Abstract
Hormonal contraception has been widely prescribed for decades. Although safety and efficacy are well-established, much uncertainty remains regarding brain effects of hormonal contraception. We systematically review human and animal studies on the brain effects of hormonal contraception which employed neuroimaging techniques such as MRI, PET and EEG, as well as animal studies which reported on neurotransmitter and other brain biochemical effects. We screened 1001 articles and ultimately extracted data from 70, comprising 51 human and 19 animal studies. Of note, there were no animal studies which employed structural or functional MRI, MRS or PET. In summary, our review shows hormonal contraceptive associations with changes in the brain have been documented. Many questions remain and more studies are needed to describe the effects of hormonal contraception on the brain.
Keywords: Hormonal birth control, neuroimaging, neurotransmitter assay, MRI, PET, EEG, brain structure, brain function, oral contraceptive, birth control, brain, contraceptive, imaging, oral contraceptive pill, OCP
1. Introduction
In 1960, the FDA approved hormonal contraceptives for use in the United States (Kao, 2000). Soon after, other nations’ governing bodies also approved use (Salles, 2020). Nearly all women in the United States have used some form of contraceptive in their lifetime, with 82% of these women choosing to take the oral contraceptive pill (OCP) (Daniels, 2013). OCPs are used to prevent pregnancy and to treat medical conditions such as polycystic ovary syndrome (PCOS), dysmenorrhea, endometriosis, uterine fibroids and other menstrual cycle or pelvic pain disorders (Allen, 2022). From a 2015–2017 CDC survey, approximately 5.9 million women in the United States alone currently use OCPs (Daniels, 2018); this number does not account for women who have used OCPs in the past. Hormonal contraceptives are well-studied -- a PubMed search for “birth control hormonal” yielded 23,999 unique results. Safety, efficacy and contraindications related to hormonal contraceptives are well-established (CDC, 2020).
Hormonal contraceptive preparations incorporate a progesterone-analog and in most cases an estrogen-analog. Mechanisms of action for their contraceptive effects have also been extensively studied, relating to impact on function of the pituitary and hypothalamus, as well as additional peripheral effects (Horvath et al., 2000). The progesterone-analog suppresses secretion of luteinizing hormone (LH), thereby preventing ovulation, and increases viscosity of cervical mucus, which inhibits sperm motility (Horvath et al., 2000). Progesterone analogs used in hormonal contraceptive preparations exhibit variable androgenic properties (Allen, 2022). The estrogen-analog also contributes to suppression of LH, suppresses secretion of follicle stimulating hormone (FSH) and alters the endometrium (Horvath et al., 2000).
The mechanistic basis of hormonal contraceptive effects on brain structure and function remains incompletely understood. However, mechanisms of endogenous estrogen and progesterone effects on the brain have been more extensively characterized. Estrogens and progesterone are produced in the ovaries and adrenal glands (Rettberg et al., 2014). Endogenous estrogens (ie. estrone, estradiol and estriol) and progesterone (including its downstream derivatives dihydroprogesterone and tetrahydroprogesterone) interact with nuclear estrogen receptor-alpha, nuclear estrogen receptor-beta, membrane bound G-protein-coupled estrogen receptor 1 (GPER), nuclear progesterone receptors and progesterone receptor membrane component 1 (PGRMC1) in the brain (Brinton et al., 2008; Rettberg et al., 2014). Estrogen receptors are in general, widely distributed and can be found in both neurons and glial cells, however, distribution of different isoforms vary (Rettberg et al., 2014). Estrogen receptor-alpha has been shown to be expressed in the hypothalamus, forebrain, hippocampus and amygdala in humans (Rettberg et al., 2014). Compared to estrogen receptor-alpha, estrogen receptor-beta is more narrowly distributed, with literature showing expression in hippocampus and cerebral cortex in rodents and humans (Rettberg et al., 2014). GPER is more recently discovered and has been shown to be expressed in the hippocampus, hypothalamus, and midbrain of rodents (Prossnitz & Barton, 2011). Nuclear progesterone receptor expression is also expressed widely across the brain (Guennoun, 2020; Schumacher et al., 2014). PGRMC1 expression has been described in rat cerebellum, cortical regions, hippocampus and hypothalamic nuclei (Toffoletto et al., 2014). Estrogen and progesterone receptors contribute to numerous downstream effects; for example, regulation of glucose transport, regulation of mitochondrial ATP production (Rettberg et al., 2014), and synapse formation (McEwen & Milner, 2017). Progesterone has also been attributed to neuroprotection and myelin repair (Guennoun, 2020).
Sex steroid effects on neurotransmitter pathways is a complex topic that requires further research to fully characterize the multilevel, interacting effects of sex steroids (Nguyen et al., 2017). Studies tentatively suggest estrogens increase serotonergic activity (Nguyen et al., 2017), however, this is only a tentative conclusion and, as other reviews have noted, many factors such as receptor type, region of the brain, and type and duration of estrogen treatment are at play (Barth et al., 2015). Additionally, estrogens are thought to modulate dopamine receptor activity; it has been shown to potentiate D1 receptors and antagonize D2 receptors (Nguyen et al., 2017). Studies also suggest progesterone can increase or decrease serotoninergic activity (Nguyen et al., 2017), suppress glutamate activity and potentiate GABA-A receptor activity (Barth et al., 2015). While literature supports the impact of sex steroids on serotonin, GABA, glutamate, and other neurotransmitter systems, there is no real consensus on the directionality (excitatory or inhibitory) and spatial localization of these effects. More research is needed on the effects of endogenous sex steroids on the brain (Barth et al., 2015; Nguyen et al., 2017). More pertinent in the context of this review, is that endogenous sex steroid effects may not extrapolate to exogenous sex steroids, such as OCPs. However, we can still refer to this information as we focus on the effects of exogenous estrogen analogs and progesterone analogs comprising hormonal contraceptive preparations in this review.
Previous reviews of human [e.g., Brønnick (Brønnick et al., 2020) and Taylor (Taylor et al., 2021)] and animal [e.g., (Porcu et al., 2019)] studies, have assessed the scientific literature and voiced the need for more research on brain effects of hormonal contraceptives. These reviews provide excellent summaries of the literature on human and animal effects of hormonal contraceptives, respectively, but they did not integrate findings across human and animal studies. The aim of this systematic review is to critically assess human and animal studies, with addition of many new studies that have not been previously reviewed, and to assess how the current literature provides insight into potential mechanisms of hormonal contraceptive effects on brain structure and function. While human studies are most clinically relevant, animal studies offer insight into underlying mechanisms, which can never be derived from in vivo human studies. Animal studies also allow for rigorous randomized experimental studies, which are challenging to conduct in humans. Combined assessment of animal and human studies can facilitate future translational studies to characterize clinically relevant mechanisms in humans.
2. Methods
2.1. Systematic review
A systematic literature search was conducted by a medical librarian (C.D.P) following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). We searched the electronic databases PubMed/MEDLINE (through October 22, 2021), Embase (through October 26, 2021), and Cochrane Library (through October 28, 2021). Two sets of searches were conducted in PubMed and Embase, the first focusing on use of imaging to evaluate brain structure and function in humans and animals, and the second research on neurotransmitters and other assays in animals. In Cochrane Library, one combined search was used.
For all databases, both controlled vocabulary and text word searches were performed using a combination of terms that included “brain structure,” “neuroanatomy,” “brain function,” “neural pathway,” “brain region,” “neural process,” “brain imaging,” “neuroimaging,” “magnetic resonance imaging,” “positron emission tomography,” “hormone,” “neurotransmitter,” “neuropeptide,” “birth control,” “contraception,” and “contraceptive.” (For the full PubMed search strategies see Appendix I.) The searches were conducted without any geographical limitation but included only English language publications and, for the second set of searches, were restricted in PubMed and Embase to studies in animals.
2.2. Study selection
All references were imported into Endnote 20 desktop version (Clarivate, Philadelphia, PA) reference management software and de-deduplication was carried out. References were then uploaded to Covidence (www.covidence.org; Veritas Health Innovation, Melbourne, Australia), an online literature review management tool. Further de-duplication was performed, followed by screening by a team of reviewers (J.S., C.D.P, R.N., R.F., R.S., L.M.) based first on the title and abstract and then on the full text. Each article was independently reviewed by two of the six reviewers and conflicts were resolved by the lead reviewer (J.S.).
Human and animal studies were included if they were conducted in females of reproductive age. Human imaging studies were required to compare healthy, naturally cycling women with those on a hormonal contraceptive. Animal studies could include any kind of neurotransmitter or neuropeptide assay. We excluded studies with no control group or that included solely men; that focused on stroke, embolism, or thrombosis in the setting of oral contraceptive use; or that reported only cognitive, behavioral, or mood tests. We excluded studies that studied animals whose ovaries had been removed because this means the control group was not “naturally cycling.” We also excluded publications that were not research studies (e.g., reviews) or that were not fully peer-reviewed (e.g., abstracts, protocols, etc.).
2.3. Data extraction
References that passed the screening process underwent data extraction by a member of the review team. Customized extraction forms were created by the librarian and lead reviewer (C.D.P and J.S.). They collected information on study and patient characteristics – including presence of randomization; type of imaging or assay used; experimental and control groups; inclusion and exclusion criteria; hormone preparation; and baseline population statistics – along with key outcomes and conclusions. No meta-analysis was performed.
3. Results
A total of 1012 references were imported into Covidence, a number that reflects the use of separate human and animal searches. After removal of 11 duplicates, we screened the titles and abstracts of 1001 articles and excluded 903 which did not meet our inclusion criteria. Full text was reviewed for the remaining 98 studies. Ultimately, 70 articles met our relatively narrow inclusion criteria and underwent data extraction. The PRISMA flow diagram is displayed in Figure 1.
Figure 1.

PRISMA diagram. The terms “wrong study type,” “wrong patient population,” “wrong intervention,” “wrong outcomes,” and “wrong setting,” refer to the patient population, intervention, outcome or setting not meeting criteria for inclusion.
3.1. Study Design Features
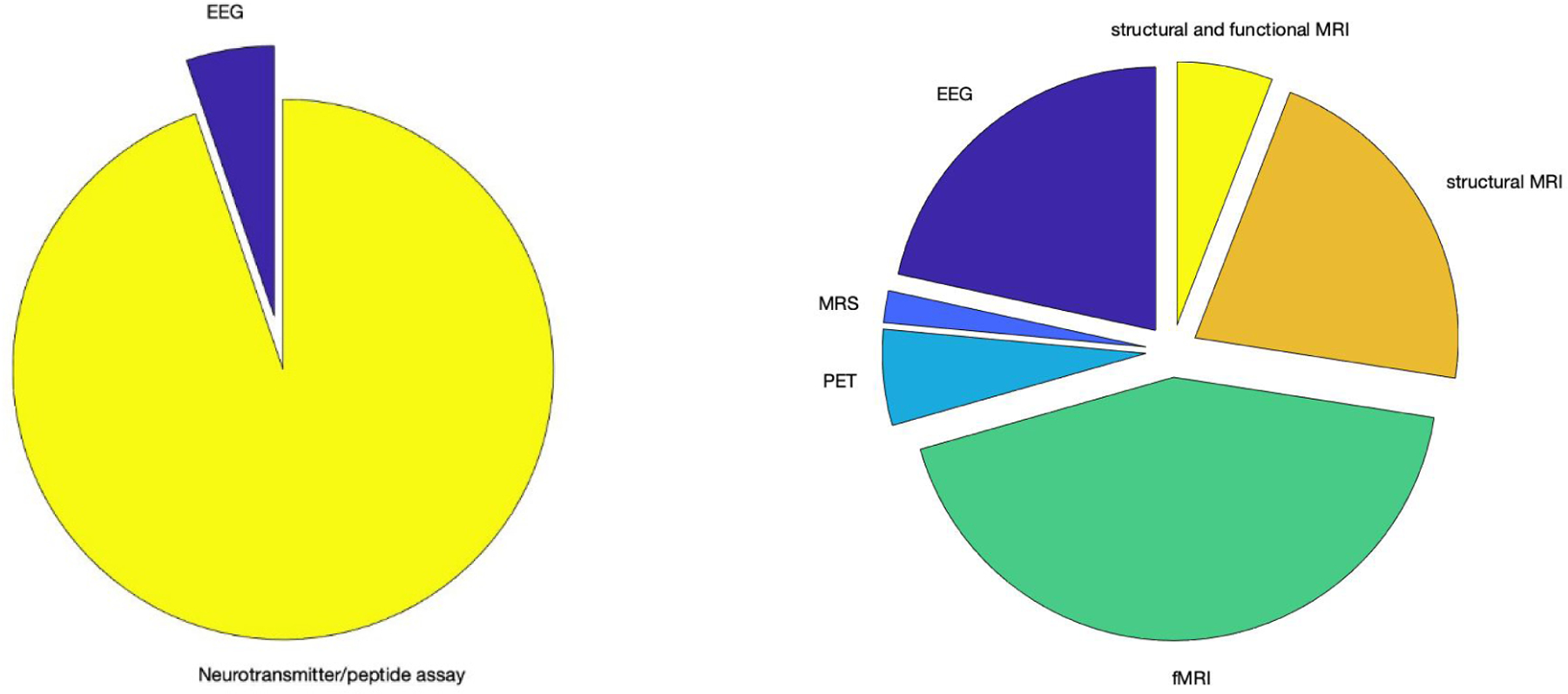
Among the 70 papers included after final data extraction, most of the human studies were published in the last decade (Figure 2). Out of the 51 human studies, 36 employed structural MRI, functional MRI, or both, 11 employed EEG, 1 employed MRS and 3 employed PET. Most of the animal studies were published in the 1970s and 1980s (Figure 2). Of the 19 animal studies we included after screening, 15 reported on rat experiments, 2 studies used both mouse and rat test subjects, one study examined guinea pigs and one examined rabbits. All but one animal study reported on biochemical assay results; one study reported on EEG (Figure 3). No animal study employed structural/functional MRI, MRS or PET.
Figure 2:

Publication rate.
Figure 3:
Experimental Methods for animal (left) and human (right) studies:
Contraceptive formulations varied within and between studies. Of 51 human studies, all but one (Basu et al., 2016) included OCP users in the experimental group. 42 studies tested a combination OCP (progestin + estrogen-analog). However, within each study, OCP formulation and dosage varied among participants, such that individual studies included participants using different OCP formulations. Between studies, formulation, dose of OCP and time on OCP varied. Of these 42 combination OCP studies, 31 studies explicitly specified ethinyl-estradiol as the estrogen component, which is expected given that this is the most prescribed estrogen-analog in OCP (Allen, 2022). For 7 studies, we were not able to determine the OCP preparation. One study focused on women who received intramuscular depo-medroxyprogesterone acetate (DMPA) injections (Basu et al., 2016). One study included combination OCP users in their experimental group but chose to include women implanted with hormone-eluting intrauterine devices (IUD) in the non-OCP control group (Larsen et al., 2020), stating that the hormone-eluting IUD is not thought to affect the ovulatory cycle (Larsen et al., 2020). The authors also performed statistical analyses to support the absence of differences between control participants with or without a hormone-eluting IUD (Larsen et al., 2020). It should be noted, however, that the levonorgesterol-eluting intrauterine device has been shown to affect ovulatory cycles for the first year after implantation, with 85% of women returning to a normal ovulatory cycle thereafter (Dinehart et al., 2020). While a case can thus be made for including hormone-eluting IUD participants in a non-OCP control group, an ideally controlled study would not have included these participants. Additionally, one study included five hormone-eluting IUD users in their experimental group, in addition to sixteen combined OCP users (Scheele et al., 2016). Dosages of estrogen-analog and progestin components were largely varied. Time on hormonal contraceptive varied greatly where reported, but was largely not specified. While Pletzer, et al. reported average OCP use in participants to be on the order of years [e.g., average of 3.8 years for anti-androgenic OCP users and 6.18 for androgenic OCP users (Pletzer et al., 2019)], we were unable to determine the length of OCP use from most papers. When specified, the most common enrollment criterion was “at least 3 months” of OCP use in the experimental group (Chung et al., 2016; Hwang et al., 2015; Merz et al., 2013; Merz et al., 2012; Petersen et al., 2014; Wen et al., 2021).
Contraceptive formulations and doses in animal studies also varied. One study delivered a progestin only hormonal contraceptive (Daabees et al., 1981), which delivered an injection of medroxyprogesterone acetate. The other 18 delivered a form of combined hormonal contraceptive, which included an estrogen component. All the formulations of combined hormonal contraceptive in animal studies were drugs available to human patients, including an estrogen component (i.e., ethinyl estradiol, mestranol) and a progestin component (ie. lynestrenol, norethindrone, norgestrel). In total, 9 studies delivered OCPs orally to animals, and 10 studies delivered the hormonal contraceptive by intramuscular/subcutaneous injection. While DMPA injections and transdermal hormonal contraceptives are available for human use, we are unable to speak to whether the bioavailability/metabolism of intramuscular/subcutaneous injections in these animal studies are comparable to oral route of delivery.
We applied the androgenicity categorization proposed by Allen (Allen, 2022) to the 41 human studies which described use of combination OCPs. Keeping with this categorization, “high” androgenicity includes progesterone analogs norgestrel and levonorgestrel and “middle”/”low” androgenicity (which we will refer to as “low”) includes norethindrone, norethindrone acetate, ethynodiol, norgestimate, desogestrel, and drospirenone (Allen, 2022). When a study does not specify progestin component name, but does provide “anti-androgen” (“low” androgenicity) or “androgenic” (“high” androgenicity) categorization, we keep with their categorization. 4 studies described use of “low” androgenicity OCPs, 8 studies described use of “high” androgenicity OCPs, 15 named OCPs that belonged to a range of androgenicity levels and 23 could not be determined because the OCP formulation was not specified.
Most human studies employed observational designs, in which participants self-reported OCP use. Only six human studies were randomized control trials (Engman et al., 2018; Gingnell et al., 2016; Gingnell et al., 2013; Petersen et al., 2019; Petersen et al., 2021; Wen et al., 2021). One EEG study (Becker et al., 1980), imaged the same participants during both a normal ovulatory cycle, phase unspecified, and after two months of OCP use. Another study, (Basu et al., 2016), imaged the same participants before and 8 weeks following DMPA injection.
3.2. Structural Magnetic Resonance Imaging (MRI)
Detailed results from structural MRI studies are summarized in Table 1. Thirteen human studies used MRI to characterize brain structure in women taking hormonal contraceptives compared to women who were naturally cycling. These studies used volumetric measures (volume – seven studies, cortical thickness – three studies), diffusion tensor imaging (DTI) measures (one study), or both volume and DTI measures (two studies). Of the 12 studies that included volumetric measures, ten evaluated regions across the whole brain. Two studies restricted their analyses to specific regions. Across studies, both greater and lesser volume or cortical thickness was associated with OCP, typically in distinct regions. Some studies revealed that the direction of volumetric change in a region was dependent on the OCP formulation. For example, (Pletzer et al., 2015) reported increases in gray matter volume in bilateral fusiform gyri, fusiform face area (FFA), para-hippocampal place area (PPA) and cerebellum with use of “low” androgenicity progestins but decrease in gray matter volume in bilateral middle and superior frontal gyri with use of “high” androgenicity progestins (Pletzer et al., 2015). The two region-specific analyses showed lower hypothalamus and pituitary volumes (Chen et al., 2021) and higher hippocampus and basal ganglia volumes (Pletzer et al., 2019) in OCP users. Three studies reported findings from DTI. One reported lower mean diffusivity in the fornix (De Bondt, Van Hecke, et al., 2013). Another reported no significant difference in fractional anisotropy (FA) (De Bondt, Jacquemyn, et al., 2013) while another study reported higher FA in the left hippocampus (Sharma, Smith, et al., 2020).
Table 1:
Structural MRI Results by Study
| SAMPLE SIZE | HORMONAL CONTRACEPTIVE FORMULATION (PROGESTIN-ONLY OR COMBINED; ANDROGENICITY) | TYPE OF ANALYSIS | METRIC | SUMMARY OF HORMONE EFFECTS COMPARED TO CONTROL | |
|---|---|---|---|---|---|
| (CHEN ET AL., 2021) | N= 50 (control=29, experimental=21) | Unable to determine | region of interest | volume | Lower hypothalamus and pituitary |
| (DE BONDT ET AL., 2016) | N= 75 (control=38, experimental= 27 high androgenicitiy,10 low androgenicity) | combined; range of androgenicities | whole brain | volume | No significant differences |
| (DE BONDT, JACQUEMYN, ET AL., 2013) | N=30 (control =15, experimental=15) | combined; unable to determine | whole brain | Volume + DTI measures | Lower anterior cingulate gyrus No significant difference in fractional anisotropy |
| (DE BONDT, VAN HECKE, ET AL., 2013) | N=30 (control =15, experimental=15) | combined; unable to determine | whole brain | DTI measures | Lower mean diffusivity in fornix |
| (LISOFSKY ET AL., 2016) | N=56 (control= 28, experimental=28) | unable to determine | whole brain | volume | Lower left amygdala/anterior parahippocampal gyrus |
| (PETERSEN & CAHILL, 2015) | N=90 (control follicular= 21, luteal=25, experimental= 22 inactive pill week, 22 active pill week) *overlapping sample from Peterson et al. 2014 |
combined; range of androgenicities *(not all participant’s OCP reported) |
whole brain | cortical thickness | Lower lateral orbitofrontal cortex and posterior cingulate cortex |
| (PETERSEN ET AL., 2019) | N= 48 (control=24, experimental=24) | Combined; high androgenicity | whole brain | cortical thickness | Lower bilateral pars triangularis, right pars opercularis, and right frontal pole |
| (PETERSEN ET AL., 2021) | N= 26 (same 26 for experimental and control) | Combined; high androgenicity | whole brain | cortical thickness | Lower bilateral pars triangularis, right pars opercularis and right frontal pole |
| (PLETZER ET AL., 2010) | N=28 (control=14, experimental=14) *this study also additionally included 14 men as a study group |
Unable to determine | whole brain | volume | Higher prefrontal cortex, precentral and postcentral gyri, parahippocampal and fusiform gyri and temporal regions |
| (PLETZER ET AL., 2015) | N=60 (control=20 , experimental=22 low androgenicity, 18 high androgenicity) | combined; range of androgenicities | whole brain | volume | Anti-androgenic progestins: Higher bilateral fusiform gyri, fusiform face area (FFA), para-hippocampal place area (PPA) and cerebellum Androgenic progestins: Lower bilateral middle and superior frontal gyri Both: Higher bilateral fusiform gyri, FFA hippocampus, parahippocampus, PPA, middle frontal gyri and anterior cingulate cortex |
| (PLETZER ET AL., 2019) | N=104 (control=52 never users of OCP, experimental=52 used one OCP for more than 3 months and have discontinued mean of 3.32 years ago) *This study also identifies 27 users of multiple OCPs in the past, chose not to include in analysis |
Unable to determine ( *not all subjects able to recall) | region of interest | volume | Higher hippocampal and basal ganglia volumes bilaterally |
| (PLETZER, 2019) | N=149(control=89, experiemental=60) * this study also additionally included 89 men as a study group |
Unable to determine | whole brain | volume | Lower right parahippocampal/fusiform gyrus |
| (SHARMA, SMITH, ET AL., 2020) | N=75 (control=48, experimental=27) | Unable to determine | whole brain | Volume + DTI measures | Lower right putamen Higher left hippocampus Higher FA left hippocampus |
3.3. Functional MRI (fMRI)
Tables 2 and 3 summarize findings from the studies which employed fMRI. Of 25 studies, 19 employed task-based fMRI, 5 employed resting-state fMRI and 1 study reported on both techniques. While task-based fMRI studies generally report on the specific structures that exhibit a significantly different response to the stimulus between experimental groups, resting fMRI studies generally report differences in connectivity within functional brain networks. The following networks were identified in the studies we reviewed. The salience network is thought to respond to a variety of emotional and sensory conditions, and can also be detected during resting-state fMRI. This network includes anterior cingulate and ventral anterior insular cortices, as well as nodes in the amygdala, hypothalamus, thalamus, and ventral striatum (Seeley, 2019). The central executive network supports to working memory, decision-making and control. It includes bilateral dorsolateral, ventrolateral and dorsomedial prefrontal cortices, and the posterior parietal cortices (Daigle et al., 2022). The default mode network is associated with internal mental processes detectable at rest, and includes posterior cingulate cortex, precuneus, medial prefrontal, and inferior parietal cortices (Ekhtiari et al., 2016). The visual network includes regions of occipital cortex, canonically associated with vision, and the somatomotor network comprises motor areas (Yeo et al., 2011). The reward network includes the anterior cingulate cortex, the orbital prefrontal cortex, the ventral striatum, the ventral pallidum, and the midbrain dopamine neuron (Haber & Knutson, 2010); however, a study included in our review defines the reward network as “the thalamus, lateral ventricle, and caudate as well as portions of the superior parietal lobule, precuneus, and dorsolateral PFC” (Sharma, Fang, et al., 2020). The subcortical limbic network includes the thalamus, hippocampus, and parahippocampal gyrus (Laird et al., 2011). The amygdala network refers to subdivisions of the amygdala (delineated as laterobasal, centromedial, and superficial) and regions including areas of the frontal and temporal lobes (Engman et al., 2018; Roy et al., 2009).
Table 2:
Resting State fMRI Results
| STUDY | SAMPLE SIZE | HORMONAL CONTRACEPTIVE TYPE (PROGESTIN-ONLY, COMBINED, BOTH ETC.; ANDROGENICITY) | STIMULUS PARADIGM | ANALYSES | IMAGING METRIC | STATISTICALLY SIGNIFICANT DIFFERENCES IN ACTIVITY BETWEEN NATURALLY CYCLING AND HORMONAL CONTRACEPTIVE GROUP |
|---|---|---|---|---|---|---|
| (PETERSEN ET AL., 2014) | N=91 (20 F, 25L, 22 active pill, 24 inactive pill user) | combined; range of androgenicities*(not all participant’s OCP reported) | Resting State | Whole brain | Correlation coefficients | Connectivity in frontal lobe (right caudate nucleus with anterior default mode network, left anterior cingulate cortex with executive control network, left middle frontal gyrus with executive control network) was lower in OCP group compared to control group (follicular phase) |
| (DE BONDT, SMEETS, ET AL., 2015) | N= 37 (control 18, experimental 19) |
combined; range of androgenicities | Resting state | ROI | Correlation coefficients | No significant difference in default mode network |
| (ENGMAN ET AL., 2018) | N=35 (control 18, experimental 17) *overlapping sample with Gingnell et al. 2013 |
combined; high androgenicity | Resting state *in women who previously reported negative mood on OCP | ROI | Correlation coefficients | Lower connectivity in amygdala network and salience network with dorsal anterior cingulate cortex (dACC) seeds in OCP users compared to control (luteal phase) |
| (LISOFSKY ET AL., 2016) | N=56 (control 28, experimental 28) | unable to determine | Resting state | Whole brain | Correlation coefficients | Connectivity between left amygdala/anterior parahippocampal gyrus and the dorsolateral prefrontal cortex changed from positive to negative connectivity with OCP use compared to the opposite was observed for the control group. |
| (SHARMA, FANG, ET AL., 2020) | N=75 (control 48, experimental 27) | unable to determine | Resting state | Whole brain | Correlation coefficients | Significant changes, both increases and decreases, in connectivity in: Salience network Central executive network Reward network Subcortical limbic network Functional connectivity between the right putamen seed and right middle frontal gyrus *No significant effects appreciated in default mode network |
| (WEN ET AL., 2021) | N=90 (control 33, experimental 57) | combined; range of androgenicities | Resting state | ROI | Correlation coefficients | Significant positive correlation between estradiol level and mean connectivity in the visual network, default mode network, somatomotor network in OCP users but not control |
Table 3:
Task-based fMRI Results
| STUDY | SAMPLE SIZE | HORMONAL CONTRACEPTIVE TYPE (PROGESTIN-ONLY, COMBINED, BOTH ETC.; ANDROGENICITY) | EXPERIMENTAL DESIGN | STIMULUS PARADIGM | ANALYSES | IMAGING METRIC | STATISTICALLY SIGNIFICANT DIFFERENCES IN ACTIVITY BETWEEN NATURALLY CYCLING AND HORMONAL CONTRACEPTIVE GROUP |
|---|---|---|---|---|---|---|---|
| (ABLER ET AL., 2013) | N= 24 (control 12, experimental 12) | combined; range of androgenicities | Block | Visual task: erotic vs neutral stimuli * cortisol given to OCP and non OCP groups |
Whole brain | BOLD signal magnitude | Activation in the precentral gyrus was lower in the OCP group compared to the follicular phase control group during picture expectation (erotic minus non-erotic) No significant differences found between the two control groups (follicular and luteal) |
| (ARNONI-BAUER ET AL., 2017) | N=29 (control=18 imaged follicular and luteal phase, experimental=11) | combined; low androgenicity | Event related | Visual task: food stimuli | ROI | BOLD signal magnitude | Activation in the amygdala (both fed and fasted states), putamen (fasted state), and prefrontal cortex (fasted state) was higher in the OCP group compared to the follicular control groups when viewing images of food. No significant effects between OCP and luteal control groups. |
| (BASU ET AL., 2016) | N=8 (control=before DMPA, experimental=after DMPA) | DMPA | Event related | Visual stimulus: food stimuli | Whole brain | BOLD signal magnitude | Activation in the following regions, organized by lobe, was higher in DMPA users compared to imaging at baseline, prior to DMPA: Frontal-cingulate cortex, paracingulate gyrus, postcentral gyrus, middle frontal gyrus, Parietal-superior parietal lobule Temporal-lingual gyrus |
| (BONENBERGE R ET AL., 2013) | N= 24 (control=12 scanned twice follicular and luteal phase, experimental=12) *overlapping sample with Abler et al. |
combined; range of androgenicities | Event related | Monetary incentive task (event related design) | Whole brain and ROI | BOLD signal magnitude | Activation in the anterior insula/inferior lateral prefrontal cortex was higher in the OCP group during monetary reward expectation No significant results in whole brain analyses |
| (CHUNG ET AL., 2016) | N= 26 (control=13 follicular phase, experimental=13) *14 male subjects also included in this study |
combined; range of androgenicities | Block | Mental arithmetic with social evaluative stress *androstenedion e given to OCP and non OCP groups |
Whole brain and ROI | BOLD signal magnitude | No significant effects found in relation to androstenedione. Within placebo group (no androstenedione given) : Activation in frontal lobe-L somatosensory cortex (L. SSC) and R pre-motor and supplementary motor area (R. pre-SMA) was lower in OCP users than non-users on whole brain analyses |
| (GINGNELL ET AL., 2013) | N= 30 (control=15, experimental – 15) | combined; high androgenicity | Block | Visual task: facial vs geometric recognition *subjects are women who previously reported negative mood on OCP |
ROI | BOLD signal magnitude | Activation of L insula, L middle frontal gyrus and bilateral inferior frontal gyri was lower in OCP group compared to control group during emotion-inducing facial recognition |
| (GINGNELL ET AL., 2016) | N=31 (control 16, experimental 15) *Same sample from Gingnell et al., 2013 |
combined; high androgenicity | Event related | Visual task: Go/No Go task with letters | Whole brain | BOLD signal magnitude | Activation in frontal lobe-right middle frontal gyrus was lower in OCP group compared to control group No significant difference in task performance |
| (HORNUNG ET AL., 2019) | N=50 (control 21, experimental 29) | combined; unable to determine | Event related | Visual task: face recognition | Whole brain | BOLD signal magnitude | No significant difference compared to control group (luteal phase) |
| (HWANG ET AL., 2015) | N=48 (control= 16 high serum estradiol women, 16 low estradiol women, experimental=16) *also included data from 37 men |
combined; unable to determine | Event related | Fear conditioning and extinction paradigm | ROI | BOLD signal magnitude | Activation in amygdala, insular cortex, middle cingulate cortex was lower in OCP group compared to control high estradiol women group during fear conditioning |
| (MARECKOVÁ ET AL., 2014) | N= 20 (control=10, experimental=10) *each imaged 4 times: menstruation, follicular phase, mid-cycle and luteal phase *not part of our review criteria but also includes adolescent study |
Combined; unable to determine | Event related | Visual task: ambiguous and angry expressions | Whole brain | BOLD signal magnitude | Activation in the right FFA was higher in the OCP group compared to control in both the ambiguous and angry condition. |
| (MERZ ET AL., 2012) | N= 90 (control=30 luteal, 30 follicular, experimental=30) *32 male subjects also analyzed in this study |
combined; unable to determine | Event related | Fear conditioning paradigm: difference conditioned and non-conditioned stimuli *cortisol pill or placebo given to OCP and non OCP groups |
Whole brain and ROI | BOLD signal magnitude | Activation in L anterior parahippocampal gyrus and left hippocampus during fear conditioning was enhanced by cortisol in the OCP group as opposed to non OCP group (luteal phase) |
| (MERZ ET AL., 2013) | N=30 (control=15 luteal, experimental=15) *20 male subjects also analyzed in this study |
combined; unable to determine | Event related | Fear conditioning-geometric shape association to electrical stimulation *salivary cortisol measures |
Whole brain and ROI | BOLD signal magnitude | Activation in R anterior parahippocampal gyrus and R amygdala was positively correlated to cortisol in the OCP group as opposed to control group (luteal phase) |
| (MIEDL ET AL., 2018) | N=53 (control=30, experimental=23) | combined; unable to determine | Event related | Visual task: traumatic stimuli | ROI | BOLD signal magnitude | Activation in the frontal lobe-insula and dorsal anterior cingulate cortex was higher in the OCP group than control during traumatic film viewing |
| (PETERSEN & CAHILL, 2015) | N=83 (control= 20 follicular phase, 23 luteal phase, experimental= 20 active pill, 20 inactive inactive pill | combined; range of androgenicities | Event related | Visual task: emotional stimuli | ROI | BOLD signal magnitude | Activation in left amygdala was lower in OCP group compared to control group (follicular phase) Activation in right amygdala was lower in OCP group compared to control group (luteal phase) |
| (PLETZER ET AL., 2014) | N=30 (control=16, experimental=14) | combined; range of androgenicities | Block | Numerical tasks: number comparison and number bisection | Whole brain | BOLD signal magnitude | Activation for within-decade (WD) and non-WD number comparison items was lower in OCP users compared to control (follicular phase) but for WD number comparison items was higher in OCP users compared to control (luteal phase) |
| (RUMBERG ET AL., 2010) | N=24 (control=12, experimental=12 ) *12 male subjects also analyzed in this study |
combined; unable to determine | Block | Verb generation test | Whole brain | BOLD signal magnitude | Activation during verb generation in the temporal lobe (right superior temporal cortex ) was higher for OCP group versus control (menstrual phase), in the frontal lobe (right inferior frontal cortex) was higher for OCP group versus control (mid-luteal phase) |
| (SCHEELE ET AL., 2016) | N=40 (control=19 both luteal and follicular,experimental=21) | combined; range of androgenicities | Event related | Visual task: face perception task *Intranasal oxytocin given to OCP and non OCP groups |
Whole brain | BOLD signal magnitude | When calculating the difference in activation of the bilateral striatum and VTA when participants viewed their partner’s face versus a familiar face, intranasal OXT increased this difference in activation in the non-OCP group but this effect was not observed in the OCP group |
| (SHARMA, SMITH, ET AL., 2020) | N=75 (control=48, experimental=27) | combined; unable to determine | Event related | Memory task with emotional pictures | ROI | BOLD signal magnitude | Frontal lobe (ie. right inferior frontal gyrus, right mid-frontal gyrus) was higher in the OCP group compared to control during memory processing of negative emotion stimuli |
| (Vincent et al., 2013) | N= 24 (each imaged 3 times in one month, control= 12, experimental=12) | combined; unable to determine | Block | Noxious stimulation | Whole brain and ROI | BOLD signal magnitude | There is lower activation in the rostral ventromedial medulla in the low testosterone subset of the OCP group compared to control and there is lower activation of the amygdala in the high testosterone subset of the OCP group compared to control in response to noxious stimuli. |
| (Wen et al., 2021) | N=90 (control=33, experimental=57) | combined; unable to determine | Event related | Fear conditioning and extinction paradigm (event related design) *Estradiol pill or placebo pill given to OCP and non OCP users |
ROI | BOLD signal magnitude and correlation coefficients | OCP and non-OCP group showed similar activation and connectivity to serum estradiol levels. Significant negative correlation between estradiol level and mean connectivity in Default mode network, Somatomotor network, Subcortical network in OCP users but not non-OCP group during extinction retention test (day 3) |
3.3.1. Resting-state fMRI
All six resting-state protocols were similar, other than the length of recording and participant instructions for eyes open or closed. Below and in Table 2, we summarize salient resting state fMRI findings, grouped by brain regions implicated:
Frontal lobe – One study showed that compared to non-OCP controls in the follicular phase, OCP users showed lower connectivity of the right caudate nucleus with the anterior portion of the default mode network (medial prefrontal cortex), of the left anterior cingulate cortex with the executive control network and of the left middle frontal gyrus in the executive control network (Petersen et al., 2014). Similarly, one study found both follicular and luteal phase non-OCP controls exhibited lower connectivity within the central executive network compared to OCP users (Sharma, Fang, et al., 2020).
Limbic system, including cingulate cortex and basal ganglia - One study showed that compared to OCP users, naturally cycling women in the follicular phase, but not the luteal phase, showed greater activity in the right caudate nucleus and left anterior cingulate cortex (Petersen et al., 2014). The salience network, reward network, subcortical limbic network and the right putamen with right middle frontal gyrus were also shown to have greater connectivity in OCP users compared to controls (Sharma, Fang, et al., 2020). Similarly, the connectivity within the amygdala network and salience network was lower with combined OCP administration, compared to controls (Engman et al., 2018) . However, this study found connectivity also varied with the normal menstrual cycle and identified stronger associations attributable to effects of endogenous hormones (Engman et al., 2018).
Frontal-limbic connectivity - When participants were imaged, started on OCP (formulation unspecified) and then imaged approximately 3 months later, dorsolateral prefrontal cortex resting-state functional connectivity with left amygdala and parahippocampal gyrus changed from positive to negative correlation , which was not observed in naturally cycling women imaged twice during their early follicular phase, also imaged 3 months apart (Lisofsky et al., 2016).
One study showed no significant association of combined OCP with connectivity in the default mode network compared to either control group (a follicular phase group and a luteal phase group (De Bondt, Smeets, et al., 2015).
One study identified a significant positive correlation between estradiol level and mean connectivity in the Default Mode Network, in the Visual network and Somatomotor Network in combined OCP users but not controls in the follicular phase (Wen et al., 2021).
3.3.2. Task-based fMRI
The task-based fMRI study paradigms reported probed fear, memory, facial recognition, and arousal to either erotic, dietary/food-related, emotional or traumatic stimuli (Table 3). The results of task-based fMRI studies are shown in Table 3.
Fear Conditioning
There were four studies that employed a fear conditioning paradigm, but no two studies employed the same paradigm/design. In two studies, the conditioned stimulus was visual light colors (blue, red, or yellow- two out of three colors were fear conditioned to an electric shock, the other wasn’t) (Hwang et al., 2015; Wen et al., 2021). Wen, et al. employed a unique exposure design, where each study participant (OCP users and non-OCP controls) was randomly assigned to receive either estradiol or placebo prior to extinction learning (Wen et al., 2021). Hwang, et al. described lower activation in amygdala, insular cortex and middle cingulate cortex of OCP users when compared to the high serum estradiol control group, and no such effect when compared to the low serum estradiol control group (Hwang et al., 2015). Wen, et al. described no difference in response to serum estradiol levels, modulated by either estradiol or placebo pill, in either OCP and non-OCP users with the only difference being a significant negative correlation of serum estradiol levels with connectivity in Default Mode Network, Somatomotor Network, Subcortical Network in OCP users, but not in the non-OCP group, during the extinction retention test (Wen et al., 2021).
In the two other fear conditioning fMRI studies, the conditioned stimulus was a geometric shape (rhomboid, square or triangle) (Merz et al., 2013; Merz et al., 2012). However, in (Merz et al., 2012), each study participant (OCP users and non-OCP controls) was randomly assigned to receive either cortisol or placebo prior to extinction learning (Merz et al., 2012). (Merz et al., 2013), showed differential activation of parahippocampal, hippocampal, and amygdala regions related to cortisol levels between OCP group and non-OCP group in luteal phase.
Visual Stimuli
Two studies imaged participants while presenting various food and non-food images. In one study, participants were shown “high-calorie sweet,” “high-calorie savory” or non-food images during the functional scan (Arnoni-Bauer et al., 2017). In another study, participants were shown “high-calorie,” “low-calorie” and non-food images during the scan (Basu et al., 2016). The Basu et al. study imaged participants before and 8 weeks after a DMPA administration. These are similar but different paradigms. Both suggest higher activation to food stimuli in the hormonal contraceptive group in differing brain regions detailed in Table 3, however, Arnoni-Bauer et al. describe no significant difference between OCP users and luteal phase control, only significant results between OCP users and follicular phase participants. On the other hand, Basu et al. only imaged participants during the luteal phase and then post-DMPA administration and did show some significant effects. Arnoni-Bauer et al. also shows activation in reward and visual regions are significantly correlated with androgen, cortisol, testosterone, and insulin levels. Basu et al. also showed no significant change in leptin, ghrelin or fat mass over the 8 weeks.
In one study, emotion-induced activation was measured by comparing scans when participants are tasked with identifying matching emotional facial expressions versus matching geometric shape dimensions (Gingnell et al., 2013). In another study, participants were asked to indicate the location of a dot probe after being shown a pair of angry, happy, fearful or neutral faces (Hornung et al., 2019). In another study, participants viewed angry and ambiguous facial expression video clips and non-biological moving circles(Marecková et al., 2014). While Gingnell et al 2013, showed lower activation of left insula, left middle frontal gyrus and bilateral inferior frontal gyri in OCP user group compared to placebo, Mareckova showed increased activation in right fusiform face area in the OCP group compared to non-OCP group and Hornung showed no significant results between OCP users and non-users.
In one study, participants were scanned while watching traumatic films, there was higher activity in the insula and dorsal anterior cingulate cortex in OCP users than non-users; estradiol levels in the non-OCP group was shown to modulate ventromedial prefrontal activity, this observation was not observed in the OCP group (Miedl et al., 2018). In another study, participants are shown images with ranging emotional intensity and it was observed in OCP users lower activation in left amygdala compared to control group (follicular phase) and lower activation in right amygdala compared to control group (luteal phase) (Petersen & Cahill, 2015).
In another study, participants were shown erotic vs neutral videos, and activation lower in precentral gyrus during picture expectation for the OCP group compared to follicular phase control group; activation was also found to be positively correlated to plasma estrogens (Abler et al., 2013). In Scheele 2016, OCP-users and non-users were given either intranasal oxytocin or placebo and were tasked with viewing the faces of their romantic partner and other familiar faces. The results showed while oxytocin increased activation of the bilateral striatum and ventral tegmental area compared to placebo in the non-OCP group, this effect was not observed in the OCP group (Scheele et al., 2016).
Cognitive
One study employed a Go/No-Go task and showed no significant difference in task performance but OCP users showed decreased activity in the right middle frontal gyrus in comparison to placebo group (Gingnell et al., 2016).
In Chung et al., participants performed a mental arithmetic task, meant to illicit a stress response and androstadienone or placebo was given to all study participants. While no significant differences, in imaging or performance, were observed between OCP users and non-users in those given androstadienone, with the placebo group, lower activation in areas of the frontal lobe was observed in the OCP users (Chung et al., 2016). In another study, participants were asked to perform either within decade or non-within-decade number comparisons and lower or higher activation was observed, depending on type of numerical task (Pletzer et al., 2014). In Sharma et al, participants performed an N-back memory task with emotional (negative, positive or neutral) images and it was shown higher frontal lobe activation during memory activation of negative stimuli (2-back > 1-back) (Sharma, Smith, et al., 2020).
In Vincent et al, participants were subjected to a noxious thermal stimulation; it was noted that activity in the rostral ventromedial medulla was lower in the low-testosterone OCP group and activity in the amygdala was lower in the high testosterone OCP group; overall activity increased with testosterone (Vincent et al., 2013).
In Bonenberger et al., participants perform a monetary incentive task and we see an increase in activation in the anterior insula/inferior lateral prefrontal cortex during monetary expectation when compared to control group (follicular phase)(Bonenberger et al., 2013). In Rumberg et al, participants were asked to think of a verb, without speaking out loud, when presented with a noun. Activation during verb generation in the temporal lobe was higher for OCP group versus control (menstrual phase), in the frontal lobe was higher for OCP group versus control (mid-luteal phase) (Rumberg et al., 2010).
3.4. Neurotransmitter Assays
Biochemical assays of neurotransmitters and neuropeptides in animals probe potential mechanisms of hormonal contraceptive effects at a level not possible in humans (Table 5). We identified 19 studies on rats, including two which reported both rat and mouse experiments, one study on rabbits, and one study in guinea pigs. Five of the 18 studies employed intramuscular injection of hormonal contraceptive preparations and 13 employed oral intake. Hormonal contraceptive effects on biochemical measures are categorized and summarized in Tables 4 and 5.
Table 5.
Neurotransmitter/neuropeptide assay detailed summary
Norethindrone= NE, ethynyl estradiol= EE, levonorgestrel=LNG, beta-endorphin immunoreactivity = beta-EI
| PAPER | ANIMAL | SUMMARY |
|---|---|---|
| ALGERI 1976 | Rat | acute and chronic administration: increased the rate of disappearance of dopamine after synthesis in the striatum chronic administration: conversion of 3H-T into 3H-DA increased in the forebrain, striatum |
| BANDYOPADHYAY 1988 | Rat | EE on acetylcholinesterase activity increased: cerebral cortex, corpus striatum decreased: hypothalamus, midbrain lynestrenol on acetylcholinesterase activity increase: cerebral cortex, hypothalamus and corpus striatum decreased: midbrain combination on acetylcholinesterase activity: increase: corpus striatum decrease: hypothalamus, midbrain |
| CHAUDHURI 1992 | Rat | Progesterone: decrease in noradrenaline of the brain stem-hypothalamus-pituitary segment of the brain Inconsistent effects observed of dopamine, 5-hydroxytryptamine, histamine |
| DAABEES 1981 | Rat | intramuscular injection of medroxyprogesterone acetate GABA and glutamate: decrease oral contraceptive steroids GABA and glutamate: increase Both treatments acetylcholine: no change serotonin: increase |
| DEY 1991 | Rat | norepinephrine in the medulla oblongata-pons, hypothalamus, midbrain-thalamus-subthalamus decreased dopamine in medulla oblongata-pons, hypothalamus, striatum-hippocampus, cortex Ascorbic acid increased in all areas except the cerebellum |
| ISLAM 1980 | Rabbit | Decreased total lipids, phospholipids, cholesterol, free fatty acids and esterified fatty acids in hypothalamus, hippocampus, nuclei of thalamus, gyrus cinguli Increase esterified fatty acids in amygdaloid nucleus Increased cholesterol in hypothalamus |
| JORI 1976 | Mouse and Rat | combined estrogen and progestin: decrease dopamine levels in the striatum of mice and rats |
| LADINSKY 1976 | Rat and Mouse | No significant effects |
| MARCHI 1974 | Rat | Decreased monoamine oxidase activity in liver and brain |
| PONZIO 1977 | Guinea Pig | Greater conversion of tyrosine to dopamine in the hypothalamus and striatum, calculated as the ratio of radioactively tagged tagged tyrosine substrate which was initially injected further conversion to norepinephrine was not observed |
| PORCU 2012 | Rat | with EE alone, LNG alone, and combination progesterone, and allopregnanolone : decreased in cerebral cortex, hippocampus LNG alone and EE/LNG combination increased the gamma2 subunit polypeptide amount in the cerebral cortex, hippocampus EE treatment alone: no change in polypeptide levels |
| RAO 1984 | Rat | 2 months of steroid treatment: GDH activity : increased in all regions of the brain AAT activity : no change in all regions of the brain GAD activity: decreased in the cerebellum, no change in other regions GABA-alphaKG aminotransferase activity: increased in all regions of the brain 6 months of steroid treatment: GDH activity : decreased in cortex, cerebellum, and brainstem (but not midbrain), AAT activity: slightly decreased in only the brainstem GAD activity : reduced in the cerebral cortex, brainstem, and midbrain (but not cerebellum GABA-T was increased in all regions of the brain |
| SASSOÈ-POGNETTO 2007 | Rat | 4 weeks hormonal contraceptive administration: uptake of a gephyrin (a GABA-receptor component) mRNA analogue : no difference in the hippocampus or cortex |
| SHETTY 1980 | Rat | estradiol, norgestrel, or combination of both: increased GABA content in the brain No change in glutamic acid decarboxylase, GABA-T estradiol alone or norgestrel alone: increase in brain pyridoxal kinase |
| SHETTY 1980 | Rat | combination: decreased brain dopamine, noradrenaline, 5-hydroxytyptamine. norgestrel treatment: decreased brain dopamine, noradrenaline, 5-hydroxytyptamine ethinyl estradiol treatment: no significant effects *brain region-specific changes in noradrenaline and 5-hydroxytyptamine based on treatment type and length |
| SIMONE 2015 | Rat | low doses EE and EE/LNG decrease in tyrosine hydroxylase mRNA and protein, increased galanin protein in the locus coeruleus reduced brain-derived neurotrophic factor mRNA in hippocampus |
| TEJWANI 1983 | Rat | NE treatment acute treatment decreased beta-EI in the striatum Combination acute treatment decreased beta-EI in the pituitary Combination acute treatment, 10-fold treatment decreased beta-EI in pituitary, hypothalamus, striatum NE treatment chronic treatment decreased beta-EI in striatum Combination chronic treatment, 10-fold treatment decreased beta-EI in pituitary and hypothalamus |
| TEJWANI 1985 | Rat | acute treatment with NE+EE : reduced beta-endorphin, dynorphin, leucine-enkephalin, methionine-enkephalin in pituitary, chronic treatment with NE+EE: increase dynorphin, leucine-enkephalin, and methionine-enkephalin in pituitary, hypothalamus, |
Table 4.
Neurotransmitter/Neuropeptide/Biochemical assay results summary
| SYSTEM PATHWAY | UPREGULATION (# OF STUDIES) | DOWNREGULATION (# OF STUDIES) | NO SIGNIFICANT EFFECTS (# OF STUDIES) | UPREGULATION AND DOWNREGULATION (# OF STUDIES) |
|---|---|---|---|---|
| DOPAMINERGIC | 1(Ponzio et al., 1977) | 6 (Chaudhuri et al., 1992; Dey et al., 1991; Jori & Dolfini, 1976; Marchi & Cugurra, 1974; Shetty & Gaitonde, 1980b; Simone et al., 2015) | 1 (Algeri et al., 1976) | |
| CHOLINERGIC | 2 (Daabees et al., 1981; Ladinsky et al., 1976) | 1 (Bandyopadhyay & Ghosh, 1988) | ||
| LIPID PATHWAYS | 1 (Islam et al., 1980) | |||
| BETA-ENDORPHIN | 2 (Tejwani et al., 1985; Tejwani et al., 1983) | |||
| BDNF | 1 (Simone et al., 2015) | |||
| GABA | 3 (Porcu et al., 2012; Rao et al., 1984; Shetty & Gaitonde, 1980a) | 1(Sassoè-Pognetto et al., 2007) | 1 (Daabees et al., 1981) | |
| SEROTONIN | 1 (Daabees et al., 1981) |
3.5. In Vivo Metabolic Imaging Methods: Positron Emission Tomography (PET)/Magnetic Resonance Spectroscopy (MRS)
We identified 3 PET studies in humans. One study showed lower global brain serotonin 4 receptor binding potential among users of 2nd and 3rd generation combined OCPs, which included progestin analogs of varying androgenicity, compared to controls who were expected to have a normal ovulatory cycle, but were not classified by menstrual cycle phase (Larsen et al., 2020). The other 2 studies showed no statistically significant differences between naturally cycling women and OCP users. One study examined D-amphetamine-induced dopamine release from 5 predefined ROIs (right pallidum, inferior frontal gyrus, bilateral ventral striatum, bilateral caudate and bilateral putamen) (Smith et al., 2019). The other measured serotonin 2A receptor binding in the cerebral cortex (Frokjaer et al., 2009).
The only MRS study reported on GABA in the prefrontal region; this study found a significant difference in the GABA+/creatine ratio, but no significant difference when GABA+ concentration was quantified, between OCP users and non-OCP women (De Bondt, De Belder, et al., 2015).
3.6. Electroencephalography (EEG)
We identified 12 EEG studies, 11 in humans and 1 in rats detailed in Table 6.
Table 6.
EEG studies detailed summary
| STUDY ID | POPULATION DESCRIPTION | HORMONAL CONTRACEPTIVE TYPE (PROGESTIN-ONLY, COMBINED, BOTH ETC.; ANDROGENICITY) | STUDY DESIGN | SAMPLE SIZE | 1–3 SENTENCE SUMMARY OF RESULTS/CONCLUSION: |
|---|---|---|---|---|---|
| (BECKER ET AL., 1980) | Human | combined; high androgenicity | no task | N= 16 (control= 8, experimental=8) | No significant results in theta, alpha frequency bands |
| (BRÖTZNER ET AL., 2014) | Human | combined; range of androgenicities | no task | N=114( control= 57 experimental=17,20, 20 for each recording session) session 1 - recordings Oct-March session 2- recordings from Nov to Mar session 3 - recordings Apr to Oct |
Inconclusive results comparing experimental and control. Significant results observed in recording 1 were not observed in recording 2 and recording 3 and vice versa. |
| (CREUTZFELDT ET AL., 1976) | Human | combined; unable to determine | no task | N= 32 (control=16, experimental=16) | The NC group had a statistically significant increase in the mean alpha frequency during the late luteal phase. This increase was not seen in the OCP group. The mean alpha frequency of the OCP group slower than that of the NC group. There was not a significant difference in the power for the alpha, beta, and theta ranges between the NC and OCP groups. The weighted mean of the alpha range was significantly different between the NC and OCP groups, but not for the beta and theta ranges. *In separate cognitive performance tasks, with not EEG recording, the reaction times for the simple tasks and arithmetic tasks in the NC group were less than those in the OCP group |
| (FLECK & POLICH, 1988) | Human | Unable to determine | auditory discrimination task - binaural 1000 Hz vs 2000 Hz tones | N= 20 (control=10, experimental=10) | No significant difference between control and experimental group in P3 or other ERP components |
| (GAUTRAY ET AL., 1974) | Human | combined; high androgenicity | no task | N= 8 1 spontaneous cycle recording 3 contraceptive cycle recording 2 spontaneous cycle and followed by a contraceptive cycle recording 1 contraceptive cycle and followed by a spontaneous cycle recording 1 spontaneous cycle, then a contraceptive cycle, and then another spontaneous cycle recording |
Results inconclusive, no statistically significant results |
| (MATSUMOTO ET AL., 1966) | Human | combined; range of androgenicities | sleep study | N=13 (control= 7, experimental=6) | There was a slow spindle during the natural sleeping EEG in all of the women taking oral contraceptives yet there was not a slow spindle in the naturally cycling women. |
| (MONCIUNSKAITE ET AL., 2019) | Human | combined; low androgenicity | visual stimuli (one second display of human images categorized as either neutral, unpleasant, pleasant, highly unpleasant, or highly pleasant) | N=70 (control=37, experimental=33) | The OC group had a significantly lesser reaction when presented with visual stimuli as compared to the NC group, especially in the reaction to highly unpleasant images. The OC group also had a significantly lower amplitude in the LPP compared to the NC group. Highly unpleasant and highly pleasant stimuli resulted in the largest amplitudes of LPP in both OC and in NC. There were also significant differences between the NC and OC groups in the post-stimulus GFP (at 222–328ms and 365–100ms), in addition to significant differences in GFP between the different stimuli categories. |
| (MUKHERJEE ET AL., 1978) | Rat | Combined; “Voldys”-no longer in market | no task-anesthesized | N=12 (control= 6, experimental= 6) | No statistically significant results. Authors note after 31 days on OCP, the frequency and amplitude of the slow waves increased and then returned to based like 31 days after OCP discontinuation. |
| (PLAMBER GER ET AL., 2021) | Human | combined; range of androgenicities | sleep study | N=62 (control=43, experimental=19) | OCP women had less REM sleep compared to NC women in the luteal phase. OCP women also had less NREM1 sleep than NC women during the follicular phase. OCP group had a higher fast spindle density compared to NC women in the follicular phase and OC women had a higher frontal fast spindle density than NC women in the follicular phase. |
| (SUGERM AN ET AL., 1970) | Human | unable to determine | no task | N=23 (control=15, experimental=8) | No statistically significant results or differences in mean energy content |
| (Ujma et al., 2017) | Human | combined; low androgenicity | sleep study | N= 30 (control=15, experimental=15) | No statistically significant results. In controls, both for slow and fast spindles, the “frontal sleep spindle amplitude was left-lateralized,” which was not seen in females using oral contraceptives. In controls, the slow and fast spindle frequency was positively correlated with progesterone levels and the fast spindle amplitude was positively correlated with estrogen levels. This was not seen in contraceptive users. |
| (WUTTKE ET AL., 1975) | Human | combined; high androgenicity | no task | N= 32 (control=16, experimental=16) | The weighted mean alpha frequency was lower in the experimental group compared to control group. In the oral contraceptive group, there were no significant changes in the weighted mean alpha frequency during the cycle whereas in the naturally cycling group, there was a slight increase in the weighted mean alpha frequency during the cycle, which was significant. There were no significant changes in the delta, theta, or beta bands. The above differences were observed in the occipito-central electrodes, but not in the left and right temporo-central EEG electrodes. |
3.6.1. Resting EEG During Sleep
Three studies reported on resting EEG during sleep. Combined OCP users had significantly less REM sleep compared to naturally cycling women in either the luteal or follicular phase of a normal menstrual cycle (Plamberger et al., 2021). Additionally, the OCP group in this study showed significantly higher frontal fast spindle density compared to naturally cycling women in the follicular phase, but not compared to women in the luteal phase (Plamberger et al., 2021). Ujma showed that both slow and fast spindle frequency varied with progesterone levels for naturally cycling women, not stratified by menstrual cycle, but spindle frequency did not exhibit the same association with progesterone level in combined OCP users (Ujma et al., 2017). In the third EEG sleep study, no significant effects were found, but it was noted that some women in the combined OCP group did not exhibit slow sleep spindles localized to the frontal or occipital areas, while all non-users did (Matsumoto et al., 1966).
3.6.2. Resting EEG During Wakefulness
Four out of six studies which analyzed awake resting state EEG from women taking hormonal contraceptive reported no significant differences compared to naturally cycling women (Becker et al., 1980; Brötzner et al., 2014; Gautray et al., 1974; Mukherjee et al., 1978; Sugerman et al., 1970). Two studies reported lower mean alpha frequency (alpha frequency defined as 8–13Hz) in the combined OCP user group compared to the OCP nonuser group (Creutzfeldt et al., 1976; Wuttke et al., 1975). However, both studies also report that the mean alpha frequency varied with menstrual cycle phase in the naturally cycling control group (Creutzfeldt et al., 1976; Wuttke et al., 1975). Wuttke and colleagues showed that mean alpha frequency increased during the luteal phase and decreased during menstruation (Wuttke et al., 1975). Meanwhile, Creutzfeldt and colleagues noted a “slight shift of the alpha peak to the right,” meaning a higher peak frequency, during the luteal phase (Creutzfeldt et al., 1976). This suggests that while the differences between hormonal contraceptive groups and controls are statistically significant, alpha frequency is influenced by the menstrual cycle itself. The higher alpha peak frequency during the luteal phase could account for the difference in mean alpha frequency between OCP and non-OCP women (Creutzfeldt et al., 1976; Wuttke et al., 1975). No group differences were reported for beta and theta frequencies (Creutzfeldt et al., 1976; Wuttke et al., 1975).
A single resting-state EEG study in anesthetized rats also showed no significant differences between the group that received oral administration of a combined megestrol acetate-ethinyl-estradiol formulation, and the non-OCP control group (Mukherjee et al., 1978).
3.6.3. Task-Based Event-Related Potentials (ERP)
In a single visual task ERP study, participants were asked to recognize “pleasant,” “neutral,” and “unpleasant” images. The authors examined the late positive potential (LPP; 400–700 ms post-stimulus, averaged over the C2, C4, CPz, CP2, CP4, Pz electrodes) (Monciunskaite et al., 2019). The OCP group, when compared to the naturally cycling group, which included follicular and luteal phase participants, exhibited a significantly lower average LPP amplitude response to all image types, but the effect was greatest for highly unpleasant images (Monciunskaite et al., 2019). A single study tested auditory task ERP, finding no difference between OCP and naturally cycling group in P3 or other ERP components (Fleck & Polich, 1988)
4. Discussion
We identified controlled animal and human studies, which describe effects of hormonal contraceptives, almost exclusively OCPs, on the brain, demonstrated using MRI, PET, MRS, biochemical assays and EEG. Aspects of hormone exposure (e.g., OCP formulation and length of time on OCPs), brain measures (e.g., MRI, PET, biochemical assays, and EEG) and study methods (e.g., observational vs. randomized designs, brain regions analyzed, and task paradigms), as well as results (e.g., opposing directionality of effects) varied across studies.
4.1. What do the results tell us? And what are their limitations?
Multiple human and animal studies identified group-level associations of hormonal contraceptive exposure with brain structure, activity, and biochemistry. Due to the variability across studies and absence of explicit replication of findings using the same experimental approach in more than one independent participant sample, these findings must be considered tentative. Nonetheless, for all these significant results to be spurious would implicate a remarkable degree of systematic confounding, selection bias or other design-related factors across many quite differently designed and executed studies. This possibility seems implausible, and therefore, it is likely, though not certain, that hormonal contraceptives affect the brain. Further standardized studies are warranted to directly replicate and confirm existing studies.
While some brain effects of hormonal contraceptives seem likely based on the existing studies, characterization of the nature of the effects, the extent to which they may be in part accounted by other factors and their relevant underlying mechanisms is much more challenging based on the limitations of the current literature. It is possible to identify limited consistency of findings across more than one study. This can be used to hypothesize biologically plausible effects which can be tested in future studies. For example, results across some of the MRI studies (e.g., (Arnoni-Bauer et al., 2017; Miedl et al., 2018; Petersen & Cahill, 2015; Pletzer et al., 2019; Sharma, Fang, et al., 2020)), are consistent with, though not proof of, hormonal contraceptives effects on frontal and limbic regions, which are in turn consistent with known patterns of estrogen and progesterone receptor expression (Guennoun, 2020; Rettberg et al., 2014). These findings may also be consistent with observed sleep patterns on EEG in OCP users ((Plamberger et al., 2021; Ujma et al., 2017)) and studies on GABAergic function under OCP effects in humans and animals ((De Bondt, De Belder, et al., 2015; Rao et al., 1984)), which is known to mediate sleep (Siegel, 2004). It again bears emphasis that these types of interpretation are only leveraging existing findings, which suggest preliminary converging data from human and animal studies, to generate relevant hypotheses. Right now, these types of interpretation can only be considered hypotheses. This is all to say, there is potential for these existing studies to fit together to tell the same conclusion, but we cannot confirm that conclusion now.
We must also consider that some studies failed to identify significant group differences. Although these null results could be due to study limitations such as power and confounding, we cannot completely rule out a null effect of hormonal contraceptives on the brain. We included more studies than prior animal (Porcu et al., 2019) and human (Brønnick et al., 2020; Taylor et al., 2021) reviews, but found the overall scope of results similar. However, by reviewing both human and animal studies, we highlight the lack of translational studies and identify additional gaps in knowledge. A systems level interpretation of potential brain effects is limited by the absence of translational studies that could inform about mechanisms of hormonal contraceptive effects on the brain. This is an important point to appreciate, because answering this question not only requires rigorous replication of existing study findings, but more so requires intentional design of translational studies.
4.1.1. MRI studies
Although much too early to draw conclusions, across MRI studies, we found a general pattern implicating hormone effects in the structure and function of the frontal lobe (e.g., inferior frontal cortex, inferior frontal gyrus, middle frontal gyrus) and limbic system (e.g., amygdala, hippocampus, parahippocampus). Both the structural and functional results are consistent with the distribution of estrogen, progesterone, and androgen receptors in the brain, with predominant expression in areas including frontal cortex, hippocampus, and amygdala (Hajszan et al., 2008; Rettberg et al., 2014). Of course, further studies are needed to test this hypothesis. It remains unproven, for example, that hormonal contraceptives affect the brain through action on endogenous sex hormones receptors in the brain. Additionally, although beyond the scope of this systematic review, it is relevant that numerous studies have identified a variable association of hormonal contraceptives with mood effects, which also implicate frontal lobe and limbic function (Robakis et al., 2019).
Variability in terminology may obscure salience of findings reported across studies. For example, the central executive network studied in (Sharma, Fang, et al., 2020) is synonymous with the central control network studied in (Petersen et al., 2014), and includes the middle frontal gyri identified as an area of structural difference in (Pletzer et al., 2010). Similarly, the default mode network studied in (Wen et al., 2021) includes the superior frontal gyri which were identified in (Pletzer et al., 2010). Conversely, variability in region naming necessitates caution in drawing cross-study conclusions regarding similarly named regions. Reporting of standardized coordinates for imaging effects is an approach that could facilitate more reliable and precise synthesis across studies.
Another limitation of MRI studies derives from the use of region-specific analyses which limit the specificity of findings, since areas not examined cannot be characterized. Studies of hypothalamus and pituitary (Chen et al., 2021), or hippocampus and basal ganglia (Pletzer et al., 2019), for example, although motivated by specific hypotheses, are limited in the extent to which they can be integrated with the broader literature. Additionally, the task-based fMRI studies we identified, by definition, employed paradigms which target specific domains of function canonically associated with limbic system and frontal lobe function. Conversely, resting-state fMRI, for which there are only 6 studies, may not target relevant brain functions. It is thus possible OCP effects on other domains of brain function remain unrevealed.
4.1.2. Neurotransmitter and neuropeptide studies
Animal neurotransmitter and neuropeptide studies suggest dopaminergic effects (8 studies), GABAergic pathway effects (4 studies), and cholinergic effects (3 studies). While these studies are not sufficient in number to draw definitive conclusions and vary in the directionality of reported effects, they do agree with the one MRS study in humans, which suggests GABAergic pathway effects as well (De Bondt, De Belder, et al., 2015).
In principle, animal biochemical studies which aim to probe neuropeptide/neurotransmitter effects could help validate and expand the results of imaging studies, by identifying biochemical mechanisms which may underly the imaging associations. A major limitation of all these studies, however, is that they reported total brain expression and provide no regional information. This factor (see further below) is a major limitation on the utility of this mechanistic information. To realize its utility, combined assessment of regional effects in both human and animal studies is needed.
4.1.3-. EEG studies
EEG studies have shown conflicting evidence for hormonal contraceptive effects on the brain. Two resting-state EEG studies suggest decreases in mean alpha frequency (Creutzfeldt et al., 1976; Wuttke et al., 1975), which is regarded as a measure of resting-wakefulness and has been associated with mood disorders (Kropotov, 2016). However, the 5 out of the 7 resting EEG studies showed no statistically significant effects, drawing into question the existence of an effect and leaving resting EEG effects indeterminate.
Sleep is a key determinant of quality of life. Sleep is also attributed to limbic system function; REM sleep in particular has been associated with memory consolidation (related to limbic function) (Blumberg et al., 2020) and sleep spindles are thought to arise from thalamic nuclei (in the limbic system) (Bandarabadi et al., 2020). EEG sleep studies, although there exists only three, suggest OCP-related sleep effects, including less REM sleep related to OCP use, and also differences in sleep spindle density, which are relevant to limbic system function (Goldstein & Walker, 2014). A cautious approach to these findings is warranted as only three EEG sleep studies have been published, of which one reported no significant effects. Therefore, more focused studies in this area can benefit from integration with structural and functional MRI, to further substantiate the role of limbic brain structures in OCP-related effects on sleep.
OCP-related ERP results related to “pleasant,” “neutral,” and “unpleasant” images also implicate frontal lobe and limbic system (Monciunskaite et al., 2019). However, this single study is insufficient to confirm the association.
Again, number and variability of methodology between studies precludes definitive conclusions, but we can identify interesting consistency of results from EEG studies with the MRI findings, which point to frontal lobe and limbic system, regions with high expression of sex hormone receptors.
Notably, many of the EEG studies were conducted in the 1960s-80s (Becker et al., 1980; Creutzfeldt et al., 1976; Fleck & Polich, 1988; Gautray et al., 1974; Matsumoto et al., 1966; Mukherjee et al., 1978; Sugerman et al., 1970; Wuttke et al., 1975). While the hypotheses tested, and recording techniques used are still relevant, hormonal contraceptive dosages have since changed. For example, 0.5mg norgestrel and 0.05mg ethinyl-estradiol, administered to the experimental group in (Becker et al., 1980; Wuttke et al., 1975) and sold at the time as Ogestrel and Ovral, have since been discontinued by the manufacturer in the US, and are no longer available for sale (GoodRx, 2022). While lower dosages of norgestrel and ethinyl-estradiol are still on the market, the relevance of results based on obsolete dosing is questionable.
4.2. Limitations related to study designs
An important limitation of published studies relates to inherent variability within experimental groups as well as the nature and potential contamination of control groups, if “naturally cycling” women have prior hormonal contraceptive exposure. Brønnick and colleagues (Brønnick et al., 2020) noted these issues related to study participant variability as well as variability in hormone formulation across groups, variability in hormonal contraceptive exposure and prior hormonal contraceptive exposure. These issues remain prevalent among the 18 human studies we have included beyond those discussed by the most recent reviews on this topic [e.g., (Brønnick et al., 2020)]. Additionally, many women have years of prior hormonal contraceptive exposure, potentially to multiple hormonal contraceptive preparations, with both duration and recency of exposure varying across individuals within a single study cohort. While these factors are potential areas of concern for confounding. We also do not yet know there are significant issues related to, for example, prior OCP exposure. To confirm the existence of effects and ascribe them specifically to OCPs, however, requires careful approaches that can control for these sources of variance. Human studies that standardize or control for specific formulations of hormonal contraceptive would offer a much clearer characterization of effects of hormonal contraceptives on the brain and especially highlight different effects across features of hormonal contraceptive preparations, such as progestin-only, combination and androgenicity. In this regard, the increase in randomized (6 studies, 2 discussed in prior reviews) and within-participant crossover (2 studies) study designs is a positive move towards addressing exposure variability. Nonetheless, no study has yet attempted to characterize prior hormonal contraceptive exposure, which could possibly obscure the exposure related to the intervention administered in a prospective trial. For example, effects of hormonal contraceptive administration during a prospective study could be blunted where experimental and control groups have significant prior exposure to hormonal contraceptives. In assessment of short-term effects of hormonal contraceptives, randomization might overcome bias due to prior exposure, to a greater or lesser extent. In the search to understand potential persistent and long-term effects of hormonal contraceptives on the brain, however, the nature and extent of lifetime exposure might become. Future studies that assess hormonal contraceptive-naïve women, begin study after a washout period or include longer term follow-up are needed to determine the existence and nature of long-term hormonal contraceptive effects. Animal studies offer a potentially powerful approach to characterize hormonal contraceptive effects in the absence of prior exposure (see further, below).
Additionally, while some studies did account for phase of menstrual cycle in the naturally cycling control group by imaging participants at multiple timepoints during the menstrual cycle, not all studies did. Though it is unlikely that so many studies have reported statistically significant group differences that stem solely from confounding by natural hormone levels in controls, it is important to account for phase of menstrual cycle in the control group. Even by attempting to account for phase of menstrual cycle, there remains an open question whether hormonal contraceptives directly affect the brain or alter endogenous hormone production, which in turn affect the brain. It is possible that both pathways exist. As we interpret existing studies, it is important to keep in mind this limitation of study design. We discuss ways to identify the underlying mechanisms related to hormonal contraceptive effects on the brain in later sections.
As noted in previous reviews, hormonal contraceptive formulation, including the androgenicity of the progestin component, may modify structural and functional brain effects in ways that remain incompletely characterized (Brønnick et al., 2020; Taylor et al., 2021). For example, a study detected differential effects of lower and higher androgenicity OCPs by defining experimental groups based on OCP preparation (Pletzer et al., 2015). However, a later study, found no significant effect by androgenicity of OCP on hippocampal and basal ganglia grey matter volumes (Pletzer et al., 2019). Other studies reported on either higher or lower androgenicity OCPs without comparison across preparation [e.g., (Arnoni-Bauer et al., 2017; Petersen et al., 2019; Petersen et al., 2021)]. Evidence on how OCP formulation may influence effects is thus limited. In studies that include participants using a range of OCP types, effects related to the specific preparation, such as androgenicity, may be obscured.
Adequate sample size is essential to ensure that true OCP effects are detected and to minimize risk for false inferences. Future studies should include larger samples and assess power a priori. It is worth mentioning here that studies from the same research group sometimes report data collected from overlapping participant groups. While the data and analyses from each study add to our knowledge on the topic, it further underscores the need for more research with larger participant populations and replication of findings in independent samples.
Previous reviews have called for more research on “the age of initiation” of hormonal contraceptive use and how that may affect the brain (Taylor et al., 2021). Another review (Brønnick et al., 2020) also points out the lack of inclusion of adolescents in hormonal contraceptive studies. Maraceková et al.(Marecková et al., 2014), included adult and adolescent experimental groups, finding increased activity in the left fusiform face area of adolescents, but not among adults. Adolescents are beyond the scope of our review. However, one study classified adults by age of OCP initiation, finding higher connectivity in the salience network in the pubertal-initiation OCP use group compared to adult initiation (Sharma, Fang, et al., 2020). While this one paper is insufficient to support definitive conclusions, it underscores the need for further research.
4.3. Direction of Future Research
To fully determine if and how hormonal contraceptives affect the brain, we must be able to synthesize results across studies and further proceed to understand the mechanisms that would lead to such an effect. While individual imaging studies may document an association between brain structure and function with hormonal contraceptive use, further research needs to address how these effects can be brought about by hormonal contraceptives. It is possible that hormonal contraceptives, which comprise synthetic sex hormones, directly bind to sex steroid receptors in the brain. It is also possible that hormonal contraceptives affect production of endogenous sex hormones, which leads to effects in the brain. It is not clear if and how neurotransmitter production and release may be affected and to what extent this may influence brain structure and function. Our systematic review identifies all human and animal imaging, EEG, and biochemical studies on this topic, but the evidence reported, while suggesting an effect of hormonal contraceptives on the brain (above), cannot confirm the existence of the effect, much less characterize its underlying mechanisms. To address the complicated but important questions on the existence and nature of hormonal contraceptives effects on the brain, it is necessary to conduct more comprehensive translational research in animals. Integration of existing human neuroimaging and animal biochemical studies is extremely limited due to the approaches that have been applied (ie. Animal biochemical studies and human imaging studies). Future studies need to bridge the knowledge gap between human and animal studies. We propose accomplishing this in the following ways:
4.4.1-. Animal neuroimaging studies, specifically in primates
Translational neuroimaging studies, which can apply similar methods to animals and humans, would allow us to place mechanistic effects from animal studies into a human anatomical and functional context. Currently, animal neuroimaging studies do not exist. The lack of studies which probe hormonal contraceptive effects in animal brains using structural and functional MRI, MRS or PET is an important gap in knowledge. Addressing this knowledge gap is a promising avenue for future research, as animal studies present fewer logistical barriers related to study design (e.g., randomization, blinding, OCP formulation, prior OCP exposure and loss to follow-up in longitudinal studies) than human studies.
Of course, the existence of corroborating results in animal studies is not guaranteed, especially in rodent test subjects, which were the primary animal model used in the studies we identified. A promising avenue of research is to apply imaging techniques in the primate brain, which is more similar to the human (Herculano-Houzel, 2009; Semendeferi & Damasio, 2000), during exposure to hormonal contraceptives. Once similarities in hormonal contraceptive effects in imaging of humans and an appropriate animal model are established, more invasive biochemical results from animals can be related to the human condition. Noninvasive imaging, such as MRI, can thus serve as a translational bridge to facilitate the characterization of effects in humans and relate them to more direct mechanistic techniques that can only be performed in animals.
4.4.2-. Animal biochemical studies with more spatial specificity
Biochemical studies in animals, which have been limited to whole brain measurement of peptide expression, suggest potential roles of neurotransmitter systems in OCP effects. However, these studies lack any information about spatial localization of effects in the brain, which is a prerequisite to drawing parallels between structural and functional imaging and biochemical studies. No animal studies have employed imaging, which can be utilized, as above, to identify similar findings across species, but can also be used guide localized sampling for biochemical assays and permit inferences about the brain regions affected by hormone exposure.
Further work in the biochemical studies in animals may require techniques with higher spatial resolution. At a minimum, dissection of structures before high-performance liquid chromatography can provide some region-specific information on peptide expression. There are however, in vivo methods; for example, multi-photon imaging techniques for in vivo quantification of neurotransmitters such as serotonin and dopamine, with the spatial specificity to the level of vesicles (Semendeferi & Damasio, 2000). This technique to quantify serotonin has been applied in rats (Maiti et al., 1997), validated with other techniques requiring dissection (Williams et al., 1999) and proven effective for large areas and whole-slice imaging (Kaushalya et al., 2008). More recently, imaging of dopamine in rat slices has also been accomplished (Sarkar et al., 2014). Identifying spatially localized biochemical effects is needed to understand human and animal hormone effects.
Neither human or animal studies examined estrogen, progesterone or testosterone receptor expression, or binding by hormonal contraceptives in the brain. This is an important area to focus research. While hormonal contraceptives likely bind to sex steroid receptors in the brain to mediate the structural, functional, and biochemical effects reported in the literature, the extent hormonal contraceptives in fact bind to brain sex steroid receptors in human, and how binding may induce downstream effects, is unknown. Moreover, how potential hormonal contraceptive effects might be modulated by endogenous sex steroids is unexplored. Endogenous estrogens (e.g., estrone, estradiol and estriol) are different from synthetic estrogen analogs comprising OCPs, and endogenous progesterone is different from synthetic progesterone analogs. Therefore, we must not assume hormonal contraceptives modulate brain structure and function in the same way or by the same mechanisms as endogenous hormones. It is technically feasible to use light sheet microscopy to perform whole-brain, ex vivo imaging of fluorescently-tagged hormonal contraceptives bound to estrogen and progesterone receptors in the animal brain (Ueda et al., 2020). Animal studies which quantify expression of and binding to brain sex steroid receptors in hormonal contraceptive users compared to non-users, considering variability in hormonal contraceptive formulation, would help advance discovery of hormonal contraceptive mechanisms in the brain.
4.4.3-. Human imaging modalities to better investigate mechanisms underlying hormonal contraceptive effects
In humans, it would be especially useful to further study the dopaminergic system, relevant to mood, which is, to date, the most implicated neurotransmitter system in animal OCP studies. Studies employing PET (Doot et al., 2019) or dopamine transporter single-photon emission computed tomography (DAT-SPECT) (Suwijn et al., 2015) to study dopamine function could be leveraged to bridge human and animal studies. To date, the only human PET study found no difference in D-amphetamine-induced dopamine release between naturally cycling women and OCP users (Smith et al., 2019). A single result such as this, however, cannot be taken as confirming a null effect.
Studies that apply multiple modalities to assess the same groups of individuals, utilizing structural MRI, fMRI, biochemical assays, EEG and PET/MRS, offer an opportunity to integrate and clarify findings. However, we identified, for example, only three studies (Lisofsky et al., 2016; Marecková et al., 2014; Sharma, Smith, et al., 2020) that used both structural and functional MRI. The amygdala, for example, is affected in both structural and functional studies (Engman et al., 2018; Lisofsky et al., 2016; Merz et al., 2013; Petersen & Cahill, 2015), and serotonin and GABA regulate neurotransmission in the amygdala (Bocchio et al., 2016; Jie et al., 2018). Furthermore, PET/MRS studies have suggested changes in these neurotransmitters related to OCPs (De Bondt, De Belder, et al., 2015; Larsen et al., 2020). Future studies can apply multiple imaging modalities to characterize effects of hormonal contraceptives on the brain more comprehensively and gain insight into underlying mechanisms.
Conclusion
After more than 60 years of hormonal contraceptive use worldwide, modest evidence points towards brain effects of OCPs. However, much remains to be replicated, discovered, and understood about the nature, mechanisms, implications, and persistence of brain effects of OCPs in women. Multimodal, longitudinal, and translational studies are needed to characterize structural, and functional effects in humans and their mechanistic basis.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Allen RH (n.d.). Combined estrogen-progestin oral contraceptives: Patient selection, counseling, and use UpToDate. Retrieved March 10, 2022, from https://www.uptodate.com/contents/combined-estrogen-progestin-oral-contraceptives-patient-selection-counseling-and-use?search=hormonal+contraception+uses&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H1553807477
- Abler B, Kumpfmüller D, Grön G, Walter M, Stingl J, & Seeringer A (2013). Neural correlates of erotic stimulation under different levels of female sexual hormones. PLoS One, 8(2), e54447. 10.1371/journal.pone.0054447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algeri S, Ponzio F, Dolfini E, & Jori A (1976). Biochemical effects of treatment with oral contraceptive steroids on the dopaminergic system of the rat. Neuroendocrinology, 22(4), 343–351. 10.1159/000122643 [DOI] [PubMed] [Google Scholar]
- Allen RH (2022). Combined estrogen-progestin oral contraceptives: Patient selection, counseling, and use UpToDate. https://www.uptodate.com/contents/combined-estrogen-progestin-oral-contraceptives-patient-selection-counseling-and-use?search=birth%20control%20androgen&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H3087783510
- Arnoni-Bauer Y, Bick A, Raz N, Imbar T, Amos S, Agmon O, Marko L, Levin N, & Weiss R (2017). Is It Me or My Hormones? Neuroendocrine Activation Profiles to Visual Food Stimuli Across the Menstrual Cycle. J Clin Endocrinol Metab, 102(9), 3406–3414. 10.1210/jc.2016-3921 [DOI] [PubMed] [Google Scholar]
- Bandarabadi M, Herrera CG, Gent TC, Bassetti C, Schindler K, & Adamantidis AR (2020). A role for spindles in the onset of rapid eye movement sleep. Nature communications, 11(1), 5247–5247. 10.1038/s41467-020-19076-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay M, & Ghosh AK (1988). Effect of prolonged use of contraceptive steroid hormones on acetylcholinesterase activity in some regions of brain in rat. Neuroendocrinology Letters, 10(3), 175–182. https://www.embase.com/search/results?subaction=viewrecord&id=L18158453&from=export [Google Scholar]
- Barth C, Villringer A, & Sacher J (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci, 9, 37. 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu T, Bao P, Lerner A, Anderson L, Page K, Stanczyk F, Mishell D Jr., & Segall-Gutierrez P (2016). The Effect of Depo Medroxyprogesterone Acetate (DMPA) on Cerebral Food Motivation Centers: A Pilot Study using Functional Magnetic Resonance Imaging. Contraception, 94(4), 321–327. 10.1016/j.contraception.2016.04.011 [DOI] [PubMed] [Google Scholar]
- Becker D, Creutzfeldt OD, Schwibbe M, & Wuttke W (1980). Electrophysiological and psychological changes induced by steroid hormones in men and women. Acta Psychiatr Belg, 80(5), 674–697. [PubMed] [Google Scholar]
- Blumberg MS, Lesku JA, Libourel P-A, Schmidt MH, & Rattenborg NC (2020). What Is REM Sleep? Current biology : CB, 30(1), R38–R49. 10.1016/j.cub.2019.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, & Capogna M (2016). Serotonin, Amygdala and Fear: Assembling the Puzzle. Frontiers in neural circuits, 10, 24–24. 10.3389/fncir.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenberger M, Groschwitz RC, Kumpfmueller D, Groen G, Plener PL, & Abler B (2013). It’s all about money: oral contraception alters neural reward processing. Neuroreport, 24(17), 951–955. 10.1097/wnr.0000000000000024 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, & Nilsen J (2008). Progesterone receptors: form and function in brain. Front Neuroendocrinol, 29(2), 313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brønnick MK, Økland I, Graugaard C, & Brønnick KK (2020). The Effects of Hormonal Contraceptives on the Brain: A Systematic Review of Neuroimaging Studies. Front Psychol, 11, 556577. 10.3389/fpsyg.2020.556577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brötzner CP, Klimesch W, Doppelmayr M, Zauner A, & Kerschbaum HH (2014). Resting state alpha frequency is associated with menstrual cycle phase, estradiol and use of oral contraceptives. Brain Res, 1577(100), 36–44. 10.1016/j.brainres.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2020). Summary of U.S. Medical Eligibility Criteria of Contraceptive Use In Center for Disease Control and Prevention (CDC). [Google Scholar]
- Chaudhuri SK, Chattopadhyay RN, Maitra SK, Ray S, & Chaudhuri S (1992). Effects of progesterone on some brain neurotransmitters in intact rats. Indian J Physiol Pharmacol, 36(4), 255–258. [PubMed] [Google Scholar]
- Chen KX, Worley S, Foster H, Edasery D, Roknsharifi S, Ifrah C, & Lipton ML (2021). Oral contraceptive use is associated with smaller hypothalamic and pituitary gland volumes in healthy women: A structural MRI study. PLoS One, 16(4), e0249482. 10.1371/journal.pone.0249482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KC, Springer I, Kogler L, Turetsky B, Freiherr J, & Derntl B (2016). The influence of androstadienone during psychosocial stress is modulated by gender, trait anxiety and subjective stress: An fMRI study. Psychoneuroendocrinology, 68, 126–139. 10.1016/j.psyneuen.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Arnold PM, Becker D, Langenstein S, Tirsch W, Wilhelm H, & Wuttke W (1976). EEG changes during spontaneous and controlled menstrual cycles and their correlation with psychological performance. Electroencephalogr Clin Neurophysiol, 40(2), 113–131. 10.1016/0013-4694(76)90157-7 [DOI] [PubMed] [Google Scholar]
- Daabees TT, El-Din MM, Zeitoun R, & Makar AB (1981). Injectable and oral contraceptive steroids in relation to some neurotransmitters in the rat brain. Biochem Pharmacol, 30(12), 1581–1585. 10.1016/0006-2952(81)90384-1 [DOI] [PubMed] [Google Scholar]
- Daigle KM, Pietrzykowski MO, Waters AB, Swenson LP, & Gansler DA (2022). Central Executive Network and Executive Function in Patients With Alzheimer’s Disease and Healthy Individuals: Meta-Analysis of Structural and Functional MRI. J Neuropsychiatry Clin Neurosci, appineuropsych20110279. 10.1176/appi.neuropsych.20110279 [DOI] [PubMed] [Google Scholar]
- Daniels KAJC (2018). Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017 https://www.cdc.gov/nchs/data/databriefs/db327-h.pdf [PubMed]
- Daniels KM, W. D.; Jones J (2013). Contraceptive Methods Women Have Ever Used: United States, 1982–2010. National Health Statistics Reports https://www.cdc.gov/nchs/data/nhsr/nhsr062.pdf [PubMed]
- De Bondt T, De Belder F, Vanhevel F, Jacquemyn Y, & Parizel PM (2015). Prefrontal GABA concentration changes in women-Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res, 1597, 129–138. 10.1016/j.brainres.2014.11.051 [DOI] [PubMed] [Google Scholar]
- De Bondt T, Jacquemyn Y, Van Hecke W, Sijbers J, Sunaert S, & Parizel PM (2013). Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain Res, 1530, 22–31. 10.1016/j.brainres.2013.07.034 [DOI] [PubMed] [Google Scholar]
- De Bondt T, Pullens P, Van Hecke W, Jacquemyn Y, & Parizel PM (2016). Reproducibility of hormone-driven regional grey matter volume changes in women using SPM8 and SPM12. Brain Struct Funct, 221(9), 4631–4641. 10.1007/s00429-016-1193-1 [DOI] [PubMed] [Google Scholar]
- De Bondt T, Smeets D, Pullens P, Van Hecke W, Jacquemyn Y, & Parizel PM (2015). Stability of resting state networks in the female brain during hormonal changes and their relation to premenstrual symptoms. Brain Res, 1624, 275–285. 10.1016/j.brainres.2015.07.045 [DOI] [PubMed] [Google Scholar]
- De Bondt T, Van Hecke W, Veraart J, Leemans A, Sijbers J, Sunaert S, Jacquemyn Y, & Parizel PM (2013). Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. Eur Radiol, 23(1), 57–64. 10.1007/s00330-012-2572-5 [DOI] [PubMed] [Google Scholar]
- Dey CD, Das PC, Das K, Patra PB, & Dasgupta S (1991). Biogenic amines and ascorbic acid in rat brain following steroid contraceptive treatment. Exp Clin Endocrinol, 97(1), 103–106. 10.1055/s-0029-1211047 [DOI] [PubMed] [Google Scholar]
- Dinehart E, Lathi RB, & Aghajanova L (2020). Levonorgestrel IUD: is there a long-lasting effect on return to fertility? Journal of Assisted Reproduction and Genetics, 37(1), 45–52. 10.1007/s10815-019-01624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doot RK, Dubroff JG, Labban KJ, & Mach RH (2019). Selectivity of probes for PET imaging of dopamine D3 receptors. Neurosci Lett, 691, 18–25. 10.1016/j.neulet.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Nasseri P, Yavari F, Mokri A, & Monterosso J (2016). Chapter 7 - Neuroscience of drug craving for addiction medicine: From circuits to therapies. In Ekhtiari H & Paulus M (Eds.), Progress in Brain Research (Vol. 223, pp. 115–141). Elsevier. 10.1016/bs.pbr.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Engman J, Sundström Poromaa I, Moby L, Wikström J, Fredrikson M, & Gingnell M (2018). Hormonal Cycle and Contraceptive Effects on Amygdala and Salience Resting-State Networks in Women with Previous Affective Side Effects on the Pill. Neuropsychopharmacology, 43(3), 555–563. 10.1038/npp.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck KM, & Polich J (1988). P300 and the menstrual cycle. Electroencephalogr Clin Neurophysiol, 71(2), 157–160. 10.1016/0168-5597(88)90076-7 [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Erritzoe D, Madsen J, Paulson OB, & Knudsen GM (2009). Gender and the use of hormonal contraception in women are not associated with cerebral cortical 5-HT 2A receptor binding. Neuroscience, 163(2), 640–645. 10.1016/j.neuroscience.2009.06.052 [DOI] [PubMed] [Google Scholar]
- Gautray JP, Eberhard A, Jalivet A, & Leygonie G (1974). Electroencephalographic changes in women treated with contraceptive steroids. Basic Life Sci, 4(Part a), 385–397. 10.1007/978-1-4684-2889-6_25 [DOI] [PubMed] [Google Scholar]
- Gingnell M, Bannbers E, Engman J, Frick A, Moby L, Wikström J, & Sundström-Poromaa I (2016). The effect of combined hormonal contraceptives use on brain reactivity during response inhibition. Eur J Contracept Reprod Health Care, 21(2), 150–157. 10.3109/13625187.2015.1077381 [DOI] [PubMed] [Google Scholar]
- Gingnell M, Engman J, Frick A, Moby L, Wikström J, Fredrikson M, & Sundström-Poromaa I (2013). Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill--a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology, 38(7), 1133–1144. 10.1016/j.psyneuen.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Goldstein AN, & Walker MP (2014). The role of sleep in emotional brain function. Annual review of clinical psychology, 10, 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GoodRx. (2022). Ogestrel, (norgestrel / ethinyl estradiol) monophasic [Google Scholar]
- Guennoun R (2020). Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int J Mol Sci, 21(15). 10.3390/ijms21155271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology, 35(1), 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, & Leranth C (2008). Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav, 53(5), 638–646. 10.1016/j.yhbeh.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S (2009). The human brain in numbers: a linearly scaled-up primate brain [Review]. Frontiers in Human Neuroscience, 3. 10.3389/neuro.09.031.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung J, Noack H, Kogler L, & Derntl B (2019). Exploring the fMRI based neural correlates of the dot probe task and its modulation by sex and body odor. Psychoneuroendocrinology, 99, 87–96. 10.1016/j.psyneuen.2018.08.036 [DOI] [PubMed] [Google Scholar]
- Horvath S, Schreiber CA, & Sonalkar S (2000). Contraception. In Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, & Wilson DP (Eds.), Endotext MDText.com, Inc.
- Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, & Milad MR (2015). Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry, 15, 295. 10.1186/s12888-015-0673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam F, Hasan M, Rizvi R, & Osman SM (1980). Microanalysis of lipids in discrete brain areas of the rabbit following intramuscular administration of steroid contraceptive. Contraception, 21(4), 434–442. 10.1016/s0010-7824(80)80020-5 [DOI] [PubMed] [Google Scholar]
- Jie F, Yin G, Yang W, Yang M, Gao S, Lv J, & Li B (2018). Stress in Regulation of GABA Amygdala System and Relevance to Neuropsychiatric Diseases. Frontiers in Neuroscience, 12, 562–562. 10.3389/fnins.2018.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori A, & Dolfini E (1976). Modifications of striatal dopamine levels by steroid contraceptive drugs in mice and rats. Neuroendocrinology, 21(1), 74–78. 10.1159/000122513 [DOI] [PubMed] [Google Scholar]
- Kao A (2000). History of oral contraception. Virtual Mentor, 2(6). 10.1001/virtualmentor.2000.2.6.dykn1-0006 [DOI] [PubMed] [Google Scholar]
- Kaushalya SK, Nag S, Ghosh H, Arumugam S, & Maiti S (2008). A high-resolution large area serotonin map of a live rat brain section. Neuroreport, 19(7), 717–721. 10.1097/WNR.0b013e3282fd6946 [DOI] [PubMed] [Google Scholar]
- Kropotov JD (2016). Chapter 2.2 - Alpha Rhythms. In Kropotov JD (Ed.), Functional Neuromarkers for Psychiatry (pp. 89–105). Academic Press. 10.1016/B978-0-12-410513-3.00008-5 [DOI] [Google Scholar]
- Ladinsky H, Consolo S, Bianchi S, Peri G, & Garattini S (1976). Lack of influence of the phase of estrus cycle or treatment with steroid contraceptive drugs on cholinergic parameters in mouse and rat brain. Pharmacology, 14(3), 232–239. 10.1159/000136600 [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, & Fox PT (2011). Behavioral Interpretations of Intrinsic Connectivity Networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SV, Köhler-Forsberg K, Dam VH, Poulsen AS, Svarer C, Jensen PS, Knudsen GM, Fisher PM, Ozenne B, & Frokjaer VG (2020). Oral contraceptives and the serotonin 4 receptor: a molecular brain imaging study in healthy women. Acta Psychiatr Scand, 142(4), 294–306. 10.1111/acps.13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisofsky N, Riediger M, Gallinat J, Lindenberger U, & Kühn S (2016). Hormonal contraceptive use is associated with neural and affective changes in healthy young women. Neuroimage, 134, 597–606. 10.1016/j.neuroimage.2016.04.042 [DOI] [PubMed] [Google Scholar]
- Maiti S, Shear JB, Williams RM, Zipfel WR, & Webb WW (1997). Measuring serotonin distribution in live cells with three-photon excitation. Science, 275(5299), 530–532. 10.1126/science.275.5299.530 [DOI] [PubMed] [Google Scholar]
- Marchi M, & Cugurra F (1974). The effect of long-term oral treatment of rats with contraceptive steroids on tissue MAO. Eur J Pharmacol, 25(3), 407–410. 10.1016/0014-2999(74)90271-4 [DOI] [PubMed] [Google Scholar]
- Marecková K, Perrin JS, Nawaz Khan I, Lawrence C, Dickie E, McQuiggan DA, & Paus T (2014). Hormonal contraceptives, menstrual cycle and brain response to faces. Soc Cogn Affect Neurosci, 9(2), 191–200. 10.1093/scan/nss128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Sato I, Ito T, & Matsuoka A (1966). Electroencephalographic changes during long term treatment with oral contraceptives. Int J Fertil, 11(2), 195–204. [PubMed] [Google Scholar]
- McEwen BS, & Milner TA (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res, 95(1–2), 24–39. 10.1002/jnr.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Stark R, Vaitl D, Tabbert K, & Wolf OT (2013). Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biol Psychol, 92(1), 82–89. 10.1016/j.biopsycho.2012.02.017 [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, & Wolf OT (2012). Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Hormones and Behavior, 62(4), 531–538. 10.1016/j.yhbeh.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Miedl SF, Wegerer M, Kerschbaum H, Blechert J, & Wilhelm FH (2018). Neural activity during traumatic film viewing is linked to endogenous estradiol and hormonal contraception. Psychoneuroendocrinology, 87, 20–26. 10.1016/j.psyneuen.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Monciunskaite R, Malden L, Lukstaite I, Ruksenas O, & Griksiene R (2019). Do oral contraceptives modulate an ERP response to affective pictures? Biol Psychol, 148, 107767. 10.1016/j.biopsycho.2019.107767 [DOI] [PubMed] [Google Scholar]
- Mukherjee C, Dey CD, & Deb C (1978). Effect of voldys, an oral contraceptive on the electrical activity of rat brain and heart. Curr Sci, 47(17), 614–616. [PubMed] [Google Scholar]
- Nguyen T-V, Ducharme S, & Karama S (2017). Effects of Sex Steroids in the Human Brain. Molecular Neurobiology, 54(9), 7507–7519. 10.1007/s12035-016-0198-3 [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, . . . Moher D (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, & Cahill L (2015). Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc Cogn Affect Neurosci, 10(9), 1266–1272. 10.1093/scan/nsv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Kearley N, Ghahremani D, Pochon JB, Fry M, Rapkin A, & London E (2019). Oral contraceptive pills reduce cortical thickness in inferior frontal gyrus. Neuropsychopharmacology, 44, 241. 10.1038/s41386-019-0546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Kearley NW, Ghahremani DG, Pochon JB, Fry ME, Rapkin AJ, & London ED (2021). Effects of oral contraceptive pills on mood and magnetic resonance imaging measures of prefrontal cortical thickness. Mol Psychiatry, 26(3), 917–926. 10.1038/s41380-020-00990-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, & Cahill L (2014). Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage, 90, 24–32. 10.1016/j.neuroimage.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamberger CP, Van Wijk HE, Kerschbaum H, Pletzer BA, Gruber G, Oberascher K, Dresler M, Hahn MA, & Hoedlmoser K (2021). Impact of menstrual cycle phase and oral contraceptives on sleep and overnight memory consolidation. Journal of Sleep Research, 30(4). 10.1111/jsr.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B (2019). Sex Hormones and Gender Role Relate to Gray Matter Volumes in Sexually Dimorphic Brain Areas. Front Neurosci, 13, 592. 10.3389/fnins.2019.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Harris T, & Hidalgo-Lopez E (2019). Previous contraceptive treatment relates to grey matter volumes in the hippocampus and basal ganglia. Sci Rep, 9(1), 11003. 10.1038/s41598-019-47446-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, & Kerschbaum HH (2010). Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res, 1348, 55–62. 10.1016/j.brainres.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, & Kerschbaum H (2015). Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Res, 1596, 108–115. 10.1016/j.brainres.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Nuerk HC, & Kerschbaum H (2014). Hormonal contraceptives masculinize brain activation patterns in the absence of behavioral changes in two numerical tasks. Brain Res, 1543, 128–142. 10.1016/j.brainres.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Ponzio F, Achilli G, & Algeri S (1977). Effect of steroid contraceptive drug treatment on the catecholamine metabolism in the guinea pig central nervous system. Med Biol, 55(4), 224–227. [PubMed] [Google Scholar]
- Porcu P, Mostallino MC, Sogliano C, Santoru F, Berretti R, & Concas A (2012). Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABA(A) receptor subunit expression and anxiety-like behavior. Pharmacol Biochem Behav, 102(2), 366–372. 10.1016/j.pbb.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Porcu P, Serra M, & Concas A (2019). The brain as a target of hormonal contraceptives: Evidence from animal studies. Front Neuroendocrinol, 55, 100799. 10.1016/j.yfrne.2019.100799 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, & Barton M (2011). The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol, 7(12), 715–726. 10.1038/nrendo.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao IM, Murthy CR, & Reddy PR (1984). Effect of contraceptive steroids on metabolism of glutamic acid & GABA in rat brain. Indian J Med Res, 80, 577–582. [PubMed] [Google Scholar]
- Rettberg JR, Yao J, & Brinton RD (2014). Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol, 35(1), 8–30. 10.1016/j.yfrne.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis T, Williams KE, Nutkiewicz L, & Rasgon NL (2019). Hormonal Contraceptives and Mood: Review of the Literature and Implications for Future Research. Current Psychiatry Reports, 21(7), 57. 10.1007/s11920-019-1034-z [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage, 45(2), 614–626. 10.1016/j.neuroimage.2008.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumberg B, Baars A, Fiebach J, Ladd ME, Forsting M, Senf W, & Gizewski ER (2010). Cycle and gender-specific cerebral activation during a verb generation task using fMRI: comparison of women in different cycle phases, under oral contraception, and men. Neurosci Res, 66(4), 366–371. 10.1016/j.neures.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Salles A (2020). Birth Control in Europe Encyclopédie d’histoire numérique de l’Europe https://ehne.fr/en/node/12236
- Sarkar B, Banerjee A, Das AK, Nag S, Kaushalya SK, Tripathy U, Shameem M, Shukla S, & Maiti S (2014). Label-free dopamine imaging in live rat brain slices. ACS Chem Neurosci, 5(5), 329–334. 10.1021/cn5000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Follesa P, Panzanelli P, Perazzini AZ, Porcu P, Sogliano C, Cherchi C, & Concas A (2007). Fluctuations in brain concentrations of neurosteroids are not associated to changes in gephyrin levels. Brain Res, 1169, 1–8. 10.1016/j.brainres.2007.06.057 [DOI] [PubMed] [Google Scholar]
- Scheele D, Plota J, Stoffel-Wagner B, Maier W, & Hurlemann R (2016). Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Soc Cogn Affect Neurosci, 11(5), 767–774. 10.1093/scan/nsv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, & Guennoun R (2014). Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol, 113, 6–39. 10.1016/j.pneurobio.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Seeley WW (2019). The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. The Journal of Neuroscience, 39(50), 9878–9882. 10.1523/jneurosci.1138-17.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, & Damasio H (2000). The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. Journal of Human Evolution, 38(2), 317–332. 10.1006/jhev.1999.0381 [DOI] [PubMed] [Google Scholar]
- Sharma R, Fang Z, Smith A, & Ismail N (2020). Oral contraceptive use, especially during puberty, alters resting state functional connectivity. Horm Behav, 126, 104849. 10.1016/j.yhbeh.2020.104849 [DOI] [PubMed] [Google Scholar]
- Sharma R, Smith SA, Boukina N, Dordari A, Mistry A, Taylor BC, Felix N, Cameron A, Fang Z, Smith A, & Ismail N (2020). Use of the birth control pill affects stress reactivity and brain structure and function. Horm Behav, 124, 104783. 10.1016/j.yhbeh.2020.104783 [DOI] [PubMed] [Google Scholar]
- Shetty KT, & Gaitonde BB (1980a). Effect of contraceptive steroids on gamma-aminobutyric acid metabolism and pyridoxal kinase activity in rat brain. Exp Neurol, 70(1), 146–154. 10.1016/0014-4886(80)90012-6 [DOI] [PubMed] [Google Scholar]
- Shetty KT, & Gaitonde BB (1980b). Effect of the chronic administration of ethinyl estradiol and norgestrel on biogenic amine(s) level and monoamine oxidase enzyme activity in rat brain. Biochem Pharmacol, 29(5), 821–825. 10.1016/0006-2952(80)90563-8 [DOI] [PubMed] [Google Scholar]
- Siegel JM (2004). The neurotransmitters of sleep. J Clin Psychiatry, 65 Suppl 16(Suppl 16), 4–7. [PMC free article] [PubMed] [Google Scholar]
- Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, & Holmes PV (2015). Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology, 62, 265–278. 10.1016/j.psyneuen.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Smith CT, Dang LC, Burgess LL, Perkins SF, San Juan MD, Smith DK, Cowan RL, Le NT, Kessler RM, Samanez-Larkin GR, & Zald DH (2019). Lack of consistent sex differences in D-amphetamine-induced dopamine release measured with [(18)F]fallypride PET. Psychopharmacology (Berl), 236(2), 581–590. 10.1007/s00213-018-5083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugerman AA, DeBruin AT, & Roth CW (1970). Quantitative EEG changes in the human menstrual cycle. Res Commun Chem Pathol Pharmacol, 1(4), 526–534. [PubMed] [Google Scholar]
- Suwijn SR, van Boheemen CJM, de Haan RJ, Tissingh G, Booij J, & de Bie RMA (2015). The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Research, 5(1), 12. 10.1186/s13550-015-0087-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, & Jacobs EG (2021). The scientific body of knowledge - Whose body does it serve? A spotlight on oral contraceptives and women’s health factors in neuroimaging. Front Neuroendocrinol, 60, 100874. 10.1016/j.yfrne.2020.100874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani GA, Vaswani KK, & Barbacci JC (1985). Effect of oral contraceptives on the rat brain and pituitary opioid peptides. Peptides, 6(3), 555–561. 10.1016/0196-9781(85)90120-2 [DOI] [PubMed] [Google Scholar]
- Tejwani GA, Vaswani KK, Barbacci JC, Richard CW 3rd, & Bianchine JR (1983). Effect of oral contraceptives on the rat brain and pituitary beta-endorphin. Life Sci, 33 Suppl 1, 519–522. 10.1016/0024-3205(83)90555-6 [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundström-Poromaa I, & Comasco E (2014). Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. Psychoneuroendocrinology, 50, 28–52. 10.1016/j.psyneuen.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Dodt HU, Osten P, Economo MN, Chandrashekar J, & Keller PJ (2020). Whole-Brain Profiling of Cells and Circuits in Mammals by Tissue Clearing and Light-Sheet Microscopy. Neuron, 106(3), 369–387. 10.1016/j.neuron.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujma P, Kristóf E, Bódizs R, Dresler M, & Genzel L (2017). Hormone and menstrual cycle effects on sleep spindle parameters in oral contraceptive users and controls. European Neuropsychopharmacology, 27, S723–S724. https://www.embase.com/search/results?subaction=viewrecord&id=L619298704&from=export [Google Scholar]
- Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, & Tracey I (2013). Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain, 154(4), 515–524. 10.1016/j.pain.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Wen Z, Hammoud MZ, Scott JC, Jimmy J, Brown L, Marin MF, Asnaani A, Gur RC, Foa EB, & Milad MR (2021). Impact of exogenous estradiol on task-based and resting-state neural signature during and after fear extinction in healthy women. Neuropsychopharmacology. 10.1038/s41386-021-01158-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Shear JB, Zipfel WR, Maiti S, & Webb WW (1999). Mucosal mast cell secretion processes imaged using three-photon microscopy of 5-hydroxytryptamine autofluorescence. Biophys J, 76(4), 1835–1846. 10.1016/s0006-3495(99)77343-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke W, Arnold P, Becker D, Creutzfeldt O, Langenstein S, & Tirsch W (1975). Circulating hormones, EEG, and performance in psychological tests of women with and without oral contraceptives. Psychoneuroendocrinology, 1(2), 141–151. 10.1016/0306-4530(75)90006-2 [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


