Abstract
Calcium pyrophosphate deposition involves deposition of calcium pyrophosphate dihydrate crystals in various joints throughout the body. The term “pseudogout” refers to an acute attack of calcium pyrophosphate crystal-induced arthritis. Though clinical presentation and joint involvement vary, involvement of the lumbar spine is rare. We present the case of a 61-year-old male who presented with 3 days of worsening lower back pain. He had elevated inflammatory markers, leukocytosis, and spinal tenderness on exam. Magnetic resonance imaging of the lumbar spine showed likely L4-L5 osteomyelitis; however, biopsy of the disk space revealed extensive calcium pyrophosphate crystal deposition. The patient was treated with prednisone taper with alleviation of symptoms. Though pseudogout of the spine is rare, our report supports literature urging clinicians to consider pseudogout when assessing elderly patients with back pain for prompt and appropriate treatment.
Background
Calcium pyrophosphate dihydrate crystal deposition disease (CPPD) is a crystalline arthropathy involving the deposition of calcium pyrophosphate crystals in connective tissues throughout the body. It affects 4%-7% of adults in the United States and Europe, with the majority of adults being over the age of 60 at diagnosis and with 95% of male prevalence [[1], [2]]. Larger joints such as the knee, wrist, elbow, and ankle joints are most affected [3]. While the pathophysiology of CPPD is not well established, proposed mechanisms often include processes leading to the upregulation of extracellular inorganic pyrophosphate causing increased crystal formation, secondary to underlying metabolic, post-traumatic, and hereditary etiologies. Most cases remain idiopathic; however, aging, osteoarthritis, hemochromatosis, hypothyroidism, hyperparathyroidism, and hypomagnesemia may increase the risk of CPPD [4].
The term pseudogout often refers to an acute attack of calcium pyrophosphate crystal-induced arthritis. Gout, another crystal-induced arthropathy, involves the deposition of monosodium urate crystals. Analysis of synovial fluid remains the gold standard for diagnosis and differentiation between these processes. In commonly affected larger joints, pseudogout is frequently part of the differential diagnosis of a warm, edematous, and tender joint. Involvement of less commonly affected joints including the spine may result in diagnostic delay, misdiagnosis, and if chronically untreated, significant morbidity and debility. Few reports have detailed CPPD spinal involvement. Most initial presentations have been managed as osteomyelitis/discitis given initial similar magnetic resonance imaging (MRI) findings including adjacent soft tissue enhancement, increased signal intensity, and extensive erosive changes.
Case presentation
A 61-year-old male with a medical history significant for chronic kidney disease, congestive heart failure, prior stroke, and peripheral vascular disease presented with worsening left knee and lower back pain of 3 days duration. He reported that both the knee and back pain began abruptly, upon awakening. He noted that his left knee “buckled under him” when he attempted to walk and was accompanied by knee swelling with dull, intermittent, throbbing pain along the medial aspect that was worsened with movement. He described the back pain as intermittent, dull, and aching in nature that was localized to the midline without radiation but worsened with any movement. He denied any prior knee or back pain, numbness, tingling, or associated stiffness. He denied any associated fever, or bladder or gastrointestinal symptomatology. He presented to the emergency department when his pain continued to increase over the next few days without noted alleviating factors. Of note, the patient had recently seen his primary care provider within the past 2 weeks due to 2 months of intermittent finger joint (involving the metacarpophalangeal joint) and toe swelling for which he was treated with prednisone 40 mg for 5 days. Rheumatoid factor was negative at this visit.
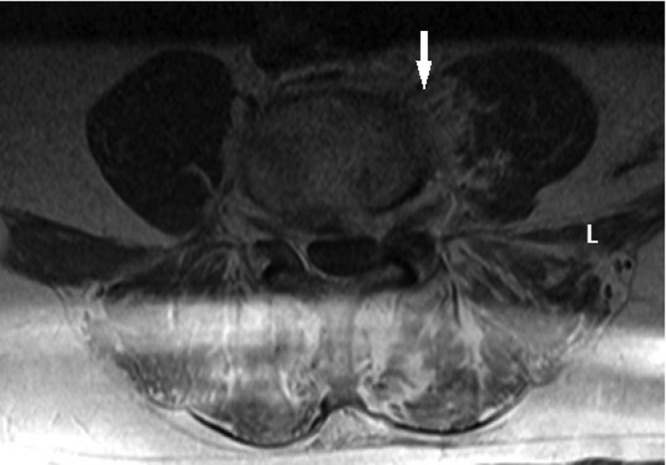
In the emergency department, the patient was found to be afebrile and with preserved vitals. On physical examination, the patient did not have evidence of significant joint effusion in the left knee; however, medial knee pain upon range of motion testing was noted. He also had paraspinal tenderness on palpation of the lumbar spine. This pain worsened with flexion, extension, and lateral movement and was worse, while the patient was in seated position. Laboratory diagnostics demonstrated leukocytosis (white blood cell count 14.7 k/ul, reference range 4.0-10.8 k/ul), and an elevated C-reactive protein (131 mg/L; reference range 0.0-3.0 mg/L) and erythrocyte sedimentation rate (66 mm/hr; reference range 0-20 mm/hr). MRI of the lumbar spine without contrast was obtained and showed disk edema extending to the L5 pedicle concordant with osteoarthropathy and superimposed infection (Fig. 1). There was also edema involving the left medial psoas muscle tracking inferiorly concordant with myositis associated with left-sided discitis, and L4-L5 osteomyelitis (Fig. 2). At this time, due to leukocytosis and elevated inflammatory markers, the patient was admitted and initiated on broad spectrum antibiotics with plans to biopsy of the L4-L5 disk space. Biopsy of the L4-L5 disk space revealed cartilage with extensive crystal deposition, consistent with calcium pyrophosphate. Cultures from this specimen were negative. The patient remained otherwise stable throughout this admission and was discharged with a prednisone taper and follow-up with his primary care physician.
Fig. 1.
T1-weighted sagittal MRI of the lumbar spine (A) and post-contrast, T1-weighted, fat-suppressed (B) sagittal MRI of the lumbar spine showing enhancement of the L4-L5 end plates and intervening disk.
Fig. 2.
Axial T2 STIR MRI of the lumbar spine at L4-L5 showing edema of the disk as well as left psoas muscle and small amount of epidural soft tissue with enhancement on T1 post-contrast imaging.
Differential diagnosis
-
1)
Pseudogout of lumbar spine
-
2)
Osteomyelitis of lumbar spine
-
3)
Degenerative disease of lumbar spine
Treatment
The patient was discharged with instructions to complete a prednisone taper beginning at 40 mg for 3 days followed by a decrease of 10 mg each day for 2 days. He was also instructed to follow-up with his primary care physician within 1 week regarding scheduling a repeat MRI in 3-4 weeks as well as referral to a rheumatologist.
Outcome and follow-up
The patient followed up with his primary care physician 1 week following his discharge. He reported improvement in his back pain and mobility. He has not followed up with rheumatology nor obtained a repeat MRI 6 months after his hospitalization given improvement in his symptoms.
Discussion
CPPD is a crystalline arthropathy that specifically involves the deposition of calcium pyrophosphate dihydrate crystals in joints throughout the body. It is associated with aging with a 15% prevalence in patients aged 65-74 years and over 40% prevalence after the age of 84 [[1], [5]]. Presenting symptoms are highly variable, with both acute and chronic presentations. Acute presentations can often mimic gout and infection with warm, erythematous, and edematous joints. Mild leukocytosis and elevated ESR and CRP levels may also be present in CPPD, as seen in our case. Synovial fluid analysis is required to make a definitive diagnosis. Traditionally, polarized light microscopy shows positively birefringent rhomboid (parallelepipedal form) crystals. However, in practice, it appears that CPPD crystals can often be smaller, weakly birefringent, and of variable shape from rod to cuboid, making synovial fluid analysis confirmation challenging [[6], [7]]. Additionally, coexistence of monosodium urate crystals along with CPP crystals can further obscure diagnostic workup. When CPPD presents in uncommon areas, including the spine, diagnosis, and workup may become difficult due to the crossover in both symptomatology and imaging, requiring further correlation between clinical, radiographic, and pathologic findings.
Spinal involvement of CPPD is rare, with cervical spine involvement more common than thoracic and lumbar spinal involvement [8]. Calcium pyrophosphate crystal deposition specifically in the cervical spine at the atlanto-axial joint and cruciform ligament is referred to as “crowned dens syndrome” in several case reports [9]. This often presents with specific physical exam findings including severe pain and limited neck rotation. Intriguingly, imaging is less likely to be confused with infectious process. In contrast, in 10 case reports of CPPD of the lumbar spine that we identified between the years 2001 and 2021, imaging was commonly confused for osteomyelitis/discitis [[8], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. In this case, initial imaging also returned with findings concerning for osteomyelitis, and the patient was managed empirically for osteomyelitis prior to the definitive diagnosis.
We also noted that post-surgical CPPD of the lumbar spine was more commonly documented than de novo spinal CPPD [12], similar to post-surgical CPPD in knee and hip joints [14]. Our patient had no prior history of spinal surgery, trauma, or CPPD in peripheral joints making this presentation even more unique among the case reports we reviewed.
Our case highlights the importance of correlating imaging findings with clinical suspicion and pathology results to avoid invasive or unnecessary treatments in elderly patients with acute back pain. CPPD can often mimic infection and gout with crossover in imaging, symptoms, and laboratory findings. Suspicion for CPPD should remain high until biopsy findings are resulted, and review for CPPD should be considered if infection is ruled out or results are equivocal.
Learning point
-
1.
CPPDs involvement of lumbar spine is rare
-
2.
CPPD spinal involvement, particularly of the lumbar spine, may be misdiagnosed as osteomyelitis
-
3.
CPPD spinal involvement should be included in the differential diagnosis of elderly patient presenting with back pain
Patient consent
Written patient consent for publication of this case was obtained.
Footnotes
Competing Interests: None.
References
- 1.Neame RL, Carr AJ, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003;62(6):513–518. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleiber Balderrama C, Rosenthal AK, Lans D, Singh JA, Bartels CM. Calcium pyrophosphate deposition disease and associated medical comorbidities: a National Cross-Sectional Study of US Veterans. Arthritis Care Res (Hoboken) 2017;69(9):1400–1406. doi: 10.1002/acr.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivera F, Andres M, Pascual E. Calcium pyrophosphate crystal deposition. Int J Clin Rheumtol. 2011;6:677–688. doi: 10.2217/ijr.11.60. [DOI] [Google Scholar]
- 4.Abhishek A, Doherty M. Pathophysiology of articular chondrocalcinosis—role of ANKH. Nat Rev Rheumatol. 2011;7:96–104. doi: 10.1038/nrrheum.2010.182. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins E, Dieppe P, Maddison P, Evison G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis. 1983;42(3):280–284. doi: 10.1136/ard.42.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagahashi M. Scanning electron microscopic observations of calcium pyrophosphate crystals of joint tissues and synovial fluid (author's transl) Nihon Seikeigeka Gakkai Zasshi. 1979;53(7):793–805. Japanese. [PubMed] [Google Scholar]
- 7.Zell M, Aung T, Kaldas M, Rosenthal AK, Bai B, Liu T, et al. Calcium pyrophosphate crystal size and characteristics. Osteoarthr Cartil Open. 2021;3(1) doi: 10.1016/j.ocarto.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loizidis G, Stern J, Baker JF. When calcium pyrophosphate deposition disease masquerades as spinal infection. J Clin Rheumatol. 2019;25(7):e118–e122. doi: 10.1097/RHU.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 9.Lee GS, Kim RS, Park HK, Chang JC. Crowned dens syndrome: a case report and review of the literature. Korean J Spine. 2014;11(1):15–17. doi: 10.14245/kjs.2014.11.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneyama H, Morishita Y, Kawano O, Yamamoto T, Maeda T. Acute attack of pseudogout with the wide lesion in lumbar spondylolytic spondylolisthesis. Case Rep Orthop. 2020;2020 doi: 10.1155/2020/4512695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ujihara T, Yamamoto K, Kitaura T, Katanami Y, Kutsuna S, Takeshita N, et al. Calcium pyrophosphate deposition disease involving a lumbar facet joint following urinary tract infection. Intern Med. 2019;58(12):1787–1789. doi: 10.2169/internalmedicine.2099-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges KJ, Bullis CL, Wanchu A, Than KD. Pseudogout of the cervical and thoracic spine mimicking infection after lumbar fusion: case report. J Neurosurg Spine. 2017;27(2):145–149. doi: 10.3171/2016.12.SPINE16979. [DOI] [PubMed] [Google Scholar]
- 13.Grobost V, Vayssade M, Roche A, Kemeny JL, Soubrier M. Axial calcium pyrophosphate dihydrate deposition disease revealed by recurrent sterile spondylodiscitis and epidural abscess. Joint Bone Spine. 2014;81(2):180–182. doi: 10.1016/j.jbspin.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Mikhael MM, Chioffe MA, Shapiro GS. Calcium pyrophosphate dihydrate crystal deposition disease (pseudogout) of lumbar spine mimicking osteomyelitis-discitis with epidural phlegmon. Am J Orthop (Belle Mead NJ) 2013;42(8):E64–E67. [PubMed] [Google Scholar]
- 15.Ogawa Y, Nagatsuma M, Kubota G, Inoue G, Eguchi Y, Orita S, et al. Acute lumbar spinal pseudogout attack after instrumented surgery. Spine (Phila Pa 1976) 2012;37(24):E1529–E1533. doi: 10.1097/BRS.0b013e31826b7977. [DOI] [PubMed] [Google Scholar]
- 16.Ziadé M, Zufferey P, So AK. Recurrent acute low back pain secondary to lumbar epidural calcification. Skelet Radiol. 2007;36(Suppl 1):S116–S119. doi: 10.1007/s00256-006-0147-8. [DOI] [PubMed] [Google Scholar]
- 17.Cameron CR, Burgess CD. Recurrent back pain and fevers. Med J Aust. 2007;186(4):208–209. doi: 10.5694/j.1326-5377.2007.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujishiro T, Nabeshima Y, Yasui S, Fujita I, Yoshiya S, Fujii H. Pseudogout attack of the lumbar facet joint: a case report. Spine (Phila Pa 1976) 2002;27(17):E396–E398. doi: 10.1097/00007632-200209010-00028. [DOI] [PubMed] [Google Scholar]