Abstract
Lupus nephritis (LN) has a high incidence in systemic lupus erythematosus (SLE) patients, but there is a lack of sensitive predictive markers. The purpose of the study was to investigate the association between the CD4+CD8+ double positive T (DPT) lymphocytes and LN. The study included patients with SLE without renal impairment (SLE-NRI), LN, nephritic syndrome (NS), or nephritis. Peripheral blood lymphocyte subsets were analyzed by flow cytometry. Biochemical measurements were performed with peripheral blood in accordance with the recommendations proposed by the National Center for Clinical Laboratories. The proportions of DPT cells in the LN group were significantly higher than that in the SLE-NRI group (t=4.012, P<0.001), NS group (t=3.240, P=0.001), and nephritis group (t=2.57, P=0.011). In the LN group, the risk of renal impairment increased significantly in a DPT cells proportion-dependent manner. The risk of LN was 5.136 times (95% confidence interval, 2.115–12.473) higher in cases with a high proportion of DPT cells than those whose proportion of DPT cells within the normal range. These findings indicated that the proportion of DPT cells could be a potential marker to evaluate LN susceptibility, and the interference of NS and nephritis could be effectively excluded when assessing the risk of renal impairment during SLE with DPT cell proportion.
Keywords: CD4+CD8+ double positive T cells, lupus nephritis, susceptibility, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease usually characterized by multiple organ damage, in which various tissues and organs can be attacked by immune complex deposition and lymphocyte infiltration. Renal impairment is the most common in SLE patients. Lupus nephritis (LN) constitutes one of the main clinical challenges in the patients and is a cause of significant morbidity and mortality[1]. The cumulative incidence of LN is relatively high in Asians (55%) and Africans (51%) with SLE. The outcome of patients with an early diagnosed LN was significantly improved in recent years[2], and thus early diagnosis and treatment of LN can greatly improve the prognosis of patients with SLE[3]. However, there is a lack of high sensitivity and specificity markers for early diagnosis of LN.
Peripheral CD4+CD8+ double positive T (DPT) lymphocytes are regarded as extrathymic cells, and these cells are rarely reported because of their small proportion in humans. In recent years, people pay more attention to DPT cells due to the improvement in detection technology. Studies have shown that increased proportions of peripheral DPT cells are observed in target organs of various autoimmune diseases and acute viral infections[4–5]. However, the role and repartition of extrathymic DPT cells remain largely uncharacterized[6].
This study applied a retrospective analysis method to analyze the association between SLE without renal impairment (SLE-NRI) and LN to find the sensitive indicators that can effectively predict the occurrence of renal impairment in patients with SLE. We found that the proportion of DPT cells could be used for evaluating LN susceptibility.
Subjects and methods
Study design and participants
This study was conducted between 2017 and 2019 in the population covered by the General Hospital of Western Theater Command. Participants were selected using a simple random sampling scheme (random number table). Of the 395 participating patients with renal impairment or SLE, 79 were diagnosed with SLE-NRI, 100 with LN, 108 with nephritic syndrome (NS), and 108 with nephritis (including glomerulonephritis and pyelonephritis).
Diagnosis of SLE-NRI and LN was made according to the American College of Rheumatology diagnostic criteria, and the criteria mainly included cheek erythema, discoid erythema, light allergy, oral ulcers, arthritis, serositis, renal lesions, neuropathy, hematological lesions, immunological abnormalities, and antinuclear antibodies. Diagnosis of NS and nephritis was made according to the World Health Organization diagnostic criteria[7–8]. The inclusion criteria for the study were patients with a definite diagnosis of SLE-NRI, LN, NS, and nephritis, disease course >1 year, age range from 10 to 85 years, and no sex restriction. Patients with tumors, low immunity, heart disease, and pregnancy/lactation, or using antihistamines were excluded.
This study used a detailed questionnaire and physical examination, which include age, blood pressure, medical history, occupation, imaging examination, B-ultrasound, peripheral blood examination, immunology examination, etc. Blood samples for further biochemical analysis were also collected from all the included patients.
The study was approved by the Ethics Committee of The General Hospital of Western Theater Command. Informed consents were obtained from all the participants.
Blood sample collection and measurements
A peripheral blood sample was collected from each participant using blood collection tubes with an inert gel and spray-dried K2EDTA (BD, USA). Blood collection tubes were centrifuged at 106.2 g for 10 minutes. The blood serum was used directly for biochemical and immunological detection, or stored at −80 °C until use. All biochemical testing reagents were purchased from Beijing Strong Biotechnologies, Inc. (China). All immunological testing reagents were purchased from EUROIMMUN Medical Diagnostics, Ltd. (China). Biochemical measurements, including total cholesterol (T-Cho), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein(a) [Lp(a)], apolipoprotein A (ApoA), apolipoprotein B (ApoB), cystatin C (Cys-C), blood urea nitrogen (BUN), serum creatinine (SCr), creatinine clearance rate (CCR), uric acid (UA), microalbuminuria (MA), urine creatinine (UCr), fibrinogen (Fib), D-dimer, and high-sensitivity C-reactive protein (hsCRP), were performed under the recommendations proposed by National Center for Clinical Laboratories. Anti-Sm antibody and anti-dsDNA antibody were measured by enzyme linked immunosorbent assay (ELISA).
Flow cytometry
Peripheral blood samples collected using blood collection tubes with spray-dried K2EDTA (BD, USA) were also used for flow cytometry. All biochemical testing reagents were purchased from BD Biosciences (USA). Peripheral blood lymphocyte subsets were analyzed on the BD FACSCanto Ⅱ Flow Cytometer (BD Biosciences). BD Multitest CD3 FITC/CD8 PE/CD45 PerCP/CD4 APC reagent (BD Biosciences) containing anti-CD3 fluorescein isothiocyanate, anti-CD8 phycoerythrin, anti-CD45 peridinin chlorophyll protein, and anti-CD4 allophycocyanin was used for identifying and determining the percentages and absolute counts of mature human T lymphocytes (CD3+), suppressor/cytotoxic (CD3+CD8+) T lymphocyte subsets, and helper/inducer (CD3+CD4+) T lymphocyte subsets in erythrocyte-lysed whole blood (Fig. 1). Briefly, the reagent cocktail (10 μL) was added to 50 μL EDTA-anticoagulated whole blood, and the sample was mixed and incubated for 30 min at room temperature in the dark. Erythrocytes were lysed by adding 500 μL of ammonium chloride hemolysis agent (BD Pharm Lyse, USA) for 15 min. Then the cells were washed, incubated with 2% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4), and measured on the flow cytometer[9].
Figure 1.
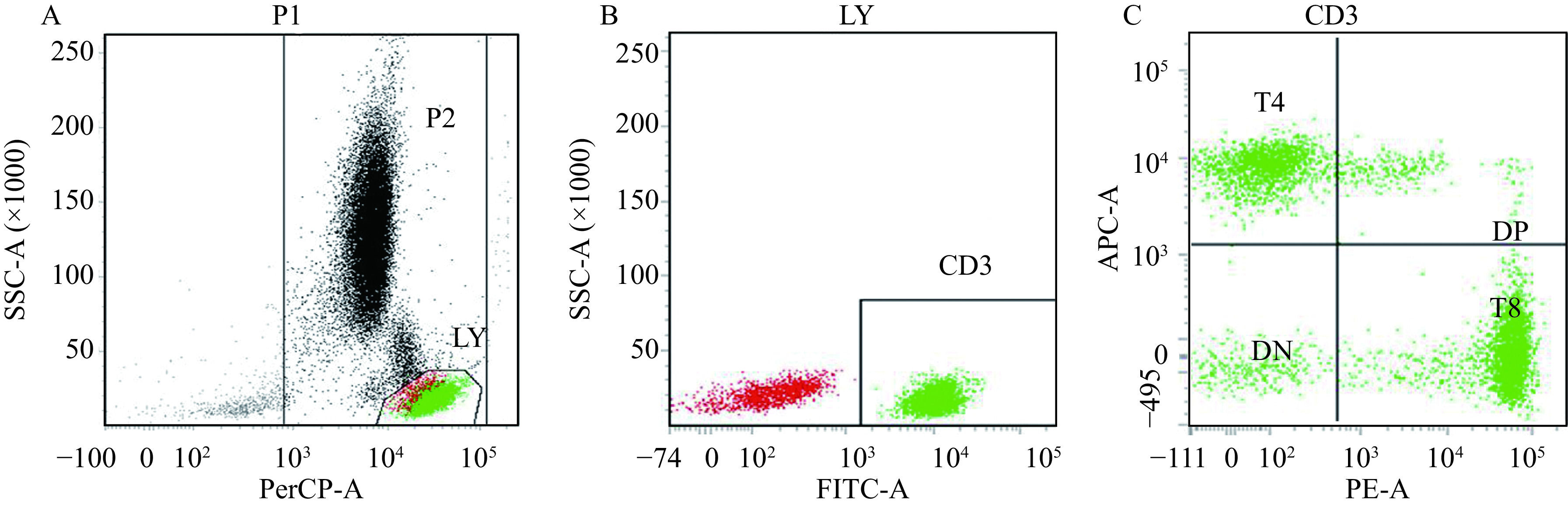
The maps of target lymphocytes and DPT cells by flow cytometry.
A: Differentiation of lymphocytes by anti-CD45 peridinin chlorophyll protein (PerCP) and side scatter (SSC). Lymphocytes are included in the polygonal check box. B: Differentiation of T lymphocytes by anti-CD3 fluorescein isothiocyanate (FITC). T lymphocytes were included in the rectangular check box. C: Differentiation of CD4+CD8+ double positive T lymphocytes by anti-CD8 phycoerythrin (PE) and anti-CD4 allophycocyanin (APC). The cruciform check box was used to divide the T4, DPT, DNP, and T8 lymphocytes. P1: all cells; P2: karyocytes; T4: CD3+CD4+CD8− T lymphocytes; T8: CD3+CD4−CD8+ T lymphocytes; Ly: lymphocytes; DP: CD4+CD8+ double positive T lymphocytes; DN: CD4−CD8− double negative T lymphocytes.
Statistical analysis
The total clinical test results were collected, including peripheral blood lymphocyte subsets and biochemical measurements. Continuous variables were expressed as mean±SD and categorical variables were reported as counts and percentages. Analyses of Student's t-tests and chi-square tests were used to test for differences between groups for continuous and categorical variables, respectively. Multivariate logistic regression analysis was used to identify independent predictors of nephropathy[10]. Analyses were performed using SPSS version 19.0 statistical software (IBM, USA). A value of P<0.05 (two-sided) was considered statistically significant.
Results
Demographic data of the enrolled subjects
A total of 395 individuals were enrolled in this study, including 154 cases of males and 241 cases of females. Of all patients, 35% were aged <30 years, 48% aged range from 30 to 50 years, 15% aged from 50 to 70 years, and 2% aged >70 years.
Test results and comparative analysis between the SLE and LN groups
Because of the high probability of LN in patients with SLE, we compared clinical profiles of the patients in the SLE-NRI and LN groups (Table 1). There was no significant difference in the proportions of sex and age between the SLE-NRI and LN groups. As compared with the SLE-NRI group, the levels of T-Cho, LDL-C, Lp(a), ApoB and Fib were significantly higher than that in the LN group in blood fatty acid and coagulative function detection. In liver and renal function biochemical examination, the levels of albumin to globulin ratio (A/G), BUN, SCr, Cys-C, UA, MA, and MA to UCr ratio (MA/UCr) were significantly higher in the LN group than that in the SLE-NRI group. In lymphocyte subsets examination, the proportion of CD3+CD4+CD8+ (DPT cells) was also significantly higher in the LN group than in the SLE-NRI group. There were no significant differences in levels of TG, HDL-C, ApoA, D-dimer, hsCRP, CD3+CD4+CD8−, CD3+CD4−CD8+, CD3+CD4−CD8−, and CD4+ to CD8+ T cells ratio (T4/T8) between the two groups. There was also no significant difference in the positive rate of anti-Sm antibody and anti-dsDNA antibody between the LN and SLE-NRI groups.
Table 1. Demographic and clinical characteristics of patients in the SLE-NRI and LN groups.
| Variables | SLE-NRI (N=79) | LN (N=100) | t/χ2 value | P-value |
| Data are presented as mean±SD, except for the data on gender and anti-Sm antibody and anti-dsDNA antibody positive rate. SLE-NRI: systemic lupus erythematosus without renal impairment; LN: lupus nephritis; T-Cho: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a); ApoA: apolipoprotein A; ApoB: apolipoprotein B; A/G: albumin/globulin; BUN: blood urea nitrogen; SCr: serum creatinine; Cys-C: cystatin C; CCR: creatinine clearance rate; UA: uric acid; MA: microalbuminuria; MA/UCr: MA/urine creatinine; Fib: fibrinogen; hsCRP: high-sensitivity C-reactive protein; T4/T8: CD4+ to CD8+ T cells ratio. | ||||
| Male [n (%)] | 11 (13.92) | 13 (13.00) | 0.065 | 0.799 |
| Age (years) | 36.54±14.15 | 40.82±13.79 | 1.557 | 0.121 |
| T-Cho (mmol/L) | 4.31±1.21 | 5.07±1.55 | 3.285 | 0.001 |
| TG (mmol/L) | 1.77±2.22 | 1.96±0.96 | 0.703 | 0.483 |
| HDL-C (mmol/L) | 1.44±0.58 | 1.48±0.55 | 0.417 | 0.667 |
| LDL-C (mmol/L) | 2.29±0.82 | 2.79±1.13 | 2.958 | 0.004 |
| Lp(a) (mg/L) | 170.81±153.08 | 294.15±310.48 | 2.601 | 0.011 |
| ApoA (g/L) | 1.30±0.36 | 1.34±0.37 | 0.454 | 0.651 |
| ApoB (g/L) | 0.76±0.30 | 0.90±0.36 | 2.343 | 0.021 |
| A/G | 1.71±0.38 | 1.51±0.44 | −5.638 | <0.001 |
| BUN (mmol/L) | 4.99±1.57 | 10.16±8.48 | 5.303 | <0.001 |
| SCr (μmol/L) | 69.63±23.84 | 135.21±140.41 | 4.076 | <0.001 |
| Cys-C (mg/L) | 0.91±0.26 | 1.87±1.22 | 6.674 | <0.001 |
| CCR (mL/minute) | 95.09±19.61 | 64.85±46.88 | −5.267 | <0.001 |
| UA (μmol/L) | 305.15±118.00 | 402.37±162.85 | 4.421 | <0.001 |
| MA (mg/L) | 29.04±68.60 | 1819.57±3357.35 | 4.092 | <0.001 |
| MA/UCr (mg/g) | 38.44±110.04 | 2400.72±5912.72 | 3.066 | 0.003 |
| Fib (g/L) | 2.87±0.98 | 3.40±1.23 | 2.717 | 0.007 |
| D-dimer (mg/L) | 1.85±1.79 | 3.22±6.50 | 1.159 | 0.250 |
| hsCRP (mg/L) | 0.99±0.98 | 1.45±2.30 | 1.617 | 0.108 |
| Anti-Sm antibody positive [n (%)] | 20 (25.32) | 27 (27.00) | 0.081 | 0.776 |
| Anti-dsDNA antibody positive [n (%)] | 24 (30.38) | 31 (31.00) | 0.001 | 0.983 |
| CD3+CD4+CD8− (%) | 32.59±9.92 | 32.15±8.89 | −0.315 | 0.753 |
| CD3+CD4−CD8+ (%) | 33.69±11.95 | 37.00±10.50 | 1.968 | 0.051 |
| CD3+CD4+CD8+ (%) | 0.40±0.35 | 0.84±0.91 | 4.012 | <0.001 |
| CD3+CD4−CD8− (%) | 3.59±2.85 | 3.55±2.45 | −0.096 | 0.924 |
| T4/T8 | 1.18±0.77 | 0.99±0.60 | −1.770 | 0.078 |
The proportion of CD4+CD8+ DPT cells was a risk factor for nephropathy in SLE patients
Multivariate logistic regression analysis was applied to analyze risk factors between the 79 SLE-NRI and 100 LN patients. As shown in Table 2, the proportion of CD4+CD8+ DPT cells was an independent risk factor for nephropathy in SLE patients (odds ratio [OR], 5.136; 95% confidence interval [CI], 2.115–12.473; P<0.001). Hyperuricemia (OR, 3.285; 95% CI, 1.597–6.757; P=0.001) and hypertriglyceridemia (OR, 2.617; 95% CI, 1.288–5.577; P=0.013) may also contribute to the deterioration of LN in patients with SLE-NRI. No significant associations between any of the other factors (hypercholesterolemia or sex) and nephropathy were observed in SLE patients.
Table 2. Multivariate logistic regression analysis of independent risk factor for renal impairment in systemic lupus erythematosus.
| Variables | 95% CI | P-value | OR value |
| Hypercholesterolemia was diagnosed when serum total cholesterol >6.2 mmol/L. Hyperuricemia was diagnosed when serum uric acid >420 μmol/L in men or >360 μmol/L in women.Blood triglyceride levels were greater than 1.7 mmol/L in hypertriglyceridemia. The cut-off value of the proportion of CD4+CD8+ double positive T (DPT) lymphocytes was 1.42%. aMale was used as the reference category for sex. CI: confidence interval; OR: odds ratio. | |||
| Hypercholesterolemia | 0.505–3.742 | 0.534 | 1.375 |
| Hypertriglyceridemia | 1.288–5.577 | 0.013 | 2.617 |
| Hyperuricemia | 1.597–6.757 | 0.001 | 3.285 |
| Sexa | 0.264–2.065 | 0.563 | 0.738 |
| CD4+CD8+ DPT | 2.115–12.473 | <0.001 | 5.136 |
The proportion of CD4+CD8+ DPT cells in the LN group was significantly higher than that in the NS group
To further determine the impact of CD4+CD8+ DPT cells on the development of LN, we compared the test results between the NS and LN groups (Table 3). As compared with the NS group, the levels of HDL-C, LDL-C, ApoA, ApoB, and Fib were significantly lower in the LN group in serum lipid and coagulative function detection. In liver and renal function biochemical examination, the level of A/G was higher in the LN group than that in the NS group, but the levels of hsCRP and MA were higher in the NS group instead. In lymphocyte subset examination, the proportion of CD3+CD4−CD8+ and CD3+CD4+CD8+ T cells was also significantly higher in the LN group than that in the NS group. The T4/T8 ratio was significantly reduced in the LN group. The positive rate of anti-Sm antibody and anti-dsDNA antibody were significantly higher in the LN group than in the NS group. There were no significant differences between the two groups in the levels of T-Cho, TG, Lp(a), BUN, SCr, Cys-C, CCR, UA, MA/UCr, D-dimer, hs-CRP, CD3+CD4+CD8−, and CD3+CD4−CD8− T cells.
Table 3. Demographic and clinical characteristics of patients in the NS and LN groups.
| Variables | NS (N=108) | LN (N=100) | t/χ2 value | P-value |
| Data are presented as mean±SD, except for the data on gender and anti-Sm antibody and anti-dsDNA antibody positive rate. NS: nephritic syndrome; LN: lupus nephritis; T-Cho: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a); ApoA: apolipoprotein A; ApoB: apolipoprotein B; A/G: albumin/globulin; BUN: blood urea nitrogen; SCr: serum creatinine; Cys-C: cystatin C; CCR: creatinine clearance rate; UA: uric acid; MA: microalbuminuria; MA/UCr: MA/urine creatinine; Fib: fibrinogen; hsCRP: high-sensitivity C-reactive protein; T4/T8: CD4+ to CD8+ T cells ratio. | ||||
| Male [n (%)] | 60 (55.56) | 13 (13.00) | 84.200 | <0.001 |
| Age (years) | 46.45±18.68 | 40.82±13.79 | −2.895 | 0.004 |
| T-Cho (mmol/L) | 7.21±3.04 | 5.07±1.55 | −0.631 | 0.529 |
| TG (mmol/L) | 2.48±1.62 | 1.96±0.96 | 1.336 | 0.183 |
| HDL-C (mmol/L) | 1.81±0.73 | 1.48±0.55 | −3.250 | 0.001 |
| LDL-C (mmol/L) | 4.18±2.20 | 2.79±1.13 | −4.82 | <0.001 |
| Lp(a) (mg/L) | 410.88±392.15 | 294.15±310.48 | −1.755 | 0.081 |
| ApoA (g/L) | 1.57±0.63 | 1.34±0.37 | −2.608 | 0.010 |
| ApoB (g/L) | 1.37±0.63 | 0.90±0.36 | −5.182 | <0.001 |
| A/G | 1.29±0.47 | 1.51±0.44 | 2.534 | 0.012 |
| BUN (mmol/L) | 8.91±6.20 | 10.16±8.48 | 1.220 | 0.224 |
| SCr (μmol/L) | 109.37±90.96 | 135.21±140.41 | 1.582 | 0.115 |
| Cys-C (mg/L) | 1.63±1.17 | 1.87±1.22 | 1.365 | 0.174 |
| CCR (mL/minute) | 66.95±26.19 | 64.85±46.88 | −0.389 | 0.698 |
| UA (μmol/L) | 408.08±122.77 | 402.37±162.85 | −0.285 | 0.776 |
| MA (mg/L) | 5212.46±7287.50 | 1819.57±3357.35 | −3.793 | <0.001 |
| MA/UCr (mg/g) | 3237.29±3670.86 | 2400.72±5912.72 | −1.138 | 0.257 |
| Fib (g/L) | 4.18±1.64 | 3.40±1.23 | −3.282 | 0.001 |
| D-dimer (mg/L) | 3.31±4.19 | 3.22±6.50 | −0.092 | 0.927 |
| hsCRP (mg/L) | 21.15±57.17 | 1.45±2.30 | −3.373 | 0.001 |
| Anti-Sm antibody positive [n (%)] | 1 (0.90) | 27 (27.00) | 27.046 | <0.001 |
| Anti-dsDNA antibody positive [n (%)] | 2 (1.90) | 31 (31.00) | 32.346 | <0.001 |
| CD3+CD4+CD8− (%) | 34.18±10.47 | 32.15±8.89 | −0.510 | 0.135 |
| CD3+CD4−CD8+ (%) | 27.58±10.31 | 37.00±10.50 | 6.525 | <0.001 |
| CD3+CD4+CD8+ (%) | 0.53±0.36 | 0.84±0.91 | 3.240 | 0.001 |
| CD3+CD4−CD8− (%) | 3.29±2.11 | 3.55±2.45 | 0.816 | 0.415 |
| T4/T8 | 1.52±1.03 | 0.99±0.60 | −4.446 | <0.001 |
The proportion of CD4+CD8+ DPT cells in the LN group was significantly higher than that in the nephritis group
To further determine the impact of CD4+CD8+ DPT cells on the development of LN, we compared the laboratory test results between the nephritis group and the LN group (Table 4). There was no significant difference in the proportions of age between these two groups. In serum lipid and coagulative function detection, there were no significant differences in levels of T-Cho, TG, HDL-C, LDL-C, Lp(a), ApoA, ApoB, A/G, Fib, D-dimer levels between the nephritis and LN groups. The level of BUN and Cys-C was higher in the LN group than that in the nephritis group, and the level of hsCRP was lower in the LN group than that in the nephritis group. The proportion of CD3+CD4−CD8+ T cells was significantly higher but the T4/T8 ratio was significantly reduced in the LN group, compared with that in the nephritis group. The positive rates of anti-Sm antibody and anti-dsDNA antibody were significantly higher in the LN group than those in the nephritis group. A high proportion (87%) of the patients in the LN group had CD4brightCD8dim T cells, and a small proportion (4%) of the patients in this group had CD4dimCD8bright T cells (Fig. 2). Meanwhile, CD4dimCD8bright T cells were found in groups of SLE-NRI, NS, and nephritis. There were no significant differences between the two groups in the levels of age, T-Cho, TG, HDL-C, LDL-C, Lp(a), ApoA, ApoB, A/G, CCR, UA, MA, MA/UCr, Fib, D-dimer, and CD3+CD4+CD8 T cells (Table 4).
Table 4. Demographic and clinical characteristics of patients in the nephritis and LN groups.
| Variables | Nephritis (N=108) | LN (N=100) | t/χ2 value | P-value |
| Data are presented as mean±SD, except for the data on gender and anti-Sm antibody and anti-dsDNA antibody positive rate. LN: lupus nephritis; T-Cho: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a); ApoA: apolipoprotein A; ApoB: apolipoprotein B; A/G: albumin/globulin; BUN: blood urea nitrogen; SCr: serum creatinine; Cys-C: cystatin C; CCR: creatinine clearance rate; UA: uric acid; MA: microalbuminuria; MA/UCr: MA/urine creatinine; Fib: fibrinogen; hsCRP: high-sensitivity C-reactive protein; T4/T8: CD4+ to CD8+ T cells ratio. | ||||
| Male [n (%)] | 70 (64.82) | 13 (13.00) | 92.582 | <0.001 |
| Age (years) | 40.85±16.46 | 40.82±13.79 | −0.377 | 0.707 |
| T-Cho (mmol/L) | 4.57±1.41 | 5.07±1.55 | 1.630 | 0.106 |
| TG (mmol/L) | 1.64±0.98 | 1.96±0.96 | 1.613 | 0.110 |
| HDL-C (mmol/L) | 1.31±0.39 | 1.48±0.55 | 1.603 | 0.112 |
| LDL-C (mmol/L) | 2.60±1.06 | 2.79±1.13 | 0.856 | 0.394 |
| Lp(a) (mg/L) | 171.11±136.54 | 294.15±310.48 | 1.834 | 0.070 |
| ApoA (g/L) | 1.29±0.32 | 1.34±0.37 | 0.557 | 0.579 |
| ApoB (g/L) | 0.87±0.37 | 0.90±0.36 | 0.430 | 0.668 |
| A/G | 1.57±0.49 | 1.51±0.44 | −0.668 | 0.505 |
| BUN (mmol/L) | 7.12±6.23 | 10.16±8.48 | 2.058 | 0.042 |
| SCr (μmol/L) | 123.38±139.95 | 135.21±140.41 | 0.446 | 0.657 |
| Cys-C (mg/L) | 1.28±0.61 | 1.87±1.22 | 2.763 | 0.007 |
| CCR (mL/minute) | 77.23±29.46 | 64.85±46.88 | −1.477 | 0.142 |
| UA (μmol/L) | 354.91±124.35 | 402.37±162.85 | 1.654 | 0.100 |
| MA (mg/L) | 1083.72±1485.24 | 1819.57±3357.35 | 1.208 | 0.230 |
| MA/UCr (mg/g) | 897.33±1537.22 | 2400.72±5912.72 | 1.437 | 0.154 |
| Fib (g/L) | 3.57±1.56 | 3.40±1.23 | −0.598 | 0.551 |
| D-dimer (mg/L) | 3.86±5.94 | 3.22±6.50 | −0.352 | 0.726 |
| hsCRP (mg/L) | 19.24±35.04 | 1.45±2.30 | −4.975 | <0.001 |
| Anti-Sm antibody positive [n (%)] | 3 (2.80) | 27 (27.00) | 30.841 | <0.001 |
| Anti-dsDNA antibody positive [n (%)] | 1 (0.90) | 31 (31.00) | 28.347 | <0.001 |
| CD3+CD4+CD8− (%) | 34.32±9.74 | 32.15±8.89 | −1.267 | 0.207 |
| CD3+CD4−CD8+ (%) | 26.02±8.13 | 37.00±10.50 | 5.934 | <0.001 |
| CD3+CD4+CD8+ (%) | 0.46±0.36 | 0.84±0.91 | 2.579 | 0.011 |
| CD3+CD4−CD8− (%) | 4.76±3.99 | 3.55±2.45 | −2.177 | 0.031 |
| T4/T8 | 1.53±0.88 | 0.99±0.60 | −4.137 | <0.001 |
Figure 2.
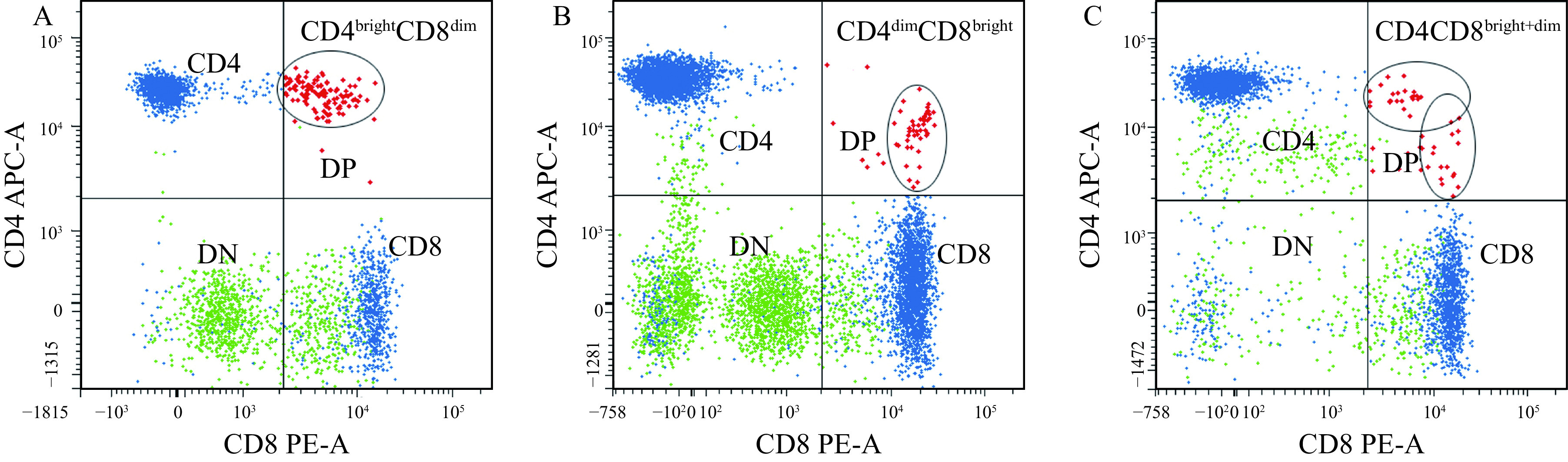
Flow cytometry maps of DPT cells with different fluorescence intensities.
These panels are representative flow cytometry maps of peripheral blood lymphocyte of the patients. A: Flow cytometry maps of CD4brightCD8dim. B: Flow cytometry maps of CD4dimCD8bright. C: Flow cytometry maps of CD4CD8bright+dim. Horizontal elliptic box represents CD4brightCD8dim. Vertical ellipse checkbox represents CD4dimCD8bright. DP: CD4+CD8+ double positive T lymphocytes; DN: CD4−CD8− double negative T lymphocytes.
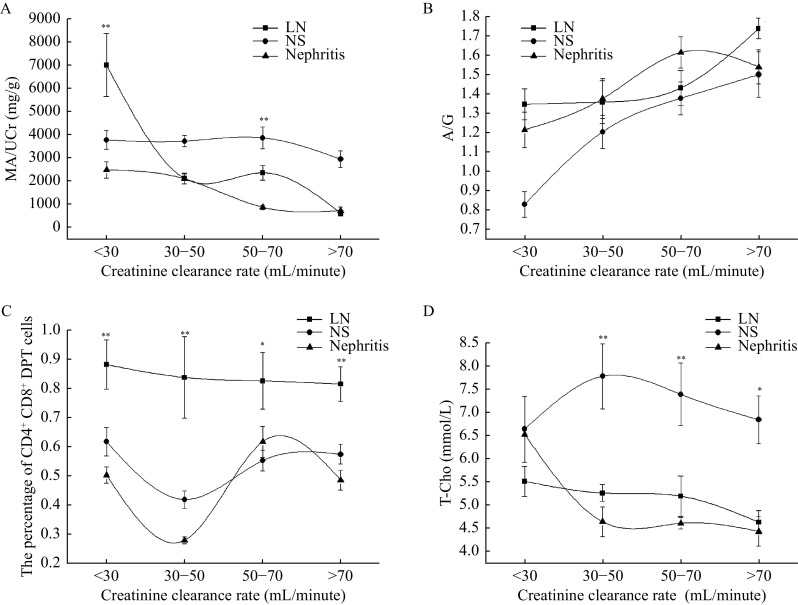
Biochemical and immunologic results in different CCR stages
To further illustrate the differences of the above-mentioned significant indexes in different renal impairment degree, the trend chart was plotted for the LN, NS, and nephritis groups in Fig. 3. We analyzed differences in individuals' biochemical and immunologic results including MA/UCr, A/G, CD3+CD4+CD8+ cells (DPT cells), and T-Cho in different CCR stages. The MA/UCr values increased as CCR decreased. When CCR >30 mL/minute, the MA/UCr values of NS group were significantly higher than those in the LN and nephritis groups (Fig. 3A). The A/G values decreased as CCR decreased, and there was no significant difference among the three groups (Fig. 3B). The proportion of DPT cells in LN group was significantly higher than that in NS and nephritis groups (Fig. 3C). In addition, the proportion of DPT cells increased as CCR decreased. In the NS group, the levels of T-Cho were not significantly different in different CCR stages, but T-Cho levels in the NS group were significantly higher than those in the LN and nephritis groups, when CCR values over 30 mL/minute (Fig. 3D). These results indicate that MA/UCr and A/G were highly dependent on renal impairment. In the LN group, the proportions of DPT cells increased in a creatinine clearance rate-dependent manner. When CCR value ranges from 30 to 70 mL/minute, the T-Cho concentration in the NS group was significantly higher than in the LN and nephritis groups. Besides, the degree of renal impairment was positively correlated with the concentration of T-Cho.
Figure 3.
The trend chart of MA/UCr, A/G, CD4+CD8+ DPT cells, and T-Cho among the LN, NS, and nephritis groups.
The trend chart of microalbuminuria to urine creatinine ratio (MA/UCr) (A), albumin to globulin ratio (A/G) (B), percentage of CD4+CD8+ DPT cells (C), and total cholesterol (T-Cho) (D) in patients with different creatinine clearance rate. The data were shown as mean±SD. Comparisons between the three groups were performed by Fisher's LSD test. *P<0.05; **P<0.01. LN: lupus nephritis; NS: nephritic syndrome.
Comparative analysis and distribution map of CD4+CD8+ DPT cells in three groups
There were significant differences between SLE-NRI and LN groups in the proportion of CD4+CD8+ DPT cells (t=4.012, P<0.001). Compared with the NS and nephritis groups, the proportions of DPT cells were significantly higher in the LN group (t=3.240, P=0.001; t=2.57, P=0.011, respectively) (Fig. 4). The CD4+CD8+ DTP proportion distribution width in the LN group was significantly greater than that in the SLE-NRI group, and the risk of renal impairment was significantly increased when the CD4+CD8+ DTP proportion >0.84%. The mean value of the NS and nephritis groups was slightly higher than that of the SLE, but it did not affect the predictive value of the DTP for the LN.
Figure 4.
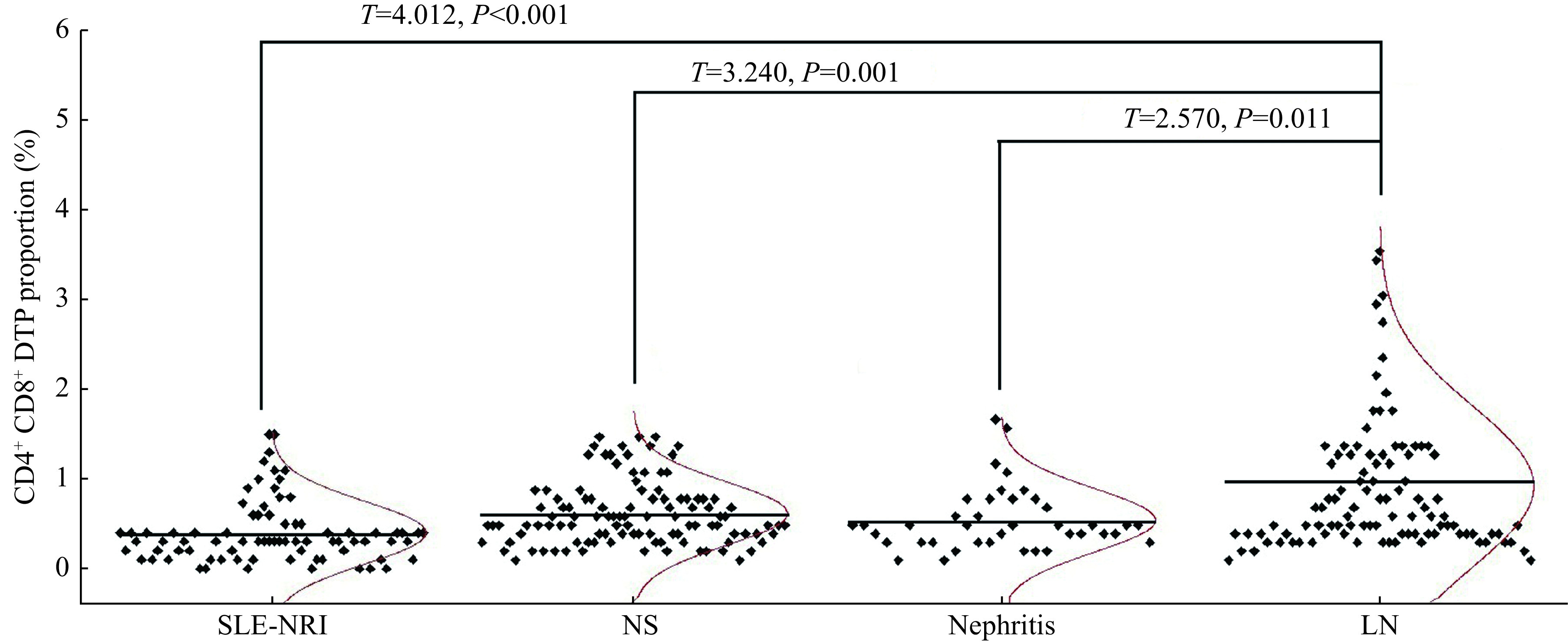
Comparative analysis and distribution map of CD4+CD8+ DPT in four groups.
The CD4+CD8+ DPT proportions in the groups of NS (N=108), nephritis (N=108), and LN (N=100) were compared with the SLE-NRI group (N=79), respectively. Comparisons between two groups were performed by Student's t-test. SLE-NRI: systemic lupus erythematosus without renal impairment; LN: lupus nephritis; NS: nephritic syndrome.
Discussion
SLE is an autoimmune disease with a worldwide distribution, always accompanied by the production of autoantibodies. LN remains a major cause of morbidity and mortality in patients with SLE[11]. Although the renal prognosis has been improved, major challenges remain in the management of disease progression and treatment of the disease due to the lack of early diagnosis method[12].
Immature T cells expressing both CD4 and CD8 were traditionally thought to be the ancestor of T lymphocytes and to undergo thymic development[13]. After T cells had transitioned to naive T cells and exited the thymus, the CD4 or CD8 could no longer be expressed. Research has shown that healthy humans exhibit low proportions of CD4+CD8+ DPT cells in peripheral blood[14]. At present, the origin and function of CD4+CD8+ DPT cells are not clear. However, several mainstream views hold that CD4+CD8+ DPT cells might trace back to immature CD4+CD8+ thymocytes, mature CD4+ single positive T cells, or mature CD8+ single positive T cells[15]. In the present study, we have found that an increase in the proportion of DPT cells is associated with renal impairment during SLE. The proportion of CD4+CD8+ DPT cells in the LN group is significantly higher than those in both NS and nephritis groups. So, when assessing the risk of renal impairment during SLE with CD4+CD8+ DPT cell proportion, we can effectively exclude the interference of NS and nephritis.
CD3+CD4−CD8+ T cells play important roles in innate and adaptive immune defense mechanisms protecting against both intrinsic and extrinsic factors, such as pathogens, viruses, and bacteria[16]. CD8+ T cells promote TNF-α, IL-6, and IFN-γ in responses to inflammation[17]. We observed that the CRP was significantly increased in both NS and nephritis groups (Table 3 and 4), which may be responsible for the increased CD3+CD4−CD8+T cell proportion. Since the difference in CRP was not significant in the LN group versus SLE group (Table 1), the CD3+CD4−CD8+ cells did not show a significant increases. As a subset of regulatory T cells, CD3+CD4−CD8− T cells have therapeutic value for autoimmune disease, depending on their regulatory effects on CD8+ T cells, CD4+ T cells, and B cells[18]. CD4+ T cells have a dual function in peripheral T cells. First, it interacts with its ligand in an antigen-independent manner to induce contact between T cells and MHCⅡ expressing cells. Second, CD4 interacts with pMHCⅡ-TCR in an antigen-dependent manner to deliver Lck kinase to the complex and thus enhance T cell sensitivity[19]. However, we did not observed any significant differences in CD3+CD4−CD8− cells and CD4+ T cells in all four groups.
In recent years, there has been more reported studies on DPT cells, and it has been found that DPT cells play an important role in the pathogenesis of autoimmune diseases and infectious diseases[20]. Wu et al have reported that the DPT cells play a key suppressive role in the production of autoantibodies in SLE[21]. In infectious diseases, DPT cells are a group of cells with a rapid response during acute HIV infection, which is different from the conventional T cell compartments[22].
In our study, CD4brightCD8dim was found to be the most common in patients with LN, and CD4dimCD8bright was extremely rare. However, CD4dimCD8bright was found in groups of SLE-NRI, NS, and nephritis. These results suggest that CD4brightCD8dim DPT cells may be a potential biomarker in the development of renal impairment in SLE. Nevertheless, specific reasons and mechanisms need to be further studied.
The serum level of anti-Sm antibody is significantly correlated with the SLE disease activity index[23]. Monitoring of anti-Sm antibody titer may help assess the disease activity in SLE[24]. Clinical studies have found that anti-dsDNA antibody exhibits a significant correlation with SLE disease activity and multiple clinical manifestations[25]. However, the immunogenicity of dsDNA depends upon its context[26]. Therefore, the anti-dsDNA and anti-Sm antibodies do not directly reflect the SLE disease activity.
In conclusion, the significant finding of an association between LN and the proportion of DPT cells suggests that the proportion of DPT cells is related to LN susceptibility in SLE patients. A high proportion of DPT cells is an independent risk factor for LN, and the risk of LN is 5.136 times higher than the normal proportion in SLE patients. Therefore, the proportion of DPT cells is a potential marker to evaluate LN susceptibility in SLE patients. Furthermore, the deteriorative degree of nephropathy becomes more obvious as the proportion of DPT cells raised. On the basis of these results, we speculate that the occurence of DPT cells are a rapid response to the production of autoantibodies in SLE. Therefore, the increased proportion of DPT cells reflects the increase of autoantibodies, the severity of disease, and the destruction of renal tissue caused by immune complex.
Acknowledgments
None.
Funding Statement
This work was supported by the Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC1415), the Special Project of Sichuan Province Traditional Chinese Medicine Administration (Grant No. 2020JC0124), the Management Project of General Hospital of Western Theater Command (Grants No. 2021-XZYG-C22 and 2021-XZYG-C21), and the Spark Young Innovative Talent Project of General Hospital of Western Theater Command.
Footnotes
CLC number: R593.242, Ducument code: A
The authors reported no conflict of interests.
Contributor Information
Yanyan Wang, Email: wangyanyanmail@126.com.
Zhongyong Jiang, Email: jiangzhongyongmail@126.com.
References
- 1.Yu F, Haas M, Glassock R, et al Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13(8):483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 2.Delarche A, Lumbroso C, Fournier A, et al Incidence and outcome of lupus nephritis in French Polynesia. Clin Nephrol. 2018;89:41–49. doi: 10.5414/CN109194. [DOI] [PubMed] [Google Scholar]
- 3.Feng X, Pan W, Liu L, et al Prognosis for hospitalized patients with systemic lupus erythematosus in China: 5-year update of the Jiangsu cohort. PLoS One. 2016;11(12):e0168619. doi: 10.1371/journal.pone.0168619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Shen H, Bai B, et al Increased CD4+CD8+ double-positive T Cell in patients with primary Sjögren's syndrome correlated with disease activity. J Immunol Res. 2021:6658324. doi: 10.1155/2021/6658324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero YT, Suárez VM, Abraham CMM, et al Immunophenotypic characterization of double positive T lymphocytes in Cuban older adults. Exp Gerontol. 2021;152:111450. doi: 10.1016/j.exger.2021.111450. [DOI] [PubMed] [Google Scholar]
- 6.Rahemtullah A, Harris NL, Dorn ME, et al Beyond the lymphocyte predominant cell: CD4+CD8+ T-cells in nodular lymphocyte predominant Hodgkin lymphoma. Leuk Lymphoma. 2008;49(10):1870–1878. doi: 10.1080/10428190802308728. [DOI] [PubMed] [Google Scholar]
- 7.Aringer M, Costenbader K, Daikh D, et al 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanouriakis A, Tziolos N, Bertsias G, et al Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14–25. doi: 10.1136/annrheumdis-2020-218272. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Z, Liu X, Chang K, et al Allyl isothiocyanate inhibits the proliferation of renal carcinoma cell line GRC-1 by inducing an imbalance between Bcl2 and Bax. Med Sci Monit. 2016;22:4283–4288. doi: 10.12659/MSM.897315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K, Jiang Z, Liu C, et al The effects of CYP2C19 genotype on the susceptibility for nephrosis in cardio-cerebral vascular disease treated by anticoagulation. Medicine. 2016;95(38):e4954. doi: 10.1097/MD.0000000000004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Gong Y, Ren H, et al The prevalence, subtypes and associated factors of hyperuricemia in lupus nephritis patients at chronic kidney disease stages 1–3. Oncotarget. 2017;8(34):57099–57108. doi: 10.18632/oncotarget.19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameh OI, Kengne AP, Jayne D, et al Standard of treatment and outcomes of adults with lupus nephritis in Africa: a systematic review. Lupus. 2016;25(11):1269–1277. doi: 10.1177/0961203316640915. [DOI] [PubMed] [Google Scholar]
- 13.Pahar B, Lackner AA, Veazey RS Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36(3):583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 14.Parel Y, Chizzolini C CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev. 2004;3(3):215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Dashtsoodol N, Shigeura T, Aihara M, et al Alternative pathway for the development of Vα14+ NKT cells directly from CD4-CD8- thymocytes that bypasses the CD4+CD8+ stage. Nat Immunol. 2017;18(3):274–282. doi: 10.1038/ni.3668. [DOI] [PubMed] [Google Scholar]
- 16.Schäfer S, Zernecke A CD8+ T cells in atherosclerosis. Cells. 2020;10(1):37. doi: 10.3390/cells10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd El-Kader SM, Al-Shreef FM Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr Health Sci. 2018;18(1):120–131. doi: 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Zheng Y, Sheng J, et al CD3+CD4-CD8- (double-negative) T cells in inflammation, immune disorders and cancer. Front Immunol. 2022;13:816005. doi: 10.3389/fimmu.2022.816005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glatzová D, Cebecauer M Dual role of CD4 in peripheral T lymphocytes. Front Immunol. 2019;10:618. doi: 10.3389/fimmu.2019.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Antón E, Egui A, Thomas MC, et al Impact of benznidazole treatment on the functional response of Trypanosoma cruzi antigen-specific CD4+CD8+ T cells in chronic Chagas disease patients. PLoS Negl Trop Dis. 2018;12(5):e0006480. doi: 10.1371/journal.pntd.0006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Cai B, Feng W, et al Double positive CD4+CD8+ T cells: key suppressive role in the production of autoantibodies in systemic lupus erythematosus. https://pubmed.ncbi.nlm.nih.gov/25488445/ Indian J Med Res. 2014;140(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- 22.Frahm MA, Picking RA, Kuruc JD, et al CD4+CD8+ T cells represent a significant portion of the anti-HIV T cell response to acute HIV infection. J Immunol. 2012;188(9):4289–4296. doi: 10.4049/jimmunol.1103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe JY, Lee SS, Kwak SG, et al Anti-Sm antibody, damage index, and corticosteroid use are associated with cardiac involvement in systemic lupus erythematosus: data from a prospective registry study. J Korean Med Sci. 2020;35(21):e139. doi: 10.3346/jkms.2020.35.e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn SS, Jung SM, Yoo J, et al Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol Int. 2019;39(11):1937–1944. doi: 10.1007/s00296-019-04445-y. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Xia Y Anti-double Stranded DNA Antibodies: Origin, Pathogenicity, and Targeted Therapies. Front Immunol. 2019;10:1667. doi: 10.3389/fimmu.2019.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rekvig OP Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clin Exp Immunol. 2015;179(1):5–10. doi: 10.1111/cei.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]