Abstract
Introduction
Through 2018, three calcitonin gene-related peptide pathway–targeted monoclonal antibodies (CGRP mAbs) had received US Food and Drug Administration (FDA) approval for migraine prevention: erenumab, fremanezumab, and galcanezumab.
Methods
This retrospective analysis evaluated adverse events (AEs) spontaneously reported to the FDA Adverse Event Reporting System (FAERS) safety surveillance database during the first 6 months post-approval of erenumab (May 2018 to November 2018), fremanezumab (September 2018 to March 2019), and galcanezumab (September 2018 to March 2019). Reporting rates (RR) per 1000 exposed patients were calculated from number of reported events (when product classified as “primary suspect”) in each AE category and estimated number of treated patients based on de-identified prescription data (IQVIA database) and were ranked on the basis of frequency for each product.
Results
RR per 1000 exposed patients for “migraine” (erenumab, 4.89; fremanezumab, 1.01; galcanezumab, 2.99), “headache” (3.32, 1.27, 3.07), and “drug ineffective” (3.68, 1.14, 1.69) were commonly reported for all three products, as were migraine-associated symptoms (“nausea”: 2.94, 0.91, 1.09) and “injection-site” reactions (“pain”: 2.94, 0.8, 4.9; “swelling”: 0.56, 0.53, 1.25; “pruritus”: 0.26, 0.63, 1.14; “erythema”: 0.58, 0.71, 1.58). “Constipation” ranked second for erenumab (4.90) but did not make the top ten events for fremanezumab (0.46) or galcanezumab (0.76); cardiovascular events did not rank in the top ten AEs for any product. The frequency of serious outcomes was low, with ≤ 2% of AEs categorized as serious across the CGRP mAbs.
Conclusion
These results aid in supporting the safety profile of CGRP mAbs in the real-world setting and may provide clinicians and patients with additional insight when considering migraine preventive treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02346-4.
Keywords: CGRP, Monoclonal antibodies, Erenumab, Fremanezumab, Galcanezumab, Adverse events, Migraine, FAERS
Key Summary Points
| Why carry out this study? |
| Although randomized controlled trials have demonstrated the safety of treatment with the calcitonin gene-related peptide pathway–targeted monoclonal antibodies (CGRP mAbs) erenumab, fremanezumab, and galcanezumab for the preventive treatment of migraine, post-marketing data are valuable for understanding the safety of these medications in a real-world setting in broad and diverse populations of patients not necessarily represented in a clinical trial setting. |
| In this study, we retrospectively evaluated data from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) to analyze real-world adverse event (AE) reporting for erenumab, fremanezumab, and galcanezumab for the 6-month periods following their respective FDA approvals. |
| What was learned from this study? |
| In this retrospective analysis, AEs of migraine/headache, drug ineffective, or injection-site reactions were commonly reported for all three products, while constipation ranked second for erenumab but did not make the top ten AEs for fremanezumab or galcanezumab. |
| The frequency of serious outcomes was low, with ≤ 2% of AEs categorized as serious across the three CGRP mAbs assessed; cardiovascular events did not rank in the top ten AEs for any of the three products. |
| These findings may inform health care providers’ decision making at the time of drug prescription for their patients. |
Introduction
Migraine is the second leading cause of years lived with disability, affecting more than one billion people globally and imposing physical, emotional, and societal burdens on populations worldwide [1, 2]. Preventive migraine treatment is recommended for patients who have frequent and/or disabling migraine headaches. This is with the goal of reducing migraine attack frequency, intensity, and duration; improving responsiveness to and avoidance of escalation in the use of acute migraine treatments; and improving performance of daily functions [3].
Various classes of medication have been used for migraine prevention, including antiepileptics, antidepressants, antihypertensives, and botulinum neurotoxins. None of these drug classes, however, targets the underlying pathophysiology of migraine [3]. Advances in understanding the pivotal role of the calcitonin gene-related peptide (CGRP) in the pathophysiology of migraine inspired the investigation of new preventive treatments targeting this pathway, including the development of monoclonal antibodies (mAbs) targeting the CGRP pathway (CGRP mAbs), for example, the CGRP ligand or the CGRP receptor [4–6]. Four mAbs, eptinezumab, fremanezumab, and galcanezumab, which target the CGRP ligand, and erenumab, which targets the CGRP receptor, have been studied in clinical trials for the prevention of episodic migraine (EM) and chronic migraine (CM) [5]. Three of these mAbs had received US Food and Drug Administration (FDA) approval by the end of 2018 for the preventive treatment of migraine. Erenumab was approved in May 2018, with approval based on results of a phase 2 CM trial and the phase 3 ARISE and STRIVE EM trials [7–9]. Subsequently, fremanezumab and galcanezumab were approved in September 2018 on the basis of results of the phase 3 HALO CM and HALO EM trials [10, 11] and the phase 3 REGAIN CM and EVOLVE-1 and EVOLVE-2 EM trials [12–14], respectively. Eptinezumab, which is administered intravenously, was approved in February 2020, a time outside the scope of the current analysis.
The three studied CGRP mAbs (erenumab, fremanezumab, and galcanezumab) are administered by subcutaneous injection, and their long half-lives permit monthly or quarterly dosing [3, 5]. The pivotal clinical studies demonstrated their efficacy in significantly reducing the frequency of migraine, with adverse event (AE) profiles similar to those seen with placebo injection [15, 16]. Published data demonstrated the safety and efficacy of long-term treatment for up to 1 year with fremanezumab or galcanezumab and 5 years with erenumab [17–21].
While randomized controlled trials provide robust information on a drug’s clinical characteristics, post-marketing data are valuable and can aid in understanding medication safety in a real-world setting in broad and diverse populations of patients.
The FDA Adverse Event Reporting System (FAERS) is a centralized, computerized information database that is used by the FDA and other pharmacovigilance experts for post-marketing drug safety surveillance and AE signal detection. It contains AE reports that the FDA has received from manufacturers as required by regulations, along with voluntary reports received directly from consumers and health care professionals [22]. Given that the data are spontaneous in nature, they are frequently used to highlight new findings or for hypothesis generation purposes. In this study, we retrospectively reviewed the FAERS data to analyze real-world AE reporting for erenumab, fremanezumab, and galcanezumab for the 6-month periods following their respective FDA approvals.
Methods
This retrospective analysis evaluated AEs spontaneously reported to FAERS during the first 6 months post-approval for patients treated with erenumab (May 17, 2018 to November 17, 2018), fremanezumab (September 14, 2018 to March 14, 2019), and galcanezumab (September 27, 2018 to March 27, 2019).
The primary objectives were (a) to determine, separately for each product, the proportions of the most frequently reported AE categories during the first 6 months post-launch, using the total number of reported cases as the denominator; and (b) to determine the reporting rates (RRs) of AE categories during the first 6 months post-launch, using the frequently reported AE categories as numerators and the estimated number of patients exposed to each of the products as denominators. The AEs were defined as preferred terms (PTs) in accordance with the Medical Dictionary for Regulatory Activities (MedDRA). The patients’ data (as denominators) came from de-identified prescription information in the IQVIA National Prescription Audit Market Dynamics database. These data were provided by permission from the IQVIA Patient Insight New to Brand database for the period of May 17, 2018 to March 27, 2019, National Prescription Audit and Longitudinal Prescriptions Data, Real World Data Sets Solutions branch, IQVIA advanced analytics, technology solutions, and clinical research services. The FAERS database includes publicly accessible AE reports data released periodically by the FDA, so no permission was required for use of those data.
The secondary objective was to determine separately for each product the proportion of serious reports among the most frequently reported AE cases.
IQVIA, a data science company that collects and provides de-identified patient prescription data and metrics in the USA and globally, provided estimates of the populations that were exposed to the CGRP mAbs during the 6-month period of interest by individual product. All data used in this retrospective analysis, including those from the IQVIA database and those from the FAERS database, were fully anonymized. Given that all data included in this study were fully de-identified, this study was exempt from institutional review board review.
Primary suspect cases included all case reports where the reporter listed the specified drug as the primary suspect associated with the reported AE of interest. A descriptive analysis provided the following information by individual product and study period: frequency distributions of all AE categories and their proportions within the total number of reported cases using the latter as the denominator; and the RR of AE categories, using the estimated number of treated patients during the 6-month observation period as the denominator. The RR represents the rate of a given AE in the population exposed to the product during the study period. They were calculated using the number of reports of a specific AE as the numerator and multiplied by 1000 to provide a standardized rate for the three products. The frequency distributions of AEs were sorted from most to least frequent in order to identify the top ten most frequent AE categories for each product. The proportion of serious AEs within the top ten AE categories for each product was calculated as well.
The analysis was not restricted to any particular AEs and included all reported MedDRA PTs for the evaluated product. Records where the product of interest was not specified as the suspect product for the AE of interest were excluded from this analysis.
Prior to analysis, the data were quality checked for items such as missing or malformed keys or duplicate reports. Figure 1 summarizes the process used to prepare the data for analysis, including removal of duplicate reports and records. The data cleaning process is described in Supplementary Material.
Fig. 1.
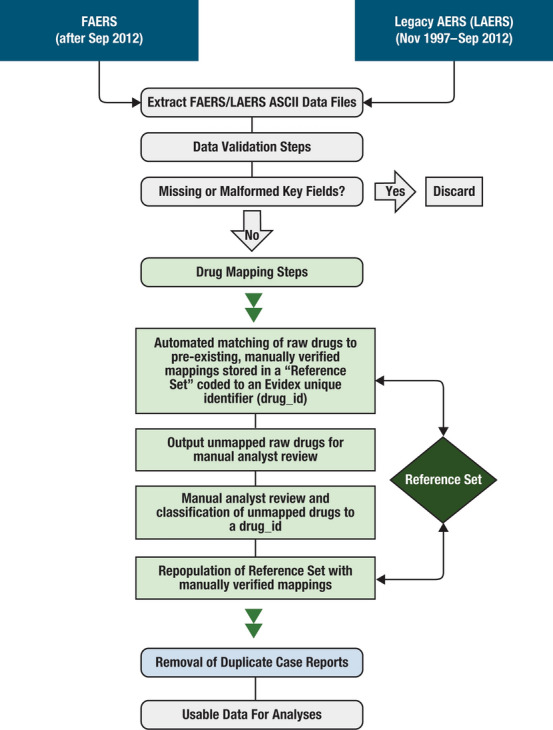
FAERS data normalization steps. AERs adverse event reporting system, ASCII American Standard Code for Information Interchange, FAERS US Food and Drug Administration Adverse Events Reporting System
Results
For erenumab, a total 5468 AEs related to the primary suspect products were reported. For fremanezumab, a total 450 primary suspect AEs were reported, and for galcanezumab, a total of 990 primary suspect AEs were reported. For erenumab, fremanezumab, and galcanezumab, the estimated total exposed populations were 116,817, 39,515, and 36,760 patients, respectively.
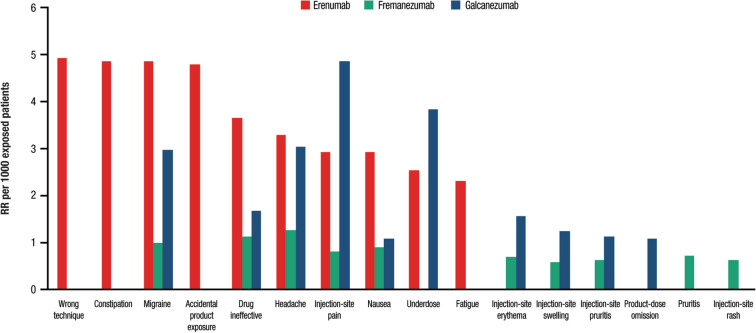
Table 1a–c and Fig. 2 show the proportions and RRs of AEs per 1000 exposed patients for each CGRP mAb ranked by frequency. For erenumab, the top ten RRs per 1000 exposed patients were as follows: “wrong technique” (4.97), “constipation” (4.90), “migraine” (4.89), “accidental product exposure” (4.83), “drug ineffective” (3.68), “headache” (3.32), “injection-site pain” (2.94), “nausea” (2.94), “underdose” (2.55), and “fatigue” (2.33). For fremanezumab, the top ten RRs per 1000 exposed patients were: “headache” (1.27), “drug ineffective” (1.14), “migraine” (1.01), “nausea” (0.91), “injection-site pain” (0.81), “pruritus” (0.73), “injection-site erythema” (0.71), “injection-site pruritus” (0.63), “injection-site rash” (0.63), and “injection-site swelling” (0.58). For galcanezumab, the top ten RRs per 1000 exposed patients were: “injection-site pain” (4.90), “underdose” (3.86), “headache” (3.07), “migraine” (2.99), “drug ineffective” (1.69), “injection-site erythema” (1.58), “injection-site swelling” (1.25), “injection-site pruritus” (1.14), “nausea” (1.09), and “product-dose omission” (1.09).
Table 1.
Top ten AE proportions and RR by primary suspect CGRP mAb and frequency rank for: (a) erenumab, (b) fremanezumab, and (c) galcanezumab
| Any AE | Serious events | ||||
|---|---|---|---|---|---|
| AE | n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) | n | Proportion of AEs (%) out of all reports |
| (a) Erenumab (n = 5468 AE reports, 116,817 estimated exposed patients; overall RR = 46.8 per 1000 exposed patients) | |||||
| Wrong technique | 580 | 10.61 | 4.97 | 13 | 0.24 |
| Constipation | 572 | 10.46 | 4.90 | 73 | 1.34 |
| Migraine | 571 | 10.44 | 4.89 | 71 | 1.30 |
| Accidental product exposure | 564 | 10.31 | 4.83 | 2 | 0.04 |
| Drug ineffective | 430 | 7.86 | 3.68 | 43 | 0.79 |
| Headache | 388 | 7.10 | 3.32 | 47 | 0.86 |
| Injection-site pain | 344 | 6.29 | 2.94 | 16 | 0.29 |
| Nausea | 343 | 6.27 | 2.94 | 48 | 0.88 |
| Underdose | 298 | 5.45 | 2.55 | 3 | 0.05 |
| Fatigue | 272 | 4.97 | 2.33 | 47 | 0.86 |
| Any AE | Serious events | ||||
|---|---|---|---|---|---|
| AE | n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) |
n | Proportion of AEs (%) out of all reports |
| (b) Fremanezumab (n = 450 AEs, 39,515 estimated exposed patients; overall RR = 11.4 per 1000 exposed patients) | |||||
| Headache | 50 | 11.11 | 1.27 | 9 | 2.00 |
| Drug ineffective | 45 | 10.00 | 1.14 | 7 | 1.56 |
| Migraine | 40 | 8.89 | 1.01 | 5 | 1.11 |
| Nausea | 36 | 8.00 | 0.91 | 10 | 2.22 |
| Injection-site pain | 32 | 7.11 | 0.81 | 3 | 0.67 |
| Pruritus | 29 | 6.44 | 0.73 | 6 | 1.33 |
| Injection-site erythema | 28 | 6.22 | 0.71 | 4 | 0.89 |
| Injection-site pruritus | 25 | 5.56 | 0.63 | 4 | 0.89 |
| Injection-site rash | 25 | 5.56 | 0.63 | 0 | 0.00 |
| Injection-site swelling | 23 | 5.11 | 0.58 | 3 | 0.67 |
| Any AE | Serious events | ||||
|---|---|---|---|---|---|
| AE | n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) |
n | Proportion of AEs (%) out of all reports |
| (c) Galcanezumab (n = 990 AEs, 36,760 estimated exposed patients; overall RR = 26.9 per 1000 exposed patients) | |||||
| Injection-site pain | 180 | 18.18 | 4.90 | 8 | 0.81 |
| Underdose | 142 | 14.34 | 3.86 | 3 | 0.30 |
| Headache | 113 | 11.41 | 3.07 | 7 | 0.71 |
| Migraine | 110 | 11.11 | 2.99 | 11 | 1.11 |
| Drug ineffective | 62 | 6.26 | 1.69 | 4 | 0.40 |
| Injection-site erythema | 58 | 5.86 | 1.58 | 1 | 0.10 |
| Injection-site swelling | 46 | 4.65 | 1.25 | 6 | 0.61 |
| Injection-site pruritus | 42 | 4.24 | 1.14 | 1 | 0.10 |
| Nausea | 40 | 4.04 | 1.09 | 5 | 0.51 |
| Product-dose omission | 40 | 4.04 | 1.09 | 2 | 0.20 |
AE adverse event, CGRP mAb calcitonin gene-related peptide pathway–targeting monoclonal antibody, RR reporting rate
The number of events is not mutually exclusive and is counted independently
Fig. 2.
Top ten adverse event RRs per 1000 exposed patients for erenumab, fremanezumab, and galcanezumab during the first 6 months after their launch. RR reporting rate
The frequency of serious outcomes was low, with ≤ 2% of AEs categorized as serious across the three CGRP mAb products (Table 1a–c). Cardiovascular AEs were rarely reported, with all individual cardiovascular AEs reported for any of the three CGRP mAbs having a RR of ≤ 0.33 per 1000 exposed patients (Table 2).
Table 2.
Proportions and RR per 1000 cases of cardiovascular AEs when recorded in ≥ 2 cases by CGRP mAb
| Erenumab (n = 5468 AEs, 116,817 estimated exposed patients) | Fremanezumab (n = 450 AEs, 39,515 estimated exposed patients) | Galcanezumab (n = 990 AEs, 36,760 estimated exposed patients) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AE | n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) |
n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) |
n | Proportion of AEs (%) out of all reports | RR (per 1000 exposed patients) |
| Chest pain | 38 | 0.69 | 0.33 | 9 | 2.00 | 0.23 | 5 | 0.51 | 0.14 |
| Blood pressure increased | 35 | 0.64 | 0.30 | 4 | 0.89 | 0.10 | 8 | 0.81 | 0.22 |
| Heart rate increased | 32 | 0.59 | 0.27 | 1 | 0.22 | 0.03 | 3 | 0.30 | 0.08 |
| Palpitations | 26 | 0.48 | 0.22 | 5 | 1.11 | 0.13 | 4 | 0.40 | 0.11 |
| Chest discomfort | 25 | 0.46 | 0.21 | 4 | 0.89 | 0.10 | 7 | 0.71 | 0.19 |
| Hypertension | 25 | 0.46 | 0.21 | 2 | 0.44 | 0.05 | 3 | 0.30 | 0.08 |
| Hot flush | 20 | 0.37 | 0.17 | 4 | 0.89 | 0.10 | 3 | 0.30 | 0.08 |
| Flushing | 18 | 0.33 | 0.15 | 4 | 0.89 | 0.10 | – | – | – |
| Cerebrovascular accident | 13 | 0.24 | 0.11 | 3 | 0.67 | 0.08 | 2 | 0.20 | 0.05 |
| Hypotension | 10 | 0.18 | 0.09 | 2 | 0.44 | 0.05 | 1 | 0.10 | 0.03 |
| Cardiac disorder | 8 | 0.15 | 0.07 | 1 | 0.22 | 0.03 | – | – | – |
| Blood pressure decreased | 6 | 0.11 | 0.05 | – | – | – | – | – | – |
| Blood pressure abnormal | 5 | 0.09 | 0.04 | – | – | – | – | – | – |
| Myocardial infarction | 5 | 0.09 | 0.04 | – | – | – | 3 | 0.30 | 0.08 |
| Angina pectoris | 3 | 0.05 | 0.03 | – | – | – | – | – | – |
| Cardiac flutter | 3 | 0.05 | 0.03 | – | – | – | – | – | – |
| Heart rate decreased | 3 | 0.05 | 0.03 | – | – | – | 2 | 0.20 | 0.05 |
| Heart rate irregular | 3 | 0.05 | 0.03 | – | – | – | – | – | – |
| Atrial fibrillation | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
| Blood pressure fluctuation | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
| Cardiovascular disorder | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
| Ischemic stroke | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
| Tachycardia | 2 | 0.04 | 0.02 | 2 | 0.44 | 0.05 | 1 | 0.10 | 0.03 |
| Transient ischemic attack | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
| Vasoconstriction | 2 | 0.04 | 0.02 | – | – | – | – | – | – |
AEs are presented as MedDRA PTs
AE adverse event, CGRP mAb calcitonin gene-related peptide pathway–targeting monoclonal antibody, MedDRA Medical Dictionary for Regulatory Activities, PT preferred term, RR reporting rate
The number of events is not mutually exclusive and is counted independently
Discussion
This retrospective analysis of the FAERS database provided information on the most common AEs reported for patients exposed to any of three CGRP mAbs during the first 6 months following launch of these products. “Migraine,” “headache,” and “drug ineffective” were commonly reported events for all three products, as were migraine-associated symptoms (such as “nausea”) and “injection-site” reactions (such as “erythema” or “swelling”). “Constipation” ranked second for erenumab but did not make the top ten events for fremanezumab or galcanezumab; cardiovascular events did not rank in the top ten AEs for any product.
In the erenumab 3- to 6-month pivotal clinical trials, the most commonly reported AEs in erenumab-treated patients (reported in ≥ 6% of patients with either dose) included nasopharyngitis (2.0–11.0%), upper respiratory tract infection (3.0–6.7%), and injection-site pain (0.3–6.0%); constipation was reported in 1.4–4.0% of patients [7–9]. Upper respiratory infections continued to be the most commonly reported AEs during the long-term clinical studies [17, 18] of this product. Recent reports from headache centers in Europe have detailed the tolerability of erenumab in clinical practice. In a retrospective review of 164 erenumab-treated patients at a UK headache center, the most frequently reported AEs following the first injection were constipation (42%) and flu/cold symptoms (32%); while most AEs were transient, 12% of patients discontinued erenumab owing to severe AEs, including severe constipation in nine patients and new-onset hypertension in one patient [23]. In a separate retrospective cohort study of 241 patients receiving erenumab at a US tertiary headache center, constipation was also the most commonly reported AE, affecting 43% of patients, followed by injection-site reactions (24%), fatigue (15%), worsening headache (12%), and dizziness (11%) [24]. Similarly, in a prospective study involving 70 erenumab-treated patients at an Italian clinic, constipation was the most frequent AE (23.9%) [25]. In a separate retrospective Italian study involving 89 erenumab-treated patients, 13.5% reported constipation [26]. No discontinuations due to constipation were reported in either study [25, 26]. The authors of these real-world studies noted the higher incidence of constipation compared with the pivotal clinical trials and speculated that this was due to patients being specifically asked about this AE or due to the frequent occurrence of comorbidities in these unselected patient populations [23, 26]. Across two other real-world studies that assessed the safety and tolerability of erenumab, constipation was the most frequently reported AE, reported by ≥ 20% of patients [26, 27]. In a recent analysis of the first 2 years of post-marketing surveillance data from the FAERS database, constipation was reported for 58% of patients [28]. In May 2021, constipation was noted as a frequent AE in the prescribing information for erenumab, with a warning included regarding the occurrence of constipation with serious complications in a postmarketing setting [29, 30].
In the fremanezumab pivotal 3-month trials, the most commonly reported AEs in the active-treatment groups (reported in ≥ 6% of patients with either dose) were injection-site events (pain in 26.0–30.0%, induration in 19.6–24.5%, and erythema in 17.9–21.0%), and these were reported at a similar frequency in the placebo groups [10, 11]. Injection-site events continued to be the most frequent AEs during long-term treatment with fremanezumab for up to 1 year [19]. Injection-site events were also among the most frequently reported AEs with active treatment (reported in ≥ 6% of patients with either dose) in the galcanezumab pivotal 6-month trials (pain in 6.0–20.5%, reaction in 3.0–7.9%, and erythema in 1.0–5.0%), with nasopharyngitis also commonly reported (2.7–8.4%) [12–14]. The same types of AE were reported during the long-term extension study that continued for an additional year after completion of the double-blind phase [20]. In a recent prospective observational cohort study of 163 patients across 13 Italian headache centers, the most commonly reported AEs with galcanezumab were gastrointestinal AEs (reported in up to 6.7% of patients) and skin reactions (reported in up to 2.5% of patients) [31].
In a network meta-analysis of randomized, controlled trials of these three CGRP mAbs (erenumab, fremanezumab, and galcanezumab) in patients with EM, safety and tolerability findings were comparable to those observed in the individual 3- to 6-month pivotal phase 3 trials of these migraine preventive treatments; only injection-site pain was reported at a significantly higher rate with CGRP mAbs than with placebo (odds ratio, 1.44; P = 0.004) [15]. Similar results were observed in a meta-analysis of randomized, controlled trials in patients with CM; only injection discomfort occurred significantly more frequently with CGRP mAbs compared with placebo (odds ratio, 2.11; P = 0.0007) [16].
CGRP receptors are present in the cardiac and vascular tissues, where CGRP acts as a vasodilator and plays an important role in regulating blood flow [32]. In particular, CGRP may act as a compensatory vasodilator during episodes of ischemia, raising the concern that CGRP blockade could transform mild or transient ischemic events into full-blown infarcts [33]. There is no evidence from short- and long-term trials to indicate that migraine preventive treatments targeting the CGRP pathway are associated with any increased risk of cardiovascular AEs; however, this remains an important question to consider with these drugs. A recent analysis pooled data from four double-blind, placebo-controlled studies of erenumab and their open-label extensions, with the aim of examining the rates of vascular AEs with erenumab versus placebo [34]. The review found no evidence of an association between erenumab treatment and vascular events. There was no increased risk of vascular AEs with erenumab versus placebo in subgroups of patients using vasoconstrictive acute migraine–specific medications (e.g., triptans or ergots) or those with common vascular risk factors at study baseline (e.g., diabetes, hypertension, or coronary artery disease), and erenumab had no relevant effect on blood pressure compared with placebo [34]. Similarly, a pooled analysis of data from three double-blind, placebo-controlled studies of galcanezumab demonstrated no changes in blood pressure, pulse, electrocardiogram findings, or increased risk of cardiovascular AEs compared with placebo during up to 6 months of treatment, including in subgroups of patients with existing cardiovascular disease, migraine with aura, or concomitant use of triptans [35]. A pooled analysis of data from five double-blind, placebo-controlled studies of fremanezumab of up to 1-year duration likewise found that cardiovascular AEs occurred infrequently and at similar rates across fremanezumab and placebo groups. The most common cardiovascular events in fremanezumab-treated patients were hypertension (2%), palpitations (< 1%), and hot flush (< 1%), with no notable changes in blood pressure or electrocardiogram parameters [36]. Of the three CGRP mAbs, erenumab is the only one to include a warning in its product label regarding cardiovascular effects, stating that development of hypertension and worsening of pre-existing hypertension have been reported in the post-marketing setting, most often within 7 days of the first dose administration [29].
The reporting of migraine symptoms or “drug ineffective” as AEs during the initial months of therapy in the current study may reflect patients’ high expectations of a new migraine treatment. While the pivotal 12-week clinical trials of the three CGRP mAbs all showed a significant reduction from baseline in the frequency of migraine days (an approximate 3- to 4-day reduction per month for patients with EM and 5- to 6-day reduction per month for patients with CM) [7–14], not all patients experienced a clinically meaningful reduction in days with migraine. The proportion of patients with EM who experienced a 50% or greater reduction in monthly migraine days from baseline (pretreatment in the initial double-blind study) was 61–65% after 1 year of treatment with erenumab [18] and 53–68% after at least 1 year of treatment with fremanezumab [19]. With galcanezumab, a 50% or greater reduction in monthly headache days was observed in 66–74% of patients with either CM or EM after at least 1 year of treatment [20]. The reduction in migraine days seen in the clinical trials was associated with significant improvements in patient-reported outcomes, such as migraine-associated disability, headache impact, and quality of life [7, 10–14, 37]. It is also noteworthy that erenumab, fremanezumab, and galcanezumab have demonstrated efficacy in patients who have experienced inadequate response with previous classes of migraine preventive drugs [38–40]. The reported AEs of “migraine,” “headache,” and “drug ineffective” could be regarded as reflecting potential treatment failure; however, preventive treatments generally reduce, but do not eliminate, the occurrence of migraine attacks, and time to improvement may vary for individual patients. Thus, patients may have reported any attacks experienced after initiating CGRP mAb treatment, reflecting a potentially higher than realistic expectation around treatment effects. Further, “drug ineffective” is the most frequently reported AE in the FAERS database, is typically reported by consumers, and is generally nonserious [41]. Treatment guidelines emphasize the importance of establishing realistic expectations when patients are introduced to a new migraine preventive regimen [3].
Limitations and Strengths
Several limitations apply to analyses of the FAERS database. It is not possible to draw clear causal inferences from the AE reports. There is no certainty that any reported AE was caused by the product in question, as it may not always be possible to provide clear-cut evidence for a causal association between a product and an AE. Not every AE or medication error is reported to the FDA, and duplicate reports from consumers, health care providers, and the sponsor may occur (our data were quality checked, and duplicates were removed) [22]. AE reports are spontaneous and are not reported in a consistent manner, and thus can be impacted by factors such as recall bias, timing and severity of AE occurrence, educational or demographic factors, media reports, product’s time on the market, or season of the year. Reports are also affected by the product’s reporters, who may range from service providers to patients to patients’ family members or friends, although the vast majority of reports (95%) go through the product manufacturers first [41]. Regardless, this may potentially reduce the uniformity and consistency of the data. The severity of the event may determine who reports and the likelihood of reporting. Reporting may be impacted by the “Weber effect,” which is the tendency for increased AE reporting during the early period following a drug’s approval; however, it has been suggested that the Weber effect is not generally observed for AE reporting in the FAERS database [42, 43]. A product may be implicated erroneously by a lay reporter, especially when other products are co-administered and/or concomitant conditions exist. In addition, the AEs recorded in the FAERS database are based on reports from patients, family members, or health care providers and, thus, may have varied on the basis of the reporter’s experience of the AE. For example, the reported AEs of “migraine” and “headache” are based only on the reporter’s perception of the AE and their own interpretation of the two terms. Furthermore, some of the AE terminologies may be open to interpretation beyond the MedDRA coded term. For instance, the AE of “wrong technique,” which was the most frequently reported for erenumab, could refer to an error during opening of the medication’s container or preparing the medication, or during its administration [44].
Despite these limitations and owing to the wide exposure to a specific drug in the real-world population and the large sample size with a wide range of AE reports, the FAERS database provides a useful aspect into the AE profile of drugs beyond the controlled phase of clinical studies and may be one of the very few sources of such information. Furthermore, spontaneous AE reports may highlight the possible occurrence of rare or severe drug reactions not previously observed. Moreover, the AE reports in the FAERS database are used by the FDA for AE monitoring, safety analysis, and decision-making purposes. With regard to this study’s data, the number of patients exposed to erenumab was approximately 3-fold the number exposed to each of the other products, fremanezumab or galcanezumab. The fact that erenumab was the first CGRP mAb approved may have contributed to this difference. Although AEs in this study were assessed as reporting rates per 1000 patients to allow for standardization across the three CGRP mAbs, the smaller sample sizes both in number of AEs and exposed population for fremanezumab or galcanezumab may have contributed to the differences in reported AEs and their RRs.
Conclusion
In the current study, “migraine,” “headache,” and “drug ineffective,” along with migraine-associated symptoms (e.g., “nausea”) and “injection-site” reactions, were commonly reported AEs for all three CGRP mAbs evaluated. Cardiovascular events were not among the top ten AEs for any product. “Constipation” was the second most commonly reported AE for erenumab but was not among the top ten AEs for fremanezumab or galcanezumab. Serious AEs were infrequent across all three CGRP mAbs. Although these results should be interpreted with caution, the findings of the current analyses from the FAERS database support the safety of CGRP mAbs as migraine preventive treatments in a medical real-world setting, which may help clinicians’ decision-making process when evaluating migraine preventive medications for their patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
This study and the journal’s Rapid Service and Open Access fees were funded by Teva Branded Pharmaceutical Products R&D, Inc.
Medial Writing and Editorial Assistance
Medical writing and editorial support were provided by Michelle Hughes, PhD, of Cello Health Communications/MedErgy (Yardley, PA), in accordance with Good Publication Practice (GPP3) guidelines, and were funded by Teva Pharmaceuticals USA, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to interpretation of the data, drafting and revising the manuscript for important intellectual content, and approved the final submitted version for publication. Shoshana Reshef contributed to the concept and design of the study and analysis of the data.
Prior Presentation
Portions of these data have been presented previously at the American Academy of Neurology (AAN) 2020 Annual Meeting, April 25-May 1, 2020, virtual; the International Society for Pharmacoepidemiology (ICPE) 2020 Annual Conference, September 16–17, 2020, virtual; the American Neurological Association (ANA) 2020 Annual Meeting, October 4–9, 2020, virtual; and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Europe 2020 Meeting, November 14–18, 2020, virtual.
Disclosures
As a consultant and/or advisory panel member, Stephen D. Silberstein receives, or has received, honoraria from Abide Therapeutics, Alder BioPharmaceuticals, Allergan, Inc., Amgen, Avanir Pharmaceuticals, Inc., Biohaven Pharmaceuticals, Cefaly, Curelator, Inc., Dr. Reddy’s Laboratories, Egalet Corporation, GlaxoSmithKline Consumer Health Holdings, LLC., eNeura Inc., electroCore Medical, LLC, Impel NeuroPharma, Inc., Lilly USA, LLC, Medscape, LLC, Novartis, Inc., Satsuma Pharmaceuticals, Supernus Pharmaceuticals, Inc., Teva Pharmaceuticals, Theranica, and Trigemina, Inc. Shoshana Reshef, Sanjay Gandhi, Verena Ramirez Campos, Yoel Kessler, and Stephen F. Thompson are employees of Teva Pharmaceuticals. Joshua M. Cohen and Michael Seminerio are former employees of Teva Pharmaceuticals and are currently affiliated with Braeburn and AbbVie, respectively. Andrew Blumenfeld has received compensation for consulting and promotional activities from Allergan, Inc., Alder BioPharmaceuticals, Amgen, Teva Pharmaceuticals, Lilly, Biohaven Pharmaceuticals, Theranica, Supernus Pharmaceuticals, Inc., and Novartis, Inc.
Compliance With Ethics Guidelines
This article is based on a database analysis and does not contain any new studies with human participants or animals performed by any of the authors; therefore, ethics approval was not required. De-identified prescription data were provided by permission from the IQVIA Patient Insight New to Brand database for the period of May 17, 2018 to March 27, 2019, National Prescription Audit and Longitudinal Prescriptions Data, Real World Data Sets Solutions branch, IQVIA advanced analytics, technology solutions, and clinical research services. The FAERS database includes publicly accessible AE reports data released periodically by the FDA, so no permission was required for use of those data.
Data Availability
The datasets generated during and/or analyzed during the current study are available from Shoshana Reshef on reasonable request.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agosti R. Migraine burden of disease: from the patient's experience to a socio-economic view. Headache. 2018;58(Suppl 1):17–32. doi: 10.1111/head.13301. [DOI] [PubMed] [Google Scholar]
- 3.American Headache Society The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18. doi: 10.1111/head.13456. [DOI] [PubMed] [Google Scholar]
- 4.Tso AR, Goadsby PJ. Anti-CGRP monoclonal antibodies: the next era of migraine prevention? Curr Treat Options Neurol. 2017;19(8):27. doi: 10.1007/s11940-017-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuteri D, Adornetto A, Rombola L, Naturale MD, Morrone LA, Bagetta G, et al. New trends in migraine pharmacology: targeting calcitonin gene-related peptide (CGRP) with monoclonal antibodies. Front Pharmacol. 2019;10:363. doi: 10.3389/fphar.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltran E, et al. Blocking CGRP in migraine patients—a review of pros and cons. J Headache Pain. 2017;18(1):96. doi: 10.1186/s10194-017-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. doi: 10.1177/0333102418759786. [DOI] [PubMed] [Google Scholar]
- 8.Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 9.Tepper S, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434. doi: 10.1016/S1474-4422(17)30083-2. [DOI] [PubMed] [Google Scholar]
- 10.Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008. doi: 10.1001/jama.2018.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 12.Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221. doi: 10.1212/WNL.0000000000006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454. doi: 10.1177/0333102418779543. [DOI] [PubMed] [Google Scholar]
- 14.Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088. doi: 10.1001/jamaneurol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H, Li GG, Nie H, Feng YY, Guo GY, Guo WL, et al. Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine—an updated systematic review and meta-analysis. BMC Neurol. 2020;20(1):57. doi: 10.1186/s12883-020-01633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han L, Liu Y, Xiong H, Hong P. CGRP monoclonal antibody for preventive treatment of chronic migraine: an update of meta-analysis. Brain Behav. 2019;9(2):e01215. doi: 10.1002/brb3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick D, Rippon GA, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39(11):1455–1464. doi: 10.1177/0333102419854082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, et al. One-year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95(5):e469–e479. doi: 10.1212/WNL.0000000000010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Silberstein SD, Yeung PP, Cohen JM, Ning X, Yang R, et al. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: a randomized study. Neurology. 2020;95:e2487–e2499. doi: 10.1212/WNL.0000000000010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camporeale A, Kudrow D, Sides R, Wang S, Van Dycke A, Selzler KJ, et al. A phase 3, long-term, open-label safety study of galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188. doi: 10.1186/s12883-018-1193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick DW, Xue F, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28(5):1716–1725. doi: 10.1111/ene.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food & Drug Administration. Questions and answers on FDA's Adverse Event Reporting System (FAERS). 2018. https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 2 Dec 2020.
- 23.Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21(1):61. doi: 10.1186/s10194-020-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanaan S, Hettie G, Loder E, Burch R. Real-world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511–1522. doi: 10.1177/0333102420946725. [DOI] [PubMed] [Google Scholar]
- 25.Russo A, Silvestro M, Scotto di Clemente F, Trojsi F, Bisecco A, Bonavita S, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. 2020;21(1):69. doi: 10.1186/s10194-020-01143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32. doi: 10.1186/s10194-020-01102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenen J, Timmermans G, Nonis R, Manise M, Fumal A, Gerard P. Erenumab for migraine prevention in a 1-year compassionate use program: efficacy, tolerability, and differences between clinical phenotypes. Front Neurol. 2021;12:805334. doi: 10.3389/fneur.2021.805334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sessa M, Andersen M. New insight on the safety of erenumab: an analysis of spontaneous reports of adverse events recorded in the US Food and Drug Administration Adverse Event Reporting System database. BioDrugs. 2021;35(2):215–227. doi: 10.1007/s40259-021-00469-8. [DOI] [PubMed] [Google Scholar]
- 29.AIMOVIG (erenumab aooe) [prescribing information]. Thousand Oaks (CA): Amgen Inc.; Revised 2019. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/aimovig/aimovig_pi_hcp_english.ashx. Accessed 12 Jan 2021.
- 30.AIMOVIG [summary of product characteristics]. Dublin (Ireland): Novartis Europharm Limited; Revised 2020. https://www.medicines.org.uk/emc/product/9380/smpc. Accessed 12 Jan 2021.
- 31.Vernieri F, Altamura C, Brunelli N, Costa CM, Aurilia C, Egeo G, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study) J Headache Pain. 2021;22(1):35. doi: 10.1186/s10194-021-01247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, de Boer I, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain. 2019;20(1):27. doi: 10.1186/s10194-019-0979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci. 2016;37(9):779–788. doi: 10.1016/j.tips.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Kudrow D, Pascual J, Winner PK, Dodick DW, Tepper SJ, Reuter U, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94(5):e497–e510. doi: 10.1212/WNL.0000000000008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakes TM, Kovacs R, Rosen N, Doty E, Kemmer P, Aurora SK, et al. Evaluation of cardiovascular outcomes in adult patients with episodic or chronic migraine treated with galcanezumab: data from three phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. Headache. 2020;60(1):110–123. doi: 10.1111/head.13684. [DOI] [PubMed] [Google Scholar]
- 36.Ning X, Faulhaber N, Lang N, Yeung P, Schiemann J, Cohen J, et al. Fremanezumab cardiovascular and cerebrovascular safety profile: pooled data from placebo-controlled and long-term studies (S17.005) Neurology. 2019;92(Suppl 15):S17.005. [Google Scholar]
- 37.Lipton RB, Tepper SJ, Reuter U, Silberstein S, Stewart WF, Nilsen J, et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–e2260. doi: 10.1212/WNL.0000000000007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–1040. doi: 10.1016/S0140-6736(19)31946-4. [DOI] [PubMed] [Google Scholar]
- 39.Mulleners WM, Kim B, Lainez MJ, Lanteri-Minet M, Aurora SK, Nichols RM, et al. A phase 3 placebo-controlled study of galcanezumab in patients with treatment-resistant migraine: results from the 3-month double-blind treatment phase of the conquer study. J Neurol Sci. 2019;405(Suppl):128. doi: 10.1016/j.jns.2019.10.1817. [DOI] [Google Scholar]
- 40.Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287. doi: 10.1016/S0140-6736(18)32534-0. [DOI] [PubMed] [Google Scholar]
- 41.Misu T, Kortepeter CM, Munoz MA, Wu E, Dal Pan GJ. An evaluation of "drug ineffective" postmarketing reports in drug safety surveillance. Drugs Real World Outcomes. 2018;5(2):91–99. doi: 10.1007/s40801-018-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arora A, Jalali RK, Vohora D. Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs. Ther Clin Risk Manag. 2017;13:1195–1203. doi: 10.2147/TCRM.S137144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM. The Weber effect and the United States Food and Drug Administration's Adverse Event Reporting System (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf. 2014;37(4):283–294. doi: 10.1007/s40264-014-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US FDA Division of Medication Errors and Technical Support Office of Surveillance and Epidemiology. Memorandum: DMETS medication error postmarketing safety review. 2007; https://www.fda.gov/media/74134/download. Accessed 8 July 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from Shoshana Reshef on reasonable request.