Abstract
Introduction
Information about patient preferences for the treatment of anaemia associated with chronic kidney disease (CKD) is scarce. Hence, our aim was to examine how patients with non-dialysis-dependent CKD valued attributes of alternative hypothetical anaemia treatments.
Methods
A discrete choice experiment (DCE) was conducted in adult patients who reported a clinical diagnosis of CKD-related anaemia. Treatment attributes included mode and frequency of administration, need for iron supplementation, risk of gastrointestinal side effects, risk of major cardiovascular events and impact on energy levels (as defined by the vitality section of the SF-6D health index). Logit models were used to analyse patients’ preferences.
Results
The DCE was completed by 200 patients in four countries. Patients preferred an oral mode of administration. Patients were willing to tolerate a 5.1% (95% CI 2.0–8.3%) increase in the risk of a major cardiovascular event and an 11.7% (95% CI 5.0–18.5%) increase in the risk of gastrointestinal side effects to switch from an at-home subcutaneous injection administered once every 2 weeks to an at-home oral pill administered three times a week. Patients were willing to tolerate a 20.3% (95% CI 15.0–25.6%) increase in the risk of gastrointestinal side effects and an 8.9% (95% CI 6.1–11.7%) increase in the risk of a major cardiovascular event to transition from ‘Sometimes having a lot of energy’ to ‘Always having a lot of energy’.
Conclusions
Patients with non-dialysis-dependent CKD-related anaemia demonstrated clear treatment preferences and were willing to accept increased gastrointestinal or cardiovascular risks in exchange for more energy or an oral treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02367-z.
Keywords: Anaemia, Chronic kidney disease, Discrete choice experiment, Oral treatment, Patient preferences
Key Summary Points
| Why carry out this study? |
| As patient preferences for anaemia treatment attributes remain uncertain, we aimed to better understand how patients with CKD value the attributes associated with anaemia treatments, including the relative importance of these attributes. |
| What was learned from the study? |
| Patients with CKD and anaemia consider the mode and frequency of administration to be an important aspect of their treatment, with a preference for oral administration over subcutaneous administration. |
| Patients with CKD and anaemia are willing to tolerate some increases in the risk of adverse events such as major cardiovascular events and gastrointestinal side effects to switch to a more convenient mode of treatment administration. |
| Energy levels are deemed to be an important aspect of patient treatment outcomes, with patients willing to tolerate some increase in side effects in exchange for more energy. |
Introduction
The prevalence of anaemia increases with progression of chronic kidney disease (CKD), from an estimated 8% in early disease to 53% in advanced disease [1]. Anaemia is a common complication in patients with moderate-to-severe CKD, and is often associated with severe cardiovascular complications and increased hospitalisation [2].
While CKD is associated with reduced quality of life, the presence of anaemia has an additional negative impact [3], thus emphasising the need for long-term individualised management of anaemia with the aim of improving quality of life in these patients [4]. First-line treatment for anaemia of CKD includes supplemental iron to address iron deficiency [5]. Some patients require additional treatment such as erythropoiesis-stimulating agents (ESAs) [5], which are either administered intravenously or injected subcutaneously. Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend considering ESA therapy once haemoglobin (Hb) concentration falls below 10.0 g/dL [5]. Despite the effectiveness of ESAs, a substantial proportion of patients fail to achieve target Hb levels [6–9]. Additionally, several studies have shown that patients with anaemia of CKD receiving high doses of ESAs to maintain Hb levels are at increased risk of cardiovascular (CV) disease [8, 10, 11].
As such, there is an unmet need for new/different drugs if patients are not meeting targets with current therapy. Furthermore, when considering new drugs, what is important to patients should be considered: for example, oral anaemia therapies have been developed recently as an alternative to injectable treatments in the CKD setting [12]. Roxadustat is the first of these to receive marketing approval in Japan and Europe, among other regions [13].
Little is known about how patients with CKD value the attributes associated with anaemia treatments, including the relative importance of these attributes. We therefore conducted a discrete choice experiment (DCE) to better understand how patients with non-dialysis-dependent (NDD) CKD regard different anaemia treatment attributes, such as mode and frequency of administration, need for additional iron supplementation, risk of major CV and gastrointestinal (GI) events, and effect on energy levels. We also aimed to assess their willingness to make trade-offs between key attributes.
Methods
Overall Study Design
This study primarily comprised a DCE survey (including a pilot phase), the design of which was informed by findings from a targeted literature review (TLR) and qualitative patient and physician interviews. The online DCE survey was conducted between November 2019 and March 2020 and elicited preferences for anaemia of CKD treatment attributes from NDD patients. The study followed best practice guidelines on preference-based methods from the International Society for Pharmacoeconomics and Outcomes Research [14].
Targeted Literature Review
The TLR was conducted in April 2018 using Embase to identify potential attributes and levels and included both quantitative and qualitative preference studies. The search terms and the inclusion and exclusion criteria for the quantitative and qualitative studies are provided in Tables S1–S3. Four relevant quantitative studies, with only one including patients with CKD-related anaemia, and seven relevant qualitative studies were identified from the search. The attribute groups from these studies included treatment procedure, severe adverse events, moderate adverse events, clinical outcomes, such as low-density lipoprotein cholesterol, efficacy in terms of relieving symptoms, such as fatigue, and the impact on quality of life. These insights informed an initial attribute list utilised within the qualitative interviews.
Qualitative Interviews
Qualitative interviews were conducted with patients and physicians, which aimed to understand the treatment attributes important to each [including the impact of subcutaneous or intravenous (IV) administration on patients’ lives], to determine how patients prioritise anaemia treatment attributes (including their willingness to accept changes in treatment benefits and risks) in exchange for access to oral treatment and to identify themes that explained patients’ preferences. The discussion guide was informed by the TLR findings and hypothetical treatment profiles were discussed with patients, comprising the following attributes: administration, need for iron supplementation, risk of vomiting or nausea, risk of a cardiovascular event, risk of other serious adverse events, cholesterol levels and anaemia-related fatigue. In addition, open-ended questions were used to assess whether there were other treatment attributes important to patients.
The qualitative interview stage of this study was approved by the Ethics and Independent Review Services (E&I) committee on 30 January 2019 (study number 18206-01). All patients provided online informed consent indicating that they understood the purpose and procedures required for the study and were willing to participate. Data were anonymous and only assigned participant identification (ID) numbers were linked to audio recordings and online survey results. All procedures were performed in accordance with the ethical standards of the relevant institutional review boards (IRBs); procedures were also performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, with the exception that study registration was not available in publicly accessible databases prior to recruitment. Patients and physicians were recruited by Global Perspectives, a research vendor that specialises in patient recruitment, using local databases, nurse and/or physician referrals, patient association referrals and social media.
Sixty-minute, semi-structured interviews were conducted with 31 NDD and 20 dialysis-dependent (DD) patients with CKD in the UK [n = 10 (NDD = 6; DD = 4)], Spain [n = 10 (NDD = 6; DD = 4)], Germany [n = 10 (NDD = 6; DD = 4)], France [n = 11 (NDD = 7; DD = 4)] and Japan [n = 10 (NDD = 6; DD = 4)] who reported a clinical diagnosis of anaemia associated with CKD and who were currently receiving ESA treatment. Full inclusion and exclusion criteria are given in Table S4.
To account for differences in perceptions of renal anaemia and its treatment for patients at different CKD stages, only patients who started ESA within 6 months prior to the interviews were included. As such, it was assumed that the majority of patients would be relatively similar to each other in terms of their renal function. In an attempt to control for differences in patient perception at different CKD stages, our inclusion criteria for this study recruited patients who had recently started ESA therapy (≤ 6 months prior to the interviews); however, this inclusion criterion was removed after 5 April 2019 due to challenges in patient recruitment.
The interviews were organised into three main sections: patients’ views on the burden of their disease impact and its treatments, a discussion of hypothetical treatment scenarios describing different modes of administration and a discussion on different treatment profiles described by the attributes listed above. The scenarios were described online in a web-assisted interview where the attributes pertinent to mode and frequency of administration for each treatment were listed. Patient interviews were conducted in the local language of the relevant country by a member of Evidera scientific staff (UK and Spain) or a scientific staff member at Global Perspectives (France, Germany and Japan). Audio recordings of the interviews were transcribed and then coded with ATLAS.ti software (version 8), using coding dictionaries informed by themes arising from the TLR and subsequently refined based on the first two transcripts. The coding dictionary was also updated as new themes emerged.
The NDD patients reported several anaemia-related symptoms, most commonly tiredness (77%, 24/31), shortness of breath (42%, 13/31), dizziness (23%, 7/31) and nausea (19%, 6/31). Patients said that these symptoms impacted their ability to do daily activities (61%, 19/31), work life (42%, 13/31), ability to exercise (26%, 8/31) and social life (13%, 4/31). Overall, 83% (19/23) of NDD patients with CKD-related anaemia preferred oral versus existing treatments (typically subcutaneous) due to the inconvenience and injection site pain associated with self-administering subcutaneous ESA. Notably, however, some patients who had previously experienced adverse GI events (such as severe nausea) as a result of oral iron expressed a preference for intravenous administration.
Ten nephrologists (two from each country) were interviewed to understand their views on the burden of CKD-related anaemia and anaemia treatment attributes that mattered to patients. Eligible nephrologists were those with at least 10 years of experience in CKD-related anaemia and currently treating patients with CKD-related anaemia. One nephrologist affiliated with a dialysis centre and one from a standard nephrology unit was sought in each country. All interviews with nephrologists, except for those with Japanese clinicians, were conducted in English by the Evidera project manager. Interviews with Japanese-speaking nephrologists were conducted in Japanese by Global Perspectives.
Clinical efficacy was identified as the most important factor by nephrologists, but they acknowledged that symptom improvement is more relevant for patients. In particular, 90% (9/10) of nephrologists reported that a reduction in tiredness was important to patients, and 70% (7/10) indicated that treatment had a positive impact on patients’ quality of life. One nephrologist identified the mode of administration as the most important factor for patients.
DCE
Patients
Patients were recruited for the DCE using various strategies, such as healthcare provider referrals, online panels and patient associations. Eligible patients were adults (aged ≥ 18 years) in the UK, France, Spain and Germany with a self-reported diagnosis of anaemia associated with CKD and who were receiving ESA treatment. Patients had to be able to read, speak, and write in the local language of the relevant country of interest. Exclusion criteria included patients who were undergoing dialysis, had received a kidney transplant or had a blood transfusion within 30 days of the online DCE; these patients were excluded to minimise the impact of other preceding treatments on the patients’ perceptions of injectable therapies. Patients who were receiving insulin therapy and those who had started receiving ESA treatment within the 6 months prior to the interview were initially excluded, but these criteria were removed on 5 April 2019 due to challenges in recruiting patients. Fulfillment of inclusion and exclusion criteria was determined by an online screening questionnaire completed by the patient.
After eligible patients provided online consent, they completed a questionnaire including the DCE, sociodemographic and clinical questions, and health literacy and numeracy questions. This study was conducted in accordance with the EU General Data Protection Regulation.
DCE Design
The DCE was designed to understand NDD patients’ valuation of different attributes of CKD-related anaemia treatments, and the trade-offs they were willing to make. Design of the DCE was informed by results from the qualitative interviews (i.e. what mattered the most to patients) that were relevant for NDD patients. Levels for the risk attributes in this patient group were informed from assessment of clinical trial data; the rates of CV and GI events in a similar patient population in a phase 3 study were 11–14% and 23%, respectively (data on file from the DOLOMITES study). Given the feedback received on the adverse events in the qualitative interviews (i.e. indicating that a 10% risk was not high for gastrointestinal side effects and suggesting that risks of 50% would be needed to impact their choices), the highest level for the risk of gastrointestinal side effects was increased to 50% to ensure that patients would trade-off with gastrointestinal side effects. The impact of cost did not inform the DCE, as anaemia treatment costs are fully reimbursed in the included study countries.
The final list of attributes and the levels of risk to include in the DCE are given in Table 1, and include the mode and frequency of administration, need for iron supplementation, risk of GI side effects, risk of major CV events and impact on energy levels. The choice of levels for energy used in this study were sourced from the vitality section of the Short Form-6D instrument described by Brazier et al. [15], which captured five grades of response; three of these were used in this study including ‘You have a lot of energy all of the time’, ‘You have a lot of energy some of the time’ and ‘You have a lot of energy none of the time’.
Table 1.
Attributes and levels in the DCE
| Attribute | Definition | Levels |
|---|---|---|
| Mode and frequency of administration |
Not all anaemia treatments are given/taken in the same way; for example, some are pills and others are injections Oral pill: A tablet would be taken once per day, but the number of days per week that they are taken varies between treatments Injection under the skin (SC injection): This requires a short needle to be injected into a pinch of skin. Common areas (sites) to inject are the abdomen, arm and thigh. There may be some pain and/or bruising at the injection site after injecting • The injection would be delivered at home, either by an HCP, yourself, a friend or a family member • If you deliver the injection yourself, you will receive training from an HCP • You would need to store the injections in a refrigerator until they are used • The benefits and risks of a treatment are not related to the mode and frequency of administration |
Oral pill, once daily, at home Oral pill, three times a week, at home SC injection, once every 2 weeks, at home (reference) SC injection, once every 4 weeks, at home |
| Need for iron supplements |
Depending on the treatment that you take, you may also need to take additional iron supplements. This can either be in oral or IV form Oral iron pill: At least one tablet would be taken once per day, over a period of 3–6 months IV iron infusions: These require a needle to be inserted into a vein, often in the arm. This must be done by an HCP in a medical facility. The infusion will take between 30 min and 3 h. There may be some pain at the injection site owing to the IV infusion • The benefits and risks of a treatment are not related to the need for iron supplements |
Intravenous iron once every 3–6 months, in a medical facility (reference) Oral iron daily, at home No iron supplements |
| Risk of gastrointestinal side effects |
Some treatments increase the risk of experiencing gastrointestinal side effects. This risk depends on the treatment that you are taking • Gastrointestinal side effects may include nausea (feeling sick), vomiting (being sick), diarrhoea (loose stools), bloating (feeling full or tight in your abdomen) or constipation (difficulty passing stools). The side effects are not severe but may be unpleasant or uncomfortable to experience. The risk of gastrointestinal effects will last for as long as you are on the treatment |
0%: 0 out of 100 people will experience gastrointestinal side effects 20%: 20 out of 100 people will experience gastrointestinal side effects 50%: 50 out of 100 people will experience gastrointestinal side effects (reference) |
| Risk of a major cardiovascular event |
Some treatments increase the risk of a major cardiovascular event. This risk depends on the treatment being taking • Major cardiovascular events include stroke (interruption in the flow of blood to the brain), heart attack, hospitalisation for severe chest pain and heart failure (wherein the heart cannot pump blood throughout the body properly). All of these events would require hospitalisation at minimum, and at worst, they could be fatal. The risk of a major cardiovascular event will last for as long as you are on the treatment |
5%: 5 out of 100 people will experience a cardiovascular event 10%: 10 out of 100 people will experience a cardiovascular event 15%: 15 out of 100 people will experience a cardiovascular event (reference) |
| Energy levels | • The main symptom of anaemia is tiredness. These treatments are meant to improve your anaemia-related tiredness and increase your energy levels. However, not all treatments will improve your energy levels in the same way. This attribute defines the frequency in which you will feel like you have a lot of energy once you are on the treatment |
You always have a lot of energy You sometimes have a lot of energy You rarely have a lot of energy (reference) |
DCE discrete choice experiment, HCP healthcare professional, IV intravenous, SC subcutaneous
The description of the injectable mode of administration given to patients also included the possibility of injection-site reactions, the potential need for training from a healthcare professional (HCP), and the need for storage and refrigeration of the medicine (Table 1).
Owing to the complexity of potential combinations of attributes and risk levels, and to avoid patients being overwhelmed with the number of hypothetical treatment profiles, only a small number of treatment profiles were included in the survey. These were chosen to ensure that all effects of interest could be estimated independently and that patients made trade-offs when choosing between treatment profiles. Patients were therefore compelled to consider risks associated with different hypothetical treatments when making their decisions.
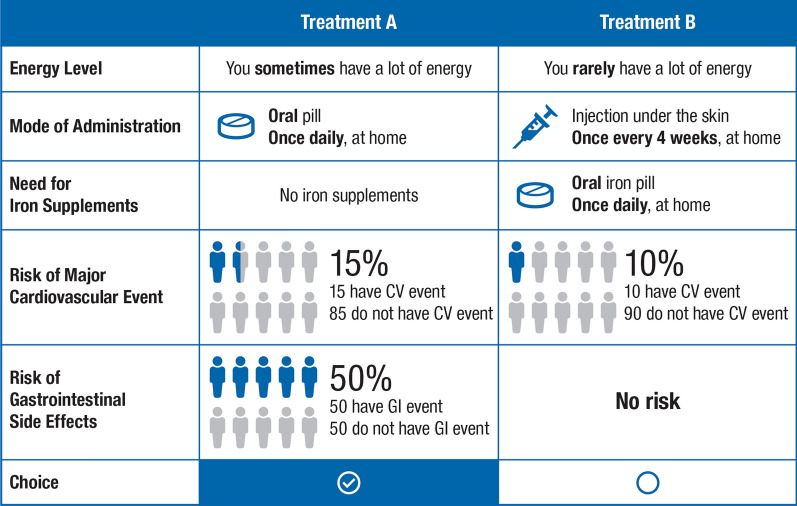
A pilot phase for the DCE survey was conducted, in which the relevance, completeness, robustness and clarity of the attributes included in the DCE were refined through cognitive interviews conducted by telephone or web-based teleconference technology with 20 patients (n = 5 each from the UK, Germany, Spain and France) with anaemia of CKD who met the eligibility criteria. The DCE was designed with the dp-optimal method using Ngene software version 1.1.2 (ChoiceMetrix, Sydney, Australia). For each DCE choice scenario, patients had to choose between two treatment alternatives (Table 2) based on the five included attributes (Fig. 1); attribute levels differed between the treatments and were varied according to the experimental design. An example DCE choice task is shown in Fig. 1; patients were asked to select their preferred treatment option between two hypothetical treatments with varied mode of administration, resultant energy levels, need for oral iron therapy and varied levels of side effects.
Table 2.
Level coding in the DCE
| Attributes | Levels |
|---|---|
| Mode and frequency of administration | Oral pill, once daily, at home |
| Oral pill, three times a week, at home | |
| Subcutaneous injection, once every 4 weeks, at home | |
| Subcutaneous injection, once every 2 weeks, at homea | |
| Need for iron supplementation | No iron supplements |
| Oral iron daily, at home | |
| IV iron once every 3–6 months, in a medical facilitya | |
| Risk of gastrointestinal side effects | 0% |
| 20% | |
| 50%a | |
| Risk of a major cardiovascular event | 5% |
| 10% | |
| 15%a | |
| Energy levels | You always have a lot of energy |
| You sometimes have a lot of energy | |
| You rarely have a lot of energya |
DCE discrete choice experiment, IV intravenous
aReference category
Fig. 1.
Example of a choice task in the DCE. The hypothetical treatment options included five attributes (energy levels, mode and frequency of administration, need for iron supplements, risk of major CV event and risk of GI side effects), and patients selected the treatment based on the level of risk they were willing to accept in exchange for an oral therapy. CV cardiovascular, DCE discrete choice experiment, GI gastrointestinal
The DCE included 24 choice tasks, covering information on all attributes, split into two blocks to reduce survey fatigue such that each participant completed 12 experimental choice tasks and preferences were assessed based on responses to all 12 tasks as the treatment profiles presented varied. This aimed to replicate real-life decision making where, for example, a treatment may offer better efficacy but be associated with higher risks. Patients also completed two non-experimental, internal validity assessment choice tasks (see below). The order of the choice questions was randomised to mitigate ordering effects. Equally, the order of the attribute groups (i.e. benefits, risks and other), and attributes within groups within the choice tasks were also randomised.
Internal Validity Assessments, Health Literacy and Numeracy, and Sociodemographic and Clinical Data
To assess the DCE’s internal validity, one set of choices was repeated to evaluate the consistency of a patient’s answers; consistency criteria were not met if respondents provided two different answers to the choice questions. A dominated-choice question was also included at the end of the DCE in which one option clearly dominated the other through every attribute, either being as good as or better than the alternative. The dominated-choice question was used to assess whether patients understood and were engaged with the discrete choice task, and respondents were deemed to lack an understanding of, or engagement with the task if they did not choose the dominant option as their preferred treatment. Following the DCE survey, patients also completed a health literacy and numeracy questionnaire, as well as a sociodemographic and clinical questionnaire.
Statistical Analysis
Statistical analyses were conducted using R version 3.6 (R Foundation, Vienna, Austria). The DCE preference data for all attributes obtained from the main survey were analysed using a multinomial logit model (MNL) based on the random utility maximisation theory [16]. The MNL estimated patient preferences for discrete changes in the treatment attributes. The estimated preferences were used to compute the maximum acceptable risk (MAR) of major CV events, MAR of GI side effects and minimum acceptable energy level that patients were willing to forego in exchange for a one-unit improvement in the other treatment dimensions. The MAR indicates the percent increase in the risk that patients are willing to accept to improve other treatment attributes by one unit, and the minimum acceptable energy level measures the willingness of patients to decrease energy levels in exchange for a one-unit improvement in the other treatment dimensions (e.g. a 1% risk decrease); 95% confidence intervals (CIs) around the MAR and minimum acceptable energy level measures were obtained with the Delta method [17]. An interacted MNL model was also used to assess the impact of participant characteristics on preferences (Supplementary Methods).
Several robustness checks were conducted to select the most appropriate reference model, such as: (1) a heteroscedastic logit model, which was used to determine whether choice data from the different countries could be pooled together; (2) a multinomial logit model with an error component tested for the presence of panel effects (i.e. multiple choice observations coming from the same respondent) in the data; and (3) sensitivity analyses based on internal validity measures. The sensitivity analysis assessed the robustness of the data to situations in which participants failed zero to four tests (i.e. five situations), using a dummy-coded multinomial logit model. The tests encompassed choice dominance (participants failed if they did not choose the dominant option); choice stability (participants failed if they provided different answers to a question that was repeated twice); dominated decision making (where a participant’s preferred treatment option was based solely on the levels of one attribute, rather than a trade-off between multiple attributes); serial non-participation (where a limited level of engagement in the DCE was displayed if a participant consistently chose an option based on its location in the choice task, e.g. always choosing option ‘B’ or an option displayed on the left) and time to complete choice tasks (the time taken for each respondent to complete each task was recorded and extreme response times—too slow or too quick—indicated a lower engagement in the DCE).
The DCE dataset had no missing values, as patients could not proceed in the survey without answering each question or item. Further details on the statistical methodology are provided in Supplementary Methods.
Results
Patient Sociodemographic and Clinical Characteristics
Of 890 patients invited to join the study, 200 consented and completed the DCE survey (Germany, n = 62; Spain, n = 53; UK, n = 52; France, n = 33). The maximum feasible sample size for the study was 200 and was sufficient for an overall sample analysis, but not sufficient for country-specific analysis.
Patient sociodemographic and clinical characteristics are presented in Table 3; additional details are provided in Tables S5–6. The mean (standard deviation [SD]) age of patients was 53.8 (12.7) years, and just over half (53%) were male. Patients had been diagnosed with CKD for an average (SD) of 6.0 (7.5) years prior to the study and had been diagnosed with anaemia of CKD for an average (SD) of 3.5 (3.4) years. The majority of patients (72%) received oral iron supplements and 12% received intravenous iron. ESA administration was predominantly subcutaneous (82% of patients), while the frequency of administration varied between three times per week to once every 6 months. Most (73%) patients did not use self-injectable medicines for other health conditions. Regarding educational background, 44% of patients had a secondary education, 26% had a college education and 16% had a postgraduate degree.
Table 3.
Sociodemographic and clinical characteristics of patients in the DCE
| Characteristics | Overall (N = 200) |
|---|---|
| Age (years), mean (SD) | 53.78 (12.73) |
| Sex (male), n (%) | 106 (53) |
| Country, n (%) | |
| UK | 52 (26) |
| Spain | 53 (26) |
| France | 33 (16) |
| Germany | 62 (31) |
| Highest education level, n (%) | |
| No formal qualifications | 27 (14) |
| Secondary education | 89 (44) |
| Higher education | 53 (26) |
| Postgraduate degree | 31 (16) |
| Time since diagnosis of CKD (years), mean (SD) | 6.02 (7.49) |
| Time since diagnosis of anaemia of CKD (years), mean (SD) | 3.45 (3.44) |
| Receive iron supplements on a regular basisa, n (%) | |
| Oral iron supplements | 145 (72) |
| Intravenous iron supplements | 25 (12) |
| No iron supplements | 34 (17) |
| Current erythropoiesis-stimulating agent treatment, n (%) | |
| Erythropoietin | 122 (61) |
| Epoetin alfa | 18 (9) |
| Epoetin beta | 29 (14) |
| Darbepoetin alfa | 40 (20) |
| Methoxy polyethylene glycol-epoetin beta | 14 (7) |
| Frequency of erythropoiesis-stimulating agent, n (%) | |
| Three times per week | 10 (5) |
| Twice per week | 45 (22) |
| Once per week | 62 (31) |
| Once every 2 weeks | 52 (26) |
| Once every month | 29 (14) |
| Otherb | 2 (1) |
| Administration of erythropoiesis-stimulating agent, n (%) | |
| Intravenous | 37 (18) |
| Subcutaneous | 163 (82) |
| Regular use of an injectable medicine for other health problems, n (%) | |
| Yes | 54 (27) |
| No | 146 (73) |
CKD chronic kidney disease, DCE discrete choice experiment, SD standard deviation
aOral iron and intravenous iron were not mutually exclusive. No iron could not be selected in addition to oral or intravenous iron
bOnce every 3 weeks and every 6 months
Internal Validity and Health Literacy and Numeracy
Only 4% of patients (n = 9) failed the dominated-choice test, in which one treatment was superior in all attributes, and 23% of patients (n = 46) failed the consistency assessment by providing two different answers to the choice questions. The 55 patients who failed the internal validity tests were not excluded from the analyses. Two-thirds (66%) of patients had adequate health literacy and 84% had adequate health numeracy.
Preferences for Attributes of Anaemia of CKD Treatments
The heteroscedastic logit model confirmed that data could be pooled across all four countries. A multinomial logit model with an error component was confirmed to have the best fit and was used as the reference model. The results from the sensitivity analysis of patient preferences indicated that the results were robust to the exclusion of participants with low validity scores.
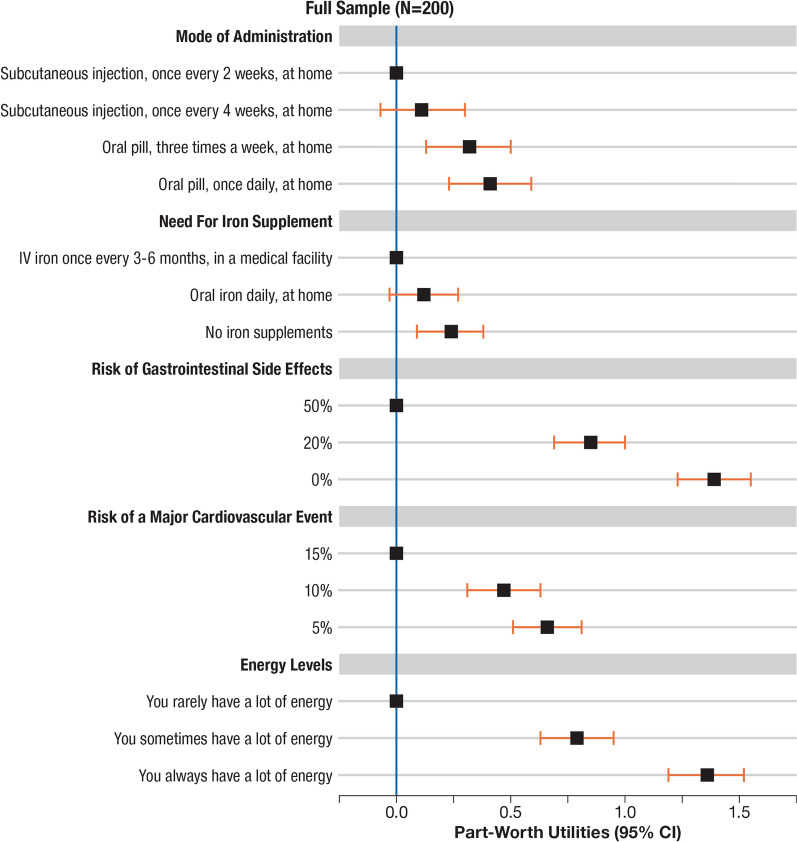
All attributes were significantly valued by patients (Fig. 2). Patients preferred lower risks and increased energy levels. Patients preferred both a reduction in the risk of GI side effects from the reference level of 50% to 20% (utility = 0.85, p < 0.001, CI 0.7–1.0) and 50% to 0% (utility = 1.4, p < 0.001, CI 1.2–1.6). Furthermore, patients preferred a reduced risk of a major CV event from the reference level of 15% to 10% (utility = 0.47, p < 0.001, CI 0.3–0.6) and 15% to 5% (utility = 0.66, p < 0.001, CI 0.5–0.8). Patients preferred an ‘oral pill at home’ either once daily or three-times weekly over the reference level ‘subcutaneous injection, once every two weeks, at home’ (once daily utility = 0.41, p < 0.001; three-times weekly utility = 0.32, p < 0.001). In addition, patients preferred ‘no iron supplements’ over the reference level, ‘intravenous iron once every 3–6 months, in a medical facility’ (utility = 0.24, p < 0.01, CI 0.1–0.4); however, no significant preference was detected between ‘oral iron daily, at home’ and the same IV reference level (once daily utility = 0.12, p > 0.05, CI 0.0–0.3).
Fig. 2.
Findings from the multinomial model with error component analysis on patient valuation of treatment attributes (N = 200). Patient preferences for treatment attributes were analysed using a multinomial logit model with an error component. CI confidence interval, IV intravenous
Trade-Offs between Attributes of CKD Treatments
Cardiovascular Risk
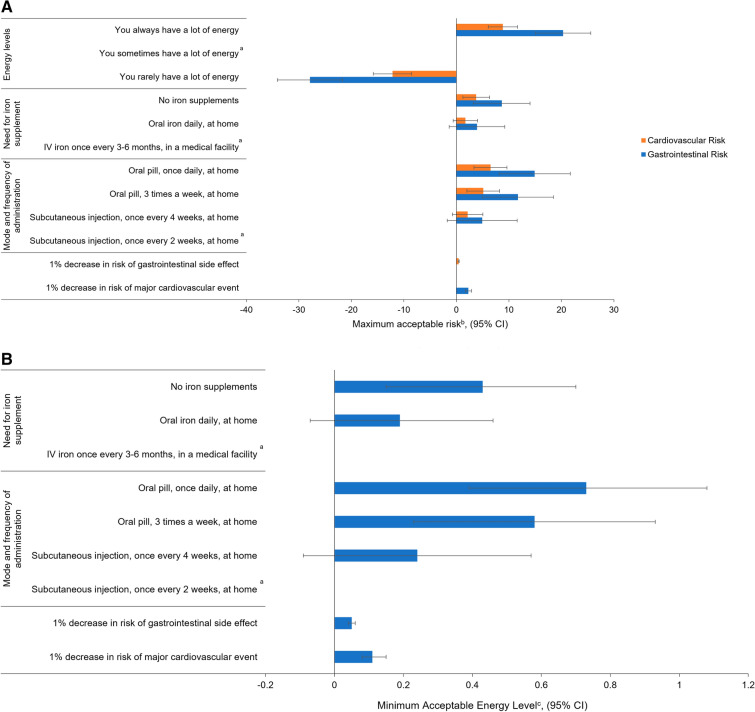
Patients were willing to accept a 5% (95% CI 2.0–8.3%) increase in the risk of a major CV event to switch from an at-home subcutaneous injection administered once every 2 weeks to an at-home oral pill taken three times a week (Fig. 3). Patients were willing to accept a 12% increase in the risk of a major CV event to move from ‘You rarely have a lot of energy’ to ‘You sometimes have a lot of energy’ (Fig. 3). Patients were willing to accept a 0.4% (95% CI 0.3–0.6%) increase in the risk of a major CV event to reduce the risk of GI side effects by 1% (Fig. 3).
Fig. 3.
Trade-offs assessed in the DCE for, A maximum acceptable riskb and B minimum acceptable energy levelc. aReference levels. bMaximum acceptable risk was used to quantify patients’ preferences using percent change in risk as a common unit of measurement. The maximum acceptable risk indicated the percent increase in the ‘risk of major cardiovascular event’ and ‘risk of gastrointestinal side effects’ that patients are willing to tolerate to improve other treatment attributes by one unit. cThe estimated preferences were also used to compute the minimum acceptable energy level that patients were willing to forego in exchange for a one-unit improvement in the other treatment dimensions. Calculated for a positive change from ‘You sometimes have a lot of energy’ to ‘You always have a lot of energy’. CI confidence interval, DCE discrete choice experiment, MAEL minimum acceptable energy level, MAR maximum acceptable risk, SE standard error
Gastrointestinal Risk
Patients were willing to accept a 12% (95% CI 5.0–18.5%) increase in the risk of GI side effects to switch from an at-home subcutaneous injection administered once every 2 weeks to an at-home oral pill administered three times a week (Fig. 3). Patients were also willing to accept a 20% increase in the risk of GI side effects to move from ‘You sometimes have a lot of energy’ to ‘You always have a lot of energy’ (Fig. 3). Further to this, from the qualitative interviews, patients that had previously experienced gastrointestinal side effects were also more averse to increases in GI risk.
Energy Levels
Switching from a subcutaneous injection every 2 weeks to an oral pill three times per week was worth 58% of the value gained moving from ‘you sometimes have a lot of energy’ to ‘you always have a lot of energy’ (Fig. 3). Switching from intravenous iron once every 3–6 months in a medical facility to daily oral iron at home was worth 19% of the value gained moving from ‘you sometimes have a lot of energy’ to ‘you always have a lot of energy’.
Preference Heterogeneity
From the interacted MNL model, patient characteristics were found to significantly influence preferences, excluding education and EPO as current ESA treatment. The influence on preferences was the largest for country, followed by current ESA frequency. Patients from the UK valued no treatment, oral mode of administration, reducing gastrointestinal risk, reducing cardiovascular risk and increasing energy levels more highly than patients in other countries (Table S7). Patients with less frequent ESA administration valued reducing cardiovascular risk and increasing energy levels more than those with more frequent ESA administration (Table S8). Patients currently receiving oral iron also gained more value from no treatment, reducing the gastrointestinal risk to 0% and increasing energy levels than those not using oral iron (Table S9).
Discussion
In this study, a targeted literature review and qualitative patient interviews were conducted to inform a DCE survey, which was then used to solicit patients’ preferences for hypothetical anaemia treatment options and determine if they were willing to trade off GI or major CV event risks in exchange for oral administration or improvement in energy levels.
The DCE survey showed that patients with anaemia consider the mode and frequency of administration to be an important aspect of their treatment. Patients expressed a preference for oral administration (either once daily or three times weekly) over subcutaneous administration at home every 2 weeks. In addition, patients on average were willing to accept a 5% increase in the risk of major CV events to exchange IV treatment for oral. Consequently, these findings suggest that, on average, patients are willing to tolerate an increase in CV risk to use an orally administered treatment. Based on qualitative insights, this may be due to potential improvements in quality of life associated with ease of use. Further to these findings, a recent study investigated the patient preferences of DD patients with CKD in Australia and Canada via DCE. Where not fully reimbursed, the cost per month was also identified as an important factor patients take into consideration in addition to the risk of side effects [18].
However, with regard to iron supplementation, no significant difference in patient preference was detected between oral iron daily at home and IV iron every 3–6 months in a medical facility; this is particularly notable, as the majority of patients were receiving oral iron on a daily basis with the associated adverse GI events. The DCE survey also indicated that patients with anaemia considered their energy levels to be an important aspect of their treatment outcomes, as they were willing to accept a 12% increase in CV risk to move from ‘rarely’ to ‘sometimes’ having a lot of energy, or a 20% increase in risk of GI side effects to move from ‘sometimes’ to ‘always’ having a lot of energy.
Patient preferences are difficult to measure in clinical practice [19]. This study’s findings—that patients were willing to tolerate certain increases in the risk of side effects when offered a more convenient mode of administration—may provide insight into patients’ willingness to accept novel treatment options. A strength of this study was that the most pertinent attributes to patients were selected through qualitative research, while quantitative research was used to assess patient preferences. Similar DCE methodology has been used previously to capture patient preferences for treatment in other therapeutic areas, such as diabetes [20], and based on the internal validity assessments, levels of consistency in our DCE results were in line with those observed in the surrounding literature [21].
Some potential limitations should be noted. The average age of patients in this study was lower than the average CKD population and had a reduced proportion of patients aged ≥ 70 years in comparison with previous studies [22]. As such, the results from this study may not be generalisable to all patients with anaemia of CKD. The sample size for each country was limited, which prevented statistical comparisons between them. Previous experience of patients with GI side effects was also not included in the DCE; as such, the impact on patient preferences was not quantitatively analysed. Most participants had been treated with ESA for longer than 6 months prior to interview; consequently, patients were likely to be in a more advanced stage of their disease. This could provide an important insight into the patients’ preference for different treatments as familiarity with the current treatment does not preclude them from exploring new treatment options.
The description of the subcutaneous injection (mode of administration) included additional considerations associated specifically with this mode of administration, such as the possibility of injection-site reactions, the potential need for training from an HCP and the need for refrigeration of the medicine, while the oral mode description did not include any additional considerations, such as the need to take treatment a specific time interval in relation to other medications.
Non-health-related benefits, such as mode of administration, are not commonly captured by traditional cost-effectiveness analyses. Evaluation of the value attributed to these benefits by patients can help guide health technology assessments and inform shared decision making at the point of care. Our findings indicate that some non-health-related aspects of treatment, such as mode of administration, were important to patients. Patient preferences and determining which mode and frequency of treatment administration would work best with the patient’s lifestyle, ought to form part of the decision-making process, which should be shared between patient and clinician. The majority of recommendations in the KDIGO guidelines that are associated with the administration of ESA and iron therapy are grade 2, indicating that patients’ values and preferences should be taken into account when choosing a treatment regimen [5]. Engaging patients as stakeholders in the healthcare decision-making process also aligns with guidance from the European Medicines Agency and the US Food and Drug Administration to better understand patients’ perspectives [23, 24]. Furthermore, regulatory and health technology assessment agencies in the US, Europe and Canada are involving patients to understand their treatment preferences and to improve transparency in the regulatory process [25]. Understanding of patient preferences to inform shared decision making may also improve treatment uptake, adherence and overall quality of life [26]. This is particularly relevant in chronic disease settings such as CKD-related anaemia, where adherence to treatment is known to be lower.
Conclusions
This study found that patients with anaemia of CKD valued non-clinical attributes of treatments in addition to clinical benefits. In particular, the mode of administration and changes in energy levels were both shown to significantly influence patient preferences. Consequently, it is imperative to ensure that benefit-risk discussion for anaemia treatments include these non-clinical features that are important to patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Writing support was provided by Katie Crosslin, PhD, and Phil Leventhal, PhD, both of Evidera/PPD, and Rhian Harper Owen, PhD, Iona Easthope, PhD, and Glen Dorrington, PhD, for Lumanity, funded by Astellas Pharma. The authors would like to thank all patients and physicians who took part in this study.
Funding
The study and writing support for this study were funded by Astellas Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualisation: Ana Filipa Alexandre, Antonia Morga, Caitlin Thomas, Tommi Tervonen, Alina Jiletcovici, Kevin Marsh; Methodology: Ana Filipa Alexandre, Antonia Morga, Caitlin Thomas, Nicolas Krucien, Tommi Tervonen, Kevin Marsh; Formal analysis and investigation: Caitlin Thomas, Nicolas Krucien, Tommi Tervonen, Kevin Marsh; Writing—review and editing: Ana Filipa Alexandre, Antonia Morga, Caitlin Thomas, Nicolas Krucien, Tommi Tervonen, Alina Jiletcovici, Kevin Marsh; Funding acquisition: Ana Filipa Alexandre, Antonia Morga; Resources: Ana Filipa Alexandre, Antonia Morga, Nicolas Krucien, Alina Jiletcovici; Supervision: Tommi Tervonen, Kevin Marsh; Project administration: Caitlin Thomas; Data curation: Nicolas Krucien; Software: Nicolas Krucien.
Disclosures
Caitlin Thomas, Tommi Tervonen, Kevin Marsh and Nicolas Krucien are employees of Evidera, which provides consulting and other research services to pharmaceutical, medical device and related organisations. In their salaried positions, they work with a variety of companies and organisations and are precluded from receiving payment or honoraria directly from these organisations for services rendered. Evidera received funds from Astellas for work related to this study. Antonia Morga, and Alina Jiletcovici are employees of Astellas Pharma. Ana Filipa Alexandre was an employee of Astellas Pharma at the time of this study.
Compliance with Ethics Guidelines
This study was approved by the Ethics and Independent Review Services (E&I) committee (study number 18206-01). All patients provided online informed consent. Data were anonymous, and only assigned participant ID numbers were linked to audio recordings and online survey results. All procedures were performed in accordance with the ethical standards of the relevant institutional review boards (IRBs); procedures were also performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, with the exception that study registration was not available in publicly accessible database prior to recruitment.
Data Availability
Researchers may request access to anonymised participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
- 1.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho IJ, Mun YC, Kwon KH, Shin GJ. Effect of anemia correction on left ventricular structure and filling pressure in anemic patients without overt heart disease. Korean J Intern Med. 2014;29:445–453. doi: 10.3904/kjim.2014.29.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 2020;21:88. doi: 10.1186/s12882-020-01746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pergola PE, Fishbane S, Ganz T. Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:272–291. doi: 10.1053/j.ackd.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease [Internet]. Kidney Int. Suppl. 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf. Accessed 22 Aug 2022.
- 6.Wong MMY, Tu C, Li Y, Perlman RL, Pecoits-Filho R, Lopes AA, et al. Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the chronic kidney disease outcomes and practice patterns study: Often unmeasured, variably treated. Clin Kidney J. 2020;13:613–624. doi: 10.1093/ckj/sfz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valderrábano F, Hörl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters J. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 2003;18:89–100. doi: 10.1093/ndt/18.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Evans M, Bower H, Cockburn E, Jacobson SH, Barany P, Carrero JJ. Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clin Kidney J. 2020;13:821–827. doi: 10.1093/ckj/sfaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minutolo R, Locatelli F, Gallieni M, Bonofiglio R, Fuiano G, Oldrizzi L, et al. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol Dial Transplant. 2013;28:3035–3045. doi: 10.1093/ndt/gft338. [DOI] [PubMed] [Google Scholar]
- 10.Drüeke TB, Locatelli F, Clyne N, Eckardt K-U, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 13.Sanghani NS, Haase VH. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26:253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 15.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/S0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 16.Paul P, Berlin C, Maessen M, Valtonen H. A comparison of regret-based and utility-based discrete choice modelling—an empirical illustration with hospital bed choice. Appl Econ. 2018;50:4295–4305. doi: 10.1080/00036846.2018.1444260. [DOI] [Google Scholar]
- 17.Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ. 2007;16:827–840. doi: 10.1002/hec.1197. [DOI] [PubMed] [Google Scholar]
- 18.Fifer S, West B, Garcia Sanchez JJ, Wittbrodt E, Bhatt P, Grandy S, et al. MO561preferences of dialysis-dependent patients for treatment of anaemia of chronic kidney disease in Australia and Canada: a discrete choice experiment. Nephrol Dial Transplant. 2021;36:gfab085.0024.
- 19.van den Broek-Altenburg E, Atherly A. Using discrete choice experiments to measure preferences for hard to observe choice attributes to inform health policy decisions. Health Econ Rev 2020;10:18. [DOI] [PMC free article] [PubMed]
- 20.Brooks A, Langer J, Tervonen T, Hemmingsen MP, Eguchi K, Bacci ED. Patient preferences for GLP-1 receptor agonist treatment of type 2 diabetes mellitus in Japan: a discrete choice experiment. Diabetes Ther. 2019;10:735–749. doi: 10.1007/s13300-019-0591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandorf ED. Did you miss something? Inattentive respondents in discrete choice experiments. Environ Resour Econ. 2019;73:1197–1235. doi: 10.1007/s10640-018-0296-y. [DOI] [Google Scholar]
- 22.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haerry D, Landgraf C, Warner K, Hunter A, Klingmann I, May M, et al. EUPATI and Patients in Medicines Research and Development: Guidance for Patient Involvement in Regulatory Processes. Front Med. 2018;5:230. [DOI] [PMC free article] [PubMed]
- 24.Patient Preference Information—Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling | FDA [Internet]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-preference-information-voluntary-submission-review-premarket-approval-applications. Accessed 2 Dec 2021.
- 25.Reed SD, Lavezzari G. International experiences in quantitative benefit-risk analysis to support regulatory decisions. Value Health. 2016;19:727–729. doi: 10.1016/j.jval.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Bouvy JC, Cowie L, Lovett R, Morrison D, Livingstone H, Crabb N. Use of patient preference studies in HTA decision making: a NICE perspective. Patient. 2020;13:145–149. doi: 10.1007/s40271-019-00408-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymised participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.