Abstract
Objectives
Monkeypox (Mpox) recent outbreak has changed in terms of predominant transmission route and typical presentation. Describing current epidemiological and clinical characteristics is crucial to identifying cases and halting transmission.
Methods
An observational study was conducted at a Peruvian tertiary-level hospital and included all individuals with Mpox virus infection between July 01 and September 03, 2022.
Results
Among 205 confirmed cases, 99% (202/205) were men, 94% (192/205) were men who have sex with men or bisexual, and 66% (136/205) were living with HIV. Regarding sexual behavior, 87% (179/205) had a sexual encounter 21 days before consultation, although only 8% (17/205) identified sexual contact with a Mpox confirmed case; 65% (133/205) had sexual intercourse with casual partners, 55% (112/205) reported a last sexual partner unknown, and 21.5% (44/205) continued having sexual intercourse with symptoms. Systemic symptoms were fever (162/205, 79%), malaise (123/205, 60%), headache (119/205, 58%), fatigue (105/205, 52%), and lymphadenopathy (111/205, 54%). The distribution of skin lesions was generalized (166/205, 81%), located in the anogenital area (160/205, 78%), polymorphic (174/205, 85%), and it was the first symptom identified in 46% (94/205) of cases. Overall, 10% (21/205) required hospitalization, of whom 85.7% (18/205) have HIV infection. Complications included bacterial superinfection (n = 18), proctitis (n = 6), balanitis (n = 4), and necrosis of skin lesions (n = 3).
Conclusion
In 2022, Mpox mainly affects men who have sex with men and People living with HIV/AIDS. It presents with skin lesions localized to the anogenital area and can lead to severe complications requiring hospitalization.
Keywords: Monkeypox virus, Human monkeypox, Disease outbreak, MSM, Men who have sex with men
Introduction
Monkeypox (Mpox) is a zoonotic infectious disease caused by an Orthopoxvirus that belongs to the same genus as classical smallpox, vaccinia, and cowpox viruses [1]. In 1970, Mpox was identified as a cause of disease in humans in Central Africa, where it has historically and widely affected rural and impoverished areas, particularly after the eradication of smallpox and the cessation of immunization in 1980 [2,3]. It was described in the United States in 2003 and has since been reported in non-endemic countries where it causes occasional outbreaks linked to travelers to endemic areas [4]. In May 2022, a non-travel-associated cluster of Mpox cases was reported in the United Kingdom and later spread to other countries across Europe [5]. Therefore, the World Health Organization defined this multinational outbreak as a Public Health Emergency of International Concern [6,7]. By the end of June, 48 cases were confirmed in seven Latin American countries, including three cases in Peru [8], a country that, to this day, has the highest number of patients with Mpox infection per million people in the region [9]. Cases initially identified were men who have sex with men (MSM) with multiple sexual partners and no history of travel to endemic countries [10], which suggested person-to-person transmission during sexual or intimate contact with infectious lesions [11]. As the predominant transmission route in the 2022 outbreak has changed, the current clinical characteristics are considered atypical [12]. Defining the spectrum of presentation of this emerging disease is crucial to identifying cases, and making a correct differential diagnosis, and will help healthcare workers maintain a high clinical suspicion [13]. This study describes the epidemiological and clinical characteristics of patients with Mpox infection treated in a hospital in Peru between July and September 2022.
Methods
Study design and population
This observational study was conducted at Hospital Nacional Arzobispo Loayza in Lima Peru. All suspected individuals infected with Mpox virus were diagnosed and treated at general or tertiary-level hospitals (Supplementary Figure 1), and Hospital Nacional Arzobispo Loayza was the one that handled most Mpox cases. Patients with a confirmed diagnosis between July 01 and September 03, 2022, were included in the study.
Procedures
Participants were recruited at the emergency department and the infectious diseases outpatient clinic. Probable cases, as defined by the National Center for Epidemiology, Prevention, and Control of Diseases (CDC-Peru), had to meet both clinical and epidemiological criteria. These included an acute rash characterized by deep and delimited lesions, usually with central umbilication, progressing from macules to papules, vesicles, pustules, and crusts, plus one or more of the following symptoms: fever, headache, myalgia, back pain, asthenia, or lymphadenopathy. In addition, direct physical contact with or exposure to a probable or confirmed case of Mpox in the 21 days preceding symptom onset or travel history to a country with confirmed cases in that same period were required [10]. These patients underwent a molecular assay (reverse transcription polymerase chain reaction) on the skin lesions. Specimens were directed to the Peruvian National Institute of Health Central Lab for processing.
Data collection and analysis
Information from case report forms and medical records was extracted and collected into an anonymous and confidential database. For numerical variables, mean or median and standard deviation or interquartile ranges were used, while absolute and relative frequencies were used for categorical variables.
Results
Epidemiological characteristics
Of 236 probable cases tested for Mpox virus between July 01 and September 03, 2022, 205 were confirmed. In the cohort, the vast majority were men (202/205, 98.5%), except for three women (Table 1 ). The population's median age was 32 years (range of 19-54 years).
Table 1.
Epidemiological characteristics of 205 patients with Mpox virus infection from a hospital in Peru between July and September 2022
| Characteristics | |
|---|---|
| Sex, n (%) | |
| Male | 202 (98.5) |
| Female | 3 (1.5) |
| Age, years (median, IQR) | 32 (28-38) |
| Sexual orientation, n (%) | |
| Men who have sex with men | 166 (81.0) |
| Bisexual | 26 (12.7) |
| Male | 25 (96.2) |
| Female | 1 (3.8) |
| Heterosexual | 13 (6.3) |
| Male | 11 (84.6) |
| Female | 2 (15.4) |
| Sexual behavior, n (%) | |
| Sexual encounter in the last 21 days | 179 (87.3) |
| Number of sex partners in the last year (median, IQR) | 3 (1 - 9) |
| Last sexual encounter with unknown partner | 112 (54.6) |
| Casual sex partners | 133 (64.9) |
| STIs history, n (%) | |
| HIV-positive | 136 (66.3) |
| Known to be taking antiretroviral treatment | 129 (94.8) |
| Self-reported history of syphilis | 67 (32.7) |
| Other self-reported STIsa | 10 (4.9) |
| Epidemiological criteria, n (%) | |
| Contact with a Mpox confirmed case | 17 (8.3) |
| Travel out of Peru's capital in the last 21 days | 27 (13.2) |
IQR, interquartile range; Mpox, monkeypox; STIs, sexually transmitted infections.
Genital warts, hepatitis C, gonorrhea, and genital herpes
Regarding sexual orientation and behavior, 93.7% (192/205) were MSM or bisexual, and 87% (179/205) reported having had a sexual encounter 21 days before the consultation date. In these patients, the median time elapsed between the last reported sexual intercourse and the onset of symptoms was seven days, with an interquartile range of 3 to 14 days. Sexual intercourse with casual partners was a frequent practice in the study population (133/205, 64.9%). On this wise, 54.6% (112/205) reported that their last sexual partner was unknown and contacted them through social networks, mobile applications, or in social spaces (parties, discos, gyms, saunas, sports fields). Only 8.3% (17/205) identified sexual contact with a confirmed Mpox diagnosis. To determine whether people with symptoms or signs of the disease continued having sexual intercourse, was made a calculation considering the date of the last sexual intercourse and the date of the onset of symptoms; it was identified that 21.5% (44/205) of the cohort had sexual intercourse with any symptom, and 8.8% (18/205) did so with rash or skin lesions.
Regarding sexually transmitted infections (STIs), 66.3% (136/205) were people living with HIV/AIDS (PLWHA), 32.7% (67/205) self-reported having had syphilis in the past, and 4.9% (10/205) have had another STIs (genital warts, hepatitis C, gonorrhea, and genital herpes).
Clinical presentation
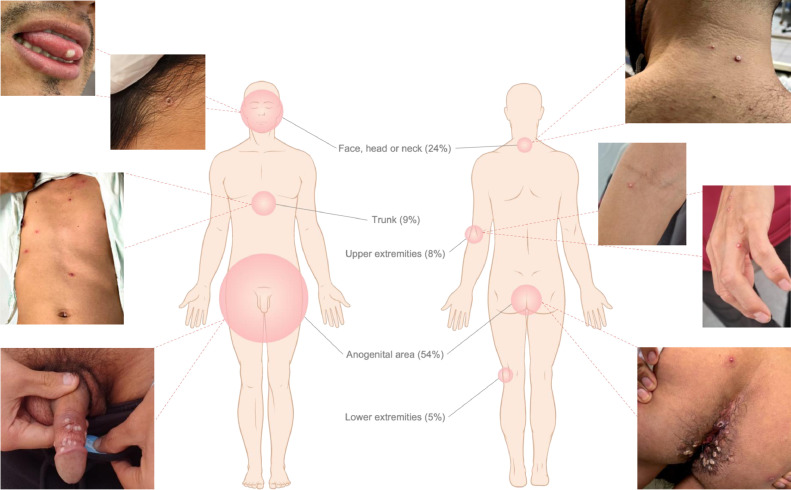
About half of the patients (111/205, 54.1%) experienced systemic symptoms before the skin lesions (Table 2 ). The most common symptom was fever (162/205, 79%), followed by malaise (123/205, 60%), headache (119/205, 58.1%), and fatigue (105/205, 51.5%). Upper respiratory symptoms such as sore throat (79/205, 38.5%), cough (7/205, 3.4%), and rhinorrhea (6/205, 2.9%) were uncommon. As for lymphadenopathy, 54.1% (111/205) had it at the time of consultation, and it was localized (98/111, 93.7%), either in the unilateral (34/111, 30.6%) or bilateral (29/111, 26.1%) inguinal region. Around 1 to 4 days later, the skin lesions or rash appeared (Figure 1 ).
Table 2.
Clinical characteristics of 205 patients with monkeypox virus infection from a hospital in Peru between July and September 2022
| Characteristics | |
|---|---|
| Systemic symptoms, n (%) | |
| Cough | 7 (3.4) |
| Rhinorrhea | 6 (2.9) |
| Sore throat | 79 (38.5) |
| Malaise | 123 (60.0) |
| Fatigue | 105 (51.5) |
| Back pain | 77 (37.6) |
| Fever | 162 (79.0) |
| Headache | 119 (58.1) |
| Chills and sweats | 69 (33.8) |
| Pruritus | 27 (13.2) |
| Lymphadenopathies, n (%) | |
| Any lymphadenopathy | 111 (54.2) |
| Lymphadenopathy distribution | |
| Localized | 98 (88.3) |
| Generalized | 13 (11.7) |
| Lymphadenopathy by region | |
| Axilar | 2 (1.8) |
| Cervical unilateral | 18 (16.2) |
| Cervical bilateral | 22 (19.8) |
| Inguinal unilateral | 34 (30.6) |
| Inguinal bilateral | 29 (26.1) |
| Submandibular | 2 (1.8) |
| Rash or skin lesions at consultation, n (%) | |
| Lesion distribution | |
| Localized | 38 (18.6) |
| Generalized | 166 (81.4) |
| Lesion location | |
| Face, head or neck | 128 (62.7) |
| Trunk | 142 (69.3) |
| Upper extremities | 111 (54.4) |
| Lower extremities | 86 (41.9) |
| Anogenital area | 160 (78.0) |
| Lesion morphology | |
| Macular | 116 (56.6) |
| Papular | 162 (79.0) |
| Vesicle | 44 (21.5) |
| Pustule | 177 (86.3) |
| Ulcer | 4 (2.0) |
| Crust | 65 (31.7) |
| Lesion pattern | |
| Monomorphic | 31 (15.1) |
| Polymorphic | 174 (84.9) |
| Rash as first symptom identified | 94 (45.9) |
| Time between last sexual encounter and onset of symptoms, days (median, IQR) | 7 (3 - 14) |
| Time between onset of systemic symptoms and appearance of skin lesions, days (median, IQR) | 2 (1 - 4) |
| Time between onset of symptoms and accessing the healthcare facility, days (mean, SD) | 6.7 ± 3.3 |
| Started with systemic symptoms | 7.4 ± 3.2 |
| Started with skin lesions | 5.9 ± 3.3 |
| Had a sexual encounter while experiencing symptoms, n (%) | 44 (21.5) |
IQR, interquartile range.
Figure 1.
Lesion location reported by patients with monkeypox virus infection as first appeared and at consultation.
The distribution of the rash was generalized in 81% (166/205), meaning that it was present in two or more body areas separated by segments of healthy skin. In those who presented with localized skin lesions, some included single lesions. Anogenital area (160/205, 78%) and trunk (142/205, 69.3%) were the most affected. Pustules (177/205, 86.3%), papules (162/205, 79%), macules (116/205, 56.6%), crusts (65/205, 31.7%), vesicles (44/205, 21.5%), and ulcers (4/205, 2%) were examined; there were observed simultaneously in different stages of progression in 84.9% (174/205) of the patients.
Additionally, the number of days between the onset of symptoms and accessing the healthcare center was calculated and defined as a diagnostic delay, which was around 6.7 days and depended on whether the first symptom was systemic (5.9 days) or rash (7.4 days).
Hospitalized patients
Twenty-one patients (21/205, 10.2%) required hospitalization; all of them were males, PLWHA (18/205, 85.7%), and receiving antiretroviral treatment (ART) (15/18, 83.3%) (Table 3 ). Of those who did not receive ART, one was diagnosed with HIV infection during hospitalization, and the other two had abandoned therapy for more than a year because of mandatory confinement during the COVID-19 pandemic. The median time of hospitalization was 8.5 days.
Table 3.
Characteristics of 21 hospitalized patients with monkeypox virus infection from a hospital in Peru between July and September 2022
| Characteristics | |
|---|---|
| Sex, n (%) | |
| Male | 21 (100) |
| Age, years (median, IQR) | 33 (30 - 43) |
| HIV-positive, n (%) | 18 (85.7) |
| Known to be taking antiretroviral treatment | 15 (83.3) |
| Days elapsed until hospitalization (median, IQR) | 6 (5 - 9) |
| Length of hospital stay, days (median, IQR) | 8.5 (5 - 11) |
| Hospital discharge, n (%) | 18 (85.7) |
| Reason for hospitalization, n (%) | |
| Cutaneous bacterial superinfection | 18 (85.7) |
| Proctitis | 6 (28.5) |
| Balanitis | 4 (19.0) |
| Necrosis of skin lesion | 3 (14.3) |
| Generalized exanthem | 2 (9.4) |
| Orchiepididymitis | 1 (4.7) |
IQR, interquartile range
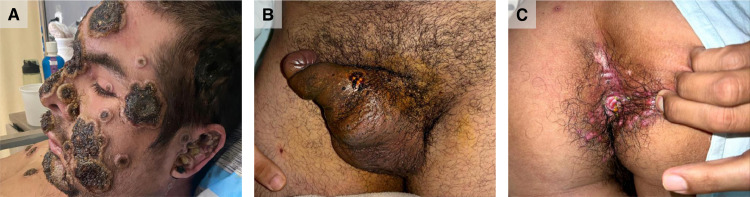
The most frequent complication was bacterial superinfection in 18 cases (18/205, 8.8%), and the most severe was necrosis of skin lesions on the face, extremities, and anogenital (Figure 2 ). The biopsy specimens from two patients revealed vasculitis of small vessels. Another complication was proctitis, which was diagnosed in 19 patients (19/205, 9.3%) during the first consultation, with six (6/19, 31.6%) of them requiring hospitalization. At the time of the study, three patients had not been discharged because of poor clinical outcomes.
Figure 2.
Complications in patients with monkeypox virus infection: (a) necrosis of skin lesions, (b) balanitis with bacterial superinfection, (c) and proctitis.
Discussion
This study describes the epidemiologic and clinical characteristics of 205 patients with a confirmed diagnosis of Mpox during the first 2 months of the epidemic in Peru. During September 2022, cases were concentrated on MSM who engaged in risky sexual behaviors. Two-thirds of patients were living with HIV, a proportion considered higher than other realities observed globally and, in the Americas, (46 and 52%, respectively) [13,14]. This situation should guide prioritizing and targeting strategies of prevention aimed at groups at high risk of contracting STIs [15]. Being aware that, otherwise, there is a risk of transmission to vulnerable groups and the general population. As was later seen in this cohort, three identified cases were women, one was a healthcare worker, and two were contacts of confirmed cases.
Patients with Mpox virus infection hardly recognized their sexual source of infection, as they could be reluctant or unable to disclose the names of their contacts because their risk exposures were previously unknown or casual [16]. Also, a fifth of them maintained sexual activity even with symptoms. If a relatively long incubation period is added to this, it becomes challenging to interrupt the chain of transmission [12]. Therefore, it is imperative to strengthen the epidemiological surveillance system to conduct case investigations, establish backward and forward contact tracing, and tailor effective countermeasures [17].
The clinical findings of this study differ from typical presentations established in endemic countries [12] but are comparable to those described in the 2022 multinational outbreak [11]. The atypical presentation consists of no systemic symptoms of onset, rash localized to the anogenital area, different stages of progression, and circumscribed lymphadenopathy. Likewise, it can vary from a mild condition with a single lesion to a generalized one complicated by bacterial superinfection or skin necrosis. The spectrum of clinical manifestations might cause delays in the recognition and diagnosis of cases by patients and physicians [18]. Consequently, educational content, case definitions, and case report forms for data collection should be focused on atypical manifestations to recognize, diagnose, treat, and isolate patients promptly [8,19].
From the outset, when cases were observed exclusively in MSM with anogenital lesions, Mpox has been negatively associated with sexual risk behaviors in this population [20]. Stigma may have had an impact on people's willingness to seek help, report symptoms, and provide trustworthy contact information, thus precluding timely diagnosis [21]. For instance, patients in this study took approximately 7 days and up to 17 days to access healthcare. Effective risk communication strategies, with assertive messages based on MSM and PLWHA participation and engagement, are needed to reduce stigma, minimize risky sexual behaviors, and maximize awareness [8,16].
Finally, although in the current outbreak, Mpox has a self-limited course and is of low mortality [11], the results of this cohort identified that one in ten patients required hospitalization, analogous to that reported in other case series [22], [23], [24]. In PLWHA, the proportion of hospitalized patients was higher when compared to those without HIV infection (13.2% vs 4.3%). Serious local complications and prolonged hospital stays have been observed in them, mainly if they had abandoned ART. More studies are needed to determine the risk factors for hospitalization, severity, and mortality.
This study has limitations. First, selection bias is possible because of the location and level of complexity of the hospital, which could have influenced the overdiagnosis of PLWHA and the complications and hospitalizations reported. However, epidemiological features from this case series match overall cases in Peru [25]. Second, patients may not remember the time of onset of systemic symptoms or other signs of the disease or may decide not to provide information about contagion. Estimates based on these variables should be interpreted with caution. Finally, although follow-up was carefully performed in hospitalized patients, information on healing time (crust formation and desquamation) and other outcomes were limited in outpatients, in whom remote follow-up was done.
Conclusion
The Mpox outbreak in Peru follows the atypical pattern described thus far, with MSM and PLWHA being disproportionately affected. Although mild presentations are frequent, severe diseases or complications are also described. In a country like Peru where access to specific antiviral treatments and vaccines is a challenge, effective prevention strategies, contact tracing, education, risk communication, and community engagement are essential to halt transmission and control the spread of the disease.
Declaration of competing interest
The authors have no competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Informed consent for the use of personal data and anonymous photographs was obtained from participants. The Research Ethics Committee of Hospital Nacional Arzobispo Loayza approved the study.
Acknowledgments
To the patients and the team of the Infectious Diseases and Epidemiology Departments at Hospital Nacional Arzobispo Loayza. RPP is a masters student studying Epidemiological Research at Universidad Peruana Cayetano Heredia supported by training grant D43 TW007393 awarded by the Fogarty International Center of the United States National Institutes of Health.
Author contributions
Dr. Sihuincha, Dr. Paredes and Dr. Lucchetti conceived and designed the analysis; Dr. Ponce, Dr. Lizarbe and Dr. Zumaeta collected the data; Dr. Martinez, Dr. Matos and Dr. Zumaeta contributed data or analysis tools; Dr. Paredes, Dr. Sihuincha and Dr. Lucchetti performed the analysis and wrote the paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.045.
Appendix. Supplementary materials
References
- 1.Petersen BW, Damon IK. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed. Bennett JE, Dolin R, Blaser MJ, editors. Elsevier; Amsterdam: 2015. Orthopoxviruses: vaccinia (smallpox vaccine), Variola (smallpox), monkeypox, and cowpox; pp. 1694–1702. [Google Scholar]
- 2.Beer EM, Bhargavi Rao V. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yinka-Ogunleye A, Aruna O, Dalhat M, Ogoina D, McCollum A, Disu Y, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan AM, Cenciarelli O, Colombe S, Alves de Sousa L, Fischer N, Gossner CM, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.36.2200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization, director. General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern, https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern; 2022 [accesed 15 August 2022].
- 7.World Health Organization. Multi-country monkeypox outbreak in non-endemic countries, https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385; 2022 [accesed 15 August 2022].
- 8.Rodriguez-Morales AJ, Lopardo G, Verbanaz S, Orduna T, Lloveras S, Azeñas-Burgoa JM, et al. Latin America: situation and preparedness facing the multi-country human monkeypox outbreak. Lancet Reg Health Am. 2022;13 doi: 10.1016/j.lana.2022.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Our world in data. Monkeypox, https://ourworldindata.org/monkeypox; 2022 [accessed 13 October 2022].
- 10.Centro Nacional de Epidemiología, prevención, y control de enfermedades. Alerta Epidemiológica, Casos de viruela del mono en Lima y riesgo de propagación a otras regiones, https://www.dge.gob.pe/epipublic/uploads/alertas/alertas_202216_01_191123.pdf; 2022 [accessed 01 August 2022].
- 11.Del Rio C, Malani PN. Update on the monkeypox outbreak. JAMA. 2022;328:921–922. doi: 10.1001/jama.2022.14857. [DOI] [PubMed] [Google Scholar]
- 12.Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi: 10.1093/ofid/ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organización Panamericana de la Salud. Viruela símica en la Región de las Américas - Evaluación de Riesgo, https://www.paho.org/es/documentos/viruela-simica-region-americas-evaluacion-riesgo; 2022 [accessed 27 September 2022].
- 14.World Health Organization. Monkeypox outbreak: global trends, https://worldhealthorg.shinyapps.io/mpx_global/#33_Case_profile_(overall); 2022 [accessed 01 September 2022].
- 15.Curran KG, Eberly K, Russell OO, Snyder RE, Phillips EK, Tang EC, et al. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1141–1147. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez Rodríguez B, Guzmán Herrador BR, Díaz Franco A, Sánchez-Seco Fariñas MP, del Amo Valero J, Aginagalde Llorente AH, et al. Epidemiologic features and control measures during monkeypox outbreak, Spain, June 2022. Emerg Infect Dis. 2022;28:1847–1851. doi: 10.3201/eid2809.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Health Emergencies Programme. Surveillance, case investigation and contact tracing for monkeypox, https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.3; 2022 [accessed 27 September 2022].
- 18.Tarín-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suñer C, Antón A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson MM, Polk CM, Fairman RT, DeWitt ME, Leonard MK, Davidson L, et al. Monkeypox testing delays: The need for drastic expansion of education and testing for monkeypox virus. Infect Control Hosp Epidemiol. 2023;44:348–349. doi: 10.1017/ice.2022.237. [DOI] [PubMed] [Google Scholar]
- 20.Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, Tittle V, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Risk communications and community engagement public health advice on understanding, preventing and addressing stigma and discrimination related to monkeypox, https://www.who.int/publications/m/item/communications-and-community-engagement-interim-guidance-on-using-inclusive-language-in-understanding–preventing-and-addressing-stigma-and-discrimination-related-to-monkeypox; 2022 [accessed 20 September 2022].
- 22.Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries - April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 25.Centro Nacional de Epidemiología. Prevención y control de enfermedades. Sala Situacional de la Viruela del Mono, https://www.dge.gob.pe/sala-monkeypox/; 2022 [accessed 03 September 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.