.Mott M A. Michigan distribution of Entomophaga maimaiga, a fungal pathogen of gypsy moth (Lepidoptera: Lymantriidae), and impact of Bacillus thuringiensis to fungal-induced mortality. M.S. dissertation. East Lansing: Michigan State University; 1998. [Google Scholar]
- 100.Murrin, F. Unpublished results.
- 101.Murrin F, Heath I B. Mithramycin staining of condensed fungal chromatin. Exp Mycol. 1982;6:303–306. [Google Scholar]
- 102.Murrin F, Heath I B, Newcomb W. The taxonomic significance of the mitotic spindle and nucleus-associated organelle of Entomophaga aulicae. Protoplasma. 1984;120:84–90. [Google Scholar]
- 103.Murrin F, Holtby J, Nolan R A, Davidson W S. The genome of Entomophaga aulicae (Entomophthorales: Zygomycetes): base composition and size. Exp Mycol. 1986;10:67–75. [Google Scholar]
- 104.Murrin F, Newcomb W, Heath I B. The ultrastructure and timing of events in the nuclear cycle of the fungus Entomophaga aulicae. J Cell Sci. 1988;90:501–516. [Google Scholar]
- 105.Murrin F, Nolan R A. Ultrastructure of the infection of spruce budworm larvae by the fungus Entomophaga aulicae. Can J Bot. 1987;65:1694–1706. [Google Scholar]
- 106.Nolan R A. An inexpensive media for mass fermentation production of Entomophaga aulicae hyphal bodies competent to form conidia. Can J Microbiol. 1993;39:588–593. [Google Scholar]
- 107.Nolan, R. A. March 1998. Growth media for entomophthoralean hyphal bodies. U.S. patent 5,728,572.
- 108.Pak S W. Studies on natural enemies of gypsy moth. M.S. thesis. Seoul, Korea: Sunggun Dwan University; 1962. . (In Korean.) [Google Scholar]
- 109.Pemberton R W, Lee J H, Reed D K, Carlson R W, Han H Y. Natural enemies of the Asian gypsy moth (Lepidoptera: Lymantriidae) in South Korea. Ann Entomol Soc Am. 1993;86:423–440. [Google Scholar]
- 110.Perry D F, Latgé J-P. Dormancy and germination of Conidiobolus obscurus azygospores. Trans Br Mycol Soc. 1982;78:221–225. [Google Scholar]
- 111.Podgwaite J. Natural disease within dense gypsy moth populations. In: Doane C C, McManus M L, editors. The gypsy moth: research toward integrated pest management. U.S. Department of Agriculture Technical Bulletin 1584. U.S. Washington, D.C.: Department of Agriculture; 1981. pp. 125–134. [Google Scholar]
- 112.Prasher D C. The Asian genotype in the 1994 gypsy moth port survey. Forest Service General Technical Report NE-213. Washington, D.C.: U.S. Department of Agriculture; 1995. p. 107. [Google Scholar]
- 113.Price P W. Insect ecology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 114.Ralph-Edwards A, Silver J C. Nucleosomal DNA repeat length and histone complement in a fungus exhibiting condensed chromatin. Exp Cell Res. 1983;148:363–376. doi: 10.1016/0014-4827(83)90159-3. [DOI] [PubMed] [Google Scholar]
- 115.Ramaseshiah, G. Personal communication.
- 116.Reardon R, Dubois N, McLane W. Bacillus thuringiensis for managing gypsy moth: a review. Forest Service Report FHM-NC-01-94. U.S. Washington, D.C.: Department of Agriculture; 1994. [Google Scholar]
- 117.Roberts D W, Campbell A S. Misc. Publ. Entomol. Soc. Amer. 1977. Stability of entomopathogenic fungi; pp. 19–76. [Google Scholar]
- 118.Samson R A, Evans H C, Latgé J-P. Atlas of entomopathogenic fungi. Berlin, Germany: Springer-Verlag KG; 1988. [Google Scholar]
- 119.Sawyer A, Griggs M, Wayne R. Dimensions, density, and settling velocity of entomophthoralean conidia: Implications for aerial dissemination of spores. J Invertebr Pathol. 1994;63:43–55. [Google Scholar]
- 120.Schaefer P W, Weseloh R M, Sun X, Wallner W E, Yan J. Gypsy moth, Lymantria (= Ocneria) dispar (L.) (Lepidoptera: Lymantriidae), in the People’s Republic of China. Environ Entomol. 1984;13:1535–1541. [Google Scholar]
- 121.Schaefer P W, Xilin Y J S, Wallner W E, Weseloh R M. Natural enemies of the gypsy moth, Lymantria dispar, in China. Sci Silvae Sin. 1984;20:434–440. . (In Chinese.) [Google Scholar]
- 122.Schneeberger N F. Gypsy moth populations plummet in 1996 while “the fungus” skyrockets. Gypsy Moth News. 1996;42:1–2. [Google Scholar]
- 123.Shimazu M. Effect of rearing humidity of host insects on the spore types of Entomophaga maimaiga Humber, Shimazu et Soper (Entomophthorales; Entomophthoraceae) Appl Entomol Zool. 1987;22:394–397. [Google Scholar]
- 124.Shimazu M, Koizumi C, Kushida T, Mitsuhashi J. Infectivity of hibernated resting spores of Entomophaga maimaiga Humber, Shimazu et Soper (Entomophthorales: Entomophthoraceae) Appl Entomol Zool. 1987;22:216–221. [Google Scholar]
- 125.Shimazu M, Soper R S. Pathogenicity and sporulation of Entomophaga maimaiga Humber, Shimazu, Soper and Hajek (Entomophthorales: Entomophthoraceae) on larvae of the gypsy moth, Lymantria dispar L. (Lepidoptera: Lymantriidae) Appl Entomol Zool. 1986;21:589–596. [Google Scholar]
- 126.Sliwa E. The occurrence of gypsy moth (Lymantria dispar) on Biebrza marshes. Sylwan. 1978;10:25–29. . (In Polish.) [Google Scholar]
- 127.Smitley D R, Bauer L S, Hajek A E, Sapio F J, Humber R A. Introduction and establishment of Entomophaga maimaiga, a fungal pathogen of gypsy moth (Lepidoptera: Lymantriidae) in Michigan. Environ Entomol. 1995;24:1685–1695. [Google Scholar]
- 128.Soper R S. Proceedings of the Symposium on Microbial Control of Spruce Budworms and Gypsy Moths. Forest Service, NE Forestry Experiment Station Report GTR-NE-100. U.S. Washington, D.C.: Department of Agriculture; 1985. Erynia radicans as a mycoinsecticide for spruce budworm control; pp. 69–76. [Google Scholar]
- 129.Soper R S, Holbrook F R, Majchrowicz I, Gordon C C. Production of Entomophthora resting spores for biological control of aphids. Univ Maine Orono Life Sci Agric Exp Stn Tech Bull. 1975;76:1–15. [Google Scholar]
- 130.Soper R S, Shimazu M, Humber R A, Ramos M E, Hajek A E. Isolation and characterization of Entomophaga maimaiga sp. nov., a fungal pathogen of gypsy moth, Lymantria dispar, from Japan. J Invertebr Pathol. 1988;51:229–241. [Google Scholar]
- 131.Speare A T, Colley R H. The artificial use of the brown-tail fungus in Massachusetts with practical suggestions for private experiments, and a brief note on a fungous disease of the gypsy caterpillar. Boston, Mass: Wright & Potter; 1912. [Google Scholar]
- 132.St. Leger R J. Biology and mechanisms of insect-cuticle invasion by deuteromycete fungal pathogens. In: Beckage N E, Thompson S N, Federici B A, editors. Parasites and pathogens of insects. 2. Pathogens. San Diego, Calif: Academic Press, Inc.; 1993. pp. 211–229. [Google Scholar]
- 133.St. Leger R J, Goettel M, Roberts D W, Staples R C. Prepenetration events during infection of host cuticle by Metarhizium anisopliae. J Invertebr Pathol. 1991;58:168–179. [Google Scholar]
- 134.Strauss G, Schurtenberger P, Hauser H. The interaction of saccharides with lipid bilayer vesicles: stabilization during freeze-thawing. Biochem Biophys Acta. 1986;858:169–180. doi: 10.1016/0005-2736(86)90303-2. [DOI] [PubMed] [Google Scholar]
- 135.Takamura N, Sato H. Transactions of the 84th Meeting of the Japanese Forestry Society. (In Japanese.) 1973. Observation on the epizootic of an Entomophthorales disease in the outbreak population of the gypsy moth. I; pp. 353–355. [Google Scholar]
- 136.Takamura N, Sato H. Transactions of the 84th Meeting of the Japanese Forestry Society. (In Japanese.) 1973. Observation on the epizootic of an Entomophthorales disease in the outbreak population of the gypsy moth. II; pp. 355–357. [Google Scholar]
- 137.Tanada Y, Fuxa J R. The pathogen population. In: Fuxa J R, Tanada Y, editors. Epizootiology of insect diseases. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 113–157. [Google Scholar]
- 138.Uziel A, Kenneth R G. Survival of primary conidia and capilliconidia at different humidities in Erynia (subgen. Zoophthora) spp. and in Neozygites fresenii (Zygomycotina, Entomophthorales), with special emphasis on Erynia radicans. J Invertebr Pathol. 1991;58:118–126. [Google Scholar]
- 139.Valenti M A. Entomophaga maimaiga: salvation from the gypsy moth or fly in the ointment? Am Entomol. 1998;44:20–22. [Google Scholar]
- 140.Vandenberg J D. Safety of four entomopathogens for caged adult honey bees (Hymenoptera: Apidae) J Econ Entomol. 1990;83:755–759. [Google Scholar]
- 141.Wagner D L, Peacock J W, Carter J L, Talley S E. Field assessment of Bacillus thuringiensis on nontarget Lepidoptera. Environ Entomol. 1996;25:1444–1454. [Google Scholar]
- 142.Wallace D R, MacLeod D M, Sullivan C R, Tyrrell D, DeLyzer A J. Induction of resting spore germination in Entomophthora aphidis by long-day light conditions. Can J Bot. 1976;54:1410–1418. [Google Scholar]
- 143.Walsh S R A. Development of molecular markers for the detection and differentiation of Entomophaga strains pathogenic for insects. Ph.D. dissertation. Toronto, Canada: University of Toronto; 1996. [Google Scholar]
- 144.Walsh S R A, Tyrrell D, Humber R A, Silver J C. DNA restriction fragment length polymorphisms in the rDNA repeat unit of Entomophaga. Exp Mycol. 1990;14:381–392. [Google Scholar]
- 145.Watson D W, Mullens B A, Petersen J J. Behavioral fever response of Musca domestica (Diptera: Muscidae) to infection by Entomophthora muscae (Zygomycetes: Entomophthorales) J Invertebr Pathol. 1993;61:10–16. [Google Scholar]
- 146.Webb R E, White G B, Thorpe K W, Talley S E. Quantitative analysis of a pathogen-induced premature collapse of a “leading-edge” gypsy moth (Lepidoptera: Lymantriidae) population in Virginia. J Entomol Sci. 1999;34:84–100. [Google Scholar]
- 147.Welton M. Fungal pathogens: forest tent caterpillar and gypsy moth. For Can For Pest Manage Inst Newsl. 1991;9:7. [Google Scholar]
- 148.Weseloh R M. Possibility for recent origin of the gypsy moth (Lepidoptera: Lymantriidae) fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) in North America. Environ Entomol. 1998;27:171–177. [Google Scholar]
- 149.Weseloh R M, Andreadis T G. Epizootiology of the fungus Entomophaga maimaiga, and its impact on gypsy moth populations. J Invertebr Pathol. 1992;59:133–141. [Google Scholar]
- 150.Weseloh R M, Andreadis T G. Mechanisms of transmission of the gypsy moth (Lepidoptera: Lymantriidae) fungus, Entomophaga maimaiga (Entomophthorales: Entomophthoraceae) and effects of site conditions on its prevalence. Environ Entomol. 1992;21:901–906. [Google Scholar]
- 151.Weseloh R M, Andreadis T G. Persistence of resting spores of Entomophaga maimaiga, a fungal pathogen of the gypsy moth, Lymantria dispar. J Invertebr Pathol. 1997;69:195–196. doi: 10.1006/jipa.1996.4645. [DOI] [PubMed] [Google Scholar]
- 152.Weseloh R M, Andreadis T G, Onstad D W. Modeling the influence of rainfall and temperature on the phenology of infection of gypsy moth, Lymantria dispar, larvae by the fungus Entomophaga maimaiga. Biol Control. 1993;3:311–318. [Google Scholar]
- 153.Wilding N, Latteur G, Dedryver C A. Evaluation of Entomophthorales for aphid control: laboratory and field data. In: Samson R A, Vlak J M, Peters D, editors. Fundamental and applied aspects of invertebrate pathology. In 4th International Colloquium on Invertebrate Pathology. 1986. pp. 159–162. [Google Scholar]
- 154.Yanbe T. Influences of the population density of the gypsy moth on the thickening growth of larch tree in the forest. Tohoku For Exp Stn Newsl. 1976;175:1–4. . (In Japanese.) [Google Scholar]
- 155.Yerger E H, Rossiter M. Natural causes and rates of early larval mortality in gypsy moths (Lepidoptera: Lymantriidae) sampled from field populations in different density states. Environ Entomol. 1996;25:1002–1011. [Google Scholar]