Abstract
Intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) are all considered “Pancreatic cystic neoplasms (PCNs)” and show a varying risk of developing into pancreatic ductal adenocarcinoma (PDAC). These lesions display different molecular characteristics, mutations, and clinical manifestations. A lack of detailed understanding of PCN subtype characteristics and their molecular mechanisms limits the development of efficient diagnostic tools and therapeutic strategies for these lesions. Proper in vivo mouse models that mimic human PCNs are also needed to study the molecular mechanisms and for therapeutic testing. A comprehensive understanding of the current status of PCN biology, mechanisms, current diagnostic methods, and therapies will help in the early detection and proper management of patients with these lesions and PDAC. This review aims to describe all these aspects of PCNs, specifically IPMNs, by describing the future perspectives.
Keywords: Pancreatic Ductal Adenocarcinoma, Pancreatic Cancer, Pancreatic precursor lesions, Cystic lesions, Cystic Neoplasm, Pancreatic Intraductal papillary Mucinous Neoplasm, Mucinous Cystic Neoplasm
Introduction
Pancreatic cancer is presently the third highest cause of cancer death in the United States and is expected to rise to second place by 2030 [1]. While it holds the 7th position worldwide, the incidence, prevalence, and mortality are even higher in countries with a high human development index (HDI) compared to low HDI [1, 2]. Pancreatic Ductal Adenocarcinoma (PDAC) accounts for almost 90% of pancreatic cancers and is a highly lethal and aggressive cancer with a 5-year survival rate of less than 10% [3]. The higher lethality and mortality of PDAC are due to its late diagnosis, at which point it is metastasized and has limited therapeutic options [4].
Moreover, PDAC is also resistant to available therapies contributing to its poor prognosis. Henceforth, the early diagnosis of this disease could facilitate better therapeutic strategies and improve the overall survival outcome of PDAC patients. Like other epithelial-type tumors, PDAC also arises from precancerous lesions. There are three central pancreatic precursor lesions (PPLs), namely, pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN) that progresses to the PDAC [5, 6]. IPMN and MCN are also pancreatic cystic lesions and have higher malignancy potential than other cystic lesions [7]. With advancements in imaging technologies, the frequency of incidental detection of these cystic lesions is rapidly increasing. Although the exact period for the progression of these lesions to PDAC is yet unknown, it does provide a window of opportunity for early diagnosis and a better prognosis.
Pancreatic Precursor Lesions
PanINs are the most common PPL to develop PDAC and have been well characterized. They are defined as asymptomatic microscopic lesions (<5mm) that arise from the interlobular pancreatic ducts [8]. These lesions are more prevalent in the head of the pancreas than in the body or tail [9]. PanINs are classed as low-grade (PanIN-1, PanIN-2) or high-grade (PanIN-3, PanIN-4) based on the degree of dysplasia (PanIN-3). Because these lesions are minuscule and usually asymptomatic, they present difficulties detecting them with current imaging modalities and diagnosing them until later stages. PanIN lesions are higher in the male and older populations, mostly found in people over the age of 65 and the prevalence increases with age [9]. The key driver genes like Kras, CDKN2A, TP53, and SMAD4 aids in the initiation and progression of PanIN lesions to PDAC[10]. Moreover, mucins like MUC1, MUC3, MUC4, and MUC5AC are also abundant in these lesions and PDAC [10]. Compared to PanIN, IPMN and MCN are detected at earlier stages, allowing the advantage of preventing them from developing the lethal disease and improving patient survival outcomes. The key characteristics and differences of these precursor lesions are also discussed in Figure 1.
Figure 1: Comparison of Pancreatic Precursor lesions.
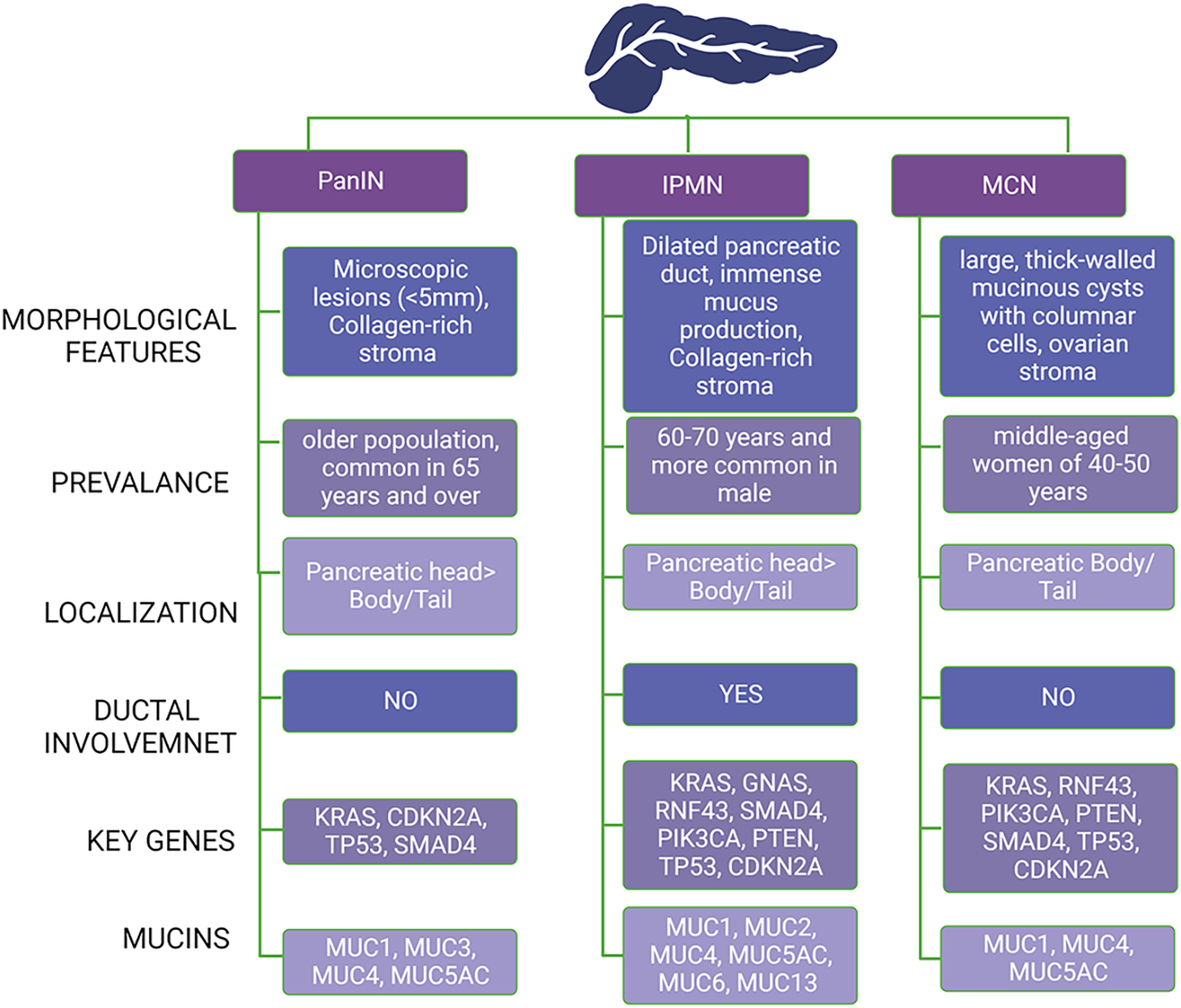
Abbreviation: PanIN: pancreatic intraepithelial neoplasia, IPMN: intraductal papillary mucinous neoplasm, MCN: mucinous cystic neoplasm, MUC: Mucin.
Mucinous Cystic Neoplasms (MCNs) are large, thick-walled mucinous cysts with columnar cells representing 2–5% of all exocrine pancreatic tumors [11]. MCN is caused by mutations in genes such as Kras, RNF43, TP53, PIK3CA, PTEN, CDKN2A, and SMAD4 [12]. MCNs are mediated by mucins such as MUC1, MUC4, and MUC5AC [13] MCNs are located in the body/tail of the pancreas and are more prevalent in middle-aged women aged 40–50 years than in males [14]. WHO reclassified MCNs based on the degree of epithelial dysplasia as MCN, low grade (low and intermediate-grade dysplasia in WHO-2010), MCN, high grade, and MCN with invasive carcinoma [15]. The significant difference between IPMN and MCN is the communication with the pancreatic duct in IPMN, unlike MCN. Furthermore, MCN has a unique feature, “ovarian stroma,” unlike the collagen-rich stroma of PanIN and IPMN. Hence, the lack of interaction with the pancreatic ductal system allows for the accurate diagnosis of MCNs compared to IPMN [16, 17]. Although MCNs have a higher potential for malignancy, especially in multilocular lesions with capillary projections and mural nodes, the diagnosis and management criteria for MCNs are more explicit and hence less controversial than IPMNs [11].
IPMN is defined as pancreatic duct dilation caused by mucus production from the epithelium. Key genes implicated in the formation and progression of IPMNs include KRAS, GNAS, RNF43, SMAD4, CDKN2A, TP53, PIK3CA, and PTEN[12]. Several mucins, including MUC1, MUC2, MUC4, MUC5AC, MUC6, and MUC13, have also been identified as possible biomarkers for IPMN [13]. IPMN accounts for 20–40% of all pancreatic cystic tumors detected radiographically and progressed to PDAC [18]. Of all the pancreatic cysts detected, 24–82% are identified as IPMN and harbor malignant potential. The prevalence of IPMN is estimated to be 3–6% in the general population and more than 10% in older adults [19, 20]. However, not everyone gets screened for pancreatic cancer, leaving all the possible cases of IPMN and other PPLs unaccounted. In addition, the risk of developing PDAC at a distance from the IPMN is increased, with a 5-year incidence reported in approximately 7% of patients under active surveillance [21, 22]. IPMNs are categorized into subgroups based on their location in the pancreatic duct, histological features, and degree of dysplasia. There are three types of IPMN based on the site of origin; main-duct IPMN (MD-IMPN), branch-duct IPMN (BD-IPMN), and mixed-duct IPMN (MX-IPMN), four groups based on histological features; gastric, intestinal, pancreatobiliary, and oncocytic, three groups based on the degree of dysplasia; low-grade IPMN, high-grade IPMN, and invasive carcinoma IPMN (IPMC) [23]. Initially, IPMN was classified into four groups based on the degree of dysplasia and had a different group for intermediate-grade IPMN. However, now both low and intermediate-grade IPMN are considered low-grade IPMN.
Characteristics of IPMN Subtypes
IPMN subtypes like Intestinal, pancreatobiliary, and oncocytic involve the main pancreatic duct, but the gastric subtype is found in branch ducts. Two distinct types of invasive carcinomas, tubular and colloid, are developed when IPMNs progress through the different dysplasia grades and become invasive [10, 24]. Intestinal IPMNs develop colloid carcinomas, while gastric and pancreatobiliary IPMNs develop tubular carcinomas [25]. BD-IPMN and gastric-type IPMN are the most common IPMN and pancreatic cysts but have less malignant potential than MD-IPMN. IPMN subtypes have distinct histological characteristics, including the following: The intestinal type of IPMN has villous papillae with cigar-shaped nuclei, basophilic cytoplasm, and mucin droplets, similar to an intestinal villous neoplasm. In addition, pancreaticobiliary -types have fern-like complex papillae composed of cells with atypical nuclei and basophilic cytoplasm; in contrast, oncocytic -types have thick phylloid papillae made up of eosinophilic cells with round enlarged cores and a loss of polarity [26]. Table 1 shows different histological subtypes of IPMN [27–29].
Table 1:
Characteristics and Correlation of IPMN subtypes
| IPMN Histological Subtype | Features | Ductal Involvement/ IPMN Type | Frequency | Atypia | Prognosis | Carcinoma Type |
|---|---|---|---|---|---|---|
| Gastric | Gastric Foveolae | Branch Duct | ~47% | Low-grade | Favorable | Tubular carcinomas |
| Pancreatobiliary | Fern-like complex papillae | Main Duct | ~33% | High-grade | Poor | Tubular carcinomas |
| Intestinal | Intestinal Villous Neoplasm | Main Duct | ~35% | High-grade | Favorable | Colloid carcinoma |
| Oncocytic | phylloid papillae | Main Duct | ~5% | High-grade | Poor | Oncocytic carcinoma |
Risk Factors for the IPMN
The lack of definite symptoms for PDAC, whether caused by PanIN or cystic neoplasms such as IPMN and MCN, makes early management and better patient outcomes for PDAC difficult. However, focusing on the risk factors associated with PC could be a supplementary route for tackling this deadly disease. Several risk factors are associated with PDAC, mainly categorized as modifiable and unmodifiable factors [30]. Mutations in numerous genes have been linked to conditions such as breast and ovarian cancer syndrome (HBOC), familial adenomatous polyposis (FAP), Peutz-Jeghers syndrome (PJS), familial atypical multiple mole melanoma syndromes (FAMMM), Lynch syndrome (HNPCC), cystic fibrosis (CF), and hereditary pancreatitis (HP) [30, 31]. Among these, PJS (132-fold), HP (52-fold), and CF (31.5-fold) have a very high risk of developing PDAC [32]. Moreover, modifiable factors, also known as environmental factors like smoking, alcohol consumption, obesity, diabetes, chronic pancreatitis, abdominal injury, and surgeries, contribute to the development of PDAC. The risk increases by 2–3 times for smoking, accounting for 25–30% of cases [31, 33], ~2 times for diabetes [31], ~1.5 for gastrointestinal injury and surgeries [34, 35], and ~1.3 for alcohol consumption of 3 or more drinks per day [31, 36]. Nevertheless, age, race, and gender are risk factors for PDAC [30, 32]. PC is more common in men compared to women[37]. The incidence and diagnosis of PDAC are higher in African Americans than in Latinos, Caucasians, and Asian Americans in the U.S [37, 38]. The average age for PDAC diagnosis is 71 years, with some developing before 60 and a few before 45 [39].
Several studies report that the incidence of IPMN is 2–4-fold higher than PC among people with a family history of PDAC [40–42]. A multicenter study that found environmental factors, family history, and medical history in 390 IPMN patients and matched controls discovered that diabetes (13.6 percent), diabetic patients with insulin use (4.9 percent), CP (3.1 percent), and a history of PDAC (5.4 percent) and peptic ulcer (7.2 percent) are risk factors for IPMN [43]. A case-controlled study examined the prevalence of IPMN in patients with extrapancreatic cancers (EPC) and a family history of cancer. It concluded that EPC was found in 16.8% of IPMN patients, 70% of which preceded [44]. Another retrospective analysis of IPMN patients with familial cancer history compared with PDAC patients showed a higher rate of extrapancreatic malignancies and cancer in families in IPMN patients [45]. Genetic and hereditary factors like HB, FAMMM, CNC Lynch syndrome, and McCune Albright Syndrome (MAS) have also been associated with IPMN [46]. Smoking history and the presence of mural modules are risk factors for malignant IPMN and PDAC [47]. However, retrospective studies [48, 49] on surgically resected IPMN samples reported smoking as a risk factor for IPMN but no correlation between smoking history and IPMN grade of dysplasia or malignant progression. Further, current smokers are also associated with the incidence of PDAC concomitant with IPMN [50]. A history of alcohol abuse and alcoholic CP are considered risks for IPMN and malignancy [51]. At the same time, another study showed no difference in IPMN patients and controls based on alcohol and tobacco intake [44]. Numerous studies have shown the correlation between IPMN and diabetes mellitus (DM), suggesting a high prevalence of DM in IPMN and DM as a risk factor for the malignant transformation of IPMN. The odds ratio (OR) for IPMN is 1.79, HGD is 2.02, and invasive carcinoma is 2.05 among diabetic patients [52]. Moreover, malignancy of IPMNs is suggested by new-onset diabetes mellitus, jaundice, and elevations in serum glucose or alkaline phosphatase level [53].
Few studies have focused on the aforementioned risk factors associated with MCNs. A multicenter study reported that male sex, larger MCNs, localized in the pancreatic head and neck, dilated duct, and mural nodule are the preoperative risk factors of MCNs [54]. Further, a clinical case report of a 27-year-old woman diagnosed with acute pancreatitis showed various changes over time and eventually identified as MCN [55].
Diagnosis and Evaluation
Standard Imaging Methods
The diagnosis of pancreatic cystic lesions (PCLs) like IPMN and MCN is incidental, often diagnosed during abdominal imaging with techniques like Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) for unrelated reasons. CT and MRI are commonly used to diagnose and assess pancreatic cysts and define characteristics like size, calcifications, nodules, septation, and interaction with the pancreatic ductal system [56]. Moreover, with more clinical significance than MRI, gadolinium-enhanced MRI with magnetic resonance cholangiopancreatography (MRCP) has also been utilized to diagnose and manage IPMN accurately [57]. A study of the diagnostic efficacy of MRI-MRCP for benign and malignant IPMN evaluation found 90.3% sensitivity, 70.4% specificity, and 81% accuracy [58].
Endoscopic Ultrasound (EUS) is a more sensitive (compared to CT or MRI) and accurate technique used to differentiate neoplastic from non-neoplastic cysts [59]. A EUS is recommended by various guidelines and is a standard care procedure for IPMN. It can determine the wall thickness, size, location of the cyst, mural nodule, dilation of the pancreatic duct, etc. [59]. EUS-guided fine needle aspiration (EUS-FNA) is also used to identify mucinous lesions and increase the diagnostic adequacy of cytology markers [59]. However, there are some limitations in using EUS-FNA as it is a high-risk- invasive procedure and can only be performed in equipped locations with an expert. While technologies like EUS and MRI/MRI-MRCP are excellent diagnostic tools for IPMN, there is still controversy about which is more accurate due to conflicting results. A multicenter retrospective investigation demonstrated little agreement between EUS and MRI-MRCP in patients with suspected BD-IPMN [60]. Another study found that MRCP and MRI have higher diagnostic accuracy than EUS for distinguishing between malignant and benign IPMN and MCN [61]; however, a study by another group found no significant difference in the diagnostic capability of MRI and EUS for malignancy of IPMN [62].
Prospective Diagnostic Imaging Tools
In the last decade, positron emission tomography- Computed Tomography (PET-CT) has become the standard imaging procedure for various tumors [63]. It contributes more to oncological applications and patient care than separate PET scans with CT. While numerous studies have focused on using PET-CT in IPMN, its role in the IPMN or any other PCL is undefined. F-18-fluorodeoxyglucose positron emission tomography (18-FDG-PET) is the metabolic imaging found to be more accurate, sensitive, and specific than the International Consensus guidelines in detecting malignant IPMN (87%, 80%, and 95% vs. 63%, 67%, and 58% respectively) [63]. Although the current IPMN management guidelines do not recommend the metabolic assessment of IPMNs, this imaging tool could be useful in evaluating IPMNs.
Diffusion-weighted MRI (DWI) is a rapidly evolving, modern-state-of-art imaging technique vital in assessing various disease progressions, including oncology [64]. While MRI and MRCP are conventional imaging methods for IPMNs, DWI could be a better option for identifying malignant IPMNs. A study conducted with 61 patients with surgically resected IPMNs undergoing enhanced MRI, DWI, and MRCP showed that DWI in combination with MRI improves diagnostic accuracy and has higher specificity and sensitivity for malignancy prediction [64]. Moreover, the combination of DWI with MRCP enhances the prediction of invasive IPMC and diagnosis of malignant IPMN. Another study found that combining DWI with MRCP with unenhanced MRI improved the prognosis of invasive IPMN and the diagnosis of malignant IPMN. The study included surgically removed IPMNs from individuals who had preoperative MRCP, unenhanced MRI, and DWI [65].
In addition to conventional EUS, several advanced endoscopic techniques, such as contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS), confocal laser endomicroscopy (nCLE), and endoscopic retrograde cholangiopancreatography (ERCP), have been developed and investigated as a potential diagnostic tool for IPMNs and other PCLs.CH-EUS uses an intravenous contrast agent based on harmonic imaging by EUS and distinguishes pancreatic masses as benign or malignant by enhancing isoenhancingis. CH-EUS showed a PPV of 62% in detecting advanced neoplasia based on the meta-analysis of 70 studies, with 2297 undergoing IPMN resection. The multiphase contrast enhancement has been successfully used to differentiate the IMPC and conventional PDAC [66]. nCLE provides real-time optical histology of the cyst wall during EUS-FNA and has more than 80% specificity and 59–95% sensitivity for the IPMN diagnosis [67]. Moreover, studies have explored the capability of nCLE in determining the type of IPMN, histological or dysplasia-based, before surgical intervention.
Standard Biomarkers
Cystic Fluid and Cytology
The fine needle aspiration (FNA) technique and EUS are usually used to collect cystic fluid from patients with pancreatic cysts. The cystic fluid is analyzed to identify mucinous cysts, differentiate between benign and malignant lesions, and further assess cells for cytology. This method has been used frequently to diagnose pancreatic cysts [27]. A meta-analysis by Suzuki et al. showed the sensitivity and specificity of EUS-FNA cytology to be 65% and 91%, respectively, for distinguishing malignant and benign IPMNs [68]. Another recent study reported that EUS-FNA diagnosed IPMN with 83.3% sensitivity, 100% specificity, and 91.7% accuracy. Moreover, EUS-FNA classified the IPMN types in 83.3% and identified nodules and/or vegetation presence in 90% of cases [69].
CA19.9 and CEA
CA19.9 is a Sialyl Lewis A glycan present on multiple glycoproteins. Carcinoembryonic antigen (CEA) is a secreted glycoprotein involved in cell adhesion [70, 71]. Various precursor lesions management guidelines recommend CA19.9 analysis in the serum level of pancreatic cystic lesions. It is used as a biomarker to evaluate and manage patients with precursor lesions like IPMN. CEA has also been used as a biomarker for various gastrointestinal carcinoma. Both CA19.9 and CEA levels in the cystic fluid of IPMN are used to differentiate it from MCN and to determine the malignancy as they are elevated in high-grade dysplasia and invasive carcinoma. A meta-analysis showed that serum CA19.9 could identify invasive and malignant IPMNs with a pooled sensitivity of 52% and 40% and specificity of 88% and 89%, respectively. CEA has a pooled sensitivity of 18% for both invasive and malignant IPMNs and a specificity of 95% and 93%, respectively [72]. However, a recent study reported that serum CEA levels have no correlation with the malignancy of IPMN. Still, serum CA19–9 > 37 U/ml is associated with invasive IPMN and concurrent pancreatic in patients with IPMN but not in high-grade dysplasia [73]. These studies still question whether these standard biomarkers in practice are accurate in diagnosing and predicting IPMN cases and their aggressiveness.
Promising Biomarkers
Mucins
Mucins are heavily glycosylated, high molecular-weight proteins produced by the epithelial tissues in the body. To date, 21 mucins have been identified and are categorized into three groups: transmembrane mucins (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, MUC21), secreted gel-forming mucins (MUC2, MUC5AC, MUC5B, MUC6, MUC19) and secreted non-gel-forming mucins (MUC7, MUC8, MUC9) [74]. The expression and role of mucins in tumor development, invasiveness, metastasis, and drug resistance have been found in many cancers [75]. The epithelial tissues of the normal pancreas express some mucins like MUC1, MUC2, MUC5AC, MUC5B, and MUC6. The de novo synthesis and the altered expression pattern of various mucins are present in both PPLs and PDAC, indicating a potential role of mucins in PPL progression to PDAC. Emerging studies have suggested that mucins have tremendous diagnostic potential as biomarkers for the early detection of cystic pancreatic lesions [75].
IPMNs are also known as mucin-producing cystic lesions. The de novo synthesis of various mucins in different IPMN subtypes differentiates malignant potential and high-risk IPMNs. A meta-analysis investigating the correlation between mucin expression and progressive malignant lesions of IPMN showed expression of MUC1, MUC2, and MUC5AC in benign lesions that increased with malignancy [76]. Elevated MUC4 and MUC2 have been observed in cystic fluid and MUC5AC in serum levels from high-risk IPMN cases [77]. A recent study showed MUC5AC as a potential biomarker for distinguishing the invasive IPMN from low-grade and non-invasive subtypes based on a simple blood test analysis of extracellular vesicles (EV) [78]. Intestinal-type IPMN shows positive MUC2, high MUC4 expression, and negative MUC1 expression, indicating high potential progression to invasive carcinoma. MUC2 is also used as a biomarker for predicting malignancy in mixed-Duct IPMN. MUC4 expression has been reported in various types of IMPM, which increases as the level of dysplasia advanced, regardless of IPMN type. Gastric-type IPMN shows negative MUC1 and MUC2 expression, implying a slight chance of gastric IPMN progressing to invasive carcinoma. Moreover, rare MUC4 expression in low-grade dysplasia that increases with advanced dysplasia has also been reported in gastric IPMN. MUC13 is also expressed in different types of IPMN [27].
The production of abnormal glycoforms of proteins/mucins is common in cystic lesions or precursors of cancers. Ohya et al. investigated the role of MUC6 and α1,4-linked N-acetylglucosamine (αGlcNAc) residues attached to MUC6 in PPL progression to PC. They demonstrated that the expression of αGlcNAc relative to MUC6 is decreased in low-grade PanIN/IPMN, indicating its role in the initiation and progression of PC [79]. Another study by Sinha et al. and group has shown that a panel of biomarkers composed of glycoforms of MUC5AC and endorepellin proteins can distinguish mucinous from non-mucinous cysts even better than that of CEA [80, 81]. Moreover, the glycan analysis of cystic fluid revealed the unique alpha-linked gastric glycoform of MUC5AC secreted by IPMN and beta-linked glycoform by most IPMNs and MCNs that can accurately diagnose mucinous cysts [82].
Other Glycans/Glycoprotein
A complete glycome study was performed on serum N-glycan patterns in IPMN patients. The study found that the expression profiles of glycans differed significantly depending on cyst size, the existence of an augmenting solid component, and the IPMN’s histological grade. In addition, they identified a signature of nine glycans for invasive IPMNs and a fucosylated triantennary glycan with the highest diagnostic value for distinguishing invasive IPMNs from non-invasive IPMNs [83]. Another study using a glycoproteomic analysis in IPMN and PC serum samples revealed that a glycoprotein, B7.1, can better differentiate between IPMN and healthy controls. In contrast, a panel including CA 19–9, IL.17E, B7.1, and DR6 could distinguish stage 1 PC from healthy controls with 100% sensitivity and 90% specificity [84].
A lectin microarray-based assessment of glycan expression in IPMN indicated that the malignant progression of IPMN is associated with fucosylation (addition of fucose sugar to the molecule). Aleuria aurantia lectin (AAL) and Aspergillus oryzae L-fucose-specific lectin (AOL), binding to fucose, significantly increased as IPMN progressed. Additionally, Fut 8 protein expression, associated with AAL and AOL, also sequentially and significantly increased [85].
Inflammation Biomarkers
Systemic inflammation markers have prognostic significance in the malignancy of various cancers [86]. The neutrophil-to-lymphocyte ratio (NLR) and Platelet-to-lymphocyte ratio (PLR) are two of these markers that can potentially identify malignancy in mucinous pancreatic cystic lesions like IPMN [87]. In recent years, numerous studies have attempted to validate the ability of serum-based NLR to distinguish IPMN malignancy is significantly higher in patients with IPMN-associated invasive cancer compared to low and high-grade IPMNs, according to studies such as Mclntyre et al. Arima et al. Watanabe et al. and Gemenetzis et al. [86, 88–90]. Another study by Hata et al. reported that NLR is higher in IPMN with invasive carcinoma and high-grade IPMN than in low-grade IPMN [91]. The combination of NLR with CA19.9 (predictive value of 78% and 96% specificity and CEA and CA19.9 (58.8% sensitivity and 76.8% specificity) improves the overall performance of NLR as a predictive biomarker for IPMN with invasive carcinoma [88, 91]. Additionally, Gemenetzis et al. showed that PLR is elevated in IPMN with invasive carcinoma but does not have the potential as a biomarker for high-grade/ invasive carcinoma IPMN [89]. However, according to the Fukuoka guideline, a high level of PLR is associated with 83.3% invasive carcinoma. Due to the varying results from different studies, NLR and PLR as potential biomarkers are debatable and require further investigations for accurate validation.
Genomics
MicroRNAs, small non-coding RNAs, are dysregulated in pancreatic cancer and PPLs. MicroRNAs like miR-155, miR-21, and miR-210 are dysregulated in IPMN [92], miR-10a-5p, and miR-221-3p are upregulated in IPMN with invasive carcinoma, while miR-148a-3p is downregulated [93]. The miRNA analysis in the cystic fluid to evaluate the IPMN malignancy discovered six miRNAs: miR-711, miR-3679-5p, miR-6126, miR-6780b-5p, miR-6798-5p, and miR-6870-5p elevated in IPMN with carcinoma compared to those with adenoma [94]. Permuth-Way and the group investigated both miRNAs and long non-coding RNAs (lncRNAs) as potential novel biomarkers for patients with IPMN [95, 96]. They showed that 8-lncRNA signature (ADARB2-AS1, ANRIL, GLIS3-AS1, LINC00472, MEG3, PANDA, PVT1, and UCA1) could distinguish malignant IPMNs from benign IPMNs. Further, when combined with plasma miRNA data and quantitative ‘radiomic’ imaging features, the accuracy of predicting IPMN pathological classification improved. In IPMN, five miRNA signatures (miR-200a-3p, miR-1185-5p, miR-33a-5p, miR-574-3p, and miR-663b) were associated with a higher probability of malignancy (high-grade dysplasia or invasive cancer). The sensitivity was 77% and 81% with the specificity of 76% and 53% for lncRNA and miRNA respectively. A comprehensive analysis of miRNA expression was done to distinguish the exosomal-miRNA signatures of pancreatic lesions. Twelve differentially expressed miRNAs between benign and malignant IPMNs, 13 between IPMN and IPMN with invasive adenocarcinoma, and 54 between IPMN and PDAC were identified [97]. A study analyzed the set of circulating miRNAs overexpressed in the tissues of pancreatic neoplasms from plasma samples. 16 miRNAs for PDAC and 14 for IPMN were found to be significantly higher than control samples, including AUC values of 0.86, 0.85, 0.85, and 0.80 for miRNAs like miR-21-5p, miR-33a-3p, miR-320a, and miR-93-5p respectively. The combination of miRNAs and miRNAs with CA19.9 further improved the diagnostic potential [98].
Metabolomics
Metabolites such as glucose and kynurenine have the potential to be novel biomarkers for distinguishing mucinous from non-mucinous pancreatic cysts [99]. The combined metabolomic and lipidomic analysis of cystic fluid revealed that this collaborative technique could discriminate between IPMN and SCN with 100% accuracy and LGD or HGD and invasive malignancy with up to 90% [100]. A study investigating the insulin resistance biomarkers for IPMN reported circulating leptin in females and branched-chain amino acids (BCAA) associated with IPMN based on dysplasia. The diagnostic accuracy of these biomarkers was further increased by 0.2 AUC when combined with the main pancreatic duct diameter and CA 19.9 [101]. Another study also showed that BCAA levels in plasma were collected 2 to 5 years before the PC diagnosis was elevated. This validates the potential of circulating BCAA as a novel biomarker for IPMN [102]. Moreover, the assessment of metabolite biomarker candidates for PC suggested that these metabolites could help detect high-risk IPMN, especially lysine, with a sensitivity of 67.8% and specificity of 86.9% [103]. A quantitative examination of IPMN and SCN plasma and cyst fluid found new markers of IPMN and its progression, including amino acids, carboxylic acids, conjugated bile acids, free and carnitine-conjugated fatty acids, purine oxidation products, and TMAO [104].
Proteomics
While more focus has been on proteins like mucins and CEA, other proteins are reported to be found in IPMN and could potentially serve as biomarkers for IPMN. Proteins like Sonic hedgehog (SHH) and Prostaglandin E2 (PGE2) were found in higher levels in IPMN. SPINK1 and pancreatic enzymes (amylase and lipase) distinguish between benign and malignant IPMN lesions. A study in Finland performed quantitative proteomics using a label-free serum to differentiate different stages of IPMN and a healthy pancreas. This study has proposed panels of protein biomarkers for the progression of IPMN from low-grade to IPMC, including proteins like apolipoproteins, complement proteins, Kininogen-1, and Retinol-binding protein-4 [105]. A meta-analysis of differences in gene expression between low-risk and high-risk IPMNs identified a 12-gene signature (downregulated: CEL, CPA1, CPB1, SERPINA4, CA2, CLU, AMBP and ALB, upregulated: CP, CEACAM5, DMBT1, and KRT6A) that could serve as a potential biomarker for identifying IPMNs with malignancy potential [106]. A quantitative proteomic profiling study detected 19 protein candidates as biomarkers for IPMN malignancy [107]. Another proteomic study identified 18 proteins with expression patterns consistent with the degree of malignancy in IPMN [108]. The proteomic analysis of cystic fluid and serum from IPMN lesions, along with additional research in this area, could create a more accurate biomarker or screening tool for IPMN [27].
Others
The pancreatic epithelial cells enter the bloodstream of patients with cystic lesions before the cancer diagnosis and are known as circulating epithelial cells (CECs) [109]. The detection of CECs could serve in identifying patients with IPMNs. Fanses et al. discovered CECs in 88% of individuals with IPMN lesions using a combination of different analytical approaches [110].
While a significant number of cystic lesions are being detected with imaging techniques, the standard practices of biomarkers and imaging tools are not adequate in differentiating IPMN lesions. The distinction of different grades of IPMN, IPMN from other cystic neoplasms, and identification of malignant potential is still a big challenge. The blood-based biomarkers like mucins, miRNAs, and different proteins discussed here are non-invasive, inexpensive, and have great potential as novel biomarkers for IPMN lesions. The combination of these biomarkers and advanced imaging tools could play an immense role in the evaluation and management of IPMN.
Consensus Guidelines for Pancreatic Cystic Neoplasm
Currently, there are four major guidelines for the treatment and management of IPMN: International Association of Pancreatology (IAP; ‘Fukuoka guidelines’), also known as International Consensus Guidelines (ICG), American College of Gastroenterology (ACG), American Gastroenterological Association (AGA), and European Study Group on Cystic Tumors of the Pancreas. All these guidelines recommend surgery and surveillance based on the size of cysts, dilated ducts, types, and their malignant potential.
International Consensus Guidelines
ICG for IPMN and MCN was established in 2006 and has since undergone two upgrades, in 2012 and 2017, to enhance the detection and treatment of malignant pancreatic cystic neoplasm. The current revised version of ICG categorized the features of cystic neoplasm as worrisome features and high-risk stigmata. The problematic features include cyst of ≥3 cm, thick cyst walls, enhancing mural nodule <5 mm, 5–9 mm main duct size, abrupt change in the MPD caliber with distal pancreatic atrophy, lymphadenopathy, an elevated serum level of CA19–9, and cyst growth at the rate of > 5 mm/2 years. The presence of any of these features requires follow-up with EUS, which, if it shows any of the signs of malignancy with the involvement of the main duct and defined mural nodule ≥5mm, requires surgical resection; otherwise, surveillance based on the size of the cyst is necessary. The presence of a 5 mm enlarged mural nodule, a 10 mm MPD, and obstructive jaundice in a patient with a cystic lesion of the pancreatic head provides a reason for surgical intervention. For MCN, surgery is recommended for all surgically fit patients with no surveillance for non-invasive MCN. However, surveillance is recommended based on the resection margin for IPMN [56].
European Study Group on Cystic Tumors of the Pancreas
The European guideline (EG) has classified the characteristics of pancreatic cystic neoplasms as relative indications and absolute indications that are considered worrisome features and high-risk stigmata in ICG. For IPMN, the absolute indications for surgery per this guideline are jaundice, enhancing mural nodule ≥5 mm, MPD ≥10 mm, HGD or cancer on cytology, and solid mass. The relative indications are acute pancreatitis, Cyst size ≥4 cm, the growth rate of cyst >5 mm/year, enhancing mural nodule <5 mm, MPD dilation 5–9.9 mm, serum CA 19.9 ≥37 U/ml, and new onset of diabetes. EG guidelines are more aggressive as it recommends surgery without EUS in the presence of a single relative indication in clinically fit patients and patients with significant comorbidities for two or more indications. Furthermore, active surveillance is recommended for patients with no absolute or relative indications for surgery every six months for a year, then annually, and every six months for patients with one relative indication with significant comorbidities through MRI/MRCP or EUS and Serum CA 19.9. In the case of MCN, cyst size ≥4 cm is the absolute indication for surgery. Regardless of cyst size, patients with any symptoms or risk factors are also recommended as the surgical option. If the cyst <4cm with no other symptoms, lifelong surveillance with MRI and EUS or both every six months for a year is recommended annually upon no change. For cysts < 3cm, EG recommends following the surveillance guidelines as IPMN as they are difficult to distinguish from other cystic lesions [59, 111]
American Gastroenterological Association
American Gastroenterological Association (AGA) guideline does not differentiate clinical features of the lesion into various categories for evaluation and management as the guideline is developed for asymptomatic lesions. AGA recommends EUS-FNA for patients with at least two high-risk features like dilated MPD, ≥ 3 cm cyst size, or solid cystic component, and then follow-up surveillance based on results. Active surveillance for patients with cyst < 3 cm without a solid component or a dilated MPD through MRI in 1 year and every 2 years is recommended. Even though AGA does not directly recommend surgery, patients with a solid component and a dilated pancreatic duct and concerning features on EUS-FNA are suggested to refer for surgery. Moreover, MRI is advised every 2 years in patients with invasive cancer or dysplasia in the surgically resected cyst. AGA proposes that the continuity of surveillance is not required after 5 years if there is no significant change in the size of the cyst or if patients are not qualified for surgery [112].
American College of Gastroenterology
American College of Gastroenterology (ACG) guidelines for pancreatic cysts only apply to patients with no history of PC or mutations for PC. ACG does not characterize the absolute indication for surgery but does recommend patients with high-risk characteristics undergo EUS-FNA and/or be referred to a multidisciplinary group for further evaluation and consideration of surgical intervention. The features include jaundice, acute pancreatitis, high serum CA 19–9 level, mural nodule or solid component either within the cyst or in the pancreatic parenchyma, dilated MPD >5 mm, a focal dilation of the pancreatic duct concerning main duct IPMN or an obstructing lesion, cysts size ≥3 cm in diameter, and presence of high-grade dysplasia or pancreatic cancer on cytology. Further, ACG strongly suggests surveillance with MRI or EUS for patients with cyst presumed IPMN or MCN and short-interval follow-up for patients with new-onset or worsening diabetes mellitus or a rapid increase in cyst size (of >3 mm/year) during surveillance. The timeline for active surveillance varies based on the cyst size. ACG recommended postoperative surveillance for all resected IPMN and MCN with PC [113].
Differences and Challenges in guidelines
An expert team created all guidelines with one purpose: accurate diagnosis, therapy, and evaluation of pancreatic cystic lesions. However, they all consider different variables for the recommendation of further evaluation, surgery, and even the timeline and necessity for surveillance. For example, only EG and ACG consider diabetes an indication of malignancy. One of the unique features of EG is that it considers cyst size ≥4 cm as a problematic feature, unlike cyst size ≥3 cm in others. However, EG is not clear for cyst size between 3–4cm as it recommends clinicians to take other factors like age, other risk factors, and patient’s preference for management. Except for all other guidelines, only ICG is mainly focused on IPMN and hence more specific. ICG, EG, and ACG strongly recommend the lifelong surveillance of patients until they are no longer fit for surgery except for AGA, which suggests discontinuation after 5 years if there is no change in the characteristics of the cyst. While lifelong surveillance provides a safety net for patients, it could be very expensive and mentally exhausting for patients. Additionally, studies have reported the development of worrisome or high-risk features in patients with BD-IPMN in surveillance after 5 years [114]. ACG recommends surveillance for all IPMN patients postoperative, and other guidelines suggest surveillance for patients with surgically resected cysts with high-grade or invasive cancer. A multi-institutional study with 1074 IPMN surgical patients recently showed reoccurrence of lesions post-surgery in 14.4% and 34.3% of lesions in the remnant pancreas occurred over 5 years after [115]. Moreover, a recent study evaluated the three major recently updated guidelines to review their accuracy in identifying malignant cysts. The study reported that ICG, EG, and AGA had a sensitivity of 66.8%, 95.5%, 80%; specificity of 26.8%, 11.3%, 43.8%; missed malignancy rate of 11.3%, 1.5%, 7.7%, and surgical overtreatment was 48.4%, 59.1%, 34.6% respectively [116]. As the guidelines contradict each other, and each comes with certain pros and cons, it is difficult to follow/recommend one and ignore another without consequences.
The variances and limitations of these guidelines provide challenges in accurately identifying both high-risk or malignant IPMN that necessitates surgical intervention and patients who merely require surveillance. This is due to a lack of better biomarkers and an understanding of the molecular biology of IPMN and its progression mechanism. To eliminate poor patient outcomes, there is an urgent need to develop a strong management strategy for IPMN and its progression prevention.
Genetic alterations in IPMN
Most of the genomic analyses in the literature have been conducted on invasive PCs, and only a few non-invasive PCs have been analyzed comprehensively. Genomic analysis in IPMN has shown Kras and GNAS to be the major driver mutations of IPMN. A study has shown that the mutations in KRAS and GNAS oncogenes occur in low-grade lesions; however, mutations in CDKN2A, TP53, RNF43, and SMAD4 occur in lesions with high-grade dysplasia or associated invasive carcinoma. Another study also performed whole-exome and targeted sequencing analyses in 18 IPMNs/MCNs and associated invasive carcinomas. This study revealed the mutation of KRAS (89%), GNAS (28%), CDKN2A (44%), TP53 (67%), SMAD4 (50%), and TGFBR2 (17%) in IPMN samples [117]. Mutations in RNF43 (56%), which plays a critical role in mucin-producing pancreatic cysts, were also identified in IPMNs. Somatic mutations in the PI3K, PIK3CA, and STK11 were observed in IPMN samples [118]. A loss of Brg1 [119], mutations in LKB1, and PTEN [120] have also been reported in the development of IPMN. Germline mutations in ATM, PTCH1, and SUFU are detected in IPMN patient samples and are at high risk of developing PDAC [121].
Key Components and associated signaling pathways
Kras, Kirsten Rat Sarcoma virus gene encodes for K-ras protein. Kras is a GTPase that acts as a molecular switch for the activation and inactivation of this protein and regulates many cellular processes. The mutations in Kras lead to the continuous GTP-bound form and, hence, Kras activation, resulting in the perpetual activation of its downstream effector proteins [122]. The oncogene-mutated Kras regulates signaling pathways like PI3K/AKT/mTOR resulting in suppression of apoptosis and Raf/MEK-ERK leading to cell proliferation and promoting cancer development [123] (Figure 2). Kras is one of the most frequently mutated oncogenes and is present in about 25% of cancers, with pancreatic cancer harboring a higher mutation in about 98% of all PC cases [124]. It is also the first mutational change associated with the development of precancerous lesions, predominantly IPMN, in the pancreas [125]. The mutations at codon 12, specifically G12D and G12V, are commonly reported in IPMN [126]. Kras mutations are also observed higher in tubular carcinoma (89%) compared to colloid carcinoma (52%) in IPMN [127]. A study examining the clinical association of IPMN and Kras showed Kras mutation in 60% gastric-type, 52.6% intestinal-type, 47.3% pancreatobiliary-type, and zero oncocytic-type IPMN. However, they found no correlation between the Kras mutations and subtypes of IPMN, the degree of dysplasia, and survival outcome [28].
Figure 2: Key signaling molecules and pathways implicated in IPMN development and progression.

Mutation in Kras results in the activation of either PI3K/AKT/mTOR-mediated or RAF/ERK-mediated target gene transcription. In addition, mutant Gnas in GPCR signaling results in cAMP/PKA-mediated activation of target genes. Dysregulation of RNF43, which is activated transcriptionally by Wnt/β-catenin signaling, is a key driver of IPMN development.
GNAS encodes for the α-subunit of guanine nucleotide-binding protein-stimulating G-protein (Gsα) that regulates the G-protein-coupled receptor (GPCR) signaling mechanism [128]. The disruption in GCPR signaling is reported as the hallmark of cancer [129]. Moreover, the frequent mutations in GNAS like R201C and R201H found in IPMN alter the GTPase activity of GSα, locking it in activated form. This leads to the continuous activation of adenyl cyclase and cAMP formation, resulting in the activation of the protein kinase A (PKA) pathway [128]. The cAMP-PKA signaling pathway has diverse downstream targets implicated in multiple cancers, including pancreatic cancer, for their role in promoting tumorigenesis [130, 131]. GNAS mutations in around 70% of IPMNs are unique and distinguish them from other PPLs developing to PDAC [132]. While the exact composition varies due to the difference in methods and patient samples, IPMNs show more frequent GNAS mutations in intestinal-IPMN compared to other histological subtypes. Molin et al. reported GNAS mutations in 100% of intestinal IPMN compared to 51% of gastric IPMN, 71% of pancreatobiliary IPMNs, and 0% of oncocytic IPMN, while Hosoda et al. found GNAS mutation in 78%% of intestinal IPMN compared to 62%% of gastric IPMN [133, 134]. Several studies revealed the initiation of IPMN due to the combined mutations in KRAS and GNAS. However, GNAS mutation alone can lead to IPMN formation and induces more intestinal-type IPMN, which have not yet been able to be mimicked in murine models. A recent study by Patra et al. demonstrated that the mutant GNAS cAMP-PKA pathway inhibits the activation of salt-inducible kinase (SIK) and promotes tumorigenesis in IPMN [135].
The whole-exome sequencing of pancreatic neoplasm cysts discovered RNF43 mutations in IPMN and MCN for the first time [136]. The higher prevalence of RNF43 mutations after KRAS and GNAS in IPMN has been reported in several studies [137–139]. Omori et al. found that the incidence of RNF43 mutation in intestinal-type IPMN is more frequent, and mutated RNF43 could be the driver in the progression of the gastric-type into the intestinal-type IPMN [139]. RNF43 mutation is also associated with GNAS mutation during the development and progression of IPMN [139, 140]. RNF43 gene encodes ring finger protein 43 and is also known as RING-type E3 ubiquitin ligase [141]. RNF43 is involved in the inactivation of the Wnt signaling pathway, which plays a critical role in embryonic development and adult tissue homeostasis [142]. The wnt/-catenin pathway, when activated, induces the expression of RNF43, which translocates to the plasma membrane, where it is ubiquitinated, internalized, and thus degrades the Wnt receptor frizzled via a negative-feedback mechanism (Figure 2) [141, 143]. Mutations in RNF43 result in the continuous activation of the Wnt pathway and its downstream molecules that are mutated in cancers, including PDAC [144].
Hedgehog (Hh) pathway plays a critical role in developing and regulating cell growth, survival, and fate [145]. The Hh pathway is complex and highly regulated with a string of ligands, transmembrane receptors, transcription factors, and signaling effectors regulating the downstream signaling cascade. The disruption of Hh/Gli signaling in cancers has developed it as the mediator of tumorigenesis and a potential therapeutic target for cancer, including PDAC [146]. High expression of Sonic hedgehog (Shh), the ligand of Hh, is detected in 68.8% of intestinal types and 92.8% of pancreaticobiliary types IPMN. The activation of the Shh pathway has been correlated with IPMN tumorigenesis in different studies [147, 148]. Shh is associated with the early stages of IPMNs and the transformation of benign to malignant forms of IPMN [149]. Moreover, germline mutation in a suppressor of fused (SUFU), a negative regulator of Gli associated with IPMN, is also at a higher risk of developing carcinomas [121]
The notch signaling pathway regulates cell fate and cell differentiation processes. This pathway is activated in cancer initiation and progression, including PDAC [150]. A study aimed to elucidate the role of Notch signaling in IPMN suggested that the Notch pathway determines the aggressiveness of IPMN as high-grade IPMN shows significantly high expression of Jagged1, a ligand of the Notch receptor [151].
Molecular Mechanism of Cystic Lesion Progression
Bussom et al. proposed a hypothetical progression mechanism of IPMN to PDAC based on the analysis of cytokines in IPMN cyst aspirates and regulatory T cells (Treg) in the peripheral blood of IPMN [152]. Any inflammation of pancreatic ductal cells causes genetic changes and, thus phenotype changes in the pancreatic duct, leading to the development of PPLs such as MCN and IPMN. In the case of IPMN, the cyst formation is accompanied by the release of cytokines like interleukin-1beta and interleukin-8. Then, Treg cells are recruited to establish a favorable tumor microenvironment as well as the survival and, consequently, the development of the metastatic potential of IPMN, a portion of which develops into PDAC (Figure 3).
Figure 3: Mechanisms for initiating IPMN and its progression to PDAC.

In response to various insults (pancreatitis, free radicals, etc.), Pancreatic ductal cells develop inflammation which, combined with a genetic mutation, leads to low-grade IPMN. Further development of these low-grade lesions into high-grade lesions requires T-regulatory cells-mediated creation of a favorable tumor microenvironment. Various driver mutations (Kras and Gnas) in the pancreatic ductal population initiate the development of low-grade IPMN. Later, RNF43, TP53, and Smad4 mutations drive these low-grade lesions into high-grade and invasive PDAC. In addition, pancreatic ductal cells with abundant Kras mutation than Gnas develop gastric subtype low-grade IPMN, which progresses into high-grade IPMN and colloid carcinoma due to mutations in LKB1, PTEN, TP53, and Smad4.
On the other hand, pancreatic ductal cells with Gnas mutation alone develop intestinal sub-type with low-grade lesions, which further progresses into high-grade IPMN and tubular carcinoma in response to mutations in RNF43 and CDX2. Low-grade IPMNs contain multiple independent clones, with heterogeneity in early driver mutations (Gnas and Kras), out of which the dominant ones expand and initiate neoplastic progression. Then, late driver gene mutations across high-grade lesions with dominant clones eventually induce the development of PDAC.
Omori et al. [153] investigated pancreatic tissues with concurrent IPMNs, PDACs, and lesions by genetic and histologic analyses for mutations in 18 pancreatic cancer genes and tumor suppressors. Based on the clonal relatedness of lesions, they proposed three mechanisms by which IPMN progress to PDAC: sequential, branch-off, and de novo. In the sequential pathway, PDAC shares the mutational profile with concurrent IPMN suggesting the direct transition of IPMN to PDAC or progressive development of PDAC from IPMN. In the branch-off pathway, some driver mutations of PDAC are shared with concurrent IPMN, indicating the common origin but divergence in the later progression period. The de novo pathway shows completely distinct mutations between PDAC and concurrent IPMN suggesting the independent development of PDAC despite IPMN (Figure 3).
Fischer et al. [154] performed comprehensive genomic sequencing of multiregional early and late-stage IPMN lesions to study the mutational diversity throughout the IPMN progression. They found multiple independent clones with heterogeneity in early driver mutations across low-grade IPMN lesions, out of which the dominant ones expand, initiating neoplastic progression. Then, the late driver genes show the convergent evolution of mutations across high-grade lesions with dominant clones, eventually inducing the development of PDAC. This study shows the polyclonal origin of IPMN in contrast to traditional monoclonal pancreatic tumor origin and the polyclonal origin of IPMN in contrast to traditional monoclonal pancreatic tumor origin and identifies the clonal evolutional model for the development of PDAC via IPMN.
Leo Mas et al. [155] proposed two hypothetical IPMN progression pathways: intestinal and non-intestinal pathways. KRAS and GNAS cause initiation, a combination of CDX2 and RNF43 mutations that causes GNAS-driven progression to colloid-type carcinoma, usually in intestinal IPMN. On the contrary, in the non-intestinal pathway, KRAS with PTEN or LKB1 mutations lead to the KRAS-driven IPMN progression with a mutation like SMAD4, TP53, or CDKN2A to tubular carcinoma (Figure 3).
The conventional route for the IPMN progression to PDAC ( similar to PanIN) is its initiation and development through the different grades of dysplasia and to PDAC. Recently, studies have proposed diverse progression mechanisms of IPMN to PDAC. However, further studies are required to validate these pathways and identify novel mechanisms of IPMN development and progression to PDAC. The characterization of these mechanisms would be of great importance for clinical utility and hence the treatment and management of IPMN patients.
Metabolic reprogramming in the development of IPMN
Recent research suggests that metabolic reprogramming is the primary hallmark of pancreatic cancer to support tumor proliferation. Glucose is processed to pyruvate via glycolysis and via mitochondrial oxidative phosphorylation in normal cells. However, pancreatic cancer cells mostly rely on aerobic glycolysis (conversion of glucose-derived pyruvate into lactate) by bypassing TCA, even in oxygen. This feature of cancer cells is called “the Warburg effect.” PDAC also relies on glutamine, which is metabolized to α-ketoglutarate by glutamate. The increased reliance of PDAC cells on glutaminolysis results in glutamine addiction. Though the metabolic alterations of PanIN-derived PDAC are well explored in the literature, the metabolic reprogramming of cystic lesions like IPMN is understudied.
Gaiser et al. performed a targeted quantitative analysis of 100 metabolites and >1000 lipid species on peri-operative pancreatic cyst fluid and pre-operative plasma samples of IPMN and serous cystic neoplasm (SCN) patients in a pancreas resection cohort. IPMN samples showed a significant alteration of lipid pathway metabolites compared to SCN [100]; however, the significance of lipid pathway metabolism in IPMN development has not yet been explored. Metabolic alterations are often associated with oncogenic mutations in PDAC. For instance, activating mutations in Kras (KrasG12D) indices aerobic glycolysis in PDAC. Though the metabolic alterations associated with IPMN mutations (BRG1, TGFBR2, RNF43, PTEN) are unknown, those related to Gnas and SMAD4 IPMN mutations were explored in the literature.
Patra et al. studied the metabolic alterations in IPMN lesions originating from concurrent GNAS and KRAS mutations. Using a genetically engineered mouse model (Kras; Gnas), the authors show that GnasR201C cooperates with KrasG12D to promote the initiation of IPMN, which requires lipid remodeling and fatty acid oxidation is driven by Sik1–3 [135, 156]. In another recent study, Nimmakayala et al. demonstrated that the induction of GnasR201C mutation under activated KrasG12D background mouse pancreas results in an increased fatty-acid β-oxidation-mediated oxidative phosphorylation, which is under the control of PGC1α, a major metabolic modulator.
Loss of function mutation of SMAD4 in PDAC is associated with IPMN; however, the metabolic reprogramming associated with SMAD4 loss in pancreatic cancer is poorly studied in the literature. Liang et al. have shown the involvement of SMAD4 mutation in metabolic reprogramming in PDAC. In SMAD4-deficient PDAC tumors, the loss of SMAD4 increases the expression of phosphoglycerate kinase 1 (PGK1), a glycolytic enzyme, leading to an increase in glycolytic flux and tumor aggressiveness. Ezrova et al. have shown that the loss of SMAD4 in PDAC inhibits mitochondrial metabolism through mitophagy. Despite these studies, the direct association of SMAD4 loss-mediated metabolic rewiring and IPMN development is unknown; a concept needs further research in the future [157, 158].
Overall, specific mutations of IPMN may induce unique metabolic alterations. While Gnas gain of function mutation in IPMN causes lipid metabolism and fatty-acid β-oxidation-mediated oxidative phosphorylation, SMAD4 loss of function mutation increases glycolysis. Further studies are required to reveal a common metabolic pathway induced by mutations in IPMN development. Identifying such a shared pathway will pave the way for developing a therapeutic strategy to target IPMN lesions.
Tumor Microenvironment and IPMN
The tumor microenvironment (TME) is an ecological niche for tumors comprising various components like immune cells, stromal cells, epithelial cells, fibroblasts, blood vessels, cancer stem cells, signaling, and extracellular matrix [159, 160]. The interaction between cells within the tumor microenvironment (TME) and/or between the TME and tumor cells promotes the growth and maintenance of the tumor. These interactions contribute to cancer cell progression, invasion, and metastasis [161]. TME is constantly at war with the immune system of its host, due to which they develop the immunosuppressive TME for tumor survival, making it challenging to respond to therapies. PDAC, like other cancers, also develops an immunosuppressive microenvironment and hence resistance to conventional chemotherapies. However, decades of research and studies on cancer immunotherapy and tumor microenvironment have paved the way for the development of TME-targeted therapies and promising outcomes.
Recently, the focus has been shifted towards the TME and immunosuppressive environment of precursor lesions of PDAC. A single-cell RNA sequencing study characterized epithelial, immune, and stromal cell phenotypes and described the immune landscape in IPMN progression to invasive carcinoma [162]. The study uncovered the presence of activated CD4+ and CD8+ T cells, Cdc2 dendritic cells, some CD19+ CD20+B cells, and myofibroblasts(myCAFs) in both low and high-grade IPMN. However, PDAC progressed from these IPMNs, myCAFs transitioned to inflammatory CAFs and cells into regulatory T cells and myeloid-derived suppressor cells. Another study with a comprehensive analysis of T cell subtypes and their spatial distribution in IPMN TME revealed a significant change in T cell composition during the transformation of high-grade IPMN to invasive carcinoma. Moreover, the evolution of the immune microenvironment from diverse T cell groups (CD8+ T cells, Th/c1, and Th/c2 along with Th9, Th/c17, Th22, and Treg cells) in low-grade IPMN to a Treg-dominated immunosuppressive state in invasive pancreatic cancer is reported [163]. The relationship between the microenvironmental factors and the histological grade of IPMN is shown by Kakizaki et. al. [164]. Stromal fibrosis and the expression of α-SMA, periostin, and galectin-1 were higher in high-grade and invasive BD-IPMN than in low-grade.
The role of the tumor microenvironment in IPMN is not well characterized compared to PDAC and PanIN-induced PDAC. More research into the microenvironment of IPMN is needed better to understand the development of PDAC from these lesions and develop therapeutic strategies that target TME.
Cellular Plasticity and stemness in Cystic Neoplasm
One of the major reasons for the high mortality of PDAC is that it is a rapid metastatic disease. In PDAC, epithelial-to-mesenchymal transition (EMT) plays a critical role in the rapid metastasis of this disease and hence the poor outcome. EMT can be defined as the transition from a stable epithelial state to a mesenchymal state capable of metastasis and invasiveness [165]. This phenotypic transition involves modifications in epigenetics and transcription factors and provides cells with higher plasticity and stemness [165, 166]. Another unique characteristic of the pancreas is its plasticity within the exocrine, known as acinar to ductal metaplasia (ADM). Acinar cells are known to lose their cellular identity and polarity, undergo morphological change and trans-differentiate to duct-like structure due to Kras mutation in acinar cells [167, 168] ADM either directly gives rise to PDAC or progresses to PanIN lesions to PDAC. The development of PDAC is heavily influenced by cancer cell plasticity and stemness, making it more complex and resistant to treatment.
The involvement of cell plasticity and stem cell factors in the case of pancreatic cystic tumors is understudied and yet unknown. However, recently several studies have focused on the cellular plasticity of acinar and ductal cells in PDAC development. Huang et.al. derived pancreatic acinar and ductal organoids from oncogene (Kras and Gnas) induced plasticity of human stem cells [169]. They found that these oncogenes show lineage tropism and plasticity as Kras was more effective in acinar cells, while Gnas was in ductal epithelia. The self-renewal potential of IPMN organoids developed with loss of PTEN and RNF43 in cooperation with Kras is also reported. Additionally, the expression of SOX9, a transcription factor of stem cells in IPMN showed changes in the expression pattern as IPMN progressed from LG to HG and invasive carcinoma suggesting a role in the malignancy of IPMN [170]. Lysine demethylase 6A (KDM6A), associated with metastasis of PDAC, is also found to regulate cell plasticity and PDAC progression [171]. In this study, PanIN and MCN show lower expression than normal, whereas IPMN shows an elevated expression of KDM6A. As stem cells and cellular plasticity remodel the entire tumor biology of cancer, it is critical to have a better understanding of how these factors influence the development of cystic neoplasm and their progression to PDAC. Hence, more studies are required in this field.
Organoids for pancreatic cystic tumors
In recent years, the use of 3-dimensional(3D) cell cultures, like organoid culture has emerged in the biological field. Organoids can be defined as a 3D cell structure, grown from either patient’s derived tissues or stem cells that recapitulate the physiological conditions of a specific organ or patient’s tumor [172]. While the use of 3D organoids was first established in 2009 by Sato et. al. [173], Clevers and group described pancreatic organoids for the first time in 2013[174]. Moreover, the protocol to establish pancreatic organoids from mouse and human PDAC tissue was reported by Clevers and Tuveson’s group in 2015 [175]. Over the years, several studies have utilized this concept and protocol to develop patient derived organoids and study this complex disease. Patients-derived PDAC organoids (PDO) have been used for personalized medicine as a model for screening treatment options for patients. Driehuis and group derived 30 PDO from different pancreas tumor types that recapitulate the genetic alteration found in these tumors and further exposed them to 76 therapeutic agents including the ones used for PDAC patients [176]. They reported the drug sensitivity profiles for different organoid lines, as observation for organoids derived from different patients following drug treatment varied, highlighting the importance of personalized medicine [176]. Another integrative study characterized the PDAC organoids using whole-genome sequencing, RNA sequencing, ATAC-seq and drug sensitivity screening to identify the chromatin accessibility signatures associated with drug sensitivity in PC [177]. Currently, Cold Spring Harbor Laboratory (CSHL), one of the largest cancer organoid facilities, is leading a clinical trial (PASS-01) to evaluate the personalized chemotherapy treatment potential for PDAC.
Recently, few studies have studied the potential of organoid culture in association with precursor lesions of PDAC. Huang et. al. [178] established a living biobank of organoids from 10 IPMNs and 7 normal pancreatic ducts and further characterized them using whole genome sequencing and transcriptome sequencing. They reported that Kras, Gnas, and RNF43 are the most mutated genes with somatic mutations found in 75%, 50%, and 37.5% IPMN organoids respectively. Moreover, CLDN18 and FOXA1 are the most significantly upregulated and downregulated genes in IPMN organoids which are consistent with the immunohistochemical analysis of FOXA1 expression in IPMNs, MCNs, and PDAC samples [178]. Another study by Beato and the group [179] established a living biobank of PDO from IPMN tumors and adjacent normal pancreatic tissue with a success rate of 81% and 87% respectively from 15 patients. The histological and molecular characterization of these PDO showed the potential to recapitulate the parental features. Consistent with the study by Huang et.al. this study also reported the key somatic mutations in Kras, Gnas, and RNF43 [179]. Other studies focused on the key driver mutations of precursor lesions and investigated their feasibility in developing organoids that resemble the precursors of PDAC. The development of genetically engineered mouse pancreatic organoids with the knockdown of PTEN and RNF43 with cooperation with overexpressed Kras mutant developed IPMN-PDAC. It showed an increase in proliferation and self-renewal potential[180]. Other two different studies [170, 181] induced human pluripotent stem cells (hPSCs) expressing key mutations like Kras and Gnas in pancreatic organoids. Huang et. al. showed that GNASR201C induces cystic growth in ductal than acinar organoids but shows no IPMN phenotypes in vivo. KRASG12D induces PanIN and early PDAC lesions in acinar organoids while developing IPMN phenotype in vivo in ductal organoids [169]. Moreover, Breunig et.al. showed the development of early PDAC/PanIN lesions in pancreatic duct-like organoids by KRASG12D while GNASR201C resulted in IPMN structure in vivo [181].
The organoid culture is a great model for the study of PC, especially to better understand the biological features of precursor lesions and progression to PDAC. This pre-clinical model provides an opportunity to test various chemotherapy resistance, drug sensitivity and predicts the therapeutic outcome, thus saving time and providing a better understanding of PDAC and its precursors tumor biology. However, there are several limitations like lack of standard organoid culture protocols, challenges in long-term passages, or expansion of organoids. Hence, further studies are necessary for this field to establish organoid 3D culture as a good pre-clinical model for pancreatic cancer.
Animal Models
The use of a genetically engineered mouse model (GEMM) provides better insights into the developmental process of PDAC from IPMN. Although many GEMMs have been used to study PC and PDAC that are developed from PanIN lesions over the years, very few have been used for IPMN studies [182, 183]. The lack of a robust mouse model has restricted the study of the underlying molecular progression mechanism of IPMN to PDAC. Here, we have assembled all the IPMN GEMMs (Table 2) and characterized their outcomes and contributions in the field (Figure 4).
Table 2 :
Various GEMMs used for IPMN Study
| GEMM Phenotype | Lesions/Tumor | Time for Lesion Development | IPMN Subtype | Metastasis | References |
|---|---|---|---|---|---|
| Ptf1a-Cre; LSL-KrasG12D; and Smad4f/f | IPMN and PDAC | 2–3 Months | Gastric | YES | Bardeesy et al., 2006 |
| P48-Cre; LSL-KrasG12D; and Ela-Tgfa | IPMN, PanIN and PDAC | 2–3 Months | Pancreatobiliary | YES | Siveke et al., 2007 |
| Pdx1-Cre; LSL-KrasG12D; and Tif1γf/f | N/A | 7 Weeks | NA | NA | Vincent et al., 2009 Vincent et al., 2012 |
| LSL-KrasG12D; Pdx1-Cre+/− and TP53INP−/− | ADM, IPMN, PanIN | 2 months | NA | NA | Saati et. al., 2013 |
| Ptf1a-Cre; LSL-KrasG12D; and Brg1f/f | IPMN, PDAC | 9 Weeks | Pancreatobiliary | NA | Von Figura et al., 2014 |
| Pdx1-Cre; LSL-KrasG12D; and Acvr1bf/f | ADM, IPMN, PanIN, PDAC | 3 Months | NA | YES | Qiu et al., 2016 |
| Pdx1-Cre; LSL-KrasG12D; and Tff2−/− | IPMN, PDAC | 6 Weeks | Gastric | YES | Yamaguchi et al., 2016 |
| Ptf1a-cre; LSL-KrasG12D; and CAG-LSL-GnasR201H; | IPMN | 5 Weeks | Pancreatobiliary and Gastric | N/A | Taki et al., 2016 |
| K5 COX-2. p48-Cre/KrasG12D | IPMN, PanIN | 1 Month | Gastric | NA | Chiblak et all., 2016 |
| P48-Cre;LSL-KrasG12D Rosa26R-LSL-rtTA-Tet-OGnasR201C | IPMN (+Dox), PanIN (−Dox) | 18 weeks | Pancreatobiliary | YES | Ideno et al., 2018 |
| Sox9-CreERT2; LSL-KrasG12D; and Ptenf/+ Sox9-CreER; LSL-KrasG12D; and Lkb1f/f | IPMN | 6–12 Months 4–8 months |
Pancreatobiliary and Oncocytic Pancreatobiliary |
NA | Kopp et al., 2018 |
| Foxa3-Cre; HER2N | IPMN, PanIN, | 8 Weeks | Pancreatobiliary and Oncocytic | Shibata et al., 2018 | |
| Ptf1a-Cre; KrasG12D; and Arid1af/f | IPMN, PDAC | 2.3 Months | Pancreatobiliary, Gastric and Oncocytic | YES | Wenjia Wang et al., 2019 Kimura et al., 2018 |
| Sox9-CreER; LSL-KrasG12D; and Lkb1f/f | IPMN, PanIN, | 2 Months | Gastric | NA | Collet et al.,2019 |
| Ptf1aCre/+; Nr5a2lox/+; Kras(G12V)+/KI | IPMN,, PanIN, PDAC | 5 Months | Pancreatobiliary | NA | Cobo et al., 2020 |
| KrasLSL-G12D/+; Jag1flox/flox; Pdx1-Cre | ADM, PanIN, SCN, IPMN | 3 Weeks | NA | NA | Chung et al., 2021 |
Figure 4: Development of Genetically Engineered Mouse Models (GEMM) for IPMN studies.
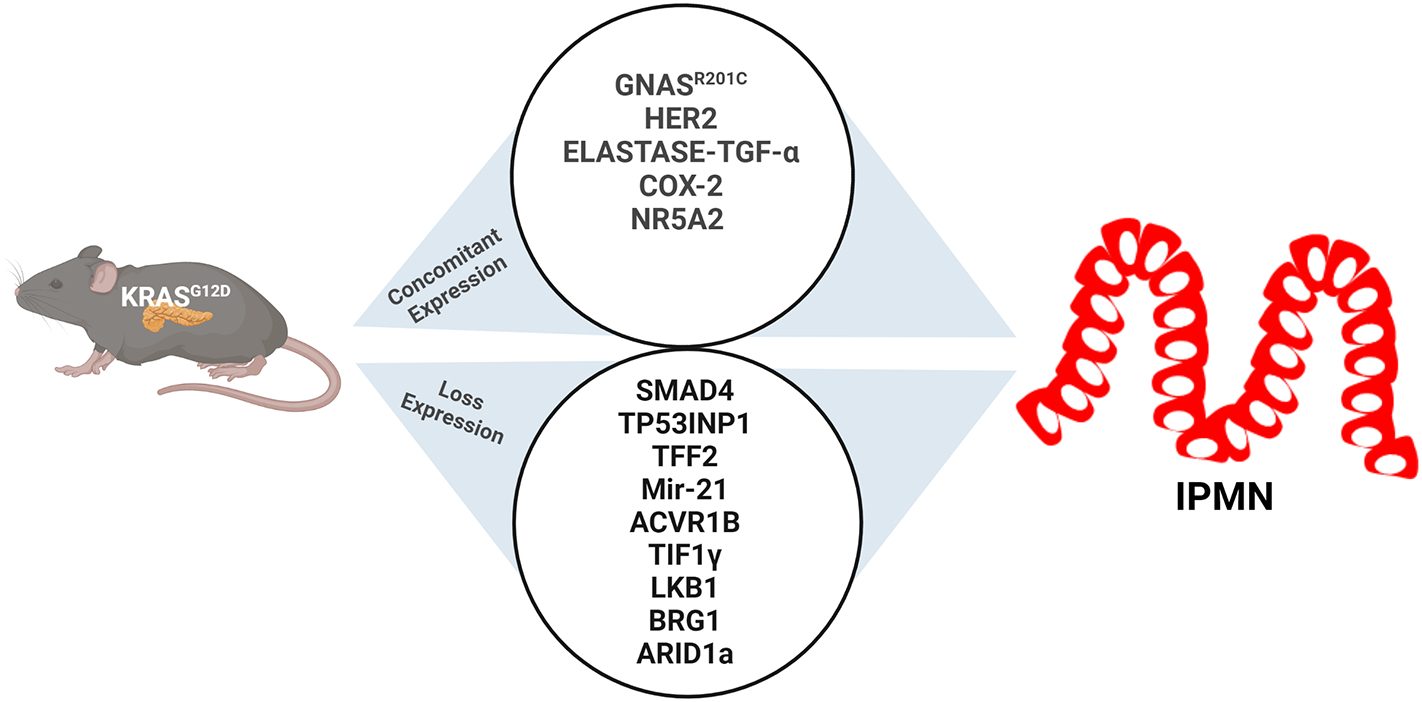
IPMN studies with GEMMs show that the concomitant expression of mutant Kras, with Gnas, HER2, Elastage-TGFα, Cox2, and NR5A2, develops IPMN lesions. On the other hand, the mutant Kras also co-operates with loss of expression mutations in Smad4, TP53, INP1, TFF2, Mir-21, ACVR1B, TIF1γ, LKB1, BRG1, and ARID1A and drives IPMN.
GEMM targeting GCPR
Taki et al. [184] showed that the synergistic expression of KRAS and GNAS elevates pancreatic tumorigenesis with cystic lesion development, imitating IPMN in the mouse model. However, this conditional transgenic mouse model, Tg (CAG-LSL-GNAS); LSL-KrasG12D; Ptf1aCre/+ shows some limitations. IPMN lesions are not developed in the presence of GNAS mutation alone. However, mutations in GNAS only and not KRAS have been shown in many studies. GNAS mutation is found in the intestinal subtype of human IPMN but was absent in this model. In addition, the study of the entire progression of IPMN to PDAC was hindered by the premature death of mice.
Ideno et al. [185] generated a GEMM, p48-Cre; LSL-KrasG12D; Rosa26R-LSL-rtTA-TetO-GnasR201C, also known as Kras; Gnas model with a main goal to elucidate the function of GNAS mutation with background expression of KRAS in IPMN progression. The mice model recapitulates the development of human IPMN lesions and the multistep progression to PDAC with the inhibition of YAP signaling.
GEMM targeting PI3K
Kopp et al. [186] targeted the PI3K pathway by disrupting PTEN in ductal-specific cells and generated two mouse models; Sox9-CreERT2; LSL-KrasG12D; Ptenf/+ and Sox9-CreER; LSL-KrasG12D; and Lkb1f/f with and without the activated KRAS. These models result in the development of different IPMN subtypes: pancreatobiliary when KRAS is present and pancreatobiliary and oncocytic when KRAS is not present. Both studies supported the ductal cell origin of IPMN and the formation of IPMN lesions associated with PTEN loss. Furthermore, the loss of PTEN association with activated KRAS is important in the formation of IPMN and its progression to PDAC.
GEMMs targeting TGFβ
Bardeesy et al. [187] developed a GEMM, Pdx1-Cre; K-Ras+/LSLG12D; Smad4lox/lox, also known as the KPSD4 model, to investigate the role of Smad4 in IPMN and progression of pancreatic cancer. This model is the first GEMM of IPMN. Smad4 has no function in the normal pancreatic epithelium, but its absence, combined with the KRAS mutation, results in the development of a tumor similar to human IPMN. The study demonstrated that Smad4 inactivation is related to limitations in IPMN initiation and its progression to high-grade lesions. Moreover, the loss of Ink4a/Arf concurrent with Smad4 deficiency was found not to have any role in IPMN and regulates distinct pathways for malignant progression and tumor suppression of PDAC.
Activin is a member of the TGFβ family, and the mutation in the activin A receptor type 1B (ACVR1B) gene is involved in PDAC. Qiu et al. investigated the role of ACVR1B in pancreatic tumorigenesis. They generated a mouse model; Pdx1-Cre; LSL-KrasG12D; and Acvr1bf/f with the loss of ACVR1B and activated KrasG12D. This model accelerated the development and progression of IPMN, indicating the possible involvement of ACVR1B as a tumor suppressor in pancreatic tumorigenesis. Moreover, the study also demonstrated the involvement of NOTCH and loss of p16 in this process [188].
Vincent et al. investigated the role of TIF1 (Transcriptional Intermediary Factor 1 gamma) in pancreatic cancer, which has been shown to regulate the TGF signaling pathway. They generated Pdx1-Cre; LSL-KrasG12D; and Tif1γf/f mice to elucidate the loss of function of TIF1 in precursor lesions of PC induced by Kras mutation [189]. Their model showed that inactivated TIF1γ cooperated with KrasG12D, resulting in the induction of human IPMNs, and these lesions also resemble the ones observed in Pdx1-Cre; K-Ras+/LSLG12D; Smad4lox/lox in Bardeesy et al. Furthermore, the study revealed TIF1’s tumor suppressive role in the pancreas and validated the importance of both TIF1 and SMAD4 in tumor progression.
The epithelial compartment that coiled glands buds off the pancreatic ducts and functions as a progenitor niche for the ductal epithelium known as the pancreatic duct gland (PDG) [190]. PDG expresses gastric mucins and trefoil factor family 2 (TFF2) and is reported to resemble the reminiscent of side-branch IPMN after the injury [190, 191]. Since the expression of TFF2 is also higher in IPMN compared to normal ductal epithelium [192], Yamaguchi et al. investigated the possible link between PDG and IPMN and the involvement of TFF2 in IPMN development [193]. They developed a TFF2 knockout model, Pdx1-Cre; LSL-KrasG12D; and Tff2−/− that resulted in the formation of IPMN. This model study revealed that PDG is the major site of epithelium proliferation, giving rise to the papillary projection characteristics of IPMN. PDG also showed the potential tumor suppression mechanism partially mediated by SMAD4 in IPMN lesions.
GEMM targeting SWI/SNF
Bhrama-related gene (Brg1) is the catalytic subunit of chromatin-remodeling SWI/SNF complexes. The mutations of Brg1 in PDAC and loss in expression have been reported in human IPMN samples [119, 194]. Von Figure et al. developed a Brg1 null mouse model; Ptf1a-Cre; LSL-KrasG12D; and Brg1f/f, which led to the development of neoplastic lesions resembling human IPMN. Their study showed that Brg1 deletion promotes IPMN development, promotes IPMN development, and suppresses the PanIN development in the context of KRAS mutation, indicating the role of Brg1 in pancreatic tumorigenesis based on the circumstance [195].
Two different groups: Wenija Wang et al. [196] and Kimura et al. [197] studied the role of AT-rich interaction domain-containing protein 1A(ARID1A), the most mutated member of SWI/SNF complex in PDAC [198]. Wang et al. established a mouse model; Ptf1a-Cre; KrasG12D; and Arid1af/f, with the deletion of Arid1a cooperated with mutant KRAS, followed by the formation of IPMN. Furthermore, Kimura et al. further validated the low expression of Arid1a in IPMN and PDAC human samples and the inhibitory role of Arid1a in IPMN and PDAC development.
GEMM targeting Wnt/β-catenin
The loss of function of liver kinase B1(LKB1) mutation is associated with mutant Kras to cause PDAC by suppressing p21-dependent growth arrest and having a poor prognosis [199]. LKB1 mutation is also commonly reported in IPMN and carries a higher risk of developing PDAC [200, 201]. Collet et al. [120] collaborated on the KRAS and LKB1 mutations and studied their involvement in developing IPMN with the creation of a new mouse model, Sox9-CreER; LSL-KrasG12D, and Lkb1f/f. They showed that the inactivation of LKB1 with the KRAS mutation led to the formation of gastric-type IPMN and supported the ductal cells as the origin of IPMN.
GEMM targeting EGFR
Siveke et al. [202] investigated the concurrent effect of growth factor, TGFa and KRAS mutation in the endogenous mouse model. The development of a GEMM, Ptf1a-Cre; K-Ras+/LSLG12D; Elastase-TGF-α led to PANin and cystic lesions resembling human IPMN. While the concomitant expression of TGFa and KRAS enhanced the progression of PanIN lesions to PDAC, the results from GEMM also showed the potential role of Egfr signaling in the development and progression mechanism of IPMN PDAC.
GEMM targeting TP53INP1
The expression of Tumor protein p53-induced protein 1 (TP53INP1), involved in cell stress response and antioxidant function, is lost in PC progression [203]. The study by Saati et al. shows that a GEMM with TP53INP1 knockout, LSL-KrasG12D; Pdx1-Cre+/− and TP53INP−/− with the concomitant Kras mutation accelerates PanIN and induces the formation of IPMN. Furthermore, they demonstrated that this phenotype could be rescued with antioxidant treatment, indicating that the loss of TP53INP1 caused an increase in oxidative stress, which resulted in the initiation of cystic lesions in collaboration with Kras [204].
GEMM targeting NR5A2
NR5A2, an orphan nuclear receptor, is a transcription factor that regulates various tumor oncogenes and suppressor genes that drive PC [205, 206].The partial loss-of-function of NR5A2; constitutive inactivation of one allele of NR52A cooperates with mutant Kras resulting in acinar-ductal metaplasia and PanIN and promoting PDAC development in the germline model [195, 207]. However, Cobo et al. in their Ptf1aCre/+; Nr5a2lox/+; Kras(G12V) +/KI model, showed that unlike in germline, the conditional heterozygosity of NR5A2 along with mutant Kras results in cystic lesions of Pancreatobiliary type via replacement of pancreatic parenchyma and develops to PDAC. Further, the model also showed that the progression of cystic lesions to PDAC accelerates due to pancreatitis [208].
GEMM targeting Cox-2
Chiblak et al. have shown the involvement of cyclooxygenase-2 (Cox-2) in the pancreatic neoplasm. They generated compound mutant mice, K5 COX-2. p48-Cre/ KrasG12D and found that the concurrent activation of Cox-2 and Kras mutant results in a cystic papillary phenotype that resembles human IPMN [209].
GEMM targeting Notch
Chung et al. investigated the role of Jag 1 loss in Kras-driven pancreatic tumorigenesis, with Notch signaling becoming more important in pancreatic cancer. They generated KrasLSL-G12D/+; Jag1flox/flox; Pdx1-Cre (KJC) mice model that develops ADM and PanIN lesions, which further transformed to IPMN serous cystic neoplasm [210]. They further developed another model, KrasLSL-G12D/+; Jag1flox/flox; Sox9-Cre, to only study the effect of Jag1 deletion in pancreatic ductal cells. This model, however, did not result in the formation of pancreatic cystic neoplasm, despite previous research showing that Lkb1, which is regulated by Jag1, in collaboration with Kras mutation, induced the formation of IPMN [211].
GEMM targeting HER2
Human epidermal growth factor-2 (HER2) is a receptor tyrosine kinase that has been linked to the oncogenesis of several cancers, including poor prognosis in PDAC [212]. Shibata et al. explored the role of pancreas-specific overexpression of HER2 alone and with Kras mutation in a GEMM. They developed two mouse models: Foxa3-Cre; HER2NT, which led to the formation of IPMN, and Ptfa-Cre; Kras; HER2NT resulting in the acceleration of PanIN development [213].
GEMMs for MCNs
The higher expression of miR-21 is found in the precursor lesions of PDAC and is upregulated as they progress [214]. While there is evidence from in vitro studies for the carcinogenesis role of miR-21, further validation in animal models is limited. Schipper et al. studied the influence of miR-21 loss in a well-established GEMM of PDAC (KPC) by developing a new GEMM with miR-21 knockout. The global loss of miR-21 in the KPC21KO (LSL-KrasG12D; p53lox/+; Pdx1-Cre; Mir-21KO/KO) model led to the development of MCN, which accelerated the tumor development resulting in the early death of 15 weeks. The tumor-suppressor role of miR-21 from this PDAC model provides possible clinical implications for miR-21-based therapeutic strategy [215].
Izeradjene et al. investigated how Smad4/Dpc4 loss of heterozygosity (LOH) affects pancreatic cancer initiation. They combined the Kras-G12D mutation with Smad4/Dpc4 haploinsufficiency to create a GEMM; KrasLSL-G12D/+; Dpc4flox/+; p48Cre/+ (KD) that developed PanIN and MCN. The model shows that Kras mutation in combination with Smad4/Dpc4 LOH develops MCN, while the combination of mutations in Kras with p53 or p16 takes the PanIN route to PDAC [211].
Mao et al. [216], in the process of developing a somatic mouse model for sporadic tumors, discovered the role of Smoothened (Smo) in the pancreatic cystic lesion. Smo is a key component of the Hh signaling pathway and regulates the Hh signaling pathway and cellular processes like cell growth, invasion, and migration in cancer [217]. Their murine model, CAGGS-CreER; R26-SmoM2 developed pancreatic lesions and were found to resemble low-grade MCN in humans. This research revealed the significance of activated Smo in the development of MCN and provided a model system for studying Hh-dependent tumorigenesis.
HIF2α is an isoform of hypoxia-inducible factor (HIF) associated with pancreatic diseases, and its deletion promotes the progression of PC [218]. Schofield et al. investigated the effects of HIF2α expression combined with Kras mutation in the development of PDAC. Two GEMMs with a stabilized expression of HIF2α and oncogenic Kras developed in this study, Ptf1a-Cre; LSL- KrasG12D; HIF2a-LSL/+ and Pdx1-Cre; LSL-KrasG12D HIF2a-LSL/+ showed the formation of cystic lesion resembling human MCN. This study provides insight into the role of hypoxia in the initiation of cystic neoplasm that develops into PDAC [219]
Sano et al. [220] studied the involvement of Wnt signaling in the Kras-mediated development of MCN in mice. They developed LSL-KrasG12D; Ptf1a-cre; elastase-tva mice and injected them with RCAS-Wnt1 to activate Wnt signaling. This model shows that the activation of Wnt signaling promotes the formation of cystic lesions resembling human MCN.
Over the years, many studies have been done on the resected IPMN samples from human patients. These studies have mainly focused on understanding the tumor biology of IPMN and the development of biomarkers. While the outcomes of these studies have provided a better understanding of IPMN and new screening methods, the underlying progression mechanism of IPMN to PDAC is still unknown. The fact that surgery is only recommended for high-risk patients presents a limitation in studying the molecular mechanism of IPMN progression with the resected samples. Hence, the development of stable GEMM for IPMN is essential.
Therapeutic Drugs and Clinical Trials for IPMN
To date, no FDA-approved therapeutic drug is available for treating IPMN and preventing its progression to PDAC. With surgical intervention and active surveillance being the only available treatment options for IPMN, it is vital to explore the drug therapy route for better outcomes for IPMN patients. Over the years, few drugs have been utilized and studied in IPMN clinical to develop non-operative options for IPMN patients (Table 3).
Table 3.
Clinical trials of various therapeutic drugs used for the treatment and preventive studies of IPMN
| Drug | Candidates | Age | Gender | Allocation | Dosage Time Period | Follow Up | Current Status |
|---|---|---|---|---|---|---|---|
| Celecoxib/COX-2 Inhibitor NCT00198081 |
Patients diagnosed with IPMN | 18 Years and older | All | Non-Randomized | Oral 200mg BID twice a day 6–8 weeks prior to surgery for surgical candidate and for 6 months for others | EUS or ERCP after 6 months | Phase-2 Terminated |
| Sulindac NCT04207944 |
Patients diagnosed with high-risk IPMN | 21–85 Years old | All | Randomized double-blind placebo controlled | Oral 200mg BID /day 3 years | Assessment of serum CMP, CBC, and CA19-9 every 6 months, EUS performed 6 months after randomization (+/− 4 weeks) and then annually. Pancreatic CT performed 1 year after randomization (+/− 4 weeks) and then annually such that EUS and CT is alternative | Phase-2 Ongoing |
| Erlotinib Hydrochloride NCT00482625 |
Patients diagnosed with IPMN, Recurrent, Stage IA, Stage IB, Stage IIA, Stage IIB, Stage III Pancreatic Cancer | 18 Years and older | All | Non-applicable (N/A) | 100mg orally once daily for 21–42 days before surgery | Surgery after treatment, protein/MUC5AC expression analysis, IPMN cells numbers, analysis of Erlotinib levels in plasma and pancreas | Phase-2 Terminated |
| Delta-Tocotrienol NCT00985777 |
Patients diagnosed with IPMN, MCN, PDAC | 18 Years and older | All | Non-applicable (N/A) | Orally twice daily. Either 100-mg, 200-mg, or 400-mg capsules for 14 consecutive days and one dose on Day of surgery | Study of Safety, bioavailability, Pharmacokinetics, and Pharmacodynamics for total 12 weeks per participants | Phase 1 Completed |
| NanoPac NCT0318899 |
Mainly Patients diagnosed with MCN and BD-IPMN | 18 Years and older | All | Non-Randomized | 6mg/ml, 10mg/ml, 15 mg/ml in a volume sufficient to fill or equal to the amount of cyst fluid aspirated in dose escalation phase. In second phase, best dose with 2 injections administered 12 weeks apart by intra-cystic injection | Patients followed for 6 months. Plasma sample collection at 1 and 2 hours after injection and subsequent visits for pharmacokinetics and cyst volume assessment by imaging | Completed |
Nonsteroidal anti-inflammatory drugs (NSAID)
NSAIDs are one of the most commonly oldest used drugs that reduce pain, fever, and inflammation. These drugs are known to inhibit cyclooxygenase-2 (COX-2), an enzyme that is an inducible form of cyclooxygenase and is expressed by inflammatory cells like macrophages [221, 222]. Cox-2 catalyzes the synthesis of prostaglandins and thromboxanes [223], and NSAIDs mediate their anti-inflammatory role by inhibiting this synthesis [222]. Previous research has linked the use of NSAIDs to a reduction in malignant progression and cancer growth because they target the cyclooxygenase enzyme, which is involved in carcinogenesis. NSAIDs like Celecoxib and Sulindac have been used in the chemoprevention of colorectal cancer and have shown prominent results in targeting COX-2 [224]. High levels of COX-2 are also found in other cancers like lung, breast, skin, prostate, bladder, head/neck, and pancreatic tumors, indicating COX-2 inhibitors as a potential therapeutic option [225]. However, studies have shown mixed results, with some reporting reduction in the risk of these cancers and some with no effect.
In terms of pancreatic cancer, Sulindac, in combination with parthenolide analog LC-1, suppresses the cancer cell growth in vitro and tumor growth in vivo by altering the cyclin D1 levels [226]. Moreover, the antitumor activity of Celecoxib is mediated through the inhibition of vascular endothelial growth factor (VGFR) and hence the angiogenesis with further reduction in cancer growth and metastasis [227]. As COX-2 inhibitors that reduce inflammation seem to be effective in pancreatic cancer, and since inflammation might be the driver of PC, Celecoxib and Sulindac have been used in clinical trials to study the IPMN Progression and prevention.
Erlotinib Hydrochloride
Erlotinib Hydrochloride is an Epidermal Growth Factor Receptor (EGFR) inhibitor, a transmembrane domain tyrosine kinase receptor [228]. The activation of EGFR leads to a cascade of pathways that regulates cell proliferation, apoptosis, angiogenesis, invasions, and metastasis [229, 230]. The EGFR expression is frequently found in epithelial tumors like non-small lung cancer, pancreatic cancer, and head and neck cancers and hence provides a rationale for targeting EGFR for cancer therapy. FDA approved Erlotinib as a chemotherapeutic agent for non-small lung cancer [231]. In combination with Gemcitabine, Erlotinib improved pancreatic cancer patients’ survival [232]. Erlotinib increases Gem-induced apoptosis in PDAC, although the mechanism by which it influences the EGFR signaling cascade and its downstream molecular mechanisms remain unknown. Erlotinib hydrochloride has been used not only in the research phase of various stages of PC but also in IPMN.
NanoPac/Paclitaxel
NanoPac is a submicron drug (nanoparticulate) particle of Paclitaxel produced using a technique known as Precipitation with Compressed Antisolvent (PCA). Supercritical carbon dioxide and acetone are employed to generate paclitaxel nanoparticles within a well-characterized particle-size distribution with PCA (ClinicalTrials.gov Identifier: NCT04314895). Paclitaxel, also known as Taxol, is a natural compound derived from Taxus brevifolia and is widely used as an anticancer drug [233]. Paclitaxel arrests the cells in the metaphase of the cell cycle, inhibiting cell mitosis and cell division and causing cell death (NCI thesaurus). Paclitaxel is an FDA-approved chemo-preventive agent used to treat ovarian, breast, and lung cancers. Kaposi’s sarcoma is also used off-label to treat various cancers like lymphoma, sarcoma, leukemia, and gastroesophageal [234]. Paclitaxel and NanoPac have been used in clinical trials for prostate, lung, and pancreatic cancers. The clinical trial for investigating the potential of Nanopac in killing cancer cells in non-surgical PDAC and preventing for progression of cystic lesions like MCN and BD-IPMN is also in the continuation phase.
Delta-Tocotrienol
Delta-Tocotrienol, or DT3 or DTT, is an antioxidant Vitamin E with anticancer effects. DTT s been used to study multiple cancers like prostate, ovarian, pancreas, colorectal, etc. [235–238]. In terms of PCs, DTT s been found to feature the induction of cell cycle arrest and apoptosis and prevent angiogenesis and invasion. Recent studies have identified DTT as a novel chemoprevention agent for PDAC [239, 240]. Moreover, studies have shown that DTT does not affect the normal pancreatic ductal cells making it a good candidate for treating PCs. The first phase of the clinical trial for DTT in patients with pancreatic ductal neoplasm has also been concluded [241].
The involvement of Kras mutation in cancer was considered untreatable for a long time, but recently, there has been much success in therapeutic response targeting Kras and its downstream signaling pathways in various cancers, including PC [242]. The discovery of Kras inhibitors like ARS-1620 by Janes et al. [243] and its superior form AMG 510 by Canon et al. [244]have shown promising results in various cancer treatments. Clinical trials like testing the autophagy hydroxychloroquine and the MEK inhibitor binimetinib (Clinicaltrials.gov Identifier: NCT04132505) and Selumetinib sulfate (Clinicaltrials.gov Identifier: NCT03040986) targeting Kras in PC are ongoing at MD Anderson. Moreover, they are in the process of targeting the KRAS activation using son of sevenless (SOS) protein inhibitors and targeting the specific mutation of Kras like KRAS G12D, the most common mutation found in IPMN by combining siRNA with an exosome called “iExosomes”. These studies provide hope for developing Kras mutation inhibitors that drive the IPMN initiation and other precancerous lesions of PCs.
Targeting the GCPR signaling and/or GNAS mutant cAMP-PKA pathway could open new opportunities for treating and preventing IPMN and other GNAS mutation-specific cancers. Currently, 475 drugs (~34% of FDA-approved drugs) act on 108 unique GPCR targets and 321 agents in clinical trials, with ~20% focusing on 66 new GPCR targets [245]. Moreover, there are ongoing studies on PKA inhibitors and the development of small-molecule PKA inhibitors [131] (Table 3).
The lack of appropriate chemoprevention strategies for PDAC and its precursor lesions leading to significant mortality and morbidity requires the development of effective therapeutic agents for this disease. This need is even more urgent for IPMN patients as most are not qualified for surgery and are in active surveillance without any treatment. The focus needs to shift towards developing novel or repurposing available chemopreventive drugs for IPMN patients. More studies in clinical settings are required to identify appropriate agent(s) and patients to be tested in human clinical trials. Developing drugs for IPMN would save most IPMN patients in surveillance from years of pain, stress, and expenses and provide better survival outcomes.
Conclusion and Overall Perspective
PCNs include IPMN, MCN, and serous cystic neoplasms that develop into PDAC. This review discusses the molecular, diagnostic, and therapeutic underpinnings of PCNs. In addition to standard diagnostic imaging tools and biomarkers (for example, CA19.9), mucins are emerging as important promising biomarkers. Current PCN diagnosis and treatment guidelines must be modified based on the updated studies. Different mutations or loss/gain of functions results in the generation of IPMNs. Most studies focused on investigating how a particular mutation or loss of function leads to IPMN. Whether a single mutation or a combination of mutations drives, the PCNs needs to be thoroughly studied in future research. The knowledge about the commonalities and unique signatures of PanIN-derived PDAC and IPMN-derived PDAC is lacking. Hence, the upcoming studies should focus on delineating these aspects of PCNs. All these will help in the prognosis, development of PCN-specific therapies, and better management of patients with PCN-derived PDAC.
Acknowledgement
The authors in this article were supported primarily by the following grants from the National Institutes of Health R01 CA273349, R01 CA263575, R01 CA256973, R01 CA201444, R01 CA210637, R01 CA228524, U01 CA200466, U01 CA210240, P01 CA217798, and Nebraska Stem Cell Grant LB606/2022-10.
Footnotes
Competing Interests
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc. The other authors declare no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 2022, 72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Ranganath R, Chu QJJoGO: Global trends in pancreas cancer among Asia-Pacific population. 2020 2020:S374–S386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. 2020, 70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Ryan DP, Hong TS, Bardeesy N: Pancreatic adenocarcinoma. The New England journal of medicine 2014, 371(11):1039–1049. [DOI] [PubMed] [Google Scholar]
- 5.Riva G, Pea A, Pilati C, Fiadone G, Lawlor RT, Scarpa A, Luchini C: Histo-molecular oncogenesis of pancreatic cancer: From precancerous lesions to invasive ductal adenocarcinoma. World journal of gastrointestinal oncology 2018, 10(10):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi J, Yokoyama Y, Kokuryo T, Ebata T, Nagino M: Cells of origin of pancreatic neoplasms. Surg Today 2018, 48(1):9–17. [DOI] [PubMed] [Google Scholar]
- 7.Matthaei H, Schulick RD, Hruban RH, Maitra A: Cystic precursors to invasive pancreatic cancer. Nature reviews Gastroenterology & hepatology 2011, 8(3):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T et al. : An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. The American journal of surgical pathology 2004, 28(8):977–987. [DOI] [PubMed] [Google Scholar]
- 9.Hruban RH, Maitra A, Goggins M: Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008, 1(4):306–316. [PMC free article] [PubMed] [Google Scholar]
- 10.Distler M, Aust D, Weitz J, Pilarsky C, Grützmann R: Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. BioMed research international 2014, 2014:474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keane MG, Afghani E: A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. 2021, 10(6):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T et al. : A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015, 149(6):1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh H, Pillai K, Morris DL: Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res 2017, 7(6):1372–1383. [PMC free article] [PubMed] [Google Scholar]
- 14.Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F et al. : Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. The American journal of surgical pathology 1999, 23(4):410–422. [DOI] [PubMed] [Google Scholar]
- 15.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH et al. : A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. The American journal of surgical pathology 2015, 39(12):1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S: International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology: official journal of the International Association of Pancreatology (IAP) 2006, 6(1–2):17–32. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Naitoh Y, Tsukamoto Y, Kozuka S, Yamao K, Nakazawa S: Mucin-producing tumor of the pancreas. 1991, 68(1):159–168. [DOI] [PubMed] [Google Scholar]
- 18.Gnoni A, Licchetta A, Scarpa A, Azzariti A, Brunetti AE, Simone G, Nardulli P, Santini D, Aieta M, Delcuratolo S et al. : Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. International journal of molecular sciences 2013, 14(10):19731–19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baiocchi GL, Portolani N, Grazioli L, Mazza G, Gheza F, Bartoli M, Vanzetti E, Giulini SM: Management of pancreatic intraductal papillary mucinous neoplasm in an academic hospital (2005–2010): what follow-up for unoperated patients? Pancreas 2013, 42(4):696–700. [DOI] [PubMed] [Google Scholar]
- 20.Laurent L, Vullierme MP, Rebours V, Maire F, Hentic O, Francoz C, Durand F, Ruszniewski P, Lévy P: Estimation of the prevalence of intraductal papillary mucinous neoplasm of the pancreas in the French population through patients waiting for liver transplantation. United European gastroenterology journal 2017, 5(4):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanno S, Obara T, Koizumi K, Nakano Y, Osanai M, Mizukami Y, Kohgo Y: Risk of additional pancreatic cancer in patients with branch duct intraductal papillary-mucinous neoplasm. Clinical journal of gastroenterology 2009, 2(6):365–370. [DOI] [PubMed] [Google Scholar]
- 22.Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A: Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008, 57(11):1561–1565. [DOI] [PubMed] [Google Scholar]
- 23.Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T, Sakurai T, Uemoto S: Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World journal of surgery 2008, 32(2):271–278; discussion 279–280. [DOI] [PubMed] [Google Scholar]
- 24.Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S, Nakamura M, Mizumoto K, Tanaka M: Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery 2010, 147(6):812–817. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S et al. : Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 2011, 60(4):509–516. [DOI] [PubMed] [Google Scholar]
- 26.Castellano-Megías VM, Andrés CI, López-Alonso G, Colina-Ruizdelgado F: Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World journal of gastrointestinal oncology 2014, 6(9):311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmicheal J, Patel A, Dalal V, Atri P, Dhaliwal AS, Wittel UA, Malafa MP, Talmon G, Swanson BJ, Singh S et al. : Elevating pancreatic cystic lesion stratification: Current and future pancreatic cancer biomarker(s). Biochimica et biophysica acta Reviews on cancer 2020, 1873(1):188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang XY, Wu Y, Li Y, Wang J, Chen J: Intraductal papillary mucinous neoplasms of the pancreas: Clinical association with KRAS. Molecular medicine reports 2018, 17(6):8061–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong Y, Wang D, Xu C, Ji Y, Jin D, Wu W, Xu X, Kuang T, Lou W: Prognostic value of histological subtype in intraductal papillary mucinous neoplasm of the pancreas: A retrospective analysis of outcome from one single center. Medicine 2017, 96(15):e6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker AE, Hernandez YG, Frucht H, Lucas AL: Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World journal of gastroenterology 2014, 20(32):11182–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capasso M, Franceschi M, Rodriguez-Castro KI, Crafa P, Cambie G, Miraglia C, Barchi A, Nouvenne A, Leandro G, Meschi T et al. : Epidemiology and risk factors of pancreatic cancer. Acta Biomed 2018, 89(9-S):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker GA, Batheja MJ, Collins JM, Silva AC, Mekeel KL, Moss AA, Nguyen CC, Lake DF, Miller LJ: Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y) 2010, 6(4):246–254. [PMC free article] [PubMed] [Google Scholar]
- 33.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB: Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008, 393(4):535–545. [DOI] [PubMed] [Google Scholar]
- 34.Lin G, Zeng Z, Wang X, Wu Z, Wang J, Wang C, Sun Q, Chen Y, Quan H: Cholecystectomy and risk of pancreatic cancer: a meta-analysis of observational studies. Cancer Causes Control 2012, 23(1):59–67. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, Nordenvall C, Nyren O, Adami HO, Permert J, Ye W: The risk of pancreatic cancer in patients with gastric or duodenal ulcer disease. International journal of cancer 2007, 120(2):368–372. [DOI] [PubMed] [Google Scholar]
- 36.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG et al. : Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev 2009, 18(3):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawla P, Sunkara T, Gaduputi V: Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019, 10(1):10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashktorab H, Kupfer SS, Brim H, Carethers JM: Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology 2017, 153(4):910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McWilliams RR, Maisonneuve P, Bamlet WR, Petersen GM, Li D, Risch HA, Yu H, Fontham ET, Luckett B, Bosetti C et al. : Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas 2016, 45(2):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C et al. : Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006, 4(6):766–781; quiz 665. [DOI] [PubMed] [Google Scholar]
- 41.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK et al. : Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004, 2(7):606–621. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC: Feasibility and yield of screening in relatives from familial pancreatic cancer families. The American journal of gastroenterology 2011, 106(5):946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capurso G, Boccia S, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, Manta R, Fabbri C, Ventrucci M et al. : Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. The American journal of gastroenterology 2013, 108(6):1003–1009. [DOI] [PubMed] [Google Scholar]
- 44.Baumgaertner I, Corcos O, Couvelard A, Sauvanet A, Rebours V, Vullierme MP, Hentic O, Hammel P, Levy P, Ruszniewski P: Prevalence of extrapancreatic cancers in patients with histologically proven intraductal papillary mucinous neoplasms of the pancreas: a case-control study. The American journal of gastroenterology 2008, 103(11):2878–2882. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Kim WY, Hwang DY, Han HS: Intraductal papillary mucinous neoplasm of the ileal heterotopic pancreas in a patient with hereditary non-polyposis colorectal cancer: A case report. World journal of gastroenterology 2015, 21(25):7916–7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardeshna DR, Rangwani S, Cao T, Pawlik TM, Stanich PP, Krishna SG: Intraductal Papillary Mucinous Neoplasms in Hereditary Cancer Syndromes. Biomedicines 2022, 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamata K, Takenaka M, Nakai A, Omoto S, Miyata T, Minaga K, Matsuda T, Yamao K, Imai H, Chiba Y et al. : Association between the Risk Factors for Pancreatic Ductal Adenocarcinoma and Those for Malignant Intraductal Papillary Mucinous Neoplasm. Oncology 2017, 93 Suppl 1:102–106. [DOI] [PubMed] [Google Scholar]
- 48.Rezaee N, Khalifian S, Cameron JL, Pawlik TM, Hruban RH, Fishman EK, Makary MA, Lennon AM, Wolfgang CL, Weiss MJ: Smoking is not associated with severe dysplasia or invasive carcinoma in resected intraductal papillary mucinous neoplasms. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2015, 19(4):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carr RA, Roch AM, Shaffer K, Aboudi S, Schmidt CM 2nd, DeWitt J, Ceppa EP, House MG, Zyromski NJ, Nakeeb A et al. Smoking and IPMN malignant progression. American journal of surgery 2017, 213(3):494–497. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T, Masuda A, Toyama H, Shiomi H, Zen Y, Sofue K, Takenaka M, Kobayashi T, Yagi Y, Yamanaka K et al. : Smoking Status and the Incidence of Pancreatic Cancer Concomitant With Intraductal Papillary Mucinous Neoplasm. Pancreas 2017, 46(4):582–588. [DOI] [PubMed] [Google Scholar]
- 51.Traverso LW, Peralta EA, Ryan JA Jr., Kozarek RA: Intraductal neoplasms of the pancreas. American journal of surgery 1998, 175(5):426–432. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno S, Nakai Y, Ishigaki K, Saito K, Oyama H, Hamada T, Suzuki Y, Inokuma A, Kanai S, Noguchi K et al. : Screening Strategy of Pancreatic Cancer in Patients with Diabetes Mellitus. Diagnostics (Basel, Switzerland) 2020, 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsson A: Hydrolysis of chyle cholesterol esters with cell-free preparations of rat liver. Biochim Biophys Acta 1976, 450(3):379–389. [DOI] [PubMed] [Google Scholar]
- 54.Postlewait LM, Ethun CG, McInnis MR, Merchant N, Parikh A, Idrees K, Isom CA, Hawkins W, Fields RC, Strand M et al. : Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms: A Multicenter Study. JAMA Surg 2017, 152(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shioyama E, Mitoro A, Ogawa H, Kubo T, Ozutsumi T, Kitagawa K, Yoshikawa M, Ueda S, Akahori T, Marugami NA et al. : A pancreatic mucinous cystic neoplasm undergoing intriguing morphological changes over time and associated with recurrent pancreatitis: A case report. Medicine 2019, 98(28):e16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL: Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2017, 17(5):738–753. [DOI] [PubMed] [Google Scholar]
- 57.Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI: Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. Journal of magnetic resonance imaging: JMRI 2009, 30(3):586–595. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Xu W, Liu Z, Ye J: MRI Combined with Magnetic Resonance Cholangiopancreatography for Diagnosis of Benign and Malignant Pancreatic Intraductal Papillary Mucinous Neoplasms. Current medical imaging reviews 2019, 15(5):504–510. [DOI] [PubMed] [Google Scholar]
- 59.Crippa S, Arcidiacono PG, De Cobelli F, Falconi M: Review of the diagnosis and management of intraductal papillary mucinous neoplasms. United European gastroenterology journal 2020, 8(3):249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uribarri-Gonzalez L, Keane MG, Pereira SP, Iglesias-García J, Dominguez-Muñoz JE, Lariño-Noia J: Agreement among Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography (MRI-MRCP) and Endoscopic Ultrasound (EUS) in the evaluation of morphological features of Branch Duct Intraductal Papillary Mucinous Neoplasm (BD-IPMN). Pancreatology : official journal of the International Association of Pancreatology (IAP) 2018, 18(2):170–175. [DOI] [PubMed] [Google Scholar]
- 61.Hwang J, Kim YK, Min JH, Jeong WK, Hong SS, Kim HJ: Comparison between MRI with MR cholangiopancreatography and endoscopic ultrasonography for differentiating malignant from benign mucinous neoplasms of the pancreas. European radiology 2018, 28(1):179–187. [DOI] [PubMed] [Google Scholar]
- 62.Choi SY, Kim JH, Yu MH, Eun HW, Lee HK, Han JK: Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: a comparison of EUS, contrast-enhanced CT and MRI. Abdominal radiology (New York) 2017, 42(5):1449–1458. [DOI] [PubMed] [Google Scholar]
- 63.Serafini S, Sperti C, Brazzale AR, Cecchin D, Zucchetta P, Pierobon ES, Ponzoni A, Valmasoni M, Moletta L: The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers 2020, 12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang KM, Kim SH, Min JH, Lee SJ, Kang TW, Lim S, Choi D: Value of diffusion-weighted MRI for differentiating malignant from benign intraductal papillary mucinous neoplasms of the pancreas. AJR Am J Roentgenol 2014, 203(5):992–1000. [DOI] [PubMed] [Google Scholar]
- 65.Kang KM, Lee JM, Shin CI, Baek JH, Kim SH, Yoon JH, Han JK, Choi BI: Added value of diffusion-weighted imaging to MR cholangiopancreatography with unenhanced mr imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. Journal of magnetic resonance imaging : JMRI 2013, 38(3):555–563. [DOI] [PubMed] [Google Scholar]
- 66.Yashika J, Ohno E, Ishikawa T, Iida T, Suzuki H, Uetsuki K, Yamada K, Yoshikawa M, Gibo N, Shimoyama Y et al. : Utility of multiphase contrast enhancement patterns on CEH-EUS for the differential diagnosis of IPMN-derived and conventional pancreatic cancer. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2021, 21(2):390–396. [DOI] [PubMed] [Google Scholar]
- 67.Keane MG, Afghani E: A Review of the Diagnosis and Management of Premalignant Pancreatic Cystic Lesions. Journal of clinical medicine 2021, 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki R, Thosani N, Annangi S, Guha S, Bhutani MS: Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: a systematic review and meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2014, 14(5):380–384. [DOI] [PubMed] [Google Scholar]
- 69.Costa D, Guerra JG, Goldman SM, Kemp R, Santos JS, Ardengh JC, Ribas C, Nassif PAN, Ribas-Filho JM: MAGNETIC RESONANCE CHOLANGIOPANCREATOGRAPHY (MRCP) VERSUS ENDOSONOGRAPHY-GUIDED FINE NEEDLE ASPIRATION (EUS-FNA) FOR DIAGNOSIS AND FOLLOW-UP OF PANCREATIC INTRADUCTAL PAPILLARY MUCINOUS NEOPLASMS. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery 2019, 32(4):e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshikawa M, Morine Y, Ikemoto T, Imura S, Higashijima J, Iwahashi S, Saito YU, Takasu C, Yamada S, Ishikawa D et al. : Elevated Preoperative Serum CEA Level Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma Through the Epithelial-Mesenchymal Transition. Anticancer research 2017, 37(3):1169–1175. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Q, Zhan T, Deng Z, Li Q, Liu Y, Yang S, Ji D, Li Y: Glycan analysis of colorectal cancer samples reveals stage-dependent changes in CEA glycosylation patterns. Clinical proteomics 2018, 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, Zhou X, Zhou D, Huang P, Yang Q et al. : Serum carcinoembryonic antigen and carbohydrate antigen 19–9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: A meta-analysis. Biomedical reports 2015, 3(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ciprani D, Morales-Oyarvide V, Qadan M, Hank T, Weniger M, Harrison JM, Rodrigues C, Horick NK, Mino-Kenudson M, Ferrone CR et al. : An elevated CA 19–9 is associated with invasive cancer and worse survival in IPMN. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2020, 20(4):729–735. [DOI] [PubMed] [Google Scholar]
- 74.Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, Kaur S, Jain M, Batra SK, Ganti AK: Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 2015, 10(1):19–27. [DOI] [PubMed] [Google Scholar]
- 75.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK: Mucins in pancreatic cancer and its microenvironment. Nature reviews Gastroenterology & hepatology 2013, 10(10):607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nissim S, Idos GE, Wu B: Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas 2012, 41(8):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, Jarnagin WR, Brennan MF, Allen PJ: Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Annals of surgical oncology 2011, 18(1):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang KS, Ciprani D, O’Shea A, Liss AS, Yang R, Fletcher-Mercaldo S, Mino-Kenudson M, Fernández-Del Castillo C, Weissleder R: Extracellular Vesicle Analysis Allows for Identification of Invasive IPMN. Gastroenterology 2021, 160(4):1345–1358.e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohya A, Yamanoi K, Shimojo H, Fujii C, Nakayama J: Gastric gland mucin-specific O-glycan expression decreases with tumor progression from precursor lesions to pancreatic cancer. Cancer science 2017, 108(9):1897–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao Z, Maupin K, Curnutte B, Fallon B, Feasley CL, Brouhard E, Kwon R, West CM, Cunningham J, Brand R et al. : Specific glycoforms of MUC5AC and endorepellin accurately distinguish mucinous from nonmucinous pancreatic cysts. Molecular & cellular proteomics : MCP 2013, 12(10):2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haab BB, Porter A, Yue T, Li L, Scheiman J, Anderson MA, Barnes D, Schmidt CM, Feng Z, Simeone DM: Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Annals of surgery 2010, 251(5):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sinha J, Cao Z, Dai J, Tang H, Partyka K, Hostetter G, Simeone DM, Feng Z, Allen PJ, Brand RE et al. : A Gastric Glycoform of MUC5AC Is a Biomarker of Mucinous Cysts of the Pancreas. PloS one 2016, 11(12):e0167070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akimoto Y, Nouso K, Kato H, Miyahara K, Dohi C, Morimoto Y, Kinugasa H, Tomoda T, Yamamoto N, Tsutsumi K et al. : Serum N-glycan profiles in patients with intraductal papillary mucinous neoplasms of the pancreas. Pancreatology : official journal of the International Association of Pancreatology (IAP) 2015, 15(4):432–438. [DOI] [PubMed] [Google Scholar]
- 84.Aronsson L, Andersson R, Bauden M, Andersson B, Bygott T, Ansari D: High-density and targeted glycoproteomic profiling of serum proteins in pancreatic cancer and intraductal papillary mucinous neoplasm. Scandinavian journal of gastroenterology 2018, 53(12):1597–1603. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe K, Ohta M, Yada K, Komori Y, Iwashita Y, Kashima K, Inomata M: Fucosylation is associated with the malignant transformation of intraductal papillary mucinous neoplasms: a lectin microarray-based study. Surgery today 2016, 46(10):1217–1223. [DOI] [PubMed] [Google Scholar]
- 86.McIntyre CA, Pulvirenti A, Lawrence SA, Seier K, Gonen M, Balachandran VP, Kingham TP, DʼAngelica MI, Drebin JA, Jarnagin WR et al. : Neutrophil-to-Lymphocyte Ratio as a Predictor of Invasive Carcinoma in Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2019, 48(6):832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou W, Rong Y, Kuang T, Xu Y, Shen X, Ji Y, Lou W, Wang D: The value of systemic inflammatory markers in identifying malignancy in mucinous pancreatic cystic neoplasms. Oncotarget 2017, 8(70):115561–115569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arima K, Okabe H, Hashimoto D, Chikamoto A, Kuroki H, Taki K, Kaida T, Higashi T, Nitta H, Komohara Y et al. : The Neutrophil-to-Lymphocyte Ratio Predicts Malignant Potential in Intraductal Papillary Mucinous Neoplasms. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2015, 19(12):2171–2177. [DOI] [PubMed] [Google Scholar]
- 89.Gemenetzis G, Bagante F, Griffin JF, Rezaee N, Javed AA, Manos LL, Lennon AM, Wood LD, Hruban RH, Zheng L et al. : Neutrophil-to-lymphocyte Ratio is a Predictive Marker for Invasive Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Annals of surgery 2017, 266(2):339–345. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe Y, Niina Y, Nishihara K, Okayama T, Tamiya S, Nakano T: Neutrophil-to-lymphocyte ratio and mural nodule height as predictive factors for malignant intraductal papillary mucinous neoplasms. Acta chirurgica Belgica 2018, 118(4):239–245. [DOI] [PubMed] [Google Scholar]
- 91.Hata T, Mizuma M, Motoi F, Ishida M, Morikawa T, Takadate T, Nakagawa K, Hayashi H, Kanno A, Masamune A et al. : Diagnostic and Prognostic Impact of Neutrophil-to-Lymphocyte Ratio for Intraductal Papillary Mucinous Neoplasms of the Pancreas With High-Grade Dysplasia and Associated Invasive Carcinoma. Pancreas 2019, 48(1):99–106. [DOI] [PubMed] [Google Scholar]
- 92.Hernandez YG, Lucas AL: MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World journal of gastrointestinal oncology 2016, 8(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuratomi N, Takano S, Fukasawa M, Maekawa S, Kadokura M, Shindo H, Takahashi E, Hirose S, Fukasawa Y, Kawakami S et al. : MiR-10a in Pancreatic Juice as a Biomarker for Invasive Intraductal Papillary Mucinous Neoplasm by miRNA Sequencing. International journal of molecular sciences 2021, 22(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shirakami Y, Iwashita T, Uemura S, Imai H, Murase K, Shimizu M: Micro-RNA Analysis of Pancreatic Cyst Fluid for Diagnosing Malignant Transformation of Intraductal Papillary Mucinous Neoplasm by Comparing Intraductal Papillary Mucinous Adenoma and Carcinoma. Journal of clinical medicine 2021, 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Permuth JB, Chen DT, Yoder SJ, Li J, Smith AT, Choi JW, Kim J, Balagurunathan Y, Jiang K, Coppola D et al. : Linc-ing Circulating Long Non-coding RNAs to the Diagnosis and Malignant Prediction of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Scientific reports 2017, 7(1):10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Permuth-Wey J, Chen DT, Fulp WJ, Yoder SJ, Zhang Y, Georgeades C, Husain K, Centeno BA, Magliocco AM, Coppola D et al. : Plasma MicroRNAs as Novel Biomarkers for Patients with Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancer prevention research (Philadelphia, Pa) 2015, 8(9):826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vicentini C, Calore F, Nigita G, Fadda P, Simbolo M, Sperandio N, Luchini C, Lawlor RT, Croce CM, Corbo V et al. : Exosomal miRNA signatures of pancreatic lesions. BMC gastroenterology 2020, 20(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vila-Navarro E, Duran-Sanchon S, Vila-Casadesús M, Moreira L, Ginès À, Cuatrecasas M, Lozano JJ, Bujanda L, Castells A, Gironella M: Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clinical and translational gastroenterology 2019, 10(4):e00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park WG, Wu M, Bowen R, Zheng M, Fitch WL, Pai RK, Wodziak D, Visser BC, Poultsides GA, Norton JA et al. : Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointestinal endoscopy 2013, 78(2):295–302.e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaiser RA, Pessia A, Ateeb Z, Davanian H, Fernández Moro C, Alkharaan H, Healy K, Ghazi S, Arnelo U, Valente R et al. : Integrated targeted metabolomic and lipidomic analysis: A novel approach to classifying early cystic precursors to invasive pancreatic cancer. Scientific reports 2019, 9(1):10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yip-Schneider MT, Simpson R, Carr RA, Wu H, Fan H, Liu Z, Korc M, Zhang J, Schmidt CM: Circulating Leptin and Branched Chain Amino Acids-Correlation with Intraductal Papillary Mucinous Neoplasm Dysplastic Grade. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2019, 23(5):966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS et al. : Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature medicine 2014, 20(10):1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore HB, Culp-Hill R, Reisz JA, Lawson PJ, Sauaia A, Schulick RD, Del Chiaro M, Nydam TL, Moore EE, Hansen KC et al. : The metabolic time line of pancreatic cancer: Opportunities to improve early detection of adenocarcinoma. American journal of surgery 2019, 218(6):1206–1212. [DOI] [PubMed] [Google Scholar]
- 104.Morgell A, Reisz JA, Ateeb Z, Davanian H, Reinsbach SE, Halimi A, Gaiser R, Valente R, Arnelo U, Chiaro MD et al. : Metabolic characterization of plasma and cyst fluid from cystic precursors to pancreatic cancer patients reveal metabolic signatures of bacterial infection. medRxiv : the preprint server for health sciences 2020. [DOI] [PubMed] [Google Scholar]
- 105.Saraswat M, Nieminen H, Joenvaara S, Tohmola T, Seppänen H, Ristimäki A, Haglund C, Renkonen R: Label-free serum proteomics and multivariate data analysis identifies biomarkers and expression trends that differentiate Intraductal papillary mucinous neoplasia from pancreatic adenocarcinoma and healthy controls. Translational Medicine Communications 2019, 4(1):6. [Google Scholar]
- 106.Saha B, Chhatriya B, Pramanick S, Goswami S: Bioinformatic Analysis and Integration of Transcriptome and Proteome Results Identify Key Coding and Noncoding Genes Predicting Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. BioMed research international 2021, 2021:1056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Do M, Kim H, Shin D, Park J, Kim H, Han Y, Jang JY, Kim Y: Marker Identification of the Grade of Dysplasia of Intraductal Papillary Mucinous Neoplasm in Pancreatic Cyst Fluid by Quantitative Proteomic Profiling. Cancers 2020, 12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Do M, Han D, Wang JI, Kim H, Kwon W, Han Y, Jang JY, Kim Y: Quantitative proteomic analysis of pancreatic cyst fluid proteins associated with malignancy in intraductal papillary mucinous neoplasms. Clinical proteomics 2018, 15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG et al. : Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014, 146(3):647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franses JW, Basar O, Kadayifci A, Yuksel O, Choz M, Kulkarni AS, Tai E, Vo KD, Arora KS, Desai N et al. : Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms. The oncologist 2018, 23(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67(5):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vege SS, Ziring B, Jain R, Moayyedi P: American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148(4):819–822; quize812–813. [DOI] [PubMed] [Google Scholar]
- 113.Elta GH, Enestvedt BK, Sauer BG, Lennon AM: ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. The American journal of gastroenterology 2018, 113(4):464–479. [DOI] [PubMed] [Google Scholar]
- 114.Crippa S, Pezzilli R, Bissolati M, Capurso G, Romano L, Brunori MP, Calculli L, Tamburrino D, Piccioli A, Ruffo G et al. : Active Surveillance Beyond 5 Years Is Required for Presumed Branch-Duct Intraductal Papillary Mucinous Neoplasms Undergoing Non-Operative Management. The American journal of gastroenterology 2017, 112(7):1153–1161. [DOI] [PubMed] [Google Scholar]
- 115.Hirono S, Shimizu Y, Ohtsuka T, Kin T, Hara K, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H et al. : Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. Journal of gastroenterology 2020, 55(1):86–99. [DOI] [PubMed] [Google Scholar]
- 116.Vanden Bulcke A, Jaekers J, Topal H, Vanbeckevoort D, Vandecaveye V, Roskams T, Weynand BA, Dekervel J, Van Cutsem E, van Malenstein H et al. : Evaluating the accuracy of three international guidelines in identifying the risk of malignancy in pancreatic cysts: a retrospective analysis of a surgical treated population. Acta gastro-enterologica Belgica 2021, 84(3):443–450. [DOI] [PubMed] [Google Scholar]
- 117.Noë M, Niknafs N, Fischer CG, Hackeng WM, Beleva Guthrie V, Hosoda W, Debeljak M, Papp E, Adleff V, White JR et al. : Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nature communications 2020, 11(1):4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schonleben F, Qiu W, Remotti HE, Hohenberger W, Su GH: PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch Surg 2008, 393(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dal Molin M, Hong SM, Hebbar S, Sharma R, Scrimieri F, de Wilde RF, Mayo SC, Goggins M, Wolfgang CL, Schulick RD et al. : Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Human pathology 2012, 43(4):585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collet L, Ghurburrun E, Meyers N, Assi M, Pirlot B, Leclercq IA, Couvelard A, Komuta M, Cros J, Demetter P et al. : Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2020, 69(4):704–714. [DOI] [PubMed] [Google Scholar]
- 121.Skaro M, Nanda N, Gauthier C, Felsenstein M, Jiang Z, Qiu M, Shindo K, Yu J, Hutchings D, Javed AA et al. : Prevalence of Germline Mutations Associated With Cancer Risk in Patients With Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2019, 156(6):1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou B, Der CJ, Cox AD: The role of wild type RAS isoforms in cancer. Seminars in cell & developmental biology 2016, 58:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simanshu DK, Nissley DV, McCormick F: RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170(1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamarsheh S, Groß O, Brummer T, Zeiser R: Immune modulatory effects of oncogenic KRAS in cancer. Nature communications 2020, 11(1):5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S, Montalto G, Cervello M, Steelman L, Abrams SL et al. : Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Advances in biological regulation 2015, 59:65–81. [DOI] [PubMed] [Google Scholar]
- 126.Nikiforova MN, Khalid A, Fasanella KE, McGrath KM, Brand RE, Chennat JS, Slivka A, Zeh HJ, Zureikat AH, Krasinskas AM et al. : Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2013, 26(11):1478–1487. [DOI] [PubMed] [Google Scholar]
- 127.Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, LaFemina J, Jarnagin WR, Berger MF, Klimstra D et al. : GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. Journal of the American College of Surgeons 2015, 220(5):845–854.e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Hayre M, Vázquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS: The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nature reviews Cancer 2013, 13(6):412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dorsam RT, Gutkind JS: G-protein-coupled receptors and cancer. Nature reviews Cancer 2007, 7(2):79–94. [DOI] [PubMed] [Google Scholar]
- 130.Reyna O, Flores Z, Nowell de Arevalo AM, Poltera AA, Beltranena F: Ultrasound detection of changes in the vitreous humor of onchocerciasis patients from Guatemala. Transactions of the Royal Society of Tropical Medicine and Hygiene 1988, 82(4):606. [DOI] [PubMed] [Google Scholar]
- 131.Zhang H, Kong Q, Wang J, Jiang Y, Hua H: Complex roles of cAMP-PKA-CREB signaling in cancer. Experimental hematology & oncology 2020, 9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH et al. : Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Science translational medicine 2011, 3(92):92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y: GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Archiv : an international journal of pathology 2015, 466(6):665–674. [DOI] [PubMed] [Google Scholar]
- 134.Molin MD, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C et al. : Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Annals of surgical oncology 2013, 20(12):3802–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patra KC, Kato Y, Mizukami Y, Widholz S, Boukhali M, Revenco I, Grossman EA, Ji F, Sadreyev RI, Liss AS et al. : Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nature cell biology 2018, 20(7):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI et al. : Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proceedings of the National Academy of Sciences of the United States of America 2011, 108(52):21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JH, Kim Y, Choi JW, Kim YS: KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. SpringerPlus 2016, 5(1):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mateos RN, Nakagawa H, Hirono S, Takano S, Fukasawa M, Yanagisawa A, Yasukawa S, Maejima K, Oku-Sasaki A, Nakano K et al. : Genomic analysis of pancreatic juice DNA assesses malignant risk of intraductal papillary mucinous neoplasm of pancreas. Cancer medicine 2019, 8(10):4565–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Omori Y, Ono Y, Kobayashi T, Motoi F, Karasaki H, Mizukami Y, Makino N, Ueno Y, Unno M, Furukawa T: How does intestinal-type intraductal papillary mucinous neoplasm emerge? CDX2 plays a critical role in the process of intestinal differentiation and progression. Virchows Archiv : an international journal of pathology 2020, 477(1):21–31. [DOI] [PubMed] [Google Scholar]
- 140.Sakamoto H, Kuboki Y, Hatori T, Yamamoto M, Sugiyama M, Shibata N, Shimizu K, Shiratori K, Furukawa T: Clinicopathological significance of somatic RNF43 mutation and aberrant expression of ring finger protein 43 in intraductal papillary mucinous neoplasms of the pancreas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2015, 28(2):261–267. [DOI] [PubMed] [Google Scholar]
- 141.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM et al. : Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488(7413):665–669. [DOI] [PubMed] [Google Scholar]
- 142.Logan CY, Nusse R: The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology 2004, 20:781–810. [DOI] [PubMed] [Google Scholar]
- 143.Tsukiyama T, Fukui A, Terai S, Fujioka Y, Shinada K, Takahashi H, Yamaguchi TP, Ohba Y, Hatakeyama S: Molecular Role of RNF43 in Canonical and Noncanonical Wnt Signaling. Molecular and cellular biology 2015, 35(11):2007–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R et al. : Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America 2013, 110(31):12649–12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Varjosalo M, Taipale J: Hedgehog signaling. Journal of cell science 2007, 120(Pt 1):3–6. [DOI] [PubMed] [Google Scholar]
- 146.Kelleher FC: Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis 2011, 32(4):445–451. [DOI] [PubMed] [Google Scholar]
- 147.Liu MS, Yang PY, Yeh TS: Sonic hedgehog signaling pathway in pancreatic cystic neoplasms and ductal adenocarcinoma. Pancreas 2007, 34(3):340–346. [DOI] [PubMed] [Google Scholar]
- 148.Satoh K, Kanno A, Hamada S, Hirota M, Umino J, Masamune A, Egawa S, Motoi F, Unno M, Shimosegawa T: Expression of Sonic hedgehog signaling pathway correlates with the tumorigenesis of intraductal papillary mucinous neoplasm of the pancreas. Oncology reports 2008, 19(5):1185–1190. [PubMed] [Google Scholar]
- 149.Paini M, Crippa S, Partelli S, Scopelliti F, Tamburrino D, Baldoni A, Falconi M: Molecular pathology of intraductal papillary mucinous neoplasms of the pancreas. World journal of gastroenterology 2014, 20(29):10008–10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, Wu GS, Wu K: Notch signaling: an emerging therapeutic target for cancer treatment. Cancer letters 2015, 369(1):20–27. [DOI] [PubMed] [Google Scholar]
- 151.Ikemoto T, Sugimoto K, Shimada M, Utsunomiya T, Morine Y, Imura S, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y et al. : Clinical role of Notch signaling pathway in intraductal papillary mucinous neoplasm of the pancreas. Journal of gastroenterology and hepatology 2015, 30(1):217–222. [DOI] [PubMed] [Google Scholar]
- 152.Bussom S, Saif MW: Intraductal papillary mucinous neoplasia (IPMN). Highlights from the “2010 ASCO Gastrointestinal Cancers Symposium”. Orlando, FL, USA. January 22–24, 2010. JOP : Journal of the pancreas 2010, 11(2):131–134. [PubMed] [Google Scholar]
- 153.Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J et al. : Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology 2019, 156(3):647–661.e642. [DOI] [PubMed] [Google Scholar]
- 154.Fischer CG, Beleva Guthrie V, Braxton AM, Zheng L, Wang P, Song Q, Griffin JF, Chianchiano PE, Hosoda W, Niknafs N et al. : Intraductal Papillary Mucinous Neoplasms Arise From Multiple Independent Clones, Each With Distinct Mutations. Gastroenterology 2019, 157(4):1123–1137.e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mas L, Lupinacci RM, Cros J, Bachet JB, Coulet F, Svrcek M: Intraductal Papillary Mucinous Carcinoma Versus Conventional Pancreatic Ductal Adenocarcinoma: A Comprehensive Review of Clinical-Pathological Features, Outcomes, and Molecular Insights. International journal of molecular sciences 2021, 22(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hollstein PE, Shaw RJ: GNAS shifts metabolism in pancreatic cancer. Nature cell biology 2018, 20(7):740–741. [DOI] [PubMed] [Google Scholar]
- 157.Ezrova Z, Nahacka Z, Stursa J, Werner L, Vlcak E, Kralova Viziova P, Berridge MV, Sedlacek R, Zobalova R, Rohlena J et al. : SMAD4 loss limits the vulnerability of pancreatic cancer cells to complex I inhibition via promotion of mitophagy. Oncogene 2021, 40(14):2539–2552. [DOI] [PubMed] [Google Scholar]
- 158.Liang C, Shi S, Qin Y, Meng Q, Hua J, Hu Q, Ji S, Zhang B, Xu J, Yu XJ: Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer. Gut 2020, 69(5):888–900. [DOI] [PubMed] [Google Scholar]
- 159.Joyce JA, Fearon DT: T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, NY) 2015, 348(6230):74–80. [DOI] [PubMed] [Google Scholar]
- 160.Mantovani A, Ponzetta A, Inforzato A, Jaillon S: Innate immunity, inflammation and tumour progression: double-edged swords. Journal of internal medicine 2019, 285(5):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang JJ, Lei KF, Han F: Tumor microenvironment: recent advances in various cancer treatments. European review for medical and pharmacological sciences 2018, 22(12):3855–3864. [DOI] [PubMed] [Google Scholar]
- 162.Hernandez-Barco YG, Bardeesy N, Ting DT: No Cell Left Unturned: Intraductal Papillary Mucinous Neoplasm Heterogeneity. Clinical cancer research: an official journal of the American Association for Cancer Research 2019, 25(7):2027–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roth S, Zamzow K, Gaida MM, Heikenwälder M, Tjaden C, Hinz U, Bose P, Michalski CW, Hackert T: Evolution of the immune landscape during progression of pancreatic intraductal papillary mucinous neoplasms to invasive cancer. EBioMedicine 2020, 54:102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kakizaki Y, Makino N, Tozawa T, Honda T, Matsuda A, Ikeda Y, Ito M, Saito Y, Kimura W, Ueno Y: Stromal Fibrosis and Expression of Matricellular Proteins Correlate With Histological Grade of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2016, 45(8):1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang S, Huang S, Sun YL: Epithelial-Mesenchymal Transition in Pancreatic Cancer: A Review. BioMed research international 2017, 2017:2646148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lu W, Kang Y: Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev Cell 2019, 49(3):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grimont A, Leach SD, Chandwani R: Uncertain Beginnings: Acinar and Ductal Cell Plasticity in the Development of Pancreatic Cancer. Cellular and molecular gastroenterology and hepatology 2022, 13(2):369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Roey R, Brabletz T, Stemmler MP, Armstark I: Deregulation of Transcription Factor Networks Driving Cell Plasticity and Metastasis in Pancreatic Cancer. Front Cell Dev Biol 2021, 9:753456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang L, Desai R, Conrad DN, Leite NC, Akshinthala D, Lim CM, Gonzalez R, Muthuswamy LB, Gartner Z, Muthuswamy SK: Commitment and oncogene-induced plasticity of human stem cell-derived pancreatic acinar and ductal organoids. Cell Stem Cell 2021, 28(6):1090–1104 e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meng F, Takaori K, Ito T, Masui T, Kawaguchi M, Kawaguchi Y, Uemoto S: Expression of SOX9 in intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2014, 43(1):7–14. [DOI] [PubMed] [Google Scholar]
- 171.Yi Z, Wei S, Jin L, Jeyarajan S, Yang J, Gu Y, Kim HS, Schechter S, Lu S, Paulsen MT et al. : KDM6A Regulates Cell Plasticity and Pancreatic Cancer Progression by Noncanonical Activin Pathway. Cellular and molecular gastroenterology and hepatology 2022, 13(2):643–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hirshorn ST, Steele N, Zavros Y: Modeling pancreatic pathophysiology using genome editing of adult stem cell-derived and induced pluripotent stem cell (iPSC)-derived organoids. Am J Physiol Gastrointest Liver Physiol 2021, 320(6):G1142–G1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang M, Liu Y, Chen YG: Generation of 3D human gastrointestinal organoids: principle and applications. Cell Regen 2020, 9(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A et al. : Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 2013, 32(20):2708–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS et al. : Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160(1–2):324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, Stigter ECA, Burgering B, Geurts V, Gracanin A et al. : Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proceedings of the National Academy of Sciences of the United States of America 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi X, Li Y, Yuan Q, Tang S, Guo S, Zhang Y, He J, Zhang X, Han M, Liu Z et al. : Integrated profiling of human pancreatic cancer organoids reveals chromatin accessibility features associated with drug sensitivity. Nature communications 2022, 13(1):2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang B, Trujillo MA, Fujikura K, Qiu M, Chen F, Felsenstein M, Zhou C, Skaro M, Gauthier C, Macgregor-Das A et al. : Molecular characterization of organoids derived from pancreatic intraductal papillary mucinous neoplasms. The Journal of pathology 2020, 252(3):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Beato F, Reveron D, Dezsi KB, Ortiz A, Johnson JO, Chen DT, Ali K, Yoder SJ, Jeong D, Malafa M et al. : Establishing a living biobank of patient-derived organoids of intraductal papillary mucinous neoplasms of the pancreas. Lab Invest 2021, 101(2):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Perkhofer L, Engler M, Gout J, Arnold F, Morawe M, Breunig M, Seufferlein T, Kleger A, Frappart PO: Pancreatic Ductal Organoids React Kras Dependent to the Removal of Tumor Suppressive Roadblocks. Stem Cells Int 2019, 2019:2079742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Breunig M, Merkle J, Wagner M, Melzer MK, Barth TFE, Engleitner T, Krumm J, Wiedenmann S, Cohrs CM, Perkhofer L et al. : Modeling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell 2021, 28(6):1105–1124 e1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guerra C, Barbacid M: Genetically engineered mouse models of pancreatic adenocarcinoma. 2013, 7(2):232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saiki Y, Horii A: Molecular pathology of pancreatic cancer. 2014, 64(1):10–19. [DOI] [PubMed] [Google Scholar]
- 184.Taki K, Ohmuraya M, Tanji E, Komatsu H, Hashimoto D, Semba K, Araki K, Kawaguchi Y, Baba H, Furukawa T: GNAS(R201H) and Kras(G12D) cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016, 35(18):2407–2412. [DOI] [PubMed] [Google Scholar]
- 185.Ideno N, Yamaguchi H, Ghosh B, Gupta S, Okumura T, Steffen DJ, Fisher CG, Wood LD, Singhi AD, Nakamura M et al. : GNAS(R201C) Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 2018, 155(5):1593–1607.e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kopp JL, Dubois CL, Schaeffer DF, Samani A, Taghizadeh F, Cowan RW, Rhim AD, Stiles BL, Valasek M, Sander M: Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154(5):1509–1523.e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D et al. : Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes & development 2006, 20(22):3130–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qiu W, Tang SM, Lee S, Turk AT, Sireci AN, Qiu A, Rose C, Xie C, Kitajewski J, Wen HJ et al. : Loss of Activin Receptor Type 1B Accelerates Development of Intraductal Papillary Mucinous Neoplasms in Mice With Activated KRAS. Gastroenterology 2016, 150(1):218–228 e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vincent DF, Yan KP, Treilleux I, Gay F, Arfi V, Kaniewski B, Marie JC, Lepinasse F, Martel S, Goddard-Leon S et al. : Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet 2009, 5(7):e1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yamaguchi J, Liss AS, Sontheimer A, Mino-Kenudson M, Castillo CF, Warshaw AL, Thayer SP: Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem cell research 2015, 15(1):190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP: Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010, 138(3):1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR: Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. The American journal of pathology 2002, 160(5):1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamaguchi J, Mino-Kenudson M, Liss AS, Chowdhury S, Wang TC, Fernández-Del Castillo C, Lillemoe KD, Warshaw AL, Thayer SP: Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice. Gastroenterology 2016, 151(6):1232–1244.e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, Maitra A, Pollack JR: Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America 2012, 109(5):E252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.von Figura G, Fukuda A, Roy N, Liku ME, Morris Iv JP, Kim GE, Russ HA, Firpo MA, Mulvihill SJ, Dawson DW et al. : The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nature cell biology 2014, 16(3):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang W, Friedland SC, Guo B, O’Dell MR, Alexander WB, Whitney-Miller CL, Agostini-Vulaj D, Huber AR, Myers JR, Ashton JM et al. : ARID1A, a SWI/SNF subunit, is critical to acinar cell homeostasis and regeneration and is a barrier to transformation and epithelial-mesenchymal transition in the pancreas. Gut 2019, 68(7):1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kimura Y, Fukuda A, Ogawa S, Maruno T, Takada Y, Tsuda M, Hiramatsu Y, Araki O, Nagao M, Yoshikawa T et al. : ARID1A Maintains Differentiation of Pancreatic Ductal Cells and Inhibits Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2018, 155(1):194–209.e192. [DOI] [PubMed] [Google Scholar]
- 198.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J et al. : Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491(7424):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morton JP, Jamieson NB, Karim SA, Athineos D, Ridgway RA, Nixon C, McKay CJ, Carter R, Brunton VG, Frame MC et al. : LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 2010, 139(2):586–597, 597.e581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bruenderman EH, Martin RC 2nd: High-risk population in sporadic pancreatic adenocarcinoma: guidelines for screening. The Journal of surgical research 2015, 194(1):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M: STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. The American journal of pathology 2001, 159(6):2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Siveke JT, Einwächter H, Sipos B, Lubeseder-Martellato C, Klöppel G, Schmid RM: Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer cell 2007, 12(3):266–279. [DOI] [PubMed] [Google Scholar]
- 203.Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT et al. : Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proceedings of the National Academy of Sciences of the United States of America 2007, 104(41):16170–16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Al Saati T, Clerc P, Hanoun N, Peuget S, Lulka H, Gigoux V, Capilla F, Béluchon B, Couvelard A, Selves J et al. : Oxidative stress induced by inactivation of TP53INP1 cooperates with KrasG12D to initiate and promote pancreatic carcinogenesis in the murine pancreas. The American journal of pathology 2013, 182(6):1996–2004. [DOI] [PubMed] [Google Scholar]
- 205.Fletterick R: NR5A2 discovering compounds that block tumor growth in PDAC. Journal of surgical oncology 2017, 116(1):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Seitz C, Huang J, Geiselhöringer AL, Galbani-Bianchi P, Michalek S, Phan TS, Reinhold C, Dietrich L, Schmidt C, Corazza N et al. : The orphan nuclear receptor LRH-1/NR5a2 critically regulates T cell functions. Science advances 2019, 5(7):eaav9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Flandez M, Cendrowski J, Cañamero M, Salas A, del Pozo N, Schoonjans K, Real FX: Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut 2014, 63(4):647–655. [DOI] [PubMed] [Google Scholar]
- 208.Cobo I, Iglesias M, Flández M, Verbeke C, Del Pozo N, Llorente M, Lawlor R, Luchini C, Rusev B, Scarpa A et al. : Epithelial Nr5a2 heterozygosity cooperates with mutant Kras in the development of pancreatic cystic lesions. The Journal of pathology 2021, 253(2):174–185. [DOI] [PubMed] [Google Scholar]
- 209.Chiblak S, Steinbauer B, Pohl-Arnold A, Kucher D, Abdollahi A, Schwager C, Höft B, Esposito I, Müller-Decker K: K-Ras and cyclooxygenase-2 coactivation augments intraductal papillary mucinous neoplasm and Notch1 mimicking human pancreas lesions. Scientific reports 2016, 6:29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chung WC, Challagundla L, Zhou Y, Li M, Atfi A, Xu K: Loss of Jag1 cooperates with oncogenic Kras to induce pancreatic cystic neoplasms. Life science alliance 2021, 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA et al. : Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer cell 2007, 11(3):229–243. [DOI] [PubMed] [Google Scholar]
- 212.Han SH, Ryu KH, Kwon AY: The Prognostic Impact of HER2 Genetic and Protein Expression in Pancreatic Carcinoma-HER2 Protein and Gene in Pancreatic Cancer. Diagnostics (Basel, Switzerland) 2021, 11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shibata W, Kinoshita H, Hikiba Y, Sato T, Ishii Y, Sue S, Sugimori M, Suzuki N, Sakitani K, Ijichi H et al. : Overexpression of HER2 in the pancreas promotes development of intraductal papillary mucinous neoplasms in mice. Scientific reports 2018, 8(1):6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.du Rieu MC, Torrisani J, Selves J, Al Saati T, Souque A, Dufresne M, Tsongalis GJ, Suriawinata AA, Carrère N, Buscail L et al. : MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clinical chemistry 2010, 56(4):603–612. [DOI] [PubMed] [Google Scholar]
- 215.Schipper J, Westerhuis JJ, Beddows I, Madaj Z, Monsma D, Hostetter G, Kiupel M, Conejo-Garcia JR, Sempere LF: Loss of microRNA-21 leads to profound stromal remodeling and short survival in K-Ras-driven mouse models of pancreatic cancer. International journal of cancer 2020, 147(8):2265–2278. [DOI] [PubMed] [Google Scholar]
- 216.Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D, McMahon AP: A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer research 2006, 66(20):10171–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jeng KS, Sheen IS, Leu CM, Tseng PH, Chang CF: The Role of Smoothened in Cancer. International journal of molecular sciences 2020, 21(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC: Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer discovery 2016, 6(3):256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schofield HK, Tandon M, Park MJ, Halbrook CJ, Ramakrishnan SK, Kim EC, Shi J, Omary MB, Shah YM, Esni F et al. : Pancreatic HIF2α Stabilization Leads to Chronic Pancreatitis and Predisposes to Mucinous Cystic Neoplasm. Cellular and molecular gastroenterology and hepatology 2018, 5(2):169–185.e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sano M, Driscoll DR, De Jesus-Monge WE, Klimstra DS, Lewis BC: Activated wnt signaling in stroma contributes to development of pancreatic mucinous cystic neoplasms. Gastroenterology 2014, 146(1):257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Barrios-Rodiles M, Chadee K: Novel regulation of cyclooxygenase-2 expression and prostaglandin E2 production by IFN-gamma in human macrophages. Journal of immunology (Baltimore, Md : 1950) 1998, 161(5):2441–2448. [PubMed] [Google Scholar]
- 222.Bindu S, Mazumder S, Bandyopadhyay U: Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical pharmacology 2020, 180:114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fitzpatrick FA: Cyclooxygenase enzymes: regulation and function. Current pharmaceutical design 2004, 10(6):577–588. [DOI] [PubMed] [Google Scholar]
- 224.Mohammed A, Yarla NS, Madka V, Rao CV: Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives. International journal of molecular sciences 2018, 19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pu D, Yin L, Huang L, Qin C, Zhou Y, Wu Q, Li Y, Zhou Q, Li L: Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Frontiers in oncology 2021, 11:637504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yip-Schneider MT, Wu H, Ralstin M, Yiannoutsos C, Crooks PA, Neelakantan S, Noble S, Nakshatri H, Sweeney CJ, Schmidt CM: Suppression of pancreatic tumor growth by combination chemotherapy with sulindac and LC-1 is associated with cyclin D1 inhibition in vivo. Molecular cancer therapeutics 2007, 6(6):1736–1744. [DOI] [PubMed] [Google Scholar]
- 227.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K: Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer research 2004, 64(6):2030–2038. [DOI] [PubMed] [Google Scholar]
- 228.Minna JD, Dowell J: Erlotinib hydrochloride. Nature reviews Drug discovery 2005, Suppl:S14–15. [DOI] [PubMed] [Google Scholar]
- 229.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK: Targeting the EGFR signaling pathway in cancer therapy. Expert opinion on therapeutic targets 2012, 16(1):15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wells A: EGF receptor. The international journal of biochemistry & cell biology 1999, 31(6):637–643. [DOI] [PubMed] [Google Scholar]
- 231.Cohen MH, Johnson JR, Chattopadhyay S, Tang S, Justice R, Sridhara R, Pazdur R: Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC). The oncologist 2010, 15(12):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Starling N, Neoptolemos J, Cunningham D: Role of erlotinib in the management of pancreatic cancer. Therapeutics and clinical risk management 2006, 2(4):435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT: Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society 1971, 93(9):2325–2327. [DOI] [PubMed] [Google Scholar]
- 234.Weaver BA: How Taxol/paclitaxel kills cancer cells. Molecular biology of the cell 2014, 25(18):2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fontana F, Marzagalli M, Raimondi M, Zuco V, Zaffaroni N, Limonta P: δ-Tocotrienol sensitizes and re-sensitizes ovarian cancer cells to cisplatin via induction of G1 phase cell cycle arrest and ROS/MAPK-mediated apoptosis. Cell proliferation 2021, 54(11):e13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fontana F, Moretti RM, Raimondi M, Marzagalli M, Beretta G, Procacci P, Sartori P, Montagnani Marelli M, Limonta P: δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell proliferation 2019, 52(3):e12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Husain K, Zhang A, Shivers S, Davis-Yadley A, Coppola D, Yang CS, Malafa MP: Chemoprevention of Azoxymethane-induced Colon Carcinogenesis by Delta-Tocotrienol. Cancer prevention research (Philadelphia, Pa) 2019, 12(6):357–366. [DOI] [PubMed] [Google Scholar]
- 238.Shen J, Yang T, Tang Y, Guo T, Guo T, Hu T, Luo F, Lin Q: δ-Tocotrienol induces apoptosis and inhibits proliferation of nasopharyngeal carcinoma cells. Food & function 2021, 12(14):6374–6388. [DOI] [PubMed] [Google Scholar]
- 239.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, Luetteke N, Hingorani SR, Sebti SM, Malafa MP: Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E δ-tocotrienol. Carcinogenesis 2013, 34(4):858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Husain K, Centeno BA, Chen DT, Hingorani SR, Sebti SM, Malafa MP: Vitamin E δ-tocotrienol prolongs survival in the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer prevention research (Philadelphia, Pa) 2013, 6(10):1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Springett GM, Husain K, Neuger A, Centeno B, Chen DT, Hutchinson TZ, Lush RM, Sebti S, Malafa MP: A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 2(12):1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mustachio LM, Chelariu-Raicu A, Szekvolgyi L, Roszik J: Targeting KRAS in Cancer: Promising Therapeutic Strategies. Cancers 2021, 13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, Chen Y, Babbar A, Firdaus SJ, Darjania L et al. : Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell 2018, 172(3):578–589.e517. [DOI] [PubMed] [Google Scholar]
- 244.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N et al. : The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575(7781):217–223. [DOI] [PubMed] [Google Scholar]
- 245.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE: Trends in GPCR drug discovery: new agents, targets and indications. Nature reviews Drug discovery 2017, 16(12):829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]


