Abstract
Neural processing differences of emotional facial expressions, while common in autism spectrum disorder (ASD), may be related to co-occurring alexithymia and interoceptive processing differences rather than autism per se. Here, we investigate relationships between alexithymia, interoceptive awareness of emotions, and functional connectivity during observation of facial expressions in youth (aged 8–17) with ASD (n = 28) compared to typically developing peers (TD; n = 37). Behaviorally, we found no significant differences between ASD and TD groups in interoceptive awareness of emotions, though alexithymia severity was significantly higher in the ASD group. In the ASD group, increased alexithymia was significantly correlated with lower interoceptive sensation felt during emotion. Using psycho-physiological interaction (PPI) analysis, the ASD group showed higher functional connectivity between the left ventral anterior insula and the left lateral prefrontal cortex than the TD group when viewing facial expressions. Further, alexithymia was associated with reduced left anterior insula-right precuneus connectivity and reduced right dorsal anterior insula-left ventral anterior insula connectivity when viewing facial expressions. In the ASD group, the degree of interoceptive sensation felt during emotion was positively correlated with left ventral anterior insula-right IFG connectivity when viewing facial expressions. However, across all participants, neither alexithymia nor interoceptive awareness of emotions predicted connectivity between emotion-related brain regions when viewing emotional facial expressions. To summarize, we found that in ASD compared to TD: 1) there is stronger connectivity between the insula and lateral prefrontal cortex; and 2) differences in interhemispheric and within left hemisphere connectivity between the insula and other emotion-related brain regions are related to individual differences in interoceptive processing and alexithymia. These results highlight complex relationships between alexithymia, interoception, and brain processing in ASD.
Keywords: autism spectrum disorder, alexithymia, interoception, functional connectivity, facial expressions, empathy
1. Introduction
Many studies on autism spectrum disorder (ASD) have explored potential differences in emotional empathy, the experience of resonating with other people’s emotional experiences (de Waal & Preston, 2017; Davis et al.,1994, Quinde-Zlibut et.al 2021; Fletcher-Watson & Bird 2019; Santiesteban et al 2021). More recently, some authors suggest that potential ASD differences in emotional empathy may be relational rather than due to a specific ASD impairment (e.g., bidirectional emotional empathy difficulties observed between typically developing [TD] and ASD individuals; Morrison et al., 2020; Edey el al., 2016; Milton et al., 2012). Further, it has been posited that emotional empathy difficulties sometimes found in ASD are not necessarily related to an ASD diagnosis, but instead may be attributed to co-occurring alexithymia, difficulty identifying and describing emotions (“alexithymia hypothesis”, Bird & Cook, 2013; Butera et al, 2022), and/or difficulties with interoception, one’s sense of the internal state of their body (“interoception hypothesis”, Quattrocki & Friston, 2014).
Further, some hypothesize that core ASD symptomatology and potential empathy differences could be due to global atypicalities in neural connectivity (Baharmand & Doesburg, 2019; Nair et al., 2020; O’Reilly et al., 2017; Valenti et al., 2020). Neural functional connectivity, a measure of the synchronization of activation among brain regions, is a proxy for the coordination of processing between brain regions. Indeed, there have been numerous papers indicating that ASD (Herbert et al., 2005; Anderson et al., 2011; Pereira et al., 2018; Jou et al., 2011; Fu et al., 2020; Wei et al., 2018; Sha et al., 2022; Floris, 2020) and alexithymia are in part due to aberrant interhemispheric connectivity (Tabibnia & Zaidel, 2005; Zaidel & Kaplan, 2007; Paul, 2021), and/or disconnectivity within a hemisphere (Bernhardt, 2014; Buchanan et al., 1980; Parker et al., 1993; Sifneos, 1988). Here, in ASD and TD groups, we explore how alexithymia and/or interoceptive sensibility may impact neural connectivity of brain regions associated with emotional empathy, when viewing facial expressions. In exploring these issues, we specifically focus on differences in inter- and intra-hemispheric connectivity between brain regions commonly involved in empathic processing.
1.1. Neural Differences in ASD When Processing Emotional Empathy
When viewing emotional facial expressions, a common probe for studying empathy, ASD groups commonly show decreased activity in the IFG compared to TD groups (bilateral/stronger in right IFG: Dapretto et al., 2006; right: Kilroy et al., 2021, Schulte-Rüther et al., 2011, left: Greimel et al., 2010, Klapwijk et al., 2016; Bastiaansen et al., 2011). In addition, in the ASD group, during social vs. nonsocial processing, the right anterior insula is hypoactive (Di Martino et al., 2009) and has atypical functional connectivity with the left fusiform gyrus (Safar et al., 2018). Such aberrant functional connectivity in ASD groups during emotional face processing has also been found between the right amygdala and several key regions, including the left middle temporal gyrus (Monk et al, 2010), the ventromedial prefrontal cortex (PFC; Monk et al, 2010; Swartz et al. 2013), and regions within the left frontal lobe (Hoffman et al., 2015). Thus, within and between hemisphere connectivity differences in ASD during empathy tasks has been found in several emotion-related brain pathways. However, there is debate as to whether these connectivity differences are best attributed to ASD group membership, co-occurring alexithymia, or potential disturbances in interoceptive processing. These possibilities are discussed further below
1.1.1. Alexithymia.
Some have argued that co-occurring alexithymia, rather than ASD group membership, may be important for understanding behavioral and neural differences in ASD (“alexithymia hypothesis”; Bird & Cook, 2013; Bird et al., 2010; Lassalle et al., 2019; Silani et al., 2008; Sivathasan et al., 2020; Zdankiewicz-Ścigała el al., 2021). About 50% of high functioning individuals with ASD have alexithymia (Hill et al., 2004; Kinnaird et al., 2019), while the incidence of alexithymia in the typical population is estimated between 5–10% (Berthoz et al., 2011; Kinnaird et al., 2019). In the neuroimaging literature, several fMRI studies on emotional empathy in ASD support the alexithymia hypothesis. One study found that alexithymia scores and trait empathy scores (combined perspective taking and empathic concern scores) significantly correlated with activity in the bilateral anterior insula and left amygdala in individuals with ASD during an emotion introspection task (as well as less activity in the left PFC, right ACC, precuneus and left temporal lobe; Silani et al., 2008). Further, alexithymia scores both correlated with activity in the left anterior insula during empathy for pain and accounted for differences between TD and ASD groups in trait empathy (Bird et al., 2010). Similarly, Lassalle et al. (2019) found when viewing pictures depicting limbs in painful or disgusting situations, neural differences between TD and ASD groups were no longer significant if alexithymia was controlled for (Lassalle et al., 2019).
Further support for the alexithymia hypothesis comes from structural connectivity studies. Alexithymia (and not an ASD diagnosis) was predictive of reduced covariance of frontal affective networks from the left insula to posterior regions, whereas ASD was predictive of covariance in the so-called theory of mind networks that include the left dorsomedial prefrontal cortex and left temporal-parietal junction (TPJ; Bernhardt et al., 2014). Further, in ASD, diffusivity differences have commonly been found in the cingulum (Travers et al. 2012) and right inferior fronto-occipital fasciculus (IFOF: connects IFG with occipital and temporal regions; Andrews et al., 2019; Roine et al., 2015; Ameis et al., 2015), and a recent study indicated that these diffusivity differences are correlated with alexithymia severity within the ASD group (Kilroy et al, 2022).
To our knowledge, similar task-based functional connectivity studies in ASD looking at the impact of alexithymia have not been performed. However, in TD populations, alexithymia has been studied in resting state functional connectivity (rsFC) and structural connectivity, with discrepant findings between many left and right brain regions, and little consensus between studies (Liemburg et al., 2012; Han et al., 2018; Kim et al., 2019; Fang et al., 2018). Since alexithymia was not measured or accounted for in many of the earlier task-based functional connectivity studies in ASD, a difference in alexithymia severity among participants with ASD could also potentially explain discrepant findings.
Finally, it has been posited that alexithymia may arise as a disconnection between right hemisphere emotion processing and left hemisphere language processing (Tabibnia & Zaidel, 2005; Zaidel & Kaplan, 2007; Hobson et al., 2019). Given that interhemispheric differences may in part underlie ASD symptomatology (Herbert et al 2005; Anderson et al 2011; Pereira et al 2018; Jou et al 2011; Deemyad 2022; Wang et al 2017; Zhu et al 2014; Lee et al 2016), and alexithymia (Goerlich-Dobre et al, 2015), here we explore this disconnectivity theory in the ASD group while considering the impact of alexithymia severity on functional connectivity during empathic processing.
1.1.2. Interoception.
Interoceptive sensibility, one’s aptitude to consciously perceive sensory experiences from the viscera, has also been linked to empathic ability and may influence differences in emotional empathy in ASD (“interoception hypothesis”; Fukushima et al., 2011; Grynberg & Pollatos, 2015). Individuals with ASD may have alterations in self-reported interoceptive sensibility, and interoceptive accuracy (e.g., heart rate tracking; for review DuBois et al., 2016), particularly in childhood and adolescence (Failla et al., 2019; Nicholson et al., 2019). The insula and the amygdala are commonly cited as involved in both interoception (Critchley et al., 2005; Kleckner et al., 2017) and emotion processing (Berthoz et al., 2002; Frewen et al., 2008; Karlsson et al., 2008). The posterior insular cortex is related to the initial perceptual stage of interoceptive stimulus representation, while the anterior insula is involved with the cognitive encoding of an interoceptive stimulus – the feelings and perceptions associated with bodily states (Uddin & Menon 2009). Further, insular activity during cardiac interoception tasks has been associated with social ability scores in adults with ASD (Social Responsiveness Scale-2 [SRS]; Failla et al., 2019).
It has been posited that lack of interoceptive awareness leads to alexithymia, and results in social difficulties (Brewer et al., 2016). In support of this hypothesis, Murphy et al. (2018) found that those with high alexithymic traits relied less on interoceptive cues for behavioral modulation, when controlling for comorbid ASD or anxiety, and studies by Shah and colleagues also indicate that interoceptive deficits should be considered a symptom of alexithymia, rather than ASD (Shah et al., 2016, 2017). However, a recent study showed no evidence for interoceptive accuracy impairments in adults with ASD, whether co-occurring alexithymia was considered (Nicholson et al., 2018).
There is a dearth of research examining functional connectivity or coactivation of regions associated with interoception and empathy in ASD. Only one study demonstrated that relative to a TD group, the ASD group showed increased functional connectivity between the right anterior insula and the dorsal ACC during a resting interoception task (resting state with instruction to focus on one’s breathing pattern; Barttfeld et al., 2012). However, it has not been explored in ASD whether interoception differences impact connectivity during empathic processing.
1.2. Hypotheses:
Here, we explore within and between hemisphere connectivity differences in ASD between neural regions commonly involved in empathic processing (regions of interest [ROIs]: anterior insula, amygdala, IFG, and ACC) and hypothesized that:
When viewing others’ facial expressions, the ASD group would have lower connectivity both between emotion-related ROIs and between ROIs and the prefrontal cortex, as compared to the TD group.
- In accord with the alexithymia and/or interoception hypotheses:
- across participants and within each group (ASD, TD), higher alexithymia and/or lower interoceptive awareness of emotion would significantly correlate with lower functional connectivity between ROIs.
- across all participants, higher alexithymia and/or lower interoceptive awareness of emotion would predict lower connectivity between neural ROIs during facial expression observation above and beyond group membership.
2. Methods
2.1. Participants.
Study participants included youth aged 8–17 (mean 12.04 ± 2.20) who were TD (n = 37, 11 female) or had a diagnosis of ASD (n = 28, 6 female). This was part of a larger brain imaging study, and many of the inclusion criteria (e.g., motor criteria) and methodology were part of that study (Kilroy et al., 2021). As ASD is a neurodevelopmental disorder and brain patterns can change with age (Edde, 2021; Snyder, 2021), we chose to focus on a youth population, in an age range that would result in high quality data from the MRI scan sessions (8–17 years old). A semi-structured interview measure of bodily sensation during emotion experience (BSE) modified from the emBODY tool (Nummenmaa et al., 2014) was collected in a subset of individuals from the total group (n = 25 TD, 23 ASD), as this measure was added later in the study. Participants were recruited through advertisements and contacts in community centers, social media sites, parent group website postings, healthcare clinics, and schools. Inclusion criteria for all groups included: IQ > 80 on at least one composite score (Verbal Comprehension Index [VCI], Perceptual Reasoning Index, Full Scale IQ–2, or the Full-Scale IQ–4) by a trained staff member on the Wechsler Abbreviated Scale of Intelligence 2nd edition (WASI-II; Wechsler, 2011); and English fluency of child and parent. All participants were screened for MRI compatibility, had no history of loss of consciousness greater than five minutes, were right-handed, and were born after 36 weeks of gestation. Each family was informed about study procedures in accordance with the protocol approved by the University of Southern California’s Institutional Review Board. Participants and parents provided their written child assent and parental consent.
TD participants were excluded if they had any psychological or neurological disorder including ADHD and generalized anxiety disorder, determined by parent report. In addition, a neurologist reviewed anatomical scans for potential neurological issues. They were also excluded if they had a first degree relative with an ASD diagnosis or scored above a t-score of 60 on the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012); indicating a risk for ASD symptoms including reduced social awareness, reciprocal social interaction, or presence of restricted or repetitive behaviors. TD participants were excluded if they received a T-score above 65 on the Conners- 3 Parent report (Conners, 2008), indicating a risk for attention deficit and hyperactivity disorder (ADHD); or a score below the 25th percentile on the Movement Assessment Battery for Children (MABC-2; Henderson et al., 2007) or probable DCD based on the Developmental Coordination Disorder Questionnaire (DCDQ; Wilson et al., 2000; Wilson et al., 2009). Many of these exclusion criteria were determined in order to keep the TD group as neurotypical as possible.
Eligible ASD participants had a previous diagnosis through a clinical diagnostic interview or diagnostic assessment, and met criteria on the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2000), the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), or both, administered by a trained researcher.
Individuals in the ASD group were excluded if they had another diagnosis of neurological or psychological disorder except for Developmental Coordination Disorder, attention deficit hyperactivity disorder (ADHD), or generalized anxiety disorder, due to their high prevalence in ASD (Mazzone et al., 2012). Eleven ASD participants had established prescriptions for psychotropic medication at the time of the study (for ADHD and anxiety). Seven were taking stimulants (dose range 1mg −72mg); 4 were taking selective serotonin reuptake inhibitors (dose range 20mg-100mg); 3 were taking antihypertensive medication (dose range 0.1 −1mg); 1 was taking antipsychotic medication (dose 40 mg). None of the TD participants reported any prescription medication use.
2.2. Assessments.
Anxiety:
The Childhood Adolescent Symptom Inventory (CASI-Anx; Sprafkin et al., 2002), a 20-item version of the parent-report, was used to assess symptoms of the six DSM-IV defined anxiety disorders. The test-retest correlation score for CAS-Anx was 0.76 with a Cronbach’s alpha of 0.87. The CASI-Anx was rated on a four-point Likert scale from “never” to “very often.”
Alexithymia Measure.
Alexithymia was measured using the 20-item self-report Alexithymia Questionnaire for Children (AQC; Rieffe et al., 2006). Items were rated on a three-point Likert scale from “not true” to “often true,” and consisted of questions like “I am often confused about the way I am feeling inside.” Two subscales are used to assess alexithymia: difficulty identifying feelings (AQC ID), and difficulty describing and communicating feelings (AQC COMM). For difficulty identifying feelings, difficulty describing feelings, and externally oriented thinking the Cronbach’s alphas were .73, .75, and .29, respectively (Rieffe et al., 2006). The third subscale, externally oriented thinking (AQC EOT) was not used in this study due to an unacceptably low Cronbach’s alpha, and because alexithymia can be reliably assessed in youth without the eight items rating the AQC EOT dimension (Loas et al., 2017). Considering this, the 2 factor total was calculated instead and used as the AQC total in this study.
Bodily Sensation of Emotion (BSE).
To measure interoceptive awareness of emotion specifically, a semi-structured interview using the emBODY tool (Nummenmaa et al., 2014) was administered. This measure was chosen due to previous reports indicating that written self-report questionnaires regarding interoceptive awareness may not map on well to task-based measures of interoception in individuals with ASD (Garfinkel et al., 2016). This interview assessed participants’ ability to retrospectively identify somatotopic patterns of physical feeling associated with each of the six basic emotions (i.e., anger, fear, disgust, happiness, sadness, and surprise). Participants were asked to color (on paper with pencil and on a computer with a mouse) a line drawing of a human body where they commonly felt increasing or decreasing sensation while experiencing different emotions. After the coloring task was completed, a qualitative interview was carried out by a trained staff member probing what types of situations elicit the given emotion, where participants feel that emotion in their body, and what type of sensation occurs within physical localizations. The interview consisted of questions such as: “What kinds of things make you feel afraid?; Can you tell me how you feel when you’re afraid?; Can you show me where in your body you feel fear, and describe that sensation?” Interviews were transcribed and coded by two raters, and a third tie-break rater for use of physical descriptions of felt emotion. All instances describing the body were coded for use of embodied language mentioning a physical sensation (e.g., “I know I am angry because I feel my head getting hot and chest-pounding” received one point as “yes” for using embodied language to describe anger). Points were collapsed across all negative emotions (BSE-neg; 4 possible points, one for each emotion).
2.3. Data Analysis
Pairwise group comparisons between TD and ASD groups were conducted on all measures using two-tailed independent samples t-tests on all measure outcome variables. Pearson partial correlation coefficients were computed to assess relationships between variables in all participants, the ASD group alone, and the TD group alone while controlling for age, gender, and VCI. For all measure analyses, extreme outlier data was removed from participants whose scores on any given measure were more than 3 times the interquartile range from the first and third quartile relative to the entire group. Scatter plots were visually analyzed for the presence of outliers to confirm the validity of correlations.
2.4. fMRI Analysis
2.4.1. fMRI Procedure, Acquisition & Preprocessing.
Functional MRI procedure, task stimuli, fMRI acquisition, and data preprocessing were done following the protocol previously published in Kilroy et al. (2021; see Supplementary Data for details). Prior to scanning, all participants completed a practice session in a mock scanner to prepare for the scanning environment and to ensure minimal head motion. Across all participants, head motion was evaluated using relative framewise displacement (FDJenk; Jenkinson et al., 2002) and participants were excluded if their mean FDJenk exceeded 0.2 mm or if more than one instance of a displacement equal or greater than the voxel size (2.5 mm) occurred while scanning (max mean FDJenk = 0.191 mm; min mean FDJenk = 0.024 mm). There were no significant differences in head motion between the two groups.
Participants were asked to watch videos while in the scanner. In a single 9-minute run, there were 5 blocks of video-stimuli per condition. Blocks consisted of emotional facial expressions (e.g., happy expression), non-emotional facial expressions (e.g., tongue to lip), or bimanual hand actions (e.g., playing the xylophone). The bimanual hand action condition was used for another study (Kilroy et al., 2021) and was not included in the analysis here. Each video was presented for 3.75 seconds followed by a 1.25 second black screen between each stimulus, there were 3 videos per block, and both male and female actors were included in each block. For further details, please see Kilroy et al., 2021.
2.4.2. Functional Connectivity Analysis: Whole Brain.
To investigate group differences in connectivity of emotional empathy regions during facial expression observation trials, psycho-physiological interaction (PPI; McLaren et al., 2012) analyses were performed. The PPI analysis was used to compare the functional correlation of a core region for interoceptive and emotion processing (left ventral anterior insula; see details in ROI selection below) to the rest of the brain during the task. PPI design matrix contained five columns of variables: (1) emotional face observation vs. rest contrast vector; (2) non-emotional face observation vs. rest contrast vector; (3) a time-series of left ventral anterior insula seed region; (4) an interaction variable that represented the interaction of emotional faces contrast vector and left ventral anterior insula seed time course; and (5) an interaction variable that represented the interaction of non-emotional faces contrast vector and left ventral anterior insula seed time course. Data for the seed region were extracted from individual time series from a 6mm spherical region centered on subject-specific peak activation in the left ventral anterior insula. Positive weights on the PPI regressor indicate increased synchrony between the seed region and other regions of the brain during the task periods compared with rest periods.
Experimental stimulus conditions were each modeled with a separate regressor derived from a convolution of the task design and a double gamma function to represent the hemodynamic response, and the temporal derivative of each task regressor was also included as an additional regressor. Subject-specific motion correction parameters were entered as nuisance regressors. Each group was entered into multivariate linear regression models for the task for exploring main effects and between-group comparisons of connectivity. In all whole-brain analyses, mean-centered age, sex and VCI were entered as covariates in the model. Individual participants’ statistical images were entered into the higher level mixed-effects analyses using FSL’s FLAME Stage 1 algorithm. Resulting group level images for all models were thresholded using FSL’s cluster probability algorithm, with a cluster-forming threshold of Z > 3.1, and a cluster size probability threshold of p < .001. To identify PPI during facial observation compared to rest, two stimulus conditions (emotional facial expressions, non-emotional facial expressions) were collapsed into one facial expression condition to determine the connectivity of all stimuli compared to resting baseline. Similar analyses were performed for each individual stimulus condition (emotional facial expressions, non-emotional facial expressions).
2.4.3. Functional Connectivity Analysis: Regions of Interest (ROIs).
ROIs were chosen using the Neurosynth database, which performs automated large-scale meta-analyses of fMRI data. Depending on anatomy, regions consisted of either 4mm (amygdala) or 6mm (all others) spheres drawn around peak activation of the anterior insula, ACC, IFGop, and amygdala in a meta-analysis of 187 studies focusing on empathy-related tasks. ROIs were only used if they overlapped with activation in the Neurosynth meta-analysis, which was not always present in both hemispheres. The left ventral anterior insula was chosen as the seed for the PPI analysis (over the right dorsal anterior insula) due to the overlap observed in that region and the main effect of our facial expression observation task. Percent signal change was extracted from these functionally defined ROIs (Figure 1) using the featquery function in FSL. For all ROI analyses, extreme outlier data was removed from 3 participants in different conditions whose mean percent signal change in the queried ROI was more than 3 times the interquartile range from the first and third quartile relative to the entire group (1 TD participant [emotional face condition and all faces condition, right IFG]; 1 ASD participant [non-emotional faces, right IFG]; 1 ASD participant [non-emotional faces and all faces condition, right AI]). For the ROI analyses, we focused on the trials where participants viewed emotional facial expressions, as most of our hypotheses are related to connectivity differences relating to emotion processing differences. Further, to maximize statistical power, the “observation of all facial expressions” condition was used as it had the highest number of trials.
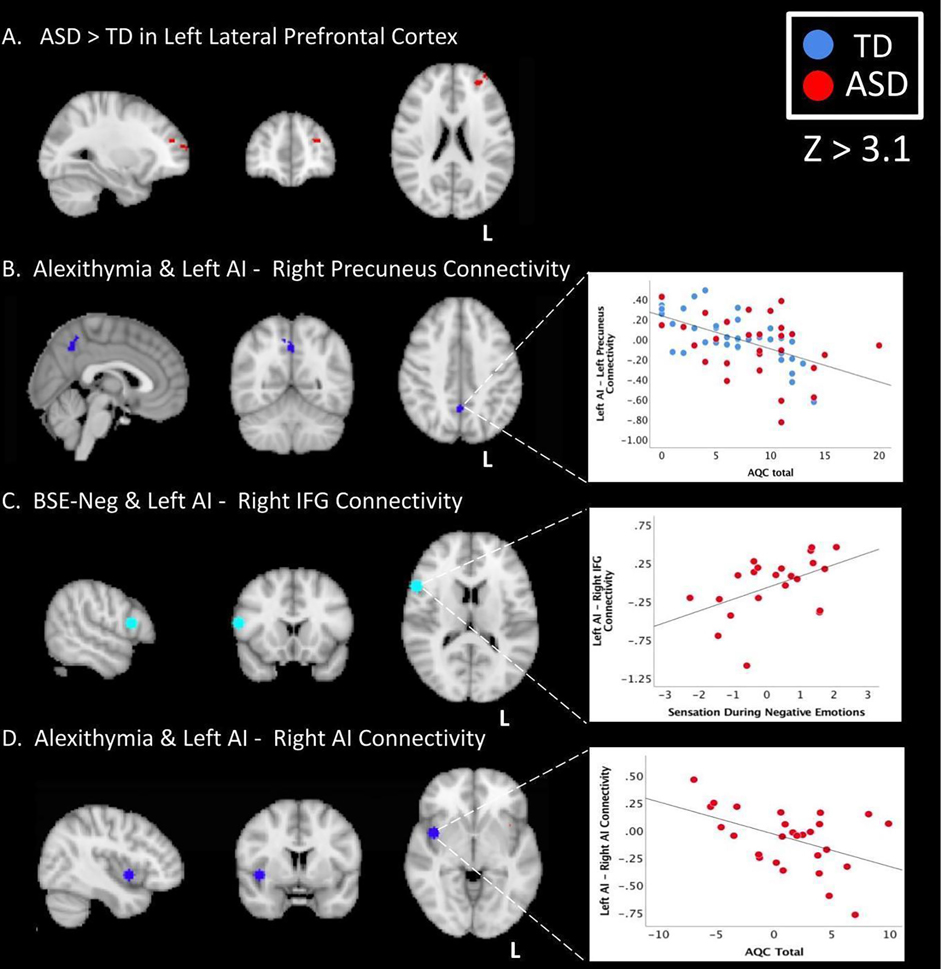
Figure 1: Neural Regions of Interest from Neurosynth Empathy Meta-Analysis Map.
A. Left inferior frontal gyrus pars opercularis (X = 17, Y = 71, Z = 43); B. Left anterior cingulate cortex (X = 49, Y = 75, Z = 50); C. Right dorsal anterior insula (X = 24, Y = 65, Z = 34); D. Left ventral anterior insula (X = 64, Y = 69, Z = 31); E. Right amygdala (X = 31, Y = 61, Z = 23).
2.4.4. ROI Connectivity Group Comparisons.
Group differences in connectivity between TD and ASD groups were assessed using two-tailed independent samples t-tests.
2.4.5. ROI Partial Correlations:
Pearson partial correlation coefficients were computed to assess relationships between measure variables with parameter estimates of ROI connectivity in all participants, the ASD group alone, and the TD group alone, while controlling for age, gender, and the VCI. Scatter plots were visually analyzed for the presence of outliers to confirm the validity of correlations. Additional partial correlations were performed to explore the relative influence of alexithymia and ASD symptomatology on emotional empathy. The impact of AQC total scores on effect size and correlation significance level was observed after controlling for AQC in partial correlation models.
To specifically test the alexithymia and interoception hypotheses in the PPI data – that differences between TD and ASD in emotional empathy processing are actually due to differences in trait alexithymia or interoceptive ability– a hierarchical multiple linear regression model was used across all participants. In order to probe the impact of alexithymia or interoceptive awareness of emotion on ROI activation during emotional face processing (ACC, IFGop, amygdala, anterior insula), nuisance regressors of age, VCI, and sex were entered into the model before interoceptive awareness of emotion or alexithymia, and group. Data were centered and variables were entered into a hierarchical multiple linear regression model in the following order: 1) age, sex, WASI-II: VCI, AQC total, group; 2) age, sex, WASI-II: VCI, BSE-neg, group. The significance of alexithymia or interoceptive awareness of emotion as a predictor was assessed before and after the addition of “group” to the model. Homoscedasticity and normality of residuals were assessed, and the VIF and tolerance statistics were used to assess independent variables for multicollinearity.
3. Results
3.1. Group Differences.
Compared to the TD group, the ASD group had significantly increased social impairment (SRS-2), alexithymia severity (AQC COMM), ADHD symptomatology (Conners-3), and anxiety (CASI-Anx). No significant differences from TD were found in the ASD group for interoceptive awareness of emotion (BSE-neg) or other symptom measures (Table 1).
Table 1.
Descriptive Statistics and Group Comparisons
| Variable | TD | ASD | t | p | ||
|---|---|---|---|---|---|---|
|
|
||||||
| M | SD | M | SD | |||
|
| ||||||
| Age | 11.927 | 2.176 | 12.199 | 2.277 | −0.488 | 0.627 |
| WASI-II FSIQ-4 | 114.570 | 13.020 | 112.820 | 17.036 | 0.469 | 0.641 |
| WASI-II VCI | 115.430 | 11.915 | 110.820 | 17.226 | 1.214 | 0.231 |
| SRS-2 | 45.680 | 5.202 | 75.820 | 9.056 | −15.757 | <.001* |
| Conners-3 | 45.340 | 2.940 | 84.110 | 8.997 | −21.521 | <.001* |
| AQC ID | 0.459 | 0.342 | 0.561 | 0.429 | −1.064 | 0.291 |
| AQC COMM | 0.638 | 0.461 | 0.921 | 0.453 | −2.472 | 0.016* |
| AQC total | 6.405 | 4.219 | 8.536 | 4.623 | −1.934 | 0.058 |
| CASI-Anx | 24.892 | 5.093 | 35.500 | 8.563 | −5.654 | <.001* |
| BSE-neg | 2.583 | 0.974 | 2.818 | 1.259 | −0.711 | 0.481 |
Group differences between TD and ASD groups. WASI-II=Wechsler Abbreviated Scale of Intelligence, Second Edition; FSIQ-4= Full Scale IQ; VCI=Verbal Comprehension Index; SRS-2=Social Responsiveness Scale, Second Edition; Conners-3=Conners 3 Attention Deficit and Hyperactivity Index; AQC ID=Alexithymia Questionnaire for Children Identifying Emotions Subscale; AQC COMM=Alexithymia Questionnaire for Children Communicating Emotions Subscale; AQC total=Alexithymia Questionnaire for Children 2 factor total; CASI-Anx=Childhood Adolescent Symptom Inventory Total; BSE-neg = bodily sensation during negative emotion.
p<.05
3.2. Partial Correlations.
Alexithymia was positively correlated with anxiety across all participants (AQC ID: r = .349, p = .006; AQC COMM: r = .402, p = .001; AQC total: r = .422, p < .001) and within the ASD group (AQC ID: r = .481, p = .020; AQC total: r = .506, p = .014). In the ASD group, sensations felt during emotional experiences was negatively correlated with the AQC total (BSE-neg: r = −.545, p = .016) and AQC ID (BSE-neg: r = −.509, p = .026) indicating that increased alexithymia is related to less interoception during emotional experiences in the ASD group. There were no significant correlations within the TD group. See Table 2 for a full list of correlations.
Table 2:
Measure Correlation R Values.
| Variables | AQC ID | AQC COMM | AQC total | CASI-Anx | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| TD | ASD | TD | ASD | TD | ASD | TD | ASD | |
|
|
||||||||
| 1. AQC ID | ||||||||
| 2. AQC COMM | 0.614*** | 0.547** | ||||||
| 3. AQC total | 0.905*** | 0.921*** | 0.892*** | 0.830*** | ||||
| 4. CASI-Anx | 0.110 | 0.481* | 0.075 | 0.391 | 0.104 | 0.506* | ||
| 5. BSE-neg | 0.358 | −0.509* | 0.351 | −0.443* | 0.401 | −0.545 | 0.041 | −0.249 |
AQC ID = Alexithymia Questionnaire for Children Identifying Emotions Subscale; AQC COMM = Alexithymia Questionnaire for Children Communicating Emotions Subscale; AQC total = Alexithymia Questionnaire for Children 2 factor total; CASI-Anx = Childhood Adolescent Symptom Inventory Total; BSE-neg = bodily sensation during negative emotion.
p < .05
p < .01
p < .001
3.3. Whole Brain Analysis
3.3.1. Between Group Differences.
The ASD group had higher connectivity between the left ventral anterior insula and the left lateral prefrontal cortex than the TD group during observation of non-emotional facial expressions (Z=3.1). No other significant whole brain connectivity differences were found (including TD>ASD differences).
3.3.2. Correlations.
Across all participants, there was a negative correlation between alexithymia severity total score and connectivity between the left anterior insula and the right precuneus during viewing of all facial expressions (Figure 2a). This relationship was also significant in parameter estimates of individual groups (TD: r = −.700, p < .001; ASD: r = −.444, p = .026; Figure 2b–d).
Figure 2. Group Differences and Significant Correlations.
(A.) Whole brain results showing ASD>TD connectivity between the left AI and left lateral prefrontal cortex (X = −28, Y = 47, Z = 21) during non-emotional facial expressions (B.) Negative whole brain correlation with AQC total score and connectivity between the left AI and right precuneus (X = 1, Y = 55, Z = 42) when viewing all facial expressions across all participants (C.) Correlation between left anterior insula and right IFGop (X = 17, Y = 71, Z = 43) connectivity with BSE-neg in the ASD group during emotional facial expressions; (D.) Correlation between AQC total score and connectivity between left anterior insula and right AI (X = 24, Y = 65, Z = 34) in the ASD group during viewing all facial expressions. L= left; AI = anterior insula, IFGop = inferior frontal gyrus pars opercularis; AQC = Alexithymia Questionnaire for Children; BSE-neg = bodily sensation experienced during negative emotions; TD = typically developing; ASD = autism spectrum disorder
3.4. ROI Analyses:
3.4.1. Between Group Differences.
There were no group differences between TD and ASD connectivity between the left ventral anterior insula and any of the predetermined ROIs (left ACC, right dorsal anterior insula, left ventral anterior insula, right IFGop, right amygdala).
3.4.2. Correlations.
Alexithymia Correlations.
In the ASD group, connectivity between right dorsal anterior insula and left ventral anterior insula was negatively correlated with AQC COMM (r = −.510, p = .011), and AQC Total (r = −.499, p = .013; Figure 2) during observation of all facial expressions.
Interoceptive Awareness of Emotion Correlations.
Across all participants and within the ASD group, BSE-neg was positively correlated with connectivity between right IFGop and left ventral anterior insula (All: r = .382, p = .012; ASD: r = .509, p = .026; Figure 2) during observation of emotional facial expressions.
3.4.3. Hierarchical Regressions.
Hierarchical linear regression models were run across all participants to assess whether alexithymia or interoceptive awareness of emotion explained variance in connectivity of ROIs during emotional facial expression observation above and beyond the influence of clinical diagnosis. Connectivity between left ventral anterior insula and any ROI was not significantly predicted by the model including age, sex, WASI-II: VCI, and AQC total, or the model including age, sex, WASI-II: VCI, AQC total, and group in the emotional facial expression fMRI task condition. During the emotional facial expression fMRI task condition, connectivity between left ventral anterior insula and right IFG, right AI, or left ACC was not significantly predicted by the model including age, sex, WASI-II: VCI, and BSE-neg, or the model including age, sex, WASI-II: VCI, BSE-neg, and group. However, the model including age, sex, WASI-II: VCI, and BSE-neg explained 20.4% of the variance in right AI-left AI connectivity. The addition of “group” to this model increased the R2 by .022 and accounted for 22.6% of the variance in right AI-left AI connectivity during emotional facial expressions. However, neither the BSE-neg variable nor the group variable were individually predictive (BSE-neg: p= .557; group p=.252) suggesting that the inclusion of BSE-neg or group does not predict the degree of functional connectivity between the right AI and left AI during observation of emotional expressions. The most significant model of right AI- left AI connectivity during observation of emotional facial expressions included age and sex alone (R2 = .191, p = .005).
4. Discussion.
Our results indicate that during a task commonly used to probe empathy (viewing facial expressions), the ASD group, relative to the TD group, showed higher functional connectivity between the left ventral anterior insula and the left lateral prefrontal cortex.
We also probed how alexithymia severity and interoception ability contributed to connectivity differences between our ROIs both within and across groups. Indeed, both alexithymia and interoception impacted functional connectivity patterns. In the ASD group, more severe alexithymia scores were associated with reduced connectivity between the left ventral anterior insula and: 1) the right precuneus; 2) the right dorsal anterior insula. Thus, in ASD, during empathic processing, increased alexithymia was associated with less connectivity across the two hemispheres. This supports the view that, in ASD, alexithymia impacts functional connectivity patterns between emotion-related brain regions during emotion processing. Further, it specifically supports the view of a potential interhemispheric disconnection syndrome in alexithymia (Tabibnia & Zaidel, 2005; Zaidel & Kaplan, 2007).
We further explored if interoception impacted functional connectivity patterns. We found that in ASD, increased interoceptive awareness of emotion (BSE-neg) was significantly related to increased connectivity between the right IFGop and the left ventral anterior insula.
Taken together, our results indicate that connectivity patterns between neural regions commonly involved in empathic processing are modulated by alexithymia and interoception. However, across all participants, neither alexithymia nor interoceptive awareness of emotion predicted neural connectivity between any of the ROIs during emotional facial expression observation, suggesting that neither the alexithymia nor interoception hypotheses had predictive power for connectivity patterns in this dataset, though future studies are necessary. The implications of these findings are discussed in depth below.
4.1. ASD vs. TD: Increased Connectivity between left ventral AI and left lateral Prefrontal Cortex
Our first aim was to explore ASD vs. TD connectivity differences in emotion processing regions when viewing facial expressions. In whole brain comparisons, the ASD group showed greater functional connectivity than the TD group between the left ventral anterior insula and the left lateral prefrontal cortex (lPFC) when observing non-emotional facial expressions. The lPFC is associated with higher-order planning, attention, task-switching, higher-order relational processing, and value estimation in decision making (Bramson et al., 2020; Hartogsveld et al., 2018; Rushworth et al., 2011). The lateral PFC has also consistently been related to emotion regulation and reappraisal, or the intentional alteration of an initial emotional response (Buhle et al., 2014; Ochsner et al., 2002; Ochsner & Gross, 2005; Ochsner et al., 2012; Wager et al., 2008). Previous studies indicate that ASD individuals attending to faces have increased activation relative to TD participants within multiple prefrontal cortex areas (Herrington et al., 2014). Additionally, individuals with ASD often experience difficulties with emotion regulation (Mazefsky et al., 2014) and can experience heightened emotional reactivity (e.g., tantrums, anxiety, and personal distress to others’ emotions; Northrup et al., 2020). Coupled with data indicating that children with ASD are less accurate at recognizing mild affective facial expressions compared to TD peers (Wong et al., 2012), connectivity between the left prefrontal and the left anterior insula in the ASD group may indicate that individuals with ASD recruit higher order regions like the lPFC to reappraise or regulate negative emotional responses elicited by less emotional facial expressions. This finding could suggest that our ASD sample is using different brain regions than our TD sample in order to respond more adaptively, and in a more emotionally regulated way, when processing ambiguous facial expressions.
4.2. Alexithymia
Our next question of interest was whether alexithymia impacts functional connectivity patterns during empathic processing, both within and across groups. We found alexithymia was related to functional connectivity during face processing in two separate analyses. First, across all participants and within groups, alexithymia scores were related to decreased connectivity between the left ventral AI and the right precuneus when viewing facial expressions. This indicates that alexithymia severity is related to decreased functional connectivity between the left ventral AI and the right precuneus, a region involved in exteroceptive and interoceptive bodily sensations (Araujo et al., 2015), self-monitoring during affect decoding (Harms et al., 2010), as well as affective mentalization (Takahashi et al., 2015; Simões et al., 2018; Stoica et al., 2020). Second, we found that connectivity between the left ventral anterior insula and right dorsal anterior insula is related to alexithymia in the ASD group when viewing emotional facial expressions. The left anterior insula - right anterior insula relationship was particularly driven by the communicating emotions subscale of the AQC.
Taken together, these results suggest that the degree of alexithymia is related to interhemispheric crosstalk between the left and right hemisphere regions important for emotional, sensory, and interoceptive processing. Indeed, previous work has posited that alexithymia may be a result of a disconnection between right hemisphere emotion processing and left hemisphere linguistic processing (Miller, 1986; Tabibnia & Zaidel, 2005; Zaidel & Kaplan, 2007), and supporting this notion, reduced white matter diffusivity has previously been found in the corpus callosum in ASD compared to TD (Andrews et al., 2019; Barnea-Goraly et al., 2010; Cheon et al., 2011; Jou et al., 2011; Kumar et al., 2010; Noriuchi et al., 2010; Shukla et al., 2011). Further, findings from individuals with callosal agenesis indicate that the corpus callosum plays a crucial role in transferring emotional information between hemispheres, with the left hemisphere potentially serving as an interpreter of affective states (Paul et al, 2021). To our knowledge, this is the first study probing the role of alexithymia in a task-based connectivity study in ASD and thus may allow for a better understanding of how alexithymia interacts with ASD. Our results indicate that in ASD, alexithymia severity is associated with functional connectivity patterns during an empathy task.
4.3. Interoceptive Awareness of Emotion
Additionally, we tested whether interoceptive processing ability impacted functional connectivity patterns during an empathy task, either across or within groups. While behaviorally we found no significant group differences for interoceptive awareness of emotion, for the ASD group, greater interoceptive awareness in the emBody interview was associated with increased connectivity between the left ventral anterior insula and the right IFGop when viewing emotional facial expressions. The anterior insula is known to integrate information from the viscera for interoceptive and affective processing (Critchley et al. 2005, Kleckner et al., 2017), while the IFGop is commonly active when viewing emotional facial actions (Dapretto et al., 2006; Kilroy et al., 2021) and also involved in affective behavior and empathic understanding (Sorella et al., 2021; Hobson et al., 2018; Rota et al., 2009; Li et al., 2020). Additionally, both regions have been associated with reductions in neural activation during emotional face processing in individuals with ASD (Dapretto et al., 2006; Kilroy et al., 2021, Di Martino et al., 2009). Thus, in the ASD group, the relationship between increased left AI-right IFG functional connectivity and greater awareness of bodily sensations may suggest that adaptive bodily awareness for processing one’s own emotions could index adaptive neural connectivity during processing of other’s emotions. Thus, similar to the alexithymia findings above, our data support the hypothesis that in ASD, functional connectivity patterns in our empathy-related ROIs are impacted by interoceptive ability.
4.4. Hierarchical Regression Analysis: Primary Predicters of Connectivity
The correlational analyses discussed previously indicated that interoception, alexithymia, and autism severity impact functional connectivity during an empathy task in ASD. To test the predictive impact of these factors on connectivity, we conducted a hierarchical regression analysis. This analysis suggested that neither alexithymia, interoceptive awareness of emotion, nor group membership are primary predictors of connectivity between any of our ROIs during this task. Thus, we did not find support for the notion that alexithymia or interoceptive awareness predict connectivity differences as the alexithymia and interoception theories would suggest. Further, the regression analyses also do not support the idea that group membership plays a predictive role in determining connectivity patterns in emotion-related ROIs – only sex and age were good predicters of connectivity patterns. Taken together, our results indicate that while alexithymia and interoception modulate connectivity between our ROIs during an empathy task, they do not predict connectivity strength between the ROIs. Future studies with increased sample sizes are needed for further exploration of the latter issue (see Limitations).
4.5. Limitations and Future Directions
The PPI approach only has the power to demonstrate effects that cannot be explained by the task or the seed time course. This may be why here we did not observe many group differences in connectivity between TD and ASD participants, or relationships between behavior and ROI connectivity. Additionally, this method is designed to probe context-related changes in connectivity, and the passive face processing task used here may have been too subtle to elicit task-based connectivity effects. Further, PPI has a general weakness of only allowing analysis of one seed region of interest in each model, unlike other functional network connectivity models which allow for more flexibility in resulting network information, and potentially unexpected overlapping networks (Xu et al., 2013). While the seed region used in the PPI analysis (left anterior insula) was hypothesis driven, it is still a limiting factor for understanding the comprehensive group differences and functional connectivity relationships that may exist outside this specific node investigated. In addition, PPI can only expose task-dependent changes in ROI connectivity and cannot determine causal relationships between regions like other methods (i.e., dynamic causal modeling; Friston et al., 2003); thus, we cannot determine the directionality of which regions are predominantly influencing others in the network. Lastly, we acknowledge that our sample size is small for running correlations between neural data and outcome measures, thus caution should be used in interpreting correlational results (Marek et al., 2022), especially regarding null results.
Second, individuals diagnosed with ASD consist of a very heterogeneous population; the relatively small sample size and homogeneity of the participants in this sample (all right-handed, IQ > 80, etc.), make generalization to the spectrum of ASD limited. As some of the main variables of interest in the study can only be measured in individuals who are verbal, future studies should further investigate the influence of VCI on the relationships reported here. Further, we did not control for ADHD in the current study; given that about 85% of individuals with ASD commonly have ADHD symptoms (Harkins et al. 2022), future studies may benefit from controlling for ADHD or comparing to an ADHD group or other deficits that are common comorbidities with ASD (e.g., anxiety).
Lastly, the assessment measures utilized in the study have limitations. The AQC was used as a gold standard tool for measuring alexithymia in youth, however, like the TAS-20 (Bagby et al., 1994), this questionnaire captures more cognitive aspects of alexithymia including identifying emotions and communicating emotions, but does not measure additional emotionalizing aspects (i.e., the degree to which a person is inclined to experience feelings and to become emotionally aroused regardless of if they can label, differentiate, and describe them or not). The latter should be further explored in ASD. Furthermore, the measures used to capture alexithymia, as well as many of the other measures utilized here, are self-report measures. Accurate self-report can be particularly challenging for individuals with ASD who may have difficulty with metacognition and self-referential insight. Future studies may consider examining these questions in a larger and more heterogeneous sample of ASD participants and employing more observational and standardized measures when possible. Additionally, future studies should include more multidimensional aspects of emotionalizing and fantasy in alexithymia as well as measures of attention regulation, body listening, and body trusting in interoceptive sensibility. Further tests of interoceptive accuracy, awareness, and sensibility should be developed and employed in this population due to the quantitative limitations of the qualitative interview data.
6. Conclusions.
Here we show that the ASD group shows increased functional connectivity between the ventral anterior insula and lateral prefrontal cortex compared to the TD group during an empathy task. Such connectivity differences may relate to emotion regulation differences common to individuals with ASD. We further show that during emotion processing, within the ASD group, functional connectivity patterns in neural regions involved with empathic processing are modulated by alexithymia severity and interoception ability. In particular, the connectivity differences mark the importance of cross hemispheric emotion processing for alexithymia (supporting the disconnectivity hypothesis of alexithymia; Zaidel & Kaplan, 2007; Tabibnia & Zaidel, 2005). However, the hierarchical regression analysis found that neither group membership, alexithymia severity, nor interoceptive ability predict connectivity differences. In fact, only sex and age were good predicters of connectivity patterns in our ROIs. These mixed results indicate that while interoception and alexithymia may modulate connectivity patterns in ASD during an empathy task, more studies with increased sample sizes and heterogeneity may be necessary to further explore these issues.
Supplementary Material
Highlights:
Individuals with autism spectrum disorder (ASD), compared to typically developing (TD) participants, showed greater functional connectivity between the left ventral anterior insula and the left lateral prefrontal cortex when observing facial expressions
In the ASD group, alexithymia severity and reduced interoceptive awareness modulated functional connectivity, with significantly reduced interhemispheric connectivity when viewing facial expressions
Across all participants, neither alexithymia nor interoceptive awareness of emotions predicted functional connectivity between emotion-related brain regions when viewing emotional facial expressions
This data calls attention to the complex relationship between autism, alexithymia, interoception, and brain connectivity
Acknowledgements.
We thank all our participants and their families. We also thank all our prior and current lab members for their contributions in participant recruitment, data collection, scoring, and manuscript formatting, in particular, Trinh Nguyen. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079432. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support came from the Department of Defense through the Idea Development Award under award number AR170062. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Credit Author Statement:
Project Conceptualization: C.B., L.A.Z.; Data Acquisition and Methodology: C.B., E.K., L.H., A.J., F.L.; Data Analysis: C.B., J.K.; Manuscript Write-Up: C.B., L.A.Z., F.L.; Manuscript Editing: C.B., J.K., E.K., L.H., A.J., F.L., L.A.Z.; Figures and Tables: C.B.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameis SH, & Catani M (2015). Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex; a journal devoted to the study of the nervous system and behavior, 62, 158–181. 10.1016/j.cortex.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Anderson JS; Druzal TJ; Froehlich A & Lainhart JE (2011); Decreased Interhemispheric Functional Connectivity in Autism. Cerebral Cortex, (21):1134–1146. 10.1093/cercor/bhq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DS, Lee JK, Solomon M, Rogers SJ, Amaral DG, & Nordahl CW (2019). A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. Journal of Neurodevelopmental Disorders, 11(1), 32. 10.1186/s11689-019-9291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Abe O, Nippashi Y, & Yamasue H (2013). Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: A meta-analysis of diffusion tensor imaging tractography studies. Molecular Autism, 4(1), 25–25. 10.1186/2040-2392-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo HF, Kaplan J, Damasio H, & Damasio A (2015). Neural correlates of different self domains. Brain and Behavior, 5(12), e00409. 10.1002/brb3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, & Taylor GJ (1994). The twenty-item Toronto Alexithymia scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- Baharmand P, & Doesburg S (2019). A Review of Altered Neurophysiology and Connectivity of the Brain in Autism Spectrum Disorder and its Impact on Common Symptoms | Simon Fraser University Science Undergraduate Research Journal. Simon Fraser University Science Undergraduate Research Journal, 4, 47–60. [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, & Reiss AL (2010). Similar white matter aberrations in children with autism and their unaffected siblings: A diffusion tensor imaging study using tract-based spatial statistics. Archives of General Psychiatry, 67(10), 1052–1060. 10.1001/archgenpsychiatry.2010.123 [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Leiguarda R, & Sigman M (2012). State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia, 50(14), 3653–3662. 10.1016/j.neuropsychologia.2012.09.047 [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, & Keysers C (2011). Age-Related Increase in Inferior Frontal Gyrus Activity and Social Functioning in Autism Spectrum Disorder. Biological Psychiatry, 69(9), 832–838. 10.1016/j.biopsych.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Valk SL, Silani G, Bird G, Frith U, & Singer T (2014). Selective Disruption of Sociocognitive Structural Brain Networks in Autism and Alexithymia. Cerebral Cortex, 24(12), 3258–3267. 10.1093/cercor/bht182 [DOI] [PubMed] [Google Scholar]
- Berthoz S, Blair R j. r., Le Clec’h G, & Martinot J-L (2002). Emotions: From neuropsychology to functional imaging. International Journal of Psychology, 37(4), 193–203. [Google Scholar]
- Berthoz S, Pouga L, & Wessa M (2011, September 12). Alexithymia from the Social Neuroscience Perspective. The Oxford Handbook of Social Neuroscience. 10.1093/oxfordhb/9780195342161.013.0060 [DOI] [Google Scholar]
- Bird G, & Cook R (2013). Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Translational Psychiatry, 3(7), e285. 10.1038/tp.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, & Singer T (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain, 133(5), 1515–1525. 10.1093/brain/awq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Cook R, & Bird G (2016). Alexithymia: A general deficit of interoception. Open Science, 3(10), 150664. 10.1098/rsos.150664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan DC, Waterhouse GJ, West SC, (1980): A Proposed Neurophysiological Basis of Alexithymia. Psychother Psychosom 1980;34:248–255. doi: 10.1159/000287465 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wage TD, Lopez R, Onyemekwu C, Kober H, Webe J, & Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex (New York, N.Y. 1991), 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera CD, Harrison L, Kilroy E, Jayashankar A, Shipkova M, Pruyser A, & Aziz-Zadeh L (2022). Relationships between alexithymia, interoception, and emotional empathy in autism spectrum disorder. Autism, 136236132211113. 10.1177/13623613221111310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon K-A, Kim Y-S, Oh S-H, Park S-Y, Yoon H-W, Herrington J, Nair A, Koh Y-J, Jang D-P, Kim Y-B, Leventhal BL, Cho Z-H, Castellanos FX, & Schultz RT (2011). Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Research, 1417, 77–86. 10.1016/j.brainres.2011.08.020 [DOI] [PubMed] [Google Scholar]
- Conners CK (2008). Conners 3rd edition: Manual (Vol. 14). Toronto, Ontario, Canada: Multi-Health Systems. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale: SRS-2. Western Psychological Services. [Google Scholar]
- Critchley HD (2005). Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology, 493(1), 154–166. 10.1002/cne.20749 [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, & Iacoboni M (2006). Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9(1), 28–30. 10.1038/nn1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44, 113126. [Google Scholar]
- Davis MH, Luce C, & Kraus SJ (1994). The Heritability of Characteristics Associated with Dispositional Empathy. Journal of Personality, 62(3), 369–391. 10.1111/j.1467-6494.1994.tb00302.x [DOI] [PubMed] [Google Scholar]
- de Waal FBM, & Preston SD (2017). Mammalian empathy: Behavioural manifestations and neural basis. Nature Reviews Neuroscience, 18(8), 498–509. 10.1038/nrn.2017.72 [DOI] [PubMed] [Google Scholar]
- Deemyad T (2022). Lateralized Changes in Language Associated Auditory and Somatosensory Cortices in Autism. Frontiers in Systems Neuroscience.16:787448. 10.3389/fnsys.2022.787448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, & Milham MP (2009). Functional Brain Correlates of Social and Non-Social Processes in Autism Spectrum Disorders: An ALE Meta-Analysis. Biological Psychiatry, 65(1), 63–74. 10.1016/j.biopsych.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Azeez A, Li X, Haque E, Biswal B (2018). Disrupted focal white matter integrity in autism spectrum disorder: A voxel-based meta-analysis of diffusion tensor imaging studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 82, 242–248. 10.1016/j.pnpbp.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois D, Ameis SH, Lai M-C, Casanova MF, & Desarkar P (2016). Interoception in Autism Spectrum Disorder: A review. International Journal of Developmental Neuroscience, 52, 104–111. 10.1016/j.ijdevneu.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Edde M, Leroux G, Altena E, & Chanraud S (2021). Functional brain connectivity changes across the human life span: From fetal development to old age. Journal of Neuroscience Research, 99(1), 236–262. 10.1002/jnr.24669 [DOI] [PubMed] [Google Scholar]
- Edey R, Cook J, Brewer R, Johnson MH, Bird G, & Press C (2016). Interaction takes two: Typical adults exhibit mind-blindness towards those with autism spectrum disorder. Journal of Abnormal Psychology, 125(7), 879–885. 10.1037/abn0000199 [DOI] [PubMed] [Google Scholar]
- Failla MD, Bryant LK, Heflin BH, Mash LE, Schauder K, Davis S, Gerdes MB, Weitlauf A, Rogers BP, & Cascio CJ (2019). Neural correlates of cardiac interoceptive accuracy across development: Implications for social symptoms in autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 13(6), 908–920. 10.1002/aur.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Li M, Mei M, Sun X, & Han D (2018). Characteristics of brain functional and structural connectivity in alexithymic students. Neuropsychiatric Disease and Treatment, 14, 2609–2615. 10.2147/NDT.S174015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-Watson S, & Bird G (2020). Autism and empathy: What are the real links? Autism, 24(1), 3–6. 10.1177/1362361319883506 [DOI] [PubMed] [Google Scholar]
- Floris DL, Wolfers T, Zabihi M,Beckmann CF, & the EU-AIMS Longitudinal European Autism Project Group. (2020) Atypical Brain Asymmetry in Autism - A Candidate for Clinically Meaning Stratification. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 6:802–812. 10.1016/j.bpsc.2020.08.008 [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA, Dozois DJA, Neufeld RWJ, Pain C, Hopper JW, Densmore M, & Stevens TK (2008). Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Journal of Abnormal Psychology, 117(1), 171–181. http://dx.doi.org.libproxy2.usc.edu/10.1037/0021-843X.117.1.171 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, & Penny W (2003). Dynamic causal modelling. NeuroImage, 19(4), 1273–1302. 10.1016/S1053-8119(03)00202-7 [DOI] [PubMed] [Google Scholar]
- Fu L, Wang Y, Fang H, Xiao X, Xiao T, Li Y, Li C, Wu Q, Chu K, Xiao C, & Ke X (2020). Longitudinal Study of Brain Asymmetries in Autism and Developmental Delays Aged 2–5 Years. Neuroscience, 432, 137–149. 10.1016/j.neuroscience.2020.02.028 [DOI] [PubMed] [Google Scholar]
- Fukushima H, Terasawa Y, & Umeda S (2011). Association between interoception and empathy: Evidence from heartbeat-evoked brain potential. International Journal of Psychophysiology, 79(2), 259–265. 10.1016/j.ijpsycho.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, & Critchley HD (2016). Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biological Psychology, 114, 117–126. 10.1016/j.biopsycho.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre KS, Votinov M, Habel U, Pripfl J, & Lamm C (2015). Neuroanatomical profiles of alexithymia dimensions and subtypes: Structural Correlates of Alexithymia Subtypes. Human Brain Mapping, 36(10), 3805–3818. 10.1002/hbm.22879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Schulte-Rüther M, Kircher T, Kamp-Becker I, Remschmidt H, Fink GR, Herpertz-Dahlmann B, & Konrad K (2010). Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. NeuroImage, 49(1), 1055–1065. 10.1016/j.neuroimage.2009.07.057 [DOI] [PubMed] [Google Scholar]
- Grynberg D, & Pollatos O (2015). Perceiving one’s body shapes empathy. Physiology & Behavior, 140, 54–60. 10.1016/j.physbeh.2014.12.026 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Zürcher NR, Rogier O, Hippolyte L, Lemonnier E, Ruest T, Ward N, Lassalle A, Gillberg N, Billstedt E, Helles A, Gillberg C, Solomon P, Prkachin KM, & Gillberg C (2014). Emotional contagion for pain is intact in autism spectrum disorders. Translational Psychiatry, 4(1), e343. 10.1038/tp.2013.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Li M, Mei M, & Sun X (2018). The functional and structural characteristics of the emotion network in alexithymia. Neuropsychiatric Disease and Treatment, 14, 991–998. 10.2147/NDT.S154601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins CM, Handen BL, & Mazurek MO (2022). The Impact of the Comorbidity of ASD and ADHD on Social Impairment. Journal of Autism and Developmental Disorders, 52(6), 2512–2522. 10.1007/s10803-021-05150-1 [DOI] [PubMed] [Google Scholar]
- Harms M, Martin A, & Wallace GL (2010). Facial Emotion Recognition in Autism Spectrum Disorders: A Review of Behavioral and Neuroimaging Studies. Neuropsychology Review, 20(3), 290–322. 10.1007/s11065-010-9138-6 [DOI] [PubMed] [Google Scholar]
- Hartogsveld B, Bramson B, Vijayakumar S, van Campen AD, Marques JP, Roelofs K, Toni I, Bekkering H, & Mars RB (2018). Lateral frontal pole and relational processing: Activation patterns and connectivity profile. Behavioural Brain Research, 355, 2–11. 10.1016/j.bbr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden D, & Barnett AL (2007). Movement Assessment Battery for Children-2 [Database record]. APA PsycTests. [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK & Caviness VS Jr (2005).Brain asymmetries in autism and developmental language disorders: a nested whole-brain analysis. Brain,129.213–226. [DOI] [PubMed] [Google Scholar]
- Herrington J, Riley ME, Grupe DW, & Schultz RT (2014). Successful Face Recognition is Associated with Increased Prefrontal Cortex Activation in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(4), 902–910. 10.1007/s10803-014-2233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Berthoz S, & Frith U (2004). Brief Report: Cognitive Processing of Own Emotions in Individuals with Autistic Spectrum Disorder and in Their Relatives. Journal of Autism and Developmental Disorders, 34(2), 229–235. 10.1023/B:JADD.0000022613.41399.14 [DOI] [PubMed] [Google Scholar]
- Hobson H, Brewer R,Catmur C, Bird G (2019). The role of language in alexithymia: Moving toward a multi-route model of alexithymia. Emotion Review.11(3):247–261. 10.1177/1754073919838528 [DOI] [Google Scholar]
- Hobson H, Hogeveen J,Brewer R, Catmur C, Gordon B, Krueger F, Chau A, Bird G, Grafman J(2018).Language and alexithymia: Evidence for the role of the inferior frontal gyrus in acquired alexithymia.Neuropsychologia.111:229–240. 10.1016/j.neuropsychologia.2017.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Brück C, Kreifelts B, Ethofer T, & Wildgruber D (2015). P8. Social brain network and autism spectrum disorder: Reduced connectivity to the frontal cortex. Clinical Neurophysiology, 126(8), e91–e92. 10.1016/j.clinph.2015.04.130 [DOI] [PubMed] [Google Scholar]
- Jones AP, Happé FGE, Gilbert F, Burnett S, & Viding E (2010). Feeling, caring, knowing: Different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry, 51(11), 1188–1197. 10.1111/j.1469-7610.2010.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, & Pelphrey KA (2011). Structural Neural Phenotype of Autism: Preliminary Evidence from a Diffusion Tensor Imaging Study Using Tract-Based Spatial Statistics. American Journal of Neuroradiology, 32(9), 1607–1613. 10.3174/ajnr.A2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JT, & Iacoboni M (2006). Getting a grip on other minds: Mirror neurons, intention understanding, and cognitive empathy. Social Neuroscience, 1(3–4), 175–183. 10.1080/17470910600985605 [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, & Stenman H (2008). Cortical activation in alexithymia as a response to emotional stimuli. The British Journal of Psychiatry, 192(1), 32–38. 10.1192/bjp.bp.106.034728 [DOI] [PubMed] [Google Scholar]
- Kilroy E, Harrison L, Butera C, Jayashankar A, Cermak S, Kaplan J, Williams M, Haranin E, Bookheimer S, Dapretto M, & Aziz-Zadeh L (2021). Unique deficit in embodied simulation in autism: An fMRI study comparing autism and developmental coordination disorder. Human Brain Mapping, 42(5), 1532–1546. 10.1002/hbm.25312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy E, Gerbella M, Cao L, Molfese P, Butera C, Harrison L, Jayashankar A, Rizzolatti G, Aziz-Zadeh L (2022) Specific tractography differences in autism as compared to developmental coordination disorder. Sci Rep 12, 19246 10.1038/s41598-022-21538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Park I, Lee YJ, Jeon S, Kim S, Lee KH, Park J, Kim H-K, Gwaq AR, Jun JY, Yoo SY, Lee SH, & Kim SJ (2019). Alexithymia and frontal–amygdala functional connectivity in North Korean refugees. Psychological Medicine, 50(2), 334–341. 10.1017/S0033291719000175 [DOI] [PubMed] [Google Scholar]
- Kinnaird E, Stewart C, & Tchanturia K (2019). Investigating alexithymia in autism: A systematic review and meta-analysis. European Psychiatry, 55, 80–89. 10.1016/j.eurpsy.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, & Chugani DC (2010). Alterations in Frontal Lobe Tracts and Corpus Callosum in Young Children with Autism Spectrum Disorder. Cerebral Cortex, 20(9), 2103–2113. 10.1093/cercor/bhp278 [DOI] [PubMed] [Google Scholar]
- Lassalle A, Zürcher NR, Porro CA, Benuzzi F, Hippolyte L, Lemonnier E, Johnels JÅ, & Hadjikhani N (2019). Influence of anxiety and alexithymia on brain activations associated with the perception of others’ pain in autism. Social Neuroscience, 14(3), 359–377. 10.1080/17470919.2018.1468358 [DOI] [PubMed] [Google Scholar]
- Lee JM, Kyeong S, Kim E & Cheon K-A. (2016) Abnormalities of Inter- and Intra-Hemispheric Functional Connectivity in Autism Spectrum Disorders: A Study Using the Autism Brain Imaging Data Exchange Database. Frontier in Neuroscience.10:191. 10.3389/fnins.2016.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li W, Zhang T, Zhang J, Jin Z, Li L (2020). Probing the role of the right inferior frontal gyrus during Pain-Related empathy processing: Evidence from fMRI and TMS. Hum Brain Mapp. 2021 Apr 1;42(5):1518–1531. doi: 10.1002/hbm.25310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg EJ, Swart M, Bruggeman R, Kortekaas R, Knegtering H, Ćurčić-Blake B, & Aleman A (2012). Altered resting state connectivity of the default mode network in alexithymia. Social Cognitive and Affective Neuroscience, 7(6), 660–666. 10.1093/scan/nss048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Braun S, Delhaye M, & Linkowski P (2017). The measurement of alexithymia in children and adolescents: Psychometric properties of the Alexithymia Questionnaire for Children and the twenty-item Toronto Alexithymia Scale in different non-clinical and clinical samples of children and adolescents. PLOS ONE, 12(5), e0177982. 10.1371/journal.pone.0177982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders, 30, 205–223. 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, Malone SM, Kandala S, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, … Dosenbach NUF (2022). Reproducible brain-wide association studies require thousands of individuals. Nature (London), 603(7902), 654–660. 10.1038/s41586-022-04492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazefsky CA, & White SW (2014). Emotion Regulation. Concepts & Practice in Autism Spectrum Disorder. Child and Adolescent Psychiatric Clinics of North America, 23(1), 15–24. 10.1016/j.chc.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Ruta L, & Reale L (2012). Psychiatric comorbidities in asperger syndrome and high functioning autism: Diagnostic challenges. Annals of General Psychiatry, 11(1), 16. 10.1186/1744-859X-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L (1986). Is alexithymia a disconnection syndrome? A neuropsychological perspective. International Journal of Psychiatry in Medicine, 16(3), 199–209. 10.2190/dae0-ewpx-r7d6-lfny [DOI] [PubMed] [Google Scholar]
- Milton DEM (2012). On the ontological status of autism: The ‘double empathy problem.’ Disability & Society, 27(6), 883–887. 10.1080/09687599.2012.710008 [DOI] [Google Scholar]
- Monk CS, Weng S-J, Wiggins JL, Kurapati N, Louro HMC, Carrasco M, Maslowsky J, Risi S, & Lord C (2010). Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry & Neuroscience : JPN, 35(2), 105–114. 10.1503/jpn.090085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, DeBrabander KM, Jones DR, Faso DJ, Ackerman RA, & Sasson NJ (2020). Outcomes of real-world social interaction for autistic adults paired with autistic compared to typically developing partners. Autism, 24(5), 1067–1080. 10.1177/1362361319892701 [DOI] [PubMed] [Google Scholar]
- Murphy J, Catmur C, & Bird G (2018). Alexithymia is associated with a multidomain, multidimensional failure of interoception: Evidence from novel tests. Journal of Experimental Psychology. General, 147(3), 398–408. 10.1037/xge0000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Jolliffe M, Lograsso YSS, & Bearden CE (2020). A Review of Default Mode Network Connectivity and Its Association with Social Cognition in Adolescents With Autism Spectrum Disorder and Early-Onset Psychosis. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Williams DM, Grainger C, Christensen JF, Calvo-Merino B, & Gaigg SB (2018). Interoceptive impairments do not lie at the heart of autism or alexithymia. Journal of Abnormal Psychology, 127(6), 612–622. 10.1037/abn0000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T, Williams D, Carpenter K, & Kallitsounaki A (2019). Interoception is Impaired in Children, But Not Adults, with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 49(9), 3625–3637. 10.1007/s10803-019-04079-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, & Kamio Y (2010). Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Research, 1362, 141–149. 10.1016/j.brainres.2010.09.051 [DOI] [PubMed] [Google Scholar]
- Northrup JB, Goodwin M, Montrenes J, Vezzoli J, Golt J, Peura CB, Siegel M, & Mazefsky C (2020). Observed emotional reactivity in response to frustration tasks in psychiatrically hospitalized youth with autism spectrum disorder. Autism : the international journal of research and practice, 24(4), 968–982. 10.1177/1362361320908108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Glerean E, Hari R, & Hietanen JK (2014). Bodily maps of emotions. Proceedings of the National Academy of Sciences, 111(2), 646–651. 10.1073/pnas.1321664111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BFM, Jones EJH, Crawley D, Charman T, Buitelaar J, Tillmann J, Murphy DG, Loth, The EU-AIMS LEAP Group (2020). Alexithymia in autism: cross-sectional and longitudinal associations with social-communication difficulties, anxiety and depression symptoms. Psychological Medicine 1–13. 10.1017/S0033291720003244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly C, Lewis JD, & Elsabbagh M (2017). Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE, 12(5), e0175870. 10.1371/journal.pone.0175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, & Johansen-Berg H (2012). Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7(5), 604–609. 10.1093/scan/nss055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Pazienza SR& Brown WS (2021) Alexithymia and somatization in agenesis of the corpus callosum. Social Cognitive and Affective Neuroscience.16(10):1071–1078. 10.1093/scan/nsab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AM, Campos BM, Coan AC & Cendes F (2018).Differences in Cortical Structures and Functional MRI Connectivity in High Functioning Autism. Frontier in Neurology, 9:539. 10.3389/fneur.2018.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki E, & Friston K (2014). Autism, oxytocin and interoception. Neuroscience & Biobehavioral Reviews, 47, 410–430. 10.1016/j.neubiorev.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinde-Zlibut JM, Williams ZJ, Gerdes M, Mash LE, Heflin BH, & Cascio C (2021). Multifaceted empathy differences in children and adults with autism. Scientific Reports, 11(1), 19503. 10.1038/s41598-021-98516-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieffe C, Oosterveld P, & Terwogt MM (2006). An alexithymia questionnaire for children: Factorial and concurrent validation results. Personality and Individual Differences, 40(1), 123–133. [Google Scholar]
- Roine U, Salmi J, Roine T et al. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Molecular Autism 6, 4 (2015). 10.1186/2040-2392-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N (2009). Self-Regulation of Regional Cortical Activity Using Real-Time fMRI: The Right Inferior Frontal Gyrus and Linguistic Processing. Human Brain Mapping.30:1605–1614. 10.1002/hbm.20621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, & Behrens TE (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–1069. 10.1016/j.neuron.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Safar K, Wong SM, Leung RC, Dunkley BT, & Taylor MJ (2018). Increased Functional Connectivity During Emotional Face Processing in Children With Autism Spectrum Disorder. Frontiers in Human Neuroscience, 12, 408–408. 10.3389/fnhum.2018.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban I, Gibbard C, Drucks H, Clayton N, Banissy MJ, & Bird G (2021). Individuals with Autism Share Others’ Emotions: Evidence from the Continuous Affective Rating and Empathic Responses (CARER) Task. Journal of Autism and Developmental Disorders, 51(2), 391–404. 10.1007/s10803-020-04535-y [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, & Piefke M (2011). Dysfunctions in brain networks supporting empathy: An fMRI study in adults with autism spectrum disorders. Social Neuroscience, 6(1), 1–21. 10.1080/17470911003708032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, van Rooij D, Anagnostou E et al. (2022) Subtly altered topological asymmetry of brain structural covariance networks in autism spectrum disorder across 43 datasets from the ENIGMA consortium. Mol Psychiatry 10.1038/s41380-022-01452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Catmur C, & Bird G (2016). Emotional decision-making in autism spectrum disorder: The roles of interoception and alexithymia. Molecular Autism, 7(1), 43. 10.1186/s13229-016-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Catmur C, & Bird G (2017). From heart to mind: Linking interoception, emotion, and theory of mind. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 93, 220–223. 10.1016/j.cortex.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, & Müller R-A (2011). Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia, 49(5), 1378–1382. 10.1016/j.neuropsychologia.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifneos PE (1988). Alexithymia and its relationship to hemispheric specialization, affect, and creativity. Psychiatric Clinics of North America, 11(3), 287–292. [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, & Frith U (2008). Levels of emotional awareness and autism: An fMRI study. Social Neuroscience, 3(2), 97–112. 10.1080/17470910701577020 [DOI] [PubMed] [Google Scholar]
- Simões M, Monteiro R, Andrade J, Mouga S, França F, Oliveira G, Carvalho P, Castelo-Branco M(2018). A Novel Biomarker of Compensatory recruitment of face emotional Imagery networks in Autism Spectrum Disorder. Frontiers in Neuroscience.12:791. 10.3389/fnins.2018.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivathasan S, Fernandes TP, Burack JA, & Quintin E-M (2020). Emotion processing and autism spectrum disorder: A review of the relative contributions of alexithymia and verbal IQ. Research in Autism Spectrum Disorders, 77, 101608. 10.1016/j.rasd.2020.101608 [DOI] [Google Scholar]
- Smith A (2009). The Empathy Imbalance Hypothesis of Autism: A Theoretical Approach to Cognitive and Emotional Empathy in Autistic Development. The Psychological Record, 59(3), 489–510. 10.1007/BF03395675 [DOI] [Google Scholar]
- Snyder W, Uddin LQ, & Nomi JS (2021). Dynamic functional connectivity profile of the salience network across the life span. Human Brain Mapping, 42(14), 4740–4749. 10.1002/hbm.25581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorella S, Grecucci A,Piretti L,Job R(2021) Do anger perception and the experience of anger share common neural mechanism? Coordinate-based meta-analytic evidence of similar and different mechanisms from functional neuroimaging studies.NeuroImage. 15: 117777. 10.1016/j.neuroimage.2021.117777 [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Salisbury H, Schneider J, & Loney J (2002). Further evidence of reliability and validity of the Child Symptom Inventory-4: Parent checklist in clinically referred boys. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 31(4), 513–524. 10.1207/S15374424JCCP3104_10 [DOI] [PubMed] [Google Scholar]
- Stoica T, & Depue B (2020). Shared Characteristics of Intrinsic Connectivity Networks Underlying Interoceptive Awareness and Empathy. Frontiers in Human Neuroscience, 14. 10.3389/fnhum.2020.571070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, & Monk CS (2013). Amygdala Habituation and Prefrontal Functional Connectivity in Youth With Autism Spectrum Disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52(1), 84–93. 10.1016/j.jaac.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, & Zaidel E (2005). Alexithymia, Interhemispheric Transfer, and Right Hemispheric Specialization: A Critical Review. Psychotherapy and Psychosomatics, 74(2), 81–92. 10.1159/000083166 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kitada R, Sasaki AT, Kawamichi H, Okazaki S, Kochiyama T, Sadato N (2015). Brain Networks of affective mentalizing revealed by the tear effect. The integrative role of the medial prefrontal cortex and precuneus. Neuroscience Research. 101:32–43. 10.1016/j.neures.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, & Alexander AL (2012). Diffusion Tensor Imaging in Autism Spectrum Disorder: A Review: Diffusion tensor imaging. Autism Research, 5(5), 289–313. 10.1002/aur.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, & Menon V (2009). The anterior insula in autism: Under-connected and under-examined. Neuroscience & Biobehavioral Reviews, 33(8), 1198–1203. 10.1016/j.neubiorev.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti M, Pino MC, Mazza M, Panzarino G, Di Paolantonio C, & Verrotti A (2020). Abnormal Structural and Functional Connectivity of the Corpus Callosum in Autism Spectrum Disorders: A Review. Review Journal of Autism and Developmental Disorders, 7(1), 46–62. 10.1007/s40489-019-00176-9 [DOI] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fu K, Chen L, Duan X,Guo X,Chen H, Wu Q, Xia W, Wu L, & Chen H (2017). Increased Gray Matter Volume and Resting-State Functional Connectivity in Somatosensory Cortex and their Relationship with Autistic Symptoms in Young Boys with Autism Spectrum Disorder.Frontiers in Physiology.8:588. 10.3389/fphys.2017.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- Wei L, Zhong S, Nie S, & Gong G (2018). Aberrant development of the asymmetry between hemispheric brain white matter networks in autism spectrum disorder. European Neuropsychopharmacology, 28(1), 48–62. 10.1016/j.euroneuro.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, & Kaplan BJ, (2009). Psychometric Properties of the Revised Developmental Coordination Disorder Questionnaire, Physical & Occupational Therapy In Pediatrics, 29:2, 182–202, DOI: 10.1080/01942630902784761 [DOI] [PubMed] [Google Scholar]
- Wilson BN, Kaplan BJ, Crawford SG, Campbell A, Dewey D, (2000). Reliability and Validity of a Parent Questionnaire on Childhood Motor Skills. Am J Occup Ther September/October 2000, Vol. 54(5), 484–493. 10.5014/ajot.54.5.484 [DOI] [PubMed] [Google Scholar]
- Wong N, Beidel DC, Sarver DE, & Sims V (2012). Facial Emotion Recognition in Children with High Functioning Autism and Children with Social Phobia. Child Psychiatry and Human Development, 43(5), 775–794. 10.1007/s10578-012-0296-z [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang S, Calhoun VD, Monterosso J, Li C-SR, Worhunsky PD, Stevens M, Pearlson GD, & Potenza MN (2013). Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. NeuroImage, 79, 62–71. 10.1016/j.neuroimage.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdankiewicz-Ścigała E, Ścigała D, Sikora J, Kwaterniak W, Longobardi C (2021) PLoS ONE. Relationship between interoceptive sensibility and somatoform disorders in adults with autism spectrum traits. The mediating role of alexithymia and emotional dysregulation. 16(8): e0255460 10.1371/journal.pone.0255460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel E, & Kaplan J (2007). Chapter 10 - The Cross-Cultural Brain. In Consciousness and Cognition (pp. 139–147). Elsevier Ltd. 10.1016/B978-012373734-2/50011-X [DOI] [Google Scholar]
- Zhu H, Fan Y Guo H, Huang D, He S (2014). Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomedical Optics Express.1261. 5(4)1262–1274. 10.1364/BOE.5.001262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.