Abstract
Since its discovery in the United States in 2017, the Asian longhorned tick (Haemaphysalis longicornis) has been detected in most eastern states between Rhode Island and Georgia. Long Island, east of New York City, a recognized high-risk area for tick-borne diseases, is geographically close to New Jersey and New York sites where H. longicornis was originally found. However, extensive tick surveys conducted in 2018 did not identify H. longicornis on Long Island. In stark contrast, our 2022 tick survey suggests that H. longicornis has rapidly invaded and expanded in multiple surveying sites on Long Island (12 out of 17 sites). Overall, the relative abundance of H. longicornis was similar to that of lone star ticks, Amblyomma americanum, a previously recognized tick species abundantly present on Long Island. Interestingly, our survey suggests that H. longicornis has expanded within the Appalachian forest ecological zone of Long Island’s north shore compared to the Pine Barrens located on the south shore of Long Island. The rapid invasion and expansion of H. longicornis into an insular environment are different from the historical invasion and expansion of two native tick species, Ixodes scapularis (blacklegged tick or deer tick) and A. americanum, in Long Island. The implications of H. longicornis transmitting or introducing tick-borne pathogens of public health importance remain unknown.
Keywords: Asian longhorned tick, Haemaphysalis longicornis, Long Island, New York
1. Introduction
Long Island, located to the east of New York City, United States, has provided optimal environmental conditions for the invasion and expansion of various tick species, increasing the chances of humans being infected with established and emerging tick-borne pathogens (Burgdorfer et al., 1982; Hilton et al., 1999; Smith et al., 1988). Historically, Long Island has experienced three waves of tick population expansions and associated increases in tick-borne infections. First, in the 1930s through 1970s, the expansion of Dermacentor variabilis (American dog tick) contributed to the increased number of infections with Rickettsia rickettsii, a causative microorganism for Rocky Mountain spotted fever in Long Island (Anastos, 1947; Benach et al., 1977; Jamnback, 1969; Vianna and Hinman, 1971). In the 1970s, significant environmental changes, such as reforestation and suburbanization, occurred in Long Island and supported a growing number of white-tailed deer populations, leading to the expansion of the second tick species, Ixodes scapularis (blacklegged tick or deer tick) (Good, 1973; Paddock and Yabsley, 2007; Rochlin et al., 2022). Consequently, cases of Lyme disease, associated with Borrelia burgdorferi sensu stricto-infected I. scapularis, exploded in Long Island (Burgdorfer et al., 1982; Grunwaldt et al., 1983; Smith et al., 1988). Likewise, human cases of babesiosis, another tick-borne disease caused by Babesia microti transmitted by I. scapularis ticks, increased markedly in the same period (Benach et al., 1978; Benach and Habicht, 1981). Since then, I. scapularis continues to remain the most medically relevant tick species, capable of transmitting multiple additional human pathogens, such as Anaplasma phagocytophilum (anaplasmosis), Borrelia miyamotoi (tick-borne relapsing fever), and Powassan virus. By the early 1990s, another tick species, Amblyomma americanum (lone star tick), rapidly expanded on Long Island to become the most abundant tick species (Ginsberg et al., 1991), impacting public health by transmitting Ehrlichia chaffeensis (ehrlichiosis) and bites being associated with alpha-gal syndrome, also known as red meat allergy (Eisen, 2020). Since the 1990s, A. americanum has remained the most abundant tick species on eastern Long Island during the spring and summer seasons (Ginsberg et al., 1991; Sanchez-Vicente et al., 2019), the most critical time of the year for human exposure to tick-borne pathogens (Piesman and Eisen, 2008). In 2018, Sanchez-Vicente et al. conducted an extensive tick survey on Long Island and documented that three tick species, D. variabilis, I. scapularis, and A. americanum, were present in significant numbers without other invasive tick species (Sanchez-Vicente et al., 2019).
In 2017, a new exotic tick species, Haemaphysalis longicornis (Asian longhorned tick), was first reported in Hunterdon County, New Jersey, about 150 km west of central Long Island (Rainey et al., 2018). Haemaphysalis longicornis is native to East and Central Asia and associated with veterinary and human diseases (Heath, 2016; Luo et al., 2015). However, it remains unknown whether H. longicornis in the United States transmits any tick-borne pathogens of public health importance. Another study conducted in 2017 reported that H. longicornis was present in suburban Westchester County, New York (Yuan et al., 2020). Subsequent studies concluded that H. longicornis successfully invaded and expanded to high densities in Westchester County and New York City by 2019 (Piedmonte et al., 2021; Tufts et al., 2021, 2019; Wormser et al., 2020). In addition, recent environmental niche modeling suggested that large parts of the eastern United States are suitable for H. longicornis (Rochlin, 2019). Indeed, as of this year, H. longicornis has been identified in 17 states (“USDA APHIS | The Asian Longhorned Tick,” n.d.). However, the local habitat suitability (i.e., sites with consistently high populations of H. longicornis in Long Island) remains uncharacterized.
Here, we report that while three established tick species (D. variabilis, I. scapularis, and A. americanum) continue to reside in Long Island, H. longicornis has successfully invaded and is in the process of rapid expansion in the northern parts of Long Island, representing the fourth wave of invasive tick species in this area into an insular environment. The primary goals of this study were to characterize changing tick communities at this early stage of H. longicornis expansion, to establish a baseline for future tick surveys, and to discuss the potential implications of high H. longicornis population densities found in some areas of Long Island.
2. Materials and Methods
2.1. Study area and sampling locations
The study was conducted in central and eastern Long Island (Suffolk County), about 80 km east of New York City (Figure 1). Sampling locations (n = 17, see Supplemental File 1 for geographic locations) and timing were similar to those from the previous comprehensive springearly summer survey in 2018 (Sanchez-Vicente et al., 2019). Specifically, five of our sites were either identical or in very close proximity, i.e. < 1 km distance to those by Sanchez-Vicente et al., 2019 (Figure 1; Supplemental File 1, sampling site description table). Additional 3 sites were located in the same upland forested/open field habitat of Long Island’s north shore in a typical Appalachian/Piedmont ecological community within less than 15 km distance from each other (Figure 1; Supplemental File 1, sampling site description table).
Figure 1.
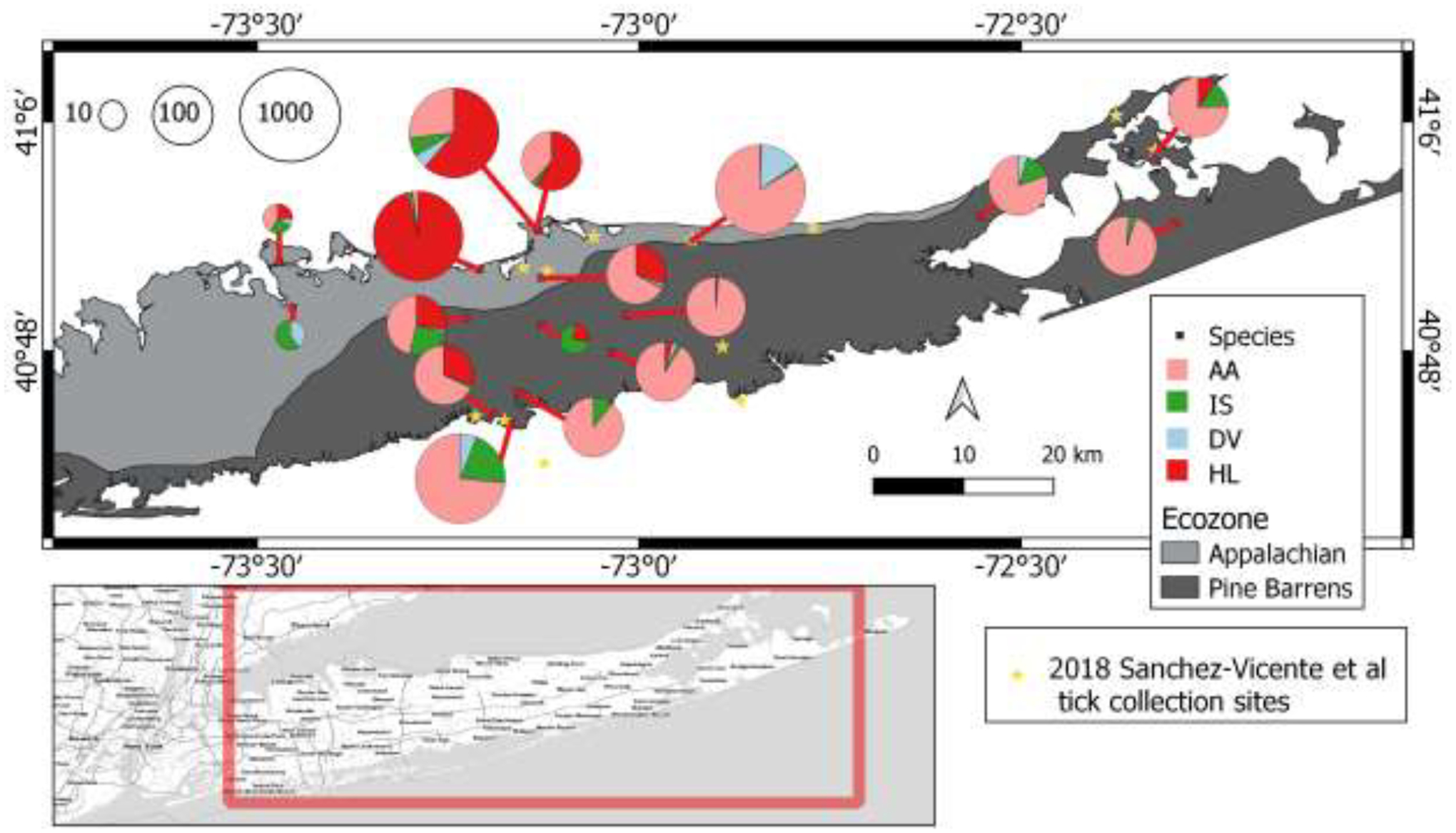
Map of the study area. (bottom) The inset shows Long Island, New York State, with the extent of the study area as a red rectangle. (top) Pie charts correspond to 2022 tick sampling locations. Each chart shows the relative species composition of Amblyomma americanum (AA), Ixodes scapularis (IS), Dermacentor variabilis (DV), and Haemaphysalis longicornis (HL). The size of the pie chart indicates the total number of ticks collected on the log10 scale (in the upper left corner). Different shades of gray correspond to two ecological zones: lighter gray indicates Appalachian forest, whereas darker gray indicates Pine Barrens. Yellow stars indicate tick survey sites described in Sanchez-Vicente et al., 2019.
In addition, we included several sites to describe ecologically distinct habitats of northern versus southern Long Island. The northern part of Long Island has northern Appalachian/Piedmont ecological communities dominated by oaks, beeches, and maples. In contrast, the southern locations support typical coastal Pine Barrens, especially in the less developed eastern regions (Bryce et al, 2010). Out of 17 sampling sites, seven were located within northern Appalachian/Piedmont ecological communities, whereas the remaining ten were located within the coastal Pine Barrens ecoregion (see Supplemental File 1).
2.2. Tick collections
Tick collections were conducted during the spring-summer season (April 3 through July 26, 2022) during the peak activity period for all tick species of medical importance, with the exception of the fall peak for the adult blacklegged ticks (Sanchez-Vicente et al., 2019). Questing ticks were collected from vegetation by flagging, using a 1 m2 white double-sided corduroy flag (Kaufman 8 wale, Robert Kaufman Fabrics, Los Angeles, USA) attached to a wooden pole (H. S. Ginsberg and Ewing, 1989a). At least 1 hour of total active flagging were carried out at each site (see Supplemental File 1 for details). The flag was checked every 5 “sweeps” or at 30 s intervals since it was noted that H. longicornis adults were easily dislodged from the flag. The collections were normalized by time rather than distance because of the differences in vegetation type, density, and presence of numerous obstacles such as fallen trees, brier thickets, and poison ivy patches that made transect sampling impractical. Major types of habitat at each site – forested, ecotone, and open grassy areas (lawns and old fields) were surveyed. Ticks were brought to the laboratory and identified to species, sex, and life stage under a high-power dissecting microscope using published keys (Egizi et al., 2019; Keirans and Litwak, 1989; Sonenshine, 1979).
2.3. Data sources and statistical analysis
A shapefile containing ecological zones for New York State was downloaded from http://ecologicalregions.info/data (Bryce et al, 2010). Weather data were accessed through www.wunderground.com. All statistical analyses were conducted using R version 3.5.3 (R Development Core Team, 2019). Tick abundance and species composition in different ecological zones were compared using generalized linear mixed effects models (GLMM) generated by the lme4 package v. 1.1–10 (Bates et al., 2013). In the model, ecological zones were included as a fixed effect and date as a random effect to account for temporal autocorrelation. The differences between combined adult and nymphal stages by species were also explored by introducing the stage as a fixed effect into the model. P-values were obtained by likelihood ratio tests comparing the full model with and without the effect in question. Post hoc tests for all analyses were performed by Tukey’s range test using the emmeans package v.1.4.7. Data visualization was performed using packages ggplot2 v.3.3.6 and ggstatplot v. 0.9.3 (Patil, 2021). Maps were processed and generated using QGIS v.3.10.8 (QGIS Association. http://www.qgis.org).
3. Results
3.1. Tick collections
Ticks were collected at 17 locations across Long Island (Figure 1) between April 3 and July 26, 2022. Combined collections comprised 3,999 adult and nymphal ticks; the numbers for each species are shown in Figure 2. Among adult ticks, A. americanum was the most commonly collected (61%), followed by I. scapularis (16%), H. longicornis (13%), and D. variabilis (10%). Among nymphs, H. longicornis was the most commonly collected (62%), followed by A. americanum (36%) and I. scapularis (2%). No D. variabilis nymphs were collected. The estimated number of ticks normalized per unit of effort (one hour) varied greatly (10 – 900 ticks per hour), depending on the species, site, and season. Specifically, the two collection sites with the most abundant H. longicornis had the highest collection rates per hour: 851 and 415 H. longicornis ticks per hour. In comparison, the two collection sites with the greatest densities of A. americanum produced 335 and 246 ticks per hour. The highest collection rates for I. scapularis and D. variabilis were 66 and 58 ticks per hour, respectively. As shown in Figure 2, the abundance of adult A. americanum, H. longicornis, and D. variabilis reached the maximal points in May or June. In contrast, the relative abundance of adult I. scapularis reached the highest point in April, with a steep decline in their population by June. Of note, adult I. scapularis exhibits two seasonal peaks on Long Island – the spring/summer peak followed by another peak in the fall (Sanchez-Vicente et al., 2019): our study period does not include the fall peak season. Nymphs of three species (A. americanum, H. longicornis, and I. scapularis) were found throughout the 4-month study period, with their peak abundance in June (Figure 2). Larvae of the same three tick species were collected intermittently: H. longicornis collected every month, A. americanum in June and July, and I. scapularis in April and July. On the other hand, our flagging failed to detect D. variabilis immatures. Interestingly, H. longicornis and A. americanum larvae populations were highly clustered, abundant (in the thousands), and intermixed in many sampling sites, making it impossible to perform quantitative analysis.
Figure 2.

(top) Totals collected for each tick species (nymphs and adults only) across all sampling sites from April to July, 2022. (bottom) Monthly collections normalized as an average number of ticks collected per one hour. A. americanum – Amblyomma americanum, D. variabilis – Dermacentor variabilis, H. longicornis – Haemaphysalis longicornis, I. scapularis – Ixodes scapularis.
3.2. Haemaphysalis longicornis distribution, abundance, and habitat association
Our current tick survey suggests that H. longicornis has successfully invaded and expanded on Long Island, with their presence in 12 of 17 collection sites (71%, Supplemental File 1). In the Appalachian forest (North shore), 6 out of 7 sites (86%) had H. longicornis, whereas this species was detected in 6 out of 10 (60%) Pine Barrens (South shore) sites. The wide distribution of H. longicornis is further evidenced by their presence in the westernmost (Caumsett State Park, Lloyd Harbor) and the easternmost (Mashomack Preserve, Shelter Island) sites of Long Island. We detected H. longicornis as early as April 3, when the high temperature was only 8°C and for the remainder of the study period. Interestingly, adult and nymphal H. longicornis were active on July 22 (the high temperature at 33°C) amid a 5-day heatwave. Our survey detected abundant and frequent nymphal H. longicornis (25 sampling events) as compared to adult (9 sampling events) and larval (7 sampling events) ticks which were always found in association with nymphal ticks.
Long Island contains great ecological diversity but is largely divided into two Level IV ecological zones – Appalachian forest (North shore) and Pine Barrens (South shore, Figure 1) (Bryce et al, 2010). Therefore, we investigated the association of H. longicornis and ecological habitats using generalized linear mixed effects models (GLMM). Since only a relatively small number of H. longicornis adults were collected, the adult and nymphal stages were combined for this analysis for both H. longicornis and A. americanum ticks. The GLMM analysis determined the ecological zone as a significant predictor of H. longicornis abundance (X2 = 7.38, P = 0.0066) and percent species composition, i.e. proportional representation of each species in the sample (X2 = 15.01, P < 0.001). Our tick survey documented significantly higher numbers of H. longicornis ticks in the Appalachian ecozone (49.9 ± 8.2 ticks per hour, mean ± S.E.) compared to Pine Barrens (3.9 ± 0.7 ticks per hour, mean ± S.E., t = 2.7, df = 32.5, P = 0.0105, Figure 3). Furthermore, H. longicornis was a dominant tick species identified in the Appalachian ecozone (42.8 ± 7.0 %, mean ± SE) but not in Pine Barrens (17.1 ± 3.0 %, mean ± SE, Z = 3.6, P = 0.0003). In comparison, our survey documented comparable numbers of A. americanum ticks in both ecozones [Appalachian (19.2 ± 3.2 ticks per hour) versus Pine Barrens (18.9 ± 3.3 ticks per hour), mean ± SE, t = 0.08, df = 32.7, P = 0.9327, Figure 3]. However, the relative abundance (i.e., number of specimens collected) of A. americanum was significantly lower in the Appalachian environment (40.1 ± 6.6 %, mean ± S.E.) compared to Pine Barrens (62.4 ± 11.0 %, mean ± S.E., Z = −3.9, P < 0.0001). Insufficient sample size due to low numbers of I. scapularis and D. variabilis, precluded similar analysis of these two tick species, We noticed unique microhabitats associated with H. longicornis. Early in the season (April – May), H. longicornis nymphal ticks were most numerous in open fields and ecotone areas (Figure 4). Of note, nymphs congregated atop dried ragweed stalks in considerable numbers. Interestingly, H. longicornis shared the open field habitat with D. variabilis. However, in more wooded habitats where the open ground was interspersed with trees, H. longicornis nymphs were frequently collected together with A. americanum adults from brush and grasses. In the Pine Barrens ecozone, H. longicornis were limited to grassy areas (Figure 4). Later in the season (June-July), the open field habitat became overgrown with tall vegetation harboring fewer H. longicornis (Figure 4). At the same time, H. longicornis nymphal and adult abundance increased in wooded areas varying from open, dry slopes – typically preferred A. americanum habitat - to heavy undergrowth (for example, by Vaccinium spp.) and densely vegetated wetlands where I. scapularis and A. americanum were also present.
Figure 3.

Comparison of H. longicornis and A. americanum in two ecological zones. The graphs in the left column show tick abundance as the number of ticks collected normalized for one hour for (A) H. longicornis and (C) A. americanum. The graphs in the right column show proportion of each species relative to all other tick species for (B) H. longicornis and (D) A. americanum. Each dot corresponds to a collection event (date and location). The violin plots illustrate the overall distribution, while the box plots show the median and 50% of the observations within the box. The box plot whiskers encompass 1.5 × interquartile range. A. americanum – Amblyomma americanum, D. variabilis – Dermacentor variabilis, H. longicornis – Haemaphysalis longicornis, I. scapularis – Ixodes scapularis.
Figure 4.

Typical Haemaphysalis longicornis microhabitat. (A) Open field in May with previous year’s ragweed (Ambrosia spp.) dry stalks harboring many H. longicornis nymphs (B) congregating at the tips. H. longicornis nymphs were found in the new growth in the fields and on the mowed path in high densities. Few adult and larval ticks were also present. (C) Early season (April) H. longicornis habitat at the ecotone (Smithtown, NY). (D) The same fields in A) later in the season (July) with tall vegetation harboring very few H. longicornis. In July, higher densities of this species were found in wooded areas nearby, including slopes with sparse undergrowth (E) more typical of Amblyomma americanum habitat. (F) Grassy open patches within wooded areas were often found to harbor H. longicornis in Pine Barrens or similar habitats (Shelter Island, NY).
4. Discussion
The Asian longhorned tick was first documented in the U.S. in 2017 (Rainey et al., 2018). However, retrospective analyses determined that H. longicornis has been present in the country since the early 2010s (Egizi et al., 2020; Rainey et al., 2018). Within one year following the initial detection in New Jersey, H. longicornis was found in nine additional states, ranging from Arkansas in the west to North Carolina in the south to Connecticut in the northeast (Beard et al., 2018). Previous work documented the established H. longicornis populations in New York City and the mainland Westchester County just north of NYC (Piedmonte et al., 2021; Tufts et al., 2019). Two extensive tick surveys on Long Island failed to detect H. longicornis in 2018 (Sanchez-Vicente et al., 2019; Yuan et al., 2020). Nonetheless, another tick survey in the same general area documented the presence of H. longicornis in 2018, albeit in very low numbers (Egizi et al., 2020). Interestingly, our preliminary tick survey in 2021 failed to collect H. longicornis at two different sites that now exhibit high populations of H. longicornis, corroborating the rapid invasion and expansion of H. longicornis observed in this and other studies.
Between 2018 and 2022, the populations of H. longicornis have undergone tremendous increase and expansion. On large geographic scales, H. longicornis has been detected in 17 states encompassing large portions of the eastern U.S. (“USDA APHIS | The Asian Longhorned Tick,” n.d.). In this study, we showed that H. longicornis has successfully invaded diverse habitats across Long Island and become a dominant tick species at several sites (Figure 1). In 2018, A. americanum dominated the spring-summer tick collections comprising 58% of the total (Sanchez-Vicente et al., 2019). In 2022, the proportion of A. americanum was reduced to 45%, whereas H. longicornis constituted up to 44% of the total. Similarly, the relative abundance of I. scapularis and D. variabilis has decreased from 32% (2018) to 11% (2022). Of note, the absolute numbers of individual tick species remained comparable between the two studies.
Our tick-sampling sites included those utilized in the 2018 study and additional locations exhibiting similar ecological settings (Figure 1; Supplemental File 1, sampling site description table). Of 17 sites surveyed in the current study, five locations were either identical or nearby (< 1 km) to those described in the 2018 study. Importantly, the 2018 study did not find any H. longicornis ticks, whereas our survey documented the presence of abundant H. longicornis in 4 out of 5 (80%) sites. Of note, these five sites are dispersed throughout the study area, implicating a rapid and successful invasion and expansion of H. longicornis in Suffolk County. Corroborating our hypothesis, we also detected the presence of abundant H. longicornis in three additional survey sites with a typical Appalachian/Piedmont ecological community on the northern shore of Long Island. In contrast, none of the 2018 survey sites with similar environmental settings (located within 15 km distance from the current survey sites) failed to support the H. longicornis settlement. Given the unprecedented rapid invasion and expansion of H. longicornis, we will continue to survey ticks in these sampling sites and report any significant changes in tick populations.
Our current study suggests that environmental factors and ecological communities contribute to the relative abundance of H. longicornis (Figures 1 and 3). Amblyomma americanum and H. longicornis constitute the most abundant tick species found on Long Island. While A. americanum was evenly distributed between the two major ecological zones (Appalachian and Pine Barrens), H. longicornis was predominantly identified in the Appalachian forest ecological zone (Figure 3). This ecozone is characterized by oak-dominated forests with tulip trees, maples, and beeches also abundant; it supports some plant communities more typical of southeastern Piedmont that reach their northern limits on Long Island (Bryce et al, 2010). Unlike the sandy outwash soils of the Pine Barrens ecozone of southern and eastern Long Island, the soils of the Appalachian forest region are composed of glacial till. The landscape is also different. The Appalachian forest occupies hills and valleys of the glacial moraines, whereas Pine Barrens has a flat topography of an outwash plain. The association of H. longicornis with heavier soils and hilly terrain has been reported in its native range in China (Liu et al., 2015).
While our study determined significant differences in H. longicornis relative abundance between the more favorable Appalachian ecozone and less suitable Pine Barrens (>10-fold normalized for effort, Figure 3), H. longicornis was present at the majority of the surveyed sites (6 out of 10). Large open grassy patches bordering artificial recreational landscaping within generally dry forested areas (e.g., Figure 4, Shelter Island, NY) often harbored H. longicornis ticks, which were absent from the surrounding woodland, corroborating a recently published study (Price et al., 2020). Within microhabitats suitable for H. longicornis, this tick species expanded to extremely high densities in our collection sites compared to other tick species. For example, we collected 468 nymphs during one 1-hr sampling session within a 50 m distance (Figure 4A). In contrast, the highest nymph count for A. americanum, a species known for its population density on Long Island (Ginsberg et al., 1991), was 131 ticks per hour of sampling. Other investigators documented similar findings where localized habitats provide exceedingly high densities of immature H. longicornis ticks (Bickerton et al., 2021; Piedmonte et al., 2021; Tufts et al., 2019). These habitat “islands” may provide a foothold for this highly invasive species in an otherwise less hospitable environment, eventually allowing H. longicornis survival and expansion in lower densities in the Pine Barrens habitat. Thus, the microhabitat characterization within broader ecological communities may provide significant insights into the understanding of the biology related to H. longicornis invasion and expansion.
Recent studies identified several species of medium to large-sized animals as the main hosts for all stages of H. longicornis in North America (Thompson et al., 2021; Tufts et al., 2021, 2019; White et al., 2021). In less urbanized regions of New York City, raccoons (Procyon lotor), opossums (Didelphis virginiana), and white-tailed deer (Odocoileus virginianus) serve as hosts for H. longicornis (Tufts et al., 2021, 2019). Two recent studies performed in New Jersey and Virginia found a broader range of host species for H. longicornis, partly due to the rural and suburban environments supporting higher diversities of mammalian hosts (Thompson et al., 2021; White et al., 2021). The mammalian fauna of Long Island has a reduced number of species compared to that of mainland New York. However, raccoons, opossums, and white-tailed deer are all abundant in the same areas, supportive of dense populations of H. longicornis. Co-localization of questing of H. longicornis and A. americanum ticks, both spatially and vertically, suggests that these two species may share white-tailed deer as a primary host. White-tailed deer have been implicated as the driver of A. americanum population and geographic expansions (Paddock and Yabsley, 2007), and this large ungulate can reach densities of up to 115 deer/km2 in some regions of Long Island (NPS, 2022). Thus, we speculate that white-tailed deer may have played a significant role in the rapid expansion of H. longicornis on Long Island, similar to the same key role of white-tailed deer in the dispersal of blacklegged ticks (Madhav et al., 2004). Further studies must identify the mammalian host species capable of supporting H. longicornis populations in these microenvironments and address the impacts on native tick species and pathogen transmission.
The host range of H. longicornis may significantly impact the transmission of tick-borne pathogens. For instance, by co-feeding on the same mammalian hosts, H. longicornis can be infected with tick-borne pathogens that are often transmitted by native tick species. At the present time, the only tick-borne pathogen that has been vectored by H. longicornis in other countries and isolated from field-collected specimens in the U.S. is Theileria orientalis Ikeda, a cattle pathogen (Thompson et al., 2020). Multiple native tick-borne human pathogens have also been detected in H. longicornis, although the role of these species in their transmission remains uncertain (Cumbie et al., 2022; Price et al., 2022, 2021; Thompson et al., 2021). For instance, H. longicornis was shown as a competent vector of R. rickettsii under laboratory conditions (Stanley et al., 2020), but it was also determined that this tick species most likely does not contribute to B. burgdorferi transmission (Breuner et al., 2020). Yet another possibility is that H. longicornis may vector exotic pathogens similar to the case of Culex pipiens (common house mosquito) and West Nile virus. Many of these possible scenarios must be scientifically tested and assessed. Without additional field and laboratory investigations, it is difficult to evaluate the epidemiological risk posed by this new and abundant tick species (Bickerton and Toledo, 2020; Wormser et al., 2020).
The speed with which H. longicornis has expanded on Long Island is unprecedented. For comparison, it took over four decades for I. scapularis to reach relatively high population densities on Long Island. Between the 1940s and 1970s, the relative abundance of I. scapularis increased moderately (Anastos, 1947; Good, 1973), with further expansions in the 1980s through 1990s (Burgdorfer et al., 1982; H S Ginsberg and Ewing, 1989b; Smith et al., 1988). Similarly, A. americanum was first detected on the island in the 1970s (Good, 1973), but the population expansion took nearly two decades to be noticeable (Ginsberg et al., 1991). In contrast, H. longicornis became a dominant or principal tick species in many areas within three years. The rapid invasion into a new area occurred despite its relative geographic isolation from the mainland U.S., no doubt facilitated by a) H. longicornis being parthenogenetic (i.e., able to reproduce asexually without males), b) its affinity for a variety of host species, and c) its shorter life-cycle as compared to the native human-biting ticks in the US (Heath, 2016; Tufts et al., 2021, 2019). Continuing surveillance is warranted to track the expansion of this invasive tick species in North America, especially in those regions that are geographically proximate to known established populations of H. longicornis.
Supplementary Material
HIGHLIGHTS.
Asian longhorned tick, Haemaphysalis longicornis, has invaded Long Island, NY.
H. longicornis has rapidly expanded on Long Island.
H. longicornis is abundantly present in the Appalachian forest ecological zone.
Acknowledgments
The authors thank the members of the Center for Infectious Disease for their daily encouragement and insightful discussions. We are grateful to Santiago Sanchez-Vicente for providing tick sampling locations for his study. We also thank the New York State Office of Parks, Recreation and Historic Preservation and the Nature Conservancy for permission to collect ticks.
Funding
The authors are grateful for ongoing research support from the National Institute of Allergy and Infectious Diseases (AI152208 and AI169287 to H.K.K.) of the National Institutes of Health and funding from the Targeted Research Opportunity Program, Renaissance School of Medicine, Stony Brook University (to M.B.F. and D.G.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- Anastos G, 1947. Hosts of certain New York ticks. Psyche (Stuttg.) 54, 178–180. [Google Scholar]
- Bates D, Maechler M, Bolker MB, Suggests mlmRev MEMS, 2013. Package `lme4’.
- Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, 2018. Multistate infestation with the exotic disease–vector tick Haemaphysalis longicornis —United States, August 2017–September 2018. Morb. Mortal. Wkly. Rep 67, 1310–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Habicht GS, 1981. Clinical characteristics of human babesiosis. J. Infect. Dis 144, 481. [DOI] [PubMed] [Google Scholar]
- Benach JL, White DJ, Burgdorfer W, Keelan T, Guirgis S, Altieri RH, 1977. Changing patterns in the incidence of Rocky Mountain Spotted Fever on Long Island (1971–1976). Am. J. Epidemiol 106, 380–387. [DOI] [PubMed] [Google Scholar]
- Benach JL, White DJ, McGovern JP, 1978. Babesiosis in Long Island. Am. J. Trop. Med. Hyg 27, 1073–1078. [PubMed] [Google Scholar]
- Bickerton M, McSorley K, Toledo A, 2021. A life stage-targeted acaricide application approach for the control of Haemaphysalis longicornis. Ticks Tick-Borne Dis 12, 101–581. 10.1016/j.ttbdis.2020.101581 [DOI] [PubMed] [Google Scholar]
- Bickerton M, Toledo A, 2020. Multiple pruritic tick bites by Asian Longhorned tick larvae (Haemaphysalis longicornis). Int. J. Acarol 46, 373–376. 10.1080/01647954.2020.1805004 [DOI] [Google Scholar]
- Breuner NE, Ford SL, Hojgaard A, Osikowicz LM, Parise CM, Rosales Rizzo MF, Bai Y, Levin ML, Eisen RJ, Eisen L, 2020. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the Lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick-Borne Dis 11, 101–311. 10.1016/j.ttbdis.2019.101311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce et al. , 2010. Ecoregions of New York (map scale 1:1,250,000) U.S. Geological Survey, Reston, Virginia. [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Cumbie AN, Trimble RN, Eastwood G, 2022. Pathogen spillover to an invasive tick species: first detection of Bourbon virus in Haemaphysalis longicornis in the United States. Pathogens 11, 454. 10.3390/pathogens11040454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egizi A, Bulaga-Seraphin L, Alt E, Bajwa WI, Bernick J, Bickerton M, Campbell SR, Connally N, Doi K, Falco RC, Gaines DN, Greay TL, Harper VL, Heath ACG, Jiang J, Klein TA, Maestas L, Mather TN, Occi JL, Oskam CL, Pendleton J, Teator M, Thompson AT, Tufts DM, Umemiya-Shirafuji R, VanAcker MC, Yabsley MJ, Fonseca DM, 2020. First glimpse into the origin and spread of the Asian longhorned tick, Haemaphysalis longicornis, in the United States. Zoonoses Public Health 67, 637–650. 10.1111/zph.12743 [DOI] [PubMed] [Google Scholar]
- Egizi AM, Robbins RG, Beati L, Nava S, vans CR, Occi JL, Fonseca DM, 2019. A pictorial key to differentiate the recently detected exotic Haemaphysalis longicornis Neumann, 1901 (Acari, Ixodidae) from native congeners in North America. ZooKeys 117–128. 10.3897/zookeys.818.30448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, 2020. Stemming the rising tide of human-biting ticks and tickborne diseases, United States. Emerg. Infect. Dis 26, 641–647. 10.3201/eid2604.191629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP, 1989a. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp. Appl. Acarol 7, 313–322. 10.1007/BF01197925 [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP, 1989b. Habitat distribution of Ixodes dammini (Acari: Ixodidae) and Lyme disease spirochetes on Fire Island, New York. J. Med. Entomol 26, 183–189. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP, O’Connell AF, Bosler EM, Daley JG, Sayre MW, 1991. Increased population densities of Amblyomma americanum (Acari: Ixodidae) on Long Island, New York. J. Parasitol 77, 493–5. [PubMed] [Google Scholar]
- Good NE, 1973. Ticks of eastern Long Island: notes on host relations and seasonal distribution. Ann. Entomol. Soc. Am 66, 240–243. [Google Scholar]
- Grunwaldt E, Barbour AG, Benach JL, 1983. Simultaneous occurrence of babesiosis and Lyme disease. N. Engl. J. Med 308, 1166. 10.1056/NEJM198305123081919 [DOI] [PubMed] [Google Scholar]
- Heath ACG, 2016. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N. Z. Vet. J 64, 10–20. [DOI] [PubMed] [Google Scholar]
- Hilton E, DeVoti J, Benach JL, Halluska ML, White DJ, Paxton H, Dumler JS, 1999. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am. J. Med 106, 404–409. 10.1016/S0002-9343(99)00046-7 [DOI] [PubMed] [Google Scholar]
- Jamnback H, 1969. Bloodsucking flies and other outdoor nuisance arthropods of New York State. Mem St Mus Sci Serv N 19, 1–90. [Google Scholar]
- Keirans JE, Litwak TR, 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi river. J. Med. Entomol 26, 435–448. 10.1093/jmedent/26.5.435 [DOI] [PubMed] [Google Scholar]
- Liu K, Zhou H, Sun R-X, Yao H-W, Li Y, Wang L-P, Mu D, Li X-L, Yang Y, Gray GC, 2015. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci. Rep 5, 9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L-M, Zhao L, Wen H-L, Zhang Z-T, Liu J-W, Fang L-Z, Xue Z-F, Ma D-Q, Zhang X-S, Ding S-J, 2015. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis 21, 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav NK, Brownstein JS, Tsao JI, Fish D, 2004. A dispersal model for the range expansion of blacklegged tick (Acari: Ixodidae). J. Med. Entomol 41, 842–852. 10.1603/0022-2585-41.5.842 [DOI] [PubMed] [Google Scholar]
- NPS, 2022. White-tailed Deer Management Plan - Fire Island National Seashore (U.S. National Park Service)
- Paddock CD, Yabsley MJ, 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol 315, 289–324. 10.1007/978-3-540-70962-6_12 [DOI] [PubMed] [Google Scholar]
- Patil I, 2021. Visualizations with statistical details: The’ggstatsplot’approach. J. Open Source Softw 6, 3167. [Google Scholar]
- Piedmonte NP, Vinci VC, Daniels TJ, Backenson BP, Falco RC, 2021. Seasonal activity of Haemaphysalis longicornis (Acari: Ixodidae) in southern New York State. J. Med. Entomol 58, 676–681. 10.1093/jme/tjaa203 [DOI] [PubMed] [Google Scholar]
- Piesman J, Eisen L, 2008. Prevention of Tick-Borne Diseases. Annu. Rev. Entomol 53, 323–343. 10.1146/annurev.ento.53.103106.093429 [DOI] [PubMed] [Google Scholar]
- Price KJ, Ayres BN, Maes SE, Witmier BJ, Chapman HA, Coder BL, Boyer CN, Eisen RJ, Nicholson WL, 2022. First detection of human pathogenic variant of Anaplasma phagocytophilum in field-collected Haemaphysalis longicornis, Pennsylvania, USA. Zoonoses Public Health 69, 143–148. 10.1111/zph.12901 [DOI] [PubMed] [Google Scholar]
- Price KJ, Graham CB, Witmier BJ, Chapman HA, Coder BL, Boyer CN, Foster E, Maes SE, Bai Y, Eisen RJ, Kyle AD, 2021. Borrelia burgdorferi sensu stricto DNA in field-collected Haemaphysalis longicornis ticks, Pennsylvania, United States. Emerg. Infect. Dis 27, 608–611. 10.3201/eid2702.201552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KJ, Witmier BJ, Eckert RA, Boyer CN, Helwig MW, Kyle AD, 2020. Distribution and density of Haemaphysalis longicornis (Acari: Ixodidae) on public lands in Pennsylvania, United States. J. Med. Entomol 58, 1433–1438. 10.1093/jme/tjaa274 [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2019. R: A language and environment for statistical computing
- Rainey T, Occi JL, Robbins RG, Egizi A, 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol 55, 757–759. [DOI] [PubMed] [Google Scholar]
- Rochlin I, 2019. Modeling the Asian Longhorned Tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol 56, 384–391. 10.1093/jme/tjy210 [DOI] [PubMed] [Google Scholar]
- Rochlin I, Egizi A, Lindström A, 2022. The original scientific description of the lone star tick (Amblyomma americanum, Acari: Ixodidae) and Implications for the species’ past and future geographic distributions. J. Med. Entomol 59, 412–420. 10.1093/jme/tjab215 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R, 2019. Polymicrobial nature of tick-Borne diseases. mBio 10, e02055–19. 10.1128/mBio.02055-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, Benach JL, White DJ, Stroup DF, Morse DL, 1988. Occupational risk of Lyme Disease in endemic areas of New York State. Ann. N. Y. Acad. Sci 539, 289–301. 10.1111/j.1749-6632.1988.tb31863.x [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 1979. Ticks of Virginia (Acari, Metastigmata) Virginia Polytechnic Institute and State University. [Google Scholar]
- Stanley HM, Ford SL, Snellgrove AN, Hartzer K, Smith EB, Krapiunaya I, Levin ML, 2020. The ability of the invasive Asian Longhorned tick Haemaphysalis longicornis (Acari: Ixodidae) to acquire and transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the agent of Rocky Mountain Spotted fever, under laboratory conditions. J. Med. Entomol 57, 1635–1639. 10.1093/jme/tjaa076 [DOI] [PubMed] [Google Scholar]
- Thompson AT, White S, Shaw D, Egizi A, Lahmers K, Ruder MG, Yabsley MJ, 2020. Theileria orientalis Ikeda in host-seeking Haemaphysalis longicornis in Virginia, U.S.A. Ticks Tick-Borne Dis 11, 101450. 10.1016/j.ttbdis.2020.101450 [DOI] [PubMed] [Google Scholar]
- Thompson AT, White SA, Shaw D, Garrett KB, Wyckoff ST, Doub EE, Ruder MG, Yabsley MJ, 2021. A multi-seasonal study investigating the phenology, host and habitat associations, and pathogens of Haemaphysalis longicornis in Virginia, USA. Ticks Tick-Borne Dis 12, 101773. [DOI] [PubMed] [Google Scholar]
- Tufts DM, Goodman LB, Benedict MC, Davis AD, VanAcker MC, Diuk-Wasser M, 2021. Association of the invasive Haemaphysalis longicornis tick with vertebrate hosts, other native tick vectors, and tick-borne pathogens in New York City, USA. Int. J. Parasitol 51, 149–157. 10.1016/j.ijpara.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts DM, VanAcker MC, Fernandez MP, DeNicola A, Egizi A, Diuk-Wasser MA, 2019. Distribution, host-seeking phenology, and host and habitat associations of Haemaphysalis longicornis ticks, Staten Island, New York, USA. Emerg. Infect. Dis 25, 792–796. 10.3201/eid2504.181541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA APHIS | The Asian Longhorned Tick [WWW Document], n.d. URL https://www.aphis.usda.gov/aphis/maps/animal-health/asian-longhorned-tick (accessed 7.23.22).
- Vianna NJ, Hinman AR, 1971. Rocky Mountain spotted fever on Long Island: epidemiologic and clinical aspects. Am. J. Med 51, 725–730. [DOI] [PubMed] [Google Scholar]
- White SA, Bevins SN, Ruder MG, Shaw D, Vigil SL, Randall A, Deliberto TJ, Dominguez K, Thompson AT, Mertins JW, Alfred JT, Yabsley MJ, 2021. Surveys for ticks on wildlife hosts and in the environment at Asian longhorned tick (Haemaphysalis longicornis)-positive sites in Virginia and New Jersey, 2018. Transbound. Emerg. Dis 68, 605–614. 10.1111/tbed.13722 [DOI] [PubMed] [Google Scholar]
- Wormser GP, McKenna D, Piedmonte N, Vinci V, Egizi AM, Backenson B, Falco RC, 2020. First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis 70, 314–316. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Llanos-Soto SG, Gangloff-Kaufmann JL, Lampman JM, Frye MJ, Benedict MC, Tallmadge RL, Mitchell PK, Anderson RR, Cronk BD, 2020. Active surveillance of pathogens from ticks collected in New York State suburban parks and schoolyards. Zoonoses Public Health 67, 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


