Abstract
Small, strained ring molecules of phenylcyclopropane carboxamide have rigid, defined conformations and unique electronic properties. For these reasons many groups, seek to use these subunits to form biologically active compounds. Herein we report a generally applicable approach for preparing a small cyclopropane ring containing 1-phenylcyclopropane carboxamide derivatives to a wide range of the different aromatic compounds by α-alkylation of 2-phenyl acetonitrile derivatives with 1, 2-dibromo ethane in good yields followed by the conversion of cyano group to acid group by the reaction with concentrated hydrochloric acid. This obtained acid derivative undergoes acid amine coupling with various Methyl 2-(aminophenoxy)acetate to form 1-Phenylcyclopropane Carboxamide. These compounds possess distinct effective inhibition on the proliferation of U937, pro-monocytic, human myeloid leukaemia cell line while these compounds did not show cytotoxic activity on these cells. The structure-activity relationships of these compounds are discussed.
Keywords: Substituted 1-phenylcyclopropane carboxamide derivatives, Anticancer, Antiproliferative agents, Cytotoxicity
1. Introduction
The unique electronic, steric and conformational properties of small rings with amide groups have led to increasing interest in methods to incorporate such structures into pharmaceutical and agrochemical industries. For example, the rigid conformation and strong C–H bonds of the cyclopropane have been stated to help achieve a range of properties, including enhanced binding and potency, increased metabolic stability, increased brain permeability, decreased plasma clearance and reduced off-target effects. The combination of small rings and aromatic fragments is particularly attractive because aryl and heteroaryl units are prevalent in compounds valuable for medicinal chemistry and the agrochemical industry. The diversely substituted 1-Phenylcyclopropane carboxamide compounds possess a wide range of pharmacological activities such as anti-inflammatory, anti-depressive, anti-arrhythmic, antitumor, antifungal, antibacterial activities [1,2].
There are some excellent reviews, which were published on the biological properties of this class of compounds including the synthesis and biological activity of novel 1-Phenylcyclopropane carboxamide derivatives [3,4].
Several methods have been developed for the synthesis of 1-phenylcyclopropane carboxylic acid derivatives, but their use is limited. The simple method was developed for 1-phenylcyclopropane carboxylic acid, α-Alkylation of 1-phenyl acetonitrile derivatives with 1, 2-dibromo ethane with the use of sodium hydroxide base in good yields, followed by the conversion of cyano group to acid group by reaction with concentrated hydrochloric acid. This obtained acid derivative will undergo acid amine coupling with coupling agent HATU (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyrdinium-3-oxide hexafluorophosphate) and various Methyl 2-(amino phenoxy) acetate to form 1-Phenylcyclopropane carboxamide derivatives [5,6].
Cancer has been known for the cellular proliferative disorder. It is one of the leading causes of death throughout the world and currently the main therapeutic options involve surgery, chemotherapy and radiotherapy. Since the beginning of cancer therapeutics and enhanced biological knowledge, synthetic chemistry has had pivotal roles in anticancer agent discovery. Even though several mechanisms are cancer cell proliferation has been established, the successful anticancer drug is still under investigation. There are several chemical compound series that have been explored but still it is a challenge to find to compound that has good anticancer properties along with related mechanisms as metastasis, cancer cell chemo resistance [[7], [8], [9]].
Previously, novel 3-alkoxymethyl/3-phenyl indole-2-carboxamide derivatives were reported for their anticancer activity. Most of the tested compounds showed moderate to excellent activity against the tested cell lines (MCF7 and HCT116) [[10], [11], [12]].
There has been always a need to get new compound series for good antiproliferative or cytotoxic compounds. The biological tests suggested the compound displayed distinct antiproliferative properties. Investigating small molecule antitumor compounds, which could decrease drug resistance and reduce unwanted side effects is more desirable. These compounds were tested further to know any unwanted cytotoxic activity on these cancer cells and found they were not cytotoxic which might be safe [13,14].
1.1. Hybrid design approach
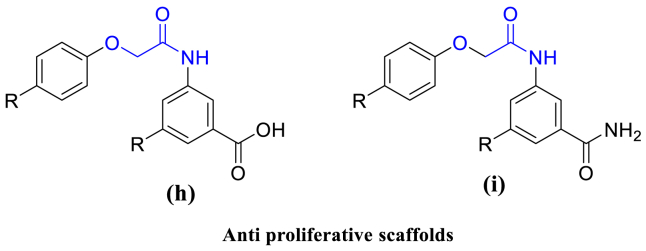
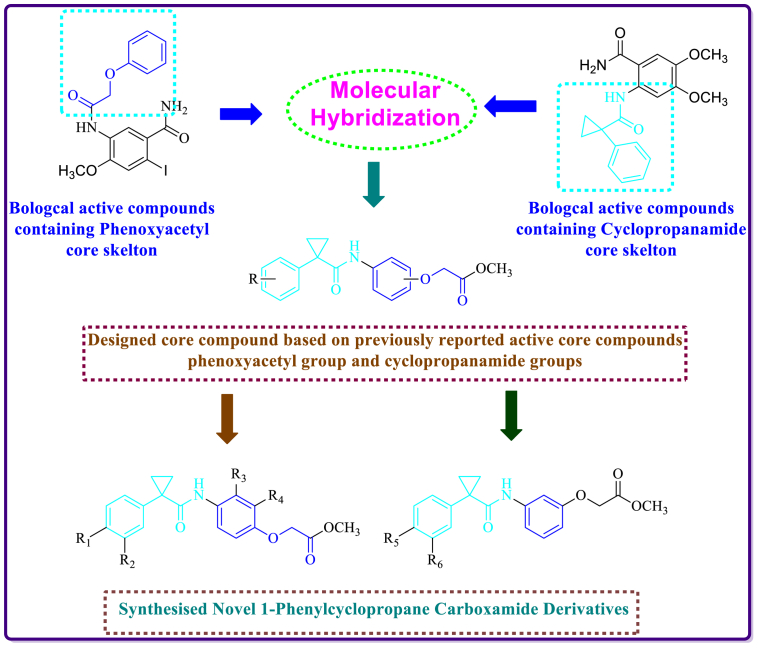
In the literature, we found several reports showing antiproliferative active compounds with phenoxyacetyl group (Fig. 3) and cyclopropanamide groups (Figs. 1 and 2). In the present paper we report the design of new compounds which contain both phenoxyacetyl group and cyclopropanamide group in one compound (Fig. 4). After designing, we have synthesized 20 compounds and screened them for antiproliferative activity [15].
Fig. 3.
Some examples of active compounds bearing the phenoxyacetamido as a core moiety having the Anti proliferative activity (h, i).
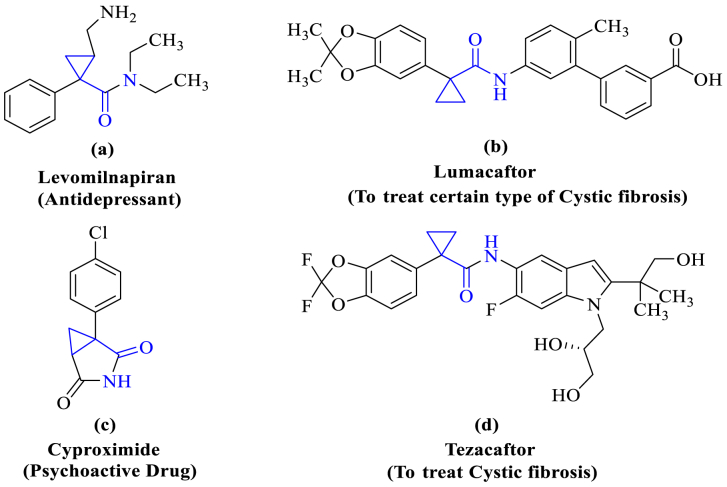
Fig. 1.
Pharmaceutical drugs in the market with 1-Phenylcyclopropane carboxamide as a core moiety having the activity like Antidepressant (a), Psychoactive (c) and to treat cystic fibrosis (b, d).
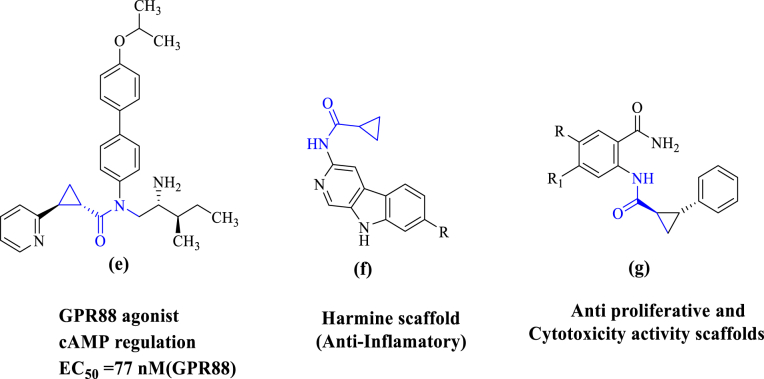
Fig. 2.
Examples of MNK1 and MNK2 inhibitors known in the literature containing cyclopropanamide as a core moiety having the activity like GPR88 agonist (e), Anti-inflammatory (f), Anti proliferative and cytotoxicity (g).
Fig. 4.
Design strategy adopted to design novel 1-phenylcyclopropane Carboxamide Derivatives.
1.2. Materials and methods
FT-IR spectra obtained on Jasco instrument with model FT/IR-4100 type A by using spectra manager software. Sample was prepared by taking 3–5 mg of compound on 250–300 mg of KBr salt.1H NMR spectra were obtained at 400 and 500 MHz with CDCl3, DMSO‑d6 as solvents. Chemical shifts are presented in parts per million downfield from Tetramethylsilane as internal standard. High-resolution mass spectrometry spectra (HRMS) were recorded by using the ESI technique, positive mode, capillary 4500, 0.4 Bar, dry gas 4.0 L min−1.
Solvents and reagents were used directly from the manufacturer or purified when required by standard procedures.
2. Experimental conditions
2.1. Synthesis of 1-phenylcyclopropane carboxylic acid
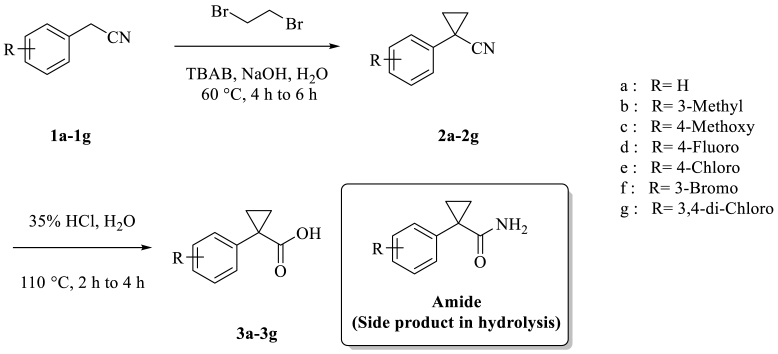
In the present work substituted 1-phenylcyclopropane carboxylic acid derivatives (3) were prepared in two steps (Scheme 1). Commercially available substituted 2-phenyl acetonitrile (1) was treated with 1,2-dibromoethane. Initially, cyclopropanation was optimized on 2-phenyl acetonitrile (1a) with different bases like KOH, NaOH, K2CO3 and Na2CO3 in water [16].
Scheme 1.
Synthesis of substituted 1-Phenylcyclopropane carboxylic acid derivatives (3a-3g).
Temperature also plays an important role in the cyclopropanation at high temperature 100 °C, the reaction yields were very less when compared to optimal temperature 60 °C (Table 1).
Table 1.
Synthesis of 1-Phenylcyclopropane acetonitrile (2a) in different reaction conditions.
| S.No | Base, water (%) | Solvent | Temperature (°C) | Time (hr) | Yield (%) |
|---|---|---|---|---|---|
| 1 | KOH, H2O (50) | – | 60 | 12 | 30 |
| 2 | KOH, H2O (50) | – | 100 | 12 | 20 |
| 3 | KOH, H2O (50) | CH3CN | 60 | 12 | 15 |
| 4 | KOH, H2O (50) | CH3CN | 100 | 12 | 10 |
| 5 | NaOH, H2O (50) | – | 60 | 12 | 45 |
| 6 | NaOH, H2O (50) | – | 100 | 12 | 40 |
| 7 | NaOH, H2O (50) | CH3CN | 60 | 12 | 35 |
| 8 | NaOH, H2O (50) | CH3CN | 100 | 12 | 30 |
| 9 | K2CO3, H2O (50) | – | 60 | 12 | 10 |
| 10 | K2CO3, H2O (50) | – | 100 | 12 | 5 |
| 11 | K2CO3, H2O (50) | CH3CN | 60 | 12 | NP |
| 12 | K2CO3, H2O (50) | CH3CN | 100 | 12 | NP |
| 13 | Na2CO3, H2O (50) | – | 60 | 12 | 15 |
| 14 | Na2CO3, H2O (50) | – | 100 | 12 | 10 |
| 15 | Na2CO3, H2O (50) | CH3CN | 60 | 12 | NP |
| 16 | Na2CO3, H2O (50) | CH3CN | 100 | 12 | NP |
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. NP = no product obtained.
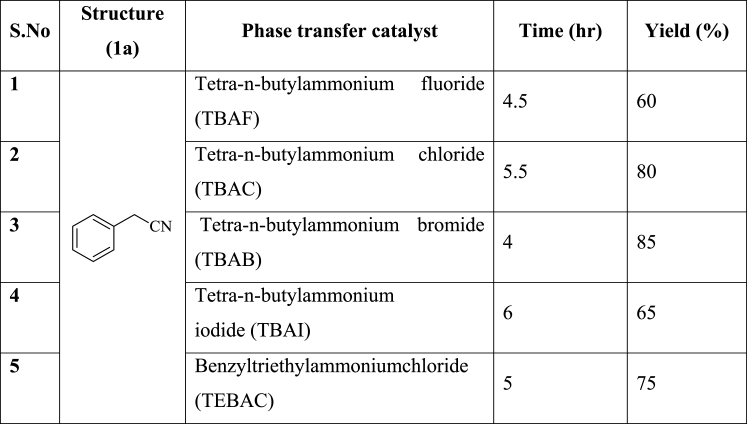
Among the different bases used, NaOH and water combinations gave good yield (45%, entry-5, Table 1). The ratio of NaOH and H2O is (50% w/v), after identifying the base and water, we tried to increase the yield by adding different phase transfer catalysts [17].
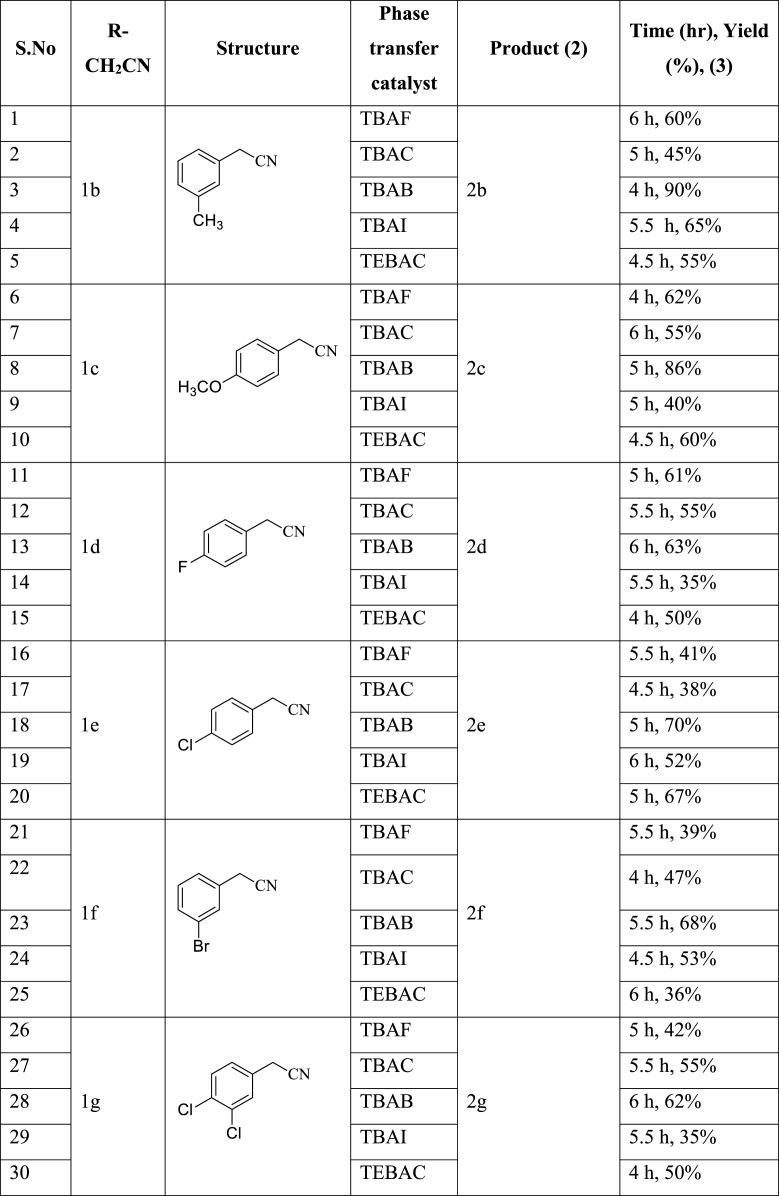
Cyclopropanation on 2-phenyl acetonitrile (1a) in presence of phase transfer catalysts completed in 4–6 h whereas 12 h is required to consume complete starting material in the absence of phase transfer catalysts. Tetra-n-butylammonium bromide (TBAB) at 60 °C over 4 h gave 1-phenylcyclopropane carbonitrile (2a) in 85% yield (Entry-3, Table 2). However, time and yield vary with different substituted 2-phenyl acetonitrile (1) derivatives (Table 3).
Table 2.
Synthesis of 1-Phenylcyclopropane acetonitrile (2a) with different phase transfer catalysts.
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. Phase transfer catalyst (0.1 eq), 1,2-dibromoethane (1.0 eq), 2-phenyl acetonitrile (1.0 eq), NaOH (2.0 eq), H2O (50% Volume with respect to weight of NaOH).
Table 3.
Reaction conditions and yields for substituted 1-Phenylcyclopropane acetonitrile derivatives (3a–3g).
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. Phase transfer catalyst (0.1 eq), 1,2-dibromoethane (1.0 eq), 2-phenyl acetonitrile (1.0 eq), NaOH (2.0 eq), H2O (50% Volume with respect to weight of NaOH), Temperature 60 °C.
1-Phenylcyclopropane acetonitrile (2a) was obtained in presence of TBAB with 85% yield (Table 2). 1-(m-tolyl)cyclopropane acetonitrile (2b) was obtained in presence of TBAB with 90% yield (Table 3). 1-(4-methoxyphenyl)cyclopropane acetonitrile (2c) was obtained in presence of TBAB with 86% yield (Table 3). 1-(4-fluorophenyl)-cyclopropane acetonitrile (2d) was obtained in presence of TBAB with 63% yield (Table 3). 1-(4-chlorophenyl)-cyclopropane acetonitrile (2e) was obtained in presence of TBAB with 70% yield (Table 3). 1-(3-bromophenyl)-cyclopropane acetonitrile (2f) was obtained in presence of TBAB with a 68% yield (Table 3). 1-(3,4-dichlorophenyl)-cyclopropane acetonitrile (2g) was obtained in presence of TBAB with 62% yield (Table 3). From Tables 2 and 3, it was concluded that cyclopropanation on 2-phenyl acetonitrile gave good yields when an electron-donating groups like methyl and methoxy were present on phenyl ring (2b: 90% and 2c:86%), whereas when electron-withdrawing groups like fluoro and chloro were present on phenyl ring this resulted in fewer yields (35%–70%: 2d, 2e, 2f, and 2g).
Substituted 1-phenylcyclopropane carbonitrile derivatives (2a–2g) further treated with an aqueous hydrochloric acid solution at 110 °C for 2–4 h to give substituted 1-phenylcyclopropane carboxylic acid derivatives (3a–3g) (Scheme 1) (Ref-1 J. Org. Chem., Vol. 40, No. 20, 1975, 2969–2970).
1-(4-methoxyphenyl)-cyclopropanecarboxylic acid (3c) obtained in good yield (Table 4, entry 3, 88%) and 1-(3,4-dichlorophenyl)-cyclopropanecarboxylic acid (3g) obtained in low yield (Table 4, entry 7, 64%). The major impurity observed in all these reactions is corresponding amides. If the reactions were continued for longer hours, the percentages of side products were increased.
Table 4.
Reaction conditions and yields for substituted 1-Phenylcyclopropane carboxylic acid derivatives (3a–3g).
| S.No | SM | Product | Time | Yield (%) | Amide (side product) |
|---|---|---|---|---|---|
| 1 | 2a | 3a | 3 h | 80% | 8% |
| 2 | 2b | 3b | 2 h | 85% | 5% |
| 3 | 2c | 3c | 2.5 h | 88% | 3% |
| 4 | 2d | 3d | 3 h | 77% | 14% |
| 5 | 2e | 3e | 3.5 h | 75% | 10% |
| 6 | 2f | 3f | 4 h | 72% | 10% |
| 7 | 2g | 3g | 4 h | 64% | 15% |
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. 1-Phenylcyclopropane acetonitrile (1.0 eq), 35% Hydrochloric acid (5.0 eq), H2O (5 vol), 110 °C.
2.2. Synthesis of substituted phenoxy acetyl derivatives
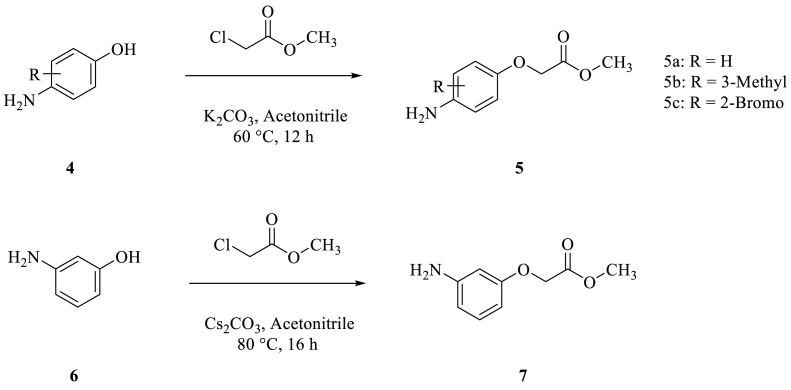
Based on the previous reports, we have identified four different phenoxy acetyl derivatives (5a, 5b, 5c and 7) for our study. These four phenoxy acetyl derivatives were prepared from 3-amino Phenol and 4-amino Phenol treated with Methyl 2-chloroacetate in acetonitrile by using different bases (Scheme 2) [18].
Scheme 2.
Synthesis of Phenoxy acetyl derivatives.
Methyl 2-(4-aminophenoxy)acetate derivatives (5a, 5b and 5c) were prepared from 4-amino Phenol derivatives (4a, 4b and 4c) when treated with Methyl 2-chloroacetate by using K2CO3 at 60 °C for 12 h. Methyl 2-(4-aminophenoxy)acetate (5a) was obtained with 75% yield (Table 5, entry 2), Methyl 2-(4-amino-3-methylphenoxy)acetate (5b) was obtained with 78% yield (Table 5, entry 5) and Methyl 2-(4-amino-2-bromophenoxy)acetate (5c) was obtained with 70% yield (Table 5, entry 8). Whereas Methyl 2-(3-aminophenoxy)acetate (7) was obtained with 79% yield from 3-amino Phenol (6) when treated with Methyl 2-chloroacetate by using CS2CO3 at 80 °C for 16 h (Table 5, entry 13).
Table 5.
Synthesis of Phenoxy acetyl derivatives with different bases.
| S.No | SM | Product | Temperature (°C) | Base | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 4a | 5a | 60 | Na2CO3 | 12 | 61 |
| 2 | 4a | 5a | 60 | K2CO3 | 12 | 75 |
| 3 | 4a | 5a | 60 | Cs2CO3 | 12 | 55 |
| 4 | 4b | 5b | 60 | Na2CO3 | 12 | 43 |
| 5 | 4b | 5b | 60 | K2CO3 | 12 | 78 |
| 6 | 4b | 5b | 60 | Cs2CO3 | 12 | 61 |
| 7 | 4c | 5c | 60 | Na2CO3 | 12 | 39 |
| 8 | 4c | 5c | 60 | K2CO3 | 12 | 70 |
| 9 | 4c | 5c | 60 | Cs2CO3 | 12 | 45 |
| 10 | 6 | 7 | 60 | Na2CO3 | 12 | 35 |
| 11 | 6 | 7 | 60 | K2CO3 | 12 | 41 |
| 12 | 6 | 7 | 60 | Cs2CO3 | 12 | 49 |
| 13 | 6 | 7 | 80 | Cs2CO3 | 16 | 79 |
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. Amino phenol (1.0 eq), Methyl 2-chloroacetate (2.0 eq), base (3.0 eq).
2.3. Synthesis of 1-phenylcyclopropane carboxamide derivatives
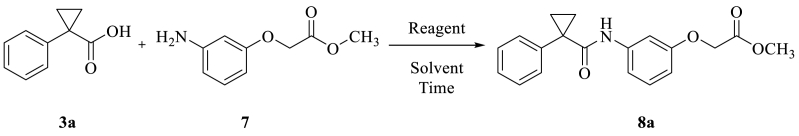
After preparing four different phenoxy acetyl derivatives (5a, 5b, 5c and 7) and seven different substituted 1-Phenylcyclopropane carboxylic acid derivatives (3a, 3b, 3c, 3d, 3e, 3f and 3g). We tried an acid-amine coupling reaction by using different amide coupling reagents with different bases. Initially, we have attempted an amide coupling reaction between 3a and 7 to get 8a (Scheme 3) [19,20].
Scheme 3.
Synthesis of 1-Phenylcyclopropane carboxamide derivatives (8a).
Among the different coupling reagents, HATU along with DIPEA in DMF obtained in good yield (Table 6, entry 8, 85%). THF solvent takes a long time to consume complete starting materials. In DMF the reactions are timeless when compared to THF. DIPEA is a better base when compared to Et3N [21,22].
Table 6.
Reaction conditions for 1-Phenylcyclopropane carboxamide derivative (8a).
| S.No | Reagent | Base | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | EDC | Et3N | THF | 20 | 41 |
| 2 | EDC | Et3N | DMF | 18 | 45 |
| 3 | EDC | DIPEA | THF | 16 | 62 |
| 4 | EDC | DIPEA | DMF | 12 | 65 |
| 5 | HATU | Et3N | THF | 20 | 46 |
| 6 | HATU | Et3N | DMF | 18 | 49 |
| 7 | HATU | DIPEA | THF | 16 | 75 |
| 8 | HATU | DIPEA | DMF | 12 | 85 |
| 9 | HBTU | Et3N | THF | 20 | 48 |
| 10 | HBTU | Et3N | DMF | 18 | 51 |
| 11 | HBTU | DIPEA | THF | 16 | 68 |
| 12 | HBTU | DIPEA | DMF | 12 | 70 |
| 13 | T3P | Et3N | THF | 20 | 47 |
| 14 | T3P | Et3N | DMF | 18 | 49 |
| 15 | T3P | DIPEA | THF | 16 | 64 |
| 16 | T3P | DIPEA | DMF | 12 | 68 |
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. Acid (1.0 eq), Amine (1.0 eq), coupling reagent (1.5 eq), base (3.0 eq), solvent (5 vol). EDC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide). HATU (1-[Bis(dimethylamino) methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluoro phosphate). HBTU (3-[Bis(dimethylamino)methyliumyl]-3H-benzotriazol-1-oxide hexafluorophosphate). T3P (Propanephosphonic acid anhydride).
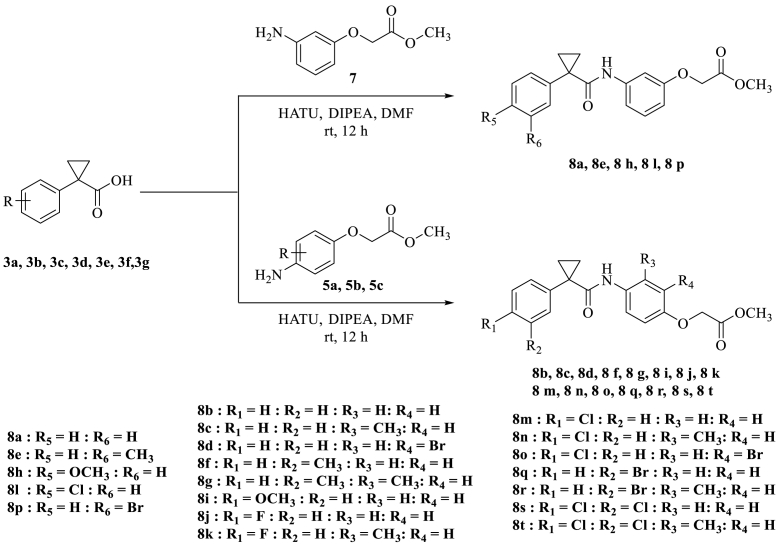
After optimizing reaction conditions for amide coupling with HATU, DIPEA in DMF for 12 h, we prepared the remaining 19 compounds by using the standard optimized condition (see Scheme 4) [23,24].
Scheme 4.
Synthesis of 1-Phenylcyclopropane carboxamide derivatives (8a–8t).
Among the twenty 1-Phenylcyclopropane carboxamide derivatives (8a–8t), compound 8o gave the best yield (Table 7, entry-15, 91%) and compound 8k gave low yield (Table 7, entry-11, 71%).
Table 7.
Synthesis of 1-Phenylcyclopropane carboxamide derivatives (8a–8t).
| S.No | Acid | Amine | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 3a | 7 | 8a | 85 |
| 2 | 3a | 5a | 8b | 72 |
| 3 | 3a | 5b | 8c | 84 |
| 4 | 3a | 5c | 8d | 81 |
| 5 | 3b | 7 | 8e | 78 |
| 6 | 3b | 5a | 8f | 76 |
| 7 | 3b | 5b | 8g | 81 |
| 8 | 3c | 7 | 8h | 83 |
| 9 | 3c | 5a | 8i | 76 |
| 10 | 3d | 5a | 8j | 79 |
| 11 | 3d | 5b | 8k | 71 |
| 12 | 3e | 7 | 8l | 79 |
| 13 | 3e | 5a | 8m | 84 |
| 14 | 3e | 5b | 8n | 88 |
| 15 | 3e | 5c | 8o | 91 |
| 16 | 3f | 7 | 8p | 72 |
| 17 | 3f | 5a | 8q | 80 |
| 18 | 3f | 5b | 8r | 83 |
| 19 | 3g | 5a | 8s | 73 |
| 20 | 3g | 5b | 8t | 88 |
*All reactions were carried out on a 10 mg scale. The reaction was monitored by TLC and LCMS. Acid (1.0 eq), Amine (1.0 eq), HATU (1.5 eq), DIPEA (3.0 eq), DMF (5 vol).
2.4. Biological activity
2.4.1. Anticancer activity evaluation
The named compound 6 was evaluated for its in vitro antiproliferative activity against U937 cells by the CellTiter-Glo Luminescent cell viability assay using Mitomycin C as a positive control. Briefly, live cells were incubated for 72 h with different concentrations of compounds. CellTiter-Glo Reagent (Promega) was added equal to the volume of the cell culture medium. Contents were mixed for 2 min on an orbital shaker for complete cell lysis. The plate was allowed to incubate at room temperature for 10 min to stabilize the luminescent signal. Luminescence signals were captured under the BMG Fluostar Omega micro plate reader using a luminescence module [27]. Viability data are calculated using the following formula: (RLU) of test cells × 100/(RLU) of control cells. Further, this cell viability was calculated for the inhibitory activity of compounds for these cells. Each dose was used to calculate the half-maximal inhibitory concentration (IC50) by Graph Pad Prism software [[25], [26], [25]].
2.4.2. Cytotoxic activity evaluation
Many therapeutic agents require multiple replication cycles for a killing effect to be evident, and dose-dependent direct cell killing can act which can be tested using an LDH-based cytotoxicity assay. When cells are insulted with compounds, the cell membrane is damaged and the cytoplasmic content containing LDH enzyme releases into the extracellular medium. LDH catalyzes the conversion of pyruvate to lactate in the presence of NADH. The increase in absorbance at 340 nm due to the oxidation of NADH is proportional to LDH activity in the sample. Briefly, a clear, flat bottom clear, the 96-well plate was plated with U937 cells and incubated with various concentrations of compounds as well as Triton X100 as positive cytotoxicity control. The plate was then incubated at 37 °C for 4 h and centrifuged for 5 min at 1000 RPM. The supernatant was harvested to another 96 well clear, flat bottom plate and LDH reagent (Cytotoxicity detection kit, Sigma) was added and incubated at room temperature for 10 min in dark. Optical density was measured at 490 nm using a BMG Fluostar Omega microplate reader. Further this optical density was calculated for the cytotoxicity activity of compounds for these cells. Each dose was used to calculate the half-maximal inhibitory concentration (IC50) by Graph Pad Prism software [[28], [29], [30]].
2.4.2.1. 1-Phenylcyclopropanecarboxylic acid (3a)
Brown solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.3 (m, 5H), 1.66 (q, J = 4.2 Hz, 3 Hz, 2H), 1.26 (q, J = 3.6 Hz, 3.3 Hz, 2H). HRMS (ESI) m/z calcd. For [C10H10O2]+:162.19 [M+H]+, found 163.2.
2.4.2.2. 1-(m-Tolyl)cyclopropanecarboxylic acid (3b)
Pale yellow solid. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.25 (m, 3H), 7.18 (m, 1H), 1.66 (q, J = 6 Hz, 3.3 Hz, 2H), 1.26 (q, J = 3.6 Hz, 3.3 Hz, 2H). HRMS (ESI) m/z calcd. For [C11H12O2]+: 176.21 [M+H]-, found 175.07.
2.4.2.3. 1-(4-Methoxyphenyl)cyclopropanecarboxylic acid (3c)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.25 (d, J = 8.7 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 1.63 (q, J = 3.9 Hz, 3.3 Hz, 2H), 1.21 (q, J = 3.6 Hz, 3.9 Hz, 2H). HRMS (ESI) m/z calcd. For [C11H12O3]+: 192.21 [M+H]+, found 193.1.
2.4.2.4. 1-(4-Fluorophenyl)cyclopropanecarboxylic acid (3d)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.25 (d, J = 6.6 Hz, 2H), 6.83 (d, J = 6.6 Hz, 2H), 1.63 (q, J = 6 Hz, 3.3 Hz, 2H), 1.21 (q, J = 3.6 Hz, 3.3 Hz, 2H). HRMS (ESI) m/z calcd. For [C10H9FO2]+: 180.18 [M+H]+, found 181.1.
2.4.2.5. 1-(4-Chlorophenyl)cyclopropanecarboxylic acid (3e)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.25 (m, 4H), 1.67 (q, J = 3.3 Hz, 3.6 Hz, 2H), 0.96 (q, J = 6 Hz, 7.2 Hz, 2H). HRMS (ESI) m/z calcd. For [C10H9ClO2]+: 196.63 [M+H]-, found 195.1.
2.4.2.6. 1-(3-Bromophenyl)cyclopropanecarboxylic acid (3f)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.48 (t, J = 1.2 Hz, 1H), 7.40 (dd, J = 1.2 Hz, 0.8 Hz, 1H), 7.25 (s, 1H), 7.17 (t, J = 7.8 Hz, 1H), 1.67 (q, J = 4.2 Hz, 3 Hz, 2H), 1.25 (q, J = 3.9 Hz, 3 Hz, 2H). HRMS (ESI) m/z calcd. For [C10H9BrO2]+: 241.08 [M+H]+, found 242.3.
2.4.2.7. 1-(3,4-Dichlorophenyl)cyclopropanecarboxylic acid (3g)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.40 (m, 2H), 7.16 (d, J = 2.1 Hz, 1H), 1.68 (q, J = 4.2 Hz, 3 Hz, 2H), 1.25 (q, J = 2.4 Hz, 1.2 Hz, 2H). HRMS (ESI) m/z calcd. For [C10H9BrO2]+: 241.08 [M+H]+, found 242.3.
2.4.2.8. Methyl 2-(3-aminophenoxy)acetate (7)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.15 (d, J = 1.5 Hz, 2H), 6.80 (d, J = 1.2 Hz, 1H), 6.23 (d, J = 1.5 Hz, 1H), 4.59 (s, 2H), 3.78 (s, 3H). HRMS (ESI) m/z calcd. For [C9H11NO3]+: 181.19 [M+H]+, found 182.3.
2.4.2.9. Methyl 2-(4-aminophenoxy)acetate (5a)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 6.80 (d, J = 2.4 Hz, 2H), 6.67 (d, J = 1.8 Hz, 2H), 4.55 (s, 2H), 3.76 (s, 3H). HRMS (ESI) m/z calcd. For [C9H11NO3]+: 181.19 [M+H]+, found 182.3.
2.4.2.10. Methyl 2-(4-amino-3-methylphenoxy)acetate (5b)
Pale yellow solid. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.75 (d, J = 9.3 Hz, 1H), 7.19 (d, J = 1.8 Hz, 1H), 6.69 (s, 1H), 4.56 (s, 2H), 3.8 (s, 3H), 2.38 (s, 3H). HRMS (ESI) m/z calcd. For [C10H13NO3]+: 195.22 [M+H]+, found 196.3.
2.4.2.11. Methyl 2-(4-amino-2-bromophenoxy)acetate (5c)
Off-white solid. 1H NMR (500 MHz, CDCl3): δ (ppm): 7.37 (s, 1H), 7.02 (d, J = 3 Hz, 1H), 6.92 (d, J = 8 Hz, 1H), 4.67 (s, 2H), 3.69 (s, 3H). HRMS (ESI) m/z calcd. For [C9H10BrNO3]+: 260.08 [M+H]+, found 261.
2.4.2.12. Methyl 2-(3-(1-phenylcyclopropane-1-carboxamido)phenoxy)acetate (8a)
84% yield. IR (KBr, cm−1): 3407, 3359, 3331, 3006, 2957, 2365, 1734, 1672, 1607, 1523, 1523, 1487, 1426, 1294, 1183, 1081, 1022, 991, 964, 907, 843, 763, 703, 680. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.43 (m, 5H), 7.13 (t, J = 4 Hz, 2H), 7.04 (s, 1H), 6.79 (d, J = 1.5 Hz, 1H), 6.60 (d, J = 1.8 Hz, 1H), 4.59 (s, 2H), 3.78 (s, 3H) 1.78 (q, J = 3.9 Hz, 3.3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.99, 169.15, 157.68, 140.16, 140.13, 129.27, 128.77, 128.57, 127.01, 112.94, 109.23, 106.43.64.5, 51.77, 31.86, 14.79. HRMS (ESI) m/z calcd. For [C19H19NO4]+: 325.36 [M+H]+, found 326.3.
2.4.2.13. Methyl 2-(4-(1-phenylcyclopropane-1-carboxamido)phenoxy)acetate (8b)
81% yield. IR (KBr, cm−1): 3446, 3402, 3007, 2971, 2364, 1732, 1668, 1524, 1436, 1412, 1282, 1219, 1141, 1093, 1067, 1002, 953, 835, 766, 706. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.39 (m, 5H), 7.25 (d, J = 2.4 Hz, 2H), 6.95 (s, 1H), 6.79 (d, J = 2.4 Hz, 2H), 4.59 (s, 2H), 3.78 (s, 3H), 1.69 (q, J = 3.6 Hz, 3 Hz, 2H), 1.15 (q, J = 3.6 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 17068, 169.26, 153.7, 140.27, 132.6, 130.28, 128.98, 128.53, 126.97, 121.8, 114.2764.75, 51.71, 31.52, 14.74. HRMS (ESI) m/z calcd. For [C19H19NO4]+: 325.36 [M+H]+, found 326.2.
2.4.2.14. Methyl 2-(3-methyl-4-(1-phenylcyclopropane-1-carboxamido)phenoxy)acetate (8c)
86% yield. IR (KBr, cm−1): 3465, 3418, 2983, 2937, 2853, 2363, 1759, 1681, 1530, 1436, 1289, 1212, 1117, 1078, 957, 868, 801, 702. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.74 (d, J = 8.7 Hz, 1H), 7.52 (d, J = 2.4 Hz, 2H), 7.42 (m, 3H), 7.26 (s, 1H), 6.7 (d, J = 2.2 Hz, 2H), 4.56 (s, 2H), 3.78 (s, 3H), 1.69 (q, J = 3.9 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.2, 169.25, 154.82, 139.82, 132.9, 130.1, 130, 128.75, 127.48, 125.54, 116, 111.64, 64.62, 51.73, 30.92, 17.27, 14.81. HRMS (ESI) m/z calcd. For [C20H21NO4]+: 339.39 [M+H]+, found 340.12.
2.4.2.15. Methyl 2-(2-bromo-4-(1-phenylcyclopropane-1-carboxamido)phenoxy)acetate (8d)
79% yield. IR (KBr, cm−1): 3383, 3008, 2954, 1735, 1667, 1586, 1496, 1437, 1289, 1209, 1061, 1022, 702. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.51 (d, J = 2.7 Hz,1H), 7.42 (m, 4H), 7.26 (t, J = 2.4 Hz, 1H), 6.94 (s, 1H), 6.72 (d, J = 8.7 Hz, 1H), 4.56 (s, 2H), 3.77 (s, 3H), 1.69 (q, J = 3.9 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171, 168.8, 150, 139.98, 133.55, 129, 128.52, 127, 125, 120.67, 113.34, 110.05, 65.43, 51.84, 31.5, 14.83. HRMS (ESI) m/z calcd. For [C19H18BrNO4]+: 404.25 [M+H]+, found 404.2.
2.4.2.16. Methyl 2-(3-(1-(m-tolyl)cyclopropane-1-carboxamido)phenoxy)acetate (8e)
84% yield. IR (KBr, cm−1): 3499, 3407, 3011, 2954, 2924, 2865, 2364, 2236, 1759, 1681, 1606, 1522, 1434, 1291, 1209, 1087, 947, 850, 780, 707, 686. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.29 (m, 2H), 7.15 (m, 5H), 6.81 (dd, J = 1.2 Hz, 1.6 Hz, 1H), 6.60 (dd, J = 1.2 Hz, 1.5 Hz, 1H), 4.59 (s, 2H), 3.78 (s, 3H), 1.68 (q, J = 3.9 Hz, 3.3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 172.25, 169.17, 158.04, 139.16, 139.07, 138.9, 131.7, 129.57, 128.49, 128.03, 112.66, 110.26, 106.01, 77.32, 77, 76.68, 65.12, 52.14, 31.18, 21.3, 16.15. HRMS (ESI) m/z calcd. For [C20H21NO4]+: 339.39 [M+H]+, found 340.7.
2.4.2.17. Methyl 2-(4-(1-(m-tolyl)cyclopropane-1-carboxamido)phenoxy)acetate (8f)
76% yield. IR (KBr, cm−1): 3448, 3403, 2953, 1757, 1677, 1529, 1509, 1437, 1411, 1257, 1213, 1078, 981, 957, 821, 791, 707. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.25 (m, 6H), 6.79 (d, J = 2.4 Hz, 2H), 4.57 (s, 2H), 3.77 (s, 3H), 1.67 (q, J = 3.6 Hz, 3 Hz, 2H), 1.13 (q, J = 3.9 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.77, 169.26, 153.7, 140.07, 137.65, 132.6, 129.68, 128.44, 127.72, 126.22, 121.8, 114.26, 64.75, 51.72, 31.41, 21.05, 14.65. HRMS (ESI) m/z calcd. For [C20H21NO4]+: 339.39 [M+H]+, found 340.6.
2.4.2.18. Methyl 2-(3-methyl-4-(1-(m-tolyl)cyclopropane-1-carboxamido)phenoxy)acetate (8g)
85% yield. IR (KBr, cm−1): 3463, 3416, 1758, 1681, 1636, 1531, 1437, 1287, 1212, 1116, 1077, 985, 802, 706. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.73 (d, J = 6.3 Hz, 1H), 7.30 (m, 2H), 7.18 (m, 1H), 6.87 (s, 1H), 6.68 (m, 2H), 4.56 (s, 2H), 3.79 (s, 3H), 2.35 (s, 3H), 1.81 (s, 3H), 1.68 (q, J = 3.6 Hz, 3 Hz, 2H), 1.15 (q, J = 3.6 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.23, 169.24, 154.76, 139.65, 137.9, 132.72, 130.76, 130.06, 128.64, 128.15, 127.11, 125.4, 116.02, 111.63, 64.61, 51.73, 30.82, 20.97, 17.23, 14.76. HRMS (ESI) m/z calcd. For [C21H23NO4]+: 353.41 [M+H]+, found 353.9.
2.4.2.19. Methyl 2-(3-(1-(4-methoxyphenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8h)
85% yield. IR (KBr, cm−1): 3408, 3373, 1725, 1666, 1605, 1532, 1514, 1498, 1436, 1415, 1297, 1247, 1195, 1165, 1030, 997, 853, 835, 782, 689. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.39 (d, J = 2.1 Hz, 2H), 7.12 (m, 3H), 6.94 (d, J = 2.1 Hz, 2H), 6.81 (d, J = 1.2 Hz, 1H), 6.60 (d, J = 1.2 Hz, 1H), 4.6 (s, 2H), 3.85 (s, 3H), 3.78 (s, 3H),1.68 (q, J = 3.9 Hz, 3 Hz, 2H), 1.12 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.5, 169.14, 158.44, 157.68, 140.05, 131.64, 130.68, 129.23, 114.05, 113, 109.23, 106.5, 64.51, 55.04, 51.76, 30.91, 14.89. HRMS (ESI) m/z calcd. For [C20H21NO5]+: 355.38 [M+H]+, found 356.14.
2.4.2.20. Methyl 2-(4-(1-(4-methoxyphenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8i)
81% yield. IR (KBr, cm−1): 3435, 3394, 1767, 1662, 1526, 1509, 1252, 1213, 1073, 1028, 835. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.4 (d, J = 2.5 Hz, 2H), 7.38 (d, J = 1.5 Hz, 2H), 6.94 (s, 2H), 6.80 (d, J = 5.5 Hz, 1H), 6.78 (d, J = 2.5 Hz, 1H), 4.57 (s, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 1.66 (q, J = 2.4 Hz, 2.1 Hz, 2H), 1.1 (q, J = 2.4 Hz, 2.1 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.18, 169.26, 158.36, 153.69, 132.52, 131.81, 130.77, 121.9, 114.21, 114.01, 64.74, 55.05, 51.72, 30.59, 14.78. HRMS (ESI) m/z calcd. For [C20H21NO5]+: 355.38 [M+H]+, found 356.2.
2.4.2.21. Methyl 2-(4-(1-(4-fluorophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8j)
81% yield. IR (KBr, cm−1): 3405, 3073, 1763, 1668, 1603, 1525, 1509, 1448, 1439, 1411, 1229, 1216, 1162, 1076, 835. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.47 (m, 2H), 7.25 (m, 2H), 7.11 (m, 2H), 6.88 (s, 1H), 6.68 (d, J = 8.7 Hz, 2H), 4.55 (s, 2H), 3.77 (s, 3H), 1.69 (q, J = 3.9 Hz, 3 Hz, 2H), 1.11 (q, J = 3.9 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.7, 169.27, 153.75, 132.51, 131.49, 131.41, 122.31, 115.39, 115.18, 114.2, 64.73, 51.73, 30.72, 14.87. HRMS (ESI) m/z calcd. For [C19H18FNO4]+: 343.35 [M+H]-, found 342.14.
2.4.2.22. Methyl 2-(4-(1-(4-fluorophenyl)cyclopropane-1-carboxamido)-3-methylphenoxy)acetate (8k)
77% yield. IR (KBr, cm−1): 3447, 3420, 1733, 1671, 1619, 1528, 1509, 1435, 1293, 1217, 1158, 1120, 1073, 1004, 955, 819. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.70 (d, J = 1.5 Hz, 1H), 7.5 (m, 2H), 7.12 (m, 2H), 6.77 (s, 1H), 6.67 (d, J = 3.3 Hz, 2H), 4.56 (s, 2H), 3.77 (s, 3H), 1.84 (s, 3H), 1.70 (q, J = 3.6 Hz, 3 Hz, 2H), 1.11 (q, J = 3.6 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.47, 169.4, 155.17, 133.93, 132.45, 132.38, 130.15, 126.48, 116.06, 115.61, 115.4, 111.7, 64.9, 51.87, 30.21, 17.54, 15.07. HRMS(ESI) m/z calcd. For [C20H20FNO4]+: 357.38 [M+H]+, found 358.5.
2.4.2.23. Methyl 2-(3-(1-(4-chlorophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8l)
75% yield. IR (KBr, cm−1): 3434, 3340, 1760, 1656, 1604, 1524, 1492, 1436, 1414, 1283, 1213, 1192, 1181, 1160, 1090, 914, 856, 832, 780, 681. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.42 (m, 4H), 7.25 (d, J = 2.1 Hz, 2H), 6.87 (s, 1H), 6.80 (d, J = 2.1 Hz, 2H), 4.58 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.11 (q, J = 3.6 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.72, 169.14, 157.7, 140.11, 139.02, 131.78, 130.98, 129.2, 128.52, 113.3, 109.3, 106.83, 64.53, 51.77, 31.18, 15.09. HRMS (ESI) m/z calcd. For [C19H18ClNO4]+: 359.8 [M+H]+, found 360.1.
2.4.2.24. Methyl 2-(4-(1-(4-chlorophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8m)
86% yield. IR (KBr, cm−1): 3524, 3408, 1763, 1671, 1525, 1509, 1448, 1437, 1411, 1256, 1239, 1214, 1174, 1074, 836, 810. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.42 (m, 4H), 7.15 (m, 2H), 6.95 (s, 1H), 6.81 (d, J = 1.2 Hz, 2H), 6.63 (d, J = 1.8 Hz, 2H), 4.60 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.13 (q, J = 3.6 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.43, 169.27, 153.8, 139.15, 132.55, 131.69, 131.16, 128.48, 122.2, 114.2, 64.76, 51.72, 30.85, 15. HRMS (ESI) m/z calcd. For [C19H18ClNO4]+: 359.8 [M+H]+, found 360.1.
2.4.2.25. Methyl 2-(4-(1-(4-chlorophenyl)cyclopropane-1-carboxamido)-3-methylphenoxy)acetate (8n)
88% yield. IR (KBr, cm−1): 3489, 3423, 2936, 1758, 1677, 1636, 1536, 1496, 1438, 1397, 1292, 1272, 1217, 1165, 1119, 1097, 1079, 958, 885, 812, 759, 723. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.67 (d, J = 9.9 Hz, 1H), 7.41 (m, 2H), 7.29 (m, 1H), 7.08 (d, J = 8.4 Hz, 1H), 6.75 (s, 1H), 6.68 (t, J = 2.7 Hz, 1H), 4.56 (s, 2H), 3.77 (s, 3H), 1.86 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.12 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.98, 169.24, 155.17, 138.9, 134.3, 131.96, 130.03, 1286, 126.91, 126.84, 115.91, 111.55, 64.61, 51.73, 30.2, 17.57, 15.53, 14.9. HRMS (ESI) m/z calcd. For [C20H20ClNO4]+: 373.83 [M+H]+, found 374.1.
2.4.2.26. Methyl 2-(2-bromo-4-(1-(4-chlorophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8o)
81% yield. IR (KBr, cm−1): 3408, 3375, 3079, 3059, 3014, 2946, 1746, 1665, 1602, 1505, 1460, 1433, 1395, 1293, 1266, 1209, 1170, 1094, 873, 837, 808, 801, 752, 744, 704, 666. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.54 (d, J = 8.4 Hz, 1H), 7.41 (m, 4H), 7.28 (d, J = 2.7 Hz, 1H), 6.85 (s, 1H), 6.74 (d, J = 2.4 Hz, 1H), 4.64 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.12 (q, J = 3.6 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.74, 168.78, 150.08, 138.79, 133.47, 131.75, 131.25, 128.49, 125.33, 120.97, 113.25, 110, 65.43, 51.84, 30.78, 15.15. HRMS (ESI) m/z calcd. For [C19H17BrClNO4]+: 438.7 [M+H]+, found 440.2.
2.4.2.27. Methyl 2-(3-(1-(3-bromophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8p)
78% yield. IR (KBr, cm−1): 3415, 3011, 2953, 2249, 1758, 1681, 1606, 1524, 1493, 1443, 1434, 1195, 1159, 912, 780, 739, 698, 687. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.63 (t, J = 1.8 Hz, 1H), 7.52 (d, J = 1.2 Hz, 1H), 7.43 (d, J = 1.2 Hz,1H), 7.30 (m, 1H), 7.14 (m, 2H), 6.96 (s, 1H), 6.82 (d, J = 1.2 Hz, 1H), 6.62 (d, J = 1.8 Hz, 1H), 4.6 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 4.2 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171, 168.95, 157.87, 141.21, 138.78, 133.75, 131.35, 130.65, 129.57, 129.43, 122.92, 112.76, 110.34, 106.14, 77.32, 77, 76.68, 64.92, 51.97, 30.83, 16.11, 15.5. HRMS (ESI) m/z calcd. For [C19H18BrNO4]+: 404.25 [M+H]+, found 404.2.
2.4.2.28. Methyl 2-(4-(1-(3-bromophenyl)cyclopropane-1-carboxamido)-3-methylphenoxy)acetate (8q)
80% yield. IR (KBr, cm−1): 3398, 2950, 2842, 1748, 1647, 1509, 1410, 1308, 1211, 1066, 1031, 1019, 697. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.64 (t, J = 1.8 Hz, 1H), 7.50 (d, J = 0.9 Hz, 1H), 7.44 (d, J = 1.2 Hz, 1H), 7.29 (m, 2H), 6.88 (s, 1H), 6.80 (d, J = 9 Hz, 2H), 4.55 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 171.33, 171.26, 169.29, 154.56, 141.62, 134.05, 131.54, 130.78, 129.8, 121.77, 114.91, 77.31, 77, 76.68, 65.58, 52.2, 30.8, 16.19, 15.68. HRMS (ESI) m/z calcd. For [C19H18BrNO4]+: 404.25 [M+H]+, found 403.9.
2.4.2.29. Methyl 2-(4-(1-(3-bromophenyl)cyclopropane-1-carboxamido)-3-methylphenoxy)acetate (8r)
84% yield. IR (KBr, cm−1): 3478, 3428, 3058, 1756, 1684, 1525, 1437, 1289, 1218, 1116, 1079, 956, 801, 696. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.68 (m, 2H), 7.50 (m, 2H), 7.3 (m, 1H), 6.75 (s, 1H), 6.68 (m, 2H), 4.56 (s, 2H), 3.77 (s, 3H), 1.87 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3.3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.3, 169.23, 155.18, 142.7, 134.2, 132.73, 130.69, 130.25, 130, 129.11, 126.76, 121.83, 115.97, 111.6, 64.65, 51.74, 30.65, 17.58, 15.82, 15.61, 14.87. HRMS (ESI) m/z calcd. For [C20H20BrNO4]+: 418.28 [M+H]+, found 418.
2.4.2.30. Methyl 2-(4-(1-(3,4-dichlorophenyl)cyclopropane-1-carboxamido)phenoxy)acetate (8s)
85% yield. IR (KBr, cm−1): 3446, 3350, 1746, 1654, 1613, 1510, 1499, 1473, 1411, 1186, 1167, 819, 709. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.58 (d, J = 1.8 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.34 (m, 1H), 7.24 (m, 2H), 6.83 (m, 3H), 4.58 (s, 2H), 3.78 (s, 3H), 1.70 (q, J = 3.9 Hz, 3 Hz, 2H), 1.15 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.04, 169.24, 153.85, 141.12, 132.45, 131.48, 131.05, 130.57, 130.18, 129.81, 122.46, 114.61, 64.74, 51.72, 30.64, 15.15. HRMS (ESI) m/z calcd. For [C19H17Cl2NO4]+: 394.25 [M+H]+, found 394.4.
2.4.2.31. Methyl 2-(4-(1-(3,4-dichlorophenyl)cyclopropane-1-carboxamido)-3-methylphenoxy) acetate (8t)
88% yield. IR (KBr, cm−1): 3432, 1763, 1686, 1619, 1524, 1290, 1211, 1118, 1079, 800. 1H NMR (300 MHz, CDCl3): δ (ppm): 7.7 (d, J = 3.9 Hz, 2H), 7.50 (d, J = 8.1 Hz, 1H), 7.37 (d, J = 1.8 Hz, 1H), 6.68 (m, 3H), 4.57 (s, 2H), 3.77 (s, 3H), 1.91 (s, 3H), 1.71 (q, J = 3.9 Hz, 3 Hz, 2H), 1.13 (q, J = 3.9 Hz, 3 Hz, 2H). 13C NMR spectrum, δc, ppm, DMSO‑d6: 170.65, 169.24, 155.38, 141, 135.07, 132.15, 131.08, 130.63, 130.54, 129.95, 127.57, 115.86, 111.52, 64.6, 51.73, 30, 17.7, 15.02. HRMS (ESI) m/z calcd. For [C20H19Cl2NO4]+: 408.28 [M+H]+, found 408.4.
3. Results and discussion
In this paper, we have described the chemistry of novel 1-phenylcyclopropane carboxamide derivatives and their biological evaluation. 1-phenyl acetonitrile will undergo α-di-alkylation with 1,2-dibromoethane in sodium hydroxide and water at temperature 50–60 °C and TBAB as a phase transfer catalyst gives 1-phenyl cyclopropane acetonitrile in 85% yield as a pure compound. The acetonitrile compound was further treated with concentrated hydrochloric acid to give the corresponding 1-phenylcyclopropane acid compound a good yield of 80%.
Substituted Methyl 2-(aminophenoxy)acetate was prepared by reacting the o-alkylation of substituted amino Phenols with Methylchloroacetate and powdered potassium carbonate or cesium carbonate at 50–60 °C.
The peptide coupling of 1-phenylcyclopropane acid compound and substituted Methyl 2-(aminophenoxy)acetate with HATU results in the 1-phenylcyclopropane carboxamide derivatives.
3.1. Biological activity evaluation
Although many anticancer drugs have been developed in the past for chemotherapy, a desirable level of the therapeutic product has not yet been possible so far. In addition, these drugs have shown resistance against several cancer and their side effects, there is still an unmet need for treating cancer. Therefore, efforts are underway to develop new cancer drugs. In this study, antiproliferative and cytotoxic activities of these newly synthesized 20 compounds were performed with human myeloid leukaemia (U937) cells. None of the newly synthesized molecules showed cytotoxicity in the LDH release assay, even at a high concentration of 100 μM tested (Table 8) while these compounds exhibited antiproliferative activities on U937 cells. (Table 9). Active compounds in antiproliferative CellTiter-Glo assay were further calculated using Graph Pad Prism (Table 10) while all the compounds were not showing activity for cytotoxicity in LDH release assay.
Table 8.
Cytotoxicity activity, Optical density at 450 nm and percent activity was determined by LDH release assay. Triton-X100 was taken as reference positive control for cytotoxicity activity while DMSO as a diluent of these compounds was considered as a negative control where there is no cytotoxicity activity was observed.
| Compound ID | OD at 450 nm at different concentrations of compounds |
Percent inhibition at different concentrations of compounds |
||||||
|---|---|---|---|---|---|---|---|---|
| 100 μM | 50 μM | 25 μM | 12.5 μM | 100 μM | 50 μM | 25 μM | 12.5 μM | |
| 8a | 0.12 | 0.123 | 0.125 | 0.112 | 1% | 2% | 2% | −1% |
| 8b | 0.108 | 0.106 | 0.103 | 0.107 | −2% | −2% | −3% | −2% |
| 8c | 0.112 | 0.114 | 0.113 | 0.116 | −1% | 0% | −1% | 0% |
| 8d | 0.103 | 0.128 | 0.115 | 0.112 | −3% | 3% | 0% | −1% |
| 8e | 0.111 | 0.111 | 0.099 | 0.101 | −1% | −1% | −4% | −3% |
| 8f | 0.114 | 0.114 | 0.11 | 0.111 | 0% | 0% | −1% | −1% |
| 8g | 0.118 | 0.122 | 0.117 | 0.118 | 0% | 1% | 0% | 0% |
| 8h | 0.109 | 0.118 | 0.128 | 0.115 | −2% | 0% | 3% | 0% |
| 8i | 0.109 | 0.107 | 0.117 | 0.103 | −2% | −2% | 0% | −3% |
| 8j | 0.111 | 0.109 | 0.113 | 0.115 | −1% | −2% | −1% | 0% |
| 8k | 0.122 | 0.113 | 0.114 | 0.117 | 4% | −1% | 0% | 0% |
| 8l | 0.123 | 0.114 | 0.112 | 0.112 | 2% | 0% | −1% | −1% |
| 8m | 0.117 | 0.119 | 0.122 | 0.113 | 0% | 1% | 1% | −1% |
| 8n | 0.113 | 0.119 | 0.111 | 0.117 | −1% | 1% | −1% | 0% |
| 8o | 0.097 | 0.107 | 0.105 | 0.115 | −4% | −2% | −2% | 0% |
| 8p | 0.115 | 0.115 | 0.11 | 0.117 | 0% | 0% | −1% | 0% |
| 8q | 0.113 | 0.117 | 0.111 | 0.108 | −1% | 0% | −1% | −2% |
| 8r | 0.109 | 0.12 | 0.111 | 0.113 | −2% | 1% | −1% | −1% |
| 8s | 0.114 | 0.112 | 0.115 | 0.136 | 0% | −1% | 0% | 4% |
| 8t | 0.107 | 0.125 | 0.11 | 0.112 | −2% | 2% | −1% | −1% |
| Positive control (Triton-X100, 0.1%) | 0.595 | 0.587 | 0.545 | 0.557 | 100% | |||
| Negative control, DMSO | 0.11 | 0.132 | 0.133 | 0.109 | 0% | |||
Table 9.
Antiproliferative activity RLU count and percent inhibition determined by Cell Titer Glo assay. Mitomycin C was taken as reference positive control for antiproliferative activity while DMSO as a diluent of these compounds was considered as a negative control where there is no antiproliferative activity was observed.
| Compound ID | RLU at different concentrations of compounds |
Percent inhibition at different concentrations of compounds |
||||||
|---|---|---|---|---|---|---|---|---|
| 200 μM | 100 μM | 50 μM | 25 μM | 200 μM | 100 μM | 50 μM | 25 μM | |
| 8a | 114 | 87 | 1204 | 1205 | 100% | 102% | 28% | 28% |
| 8b | 101 | 93 | 964 | 1015 | 101% | 102% | 44% | 41% |
| 8c | 95 | 93 | 677 | 1069 | 101% | 102% | 63% | 37% |
| 8d | 94 | 109 | 1263 | 1401 | 101% | 100% | 24% | 15% |
| 8e | 108 | 732 | 956 | 935 | 101% | 59% | 45% | 46% |
| 8f | 119 | 1150 | 1079 | 1109 | 100% | 32% | 36% | 34% |
| 8g | 109 | 284 | 1249 | 1357 | 100% | 89% | 25% | 18% |
| 8h | 123 | 145 | 420 | 1378 | 100% | 98% | 80% | 17% |
| 8i | 99 | 423 | 1060 | 1213 | 101% | 80% | 38% | 28% |
| 8j | 95 | 484 | 1042 | 1807 | 101% | 76% | 39% | −12% |
| 8k | 85 | 106 | 509 | 1147 | 102% | 101% | 74% | 32% |
| 8l | 87 | 104 | 174 | 680 | 102% | 101% | 96% | 63% |
| 8m | 100 | 125 | 892 | 1039 | 101% | 99% | 49% | 39% |
| 8n | 104 | 104 | 1138 | 1212 | 101% | 101% | 33% | 28% |
| 8o | 93 | 79 | 463 | 838 | 102% | 102% | 77% | 52% |
| 8p | 82 | 98 | 721 | 1075 | 102% | 101% | 60% | 37% |
| 8q | 89 | 84 | 955 | 1231 | 102% | 102% | 45% | 26% |
| 8r | 105 | 119 | 359 | 1225 | 101% | 100% | 84% | 27% |
| 8s | 81 | 93 | 666 | 1023 | 102% | 102% | 64% | 40% |
| 8t | 108 | 90 | 645 | 1132 | 101% | 102% | 65% | 33% |
| Positive control (Mitomycin C, 10 μM) | 142 | 143 | 139 | 140 | 100% | |||
| Negative control, DMSO | 1985 | 1651 | 1758 | 1507 | 0% | |||
Table 10.
Calculated antiproliferative activity (GI50) and cytotoxicity activity (LC50) values determined by Graph Pad Prism analysis using percent inhibition as well as percent activity respectively of the compounds.
| Compound ID | Antiproliferative (U937) GI50 (uM) | Cytotoxicity LC50 (uM) |
|---|---|---|
| 8a | 5.6 | >100 |
| 8b | 4.2 | >100 |
| 8c | 3.3 | >100 |
| 8d | 6.1 | >100 |
| 8e | >200 | >100 |
| 8f | >200 | >100 |
| 8g | 6.8 | >100 |
| 8h | 3.6 | >100 |
| 8i | 7.9 | >100 |
| 8j | 5.9 | >100 |
| 8k | 3.3 | >100 |
| 8l | 2.1 | >100 |
| 8 m | 3.9 | >100 |
| 8n | 6 | >100 |
| 8o | 1.6 | >100 |
| 8p | 3.5 | >100 |
| 8q | 5.3 | >100 |
| 8r | 3.2 | >100 |
| 8s | 3 | >100 |
| 8t | 3.6 | >100 |
4. Conclusion
In synthetic organic chemistry, the development of novel approaches for the synthesis of 1-phenylcyclopropane carboxamide derivatives along with their bioactivities examination is known to be an important and continuing challenge. Here we have reported the synthesis and characterization of novel 1-phenylcyclopropane carboxamide derivatives, these compounds were screened for biological activities. We have accomplished by a linear strategy utilizing simple, efficient and involve mild reaction conditions and higher yields. This will add as an attractive procedure for the existing armory of 1-phenylcyclopropane carboxamide derivatives.
Funding
No funding is available.
Availability of data and material
All the experimental data was included in the manuscript.
Author contributions
Mr. Panasa Mahesh: Performed the experiments; Dr. Parameswari Akshinthala: Contributed reagents and materials; Dr. Ashok Reddy Ankireddy: Designed the experiments; Dr. Naresh Kumar Katari: Conceived the experiments; Dr. Lavleen Kumar Gupta: Analysis tools; Ms. Deepali Srivastava: Analysis data; Prof. Sreekantha Babu Jonnalagadda: Interpreted the data, Edit and Proof reading; Prof. Rambabu Gundla: Analyzed the data and Wrote the paper.
Competing interest/Competing interests
The authors declare that they have no competing interests/competing interests.
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Consent for publication
We authorize to publish the article without any conflict.
Acknowledgements
The authors wish to thank the management of a Department of Chemistry, GITAM deemed to be University for the research facilities.
Contributor Information
Naresh Kumar Katari, Email: nkatari@gitam.edu.
Sreekantha Babu Jonnalagadda, Email: Jonnalagaddas@ukzn.ac.za.
Rambabu Gundla, Email: rgundla@gitam.edu.
References
- 1.Liessi N., Cicero E., Pesce E., Arkel M., Salis A., Tomati V., Paccagnella M., Damonte G., Tasso B., Galietta L.J.V., Pedemonte N., Fossa P., Millo E. Synthesis and biological evaluation of novel thiazole- VX-809 hybrid derivatives as F508del correctors by QSAR-based filtering tools. Eur. J. Med. Chem. 2018;144:179–200. doi: 10.1016/j.ejmech.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M. Brian. Amide, carbamate and urea derivatives having glutamate receptor function potentiating activity. Eur. Pat. Appl. 2000 [Google Scholar]
- 3.Yongjia S., Gao Q., Xu S. Chiral bidentate boryl ligand enabled iridium-catalyzed enantioselective C(sp3)−H borylation of cyclopropanes. J. Am. Chem. Soc. 2019;141(27):10599–10604. doi: 10.1021/jacs.9b04549. [DOI] [PubMed] [Google Scholar]
- 4.Peng-Xiang S., Hu L., Shao Q., Hong K., Yu J.Q. Pd(II)-Catalyzed enantioselective C(sp3)−H arylation of free carboxylic acids. J. Am. Chem. Soc. 2018;140(21):6545–6549. doi: 10.1021/jacs.8b03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingwei Z., Yimin H., Ke E., Xuefei T., Hong C.S., Xuhong Q. Efficient cyclopropanation of aryl/heteroaryl acetates and acetonitriles with vinyl diphenyl sulfonium triflate. Tetrahedron Lett. 2018;59(14):1443–1445. [Google Scholar]
- 6.Dunn M., Jamie M., Kuethe J.T., Orr R.K., Tudge M., Campeau L.C. The development of a palladium-catalyzed α-arylation of cyclopropyl nitriles. Org. Lett. 2014;16(24):6314–6317. doi: 10.1021/ol5030426. [DOI] [PubMed] [Google Scholar]
- 7.Neidle S., Thurston D.E. Chemical approach to the discovery and development of cancer therapies. Nat. Rev. Cancer. 2005:285–296. doi: 10.1038/nrc1587. [DOI] [PubMed] [Google Scholar]
- 8.Desplat V., Moreau S., Gay A., Fabre S., Thiolat D., Massip S., Macky G., Godde F., Mossalayi D., Jarry C., Guillon J. Synthesis and evaluation of the antiproliferative activity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt kinase. Part II. J. Enzym. Inhib. Med. Chem. 2010;25(2):204–215. doi: 10.3109/14756360903169881. [DOI] [PubMed] [Google Scholar]
- 9.Ty N., Kaffy J., Arrault A., Thoret S., Pontikis R., Dubois J., Allory L., Florent J. Synthesis and biological evaluation of cis-locked vinylogous combretastatin-A4 analogues: derivatives with a cyclopropyl-vinyl or a cyclopropyl-amide bridge. Bioorg. Med. Chem. Lett. 2009;19:1318–1322. doi: 10.1016/j.bmcl.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Dreas A., Mikulski M., Milik M., Fabritius C., Brzozka K., Rzymski T. Mitogen-activated Protein Kinase (MAPK) interacting kinases 1 and 2 (MNK1 and MNK2) as targets for cancer therapy: recent progress in the development of MNK inhibitors. Curr. Med. Chem. 2017;24:3025–3053. doi: 10.2174/0929867324666170203123427. [DOI] [PubMed] [Google Scholar]
- 11.Teruya T., Konishi T., Uechi S., Tamaki H., Tako M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus TOKIDA in U937 cells. Int. J. Biol. Macromol. 2007;41:221–226. doi: 10.1016/j.ijbiomac.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu W., Liu X., Tian L., Gao Y., Liu W., Chen H., Jiang X., Xu Z., Ding H., Zhao Q. Design, synthesis and biological evaluation of harmine derivatives as potent GSK-3b/DYRK1A dual inhibitors for the treatment of Alzheimer's disease. Eur. J. Med. Chem. 2021;222 doi: 10.1016/j.ejmech.2021.113554. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher G.C., Brokx R.D., Denny T.A., Hembrough T.A., Plum S.M., Fogler W.E., Sidor C.F., Bray M.R. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol. Cancer Therapeut. 2010;10(1):126–137. doi: 10.1158/1535-7163.MCT-10-0574. [DOI] [PubMed] [Google Scholar]
- 14.Kuo C., Shih M., Kuo Y., Chiang W. Antagonism of free-radical-induced damage of adlay seed and its antiproliferative effect in human histolytic lymphoma U937 monocytic cells. J. Agric. Food Chem. 2001;49:1564–1570. doi: 10.1021/jf001215v. [DOI] [PubMed] [Google Scholar]
- 15.Liu J., Gong Y., Shi J., Hao X., Wang Y., Zhou Y., Hou Y., Liu Y., Ding S., Chen Y. Design, synthesis and biological evaluation of novel N-[4-(2-fluorophenoxy)pyridin-2-yl]cyclopropanecarboxamide derivatives as potential c-Met kinase inhibitors. Eur. J. Med. Chem. 2020;194 doi: 10.1016/j.ejmech.2020.112244. [DOI] [PubMed] [Google Scholar]
- 16.Shen Peng-Xiang, Hu Liang, Jin-Quan Yu. Pd(II)-catalyzed enantioselective C(sp3)-H arylation of free carboxylic acids. J. Am. Chem. Soc. 2018;140(21):6545–6549. doi: 10.1021/jacs.8b03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbasiewicz M., Marciniak K., Fedorynski M. Phase transfer alkylation of arylacetonitriles revisited. Tetrahedron Lett. 2006;47:3871–3874. [Google Scholar]
- 18.Cherian J., Nacro K., Poh Z., Guo S., Athisayamani Jeyaraj D., Wong Y., Ho M., Yang H., Joy J., Kwek Z., Liu B., Wee J., Ong E., Choong M., Poulsen A., Lee M., Pendharkar V., Ding L., Manoharan V., Chew Y., Sangthongpitag K., Lim S., Tiong Ong S., Hill J., Keller T.H. Structure-activity relationship studies of mitogen activated protein kinase interacting kinase (MNK) 1 & 2 and BCRABL1 inhibitors targeting chronic myeloid leukemic cells. J. Med. Chem. 2016;59(7):3063–3078. doi: 10.1021/acs.jmedchem.5b01712. [DOI] [PubMed] [Google Scholar]
- 19.Aay N., Aoyama R.G., Arcalas A., Chan W.K.V., Hongwang D., Kearney P., Koltun E.S., Nachtigall J.A., Pack M., Richards S.J. Inhibitors of glucosylceramide synthase. PCT. Appl. 2010 [Google Scholar]
- 20.Huang A., Moretto A., Janz K., Lowe M., Bedard P.W., Tam S., Di L., Clerin V., Sushkova N., Tchernychev B., Tsao D.H.H., Keith J.C., Jr., Shaw G.D., Schaub R.G., Wang Q., Kaila N. Discovery of 2-[1-(4-chlorophenyl)cyclopropyl]-3-hydroxy-8-(trifluoromethyl)quinoline-4-carboxylic acid (PSI-421), a P-selectin inhibitor with improved pharmacokinetic properties and oral efficacy in models of vascular injury. J. Med. Chem. 2010;53(16):6003–6017. doi: 10.1021/jm9013696. [DOI] [PubMed] [Google Scholar]
- 21.Kaila N., Janz K.M., Huang A., Moretto A.F., Bedard P.W. Methods and compositions for selectin inhibition. PCT. Appl. 2008 [Google Scholar]
- 22.Hartung I., Ince S., Kettschau G., Thierauch K.H., Briem H., Ter Laak A.M., Lienau P. Substituted Arylpyrazolopyridines and salts thereof, pharmaceutical compositions comprising same, methods of preparing same and uses of same. PCT Appl. 2007 [Google Scholar]
- 23.Pandya Vrajesh B., Parmar Bhavesh M. Novel antithrombotic agents. PCT Appl. 2011 [Google Scholar]
- 24.Wong Y.C., Ke Z., Yeung Y.Y. Lewis basic sulfide catalyzed electrophilic bromocyclization of cyclopropylmethyl amide. Org. Lett. 2015;17(20):4944–4947. doi: 10.1021/acs.orglett.5b02557. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed F.A.M., Gomaa H.A.M., Hendawy O.M., Ali A.T., Farghaly H.S., Gouda A.M., Abdelazeem A.H., Abdulrahman M.H., Trembleau L., Youssif B.G.M. Design, synthesis, and biological evaluation of novel EGFR inhibitors containing 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity. Bioorg. Chem. 2021;112 doi: 10.1016/j.bioorg.2021.104960. [DOI] [PubMed] [Google Scholar]
- 26.Abdelrahman M.H., Aboraia A.S., Youssif B.G.M., Elsadek B.E.M. Design, synthesis and pharmacophoric model building of new 3-alkoxymethyl/3-phenyl indole-2-carboxamides with potential antiproliferative activity. Chem. Biol. Drug Des. 2017;90(1):64–82. doi: 10.1111/cbdd.12928. [DOI] [PubMed] [Google Scholar]
- 27.Crouch S.P.M., Kozlowski R., Slater K.J., Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 28.Raffa D., Plescia F., Maggio B., Valeria Raimondi M., D'Anneo A., Lauricella M., Daidone G. Anthranilamide-based 2-phenylcyclopropane-1-carboxamides, 1,1'-biphenyl-4-carboxamides and 1,1'-biphenyl-2-carboxamides: synthesis biological evaluation and mechanism of action. Eur. J. Med. Chem. 2017;132:262–273. doi: 10.1016/j.ejmech.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Li Y., Sheng C., ZhiliangLv, Dong G., Wang T., Liu J., Zhang M., Li L., Zhang Tao, Geng D., Niu C., Li Ke. Design and synthesis of cyclopropylamide analogues of combretastatin-A4 as novel microtubule-stabilizing agents. J. Med. Chem. 2013;56:685–699. doi: 10.1021/jm301864s. [DOI] [PubMed] [Google Scholar]
- 30.Sun W., Liu K., Chul Ryu H., Kang D., Kim Y., Kim M., Cho Y., Rahul S., Shivaji B., Thorat A., Kim H., Pearce L.V., Pavlyukovets V., Tran R., Morgan M.A., Lazar J., Ryder C.B., Toth A., Blumberg P.M., Lee J. 2-(4-Methylsulfonylaminophenyl) propanamide TRPV1 antagonists: structure–activity relationships in the B and C-regions. Bioorg. Med. Chem. 2012;20:1310–1318. doi: 10.1016/j.bmc.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the experimental data was included in the manuscript.