Abstract
Laccase is a multi-copper enzyme widely expressed in fungi, higher plants, and bacteria which facilitates the direct reduction of molecular oxygen to water (without hydrogen peroxide production) accompanied by the oxidation of an electron donor. Laccase has attracted attention in biotechnological applications due to its non-specificity and use of molecular oxygen as secondary substrate. This review discusses different applications of laccase in various sectors of food, paper and pulp, waste water treatment, pharmaceuticals, sensors, and fuel cells. Despite the many advantages of laccase, challenges such as high cost due to its non-reusability, instability in harsh environmental conditions, and proteolysis are often encountered in its application. One of the approaches used to minimize these challenges is immobilization. The various methods used to immobilize laccase and the different supports used are further extensively discussed in this review.
Keywords: Laccase, Applications, Immobilization
1. Introduction
Laccase (EC 1.10.3:2) is a multi-copper enzyme that was first discovered in the discharge of Rhus Verniciflua by Yoshida in 1883 and later characterized as a fungal enzyme (metal containing oxidase) by Bertrand in 1985 [1,2]. The enzyme has been widely identified in fungi and higher plants, however it was only recently (1993) isolated in bacteria with the first bacterial laccase identified from Azospirillum lipoferum [3, 4]. All the laccases described to date are glycoproteins (monomeric, dimeric, and tetrameric) that show a great deal of divergence [5]. Although the catalytic site is conserved, diversity is observed in the rest of the molecule structure and sugar moiety [6].
Laccase catalyses direct reduction of molecular oxygen to water, through a one electron oxidation of aromatic substrates without formation of hydrogen peroxide intermediate, by one electron abstraction [7, 8, 9]. Molecules containing an aromatic ring substituted with an electron withdrawing groups such as phenols, cresols, chlorophenols, aryldiamines, aromatic amines, and polymethoxybenzenes all undergo oxidation in the presence of laccase [10, 11]. Upon oxidation, free cationic radicals are formed that further undergo catalysed oxidation to form quinones or non-enzymatic reactions such as hydration and polymerization occur to form high molecular weight insoluble components such as dimers, oligomers, or polymers [12, 13, 14, 15]. Thus, laccase shows broad specificity of its substrates and has gained popularity in detoxification processes of aquatic and xenobiotics, industrial effluents, and biotechnological industrial applications [16, 17].
Physical methods such as circular dichroism and Electron paramagnetic resonance (EPR) have been used to study laccase structure. It has been observed that the metallic centres are relatively stable but the secondary structures differ due to varying amino acid sequence and/or composition of the carbohydrate moiety [6]. Due to the diversity in the enzyme secondary structures, the substrates oxidized by laccase vary from one laccase to another ranging from inorganic or organic metal complexes, ferrocyanides, anilines, benzenethiols, and phenols to other redox inorganic, organic, or biological compounds [18, 19]. For example Xu et al. [20], demonstrated that the catalytic properties of laccase from the fungi Rhizoctonia sp. and Myceliophthora thermophile as well their inhibition were altered by type 1 copper site directed mutagenesis.
Laccase catalytic reactions occur in ambient temperatures, use molecular oxygen as the secondary substrate reducing it by a four electron mediator-less mechanism, do not require any other cofactors and only water is produced as the by-product [21, 22]. Due to their extracellular nature, laccases can tolerate high pollutant concentrations in substrates [23]. These advantages and the ability to produce laccase by simple and inexpensive methods make it desirable in various industrial and environmental applications including organic synthesis, degradation of toxic organic compounds, biosensors, and immunoassays [24, 25, 26]. As a result, it has attracted research interest in order to understand its mechanism and obtain a scientific basis for its employment in biotechnological applications [27].
However, with all the advantages of using laccase, it has its own shortcomings including very low stability in harsh conditions and non-recoverability which limits its application at an industrial scale [28]. Recently, attention has been placed on the enzyme immobilization in a confined space or on a support to improve and optimize its performance for commercial use as a biocatalyst and facilitate its reuse [28, 29]. This review explores the various sources of laccase in nature, its structures and proposed mechanisms of catalytic activity, its roles in nature, and its industrial and environmental applications. The immobilization techniques that have been employed over the years to improve its stability and reusability as well as the various immobilization supports are also extensively discussed. The various sections discussed in this review about laccase enzyme are schematically represented in Figure 1.
Figure 1.
A schematic representation of what is covered in this review about laccase enzyme.
2. Structure and reaction mechanism of laccase enzyme
Laccase is a blue oxidase glycoprotein (monomeric, dimeric, or tetrameric) with an amino acid chain over 500 amino acids and carbohydrate moiety of 10–45% of the protein molecule by weight [28, 30]. Different carbohydrates including glucose, hexose amine, mannose, galactose, fructose, xylose and arabinose make up the carbohydrate moiety (glycans), are bound to the polypeptide of the enzyme through an N-linkage and vary depending on the source of the enzyme [31, 32]. The binding of the carbohydrate moieties to the enzyme defines the structure of the enzyme through a specific folding which provides protection against proteolytic and elevated temperature degradations [32].
More than one laccase are often identified in a single fungus with different molecular weights, pH optima, sugar moiety, and substrate specificity [6]. For example, Kim et al. [33] discovered a second extracellular laccase from Crypthonectria parasitica by deleting laccase 1 using recombinant DNA techniques and growing the mutant strain in tannic acid. Wahleithner et al. [34] identified four distinct laccase genes in the fungus Rhizoctonia solani and three of which had been expressed in another fungus Aspergillus oryzae. Marbach et al. [35], identified two molecular forms of laccase induced by different phenolics from Botrytis cinera with different sugar content, isoelectric focusing pattern, amino acid composition, and molecular weight. The enzyme induced by grape juice had a molecular weight of 38,000 and 80% sugar while that induced by gallic acid had molecular weight of 36,000 and 70% sugar. Fukushima and Kirk [36] identified two laccases from the fungus Ceriporiopsis subvermispora with two isoelectric points (3.4 and 4.8), molecular masses of 71 and 68 kDa, half-lives of 120 and 50 min at 60 °C, and carbohydrate content of 15 and 10%, respectively.
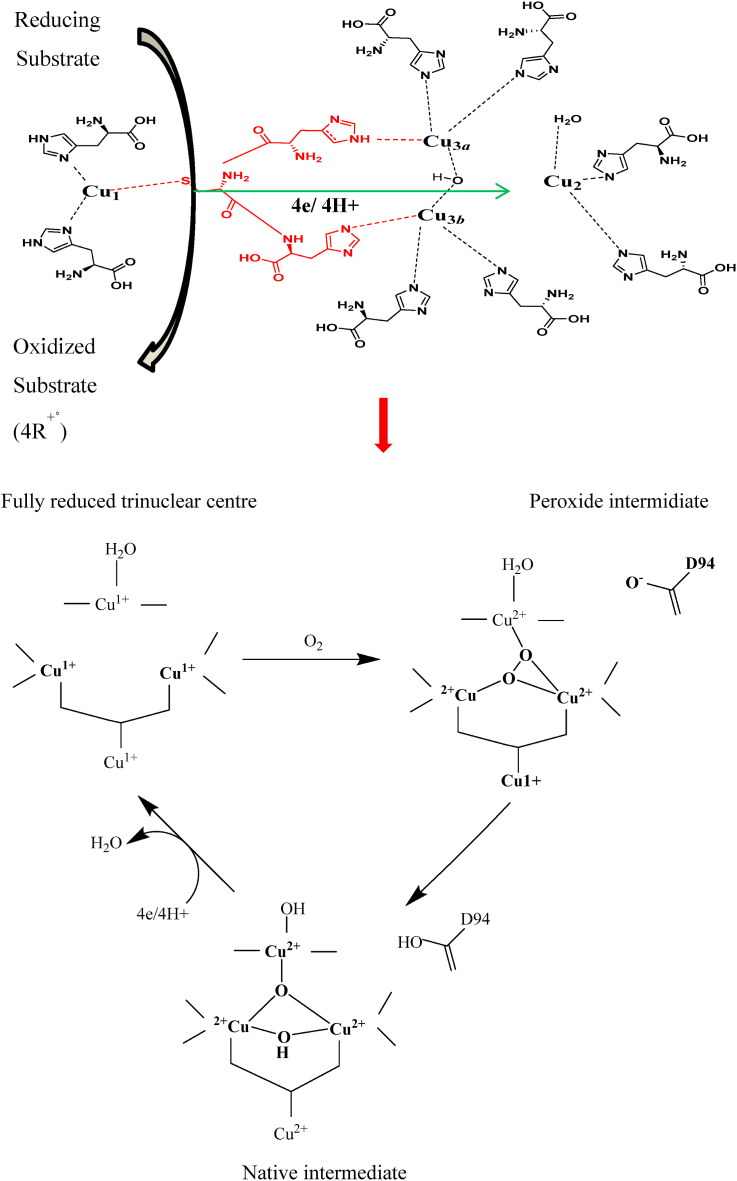
The active holoenzyme form of laccase consists of four copper atoms that are classified into three types depending on the features of their surroundings, their accessibility to solvents, and characteristic electron paramagnetic resonance (EPR) signals [37, 38]. The copper atoms are classified into type 1 (blue), type 2 (normal) and type 3 (coupled binuclear) copper with two copper atoms belonging to the type 3 site [30, 39]. Type 1 and type 2 copper have strong electronic adsorption and well characterized EPR signals and type 3 copper atoms are antiferromagnetically coupled through a binding ligand which makes the EPR signal undetectable but give a weak absorbance in the near UV region at 330 nm [30, 40]. Type 1 copper is responsible for the intense blue colour of the enzyme due to a ligand-to-metal charge transfer absorption of the copper cysteine-bond [41, 42]. It is further available to interact freely with solvents (water inclusive), can be removed from the enzyme molecule by various copper complexes, and can displaced by mercury or cobalt with a great loss in activity [30]. Type 2 copper coordinates two Histidine (His)-N and an oxygen atom as OH− while each copper of type 3 coordinates three His residues [43, 44]. The type 1 and type 2/3 copper are bridged by a His-cysteine (Cys)-His tripeptide bridge that acts as an intramolecular electron transfer highway [45].
Substrates attach to the binding site of laccase by hydrophobic interaction and all the copper ions are presumed to be involved in the catalytic mechanism [46, 47]. As demonstrated in Figure 2, Type 1 and 2 are presumed to participate in electron capture and transfer while type 2 and 3 are involved in binding with oxygen or oxygen uptake [38, 48]. The catalysis process of laccase comprises of type 1 copper reduction by the reducing substrate, internal electron transfer from type 1 to type 2 and 3 trinuclear structure, and ultimate activation and reduction of oxygen to water at the type 2 and 3 copper trinuclear structure (Figure 2) [42, 49, 50]. The O2 molecule binds to the trinuclear cluster for asymmetric activation and it is presumed that the O2 binding compartment restricts access of other oxidizing agents other than oxygen [30, 49].
Figure 2.
A schematic representation of the different amino acids that coordinate with the copper at the laccase site and the reaction mechanism for the laccase catalytic activity.
Laccase-like enzymes that lack typical absorption at 600 nm have been identified. For example, Palmeri et al. [41], reported a white laccase from P. ostreatus that contained one copper, two zinc, and one iron atoms per mole of enzyme and did not have an absorbance at 600 nm hence the classification of white laccase. The enzyme is considered a laccase due to its identical structure to other laccases and the fact that it uses oxygen as the oxidizing substrate [51]. Leotievsky et al. [52], identified yellow laccase from solid-state culture of ligninolytic fungi as a result of binding lignin-derived molecules by the enzyme protein. The yellow laccases show high homology with the blue laccases when their N-terminal amino acids sequences are compared. They have similar copper content to the typical blue laccase but don't maintain their copper centres in the oxidized state under anaerobic conditions, that is, the copper in the yellow laccase appears in the reduced form. The change in this enzyme property is attributed to binding of low molecular mass phenolic material arising from lignin degradation or heterogeneity induced by glycosylation [51].
Laccases are inhibited by metal ions such as Hg2+, Fe2+, Ag+, Li+ and Pb2+ [53, 54, 55], humic acid [56], cationic quaternary ammonium detergents and small anions like halides [57, 58, 59], azide [60, 61], and hydroxyl [62]. The inhibition process involves amino acid residue modification, copper chelation or conformational changes, and binding of small anions on type 2 and 3 copper hence interrupting internal electron transfer [58, 59]. Enzyme inhibition due to organic solvents occurs as a result of hydrophobic interactions between the solvent and non-polar groups of laccase and substitution of hydration water within the enzyme with the organic solvent. This happens because the binding site of the organic substrate in the enzyme is close to the surface of enzyme globule hence readily accessible for the solvent [63, 64]. Halides bind to the type 2 and 3 copper of the enzyme and inhibit electron transfer from type 1 copper to the trinuclear structure thus leading to enzyme catalytic decay [65]. Metal ions competitively inhibit laccase due to their strong affinity to thiol groups in laccase but have no impact on the enzyme active centre [66]. Interestingly, the presence of divalent metal ions has shown to improve laccase activity due to their competition with Cu2+ in the electron transport system which improves the enzyme-substrate relationship [67].
With the wide range of substrates that can be oxidized by laccase, addition of mediators such as 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) ABTS [68, 69], N-hydroxybenzotriazole (HOBT) [70], and 2,2,5,5-tetramethyl-4-piperidin-1-oxyl radical (TEMPO) [71, 72] to a specific laccase enzyme can further increase the substrate range to include non-phenolic compounds. Mediators are low-molecular-weight organic compounds that are oxidized by laccase to form highly active cation radicals which oxidize the non-phenolic compounds that are usually resistant or impervious to laccase oxidation through the normal shuttling of electrons from the substrate to the enzyme [73, 74, 75]. For example, Peralta-Zamora et al. [76], used HOBT as a mediator in the decolourisation of azo and indigo dyes (usually not laccase substrates) using free laccase and laccase immobilized on silica modified with imidazole. No decolourisation was detected in the absence of HOBT but achieved 30% and 45% decolourisation efficiency for free and immobilized laccase respectively within 30 min after adding 0.4 mL of HOBT. Dodor et al. [77], used T. versicolor laccase immobilized on kaolinite for the catalytic oxidation of anthracene and benzo [a]pyrene in the presence of ABTS as a mediator. Between 17% and 19% oxidation were achieved without a mediator, but after addition of ABTS the oxidation efficiency increased to 80% and 85% for anthracene and benzo [a]pyrene respectively. Gu et al. [78], covalently immobilized laccase on ABTS-encapsulated cellulose beads for indole degradation. While free laccase hardly degrades indole, the biocatalyst achieved a degradation rate of up to 99.7%. Reyes et al. [79], demonstrated that the number of decolourised dyes by laccase increased from 13 to 26 out of 38 dyes when 1 mM HOBT was added as a mediator and increased rate of decolourisation was observed. Laccase immobilized on concanavalin A-activated Fe3O4 nanoparticles demonstrated an exponential increase in the rate of removal of sulfonamide antibiotics when syringic acid was used as a mediator [80].
However, the benefits of mediators are sometimes negated by their disadvantages such as toxicity, cost, and the ability of radical species from mediator compounds to undergo chemical reactions with aromatic side-chains of laccase thereby inactivating the enzyme [81, 82]. For example, Skoronski et al. [83], observed that it was impossible to reuse Aspergillus sp. laccase immobilized on chitosan during syringaldazine bioconversion. More so, the approach used in introducing a mediator has an impact on the performance of the biocatalyst. For example, when laccase and acetylacetone (mediator) were co-immobilized through initiated polymerization into a hydrogel, the biocatalyst displayed a high substrate conversion of malachite green as compared to the sole immobilized laccase and immobilized laccase with an external mediator [84]. Therefore, factors such as good laccase substrate with high redox potential intermediate and with stable reduced and oxidized forms that do not inhibit enzymatic reaction should be considered when selecting a redox mediator [85].
3. Role of laccase in nature
Due to the large amounts of laccase produced by lignin degrading (wood rotting) fungi, it has been presumed that the main role of laccase in nature is the polymerization and degradation of lignin [63, 86]. Laccase mediated degradation of lignin starts with the loss of an electron from the phenolic hydroxyl groups of lignin to produce phenoxy radicals which spontaneously re-organizes to aid either α-carbon oxidation or Cα-Cβ bond cleavage of alkyl side chains of the polymer to produce low molecular weight and/or polymeric products due to polymerizing activity of the enzyme [30, 87]. Fungal laccases are argued to be involved in the regeneration of tobacco protoplasts [88], lignification of cell walls [6, 89], morphogenesis of rhizomorphs [37], sclerotization and melanogenesis processes in insects [90, 91, 92], fungal virulence, sporulation and plant pathogenesis [49, 93].
Laccase has been suggested to play a role in humus synthesis. Laccase catalyses the cleavage of lignin and non-lignin macromolecules into their monomeric structural units which react with other compounds to form humic substances of varying complexity [49, 94]. Additionally, laccase catalyzes coupling reactions between phenolic compounds and humic acids leading to the binding of pollutants on organic matter of soil hence reducing their bioavailability and toxicity [11, 32]. Because of this, laccases have been investigated for applications in detoxification of pollutants in soil environments. Polluted soils can be cleaned up by laccase-mediated incorporation of pollutants into humus through catalytic coupling of xenobiotic compounds and derivatives to humic substance or humic like compounds [49].
4. Sources and production of laccase
Laccases have been identified in fungi [13, 95], higher plants [96, 97], bacteria [49, 98], and insects [99, 100]. The laccases extracted from the various sources have shown different biochemical properties dependent on the source due to the different roles they play in these organisms [101]. In plants laccases aid in lignin polymer formation, in fungi morphogenesis, stress defence, and lignin degradation, while in insects it participates in stress defence, the biosynthesis of brown melanin like pigment, and protection from UV light and hydrogen peroxide [102]. The laccase from plants and fungi is mainly extracellular while that from bacteria is mostly intracellularly localized [103]. Laccases from fungi have lower optimum pH, ranging from 3.6 – 5.2, than plant laccases that have optimum pH in the range 6.8–7.4. The low optimum pH of fungal laccases is due to their adaptability to grow under acidic conditions, while the plant laccase being intracellular have their pH optima nearer to the physiological range. As a result, the functions of laccases obtained from these sources differ in that fungal laccases mainly degrade toxic phenolic compounds while plant laccases are mainly involved in synthetic process such as lignin formation [5].
Different fungi have been used for the production of laccase including Agaricus sp. [93], Trametes sp. [104, 105], Cerrena unicolor [106, 107], Dichomitus squalens [108], Aspergillus oryzae [109, 110], Pleurotus ostreatus [111, 112], Coriolus hirsitus [113], Coriolopsis sp. [114, 115], Rigidoporus lignosus [116], Neurospa crassa [31], Pleurotus florida [117], Cyathus bulleri [118], Ganoderma lucidum [119], Funalia trogii [120], Paraconiothyrium variabile [121] and Trichoderma harzianum [122]. Laccase producing fungi are mainly from three genera, that is, ascomycetes [14], deuteromycetes [123] and basidiomycetes [13]. The basidiomycetes class includes various wood and litter decomposing and soil inhabiting fungi that have been broadly used to produce laccases with variable induction mechanisms, degrees of polymorphism, and physiochemical and kinetic properties due to their efficiency in lignin degradation [108, 112].
Recently, some bacteria have been used as sources of laccase e.g. B. subtillis [124, 125], γ-Proteobacterium JB commonly found in industrial waste water [126], Streptomyces coelicolor [127], Thermus thermophilus [27], Weissella viridescens [128], Shewanella putrefaciens [103], Alcaligenes faecalis [129], Sphingobacterium ksn-11 [130], Brevibacterium halotolerans N11 [131], and E. coli [27]. Laccases obtained from bacteria have unique characteristics compared to the fungal and higher plant laccases which gives them greater advantages in terms of industrial applications. For example, the laccase obtained from T. thermophilus demonstrated a very high optimal reaction temperature of 92 °C and a half-life of thermal inactivation at 80 °C of over 14 h, making it the most thermophilic laccase reported thus far [27]. Endospore laccases from bacteria serve microorganisms to survive harsh conditions hence the enzyme is expected to withstand harsh conditions such as high temperature, extreme pH, and presence of ionic salts which is beneficial for industrial applications [130, 132].
In plants, laccases have been obtained from maize seeds [133], Rhus vernicifera [25], papaya (Carica papaya) leaves [67], Sycamore Maple (Acer pseudoplatanus) [96], and vegetables such as cabbages, turnip, potatoes, pears, and apples [5, 30]. For example, Bailey et al. [133], extracted laccase from maize seeds. A portion of the extractable laccases was in an inactive form and was activated by treatment with copper and chloride.
Laccase is obtained through fermentation of laccase producing organisms. However, the enzyme is usually produced in small amounts and its productivity is increased by introduction of inducers or altering cultivation conditions [134]. The two most critical components of nutritional medium are nitrogen and carbon [42]. Presence or absence of inducers, induction time, nature and composition of culture medium, carbon to nitrogen ratio, type of culture conditions (pH, temperature, aeration), and genetic manipulation determine the amount and type of laccase to be obtained [135, 136]. Inducers such as 2,5-xylidine [135], benzyl alcohol [49], or xylan [93], their chemical nature, quantity and time of their addition influence laccase production to a great extent. For example, when Osiadacz et al. [18], added 10 μM pyrogallol as inducer on the fourth day to the submerged culture, there was a three-fold increase in laccase production from Trametes versicolor. Therefore, medium optimization is an important factor to ensure maximum enzyme yield with minimal production costs.
Industrial and agricultural byproducts such as corn cobs [137], olive oil mill waste [138], oak dust [139], banana peel [140], pistachio shell [141], tea residues [142], spent coffee, rice straw, and wheat bran [143] have been used as excellent substrates in the fermentation process due to their complex composition of carbon, nitrogen, and mineral supplies suitable for the growth of microorganisms. They also possess natural polymers including cellulose, lignin, pectin, and starch which provide suitable substrates during the fermentation process [144, 145, 146]. In fact, fermentation of some white-rot fungi in the presence of lignocellulosic residues significantly stimulated laccase secretion without any supplementation with inducers [147]. For example, laccase was produced by cultivation of T. versicolor on barley husks and egg shells in solid state conditions without the need for additional carbon and nitrogen sources or mineral compounds [66]. The eggshells acted as supports for microorganism growth and improved the porosity of nutrient media which enabled more oxygen to diffuse deeper inside the reaction mixture. More so, the egg shells were a source of calcium which acted as a laccase inducer. Production of laccase from various sources has been extensively reviewed elsewhere [148].
5. Applications of laccase
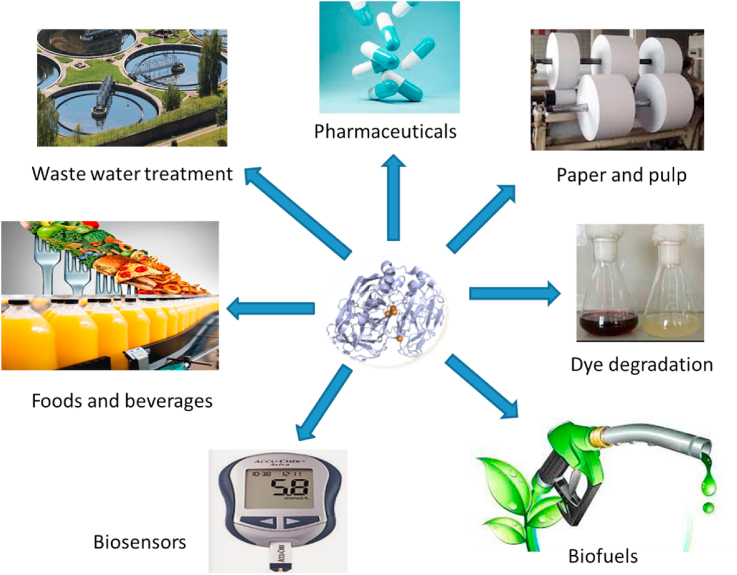
Laccases have attracted attention as biocatalysts in a wide range of biotechnological applications due to their non-specificity and their use of molecular oxygen as an electron acceptor [32, 149]. They have been used as biocatalysts in wastewater treatment of textile, paper and pulp, petrochemical effluents, food industries, medical diagnosis and manufacturing, lignin modification, and as a bioremediation agent for herbicides and pesticides [150, 151]. Laccases are one of the most promising enzymes for decontamination of phenol polluted systems and biotechnological applications, they have been explored in the removal of toxic compounds from aquatic and terrestrial systems [87], production treatment of beverages [152], region-specific bio-transformations [153], aerobic oxidation of benzyl alcohols [154], analytical tools and biosensors [49], and commercial polymerization of lignosulfates for further applications as surfactants, dispersants and plasticizers in the cement and concrete industry [26]. In this section the various applications of laccase enzyme in waste water treatment, biosensors, paper and pulp industry, food industry, biofuel cells, and pharmaceutical industry are represented in Figure 3 and further discussed.
Figure 3.
Applications of laccase enzyme.
5.1. Waste water treatment
Enzymatic bioconversion technologies have attracted growing interests in the field of wastewater treatment due to their ability to eliminate toxic compounds at mild conditions with less biodegradation by-products [155]. The use of enzymes for wastewater treatment is advantageous due to the substrate specificity of enzymes, reduced susceptibility of the biocatalyst to shock-loading effects, high rates of reaction, efficient use of oxidants, and effective treatment at low substrate concentrations [156]. The removal of phenolic compounds from industrial effluents and the environment is of great importance as these compounds are highly toxic to aquatic organisms and account for the biggest percentage of organic pollutants [157, 158]. Phenolic compounds form a significant percentage of industrial effluents due to their wide use in many industrial processes such as manufacture of plastics and resins, wood preservation, petroleum refining, paper, dyes, pesticides, textiles, drugs, and anti-oxidants [159, 160, 161]. Majority of these compounds are not removed from the liquid portion by waste water treatment plants and they are transferred into the sludge. More so, they are not degraded by the active sludge in treatment plants hence end up in the environment via waste water effluents and in bio-solids through agricultural applications [162, 163]. These compounds have been related to health problems and environmental pollution due to their inherent toxicity [164]. Consequently, the need to degrade or transform these compounds into degradable or non-toxic ones using enzymes is of great interest [165].
The ability of laccase to degrade a variety of phenolic compounds without harsh side effects has attracted attention in detoxification of polluted waste waters [166, 167]. The enzyme doesn't require exotic co-substrates, uses readily available oxygen as electron acceptor, and the free radicals formed by laccase oxidation bypass the steps involved in formation of carcinogenic amines [168]. Mostly insoluble products are formed when phenol derivatives are oxidized by laccase and can easily be removed by filtration or sedimentation [157, 169]. Laccase has proved to oxidize recalcitrant chemicals from the environment which are difficult to degrade to more bioavailable or harmless and stable water insoluble polymers [29, 83]. For example, High-performance liquid chromatography (HPLC) indicated that the main product of anthracene oxidation by laccase was 9,10-anthraquinone which is less toxic than its precursor [170]. As a result, laccase has been widely used in decontamination and restoration of hazardous chemical-contaminated waters [83, 114]. The mechanisms of free and immobilized laccase in detoxification of phenolic compounds have been reviewed elsewhere [171].
Laccase has been utilized in decolourisation of effluents from olive oil mills [116, 172], molasses wastewaters and baker's yeast effluents [173], and textile, paper and pulp effluents [174]. Degradation of pharmaceutically active compounds such as Diclofenac [175, 176], Tetracycline [177], Sulfamethoxazole [178, 179], Chlortetracycline [180], and carbamazepine [181, 182] from the environment using laccase has been reported. A number of researchers have reported successful laccase mediated degradation of endocrine disrupting chemicals such as bisphenols [114, 183, 184, 185], alkylphenols [186], Polycyclic aromatic hydrocarbons (PAHs) [187, 188, 189], benzidine-based dyes [190], and synthetic estrogenic compounds [156]. Herbicides, pesticides, and insecticides such as 2,4-dichlorophenol [191], Chlorpyrifos [192, 193], lindane [23], pirimicarb [194], triclosan [195], pentachlorophenol [196], as well as other phenolic compounds like naphthalene [17], phenol, p-chlorophenol, and catechol [197, 198, 199] have also been successfully degraded by laccase (Table S1). The application of laccase in waste water treatment and detection of harmful pollutants in the environment has been reviewed elsewhere [200, 201, 202, 203, 204].
Figure 4 shows the different drugs, pesticides, and organic pollutants that have been studied for possible degradation by laccase immobilized on various supports. Different drugs such as tetracycline [177, 205, 206], diclofenac [176, 207], estrogens including estrone, 17β-estradiol, 17α-ethinylestradiol [208, 209], sulfa drugs including sulfamethoxazide, sulfadiazine, sulfamethazine and sulfathiazole [80, 210, 211], and carbamazepine [181, 182, 212]. Among other drugs explored are paracetamol [163, 213], hydroquinone [214] and antibiotics such as ampicillin, erythromycin [177], amoxicillin and ciproflaxin [215]. Laccase immobilized on nanoparticles (NPs), nanocomposites (NCs) and metal organic frameworks (MOFs) show the highest degradation efficiencies of over 80% even with short incubation time (0–6 h) as compared to other supports like polymers, silica, and clay. This could be due the high surface area to volume ratios provided by the NPs and NCs which enhances the catalysis of the enzyme [216]. Also immobilization of the enzyme on MOFs is usually by entrapment based on enzyme-MOF affinity which has minimal enzyme denaturation and high enzyme loading hence facilitating high enzyme activity [217].
Figure 4.
A graph showing the percentage degradation of various drugs, pesticides, herbicides and other organic pollutants that have been degraded using laccase enzyme immobilized on various supports with different times of exposure, that is, 0–6 h, 6–24 h and over 24 h.
However, the degradation rate of carbamazepine is low (less than 60%) regardless of reaction time or immobilization support used. Degradation in presence of mediators in the drug solution or co-immobilized on the support can be explored to improve the degradability of this drug since the degradation has been studied exclusively at room temperature in stirred reactors systems [155, 181, 218].
For the case of pesticides and other organic pollutants, bisphenols (A,B and F) [183, 219, 220], chlorophenols [22, 221, 222], triclosan [64, 147, 195], anthracene [77, 223, 224], and other phenolic compounds such as naphthalene [17], catechol [225], phenol [226], and aminophenols [227]. Other pesticides explored include pyrometryn, atrazine [228], indole [223] and chlorpyrifos [192]. As compared to drugs, laccase shows generally higher degradation efficiency for pesticides and other organics regardless of immobilization support or reaction time. This could be due to the phenolic structure of these compounds that provide wonderful substrates for the catalytic activity of the enzyme [10, 11]. It is evident that increasing reaction time from 0-6 h to 6–24 h increases the overall performance of the enzyme in degrading pesticides from below 60% to above 60% with majority above 80% efficiency in degradation. Increasing reaction time above 24 h has limited effect on the performance of the biocatalyst and other options such as use of mediators should be explored.
5.2. Dye degradation
Synthetic dyes have gained popularity in the textile, paper, cosmetics, leather dyeing, colour photography, pharmaceutical, and food industries due to their low cost, ease of synthesis, and colour variety [111, 162]. They are classified according to chemical structure of the chromophore (molecule responsible for their colour) groups as azo, anthraquinone, heterocyclic, triphenylmethane, xanthene, acridine, or phthalocyanine [229]. Azo dyes constitute of the largest percentage accounting for 60–70% of all the dyes in the textile industry [230, 231] followed by the anthraquinones [232]. Since the dyes are designed to meet various colouring requirements, they have very stable chemical structures that are difficult to degrade hence causing serious environmental pollution [198, 233]. They are obstinate to microbial degradation and conventional treatment methods, affecting water transparency and gas solubility leading to reduced dissolved oxygen and may be transformed to carcinogenic compounds under anaerobic conditions [234, 235, 236].
The textile and dye industry is the major source of water pollution with its effluents containing 10–30% of the initial dyestuffs that are difficult to treat due to their non-biodegradable nature and complex molecular structures [168, 237]. The most crucial step in dye degradation by laccases is the cleavage of their chromophores which renders the dye fragments more susceptible to biodegradation by other less specialized biocatalysts [111, 234]. The chemical structure and type of substitute group such as azo, nitro, and sulfo groups of the reactive dye molecule determine the dye decolourisation rate [235, 237]. Biological treatment processes of effluent dye are inefficient because the dyes are highly resistant to biological oxidation and they poorly adsorb on activated sludge [66]. Decolourisation using physical or chemical methods such as adsorption, precipitation, chemical degradation, and photo-degradation has financial and methodological disadvantages, is time-consuming, and mostly ineffective [238]. With the stringent regulations and restrictions established for effluent discharge into the ecosystem, a number of studies have focused on microorganisms, which are able to decolourise and biodegrade these dyes [239, 240, 241, 242]. Enzymatic treatment of dye effluents has gained attention because of the mild reaction conditions involved without generating any secondary pollution like toxic sludge [243, 244].
Laccases have emerged as an attractive enzyme for dye degradation because of their ability to mediate coupling reactions which form a basis for dye removal [43]. Most of the commercially relevant dyes have similar structures to lignin substructures which likely enables the enzyme to act on the compounds chromophore hence decolourising a wide range of synthetic and organic dyes even at environmentally relevant concentrations [245, 246]. Laccases have decolourised a wide spectrum of dyes (Table S1) including Malachite green (MG) [247, 248], Congo red [249], Crystal violet dye [244], Direct blue [250], Procion red MX-5B [231], Reactive red [198, 251], reactive blue [252], Phenol red [253], Acid blue [241], Acid orange [121], Remazol brilliant blue R [232], Reactive green, Reactive brown, Cibacron blue [254], bromaminic acid [255], Direct green [256], Bismark brown R, and Lanaset grey G [115]. The application of laccase enzyme in degradation dyes has been extensively reviewed elsewhere [51, 257, 258, 259].
Although bacterial laccase has not been widely explored, they demonstrate overall high degradation efficiencies of over 60% regardless of the immobilization support and reaction time (Figure 5). Amongst the fungi, Aspergillus sp., M. thermophilia, and Cerrena sp. laccases show higher degradation on all the supports except for biomass immobilized that give less than 40% efficiency. The most explored fungus for laccase production is Trametes sp. and their laccases provide desirable degradation rates overall for dyes and industrial effluents in the free and immobilized state.
Figure 5.
A graph showing laccase immobilized on various supports and its efficiency in degrading synthetic dyes, textile effluents, olive mill waste water and paper industry effluents at different hours of exposure, that is, 0–6 h, 6–24 h and over 24 h.
Even though laccase-NPs/NCs biocatalysts showed high degradation rates for drugs and pesticides at in short incubation times, a longer time (6–24 h) is needed for them to achieve higher degradation efficiencies in dyes and industrial effluents (Figure 5). Polymers have been the most extensively used supports for laccase immobilization and subsequently its application in waste water treatment and their biocatalysts show desirable degradation efficiencies. As observed earlier, increasing reaction or contact time of polymer-laccase biocatalyst with waste water beyond 24 h has no impact on the improvement of the performance of the biocatalyst. In fact, their degradation capacities decrease and degradation efficiencies of below 40% have been recorded for over 24 h incubation. Other supports such as clay, silica, alumina, zeolite and glass provide desirable platforms for the catalytic performance of laccase. Addition of mediators and degradation at laccase optimum pH and temperature can be utilized to improve and facilitate complete degradation of dyes and industrial effluents by laccase. The different supports used to immobilize laccase from various sources and their efficiency in degradation of dyes, paper and textile effluents and olive mill waste water have been showed in Figure 5.
5.3. Paper and pulp industry
Laccase is used in the pulp and paper industry for lignin degradation, deinking, pitch control, effluent detoxification, bio-pulping, and grafting on fibres to improve the physical, chemical, and mechanical properties of paper [260]. In order to obtain high quality paper, structurally embedded lignin responsible for the dark colour should be removed after cooking. This is traditionally done by bleaching with chlorine based chemical agents which results in production of various chloro-organic derivatives that are toxic once released in the environment [261]. Laccase offers a green alternative in bioleaching of pulp to conventional and environment non-friendly chlorine and chlorine based bleaching and has no reductive effect on the final yield of pulp as compared to other enzymes (xylanases and mannanases) [262]. Use of laccase in the pulp industry has showed improved pulp properties, reasonable decrease in kappa number (residual lignin content), increased paper brightness, and reduced chlorine consumption [102]. Laccase has also been used in lignin removal from biomass such as kraft pulp [234] and olive pomace [226] to help in its recycling and enhance the efficiency of cellulose extraction and hydrolysis. The applications of laccase in the paper and pulp industry have been extensively reviewed elsewhere [263, 264, 265].
5.4. Food and pharmaceutical industries
Laccase has found application in food industry as a food additive for stabilization of beverages, wines and beers [266, 267, 268], clarification of fruit juices [269, 270, 271], baking [272, 273, 274, 275], sugar beet pectin gelation [276], and improvement of food sensory parameters [277]. It is also used in the detection of phenolic compounds in beverages, wines and beers [278], as well as bioremediation of food industry waste water. The application of laccase in the food industry has been extensively reviewed elsewhere [277, 279, 280].
Laccase has been used to mediate coupling reactions for synthesis and modification of pharmaceutical products and food supplements such as Trans-Resveratrol [281], degradation of pharmaceuticals from waste water (Figure 4) [218, 282] and catalytic synthesis of anti-cancer drugs and antimicrobial applications [283]. For example, Sampaio et al. [284], used entrapped M. thermophila laccase in bacterial nanocellulose membrane (from Gluconacetobacter xylinum) in wound dressing application. The bioconjugate demonstrated cytotoxicity acceptable for wound dressing applications and 92% and 28% antimicrobial activity against gram positive and gram negative bacteria respectively. The antimicrobial activity of laccase against gram positive and gram negative bacteria was attributed to the electrochemical mode of action to penetrate cell wall of the microorganisms, thereby causing leakage of essential metabolites and physically disrupting other microbial key cell functions.
5.5. Biofuel cells
The fascinating character of direct four electron reduction of oxygen to water by laccase at high electrode potentials has promoted its application in the cathode compartment of biofuel cells [285, 286, 287]. Compared to metallic catalysts, laccase has unique advantages such as catalytic efficiency at high redox potentials, clean oxygen reduction without the formation of hydrogen peroxide intermediate, and relatively low cost [65, 288]. The effective immobilization of laccase and its mediator on electrodes as required is the key step in construction of biofuel cells [289]. Efficient electron transfer between the active site of laccase and the electrode and efficient supply of laccase with oxygen are the prerequisites for high performance of the biofuel cell [285].
Biofuel cells using enzymes as biocatalysts are emerging as the new non-polluting and renewable electricity sources that respect the standards of “green energy’’ [124]. Enzymatic biofuel cells take advantage of selectivity of enzymes to oxidize specific substrate and reduce oxygen in order to obtain power output from physiological fluids [290, 291]. Due to the selectivity of enzymes, separation of electrodes in an enzymatic biofuel cell is not required hence the anode and cathode can all be immersed in one membrane containing the fuel and oxidant. This allows for fabrication of miniaturized fuel cells as small as the micrometre scale [292]. For example, Barrière et al. [293], used an osmium-based redox polymer for laccase-mediated reduction of oxygen coupled to glucose oxidase-mediated oxidation of glucose to form a membrane-less biofuel cell. At the biocathode, laccase is wired on to an electrode surface to achieve oxygen reduction through either mediated electron transfer (MET) or direct electron transfer (DET) [290]. MET involves integration of redox mediators that shuttle electrons between the active sites of the enzyme and the electrode while DET allows electron transfer between laccase and electrode via fast tunnelling in absence of a mediator [45, 294].
In MET, the mediator (e.g. Dopamine [295] and ABTS [291]) is either free in solution or immobilized with the enzyme on the electrode [296]. For example, laccase was immobilized on three-dimensional graphene networks (3D-GNs) functionalized with dopamine and 3,4,9,10-perylene tetra carboxylic acid (PTCA) which acted as mediator and spacer respectively on the GCE. The modified electrode was used as a cathode in a glucose/O2 biofuel cell with a maximum power density of 112 μWcm-2 and a short circuit current of 0.96 mAcm-2 [289]. Barrière et al. [297], fabricated a biofuel cell consisting of a glucose-oxidase based anode and laccase based cathode. The enzymes were immobilized on graphite electrodes using osmium-based redox polymers as mediators and the biofuel cell gave maximum power density of 16 μW/cm2 at a cell voltage of 0.25 V. The redox mediators should have excellent reversible electron transfer properties, potentials close to the redox potential of the enzyme active site, and ensure sufficient driving force to optimize bioelectrocatalytic activity [285]. Even though utilization of redox mediators results in higher current densities, MET-based systems often experience mediator leaching, oxygen sensitivity of the mediators, and loss of open-circuit potential [45, 298]. As a result DET-based systems are preferred for high power biofuel cells that can be used in portable power applications [298].
In DET-based cell, the reduction of oxygen relies on the close proximity (within 1.5 nm) of the enzyme active site and the electrode surface [45, 299]. Electron transfer occurs during catalytic transformation of the substrate and its kinetics is determined by the orientation of the enzyme on the electrode surface, potential difference, and distance between the enzyme active site and the electrode surface [298, 300]. DET is optimized when active site of the enzyme molecules are most exposed to the electrode surface and can be improved by modifying the electrode surface with conductive nano-elements like carbon nanotubes and metal and metal oxide nanoparticles [301]. Electrode surfaces modified with nanoparticles such as nano-Gold (nAu) provide a microenvironment similar to that of redox-enzymes in native systems and allow freedom in enzyme orientation since electron transfer proceeds through the conducting tunnels of the nanoparticles [302]. DET is preferred because the biofuel cell voltage is maximized since enzymatic catalysis is performed at apparent redox potentials of the enzymes at the cathode and anode. Also the absence of redox mediators simplifies the fabrication process of the cell, increases cell stability, and minimizes toxicity issues that could arise from the leaching of mediators [285, 303]. Gellet et al. [298], used Rhus vernificera laccase to develop an air-breathing biocathode that employed DET for application in a proton exchange membrane hydrogen/air fuel cell and a direct methanol fuel cell (DMFC) with an anion exchange membrane. The biocatalyst provided high operational current density of 50 mA/cm2, maximum power density of 8.5 mW/cm2, and a lifetime of 290 h in a 40% methanol DMFC. A stable current for 350 discontinuous h when operated for 8 h daily was achieved in the hydrogen/air fuel cell.
5.6. Biosensors
The release of phenolic compounds by a large number of industries necessitates their detection and quantification in the environment. Since these compounds are toxic and persistent in the environment, their quantification is important when evaluating the total toxicity of an environmental sample [304, 305]. Phenolic compounds are also present in fruits and vegetables and their products such as juices and wines. The polyphenol content in juices and wines affects their quality in terms of colour, flavour, stability, and aging behaviour hence the need for their quantification [306]. Most analytical methods used to qualitatively and quantitatively determine polyphenols are expensive, time consuming, and require several operation steps, costly reagents, and separation steps with large amounts of environmentally unfriendly effluents [304, 307].
Biosensors modified with laccase have been developed for detection of laccase electron donors (phenolic compounds) [116, 308] and monitoring oxygen and laccase inhibitors [24]. The construction of laccase-based biosensors is relatively simple as it doesn't require hydrogen peroxide as a co-substrate or any other co-factor for its catalytic activity [164, 309]. The biosensors are based on the principle of reduction of oxygen by laccase to water hence monitor the consumption of oxygen during oxidation of the analyte [43, 308]. The response time is dependent on the enzymatic reaction kinetics, the diffusion and solubility of the substrates at the electrode, and the electrochemical reduction of the oxidized mediator at the electrode surface [310].
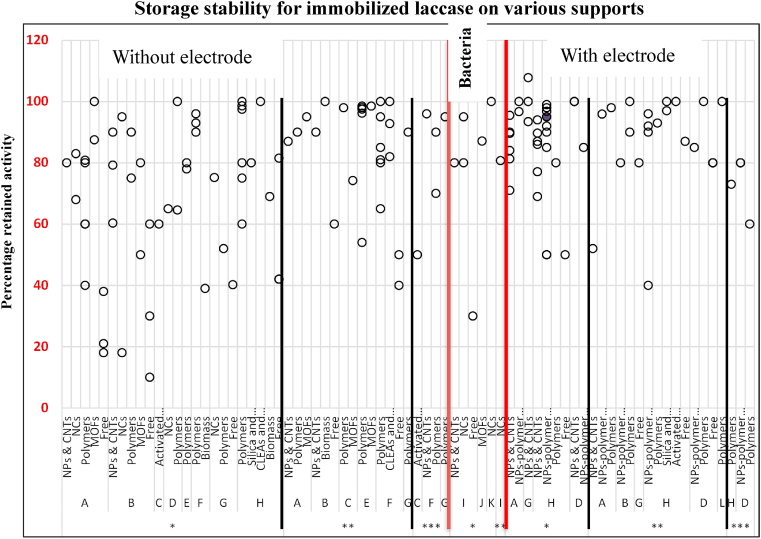
Laccase-based biosensors have been fabricated for detection of various compounds including Catechol and its derivatives [164, 305, 311], guaiacol [302], hydroquinone [312], adrenaline [296], Methyldopa [313], Rutin (flavonoid) in pharmaceutical formulations [314], methomyl (insetcide) in vegetable extracts [109], azo-dye tartrazine [15], and industrial kraft lignin [315] as demonstrated in Table 1. For example, Jarosz-Wilkotazka et al. [308], immobilized C. unicolor laccase on the surface of graphite electrode by physical adsorption and the electrode was inserted into a flow-injection system to serve as a biosensor for detection of polyphenols including flavonoids. The biosensor was more sensitive to bulky highly polymerized polyphenolic structures. The laccase biosensors demonstrate great stability in reusability and storage over long periods. For example, laccase/Nafion biosensor demonstrated 92% activity after 98 days storage [1] and a carbon/laccase biosensor for catechol retained 100% after 90 days storage at room temperature and after 25 assays [316]. Chawla et al. [317], for detection of guaiacol, reported retained activity of 85% on a laccase/NiNPs/MWCNTs/PANI/Au biosensor in a space of 4 months and 80% after 300 assays over 7 months for laccase/CuNPs/CS/MWCNTs/PANI/Au biosensor [318]. Similarly, Cadorin-Fernandes et al. [109], reported 100% after 450 assays over 2 months for laccase/sol-gel/carbon ceramic electrode for detection of methomyl.
Table 1.
Application of laccase in fabrication of biosensors for detection of organic pollutants.
| Laccase source | Immobilization support | Electrode used | Analyte | Detection range (μM) | LOD (μM) | Response time (s) | Storage stability and reusability | Ref |
|---|---|---|---|---|---|---|---|---|
| A. oryzae | AuNPs/poly (allylamine hydrochloride) (PAH) | Carbon paste | Dopamine | 0.49–23.0 | 0.26 | [319] | ||
| - | AuNPs-MoS2 | Glassy carbon electrode (GCE) | Catechol | 2 to 2000 | 2 | 100 | 90% activity after 30 days, 97% after 10 cycles and RSD of 1.2% and 0.7% for 10 assays and 5 electrodes | [320] |
| A. oryzae | PEI-AuNPs | GCE | Catechol | 0.36–11.00 | 0.03 | 80% after 90 days and 150 assays | [302] | |
| Guaiacol | 0.79–17.42 | 0.03 | ||||||
| Pyrogallol | 1.74–19.60 | 0.14 | ||||||
| Hydroquinone | 2.90–22.00 | 0.21 | ||||||
| C. unicolor | poly (N-isopropylacrylamide) gel | Indium Tin Oxide (ITO) | Oxygen in solutions | Reduction signals of 0.79 V and 0.38 V at T1 and T2 respectively | [321] | |||
| T. Versicolor | poly (ethyleneimine) (PEI) microcapsules with p-phenylenediamine (PPD) mediator | GCE | Oxygen in solutions | 73% after 6 months of storage | [322] | |||
| T. versicolor | Fe3O4@Au nanoparticles | Catechol | 5.0–70.0 | 2 | 2400 | [199] | ||
| Au/Mxene NPs | GCE | Catechol | 0.05–0.15 | 0.05 | 10.98 | 89.6% after 15 days of storage and RSD of 2.21% for 9 assays | [323] | |
| T. versicolor | Bacterial cellulose-AuNPs | GCE | Hydroquinone | 0.03–0.1 | 0.00571 | 96% after 3 months storage, RSD of 2.65% for 3 electrodes and 3.17% for 3 assays | [324] | |
| R.vernicifera sp. | TiO2–CuC NFs | GCE | Hydroquinone | 1–89.8 | 3.65 | 5 | 107.8% after 1 week and 93.45% after 1 month. RSD of 2.69% and 1% for 3 biosensors and 9 assays respectively | [312] |
| T. versicolor | MnONPs-graphene nanoplates nanocomposite | Caffeic acid | 5–320 | 1.9 | 77.1% after 1 week, RSD of 3.97% and 1.84% for 10 biosensors and 3 assays | [325] | ||
| Cu/C NFs | GCE | Catechol | 9.95–9760 | 1.18 | 5 | 100% and 95.9% after 4 and 22 days, RSD of 4.35% for 3 assays | [326] | |
| T. versicolor | Nafion | DROPSENS cells screen printed electrodes | Catechol | 1.2–120 | 0.43 | 100 | 92% after 98 days, RSD 6.08% for 9 electrodes | [327] |
| Caffeic acid | 3–15 | 2.5 | ||||||
| Chlorogenic acid | 3–15 | 2.8 | ||||||
| Gallic acid | 2–7 | 1.55 | ||||||
| Rosmarinic acid | 3–15 | 2.4 | ||||||
| T. versicolor | Mg-MCM-41/PVA | Gold | Catechol | 0.94–10.23 | 0.00531 | 14 | 90% after 30 days, RSD 5.2% and 4.6% for 10 electrodes and 30 assays | [328] |
| T. versicolor | PANI/CMC/cellulose | GCE | Catechol | 0.497–2270 | 0.374 | 8 | 98% after 2 weeks, RSD of 2.23% and 3.03% for 5 assays and 5 electrodes | [329] |
| T. versicolor | PANI | Screen printed electrode (SPE) | Bisphenol A | 0.004–4 | 0.004 | 100 | [330] | |
| MWCNTs-Ag@ZnO nanocomposites | Carbon SPE | Bisphenol A | 0.5–2.99 | 0.6 | 25 | 71.02% after 10 days, RSD of 0.86% for 3 assays | [331] | |
| T. versicolor | Carbon black | Carbon SPE | Catechol | 2.5–50 | 2 | 0.5 | 100% after 90 days storage at roomtemperature, 100% after 25 assays | [316] |
| T. versicolor | MWCNTs | Carbon SPE | Bisphenol A | 0.84–12 | 0.84 | 87% after 1 month, RSD 5.3% for 3 electrodes | [185] | |
| Coriolus hirsuta | MWCNTs | Carbon SPE | Catechol | 0.0–0.102 | 5 | 100% after 20 days storage, 77% after 10 cycles | [332] | |
| T. versicolor | CS/silica-magnetic MWCNT | Carbon paste | Catechol | 0.1–165 | 0.034 | 50 | 90% after 45 days, RSD 3.01% for 35 assays over 29 days | [333] |
| T. versicolor | CS-MWCNTs nanocomposite | GCE | Catechol | 0.1–50 | 0.02 | 92% and 85% after 1 week and 1 month respectively. RSD 3.03% for 20 assays | [334] | |
| Ganoderma lucidium | NiNPs/cMWCNTs/PANI | Gold | Guaiacol | 0.1–500 | 0.05 | 8 | 85% after 200 cycles in a space of 120 days | [317] |
| T. versicolor | MWCNT-COOH/AuNPs-SDBS-PEDOT | GCE | Catechol | 0.1–0.5 | 0.11 | [335] | ||
| 11.99–94.11 | 12.26 | |||||||
| T. versicolor | MWCNTs | Carbon paste | Pirimicarb | 0.99–11.5 | 0.18 | 1200 | [194] | |
| P. ostreatus | SWCNT/C-quantum dots | GCE | 17a-ethynylestradiol | 0.05–7 | 0.004 | 96.8% after 10 cycles | [336] | |
| Reduced GO (rGO)- Sb2O5 | Estriol | 0.025–1.03 | 0.011 | 4 | 84% and 52% after 1 and 2 months storage. RSD of 2.84% and 4.37% for 10 assays and 5 electrodes | [337] | ||
| Magnetic graphene/PANI (PANI/MG) | GCE | Hydroquinone | 0.4–337.2 | 2.94 | 5 | 96.7% after 2 weeks storage. RSD of 4.3% and 1.95 for 3 electrodes and 20 assays | [338] | |
| T. versicolor | Graphene-Cellulose Microfiber | Carbon SPE | Catechol | 0.2–209.7 | 0.085 | 2 | 96.8% after 132 h. RSD 2.6% for 5 electrodes | [339] |
| R. vernicifera | AuNPs-graphene nanoplates | Carbon SPE | Hydroquinone | 4–130 | 1.5 | 100% after 5 days. RSD of 2% and 3% for 5 assays and electrodes respectively | [278] | |
| T. versicolor | ∗Mercury | 49.9–600 | 74.8 | [55] | ||||
| T. versicolor | rGO | GCE | Dopamine | 0.0–3 | 0.091 | 89.7% after 30 days storage, RSD 2.1% for 3 electrodes | [340] | |
| T. versicolor | N-doped carbon hollow spheres (NCHS)/CS composite film | GCE | Calcium lignosulfonate (lignin in kraft effluent) | 370–19000 | 120 | 95% after 1 week storage | [315] | |
| T. versicolor | polyazetidine prepolymer (PAP)/MWCNTs | SPE | Catechol | 0.64–20.73 | 0.18 | 10 | [341] | |
| T. versicolor | Nafion/MWCNTs | SPE | Catechol | 1.36–65.45 | 0.45 | 10 | [341] | |
| T. versicolor | MWCNTs | SPE | Catechol | 2.36–134.73 | 0.73 | 10 | [341] | |
| Agaricus bisporus | AuNPs | Gold disk electrode (GDE) | Dopamine | 0.5–13.0 | 0.029 | 100% after 1 week, RSD 2.7% and 3.2% for 7 assays and 5 electrodes | [101] | |
| 47.0–430.0 | ||||||||
| AuNPs | Carbon SPE | Tartrazine | 0.2–14 | 0.04 | 120 | 95.5% and 81.3% after 10 days and 1 month. RSD of 2.37% and 8.54% for 5 assays and electrodes respectively | [15] | |
| Montmorillonite | GCE | Catechol | 1–10 | 0.89 | 10 | RSD of 3.58% for 10 assays | [342] | |
| T. versicolor | 3,3′-Dithiodipropionic acid di (Nsuccinimidyl ester) (DTSP) | Gold | Hydroquinone | 3–15 | 0.89 | 50 | 96% and 93% after 50 cycles and 6 weeks storage. RSD of 3.1% for 5 electrodes | [343] |
| T. versicolor | (3-mercaptopropyl)-trimethoxysilane (MPTS) | Gold | Hydroquinone | 0.9–20 | 0.25 | 50 | 99% and 97% after 50 cycles and 6 weeks storage. RSD of 3.0% for 5 electrodes | [343] |
| T. versicolor | Gold | Hydroquinone | 3–15 | 0.91 | 50 | 89% and 87% after 50 cycles and 6 weeks storage. RSD of 2.6% for 5 electrodes | [343] | |
| T. versicolor | PANI | GCE | Catechol | 3.2–19.6 | 2.07 | [311] | ||
| T. versicolor | MWCNTs-silica spheres | SPE | Dopamine | 1.3–85.5 | 0.42 | 91% and 86% after 10 and 30 days storage. RSD of 4.7% for 5 assays | [344] | |
| T. versicolor | Titania NPs | Graphite | Catechol | 0.75–150 | 0.75 | 60 | 94% and 69% after 7 and 22 days. RSd of 7% for 6 assays | [164] |
| T. versicolor | polyazetidine prepolymer (PAP)-MWCNTs nanocomposite | SPE | Dopamine | 0.80–4.13 | 0.24 | 50% after 6 days | [310] | |
| Catechol | 0.07–2.28 | 0.02 | ||||||
| Gallic acid | 1.07–49.16 | 0.32 | ||||||
| Caffeic acid | 0.03–0.79 | 0.01 | ||||||
| T. hirsuta | polyazetidine prepolymer (PAP)-MWCNTs nanocomposite | SPE | Dopamine | 0.03–6.00 | 0.008 | 50% after 6 days | [310] | |
| Catechol | 0.01–0.95 | 0.003 | ||||||
| Gallic acid | 0.79–19.9 | 0.24 | ||||||
| Caffeic acid | 0.03–0.60 | 0.01 | ||||||
| T. versicolor | GCE | Gallic acid | 0.24–117.6 | 0.24 | 100 | 50% fater 5 days. RSD of 2.8% for 5 assays | [306] | |
| Caffeic acid | 0.0056–0.56 | 0.56 | ||||||
| Ganoderma sp. | CuNPs/CS/MWCNTs/PANI | Gold | Guaiacol | 1–500 | 0.156 | 4 | 80% after 300 assays over 7 months. RSD of 2.6% for 6 assays | [318] |
| T. versicolor | Mesoporous silica | GCE | Catechol | 2.0–100 | 2 | 120 | 100% after 50 days storage | [305] |
| T. versicolor | CS-MWCNTs film | Gold | Rosmarinic acid | 0.91–12.1 | 0.233 | 120 | 90% after 15 cycles. RSD of 5.62% for 10 assays | [345] |
| Caffeic acid | 0.735–10.5 | 0.151 | ||||||
| Chlorogenic acid | 0.793–6.71 | 0.161 | ||||||
| Gallic acid | 0.79–2.1 | 0.79 | ||||||
| Pycnoporus sanguineus | CuTAPc-Fe3O4 NPs | Sensor head with oxygen sensing membrane | Adrenaline | 0.2–0.9 | 0.01 | 30 | 85% after 1 month storage | [296] |
| 0.01–0.09 | ||||||||
| A. oryzae | Sol-gel | Carbon ceramic electrode (CCE) | Methomyl | 0.5–12.2 | 0.2 | 100% for 450 assays over 2 months. RSD of 5.7% and 4.3% for 10 assays and 5 electrodes | [109] | |
| A. oryzae | CS-tripolyphosphate microspheres | Carbon paste | Rutin | 0.6–3.92 | 0.0623 | 930 assays over 320 days | [314] | |
| 5.83–13.1 | 0.712 | RSD 3.1% for 9 assays | ||||||
| A. oryzae | Cellulose acetate | Carbon paste | Methyldopa | 34.8–370.3 | 5.5 | 90% after 60 days. RSD of 1.5% and 4.3% for 6 assays and 5 electrodes | [313] | |
| T. versicolor | Cu-ordered mesoporous carbon/CS | Gold | Catechol | 0.67–15.75 | 0.67 | 99% and 95% after 15 and 30 days storage. RSD 2.01% for 6 assays | [307] | |
| Ganoderma sp | Epoxy resin membrane | Platinum | Guaiacol | 0.5–50 | 0.3 | 30 | 60% after 200 assays in 10 months. RSD | [304] |
| Polyphenols in fruit juices | 0.81–1.92 | |||||||
| Polyphenols in alcoholic beverages | 1.9–3.0 | |||||||
| Rigidoporus lignosus | Hydrophilic matrix | GCE | Hydroquinone | 0–500 | 60 | 100% after 100 working days | [116] | |
| Catechol | 0–500 | |||||||
| Caffeic acid | 0–500 | |||||||
| Catechin | 0–100 | |||||||
| Quercetin | 0–100 | |||||||
| Guaiacol | 0–500 | |||||||
| Vanillic acid | 0–500 | |||||||
| Pyrogallol | 0–500 | |||||||
| Gallic acid (in olive oil mill waste water) | 0–500 | |||||||
| T. versicolor | Methylene blue-mesoporous silica | Gold | Catechol | 4–87.98 | 0.331 | 4 | [346] | |
| Ganoderma sp | AgNPs/MWCNTs/PANI | Gold | Phenolic content in tea, alcoholic beverages and pharmaceutical formulations | 0.1–500 | 0.1 | 6 | 80% after 200 assays in 4 months. RSD of 2.3% for 5 assays | [347] |
| γ-Proteobacterium JB | Nitrocellulose membrane | Catechol | 40–90 | 100% after 3 months | ||||
| Catechin | 40–60 | |||||||
| L-methyl DOPA | 30–70 | |||||||
| T. versicolor | Zn–Cr–ABTS | GCE | Dissolved oxygen | 0.06–4 | 0.06 | RSD 8% for 6 assays | [24] | |
| T. versicolor | Polytetrafluoroethylene membranes | Oxygen electrode | Guaiacol | 91.3–400 | 91.3 | 40% and 40% after 50 assays and 20 days | [160] | |
| 3-methyl2-benzothiazolinone hydrazone (MBTH) films | Catechol | 500–8000 | 330 | 600 | 98% after 2 months storage. RSD 5.3% for 8 electrodes | [348] | ||
| Denilite | Platinum | Hydroquinone | 0.2–35 | 0.05 | 2 | 80% after 60 days. RSD 3.1% for 7 assays | [349] | |
| Homogentisic acid | 1–50 | 0.3 | ||||||
| T. versicolor | PVP-CLECs | Gold | Phenols | 50–1000 | 120 | 40% after 30 cycles | [309] | |
| T. versicolor | PANI | Platinum | Phenol | 0.4–6 | 0.4 | 300 | [159] | |
| Catechol | 0.2–1 | 0.2 | ||||||
| L-DOPA | 2–20 | 2 | ||||||
| A. niger | PANI | Platinum | Phenol | 0.4–4 | 0.4 | 300 | [159] | |
| Catechol | 0.4–15 | |||||||
| L-DOPA | 0.4–6 | |||||||
| Agaricus bisporus | PANI | Platinum | Phenol | 1–10 | 1 | 300 | [159] | |
| Catechol | 0.4–1.6 | 0.4 | ||||||
| L-DOPA | 1–10 | 1 | ||||||
| T. versicolor | Graphite | Catechol | 1–10 | 0.23 | RSD 1% and 11% for 12 assays and 6 electrodes | [87] | ||
| R. vernicifera | Platinum | ABTS | 0.5–15 | 0.5 | 3 | 80% after 2 months | [86] | |
| p-phenylenediamine | 0.5–20 | 0.05 | ||||||
| T. hirsute | Nafion | GCE | Hydroquinone | 0.1–3 | 0.035 | 87 | 80% after 5 days | [350] |
| Denilite | Platinum | p-phenylenediamine | 0.14–29 | 0.045 | 2 | 80% after 2 months. RSD for 2.8% and 2.6% for 7 assays for PPD and PAP respectively | [46] | |
| p-aminophenol (PAP) | 0.12–22 | 0.04 | ||||||
| C. unicolor | Graphite | Caffeic acid | 1–10 | 0.56 | [308] | |||
| Prodelphinidin B3 | 1–10 | 0.43 | ||||||
| Epicatechin gallate | 1–10 | 0.54 | ||||||
| Catechin | 4–40 | 4.36 | ||||||
| Epicatechin | 2–60 | 2.44 | ||||||
| Denilite | GCE | PPD | 0.15–30 | 0.04 | 2 | 80% after 2 months | [351] | |
| Botryris cinerea | Gelatin | O2 sensing electrode | Hydroquinone | 0–8000 | 96% after 500 assays | [352] |
used UV-Vis spectroscopy using Caffeic acid as an electron donor.
In order to improve the biosensor characteristics such as minimize laccase leakage and improve laccase-electrode electron movement, a variety of materials have been co-immobilized with laccase on the electrode either by crosslinking or physical adsorption [164]. For example, Rawal et al. [353], covalently immobilized Ganoderma sp. laccase on a silver nanoparticles (AgNPs)/carboxylated multiwalled carbon nanotubes (cMWCNT)/polyaniline (PANI) layer modified gold (Au) electrode and used it as biosensor for detection of phenolic content in tea, alcoholic beverages, and pharmaceutical formulations. The biosensor gave a linear range, response time, and detection limit of 0.1–500 μM, 6 s, and 0.1 μM, respectively and retained 80% of its activity after 200 reuses for a period of over 4 months. The PANI provided a protective microenvironment that sheltered the enzyme from leakage and the external environment. Wu et al. [354], assembled laccase and ABTS on graphene surface and the bioconjugate was deposited on a GCE for detection of extracellular oxygen released from human erythrocytes. The GC/rGO/Lac biosensor was applied to the detection of dopamine in synthetic urine and plasmatic serum samples, achieving a detection limit of 91.0 nmol L−1 [340]. Jabbari et al. [355], covalently immobilized laccase on surface plasmon resonance (SPR) carboxymethyldextran chip to promote its activity towards syringaldazine while at the same time making it inert to ABTS. This showed that the specificity and activity of laccase can be enhanced towards a target metabolite. Applications of laccase in biosensors has been extensively reviewed elsewhere [356, 357, 358].
6. Immobilization of laccase
Despite the many advantages associated with using laccase in industrial settings, limitations such as enzyme instability in varying environmental conditions like pH, ionic strength and temperature, proteolysis, inactivation by inhibitors, and the difficulty to separate the enzyme from the reaction mixture limits its further use in industrial applications [215, 359, 360]. Moreover the high cost of continually discarding the enzyme with treated solutions may be particularly prohibitive [361]. For the potential use of laccase to be increased and its reuse and stability in harsh conditions to be achieved, immobilization is necessary [362, 363]. It is the key to optimizing the operational performance of laccase in industrial processes and especially in non-aqueous media [364]. Immobilized enzymes mimic their natural mode in living cells where they are mostly attached to organelle structures, membranes, and cellular cytoskeleton there by improving their stability [115, 365]. The extent of stabilization depends on the enzyme structure, the immobilization methods, and type of support [366].
Enzyme immobilization limits its freedom to undergo drastic conformational changes which equips it with characteristics such as resistance to thermal and pH changes, improved activity, prolonged half-life, greater variety of bioreactor designs, ease of separation from reaction medium, and reusability for longer periods of time hence reduced cost [367, 368, 369, 370]. For example, When Mukhopadhyay et al. [371], immobilized laccase from a psychrophilic bacteria on Cu2O nanoparticles (CuONPs), the enzyme retained high activity at 4 °C which is lower than its optimum temperature (10 °C). And when lipid functionalized single walled carbon nanotubes (SWNTs) were attached to the CuONPs, it retained activity in very low (4 °C) and high (80 °C) temperatures and great stability under repeated freeze-thaw cycles. Lloret et al. [208], encapsulated M. thermophilia laccase in a sol-gel matrix based on methyltrimethoxysilane and tetramethoxysilane and the enzyme demonstrated improved pH and thermal stability by 10–30%, increased tolerance to different inactivating agents such as acetone, methanol, zinc chloride, calcium chloride, and sodium nitride by 20–40% and preserved up to 80% activity after 10 cycles. Yinghui et al. [372], immobilized laccase on carboxylated crosslinked PVA particles activated by N-hydroxysuccinimide (NHS) in aqueous solution. The bioconjugate showed increased stability in extreme pH conditions where it retained 95% activity when incubated at pH 2 and 13 for 4 and 1 h respectively as compared to the free enzyme that had an optimum pH of 2.4.
The reuse and ease of separation of enzymes after immobilization allow use of enzymes in continuous bioreactor operations which is useful in the production of fine chemicals and bio-treatment of industrial and agricultural wastes [373, 374]. It also enables recycling and reuse of enzymatically treated effluents which drastically decreases water consumption and reduces pollution [111, 375]. The properties of the immobilized enzyme are determine by the properties of the support (such as hydrophobicity, surface charge, density of binding sites, and level of support activation) and the immobilization procedure while the operational efficiency of any immobilization system depends on the amount of enzyme retained by the system [359, 376, 377]. Also, the stability of an immobilized enzyme upon storage and repeated use determine its effectiveness in continuous processes [237]. It is evident that enzymes lose much of their initial activity after immobilization due to changes in diffusion rate, variations in the microenvironment, and non-biospecific interactions especially when binding takes place at the enzyme active site or its vicinity since maximal activity is associated with protein flexibility [241, 379]. For example, Gutiérrez-Sánchez et al. [380], observed that covalent immobilization of laccase on aminophenyl-modified carbon nanofibers/carbon nanotubes electrode via amide bonds with the aspartic/glutamic carboxylic acid residue close to the type 1 copper site provided unfavourable laccase orientation for DET.
A suitable support matrix with appropriate structural characteristics and an immobilization strategy that maximizes enzyme-matrix interactions for catalytic efficiency should be considered during immobilization for a stable and active biocatalyst [316, 381]. The enzyme-support interaction is significantly influenced by the properties of the enzyme and the support matrix [382]. There is no universal support matrix that can immobilize all kinds of enzymes and the choice of support and immobilization method depends on the specific features of the enzyme and the application it is devoted to [270, 383, 384]. The ideal support for immobilization is expected to be inert, stable, compatible with laccase, and resistant to microbial attack and mechanical force [313, 383, 385]. The morphology, composition, hydrophobicity, specific surface area and functional surface group of the support material determine the immobilization yield and immobilization efficiency of the biocatalyst which in turn affect its performance [256, 386]. For instance, hydrophilic membranes are preferred as immobilization supports for catalysis under isothermal conditions (because they provide a better microenvironment for enzymes) while hydrophobic membranes are preferred for non-isothermal conditions [190]. Also, the charge and charge density on the support surface are important as they can alter the enzyme activity upon immobilization due to electrostatic interaction [387]. Therefore for a successful immobilization, a suitable carrier and immobilization procedure that maximize the catalytic and non-catalytic needs of a biocatalyst have to be considered [379, 388, 389]. For a successful biocatalyst, enzyme loading, activity and stability, specific activity of the bound enzyme, and storage stability have to be maximized [390].
A good immobilization strategy should maximize the catalytic (stability, selectivity, and space time yield) and non-catalytic needs (separation, control, and down-streaming processing) for a given application [391]. This entails selecting a suitable carrier, considering the nature of the enzyme, and conditions of immobilization [392]. For example, Tastan et al. [160], compared immobilization of laccase on polytetrafluoroethylene (PTFE) membranes using entrapment to gelatin and covalent immobilization to the surface using carbodiimide coupling. For covalent immobilization, the functional groups were formed on the PTFE surface by radiofrequency (RF) plasma treatment followed by polymer grafting with polyacrylamide (pAAm) and polyacrylic acid (pAAc) polymers. It was observed that, although the membrane with entrapped enzyme displayed high activity, it had poor mechanical stability, narrow working pH range, and low storage life while the covalently bound membrane showed high stability and reusability. Qiu et al. [393], immobilized laccase on nanoporous gold via three approaches: physical adsorption, electrostatic attraction, and covalent coupling. Physical adsorption gave the best results because of the covalent linkage between the nanoscale gold surface and the amino groups of the residue amino acids of laccase. Adhami et al. [374], compared covalent immobilization of laccase from three different fungi, viz. Cerrena unicolor, Heterobasidion annosum and Trametes versicolor on DEAE-Granocel 500. C. unicolor laccase showed the best binding efficiency to the carrier, good enzyme activity, and thermal and storage stability. On the other hand, T. versicolor laccase immobilized on hexagonal mesoporous silica nanoparticles demonstrated higher activity across a broader pH range as compared to when it was immobilized on Kaolinite under the same conditions [166].
Immobilization sometimes leads to alteration of enzyme properties such as catalytic efficiency and optimum pH and temperature. The enzyme kinetic parameters are altered as well facilitated by changes in affinity of substrate to an enzyme due to steric hindrances, partitioning, and diffusion effects of the substrate and decreased protein flexibility [162, 197, 394]. For example, Lante et al. [395], adsorbed laccase on a spiral-wound asymmetric polyether-sulphone membrane, the optimum pH changed from 6.3 to 6.6 due to the presence of ionized groups on the support. The change in pH activity of the immobilized enzyme is due to electrostatic interactions especially for charged supports which lead to the unequal partitioning of H+ and OH− concentrations between the microenvironment of the immobilized enzyme and the bulk phase [107, 161, 390]. When Abdullah et al. [348], immobilized laccase on chitosan-nafion/sol-gel silicate MBTH and used it as a biosensor for catechol, guaniacol, o-cresol and m-cresol, the sensor was responsive only to catechol and the immobilized enzyme had no catalytic effect on the remaining substrates. The non-reactivity of immobilized laccase is attributed to the hydrophobic hybrid Nafion/sol-gel silicate film used for immobilization which restricts the interaction of the enzymes active site with certain phenolic compounds through steric hindrance and change in stability of the phenoxy radical produced.
However, immobilization may not offer any improvement on the enzyme properties. For example, when Abadulla et al. [104], immobilized T. hirsuta laccase on APTES silanized alumina pellets with GA cross-coupling, immobilization showed insignificant change in enzyme half-life, thermal stability and resistance against inhibitors. In some instances, the immobilization has shown to reduce the enzyme properties. For example, Zille et al. [375], immobilized Thapsia. villosa laccase on APTES silanized alumina spherical pellets with GA crosslinking and used it for decolourisation of industrial dye effluent. It was observed that decolourisation was predominantly due to physical adsorption of the dye on the immobilization support (79%) and not by the catalytic activity of laccase (4%) and the free enzyme demonstrated more stability than the immobilized enzyme. The high amounts of salts (especially sodium chloride) in dyeing effluents enhanced electrostatic coupling of the anionic dyes and the positively charged proteins thereby forming stable dye/enzyme aggregates for free laccase. However, the restricted enzyme structure limited accessibility for interaction of the enzyme with salts and anionic dyes.
Immobilization conditions such as pH, temperature, concentration of crosslinking agent, concentration of enzyme, and type of solvent also affect the activity of the biocatalyst [359]. Different solvents affect the substrate-enzyme interactions and conformational mobility and structure of catalyst [396]. For example, Addition of organic solvents (20% ethanol, acetone and acetonitrile) in laccase solution during laccase immobilization on SWCNTs registered 600%, 400%, and 350% increase in maximum reductive current, respectively, when the biocatalyst was used in mediatorless electro-reduction of oxygen [45]. It was observed that ethanol promoted laccase-SWCNTs contact leading to favourable enzyme orientation on SWNTs. An increase in enzyme concentration during immobilization offers increased bioconjugate activity up to a maximum beyond which increasing the enzyme concentration leads to decreased bioconjugate activity [397, 398]. This is because an increased enzyme concentration leads to increased enzyme loading up to a point beyond which high enzyme loadings produces intermolecular space hindrance of the immobilized enzyme which screens the active site of the enzyme and restrains the dispersion of the enzyme or damages the active sites [359, 399]. This was demonstrated by Fortes et al. [400], where beyond a given enzyme concentration, the enzymatic activity decreased with increases in enzyme concentration. This was attributed to support saturation due to excess enzyme in solution leading to steric hindrance and restrained dispersion of substrate and products. Figure 6 represents the various immobilization techniques and immobilization supports that have been explored for laccase enzyme.
Figure 6.
Various techniques and supports used for immobilization of laccase enzyme.
6.1. Immobilization techniques
There are five major techniques employed in immobilization of laccase namely, physical adsorption, entrapment, encapsulation, covalent bonding, and crosslinking (Figure 6). The techniques are classified as physical (entrapment, encapsulation and adsorption) and chemical (covalent and crosslinking). These methods vary in cost and ease of preparation, stability, catalytic efficiency, and physical properties of the resultant biocatalyst [401]. The different immobilization techniques with their advantages and disadvantages as well as the type and strength of interaction between enzyme and support for each technique have been summarized in Table 2.
Table 2.
Characteristics of the various immobilization techniques.
| Immobilization technique | Type of interaction | Strength of interaction | Advantages | Disadvantages |
|---|---|---|---|---|
| Entrapment | Ionic interactions, hydrophobic interactions, covalent bonds | weak/strong | No enzyme modification, ease of preparation, minimal loss of enzyme activity | Pose diffusion limitations to substrates and products, high enzyme leakage, difficult to implement at industrial level |
| Encapsulation | Ionic interactions, hydrophobic interactions | Weak | No enzyme modification, protection of the enzyme, minimal loss of enzyme activity, minimal enzyme leakage | Pose diffusion limitations to substrates and products, less concentration of enzyme |
| Adsorption | Epoxy groups hydrogen bonds, ionic interactions, hydrophobic interactions | Weak | No enzyme modification, simple and inexpensive reusability of the support, minimal diffusion limitations for substrates and products | May pose enzyme leakage, probable activity loss of enzyme, lower efficacy |
| Covalent binding | covalent bonds | strong | Strong and stable interactions, multipoint attachment, minimal enzyme leakage | Costly to prepare, may pose diffusion limitations for substrates and products, activity loss of enzymes, enzyme once denatured the support and enzyme are discarded |
| Cross-linking | covalent bonds | strong | No support needed, high strength of interactions | Poor stability, structural modification of enzyme by crosslinker leading to activity loss |
Immobilization with physical techniques involves relatively weak and reversible interactions with the support [365]. They offer less disturbance to the enzyme structure and retain enzyme properties to those in solution [19]. However, the weak interactions between the enzyme and support cause enzyme leaching which results in activity loss and contamination of the surrounding media [212]. And since there is no control over the parking density of the enzyme on the support, the activity of the biocatalyst can be reduced due to overcrowding of the enzyme [365].
6.1.1. Entrapment
Entrapment is the physical confinement of an enzyme in the micro-spaces of porous hollow fiber, spun fiber, insoluble gel matrix, and/or a reverse micelle without chemically binding to the support [402]. This approach allows preservation of the enzymes’ three-dimensional conformation and optimum operating parameters since there is no interaction between the enzyme active site and support upon immobilization [245]. It also offers practical convenience of simple regeneration by removal of deactivated enzyme reloading with fresh active catalyst [367]. It is the most preferred technique in industries because it is a mild process, easier to operate, and provides minimal structural changes to the enzyme hence retaining the enzyme activity [200, 403]. For instance, When Makas et al. [367], entrapped laccase in K-carrageenan based semi-interpenetrating polymer networks, the optimum pH and temperature remained unchanged and the enzyme displayed high stability of 82% activity after 42 days of storage and over 50% activity after 10 reuses.
During this process, a suitable support is required to trap the enzyme molecules or enzymatic preparations within a matrix [404]. The success of this technique is greatly dependent on the size of the pores of the support as pores smaller or equal to the size of the enzyme yield lower loadings and the enzyme simply adsorbs onto the external surface while pore sizes that are much bigger than the enzyme size encourage leaching of the enzyme from the support [405]. In instances where the pore size is relatively larger than the size of the enzyme, adsorption of the enzyme to a porous support is often followed by crosslinking to minimize leaching [406].
6.1.2. Encapsulation
Microencapsulation is a form of entrapment where the enzyme is entrapped in the core of micron-sized spheres made from semipermeable hollow (microcapsule) or solid (microbeads) material [40, 407]. The core of the capsule provides an aqueous environment which support enzymatic activity and the capsule (wall) itself serves as a container through which only the substrates can diffuse [322]. This method ensures the enzyme is fully embed in the supports and prevents interference that can arise from interaction of the enzyme with the external interface [408]. Microencapsulation is especially favourable in electrodes as high amounts of enzyme can be immobilized and the polymer wall properties can be tuned to prevent diffusion of inhibiting molecules in the capsule [322]. This method is economical and allows encapsulation of large volumes of the enzyme but is limited by diffusion limitation of the substrate across the membrane and accumulation or repulsion of the substrate by the membrane [200, 322].
Approaches such as layer by layer (LbL) and self-assembly using polyelectrolytes have been used to encapsulate laccase [322]. The LbL technique involves deposition of enzymes between films in multilayer system through alternating layer by layer deposition of support and enzyme. The advantages with this technique are that a variety of materials can be incorporated into the layers with controlled thickness of the biocatalyst [85, 224]. The enzyme activity is retained without compromising its stability and it can easily be reloaded when necessary [409]. Laccase has been encapsulated in materials like nanocellulose [200], thin silicate films [69], graphene oxide-laccase nanoassemblies [224], self-assembled monolayer (SAM) - 3-mercaptopropionic acid [101], and sol-gels [109, 164].
6.1.3. Adsorption
The adsorption technique involves attachment of laccase on the exterior surface of a support through hydrogen bonding, electrostatic forces, or hydrophobic effects and solely depends on the isoelectric points of the enzyme and support [200, 405]. For electrostatic attraction, the charge on the surface of the carrier should be opposite to the charge of the enzyme and these are determined by the isoelectric point of the enzyme, pH of the buffer, and ionic strength of the medium [383, 393]. Because this technique is associated with low linking energy between enzyme and support, the enzyme is susceptible to leaching during washings and changing pH, temperature, and ionic strength [391]. The active site of the support may be blocked by the support greatly reducing the enzyme activity [410].
To minimize these limitations, metal-chelated and bioaffinity adsorption have gained popularity in immobilizing laccase. Metal chelated adsorption is based on the covalent conjugation of metal ions and His or Cys residues at the surface of the enzyme [411]. This approach is preferred because of its simple operation, stability, reversibility, and oriented enzyme immobilization for spatial accessibility into the substrate [196, 213]. For example, when laccase was immobilized on Cu2+ chelated zinc oxide nanoparticles (ZnONPs) and manganese oxide nanoparticles (MnO2NPs) through metal affinity adsorption, the immobilized laccase demonstrated almost double the catalytic activity towards degradation of alizarin red S dye (95% for lac-ZnONPs and 85% for lac-MnO2NPs) than the free laccase [412]. Wang et al. [397], achieved 92.5% activity and a retained activity of 86.6% after 10 successive batch oxidation of catechol when T. versicolor laccase was immobilized on Cu2+ chelated magnetic mesoporous silica nanoparticles via metal affinity adsorption at pH 3.0. Wang et al. [413], immobilized laccase on amidoxime polyacrylonitrile nanofibrous membranes (AOPAN) chelated with four metal ions (Fe3+, Cu2+, Ni2+, Cd2+) individually and as a mixture of the metal ions. The membrane chelated with four mixed metal ions showed the best properties followed by the individual metal ions in the order Fe–AOPAN–Lac > Cu–AOPAN–Lac > Ni–AOPAN–Lac > Cd–AOPAN–Lac. The stability of the immobilized enzyme depended on the size and polarizability of the metal ions. The saturation of laccase His structures with metal ions stabilized the enzyme's three-dimensional structure through special covalent coordinate bonds which minimized enzyme distortion and desorption [184, 414].
Bioaffinity based adsorption utilizing the affinity of laccase carbon moieties to the support surface has been explored. For example, Li et al. [415], exploited the specific affinity of Concanavalin A (Con A) for glycosyl to immobilize horseradish peroxidase and laccase on the surface of single walled carbon nanotubes (SWNTs) through self-assembly. The SWNTs were incubated with n-dodecyl β-D maltoside (DM) to form SWNTs-DM complex which was activated with Con A for enzyme immobilization. DM and the enzymes were bound on Con A step by step via the affinity force to form a sandwich structure hence indirectly immobilizing the enzymes on the SWNTs. This approach allows enzymes to be directly adsorbed from crude homogenates which bypasses the cost of enzyme purification with fewer immobilization residues generated [391].
6.1.4. Covalent
Covalent immobilization methods include enzyme attachment to matrix by covalent bonding, crosslinking between enzyme and matrix and enzyme crosslinking by multifunctional reagents. Covalent immobilization to a support involves interaction between an activated group on the support and a functional group on the enzyme (usually β-amino groups of basic residues) [11]. Different functional groups such as carboxyl [379], hydroxyl [390], amino [17, 166], and epoxy groups are added or activated on the support surface which form covalent bond with the enzyme. For example, Salami et al. [416], functionalized silica gel with epoxy groups by dispersing it in dry toluene followed by addition of glycidyloxypropyltrimethoxysilane (3-GPTMS) and triethylamine Et3N solutions. The epoxy functionalized silica gel was then added to laccase solution for immobilization. Kashefi et al. [417], introduced amino functional groups on graphene oxide (GO) nanosheets by reacting them with APTES in ethanol solution. Glutaraldehyde was then added as a crosslinking agent between the amino residues on laccase and the modified GO.
Protocol for covalent immobilization begins with surface modification or activation, for example carbodiimide activation, silanization of inorganic supports, and derivatization of amino groups to arylamine groups using p-nitrobenzoyl chloride or to aldehyde groups using glutaraldehyde [405, 418]. The most common is silanization where the support is coated with organic functional groups using an organofunctional silane reagent [419]. Carbodiimide activation is also used often to form epoxy groups on the surface especially when a carboxyl group is expected to react with the amino groups on the enzyme [19]. Other support activation methods such as plasma polymerization of the support [418] and activation with glutaraldehyde have been reported [378]. The added functional groups on the support surface are then derivatized using coupling or crosslinking agents to react with the amino groups on the enzyme surface to form covalent bonds [404, 419]. For example Kuznetsov et al. [420], during the covalent immobilization of laccase on a GCE modified the surface of the electrode with carboxyl groups by treating it with oxygen plasma and used condensing N-cyclohexy-N’-[b-(N-methylmorpholino)ethyl] carbodiimide p-toluene-sulfonate salt (CMC) to activate the carboxyl groups. Laccase immobilization was then achieved by incubating the modified electrode in PBS containing laccase enzyme. Merle et al. [68], explored the aspect of molecular recognition for covalent immobilization of laccase on aminopolypyrrole film modified carbon electrodes. The enzyme and aminopropyrrole modified electrode were biotinylated by reaction with excess biotin-amidocarproate NHS ester solution. The biotinylated electrode was immersed in avidin solution followed by immersion in biotinylated laccase solution to form covalent bonds. Labus et al. [418], modified the surface cellulosic and polyamide microfiltration membrane by plasma polymerization of allylamine, allyl alcohol, and acrylic acid to introduce amine, hydroxyl, and carboxylic functional groups for covalent immobilization of C. unicolor laccase and A. bisporus tyrosinase. The hydroxyl groups were then activated with divinyl sulfone, carbodiimide, or glutaraldehyde.
Various coupling agents such as glutaraldehyde, tripolyphosphate [314], Sebacoyl chloride [40], EDC [421], and/or NHS [44, 341, 422] are used to bind the activated support surface and the enzyme. Glutaraldehyde (GA) is one of the most commonly used crosslinking agents during covalent immobilization and also acts as a spacer arm due to its five carbon length [114, 373]. It reacts with the amine groups of enzymes and supports through formation of Schiff's bases and Michael's adducts [423, 424, 425]. One aldehyde group of GA reacts with the amino groups on the surface of the support to form imino groups and the other interacts with the amino groups of the enzyme [388, 399]. GA promotes multi point attachments and enzyme rigidification hence the amine content of the carrier, enzyme type and concentration, pH, and reaction time have to be considered when using it [219]. It has been observed that activity of immobilized enzyme increases with increasing concentration of GA due to the increasing amount of free aldehyde groups on the surface to cause higher loading [359, 388]. At higher glutaraldehyde concentrations, there are extensive interactions of individual enzymes with aldehyde groups which changes the enzyme conformation leading to excessive crosslinking between enzyme and support, aggregation and precipitation of the enzyme, hence drop in activity [348, 399]. Moreover, very high concentrations of GA can induce GA-GA interactions rather than laccase-glutaraldehyde interactions, since GA is a bifunctional reagent [426].
Crosslinking with GA leads to a dimeric or trimeric structure which results in creation of a spacer between the matrix and protein surfaces. The spacer distances the enzyme from the support surface which allows access of the substrate to the enzyme [390]. Patel et al. [386], compared covalent immobilization of laccase on silica nanoparticles by functionalization with different functional groups, that is, aldehyde using glutaraldehyde, carbodiimide using 1-cyclohexyl-3-(2-morpholinoethyl) carbodiimide metho-p-toluenesulfonate, and cyanogen using cyanogen bromide under the same conditions. Aldehyde functionalized support gave the highest immobilization yield and efficiency (73.2% and 78.4% respectively) followed by carbodiimide (64.5% and 64.2% respectively) and lastly cyanogen (56.45% and 61.4% respectively). However, the high reactivity of GA and its small size enable it to diffuse into the internal structure of the enzyme thereby causing loss in enzyme activity [32]. GA has also been proven to be toxic to aquatic species by reducing reproduction and impeding growth and to human health. When used for crosslinking it may result in leaching from the biocatalysts to the receiving environment where it can cause adverse effects to the aquatic ecosystems [427].
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) is another commonly used crosslinking agent. It is a water-soluble, zero-length heterobifunctional crosslinking reagent that reacts with carboxylates to form highly reactive O-acylisourea intermediates that spontaneously react with the amine groups of the enzyme to form amide bonds [421, 428]. It is a zero-length crosslinker and therefore does not generate extra linkage but dissolves as urea. However, it can promote intramolecular bonding between carboxylates and amines groups of the enzyme instead of intermolecular bonding between enzyme and support. It is also unstable in aqueous solutions as the O-acylisourea is cleaved to an iso-urea before amide coupling which leads to regeneration of the carboxylate [32]. It has also been used as an activating agent in conjunction with other crosslinkers such as chitosan [427] and NHS [65].
Other coupling agents such as NHS [365], glyoxal [32], genipin [429] and epichlorohydrin [366] have been reported. NHS as a crosslinker generates an NHS ester on the enzyme which the reacts with the amine-bearing supports to form covalent bonds [365]. Glyoxal is a dialdehyde often used as a surrogate to GA due to its lower toxicity and reduced steric hindrance [32]. Genipin, a natural crosslinker, reacts with primary amine groups under mild conditions and exhibits biocompatibility with low toxicity [429].
Depending on the enzyme and support properties, different coupling reactions can offer superior immobilization yield and efficiency for laccase. For example, Quan and Shin, 2004 [351] compared four covalent immobilization approaches for laccase on a glassy carbon and platinum electrodes. The first and second approach involved peptide coupling using EDC/NHS of laccase on 1,5-pentanediol modified or bare electrodes. The third approach involved immobilization on hydroxyl functionalized electrode with cyanuric chloride as a coupling agent while the fourth method used APTES silanized electrode with GA coupling. The APTES/GA immobilization technique was the most effective with respect to long term stability and fast response in detection of p-phenyldiamine substrate. On the other hand, when Costa et al. [430], compared different approaches with combinations of methods involving hydrothermal oxidation with nitric acid, treatment with APTES, GA, EDC, and NHS for laccase immobilization on multi-walled carbon nanotubes (MWNTs), the MWNTs functionalized with HNO3 and treated with EDC/NHS gave the best thermal stability and immobilization efficiency and yield.
During covalent immobilization, laccase activity may be hindered due to the involvement of amino acid residues near the active site in bonding. To minimize this, small molecules to act as spacer arms are added [39, 80, 85]. Spacers such as β-alanine and polyethyleneimine (PEI) improve the enzyme loading capacity, microenvironment, orientation, and conformational flexibility of the enzyme and in turn improve biocatalytic activity [414, 431]. For instance, Arica et al. [373], compared direct immobilization of laccase on poly (GMA/EGDMA) and use of DAH-GA spacer-arm to move the enzyme away from the support and the immobilization with a spacer arm gave a higher activity yield of 83% as compared to no spacer-arm used of 53%. The low activity of the enzyme immobilized directly on the polymeric support was attributed to steric hindrance between the support surface and immobilized enzyme.
Covalent immobilization techniques provide strong stable enzyme attachment with reduced leaching and deactivation rates [362, 432]. For example, Pang et al., compared immobilization of T. versicolor laccase on carbon nanotubes by adsorption and covalent immobilization achieved by functionalization of the nanotubes with 1-aminopyrene and GA crosslinking. The biocatalysts were used in oxygen reduction and it was observed that the covalently immobilized enzyme displayed higher electrocatalytic activity and better stability than the adsorbed laccase. As a result this method is preferred for long-lasting, real-scale, and continuous applications [426].
The other advantage with covalent immobilization is that the bonding between enzyme and support can be oriented so as to achieve better biocatalyst activity [80]. For example, Rotková et al. [433], explored oriented (through carbohydrate moieties) and non-oriented (through NH2 groups on the laccase surface) covalent immobilization of laccase on magnetic bead cellulose (size 125–250 μm). For non-oriented immobilization, the magnetic macroporous bead cellulose were oxidized with sodium periodate followed by incubation with laccase in the presence of Sodium cyanoborohydride. In the case of oriented immobilization, the magnetic beads were functionalized with hydrazide functional groups and laccase was oxidized with sodium periodate. The oxidized enzyme was then incubated with the functionalized carrier to form covalent bonds. Although both approaches led to formation of stable hydrazone based bonds, the oriented approach achieved an enzyme activity that was almost three times (0.63 I.U./1 ml of settled carrier) the activity of the non-oriented immobilization approach (0.22 I.U./1 ml of settled carrier).
However, during covalent immobilization, the native structure of the enzyme is usually affected due to the multipoint attachments and the enzyme activity often reduces due to the rigorous treatment with toxic coupling reagents [322, 423, 434]. For example, when Zhu et al. [360], immobilized T. versicolor laccase on magnetic mesoporous silica spheres by physical adsorption and covalent attachment method (using –NH2 activation and GA crosslinking), the retained enzyme activity for covalent attachment was much lower (56.9%) compared to that of physical adsorption (79.4%). In some instances, the activated groups on the support surface and enzymes may not be compatible leading to very low immobilization yield. For example, Russo et al. [435], achieved only 7% immobilization yield when laccase was immobilized via covalent binding between amino, thiol, and carboxyl groups of the enzyme and the epoxy residues of Epoxy-activated acrylic beads, EUPERGIT C250 L. More so, the covalent bonds formed via amino groups of the enzyme reduce its conformational flexibility and may result in higher activation energy for the molecule to reorganize the proper conformation of the binding substrate [373].
6.1.5. Crosslinking of enzymes
The main challenge with carrier bound immobilization is the dilution of catalytic activity as the biggest portion of the biocatalyst is occupied by the support leading to lower volumetric and space-time yields hence lower catalyst productivity [147, 436]. Moreover high enzyme loading on the support often results in loss of activity and instability towards leaching in aqueous media [437]. Also cross linking reactions between enzyme and support are difficult to control which often leads to structural denaturation of the enzyme molecules [404]. To minimize these effects, self-immobilization techniques where laccase is incubated with crosslinking agents have been explored [81, 436]. And since the molecular weight of the crosslinking agent is negligible in relation that of the enzyme, the biocatalyst can essentially comprise of 100 % active enzyme [147]. Therefore, the immobilized enzyme exhibits operational stability and reusability with efficiencies close to those of the free enzyme [103, 438].
6.1.5.1. Crosslinked enzyme crystals (CLECs)
Crosslinked enzyme crystal (CLECs) are prepared by controlled precipitation of enzymes into micro crystals followed by crosslinking using bifunctional reagents to form strong covalent bonds between amino groups of lysine residues within and between the enzyme molecules [32, 438]. The protein in CLECs are stabilized by links in the three-dimensional structure unlike immobilization on a support in which the enzyme is linked to the support by multi-point attachment to a two dimensional solid surface [309]. CLECs display stability in organic solvents, shear stress and temperature and storage conditions [439]. The stability of CLECs in organic solvents is attributed to cross linking which increases the rigidity of the enzyme molecules and reduces the unfolding of the three-dimensional structure of the enzyme by the organic solvents [309, 440]. For example, CLECs showed higher activity in organic solvents (toluene, hexane, cyclohexane, and isooctane), 4-fold thermal stability, and a half-life of 123 min compared to the 24 min of the free enzyme at 60 °C [441]. During the conjugation reaction of catechin with poly (alkylamine), Gogoi et al. [442], compared the catalytic activity of free laccase, crosslinked enzyme crystals of laccase, and laccase immobilized on Celite 545 support. CLECs displayed the highest stability in the organic solvents as well as catalytic activity. Their operational stability, controllable particle size, and ease of recycling, coupled with their high catalytic and volumetric productivities make CLECs ideal as industrial biocatalysts [443].
One major drawback of CLECs is high purity enzymes are needed for crystallization which translates to high costs [439, 444]. Also the multi-step enzyme purification is tedious and time consuming and enzyme purification and stabilization should ideally be achieved simultaneously [155]. As a result, crosslinked enzyme aggregates (CLEAs) have gained popularity. The CLEAs are prepared by precipitation of the enzyme from aqueous buffer (using precipitating agents like ammonium sulfate and polyethylene glycol [429, 445] followed by crosslinking of the aggregates with crosslinking agents such as glutaraldehyde [28, 445], genipin [429], chitosan and EDC [427]. Cross-linking prevents the solubilization and possible loss of the aggregates after removing the precipitating agent [64]. This technique combines purification (precipitation) and immobilization (crosslinking) into a single operation and can be performed with crude preparations hence low costs are associated with it [103, 437, 446]. The precipitated aggregates are held together by non-covalent bonding without alteration of the enzyme structure and subsequent crosslinking makes the aggregates permanently insoluble while maintaining their pre-organized superstructure hence preserving catalytic activity [365, 447]. CLEAs demonstrate simple and low cost preparation, they are easy to recover and reuse, require no carrier, show high activity in organic solvents or unfavourable conditions, and are stable towards leaching in aqueous media [405, 448]. For example, Cabana et al. [64], produced C. polyzona laccase CLEAs using polyethylene glycol as precipitant and glutaraldehyde as a cross-linking agent. The CLEAs were used to eliminate EDCs such as nonylphenol, bisphenol A, and triclosan in a fluidized bed reactor and they displayed desirable thermal stability the cross-linking (presence of multiple links on the protein surface) prevented the unfolding of laccase under thermal stress conditions and removed all the EDCs at concentrations up to 5 mg/L. T. versicolor laccase CLEAs retained 80% of their activity after 70 days of storage at 4 °C and demonstrated 95% decolourisation efficiency for malachite green, bromothymol blue, and methyl red dyes [449]. Cerrena sp. laccase CLEAs demonstrated improved tolerance to NaCl, metal ions, and organic solvents [450]. Moreover, two or more enzymes can be precipitated simultaneously or sequentially to catalyze multiple biotransformation reactions [410].
Even though CLEAs show promises, their irregular shapes and sizes affect their recovery after reactions and diffusion of substrates to the active site [448]. Improvement of their properties has been studied and CLEAs of improved properties have been reported. For example, Nguyen et al. [448], synthesized hollow CLEAs of laccase (h-CLEAs) by employing a millifluidic reactor with two coaxial glass capillaries containing acetate buffer/laccase solution and acetonitrile/glutaraldehyde solution respectively. h-CLEAs were formed by rapid precipitation at the confluence zone where the two solutions met. Kumar et al. [451], reported synthesis of porous CLEAs through a three-phase partitioning technique where laccase and starch were co-precipitated, crosslinked with GA and the starch removed by α-amylase to create pores in the CLEAs. Entrapped CLEAs have been synthesized by using a combinatorial strategy of cross-linking and entrapping in mesoporous silica to form E-CLEAs [436]. Magnetic CLEAs formed through modification of CLEAs using functionalized and non-functionalized magnetic nanoparticles for increased activity, stability, and easy separation from the reaction mixture using a magnetic field have been reported [401, 452]. Laccase CLEAs have been extensively reviewed elsewhere [447].
Immobilization of individual enzyme molecules through covalent bonding to cross-linked polymer matrix to form single enzyme nanoparticles (SENs) has also been explored for laccase. For example, Şahutoğlu et al. [453], immobilized T. versicolor laccase as SENs via acryloyl chloride modification followed by in-situ polyacrylamide polymerization in water phase to form spherical 50 nm SENs. The multipoint enzyme attachment of the enzyme and the presence of the polymeric network enhance the conformational stability of the enzyme and the polymeric network is sufficiently porous to allow migration of the substrates to the active site [32].
7. Supports used for laccase immobilization
A suitable support material for enzyme immobilization should be compatible with the enzyme, inert in process solutions, and facilitate substrate diffusion to accomplish the biocatalytic reaction [85, 376]. Therefore factors such as volume, porosity, shape, surface properties, stability in given reaction conditions, and cost are considered when selecting a support for immobilization [51]. For inert supports, their surfaces are modified to offer functional groups for protein binding [454]. Various materials have been explored over the years as supports for laccase enzyme (Figure 6, Table S2) [455]. The supports are classified into porous and nonporous supports (based on the structure) or organic and inorganic supports (based on their composition). Porous supports offer high enzyme loading with diffusion limitations especially for large molecular weight substrates [373] while nonporous supports have low diffusion limitations but suffer from low surface areas for enzyme immobilization [424]. Different materials have been explored as supports for immobilization of laccase enzyme (Table S2) and have been extensively discussed in this section.
7.1. Inorganic supports
Inorganic supports are reported to outperform organic materials in various aspects including microbial corrosion resistance, anti-swelling ability, mechanical and chemical stability, and better reusability [178, 249]. Various inorganic supports such as aluminium hydroxide [94], silica [17, 456], ceramics [362], Celite [457], perlite [423], and clay such as bentonite [129] and montmorillonite [342] have been utilized in immobilization of laccase.
7.1.1. Glass/silica
Silica has been widely used as a choice for immobilization because it is neutral, non-toxic, and occurs in the natural environment [188]. It is endowed with properties such as tunable particle size, porosity, surface area, transparency, chemical stability, and convenient preparation [22]. Various forms of silica including activated glass beads [249], perlite (amorphous volcanic glass) [423], porous silica beads [121, 458], silica gel [197, 365], controlled porosity glass/silica beads [26, 147, 210], and ordered mesoporous silica (OMS) such as SBA-15 [74,459] have been used for laccase immobilization.
In the past decade, much work has been undertaken in the synthesis of mesoporous silicates and in the immobilization of enzymes onto these supports. Mesoporous silica materials have attracted attention as catalyst supports due to their large specific surface area, mechanical stability, water insolubility, biocompatibility, adjustable pore size, highly ordered structure, and resistance to microbial attack [397, 406, 444]. More so, their surfaces can be chemically modified with various functional groups to enable electrostatic attraction between the support and enzyme while offering thermal and chemical stability [360, 365]. For example, functionalized ionic liquid supports were grafted onto mesoporous silica molecular sieves to obtain modified mesoporous silica with imidazole functional group for laccase adsorption [460]. Enzymes can be immobilized in mesoporous materials by different methods: adsorption, covalent attachment, or encapsulation.
The most important aspect when adsorbing enzymes on ordered mesoporous silica (OMS) materials is the pore opening diameter. Enzymes larger that the pore diameter cannot fit through the pore openings while pore opening way larger than the enzyme leads to enzyme leaching [461]. Encapsulation of enzymes in these supports renders them more mechanically robust and stable over time and covalent bonding is not required hence the native properties of the enzyme are maintained [305, 346]. Adsorption of the enzyme is suitable when the pore size is close to the enzyme dimension while covalent attachment is advisable when the pore sizes are far larger than the enzyme dimensions [462]. For large pore sizes, the silanol groups on the surface of the OMS can be activated with functional groups to strengthen the van der Waals interactions or serve as anchoring points for covalent immobilization [405, 461]. For example, Yang et al. [222], introduced amino functional groups in the pores of mesoporous silica using APTES and then crosslinked the activated carrier with GA prior to reaction with laccase solution.
7.1.2. Aluminium oxide
Aluminium oxide (Al2O3) is one of the metal oxides characterized with well-developed porous structures of varying pore diameters (ranging from 2 to 10 nm). It demonstrates high crystallinity, porosity, thermal, and mechanical stability [463]. Aluminium oxide in the form of pellets [234], mesoporous [463], nanoporous [464], and zeolite [198, 465] has been explored for laccase immobilization due to its high surface area, porosity, chemical, and mechanical stability. For example, Bruera et al. [464], adsorbed laccase on nanoporous aluminum oxide for treatment of black liquor. An immobilization yield of 18.1% was achieved and the biocatalyst retained 95.6% of its initial activity after consecutive runs and a 40 fold increase in removal of black liquor as compared to the free enzyme. Wehaidy et al. [466], immobilized laccase on nanoporus zeolite-x with an immobilization efficiency of 83% and 100% residual activity after 7 reuse cycles. When laccase was adsorbed in mesoporous aluminium oxide, the enzyme retained 65% and 60% of its initial activity after 30 days of storage and 10 reaction cycles respectively [463].
7.1.3. Nanoporous gold
Nanoporous gold (NPG) is a new type of porous inorganic material that has attracted attention in immobilization of enzymes due to its large surface area, its truly support-free, simple, and reproducible preparation methodology, tunable pore size, and clean nanostructured surfaces [368, 467]. It is prepared by etching alloys of gold and a less noble metal such as Ag, Sn, Cu, and Zn to form interconnected ligaments with gaps between them [468, 469, 470]. It is highly stable, highly conductive, has large surface areas, has easily functionalized nanostructured surfaces, and its pore sizes can be tuned from a few nanometers to several microns by adjusting the preparation method [469, 471]. It has been observed that enzymes confined within the NPG structure display stability to high temperatures and organic solvents due to the protective environment provide by the nanopores and biofouling can be greatly reduced since large proteins are restricted from accessing the internal pores [472]. For example, laccase was adsorbed on the surface of NPG and used in a biofuel cell for DET. The optimum temperature of the enzyme increased from 40 to 60 °C and highly efficient DET on a GCE was attained. Interestingly, the electrode showed no obvious changes in response after one month of storage at 4 °C [368].
Another advantage of NPG is that SAMs are more stable as they benefit from its defective sites, ligament surface, and lattice strain [472]. For example, Hakamada et al. [473], modified NPG with SAM of 4-aminothiophenol and used it for laccase immobilization. The SAM-modified NPG showed stronger bonding towards laccase and demonstrated higher thermal stability than the unmodified NPG.
7.1.4. Clay
The use of a natural supports such as clay or soil is desirable since it poses no environmental risk and is therefore beneficial for terrestrial bioremediation [126]. Clays are low-cost, eco-friendly, recyclable, have low mass transfer, demonstrate microbial corrosion resistance capacity, and their surfaces can be tuned through activation with desired functional groups and/or etching [474]. They present excellent properties such as high porosity, cationic exchange capacity, intercalation capacity, and swelling response [475, 476]. Various forms of clay such as bentonite [474, 475], sepiolite [477], smectite [476], kaolinite [77], and light expanded clay aggregates (LECA) [252, 478, 479] have been explored for laccase immobilization. For example, Dodor et al. [77], Immobilized T. versicolor laccase on kaolinite and used for the catalytic oxidation of PAHs, anthracene and benzo [a]pyrene, in the presence of ABTS as a mediator. The immobilized enzyme showed a broader optimum pH range of 4–6 as opposed to 4.5 of the free enzymes, storage stability of 100% activity after 4 months at 4 °C, stability in presence of sodium azide inhibitor, and increased the optimum operating temperature from 40 °C to 60 °C and 70% activity at 80 °C. Clay has also been mixed with other materials such as polymers and nanoparticles to improve on the performance of the biocatalyst. For example, Mehandia et al. [475], entrapped Alcaligenes faecalis laccase in chitosan-bentonite clay composite beads and achieved 88.4% immobilization yield with no leakage observed during washing or storage. Combinations of smectite nanoclay with carbon based nanomaterials (graphene oxide, carbon nanotubes and adamantylamine) were used for laccase immobilization with an immobilization yield of up to 85% and minimal alteration of the enzyme structure [476].
7.2. Organic supports
In this section, the various organic supports that have been explored for immobilization of laccase enzyme are discussed.
7.2.1. Carbon
Carbon has been explored as an enzyme support material due to is good mechanical strength, availability, easy modification with different functional groups such as carboxyl, amino, and phenolic-OH for enzyme coupling, and is already used in food and waste treatment industries [480]. Carboxylic functional groups can be easily formed on the surface of carbonaceous materials which provide ideal anchoring points for physical attachment and covalent bonding of enzymes on their surface [212]. Carbon can be obtained from wastes from the food industry hence provides cheaper alternatives for enzyme immobilization [150]. Various forms of carbon including graphite and its oxide [87], copper-containing ordered mesoporous carbon (Cu-OMC) [307], carbon fibers [481], magnetic bimodal mesoporous carbon [162], activated carbon [454, 480], and carbon nanotubes [482, 483, 484] have been used for laccase immobilization.
Carbon nanomaterials such as graphene oxide, SWNT, and MWNT are promising enzyme supports because of their chemical inertness, biocompatibility, and electrical conductivity [225, 381]. Graphene is a two dimensional monoatomic thick carbon material with high electrical and thermal conductivity, chemical stability, superior mechanical properties, nontoxicity, and high transparency usually derived from ordinary graphite [485, 486]. The two dimensional plate like structure of graphene offers a large surface area accompanied with incredible flexibility and great mechanical properties [289, 487]. Graphene offers low energy dynamics of electrons with atomic thickness, high carrier mobilities, abundant oxygen-containing functionalities, and water solubility making it an attractive support for laccase immobilization [224, 354]. The surfaces of graphene can be modified or derivatized to obtain congeners such as graphene oxide and reduced graphene oxide nanomaterials [485] and hydrogels or xerogels [488]. The use of graphene oxide and reduced graphene oxide to immobilize laccase have provided simple and less aggressive techniques [340, 377]. Due to its high hydrophilicity and tendency to agglomerate, graphene and its derivatives are combined with other nanomaterials such as Fe3O4 [382,489,490], CuFe2O4 [486], zeolite [491], PANI [338], MWNTs [492, 493], cellulose [494], zirconium-metal organic framework (Zr-MOF) [214], and Sb2O5 [337] to aid its recovery. For example, Palanisamy et al. [339], adsorbed laccase on a screen printed electrode modified with graphene-cellulose microfiber composite for detection of catechol. A sensitive and selective biosensor was developed with a limit of detection of 85 nM, response time of 2s, linear response range up to 209.7 μM, and sensitivity of 0.932 μAμM−1 cm−2. Xu et al. [495], adsorbed laccase on a membrane developed by depositing GO nanosheets by layer-by-layer approach on a polyethersulfone support to obtain a biocatalyst with excellent stability towards temperature and pH over a long time.
Carbon nanotubes (CNTs) are nanowires based on tubular graphene layers of varying diameter, length, and chirality consisting of one (SWNT) or more (MWNT) nanotubes. They offer high adsorption capacity, large specific surface area, high enzyme loading, high conductivity, and they can easily be functionalized to obtain specific properties [496, 497]. CNTs easily bind to the enzyme noncovalently through hydrophobic interactions, formation of π–π stacks between enzyme residues and their surfaces, ampiphilic binding, electrostatic interactions, and hydrogen bonding between the enzyme amino groups and oxidized groups on their surfaces [285, 498]. For example, laccase was noncovalently immobilized on SWNT using a molecular tether (1-pyrene butanoic acid, succinimidylester) that formed an amide bond with the amine groups on laccase surface and coordinated to the SWNTs through π–π stacking with pyrene [499].
The unique tubular geometrical structure, superb electrical conductivity, high thermal conductivity, and superior tensile strength have made MWNT excellent enzyme carriers [176, 381]. MWNT can also be oxidized to produce carboxylated MWNT which have abundant oxygen-containing surface functional groups such as hydroxyl, carboxylic, and epoxy groups to enable the facile binding of various molecules including proteins, enzymes, DNA, surfactants, and metals, etc., with or without coupling agents [482]. They have been utilized as electron mediators to facilitate electron transfer between electrodes and enzymes due to their high electrical conductivity [332, 344]. However, immobilization of laccase on MWNT has been reported to have limited impact on the thermal stability of the enzyme [498]. MWNT have been combined with other compounds such as cellulose nitrate, agarose, polyvinyl alcohol (PVA) [381], polynorbornene-pyrene [500], electrospun fibers [501], chitosan [334], mesoporous silica [502], poly (3,4-ethylenedioxythiophene) [335], nanoparticles such as Ni [317], Au [335], and anthraquinone moieties [290, 503] to increase their stability through reduced agglomeration for continuous or multibatch processes. For example, laccase was adsorbed on a MWNT/Ag-nZnO composite by dropcasting laccase on a screen printed electrode modified with MWNTs that are functionalized with silver doped zinc oxide nanoparticles (nAg-ZnO) for detection of bisphenol A [331].
7.2.2. Agricultural waste
Use of synthetic support materials can not only be expensive but also present a concern for their disposal. As a result investigations focused on easy to find, inexpensive, biodegradable, and easily functionalized supports have led to the adaptation of agro-industrial wastes as enzyme supports [28]. Various agricultural products and wastes including eggshell membranes [504], coconut fiber [269, 505], Luffa cylindrica fiber [209], chicken feathers [506], pallet wood [507], pistachio nut shell [508], maize starch [228], spent grain [509, 510], biochar [181], and bacteria [511] have been used for laccase immobilization. For example, Montoya et al. [150], produced mesoporous carbon supports from various wastes, that is, pecan nut shells, peach stones, pistachio shells, and pine nut shells using activating agents to promote mesoporosity. Cristóvão et al. [391], adsorbed laccase on green coconut fibre for dye decolourisation and obtained an immobilization yield of up to 69% and recovery yield of up to 45%. Da silva et al. [512], immobilized laccase on beer spent grain (SG) and digested beer spent grain (DSG) by adsorption and covalent bonding through modification of DSG of the supports with glycidol and glycidol followed by ethylenediamine. The DSG was obtained by acid/base treatment of SG. Modification of the supports to obtain covalent immobilization significantly decreased the laccase activity and immobilization yield. A high immobilization yield of up to 90% was obtained through adsorption and the half-life of laccase increased from 0.64 to 1.1 h at 70 °C. Nanocellulose for laccase immobilization has also been synthesized from wastes such as quinoa husks [247] and sugar mill bagasse.
Biochar is a carbonaceous material obtained from hydrothermal and thermochemical methods of biomass conversion and offers as a large specific surface area, good dispersibility, and biocompatibility for stable and high-load enzyme immobilization [513, 514, 515]. The raw materials used for its production and the production conditions affect the chemical and physical properties of the biochar produced and hence its utilization in enzyme immobilization [516]. In addition to being inexpensive and easily available, these methods also provides the potential for waste valorization [426]. For example, Naghdi et al. [212], immobilized laccase on pinewood nanobiochar functionalized through acidic treatment (HCl, H2SO4 and HNO3). When laccase was covalently immobilized on citric acid pretreated micro biochar from pine wood, pig manure, and almond shell using glutaraldehyde, it was observed that feed stock selection and method of production affected biochar properties (texture, morphology, surface area and cationic nature) and consequently the immobilization efficiency [207, 426]. Since the enzyme immobilization efficiency was dependent on ionic strength and cationic nature of the biochar, biochar from pig manure offered the best support due to its high cationic exchange capacity compared to the wood based biochar [426]. Immobilization of laccase on biochar has been extensively reviewed elsewhere [514, 517].
7.2.3. Polymers
Polymer based carriers have attracted attention in enzyme immobilization because of their easy surface modification where various reactive groups can be anchored on the matrix and their morphology can be tuned during the polymerization step [379]. Properties such as surface area, functional group density, and porosity can be tailored to meet the specific needs for immobilization [389]. A variety of polymeric support materials including natural (chitin, chitosan, agarose, and cellulose derivatives) and synthetic (nylon, polysiloxane, polyvinyl alcohol [431], poly (glycidyl methacrylate), and polyacrylic polymers) have been prepared in various geometric forms and used for the immobilization of laccase. Biological or natural polymers have demonstrated advantages such as low cost, nontoxicity, and biocompatibility with enzymes through interaction with their functional groups [122]. However these supports suffer from low mechanical strength and are liable to microbial degradation necessitating the need for synthetic polymers [245]. The various polymers that have been utilized in laccase immobilization are discussed in this section.
7.2.3.1. Natural polymers
Various natural polymers have been used as supports for laccase immobilization and have been discussed in the following section [518].
7.2.3.1.1. Chitosan
Chitosan (CS) is a partially deacetylated polymer that is obtain via alkaline deacetylation of chitin [129]. Chitin is a biodegradable and non-toxic polysaccharide mainly obtained from marine and terrestrial invertebrates [366, 519]. The degree of deacetylation, concentration, and molecular mass greatly determine the solubility of CS and in turn the properties of the enzyme conjugates [520]. CS is endowed with attractive properties including hydrophilicity, biodegradability, biocompatibility, nontoxicity, and many large amine groups [314, 318, 399]. In addition, CS has demonstrated resistance to microbial degradation compared to other organic supports and is obtained from chitin, the second most abundant natural material on earth after cellulose [521]. This implies that CS is widely commercially available at low cost making it an ideal carrier for enzymes [25, 522]. The amine and OH groups on its surface give it excellent affinity to a variety of enzymes and solubility in mildly acidic aqueous solutions making it a good immobilization support [421, 523]. The presence of amino and OH functional groups on chitosan have allowed it to be used as a coating on other enzyme supports such as alginate beads [235] to improve their biocompatibility with laccase [345]. For example, during the entrapment of P. ostreatus laccase on copper alginate beads, enzyme retention during dye decolourisation was greatly improved when the copper alginate beads were coated with chitosan [524]. Immobilization of enzymes on CS has been shown to protect the enzyme from metal ion inhibitors [359]. The polymer has been used in various forms and shapes such as powder [525], membranes [526], beads [67, 404, 527] and hydrogel [528, 529] for immobilization of laccase.
Due to its soft texture, CS is usually cross linked with coupling agents such as GA to minimize dissolution in acidic conditions or incorporated with organic or inorganic materials to improve it mechanical stability and immobilization efficiency [28, 129]. For example, Cu (II)-chelated chitosan-graft-poly (glycidyl methacrylate) nanoparticles were prepared using poly (ethylene imine) (PEI) which acted as a spacer arm and metal chelator for laccase immobilization by coordination. The high density of amino functional groups on PEI provided the ideal conditions for chelating metal ions [521]. Addition of bentonite clay laccase solution to CS to form CS-clay laccase composite beads improved the density, surface area, pore volume, and pore size of the support hence yielding improved reusability, thermal-, and operational stability of the biocatalyst [129]. Laccase immobilized on glutaraldehyde-activated chitosan [170], CS/CeO2 microspheres [523], itaconic acid grafted and Cu(II) ion chelated CS membrane [526], CS coated magnetic nanoparticles [192], Alginate/CS microspheres [40], N-doped carbon hollow spheres/CS composite [315], MWNT-CS [345], halloysite nanotubes (HNTs)/CS microspheres [530], HNTs/Fe3O4/CS composite [179], magnetic multi-walled carbon nanotube-chitosan/silica hybrid membranes [333], and polyethyleneimine grafted chitosan films [531] has been reported.
7.2.3.1.2. Cellulose
Cellulose is the most abundant natural polymer and is nontoxic, biodegradable, hydrophilic, and biocompatible making it an excellent support for enzyme immobilization [313]. In addition, its derivatives such as cellulose acetate, cellulose nitrate, and carboxymethyl cellulose also offer excellent biocompatibility [168, 532]. Cellulose usually varies in morphology, size, crystallinity, and surface functional groups depending on the source of agricultural waste used during synthesis [247]. Laccase has been immobilized on cellulose based carriers such as cellulose acetate [313], nanocrystals [533], cellulose based Granocel carriers [8, 379] and solubilized cellulose, and PAMAM films [534]. For example, Ghodake et al. [211], extracted α-cellulose fibers form waste-paper biomass and super-magnetized them with Fe3O4 followed by grafting with CS and thiol groups for laccase immobilization.
Even though most cellulose has been isolated from plants, several bacteria from the genus Gluconacetobacter have recently been reported to produce a unique form of cellulose commonly called bacterial cellulose (BC) [535]. BC has demonstrated excellent characteristics such as tridimensional and fibrillar nanostructure, high water absorption capacity, and excellent mechanical strength which differ from plant cellulose [535, 536]. Since BC is nontoxic, biocompatible, bioavailable, non-carcinogenic, and easily biodegradable, it has been explored in enzyme immobilization for biomedical applications such as wound dressing, burn treatment, and tissue regeneration [284]. Due to its fibrillar nanostructure, large surface area, and high porosity, BC is expected to easily entrap enzymes and achieve high enzyme immobilization yield and efficiency [536]. For example, Li et al. [324], hybridized BC nanofibers by depositing gold nanoparticles (AuNPs). Laccase was then adsorbed to the hybrid followed by addition of Nafion. The biocatalyst was used as a biosensor for detection hydroquinone with a low detection limit of 5.71 nM and a wide linear range of 30–100 nM. Laccase adsorbed on BC aerogels for degradation organic dyes degraded 94.5% and 85.2% of reactive red and 2,4 dichlorophenol respectively within 4 h and retained 76% activity after 5 reuse cycles [537].
Enzyme immobilization on cellulose supports is through simple physical adsorption or weak inter ionic interactions between the enzyme and activated hydroxyl groups on cellulose [534]. Drozd et al. [538], reported that exposing BC to a rotating magnetic field prior to laccase immobilization improved the thermal stability of the biocatalyst by 10 °C and activity retention after 8 cycle by 15%.
7.2.3.1.3. Agarose/sepharose
Agarose based gels are used as matrices for enzyme immobilization due to their biocompatibility, hydrophilicity, and presence of hydroxyl groups on the surface that can be activated for covalent bonding with enzymes [72, 539]. For example, Brugnari et al. [540], immobilized P. ostreatus laccase on MANAE-agarose for degradation of bisphenol A. An immobilization yield of 100% and 138% activity of the immobilized enzyme compared to its free form were obtained. The biocatalyst also demonstrated thermal and storage stability, longer half-life and retained 90% of its initial activity after 15 reuse cycles. Laccase immobilized in sepharose-CL-6B glass beads has also showed great stability in organic solvents such as hexane, tetrahydrofuran, and dioxane [539]. Agarose activated with functional groups such as hydroquinone and β-benzoquinone [10], glyoxal [541], thiolsulfinate [542], aldehydes [165], and anti-laccase antibodies [253] have been explored for laccase immobilization.
7.2.3.1.4. Alginate
Alginate is a natural polymer easily converted to hydrogel via crosslinking its carboxyl groups with solutions of cations such as Ca2+, Ba2+, and Cu2+ [115, 157]. The polymer is nontoxic and biodegradable and entrapment in alginate beads is one of the cheapest, fastest, and simplest methods to develop efficient and stable laccase [137, 250]. Laccase has been entrapped in various alginate beads such as calcium alginate–chitosan beads and sol–gel matrices [122], Calcium alginate beads [115], and copper alginate beads. For example, Domínguez et al. [136], entrapped T. hirsuta laccase in calcium alginate beads for decolourisation of indigo carmine and phenol red in an airlift bioreactor. 96% and 69% decolourisation efficiency were achieved for indigo carmine and phenol red, respectively, after 24 h and the bioreactor operated uninterrupted for 40 days without any significant changes in its activity. The bead sizes allowed movement of air bubbles throughout the reactor bed giving suitable aeration for the microorganism and avoiding clogging problems which would hinder mass and oxygen transfer rates. Niladevi and Prema, 2008 [157] entrapped Streptomyces psammoticus laccase on Ca and Cu-alginate beads and Cu-alginate provided higher immobilization yield (61%) than Ca-alginate (42%). This was because Cu has a higher affinity for alginate than Ca and strongly complexes within the polymer network. The Ca-alginate beads also had higher porosity and lower chemical stability leading to higher enzyme leakage than Cu-alginate beads. However, Lassouane et al. [543], observed that crosslinking of laccase with GA prior to entrapment in Ca-alginate beads increased the immobilization yield by 30% and reduced leakage by 7 fold.
7.2.3.1.5. Gelatin
Gelatin has abundant free amino, hydroxyl, and carboxyl functions in molecule chains and GA can interact with free amino groups in gelatin or the enzymes, which makes water easily exude from the beads during the crosslinking and prevents the leakage of immobilized enzymes from the beads. Owing to its low intensity and high brittleness, gelatin is rarely used alone and is often used after crosslinking, grafting, or blending [544].
7.2.3.2. Synthetic polymers
Various synthetic polymers such as Polyacrylonitrile [183, 545], polyethersulfone [546], Polypropylene [190], polyamide [376], polyhydroxybutyrate [547], polyurea [548], poly (ethyleneimine) [350], poly (ethylene terephthalate) (PET) [432], polypyrrole [68], polypropylene chloride [549], polymethacrylate [229], and Polyacrylamide gel [18] have been used for immobilization of laccase. The most commonly used synthetic polymers for laccase immobilization are discussed in this section.
7.2.3.2.1. Eupergit
Eupergit is an epoxy activated polymer that binds to enzymes via epoxide groups [172, 550]. Its oxirane groups function as active components for covalent binding of ligands containing amino, mercapto, or hydroxyl groups [48, 551]. Due to their hydrophilicity, wide pore distribution, and good hydrodynamic properties, they offer a simple coupling procedure to the enzymes [552]. Hublik and Schinner [48] immobilized P. ostreatus laccase on Eupergit C. benzoate and used the bioconjugate for continuous elimination of 2, 6-dimethoxyphenol in a packed bed reactor. No substrate was detected in the filtrate and the bioconjugate showed increased activity and great stability with a 2% registered loss after 10 days. When laccase-eupergit bioconjugate was used in a fluidized bed reactor for the removal of estrogens, prolonged enzyme stability of over 16 days was attained [553].
7.2.3.2.2. Polyaniline
Polyaniline, a conducting polymer, has been explored for laccase immobilization particularly for biosensor and biofuel cell applications [311]. It is environmentally safe, can be cheaply manufactured, can act as a direct mediator for electron transfer in redox reactions, and exhibits high conductivity and thermal stability [330]. For instance, Mani et al. [554], compared the catalytic oxygen reduction in microbial fuel cell of laccase immobilized by crosslinking with PANI, entrapment in Cu-alginate, and encapsulation in Nafion micelles. The PANI-laccase showed the highest stability, activity, and reusability. PANI has been used in various forms such as films [159], nanofibers [330], and printable ink [555] for laccase immobilization. For example, Timur et al. [159], adsorbed T. versicolor laccase on a PANI modified thick film electrode and used it as a biosensor for detection of phenol, catechol, and L-DOPA. Laccase was also immobilized on porous PANI nanofibers in a three-step process of enzyme adsorption, precipitation, and crosslinking for detection of phenolic compounds. The biocatalyst retained 100% of its initial activity after 6 days of storage at room temperature and 74 after 3 h of incubation at 60 °C [556]. When the biocatalyst was used as a cathode in an ABTS mediated biofuel cell with glucose oxidase-PANI nanofibers as the anode, the biofuel cell demonstrated a power density of 37.4 μW/cm2 and retained 54% and 70% of its initial power density after 28 days of storage at room temperature and 4 °C, respectively. PANI has been combined with other compounds such as poly (methyl methacrylate) [557], magnetic graphene [338], MWNTs [558], graphene oxide [559], and cellulose [329].
7.2.3.2.3. Poly (vinyl alcohol) (PVA)
PVA based materials have been used to entrap enzymes especially for biosensor applications due to their low cost, stability, recyclability, biocompatibility, and easy manipulation with different activation methods for enzyme immobilization [377, 560, 561]. Also PVA polymers are reported to have high mechanical strength which is an important attribute for an enzyme support [118]. Different forms of PVA such as particles [372], beads [118], cryogels [431], hydrogels [562, 563], and microspheres [560] have been used to entrap laccase. For example, Gonzalez-Coronel et al. [434], entrapped laccase in Lentikats (lentil shaped particles of PVA) and obtained 89% activity recovery, 0% leaching, and 90% retained activity after 84 h. Laccase entrapped in PVA microspheres demonstrated stability on organic solvents (methanol, ethanol, acetone, and DMSO) for concentrations up to 40% [560]. However, PVA is susceptible to deformation at higher temperatures hence the biocatalyst can only be used for low temperature reactions [560]. To overcome that, PVA has been combined with other supports such as halloysite nanotubes [564], metal organic frameworks [561], alginate [377], xylan [565] and mesoporous silica [328].
7.2.3.2.4. Nafion
Nafion is a polyanion with a hydrophobic fluorocarbon backbone and hydrophilic cation-exchange sites making it moderately hydrophobic [348, 566]. It is usually used in biosensors and biofuel cells to provide a high adsorption for laccase and to prevent enzyme leaching from the electrode surface as well as undesirable side reactions [567, 568, 569]. Nafion is preferred in biosensors because its negatively charged polyelectrolyte matrix eliminates the influence of interfering signals hence improving the sensitivity of the sensor [323]. For example, Litescu et al. [327], drop-casted a laccase solution on a screen printed electrode, allowed it to dry with forced air followed by immobilization of a Nafion membrane to protect the enzyme from leaching. Đurđić et al. [325], drop-coated laccase on a screen printed carbon electrode modified with graphene nanoplates @MnO2 nanoparticles, followed by drop-coating of Nafion solution and allowing to dry. Nafion has been reported to improve the long-term stability, enhance the adhesion and binding force, and the selectivity of a biosensor [326]. Choi et al. [570], dispersed MWNTs in Nafion solution to form MWNTs/Nafion composite which was then used to immobilize laccase covalently using EDC/NHS. The MWNTs/Nafion nanocomposite provided a microporous structure with a large surface area for laccase immobilization.
7.2.3.2.5. Mixtures and hybrids of polymers
Because polymers suffer from low mechanical strength and ease of microbial attack, new techniques have been developed to enhance their mechanical properties such as crosslinking, mixing the polymers with other polymers or inorganic materials [198], graft copolymerization, and use of interpenetrating polymer networks (IPNs). Supports of polymers mixed with other materials such as Alginate-carbon beads [571], polyacrylamide-alginate cryogels [572], alginate-gelatin Mixed Gel [162], polyvinyl-alginate-silicon dioxide matrix [130], poly (amido amine) (PAMAM) dendrimers [534], gelatin blended with polyethylene glycol [544], butyl acrylate, and ethylene glycol [390] have been reported for laccase immobilization with improved immobilization yields and efficiencies compared to the supports from polymers alone. Graft copolymerization is the addition of functional groups to a polymer through covalent bonding of monomers onto the polymer chain to improve its mechanical properties and stability [521, 546]. The properties of the resultant copolymer combine properties of the prepolymer and the grafted molecule and these properties can be regulated by varying the concentrations of initiators, crosslinkers, and the prepolymer [573]. IPNs are combinations of two polymers in network form in which one polymer is synthesized and/or crosslinked in the presence of another [245, 402].
Smart polymers have also been utilized to immobilize laccase with the hope of retaining its activity over a wider range of pH, temperature, and ionic strength [365]. For example, Klis et al. [321], covalently immobilized laccase on poly (N-isopropyacrylamide) gel attached to a dimethoxyloxyvinylsilnae silanized ITO electrode, and determined its redox and catalytic properties at varying sizes of the gel matrix. It was observed that the catalytic efficiency decreased with shrinking gel size but the enzyme structure remained unchanged.
Crosslinking of polymers to form gels (hydrogels and cryogels) for entrapment of laccase has also been reported as the gels present improved resistance to thermal and chemical inactivation, remarkable storage, and operational stability [573]. For example, laccase was covalently immobilized on a composite of methyl methacrylate (MMA)/butyl acrylate (BA) copolymer latexes, and rGO hydrogels. The composite hydrogels were synthesized by self-assembly of graphene oxide nanoparticles in the polymer latex matrix where the polymer nanoparticles were adsorbed on the rGO surface [487]. Hydrogels are soft and highly hydrated polymeric networks synthesized via covalent or ionic crosslinking of monomers with or without metal ions [574]. Entrapment of enzymes in hydrogel leads to secondary interactions between the enzyme and the polymeric matrix which may cause a shift of the enzyme maximum activity to a higher pH than the free enzyme [402]. And since the retention and expression of enzyme activity are closely related to the particle size, porosity, and polarity of the supporting matrix, the structure of the hydrogel needs to be optimized for maximum enzyme activity [573]. Electron-beam grafting techniques have also been explored to form gels in the absence of initiators and crosslinkers. For example, Jahangiri et al. [575], used electron beam irradiation on a mixture of poly (vinylidene fluoride) membranes and laccase to form membrane-laccase bioreactors and on a mixture of macroporous polymeric gels, laccase, syringaldehyde, and ABTS to form cryogel bioreactors for degradation of bisphenol A. By using high-energy electron radiation cryogels can be synthesized without additional initiators or cross-linkers within 10 min (excl. freezing time) as larger bulk materials allowing furthermore the simultaneous incorporation of macromolecules in a one-step process.
7.3. Electrodes
Laccase has been immobilized on electrodes for application in biosensors and biofuel cells [3]. An immobilization strategy that maximizes coverage of enzyme on the electrode surface with high operational stability and ensures maximum electron transfer between the redox sites of the enzyme and the electrode surface and minimum loss of enzyme from the electrode surface through leaching are the most important aspects considered during immobilization [301, 344]. Therefore the development of biosensors based on enzyme modified electrode surfaces in such a way that the enzymes’ full activity and stability are retained has become an important aspect in various fields ranging from environmental analysis to clinical diagnosis [343, 481]. Immobilization of enzymes on electrode surfaces should ensure that the redox centres of the enzyme are as close as possible to the electrode surface to allow rapid electron transfer between the enzyme and electrode [101, 322]. The immobilization method is also an important factor as it should provide proper orientation of the enzyme efficient electronic contact with the electrode [576].
Various immobilization techniques such as physical adsorption and covalent immobilization have been explored. For example, Haghighi et al. [87], adsorbed T. versicolor laccase on spectrographic graphite electrode for use in an amperometric flow through cell to monitor phenolic compounds in a single line flow injection system. However, when enzymes are directly adsorbed on electrodes, there is poor electronic communication between the enzyme redox centers and the electrode surface hence electron mediators that can shuttle electrons between the enzyme and electrode conductive surface are added [39, 292, 576]. Chemical modification of electrode surfaces with appropriate functional groups for covalent linkage has also been explored [44, 577]. For example, Quan et al. [46], covalently immobilized laccase on silane modified platinum electrode surface and used it as a biosensor for ABTS, p-phenylenediamine (PPD), and p-aminophenol (PAP). The sensor gave a response time of 2 s for PPD and PAP and 6 s for ABTS. When the pH of the media was changed, the redox potentials for PPD and PAP changed by 60 mV per 1 unit of pH and no change in redox behaviour for ABTS was observed. Freire et al. [481], immobilized laccase on carbon-fibre electrodes using physical adsorption, GA, carbodiimide, and carbodiimide/GA approaches. The highest biosensor activity was obtained using carbodiimide/GA for coupling laccase to carboxyl groups on carbon fibres.
Entrapping laccase in other materials including Montmorillonite [342], Nafion [164], and PANI [318] is utilized to prevent leaching of the enzyme into the solution and cracking of the enzyme matrix. For example Li et al. [578], entrapped laccase in Poly (3,4-Ethylenedioxythiophene) (PEDOT) on a Pt electrode through electropolymerization of EDOT and laccase solution from 0.2 to 1.3 V at a scan rate of 100 mV/s. Rochefort et al. [322], used interfacial condensation of polyethyleneimine (PEI) to prepare microcapsules containing laccase. The laccase was mixed with PEI in an aqueous phase that was emulsified in an organic phase prior to addition of a water-insoluble crosslinking agent. The microcapsules were adsorbed onto a GCE and the charged micro-environment offered by the microcapsules led to a shift in optimum pH and half saturation constant (Km) of the enzyme and storage stability of 73% activity after six months of storage. Palys et al. [579], entrapped laccase in poly-o-phenylenediamine (POPDA) on a GCE and the POPDA acted as an immobilizing matrix as well as a redox mediator during reduction of molecular oxygen. This approach displayed minimal distortion of the three-dimensional structure of the enzyme and gave comparable current density values to those obtained when other mediators were used and higher values compared to mediator-less electrode systems.
For efficient contact between the enzyme and electrodes, mediators are sometimes added and are either dissolved in the analyte solution or co-immobilized together with the enzyme [580, 581]. For example, Nogala et al. [582], co-immobilized laccase and ABTS on a silicate carbon ceramic electrode for biolectrocatalytic reduction of dioxygen. This allowed close contact of the mediator and enzyme hence efficient electro transfer and electrocatalysis was achieved. However, the electrode suffered immensely from laccase deactivation by ABTS radical. Brunel et al. [292], entrapped laccase and ABTS on polypyrrole polymer through electropolymerization by controlled-potential electrolysis in the presence of a mixture of biomolecules and monomer solution and used it as a cathode in a glucose/O2 biofuel cell. When laccase was immobilized on mesoporous silica (MCM-41) support modified with methylene blue dye (MB) as a mediator and used as a biosensor for detection of phenols, the MB-MCM-41 modified electrode displayed a shorter response time (less than 4s) than the MCM-41 modified electrode without MB as a mediator (15 s) [346]. Laccase-mediator co-immobilized bioconjugates have been reviewed elsewhere [583]. The advantages of systems with co-immobilized mediators are that the rate of reaction is not limited by the mediator diffusion leading to higher bioelectrocatalytic current values, there is minimal pollution from residual enzyme and mediator in solution, an appropriate choice of mediator can be used to tune the potential of the reaction, and there is facilitated recovery and reuse of the mediator [579, 580, 584]. However, drawbacks such as high cost, irreversible oxidation/reduction of mediators, leaching into the reaction system, and sometimes generation of toxic byproducts have to be considered prior to selection of a mediator [575].
Other than electrodes, laccase has been explored in bioactive paper. For example, Savolainen et al. [585], entrapped Trametes sp. laccase in PEI microcapsules via interfacial polycondesation of PEI with sebacoyl chloride cross linker. The microcapsules were incorporated into an ink and coated on paper substrate to form bioactive paper. A similar approach was utilized by Guerrero et al. [407], and in both cases, it was observed that the use of microencapsulation allows better activity retention of laccase on papers over time at room temperature and decrease the rate of laccase inhibition in presence of inhibitors.
7.4. Metal organic frameworks (MOFs)
MOFs are mesoporous structures consisting of metal ions or clusters coordinated to organic ligands [365]. They are synthesized by diffusion of a combination of metals and organic skeletons [586]. They are associated with ultrahigh and tuneable porosities, large surface areas, multiple functional sites, structural flexibility, chemical, and thermal stability [587]. More so, their organic bridging ligand can be synthetically modified to introduce desired functional groups to the framework [588, 589]. MOFs have been synthesized with various metals and metal oxides such as copper [586], iron [590], zirconium [588], and zeolite [587] for laccase immobilization. Ladole et al. [591], reported synthesis of magnetic MOFs (MMOFs) consisting of peroxidase and magnetic nanoparticles. Laccase was embedded in the MMOFs to form a biocatalyst of 100 nm average diameter, high thermal stability, and recyclability of 89% residual activity after 10 cycles, and storage stability of 100% residual activity after 30 days. Entrapment of laccase in nanocrystalline NH2-MIL-53 (Al) demonstrated high enzyme loading, permanent retention of the enzyme and stability. The bioconjugate was used for the removal of bisphenol A from water and it achieved 100% removal after 3 min [220]. Immobilization of laccase on MOFs has been extensively reviewed elsewhere [592].
7.5. Nanomaterials
Various inorganic and organic nanomaterials such as silica nanoparticles [593], Manganese ferrite nanoparticles [594], copper ferrite nanoparticles [595], Titanium oxide nanoparticles [256], Halloysite nanotubes (HNTs) [178, 295], Graphene nanosheets [110], ZnO nanoparticles [596], carbon nanotubes and nanospheres [206, 310, 415], gold nanoparticles, and metal oxide nanoparticles have been used as carriers to immobilize enzymes. The advantages associated with immobilizing enzymes on nanomaterials are: they provide high surface area for enzyme attachment and high effective enzyme loading, high mechanical resistance, and their similar size to enzymes ensures minimal diffusion limitations and a reduced mass transfer resistance [75, 192, 597]. Therefore, they provide an ideal solution to the challenges encountered in optimization of enzyme immobilization on nonporous supports and provide a transition between homogenous and heterogeneous catalysis [398]. These nanomaterial are available in different sizes, shapes and compositions, and retain their characteristics after functional activation even when very harsh chemical modifications are carried out [386]. More so, when combined with enzymes, they do not interfere with the active site hence preserving the biological activity of the enzyme [412].
Magnetic nanoparticles (MNPs) have been widely studied due to their superparamagnetism and ease of separation from the reaction mixture using an applied magnetic field [192, 433, 598]. This improves their reutilization since there is no need to for energy and time consuming centrifugation and filtration steps and the enzymes are subjected to very low mechanical stress compared to when nonmagnetic nanomaterials are used [400, 414, 599]. Nevertheless, bare magnetic nanoparticles are highly susceptible to acidic and oxidative conditions and when directly exposed to the biological system, they influence the stability and activity of the enzyme [227, 446, 600]. To improve their functionality, they are entrapped in other compounds such as chitosan [184, 446], carbon [227], PEI [302, 414], and graphene oxide [422] and then functionalized for enzyme bonding. For example, Kalkan et al. [599], coated Fe3O4 (magnetic) nanoparticles with chitosan followed by activating the hydroxyl groups of chitosan with carbodiimide or cyanuric chloride for covalent binding with laccase. Fernandes et al. [75], functionalized silica-coated magnetite NPs with (trimethoxysilylpropyl)ethylenediamine triacetic acid (EDTA-TMS) and chelated with copper (II) ions as a metal ligand for laccase immobilization. PEI modified Fe3O4 nanoparticles (Fe3O4–NH2–PEI NPs) were chelated with Cu2+ to immobilize laccase through metal affinity adsorption [414].
Silica based nanoparticles are also highly suitable for enzyme immobilization due to their environmental friendliness, high biocompatibility, and resistance towards organic solvents and microbial attacks [386, 593]. Silica based nanoparticles have been widely reported for immobilization of laccase enzyme [74, 114, 188, 281, 462]. Laccase was immobilized by sorption on amino-modified silica nanoparticles with subsequent covalent crosslinking using glutaraldehyde. An immobilization efficiency of 98.2% was obtained and subsequently retained 63% activity after 32 days incubation in phosphate buffer at pH 7 and 77% after 25 days incubation in real waste water [158].
Electrospun nanofibers from polymeric materials such as cellulose [168], poly (ε-caprolactone) [601], polyacrylonitrile [251], and Poly (lactic-co-glycolic acid) [175] have gained attention as well due to their large surface area, high porosity biocompatibility with tissue and cells, and a subcellular size that makes them good carriers for enzymes [521, 602]. These nanofibers offer minimal mass transfer limitations, low environmental risk, and their surface and texture can be modified to suit a specific enzyme [191, 200]. Zdarta et al, [205] immobilized laccase on poly (methyl methacrylate) (PMMA)/Fe3O4 electrospun nanofibers through covalent binding and entrapment. The biocatalyst exhibited excellent pH, thermal and storage stability, and high enzyme loading and reusability where 80% activity was retained after 40 days of storage and 5 successive biocatalytic cycles.
Composites consisting of two or more nanomaterials have also been studied for laccase immobilization. For example, Poly (hydroxyethyl methacrylate-N-methacryloly-(l)-histidinemethylester)-copper (II) [PHEMAH-Cu2+] nanospheres [398], TiO2-Montmorillonite (MT) complexes [385], Fe3O4/SiO2 nanoparticles [230, 231], Polyacrylonitrile-biochar composite nanofibrous membrane [180], SWNT/nanocrystalline carbon quantum dots [336], CS/PVA composite nanofibrous membranes [191], ZnO/SiO2 nano-composited [243], Zinc tetraaminophthalocyanine-Fe3O4 nanoparticle composite [394], and copper tetra-aminophthalocyanine (CuTAPc)-Fe3O4 [296,392,603].
Nonmagnetic nanoparticles have been combined with other processes to improve their reusability especially in waste water treatment [597]. For example titanium oxide nanoparticles have been mixed with polyvinylidene fluoride (PVDF) [169] and polyethersulfone (PES) membranes [597]. Cao et al. [428], coated a polydopamine/gold nanoparticles hybrid layer on the surface of silica shell encapsulated n-docosane through surfactant-assisted self-assembly and in-situ reduction of Au ions to form self-thermoregulatory microcapsules for laccase immobilization.
As shown in Figure 7, immobilization of laccase on supports improves its storage stability as immobilized laccase generally retains over 80% as compared to free laccase that retains less than 40% over time. Most of the laccase completely lost its activity within a period of 4 months (not shown on the graph). It is noteworthy that further immobilization of the biocatalyst on electrodes further enhances the storage stability with retained activities of over 80% for over 4 months. NPs and NCs facilitate high enzyme activity retention regardless of the laccase source even for periods of over 4 months. This could be due to their small size that allows minimal diffusion limitations hence enabling the enzyme to behave as if it were free and at the same time with the protection from denaturation by the polymer.
Figure 7.
A graph showing the storage stability (percentage retained activity after storage) of free and immobilized laccase on various supports after 1 month, 1–4 months and over 4 months.
On top of storage stability, the reusability of the biocatalyst also has to be considered for industrial applications. Although polymers provide favourable storage stability over time, the reusability is limited to a few cycles (less than 50). This can be improved by impregnating the polymers with metal NPs to form metal-polymer nanocomposites to allow free expression of the enzyme with maximum protection from the polymer. This is evident in Figure 8 as the NCs and NPs supports allow reusability for the enzyme retaining high activity for over 50 cycles. Furthermore, immobilization of the supports on electrodes shows remarkable retained activities for more cycles. For industrial applications, a support matrix that retains the enzyme activity, allows good storage stability and reusability for longer periods of time is desirable and all these factors need to be put into consideration when selecting an immobilization support.
Figure 8.
A graph showing reusability (retained percentage activity) of laccase immobilized on various supports after 1–5 cycles, 5–10 cycles, 10–50 cycles and over 50 cycles.
8. Co-immobilization of laccase with other enzymes and substrates
Simultaneous immobilization of laccase with another or more enzymes has been explored to enhance the sensitivity and extend the substrate spectrum of biosensors [604, 605]. For example, Montereali et al. [606], reported a lower limit of detection and a wider range of phenolic compounds detected when a biosensor of co-immobilized laccase and tyrosinase on a ferrocene modified graphite screen printed electrode was used as compared to the individual laccase and tyrosinase biosensors. Moeder et al. [403], co-immobilized laccase and horse radish peroxidase (HRP) on a microporous polypropylene hollow fibre membrane by physical entrapment and the bioconjugate was used in the degradation of 4-ethylphenol, 3,4-dimethylphenol, tetralol, and 4-hydroxybiphenyl from water samples. Quan et al. [607], co-immobilized tyrosinase and laccase on a platinum electrode for use as a biosensor for PPD and p-chlorophenol. The sensitivity of the bi-enzyme sensor increased by 70% for PPD compared to only when the laccase sensor was used but the sensitivity of p-chlorophenol decreased by 40%. The increase in sensitivity towards PPD was due to additional catalytic function of the co-immobilized tyrosinase while the decrease in p-chlorophenol sensitivity was due to the blocking effect of the co-immobilized laccase which hinders mass transport through the immobilization layer. Similarly, ElKaoutit et al. [604], co-immobilized laccase and tyrosinase phenoxidase Sonogel–Carbon electrode using glutaric dialdehyde as crosslinker and nafion-ion exchanger as a protective additive. The electrode was used in a biosensing system to detect polyphenols in beer.
Co-immobilization of laccases from different sources has also been explored. For example, Vera et al. [608], co-immobilized laccase from Aspergillus sp., M. thermophile, and T. versicolor on poly (glycidyl methacrylate) microspheres. The biocatalyst showed a wide optimal pH range (2.0–7.0), high thermal, mechanical, and storage stabilities compared to the free and single-immobilized laccases.
9. Concluding remarks and future perspectives
Laccase has been widely studied due to its availability in fungi, plants, microorganisms, and insects. Since the enzyme is obtained from various sources, it demonstrates diversity in the composition of its carbohydrate moiety thus having varying secondary structures. This in turn affects the orientation of the enzyme active site hence different substrates are oxidized by different laccases from various sources. Nevertheless, laccase generally oxidizes a wide range of ortho and para substituted phenolic compounds using only molecular oxygen as the secondary substrate. This unique feature has allowed the study of the enzyme's use in various biotechnological applications including waste water treatment, dye decolourisation, paper and pulp industry, biofuel cells, pharmaceutical industry, and biosensors. However, most of the previous studies focused on the degradation of pollutants in aqueous solution which does not fully represent real industrial or environmental settings. Although the enzyme shows promising results in degradation of single organic pollutants, the complex physicochemical properties of the industrial effluents and surface water in the environment may not favour the smooth catalytic operation of the enzyme. The performance of the immobilized enzyme therefore needs to be evaluated in real wastewater, industrial effluents, and environmental conditions. More so, most of the studies have been done under batch conditions and yet continuous operations are preferred especially in industrial settings. It is therefore imperative to evaluate the performance of the biocatalyst under continuous operation while determining the optimal operational conditions for the process before industrial application can be realised.
Various supports ranging from organic to inorganic, porous to non-porous, and agricultural and industrial wastes have been used to immobilize laccase as discussed above. Since the performance of the biocatalyst depends on the type and structure of the enzyme, nature of the support, immobilization method and conditions, and intended use of the biocatalyst, consideration have to be made to maximize the catalytic and non-catalytic characteristics of the final biocatalyst.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Ms Hilda Dinah Kyomuhimbo and Hendrik G Brink was supported by National Research Foundation (NRF) of South Africa [MND210426597525 and 145848].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2023.e13156.
Appendix ASupplementary data
The following is the supplementary data related to this article:
References
- 1.Alcalde M. In: Ind. Enzym. Struct. Funct. Appl. Polaina J., MacCabe A.P., editors. Springer Netherlands; Dordrecht, Laccases: 2007. pp. 461–476. (Biological Functions, Molecular Structure and Industrial Applications). [Google Scholar]
- 2.Thurston C.F.Y. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 3.Hyung K.H., Jun K.Y., Hong’ H.-G., Kim’ H.S. Immobilization of laccase onto the gold electrode using -mercaptopropionate. Bull. Kor. Chem. Soc. 1997;18:564–566. [Google Scholar]
- 4.Held C., Kandelbauer A., Schroeder M., Cavaco-Paulo A., Guebitz G.M. Biotransformation of phenolics with laccase containing bacterial spores. Environ. Chem. Lett. 2005;3:74–77. [Google Scholar]
- 5.Madhavi V., Lele S.S. Laccase: properties and applications. Bioresources. 2009;4:1694–1717. [Google Scholar]
- 6.Mayer A.M., Staples R.C. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- 7.Ryan S., Schnitzhofer W., Tzanov T., Cavaco-Paulo A., Gübitz G.M. An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzym. Microb. Technol. 2003;33:766–774. [Google Scholar]
- 8.Rekuć A., Jastrzembska B., Liesiene J., Bryjak J. Comparative studies on immobilized laccase behaviour in packed-bed and batch reactors. J. Mol. Catal. B Enzym. 2009;57:216–223. [Google Scholar]
- 9.Wan Y., Du Y., Miyakoshi T. Enzymatic catalysis of 2,6-dimethoxyphenol by laccases and products characterization in organic solutions. Sci. China, Ser. B: Chem. 2008;51:669–676. [Google Scholar]
- 10.Mateescu M.-A., Agostinelli E., Weltrowska G., Weltrowski M., Mondovi B. Specific immobilization of laccase on p-benzoquinone-activated supports. Biol. Met. 1990;3:98–102. [Google Scholar]
- 11.Jolivalt C., Brenon S., Caminade E., Mougin C., Pontié M. Immobilization of laccase from Trametes versicolor on a modified PVDF microfiltration membrane: characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membr. Sci. 2000;180:103–113. [Google Scholar]
- 12.Minussi R.C., Pastore G.M., Durán N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002;13:205–216. [Google Scholar]
- 13.Luterek J., Gianfreda L., Wojtaś-Wasilewska M., Cho N.S., Rogalski J., Jaszek M., Malarczyk E., Staszczak M., Fink-Boots M., Leonowicz A. Activity of free and immobilized extracellular Cerrena unicolor laccase in water miscible organic solvents. Holzforschung. 1998;52:589–595. [Google Scholar]
- 14.Claus H., Faber G., König H. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl. Microbiol. Biotechnol. 2002;59:672–678. doi: 10.1007/s00253-002-1047-z. [DOI] [PubMed] [Google Scholar]
- 15.Mazlan S.Z., Lee Y.H., Hanifah S.A. A new laccase based biosensor for tartrazine. Sensors. 2017;17:2859. doi: 10.3390/s17122859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogalski J., Dawidowicz A., Jóźwik E., Leonowicz A. Immobilization of laccase from Cerrena unicolor on controlled porosity glass. J. Mol. Catal. B Enzym. 1999;6:29–39. [Google Scholar]
- 17.Fernando Bautista L., Morales G., Sanz R. Immobilization strategies for laccase from Trametes versicolor on mesostructured silica materials and the application to the degradation of naphthalene. Bioresour. Technol. 2010;101:8541–8548. doi: 10.1016/j.biortech.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Osiadacz J., Al-Adhami A.J.H., Bajraszewska D., Fischer P., Peczyñska-Czoch W. On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J. Biotechnol. 1999;72:141–149. [Google Scholar]
- 19.Durán N., Rosa M.A., D’Annibale A., Gianfreda L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzym. Microb. Technol. 2002;31:907–931. [Google Scholar]
- 20.Xu F., Berka R.M., Wahleithner J.A., Nelson B.A., Shuster J.R., Brown S.H., Palmer A.E., Solomon E.I. Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem. J. 1998;334:63–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgieva S., Godjevargova T., Portaccio M., Lepore M., Mita D.G. Advantages in using non-isothermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera. J. Mol. Catal. B Enzym. 2008;55:177–184. [Google Scholar]
- 22.Qiu L., Huang Z. The treatment of chlorophenols with laccase immobilized on sol–gel-derived silica. World J. Microbiol. Biotechnol. 2010;26:775–781. [Google Scholar]
- 23.Bebić J., Banjanac K., Ćorović M., Milivojević A., Simović M., Marinković A., Bezbradica D. Immobilization of laccase from Myceliophthora thermophila on functionalized silica nanoparticles: optimization and application in lindane degradation. Chin. J. Chem. Eng. 2020;28:1136–1144. [Google Scholar]
- 24.Mousty C., Vieille L., Cosnier S. Laccase immobilization in redox active layered double hydroxides: a reagentless amperometric biosensor. Biosens. Bioelectron. 2007;22:1733–1738. doi: 10.1016/j.bios.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Yang W.Y., Min D.Y., Wen S.X., Jin L., Rong L., Tetsuo M., Bo C. Immobilization and characterization of laccase from Chinese Rhus vernicifera on modified chitosan. Process Biochem. 2006;41:1378–1382. [Google Scholar]
- 26.Areskogh D., Henriksson G. Immobilisation of laccase for polymerisation of commercial lignosulphonates. Process Biochem. 2011;46:1071–1075. [Google Scholar]
- 27.Liu X., Gillespie M., Ozel A.D., Dikici E., Daunert S., Bachas L.G. Electrochemical properties and temperature dependence of a recombinant laccase from Thermus thermophilus. Anal. Bioanal. Chem. 2011;399:361–366. doi: 10.1007/s00216-010-4345-9. [DOI] [PubMed] [Google Scholar]
- 28.Alvarado-Ramírez L., Rostro-Alanis M., Rodríguez-Rodríguez J., Castillo-Zacarías C., Sosa-Hernández J.E., Barceló D., Iqbal H.M.N., Parra-Saldívar R. Exploring current tendencies in techniques and materials for immobilization of laccases – a review. Int. J. Biol. Macromol. 2021;181:683–696. doi: 10.1016/j.ijbiomac.2021.03.175. [DOI] [PubMed] [Google Scholar]
- 29.Nair R.R., Demarche P., Agathos S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. N. Biotech. 2013;30:814–823. doi: 10.1016/j.nbt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Shraddha, Shekher R., Sehgal S., Kamthania M., Kumar A. Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011;1–11 doi: 10.4061/2011/217861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaropolov A.I., Skorobogat’K V., Vartanov S.S. Catalytic .mechanism, and applicability. Appl. Biochem. Biotechnol, Properties. 1994;49:257–280. [Google Scholar]
- 32.Ba S., Arsenault A., Hassani T., Jones J.P., Cabana H. Laccase immobilization and insolubilization: from fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit. Rev. Biotechnol. 2013;33:404–418. doi: 10.3109/07388551.2012.725390. [DOI] [PubMed] [Google Scholar]
- 33.Kim D.H., Rigling D., Zhang L., Van Alfen N.K. A new extracellular laccase of Cryphonectria parasitica is revealed by deletion of lac 1. Mol. Plant Microbe Interact. 1995;8:259–266. [Google Scholar]
- 34.Wahleithner J.A., Xu F., Brown K.M., Brown S.H., Golightly E.J., Halkier T., Kauppinen S., Pederson A., Schneider P. The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr. Genet. 1996;29:395–403. doi: 10.1007/BF02208621. [DOI] [PubMed] [Google Scholar]
- 35.Marbach I., Harel E., Mayer A.M. Phytochemistry. Molecular properties of extracellular Botrytis cinerea laccase. 1984;23:2713–2717. [Google Scholar]
- 36.Fukushima Y., Kirk T.K. Laccase component of the Ceriporiopsis subvermispora lignin-degrading system. Appl. Environ. Microbiol. 1995;61:872–876. doi: 10.1128/aem.61.3.872-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J., Kwan H.S. Characterization, molecular cloning, and differential expression analysis of laccase genes from the edible mushroom lentinula edodes. Appl. Environ. Microbiol. 1999;65:4908–4913. doi: 10.1128/aem.65.11.4908-4913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durán N., Esposito E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl. Catal. B Environ. 2000;28:83–99. [Google Scholar]
- 39.Klis M., Maicka E., Michota A., Bukowska J., Sek S., Rogalski J., Bilewicz R. Electroreduction of laccase covalently bound to organothiol monolayers on gold electrodes. Electrochim. Acta. 2007;52:5591–5598. [Google Scholar]
- 40.Zhang Y., Rochefort D. Activity, conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine) microcapsules for immobilization in paper. Process Biochem. 2011;46:993–1000. [Google Scholar]
- 41.Palmieri G., Giardina P., Bianco C., Scaloni A., Capasso A., Sannia G. A novel white laccase from Pleurotus ostreatus. J. Biol. Chem. 1997;272:31301–31307. doi: 10.1074/jbc.272.50.31301. [DOI] [PubMed] [Google Scholar]
- 42.Singh G., Bhalla A., Kaur P., Capalash N., Sharma P. Laccase from prokaryotes: a new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011;10:309–326. [Google Scholar]
- 43.Kudanga T., Nyanhongo G.S., Guebitz G.M., Burton S. Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzym. Microb. Technol. 2011;48:195–208. doi: 10.1016/j.enzmictec.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Pita M., Gutierrez-Sanchez C., Olea D., Velez M., Garcia-Diego C., Shleev S., Fernandez V.M., De Lacey A.L. High redox potential cathode based on laccase covalently attached to gold electrode. J. Phys. Chem. C. 2011;115:13420–13428. [Google Scholar]
- 45.Wu F., Su L., Yu P., Mao L. Role of organic solvents in immobilizing fungus laccase on single-walled carbon nanotubes for improved current response in direct bioelectrocatalysis. J. Am. Chem. Soc. 2017;139:1565–1574. doi: 10.1021/jacs.6b11469. [DOI] [PubMed] [Google Scholar]
- 46.Quan D., Kim Y., Shin W. Characterization of an amperometric laccase electrode covalently immobilized on platinum surface. J. Electroanal. Chem. 2004;561:181–189. [Google Scholar]
- 47.Guzik U., Hupert-Kocurek K., Wojcieszyńska D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules. 2014;19:8995–9018. doi: 10.3390/molecules19078995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hublik G., Schinner F. Characterization and immobilization of the laccase from Pleurotus ostreatus and its use for the continuous elimination of phenolic pollutants. Enzym. Microb. Technol. 2000;27:330–336. doi: 10.1016/s0141-0229(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 49.Gianfreda L., Xu F., Bollag J.-M. Laccases: a useful group of oxidoreductive enzymes. Ann. Finance. 1999;3:1–26. [Google Scholar]
- 50.Shleev S., Pita M., Yaropolov A.I., Ruzgas T., Gorton L. Direct heterogeneous electron transfer reactions of Trametes hirsuta laccase at bare and thiol-modified gold electrodes. Electroanalysis. 2006;18:1901–1908. [Google Scholar]
- 51.Deska M., Kończak B. Immobilized fungal laccase as “green catalyst” for the decolourization process – state of the art. Process Biochem. 2019;84:112–123. [Google Scholar]
- 52.Leontievsky A.A., Vares T., Lankinen P., Shergill J.K., Pozdnyakova N.N., Myasoedova N.M., Kalkkinen N., Golovleva L.A., Cammack R., Thurston C.F., Hatakka A. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol. Lett. 1997;156:9–14. doi: 10.1111/j.1574-6968.1997.tb12698.x. [DOI] [PubMed] [Google Scholar]
- 53.Xu X., Huang X., Liu D., Lin J., Ye X., Yang J. Inhibition of metal ions on Cerrena sp. laccase: kinetic, decolorization and fluorescence studies. J. Taiwan Inst. Chem. Eng. 2018;84:1–10. [Google Scholar]
- 54.Rodríguez Couto S., Sanromán Ma., Gübitz G.M. Influence of redox mediators and metal ions on synthetic acid dye decolourization by crude laccase from Trametes hirsuta. Chemosphere. 2005;58:417–422. doi: 10.1016/j.chemosphere.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 55.Juárez-Gómez J., Rosas-Tate E.S., Roa-Morales G., Balderas-Hernández P., Romero-Romo M., Ramírez-Silva M.T. Laccase inhibition by mercury: kinetics, inhibition mechanism, and preliminary application in the spectrophotometric quantification of mercury ions. J. Chem. 2018 [Google Scholar]
- 56.Keum Y.-S., Li Q.X. Copper dissociation as a mechanism of fungal laccase denaturation by humic acid. Appl. Microbiol. Biotechnol. 2004;64:588–592. doi: 10.1007/s00253-003-1460-y. [DOI] [PubMed] [Google Scholar]
- 57.Enaud E., Trovaslet M., Naveau F., Decristoforo A., Bizet S., Vanhulle S., Jolivalt C. Laccase chloride inhibition reduction by an anthraquinonic substrate. Enzym. Microb. Technol. 2011;49:517–525. doi: 10.1016/j.enzmictec.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Kepp K.P. Halide binding and inhibition of laccase copper clusters: the role of reorganization energy. Inorg. Chem. 2015;54:476–483. doi: 10.1021/ic5021466. [DOI] [PubMed] [Google Scholar]
- 59.Di Bari C., Mano N., Shleev S., Pita M., De Lacey A.L. Halides inhibition of multicopper oxidases studied by FTIR spectroelectrochemistry using azide as an active infrared probe. J. Biol. Inorg Chem. 2017;22:1179–1186. doi: 10.1007/s00775-017-1494-8. [DOI] [PubMed] [Google Scholar]
- 60.Johannes C., Majcherczyk A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000;78:193–199. doi: 10.1016/s0168-1656(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 61.Johnson D.L., Thompson J.L., Brinkmann S.M., Schuller K.A., Martin L.L. Electrochemical characterization of purified Rhus vernicifera laccase: voltammetric evidence for a sequential four-electron transfer. Biochemistry. 2003;42:10229–10237. doi: 10.1021/bi034268p. [DOI] [PubMed] [Google Scholar]
- 62.Raseda N., Hong S., Kwon O.Y., Ryu K. Kinetic evidence for the interactive inhibition of laccase from Trametes versicolor by pH and chloride. J. Microbiol. Biotechnol. 2014;24:1673–1678. doi: 10.4014/jmb.1408.08012. [DOI] [PubMed] [Google Scholar]
- 63.Rogalski J., Jóźwik E., Hatakka A., Leonowicz A. Immobilization of laccase from Phlebia radiata on controlled porosity glass. J. Mol. Catal. Chem. 1995;95:99–108. [Google Scholar]
- 64.Cabana H., Jones J.P., Agathos S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007;132:23–31. doi: 10.1016/j.jbiotec.2007.07.948. [DOI] [PubMed] [Google Scholar]
- 65.Szamocki R., Flexer V., Levin L., Forchiasin F., Calvo E.J. Oxygen cathode based on a layer-by-layer self-assembled laccase and osmium redox mediator. Electrochim. Acta. 2009;54:1970–1977. [Google Scholar]
- 66.Tišma M., Šalić A., Planinić M., Zelić B., Potočnik M., Šelo G., Bucić-Kojić A. Production, characterisation and immobilization of laccase for an efficient aniline-based dye decolourization. J. Water Proc. Eng. 2020;36 [Google Scholar]
- 67.Jaiswal N., Pandey V.P., Dwivedi U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. Int. J. Biol. Macromol. 2016;86:288–295. doi: 10.1016/j.ijbiomac.2016.01.079. [DOI] [PubMed] [Google Scholar]
- 68.Merle G., Brunel L., Tingry S., Cretin M., Rolland M., Servat K., Jolivalt C., Innocent C., Seta P. Electrode biomaterials based on immobilized laccase. Application for enzymatic reduction of dioxygen. Mater. Sci. Eng. C. 2008;28:932–938. [Google Scholar]
- 69.Zawisza I., Rogalski J., Opallo M. Electrocatalytic reduction of dioxygen by redox mediator and laccase immobilized in silicate thin film. J. Electroanal. Chem. 2006;588:244–252. [Google Scholar]
- 70.Champagne P.-P., Ramsay J.A. Reactive blue 19 decolouration by laccase immobilized on silica beads. Appl. Microbiol. Biotechnol. 2007;77:819–823. doi: 10.1007/s00253-007-1208-1. [DOI] [PubMed] [Google Scholar]
- 71.Brandi P., D’Annibale A., Galli C., Gentili P., Pontes A.S.N. In search for practical advantages from the immobilisation of an enzyme: the case of laccase. J. Mol. Catal. B Enzym. 2006;41:61–69. [Google Scholar]
- 72.Mendoza L., Mamo G., Flores N., Gimenez A., Hatti-Kaul R. Laccase mediator system for activation of agarose gel: application for immobilization of proteins. J. Mol. Catal. B Enzym. 2011;68:270–274. [Google Scholar]
- 73.Husain Q. Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit. Rev. Biotechnol. 2006;26:201–221. doi: 10.1080/07388550600969936. [DOI] [PubMed] [Google Scholar]
- 74.Hu X., Wang P., Hwang H. Oxidation of anthracene by immobilized laccase from Trametes versicolor. Bioresour. Technol. 2009;100:4963–4968. doi: 10.1016/j.biortech.2009.03.089. [DOI] [PubMed] [Google Scholar]
- 75.Fernandes R.A., Daniel-da-Silva A.L., Tavares A.P.M., Xavier A.M.R.B. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem. Eng. Sci. 2017;158:599–605. [Google Scholar]
- 76.Peralta-Zamora P., Pereira C.M., Tiburtius E.R.L., Moraes S.G., Rosa M.A., Minussi R.C., Durán N. Decolorization of reactive dyes by immobilized laccase. Appl. Catal. B Environ. 2003;42:131–144. [Google Scholar]
- 77.Dodor D.E., Hwang H.-M., Ekunwe S.I.N. Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzym. Microb. Technol. 2004;35:210–217. [Google Scholar]
- 78.yaohua G., ping X., feng J., keren S. Co-immobilization of laccase and ABTS onto novel dual-functionalized cellulose beads for highly improved biodegradation of indole. J. Hazard Mater. 2019;365:118–124. doi: 10.1016/j.jhazmat.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 79.Reyes P., Pickard M.A., Vazquez-Duhalt R. Hydroxybenzotriazole increases the range of textile dyes decolorized by immobilized laccase. Biotechnol. Lett. 1999;21:875–880. [Google Scholar]
- 80.Shi L., Ma F., Han Y., Zhang X., Yu H. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J. Hazard Mater. 2014;279:203–211. doi: 10.1016/j.jhazmat.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 81.Jordaan J., Mathye S., Simpson C., Brady D. Improved chemical and physical stability of laccase after spherezyme immobilisation. Enzym. Microb. Technol. 2009;45:432–435. [Google Scholar]
- 82.Sondhi S., Kaur R., Kaur S., Kaur P.S. Immobilization of laccase-ABTS system for the development of a continuous flow packed bed bioreactor for decolorization of textile effluent. Int. J. Biol. Macromol. 2018;117:1093–1100. doi: 10.1016/j.ijbiomac.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Skoronski E., Fernandes M., Magalhães M.D.L.B., Da Silva G.F., João J.J., Soares C.H.L., Júnior A.F. Substrate specificity and enzyme recycling using chitosan immobilized laccase. Molecules. 2014;19:16794–16809. doi: 10.3390/molecules191016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun H., Huang W., Yang H., Zhang S. Co-immobilization of laccase and mediator through a self-initiated one-pot process for enhanced conversion of malachite green. J. Colloid Interface Sci. 2016;471:20–28. doi: 10.1016/j.jcis.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Fernández-Fernández M., Sanromán M.Á., Moldes D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013;31:1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 86.De Quan, Kim You-Sung, Shin, Woonsup, Yang Dang-Wei. Assembly of laccase over platinum oxide surface and application as an amperometric biosensor. Bull. Kor. Chem. Soc. 2002;23:385–390. [Google Scholar]
- 87.Haghighi B., Gorton L., Ruzgas T., Jönsson L.J. Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta. 2003;487:3–14. [Google Scholar]
- 88.Papadakis A.K., Roubelakis-Angelakis K.A. Oxidative stress could be responsible for the recalcitrance of plant protoplasts. Plant Physiol. Biochem. 2002;40:549–559. [Google Scholar]
- 89.Bleve G., Lezzi C., Mita G., Rampino P., Perrotta C., Villanova L., Grieco F. Molecular cloning and heterologous expression of a laccase gene from Pleurotus eryngii in free and immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2008;79:731. doi: 10.1007/s00253-008-1479-1. [DOI] [PubMed] [Google Scholar]
- 90.Sugumaran M., Barek H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016;17:1753. doi: 10.3390/ijms17101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun K., Li S., Si Y., Huang Q. Advances in laccase-triggered anabolism for biotechnology applications. Crit. Rev. Biotechnol. 2021;41:969–993. doi: 10.1080/07388551.2021.1895053. [DOI] [PubMed] [Google Scholar]
- 92.Asano T., Seto Y., Hashimoto K., Kurushima H. Mini-review an insect-specific system for terrestrialization: laccase-mediated cuticle formation. Insect Biochem. Mol. Biol. 2019;108:61–70. doi: 10.1016/j.ibmb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Kaluskar V.M., Kapadnis B.P., Jaspers Ch., Penninckx M.J. Production of laccase by immobilized cells of Agaricus sp.: induction effect of xylan and lignin derivatives. Appl. Biochem. Biotechnol. 1999;76:161–170. doi: 10.1385/abab:76:3:161. [DOI] [PubMed] [Google Scholar]
- 94.Ahn M.-Y., Zimmerman A.R., Martínez C.E., Archibald D.D., Bollag J.-M., Dec J. Technol, Characteristics of Trametes villosa laccase adsorbed on aluminum hydroxide. Enzym. Microb. Technol. 2007;41:141–148. [Google Scholar]
- 95.Hollmann F., Gumulya Y., Tölle C., Liese A., Thum O. Evaluation of the laccase from Myceliophthora thermophila as industrial biocatalyst for polymerization reactions. Macromolecules. 2008;41:8520–8524. [Google Scholar]
- 96.Sterjiades R., Dean J.F.D., Eriksson K.-E.L. Laccase from Sycamore Maple (acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 1992;99:1162–1168. doi: 10.1104/pp.99.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J., Wang C., Zhu M., Yu Y., Zhang Y., Wei Z. Generation and characterization of transgenic poplar plants overexpressing a cotton laccase gene. Plant Cell Tissue Organ Cult. 2008;93:303. [Google Scholar]
- 98.Kumar D., Khullar A., Sharma N., Gupta N. Immobilization of laccase from Bacillus subtilis DS on chitosan beads and standardization of process for biodegradation of textile dyes. J. Chem. Technol. Biotechnol. 2022;97:466–473. [Google Scholar]
- 99.Yatsu J., Asano T. Cuticle laccase of the silkworm, Bombyx mori: purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem. Mol. Biol. 2009;39:254–262. doi: 10.1016/j.ibmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Dittmer N.T., Gorman M.J., Kanost M.R. Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2009;39:596–606. doi: 10.1016/j.ibmb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shervedani R.K., Amini A. Direct electrochemistry of dopamine on gold—Agaricus bisporus laccase enzyme electrode: characterization and quantitative detection. Bioelectrochemistry. 2012;84:25–31. doi: 10.1016/j.bioelechem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Sharma D., Chaudhary R., Kaur J., Arya S.K. Greener approach for pulp and paper industry by Xylanase and Laccase. Biocatal. Agric. Biotechnol. 2020;25 [Google Scholar]
- 103.Sinirlioglu Z.A., Sinirlioglu D., Akbas F. Preparation and characterization of stable cross-linked enzyme aggregates of novel laccase enzyme from Shewanella putrefaciens and using malachite green decolorization. Bioresour. Technol. 2013;146:807–811. doi: 10.1016/j.biortech.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 104.Abadulla E., Tzanov T., Costa S., Robra K.-H., Cavaco-Paulo A., Gübitz G.M. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000;66:3357–3362. doi: 10.1128/aem.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Almansa E., Kandelbauer A., Pereira L., Cavaco-Paulo A., Guebitz G.M. Influence of structure on dye degradation with laccase mediator systems. Biocatal. Biotransform. 2004;22:315–324. [Google Scholar]
- 106.Rekuć A., Bryjak J., Szymańska K., Jarzębski A.B. Laccase immobilization on mesostructured cellular foams affords preparations with ultra high activity. Process Biochem. 2009;44:191–198. [Google Scholar]
- 107.Nam–Seok C.H.O., Hee–Yeon C.H.O., Soo–Jeong S.H.I.N., Yun–Jeong C.H.O.I., Andrzej L.E.O.N., Shoji O.H.G.A. Production of fungal laccase and its immobilization and stability. J. Fac. Agr. 2008;53:13–18. OWICZ. [Google Scholar]
- 108.Šušla M., Novotný Č., Svobodová K. The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures. Bioresour. Technol. 2007;98:2109–2115. doi: 10.1016/j.biortech.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Cadorin Fernandes S., Cruz Vieira I., Barbosa A.M.J., Souza Ferreira V. Methomyl detection by inhibition of laccase using a carbon ceramic biosensor. Electroanalysis. 2011;23:1623–1630. [Google Scholar]
- 110.Skoronski E., Souza D.H., Ely C., Broilo F., Fernandes M., Fúrigo A., Ghislandi M.G. Immobilization of laccase from Aspergillus oryzae on graphene nanosheets. Int. J. Biol. Macromol. 2017;99:121–127. doi: 10.1016/j.ijbiomac.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 111.Katuri K.P., Venkata Mohan S., Sridhar S., Pati B.R., Sarma P.N. Laccase-membrane reactors for decolorization of an acid azo dye in aqueous phase: process optimization. Water Res. 2009;43:3647–3658. doi: 10.1016/j.watres.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 112.Velu C., Veeramani E., Suntharam S., Kalimuthu K. Insilico screening and comparative study on the effectiveness of textile dye decolourization by crude laccase immobilised alginate encapsulated beads from Pleurotus ostreatus. J. Bioprocess. Biotech. 2011;1 [Google Scholar]
- 113.Karamyshev A.V., Shleev S.V., Koroleva O.V., Yaropolov A.I., Sakharov I.Y. Laccase-catalyzed synthesis of conducting polyaniline. Enzym. Microb. Technol. 2003;33:556–564. [Google Scholar]
- 114.Galliker P., Hommes G., Schlosser D., Corvini P.F.-X., Shahgaldian P. Laccase-modified silica nanoparticles efficiently catalyze the transformation of phenolic compounds. J. Colloid Interface Sci. 2010;349:98–105. doi: 10.1016/j.jcis.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 115.Daâssi D., Rodríguez-Couto S., Nasri M., Mechichi T. Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. Int. Biodeterior. Biodegrad. 2014;90:71–78. [Google Scholar]
- 116.Vianello F., Ragusa S., Cambria M.T., Rigo A. A high sensitivity amperometric biosensor using laccase as biorecognition element. Biosens. Bioelectron. 2006;21:2155–2160. doi: 10.1016/j.bios.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 117.Poonkuzhali K., Palvannan T. Comparison of biopolymers for immobilization of laccase: ecotoxicity assessment of azo dye. Indian J. Biotechnol. 2013;12:395–401. [Google Scholar]
- 118.Chhabra M., Mishra S., Sreekrishnan T.R. Immobilized laccase mediated dye decolorization and transformation pathway of azo dye acid red. J. Environ. Health Sci. Eng. 2015;27(13):1–9. doi: 10.1186/s40201-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaudhary R., Hooda S., Beniwal S., Sindhu A. Partial purification and immobilization of laccase isolated from medicinal mushroom (Ganoderma lucidum) Indian J. Chem. Technol. 2016;23:313–317. [Google Scholar]
- 120.Birhanli E., Erdogan S., Yesilada O., Onal Y. Laccase production by newly isolated white rot fungus Funalia trogii: effect of immobilization matrix on laccase production. Biochem. Eng. J. 2013;71:134–139. [Google Scholar]
- 121.Mirzadeh S.-S., Khezri S.-M., Rezaei S., Forootanfar H., Mahvi A.H., Faramarzi M.A. Decolorization of two synthetic dyes using the purified laccase of Paraconiothyrium variabile immobilized on porous silica beads. J. Environ. Health Sci. Eng. 2014;12:6. doi: 10.1186/2052-336X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bagewadi Z.K., Mulla S.I., Ninnekar H.Z. Purification and immobilization of laccase from Trichoderma harzianum strain HZN10 and its application in dye decolorization. J. Genet. Eng. Biotechnol. 2017;15:139–150. doi: 10.1016/j.jgeb.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rekadwad B., Khobragade C. In: Microb. Appl. Vol2 Biomed. Agric. Ind. Kalia V.C., editor. Springer International Publishing; Cham, Fungi Imperfecti Laccase: 2017. pp. 203–212. (Biotechnological Potential and Perspectives). [Google Scholar]
- 124.Beneyton T., El Harrak A., Griffiths A.D., Hellwig P., Taly V. Commun, Immobilization of CotA, an extremophilic laccase from Bacillus subtilis, on glassy carbon electrodes for biofuel cell applications. Electrochemistry (Tokyo, Jpn.) 2011;13:24–27. [Google Scholar]
- 125.Khakshoor M., Makhdoumi A., Asoodeh A., Hosseindokht M.R. Co-immobilized spore laccase/TiO2 nanoparticles in the alginate beads enhance dye removal by two-step decolorization. Environ. Sci. Pollut. Res. 2021;28:6099–6110. doi: 10.1007/s11356-020-10901-1. [DOI] [PubMed] [Google Scholar]
- 126.Singh G. Characterization of immobilized laccase from γ-proteobacterium JB: approach towards the development of biosensor for the detection of phenolic compounds. Indian J. Sci. Technol. 2010;3:48–53. [Google Scholar]
- 127.Yadav D., Ranjan B., Mchunu N., Le Roes-Hill M., Kudanga T. Enzymatic treatment of phenolic pollutants by a small laccase immobilized on APTES-functionalised magnetic nanoparticles. Biotec. 2021;11:302. doi: 10.1007/s13205-021-02854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nadaroglu H., Mosber G., Gungor A.A., Adıguzel G., Adiguzel A. Biodegradation of some azo dyes from wastewater with laccase from Weissella viridescens LB37 immobilized on magnetic chitosan nanoparticles. J. Water Proc. Eng. 2019;31 [Google Scholar]
- 129.Mehandia S., Sharma S.C., Arya S.K. Immobilization of laccase on chitosan-clay composite beads to improve its catalytic efficiency to degrade industrial dyes. Mater. Today Commun. 2020;25 [Google Scholar]
- 130.Neelkant K.S., Shankar K., Jayalakshmi S.K., Sreeramulu K. Purification, biochemical characterization, and facile immobilization of laccase from Sphingobacterium ksn-11 and its application in transformation of diclofenac. Appl. Biochem. Biotechnol. 2020;192:831–844. doi: 10.1007/s12010-020-03371-1. [DOI] [PubMed] [Google Scholar]
- 131.Reda F.M., Hassan N.S., El-Moghazy A.-N. Decolorization of synthetic dyes by free and immobilized laccases from newly isolated strain Brevibacterium halotolerans N11 (KY883983) Biocatal. Agric. Biotechnol. 2018;15:138–145. [Google Scholar]
- 132.Hullo M.-F., Moszer I., Danchin A., Martin-Verstraete I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001;183:5426–5430. doi: 10.1128/JB.183.18.5426-5430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bailey M.R., Woodard S.L., Callaway E., Beifuss K., Magallanes-Lundback M., Lane J.R., Horn M.E., Mallubhotla H., Delaney D.D., Ward M., Van Gastel F., Howard J.A., Hood E.E. Improved recovery of active recombinant laccase from maize seed. Appl. Microbiol. Biotechnol. 2004;63:390–397. doi: 10.1007/s00253-003-1362-z. [DOI] [PubMed] [Google Scholar]
- 134.Birhanli E., Yesilada O. Enhanced production of laccase in repeated-batch cultures of Funalia trogii and Trametes versicolor. Biochem. Eng. J. 2010;52:33–37. [Google Scholar]
- 135.Cordi L., Minussi R.C., Freire R.S., Durán N. Fungal laccase: copper induction, semi-purification, immobilization, phenolic effluent treatment and electrochemical measurement. Afr. J. Biotechnol. 2007;6:1255–1259. [Google Scholar]
- 136.Domínguez A., Couto S.R., Sanromán M.Á. Dye decolorization by Trametes hirsuta immobilized into alginate beads. World J. Microbiol. Biotechnol. 2005;21:405–409. [Google Scholar]
- 137.Noreen S., Asgher M., Hussain F., Iqbal A. Performance improvement of Ca-alginate bead cross- linked laccase from Trametes versicolor IBL-04. Bioresources. 2016;11:558–572. [Google Scholar]
- 138.Kahraman S., Yeilada O. Vol. 46. 2001. pp. 133–139. (Industrial and Agricultural Wastes as Substrates for Laccase Production by white-rot Fungi). [DOI] [PubMed] [Google Scholar]
- 139.Martínez-Morales F., Bertrand B., Pasión Nava A.A., Tinoco R., Acosta-Urdapilleta L., Trejo-Hernández M.R. Production, purification and biochemical characterization of two laccase isoforms produced by Trametes versicolor grown on oak sawdust. Biotechnol. Lett. 2015;37:391–396. doi: 10.1007/s10529-014-1679-y. [DOI] [PubMed] [Google Scholar]
- 140.Sathishkumar P., Murugesan K., Palvannan T. Production of laccase from Pleurotus Florida using agro-wastes and efficient decolorization of Reactive blue. J. Basic Microbiol. 2010;198(50):360–367. doi: 10.1002/jobm.200900407. [DOI] [PubMed] [Google Scholar]
- 141.Sadeghian-Abadi S., Rezaei S., Yousefi-Mokri M., Faramarzi M.A. Enhanced production, one-step affinity purification, and characterization of laccase from solid-state culture of Lentinus tigrinus and delignification of pistachio shell by free and immobilized enzyme. J. Environ. Manag. 2019;244:235–246. doi: 10.1016/j.jenvman.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 142.Xu L., Sun K., Wang F., Zhao L., Hu J., Ma H., Ding Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020;270 doi: 10.1016/j.jenvman.2020.110904. [DOI] [PubMed] [Google Scholar]
- 143.El-Batal A.I., ElKenawy N.M., Yassin A.S., Amin M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015;5:31–39. doi: 10.1016/j.btre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hatvani N., Mécs I. Production of laccase and manganese peroxidase by Lentinus edodes on malt-containing by-product of the brewing process. Process Biochem. 2001;37:491–496. [Google Scholar]
- 145.Wang F., Xu L., Zhao L., Ding Z., Ma H., Terry N. Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: a review. Microorganisms. 2019;7:665. doi: 10.3390/microorganisms7120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Agrawal K., Verma P. In: Biotechnol. Appl. Hum. Health. Sadhukhan P.C., Premi S., editors. Springer; Singapore, Laccase-: 2020. pp. 87–93. (Mediated Synthesis of Bio-Material Using Agro-Residues). [Google Scholar]
- 147.Songulashvili G., Jimenéz-Tobón G.A., Jaspers C., Penninckx M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012;116:883–889. doi: 10.1016/j.funbio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Patel N., Shahane S., Shivam, Majumdar R., Mishra U. Mode of action, properties, production, and application of laccase: a review. Recent Pat. Biotechnol. 2019;13:19–32. doi: 10.2174/1872208312666180821161015. [DOI] [PubMed] [Google Scholar]
- 149.Ren D., Wang Z., Jiang S., Yu H., Zhang S., Zhang X. Recent environmental applications of and development prospects for immobilized laccase: a review. Biotechnol. Genet. Eng. Rev. 2020;36:81–131. doi: 10.1080/02648725.2020.1864187. [DOI] [PubMed] [Google Scholar]
- 150.Ramírez-Montoya L.A., Hernández-Montoya V., Montes-Morán M.A., Cervantes F.J. Correlation between mesopore volume of carbon supports and the immobilization of laccase from Trametes versicolor for the decolorization of Acid Orange 7. J. Environ. Manag. 2015;162:206–214. doi: 10.1016/j.jenvman.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 151.Khatami S.H., Vakili O., Movahedpour A., Ghesmati Z., Ghasemi H., Taheri-Anganeh M. Laccase: various types and applications. Biotechnol. Appl. Biochem. 2022:1–15. doi: 10.1002/bab.2313. [DOI] [PubMed] [Google Scholar]
- 152.Piacquadio P., Stefano G.D., Sammartino M., Sciancalepore V. Phenols removal from apple juice by laccase immobilized on Cu 2+ -chelate regenerable carrier. Biotechnol. Tech. 1997;11:515–517. [Google Scholar]
- 153.Baratto L., Candido A., Marzorati M., Sagui F., Riva S., Danieli B. Laccase-mediated oxidation of natural glycosides. J. Mol. Catal. B Enzym. 2006;39:3–8. [Google Scholar]
- 154.Mogharabi-Manzari M., Amini M., Abdollahi M., Khoobi M., Bagherzadeh G., Faramarzi M.A. Co-Immobilization of laccase and TEMPO in the compartments of mesoporous silica for a green and one-pot cascade synthesis of coumarins by knoevenagel condensation. ChemCatChem. 2018;10:1542–1546. [Google Scholar]
- 155.Ji C., Nguyen L.N., Hou J., Hai F.I., Chen V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Separ. Purif. Technol. 2017;178:215–223. [Google Scholar]
- 156.Cardinal-Watkins C., Nicell J.A. Enzyme-Catalyzed oxidation of 17 β -estradiol using immobilized laccase from Trametes versicolor. Enzym. Res. 2011:1–11. doi: 10.4061/2011/725172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Niladevi K.N., Prema P. Immobilization of laccase from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World J. Microbiol. Biotechnol. 2008;24:1215–1222. [Google Scholar]
- 158.Zimmermann Y.-S., Shahgaldian P., Corvini P.F.X., Hommes G. Sorption-assisted surface conjugation: a way to stabilize laccase enzyme. Appl. Microbiol. Biotechnol. 2011;92:169–178. doi: 10.1007/s00253-011-3534-6. [DOI] [PubMed] [Google Scholar]
- 159.Timur S., Pazarlıoǧlu N., Pilloton R., Telefoncu A. Thick film sensors based on laccases from different sources immobilized in polyaniline matrix. Sensor. Actuator. B Chem. 2004;97:132–136. [Google Scholar]
- 160.Tastan E., Önder S., Kok F.N. Immobilization of laccase on polymer grafted polytetrafluoroethylene membranes for biosensor construction. Talanta. 2011;84:524–530. doi: 10.1016/j.talanta.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 161.Rekuć A., Bryjak J., Szymańska K., Jarzębski A.B. Very stable silica-gel-bound laccase biocatalysts for the selective oxidation in continuous systems. Bioresour. Technol. 2010;101:2076–2083. doi: 10.1016/j.biortech.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y., Zeng Z., Zeng G., Tang L., Pang Y., Li Z., Liu C., Lei X., Wu M., Ren P., Liu Z., Chen M., Xie G. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012;115:21–26. doi: 10.1016/j.biortech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 163.García-Morales R., García-García A., Orona-Navar C., Osma J.F., Nigam K.D.P., Ornelas-Soto N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018;6:710–717. [Google Scholar]
- 164.Romero-Arcos M., Garnica-Romo M.G., Martínez-Flores H.E. Electrochemical study and characterization of an amperometric biosensor based on the immobilization of laccase in a nanostructure of TiO2 synthesized by the sol-gel method. Materials. 2016;9:543. doi: 10.3390/ma9070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Addorisio V., Sannino F., Mateo C., Guisan J.M. Oxidation of phenyl compounds using strongly stable immobilized-stabilized laccase from Trametes versicolor. Process Biochem. 2013;48:1174–1180. [Google Scholar]
- 166.Hu X., Zhao X., Hwang H. Comparative study of immobilized Trametes versicolor laccase on nanoparticles and kaolinite. Chemosphere. 2007;66:1618–1626. doi: 10.1016/j.chemosphere.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Nieto M., Nardecchia S., Peinado C., Catalina F., Abrusci C., Gutiérrez M.C., Luisa Ferrer M., del Monte F. Enzyme -induced graft polymerization for preparation of hydrogels: synergetic effect of laccase -immobilized-cryogels for pollutants adsorption. Soft Matter. 2010;6:3533–3540. [Google Scholar]
- 168.Sathishkumar P., Kamala-Kannan S., Cho M., Kim J.S., Hadibarata T., Salim M.R., Oh B.-T. Laccase immobilization on cellulose nanofiber: the catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014;100:111–120. [Google Scholar]
- 169.Hou J., Dong G., Ye Y., Chen V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014;469:19–30. [Google Scholar]
- 170.Apriceno A., Bucci R., Girelli A.M. immobilization of laccase from Trametes versicolor on chitosan macrobeads for anthracene degradation. Anal. Lett. 2017;50:2308–2322. [Google Scholar]
- 171.Rostami A., Abdelrasoul A., Shokri Z., Shirvandi Z. Applications and mechanisms of free and immobilized laccase in detoxification of phenolic compounds — a review. Kor. J. Chem. Eng. 2022;39:821–832. [Google Scholar]
- 172.Berrio J., Plou F.J., Ballesteros A., Martínez Á.T., Martínez M.J. Immobilization of pycnoporus coccineus laccase on Eupergit C: stabilization and treatment of olive oil mill wastewaters. Biocatal. Biotransform. 2007;25:130–134. [Google Scholar]
- 173.Georgiou R.P., Tsiakiri E.P., Lazaridis N.K., Pantazaki A.A. Decolorization of melanoidins from simulated and industrial molasses effluents by immobilized laccase. J. Environ. Chem. Eng. 2016;4:1322–1331. [Google Scholar]
- 174.D’Annibale A., Rita Stazi S., Vinciguerra V., Di Mattia E., Giovannozzi Sermanni G. Characterization of immobilized laccase from Lentinula edodes and its use in olive-mill wastewater treatment. Process Biochem. 1999;34:697–706. [Google Scholar]
- 175.Sathishkumar P., Chae J.-C., Unnithan A.R., Palvannan T., Kim H.Y., Lee K.-J., Cho M., Kamala-Kannan S., Oh B.-T. Laccase-poly(lactic-co-glycolic acid) (PLGA) nanofiber: highly stable, reusable, and efficacious for the transformation of diclofenac. Enzym. Microb. Technol. 2012;51:113–118. doi: 10.1016/j.enzmictec.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 176.Xu R., Tang R., Zhou Q., Li F., Zhang B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem. Eng. J. 2015;262:88–95. [Google Scholar]
- 177.Yang J., Lin Y., Yang X., Ng T.B., Ye X., Lin J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard Mater. 2017;322:525–531. doi: 10.1016/j.jhazmat.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 178.Kadam A.A., Jang J., Lee D.S. Supermagnetically tuned halloysite nanotubes functionalized with aminosilane for covalent laccase immobilization. ACS Appl. Mater. Interfaces. 2017;9:15492–15501. doi: 10.1021/acsami.7b02531. [DOI] [PubMed] [Google Scholar]
- 179.Kadam A.A., Shinde S.K., Ghodake G.S., Saratale G.D., Saratale R.G., Sharma B., Hyun S., Sung J.-S. Chitosan-grafted halloysite nanotubes-Fe3O4 composite for laccase-immobilization and sulfamethoxazole-degradation. Polymers. 2020;12:2221. doi: 10.3390/polym12102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Taheran M., Naghdi M., Brar S.K., Knystautas E.J., Verma M., Surampalli R.Y. Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile-biochar composite nanofibrous membrane. Sci. Total Environ. 2017;606:315–321. doi: 10.1016/j.scitotenv.2017.06.185. [DOI] [PubMed] [Google Scholar]
- 181.Taheran M., Naghdi M., Brar S.K., Knystautas E.J., Verma M., Surampalli R.Y. Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain. Chem. Eng. 2017;5:10430–10438. [Google Scholar]
- 182.Simón-Herrero C., Naghdi M., Taheran M., Kaur Brar S., Romero A., Valverde J.L., Avalos Ramirez A., Sánchez-Silva L. Immobilized laccase on polyimide aerogels for removal of carbamazepine. J. Hazard Mater. 2019;376:83–90. doi: 10.1016/j.jhazmat.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 183.Nicolucci C., Rossi S., Menale C., Godjevargova T., Ivanov Y., Bianco M., Mita L., Bencivenga U., Mita D.G., Diano N. Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation. 2011;22:673–683. doi: 10.1007/s10532-010-9440-2. [DOI] [PubMed] [Google Scholar]
- 184.Lin J., Liu Y., Chen S., Le X., Zhou X., Zhao Z., Ou Y., Yang J. Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal. Int. J. Biol. Macromol. 2016;84:189–199. doi: 10.1016/j.ijbiomac.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 185.Bravo I., Prata M., Torrinha Á., Delerue-Matos C., Lorenzo E., Morais S. Laccase bioconjugate and multi-walled carbon nanotubes-based biosensor for bisphenol A analysis. Bioelectrochemistry. 2022;144 doi: 10.1016/j.bioelechem.2021.108033. [DOI] [PubMed] [Google Scholar]
- 186.Catapane M., Nicolucci C., Menale C., Mita L., Rossi S., Mita D.G., Diano N. Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J. Hazard Mater. 2013;248:337–346. doi: 10.1016/j.jhazmat.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 187.Chang Y.-T., Lee J.-F., Liu K.-H., Liao Y.-F., Yang V. Immobilization of fungal laccase onto a nonionic surfactant-modified clay material: application to PAH degradation. Environ. Sci. Pollut. Res. 2016;23:4024–4035. doi: 10.1007/s11356-015-4248-6. [DOI] [PubMed] [Google Scholar]
- 188.Corvini P.F.X., Shahgaldian P. LANCE: laccase-nanoparticle conjugates for the elimination of micropollutants (endocrine disrupting chemicals) from wastewater in bioreactors. Rev. Environ. Sci. Biotechnol. 2010;9:23–27. [Google Scholar]
- 189.Domínguez A., Gómez J., Lorenzo M., Sanromán Á. Enhanced production of laccase activity by Trametes versicolor immobilized into alginate beads by the addition of different inducers. World J. Microbiol. Biotechnol. 2007;23:367–373. [Google Scholar]
- 190.Georgieva S., Godjevargova T., Mita D.G., Diano N., Menale C., Nicolucci C., Carratelli C.R., Mita L., Golovinsky E. Non-isothermal bioremediation of waters polluted by phenol and some of its derivatives by laccase covalently immobilized on polypropylene membranes. J. Mol. Catal. B Enzym. 2010;66:210–218. [Google Scholar]
- 191.Xu R., Zhou Q., Li F., Zhang B. Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem. Eng. J. 2013;222:321–329. [Google Scholar]
- 192.Das A., Singh J., K.n. Y. Laccase immobilized magnetic iron nanoparticles: fabrication and its performance evaluation in chlorpyrifos degradation. Int. Biodeterior. Biodegrad. 2017;117:183–189. [Google Scholar]
- 193.Srinivasan P., Selvankumar T., Paray B.A., Rehman M.U., Kamala-Kannan S., Govarthanan M., Kim W., Selvam K. Chlorpyrifos degradation efficiency of Bacillus sp. laccase immobilized on iron magnetic nanoparticles. 3 Biotech. 2020;10:366. doi: 10.1007/s13205-020-02363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oliveira T.M.B.F., Fátima Barroso M., Morais S., de Lima-Neto P., Correia A.N., Oliveira M.B.P.P., Delerue-Matos C. Biosensor based on multi-walled carbon nanotubes paste electrode modified with laccase for pirimicarb pesticide quantification. Talanta. 2013;106:137–143. doi: 10.1016/j.talanta.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 195.Jia Y., Chen Y., Luo J., Hu Y. Immobilization of laccase onto meso-MIL-53(Al) via physical adsorption for the catalytic conversion of triclosan. Ecotoxicol. Environ. Saf. 2019;184 doi: 10.1016/j.ecoenv.2019.109670. [DOI] [PubMed] [Google Scholar]
- 196.Wang Y., Zhang D., He F.R., Chen X.C. Immobilization of laccase by Cu2+ chelate affinity interaction on surface modified magnetic silica particles and its use for the removal of pentachlorophenol. Chin. Chem. Lett. 2012;23:197–200. [Google Scholar]
- 197.Mohammadi M., As’habi M.A., Salehi P., Yousefi M., Nazari M., Brask J. Immobilization of laccase on epoxy-functionalized silica and its application in biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2018;109:443–447. doi: 10.1016/j.ijbiomac.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 198.Celikbicak O., Bayramoglu G., Yılmaz M., Ersoy G., Bicak N., Salih B., Arica M.Y. Immobilization of laccase on hairy polymer grafted zeolite particles: degradation of a model dye and product analysis with MALDI–ToF-MS. Microporous Mesoporous Mater. 2014;199:57–65. [Google Scholar]
- 199.Karami C., Taher M.A. A catechol biosensor based on immobilizing laccase to Fe3O4@Au core-shell nanoparticles. Int. J. Biol. Macromol. 2019;129:84–90. doi: 10.1016/j.ijbiomac.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 200.Datta S., Veena R., Samuel M.S., Selvarajan E. Immobilization of laccases and applications for the detection and remediation of pollutants: a review. Environ. Chem. Lett. 2021;19:521–538. [Google Scholar]
- 201.Unuofin J.O., Okoh A.I., Nwodo U.U. Aptitude of oxidative enzymes for treatment of wastewater pollutants: a laccase perspective. Molecules. 2019;24:2064. doi: 10.3390/molecules24112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou W., Zhang W., Cai Y. Laccase immobilization for water purification: a comprehensive review. Chem. Eng. J. 2021;403 [Google Scholar]
- 203.Singh D., Gupta N. Microbial Laccase: a robust enzyme and its industrial applications. Biologia. 2020;75:1183–1193. [Google Scholar]
- 204.Bilal M., Rasheed T., Nabeel F., Iqbal H.M.N., Zhao Y. Hazardous contaminants in the environment and their laccase-assisted degradation – a review. J. Environ. Manag. 2019;234:253–264. doi: 10.1016/j.jenvman.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 205.Zdarta J., Jankowska K., Bachosz K., Kijeńska-Gawrońska E., Zgoła-Grześkowiak A., Kaczorek E., Jesionowski T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: effect of process conditions and catalytic pathways. Catal. Today. 2020;348:127–136. [Google Scholar]
- 206.Shao B., Liu Z., Zeng G., Liu Y., Yang X., Zhou C., Chen M., Liu Y., Jiang Y., Yan M., Hazard J. Immobilization of laccase on hollow mesoporous carbon nanospheres: noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal. Materials. 2019;362:318–326. doi: 10.1016/j.jhazmat.2018.08.069. [DOI] [PubMed] [Google Scholar]
- 207.Lonappan L., Liu Y., Rouissi T., Brar S.K., Verma M., Surampalli R.Y. Adsorptive immobilization of agro-industrially produced crude laccase on various micro-biochars and degradation of diclofenac. Sci. Total Environ. 2018;641:1251–1258. doi: 10.1016/j.scitotenv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 208.Lloret L., Eibes G., Feijoo G., Moreira M.T., Lema J.M., Hollmann F. Immobilization of laccase by encapsulation in a sol–gel matrix and its characterization and use for the removal of estrogens. Biotechnol. Prog. 2011;27:1570–1579. doi: 10.1002/btpr.694. [DOI] [PubMed] [Google Scholar]
- 209.Lacerda M.F.A.R., Lopes F.M., Sartoratto A., Ponezi A.N., Thomaz D.V., Schimidt F., Santiago M.F. Stability of immobilized laccase on Luffa Cylindrica fibers and assessment of synthetic hormone degradation. Prep. Biochem. Biotechnol. 2019;49:58–63. doi: 10.1080/10826068.2018.1525568. [DOI] [PubMed] [Google Scholar]
- 210.Rahmani K., Faramarzi M.A., Mahvi A.H., Gholami M., Esrafili A., Forootanfar H., Farzadkia M. Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int. Biodeterior. Biodegrad. 2015;97:107–114. [Google Scholar]
- 211.Ghodake G.S., Shinde S.K., Saratale G.D., Saratale R.G., Kim M., Jee S.-C., Kim D.-Y., Sung J.-S., Kadam A.A. α-Cellulose fibers of paper-waste origin surface-modified with Fe3O4 and thiolated-chitosan for efficacious immobilization of laccase. Polymers. 2021;13:581. doi: 10.3390/polym13040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Naghdi M., Taheran M., Brar S.K., Kermanshahi-pour A., Verma M., Surampalli R.Y. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 2017;585:393–401. doi: 10.1016/j.scitotenv.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 213.Bao C., Wang Y., Xu X., Li D., Chen J., Guan Z., Wang B., Hong M., Zhang J., Wang T., Zhang Q. Reversible immobilization of laccase onto glycopolymer microspheres via protein-carbohydrate interaction for biodegradation of phenolic compounds. Bioresour. Technol. 2021;342 doi: 10.1016/j.biortech.2021.126026. [DOI] [PubMed] [Google Scholar]
- 214.Li G., Pang S., Wu Y., Ouyang J. Enhanced removal of hydroquinone by graphene aerogel-Zr-MOF with immobilized laccase. Chem. Eng. Commun. 2018;205:698–705. [Google Scholar]
- 215.Guardado A.L.P., Druon-Bocquet S., Belleville M.-P., Sanchez-Marcano J. A novel process for the covalent immobilization of laccases on silica gel and its application for the elimination of pharmaceutical micropollutants. Environ. Sci. Pollut. Res. 2021;28:25579–25593. doi: 10.1007/s11356-021-12394-y. [DOI] [PubMed] [Google Scholar]
- 216.Ding S., Cargill A.A., Medintz I.L., Claussen J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015;34:242–250. doi: 10.1016/j.copbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 217.Molina M.A., Gascón-Pérez V., Sánchez-Sánchez M., Blanco R.M. Sustainable one-pot immobilization of enzymes in/on metal-organic framework materials. Catalysts. 2021;11:1002. [Google Scholar]
- 218.Masjoudi M., Golgoli M., Ghobadi Nejad Z., Sadeghzadeh S., Borghei S.M. Pharmaceuticals removal by immobilized laccase on polyvinylidene fluoride nanocomposite with multi-walled carbon nanotubes. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128043. [DOI] [PubMed] [Google Scholar]
- 219.Demarche P., Junghanns C., Mazy N., Agathos S.N. Design-of-experiment strategy for the formulation of laccase biocatalysts and their application to degrade bisphenol A. N. Biotech. 2012;30:96–103. doi: 10.1016/j.nbt.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 220.Molina M.A., Díez-Jaén J., Sánchez-Sánchez M., Blanco R.M. One-pot laccase@MOF biocatalysts efficiently remove bisphenol A from water. Catal. Today. 2022;391:265–271. [Google Scholar]
- 221.Bayramoğlu G., Yakup Arıca M. Immobilization of laccase onto poly(glycidylmethacrylate) brush grafted poly(hydroxyethylmethacrylate) films: enzymatic oxidation of phenolic compounds. Mater. Sci. Eng. C. 2009;29:1990–1997. [Google Scholar]
- 222.Yang B., Tang K., Wei S., Zhai X., Nie N. Preparation of functionalized mesoporous silica as a novel carrier and immobilization of laccase. Appl. Biochem. Biotechnol. 2021;193:2547–2566. doi: 10.1007/s12010-021-03556-2. [DOI] [PubMed] [Google Scholar]
- 223.Qiu X., Wang S., Miao S., Suo H., Xu H., Hu Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123353. [DOI] [PubMed] [Google Scholar]
- 224.Patila M., Kouloumpis A., Gournis D., Rudolf P., Stamatis H. Laccase-functionalized graphene oxide assemblies as efficient nanobiocatalysts for oxidation reactions. Sensors. 2016;16:287. doi: 10.3390/s16030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pang R., Li M., Zhang C. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: diffusional limitation investigation. Talanta. 2015;131:38–45. doi: 10.1016/j.talanta.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 226.Amin R., Khorshidi A., Shojaei A.F., Rezaei S., Faramarzi M.A. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018;114:106–113. doi: 10.1016/j.ijbiomac.2018.03.086. [DOI] [PubMed] [Google Scholar]
- 227.Lin J., Wen Q., Chen S., Le X., Zhou X., Huang L. Synthesis of amine-functionalized Fe3O4@C nanoparticles for laccase immobilization. Int. J. Biol. Macromol. 2017;96:377–383. doi: 10.1016/j.ijbiomac.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 228.Chen X., Zhou Q., Liu F., Peng Q., Bian Y. Performance and kinetic of pesticide residues removal by microporous starch immobilized laccase in a combined adsorption and biotransformation process. Environ. Technol. Innovat. 2021;21 [Google Scholar]
- 229.Kunamneni A., Ghazi I., Camarero S., Ballesteros A., Plou F.J., Alcalde M. Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem. 2008;43:169–178. [Google Scholar]
- 230.Wang H., Zhang W., Zhao J., Xu L., Zhou C., Chang L., Wang L. Rapid decolorization of phenolic azo dyes by immobilized laccase with Fe3O4/SiO2 nanoparticles as support. Ind. Eng. Chem. Res. 2013;52:4401–4407. [Google Scholar]
- 231.Dai J., Wang H., Chi H., Wang Y., Zhao J. Immobilization of laccase from Pleurotus ostreatus on magnetic separable SiO2 support and excellent activity towards azo dye decolorization. J. Environ. Chem. Eng. 2016;4:2585–2591. [Google Scholar]
- 232.Osma J.F., Toca-Herrera J.L., Rodríguez-Couto S. Transformation pathway of remazol brilliant blue R by immobilised laccase. Bioresour. Technol. 2010;101:8509–8514. doi: 10.1016/j.biortech.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 233.Uygun M., de la Asunción-Nadal V., Evli S., Uygun D.A., Jurado-Sánchez B., Escarpa A. Dye removal by laccase-functionalized micromotors. Appl. Mater. Today. 2021;23 [Google Scholar]
- 234.Kandelbauer A., Maute O., Kessler R.W., Erlacher A., Gübitz G.M. Study of dye decolorization in an immobilized laccase enzyme-reactor using online spectroscopy. Biotechnol. Bioeng. 2004;87:552–563. doi: 10.1002/bit.20162. [DOI] [PubMed] [Google Scholar]
- 235.Lu L., Zhao M., Wang Y. Immobilization of laccase by alginate–chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol. 2007;23:159–166. [Google Scholar]
- 236.Rasera K., Ferla J., Dillon A.J.P., Riveiros R., Zeni M. Immobilization of laccase from Pleurotus sajor-caju in polyamide membranes. Desalination. 2009;245:657–661. [Google Scholar]
- 237.Bayramoğlu G., Yilmaz M., Yakup Arica M. Reversible immobilization of laccase to poly(4-vinylpyridine) grafted and Cu(II) chelated magnetic beads: biodegradation of reactive dyes. Bioresour. Technol. 2010;101:6615–6621. doi: 10.1016/j.biortech.2010.03.088. [DOI] [PubMed] [Google Scholar]
- 238.Zhou W., Zhang W., Cai Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal. Separ. Purif. Technol. 2022;294 [Google Scholar]
- 239.Erkurt E.A., Ünyayar A., Kumbur H. Decolorization of synthetic dyes by white rot fungi, involving laccase enzyme in the process. Process Biochem. 2007;42:1429–1435. [Google Scholar]
- 240.˙ilk Sedef, Dem˙ircan Deniz, Saglam Semran, Saglam Necdet, Zakir M., Rzayev O. Immobilization of laccase onto a porous nanocomposite: application for textile dye degradation. Turk. J. Chem. 2016;40:262–278. [Google Scholar]
- 241.Jořenek M., Zajoncová L. Immobilization of laccase on magnetic carriers and its use in decolorization of dyes. Chem. Biochem. Eng. Q. 2015;29:457–466. [Google Scholar]
- 242.Zhang Y., Hu P., Muhammad Y., Tang Y., Shao S., Gao Z., Wang J., Wang R., Hu Y., Kuang L., Zhao Z., Zhao Z. High-density immobilization of laccase on hollow nano-sphere NH2-MIL88(Fe) host with interfacial defects to improve enzyme activity and stability for remazol brilliant blue R decolorization. Chem. Eng. J. 2021;405 [Google Scholar]
- 243.Li W.-X., Sun H.-Y., Zhang R.-F. Immobilization of laccase on a novel ZnO/SiO 2 nano-composited support for dye decolorization. IOP Conf. Ser. Mater. Sci. Eng. 2015;87 [Google Scholar]
- 244.Abd El Aty A.A., Mostafa F.A., Hassan M.E., Hamed E.R., Esawy M.A. Covalent immobilization of Alternaria tenuissima KM651985 laccase and some applied aspects. Biocatal. Agric. Biotechnol. 2017;9:74–81. [Google Scholar]
- 245.Yamak O., Kalkan N.A., Aksoy S., Altinok H., Hasirci N. Semi-interpenetrating polymer networks (semi-IPNs) for entrapment of laccase and their use in Acid Orange 52 decolorization. Process Biochem. 2009;44:440–445. [Google Scholar]
- 246.Faraco V., Pezzella C., Miele A., Giardina P., Sannia G. Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation. 2009;20:209–220. doi: 10.1007/s10532-008-9214-2. [DOI] [PubMed] [Google Scholar]
- 247.Ariaeenejad S., Motamedi E., Salekdeh G.H. Highly efficient removal of dyes from wastewater using nanocellulose from quinoa husk as a carrier for immobilization of laccase. Bioresour. Technol. 2022;349 doi: 10.1016/j.biortech.2022.126833. [DOI] [PubMed] [Google Scholar]
- 248.Qiao W., Liu H. Enhanced decolorization of malachite green by a magnetic graphene oxide-CotA laccase composite. Int. J. Biol. Macromol. 2019;138:1–12. doi: 10.1016/j.ijbiomac.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 249.Sadighi A., Faramarzi M.A. Congo red decolorization by immobilized laccase through chitosan nanoparticles on the glass beads. J. Taiwan Inst. Chem. Eng. 2013;44:156–162. [Google Scholar]
- 250.Sanlıer S.H., Gider S., Köprülü A. Immobilization of laccase for biotechnology applications. Artif. Cell Nanomed. Biotechnol. 2013;41:259–263. doi: 10.3109/10731199.2012.731414. [DOI] [PubMed] [Google Scholar]
- 251.Zhang P., Wang Q., Zhang J., Li G., Wei Q. Preparation of amidoxime-modified polyacrylonitrile nanofibers immobilized with laccase for dye degradation. Fibers Polym. 2014;15:30–34. [Google Scholar]
- 252.Alam R., Ardiati F.C., Solihat N.N., Alam M.B., Lee S.H., Yanto D.H.Y., Watanabe T., Kim S. Biodegradation and metabolic pathway of anthraquinone dyes by Trametes hirsuta D7 immobilized in light expanded clay aggregate and cytotoxicity assessment. J. Hazard Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124176. [DOI] [PubMed] [Google Scholar]
- 253.Zofair S.F.F., Arsalan A., Khan M.A., Alhumaydhi F.A., Younus H. Immobilization of laccase on Sepharose-linked antibody support for decolourization of phenol red. Int. J. Biol. Macromol. 2020;161:78–87. doi: 10.1016/j.ijbiomac.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 254.Uygun M., Chem J. 2013. Preparation of Laccase Immobilized Cryogels and Usage for Decolorization. [Google Scholar]
- 255.Stanescu M.D., Sanislav A., Ivanov R.V., Hirtopeanu A., Lozinsky V.I. Immobilized laccase on a new cryogel carrier and kinetics of two anthraquinone derivatives oxidation. Appl. Biochem. Biotechnol. 2011;165:1789–1798. doi: 10.1007/s12010-011-9395-8. [DOI] [PubMed] [Google Scholar]
- 256.Mohajershojaei K., Mahmoodi N.M., Khosravi A. Immobilization of laccase enzyme onto titania nanoparticle and decolorization of dyes from single and binary systems. Biotechnol. Bioproc. Eng. 2015;20:109–116. [Google Scholar]
- 257.Senthivelan T., Kanagaraj J., Panda R.C. Recent trends in fungal laccase for various industrial applications: an eco-friendly approach - a review. Biotechnol. Bioproc. Eng. 2016;21:19–38. [Google Scholar]
- 258.Mishra A., Kumar S., Bhatnagar A. In: Microb. Wastewater Treat. Shah M.P., Rodriguez-Couto S., editors. Elsevier; 2019. pp. 127–151. (Chapter 7, Potential of Fungal Laccase in Decolorization of Synthetic Dyes). [Google Scholar]
- 259.Singh R.L., Singh P.K., Singh R.P. Enzymatic decolorization and degradation of azo dyes –A review. Int. Biodeterior. Biodegrad. 2015;104:21–31. [Google Scholar]
- 260.Virk A.P., Sharma P., Capalash N. Use of laccase in pulp and paper industry. Biotechnol. Prog. 2012;28:21–32. doi: 10.1002/btpr.727. [DOI] [PubMed] [Google Scholar]
- 261.Sadhasivam S., Savitha S., Swaminathan K. Deployment of Trichoderma harzianum WL1 laccase in pulp bleaching and paper industry effluent treatment. J. Clean. Prod. 2010;18:799–806. [Google Scholar]
- 262.Singh G., Kaur K., Puri S., Sharma P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl. Microbiol. Biotechnol. 2015;99:155–164. doi: 10.1007/s00253-014-6219-0. [DOI] [PubMed] [Google Scholar]
- 263.Singh G., Arya S.K. Utility of laccase in pulp and paper industry: a progressive step towards the green technology. Int. J. Biol. Macromol. 2019;134:1070–1084. doi: 10.1016/j.ijbiomac.2019.05.168. [DOI] [PubMed] [Google Scholar]
- 264.Singh G., Capalash N., Kaur K., Puri S., Sharma P., Dhillon G.S., Kaur S., editors. Agro-Ind. Wastes Feedstock Enzyme Prod. Academic Press; San Diego: 2016. pp. 157–172. (Chapter 7 - Enzymes: Applications in Pulp and Paper Industry). [Google Scholar]
- 265.Gupta G.K., Dixit M., Kapoor R.K., Shukla P. Xylanolytic enzymes in pulp and paper industry: new technologies and perspectives. Mol. Biotechnol. 2022;64:130–143. doi: 10.1007/s12033-021-00396-7. [DOI] [PubMed] [Google Scholar]
- 266.Chen P., Chen W., Jiang S., Zhong Q., Chen H., Chen W. Synergistic effect of laccase and sugar beet pectin on the properties of concentrated protein emulsions and its application in concentrated coconut milk. Molecules. 2018;23:2591. doi: 10.3390/molecules23102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Minussi R.C., Rossi M., Bologna L., Rotilio D., Pastore G.M., Durán N. Phenols removal in musts: strategy for wine stabilization by laccase. J. Mol. Catal. B Enzym. 2007;45:102–107. [Google Scholar]
- 268.Dhillon G.S., Kaur S., Brar S.K., Verma M. Flocculation and haze removal from crude beer using in-house produced laccase from Trametes versicolor cultured on brewer’s spent grain. J. Agric. Food Chem. 2012;60:7895–7904. doi: 10.1021/jf301747z. [DOI] [PubMed] [Google Scholar]
- 269.Bezerra T.M. de S., Bassan J.C., Santos V.T. de O., Ferraz A., Monti R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem. 2015;50:417–423. [Google Scholar]
- 270.Lettera V., Pezzella C., Cicatiello P., Piscitelli A., Giacobelli V.G., Galano E., Amoresano A., Sannia G. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2016;196:1272–1278. doi: 10.1016/j.foodchem.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 271.Wang F., Owusu-Fordjour M., Xu L., Ding Z., Gu Z. Immobilization of laccase on magnetic chelator nanoparticles for apple juice clarification in magnetically stabilized fluidized bed. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00589. (accessed June 16, 2022) https://www.frontiersin.org/article/10.3389/fbioe.2020.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Manhivi V.E., Amonsou E.O., Kudanga T. Laccase-mediated crosslinking of gluten-free amadumbe flour improves rheological properties. Food Chem. 2018;264:157–163. doi: 10.1016/j.foodchem.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 273.Selinheimo E., Kruus K., Buchert J., Hopia A., Autio K. Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J. Cereal. Sci. 2006;43:152–159. [Google Scholar]
- 274.Flander L., Rouau X., Morel M.-H., Autio K., Seppänen-Laakso T., Kruus K., Buchert J. Effects of laccase and xylanase on the chemical and rheological properties of oat and wheat doughs. J. Agric. Food Chem. 2008;56:5732–5742. doi: 10.1021/jf800264a. [DOI] [PubMed] [Google Scholar]
- 275.Ayala-Soto F.E., Serna-Saldívar S.O., Welti-Chanes J. Effect of arabinoxylans and laccase on batter rheology and quality of yeast-leavened gluten-free breads. J. Cereal. Sci. 2017;73:10–17. [Google Scholar]
- 276.Chen B., McClements D.J., Gray D.A., Decker E.A., Agric J. Stabilization of soybean oil bodies by enzyme (laccase) cross-linking of adsorbed beet pectin coatings. Food Chem. 2010;58:9259–9265. doi: 10.1021/jf102082u. [DOI] [PubMed] [Google Scholar]
- 277.Chaurasia P.K., Bharati S.L., Grumezescu A.M. In: Soft Chem. Food Ferment. Holban A.M., editor. Academic Press; 2017. pp. 299–335. (Chapter 11 - Significance of Laccases in Food Chemistry and Related Bioremediation). [Google Scholar]
- 278.Zrinski I., Pungjunun K., Martinez S., Zavašnik J., Stanković D., Kalcher K., Mehmeti E. Evaluation of phenolic antioxidant capacity in beverages based on laccase immobilized on screen-printed carbon electrode modified with graphene nanoplatelets and gold nanoparticles. Microchem. J. 2020;152 [Google Scholar]
- 279.Osma J.F., Toca-Herrera J.L., Rodríguez-Couto S. Uses of laccases in the food industry. Enzym. Res. 2010:1–8. doi: 10.4061/2010/918761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mayolo-Deloisa K., González-González M., Rito-Palomares M. Laccases in food industry: bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020;8:1–8. doi: 10.3389/fbioe.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang H., Xun E., Wang J., Chen G., Cheng T., Wang Z., Ji T., Wang L. Immobilization of laccase for oxidative coupling of trans-resveratrol and its derivatives. Int. J. Mol. Sci. 2012;13:5998–6008. doi: 10.3390/ijms13055998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.de Cazes M., Belleville M.-P., Mougel M., Kellner H., Sanchez-Marcano J. Characterization of laccase-grafted ceramic membranes for pharmaceuticals degradation. J. Membr. Sci. 2015;476:384–393. [Google Scholar]
- 283.Camacho Córdova D.I., Morales Borges R., Arizaga G.G.C., Wypych F., Krieger N. Immobilization of laccase on hybrid layered double hydroxide. Quím. Nova. 2009;32:1495–1499. [Google Scholar]
- 284.Sampaio L.M.P., Padrão J., Faria J., Silva J.P., Silva C.J., Dourado F., Zille A. Laccase immobilization on bacterial nanocellulose membranes: antimicrobial, kinetic and stability properties. Carbohydr. Polym. 2016;145:1–12. doi: 10.1016/j.carbpol.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 285.Le Goff A., Holzinger M., Cosnier S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell. Mol. Life Sci. 2015;72:941–952. doi: 10.1007/s00018-014-1828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ghosh B., Saha R., Bhattacharya D., Mukhopadhyay M. Laccase and its source of sustainability in an enzymatic biofuel cell. Bioresour. Technol. Rep. 2019;6:268–278. [Google Scholar]
- 287.Zhang Y., Lv Z., Zhou J., Xin F., Ma J., Wu H., Fang Y., Jiang M., Dong W. Application of eukaryotic and prokaryotic laccases in biosensor and biofuel cells: recent advances and electrochemical aspects. Appl. Microbiol. Biotechnol. 2018;102:10409–10423. doi: 10.1007/s00253-018-9421-7. [DOI] [PubMed] [Google Scholar]
- 288.Pang H.L., Liu J., Hu D., Zhang X.H., Chen J.H. Immobilization of laccase onto 1-aminopyrene functionalized carbon nanotubes and their electrocatalytic activity for oxygen reduction. Electrochim. Acta. 2010;55:6611–6616. [Google Scholar]
- 289.Zhang Y., Chu M., Yang L., Tan Y., Deng W., Ma M., Su X., Xie Q. Three-dimensional graphene networks as a new substrate for immobilization of laccase and dopamine and its application in glucose/O2 biofuel cell. ACS Appl. Mater. Interfaces. 2014;6:12808–12814. doi: 10.1021/am502791h. [DOI] [PubMed] [Google Scholar]
- 290.Bourourou M., Elouarzaki K., Lalaoui N., Agnès C., Le Goff A., Holzinger M., Maaref A., Cosnier S. Supramolecular immobilization of laccase on carbon nanotube electrodes functionalized with (Methylpyrenylaminomethyl)anthraquinone for direct electron reduction of oxygen. Chem. Eur J. 2013;19:9371–9375. doi: 10.1002/chem.201301043. [DOI] [PubMed] [Google Scholar]
- 291.Luo H., Jin S., Fallgren P.H., Park H.J., Johnson P.A. A novel laccase-catalyzed cathode for microbial fuel cells. Chem. Eng. J. 2010;165:524–528. [Google Scholar]
- 292.Brunel L., Denele J., Servat K., Kokoh K.B., Jolivalt C., Innocent C., Cretin M., Rolland M., Tingry S. Oxygen transport through laccase biocathodes for a membrane-less glucose/O2 biofuel cell. Electrochem. Commun. 2007;9:331–336. [Google Scholar]
- 293.Barrière F., Ferry Y., Rochefort D., Leech D. Targetting redox polymers as mediators for laccase oxygen reduction in a membrane-less biofuel cell. Electrochem. Commun. 2004;6:237–241. [Google Scholar]
- 294.Meredith M.T., Minson M., Hickey D., Artyushkova K., Glatzhofer D.T., Minteer S.D. Anthracene-modified multi-walled carbon nanotubes as direct electron transfer scaffolds for enzymatic oxygen reduction. ACS Catal. 2011;1:1683–1690. [Google Scholar]
- 295.Chao C., Liu J., Wang J., Zhang Y., Zhang B., Zhang Y., Xiang X., Chen R. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces. 2013;5:10559–10564. doi: 10.1021/am4022973. [DOI] [PubMed] [Google Scholar]
- 296.Huang J., Fang H., Liu C., Gu E., Jiang D. A novel fiber optic biosensor for the determination of adrenaline based on immobilized laccase catalysis. Anal. Lett. 2008;41:1430–1442. [Google Scholar]
- 297.Barrière F., Kavanagh P., Leech D. A laccase–glucose oxidase biofuel cell prototype operating in a physiological buffer. Electrochim. Acta. 2006;51:5187–5192. [Google Scholar]
- 298.Gellett W., Schumacher J., Kesmez M., Le D., Minteer S.D. High current density air-breathing laccase biocathode. J. Electrochem. Soc. 2010;157:B557. [Google Scholar]
- 299.Giroud F., Minteer S.D. Anthracene-modified pyrenes immobilized on carbon nanotubes for direct electroreduction of O2 by laccase. Electrochem. Commun. 2013;34:157–160. [Google Scholar]
- 300.Guan D., Kurra Y., Liu W., Chen Z. A click chemistry approach to site-specific immobilization of a small laccase enables efficient direct electron transfer in a biocathode. Chem. Commun. 2015;51:2522–2525. doi: 10.1039/c4cc09179e. [DOI] [PubMed] [Google Scholar]
- 301.Gutiérrez-Sánchez C., Pita M., Vaz-Domínguez C., Shleev S., De Lacey A.L. Gold nanoparticles as electronic bridges for laccase-based biocathodes. J. Am. Chem. Soc. 2012;134:17212–17220. doi: 10.1021/ja307308j. [DOI] [PubMed] [Google Scholar]
- 302.Brondani D., de Souza B., Souza B.S., Neves A., Vieira I.C. PEI-coated gold nanoparticles decorated with laccase: a new platform for direct electrochemistry of enzymes and biosensingapplications. Biosens. Bioelectron. 2013;42:242–247. doi: 10.1016/j.bios.2012.10.087. [DOI] [PubMed] [Google Scholar]
- 303.Stolarczyk K., Nazaruk E., Rogalski J., Bilewicz R. Nanostructured carbon electrodes for laccase-catalyzed oxygen reduction without added mediators. Electrochim. Acta. 2008;53:3983–3990. [Google Scholar]
- 304.Chawla S., Rawal R., Shabnam, Kuhad R.C., Pundir C.S. An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal. Methods. 2011;3:709–714. doi: 10.1039/c0ay00679c. [DOI] [PubMed] [Google Scholar]
- 305.Shimomura T., Itoh T., Sumiya T., Hanaoka T., Mizukami F., Ono M. Amperometric detection of phenolic compounds with enzyme immobilized in mesoporous silica prepared by electrophoretic deposition. Sensor. Actuator. B Chem. 2011;153:361–368. [Google Scholar]
- 306.Gamella M., Campuzano S., Reviejo A.J., Pingarrón J.M. Electrochemical estimation of the polyphenol index in wines using a laccase biosensor. J. Agric. Food Chem. 2006;54:7960–7967. doi: 10.1021/jf061451r. [DOI] [PubMed] [Google Scholar]
- 307.Xu X., Guo M., Lu P., Wang R. Development of amperometric laccase biosensor through immobilizing enzyme in copper-containing ordered mesoporous carbon (Cu-OMC)/chitosan matrix. Mater. Sci. Eng. C. 2010;30:722–729. [Google Scholar]
- 308.Jarosz-Wilkołazka A., Ruzgas T., Gorton L. Use of laccase-modified electrode for amperometric detection of plant flavonoids. Enzym. Microb. Technol. 2004;35:238–241. [Google Scholar]
- 309.Roy J.J., Abraham T.E., Abhijith K.S., Kumar P.V.S., Thakur M.S. Biosensor for the determination of phenols based on Cross-Linked Enzyme Crystals (CLEC) of laccase. Biosens. Bioelectron. 2005;21:206–211. doi: 10.1016/j.bios.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 310.Tortolini C., Di Fusco M., Frasconi M., Favero G., Mazzei F. Laccase–polyazetidine prepolymer–MWCNT integrated system: biochemical properties and application to analytical determinations in real samples. Microchem. J. 2010;96:301–307. [Google Scholar]
- 311.Nazari M., Kashanian S., Rafipour R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015;145:130–138. doi: 10.1016/j.saa.2015.01.126. [DOI] [PubMed] [Google Scholar]
- 312.Yang J., Li D., Fu J., Huang F., Wei Q., Electroanal J. TiO2-CuCNFs based laccase biosensor for enhanced electrocatalysis in hydroquinone detection. Chem. 2016;766:16–23. [Google Scholar]
- 313.Moccelini S.K., Franzoi A.C., Vieira I.C., Dupont J., Scheeren C.W. A novel support for laccase immobilization: cellulose acetate modified with ionic liquid and application in biosensor for methyldopa detection. Biosens. Bioelectron. 2011;26:3549–3554. doi: 10.1016/j.bios.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 314.Fernandes S.C., de Oliveira I.R.W.Z., Fatibello-Filho O., Spinelli A., Vieira I.C. Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate. Sensor. Actuator. B Chem. 2008;133:202–207. [Google Scholar]
- 315.Li Y., Cao X., Qian X., Chen Y., Liu S., Electroanal J. Immobilization of laccase in N-doped carbon hollow spheres/chitosan composite film for electrochemical detection of kraft lignin. Chem. 2012;686:7–11. [Google Scholar]
- 316.Castrovilli M.C., Bolognesi P., Chiarinelli J., Avaldi L., Cartoni A., Calandra P., Tempesta E., Giardi M.T., Antonacci A., Arduini F., Scognamiglio V. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112299. [DOI] [PubMed] [Google Scholar]
- 317.Chawla S., Rawal R., Sharma S., Pundir C.S. An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices. Biochem. Eng. J. 2012;68:76–84. [Google Scholar]
- 318.Chawla S., Rawal R., Pundir C.S. Fabrication of polyphenol biosensor based on laccase immobilized on copper nanoparticles/chitosan/multiwalled carbon nanotubes/polyaniline-modified gold electrode. J. Biotechnol. 2011;156:39–45. doi: 10.1016/j.jbiotec.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 319.Regina Silva T., Cruz Vieira I. A biosensor based on gold nanoparticles stabilized in poly(allylamine hydrochloride) and decorated with laccase for determination of dopamine. Analyst. 2016;141:216–224. doi: 10.1039/c5an01784j. [DOI] [PubMed] [Google Scholar]
- 320.Zhang Y., Li X., Li D., Wei Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf. B Biointerfaces. 2020;186 doi: 10.1016/j.colsurfb.2019.110683. [DOI] [PubMed] [Google Scholar]
- 321.Klis M., Karbarz M., Stojek Z., Rogalski J., Bilewicz R. Thermoresponsive poly(N-isopropylacrylamide) gel for immobilization of laccase on indium tin oxide electrodes. J. Phys. Chem. B. 2009;113:6062–6067. doi: 10.1021/jp8094159. [DOI] [PubMed] [Google Scholar]
- 322.Rochefort D., Kouisni L., Gendron K. Physical immobilization of laccase on an electrode by means of poly(ethyleneimine) microcapsules. J. Electroanal. Chem. 2008;617:53–63. [Google Scholar]
- 323.Chandran M., Aswathy E., Shamna I., Vinoba M., Kottappara R., Bhagiyalakshmi M. Laccase immobilized on Au confined MXene based electrode for electrochemical detection of catechol. Mater. Today Proc. 2021;46:3136–3143. [Google Scholar]
- 324.Li G., Sun K., Li D., Lv P., Wang Q., Huang F., Wei Q. Biosensor based on bacterial cellulose-Au nanoparticles electrode modified with laccase for hydroquinone detection. Colloids Surf. A Physicochem. Eng. Asp. 2016;509:408–414. [Google Scholar]
- 325.Đurđić S., Stanković V., Vlahović F., Ognjanović M., Kalcher K., Veličković T.Ć., Mutić J., Stanković D.M. Laccase polyphenolic biosensor supported on MnO$\less$sub$\greater$2$\less$/sub$\greater$@GNP decorated SPCE: preparation, characterization, and analytical application. J. Electrochem. Soc. 2021;168 [Google Scholar]
- 326.Fu J., Qiao H., Li D., Luo L., Chen K., Wei Q. Laccase biosensor based on electrospun copper/carbon composite nanofibers for catechol detection. Sensors. 2014;14:3543–3556. doi: 10.3390/s140203543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Litescu S.C., Eremia S.A.V., Bertoli A., Pistelli L., Radu G.-L. Laccase-nafion based biosensor for the determination of polyphenolic secondary metabolites. Anal. Lett. 2010;43:1089–1099. [Google Scholar]
- 328.Dai Z., Guo M.Q., Wang X.J., Wang H.F., Chen W.Y. Development of amperometric laccase biosensor through immobilizing enzyme in magnesium-containing mesoporous silica sieve (Mg-MCM-41)/polyvinyl alcohol matrix. J. Nanomater. 2014;2014:1–8. [Google Scholar]
- 329.Fu J., Pang Z., Yang J., Huang F., Cai Y., Wei Q. Fabrication of polyaniline/carboxymethyl cellulose/cellulose nanofibrous mats and their biosensing application. Appl. Surf. Sci. 2015;349:35–42. [Google Scholar]
- 330.Jo E., Lee J. Polyaniline-nanofiber-modified screen-printed electrode with intermediate dye amplification for detection of endocrine disruptor bisphenol A. Microchem. J. 2020;155 [Google Scholar]
- 331.Kunene K., Sabela M., Kanchi S., Bisetty K. High performance electrochemical biosensor for bisphenol A using screen printed electrodes modified with multiwalled carbon nanotubes functionalized with silver-doped zinc oxide. Waste Biomass Valorization. 2020;11:1085–1096. [Google Scholar]
- 332.Othman A.M., Wollenberger U. Amperometric biosensor based on coupling aminated laccase to functionalized carbon nanotubes for phenolics detection. Int. J. Biol. Macromol. 2020;153:855–864. doi: 10.1016/j.ijbiomac.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 333.Pang Y., Zeng G., Tang L., Zhang Y., Li Z., Chen L. Laccase biosensor using magnetic multiwalled carbon nanotubes and chitosan/silica hybrid membrane modified magnetic carbon paste electrode. J. Cent. South Univ. Technol. 2011;18:1849–1856. [Google Scholar]
- 334.Tan Y., Deng W., Ge B., Xie Q., Huang J., Yao S. Biofuel cell and phenolic biosensor based on acid-resistant laccase–glutaraldehyde functionalized chitosan–multiwalled carbon nanotubes nanocomposite film. Biosens. Bioelectron. 2009;24:2225–2231. doi: 10.1016/j.bios.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 335.Albayati S.A.R., Kashanian S., Nazari M., Rezaei S. Novel fabrication of a laccase biosensor to detect phenolic compounds using a carboxylated multiwalled carbon nanotube on the electropolymerized support. Bull. Mater. Sci. 2019;42:187. [Google Scholar]
- 336.Canevari T.C., Cincotto F.H., Nakamura M., Machado S.A.S., Toma H.E. Efficient electrochemical biosensors for ethynylestradiol based on the laccase enzyme supported on single walled carbon nanotubes decorated with nanocrystalline carbon quantum dots. Anal. Methods. 2016;8:7254–7259. [Google Scholar]
- 337.Cincotto F.H., Canevari T.C., Machado S.A.S., Sánchez A., Barrio M.A.R., Villalonga R., Pingarrón J.M. Reduced graphene oxide-Sb2O5 hybrid nanomaterial for the design of a laccase-based amperometric biosensor for estriol. Electrochim. Acta. 2015;174:332–339. [Google Scholar]
- 338.Lou C., Jing T., Zhou J., Tian J., Zheng Y., Wang C., Zhao Z., Lin J., Liu H., Zhao C., Guo Z. Laccase immobilized polyaniline/magnetic graphene composite electrode for detecting hydroquinone. Int. J. Biol. Macromol. 2020;149:1130–1138. doi: 10.1016/j.ijbiomac.2020.01.248. [DOI] [PubMed] [Google Scholar]
- 339.Palanisamy S., Ramaraj S.K., Chen S.-M., Yang T.C.K., Yi-Fan P., Chen T.-W., Velusamy V., Selvam S. A novel laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol. Sci. Rep. 2017;7 doi: 10.1038/srep41214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kohori N.A., da Silva M.K.L., Cesarino I. Evaluation of graphene oxide and reduced graphene oxide in the immobilization of laccase enzyme and its application in the determination of dopamine. J. Solid State Electrochem. 2018;22:141–148. [Google Scholar]
- 341.Tortolini C., Rea S., Carota E., Cannistraro S., Mazzei F. Influence of the immobilization procedures on the electroanalytical performances of Trametes versicolor laccase based bioelectrode. Microchem. J. 2012;100:8–13. [Google Scholar]
- 342.Songurtekin D., Yalcinkaya E.E., Ag D., Seleci M., Demirkol D.O., Timur S. Histidine modified montmorillonite: laccase immobilization and application to flow injection analysis of phenols. Appl. Clay Sci. 2013;86:64–69. [Google Scholar]
- 343.Casero E., Petit-Domínguez M.D., Vázquez L., Ramírez-Asperilla I., Parra-Alfambra A.M., Pariente F., Lorenzo E. Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta. 2013;115:401–408. doi: 10.1016/j.talanta.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 344.Li Y., Zhang L., Li M., Pan Z., Li D. A disposable biosensor based on immobilization of laccase with silica spheres on the MWCNTs-doped screen-printed electrode. Chem. Cent. J. 2012;6:103. doi: 10.1186/1752-153X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Diaconu M., Litescu S.C., Radu G.L. Laccase–MWCNT–chitosan biosensor—a new tool for total polyphenolic content evaluation from in vitro cultivated plants. Sensor. Actuator. B Chem. 2010;145:800–806. [Google Scholar]
- 346.Xu X., Lu P., Zhou Y., Zhao Z., Guo M. Laccase immobilized on methylene blue modified mesoporous silica MCM-41/PVA. Mater. Sci. Eng. C. 2009;29:2160–2164. [Google Scholar]
- 347.Rawal R., Chawla S., Pundir C.S. Polyphenol biosensor based on laccase immobilized onto silver nanoparticles/multiwalled carbon nanotube/polyaniline gold electrode. Anal. Biochem. 2011;419:196–204. doi: 10.1016/j.ab.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 348.Abdullah J., Ahmad M., Heng L.Y., Karuppiah N., Sidek H. An optical biosensor based on immobilization of laccase and MBTH in stacked films for the detection of catechol. Sensors. 2007;7:2238–2250. doi: 10.3390/s7102238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Quan D., Shin W. Amperometric detection of catechol and catecholamines by immobilized laccase from DeniLite. Electroanalysis. 2004;16:1576–1582. [Google Scholar]
- 350.Yaropolov A.I., Shleev S.V., Morozova O.V., Zaitseva E.A., Marko-Varga G., Emneus J., Gorton L. An amperometric biosensor based on laccase immobilized in polymer matrices for determining phenolic compounds. J. Anal. Chem. 2005;60:553–557. [Google Scholar]
- 351.Quan D., Shin W. Modification of electrode surface for covalent immobilization of laccase. Mater. Sci. Eng. C. 2004;24:113–115. [Google Scholar]
- 352.Zouari N., Romette J.-L., Thomas D. Laccase electrode for the continuous-flow determination of phenolic compounds. Biotechnol. Tech. 1994;8:503–508. [Google Scholar]
- 353.Rawal R., Chawla S., Pundir C.S. Polyphenol biosensor based on laccase immobilized onto silver nanoparticles/multiwalled carbon nanotube/polyaniline gold electrode. Anal. Biochem. 2011;419:196–204. doi: 10.1016/j.ab.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 354.Wu X., Hu Y., Jin J., Zhou N., Wu P., Zhang H., Cai C. Electrochemical approach for detection of extracellular oxygen released from erythrocytes based on graphene film integrated with laccase and 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Anal. Chem. 2010;82:3588–3596. doi: 10.1021/ac100621r. [DOI] [PubMed] [Google Scholar]
- 355.Jabbari S., Dabirmanesh B., Khajeh K. Specificity enhancement towards phenolic substrate by immobilization of laccase on surface plasmon resonance sensor chip. J. Mol. Catal. B Enzym. 2015;121:32–36. [Google Scholar]
- 356.Rodríguez-Delgado M.M., Alemán-Nava G.S., Rodríguez-Delgado J.M., Dieck-Assad G., Martínez-Chapa S.O., Barceló D., Parra R. Laccase-based biosensors for detection of phenolic compounds. Trends Anal. Chem. 2015;74:21–45. [Google Scholar]
- 357.Castrovilli M.C., Bolognesi P., Chiarinelli J., Avaldi L., Calandra P., Antonacci A., Scognamiglio V. The convergence of forefront technologies in the design of laccase-based biosensors – an update. Trends Anal. Chem. 2019;119 [Google Scholar]
- 358.Kadam A.A., Saratale G.D., Ghodake G.S., Saratale R.G., Shahzad A., Magotra V.K., Kumar M., Palem R.R. Recent advances in the development of laccase-based biosensors via nano-immobilization techniques. J.-S. Sung, Chemosensors. 2022;10:58. [Google Scholar]
- 359.Jiang D.-S., Long S.-Y., Huang J., Xiao H.-Y., Zhou J.-Y. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005;25:15–23. [Google Scholar]
- 360.Zhu Y., Kaskel S., Shi J., Wage T., van Pée K.-H. Immobilization of Trametes versicolor laccase on magnetically separable mesoporous silica spheres. Chem. Mater. 2007;19:6408–6413. [Google Scholar]
- 361.Patel S.K.S., Gupta R.K., Kim S.-Y., Kim I.-W., Kalia V.C., Lee J.-K. Laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Rhus vernicifera Indian J. Microbiol. 2021;61:45–54. doi: 10.1007/s12088-020-00912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Plagemann R., Jonas L., Kragl U. Ceramic honeycomb as support for covalent immobilization of laccase from Trametes versicolor and transformation of nuclear fast red. Appl. Microbiol. Biotechnol. 2011;90:313–320. doi: 10.1007/s00253-010-3038-9. [DOI] [PubMed] [Google Scholar]
- 363.Amari A., Alzahrani F.M., Alsaiari N.S., Katubi K.M., Rebah F.B., Tahoon M.A. Magnetic metal organic framework immobilized laccase for wastewater decolorization. Processes. 2021;9:774. [Google Scholar]
- 364.Sheldon R.A. Enzyme immobilization: the quest for optimum performance. Adv. Synth. Catal. 2007;349:1289–1307. [Google Scholar]
- 365.Homaei A.A., Sariri R., Vianello F., Stevanato R. Enzyme immobilization: an update. J. Chem. Biol. 2013;6:185–205. doi: 10.1007/s12154-013-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bayramoglu G., Yilmaz M., Yakup Arica M. Preparation and characterization of epoxy-functionalized magnetic chitosan beads: laccase immobilized for degradation of reactive dyes. Bioproc. Biosyst. Eng. 2010;33:439–448. doi: 10.1007/s00449-009-0345-6. [DOI] [PubMed] [Google Scholar]
- 367.Makas Y.G., Kalkan N.A., Aksoy S., Altinok H., Hasirci N. Immobilization of laccase in κ-carrageenan based semi-interpenetrating polymer networks. J. Biotechnol. 2010;148:216–220. doi: 10.1016/j.jbiotec.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 368.Qiu H., Xu C., Huang X., Ding Y., Qu Y., Gao P., Phys J. Adsorption of laccase on the surface of nanoporous gold and the direct electron transfer between them. Chem. Can. 2008;112:14781–14785. [Google Scholar]
- 369.Vera M., Rivas B.L. Immobilization of Trametes versicolor laccase on different PGMA-based polymeric microspheres using response surface methodology: optimization of conditions. J. Appl. Polym. Sci. 2017;134 [Google Scholar]
- 370.Mahmoodi N.M., Saffar-Dastgerdi M.H., Hayati B. Environmentally friendly novel covalently immobilized enzyme bionanocomposite: from synthesis to the destruction of pollutant. Compos. B Eng. 2020;184 [Google Scholar]
- 371.Mukhopadhyay A., Dasgupta A.Kr., Chakrabarti K. Enhanced functionality and stabilization of a cold active laccase using nanotechnology based activation-immobilization. Bioresour. Technol. 2015;179:573–584. doi: 10.1016/j.biortech.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 372.Yinghui D., Qiuling W., Shiyu F. Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett. Appl. Microbiol. 2002;35:451–456. doi: 10.1046/j.1472-765x.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 373.Arıca M.Y., Altıntas B., Bayramoğlu G. Immobilization of laccase onto spacer-arm attached non-porous poly(GMA/EGDMA) beads: application for textile dye degradation. Bioresour. Technol. 2009;100:665–669. doi: 10.1016/j.biortech.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 374.Al-Adhami A.J.H., Bryjak J., Greb-Markiewicz B., Peczyńska-Czoch W. Immobilization of wood-rotting fungi laccases on modified cellulose and acrylic carriers. Process Biochem. 2002;37:1387–1394. [Google Scholar]
- 375.Zille A., Tzanov T., Gübitz G.M., Cavaco-Paulo A. Immobilized laccase for decolourization of Reactive Black 5 dyeing effluent. Biotechnol. Lett. 2003;25:1473–1477. doi: 10.1023/a:1025032323517. [DOI] [PubMed] [Google Scholar]
- 376.Silva C., Silva C.J., Zille A., Guebitz G.M., Cavaco-Paulo A. Laccase immobilization on enzymatically functionalized polyamide 6,6 fibres. Enzym. Microb. Technol. 2007;41:867–875. [Google Scholar]
- 377.Noreen S., Perveen S., Shafiq N., Aslam S., Iqbal H.M.N., Ashraf S.S., Bilal M. Laccase-loaded functionalized graphene oxide assemblies with improved biocatalytic properties and decolorization performance. Environ. Technol. Innovat. 2021;24 [Google Scholar]
- 378.Tavares A.P.M., Rodríguez O., Fernández-Fernández M., Domínguez A., Moldes D., Sanromán M.A., Macedo E.A. Immobilization of laccase on modified silica: stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. Bioresour. Technol. 2013;131:405–412. doi: 10.1016/j.biortech.2012.12.139. [DOI] [PubMed] [Google Scholar]
- 379.Rekuć A., Kruczkiewicz P., Jastrzembska B., Liesiene J., Peczyńska-Czoch W., Bryjak J. Laccase immobilization on the tailored cellulose-based Granocel carriers. Int. J. Biol. Macromol. 2008;42:208–215. doi: 10.1016/j.ijbiomac.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 380.Gutiérrez-Sánchez C., Jia W., Beyl Y., Pita M., Schuhmann W., De Lacey A.L., Stoica L. Enhanced direct electron transfer between laccase and hierarchical carbon microfibers/carbon nanotubes composite electrodes. Comparison of three enzyme immobilization methods. Electrochim. Acta. 2012;82:218–223. [Google Scholar]
- 381.Othman A.M., González-Domínguez E., Sanromán Á., Correa-Duarte M., Moldes D. Immobilization of laccase on functionalized multiwalled carbon nanotube membranes and application for dye decolorization. RSC Adv. 2016;6:114690–114697. [Google Scholar]
- 382.Patel S.K.S., Choi S.H., Kang Y.C., Lee J.-K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl. Mater. Interfaces. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 383.Wang Q., Cui J., Li G., Zhang J., Li D., Huang F., Wei Q. Laccase immobilized on a PAN/adsorbents composite nanofibrous membrane for catechol treatment by a biocatalysis/adsorption process. Molecules. 2014;19:3376–3388. doi: 10.3390/molecules19033376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Vasil’eva I.S., Morozova O.V., Shumakovich G.P., Yaropolov A.I. Synthesis of electroconductive polyaniline using immobilized laccase. Appl. Biochem. Microbiol. 2009;45:27–30. [PubMed] [Google Scholar]
- 385.Wang Q., Peng L., Li G., Zhang P., Li D., Huang F., Wei Q. Activity of laccase immobilized on TiO2-montmorillonite complexes. Int. J. Mol. Sci. 2013;14:12520–12532. doi: 10.3390/ijms140612520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Patel S.K.S., Kalia V.C., Choi J.-H., Haw J.-R., Kim I.-W., Lee J.K. Immobilization of laccase on $SiO_2$ nanocarriers improves its stability and reusability. J. Microbiol. Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 387.Spinelli D., Fatarella E., Di Michele A., Pogni R. Immobilization of fungal (Trametes versicolor) laccase onto Amberlite IR-120 H beads: optimization and characterization. Process Biochem. 2013;48:218–223. [Google Scholar]
- 388.Zhang J., Xu Z., Chen H., Zong Y. Removal of 2,4-dichlorophenol by chitosan-immobilized laccase from Coriolus versicolor. Biochem. Eng. J. 2009;45:54–59. [Google Scholar]
- 389.Mazlan S.Z., Hanifah S.A. Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int. J. Polym. Sci. 2017;2017 [Google Scholar]
- 390.Bryjak J., Kruczkiewicz P., Rekuć A., Peczyńska-Czoch W. Laccase immobilization on copolymer of butyl acrylate and ethylene glycol dimethacrylate. Biochem. Eng. J. 2007;35:325–332. [Google Scholar]
- 391.Cristóvão R.O., Tavares A.P.M., Brígida A.I., Loureiro J.M., Boaventura R.A.R., Macedo E.A., Coelho M.A.Z. Immobilization of commercial laccase onto green coconut fiber by adsorption and its application for reactive textile dyes degradation. J. Mol. Catal. B Enzym. 2011;72:6–12. [Google Scholar]
- 392.Xiao H., Huang J., Liu C., Jiang D. Immobilization of laccase on amine-terminated magnetic nano-composite by glutaraldehyde crosslinking method. Trans. Nonferrous Metals Soc. China. 2006;16:s414–s418. [Google Scholar]
- 393.Qiu H., Xu C., Huang X., Ding Y., Qu Y., Gao P. Immobilization of laccase on nanoporous gold: comparative studies on the immobilization strategies and the particle size effects. J. Phys. Chem. C. 2009;113:2521–2525. [Google Scholar]
- 394.Huang J., Liu C., Xiao H., Wang J., Jiang D., Gu E. Zinc tetraaminophthalocyanine-Fe3O4 nanoparticle composite for laccase immobilization. Int. J. Nanomed. 2007;2:775–784. [PMC free article] [PubMed] [Google Scholar]
- 395.Lante A., Crapisi A., Krastanov A., Spettoli P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem. 2000;36:51–58. [Google Scholar]
- 396.Ruiz A.I., Malave A.J., Felby C., Griebenow K. Improved activity and stability of an immobilized recombinant laccase in organic solvents. Biotechnol. Lett. 2000;22:229–233. [Google Scholar]
- 397.Wang F., Guo C., Yang L., Liu C.-Z. Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour. Technol. 2010;101:8931–8935. doi: 10.1016/j.biortech.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 398.Çorman M.E., Öztürk N., Bereli N., Akgöl S., Denizli A. Preparation of nanoparticles which contains histidine for immobilization of Trametes versicolor laccase. J. Mol. Catal. B Enzym. 2010;63:102–107. [Google Scholar]
- 399.Fang H., Huang J., Ding L., Li M., Chen Z. Preparation of magnetic chitosan nanoparticles and immobilization of laccase. J. Wuhan Univ. Technol.-Materials Sci. Ed. 2009;24:42–47. [Google Scholar]
- 400.Fortes C.C.S., Daniel-da-Silva A.L., Xavier A.M.R.B., Tavares A.P.M. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. Process Intensif. 2017;117:1–8. [Google Scholar]
- 401.Yang J., Wang Z., Lin Y., Ng T.B., Ye X., Lin J. Immobilized Cerrena sp. laccase: preparation, thermal inactivation, and operational stability in malachite green decolorization. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-16771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Gökgöz M., Altinok H. Immobilization of laccase on polyacrylamide and polyacrylamide – κ – carragennan-based semi-interpenetrating polymer networks. Artif. Cell Blood Substit. Biotechnol. 2012;40:326–330. doi: 10.3109/10731199.2012.658469. [DOI] [PubMed] [Google Scholar]
- 403.Moeder M., Martin C., Koeller G. Degradation of hydroxylated compounds using laccase and horseradish peroxidase immobilized on microporous polypropylene hollow fiber membranes. J. Membr. Sci. 2004;245:183–190. [Google Scholar]
- 404.Zheng F., Cui B.-K., Wu X.-J., Meng G., Liu H.-X., Si J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016;110:69–78. [Google Scholar]
- 405.Tran D.N., Balkus K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011;1:956–968. [Google Scholar]
- 406.Zhao M., Wang Y., Liu Z., Cui D., Bian X. Properties of immobilized laccase on mesostructured cellular foam silica and its use in dye decolorization. J. Macromol. Sci. Part A. 2011;48:447–453. [Google Scholar]
- 407.Guerrero M.P., Bertrand F., Rochefort D. Activity, stability and inhibition of a bioactive paper prepared by large-scale coating of laccase microcapsules. Chem. Eng. Sci. 2011;66:5313–5320. [Google Scholar]
- 408.Dai Y., Niu J., Liu J., Yin L., Xu J. In situ encapsulation of laccase in microfibers by emulsion electrospinning: preparation, characterization, and application. Bioresour. Technol. 2010;101:8942–8947. doi: 10.1016/j.biortech.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 409.Sarma R., Islam Md.S., Miller A.-F., Bhattacharyya D. Layer-by-Layer-Assembled laccase enzyme on stimuli-responsive membranes for chloro-organics degradation. ACS Appl. Mater. Interfaces. 2017;9:14858–14867. doi: 10.1021/acsami.7b01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Asgher M., Shahid M., Kamal S., Iqbal H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Catal. B Enzym. 2014;101:56–66. [Google Scholar]
- 411.Wang Y., Chen X., Liu J., He F., Wang R. Immobilization of laccase by Cu2+ chelate affinity interaction on surface-modified magnetic silica particles and its use for the removal of 2,4-dichlorophenol. Environ. Sci. Pollut. Res. 2013;20:6222–6231. doi: 10.1007/s11356-013-1661-6. [DOI] [PubMed] [Google Scholar]
- 412.Rani M., Shanker U., Chaurasia A.K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: degradation of alizarin red S dye. J. Environ. Chem. Eng. 2017;5:2730–2739. [Google Scholar]
- 413.Wang Q., Cui J., Li G., Zhang J., Huang F., Wei Q. Laccase immobilization by chelated metal ion coordination chemistry. Polymers. 2014;6:2357–2370. [Google Scholar]
- 414.Xia T.-T., Liu C.-Z., Hu J.-H., Guo C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem. Eng. J. 2016;295:201–206. [Google Scholar]
- 415.Li Y., Huang X., Qu Y. A strategy for efficient immobilization of laccase and horseradish peroxidase on single-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2013;88:2227–2232. [Google Scholar]
- 416.Salami F., Habibi Z., Yousefi M., Mohammadi M. Covalent immobilization of laccase by one pot three component reaction and its application in the decolorization of textile dyes. Int. J. Biol. Macromol. 2018;120:144–151. doi: 10.1016/j.ijbiomac.2018.08.077. [DOI] [PubMed] [Google Scholar]
- 417.Kashefi S., Borghei S.M., Mahmoodi N.M. Covalently immobilized laccase onto graphene oxide nanosheets: preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2019;276:153–162. [Google Scholar]
- 418.Labus K., Gancarz I., Bryjak J. Immobilization of laccase and tyrosinase on untreated and plasma-treated cellulosic and polyamide membranes. Mater. Sci. Eng. C. 2012;32:228–235. [Google Scholar]
- 419.Cabana H., Alexandre C., Agathos S.N., Jones J.P. Immobilization of laccase from the white rot fungus Coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresour. Technol. 2009;100:3447–3458. doi: 10.1016/j.biortech.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 420.Kuznetsov B.A., Shumakovich G.P., Koroleva O.V., Yaropolov A.I. On applicability of laccase as label in the mediated and mediatorless electroimmunoassay: effect of distance on the direct electron transfer between laccase and electrode. Biosens. Bioelectron. 2001;16:73–84. doi: 10.1016/s0956-5663(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 421.Cabana H., Ahamed A., Leduc R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour. Technol. 2011;102:1656–1662. doi: 10.1016/j.biortech.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 422.Chen J., Leng J., Yang X., Liao L., Liu L., Xiao A. Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules. 2017;22:221. doi: 10.3390/molecules22020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Pezzella C., Russo M.E., Marzocchella A., Salatino P., Sannia G. Immobilization of a Pleurotus ostreatus laccase mixture on perlite and its application to dye decolourisation. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/308613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Karagoz B., Bayramoglu G., Altintas B., Bicak N., Yakup Arica M. Amine functional monodisperse microbeads via precipitation polymerization of N-vinyl formamide: immobilized laccase for benzidine based dyes degradation. Bioresour. Technol. 2011;102:6783–6790. doi: 10.1016/j.biortech.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 425.Ammann E.M., Gasser C.A., Hommes G., Corvini P.F.-X. Immobilization of defined laccase combinations for enhanced oxidation of phenolic contaminants. Appl. Microbiol. Biotechnol. 2014;98:1397–1406. doi: 10.1007/s00253-013-5055-y. [DOI] [PubMed] [Google Scholar]
- 426.Lonappan L., Liu Y., Rouissi T., Pourcel F., Brar S.K., Verma M., Surampalli R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018;351:985–994. [Google Scholar]
- 427.Arsenault A., Cabana H., Jones J.P. Laccase-based CLEAs: chitosan as a novel cross-linking agent. Enzym. Res. 2011:1–10. doi: 10.4061/2011/376015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Cao P., Liu H., Wu D., Wang X. Immobilization of laccase on phase-change microcapsules as self-thermoregulatory enzyme carrier for biocatalytic enhancement. Chem. Eng. J. 2021;405 [Google Scholar]
- 429.Hong J., Jung D., Park S., Oh Y., Oh K.K., Lee S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021;169:541–550. doi: 10.1016/j.ijbiomac.2020.12.136. [DOI] [PubMed] [Google Scholar]
- 430.Costa J.B., Lima M.J., Sampaio M.J., Neves M.C., Faria J.L., Morales-Torres S., Tavares A.P.M., Silva C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019;355:974–985. [Google Scholar]
- 431.Stanescu M.D., Fogorasi M., Shaskolskiy B.L., Gavrilas S., Lozinsky V.I. New potential biocatalysts by laccase immobilization in PVA cryogel type carrier. Appl. Biochem. Biotechnol. 2010;160:1947–1954. doi: 10.1007/s12010-009-8755-0. [DOI] [PubMed] [Google Scholar]
- 432.Irena G., Jolanta B., Karolina Z. Chemical modification of poly(ethylene terephthalate) and immobilization of the selected enzymes on the modified film. Appl. Surf. Sci. 2009;255:8293–8298. [Google Scholar]
- 433.Rotková J., Šuláková R., Korecká L., Zdražilová P., Jandová M., Lenfeld J., Horák D., Bílková Z. Laccase immobilized on magnetic carriers for biotechnology applications. J. Magn. Magn Mater. 2009;321:1335–1340. [Google Scholar]
- 434.Gonzalez-Coronel L.A., Cobas M., Rostro-Alanis M. de J., Parra-Saldívar R., Hernandez-Luna C., Pazos M., Sanromán M.Á. Immobilization of laccase of Pycnoporus sanguineus CS43. N. Biotech. 2017;39:141–149. doi: 10.1016/j.nbt.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 435.Russo M.E., Giardina P., Marzocchella A., Salatino P., Sannia G. Assessment of anthraquinone-dye conversion by free and immobilized crude laccase mixtures. Enzym. Microb. Technol. 2008;42:521–530. [Google Scholar]
- 436.Fathali Z., Rezaei S., Faramarzi M.A., Habibi-Rezaei M. Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int. J. Biol. Macromol. 2019;122:359–366. doi: 10.1016/j.ijbiomac.2018.10.147. [DOI] [PubMed] [Google Scholar]
- 437.Matijošytė I., Arends I.W.C.E., de Vries S., Sheldon R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010;62:142–148. [Google Scholar]
- 438.Perveen S., Noreen S., Shahid S., Mehboob H., Aslam S., Iqbal H.M.N., Bilal M. Carrier-free cross-linked laccase crystals for biocatalytic degradation of textile industrial effluents. Appl. Biochem. Biotechnol. 2022;194:1775–1789. doi: 10.1007/s12010-021-03795-3. [DOI] [PubMed] [Google Scholar]
- 439.Sheldon R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs) Appl. Microbiol. Biotechnol. 2011;92:467–477. doi: 10.1007/s00253-011-3554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Patel I., Ludwig R., Mueangtoom K., Haltrich D., Rosenau T., Potthast A. Vol. 63. 2009. pp. 715–720. (Comparing Soluble Trametes Pubescens Laccase and Cross-Linked Enzyme Crystals (CLECs) for Enzymatic Modification of Cellulose 10th EWLP, Stockholm, Sweden, August 25–28, 2008). [Google Scholar]
- 441.Roy J.J., Abraham T.E. Preparation and characterization of cross-linked enzyme crystals of laccase. J. Mol. Catal. B Enzym. 2006;38:31–36. [Google Scholar]
- 442.Gogoi P., Hazarika S., Dutta N.N., Rao P.G. Kinetics and mechanism on laccase catalyzed synthesis of poly(allylamine)–catechin conjugate. Chem. Eng. J. 2010;163:86–92. [Google Scholar]
- 443.Sheldon R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011;15:213–223. [Google Scholar]
- 444.Hartmann M., Jung D. Biocatalysis with enzymes immobilized on mesoporous hosts : the status quo and future trends. J. Mater. Chem. 2010;20:844–857. [Google Scholar]
- 445.Bilal M., Noreen S., Asgher M., Parveen S. Development and characterization of cross-linked laccase aggregates (Lac-CLEAs) from Trametes versicolor IBL-04 as ecofriendly biocatalyst for degradation of dye-based environmental pollutants. Environ. Technol. Innovat. 2021;21 [Google Scholar]
- 446.Zheng X., Wang Q., Jiang Y., Gao J. Biomimetic synthesis of magnetic composite particles for laccase immobilization. Ind. Eng. Chem. Res. 2012;51:10140–10146. [Google Scholar]
- 447.Velasco-Lozano S., López-Gallego F., Mateos-Díaz J.C., Favela-Torres E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement – a review. Biocatalysis. 2016;1:166–177. [Google Scholar]
- 448.Nguyen L.T., Seow N., Yang K.-L. Hollow cross-linked enzyme aggregates (h-CLEA) of laccase with high uniformity and activity. Colloids Surf. B Biointerfaces. 2017;151:88–94. doi: 10.1016/j.colsurfb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 449.Vršanská M., Voběrková S., Jiménez Jiménez A.M., Strmiska V., Adam V. Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and fomes fomentarius for the decolourisation of synthetic dyes. Int. J. Environ. Res. Publ. Health. 2018;15:23. doi: 10.3390/ijerph15010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Yang J., Xu X., Yang X., Ye X., Lin J. Cross-linked enzyme aggregates of Cerrena laccase: preparation, enhanced NaCl tolerance and decolorization of remazol brilliant blue reactive. J. Taiwan Inst. Chem. Eng. 2016;65:1–7. [Google Scholar]
- 451.Vinoth Kumar V., Prem Kumar M.P., Thiruvenkadaravi K.V., Baskaralingam P., Senthil Kumar P., Sivanesan S. Preparation and characterization of porous cross linked laccase aggregates for the decolorization of triphenyl methane and reactive dyes. Bioresour. Technol. 2012;119:28–34. doi: 10.1016/j.biortech.2012.05.078. [DOI] [PubMed] [Google Scholar]
- 452.Primožič M., Kravanja G., Knez Ž., Crnjac A., Leitgeb M. Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio)degradation. J. Clean. Prod. 2020;275 [Google Scholar]
- 453.Şahutoğlu A.S., Akgül C. One-phase synthesis of single enzyme nanoparticles (SENs) of Trametes versicolor laccase by in situ acrylamide polymerisation. Biocatal. Biotransform. 2020;38:64–74. [Google Scholar]
- 454.Nguyen L.N., Hai F.I., Dosseto A., Richardson C., Price W.E., Nghiem L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresour. Technol. 2016;210:108–116. doi: 10.1016/j.biortech.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 455.Zdarta J., Meyer A.S., Jesionowski T., Pinelo M. Developments in support materials for immobilization of oxidoreductases: a comprehensive review. Adv. Colloid Interface Sci. 2018;258:1–20. doi: 10.1016/j.cis.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 456.Solís-Oba M., Ugalde-Saldívar V.M., González I., Viniegra-González G. An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J. Electroanal. Chem. 2005;579:59–66. [Google Scholar]
- 457.Leontievsky A., Myasoedova N., Golovleva L., Bucke C., Evans C. Transformation of 2,4,6-trichlorophenol by free and immobilized fungal laccase. Appl. Microbiol. Biotechnol. 2001;57:85–91. doi: 10.1007/s002530100756. [DOI] [PubMed] [Google Scholar]
- 458.Champagne P.-P., Ramsay J.A. Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresour. Technol. 2010;101:2230–2235. doi: 10.1016/j.biortech.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 459.Piao M., Zou D., Ren X., Gao S., Qin C., Piao Y. High efficiency biotransformation of bisphenol A in a fluidized bed reactor using stabilized laccase in porous silica. Enzym. Microb. Technol. 2019;126:1–8. doi: 10.1016/j.enzmictec.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 460.Zhang H., Zhang X., Wang B., Zeng X., Guo H., Ren B., Yang X. Immobilization of laccase onto functionalized ionic liquid-modified mesoporous silica SBA-15. Biocatal. Biotransform. 2021:1–10. 0. [Google Scholar]
- 461.Khanmohammadi F., Molina M.A., Blanco R.M., Azizi S.N., Márquez-Álvarez C., Díaz I. SBA-15 with short channels for laccase immobilization. Microporous Mesoporous Mater. 2020;309 [Google Scholar]
- 462.Gascón V., Díaz I., Márquez-Álvarez C., Blanco R.M. Mesoporous silicas with tunable morphology for the immobilization of laccase. Molecules. 2014;19:7057–7071. doi: 10.3390/molecules19067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kołodziejczak-Radzimska A., Budna A., Ciesielczyk F., Moszyński D., Jesionowski T. Laccase from Trametes versicolor supported onto mesoporous Al2O3: stability tests and evaluations of catalytic activity. Process Biochem. 2020;95:71–80. [Google Scholar]
- 464.Bruera F.A., Kramer G.R., Velázquez J.E., Sadañoski M.A., Fonseca M.I., Ares A.E., Zapata P.D. Laccase immobilization on nanoporous aluminum oxide for black liquor treatment. Surface. Interfac. 2022;30 [Google Scholar]
- 465.Taghizadeh T., Talebian-Kiakalaieh A., Jahandar H., Amin M., Tarighi S., Faramarzi M.A. Biodegradation of bisphenol A by the immobilized laccase on some synthesized and modified forms of zeolite Y. J. Hazard Mater. 2020;386 doi: 10.1016/j.jhazmat.2019.121950. [DOI] [PubMed] [Google Scholar]
- 466.Wehaidy H.R., Abdel-Naby M.A., El-Hennawi H.M., Youssef H.F. Nanoporous Zeolite-X as a new carrier for laccase immobilization and its application in dyes decolorization. Biocatal. Agric. Biotechnol. 2019;19 [Google Scholar]
- 467.Salaj-Kosla U., Pöller S., Schuhmann W., Shleev S., Magner E. Direct electron transfer of Trametes hirsuta laccase adsorbed at unmodified nanoporous gold electrodes. Bioelectrochemistry. 2013;91:15–20. doi: 10.1016/j.bioelechem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 468.Stine K.J. Enzyme immobilization on nanoporous gold: a review. Biochem. Insights. 2017;10 doi: 10.1177/1178626417748607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Stine K.J., Jefferson K., Shulga O.V. In: Enzyme Stab. Immobil. Methods Protoc. Minteer S.D., editor. Humana Press; Totowa, NJ: 2011. pp. 67–83. (Nanoporous Gold for Enzyme Immobilization). https://doi.org/10.1007/978-1-60761-895-9_7. [Google Scholar]
- 470.Collinson M.M. Nanoporous gold electrodes and their applications in analytical chemistry. Anal. Chem. 2013;2013:1–21. [Google Scholar]
- 471.Qiu H., Li Y., Ji G., Zhou G., Huang X., Qu Y., Gao P. Immobilization of lignin peroxidase on nanoporous gold: enzymatic properties and in situ release of H2O2 by co-immobilized glucose oxidase. Bioresour. Technol. 2009;100:3837–3842. doi: 10.1016/j.biortech.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 472.Xiao X., Si P., Magner E. An overview of dealloyed nanoporous gold in bioelectrochemistry. Bioelectrochemistry. 2016;109:117–126. doi: 10.1016/j.bioelechem.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 473.Hakamada M., Takahashi M., Mabuchi M. Enhanced thermal stability of laccase immobilized on monolayer-modified nanoporous Au. Mater. Lett. 2012;66:4–6. [Google Scholar]
- 474.Wen X., Zeng Z., Du C., Huang D., Zeng G., Xiao R., Lai C., Xu P., Zhang C., Wan J., Hu L., Yin L., Zhou C., Deng R. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere. 2019;222:865–871. doi: 10.1016/j.chemosphere.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 475.Mehandia S., Ahmad S., Sharma S.C., Arya S.K. Decolorization and detoxification of textile effluent by immobilized laccase-ACS into chitosan-clay composite beads using a packed bed reactor system: an ecofriendly approach. J. Water Proc. Eng. 2022;47 [Google Scholar]
- 476.Patila M., Athanasiou P.E., Kortessis L., Potsi G., Kouloumpis A., Gournis D., Stamatis H. Immobilization of laccase on hybrid super-structured nanomaterials for the decolorization of phenolic dyes. Processes. 2022;10:233. [Google Scholar]
- 477.Olshansky Y., Masaphy S., Root R.A., Rytwo G. Immobilization of Rhus vernicifera laccase on sepiolite; effect of chitosan and copper modification on laccase adsorption and activity. Appl. Clay Sci. 2018;152:143–147. [Google Scholar]
- 478.Anita S.H., Ardiati F.C., Oktaviani M., Sari F.P., Nurhayat O.D., Ramadhan K.P., Yanto D.H.Y. Immobilization of laccase from Trametes hirsuta EDN 082 in light expanded clay aggregate for decolorization of Remazol Brilliant Blue R dye. Bioresour. Technol. Rep. 2020;12 [Google Scholar]
- 479.Ardiati F.C., Yanto D.H.Y., Anita S.H., Watanabe T. Immobilization of Trametes hirsuta D7 in light expanded clay aggregate for decolorization of synthetic dye. IOP Conf. Ser. Earth Environ. Sci. 2019;308 [Google Scholar]
- 480.Davis S., Burns R.G. Covalent immobilization of laccase on activated carbon for phenolic effluent treatment. Appl. Microbiol. Biotechnol. 1992;37:474–479. [Google Scholar]
- 481.Freire R.S., Durán N., Kubota L.T. Effects of fungal laccase immobilization procedures for the development of a biosensor for phenol compounds. Talanta. 2001;54:681–686. doi: 10.1016/s0039-9140(01)00318-6. [DOI] [PubMed] [Google Scholar]
- 482.Park J.H., Xue H., Jung J.S., Ryu K. Immobilization of laccase on carbon nanomaterials. Kor. J. Chem. Eng. 2012;29:1409–1412. [Google Scholar]
- 483.Ding S.-N., Holzinger M., Mousty C., Cosnier S. Laccase electrodes based on the combination of single-walled carbon nanotubes and redox layered double hydroxides: towards the development of biocathode for biofuel cells. J. Power Sources. 2010;195:4714–4717. [Google Scholar]
- 484.Zhang W., Yang Q., Luo Q., Shi L., Meng S. Laccase-Carbon nanotube nanocomposites for enhancing dyes removal. J. Clean. Prod. 2020;242 [Google Scholar]
- 485.Yoon T., Baek I., Lee S., Choi H., Yoon S., Lee H., Ung Kim S., Na S. Immobilization of laccase on a graphene interface: direct electron transfer and molecular dynamics study. Appl. Surf. Sci. 2020;521 [Google Scholar]
- 486.Rouhani S., Rostami A., Salimi A., Pourshiani O. Graphene oxide/CuFe2O4 nanocomposite as a novel scaffold for the immobilization of laccase and its application as a recyclable nanobiocatalyst for the green synthesis of arylsulfonyl benzenediols. Biochem. Eng. J. 2018;133:1–11. [Google Scholar]
- 487.Ormategui N., Veloso A., Leal G.P., Rodriguez-Couto S., Tomovska R. Design of stable and powerful nanobiocatalysts, based on enzyme laccase immobilized on self-assembled 3D graphene/polymer composite hydrogels. ACS Appl. Mater. Interfaces. 2015;7:14104–14112. doi: 10.1021/acsami.5b03325. [DOI] [PubMed] [Google Scholar]
- 488.Rodriguez-Couto S., Arzac A., Leal G.P., Tomovska R. Reduced graphene oxide hydrogels and xerogels provide efficient platforms for immobilization and laccase production by Trametes pubescens. Biotechnol. J. 2014;9:578–584. doi: 10.1002/biot.201300474. [DOI] [PubMed] [Google Scholar]
- 489.Rouhani S., Azizi S., Kibechu R.W., Mamba B.B., Msagati T.A.M. Laccase immobilized Fe3O4-graphene oxide nanobiocatalyst improves stability and immobilization efficiency in the green preparation of sulfa drugs. Catalysts. 2020;10:459. [Google Scholar]
- 490.Samak N.A., Tan Y., Sui K., Xia T.-T., Wang K., Guo C., Liu C. CotA laccase immobilized on functionalized magnetic graphene oxide nano-sheets for efficient biocatalysis. Mol. Catal. 2018;445:269–278. [Google Scholar]
- 491.Mahmoodi N.M., Saffar-Dastgerdi M.H. Clean Laccase immobilized nanobiocatalysts (graphene oxide - zeolite nanocomposites): from production to detailed biocatalytic degradation of organic pollutant. Appl. Catal. B Environ. 2020;268 [Google Scholar]
- 492.Lalaoui N., Le Goff A., Holzinger M., Mermoux M., Cosnier S. Wiring laccase on covalently modified graphene: carbon nanotube assemblies for the direct bio-electrocatalytic reduction of oxygen. Chem. Eur J. 2015;21:3198–3201. doi: 10.1002/chem.201405557. [DOI] [PubMed] [Google Scholar]
- 493.Aguila S.A., Shimomoto D., Ipinza F., Bedolla-Valdez Z.I., Romo-Herrera J., Contreras O.E., Farías M.H., Alonso-Núñez G. A biosensor based on Coriolopsis gallica laccase immobilized on nitrogen-doped multiwalled carbon nanotubes and graphene oxide for polyphenol detection. Sci. Technol. Adv. Mater. 2015;16 doi: 10.1088/1468-6996/16/5/055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Ruiz-Palomero C., Benítez-Martínez S., Soriano M.L., Valcárcel M. Fluorescent nanocellulosic hydrogels based on graphene quantum dots for sensing laccase. Anal. Chim. Acta. 2017;974:93–99. doi: 10.1016/j.aca.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 495.Xu H.-M., Sun X.-F., Wang S.-Y., Song C., Wang S.-G. Development of laccase/graphene oxide membrane for enhanced synthetic dyes separation and degradation. Separ. Purif. Technol. 2018;204:255–260. [Google Scholar]
- 496.Silva C.G., Tavares A.P.M., Dražić G., Silva A.M.T., Loureiro J.M., Faria J.L. Controlling the surface chemistry of multiwalled carbon nanotubes for the production of highly efficient and stable laccase-based biocatalysts. ChemPlusChem. 2014;79:1116–1122. [Google Scholar]
- 497.Bourourou M., Elouarzaki K., Holzinger M., Agnès C., Goff A.L., Reverdy-Bruas N., Chaussy D., Party M., Maaref A., Cosnier S. Freestanding redox buckypaper electrodes from multi-wall carbon nanotubes for bioelectrocatalytic oxygen reduction via mediated electron transfer. Chem. Sci. 2014;5:2885. –2888. [Google Scholar]
- 498.Tavares A.P.M., Silva C.G., Dražić G., Silva A.M.T., Loureiro J.M., Faria J.L. Laccase immobilization over multi-walled carbon nanotubes: kinetic, thermodynamic and stability studies. J. Colloid Interface Sci. 2015;454:52–60. doi: 10.1016/j.jcis.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 499.Trohalaki S., Pachter R., Luckarift H.R., Johnson G.R. Immobilization of the laccases from Trametes versicolor and Streptomyces coelicolor on single-wall carbon nanotube electrodes: a molecular dynamics study. Fuel Cell. 2012;12:656–664. [Google Scholar]
- 500.Cosnier S., Haddad R., Moatsou D., O’Reilly R.K. Biofunctionalizable flexible bucky paper by combination of multi-walled carbon nanotubes and polynorbornene-pyrene – application to the bioelectrocatalytic reduction of oxygen. Carbon. 2015;93:713–718. [Google Scholar]
- 501.Dai Y., Yao J., Song Y., Liu X., Wang S., Yuan Y. Enhanced performance of immobilized laccase in electrospun fibrous membranes by carbon nanotubes modification and its application for bisphenol A removal from water. J. Hazard Mater. 2016;317:485–493. doi: 10.1016/j.jhazmat.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 502.Habimana P., Gao J., Mwizerwa J.P., Ndayambaje J.B., Liu H., Luan P., Ma L., Jiang Y. Improvement of laccase activity via covalent immobilization over mesoporous silica coated magnetic multiwalled carbon nanotubes for the discoloration of synthetic dyes. ACS Omega. 2021;6:2777–2789. doi: 10.1021/acsomega.0c05081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Sorrentino I., Stanzione I., Piscitelli A., Giardina P., Goff A.L. Carbon-nanotube-supported POXA1b laccase and its hydrophobin chimera for oxygen reduction and picomolar phenol biosensing. Biosens. Bioelectron. X. 2021;8 [Google Scholar]
- 504.Girelli A.M., Scuto F.R. Eggshell membrane as feedstock in enzyme immobilization. J. Biotechnol. 2021;325:241–249. doi: 10.1016/j.jbiotec.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 505.Cristóvão R.O., Silvério S.C., Tavares A.P.M., Brígida A.I.S., Loureiro J.M., Boaventura R.A.R., Macedo E.A., Coelho M.A.Z. Green coconut fiber: a novel carrier for the immobilization of commercial laccase by covalent attachment for textile dyes decolourization. World J. Microbiol. Biotechnol. 2012;28:2827–2838. doi: 10.1007/s11274-012-1092-4. [DOI] [PubMed] [Google Scholar]
- 506.Suman S.K., Patnam P.L., Ghosh S., Jain S.L. Chicken feather derived novel support material for immobilization of laccase and its application in oxidation of veratryl alcohol. ACS Sustain. Chem. Eng. 2019;7:3464–3474. [Google Scholar]
- 507.Torán J., Blánquez P., Caminal G. Comparison between several reactors with Trametes versicolor immobilized on lignocellulosic support for the continuous treatments of hospital wastewater. Bioresour. Technol. 2017;243:966–974. doi: 10.1016/j.biortech.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 508.Przystaś W., Zabłocka-Godlewska E. Improvement of efficiency of dye removal by immobilization of fungal biomass. Ecol. Chem. Eng. A. 2020;27:1–11. [Google Scholar]
- 509.Girelli A.M., Pambianco E., Scuto F.R. Sustainable recycling of spent grain for laccase immobilization as dyes removal tool. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 510.Girelli A.M., Scuto F.R. Spent grain as a sustainable and low-cost carrier for laccase immobilization. Waste Manag. 2021;128:114–121. doi: 10.1016/j.wasman.2021.04.055. [DOI] [PubMed] [Google Scholar]
- 511.Wang W., Zhang Z., Ni H., Yang X., Li Q., Li L. Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microb. Cell Factories. 2012;11:75. doi: 10.1186/1475-2859-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.da Silva A.M., Tavares A.P.M., Rocha C.M.R., Cristóvão R.O., Teixeira J.A., Macedo E.A. Immobilization of commercial laccase on spent grain. Process Biochem. 2012;47:1095–1101. [Google Scholar]
- 513.Zhang Y., Piao M., He L., Yao L., Piao T., Liu Z., Piao Y. Immobilization of laccase on magnetically separable biochar for highly efficient removal of bisphenol A in water. RSC Adv. 2020;10:4795–4804. doi: 10.1039/c9ra08800h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Adamian Y., Lonappan L., Alokpa K., Agathos S.N., Cabana H. Recent developments in the immobilization of laccase on carbonaceous supports for environmental applications - a critical review. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.778239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Zhang H., Hay A.G., Hazard J. Magnetic biochar derived from biosolids via hydrothermal carbonization: enzyme immobilization, immobilized-enzyme kinetics, environmental toxicity. Materials. 2020;384 doi: 10.1016/j.jhazmat.2019.121272. [DOI] [PubMed] [Google Scholar]
- 516.Li N., Xia Q., Niu M., Ping Q., Xiao H. Immobilizing laccase on different species wood biochar to remove the chlorinated biphenyl in wastewater. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Pandey D., Daverey A., Arunachalam K. Biochar: production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020;255 [Google Scholar]
- 518.Shokri Z., Seidi F., Karami S., Li C., Saeb M.R., Xiao H. Laccase immobilization onto natural polysaccharides for biosensing and biodegradation. Carbohydr. Polym. 2021;262 doi: 10.1016/j.carbpol.2021.117963. [DOI] [PubMed] [Google Scholar]
- 519.Zdarta J., Machałowski T., Degórska O., Bachosz K., Fursov A., Ehrlich H., Ivanenko V.N., Jesionowski T. 3D chitin scaffolds from the marine demosponge aplysina archeri as a support for laccase immobilization and its use in the removal of pharmaceuticals. Biomolecules. 2020;10:646. doi: 10.3390/biom10040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Delanoy G., Li Q., Yu J. Activity and stability of laccase in conjugation with chitosan. Int. J. Biol. Macromol. 2005;35:89–95. doi: 10.1016/j.ijbiomac.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 521.Alver E., Metin A.Ü. Chitosan based metal-chelated copolymer nanoparticles: laccase immobilization and phenol degradation studies. Int. Biodeterior. Biodegrad. 2017;125:235–242. [Google Scholar]
- 522.de Araújo J.H.B., Uemura V.O., de Moraes F.F., Barbosa A. de M., Zanin G.M. A comparative study on fungal laccases immobilized on chitosan. Braz. Arch. Biol. Technol. 2005;48:1–6. [Google Scholar]
- 523.Lin J., Fan L., Miao R., Le X., Chen S., Zhou X. Enhancing catalytic performance of laccase via immobilization on chitosan/CeO2 microspheres. Int. J. Biol. Macromol. 2015;78:1–8. doi: 10.1016/j.ijbiomac.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 524.Palmieri G., Giardina P., Sannia G. Laccase-mediated remazol brilliant blue R decolorization in a fixed-bed bioreactor. Biotechnol. Prog. 2005;21:1436–1441. doi: 10.1021/bp050140i. [DOI] [PubMed] [Google Scholar]
- 525.Ma H.-F., Meng G., Cui B.-K., Si J., Dai Y.-C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: laccase immobilization and characterization. Chem. Eng. Res. Des. 2018;132:664–676. [Google Scholar]
- 526.Bayramoglu G., Gursel I., Yilmaz M., Arica M.Y. Immobilization of laccase on itaconic acid grafted and Cu(II) ion chelated chitosan membrane for bioremediation of hazardous materials. J. Chem. Technol. Biotechnol. 2012;87:530–539. [Google Scholar]
- 527.Bilal M., Jing Z., Zhao Y., Iqbal H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019;19 [Google Scholar]
- 528.Qu B., Luo Y. Chitosan-based hydrogel beads: preparations, modifications and applications in food and agriculture sectors – a review. Int. J. Biol. Macromol. 2020;152:437–448. doi: 10.1016/j.ijbiomac.2020.02.240. [DOI] [PubMed] [Google Scholar]
- 529.Vazquez-Duhalt R., Tinoco R., D’Antonio P., Topoleski L.D.T., Payne G.F. Enzyme conjugation to the polysaccharide chitosan: smart biocatalysts and biocatalytic hydrogels. Bioconjugate Chem. 2001;12:301–306. doi: 10.1021/bc000095u. [DOI] [PubMed] [Google Scholar]
- 530.Yao J., Wang Q., Wang Y., Zhang Y., Zhang B., Zhang H. Immobilization of laccase on chitosan–halloysite hybrid porous microspheres for phenols removal. Desalination Water Treat. 2015;55:1293–1301. [Google Scholar]
- 531.Metin A.Ü. Immobilization of laccase onto polyethyleneimine grafted chitosan films: effect of system parameters. Macromol. Res. 2013;21:1145–1152. [Google Scholar]
- 532.Wu R., Liu F., Dong Q., Huang Y., Qiu Y., Sun Y., Su E. Combination of adsorption and cellulose derivative membrane coating for efficient immobilization of laccase. Appl. Biochem. Biotechnol. 2021;193:446–462. doi: 10.1007/s12010-020-03446-z. [DOI] [PubMed] [Google Scholar]
- 533.Xing X., Han Y., Jiang Q., Sun Y., Wang X., Qu G., Sun G., Li Y. Immobilization of laccases onto cellulose nanocrystals derived from waste newspaper: relationship between immobilized laccase activity and dialdehyde content. Cellulose. 2021;28:4793–4805. [Google Scholar]
- 534.Bagheri M., Rodríguez H., Swatloski R.P., Spear S.K., Daly D.T., Rogers R.D. Ionic liquid-based preparation of Cellulose−Dendrimer films as solid supports for enzyme immobilization. Biomacromolecules. 2008;9:381–387. doi: 10.1021/bm701023w. [DOI] [PubMed] [Google Scholar]
- 535.Frazão C.J.R., Silva N.H.C., Freire C.S.R., Silvestre A.J.D., Xavier A.M.R.B., Tavares A.P.M. Bacterial cellulose as carrier for immobilization of laccase: optimization and characterization. Eng. Life Sci. 2014;14:500–508. [Google Scholar]
- 536.Chen L., Zou M., Hong F.F. Evaluation of fungal laccase immobilized on natural nanostructured bacterial cellulose. Front. Microbiol. 2015;6:e1245. doi: 10.3389/fmicb.2015.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Wu D., Lv P., Feng Q., Jiang Y., Yang H., Alfred M., Wei Q. Biomass-derived nanocellulose aerogel enable highly efficient immobilization of laccase for the degradation of organic pollutants. Bioresour. Technol. 2022;356 doi: 10.1016/j.biortech.2022.127311. [DOI] [PubMed] [Google Scholar]
- 538.Drozd R., Rakoczy R., Wasak A., Junka A., Fijałkowski K. The application of magnetically modified bacterial cellulose for immobilization of laccase. Int. J. Biol. Macromol. 2018;108:462–470. doi: 10.1016/j.ijbiomac.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 539.Milstein O., Hüttermann A., Majcherczyk A., Schulze K., Fründ R., Lüdemann H.-D. Transformation of lignin-related compounds with laccase in organic solvents. J. Biotechnol. 1993;30:37–48. [Google Scholar]
- 540.Brugnari T., Pereira M.G., Bubna G.A., de Freitas E.N., Contato A.G., Corrêa R.C.G., Castoldi R., de Souza C.G.M., Polizeli M. de L.T. de M., Bracht A., Peralta R.M. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018;634:1346–1351. doi: 10.1016/j.scitotenv.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 541.Fernández-Fernández M., Moldes D., Domínguez A., Sanromán M.Á., Tavares A.P.M., Rodríguez O., Macedo E.A. Stability and kinetic behavior of immobilized laccase from Myceliophthora thermophila in the presence of the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. Biotechnol. Prog. 2014;30:790–796. doi: 10.1002/btpr.1910. [DOI] [PubMed] [Google Scholar]
- 542.Gioia L., Rodríguez-Couto S., Menéndez M. del P., Manta C., Ovsejevi K. Reversible covalent immobilization of Trametes villosa laccase onto thiolsulfinate-agarose: an insoluble biocatalyst with potential for decoloring recalcitrant dyes. Biotechnol. Appl. Biochem. 2015;62:502–513. doi: 10.1002/bab.1287. [DOI] [PubMed] [Google Scholar]
- 543.Lassouane F., Aït-Amar H., Amrani S., Rodriguez-Couto S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019;271:360–367. doi: 10.1016/j.biortech.2018.09.129. [DOI] [PubMed] [Google Scholar]
- 544.Wang P., Fan X., Cui L., Wang Q., Zhou A. Decolorization of reactive dyes by laccase immobilized in alginate/gelatin blent with PEG. J. Environ. Sci. 2008;20:1519–1522. doi: 10.1016/s1001-0742(08)62559-0. [DOI] [PubMed] [Google Scholar]
- 545.Vieira Y.A., Gurgel D., Henriques R.O., Machado R.A.F., de Oliveira D., Oechsler B.F., Furigo Junior A. A perspective review on the application of polyacrylonitrile-based supports for laccase immobilization. Chem. Rec. 2022;22 doi: 10.1002/tcr.202100215. [DOI] [PubMed] [Google Scholar]
- 546.Misra N., Kumar V., Goel N.K., Varshney L. Laccase immobilization on radiation synthesized epoxy functionalized polyethersulfone beads and their application for degradation of acid dye. Polymer. 2014;55:6017–6024. [Google Scholar]
- 547.Bello-Gil D., Roig-Molina E., Fonseca J., Sarmiento-Ferrández M.D., Ferrándiz M., Franco E., Mira E., Maestro B., Sanz J.M. An enzymatic system for decolorization of wastewater dyes using immobilized CueO laccase-like multicopper oxidase on poly-3-hydroxybutyrate. Microb. Biotechnol. 2018;11:881–892. doi: 10.1111/1751-7915.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Jiang X., Yu Y., Li X., Kong X.Z. High yield preparation of uniform polyurea microspheres through precipitation polymerization and their application as laccase immobilization support. Chem. Eng. J. 2017;328:1043–1050. [Google Scholar]
- 549.Arica M.Y., Salih B., Celikbicak O., Bayramoglu G. Immobilization of laccase on the fibrous polymer-grafted film and study of textile dye degradation by MALDI–ToF-MS. Chem. Eng. Res. Des. 2017;128:107–119. [Google Scholar]
- 550.Lloret L., Hollmann F., Eibes G., Feijoo G., Moreira M.T., Lema J.M. Immobilisation of laccase on Eupergit supports and its application for the removal of endocrine disrupting chemicals in a packed-bed reactor, Biodegradation. Chem. Eng. Res. Des. 2012;23:373–386. doi: 10.1007/s10532-011-9516-7. [DOI] [PubMed] [Google Scholar]
- 551.Assalin M.R., Rosa M.A., Durán N. Trametes versicolour laccase immobilization by covalent binding and its application in Kraft E1 effluent pre-treated with ozone. Biocatal. Biotransform. 2022:1–9. 0. [Google Scholar]
- 552.D’Annibale A., Stazi S.R., Vinciguerra V., Giovannozzi Sermanni G. Oxirane-immobilized Lentinula edodes laccase: stability and phenolics removal efficiency in olive mill wastewater. J. Biotechnol. 2000;77:265–273. doi: 10.1016/s0168-1656(99)00224-2. [DOI] [PubMed] [Google Scholar]
- 553.Lloret L., Eibes G., Feijoo G., Moreira M.T., Lema J.M. Continuous operation of a fluidized bed reactor for the removal of estrogens by immobilized laccase on Eupergit supports. J. Biotechnol. 2012;162:404–406. doi: 10.1016/j.jbiotec.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 554.Mani P., Fidal V.T., Keshavarz T., Chandra T.S., Kyazze G. Laccase immobilization strategies for application as a cathode catalyst in microbial fuel cells for azo dye decolourization. Front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.620075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Wang X., Sjöberg-Eerola P., Immonen K., Bobacka J., Bergelin M. Immobilization of Trametes hirsuta laccase into poly(3,4-ethylenedioxythiophene) and polyaniline polymer-matrices. J. Power Sources. 2011;196:4957–4964. [Google Scholar]
- 556.Kim J.H., Hong S.-G., Sun H.J., Ha S., Kim J. Precipitated and chemically-crosslinked laccase over polyaniline nanofiber for high performance phenol sensing. Chemosphere. 2016;143:142–147. doi: 10.1016/j.chemosphere.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 557.Jankowska K., Zdarta J., Grzywaczyk A., Kijeńska-Gawrońska E., Biadasz A., Jesionowski T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109332. [DOI] [PubMed] [Google Scholar]
- 558.Kashyap D., Kim C., Kim S.Y., Kim Y.H., Kim G.M., Dwivedi P.K., Sharma A., Goel S. Multi walled carbon nanotube and polyaniline coated pencil graphite based bio-cathode for enzymatic biofuel cell. Int. J. Hydrogen Energy. 2015;40:9515–9522. [Google Scholar]
- 559.Hui J., Jiang X., Xie H., Chen D., Shen J., Sun X., Han W., Li J., Wang L. Laccase-catalyzed electrochemical fabrication of polyaniline/graphene oxide composite onto graphite felt electrode and its application in bioelectrochemical system. Electrochim. Acta. 2016;190:16–24. [Google Scholar]
- 560.Bai X., Gu H., Chen W., Shi H., Yang B., Huang X., Zhang Q. Immobilized laccase on activated poly(vinyl alcohol) microspheres for enzyme thermistor application. Appl. Biochem. Biotechnol. 2014;173:1097–1107. doi: 10.1007/s12010-014-0913-3. [DOI] [PubMed] [Google Scholar]
- 561.Peng J., Wu E., Lou X., Deng Q., Hou X., Lv C., Hu Q. Anthraquinone removal by a metal-organic framework/polyvinyl alcohol cryogel-immobilized laccase: effect and mechanism exploration. Chem. Eng. J. 2021;418 [Google Scholar]
- 562.Stloukal R., Watzková J., Gregušová B. Dye decolorisation by laccase immobilised in lens-shaped poly(vinyl alcohol) hydrogel capsules. Chem. Pap. 2014;68:1514–1520. [Google Scholar]
- 563.Herkommerová K., Zemančíková J., Sychrová H., Antošová Z. Immobilization in polyvinyl alcohol hydrogel enhances yeast storage stability and reusability of recombinant laccase-producing S. cerevisiae. Biotechnol. Lett. 2018;40:405–411. doi: 10.1007/s10529-017-2485-0. [DOI] [PubMed] [Google Scholar]
- 564.Chao C., Guan H., Zhang J., Liu Y., Zhao Y., Zhang B. Immobilization of laccase onto porous polyvinyl alcohol/halloysite hybrid beads for dye removal. Water Sci. Technol. 2017;77:809–818. doi: 10.2166/wst.2017.594. [DOI] [PubMed] [Google Scholar]
- 565.Bankeeree W., Prasongsuk S., Imai T., Lotrakul P., Punnapayak H. A novel xylan-polyvinyl alcohol hydrogel bead with laccase entrapment for decolorization of reactive black. Bioresources. 2016;5(11):6984–7000. [Google Scholar]
- 566.Shrier A., Giroud F., Rasmussen M., Minteer S.D. Operational stability assays for bioelectrodes for biofuel cells: effect of immobilization matrix on laccase biocathode stability. J. Electrochem. Soc. 2014;161:H244. [Google Scholar]
- 567.Stolarczyk K., Sepelowska M., Lyp D., Żelechowska K., Biernat J.F., Rogalski J., Farmer K.D., Roberts K.N., Bilewicz R. Hybrid biobattery based on arylated carbon nanotubes and laccase. Bioelectrochemistry. 2012;87:154–163. doi: 10.1016/j.bioelechem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 568.Chomicz R., Bystrzejewski M., Stolarczyk K. Carbon-encapsulated iron nanoparticles as a magnetic modifier of bioanode and biocathode in a biofuel cell and biobattery. Catalysts. 2021;11:705. [Google Scholar]
- 569.Bogdanovskaya V.A., Arkad’eva I.N., Osina M.A. Bioelectrocatalytic oxygen reduction by laccase immobilized on various carbon carriers. Russ. J. Electrochem. 2017;53:1323–1333. [Google Scholar]
- 570.Choi S.D., Choi J.H., Kim Y.H., Kim S.Y., Dwivedi P.K., Sharma A., Goel S., Kim G.M. Enzyme immobilization on microelectrode arrays of CNT/Nafion nanocomposites fabricated using hydrogel microstencils. Microelectron. Eng. 2015;141:193–197. [Google Scholar]
- 571.Khani Z., Jolivalt C., Cretin M., Tingry S., Innocent C. Alginate/carbon composite beads for laccase and glucose oxidase encapsulation: application in biofuel cell technology. Biotechnol. Lett. 2006;28:1779–1786. doi: 10.1007/s10529-006-9160-1. [DOI] [PubMed] [Google Scholar]
- 572.Yavaşer R., Karagözler A.A. Laccase immobilized polyacrylamide-alginate cryogel: a candidate for treatment of effluents. Process Biochem. 2021;101:137–146. [Google Scholar]
- 573.Sun H., Yang H., Huang W., Zhang S. Immobilization of laccase in a sponge-like hydrogel for enhanced durability in enzymatic degradation of dye pollutants. J. Colloid Interface Sci. 2015;450:353–360. doi: 10.1016/j.jcis.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 574.Liu J., Shen X., Zheng Z., Li M., Zhu X., Cao H., Cui C. Immobilization of laccase by 3D bioprinting and its application in the biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2020;164:518–525. doi: 10.1016/j.ijbiomac.2020.07.144. [DOI] [PubMed] [Google Scholar]
- 575.Jahangiri E., Reichelt S., Thomas I., Hausmann K., Schlosser D., Schulze A. Electron beam-induced immobilization of laccase on porous supports for waste water treatment applications. Molecules. 2014;19:11860–11882. doi: 10.3390/molecules190811860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Mazur M., Krysiński P., Michota-Kamińska A., Bukowska J., Rogalski J., Blanchard G.J. Immobilization of laccase on gold, silver and indium tin oxide by zirconium–phosphonate–carboxylate (ZPC) coordination chemistry. Bioelectrochemistry. 2007;71:15–22. doi: 10.1016/j.bioelechem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 577.Vaz-Domínguez C., Pita M., de Lacey A.L., Shleev S., Cuesta A., Phys J. Combined ATR-SEIRAS and EC-STM study of the immobilization of laccase on chemically modified Au electrodes. Chem. Can. 2012;116:16532–16540. [Google Scholar]
- 578.Li Y., Wu T., Ali M.A., Alhemaid F.M.A. Immobilization of laccase into poly(3,4- ethylenedioxythiophene) assisted biocathode for biofuel cell applications. Int. J. Electrochem. Sci. 2012;7:11400–11413. [Google Scholar]
- 579.Palys B., Bokun A., Rogalski J. Poly-o-phenylenediamine as redox mediator for laccase. Electrochim. Acta. 2007;52:7075–7082. [Google Scholar]
- 580.Pałys B., Marzec M., Rogalski J. Poly-o-aminophenol as a laccase mediator and influence of the enzyme on the polymer electrodeposition. Bioelectrochemistry. 2010;80:43–48. doi: 10.1016/j.bioelechem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 581.Gao Z., Yi Y., Zhao J., Xia Y., Jiang M., Cao F., Zhou H., Wei P., Jia H., Yong X. Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization. Bioresour. Bioprocess. 2018;5:27. [Google Scholar]
- 582.Nogala W., Rozniecka E., Zawisza I., Rogalski J., Opallo M. Immobilization of ABTS – laccase system in silicate based electrode for biolectrocatalytic reduction of dioxygen. Electrochem. Commun. 2006;8:1850–1854. [Google Scholar]
- 583.Gu Y., Yuan L., Jia L., Xue P., Yao H. Recent developments of a co-immobilized laccase–mediator system: a review. RSC Adv. 2021;11:29498–29506. doi: 10.1039/d1ra05104k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Shan H., Wang X., Ge Y., Li Z. Homologous amino acids promoted co-immobilization of laccase and mediator onto geopolymer microspheres for enhancing degradation of dyes in water. J. Hazard Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127107. [DOI] [PubMed] [Google Scholar]
- 585.Savolainen A., Zhang Y., Rochefort D., Holopainen U., Erho T., Virtanen J., Smolander M. Biomacromolecules, printing of polymer microcapsules for enzyme immobilization on paper substrate. Biomacromolecules. 2011;12:2008–2015. doi: 10.1021/bm2003434. [DOI] [PubMed] [Google Scholar]
- 586.Zhong Z., Pang S., Wu Y., Jiang S., Ouyang J., Chem J., Biotechnol Technol. Synthesis and characterization of mesoporous Cu–MOF for laccase immobilization. J. Appl. Chem. Biotechnol. 2017;92:1841–1847. [Google Scholar]
- 587.Wang J., Yu S., Feng F., Lu L. Simultaneous purification and immobilization of laccase on magnetic zeolitic imidazolate frameworks: recyclable biocatalysts with enhanced stability for dye decolorization. Biochem. Eng. J. 2019;150 [Google Scholar]
- 588.Pang S., Wu Y., Zhang X., Li B., Ouyang J., Ding M. Immobilization of laccase via adsorption onto bimodal mesoporous Zr-MOF. Process Biochem. 2016;51:229–239. [Google Scholar]
- 589.Gascón V., Márquez-Álvarez C., Blanco R.M. Efficient retention of laccase by non-covalent immobilization on amino-functionalized ordered mesoporous silica. Appl. Catal. Gen. 2014;482:116–126. [Google Scholar]
- 590.Tocco D., Carucci C., Todde D., Shortall K., Otero F., Sanjust E., Magner E., Salis A. Enzyme immobilization on metal organic frameworks: laccase from Aspergillus sp. is better adapted to ZIF-zni rather than Fe-BTC. Colloids Surf. B Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112147. [DOI] [PubMed] [Google Scholar]
- 591.Ladole M.R., Pokale P.B., Patil S.S., Belokar P.G., Pandit A.B., Technol Bioresour. Laccase immobilized peroxidase mimicking magnetic metal organic frameworks for industrial dye degradation. Bioresour. Technol. 2020;317 doi: 10.1016/j.biortech.2020.124035. [DOI] [PubMed] [Google Scholar]
- 592.Huang W., Zhang W., Gan Y., Yang J., Zhang S. Laccase immobilization with metal-organic frameworks: current status, remaining challenges and future perspectives. Crit. Rev. Environ. Sci. Technol. 2022;52:1282–1324. [Google Scholar]
- 593.Hu J., Yuan B., Zhang Y., Guo M. Immobilization of laccase on magnetic silica nanoparticles and its application in the oxidation of guaiacol, a phenolic lignin model compound. RSC Adv. 2015;5:99439–99447. [Google Scholar]
- 594.Mahmoodi N.M., Arabloo M., Abdi J. Laccase immobilized manganese ferrite nanoparticle: synthesis and LSSVM intelligent modeling of decolorization. Water Res. 2014;67:216–226. doi: 10.1016/j.watres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 595.Muthuvelu K.S., Rajarathinam R., Selvaraj R.N., Rajendren V.B. A novel method for improving laccase activity by immobilization onto copper ferrite nanoparticles for lignin degradation. Int. J. Biol. Macromol. 2020;152:1098–1107. doi: 10.1016/j.ijbiomac.2019.10.198. [DOI] [PubMed] [Google Scholar]
- 596.Isanapong J., Pornwongthong P. Immobilized laccase on zinc oxide nanoarray for catalytic degradation of tertiary butyl alcohol. J. Hazard Mater. 2021;411 doi: 10.1016/j.jhazmat.2021.125104. [DOI] [PubMed] [Google Scholar]
- 597.Hou J., Dong G., Ye Y., Chen V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membr. Sci. 2014;452:229–240. [Google Scholar]
- 598.Balaji Nagarajan, Sathish Kumar Kannaiyan, Seenuvasan Muthulingam, Vinodhini Govindasamy, Madhava Anil Kumar J. Immobilization of laccase onto micro-emulsified magnetic nanoparticles for enhanced degradation of a textile recalcitrant. Environ. Biol. 2016;37:1489–1496. [Google Scholar]
- 599.Kalkan N.A., Aksoy S., Aksoy E.A., Hasirci N. Preparation of chitosan-coated magnetite nanoparticles and application for immobilization of laccase. J. Appl. Polym. Sci. 2012;123:707–716. [Google Scholar]
- 600.Shanmugam S., Krishnaswamy S., Chandrababu R., Veerabagu U., Pugazhendhi A., Mathimani T. Optimal immobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel. 2020;273 [Google Scholar]
- 601.Canbolat M.F., Savas H.B., Gultekin F. Enzymatic behavior of laccase following interaction with γ-CD and immobilization into PCL nanofibers. Anal. Biochem. 2017;528:13–18. doi: 10.1016/j.ab.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 602.Wu D., Feng Q., Xu T., Wei A., Fong H. Electrospun blend nanofiber membrane consisting of polyurethane, amidoxime polyarcylonitrile, and β-cyclodextrin as high-performance carrier/support for efficient and reusable immobilization of laccase. Chem. Eng. J. 2018;331:517–526. [Google Scholar]
- 603.Huang J., Xiao H., Li B., Wang J., Jiang D. Immobilization of Pycnoporus sanguineus laccase on copper tetra-aminophthalocyanine–Fe3O4 nanoparticle composite. Biotechnol. Appl. Biochem. 2006;44:93–100. doi: 10.1042/BA20050213. [DOI] [PubMed] [Google Scholar]
- 604.ElKaoutit M., Naranjo-Rodriguez I., Temsamani K.R., de la Vega M.D., de Cisneros J.L.H.-H. Dual laccase–tyrosinase based sonogel–carbon biosensor for monitoring polyphenols in beers. J. Agric. Food Chem. 2007;55:8011–8018. doi: 10.1021/jf0711136. [DOI] [PubMed] [Google Scholar]
- 605.Kochana J., Nowak P., Jarosz-Wilkołazka A., Bieroń M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J. 2008;89:171–174. [Google Scholar]
- 606.Montereali M.R., Seta L.D., Vastarella W., Pilloton R. A disposable Laccase–Tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine. J. Mol. Catal. B Enzym. 2010;64:189–194. [Google Scholar]
- 607.Kim You-Sung, Shin Woonsup. Sensing characteristics of tyrosinase immobilized and tyrosinase, laccase Co-immobilized platinum electrodes. Bull. Kor. Chem. Soc. 2004;25:1195–1201. [Google Scholar]
- 608.Vera M., Fodor C., Garcia Y., Pereira E., Loos K., Rivas B.L. Multienzymatic immobilization of laccases on polymeric microspheres: a strategy to expand the maximum catalytic efficiency. J. Appl. Polym. Sci. 2020;137 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.