Abstract
Δ9-tetrahydrocannabinol (THC) and its sibling, cannabidiol (CBD), are produced by the same Cannabis plant and have similar chemical structures but differ dramatically in their mechanisms of action and effects on brain functions. Both THC and CBD exhibit promising therapeutic properties, however, impairments and increased incidence of mental health diseases are associated with acute and chronic THC use, respectively, and significant side effects are associated with chronic use of high dose CBD. This review covers recent molecular and preclinical discoveries concerning the distinct mechanisms of action and bioactivities of THC and CBD, and their impact on human behavior and diseases. These discoveries provide a foundation for the development of cannabinoid-based therapeutics for multiple devastating diseases and assure their safe use in the growing legal market of Cannabis-based products.
In brief:
This review by Nephi Stella discusses the recent molecular and preclinical breakthroughs made in our understanding of THC’s and CBD’s influence on human behaviors and neuropathological processes, and the key results that are fostering the development of transformative cannabinoid-based therapies.
1]. Introduction
The Cannabis plant synthesizes phytocannabinoids (phyto-CB), including the bioactive compounds THC and CBD (Figure 1). THC is often referred to as the principal “psychotropic” compound produced by Cannabis and CBD as the principal “non-psychotropic” compound. Since both compounds influence brain functions, this simple distinction is not valid, and this review addresses the effects of both. The recent legalization of medical and adult use of products that contain phyto-CBs by several countries, including the US, Canada, and the Netherlands, represents a historical shift in policy that was led by patient advocates who use Cannabis products medicinally, and by Cannabis industry advocates. Both groups sought to encourage a legal market that could improve well-being and reduce use of other harmful substances (for example opioids)1,2. A milestone in the acceptance of CBD as a therapeutic modality was its FDA approval in the treatment of refractory epilepsies. Recent studies suggest promising therapeutic indications for other neurologic diseases where CBD also modulates neuronal function.
Figure 1: Cannabinoid compounds.
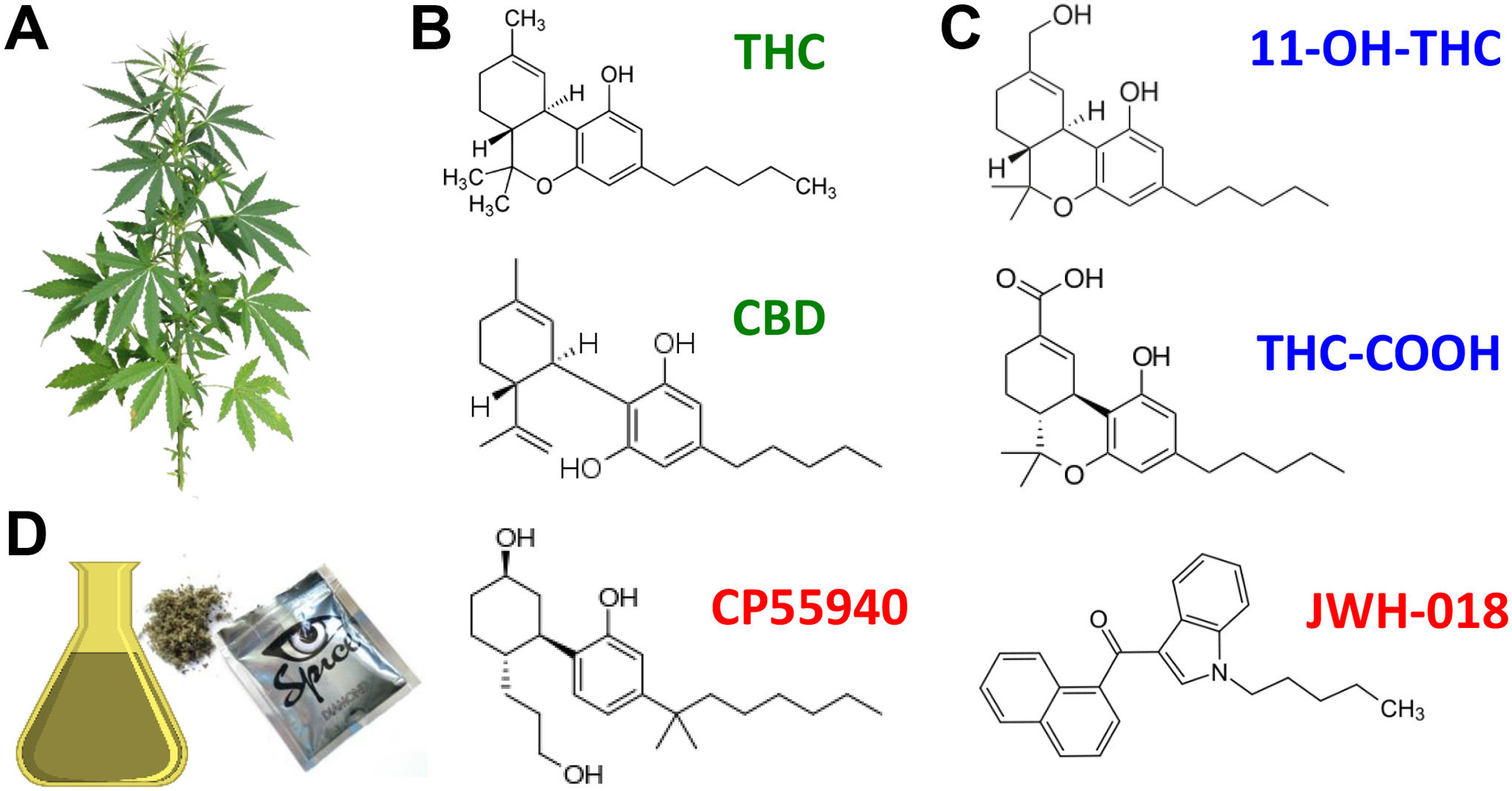
A] Cannabis plant. B] Phytocannabinoids THC and CBD (green) produced by the plant, C] THC metabolites 11-OH-THC and THC-COOH (blue) produced by P450 enzymes expressed in liver and brain and D] artificial cannabinoids, CP55940 and JWH-018 (active ingredient in the illegal market product, “Spice”) (red).
Concomitantly, public health professionals have expressed concerns about the increasing acceptance of a substance with significant side-effects, especially THC-associated mental health diseases1,3. This concern is heightened by the escalation in THC concentrations found in Cannabis plants attributable to increased understanding of Cannabis genetics, improved cultivation methods, and optimization of extraction yields (Box 1)4. Taking Washington state between October 2014 and September 2016 as an example, the average levels of THC in extracts reached 75–80%, which is 3 fold more than Cannabis flowers (20.6%) during the same period5,6. Furthermore, while traditional Cannabis flowers still accounted for most of the sales in Washington State at that time (i.e., 66.6%), the market share of THC extracts for inhalation increased by 150%5. Public health professionals in the US have also noted the increasing perception of THC-based products as “safe” recreational drugs, particularly in 16% of individuals ages 12–17 and in 52% of individuals ages 18–25 years (NIDA, News release in 8/22/22). These alarming statistics indicate increased use of products that contain high levels of THC, a scenario that may have important health consequences, particularly on brain function and behavior in vulnerable individuals such as adolescents.
Box 1. Inheritance of cannabinoid compounds synthesized by the plant.
THC concentration in plant: Best explained by both additive and dominance composite genetic effects
CBD concentration in plant: Best explained by cytoplasmic genomes and additive genes225,330.
Enzymes that synthesize THC and CBD: single-nucleotide polymorphisms in the coding regions determine plant chemotype225.
Current understanding: Both genetic effects and cytogenetic contributions influence the THC:CBD ratios produced by the Cannabis plant330.
Outcome: Facilitated effective crop breeding to select cultivars that produce defined amounts of THC and CBD.
The research summarized in this review is discussed in the context of the known differences in the pharmacodynamic (PD) and pharmacokinetic (PK) profiles of THC and CBD. The body of work reviewed provides a foundation for translating research into therapies for a variety of diseases and conditions.
2]. Pharmacokinetics of THC: Differences among routes of administration
Humans administer THC-containing products by smoking, vaporizing and dabbing (a slang term that refers to the process of consuming THC products that come in a variety of textures, including resin); as well as by drinking and eating6,7. When measuring THC bioactivity, studies need to indicate 1) the route of administrations, 2) how much THC was administered, and 3) the regimen of THC use (e.g., times a day, a week, or a month). Below is our current understanding of the PK profile of THC in humans and rodents, accounting for those three factors. The PK profile of a drug depends on four key physiological events: absorption, distribution, metabolism, and excretion, typically referred to as ADME. Thus, our understanding of how the drug’s concentration changes in selected tissues over time combined with knowledge of how that might correlate with the drug’s activity at selected protein targets (i.e., its PD) provides a clearer picture of its in vivo bioactivity.
2.1]. THC PK in humans.
Inhalation of Cannabis-based cigarettes has historically been the most common route of THC administration by humans. It leads to rapid THC absorption that peaks within minutes in blood and is not subject to first pass liver metabolism8. Considering that Cannabis-based cigarette currently average ≈20% of THC/total weight and that a cigarette weigh ≈1 g, then a full cigarette contains ≈200 mg THC6. Thus, a human adult of ≈70 Kg who smokes a 1/4 of a cigarette absorbs ≈0.3 mg/kg of THC (considering ≈50% pyrolytic loss because of burning the product). Human PK studies show that one inhalation of THC (0.3 mg/kg) results in vastly variable levels of THC in blood within the initial minutes, depending on the individual: 1.6–160 ng/ml of THC (≈ 5–500 nM)9. The hydrophobic nature of THC allows its distribution into most tissues, including the brain10. Thus, THC passes the blood brain barrier, briefly accumulates in brain parenchyma, and then slowly declines because of THC metabolism and excretion. THC undergoes extensive liver metabolism, and its clearance is restricted by fatty acid binding proteins11. Of note, while over 80 metabolites of THC have been identified, only a handful remain bioactive, including THC’s first metabolite 11-OH-THC (more potent than Δ9-THC) and 11-nor-Δ9-THC-9-carboxylic acid (less potent than Δ9-THC)12.
Humans are increasingly administering THC through eatables and drinks. In contrast to inhalation, oral THC absorption is slower and maximum THC concentrations are reached 1–3 h after dosing13. For example, oral consumption of 15 mg THC (≈200 μg/kg) results in ≈9 ng of THC/mL of blood (≈ 30 nM) after 3h14. Considering the current average amount of THC in edibles (10 mg/serving), its oral consumption by a human adult will result in ≈20 nM of THC in blood after 3 h. Orally administered THC and its bioactive metabolites decline slowly (half-life 20–60 h for THC), suggesting prolonged exposure and lingering bioactivity even after a single ingestion14. Thus, the bioactivity of THC fluctuates depending on its route of administration, rate and extent of absorption, metabolism, and excretion, all of which can significantly vary between individuals (Figure 2A–B)10.
Figure 2: THC metabolism, PK, bioactivity and regiments.
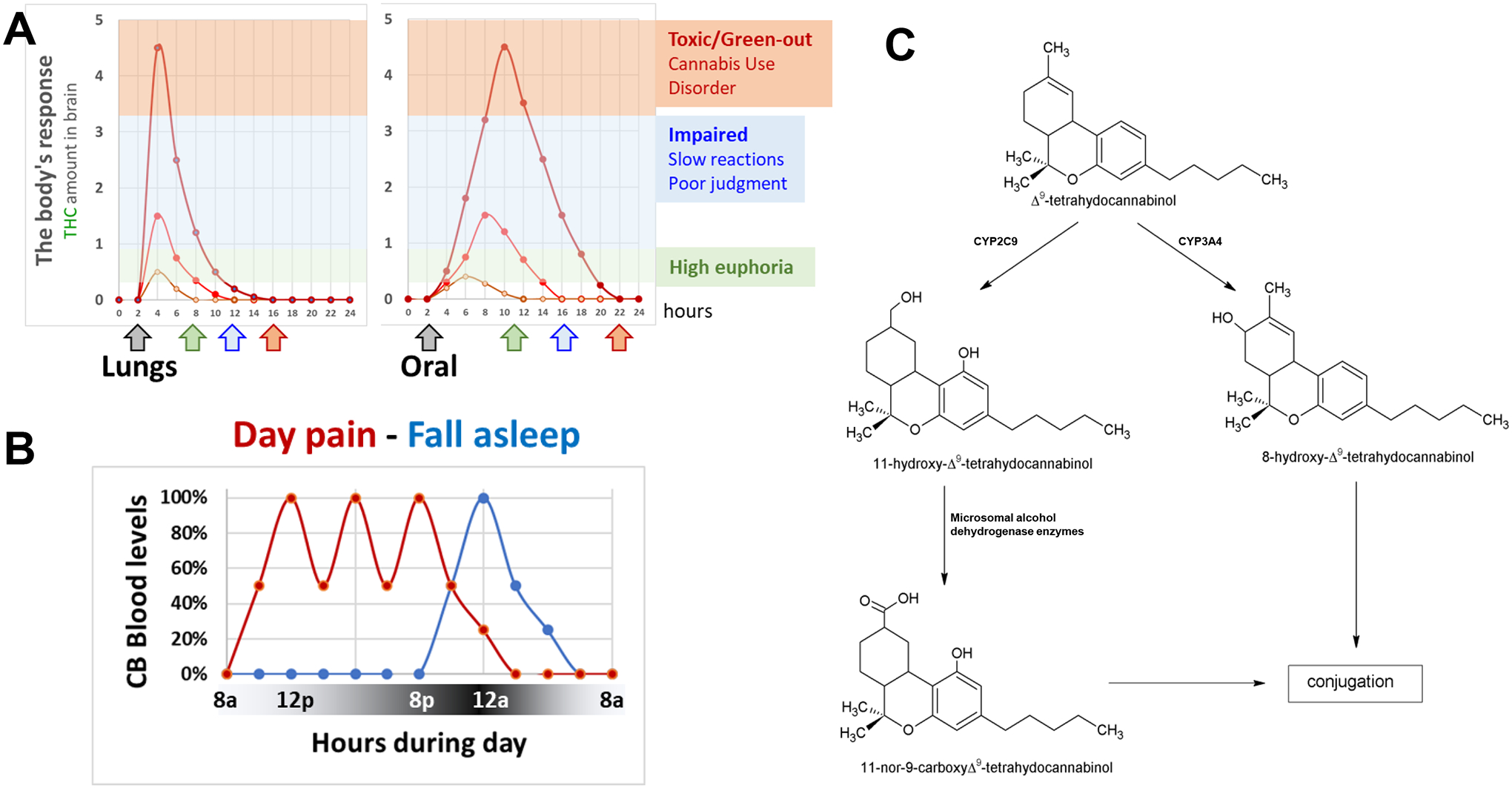
A] Model examples of the PK profiles of THC when inhaled (lungs) or consumed (oral). Examples of resulting behavioral responses associated with low dose THC producing the “high”/euphoria (green arrow), higher dose THC that impairs behavior such as reaction time and judgment (blue arrow), and even higher dose THC (red arrow) that might lead to Cannabis Use Disorder in vulnerable populations, and can be toxic and associated with physiological and psychological panic attack (commonly referred to as “to green-out”; symptoms include sweating, nausea, heart palpitations, hypervigilance, paranoia, and the fear or feeling that you might be dying or about to die). B] Model examples of the PK profile of THC when used to treat pain during the day using several administrations (red line) or to fall asleep with one administration (blue line). A] THC is either metabolized by CYP2C9 that produces 11-OH-THC, which is then metabolized by microsomal alcohol dehydrogenase, or CYP3A4 that produces 8-OH-THC. Conjugation represents the next metabolic step.
2.2]. THC PK in rodents.
As a preclinical model system for PK studies, rodents provide important information but also have key limitations, including a very active liver metabolism compared to humans. The PK and bioactivity of THC in rodents has been studied when administered by i.p., i.v. and s.c. injections, as well as by smoke exposure and oral gavage, routes of administration that allow for precise dosing yet are not used by humans7,15,16. For example, i.p. injections into adult mice with THC (5 mg/kg) result in 600 pmol/ml of THC (≈ 2 nM) in blood within 15–30 min and 1000 pmol/g of THC in brain (≈3 nM) after 2h17,18. However, it is important to emphasize that a portion of the THC injected via i.p. will undergo first pass liver metabolism and thus not all the injected THC will reach the brain. Obvious limitations of injections, smoke exposure and oral gavage are the absence of self-administration and the mild stress response by rodents from administration procedures.
Oral self-administration of THC by rodents is easily achieved when formulating THC in either gelatin or dough, and consumption results in THC brain concentrations that are bioactive19–21. For example, consumption of 2–3 mg THC-gelatin during 1h results in 2–4 ng/ml THC in blood (≈ 6–12 nM)19. Of note, rodents self-administer less gelatin containing high dose THC compared to control-gelatin, suggesting an avoidance of the taste/odor of THC19. Inhalation of THC by rodents involves either burning Cannabis cigarettes or vaporizing THC extract in an airtight environment similar to an operant chamber 18,22,23. THC vapor self-administration by rats is achieved by training them to nose poke for the vapor, which leads to increased motivation to take more THC vapor24. For example, 30 min inhalation exposure of 25 mg/ml results in 40 pmol/ml (≈ 30 nM) in blood that then rapidly decays24.
THC is metabolized by CYP2C3 and CYP3A4, P450 enzymes expressed in liver and brain tissues, which produce similar metabolites in rodents and humans (Figures 2C and 3A)10. When reaching the brain, THC rapidly crosses the blood brain barrier via ABC transporters P-glycoprotein and breast cancer resistance protein25–27. A recent study showed an association between an ABCB1 polymorphism in humans and increased THC metabolism and frequency of use; yet there was no difference in the self-reported subjective high associated with smoking THC (12.5% in a cigarette) suggesting a nonlinear relation between THC metabolism and subjective high28. In summary, the development of experimental approaches that better recapitulate human use of THC-containing products, combined with our growing understanding of the molecular mechanisms that control the overall PK profile of THC in rodents, provide initial mechanistic understanding of the link between the PK profile of THC and its bioactivity.
Figure 3: THC inactivation by CYP2C and biased signaling at CB1R.
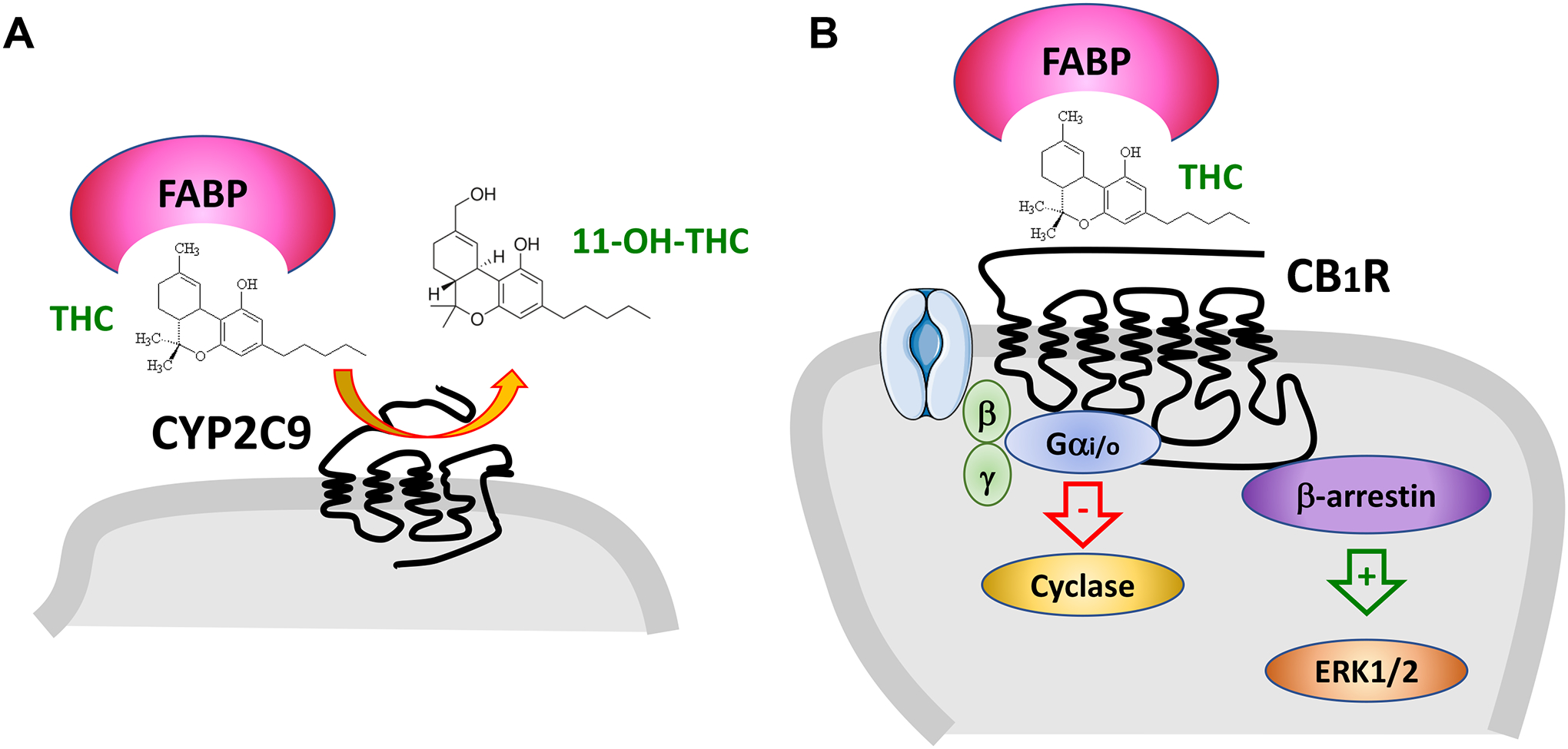
A] THC is metabolized to 11-OH-THC by the P450 enzyme CYP2C9. Fatty acid binding proteins (FABP) assist THC in being metabolized. B] THC activates CB1R that couple to Gαi proteins that inhibit adenylyl cyclase, Gbg proteins that regulate ion channels and b-arrestin that regulates kinase signaling. Different agonist will often preferentially modulate one of these signaling pathway. FABPassist THC in activating CB1R.
3]. THC pharmacodynamics: Modulation of multiple targets
THC modulates that activity of multiple G protein-coupled receptors (GPCRs) and ion channels as shown by cell culture studies, and most of these targets mediate THC’s bioactivity in vivo as demonstrated by fully blocking THC’s bioactivity in rodent model systems using genetic deletion and/or antagonism. In this section, emphasis is on the concentrations of THC required to modulate these targets, especially concentrations of THC in the brain.
3.1]. Cannabinoid receptor 1 (CB1R).
This GPCR was the first receptor shown to be modulated by THC and is the target that THC activates with the highest potency (≈30 nM) (Figure 4A)29. CB1R mediates THC’s psychotropic effects in humans as shown with the CB1R antagonist, SR14161730. CB1R is involved in cell division and neuronal pathfinding during development and remains expressed by most neuronal and glial cell subtypes in the adult brain, although at remarkably different expression levels depending on the cell type. For example, CB1R is expressed at high levels by GABAergic neurons, at intermediate levels by glutamatergic neurons, and at low levels by microglia31. This receptor signals through Gi/o and β-arrestin, and sometimes through Gs in neurons, microglia, and oligodendrocytes, and through Gq in astrocytes (Figure 3B)32,33. However, coupling of CB1R to different signaling mechanisms may vary with overexpression in heterologous systems34. CB1R interacts with cytosolic CRIP1a and BiP proteins, and this interaction controls signaling biased toward second messenger pathways in a neuron population-selective manner35,36. THC activates CB1R as a partial agonist and modulates neuronal functions by reducing presynaptic neurotransmitter release (e.g., glutamate, GABA and acetylcholine), inducing synaptic plasticity and adjusting energy metabolism and cell phenotype through changes in gene expression37. CB1R expressed by astrocytes, oligodendrocytes and microglia controls energy metabolism and cell phenotype38–40. Thus, THC influences the function of most neuronal and glial cells in the brain by modulating their CB1R cell-specific signaling.
Figure 4: Molecular targets modulated by THC.
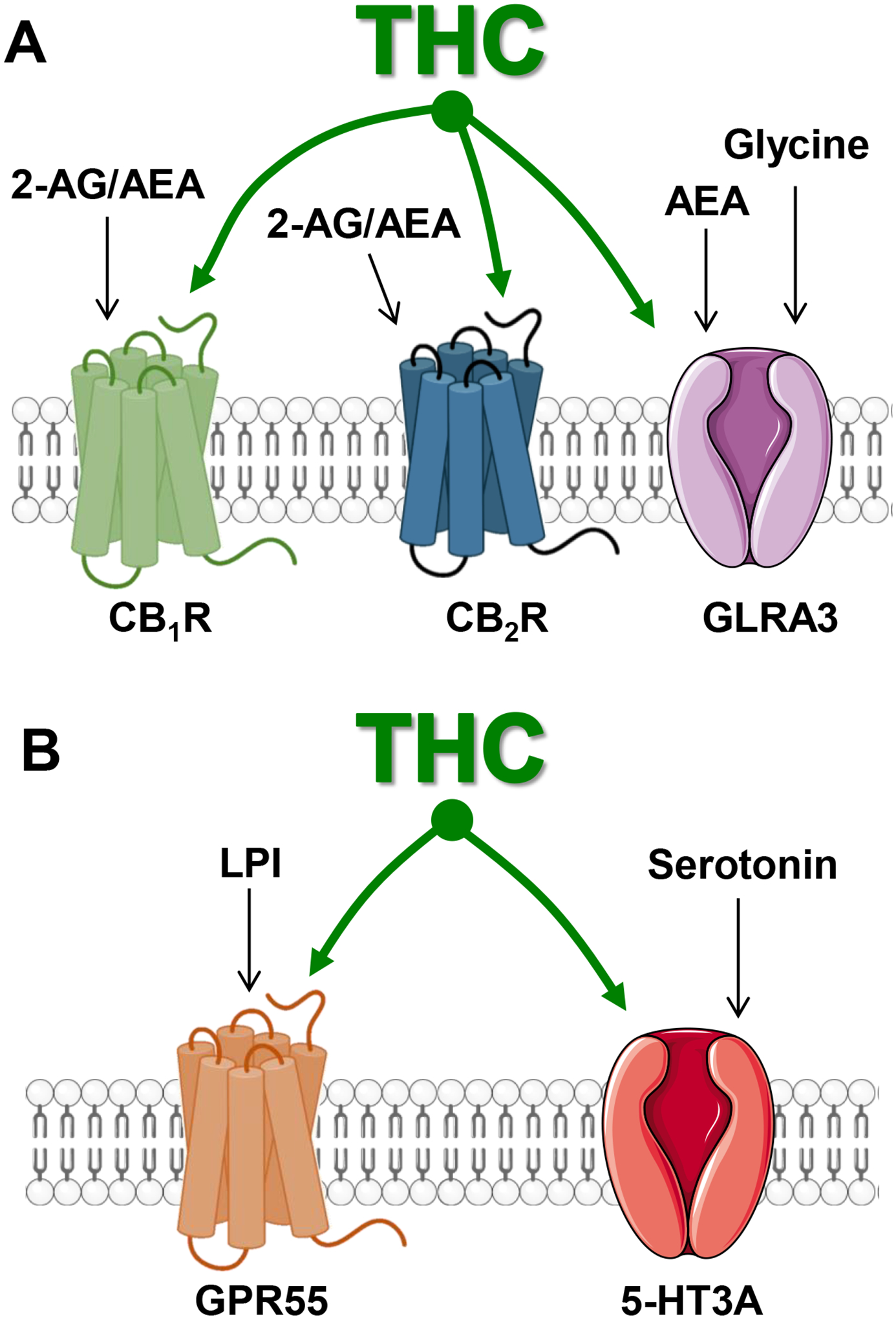
A] THC modulates CB1R and CB2R that are endogenously activated by 2-AG and AEA, and GLRA3 receptors that are endogenously activated by glycine, as demonstrated by in vivo genetic and antagonist experiments. B] THC modulates GPR55 that is endogenously activated by lysophosphatidyl inositol (LPI) and 5-HT3A receptors that are endogenously activated by serotonin, as suggested by cell culture experiments.
3.2]. Cannabinoid receptor 2 (CB2R).
CB2R is expressed at low levels in the healthy brain and predominantly by endothelial cells forming the blood brain barrier41,42. Its expression is greatly upregulated under select pathological states, (e.g., activated microglia in the mouse model of experimental autoimmune encephalomyelitis)43. While both genetic and pharmacological evidence indicate that CB2R directly regulates neuronal functions, its expression by neurons remains inconclusive because of the absence of reagents such as selective antibodies44. CB2R signals through Gi/o and β-arrestin in microglia and oligodendrocytes, and sometimes through Gq in endothelial cells38,45. Thus, THC’s bioactivity mediated by CB2R includes glial cell activation and endothelial cell function, though it is undetermined whether modulation of neurons is direct or indirect through these cell types (Figure 4A).
3.3]. Endocannabinoid-dependent modulation of CB1R/CB2R.
CB1R and CB2R are endogenously activated by two signaling lipids, anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), that are produced and inactivated by neuronal and glial cells46 (Figure 5). These endocannabinoids (eCB) are produced on-demand in response to select stimuli that increase cellular activity (typically by increasing the calcium-dependent activity of membrane-associated lipases)47. AEA is mainly produced by N-acetylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD), whereas 2-AG is mainly produced by diacyl glycerol lipase (DAGL)48. Thus, AEA and 2-AG are released from plasma membranes and act in an autocrine and paracrine manner to modulate CB1R/CR2R signaling.
Figure 5: Endocannabinoid signaling.
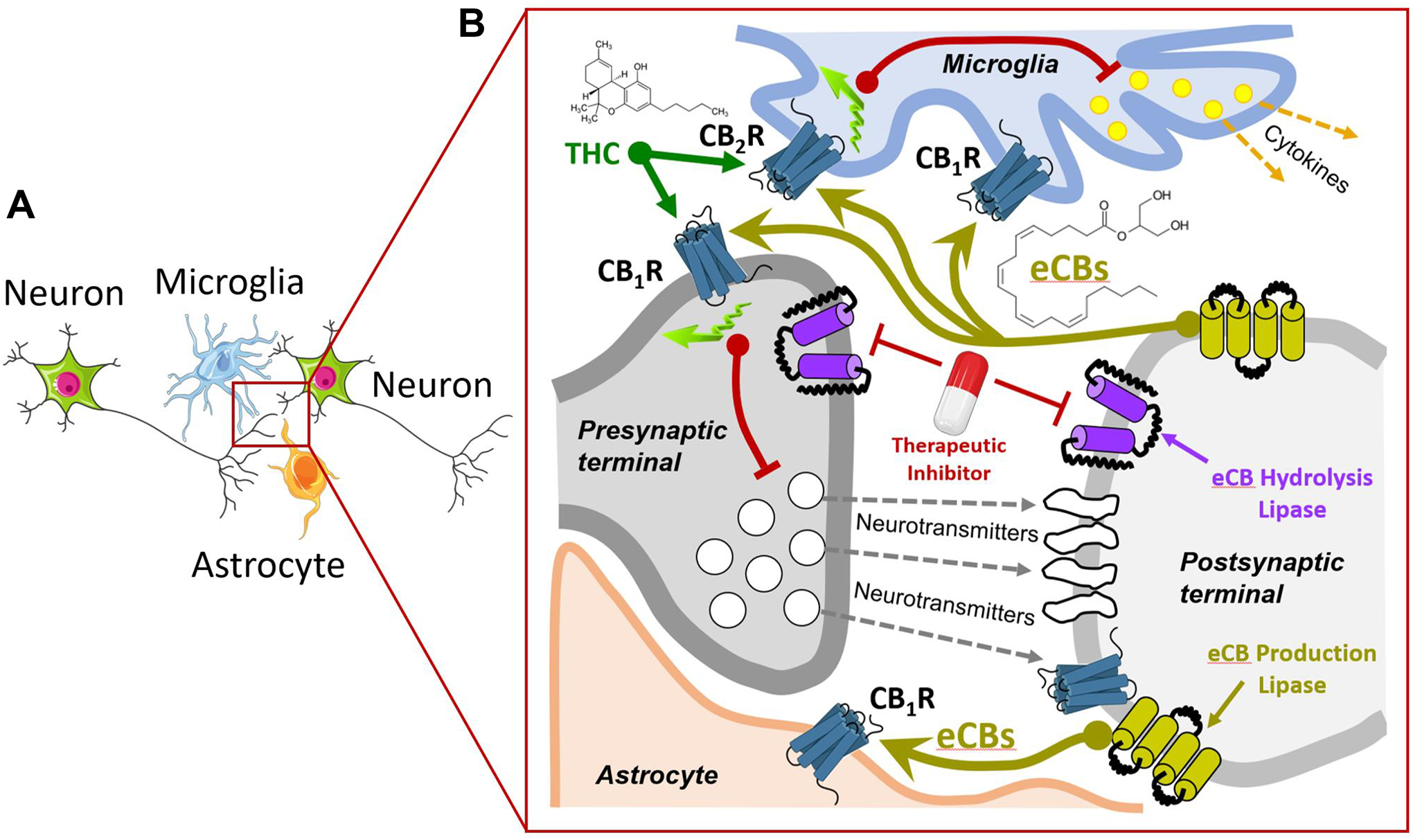
A] Focus on a synapse between two neurons with adjacent a microglia and an astrocyte (tripartite synapse). B] Presynaptic release of neurotransmitters (gray dotted arrows) stimulates post-synaptic receptors and activate lipases involved in eCB production (light green arrow). Released eCB activate presynaptic CB1R and inhibit neurotransmitter release (red line), CB1R expressed by astrocytes and regulate their energy metabolism, CB1R/CB2R expressed by microglia and inhibit cytokine production. THC modulates these receptors. Therapeutic inhibitors that target eCB hydrolysis result in increasing local eCB levels and activity at their targets.
The amount of AEA and 2-AG that reach and activate CB1R/CR2R is regulated by at least five mechanisms: 1] enzymes that produce eCBs, 2] eCB transport from their synthesis to their target receptors, which involves fatty acid binding proteins, 3] the number of functional CB receptors at the membrane and their biased signaling, 4] the clearance of eCBs by uptake through the plasma membrane and 5] eCB inactivation by enzymatic hydrolysis. Specifically, presynaptic fatty acid amide hydrolase (FAAH) hydrolyzes AEA, whereas presynaptic monoacylglycerol lipase (MAGL) and post-synaptic α/β-hydrolase domain 6 (ABHD6) hydrolyze 2-AG49,50. Importantly, these proteins are expressed by neuronal, glial, and endothelial cells; their contribution in controlling eCB signaling is being studied51. Thus, eCB signaling encompasses CB1R/CB2R, AEA/2-AG and the enzymes that produce and inactivate these two main eCBs, exhibit different potencies at CB1R/CB2R and provide parallel signaling systems to regulate multiple brain functions.
The PD differences between 2-AG and AEA include their potency and efficacy at CB1R/CB2R; 2-AG acts as a low potency full agonist compared to the high potency and partial agonist AEA. Considering that THC has a higher potency at CB1R/CB2R and acts as a partial agonist/antagonist, respectively, it may both partially activate free CB1R/CB2R and compete for eCB activity at CB1R/CR2R52. Thus, THC more likely mimics AEA’s bioactivity and reduces 2-AG’s bioactivity. A notable difference is that THC modulates CB1R/CB2R signaling in the entire brain as compared to the localized, activity-dependent, increases in AEA/2-AG levels and ensuing activation of CB1R/CR2R. Furthermore, CB1R, and probably CB2R, represent molecular hubs/coincidence detectors that respond to several endogenous signaling molecules through allosteric binding sites modulating their activity53,54. Specifically, an allosteric binding site is a distinct protein domain from the orthosteric site that binds the primary endogenously produced ligand and modulates the protein’s activity. Thus, there are both positive and negative allosteric modulators. A remarkable example is pregnenolone, which acts as a negative allosteric modulator of CB1R; its synthesis is CB1R-dependent, an endogenous negative feedback loop mechanism thought to protect the brain from CB1R overactivation55. Significantly, this allosteric modulation site on CB1R represents a very promising target for the treatment of several neurological and psychiatric diseases56.
3.4]. Glycine Receptor.
The currents mediated by this ligand-gated chloride ion channels are enhanced by AEA and THC (EC50s = 0.1–1 μM and 3 μM, respectively) and are thought to act through a positive allosteric mechanism (Figure 3)57,58. Accordingly, the commonly studied THC-induced analgesia is absent in α3GlyR knockout mice and intact in CB1R/CB2R knockout mice59. This research showed that a commonly studied THC bioactivity, here analgesia, may also be partially mediated by glycine receptors and that the selective targeting of this mechanism may produce specific analgesic benefit distinct from the analgesia mediated through CB1R and CB2R (see below). These results also uncover a new coincidence detector mechanism mediated by the synchronized release of glycine and AEA onto cells expressing glycine receptors. Such a coincidence detector mechanism likely contributes to locally inhibiting neuronal activity (Figure 4A).
3.5]. Other possible targets.
THC modulates the activity of at least two additional GPCRs when tested in cells in culture. THC (1–5 μM) is a partial agonist at post-synaptic GPR55 and reduces excitatory transmission (Figure 4B)60,61. GPR55 is endogenously activated by the lipid signaling molecule, lysophosphatidic acid, and possibly the small peptide PACAP-2762,63. GPR55 couples to Gq and β-arrestin and is expressed by neurons, astrocytes, and microglia64–66. Thus, by only partially activating GPR55, THC might prevent endogenous activation of GPR55 and reduce excitatory transmission. Cell culture evidence suggests that AEA decreases and THC increases 5-HT3AR currents (EC50s ≈ 300 nM and 30 μM, respectively) (Figure 4B)67,68.
In summary, the strong evidence above shows that the nanomolar concentrations of THC reached in the brain modulate the activities of multiple proteins, including CB1R, CB2R and GlyR, and possibly GPR55 and 5-HT3AR. This poly-pharmacology profile of THC suggests that selective activation of each target will not fully recapitulate THC’s bioactivity, and that selectively modulating a target (excluding CB1R) may provide specific therapeutic benefits and avoid THC’s associated psychotropic effects (see below).
4]. THC bioactivity: From humans to rodents and back
The landmark discovery of the chemical synthesis of THC and CBD in the 1960s enabled preclinical and human research on its bioactivity69. Early studies aimed at comparing the bioactivity of injected THC versus smoked cannabis in humans established that both trigger similar somatic, perceptual, and cognitive changes. Specifically, both smoking a Cannabis cigarette containing THC (12 mg) or injecting THC i.v. (1–6 mg) trigger a rapid succession of symptoms, starting with numbness and tingling of the extremities, light-headedness, “floating” feelings, and loss of concentration, followed by euphoria, palpitation, sweating, tremulousness and weakness that lasts several hours70. Subjects also report cognitive impairment described as difficulty in paying attention, difficulty in expressing oneself, mental confusion and loss of sense of time (e.g., one min feels like several min)70,71 (Table 1). Below are the main behavioral changes commonly measured in rodents exposed to THC, and their relevance to THC’s bioactivity in humans.
Table 1: Psychotropic bioactivity of THC in humans.
Examples of changes in human somatic, perceptual, and cognitive functions triggered by acute THC use.
| somatic | perceptual | cognitive |
|---|---|---|
| light-headedness | euphoria | introspective states |
| “floating” feelings | loss of time sense | rapid flow of thoughts |
| pulse rate increases | increased body | dreamy |
| palpitation | awareness | loss of concentration |
| sweating | distortions of vision | disrupted memory |
| tremulousness | decreased hearing | anxiety |
| weakness | decreased paying attention | incoordination |
| numbness | mental confusion | sleepiness |
| dizziness | difficulty in thinking | |
| blurring | difficulty in speaking | |
| fatigue | difficulty in reading | |
| difficulty in remembering |
4.1]. Cannabimimetic responses in rodents.
Early studies outlined the broad bioactivity of THC (1–30 mg/kg, i.p.) in rodents and showed progressive reduction in spontaneous locomotion; hypothermia; hypersensitivity to tactile and auditory stimuli; ataxia; and sedation, all of which were paralleled by increases in serotonin and noradrenalin levels in the brain72,73. The first experimental approach commonly used to test the bioactivity of cannabinoids in rodents is referred to as the “classic tetrad behavior test,” which includes four easy-to-measure physiological and behavioral changes: hypothermia, hypolocomotion, analgesia, and catalepsy (Figure 6A)74. Most, but not all, cannabimimetic responses are mediated by CB1R expressed by select cell types, principally glutamatergic neurons and astrocytes (see below)75–81. Furthermore, THC bioactivity, impairing effects, and therapeutic effects differ significantly depending on the age and sex of mice. For example, THC (5 mg/kg) reduces spontaneous locomotor activity and is anxiogenic in adult mice but not adolescents and exhibits stronger bioactivity in females17,24,82. These behavioral measures in rodents have evident translational value in understanding THC’s bioactivity in humans.
Figure 6: Examples of THC bioactivity in rodents.
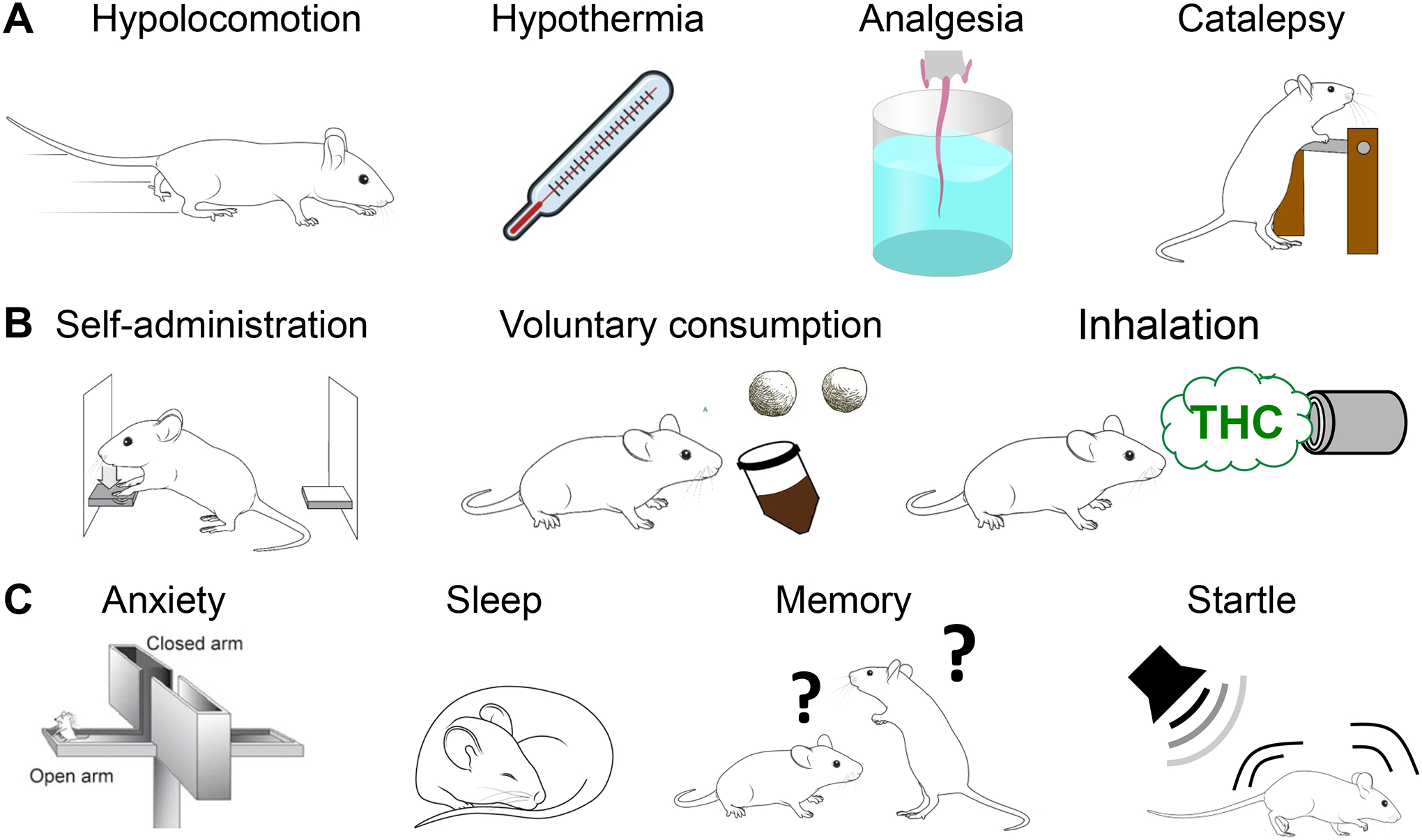
A] “Classic tetrad” behaviors triggered by CB1R agonists. B] Recent experimental approaches to study THC self-administration. C] Behavioral changes induced by THC in rodents with translational relevance to humans.
Oral consumption and vapor inhalation of THC can be reliably self-administered by rodents (Figure 6B). Voluntary oral self-administration of THC by rats and mice results in significant acute CB1R-dependent cannabimimetic responses19–21,83. Rats trained to nose poke for THC vapor show high rates of responding and motivation to take vapor22,84. Significantly, rats and mice can reliably discriminate between vehicle and THC injections through a CB1R-dependent mechanism (e.g., i.p. injections of THC (10 mg/kg) versus vehicle injection in a 2-lever drug discrimination test for sucrose reward)85.
THC chronic administration results in sex-specific tolerance to its bioactivity, principally because of receptor desensitization dependent on CB1R phosphorylation by a G protein-coupled receptor kinase and CRIP1A interaction36,86–88. In rodents, THC tolerance is associated with altered sleep during spontaneous cannabinoid withdrawal, a mechanism mediated by impaired dopamine function89,90. Thus, rodents will self-administer THC, can discriminate its bioactivity from control treatment, and exhibit increasing tolerance to its bioactivity, providing direct translational value to understanding THC’s bioactivity in humans.
4.2]. Impairing effects triggered by THC.
Impairment is defined as a state or condition associated with marked reduction in an individual’s ability to control physical and mental functions. The importance of measuring impairment is critical. Motor vehicle accidents are a leading cause of mortality across the world. Accordingly, both simulated and real driving studies that performed a double-blind, placebo-controlled testing of THC administration (20 mg in a cigarette) show significant reduction in driving stability as measured by inappropriate line crossings91,92. Can such impaired behavior be studied in rodents using simplified approaches?
Behaviors in rodents used to measure impairment from THC include locomotion, motor control, reaction time and the startle response to sudden noise. THC affects spontaneous locomotion of mice in an open field through a CB1R-dependent mechanism that changes with dose: a low dose increases locomotion and a high dose reduces locomotion93,94. CB1R KO mice show worse motor coordination than WT mice as measured by the Rotarod performance test95,96. Impaired motor coordination is thought to result from dysfunction of the cortico-striatal neuronal circuits97. CB1R is expressed at different levels in striatum: higher levels by GABAergic medium spiny neurons and parvalbumin-expressing interneurons, and lower levels by cortical neurons that project to the striatum98–106. Evidence suggests that CB1Rs expressed in cortico-striatal terminal and in medium spiny neurons control motor coordination skills. For example, brain slice electrophysiological studies show that cannabinoids dampen cortico-striatal activity through both CB1R and CB1R/D2 receptor heteromers located on cortical axon terminals abutting D2 receptor-expressing medium spiny neurons98,106–110. CB1R expressed by medium spinal neurons are involved in the initial learning of motor coordination skills measured on a Rotarod96. Thus, rodent studies point to CB1R expressed by the cortico-striatal pathway as playing a prominent role in the control of spontaneous locomotion and motor coordination and show that THC impairs this mechanism.
Startle is defined as a largely unconscious defensive response to sudden or threatening stimuli, such as sudden noise or sharp movement. In mice, THC (10 mg/kg) decreases the startle response as measured by the reflex response to loud acoustic stimulation via CB1R- and 5HT2A-dependent mechanisms111,112. Furthermore, THC (10 mg/kg) reduces reaction time tasks in rats as measured by the trial-unique, delayed nonmatching-to-location task, an index of executive function involving working memory and attention111,113.
A recent human study showed that THC (36 mg, oral) impacts neuronal connectivity in prefrontal cortex (PFC), as measured by functional near infrared spectroscopy (fNIRS), a commonly used neural signature of impairment in humans114. CB1R is expressed at different levels by distinct neuronal subpopulations in the PFC of rodents: higher levels by GABAergic projecting neurons and interneurons, and lower levels by projecting glutamatergic neurons115. Activation of CB1R in rodent PFC slice electrophysiology recording shifts the balance between excitation and inhibition towards excitation, and THC administration to mice results in a remarkable up-regulation in CB1R expression and down-regulation of BNDF in the PFC116–118. Accordingly, the sensitive balance of glutamatergic and GABAergic transmission within the PFC is particularly vulnerable to THC that down-regulates CB1R function119–121. These rodent studies enhance our understanding of a molecular mechanism associated with THC triggered impairment that affects CB1R signaling in the PFC.
Acute oral consumption of THC (20 mg/kg) impairs memory functions for hours in humans122. This finding was validated in a double blind trial that tested 7.5–15 mg oral THC on visual working memory123. In mice, THC impacts spatial reference and working memory in a sex-dependent manner124,125. Mechanistically, THC disrupts network neuronal spiking patterns and temporal firing synchrony in the hippocampus, a response that is mediated through CB1R expressed in astrocytes, PKC and COX2 signaling in addition to hyperpolarization activated cyclic nucleotide-gated channels126–131.
Thus, THC triggers a broad range of well-defined behavioral changes in rodents, some of which are considered close proxies of human behavior and cognitive tasks, including memory processing, motor coordination, and startle responses (Figures 6B–C)132–135. Importantly, the bioactivity of THC in rodents shifts depending on the dose. For example, while THC (1 mg/kg) increases food consumption and locomotion and has anxiolytic effects, higher dose THC (5 mg/kg) reduces locomotion and produces anxiogenic and aversive effects24,111,113. Such a biphasic response to THC is also apparent in humans: low doses of THC alter perception, produce euphoria, impair verbal fluency, and impair working memory while higher doses increase anxiety. Rapid-onset high doses trigger schizophrenia-like symptoms in some individuals136. In summary, some of the cardinal behavioral impairments triggered by THC in humans can be studied in rodents, and thus have evident translational value.
4.3]. Mental health disorders associated with THC use: epidemiology and preclinical studies.
Chronic use of high amounts of THC impacts brain function and is associated with increased incidence of mental health disorders. I will highlight two predominant disorders: cannabis use disorder (CUD) and psychosis or schizophrenia137. The impacts of THC during pregnancy and in the elderly have been recently reviewed and are not covered here10,138–140.
Based on self-reporting surveys between 2016 and 2017 across the US, Cannabis dependence was the highest among young adults (16.7%)1. The public health community is concerned that use of high dose THC-base products often increases during adolescence, may contribute to transitioning from experimental use to frequent use, and may increase impulsive/risky behaviors141–145. CUD is characterized by impaired memory processing, decision-making, school/work performance, and social functioning. Chronic use of high concentration THC product is associated with progression to first CUD symptoms in adolescents146,147. Adolescents with CUD also have predicted lower intelligence quotients and slower cognitive function as measured by full-scale IQ and reaction time testing148. The largest online drug survey world-wide, the Global Drug Survey, along with the US National Comorbidity Survey database, indicate that acute Cannabis use can trigger psychotic symptoms as defined by the occurrence of hallucinations and/or paranoia in select individuals, including young adults who regularly use high amounts of Cannabis and individuals diagnosed with psychosis149,150. Correspondingly, i.v. injection of THC (2.5 and 5 mg) produces a broad range of transient symptoms and cognitive deficits in healthy individuals that resemble some aspects of endogenous psychoses136,151. Furthermore, daily use of Cannabis-based products with high THC increases the risk of developing psychotic disorders, including schizophrenia, and is related to earlier onset of symptoms compared to people who do not use Cannabis152–155. Thus, daily use of products with high THC levels during adolescence may have a strong impact during adolescence itself and later in adulthood in some individuals, and, in particular, may increase the incidence of CUD, psychosis, and schizophrenia153,156.
A recent flurry of results gathered in rodents outline the impact of THC use on adolescent rodent behavior and on later adulthood behavior. Administration of THC (3 mg/kg, i.p. daily for 3 weeks) to adolescent rats is associated with anxiety-like and impaired locomotor behavior in adulthood, indicated by increased repetitive and compulsive-like behaviors measured by the Nestlet shredding task15. Confirming and extending this result, administration of THC (0.3–3 mg/kg, twice daily for 10 days) to adolescent rats is associated with anxiety-like behavior in adulthood as measured by the elevated plus maze, increased spontaneous locomotion in open-field, and no change in attentional set-shifting performance116. Administration of adolescent mice with THC (3–8 mg/kg, daily for 3 weeks) impairs recognition memory, working memory, and novel object recognition in adulthood15,157,158. THC exposure during adolescence does not influence adult nicotine self-administration, extinction, and reinstatement, nor does it influence conditioned place preference to THC itself, pre-pulse inhibition or attentional set-shifting performance in adulthood116,159,160. In a rat model of anxiety-like behavior, acute intra-PFC infusions of THC produce anxiogenic effects while producing no observable impairments161. Finally, glial cells are also involved in the adolescent brain response to THC. For example, microglia-mediated phagocytosis of newborn cells during development is controlled by eCB signaling and sculpts sex differences in juvenile rodent social play162. A recent study based on analyzing microglia phenotype by RNA sequencing showed that daily low dose administration of THC to adolescent mice markedly changed microglia activation and function, and impaired the stress behavior caused by social defeat in repeated resident-intruder encounters, an experimental paradigm that produces a depression-like phenotype, anxiety, and social avoidance163. Thus, the influence of THC on the developing brain appears to impact multiple neuronal and glial cell functions that affect adulthood behaviors39,164.
Whether THC acts as a gateway drug, predisposing individuals to want to take ‘harder’ drugs such as opiates or psychostimulants, has been a longstanding debate. CB1Rs are densely expressed in brain areas involved in reward, addiction, and executive function, including the amygdala, PFC, nucleus accumbens and VTA (Figure 7)165. Because adolescence represents a vulnerable time of marked neuronal development, it is possible that THC use during this period changes an underlying neurophysiology that makes drugs that are already reinforcing even more rewarding. Several rodent studies proved this hypothesis correct. Adult rats show increased heroin self-administration when exposed to THC during adolescence (1.5 mg/kg, 8 injections over 21 days), a response that relies on increased pro-enkephalin expression in the nucleus accumbens166–168. Adult rats also show increased cocaine self-administration when exposed to THC during adolescence (1 mg/kg, i.p., 18 days)169. These effects of THC are likely mediated by CB1R. Indeed, CB1R is involved in the development of the rewarding properties of psychostimulants mediated by dopamine, including amphetamine and cocaine, as shown by CB1R genetic deletion and SR1 treatment170–173. The dopaminergic system that originates in the midbrain and projects to select areas of the forebrain to regulate motivated behaviors is particularly sensitive to chronic THC exposure, and its dysfunction is associated with drug addiction19,174. THC exposure (2.5–10 mg/kg, daily for 10 days) during adolescence in rats affects the functionality of dopamine receptors and dysregulates glutamate receptor expression in adulthood175–178. These results paint a grim picture in which THC exposure in rats during adolescence delays the maturation of the dopamine and glutamatergic neurons and impairs their ability to control motivated and reward-triggered behaviors.
Figure 7: CB1R-dependent impact of THC on the developing brain during adolescence.
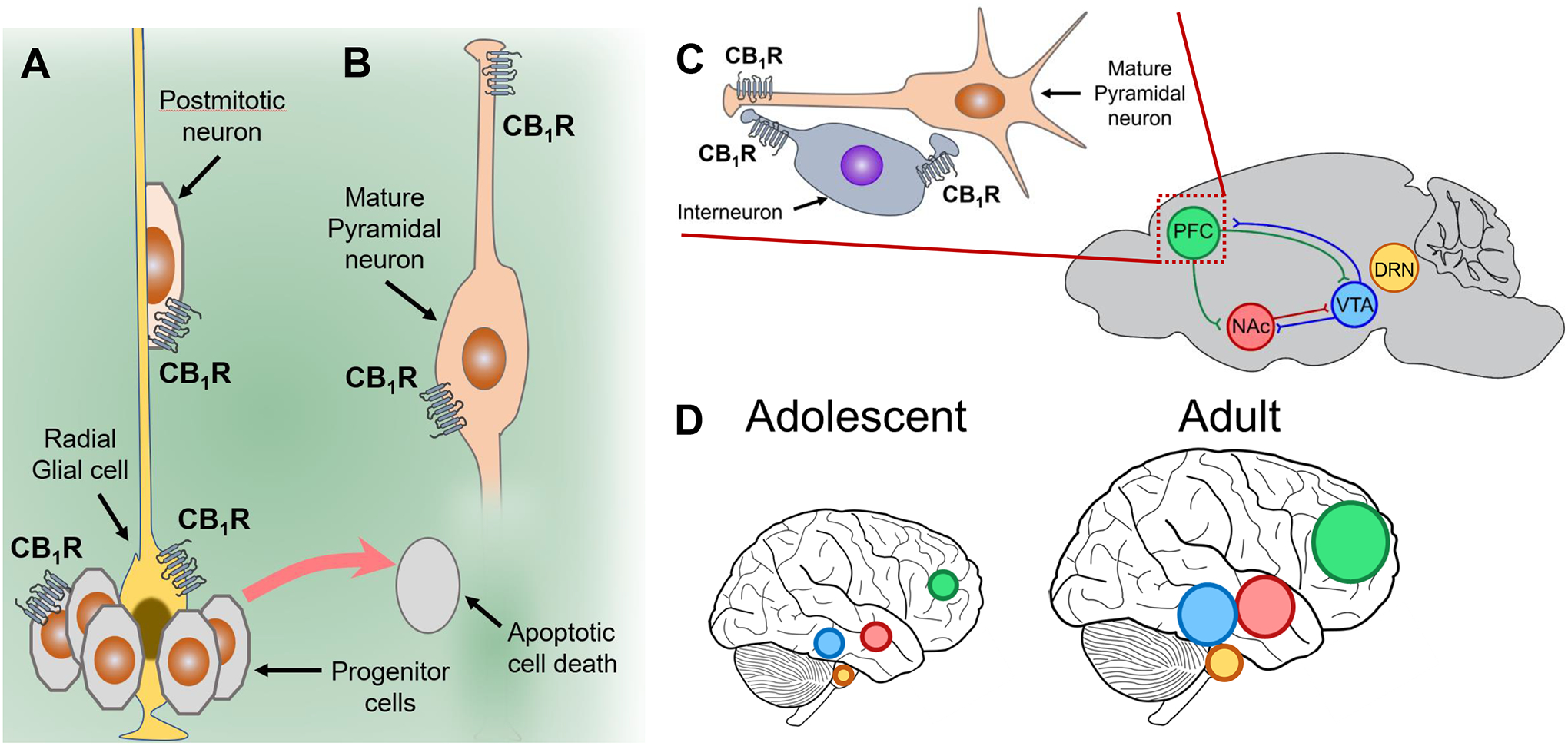
A] CB1R are expressed by progenitor cells and their activation by THC can trigger apoptoptic cell death, and impair the development of radial glial cells and postmitotic neurons. B] THC activation of CB1R expressed by mature neurons affects their phenotype and neurotransmitter release. C] CB1R expressed by mature pyramidal neurons and inhibitory interneurons control the excitatory/inhibitory transmission balance in several brain areas, including prefrontal cortex (PFC), nucleus accumbens (Nac), ventral tegmentum area (VTA). THC also interacts with 5HT1A receptors in the dorsal raphe nucleus (DRN). D] PFC, Nac and VTA in human adolescents undergoes significant development and maturation into adulthood.
5]. THC therapeutic indications: Favorable evidence
Despite the negatives discussed above, THC shows promising therapeutic efficacy. Considering the multiple pathways that THC modulates, an understanding how each target might be involved in THC’s therapeutic response will enable the development of selective small molecules with improved therapeutic index compared to THC. Below I outline preclinical evidence for use of THC in the treatment of pain, sleep disorders, seizures, multiple sclerosis, and Huntington’s disease.
Probably the original and oldest therapeutic use of THC was for its analgesic properties in chronic pain179,180. We now know that acute inhalation of THC (0.5–1 mg) produces greater analgesia compared to placebo in chronic pain patients and causes only mild side effects that resolve spontaneously181. Based on our understanding of THC’s molecular and cellular mechanism of action, preclinical studies are optimizing this analgesic activity180,182. The antinociceptive efficacy of THC in rodent models of both pathological and injury-related persistent pain are well established; in addition, the expression pattern of most of its targets in pain pathways have been precisely mapped. Furthermore, the opioid-sparing effects and antinociceptive synergy with nonopioid analgesics have been described in detail, and co-administration of heroin and THC (50 mg/ml) by inhalation produces additive effects on thermal nociception in rats180,183,184. Mechanistically, oral THC (≈ 3 mg/kg) reduces neuropathic pain in mouse injury via a CB1R and through CB2R in the mouse model of chemotherapy-induced neuropathy without the development of tolerance20,83,185. Of particular relevance for the therapeutic relevance of this mechanism, rats with nerve injury self-medicate with CB2R small molecule agonists to attenuate a neuropathic pain state186. Thus, THC induces analgesia by interacting with at least 3 targets: CB1R, CB2R and GlyR, and if this depends on the drug or experimental paradigm used selective, emphasizing that the selective targeting these molecular components involved in pain sensation represents independent therapeutic approach. Dedicating resources to the optimization of cannabinoid-based analgesics is particularly relevant to the goal of reducing the use of opioid-mediated analgesics that are addictive and that represent a current major health problem187.
The effect of THC on sleep quality has been explored since increased sleepiness is often reported following THC use. Low dose THC enhances subjective sleep quality, which is measured by the insomnia severity index via a clinically validated questionnaire, whereas high dose THC increases insomnia symptoms188,189. One consistent finding is the short-term benefit of THC to treat sleep apnea, most likely due to its modulatory effect on serotonin transmission in key brain areas190. However, chronic use of Cannabis is associated with rapid habituation to its sleep inducing and enhancing properties, and represents a primary reason for the lapse/relapse of using Cannabis191. Specifically, abrupt Cannabis use cessation among heavy users impairs sleep, may sabotage attempts to quit Cannabis use and raises the risk of relapse and emotional behavior (e.g., irritability and anhedonia)192–194. One of the most intriguing results is found in individuals suffering from post-traumatic stress disorder who experience both emotional and behavioral symptoms from previous traumatic events. Here THC (5 mg/oral) improves subjective measures of sleep quality and reduces the frequency of nightmares in 70% of the subjects195–197. Thus, THC may decrease sleep latency initially but could impair sleep quality long-term, depending on the individual191,194. Studies on the effect of THC on sleep in rodents show that mice exhibit altered sleep during spontaneous THC withdrawal through a CB1R-dependent mechanism and changes in striatal dopamine signaling89,198. CB1R-KO mice spend more time awake during the dark (active) period but not during the light (rest) period, thus enhancing the day-night variation of wake-sleep hours compared to wildtype mice199.
There is also evidence that THC may be useful for the treatment of seizures, as shown in several rodent models of epilepsy. THC (1–10 mg/kg) reduces both electrically and chemically induced seizures in rats through a CB1R-dependent mechanism200,201. Accordingly, THC and CB1R agonists reduce excitatory transmission and hippocampal overexcitation known to causes seizures127,202–204. Of note, CB1R expression is both upregulated and involved in the development of rodent models of seizures, suggesting increased therapeutic efficacy when selectively targeting this mechanism. However, considering THC’s side effects, few studies followed up on the therapeutic efficacy of THC as an antiepileptic drug, and the field moved to targeting the eCB signaling and the allosteric site of CB1R205,206. For example, inhibition of MAGL increases 2-AG levels and reduces seizures through a CB1R-dependent mechanism in chemically induced seizures in rats207,208. Treatment of a rat genetic model of childhood epilepsy with the small molecule allosteric CB1R modulators, GAT211 and GAT229, reduces in vivo cortical spikes and wave discharges, a measure of absence seizures that are characterized in humans by staring blankly into space for a few seconds209.
Several clinical trials of THC for the treatment of multiple sclerosis (MS) showed limited therapeutic efficacy at reducing spasticity and pain210,211. However, n a mouse model of MS (experimental autoimmune encephalomyelitis; EAE) activation of CB1R expressed by neurons and CB2R expressed by immune cells reduced disease severity212–215. The therapeutic promise of targeting CB2R is underscored by a 200-fold upregulation of its expression in microglia in the EAE mouse model43. Thus, preclinical studies suggest that the mild therapeutic properties of THC will require optimization, for example by testing CB1R/CB2R allosteric modulators.
THC was tested therapeutically for Huntington’s disease (HD) as part of a clinical trial and showed no significant symptomatic effects when prescribed at 3 mg/kg administered via sublingual spray for 12-weeks216. This lack of therapeutic efficacy should be interpreted in the context of the pronounced down-regulation of CB1R expression in striatum, a predominant brain structure that undergoes neurodegeneration in HD101. CB1R expression is also downregulated in multiple genetic mouse models of HD, and CB1R genetic rescue prevents the loss of striatal synaptic contacts95,217–219. THC exhibits only small therapeutic efficacy in mouse models of HD, indicating that either THC treatment requires optimization or that we need to consider targeting eCB signaling220,221. For example, MAGL expressed in astrocytes controls mutant huntingtin-induced damage of striatal neurons as shown by the therapeutic efficacy of MAGL inhibition220. Remarkably, THC treatment is associated with significant neuroprotection in mouse models of HD through a CB1R/CB2R-independent mechanism of action; a result that is encouraging and prompts further investigations222.
In summary, human trials and preclinical rodent models of chronic pain suggest promising therapeutic properties of THC that would benefit from optimization, but preclinical rodent models of insomnia, epilepsy, MS, and HD suggest that THC therapy would require significant optimization, most likely focusing on non-CB1R-dependent mechanisms.
6]. CBD PK/PD: Similarities to and differences from THC
Since the legalization of CBD-containing products by several countries, including the US in 2018, there has been a striking increase in the human use of CBD-containing tinctures, drinks and edibles223,224. Selective cross breeding of Cannabis has resulted in a plant producing high levels of CBD and low levels of THC (below 0.3%)225. In the US, this plant is now legally referred to as hemp. Concomitantly, peer-reviewed studies have reported behavioral and therapeutic effects of CBD in preclinical and human trials and have increased our understanding of the behavioral changes induced by CBD administered as a single agent, its mechanism of action and its therapeutic promise. Again, to measure CBD bioactivity in humans, studies need to provide information on route of administration, how much CBD was used, and its regimen of use. Our understanding of the PK profile of CBD, and the multiple protein activities that it modulates provides a clearer picture of its bioactivity in vivo.
6.1]. CBD PK in humans and rodents.
The PK profile of CBD in humans when administered by different routes of administrations has only recently been studied in detail. In humans, inhaled and oral CBD (10 mg) results in blood levels reaching maximal concentration values of 105 and 14 ng/ml, respectively (≈330 and 45 nM); values that are comparable to those obtained with inhaled and oral THC (10 mg)226. Oral CBD undergoes first hepatic metabolism that forms 7-COOH-CBD with a more pronounced and prolonged exposure profile than inhaled CBD226. Whether 7-COOH-CBD remains bioactive is unknown. The therapeutic potential of oral CBD formulations is limited by poor bioavailability and extensive first-pass hepatic metabolism. For example, following oral administration, 7-COOH-CBD blood concentration is ~40-fold higher than CBD227. Significantly, inhalation as a route of administration has several advantage compared to oral: it bypasses the PK variability attributed to irregular gastrointestinal absorption and first-pass hepatic metabolism, and efficiently delivers CBD into systemic circulation227. In rodents, i.p. and oral gavage delivery of CBD results in a PK profile that parallels the PK profile of THC17,228. Because of the similarity of its chemical structure to THC, CBD is also metabolized by CYP3A4, and thus CBD competes for THC metabolism by this enzyme and augments THC levels by reducing its conversion to 11-OH-THC229. Of note, the benzodiazepine, clobazam, is also metabolized by CYP3A4, and thus CBD treatment augments the exposure of clobazam; and yet this molecular interaction does not significantly affect the bioactivities of CBD and clobazam (see below)230,231. In summary, the PK profile of CBD delivered orally and i.p. parallels the PK profile of THC in both humans and rodents, but co-administration of CBD with THC and other select medications impacts their respective PK profiles14.
6.2]. CBD modulates the activities of multiple proteins.
CBD exhibits an even broader poly-pharmacology profile than THC, with only few common targets. The discussion below starts with those targets validated by genetic deletion or antagonism in vivo and ends with those that were reported using heterologous expression systems, systematically emphasizing the concentrations of CBD required to modulate these targets in the context of the concentrations it reaches in brain.
CBD modulates the activity of several GPCRs. Early studies established that CBD binds poorly to the orthosteric site of CB1R, and yet it antagonizes CB1R signaling (Figure 8)29,232. Studies of CB1R tertiary structure and signaling suggest that CBD is a negative allosteric modulator of CB1R, most likely by binding to its N termini, one of the longest among class A GPCRs53,233. Specifically, the current structure-based model of CB1R transition between inactive and active state posits that CBD might bind to the N termini of CB1R, resulting in a change in the 3 dimensional structure of the orthosteric binding site and in THC’s and 2-AG’s potencies233. In line with this model, CBD (0.1–1 μM) reduces the efficacies and potencies of both 2-AG and THC by activating CB1R signaling in neurons in cell culture, and inhibits eCB-mediated synaptic plasticity without influencing excitatory transmission234–236. While these results suggest that CBD inhibits CB1R signaling by directly interacting with an allosteric binding site on this target, a likely mechanism for some of CBD’s therapeutic properties and diminishing the impairing consequences linked to CB1R activation, direct demonstration of CBD binding to CB1R is still needed (see below).
Figure 8: Molecular targets modulated by CBD.
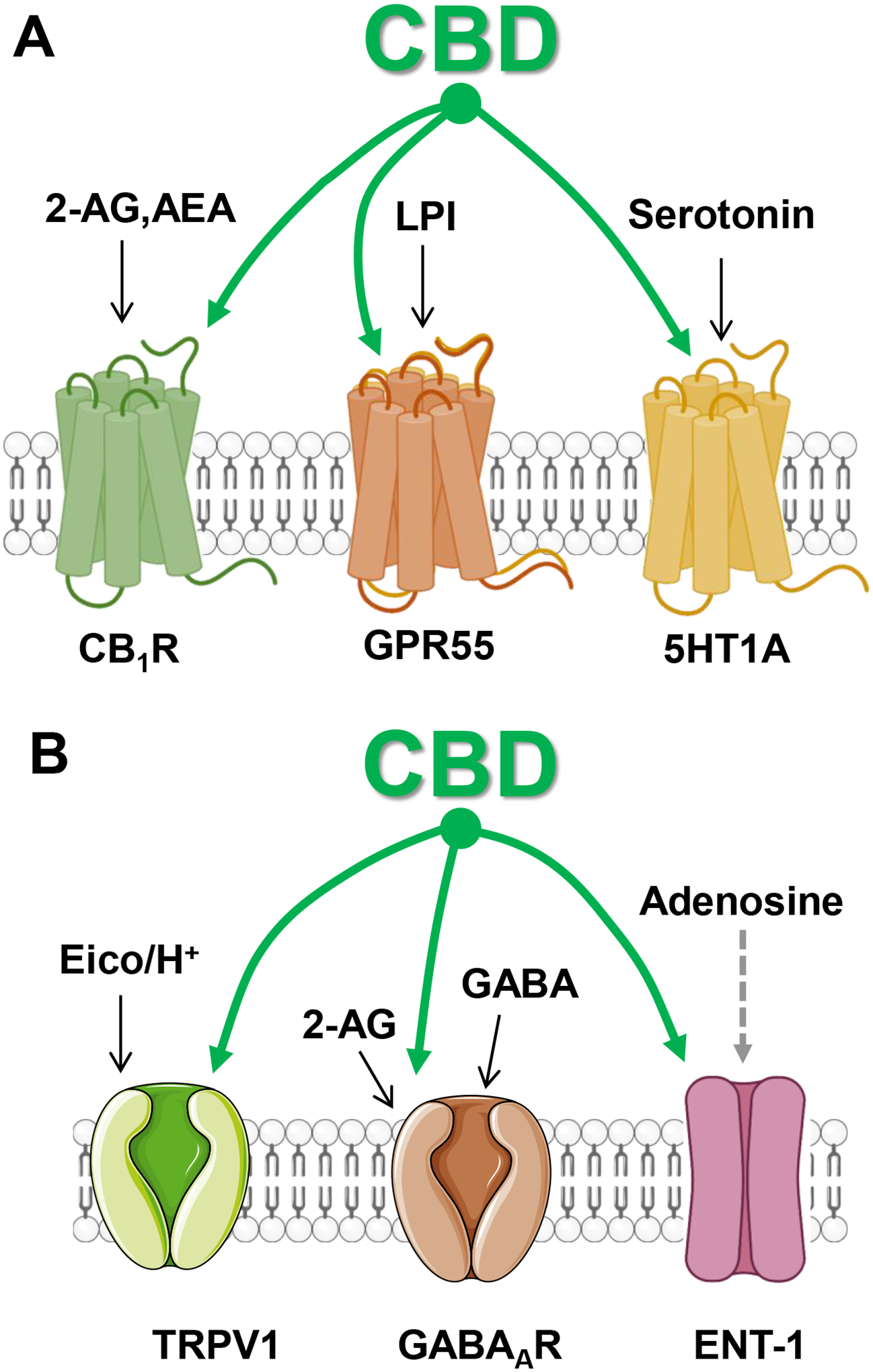
A] CBD is a negative allosteric modulator of CB1R that is endogenously activated by 2-AG and AEA, an antagonist of GPR55 that is endogenously activated by lysophosphatidyl inositol (LPI) and activate 5HT1A that is endogenously activated by serotonin. B] CBD is an agonist at TRPV1 that is activated by eicosanoids (Eico) and changes in H+, a positive allosteric modulator of GABAAR that is activated by GABA and endogenously allosterically modulated by 2-AG, and a blocker of ENT-1 transporters that carry adenosine.
CBD (1–5 μM) is a partial agonist at GPR55, a GPCR involved in the regulation of cell division and neuronal pathfinding during development, and regulates neurotransmission in the adult brain (Figure 8)237,238. By partially activating GPR55 and antagonizing its endogenous activation by lysophosphatidyl inositol, CBD most likely increases GABAergic inhibitory neurotransmission in the hippocampus and dampens hippocampal overexcitation in epilepsy61,239–241 (Figure 9). In vitro experiments suggest that CBD antagonizes 5-HT1A receptors; but only when reaching 100 μM, a surprising result considering that some of CBD’s bioactivity is mediated through this GPCR in vivo (see below)242.
Figure 9: LPI-GPR55 Endogenous signaling.
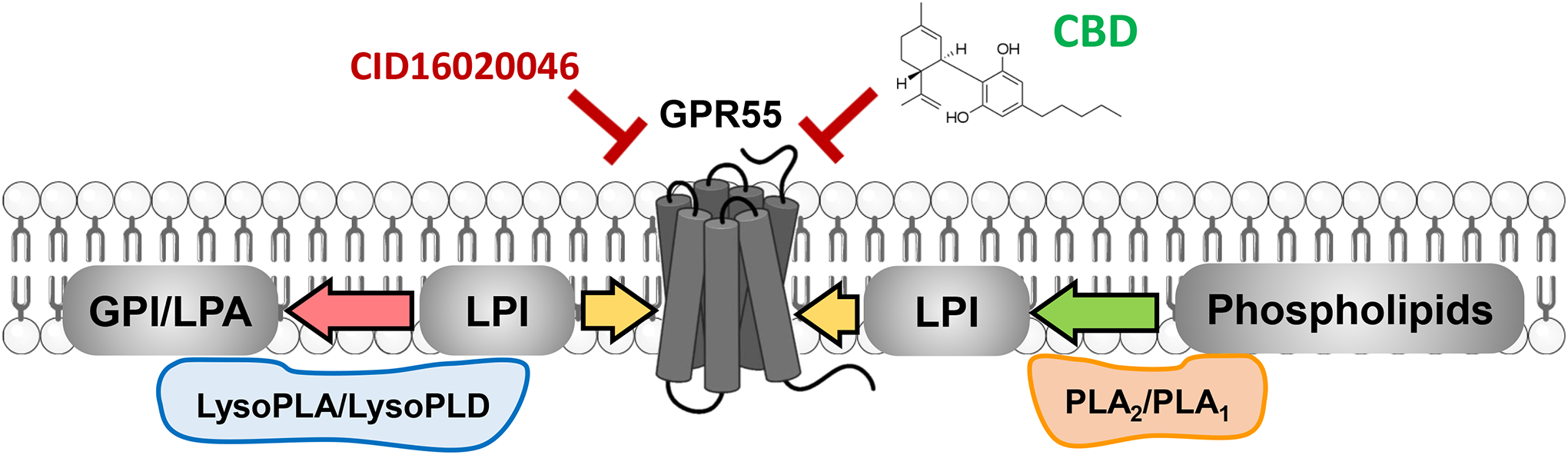
LPI is produced by PLA1/PLA2, activates GPR55 and is inactivated by LysoPLA and LysoPLD. CBD antagonizes GPR55, as well as the antagonist CID160200465.
Cell culture evidence suggests that additional GPCRs are modulated by CBD. Computational and cell culture functional validation suggest that CBD (10 μM) antagonizes orexin 1 receptor of type 1 (OX1R), a key regulator in arousal and the sleep/wake cycle, as well as motivation and reward processes243. CBD acts as a negative allosteric modulate at mu- and delta-opioid receptors, though this effect occurs at 30–100 μM244. Considering that CBD reaches at maximum of 3 μM in the brain following 120 mg/kg i.p. injections, the involvement of these targets in mediating CBD’s bioactivity still requires in vivo validation228.
CBD modulates the activity of ion channels and protein transporters (Figure 8). Of relevance to the sedative response to high dose CBD, we now have a good understanding of the molecular mechanism by which CBD increases GABAAR currents. Several endogenous mechanisms increase tonic GABAAR currents through allosteric modulation, including neurosteroids and 2-AG, and thus contribute to enhancing inhibitory neurotransmission49,245. Cell culture evidence shows that 2-AG (0.3–30 μM) and CBD (3–30 μM) allosterically increase GABAAR currents by 4-fold though a mechanism that does not involve the benzodiazepine and neurosteroid binding sites230,246–248. While the resolution of the GABAAR crystal structure outlined an allosteric site in hydrophobic tails that is distinct from the site engaged by neurosteroids and possibly cholesterol, whether CBD acts by binding to this site has not been directly tested249,250. CBD potentiation of tonic GABAAR currents could also be responsible for dampening hyperexcitation and seizures and may cause sedation at high concentrations251. These studies suggest that small molecules that directly target allosteric sites on GABAAR engaged by 2-AG and possibly CBD, or by inhibiting 2-AG hydrolysis, (e.g., with ABHD6 inhibitors) represent promising therapeutic approaches to reduce enhanced excitatory transmission and treat select neurological diseases, including epilepsy49.
CBD, acting through transient receptor potential cation channel subfamily V member 1 (TRPV1), is anxiolytic in rats, and analgesic in a rat model of acute inflammation (Figure 8)252,253. Cell culture studies suggest that CBD activates and desensitizes TRPV1254. Computational approaches suggest that upregulation of TRPV channels might unmask CBD bioactivity at TPRV2, TRPV3, TRPV4, TRPA1, and TRPM8255,256. While the molecular mechanism by which CBD might regulate TRP channels remains unknown, better understanding of its TRPV1-mediated bioactivity in vivo represents a growing area of study.
CBD is a competitive inhibitor of the equilibrative nucleoside transporter-1 (IC50 < 200 nM), a primary inactivation mechanism for the signaling function of extracellular adenosine that is expressed by neurons and glia257–259. Thus, inhibition of this transporter by CBD blocks adenosine reuptake by neurons and glia increases local adenosine levels and its ensuing activity at its receptors, A2a. Through this mechanism of action, CBD influences several behaviors, including reducing anxiety (see below). Cell culture studies suggest that CBD (3–10 μM) inhibits currents mediated by the voltage-gated sodium channel Nav1.4 and the voltage-dependent anion channel 1 in mitochondria260–262.
Thus, CBD modulates the activity of several GPCRs, ion channels and transporters at concentrations that are within the range of the concentrations reached in the brain when treated in vivo. The involvement of most of these targets in the bioactivity CBD exhibits in vivo remain to be demonstrated but should guide the development of novel therapeutics.
7]. CBD bioactivity in humans and rodents and back
CBD has been considered non-psychotropic because, in humans, oral consumption of CBD up to 300 mg and i.v. injection of CBD up to 30 mg are both felt as being inactive263,264. While recent human studies confirmed that even high doses of oral CBD do not cause THC-like or Cannabis-like effects265, additional studies showed that low and high dose CBD does influence human behaviors and thus CBD should be considered psychoactive. For example, inhaled CBD (12.5 mg) enhances verbal episodic memory in healthy young participants266.
CBD also influences rodent behavior when administered as a single agent and at low dose. CBD (10 mg/kg, i.p.) impairs memory reconsolidation in rats through a CB1R-dependent mechanism and possibly by reducing Zif268/Egr1 expression, a transcription factor proxy for synaptic plasticity related to reconsolidation267,268. CBD (20 mg/kg, i.p.) affects motor behavior in rats as indicated by reduction in vertical activity (i.e., rearing which is a context-sensitive behavior) through a 5-HT1A-dependent mechanism269,270. Acute infusions of CBD (100 ng over 5 min) into the PFC in rats impairs attentional set-shifting and spatial working memory, without interfering with anxiety or sociability behaviors161. Mechanistically, single and repeated exposure to CBD (5–30 mg/kg, i.p.) differently modulate BDNF expression and signaling in rat cortex and striatum271. Thus, rodent studies emphasize that CBD influences key brain functions, including motor behavior and memory reconsolidation, and emphasizes its psychoactivity at low dose.
7.1. CBD therapeutic indications.
The successful clinical trial demonstrating CBD’s antiseizure properties in young adults with refractory seizures represents yet another landmark in the study of its bioactivity. Specifically, a multinational, randomized, double-blind trial of adjunctive CBD (300 mg/kg, oral) versus placebo showed significant reduction in seizures in adolescent patients with Dravet syndrome and Lennox-Gastaut syndrome and caused only mild side-effects240,272. The early finding that CBD metabolism competes with clobazam metabolism at CYP3A4 led to speculation that the antiseizure efficacy of CBD may simply reflect CBD augmenting clobazam exposure and intrinsic activity; however, human studies indicated that CBD reduces seizure frequency both with and without concomitant clobazam, and that these two antiepileptic treatments appear to produce additive benefit231,273. Thus, Combination Therapy treatments using CBD and low dose clobazam represents a promising approach to control seizures while reducing the side effects linked to clobazam231,273–276.
Rodent studies provided an initial mechanistic understanding of the anti-seizure qualities of CBD. CBD (30–100 mg/kg) reduces epileptiform and seizure responses as measured by electrophysiological recordings of rodents’ hippocampal slices and dampens seizures in rodent model systems of chemically induced seizures as well as in Scna1+/− mice, a genetic mouse model of Dravet Syndrome239,277–279. Furthermore, CBD potently inhibits CYP3A4 mediated metabolism of clobazam in Scn1a+/− mice. CBD-clobazam combination treatment achieves greater antiseizure responses, but only when an anti-seizure dose of CBD is used230. Thus, in this preclinical mouse model of Dravet syndrome, CBD and clobazam produce additive effects, a dual mechanism likely to involve competition for metabolism and independent potentiation of GABAAR, results that are guiding the design of future human clinical trials230. Of note, while CBD use as single agent at high concentrations does not produce side effects in the clinical trial setting, the effects of its use in combination with other medications and their mechanistic interactions remains largely unknown.
Two additional therapeutic opportunities with CBD may be in anxiety and sleep quality280. Early evidence in double-blind randomized design trials suggested that CBD (400–600 mg, oral, acute) decreases anxiety as measure by survey and functional neuroimaging (i.e., regional cerebral blood flow at rest) in naive patients with generalized social anxiety disorder281,282. However, several trials challenged this result, including a randomized double-blind, placebo-controlled trial that tested acute oral CBD (600 mg) and measured emotional processing and neuroimaging283. Remarkably, lower dose CBD might represent a more promising approach as suggested by a single patient case reports and small retrospective study with CBD (18–25 mg, oral, chronic) reporting reduced sustained anxiety and improved sleep284,285. Based on this premise, CBD treatment might help with anxiety and sleep, but significant optimization besides dose adjustment will be required. Preclinical evidence in rats might provide important clues as direct injection of CBD into the PFC induces antidepressant-like effect, and injection into the periaqueductal gray blocks panic-like response by activating 5-HT1A receptors270,286–288.
CBD appears to have analgesic properties, at least when used at high dose. In humans, CBD (300 mg/oral/daily) prevents acute and transient chemotherapy-induced peripheral neuropathy, but CBD (30 mg/kg) does not affect pain linked to irritable bowel syndrome289,290. In line with these human results, acute CBD in rodents significantly attenuates pain-associated behaviors in neuropathic pain models, but yields mixed results in inflammatory pain models182. CBD (0.3–30 mg/kg, i.p.) effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a rat neuropathic pain model, a response that involves CB1R and TRPV1291. Furthermore, the GRP55 antagonist, CID16020046, exhibits promising antinociceptive properties in a rat formalin test, suggesting the involvement of a CBD-GPR55 molecular interaction61,292. Thus, CBD has evident analgesic properties that involve multiple protein targets (Figure 8).
What are the next most promising therapeutic indications for CBD? Monitoring practice-based evidence in patients can provide clues for new therapeutic approaches. The first striking example is autism spectrum disorder, which is characterized by persistent deficits in social communication; restricted and repetitive patterns of behavior, interests, or activities; and often intellectual disabilities293,294. CBD (4–7 mg/kg/day for 6–9 months) is associated with some level of improvement in attention deficit, hyperactivity, communication deficits, social interaction deficits and cognitive deficits, as well as sleep, a prevalent co-morbidity of this syndrome295. CBD (600 mg/oral) alters regional low-frequency activity and functional connectivity in the brain of adults diagnosed with autism spectrum disorder as measured by fMRI signals296. A telling example provided by preclinical studies is that CBD bioactivity is also often biphasic, producing one set of behavioral changes at low doses and exhibiting distinctly different bioactivity at high doses. Specifically, CBD (5–20 mg/kg) improves autistic-like social interaction deficits in adult Scn1a+/− mice, a preclinical model of Dravet Syndrome, and only at a higher dose (100 mg/kg) reduces seizures in adolescent Scn1a+/− mice206,239. Furthermore, CBD (5 mg/kg) attenuates aggressive behavior induced by social isolation in mice through CB1R and 5-HT1A receptor-dependent mechanisms297.
The second example of CBD’s potential is for the treatment of drug abuse, especially opioid addiction298. Human trial testing of CBD showed that it reduces cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder299. Similarly, CBD shows promising therapeutic efficacy in several rodent models of addiction. CBD (5–20 mg/kg) reduces heroin-triggered anxiety-like behavior, motor activity, and somatic signs in mice300. Mechanistically, this treatment normalizes the altered expression profile of the mu-opioid receptor, proopiomelanocortin, and CB1R expression in the VTA of mice exposed to spontaneous heroin withdrawal300. Thus, CBD has promising adjunctive management properties for opioid withdrawal301. CBD (20 mg/kg) 1) attenuated the rewarding effects of cocaine in rats via CB2R, 5-HT1A and TRPV1 receptor mechanisms, 2) decreased motivation for cocaine in a behavioral economics paradigm in mice (i.e., reduced cocaine intake when administered during the acquisition phase), and 3) normalized the expression of GluRA2 and pERK1/2 in amygdala302,303. Mechanistically, CB1R antagonism reverses the effects of CBD on cue- and stress-induced reinstatement of cocaine-seeking behavior in mice, suggesting that CBD acts by allosterically reducing CB1R signaling and its role in the control of psychostimulant triggered hyperlocomotion96,304. Accordingly, CBD (10 μg/5 μL) infused in the lateral ventricle during the drug conditioning phase impairs the rewarding effects of methamphetamine305. Finally, rodent studies suggest that CBD might also help with alcohol addiction. For example, CBD (10 mg/kg, acute) attenuates ethanol-induced place preference and reduces aggressivity in group-housed rats306. Together, this growing preclinical evidence suggests that CBD could potentially be added to the standard detoxification regimen and mitigate acute or protracted withdrawal-related symptoms for several drugs of abuse, including opioids, psychostimulants, and alcohol. Clearly, the therapeutic potential of CBD is only starting to be revealed and preclinical studies are poised to help identify new medical indications.
7.2]. Is CBD use associated with side effects?
It is noteworthy that CBD does not produce overt impairment as a single agent, and thus exhibits a broader therapeutic index than THC (even at relatively high doses). For example, the recent testing of CBD’s therapeutic efficacy in multiple human clinical trials reported only mild and transient side-effects in some patients (e.g., somnolence, decreased appetite, diarrhea, and fatigue)265. Inhaled CBD (400 mg) followed by a one-week washout period increases the duration and intensity of measures of mismatch negativity amplitude, an auditory event-related potential that occurs when a sequence of repetitive sounds is interrupted by an occasional sound that differs in frequency or duration307. Furthermore, an observational study on the impact of long-term consumption of oral CBD (40–60 mg/day for 30 days) suggested no increased prevalence of liver toxicity in these self-medicating patients308. Thus, these studies indicate that a handful of reversible side effects may occur in some individuals who take high doses of CBD (e.g., 400 mg/day for several weeks) and that using 10-fold lower doses (e.g., 40 mg/day for several weeks) may be safe while still significantly influencing behavior.
7.3]. Interactions between CBD and THC bioactivities.
As early as the 1970s, evidence suggested that CBD (40 mg, oral, acute) might interact with THC (20 mg, oral, acute) in humans. For example, CBD delays the onset and prolongs the psychotropic effects of THC309,310. We now know that different doses and routes of administration of CBD have different effects on THC’s bioactivity. In humans, inhaled CBD (400 mg) attenuates the duration and intensity of THC (12 mg)-induced changes in mismatch negativity amplitude, the measure of auditory sensory perception307. However, inhaled CBD (125 mg) does not curb THC (125 mg)-induced impairment of driving and cognition as measured by simulated driving and cognitive performance tests, and, in some circumstances CBD may even exacerbate THC-induced impairment311. Furthermore, inhaled CBD (30 mg) does not protect against the acute adverse effects of cannabis as measured by delayed verbal recall, psychotic symptoms and subjective measures312. Oral CBD (600 mg) attenuates both THC (1.5 mg, i.v.)-elicited paranoid symptoms and memory impairments.
The ability of CBD to dampen specific THC’s bioactivities in rodents is well documented and depends on the dose administered. For example, in rats, CBD (3mg/kg, i.p.) mitigates the negative anxiogenic effects experienced from THC (1mg/kg, i.p.), a response that correlates with increase dopamine levels in the PFC313. CBD (3 mg/kg, i.p.) prevents the THC (3 mg/kg, i.p.)-induced downregulation of BDNF function in the hippocampus314. However, CBD fails to reverse hypothermia or locomotor suppression induced by THC (30 mg/kg, i.p.) in rats315.
Can CBD help mitigate the detrimental effects of THC in the developing brain? Multiple preclinical studies suggest yes. In adolescent mice treated for 15 days (PND 28–48) with daily i.p. injections of THC (3 mg/kg), CBD (3 mg/kg) reduced the impact of THC15. Specifically, THC triggers immediate and long-term impairments in working memory measured by the novel object recognition task; increases adulthood anxiety measured on the elevated plus maze; and increases repetitive and compulsive-like behaviors measured with the Nestlet shredding task and marble burying15. All of these THC-induced behavioral abnormalities were dampened by the coadministration of CBD while CBD as a single agent did not generate behavioral outcomes15. Treatment of adolescent female rats (PND 35–45, 15 days, twice daily) with increasing doses of THC (2.5, 5 and 10 mg/kg) impairs emotional behaviors in adulthood as measured by the swim test, sucrose intake, palatable food intake, and elevated plus maze300,316. However, CBD mitigates select long-term behavioral alterations and molecular changes in the PFC300,316. Intra-PFC injection of CBD reverses the cognitive impairments induced by acute glutamatergic antagonism within the PFC, and blocks the anxiogenic properties of THC316. These results suggest that CBD mitigates some of the long-term behavioral alterations induced by adolescent THC exposure and diminishes the long-term changes in PFC molecular components.
In summary, multiple, independent, preclinical studies have shown that the effects of THC use during adolescence and adulthood are lessened by CBD. The molecular mechanism by which CBD decreases THC’s effect on the brain remains to be established in vivo, but likely involves the negative allosteric modulation of CB1R. Further studies are urgently needed to help develop therapeutics for such conditions as CUD and THC-triggered psychosis and schizophrenia.
8]. OUTLOOK
This review raises questions that point to future research directions. First, we need more, large, placebo-controlled studies to solidify the therapeutic properties of both THC and CBD, as well as for the interaction of their bioactivities using several ratios and for clinical indications suggested by preclinical studies. This commitment will result in the development of multiple new transformative therapeutic options for the treatment of devastating diseases that remain without standard care options. Second, we need better definitions of “psychotropic”, “non-psychotropic” and “impairing.” In particular, we need to better understand to what extent individuals can self-titrate. For example, individuals that smoke Cannabis containing THC, Cannabis containing THC-CBD or vaping THC concentrates self-titrate to reach comparable intoxicating levels317. Remarkably, self-titration of THC is also measured in rodents19,20. Accordingly, it is critical to develop reliable measures of acute THC and CBD bioactivity in preclinical models and humans when administered using common routes (inhaled and oral). Considering that 11-OH-Δ9-THC exhibits comparable activity at CB1R and accumulation in the brain to THC, could this THC metabolite significantly contribute to THC’s bioactivity profile when administered orally? What is the role of P450 enzymes expressed in the brain that metabolize THC and CBD318? How does the bioactivity of artificial cannabinoid agonists compare with THC? For example, what is the bioactivity of JWH-018, a high-affinity and full agonist at CB1R, often infused in illegal products such as Spice and K2, that can be abused176,319?
It remains possible that chronic use of high dose CBD may produce side effects in vulnerable individuals, including during development and when used in combination with other medications. For example, considering that CBD modulates the activity of proteins involved in neuronal pathfinding and maturation, such as CB1R and GPR55, we still do not know what the impact of high dose CBD is on brain development? What medication interact pharmacologically with CBD? We have started to outline the pharmacological interactions between THC and other psychoactive drugs, such as opioids, but we still lack research on CBD’s pharmacological interactions. For example, considering that CBD allosterically modulates GABAAR, does co-administration of benzodiazepine and CBD increase concentration difficulties, confusion, sedation, and motor incoordination? To answer these questions, we need to further define the single agent bioactivities of CBD at various doses and administered through distinct routes, determine its selectivity at specific targets, and consider whether CBD tissue exposure reaches concentrations that will significantly engage and modulate these targets in vivo.
THC and CBD as single agents do not represent the full bioactive profile of Cannabis which contains additional bioactive phyto-CBs and ingredients, such as terpenes320. Thus, another blooming area of research is the bioactivity, mechanism of action, and effects of THC analogues that are produced by Cannabis at low levels and that activate CB1R similarly to THC, such as Δ8-THC and Δ9-tetrahydrocannabiphorol. These analogues can be concentrated via extraction procedures321,322. For example, Δ8-THC (1–6 mg) triggers comparable but less potent behavioral changes in healthy human adults than Δ9-THC at similar dose71,323. This research is particularly important considering the recent increase in the US of products that contain Δ8-THC instead of Δ9-THC324.
9]. Conclusions
Research on the bioactivity of THC and CBD started in the 1960s, initially focusing on THC, and more recently rediscovering CBD. We now have a foundational understanding of their distinct molecular mechanisms of action, and how they modify neuronal and glial cell functions in the brain over an individual’s lifetime. Key results, gathered in well-controlled human studies and validated in preclinical model systems, have set the stage for the optimization and development of novel, phyto-CB-based therapeutics for a broad array of devastating neurological and psychiatric diseases. This review summarizes impairing consequences and side effects alongside potential therapeutics, emphasizing the importance of establishing THC and CBD dosages and regimens based on bioactivity (Figure 10). Considering the wide differences and diversity in the molecular targets engaged by THC and CBD, phyto-CB-based therapeutics will require optimization for each medical indication, from sleep disorders, anxiety and drug abuse to refractory childhood epilepsy and autism.
Figure 10: THC and CBD bioactivity occurs along continuum.
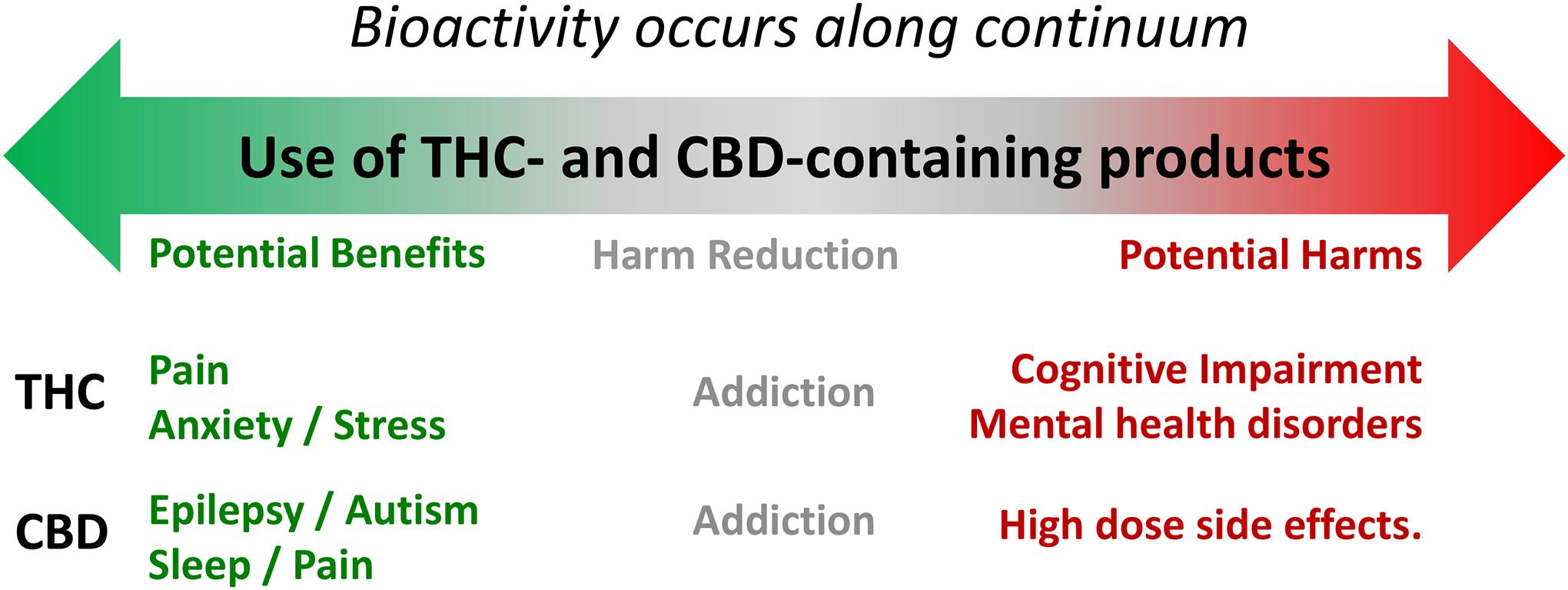
THC exhibits promising therapeutic response for the treatment of chronic pain (green), , potential harm reduction properties in the context of addiction (gray), and triggers impairing effects and enhances the incidence of mental health disorders (red). CBD exhibits promising therapeutic response for the treatment of epileptic seizures, autism, sleep quality and chronic pain, potential harm reduction properties in the context of addiction, and triggers side effects when used at high dose.
Recent development of molecular tools and experimental approaches, validated in rodents, create an opportunity to provide practical and translatable results for humans. Molecular imaging technologies now enable the visualization of targets engaged by phyto-CBs, including PET ligands that target and image CB1R in vivo and can be leveraged to study CB1R pharmacological interaction with other psychoactive drugs, such as nicotine and alcohol325,326. Biosensors that selectively measure changes in THC, CBD and eCB levels in tissue are paving the way to establish the precise real-time changes of these agents in different tissues and how that relates to their bioactivities327–329.
Much of the Cannabis and cannabinoid research over the past 50 years was done in reverse: listening to users to understand bioactivity and to patients to recognize therapeutic benefits before carrying out the needed trials. A perfect illustration of such a research trajectory was the development of CBD-containing therapeutics for the treatment of epilepsies: patients and their families spurred the study and optimization of the anti-seizure properties of CBD, and this novel therapeutic approach was eventually proven in preclinical studies and human trials. The current thriving and innovative field of Cannabis and cannabinoid research is reversing this trajectory. We are poised to optimize cannabinoids, develop multiple lines of transformative medications, and guide their safe use in the growing legal market of Cannabis-based products.
Acknowledgements
National Institute of Health (DA051558, DA047626 and NS118130 to N.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: N.S. is employed by the University of Washington, Seattle, and by Stella Consulting LLC. The terms of this arrangement have been reviewed and approved by the University of Washington in accordance with its policies governing outside work and financial conflicts of interest in research.
References
- 1.Carlini BH & Schauer GL Cannabis-only use in the USA: prevalence, demographics, use patterns, and health indicators. Journal of cannabis research 4, 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences, E. & Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. (2017). [PubMed]
- 3.Hines LA et al. Association of high-potency cannabis use with mental health and substance use in adolescence. JAMA psychiatry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasin DS et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA psychiatry 74, 579–588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smart R, Caulkins JP, Kilmer B, Davenport S & Midgette G Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state. Addiction 112, 2167–2177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman TP & Lorenzetti V ‘Standard THC units’: a proposal to standardize dose across all cannabis products and methods of administration. Addiction 115, 1207–1216 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Barrus DG, Lefever TW & Wiley JL Evaluation of reinforcing and aversive effects of voluntary Δ9-tetrahydrocannabinol ingestion in rats. Neuropharmacology 137, 133–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newmeyer MN et al. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clinical Chemistry 62, 1579–1592 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Marsot A et al. Comparison of cannabinoid concentrations in plasma, oral fluid and urine in occasional cannabis smokers after smoking cannabis cigarette. Journal of Pharmacy & Pharmaceutical Sciences 19, 411–422 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Grant KS, Petroff R, Isoherranen N, Stella N & Burbacher TM Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther 182, 133–151, doi: 10.1016/j.pharmthera.2017.08.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H et al. Structural and functional interaction of δ9-tetrahydrocannabinol with liver fatty acid binding protein (FABP1). Biochemistry 57, 6027–6042 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Dinis-Oliveira RJ Metabolomics of Δ9-tetrahydrocannabinol: implications in toxicity. Drug metabolism reviews 48, 80–87 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Parikh N, Kramer WG, Khurana V, Smith CC & Vetticaden S Bioavailability study of dronabinol oral solution versus dronabinol capsules in healthy volunteers. Clinical pharmacology: advances and applications 8, 155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karschner EL, Darwin WD, Goodwin RS, Wright S & Huestis MA Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clinical chemistry 57, 66–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M et al. Chronic adolescent Δ9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis and cannabinoid research 2, 235–246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dow-Edwards D & Zhao N Oral THC produces minimal behavioral alterations in preadolescent rats. Neurotoxicology and teratology 30, 385–389 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torrens A et al. Comparative pharmacokinetics of Δ9-tetrahydrocannabinol in adolescent and adult male mice. Journal of Pharmacology and Experimental Therapeutics 374, 151–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manwell LA et al. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part I: Development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. Journal of pharmacological and toxicological methods 70, 120–127 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Kruse LC, Cao JK, Viray K, Stella N & Clark JC Voluntary oral consumption of Δ9-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham AD et al. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 45, 1105–1114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smoker MP, Mackie K, Lapish CC & Boehm II SL Self-administration of edible Δ9-tetrahydrocannabinol and associated behavioral effects in mice. Drug and alcohol dependence 199, 106–115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freels TG et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. Journal of Neuroscience 40, 1897–1908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taffe MA, Creehan KM, Vandewater SA, Kerr TM & Cole M Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Experimental and clinical psychopharmacology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz C et al. Pharmacokinetic and pharmacodynamic properties of aerosolized (“vaped”) THC in adolescent male and female rats. Psychopharmacology 238, 3595–3605 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narimatsu S, Watanabe K, Yamamoto I & Yoshimura H Sex difference in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochemical pharmacology 41, 1187–1194 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Spiro AS, Wong A, Boucher AA & Arnold JC Enhanced brain disposition and effects of Δ9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PloS one 7, e35937 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonhomme-Faivre L, Benyamina A, Reynaud M, Farinotti R & Abbara C PRECLINICAL STUDY: Disposition of Δ9 tetrahydrocannabinol in CF1 mice deficient in mdr1a P-glycoprotein. Addiction biology 13, 295–300 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Matheson J et al. Association between ABCB1 rs2235048 Polymorphism and THC Pharmacokinetics and Subjective Effects following Smoked Cannabis in Young Adults. Brain Sciences 12, 1189 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC & Bonner TI Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Huestis MA et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of general psychiatry 58, 322–328 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Lutz B Neurobiology of cannabinoid receptor signaling. Dialogues in Clinical Neuroscience 22, 207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning JJ, Green HM, Glass M & Finlay DB Pharmacological selection of cannabinoid receptor effectors: Signalling, allosteric modulation and bias. Neuropharmacology 193, 108611 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Leo LM & Abood ME CB1 cannabinoid receptor signaling and biased signaling. Molecules 26, 5413 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horváth VB et al. Optimization of the Heterologous Expression of the Cannabinoid Type-1 (CB1) Receptor. Frontiers in Endocrinology 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costas-Insua C et al. Identification of BiP as a CB1 receptor-interacting protein that fine-tunes cannabinoid signaling in the mouse brain. Journal of Neuroscience 41, 7924–7941 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith TH et al. Cannabinoid receptor–interacting protein 1a modulates CB1 receptor signaling and regulation. Molecular pharmacology 87, 747–765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busquets-Garcia A, Bains J & Marsicano G CB1 receptor signaling in the brain: extracting specificity from ubiquity. Neuropsychopharmacology 43, 4–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stella N Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030, doi: 10.1002/glia.20983 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eraso-Pichot A et al. Endocannabinoid signaling in astrocytes. Glia (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina-Holgado E et al. Endocannabinoid signaling in oligodendroglia. Glia (2022). [DOI] [PubMed] [Google Scholar]
- 41.Vendel E & de Lange E Functions of the CB1 and CB2 receptors in neuroprotection at the level of the blood–brain barrier. Neuromolecular medicine 16, 620–642 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Chung YC et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Experimental & molecular medicine 48, e205–e205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ & Dittel BN Modulation of the cannabinoid CB receptor in microglial cells in response to inflammatory stimuli. J Neurochem (2005). [DOI] [PubMed] [Google Scholar]
- 44.Van Sickle MD et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332, doi: 10.1126/science.1115740 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Zoratti C, Kipmen-Korgun D, Osibow K, Malli R & Graier WF Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. British journal of pharmacology 140, 1351–1362 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H-C & Mackie K Review of the endocannabinoid system. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6, 607–615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araque A, Castillo PE, Manzoni OJ & Tonini R Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janssen FJ & van der Stelt M Inhibitors of diacylglycerol lipases in neurodegenerative and metabolic disorders. Bioorganic & medicinal chemistry letters 26, 3831–3837 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Cao JK, Kaplan J & Stella N ABHD6: Its Place in Endocannabinoid Signaling and Beyond. Trends in pharmacological sciences (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Egmond N, Straub VM & van der Stelt M Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annual Review of Pharmacology and Toxicology 61, 441–463 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Bernal-Chico A et al. Endocannabinoid signaling in brain diseases: Emerging relevance of glial cells. Glia (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley BG & Thayer SA Delta 9-tetrahydrocannabinol antagonizes endocannabinoid modulation of synaptic transmission between hippocampal neurons in culture. Neuropharmacology 46, 709–715 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Shao Z et al. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nature chemical biology 15, 1199–1205 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Hua T et al. Activation and signaling mechanism revealed by cannabinoid receptor-Gi complex structures. Cell 180, 655–665. e618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallée M et al. Pregnenolone can protect the brain from cannabis intoxication. science 343, 94–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busquets-Garcia A et al. Pregnenolone blocks cannabinoid-induced acute psychotic-like states in mice. Molecular psychiatry 22, 1594–1603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong W, Wu X, Lovinger DM & Zhang L A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. Journal of Neuroscience 32, 5200–5208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z et al. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochemical pharmacology 76, 1014–1023 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Xiong W et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol 7, 296–303, doi:nchembio.552 [pii] 10.1038/nchembio.552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauckner JE et al. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 105, 2699–2704, doi:0711278105 [pii] 10.1073/pnas.0711278105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sylantyev S, Jensen TP, Ross RA & Rusakov DA Cannabinoid-and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proceedings of the National Academy of Sciences 110, 5193–5198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lingerfelt MA et al. Identification of crucial amino acid residues involved in agonist signaling at the GPR55 receptor. Biochemistry 56, 473–486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foster SR et al. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell 179, 895–908. e821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kapur A et al. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem 284, 29817–29827, doi:M109.050187 [pii] 10.1074/jbc.M109.050187 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietr M et al. Differential changes in GPR55 during microglial cell activation. FEBS Lett 583, 2071–2076, doi: 10.1016/j.febslet.2009.05.028 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Kallendrusch S et al. The G protein-coupled receptor 55 ligand l-α-lysophosphatidylinositol exerts microglia-dependent neuroprotection after excitotoxic lesion. Glia 61, 1822–1831 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Barann M et al. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. British journal of pharmacology 137, 589–596 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong W, Hosoi M, Koo B-N & Zhang L Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Molecular pharmacology 73, 314–322 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Gaoni Y & Mechoulam R Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc 86, 1646–1647 (1964). [Google Scholar]
- 70.Hollister LE Tetrahydrocannabinol isomers and homologues: contrasted effects of smoking. Nature 227, 968–969 (1970). [DOI] [PubMed] [Google Scholar]
- 71.Hollister LE & Gillespie H Delta-8-and delta-9-tetrahydrocannabinol; Comparison in man by oral and intravenous administration. Clinical Pharmacology & Therapeutics 14, 353–357 (1973). [DOI] [PubMed] [Google Scholar]
- 72.Holtzman D, Lovell RA, Jaffe JH & Freedman DX 1-Δ9-tetrahydrocannabinol: neurochemical and behavioral effects in the mouse. Science 163, 1464–1467 (1969). [DOI] [PubMed] [Google Scholar]
- 73.Beardsley PM, Scimeca JA & Martin BR Studies on the agonistic activity of! D9–21-tetrahydrocannabinol in mice, dogs and rhesus monkeys and its interactions with! D–9-tetrahydrocannabinol. The Journal of Pharmacology and Experimental Therapeutics (1987). [PubMed] [Google Scholar]
- 74.Metna-Laurent M, Mondésir M, Grel A, Vallée M & Piazza PV Cannabinoid-induced tetrad in mice. Current protocols in neuroscience 80, 9.59. 51–59.59. 10 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Monory K et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol 5, e269, doi:07-PLBI-RA-0386 [pii] 10.1371/journal.pbio.0050269 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Häring M, Kaiser N, Monory K & Lutz B Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PloS one 6, e26617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han X et al. CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ 9-tetrahydrocannabinol (Δ 9-THC)-induced aversive effects in mice. Scientific reports 7, 1–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner BD et al. Cannabinoid type 1 receptors in A2a neurons contribute to cocaine-environment association. Psychopharmacology, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lafenetre P, Chaouloff F & Marsicano G Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology 57, 715–721, doi:S0028–3908(09)00219–6 [pii] 10.1016/j.neuropharm.2009.07.014 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Bellocchio L et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13, 281–283, doi:nn.2494 [pii] 10.1038/nn.2494 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Soria-Gomez E et al. Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron (2021). [DOI] [PubMed] [Google Scholar]
- 82.Kasten CR, Zhang Y & Boehm SL Acute cannabinoids produce robust anxiety-like and locomotor effects in mice, but long-term consequences are age-and sex-dependent. Frontiers in behavioral neuroscience 13, 32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell VA et al. Oral efficacy of Δ (9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model. Neuropharmacology 189, 108529 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Bruijnzeel AW et al. Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PloS one 11, e0153327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vann RE et al. Discriminative stimulus properties of Δ9-tetrahydrocannabinol (THC) in C57BL/6J mice. European journal of pharmacology 615, 102–107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bass CE & Martin BR Time course for the induction and maintenance of tolerance to Δ9-tetrahydrocannabinol in mice. Drug and alcohol dependence 60, 113–119 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Morgan DJ et al. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. Journal of Neuroscience 34, 5152–5163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parks C, Jones BC, Moore BM & Mulligan MK Sex and Strain Variation in Initial Sensitivity and Rapid Tolerance to Δ9–Tetrahydrocannabinol. Cannabis and Cannabinoid Research 5, 231–245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Missig G et al. Altered sleep during spontaneous cannabinoid withdrawal in male mice. Behavioural Pharmacology 33, 195–205 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kesner AJ et al. Sex-dependent changes in murine striatal dopamine release, sleep, and behavior during spontaneous Δ-9-tetrahydrocannabinol abstinence. bioRxiv (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Micallef J et al. Cannabis smoking impairs driving performance on the simulator and real driving: a randomized, double-blind, placebo-controlled, crossover trial. Fundamental & clinical pharmacology 32, 558–570 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Windle SB et al. Association between legalization of recreational cannabis and fatal motor vehicle collisions in the United States: an ecologic study. Canadian Medical Association Open Access Journal 9, E233–E241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ledent C et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283, 401–404 (1999). [DOI] [PubMed] [Google Scholar]
- 94.Wiley JL Sex-dependent effects of Δ9-tetrahydrocannabinol on locomotor activity in mice. Neuroscience letters 352, 77–80 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Blazquez C et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 134, 119–136, doi:awq278 [pii] 10.1093/brain/awq278 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Bonm AV et al. Control of exploration, motor coordination and amphetamine sensitization by cannabinoid CB1 receptors expressed in medium spiny neurons. European Journal of Neuroscience (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walker FO in Lancet Vol. 369 218–228 (Elsevier, 2007). [DOI] [PubMed] [Google Scholar]
- 98.Wang W et al. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol 590, 3743–3769, doi: 10.1113/jphysiol.2012.235200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bamford NS, Wightman RM & Sulzer D Dopamine’s Effects on Corticostriatal Synapses during Reward-Based Behaviors. Neuron 97, 494–510, doi: 10.1016/j.neuron.2018.01.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M & Watanabe M Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89, 309–380, doi:89/1/309 [pii] 10.1152/physrev.00019.2008 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Glass M, Dragunow M & Faull RLM Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318 (1997). [DOI] [PubMed] [Google Scholar]
- 102.Tsou K, Brown S, Sanudo-Pena MC, Mackie K & Walker JM Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 (1998). [DOI] [PubMed] [Google Scholar]
- 103.Davis MI et al. The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS One 13, e0191436, doi: 10.1371/journal.pone.0191436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hohmann AG & Herkenham M Localization of cannabinoid CB1 receptor mRNA in neuronal subpopulations of rat striatum: A double-label in situ hybridization study. Synapse 37, 71–80 (2000). [DOI] [PubMed] [Google Scholar]
- 105.Hu SS-J & Mackie K Distribution of the endocannabinoid system in the central nervous system. Endocannabinoids, 59–93 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Uchigashima M et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 27, 3663–3676, doi:27/14/3663 [pii] 10.1523/JNEUROSCI.0448-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin HH & Lovinger DM Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc. Nat. Acad. Sci 103, 8251–8256, doi: 10.1073/pnas.0510797103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bamford NS et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42, 653–663 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Pickel VM, Chan J, Kearn CS & Mackie K Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. The Journal of comparative neurology 495, 299–313, doi: 10.1002/cne.20881 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marcellino D et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 54, 815–823, doi: 10.1016/j.neuropharm.2007.12.011 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Nagai H et al. Antipsychotics improve Δ9-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacology Biochemistry and Behavior 84, 330–336 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Marques AM et al. Effects of combined 5-HT2A and cannabinoid receptor modulation on a schizophrenia-related prepulse inhibition deficit in mice. Psychopharmacology 237, 1643–1655 (2020). [DOI] [PubMed] [Google Scholar]
- 113.Barnard IL et al. The effects of acute Cannabis smoke or Δ9-THC injections on the trial-unique, nonmatching-to-location and five-choice serial reaction time tasks in male Long-Evans rats. Neurobiology of learning and memory 192, 107624 (2022). [DOI] [PubMed] [Google Scholar]
- 114.Gilman JM et al. Identification ofΔ 9-tetrahydrocannabinol (THC) impairment using functional brain imaging. Neuropsychopharmacology, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fortin DA & Levine ES Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cerebral Cortex 17, 163–174 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Poulia N et al. Detrimental effects of adolescent escalating low-dose Δ9-tetrahydrocannabinol leads to a specific bio-behavioural profile in adult male rats. British Journal of Pharmacology 178, 1722–1736 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Marsicano G et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003). [DOI] [PubMed] [Google Scholar]
- 118.Den Boon FS et al. Activation of type-1 cannabinoid receptor shifts the balance between excitation and inhibition towards excitation in layer II/III pyramidal neurons of the rat prelimbic cortex. Pflügers Archiv-European Journal of Physiology 467, 1551–1564 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Caballero A, Granberg R & Tseng KY Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews 70, 4–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lui JH et al. Differential encoding in prefrontal cortex projection neuron classes across cognitive tasks. Cell 184, 489–506. e426 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morgunova A & Flores C in Seminars in Cell & Developmental Biology. (Elsevier; ). [Google Scholar]
- 122.Tinklenberg J, Melges F, Hollister L & Gillespie H Marijuana and immediate memory. Nature 226, 1171–1172 (1970). [DOI] [PubMed] [Google Scholar]
- 123.Adam K, Doss MK, Pabon E, Vogel EK & de Wit H Δ9-Tetrahydrocannabinol (THC) impairs visual working memory performance: a randomized crossover trial. Neuropsychopharmacology 45, 1807–1816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varvel S, Hamm R, Martin B & Lichtman A Differential effects of Δ9-THC on spatial reference and working memory in mice. Psychopharmacology 157, 142–150 (2001). [DOI] [PubMed] [Google Scholar]
- 125.Cha YM, Jones KH, Kuhn CM, Wilson WA & Swartzwelder HS Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behavioural pharmacology 18, 563–569 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Chen R et al. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 155, 1154–1165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Robbe D et al. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci 9, 1526–1533, doi:nn1801 [pii] 10.1038/nn1801 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Hebert-Chatelain E et al. A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Maroso M et al. Cannabinoid control of learning and memory through HCN channels. Neuron 89, 1059–1073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Busquets-Garcia A et al. Hippocampal protein kinase C signaling mediates the short-term memory impairment induced by delta9-tetrahydrocannabinol. Neuropsychopharmacology 43, 1021–1031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hampson RE & Deadwyler SA Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of Neuroscience 20, 8932–8942 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tournier BB & Ginovart N Repeated but not acute treatment withΔ 9-tetrahydrocannabinol disrupts prepulse inhibition of the acoustic startle: Reversal by the dopamine D2/3 receptor antagonist haloperidol. European Neuropsychopharmacology 24, 1415–1423 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Dar MS Cerebellar CB1 receptor mediation of Δ9-THC-induced motor incoordination and its potentiation by ethanol and modulation by the cerebellar adenosinergic A1 receptor in the mouse. Brain research 864, 186–194 (2000). [DOI] [PubMed] [Google Scholar]
- 134.Saravia R et al. Concomitant THC and stress adolescent exposure induces impaired fear extinction and related neurobiological changes in adulthood. Neuropharmacology 144, 345–357 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Calabrese EJ & Rubio-Casillas A Biphasic effects of THC in memory and cognition. European journal of clinical investigation 48, e12920 (2018). [DOI] [PubMed] [Google Scholar]
- 136.D’Souza DC et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29, 1558–1572 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Mennis J, Stahler GJ & McKeon TP Young adult cannabis use disorder treatment admissions declined as past month cannabis use increased in the US: An analysis of states by year, 2008–2017. Addictive behaviors 123, 107049 (2021). [DOI] [PubMed] [Google Scholar]
- 138.Antonia M & Viviana T Behavioral consequences of pre/peri-natal Cannabis exposure. Cannabis and the Developing Brain Elsevier; (2022). [Google Scholar]
- 139.Roberto F & Miriam M Effects of prenatal THC exposure on the mesolimbic dopamine system: Unveiling an endophenotype of sensory information processing deficits. Cannabis and the Developing Brain Elsevier; (2022). [Google Scholar]
- 140.Di Marzo V, Stella N & Zimmer A Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16, 30–42, doi: 10.1038/nrn3876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Albaugh MD et al. Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA psychiatry (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barrington-Trimis JL et al. Risk of persistence and progression of use of 5 cannabis products after experimentation among adolescents. JAMA network open 3, e1919792–e1919792 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hammond CJ, Chaney A, Hendrickson B & Sharma P Cannabis use among US adolescents in the era of marijuana legalization: a review of changing use patterns, comorbidity, and health correlates. International review of psychiatry 32, 221–234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knapp AA et al. Emerging trends in cannabis administration among adolescent cannabis users. Journal of Adolescent Health 64, 487–493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hines LA et al. Association of high-potency cannabis use with mental health and substance use in adolescence. JAMA psychiatry 77, 1044–1051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gunn RL, Aston ER, Sokolovsky AW, White HR & Jackson KM Complex cannabis use patterns: Associations with cannabis consequences and cannabis use disorder symptomatology. Addictive behaviors 105, 106329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arterberry BJ, Padovano HT, Foster KT, Zucker RA & Hicks BM Higher average potency across the United States is associated with progression to first cannabis use disorder symptom. Drug and alcohol dependence 195, 186–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Camchong J, Lim KO & Kumra S Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cerebral cortex 27, 1922–1930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schoeler T, Ferris J & Winstock AR Rates and correlates of cannabis-associated psychotic symptoms in over 230,000 people who use cannabis. Translational psychiatry 12, 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Betz LT, Penzel N & Kambeitz J A network approach to relationships between cannabis use characteristics and psychopathology in the general population. Scientific reports 12, 1–10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morrison P et al. The acute effects of synthetic intravenous Δ9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychological medicine 39, 1607–1616 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Van der Steur SJ, Batalla A & Bossong MG Factors moderating the association between cannabis use and psychosis risk: a systematic review. Brain sciences 10, 97 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Di Forti M et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophrenia bulletin 40, 1509–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Di Forti M et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. The Lancet Psychiatry 6, 427–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pierre JM, Gandal M & Son M Cannabis-induced psychosis associated with high potency “wax dabs”. Schizophrenia research 172, 211–212 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Gobbi G et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA psychiatry 76, 426–434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jouroukhin Y et al. Adolescent Δ9-tetrahydrocannabinol exposure and astrocyte-specific genetic vulnerability converge on nuclear factor-κB–cyclooxygenase-2 signaling to impair memory in adulthood. Biological psychiatry 85, 891–903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen H-T & Mackie K Adolescent Δ9-Tetrahydrocannabinol Exposure Selectively Impairs Working Memory but Not Several Other mPFC-Mediated Behaviors. Frontiers in psychiatry 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hempel BJ, Wakeford AG, Clasen MM, Friar MA & Riley AL Delta-9-tetrahydrocannabinol (THC) history fails to affect THC’s ability to induce place preferences in rats. Pharmacology Biochemistry and Behavior 144, 1–6 (2016). [DOI] [PubMed] [Google Scholar]
- 160.Flores Á, Maldonado R & Berrendero F THC exposure during adolescence does not modify nicotine reinforcing effects and relapse in adult male mice. Psychopharmacology 237, 801–809 (2020). [DOI] [PubMed] [Google Scholar]
- 161.Szkudlarek HJ et al. Δ-9-Tetrahydrocannabinol and cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology 44, 817–825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.VanRyzin JW et al. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron 102, 435–449. e436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee H-L et al. Frequent low-dose Δ9-tetrahydrocannabinol in adolescence disrupts microglia homeostasis and disables responses to microbial infection and social stress in young adulthood. Biological psychiatry (in press) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Erica Z, Cristina M, Marina G, Tiziana Rubino & Daniela P Correlates and consequences of cannabinoid exposure on adolescent brain remodeling: Focus on glial cells and epigenetics. Cannabis and the Developing Brain Elsevier (2022). [Google Scholar]
- 165.Parsons LH & Hurd YL Endocannabinoid signalling in reward and addiction. Nature Reviews Neuroscience 16, 579–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ellgren M, Spano SM & Hurd YL Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32, 607–615 (2007). [DOI] [PubMed] [Google Scholar]
- 167.Tomasiewicz HC et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological psychiatry 72, 803–810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lecca D et al. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology 166, 107974 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Friedman AL, Meurice C & Jutkiewicz EM Effects of adolescent Δ9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague–Dawley rats. Experimental and clinical psychopharmacology 27, 326 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Cheer JF et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. Journal of Neuroscience 27, 791–795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Corbillé A-G et al. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. Journal of Neuroscience 27, 6937–6947 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Covey DP, Bunner KD, Schuweiler DR, Cheer JF & Garris PA Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. European Journal of Neuroscience 43, 1661–1673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Deng L, Viray K, Singh S, Cravatt B & Stella N ABHD6 Controls Amphetamine-Stimulated Hyperlocomotion: Involvement of CB1 Receptors. Cannabis and Cannabinoid Research (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.English A, Land BB & Stella N Impact of adolescent THC exposure on later adulthood: Focus on mesocorticolimbic function and behaviors. Cannabis and the Developing Brain. Elsevier (2022). [Google Scholar]
- 175.Pickel VM et al. Chronic adolescent exposure toΔ 9-tetrahydrocannabinol decreases NMDA current and extrasynaptic plasmalemmal density of NMDA GluN1 subunits in the prelimbic cortex of adult male mice. Neuropsychopharmacology 45, 374–383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Renard J et al. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Scientific reports 7, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Renard J, Norris C, Rushlow W & Laviolette SR Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neuroscience & Biobehavioral Reviews 75, 157–165 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Rubino T et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiology of disease 73, 60–69 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Buxbaum D Analgesic activity of Δ9-tetrahydrocannabinol in the rat and mouse. Psychopharmacologia 25, 275–280 (1972). [DOI] [PubMed] [Google Scholar]
- 180.Fisher E et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: a protocol for an overview of systematic reviews and a systematic review of randomised controlled trials. Pain reports 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Almog S et al. The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: A randomized, double-blinded, placebo-controlled trial. European Journal of Pain 24, 1505–1516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soliman N et al. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain 162, S26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Finn DP et al. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162, S5–S25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gutierrez A et al. Effects of combined THC and heroin vapor inhalation in rats. Psychopharmacology 239, 1321–1335 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deng L et al. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry 77, 475–487, doi: 10.1016/j.biopsych.2014.04.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gutierrez T, Crystal JD, Zvonok AM, Makriyannis A & Hohmann AG Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain. Pain 152, 1976–1987 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee MT, Mackie K & Chiou LC Alternative pain management via endocannabinoids in the time of the opioid epidemic: Peripheral neuromodulation and pharmacological interventions. British Journal of Pharmacology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Belendiuk KA, Babson KA, Vandrey R & Bonn-Miller MO Cannabis species and cannabinoid concentration preference among sleep-disturbed medicinal cannabis users. Addictive Behaviors 50, 178–181 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Fabbri M et al. Measuring subjective sleep quality: a review. International journal of environmental research and public health 18, 1082 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Prasad B, Radulovacki MG & Carley DW Proof of concept trial of dronabinol in obstructive sleep apnea. Frontiers in Psychiatry 4, 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kuhathasan N et al. The use of cannabinoids for sleep: A critical review on clinical trials. Experimental and clinical psychopharmacology 27, 383 (2019). [DOI] [PubMed] [Google Scholar]
- 192.Bonn-Miller MO, Babson KA & Vandrey R Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug and alcohol dependence 136, 162–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD & Bursac Z Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. Journal of substance abuse treatment 35, 362–368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Monti JM & Pandi-Perumal SR Clinical Management of Sleep and Sleep Disorders With Cannabis and Cannabinoids: Implications to Practicing Psychiatrists. Clinical Neuropharmacology 45, 27–31 (2022). [DOI] [PubMed] [Google Scholar]
- 195.Roitman P, Mechoulam R, Cooper-Kazaz R & Shalev A Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clinical drug investigation 34, 587–591 (2014). [DOI] [PubMed] [Google Scholar]
- 196.Fraser GA The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS neuroscience & therapeutics 15, 84–88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jetly R, Heber A, Fraser G & Boisvert D The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 51, 585–588 (2015). [DOI] [PubMed] [Google Scholar]
- 198.Kesner AJ et al. Changes in striatal dopamine release, sleep, and behavior during spontaneous Δ-9-tetrahydrocannabinol abstinence in male and female mice. Neuropsychopharmacology, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Silvani A et al. Multiple sleep alterations in mice lacking cannabinoid type 1 receptors. PloS one 9, e89432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Corcoran ME, McCaughran JA Jr & Wada JA Acute antiepileptic effects of Δ9-tetrahydrocannabinol in rats with kindled seizures. Experimental neurology 40, 471–483 (1973). [DOI] [PubMed] [Google Scholar]
- 201.Wallace MJ, Blair RE, Falenski KW, Martin BR & DeLorenzo RJ The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 307, 129–137 (2003). [DOI] [PubMed] [Google Scholar]
- 202.Krook-Magnuson E et al. In vivo evaluation of the dentate gate theory in epilepsy. The Journal of physiology 593, 2379–2388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dengler CG & Coulter D Normal and epilepsy-associated pathologic function of the dentate gyrus. Progress in brain research 226, 155–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shen M & Thayer SA Ð9-Tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Molecular Pharmacology 55, 8–13 (1999). [DOI] [PubMed] [Google Scholar]
- 205.Whalley BJ et al. Species-specific susceptibility to cannabis-induced convulsions. British Journal of Pharmacology 176, 1506–1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huizenga MN, Fureman BE, Soltesz I & Stella N Proceedings of the Epilepsy Foundation’s 2017 Cannabinoids in Epilepsy Therapy Workshop. Epilepsy & Behavior 85, 237–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zareie P, Sadegh M, Palizvan MR & Moradi-Chameh H Anticonvulsive effects of endocannabinoids; an investigation to determine the role of regulatory components of endocannabinoid metabolism in the Pentylenetetrazol induced tonic-clonic seizures. Metabolic brain disease 33, 939–948 (2018). [DOI] [PubMed] [Google Scholar]
- 208.Von Rüden E, Bogdanovic R, Wotjak C & Potschka H Inhibition of monoacylglycerol lipase mediates a cannabinoid 1-receptor dependent delay of kindling progression in mice. Neurobiology of disease 77, 238–245 (2015). [DOI] [PubMed] [Google Scholar]
- 209.Roebuck AJ et al. Positive allosteric modulation of type 1 cannabinoid receptors reduces spike-and-wave discharges in Genetic Absence Epilepsy Rats from Strasbourg. Neuropharmacology 190, 108553 (2021). [DOI] [PubMed] [Google Scholar]
- 210.van Amerongen G et al. Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clinical therapeutics 40, 1467–1482 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Di Marzo V & Centonze D Placebo effects in a multiple sclerosis spasticity enriched clinical trial with the oromucosal cannabinoid spray (THC/CBD): dimension and possible causes. CNS neuroscience & therapeutics 21, 215–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maresz K et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med 13, 492–497, doi:nm1561 [pii] 10.1038/nm1561 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Lyman W, Sonett J, Brosnan C, Elkin R & Bornstein M Δ9-Tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. Journal of neuroimmunology 23, 73–81 (1989). [DOI] [PubMed] [Google Scholar]
- 214.Baker D et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404, 84–87 (2000). [DOI] [PubMed] [Google Scholar]
- 215.Pryce G et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126, 2191–2202 (2003). [DOI] [PubMed] [Google Scholar]
- 216.López-Sendón Moreno JL et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. Journal of neurology 263, 1390–1400 (2016). [DOI] [PubMed] [Google Scholar]
- 217.Naydenov AV et al. Genetic rescue of CB1 receptors on medium spiny neurons prevents loss of excitatory striatal synapses but not motor impairment in HD mice. Neurobiol Dis 71, 140–150, doi: 10.1016/j.nbd.2014.08.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Woodman B et al. The Hdh(Q150/Q150) knock-in mouse model of HD and the R6/2 exon 1 model develop comparable and widespread molecular phenotypes. Brain Res Bull 72, 83–97, doi:S0361–9230(06)00352–2 [pii] 10.1016/j.brainresbull.2006.11.004 (2007). [DOI] [PubMed] [Google Scholar]
- 219.Horne EA et al. Downregulation of cannabinoid receptor 1 from neuropeptide Y interneurons in the basal ganglia of patients with Huntington’s disease and mouse models. Eur J Neurosci 37, 429–440, doi: 10.1111/ejn.12045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ruiz-Calvo A et al. Astroglial monoacylglycerol lipase controls mutant huntingtin-induced damage of striatal neurons. Neuropharmacology (2019). [DOI] [PubMed] [Google Scholar]
- 221.Chiarlone A et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proceedings of the National Academy of Sciences 111, 8257–8262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sagredo O et al. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J Neurosci Res 89, 1509–1518, doi: 10.1002/jnr.22682 (2011). [DOI] [PubMed] [Google Scholar]
- 223.Liebling JP, Clarkson NJ, Gibbs BW, Yates AS & O’Sullivan SE An analysis of over-the-counter cannabidiol products in the United Kingdom. Cannabis and Cannabinoid Research 7, 207–213 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spindle TR, Bonn-Miller MO & Vandrey R Changing landscape of cannabis: novel products, formulations, and methods of administration. Current opinion in psychology 30, 98–102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Melzer R, McCabe PF & Schilling S Evolution, genetics and biochemistry of plant cannabinoid synthesis: a challenge for biotechnology in the years ahead. Current Opinion in Biotechnology 75, 102684 (2022). [DOI] [PubMed] [Google Scholar]
- 226.Bergeria CL et al. Pharmacokinetic Profile of Δ9-Tetrahydrocannabinol, Cannabidiol, and Metabolites in Blood Following Vaporization and Oral Ingestion of Cannabidiol Products. Journal of Analytical Toxicology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Devinsky O, Kraft K, Rusch L, Fein M & Leone-Bay A Improved bioavailability with dry powder cannabidiol inhalation: A phase 1 clinical study. Journal of pharmaceutical sciences 110, 3946–3952 (2021). [DOI] [PubMed] [Google Scholar]
- 228.Deiana S et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 219, 859–873 (2012). [DOI] [PubMed] [Google Scholar]
- 229.Bornheim LM & Grillo MP Characterization of cytochrome P450 3A inactivation by cannabidiol: possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chemical research in toxicology 11, 1209–1216 (1998). [DOI] [PubMed] [Google Scholar]
- 230.Anderson LL et al. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 60, 2224–2234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Geffrey AL, Pollack SF, Bruno PL & Thiele EA Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 56, 1246–1251 (2015). [DOI] [PubMed] [Google Scholar]
- 232.Thomas BF, Gilliam AF, Burch DF, Roche MJ & Seltzman HH Comparative receptor binding analyses of cannabinoid agonists and antagonists. Journal of Pharmacology and Experimental Therapeutics 285, 285–292 (1998). [PubMed] [Google Scholar]
- 233.Jakowiecki J et al. Allosteric Modulation of the CB1 Cannabinoid Receptor by Cannabidiol—A Molecular Modeling Study of the N-Terminal Domain and the Allosteric-Orthosteric Coupling. Molecules 26, 2456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Straiker A, Dvorakova M, Zimmowitch A & Mackie K Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Molecular pharmacology 94, 743–748 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Laprairie RB, Bagher AM, Kelly ME, Dupre DJ & Denovan-Wright EM Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem 289, 24845–24862, doi: 10.1074/jbc.M114.557025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Laprairie R, Bagher A, Kelly M & Denovan-Wright E Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British journal of pharmacology 172, 4790–4805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hill JD, Zuluaga-Ramirez V, Gajghate S, Winfield M & Persidsky Y Activation of GPR55 increases neural stem cell proliferation and promotes early adult hippocampal neurogenesis. British journal of pharmacology 175, 3407–3421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ross RA The enigmatic pharmacology of GPR55. Trends in pharmacological sciences 30, 156–163 (2009). [DOI] [PubMed] [Google Scholar]
- 239.Kaplan JS, Stella N, Catterall W & Westenbroek RE Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences 114, 11229–11234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Devinsky O et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. New England Journal of Medicine 376, 2011–2020 (2017). [DOI] [PubMed] [Google Scholar]
- 241.Chuang S-H, Westenbroek RE, Stella N & Catterall WA Combined Antiseizure Efficacy of Cannabidiol and Clonazepam in a Conditional Mouse Model of Dravet Syndrome. J Exp Neurol 2, 81 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Martínez-Aguirre C et al. Cannabidiol acts at 5-HT1A receptors in the human brain: relevance for treating temporal lobe epilepsy. Frontiers in Behavioral Neuroscience 14, 611278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vitale RM et al. Identification and Characterization of Cannabidiol as an OX1R Antagonist by Computational and In Vitro Functional Validation. Biomolecules 11, 1134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kathmann M, Flau K, Redmer A, Tränkle C & Schlicker E Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. Naunyn-Schmiedeberg’s archives of pharmacology 372, 354–361 (2006). [DOI] [PubMed] [Google Scholar]
- 245.Rudolph U & Knoflach F Beyond classical benzodiazepines: novel therapeutic potential of GABA A receptor subtypes. Nature reviews Drug discovery 10, 685 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bakas T et al. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacological research 119, 358–370 (2017). [DOI] [PubMed] [Google Scholar]
- 247.Golovko T et al. Control of inhibition by the direct action of cannabinoids on GABAA receptors. Cerebral cortex 25, 2440–2455 (2014). [DOI] [PubMed] [Google Scholar]
- 248.Sigel E et al. The major central endocannabinoid directly acts at GABA(A) receptors. Proc Natl Acad Sci U S A 108, 18150–18155, doi:1113444108 [pii] 10.1073/pnas.1113444108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Puthenkalam R et al. Structural studies of GABAA receptor binding sites: which experimental structure tells us what? Frontiers in molecular neuroscience 9, 44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu S et al. Structure of a human synaptic GABA A receptor. Nature 559, 67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mitchell SJ & Silver RA Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445 (2003). [DOI] [PubMed] [Google Scholar]
- 252.Campos AC & Guimarães FS Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Progress in Neuro-Psychopharmacology and Biological Psychiatry 33, 1517–1521 (2009). [DOI] [PubMed] [Google Scholar]
- 253.Costa B, Giagnoni G, Franke C, Trovato AE & Colleoni M Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. British journal of pharmacology 143, 247–250 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Iannotti FA et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS chemical neuroscience 5, 1131–1141 (2014). [DOI] [PubMed] [Google Scholar]
- 255.Mlost J, Kędziora M & Starowicz K Computational Approach Reveals Pronociceptive Potential of Cannabidiol in Osteoarthritis: Role of Transient Receptor Potential Channels. Pharmaceuticals 14, 964 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Muller C & Reggio PH An analysis of the putative CBD binding site in the ionotropic cannabinoid receptors. Frontiers in cellular neuroscience 14, 435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Carrier EJ, Auchampach JA & Hillard CJ Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 103, 7895–7900, doi:0511232103 [pii] 10.1073/pnas.0511232103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hinton DJ, Lee MR, Jang JS & Choi DS Type 1 equilibrative nucleoside transporter regulates astrocyte-specific glial fibrillary acidic protein expression in the striatum. Brain and behavior 4, 903–914 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pastor-Anglada M & Pérez-Torras S Who is who in adenosine transport. Frontiers in pharmacology 9, 627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rimmerman N et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell death & disease 4, e949–e949 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Huang C-W, Lin P-C, Chen J-L & Lee M-J Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1. 4 in Its Slow-Inactivated State and Inhibits Sodium Current. Biomedicines 9, 1141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ghovanloo M-R et al. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. Journal of Biological Chemistry 293, 16546–16558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hollister LE Cannabidiol and cannabinol in man. Experientia 29, 825–826 (1973). [DOI] [PubMed] [Google Scholar]
- 264.Perez-Reyes M, Timmons MC, Davis K & Wall E A comparison of the pharmacological activity in man of intravenously administered 1368–11368-11368–1, cannabinol, and cannabidiol. Experientia 29, 1368–1369 (1973). [DOI] [PubMed] [Google Scholar]
- 265.Devinsky O et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. The Lancet Neurology 15, 270–278 (2016). [DOI] [PubMed] [Google Scholar]
- 266.Hotz J, Fehlmann B, Papassotiropoulos A, de Quervain DJ & Schicktanz NS Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. Journal of Psychiatric Research 143, 327–333 (2021). [DOI] [PubMed] [Google Scholar]
- 267.Bayer H et al. Medial prefrontal cortex mechanisms of cannabidiol-induced aversive memory reconsolidation impairments. Neuropharmacology 205, 108913 (2022). [DOI] [PubMed] [Google Scholar]
- 268.Marsicano G et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003). [DOI] [PubMed] [Google Scholar]
- 269.Espejo-Porras F, Fernández-Ruiz J, Pertwee RG, Mechoulam R & García C Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology 75, 155–163 (2013). [DOI] [PubMed] [Google Scholar]
- 270.Linge R et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 103, 16–26 (2016). [DOI] [PubMed] [Google Scholar]
- 271.Mottarlini F et al. Single and Repeated Exposure to Cannabidiol Differently Modulate BDNF Expression and Signaling in the Cortico-Striatal Brain Network. Biomedicines 10, 1853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mandelbaum DE Cannabidiol in patients with treatment-resistant epilepsy. The Lancet Neurology 15, 544–545 (2016). [DOI] [PubMed] [Google Scholar]
- 273.Savage TE et al. Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy Research 160, 106263 (2020). [DOI] [PubMed] [Google Scholar]
- 274.Devinsky O et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. New England Journal of Medicine 378, 1888–1897 (2018). [DOI] [PubMed] [Google Scholar]
- 275.Barker MJ, Greenwood KM, Jackson M & Crowe SF Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Archives of Clinical Neuropsychology 19, 437–454 (2004). [DOI] [PubMed] [Google Scholar]
- 276.Chamberlain JM et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. Jama 311, 1652–1660 (2014). [DOI] [PubMed] [Google Scholar]
- 277.Jones NA et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. Journal of Pharmacology and Experimental Therapeutics 332, 569–577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Consroe P & Wolkin A Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. Journal of Pharmacology and Experimental Therapeutics 201, 26–32 (1977). [PubMed] [Google Scholar]
- 279.Patra PH et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia 60, 303–314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wright M, Di Ciano P & Brands B Use of cannabidiol for the treatment of anxiety: a short synthesis of preclinical and clinical evidence. Cannabis and cannabinoid research 5, 191–196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Crippa JAS et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of psychopharmacology 25, 121–130 (2011). [DOI] [PubMed] [Google Scholar]
- 282.Bergamaschi MM et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36, 1219–1226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bloomfield MA et al. The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacology 239, 1539–1549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shannon S & Opila-Lehman J Cannabidiol oil for decreasing addictive use of marijuana: a case report. Integrative Medicine: A Clinician’s Journal 14, 31 (2015). [PMC free article] [PubMed] [Google Scholar]
- 285.Shannon S, Lewis N, Lee H & Hughes S Cannabidiol in anxiety and sleep: a large case series. The Permanente Journal 23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sartim AG, Guimarães FS & Joca SRL Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—Possible involvement of 5-HT1A and CB1 receptors. Behavioural brain research 303, 218–227 (2016). [DOI] [PubMed] [Google Scholar]
- 287.de Paula Soares V et al. Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behavioural brain research 213, 225–229 (2010). [DOI] [PubMed] [Google Scholar]
- 288.Franzen JM et al. Cannabidiol attenuates fear memory expression in female rats via hippocampal 5-HT1A but not CB1 or CB2 receptors. Neuropharmacology, 109316 (2022). [DOI] [PubMed] [Google Scholar]
- 289.Nielsen SW et al. Oral cannabidiol for prevention of acute and transient chemotherapy-induced peripheral neuropathy. Supportive Care in Cancer, 1–11 (2022). [DOI] [PubMed] [Google Scholar]
- 290.van Orten-Luiten A-CB, De Roos NM, Majait S, Witteman BJ & Witkamp RF Effects of Cannabidiol chewing gum on perceived pain and well-being of irritable bowel syndrome patients: a placebo-controlled crossover exploratory intervention study with symptom-driven dosing. Cannabis and cannabinoid research 7, 436–444 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Silva-Cardoso GK et al. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: possible role of CB1 and TRPV1 receptors. Neuropharmacology 197, 108712 (2021). [DOI] [PubMed] [Google Scholar]
- 292.Okine BN et al. Antinociceptive effects of the GPR55 antagonist CID16020046 injected into the rat anterior cingulate cortex. Neuroscience 443, 19–29 (2020). [DOI] [PubMed] [Google Scholar]
- 293.Leas EC et al. Self-reported cannabidiol (CBD) use for conditions with proven therapies. JAMA network open 3, e2020977–e2020977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fusar-Poli L et al. Cannabinoids for people with ASD: a systematic review of published and ongoing studies. Brain sciences 10, 572 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fleury-Teixeira P, Caixeta FV, Ramires da Silva LC, Brasil-Neto JP & Malcher-Lopes R Effects of CBD-enriched cannabis sativa extract on autism spectrum disorder symptoms: an observational study of 18 participants undergoing compassionate use. Frontiers in neurology, 1145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pretzsch CM et al. The effect of cannabidiol (CBD) on low-frequency activity and functional connectivity in the brain of adults with and without autism spectrum disorder (ASD). Journal of Psychopharmacology 33, 1141–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hartmann A et al. Cannabidiol attenuates aggressive behavior induced by social isolation in mice: Involvement of 5-HT1A and CB1 receptors. Progress in Neuro-Psychopharmacology and Biological Psychiatry 94, 109637 (2019). [DOI] [PubMed] [Google Scholar]
- 298.Hurd YL Leading the next CBD wave—safety and efficacy. JAMA psychiatry 77, 341–342 (2020). [DOI] [PubMed] [Google Scholar]
- 299.Hurd YL et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. American Journal of Psychiatry 176, 911–922 (2019). [DOI] [PubMed] [Google Scholar]
- 300.Navarrete F, Gasparyan A & Manzanares J CBD-mediated regulation of heroin withdrawal-induced behavioural and molecular changes in mice. Addiction Biology 27, e13150 (2022). [DOI] [PubMed] [Google Scholar]
- 301.Kudrich C, Hurd YL, Salsitz E & Wang A-L Adjunctive Management of Opioid Withdrawal with the Nonopioid Medication Cannabidiol. Cannabis and Cannabinoid Research (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Alegre-Zurano L et al. Cannabidiol decreases motivation for cocaine in a behavioral economics paradigm but does not prevent incubation of craving in mice. Biomedicine & Pharmacotherapy 148, 112708 (2022). [DOI] [PubMed] [Google Scholar]
- 303.Galaj E, Bi G-H, Yang H-J & Xi Z-X Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology 167, 107740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Luján MÁ, Alegre-Zurano L, Martín-Sánchez A, Cantacorps L & Valverde O CB1 receptor antagonist AM4113 reverts the effects of cannabidiol on cue and stress-induced reinstatement of cocaine-seeking behaviour in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry 113, 110462 (2022). [DOI] [PubMed] [Google Scholar]
- 305.Sharifi A et al. Cannabidiol impairs the rewarding effects of methamphetamine: Involvement of dopaminergic receptors in the nucleus accumbens. Progress in Neuro-Psychopharmacology and Biological Psychiatry 113, 110458 (2022). [DOI] [PubMed] [Google Scholar]
- 306.Andaloussi ZIL, Lauer W, Zulu SS, Taghzouti K & Abboussi O Acute cannabidiol treatment attenuates ethanol-induced place preference and reduces aggressivity in group-housed male rats. Pharmacology Biochemistry and Behavior 211, 173290 (2021). [DOI] [PubMed] [Google Scholar]
- 307.Greenwood L-M et al. Acute effects of Δ9-tetrahydrocannabinol and cannabidiol on auditory mismatch negativity. Psychopharmacology, 1–16 (2021). [DOI] [PubMed] [Google Scholar]
- 308.Kaufmann R, Aqua K, Lombardo J & Lee M Observed Impact of Long-term Consumption of Oral Cannabidiol on Liver Function in Healthy Adults. Cannabis and Cannabinoid Research (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A & Carlini EA Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. European journal of pharmacology 28, 172–177 (1974). [DOI] [PubMed] [Google Scholar]
- 310.Hollister LE & Gillespie H Interactions in man of delta-9-tetrahydrocannabinol; II. Cannabinol and cannabidiol. Clinical Pharmacology & Therapeutics 18, 80–83 (1975). [DOI] [PubMed] [Google Scholar]
- 311.Arkell TR et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 236, 2713–2724 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Englund A et al. Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different CBD: THC ratios. Neuropsychopharmacology, 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Salviato BZ et al. Female but not male rats show biphasic effects of low doses of Δ9-tetrahydrocannabinol on anxiety: can cannabidiol interfere with these effects? Neuropharmacology 196, 108684 (2021). [DOI] [PubMed] [Google Scholar]
- 314.Winstone J, Shafique H, Clemmer ME, Mackie K & Wager-Miller J Effects of Tetrahydrocannabinol and Cannabidiol on Brain-Derived Neurotrophic Factor and Tropomyosin Receptor Kinase B Expression in the Adolescent Hippocampus. Cannabis and Cannabinoid Research (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Taffe MA, Creehan KM & Vandewater SA Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Δ9-tetrahydrocannabinol in S prague-D awley rats. British journal of pharmacology 172, 1783–1791 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gabaglio M, Zamberletti E, Manenti C, Parolaro D & Rubino T Long-Term Consequences of Adolescent Exposure to THC-Rich/CBD-Poor and CBD-Rich/THC-Poor Combinations: A Comparison with Pure THC Treatment in Female Rats. International Journal of Molecular Sciences 22, 8899 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Cuttler C, LaFrance EM & Stueber A Acute effects of high-potency cannabis flower and cannabis concentrates on everyday life memory and decision making. Scientific Reports 11, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zelasko S, Arnold WR & Das A Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins & other lipid mediators 116, 112–123 (2015). [DOI] [PubMed] [Google Scholar]
- 319.Atwood BK, Huffman J, Straiker A & Mackie K JWH018, a common constituent of ‘Spice’herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. British journal of pharmacology 160, 585–593 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sexton M, Shelton K, Haley P & West M Evaluation of cannabinoid and terpenoid content: cannabis flower compared to supercritical CO2 concentrate. Planta medica 84, 234–241 (2018). [DOI] [PubMed] [Google Scholar]
- 321.Citti C et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Scientific reports 9, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Dvorakova M et al. A Critical Evaluation of Terpenoid Signaling at Cannabinoid CB1 Receptors in a Neuronal Model. Molecules 27, 5655 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tagen M & Klumpers LE Review of delta-8-tetrahydrocannabinol (Δ8-THC): Comparative pharmacology with Δ9-THC. British journal of pharmacology 179, 3915–3933 (2022). [DOI] [PubMed] [Google Scholar]
- 324.Thapa D et al. The cannabinoids Δ8THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation. Cannabis and cannabinoid research 3, 11–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Terry G et al. Positron emission tomography imaging using an inverse agonist radioligand to assess cannabinoid CB1 receptors in rodents. Neuroimage 41, 690–698 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hirvonen J et al. Decreased cannabinoid CB1 receptors in male tobacco smokers examined with positron emission tomography. Biological psychiatry 84, 715–721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cao S et al. Defining molecular glues with a dual-nanobody cannabidiol sensor. Nature communications 13, 1–14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dong A et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nature biotechnology, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Day AL et al. Unintended specificity of an engineered ligand-binding protein facilitated by unpredicted plasticity of the protein fold. Protein Engineering, Design and Selection 31, 375–387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Campbell LG, Dufresne J & Sabatinos SA Cannabinoid inheritance relies on complex genetic architecture. Cannabis and Cannabinoid Research 5, 105–116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


