Abstract
Objectives:
This study examined differences in accelerated biological aging among non-Hispanic Blacks, Hispanics, and non-Hispanic Whites in the United States, and assessed whether including life course socioeconomic conditions attenuated observed racial/ethnic differences.
Methods:
Data came from the Venous Blood Collection Subsample of the Health and Retirement Study. We used a comprehensive summary measure of biological age (BA-22). We determined whether key lifetime socioeconomic conditions contributed to racial/ethnic differences in biological aging.
Results:
Findings indicated that non-Hispanic Blacks and Hispanics have accelerated aging, and non-Hispanic Whites have decelerated aging. Racial/ethnic differences were strongly tied to educational attainment. We also observed a significant difference by birthplace for Hispanics. US-born Hispanics had accelerated biological aging, whereas foreign-born Hispanics did not. In age-stratified analyses, these racial/ethnic differences were found for adults 56–74, but not for adults 75+.
Conclusions:
These findings provide insight into biological differences underlying racial/ethnic disparities in health.
Keywords: accelerated biological aging, HRS, racial/ethnic disparities
Introduction
Racial/ethnic disparities in health outcomes persist in the United States. Underlying these disparities are differences in physiological state that can be characterized as biological, which are linked to adverse health outcomes. Non-Hispanic Blacks and Hispanics have been found to have greater biological risk than non-Hispanic Whites across multiple studies and biomarkers (Crimmins et al., 2007; García & Ailshire, 2019; Seeman et al., 2008). Researchers have found that racial/ethnic differences in biological risk can help explain health disparities (Beckie, 2012; Kamin Mukaz Debora et al., 2020). Higher levels of biological risk for racial/ethnic minorities have been linked to an elevated risk of disability, disease, and death (Cobb et al., 2016; Crimmins et al., 2007; Debora et al., 2020).
While individual biomarkers or system specific profiles allow researchers to assess how individual biological mechanisms lead to poor health outcomes and mortality, recent work in the health and social sciences has utilized multiple biological indicators to capture multi-system physiological dysregulation and summarize it in measures of biological age (Belsky et al., 2015; Klemera & Doubal, 2006; Levine, 2013). Biological age reflects physiological functioning relative to the average physiological state at a specific chronological age; and the difference between an individual’s or group’s biological and chronological age reflects either an accelerated or a delayed aging process. For instance, a 65-year-old with a biological age of 70 has aged physiologically faster than the average 65-year-old in the population. The original formulation of biological age was based on 10 markers, chosen because of availability and their link to age (Levine, 2013). Recent formulations of similar measures have included more, or a broader set of, measures (e.g. phenotypic age, the pace of aging, etc.) (Belsky et al., 2015; Liu et al., 2019). In this study, we used an expanded measure of biological age based on 22 markers (BA-22) which incorporated a number of newly available markers specifically identified as markers of aging and collected because of their relationship to aging health that have not previously been used in a population based study of racial/ethnic differences (Crimmins et al., 2021; Justice et al., 2018).
Biological age provides a summary measure to directly evaluate physiological differences related to aging for disadvantaged groups. Prior work using a more limited biological age measure has found that at a given age non-Hispanic Blacks appear older biologically than non-Hispanic Whites (Levine & Crimmins, 2014). Thus, Non-Hispanic Blacks are thought to have accelerated biological aging. Racial differences in physiological dysfunction have been used to understand racial disparities in health and mortality. For instance, differences in biological age between non-Hispanic Blacks and non-Hispanics Whites explained the Black-White disparity in mortality (Levine & Crimmins, 2014). While this research has made significant advancements in our understanding of physiological functioning differences between non-Hispanic Blacks and non-Hispanic Whites, these findings have not been extended to Hispanics. To the best of our knowledge, no research to date has compared differences in biological age between Hispanics and other racial/ethnic groups. Hispanics have been found to have lower mortality than expected given their socioeconomic position in society (commonly referred to as the “Hispanic paradox”), but have high rates of disability and chronic diseases (Crimmins et al., 2007; García & Ailshire, 2019). These divergent health patterns do not give a clear indication of whether Hispanics experience faster overall biological aging and how it may compare to other groups.
Additionally, birthplace has differentiated Hispanic health in the United States. Foreign-born Hispanics have a lower risk of mortality, disability, and other chronic health conditions than US-born Hispanics. Studies evaluating physiological risk across specific domains have also found this advantage among foreign-born Hispanics (Peek et al., 2010; Salazar et al., 2016). However, to the best of our knowledge, no study to date has evaluated differences in biological age by birthplace for Hispanics. Scientific questions remain as to whether foreign-born Hispanics will have similar aging patterns as non-Hispanic Whites (the group with the slowest biological aging) or be somewhere in-between US-born Hispanics and non-Hispanic Whites. Evaluating differences in biological aging by birthplace will help determine whether foreign-born Hispanics are biologically younger than US-born Hispanics. If so, this would provide some insight into the downstream health advantages observed for the older adult Hispanic population.
Racial/ethnic differences in biological aging may attenuate at the oldest ages. Prior research on mortality has documented a significant decline in the increased mortality risk for non-Hispanic Blacks when compared to non-Hispanic Whites across older adulthood, reaching an age where no racial/ethnic differences are observed and, thereafter, where non-Hispanic Blacks have lower mortality risk than non-Hispanic Whites (Lariscy, 2017; Manton et al., 1979; Masters, 2012). This demographic phenomenon has been referred to as the “mortality crossover”. Mortality crossover reflects compositional changes in the population where individuals with the greatest mortality risk within racial/ethnic groups experience death at earlier ages, making the surviving populations selective and more similar in health profiles. When evaluating biological aging, mortality processes are an important consideration because of the strong positive association between accelerated biological aging and mortality in older adulthood (Liu et al., 2019). As a result, a cross-sectional examination of biological aging could show smaller differences between racial/ethnic groups because of differential morality processes experienced by those populations. In fact, a prior study evaluating differences in biological aging, using a cross-sectional, nationally representative sample, observed that the greatest racial difference in biological aging was found in midlife, with smaller differences at older ages (Levine & Crimmins, 2014). For this reason, to understand racial/ethnic biological aging differences in a cross-sectional sample, it is important to consider changes across older adulthood.
Biological aging has been linked to socioeconomic factors across the life course. Previous work has found that individual and group differences in biological risk can be explained by differences in socioeconomic resources in childhood and adulthood (Crimmins et al., 2009; Seeman et al., 2008). In childhood, adverse socioeconomic conditions have been found to be strongly associated with similar indicators of biological aging (Berg et al., 2017; Danese & McEwen, 2012; Su et al., 2015). In part, the effects of poor childhood conditions can permanently alter physiological dysregulation (Friedman et al., 2015; Yang et al., 2017). However, these early life circumstances also influence adult socioeconomic achievement which has a role in physiological dysregulation as well (Milaniak & Jaffee, 2019; Steptoe & Zaninotto, 2020; Yang et al., 2020). Therefore, in evaluating differences in biological aging, both childhood and adulthood circumstances should be considered. Prior research has not evaluated how biological age may be impacted by lifetime SES factors.
Socioeconomic circumstances may provide an important explanation of accelerated biological aging among racial/ethnic minorities. Prior studies have attributed racial/ethnic differences in specific biological risk profiles to the adverse socioeconomic circumstances disproportionately experienced across life by non-Hispanic Blacks and Hispanics (Herd et al., 2012). Socially disadvantaged racial/ethnic groups experience pernicious physical, social, and economic environments from childhood to late adulthood that can lead to accelerated biological aging throughout life, commonly referred to as the “weathering hypothesis” (Ferraro et al., 2017; Geronimus et al., 2006; Jones et al., 2019; Simons et al., 2018). Given that biological aging is tied to a lifetime of experiences, childhood and adulthood socioeconomic adversity most likely contributes to faster aging. Because non-Hispanic Blacks and Hispanics experience greater levels of adversity at all life stages, the inclusion of life course information is likely to explain some of the racial/ethnic differences in biological aging.
We used the Health and Retirement Study to examine 1) racial/ethnic differences in biological aging for older adults and whether birthplace is a factor for Hispanics, 2) whether the differences in biological aging can be attributed to adverse childhood or adulthood socioeconomic conditions, or both, and 3) whether the racial/ethnic differences in biological age differ between younger-old and older-old age groups (56–74 and 75+). Overall, we hypothesized that non-Hispanic Blacks and Hispanics will age faster than non-Hispanic Whites, that US-born Hispanics will have faster accelerated biological aging than foreign-born Hispanics, that the difference will be greatest for ages 56–74, and that the racial/ethnic differences will largely be linked to socioeconomic differences across the life course. Our research contributes to the literature in two notable ways: addressing whether accelerated biological aging is found among Hispanics (a rapidly growing group of older adults) and evaluating how life course SES factors combine to impact accelerated biological aging observed among key racial/ethnic groups in the United States.
Data and Methods
To evaluate racial/ethnic differences in biological aging, we used data from the Health and Retirement Study (HRS). The HRS is a biennial longitudinal study of adults older than 50 in the United States, which collected venous blood data in 2016 from respondents 56 years of age and older. The venous blood study (VBS) data are the main source of the biological indicators used in our measure of biological age (BA-22). VBS collection was limited to respondents who had completed a prior interview, were not living in a nursing home, did not have a proxy respondent, and agreed to and completed subsequent blood collection (Crimmins et al., 2017).
To collect the venous blood sample, approximately 2 months after completing the 2016 interview, phlebotomists went to respondents’ homes. Blood was centrifuged in the field and shipped cold to the Advanced Research & Diagnostics Laboratory at the University of Minnesota. Most samples arrived within a 24-hour window. Nineteen of the biomarkers were estimated from assays performed on the VBS data. However, three of the markers included in the BA-22 measure were collected by interviewers either 2 years prior in the 2014 core interview, or 2 months prior in the 2016 core interview. These three markers are blood pressure, peak flow which is a measure of lung function, and HbA1c measured from dried blood spots.
In total, the VBS sample contained over 9,189 respondents, who were assigned a non-zero weight. This sample was further reduced to 4,134 (or 45% of the sample) due to missing biomarker information. After accounting for missing biomarker information, we did not have additional missing values for covariates. Of the 4,134 respondents, 2,869 identified as White, 674 identified as Black, and 591 identified as Hispanic. Compared to the missing sample in the VBS, the analytic sample was slightly younger (68.1 years old compared to 69.2, p<.001) and more likely to be female (55.3 vs. 53.1, p<.05). The analytical sample had fewer people with less than 12 years of education (12.4% vs. 16.6%, p<.001) and was more likely to be non-Hispanic White (82.1% vs. 78.8%, p<.001) (see supplementary Table 1).
BA-22.
The BA-22 measurement combined 10 markers in the original biological age measure developed by Levine (2013) with 12 additional markers. Compared to the prior version of biological age, the expanded measure of biological age (BA-22) used in this study incorporates additional markers that represent other physiological systems that were not available in prior data; therefore, the additional measure can be considered a more robust assessment of biological aging. The additional 12 markers were chosen because they were either included in a subsequent measure of Phenotypic Age designed to predict mortality (Liu et al., 2019) or they were part of a set of aging biomarkers known as the Targeting Aging with Metformin (TAME) assays which were suggested by a panel of aging specialists as appropriate measures for clinical trials to examine the potential for delaying aging (Justice et al., 2018). In a recent study, a comparison of the original Biological Age, Phenotypic Age, the TAME assays, and BA-22 showed that BA-22 performed better in predicting age-related health outcomes (Crimmins et al. 2021). The biomarkers used in BA-22 are detailed in Table 1. These biomarkers included standard clinical markers often collected while providing clinical care and a number of markers typically used in research: cardiovascular indicators (systolic blood pressure, NT-proBNP), metabolic markers (total cholesterol, HbA1c, IGF 1), kidney functioning (cystatin C, BUN), markers of inflammation and infection [cytomegalovirus or CMV IgG level, C-reactive protein, albumin, 5 cytokines (IL6, TNFRI, IL-10, IL-1Ra, and TGFB), white blood cell count], immune functioning (percent lymphocytes, CD4/CD8), hematological markers (mean cell volume, red cell width), and markers of organ functioning (alkaline phosphatase, expiratory peak flow).
Table 1.
Demographic Description of the Analytical Sample, HRS 2016 (N=4,134)
| Variable | Mean or % |
|---|---|
| Age | |
| Mean Age | 68.1 (SD=8.3) |
| %56–74 years | 77.5 |
| %75+ years | 22.5 |
| %Female | 55.3 |
| Race/ethnicity | |
| %Non-Hispanic White | 82.1 |
| %Non-Hispanic Black | 8.9 |
| %Hispanic | 9.1 |
| %US-born | 4.0 |
| %Foreign-born | 5.1 |
| Education | |
| %<=11 years | 12.4 |
| %12 years | 30.5 |
| %13–15 years | 25.8 |
| %16+ years | 31.4 |
| %No health insurance | 3.2 |
| % Some Childhood SES Adversity | 60.3 |
The BA-22 measurement was derived using a similar methodological technique to that used in the Levine measure (also known as the Klemera Doubal approach), but was developed using the HRS data from 2016 for adults 56+ years (Levine, 2013; Crimmins et al. 2021). Specifically, BA-22 was derived from the following equation:
In this equation, chronological age (CA) was regressed on the m biological measures. The intercept (qj), slope (kj) and the mean squared error (sj2) were all estimated from the regression equation. Biological age was then estimated by substituting the xj value for each respondent. The BA-22 was designed so that on average it equals average chronological age.
After obtaining BA-22, we regressed biological age on chronological age to obtain measures of accelerated (or decelerated) aging for each individual. From the regression model, we took the residual differences between the regression estimation and chronological age, which could have negative or positive values. Negative values indicated slower biological aging, whereas positive values indicated faster biological aging. Because we evaluated average differences in biological aging between racial/ethnic groups and non-Hispanic Blacks and Hispanics have faster biological aging on average, we called this measure accelerated biological aging.
Race/Ethnicity.
To evaluate physiological aging differences by racial/ethnic group, we used two categorical variables. The first was a three-category variable (non-Hispanic Whites, non-Hispanic Blacks, Hispanics) to evaluate overall racial/ethnic differences. Respondents who were categorized as “Other” were not included. We then created a four-category variable to observe the effect of birthplace as well as race/ethnicity on biological aging for Hispanics; we classified Hispanics into foreign-born and US-born. Both measures were used in our analysis.
Childhood Adversity.
We used a two-category indicator of any childhood socioeconomic adversity: (0) no childhood socioeconomic adversity and (1) some childhood socioeconomic adversity. Adversities were based on the following six items: while you were growing up, 1) did financial difficulties ever cause you or your family to move to a different place?; 2) Was there time when you or your family received help from relatives because of financial difficulties?; 3) Would you say your family during that time was pretty well off financially, about average, or poor? (0 for pretty well off or about average and 1 for poor); 4) Father had 8 years of education or less; 5) Mother had 8 years of education or less; and 6) Was there a time of several months or more when you father had no job? If respondents were missing on mother’s or father’s education, we categorized them as having low parental education, which follows previous HRS studies that found missingness strongly associated with low parental education (Luo & Waite, 2005; Zhang et al., 2016). As a sensitivity test, we refitted the models with the assumption that missing mother’s and father’s education was indicative of greater than 8 years of education. The results remained unchanged.
Education.
Education was categorized based on years of schooling: 11 years or less, 12 years, 13–15 years, and 16 years or more.
Health Insurance.
Our health insurance measure indicated whether the respondent currently had health insurance or not.
Other Variables.
Age and sex were controlled in each equation, so that the equations reflected the association of each covariate with accelerated BA-22 assuming a similar distribution of age and sex.
Statistical Analysis
First, we examined the means for each biomarker in BA-22 by racial/ethnic group. We tested for statistically significant differences between non-Hispanic Whites and non-Hispanic Blacks and Hispanics. Second, we calculated the differences in accelerated BA-22 for non-Hispanic Whites, Non-Hispanic Blacks, and Hispanics. Third, we estimated a set of 4 nested linear regression models to evaluate racial/ethnic differences in acceleration in BA-22, and whether key life course socioeconomic conditions explained part of the association. The first model contained only race/ethnicity with age and sex controls (without birthplace for Hispanics). The second model was similar to the first but included foreign-born status for Hispanics. The third model added childhood socioeconomic adversity. The fourth model added education. Lastly, the fifth model added currently having insurance. Using OLS regression, we were able to evaluate the changes in racial/ethnic differences in accelerated biological age due to life course socioeconomic circumstances by comparing the change in the racial/ethnic coefficients with each subsequent model. If the coefficients for non-Hispanic Blacks and Hispanics were reduced, then we would find evidence that the racial/ethnic difference in accelerated biological age could be partly or fully linked to the differences in life course socioeconomic conditions.
Next, we fitted age-stratified OLS regression models (for ages 56–74 and 75+) with the same sequence of 4 models (outlined prior). Results from these models were used to determine whether the racial/ethnic differences in biological age varied across the older ages. Additionally, we tested differences in individual biomarkers between US- and foreign-born Hispanics (shown in Supplemental Table 2). All analyses were completed in SAS 9.4. We used sample weights provided by the HRS to adjust for sampling probability, non-response, and complex survey design.
Results
Sample Characteristics.
The average age of the sample was 68.1 years old, and age ranged from 56 to 90 (shown in Table 1). About three-quarters (77.5%) of the respondents were ages 56–74. They were also mostly non-Hispanic White (82.1%). Non-Hispanic Blacks and Hispanics consisted of nearly identical proportions: 8.9% and 9.1%. A majority of Hispanics were foreign-born (56.0% of Hispanics or 5.1% of the total sample). The analytical sample was relatively well educated: 12.4% had less than 12 years of education, 30.5% had 12 years, 25.8% have 13–15 years, and 31.4% had 16+ years. Very few respondents had no health insurance: 3.2%. A majority of the sample had experienced some form of childhood socioeconomic adversity: 60.3%.
Differences in Individual Markers by Racial/Ethnic Group.
The individual biomarkers used in the BA-22 measure provided evidence of worse biological functioning for non-Hispanic Blacks and Hispanics when compared to non-Hispanic Whites (Table 2). Compared to non-Hispanic Whites, non-Hispanic Blacks and Hispanics presented higher systolic blood pressure, higher HbA1c levels, lower BUN, higher CMV levels, higher logged CRP, lower albumin, higher IL6, higher IL-RA, higher lymphocyte percentage, lower CD4/CD8 count, lower mean cell volume, higher alkaline phosphatase, and lower peak flow. Some biomarker differences were only found between non-Hispanic Blacks and non-Hispanic Whites: non-Hispanic Blacks presented higher cystatin C levels, higher IL-10 levels, lower white blood cell count, and higher red cell distribution width than non-Hispanic Whites. When compared to non-Hispanic Whites, we found no differences in NT-PROBNP, cholesterol, TNFRI, and TGFB levels for either non-Hispanic Blacks or Hispanics.
Table 2.
Means for 22 Biomarkers used in the BA-22 by Race/ethnicity, HRS 2016
| Non-Hispanic White (N=2,869) |
Non-Hispanic Black (N=674) |
Hispanic (N=591) |
|
|---|---|---|---|
| Cardiovascular | |||
| Systolic Blood Pressure (mmHg)1,2 | 126.9 | 131.4*** | 129.3* |
| NT-PROBNP (pg/mL) (logged) | 4.6 | 4.4 | 4.5 |
| Metabolic | |||
| Total cholesterol (mg/dL)2 | 192.3 | 188.4 | 190.5 |
| HbA1c (%)1 | 5.8 | 6.2* | 6.3*** |
| IGF 1 (ng/mL)2 | 108.0 | 108.9 | 88.9*** |
| Kidney Functioning | |||
| Cystatin C (mg/L) | 1.1 | 1.2* | 1.1 |
| BUN (mg/dL)2 | 17.5 | 16.8* | 16.3*** |
| Infection and Inflammation | |||
| CMV (COI)2 | 246.2 | 422.3*** | 436.2*** |
| CRP (mg/L) (logged) | −1.6 | −1.3*** | −1.2** |
| Albumin (g/dL) | 4.0 | 3.8*** | 3.9*** |
| IL6 (pg/mL) (logged) | 1.3 | 1.6*** | 1.4* |
| TNFRI (pg/mL)2 | 1730.0 | 1715.0 | 1674.3 |
| IL-10 (pg/mL)2 | 3.6 | 3.9** | 3.8 |
| IL-1Ra (pg/mL)2 | 573.3 | 534.4* | 520.2** |
| TGFB (pg/mL)2 | 48165.6 | 48378.1 | 49237.6 |
| White blood cell count2 | 6.5 | 6.1*** | 6.7 |
| Immune Functioning | |||
| Lymphocyte %2 | 29.2 | 35.0*** | 32.2*** |
| CD4/CD8 count2 | 4.0 | 3.2*** | 3.3*** |
| Hematological | |||
| Mean Cell Volume2 | 93.6 | 89.8*** | 92.6** |
| Red cell distribution width | 13.8 | 14.6*** | 13.8 |
| Organ Functioning | |||
| Alkaline phosphatase (U/L)2 | 77.7 | 85.2*** | 90.0*** |
| Peak Flow (L/min)2 | 381.2 | 318.4*** | 330.0*** |
| Demographics | |||
| Mean Age | 68.3 | 67.4 | 67.0* |
| %Female | 54.2% | 60.0%* | 61.5%** |
| BA-22 | 67.7 | 68.6 | 68.0 |
p<.001
p<.01
p<.05 indicates difference from white.
Note: All markers are top coded at the 99%ile.
2014 and 2016 combined.
Due to small but significant effect of these markers on health outcomes in regression equations, we divide these markers by 100 to visualize the effects of these markers on health outcomes in later analyses.
Differences in Accelerated Biological Aging.
Mean differences in accelerated biological age are presented in Figure 1. Non-Hispanic Blacks and Hispanics were found to have accelerated biological aging. Non-Hispanic Black men’s acceleration in BA-22 was on average 1.89 years, while non-Hispanic Black women were .79 years older. Hispanic men were 1.95 years older biologically, and Hispanic women were 0.46 older biologically. In contrast, non-Hispanic Whites had decelerated aging. On average, non-Hispanic White men were −0.53 years biologically younger than their chronological age, while White women were −0.56 years younger. On average, non-Hispanic Blacks and Hispanics were biologically older than non-Hispanic Whites. Non-Hispanic Black men were 2.40 years older than non-Hispanic White men. Hispanic men were 2.48 years older than non-Hispanic White men. Black women were 1.35 years older than White women. Hispanic women were 1.02 years older than White women.
Figure 1.
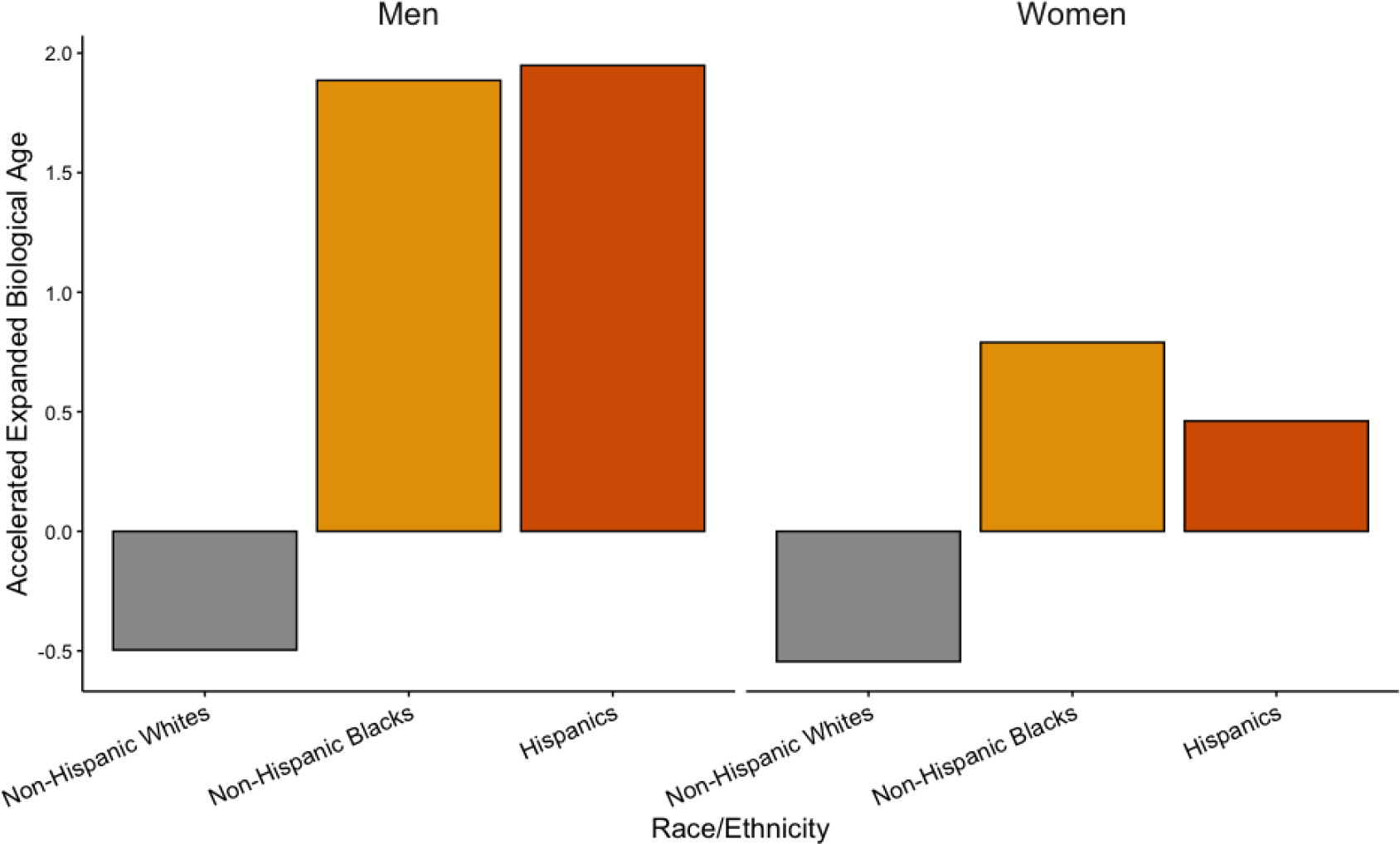
Mean Accelerated Expanded Biological Age by Race/Ethnicity and Gender, HRS 2016
Life Course Socioeconomic Contributions to the Racial/Ethnic Difference in BA-22.
The results from the nested regression equations predicting racial/ethnic differences in accelerated BA-22 are shown in Table 3. In Model 1, we tested racial/ethnic differences in accelerated biological age after netting out any additional effect of age and gender. Again, we found strong evidence that non-Hispanic Blacks and Hispanics are aging faster than non-Hispanic Whites (b=1.80 years, p<.001 and b=1.61 years, p<.001, respectively).
Table 3.
Coefficients from Nested OLS Regressions of Accelerated BA-22 (in years) on Demographic and Socioeconomic Characteristics: HRS, 56+ (N=4,134)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Age | 0.01 | 0.01 | −0.01 | −0.03* | −0.03 |
| Female | −0.26 | −0.26 | −0.31 | −0.46 | −0.43 |
| Race/Ethnicity (Non-Hispanic white as reference) | |||||
| Non-Hispanic Black | 1.80*** | 1.80*** | 1.53*** | 0.77 | 0.73 |
| Hispanic | 1.61*** | ||||
| US-born Hispanic | 2.22*** | 1.73** | 0.73 | 0.67 | |
| Foreign-born Hispanic | 1.12* | 0.58 | −1.17* | −1.34* | |
| Some Childhood SES adversity | 1.52*** | 0.97*** | 0.96*** | ||
| Education (11 or less years as reference) | |||||
| 12 years | −2.15*** | −2.16*** | |||
| 13–15 years | −3.30*** | −3.29*** | |||
| 16+ years | −4.80*** | −4.78*** | |||
| No Health Insurance | 1.37* | ||||
| R2 | 0.01 | 0.01 | 0.01 | 0.05 | 0.05 |
p<.001
p<.01
p<.05
Next, we assessed the role of birthplace for Hispanics in Model 2. When birthplace was assessed, we found that both US-born and foreign-born Hispanics had increased accelerated biological aging compared to non-Hispanic Whites (b=2.22, p<.001 for US-born Hispanics; b=1.12, p<.05 for foreign-born Hispanics).
Model 3 assessed the role of childhood socioeconomic conditions on the racial/ethnic differences. Childhood SES adversity was associated with accelerated biological aging (b=1.52, p<.001). Compared to Model 2, the coefficients for race/ethnicity slightly decreased for non-Hispanic Blacks (b=1.53, p<.001) and US-born Hispanics (b=1.73, p<.01). Foreign-born Hispanics no longer had increased accelerated biological aging.
In Model 4, we examined the role of education. Greater levels of education were associated with decelerated biological aging. Compared to respondents with less than 12 years of education, respondents with 12 years of education were 2.15 years younger biologically (b=−2.15, p<.001), with 13–15 years of education were 3.30 years younger (b=−3.30, p<.001), and respondents with 16+ years of education were 4.80 years younger (b=−4.80, p<.001). After controlling for education, non-Hispanic Blacks and Hispanics were no longer statistically significantly different in accelerated biological age from non-Hispanic Whites. However, we found that foreign-born Hispanics had slower accelerated biological aging, after education adjustments (b=−1.17, p<.05). Additionally, the effect of some childhood socioeconomic adversity continued to be associated with accelerated aging, but decreased in magnitude (b=.96, p<.001).
Lastly, we examined the role of not having health insurance for older adults. We found that not having health insurance did not impact the associations between BA-22 and other covariates, although not having insurance was positively related to BA-22 (b=1.37, p<.05).
Racial/Ethnic Differences by Age Groups.
Next, we fitted age-stratified regression models to observe whether the racial/ethnic gap in BA-22 differed for adults aged 56–74 and 75+. We found racial/ethnic differences in BA-22 for adults 56–74, but we did not find racial/ethnic differences for adults 75+. For adults 56–74, non-Hispanic Blacks and Hispanics were biologically older than non-Hispanic Whites (b=2.13 and 1.81, p<.001). We also observed differences by birthplace for Hispanics. US-born Hispanics were biologically older (b=2.79, p<.001) than non-Hispanic Whites. We found no statistically significant difference between foreign-born Hispanics and non-Hispanic Whites (although this may, in part, be reflective of sample size). After adjusting for socioeconomic conditions, the changes in the racial/ethnic coefficients across nested models were similar to those found in the full model; however, we found one notable deviation: Non-Hispanic Blacks continued to have accelerated biological aging when compared to non-Hispanic Whites, even though this racial difference was reduced by 47% between Model 1 and Model 5.
Discussion
This study evaluated racial/ethnic differences in Accelerated BA-22 for older adults, and whether these differences could be explained by childhood and adult socioeconomic conditions. Compared to non-Hispanic Whites, non-Hispanic Blacks and US-born Hispanics were found to have accelerated biological aging, which is in line with other work in the HRS that has found greater biological risk among racial/ethnic minorities (Boen, 2020; Brown et al., 2017). Our research extended prior work to Hispanics and evaluated the potential role of socioeconomic conditions.
Our results showed that non-Hispanic Blacks and Hispanics are experiencing accelerated biological aging, which may lead to non-Hispanic Blacks and Hispanics being more susceptible to health complications in later life. However, accelerated biological aging may not be equally tied to adverse health outcomes across racial/ethnic groups. Research has consistently found that Hispanics have a mortality risk lower than or equal to non-Hispanic Whites (“Hispanic Paradox”) (Doza et al.; Lariscy et al., 2015). Therefore, while prior work has used biological aging to explain Black-White mortality differences (Levine & Crimmins, 2014), it may play a smaller role for Hispanics. The health consequences of physiological dysregulation for non-Hispanic Blacks and Hispanics may differ. This difference may arise from a variety factors, such as differences in exposure to other mortality risks that may be compounded by physiological dysregulation and the progression and development of disease from physiological changes. Future mortality research should evaluate racial/ethnic differences in the health consequences of physiological dysregulation more closely to better understand the mechanisms that link it to morality.
Biological aging has been tied to childhood and adulthood socioeconomic conditions. Childhood adversity has been found to be strongly associated with increased physiological dysregulation (Solís et al., 2015). Lower levels of education or poverty in adulthood also can contribute to increased biological risk (Crimmins et al., 2009; Yang et al., 2020). These conditions have been found to be additive: poor socioeconomic conditions at each life stage adversely impact biological systems, leading to an accumulation of physiological dysregulation in later life (Yang et al., 2020). Put differently, accelerated biological aging reflects the cumulative biological experience of each individual. Given worse life course socioeconomic conditions for disadvantaged racial/ethnic groups, we evaluated whether childhood or adult socioeconomic conditions or both contributed to the racial/ethnic differences in accelerated biological aging. Differences in childhood adversity did not greatly impact the racial/ethnic differences in accelerated biological aging. In contrast, including education attenuated the racial differences, which is similar to findings in other studies for younger adults (Geronimus et al., 2006). Thus, this finding indicates that differences in adult life course circumstances may be more consequential than early life for accelerated biological aging patterns observed for non-Hispanic Blacks and Hispanics in older adulthood.
However, this association cannot be completely attributed to education without further study. Education is linked to many life circumstances, reflecting and reinforcing social stratification. For example, discrimination leads to greater physiological dysregulation among non-Hispanic Blacks and Hispanics (Boen, 2020; Cave et al., 2020; Korous et al., 2017; Paradies et al., 2015), but may also impact educational attainment: higher levels of perceived discrimination are associated with lower educational attainment (Chavous et al., 2008; Neblett et al., 2016). Therefore, education may reflect other pathways that are not solely linked to socioeconomic conditions. Future longitudinal work with discrimination measures will be better equipped to identify other mechanisms.
We also showed the racial/ethnic differences in accelerated biological age may differ across age. The racial/ethnic differences in accelerated biological aging were evident from 56–74, but not for 75+. This is consistent with other studies that showed a decrease or convergence in racial/ethnic differences for biological risk profiles or biological age for the 75+ population (Crimmins et al., 2009; Levine & Crimmins, 2014). This convergence is likely attributable to selective survival. People with greater biological risk or who are biologically older have a greater mortality risk (Levine, 2013; Yashin et al., 1985). As a result, individuals who have increased physiological dysregulation are “selected out” of the population; the racial/ethnic differences decrease or converge. Therefore, for the older adult racial/ethnic groups may not be directly comparable, since each group is subject to the different mortality processes.
However, it is also important to note that selectivity may not be the only factor. Racial/ethnic differences in biological aging may also converge due to differences in the pace of aging as well, especially among the survivors for each group. For example, if the surviving non-Hispanics Blacks and Hispanics have slower rates of aging than the surviving groups of non-Hispanic Whites, the convergence between the groups may occur earlier than if determined only by mortality processes. However, we could not assess the pace of aging and mortality effects on the convergence of biological age of Non-Hispanic Blacks and non-Hispanic Whites because our data were cross-sectional. When longitudinal data is available, future work should consider within group “pace of aging” to better understand this dynamic.
Additionally, we found that birthplace had a role in understanding biological aging differences for non-Hispanic Whites and Hispanics. US-born Hispanics experienced greater accelerated biological age than non-Hispanic Whites, while foreign-born Hispanics were to a lesser extent biologically older as well. However, after adjusting for life course socioeconomic conditions, foreign-born Hispanics would appear biologically younger than non-Hispanic Whites. These findings are consistent with other studies that have shown foreign-born Hispanics with better health risk profiles than US-born Hispanics, and would have better health profiles than non-Hispanic Whites had they had similar socioeconomic conditions throughout life (Salazar et al., 2016).
Differences in biological aging between foreign-born and US-born have been theorized to be linked to acculturation and immigration-related processes. Acculturation (or immigrant adaptation process) has been linked to worse biological dysregulation across multiple studies, which may partly explain the worse biological functioning among US-born Hispanics who have higher levels of acculturation (Abraído-Lanza et al., 1999; Niño & Hearne, 2020; Riosmena et al., 2012; Scholaske et al., 2021). Additionally, biological aging patterns among foreign-born Hispanics may be partly attributable to immigration-related processes. Foreign-born Hispanics who move to the United States are likely to be healthier (Cunningham et al., 2008) and have favorable health behaviors (Blue & Fenelon, 2011), while also unhealthy migrants may return to countries of origin—also referred to as the “salmon bias” (Diaz et al., 2016; Markides & Eschbach, 2005). However, research has not consistently supported the “salmon bias” hypothesis (Abraído-Lanza et al., 1999; Diaz et al., 2016). To better understand how immigration patterns impact biological aging, a future study should examine the biological age of Hispanics who returned to their countries of origin. Thus, part of slower biological aging for foreign-born Hispanic may reflect compositional changes that make this group select.
Despite its strength, the study has some limitations. First, the full set of biomarker information used in the BA-22 measure was cross-sectional. We could not access age-trajectories, which would provide information on racial/ethnic differences in the pace of aging. Second, due to missing biomarker information, our sample was reduced by 45%. We tested for statistically significant differences between the analytical sample and those excluded. The analytical sample had significantly fewer adults with low levels of education, who were also more likely to be Black and Hispanic. As a result, the racial/ethnic differences presented in our study are most likely conservative, given that education is an important explanatory factor. Lastly, our measurement of childhood socioeconomic adversity was developed to retain the maximum number of sample participants. While we originally explored a variable with more levels of adversity, we did not present these results because the sample size was limited in higher categories. Future work with more dimensional measurements of childhood adversity and larger samples should consider the importance of adversity levels more thoroughly.
Conclusions
We found evidence of faster biological aging for non-Hispanic Blacks and Hispanics. These differences, however, should not be viewed as immutable nor biologically inherent. We found strong evidence that the racial/ethnic gap in biological aging is explained by differences in life course socioeconomic conditions. We also found further evidence of the “Hispanic Paradox” among foreign-born Hispanics. To better understand racial/ethnic differences in biological aging, future studies using longitudinal data should consider how selection processes (from mortality and out-migration) impact the assessment of biological aging among these groups and evaluate whether changes in biological aging are occurring at similar rates across racial/ethnic groups. Additionally, to better understand health disparities, studies should also evaluate how biological aging is associated with later life health outcomes, and whether the associations are similar across racial/ethnic groups. Overall, accelerated BA-22 provides another indicator of how race/ethnicity is linked to adverse health outcomes in the United States and provides support that racial/ethnic differences in biological aging are closely tied to socioeconomic circumstances throughout life.
Supplementary Material
Table 4.
Coefficients from Age-Stratified Nested OLS Regression of Accelerated BA-22 (in years) on Demographic and Socioeconomic Characteristics: HRS, 56–74 and 75+
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Ages 56–74 (N=2,786) | |||||
| Female | −0.29 | −0.29 | −0.35 | −0.53 | −0.50 |
| Race/Ethnicity (Non-Hispanic White as reference) | |||||
| Non-Hispanic Black | 2.13*** | 2.13*** | 1.86*** | 1.07* | 1.01* |
| Hispanic | 1.81*** | ||||
| US-born Hispanic | 2.79*** | 2.24** | 1.31 | 1.23 | |
| Foreign-born Hispanic | 1.08 | 0.52 | −1.41* | −1.62* | |
| Some Childhood SES adversity | 1.44*** | 0.89** | 0.89** | ||
| Education (11 or fewer years as reference) | |||||
| 12 years | −2.70*** | −2.71*** | |||
| 13–15 years | −3.49*** | −3.49*** | |||
| 16+ years | −5.20*** | −5.18*** | |||
| No Health Insurance | 1.41* | ||||
| R2 | 0.01 | 0.01 | 0.02 | 0.05 | 0.05 |
| Ages 75+ (N=1,348) | |||||
| Female | −0.19 | −0.20 | −0.24 | −0.29 | −0.29 |
| Race/Ethnicity (Non-Hispanic White as reference) | |||||
| Non-Hispanic Black | 0.51 | 0.51 | 0.32 | −0.36 | −0.37 |
| Hispanic | 0.88 | ||||
| US-born Hispanic | 0.09 | −0.09 | −1.28 | −1.32 | |
| Foreign-born Hispanic | 1.72 | 1.49 | 0.22 | 0.07 | |
| Some Childhood SES adversity | 1.51* | 0.84 | 0.83 | ||
| Education (11 or less years as reference) | |||||
| 12 years | −1.12 | −1.11 | |||
| 13–15 years | −3.32*** | −3.29*** | |||
| 16+ years | −3.78*** | −3.78*** | |||
| No Health Insurance | 5.43 | ||||
| R2 | 0.00 | 0.00 | 0.00 | 0.03 | 0.03 |
Acknowledgements
Author’s Contributions: Mateo P. Farina directed the analysis and drafted the manuscript. Jung Ki Kim completed the analysis and edited the manuscript. Eileen M. Crimmins directed the analysis and edited the manuscript. All authors participated in the revisions.
Funding
This analysis was supported by funds from the National Institutes of Health (grant numbers T32AG000037, R01 AG060110, P30 AG017265, P30 AG043073). The Health and Retirement Study is supported by the National Institute on Aging (grant number U01-AG009740).
References
- Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, & Turner JB (1999). The Latino mortality paradox: A test of the “salmon bias” and healthy migrant hypotheses. American Journal of Public Health, 89(10), 1543–1548. 10.2105/AJPH.89.10.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argeseanu Cunningham S, Ruben JD, & Venkat Narayan KM (2008). Health of foreign-born people in the United States: A review. Health & Place, 14(4), 623–635. 10.1016/j.healthplace.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Beckie TM (2012). A Systematic Review of Allostatic Load, Health, and Health Disparities. Biological Research For Nursing, 14(4), 311–346. 10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, & Moffitt TE (2015). Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences of the United States of America, 112(30), E4104–4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg MT, Simons RL, Barr A, Beach SRH, & Philibert RA (2017). Childhood/Adolescent stressors and allostatic load in adulthood: Support for a calibration model. Social Science & Medicine, 193, 130–139. 10.1016/j.socscimed.2017.09.028 [DOI] [PubMed] [Google Scholar]
- Blue L, & Fenelon A (2011). Explaining low mortality among US immigrants relative to native-born Americans: The role of smoking. International Journal of Epidemiology, 40(3), 786–793. 10.1093/ije/dyr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen C (2020). Death by a Thousand Cuts: Stress Exposure and Black–White Disparities in Physiological Functioning in Late Life. The Journals of Gerontology: Series B, 75(9), 1937–1950. 10.1093/geronb/gbz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Needham B, & Ailshire J (2017). Telomere Length Among Older U.S. Adults: Differences by Race/Ethnicity, Gender, and Age. Journal of Aging and Health, 29(8), 1350–1366. 10.1177/0898264316661390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave L, Cooper MN, Zubrick SR, & Shepherd CCJ (2020). Racial discrimination and child and adolescent health in longitudinal studies: A systematic review. Social Science & Medicine, 250, 112864. 10.1016/j.socscimed.2020.112864 [DOI] [PubMed] [Google Scholar]
- Chavous TM, Rivas-Drake D, Smalls C, Griffin T, & Cogburn C (2008). Gender matters, too: The influences of school racial discrimination and racial identity on academic engagement outcomes among African American adolescents. Developmental Psychology, 44(3), 637–654. 10.1037/0012-1649.44.3.637 [DOI] [PubMed] [Google Scholar]
- Cobb RJ, Thomas CS, Laster Pirtle WN, & Darity WA (2016). Self-identified race, socially assigned skin tone, and adult physiological dysregulation: Assessing multiple dimensions of “race” in health disparities research. SSM - Population Health, 2, 595–602. 10.1016/j.ssmph.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Faul J, Thyagarajan B, & Weir D (2017). Venous blood collection and assay protocol in the 2016 Health and Retirement Study Venous Blood Study (VBS). https://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf [Google Scholar]
- Crimmins EM, Kim JK, Alley DE, Karlamangla A, & Seeman T (2007). Hispanic Paradox in Biological Risk Profiles. American Journal of Public Health, 97(7), 1305–1310. 10.2105/AJPH.2006.091892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, & Seeman TE (2009). Poverty and Biological Risk: The Earlier “Aging” of the Poor. The Journals of Gerontology: Series A, 64A(2), 286–292. 10.1093/gerona/gln010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Thyagarajan B, Kim JK, Weir D, & Faul J (2021). Quest for a summary measure of biological age: The health and retirement study. GeroScience. 10.1007/s11357-021-00325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106(1), 29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Diaz CJ, Koning SM, & Martinez-Donate AP (2016). Moving Beyond Salmon Bias: Mexican Return Migration and Health Selection. Demography, 53(6), 2005–2030. 10.1007/s13524-016-0526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doza A, Jensen GA, & Tarraf W (n.d.). Racial/Ethnic Differences in Mortality in Late Midlife: Have They Narrowed in Recent Years? The Journals of Gerontology: Series B. 10.1093/geronb/gbaa175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrique W. Neblett J, Philip CL, Cogburn CD, & Sellers RM (2016). African American Adolescents’ Discrimination Experiences and Academic Achievement: Racial Socialization as a Cultural Compensatory and Protective Factor: Journal of Black Psychology. 10.1177/0095798406287072 [DOI] [Google Scholar]
- Ferraro KF, Kemp BR, & Williams MM (2017). Diverse Aging and Health Inequality by Race and Ethnicity. Innovation in Aging, 1(1). 10.1093/geroni/igx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Karlamangla AS, Gruenewald T, Koretz B, & Seeman TE (2015). Early Life Adversity and Adult Biological Risk Profiles. Psychosomatic Medicine, 77(2), 176–185. 10.1097/PSY.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García C, & Ailshire JA (2019). Biological Risk Profiles Among Latino Subgroups in the Health and Retirement Study. Innovation in Aging, 3(2). 10.1093/geroni/igz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. American Journal of Public Health, 96(5), 826–833. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd P, Karraker A, & Friedman E (2012). The Social Patterns of a Biological Risk Factor for Disease: Race, Gender, Socioeconomic Position, and C-reactive Protein. The Journals of Gerontology: Series B, 67(4), 503–513. 10.1093/geronb/gbs048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL, Gilman SE, Cheng TL, Drury SS, Hill CV, & Geronimus AT (2019). Life Course Approaches to the Causes of Health Disparities. American Journal of Public Health, 109(Suppl 1), S48–S55. 10.2105/AJPH.2018.304738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SB, Barzilai N, & Kuchel GA (2018). A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. GeroScience, 40(5–6), 419–436. 10.1007/s11357-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debora Kamin Mukaz, Zakai Neil A, Salvador Cruz-Flores, McCullough Louise D, & Mary Cushman. (2020). Identifying Genetic and Biological Determinants of Race-Ethnic Disparities in Stroke in the United States. Stroke, 51(11), 3417–3424. 10.1161/STROKEAHA.120.030425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemera P, & Doubal S (2006). A new approach to the concept and computation of biological age. Mechanisms of Ageing and Development, 127(3), 240–248. 10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Korous KM, Causadias JM, & Casper DM (2017). Racial discrimination and cortisol output: A meta-analysis. Social Science & Medicine, 193, 90–100. 10.1016/j.socscimed.2017.09.042 [DOI] [PubMed] [Google Scholar]
- Lariscy JT (2017). Black-White Disparities in Adult Mortality: Implications of Differential Record Linkage for Understanding the Mortality Crossover. Population Research and Policy Review, 36(1), 137–156. 10.1007/s11113-016-9415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariscy JT, Hummer RA, & Hayward MD (2015). Hispanic Older Adult Mortality in the United States: New Estimates and an Assessment of Factors Shaping the Hispanic Paradox. Demography, 52, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME (2013). Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? The Journals of Gerontology: Series A, 68(6), 667–674. 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, & Crimmins EM (2014). Evidence of accelerated aging among African Americans and its implications for mortality. Social Science & Medicine, 118, 27–32. 10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kuo P-L, Horvath S, Crimmins E, Ferrucci L, & Levine M (2019). A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLOS Medicine, 16(2), e1002760. 10.1371/journal.pmed.1002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, & Waite LJ (2005). The Impact of Childhood and Adult SES on Physical, Mental, and Cognitive Well-Being in Later Life. The Journals of Gerontology: Series B, 60(2), S93–S101. 10.1093/geronb/60.2.S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Poss SS, & Wing S (1979). The Black/White Mortality Crossover: Investigation from the Perspective of the Components of Aging. The Gerontologist, 19(3), 291–300. 10.1093/geront/19.3.291 [DOI] [PubMed] [Google Scholar]
- Markides KS, & Eschbach K (2005). Aging, Migration, and Mortality: Current Status of Research on the Hispanic Paradox. The Journals of Gerontology: Series B, 60(Special_Issue_2), S68–S75. 10.1093/geronb/60.Special_Issue_2.S68 [DOI] [PubMed] [Google Scholar]
- Masters RK (2012). Uncrossing the U.S. Black-White Mortality Crossover: The Role of Cohort Forces in Life Course Mortality Risk. Demography, 49(3), 773–796. 10.1007/s13524-012-0107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaniak I, & Jaffee SR (2019). Childhood socioeconomic status and inflammation: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 78, 161–176. 10.1016/j.bbi.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Niño MD, & Hearne BN (2020). Dimensions of acculturation and biological dysregulation among Latina/os: The role of ethnic background, gender, and immigrant generation. Ethnicity & Health, 0(0), 1–17. 10.1080/13557858.2020.1821175 [DOI] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, Gupta A, Kelaher M, & Gee G (2015). Racism as a Determinant of Health: A Systematic Review and Meta-Analysis. PLOS ONE, 10(9), e0138511. 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek MK, Cutchin MP, Salinas JJ, Sheffield KM, Eschbach K, Stowe RP, & Goodwin JS (2010). Allostatic Load Among Non-Hispanic Whites, Non-Hispanic Blacks, and People of Mexican Origin: Effects of Ethnicity, Nativity, and Acculturation. American Journal of Public Health, 100(5), 940–946. 10.2105/AJPH.2007.129312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riosmena F, Wong R, & Palloni A (2012). Migration Selection, Protection, and Acculturation in Health: A Binational Perspective on Older Adults. Demography, 50(3), 1039–1064. 10.1007/s13524-012-0178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar CR, Strizich G, Seeman TE, Isasi CR, Gallo LC, Avilés-Santa LM, Cai J, Penedo FJ, Arguelles W, Sanders AE, Lipton RB, & Kaplan RC (2016). Nativity differences in allostatic load by age, sex, and Hispanic background from the Hispanic Community Health Study/Study of Latinos. SSM - Population Health, 2, 416–424. 10.1016/j.ssmph.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholaske L, Wadhwa PD, & Entringer S (2021). Acculturation and biological stress markers: A systematic review. Psychoneuroendocrinology, 132, 105349. 10.1016/j.psyneuen.2021.105349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, & Karlamangla A (2008). Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994). Social Science & Medicine, 66(1), 72–87. 10.1016/j.socscimed.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Barr AB, Simons LG, Gibbons FX, & Philibert RA (2018). Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Developmental Psychology, 54(10), 1993–2006. 10.1037/dev0000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís CB, Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, Lang T, & Delpierre C (2015). Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proceedings of the National Academy of Sciences, 112(7), E738–E746. 10.1073/pnas.1417325112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, & Zaninotto P (2020). Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proceedings of the National Academy of Sciences, 117(26), 14911–14917. 10.1073/pnas.1915741117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, & Harshfield GA (2015). Adverse Childhood Experiences and Blood Pressure Trajectories from Childhood to Young Adulthood: The Georgia Stress and Heart Study. Circulation, 131(19), 1674–1681. 10.1161/CIRCULATIONAHA.114.013104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Gerken K, Schorpp K, Boen C, & Harris KM (2017). Early-Life Socioeconomic Status and Adult Physiological Functioning: A Life Course Examination of Biosocial Mechanisms. Biodemography and Social Biology, 63(2), 87–103. 10.1080/19485565.2017.1279536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Schorpp K, Boen C, Johnson M, & Harris KM (2020). Socioeconomic Status and Biological Risks for Health and Illness Across the Life Course. The Journals of Gerontology: Series B, 75(3), 613–624. 10.1093/geronb/gby108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Manton KG, & Vaupel JW (1985). Mortality and aging in a heterogeneous population: A stochastic process model with observed and unobserved variables. Theoretical Population Biology, 27(2), 154–175. 10.1016/0040-5809(85)90008-5 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hayward MD, & Yu Y-L (2016). Life Course Pathways to Racial Disparities in Cognitive Impairment among Older Americans. Journal of Health and Social Behavior, 57(2), 184–199. 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


