Abstract
The thymus is a hormone sensitive organ, which involutes with age in response to production of sex steroids. Thymic involution leads to a decrease in the generation of recent thymic emigrants (RTE), resulting in a reduced response to immune challenges such as cancer. Interestingly, the standard of care for prostate cancer patients is androgen deprivation therapy, which leads to thymic regeneration and an increase in thymic output. It remains unknown whether these newly produced T cells can contribute to the anti-tumor immne response. The present study defines the kinetics of thymic regeneration in response to ADT in mice, determining that thymic epithelial cell (TEC) proliferation is critical for the increase in RTE output. Using a novel mouse model to track RTE in vivo, we demonstrate that these newly generated RTE can traffic to tumors where they become activated and produce effector cytokines at levels similar to more mature T cells. Collectively, these data suggest that RTE produced from ADT-induced thymic regeneration could be harnessed for the anti-tumor immune response.
Introduction
The thymus is critical in establishing and maintaining a diverse peripheral T cell pool, which is essential for mounting an effective immune response against a variety of pathogens. However, the thymus degenerates with age in part due to an increase in circulating sex steroids that begins in puberty (1). Thymic decline results in a decrease in thymic cellularity and a reduction in the export of naïve CD4+ and CD8+ T cells, called recent thymic emigrants (RTE) (2). Decreased production of RTE causes expansion of existing memory T cells (3–5), and decreases the diversity of the TCR repertoire (4, 6), which is thought to contribute to age-related reductions in the response to vaccines, infections, and cancer (7). Further, this decline in TCR repertoire diversity may make it more difficult for patients to respond to immunotherapies, such as checkpoint blockade (8).
The effect of sex steroids on the thymus can be reversed through androgen deprivation therapy (ADT), which can be achieved with bilateral orchiectomy or gonadotropin-releasing hormone (GnRH) agonists or antagonists (1, 9, 10). Interestingly, the standard of care for prostate cancer patients is ADT, which results in thymic regeneration and an increase in thymic output (9, 10). Though increased thymic output following ADT might be predicted to improve immunity, some evidence suggests that RTE require further maturation in the peripheral secondary lymphoid organs to be fully functional (11, 12). Therefore, it is unclear whether RTE produced in the context of ADT can contribute to anti-tumor responses.
In the present study, we define the kinetics of thymic regeneration after ADT in male mice and highlight the importance of thymic epithelial cell proliferation as a driver of thymic regeneration. Using a novel mouse model to track RTE in vivo, we demonstrate that RTE produced as a result of ADT can traffic to tumors. Importantly, the RTE found in tumors were functional, expressed activation markers and produced similar levels of cytokines as non-RTE, suggesting that further maturation is not required in this context. Together, this evidence suggests that RTE produced as a result of ADT can be harnessed for anti-tumor responses.
Materials and Methods
Animals and tumor models
C57BL/6 (stock #000664), RIP-mOVA (stock #005431), OTI (stock #003831), C57Bl/6;CD90.1 (also known as Thy1.1, stock #000406), C57BL/6;CD45.1 (also known as B6.SJL, stock #002014), Rag2-GFP (stock #005688), TCRδ-CREERT2 (stock #031679) and Rosa26-tdTomato (stock #007914) were purchased from the Jackson Laboratory. All animals were maintained under specific pathogen-free conditions in the Oregon Health & Science University (Portland, OR) animal facility. Sexually mature 12-week old males were used in all the experiments described. Murine Pten−/−;p53−/−;Smad4−/− (PPSM) castration resistant prostate tumor model (gift of Ronald DePinho) and TrampC1-OVA (gift of Michael Gough) were propagated in vitro using complete media, RPMI 1640 (Lonza) containing 0.292 ng/ml glutamine, 100 U/ml streptomycin/penicillin, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 10mM HEPES (Sigma-Aldrich). All cell lines were tested and confirmed to be Mycoplasma and endotoxin-free using the MycoAlert Detection kit (Lonza) and the Endosafe-PTS system (Charles River Laboratories). All culture media reagents were purchased from Hyclone Laboratories unless noted otherwise. All animal experiments were approved by the Institutional Animal Care and Use Committee of OHSU.
Orchiectomy surgeries and androgen deprivation therapy treatments.
Orchiectomy surgeries were performed as previously described (13). Briefly, male mice were anesthetized with isofluorane, and a small midline ventral skin and muscle incision made. Testes were pulled out through the incision, and the spermatic cords were sectioned and cauterized, and testes removed along with surrounding fatty tissue. The muscle layer and skin were closed with absorbable suture. For antigen deprivation therapy, mice were treated with 0.5 μg of degarelix (Medchem Express) by S.C. injection once every 14 days. Enzalutamide (Medchem Express) was administered daily for 2 weeks in the feed at 50 mg/kg in Purina chow 5053, Research Diet Inc. (~ 0.25 mg/mouse/day). Abiraterone (Medchem Express) was administered by oral gavage at 0.5 mmol/kg/d in vehicle (5% benzyl alcohol, 95% safflower oil), daily for 2 weeks.
Tumor challenge and adoptive transfers
1×106 PPsm tumor cells were injected on the hind flanks of C57BL/6, B6;SJL, and 1×106 TrampC1-OVA were injected on the hind flanks of RipmOVA male mice (3–5 per group). On day 10 for PPsm or day 17 for TrampC1-OVA tumor bearing animals, 1×105 flow cytometry sorted Rag2-GFP+ (RTE), Rag2-GFP– (non-RTE), OTI;Thy1.1;Rag2-GFP+ (OTI RTE) or OTI;Thy1.1;Rag2-GFP– (OTI non-RTE), were adoptively transferred by i.v. injection in the tumor bearing animals. Tumor draining lymph nodes (dLN) and tumors were harvested 5 days post adoptive transfer. For RTE time-stamping experiments, 1×106 PPsm tumor cells were injected in the hind flanks of TCRg-CREERT2-R26-tdTomato animals. 7 days post tumor implantation, half of the animals were orchiectomized. 2 days post orchiectomy, all animals were treated with 2mg Tamoxifen by oral gavage daily for 14 days. Tumor dLN (inguinal) and tumors were harvested 21 days post orchiectomy.
TEC, thymocytes and lymphocyte isolation
TEC were isolated and enriched as previously described (14). For thymocyte isolation, thymi were harvested and processed to obtain single-cell suspensions using frosted ends of microscope slides. Cells were rinsed with PBS containing 1% FBS and 4 mM EDTA. Tumor infiltrating lymphocytes (TIL) were harvested by dissection of tumor tissue into small fragments in a 50-cc conical tube followed by digestion at room temperature in a bacterial shaker at 180 rpm for 30 min in 1 mg/ml collagenase type IV (Worthington Biochemicals), 100 μg/ml hyaluronidase (Sigma-Aldrich) and 20 mg/ml DNAse (Roche) in PBS. Cells were then further disrupted with a 1-cc syringe plunger through a 70-μm nylon cell strainer (BD Biosciences), and filtered to obtain a single cell suspension. Tumor dLN (inguinal) were harvested and processed to obtain single-cell suspensions using frosted ends of microscope slides. Cells were rinsed with PBS containing 1% FBS and 4 mM EDTA.
Flow cytometry and cell sorting
For flow cytometry analysis, cells were incubated for 20 min on ice with e506 fixable viability dye and the following antibodies: TCRβ (H57–597), CD4 (RM4–5), CD8 (53–6.7), TCRγδ (eBioGL3), NK1.1 (PK136), CD44 (IM7), CD69 (H1.2F3), Epcam (G8.8), CD45 (30-F11), PD1 (J43), CD45.2 (104), CD45.1 (A20) and Thy1.1 (HIS51). Intracellular proteins FoxP3 (FJK-16s), Ki67 (SolA15), IFNγ (XMG1.2) and TNFα (MP6-CT22) were detected using the FoxP3 Transcription Factor Concentrate and Diluent from eBioscience. All antibodies and viability dyes were purchased from eBioscience, Biolegend, or BD Biosciences. Data were collected with a Fortessa flow cytometer (BD Biosciences) and analyzed using Flowjo software (Tree Star). Unless noted otherwise in the figure legend, cells were gated through live/TCRβ+ gates for analysis. T cell sorts were performed on an Aria (BD Biosciences) with an 85 micron nozzle.
In vitro activation and intracellular cytokine staining
Splenocytes and bulk TIL were plated at 1×106 cells/well in 96-well plates and stimulated for 5h with PMA (80 nM) and ionomycin (1.3 μM) or 1nM SIINFEKL peptide for OTI re-stimulation, in the presence of brefeldin A (BFA). Cells were then stained for surface markers, fixed and permeabilized using either the BD Cytofix/CytoPerm or eBioscience Foxp3 kit, and stained for intracellular cytokines.
Statistical analysis
Statistical analysis was performed using unpaired two-tailed Student t test (for comparison between two groups) or One-way ANOVA with Tukey multiple comparision (for comparison between multiple groups) using GraphPad Prism 8 (GraphPad Software). Error bars represent SEM. Statistical tests and p values are specified for each panel in the respective figure legends, and p values < 0.05 were considered significant. Biological replicates (individual animals) for each experiment are indicated in the figure legends.
Study Approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Earle A. Chiles Research Institute at Portland Providence Cancer Center or the Institutional Animal Care and Use Committee of OHSU.
Results
ADT promotes thymic regeneration through proliferation of TEC but not thymocytes.
It is well established that ADT leads to regeneration of the thymus in mice and humans (1, 9, 10, 15), but the kinetics of this regeneration process have not been explored in detail. Sexually mature (12-week old) male mice were orchiectomized, and thymi harvested 2-, 5- and 12-weeks post orchiectomy. Thymic weight and cellularity increased over time after orchiectomy (Fig. 1A, B), peaking at 5 weeks. As a comparison, thymic weight and cellularity in intact male mice at ages 2, 5 and 12-weeks remained unchanged (supplemental Fig. 1A and B). A known mechanism of age-related thymic atrophy results from a reduction of the thymic epithelial space and reduced numbers of TEC (16). We indeed observed a gradual reduction in Epcam+CD45− TEC numbers in the thymi of intact male mice at 5 and 12 weeks of age compared to 2-week-old neonates (supplemental Fig. 1C and D), reflecting thymic involution. Following orchiectomy, we observed a sharp increase in TEC numbers in the thymus at 5 weeks post-surgery (Fig. 1C), with increased numbers maintained up to 12 weeks. To establish whether the increase in TEC numbers was due to proliferation, we assessed Ki67 expression in TEC over time after orchiectomy. The frequency of proliferating TEC increased after orchiectomy, peaking at 5 weeks post-surgery (Fig. 1D and E) to reach proliferation levels similar to 2-week old neonates (supplemental Fig. 1E).
Figure 1. ADT promotes thymic regeneration through proliferation of TEC but not thymocytes.
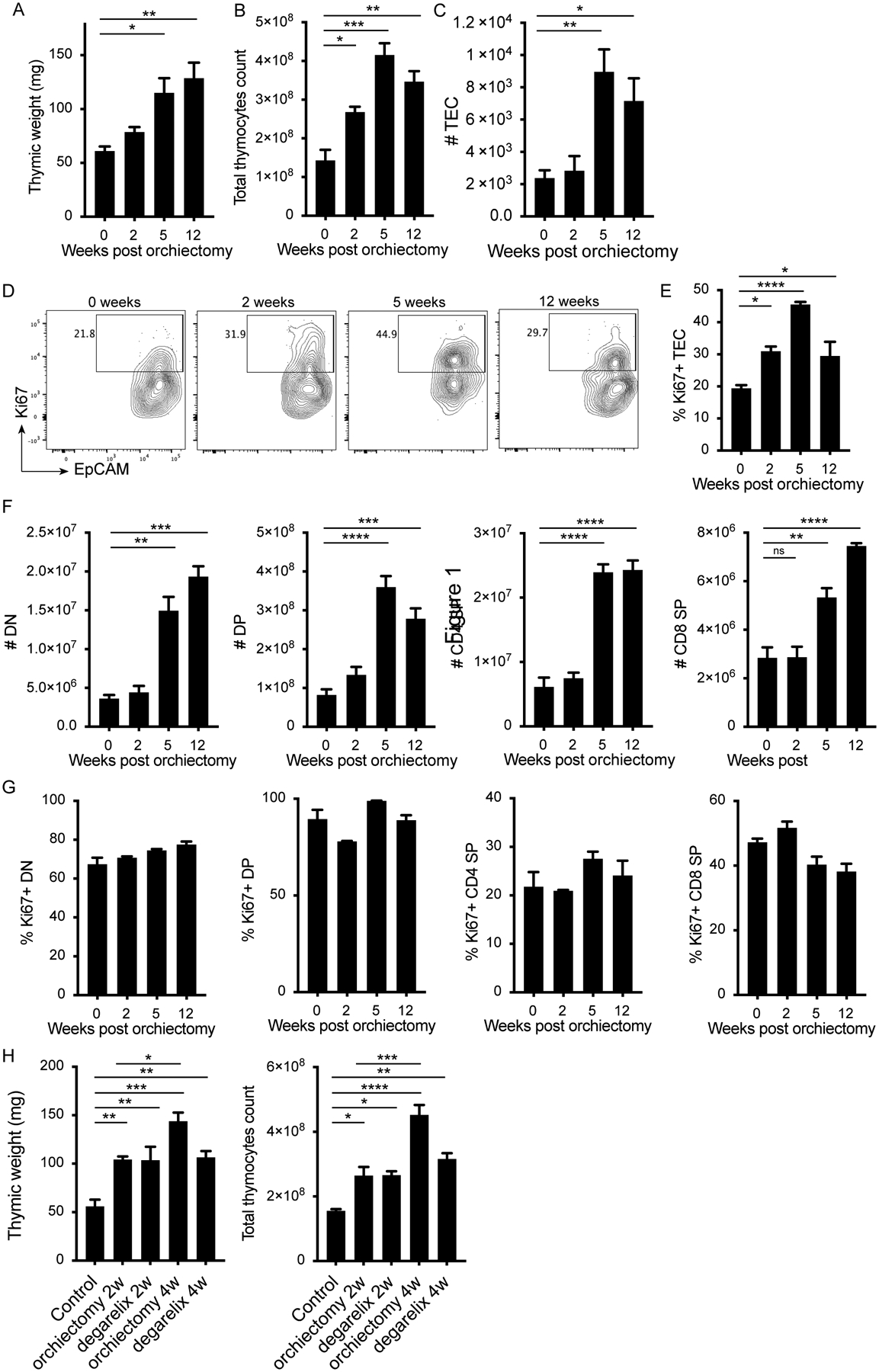
12-week old male mice were orchiectomized, and thymi were harvested at 2, 5 and 12 weeks later. A) Thymic weight, B) total thymocyte count and C) number of thymic epithelial cells (TEC) at time of harvest. D) Representative flow plots gated on TECs (CD45−Epcam+) and showing EpCAM and Ki67 expression, and E) quantification of percent Ki67+ TEC at 0, 2, 5 and 12 weeks post orchiectomy. F) Numbers and G) percent Ki67+ of DN, DP, CD4 SP and CD8 SP in the thymus at the indicated times. H) 12 week old male mice were orchiectomized or chemically castrated using degarelix, and thymi were harvested 2 or 4 weeks later. Graphs show thymic weight and total thymocyte counts after the indicated treatments. 3 animals per group. Data representative of 3 experiments. One-way ANOVA with Tukey multiple comparison, * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
Given the large increase in thymic cellularity post orchiectomy (Fig. 1B), we sought to identify which thymocyte subsets accounted for this increase during thymic regeneration and to elucidate the kinetics of this process. We observed a gradual increase in the number of double negative (DN) and double positive (DP) thymocytes over time after orchiectomy, as well as a gradual increase in CD4 and CD8 single positive (SP) thymocytes, with a 3 to 4-fold increase at 12 weeks post orchiectomy compared to pre-surgery (Fig. 1F). SP, DP, CD4 SP and CD8 SP thymocytes did not display increased expression of Ki67 after orchiectomy, contrary to observations made in TEC (Fig. 1G). This suggests that the increase in cell number was due to enhanced positive selection rather than proliferation of existing thymocytes in the thymus. We also observed a gradual increase in the number of γδ T cells, NKT cells and regulatory T cells (Tregs) over time after orchiectomy (supplemental Fig. 1G, H, I), without an increase in proliferation as measured by Ki67 expression (supplemental Fig. 1J, K, L). These data establish that although thymic regeneration is measurable as early as two weeks post orchiectomy, this process continues over time and increased thymic weight and cellularity is maintained up to 12 weeks post-surgery.
ADT is the mainstay of treatment for advanced prostate cancer patients, and thymic regeneration associated with such treatment has been reported (1, 9, 10, 15). Degarelix, a GnRH antagonist, is used clinically to reduce circulating testosterone levels. We compared the thymic regeneration achieved with orchiectomy to degarelix treatment in 12-week-old male mice. At 2 weeks post orchiectomy or degarelix treatment, the increase in thymic weight and total thymocyte number was similar between the two treatments compared to untreated control animals (Fig. 1H). Thymic weight and cellularity continued to increase in orchiectomized animals between 2 and 4 weeks whereas degrelix treated animals achieved maximal regeneration after 2 weeks (Fig. 1H). Second generation androgen receptor (AR) inhibitors such as enzalutamide or abiraterone are often added to GnRH inhibition to reduce AR signaling and adrenal androgens. The addition of either of these small molecules to degarelix did not significantly increase thymus weight over degarelix alone (supplemental Fig. 1M). We did observe a further increase in total thymocyte numbers 2 weeks post treatment (supplemental Fig. 1N).
RTE traffic to the tumor and display an activated phenotype
Given our observations and previously reported detection of RTEs in the blood of patients treated with ADT (2), we asked whether these new T cells could traffic into the tumor. To answer this question, we utilized Rag2-GFP mice, in which GFP is expressed under the control of the Rag2 promoter, fluorescently tagging RTE in the periphery for ~5 days after exiting the thymus (12). We sorted TCRβ+Rag2GFP+ T cells from the spleens of Rag2-GFP male mice as a source of RTE (supplemental Fig. 2), and adoptively transferred these RTE into congenic (CD45.1+) male recipient mice harboring a transplantable mouse prostate tumor (Pten−/−;p53−/−;Smad4−/−, referred to as PPSM) (13, 17). Sorted Rag2GFP+ cells (RTE) were adoptively transferred 10 days post tumor implantation (Fig. 2A). dLN and tumors were harvested 5 days post adoptive transfer, and the presence of adoptively transferred RTE was assessed. CD45.2+ adoptively transferred RTE were recovered in both the dLN and tumors (Fig. 2B), highlighting that RTE are able to traffic to the tumor site within 5 days despite recent emigration from the thymus. Notably, the CD45.2+ T cells had lost Rag2-GFP expression in both the dLN (Fig. 2C) and the tumor (Fig. 2D) at time of harvest, reflecting time since undergoing positive selection. In the dLN, these cells expressed low levels of activation markers PD-1, CD44 and CD69, similar to host T cells (Fig. 2C). In the tumor, the RTE expressed PD-1, CD44 and CD69, markers consistent with activation. However, RTE expressed lower levels of PD-1 when compared to host T cells in the tumor (Fig. 2D and E). Together, these data demonstrate that RTE traffic to the tumor and exhibit an activated phenotype.
Figure 2. RTE traffic to the tumor and display an activated phenotype.
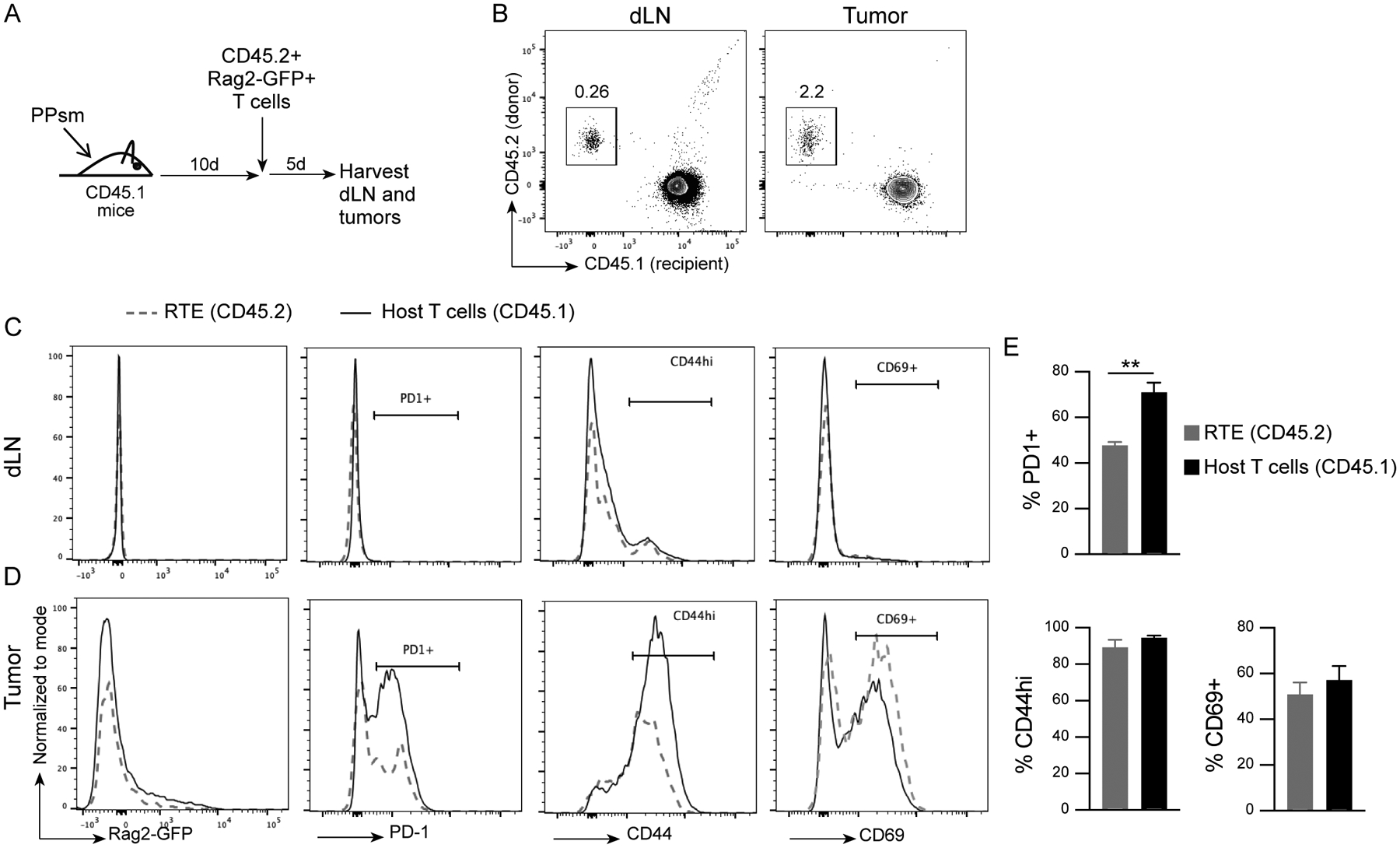
A) Experimental design. B) Representative cytogram (gated on live, TCRβ+) showing CD45.1+ (host) and CD45.2+ (adoptively transferred cells) staining in the dLN and tumor. C-D) Representative histograms, gated on live, TCRβ+, CD45.1+ (host T cells) or CD45.2+ (RTE), showing Rag2-GFP, PD1, CD44 and CD69 expression in host CD8 T cells and adoptively transfered RTE CD8 T cells in dLN (C) and tumor (D). E) Quantification of percent PD1+, CD44hi and CD69+ RTE and host T cells in the tumor. 3 animals per group. Data representative of 3 experiments. Unpaired two-tailed Student t test, * P<0.05, ** P<0.01.
Tumor-specific RTE contribute to the anti-tumor immune response
Previous work suggests that RTE require further maturation in the peripheral secondary lymphoid organs (11) to be fully functional. Our findings that RTE can traffic to the tumor and appear activated is therefore novel and surprising. To further confirm that RTE have the ability to participate in the anti-tumor immune response, we tested whether tumor-specific CD8 RTE function as well as tumor-specific mature CD8 T cells sorted from the periphery. We generated OTI;Rag2-GFP;Thy1.1 animals, and sorted OTI RTE (Rag2-GFP+) and OTI non-RTE (Rag2-GFP-) from the spleens of these animals. Sorted OTI RTE or OTI non-RTE were adoptively transferred into OVA-expressing tumor-bearing male mice (TrampC1-Ova), allowing for direct comparison of tumor-antigen specific RTE and non-RTE T cell function in the tumor. Five days post adoptive transfer, dLN and tumors were harvested (Fig. 3A). Both RTE and non-RTE Thy1.1+ cells were recovered in dLN and tumors (Fig. 3B). The number of recovered RTE was slightly lower compared to the number of non-RTE in the dLN, but was equivalent in the tumor (Fig. 3C), demonstrating that tumor-specific CD8 RTE have a similar ability to traffic to the tumor as non-RTE. As expected, the RTE had lost Rag2-GFP expression 5 days post transfer (Fig. 3D). Both the RTE and non-RTE OTI T cells displayed an activated phenotype compared to endogenous CD8 T cells in the dLN and tumor. RTE expressed similar levels of activation markers CD44 and PD-1 compared to non-RTE in both the dLN and tumor (Fig. 3D and E). The majority of both the RTE and non-RTE were proliferating in the dLN and tumors, with a small reduction in the frequency of Ki67+ RTE in the tumor compared to the non-RTE (Fig. 3F). These data further establish that tumor-specific CD8 RTE are similarly able to traffic to the tumor and exhibit an activated phenotype comparable to mature tumor-specific non-RTE CD8 T cells.
Figure 3. Tumor-specific RTE contribute to the anti-tumor immune response.
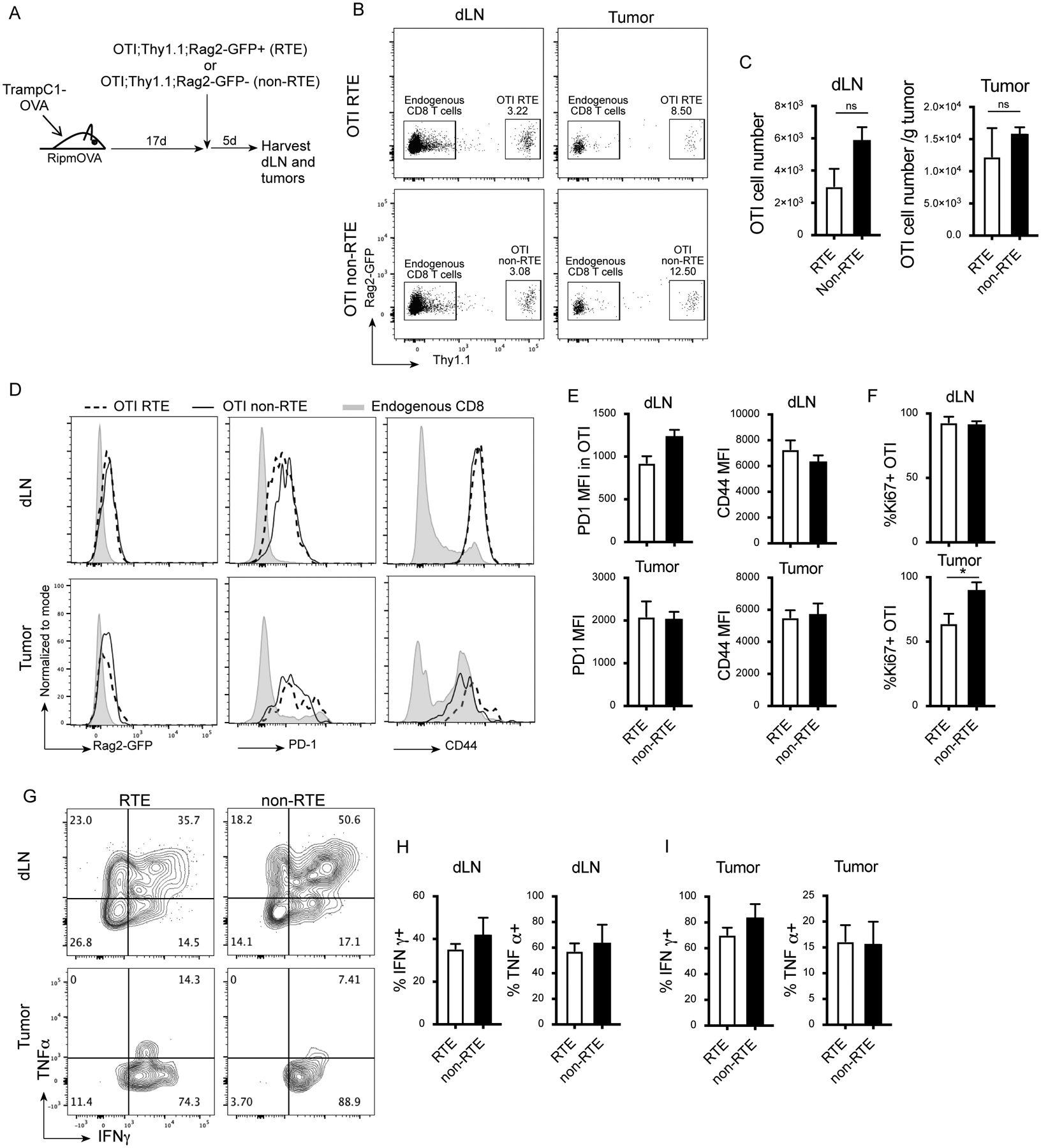
A) Experimental design. B) Representative flow plots (gated on live, TCRβ+) showing Rag2-GFP and Thy1.1 staining in the dLN and tumor of animals that received adoptive transfer of RTE OTI or non-RTE OTI. C) Cell number of recovered OTI cells in the dLN and tumor. D) Representative histograms (gated on live, TCRβ+, CD8+, Thy1.1+ or Thy1.1-) showing Rag2-GFP, PD1 and CD44 expression in the OTI cells and host endogenous CD8 T cells. E) Quantification of PD1 and CD44 MFI in OTI the dLN and tumor. F) Percent Ki67+ OTI cells. G-I) dLN cells and tumor infiltrating lymphocytes (TIL) were stimulated in vitro with SIINFEKL peptide and stained for intracellular cytokines. G) Representative flow plots (gated on live, TCRβ+, CD8, Thy1.1+) showing IFNγ and TNFα expression adoptively transferred RTE and non-RTE in the dLN and tumor. H-I) Quantification of percent IFNγ+ and TNFα+ among adoptively transferred RTE and non-RTE in the dLN (H) and tumor (I). 3 animals per group. Data are representative of 2 experiments. Unpaired two-tailed Student t test, * P<0.05.
Activation markers on the RTE could be due to bystander activation in the tumor rather than direct activation by tumor antigen, and these cells might still be too immature to become fully activated and participate in the anti-tumor immune response. Therefore, we re-stimulated the RTE and non-RTE ex vivo with SIINFEKL peptide 5 days post adoptive transfer. The RTE produced IFNγ and TNFα cytokines at comparable levels to the non-RTE (Fig. 3G–I), suggesting that the RTE can actively participate in the anti-tumor immune response.
Orchiectomy leads to increased numbers of functional RTE in the tumor.
We have shown that orchiectomy leads to thymic regeneration and increases thymocyte numbers, and that adoptively transferred RTE have the ability to traffic to the tumor and become activated. We therefore sought to directly test whether the RTE released following orchiectomy can traffic to tumors and participate in anti-tumor immune responses. To evaluate this, we initially implanted PPSM tumors in the flanks of Rag2-GFP male mice. Seven days after tumor implantation, animals were orchiectomized. Thymus, dLN, and tumors were harvested 2 weeks post-surgery. The thymic weights were increased 2 weeks post orchiectomy (Supplemental Fig. 2B), consistent with thymic regeneration. To assess a potential increase in RTE release from the thymus post orchiectomy, we looked at the frequency and total number of Rag2-GFP+ T cells in the dLN and tumors. We did not observe an increase in GFP+ T cells in orchiectomized animals compared to intact animals (supplemental Fig. 2C–D). However, these data are confounded by the fact that RTE lose Rag2-GFP expression within 5 days of exiting the thymus, making it impossible to track them long-term in this system.
In order to follow RTE long-term, we developed a fate mapping system to allow for permanent marking of RTE following orchiectomy. TCRδ-CREERT2 mice have a tamoxifen-inducible Cre recombinase under the control of the TCRδ chain gene promotor. TCRδ expression starts in the thymus at the DN stage, and is lost in mature αβ T cells due to somatic deletion at the DP stage (18). Therefore, TCRδ is only expressed in αβ T cells in the thymus. We generated TCRδ-CREERT2;Rosa26-tdTomato animals, and implanted them with PPSM tumors. Ten days post tumor implantation, animals were orchiectomized to initiate thymic regeneration. Tamoxifen was administered daily for 14 days starting 2 days post orchiectomy, allowing for CRE expression in newly generated thymocytes and permanent expression of tdTomato in newly released RTE. dLN and tumors were harvested 21 days post orchiectomy (Fig. 4A). In both the dLN (Fig. 4B and C) and the tumor (Fig. 4B and D), a 2-fold increase in frequency and number of tdTomato+ CD8 T cells were recovered in orchiectomized animals compared to intact animals, reflecting an increase in the release of RTE following orchiectomy. In the dLN, the tdTomato+ CD8 T cells from both intact and orchiectomized animals showed very low levels of activation markers PD-1 and CD44, comparable to levels in tdTomato- T cells (supplemental Fig. 3A and B). In the tumor, tdTomato+ CD8 T cells from both intact and orchiectomized animals expressed PD-1 and CD44, although at lower levels than the tdTomato- CD8 T cells (supplemental Fig. 3A and C). Strikingly, the TdTomato+ CD8 T cells from both intact and orchiectomized animals were able to produce IFNγ and TNFα comparable to the more mature tdTomato- CD8 T cells in the dLN and tumor (Fig. 4E–G). Finally, orchiectomized animals had higher numbers of CD8 RTE expressing IFNγ and TNFα in the tumor than did intact mice (Fig. 4H and I). We observed a similar increase in the proportion and number of tdTomato+ CD4 T cells post orchiectomy in both the dLN and tumor (supplemental Fig. 4A–C). TdTomato+ CD4 T cells were able to produce IFNγ and TNFα cytokines in the dLN and tumor (supplemental Fig. 4D–F), and the umber of CD4 RTE expressing IFNγ and TNFα in the tumor was higher in orchiectomized animals than in intact animals (supplemental Fig. 4G and H). These data establish that RTE generated during thymic regeneration following orchiectomy are able to participate in the anti-tumor immune response.
Figure 4. Orchiectomy leads to increased numbers of functional CD8+ RTE in the tumor.
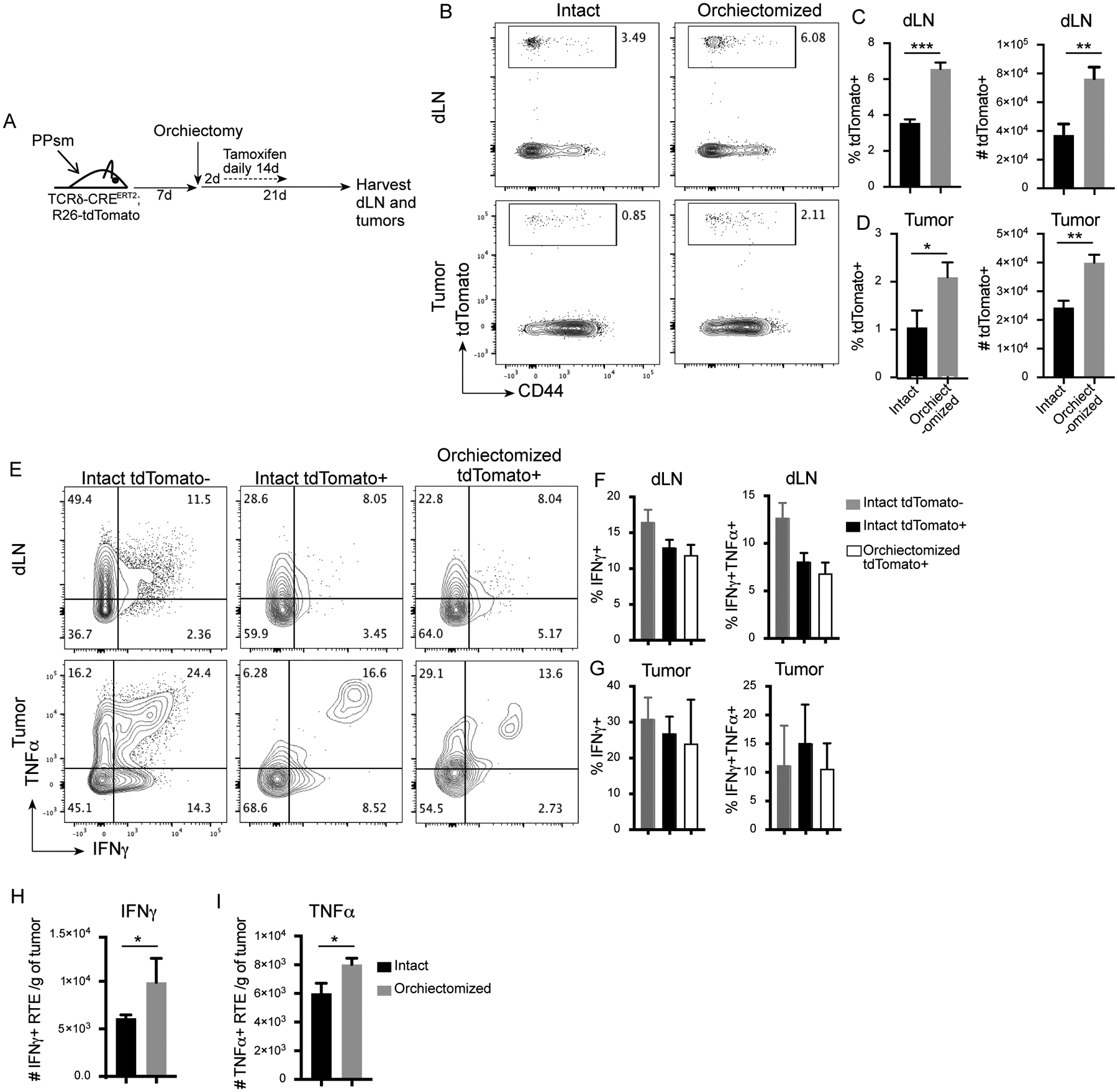
A) Experimental design. B) Representative flow plots (gated on live, TCRβ+, CD8+) showing CD44 and tdTomato expression in the dLN and tumor of intact and orchiectomized animals. Quantification of percent and numbers of tdTomato+ CD8 T cells in the C) dLN and D) tumor (numbers are per gram of tumor). E) Representative flow plots gated on live, TCRβ+, CD8, tdTomato+ or - showing IFNγ and TNFα expression. Intact TdTomato+ cells are not shown, but look identical to intact TdTomato- cells. F, G) Quantification of percent IFNγ+ and IFNγ+TNFα+ in the dLN (F) and tumor (G). H, I) Number of CD8+ RTE expressing IFNγ (H) and TNFα (I) per gram of tumor. 3 animals per group. Data representative of 3 repeat experiments. Unpaired two-tailed Student t test, * P<0.05, ** P<0.01, *** P<0.001.
Discussion
Thymic involution prevents aged hosts from mounting an effective response to vaccination and invading pathogens (19). We show in this report that both orchiectomy and ADT of male mice leads to proliferation of TEC, which correlates with an increase in thymic output of RTE. We demonstrate that RTE produced as a result of ADT can traffic to tumors where they are activated and produce levels of cytokines comparable to non-RTEs. Together, this evidence suggests that ADT induces an increase in RTEs that can participate in the anti-tumor response.
The impact of androgens on the function of the thymus has been well established. AR expression in the thymus has been demonstrated using ligand-binding assays in homogenate from whole thymus tissue in rats and humans (20, 21). More recent studies found AR expression in both thymocytes (22, 23) and thymic epithelial cells (TEC), with more abundant expression in TEC (24). We and others have demonstrated that blocking AR results in thymic regeneration (1, 9, 10, 15). Our findings indicate that this regeneration may be due to the effect of androgens on the TEC as orchiectomy led to the proliferation of TEC, but not thymocytes. This is in agreement with previous reports where the authors determined that the effect of androgens on TEC resulted in thymic enlargement (1, 25). A recent report in a murine model of prostate cancer demonstrated that ADT increased thymic area as well as output (10). Reports in prostate cancer patients similarly demonstrated that the increase in RTE after ADT was due to an increase in thymic output rather than an increase in proliferation (2, 9). However, at least one study demonstrated an increase in thymocyte proliferation in response to orchiectomy (15).
RTE are thought to undergo further maturation in the periphery (11, 12), which, until now, has led to uncertainty regarding whether the RTE generated from ADT can meaningfully contribute to an immune response. Our data clearly show that RTE produced from ADT can traffic to tumors and participate in the anti-tumor immune response through the production of cytokines. Moreover, our data provide a potential mechanism to explain the positive effects of castration on disease outcome in a number of infection and tumor models. Orchiectomy of old mice that were then infected with human HSV-1 led to increased T cell activation in the draining lymph node as well as an increase in the specific lysis of HSV-1-infected cells (9). In an influenza A virus infection model, orchiectomy led to better viral control, which was due to an increase in virus-specific CD8+ T cell number and function (26). Other studies have shown that orchiectomized animals were protected from both chemically induced and transplantable tumors models, an effect that was dependent upon the thymus (26, 27). It’s intriguining to consider the possibility of combining ADT with cancer vaccines to harness RTEs and their lack of long-term exposure to inflammatory cytokines. In fact, the lower expression of PD1 on RTEs within the tumor compared to non-RTEs further suggests that mechanisms to exploit the presence of these ‘young’ cells could enhance anti-tumor immunity. Finally, the use of androgen inhibition to regenerate the thymus not only induces a new wave of T cells, but also relieves androgen mediated immune suppression (28–30); a phenomena that we believe would impact RTE and non-RTE alike.
Although the increased output of functionally competent RTEs likely plays a role in improved T cell responses following orchiectomy, our data do not preclude other mechanisms by which removal of androgens could boost T cell immunity. There may be a direct impact of the removal of androgens on peripheral T cells as AR is expressed by these cells (13, 31). Further, we cannot discount the effect of ADT on hematopoeitic stem cells as hematopoiesis also increases with ADT (9, 32). Additionally, there is evidence that removal of androgens increases antigen presentation, which could also contribute to the reported findings (33).
Simple blockade of sex steroid production can return the thymus to its prepubertal state, allowing the thymus to utilize its inherent regenerative capacity to produce T cells without the addition of exogenous cytokines. Therefore, there is no evidence of the development of pathological conditions, such as autoimmunity, resulting from this strategy. Further, we do not think that the importance of sex hormones in regulating thymic output is limited to males as ovariectomy in aged female mice also led to an increase in thymic cellularity and output (26, 34). Further studies should be conducted to determine the role of hormones in regulating thymic output in females.
Collectively, we demonstrate that both physical and chemical castration lead to comparable levels of thymic regeneration, which results in an increase in RTE. These RTE traffic to tumors, where they become activated and produce effector cytokines comparable to non-RTEs. Together, these data have implications for enhancing responses to tumor-vaccines, chemotherapy, immunotherapies, and for increasing immune reconstitution following chemotherapy.
Supplementary Material
Key Points.
Androgen deprivation therapy results in increased recent thymic emigrants (RTE).
RTE traffic to prostate tumors.
RTE become activated and produce effector cytokines in the tumor microenvironment.
Acknowledgements:
We are grateful to the OHSU Department of Comparative Medicine for outstanding animal husbandry and the OHSU Flow Cytometry Core for their support.
Funding:
OHSU Foundation; Knight Cancer Institute 2019-CCSG-24
References:
- 1.Olsen NJ, Olson G, Viselli SM, Gu X, and Kovacs WJ. 2001. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 142: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 2.Sempowski GD, Gooding ME, Liao HX, Le PT, and Haynes BF. 2002. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol 38: 841–848. [DOI] [PubMed] [Google Scholar]
- 3.Callahan JE, Kappler JW, and Marrack P. 1993. Unexpected expansions of CD8-bearing cells in old mice. J Immunol 151: 6657–6669. [PubMed] [Google Scholar]
- 4.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, and Moss PA. 2007. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 81: 7759–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricalton NS, Roberton C, Norris JM, Rewers M, Hamman RF, and Kotzin BL. 1998. Prevalence of CD8+ T-cell expansions in relation to age in healthy individuals. J Gerontol A Biol Sci Med Sci 53: B196–203. [DOI] [PubMed] [Google Scholar]
- 6.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, and Goronzy JJ. 2005. The influence of age on T cell generation and TCR diversity. J Immunol 174: 7446–7452. [DOI] [PubMed] [Google Scholar]
- 7.Palmer DB 2013. The effect of age on thymic function. Front Immunol 4: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale A, De Luca CD, Locatelli F, and Velardi E. 2021. Thymic Function and T-Cell Receptor Repertoire Diversity: Implications for Patient Response to Checkpoint Blockade Immunotherapy. Front Immunol 12: 752042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, and Boyd RL. 2005. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 175: 2741–2753. [DOI] [PubMed] [Google Scholar]
- 10.Terrisse S, Goubet AG, Ueda K, Thomas AM, Quiniou V, Thelemaque C, Dunsmore G, Clave E, Gamat-Huber M, Yonekura S, Ferrere G, Rauber C, Pham HP, Fahrner JE, Pizzato E, Ly P, Fidelle M, Mazzenga M, Costa Silva CA, Armanini F, Pinto F, Asnicar F, Daillère R, Derosa L, Richard C, Blanchard P, Routy B, Culine S, Opolon P, Silvin A, Ginhoux F, Toubert A, Segata N, McNeel DG, Fizazi K, Kroemer G, and Zitvogel L. 2022. Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J Immunother Cancer 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boursalian TE, Golob J, Soper DM, Cooper CJ, and Fink PJ. 2004. Continued maturation of thymic emigrants in the periphery. Nat Immunol 5: 418–425. [DOI] [PubMed] [Google Scholar]
- 12.Houston EG Jr., Nechanitzky R, and Fink PJ. 2008. Cutting edge: Contact with secondary lymphoid organs drives postthymic T cell maturation. J Immunol 181: 5213–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, Thomas GV, Caruso B, Thompson RF, Wood MA, Hipfinger C, Hammond SA, Graff JN, Xia Z, and Moran AE. 2022. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Y, and Hogquist KA. 2014. Isolation, identification, and purification of murine thymic epithelial cells. J Vis Exp: e51780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, and Boyd RL. 2005. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol 175: 2982–2993. [DOI] [PubMed] [Google Scholar]
- 16.Aw D, Taylor-Brown F, Cooper K, and Palmer DB. 2009. Phenotypical and morphological changes in the thymic microenvironment from ageing mice. Biogerontology 10: 311–322. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, Jiang S, Chang Q, Spring DJ, Sharma P, Zebala JA, Maeda DY, Wang YA, and DePinho RA. 2017. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543: 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Wu J, Jiao Y, Bock C, Dai M, Chen B, Chao N, Zhang W, and Zhuang Y. 2015. Differential Requirements of TCR Signaling in Homeostatic Maintenance and Function of Dendritic Epidermal T Cells. J Immunol 195: 4282–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehm T, and Swann JB. 2013. Thymus involution and regeneration: two sides of the same coin? Nat Rev Immunol 13: 831–838. [DOI] [PubMed] [Google Scholar]
- 20.Grossman CJ, Nathan P, Taylor BB, and Sholiton LJ. 1979. Rat thymic dihydrotestosterone receptor: preparation, location and physiochemical properties. Steroids 34: 539–553. [DOI] [PubMed] [Google Scholar]
- 21.McCruden AB, and Stimson WH. 1984. Androgen receptor in the human thymus. Immunol Lett 8: 49–53. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs WJ, and Olsen NJ. 1987. Androgen receptors in human thymocytes. J Immunol 139: 490–493. [PubMed] [Google Scholar]
- 23.Viselli SM, Olsen NJ, Shults K, Steizer G, and Kovacs WJ. 1995. Immunochemical and flow cytometric analysis of androgen receptor expression in thymocytes. Mol Cell Endocrinol 109: 19–26. [DOI] [PubMed] [Google Scholar]
- 24.Kumar N, Shan LX, Hardy MP, Bardin CW, and Sundaram K. 1995. Mechanism of androgen-induced thymolysis in rats. Endocrinology 136: 4887–4893. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelmson AS, Lantero Rodriguez M, Johansson I, Svedlund Eriksson E, Stubelius A, Lindgren S, Fagman JB, Fink PJ, Carlsten H, Ekwall O, and Tivesten Å. 2020. Androgen Receptors in Epithelial Cells Regulate Thymopoiesis and Recent Thymic Emigrants in Male Mice. Front Immunol 11: 1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heng TS, Reiseger JJ, Fletcher AL, Leggatt GR, White OJ, Vlahos K, Frazer IH, Turner SJ, and Boyd RL. 2012. Impact of sex steroid ablation on viral, tumour and vaccine responses in aged mice. PLoS One 7: e42677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro JE 1974. Orchidectomy and the immune response. III. The effect of orchidectomy on tumour induction and transplantation in mice. Proc R Soc Lond B Biol Sci 186: 387–398. [DOI] [PubMed] [Google Scholar]
- 28.Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, Thomas GV, Caruso B, Thompson RF, Wood MA, Hipfinger C, Hammond SA, Graff JN, Xia Z, and Moran AE. 2022. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon H, Schafer JM, Song NJ, Kaneko S, Li A, Xiao T, Ma A, Allen C, Das K, Zhou L, Riesenberg B, Chang Y, Weltge P, Velegraki M, Oh DY, Fong L, Ma Q, Sundi D, Chung D, Li X, and Li Z. 2022. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci Immunol 7: eabq2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Jin J, Yang Y, Sun H, Wu L, Shen M, Hong X, Li W, Lu L, Cao D, Wang X, Sun J, Ye Y, Su B, and Deng L. 2022. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity 55: 1268–1283.e1269. [DOI] [PubMed] [Google Scholar]
- 31.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, and Wunderlich F. 1999. Functional testosterone receptors in plasma membranes of T cells. Faseb j 13: 123–133. [DOI] [PubMed] [Google Scholar]
- 32.Khong DM, Dudakov JA, Hammett MV, Jurblum MI, Khong SM, Goldberg GL, Ueno T, Spyroglou L, Young LF, van den Brink MR, Boyd RL, and Chidgey AP. 2015. Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Reports 4: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh YT, Gray A, Higgins SA, Hubby B, and Kast WM. 2009. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 69: 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perisić M, Arsenović-Ranin N, Pilipović I, Kosec D, Pesić V, Radojević K, and Leposavić G. 2010. Role of ovarian hormones in age-associated thymic involution revisited. Immunobiology 215: 275–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


