Abstract
The activation of lymphocytes in patients with lupus and in mouse models of the disease is coupled with an increased cellular metabolism in which glucose plays a major role. The pharmacological inhibition of glycolysis with 2DG reversed the expansion of Tfh cells and GC B cells in lupus-prone mice, as well as the production of autoantibodies. The response of foreign antigens was however not affected by 2DG in these mice, suggesting that B and CD4+ T cell activation by autoantigens is uniquely sensitive to glycolysis. Here, we tested this hypothesis with monoclonal B cells and CD4+ T cells specific for lupus-relevant autoantigens. AM14 Tg B cells are activated by IgG2a/chromatin immune complexes and they can receive cognate help from chromatin-specific 13C2 CD4+ T cells. We showed that activation of AM14 B cells by their cognate antigen PL2-3 induced glycolysis, and that the inhibition of glycolysis reduced their activation and differentiation into antibody-forming cells, in the absence or presence of T cell help. The dependency of autoreactive B cells on glycolysis is in sharp contrast with the previously reported dependency of NP-specific B cells on fatty acid oxidation. Contrary to AM14 B cells, the activation and differentiation of 13C2 T cells into Tfh cells was not altered by 2DG, which differs from polyclonal CD4+ T cells from lupus-prone mice. These results further define the role of glycolysis in the production of lupus autoantibodies demonstrate the need to evaluate the metabolic requirements of antigen-specific B and T cells.
Keywords: lupus, autoantibodies, glucose, B cells, CD4+ T cells
Introduction
High affinity class-switched autoantibodies (autoAb) are the major pathogenic effectors in lupus. They are generated by B cells that differentiate through either the germinal center (GC) or the extrafollicular (EF) pathways (1–3). A high frequency of GC B and follicular helper CD4+ T (Tfh) cells has been reported in several mouse models of lupus, and pharmacologic or genetic targeting of the GC, such as through IL-21 (4, 5) is therapeutic in these models. The frequency of Tfh cells is also expanded in lupus in direct correlation with disease activity (6–8).
We and others have shown that the cellular metabolism of immune cells was altered in lupus patients and mice, and that some of these alterations offer therapeutic targets (9, 10). Although the field of immunometabolism has exploded in the last decade, less is known about the metabolic programs that sustain GC B cells and Tfh cells (11). Hypoxia and mTOR activation are major determinants of GC B cell metabolism (11). But surprisingly, since mTOR and hypoxia are usually associated with glycolysis, the differentiation of B cells into GC B cells in response to protein immunization and antibody affinity maturation through somatic hypermutations is associated with oxidative phosphorylation (OXPHOS) (12), and more specifically on fatty acid oxidation (FAO) (13). Although Tfh cells have been considered metabolically quiescent (14, 15), they require both mTORC1 and mTORC2 signaling (16). Moreover, the proliferation of Tfh cells in response to cognate interactions with GC B cells following protein immunization is sustained by a coordinated metabolic reprogramming with an upregulation of OXPHOS, glycolysis and the Myc pathway (16, 17). Using 2-deoxy-D-glucose (2DG) to inhibit glycolysis in a lupus-prone mouse model, we have shown that the production of anti-dsDNA IgG and corresponding expansion of spontaneous GC B cells and Tfh cells were glucose-dependent. This was different from the humoral response and the expansion of GC B cells and Tfh cells in response to immunization with a nominal antigen or influenza, which were little affected by 2DG (18). In contrast to glycolysis inhibition, both autoAbs and immunization-induced antibodies were eliminated by the pharmacological inhibition of glutaminolysis (18). This led us to hypothesize that autoreactive GC B and Tfh cells that are responsible for the production of autoAb have a different glucose requirement than GC B and Tfh cells involved in the humoral response to exogenous protein antigens. This hypothesis had not been tested yet due to the integral interdependency of GC B and Tfh differentiation and survival, and even more importantly, in the absence of a model to track autoreactive B and T cells with a lupus-relevant autoantigen.
BCR and TCR transgenic (Tg) models have been used to define the mechanisms of tolerance. Specific BCR Tg models have dissected the mechanisms of B cell tolerance to lupus autoantigens. Among these, the well-characterized AM14 Tg B cell model offers a unique system in which the presence of the autoantigen can be controlled and BCR signaling is coupled with TLR9 activation (19). AM14 B cells combine a Tg HC to Vk8 light chain to produce anti-IgG2aa rheumatoid factor (RF) antibodies, which bind IgG2aa/chromatin immune complexes (20). AM14 B cells are clonally ignorant on a non-autoimmune background, but they break tolerance and differentiate into RF-producing antibody forming cells (AFC) when expressed in lupus-prone models (21, 22). The differentiation into RF AFC can also be induced on a non-autoimmune background in an antigen-specific manner with the anti-chromatin IgG2aa hybridoma PL2-3 (23). Anti-chromatin CD4+ T cell clones, such as 13C2, have been shown to differentiate into Tfh cells in the presence of PL2-3-stimulated AM14 B cells (23). In this study, we performed adoptive transfers of AM14 B cells and 13C2 T cells to assess the role of glycolysis in lupus-relevant autoreactive antigen-specific B and CD4+ T cells using the pharmacologic inhibitor 2DG. We compared AM14 B cells activated specifically by PL2-3 or non-specifically with a TLR7 agonist, R848. We also assessed the requirement of AM14 B cells for glycolysis in an induced lupus-like environment. Finally, we investigated the effect of 2DG on antigen-specific CD4+ T cells in response to protein immunization. We found that autoreactive AM14 B cells use glycolysis to respond to PL2-3, unless they get activated during homeostatic expansion. Moreover, the GC stage was consistently sensitive to the inhibition of glycolysis for both AM14 B cells as well as polyclonal B cells in mice stimulated with PL2-3, R848 or both. In contrast, neither the activation of autoreactive 13C2 T cells or OVA-specific CD4+ T cells, including the differentiation into Tfh cells, depended on glycolysis. These results differ from the results obtained both with NP-specific B cells (13) and with total spontaneous CD4+ T cells from lupus models (18, 24), and demonstrate the value of directly assessing the metabolic requirements of autoreactive lymphocytes.
Methods
Mice
AM14 Vκ8R (AM14), DO11.10 Tcrα−/−Rag2−/− (DO11.10) and BALB/c.Rag2−/− (BALB/c.Rag) mice on BALB/c background have been described previously (23). C57BL/6 (B6) and BALB/c mice were originally obtained from the Jackson Laboratory. B6.Cg-Tg(TcraTcrb)425Cbn/J mice on a Thy1a background (CD90.1+ OT-II) were graciously provided by Dr. Stephen Schoenberger (La Jolla Institute for Allergy and Immunology). Experiments were performed with 2 – 3 months old male and female mice that were gender-matched within each cohort. Each experiment was performed at least on one cohort of each gender, and the results were pooled since no differences were observed between genders. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Florida and the University of Texas Health San Antonio.
Generation of retrogenic (Rg) 13C2 mice
13C2 Rg mice were produced as previously described (23). Briefly, bone marrow (BM) harvested from BALB/c.Rag mice was enriched for hematopoietic progenitor cells using an EasySep kit (Stemcell Technologies). Cells were cultured overnight in 20% FBS complete DMEM with 20 ng/ml IL-3 (Thermofisher), 50 ng/ml IL-6 (Shenandoah Biotechnology), and 100 ng/ml stem cell factor (Invitrogen). Platinum-E cells (Cell Biolabs) were transfected with pCL-Ecohelper and 13C2 pMIG II plasmids using Lipojet (Signagene) kit and replaced to 20% DMEM media 7 h later. Cells were grown at 32°C for up to 48 h. After 24 h, the 13C2 TCR-encoded retrovirus (RV) containing medium was harvested and refilled with fresh media. The harvested RV media was added to the BM cells (1×106 cells per 1 ml of RV supernatant) with polybrene (6 µ g/ml, Sigma-Aldricht) and the cells were spinoculated at 2,200 rpm for 90 min at 32°C in a 6 well-plate. The process was repeated with RV supernatant collected at 48 h. The transfected BM cells were then rested in fresh medium and 5 – 10 × 106 cells were injected into DO11.10 mice that had been irradiated 3 h prior with 4.5 Gy. 13C2 GFP+ T cells were FACS-sorted from Rg mice 8 weeks after BM transfer to be used in experiments.
Adoptive transfers
10 × 106 AM14 B cells enriched by negative selection with magnetic beads (Miltenyi Biotec) were injected intravenously (iv) alone or with 1 – 3 × 105 13C2 GFP+ T cells into BALB/c, BALB/c.Rag or DO11.10 recipient mice as indicated in the text for each experiment. Recipient mice were either immunized with 200 μg of PL2-3 or control PL2-8 antibody (25), or treated with the TLR7 ligand resiquimod (R848; Tocris) by topical application of 100 μg in 100 μl acetone to the right ear on days 0, 2, and 4. Some mice were treated with 2DG (Millipore Sigma) in drinking water (6 g / l) for the duration of the experiment. Other mice were treated with 0.5 mg lonidamine (LND, Tocris) in intra-peritoneal injections on days 0 and 1. Control mice received plain drinking water. In other experiments, recipients of AM14 B cells were treated with R848 starting two weeks before PL2-3 immunization, then treated or not with 2DG starting with PL2-3 immunization. To characterize the response of antigen-specific CD4+ T cells to glycolysis inhibition, 1 – 2 × 105 FACS-sorted CD90.1+ OT-II T cells were iv-injected into B6 recipients immunized with 50 μg NP16-OVA (Biosearch Technologies) in alum (Thermo Fisher Scientific) treated or not with 2DG starting with immunization. Finally, 3 × 105 bead-purified DO11.10 CD4+ T cells were injected to AM14 mice immunized with NP16-OVA/alum. Control mice included cell recipients that did not receive any treatment or immunization, as well as mice that did not receive any cells but were treated / immunized. All mice were sacrificed for analysis at day 7 after cell transfer. PL2-3 and PL-8 hybridomas were grown and purified in-house as previously described (22).
In vitro assays
Bead-purified AM14 B cells were cultured at 106 cells/ml in complete RPMI with 10% fetal calf serum and 200 mM L-glutamine in the presence of PL2-3, R848 (0.1 μg / ml each), or medium alone for 16 h. The mitochondrial stress assays were conducted on an XF96 extracellular flux analyzer (Agilent) as previously described (24). Basal oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were calculated as the average of the 3 measurements before the addition of oligomycin. The glycolysis stress assay and the calculations for glycolysis, glycolytic capacity and glycolytic reserve were performed according to manufacturer’s instructions. Both mitochondrial and glycolysis stress assays were performed with 4 – 6 technical replicates per sample. To assess the glucose requirement for antibody production by AM14 B cells in the presence of cognate T cell help, 3 × 106 AM14 B cells and 3 × 105 T cells labelled with Cell Trace Violet (Thermo Fisher Scientific) were cultured in 12-well plates in 10% complete Click’s media for 5 days as previously described (23). On the third day, half of the media were replaced with fresh 10% complete Click’s media and 10 ng/ml human IL-2 (Peprotech) with 2DG (50 μM) or control media. To assess the effect of glycolysis inhibitors, 3 × 106 AM14 B cells were cultured as above with IL-2 in the presence of 3-bromopyruvate (3-BP, 50 μM, Sigma-Aldrich), LND (200 uM), or koningic acid (KA, 4 uM, ABCAM). Etomoxir (4 uM, Sigma-Aldrich) was used to inhibit FAO.
Antibody measurements
4–44 antibodies were measured by ELISA as previously described (23) with biotinylated anti-4–44 Ab, which recognizes the AM14 RF clonotype (26), in sera diluted 1:100 or supernatant diluted 1:10. Total IgM ELISA was performed as previously described (27). ELISPOT assays were performed as previously described (22). NP-specific Abs were measured by ELISA using plates coated with NP25-BSA (Biosearch Tech.), followed by incubation with 1:1000 diluted serum samples and developed with alkaline phosphatase–conjugated goat anti-mouse IgG1 (Southern Biotech). All samples were run in duplicate.
Flow cytometry
Single-cell suspensions were prepared using standard procedures from spleen and bone marrow (BM). After RBC lysis, cells were blocked with anti-CD16/32 (2.4G2) and stained in FACS staining buffer (2.5% FBS, 0.05% sodium azide in PBS). Dead cells were excluded with fixable viability dye (eBioscience). Fluorochrome-conjugated antibodies were used to detect B220 (RA3-6B2), BCL-2 (BCL/10C4), BCL6 (K112-91), CD4 (RAM4-5), CD5 (53–7.3), CD11c (N418), CD19 (6D5), CD21 (eBio4E3), CD23 (B3B4), CD25 (PC61.5), CD44 (IM7), CD45.1 (A20), CD62L (MEL-14), CD90.1 (HIS51), CD93 (AA4.1), CD95 (Jo2), CD138 (281–2), IgD (11–26c.2a), IgM (II/41), IL-2 (JES6-5H4), IFNγ (XMG1.2), Ki-67 (SolA15), Ly-77 (GL7), pAKT1 Ser473 (SDRNR), p4E-BP1 (236B4), PD-1 (RMP1-30), PSGL-1/CD162 (2PH1), T-bet (eBio4B10) and TCR DO11.10 (KJ1-26). These antibodies were purchased from BD Biosciences, eBioscience, BioLegend, and R&D Systems. AM14 B cells were identified as surface and intracellular double positive 4–44+ B cells (Sup. Fig. 1A). Class-switched (CS) B cells were defined as IgM− IgD− double negative B cells (Sup. Fig. 1B). Plasmablasts (PB) were defined as CD19+B220+CD138+, plasma cells (PC) as CD19lowB220lowCD138+ and GC B cells were gated as B220+CD95+GL7+ as previously described (28). Tbet+CD11c+ age-related B cells (ABC) were gated as CD5loCD93loCD21loCD23loT-bet+CD11c+/− (Sup. Fig. 1A). Tfh cells were gated either as CD4+PD-1+BCL-6+ (BCL-6+ Tfh) or CD4+PD-1+PSGL-1neg (PSGL-1neg Tfh). Extrafollicular T cells (Tef) were gated as CD4+ PD-1+ PSGL-1+ (Sup. Fig. 1C). NP-specific B cells were detected by an additional stain with PE-conjugated NP (Biosearch Tech.). Fixation with permeabilization using the FOXP3 Fix/Perm Buffer Set (Biolegend) was performed for intracellular stains. Stimulation with the Leukocyte Activation Cocktail (BD Biosciences) for 5 h was used to detect intracellular cytokines. Total splenocytes (1 × 106) were incubated with 20 μM 2-NBDG or 2 μM Bodipy (both from Sigma) for 20 min at 37°C to measure glucose uptake or total cellular lipids, respectively. Data was collected on an LSR Fortessa (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.0 software. Graphs show mean and standard error of the mean (SEM) for each group. When data was normally distributed, results were compared using one-way ANOVA with Dunnett’s multiple test corrections or two-tailed unpaired or paired t tests. Non-normally distributed data was analyzed with Brown-Forsythe and Welch ANOVA tests.
Results
Metabolism of autoreactive B cell activation in vitro
We first evaluated the metabolism of purified AM14 B cells stimulated in vitro with their cognate antigen PL2-3 or with a non-antigen specific TRL7 agonist, R848. Both stimuli greatly increased respiration and glycolysis, PL2-3 being more potent (Fig. 1A-B). Furthermore, PL2-3 stimulation induced a more glycolytic state as shown by a lower respiration to glycolysis ratio as compared to R848 (Fig. 1C). This was confirmed with a glycolysis stress assay (Fig. 1D) that showed that PL-3 induced a higher glycolysis, glycolytic capacity and glycolytic reserve than R848 (Fig. 1E). To assess whether glycolysis was required to produce cognate antibody, we co-cultured AM14 B cells and 13C2 CD4+ T cells in the presence of PL2-3 with or without 2DG at a concentration (50 μM) that had no consequence on cell survival (data not shown) or proliferation of either B or T cells (Fig. 1F). As expected (23), the production of 4–44 antibody in these conditions required PL2-3 stimulation. More interestingly, the inhibition of glycolysis decreased the differentiation of AM14 B cells into 4–44 IgM and IgG2a producing cells by about 2 and 4 folds, respectively (Fig. 1G). 2DG caused 13C2 T cells to produce increased IL-2 and decreased IFNγ (Fig. 1H). To validate the results obtained on AM14 B cell activation in the presence of 2DG, which competes with glucose to bind to hexokinase (HK), we tested other glycolysis inhibitors: lonidamine (LND), an HK inhibitor, koningic acid (KA), a GAPDH inhibitor, and bromopyruvate (3-BP), which inhibits multiple steps of glycolysis, including HK and GAPDH. AM14 B cells stimulated with either PL2-3 or R848 produced less antibody, especially measured as total IgM, in the presence of LND, KA and 3-BP (Sup. Fig. 2A and B). In these conditions, these glycolysis inhibitors showed a stronger effect than 2DG. LND also reduced the frequency of CD138+ plasmablasts in the culture (Sup. Fig. 2C). We next assessed the effect of these inhibitors on mTOR activation. None of the inhibitors changed the expression of p4E-BP1, which is phosphorylated by mTORC1 (Sup. Fig. 2D). However, the four glycolysis inhibitors decreased the expression of pAKT1 (Ser 473), which is phosphorylated by mTORC2, with a potential reduced effect of 3-BP on PL2-3-activated AM14 B cells (Sup. Fig. 2E and F). These results indicate that glycolysis inhibitors reduce mTORC2 activation in AM14 B cells. mTORC2 is essential for cell growth and survival, largely by promoting glycolysis and the pentose phosphate pathway, including by phosphorylating HK2 (29). Contrary to the concerted effects of glycolysis inhibitors, etomoxir had no effect on the production of antibody and mTORC2 activation in AM14 B cells (Sup. Fig. 2A-F). Overall, these results indicate that the stimulation of AM14 B cells with their cognate antigen induced a higher glycolysis as compared to the TLR7 stimulation. Further, glycolysis but not FAO is required for the optimal production of RF antibodies and T cell activation in cognate B / T cell co-cultures.
Figure 1. Metabolism of AM14 B cells stimulated in vitro.
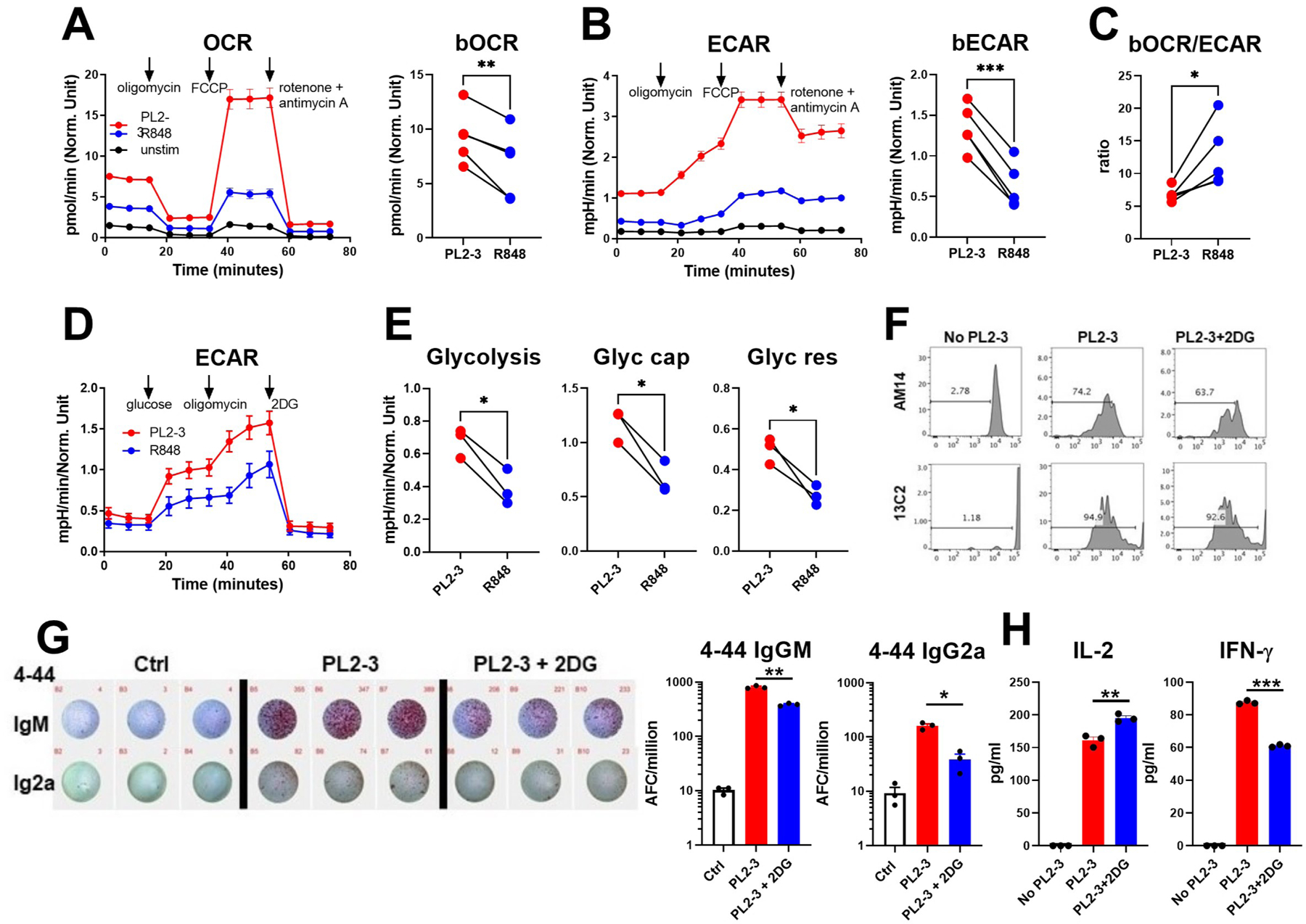
A - B. Mitochondrial stress test performed on AM14 B cells stimulated with PL2-3, R848 or unstimulated for 16 h. Representative time-course plots and quantitation of basal OCR (A) and basal ECAR (B), which each correspond to the first three time-points on the time-course plots before the addition of oligomycin (ATP synthase inhibitor, first arrow). The subsequent addition of the OXPHOS uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP, second arrow) result in maximal respiration and glycolysis. The final addition of rotenone (complex I inhibitor) and antimycin A (complex III inhibitor) shuts down the electron transport chain. C. Basal OCR/ECAR ratio. D. Glycolysis stress test in which glycolysis is induced by the addition of glucose (first arrow), followed by the addition of oligomycin (second arrow) that induces maximal glycolysis, then 2DG that blocks glycolysis. E. Glycolysis (increase in ECAR value after the addition of glucose relative to background), glycolytic capacity (increase in ECAR value after the addition of oligomycin relative to background), and glycolytic reserve (difference between glycolytic capacity and glycolysis) calculated from data shown in D. A - E: n = 3 – 4, with different mice used in the two assays. F - H. Effect of 2DG on AM14 B cells and 13C2 T cells co-cultured with PL2-3. F. Representative FACS plots showing CTV dilution as a measure of proliferation in AM14 B cells (top) and 13C2 T cells (bottom). G. Representative 4–44 IgM and IgG2a ELISPOTs, with each well representing a mouse with 2 × 106 cells. Cells in wells in a same column (IgM and IgG2a) come from the same mouse. Corresponding quantitation on the right. H. IL-2 and IFNγ production in the culture supernatants, n = 3. Paired t tests, *P < 0.05; **P < 0.01. ***P < 0.001.
The inhibition of glycolysis does not impair cognate AM14 AFC differentiation in lymphocyte-deficient mice
We evaluated the requirement of AM14 B cells for glycolysis upon stimulation without bystander B cells and T cells, after adoptive transfer to BALB/c.Rag mice treated with PL2-3 or R848, in the presence or not of 2DG until the day of sacrifice. Recipient controls did not receive any stimulus. 2DG reduced the number of splenocytes in all conditions, regardless of the presence or nature of the stimulation (Fig. 2A). Differentiation of AM14 B cells into 4–44 IgM AFC was greatly increased with both kinds of stimulations, but higher in response to PL2-3, especially as reflected by 4–44 IgM serum levels. 2DG had no impact 4–44 IgM AFC differentiation and 4–44 IgM production induced by PL2-3, but the number of R848-induced 4–44 IgM AFC was reduced by 2DG (Fig. 2B-C). At the cellular level, 2DG decreased the number of AM14 B cells stimulated with either PL2-3 or R848, and in R848-treated animals (Fig. 2D). As expected, both treatments lead to the proliferation of first IgD+, then IgDlow AM14 B cells (Fig. 2E-F), and differentiation into PBs (Fig. 2G-H). 2DG had no effect on the proliferation of IgD+ cells in either PL2-3 or R848-treated mice, but it reduced proliferation of the IgDlow subset by 3-folds (Fig. 2F). 2DG also decreased the frequency and number of R848-induced AM14 PBs, but significantly reduced on the number of PL2-3-induced PBs (Fig. 2G-H). To assess the dependency of total AM14 B cells or AM14 PBs on glucose and lipid metabolism, their uptake was quantified ex-vivo with 2NBDG and bodipy, respectively in B cells from 7 d-treated animals. Both stimulations increased glucose uptake in total AM14 B cells. This was not impacted by 2DG, which block glucose phosphorylation but not its import. Glucose uptake was 2 to 3-fold higher in AM14 PBs than total AM14 cells regardless of stimulation or glucose inhibition (Fig. 2K). On the other hand, lipid uptake was not increased by either stimulation in total AM14 cells, but AM14 PBs had almost 2-fold higher lipid uptake than total AM14 cells (Fig. 2L). 2DG increased lipid uptake only in the AM14 B cells from R848-treated mice, corresponding to the greater inhibitory effect 2DG in these cells. Thus, in the absence of bystander B cells and T cells, RF production by AM14 B cells stimulated by PL2-3 was not dependent on glycolysis, although their activation increased glucose uptake. 2DG affected PL2-3 stimulated AM14 B cells upstream of AFC differentiation, by reducing the number of total AM14 B cells and PBs, as well as their differentiation into proliferating IgDlow AM14 B cells. Finally, 2DG has a greater effect of R848-stimulated AM14 B cells in these conditions.
Figure 2. The inhibition of glycolysis does not impair cognate AM14 AFC differentiation in lymphocyte-deficient mice.
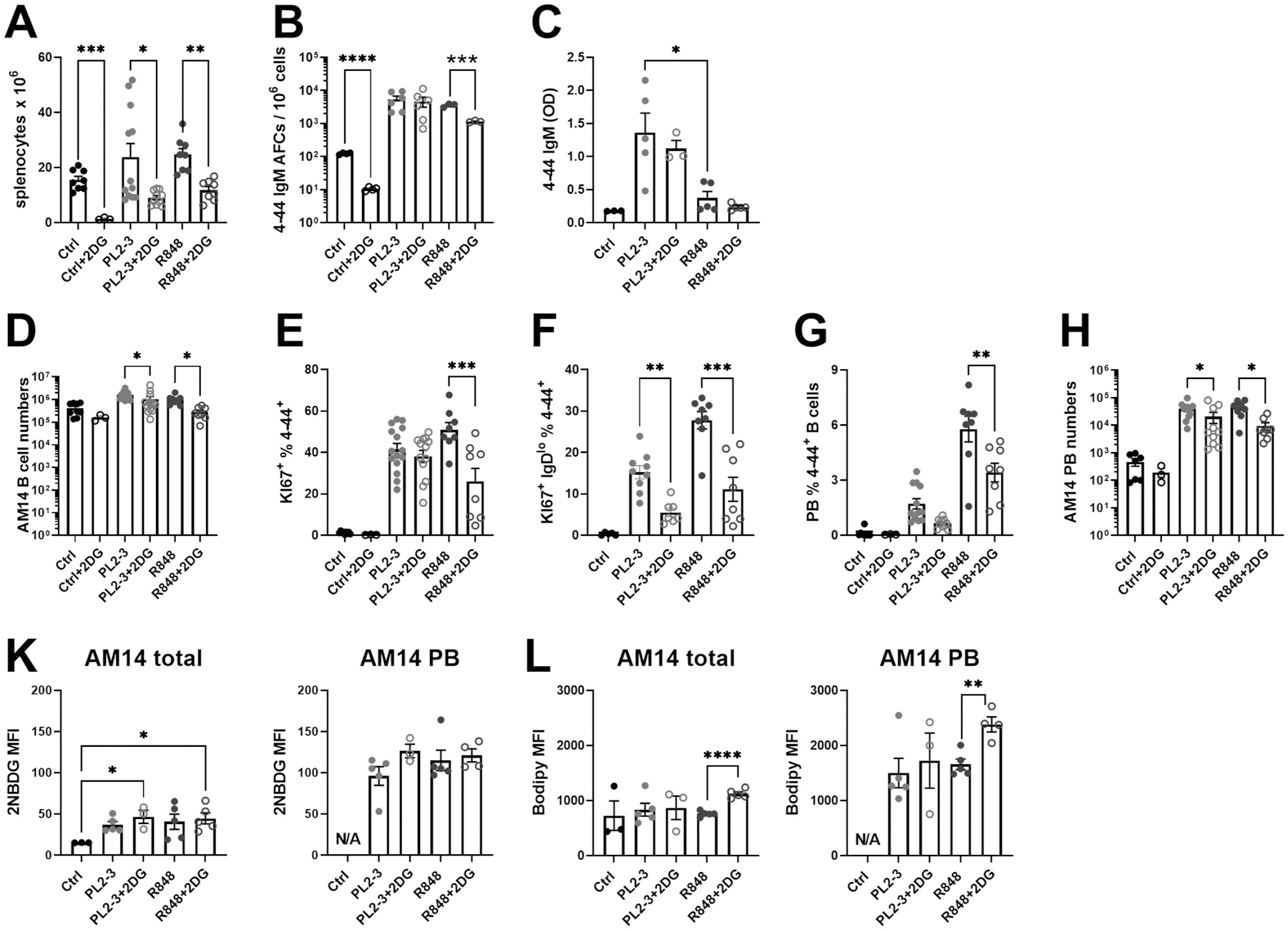
AM14 B cells were transferred into BALB/c.Rag mice immunized with PL2-3 or R848, and treated or not with 2DG for 7 d. A. Absolute numbers of splenocytes. B. 4–44 IgM ELISPOTs. C. Serum 4–44 IgM. D. Absolute AM14 B cell numbers. E. Frequency of proliferating Ki-67+ AM14 B cells. F. Frequency of IgDlowKi-67+ among AM14 B cells. Frequency (G) and number (H) of PBs among AM14 B cells. K. Ex vivo 2NBDG uptake in total AM14 cells (left) or AM14 PBs (right). L. Bodipy uptake in total AM14 cells (left) or AM14 PBs (right). Data was pooled from 2 – 4 experiments. Each point represents a mouse and graphs show means and SEM. Statistics were calculated with 1-way ANOVA with multiple comparison tests. *P < 0.05; **P < 0.01. ***P < 0.001.
The inhibition of glycolysis impairs the cognate activation of AM14 B cells in a non-lymphopenic environment
We evaluated whether AM14 B cells require glycolysis to expand and differentiate into PBs after adoptive transfer into DO11.10 mice, which have polyclonal B cells and OVA-specific CD4+ T cells. Recipient mice were stimulated with PL2-3 or R848 and were treated or not with 2DG as in Fig. 2. Control recipient mice were unstimulated. 2DG decreased the amount of 4–44 IgM AFCs relative to total IgM AFCs as well as the amount of serum 4–44 IgM induced by PL2-3 (Fig. 3A). Treatment of recipient mice with LND confirmed that glycolysis is required by AM14 B cells to produce antibody in response to PL2-3 immunization (Sup. Fig. 2G). PL2-3 induced the production of a greater amount of 4–44 IgM than R848, and the latter was not affected by 2DG. As previously shown (23), PL2-3 decreased the frequency of AM14 B cells among total B cells as compared to untreated mice, but this was not observed with the R848 treatment (Fig. 3B). In either condition, 2DG did not decrease the frequency of total AM14 B cells. Both stimulations induced a robust proliferation of AM14 B cells. As shown in BALB/c.Rag recipients (Fig. 2H-G), 2DG did not alter the proliferation of IgD+ AM14 B cells, but again reduced the frequency of proliferating IgDlow AM14 B cells by almost 3-fold with both types of stimulation (Fig. 3C-D). PL2-3 immunization induced the differentiation of more PBs than R848 (Fig. 3E-F). In contrast to BALB/c.Rag recipients (Fig. 2J-K) however, 2DG reduced the AM14 PB frequency by 2-fold- in DO11.10 mice treated with either PL2-3 or R848 (Fig. 3E-F). PL2-3 stimulation was specific to AM14 B cells since it reduced the frequency of endogenous polyclonal GC B cells and PCs (Fig. 3G-H). In contrast, R848 enhanced the differentiation of endogenous GC B cells and PCs, which were both reduced by 2DG. Finally, PL2-3 increased mTORC2 activation in AM14 B cells by 3-folds as compared to endogenous B cells (Sup. Fig. 2H). Contrary to the direct effect of glycolysis inhibitors on purified AM14 B cells (Sup. Fig. 2F), 2DG or LND did not reduce pAKT1 levels in either AM14 or host B cells. These results suggest that PL2-3 activates mTORC2, which supports their growth and differentiation of AM14 B cells, but the inhibition of glycolysis reduced antibody production without a reduction in mTORC2 activation in these cells.
Figure 3. The inhibition of glycolysis alters the cognate activation of AM14 B cells in a non-lymphopenic environment.
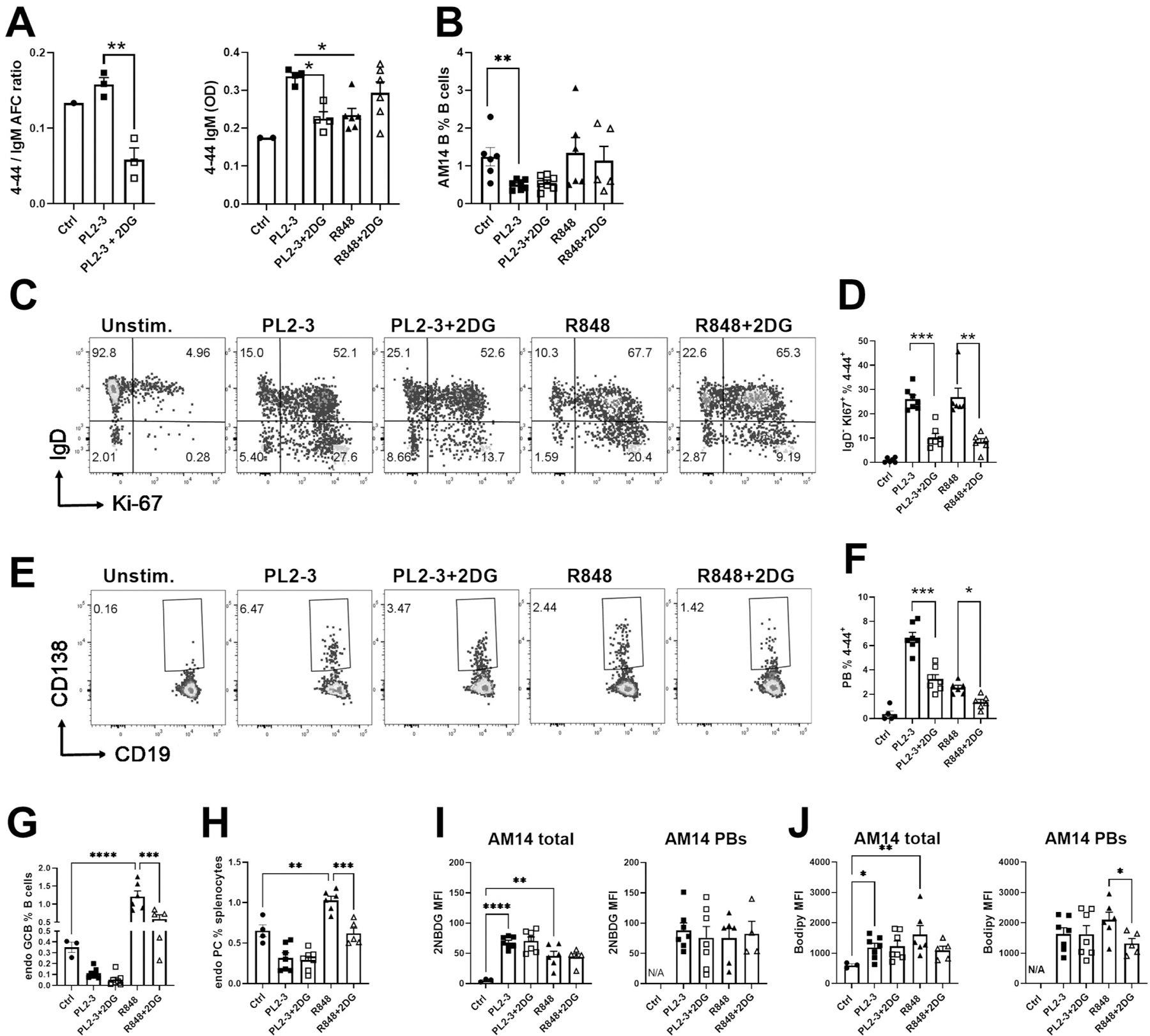
AM14 B cells were transferred into DO11.10 mice immunized with PL2-3 or R848, and treated or not with 2DG for 7 d. A. 4–44 IgM to total IgM AFC ratio measured by ELISPOTs (left) and serum 4–44 IgM (right). B. AM14 B cell frequency among total B cells. C. Representative FACS plots of AM14 B cells stained with IgD and Ki-67. D. Frequency of IgDlowKi-67+ among AM14 B cells. E. Representative FACS plots of AM14 PBs gated with CD19 and CD138. F. Frequency of PBs among AM14 B cells. Frequency of non-AM14 endogenous (endo) GC B cells (G) and PCs (H). 2NBDG (I) and bodipy (J) uptake in total AM14 cells (left) or AM14 PB (right). Data was pooled from 3 experiments. Each point represents a mouse and graphs show means and SEM. Statistics were calculated with 1-way ANOVA with multiple comparison tests. *P < 0.05; **P < 0.01. ***P < 0.001, ****P < 0.001.
Both stimulations greatly enhanced glucose and lipid uptake by total AM14 B cells transferred in DO11.10 mice, which was unaffected by 2DG (Fig. 3I-J). This indicates that activated AM14 B cells may use both source of energy in a non-lymphopenic environment. In contrast to BALB/c.Rag recipients, AM14 PBs in DO11.10 mice had glucose and lipid uptake similar to that of total AM14 cells and 2DG slightly reduced lipid uptake in R848-induced PBs (Fig. 3J). Hence, in the presence of polyclonal B cells and CD4+ T cells of irrelevant specificity, the production of RF by AM14 B cells depended on glycolysis when they were stimulated by their cognate antigen PL2-3 but not by non-specific TLR7, despite a similar glucose uptake in both conditions. As in the lymphopenic environment however, the differentiation of proliferating IgDlow AM14 B cells was highly dependent on glycolysis in both types of stimulation.
The inhibition of glycolysis impairs the cognate activation of TLR7-stimulated AM14 B cells
To determine whether glycolysis also supports the differentiation of AM14 B cells activated by PL2-3 in a lupus-like environment, we pretreated DO11-10 recipient mice for 4 weeks with R848, which induces the production of type I IFN and autoimmune phenotypes in BALB/c mice (30). During the fifth week, mice received AM14 B cells and PL2-3, and were treated or not with 2DG. Control mice were immunized with PL2-3 without R848 pre-treatment. Induction of autoimmune phenotypes by R848 was confirmed by splenomegaly (Fig. 4A), production of anti-dsDNA IgG, as well as by an increased frequency of endogenous GC B cells, PBs and PCs (Sup. Fig. 3A-C), some of which (splenomegaly and PBs/PCs) were reduced by the short 2DG treatment. R848 increased the frequency of AM14 B cells as well as the frequency and number of AM14 PBs (Fig. 4B-C), corresponding to an increased 4–44 IgM production (Fig. 4D). The R848 treatment also enhanced the frequency of proliferating IgDlow AM14 B cells (Fig. 4E), which was highly correlated with 4–44 IgM production (Fig. 4F). This expansion coincided with the migration of AM14 B cells to the T cell zone where they decreased their IgD expression (Fig. 4G). The 2DG treatment during PL2-3 activation decreased all these parameters, except the differentiation of AM14 cells into PBs, which was less glucose-dependent (Fig. 4C). Further, spleen histology showed that the 2DG treatment promoted the retention of AM14 B cells in the follicles where they maintained IgD expression (Fig. 4G), confirming the flow cytometry results. Interestingly, 2DG eliminated the R848 effect, with values obtained from R848 and 2DG-treated PL2-3 immunized mice similar to those obtained from PL2-3-immunized only control mice for most phenotypes. Finally, the R848 treatment greatly expanded the ABC subset (Sup. Fig. 1A) both among endogenous (Sup. Fig. 2D) and AM14 B cells (Fig. 4H). TRL7 activation promotes the differentiation of ABCs, which produce autoantibodies, and are found in high numbers in SLE patients (31, 32). The absolute numbers of endogenous and AM14 ABCs were highly correlated to each other (Fig. 4I), but there was no correlation between the number of AM14 ABCs and the production of 4–44 IgM (data not shown). This suggests that the expansion of the number of AM14 ABCs resulted from the global effect of R848 on ABCs. 2DG reduced the number of both AM14 (Fig. 4H) and endogenous ABCs (Sup. Fig. 3D). Similar results were obtained when AM14 B cells were stimulated with PL2-3 after transfer into R848 pre-treated BALB/c mice. As expected (30), R848 induced autoimmune phenotypes in these mice, which was reversed by 2DG (Sup. Fig. 3E-H). R848 also expanded AM14 B cells, their differentiation into PBs and the production of 4–44 IgM, all of which were greatly reduced by 2DG (Sup. Fig. 3I-K). The frequency of IgD−Ki67+ AM14 B cells, which again correlated with 4–44 IgM production, was also expanded by R848 and reduced by 2DG (Sup. Fig. 3L-M). Finally, R848 expanded the number of ABCs cells, including AM14 ABCs in the BALB/c recipients, but only the later were affected by 2DG (Sup. Fig. 3N-P). Overall, these results show that the antigen-specific activation of AM14 B cells is enhanced in a lupus-like interferogenic environment, but this enhancement is lost when glycolysis is inhibited. The differentiation into ABCs, which may correspond to an extrafollicular response is less glucose-dependent as both AM14 and polyclonal endogenous ABCs were less affected by 2DG than the conventional B cells.
Figure 4. The inhibition of glycolysis impairs the cognate activation of TLR7 stimulated AM14 B cells.
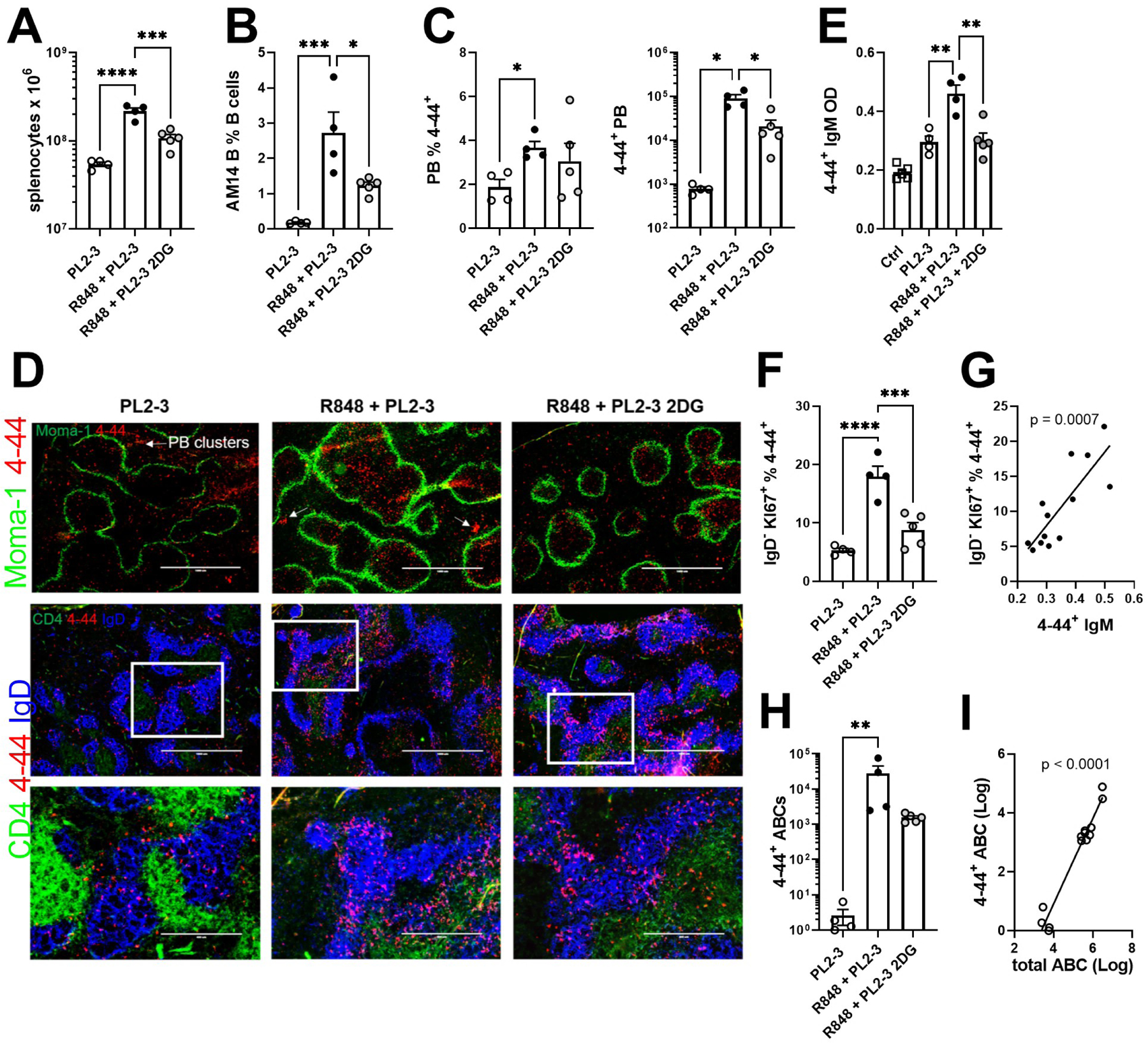
AM14 B cells were transferred into DO11.10 mice immunized with PL2-3. Some recipients were pre-treated with R848 (R848 + PL2-3), then treated or not with 2DG starting with PL2-3 immunization. A. Absolute numbers of splenocytes. B. AM14 B cell frequency among total B cells. C. Frequency and number of AM14 PBs. D. Serum 4–44+ IgM. E. Frequency of IgDlowKi-67+ cells among AM14 B cells. F. Correlation between the frequency of IgDlowKi-67+ AM14 B cells and 4–44+ IgM serum levels. G. Representative spleen histology from each of the three treatment groups. AM14 B cells were stained with 4–44-PE. Top row (4X): the location of the marginal sinus is shown with MOMA-1-FITC. Middle (4X) and bottom (10X) rows: sections are stained with IgD-APC (blue) and CD4-FITC. The bottom row images correspond to the gates shown in the middle row. H. Absolute numbers of AM14 ABCs. I. Correlation between the numbers of AM14 ABCs and total ABCs. Data were pooled from 2 experiments. Each point represents a mouse and graphs show means and SEM. The statistics correspond to 1-way ANOVA with multiple comparison tests or to Pearson’s tests (G and I). *P < 0.05; **P < 0.01. ***P < 0.001, ****P < 0.001.
The inhibition of glycolysis impairs the cognate activation of AM14 B cells in the presence of T cell help
We evaluated the requirement of AM14 B cells for glycolysis in DO11.10 mice treated with PL2-3 in the presence of cognate 13C2 T cells, which enhance the GC and PB differentiation of AM14 B cells (23). The combination of PL2-3 and 13C2 T cells induced strong 4–44 IgM as well as 4–44 IgG2a AFC responses, which were reduced by almost 2-fold by 2DG (Fig. 5A). 2DG reduced the number of splenocytes in PL2-3-treated mice (Fig. 5B), but not the frequency of AM14 B cells, which was similar between PL2-3 and PL2-8 control treatment with 13C2 T cell help (Fig. 5C). As previously shown (23), AM14 GC B responses were elicited by PL2-3 but not PL2-8 in the presence of 13C2 T cells and 20–30% of these GC B cells were class-switched (CS, Sup. Fig 1B, Fig. 5D-E). The inhibition of glycolysis decreased both the frequency of total and CS AM14 GC B cells (Fig. 5D-E). Again, 2DG did not change the frequency of proliferating IgD+ AM14 B cells, but it reduced the frequency of proliferating IgDlow cells by 3 folds (Fig. 5F-G), as well as the frequency of PBs among AM14 B cells (Fig 5H-I). 2DG did not change the frequency of endogenous polyclonal total B cells, but it reduced the frequency of endogenous GC B cells (Fig. 5J-K). These results show that the T cell-dependent activation of autoreactive B cells, their differentiation into GC B cells and AFCs is strongly promoted by glycolysis. This could indicate an intrinsic requirement of AM14 B cells for glycolysis in these conditions as well as glycolysis promoting effecting cognate T cell help.
Figure 5. The inhibition of glycolysis impairs the cognate activation of AM14 B cells in the presence of T cell help.
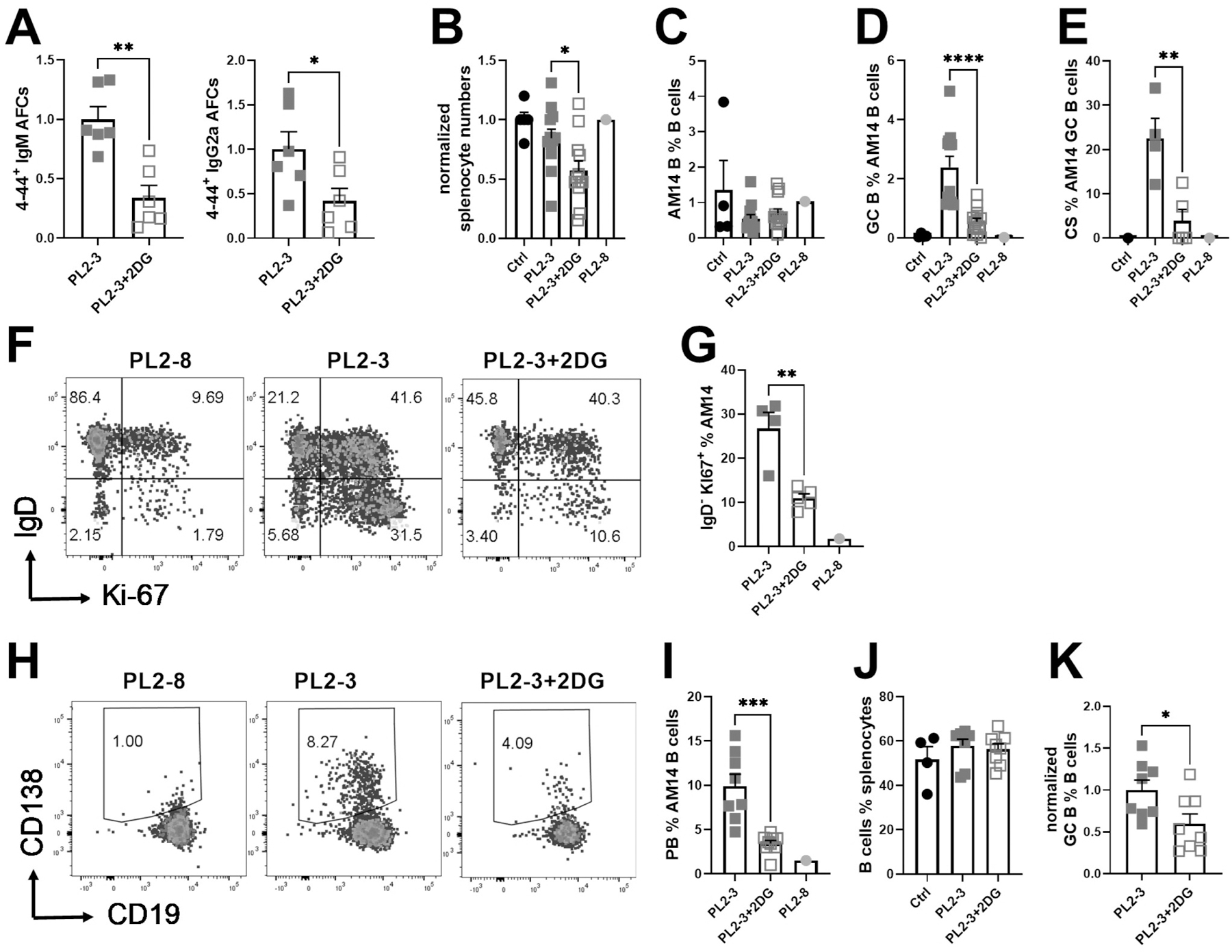
AM14 B cells and 13C2 T cells were co-transferred into DO11.10 mice immunized with PL2-3 and treated or not with 2DG for 7 d. A. 4–44 IgM and IgG2a ELISPOTs. B. Numbers of splenocytes. C. AM14 B cell frequency among total B cells. D. Frequency of GC B cells among AM14 B cells E. CS cells frequency among GC AM14 B cells. F. Representative FACS plots of AM14 B cells stained with IgD and Ki-67. G. Frequency of IgDlow Ki-67+ among AM14 B cells. H. Representative FACS plots of AM14 PBs gated with CD19 and CD138. I. PB frequency among AM14 B cells. J. Frequency of endogenous B cells. K. Frequency of endogenous GC B cells among endogenous. Data were pooled from 3 experiments and were normalized to the mean PL2-3 values for each experiment as 1 in A, B and K. Each point represents a mouse and graphs show means and SEM. Comparisons between groups were performed with t tests. *P < 0.05; **P < 0.01. ***P < 0.001, ****P < 0.001.
13C2 T cell activation by AM14 B cells does not require glycolysis
We characterized 13C2 T cells transferred along with AM14 B cells into DO11.10 mice that received PL2-3 or PL2-8, and that were treated or not with 2DG. As expected, the frequency of 13C2 T cells (Sup. Fig 1C) and their proliferation increased with PL2-3 but not with control PL2-8, confirming the specificity of the activation (Fig. 6A-B). PL2-3 also enhanced IL-2 production by 13C2 cells, but not that of IFNγ (Fig. 6C). Unexpectedly, however, 2DG did not reduce PL2-3-induced 13C2 frequency, proliferation, or IL-2 production (Fig. 6A-C). Moreover, there was a trend for an enhanced expression of BCL-2 in 13C2 T cells from 2DG-treated mice, suggesting an enhanced survival (Fig. 6D). PL2-3 elicited Tfh differentiation in 13C2 T cells, gated either as PD-1+ BCL-6+ or PD-1+ PSGL-1neg (Sup. Fig 1C and Fig. 6E). These Tfh cells were highly proliferative as compared to those from PL2-8-treated mice (Fig. 6F). The inhibition of glycolysis did not reduce the frequency or proliferation of 13C2 Tfh cells (Fig. 6E-F), their BCL-2 expression or IL-2 production (Fig. 6G-H). PL2-3 also enhanced the frequency of 13C2 Tef cells gated as CD4+ PD-1+ PSGL-1+, as well as their IL-2 production. As for 13C2 Tfh cells, 13C2 Tef cells were not sensitive to 2DG (Fig. 6I-J). Finally, the frequency of Tfh cells and cytokine production among endogenous OVA-specific DO11.10 T cells were unchanged following PL2-3 immunization and these responses were not altered by 2DG (Fig. 6K-M). These results show that antigen-specific autoreactive T cells do not require glycolysis to differentiate into Tfh cells in response to the activation of their cognate autoreactive B cells that are themselves glucose-sensitive.
Figure 6. 13C2 T cell activation does not require glycolysis.
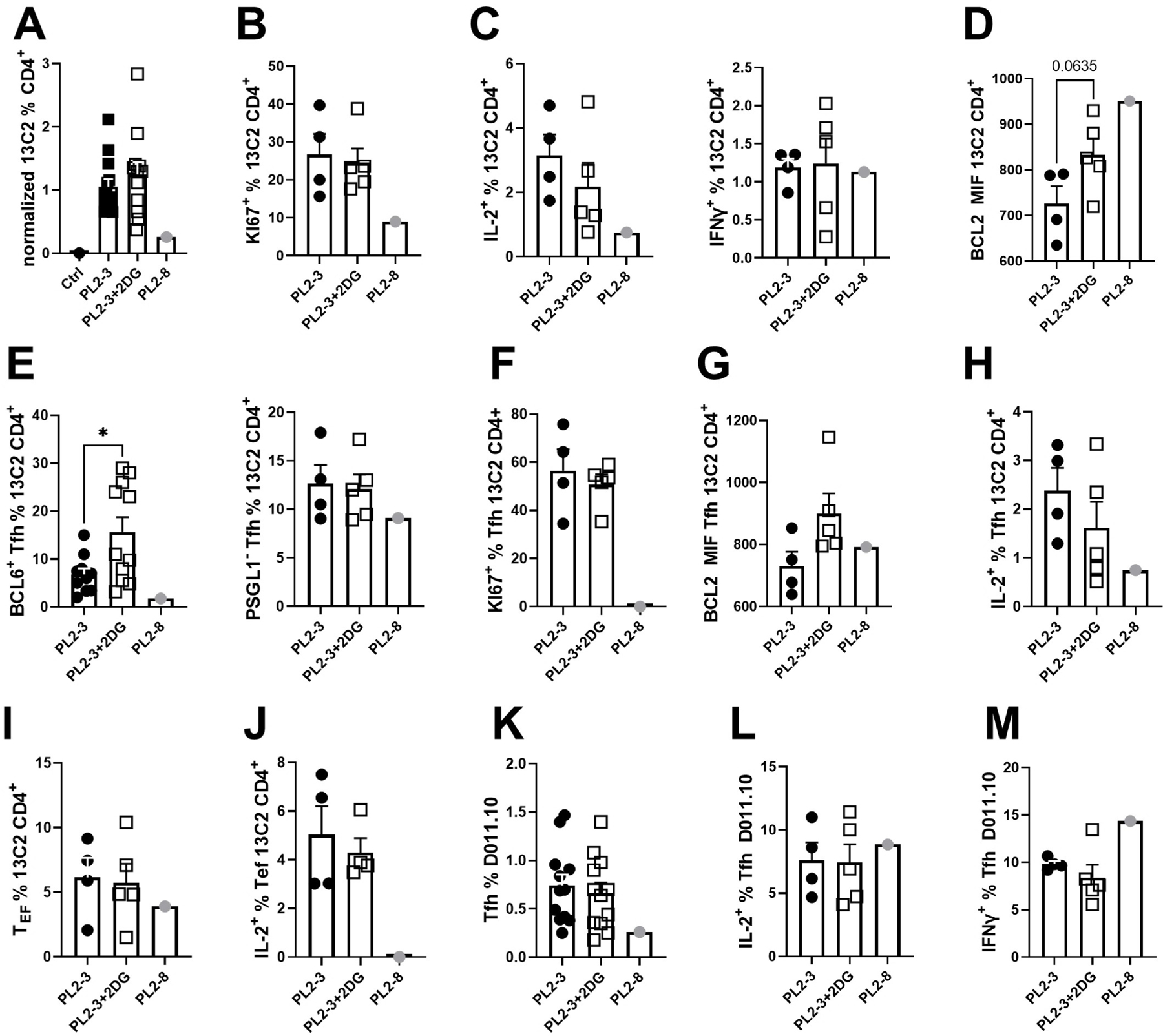
AM14 B cells and 13C2 T cells were co-transferred into DO11.10 mice immunized with PL2-3 and treated or not with 2DG for 7 d. A. Frequency of 13C2 T cells among total CD4+ T cells. B. Proliferation of 13C2 T cells. C. IL-2 and IFNγ production by 13C2 T cells. D. BCL-2 expression by 13C2 cells. E. Frequency of Tfh cells among 13C2 T cells gated as PD-1+Bcl-6+ (left) or PD-1+PSGL-1low (right) cells. Proliferation (F) and BCL-2 expression (G) in Tfh 13C2 T cells. H. IL-2 production by Tfh 13C2 T cells. I. Extra-follicular (Tef) cell frequency among 13C2 T cells. J. IL-2 production by Tef 13C2 T cells. K. Frequency of endogenous DO11.10 T cells. K. Tfh frequency among DO11.10 cells. IL-2 (L) and IFNγ (M) production by DO11.10 Tfh cells. Data were pooled from 3 experiments and values were normalized to the mean PL2-3 values as 1 in A. Each point represents a mouse and graphs show means and SEM. Comparisons between groups were performed with t tests. *P < 0.05.
Glycolysis is dispensable for antigen-specific CD4+ T cells activated by exogenous antigen
To further investigate the glycolysis requirements of antigen-specific CD4+ T cells, OVA-specific CD90.1+ OT-II T cells were transferred into B6 recipients, which were immunized 24 h later with NP16-OVA in alum and treated or not with 2DG for 7 d. Control recipients were not immunized. The number of OT-II cells and their proliferation increased upon immunization, as expected, but it was not affected by the 2DG treatment (Fig. 7A-B). The frequency of effector memory Tem cells (Sup. Fig. 1C) or that of highly proliferating Tfh OT-II cells induced by immunization were also not affected by 2DG (Fig. 7C-E). However, 2DG reduced the frequency of NP-specific GC B cells and PCs (Fig. 7F-G). This contrasts with the non-glycolytic NP-specific B1-8 cells (13), which might be due to differences between the transgenic BCR in B1-8 cells and the polyclonal B cells in this experiment. Despite a reduced GC B cell frequency, the class-switching among NP+ GC B and PC cells was similar after 2DG treatment (Fig. 7H-I), which corresponded to unchanged levels of serum NP-specific IgG1 (Fig. 6J). Finally, endogenous polyclonal CD4+ T cell responses were unaffected by either NP-OVA immunization or 2DG treatment (Fig. 7K-L).
Figure 7. Glycolysis is dispensable for foreign antigen-specific CD4+ T cells in response to immunization.
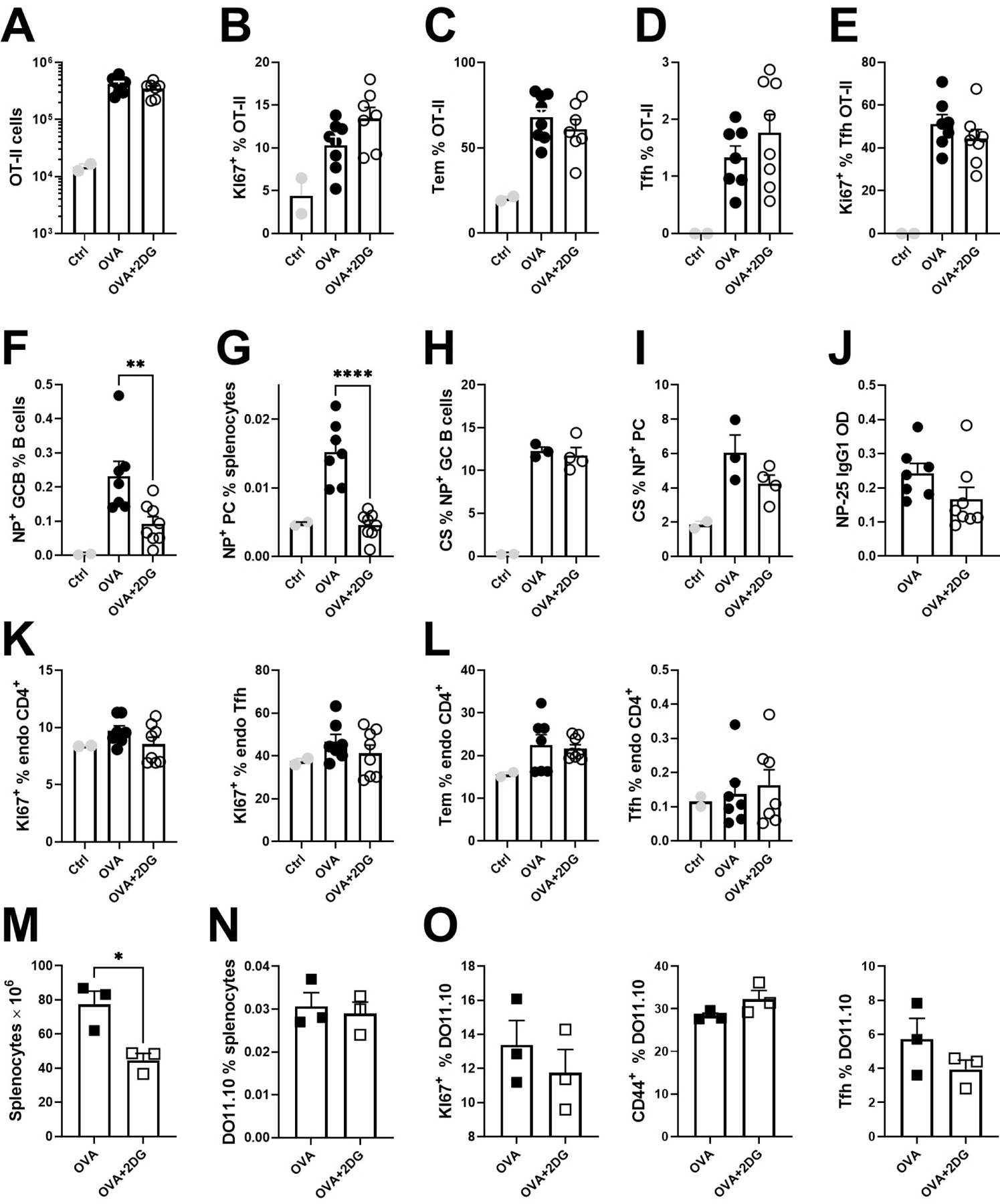
A – L. CD45.1+ OT-II T cells were transferred into B6 mice immunized with NP-OVA in alum, and treated or not with 2DG for 7 d. A. Numbers of OT-II cells. B. Frequency of proliferating OT-II cells. C. Frequency of Tem cells among OT-II cells. D. Frequency of Tfh cells among OT-II cells. E. Frequency of proliferating OT-II Tfh cells. Frequency of NP+ GC B (F) and NP+ PC (G) cells. Frequency of CS in NP+ GC B (H) and PC (I) cells. J. Serum levels of anti-NP25 IgG1. K. Proliferation in endogenous total CD4+ or Tfh cells. L. Frequency of Tem and Tfh cells among endogenous B6 CD4+ T cells. M – O. DO11-10 T cells were transferred into AM14 mice immunized with NP-OVA in alum, and treated or not with 2DG for 7 d. M. Numbers of splenocytes in AM14 recipient mice. Frequency of total DO11.10 T cells among splenocytes (N), as well as proliferating (left), CD44+ (middle) and Tfh (right) cells among DO11.10 T cells (O). (A – L) data were pooled from two experiments. Each point represents a mouse and graphs show means and SEM. Statistics were calculated with 1-way ANOVA with multiple comparison tests. *P < 0.05; **P < 0.01. ***P < 0.001.
Finally, we investigated the role of glycolysis in antigen-specific T cells independently from cognate B cell help with DO11.10 T cells injected to AM14 recipients that were immunized 24 h later with NP16-OVA in alum. In this setting, DO11-10 T cells get activated by their cognate antigen without help from monoclonal AM14 B cells. Despite decreased splenocyte numbers that confirmed the global effect of 2DG (Fig. 7N), the frequency of DO11.10 cells, their proliferation and differentiation into Tem and Tfh cells were unchanged by the inhibition of glycolysis (Fig. 7O-P). Overall, these results show that antigen-specific CD4+ T cells are glycolysis-independent upon immunization with their cognate exogenous antigen, independently from B cells.
Discussion
Immune effector functions are tightly dependent on metabolic reprogramming, with increasingly recognized levels of cell and context-specific complexity (33, 34). Glycolysis is required for BCR-mediated growth through the PI3K pathway (35), but the GC stage of non-autoimmune B cell differentiation in response to protein immunization depends on OXPHOS, and more specifically on FAO (12, 13). In a spontaneous mouse model of lupus, the activation of CD4+ T cell and B cell is coupled with an enhanced glycolysis and mitochondrial metabolism (24, 36). The differential effect of 2DG on spontaneous and immunization-induced responses in lupus-prone mice has suggested that autoreactive T and B cells may be driven by specific metabolic programs (9) (36). In support of this hypothesis, spontaneous Tfh cells and virus-induced Tfh cells present a different transcriptional signature that includes several metabolic pathways in two lupus-prone mouse models (18) (37). A comparison of the spontaneous / autoreactive and immunization-induced transcriptomes has not been reported so far for GC B cells, but it is likely that differences would also been found. The caveat in the interpretation of these results is that spontaneously expanded activated B and CD4+ T cells in autoimmune mice have been used as a proxy for autoreactive lymphocytes, although they correspond to complex mixed populations. Therefore, the specific metabolism of autoreactive B cells and CD4+ T cells has never been evaluated. To address this issue, we used RF-producing AM14 B cells (19) and their cognate chromatin-specific 13C2 CD4+ T cells (23).
We showed that the cognate activation of purified AM14 B cells with PL2-3 in vitro greatly increased their metabolism relative to their activation with a TLR7 agonist. This finding is consistent with results obtained with polyclonal B cells showing that the combination of BCR and TLR signaling induced a greater mitochondrial activity and glycolysis that either signal alone (38). In addition, we found that PL-3 increased glycolysis relative to R848. The combination of BCR and TLR stimulation, the type of stimulation induced by PL2-3, did not increase glycolysis over respiration in polyclonal B cells (38), indicating that this feature of PL2-3-activated AM14 B cells may not be shared among all B cells. Consistent with the high glycolytic activity triggered by PL2-3, the differentiation of AM14 B cells into AFCs induced in vitro by PL2-3 with 13C2 T cell help was reduced by the inhibition of glycolysis. These results are consistent with the antibody production by LPS-activated polyclonal B cells or by T-independent immunization being eliminated by 2DG (39) (36). Antibody production by 2DG-treated PL2-3-stimulated AM14 B cells was still much higher than in unstimulated controls, arguing that these B cells can use multiple energy sources, including glucose, for an antigen-specific differentiation in the presence of cognate T cell help. Etomoxir, however, did not reduced antibody production by AM14 B cells, suggesting that this energy source is not FAO.
The glycolytic requirement for AM14 B cell differentiation into AFCs was further investigated in adoptive transfer models with different settings. In BALB/c.Rag recipients, the PB and AFC differentiation of AM14 B cells stimulated by PL2-3 and their RF production was not reduced by 2DG. Chronic exposure to BAFF, a condition found in lupus (40), makes B cells more glycolytic and activates their AKT/mTOR pathway (39, 41). In lymphopenic mice, AM14 B cells were likely to be exposed to high levels of BAFF when stimulated by PL2-3, and strong BAFF-R signaling may bypass at least partially their requirement for glycolysis. Their R848 activation in the same setting was however more sensitive to 2DG, suggesting that different signaling pathways trigger different metabolic program. Activation by PL2-3 or R848 in BALB/c.Rag mice increased glucose uptake in AM14 B cells, but there was no compensatory increase in fatty acid intake by AM14 B cells in 2DG-treated mice, suggesting that fatty acids were not used as an alternate source of energy, which supports the etomoxir results. In non-lymphopenic DO11-10 mice, the activation and RF production of PL2-3-stimulated AM14 B cells was reduced to baseline by 2DG, suggesting that glycolysis is the main energy source. 2DG also reduced the activation of R848-stimulated AM14 B cells, but it did not reduce their RF production. This is consistent with R848 increasing glycolysis to a lower extent than PL2-3 in AM4 B cells. Finally, AM14 B cells in DO11-10 mice upregulated both their glucose and fatty acid uptake when stimulated with either PL2-3 or R848, but fatty acids were not able to compensate for the inhibition of glycolysis.
Although AM14 B cells have been used as a model of autoreactivity, short-term adoptive transfers in DO11-10 mice do not represent an autoimmune environment. This issue was addressed with the strong autoimmune response induced by TRL7 activation on a BALB/C background (30). Consistent with the strong TLR7 signal activating AM14 B cells in MRL/lpr mice (42), a pre-treatment with R848 greatly enhanced the activation and differentiation of PL2-3-stimulated AM14 B cells, as well as their RF production in both BALB/c and DO11-10 mice. All these phenotypes were greatly reduced by 2DG. Therefore, the cognate AM14 B cell activation and RF production rely on glycolysis in both a non-autoimmune and lupus-like environment. The R848 pre-treatment also induced a strong ABC response that was similar in AM14 and endogenous B cells. Although 2DG reduced the number of AM14 and total ABCs, it did not reduce their frequency relative to total B cells. Extra-follicular activation is a major pathway for autoreactive B cells (2, 3). Since ABCs largely correspond to TLR7-activated extra-follicular B cells, this result suggest that this pathway may be less glucose-dependent than the GC-pathway, a hypothesis that requires to be formally tested.
An inhibitory effect of 2DG was also observed in AM14 B cells activated by PL2-3 in the presence of 13C2 T cells in DO11-10 mice. Here, in addition to the phenotypes observed in the absence of T cells, 2DG reduced GC B cell differentiation and class-switching. Thus, our results show that AM14 B cells are glycolytic, especially when activated by their cognate antigen, and that their differentiation into AFCs to produce RF depends on glycolysis, except when differentiating in a lymphopenic environment or possibly through the ABC pathway. Not all differentiation stages were sensitive to the inhibition of glycolysis, since the frequency of total AM14 B cells and the first step of proliferation of IgD+ positive cells were not affected by 2DG. The initial activation of resting cells may use fatty acids and/or glutamine in addition to glucose while the latter differentiation to IgDlow cells into GC B cells and PBs was greatly dependent on glucose. The mechanism responsible for autoreactive AM14 B cells relying on glycolysis while NP-specific B1-8 cells, mainly in the GC subset, depending on fatty acid oxidation is yet unknown. The chronicity of autoantigen stimulation is unlikely to be involved in our model in which PL2-3 was delivered over one week. A major difference between AM14 and B1-8 B cells is that the former are dually activated by the BCR and TLR9, which could intrinsically deliver a more glycolytic signal than either BCR or TLR alone. In addition, cognate antigen-specific 13C2 T cells, which are essential for the generation of AM14 GC B cells, may provide robust activating signals that render AM14 GC B cells glycolytic in contrast to B1-8 GC B cells, which are generated in the presence of polyclonal CD4+ T cells. This hypothesis needs to be rigorously tested, and the molecular pathways linking these modes of B cell activation to metabolic programing identified.
The AM14 B cells and 13C2 T cells co-transfer provided the opportunity to assess the response of autoreactive T cells to 2DG treatment. To our surprise, the inhibition of glycolysis did not reduce 13C2 cell proliferation, survival, IL-2 and IFNγ production or differentiation into Tfh and Tef cells, although, in the same mice, GC B cell differentiation and class-switching was reduced. It is possible that the Tfh 13C2 cells from 2DG-treated mice differ functionally from the untreated controls in a manner that we have not yet identified. We further explored the effect of glycolysis inhibition on the acute short-term activation of antigen-specific CD4+ T cells in response to immunization. As with autoreactive 13C2 T cells, OVA-specific T cells proliferated and differentiated into Tfh cells to the same extent in mice treated with 2DG and controls, which confirmed results previously obtained with polyclonal T cells as well as influenza-specific CD4+ T cells (18). These results show that contrary to what we have reported with polyclonal CD4+ T cells in lupus-prone mice (18), the differentiation of autoreactive antigen-specific Tfh cells, at least in the transfer model that we have used, is not limited by the inhibition of glycolysis.
A limitation of the study is that it largely relied on 2DG, a pharmacological inhibition of glycolysis that may have off-target effects. 2DG also inhibits N-glycosylation (43), induces endoplasmic reticulum stress (44), and restricts glutamine utilization by mitochondria (45), all of which may alter immune cell function. This limitation was partially addressed on purified AM14 B cells with three other inhibitors of glycolysis that have been used in the cancer field, as well as in vivo with LND, a drug that targets HK, the enzyme for which 2DG competes with glucose for binding. The effect of the inhibitors of purified B cells suggest an intrinsic requirement for glycolysis. The complexity of the AM14/13C2 model limits the feasibility of cell-specific gene targeting to probe metabolic pathways. Thus, other strategies will be required to address the metabolic requirement of antigen-specific autoreactive B cells and T cells in a cell-intrinsic manner.
Supplementary Material
Key points.
The differentiation of lupus autoantigen-specific B cells rely on glycolysis
These B cells are more glycolytic under antigen-specific than TLR7 stimulation
The activation of chromatin-specific CD4 T cells does not require glycolysis
Acknowledgments.
We thank the Morel lab members for technical assistance and discussion.
This work is supported by a grant from the National Institutes of Health R37AI128901 to LM.
References
- 1.Malkiel S, Barlev AN, Atisha-Fregoso Y, Suurmond J, and Diamond B. 2018. Plasma cell differentiation pathways in systemic lupus erythematosus. Front Immunol 9: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenks SA, Cashman KS, Woodruff MC, Lee FE, and Sanz I. 2019. Extrafollicular responses in humans and SLE. Immunol Rev 288: 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsner RA, and Shlomchik MJ. 2020. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53: 1136–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, Leonard WJ, and Roopenian DC. 2009. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Nat Acad Sci USA 106: 1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JY, Seth A, Kashgarian M, Terrillon S, Fung E, Huang L, Wang LC, and Craft J. 2017. Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J Immunol 198: 2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft JE 2012. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 8: 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SJ, Lee K, and Diamond B. 2018. Follicular helper T cells in systemic lupus erythematosus. Front Immunol 9: 1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mountz JD, Hsu HC, and Ballesteros-Tato A. 2019. Dysregulation of T follicular helper cells in lupus. J Immunol 202: 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng X, Brown J, Choi SC, Li W, and Morel L. 2020. Metabolic determinants of lupus pathogenesis. Immunol Rev 295: 167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piranavan P, Bhamra M, and Perl A. 2020. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi SC, and Morel L. 2020. Immune metabolism regulation of the germinal center response. Exp Mol Med 52: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Wang Y, Manakkat Vijay GK, Fu S, Nash CW, Xu D, He D, Salomonis N, Singh H, and Xu H. 2021. Coupled analysis of transcriptome and BCR mutations reveals role of OXPHOS in affinity maturation. Nat Immunol 22: 904–913. [DOI] [PubMed] [Google Scholar]
- 13.Weisel FJ, Mullett SJ, Elsner RA, Menk AV, Trivedi N, Luo W, Wikenheiser D, Hawse WF, Chikina M, Smita S, Conter LJ, Joachim SM, Wendell SG, Jurczak MJ, Winkler TH, Delgoffe GM, and Shlomchik MJ. 2020. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat Immunol 21: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, and Weinmann AS. 2014. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol 15: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, and Craft J. 2015. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43: 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, Cloer C, Kishton RJ, Gao X, Youngblood B, Do M, Li MO, Locasale JW, Rathmell JC, and Chi H. 2016. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 45: 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkenschlager J, Finkin S, Ramos V, Kraft J, Cipolla M, Nowosad CR, Hartweger H, Zhang W, Olinares PDB, Gazumyan A, Oliveira TY, Chait BT, and Nussenzweig MC. 2021. Dynamic regulation of TFH selection during the germinal centre reaction. Nature 591: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SC, Titov AA, Abboud G, Seay HR, Brusko TM, Roopenian DC, Salek-Ardakani S, and Morel L. 2018. Inhibition of glucose metabolism selectively targets autoreactive follicular helper T cells. Nat Commun 9: 4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, and Weigert MG. 1993. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol 5: 1329–1341. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, and Marshak-Rothstein A. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607. [DOI] [PubMed] [Google Scholar]
- 21.Hannum LG, Ni D, Haberman AM, Weigert MG, and Shlomchik MJ. 1996. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J Exp Med 184: 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang A, Niu H, Cullen J, Choi SC, Zheng YY, Wang H, Shlomchik MJ, and Morel L. 2014. Activation of rheumatoid factor-specific B cells is antigen dependent and occurs preferentially outside of germinal centers in the lupus-prone NZM2410 mouse model. J Immunol 193: 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles JR, Neves AT, Marshak-Rothstein A, and Shlomchik MJ. 2017. Autoreactive helper T cells alleviate the need for intrinsic TLR signaling in autoreactive B cell activation. JCI Insight 2: e90870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, and Morel L. 2015. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7: 274ra218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herlands RA, William J, Hershberg U, and Shlomchik MJ. 2007. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol 37: 3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, and Shlomchik MJ. 1999. Autoantigen-specific B cell activation in Fas-deficient rheumatoid factor immunoglobulin transgenic mice. J Exp Med 190: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SC, Li W, Zhang X, Kanda N, Zeumer-Spataro L, Teng X, and Morel L. 2022. Pharmacologically inferred glycolysis and glutaminolysis requirement of B cells in lupus-prone mice. J Immunol 208: 2098–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SC, Hutchinson TE, Titov AA, Seay HR, Li S, Brusko TM, Croker BP, Salek-Ardakani S, and Morel L. 2016. The lupus susceptibility gene Pbx1 regulates the balance between follicular helper T cell and regulatory T cell differentiation. J Immunol 197: 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Xu W, Li G, and Cui W. 2018. Weighing In on mTOR Complex 2 Signaling: The Expanding Role in Cell Metabolism. Oxid Med Cell Longev 2018: 7838647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokogawa M, Takaishi M, Nakajima K, Kamijima R, Fujimoto C, Kataoka S, Terada Y, and Sano S. 2014. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic lupus erythematosus. Arthritis Rheumatol 66: 694–706. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Zhang H, Liu S, Xia F, Kang Z, Zhang Y, Liu Y, Xiao H, Chen L, Huang C, Shen N, Xu H, and Li F. 2019. Excessive CD11c(+)Tbet(+) B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus. Proc Natl Acad Sci USA 116: 18550–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FE, Boss JM, Lund FE, and Sanz I. 2018. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49: 725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geltink RIK, Kyle RL, and Pearce EL. 2018. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol 36: 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makowski L, Chaib M, and Rathmell JC. 2020. Immunometabolism: From basic mechanisms to translation. Immunol Rev 295: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, and Chiles TC. 2006. Antigen receptor–mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 107: 4458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SC, Li W, Zhang X, Kanda N, Zeumer-Spataro L, Teng X, and Morel L. 2022. Pharmacologically inferred glycolysis and glutaminolysis requirement of B cells in lupus-prone mice. J Immunol 208: 2098–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong X, Antao OQ, Song W, Sanchez GM, Zembrzuski K, Koumpouras F, Lemenze A, Craft J, and Weinstein JS. 2021. Type I Interferon-Activated STAT4 Regulation of Follicular Helper T Cell-Dependent Cytokine and Immunoglobulin Production in Lupus. Arthritis Rheumatol 73: 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, Dahlstrom E, Dorward DW, Skinner J, Sack MN, and Pierce SK. 2018. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 19: 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, and Rathmell JC. 2014. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 192: 3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent FB, Morand EF, Schneider P, and Mackay F. 2014. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 10: 365–373. [DOI] [PubMed] [Google Scholar]
- 41.Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, Hilbert DM, and Thompson CB. 2008. Multiple signaling pathways promote B lymphocyte stimulator–dependent B-cell growth and survival. Blood 111: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herlands RA, Christensen SR, Sweet RA, Hershberg U, and Shlomchik MJ. 2008. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 29: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andresen L, Skovbakke SL, Persson G, Hagemann-Jensen M, Hansen KA, Jensen H, and Skov S. 2012. 2-deoxy D-glucose prevents cell surface expression of NKG2D ligands through inhibition of N-linked glycosylation. J Immunol 188: 1847–1855. [DOI] [PubMed] [Google Scholar]
- 44.Xi H, Kurtoglu M, and Lampidis TJ. 2014. The wonders of 2-deoxy-D-glucose. IUBMB Life 66: 110–121. [DOI] [PubMed] [Google Scholar]
- 45.Pantic B, Ives D, Mennuni M, Perez-Rodriguez D, Fernandez-Pelayo U, Lopez de Arbina A, Muñoz-Oreja M, Villar-Fernandez M, Dang TJ, Vergani L, Johnston IG, Pitceathly RDS, McFarland R, Hanna MG, Taylor RW, Holt IJ, and Spinazzola A. 2021. 2-Deoxy-D-glucose couples mitochondrial DNA replication with mitochondrial fitness and promotes the selection of wild-type over mutant mitochondrial DNA. Nat Commun 12: 6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


