Abstract
Background:
Medical and surgical treatment for musculoskeletal sarcoma (MSS) place survivors at risk for impairments in muscle properties including muscle strength, muscle size, and neuromuscular activation. The purpose of this study was to explore muscle properties, gross motor performance, and quality of life (QoL) and the changes in response to a 6-week functional strengthening intervention (PT-STRONG) in MSS survivors of childhood cancer (CCS).
Methods:
Eight lower extremity MSS CCS (13–23 years old) performed baseline testing and three completed PT-STRONG. Participants completed measurements of knee extension strength using handheld dynamometry, vastus lateralis (VL) and rectus femoris (RF) muscle thickness using ultrasonography at rest, and neuromuscular activation using electromyography during strength testing and a step-up task. Participants also completed gross motor and QoL assessments.
Results:
Compared with the non-surgical limb, MSS CCS had lower surgical limb knee extension strength, VL muscle thickness, and RF step-up muscle rate of activation (RoA). Compared with normative values, MSS CCS had decreased bilateral knee extension strength, gross motor performance, and physical QoL. Positive correlations among muscle strength, muscle thickness, and gross motor performance were identified. After PT-STRONG, MSS CCSS had improvements in VL muscle thickness, VL and RF step-up RoA, gross motor performance, and physical QoL.
Conclusions:
Positive association between larger muscle thickness with greater knee extension strength, and higher knee extension strength with better gross motor performance indicate that comprehensive physical therapy assessment and interventions that identify and target impairments in muscle properties to guide clinical decision making should be considered for MSS CCS into survivorship.
Keywords: Pediatrics, Oncology, Rehabilitation science, Muscle architecture, Biomechanics, Physiology
1. INTRODUCTION
Musculoskeletal sarcomas (MSS) of the bone, such as osteosarcoma and Ewing sarcoma, generally arise from transformed mesenchymal connective tissue cells. MSS most commonly occur by 25 years of age in the long bones of the lower and upper extremities (i.e., femur, tibia, and humerus) or the pelvis [1]. Although MSS account for a small percentage (3.4%) of childhood cancer and affect approximately 750 children and adolescents per year in the USA, these MSS survivors of childhood cancer (CCS) have a high likelihood of recovery and survival [2,3]. Improvements in chemotherapeutic, radiation, and surgical management in MSS CCS have increased long-term survivorship, with a 5-year overall survival rate reaching 70% [2,3]. Due to the complex medical and surgical management, MSS CCS are at risk for impaired muscle properties (muscle strength, muscle size, and neuromuscular activation) of the surgical and non-surgical limbs [4–8], limited gross motor performance [4,5,7–18], and lower health-related quality of life (QoL) [13,16,18–22].
The evolving medical and surgical treatments for MSS have the goal of not only improving survival but also preserving the individual’s long-term physical function and participation in school, work, and recreation. Medical treatments typically consist of six or more months of chemotherapy combined with surgical tumor resection. The chemotherapeutic agents used for MSS affect both cancerous and normal cells and cause short- and long-term side effects in multiple body systems [23–25]. In addition to chemotherapy, MSS CCS undergo a wide surgical resection of the tumor, which may consist of limb amputation, rotationplasty, or limb-sparing surgery (LSS). LSS is the most common surgical procedure for local control for 90% of patients with MSS [26,27]. LSS includes oncologic resection of the bone and/or soft tissue tumor, reconstruction of resected bone segments, and muscular or tendon re-routing [26]. To ensure the entire tumor is removed, the resection of both cancerous tissues and healthy tissues is required [26]. During an LSS surgery, the resected bone segments are reconstructed using an endoprosthesis, hardware, autograft, and/or allograft, and tendons and muscle are re-routed to optimize the functional potential of the limb [26]. For example, the common LSS procedure for a proximal tibial MSS involves resection of the tumor, bone, and surrounding soft tissue, endoprosthetic replacement of the resected tibia and knee joint, reconstruction of the patellar tendon attachment to the endoprosthesis, and a medial gastrocnemius muscle flap [26].
Due to the multimodal medical and surgical treatment, MSS CCS are at risk for impairments in quadriceps muscle strength due to multiple underlying mechanisms beyond surgical loss of muscle such as impairments in muscle size and neuromuscular activation. Specific chemotherapy agents (i.e., doxorubicin), cancer cachexia, pain, immobilization, and disuse have been associated with changes in skeletal muscle properties such as muscle atrophy, weakness, and impaired neuromuscular activation [8,28–31]. MSS CCS are also at risk for alterations in neuromuscular activation due to changes in the knee joint kinematic patterns (i.e. stiff knee gait) that may affect quadriceps rate of muscle activation (RoA) and knee co-contraction index (CCI) [32]. Given that MSS of the lower extremities are most commonly located in the distal femur or proximal tibia, the function of the quadriceps muscles is at high risk for impairment. The vastus lateralis (VL) and rectus femoris (RF) muscles are typically conserved after LSS and are critical to gross motor tasks such as walking, running, and jumping. The VL is the primary extender of the knee, and the biarticular RF performs hip flexion and knee extension, the movements required to kick a ball or climb stairs [33,34]. Therefore, VL and RF muscle properties including muscle strength as measured by hand-held dynamometry (HHD) [35,36], muscle thickness measured by ultrasonography (US) [35,37,38], and neuromuscular activation measured by electromyography (EMG) may relate to gross motor performance in MSS CCS and have yet to be comprehensively explored. In other complex childhood health conditions such as cerebral palsy and acute lymphoblastic leukemia, relationships have been reported among quadriceps muscle thickness, gross motor performance, and QoL [37,39,40].
Previous MSS CCS exercise intervention studies have focused on pre-surgical rehabilitation and increasing physical activity levels during medical treatment [6,41]. Despite the benefits of pre-surgical interventions, significant impairments in muscle strength and gross motor performance persist well beyond medical and surgical treatment [4–6,8–10,12,14–16,39]. Therefore, current efforts are focused on developing exercise interventions to effectively mitigate these declines into survivorship. Functional strength training targets lower extremity muscles that are required to produce functional movements such as walking, rising from a chair, and climbing stairs. Previous studies in healthy children and acute lymphoblastic leukemia CCS using functional strengthening interventions have demonstrated a two times per week 6-week program effectively increased strength and improve gross motor performance [31,42]. Building on this evidence, we sought to determine whether a functional strengthening intervention would be effective in MSS CCS.
Therefore, this study aimed to explore the muscle properties (muscle strength, muscle size, and neuromuscular activation) of the lower extremity that underwent surgery (surgical limb) and the contralateral lower extremity (non-surgical limb) and the relationships among muscle properties, gross motor performance, and QoL in adolescent and young adult MSS CCS. We hypothesized that MSS CCS would present with decreased knee extension strength, smaller muscle thickness, lower VL and RF muscle RoA, and increased knee CCI in the surgical limb compared with the non-surgical limb, and poorer gross motor performance and QoL compared with a normative population. We also hypothesized that muscle size and neuromuscular activation would be correlated with muscle strength and gross motor performance and that a 6-week functional strengthening intervention would increase knee extension strength, increase VL and RF muscle thickness and RoA, decrease knee CCI, and improve gross motor performance.
2. METHODS
2.1. Study Participants
Eight adolescent and young adult MSS CCS participants were recruited from Pediatric Hematology/Oncology Clinics at the University of Maryland Medical Center, Children’s National Hospital, and Johns Hopkins Hospital between July 2018 and June 2022 for participation in this study at the University of Maryland, School of Medicine, Department of Physical Therapy & Rehabilitation Science. Participants were eligible if they were adolescents or young adults between 13 and 26 years old, had a previous diagnosis of osteosarcoma or Ewing sarcoma of the femur or tibia, underwent lower extremity surgery more than 1 year before enrolling in this study, and completed chemotherapy prior to enrollment in this study. Participant exclusion criteria included a diagnosis of a neurological or muscle disorder not related to a diagnosis of MSS, a history of a serious lower-extremity injury or surgery 6 months before the enrollment in the study, or currently pregnant based on participant report. This study was approved by the University of Maryland, Baltimore institutional review board (HP-00078661), and was performed in line with the principles of the Declaration of Helsinki. All participants <18 years of age provided informed assent and caregivers provided informed consent. All participants ≥18 years of age provided informed consent.
2.2. Study Procedures
The initial baseline assessment, performed in a single session, included measurements of knee extension strength, quadriceps muscle thickness, gross motor performance, and QoL. Participants were given the option to participate in the intervention component of this study. The intervention group participants (n = 3) were asked to attend treatment sessions two times per week for 6 weeks (12 sessions in total). The non-intervention group participants were instructed to continue with their usual activity. Post-intervention assessments (post-test) consisted of the identical measures performed at baseline. To maintain consistency and reduce bias in data collection, the same assessor performed baseline and post-test outcome measures for all participants and a different study team member performed all of the intervention sessions.
2.3. Outcome Measures
2.3.1. Muscle Properties
2.3.1.1. Knee extension strength
Knee extension joint force at the distal tibia was measured using an HHD (Lafayette Manual Muscle Testing System model 01165, Lafayette Instrument, Lafayette, IN, USA) in the seated position with both knees flexed to 90° (seated 90°) and in the supine position with both knees supported at 35° of flexion (supine 35°) as measured by a standard goniometer [30,43,44]. The HHD was placed on the anterior tibia 2 cm proximal to the intermalleolar line. The participants were instructed to take a normal inspiration and slow expiration during maximum voluntary isometric contractions (MVIC) with the instructions, “hold the position you are placed in and kick as fast and as hard as you can into the testing device.” Participants performed two familiarization trials of sub-maximal effort followed by three MVIC trials of 5 s each with at least a 30-s but no more than a 2-min rest break between trials. Knee extension force was recorded as the average of the highest two force values in kilograms (kg) [35]. To account for the difference in lever arms for the participant with a transtibial amputation, the surgical limb force was normalized by multiplying the ratio of the length of the non-surgical lower leg divided by the length of the surgical lower leg. Muscle strength measurement of knee extension using HHD has excellent intra-rater and inter-rater reliability [35,45].
2.3.1.2. Muscle Size
Resting quadriceps muscle thickness was measured by two-dimensional B-mode US (GE Logiq v9, GE Healthcare, Milwaukee, WI, USA; Whale Sigma P5, Whale Imaging Inc., Waltham, MA, USA) of the VL at 50% of the distance from the inferior margin of the greater trochanter to the tibial tuberosity and the RF at 40% of the distance from the anterior superior iliac spine to the tibial tuberosity [35]. Images were acquired using a 5- to 12-MHz frequency, 38-mm linear array probe placed parallel to the long axis of the muscle and perpendicular to the skin surface. Three images of each muscle were collected with the participant positioned supine with the hips and knees in neutral positions. The muscles were verified to be at rest using real-time surface EMG (iWorx, Dover, NH, USA). Off-line analysis of muscle thickness was performed using a custom MATLAB code and ImageJ v1.52s (National Institutes of Health, Bethesda, MD, USA) [35]. The muscle thickness was measured using the average of four measurements of the distance between the deep and superficial aponeuroses taken at the left (distal) and the right (proximal) borders of the US images [35]. These methods for VL and RF muscle thickness using US have excellent intra-rater and inter-rater reliability [35].
2.3.1.3. Neuromuscular Activation
Muscle activity was measured during knee extension in supine 35° MVIC and during a step-up task using the iWorx IX-BIO4 EMG system (iWorx, Dover, NH, USA). This position of knee flexion for MVIC testing was selected based on previous evidence for VL and RF EMG activity to be at their greatest [46]. The participants performed the step-up task by standing in front of a standard stair and stepping onto the stair with one limb followed by the other limb. Surface electrodes were placed on the VL, RF, biceps femoris (BF), and semitendinosus (ST) muscles of the limb that was performing the MVIC or step-up task. The electrodes were bipolar, disposable, pre-gelled, 1-cm-diameter, Ag/AgCl self-adhesive, circular snap electrodes with 20 mm interelectrode spacing (Noraxon, Scottsdale, AZ, USA). Before electrode placement, the skin was cleaned with alcohol. For VL, the electrodes were placed at 60% of the distance between ASIS and tibial tuberosity. The electrodes were placed on the muscle belly of RF at 50% of the distance between the anterior superior iliac spine and tibial tuberosity. The electrodes were placed for the BF and ST at 50% of the distance between the ischial tuberosity and the lateral and medial epicondyle, respectively. A reference electrode was positioned on the anterior tibial bone of the ipsilateral limb.
2.3.1.3.1. Muscle RoA
EMG data were analyzed using Visual 3D (C-Motion Research Biomechanics, Germantown, MD, USA) and band-pass filtered 10–500 Hz. EMG data from the two trials corresponding with the two highest torque values for the MVIC and the two trials of the step-up task were averaged and used for analysis. The EMG amplitude was calculated as the root mean square of the EMG signal. The baseline EMG means and standard deviations (SD) were calculated over 500 ms before EMG activity for each contraction [47]. The EMG onset was identified as the time at which the signal increased by at least 2 SD over the baseline mean and verified visually. The RoA was calculated across epochs of 0–50 ms (RoA50), 0–100 ms (RoA100), 0–150 ms (RoA150), and 0–200 ms (RoA200) [47]. The data were normalized to 150 ms around the maximal EMG amplitude, the highest value within the active signal.
2.3.1.3.2. Knee CCI
Knee CCI was calculated for both the MVIC supine 35° and the step-up tasks. A 500-ms window around the maximal EMG amplitude was used to calculate the CCI and normalized to the peak amplitude during this MVIC knee extension task for VL and RF, and during an MVIC knee flexion task in sitting with the knee flexed to 90° for BF and ST [48]. These testing positions selected for normalization were based on knee flexion angles associated with the highest VL, RF, BF, and ST EMG activity [46,49]. CCI between agonist and antagonist muscles was calculated by applying the CCI equation [50]: . In this equation, Iant is the sum of the EMG activity from antagonist muscles and Itot is the sum of the EMG activity from the agonist and antagonist muscles. A CCI value of 0% represents pure agonist activation and 100% represents total co-contraction [50,51]
2.3.2. Gross Motor Performance
Gross motor performance was assessed using the functional mobility assessment (FMA), which includes the Timed Up and Down Stairs (TUDS), the Timed Up and Go (TUG), and the 9-min Run–Walk (9RW) tests. The FMA is valid and has excellent intra-rater and inter-rater reliability in MSS CCS [52]. Participants performed the TUDS by walking up and down a flight of stairs [53]. Participants performed the TUG by rising from a chair, walking three meters, turning around, returning to the chair, and sitting down [53,54]. A stopwatch was used to record the time for the TUDS and TUG in seconds [53,54]. The 9RW test was performed using standard procedures over a 65-feet walkway and the distance (m) the participant completed in 9 min was recorded [52,55]. These timed tests have established excellent intra-rater, inter-rater, and test–rest reliability [56,57]. At rest and after each timed test, heart rate (HR) was measured using a finger pulse oximeter (Zacurate, Stafford, TX, USA) and rate of perceived exertion was reported using the Borg Scale (6–20) in which 7 is labeled is ‘very, very light’ and 19 as ‘very, very hard’ [58]. The physiological cost index (PCI) was calculated using the data from the 9RW test and the formula: . A total FMA score was calculated using the standard procedure [53].
2.3.3. QoL Questionnaire
The Short Form-36, version 2 (SF-36v2) is an established, reliable, and validated measure of health-related QoL [59]. The questionnaire consists of 36 items with eight subscales (physical functioning, role limitations due to physical problems, general health perceptions, vitality, social functioning, role limitations due to emotional problems, general mental health, health transitions) and two composite domains (physical health (PCS) and mental health (MCS)). A higher score on these scales indicates a higher perceived QoL. A score of 50 on the PCS and MCS is representative of the USA general population norm [59].
2.4. PT-STRONG Physical Therapy Intervention
Adolescent and young adult MSS CCS participants assigned to the intervention group were asked to perform in-person exercise sessions with a physical therapist two times per week for six weeks. Each therapy session included: (1) Discussion: session goals of repetitions and sets; (2) Warm-up: the participant walked overground for 5 min (at least 11 of 20 ‘light’ on the Borg scale); (3) Stretching: the participant performed gentle trunk (flexors/extensors, lateral flexors), hip (flexors/extensors, internal/external rotators), knee (flexors/extensors), and ankle (dorsiflexors/plantarflexors) stretching exercises for three repetitions of 10 s each; (4) Strengthening exercises: the participant performed rapid functional strengthening exercises, which included body squats, step-ups, and step-downs. During these exercises, the physical therapist ensured the participant used proper body mechanics. Exercises were progressed by the number of repetitions (six to 15), followed by the number of sets (one to three), and then by the range (squat depth and step height); (5) Cool-down: the participant walked overground for 5 min (11 on the Borg scale). During exercises, the physical therapist provided verbal cues and feedback for proper body position, but only provided hands-on contact to maintain safety, not to physically assist with performing the movement. In each session, the physical therapist advanced the program based on the participant’s progress with a goal of three sets of 15 repetitions for squatting and stepping. The physical therapist kept a weekly log to document the number of sets and repetitions of each exercise performed.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS (IBM Inc., Chicago, IL, USA). Descriptive data are presented as mean ± SD. The normality of the data was assessed using the Shapiro–Wilk test. Differences between limbs at baseline in muscle strength, size, and neuromuscular activation during MVIC and the step-up task were assessed using paired t-tests. Differences between established normative values for knee extension force in seated 90°, the 9RW distance, TUG time, TUDS time, total FMA score, and SF-36v2 PCS and MCS were assessed using a one-sample t-test compared with a standardized population [53,59,60]. Relationships between baseline data were assessed using the Spearman rank correlation coefficient (rs). The level of significance was set at P≤0.05. The sample size was determined by a priori power analysis and an interim post hoc power analysis that assumed that detecting a sample of n = 5 participants would have sufficient power to detect a moderate-to-large difference between groups for knee extension strength (G*Power v.3.1.9.4, Germany). Differences between baseline and post-test surgical limb outcomes for the intervention group are reported as a percentage change. The minimal detectable change (MDC95) was calculated using the baseline data for surgical limb knee extension force, VL and RF muscle thickness, RoA, knee CCI, TUDS, TUG, and 9RW tests using the formula , in which SEM is the standard error of the mean [61].
3. RESULTS
3.1. Participants
Eight MSS CCS participants with a mean age of 17.34 ± 3.33 years (five males and three females) were recruited, enrolled, and completed baseline assessments. All participants were survivors of a primary bone sarcoma of the femur (n = 4) or tibia (n = 4), with six having a diagnosis of conventional high-grade osteosarcoma and two participants (participants 4 and 8) having a positive BCOR gene rearrangement, a small round cell sarcoma with similarities to Ewing sarcoma [62]. For medical management, all the participants received a combination of chemotherapy including doxorubicin plus methotrexate, cisplatin, vincristine, and/or Cytoxan. At the time of enrollment in our study, the average time since completion of chemotherapy was 3.55 ± 2.30 years, and since surgery was 4.09 ± 2.00 years. For local control, seven of the eight (87.5%) participants underwent an LSS, and one participant underwent a transtibial amputation. Table 1 summarizes the medical and surgical history of the MSS CCS participants.
Table 1.
Medical and surgical history of study participants.
| Tumor location | Surgery | Time since surgery | Time since completion of chemotherapy | Known complications | PT-STRONG intervention | |
|---|---|---|---|---|---|---|
| 1 | Left proximal femur | Limb salvage fibular allograft | Over 6 years | 6.09 years | None | No |
| 2 | Right distal femur | Limb salvage expandable endoprosthesis | 5.78 years | 5.44 years | Significantly limited knee flexion range of motion | Yes |
| 3 | Right proximal tibia | Limb salvage endoprosthesis with medial gastrocnemius muscle flap | 1.66 years | 1.10 years | Right toes amputation due to necrosis | Yes |
| 4 | Left proximal tibial | Limb salvage endoprosthesis with medial gastrocnemius muscle flap | 1.74 years | 0.37 years | None | No |
| 5 | Right distal femur | Limb salvage endoprosthesis | 5.94 years | 5.51 years | None | No |
| 6 | Left distal femur | Limb salvage endoprosthesis | 1.98 years | 1.48 years | Poor wound healing requiring rotational gastrocnemius muscle flap and skin graft | Yes |
| 7 | Right distal femur | Limb salvage endoprosthesis | 5.53 years | 5.16 years | Post-surgical foot drop; uses an ankle-foot orthosis | No |
| 8 | Right distal tibia | Transtibial amputation | 4.04 years | 3.26 years | Wound debridement; uses a prosthetic limb | No |
All MSS CCS participants completed the baseline data testing for knee extension strength in seated 90°, VL muscle thickness, gross motor performance, and QoL measures. However, only four of the participants performed strength testing in supine 35°, RF muscle thickness, quadriceps neuromuscular activation measurements as these exploratory outcomes were added to the original study design after further examination of previous findings. The participant with a transtibial amputation did not complete neuromuscular activation measures during the step-up task due to restrictions from the prosthetic sock and socket fitting comfortably over the electrodes. Four of the participants were assigned to the intervention group. Three of the four participants completed >80% of the intervention sessions and these data were analyzed for baseline to post-test changes.
3.2. Muscle Properties
Compared with the non-surgical limb, knee extension force (seated 90°; P<0.001), muscle thickness (vastus lateralis; P<0.001), and rectus femoris RoA150–200 during the step-up task (P=0.016–0.038) were significantly lower in the surgical limb (Table 2). Compared with normative values [60], MSS CCS demonstrated decreased knee extension force for both the surgical and non-surgical limbs (seated 90°; P<0.001).
Table 2.
Measured muscle properties.
| Surgical limb | Non-surgical limb | n | P | |
|---|---|---|---|---|
| Knee extension force | ||||
| Seated 90°, kg | 10.70 (5.59) | 21.34 (6.02) | 8 | <0.001** |
| Supine 35°, kg | 10.39 (5.36) | 14.05 (5.64) | 4 | 0.076 |
| Muscle thickness | ||||
| Vastus lateralis, cm | 1.52 (0.45) | 2.19 (0.54) | 8 | <0.001** |
| Rectus femoris, cm | 1.70 (0.67) | 2.56 (0.48) | 4 | 0.126 |
| Neuromuscular activation | ||||
| MVIC - vastus lateralis | ||||
| RoA50, s−1 | 1.00 (0.84) | 1.41 (0.90) | 4 | 0.630 |
| RoA100, s−1 | 0.89 (0.84) | 1.18 (0.76) | 4 | 0.716 |
| RoA150, s−1 | 0.91 (0.78) | 1.11 (0.69) | 4 | 0.779 |
| RoA200, s−1 | 0.79 (0.71) | 0.99 (0.51) | 4 | 0.709 |
| MVIC - rectus femoris | ||||
| RoA50, s−1 | 0.93 (0.72) | 0.93 (0.49) | 4 | 0.998 |
| RoA100, s−1 | 0.95 (0.69) | 0.94 (0.48) | 4 | 0.994 |
| RoA150, s−1 | 0.98 (0.69) | 0.96 (0.49) | 4 | 0.963 |
| RoA200, s−1 | 0.99 (0.60) | 0.94 (0.58) | 4 | 0.925 |
| MVIC - Knee CCI | 0.51 (0.28) | 0.61 (0.09) | 4 | 0.391 |
| Step-Up - vastus lateralis | ||||
| RoA50, s−1 | 5.44 (5.09) | 9.35 (2.71) | 3 | 0.312 |
| RoA100, s−1 | 3.68 (2.34) | 6.81 (1.23) | 3 | 0.187 |
| RoA150, s−1 | 3.25 (1.07) | 5.29 (0.96) | 3 | 0.192 |
| RoA200, s−1 | 2.85 (0.36) | 4.13 (0.75) | 3 | 0.159 |
| Step-Up - rectus femoris | ||||
| RoA50, s−1 | 8.85 (6.66) | 14.41 (1.75) | 3 | 0.276 |
| RoA100, s−1 | 5.25 (1.90) | 8.19 (1.08) | 3 | 0.152 |
| RoA150, s−1 | 3.52 (0.42) | 5.60 (0.51) | 3 | 0.038* |
| RoA200, s−1 | 2.64 (0.10) | 4.16 (0.25) | 3 | 0.016* |
| Step-up - Knee CCI | 0.79 (0.46) | 0.41 (0.14) | 3 | 0.369 |
Mean (standard deviation (SD)). CCI, co-contraction index; MVIC, maximal voluntary isometric contraction measured in supine with knees flexed to 35°; RoA, rate of muscle activation.
3.3. Gross Motor Performance
Compared with normative values [53], MSS CCS presented with the TUDS (MSS CCS: 17.31 ± 11.41s vs. normative: 6.18 ± 0.8 s; P=0.030), TUG (MSS CCS: 7.63 ± 2.45 s vs. normative: 3.78 ± 0.6 s; P=0.003), 9RW (MSS CCS: 706.53 ± 215.30 m vs. normative: 1268.28 ± 272.19 m; P<0.001), and FMA total scores (MSS CCS: 49.75 ± 2.43 vs. normative: 59.00 ± 3.00; P<0.001).
3.4. QoL
On average, MSS CCS reported scores of 44.52 (4.06) and 57.87 (16.58) on the SF36v2 PCS and MCS scores, respectively. These reported scores are lower than normative values for PCS (P=0.007) and similar to normative values for MCS [59].
3.5. Relationships
Significant relationships were identified between knee extension strength and muscle thickness (rs = 0.76–0.95; P<0.001–0.03; Figure 1(a)–(d)), surgical limb knee extension strength and the TUDS (rs = −0.71; P=0.05; Figure 2), and non-surgical limb knee extension strength and the TUG (rs = −0.71; P=0.05) and 9RW (rs = 0.76; P=0.03) tests (Figure 3). No significant relationships between gross motor performance and QoL were detected.
Figure 1.

Relationships between knee extension strength and muscle thickness.
Figure 2.

Relationships between surgical limb knee extension strength and the Timed Up and Down Stairs test.
Figure 3.
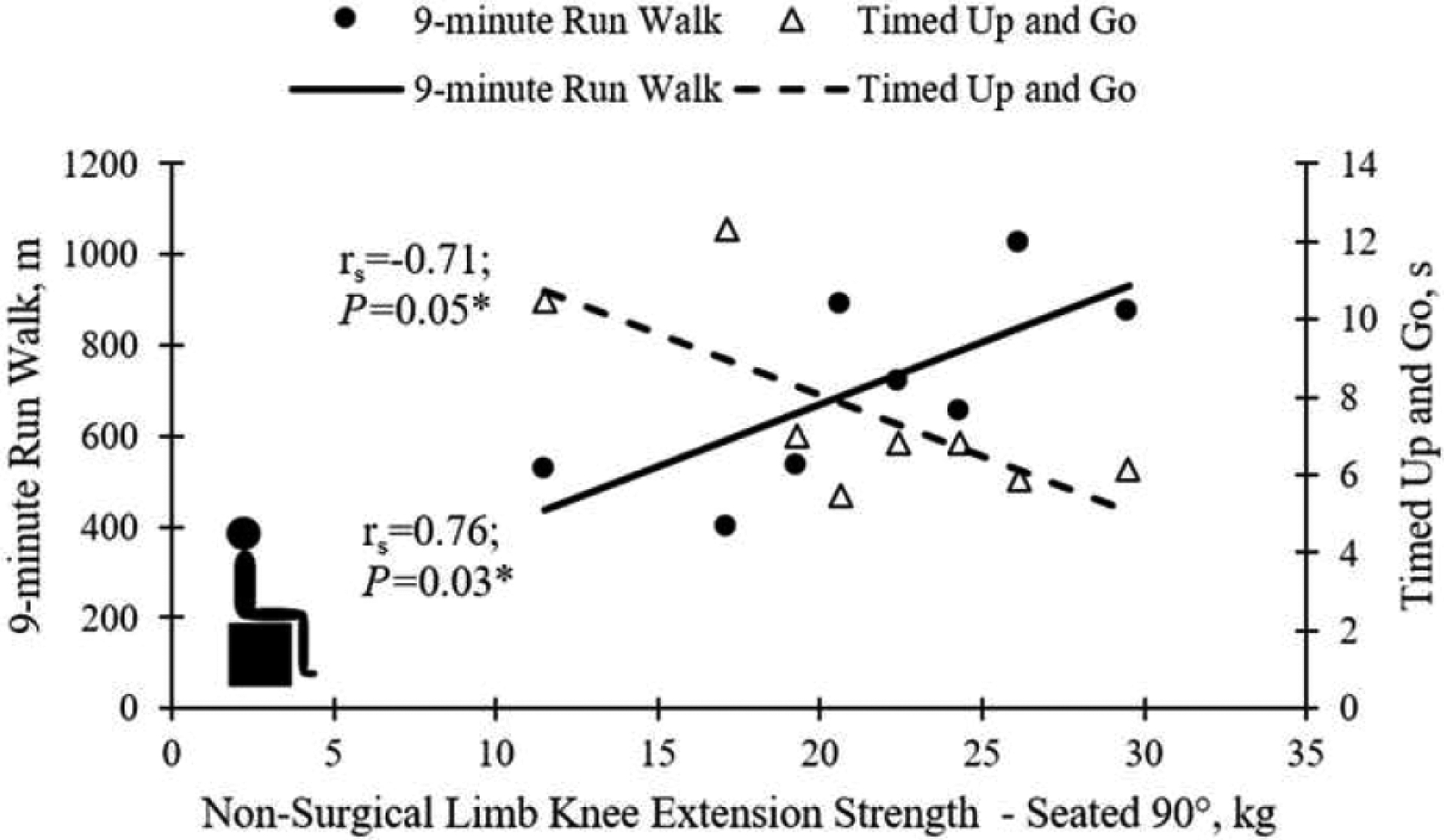
Relationships between non-surgical limb knee extension strength and the Timed up and Go (open triangles; dotted line) and 9-min Run–Walk (filled circles; solid line) tests.
3.6. PT-STRONG Intervention
Three MSS CCS participants completed 100% of the 12 PT-STRONG intervention sessions. Two of the three participants demonstrated improvements between baseline and post-test in surgical limb knee extension strength at seated 90° and VL muscle thickness (Table 3). The VL muscle thickness increase for Participant no. 6 exceeded the MDC95. On average, the participants that completed PT-STRONG improved in gross motor performance measures by decreasing the time to complete the TUDS by 1.80 s and TUG by 1.86 s and increasing the distance walked on the 9RW by 41.86 m (Table 3). The improved performance on the TUG for Participants no. 3 and no. 6 exceeded the MDC95 (Table 3). On average, the participants that completed PT-STRONG improved SF-36v2 PCS scored by 10.74 points and decreased MCS scores by 14.67 points.
Table 3.
PT-STRONG intervention outcome measures.
| Participant no. 2 | Participant no. 3 | Participant no. 6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | MD C95 | Baseline | Post-test | Difference | Baseline | Post-test | Difference | Baseline | Post-test | Difference |
| Knee extension force | ||||||||||
| Seated 90°, kg | 1.98 | 13.02a | 10.75a | −17.42% | 2.68 | 2.72 | +1.69% | 6.26 | 6.80 | +8.67% |
| Supine 35°, kg | 7.39 | – | – | – | – | – | – | 5.75 | 6.88 | +19.79% |
| Muscle thickness | ||||||||||
| Vastus lateralis, cm | 0.16 | 1.34 | 1.16 | −12.90% | 1.04 | 1.09 | +4.80% | 0.75 | 0.92 | +22.38% * |
| MVIC - vastus lateralis | ||||||||||
| RoA50, s−1 | 1.16 | – | – | – | – | – | – | 0.71 | 0.91 | +26.85% |
| RoA100, s−1 | 1.16 | – | – | – | – | – | – | 0.46 | 0.63 | +37.46% |
| RoA150, s−1 | 1.08 | – | – | – | – | – | – | 0.33 | 0.82 | +146.50% |
| RoA200, s−1 | 0.98 | – | – | – | – | – | – | 0.25 | 0.85 | +240.04% |
| MVIC - rectus femoris | ||||||||||
| RoA50, s−1 | 0.99 | – | – | – | – | – | – | 0.44 | 0.73 | +63.05% |
| RoA100, s−1 | 0.95 | – | – | – | – | – | – | 0.38 | 0.71 | +85.61% |
| RoA150, s−1 | 0.95 | – | – | – | – | – | – | 0.49 | 0.63 | +28.80% |
| RoA200, s−1 | 0.83 | – | – | – | – | – | – | 0.51 | 0.57 | +12.52% |
| MVIC - knee CCI | 0.39 | – | – | – | – | – | – | 0.42 | 0.19 | −55.14% |
| Step-Up - vastus lateralis | ||||||||||
| RoA50, s−1 | 8.10 | – | – | – | – | – | – | 6.40 | 15.74 | +145.99% |
| RoA100, s−1 | 3.73 | – | – | – | – | – | – | 3.82 | 9.35 | +145.06% * |
| RoA150, s−1 | 1.70 | – | – | – | – | – | – | 2.89 | 6.48 | +124.43% * |
| RoA200, s−1 | 0.57 | – | – | – | – | – | – | 2.63 | 4.68 | +77.97% * |
| Step-Up - rectus femoris | ||||||||||
| RoA50, s−1 | 10.60 | – | – | – | – | – | – | 9.74 | 16.24 | +66.71% |
| RoA100, s−1 | 3.03 | – | – | – | – | – | – | 5.40 | 10.18 | +88.48% * |
| RoA150, s−1 | 0.67 | – | – | – | – | – | – | 3.51 | 6.62 | +88.82% * |
| RoA200, s−1 | 0.16 | – | – | – | – | – | – | 2.90 | 4.30 | +48.47% * |
| Step-up - knee CCI | 0.73 | – | – | – | – | – | – | 0.66 | 0.52 | −20.91% |
| Timed Up and Down Stairs, s | 4.03 | 15.43 | 12.38 | −19.77% | 41.92 | 41.94 | +0.05% | 15.90 | 13.55 | −14.78% |
| Timed Up and Go, s | 0.87 | 6.82 | 6.00 | −12.02% | 10.52 | 9.15 | −13.02%* | 12.33 | 8.93 | −27.58%* |
| 9-min Run-Walk, m | 76.13 | 722.99 | 789.74 | +9.23% | 531.57 | 534.92 | +0.63% | 401.12 | 456.59 | +13.83% |
Bold text indicates improvement in outcome measure. CCI, co-contraction index; MVIC, maximum voluntary isometric contractions; RoA, rate of muscle activation.
Participant no. 2 had limited knee flexion range of motion; therefore, the strength testing was performed at end-range of knee flexion (baseline: 58°; post-test: 60°).
The difference between baseline and post-test exceeded the minimally detectable change (MDC95).
Of the three participants that completed the intervention, only Participant no. 6 completed knee extension strength at supine 35° and neuromuscular activation testing. Compared with baseline, this participant demonstrated increased surgical limb knee extension strength in supine 35°, decrease in RF muscle thickness, and increased VL and RF RoA, and decreased knee CCI during MVIC and step-up tasks (Table 3). The increases in VL RoA50–200 and RF RoA100–200 during the step-up task exceeded the MDC95 (Table 3).
4. DISCUSSION
This study quantified the muscle properties (muscle strength, muscle size, and neuromuscular activation) of the surgical and non-surgical limbs and assessed relationships among muscle properties, gross motor performance, and QoL in adolescent and young adult MSS CCS. Participants in this study presented with decreased muscle strength, muscle thickness, and RoA in the surgical limb compared with their non-surgical limb and poorer gross motor performance and physical QoL compared with a normative population. At baseline, a positive correlation between muscle strength, muscle size, and gross motor performance was identified. We showed that our 6-week PT-STRONG functional strengthening intervention produced individual improvements in MSS CCS for VL muscle thickness, VL and RF RoA during a step-up task, gross motor performance on the TUG, and physical QoL.
MSS CCS presented with decreased bilateral knee extension strength with significantly less strength in the surgical limb compared with the non-surgical limb, consistent with previously reported data [4–6]. This current study identified a 26.1–49.9% between-limb deficit in knee extension strength. Tsauo et al. [4] reported ratios of muscle strength between MSS CCS surgical and non-surgical limbs ranging from 37.4 to 47.5% for knee extension. Muscle strength deficits have not only been identified in the surgical limb but also have been identified in the non-surgical limb [5,6]. The results of our study identified a 72.7% decrease in surgical limb and a 45.6% decrease in non-surgical limb knee extension compared with a normative population. A case series by Beebe et al. [5] examined four children (aged 9–11 years) and reported a decrease in non-surgical knee force production by 63% compared with normative values. Corr et al. [6] also found knee extension strength deficits in the non-operative limb and these strength deficits declined from baseline to post-surgery and did not recover by 20–22 weeks post-surgery. These findings, along with the results of this study, support that MSS CCS have strength deficits that persist well beyond the initial post-operative period.
The relationships between the properties of skeletal muscle have not been previously defined in MSS CCS. The participants in this study had lower surgical limb VL and RF thickness compared with the non-surgical limb. Both the VL and RF muscle thickness measurements were found to correlate with knee extension strength. These findings are consistent with previous reports in children and adolescents with cerebral palsy and adults with osteoarthritis and after total knee arthroplasty, which are populations with known knee joint kinematic changes and decreased activity levels [37,63–65]. Compared with those without health conditions, children and adolescents with cerebral palsy have lower VL and RF muscle thickness, and positive relationships between muscle thickness and knee extension strength have been reported [37,63]. In adults after total knee arthroplasty RF muscle thickness is lower on the surgical limb compared with the non-surgical limb and control participants, and relationships between higher muscle thickness with greater knee extension muscle strength have been identified [64]. Quadriceps muscle thickness is negatively correlated with pain levels and positively related to patient-reported physical function and gross motor performance, including the TUG test, in adults with osteoarthritis and post-total knee arthroplasty [64,65]. Therefore, utilizing US muscle thickness measurements may be a beneficial alternative for MSS CCS, especially if pain or medical restrictions prevent formal muscle strength testing [38].
The MSS CCS in our study presented with limited gross motor performance and lower physical QoL compared with normative values on the FMA, TUDS, TUG, 9RW, and SF-36v2 PCS. These findings are consistent with previously reported performance on these measures in a larger cohort of MSS CCS [10,39,52]. Marchese et al. [39] identified relationships between hip and knee joint range of motion and performance on the TUDS, TUG, 9RW, and SF-36v2 PCS. Relationships between increased knee extension strength and better gross motor performance were present for the MSS CCS. Surgical limb knee extension strength was correlated with better performance on the TUDS. Non-surgical limb knee extension strength was related to a shorter time to complete the TUG and farther distance walked on the 9RW test. These findings are reflective of the lower extremity demands required for walking and climbing stairs. During walking, adaptive gait deviations (i.e., hip Trendelenburg, vaulting) can compensate for a lack of surgical limb function [5]. Okita et al. [66] reported that MSS CCS who underwent LSS increased bilateral hip, bilateral ankle, and non-surgical limb knee power to increase walking speed. However, the participants in this study performed stair climbing with a reciprocal pattern, which requires the isolated surgical limb to extend to ascend the stairs. Given that these participants also demonstrated decreased bilateral knee extension strength, performing a variety of functional tasks that require muscular challenges to both single and double limbs should be considered. The findings from these assessments could help support the development of physical therapy interventions and goals for MSS CCS.
In response to a 6-week PT-STRONG intervention, individual improvements in VL muscle thickness, VL and RF RoA during a step-up task, gross motor performance on the TUG, and physical QoL but not mental QoL were identified in MSS CCS. These findings add to the evidence that exercise can elicit improvements in these patients. The decreased time to perform the TUG for two of the participants dropped below 10 s, which is the cut-off score sensitive to detecting near-falling risk in older adults with hip osteoarthritis [67]. These participants also decreased their TUG time by greater than 0.87 s, which is the MDC95 calculated from the baseline data. Previous intervention studies in CCS demonstrated positive improvements in knee extension strength [68,69], lower extremity RoA [31], and gross motor performance in response to an exercise intervention [31,69]. In MSS CCS, Winter et al. [41] demonstrated that an individualized exercise intervention can improve post-surgical physical activity levels. Participant no. 2 demonstrated decreased knee extension strength and decreased VL muscle thickness post-intervention. Although the reason for the decline in this participant is unknown, it may be due to the participant’s baseline limitations in knee flexion range of motion, which improved from baseline to post-test. The decreased knee extension force and decreased VL muscle thickness may represent reduced muscle stiffness due to the static and dynamic stretching effects of the intervention, which were not specifically measured in this study [70]. Nonetheless, muscle property and gross motor performance deficits in MSS CCS may be amendable to physical therapy interventions. Specifically, our PT-STRONG intervention included body-weight resisted functional strengthening and rapid squat and step-up tasks that targeted muscle size through hypertrophy and neuromuscular activation through challenging RoA, respectively.
Despite the clinically relevant and sensitive outcome measures used in this study, the small sample size arising from the rarity of MSS diagnosis is a limiting factor in generalizing these findings to the broader population. To this end, while this study identifies that MSS CCS at risk for impairments in muscle properties and gross motor performance that may be responsive to exercise intervention, an expansion of this study is warranted before generalizing these results. The control condition for the exploratory outcomes in this study was the participant’s non-surgical limb, which also demonstrated deficits, therefore greater impairments may have been identified had these participants been compared with healthy controls. For the SF-36v2, the participants in this study were compared with the USA general population (>18 years old). In a study by Mones et al. [71], they reported a mean PCS score of 90.57 and MCS score of 89.33 in a cohort of healthy adolescent controls (n = 174; age = 12–18 years). Therefore, the normative sample used for comparison in this study may be conservative and underrepresent the degree of QoL differences among adolescent MSS CCS and their peers. Additional studies with larger samples of MSS CCS and healthy age- and sex-matched controls should be performed to further explore the dosage of training and the effects of an intervention on muscle properties and gross motor performance.
5. CONCLUSIONS
Adolescent and young adult MSS CCS present with decreased quadriceps muscle strength, muscle size, neuromuscular activation, gross motor performance, and physical QoL, which may be amendable to physical therapy interventions. Due to the interplay between skeletal muscle properties and gross motor performance, comprehensive physical therapy assessments that identify impairments in muscle properties, such as muscle thickness and neuromuscular activation, and interventions that target these impairments may enhance the rehabilitation of MSS CCS well into survivorship. The results of these assessments should be used to guide clinical decision-making to promote physical performance and participation in daily activities.
Acknowledgements
The authors would like to acknowledge the participants and their families for dedicating their time to our research.
Funding
The work related to this manuscript has been funded in part by the Snyder Research Grant from the Foundation for Physical Therapy Research to V.M. and a NIAMS-funded predoctoral fellowship to K.R. (T32AR007592).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant conflicts of interest to report.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the University of Maryland, Baltimore institutional review board (HP-00078661) on January 23, 2018.
Consent to Participate: All participants <18 years of age provided informed assent and caregivers provided informed consent. All participants ≥ 18 years of age provided informed consent.
References
- [1].Heare T, Hensley MA, Dell’Orfano S. Bone tumors: Osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr 2009;21:365–72. 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- [3].Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N. Children’s Oncology Group’s 2013 blueprint for research: Bone tumors. Pediatr Blood Cancer 2013;60:1009–15. 10.1002/pbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsauo JY, Li WC, Yang RS. Functional outcomes after endoprosthetic knee reconstruction following resection of osteosarcoma near the knee. Disabil Rehabil 2006;28:61–6. 10.1080/09638280500164008. [DOI] [PubMed] [Google Scholar]
- [5].Beebe K, Song KJ, Ross E, Tuy B, Patterson F, Benevenia J. Functional outcomes after limb-salvage surgery and endoprosthetic reconstruction with an expandable prosthesis: A report of 4 cases. Arch Phys Med Rehabil 2009;90:1039–47. 10.1016/j.apmr.2008.12.025. [DOI] [PubMed] [Google Scholar]
- [6].Corr AM, Liu W, Bishop M, Pappo A, Srivastava DK, Neel M, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabil Oncol 2017;35:38–45 10.1097/01.REO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fernandez-Pineda I, Hudson MM, Pappo AS, Bishop MW, Klosky JL, Brinkman TM, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. J Cancer Surviv 2017;11:1–12. 10.1007/s11764-016-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carty CP, Bennett MB, Dickinson IC, Steadman P. Electromyographic assessment of gait function following limb salvage procedures for bone sarcoma. J Electromyogr Kinesiol 2010;20:502–7. 10.1016/j.jelekin.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [9].Carty CP, Dickinson IC, Watts MC, Crawford RW, Steadman P. Impairment and disability following limb salvage procedures for bone sarcoma. Knee 2009;16:405–8. 10.1016/j.knee.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [10].Ginsberg JP, Rai SN, Carlson C, Meadows AT, Hinds PS, Spearing EM, et al. A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr Blood Cancer 2007;49:964–9. 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- [11].Bekkering WP, van Egmond-van Dam JC, Bramer JAM, Beishuizen A, Fiocco M, Dijkstra PDS. Quality of life after bone sarcoma surgery around the knee: A long-term follow-up study. Eur J Cancer Care (Engl) 2017;26:1–9. 10.1111/ecc.12603. [DOI] [PubMed] [Google Scholar]
- [12].Carty CP, Bennett MB, Dickinson IC, Steadman P. Assessment of kinematic and kinetic patterns following limb salvage procedures for bone sarcoma. Gait Posture 2009;30:547–51. 10.1016/j.gaitpost.2009.08.234. [DOI] [PubMed] [Google Scholar]
- [13].Marchese VG, Spearing E, Callaway L, Rai SN, Zhang L, Hinds PS, et al. Relationships among range of motion, functional mobility, and quality of life in children and adolescents after limb-sparing surgery for lower-extremity sarcoma. Pediatr Phys Ther 2006;18:238–44. 10.1097/01.pep.0000232620.42407.9f. [DOI] [PubMed] [Google Scholar]
- [14].Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, Mertens A, et al. Twenty years of follow-up of survivors of childhood osteosarcoma. Cancer 2011;117:625–34. 10.1002/cncr.25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bekkering WP, Vliet Vieland TP, Kooperman HM, Schaap GR, Schreuder HWB, Beishuizen A,et al. Functional ability and physical activity in children and young adults after limb-salvage or ablative surgery for lower extremity bone tumors. J Surg Oncol 2011;103:276–82. 10.1002/jso. [DOI] [PubMed] [Google Scholar]
- [16].Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med 2005;143:639–48. 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- [17].Bekkering WP, Vliet Vieland TP, Kooperman HM, Schapp GR, Beishuizen A, Anninga JK, et al. A prospective study on quality of life and functional outcome in children and adolescents after malignant bone tumor surgery. Pediatr Blood Cancer 2012;58:978–85. 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- [18].Bekkering WP, Vliet Vieland TP, Kooperman HM, Schaap GR, Schreuder HWB, Beishuizen A, et al. Quality of life in young patients after bone tumor surgery around the knee joint and comparison with healthy controls. Pediatr Blood Cancer 2010;54:738–45. 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- [19].Nagarajan R, Clohisy DR, Neglia JP, Yasui Y, Mitby PA, Sklar C, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: The Childhood Cancer Survivor Study. Br J Cancer 2004;91:1858–65. 10.1038/sj.bjc.6602220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Am Med Assoc 2003;290:1583–92. 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- [21].Marina N, Hudson MM, Jones KE, Mulrooney DA, Avedian R, Donaldson SS, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: A report from the childhood cancer survivor study. Arch Phys Med Rehabil 2013;94:1062–73. 10.1016/j.apmr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. New Engl J Med 2006;355:1572–82. 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- [23].Wilson C, Gawade P, Ness K. Impairments that influence physical function among survivors of childhood cancer. Children 2015;2:1–36. 10.3390/children2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gilchrist L, Tanner L. Gait patterns in children with cancer and vincristine neuropathy. Pediatr Phys Ther 2016;28:16–22. 10.1097/PEP.0000000000000208. [DOI] [PubMed] [Google Scholar]
- [25].Mizrahi D, Fardell JE, Cohn RJ, Partin RE, Howell CR, Hudson MM, et al. The 6-minute walk test is a good predictor of cardiorespiratory fitness in childhood cancer survivors when access to comprehensive testing is limited. Int J Cancer 2020;147:847–55. 10.1002/ijc.32819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Levin AS, Arkader A, Morris CD. Reconstruction following tumor resections in skeletally immature patients. J Am Acad Orthop Surg 2017;25:204–13. 10.5435/JAAOS-D-15-00619. [DOI] [PubMed] [Google Scholar]
- [27].Shehadeh A, Dahleh M el, Salem A, Sarhan Y, Sultan I, Henshaw RM, et al. Standardization of rehabilitation after limb salvage surgery for sarcomas improves patients’ outcome. Hematol Oncol Stem Cell Ther 2013;6:105–11. 10.1016/j.hemonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- [28].Gilliam LAA, St. Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxid Redox Signal 2011;15:2543–63. 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Van Norren K, Van Helvoort A, Argilés JM, Van Tuijl S, Arts K, Gorselink M, et al. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer 2009;100:311–4. 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marchese V, Rock K, York T, Creath R, Gray V. Neuromuscular mechanisms that contribute to gross motor performance in survivors of childhood acute lymphoblastic leukemia. J Pediatr Rehabil Med 2021;14:415–23. 10.3233/PRM-200784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marchese V, Rock K, York T, Ruble K, Gray VL. The efficacy of targeted exercise on gross motor and neuromuscular performance in survivors of childhood leukemia: A pilot study. Front Pediatr 2022;10:4–11. 10.3389/fped.2022.891650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davidson BS, Judd DL, Thomas AC, Mizner RL, Eckhoff DG, Stevens-Lapsley JE. Muscle activation and coactivation during five-time-sit-to-stand movement in patients undergoing total knee arthroplasty. J Electromyogr Kinesiol 2013;23:1485–93. 10.1016/j.jelekin.2013.06.008. [DOI] [PubMed] [Google Scholar]
- [33].Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res 1998;16:136–43. 10.1002/jor.1100160123. [DOI] [PubMed] [Google Scholar]
- [34].Bordoni B, Varacallo M. Anatomy, Bony Pelvis and Lower Limb, Thigh Quadriceps Muscle. 2022. [PubMed] [Google Scholar]
- [35].Rock K, Nelson C, Addison O, Marchese V. Assessing the reliability of handheld dynamometry and ultrasonography to measure quadriceps strength and muscle thickness in children, adolescents, and young adults. Phys Occup Ther Pediatr 2021;41:540–54. 10.1080/01942638.2021.1881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van den Beld WA, van der Sanden GAC, Sengers RCA, Verbeek ALM, Gabreëls FJM. Validity and reproducibility of hand-held dynamometry in children aged 4–11 years. J Rehabil Med 2006;38:57–64. 10.1080/16501970510044043. [DOI] [PubMed] [Google Scholar]
- [37].Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys Ther 2010;90:1619–30. 10.2522/ptj.20090377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nelson CM, Marchese V, Rock K, Henshaw RM, Addison O, Nelson CM. Alterations in muscle architecture: A review of the relevance to individuals after limb salvage surgery for bone sarcoma 2020;8:1–10. 10.3389/fped.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marchese VG, Spearing E, Callaway L, Rai SN, Zhang L, Hinds PS, et al. Relationships among range of motion, functional mobility, and quality of life in children and adolescents after limb-sparing surgery for lower-extremity sarcoma. Pediatr Phys Ther 2006;18:238–44. 10.1097/01.pep.0000232620.42407.9f. [DOI] [PubMed] [Google Scholar]
- [40].Shelly A, Davis E, Waters E, Mackinnon A, Reddihough D, Boyd R, et al. The relationship between quality of life and functioning for children with cerebral palsy. Dev Med Child Neurol 2008;50:199–203. 10.1111/j.1469-8749.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- [41].Winter CC, Müller C, Hardes J, Gosheger G, Boos J, Rosenbaum D. The effect of individualized exercise interventions during treatment in pediatric patients with a malignant bone tumor. Support Care Cancer 2013;21:1629–36. 10.1007/s00520-012-1707-1. [DOI] [PubMed] [Google Scholar]
- [42].Wyon MA, Smith A, Koutedakis Y. A comparison of strength and stretch interventions on active and passive ranges of movement in dancers: A randomized controlled trial. J Strength Cond Res 2013;27:3053–9. 10.1519/JSC.0b013e31828a4842. [DOI] [PubMed] [Google Scholar]
- [43].Katoh M Reliability of isometric knee extension muscle strength measurements made by a hand-held dynamometer and a belt: A comparison of two types of device. J Phys Ther Sci 2015;27:851–4. 10.1589/jpts.27.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Walsworth M, Schneider R, Schultz J, Dahl C, Allison S, Underwood F, et al. Prediction of 10 repetition maximum for short-arc quadriceps exercise from hand-held dynomometer and anthropometric measurements. J Orthop Sports Phys Ther 1998;28:97–104. [DOI] [PubMed] [Google Scholar]
- [45].Eek MN, Kroksmark AK, Beckung E. Isometric muscle torque in children 5 to 15 years of age: Normative data. Arch Phys Med Rehabil 2006;87:1091–9. 10.1016/j.apmr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [46].Babault N, Pousson M, Michaut A, van Hoecke J. Effect of quadriceps femoris muscle length on neural activation during isometric and concentric contractions. J Appl Physiol 2003;94:983–90. 10.1152/japplphysiol.00717.2002. [DOI] [PubMed] [Google Scholar]
- [47].Lanza MB, Rock K, Marchese V, Addison O, Gray VL. Hip abductor and adductor rate of torque development and muscle activation, but not muscle size, are associated with functional performance. Front Physiol 2021;12:744153. 10.3389/fphys.2021.744153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Katsavelis D, Threlkeld AJ. Quantifying thigh muscle co-activation during isometric knee extension contractions: Within- and between-session reliability. J Electromyogr Kinesiol 2014;24:502–7. 10.1016/j.jelekin.2014.04.004. Quantifying. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Onishi H, Yagi R, Oyama M, Akasaka K, Ihashi K, Handa Y. EMG-angle relationship of the hamstring muscles during maximum knee flexion. J Electromyogr Kinesiol 2002;12:399–406. 10.1016/S1050-6411(02)00033-0. [DOI] [PubMed] [Google Scholar]
- [50].Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol 1985;25:135–49. [PubMed] [Google Scholar]
- [51].Banks CL, Huang HJ, Little VL, Patten C. Electromyography exposes heterogeneity in muscle co-contraction following stroke. Front Neurol 2017;8:1–11. 10.3389/fneur.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marchese VG, Rai SN, Carlson CA, Hinds PS, Spearing EM, Zhang L, et al. Assessing functional mobility in survivors of lower-extremity sarcoma: Reliability and validity of a new assessment tool. Pediatr Blood Cancer 2007;49:183–9. 10.1002/pbc.20932. [DOI] [PubMed] [Google Scholar]
- [53].Marchese VG, Oriel KN, Fry JA, Kovacs JL, Weaver RL, Reilly MM, et al. Development of reference values for the functional mobility assessment. Pediatr Phys Ther 2012;24:224–30. 10.1097/PEP.0b013e31825c87e7. [DOI] [PubMed] [Google Scholar]
- [54].Nicolini-Panisson RD, Donadio MVF. Timed “Up & Go” test in children and adolescents. Rev Paul Pediatr 2013;31:377–83. 10.1590/s0103-05822013000300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [56].Zaino CA, Marchese VG, Westcott SL. Timed up and down stairs test: Preliminary reliability and validity of a new measure of functional mobility. Pediatr Phys Ther 2004;16:90–8. 10.1097/01.PEP.0000127564.08922.6A. [DOI] [PubMed] [Google Scholar]
- [57].Williams EN, Carroll SG, Reddihough DS, Phillips BA, Galea MP. Investigation of the timed ‘Up & Go’ test in children. Dev Med Child Neurol 2005;47:518–24. 10.1017/S0012162205001027. [DOI] [PubMed] [Google Scholar]
- [58].Borg G Borg’s perceived exertion and pain scales. Champaign, IL, US: Human Kinetics; 1998. [Google Scholar]
- [59].Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Lincoln: Quality Metric Inc; 2000. [Google Scholar]
- [60].Beenakker EAC, van der Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromusc Disord 2001. 11(5):441–6. 10.1016/S0960-8966(01)00193-6. [DOI] [PubMed] [Google Scholar]
- [61].Haley SM, Fragala-pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther 2006;86:735–43. [PubMed] [Google Scholar]
- [62].Kao Y-C, Owosho AA, Sung Y-S, Zhang L, Fujisawa Y, Lee J-C, et al. BCOR-CCNB3 Fusion positive sarcomas: A clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am J Surg Pathol 2018;42:604–15. 10.1097/PAS.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol 2009;51:800–6. 10.1111/j.1469-8749.2009.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kitsuda Y, Tanimura C, Inoue K, Park D, Osaki M, Hagino H. Effectiveness of ultrasonographic skeletal muscle assessment in patients after total knee arthroplasty. Osteoporos Sarcopenia 2019;5:94–101. 10.1016/j.afos.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gellhorn AC, Stumph JM, Zikry HE, Creelman CA, Welbel R. Ultrasound measures of muscle thickness may be superior to strength testing in adults with knee osteoarthritis: A cross-sectional study. BMC Musculoskelet Disord 2018;19:1–8. 10.1186/s12891-018-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Okita Y, Tatematsu N, Nagai K, Nakayama T, Nakamata T, Okamoto T, et al. The effect of walking speed on gait kinematics and kinetics after endoprosthetic knee replacement following bone tumor resection. Gait Posture 2014;40:622–7. 10.1016/j.gaitpost.2014.07.012. [DOI] [PubMed] [Google Scholar]
- [67].Arnold CM, Faulkner RA. The history of falls and the association of the timed up and go test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatr 2007;7:1–9. 10.1186/1471-2318-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2004;42:127–33. 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- [69].Stössel S, Neu MA, Wingerter A, Bloch W, Zimmer P, Paret C, et al. Benefits of exercise training for children and adolescents undergoing cancer treatment: Results from the randomized controlled MUCKI trial. Front Pediatr 2020;8:1–10. 10.3389/fped.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Weppler CH, Magnusson SP. Increasing muscle extensibility: A matter of increasing length or modifying sensation? Phys Ther 2010;90:438–49. 10.2522/ptj.20090012. [DOI] [PubMed] [Google Scholar]
- [71].Mones HM, Hassan MK, Ahmed BAAH. Health-related quality of life of adolescents with sickle cell disease on hydroxyurea: A case–control study. J Appl Hematol 2022;13:13–21. 10.4103/joah.joah_7_21. [DOI] [Google Scholar]


