Abstract
Introduction
We sought to synthesize published empirical studies that elicited and characterized societal valuations of orphan drugs and the attributes that may drive different valuations for orphan drugs versus other treatments.
Methods
We conducted a systematic literature review (SLR) in MEDLINE and EMBASE databases up to November 2, 2020. Search terms covered societal preferences and attributes of orphan drugs (e.g., disease prevalence, severity, burden, unmet needs, and benefits).
Results
We identified 38 eligible publications: 33 societal preference studies and 5 reviews discussing societal valuations and attributes of orphan drugs. Most publications suggested that a majority of respondents favored allocating funds to more prevalent diseases. However, trade-off studies and discrete-choice experiments found that survey participants chose to allocate resources to orphan drugs even when the cost per unit of health benefit was greater than for therapies for more prevalent diseases. Overall, 19 of 27 studies assessing severity in treatment valuation revealed that respondents prioritized patients with severe diseases over those with milder ones for equal health benefits. Members of the general public tended to prefer treatments for diseases with no alternative or when existing alternatives had limited efficacy over diseases with clear therapeutic alternatives. There was evidence that individuals preferred sharing resources, so no patient was left without treatment.
Conclusions
Our SLR indicates the general public typically attaches greater value to orphan drugs than to other treatments for common diseases. This is not because of rarity per se, but primarily because of disease severity and lack of therapeutic alternatives typically associated with rare diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02359-z.
Keywords: Orphan drugs, Rare diseases, Societal preferences, Systematic literature review, Treatments for rare diseases, Valuation
Plain Language Summary
Orphan drugs are drugs serving a substantial public health need by treating life-threatening or chronically debilitating medical conditions affecting a small number of people with very high unmet needs. We reviewed 38 published studies looking at drug characteristics that may cause people to value orphan drugs differently versus treatments for common conditions. Most people surveyed in these publications favored health care funds going to more prevalent diseases. However, some people preferred funding orphan drugs even when the cost versus health benefit was higher compared with treatments for more common diseases. The majority of studies that investigated the impact of disease severity on the valuation of treatments found that people prioritized patients with severe disease over those with milder disease, for the same extent of health benefit. People also preferred funding treatments for diseases that have no alternative treatments, or treatments with limited benefits, over treatments for diseases with many treatments or more effective treatments. We also found evidence of a societal preference for shared resources, meaning that no patient would be left without treatment, including those who receive limited benefits from health care resources, even if this does not lead to the maximization of health benefits across society. In conclusion, our literature review indicated that the general public attaches greater value to orphan drugs versus treatments for more common diseases, not because of rarity per se, but largely because the rare diseases treated by orphan drugs are often severe and have no or few treatment options.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02359-z.
Key Summary Points
| Several criteria are considered for orphan drug designations, and the most important factor driving acceptance of greater costs from health authorities’ perspective appears to be disease rarity. |
| This literature review suggests that study participants and respondents from the general public generally attach greater value to treatments for rare diseases than to treatments for common diseases. |
| The excess value of orphan drugs does not appear to be related to disease rarity per se. With all else equal, members of the general public are willing to allocate resources preferentially to treatment of patients with a rare disease, but only in those cases where these patients are severely ill and/or have a great unmet need or potential to benefit from treatment. |
Introduction
Orphan drugs include therapies for life-threatening or chronically debilitating rare diseases affecting no more than 5 in 10,000 people in the European Union [1]. No existing treatment provides satisfactory or significant benefit for these diseases, according to the European Medicines Agency (EMA) orphan drug designation criteria [1]. Established legislation aimed at promoting treatments for rare diseases, such as the 1983 US Orphan Drug Act [2, 3], the 2000 European Commission Regulation on orphan medicinal products [4], and the Joint Evaluations of Regulation of the European Parliament (for orphan medicinal products in 1999 and for pediatric medicinal products in 2006) [4], has possibly resulted in an increased number of orphan drugs in the last decade [5].
To offset the financial pressures and developmental challenges associated with treatments for rare diseases arising from small patient populations, health technology assessment (HTA) and payer organizations have updated their requirements for orphan drug appraisal during the last decade. Even though orphan drugs broadly undergo the same HTA process as other treatments, focusing on a drug’s performance by efficacy, safety, and economic considerations (cost effectiveness and budget impact), orphan drugs may benefit from specific considerations in some countries [6].
In France, for example, orphan drugs are fast-tracked, reducing assessment timelines from 90 to 15 days, and products with a budget impact below €20 million per year are exempted from health economic assessments [7]. In Germany, for orphan drugs authorized by the EMA, there is de facto “proven benefit” and free pricing during the first year [7]. For countries using evidence from cost-effectiveness analyses, greater incremental cost-effectiveness ratios (ICERs) have been accepted for rare disease treatments compared with non-rare disease treatments. For instance, while the cost-effectiveness threshold set by the National Institute for Health and Care Excellence (NICE) in the UK is of £20,000–£30,000 per quality-adjusted life year (QALY) for the standard technology assessment process, it is £100,000 per QALY for highly specialized technologies [8, 9].
In other countries, such as The Netherlands, orphan drugs may benefit from greater ICER thresholds based on severity of illness. The Zorginstituut Nederland pays up to €20,000 per QALY for the least severe diseases and up to €80,000 per QALY for the most severe diseases [9–11]. The existence of mechanisms that allow for greater prices for orphan drugs raises the question of whether it is justifiable for payers to accept such price premiums [12].
Drug pricing and, more generally, health care decision-making should be assessed based on “value” [13, 14]. Côté et al. [15] argued that HTA organizations, manufacturers, patients, and society must engage in further discussions about how to assess value in the context of orphan drugs. Many studies have looked at societal preferences related to the allocation of health care resources and at what drives the value of health care treatment from the perspective of society to inform the development of decision frameworks. However, there is no review summarizing the learnings from these studies regarding society’s value of orphan drugs relative to other treatments.
The objective of this systematic literature review (SLR) was to better understand whether societal values support greater prices for orphan drugs than other treatments by investigating published reviews and empirical studies that assessed societal preferences related to attributes characterizing orphan drugs. A special emphasis was also placed on the additional value and attributes of orphan drugs that drive this societal preference.
Methods
Search Strategy
PRISMA guidelines were used for the design of the review. We searched two databases, Ovid MEDLINE and EMBASE, up to November 2, 2020. No chronological or geographical restrictions were applied. Search terms were developed to cover societal preferences for health care priority setting in the context of rare diseases (Supplemental Table S1). The search strategy encompassed all the attributes pertaining to orphan drugs according to EMA designation criteria (i.e., rarity, severity, lack of alternative, or significant benefit). In line with the EMA grounds for significant benefit and added value, the search also included a focus on improved mortality and morbidity and health-related quality of life (HRQOL) [16, 17]. In particular, key words related to cure and life extension as specific types of “significant benefit” were included. Further records were identified opportunistically with recommendations from experts in the field and from citations from the assembled articles. Publications not reported in English were excluded from the review during the screening and eligibility assessment. The complete search strategy, along with search terms, is provided in Supplemental Table S1.
Selection of Studies and Eligibility Criteria
Two reviewers independently screened the titles and abstracts of all records identified by the search strategy and then reviewed full texts of eligible records for inclusion. All discrepancies were resolved by a third reviewer. Reviews and quantitative preference studies were included if they assessed societal valuations for orphan drugs and treatments of rare diseases in general or societal preferences between competing criteria applicable to a priority setting for health care interventions (Table 1). In particular, we included any record that assessed whether a greater health improvement in a small population had a different value compared with a lesser improvement in a large population, with an equivalent aggregated benefit in health units. We designated this concept the “relative health improvement value.” Disease- or treatment-specific studies, and therefore patient preference studies, were excluded because this SLR was designed to synthesize evidence on relative value of treatments of rare diseases, specifically orphan drugs, compared with other health care interventions from a societal perspective.
Table 1.
Eligibility criteria
| Criteria | Inclusion criteria |
|---|---|
| Population | General population |
| Intervention |
Rare diseases and/or ODs Any condition or treatment presenting at least 1 attribute of ODs, according to the EMA designation criteria, namely: Disease rarity (prevalence) Disease severity and burden Disease unmet need (absence of alternatives or absence of a satisfactory alternative) Treatment added value by significant benefit brought to those affected by the condition Note that the intervention may not be explicitly defined |
| Comparator | Any other health care intervention (may not be explicitly defined) |
| Outcome | Preference values, willingness to pay |
| Study design |
Preference studies conducted in the context of general and non-specific health care settings (excluding any disease- or treatment-specific study) Reviews of preference studies Quantitative methods such as choice-based methods, trade-off methods, and ranking/rating exercises |
| Other restrictions | Articles published in English |
EMA European Medicines Agency; ODs orphan drugs
Data Extraction and Synthesis
For each reference selected, we reported information such as the type of article (preference study or review), country or geographic area, perspective adopted for preference elicitation (personal or societal), and elicitation method used (rating, ranking, choice-based methods, or trade-off tasks) as well as the attributes obtained. The identified attributes were classified into four categories: disease and patient characteristics, treatment and health benefit characteristics, economic considerations, and other contextual factors. In addition, we examined results related to attributes characterizing rare diseases and/or orphan drugs to assess their implications for the prioritization of orphan drugs in pricing and reimbursement decisions. Two independent reviewers classified these results into three categories: (1) supporting greater valuation of orphan drugs, (2) not supporting greater valuation of orphan drugs, and (3) ambivalent.
This analysis is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; therefore, ethics committee approval is not required.
Results
Study Selection and Characteristics
Based on the search strategies, 894 records were identified (Fig. 1); 38 met the inclusion criteria and were included in the SLR (Table 2). Fifteen publications directly addressed societal valuation of orphan drugs while the remaining covered only some of their attributes. The full list of attributes for each study is presented in Supplemental Table S2. Most were societal preference studies (n = 33), and the remainder (n = 5) were literature reviews. The studies included covered 3 decades (1996–2020), with most (n = 33; 87%) published after 2010. The most frequently represented countries were Australia (n = 9) and Canada (n = 6).
Fig. 1.
PRISMA flow chart. ODs orphan drugs. aHand searching is a manual method of manually scanning select journals from cover to cover, page by page, for relevant articles in case they were missed during indexing. This methodical process searches a journal’s entire contents (e.g., articles, editorials, letters from readers) to identify relevant studies and complete the non-indexed searching in the databases
Table 2.
Summary of study characteristics and results of valuation of treatments for rare diseases attributes
| Publication details | Country | Participants | Perspective adopted | Assessment method | (1) Disease rarity | (2) Disease severity/burden | (3) Disease unmet need | (4) Relative health improvement | Results consistent with attribute valuation and prioritization |
|---|---|---|---|---|---|---|---|---|---|
| Bae et al. (2020) [23] | Korea | Society | Not specified | TO | (−) | NR | (+ /−) | NR |
(1) When comparing rare and common diseases, only 22.4% of the respondents agreed that if all other conditions were the same, patients in both disease groups should be equally supported. The majority (59.8%) allocated more to common diseases (3) When comparing diseases with and without alternative therapies, 33.5% answered that they would support the two diseases equally and 45.0% responded that they would support patients who do not have alternative therapies. Assuming that the effects of the two groups are different, 42.9% said that a larger budget should be allocated to diseases that have more effective treatments, even though they already have a few alternatives. Assuming a situation where the costs of the two groups are different, 43.3% allocated the budget to the disease with alternative treatments available that were less expensive |
| Toumi et al. (2020) [33] | France | Society | Health care DM | DCE | (−) | ( ±) | (+) | (+ /−) |
(1) Respondents were willing to treat more patients at a given total cost (2) Disease characteristics (disability and mortality) had only a very moderate impact on participant choices. Although there existed a trend toward greater preferences for more disabling diseases, neither moderate nor severe initial disability reached the threshold for statistical significance in the comparison vs. mild disease disability. For disease mortality, significantly greater preferences were observed only for diseases with the shortest life expectancy (20 years) compared with diseases that did not shorten patient life (0.290, P = .0033) (3) The respondents were more likely to choose treatments without available alternative options or when the effectiveness of alternative options was limited compared with treatments with effective alternatives available (4) The incremental value per LY was greater for a gain of 30 years (0.04/year) than for a gain of 10 years (0.02/year), but less than for a gain of 2 years (0.10/year) |
| Hampson et al. (2019) [24] | UK | Society | Health care DM | DCE | (−) | (−) | NR | (−) |
(1) Respondents preferred to treat greater numbers of patients (utility for treating 200 patients: 0.517 vs. treating 800: 1.414, P < .01). The marginal utility per patient treated does not appear to be significantly greater for smaller patient numbers (2) The results suggest that the worse the outlook of a group of patients is with current care, the less likely it was that respondents would choose to treat that group (weight of dying in 10 years − 0.804 versus dying in 40 years − 1.516, P < .01) (4) The utility increment for 1 LY gained appears to be independent of the magnitude of the LE improvement. Whether a treatment was a cure or not did not appear to influence respondents’ choices |
| Chim et al. (2019) [22] | Australia | Society | Not specified | Ranking | (−) | (+) | (+ /−) | NR |
(1) “Rare disease therapies” received the least number of highest-priority rankings (1.7%) (2) Of all respondents, 1213 (39.4%) considered disease severity to be the most important prioritization criterion for funding a new medicine (3) Medicines targeting a disease for which there is no alternative treatment available received highest priority from 8.6% of respondents |
| Reckers-Droog et al. (2019) [42] | The Netherlands | Society | Citizen | Simple choice task + TO | NR | (+) | NR | NR | (2) Most respondents (47.4–58.9%) attached a greater weight (median of ratios: 2.46–3.50) to reimbursing treatment for relatively more severely ill and younger patients when preferences for both were elicited separately. Moreover, the value attached to a health improvement for patients with severe illness is approximately twice as great as the value for patients with moderate illness, and the value attached to a health improvement for patients with moderate illness is approximately twice as great as the value for patients with mild illness |
| Olofsson et al. (2019) [55] | Sweden | Society | Personal | Contingent valuation | NR | NR | NR | (+ /−) | (4) The VSL in the risk-elimination scenario was significantly greater than the VSL in the risk-reduction scenario when the size of the risk reduction/elimination was larger (10 per 100,000). When risk reduction/elimination was smaller (5 per 100,000), differences were reduced and only of borderline significance for ALS (P = .047) and fatal road traffic accidents (P = .054). Eliminating risk was associated with a premium of approximately 20% |
| Richardson et al. (2019) [43] | Review | (−) | NR | NR | NR | (1) “Survey evidence indicates no preference for the special treatment of RDs when the choice is between rare and common diseases… Rarity per se is not viewed as a reason for special treatment” | |||
| Richardson et al. (2018) [44] | Australia | Society | Citizen | CSPC | NR | (+) | NR | NR | (2) The urgency of the patient’s illness had a significant effect, resulting in almost twice the allocation of LY to the patient with an LE of 2 years compared with patients with an LE of 10 years (5.8 vs. 3.2 years). Although survey respondents reacted as expected to differences in the cost effectiveness of services and to differences in the severity of the patient’s condition, they continued to allocate some part of the notional budget to the patient who would receive no support in a health-maximizing or severity-focused health service. The result is not incompatible with broader economic theory because it is consistent with reciprocal altruism from which individuals may benefit. Nevertheless, it is inconsistent with the present theory and practice of economic evaluation |
| Richardson, et al. (2018) [45] | Australia | Society | Personal | Rating + choice task | NR | (+) | NR | NR | (2) Respondents selected more than utility-maximizing insurance for protection against severe health states: insurance selected by survey participants gave greater coverage for severe health states than the insurance that maximized utility. The differences were quantitatively large. Evidence presented here supports the hypothesis that uncertainty will result in a “severity effect,” a personal preference for greater spending on severe health states than would occur if a health authority allocates its budget to maximize QALYs or total utility as presently measured |
| Bourke et al. (2018) [39] | UK | Society, patients, caregivers, HCP, DM | Health care DM | TO + DCE | (+ /−) | NR | (+) | NR |
(1) According to the TO, on the basis of equal cost and benefit per patient, there were more members of the general public who favored treating patients with rare diseases than with common diseases. When the cost was 10 times greater for patients with rare diseases, more members of the general public favored treating patients with common diseases, but the ratio of mean of 0.43 suggests members of the general public on average would be willing to allocate 4.3 times more money per patient with rare disease than per patient with common disease. According to the DCE, survey participants would prefer the NHS to fund treatments for patients with common diseases (including equal cost per patient and equal benefit) (3) The DCE demonstrates that respondents would prefer the NHS to fund treatments for patients without treatment alternatives (OR = 1.08) |
| Magalhaes et al. (2018) [25] | Canada | Society | Health care DM | Ranking + TO | (−) | (+) | (+) | (+) |
(1) When deciding whether to prioritize a small and more severely ill population (100 patients) or a large and less severely ill population (10,000 patients), respondents began by choosing the larger number. This was expected because in the first few questions the large and small populations were both in severe states of health (2) As the large number of patients was moved to less severe conditions, however, the presumption of priority to the larger population weakened. Participants expressed a willingness to some extent to sacrifice numbers for the sake of prioritizing the worst off (3) Lack of alternative treatments or options to manage symptoms, particularly pain, took priority over large numbers (4) The findings of the study indicate that respondents would support giving priority to a smaller but more severely ill patient population over a larger patient population when prioritizing the needs of the few is lifesaving, extends life enough to give hope of future improvement, and relieves otherwise intractable symptoms, especially pain |
| Song et al. (2018) [51] | Korea | Society | Personal | Contingent valuation | NR | (+) | NR | (+) |
(2) The WTP/QALY based on double-bounded dichotomous choice method was about two times greater for scenarios with severe illness compared with scenarios with moderate illness, except when the treatment was curative (in which situation, WTP appeared to reach a plateau) (4) The WTP for prolonged treatment effects lasting 5 and 10 years and cure scenarios were 1.4, 1.8, and 2.3 times greater, respectively, than in the non-cure scenario (where the treatment effect instantly stopped when treatment was discontinued) |
| Schlander et al. (2018) [37] | Switzerland | Society | Not specified | DCE | (+) | NR | NR | NR | (1) The study provides support for the importance of health state before and after intervention, age, disease prevalence (rarity) and availability of social health insurance as driver of societal preference |
| Richardson et al. (2017) [20] | Australia | Society | Personal + health care DM | Rating + TO | NR | (+) | NR | (−) |
(2) The study supports the “severity hypothesis” that implies that a health service increasing a patient’s utility by a fixed amount will be valued greater when the initial health state is more severe (i.e., a social preference for additional priority for services for severe health states). Weights vary significantly from almost 3.0, in which there is maximum severity and a minimum increment to utility, to 0.3, in which there is minimum severity (4) The ratios of change in societal value over change in health state utility are larger for smaller utility increments |
| McKie et al. (2017) [46] | Australia | Society | Health care DM | Rating + TO | NR | (+) | NR | NR | (2) “Funding of drugs improving the condition of those who will be left more severely ill or disabled in absence of treatment favored by 51% of respondents.” For budget allocation, respondents tended to choose to improve the condition of the worst-off irrespective of the patient’s past health state and even though this meant a reduction in health benefits |
| Polisena et al. (2017) [53] | Canada | Society | Not specified | Rating + ranking | (+ /−) | (+) | (+ /−) | NR |
(1) “79.2% of respondents agreed with equal access to ODs across Canada, and 73.0% agreed with OD funding if additional expenses are justified in the OD’s cost effectiveness. Approximately one-half agreed to pay for ODs independent of their effectiveness.” (2) 26.4% of respondents selected severity of symptoms as their top priority (3) Less than 25% of respondents selected a lack of current treatment as their top priority |
| Upton et al. (2017) [38] | Review | (+ /−) | NR | NR | NR | (1) The results suggest that most respondents do not specifically value rarity, splitting funding when the diseases are of equal cost. However, when the rare disease is more costly, 65.4% of respondents favored giving equal or more than equal funding to the rare disease, sacrificing some utility for equity | |||
| Funagoshi et al. (2017) [26] | Japan | Society, HCP | Not specified | Rating | (−) | (+ /−) | (+ /−) | NR |
(1) Participants rated 26 criteria on a 7-point scale. Disease rarity was ranked 22nd via mean score based on ratings from the public (2) Disease severity was ranked 16th (5.10 points) (3) Treatment alternatives received a mean rating equal to 5.27, ranking 11th |
| Richardson et al. (2017) [36] | Australia | Society | Citizen | TO | (+) | (+) | NR | NR |
(1) A large majority of respondents agreed with the statement: “It is OK to reduce services to the majority by a little to cover the cost of very expensive services needed by the few people with rare diseases.” In each PRO scenario, there was a significant allocation of resources to services for illness A (rare disease) despite the lowering of total health (2) A large majority of respondents agreed with the statement: “The severity of illness, not the cost of treatment, should determine priority. If services for severe illnesses are very costly, the cost should be shared across the whole community.” |
| Shiroiwa et al. (2016) [27] | Japan | Society | Health care DM | TO + DCE | (−) | (+ /−) | (−) | NR |
(1) Only 13.3% of the respondents preferentially allocated medical resources to interventions for the treatment of rare diseases (2) More than one-third of respondents preferentially allocated medical resources to interventions for treatment, severe diseases, and hereditary diseases, whereas approximately 35–45% selected equal allocation. According to the DCE, the most important attribute was age, followed by treatment (vs. prevention) and disease severity (P < .001) (3) There was no statistically significant preference for a lack of prior medical care |
| Ramalle-Gomara et al. (2015) [35] | Spain | Society, HCP (students, mean age 24 years) | Not specified | Rating | (+) | (+ /−) | NR | NR |
(1) Participants agreed with the statement: “Patients with rare diseases should have the same right to treatment as others, even if the treatments are more expensive” (average score of 4.6 on the Likert scale from 1-completely disagree to 5-completely agree) (2) Participants tended to agree that “the health budget should be used so that patients with the most serious illnesses receive treatment, even if they don’t experience the largest health improvements (average score of 3.4 points on a scale from 1 to 5), but also that “the health budget should be used so that patients who can receive the largest improvements receive treatment, even if they don’t have serious illnesses” (average score of 3.3) |
| Gu et al. (2015) [40] | Review | NR | (+) | NR | NR | (2) The empirical evidence is consistent with 16 of 19 studies, suggesting that the general public is largely willing to give priority to a patient with more severe disease. For these studies, three further highlight that severity may be one of the most important attributes to use in health care priority setting | |||
| Dragojlovic et al. (2015) [28] | Canada | Society | Health care DM | Simple task choice + TO | (−) | NR | NR | NR | (1) Overall, between 23.8% and 30.4% of respondents expressed indifference between allocating treatments for patients with rare or common diseases, depending on the considered scenarios. The proportion of respondents preferring to fund the common-disease patients was greater than the proportion preferring to fund the rare disease patients, even when costs per patient were equal for both patient groups |
| Kolasa et al. (2015) [52] | Poland | Society | Not specified | TO + DCE | NR | (+ /−) | NR | (+) |
(2) The first experiment revealed that the majority of senior respondents did not trade patients who were less severely ill against those who were more severely ill. However, most junior respondents preferred to treat more less severely ill patients as a compensation for the loss of 10 more severely ill patients for equal magnitude of benefit. The median junior respondent was willing to treat 20 to 100 less severely ill patients to compensate for the loss for 10 more severely ill patients; the exact number varies according to difference of severity (4) Both juniors and seniors tended to select more patients with smaller health gain as a compensation for the loss of 10 individuals with a bigger potential to benefit. In the case of seniors, the societal value of a health improvement was roughly proportional to the utility gain. In the case of junior respondents, the societal value of a larger utility gain was more than proportionately greater than the societal value of a smaller utility gain. For example, four patients should be treated with a utility gain of 0.2 to compensate for the loss of one patient with a potential utility gain of 0.4 |
| Wiss et al. (2014) [54] | Sweden | Society | Not specified | Simple task choice | (+ /−) | NR | NR | NR | (1) For equal-cost scenarios, 42.3% were indifferent between the rare disease and the common-disease groups; 23.9% prioritized rare disease and 33.4% prioritized common disease. When questions were framed to be on an individual vs. a group level, respondents were significantly (P < .001) more likely to be indifferent. Proportion dominance increased respondents’ preferences to prioritize rare diseases (P < .001). Authors concluded: “We see no strong support that a societal preference for rarity exists. However, we observe psychological effects influencing the judgments that individuals make when setting priorities related to rare diseases” |
| Rizzardo et al. (2014) [29] | Canada | Society | Not specified | Ranking + AHP | (−) | (+) | (−) | NR |
(1) Rarity had a relative weight of 0.02, the second least important value (2) and (3) the factors selected in the top 5 for the majority of our respondents were: The ability of the drug to improve health-related quality of life (66%) and quantity of life (50%), ability of drug to work (61%), severity of disease (60%), and safety (65%). These values were weighted 4–5-fold more important than those ranked least important: unmet need, socioeconomic status, rarity, and adherence |
| Rizzardo et al. (2014) [30] | Canada | Society | Not specified | TO | (−) | NR | NR | NR | (1) Canadians prefer to use resources to fund treatment of rare diseases over other societal benefits, including recreation and education; however, they prefer to maximize health care resources to benefit the greatest number of patients. Rare diseases are valued by Canadians only when the opportunity cost to treat them does not take away from common-disease treatments |
| Linley et al. (2013) [31] | UK | Society | Health care DM | Simple choice task + TO | (−) | (+) | (+) | NR |
(1) Respondents preferred treatment of common disease or equally distributed resources over treatments of rare disease (all equal, smaller health improvement, and twice the costs of the common-disease population scenario) (2) There was a societal preference for treating patients with severe disease (59.6%). This preference still existed when costs were twice as great as for moderate disease (60.9%). When the health improvement was smaller for rare disease patients, equal proportions of respondents preferred treatments of rare and common diseases (3) There was a societal preference for treating diseases with treatment alternatives (56.5%). This preference still existed when costs were twice as much as those for moderate disease (60.4%). When the health improvement was smaller for the disease without treatment alternative, a relative majority of respondents still preferred to treat the disease without treatment alternative |
| Desser et al. (2013) [32] | Norway | Society | Citizen | TO | (−) | NR | NR | NR | (1) There was a stronger preference for treating common disease. In an equal-cost scenario, a large majority of respondents divided resources evenly between rare and common-disease patients, but more of the remaining respondents favored treating the common-disease group, a result that may reflect the much larger probability of suffering from a common disease. The scenario varying the opportunity cost of the rare disease in survey versions V1–V4 (25:1, 8:1, 4:1, and 1:8, respectively) affected the share of funds allocated to treating the rare disease patient group |
| Mentzakis et al. (2011) [21] | Canada | Society | Health care DM | DCE | (−) | (+) | NR | NR |
(1) “A subset of people actually appears willing to have government pay less for drugs used to treat rare diseases; however, another subset makes no distinction based on frequency of disease. […] People do not appear willing to pay more per LY gained for those who suffer from a rare disease than those who suffer from a common disease.” (2) The significantly positive coefficients for severity indicate a societal preference for treating diseases with “serious impact” rather than diseases with “moderate impact.” The gain in societal value from treating a disease with serious impact rather than a disease with moderate impact is equivalent to approximately 6–7 LYs gained |
| Richardson et al. (2011) [41] | Australia | Society | Not specified | TO | NR | (+) | NR | (−) |
(2) Study presents data consistent with the view that, after considering health improvement, health programs that do not leave patients in severe health states are preferred. The ratio of societal value to utility gain was greater for patients whose initial health state was more severe (e.g., the ratio ranged from 1.1 for a utility gain of 0.2 in a patient with an initial utility of 0.8 to 2.4 for the same utility gain in a patient with an initial utility of 0) (4) The ratio of societal value to utility gain was greater for patients’ smaller health gain (2.4 for a utility gain of 0.2 vs. 1.0 for a utility gain of 1.0, starting from an initial utility of 0) |
| Bae et al. (2010) [47] | Korea | Society | Citizen | Ranking + simple choice task | NR | (+) | NR | NR | (2) Severity of disease was the most important criterion of priority setting for participants. The majority support the idea that the most disadvantaged should have the greatest priority, even though their health gain is less than that for others |
| Desser et al. (2010) [34] | Norway | Society | Not specified | TO | (−) | NR | NR | NR | (1) Despite strong general support for statements expressing a desire for equal treatment rights for patients with rare diseases, there was little evidence that a societal preference for rarity exists if treatment of patients with rare diseases is at the expense of treatment of those with common diseases. When treatment costs were equal, 64.9% of respondents were indifferent. When treatment of rare diseases was 4 times greater, 47.3% were indifferent, and 45.3% were in favor of treating common diseases |
| Shah (2009) [48] | Review | NR | (+) | NR | NR | (2) The use of severity as a priority-setting criterion is supported by a large number of empirical studies of popular preferences | |||
| Whitty et al. (2009) [49] | Australia | Society, DM | Not specified | DCE | NR | (+) | NR | NR | (2) Both the general public and decision-makers were more likely to fund a pharmaceutical that was used for the treatment of severe illness |
| Oddsson (2003) [18] | Iceland | Society, HCP, DM | Personal + citizen (patient perspective or planner) | Simple choice task | NR | (−) | NR | (−) |
(2) When offered a choice between prioritizing according to treatment effect, prioritizing according to disease severity, or an equal split of resources, 52% preferred an equal split of limited resources and were reluctant to prioritize either treatment-effect or disease-severity level. Only 10–14% of members of the public wanted to prioritize treatments according to disease severity (4) When offered a choice between prioritizing according to treatment effect or an equal split of resources, only 14–18% chose to prioritize funding according to treatment effect |
| Ubel (1999) [19] | US | Society | Personal + citizen | Simple choice task | NR | (+) | NR | NR | (2) Results were largely dependent on the wording of questions, but authors concluded that this study adds to evidence suggesting that many members of the general public place priority on allocating resources to severely ill patients, even when they would benefit less from treatment than others would |
| Nord (1996) [50] | Review | NR | (+) | NR | NR | (2) Various convenience samples and small population samples compared programs for curing severely ill and less severely ill patients by person trade-offs. The preference for treating the severely ill was very strong | |||
Valuation of treatment of rare diseases’ attributes: (+) finding in favor of prioritization, (−) finding not in favor of prioritization, (+ /−) ambivalent finding
AHP analytic hierarchy process, ALS amyotrophic lateral sclerosis, CSPC constant sum paired comparison, DCE discrete-choice experiment, DM decision-maker, HCP health care provider, LE life expectancy, LY life year, NHS National Health Service, NR not reported, ODs orphan drugs, OR odds ratio, PRO patient-reported outcome, QALY quality-adjusted life year, RD rare disease, TO trade-off methods, VSL value of a statistical life, WTP willingness to pay
Methodology to Assess Societal Valuation
Preferences were elicited from the included studies (Fig. 2) using trade-offs (n = 13) and choice-based tasks (n = 14). The former included person, time, and benefit trade-offs, as well as resource allocation tasks, whereas the latter were discrete-choice experiments (DCEs; n = 6) and simple choice questions (n = 8). In earlier studies, preferences were most often assessed based on simple choice questions (i.e., asking participants choose their most preferred option). In more recent studies, advanced methods such as trade-offs and DCEs were more commonly used.
Fig. 2.
Methods used for societal preference elicitation
Questions asked from respondents in reviewed studies were framed with different perspectives, specifically: (1) a personal perspective in which the respondent imagined he/she could be one of the patients directly affected by a treatment scenario; (2) the citizen perspective, in which the respondent imagined he/she was a representative of a societal body advising the government; or (3) the health care decision-maker perspective, in which the respondent imagined him/herself as a health care authority allocating budgets or resources. For the 33 studies, 13 did not provide any information about the question context and thus could not be classified. Twenty-three perspectives were identified in the remaining 20 studies (three included more than one perspective) [18–20]. Ten of the 23 perspectives were classified as health care decision-maker, 6 as personal, and 7 as citizen (Table 2).
Valuation of Rare Diseases and Treatment of Rare Diseases Attributes
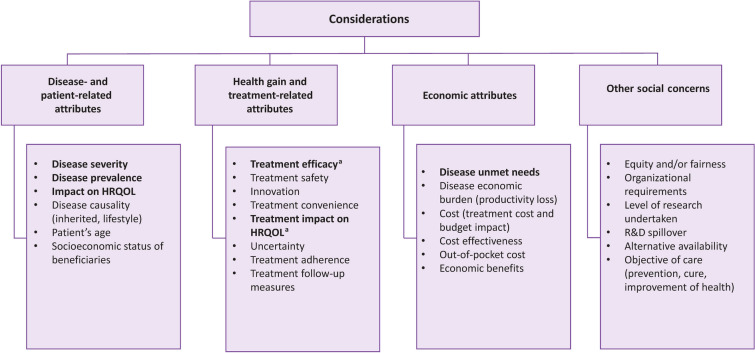
A total of 28 attributes, with a minimum of 2 and a maximum of 18 attributes per study (Fig. 3), was identified. Findings related to disease and patient characteristics and treatments were examined in detail. We reviewed studies that examined the characteristics of health care interventions that affected population preferences to determine whether they included the characteristics that define orphan drugs. Figure 3 summarizes all the characteristics reported in those publications, some of which relate to orphan drugs. In our analysis, we focused only on the characteristics related to the definition of orphan drugs according to the EMA orphan designation criteria.
Fig. 3.
Determinants of population preferences for health care priority setting. HRQOL health-related quality of life, OD orphan drug, R&D research and development, SLR systematic literature review. All attributes assessed in societal preference studies as identified by the SLR are listed here; however, according to the European Medicines Agency definition of ODs, the article focused on those in bold. aTreatment efficacy and impact on HRQOL were assessed under the same notion as proxy of “substantial benefit.”
Disease Prevalence
The systematic search identified 22 studies that addressed the valuation of disease prevalence from a societal perspective. Most of these studies (n = 15) favored allocating funds to more prevalent diseases, even in scenarios in which numbers of patients eligible to be treated were the same for rare and common diseases [21–34]. However, some studies (n = 3) suggested that treatments for less prevalent diseases should be prioritized [35–37]. For example, 57% of respondents in a budget allocation survey conducted in the Australian population agreed that it was “OK to reduce services to the majority by a little to cover the cost of very expensive services needed by the few people with rare illnesses.” In each trade-off scenario, there was a significant allocation of resources to services for the rare disease, despite the reduction in total health [36]. In another trade-off survey conducted in Spain, the majority of respondents stated that the government should systematically reimburse orphan drugs, regardless of prices and, to some extent, effectiveness [35]. According to a study that assessed public opinion regarding criteria for drug reimbursement, respondents did not specifically value disease rarity per se, but 65% of respondents favored giving equal or more funding to the rare disease when the therapy was more costly [34, 38]. Bourke et al. [39] reported that even if a majority (51%) supported prioritizing the treatment of a more common disease when the price of therapy for the rare disease was 10 times greater, 23% of study participants would prioritize treatment of the rare disease. Members of the general public were willing to spend on aggregate 4.3 times as much money per patient with a rare disease as per patient with a common disease for the same magnitude of health benefit [39].
A study conducted in the UK reported that preferences differed when assessed using different methodologies (e.g., a trade-off task or a DCE) for the same sample of respondents. According to the trade-off task, based on equal cost and benefit per patient, more respondents favored treating patients with rare diseases than patients with common diseases [39]. However, according to a DCE conducted with the same group of participants, they preferred that the National Health Service fund treatments for patients with common diseases, all other attributes equal (i.e., disease severity, treatment benefit, availability of treatment alternatives, improvements to everyday life, cost) [39].
More generally, in DCEs that defined therapeutic scenarios by prevalence of the treated disease and other attributes (severity of the condition and/or existence of alternative therapies), there was consistently no preference for prioritizing the rare disease, all other attributes equal. Often there was a preference for treating the more common diseases [21, 24, 38, 39].
Disease Severity and the Related Personal Burden
The SLR identified 26 studies assessing societal preferences for disease severity and related personal burden. A majority (n = 20) suggested that the general public was willing to prioritize the most disadvantaged [19–22, 25, 29, 31, 33, 36, 40–50]. According to a published literature review, members of the general public often gave priority to patients with more severe disease, regardless of size of the health gain or the cost of treatment [40]. Some studies reported that survey respondents were willing to prioritize more severe diseases over potential health gains [36, 43, 44]. A trade-off study from Australia supported the view that members of the general public prefer health programs that do not leave patients in severe health states. The ratio of increased societal value to increased patient utility gain was greater for those whose initial health state was more severe (e.g., the ratio ranged from 1.1 for a patient with an initial utility of 0.8 and a utility gain of 0.2 to 2.4 for a patient with an initial utility of 0.0 who also had a utility gain of 0.2) [40]. Studies that quantified the value of treatment for patients with the most severe diseases relative to those with least severe diseases consistently demonstrated a ratio of 2.0 or greater [31, 42, 45, 51]. Kolasa and Lewandowski [52] reported heterogeneity between older and younger respondents. A majority of older respondents did not trade off the treatment of patients who were less severely ill for those who were more severely ill [52]. However, the median junior respondent was willing to treat 20–100 less severely ill patients to compensate for the loss of 10 more severely ill patients, suggesting a ratio of value of more than 2.0. According to Reckers-Droog et al. [42], 45 children with moderate disease (a severity score of 50; scale 0–100) should be treated to reach the social value of an equivalent health benefit in one child with severe disease (a severity score of 80). In addition, 32 children with mild disease (a severity score of 20; scale 0–100) should be treated to reach the social value of an equivalent health benefit in one child with moderate disease (a severity score of 50) [42].
A few studies (n = 7) found severity to be of lesser importance [18, 24, 26, 27, 33, 35, 52]. In a study conducted in Japan, a ranking exercise on a seven-point Likert scale revealed that disease severity ranked 16 of 26 attributes in importance and was considered lesser or of no importance compared with other criteria for prioritization and decision-making in drug reimbursement [26].
The willingness to prioritize treatment of severe disease could be relevant to risk aversion, although this is not the only explanatory factor [40]. The preference for treating severely ill patients was even greater when preferences were assessed from a social perspective rather than from a personal perspective (i.e., when related to the treatment of others vs. self-interest in the prioritization and distribution of treatments) [41, 42].
Some studies also considered interactions between disease severity and other characteristics of a treatment or a targeted disease. The preference for treating patients with severe disease was dependent on cost. Richardson et al. [44] found that when the cost per life-year (LY) gained was identical for patients with life expectancies (LEs) of 2 and 10 years, the allocation for patients with shorter LEs was approximately twice as great as for patients with longer LEs. When the cost per LY gained was twice as great for individuals with shorter LEs, study participants allocated roughly the same number for both patient profiles (i.e., again twice the budget for patients with shorter LEs) [44].
Reckers-Droog et al. [42] considered the interaction between severity and age in a US person trade-off (PTO) exercise. Respondents in this study were generally willing to allocate more resources for more severely ill patients and for patients who were younger. The ratio of the median number of more severely ill patients compared with the median number of less severely ill was approximately the same whether patients were children, adults, or elderly [42]. However, the societal valuation of disease severity may depend on whether the patient’s lifestyle contributed to the occurrence of the disease. Notably, according to Gu et al. [40], the general public would assign lesser priority to those considered in some way responsible for their ill health.
Unmet Need
Ten of the 38 studies assessed preferences related to the presence of alternative treatments as a criterion in priority setting. Four studies that used ranking or rating exercises suggested that lack of a treatment alternative was not an important attribute [22, 26, 29, 53]. However, for the seven studies that used DCEs or trade-off exercises, most (n = 5) concluded that the public attached more value to therapies for diseases in which no alternative exists or in which the alternatives have limited efficacy compared with treatments for diseases with effective alternatives [23, 25, 31, 33, 39]. According to a PTO study conducted in the UK [31], 57% of the study respondents chose to prioritize patients without a treatment alternative, all other factors being equal. This preference remained when costs were twice as great as for a treatment with several alternatives available (60% of respondents). Study participants were twice as likely to choose a treatment scenario without an alternative and of lesser health benefit than choose a treatment scenario with several alternatives and considerable health benefit [31]. A study from South Korea with a similar design provided more nuanced results. A relative majority of respondents, 45%, prioritized the treatment without therapeutic alternatives, and 22% supported the treatments with alternatives (remaining respondents were indifferent) [23]. However, when the treatment without an alternative was more costly or less effective, the treatment with alternatives was preferred by most respondents [23]. Two DCEs demonstrated a significant preference for treatments without alternatives, with odds ratios of 1.1–1.3 compared with therapies with available alternatives. Other studies illustrated that a sizeable percentage of respondents, even if not always the majority, were more concerned about equity than efficacy (i.e., ensuring that patients with unmet needs also received treatment) [31, 35, 36, 38, 53, 54].
Relative Health Improvement
Reviewed publications reported that larger health and HRQOL gains were universally preferred over smaller gains when costs were similar [25, 29, 51, 52]. However, there was a notable exception. Using a simple choice task, Oddsson [18] discovered that only 14–18% of respondents chose to prioritize funding according to treatment effect when offered a choice between prioritizing and an equal split of resources. When considering whether the relative social value per unit of health gain was affected by the size and duration of the gain, the evidence was inconsistent. Of the nine studies that addressed this question, three provided positive evidence [25, 51, 52], four were negative [18, 20, 24, 41], and two were inconclusive [33, 55]. According to a contingent valuation study from South Korea, the societal willingness to pay for long-lasting treatment effects and curative scenarios was two times greater than non-cure scenarios [51]. This preference was partly corroborated by a trade-off study from Poland in which both junior (students) and senior (adult) respondents selected more patients with a smaller health gain to compensate for the loss of ten individuals with a greater potential benefit. In the case of junior respondents, the societal value of a larger health gain was more than proportionately greater than the societal value of a smaller utility gain [52]. However, in the case of senior respondents, the societal value of a health improvement was roughly proportional to the utility gain. These results for senior respondents were corroborated by a UK DCE by Hampson et al. [24], which explored the general public’s preferences across large and small health gains. In addition, results from this study demonstrated that whether a treatment was or was not a cure (restoring patients to normal health and full HRQOL) did not influence respondents’ choices beyond the influence of health gains themselves [23]. Furthermore, the incremental value associated with one LY gained was independent of the magnitude of the LE improvement. Finally, some studies using trade-off exercises noted that the ratio of societal value to utility gain was greater for patients with smaller health gains [19, 45]. For example, Richardson et al. [41] estimated a ratio of societal value to utility gain of 2.4 when utility increased from 0.0 to 0.02 relative to an increase from 0.0 to 1.0.
Discussion
Our SLR demonstrates that members of the general public attach greater value to orphan drugs compared with other treatments with equivalent health benefits. This is not because of disease rarity per se, but primarily because disease severity and a lack of therapeutic alternatives were typically associated with rare diseases. Many studies we reviewed suggested that a majority of respondents favored allocating funds to more prevalent diseases. However, all trade-off studies found that a sizable percentage of participants chose to allocate resources to orphan drugs and treatment of rare diseases, even when the cost per unit of health benefit was greater compared with therapies for more prevalent diseases, to such an extent that the resulting average valuation of an orphan drug was greater. Respondents who were indifferent to funding an expensive treatment for a rare disease or funding a less expensive treatment for a common disease were also effectively willing to pay more for patients with rare diseases [31, 32, 34]. Study participants also preferred sharing resources, so that no patient was left without treatment. Therefore, there appears a clear willingness from members of the general public to accept the opportunity cost of funding more expensive therapies for those with rare diseases.
We referred to the EMA criteria to define an orphan drug: rarity, severity, unmet need, and significant benefit. Decision-makers and payers have accepted greater costs for orphan drugs, and this acceptance was mostly driven by disease rarity [56]. However, across DCEs, rarity was not valued per se from a societal perspective [21, 24, 33, 37–39]. Members of the general public preferred that health care funds be used to treat common diseases and benefit more patients overall. According to these DCEs, the excess value attached to treatments for rare diseases was attributed to severity of disease and a lack of therapeutic alternatives [21, 25, 33, 42]. Indeed, most studies evaluating disease severity and burden as criteria for treatment valuation supported prioritization of therapy for patients with severe illness. This suggested that a health service that increased a patient’s utility should be valued more when the initial health state is more severe [20]. Unmet needs and a dearth of therapeutic alternatives were not the top criteria for prioritizing treatments according to rating and ranking studies. However, these criteria significantly influenced the valuation of treatments according to choice-based studies [31, 33, 39]. Lastly, members of the general public attached more value to greater health gains, but it was unclear whether the social value per unit of health outcome gained was greater for larger gains than for smaller ones [18, 24, 25, 33, 38, 45, 49–51].
The extent of excess value that members of the general public attached to orphan drugs compared with other treatments varied significantly between studies. The valuation of a unit of health outcome gained based on trade-off and contingent valuation studies was at least double for patients with severe disease versus those with mild disease. Fewer studies assessed the societal value attached to treatments without alternatives compared with studies observing severity, and the quantifications were not clear.
Within the conventional cost-utility analysis framework, the valuation of a health improvement is independent of the characteristics of the recipient. However, our review illustrates that members of the general public expressed a strong preference for allocating resources to patients with poorer initial health states or those without therapeutic alternatives for health improvements of equal magnitude. To account for such preferences, equity weights may be assigned to QALYs, or the cost-effectiveness threshold may be increased for some patient groups [57]. However, the social value of orphan drugs per unit of health benefit is difficult to quantify and, therefore, based on evidence from the stated preference studies, obtaining equity weights or determining new cost-effectiveness thresholds for orphan drugs is also difficult. Many respondents may prefer sharing health care resources between patients rather than prioritizing some groups over others [18, 38]. Members of the general public appeared willing to allocate some resources to patients with the worst health and no therapeutic alternative regardless of the opportunity cost [23, 36]. Richardson et al. [44] reported a social preference for sharing, possibly in the absence of severity, unmet treatment needs, or other attributes of patients receiving health care, which may be the result of an expected social reciprocity.
We reported in greater detail the results of choice-based studies (DCEs and trade-off exercises) as opposed to ranking studies (where patients were asked to rank criteria in terms of importance). We considered choice-based studies to have greater validity. DCEs were of particular interest for disentangling different criteria contributing to the value of rare diseases (e.g., to determine if members of the general public attached value to rarity independent of severity). We mostly referred to trade-off studies when looking at the extent of additional value of orphan drugs relative to other drugs.
There are several limitations of this literature review. We used EMBASE and MEDLINE databases, but not EconLit, and we reviewed English-language publications only. Disease-specific preference and valuation studies were not reviewed, and the classification of studies into categories (supporting treatment prioritization according to an attribute, not supporting, or ambivalent) was somewhat subjective, since results were often not clear cut. However, to reduce subjectivity, all findings were classified independently by two reviewers, with discrepancies resolved by a third reviewer when required.
The studies included in our SLR also have their own inherent limitations that should be acknowledged. For example, attributes were so succinctly described in several studies that they may not have been fully understood by respondents. Consequently, respondents may have found it difficult to appreciate the full meaning of an attribute when it was not placed in the context of a realistic choice. For trade-off studies, the interpretation of results can be complex. Several authors of trade-off studies reported only distribution of respondents according to the patient group to which most funds were allocated and not the numbers of patients allocated treatment in each group. When mean numbers of patients were reported, a small number of respondents provided very large numbers of patients (e.g., very large numbers of patients with mild disease to get treated to offset loss of benefit for patients with severe disease), which influenced mean values significantly. Furthermore, in choice studies, it was not clear to what extent patients understood and integrated all important information. In some PTO studies, respondents were asked to allocate resources between treatments for patients with and without therapeutic alternatives and generally prioritized patients without therapeutic alternatives. Respondents may have assumed that patients could still receive treatment if there were therapeutic alternatives, although they were instructed that these patients would be denied treatment [31]. For DCEs, the additive utility model, which is commonly applied, can lead to unrealistic interpretations. For example, treatments may have a strictly positive value even with zero efficacy [21, 33]. Further research should consider DCEs with multiplicative models and trade-off studies to report numbers of patients (not just favored programs). Trade-offs could be framed by an average reduction of health benefit for patients with common diseases rather than by reductions in numbers of patients with any health benefit.
Conclusions
This SLR suggests that members of the general public generally attach greater value to orphan drugs than to other treatments. The percentage of the general public who would prioritize orphan drugs over other therapies varies between countries and studies. However, in all trade-off studies, a significant percentage of society would allocate resources for orphan drugs, even if these are more expensive than other treatments. This drives the average valuation of orphan drugs to a substantially greater degree compared with treatments for more common diseases. The excess value of orphan drugs does not appear to be related to disease rarity per se, but rather that members of the general public are willing to allocate resources preferentially to patients with severe diseases that have no treatment alternatives, even if such therapies are expensive.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Daniel C. Malone, PhD, for his assistance in interpretation of our findings and for critical review of the manuscript.
Funding
Support for this manuscript was funded by Novartis Gene Therapies, Inc., Bannockburn, IL, USA. The funder had no role in the design and conduct of the study or the collection, management, analysis, and interpretation of the data. The study sponsor is funding the journal’s Rapid Service and Open Access fees.
Medical Writing/Editorial Assistance
Medical writing assistance and editorial support were provided by Leonard Lionnet, PhD, of Kay Square Scientific, LLC, Newtown Square, PA, USA. This support was funded by Novartis Gene Therapies, Inc., Bannockburn, IL, USA.
Author Contributions
Conceptualization of the work: Omar Dabbous, Mondher Toumi, Marine Sivignon. Development and validation of the review protocol: all authors. Conduct of the literature review: Lylia Chachoua, Samuel Aballéa, Marine Sivignon. Interpretation of findings from the literature: all authors. Drafting of the manuscript: Lylia Chachoua, Samuel Aballéa, Marine Sivignon. Reviewing of the manuscript: all authors. Design and editing of figures: Lylia Chachoua, Samuel Aballéa. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have read and given their approval for this version to be published.
Disclosures
Omar Dabbous is an employee of Novartis Gene Therapies, Inc., and owns stock or other equities; Lylia Chachoua and Marine Sivignon are employees of Creativ-Ceutical and consultants for Novartis Gene Therapies, Inc.; Samuel Aballéa and Mondher Toumi were employees of Creativ-Ceutical and consultants for Novartis Gene Therapies, Inc., at the time of the study and manuscript development; Steven Simoens has served as a consultant for the Belgian Health Care Knowledge Centre (research about market access of orphan drugs), Celgene (participated in an orphan drug roundtable), Genzyme (now Sanofi) (research about market access of orphan drugs), Innoval Working Group on Ultra-Rare Disorders (member of group), International Working Group on Orphan Drugs (member of group), ISPOR Rare Disease Special Interest Group’s Challenges in Research and Health Technology, Assessment of Rare Disease Technologies Working Group (member of group); Stavros Petrou receives support as a UK National Institute for Health Research Senior Investigator (NF-SI-0616-10103) and from the UK National Institute for Health Research Applied Research Collaboration Oxford and Thames Valley; Ulf Persson and Jeff Richardson have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.European Medicines Agency. Orphan designation: overview. https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation-overview. Accessed 10 June 2021.
- 2.Kesselheim AS. B, Innovation and the Orphan Drug Act, 1983–2009: regulatory and clinical characteristics of approved orphan drugs. In: Field MJ, Boat TF, editors. Rare diseases and orphan products: accelerating research and development. Washington, DC: National Academies Press (US); 2010. pp. 291–308. [PubMed] [Google Scholar]
- 3.Swann J. The story behind the Orphan Drug Act. 2018. https://www.fda.gov/industry/orphan-products-development-events/story-behind-orphan-drug-act. Accessed 15 Feb 2022.
- 4.European Medicines Agency. Legal framework: orphan designation. https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation/legal-framework-orphan-designation. Accessed 15 Feb 2022.
- 5.Brown DG, Wobst HJ. A decade of FDA-approved drugs (2010–2019): trends and future directions. J Med Chem. 2021;64(5):2312–2338. doi: 10.1021/acs.jmedchem.0c01516. [DOI] [PubMed] [Google Scholar]
- 6.Nicod E, Whittal A, Facey K. Impact HTA (2018–2020) (Improved Methods and ACtionable Tools for enhancing HTA). WP10—HTA appraisal of orphan medicinal products (medicines for rare diseases). Country vignettes. 2020. https://www.impact-hta.eu/country-vignettes. Accessed 22 July 2021.
- 7.Young KE, Soussi I, Hemels M, Toumi M. A comparative study of orphan drug prices in Europe. J Mark Access Health Policy. 2017;5(1):1297886. doi: 10.1080/20016689.2017.1297886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Interim process and methods of the highly specialised technologies programme updated to reflect 2017 changes. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-highly-specialised-technologies-guidance/HST-interim-methods-process-guide-may-17.pdf. Accessed 10 June 2021. [PubMed]
- 9.Versteegh MM, Ramos IC, Buyukkaramikli NC, Ansaripour A, Reckers-Droog VT, Brouwer WBF. Severity-adjusted probability of being cost effective. Pharmacoeconomics. 2019;37(9):1155–1163. doi: 10.1007/s40273-019-00810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blonda A, Denier Y, Huys I, Simoens S. How to value orphan drugs? A review of European value assessment frameworks. Front Pharmacol. 2021;12:631527. doi: 10.3389/fphar.2021.631527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuijten M, Van Wilder P. The impact of early phase price agreements on prices of orphan drugs. BMC Health Serv Res. 2021;21(1):222. doi: 10.1186/s12913-021-06208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ollendorf DA, Chapman RH, Pearson SD. Evaluating and valuing drugs for rare conditions: no easy answers. Value Health. 2018;21(5):547–552. doi: 10.1016/j.jval.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Côté A, Keating B. What is wrong with orphan drug policies? Value Health. 2012;15(8):1185–1191. doi: 10.1016/j.jval.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Jommi C, Armeni P, Costa F, Bertolani A, Otto M. Implementation of value-based pricing for medicines. Clin Ther. 2020;42(1):15–24. doi: 10.1016/j.clinthera.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Côté S, Gaudig M, Nielsen SK, Shields GE, Britton JA. Challenges for assessing the economic value of orphan drugs—a literature review of current and alternative approaches. Value Health. 2015;18(7):A681. [Google Scholar]
- 16.European Medicines Agency. Demonstrating significant benefit of orphan medicines: concepts, methodology and impact on access. https://www.ema.europa.eu/en/documents/report/workshop-report-demonstrating-significant-benefit-orphan-medicines-concepts-methodology-impact_en.pdf. Accessed 10 June 2021.
- 17.Vreman RA, de Ruijter AS, Zawada A, et al. Assessment of significant benefit for orphan medicinal products by European regulators may support subsequent relative effectiveness assessments by health technology assessment organizations. Drug Discov Today. 2020;25(7):1223–1231. doi: 10.1016/j.drudis.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Oddsson K. Assessing attitude towards prioritizing in healthcare in Iceland. Health Policy. 2003;66(2):135–146. doi: 10.1016/s0168-8510(02)00211-7. [DOI] [PubMed] [Google Scholar]
- 19.Ubel PA. How stable are people’s preferences for giving priority to severely ill patients? Soc Sci Med. 1999;49(7):895–903. doi: 10.1016/s0277-9536(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 20.Richardson J, Iezzi A, Maxwell A. How important is severity for the evaluation of health services: new evidence using the relative social willingness to pay instrument. Eur J Health Econ. 2017;18(6):671–683. doi: 10.1007/s10198-016-0817-y. [DOI] [PubMed] [Google Scholar]
- 21.Mentzakis E, Stefanowska P, Hurley J. A discrete choice experiment investigating preferences for funding drugs used to treat orphan diseases: an exploratory study. Health Econ Policy Law. 2011;6(3):405–433. doi: 10.1017/S1744133110000344. [DOI] [PubMed] [Google Scholar]
- 22.Chim L, Salkeld G, Kelly PJ, Lipworth W, Hughes DA, Stockler MR. Community views on factors affecting medicines resource allocation: cross-sectional survey of 3080 adults in Australia. Aust Health Rev. 2019;43(3):254–260. doi: 10.1071/AH16209. [DOI] [PubMed] [Google Scholar]
- 23.Bae EY, Lim MK, Lee B, Bae G. Who should be given priority for public funding? Health Policy. 2020;124(10):1108–1114. doi: 10.1016/j.healthpol.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Hampson G, Mott D, Devlin N, Shah K. Public preferences for health gains and cures: a discrete choice experiment. https://www.ohe.org/system/files/private/publications/OHE%20Consulting%20Report%20-%20Health%20Gains%20and%20Cures%20JAN%202019.pdf. Accessed 10 June 2021.
- 25.Magalhaes M. Can severity outweigh smaller numbers? A deliberative perspective from Canada. Value Health. 2018;21(5):532–537. doi: 10.1016/j.jval.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Funagoshi M, Murasawa H, Shimozuma K. Identification of important criteria for drug reimbursement decision-making and their relative importance. Value Health. 2017;20(9):A661. [Google Scholar]
- 27.Shiroiwa T, Saito S, Shimozuma K, Kodama S, Noto S, Fukuda T. Societal preferences for interventions with the same efficiency: assessment and application to decision making. Appl Health Econ Health Policy. 2016;14(3):375–385. doi: 10.1007/s40258-016-0236-3. [DOI] [PubMed] [Google Scholar]
- 28.Dragojlovic N, Rizzardo S, Bansback N, Mitton C, Marra CA, Lynd LD. Challenges in measuring the societal value of orphan drugs: insights from a Canadian stated preference survey. Patient. 2015;8(1):93–101. doi: 10.1007/s40271-014-0109-5. [DOI] [PubMed] [Google Scholar]
- 29.Rizzardo S, Bansback N, Mitton C, Lynd L. Values of the Canadian public towards pharmaceutical reimbursement decisions. Value Health. 2014;17(3):A234. [Google Scholar]
- 30.Rizzardo S, Bansback N, Mitton C, Marra C, Lynd L. How Canadians value rare diseases given their opportunity cost? Value Health. 2014;17(3):A4. [Google Scholar]
- 31.Linley WG, Hughes DA. Societal views on NICE, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22(8):948–964. doi: 10.1002/hec.2872. [DOI] [PubMed] [Google Scholar]
- 32.Desser AS, Olsen JA, Grepperud S. Eliciting preferences for prioritizing treatment of rare diseases: the role of opportunity costs and framing effects. Pharmacoeconomics. 2013;31(11):1051–1061. doi: 10.1007/s40273-013-0093-y. [DOI] [PubMed] [Google Scholar]
- 33.Toumi M, Millier A, Cristeau O, Thokagevistk-Desroziers K, Dorey J, Aballéa S. Social preferences for orphan drugs: a discrete choice experiment among the French general population. Front Med (Lausanne) 2020;7:323. doi: 10.3389/fmed.2020.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desser AS, Gyrd-Hansen D, Olsen JA, Grepperud S, Kristiansen IS. Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. BMJ. 2010;341:c4715. doi: 10.1136/bmj.c4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalle-Gomara E, Ruiz E, Quinones C, Andrés S, Iruzubieta J, Gil-de-Gómez J. General knowledge and opinion of future healthcare and non-healthcare professionals on rare diseases. J Eval Clin Pract. 2015;21(2):198–201. doi: 10.1111/jep.12281. [DOI] [PubMed] [Google Scholar]
- 36.Richardson J, Iezzi A, Chen G, Maxwell A. Communal sharing and the provision of low-volume high-cost health services: results of a survey. Pharmacoecon Open. 2017;1(1):13–23. doi: 10.1007/s41669-016-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlander M, Telser H, Fischer B, Rechenberg TV, Schaefer R, ESPM Project Group Drivers of social value exceed length and quality of life: evidence from Switzerland. Value Health. 2018;21(suppl 1):S115. [Google Scholar]
- 38.Upton CM, Wordsworth J, Cork D, Ralston S. Assessment of public opinion regarding the ethics of NICE CDF, HST, and end-of-life criteria for drug reimbursement. Value Health. 2017;20(9):A697. [Google Scholar]
- 39.Bourke SM, Plumpton CO, Hughes DA. Societal preferences for funding orphan drugs in the United Kingdom: an application of person trade-off and discrete choice experiment methods. Value Health. 2018;21(5):538–546. doi: 10.1016/j.jval.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Gu Y, Lancsar E, Ghijben P, Butler JRG, Donaldson C. Attributes and weights in healthcare priority setting: a systematic review of what counts and to what extent. Soc Sci Med. 2015;146:41–52. doi: 10.1016/j.socscimed.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Richardson JRJ, McKie J, Peacock SJ, Iezzi A. Severity as an independent determinant of the social value of a health service. Eur J Health Econ. 2011;12(2):163–174. doi: 10.1007/s10198-010-0249-z. [DOI] [PubMed] [Google Scholar]
- 42.Reckers-Droog V, van Exel J, Brouwer W. Equity weights for priority setting in healthcare: severity, age, or both? Value Health. 2019;22(12):1441–1449. doi: 10.1016/j.jval.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Richardson J, Schlander M. Health technology assessment (HTA) and economic evaluation: efficiency or fairness first. J Mark Access Health Policy. 2019;7(1):1557981. doi: 10.1080/20016689.2018.1557981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson J, Iezzi A, Maxwell A. Sharing and the provision of “cost-ineffective” life-extending services to less severely ill patients. Value Health. 2018;21(8):951–957. doi: 10.1016/j.jval.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Richardson J, Iezzi A, Maxwell A. Uncertainty and the undervaluation of services for severe health states in cost-utility analyses. Value Health. 2018;21(7):850–857. doi: 10.1016/j.jval.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 46.McKie J, Richardson J. Social preferences for prioritizing the treatment of severely ill patients: the relevance of severity, expected benefit, past health and lifetime health. Health Policy. 2017;121(8):913–922. doi: 10.1016/j.healthpol.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Bae EY, Lim MK, Choi SE, Lee TJ. The public’s preference on the priorities in healthcare. Value Health. 2010;13(7):A534. [Google Scholar]
- 48.Shah KK. Severity of illness and priority setting in healthcare: a review of the literature. Health Policy. 2009;93(2–3):77–84. doi: 10.1016/j.healthpol.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Whitty JA, Scuffham PA, Rundle-Thiele SR. A discrete choice experiment comparing public and decision-maker stated preferences for pharmaceutical subsidy decisions. Value Health. 2009;12(7):A230. [Google Scholar]
- 50.Nord E. Health status index models for use in resource allocation decisions: a critical review in the light of observed preferences for social choice. Int J Technol Assess Health Care. 1996;12(1):31–44. doi: 10.1017/s0266462300009363. [DOI] [PubMed] [Google Scholar]
- 51.Song HJ, Lee EK. Evaluation of willingness to pay per quality-adjusted life year for a cure: a contingent valuation method using a scenario-based survey. Medicine (Baltimore) 2018;97(38):e12453. doi: 10.1097/MD.0000000000012453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolasa K, Lewandowski T. Does it matter whose opinion we seek regarding the allocation of healthcare resources? - a case study. BMC Health Serv Res. 2015;15:564. doi: 10.1186/s12913-015-1210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polisena J, Burgess M, Mitton C, Lynd LD. Engaging the Canadian public on reimbursement decision-making for drugs for rare diseases: a national online survey. BMC Health Serv Res. 2017;17(1):372. doi: 10.1186/s12913-017-2310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiss J, Levin LA. Preferences for prioritizing patients with rare diseases: a survey of the general population in Sweden. Value Health. 2014;17(7):A325–A326. doi: 10.1016/j.jval.2014.08.582. [DOI] [PubMed] [Google Scholar]
- 55.Olofsson S, Gerdtham UG, Hultkrantz L, Persson U. Dread and risk elimination premium for the value of a statistical life. Risk Anal. 2019;39(11):2391–2407. doi: 10.1111/risa.13341. [DOI] [PubMed] [Google Scholar]
- 56.Medic G, Korchagina D, Young KE, et al. Do payers value rarity? An analysis of the relationship between disease rarity and orphan drug prices in Europe. J Mark Access Health Policy. 2017;5(1):1299665. doi: 10.1080/20016689.2017.1299665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bleichrodt H, Diecidue E, Quiggin J. Equity weights in the allocation of health care: the rank-dependent QALY model. J Health Econ. 2004;23(1):157–171. doi: 10.1016/j.jhealeco.2003.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.