Abstract
This study was conducted to understand the impact of including full fat high-oleic soybean meal in layer hen diets on nutrient digestibility and added nutritional value in eggs. Forty-eight layers (∼36 wk old) were randomly assigned to one of 4 isonitrogenous (18.5% crude protein) treatment diets with 12 replicate birds per treatment in a 3-wk study. Treatments were 1) solvent extracted defatted soybean meal + corn diet, 2) dry extruded defatted soybean meal + corn, 3) full-fat soybean meal + corn, 4) high-oleic full-fat soybean meal + corn diet. Apparent ileal digestibility of crude fat (CF) and crude protein (CP) were determined using celite (∼2%) as an indigestible marker. Tibia strength and egg quality parameters (egg weight, shell strength, Haugh unit, shell color, and yolk color) were recorded during the study. Fatty acid profiles, including the monounsaturated fatty acid, oleic acid (C18:1, cis), in eggs and adipogenic tissue (liver, muscle, and fat pad) were measured using gas chromatography (GC-FID). Digestibility values of CF ranged from 71 to 84% and CP varied from 67 to 72% for treatment diets, with treatment mean values being no different (P > 0.05) between treatment diets. No differences between treatment diets in tibia strength or egg quality parameters (egg weight, shell strength, and Haugh unit) were observed (P > 0.05) except for yolk color. Similarly, there were no differences in the total lipids in egg yolk (P > 0.05) between treatment diets. However, oleic acid percentage of total lipid in egg and tissue was significantly higher (P < 0.001) in hens given the high-oleic full-fat soybean meal diet than in other treatment groups. No difference was observed in oleic acid percentage of total lipid in egg between the other 3 treatment diets (P > 0.05). Overall, the results exhibited that the eggs and tissue of layer hens fed the full-fat high-oleic acid soybean meal diet were higher in oleic acid while the CF and CP digestibility remained similar to the digestibility of the other diets.
Key words: high-oleic soybean meal, digestibility, egg quality parameters, oleic acid, layer hen
INTRODUCTION
Conventionally, defatted soybean meal and supplemental dietary vegetable oil are common ingredients in poultry and livestock feed, with the US poultry and swine industries utilizing more than two-thirds of 60% of the commercial soybean meal produced annually in the nation (US Soybean Board, 2020; SoyStats, 2022). However, full-fat soybean meal could potentially replace these 2 feed ingredients in animal food production. Expansion and development of the soybean germplasm through many soybean breeding programs has led to the development of high-oleic (HO) soybean cultivars. These cultivars have a lipid profile of 80% oleic and 1.5% linoleic acid, as compared to normal-oleic soybeans, which have a lipid profile of 30% oleic and 7% linoleic acid. Studies have shown that a higher level of the monounsaturated fatty acid, oleic acid, in oilseeds, such as soybeans and peanuts, have different advantages (Braddock et al., 1995; Scarth and McVetty, 1999; Liu and Iassonova, 2012; Knowlton, 2022). Oleic acid extends product shelf-life compared to products made with normal-oleic oilseeds by preventing oxidative rancidity of dietary fats within the feed or finished product (Braddock et al., 1995).
Many feeding studies have demonstrated that poultry can effectively utilize full-fat soybean meal prepared from whole ‘normal-oleic’ soybeans without performance deficits (Erdaw et al., 2016, 2017, 2018). However, studies that examined the use of full-fat ‘high-oleic’ (HO) soybean meal in the diet of poultry are limited, even though the performance benefits of full-fat HO soybean meal have already been demonstrated in species other than poultry (Weld and Armentano, 2018). Therefore, this study aimed to examine the protein and fat digestibility and energy utilization of full-fat HO soybean meal as a feed ingredient for an egg-producing laying hen strain. The present research also studied the effects of including full-fat HO soybean meal in layer hen diets on hen bone health, blood chemistry, egg quality parameters, fatty acid profile of the lipogenic tissue and egg, and hen performance.
MATERIALS AND METHODS
This study was conducted in the bird wing of Prestage Department of Poultry Science at North Carolina State University (Raleigh, NC). All methods and procedures used for animal research in this digestibility trial were approved by the North Carolina State University Institutional Animal Care and Use Committee (19-761-07-A) following an accredited internal research animal protocol review in accordance with the standards within the “Guide for the Care and Use of Agricultural Animals in Research and Teaching” set forth by the American Dairy Science Association, the American Society of Animal Science, and the Poultry Science Association.
Experimental Design, Animal Husbandry, and Dietary Treatments
Four corn-soy based dietary treatments were formulated to be isonitrogenous (18.5% CP) and isocaloric (ME 2,927 kcal/kg) using Concept 5 (level 2, version 10.0) software (Table 1). The diets varied by treatment in how the soybean meal was processed: Treatment 1 (T1) used solvent-extracted defatted soybean meal; Treatment 2 (T2) used extruded-expelled defatted soybean meal; Treatment 3 (T3) used full fat normal-oleic soybean meal; and Treatment 4 (T4) used full fat HO soybean meal. Particle sizes of all experimental diets were between approximately 800 and 1,000 µm, and chemical composition was determined by an AOAC-approved commercial lab (Table 1).
Table 1.
Composition of formulated experimental laying hen diets1.
| Feed Ingredient, % | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 |
|---|---|---|---|---|
| Yellow corn | 57.9 | 58.1 | 57.9 | 58.4 |
| Soybean Meal | 20.6 | 21.4 | 24.6 | 23.9 |
| Calcium carbonate | 9.7 | 9.7 | 9.7 | 9.7 |
| Dicalcium phosphate | 1.6 | 1.6 | 1.6 | 1.6 |
| Corn gluten meal | 3.8 | 3.8 | 3.2 | 3.5 |
| Sodium bicarbonate | 0.2 | 0.15 | 0.2 | 0.2 |
| Sodium chloride | 0.3 | 0.25 | 0.23 | 0.25 |
| DL-Methionine | 0.12 | 0.12 | 0.12 | 0.12 |
| Pro Fam 974 (Soy Protein) | 2.0 | 2.0 | 2.0 | 2.0 |
| Soybean Oil | 3.5 | 2.5 | 0 | 0 |
| Santoquin2 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.07 | 0.06 | 0.06 | 0.07 |
| Trace mineral premix3 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin premix4 | 0.05 | 0.05 | 0.05 | 0.05 |
| Selenium premix5 | 0.05 | 0.05 | 0.05 | 0.05 |
| Metabolizable energy (kcal/kg) | 2927 | 2927 | 2927 | 2927 |
Four experimental isonitrogenous (18.5% crude protein) diets were formulated: Trmt1-Control = conventional diet containing solvent extracted defatted soybean meal and corn; Trmt2-EENO = diet containing extruded expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO = diet containing full fat high-oleic soybean meal and corn.
Santoquin® = Feed antioxidant and preservative to prevent fat oxidation in stored feed (Novus International, St. Charles, MO, USA).
Mineral premix provides per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt.
Vitamin premix provides per kg of diet: 13,200 IU vitamin A, 4000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
Selenium premix = 1 mg Selenium premix provides 0.2 mg Se (as Na2SeO3).
Forty-eight-layer hens (White shavers, ∼36 wk old) were randomly assigned to one of the 4 treatment diets (T1–T4) to determine the apparent ileal digestibility (crude protein, crude fat, and metabolizable energy) of each diet. Birds were individually housed in PVC-coated wire cages (30.5 cm × 45.7 cm) providing 1393.9 cm2 per hen. There were 12 cages per treatment with each cage being a replicate. A 1-week acclimation period to new cages was provided before transferring to experimental treatments. The study was conducted for a 3-wk period with ad libitum feeding of feed and water. Acid insoluble ash (AIA) (Celite, 2%) was added to each diet as a digestibility marker. This was to evaluate the nutrient digestibility with partial excreta collection (Huang et al., 2006).
Digesta and Excreta Collection
At the end of the experimental period, the birds were humanely sacrificed, and the contents of the lower half of the ileum to the ileocecal junction (digesta) were removed by gently squeezing at d 21 of the experimental period. Samples were put on ice immediately after collection, then frozen and stored at −20°C. Excreta samples were directly collected each day for 3 days by placing aluminum pans beneath each individual cage. Collected ileal digesta and excreta were then oven dried at 70°C for 24 h and ground through a 1-mm screen prior to analysis. Gross energy in the feed and dried excreta samples was measured as discussed in Toomer et al. (2020a) using an adiabatic oxygen bomb calorimeter (IKA model C5003onnected to compressed oxygen with NESLAB Refrigerated Re-circulator CFT-25).
The AIA in feed, digesta, and excreta samples (1 g each) was measured by first boiling samples in 4 N HCl for 10 m then filtering the resulting slurry through ash-less filter paper (Whatman No.541). DI water was used to wash the residue free of acid, followed by drying in the muffle furnace overnight at 600°C. Percent recovery of AIA (initial weight-final weight/initial weight × 100) was measured as the marker concentration in samples.
Feed, ileal digesta, and excreta samples were also analyzed for proximate analysis of crude protein (CP) and crude fat (CF) using an AOAC-approved service lab (ATC Scientific, Little Rock, AR). Nitrogen level was measured through combustion of homogenized samples using an Elemental N cube analyzer (Elemnatar Americas, Mt. Laurel, PA) following AOAC 990.03 methods, then the total protein in the sample was calculated using Kjeldahl conversion factor of 6.25. Crude fat content was measured gravimetrically after Soxhlet extraction using diethyl ether as discussed in Toomer et al. (2020a).
Digestibility coefficients (DC) for digesta and excreta were calculated based on the marker concentration in the diets, digesta, and excreta. This equation as discussed in Maharjan et al., (2019) was used to calculate the DC for both CP and CF (Maharjan et al., 2019).
Where Ci is the concentration of AIA in the diet, Co is the concentration of AIA in the digesta or excreta, Xo is the CP or CF content of digesta or excreta, and Xi is the CP or CF content of the diet. All values for Ci, Co, Xo, and Xi were expressed as % DM basis. Digestibility coefficient values for total CP or CF were determined.
The nitrogen-corrected apparent metabolizable energy, AMEn, in treatment diets was measured using the following expression:
Egg Quality Parameter Measurements
Shell eggs were collected, enumerated, and weighed daily. Egg quality parameters were determined (12 eggs/treatment) in the Egg Quality Lab at the Prestage Department of Poultry Science, NC State University (Raleigh, NC). Egg quality parameters measured included albumin height, Haugh unit, yolk color, and shell strength utilizing the methods as discussed in Toomer et al. (2019). Briefly, albumen height was measured and Haugh unit (HU; Haugh, 1937) was calculated using the TSS QCD system (Technical Services and Supplies, Dunnington, York, UK) to determine egg albumen quality. Yolk color was determined by using the TSS QCD System yolk color scan, which was calibrated to the DSM Yolk Color Fan, a color index from 1 to 15 (with 1 being the least intense color) to identify the yolk color density (Vuilleumier, 1969). Eggshell strength was tested using a TA-HD plus texture analyzer (Texture Technologies Corp. and Stable Micro Systems Ltd., Hamilton, MA).
Fatty Acid Analysis of Lipogenic Tissues and Egg egg Samples
The fatty acid profiles of the chicken breast meat, liver, and fat pad, and egg composites collected at termination were analyzed by extracting the total fat of the homogenized samples using the method described in Folch et al. (1957). In brief, 2- to 10-gram samples were weighed and transferred to a blender jar with 40 mL of methanol followed by 40 mL chloroform. The jar was fitted to a Waring Laboratory Blender (Dynamic Corp of America, Greenwich, CT). After blending for 2 min, 40 mL of chloroform was added, and the sample was blended for 30 s. Finally, 40 mL of water was added to the blender jar, and the sample was blended for another 30 s. The contents of the blender were decanted to a centrifuge bottle. The samples were centrifuged for 10 min at 1,000 rpm in an IEC Model K centrifuge (Irvine, CA) to form layers. An aliquot of 10 to 20 mL from the bottom layer (chloroform) was pipetted into a pre-weighed aluminum dish. The solvent was removed under a gentle stream of nitrogen gas. The dish and residue were weighed, and the weight of the residue was used to calculate the total fat content of the original sample. All solvents used were of Optima-grade from Thermo Fisher Scientific Corporation (Fair Lawn, NJ).
The residue from the weighing dish was then transferred to a screw-capped glass tube using 1 mL chloroform. The solvent was removed under a gentle stream of nitrogen gas. A 1 mL portion of 0.5 N sodium hydroxide in water was added to the tube to hydrolyze the triglycerides. The tubes were incubated for 10 min at 80°C in a water bath. The tubes were cooled, and a 1 mL of 14% Boron Trifluoride in Methanol (Sigma Aldrich Corporation, St. Louis, MO) was added to form methyl esters from the liberated fatty acids. The tubes were capped and heated in the water bath for an additional minute. The tubes were cooled, and a 1 mL portion of water followed by a 1 mL portion of hexane was added. The tubes were vortexed for 15 min to mix and extract the fatty acid methyl esters into the hexane. The tubes were allowed to stand at room temperature to form layers. The hexane layer was removed and passed through a few grains of sodium sulfate to remove any water present. Gas liquid chromatography was performed using a Perkin Elmer XL Autosampler chromatography system (Perkin Elmer, Shelton, CT) equipped with a BPX-070 (SGE, Austin, TX) type capillary column (30 m × 0.25 mm internal diameter, 0.25 µm film) and flame ionization detector. The operating conditions were as follows: injector temperature, 220°C; detector temperature, 235°C; helium flow, 40 mL/min. The oven temperature was increased from 60 to 180°C at a rate of 4°C/min and then increased to 235°C at a rate of 10°C/min. To identify the individual fatty acids in the chromatogram, a fatty acid standard mixture (Supelco 37 Component FAME Mix, Sigma Aldrich) was used. The fatty acids identified were expressed as a percentage of the total fatty acids in the samples (g/100 g). The total fat values were used to calculate the fatty acids in the original samples and expressed as g of fatty acids/100 g of sample.
Tibia Bone Strength and Blood Parameter Profiling
Tibia bones (12 per treatment) were collected from all birds that were used for ilea digesta collection in the nutrient digestibility study. The bones collected were preserved at −20°C until breaking strength were measured. Tibia bones were first thawed and then the breaking strength in the midpoint of the bone placed the same orientation was measured in terms of bending moment (kg/m2) and peak force (kg) for all treatment diets in the Egg Quality Lab, Prestage Department of Poultry Science, NC State University (Raleigh, NC). Before sacrificing the birds, blood samples were collected from the brachial vein and blood biochemical parameters were measured, such as pCO2, HCO3, TCO2, Ca2+, pH, pO2, SO2, Na+, K+, glucose, hematocrit, and hemoglobin, using I-Stat (instrument # 704930, Abbott Laboratories).
Statistical Analysis
Each hen in an individual cage acted as the experimental unit for the response variables measured. Within each treatment, data were checked for outliers. Outliers were not automatically rejected but were checked for plausibility. The data obtained for all variables measured (CP and CF digestibility values, AMEn, egg quality parameters, total yolk lipids, and % fatty acids) were analyzed using one-way ANOVA and JMPro 15 software (SAS Institute, Inc., Cary, NC). Means were compared using the Student's t test and were considered significantly different at P < 0.05.
RESULTS
While all four experimental diets were formulated to be isocaloric (2,927 kcal/kg) and isonitrogenous (18.5 % crude protein), the crude fat was lowest in Treatment 4 (Table 2). The calculated AMEn values were very similar between the four experimental diets (P = 0.4206). Dietary treatment 4 had the lowest saturated fatty acid values relative to the other dietary treatments. Dietary treatment 4 also had the lowest omega 3, linoleic, and omega 6 values relative to the other dietary treatments. Oleic acid and total omega 9 values were highest in dietary treatment 4 (Table 2).
Table 2.
Chemical analysis of experimental layer diets1.
| Analyzed values | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 |
|---|---|---|---|---|
| Crude fat2, % | 5.28 | 5.76 | 4.63 | 6.09 |
| Crude protein, % | 19.06 | 18.21 | 17.16 | 17.30 |
| Gross energy, kcal/kg | 3,613 | 3,645 | 3,685 | 3,510 |
| AMEn, kcal/kg | 2,578 | 2,518 | 2,524 | 2,622 |
| *Palmitic (C16:0), % | 11.2 | 11.03 | 11.15 | 7.72 |
| *Stearic (C18:0), % | 3.58 | 3.56 | 3.42 | 2.94 |
| *Saturated fat, % | 15.95 | 15.83 | 15.79 | 12.02 |
| *Omega 3 fatty acids, % | 7.47 | 7.7 | 7.74 | 2.66 |
| *Omega 6 fatty acids, % | 55.06 | 54.9 | 55.15 | 11.46 |
| *Omega 9 fatty acids, % | 21.01 | 21.08 | 20.82 | 73.33 |
| *Trans fats, % | 0.02 | 0.02 | 0.05 | 0.02 |
| *Oleic acid (C18:1), % | 20.54 | 20.65 | 20.36 | 72.87 |
| *Linoleic (C18:2), % | 54.94 | 54.75 | 54.99 | 11.37 |
Dietary treatments: Treatment 1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment 2-EENO = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment 3-FFNO = diet containing full-fat normal-oleic soybean meal and corn; Treatment 4 -FFHO = diet containing full-fat high-oleic soybean meal and corn. The four dietary treatments were chemically analyzed by an AOAC-certified lab (ATC Scientific, Little Rock, AR) using standard AOAC-approved methods.
Crude Fat content = g crude fat/g total sample weight × 100.
Fatty acid content = g of fatty acid/g total lipid content × 100.
There were no significant differences between treatments in the following hen performance parameters: initial body weights, final body weights, feed conversion ratio (FCR), average hen house egg production, or average daily egg weights (Table 3). Interestingly, hens of Treatment 2 consumed significantly less feed as compared to the control (P < 0.01), which was not reflected in differences in FCR between the treatment groups. There were no significant treatment differences in the egg quality parameters measured: egg weights, Haugh unit (HU), albumen height, shell color or shell strength (Table 4). However, yolk color was significantly darker in eggs produced by hens fed Treatment 1 and Treatment 2 compared to Treatment 3 and Treatment 4 (P < 0.05).
Table 3.
Comparative body weights of hens fed a full-fat high-oleic soybean meal diet for 3 weeks.
| Treatments1 | BW Wk0 | BW Wk3 | Mean daily feed consumption2 | FCR3 | Mean hen house egg production4 (%) | Mean daily egg Wt5 (g) |
|---|---|---|---|---|---|---|
| Treatment 1 | 1568 | 1693 | 109.0a | 1.727 | 92.71 | 63.16 |
| Treatment 2 | 1565 | 1628 | 96.34b | 1.601 | 96.35 | 60.20 |
| Treatment 3 | 1589 | 1660 | 101.1ab | 1.660 | 93.75 | 60.98 |
| Treatment 4 | 1618 | 1695 | 102.5ab | 1.641 | 94.27 | 62.52 |
| SEM | 46.7 | 45.9 | 3.144 | 0.046 | 2.292 | 1.383 |
| P-value⁎ | 0.656 | 0.417 | 0.003 | 0.065 | 0.451 | 0.136 |
Four experimental isonitrogenous (18.5% crude protein) diets were formulated: Trmt1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Trmt2-EENO = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full-fat normal-oleic soybean meal and corn; Trmt4-FFHO = diet containing full-fat high-oleic soybean meal and corn
Average Daily Feed Consumption = amount of feed consumed per week by each treatment group divided by 7 days.
Feed conversion ratio (FCR) = total egg weights (in grams) for each treatment/g total feed consumed by that treatment group over the 3-week feeding trial.
Mean Hen House Egg Production = total number of eggs produced divided by total number of hens present over the 3-week feeding trial.
Mean Daily Egg Weight = average mass of the eggs produced per week by each treatment group divided by 7 days.
Means within the same column lacking a common superscript differ significantly (P < 0.05).
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Table 4.
Comparative egg quality of eggs produced from hens fed full-fat high-oleic soybean meal diet.
| Treatments1 | Egg Wt (g) | Haugh Unit (HU) | Albumen Ht (mm) | Shell color (%) | Shell Sth (g force) | Yolk color (1-15) |
|---|---|---|---|---|---|---|
| Treatment 1 | 61.2 | 92.1 | 8.66 | 81.8 | 4936 | 8.50a |
| Treatment 2 | 59.4 | 91.6 | 8.46 | 82.3 | 5285 | 8.75a |
| Treatment 3 | 59.4 | 96.1 | 9.39 | 83.9 | 4772 | 7.83b |
| Treatment 4 | 61.2 | 94.6 | 9.11 | 81.8 | 4432 | 7.83b |
| SEM | 1.15 | 1.89 | 0.398 | 0.689 | 229 | 0.248 |
| P-value⁎ | 0.495 | 0.332 | 0.364 | 0.117 | 0.081 | 0.022 |
Dietary treatments: Trmt1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Trmt2-EENO = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO=diet containing full fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, 12 eggs were collected from each treatment group for quality assessment using Technical Services and Supplies QCD system, with calibration with the DSM Color Fan for yolk color. Egg wt, egg weight; HU, Haugh Unit; Albumen Ht, albumen height; Shell Sth, shell strength and Yolk color = index 1-15 (lightest to darkest color intensity).
Means within the same column lacking a common superscript differ significantly (P < 0.05).
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Eggs produced by hens fed Treatment 4 had significantly lower palmitic and stearic saturated fatty acid levels compared to the other treatment groups (Table 5, P < 0.0001). Also, eggs produced from hens fed Treatment 4 had significantly lower levels of linoleic and linolenic acid compared to other treatments (P < 0.0001). As expected, eggs produced from hens fed the full-fat high-oleic soybean meal (Treatment 4) had the highest level of oleic acid relative to the other treatment groups (P < 0.0001). There were no significant differences in crude fat or pentadecanoic acid levels in eggs between the treatment groups (Table 5) or between liver samples collected from each of the treatment groups (Table 6).
Table 5.
Comparative lipid and fatty acid analysis of eggs produced from hens fed a full-fat high-oleic soybean meal diet.
| Treatments1 |
||||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | SEM | P-value* | |
| Crude fat, %2 | 34.00 | 33.31 | 33.14 | 33.47 | 0.348 | 0.685 |
| Palmitic % (C16:0) | 24.16a | 23.32b | 23.20b | 22.05c | 0.137 | <0.0001 |
| Stearic % (C18:0) | 9.77a | 9.73a | 10.13a | 7.56b | 0.193 | <0.0001 |
| Oleic % (C18:1) | 35.96b | 35.32bc | 34.80c | 50.67a | 0.28 | <0.0001 |
| Linoleic % (C18:2) | 21.0c | 22.51b | 23.25a | 11.32d | 0.243 | <0.0001 |
| Linolenic % (C18:3) | 0.943b | 1.135a | 1.108a | 0.427c | 0.353 | <0.0001 |
| Pentadecanoic % (C15:0) | 0.063 | 0.073 | 0.065 | 0.070 | 0.017 | 0.929 |
Dietary treatments: Treatment 1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, 12 eggs were collected from each treatment group for chemical analysis.
Crude Fat content = g crude fat/g total sample weight × 100; Fatty acid content = g of fatty acid/g total lipid content × 100.
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Means within the same row lacking a common superscript differ significantly (P < 0.05).
Table 6.
Comparative lipid and fatty acid analysis of liver produced from hens fed a full-fat high-oleic soybean meal diet.
| Treatments1 |
||||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | SEM | P-value* | |
| Crude Fat, %2 | 9.718 | 8.772 | 7.853 | 10.327 | 0.969 | 0.318 |
| Palmitic % (C16:0) | 22.80a | 22.19a | 21.57a | 20.39b | 0.574 | 0.003 |
| Stearic % (C18:0) | 11.48a | 11.75a | 12.61a | 9.903b | 0.436 | <0.0001 |
| Oleic % (C18:1) | 35.29b | 35.16b | 31.62c | 47.53a | 1.20 | <0.0001 |
| Elaidic % (C18:1trans) | 0 | 0 | 0 | 0 | 0 | N/A |
| Linoleic % (C18:2) | 19.67a | 20.73a | 22.38a | 11.03b | 1.093 | <0.0001 |
| Linolenic % (C18:3) | 0.727a | 0.825a | 0.910a | 0.248b | 0.087 | <0.0001 |
| Pentadecanoic % (C15:0) | 0.060 | 0.063 | 0.063 | 0.042 | 0.024 | 0.772 |
Dietary treatments: Treatment 1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, liver, fat pad, and Pectoralis major breast muscle samples (6 per treatment) were collected for chemical analysis.
Crude Fat content = g crude fat/g total sample weight × 100; Fatty acid content = g of fatty acid/g total lipid content × 100.
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Means within the same row lacking a common superscript differ significantly (P < 0.05).
In parallel, saturated stearic (P < 0.0001) and palmitic (P < 0.01) acid levels were significantly lower in liver samples collected from Treatment 4 hens. Also, linoleic and linolenic acid levels were lowest in liver samples from hens of Treatment 4 (P < 0.0001). Oleic acid content was highest in liver samples collected from Treatment 4 hens (P < 0.0001).
There were no significant treatment differences in crude fat, palmitic acid, or stearic acid levels in the Pectoralis major muscle (Table 7). Pectoralis major muscle samples from hens fed Treatment 4 had significantly lower linoleic acid (P < 0.01) and linolenic acid (P < 0.05) levels in comparison to samples collected from Treatment 3, but the levels were similar to other treatment groups (Table 7). Pentadecanoic acid levels were below the detection level in muscle samples collected from Treatment 2 and Treatment 3, while pentadecanoic acid levels were very low (<0.125%) in the other treatment groups (P < 0.05).
Table 7.
Comparative lipid and fatty acid analysis of Pectoralis major muscle produced from hens fed a full-fat high-oleic soybean meal diet.
| Treatments1 |
||||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | SEM | P-value* | |
| Crude fat, %2 | 1.248 | 1.0717 | 1.2567 | 1.295 | 0.0079 | 0.232 |
| Palmitic % (C16:0) | 20.15 | 20.40 | 20.10 | 19.87 | 0.6451 | 0.880 |
| Stearic % (C18:0) | 7.64 | 6.95 | 6.86 | 6.70 | 0.025 | 0.068 |
| Oleic % (C18:1) | 26.15b | 25.77b | 26.255b | 31.742a | 0.92 | 0.0004 |
| Elaidic % (C18:1trans) | 0 | 0 | 0 | 0 | 0 | N/A |
| Linoleic % (C18:2) | 24.76ab | 23.81b | 26.88a | 22.78b | 1.056 | 0.007 |
| Linolenic % (C18:3) | 1.053ab | 1.076ab | 1.378a | 0.999b | 0.129 | 0.034 |
| Pentadecanoic % (C15:0) | 0.098 | 0 | 0 | 0.037 | 0.129 | 0.033 |
Dietary treatments: Treatment 1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, liver, fat pad, and Pectoralis major breast muscle samples (6 per treatment) were collected for chemical analysis.
Crude Fat content = g crude fat/g total sample weight × 100; Fatty acid content = g of fatty acid/g total lipid content × 100.
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Means within the same row lacking a common superscript differ significantly (P < 0.05).
There were no significant differences in crude fat or stearic acid levels in fat pad samples between the groups (Table 8). Palmitic saturated fatty acid levels were lower in fat pads from hens fed Treatment 4 compared to the control and Treatment 1 but were similar to the other treatment groups (P < 0.05). Oleic acid levels were the highest in fat pad samples collected from hens fed Treatment 4 compared to the other treatment groups (P < 0.01). Linoleic and linolenic acid levels were lowest in fat pads collected from Treatment 4 hens relative to all other treatment groups (P < 0.01). Pentadecanoic acid levels were highest in fat pad samples from hens fed Treatment 3 and Treatment 4 as compared to samples from Treatment 1 and Treatment 2 (P < 0.01).
Table 8.
Comparative lipid and fatty acid analysis of fat pad produced from hens fed a full-fat high-oleic soybean eal diet.
| Treatments1 |
||||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | SEM | P-value* | |
| Crude Fat, %2 | 88,8 | 90.4 | 80.2 | 85.0 | 3.43 | 0.187 |
| Palmitic % (C16:0) | 15.90a | 14.64ab | 14.76ab | 14.27b | 0.3752 | 0.034 |
| Stearic % (C18:0) | 5.231 | 5.162 | 5.221 | 4.776 | 0.2816 | 0.342 |
| Oleic % (C18:1) | 36.6b | 39.3b | 38.0b | 45.7a | 1.76 | 0.003 |
| Elaidic % (C18:1trans) | 0 | 0 | 0 | 0 | 0 | N/A |
| Linoleic % (C18:2) | 35.99a | 35.05a | 35.85a | 29.64b | 1.09 | 0.001 |
| Linolenic % (C18:3) | 2.187a | 2.081ab | 2.297a | 1.525b | 0.141 | 0.005 |
| Pentadecanoic % (C15:0) | 0.069b | 0.070b | 0.089a | 0.092a | 0.005 | 0.002 |
Dietary treatments: Treatment 1-Control = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, liver, fat pad, and Pectoralis major breast muscle samples (6 per treatment) were collected for chemical analysis.
Crude Fat content = g crude fat/g total sample weight × 100. Fatty acid content = g of fatty acid/g total lipid content × 100.
P-value = statistically significant differences are identified by P < 0.05 in analysis of variance (ANOVA).
Means within the same row lacking a common superscript differ significantly (P < 0.05).
There were no significant treatment differences in tibial bone bending moment or tibial peak force (Table 9). Moreover, there were no significant differences between treatments in the following blood chemistry measurements: pH, pO2, SO2, Na+, K+, glucose, hematocrit, or hemoglobin (data not shown). Also, there were no significant differences in blood TCO2 or HCO3− between treatments. Blood calcium was significantly higher in hens fed Treatment 3 relative to the other treatment groups (P < 0.01). Blood pCO2 was higher in hens fed Treatment 3 relative to Treatment 4, while similar to levels in hens of Treatment 1 and Treatment 2 (P < 0.05).
Table 9.
Comparative bone and blood analysis of fat pad produced from hens fed a full-fat high-oleic soybean meal diet.
| Treatments1 | Bone |
Blood |
||||
|---|---|---|---|---|---|---|
| Bending moment (kg/m2) | Peak force (g) | pCO2 (mm Hg) | HCO3 (mEq/L) | TCO2 (mEq/L) | iCa2+ (mmol/L) | |
| Treatment 1 | 0.050 | 15.4 | 34.4ab | 23.8 | 25.0 | 1.59b |
| Treatment 2 | 0.055 | 16.0 | 31.0ab | 23.1 | 24.0 | 1.57b |
| Treatment 3 | 0.056 | 17.3 | 38.4a | 27.6 | 28.7 | 1.88a |
| Treatment 4 | 0.049 | 15.2 | 29.6b | 23.0 | 23.7 | 1.48b |
| SEM | 0.004 | 1.31 | 2.53 | 1.63 | 1.62 | 0.067 |
| P-value | 0.214 | 0.418 | 0.032 | 0.061 | 0.051 | 0.002 |
Dietary treatments: Treatment 1-Contro l = conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four isocaloric, isonitrogenous dietary treatments (12 replicate cages/treatment). At termination, tibial bone and blood samples were collected for analysis using IACUC-approved methods. Tibia bone strength was measured in terms of bending moment (kg/m2) and peak force (kg) and was not different between treatment diets (P < 0.05).
Means within the same column lacking a common superscript differ significantly (P < 0.05). Blood parameters measured: HCO3 = bicarbonate, pCO2 = partial pressure of carbon dioxide, TCO2 = total carbon dioxide, iCa2+ = ionized calcium.
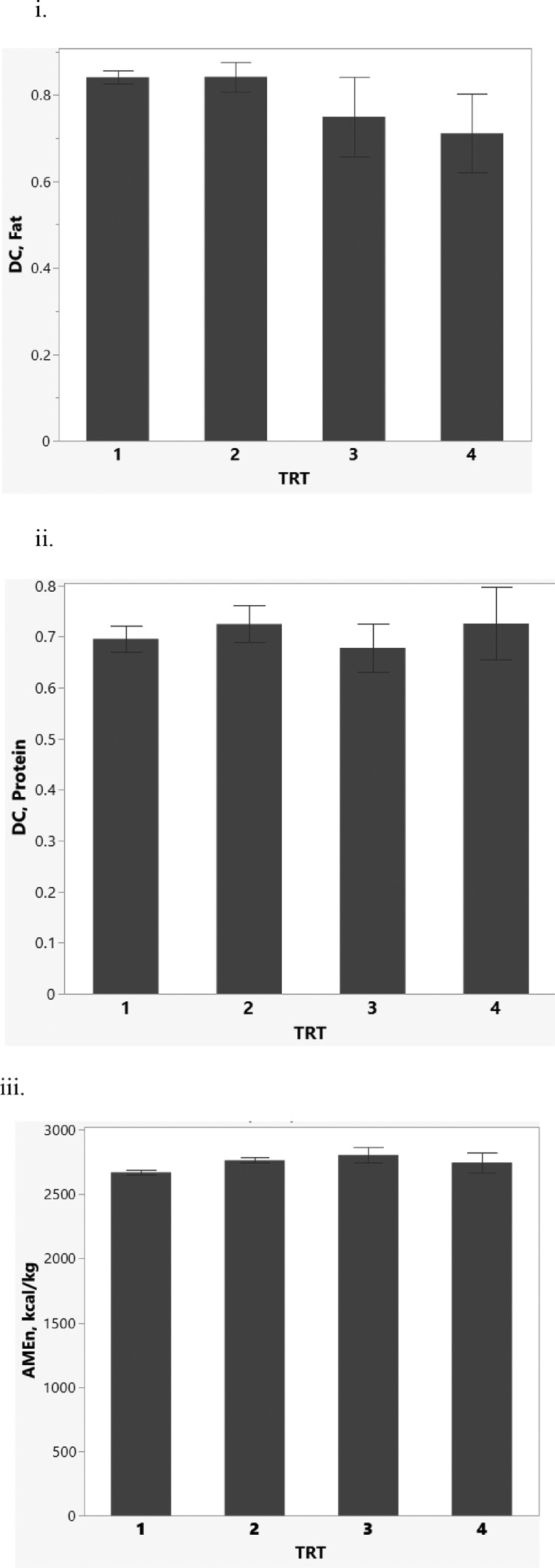
There were no significant dietary treatment differences (P > 0.05) in digestibility coefficients for crude fat (Figure 1. i.), crude protein (Figure 1.ii.), or apparent metabolizable energy corrected for nitrogen (Figure 1.iii.). This implies that all experimental diets provided similar levels of dietary fat, protein, and energy.
Figure 1.
Apparent ileal nutrient digestibility of hens for treatment (TRT) diets1. Digestibility coefficients (DC) for crude fat (i) and crude protein (CP) (ii) were not different (P > 0.05) between treatment diets. Nitrogen-corrected apparent metabolizable energy (AMEn) values for the diets were not different (P > 0.05). 1Four isonitrogenous, isocaloric treatments with a 2% inclusion of Celite were fed to: Treatment 1-Control=conventional diet containing solvent-extracted defatted soybean meal and corn; Treatment2 = diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Treatment3 = diet containing full-fat normal-oleic soybean meal and corn; Treatment4 = diet containing full-fat high-oleic soybean meal and corn. Forty-eight White Shaver hens were individually housed and randomly assigned to one of four dietary treatments (12 replicate cages/treatment). Fecal samples were collected for each individual bird, and ileal contents and feed samples were collected for analysis using standard methodologies. Each bar represents the average ± standard error of the mean. No differences (P > 0.05) were observed between treatments. ANo differences were observed in fat digestibility between treatment diets (P > 0.05). No differences were observed in protein digestibility between treatment diets (P > 0.05). Dietary apparent metabolizable energy was not different (P > 0.05) between treatment diets.
DISCUSSION
The US Poultry industry utilizes approximately 67% of US commercial defatted soybean meal annually (American Soybean Association, 2020). Soybean meal is a primary protein supplement ingredient in typical North American poultry diets. It contains large quantities (∼ 45% of total amino acids) of essential amino acids (Bernard, 2016). However, this study investigated the performance impacts of differences in lipid profile of SBM in layer hen diets originated from variously processed (defatted versus full fat) soybean cultivars (normal or high oleic acid). More specifically, the research explored the potential utilization of full-fat HO soybean meal as an ingredient in layer hen diets to understand its impact on hen performance, nutrient digestibility, and fatty acid egg enrichment.
Protein and fat digestibility values for high-oleic acid ingredients in poultry diets could be affected by the ingredient inclusion rates and poultry species. Studies testing the utilization by broiler chickens of high-oleic acid sunflower seed oil at various inclusion rates showed a negative effect of high oleic acid levels on protein and fat digestibility, and thus the performance (Rodriguez et al., 2005; Brenes et al., 2008). Another study with broilers varied the oleic acid content in peanuts and found no differences in protein and fat digestibility (Toomer et al., 2020a). Poultry feeding trials in layer hens with whole, unblanched, high-oleic peanuts demonstrated that eggs produced from layers fed high-oleic peanuts had a roughly 2-fold increase in yolk color (P < 0.0001), β-carotene (P < 0.0001), and oleic fatty acid (P < 0.0001) content. There was also a reduction in saturated and trans-fatty acids (P < 0.0001) in eggs and chicken breast produced by birds fed whole high-oleic peanuts (Toomer et al., 2019,2020b). These results were very promising, but there was a paucity of published feeding trials with poultry to determine the effects of full-fat, HO soybean meal on performance, nutritional value, and quality of eggs produced.
The findings of this study support the potential use of full-fat, HO soybean meal as a feed ingredient to enhance performance of food animals and enrich the nutritional value of the products produced for human consumption. This study also demonstrated that fatty acid composition of the diet of laying hens could be reflected in fatty acid composition of body tissue, such as in muscle and liver, and in eggs. Eggs from hens fed the HO soybean meal diet had a significantly higher amount of oleic acid compared to other treatment diets, which that could potentially provide cardiovascular health benefits. Moreover, consumers will benefit from the expansion of the use of high-oleic soybean cultivars for oil extraction given that high-oleic soybean oil has zero transfats and 20% less saturated fat than conventional soybean oil (Successful Farming, 2017). Interestingly, most conventional soybean oil must be hydrogenated to extend product shelf-life. However, in 2018 the US Food and Drug Administration banned partially hydrogenated oils that contain artificial trans-fats in food products, leading to an approximately 4-billion-pound loss of soy oil demand annually (Successful Farming, 2017).
Overall, the results of this study exhibited that the monounsaturated oleic acid profile in eggs was significantly improved without impacting other egg quality parameters by including full-fat HO SBM in layer diets. Additionally, the digestibility for CF and CP in this diet were similar to layer diets with defatted SBM or normal-oleic full-fat SBM. The findings of this study parallel a period of great expansion of the soybean cultivars. The resulting release of numerous soybean lines with the HO phenotypic trait will greatly influence future uses of these new cultivars within animal production (including poultry) and will have a direct economic impact on US Soybean producers, the processing industry, the animal production industry, and human health.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following: The United States Soybean Board for funding for this project (1930-362-0618), Mr. Philip Lobo and the US Soybean Board-Animal Nutrition Working group for providing their guidance and leadership, Dr. Muquarrab Qureshi, Location Coordinator, ARS Raleigh Location & Research Leader, FSMQH for administrative support and guidance, the Soybean & Nitrogen Fixation Unit for the production and donation of the soybean cultivars, the Animal & Poultry Waste Processing Facility for processing of soybean cultivars, the Staff of the Prestage Department of Poultry Science, the NC State University Feed Mill, and Chicken Education Unit for assistance with animal care & husbandry and feed manufacturing, and the FSMQH-ARS for their contributions to this study. This work was also supported by appropriated funds from the Agricultural Research Service, US Department of Agriculture (CRIS 6070-43440-013-00D).
DISCLOSURES
The authors have no conflicts of interest to report.
Footnotes
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture or North Carolina Agricultural Research Service, nor does it imply approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
References
- American Soybean Association, 2020. The global rise of protein: U.S. Soy's growth through the next decade. Accessed online at: https://soygrowers.com/the-global-rise-of-protein-u-s-soys-growth-through-the-next-decade/. Accessed July 20, 2022.
- Bernard, J.K. (2016). Oilseed and oilseed meals. Reference module in food science.
- Braddock J.C., Sims C.A., O'keefe S.F. Flavor and oxidative stability of roasted high oleic acid peanuts. J. Food. Sci. 1995;60:489–493. [Google Scholar]
- Brenes A., Centeno C., Viveros A., Arija I. Effect of enzyme addition on the nutritive value of high oleic acid sunflower seeds in chicken diets. Poult. Sci. 2008;87:2300–2310. doi: 10.3382/ps.2008-00130. [DOI] [PubMed] [Google Scholar]
- Erdaw M.M., Perez-Maldonado R.A., Bhuiyan M., Iji P.A. Physicochemical properties and enzymatic in vitro nutrient digestibility of full-fat soybean meal. J. Food. Agric. Environ. 2016;14:85–91. [Google Scholar]
- Erdaw M.M., Perez-Maldonado R.A., Iji P.A. Apparent and standardized ileal nutrient digestibility of broiler diets containing varying levels of raw full-fat soybean and microbial protease. J. Anim. Sci. Technol. 2017;59:1–11. doi: 10.1186/s40781-017-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdaw M.M., Perez-Maldonado R.A., Iji P.A. Physiological and health-related response of broiler chickens fed diets containing raw, full-fat soya bean meal supplemented with microbial protease. J. Anim. Physiol. Anim. Nutr. 2018;102:533–544. doi: 10.1111/jpn.12785. [DOI] [PubMed] [Google Scholar]
- Folch J.M., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:495–509. [PubMed] [Google Scholar]
- Huang K.H., Li X., Ravindran V., Bryden W.L. Comparison of apparent ileal amino acid digestibility of feed ingredients measured with broilers, layers, and roosters. Poult. Sci. 2006;85:625–634. doi: 10.1093/ps/85.4.625. [DOI] [PubMed] [Google Scholar]
- Knowlton S. High. Oleic. Oils. AOCS Press; 2022. High-oleic soybean oil; pp. 53–87. [Google Scholar]
- Liu L., Iassonova D. High-oleic canola oils and their food applications. AOCS. Inform. 2012:9–11. [Google Scholar]
- Maharjan P., Mayorga M., Hilton K., Weil J., Beitia A., Caldas J., England J., Coon C. Non-cellulosic polysaccharide content in feed ingredients and ileal and total tract non-cellulosic polysaccharide digestibility in 21-and 42-day-old broilers fed diets with and without added composite enzymes. Poult. Sci. 2019;98:4048–4057. doi: 10.3382/ps/pez079. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.L., Ortiz L.T., Alzueta C., Rebole A., Trevino J. Nutritive value of high-oleic acid sunflower seed for broiler chickens. Poult. Sci. 2005;84(3):395–402. doi: 10.1093/ps/84.3.395. [DOI] [PubMed] [Google Scholar]
- Scarth R., McVetty P.B. Designer oil canola–a review of new food-grade Brassica oils with focus on high oleic, low linolenic types. In Proc. 10th International Rapeseed Congress, Canberra, Australia (pp. 26-29); Canberra, Australia; 1999. [Google Scholar]
- Soystats, 2022. U.S. Soybean Meal: Use by Livestock 2020/2021 Marketing Year. USB Soybean Meal Demand Assessment Reports.
- Successful Farming . 2017. High-Oleic Soybeans Have Promise-A New Horizon for Soybeans is on the way via High-Oleic Soybean Oil.https://www.agriculture.com/crops/soybeans/high-oleic-soybeans-have-promise Available online at. Accessed July 20, 2022. [Google Scholar]
- Toomer O.T., Hulse-Kemp A.M., Dean L.L., Boykin D.L., Malheiros R., Anderson K.E. Feeding high-oleic peanuts to layer hens enhances egg yolk color and oleic fatty acid content in shell eggs. Poult. Sci. 2019;98:1732–1748. doi: 10.3382/ps/pey531. [DOI] [PubMed] [Google Scholar]
- Toomer O.T., Livingston M., Wall B., Sanders E., Vu T., Malheiros R.D., Livingston K.A., Carvalho L.V., Ferket P.R., Dean L.L. Feeding high-oleic peanuts to meat-type broiler chickens enhances the fatty acid profile of the meat produce. Poult. Sci. 2020;99:2236–2245. doi: 10.1016/j.psj.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomer O.T., Sanders E., Vu T.C., Malheiros R.D., Redhead A.K., Livingston M.L., Ferket P.R. The effects of high-oleic peanuts as an alternative feed ingredient on broiler performance, ileal digestibility, apparent metabolizable energy, and histology of the intestine. Transl. Anim. Sci. 2020;4:txaa137. doi: 10.1093/tas/txaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Soybean Board, 2020. Soybean meal: the one-stop solution for nutrition. Available online at: https://www.unitedsoybean.org/hopper/soybean-meal-the-one-stop-solution-for-nutrition/. Accessed July 19, 2022.
- Vuilleumier J.P. The ‘Roche yolk colour fan’—An instrument for measuring yolk colour. Poult. Sci. 1969;48:767–779. [Google Scholar]
- Weld K.A., Armentano L.E. Feeding high oleic acid soybeans in place of conventional soybeans increases milk fat concentration. J. Dairy. Sci. 2018;101:9768–9776. doi: 10.3168/jds.2018-14498. [DOI] [PubMed] [Google Scholar]