Summary
Pleasurable touch is paramount during social behavior, including sexual encounters. However, the identity and precise role of sensory neurons that transduce sexual touch remains unknown. A population of sensory neurons labeled by developmental expression of the G-protein coupled receptor Mrgprb4 detect mechanical stimulation in mice. Here, we study the social relevance of Mrgprb4-lineage neurons and reveal that these neurons are required for sexual receptivity and sufficient to induce dopamine release in the brain. Even in social isolation, optogenetic stimulation of Mrgprb4-lineage neurons through the back skin is sufficient to induce a conditioned place preference and a striking dorsiflexion resembling the lordotic copulatory posture. In the absence of Mrgprb4-lineage neurons, female mice no longer find male mounts rewarding: sexual receptivity is supplanted by aggression and a coincident decline in dopamine release in the nucleus accumbens. Together, these findings establish that Mrgprb4-lineage neurons initiate a skin-to-brain circuit encoding the rewarding quality of social touch.
Graphical Abstract
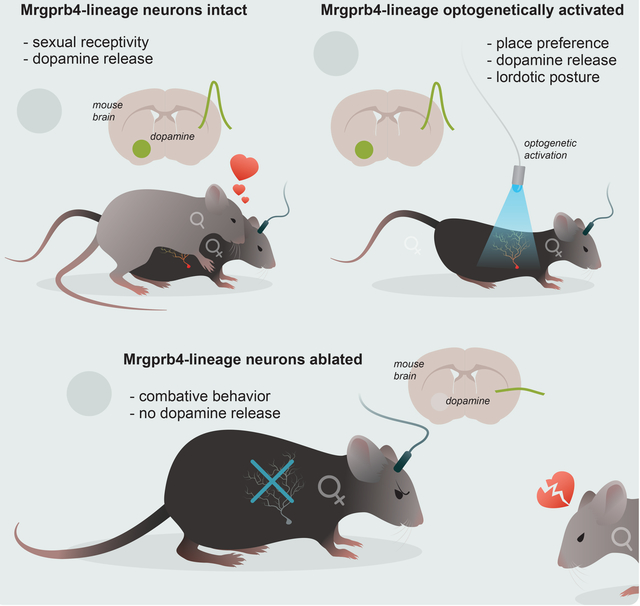
In Brief
Mrgprb4-lineage touch neurons in the skin are required for sexual receptivity and dopamine release that makes this type of social touch rewarding.
Introduction
The pleasure of a partner’s caress or a child’s embrace begins with mechanical signals transduced by neurons in our skin. Despite the centrality of socially rewarding touch in our daily lives, the neurons in the skin that detect social touch and shape the valence of perception generated in the brain, remain unknown. This gap in knowledge is critical, especially when considering the nature of neurodevelopmental disorders like autism spectrum disorder, where gentle touch and socially rewarding behaviors are aversive1, 2, 3.
How might touch generate reward during social interactions? The mesolimbic pathway consists of ventral tegmental area (VTA) neurons that release dopamine into the nucleus accumbens (NAc) to promote reward-learning and reinforcement4–8. VTA dopamine signaling drives social behaviors such as exploring a non-familiar conspecific, same-sex social interactions, or play behavior between female rats9–11. Although a role for VTA dopamine neurons in promoting social behaviors has been identified, except for a handful of studies12, the sensory neurons and ascending pathways that encode social stimuli – such as tactile input – and activate VTA dopamine neurons, remain obscure.
A class of sensory neurons in humans linked to gentle stroking are termed C-tactile afferents13, 14, and there appear to be analogous populations of these neurons in the mouse15–17. One such class of neurons in mice express the G-protein coupled receptor Mrgprb4 and share anatomical and physiological similarities with human C-tactile afferents18, 19. Prior studies demonstrate that hairy skin-innervating C-mechanoreceptors labeled in the Mrgprb4Cre mouse line respond to gentle stroking and produce a conditioned place preference, suggesting that their activation is rewarding18, 19. These findings prompted us to ask whether sensory neurons marked by the Mrgprb4Cre mouse line might be important for promoting ethologically relevant rewarding touch that engages the mesolimbic reward pathway. Here we used a combination of mouse genetics, slice electrophysiology, circuit tracing, behavioral paradigms, and in vivo brain imaging to elucidate the skin-to-brain circuits responsible for mediating the rewarding aspect of social touch.
Results
Focalized activation of Mrgprb4-lineage neurons in the skin is rewarding and induces lordosis-like posture
We reasoned that focal activation of Mrgprb4-lineage neurons in the back skin might mimic touch among mice, so we used the blue light sensitive ion channel channelrhodopsin (ChR2) to focally stimulate Mrgprb4+ neurons in vivo. To accomplish this goal, we used an Mrgprb4Cre driver that functions as a lineage tracer to express a ChR2-eYFP fusion protein in Mrgprb4-lineage neurons (Fig 1a), and crossed these Mrgprb4Cre mice to RosaChR2/ChR2 mice for two generations to generate Mrgprb4Cre; RosaChR2/ChR2 mice. We confirmed this genetic targeting strategy with RNAscope in situ hybridization and immunofluorescence (Fig 1b,c; Fig 2b). Because Mrgprb4 expression begins at ~P4–5 in a population of progenitors broader than the Mrgprb4-expressing population of adult neurons20, we used RNAscope double in situ hybridization to characterize the expression of ChR2-eYFP in Mrgpra3+, Mrgprc11+, and Mrgprd+ populations of dorsal root ganglion (DRG) neurons, which are known to share lineage with Mrgprb4+ neurons (Fig 1b,c). We found the expression of ChR2 across these populations consistent with the expected developmental expression of Mrgprb420, 21 (Fig 1b,c), thus accounting for all ChR2-eYFP+ cells in Mrgprb4Cre;RosaChR2/ChR2 mice. Based on recent single cell RNAseq data in the mouse DRG22, we performed additional sets of RNAscope double in situ hybridization experiments to further characterize the Mrgprb4-lineage neurons by probing for genes across transcriptomically defined clusters including Pou4f2, Trpa1, Ret, TH, Calca, Gfra1, Trpv1, Scn10a, and Calca (Fig 1b,c). Notably, there was minimal overlap between eYFP and TH, a marker of other C-LTMRs22–24, confirming that Mrgprb4-lineage neurons are a distinct population of C-fibers. Moreover, consistent with prior neuroanatomy targeting Mrgprb4+ neurons19, we observed sensory afferent endings in the hairy skin associated with, and in close apposition to, hair follicles (Fig. 1d). Finally, to confirm and extend prior studies18, 19, 25, we probed the mechanical sensitivity of Mrgprb4-lineage neurons using whole-cell patch clamp recordings from acutely dissociated DRG neurons. We thereby confirmed that Mrgprb4-lineage neurons are mechanosensitive, exhibiting mechanically evoked currents that increased with membrane indentation (Fig 1e,f).
Figure 1: Genetic targeting strategy to optogenetically manipulate Mrgprb4-lineage touch neurons.
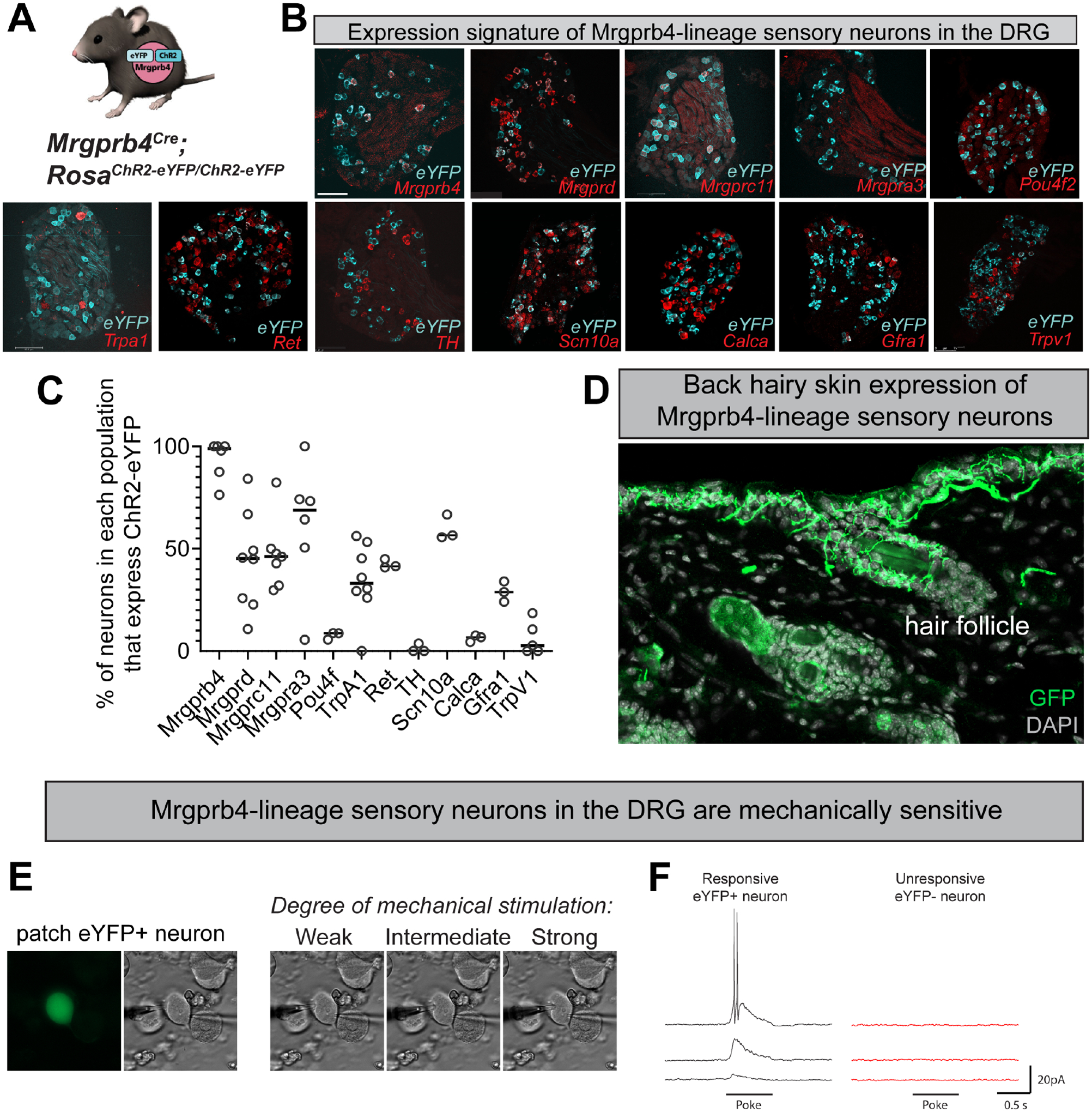
A) Mrgprb4Cre mice express ChR2-eYFP in a Cre-dependent manner. B) RNAscope in situ hybridization in DRG to quantify the expression of ChR2 in Mrgprb4+, Mrgprd+, Mrgprc11+, Mrgpra3+, Pou4f2+, Trpa1+, Ret+, TH+, Scn10a+, Calca+, Gfra1+, and Trpv1+ cells. Scale bars represent 100 μm. C) Percent expression of eYFP in different populations of DRG neurons from B. D) Expression of ChR2-eYFP in Mrgprb4-lineage nerve terminal endings in the hairy skin of the mouse back, labeled via immunofluorescence. E-F) Whole cell patch clamp recordings from individual eYFP+ Mrgprb4-lineage neurons demonstrating mechanical sensitivity. Increase in current response with increased mechanical stimulation.
Figure 2: Mrgprb4-lineage neurons activate downstream spinal circuits including Gpr83+ spinoparabrachial projection neurons.
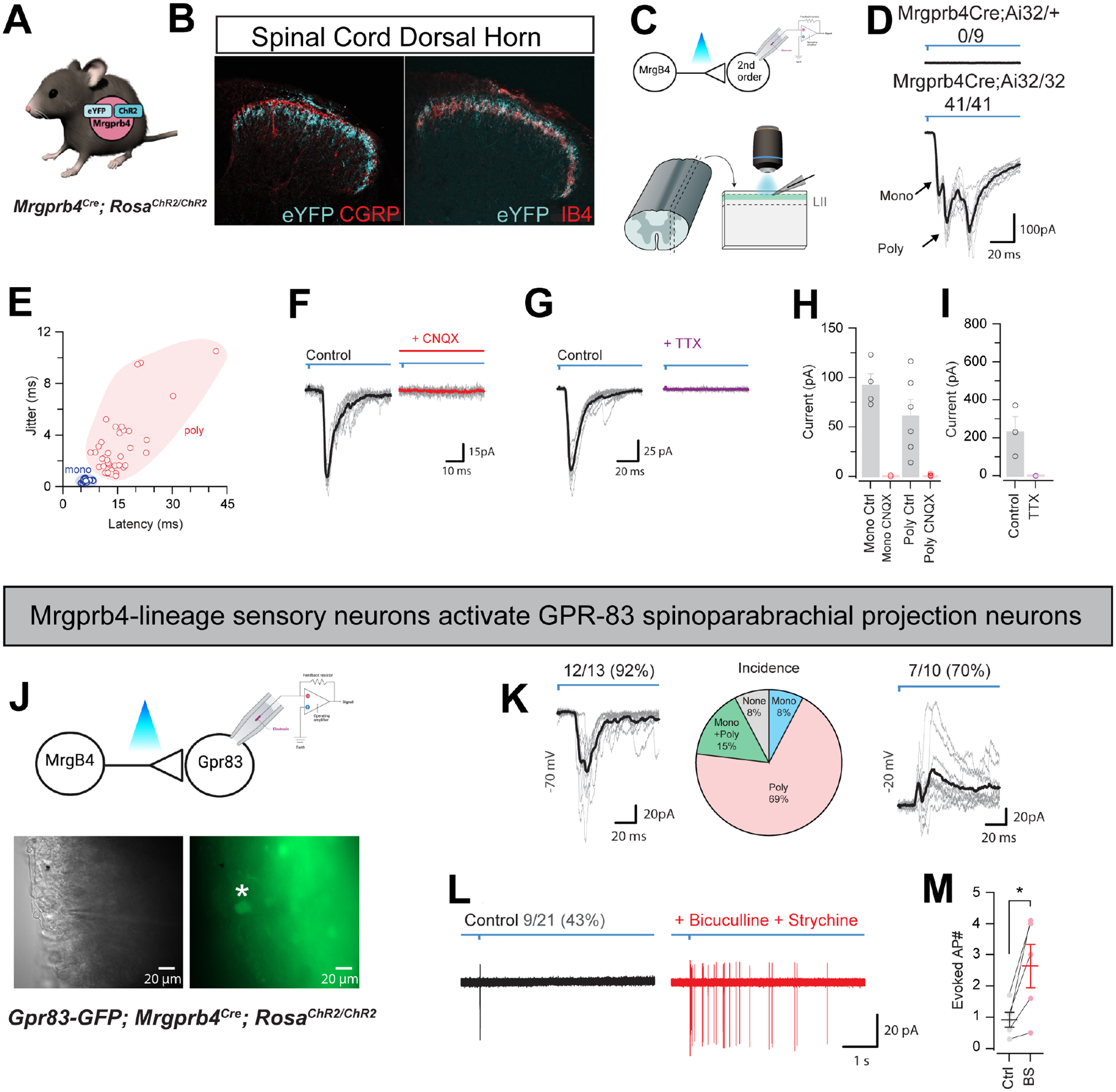
A) Mrgprb4Cre mice express ChR2-eYFP in a Cre-dependent manner. B) Immunofluorescence staining in spinal cord dorsal horn. Mrgprb4+ terminals innervate lamina II, inferior to CGRP+ terminals in lamina I and overlapping with IB4+ terminals in lamina II. Scale bars represent 100 μm. C) Schematic illustrating spinal cord slice electrophysiological recordings from lamina II during optogenetic stimulation of terminals. D) Mono- or poly-synaptic light induced currents only in Mrgprb4Cre; RosaChR2/ChR2 mice. E) Quantification of light induced currents from Mrgprb4Cre; RosaChR2/ChR2 mice with jitter used to determine mono- or poly-synaptic transmission. F) Light-induced currents in Mrgprb4Cre; RosaChR2/ChR2 mice are abolished with CNQX. G) Light-induced currents are abolished in Mrgprb4Cre; RosaChR2/ChR2 mice with TTX. H,I) Quantification of data presented in F,G. J) Schematic, brightfield, and fluorescent images illustrating recording from GFP+ GPR83+ neurons during optogenetic stimulation of Mrgprb4-lineage neurons. K-M) Characterization of light-induced inputs to Gpr83+ putative spinoparabrachial projection neurons. K) Left: Whole-cell voltage clamp recording at −70 mV demonstrating excitatory inputs, Middle: Incidence of a monosynaptic only, polysynaptic only, or mono + polysynaptic excitatory input, Right: Whole-cell voltage clamp recording at −20 mV demonstrating inhibitory inputs. L) Cell-attached recording showing light-evoked action potential discharge under normal conditions (left) and following addition of bicuculline and strychnine (right). M) Quantification of data presented in L.
With confirmation of our genetic targeting strategy, we next asked if we could induce light-evoked, synaptically driven currents to second-order neurons downstream in the dorsal horn of the spinal cord (Fig 2a–i). Recording from LII of the spinal cord of Mrgprb4Cre;RosaChR2/+ heterozygous mice yielded no light-induced currents, while all recordings from Mrgprb4Cre;RosaChR2/ChR2 homozygotes exhibited mono- and/or poly-synaptic inputs (Fig 2d,e). Both mono and polysynaptic inputs could be abolished by application of CNQX, demonstrating glutamatergic synaptic transmission between Mrgprb4-lineage neurons and second-order neurons (Fig 2f–i). Mrgprb4-lineage neuron photostimulation evoked action potential (AP) discharge in ~38% of postsynaptic neurons. AP discharge was particularly prevalent in tonic firing and initial bursting neurons, previously associated with inhibitory interneurons (see Figure S1 for further characterization of spinal neurons downstream of Mrgprb4-lineage neurons).
Recent studies demonstrated that GPR83+ spinoparabrachial (SPB) neurons constitute an affective touch circuit and that these spinal cord projection neurons receive direct input from Mrgprb4-lineage neurons26. To confirm and extend these findings, we crossed the Gpr83-GFP line with Mrgprb4Cre;RosaChR2/ChR2 mice and optogenetically activated the terminals of Mrgprb4-lineage neurons while recording from Gpr83-GFP putative SPB neurons (Fig 2j). We targeted the GFP+ cells within Lamina I, as these were most likely projection neurons, and we avoided recording from the clearly distinguishable Lamina II GFP+ neurons, which were likely interneurons26. When holding the membrane potential at −70mV, we noticed that most GFP+ SPB neurons received polysynaptic EPSCs (Fig 2k). When we held the membrane potential at −20mV to record inhibitory currents, the majority of cells also received polysynaptic inhibitory input (Fig 2k, right). Using cell-attached recordings of lamina I GFP+ SPB neurons, we observed that 1ms photo-stimulation of Mrgprb4-lineage neurons evoked action potentials in 43% of cells (Fig 2l). Blocking inhibitory transmission with GABA and glycine receptor antagonists, bicuculline and strychnine, we observed enhanced responses in the GFP+ SPB neurons to Mrgprb4-lineage neuron photo-stimulation.
We next sought to determine the valence of focal activation of Mrgprb4-lineage neurons in the back skin of adult females. We developed a conditioned place preference assay with laser light administered transdermally through the shaved back skin: one chamber had ChR2-activating blue light, and the other had non-stimulating green light to control for any effect of a visible light beam (Fig 3b). Interestingly, we observed that Mrgprb4Cre; RosaChR2/ChR2 females had a significant preference for the chamber associated with blue light stimulation after training compared to Cre-negative littermates, suggesting that focal activation of Mrgprb4-lineage neurons in the back skin is inherently rewarding (Fig 3c,d). Because approximately 45% of Mrgprd+ neurons express ChR2 in the Mrgprb4-lineage line (Fig.1c), we sought to confirm the specificity of this result to Mrgprb4-lineage neurons by repeating these experiments with MrgprdCre-ERT2; RosaChR2/ChR2 mice. The MrgprdCre-ERT2 mouse line has an inducible Cre, allowing us to treat these mice with tamoxifen after P10, thereby labeling only the adult Mrgprd+ population that does not co-express Mrgprb4. We and others have previously demonstrated that optogenetic activation of Mrgprd+ neurons is neutral at baseline conditions27–29, and we recapitulated those findings here (Fig 3e,f). We did not observe conditioned place preference for activation of Mrgprb4-lineage neurons in the back skin of adult male mice (Figure S2). Future studies are necessary to examine the role of Mrgprb4-lineage neurons in male social behaviors, but for this study we focus on female social behaviors.
Figure 3: Focalized activation of Mrgprb4-lineage neurons in the back skin is rewarding and induces a lordosis-like posture in female mice.
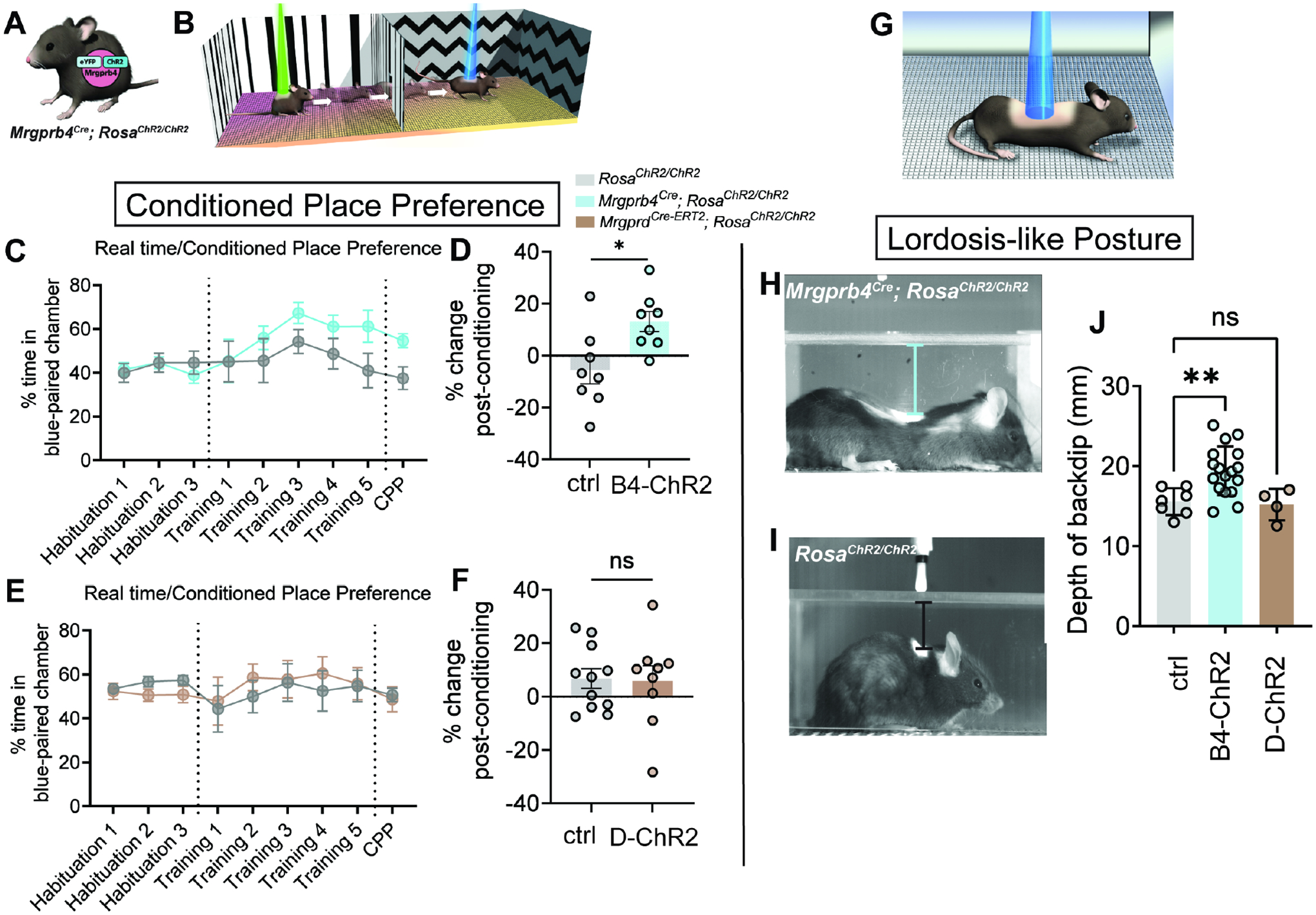
A) Mrgprb4Cre; RosaChR2/ChR2 females and Cre-negative littermates are used in this assay B) Schematic illustrating our real time/conditioned place preference assay. C) Mrgprb4Cre; RosaChR2/ChR2 females gradually spend more time in the blue laser chamber compared to green laser chamber during the training days (lasers on) and D) exhibit a significantly greater change in time spent in the chamber they learned to associate with blue light on the test day (lasers off) compared to Cre-negative littermate controls. (*p=0.0139, unpaired t-test.) E,F) MrgprdCRE-ERT2; RosaChR2/ChR2 females do not develop a preference for the blue laser-paired chamber. H,I) Stills from high speed videography to closely examine the behavior during the preferable transdermal optogenetic activation of Mrgprb4-lineage neurons. Mrgprb4Cre; RosaChR2/ChR2 females (H) exhibit a striking lordosis-like dorsiflexion in response to Mrgprb4-lineage neuron activation, a behavior absent from (I) RosaChR2/ChR2 female littermates and MrgprdCRE-ERT2; RosaChR2/ChR2 females. J) This posture is quantified as the back’s maximum distance from the chamber ceiling over the course of 20s optogenetic stimulation (**p=0.0065, one-way ANOVA). All optogenetic stimuli are 35mW, pulsed at 10Hz sin wave.
We next sought to determine whether a stereotyped behavior could be evoked by optogenetic activation of Mrgprb4-lineage neurons in freely behaving female mice, as an evoked behavior may reveal their role in natural behaviors. We combined optogenetic stimulation with high-speed videography to capture behavior at high spatial and temporal resolution (Fig 3g–j). Intriguingly, a lordosis-like posture, involving robust dorsiflexion, appeared as a stereotyped response to selective activation of Mrgprb4-lineage neurons in the back skin (Video S1,S2; Fig 3h–j). The posture is not observed upon optogenetic activation in the same experimental chambers of either Mrgprd+ neurons (Fig 3j) or Mrgpra3+ neurons (Figure S2), two populations of neurons that share lineage with Mrgprb4. Interestingly, we observed a similar back lowering phenotype to blue light in Mrgprb4Cre; RosaChR2/ChR2 male mice (Figure S2), however, because the same stimulus does not evoke a conditioned place preference, the valence associated with this posture in males remains unclear. While expression of Mrgprb4 is not sexually dimorphic (Figure S2), we speculate that sexually divergent central circuits underlie behavioral assignment and valence distinctions when activating Mrgprb4-lineage neurons.
Because the earlier study on Mrgprb4Cre neurons used viral strategies to manipulate or observe the activity of Mrgprb4Cre neurons18, we sought to determine whether we could replicate the opto-induced dorsiflexion with a viral strategy. In our genetic approach we label neurons with a developmental history of expressing Mrgprb4, while a viral approach is expected to be more specific to the smaller Mrgprb4+ adult population. To address this, we followed previous approaches of injecting effector proteins into DRG neurons into pups at P0-P3 with intraperitoneal injections of a Cre-dependent virus18, 30. We made a virus packaged with the AAV-PHP.S serotype for efficient infection of peripheral sensory neurons31, and we used a Cre-dependent red-orange shifted excitatory opsin, ChRmine32. The efficiency of targeting the Mrgprb4 population that is only ~2% of all DRG neurons19 is not trivial, and our viral strategy labeled 36% of Mrgprb4+ neurons (Figure S3). Targeting DRG neurons virally is far more challenging than neurons in the brain, since the dozens of sensory ganglia in a mouse cannot all be directly targeted, and thus systemic, and less efficient intraperitoneal injections are typically used. When Mrgprb4Cre mice were injected with the Cre-dependent ChRmine and orange laser light was pulsed to their shaven back skin, we did not see the lordosis-like posture we observed using Mrgprb4Cre; RosaChR2/ChR2 mice (Fig 3h). We did however, observe touch-like responses such as turning and orienting towards the light beam on their back (Figure S3). We see two explanations for the lack of dorsiflexion with the viral targeting strategy: 1) Viral targeting yields low transfection efficiency in an already small population of DRG neurons, and 2) there is a distinct behavioral role for neurons with a developmental history of Mrgprb4 expression versus those that retain expression of Mrgprb4 throughout life, in particular for the lordosis-like behavior. Motivated to understand the lordosis-like posture (Fig 3h), and drawn by the more consistent and robust expression of Cre-dependent effector proteins, we proceeded with the genetic approach.
Mrgprb4-lineage neurons are required for female sexual receptivity
Next, we interrogated the role of Mrgprb4 neurons in a natural behavior in which female rodents display prominent dorsiflexion: lordosis, the female sexually receptive posture which includes dorsiflexion in response to male tactile input to the back and flanks33–36. To test this idea, we ablated the population using Cre-dependent expression of diphtheria toxin subunit A (DTA) (Figure 4a; Figure S4). We used RNAscope in situ hybridization to confirm the selective elimination of Mrgprb4-lineage neurons. (Figure S5b). Mrgprb4Cre; RosaDTA mice did not exhibit any motor abnormalities that might confound the interpretation of our results, and pain and somatosensory detection in the hairy skin appear largely normal (Figure S4).29, 37, 38. (Figure S4). To determine if the Mrgprb4-lineage neurons are required in females to detect male mounts and respond with sexual receptivity, we conducted a lordosis quotient assay34, 39. To control for fluctuations in the estrous cycle, we ovariectomized females (OVX) and subsequently treated them with both estradiol and progesterone to mimic a state of behavioral estrus at the time of the assay.40 (Fig 4b). Healthy females have been shown to increase their sexual receptivity with sexual experience, and therefore LQ assays are sometimes conducted over multiple trials, which we did here41. On the first trial, Mrgprb4Cre; RosaDTA and control females both exhibited moderate levels of sexual receptivity (Fig 4d,e,h,i). While control females increased in receptivity on subsequent trials, strikingly, Mrgprb4Cre; RosaDTA females’ receptivity plummeted on the second pairing, and remained minimal for the third pairing (Fig 4d,e,h,i).
Figure 4: Mrgprb4-lineage neurons are required for female sexual receptivity.
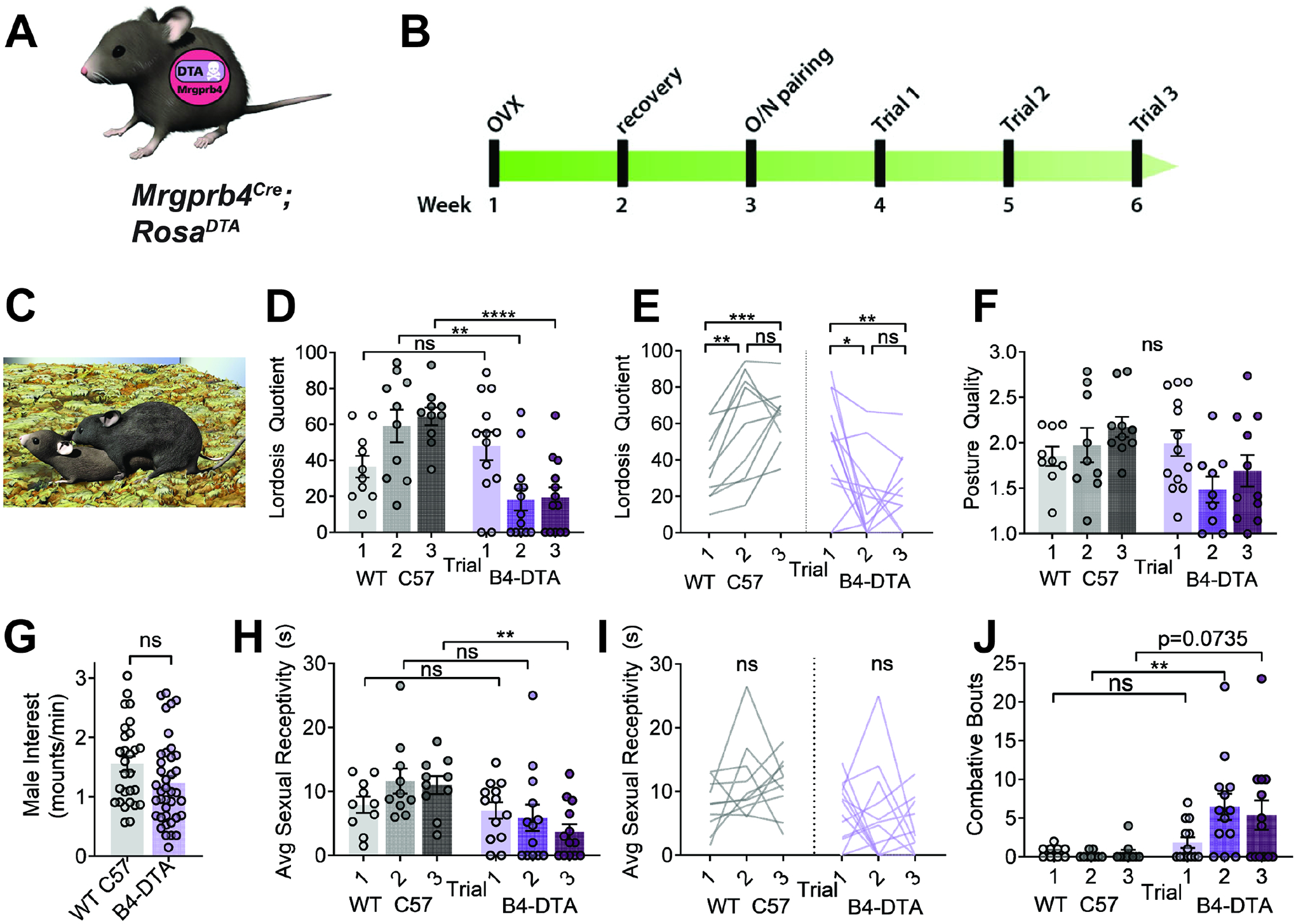
A) Graphic representing Mrgprb4Cre; RosaDTA mouseline, expressing DTA in a Cre-dependent manner to ablate Mrgprb4-lineage neurons. B) Timeline for assessing sexual receptivity with lordosis quotient (LQ) assay: all females are given two weeks to recover after ovariectomy (OVX), at which point estradiol and progesterone are administered to put them in behavioral estrus for an overnight pairing with a male. Hormones replaced prior to each LQ trial. C) Assays conducted in the male home cage in the dark cycle. Graphic depicts a female in sexually receptive lordosis posture in response to male mount D,E) Lordosis quotient scores for 3 sequential trials for WT C57 and Mrgprb4Cre; RosaDTA females. (Two-Way Repeated Measures ANOVA) D) On trials 2 and 3, Mrgprb4Cre; RosaDTA females exhibited significantly reduced sexual receptivity compared to WT females at the same trials (Trial 2 **p=0.0052; Trial 3 ****p<0.0001, Sidak’s multiple comparison test) E) Changes in individual mice across the three trials. WT females exhibit significant increase in sexual receptivity from trial 1 to trial 2 (**p=0.0055) and from trial 1 to trial 3 (***p=0.0009), whereas Mrgprb4Cre; RosaDTA females exhibit significant decrease in sexual receptivity from trial 1 to trial 2 (*p=0.0237) and from trial 1 to trial 3 (**p=0.0059), Tukey’s multiple comparisons test). F) Posture quality assessed on a scale from 1–3, where 1 is the minimum receptive posture and 3 is the most robust posture (details in methods) (ns, Two-Way Repeated Measures Mixed-effects analysis). G) Male mounting frequency was no different between Mrgprb4Cre; RosaDTA females and controls (ns, independent samples t-test). H) Average sexual receptivity, or duration maintained a receptive posture. (**p=0.0021, Two-Way Repeated Measures ANOVA, Sidak’s multiple comparisons test). I) Changes in sexual receptivity in individual mice across trials (ns, Tukey’s multiple comparisons test). J) Total number of combative bouts observed for each female in each trial. (**p=0.0096, Two-Way Repeated Measures Mixed-effects analysis, Sidak’s multiple comparisons test)
While the neurobiology of why females increase receptivity upon repeated exposure to males is unknown41, it may indicate a rewarding component of sexual encounters that positively reinforces the behavior for females, driving them to engage more receptively on subsequent trials. Our observed decline in receptivity revealed that, without Mrgprb4-lineage neurons, sexual touch becomes negatively reinforcing. Importantly, as evidenced by the number of Mrgprb4Cre; RosaDTA females exhibiting quality lordosis postures on their first trial, the Mrgprb4Cre; RosaDTA females are capable, but choosing not to display lordosis on subsequent trials (Fig 4f). Concomitant with the sharp decline in sexual receptivity in Mrgprb4Cre; RosaDTA females, these females now displayed combative behavior towards the males (Fig 4j), who mounted with the same frequency regardless of female genotype (Fig 4g). This phenotype is striking, as female mice primed for behavioral estrus are not naturally aggressive40, 42. The decline in receptivity despite demonstrated ability to dorsiflex in response to touch, combined with combative behavior towards males, together indicate a role for the Mrgprb4-lineage neurons in the more affective components of touch.
Optogenetic-induced dorsiflexion posture is not modulated by ovarian hormones
Decades of work have established that rodent lordosis is tightly modulated by levels of estrogen and progesterone35, 43, 44. Thus, we tested whether the optogenetically induced posture was modulated by ovarian hormones. We found that the dorsiflexion posture during 20s of optogenetic stimulation did not significantly vary across natural (measured by vaginal lavage) or experimentally manipulated (OVX + hormone replacement) estrous states (Figure S5). Thus, the dorsiflexion induced by optogenetic activation of Mrgprb4-lineage neurons does not indicate sexual receptivity.
Optogenetic activation of Mrgprb4-lineage neurons is sufficient to induce dopamine release in the mesolimbic reward pathway
Given the above results, we hypothesized that Mrgprb4-lineage neurons transduce rewarding sensation during sexual encounters. We examined both back and anogenital skin for Mrgprb4+ nerve endings, areas where male tactile input is expected to occur during sexual behavior. Consistent with prior neuroanatomical studies19, Mrgprb4+ terminals innervated all examined hairy skin, including the back (Fig 5c), underbelly (Figure S5), and skin surrounding the genitalia in both sexes (Fig 5g, Figure S2), but were absent from the internal lining of the vaginal wall (Figure S5). We sought to determine whether and how these afferents could signal to central reward centers, such as ventral tegmental area (VTA) release of dopamine into nucleus accumbens (NAc), which has been associated with sexual reward45–47 48, 49. Recent work has demonstrated that Mrgprb4+ neurons synapse onto GPR83+ projection neurons in the dorsal horn, which project to lateral parabrachial nucleus (lPBN) among other areas26; see our review50. Indeed, we confirm the synaptic connectivity between Mrgprb4-lineage neurons and GPR83+ projection neurons in the spinal cord (Fig 2j–m). We therefore sought to determine whether neurons in the lPBN served as a bridge between GPR83+ projection neurons and the VTA. To address this question, we used viral tracing strategies to examine whether there were lPBN neurons that both received GPR83 input and projected to the VTA. To target GPR83+ projection neurons, we utilized combinatorial CRE-DOG viruses, whereby viral expression of a split Cre combines into a functional Cre in the presence of GFP51. We injected pAAV.CAG.LSL.tdTomato and combinatorial CRE-DOG viruses into the lower thoracic spinal cord of GPR83-eGFP animals to label GPR83+ projection neurons with tdTomato (Figure S6a). By injecting a BFP retrograde virus into VTA of the same animals (Figure S6a,b), we found that BFP+ (VTA-projecting) cell bodies in lPBN had visible overlap with spinoparabrachial GPR83+ (red) terminals (Figure S6c, c’). These results offer a putative neuroanatomical pathway emerging from the skin and spinal cord to the mesolimbic reward center.
Figure 5: Activation of Mrgprb4-lineage neurons triggers dopamine release in the nucleus accumbens.
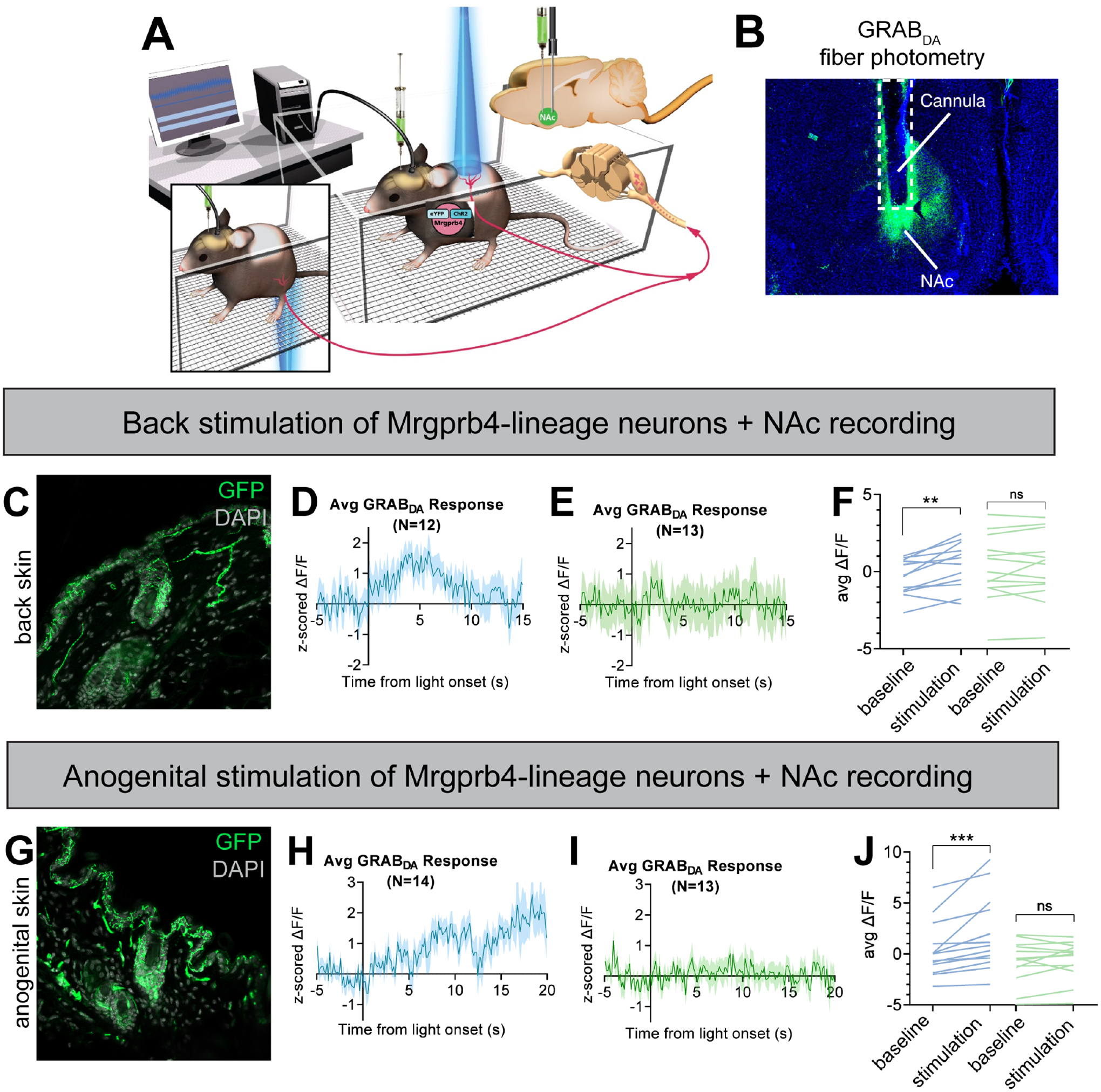
A) Schematic depicting the experimental setup. Female Mrgprb4Cre; RosaChR2/ChR2 mice with shaved backs, which had been injected with GRABDA to the NAc two weeks prior to testing, were placed under plastic chambers on a mesh platform. Pulsed blue (stimulating) or green (control) laser light (35mW, 10Hz sin wave) was shined to either the back skin (C-F) or skin surrounding the vagina (G-J) while recording GRABDA signals. B) Cannula placement and GRABDA expression in NAc C) ChR2-eYFP expression in Mrgprb4-lineage terminals in the back skin. D) Average GRABDA signal as z-score upon blue light (D) or green light (E) stimulation to the back. F) Average deltaF/F signals during pre-stimulation baseline (−5–0s) and during stimulation (0–6s) for each animal. Repeated measures two-way ANOVA revealed significant interaction between wavelength and time (p=0.0161) and Sidak’s multiple comparison test revealed significant difference in blue light (**p=0.0014) but not green light (p=0.9411) pre-stim compared to during stimulation. G-J) Average GRABDA signal as z-score upon blue light (H) or green light (I) stimulation to the anogenital region. J) Average deltaF/F signals during pre-stimulation baseline (−5–0s) and during stimulation (13–20s) for each animal. Repeated measures two-way ANOVA revealed significant interaction between wavelength and time (p=0.0110) and Sidak’s multiple comparison test revealed significant difference in blue light (***p=0.0003) but not green light (p=0.8547) pre-stim compared to during stimulation.
To determine whether this anatomical circuit was functionally connected, we coupled fiber photometry with transdermal optogenetic stimulation to test whether activation of Mrgprb4-lineage neurons is sufficient to induce dopamine release in NAc (Fig 5a). We measured dopamine release using the GRABDA dopamine sensor, which we injected into the NAc of Mrgprb4Cre; RosaChR2/ChR2 females (Fig 5a,b). The GRABDA sensor is a GPCR-based approach where dopamine release and binding to its cognate receptor generates fluorescence with subcellular resolution and sub-second kinetics52. Mrgprb4Cre; RosaChR2/ChR2 females expressing the GRABDA dopamine sensor in NAc were given either 30s transdermal optogenetic stimulation to the back or anogenital region or the same treatment with non-stimulating green light while dopamine signals were recorded. Interestingly, we observed a significant increase in the z-scored deltaF/F from baseline to the first 6 seconds of blue-light stimulation to the back, but not at these same timepoints with non-stimulating green light application (Fig 5d–f). We next turned to the Mrgprb4-lineage neurons innervating the skin surrounding the genitalia. Intriguingly, blue light stimulation to skin surrounding the genitalia, but not green, was sufficient to raise z-scored deltaF/F significantly from baseline levels (Fig 5h–j). With blue light stimulation to the genitalia, sometimes the mouse investigated the area where it had been “touched”, but a lordosis-like posture was never observed (Video S3). This demonstrates that the Mrgprb4-lineage neurons in the back and anogenital skin are sufficient, in otherwise socially-isolated mice, to induce dopamine release in NAc.
Mrgprb4-lineage neurons are required for dopamine release during sexual behavior
Based on the results above, we hypothesized that Mrgprb4-lineage neurons drive dopamine release during sexual behaviors. To test this hypothesis, we injected Mrgprb4Cre; RosaDTA females and littermate controls with the GRABDA dopamine sensor to the NAc to examine any differences in dopamine release during sexual behavior in the absence of Mrgprb4-lineage neurons (Fig 6a). We measured dopamine activity in the seconds surrounding mounts on their first trial, and found that while Cre-negative littermate controls exhibited a significant increase in dopamine release immediately following mount initiation, this dopamine release was missing in Mrgprb4Cre; RosaDTA females (Fig 6b–d). Thus, the Mrgprb4-lineage neurons are indeed required for the sexual reward that occurs at mount onset.
Figure 6: Mrgprb4-lineage neurons are required for dopamine release in the nucleus accumbens during sexual mounts.
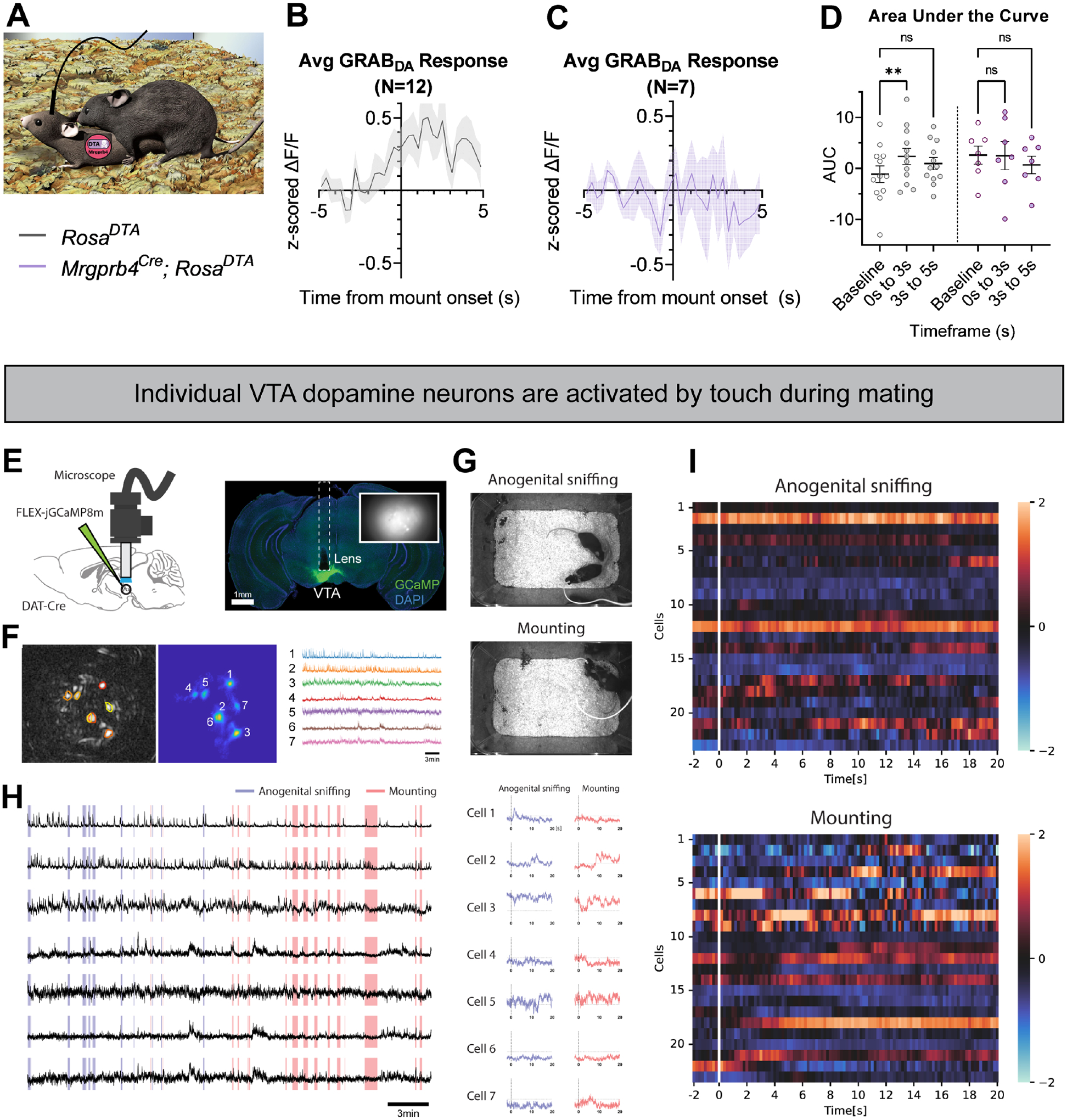
A) Graphic depicting experimental setup. Female Mrgprb4Cre; RosaDTA mice or littermate RosaDTA controls, which had been injected with GRABDA to the NAc over two weeks prior to testing were paired with males for a mating assay. Females were ovariectomized and hormone primed to be in a state of behavioral estrus for testing. GRABDA signals were recorded for the entire pairing and analyzed surrounding male mounts. B) Average z-score traces surrounding mount onset (T=0) for littermate RosaDTA controls (gray) (N=12) and C) Mrgprb4Cre; RosaDTA females (purple) (N=7) D) Area under the curve for Z-scored signals at pre-mount baseline (−5 to −2s), 0–3s post-mount, and 3–5s post-mount for each animal. Repeated measures two-way ANOVA revealed a significant interaction between genotype and time (p=0.037), and Sidak’s multiple comparison test revealed significant difference in GRABDA signal between baseline and 0–3s post mount for control (**p=0.0024) but not Mrgprb4Cre; RosaDTA females (p=0.9945). E) Schematic of a miniaturized one-photon fluorescence microscope and calcium imaging. A DAT-Cre animal was unilaterally injected with jGCaMP8m virus into the left hemisphere of the ventral tegmental area (VTA; AP:−3.20 mm, ML: −0.40 mm, DV: −4.40 mm). DAPI-stained (blue) coronal section of DAT-Cre mouse showing GCaMP8m fluorescence (green) and the location of a 0.6mm-diameter gradient index (GRIN) lens implant (dashed lines). The inset image indicates the imaging field from the lens. F) A cell contour map (left) and CNMFe-processed image of the neurons located in the VTA (middle) with corresponding raw calcium traces (right). G) Sample video frames of anogenital sniffing and mounting. The animal poses were manually annotated on a frame-by-frame by two human annotators. H) Raw calcium traces from 7 dopamine cells in VTA (black lines) with two manual behavior annotations: anogenital sniffing (blue; 14 events) and mounting (red; 16 events). Median z-scored calcium signals of each dopamine cell at the time of anogenital sniffing (blue; left) or mounting (red; right). I) Representative calcium activity of VTA dopamine neurons during anogenital sniffing or mounting (n=23 cells, 3 mice).
Lastly, we investigated whether individual VTA dopamine neurons were activated by different types of sexual touch in females, specifically anogenital sniffing and mounting (Fig 6g). We introduced microendscopic calcium imaging of VTA dopamine neurons in freely behaving female mice and recorded the activity during mating behaviors (Fig 6e,f). Indeed we found that the individual dopamine neurons exhibited distinct patterns of activity depending on the type of touch, with some responding to anogenital sniffing, some to mounting, some to both, and some to neither (Fig 6h, i). These data suggest that different populations of dopamine neurons in VTA are related to anogenital sniffing versus mounting, implying heterogenous dopamine responses for rewarding social touch. We speculate that some of these touch inputs arise from direct input of Mrgprb4-lineage touch neurons.
Discussion
Although affective social touch begins at the skin’s surface, the molecular identity of sensory neurons in the skin that detect socially relevant signals, and the ascending pathways that connect them to reward centers in the brain have remained poorly defined. Moreover, because touch itself is highly heterogeneous (i.e., discriminative touch to detect texture with our fingertips versus the affiliative touch during a hug from a friend), sensory perception is likely generated by different sets of neurons to provide specificity16, 22, 24, 53–57. Might there be a specific subset of DRG neurons with a predominant role in rewarding social touch, including sexual receptivity? One class of DRG neurons termed C-low threshold mechanoreceptors (C-LTMRs), or C-tactile afferents in humans, are implicated in detecting gentle strokes across the skin’s surface, including interpretation of these signals as erotic, although in a sex-dependent manner13, 58. Although a limited number of papers in the mouse identify molecular populations of C-LTMRs, including their neuroanatomy and roles in somatosensation during baseline and chronic pain states15, 24, 59–64, the field is beginning to emerge with exciting new findings. For example, two recent studies in mice identify different classes of C-LTMRs and show that animals prefer their activation and modulating these neurons alters social behaviors23, 65.
Here, beginning with mouse sensory neurons that are anatomically and physiologically similar to human C-tactile afferents18, 19, we propose a behavior-first approach. We demonstrate that Mrgprb4-lineage neurons are critical for specific social behaviors and for signaling social reward to the brain. Focal activation of Mrgprb4-lineage neurons yields a striking dorsiflexion posture resembling mammalian lordosis, representing a behavioral response to optogenetic activation of social touch neurons. However, their role in female sexual behavior is not simply in facilitating lordosis; rather, the neurons convey an affective sensation that positively reinforces sexual receptivity, and without them, male advances during sexually receptive hormonal states become aversive. This finding suggests that Mrgprb4-lineage neurons contribute to the perceived valence of sexual encounters in females by encoding the rewarding aspect of male sexual touch. We demonstrate this functional link from Mrgprb4-lineage neurons in the skin to reward circuitry in the brain through fiber photometry with the GRABDA dopamine sensor. Transdermal optogenetic activation of Mrgprb4-lineage neurons in the back skin or skin surrounding the female genitalia is sufficient to induce dopamine release in the NAc. This is the first report of molecularly defined somatosensory neurons triggering activation of a brain reward center. Finally, we link this stimulation of rewarding touch to natural tactile reward by demonstrating that Mrgprb4-lineage neurons are required for this same dopamine release at the onset of a male mount.
In summary, the work described here has revealed the sufficiency of peripheral inputs to regulate social reward independent of context and other sensory cues. Since ventral tegmental dopaminergic neurons that project to the NAc are themselves functionally heterogeneous66, 67, it is possible that some of these neurons might be tuned for encoding rewarding touch, and our microendoscopy results in the VTA support such a hypothesis. Future studies are warranted to tease apart whether Mrgprb4-lineage neurons engage unique ensembles of VTA dopamine neurons, and in what behavioral contexts. Thus, it will also be interesting to determine if Mrgprb4-lineage neurons are involved in other behaviors that integrate social touch, such as maternal care or even play or bonding between animals. We believe this study draws new attention to the importance of elucidating skin-brain circuits. Moreover, this work points towards the therapeutic potential of peripheral manipulations for enhancing intact or impaired social reward systems, including sexual dysfunction, or simulating social reward during periods of isolation.
Limitations of the study
In this work we perform circuit-based studies using the Mrgprb4Cre mouse line where we activate or ablate the entire population of neurons in behavioral settings. Moreover, we demonstrate in vitro that Mrgprb4-lineage neurons are mechanosensitive. Important questions thus remain regarding the biological basis of mechanical transduction in Mrgprb4-lineage neurons: 1) what is the mechano-sensor in these cells that drives activation of these neurons in natural scenarios? and 2) are there unique neuropeptides beyond the canonical excitatory neurotransmitter glutamate that drive activation of this circuit? In regards to mechano-sensors, the Piezo2 mechanosensory ion channel seems fitting, but recent single-cell RNAseq data demonstrate that almost no Piezo2 mRNA is co-expressed in Mrgprb4 neurons22. Thus, further studies in the field that combine RNA sequencing, physiology, calcium imaging, and other biophysical approaches are needed to determine the molecular nature of mechanosensation in Mrgprb4 neurons, and to what extent, if any, the Mrgprb4 receptor itself may be involved in signal transduction in these neurons.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by lead contact, Dr. Ishmail Abdus-Saboor (ia2458@columbia.edu)
Materials Availability
This study did not generate new unique reagents. All mouselines were obtained from Jackson Laboratories, and viruses from Addgene, except the pAAV-Ef1a-DIO-ChRminemScarlet-3xHA (PHP.S) virus which was custom generated by core facilities at the Zuckerman Mind Brain Behavior Institute. Any inquiries for particular mouse crosses generated from Jackson Laboratories strains can be directed to the Lead Contact.
Data and Code Availability
All data reported in this study will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Subject Details
All testing was performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, Columbia University, and Rutgers University. Mice born into our colony on a C57bl/6J background were maintained in conventional housing with food and water available ad libitum when not being tested, on a 12 hour light cycle beginning at 7:00, and all testing unless otherwise noted occurred during the light cycle in a room directly adjacent to the housing room in the animal facility. Mouse lines used in this study are located at Jackson Laboratories: C57BL/6J (Stock No: 000664), Mrgprb4Cre (Stock No: 021077), MrgprdCre-ERT2 (Stock No: 031286), RosaChR2-eYFP (Stock No: 024109), RosaDTA (Stock No: 009669). The MrgprA3Cre line was generously provided by Dr. Xinzhong Dong at Johns Hopkins University School of Medicine. Breeding was conducted as follows: for optogenetic experiments: Mrg(X)Cre; RosaChR2/ChR2 X RosaChR2/ChR2. For ablation experiments: Mrgprb4Cre X RosaDTA/DTA. Both sexes of mice were tested and all experiments were performed in adult animals at least 6–8 weeks old. Viral injection experiments began with Postnatal day 0 pups.
Method Details
RNAscope in situ hybridization
Brains were harvested immediately following transcardial perfusion and post-fixed in 4% PFA in PBS at 4°C for 24h. Brains were subsequently submerged in 30% sucrose in PBS at 4°C for 18h or until sunk, flash frozen in cryomold on dry ice in OCT, sectioned at 12μm directly onto Superfrost Plus Slides and stored in foil-wrapped slide box at −80°C until beginning Fixed Frozen RNAscope protocol (ACD).
Entire spinal columns were harvested immediately following transcardial perfusion and post-fixed in 4% PFA in PBS at 4°C for 24h. Either spinal cord or DRG were subsequently dissected and submerged in 30% sucrose overnight or until sunk. Tissue was flash frozen in cryomold on dry ice in OCT, sectioned at 12μm directly onto Superfrost Plus Slides, and stored in foil-wrapped slide box at −80°C until beginning Fresh Frozen RNAscope protocol (ACD). Note the fixation step was skipped in ACD’s Fresh Frozen protocol for these tissues.
Immunohistochemistry
Brains, spinal cord, and DRG were collected and prepared in the same way as in situ hybridization. 30μm cryosections were cut directly onto Superfrost Plus Slides. Slides were frozen overnight in a slide box at −80°C. Slides were washed 3× 10min in PBS; 30 minutes in PBST; 1 hour in PBST with 5% normal donkey serum. Primary antibody, 1:1000 in PBST with 5% normal donkey serum, was applied to slides in a humidified chamber overnight at room temperature. Secondary antibody, 1:400 in PBST with 5% normal donkey serum, was applied to slides in a humidified chamber 1–2 hours at room temperature. Slides were washed 3× 10 minutes in PBS before applying mounting media and coverslip.
Transdermal optogenetic activation of Mrgprb4-lineage neurons
8–16 week old female mice were habituated to mesh platform under a plastic chamber for 1 hour on each of two days prior to behavioral testing. Experimenter was present with lights, camera, and laser running during habituation to mimic entire sensory experience of test day. On the day of testing, the mice were habituated to the chambers for an additional 20 minutes before stimulation. 35mW blue laser light pulsed at 10 Hz sin wave was shined through the ceiling of the chamber to the shaved backs of mice for 20 seconds during high-speed video captured at 750fps. For female mice, testing was performed such that each female was tested once daily for five consecutive days. The average of these five trials for each mouse is plotted in Fig 3. Because no variation with the estrous cycle was observed (Supplemental Fig 5), all other testing of transdermal optogenetic stimulation to the back was conducted on a single test day after habituation
Labeling Mrgprb4 neurons with viral injection of excitatory opsin and scoring touch-like behaviors
As an alternative strategy to our genetic approach, we intraperitoneally injected 30μl (8.70E+12 vg/mL) of pAAV-Ef1a-DIO-ChRmine-mScarlet-3xHA (PHP.S) at postnatal days 0–3. Behavioral testing began 6–8 weeks following injection. Animals were habituated to the presence of orange laser light and the experimenter as described above. On the day of testing, orange laser light (598nm) was pulsed through the shaved back skin similarly to what was done for transdermal optogenetic activation of the lineage population. Behavioral videos were scored by a blinded observer for instances where the mouse either looked at the light or reoriented its body as a result of the light stimulation. These were considered touch-like behaviors because the mouse reacted as if it had been touched to the back. Moreover, these behaviors were never observed in littermate control animals.
Tamoxifen injection for MrgprdCre-ERT2; RosaChR2/ChR2 mice
To induce expression of Cre recombinase in MrgprdCre-ERT2; RosaChR2/ChR2 mice, we intraperitoneally injected 0.5mg tamoxifen in 100μl sunflower seed oil in mice aged P10 or older. We repeated this injection daily for three days, so each mouse received three 0.5mg doses. Behavioral testing began at least two weeks after the third injection to allow for Cre mediated recombination and expression of the opsin protein.
Quantification of the back dip
Optogenetic-induced dorsiflexion posture was calculated as the maximum back dip from the ceiling of the behavioral chamber for the duration of the 20s stimulation. High speed videos were analyzed in ImageJ to track the lowest point of the back throughout the 20s stimulation. The lowest y value was subtracted from the y value of the top of the chamber, and the value was converted from pixels to millimeters by determining the pixel height of the 4.5cm chamber for each video. It was decided that this was the most quantifiable way to characterize the dip. While duration or frequency of response require a subjective determination of when a back dip starts and stops – and therefore can be more challenging to compare to control animals – the back dip depth is the most objective measure because it allows us to compare between natural movement of the spine in controls, small back dips, and most drastic back dips. This measure encapsulates the full variability of response.
Conditioned Place Preference
8–14 week old Mrgprb4Cre; RosaChR2/ChR2 females or MrgprdCRE-ERT2; RosaChR2/ChR2 and Cre-negative RosaChR2/ChR2 female littermates underwent a 9 day conditioned place preference paradigm. The apparatus, originally designed at the University of Iowa and described previously68, consisted of two chambers, one paired with almond extract and a textured floor, the other coconut extract and a smooth floor. Additionally, one chamber wall had stripes, while the other had polka dots. These olfactory and visual stimuli were present throughout the 9 day paradigm to aid in the mouse’s encoding of different chambers. Days 1–3 were habituation: each mouse was allowed 20 minutes to explore the two chambered apparatus with no optogenetic stimulation. Days 4–8 were training: each mouse was allowed 20 minutes to freely move about the two-chambered apparatus, now receiving laser light stimulation to the back. In one chamber the mouse received 10Hz pulsed sin wave 35mW blue laser light to the shaved back, and the other chamber non-stimulating green light of the same parameters. Experimenter held the laser lights ~1cm from the back skin for the duration that the mouse was in the chamber. The lasers were held with an extendable alligator clip to avoid casting body shadow on the chambers. Because the back was the only shaved body region, and represents the largest body area, we believe our results are driven by the activation of Mrgprb4-lineage neurons in the back skin, as opposed to rare moments the laser passed over the ear, foot, or tail. Day 9 was test day: each mouse was allowed 20 minutes to freely move about the two-chambered apparatus in the absence of any laser light stimulation. Video was scored manually by blinded experimenter. Baseline preference was calculated for each mouse by averaging the duration spent in each chamber across the three habituation days. The conditioned preference was calculated for each mouse as the duration spent in each chamber on the test day. Percent change was calculated as percent time in blue light chamber after training – percent time in blue light chamber before training.
Determination of natural estrous state
Natural estrous cycle was determined by vaginal lavage. Immediately following behavioral testing, the vagina was flushed with 20μl ultrapure water which was then pipetted onto a Superfrost plus slide for examination under dissecting scope. Mice were tested mid-morning each day to ensure the most accurate tracking. Lavage samples were assessed as described69, and estrous state was recorded throughout the week to ensure normal cycling. Data from mice with lavage samples that could not be fit into a typical four or five day cycling pattern were excluded.
Ovariectomy Surgery
Ovariectomies were performed on 8 week old female mice under 1.5–2% isoflurane using proper sterile technique. 5mg/kg oral or intraperitoneal meloxicam and 2mg/kg subcutaneous bupivacaine (at incision sites) were administered before surgery. A 0.5cm incision is made 1cm lateral to spinal cord, at the point where ribcage ends. An equivalent incision was made through the muscle wall. The white fat pad was exposed to identify the ovary, which was cauterized with a hemostat and scraped off with a scalpel. The fat pad was reinserted and 1–2 sutures closed the muscle wall. 2–4 sutures closed the skin. Meloxicam was administered 24 hours after surgery and mice were allowed two weeks to recover before behavioral testing.
Lordosis Quotient Assay
10–14 week old ovariectomized females underwent two overnight pairings with stud males two weeks following surgery. To mimic behavioral estrus state at the time of pairing, females were subcutaneously injected with 0.5 μg estradiol benzoate in sunflower seed oil 52 hours before pairing, and with 800 μg progesterone in sunflower seed oil 4 hours before pairing. The females received the same hormone treatment prior to the lordosis quotient assay. The three trials were spaced 1–3 weeks apart to allow circulating hormones to reset before the next hormone priming. Lordosis quotient assay was conducted in male home cage, 1–2 hours into the dark cycle. Video of the assay was recorded for 20 minutes or 20 attempted male mounts, whichever came first. Lordosis quotient was calculated as the number of female receptive responses divided by the number of attempted male mounts. A receptive response for the female was scored as all four limbs securely on the cage floor with no combative or escape behaviors. If the first male did not mount within 10 minutes, the female was moved to another male’s cage. For any given trial, up to three males may have been used. Receptive postures were scored for quality on a scale from 1–3 as follows: 1: limbs on the ground with no attempts to escape, neither dorsiflexion nor upturned nose. 2: Some dorsiflexion, no upturned nose. 3: Robust dorsiflexion and/or upturned nose. Posture scores were averaged within each trial.
Intracranial viral injections and optic fiber implantation
7–10 week old females were pretreated with 5mg/kg oral or intraperitoneal meloxicam before surgery. They were anesthetized in a chamber with 3% isoflurane before being placed in a stereotaxic frame and kept anesthetized with 1.5–2% isoflurane. Skull was exposed and leveled before drilling at the appropriate coordinates. 200nL pAAV-hSyn-GRAB_DA1h or 200nL pAAV-hSyn-GRAB_DA2mwas injected unilaterally into NAc (AP: +1.0 mm relative to Bregma, ML: ±1.2 mm relative to Bregma, DV: 4.6 mm from the brain surface) using a backfilled glass needle and syringe pump (PHD Ultra, Harvard Apparatus), or nanoject. Skin was either sutured for one week recovery before implanting optic fiber (Doric, MFC_200/230–0.57_6mm_MF1.25_FLT) 200μm above injection site, or optic fiber was inserted immediately following injection. Three skull screws were inserted in the skull to stabilize the implant. A small amount of Metabond cement (Parkell) was used to bond the fiber, skull, and skull screws. Dental cement was applied on top of dried Metabond to create a stable implant structure. Mice were given 1 week to recover from implant surgery and 2 weeks to recover from injection and implant combined surgeries before behavioral testing. Meloxicam was administered 24 hours after surgery during post-operative monitoring.
Viral injections and GRIN lens implantation
A DAT-Cre animal was unilaterally injected with 300nL of AAV1-syn-FLEX-jGCaMP8m (titer: 1.6E13 GC/mL; Addgene #162378-AAV1) into the left hemisphere of the ventral tegmental area (VTA; AP:−3.20 mm, ML: −0.40 mm, DV: −4.40 mm). A 0.6mm-diameter gradient index (GRIN) lens (1050–004413, 7.3mm length, NA 0.5; Inscopix, Mountain View, CA, USA) was implanted in the same place of the virus injection site. Once in place, the lens was secured to the skull with super glue (Loctite, LOC1255800) and dental cements (Ortho-Jet™ Liquid, Black, Lang Dental, Wheeling, Illinois, USA). The imaging field of view was inspected and allowed to clear for at least five days prior to imaging and behavioral experiments.
Fiber Photometry
Zirconia sleeves (Doric) were used to connect the optic fiber implant to the patch cord. Signals were recorded using a real-time processor (RZ10X, TDT) and extracted in real time using Synapse software (TDT). A 465nm LED was used to excite the GRABDA while a 405nm LED was used as isosbestic to measure and control for changes in fluorescence due to photobleaching and movement artifacts.
For fiber photometry during transdermal optogenetic activation experiments, mice were placed on the mesh platform in plastic chambers with a 7mm slot cut into the ceiling to allow the cord to connect from the head to the computer. Habituation to the chambers was as previously described, except the fiber was attached to the implanted cannula after the first habituation day. After 30 second baseline recording, 10Hz pulsed sin wave at 35mW blue light was shined through the mesh platform to the shaved back skin or vaginal area at a 2–3cm distance for 30 seconds. The order in which the mice received stimulation (blue vs green; anogenital vs back) was counterbalanced.
For fiber photometry during sexual behavior, females were ovariectomized and hormone primed in the same way as the lordosis quotient assay without photometry. The recordings presented are equivalent with trial 1 from the lordosis quotient assay. (First assay after an overnight pairing the week prior). Females were placed in male home cage 1 hour into the dark cycle for 10 minutes or 10 mounts, whichever occurred first. If the male did not mount in the first 5 minutes, the female was placed in another male cage, for up to three total males.
Microendoscope Imaging
Mice were placed in their home cages and allowed to habituate with the head-mounted dummy scope for at least three days (15min each session). Before starting mating assays, the mice were briefly anaesthetized using a mixture of isoflurane and oxygen and the mini-epifluorescence microscope was attached to the baseplate. A period of 20–30 minutes was allowed to recover in the home cage before experiments started. Calcium imaging data were acquired using Inscopix Data Acquisition Software (version 1.3.0) at a frame rate of 20 Hz, 1mW/mm2 LED power (475nm) with 4–5x gain, depending on the brightness of GCaMP expression. Image acquisition parameters were set to the same values between sessions to be able to compare the activity recorded. Females were ovariectomized and hormone-primed as described for other mating assays.
Microendoscope fluorescence trace extraction
Imaging frames were spatially downsampled by a factor of four in the x and y dimensions. Motion artifacts were corrected using Inscopix Data Processing software (version 1.6.0). To extract calcium activity traces of individual cells, we used the constrained non-negative matrix factorization for microendoscopic data (CNMF-E) algorithm70. Segmented cells were manually inspected and non-cell-like shapes or cells with activity traces with non-calcium like events were discarded. Accepted cells and their activity traces were exported to MATLAB or Python for further analysis.
Electrophysiology
For all electrophysiology recordings, tissue was transferred to a recording chamber and continually superfused with ACSF bubbled with Carbogen (95% O2 and 5% CO2) to achieve a pH of 7.3–7.4. All recordings were made at room temperature (22–24°C) and neurons visualized using a Zeiss Axiocam 506 color camera. Recordings were acquired in cell-attached (holding current = 0mV), voltage-clamp (holding potential −70mV), or current-clamp (−60mV) configuration. Patch pipettes (3–7 MΩ) were filled with a potassium gluconate-based internal solution containing (in mM): 135 C6H11KO7, 8 NaCl, 10 HEPES, 2 Mg2ATP, 0.3 Na3GTP, and 0.1 EGTA, pH 7.3 (with KOH). No liquid junction potential correction was made, although this value was calculated at 14.7 mV (22 °C). All data were amplified using a MultiClamp 700B amplifier, digitized online (sampled at 20 kHz, filtered at 5 kHz) using an Axon Digidata 1550B, and acquired using Clampex software. After obtaining the whole-cell recording configuration, series resistance, input resistance, and membrane capacitance were calculated (averaged response to −5mV step, 20 trials, holding potential −70mV).
Acute slice
Recordings were made from Mrgprb4Cre;RosaChR2/+ or Mrgprb4Cre;RosaChR2/ChR2 mice (female; age 3.9 ± 0.6 weeks). Mice were anaesthetized with ketamine (100 mg/kg i.p), decapitated, and spinal cord (T10-L2) rapidly removed in ice-cold sucrose substituted artificial cerebrospinal fluid (sACSF) containing (in mM): 250 sucrose, 25 NaHCO3, 10 glucose, 2.5 KCl, 1 NaH2PO4, 6 MgCl2, and 1 CaCl2. Sagittal slices (200μm thick) were prepared using a vibrating microtome (Leica VT1200S). Slices were incubated for at least 1hr at 22–24°C in an interface chamber holding oxygenated ACSF containing (in mM): 118 NaCl, 25 NaHCO3, 10 glucose, 2.5 KCl, 1 NaH2PO4, 1 MgCl2, and 2.5 CaCl2. Slices were illuminated using an X-CITE 120LED Boost High-Power LED Illumination System that allowed visualization of GFP fluorescence and ChR2 photostimulation using FITC filter set. Photostimulation intensity was suprathreshold (24 mW), duration 1 ms (controlled by transistor-transistor logic pulses). AP discharge patterns were assessed in the current clamp from a membrane potential of −60 mV by delivering a series of depolarizing current steps (1 s duration, 20 pA increments, 0.1 Hz)
DRG cell culture
Recordings were made from Mrgprb4Cre;RosaChR2/GFP mice (male and female; age 10.3 ± 0.1 wks). Mice were anaesthetized with ketamine (100 mg/kg i.p), decapitated, and DRGs dissected in ice-cold HBSS solution. DRGs were then incubated in a mix of collagenase type XI (900 μ/ml) and dispase (3 mg/ml) in INCmix medium (in mM: 155 NaCl, 1.5 K2PO4, 10 HEPES, and 5 glucose buffered to pH 7.4) containing 1% penicillin and streptomycin for 45–60 mins at 37°C in 5% CO2. After enzyme digestion a gentle mechanical dissociation was performed by aspiration/expulsion using a 1ml pipette and passed through a 70μm nylon filter. Enzymes were stopped by adding Ca2+- and Mg2+-free HBSS medium containing 1% MEM Vitamin Solution, 10% FBS, and 1% penicillin and streptomycin, followed by centrifugation at 300G for 8 minutes. The pellets were resuspended in MEM supplemented with 1% MEM Vitamin Solution, 10% FBS, and 1% penicillin and streptomycin. Cell suspensions were plated on poly-l-lysine-coated glass coverslips and maintained at 37°C in 5% CO2 for 24hrs. Graded mechanical stimulation of DRG neuron cell bodies was performed using a glass pipette driven by a Siskiyou MX7600 motorized micromanipulator. The pipette was positioned on a cell body at a 45° angle and opposite to the recording pipette.
Viral Tracing
(N-terminal and C-terminal fragments AAV-EF1a-N-CretrcintG and AAV-EF1a-C-CreintG, Addgene # 69571 and # 69570) We injected pAAV.CAG.LSL.tdTomato (Addgene #100048) and combinatorial CRE-DOG viruses into the lower thoracic spinal cord of GPR83-eGFP animals to label GPR83+ projection neurons with tdTomato (Figure S6a). By injecting a BFP retrograde virus (pENN.AAV.CB7.CI.mCerulean.WPRE.RBG) (Addgene#105557-AAV9) into VTA of the same animals (Figure S6a,b),
Quantification and Statistical Analysis
Statistical analysis, tests used, n, and parameters for determining significance can be found in the figure legends. Unless otherwise noted, for all repeated measures behavioral testing, Two-Way Repeated Measures ANOVAs were performed, followed by multiple comparisons testing if a main effect or interaction was observed. If there were missing values, a mixed-effects model was used instead. When only one variable was tested (e.g. genotype but not time/trial), a One-Way ANOVA (if more than two groups) or an independent samples t-test (if only two groups) was performed.
Fiber Photometry Analysis
TDT folders were imported directly into Fiber photometry Modular Analysis Tool (pMAT) software for analysis71. Fiber photometry during transdermal optogenetics: Z-scored deltaF/F was calculated in pMAT by normalizing to the median of 5s baseline sampling window (−10 – −5s), bin constant: 150ms. These data were exported in a csv file from pMAT into GraphPad prism where the average trace across animals was plotted with shaded region representing SEM. These data were then normalized to the mean of the plotted baseline for visualization. The average raw deltaF/F in time bins was calculated for each animal and plotted in GraphPad prism to visualize changes from baseline to stim in individual animals and for statistical anaylsis. Mice were excluded if cannula placement or viral injection was not found in NAc after immunofluorescence. Fiber photometry during sexual behavior: Z-scored deltaF/F was calculated in pMAT by normalizing to the median of 3s baseline sampling window (−5 – −2s), bin constant: 300ms. These data were exported in a csv file from pMAT into GraphPad prism where the average trace across animals was plotted with shaded region representing SEM. These data were then normalized to the mean of the plotted baseline for visualization. Area under the curve was exported as csv from pMAT and plotted in GraphPad prism for statistical analysis.
Supplementary Material
Supplemental Figure 1: Characterizing physiological properties of dorsal horn spinal cord neurons that receive synaptic input from Mrgprb4-lineage neurons, Related to Figure 2. A) Schematic of the spinal cord slice preparation to activate Mrgprb4+ terminals with blue light in the superficial dorsal horn and record from synaptically connected neurons. B) Grouped data of mono and polysynaptic EPSC amplitudes. C) Incidence of a monosynaptic only, polysynaptic only, or mono + polysynaptic input. D) Optically evoked action potential (AP). 15/39 neurons tested fired AP’s following 1ms photostimulation. E) Latency of EPSCs (mono and polysynaptic) in these neurons, compared to the latency of the first evoked AP. Of these 15 neurons, 7 display characteristics of AP’s evoked by direct Mrgprb4 afferent input, 8 display characteristics of AP’s evoked by indirect (polysynaptic) Mrgprb4 inputs. F,G) In a subset of recordings AP discharge was characterized in post-synaptic neurons (n = 36). 2/14 Tonic firing (TF) neurons received mono input only, 4/14 poly only, and 8/14 both. 0/15 Delayed firing (DF) neurons received mono input only, 12/15 received poly only, and 3/15 both. 1/4 Initial bursting (IB) neurons received mono input only, 1/4 received poly only, and 2/4 both. 0/3 Single spikers (SS) received mono input only, 1/3 received poly only, and 2/3 both. H) TF neurons receive the strongest monosynaptic inputs, followed by IB’s. I) Polysynaptic currents are similar across all types. J) AP incidence is highest in TF neurons.
Supplemental Figure 2: Optogenetic behaviors stimulating either MrgprA3 neurons or Mrgprb4-lineage neurons in males, Related to Figure 3. A) Schematic showing experimental setup with blue light applied to the shaved back skin, which elicits nearly immediate scratching bouts, and not the back lowering dorsiflexion phenotype observed when the same experiments are performed with Mrgprb4-lineage neurons. B) Quantification of scratching bouts to 5 minutes of blue or green laser light (negative control). Unpaired student’s t-test, **** P ≤ 0.0001. Individual circles represent a single mouse. C,D) Optogenetic activation of Mrgprb4-lineage neurons in the shaved backs of Mrgprb4Cre; RosaChR2/ChR2 but not Cre-negative littermate controls yields dorsiflexion posture (blue light 35mW pulsed at 10Hz sin wave). E) Maximal backdip from the ceiling of the chamber over the course of 20s stimulation. P<0.0001, independent samples t-test. E-G) No significant difference in the abundance of Mrgprb4+ neurons in thoracic DRGs of male (E) vs female (F) mice. (ns, Independent samples t-test) H) Male mice exhibit no significant difference in preference for blue light chamber compared to Cre-negative littermate controls (ns, independent samples t-test). Same conditioned place preference paradigm described in Fig 3a–f. I) Mrgprb4-lineage neurons innervate the male genital skin. Immunofluorescence staining for ChR2-eYFP+ terminals.
Supplemental Figure 3: A viral strategy to target Mrgprb4+ sensory neurons reveals touch-like behaviors and no dorsiflexion, Related to Figure 3. A) Both experimental and control litter pups were injected with 30microliters of cre-dependent opsin virus between P0-P3. B) 36% of Mrgprb4+ neurons were labeled with the cre-dependent opsin Chrmine. C) Tissue section through a DRG showing ChRmine virus typically labeling 1 of every 3 Mrgprb4+ neurons. D) Typical orange laser light induced behaviors where animals would orient towards the light searching, appearing to search for the source of the touch. E) Quantification of number of touch-like responses during 20 seconds of pulsing laser light. Control animals never appeared to care about the light or alter behavior. All scoring was done blind to genotype, conducted by different investigators. p=0.0085, unpaired t-test.
Supplemental Figure 4: Ablating Mrgprb4-lineage neurons does not alter motor function or pain threshold, Related to Figure 4. A) RNAscope in situ hybridization of DRGs from WT C57 control and Mrgprb4Cre; RosaDTA female. Scale bars = 75μm. B) Quantification of ablation in A. Cell bodies expressing Mrgprb4 are absent upon DTA expression while Trpv1+ cells remain intact. C,D) Mrgprb4Cre; RosaDTA mice exhibit no motor deficits in open field (C, unpaired t-test) or rotarod (D, Two-Way ANOVA) assays. E-H) Mrgprb4Cre; RosaDTA mice exhibit no differences in pain sensitivities to innocuous or noxious stimuli applied to the hairy skin compared to controls. Note: In panel G B4DTA animals have an increased distance traveled to the innocuous air puff suggesting that some subtle aspect of somatosensation might be altered in these animals. However, since they display no pain behaviors to the air puff, and normal pain behaviors to pinprick, we can conclude their pain threshold is normal. In H, the composite nocifensive score captures four affective pain behaviors including jumping, paw guarding, paw shaking, and eye grimace.
Supplemental Figure 5. Optogenetic posture is independent of estrous state and neuroanatomy of Mrgprb4+ sensory endings, Related to Figure 4. A) No differences in the depth of the optogenetically induced back dip across stages of the estrous cycle. Circles represent individual mice, data plotted as mean +/− SEM. B) Neither ovariectomizing (OVX) females, nor replacing estrogen and progesterone exogenously to mimic behavioral estrus, significantly modulates the optogenetically induced back dip. Dots represent individual mice. All data analyzed by one way ANOVA followed by Tukey’s post-hoc comparisons. C) Nerve terminal endings in red are seen in the underbelly skin. D) Nerve terminals for Mrgprb4+ touch neurons are not seen in the internal lining of the vaginal wall, which is non-hairy skin.
Supplemental Figure 6: Viral tracing of putative skin-to-brain circuit from Mrgprb4-lineage neurons to VTA, Related to Figure 5. A) Schematic depicting experimental approach. AAV-LSL-Tomato and CRE-DOG AAVs (AAV-EF1a-C-CreintG and AAV-EF1a-N-CretrcintG) were injected into spinal cord of GPR83-eGFP mice in order to express Cre in and label GPR83+ projection neurons with Cre-dependent tdTomato. AAV retro-BFP was injected into the VTA of the same mice in order to label cells projecting to the VTA with BFP. B) In blue: The retro-BFP+ endings in VTA. C) In blue: the retro-BFP+ somas of neurons that project to the VTA. In red: the GPR83+ projections from lower thoracic spinal cord. C’) Magnified view of C: The red GPR83+ projection neurons make contact with blue somas that project to the VTA.
Supplemental Video 1: Optogenetic back stimulation of Mrgprb4-lineage touch neurons in Mrgprb4-Cre; ChR2 female mice, Related to Figure 3. Pulsing blue laser light at 10Hz to the shaven back skin results in back lowering phenotype that is reminiscent of the lordotic copulatory posture.
Supplemental Video 2: Optogenetic back stimulation of Cre-negative female control littermates, Related to Figure 3. Pulsing blue laser light at 10Hz to the shaven back skin of control mice does not result in back lowering phenotype. Note the animal is alerted to the presence of the experimenter but does not alter its behavior in any noticeable robust manner.
Supplemental Video 3: Optogenetic anogenital stimulation of Mrgprb4-lineage touch neurons in Mrgprb4-Cre; ChR2 female mice, Related to Figure 5. Pulsing blue laser light at 10Hz to the anogenital skin does not trigger any noticeable robust behaviors, or lordosis-like postures.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-CGRP | Immunostar | Cat#24112 |
| Griffonia anti-IB4 | Life Tech | Cat#I21411 |
| Chicken anti-GFP | Life Tech | Cat#A10262 |
| Rabbit anti-RFP | Rockland | Cat#600401379 |
| Rabbit anti-HA-Tag | Cell Signaling | Cat#3724 |
| Bacterial and virus strains | ||
| pAAV.CAG.LSL.tdTomato | Addgene | Plasmid#100048 |
| AAV-EF1a-N-CretrcintG | Addgene | Plasmid#69571 |
| AAV-EF1a-C-CreintG | Addgene | Plasmid#69570 |
| AAV1-syn-FLEX-jGCaMP8m | Addgene | Plasmid#162378-AAV1 |
| pENN.AAV.CB7.Cl.mCerulean.WPRE.RBG | Addgene | Plasmid#105557-AAV9 |
| pAAV-hSyn-GRAB_DA2m | Addgene | Plasmid#140553 |
| pAAV-hSyn-GRAB_DA1h | Addgene | Plasmid#113050 |
| pAAV-Ef1a-DIO-ChRmine-mScarlet-3xHA (PHP.S) | Dr. David Ng | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Bicuculline | Alomone | Cat #: B-137 |
| Strychnine | Sigma | Cat #: S8753-25G |
| CNQX | Alomone | Cat #: C-141 |
| TTX | Caymen Chemical | Cat #: 14964 |
| Estradiol benzoate | Sigma | Cat#E1600000 |
| Progesterone | Sigma | Cat#p0130-100g |
| Tamoxifen | Sigma | Cat#T5648-1G |
| Critical commercial assays | ||
| RNAScope Probe: eYFP | ACD | Cat#312131 |
| RNAScope Probe: Mrgprb4 | ACD | Cat#435781 |
| RNAScope Probe: Mrgprd | ACD | Cat#417921 |
| RNAScope Probe: Mrgprc11 | ACD | Cat#488771 |
| RNAScope Probe: Mrgpra3 | ACD | Cat#548161 |
| RNAScope Probe: Pou4f2 | ACD | Cat#558481 |
| RNAScope Probe: Trpa1 | ACD | Cat#400211 |
| RNAScope Probe: Ret | ACD | Cat#431791 |
| RNAScope Probe: Th | ACD | Cat#317621 |
| RNAScope Probe: Scn10a | ACD | Cat#426011 |
| RNAScope Probe: Calca | ACD | Cat#578771 |
| RNAScope Probe: Gfra1 | ACD | Cat#431781 |
| RNAScope Probe: Trpv1 | ACD | Cat#313331 |
| RNAScope Intro Pack for Multiplex Fluorescence | ACD | Cat#323136 |
| RNAScope Multiplex Fluorescent Reagent Kit v2 | ACD | Cat#323100 |
| Opal 520 Reagent Pack | Perkin Elmer | FP1487001KT |
| Opal 570 Reagent Pack | Perkin Elmer | FP1488001KT |
| Opal 690 Reagent Pack | Perkin Elmer | FP1497001KT |
| Experimental models: Organisms/strains | ||
| Mouse: Mrgprb4tdTomato-Cre | Jackson Laboratories | Strain #: 021077 |
| Mouse: Rosa26Ai32-eYFP | Jackson Laboratories | Strain #: 012569 |
| Mouse: Tg(Gpr83-EGFP)DV46Gsat/Mmucd | MMRRC, UC Davis | Stock #: 010442-UCD |
| Mouse: Rosa26DTA | Jackson Laboratories | Strain #: 009669 |
| Mouse: DATIREScre | Jackson Laboratories | Strain #: 006660 |
| Mouse: MrgprdCreERT2 | Jackson Laboratories | Strain #: 031286 |
| Mouse: Mrgpra3Cre-EGFP | Dr. Xinzong Dong | N/A |
| Software and algorithms | ||
| Synapse software | TDT | N/A |
| Clampex software | Molecular Devices | N/A |
| PAWS software | Jones et al.37 | N/A |
| ProAnalyst | Xcitex | N/A |
| pMAT software | Bruno et al.71 | https://github.com/djamesbarker/pMAT |
| CNMF-E | Zhou et al.70 | N/A |
| Inscopix Data Processing Software | Inscopix | Version 1.6.0 |
| Inscopix Data Acquisition Software | Inscopix | Version 1.3.0 |
| Other | ||
| Zeiss Axiocam 506 color camera | Zeiss | N/A |
| MultiClamp 700B amplifier | Molecular Devices | N/A |
| Axon Digidata 1550B | Molecular Devices | N/A |
| Siskiyou MX7600 Motorized Micromanipulator | Siskiyou | MX7600 |
| Conditioned Place Preference Chamber | Rossi et al.68 | N/A |
| FastCAM UX100 high speed camera | Photron | 800K-M-4GB |
| 473 nm blue laser light | Shanghai Laser and Optics, China | BL473T8-150FC/ADR-800A |
| 532 nm green laser | Shanghai Laser and Optics Century, Shanghai, China | GL532T8-1000FC/ADR-800A |
| 593 nm continuous wave diode pumped solid-state orange laser | CrystalLaser LC | CL593-050-O |
| 10MHZ Function Waveform Generator | Agilent | 33210A |
| FC/PC Optogenetic patch cable | ThorLabs | M72L01 |
| TDT Fiber Photometry RZ10X Real Time Processor | TDT | RZ10X |
| TDT Experiment Control Workstation | TDT | WS-4 |
| Mono Fiber-optic Cannula | Doric | MFC_200/230-0.57_6mm_MF1.25_FLT |
| Super glue | Loctite | LOC1255800 |
| Fiber Photometry Fluorescence Mini Cubes – 6 ports | Doric | FMC6_IE(400-410)_E1(460-490)_F1(500-540)_E2(555-570)_F2(580-680)_S |
| Zirconia mating sleeves | Doric | SLEEVE_ZR_1.25_BK |
| Mono Fiber-optic Patch Cords | Doric | MFP_200/230/900-0.57_1m_FCM-MF1.25(F)_LAF |
| Metabond Quick Adhesive Cement System | Parkell | SKU: S380 |
| Ortho-Jet Liquid Dental cement | Lang Dental | N/A |
| GRIN lens 7.3mm length, NA 0.5 | Inscopix | 1050-004413 |
| Laser power meter | ThorLabs | s120c |
| Syringe pump | Harvard Apparatus | PHD Ultra |
Highlights.
Activation of Mrgprb4-lineage touch neurons induces lordosis-like posture
Activation of Mrgprb4-lineage touch neurons is rewarding
Mrgprb4-lineage touch neurons are required for female sexual receptivity
Mrgprb4-lineage touch neurons engage dopaminergic neurons during social behavior
Acknowledgments
We thank members of the Abdus-Saboor, Costa, Abraira labs for helpful discussion and comments on this manuscript. We thank Oliver Hobert, Richard Axel, and Charles Zuker for helpful comments on this work and manuscript. We thank Nitsan Goldstein and members of the Betley lab for assistance with fiber photometry set up. We thank David Barker for suggestions with pMAT software. We thank members of LJE’s thesis committee, especially Lori Flannagan-Cato for helpful suggestions on experiments, and Greg Corder for providing space for revisions experiments. We thank Qin Liu for sharing Mrgprb4Cre; RosaChR2/ChR2 mice with us and Wenqin Luo. We thank David Ng and the Zuckerman Mind Brain Behavior Institute molecular virology core for generating ChRmine viruses for this study. We thank Gabriela Martins and Helio Rodrigues for assistance with setting up an Inscopix microendoscopy rig. We thank Janet Sinn-Hanlon for illustrations. We thank the Marlin lab for sharing an anti-HA antibody. We thank Darcy Peterka for sharing an orange laser with us. LJE and MDS were supported by NIH NRSA grants from NINDS and NCCIH. AF is supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. IAS and lab members acknowledge support from startup funds provided by the University of Pennsylvania and Columbia University, National Institute of Health grants K99/R00 and DP2 New Innovator, and fellowships from the Rita Allen Foundation, Pew Charitable Trust, Brain Research Foundation, Chan Zuckerberg Initiative, and Alfred P. Sloan Foundation. VEA, MB, and MG acknowledge support from startup funds from Rutgers University, Pew Charitable Trust, NIH R01 and K01 grants from NINDS, and Whitehall Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References:
- 1.Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, Ye M, Chirila AM, Emanuel AJ, Rankin G, Fame RM, Lehtinen MK, Feng G, Ginty DD. Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell. 2019;178(4):867–86 e24. Epub 2019/08/10. doi: 10.1016/j.cell.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell. 2016;166(2):299–313. Epub 2016/06/14. doi: 10.1016/j.cell.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peled-Avron L, Shamay-Tsoory SG. Don’t touch me! autistic traits modulate early and late ERP components during visual perception of social touch. Autism Res. 2017;10(6):1141–54. Epub 2017/03/25. doi: 10.1002/aur.1762. [DOI] [PubMed] [Google Scholar]
- 4.Hart AS, Rutledge RB, Glimcher PW, Phillips PE. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci. 2014;34(3):698–704. Epub 2014/01/17. doi: 10.1523/JNEUROSCI.2489-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570(7759):65–70. Epub 2019/05/24. doi: 10.1038/s41586-019-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz W Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016;17(3):183–95. Epub 2016/02/13. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starkweather CK, Babayan BM, Uchida N, Gershman SJ. Dopamine reward prediction errors reflect hidden-state inference across time. Nat Neurosci. 2017;20(4):581–9. Epub 2017/03/07. doi: 10.1038/nn.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–94. Epub 2004/05/21. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 9.Northcutt KV, Nguyen JM. Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behav Neurosci. 2014;128(2):178–86. Epub 2014/04/30. doi: 10.1037/a0035964. [DOI] [PubMed] [Google Scholar]
- 10.Bariselli S, Hornberg H, Prevost-Solie C, Musardo S, Hatstatt-Burkle L, Scheiffele P, Bellone C. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun. 2018;9(1):3173. Epub 2018/08/11. doi: 10.1038/s41467-018-05382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–51. Epub 2014/06/21. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Li J, Yang Q, Xie YK, Wen YL, Xu ZZ, Li Y, Xu T, Wu ZY, Duan S, Xu H. Basal forebrain mediates prosocial behavior via disinhibition of midbrain dopamine neurons. Proc Natl Acad Sci U S A. 2021;118(7). Epub 2021/02/11. doi: 10.1073/pnas.2019295118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci. 2014;34(8):2879–83. Epub 2014/02/21. doi: 10.1523/JNEUROSCI.2847-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawling R, Cannon PR, McGlone FP, Walker SC. C-tactile afferent stimulating touch carries a positive affective value. PLoS One. 2017;12(3):e0173457. Epub 2017/03/11. doi: 10.1371/journal.pone.0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci. 2013;33(3):870–82. Epub 2013/01/18. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handler A, Ginty DD. The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci. 2021;22(9):521–37. Epub 2021/07/28. doi: 10.1038/s41583-021-00489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, Alonso S, Francois A, Barrere C, Seal R, Landry M, Eschallier A, Alloui A, Bourinet E, Delmas P, Le Feuvre Y, Moqrich A. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 2013;5(2):378–88. Epub 2013/10/22. doi: 10.1016/j.celrep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Vrontou S, Wong AM, Rau KK, Koerber HR, Anderson DJ. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature. 2013;493(7434):669–73. Epub 2013/02/01. doi: 10.1038/nature11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10(8):946–8. Epub 2007/07/10. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci. 2008;28(1):125–32. Epub 2008/01/04. doi: 10.1523/JNEUROSCI.4472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lou S, Pan X, Huang T, Duan B, Yang FC, Yang J, Xiong M, Liu Y, Ma Q. Incoherent feed-forward regulatory loops control segregation of C-mechanoreceptors, nociceptors, and pruriceptors. J Neurosci. 2015;35(13):5317–29. doi: 10.1523/JNEUROSCI.0122-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577(7790):392–8. Epub 2020/01/10. doi: 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huzard D, Martin M, Maingret F, Chemin J, Jeanneteau F, Mery PF, Fossat P, Bourinet E, Francois A. The impact of C-tactile low-threshold mechanoreceptors on affective touch and social interactions in mice. Sci Adv. 2022;8(26):eabo7566. Epub 20220629. doi: 10.1126/sciadv.abo7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–27. Epub 2011/12/27. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi S, Otsuguro K. A mechanically activated ion channel is functionally expressed in the MrgprB4 positive sensory neurons, which detect stroking of hairy skin in mice. Neurosci Lett. 2017;653:139–45. Epub 20170522. doi: 10.1016/j.neulet.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal NU, Zhang D, DeLisle MM, Wolfson RL, Bai L, Santiago C, Gong S, Goulding M, Heintz N, Koerber HR, Ross SE, Ginty DD. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587(7833):258–63. Epub 2020/10/30. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warwick C, Cassidy C, Hachisuka J, Wright MC, Baumbauer KM, Adelman PC, Lee KH, Smith KM, Sheahan TD, Ross SE, Koerber HR. MrgprdCre lineage neurons mediate optogenetic allodynia through an emergent polysynaptic circuit. Pain. 2021;162(7):2120–31. doi: 10.1097/j.pain.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, Seguela P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain. 2017;158(12):2329–39. doi: 10.1097/j.pain.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 29.Abdus-Saboor I, Fried NT, Lay M, Burdge J, Swanson K, Fischer R, Jones J, Dong P, Cai W, Guo X, Tao YX, Bethea J, Ma M, Dong X, Ding L, Luo W. Development of a Mouse Pain Scale Using Sub-second Behavioral Mapping and Statistical Modeling. Cell Rep. 2019;28(6):1623–34 e4. doi: 10.1016/j.celrep.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kuhnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, Patapoutian A. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. 2018;10(462). doi: 10.1126/scitranslmed.aat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20(8):1172–9. Epub 20170626. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshel JH, Kim YS, Machado TA, Quirin S, Benson B, Kadmon J, Raja C, Chibukhchyan A, Ramakrishnan C, Inoue M, Shane JC, McKnight DJ, Yoshizawa S, Kato HE, Ganguli S, Deisseroth K. Cortical layer-specific critical dynamics triggering perception. Science. 2019;365(6453). Epub 20190718. doi: 10.1126/science.aaw5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodersten P, Larsson K. Lordosis behavior and mounting behavior in male rats: effects of castration and treatment with estradiol benzoate or testosterone propionate. Physiol Behav. 1975;14(2):159–64. Epub 1975/02/01. doi: 10.1016/0031-9384(75)90160-2. [DOI] [PubMed] [Google Scholar]
- 34.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–10. [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson K, Sodersten P, Beyer C, Morali G, Perez-Palacios G. Effects of estrone, estradiol and estriol combined with dihydrotestosterone on mounting and lordosis behavior in castrated male rats. Horm Behav. 1976;7(4):379–90. Epub 1976/12/01. doi: 10.1016/0018-506x(76)90009-x. [DOI] [PubMed] [Google Scholar]
- 36.Kow LM, Montgomery MO, Pfaff DW. Triggering of lordosis reflex in female rats with somatosensory stimulation: quantitative determination of stimulus parameters. J Neurophysiol. 1979;42(1 Pt 1):195–202. Epub 1979/01/01. doi: 10.1152/jn.1979.42.1.195. [DOI] [PubMed] [Google Scholar]
- 37.Jones JM, Foster W, Twomey CR, Burdge J, Ahmed OM, Pereira TD, Wojick JA, Corder G, Plotkin JB, Abdus-Saboor I. A machine-vision approach for automated pain measurement at millisecond timescales. Elife. 2020;9. Epub 2020/08/08. doi: 10.7554/eLife.57258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toussaint AB, Foster W, Jones JM, Kaufmann S, Wachira M, Hughes R, Bongiovanni AR, Famularo ST, Dunham BP, Schwark R, Karbalaei R, Dressler C, Bavley CC, Fried NT, Wimmer ME, Abdus-Saboor I. Chronic paternal morphine exposure increases sensitivity to morphine-derived pain relief in male progeny. Sci Adv. 2022;8(7):eabk2425. Epub 20220216. doi: 10.1126/sciadv.abk2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy EA, Maqsudlu A, Bass M, Georghiou S, Cherry JA, Baum MJ. DREADD-induced silencing of the medial amygdala reduces the preference for male pheromones and the expression of lordosis in estrous female mice. Eur J Neurosci. 2017;46(4):2035–46. Epub 2017/07/06. doi: 10.1111/ejn.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye CA, Walf AA, Kohtz AS, Zhu Y. Progesterone-facilitated lordosis of estradiol-primed mice is attenuated by knocking down expression of membrane progestin receptors in the midbrain. Steroids. 2014;81:17–25. Epub 2013/11/26. doi: 10.1016/j.steroids.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marco-Manclus P, Paredes RG, Portillo W. Sexual experience with a known male modulates c-Fos expression in response to mating and male pheromone exposure in female mice. Physiol Behav. 2020;222:112906. Epub 20200520. doi: 10.1016/j.physbeh.2020.112906. [DOI] [PubMed] [Google Scholar]
- 42.Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31(15):5574–8. Epub 2011/04/15. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanase M, Gorski RA. Sites of estrogen and progesterone facilitation of lordosis behavior in the spayed rat. Biol Reprod. 1976;15(4):536–43. Epub 1976/11/01. doi: 10.1095/biolreprod15.4.536. [DOI] [PubMed] [Google Scholar]
- 44.White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45(3):201–8. Epub 2004/03/30. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Dai B, Sun F, Tong X, Ding Y, Kuang A, Osakada T, Li Y, Lin D. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 2022;40(8):111246. doi: 10.1016/j.celrep.2022.111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693(1–2):21–30. Epub 1995/09/25. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18(7):1997–2001. Epub 2003/11/19. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- 48.Beny-Shefer Y, Zilkha N, Lavi-Avnon Y, Bezalel N, Rogachev I, Brandis A, Dayan M, Kimchi T. Nucleus Accumbens Dopamine Signaling Regulates Sexual Preference for Females in Male Mice. Cell Rep. 2017;21(11):3079–88. Epub 2017/12/16. doi: 10.1016/j.celrep.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 49.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21(9):3236–41. Epub 2001/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias LJ, Abdus-Saboor I. Bridging skin, brain, and behavior to understand pleasurable social touch. Curr Opin Neurobiol. 2022;73:102527. Epub 20220419. doi: 10.1016/j.conb.2022.102527. [DOI] [PubMed] [Google Scholar]
- 51.Tang JC, Rudolph S, Dhande OS, Abraira VE, Choi S, Lapan SW, Drew IR, Drokhlyansky E, Huberman AD, Regehr WG, Cepko CL. Cell type-specific manipulation with GFP-dependent Cre recombinase. Nat Neurosci. 2015;18(9):1334–41. Epub 20150810. doi: 10.1038/nn.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell. 2018;174(2):481–96 e19. Epub 2018/07/17. doi: 10.1016/j.cell.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Severson KS, Xu D, Van de Loo M, Bai L, Ginty DD, O’Connor DH. Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents. Neuron. 2017;94(3):666-+. doi: 10.1016/j.neuron.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462(7273):651–5. Epub 2009/11/17. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodgers CC, Nogueira R, Pil BC, Greeman EA, Park JM, Hong YK, Fusi S, Bruno RM. Sensorimotor strategies and neuronal representations for shape discrimination. Neuron. 2021;109(14):2308-+. doi: 10.1016/j.neuron.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang QY, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, DeLisle MM, Iskols M, Rhyins J, Kim SJ, Cattel SJ, Regehr W, Harvey CD, Drugowitsch J, Ginty DD. Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science. 2020;368(6497):1330-+. doi: ARTN eabb2751 10.1126/science.abb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509(7502):617–21. Epub 2014/04/11. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendas J, Georgiadis JR, Ritschel G, Olausson H, Weidner K, Croy I. C-Tactile Mediated Erotic Touch Perception Relates to Sexual Desire and Performance in a Gender-Specific Way. J Sex Med. 2017;14(5):645–53. Epub 20170331. doi: 10.1016/j.jsxm.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Urien L, Gaillard S, Lo Re L, Malapert P, Bohic M, Reynders A, Moqrich A. Genetic ablation of GINIP-expressing primary sensory neurons strongly impairs Formalin-evoked pain. Sci Rep. 2017;7:43493. Epub 2017/02/28. doi: 10.1038/srep43493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seal RP, Wang XD, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462(7273):651–5. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li L, Ginty DD. The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife. 2014;3:e01901. Epub 2014/02/27. doi: 10.7554/eLife.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuehn ED, Meltzer S, Abraira VE, Ho CY, Ginty DD. Tiling and somatotopic alignment of mammalian low-threshold mechanoreceptors. Proc Natl Acad Sci U S A. 2019;116(19):9168–77. Epub 2019/04/19. doi: 10.1073/pnas.1901378116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francois A, Schuetter N, Laffray S, Sanguesa J, Pizzoccaro A, Dubel S, Mantilleri A, Nargeot J, Noel J, Wood JN, Moqrich A, Pongs O, Bourinet E. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015;10(3):370–82. Epub 2015/01/21. doi: 10.1016/j.celrep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 64.Bohic M, Marics I, Santos C, Malapert P, Ben-Arie N, Salio C, Reynders A, Le Feuvre Y, Saurin AJ, Moqrich A. Loss of bhlha9 Impairs Thermotaxis and Formalin-Evoked Pain in a Sexually Dimorphic Manner. Cell Rep. 2020;30(3):602–10 e6. Epub 2020/01/23. doi: 10.1016/j.celrep.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Miao W, Ji E, Huang S, Jin S, Zhu X, Liu MZ, Sun YG, Xu F, Yu X. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron. 2022. Epub 20220112. doi: 10.1016/j.neuron.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18(2):73–85. Epub 2017/01/06. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 67.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855–62. Epub 2011/06/11. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi HL, Raj NR, Marquez de Prado B, Kuburas A, Luu AKS, Barr GA, Recober A. Trigeminal Pain Responses in Obese ob/ob Mice Are Modality-Specific. Neuroscience. 2019;415:121–34. Epub 20190708. doi: 10.1016/j.neuroscience.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 69.Cora MC, Kooistra L, Travlos G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol Pathol. 2015;43(6):776–93. Epub 2015/03/06. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, Kheirbek MA, Sabatini BL, Kass RE, Paninski L. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife. 2018;7. Epub 20180222. doi: 10.7554/eLife.28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruno CA, O’Brien C, Bryant S, Mejaes JI, Estrin DJ, Pizzano C, Barker DJ. pMAT: An open-source software suite for the analysis of fiber photometry data. Pharmacol Biochem Behav. 2021;201:173093. Epub 2021/01/02. doi: 10.1016/j.pbb.2020.173093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Characterizing physiological properties of dorsal horn spinal cord neurons that receive synaptic input from Mrgprb4-lineage neurons, Related to Figure 2. A) Schematic of the spinal cord slice preparation to activate Mrgprb4+ terminals with blue light in the superficial dorsal horn and record from synaptically connected neurons. B) Grouped data of mono and polysynaptic EPSC amplitudes. C) Incidence of a monosynaptic only, polysynaptic only, or mono + polysynaptic input. D) Optically evoked action potential (AP). 15/39 neurons tested fired AP’s following 1ms photostimulation. E) Latency of EPSCs (mono and polysynaptic) in these neurons, compared to the latency of the first evoked AP. Of these 15 neurons, 7 display characteristics of AP’s evoked by direct Mrgprb4 afferent input, 8 display characteristics of AP’s evoked by indirect (polysynaptic) Mrgprb4 inputs. F,G) In a subset of recordings AP discharge was characterized in post-synaptic neurons (n = 36). 2/14 Tonic firing (TF) neurons received mono input only, 4/14 poly only, and 8/14 both. 0/15 Delayed firing (DF) neurons received mono input only, 12/15 received poly only, and 3/15 both. 1/4 Initial bursting (IB) neurons received mono input only, 1/4 received poly only, and 2/4 both. 0/3 Single spikers (SS) received mono input only, 1/3 received poly only, and 2/3 both. H) TF neurons receive the strongest monosynaptic inputs, followed by IB’s. I) Polysynaptic currents are similar across all types. J) AP incidence is highest in TF neurons.
Supplemental Figure 2: Optogenetic behaviors stimulating either MrgprA3 neurons or Mrgprb4-lineage neurons in males, Related to Figure 3. A) Schematic showing experimental setup with blue light applied to the shaved back skin, which elicits nearly immediate scratching bouts, and not the back lowering dorsiflexion phenotype observed when the same experiments are performed with Mrgprb4-lineage neurons. B) Quantification of scratching bouts to 5 minutes of blue or green laser light (negative control). Unpaired student’s t-test, **** P ≤ 0.0001. Individual circles represent a single mouse. C,D) Optogenetic activation of Mrgprb4-lineage neurons in the shaved backs of Mrgprb4Cre; RosaChR2/ChR2 but not Cre-negative littermate controls yields dorsiflexion posture (blue light 35mW pulsed at 10Hz sin wave). E) Maximal backdip from the ceiling of the chamber over the course of 20s stimulation. P<0.0001, independent samples t-test. E-G) No significant difference in the abundance of Mrgprb4+ neurons in thoracic DRGs of male (E) vs female (F) mice. (ns, Independent samples t-test) H) Male mice exhibit no significant difference in preference for blue light chamber compared to Cre-negative littermate controls (ns, independent samples t-test). Same conditioned place preference paradigm described in Fig 3a–f. I) Mrgprb4-lineage neurons innervate the male genital skin. Immunofluorescence staining for ChR2-eYFP+ terminals.
Supplemental Figure 3: A viral strategy to target Mrgprb4+ sensory neurons reveals touch-like behaviors and no dorsiflexion, Related to Figure 3. A) Both experimental and control litter pups were injected with 30microliters of cre-dependent opsin virus between P0-P3. B) 36% of Mrgprb4+ neurons were labeled with the cre-dependent opsin Chrmine. C) Tissue section through a DRG showing ChRmine virus typically labeling 1 of every 3 Mrgprb4+ neurons. D) Typical orange laser light induced behaviors where animals would orient towards the light searching, appearing to search for the source of the touch. E) Quantification of number of touch-like responses during 20 seconds of pulsing laser light. Control animals never appeared to care about the light or alter behavior. All scoring was done blind to genotype, conducted by different investigators. p=0.0085, unpaired t-test.
Supplemental Figure 4: Ablating Mrgprb4-lineage neurons does not alter motor function or pain threshold, Related to Figure 4. A) RNAscope in situ hybridization of DRGs from WT C57 control and Mrgprb4Cre; RosaDTA female. Scale bars = 75μm. B) Quantification of ablation in A. Cell bodies expressing Mrgprb4 are absent upon DTA expression while Trpv1+ cells remain intact. C,D) Mrgprb4Cre; RosaDTA mice exhibit no motor deficits in open field (C, unpaired t-test) or rotarod (D, Two-Way ANOVA) assays. E-H) Mrgprb4Cre; RosaDTA mice exhibit no differences in pain sensitivities to innocuous or noxious stimuli applied to the hairy skin compared to controls. Note: In panel G B4DTA animals have an increased distance traveled to the innocuous air puff suggesting that some subtle aspect of somatosensation might be altered in these animals. However, since they display no pain behaviors to the air puff, and normal pain behaviors to pinprick, we can conclude their pain threshold is normal. In H, the composite nocifensive score captures four affective pain behaviors including jumping, paw guarding, paw shaking, and eye grimace.
Supplemental Figure 5. Optogenetic posture is independent of estrous state and neuroanatomy of Mrgprb4+ sensory endings, Related to Figure 4. A) No differences in the depth of the optogenetically induced back dip across stages of the estrous cycle. Circles represent individual mice, data plotted as mean +/− SEM. B) Neither ovariectomizing (OVX) females, nor replacing estrogen and progesterone exogenously to mimic behavioral estrus, significantly modulates the optogenetically induced back dip. Dots represent individual mice. All data analyzed by one way ANOVA followed by Tukey’s post-hoc comparisons. C) Nerve terminal endings in red are seen in the underbelly skin. D) Nerve terminals for Mrgprb4+ touch neurons are not seen in the internal lining of the vaginal wall, which is non-hairy skin.
Supplemental Figure 6: Viral tracing of putative skin-to-brain circuit from Mrgprb4-lineage neurons to VTA, Related to Figure 5. A) Schematic depicting experimental approach. AAV-LSL-Tomato and CRE-DOG AAVs (AAV-EF1a-C-CreintG and AAV-EF1a-N-CretrcintG) were injected into spinal cord of GPR83-eGFP mice in order to express Cre in and label GPR83+ projection neurons with Cre-dependent tdTomato. AAV retro-BFP was injected into the VTA of the same mice in order to label cells projecting to the VTA with BFP. B) In blue: The retro-BFP+ endings in VTA. C) In blue: the retro-BFP+ somas of neurons that project to the VTA. In red: the GPR83+ projections from lower thoracic spinal cord. C’) Magnified view of C: The red GPR83+ projection neurons make contact with blue somas that project to the VTA.
Supplemental Video 1: Optogenetic back stimulation of Mrgprb4-lineage touch neurons in Mrgprb4-Cre; ChR2 female mice, Related to Figure 3. Pulsing blue laser light at 10Hz to the shaven back skin results in back lowering phenotype that is reminiscent of the lordotic copulatory posture.
Supplemental Video 2: Optogenetic back stimulation of Cre-negative female control littermates, Related to Figure 3. Pulsing blue laser light at 10Hz to the shaven back skin of control mice does not result in back lowering phenotype. Note the animal is alerted to the presence of the experimenter but does not alter its behavior in any noticeable robust manner.
Supplemental Video 3: Optogenetic anogenital stimulation of Mrgprb4-lineage touch neurons in Mrgprb4-Cre; ChR2 female mice, Related to Figure 5. Pulsing blue laser light at 10Hz to the anogenital skin does not trigger any noticeable robust behaviors, or lordosis-like postures.
Data Availability Statement
All data reported in this study will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


