Abstract
The plasma membrane glucose transporter-2 (GLUT2) monitors brain cell uptake of the critical nutrient glucose, and functions within astrocytes of as-yet-unknown location to control glucose counter-regulation. Hypothalamic astrocyte-neuron metabolic coupling provides vital cues to the neural glucostatic network. Current research utilized an established hypothalamic primary astrocyte culture model along with gene knockdown tools to investigate whether GLUT2 imposes sex-specific regulation of glucose/energy sensor function and glycogen metabolism in this cell population. Data show that GLUT2 stimulates or inhibits glucokinase (GCK) expression in glucose-supplied versus -deprived male astrocytes, but does not control this protein in female. Astrocyte 5’-AMP-activated protein kinaseα1/2 (AMPK) protein is augmented by GLUT2 in each sex, but phosphoAMPKα1/2 is coincidently up- (male) or down- (female) regulated. GLUT2 effects on glycogen synthase (GS) diverges in the two sexes, but direction of this control is reversed by glucoprivation in each sex. GLUT2 increases (male) or decreases (female) glycogen phosphorylase-brain type (GPbb) protein during glucoprivation, yet simultaneously inhibits (male) or stimulates (female) GP-muscle type (GPmm) expression. Astrocyte glycogen accumulation is restrained by GLUT2 when glucose is present (male) or absent (both sexes). Outcomes disclose sex-dependent GLUT2 control of the astrocyte glycolytic pathway sensor GCK. Data show that glucose status determines GLUT2 regulation of GS (both sexes), GPbb (female), and GPmm (male), and that GLUT2 imposes opposite control of GS, GPbb, and GPmm profiles between sexes during glucoprivation. Ongoing studies aim to investigate molecular mechanisms underlying sex-dimorphic GLUT2 regulation of hypothalamic astrocyte metabolic-sensory and glycogen metabolic proteins, and to characterize effects of sex-specific astrocyte target protein responses to GLUT2 on glucose regulation.
Keywords: GLUT2, glycogen phosphorylase, glucokinase, AMPK, glycogen, sex differences
Introduction:
Glucose is the primary energy fuel delivered to the brain, where it is consumed at a disproportionately high rate relative to other organs. Decrements in blood glucose concentrations imperil high energy-demand neuron functions, including maintenance of transmembrane electrolyte gradients and synaptic transmission, and thus pose a risk for neurological impairment or injury to vulnerable nerve cell populations. The brain glucostatic regulatory network receives dynamic sensory feedback from metabolic detectors located in discrete locations in the central nervous system (CNS) and peripheral organs and blood vessels. The hypothalamus, the principal autonomic motor center in the brain, exerts final common control of autonomic, neuroendocrine, and behavioral outflow that maintains blood glucose profiles within an optimal physiological range.
Several metabolic-sensory mechanisms are employed in the brain, including monitoring of glucose at the key junctures of cellular uptake and entry into the glycolytic pathway. The facilitative glucose transporter glucose transporter-2 (GLUT2) is an integral SLC2 gene-encoded membrane protein that belongs to the major facilitator transporter superfamily [1–3]. GLUT2 is distinguished from other GLUT family proteins by its low affinity for glucose, e.g. Km = 17 mM, which infers that GLUT2 glucose transport varies with physiological changes in glucose concentrations and does not limit glucose utilization [4]. GLUT2 is expressed in multiple brain cell types, including neurons and astrocytes [5,6]. This unique glucose transporter is implicated in CNS glucose sensing by reports that GLUT2 function within the astrocyte cell compartment is critical for neuroglucopenia-stimulated counter-regulatory hormone secretion [7]. Yet, the neuroanatomical distribution of astrocytes that utilize GLUT2 sensory information to shape glucose-regulation remains unknown.
Astrocytes actively participate in neuro-metabolic homeostasis through maintenance of a glycogen energy reserve that can be disassembled to yield the oxidizable metabolic fuel L-lactate for trafficking to neurons [8–12]. Hypothalamic astrocytes control glucose-regulatory neurotransmitter signaling through 5’-AMP-activated protein kinase (AMPK)-dependent glycogen-derived lactate signaling [13–15]. Hypothalamic astrocytes express GLUT2 [16]. Current research utilized an established rat hypothalamic astrocyte primary cell culture model [17–19] to investigate the hypothesis that GLUT2 may regulate energy stability and glycogen metabolism in these glial cells according to sex. Consideration here of potential sex differences in hypothalamic astrocyte target protein control by GLUT2, within the context of U.S. National Institutes of Health current policy emphasis on consideration of sex as a critical biological variable, was impelled by evidence that glycogen mass differs significantly between male versus female astrocytes [16,17]. Studies described herein employed siRNA gene silencing tools, in conjunction with Western blotting and uHPLC-electrospray ionization-mass spectrometry, to examine if and how GLUT2 gene knockdown may affect expression profiles of the specialized hexokinase glucokinase (GCK; rate-limiting glycolytic pathway enzyme and glucose sensor [20]), total and activated, i.e. phosphorylated AMPKα1/2 (pAMPK), and glycogen metabolic enzymes and net glycogen amassment, respectively, in glucose-supplied versus glucose-deprived astrocytes from each sex.
Glycogen metabolism is controlled by antagonistic actions of glycogen synthase (GS) and glycogen phosphorylase (GP), which correspondingly catalyze glycogen synthesis or glycogenolysis, GP is converted from a non-active to an active conformation by Ser-14 phosphorylation and/or binding of the allosteric activator AMP. GP-muscle type (GPmm) and GP-brain (GPbb) type isoforms are expressed in the brain [21]. Our studies document co-expression of these GP variants in hypothalamic astrocytes [19,22]. GPmm and GPbb exhibit significant differences in activation by the effectors described above, as phosphorylation causes complete versus partial activation of GPmm or GPbb, respectively, whereas AMP more potently activates GPbb compared to GPmm and is required for maximal GPbb Km and function [21]. Reports that GPmm and GPbb respectively mediate noradrenergic or glucoprivic induction of astrocyte glycogen glycogenolysis [23] support the notion that these isoforms confer physiological stimulus-specific regulation of glycogen mobilization in the brain. Present studies addressed the premise that GLUT2 may selectively regulate glucoprivic-sensitive GPbb expression in hypothalamic astrocytes, and that such control may differ in magnitude or direction between sexes.
Glucokinase regulatory protein (GKRP) controls GCK enzyme activity and subcellular localization via formation of GKRP-GCK complexes [24–26]. Glucose and GKRP compete for binding to GCK. Few studies have focused on the neuroanatomical distribution of GKRP in brain [27,28], and the identity of brain cell types that express this regulatory enzyme is not known. In hepatocytes, GKRP-GCK complexes that form when intracellular glucose levels decline translocate from the cytoplasm to the nucleus, resulting in compartmentalization of deactivated GCK [28b]. It is unclear if GKRP-GCK complexes formed in distinctive brain cell types in response to glucose deficiency are located in the cytoplasm and/or nucleus. A corollary objective of current work was to determine if GKRP protein is expressed in hypothalamic astrocytes of each sex; investigate whether total cell content of this protein is affected similarly or differently by glucoprivation in these cells from male versus female; and examine whether GKRP expression profiles are governed by GLUT2 when glucose is present and/or absent in one or both sexes.
Materials and Methods:
Primary astrocyte cell cultures:
High-purity primary astrocyte cultures were prepared from dissected adult (2–3 months of age) male or female rat hypothalami, as described [17–19]. All animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, 8th Edition, with ULM Institutional Animal Care and Use Committee approval. In brief, whole-hypothalamus tissue blocks were digested by 2.5% trypsin (prod. no. 15090–046; Thermo-Fisher Scientific, Waltham, MA) treatment and pipet-dissociated into a single-cell suspension in DMEM high-glucose media [prod. no. 12800–017; ThermoFisherScientific (ThermoFisherSci), Waltham, MA] augmented with 10.0% heat-inactivated fetal bovine serum (FBS; GE Healthcare Bio-Sciences, Pittsburgh, PA) and 1.0% penicillin/streptomycin (prod. no. 15140–122, ThermoFisherSci.). For each astrocyte collection, dissociated cells from three hypothalami were pooled by suspension in an adjusted media volume of 20 mL astrocyte plating media, plated in Poly-D-lysine (prod. no. A-003-E, MilliporeSigma, Burlington, MA) - coated T75 culture flasks at a concentration of 50 μg/mL, and incubated at 37 °C in a humidified environment in the presence of 5% CO2 with media changes every 2–3 days post-plating. After two weeks, microglia and oligodendrocytes were removed as reported [17–19]. Purified astrocytes were incubated for 12–14 days before plating (1 × 106 cells/100 mm2) in poly-D-lysine – coated culture dishes and growth ahead of experimentation. Routine Western blot and immunofluorescence cytochemical detection of the astrocyte marker protein glial fibrillary acidic protein (GFAP) showed that culture purity exceeded 95% [17–19].
Experimental Design:
Cultures reaching approximately 70% confluency were incubated (18 hr) in DMEM high-glucose media containing 5.0% charcoal-stripped FBS (prod. no. 12676029; ThermoFisherSci.). Astrocytes from each sex were pretreated by 72 hr incubation with Accell™ controlled non-targeting pool (scramble; SCR) siRNA (prod. no. D-001910–10-20, 5.0 nM; Horizon Discovery, Waterbeach, UK) or Accell™ rat GLUT2si RNA (prod. no. A-099803–14-0010, 5.0 mM, Horizon Disc.) dissolved in siRNA buffer (prod. no. B-002000-UB-100; Horizon Discovery) in high-glucose DMEM media. Optimization of GLUT2 siRNA dosing was achieved by Western blot analysis of gene product knockdown caused by graded, i.e. 0.2, 1.0, 5.0 or 25.0 nM titrant doses. SCR or GLUT2 siRNA-pretreated astrocytes were incubated for 8 hr with 10 nM 17β-estradiol – supplemented HBSS media [17] containing 5.5 mM (G5.5) or 0 mM (G0) glucose. Cells were detached for suspension in lysis buffer, e.g. 2.0% sodium dodecyl sulfate, 10.0% glycerol, 5% β-mercaptoethanol, 1 mM sodium orthovanadate, 60 mM Tris-HCl, pH 6.8, or ultra-pure water for Western blot protein or uHPLC-electrospray ionization-mass spectrometric (LC-ESI-MS) glycogen analyses, respectively.
Western Blot Analysis:
Astrocyte cell pellets were heat-denatured (10 min, 95°C), sonicated, centrifuged, and diluted with 2x Laemmli buffer. Lysate sample protein concentrations were determined by NanoDrop spectrophotometry (prod. no. ND-ONE-W, ThermoFisherSci.). For each protein of interest, sample aliquots of equivalent protein mass for each treatment group were loaded for separation in Bio-Rad Stain Free 10% acrylamide gels (prod. no 161–0183, Bio-Rad, Hercules, CA). After UV gel activation (1 min) in a Bio-Rad ChemiDoc™ Touch Imaging System, proteins were transblotted to 0.45-μm PVDF-Plus membranes (prod. no. 1212639; Data Support Co., Panorama City, CA). For each sex, protein analyses were carried out in triplicate independent experiments, involving a minimum of three separate astrocyte collections. Membrane buffer washes and antibody incubations were performed by Freedom Rocker™ Blotbot® automation (Next Advance, Inc., Troy NY). After blocking (2 hr) with Tris-buffered saline (TBS), pH 7.4, containing 0.2 % Tween-20 (prod. no. 9005–64-5; VWR, Radnor, PA) and 2% bovine serum albumin (BSA) (prod. no. 9048–46-8; VWR), membranes were incubated (24–48 hr; 4°C) with primary antisera raised in rabbit against GLUT2 (prod. no. PA5–97263; 1:1000, ThermoFisherSci.; RRID: AB_2809065), GCK (prod. no. bs-1796R; 1:1500; Bioss Antibodies, Inc., Woburn, MA), GKRP (prod. no. NBP2–03396; 1:1500; Novus Biologicals, Littleton, CO), AMPKα1/2 (AMPK; prod. no. 2532S; 1:2000; Cell Signaling Technology, Danvers, MA).; RRID: AB-330331), phosphoAMPKα1/2-Thr 172 (pAMPK; prod. no. 2535S; 1:1600; Cell Signal. Technol.; RRID: AB_331250), GS (prod. no. 3893S; 1: 2,000; Cell Signal. Technol.; RRID: AB_2279563); GPbb (prod. no. NBP1–32799; 1:2,000; Novus Biol.; RRID: AB-2253353), or GPmm (prod. no. NBP2–16689; 1: 2,000; Novus Biol.), as described [22]. Membranes were sequentially incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (prod. no. NEF812001EA; 1:4,000; PerkinElmer, Waltham, MA) for 1 hr, followed by SuperSignal West Femto maximum sensitivity chemiluminescent substrate (prod. no. 34096; ThermoFisherSci.). Target protein optical density (O.D.) signals were quantified in the ChemiDoc™ Touch Imaging System described above, and normalized to total in-lane protein, e.g. all protein electrophoresed in the individual sample lane, using Bio-Rad proprietary stain-free imaging gel technology and Image Lab™ 6.0.0 software, as reported [13,14,17–19]. Precision plus protein molecular weight dual color standards (prod. no. 161–0374, Bio-Rad) were included in each Western blot analysis.
Astrocyte LC-ESI-MS Glycogen Analysis:
Astrocyte glycogen concentrations were measured as reported [17–19,29]. Cell lysate supernatant 10 μL aliquots were hydrolyzed by incubation (2 hr) with 10 μL 0.5 mg/mL amyloglucosidase and 10 μL 0.1M sodium acetate, pH 5.0, followed by heating (100 °C, 5 min), then cooling to room temperature. Glycogen was measured in a ThermoFisherScientific Vanquish UHPLC + System equipped with ThermoScientific™ Dionex™ Chromeleon™ 7 Chromatography Data System software [29]. Column and autosampler temperatures were maintained at 35°C and 15°C, respectively. The auto-sampler needle was washed (10 sec) with 10% (v/v) methanol. Hydrolyzed and non-hydrolyzed samples were derivatized with 100 μL 0.5 M 1-phenyl-3-methyl-5-pyrazolone (PMP) reagent supplemented with 0.3 M NaOH, then acidified with 400 μL 0.75% formic acid and extracted with chloroform to remove excess PMP. Sample supernatant (400 μL) was vacuum-concentrated to remove organic solvents, frozen at −80 °C, and lyophilized. Lyophilization product was diluted to 1.0 mL with 10 mM ammonium acetate, bath-sonicated (30 sec), and centrifuged. Supernatant aliquots (250 μL) were transferred to 350 μL inserts, which were placed in 2 mL Surestop vials in an autosampler tray. D-(+)-Glucose-PMP was resolved using a Shodex™ Asahipak™ NH2P-40 3E column with acetonitrile:10 mM ammonium acetate (75:25 v/v; 0.2 mL/min) as mobile phase. D-(+)-Glucose-PMP ion chromatograms were extracted from Total Ion Current (TIC) at m/z 510.2 to generate area-under-the curve data. Critical LC-ESI-MS parameters such as sheath gas pressure (SGP; 25 psig), auxiliary gas pressure (AGP; 4.6 psig), sweep gas pressure (SWGP; 0.5 psig), vaporizer temperature (VT; 150 °C), ion transfer tube temperature (ITT; 150 °C), source voltage (−2000V), foreline pressure (1.76 Torr; auto-set by instrument- and variable), source gas (nitrogen; Genius NM32LA 110V, 10–6520; Peak Scientific, Inchinnan, Scotland), and mass peak area detection algorithm (ICIS/Genesis) were maintained at pre-determined optimum settings.
Statistics:
Mean normalized protein O.D. and glycogen values were evaluated between treatment groups by three-way analysis of variance and Student Newman Keuls post-hoc test. Differences of p<0.05 were considered significant. In each figure, statistical differences between specific pairs of treatment groups are denoted as follows: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Results:
Current studies employed an siRNA knockdown approach to examine whether diminished hypothalamic astrocyte GLUT2 expression has corresponding effects on nutrient/energy sensor and glycogen metabolic protein profiles in this cell type. Results presented in Figure 1 depict effects of GLUT2 gene knockdown on GLU2 protein expression in hypothalamic astrocyte primary cultures derived from male (at left; gray bars) versus female (at right; white bars) rats. Data show that in each sex, GLUT2 protein profiles were significantly reduced in primary cultures pretreated with GLUT2 versus SCR siRNA prior to incubation in the presence of 5.5 [G5.5/GLUT siRNA (horizontal-striped bars; M: gray, F: white) versus G5.5/SCR siRNA (solid bars; M: gray, F: white)] or 0 [G0/GLUT siRNA (cross-hatched bars; M: gray, F: white) versus G/SCR siRNA (diagonal-striped bars; M: gray, F: white)] mM glucose. These data verify the efficacy of the GLUT2 siRNA tool used here for down-regulation of the gene product GLUT2 in hypothalamic astrocytes of each sex.
Figure 1.
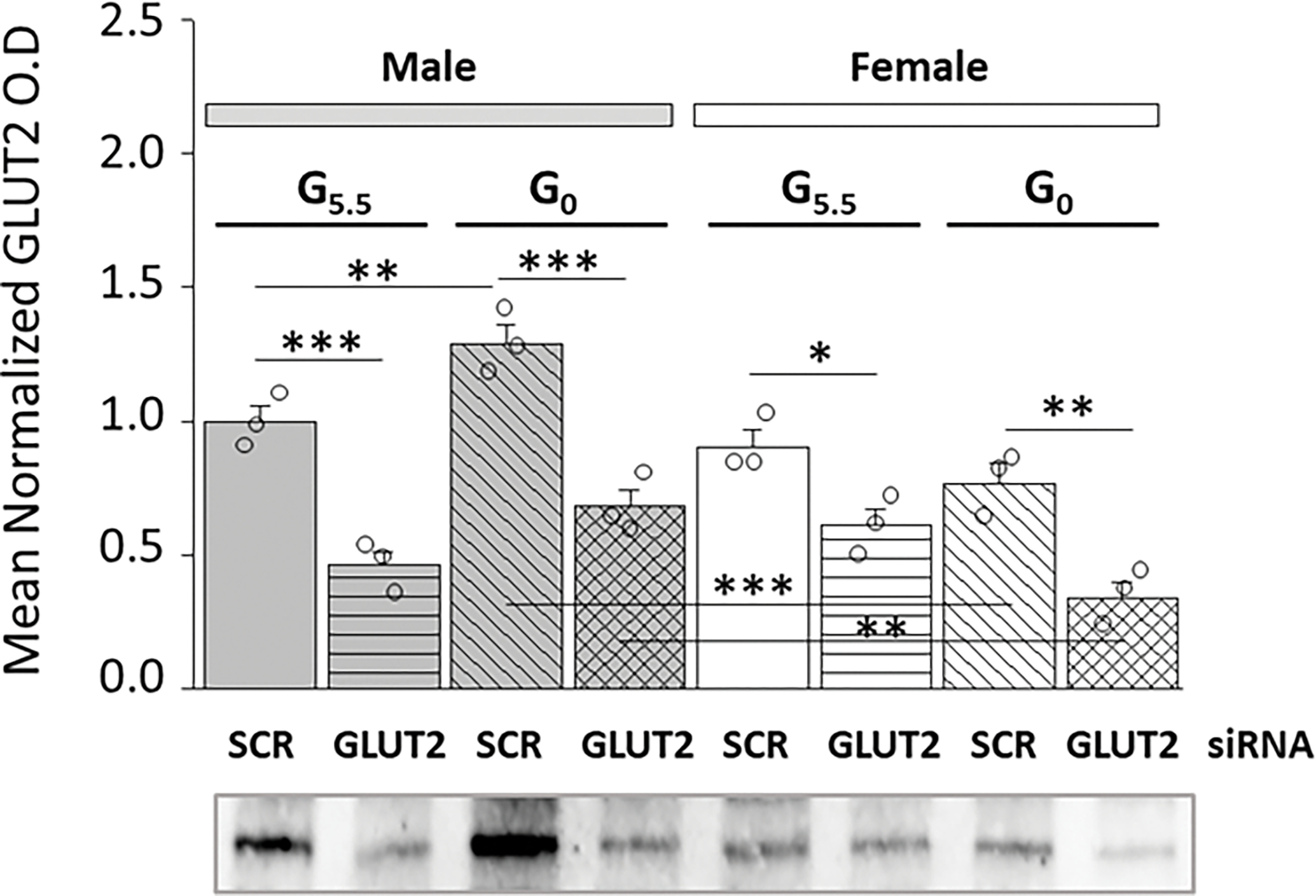
Effects of Glucose Transporter-2 (GLUT2) GLUT2 siRNA on Male and Female Hypothalamic Primary Astrocyte GLUT2 Protein Expression. Confluent male and female rat hypothalamic primary astrocyte cultures were steroid-deprived (18 hr) before pretreatment (72 hr) with control, i.e. scramble (SCR) short-interfering RNA (siRNA) or siRNA designed to knockdown rat GLUT2. Cells were then incubated (4 hr) in the presence of 5.5 (G5.5) or 0 (G0) mM glucose. Astrocyte lysate aliquots were analyzed in triplicate by stain-free Western blot for GLUT2 protein content. Target protein optical density (O.D.) measures obtained in a Bio-Rad ChemiDoc™ Touch Imaging System were normalized to total in-lane protein, e.g. all protein electrophoresed in the individual sample lane using Bio-Rad Image Lab™ 6.0.0 software. Data depict mean normalized GLUT2 protein O.D. values ± S.E.M. for male (M; gray bars, at left) and female (F; white bars, at right) G5.5- or G0-exposed astrocytes pretreated with SCR versus GLUT2 siRNA. Treatment groups were comprised of male and female astrocytes treated with G5.5/SCR siRNA [solid bars (gray, M; white, F)]; G5.5/GLUT2 [horizontal-striped bars (gray, M; white, F)]; G0/SCR siRNA [diagonal-striped bars (gray, M; white, F)]; or G0/GLUT2 siRNA [cross-hatched bars (gray, M; white, F)]. Mean normalized O.D. data were analyzed by three-way ANOVA and Student-Neuman-Keuls post-hoc test, using GraphPad Prism (Volume 8) software: F7,16=23.17, p<0.001, 1-β=1.000; Sex main effect: F1,16=21.74, p<0.001; siRNA main effect: F1,16=107.66, p<0.001; Glucoprivic (GP) main effect: F1,16=0.25, p=0.626; Sex/siRNA interaction: F1,16=5.18, p=0.037; Sex/GP interaction: F1,8=25.76, p<0.001; siRNA/GP interaction: F1,16=1.50, p=0.239; Sex/siRNA/GP interaction: F1,16=0.08, p=0.780 (Supplementary Table 1). Statistical differences between discrete pairs of treatment groups are denoted as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Current studies investigated the premise that the plasma membrane glucose sensor GLUT2 may regulate GCK, a critical intracellular checkpoint at which glucose is phosphorylated ahead of entry into the glycolytic pathway or glycogen shunt. Data shown in Figure 2 illustrate effects of GLUT2 siRNA pretreatment on GCK (Figure 2A) or GKRP (Figure 2B) protein expression patterns in male versus female glucose-supplied or glucose-deprived astrocytes. Outcomes presented in Figure 2A show that GCK protein profiles were augmented by GLUT2 siRNA in glucose-suppled male astrocytes [G5.5/GLUT siRNA (gray horizontal-striped bar) versus G5.5/SCR siRNA (gray solid bar)], but were unaffected by GLUT2 gene repression in the female when glucose is present. In each sex, astrocytes incubated in the absence of glucose showed marked suppression of GCK protein relative to glucose-supplied glial cells [G0/SCR siRNA (horizontal-striped bars; M: gray, F: white) versus G5.5/SCR siRNA (solid bars; M: gray, F: white)]. GLUT2 gene knockdown elevated GCK protein levels in glucose-deprived male [G0/GLUT siRNA (gray cross-hatched bar) versus G0/SCR siRNA (gray diagonal-striped bar)] and female [G0/GLUT siRNA (white cross-hatched bar) versus G0/SCR siRNA (white diagonal-striped bars] astrocytes. As shown in Figure 2B, GLUT2 enhances GKRP expression in male, but not female glucose-supplied astrocytes. Glucose starvation did not modify GKRP profiles in male astrocytes, but suppressed this protein in female. GLUT2 knockdown decreased or increased GKRP protein levels in glucose-deprived male or female astrocytes, respectively. Outcomes here indicate that GLUT2 stimulates GCK protein in male only in the presence of glucose, but inhibits this protein in both sexes in the absence of this nutrient. Data also show that GLUT2 up-regulates male hypothalamic astrocyte GKRP protein expression regardless of glucose status, and that is protein is controlled by GLUT2 control in the female upon withdrawal of glucose.
Figure 2.
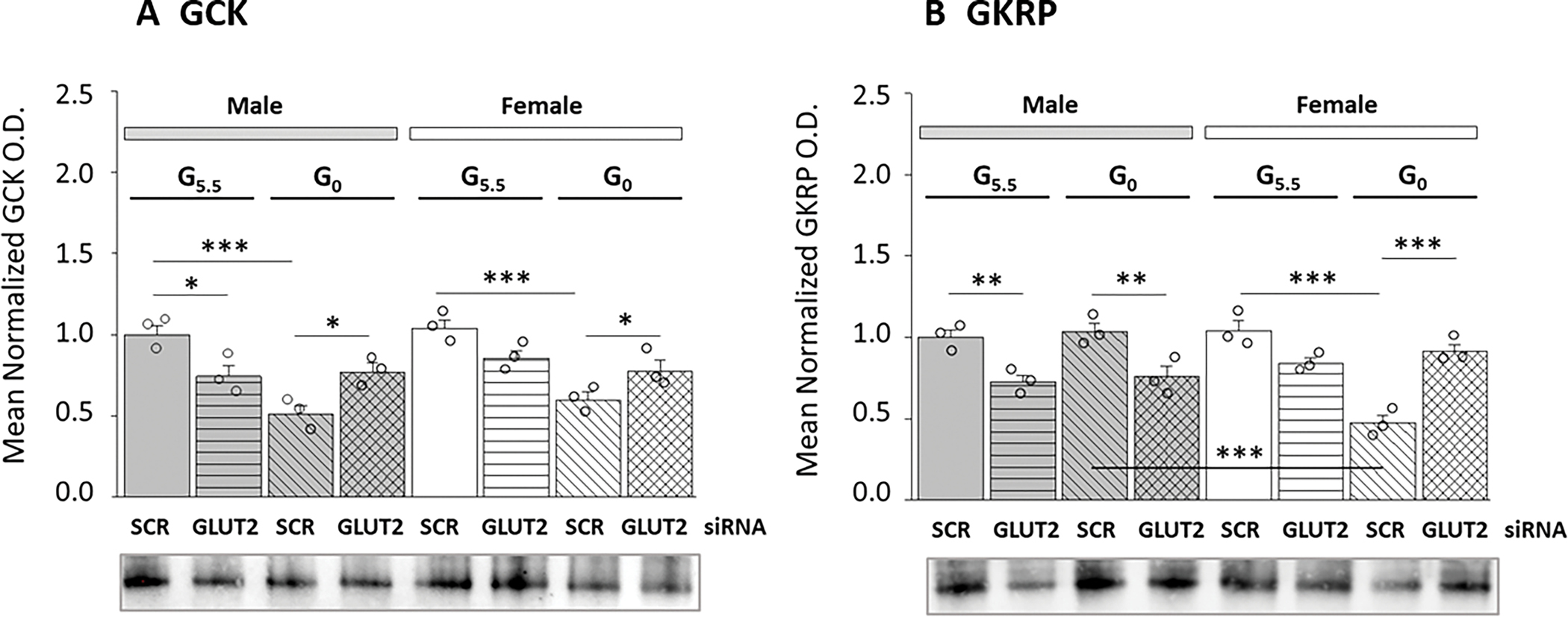
GLUT2 Regulation of Glucokinase (GCK) and Glucokinase Regulatory Protein (GKRP) Protein Expression in Male and Female Hypothalamic Astrocytes Incubated with 5.5 or 0 mM Glucose. Mean O.D. data illustrate mean normalized GCK (Figure 2A) or GKRP (Figure 2B) O.D. measures ± S.E.M. for male astrocyte [M-G5.5/SCR siRNA, M-G5.5/GLUT2 siRNA, M-G0/SCR siRNA, M-G0/GLUT2 siRNA] and female astrocyte [F-G5.5/SCR siRNA; F-G5.5/GLUT2 siRNA; F-G0/SCR siRNA; F-G0/GLUT2 siRNA] treatment groups (n=3/treatment group). GCK or GKRP data were analyzed by three-way ANOVA and Student-Newman-Keuls using GraphPad Prism (Volume 8): GCK: F7,16=10.17, p<0.001, 1-β=1.000; Sex main effect: F1,16=2.24, p=0.154; siRNA main effect: F1,16=0.003, p=0.958; Glucoprivic (GP) main effect: F1,16=38.02, p<0.001; Sex/siRNA interaction: F1,16=0.01, p=0.946; Sex/GP interaction: F1,8=0.15, p=0.704; siRNA/GP interaction: F1,16=29.86, p<0.001; Sex/siRNA/GP interaction: F1,16=0.93, p=0.349; GKRP: F7,16=16.06, p<0.001, 1-β=1.000; Sex main effect: F1,16=3.33, p=0.086; siRNA main effect: F1,16=5.29, p=0.035; Glucoprivic (GP) main effect: F1,16=9.51, p=0.007; Sex/siRNA interaction: F1,16=33.71, p<0.001; Sex/GP interaction: F1,8=17.26, p<0.001; siRNA/GP interaction: F1,16=21.58, p<0.001; Sex/siRNA/GP interaction: F1,16=21.73, p<0.001 (Supplementary Table 1). Statistical differences between specific treatment group pairs are denoted as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Present research examined whether GLUT2 controls expression and/or phosphorylation of the energy sensor protein AMPK in hypothalamic astrocytes. Figure 3 illustrates GLUT2 gene knockdown effects on astrocyte AMPK (Figure 3A) and pAMPK (Figure 3B) protein expression in male versus female hypothalamic astrocytes. Data indicate that GLUT2 siRNA decreased AMPK protein levels in glucose-supplied or -deprived astrocytes of each sex (Figure 3A). Glucose starvation elevated male astrocyte AMPK content, but did not modify this protein profile in the female. As shown in Figure 3B, baseline astrocyte pAMPK levels were higher in male versus female. The direction of GLUT2 control of pAMPK expression in glucose-supplied or –deprived astrocytes is sex-specific as GLUT2 siRNA decreased (male) or increased (female) pAMPK protein content regardless of the presence or absence of glucose, inferring that this sensor respectively augments or suppresses pAMPK protein in male versus female. Results show that GLUT2 signaling up-regulates total AMPK protein content in astrocytes of each sex, but that this plasma membrane sensor imposes divergent, sex-specific control of pAMPK, the activated form of AMPK.
Figure 3.
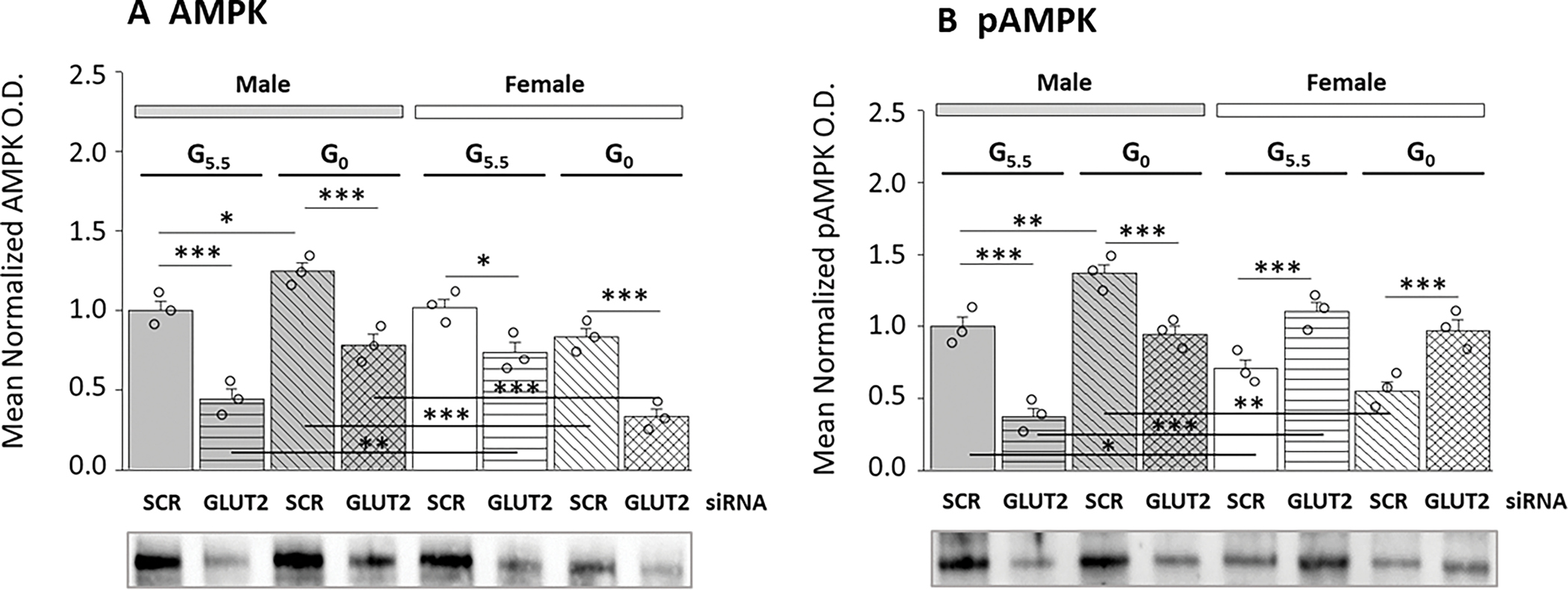
Hypothalamic Astrocyte 5-AMP-Activated Protein Kinaseα1/2 (AMPK) and Phosphorylated AMPKα1/2 (pAMPK) Protein Expression Profiles: Effects of SCR versus GLUT2 siRNA Pretreatment. Data illustrate mean normalized AMPK (Figure 3A) and pAMPK (Figures 3B) protein O.D. values ± S.E.M. for treatment groups of male and female astrocytes treated with G5.5/SCR siRNA [solid bars (gray, M; white, F)]; G5.5/GLUT2 [horizontal-striped bars (gray, M; white, F)]; G0/SCR siRNA [diagonal-striped bars (gray, M; white, F)]; or G0/GLUT2 siRNA [cross-hatched bars (gray, M; white, F)] (n=3/ group). Statistical analyses performed using GraphPad Prism (Volume 8) yielded the following outcomes for AMPK [F7,16=27.09, p<0.001, 1-β=1.000; Sex main effect: F1,16=11.64, p=0.004; siRNA main effect: F1,16=120.25, p<0.001; Glucoprivic (GP) main effect: F1,16=0.001, p=0.973; Sex/siRNA interaction: F1,16=2.12, p=0.165; Sex/GP interaction: F1,8=51.41, p<0.001; siRNA/GP interaction: F1,16=0.54, p=0.472; Sex/siRNA/GP interaction: F1,16=3.66, p=0.074] and pAMPK [F7,16=24.34, p<0.001, 1-β=1.000; Sex main effect: F1,16=3.97, p=0.063; siRNA main effect: F1,16=1.70, p=0.210; Glucoprivic (GP) main effect: F1,16=12.91, p=0.002; Sex/siRNA interaction: F1,16=103.75, p<0.001; Sex/GP interaction: F1,8=45.54, p<0.001; siRNA/GP interaction: F1,16=1.52, p=0.236; Sex/siRNA/GP interaction: F1,16=1.03, p=0.335] data (Supplementary Table 1). Statistical differences between specific treatment group pairs are denoted as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
A substantial fraction of glucose internalized by astrocytes passes through the glycogen shunt, which involves sequential glucose incorporation into and liberation from this polymer, prior to glycolytic catabolism. Current work addressed the premise that GLUT2 controls expression levels of enzyme proteins that incorporate or disassemble hypothalamic astrocyte glycogen and net glycogen accumulation. Data in Figure 4 show effects of GLUT2 siRNA pretreatment on male versus female hypothalamic astrocyte glycogen metabolic enzyme protein expression (Figures 4A–C) and glycogen content (Figure 4D) after incubation in media containing 5.5 or 0 mM glucose. Data show that baseline GS protein levels were relatively higher in female compared to male astrocytes. GLUT2 gene repression respectively diminished or augmented GS protein content in male versus female astrocytes (Figure 4A). Glucoprivation decreased (male) or elevated (female) GS protein expression according to sex. GLUT2 input suppressed or elevated GS levels in glucose-deprived male versus female astrocytes. As shown in Figure 4B, baseline GPbb protein levels were higher in male compared to female astrocytes. GLUT2 siRNA significantly decreased this profile in male, but did not modify GPbb protein expression in the other sex. Glucoprivation also decreased GPbb protein in the male, yet had no impact on this protein profile in the female. GLUT2 input was observed to enhance GPbb expression in glucose-deprived male astrocytes, yet to suppression this protein profile in glucose-starved female astrocytes. GPmm protein levels were greater in female versus male astrocytes, and were up-regulated by GLUT2 gene knockdown in each sex (Figure 4C). Results show that GLUT2 imposes divergent control of hypothalamic astrocyte GS protein expression in the two sexes, and that in each sex the direction of this control is determined by glucose status. Outcomes also document differential expression of the GP isoforms GPbb and GPmm between the two sexes, and sex-specific regulation of each isoform by GLUT2.
Figure 4.
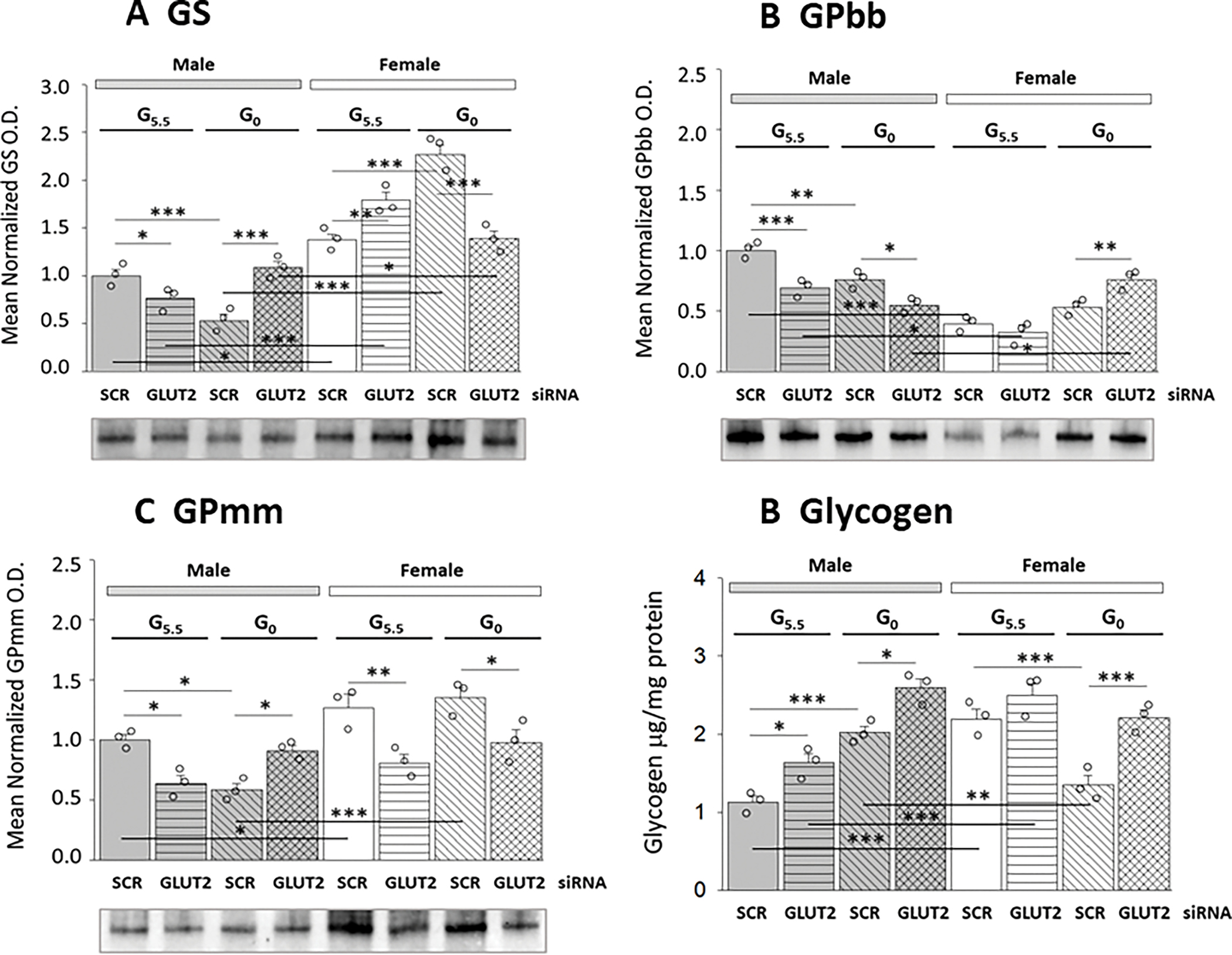
GLUT2 Regulation of Glycogen Metabolic Enzyme Protein Expression Profiles and Glycogen Mass in Glucose-Supplied or Glucose-Deprived Male and Female Hypothalamic Primary Astrocyte Cultures. Male and female hypothalamic astrocytes pretreated with SCR or GLUT2 siRNA were incubated in the presence of 5.5 (G5.5) or 0 (G0) mM glucose. Data from cell lysate Western blots are presented as mean normalized glycogen synthase (GS; Figure 4A), glycogen phosphorylase-brain type (GPbb; Figure 4B), and glycogen phosphorylase-muscle type (GPmm; Figure 4C protein O.D. measures ± S.E.M. for male and female astrocytes from G5.5/SCR siRNA; G5.5/GLUT2 siRNA; G0/SCR siRNA; and G0/GLUT2 siRNA treatment groups (n=3/group). Results from uHPLC-electrospray ionization-mass spectrometry glycogen analyses are depicted in Figure 4D as mean normalized astrocyte glycogen measures ± S.E.M. for male astrocyte treatment groups (n=3/group), at left [M-G5.5/SCR siRNA; M-G5.5/GLUT2 siRNA; M-G0/SCR siRNA; M-G0/GLUT2 siRNA] or female rats, at right [F-G5.5/SCR siRNA; F-G5.5/GLUT2 siRNA; F-G0/SCR siRNA; F-G0/GLUT2 siRNA]. GraphPad Prism (Volume 8) analysis of data yielded the following results: GS: F7,16=59.02, p<0.001, 1-β=1.000; Sex main effect: F1,16=279.25, p<0.001; siRNA main effect: F1,16=0.46, p=0.507; Glucoprivic (GP) main effect: F1,16=2.66, p=0.122; Sex/siRNA interaction: F1,16=14.29, p=0.002; Sex/GP interaction: F1,8=9.47, p=0.007; siRNA/GP interaction: F1,16=5.62, p=0.031; Sex/siRNA/GP interaction: F1,16=101.38, p<0.001; GPbb: F7,16=26.78, p<0.001, 1-β=1.000; Sex main effect: F1,16=67.51, p<0.001; siRNA main effect: F1,16=9.34, p=0.007; Glucoprivic (GP) main effect: F1,16=2.34, p=0.145; Sex/siRNA interaction: F1,16=32.38, p<0.001; Sex/GP interaction: F1,8=62.52, p<0.001; siRNA/GP interaction: F1,16=10.62, p=0.005; Sex/siRNA/GP interaction: F1,16=2.77, p=0.116; GPmm: F7,16=12.57, p<0.001, 1-β=1.000; Sex main effect: F1,16=34.29, p<0.001; siRNA main effect: F1,16=16.14, p<0.001; Glucoprivic (GP) main effect: F1,16=0.30, p=0.593; Sex/siRNA interaction: F1,16=13.56, p=0.002; Sex/GP interaction: F1,8=3.37, p=0.085; siRNA/GP interaction: F1,16=12.59, p=0.002; Sex/siRNA/GP interaction: F1,16=7.77, p=0.013; glycogen: F7,16= 21.92, p<0.001, 1-β=1.000; Sex main effect: F1,16=7.31, p=0.015; siRNA main effect: F1,16=48.45, p<0.001; Glucoprivic (GP) main effect: F1,16=5.14, p=0.038; Sex/siRNA interaction: F1,16=0.06, p=0.803; Sex/GP interaction: F1,8=86.40, p<0.001; siRNA/GP interaction: F1,16=3.79, p=0.069; Sex/siRNA/GP interaction: F1,16=0.15, p=0.152 (Supplementary Table 1). Statistical differences between treatment group pairs is indicated as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Data in Figure 4D show that astrocyte glycogen content in the female exceeds that measured in the male, and is regulated by GLUT2 in the latter sex only. Glucose starvation caused opposite adjustments in glycogen. i.e. up-regulation in the male versus down-regulation in the female. GLUT2 gene knockdown further amplified glycogen levels in the male and reversed glucoprivic diminution of glycogen in the female. Outcomes indicate that GLUT2 acts to curtain glycogen accumulation in male astrocytes whether glucose is present or absent, but mediates breakdown of this fuel reserve in the female in response to glucose withdrawal.
Discussion:
Current studies investigated the hypothesis that the plasma membrane glucose sensor GLUT2 exerts sex-dimorphic control of hypothalamic astrocyte glycolytic pathway function, energy stability, and glycogen metabolism. Data show that GLUT2 regulates GCK protein in male, but not female astrocytes. Results provide unique documentation of hypothalamic astrocyte GKRP expression. Baseline GKRP profiles were altered to a statistically significant extent by GLUT2 knockdown in male astrocytes only, whereas glucoprivic patterns of GKRP expression reflect divergent, i.e. stimulatory (male) versus inhibitory (female) GLUT input in the two sexes. GLUT2 up-(male) or down- (female) regulates hypothalamic astrocyte pAMPK expression in the presence or absence of glucose. GLUT2 regulation of the glycogen metabolic enzymes GS (male and female), GPbb (female), and GPmm (male) is dependent upon glucose status. In the absence of glucose, GLUT2 imposes contrary effects on glycogen enzyme protein expression and glycogen accumulation in male versus female. Further research is necessary to identify molecular mechanisms that enact sex-dimorphic GLUT2 control of hypothalamic astrocyte glucose catabolism, energy status, and glycogen metabolism. Current findings supply an impetus to investigate, from a systems-level perspective, how sex-specific GLUT2 regulation of these critical hypothalamic astrocyte functions may impact glucose-regulatory transmitter signaling and systemic glucose homeostasis [30,31]. Studies utilizing in vivo experimental approaches will thus be needed to validate the physiological relevance of hypothalamic astrocyte GLUT2 glucose monitoring in situ. Astrocytes are known substrates for glucose-sensitive endocrine and neurochemical regulatory signals. It would be insightful to learn if hypothalamic astrocytes function to integrate intrinsic GLUT2-derived cues on brain tissue glucose concentrations with metabolic cues originating outside the hypothalamus.
The large cytoplasmic loop domain of GLUT2 is implicated in metabolic detection of glucose independent of its transport [32]. While function of this cytoplasmic loop is critical for GLUT2-controlled feeding [33], there is scarce knowledge of the identity of the cell type(s) that provide sensory input from this glucose sensor to neural pathways that control systemic metabolic homeostasis or how those cells transduce GLUT2-sensed glucose volume into regulatory signals. Data reported here show that this sensor is present in pure hypothalamic astrocyte cultures derived from each sex, and that its expression levels are evidently controlled by glucose. This in vitro experimental model will provide a unique opportunity for ongoing investigation to determine the molecular basis, including potential signal transduction mechanisms for GLUT2 cytoplasmic loop glucose screening and to determine how glucose volume information may shape astrocyte release of regulatory glio-transmitters, including the substrate fuel lactate. Further experimentation involving whole-animal models will be necessary to verify that GLUT2 is present in this brain cell type in vivo, and to investigate whether glucose-sensitive endocrine (e.g. glucocorticoid [33b]) and/or neurochemical (e.g. norepinephrine [13]) signals known to act on astrocytes impose similar or differential control of GLUT2 expression in the two sexes.
Prior published research showed that GLUT2 and GCK are co-localized to glucose-regulatory hypothalamic loci [34,35]. Current work extends those findings with evidence for cellular-level co-expression of these membrane and metabolic pathway glucose sensors. It remains to be clarified whether GLUT2 and GCK proteins co-exist in additional hypothalamic cell types, e.g. neurons, tanycytes, etc. Present data provide unique proof of a regulatory link between membrane and cytoplasmic glucose sensors, which is operative in one, but not both sexes. Ongoing studies seek to ascertain why this control occurs solely in the male, if the GLUT2 cytoplasmic loop domain is required, and whether transcriptional and/or post-translational mechanisms are involved in this control. While astrocytes express additional GLUTs, including GLUT1 [36], glycolytic pathway function in the male astrocyte may be linked to transporter-specific glucose uptake and screening. Glucose withdrawal, achieved here using established experimental protocols [37–40], caused a stimulatory-to-inhibitory switch in direction of GLUT2 control of male astrocyte GCK expression, findings that emphasize that this sensor alters glycolytic pathway function in detected loss of glucose availability. It should be noted that this in vitro substrate fuel manipulation deviates from pathophysiological insulin dosage-dependent diminution of brain tissue glucose levels in vivo that may occur as a result of inadvertent iatrogenic insulin-induced hypoglycemia. Thus, ongoing work aims to determine if male astrocyte GLUT2 has the capacity to detect small, physiological-like decrements or increments in glucose availability and, moreover, utilize that sensory information to impose graded regulatory effects on expression of GCK and other target proteins. In light of current findings that female astrocyte GCK is refractory to GLUT2 control, down-regulation of glycolytic glucose catabolism in this sex during glucoprivation may be mediated by other factors including non-GLUT2-mediated glucose uptake.
Present results are among the first to ascribe GKRP expression to a distinct brain cell type, and document GLUT2 regulation of this protein in cells acquired from male, not female. GKRP profiles exhibit dissimilar responses to glucoprivation (unchanged in male; diminished in female) due in part to stimulatory (male) versus inhibitory (female) input from GLUT2. A plausible interpretation of these data is glucose-deprived male versus female astrocytes may correspondingly exhibit no net change or down-regulated numbers of glucose-free GCK molecules, despite decreased GCK protein expression in each sex. These findings raise the intriguing prospect that during glucose starvation, male astrocytes may undertake a lower rate of de novo glucose synthesis from non-carbohydrate substrates compared to female, thus resulting in dissimilar ratios of glucose-occupied versus -unoccupied GCK in the two sexes. GKRP functions in cultured liver hepatocytes as a nuclear retention factor, acting to sequester non-glucose – bound, i.e. inactive GCK from the cytoplasmic compartment [28b]. While current evidence for sex-specific hypothalamic astrocyte GKRP protein responses to glucose deprivation infers that GCK-GKRP complexation rates may vary according to sex under in vitro conditions, present data do not shed light on the subcellular distribution of these complexes in these glial cells during glucose deficiency.
Current data show that GLUT2 increases total AMPK protein expression in each sex, but imposes opposite, sex-specific (stimulatory in male; inhibitory in female) control of activated, e.g. pAMPK profiles. These findings constitute first-of-its kind evidence for membrane glucose sensor regulation of AMPK protein expression and activity. The mechanisms by which GLUT2 may regulate AMPK transcription and/or translation/posttranslational processing remain unclear. Data here show that glucoprivation increased AMPK expression in male, but not female astrocytes, despite GLUT2 stimulatory tone in each sex. These findings infer that facilitative GLUT2 signaling may be offset by suppressive cues to a relatively greater extent in female versus male. While current results suggest that GLUT2 may enhance or diminish astrocyte energy instability in male versus female astrocytes, respectively, regardless of glucose status, this interpretation remains speculative in the absence of quantitative measures of GLUT2 knockdown effects on the cellular AMP/ATP ratio. It can be speculated that GLUT2 may control hypothalamic astrocyte energy production differently in each sex, involving actions as-yet-unknown targets within mitochondrial tricarboxylic acid (TCA) cycle and oxidative respiratory pathways as well as substrates that govern energy utilization. Alternatively, sex-based dissimilarities in GLUT2-dependent patterns of pAMPK expression may reflect, in part, divergent levels of pyruvate introduced to the TCA cycle from the glycolytic pathway. As discussed above, further research is needed to examine how astrocyte GLUT2 sensing of hypothalamic tissue glucose decrements may regulate pAMPK expression in each sex in vivo.
Present outcomes provide novel evidence for GLUT2 regulation of hypothalamic astrocyte glycogen metabolism. Current evidence that female astrocyte GS protein levels exceed those in male correlates with greater glycogen content in the female. GLUT2 imposes divergent control of GP profiles in male (stimulatory) and female (inhibitory) astrocytes, which is reversed in direction in each sex during glucoprivation. Notably, glucose starvation down- or up-regulates GP expression in male versus female astrocytes alongside augmentation or diminution of glycogen, inferring that in each sex, glucose deficiency may have opposite regulatory effects on GS expression profiles versus enzyme specific activity. Evidence here that GLUT2 knockdown prevents GS protein adaptation to glucoprivation in each sex implicates this sensor in glycogen assembly under those conditions. Present work affirms recent reports that expression profiles of hypothalamic astrocyte GP isoforms vary between the two sexes, as AMP-sensitive GPbb profiles were elevated in male versus female, whereas female astrocytes exhibited a greater abundance of phosphorylation-sensitive GPmm than male [19]. Data here document sex-specific GLUT2 control of hypothalamic astrocyte GP variant expression as GLUT2 stimulates GPbb protein expression in male regardless of glucose status, but inhibits GPbb profiles in glucose-deprived female cells. Yet, this glucose sensor up-regulates GPmm protein expression in the female irrespective of glucose status, but increases or suppresses GPmm levels in male when glucose is respectively present or absent. Additional research effort is required to determine if GLUT2 regulates these protein profiles independently of effects on other target proteins, namely activated AMPK.
Current data show that GLUT2 imposes sex-dimorphic control of total glycogen content of primary hypothalamic astrocyte cell cultures. This glucose sensor curbs astrocyte total glycogen content in glucose-supplied or -deprived male astrocytes, reflecting an overall negative impact on the ratio of glycogen synthesis versus breakdown, thereby limiting glucose storage. In contrast, in the female, this sensor does not regulate glycogen mass except in the absence of glucose, when it drives glucoprivic mobilization of this energy reserve. Thus, during glucose sufficiency, cultured male astrocytes may exhibit relatively higher rates of release of glucosyl units from glycogen for TCA cycle conversion to lactate compared to female, due to GLUT2 sensory signaling [Figure 5]. Outcomes that show discordance between glucoprivic patterns of astrocyte glycogen metabolic enzyme protein expression versus direction of change in glycogen accumulation infer that GLUT2 may impose divergent control of net cellular levels versus enzymatic activity of those proteins. Current evidence for GLUT2-associated adjustments in GS and GP isoform protein profiles does not disclose if or how specific activities of these enzymes may be governed by GLUT2. GS is active in the non-phosphorylated state and is allosterically activated by glucose 6-phosphate, whereas GP variants are activated by phosphorylation or AMP allosteric effects (discussed in the Introduction). It is unclear if or how GLUT2 may regulate these post-transcriptional modifications in either sex. Information on whether GLUT2 exerts sex-specific effects on expression of activated GS, GPbb, and GPmm relative to total enzyme protein levels would provide useful insight on potential sensor control of glycogen turnover aside from mass. It is unclear if astrocyte glycogen is a common substrate for GPbb- versus GPmm-mediated disassembly, or if it is spatially organized into distinct segments that are dismantled by a single GP variant. Further studies are needed to examine whether GLUT2 causes similar or different adjustments in GPbb versus GPmm enzyme activity, and to investigate how changes in individual GP isoform specific activity may affect net glycogen content. However, it should be noted that efforts to resolve this critical issue are currently impeded by the lack of available antibody-based analytical tools for quantification of GP variant phosphorylation status.
Figure 5.
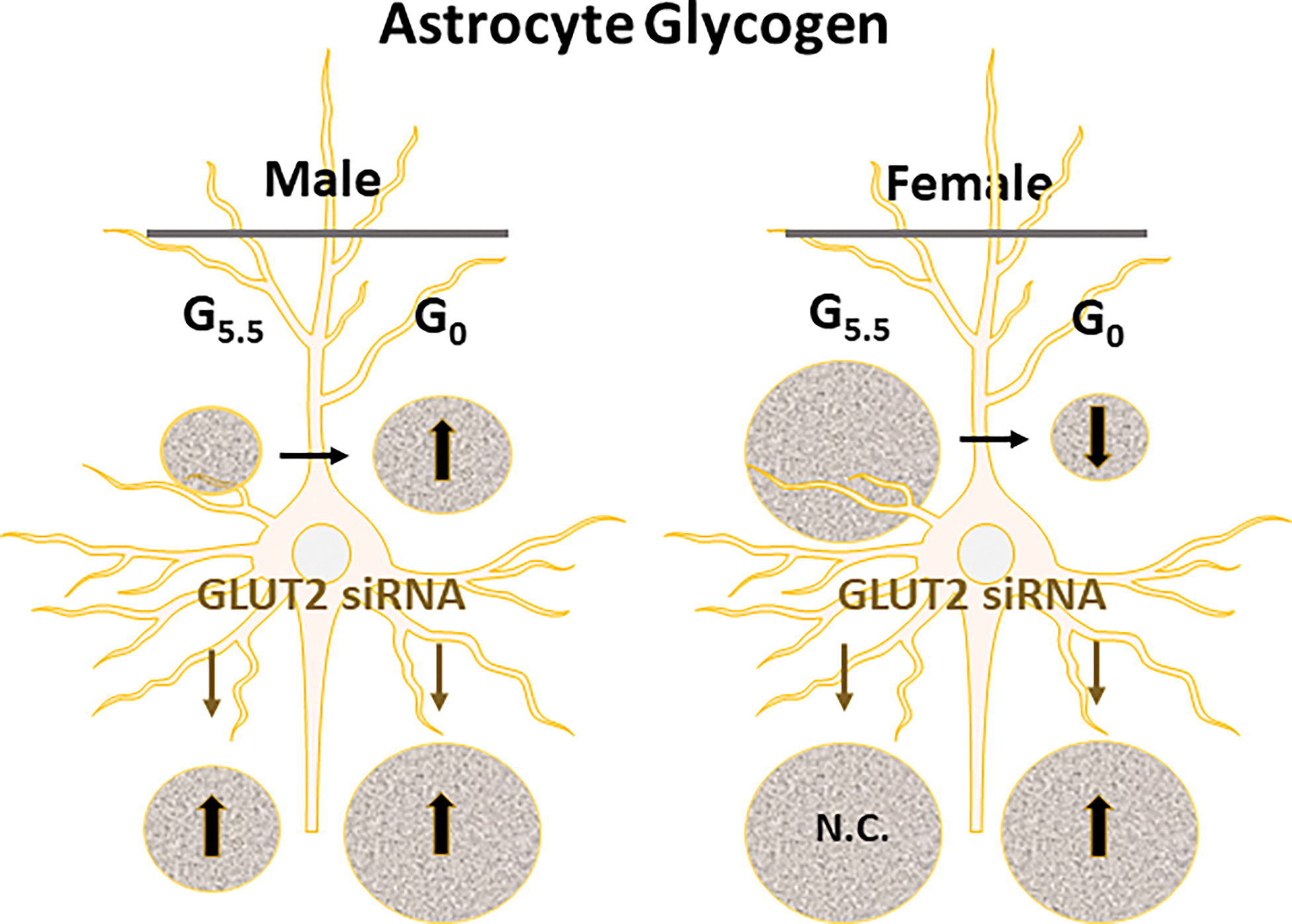
Summary of Glucose-Dependent GLUT2 Gene Knockdown Effects on Male versus Female Primary Hypothalamic Astrocyte Glycogen Content. Abbreviations: G5.5: 5.5 mM media glucose concentration; G0: 0 mM media glucose concentration; GLUT2: glucose transporter-2; N.C.: no change. Symbols: ↑, treatment-induced increase in astrocyte glycogen mass; ↓, treatment-induced decrease in astrocyte glycogen content.
Astrocyte-nerve cell metabolic coupling, involving trafficking of the oxidizable substrate fuel lactate, is indispensable for optimal neuronal energy stability [41–43], and is a critical metabolic variable that shapes hypothalamic control of glucose homeostasis [14,15]. Exportable lactate is generated by catabolism of glucose acquired from either the extracellular environment or liberated from glycogen [Figure 6]. Observations here of GLUT2 stimulation of male but not female GCK protein in glucose-supplied astrocytes suggest that glucose phosphorylation rate, hence the volume of glucose available for either glycolytic pathway catabolism or routing through the glycogen shunt may vary between sexes due to GLUT2 sensory signaling. Current observations of sex-contingent GLUT2 regulation of hypothalamic astrocyte glycogen accumulation in vitro under conditions of glucose provision or withdrawal infer that this sensor may thus control entry of glycogen-derived glucose into the glycolytic pathway according to sex, and thereby affect lactate production volume differently in male versus female. Notably, GLUT2-dependent patterns of lactate yield during glucoprivation may likely be sex-dimorphic as this sensor apparently curbs glycogen amassment in the male to prevent minimization of glucose discharge, but functions as the regulatory impetus for glucoprivic disassembly of glycogen in glucose-deprived female astrocytes. The premise that glycogen-derived lactate stream is a principal or sole signal that links hypothalamic astrocyte GLUT2 glucose monitoring with relevant glucose-regulatory neurons will require validation using in vivo experimental approaches. Alternatively, a bioactive fragment of the astrocyte gliopeptide diazepam-binding inhibitor (DBI) octadecaneuropeptide (ODN; DBI33–50) is known to act on the glucose-regulatory network to control blood glucose levels [44], but it is unclear if GLUT2 regulates this peptide signal. There should also be consideration given to the potential for sex differences in target nerve cell receptivity to GLUT2-driven astrocyte metabolic signals. There is a need, for example, to investigate whether plasma membrane monocarboxylate transporter-2 [45] and/or G protein-coupled lactate receptor GPR81 [46,47] protein expression by hypothalamic glucose-regulatory neurons is equivalent or different between male versus female during either glucose abundance or insufficiency.
Figure 6.
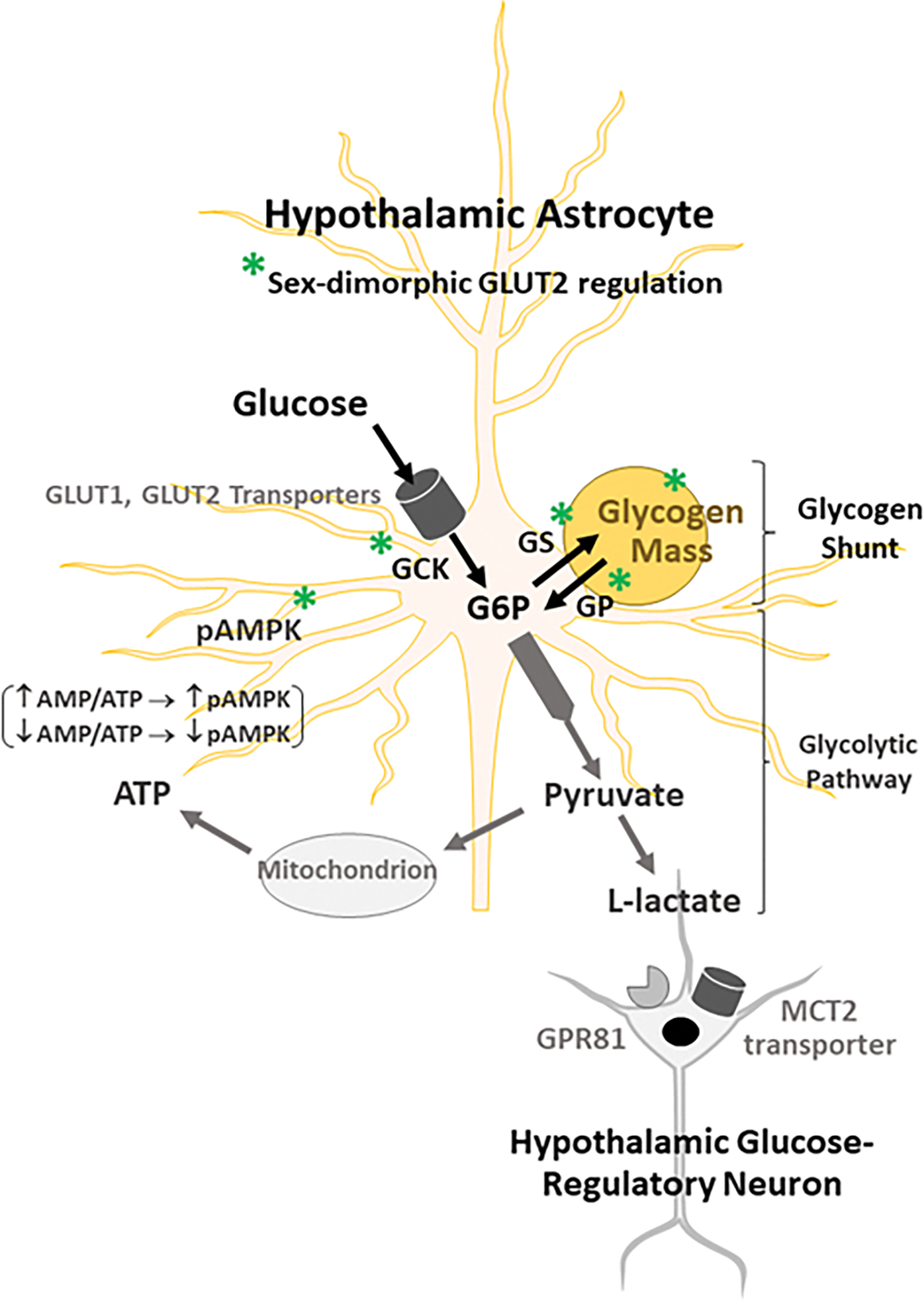
Schematic Organization of GLUT2-Sensitive Hypothalamic Astrocyte Nutrient/Energy Sensors and Glycogen Metabolic Targets. Abbreviations: AMPK: 5’-AMP-activated protein kinase; ATP: adenosine triphosphate; GLUT1: glucose transporter-1; GLUT2: glucose transporter-2; G6P: glucose-6-phosphate; GCK: glucokinase (hexokinase IV); GP: glycogen phosphorylase; GPR81: plasma membrane lactate receptor; MCT2: monocarboxylate transporter 2; pAMPK: phosphoAMPK. Symbols: ↑: increase; ↓: decrease. Illustrated here is GLUT2-dependent GCK-mediated phosphorylation of glucose following astrocyte uptake by GLUT1/GLUT2 activity. Potential sex dissimilarities in glucose volume designated for entry into the glycolytic pathway or the glycogen shunt, as well as differences in ratio of glucose incorporation into versus liberation from glycogen would likely affect net yield of lactate available for trafficking as a regulatory signal to glucose-regulatory neurons.
In summary, current studies provide novel evidence that GLUT2 regulates hypothalamic astrocyte metabolic-sensory function and glycogen metabolism unique to each sex under in vitro culture conditions. Results document sex-dependent GLUT2 control of the astrocyte glycolytic pathway rate-limiting enzyme and glucose sensor GCK in primary hypothalamic astrocyte cultures. Moreover, GLUT2 imposes opposite effects on activated AMPK expression in male (stimulatory) versus female (inhibitory). Data show that glucose status determines GLUT2 regulation of GS protein in each sex and GPbb and GPmm profiles in female or male, respectively, and reveal divergent sensor control of GS, GPbb, and GPmm expression between the two sexes during glucoprivation. GLUT2 limits astrocyte glycogen content in the male regardless of glucose status and mediates glucoprivic diminution of this energy reserve in the female. Further research is required to characterize molecular mechanisms underlying sex-dimorphic GLUT2 regulation of hypothalamic astrocyte target proteins and to determine how GLUT2 regulation of these astrocyte functions may affect neural regulation of glucose homeostasis in each sex.
Supplementary Material
Funding Source:
This research was supported by NIH grant DK 109382.
Abbreviations:
- AMPK
5’-AMP-activated protein kinaseα1/2
- CNS
central nervous system
- GCK
glucokinase
- GKRP
glucokinase regulatory protein
- GLUT2
glucose transporter-2
- GPbb
glycogen phosphorylase-brain type
- GPmm
glycogen phosphorylase-muscle type
- GS
glycogen synthase
- LC-ESI-MS
uHPLC-electrospray ionization-mass spectrometry
- pAMPK
phosphoAMPKα1/2
Footnotes
Conflict of Interest: The authors have no conflicts interest to declare.
Declarations:
Statement of Ethics: Studies performed here were approved by the University of Louisiana Monroe Institutional Animal Care and Use Committee, reference no. 19AUG-KPB-01, in accordance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals, 8th Edition.
CRediT Statement:
Madhu Babu Pasula: conceptualization, investigation, formal analysis, validation, data curation, writing – original draft, writing – review and editing, visualization; Prabhat R. Napit: conceptualization, methodology; Abdulrahman Alhamyani: conceptualization, methodology; Khaggeswar Bheemanapally: methodology, software, formal analysis, validation, data curation, visualization; Paul W. Sylvester: Resources, Writing – Review and Editing; Karen P. Briski: conceptualization, writing – original draft, writing – review and editing, supervision, project administration, funding acquisition
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Thorens B, Mueckler M (2010) Glucose transporters in the 21st century. Amer J Physiol Endocrinol Metab 298: E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood IS, Trayhurn P (2003) Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Brit J Nutr 89: 3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 3.Holman GD (2020) Structure, function, and regulation of mammalian glucose transporters of the SLC2 family. Pflügers Archiv - Eur J Physiol 472: 1155–1175. doi: 10.1007/s00424-020-02411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34: 121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arluison M, Quignon M, Nguyen P, Thorens B, Leloup C, Penicaud L. (2004) Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain--an immunohistochemical study. J Chem Neuroanat 28(3):117–36. doi: 10.1016/j.jchemneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Mounien L, Marty N, Tarussio D, Metref S, Genoux D, Preitner F, Foretz M, Thorens B (2010) Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. FASEB J 24:1747–58. doi: 10.1096/fj.09-144923. [DOI] [PubMed] [Google Scholar]
- 7.Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B (2005) Regulation of glucagon secretion by glucose transporter type 2 (GLUT2) and astrocyte-dependent glucose sensors. J Clin Invest 115: 3543–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stobart JL, Anderson CM (2013) Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Cell Neurosci 7:1–21. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argente-Arizón P, Guerra-Cantera S, Garcia-Segura LM, Argente J, Chowen JA (2017) Glial cells and energy balance. J Mol Endocrinol 58:R59–R71. doi: 10.1530/JME-16-0182. [DOI] [PubMed] [Google Scholar]
- 10.Douglass JD, Dorfman MD, Thaler JPI(2017) Glia: silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 60:226–236. doi: 10.1007/s00125-016-4181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald AJ, Robb JL, Morrissey NA, Beall C, Ellacott KLJ (2019) Astrocytes in neuroendocrine systems: An overview. J Neuroendocrinol 31:e12726. doi: 10.1111/jne.12726. [DOI] [PubMed] [Google Scholar]
- 12.Zhou YD (2018) Glial Regulation of Energy Metabolism. Adv Exp Med Biol 1090: 105–121. doi: 10.1007/978-981-13-1286-1_6. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MMH, Alhamami HN, Briski KP (2019) Norepinephrine regulation of ventromedial hypothalamic nucleus metabolic transmitter biomarker and astrocyte enzyme and receptor expression: impact of 5’-AMP-activated protein kinase. Brain Res. 1711:48–57. doi: 10.1016/j.brainres.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood ASMH, Bheemanapally K, Mandal SK, Ibrahim MMH, Briski KP (2019) Norepinephrine control of ventromedial hypothalamic nucleus glucoregulatory neurotransmitter expression in the female rat: role of monocarboxylate transporter function. Mol Cell Neurosci 95:51–58. doi: 10.1016/j.mcn.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bheemanapally K, Alhamyani AR, Ibrahim MMH, Briski KP (2021) Ventromedial hypothalamic nucleus glycogen phosphorylase regulation of metabolic-sensory neuron AMPK and neurotransmitter protein expression: Role of L-lactate. Amer J Physiol Regul Integr Comp Physiol 320:R791–R799. doi: 10.1152/ajpregu.00292.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuente-Martín E, García-Cáceres C, Argente-Arizón P, Díaz F, Granado M, Freire-Regatillo A, Castro-González D, Ceballos ML, Frago LM, Dickson SL, Argente J, Chowen JA (2016) Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes. Sci Rep 6:23673. doi: 10.1038/srep23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim MMH, Bheemanapally K, Sylvester PW, Briski KP (2020a) Sex-specific estrogen regulation of hypothalamic astrocyte estrogen receptor expression and glycogen metabolism in rats. Mol Cell Endocrinol 504:110703. doi: 10.1016/j.mce.2020.110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim MMH, Bheemanapally K, Sylvester PW, Briski KP (2020b) Sex differences in glucoprivic regulation of glycogen metabolism in hypothalamic primary astrocyte cultures: role of estrogen receptor signaling. Mol Cell Endocrinol 518:111000. doi: 10.1016/j.mce.2020.111000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim MMH, Bheemanapally K, Sylvester PW, Briski KP (2020c) Norepinephrine regulation of adrenergic receptor expression, 5’ AMP-activated protein kinase activity, and glycogen metabolism and mass in male versus female hypothalamic primary astrocyte cultures. ASN Neuro 12:1759091420974134. doi: 10.1177/1759091420974134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matschinsky FM, Wilson DF (2019) The central role of glucokinase in glucose homeostasis; a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front Physiol doi: 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau OW, Fontes JD, Carlson GM (2018) The regulation of glycogenolysis in the brain. J Biol Chem 293:7099–7107. doi: 10.1074/jbc.r117.803023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhamyani AR, Napit PR, Bheemanapally K, Ibrahim MMH, Sylvester PW, Briski KP (2022) Glycogen phosphorylase isoform regulation of glucose and energy sensor expression in male versus female hypothalamic astrocyte primary cultures. Mol Cell Endocrinol 111698. doi: 10.1016/j.mce.2022.111698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller MS, Pedersen S, Walls AB, Waagepetersen HS, Bak LK (2014) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63: 154–162. doi: 10.1002/glia.22741. [DOI] [PubMed] [Google Scholar]
- 24.Agius L (2008) Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 414: −18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 25.Agius L (2016) Hormonal and metabolite regulation of hepatic glucokinase. Annu Rev Nutr 36:389–415. doi: 10.1146/annurev-nutr-071715-051145. [DOI] [PubMed] [Google Scholar]
- 26.Sternisha SM, Miller BG (2019) Molecular and cellular regulation of human glucokinase. Arch Biochem Biophys 663:299–213. doi: 10.1016/j.abb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez E, Roncero I, Chowe1n JA, Vasquez P, Blazquez E (2002) Evidence that glucokinase regulatory protein is expressed and interacts with glucokinase in rat brain. J Neurochem 80:45–53. doi: 10.1046/j.0022-3042.2001.00677.x. [DOI] [PubMed] [Google Scholar]
- 28.Roncero I, Sanz C, Alvarez E, Vázquez P, Barrio PA, Blázquez E (2009) Glucokinase and glucokinase regulatory proteins are functionally coexpressed before birth in the rat brain. J Neuroendocrinol 21:973–981. doi: 10.1111/j.1365-2826.2009.01919.x. [DOI] [PubMed] [Google Scholar]; 28b. de la Iglesia N, Veiga de Cunha M, Van Schaftingen E, Guinovart JJ, Ferrer JC (1999) Glucokinase regulatory protein is essential for the proper subcellular localization of liver glucokinase. FEBS Lett. 456:332–338. doi: 10.1016/S0014-5793(99)00971-0. [DOI] [PubMed] [Google Scholar]
- 29.Bheemanapally K, Ibrahim MMH, Briski KP (2020) Combinatory high-resolution microdissection/ultra-performance liquid chromatographic-mass spectrometry approach for small tissue volume analysis of rat brain glycogen. J Pharmaceut Biomed Anal 178: 112884. doi: 10.1016/j.jpba.2019.112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leloup C, Allard C, Carneiro L, Fioramonti X, Collins S, Penicaud L (2016) Glucose and hypothalamic astrocytes: more than a fueling role? Neuroscience 323:110–120. doi: 10.1016/j.neuroscience.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 31.Watts AG, Donovan CM (2010) Sweet talk in the brain: Glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 31:32–43, doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillemain G, Loizeau M, Pinçon-Raymond M, Girard J, Leturque A (2000) The large intracytoplasmic loop of the glucose transporter GLUT2 is involved in glucose signaling in hepatic cells. J Cell Sci 113:841–847. doi: 10.1242/jcs.113.5.841. [DOI] [PubMed] [Google Scholar]
- 33.Stolarczyk E, Guissard C, Michau A, Even PC, Grosfeld A, Serradas P, Lorsignol A, Pénicaud L, Brot-Laroche E, Leturque A, Le Gall M (2010) Detection of extracellular glucose by GLUT2 contributes to hypothalamic control of food intake. Amer J Physiol Endocrinol Metab 298:E1078–1087. doi: 10.1152/ajpendo.00737.2009. [DOI] [PubMed] [Google Scholar]; 33b. Vielkind U, Walencewicz A, Levine M, Churchill Bohn M (1990) Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res 27:360–373. [DOI] [PubMed] [Google Scholar]
- 34.Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat P, Ferre P, Penicaud L (1994) Glucose ransporter 2 (GLUT 2): expression in specific brain nuclei. Brain Res 638:221–226. doi: 10.1016/0006-8993(94)90653-x. [DOI] [PubMed] [Google Scholar]
- 35.Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K, Matschinsky FM, Magnuson MA (1994) Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem 269:3641–3654. [PubMed] [Google Scholar]
- 36.Koespell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch 472:1299–1343. doi: 10.1007/s00424-020-02441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson RA, Choi DW. Glial Glycogen Stores Affect Neuronal Survival during Glucose Deprivation in Vitro. J. Cereb. Blood Flow Metab. 1993; 13: 162–169. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones 2006; 11: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández-Fonseca K, Massieu L, García de la Cadena S, Guzmán C, Camacho-Arroyo I. Neuroprotective role of estradiol against neuronal death induced by glucose deprivation in cultured rat hippocampal neurons. Neuroendocrinology 2012; 96: 41–50. [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulos MC, Koumenis IL, Yuan TY, Giffard RG. Increasing Vulnerability of Astrocytes to Oxidative Injury with Age despite Constant Antioxidant Defenses. Neuroscience. 1998; 82: 915–925 Schildge S, Bohrer C, Beck K, Schachtrup. JOVE 2013 (Jan 19); 50079. 10.3791/50079. [DOI] [PubMed] [Google Scholar]
- 41.Murat CB, García-Cáceres C (2021) Astrocyte gliotransmission in the regulation of systemic metabolism. Metabolites 11(11):732. doi: 10.3390/metabo11110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonvento G, Bolaños JP (2021) Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 33(8):1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Beard E, Lengacher S, Dias S, Magistretti PJ, Finsterwald C (2022) Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front Physiol 12:825816. doi: 10.3389/fphys.2021.825816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanfray D, Arthaud S, Ouellet J, Compère V, Do Rego JL, Leprince J, Lefranc B, Castel H, Bouchard C, Monge-Roffarello B, Richard D, Pelletier G, Vaudry H, Tonon MC, Morin F (2013) Gliotransmission and brain glucose sensing: critical role of endozepines. Diabetes 62:801–810. doi: 10.2337/db11-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 47.Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res 93:1045–1055. doi: 10.1002/jnr.23593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


