Abstract
Background:
This study sought to determine the isoform-specific role of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) in the endothelium-mediated vascular dysfunction associated with aging.
Methods:
Endothelium-dependent (intraluminal flow- and acetylcholine (ACh)-induced) vasodilation in human skeletal muscle feed arteries (SMFAs) of young (24±1 yrs, n=16), middle aged (45±1 yrs, n=18), and old (76±2 yrs, n=21) subjects was assessed, in vitro, with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin). To identify the role of nitric oxide (NO) bioavailability in these responses, NO synthase blockade (L-NG-monomethyl arginine citrate (L-NMMA)) was utilized. SMFA NOX1, NOX2, and NOX4 protein expression was determined by Western blot.
Results:
Age related endothelium-dependent vasodilatory dysfunction was evident in response to flow (Young: 69±3; Middle aged: 51±3; Old: 27±3%, P<0.05) and ACh (Young: 89±2; Middle aged: 72±3; Old: 45±4 %, P<0.05). NOX1 inhibition had no effect on SMFA vasodilation, while NOX2 inhibition restored flow- and ACh-induced vasodilation in the middle aged and the old SMFAs (Middle aged + gp91: 69±3; 86±3, Old + gp91: 65±5; 83±2 %, P < 0.05), and NOX4 inhibition tended to restore these vasodilatory responses in these two groups, but neither achieved statistical significance (P≈0.06). L-NMMA negated the restorative effects of NOX2 and NOX4 blockade. Only NOX2 and NOX4 protein expression was significantly greater in the two older groups and inversely related to vascular function (r=0.48–0.93, P<0.05).
Conclusions:
NOX2 and, to a lesser extent, NOX4 appear to play an important, likely NO-mediated, role in age-related endothelial dysfunction.
Keywords: NOX, human skeletal muscle feed artery, shear stress, NO bioavailability
INTRODUCTION
Aging is associated with a decline in blood flow and oxygen delivery to contracting skeletal muscle, which can result in attenuated exercise capacity and, ultimately, diminished physical function and mobility (Proctor et al., 1998; Lawrenson et al., 2003; Nyberg et al., 2012). There are many factors potentially responsible for the age-associated reduction in blood flow to skeletal muscle, such as augmented skeletal muscle sympathetic nerve activity and alterations in local metabolites that influence vascular tone. However, growing evidence supports the tenet that the progressive decline in blood flow to skeletal muscle is, at least in part, due to endothelial dysfunction with advancing age (Saltin et al., 1998; Crecelius et al., 2010; Trinity et al., 2013; Hearon & Dinenno, 2016). Indeed, endothelial dysfunction in the form of decreased endothelial nitric oxide synthase (eNOS) and, subsequently, attenuated nitric oxide (NO) bioavailability, can greatly influence skeletal muscle blood flow (DeSouza et al., 2000; Muller-Delp et al., 2002; Trott et al., 2011; El Assar et al., 2013; Park et al., 2016; Park et al., 2018). Among the multiple factors that influence both NO production and degradation are reactive oxygen species (ROS). These ROS play a major role in attenuating NO bioavailability and the impaired endothelial function that has been documented in skeletal muscle feed arteries (SMFAs) with advancing age (Park et al., 2016; Park et al., 2018).
Throughout the vasculature, there are a variety of enzyme systems that contribute to ROS formation, including cytochrome P-450 oxygenase, xanthine oxidase, and the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzymes of the respiratory chain (Laurindo et al., 1994; Csiszar et al., 2002; Trott et al., 2009; Wind et al., 2010; Trott et al., 2011). With the latter proposed as a promising therapeutic target for age-related vascular dysfunction. Indeed, Trott et al. (Trott et al., 2011) reported that in rats the protein expression of NOX subunit, gp91phox, was significantly greater than in their young counterparts and apocynin, a commonly used broad NOX inhibitor, restored the attenuated flow-mediated vasodilation in the SMFAs of the old rats. Another animal study documented that old mice exhibited greater protein expression of a different NOX subunit, p67phox, than young mice and apocynin, again, restored the age-related impairment in carotid artery vasodilation (Sindler et al., 2011). In humans, Donato et al. (Donato et al., 2007) identified that the increased expression of NOX subunit, p47phox, which when translocated to membrane bound NOX subunits, stimulates superoxide (O2−) production, contributes to endothelial oxidative stress, and, ultimately, leads to impairment of endothelial-dependent vasodilation with advancing age.
The NOX family of enzymes includes NOX1-5 and DUOX1-2, but among them, 3 of the NOX isoforms, NOX1, NOX2, and NOX4 are most commonly expressed in vascular endothelial cells, which are strongly linked to the pathogenesis of vascular diseases (Sorescu et al., 2002; Hwang et al., 2003; Lassègue & Clempus, 2003; Ago et al., 2004; Guzik et al., 2006; Larsen et al., 2009; Wind et al., 2010). NOX2 appears to be the most important NOX isoform in vascular pathology. Indeed, NOX2 expression is elevated in the vasculature with both hypertension and atherosclerosis (Guzik et al., 2006; DeMarco et al., 2008; Park et al., 2008) and the NOX2 inhibitor, gp91, significantly attenuated bradykinin-induced ROS production in intact human coronary arterioles (Larsen et al., 2009). NOX1 and NOX4 are also upregulated in a variety of vascular pathologic models (Muller-Delp et al., 2002; Nishiyama et al., 2004; Dikalova et al., 2005; Yoshida & Tsunawaki, 2008; Ismail et al., 2009; Jaulmes et al., 2009; Meijles & Pagano, 2016). Interestingly, although the upregulation in NOX enzymes appears to play a critical role in age-related vascular disease and oxidative stress, a true parsing out of the role of these NOX isoforms with respect to endothelial function and age has yet to be performed. Furthermore, the specific roles of NOX1, NOX2, and NOX4 are unknown in human SMFAs.
Therefore, utilizing the combination of the pressure myography technique to study vascular function and the incubation of human SMFAs in isoform-specific NOX inhibitors, this study sought to determine the role of NOX1, NOX2, and NOX4 in age-related endothelial dysfunction. We tested the hypothesis that the treatment of human SMFAs with a NOX1 inhibitor (ML090), a NOX2 inhibitor (gp91), and a NOX4 inhibitor (plumbagin) would, by increasing NO bioavailability, correct age-related endothelial dysfunction.
METHODS
Subjects and general procedures:
SMFAs, from the axillary and inguinal regions, were obtained from young (<35 yrs), middle aged (>40 and <60 yrs), and old (>65 yrs) subjects during excisions of clinical state I or II primary melanoma. All subjects were free from metastatic cancer and not undergoing chemotherapy. Although there were no other specific exclusion criteria for this study, all medical conditions and medications were noted. All procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City VA Medical Center (IRB# 32786), carried out in accordance with the Declaration of Helsinki, and written informed consent was obtained from all subjects prior to surgery.
Vessel harvest and preparation:
Briefly, as described previously (Park et al., 2016; Park et al., 2018), SMFAs were obtained during a sentinel node biopsy surgery, for melanoma, at the Huntsman Cancer Hospital. Specifically, SMFAs were harvested during the dissection to locate sentinel lymph nodes, most commonly within the axilla or superficial inguinal subcutaneous regions. SMFAs were ligated, excised, immediately placed on iced normal physiological saline solution (PSS) (mM: 145.0 NaCl, 4.7 KCL, 2.0 CaCl2, 1.17 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer and 10 g/ L of BSA at pH 7.4), and transferred to the laboratory within 15 min of harvesting.
Vasodilation assessments:
Briefly, as described previously (Park et al., 2016; Park et al., 2018), cleaned SMFAs were cannulated at both ends with micropipette tips and placed in the pressure myography organ baths (110p; DMT Systems, Aarhus, Denmark) containing PSS at 37°C. Two pieces of the same vessel were placed in separate bath and both were incubated for 1 hour in this PSS, either with or without the addition of either ML090, gp91, or plumbagin (all 1 μmol/L) (Smith et al., 2015). With an intralumintal pressure of 60 mmHg, baseline vessel outer diameters were then recorded using transillumiation and an inverted microscope with a video camera (TS100; Nikon Eclipse, Melville, NY, USA). It should be noted that all vessel diameters were meaured as outer diameter, rather than the typical inner diameter, due to opaqueness of the relatively thick SMFA smooth muscle. Following pre-constriction with phenylephrine (PE) (10−6 to 10−4 M: Sigma-Aldrich Corp.) to approximately 70% of the maximum PE response, vasodilation (%) was assessed in response to three stimuli: First, the endothelium-mediated vasodilatory response to flow-induced shear stress was measured by establishing intraluminal flow in the SMFAs. This was achieved by altering the heights of the independent fluid reservoirs, contiguous with both cannulated ends of the SMFAs, in equal and opposite directions so that a pressure difference was developed across the vessel without altering mean intraluminal pressure (≈60 mmHg) at the midpoint of the artery (Kuo et al., 1991; Park et al., 2018). Three pressure differences of 15, 30, and 45 mmHg, which yielded an approximate flow rate of 15, 30 and 45 μl/min, were utilized for the flow experiments. Each flow rate was maintained for 5 min to allow the SMFAs to reach a steady state diameter. Second, to assess endothelium-dependent vasodilation pharmacologically, an acetylcholine (ACh) dose response curve (10−7 to 10−3 M) was performed by, cumulatively, increasing the concentration of the ACh incubation. Third, endothelium-independent vasodilation was assessed in the SMFAs, cumulatively, increasing the concentration of sodium nitroprusside (SNP) over the range of 10−8 to 10−3 M. Additionally, to determine the contribution of eNOS and NO bioavailability to endothelium-mediated vasodilation of the SMFAs with and without ML090, gp91, and plumbagin incubation, flow and ACh dose-response curves were performed with and without NOX isoform-specific blockade in the presence and absence of N-monomethyl-L-arginine (L-NMMA) (10−3 M, 30 min), as previously described (Park et al., 2016; Park et al., 2018).
Calculations:
The vasodilator response was assessed as percent dilation calculated using the following equation:
Where DDose is the recorded diameter at a given dose/flow rate, DB is the baseline diameter before the dose-response curve assessement, and DM is the maximal diameter.
Immunoblotting:
Briefly, SMFAs were homogenized in a lysis buffer (Santa Cruz Biotech, Santa Cruz, CA) including a protease inhibitor cocktail, 10 mM sodium fluoride and 1 mM phenyl methyl sulfonyl fluoride (PMSF) (all listed chemicals were from Santa Cruz Biotech, Santa Cruz, CA). Protein concentration was determined using the Bradford technique. 30 μg of homogenate was separated by polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and incubated with primary and secondary antibodies directed against the proteins of interest. Membranes were imaged on a ChemiDoc XRS (Bio-Rad) and quantified with Image Lab software (Bio-Rad). The specific antibodies used to detect SMFA proteins included: NOX1 antibody (ab213634, Abcam, Cambridge, MA), NOX2 antibody (9570, Cell Signaling, Boston, MA), and NOX4 antibody (610296, BD Transduction, San Jose, CA). Protein abundance was normalized to GAPDH (ab9485, Abcam, Cambridge, MA), which served as a loading control.
Immunofluorescence:
Visualization of the exact locus of NOX1, NOX2, and NOX4 on the SMFAs was achieved by immunofluorescence (primary antibody: anti-NOX1 antibody (ab55831), anti-NOX2/gp91phox antibody (ab80508), and anti-NOX 4 antibody (ab79971) (Abcam, Cambridge, UK) utilizing an Imager A2 microscope with Axiocam and accompanying software (Zeiss, Jena, Germany).
Statistical analyses:
Statistical analyses were performed using GraphPad Prism 7 Software (La Jolla, CA). Two-way repeated measures ANOVA was used to assess relative changes in vessel diameter with and without ML090, gp91, and plumbagin in response to flow, ACh, and SNP. Two-way repeated measures ANOVA was used to assess changes in vessel diameter with and without ML090, gp91, and plumbagin and with and without L-NMMA in response to flow and ACh. When necessary, a Tukey’s post hoc test was used to identify significant differences. For all other comparisons, one-way ANOVA was used and, if necessary, a Tukey’s post hoc test was employed to identify significant differences. The relationship between variables were assessed with Pearson Product-Moment Correlations. For all analyses, a p-value of < 0.05 was considered significantly different. All data are expressed as mean ± SD.
RESULTS
Subject characteristics:
From the 55 SMFAs that were harvested, 16 were from young subjects (24 ± 4 yrs), 18 were from middle aged subjects (45 ± 4 yrs), and 21 were from old subjects (76 ± 6 yrs). The subject characteristics, obtained from the preoperative examination of medical records, are presented in Table 1. Note that users of cancer-related medications were excluded from the study. Also, it should be noted that all blood chemistry and complete blood count results (Table 1) were within the normal range, suggesting that the subjects who participated in this study were relatively healthy.
Table 1.
Subject characteristics
| Young | Middle aged | Old | |
|---|---|---|---|
| n | 16 | 18 | 21 |
| Age (year) | 24±4 | 45±4* | 76±6 * |
| Sex (male/female) | 10/6 | 11/7 | 13/8 |
| Height (cm) | 184±12 | 178±14 | 178±14 |
| Body mass (kg) | 82±11 | 80±13 | 83±12 |
| BMI (kg m−2) | 19±6 | 22±7 | 24±8 |
| Systolic blood pressure (mmHg) | 124±14 | 123±17 | 128±16 |
| Diastolic blood pressure (mmHg) | 83±8 | 86±10 | 87±11 |
| Medications (users/n) | |||
| Diuretics | 0/16 | 3/18 | 4/21 |
| Angiotensin- converting enzyme inhibitors | 0/16 | 1/18 | 2/21 |
| Diabetic drugs | 0/16 | 0/18 | 3/21 |
| Statins | 0/16 | 2/18 | 2/21 |
BMI, body mass index. Data are expressed as mean ± SD or number of subjects (of the total number; n). Data were analyzed using one-way ANOVA with a Tukey’s Post Hoc test and are presented as mean ± SD
Significantly different from young subjects, P<0.05
The vasodilatory response to flow, ACh, and SNP and the impact of ML090, gp 91, and plumbagin:
The phenylephrine (PE)-induced pre-constriction of the SMFAs prior to the flow stimulus was similar between groups (Young: 59 ± 8 %, Middle aged: 57 ± 8 %, Old: 60 ± 10, P = 0.795). Vasodilation, in response to intraluminal flow was progressively and significantly different between age groups with the old exhibiting the greatest attenuation compared to the young (45 ul/min: Young: 70 ± 8, Middle aged: 51± 9, Old: 27 ± 6%, P < 0.001) (Figure 1A). There was no impact of ML090, GP91, or plumbagin on the flow-induced vasodilatory response in the young (Figure 2A). ML090 did not significantly alter the attenuated vasodilatory response to flow in the middle aged and the old (Middle aged + ML090: 52 ± 11, Old + ML090: 32 ± 8%) (Figure 2B and 2C). In contrast, attenuated vasodilatory response to flow in the middle aged and the old was restored to that of the young by gp91 (Middle aged + gp91: 69 ± 11, Old + gp91: 65 ± 9%, P < 0.001) (Figure 2A, 2B, and 2C). Additionally, plumbagin completedly restored the vasodilatory response to flow in the middle aged to that of the young, but only partially restored the old to that of the young (Middle aged + plumbagin: 63 ± 9, P < 0.05, Old + plumbagin: 45 ± 7%, P = 0.062) (Figure 2A, 2B, and 2C). The effect of gp91 and plumbagin in the middle aged and the old was also evident at the lower intraluminal flow rates of 15 and 30 ul/min (Figure 2B and 2C).
Figure 1. Flow (panel A) and acetylecholine (ACh) (panel B) evoked vasodilator dose response curves of skeletal muscle feed arteries from young, middle aged, and old subjects.
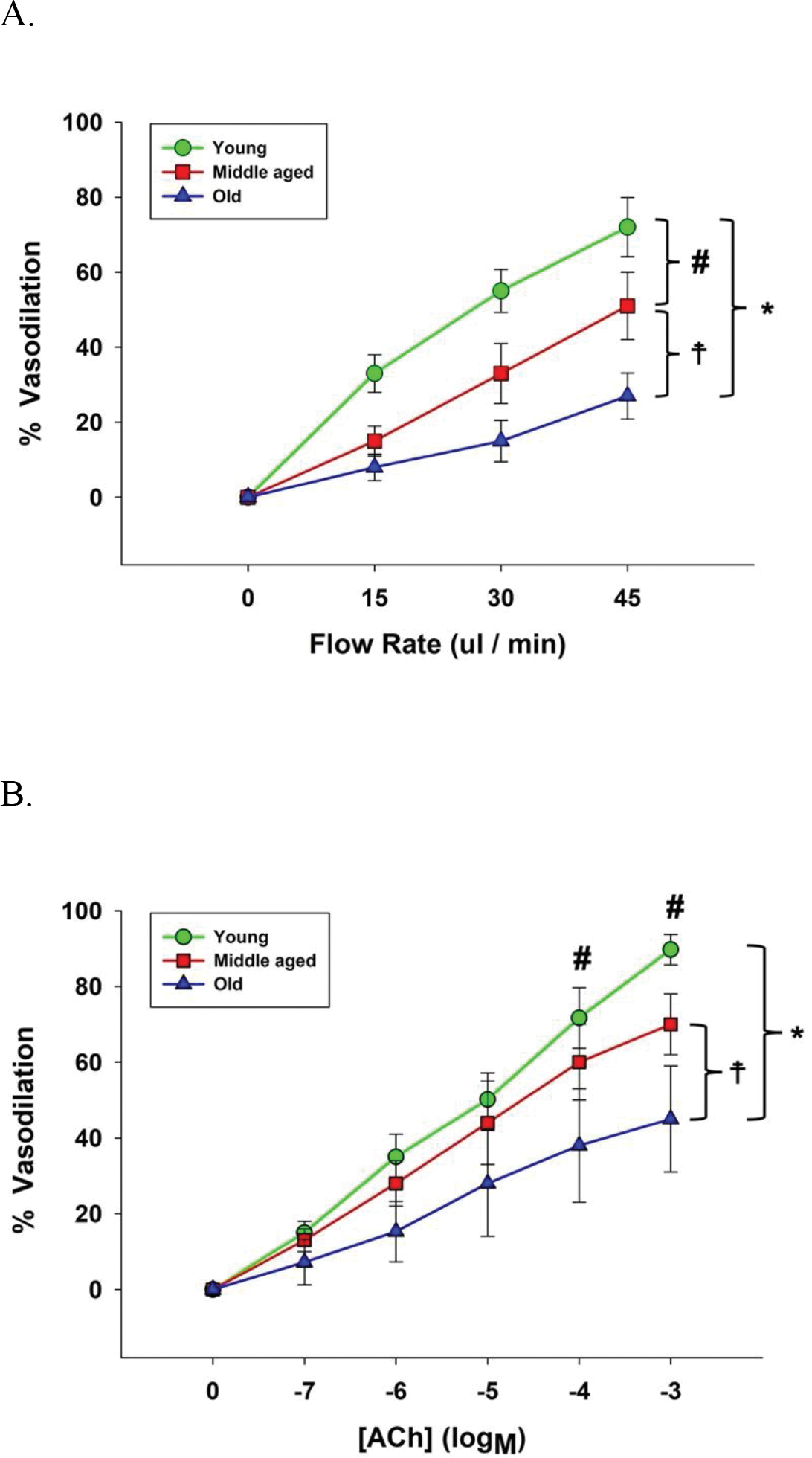
Data were analyzed using two-way repeated-measure ANOVA with a Tukey’s post hoc test and are presented as mean ± SD. n = 16 young, 18 middle aged, and 21 old subjects. * Significant difference between young and old, P<0.05. # Significant difference between young and midle aged, P<0.05. ☨ Significant difference between middle aged and old, P<0.05.
Figure 2. Flow (panels A-C) and acetylecholine (ACh) (panels D-F) evoked vasodilator dose response curves of skeletal muscle feed arteries from young, middle aged, and old subjects with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin).
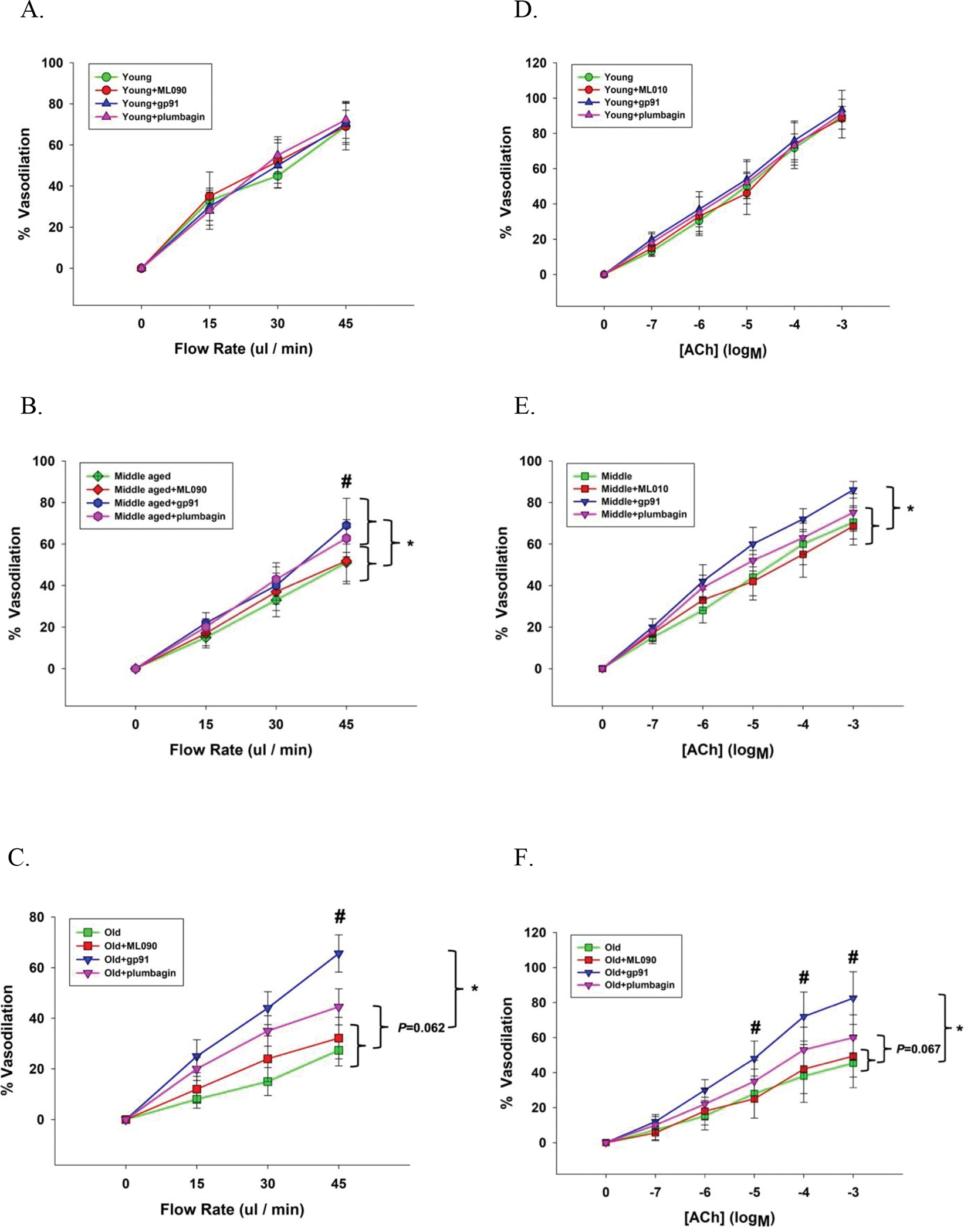
Data were analyzed using two-way repeated-measure ANOVA with a Tukey’s post hoc test and are presented as mean ± SD. n = 16 young, 18 middle aged, and 21 old subjects. * Significant difference between with and without NOX2 inhibition (gp91), P<0.05. # Significant difference between NOX2 inhibition (gp91) and NOX4 inhibition (plumbagin).
The PE-induced pre-constriction of the SMFAs prior to the ACh and SNP dose response curves were similar between groups (Young: 69 ± 9 %; Middle aged: 68 ± 10 %; Old : 69 ± 9 %, P = 0.812). Vasodilation in response to ACh was progressively and significantly different between age groups with the old exhibiting the greatest attenuation compared to the young (10−3 M: Young: 89 ± 4, Middle aged: 70 ± 8, Old: 45 ± 11 %, P < 0.001) (Figure 1B). There was no impact of ML090, GP91, or plumbagin on the ACh-induced vasodilatory response in the young (Figure 2D). ML090 did not significantly alter the attenuated vasodilatory response to the ACh in the middle aged and the old (Middle aged + ML090: 69 ± 9, Old + ML090: 49 ± 12%) (Figure 2D, 2E, and 2F). In contrast, the attenuated response to ACh in the middle aged and the old was restored to that of the young by gp91 (Middle aged + gp91: 86 ± 4, Old + gp91: 83 ± 11%, P < 0.001) (Figure 2D, 2E, and 2F). Additionally, the vasodilatory response to ACh in the middle aged and the old was partially restored to that of the young by plumbagin (Middle aged + plumbagin: 75 ± 8, P = 0.061, Old + plumbagin: 60 ± 13%, P = 0.067) (Figure 2D, 2E, and 2F). The effect of gp91 and plumbagin in the old was clearly evident across the whole ACh dose response curve (Figure 2D, 2E, and 2F).
NO bioavailability and vasodilatory function:
L-NMMA, a NOS inhibitor, ablated the flow- and ACh-induced vasodilation in the presence of the NOX1, NOX2, and NOX3 inhibitors (Figure 3), and, therefore, the NOX2 and NOX4 inhibitor-induced recovery of both flow- and ACh-mediated endothelium-dependent vasodilation in the middle aged and the old (Figure 3B, 2C, 2E, and 2F). With both vasodilatory stimuli, flow and ACh, in the presence of L-NMMA, the vasodilatory response in the young and middle aged SMFAs was reduced to that of the older vessels (Figure 3).
Figure 3. Flow (panels A-C) and acetylcholine (ACh) (panels D-F) evoked vasodilator dose response curves of skeletal muscle feed arteries from young, middle aged, and old subjects both with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin) and with and without nitric oxide synthase blockade (L-NMMA).
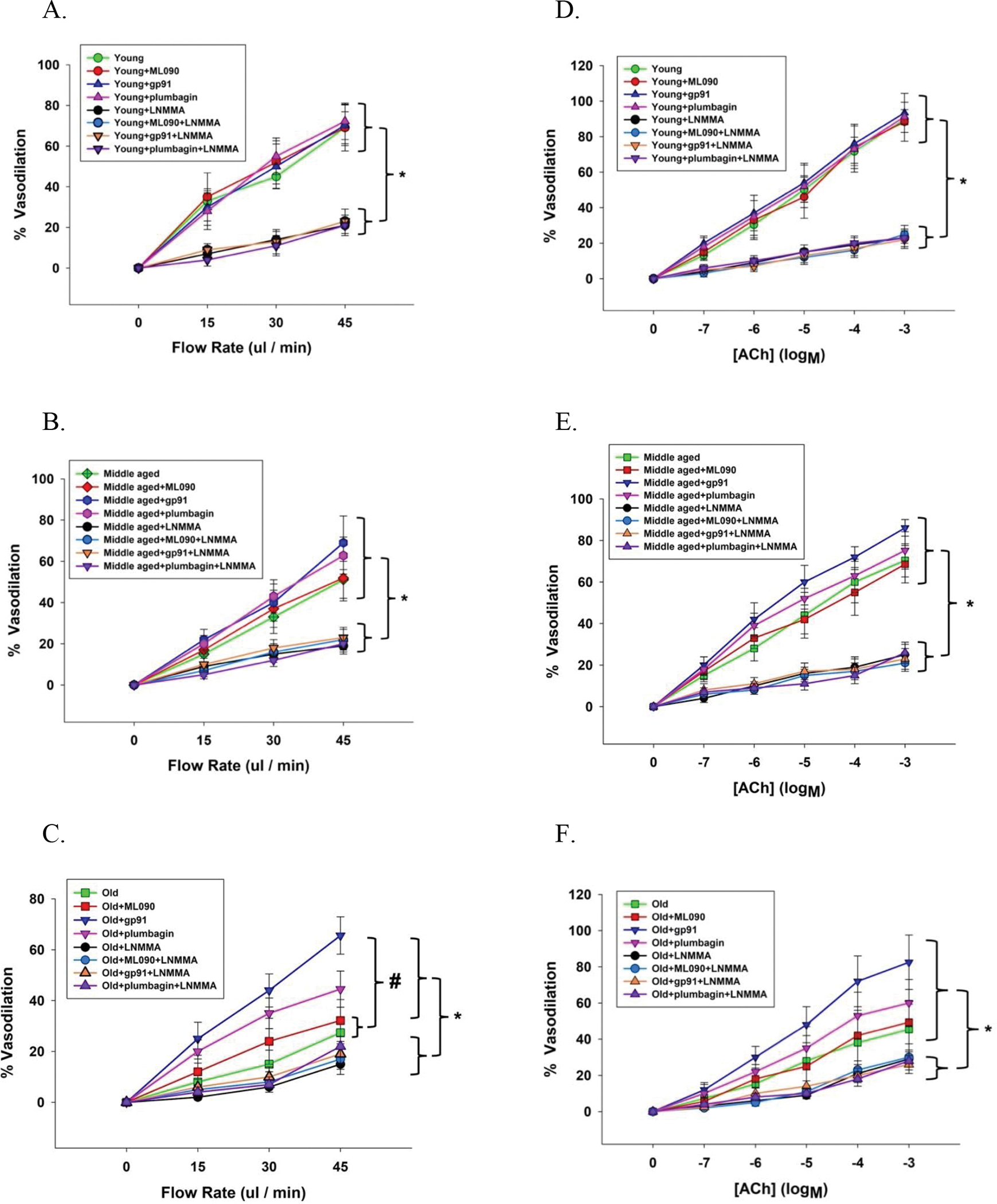
Data were analyzed using two-way repeated-measure ANOVA with a Tukey’s Post Hoc test and are presented as mean ± SD. n = 15 young, 15 middle aged and 16 old subjects. * Significant difference between with and without nitric oxide synthase blockade (L-NMMA), P < 0.05. Panel C: # Significant difference between old and old + ML090 incubation compared to old + gp91 incubation, P < 0.05.
Endothelial-independent vasodilatory function:
Assessed by the vasodilatory response to the highest dose of SNP (10−3 M), endothelial-independent vasodilatory function (Young: 92 ± 8, Young + ML090 : 93 ± 7, Young + gp91: 98 ± 7, Young + plumbagin: 95 ± 8, Middle aged: 92 ± 7, Middle aged + ML090: 94 ± 5, Middle aged + gp91: 96 ± 6, Middle aged + plumbagin: 96 ± 8, Old: 96 ± 7, Old + ML090: 92 ± 4, Old + gp91: 98 ± 8, and Old + plumbagin: 97 ± 7%) (Figure 4) and additionally, across the whole dose response curve, was not significantly different between young, the middle aged, and the old with ML090, gp91, and plumbagin (Figure 4).
Figure 4. Sodium nitroprusside (SNP) evoked vasodilator dose response curves of skeletal muscle feed arteries from young, middle aged, and old subjects with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin).
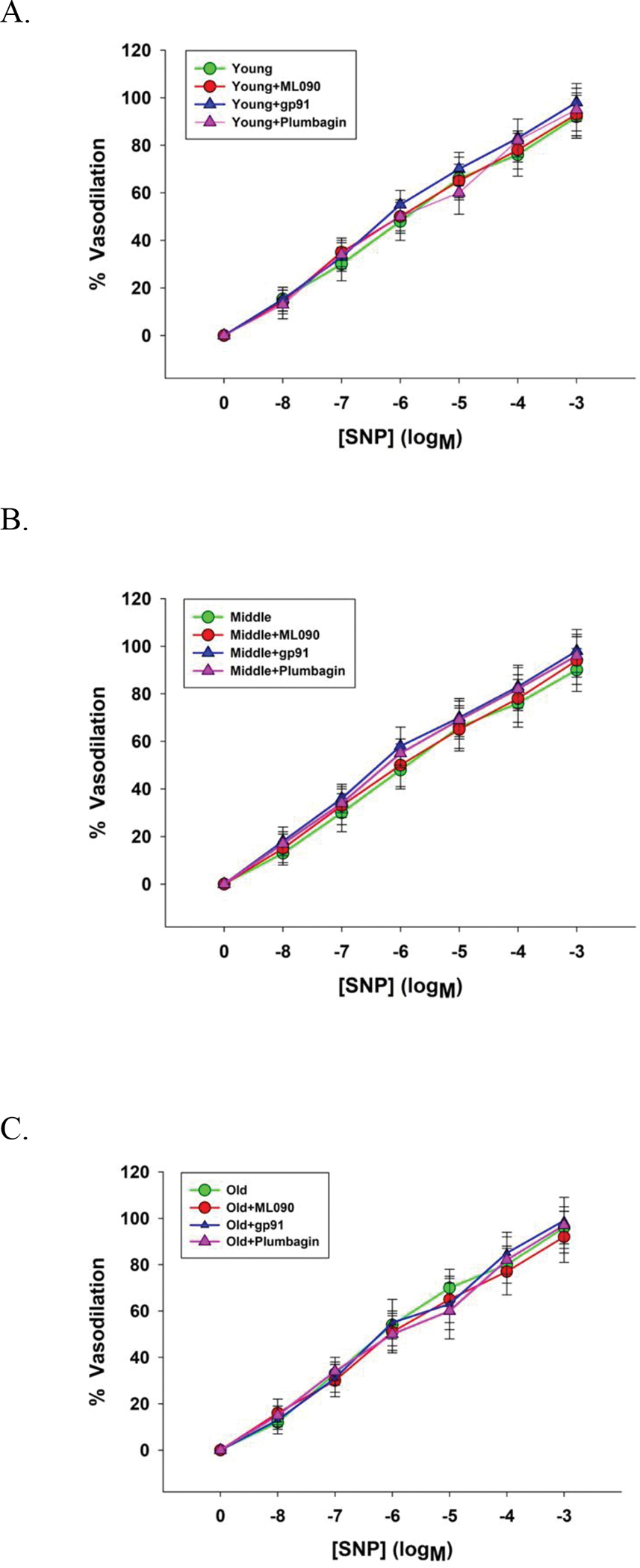
Data were analyzed using two-way repeated-measure ANOVA with a Tukey’s post hoc test and presented as mean ± SD. n = 16 young, 18 middle aged, and 21 old subjects.
NOX, NO bioavailability, and vasodilatory function:
The relative abundance of NOX1 was not significantly different in the young, the middle aged, and the old SMFAs (Figure 5A). However, the relative abundance of NOX2 and NOX4 protein expression in the SMFAs was significantly greater in the middle aged and old compared to the young (Figure 5B and 5C). Furthermore, basal NOX2 and NOX4 protein expression, but not NOX1, were significantly negatively correlated with vasodilation evoked by flow and ACh (Figure 6). However, the change in endothelium-dependent vasodilation resulting from the incubation of the SMFAs in NOX2 (gp91) and NOX4 (plumbagin) inhibitors ablated the statistically significant relationship between flow and ACh-induced vasodilation and NOX2 and NOX4 expression (Figure 6B, 6C, 6E, and 6F). Furthermore, highlighting the role of NO bioavilability on the impact of these NOX isoforms and vascular function, L-NMMA ablated the previously significant relationship between maximal vasodilation evoked by both flow and ACh, and NOX2 and NOX4 protein expression, while simply lowering the degree of vasodilation across the unrelated NOX1 protein expression levels (Figure 7B, 7C, 7E, and 7F).
Figure 5. The relative abundance of NOX1, NOX2, and NOX4 protein in skeletal muscle feed arteries of young (Y), middle aged (MA), and old (O) subjects.
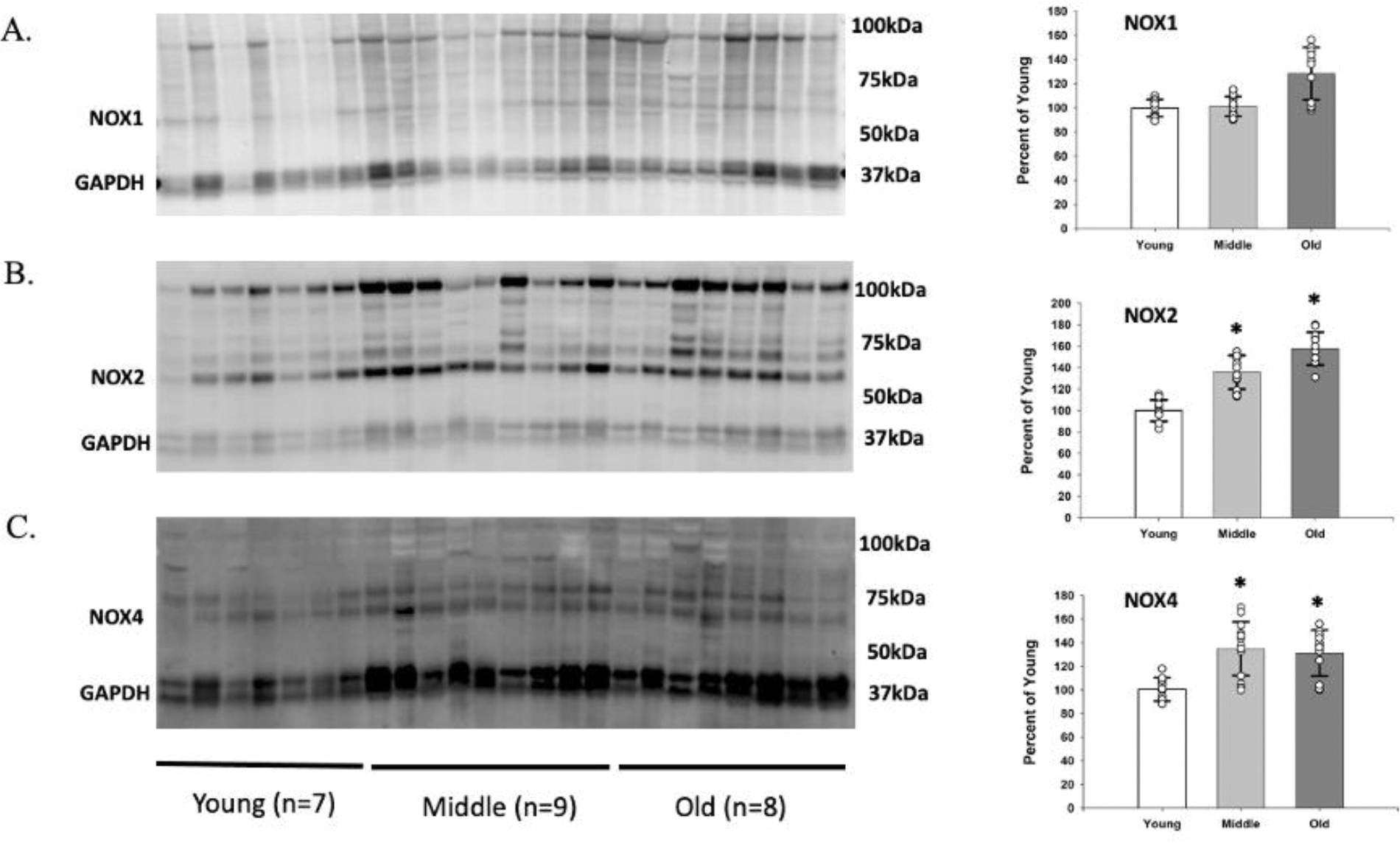
Data were analyzed using one-way ANOVA with a Tukey’s Post Hoc test and are presented as mean ± SD. n = 10 young, 14 middle aged, and 12 old subjects. * Significant difference between middle aged and old compared to the young, P<0.05.
Figure 6. The relationship between NOX1, NOX2, and NOX4 protein expression in skeletal muscle feed arteries of young (Y), middle aged (MA), and old (O) subjects and vasodilation evoked by flow (panels A-C) and acetylcholine (ACh) (panels D-F) with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin).
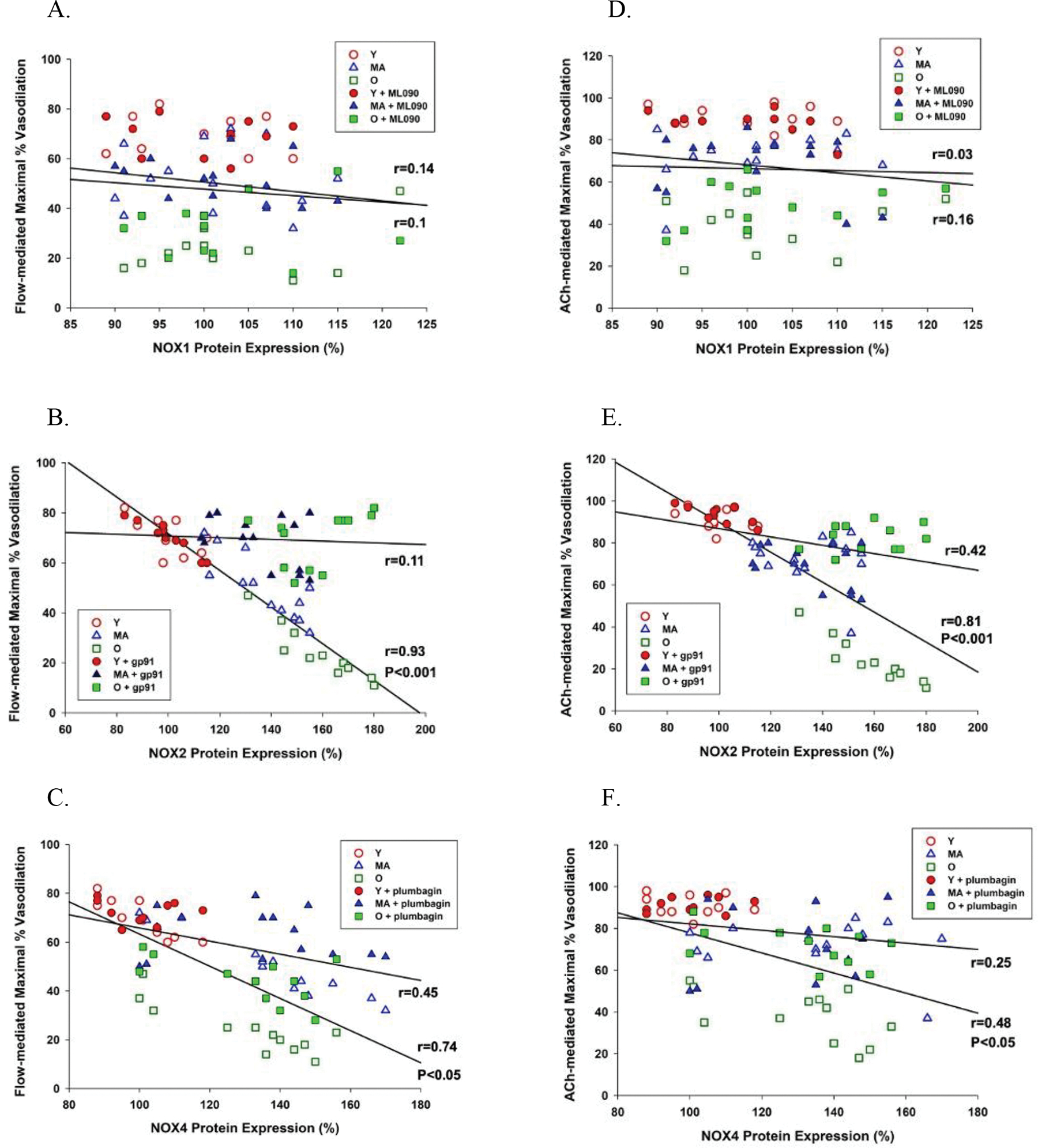
n = 10 young, 14 middle aged, and 12 old subjects. The relationship between variables was assessed with a Pearson Product-Moment Correlation.
Figure 7. The relationship between NOX1, NOX2, and NOX4 protein expression in skeletal muscle feed arteries of young (Y), middle aged (MA), and old (O) subjects and vasodilation evoked by flow (panels A-C) and acetylcholine (ACh) (panels D-F) with and without nitric oxide synthase blockade (L-NMMA).
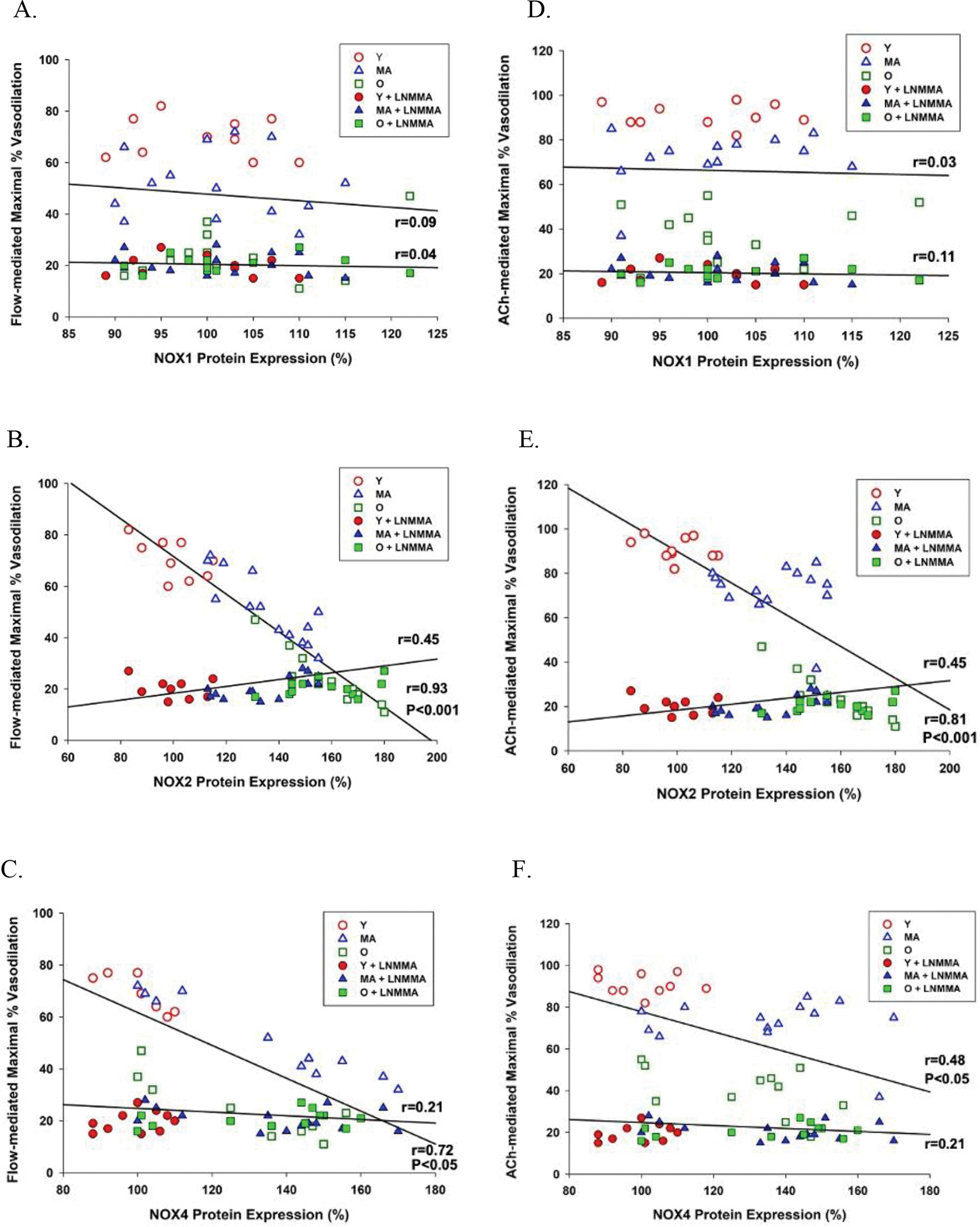
The relationship between variables was assessed with a Pearson Product-Moment Correlation.
Finally, there was a very clear and significant relationship between the age of the SMFA donor and the magnitude of effect of NOX2 (GP91) and NOX4 (plumbagin) inhibition on both flow- (NOX2, r=0.93, P<0.001; NOX4, r=0.76, P<0.05) and ACh-induced (NOX2, r=0.92, P<0.001; NOX4, r=0.82, P<0.001) vasodilation (Figure 8A and 8B). This was not the case for NOX1 (ML090) inhibition, for which the relationship between age and the magnitude of effect of the NOX1 inhibition on both flow- and ACh-induced vasodilation failed to achieve significance (Figure 8A and 8B).
Figure 8. The relationship between age and NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin) inhibition-induced change in flow-mediated vasodilation.
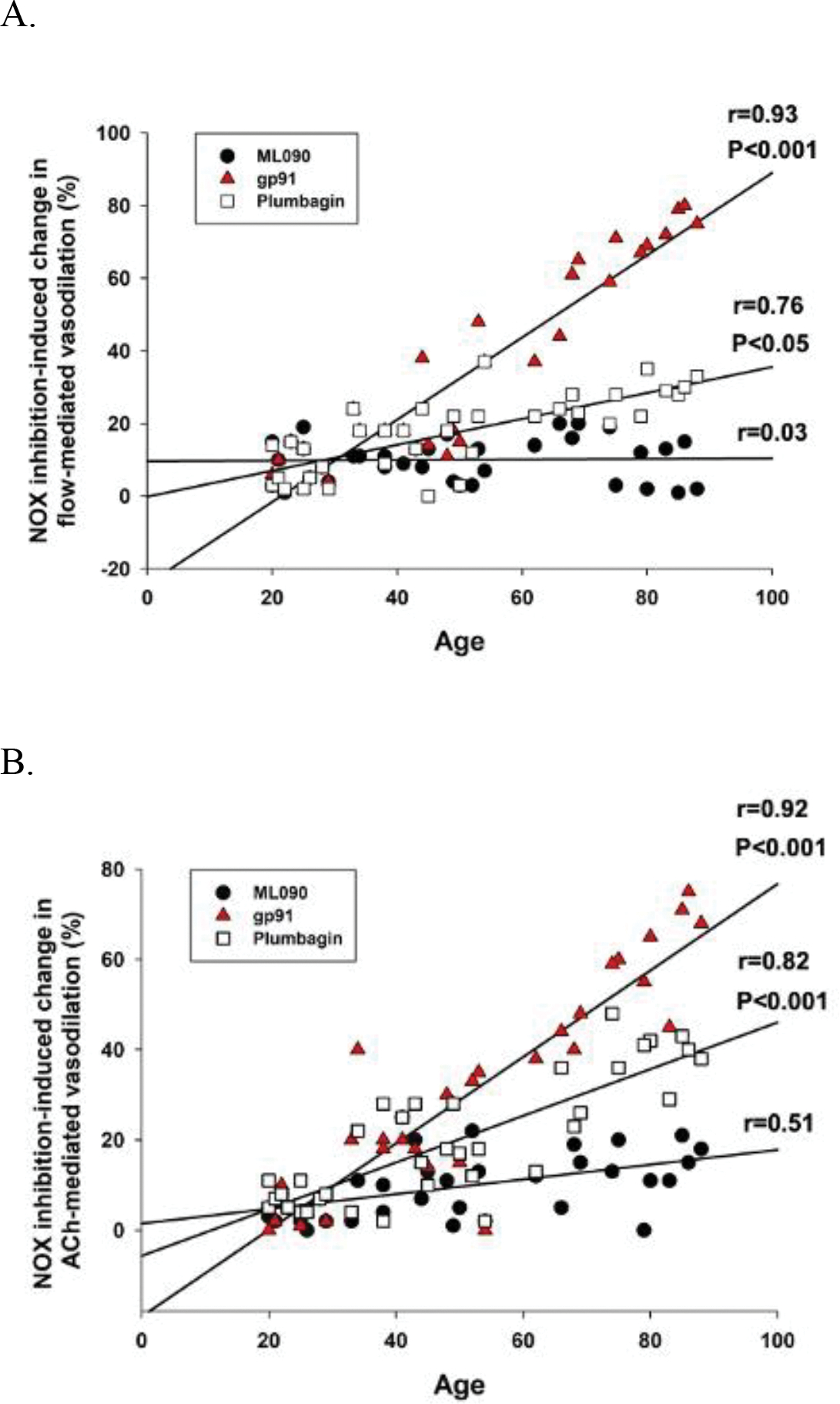
The relationship between variables was assessed with a Pearson Product-Moment Correlation.
DISCUSSION
The isoform-specific role of NOX in age-related endothelium-mediated vascular dysfunction in humans is not well understood and, therefore, this study sought to determine the role of the lead candidates NOX1, NOX2, and NOX4. This was achieved by assessing endothelium-dependent (intraluminal flow- and ACh-induced) vasodilation in human SMFAs of young, middle aged, and old subjects, in vitro, with and without the inhibition of NOX1 (ML090), NOX2 (gp91), and NOX4 (plumbagin). Additionally, NO synthase blockade (L-NMMA) was utilized to identify the impact of NO bioavailability on these responses. SMFA NOX1, NOX2, and NOX4 protein expression was also measured. Following the initial confirmation of, age-related, endothelium-dependent vasodilatory dysfunction in response to both flow and ACh, there are several novel and important findings of this study: In contrast to NOX1 inhibition, the attenuated endothelium-induced vasodilation response in SMFAs from the middle aged and old subjects, compared to young, was restored by NOX2 inhibition and, although the tendency for NOX4 inhibition, to achieve the same outcome, did not reach statistical significance, the magnitude of effect of blocking NOX2 and NOX4 were both significantly and positively related to age. In keeping with these findings, NOX2 and NOX4 protein expression, but not NOX1, was significantly greater in the two older groups and inversely related to endothelium-mediated vascular function. Finally, nitric oxide synthase blockade negated both the restorative effects of NOX2 and NOX4 blockade on endothelium-mediated vascular function and the relationship between NOX2 and NOX4 protein expression and endothelium-mediated vascular function, implying an NO-mediated mechanism. Therefore, in humans, NOX2 and, to a lesser extent, NOX4 appear to play an important, likely NO-mediated, role in age-related endothelial dysfunction and could be critical therapeutic targets to maintain vascular health.
SMFAs, NO, age-related vascular dysfunction, and redox balance
Skeletal muscle feed arteries (SMFAs) are of particular importance as they are primary regulators of O2 delivery and total blood flow to the skeletal muscle at rest and during exercise (Spier et al., 2007; Calbet & Joyner, 2010; Ives et al., 2012). With aging, impaired endothelial function in the skeletal muscle resistance vasculature is primarily due to attenuated NO bioavailability (DeSouza et al., 2000; Muller-Delp et al., 2002; Spier et al., 2007). Previously, our group has demonstrated that endothelial-dependent vasodilatory function in old SMFAs was significantly attenuated compared to young SMFAs, which, indeed, appeared to be closely linked to attenuated NO bioavailability, as evidenced by a decrease in the ratio of p-eNOS to total eNOS protein expression in the old SMFAs (Park et al., 2016; Park et al., 2018). As with these previous studies, the current investigation, again, confirms that both SMFA flow- and ACh-mediated vasodilation, indicative of endothelium-dependent vasodilation, are significantly and progressively attenuated with advancing age (Figure 1).
Although there is considerable evidence that skeletal muscle antioxidant capacity, typically, increases with advancing age in attempt to maintain redox balance (Ji, 1993), our previous studies (Park et al., 2016; Park et al., 2018) provided, mechanistic, evidence that ROS are still likely playing a significant role in age-related vascular dysfunction. Specifically, there were greater superoxide (O2−) levels in SMFAs from the old compared to young subjects, as measured, directly, by EPR spectroscopy (Park et al., 2016; Park et al., 2018). Increased O2− with advancing age attenuates NO bioavailability by rapidly reacting with NO to form peroxynitrite (ONOO−). Of note, O2− does not only directly lower NO levels by such a reaction, but the product of O2− + NO, ONOO−, can, indirectly, attenuate NO bioavailability by uncoupling eNOS (van der Loo et al., 2000; Seals et al., 2011; Bachschmid et al., 2013). Interestingly, in one our prior studies with SMFAs (Park et al., 2018) we documented a strong positive relationship between vascular mitochondrial respiratory capacity, which was lower in the old, and endothelial mediated vasodilation. Furthermore, and with implications for redox balance, a direct intervention with a mitochondrial-targeted antioxidant, MitoQ, significantly improved endothelium-dependent vasodilation in the old, but not the young (Park et al., 2018). Thus, at least one component of the age-related decline in vasodilatory function appears to be linked to attenuated vascular mitochondrial respiratory function, likely by augmented free radicals.
NOX, ROS, and vascular function
Although NADPH oxidase is not the sole source of ROS in the vasculature, others include NADPH oxidase, uncoupled eNOS, xanthine oxidase, and mitochondria, in both humans and animals (Csiszar et al., 2002; Jacobson et al., 2007; Park et al., 2020), NADPH oxidase-derived ROS have been documented to increase with advancing age and are associated with vascular endothelial dysfunction (Donato et al., 2007; López-Sepúlveda et al., 2011). However, at this point, it is also interesting to recognize that ROS do not only yield a negative outcome, as redox signaling is key to many important physiological processes, including, in some cases, vascular function. For example, our group previously documented that an oral antioxidant cocktail, which attenuated free radical levels, resulted in an impairment in vascular function in young healthy subjects (Richardson et al., 2007) while restoring vascular function in the elderly (Donato et al., 2010). Similarly, Sindler et al. (Sindler et al., 2013) reported that the inhibition of NADPH oxidase-derived ROS attenuated endothelial-dependent vasodilation in skeletal muscle arterioles in young and old rats. These differing effects of ROS may due to the milieu surrounding the vessel or the size and location of the vessels (Richardson et al., 2007; Trott et al., 2011; Sindler et al., 2013). Thus, NADPH oxidase-derived ROS could, conceivably, have either a positive or negative effect on vascular endothelial function, dependent upon a variety of factors.
NOX isoforms and human SMFAs
There are seven different NOX enzymes, with only three, NOX1, NOX2, and NOX4, expressed in the vasculature and tightly linked to the pathogenesis of vascular diseases (Sorescu et al., 2002; Hwang et al., 2003; Lassègue & Clempus, 2003; Ago et al., 2004; Guzik et al., 2006; Park et al., 2008; Larsen et al., 2009; Wind et al., 2010). NOX2 expression is elevated in the vasculature with hypertension and atherosclerosis (Guzik et al., 2006; DeMarco et al., 2008; Park et al., 2008) and a NOX2 inhibitor, gp91, significantly attenuated bradykinin-induced ROS production in intact human coronary arterioles (Larsen et al., 2009). NOX1 and NOX4 are also upregulated in a variety of vascular pathologic models (Lassègue & Clempus, 2003; Nishiyama et al., 2004; Dikalova et al., 2005; Jacobson et al., 2007; Park et al., 2008; Yoshida & Tsunawaki, 2008; Ismail et al., 2009; Jaulmes et al., 2009; Meijles & Pagano, 2016). Interestingly, the current data indicate that NOX2 and NOX4 protein expression, but not NOX1, increases progressively with age in human SMFAs (Figure 5). As the NADPH oxidases are well recognized as major contributors to vascular ROS production, there is a clear need to develop a better understanding of the specific roles of these NOX enzymes, as they promise to be a powerful therapeutic target for age-related vascular dysfunction (Laurindo et al., 1994; Csiszar et al., 2002; Trott et al., 2009; Trott et al., 2011).
NOX and age-related vascular dysfunction in human SMFAs
Much of the research focused upon vascular NADPH oxidases was muddied by the common use of the supposed NADPH oxidase inhibitor apocynin (4’-hydroxy-3’ methoxyacetophenone). Indeed, apocynin has recently been documented to, predominantly, act as an antioxidant in endothelial and vascular smooth muscle cells and, therefore, probably should not be used as a NOX inhibitor in vascular systems (Heumüller et al., 2008). Based upon this work, it is not surprising that the mechanism of action and, therefore, the NOX isoform specificity of apocynin, was poorly described in prior studies. With this concern regarding the use of apocynin to study NOX in the vasculature as an important back drop, it is still interesting to consider the highly germane findings of Trott et al. (Trott et al., 2011) who found that, in the soleus feed arteries from old rats, both flow- and ACh-mediated vasodilation were significantly attenuated with aging, but apocynin greatly improved this function. Although using different, likely less controversial, and NOX isoform specific, inhibitors the current study revealed similar findings. Specifically, endothelial-dependent vasodilation, both flow- and ACh-mediated vasodilation, were attenuated in the middle aged and the old SMFAs compared to their young counterparts and incubation in a NOX2 inhibitor, gp91, completely restored endothelium-dependent vasodilation in the middle aged and the old SMFAs (Figure 2A–F). Although less impressive, a similar trend was apparent with incubation in a NOX4 inhibitor, plumbagin, with the vasodilatory response to both flow and ACh partially restored in the middle aged and the old subjects (Figure 2A–F). There was no such effect with the inhibition of NOX1 with ML090 (Figure 2A–F). Furthermore, consistent with prior studies, there was no evidence of an age-related deficit in endothelium-independent vasodilation and so there was no impact of NOX inhibition (2A-C)
NOX2 and NOX4, age-related vascular dysfunction, and NO bioavailability in human SMFAs
As already described, the incubation of SMFAs from the middle aged and old with a NOX2 inhibitor, gp91, and, to a lesser extent, NOX4 inhibitor, plumbagin, effectively reversed the age-related endothelium-dependent vascular dysfunction (Figure 2A–F). However, in addition, the significant strong negative relationship between NOX2 and NOX4 protein expression and endothelium-dependent vasodilation evoked by flow and ACh was ablated by incubation with the NOX2 and NOX4 inhibitors (Figure 6B, 6C, 6E, and 6F). These findings are both important and convincing because they confirm that not only are NOX2 and NOX4 closely associated with endothelium-dependent vasodilation, but, also, that their inhibition greatly attenuates this relationship, more suggestive of cause and effect. Furthermore, this study documents that both the NOX2 and NOX4 inhibition-facilitated restoration of the age-related vasodilatory dysfunction (Figure 2A–F) was mediated by NO bioavailability, as evidenced by the ablation of the positive vascular effect of the NOX2 and NOX4 inhibitors, in the middle aged and the old SMFAs, when combined with NOS blockade, L-NMMA (Figure 3B, 3C, 3E, and 3F). Also, in a similar fashion to the effect of NOX2 and NOX4 inhibition (Figure 6B, 6C, 6E, and 6F), L-NMMA ablated the significant strong negative relationship between NOX2 and NOX4 protein expression and flow- and ACh-mediated vasodilation (Figure 7B, 7C, 7E, and 7F). This finding further demonstrates that NO bioavailability is strongly linked to the NOX2 and NOX4 isoforms and attenuated NO bioavailability plays a critical role in the vascular dysfunction occurring with advancing age. Finally, the significant and strong correlation between age of the SMFA donor and the effect of NOX2 and NOX4 inhibition on endothelium-dependent vasodilation further illustrates that NOX2 and, to a lesser extent, NOX4 appear to play an important role in age-related endothelial dysfunction (Figure 8A and 8B).
Conclusions
The attenuated endothelium-induced vasodilation response in SMFAs from the middle aged and old subjects compared to young was restored by NOX2 inhibition and, although the tendency for NOX4 inhibition to achieve the same outcome did not reach statistical significance, the magnitude of effect of blocking NOX2 and NOX4 were both significantly and positively related to age. Therefore, NOX2 and, to a lesser extent, NOX4 appear to play an important, likely NO-mediated, role in age-related endothelial dysfunction and could be critical therapeutic targets to maintain vascular health.
Supplementary Material
Table 2.
Vessel outer diameter at baseline, with PE, flow (45 ul/min), and with ACh (10−3 M)
| Group and treatment | Baseline Outer diameter | Outer diameter with PE (10−6 to 10−4 M) | Outer diameter with Flow (45 ul/min) | Outer diameter with ACh (10−3 M) |
|---|---|---|---|---|
| Young | 306±105 | 263±92 | 292±101 | 302±104 |
| Young + ML090 | 307±104 | 261±94 | 292±99 | 301±99 |
| Young + gp91 | 304±100 | 264±93 | 292±101 | 309±107 |
| Young + plumbagin | 304±100 | 264±92 | 293±100 | 295±88 |
| Middle aged | 308±115 | 271±18 | 285±110 | 294±99 |
| Middle aged + ML090 | 306±64 | 267±67 | 286±67 | 294±98 |
| Middle aged + gp91 | 296±98 | 261±89 | 289±94 | 307±101 |
| Middle + plumbagin | 308±84 | 268±88 | 287±84 | 298±97 |
| Old | 302±61 | 270±61 | 275±60 | 285±108 |
| Old + ML090 | 302±53 | 268±55 | 278±54 | 285±115 |
| Old + gp91 | 300±77 | 262±79 | 287±80 | 306±113 |
| Old + plumbagin | 298±52 | 264±52 | 279±51 | 300±111 |
Vessel characteristics. Vessel outer diameter (μm) at baseline (intraluminal pressure ≈60 mmHg), incubation with phenylephrine (PE) (10−6 to 10−4 M), with Flow(45 ul/min), and with acetylcholine (ACh) (10−3 M). Data are expressed as mean ± SD.
KEY POINTS.
This study sought to determine the isoform-specific role of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) in the endothelium-mediated vascular dysfunction associated with aging.
Age related endothelium-dependent vasodilatory dysfunction was evident in skeletal muscle feed arteries (SMFAs) in response to both flow and acetylcholine.
NOX2 inhibition (gp91) restored endothelium-dependent vasodilation in the middle aged and the old SMFAs, and NOX4 inhibition (plumbagin) tended to restore these vasodilatory responses in these two groups. Nitric oxide synthase inhibition (L-NMMA) negated the restorative effects of NOX2 and NOX4 blockade.
NOX2 and NOX4 protein expression was significantly greater in the two older groups and inversely related to vascular function.
NOX2 and, to a lesser extent, NOX4 appear to play an important, likely NO-mediated, role in age-related endothelial dysfunction and could be important therapeutic targets to maintain vascular health with aging.
Acknowledgements
The authors wish to thank all the subjects who partook in this study.
Sources of Funding
This work was funded, in part, by the National Heart, Lung, and Blood Institute at the National Institute of Health (PO1 HL1091830 and T32 HL139451) and the Veteran’s Administration Rehabilitation Research and Development Service (E6910-R, E1697-R, E1433-P, E9275-L and E1572-P).
Abbreviations
- Y
Young
- MA
Middle aged
- O
Old
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- eNOS
Endothelial nitric oxide synthase
- SMFAs
Skeletal muscle feed arteries
- ACh
Acetylcholine
- SNP
Sodium nitroprusside
- L-NMMA
N-monomethyl-L-arginine
Biography

Oh Sung Kwon is an Assistant Professor in the Department of Kinesiology at the University of Connecticut. He received his PhD in Exercise Physiology (2013) from the University of Florida under the supervision of Professor Scott K. Powers. Subsequently, Oh Sung completed a Postdoctoral Fellow at the Utah Vascular Research Laboratory in the University of Utah under the guidance of Professor Russell Richardson. His research focus is on the integration of ageing, skeletal muscle and cardiovascular function, especially the role of mitochondria and free radical production in the attenuation of endothelial function with advancing age.
Footnotes
Conflic of Interest
No conflict of interest.
REFERENCES
- Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H & Iida M. (2004). Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109, 227–233. [DOI] [PubMed] [Google Scholar]
- Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, Pimental D & Loo B. (2013). Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45, 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA & Joyner MJ. (2010). Disparity in regional and systemic circulatory capacities: do they affect the regulation of the circulation? Acta Physiol (Oxf) 199, 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF & Dinenno FA. (2010). Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299, H1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A & Kaley G. (2002). Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90, 1159–1166. [DOI] [PubMed] [Google Scholar]
- DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR & Dellsperger KC. (2008). Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 294, H2659–2668. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H & Seals DR. (2000). Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD & Griendling KK. (2005). Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112, 2668–2676. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE & Seals DR. (2007). Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Bailey DM, Wray DW & Richardson RS. (2010). Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298, H671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Assar M, Angulo J & Rodríguez-Mañas L. (2013). Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65, 380–401. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG & Channon KM. (2006). Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26, 333–339. [DOI] [PubMed] [Google Scholar]
- Hearon CM Jr & Dinenno FA. (2016). Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594, 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K & Brandes RP. (2008). Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51, 211–217. [DOI] [PubMed] [Google Scholar]
- Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG & Jo H. (2003). Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278, 47291–47298. [DOI] [PubMed] [Google Scholar]
- Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T & Hoidal J. (2009). NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296, L489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA & Richardson RS. (2012). Human skeletal muscle feed arteries: evidence of regulatory potential. Acta Physiol (Oxf) 206, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G & Sun D. (2007). Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293, H1344–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulmes A, Sansilvestri-Morel P, Rolland-Valognes G, Bernhardt F, Gaertner R, Lockhart BP, Cordi A, Wierzbicki M, Rupin A & Verbeuren TJ. (2009). Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb Res 124, 439–446. [DOI] [PubMed] [Google Scholar]
- Ji LL. (1993). Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc 25, 225–231. [PubMed] [Google Scholar]
- Kuo L, Chilian WM & Davis MJ. (1991). Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol 261, H1706–1715. [DOI] [PubMed] [Google Scholar]
- Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA Jr. & Gutterman DD. (2009). Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 29, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassègue B & Clempus RE. (2003). Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285, R277–297. [DOI] [PubMed] [Google Scholar]
- Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O & da Luz PL. (1994). Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 74, 700–709. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P & Richardson RS. (2003). Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285, H1023–1031. [DOI] [PubMed] [Google Scholar]
- López-Sepúlveda R, Gómez-Guzmán M, Zarzuelo MJ, Romero M, Sánchez M, Quintela AM, Galindo P, O’Valle F, Tamargo J, Pérez-Vizcaíno F, Duarte J & Jiménez R. (2011). Red wine polyphenols prevent endothelial dysfunction induced by endothelin-1 in rat aorta: role of NADPH oxidase. Clin Sci (Lond) 120, 321–333. [DOI] [PubMed] [Google Scholar]
- Meijles DN & Pagano PJ. (2016). Nox and Inflammation in the Vascular Adventitia. Hypertension 67, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW & Delp MD. (2002). Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283, H1662–1672. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S & Abe Y. (2004). The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol 15, 306–315. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y & Mortensen SP. (2012). Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590, 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kwon OS, Park SY, Weavil JC, Hydren JR, Reese V, Andtbacka RHI, Hyngstrom JR & Richardson RS. (2020). Vasodilatory and vascular mitochondrial respiratory function with advancing age: evidence of a free radically mediated link in the human vasculature. Am J Physiol Regul Integr Comp Physiol 318, R701–r711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD & Richardson RS. (2016). Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol 310, H217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP & Richardson RS. (2018). Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 222. [DOI] [PubMed] [Google Scholar]
- Park YM, Lim BH, Touyz RM & Park JB. (2008). Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J Korean Med Sci 23, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL & Joyner MJ. (1998). Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol (1985) 85, 68–75. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S & Bailey DM. (2007). Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292, H1516–1522. [DOI] [PubMed] [Google Scholar]
- Saltin B, Rådegran G, Koskolou MD & Roach RC. (1998). Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162, 421–436. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ. (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ & Seals DR. (2011). Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD & Muller-Delp JM. (2013). Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Kruzliak P, Adamcikova Z & Zulli A. (2015). Role of Nox inhibitors plumbagin, ML090 and gp91ds-tat peptide on homocysteine thiolactone induced blood vessel dysfunction. Clin Exp Pharmacol Physiol 42, 860–864. [DOI] [PubMed] [Google Scholar]
- Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR & Griendling KK. (2002). Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Stallone JN, Dominguez JM 2nd, & Muller-Delp JM. (2007). Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292, H3119–3127. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V & Richardson RS. (2013). Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305, R893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH & Woodman CR. (2009). Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985) 106, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Seawright JW, Luttrell MJ & Woodman CR. (2011). NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol (1985) 110, 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V & Lüscher TF. (2000). Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192, 1731–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K & Schmidt HH. (2010). Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56, 490–497. [DOI] [PubMed] [Google Scholar]
- Yoshida LS & Tsunawaki S. (2008). Expression of NADPH oxidases and enhanced H(2)O(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int Immunopharmacol 8, 1377–1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


