Abstract
Objective:
To determine whether a user-controlled sperm concentration test compared to standard semen analysis can effectively monitor spermatogenesis suppression for male contraception.
Design:
Single center, prospective sub-study of the ongoing clinical trial: “Study of Daily Application of Nestorone® and Testosterone Combination Gel for Male Contraception.”
Setting:
Research institute at an academic medical center.
Participants:
Couples participating in the male contraceptive clinical trial.
Interventions:
None
Main Outcome Measure:
The ability by participants to monitor sperm suppression to a threshold compatible with contraceptive efficacy utilizing a user-controlled test verified by sperm concentration determined by standard laboratory methods.
Results:
Thirty-eight men participating in a hormonal male contraceptive clinical trial provided multiple samples during spermatogenesis suppression for this sub-study. Participants, employing a user-controlled test, correctly identified the absence of sperm (a negative test) in 100% of their laboratory-confirmed azoospermic samples (n= 122). Participants also identified 100% of samples (n=73) with sperm >0.2 million/ml as positive. Sperm counts between 0.01 and 0.2 million/ml were identified as negative in 96% of samples. Trial participants noted the overall ease of using the test with respect to sample preparation, test timing, and result interpretation, and that they could accurately use this test at home without difficulty.
Conclusion:
Participants undergoing spermatogenesis suppression in a hormonal male contraceptive trial performed user-controlled test to determine whether their semen sperm concentration was ≤ or >0.2 million sperm/ml. Compared to standard semen analyses, participants correctly identified 100% of samples with sperm counts >0.2 million/ml as positive (Sensitivity 100%). A positive result when the couple is using a male contraceptive method triggers the need for semen analysis by a laboratory while the couple uses another method of contraception. Participants correctly diagnosed samples ≤0.2 million sperm/ml as negative in 99% of samples (Specificity 99%). A negative result indicates a sperm concentration ≤0.2 million/ml, well below the threshold of ≤1 million/ml offering contraceptive efficacy demonstrated by prior studies. At-home sperm concentration test would minimize the need for users to return to the clinic to monitor suppression of spermatogenesis, decreasing cost and burden of male contraception trials and increasing practicality of the method.
(Clinical Trial registration: NCT: 03452111)
Keywords: Male contraception, hormonal male contraception, spermatogenesis suppression, at-home sperm concentration test
Capsule
User-controlled tests identify severe oligozoospermia or azoospermia associated with contraceptive efficacy, minimize need for clinic visits to monitor spermatogenesis suppression, reducing costs and burdens in male contraceptive development and increasing practicality of developed male methods.
Introduction
Despite available methods of contraception, the rate of unintended pregnancy remains at 45%. Men and women express willingness to add use of male contraceptive methods if products are developed. After decades of research, a novel transdermal hormonal gel has reached late phase clinical development with high likelihood of progressing to a commercial product for effective, reversible male contraception (1). Additional products in the pipeline for male contraception (both hormonal and non-hormonal) are at earlier stages of development (2). Among the developmental challenges is the need for large clinical trials with frequent monitoring to assess sperm suppression, maintenance during efficacy, and return to normal sperm production in recovery. A validated tool that would assist with these assessments will reduce cost and accelerate male contraceptive development.
In the hormonal approach, sperm suppression is achieved by administering an exogenous progestin, which causes negative feedback on the hypothalamic-pituitary-testicular axis and reduces the intratesticular testosterone (T) concentrations (2–4) below the amount needed to support spermatogenesis (5–7). Exogenous T is administered to maintain serum T levels in the adult male reference range to support sexual function, secondary sexual characteristics, and androgen-dependent effects in non-gonadal tissues but is insufficient to support spermatogenesis (8). The suppressive effects are reversible upon treatment cessation (9).
A transdermal gel containing a progestin (Nestorone, NES) and T is currently undergoing evaluation for safety and contraceptive efficacy in a Phase IIb clinical trial in couples at risk of pregnancy. We are determining pregnancy rates when the sperm concentration is suppressed to ≤ 1 million/ml, at which point the couple begins to use the experimental gel as the sole method of contraception. Prior efficacy trials of hormonal male contraceptive prototypes in couples have demonstrated that suppression of sperm concentration to ≤ 1 million/ml (independent of motility) is sufficient to prevent pregnancy in the female partner at rates equal to or better than typical use failure rates of pills approved for female contraception (10–17). Our prior study showed that at week 16 to 20 after application of NES/T most men (> 80%) would have a sperm concentration (0.2 million/ml)(18). In prior studies sperm motility was markedly suppressed when concentration was ≤1.0 million/ml rendering motility assessment unnecessary (18–20).
Currently, the gold standard method to measure sperm concentration requires counting spermatozoa in a hemocytometer under the microscope performed by a trained technologist (21, 22). In our Phase IIb efficacy trial, participants provide ~24 samples throughout screening, suppression, efficacy, and recovery periods (approximately 2 years). For a trial recruiting subjects with a goal to have 200 couples complete a year of efficacy, the estimated number of sperm counts is ~7200 (including screen failures and subjects who discontinue early). Pivotal Phase III trials may require numbers equivalent to the 20,000 cycles required for a new drug for female contraception; thus, subject recruitment would be 4 to 5 fold higher than is needed for Phase II. Large-scale efficacy trials for current or future male contraceptive agents that function via suppressing spermatogenesis will benefit from a low cost, practical method of monitoring sperm concentration, reducing cost of studies and burden on the participants. To this end, the ongoing hormonal male contraceptive efficacy of daily application NES/T gel study includes an approved sub-study to determine if a user-controlled test performed by participants would provide accurate evidence of spermatogenesis suppression, confirming that a man has or has not reached a threshold that is compatible with contraceptive effectiveness. Sperm concentration is verified from the same sample by a laboratory technologist using the standardized method to count the number of spermatozoa with a hemocytometer under a microscope (21). We also describe preliminary data on participants’ attitudes and assessments of self-testing using an at-home test for hormonal male contraceptive monitoring of spermatogenesis suppression.
Participants and Methods
Study participants
The ongoing Phase IIb efficacy trial: “Study of Daily Application of Nestorone® (NES) and Testosterone (T) Combination Gel for Male Contraception” (www.clinicaltrials.gov, NCT 03452111) is being conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in collaboration with the Population Council. The main study is ongoing in the NICHD Contraceptive Clinical Trials Network (CCTN) and includes multiple clinical sites in the United States and international sites. A substudy was added to the main study protocol (Version 8) with the goal to investigate the ability by participants to identify if their sperm count is below a threshold (e.g., <1 million/ml) required for effective contraception utilizing a user-controlled at-home test, compared to sperm concentration determined by standard laboratory methods. Couples that met the eligibility criteria of the main study at one clinical site, the Lundquist Institute at Harbor-UCLA Medical Center, were invited to participate in this sub-study. Actual sperm counts were confirmed by the technician-dependent standard semen analysis. In the main study, couples use another method of contraception during a suppression phase and enter an efficacy phase when two consecutive sperm concentration reached ≤ 1 million/ml, after which the couple uses the combined NES/ T gel as their only method of contraception. The purpose of the sub-study was explained to both the male and female partners after they passed screening and were enrolled in the main clinical trial. The Sub-study described in the main study (Version8) was approved by the Central Institutional Review Board (Advarra Integrated Solutions) as well as the local IRB at The Lundquist Institute. The male partner gave written, informed consent for participation in the sub-study using a consent form specific for the sub-study.
Study Design
Hormonal male contraception is highly effective when sperm concentrations were suppressed to <1 million sperm/ml. The main clinical trial protocol for semen analysis followed the WHO laboratory manual for the examination and processing of human semen, 5th Edition (21). In this sub-study, we also used a commercially available user-controlled test (SpermCheck-V) (23). The test information claimed that the test can detect sperm concentration >0.25 million/ml, giving an effectiveness margin between 0.25 to 1 million sperm/ml. This user-controlled test for sperm concentration has not been validated for monitoring suppression of spermatogenesis in male contraceptive studies. The SpermCheck-V results were not used as a suppression parameter in the ongoing clinical study. Sub-study participants provided semen samples according to the main clinical trial protocol and processed a small portion of their own semen sample using the SpermCheck-V test under observation of the laboratory technologist. The same sample was analyzed by standard methodology of assessing sperm concentration by a trained technologist but the results were not made available to participants. Participants used SpermCheck-V tests at the following time points when possible: (1) start of treatment to provide a positive control; (2) during the suppression phase after their sperm concentration decreased to <5 million/ml, (usually about 4 to 8 weeks after starting gel application; 3) entry into the efficacy phase (as determined by two semen samples with a sperm concentration of ≤1 million/ml by hemocytometer); and (4) at 4 or 8 weeks after entry into the efficacy phase. Efforts focused on analyzing samples from all participants enrolled in the sub-study with sperm concentrations in the critical range of 0.01 million/ml to ~ 1 million/ml and results were verified by standard method of assessing sperm concentration by a technologist.
SpermCheck-V Test
SpermCheck-V is a sensitive lateral flow immune-chromatographic device that detects a sperm specific acrosome protein SP-10 (23–26). The SpermCheck-V test was designed and optimized for a threshold of detection of ≥0.25 million sperm/ml. It was validated in 144 post-vasectomy samples with a sensitivity of 93% and a specificity of 97% (23). Two batches of the commercially available SpermCheck-V Kits were purchased from vendors or directly from the manufacturer, and a third batch was a gift from the manufacturer (DNA Diagnostics Center, Fairfield, OH). While we were unable to study batch variations in our laboratory because batches were purchased sequentially for the sub-study, we requested the manufacturer to re-test batch variability. The manufacturer’s test report showed that all three batches were comparable and detected a positive result for sperm concentration between 0.25 and 0.5 million /ml. In addition, batch quality control was measured by the density of the test line (DXpress Digital Reader, LifeSign) using known sperm concentrations verified by the gold standard using microscope counting. At a concentration of 0.25 million/ml and 0.4 million/ml, the coefficients of variation were 13.48 and 12.15% respectively (five samples were used with each batch).
Study participants (n=38) performed SpermCheck-V on 217 samples according to the manufacturer’s instructions (https://www.spermcheck.com/wp-content/uploads/2015/12/P-5192-B_SCV2_MPS-2013-12-06.pdf). Participants performed the test after semen liquefaction (usually 30 to 60 minutes at room temperature and within 3 hours after ejaculation). The sperm transfer device provided in the kit was filled with semen to the frosted line and the sample was added to the SpermCheck-V Solution bottle and mixed gently. After two minutes, five drops of the mixture were added to sample well in the cartridge and the result was read at 7 minutes (23). Participants photographed the SpermCheck-V cartridge test results using an iPhone at 7 minutes and photographs were stored in REDCap® database (https://www.project-redcap.org/). The male participant interpreted the test results: positive results exhibited both control and test lines that were visible on SpermCheck-V device (Fig. 1 A, B, and C). If a control line appeared, but the test line was invisible, this was a negative result indicating the sperm concentration was ≤0.25 million/ml according to manufacturer claims (Fig. 1 D and E). If a control line was not visible, the test was invalid thus requiring a repeat test.
Figure 1.
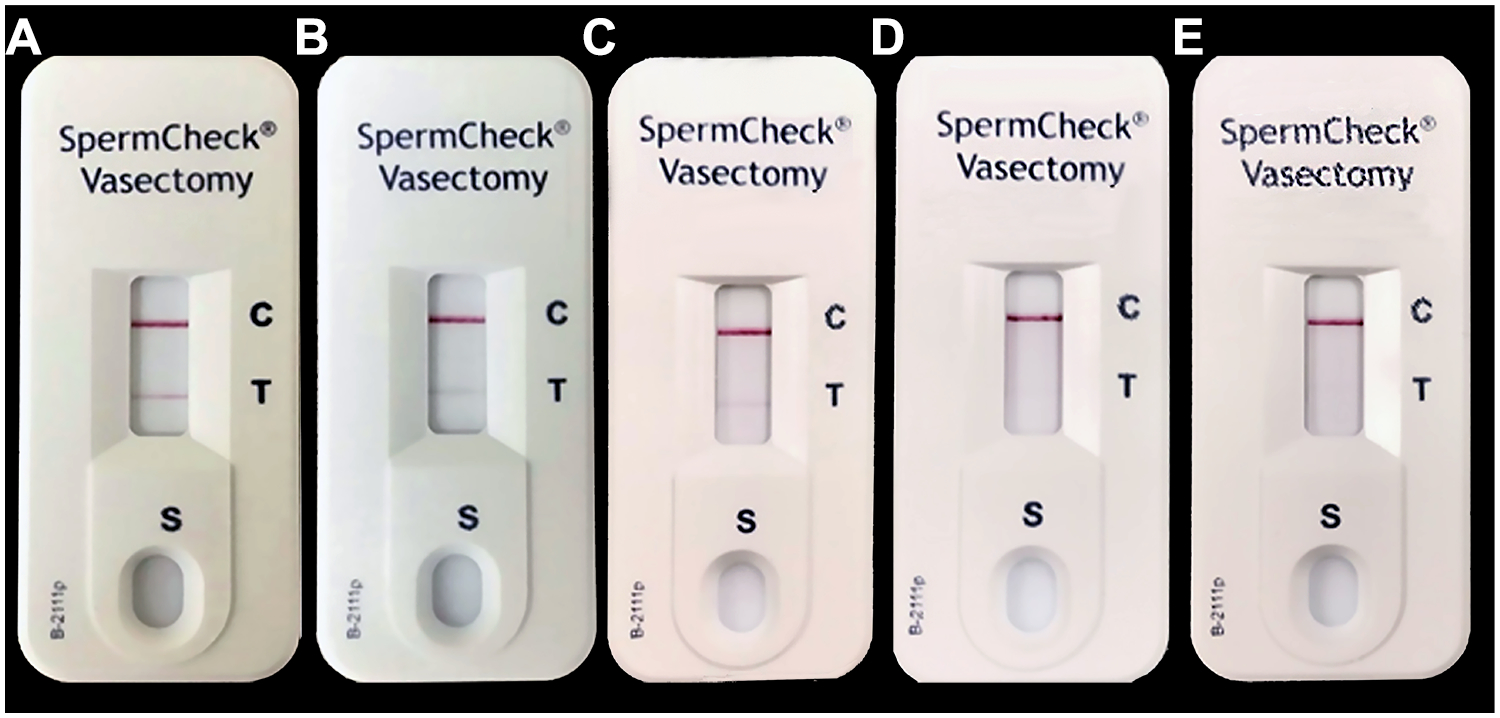
SpermCheck-V test results in representative semen samples from trial participants in sperm suppression phase (sperm concentrations <5 million/ml). The upper positive control line (C) indicates all SpermCheck-V test procedures were performed correctly. The lower positive test line (T) indicates sperm concentrations were above the threshold (>0.2 million/ml) in A (4.8 million/ml), B (1.13 million/ml), and C (0.23 million/ml. There is no line (negative result) visible when sperm concentrations were 0.18 million/ml in D and zero (azoospermia) in E. SpermCheck-V test results were read and photographs were taken at 7 minutes after loading samples into test cartridge.
Sperm concentration by hemocytometer method
Sperm concentration was determined in the Andrology laboratory at The Lundquist Institute by technologists following the methods detailed in the “WHO laboratory manual for the examination and processing of human semen” without modifications (21) (https://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf?sequence=1&isAllowed=y, pages 32 to 52). Semen samples were diluted with sperm immobilizing solution, two aliquots were loaded into each chamber of the improved Neubauer hemocytometer, the number of sperm were assessed in the center grid of both chambers under 20X phase microscopy until 200 sperm were counted, and the average number of sperm in both chambers were used provided the difference between the two chamber is <15%. If the sperm concentration was low, all nine grids of the hemocytometer were assessed until 200 cells were counted taking at least 30 minutes per chamber. If there are <25 spermatozoa counted in all nine grids of the hemocytometer chamber, the concentration is <56,000 sperm per ml. This is the lower limit of quantification using a hemocytometer for an acceptable sampling error of ± 20% (21, 27).
Acceptability survey
Upon completion of their last SpermCheck-V test, 20 trial participants completed a 10-item survey. The survey consisted of nine Likert-type queries on the user’s perceived ease or difficulty understanding how to perform, read, and interpret the SpermCheck-V test results; how confident they were in interpreting the test at varying sperm concentration levels; and how satisfied they were with using the test kit. The last item asked if the participant would prefer to verify sperm suppression via an at-home test kit like SpermCheck-V versus conventional lab-based semen analysis (see Survey in Supplemental text).
Study Size and Statistical analysis
We computed the total number of semen samples required based on the precision in estimating the confidence interval for the sensitivity or specificity. Two hundred semen samples provide a margin of 6 to 10% error, with a sensitivity or specificity set at 90% and an assumed prevalence of 70% of the sperm concentration assessed would be ≤0.25 during spermatogenesis suppression based on a prior study of NES/T gel in heathy men (18), accounting for a potential within-participant clustering effect. The accuracy of SpermCheck-V was calculated using sensitivity and specificity, along with 95% confidence intervals (CI). The within-participant clustering effect was considered in the variance estimation when computing the confidence interval (28, 29). We additionally evaluated the impact of reducing the threshold value to 0.20 million/ml as a stricter threshold for contraceptive efficacy to determine whether this will provide better specificity and sensitivity. Lowering the threshold to 0.2 million/ml will further reduce the chance of a negative test when sperm concentrations were not suppressed to a level equivalent to contraceptive efficacy. Positive and negative predictive values, and accuracy, were computed using the prevalence of our sample (32% for test result >0.25 million/ml, 33% for test result >0.20 million/ml). All data analyses were performed using SAS 9.4 (Cary, NC: SAS Institute Inc).
Results
Participants assessment of sperm concentration suppression using the user-controlled test
Thirty-eight couples participated in this sub-study and each male participant provided two to ten samples. Participants performed sperm concentration assessments using SpermCheck-V in 217 samples. Participants identified 144 samples as negative. They identified 73 samples as positive. Technologist analysis confirmed that 98.6% of the positive samples were >0.2 million/ml. One sample with a laboratory derived concentration of 0.04 million/ml produced a very faint line (Table 1). Thus, using a sperm threshold of ≤0.2 million/ml, participants identified 100% of samples in which sperm counts were >0.2 million/ml as positive. A negative result indicated a sperm level that was <0.2 million/ml which is compatible with high contraceptive efficacy. Based on these results, a subject who obtained a negative result would have confidence that sperm were suppressed to an effective contraceptive level (<0.2 million/ml).
Table 1.
Sperm concentration distribution and participant assessment by SpermCheck-V (SC) with threshold positive as 0.2 million/ml (from 38 participants)
| Sperm concentration (million/ml) | Number of samples | Participant identified as negative | Participant Identified as positive |
|---|---|---|---|
| 0 | 122 | 122 | 0 |
| 0.1 to <0.2 | 23 | 22 | 1 |
| >0.2 to 1 | 21 | 0 | 21 |
| >1 | 51 | 0 | 51 |
| Total | 217 | 144 | 73 |
User-controlled sperm concentration threshold were verified by sperm concentration assessed by hemocytometer
Positive test lines were consistently absent (negative test result) in all 122 azoospermic samples and in 22 of 23 severely oligozoospermic samples (sperm concentrations >0.01 and ≤0.2 million/ml) (Fig. 1). For samples with confirmed sperm concentration between 0.01 and 0.25 million/ml, one sample showed a very faint positive test line at a sperm concentration of 0.04 million/ml.
The manufacturer indicated a threshold of 0.25 million/ml. We found that the two samples that fell between 0.2 and 0.25 million/ml (0.21, and 0.23 million/ml by quantitative analysis) showed a faint positive line. Of note, the acceptable counting error using the hemocytometer at such low concentrations is ± 20% (21, 22). The diagnostic accuracy of SpermCheck-V in our laboratory according to manufacturer’s recommendation for distinguishing sperm >0.25 million/ml was 98.4% (95% CI: 95.4%, 99.7%), the sensitivity was 100% (95% CI: 94.8%, 100%) and the specificity was 97.3% (95% CI: 94.9%, 99.9%). The Positive Predictive Value (PPV) was 94.5% (95% CI: 86.8%, 97.8%), and the Negative Predictive was 100% (Table 2, left clear panel).
Table 2.
Verification of SpermCheck-V results versus hemocytometer-derived sperm concentration using a threshold of positive at 0.25 Versus 0.2 million/ml
| Number of samples | Hemocytometer-derived Sperm Concentration (Gold Standard) | ||||
|---|---|---|---|---|---|
| SpermCheck-V Results | Sperm concentration >0.25 | Sperm concentration ≤ 0.25 | Sperm concentration >0.20 | Sperm concentration ≤0.20 | |
| Positive | 73 | 69 | 4 | 72 | 1 |
| Negative | 144 | 0 | 144 | 0 | 144 |
| Total | 217 | 69 | 148 | 72 | 145 |
| Diagnostic accuracy | Value | 95% CI | Value | 95% CI | |
| Sensitivity | 100% | 0.948 to 1.000 | 100% | 0.95 to 1.000 | |
| Specificity | 97.3% | 0.932 to 0.993 | 99.3% | 0.962 to 0.999 | |
| Accuracy | 98.2% | 0.954 to 0.995 | 99.5% | 0.975 to 0.999 | |
In view of our detection of a positive result with the two samples that fell between 0.2 and 0.25 million/ml, it appears that the actual threshold for a positive result is >0.2 million/ml. Using this lower threshold of 0.2 million/ml to define a positive, the accuracy improved to 99.5 % (95% CI:97.5%, 99.9%), the Sensitivity was 100% (95% CI: 95.0%, 100%), and the Specificity was 99.3% (95% CI: 97.9%, 100%). The Positive Predictive Value (PPV) was 98.6% (95% CI: 91.08%, 99.8%), and the Negative Predictive was 100% (Table 2, Right grey panel). It should be noted the that 95% CI for the 0.2 million/ml threshold overlapped with the 95% CI of the 0.25 million/ml threshold for sperm concentration. These results suggest that participants in this hormonal male contraceptive trial with NES/T gel can use this test at home to monitor their spermatogenesis suppression to a threshold near azoospermia (≤ 0.2 million/ml) with 99.3% specificity and 100% sensitivity. SpermCheck-V test was as effective as using the hemocytometer by a technician in a laboratory for the purposes of determining adequate spermatogenesis suppression for male contraception to allow the participants to enter the efficacy phase where the couple used NES/T gel daily application by the male partner as the sole method of contraception (Supplemental Figure 1).
Acceptability Survey
Preliminary data from this pilot study of twenty participants who performed the SpermCheck-V using their own semen samples completed the acceptability survey (Table 3). With respect to ease-of-use of the SpermCheck-V, more than 90% of respondents reported that it was easy or very easy to understand how to use the test. None of the respondents reported not being confident about interpreting results at any sperm concentration. Nearly all respondents reported satisfaction with the test and 80% would prefer an at-home test like SpermCheck-V to a clinic visit with laboratory-based semen analysis. These data will be verified in future larger scale study.
Table 3:
SpermCheck-V acceptability survey results (N=20)
| Difficult or very difficult n (%) | Neither easy nor difficult n (%) | Easy or very easy n (%) | |
|---|---|---|---|
| Understanding how to use test | 0 (0) | 0 (0) | 20 (100) |
| Preparing semen sample | 0 (0) | 0 (0) | 20 (100) |
| Following timing of test | 0 (0) | 0 (0) | 20 (100) |
| Interpreting test results | 0 (0) | 1 (5.0) | 19 (95.0) |
| Not confident | Neither confident nor unconfident | Confident | |
| Interpreting test with NORMAL sperm count | 0 (0) | 1 (5.0) | 19 (95.0) |
| Interpreting test with VERY LITTLE sperm | 0 (0) | 1 (5.0) | 19 (95.0) |
| Interpreting test with NO sperm | 0 (0) | 2 (10.0) | 18 (90.0) |
| Unlikely or very unlikely | Neither likely nor unlikely | Likely or very likely | |
| Likelihood of abstaining or using another contraceptive if they had VERY LITTLE sperm | 7 (35.0) | 3 (15.0) | 10 (50.0) |
| Unsatisfied or very unsatisfied | Neither satisfied nor unsatisfied | Satisfied or very satisfied | |
| Satisfaction with at-home test | 0 (0) | 1 (5.0) | 19 (95.0) |
| At-home test kit | Professional semen analysis | ||
| “When using a hormonal male contraceptive method, how would you prefer to have your semen tested to make sure that you could not get a woman pregnant?” | 15 (79.0) | 4 (21.1) |
Discussion
In male contraceptive development where the hormonal or non-hormonal entity acts by suppression of spermatogenesis, monitoring of sperm concentration is critical for assessment of the success of the method. Our results indicate that participants can successfully use a home test to distinguish between sperm counts above or below a threshold of 0.2 million/ml. All participants with a negative SpermCheck-V result had a sperm count that was well below the level that provides effective contraception (< 1 million/ml) based on clinical contraceptive efficacy studies (12–15). Only one sample with a confirmed sperm count between 0.01–0.2 million/ml produced a faint positive result. This hyper-sensitive low sperm result would cause the subject to either get a confirmatory quantitative count or repeat the test later but would not inaccurately place the couple at risk of pregnancy. Participants correctly identified 100% of instances in which sperm concentrations were higher than 0.2 million/ml. In male hormonal contraception, a positive result may still be compatible with effective contraception if the actual sperm count is below 1 million/ml. However, in a case where the participant has reached azoospermia or very severe oligospermia as confirmed by a negative test result, obtaining a subsequent positive test result would alert the participant to the possibility that spermatogenesis has escaped suppression, and it may be prudent for the male to obtain a quantitative count to see if the level exceeds 1 million/ml. This warning would allow the couple to use a back-up method until confirmation of the count could be obtained or until a subsequent home test was again negative.
In large phase 2 and phase 3 male contraceptive studies, semen analysis requires the male participant to come to the clinic site. The semen analysis is performed by a trained laboratory technician in a laboratory equipped with hemocytometers and a phase contrast microscope. This technological requirement will severely restrict the ability of many centers to participate in male contraceptive efficacy studies especially in under-resourced countries. The availability of a validated user-controlled, at-home test with the ability to detect sperm threshold below 1 million/ml (such as SpermCheck-V) will increase convenience for participants, reduce costs for study sponsors, and enhance the ability to recruit centers for phase 3 pivotal studies needed for regulatory approval and marketing of the product. Once a method is approved, a validated user-controlled test would allow couples to assess the suppression of spermatogenesis to an effective level of protection against pregnancy during actual use of an approved product.
Determination of low sperm concentration ≤1 million/ml is a labor-intensive procedure that requires counting all nine grids on both chambers of the hemocytometer under a microscope by a trained technologist (21). The method is costly, cumbersome, and dependent on availability of trained technologists in an andrology laboratory when using this standard method for assessing sperm concentration. Our results show that SpermCheck-V could be used to monitor sperm suppression to the contraceptive threshold of ≤0.2 million/ml. Once a negative result is obtained, the couple can rely on male contraception as the sole method (15, 18–20). We predict that efficacy would be high because the threshold of ≤0.2 million/ml is well under ≤1 million/ml that has been shown to be effective for male contraception. Importantly, in this study, we used a mobile phone to take pictures of SpermCheck-V device at 7 minutes as a record indicative of a positive or negative result. In future hormonal male contraceptive clinical trials, the user can send the SpermCheck-V picture through a mobile phone to investigators/laboratory for evaluation and tracking and a central repository for storage of the in-home test results. We speculate that the density of the test line may be quantified using mobile phone-based image analysis software in the future (30).
The American Urological Association (AUA), the British Society of Urological Surgeons, and British Andrology Society vasectomy guidelines state that vasectomy is successful if ≤0.1 million non-motile sperm/ml are present in post-vasectomy semen samples (31, 32). After allowing time for sperm clearance from the reproductive tract, pregnancy risk is very low after a single semen sample confirming azoospermia, only non-motile sperm, or ≤0.1 million/ml non-motile sperm after vasectomy in retrospective studies (33–35). Studies on the risk of pregnancy after vasectomy using thresholds higher than 0.1 million motile sperm/ml sperm is not available. A recent report on measuring sperm concentration in over 9000 semen samples indicated that using the World Health Organization Laboratory Manual for the Examination and Processing of Human Semen (21) was sufficiently robust to confirm success of vasectomy and efforts to detect occasional motile sperm were futile (36). Another study questioned the requirement of assessing sperm motility in post-vasectomy semen analyses (over 6000 post-vasectomy semen sample) given the low likelihood of finding motile sperm at very low sperm concentrations (when sperm concentration was <1 million or < 0.25 million/ml, only 0.5 and 0.3% of samples had motile sperm) (37). Estimating sperm concentration to ≤ 0.1 million/ml without assessment of motility is likely to be sufficient to classify success/failure of vasectomy. However, the guidelines that define failure of vasectomy may not be applicable to male contraceptive development in which spermatogenesis is markedly suppressed by a hormonal mechanism and studies show that higher thresholds up to <1 million/ml provide effective contraceptive efficacy (12–15).
We demonstrated that all participants from a hormonal male contraceptive efficacy trial could process their own semen samples with ease using the SpermCheck-V test. They could accurately interpret the results. This user-controlled kit could be an effective and accurate tool in male contraceptive efficacy trials to monitor suppression of sperm concentration to a very low threshold ≤0.2 million/ml. We have observed that most men in our study maintain their sperm below this threshold if they use the contraceptive gel consistently. Our next step is to translate and assess this user-controlled test as an in-home test (performed independently by participants) for hormonal male contraception monitoring to ensure the negative predictive value remains near 100%. This second substudy has just started. If the results are confirmed, this at-home test for the estimation of sperm concentration will be invaluable for the pivotal, large-scale efficacy trials required for regulatory approval of a male contraceptive product. Using this or a similar at-home test to detect sperm concentrations ≤1 million/ml will markedly reduce the number of visits and the burden on participants during the long study period required to monitor efficacy of methods that suppress spermatogenesis.
We envisaged that once a product that suppresses spermatogenesis is approved for use as a male contraceptive, couples begin to use the product and monitor decrease in sperm output by an at-home sperm concentration test. The results from this study demonstrate convincingly that obtaining a negative test result, the couple may stop other methods of contraception and rely on the male method because the threshold for this test is 0.2 million/ml below the current threshold of 1 million/ml to be effective in preventing pregnancy in the female. Presumably, a negative test could be verified by a laboratory determined sperm concentration, but it could cut down on the need for obtaining laboratory-based sperm counts while the test line was still visible. Moreover, once reaching that <0.2 million/ml threshold, the test has utility in reassuring couples that they are still suppressed to a sufficient degree or alerting couples that they may need to get a laboratory sperm concentration test and possibly use a backup method until they get the results of the lab count. The female partner could witness or perform the test for the male partner, providing further assurance for the couple that an effective level of suppression is achieved or maintained.
In approving a product that suppresses spermatogenesis for male contraception, the instructions will likely advise the user to obtain a laboratory-based sperm concentration after some weeks of product use to ensure that sperm concentrations are sufficiently suppressed to the contraceptive level. In the current clinical trial, nearly all men are fully suppressed by 24 weeks, and most are suppressed by 16 weeks if they use the product consistently. However, a substantial number of men would have sperm concentrations at ≤ 0.2 million/ml by 4, 6, or 8 weeks. If they can use SpermCheck V at home, a negative result would allow them to use the male contraceptive product much sooner than the threshold for most men which is likely to be set conservatively at 12, 16, 20 or 24 weeks. The use of this user-controlled test will make the method much more convenient to users by reducing the time to enter efficacy without having to come to a clinic for monthly sperm counts or waiting an unnecessarily long time to get their first sperm concentration by a laboratory.
In summary, an at-home test for monitoring sperm concentration would be very useful for couples using a male contraceptive method that works by inhibiting spermatogenesis resulting in marked suppression of sperm output. A negative result with SpermCheck-V indicates that the sperm concentration is ≤0.2 million/ml; this level would provide highly effective contraception for the couple. Validation of this test will minimize the need for clinic visits for semen analyses, reduce costs for developers and reduce burden on participants and clinical sites. This will accelerate the pace of male contraceptive development and increase practicality of monitoring persistent suppression of spermatogenesis, making hormonal male contraceptive an even more practical and attractive family planning approach for a couple.
Supplementary Material
Acknowledgments
This study was supported by the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. Contract HHSN275220130024I Task Order HHSN 2750007, and the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR001881. The authors thank our coordinators Michael Massone, Xiaodan Han and Elizabeth Ruiz and the staff of the Clinical and Translational Research Center, The Lundquist Institute at Harbor-UCLA Medical Center for their help in coordinating the semen collection, semen analysis and SpermCheck-V in this study and Sima Baravarian for assisting with the laboratory hemocytometer semen analysis for this study. Most importantly, the authors thank the couples who participated enthusiastically in this study. The authors thanked DNA Diagnostic Center for providing the third batch of SpermCheck-V and the quality control data of all the three batches in this study.
Funding Statement:
The authors received support for this study from the Contraceptive Clinical Trials Network NIH Contract HHSN275220130024I Task Order HHSN 2750007, and P50 HD098593, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Institutes of Health, Bethesda, USA, and UCLA CTSI Grant UL1TR001881 from the National Center for Advancing Translational Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Yanhe Lue, Ronald Swerdloff, Youngju Pak, Brian T. Nguyen, Fiona Yuen, Peter Y. Liu, and Christina Wang have no disclosure. Diana L. Blithe is a US government employee. The distributor of the SpermCheck kits, DNA Diagnostic Center, did not contribute to the design, conduct of the study nor the writing or approval of the manuscript.
Attestation statements: The subjects in this trial have not concomitantly been involved in other randomized trial. Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Clinical Trial registration: NCT: 03452111
Data Sharing Statement:
The raw data on SpermCheck-V tests and the sperm concentration estimated in the laboratory will be make available upon request to the corresponding author. The study is funded by the National Institutes of Health and data sharing is mandatory.
REFERENCES
- 1.Page S, Blithe D, Wang C. Hormonal male contraception: getting to market. Frontiers in endocrinology 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Festin MP, Swerdloff RS. Male Hormonal Contraception: Where Are We Now? Current obstetrics and gynecology reports 2016;5:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirumalai A, Amory JK. Emerging approaches to male contraception. Fertil Steril 2021;115:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen F, Nguyen BT, Swerdloff RS, Wang C. Continuing the search for a hormonal male contraceptive. Best Pract Res Clin Obstet Gynaecol 2020;66:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl 2001;22:640–5. [PubMed] [Google Scholar]
- 6.Jarow JP, Wright WW, Brown TR, Yan X, Zirkin BR. Bioactivity of androgens within the testes and serum of normal men. J Androl 2005;26:343–8. [DOI] [PubMed] [Google Scholar]
- 7.Maddocks S, Hargreave TB, Reddie K, Fraser HM, Kerr JB, Sharpe RM. Intratesticular hormone levels and the route of secretion of hormones from the testis of the rat, guinea pig, monkey and human. Int J Androl 1993;16:272–8. [DOI] [PubMed] [Google Scholar]
- 8.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl 2004;25:931–8. [DOI] [PubMed] [Google Scholar]
- 9.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet 2006;367:1412–20. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 1990;336:955–9. [PubMed] [Google Scholar]
- 11.World Health Organization Task Force on the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 1996;65:821–9. [PubMed] [Google Scholar]
- 12.Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 2003;88:4659–67. [DOI] [PubMed] [Google Scholar]
- 13.Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 2003;88:562–8. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009;94:1910–5. [DOI] [PubMed] [Google Scholar]
- 15.Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI et al. Efficacy and Safety of an Injectable Combination Hormonal Contraceptive for Men. J Clin Endocrinol Metab 2016:jc20162141. [DOI] [PubMed] [Google Scholar]
- 16.Aaltonen P, Amory JK, Anderson RA, Behre HM, Bialy G, Blithe D et al. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl 2007;28:362–3. [DOI] [PubMed] [Google Scholar]
- 17.Sundaram A, Vaughan B, Kost K, Bankole A, Finer L, Singh S et al. Contraceptive Failure in the United States: Estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health 2017;49:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab 2012;97:3476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalo IT, Swerdloff RS, Nelson AL, Clevenger B, Garcia R, Berman N et al. Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab 2002;87:3562–72. [DOI] [PubMed] [Google Scholar]
- 20.Kamischke A, Heuermann T, Kruger K, von Eckardstein S, Schellschmidt I, Rubig A et al. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab 2002;87:530–9. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO laboratory manual for the examination and processing of human semen. Fifth ed. Geneva, Swtizerland: World Health Organization, 2010. [Google Scholar]
- 22.World Health Organization. WHO laboratory manual for the examination and processing of human semen. Sixth ed. Geneva, Switzerland: WHO, 2021. [Google Scholar]
- 23.Klotz KL, Coppola MA, Labrecque M, Brugh VM III, Ramsey K, Kim KA et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol 2008;180:2569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herr JC, Woodward MP. An enzyme-linked immunosorbent assay (ELISA) for human semen identification based on a biotinylated monoclonal antibody to a seminal vesicle-specific antigen. J Forensic Sci 1987;32:346–56. [PubMed] [Google Scholar]
- 25.Herr JC, Wright RM, John E, Foster J, Kays T, Flickinger CJ. Identification of human acrosomal antigen SP-10 in primates and pigs. Biol Reprod 1990;42:377–82. [DOI] [PubMed] [Google Scholar]
- 26.Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, Shetty J et al. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod 2010;25:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper TG, Hellenkemper B, Jonckheere J, Callewaert N, Grootenhuis AJ, Kersemaekers WM et al. Azoospermia: virtual reality or possible to quantify? J Androl 2006;27:483–90. [DOI] [PubMed] [Google Scholar]
- 28.Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48:577–85. [PubMed] [Google Scholar]
- 29.Cochran W Sampling Techniques. 3rd Edition ed. New York: John Wiley and Sons, 1997. [Google Scholar]
- 30.Onofre J, Geenen L, Cox A, Van Der Auwera I, Willendrup F, Andersen E et al. Simplified sperm testing devices: a possible tool to overcome lack of accessibility and inconsistency in male factor infertility diagnosis. An opportunity for low- and middle-income countries. Facts Views Vis Obgyn 2021;13:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharlip IM, Belker AM, Honig S, Labrecque M, Marmar JL, Ross LS et al. AUA Clinical Guideline: Vasectomy. https://wwwauanetorg/guidelines/vasectomy-(2012-reviewed-for-currency-2015) 2015.
- 32.Hancock P, Woodward BJ, Muneer A, Kirkman-Brown JC. 2016 Laboratory guidelines for postvasectomy semen analysis: Association of Biomedical Andrologists, the British Andrology Society and the British Association of Urological Surgeons. J Clin Pathol 2016;69:655–60. [DOI] [PubMed] [Google Scholar]
- 33.Edwards IS. Earlier testing after vasectomy, based on the absence of motile sperm. Fertil Steril 1993;59:431–6. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien TS, Cranston D, Ashwin P, Turner E, MacKenzie IZ, Guillebaud J. Temporary reappearance of sperm 12 months after vasectomy clearance. Br J Urol 1995;76:371–2. [DOI] [PubMed] [Google Scholar]
- 35.Korthorst RA, Consten D, van Roijen JH. Clearance after vasectomy with a single semen sample containing < than 100 000 immotile sperm/mL: analysis of 1073 patients. BJU Int 2010;105:1572–5. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson M, Pooley K, Kohut T, Atkinson M. Is azoospermia the appropriate standard for post-vasectomy semen analysis? Or an unachievable goal of best practice laboratory guidelines. Hum Fertil (Camb) 2020;23:268–74. [DOI] [PubMed] [Google Scholar]
- 37.McMartin C, Lehouillier P, Cloutier J, Singbo N, Labrecque M. Can a Low Sperm Concentration without Assessing Motility Confirm Vasectomy Success? A Retrospective Descriptive Study. J Urol 2021;206:109–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data on SpermCheck-V tests and the sperm concentration estimated in the laboratory will be make available upon request to the corresponding author. The study is funded by the National Institutes of Health and data sharing is mandatory.


